Abstract
Sustainable food production is a grand challenge facing the global economy. Traditional agricultural practice requires numerous interventions, such as application of nutrients and pesticides, of which only a fraction are utilized by the target crop plants. Controlled release systems (CRSs) designed for agriculture could improve targeting of agrochemicals, reducing costs and improving environmental sustainability. CRSs have been extensively used in biomedical applications to generate spatiotemporal release patterns of targeted compounds. Such systems protect encapsulant molecules from the external environment and off-target uptake, increasing their biodistribution and pharmacokinetic profiles. Advanced ‘smart’ release designs enable on-demand release in response to environmental cues, and theranostic systems combine sensing and release for real-time monitoring of therapeutic interventions. This review examines the history of biomedical CRSs, highlighting opportunities to translate biomedical designs to agricultural applications. Common encapsulants and targets of agricultural CRSs are discussed, as well as additional demands of these systems, such as need for high volume, low cost, environmentally friendly materials and manufacturing processes. Existing agricultural CRSs are reviewed, and opportunities in emerging systems, such as nanoparticle, ‘smart’ release, and theranostic formulations are highlighted. This review is designed to provide a guide to researchers in the biomedical controlled release field for translating their knowledge to agricultural applications, and to provide a brief introduction of biomedical CRSs to experts in soil ecology, microbiology, horticulture, and crop sciences.
1 Introduction
Biomedical controlled release systems (CRSs) have made significant advances since their introduction ∼70 years ago (Ullyot et al., 2000). Biomedical CRSs can be manufactured from a variety of materials with controlled release spanning hours to years. They can be programmed to release on demand, at a set rate, or in response to changing environmental conditions. Manufacturing methods for these materials are well established, and several formulations have reached the clinic (Moghanjoughi et al., 2016). Meanwhile, there is a growing and compelling need for sustainable food production, highlighted by the National Academies as one of the ten grand challenges for the 21st century (National_Academies_of_Sciences_Engineering_and_Medicine, 2019). We believe the time is right to translate learnings from biomedical CRSs to agriculture. This review discusses the potential for CRSs in agriculture. We begin by providing a brief history of biomedical CRSs detailing similarities and differences between agricultural CRS engineering design criteria. Then, we outline the need for CRSs in agriculture, including the types of organisms and encapsulants to be targeted. We discuss the challenges that must be met by agricultural CRSs and describe opportunities to meet these challenges by leveraging knowledge gained from biomedical CRS systems. We then discuss existing agricultural CRSs, including common materials and manufacturing methods. We conclude by highlighting the rich opportunities for growth, particularly in nanoparticle, ‘smart’ release, and theranostic agricultural CRS formulations.
2 Biomedical controlled release systems
2.1 History of controlled drug delivery systems
Biomedical CRSs were first developed to increase therapeutic efficacy and safety by controlling the time, place, and rate of drug release (Jain and Jain, 2008). The history of CRSs traces its origins to 1952 with Smith, Kline & French’s Spansule, sustained release capsule technology that used granules with different dissolution rates to achieve release over 12 h (Ullyot et al., 2000). In 1964, Dr. Judah Folkman published the first article on “zero order” reservoir drug delivery systems that released drugs at a constant rate (Folkman and Long, 1964). As head of Alza Corp’s Scientific Advisory Board, Folkman (and others) would usher in the era of macroscopic CRSs throughout the 1970’s and 1980’s (Hoffman, 2008). These macroscopic devices exhibited constant drug release profiles and included intrauterine devices (IUDs), ocular inserts, and transdermal patches. In the mid to late 1980’s, the CRS field expanded to include drug-loaded, biodegradable polymer microparticles based on polyesters (Hoffman, 2008). These degradable microparticles exhibited sustained delivery rates that gradually decreased with time. These technologies have paved the way for nanomedicine CRSs, such as micelles, liposomes, and dendrimers (Bobo et al., 2016).
In this review, we will confine our discussion to polymers and polymer hydrogels used in biomedical CRSs with potential for crossover utilization in agriculture, later highlighting materials unique to agricultural CRSs. These systems can undergo the zero order release of Folkman (i.e., passive release) or follow degradational release patterns of microparticles (Figure 1). Additionally, release may be initiated by a specific environmental cue, so-called “smart” or stimuli-responsive release systems. These designs in particular would have advantages for agricultural CRSs, enabling release in response to plant, pathogen, or beneficial microbe signals.
FIGURE 1
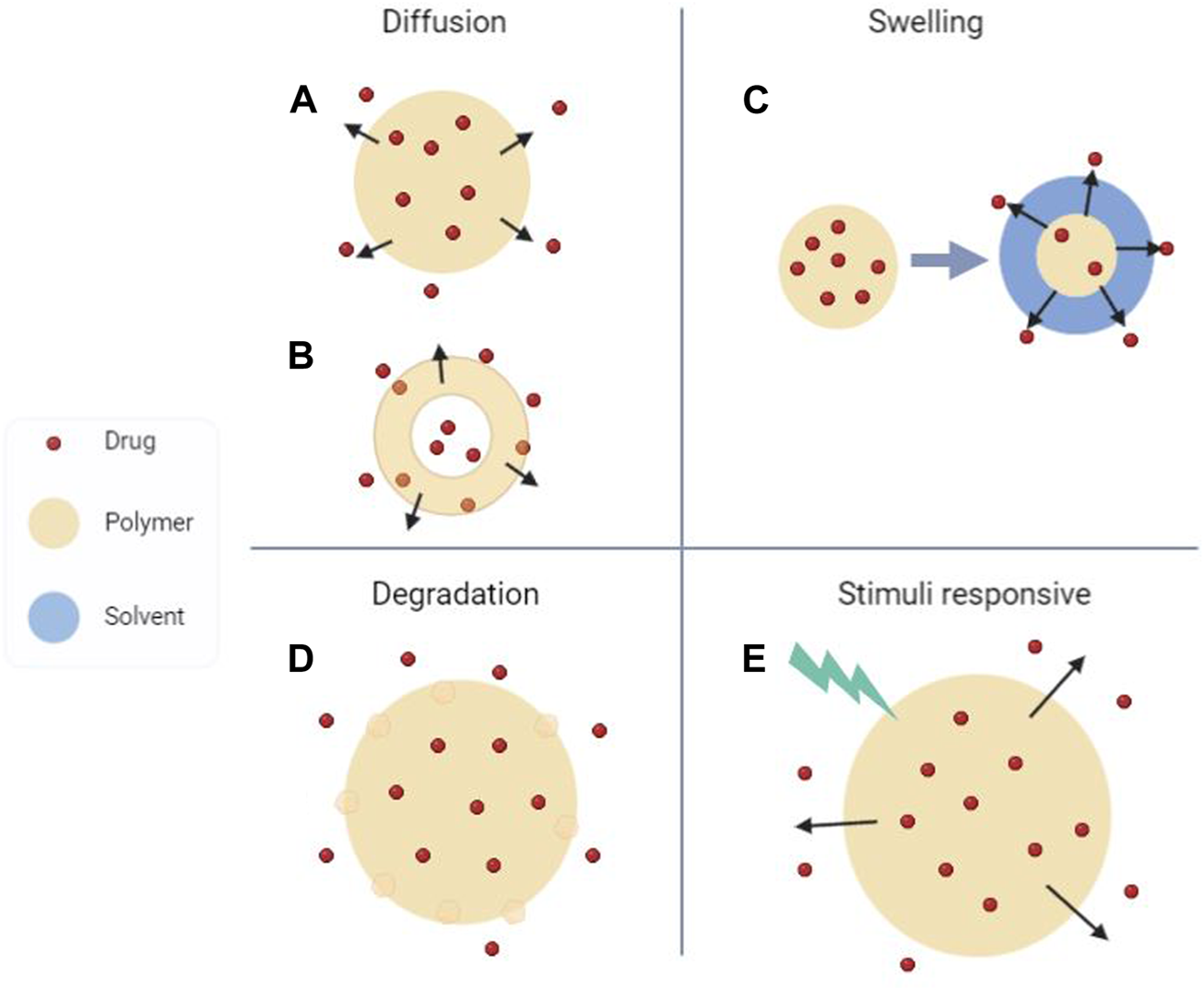
Categorization of controlled release systems (CRSs) according to release mechanism. (A and B) Diffusion controlled systems can be (A) matrix or (B) reservoir based. (C) Polymer matrices can swell to release drugs. (D) Chemical degradation leads to release via surface erosion. (E) Stimuli-triggered “smart” release systems rely on external or environmental cues such as temperature, pH, and enzymatic activity to alter matrix properties. Created using BioRender.
2.2 Release by solute diffusion and polymer matrix swelling
CRSs comprised of non-degradable polymers can be categorized as either “reservoir” or “matrix” devices (Figures 1A,B). The most common release mechanism for these systems is diffusion (Fu and Kao, 2010). Reservoir devices typically are comprised of an inert coating that acts as a rate-controlling membrane against drug diffusion. As such, the thickness and permeability of the membrane dictates the drug release rate. This design is similar to agricultural seed coatings. Matrix devices consist of a polymer matrix in which the drug is either dissolved or dispersed. The release rate decreases with time, and is controlled by Fickian diffusion (Crank, 1975) based on drug concentration gradient and diffusivity (Liechty et al., 2010). In devices for which the encapsulant concentration far exceeds the solubility limit, zero order, constant release rates can be achieved until the concentration falls below this limit (Higuchi, 1961).
Non-degradable polymers can also exhibit drug release via swelling mechanisms (Figure 1C). Hydrogels are hydrophilic polymer networks capable of swelling with large amounts of water to release an entrapped drug. In these designs, water-soluble drugs are initially loaded into dry hydrogels in the glassy state. Drug release is then triggered by the addition of aqueous media that rapidly swells the matrix. As the hydrogel swells, it undergoes a transition between glassy and rubbery states, leading to polymer chain relaxation and drug diffusion into the surrounding media through simultaneous swelling-diffusion mechanisms (Lee, 1985; Liechty et al., 2010). This particular design could be attractive for translation to agricultural CRS as it would permit CRS transport in a non-hydrated state, which could enhance ease of shipping.
2.3 Release based on degradation
One of the most popular methods of drug release relies on controlled degradation of cross-links within the matrix (Figure 1D), typically through hydrolysis or enzymolysis (Treiser et al., 2013). Whereas all polymers degrade over time, polymers classified as degradable polymers exhibit degradation within the timeframe of their expected shelf life (Treiser et al., 2013). In this context, the terms degradation and erosion are often used interchangeably; however, it should be noted that degradation differs from erosion in that it involves changes to the chemical structure, whereas erosion involves physical changes in the shape and size of the matrix (Treiser et al., 2013).
Degradable polymers can be natural or synthetic, but synthetic polymers have been more widely studied because of the ability to engineer and predict their properties and control their batch-to-batch uniformity (Nair and Laurencin, 2007). The most commonly used degradable, synthetic polymers are polyesters: poly (glycolic acid) (PGA), poly (lactic acid) (PLA), and their combination poly (lactic-co-glycolic acid) (PLGA) (Figure 2). PGA represents the simplest linear, aliphatic polyester and has low solubility in organic solvents because of its high crystallinity (Vroman and Tighzert, 2009). Because of its excellent ability to form fibers, PGA was used to form the first biodegradable synthetic suture, DEXONTM, approved by the FDA in 1969 (Nair and Laurencin, 2007). However, PGA degrades very rapidly and generates acidic degradation products that limit its biomedical usage.
FIGURE 2
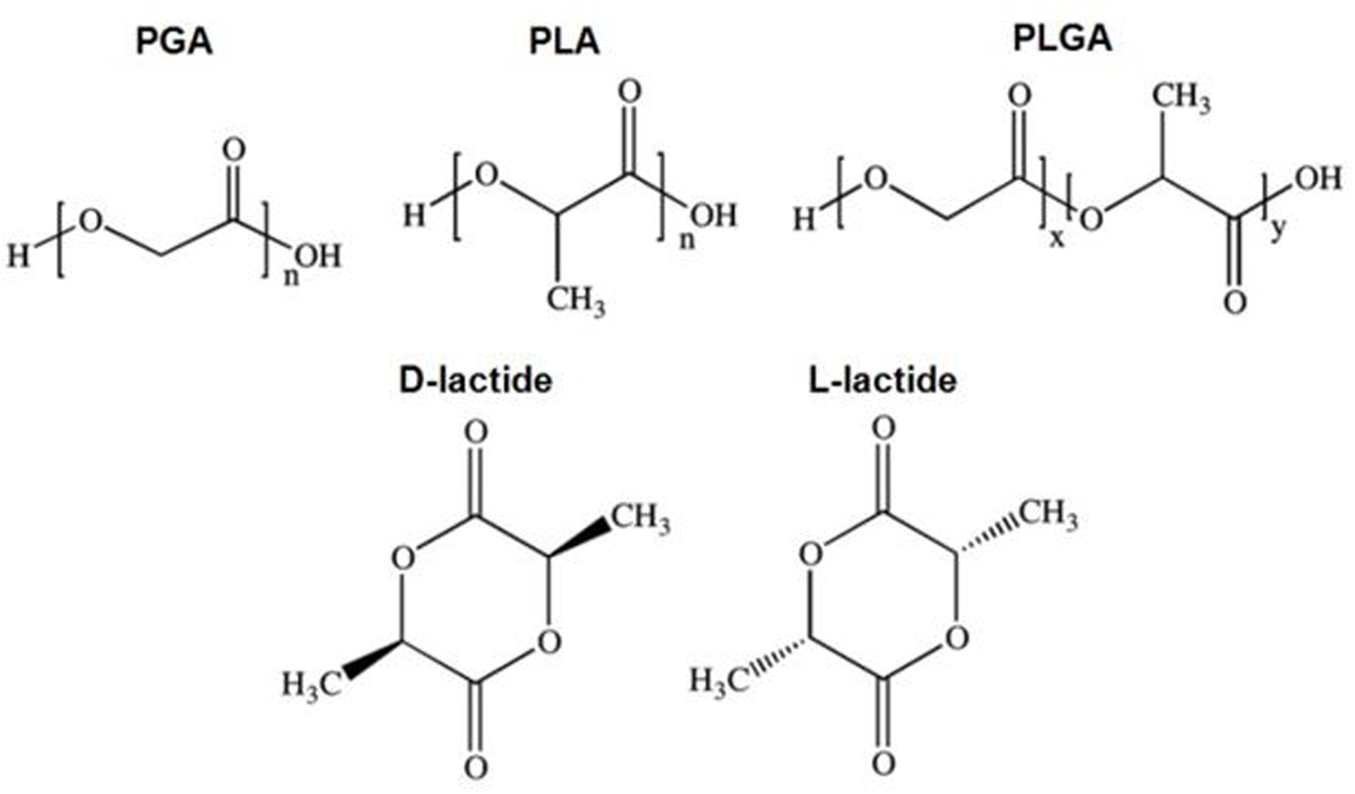
Chemical structures of polyglycolic acid (PGA), polylactic acid (PLA), and poly (lactic-co-glycolic acid) PLGA and its stereoisomers D-lactide and L-lactide. Reprinted with CC BY 4.0 permission from C. S Miranda, A. R. M Ribeiro, N. C Homem, H. P Felgueiras. Spun Biotextiles in Tissue Engineering and Biomolecules Delivery Systems. Antibiotics (2020) 174.
Unlike glycolic acid, lactic acid is a chiral molecule, meaning that it exists as two stereoisomers, D-lactide and L-lactide. Polymerization of D-lactide and L-lactide monomers forms semi-crystalline polymers, whereas polymerization of racemic or equimolar D,L-lactide forms amorphous polymers (Nair and Laurencin, 2007). In general, poly (L-lactic acid) (PLLA) is used over its pure D-lactide counterpart because its hydrolysis yields L-lactide, which is the naturally occurring isomer of lactic acid (Nair and Laurencin, 2007; Treiser et al., 2013). Semicrystalline PLLA exhibits good mechanical strength and toughness making it ideal for orthopedic devices, whereas the amorphous poly (DL-lactide) (PDLLA) is more suited for CRSs because it is of low strength and quicker degradation rate (Nair and Laurencin, 2007; Treiser et al., 2013).
To improve biomedical applicability of PGA, the copolymer poly (lactic-co-glycolic acid) (PLGA) combines features of PGA and PLA. Compared to PGA, PLA has methyl side groups making it more hydrophobic, thus reducing backbone hydrolysis compared to PGA. This has the effect of slowing degradation rate and subsequent drug release. The physico-mechanical properties of PLGA can be tuned by altering the ratio of lactic to glycolic acid, but the relationship is not linear (Jain, 2000). For example, 50:50 PLGA copolymers degrade much quicker than either PGA or PLA (Jain, 2000; Nair and Laurencin, 2007; Treiser et al., 2013). Whereas a PLGA co-polymer made of 10:90 LA:GA PLGA ratio has been used in resorbable suture designs (VicrylTM); PLGA has also been used for meshes and skin grafts (Jain, 2000). The ability to control degradation rates by tuning monomer ratios and polymer molecular weight has strongly contributed to the popularity of PLGA in the biomedical field, including drug delivery. In particular, increasing the proportion of lactic acid in PLGA increases copolymer hydrophobicity, which can be used to reduce water penetration and slow release rate or to better match drug hydrophobicity to increase drug encapsulation efficiency (Hines and Kaplan, 2013).
