- 1Romanian National Pediatric Oncology and Hematology Registry, Romanian Society of Pediatric Oncology and Hematology, Bucharest, Romania
- 2Cancer Epidemiology Education in Special Populations (CEESP), City University of New York, New York, NY, United States
- 3Department of Community Health and Social Medicine, City University of New York School of Medicine, New York, NY, United States
Introduction: Pediatric cancer survival is increasing over time in European countries. However, there are survival differences in survival between Eastern and Western European member countries. The available mortality data based on the Romanian National Statistics Institute reports to Eurostat place Romania among the European countries with the highest child cancer mortality rates. The current study aims to investigate pediatric cancer survival and mortality outcomes in Romania, using the Romanian national-level cancer registry data. The Registry results add to the literature to illustrate the profile of pediatric cancers in Eastern Europe.
Methods: The study included 4,144 cancer patients aged 0–19 years, whose data were collected in the Romanian National Pediatric Oncology and Hematology Registry. These data comprise all the new cases diagnosed in Romanian pediatric cancer facilities from January 1, 2010, to December 31, 2019. Survival probabilities were examined according to patient characteristics, such as tumor type, demography, geography and place of residence. The Chi-square test (Fisher Exact Test) was used to compare patients' personal and clinical characteristics by rural/urban designation. The Cox proportional hazards regression model was used to estimate the hazard ratios and 95% confidence intervals by rural/urban designation, economic development region, and selected cancer subtypes, according to the International Classification of Childhood Cancer, 3rd edition. The mean follow-up time was 6.09 ± 3.84 years. To calculate the 5-year survival rates, the study period ended on December 31, 2017, and the sample size was restricted to 3,308. A predictive model using multivariable logistic regression was used to assess the age group and rural-urban survival probabilities as well as survival probabilities for major cancer subtypes.
Results: The 5-year overall survival probability for the 0–14 and 15–19 age groups was 73% (95% CI: 71, 75) and 69% (65%, 72%) respectively. Categorized further by smaller age groups for the 0–14 age group, the survival rates were 75% (0–4 years), 73% (5–9 years) and 69% (10–14 years). Hodgkin lymphoma (92%), nephroblastoma and other nonepithelial renal tumors (89 %), and lymphoid leukemias (80%) had the highest survival rates among all the seven major cancer subtypes in the 0–14 years population. The worst survival was observed for CNS tumors (62%), rhabdomyosarcoma (62%), neuroblastoma (67%), and bone tumors (52%). As compared to pediatric cancer patients residing in urban areas, significantly more rural patients died from cancer (32.6% vs. 22.4%, p < 0.0001).
Discussion/conclusion: This is Romania's first pediatric cancer survival study based on well-validated national cancer registry data. The Romanian Pediatric Cancer Registry continues to shed light on the profile of pediatric cancers in Romania. Overall survival rates in Romania were lower than survival rates reported from the EU-15 countries. Rural patients had lower survival than urban patients. Future studies should investigate the relationship between patients' clinical and socioeconomic characteristics and survival outcomes. Further research is also needed to investigate recurrence and secondary malignancies among this population.
1 Introduction
Pediatric cancer survival is increasing over time in European countries, whereas the survival varies by country (1). East-West survival differences documented in the EUROCARE-6 study both in adults as well as in children are a major concern, addressed by current European Union policies (2). Differences in cancer registration and reporting among Eastern European countries further complicate the comparison.
This is particularly the case of Romania, an Eastern European country with a total population of 20 million, where national registration of cancer is still in a development process, and with regional registries achieving different coverage rates and levels of activity There is a paucity of internationally available published data on the national incidence and survival of pediatric cancer patients in Romania. This is why, in 2010, the Romanian Society of Pediatric Oncology and Hematology decided to establish a national childhood cancer registry, with all the pediatric oncology facilities in the country reporting-in new cases. Romania population numbers 4.1 million children and adolescents (aged 0–19) (representative of 21% of the total) (3, 4). Data collected via the Romanian National Pediatric Oncology and Hematology Registry (RNPOHR) show that pediatric cancer makes up <1% of all new cancer cases in Romania, similar to the global data. According to GLOBOCAN, ~525 Romanians aged 0–19 are estimated to develop cancers annually, of which, according to the RNPOHR, cca 420 are undergoing treatment in the Romanian pediatric oncology network (5, 6).
The age-standardized incidence rate of cancers between 2010–2021 in Romanian children and adolescents (0–19) reported by RNPOHR was 9.72 per 100,000 and 11.02 per 100,000 in the 0–14 population (European Standard Population 2016). In 2022, RNPOHR reported 461 new cases compared to 545 estimated by GLOBOCAN, with an actual crude incidence rate of 11.16 per 100,000 vs. 13.8 per 100,000 estimated (5, 6).
Age and gender distribution of pediatric cancer cases tend to follow similar patterns to the 0–14 European population for all the three 5-year age groups (0–4 years: 32.4%, 5–9 years: 22.3% 10–14 years: 23.3%), but with a notable difference for the 15–19 age group. The latter represents the largest share of EU cases, whereas in Romania this is the smallest group across all ages (22%). The male-to-female ratio for new cases is 1.27/1, similar to global trends (6–8).
Leukemia (30%), lymphoma (15%) and central nervous system (CNS) tumors (14%) top the cancer diagnosis in the Romanian pediatric population from 2010–2021, indicating a similar distribution to the European trends (6–8).