2.4 Smart release systems
The term “smart” release refers to stimuli-responsive materials that release target molecules in the presence of a specific biological or environmental cue, such as a change in pH (Falamarzian and Varshosaz, 1998), temperature (Chen and Hoffman, 1995), or the availability of a biomolecule (Mann et al., 2001). CRSs that respond to pH or temperature changes are typically based on polymer systems that undergo a phase transition in response to these cues, whereas biomolecular smart release is usually based on the presence of an enzyme cleaving a substrate in the CRS material. Smart release systems can improve drug targeting because release only occurs under certain conditions, improving therapeutic benefit while reducing systemic side effects (Moghanjoughi et al., 2016). A unique advantage of these materials is that release rates are often reversible. As materials cycle between swollen and collapsed states, release can be turned “on” and “off” (Moghanjoughi et al., 2016).
2.4.1 Temperature-responsive CRSs
Temperature responsive smart materials are typically polymers that exhibit changes in aqueous solubility in response to small alterations in temperature. Often these materials exhibit a lower or upper critical solution temperature (LCST or UCST) that demarks a rapid transition from a swollen to collapsed state (Yoshida et al., 1995). The most widely studied temperature responsive smart material is the polymer poly (N-isopropylacrylamide) (PNIPAm), which undergoes a reversible phase transition (LCST) in water around 32–34°C (Schild, 1992). Below the LCST the polymer is soluble and swollen and above the LCST it rapidly collapses (Dong and Hoffman, 1986), releasing encapsulants. With initial collapse, water and the drug are expelled in a burst, followed by a slower diffusion of the drug from the shrunken gel in what is defined as pulsatile release (Moghanjoughi et al., 2016). PNIPAm has been of particular interest for biomedical applications because its LCST is close to body temperature of 37°C. To further tune its response, the LCST of PNIPAm can be adjusted by copolymerization with more hydrophilic monomers that alter swelling/collapse behaviors (Moghanjoughi et al., 2016). Collapse kinetics can be enhanced by grafting chains of either free PNIPAm or poly (ethylene glycol) PEG to cross-linked PNIPAm gels (Kikuchi and Okano, 2002).
2.4.2 pH-responsive CRSs
Another widely studied category of smart release CRSs relies on pH changes to induce material conformational or phase changes. This pH-responsive drug release can occur based on biological pH variations found throughout the body, such as in the gastrointestinal tract or via locally induced pH changes, such as those found in the tumor microenvironment or cellular lysosomes (Gupta et al., 2002). pH-responsive systems are typically composed of polymers or polymer networks, i.e., hydrogels, that display large numbers of ionic side (pendant) groups (Moghanjoughi et al., 2016). Some of the most commonly studied ionic polymers include poly (acrylic acid) (PAA), poly (methacrylic acid) (PMAA), poly (ethyleneimine) (PEI), poly (amidoamine) (PAMAM), poly (diethylaminoethyl methacrylate) (PDEAEMA), and poly (dimethylaminoethyl methacrylate) (PDMAEMA) (Peppas et al., 2013; Moghanjoughi et al., 2016) (Figure 3). In aqueous media of the appropriate ionic strength and pH, the pendant groups ionize, changing their hydrophobicity/philicity and therefore water solubility. This can induce material collapse/swelling enabling on-demand drug release (Peppas et al., 2013). Anionic polymers swell at high pH, whereas cationic polymers swell at low pH. Swelling is caused by the presence of mobile counter-ions that electrostatically balance the fixed charges—even with small changes in pH, the shift in osmotic pressure of the counter-ions can lead to significant changes in the water content and mesh size of the ionic polymer network (Peppas et al., 2013).
FIGURE 3
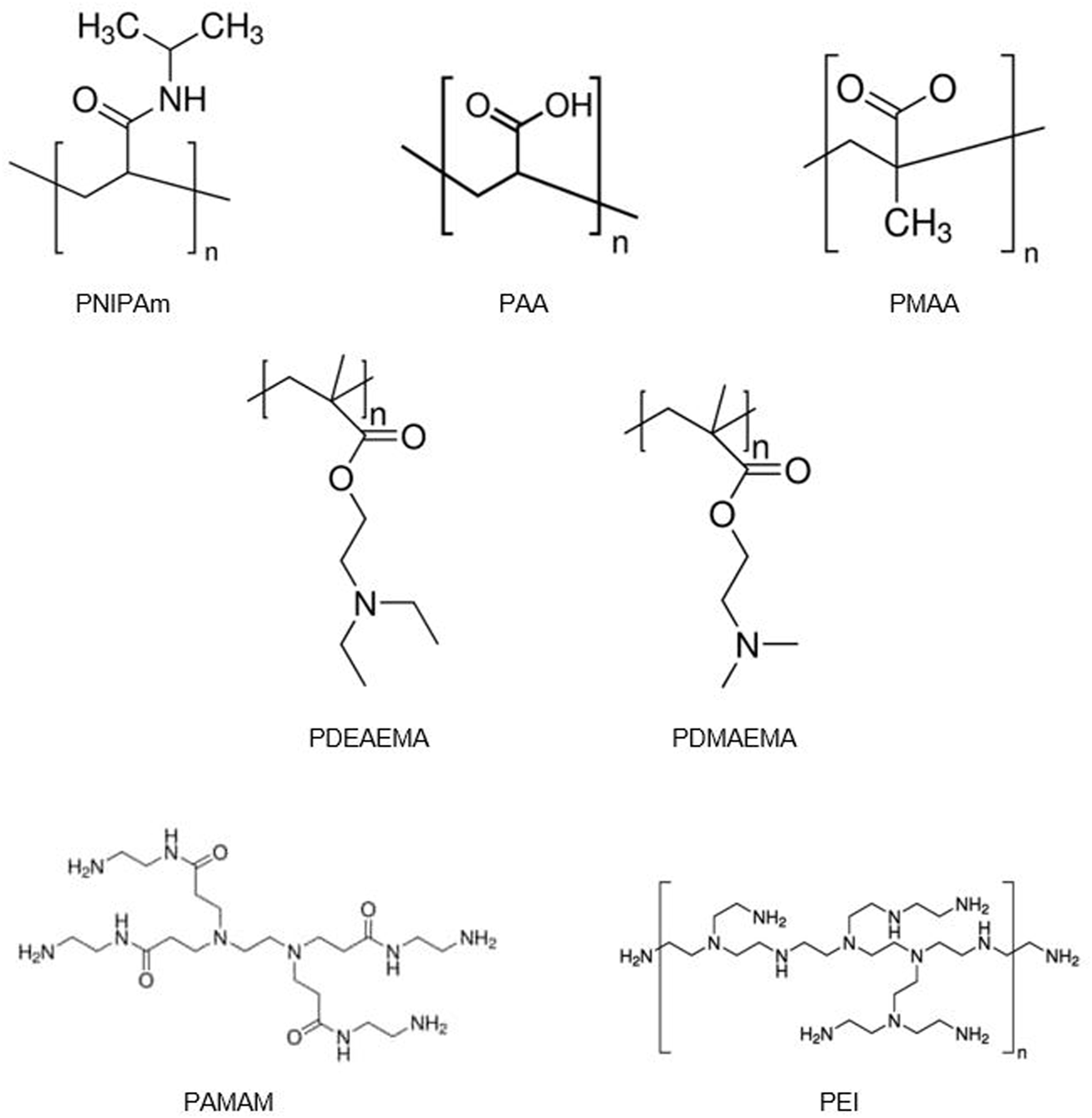
Chemical structures of synthetic polymers commonly used as smart release systems, including poly (N-isopropylacrylamide) (PNIPAm), poly (acrylic acid) (PAA), poly (methacrylic acid) (PMAA), poly (diethylaminoethyl methacrylate) (PDEAEMA), poly (dimethylaminoethyl methacrylate) (PDMAEMA), poly (amidoamine) (PAMAM), and poly (ethyleneimine) (PEI).
2.4.3 Enzyme responsive CRSs
Enzymatic-responsive CRSs are typically based on enzyme-substrate systems (Mann et al., 2001). The substrate, consisting of a cleavable peptide, protein, biomolecule, or chemical molecule, is incorporated into a polymeric material. When the enzyme is present, it acts on the substrate, cleaving it. Similar to hydrolytically degradable materials, this increases the material pore size, enhancing diffusion rates. However, in contrast to these systems, cleavage and therefore diffusion only takes place (or is increased) when the enzyme is present. Examples of this approach include hydrogels responsive to the presence of matrix metalloproteinases (MMPs) commonly secreted by migrating cells (Mann et al., 2001; Leight et al., 2013) and glucose responsive systems that release insulin (Brown et al., 1986). Hydrogels employed as host matrices for substrates are typically composed of inert, biocompatible materials, such as poly (ethylene glycol) or alginate.
3 The potential for controlled release systems in agriculture
The long history of biomedical CRSs can inform agricultural applications, which would benefit from enhancements in controlled delivery. Agricultural inputs are generally categorized as fertilizer or pesticides, and are applied in millions of tons worldwide each year (Food and Agriculture, 2022). Reducing these inputs has long been desirable as they 1) require fossil fuels for production, 2) increase farm expenses, 3) can have off target effects in the farm field (e.g. (Matsuda et al., 2020; Meena et al., 2020; Siviter and Muth, 2020; Raven and Wagner, 2021)) and 4) can leave the field in water or as a gas resulting in nutrient overloading in waterways (eutrophication) or increases in atmospheric greenhouse gases. Current nutrient and pesticide applications tend to be untargeted and reactive. This is for good reason: farmers frequently cannot detect nutrient deficiencies or pathogens until after a crop plant has detected it and failed to handle it. Farmers identify this failure in crop plants when they observe disease symptoms and changes in growth rate or leaf coloration indicative of nutrient deficiencies. Thus, farmers and land managers are gaining information about issues in their systems almost third hand. Precision farming aims to reduce time to detection by gaining information before or concurrently with the plant (e.g., via soil nutrient testing or advanced imaging); however, this often requires significant expense (e.g., multiple soil tests or purchasing imaging equipment). By contrast, if engineers could develop controlled release systems (CRSs) responsive to organisms in the environment, farmers could potentially target nutrient deficiencies or pathogens before they threaten the crop or yields.
Whereas agricultural inputs are numerous, here we focus on the most abundant classes: nitrogen (N) and phosphorus (P) fertilizer and pesticides targeting bacterial and fungal pathogens and herbivores. Next to carbon, N is the nutrient most in demand by all living organisms. N is needed to produce the building blocks of life (e.g., nucleic acids, amino acids, and proteins), and most N in aboveground living organisms is sourced from plants. To provide N for crops, worldwide in 2019, 107 million tons of N fertilizer were applied to agricultural fields (Food and Agriculture, 2022). Yet, only an estimated 46% of applied N is utilized by crop plants (Zhang et al., 2021a). Much of the remaining 54% is converted by soil microbes into other forms of N, including gasses such as nitrous oxide (N2O) that make significant contributions to global change.
Following N, P is considered the second most limiting nutrient for crops. In 2019, 43 million tons of phosphate were added to agricultural settings worldwide (Food and Agriculture, 2022), but less than half of this applied phosphate reaches crop roots (Syers et al., 2008). Broad phosphate application recommendations are high despite variations in soil ability to retain phosphorus (P) because of geochemical, biological, and hydrological characteristics (Kleinman, 2017). Excess P can leave agricultural systems via run-off, and persist long term in waterways (Powers et al., 2016) and sediments, resulting in long term patterns of eutrophication, i.e., toxic algal blooms, and poisoned water (Schindler, 2012). Research to mitigate P application has focused on Ecosystem Service payouts (Macintosh et al., 2019) and plant breeding for increased P use efficiency (e.g. (Schindler, 2012; de Souza et al., 2020)). Similarly, worldwide 969,061 tons of fungicide and bactericide and 698,169 tons of insecticides were added to agricultural settings in 2019 (Food and Agriculture, 2022). All of these agrochemicals have non-target effects. Like P, fungicides (Zubrod et al., 2019; de Souza et al., 2020) can leave agricultural systems via run-off and persist in waterways and sediments (Schindler, 2012; de Souza et al., 2020). Additionally, fungicides frequently persist in soil long term, and therefore are likely having non-target effects on soil organisms both good and bad (Silva et al., 2019). Insecticides and bactericides also have non-target effects that can lead to the loss of beneficial insects and soil microbes (e.g. (Matsuda et al., 2020; Meena et al., 2020; Siviter and Muth, 2020; Raven and Wagner, 2021)).
Thus, there are significant opportunities for CRSs in agriculture to manage delivery of nutrients, like P and N, and pesticides. However, in addition to compound delivery, agricultural CRSs also afford the opportunity to manage soil water content, an application more specific to this field (Chu et al., 2006; Mazloom et al., 2020). Soil water content influences the growth of crop plants and soil organisms, as well as the effectiveness of fertilizer and pesticide applications. Water can be introduced into agricultural systems naturally (e.g., via rainfall) or via irrigation. High soil water contents lead to low-oxygen environments that inhibit the growth of plants and aerobic microorganisms and contribute to fertilizer or pesticide run-off, leading to off-target effects. In contrast, low soil water content leads to drought and can limit the delivery of fertilizers or pesticides that move through soil water. Therefore, proper maintenance of soil water content is crucial for improved crop yields and water use efficiencies.
CRSs provide opportunities to meet these challenges. The most common CRS design consists of a matrix material, usually polymer or lipid, that encapsulates the compound(s) to be delivered into the aqueous phase. The encapsulant can be water soluble or insoluble and can be a small molecule or a large nanoparticle. Typically, release is achieved by passive diffusion through the porous matrix or hydrolytic degradation, although many advanced designs are possible. The advantages of CRSs are numerous. They protect the encapsulant from degradation and off-target uptake; they can enhance diffusion of the encapsulant, enabling it to reach its target before activation; and, they control access to the encapsulant, delivering it at a desired time and rate. Application of CRSs to agriculture could reduce fertilizer and pesticide applications, lowering costs and improving environmental sustainability, and could also enhance water management. However, CRSs have primarily been designed for biomedical applications.
4 Potential agricultural CRS targets
The agricultural milieu is a complex environment with many contributors that can provide both positive and negative impacts (Figure 4). Each of these serves as a potential target for CRSs. In addition, the soil itself is a multiphase mixture that can influence local biology through changes in pH, hydration, and porosity, and CRSs also offer the potential to alter some of these characteristics. Here, we review key potential targets for agricultural CRSs (Figure 5).
FIGURE 4
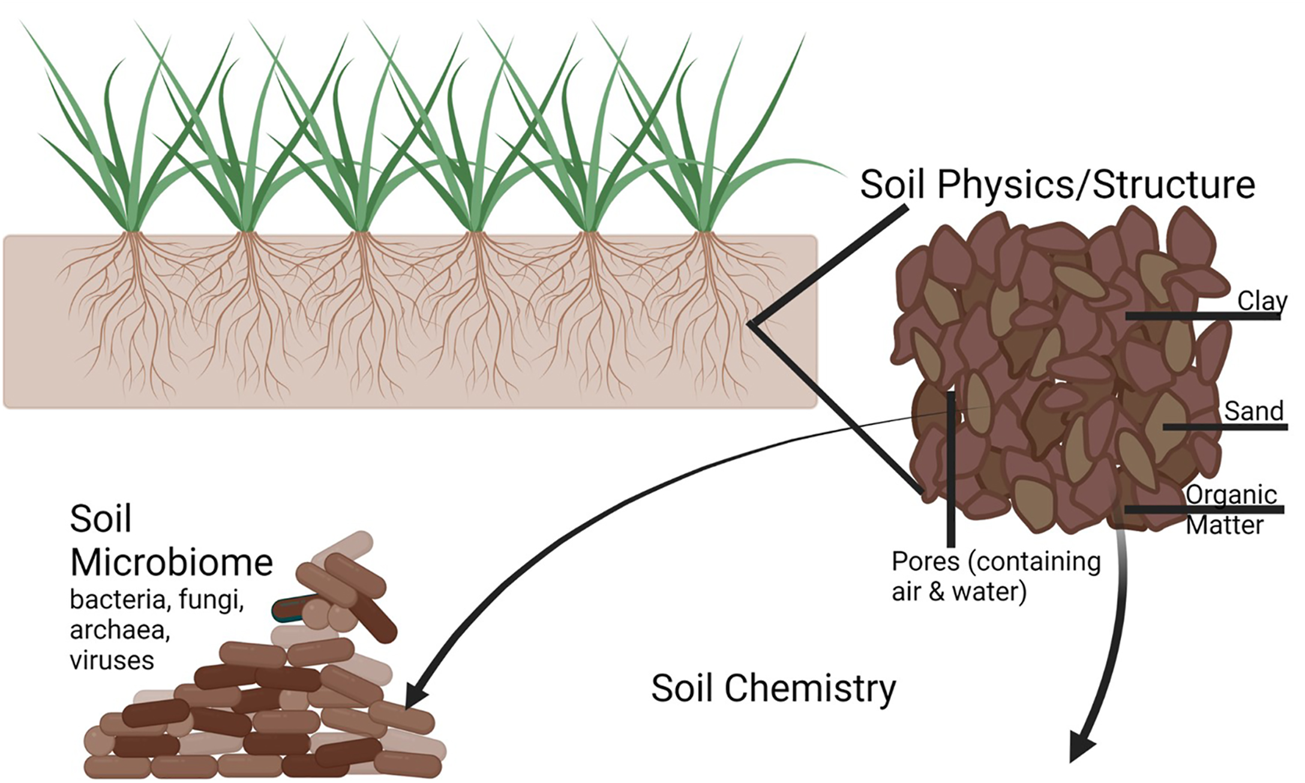
A depiction of the complexity of soil including the soil physics/structure consisting of aggregates of clay, sand, and organic matter with water and air containing pores between them; different soil chemistry constituents (e.g., nutrients, carbon, and signaling compounds) released into the pore spaces by plants, microbes, and other organisms; and the soil microbiome consisting of bacterial, fungal, archaeal, oomycete, protozoan, and viral constituents that contribute to decomposition, pathogenic interactions, and beneficial interactions. Partially created using BioRender.com.
FIGURE 5
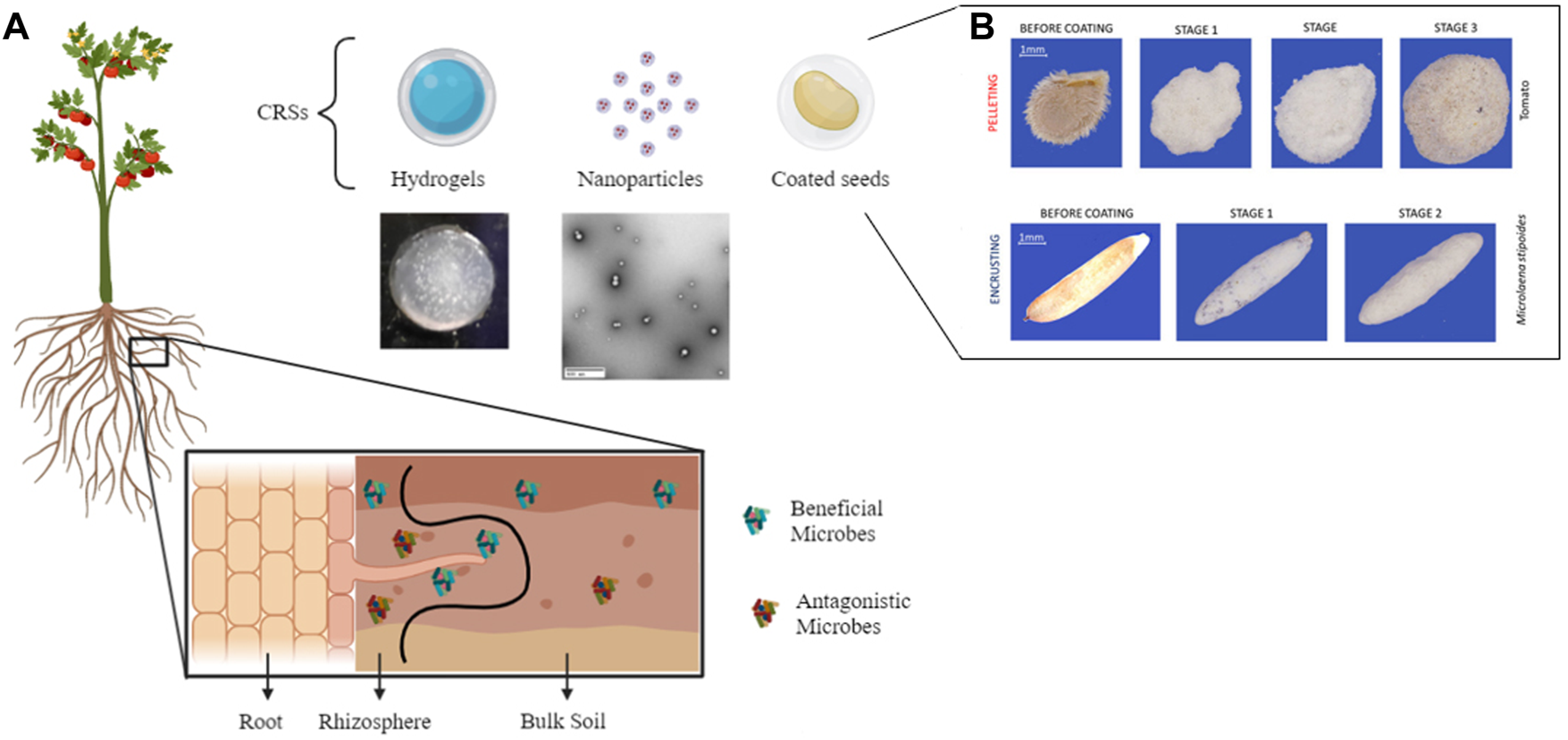
(A) Potential targets of controlled release systems (CRSs) in agriculture include plant roots and the beneficial microbes and pathogens found throughout the rhizosphere adjacent to plant roots and in bulk soil. CRSs may take many forms such as hydrogels (i.e., water swollen polymer networks) and nanoparticles that could be implemented in films, inserts, or as components of (B) seed coatings. Examples shown are of Example of tomato and Microlaena stipoides (Australian grass) coated seeds. (A) Created using Biorender.com. (B) Reprinted without edit under Creative Commons license from Pedrini, S., K. Bhalsing, A.T. Cross, and K.W. Dixon, Protocol Development Tool (PDT) for seed encrusting and pelleting. Seed Science and Technology, 2018. 46 (2): p. 393–405 (Pedrini et al., 2018).
4.1 Plants
Plants are obvious potential targets for CRSs, as most agricultural inputs target plants. Farmers add fertilizer to plants to improve plant growth, and spray plants with herbicides and pesticides to improve their disease and herbivore resistance. Biologists know more about crop plants than most of the other organisms in agricultural systems. For many of the more common agricultural and horticultural crops, basic root architecture (e.g. (Guo et al., 2020; Maqbool et al., 2022; Viana et al., 2022)), chemicals exuded into the soil (exudates) surrounding plant roots (rhizosphere) (e.g. (Weston and Mathesius, 2013; Schmelz et al., 2014; Tsunoda and van Dam, 2017)) are known, and biologists can manipulate many of the underlying genetics for these traits (e.g., (Atkinson et al., 2014; Soyano et al., 2019; Guo et al., 2020; Huang et al., 2021). This data is available for the top crops per area cultivated worldwide in 2020: cereals (wheat, maize, rice, barley, sorghum, and millet), legumes (soybeans and dry beans), and oil rapeseed (Food and Agriculture, 2022) and their close relatives. This biological knowledge could be used to develop CRSs or to engineer plant-CRS partnerships. However, crop and horticultural plants are often grown in dense monocultures, and therefore have the greatest biomass in these systems. Thus, targeting nutrients or other inputs to plants in need could prove challenging if a controlled release compound could be taken up by a near neighbor. An ideal CRS would detect plant distress signals and release nutrients directly in response to the plant releasing the distress signal.
4.2 Beneficial microbes
Beneficial soil microbes are another potential target for CRSs. There are many types of beneficial microbes, including plant growth promoting rhizobacteria (PGPR), plant growth promoting fungi (PGPF), endophytes, entomopathogens, rhizobia, and arbuscular mycorrhizal (AM) fungi. These microbes play different roles in soil that benefit plants, including delivering nutrients to host plants, fixing atmospheric nitrogen, decomposing organic matter, suppressing plant pests and diseases, and improving soil structure (Hayat et al., 2010). For example, rhizobia are beneficial soil bacteria that associate with leguminous crops and fix nitrogen for host plants (Peter et al., 1996). Phosphate solubilizing bacteria, including a wide range of species from the genera Pseudomonas, Bacillus and Rhizobium, can turn immobile mineral P into its soluble form that is absorbable by plants (Chen et al., 2006). Some species in Pseudomonas and Bacillus also control pests and pathogens (Pineda et al., 2010; Pineda et al., 2013; Pieterse et al., 2014; Shikano et al., 2017; Gruden et al., 2020). Thus, promoting beneficial soil microbes has the potential to enhance crop growth.
To our knowledge, there are no current CRSs targeting beneficial soil microbes. To demonstrate the potential of CRSs for beneficial microbes, we provide an example using arbuscular mycorrhizal (AM) fungi as a case study. AM fungi are a group of obligate symbionts in Glomeromycota that can associate with over 80% of vascular plants. They provide multiple benefits to host plants and receive photosynthetic carbon in return. AM fungi can promote plant growth by providing soil mineral nutrients to host plants, improving plant biotic and abiotic stress tolerance, and producing secondary metabolites to promote plant growth or improve soil structure (Bennett and Groten, 2022). Thus, AM fungi have significant effects on phytobiomes (the plant along with all other organisms living in, on, or around the plant) in both natural and agricultural systems.
Sustainable agriculture would benefit from a greater reliance on AM fungi for nutrient delivery to crops, but promoting the AM fungal-plant interaction in agriculture has proven difficult in the face of tillage, heavy fertilization, and monocultures (Bennett et al., 2013). However, CRSs could be used to encourage AM fungal-plant interactions. For example, CRSs could be designed to release signaling compounds, such as strigolactones and flavonoids, that attract AM fungi to colonize host plants. Strigolactones are phytohormones that can induce AM fungal spore germination, hyphal branching, and production of chitin oligosaccharides which stimulate the response of host plants (Lanfranco et al., 2018a). Flavonoids released by plants also promote the colonization of both rhizobia and AM fungi (Steinkellner et al., 2007; Oldroyd, 2013). General release of these compounds into the soil could have negative effects, such as inducing AM fungal spores to germinate without a suitable host. However, if release of strigolactones and flavonoids from CRSs could be coupled with plant release of these compounds, the plant-derived signal to enhance plant-fungal associations could be boosted. Thus, CRSs could promote the AM fungal-plant interaction and sustainable agriculture by reducing fertilizer needs.
4.3 Antagonistic microbes
Antagonistic microbes and herbivores have long presented challenges in agriculture and horticulture. A recent estimate of crop losses to pests and pathogens in five of the major crops worldwide (wheat, rice, maize, potato, and soybean) revealed losses ranging from 8–41% and with higher losses in areas of food insecurity (Savary et al., 2019). Mechanisms for controlling pests and pathogens are thus of significant interest. Soils host a number of antagonistic microbes, many of which infect a wide range of plants or have dramatic impacts on important crop species. Here, we highlight some of the most damaging species found in soil as potential targets for CRS management. Botrytis cinerea, which causes grey mold, leads to significant losses because of its wide host range and host structural use (e.g., stems, leaves, and fruits). Among above and belowground pathogens, B. cinerea is the second most important fungal pathogen worldwide (Dean et al., 2012). Another potential target is the fungal pathogen Fusarium oxysporum, which can infect over 100 plant hosts, also has a wide host structural use, and frequently causes significant losses in horticultural systems (Michielse and Rep, 2009). Among above and belowground pathogens, F. oxysporum is considered the 5th most important fungal pathogen worldwide (Dean et al., 2012). Another potential target, the oomycete pathogen Phytopthera infestens, is the causal agent of late blight in potatoes—the pathogen known to have caused the Irish Potato famine in the mid-1800s. P. infestans can infect multiple plant hosts in the Solanaceae (i.e., nightshade) family; is found above and belowground, and can persist in soil as oospores for many years. To this day, farmers have few effective means of eliminating late blight once it has infected a potato field. The most effective means of controlling P. infestans is to grow resistant cultivars. All of these pathogens present opportunities for CRS management. CRSs could be designed to release fungicides or pesticides upon detection of metabolites associated with these pathogens; thereby, controlling the pathogen in soil before it reaches a host plant.
5 Commercial and emerging agricultural controlled release systems
5.1 Applications
5.1.1 Nutrient and pesticide delivery
Nutrient delivery, particularly to plants, has been a primary focus of the agricultural controlled release market. The first CRS for the agricultural market was Osmocote (Scotts Company), introduced by the Archer Daniels Midland company in 1960 for nutrient delivery (Lafaille et al., 2018). These fertilizers are comprised of plant nutrients in an encapsulated form that delays availability for plant uptake, therefore extending the window in which the fertilizer is available for use after a single application (Fu et al., 2018). The use of CRS encapsulated fertilizers has many potential benefits, including delivery of nutrients to crops, maintaining water availability, and reducing greenhouse gas emissions by limiting the availability of applied nitrogen for denitrification and N2O production (Jariwala et al., 2022). In addition, by controlling fertilizer release into soil, CRS encapsulated fertilizers can prevent shifts in soil pH, as overfertilization leads to acidification (Jariwala et al., 2022). Controlled release fertilizers like Osmocote operate by limiting access to water to limit encapsulant solubilization or through slow coating hydrolysis to limit release over time. Coating fertilizers with multiple materials, including CRSs, is a growing area (Jariwala et al., 2022; marketresearch, 2022a); and the industrial leaders in this area are BASF Corporation, DuPont Pioneer, and Invista. New products in this market include U-COAT (Bio-on), a controlled release coating of urea fertilizer produced using proprietary bioplastic. Graphene based nanocarriers are also gaining popularity for the delivery of both fertilizers and pesticides (Bhattacharya et al., 2022). Seed coatings are another fast growing sector of agricultural CRSs (Farooq et al., 2012; Pedrini et al., 2017; Afzal et al., 2020). Seed coatings are used to deliver nutrients, pesticides and fungicides, and beneficial microbes to germinating seeds (Farooq et al., 2012; Pedrini et al., 2017; Rocha et al., 2019; Afzal et al., 2020; Cardarelli et al., 2022). Engineered nanomaterials are also being used as seed treatments (De La Torre-Roche et al., 2020) and pesticides (marketresearch, 2022b). However, most of these nanomaterials are not used in combination with CRSs.
Controlled release pesticides, particularly incorporating nanomaterials, are also a growing agricultural sector (Chaud et al., 2021; Hou et al., 2021), but they lag behind other CRSs because of ecotoxicology concerns and the potential to promote pesticide resistance. These products vary in composition, but often comprise a smaller part of the market than other formulations as the controlled release aspect increases the cost of the product (Li et al., 2021). The first pesticide CRS was microencapsulated methyl parathion introduced by the Pennwalt Corporation in 1974, and the majority of products currently on the market are microencapsulated (Li et al., 2021). Advances in this area include the use of nanoparticles as disease sensors (Singh et al., 2022) (that could be partnered with controlled release systems, see below theranostics). We see an opportunity for the expansion of CRSs for targeted pesticide release in soil and on crop plants, particularly in combination with nanotechnology.
5.1.2 Modulating soil water content
In addition to agrochemical delivery, controlling hydration is a target for agricultural CRSs. This application may be unique to agricultural systems in that water itself is the delivered molecule. CRSs, and in particular hydrogels, have been used in agricultural to provide water in drought conditions (Chaudhary et al., 2020; Mazloom et al., 2020; Gao et al., 2021). Hydrogel carriers, comprised mostly of water (Ullah et al., 2015), have been evaluated for their ability to retain soil water and release it when soil gets dry (Romero et al., 2016). Also, hydrogels can deliver water and plant nutrients (N or P) into soil simultaneously, making them more efficient carriers (Dhanapal et al., 2021; Hu et al., 2021). Hydrogels can release water for 6–30 days, depending on the soil type (Chaudhary et al., 2020; Zhang et al., 2021b; Dhanapal et al., 2021). This approach has longer impact than most irrigation methods and could save human, equipment, and energy costs. Also, CRSs can be “smart” and designed to release water under conditions other than soil humidity (Azevedo and Bertonha, 2008; Fujita et al., 2022). Thus, CRSs may be designed as a constant, economical, and precise water supply, complementary to traditional agricultural irrigation systems, while delivering fertilizers or pesticides simultaneously. Furthermore, CRSs may be able to deliver water differently to disparate targets in soil, and be applied to different crop rotations or intercropping systems.
5.2 Materials for agricultural CRSs
As discussed above, synthetic polymers, such as PLGA, pose potential toxic hazards when applied in agriculture. As such, natural polymers have garnered much interest because of their inherent biocompatibility and lower potential for environmental toxicity. Of these, cellulose, chitosan, and alginate are the most commonly studied for mutual biomedical and agricultural CRS use. These materials are abundant, low-cost, and have high biocompatibility. In addition, other plant-derived materials, such as zein, have been more popular in agricultural CRSs with more limited use in biomedical settings. Here, we discuss the most common agricultural CRS materials (Figure 6).