The geographic distribution of registry-reported cases does not infer a significant difference in incidence according to the place of residence (urban vs. rural) with an age-standardized rate (ASR) of 10.67 per 100,000 in urban areas to 9.31 per 100,000 in rural areas (0–19 years, European Standard Population 2016) (6–8). Still, some regional differences were noted in the number of pediatric cancer cases across economic development regions (North-East, Bucharest-Ilfov, Center, North-West, South-East, South-Muntenia, South-West Oltenia, and West). These eight economic development regions were created in Romania after EU accession to address disparities in development between geographic areas, via strategic allocation of national resources and capitalization of the local and regional resources. However, significant differences persist today, with the wealthiest region (Bucharest-Ilfov) having a per-capita GDP almost four times higher than the poorest region (North-East) (9, 10). The highest regional incidence (in the 0–19 year) appears to be sourced in the North-West region with an ASR of 11.25 per 100,000, while the lowest incidence is observed in the South-Muntenia and West regions, of 8.9 per 100,000 (6). This may reflect the differing size of the pediatric population and possible differences in health infrastructure and the accessing care behavior of the patient population toward the different pediatric oncology centers across regions.
To date, little is known about the survival outcomes of Romanian children and adolescents with cancer at the national level, and no studies have yet compared pediatric cancer survival in Romania to other EU countries (including within the East European region). The existing Romanian studies in pediatric cancers were limited to a single institute/single site (with focus on hematologic, brain, and solid tumors (11–13). The CONCORD-3: analysis based on data from 322 population-based registries in 71 countries is the single international registry-based study to mention survival in child cancer in Romania, for brain tumors and acute lymphoblastic leukemia. However, the results, of a 60.1% (95% CI: 31.6, 81.5) overall survival rate in brain cancers and 53.9% (95% CI: 28.2, 79.6) were based on a very small number of cases (19, respectively 21 cases) reported for the 2010–2014 period by the Cluj Regional Population Cancer Registry (14). The available mortality data based on the Romanian National Statistics Institute reports to Eurostat and published on the European Cancer Inequalities Registry website reflects all deaths by cancer in the 0–19 population occurring on the Romanian territory. The 2021 presented data place Romania among the EU members with the highest cancer mortality in children and adolescents with a rate of 3.8 per 100,000, compared to the 2.8 EU average. Significant disparities between member countries can be noted, with the highest rates recorded for Lithuania (4.5 per 100,000), Greece (4.3 per 100,000) and Bulgaria (4.1 per 100,000) in contrast with France and Germany (2.6 per 100,000) and Italy, Finland and Hungary (2.7 per 100,000). However, these data are not fully informative of the performance of the Romanian pediatric cancer care system as it doesn't distinguish between cases treated in Romanian pediatric oncology facilities and cases treated abroad as well as cases (18+ years) treated in adult cancer facilities (14, 15).
More so, there have been no studies of the determinants of survival outcomes in Romania and their impact on the potential differences to other EU member countries. The significant regional disparity across economic development regions, most likely results in differing availability of pediatric specialty care and lab/imaging services across economic development regions as well as rural and urban areas (9). Additionally, survival outcomes could also likely differ between rural and urban pediatric cancer patients due to a huge rural-urban disparity in the healthcare infrastructure (the health facilities and the number of hospitals in urban vs. rural = 4.5: 1 and 8.9:1) in Romania (16, 17).
Given the scarcity of quality pediatric cancer survival data in Romania in the current literature, we took advantage of the only existing national-level data from the RNPOHR with a linkage to economic development regions and rural-urban designation information (6).
We aimed to fill the current information gap by conducting a retrospective cohort study in Romanian children and adolescents (0–19 years) diagnosed with cancers spanning over 10 years and examining the potential disparity in survival outcomes of these patients, both from an international European perspective as well as among population groups in Romania.
2 Methods
2.1 Data sources and data collection
This study was conducted in Romania. Reporting of cancers can be historically traced in Romania to the 80's. With Romania's EU accession in 2007, a Ministerial Order updated the rules and procedures of cancer reporting, to ensure compliance of the cancer reporting process, data collection, classification, and codification with the standards from the European Network of Cancer Registers and the International Agency for Research on Cancer. The same regulatory document established regional cancer registers rooted in each of the eight development regions (18).
However, insufficient regulatory reinforcement and funding hampered the development and reporting output of the regional cancer registry network.
Given the rarity of pediatric cancers, the national pooling of cases is critical, as well as the adaptation of the reporting process to childhood cancer specificities. Therefore, in 2010 the Romanian Society of Pediatric Oncology and Hematology established the RNPOHR which collects data for analysis purposes for all the cases taken up by Romanian pediatric oncology facilities. RNPOHR receives data from 13 registrars from respective pediatric oncology centers across the nation (6). Trained personnel from these centers report the cancer diagnosis by filling out forms based on the ENCR minimum recommended dataset. Since 2018, reporting has been performed via a dedicated secured digital platform and the dataset has been expanded/customized to fit pediatric cancer registration requirements (such as automatic assignment to ICCC-3 tumor categories, Toronto staging system etc.). Staff at the centers also collect other clinical and demographic information of patients to establish a complete profile for patients. Specifically, tumors are classified according to the International Classification of Disease and Oncology, 3rd edition (ICD-O-3), and the International Classification of Childhood Cancer, 3rd edition (ICCC-3). ICCC-3 assignment is based on the morphological and topographic coding of tumor according to ICD-O-3 classification. Twelve primary ICCC-3 cancer types included leukemia, lymphoma, CNS tumor, neuroblastoma, soft tissue and other extraosseous sarcomas, retinoblastoma, renal tumor, hepatic tumor, malignant bone tumor, germ cell tumors, other malignant epithelial neoplasms and malignant melanomas, and other and unspecified malignant neoplasms (19, 20).