FIGURE 6
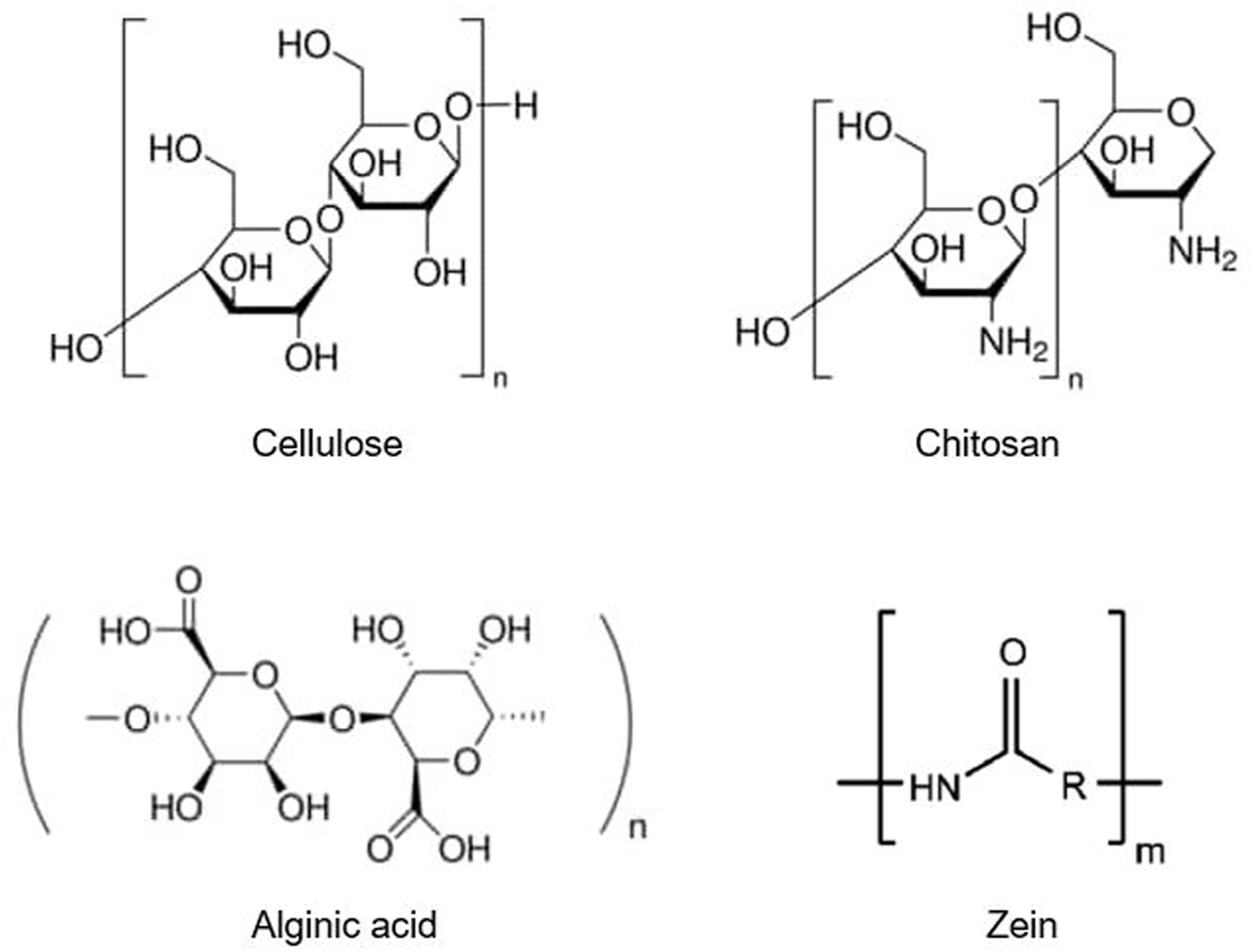
Chemical structures of biopolymers commonly used in agricultural controlled release systems, including cellulose, chitosan, alginate, and zein.
5.2.1 Cellulose
Cellulose is the most abundant biopolymer and is mostly found as the main component in lignocellulosic plants and natural fibers including cotton and linen (Ghorbani et al., 2018). Celluloses are polysaccharides comprised of repeating glucose units with a high degree of crystallinity. The intricate, multi-level, hierarchical structure of cellulose provides it with functionality, flexibility, and a high strength-to-mass ratio, making it attractive for biomedical applications (Seddiqi et al., 2021). Whereas pure cellulose has less favorable properties, such as its insolubility in water, substitution of its native hydroxyl groups with alternative functional groups allows for modulation of its properties (Seddiqi et al., 2021). Indeed, water-soluble cellulose derivatives methyl cellulose (MC), carboxymethyl cellulose (CMC), and hydroxypropyl cellulose (HPC) have been used in tissue engineering, wound healing, and drug delivery (Seddiqi et al., 2021). These materials also have cross-over use as agricultural CRS matrices. Cellulose-based superabsorbent hydrogels have been explored as soil conditioners, as these hydrogels can hold water in capacities of hundreds to thousand times more than their dry mass. For example, CMC and acrylamide composite hydrogels exhibited up to 6,000% swelling with water retention up to 1 month (Ibrahim et al., 2007). Moreover, the inherent biodegradability of cellulose via cellulases secreted by the soil microbiome (Béguin and Aubert, 1994) and lack of environmental toxicity (Seddiqi et al., 2021) make cellulose an ideal candidate for agricultural applications.
5.2.2 Chitosan
Chitosan is the second most abundant natural polysaccharide and is obtained by partial deacetylation of chitin, which is mainly found in the exoskeletons of crustaceans (Ramli, 2019). Chitin consists of repeating N-acetyl glucosamine units, whereas chitosan is generated by partial deacetylation to yield repeating units of N-acetyl glucosamine and glucosamine. The conversion of water-insoluble chitin to chitosan confers solubility in dilute acid solutions. Chitosan is more commonly employed as it is biodegradable, non-toxic, and has some antibacterial properties (Divya and Jisha, 2018). Additionally, as a cationic polymer, chitosan enables bio-adhesion, cellular transfection, and possesses anti-inflammatory properties, making it a highly attractive biopolymer for biomedical applications (Bandara et al., 2020). Chitosan has been used extensively in contact lenses, wound dressings with FDA approval, and in drug delivery as a carrier for proteins and biomolecules (Jiang et al., 2014). Chitosan has also been employed in environmental engineering to remove heavy metals from industrial wastewater because of its chelating properties, and has also been used in cosmetics because of its antimicrobial and antifungal properties (Jiang et al., 2014). Chitosan has direct biological activity on plants, enhancing their metabolic response and pathogen defense (El Hadrami et al., 2010), but has also been used widely for controlled release of fertilizers and pesticides (Divya and Jisha, 2018; Kumaraswamy et al., 2018), and to a lesser extent for managing abiotic stress in plants (Bandara et al., 2020). The degradation of chitosan largely depends on the degree of deacetylation, and the in vitro degradation of chitosan via oxidation or enzymatic hydrolysis has been well-established. In living organisms, chitosan is likely degraded by lysozymes to non-toxic oligosaccharides that can be excreted (Szymańska and Winnicka, 2015). Chitosan is also readily biodegraded in agricultural settings, as evidenced by complete degradation of chitosan films in industrial compost, gardening soil, and vineyard soil within 14 days (Oberlintner et al., 2021).
5.2.3 Alginate
Alginates are naturally occurring anionic polysaccharides derived from kelp or brown algae that are biocompatible, biodegradable, non-toxic, and readily form gels. Whereas alginic acid is insoluble in water, its monovalent salts, such as sodium alginate, are water-soluble and form viscous solutions that can readily interact with polyvalent cations (e.g., calcium) to form ionic hydrogels (Zhang et al., 2021c). Such alginate hydrogels have been extensively explored for biomedical applications like wound healing (moist wound dressing), drug delivery (encapsulation of active substances for controlled release), and tissue engineering (scaffolding material) (Zhang et al., 2021c). In agriculture, alginate has been used to encapsulate, protect, and release growth-promoting microorganism soil inoculants (Martínez-Cano et al., 2022). Alginate has also been used to deliver nutrients (Fan et al., 2019). However, it is more commonly applied as a co-polymer than in its pure form (Al Rohily et al., 2020). For example, anionic sodium alginate and cationic chitosan have been combined to form copolymers with improved mechanical properties (Iwasaki et al., 2004). Alginate-chitosan beads have been used to encapsulate micronutrients, increasing their stability against temperature, moisture, and acidic pH changes (Han et al., 2008). Degradation of alginate occurs via partial oxidation and strongly depends on the alginate degree of oxidation, temperature, pH, and molecular weight (Lee and Mooney, 2012). Interestingly, degraded alginate can promote plant growth and even provide protection against salinity stress (Hien et al., 2000; Salachna et al., 2018).
5.2.4 Zein
Zein is the predominant protein found in corn and has been widely used in biodegradable plastics, fibers, and textiles (Shukla and Cheryan, 2001). Zein is biocompatible, biodegradable, relatively cheap to produce, and is considered generally regarded as safe by the FDA (Kacsó et al., 2018). Furthermore, zein is inherently hydrophobic and can self-assemble into nanoparticles, making it an attractive delivery vehicle. While zein has been used for biomedical applications such as drug delivery and tissue engineering, it has not been as widely used compared to cellulose, chitosan, or alginate. Instead, zein has been of more interest in agriculture for encapsulation of food products such as essential oil components for increased stability (Chen et al., 2015) and more recently as pesticide carriers. While pesticides suffer from poor stability and rapid degradation, encapsulating repellents with zein nanoparticles exhibited effective protection from UV degradation, decreased phytotoxicity, and served as effective barriers against pests (Oliveira et al., 2018).
5.3 Manufacturing methods
The most common formulations for agricultural CRS are micro-capsule suspensions encapsulating agrochemicals (Hack et al., 2012). Emerging technologies include nanoparticles comprised of polymers, starches, and polysaccharides (Moulick et al., 2020; Shakiba et al., 2020; Vega-Vasquez et al., 2020) (see below). Both are manufactured via similar processes. Given that agricultural applications will likely require kg-scale quantities of these materials in a given field application, it is crucial that scalable methods for their manufacture exist. Traditional methods have focused on bottom-up approaches that assemble molecules at the molecular scale, offering excellent control over final size and shape. However, these methods are limited in their ability to generate large quantities and the slow kinetics of assembly. Hence, there has been a recent shift in academic focus towards rapid and scalable approaches that are more suitable to the high volume, low cost demands of agricultural CRSs (Figure 7). In this section, we focus discussion on common methods of micro/nanoparticle synthesis amenable to current and emerging agricultural CRSs.
FIGURE 7
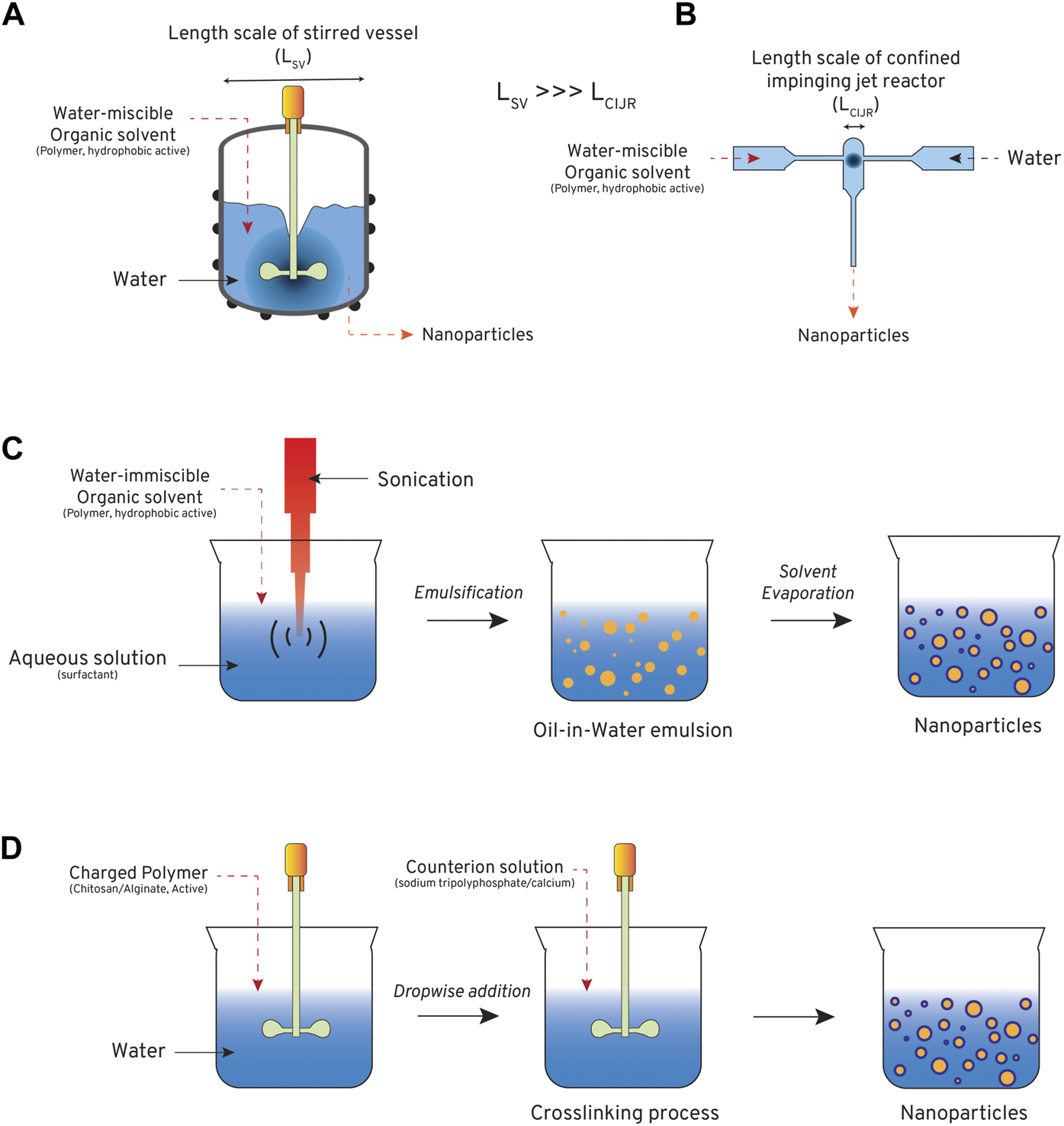
Methods for forming nanoparticle controlled release systems (CRSs): (A) batch nanoprecipitation in large, stirred vessels, (B) flash nanoprecipitation in a confined impinging jet mixer, (C) batch emulsion-evaporation techniques, and (D) batch ionotropic gelation for crosslinking charged matrices.
5.3.1 Nanoprecipitation
Nanoprecipitation, also known as solvent displacement or anti-solvent precipitation, is a facile technique for producing micro/nanoparticles. The technique was first reported by Fessi in 1989 to produce drug-loaded polymer nanocapsules (Fessi et al., 1989). Over the years, nanoprecipitation has gained prominence because of its simplicity of operation and low energy requirements. In nanoprecipitation, dissolved solutes are precipitated as particles through rapid change in solvent quality generated by the addition of miscible anti-solvent or ionic, pH, or temperature manipulation (Hornig et al., 2009; Zhou et al., 2017; D'Addio and Prud’homme, 2011; Zhu et al., 2010) (Figure 7A). This approach has been used primarily to generate CRSs for pesticide delivery (Boehm et al., 2003; Yearla and Padmasree, 2016). CRS formulations offer many benefits over their commercial counterparts, including enhanced penetration across the leaf (Boehm et al., 2003), increased systemic delivery to the plant (Boehm et al., 2003), and higher efficacy (Yearla and Padmasree, 2016).
Toward agricultural implementation, nanoprecipitation approaches can be translated to commercial scales through a modified approach known as flash nanoprecipitation (FNP) (Johnson and Prud’homme, 2003; Feng et al., 2019a). FNP converts nanoprecipitation from a batch to continuous process by the addition of cross flows in a confined micromixer geometry (Figure 7B), such as the confined impinging jet mixer (Han et al., 2012), multi-inlet vortex mixer (Liu et al., 2008), or jet mixing reactor (Ranadive et al., 2019). FNP can generate production rates of 3–10 kg/day using an apparatus that can fit onto a standard lab workbench (Lim et al., 2014; Feng et al., 2019b). Production can also be enhanced by scale-out, running parallel FNP unit operations to maximize throughput (Sealy, 2021). Similar to nanoprecipitation, FNP has been used to generate agricultural CRS delivering pesticides (Chen et al., 2018a; Chun and Feng, 2021), revealing similar performance to other manufacturing methods (Chen et al., 2018a) and improved performance against unencapsulated compounds (Chun and Feng, 2021).
5.3.2 Emulsion evaporation
Emulsion methods are based on self-assembly phenomena that occur at the oil-water (O-W) interface, and are the most common CRS manufacturing method in current use (Hack et al., 2012). In emulsion-evaporation, agrochemicals, CRS matrices, and other organic components (e.g., excipients) are dissolved in a water-immiscible, volatile organic solvent, such as dichloromethane, chloroform, or ethyl acetate. The oil phase is emulsified with a water phase containing surfactants using an ultrasonic probe or a high-speed homogenizer (Figure 7C). After a O-W emulsion forms, the organic solvent is removed by evaporation. This leads to micro/nanoparticle formation via self-assembly. Emulsion-evaporation has been widely reported in the academic literature (Zhang et al., 2013; Kumar et al., 2014; Pereira et al., 2014; Liang et al., 2017; Shen et al., 2018; Salinas et al., 2021; Mendez et al., 2022), primarily for pesticide delivery, and shows similar encapsulation efficiency to nanoprecipitation approaches (Zhang et al., 2013). However, application is limited by the energy-intensive homogenization/sonication process and the need for additional solvent removal steps. Furthermore, many of the organic solvents employed may pose ecological toxicity if not removed, which limit enthusiasm for this approach.
5.3.3 Ionotropic gelation
Ionotropic gelation involves CRS formation through crosslinking or electrostatic interactions between a charged matrix and an oppositely charged ionic species (Figure 7D). Common examples include crosslinking of sodium alginate with calcium ions or chitosan crosslinking with sodium tripolyphosphate; both are natural polymers that have been studied for agrochemical delivery. This approach has been used to generate CRSs for delivery of a wide variety of agrochemicals, including plant regulators (Santo Pereira et al., 2017; Valderrama et al., 2020), insecticide metabolites (Namasivayam et al., 2018), herbicides (Maruyama et al., 2016; Ghaderpoori et al., 2020), and fungicides (Maluin et al., 2019). These materials provide sustained release that can extend activity of agrochemicals in the field (Artusio et al., 2021).
5.4 Emerging agricultural controlled release systems
5.4.1 Nanoparticles
One of the largest areas of growth for agricultural CRSs has been the explosion of nanoparticle delivery devices, which have also been widely used for medical drug delivery (Mura et al., 2013). Nanoparticles can be comprised of natural or synthetic, organic or inorganic materials with one aspect from 1–100 nm. Typical technologies for controlled release are ∼20–80 nm in diameter. Their small size is a significant advantage as these small particles can more readily diffuse through soils. Furthermore, nanoparticle surfaces can be modified (e.g., charge neutral) to reduce non-specific accumulation. Nanoparticles protect their encapsulated compounds and increase solubility of poorly soluble compounds (Moghanjoughi et al., 2016). Nanoscale interactions also provide an opportunity to manage soil organisms at their length scale, before they interact with the crop. Thus, nanoparticles could reduce agrochemical non-target uptake or degradation, reducing costs and environmental impacts.