For each case, the information retrieved from different sources is aggregated based on the personal identification number. Each cancer case is verified, coded and finalized by one trained cancer registrar in the central setting of the Registry. In addition, each report is subject to an automatic validation procedure via Version 2.2.8 of the JRC-ENCR Quality Check Software.
RNPOHR has an estimated coverage of 80% of the total national number of new cases. The cases that escape capture are likely patients aged 17 and more who are taken up by adult cancer facilities as well as the cases that seek care across borders without accessing the national pediatric cancer care network. Also, the RNPHOR does not automatically receive death certificate (DC) notifications from the Office of Vital Statistics, nor from pathology labs. Ascertaining of the vital status is possible only for reported cases included in the registry database. 93.5% of the registry cases are microscopically verified.
2.2 Study population
The study population consisted of individuals who were diagnosed with cancer between January 1, 2010, and December 31, 2019. Inclusion criteria were further refined to those individuals 19 years of age or less with cancer confirmed on pathology. Exclusion criteria were those individuals who were over 19 years old or who were not confirmed with a cancer diagnosis. After applying inclusion and exclusion criteria, a total of 4,144 individuals were included in the final analysis. To ideally calculate the 5-year survival rate, one should follow up the cohort for at least 5 years. In our data, we have some patients with follow-up time as less as 3 years. To best describe the survival situation in Romanian pediatric cancer patients, we estimated a 5-year survival rate as well as a 3-year survival rate. Therefore we further restricted the sample to the ones who were diagnosed by December 31, 2017 (n = 3,308) to estimate the 5-year survival probability in total, by specific cancer subtype, age group, and rurality. The subjects' mortality follow-up time was assessed from the date of cancer diagnosis through December 31, 2022, or their death date, whichever came first. Respondents without matched death records for the entire follow-up period were considered alive.
Because the survival differs by cancer type, a sub-analysis was done in 2,432 individuals focusing on the common cancer subtypes such as lymphoid leukemias, Hodgkin lymphomas, astrocytomas and intracranial and intraspinal embryonal tumors, neuroblastoma and ganglioneuroblastoma, nephroblastoma and other nonepithelial renal tumors, osteosarcomas and Ewing tumor and related sarcomas of bone, and rhabdomyosarcomas.
2.3 Covariates
The study included the following sociodemographic variables: age (year), sex (male/female), geography (rural/urban), and economic development regions (North-East, Bucharest-Ilfov, Center, North-West, South-East, South-Muntenia, South-West Oltenia, and West). Patients' clinical information was retrieved from patients' medical records, including documented 12 primary ICCC-3 cancer types, seven prevalent ICCC cancer subtypes (lymphoid leukemias, Hodgkin lymphomas, astrocytomas and intracranial and intraspinal embryonal tumors, neuroblastoma and ganglioneuroblastoma, nephroblastoma and other nonepithelial renal tumors, osteosarcomas and Ewing tumor and related sarcomas of bone, and rhabdmyosarcomas), behavior (borderline and in situ vs. malignant), and reported treatment (unimodal including no information, surgery, chemotherapy, radiotherapy, immunotherapy, and other therapy vs. multimodal including a combination of the reported treatments).
Rural-urban designation was coded according to the Information System of the Register of the Territorial Administration (SIRUTA), a European classification system. SIRUTA classifies the 41 counties and the municipality of Bucharest in Romania into urban (large cities) and rural (villages, communes, and small towns) areas (21). For the analysis purpose, small towns which share more infrastructure and economic similarities with villages and communes, were included in the rural group, thus accounting for the slightly higher share of the rural patients in the study population.
2.4 Statistical analysis
Data collected was analyzed using SAS version 9.4, and all results were reported at a two-tailed significance level of 0.05. The Chi-square test (Fisher Exact Test) was used to compare patients' characteristics by the treatment type, completion status of treatment, and treatment adherence. For numerical variables such as age, t test was used for comparing the patients' demographic, lifestyle, and clinical information by rural/urban designation.
Overall survival (OS) was calculated for major and minor age groups, place of residence (urban vs. rural), and selected ICCC-3 tumor categories and subcategories, but event-free survival (EFS) could not be determined due to data incompleteness for relapse and progression. Survival probabilities were analyzed at 5 years using the Kaplan-Meyer method for cases diagnosed between 2010 and 2017. Overall survival estimates are calculated, totally, by group age (0–14, 15–19), by sex and by ICCC3 selected categories.
Univariable and multivariable-adjusted Cox proportional hazards regression models were used to estimate the hazard ratios and 95% confidence intervals by rural/urban designation, development region, and major cancer subtype. In the univariable model, sociodemographic, lifestyle, and clinical covariates, such as age, sex, geography, development regions, treatment types, and primary cancer types were included. A backward stepwise selection procedure was adopted in the multivariable model to include the most relevant and significant covariates. A predictive model using multivariable logistic regression was applied to assess the survival probability in rural and urban areas.