Nanoparticle CRSs can be made from a variety of materials (Vega-Vasquez et al., 2020), but polymer materials are the most popular. Polymers can be natural (e.g., cellulose, chitosan) or synthetic (e.g., polyacrylamide) materials (Aouada et al., 2011), and in agricultural applications natural polysaccharides are most commonly employed (Barclay et al., 2019). Macroscale polymer materials have been used for fertilizer, pesticide, and herbicide delivery, as soil conditioners, and to increase water absorbency (Milani et al., 2017). These materials are biodegradable (e.g., by chelation of alginate crosslinking ions (Ullah et al., 2015) or enzymatic oxidation and photocatalyzed Fenton reactions in polyacrylamide materials (Stahl et al., 2000)), non-toxic (Chen et al., 2018b), and can benefit soil by promoting drought tolerance via increased water absorption and by carbon fixation via reduced anti-oxidant activity (Islam et al., 2011). Nanoparticle versions of these materials are generated via self-assembly of individual chains into complexes that can have disordered or highly defined structures (Mai and Eisenberg, 2012). Although similar phase transformations also manifest in macroscale materials, unique structures such as micelles and liposomes emerge at the nanoscale that may increase solubility or afford better protection for agrochemicals. However, despite their promise, translation of nanoparticle-based formulations to commercial applications is limited by the lack of adequate toxicity studies, poorly defined regulatory structure, consumer distrust, and other economic feasibility roadblocks (Pérez-de-Luque, 2017).
5.4.2 Materials derived from agricultural waste
Another area of rapid growth is the use of materials derived from agricultural waste as CRS matrices, toward a circular agricultural economy. Chitin and chitosan, already common biomedical CRSs (Shen et al., 2016), are attracting increased attention because of their environmental compatibility. Chitin and chitosan are derived from the shells of marine crustaceans, generating up to 80,000 waste tons per year (Divya and Jisha, 2018). Other waste polymers include those comprising plant cell walls, such as celluloses, hemicelluloses, and lignin. These materials are derived from woods and grasses, and their biomass waste is estimated at 5 billion tons per year (Naidu et al., 2018). Celluloses comprise 30–50% of this waste, with the remainder hemicellulose (15–35%) and lignin (10–20%). Like celluloses, hemicelluloses are polysaccharides, but are comprised of a greater variety of sugars, are non-crystalline, and have shorter chains with more branching. Lignin is composed of phenylpropane molecules displaying a variety of linkages (Naidu et al., 2018). All of these materials are hydrophobic and require processing to generate pure feedstocks for downstream applications. However, because of their derivation from plants, they can usually be degraded by enzymes in the soil microenvironment, making them an attractive option for agricultural CRSs (Yadav et al., 2022). Materials commonly employed include hydroxypropyl methylcellulose (HPMC) (Chen and Chen, 2019), carboxymethyl cellulose (CMC) (Elbarbary and Ghobashy, 2017; Fujita et al., 2022), ethylcellulose (EC) (Sopeña et al., 2011), and lignin (Mazloom et al., 2020; Mendez et al., 2022).
Aside from bio-polymers, other common waste materials targeted for agricultural CRSs include zein (Salinas et al., 2021), the primary protein of the maize plant, and biochar (Sashidhar et al., 2020). Given the growing global focus on sustainability, it is likely that interest in converting waste materials to CRSs will continue to grow. Because the amounts of material required for agricultural CRSs are much higher than those needed for biomedical therapy (e.g., treating a field vs. a patient), the low-cost and biocompatibility of these materials makes them an attractive option. However, it will be important to control for variability in feedstock composition and develop sustainable manufacturing techniques to process these materials.
6 Challenges in agricultural controlled release
Current agricultural CRSs focus primarily on nutrient or pesticide delivery, and most systems employ passive or degradational release mechanisms that are not targeted to changes in the soil microenvironment. Thus, we believe there are significant opportunities to adapt advances in biomedical CRSs to agriculture. However, translating biomedical CRSs to agriculture is not necessarily straightforward. Whereas many challenges are similar in both systems, the scale of application, cost, commercial considerations, and the unique milieu of the soil microenvironment, make agriculture CRSs a distinct class of materials. In this section, we outline challenges of translating biomedical CRSs to agriculture, highlighting shared and dissimilar challenges that must be surmounted.
One of the biggest differences between biomedical and agricultural CRSs is cost and scale. Whereas healthcare applications typically require mg to g scales with a high cost tolerance, agricultural applications require kg to ton scales with very low cost tolerance. World annual fertilizer (N, P and K) consumption is 146.4 kg per hectare of arable land (Food and Agriculture, 2022). Depending on the fertilizer type, the average cost is $227–333 per metric ton (data from 2017–2020) (the World Bank Group Commodity Markets Outlook, 20222022), resulting in the average annual cost for fertilizer applications of $33.2–48.7 per hectare of arable land. NPK fertilizers are available as granular solid or liquid formulation, and farmers employ various sprayer systems for field applications, with multiple application cycles throughout the year depending on crop and land requirements. Given 166 million hectares of agricultural land in US alone, there is an annual demand of ∼24.2 million tons of fertilizer. Agricultural CRSs will need to be produced at the multi-ton scale and at similar price points to be competitive with current agricultural practice. However, it is likely that the use of CRSs would decrease the required number of field applications and agricultural run-off, which would need to be considered in establishing commercial viability. Regarding scaling issues, several of the scalable nanomanufacturing approaches discussed above offer both scale-up (larger, single manufacturing units) and scale-out (multiple smaller manufacturing units operating in parallel) opportunities. Agricultural CRSs will also need to be compatible with current agricultural application processes, such as seed coatings, soil inserts (horticulture), and spreaders. However, the number of application cycles and timing would likely be altered by the slower and/or triggered release patterns of CRSs. One intriguing possibility is potential integration with precision agriculture systems for release on demand from CRS depots.
In addition to cost, scale, and usability, similar to biomedical CRSs, biocompatibility is tantamount. However, in agriculture this encompasses a broader concept. Whereas biomedical systems are primarily concerned with cell, tissue, and organ level toxicity, agricultural CRSs must also consider ecotoxicology. Of great concern is the potential for CRSs to have negative effects on the soil ecosystem. For example, common biomedical CRS materials, such as PEG and PLGA, are difficult to degrade to monomers, have monomers that could persist in soils, or can acidify soil (Plaut and Federman, 1985; Nishu et al., 2020). Even for some currently accepted agricultural materials (e.g., polyacrylamide), there is debate as to the long-term environmental consequences (Chen and Chen, 2019). Toxic degradation products from CRSs can accumulate through the food chain, potentially including crop plants, which could impact human consumption. Therefore, there is a need to check the physical, chemical, and biological safety of CRS materials before using them in agriculture.
Assuming compatibility with the soil environment, the next challenge is the soil itself. Soil is a multi-phase media with solid, gas, and liquid components, as well as dissolved chemicals and organisms (Figure 4). The complexity of the soil system makes it difficult to control. As a result, soil may decrease CRS effectiveness. CRSs may be degraded before they reach their targets because of changes in soil physical or chemical conditions (e.g., local acidification). To date, most CRSs applied in agriculture have utilized either passive release or smart release controlled by environmental properties (i.e., temperature, pH, and soil moisture) (Jariwala et al., 2022). The latter approach demands environmental stability for constant and uniform release. A small environmental change, such as a sudden rain, a short period of extreme cold/hot weather, or human disturbance, and could reduce CRS effectiveness. A second challenge of the soil environment is the presence of chelators, sometimes even the soil particles themselves, that can bind CRS encapsulants, which are often ionic P or N forms (Marschner et al., 2011). Thus, soil can prevent CRS contents from reaching their targets.
After surviving physical and chemical challenges found in soil, CRSs may encounter another challenge: non-target organisms. Similar to the delivery of drugs in gut systems (Fan and Pedersen, 2021), the biological diversity of the soil microbiome may result in decreased delivery of encapsulants to targets (Marschner et al., 2011). This could occur through a number of mechanisms: changes in the soil environment, enzymatic digestion, or off-target uptake of the compound. Off-target uptake could have particularly negative consequences on the agricultural system. If beneficial compounds are delivered to neutral organisms, only target organism access is impacted, but if they are delivered to antagonistic microbes (e.g., pathogens), increased disease incidence and decreased yields may occur. Similarly, releasing pesticides to non-targeted beneficial microbes could reduce crop production. To increase targeting efficiency, the composition of the target environment (e.g., plants, soil composition, soil microbiome) must be considered when selecting and designing CRS materials.
However, understanding of the soil microbiome and the chemical environment generated by root exudates and the microbiota secretome is limited, in part by its complexity. Organisms living in soil often have to navigate multiple habitats (e.g., air, water, and solid media) as well as interact with other organisms and/or find hosts. Navigation is often mediated by the metabolome of focal organisms and interaction with the metabolome of neighbors. For example, plants manipulate their rhizosphere microbiome composition via release of metabolites in their exudates (e.g. (Tsunoda and van Dam, 2017; Vives-Peris et al., 2020; Rizaludin et al., 2021)), and microbes communicate and compete via the release of metabolites (Weisskopf et al., 2021; Avalos et al., 2022). However, whereas the importance of the soil metabolome has been established, its content is less understood. Plant metabolomes are the most frequently studied ((Weston and Mathesius, 2013; Schmelz et al., 2014; Tsunoda and van Dam, 2017), but knowledge is primarily limited to crop species and model systems. The metabolomes of beneficial or pathogenic soil microbes, which could be used to target them in situ, are not well understood. This is further complicated by the difficulties in culturing soil microbes—whereas microbiologists can now culture the majority of microbes found in gut systems, the ability to culture soil microbes lags far behind. Thus, there are multiple opportunities for genomics, transcriptomics, and metabolomics to contribute to expanding our knowledge of soil organisms and identifying specific metabolites produced by plants and microbes that could be used to improve agricultural CRS design.
7 Translating biomedical CRS diffusion models to agriculture CRSs
Once compatibility with the soil ecosystem has been established, the next challenge is understanding compound release, which is doubly challenging for agricultural CRSs. First, the encapsulant must be released from the carrier itself into the soil phase. Then, the encapsulant must navigate through complex, multiphase soil media to reach its intended target. Fortunately, many of the diffusion models developed for biomedical engineering can be readily translated to agricultural systems (Table 1) (Bruschi and Bruschi, 2015; Paarakh et al., 2019; Trucillo, 2022). Many common agricultural CRSs consist of solid granules coated with polymers (Guilherme et al., 2015). These materials are akin to biomedical tablet formulations that can be described using zero order kinetics or Hixson-Crowell models. Agriculture controlled release is characterized by a lag period, often seen in biomedical CRSs, followed by a period of constant release (zero order release) when the solute concentration greatly exceeds solubility limits, and then a gradual decay as the carrier concentration falls below the solubility limits of the soil media because of the declining diffusional driving force (Hixson-Cromwell) (Guilherme et al., 2015). However, mathematical models have also been developed specifically for agricultural CRS (Fickian Three Stage) based on a direct solution of Fick’s second law. These models are suitable for compositions in which the encapsulant is a solid, initially present in large excess relative to its solubility in the fluid phase.
TABLE 1
| Model | Formulation | Equation | Release Type | Assumptions | Ref. |
|---|---|---|---|---|---|
| Zero Order | Coated Granules | Fickian Diffusion | Initial solute concentration > than solubility in soil low solubility | Crank, (1975) | |
| Hixson-Crowell | Coated Granules | Solute dissolution from a surface | Solute dissolution occurs in a plane parallel to the CRS surface Geometric form is unchanged by dissolution | Hixson and Crowell, (1931) | |
| Fickian Three Stage | Coated Granules | Stage 1: Stage 2: Stage 3: | Fickian Diffusion | Water saturation of the matrix Initial solute concentration > than solubility in soil | Du et al. (2004) |
| Fickian (short times, sphere) | Matrix | Fickian Diffusion | Only describes first 10–15% of release data | (Crank, 1975; Ritger and Peppas, 1987) | |
| Higuchi | Matrix | Fickian Diffusion | Matrix is not altered by water contact Matrix is saturated with drug Unidirection diffusion (no edge effects) Matrix is much larger than encapsulant size Matrix swelling and dissolution is negligible Perfect sink conditions in the bulk phase are maintained | Higuchi, (1961) | |
| Peppas | Matrix | Multi | Good for ∼ first 60% of release data. Known matrix geometry Degradation and diffusion components are time matched | (Korsmeyer et al., 1983; Ritger and Peppas, 1987) |
Biomedical diffusion models.
solute release or dissolved, initial solute in soil (usually zero), zero order release constant, time, initial solute mass, solute mass at time (t), constant of proportionality, solute density, radius of diffusion, the solute diffusion coefficient, lag time, diffusion length, saturated solute concentration, time to complete dissolution of the solute, Higuchi release constant, effective diffusion constant, diffusional exponent.
Alternatively, there has been interest in developing agriculture CRSs that encapsulate soluble compounds in polymer or sugar-based matrices. In these systems, the encapsulant is usually present at a concentration below its solubility limit and is usually dispersed throughout the matrix. Like biomedical CRSs, these CRSs can exhibit passive, degradational, or smart/triggered release. However, the most common materials are nanoparticles that employ passive, or more commonly, degradational release (Shakiba et al., 2020). Release in these systems usually includes a passive, concentration-gradient driven component that is accelerated by degradation of the matrix material. As the matrix degrades, the pore sizes change. Thus, the diffusion coefficient, D, is a function of time. These types of release correspond to either pure Fickian behaviors (Crank, 1975) or anomalous diffusion that can be understood using one of several existing biomedical CRS models (Table 1).
In a standard Fickian model with no degradation, release as measured by , the amount of release at time, t, relative to the total amount release at the endpoint, is proportional to . The Higuchi model (Higuchi, 1961) improves upon this slightly by lumping together other terms in the Fickian solution into a proportionality constant. Models that capture anomalous diffusion behaviors improve upon these by recognizing that D is a function of time, the most popular of which is the Peppas (Korsmeyer et al., 1983) equation. The Peppas equation defines release by the effective diffusion constant, k, and the diffusional exponent, n. The Peppas equation has the advantage of being applicable to a wide variety of systems regardless of their mechanism of release, and is in fact often used to determine the release type, which correlates with the value of n. Depending on geometry, n is ∼0.5 for Fickian release, between ∼0.5 and 1 for anomalous diffusion driven by degradation or time-dependent swelling, and for Case II transport characteristic of glassy polymer relaxation. These models are equally applicable for biomedical and agricultural CRSs.
Once a compound has been released from the CRS, it must navigate through soil to reach its target. In biomedical systems, this may mean navigating through blood, tissue, or fluid filled spaces. In agriculture, this primarily means navigating through the soil. Similar to biomedical environments, soil can exist in a myriad of forms and compositions (Wiesmeier et al., 2019), exhibiting varying hydration, porosity, and chelation strengths that effect diffusion. Fortunately, soil diffusion models have been well developed in the context of petrochemical engineering and geological sciences (Ghanbarian et al., 2013; Hunt and Sahimi, 2017). Diffusion through the complex soil network is captured using a tortuosity parameter ():where is the porosity, is the diffusion constant in liquid media, and is the value observed in soil. Porosity can be modeled from percolation theory (Hunt and Sahimi, 2017), with the Rieu and Sposito (RS) model commonly used (Rieu and Sposito, 1991).
As with biomedical CRSs, it is important to consider several factors when using these models to determine the release rate in an actual use setting. Many systems are characterized in vitro where infinite sink conditions are maintained (i.e., matrix volume is 1:10 volume of the bulk phase). This assumption may not be the case if a compound is released into a sterile environment. Mixing in soil, which is a multiphase mixture, is much lower than in pure liquid phases, but may be closer to the poor mixing conditions experienced in tissues. If mixing is poor, a compound may build up in the bulk phase despite sink conditions. A second problem, also experienced by biomedical CRSs, is off-target removal of the compound from the bulk phase. In soil, a compound could be removed via chelation (Marschner et al., 2011), hydrolytic or enzymatic degradation, or off-target uptake by another organism (Canarini et al., 2019). In this sense, agricultural delivery has many parallels to challenging gastrointestinal biological delivery environments, such as the stomach or gut (Fan and Pedersen, 2021). All of these factors may alter CRS performance relative to in vitro testing results.
8 Discussion: Opportunities to translate biomedical CRSs to agriculture
8.1 Smart release CRSs for agriculture
Currently, most agricultural CRSs rely on passive transport or biodegradation for delivery. However, smart release strategies offer many advantages in that they can directly respond to conditions in the soil environment. Agricultural smart release strategies are relatively new, with virtually no reports prior to 2015 (Calabi-Floody et al., 2018; Camara et al., 2019). Most agricultural smart release systems are based on pH or temperature changes, with only 10% utilizing enzymatic degradation (Camara et al., 2019). Agricultural pH- or temperature-responsive CRSs are comprised of different materials than biomedical CRSs, largely because the temperature and pH conditions in agricultural fields differ widely from those found in the body. However, there is also emphasis on the use of low cost, ecological friendly materials. Common materials employed include chitosan, alginate, and polydopamine; specific functionalizations can also be added to generate pH- and temperature-responsive behaviors (Camara et al., 2019). It is likely that development of pH- and temperature-responsive CRSs will continue to grow; however, these are relatively untargeted in their response. Temperature changes could reflect seasonal variations and pH changes could result from fertilization or other interventions.