Ethical approval was obtained from the Romanian Institutional Review Board of the Romanian Society for Pediatric Oncology and Hematology and the CUNY Institutional Review Boards.
3 Results
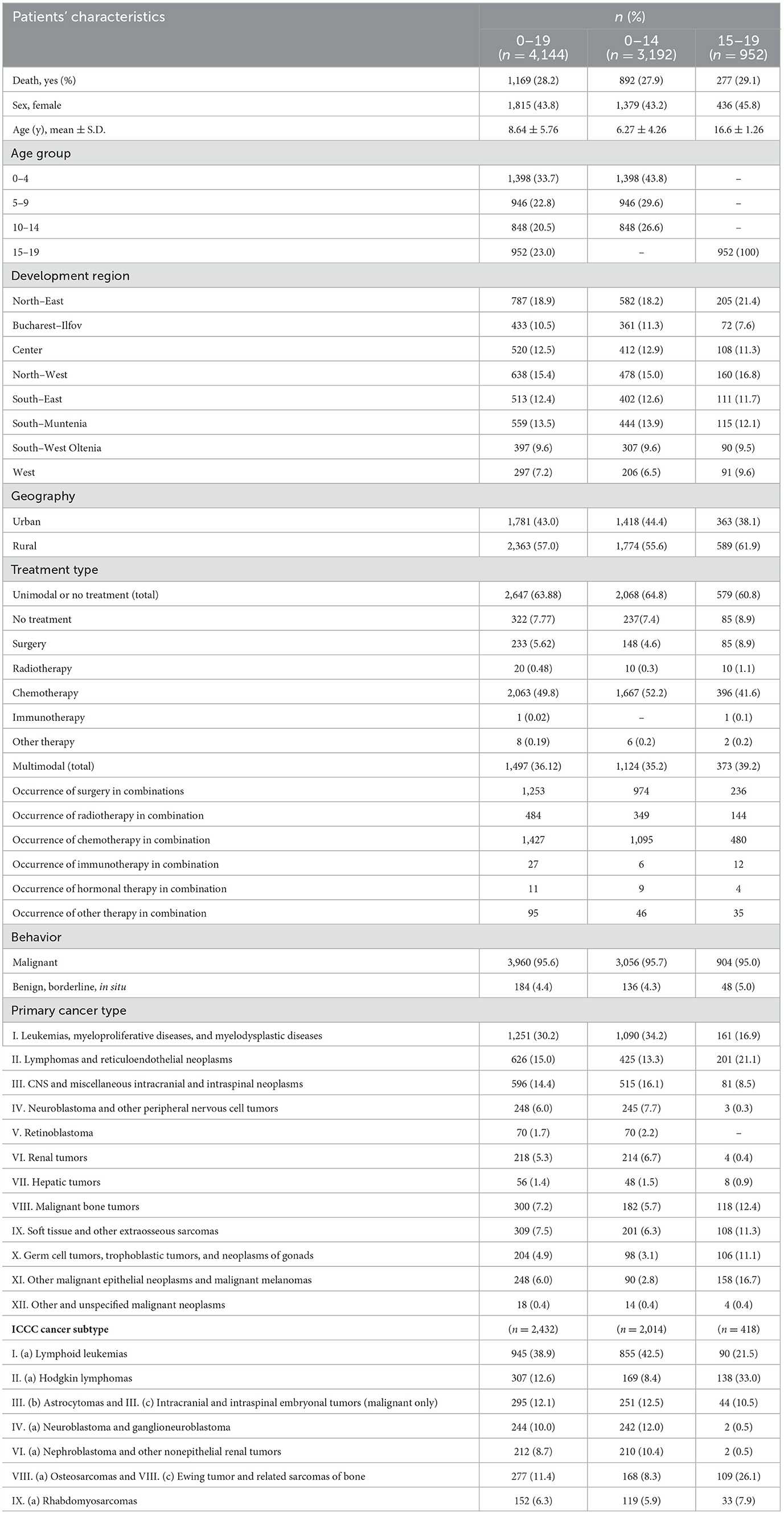
The current study includes 4,144 patients (0–19 y) diagnosed with cancer and reported to the cancer registry with a follow-up of 6.09 ± 3.84 years (Table 1). The mean age of the patients is 8.64 ± 5.76 years. Considering the minor age groups, the majority are 0–4 years (33.7%, n = 1,398), followed by 15–19 years (23%, n = 952), with patients stratified in the 10-14 years group representing only 20.5% (n = 848). The majority of patients reside in the North-East (18.9%, n = 787), North-West (15.4%, n = 638), and South-Muntenia regions (13.5%, n = 559). The lowest percent of subjects are from the West region, with only 7.2% (n = 297) of the total sample. Of the 4,144 subjects, 57% (n = 2,363) are from rural areas, 28.2% (n = 1,169) died, and 44% (n = 1,815) are female.
Referring to the treatment type, 63.88% (n = 2,647) followed unimodal therapy and 36.12% (n = 1,497) were treated by multimodal therapy. Chemotherapy (49.8%, n = 2,063) was the most used unimodal treatment, while only 1 patient record (0.02%) reported treatment by immunotherapy. Surgery and chemotherapy (22%, n = 1,253) were the most common multimodal treatment combination. Considering therapy modes' frequency of utilization, chemotherapy has the most occurrences in multimodal combinations, followed almost at parity by surgery and to a lesser extent, by radiotherapy. Occurrence rates of other systemic therapies (such as targeted therapies or hormone therapies) were insignificant. Due to inconsistent reporting, information on hematopoietic stem cell transplantation could not be included.
Most cancer diagnoses are of malignant behavior (95.6%, n = 3,960), while the rest of the cases are mostly benign CNS tumors. Considering the distribution of the sample according to the main ICCC-3 groups, most of the subjects are diagnosed with leukemias, myeloproliferative diseases, and myelodysplastic diseases (30.2%, n = 1,251), followed by lymphomas and reticuloendothelial neoplasms (15%, n = 626) and CNS and miscellaneous intracranial and intraspinal neoplasms (14.4%, n = 596). Only 1.4% (n = 56) of the patients are diagnosed with hepatic tumors and 1.7% (n = 70) with retinoblastomas, while the lowest percentage (0.4%, n = 18) is represented by other and unspecified malignant neoplasms. As for ICCC-3 subcategories, lymphoid leukemias (38.9%, n = 945), Hodgkin lymphomas (12.6%, n = 307), and astrocytomas and intracranial and intraspinal embryonal tumors (12.1%, n = 295) are the most diagnosed cancers in these children and adolescents.
The distribution of the patient's characteristics represented in Table 1 is similar between 0–14 and 15–19 age groups; however, a higher percentage of adolescent patients resided in rural areas (61.9% vs. 55.6%).
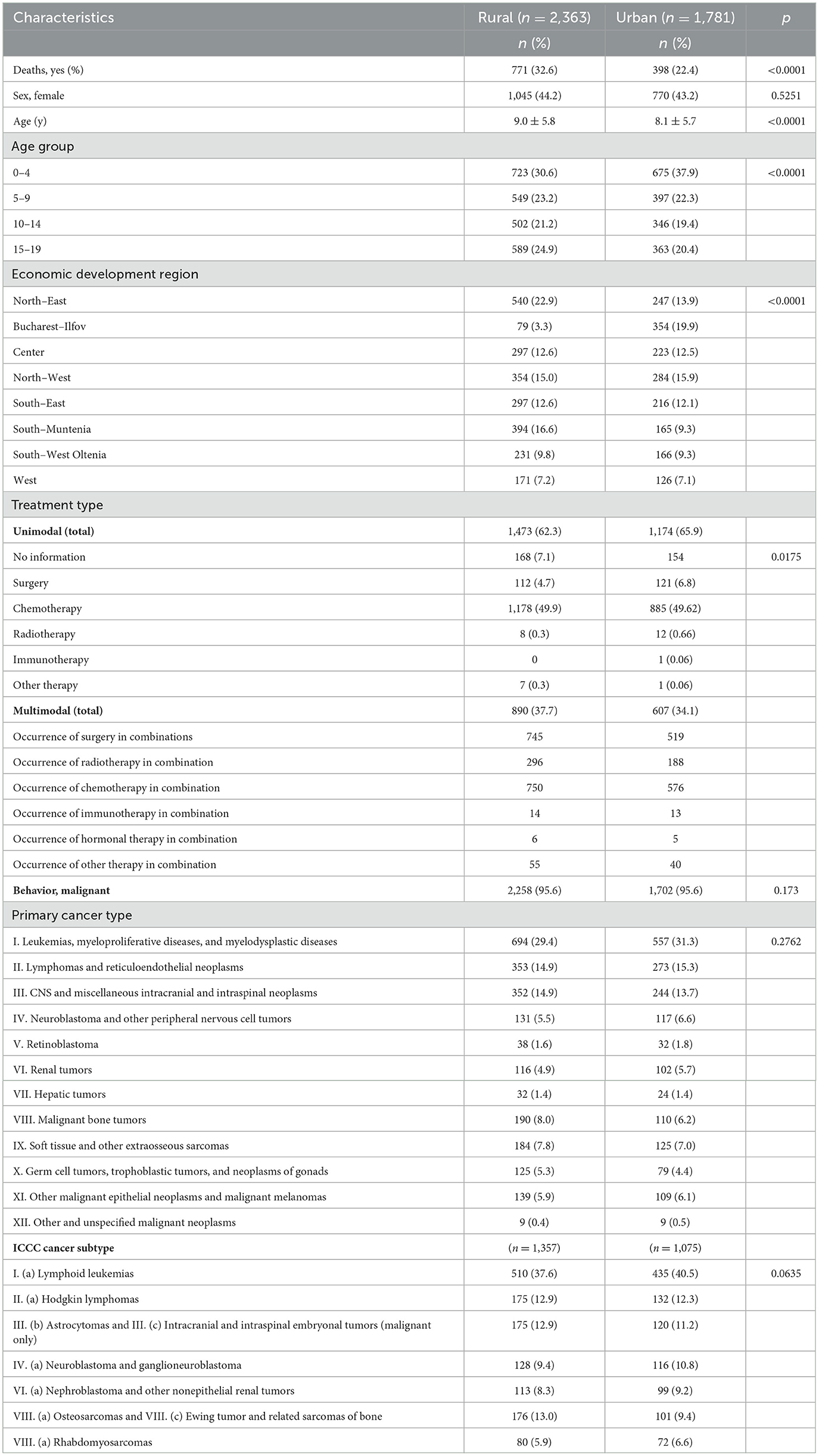
Table 2 compares patients' personal, lifestyle, and clinical characteristics by rural and urban designation. More patients diagnosed with cancer are living in rural areas (57%, n = 2,363), opposed to those who reside urban areas (43%, n = 1,781). As compared to the patients residing in urban areas, more rural residents died from cancers (32.6%, n = 771 vs. 22.4%, n = 398, p < 0.0001). The subjects' gender doesn't seem to be statistically significant related to the place of residence (p = 0.52), but older patients reside in rural areas (9 ± 5.8 years), as opposed to those who reside in urban regions (8.1 ± 5.7 years), p < 0.0001. The subjects stratified in the 5–9, 10–14 and 15–19 age groups live in rural territories (n = 549, 23.2%, n = 502, 21.2%, and n = 589, 24.9%, respectively), while only those aged between 0 and 4 years live mostly in urban environments (n = 675, 37.9%), p < 0.0001.