By far, the largest opportunity for smart agricultural CRSs lies in enzyme-responsive systems. Enzymatic-responsive CRSs for agriculture are emergent, despite the fact that all soil organisms produce enzymes that degrade substrates in their microenvironment. Examples of agricultural enzymatic-responsive CRSs include cellulose materials responsive to cellulases and starches responsive to amylases (Camara et al., 2019). However, development of these systems has been limited by a lack of knowledge of the plant and microbial secretomes (e.g. (Kamel et al., 2017; Lanfranco et al., 2018b; Vincent et al., 2020)). Advances in agricultural enzymatic-responsive CRSs will require concomitant advances in plant and microbe ‘-omics’. A further difficulty is the complexity of the soil microbiome. For example, all fungi emit hydrolases (Béguin and Aubert, 1994) that break down plant cell walls. Thus, targeting a specific (e.g., pathogenic) fungal class would require complex materials optimization and would likely require utilization of several enzyme-substrate combinations. If these barriers can be overcome, smart release CRSs offer many potential benefits with the potential to manage the microbiome at the scale of the soil microbes themselves.
8.2 Agricultural theranostics
Another significant opportunity for advancing CRSs is the potential for developing agricultural equivalents of biomedical theranostic systems. Theranostics are a class of materials that can both sense and treat a disease. This is often achieved using materials that exhibit “smart” release (see above) in combination with built in reporter elements. For example, a smart hydrogel could incorporate a fluorescent reporter that is quenched until cleaved by an enzyme (Leight et al., 2013), releasing a drug and restoring fluorescence of the reporter.
Such materials would be complementary to existing practices in “precision farming”, which focus on more precise application of agrichemical inputs. Precision farming technologies, such as drones, can sense a need for agrochemicals at the centimeter scale (e.g. (de Jesus Colwell et al., 2021)). Whereas these technologies have great potential, they require significant infrastructure investment that can limit application for some stakeholders. Additionally, there is still a minimum scale at which agrochemical application occurs—typically about a meter, much larger than soil organisms (∼micrometers in size). Finally, precision farming is still reactive: agrochemicals are applied when deficiencies or pathogens are detected. Current state-of-the-art technology allows for earlier antagonist detection; however, damage to the crop must occur before a farmer can react with agrochemical applications. Thus, current methods to control agrochemical delivery are expensive, limited in scale, and are necessarily reactive.
Developing theranostic CRSs for agriculture similar to their biomedical counterparts will require significant advances in soil sensing technologies. Because of the poor light transmissibility of soil and potential toxicity, theranostic detection methods used in biomedicine based on fluorescence or radiological signals are not appropriate for farming (El-Sawy et al., 2018). Agricultural techniques such as reflectance spectroscopy for monitoring plant health (Katsoulas et al., 2016) or wireless sensor networks for monitoring soil conditions (Ojha et al., 2015) could be integrated with CRS depots that release agrochemicals into the soil upon activation. However, these are only modest improvements over existing precision farming methods. Some progress may be obtained from the burgeoning field of nanoparticle disease sensors (Singh et al., 2022). However, true agricultural theranostics require materials that sense a condition in the soil and release an ameliorating compound in response. Existing smart materials that respond to pH and temperature changes offer promise as theranostics (Camara et al., 2019), but they are non-specific. Ideally, concomitant advances in enzymatically responsive systems or those requiring multiple cues for activation would be used. Additionally, most theranostics report sensing results to the external world, but currently used stimuli-responsive materials lack this capability. One can envision smart materials that incorporate electrochemical or optical sensing components (e.g., co-encapsulated metallic nanoparticles) that could be coupled with inground sensors for read-out. Combined with Internet of Things (IoT) connectivity, such an approach could compete with precision farming technologies. However, at present, this remains an area of great opportunity.
9 Conclusion
Given the increasing challenge of feeding a growing population, the translation of biomedical CRS technologies to agricultural delivery practices will likely play an important role in sustainable food production. With the potential to target crop plants, the soil microbiome, or modulate the properties of soil, CRSs offer many advantages. CRSs can protect compounds from the environment, provide spatiotemporal control of their release, and can limit agricultural waste, run-off, and concomitant eutrophication (Schindler, 2012; Paerl et al., 2016). However, agricultural CRSs are underdeveloped compared to biomedical CRS technologies. Currently, CRSs are primarily employed as seed and fertilizer coatings (marketresearch, 2022a; marketresearch, 2022b), whereas hydrogel and nanoparticle formulations are still being developed. Most agricultural systems rely on passive or degradational release, whereas smart, stimuli-responsive materials are still being realized (Calabi-Floody et al., 2018; Moulick et al., 2020) and advanced theranostic approaches remain beyond reach. Nevertheless, agricultural and biomedical CRSs share many features. They employ similar materials and manufacturing methods (although agricultural CRSs favor inexpensive natural materials composed of starches or sugars) and their release is governed by similar mathematical frameworks. However, advances in understanding the soil microenvironment and plant molecule biology are needed to realize advanced technologies, like smart CRSs and theranostics. Additionally, sensing methods that can penetrate soil and are compatible with large-scale, IoT sensing networks are vital. These advances will likely require a convergent research approach that combines existing experience in biomedical CRSs with expertise in soil ecology, agricultural science, horticulture, and microbiology. Agricultural CRSs are thus at a precipice, with the potential to offer solutions to the urgent problems of providing a sustainable food supply while minimizing pollution. However, it is only through combined efforts across multiple disciplines that their promise will be achieved.
Statements
Author contributions
PL: Writing-Original Draft, Writing-Review and Editing, Visualization; XL: Writing-Original Draft, Writing, Review and Editing, Visualization; FK: Writing-Original Draft, Writing-Review and Editing, Visualization; AB: Writing-Conceptualization, Original Draft, Writing-Review and Editing, Visualization, Supervision; JW: Writing-Conceptualization, Original Draft, Writing-Review and Editing, Visualization, Supervision.
Funding
This research was supported by funding from the Ohio State University Sustainability Institute NSF SitS 2226740.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Afzal I. Javed T. Amirkhani M. Taylor A. G. (2020). Modern seed technology: Seed coating delivery systems for enhancing seed and crop performance. Agriculture10, 526. 10.3390/agriculture10110526
2
Al Rohily K. El-Hamshary H. Ghoneim A. Modaihsh A. (2020). Controlled release of phosphorus from superabsorbent phosphate-bound alginate-graft-polyacrylamide: Resistance to soil cations and release mechanism. ACS Omega5, 32919–32929. 10.1021/acsomega.0c03740
3
Aouada F. A. Moura M. R. d. Lopes da Silva W. T. Muniz E. C. Mattoso L. H. C. (2011). Preparation and characterization of hydrophilic, spectroscopic, and kinetic properties of hydrogels based on polyacrylamide and methylcellulose polysaccharide. J. Appl. Polym. Sci.120, 3004–3013. 10.1002/app.33425
4
Artusio F. Casà D. Granetto M. Tosco T. Pisano R. (2021). Alginate nanohydrogels as a biocompatible platform for the controlled release of a hydrophilic herbicide. Processes9, 1641. 10.3390/pr9091641
5
Atkinson J. A. Rasmussen A. Traini R. Voß U. Sturrock C. Mooney S. J. et al (2014). Branching out in roots: Uncovering form, function, and regulation. Plant Physiol.166, 538–550. 10.1104/pp.114.245423
6
Avalos M. Garbeva P. Vader L. van Wezel G. P. Dickschat J. S. Ulanova D. (2022). Biosynthesis, evolution and ecology of microbial terpenoids. Nat. Prod. Rep.39, 249–272. 10.1039/d1np00047k
7
Azevedo T. L. F. Bertonha A. (2008). The water holding capacity of hydrogel as affected by concentrations of different salts. Leuven, Belgium: International Society for Horticultural Science ISHS, 655–660.
8
Bandara S. Du H. Carson L. Bradford D. Kommalapati R. (2020). Agricultural and biomedical applications of chitosan-based nanomaterials. Nanomater. (Basel)10.
9
Barclay T. G. Day C. M. Petrovsky N. Garg S. (2019). Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym.221, 94–112. 10.1016/j.carbpol.2019.05.067
10
Béguin P. Aubert J. P. (1994). The biological degradation of cellulose. FEMS Microbiol. Rev.13, 25–58. 10.1111/j.1574-6976.1994.tb00033.x
11
Bennett A. E. Daniell T. J. White P. J. (2013). “Benefits of breeding crops for yield response to soil organisms,” in Molecular microbial biology of the rhizosphere. Editor de BruijnF. J. (Hoboken, New Jersey, USA: Wiley-Blackwell), 17–27.
12
Bennett A. E. Groten K. (2022). The costs and benefits of plant–arbuscular mycorrhizal fungal interactions. Annu. Rev. Plant Biol.73, 649–672. 10.1146/annurev-arplant-102820-124504
13
Bhattacharya N. Cahill D. M. Yang W. Kochar M. (2022). Graphene as a nano-delivery vehicle in agriculture – current knowledge and future prospects. Crit. Rev. Biotechnol., 1–19. 10.1080/07388551.2022.2090315
14
Bobo D. Robinson K. J. Islam J. Thurecht K. J. Corrie S. R. (2016). Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res.33, 2373–2387. 10.1007/s11095-016-1958-5
15
Boehm A. Martinon I. Zerrouk R. Rump E. Fessi H. (2003). Nanoprecipitation technique for the encapsulation of agrochemical active ingredients. J. Microencapsul.20, 433–441. 10.1080/0265204021000058410
16
Brown L. Siemer L. Munoz C. Langer R. (1986). Controlled release of insulin from polymer matrices. in vitro kinetics. Diabetes35, 684–691. 10.2337/diab.35.6.684
17
Bruschi M. L. (2015). “Chapter 5 - mathematical models of drug release,” in Strategies to modify the drug release from pharmaceutical systems. Editor BruschiM. L. (Waltham, MA: Woodhead Publishing), 63–86.
18
Calabi-Floody M. Medina J. Rumpel C. Condron L. M. Hernandez M. Dumont M. et al (2018). Smart fertilizers as a strategy for sustainable agriculture. Adv. Agron.147, 119–157. 10.1016/bs.agron.2017.10.003
19
Camara M. C. Campos E. V. R. Monteiro R. A. Pereira A. D. S. Proenca P. L. D. Fraceto L. F. (2019). Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol.17, 100. 10.1186/s12951-019-0533-8
20
Canarini A. Kaiser C. Merchant A. Richter A. Wanek W. (2019). Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci.10, 157. 10.3389/fpls.2019.00157
21
Cardarelli M. Woo S. L. Rouphael Y. Colla G. (2022). Seed treatments with microorganisms can have a biostimulant effect by influencing germination and seedling growth of crops. Plants11, 259. 10.3390/plants11030259
22
Chaud M. Souto E. B. Zielinska A. Severino P. Batain F. Oliveira-Junior J. et al (2021). Nanopesticides in agriculture: Benefits and challenge in agricultural productivity, toxicological risks to human health and environment. Toxics9, 131. 10.3390/toxics9060131
23
Chaudhary J. Thakur S. Sharma M. Gupta V. K. Thakur V. K. (2020). Development of biodegradable agar-agar/gelatin-based superabsorbent hydrogel as an efficient moisture-retaining agent. Biomolecules10, 939. 10.3390/biom10060939
24
Chen G. H. Hoffman A. S. (1995). Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature373, 49–52. 10.1038/373049a0
25
Chen H. Zhang Y. Zhong Q. (2015). Physical and antimicrobial properties of spray-dried zein–casein nanocapsules with co-encapsulated eugenol and thymol. J. Food Eng.144, 93–102. 10.1016/j.jfoodeng.2014.07.021
26
Chen J. Lu S. Y. Zhang Z. Zhao X. X. Li X. M. Ning P. et al (2018). Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Total Environ.613, 829–839. 10.1016/j.scitotenv.2017.09.186
27
Chen K. Fu Z. Wang M. Lv Y. Wang C. Shen Y. et al (2018). Preparation and characterization of size-controlled nanoparticles for high-loading λ-cyhalothrin delivery through flash nanoprecipitation. J. Agric. Food Chem.66, 8246–8252. 10.1021/acs.jafc.8b02851
28
Chen Y.-C. Chen Y.-H. (2019). Thermo and pH-responsive methylcellulose and hydroxypropyl methylcellulose hydrogels containing K2SO4 for water retention and a controlled-release water-soluble fertilizer. Sci. Total Environ.655, 958–967. 10.1016/j.scitotenv.2018.11.264
29
Chen Y. P. Rekha P. D. Arun A. B. Shen F. T. Lai W. A. Young C. C. (2006). Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol.34, 33–41. 10.1016/j.apsoil.2005.12.002
30
Chu M. Zhu S. Q. Li H. M. Huang Z. B. Li S. Q. (2006). Synthesis of poly(acrylic acid)/sodium humate superabsorbent composite for agricultural use. J. Appl. Polym. Sci.102, 5137–5143. 10.1002/app.24661
31
Chun S. Feng J. (2021). Preparation of abamectin nanoparticles by flash nanoprecipitation for extended photostability and sustained pesticide release. ACS Appl. Nano Mat.4, 1228–1234. 10.1021/acsanm.0c02847
32
Crank J. (1975). The mathematics of diffusion. Oxford: Oxford University Press.
33
D'Addio S. M. Prud'homme R. K. (2011). Controlling drug nanoparticle formation by rapid precipitation. Adv. Drug Deliv. Rev.63, 417–426. 10.1016/j.addr.2011.04.005
34
de Jesus Colwell F. Souter J. Bryan G. J. Compton L. J. Boonham N. Prashar A. (2021). Development and validation of methodology for estimating potato canopy structure for field crop phenotyping and improved breeding. Front. Plant Sci.12, 612843. 10.3389/fpls.2021.612843
35
De La Torre-Roche R. Cantu J. Tamez C. Zuverza-Mena N. Hamdi H. Adisa I. O. et al (2020). Seed biofortification by engineered nanomaterials: A pathway to alleviate malnutrition?J. Agric. Food Chem.68, 12189–12202. 10.1021/acs.jafc.0c04881
36
de Souza R. M. Seibert D. Quesada H. B. Bassetti F. d. J. Fagundes-Klen M. R. Bergamasco R. (2020). Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf. Environ. Prot.135, 22–37. 10.1016/j.psep.2019.12.035
37
Dean R. Van Kan J. A. L. Pretorius Z. A. Hammond-Kosack K. E. Di Pietro A. Spanu P. D. et al (2012). The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol.13, 414–430. 10.1111/j.1364-3703.2011.00783.x
38
Dhanapal V. Subhapriya P. Nithyanandam K. P. Kiruthika M. V. Keerthana T. Dineshkumar G. (2021). Design, synthesis and evaluation of N, N1-methylenebisacrylamide crosslinked smart polymer hydrogel for the controlled release of water and plant nutrients in agriculture field. Mater. Today Proc.45, 2491–2497. 10.1016/j.matpr.2020.11.101
39
Divya K. Jisha M. S. (2018). Chitosan nanoparticles preparation and applications. Environ. Chem. Lett.16, 101–112. 10.1007/s10311-017-0670-y
40
Dong L. C. Hoffman A. S. (1986). Thermally reversible hydrogels: III. Immobilization of enzymes for feedback reaction control. J. Control. Release4, 223–227. 10.1016/0168-3659(86)90006-4
41
Du C. Zhou J. Shaviv A. Wang H. (2004). Mathematical model for potassium release from polymer-coated fertiliser. Biosyst. Eng.88, 395–400. 10.1016/j.biosystemseng.2004.03.004
42
El Hadrami A. Adam L. R. El Hadrami I. Daayf F. (2010). Chitosan in plant protection. Mar. Drugs8, 968–987. 10.3390/md8040968
43
El-Sawy H. S. Al-Abd A. M. Ahmed T. A. El-Say K. M. Torchilin V. P. (2018). Stimuli-responsive nano-architecture drug-delivery systems to solid tumor micromilieu: Past, present, and future perspectives. ACS Nano12, 10636–10664. 10.1021/acsnano.8b06104
44
Elbarbary A. M. Ghobashy M. M. (2017). Controlled release fertilizers using superabsorbent hydrogel prepared by gamma radiation. Radiochim. Acta105, 865–876. 10.1515/ract-2016-2679
45
Falamarzian M. Varshosaz J. (1998). The effect of structural changes on swelling kinetics of polybasic/hydrophobic pH-sensitive hydrogels. Drug Dev. Ind. Pharm.24, 667–669. 10.3109/03639049809082369
46
Fan Y. Pedersen O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol.19, 55–71. 10.1038/s41579-020-0433-9
47
Fan Y. Xu J. Gao X. Fu X. Yang X. (2019). Effect of alginate on the release of amide nitrogen for soilless cultivation applications. Sci. Hortic.256, 108545. 10.1016/j.scienta.2019.108545
48
Farooq M. Wahid A. Siddique K. H. M. (2012). Micronutrient application through seed treatments - a review. J. Soil Sci. Plant Nutr.12, 125–142. 10.4067/s0718-95162012000100011
49
Feng J. Markwalter C. E. Tian C. Armstrong M. Prud'homme R. K. (2019). Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. J. Transl. Med.17, 200. 10.1186/s12967-019-1945-9
50
Feng J. Markwalter C. E. Tian C. Armstrong M. Prud’homme R. K. (2019). Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. J. Transl. Med.17, 200–209. 10.1186/s12967-019-1945-9
51
Fessi H. Puisieux F. Devissaguet J. P. Ammoury N. Benita S. (1989). Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. X.55, R1–R4. 10.1016/0378-5173(89)90281-0
52
Folkman J. Long D. M. (1964). The use of silicone rubber as a carrier for prolonged drug therapy. J. Surg. Res.4, 139–142. 10.1016/s0022-4804(64)80040-8
53
Food and Agriculture Food and agriculture organization of the United_Nations: Statistics division. FAOSTAT 2022. Available at: https://www.fao.org/faostat/en/.Accessed.