Taking into account the economic development regions, a notable difference was observed in the North-East area, where 22.9% (n = 540) of the patients live in rural territories, whereas only 13.9% (n = 247) inhabit the urban regions and in South-Muntenia area, where 16.6% (n = 394) of the subjects live in rural areas, while 9.3% (n = 165) inhabit the urban regions. Bucharest-Ilfov, a highly urbanized area around the capital city (Bucharest), gives the highest per cent of urban inhabitants (19.9%, n = 354), as opposed to 3.3% (n = 79) who live in rural areas. The distribution of the development regions is significantly different in rural areas vs. urban areas (p < 0.0001).
Unimodal treatment type was used in 62.3% (n = 1,473) for children from rural areas and in 65.9% (n = 1,174) for those residing in urban areas. The most common used unimodal treatment type in both rural and urban patients is chemotherapy (49.9%, n = 1,178, vs. 49.62%, n = 885). Multimodal treatment was administered to 37.7% (n = 890) patients from rural territories and 34.1% (n = 607) to patients from urban areas. The most commonly used multimodal therapy was chemotherapy combined with surgery in 31.5% (n = 745) of the subjects residing in rural regions and in 29.1% (n = 519) of the subjects residing in urban regions. As expected, no correlations were found regarding the geography and behavior (p = 0.17), main ICCC-3 categories (p = 0.27) and ICCC-3 subcategories (p = 0.063).