54
Fu J. Wang C. Chen X. Huang Z. Chen D. (2018). Classification research and types of slow controlled release fertilizers (SRFs) used - a review. Commun. Soil Sci. Plant Anal.49, 2219–2230. 10.1080/00103624.2018.1499757
55
Fu Y. Kao W. J. (2010). Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv.7, 429–444. 10.1517/17425241003602259
56
Fujita S. Tazawa T. Kono H. (2022). Preparation and enzyme degradability of spherical and water-absorbent gels from sodium carboxymethyl cellulose. Gels8, 321. 10.3390/gels8050321
57
Gao L. Luo H. Wang Q. Hu G. Xiong Y. (2021). Synergistic effect of hydrogen bonds and chemical bonds to construct a starch-based water-absorbing/retaining hydrogel composite reinforced with cellulose and poly(ethylene glycol). ACS Omega6, 35039–35049. 10.1021/acsomega.1c05614
58
Ghaderpoori M. Jafari A. Nazari E. Rashidipour M. Nazari A. Chehelcheraghi F. et al (2020). Preparation and characterization of loaded paraquat-polymeric chitosan/xantan/tripolyphosphate nanocapsules and evaluation for controlled release. J. Environ. Health Sci. Eng.18, 1057–1066. 10.1007/s40201-020-00527-3
59
Ghanbarian B. Hunt A. G. Ewing R. P. Sahimi M. (2013). Tortuosity in porous media: A critical review. Soil Sci. Soc. Am. J.77, 1461–1477. 10.2136/sssaj2012.0435
60
Ghorbani S. Eyni H. Bazaz S. R. Nazari H. Asl L. S. Zaferani H. et al (2018). Hydrogels based on cellulose and its derivatives: Applications, synthesis, and characteristics. Polym. Sci. Ser. A60, 707–722. 10.1134/s0965545x18060044
61
Gruden K. Lidoy J. Petek M. Podpečan V. Flors V. Papadopoulou K. K. et al (2020). Ménage à Trois: Unraveling the Mechanisms Regulating Plant–Microbe–Arthropod Interactions. Trends Plant Sci.25, 1215–1226. 10.1016/j.tplants.2020.07.008
62
Guilherme M. R. Aouada F. A. Fajardo A. R. Martins A. F. Paulino A. T. Davi M. F. T. et al (2015). Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J.72, 365–385. 10.1016/j.eurpolymj.2015.04.017
63
Guo W. Chen L. Herrera-Estrella L. Cao D. Tran L.-S. P. (2020). Altering plant architecture to improve performance and resistance. Trends Plant Sci.25, 1154–1170. 10.1016/j.tplants.2020.05.009
64
Gupta P. Vermani K. Garg S. (2002). Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today7, 569–579. 10.1016/s1359-6446(02)02255-9
65
Hack B. Egger H. Uhlemann J. Henriet M. Wirth W. Vermeer A. W. et al (2012). Advanced agrochemical formulations through encapsulation strategies?Chem. Ing. Tech.84, 223–234. 10.1002/cite.201100212
66
Han J. Guenier A. S. Salmieri S. Lacroix M. (2008). Alginate and chitosan functionalization for micronutrient encapsulation. J. Agric. Food Chem.56, 2528–2535. 10.1021/jf703739k
67
Han J. Zhu Z. Qian H. Wohl A. R. Beaman C. J. Hoye T. R. et al (2012). A simple confined impingement jets mixer for flash nanoprecipitation. J. Pharm. Sci.101, 4018–4023. 10.1002/jps.23259
68
Hayat R. Ali S. Amara U. Khalid R. Ahmed I. (2010). Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol.60, 579–598. 10.1007/s13213-010-0117-1
69
Hien N. Q. Nagasawa N. Tham L. X. Yoshii F. Dang V. H. Mitomo H. et al (2000). Growth-promotion of plants with depolymerized alginates by irradiation. Radiat. Phys. Chem. Oxf. Engl. 1993.59, 97–101. 10.1016/s0969-806x(99)00522-8
70
Higuchi T. (1961). Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci.50, 874–875. 10.1002/jps.2600501018
71
Hines D. J. Kaplan D. L. (2013). Poly(lactic-co-glycolic) acid-controlled-release systems: Experimental and modeling insights. Crit. Rev. Ther. Drug Carr. Syst.30, 257–276. 10.1615/critrevtherdrugcarriersyst.2013006475
72
Hixson A. W. Crowell J. H. (1931). Dependence of reaction velocity upon surface and agitation. Ind. Eng. Chem.23, 1160–1168. 10.1021/ie50262a025
73
Hoffman A. S. (2008). The origins and evolution of “controlled” drug delivery systems. J. Control. Release132, 153–163. 10.1016/j.jconrel.2008.08.012
74
Hornig S. Heinze T. Becer C. R. Schubert U. S. (2009). Synthetic polymeric nanoparticles by nanoprecipitation. J. Mat. Chem.19, 3838. 10.1039/b906556n
75
Hou Q. Zhang H. Bao L. Song Z. Liu C. Jiang Z. et al (2021). NCs-delivered pesticides: A promising candidate in smart agriculture. Int. J. Mol. Sci.22, 13043. 10.3390/ijms222313043
76
Hu Z.-Y. Chen G. Yi S.-H. Wang Y. Liu Q. Wang R. (2021). Multifunctional porous hydrogel with nutrient controlled-release and excellent biodegradation. J. Environ. Chem. Eng.9, 106146. 10.1016/j.jece.2021.106146
77
Huang X. Hilscher J. Stoger E. Christou P. Zhu C. (2021). Modification of cereal plant architecture by genome editing to improve yields. Plant Cell Rep.40, 953–978. 10.1007/s00299-021-02668-7
78
Hunt A. G. Sahimi M. (2017). Flow, transport, and reaction in porous media: Percolation scaling, critical-path analysis, and effective medium approximation. Rev. Geophys.55, 993–1078. 10.1002/2017rg000558
79
Ibrahim S. M. El Salmawi K. M. Zahran A. H. (2007). Synthesis of crosslinked superabsorbent carboxymethyl cellulose/acrylamide hydrogels through electron-beam irradiation. J. Appl. Polym. Sci.104, 2003–2008. 10.1002/app.25916
80
Islam M. R. Xue X. Z. Mao S. S. A. Ren C. Z. Eneji A. E. Hu Y. G. (2011). Effects of water-saving superabsorbent polymer on antioxidant enzyme activities and lipid peroxidation in oat (Avena sativa L.) under drought stress. J. Sci. Food Agric.91, 680–686. 10.1002/jsfa.4234
81
Iwasaki N. Yamane S.-T. Majima T. Kasahara Y. Minami A. Harada K. et al (2004). Feasibility of polysaccharide hybrid materials for scaffolds in cartilage tissue engineering: Evaluation of chondrocyte adhesion to polyion complex fibers prepared from alginate and chitosan. Biomacromolecules5, 828–833. 10.1021/bm0400067
82
Jain K. K. (2008). “Drug delivery systems - an overview,” in Drug delivery systems. Editor JainK. K. (Totowa, NJ: Humana Press), 1–50.
83
Jain R. A. (2000). The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials21, 2475–2490. 10.1016/s0142-9612(00)00115-0
84
Jariwala H. Santos R. M. Lauzon J. D. Dutta A. Wai Chiang Y. (2022). Controlled release fertilizers (CRFs) for climate-smart agriculture practices: A comprehensive review on release mechanism, materials, methods of preparation, and effect on environmental parameters. Environ. Sci. Pollut. Res.29, 53967–53995. 10.1007/s11356-022-20890-y
85
Jiang T. James R. Kumbar S. G. Laurencin C. T. (2014). “Chapter 5 - chitosan as a biomaterial: Structure, properties, and applications in tissue engineering and drug delivery,” in Natural and synthetic biomedical polymers. Editors KumbarS. G.LaurencinC. T.DengM. (Oxford: Elsevier), 91–113.
86
Johnson B. K. Prud'homme R. K. (2003). Flash NanoPrecipitation of organic actives and block copolymers using a confined impinging jets mixer. Aust. J. Chem.56, 1021–1024. 10.1071/ch03115
87
Kacsó T. Neaga I. O. Erincz A. Astete C. E. Sabliov C. M. Oprean R. et al (2018). Perspectives in the design of zein-based polymeric delivery systems with programmed wear down for sustainable agricultural applications. Polym. Degrad. Stab.155, 130–135. 10.1016/j.polymdegradstab.2018.07.014
88
Kamel L. Tang N. Malbreil M. San Clemente H. Le Marquer M. Roux C. et al (2017). Corrigendum: The comparison of expressed candidate secreted proteins from two arbuscular mycorrhizal fungi unravels common and specific molecular tools to invade different host plants. Front. Plant Sci.8, 2065. 10.3389/fpls.2017.02065
89
Katsoulas N. Elvanidi A. Ferentinos K. P. Kacira M. Bartzanas T. Kittas C. (2016). Crop reflectance monitoring as a tool for water stress detection in greenhouses: A review. Biosyst. Eng.151, 374–398. 10.1016/j.biosystemseng.2016.10.003
90
Kikuchi A. Okano T. (2002). Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev.54, 53–77. 10.1016/s0169-409x(01)00243-5
91
Kleinman P. J. A. (2017). The persistent environmental relevance of soil phosphorus sorption saturation. Curr. Pollut. Rep.3, 141–150. 10.1007/s40726-017-0058-4
92
Korsmeyer R. W. Gurny R. Doelker E. Buri P. Peppas N. A. (1983). Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. X.15, 25–35. 10.1016/0378-5173(83)90064-9
93
Kumar S. Bhanjana G. Sharma A. Sidhu M. Dilbaghi N. (2014). Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym.101, 1061–1067. 10.1016/j.carbpol.2013.10.025
94
Kumaraswamy R. V. Kumari S. Choudhary R. C. Pal A. Raliya R. Biswas P. et al (2018). Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Biol. Macromol.113, 494–506. 10.1016/j.ijbiomac.2018.02.130
95
Lafaille N. (2018). The development of fertilizer from the early years to today©. Acta Hortic.1212, 213–216.
96
Lanfranco L. Fiorilli V. Gutjahr C. (2018). Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol.220, 1031–1046. 10.1111/nph.15230
97
Lanfranco L. Fiorilli V. Venice F. Bonfante P. (2018). Strigolactones cross the kingdoms: Plants, fungi, and bacteria in the arbuscular mycorrhizal symbiosis. J. Exp. Bot.69, 2175–2188. 10.1093/jxb/erx432
98
Lee K. Y. Mooney D. J. (2012). Alginate: Properties and biomedical applications. Prog. Polym. Sci.37, 106–126. 10.1016/j.progpolymsci.2011.06.003
99
Lee P. I. (1985). Kinetics of drug release from hydrogel matrices. J. Control. Release2, 277–288. 10.1016/0168-3659(85)90051-3
100
Leight J. L. Alge D. L. Maier A. J. Anseth K. S. (2013). Direct measurement of matrix metalloproteinase activity in 3D cellular microenvironments using a fluorogenic peptide substrate. Biomaterials34, 7344–7352. 10.1016/j.biomaterials.2013.06.023
101
Li N. Sun C. Jiang J. Wang A. Wang C. Shen Y. et al (2021). Advances in controlled-release pesticide formulations with improved efficacy and targetability. J. Agric. Food Chem.69, 12579–12597. 10.1021/acs.jafc.0c05431
102
Liang J. Yu M. Guo L. Cui B. Zhao X. Sun C. et al (2017). Bioinspired development of P (St–MAA)–avermectin nanoparticles with high affinity for foliage to enhance folia retention. J. Agric. Food Chem.66, 6578–6584. 10.1021/acs.jafc.7b01998
103
Liechty W. B. Kryscio D. R. Slaughter B. V. Peppas N. A. (2010). Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng.1, 149–173. 10.1146/annurev-chembioeng-073009-100847
104
Lim J.-M. Swami A. Gilson L. M. Chopra S. Choi S. Wu J. et al (2014). Ultra-high throughput synthesis of nanoparticles with homogeneous size distribution using a coaxial turbulent jet mixer. ACS Nano8, 6056–6065. 10.1021/nn501371n
105
Liu Y. Cheng C. Prud’homme R. K. Fox R. O. (2008). Mixing in a multi-inlet vortex mixer (MIVM) for flash nano-precipitation. Chem. Eng. Sci.63, 2829–2842. 10.1016/j.ces.2007.10.020
106
Macintosh K. A. Doody D. G. Withers P. J. A. McDowell R. W. Smith D. R. Johnson L. T. et al (2019). Transforming soil phosphorus fertility management strategies to support the delivery of multiple ecosystem services from agricultural systems. Sci. Total Environ.649, 90–98. 10.1016/j.scitotenv.2018.08.272
107
Mai Y. Eisenberg A. (2012). Self-assembly of block copolymers. Chem. Soc. Rev.41, 5969–5985. 10.1039/c2cs35115c
108
Maluin F. N. Hussein M. Z. Yusof N. A. Fakurazi S. Idris A. S. Zainol Hilmi N. H. et al (2019). Preparation of chitosan–hexaconazole nanoparticles as fungicide nanodelivery system for combating Ganoderma disease in oil palm. Molecules24, 2498. 10.3390/molecules24132498
109
Mann B. K. Gobin A. S. Tsai A. T. Schmedlen R. H. West J. L. (2001). Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: Synthetic ECM analogs for tissue engineering. Biomaterials22, 3045–3051. 10.1016/s0142-9612(01)00051-5
110
Maqbool S. Hassan M. A. Xia X. York L. M. Rasheed A. He Z. (2022). Root system architecture in cereals: Progress, challenges and perspective. Plant J.110, 23–42. 10.1111/tpj.15669
111
marketresearch (2022). The business research company chemical fertillizers market global briefing. Available at: https://www.marketresearch.com/Business-Research-Company-v4006/Chemical-Fertilizers-Global-Briefing-31503799/.
112
marketresearch (2022). The business research company pesticide and other agricultural chemicals market global briefing. Available at: https://www.marketresearch.com/Business-Research-Company-v4006/Pesticide-Agricultural-Chemicals-Global-Briefing-31503819/.
113
Marschner P. Crowley D. Rengel Z. (2011). Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis – model and research methods. Soil Biol. Biochem.43, 883–894. 10.1016/j.soilbio.2011.01.005
114
Martínez-Cano B. Mendoza-Meneses C. J. García-Trejo J. F. Macías-Bobadilla G. Aguirre-Becerra H. Soto-Zarazúa G. M. et al (2022). Review and perspectives of the use of alginate as a polymer matrix for microorganisms applied in agro-industry. Molecules27, 4248. 10.3390/molecules27134248
115
Maruyama C. R. Guilger M. Pascoli M. Bileshy-José N. Abhilash P. Fraceto L. F. et al (2016). Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci. Rep.19768, 1–15. 10.1038/srep19768
116
Matsuda K. Ihara M. Sattelle D. B. (2020). Neonicotinoid insecticides: Molecular targets, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol.60, 241–255. 10.1146/annurev-pharmtox-010818-021747
117
Mazloom N. Khorassani R. Zohury G. H. Emami H. Whalen J. (2020). Lignin-based hydrogel alleviates drought stress in maize. Environ. Exp. Bot.175, 104055. 10.1016/j.envexpbot.2020.104055
118
Meena R. S. Kumar S. Datta R. Lal R. Vijayakumar V. Brtnicky M. et al (2020). Impact of agrochemicals on soil microbiota and management: A review. Land9, 34. 10.3390/land9020034
119
Mendez O. E. Astete C. E. Cueto R. Eitzer B. Hanna E. A. Salinas F. et al (2022). Lignin nanoparticles as delivery systems to facilitate translocation of methoxyfenozide in soybean (Glycine max). J. Agric. Food Res.7, 100259. 10.1016/j.jafr.2021.100259
120
Michielse C. B. Rep M. (2009). Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol.10, 311–324. 10.1111/j.1364-3703.2009.00538.x
121
Milani P. Franca D. Balieiro A. G. Faez R. (2017). Polymers and its applications in agriculture. Polimeros27, 256–266. 10.1590/0104-1428.09316
122
Moghanjoughi A. A. Khoshnevis D. Zarrabi A. (2016). A concise review on smart polymers for controlled drug release. Drug Deliv. Transl. Res.6, 333–340. 10.1007/s13346-015-0274-7
123
Moulick R. G. Das S. Debnath N. Bandyopadhyay K. (2020). Potential use of nanotechnology in sustainable and 'smart' agriculture: Advancements made in the last decade. Plant Biotechnol. Rep.14, 505–513. 10.1007/s11816-020-00636-3
124
Mura S. Nicolas J. Couvreur P. (2013). Stimuli-responsive nanocarriers for drug delivery. Nat. Mat.12, 991–1003. 10.1038/nmat3776
125
Naidu D. S. Hlangothi S. P. John M. J. (2018). Bio-based products from xylan: A review. Carbohydr. Polym.179, 28–41. 10.1016/j.carbpol.2017.09.064
126
Nair L. S. Laurencin C. T. (2007). Biodegradable polymers as biomaterials. Prog. Polym. Sci.32, 762–798. 10.1016/j.progpolymsci.2007.05.017
127
Namasivayam S. K. R. Bharani R. A. Karunamoorthy K. (2018). Insecticidal fungal metabolites fabricated chitosan nanocomposite (IM-CNC) preparation for the enhanced larvicidal activity-An effective strategy for green pesticide against economic important insect pests. Int. J. Biol. Macromol.120, 921–944. 10.1016/j.ijbiomac.2018.08.130
128
National_Academies_of_Sciences_Engineering_and_Medicine (2019). Environmental engineering for the 21st century: Addressing grand challenges. Washington, DC: The National Academies Press.