The overall 5-year survival rate for all the cases diagnosed between 2010 to 2017 in the 0–19 years population (n = 3,308) was 72% (95% CI: 71, 75) during the study period.
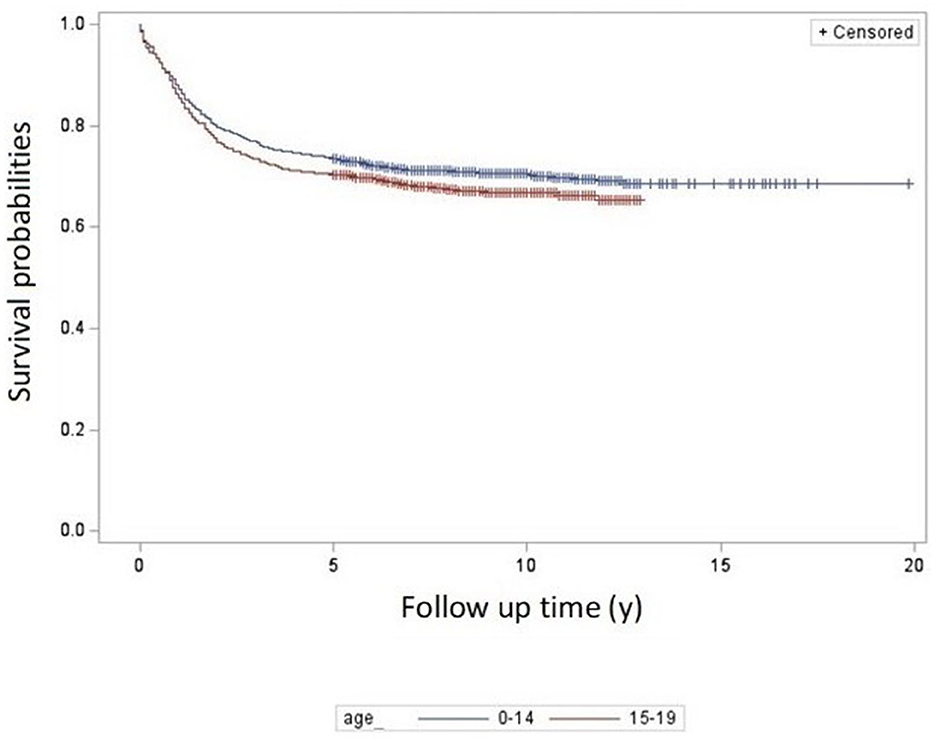
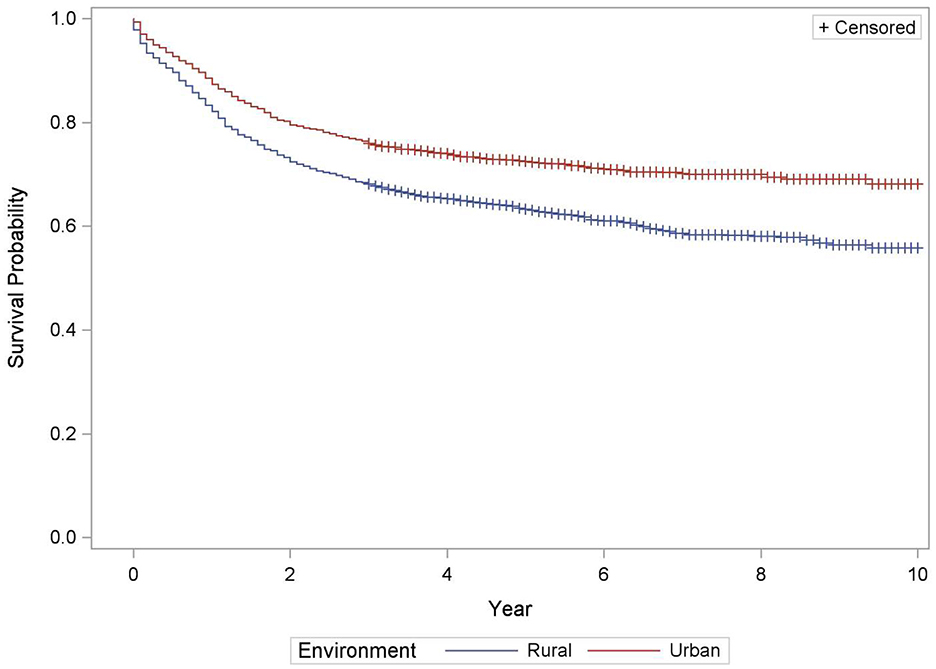
The 5-year overall survival probability for 0–14 and 15–19 age groups was 73% (95% CI: 71, 75) and 69% (95% CI: 65, 72) respectively (Figure 1). Categorized further by smaller age groups for 0–14 age group, the survival rates were 75% (0–4 years), 73% (5-9 years) and 69% (10–14 years).
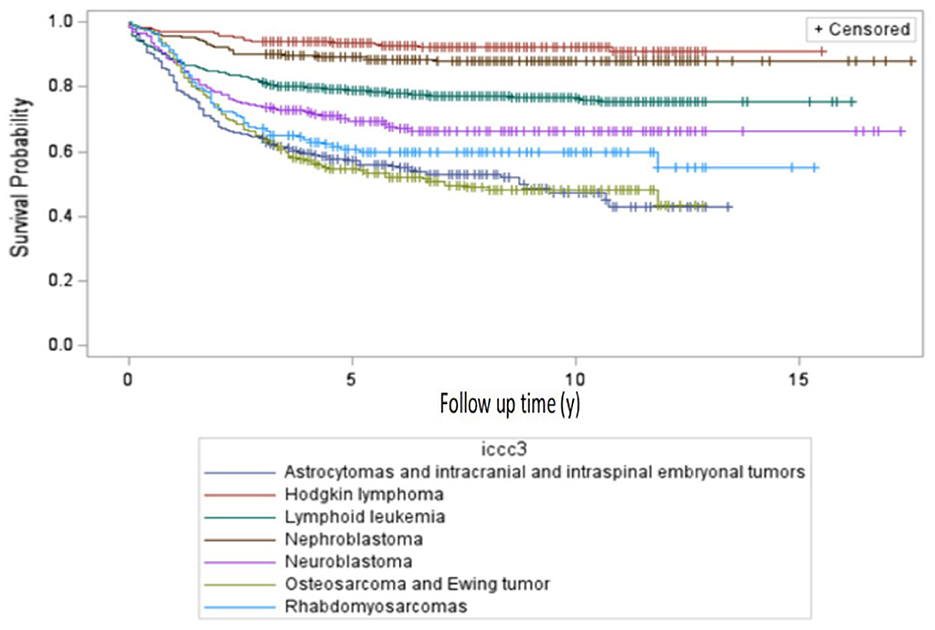
For an international comparability purpose, survival analysis by selected ICCC-3 tumor categories was performed for the 0–14-year group. Crude 5-year overall survival rates showed that Hodgkin lymphoma (92%, 95% CI: 87, 96), nephroblastoma and other nonepithelial renal tumors (89%, 95% CI: 84, 94), and lymphoid leukemias (80%, 95% CI: 77, 83) had the highest survival rates among all the seven major cancer subtypes. The worst survival was observed for CNS tumors (62%, 95% CI: 57, 68), rhabdomyosarcoma (62%, 95% CI: 53, 72), bone tumors (52%, 95% CI: 47, 63), and neuroblastoma (67%, 95% CI: 61, 74), as shown in Table 3. The 5-year survival probabilities in the 0-19 years group is portrayed in Figure 2.