129
Nishu S. D. Park S. Ji Y. Han I. Key J. Lee T. K. (2020). The effect of engineered PLGA nanoparticles on nitrifying bacteria in the soil environment. J. Industrial Eng. Chem.84, 297–304. 10.1016/j.jiec.2020.01.011
130
Oberlintner A. Bajić M. Kalčíková G. Likozar B. Novak U. (2021). Biodegradability study of active chitosan biopolymer films enriched with Quercus polyphenol extract in different soil types. Environ. Technol. Innovation21, 101318. 10.1016/j.eti.2020.101318
131
Ojha T. Misra S. Raghuwanshi N. S. (2015). Wireless sensor networks for agriculture: The state-of-the-art in practice and future challenges. Comput. Electron. Agric.118, 66–84. 10.1016/j.compag.2015.08.011
132
Oldroyd G. E. D. (2013). Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol.11, 252–263. 10.1038/nrmicro2990
133
Oliveira J. L. d. Campos E. V. R. Pereira A. E. S. Pasquoto T. Lima R. Grillo R. et al (2018). Zein nanoparticles as eco-friendly carrier systems for botanical repellents aiming sustainable agriculture. J. Agric. Food Chem.66, 1330–1340. 10.1021/acs.jafc.7b05552
134
Paarakh M. P. Jose P. A. Setty C. M. Christoper G. V. P. (2019). Release kinetics - concepts and applications. Inter.J. Phar. Res. Tech.8, 12–20.
135
Paerl H. W. Gardner W. S. Havens K. E. Joyner A. R. McCarthy M. J. Newell S. E. et al (2016). Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae54, 213–222. 10.1016/j.hal.2015.09.009
136
Pedrini S. Bhalsing K. Cross A. T. Dixon K. W. (2018). Protocol Development Tool (PDT) for seed encrusting and pelleting. Seed Sci. Technol.46, 393–405. 10.15258/sst.2018.46.2.21
137
Pedrini S. Merritt D. J. Stevens J. Dixon K. (2017). Seed coating: Science or marketing spin?Trends Plant Sci.22, 106–116. 10.1016/j.tplants.2016.11.002
138
Peppas N. A. Hoffman A. S. (2013). “Chapter I.2.5 - hydrogels,” in Biomaterials science. Editors RatnerB. D.HoffmanA. S.SchoenF. J.LemonsJ. E.. Third Edition (Academic Press), 166–179.
139
Pereira A. E. Grillo R. Mello N. F. Rosa A. H. Fraceto L. F. (2014). Application of poly(epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J. Hazard. Mat.268, 207–215. 10.1016/j.jhazmat.2014.01.025
140
Pérez-de-Luque A. (2017). Interaction of nanomaterials with plants: What do we need for real applications in agriculture?Front. Environ. Sci.5, 12. 10.3389/fenvs.2017.00012
141
Peter J. Young W. Haukka K. E. (1996). Diversity and phylogeny of rhizobia. New Phytol.133, 87–94. 10.1111/j.1469-8137.1996.tb04344.x
142
Pieterse C. M. J. Zamioudis C. Berendsen R. L. Weller D. M. Wees S. C. M. V. Bakker P. A. H. M. (2014). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol.52, 347–375. 10.1146/annurev-phyto-082712-102340
143
Pineda A. Dicke M. Pieterse C. M. J. Pozo M. J. (2013). Beneficial microbes in a changing environment: Are they always helping plants to deal with insects?Funct. Ecol.27, 574–586. 10.1111/1365-2435.12050
144
Pineda A. Zheng S. J. van Loon J. J. Pieterse C. M. Dicke M. (2010). Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci.15, 507–514. 10.1016/j.tplants.2010.05.007
145
Plaut Z. Federman E. (1985). A simple procedure to overcome polyethelene glycol toxicity on whole plants. Plant Physiol.79, 559–561. 10.1104/pp.79.2.559
146
Powers S. M. Bruulsema T. W. Burt T. P. Chan N. I. Elser J. J. Haygarth P. M. et al (2016). Long-term accumulation and transport of anthropogenic phosphorus in three river basins. Nat. Geosci.9, 353–356. 10.1038/ngeo2693
147
Ramli R. A. (2019). Slow release fertilizer hydrogels: A review. Polym. Chem.10, 6073–6090. 10.1039/c9py01036j
148
Ranadive P. Parulkar A. Brunelli N. A. (2019). Jet-mixing reactor for the production of monodisperse silver nanoparticles using a reduced amount of capping agent. React. Chem. Eng.4, 1779–1789. 10.1039/c9re00152b
149
Raven P. H. Wagner D. L. (2021). Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. U. S. A.118, e2002548117. 10.1073/pnas.2002548117
150
Rieu M. Sposito G. (1991). Fractal fragmentation, soil porosity, and soil water properties: II. Applications. Soil Sci. Soc. Am. J.55, 1239–1244. 10.2136/sssaj1991.03615995005500050007x
151
Ritger P. L. Peppas N. A. (1987). A simple equation for description of solute release I: Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release5, 23–36. 10.1016/0168-3659(87)90034-4
152
Rizaludin M. S. Stopnisek N. Raaijmakers J. M. Garbeva P. (2021). The chemistry of stress: Understanding the ‘cry for help’ of plant roots. Metabolites11, 357. 10.3390/metabo11060357
153
Rocha I. Ma Y. Souza-Alonso P. Vosátka M. Freitas H. Oliveira R. S. (2019). Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci.10, 1357. 10.3389/fpls.2019.01357
154
Romero M. R. Wolfel A. Igarzabal C. I. A. (2016). Smart valve: Polymer actuator to moisture soil control. Sensors Actuators B Chem.234, 53–62. 10.1016/j.snb.2016.04.104
155
Salachna P. Grzeszczuk M. Meller E. Soból M. (2018). Oligo-alginate with low molecular mass improves growth and physiological activity of eucomis autumnalis under salinity stress. Molecules23, 812. 10.3390/molecules23040812
156
Salinas F. Astete C. E. Waldvogel J. H. Navarro S. White J. C. Elmer W. et al (2021). Effects of engineered lignin-graft-PLGA and zein-based nanoparticles on soybean health. NanoImpact23, 100329. 10.1016/j.impact.2021.100329
157
Santo Pereira A. E. Silva P. M. Oliveira J. L. Oliveira H. C. Fraceto L. F. (2017). Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Colloids Surfaces B Biointerfaces150, 141–152. 10.1016/j.colsurfb.2016.11.027
158
Sashidhar P. Kochar M. Singh B. Gupta M. Cahill D. Adholeya A. et al (2020). Biochar for delivery of agri-inputs: Current status and future perspectives. Sci. Total Environ.703, 134892. 10.1016/j.scitotenv.2019.134892
159
Savary S. Willocquet L. Pethybridge S. J. Esker P. McRoberts N. Nelson A. (2019). The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol.3, 430–439. 10.1038/s41559-018-0793-y
160
Schild H. G. (1992). Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci.17, 163–249. 10.1016/0079-6700(92)90023-r
161
Schindler D. W. (2012). The dilemma of controlling cultural eutrophication of lakes. Proc. R. Soc. B279, 4322–4333. 10.1098/rspb.2012.1032
162
Schmelz E. A. Huffaker A. Sims J. W. Christensen S. A. Lu X. Okada K. et al (2014). Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J.79, 659–678. 10.1111/tpj.12436
163
Sealy A. Manufacturing moonshot: How Pfizer makes its millions of Covid-19 vaccine doses. Available at: https://www.cnn.com/2021/03/31/health/pfizer-vaccine-manufacturing/index.html (Accessed Jul01, 2022).
164
Seddiqi H. Oliaei E. Honarkar H. Jin J. Geonzon L. C. Bacabac R. G. et al (2021). Cellulose and its derivatives: Towards biomedical applications. Cellulose28, 1893–1931. 10.1007/s10570-020-03674-w
165
Shakiba S. Astete C. E. Paudel S. Sabliov C. M. Rodrigues D. F. Louie S. M. (2020). Emerging investigator series: Polymeric nanocarriers for agricultural applications: Synthesis, characterization, and environmental and biological interactions. Environ. Sci. Nano7, 37–67. 10.1039/c9en01127g
166
Shen X. Shamshina J. L. Berton P. Gurau G. Rogers R. D. (2016). Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem.18, 53–75. 10.1039/c5gc02396c
167
Shen Y. Zhu H. Cui J. Wang A. Zhao X. Cui B. et al (2018). Construction of lambda-cyhalothrin nano-delivery system with a high loading content and controlled-release property. Nanomater. (Basel).8, 1016. 10.3390/nano8121016
168
Shikano I. Rosa C. Tan C.-W. Felton G. W. (2017). Tritrophic interactions: Microbe-mediated plant effects on insect herbivores. Annu. Rev. Phytopathol.55, 313–331. 10.1146/annurev-phyto-080516-035319
169
Shukla R. Cheryan M. (2001). Zein: The industrial protein from corn. Industrial Crops Prod.13, 171–192. 10.1016/s0926-6690(00)00064-9
170
Silva V. Mol H. G. J. Zomer P. Tienstra M. Ritsema C. J. Geissen V. (2019). Pesticide residues in European agricultural soils – a hidden reality unfolded. Sci. Total Environ.653, 1532–1545. 10.1016/j.scitotenv.2018.10.441
171
Singh P. M. Tiwari A. Maity D. Saha S. (2022). Recent progress of nanomaterials in sustainable agricultural applications. J. Mat. Sci.57, 10836–10862. 10.1007/s10853-022-07259-9
172
Siviter H. Muth F. (2020). Do novel insecticides pose a threat to beneficial insects?Proc. R. Soc. B287, 20201265. 10.1098/rspb.2020.1265
173
Sopeña F. Villaverde J. Maqueda C. Morillo E. (2011). Photostabilization of the herbicide norflurazon microencapsulated with ethylcellulose in the soil-water system. J. Hazard. Mat.195, 298–305. 10.1016/j.jhazmat.2011.08.039
174
Soyano T. Shimoda Y. Kawaguchi M. Hayashi M. (2019). A shared gene drives lateral root development and root nodule symbiosis pathways in Lotus. Science366, 1021–1023. 10.1126/science.aax2153
175
Stahl J. D. Cameron M. D. Haselbach J. Aust S. D. (2000). Biodegradation of superabsorbent polymers in soil. Environ. Sci. Pollut. Res.7, 83–88. 10.1065/espr199912.014
176
Steinkellner S. Lendzemo V. Langer I. Schweiger P. Khaosaad T. Toussaint J.-P. et al (2007). Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant-fungus interactions. Molecules12, 1290–1306. 10.3390/12071290
177
Syers J. K. Johnston A. E. Curtin D. (2008). Efficiency of soil and fertilizer phosphorus use: Reconciling changing concepts of soil phosphorus behaviour with agronomic information. Rome: Food and Agricultural Organization of the United Nations.
178
Szymańska E. Winnicka K. (2015). Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs13, 1819–1846. 10.3390/md13041819
179
the World Bank Group Commodity Markets Outlook (20222022). The impact of the war in Ukraine on commodity markets. Washington, D.C.: World Bank.
180
Treiser M. Abramson S. Langer R. Kohn J. (2013). “Chapter I.2.6 - degradable and resorbable biomaterials,” in Biomaterials science. Editors RatnerB. D.HoffmanA. S.SchoenF. J.LemonsJ. E.. Third Edition (Academic Press), 179–195.
181
Trucillo P. (2022). Drug carriers: A review on the most used mathematical models for drug release. Processes10, 1094. 10.3390/pr10061094
182
Tsunoda T. van Dam N. M. (2017). Root chemical traits and their roles in belowground biotic interactions. Pedobiologia65, 58–67. 10.1016/j.pedobi.2017.05.007
183
Ullah F. Othman M. B. H. Javed F. Ahmad Z. Akil H. M. (2015). Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C57, 414–433. 10.1016/j.msec.2015.07.053
184
Ullyot G. E. Ullyot B. H. Slater L. B. (2000). The metamorphosis of smith-kline & French laboratories to Smith kline beecham: 1925-1998. Bull. Hist. Chem.25, 16–20.
185
Valderrama A. Lay J. Flores Y. Zavaleta D. Delfín A. R. Delfin A. R. (2020). Factorial design for preparing chitosan nanoparticles and its use for loading and controlled release of indole-3-acetic acid with effect on hydroponic lettuce crops. Biocatal. Agric. Biotechnol.26, 101640. 10.1016/j.bcab.2020.101640
186
Vega-Vasquez P. Mosier N. S. Irudayaraj J. (2020). Nanoscale drug delivery systems: From medicine to agriculture. Front. Bioeng. Biotechnol.8, 79. 10.3389/fbioe.2020.00079
187
Viana W. G. Scharwies J. D. Dinneny J. R. (2022). Deconstructing the root system of grasses through an exploration of development, anatomy and function. Plant, Cell Environ.45, 602–619. 10.1111/pce.14270
188
Vincent D. Rafiqi M. Job D. (2020). The multiple facets of plant-fungal interactions revealed through plant and fungal secretomics. Front. Plant Sci.10, 1626. 10.3389/fpls.2019.01626
189
Vives-Peris V. de Ollas C. Perez-Clemente R. M. (2020). Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep.39, 3–17. 10.1007/s00299-019-02447-5
190
Vroman I. Tighzert L. (2009). Biodegradable polymers. Materials2, 307–344. 10.3390/ma2020307
191
Weisskopf L. Schulz S. Garbeva P. (2021). Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol.19, 391–404. 10.1038/s41579-020-00508-1
192
Weston L. A. Mathesius U. (2013). Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol.39, 283–297. 10.1007/s10886-013-0248-5
193
Wiesmeier M. Urbanski L. Hobley E. Lang B. von Lutzow M. Marin-Spiotta E. et al (2019). Soil organic carbon storage as a key function of soils - a review of drivers and indicators at various scales. Geoderma333, 149–162. 10.1016/j.geoderma.2018.07.026
194
Yadav V. K. Gupta N. Kumar P. Dashti M. G. Tirth V. Khan S. H. et al (2022). Recent advances in synthesis and degradation of lignin and lignin nanoparticles and their emerging applications in nanotechnology. Materials15, 953. 10.3390/ma15030953
195
Yearla S. R. Padmasree K. (2016). Exploitation of subabul stem lignin as a matrix in controlled release agrochemical nanoformulations: A case study with herbicide diuron. Environ. Sci. Pollut. Res.23, 18085–18098. 10.1007/s11356-016-6983-8
196
Yoshida R. Uchida K. Kaneko Y. Sakai K. Kikuchi A. Sakurai Y. et al (1995). Comb-type grafted hydrogels with rapid de-swelling response to temperature-changes. Nature374, 240–242. 10.1038/374240a0
197
Zhang H. Cheng J. Ao Q. (2021). Preparation of alginate-based biomaterials and their applications in biomedicine. Mar. Drugs19, 264. 10.3390/md19050264
198
Zhang H. Liu Z. Mai J. Wang N. Liu H. Zhong J. et al (2021). A smart design strategy for super-elastic hydrogel with long-term moisture, extreme temperature resistance, and non-flammability. Adv. Sci.8, 2100320. 10.1002/advs.202100320
199
Zhang J. Li M. Fan T. Xu Q. Wu Y. Chen C. et al (2013). Construction of novel amphiphilic chitosan copolymer nanoparticles for chlorpyrifos delivery. J. Polym. Res.20, 107–111. 10.1007/s10965-013-0107-7
200
Zhang X. Zou T. Lassaletta L. Mueller N. D. Tubiello F. N. Lisk M. D. et al (2021). Quantification of global and national nitrogen budgets for crop production. Nat. Food2, 529–540. 10.1038/s43016-021-00318-5
201
Zhou J. Ni R. Chau Y. (2017). Polymeric vesicle formation via temperature-assisted nanoprecipitation. RSC Adv.7, 17997–18000. 10.1039/c7ra01959a
202
Zhu Z. Margulis-Goshen K. Magdassi S. Talmon Y. Macosko C. W. (2010). Polyelectrolyte stabilized drug nanoparticles via flash nanoprecipitation: A model study with beta-carotene. J. Pharm. Sci.99, 4295–4306. 10.1002/jps.22090
203
Zubrod J. P. Bundschuh M. Arts G. Brühl C. A. Imfeld G. Knäbel A. et al (2019). Fungicides: An overlooked pesticide class?Environ. Sci. Technol.53, 3347–3365. 10.1021/acs.est.8b04392
Summary
Keywords
controlled release, agriculture, fertilizer, pesticide, nanoparticle
Citation
Lee P, Lin X, Khan F, Bennett AE and Winter JO (2022) Translating controlled release systems from biomedicine to agriculture. Front. Front. Biomater. Sci. 1:1011877. doi: 10.3389/fbiom.2022.1011877
Received
04 August 2022
Accepted
11 October 2022
Published
25 October 2022
Volume
1 - 2022
Edited by
Silviya Petrova Zustiak, Saint Louis University, United States
Reviewed by
Joshua Widhalm, Purdue University, United States
Ana Mora Boza, Georgia Institute of Technology, United States
Updates
Copyright
© 2022 Lee, Lin, Khan, Bennett and Winter.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica O. Winter, winter.63@osu.edu
This article was submitted to Delivery Systems and Controlled Release, a section of the journal Frontiers in Biomaterials Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.