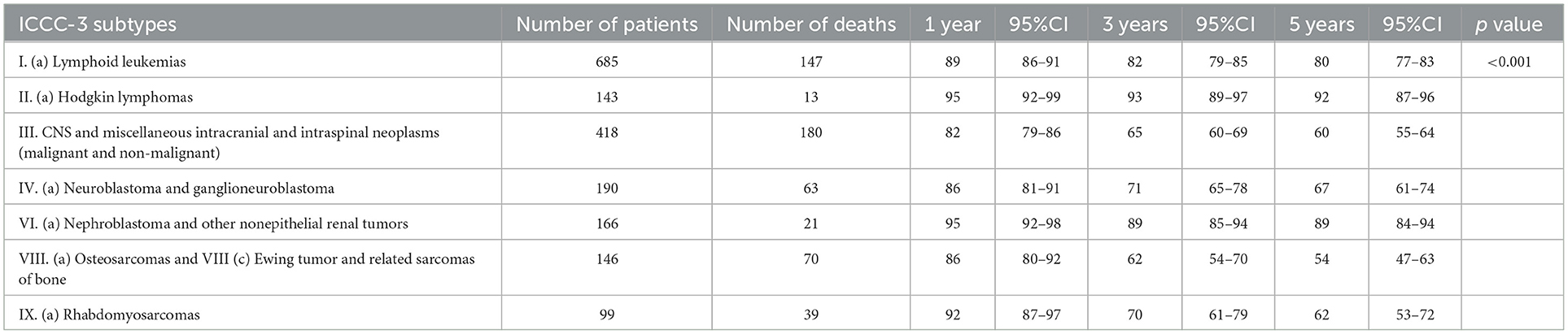
Table 3. Crude 1-, 3- and 5-year survival in patients aged 0–14, diagnosed in 2010–2017 (number of patients = 1,847).
We also examined the comparative death-risk by selected tumor category in the entire study population (aged 0–19, diagnosed in the 2010–19 period). Table 4 shows that Hodgkin lymphoma (0.96, 95% CI: 0.93, 0.98), nephroblastoma and other nonepithelial renal tumors (0.91, 95% CI: 0.86, 0.95), and lymphoid leukemias (0.84, 95% CI: 0.80, 0.87) had the highest survival rates among all seven major cancer subtypes. Patients with Hodgkin lymphomas and nephroblastoma and other nonepithelial renal tumors had 75% (HR = 0.25, 95% CI: 0.16, 0.39) and 40% (HR = 0.6, 95% CI: 0.38, 0.94) reduced risk of mortality from cancers compared to patients with lymphoid leukemias. The mortality risks for patients with the other four subtype cancers were all higher than for the patients with lymphoid leukemias: astrocytoma and intracranial and intraspinal embryonal tumors (HR = 2.46, 95% CI: 1.9, 3.19), osteosarcomas and Ewing tumor (HR = 2.04, 95% CI: 1.58, 2.63), rhabdomyosarcomas (HR = 1.97, 95% CI: 1.44, 2.69), and neuroblastoma (HR = 1.82, 95% CI: 1.37, 2.4).
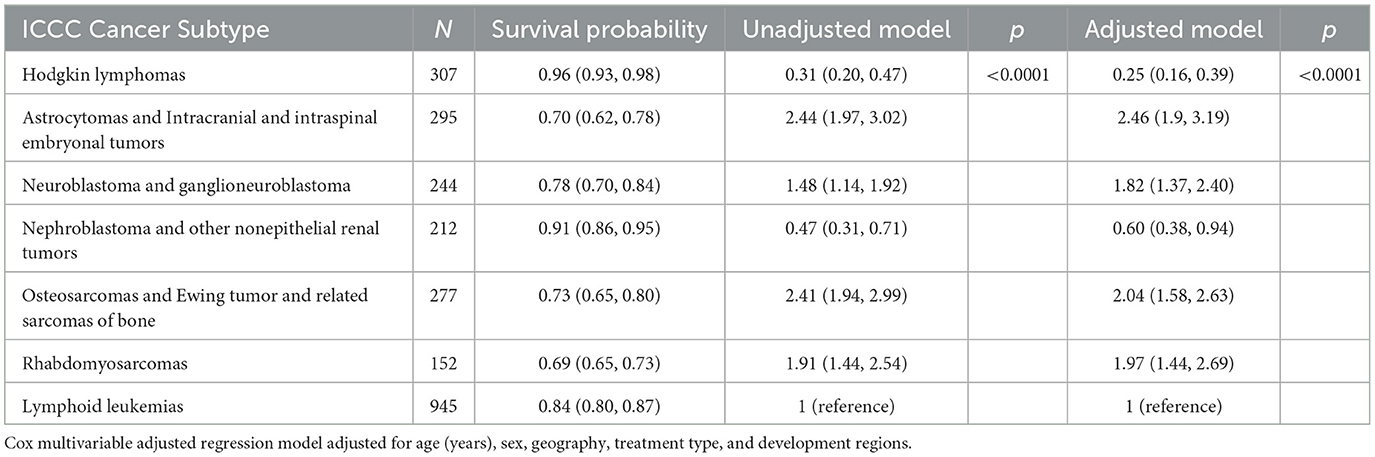
Table 4. Survival probability (95% CI) and unadjusted/adjusted hazard ratios (95% CI) by major ICCC−3 cancer subtype, number of patients = 2,432.
Table 5 depicts the survival of pediatric cancer by rural-urban designation. As compared to urban residents, the risk of dying from cancer in rural residents was 47% higher than urban counterparts (HR = 1.47, 95% CI: 1.29, 1.67), after adjusting for other covariates, such as age in years, gender, development regions, treatment type and primary cancer type (p < 0.0001). The survival rate for rural residents was significantly lower than urban residents (69%, 95% CI: 66, 72) vs. 78%, 95% CI: 75, 80; Figure 3).
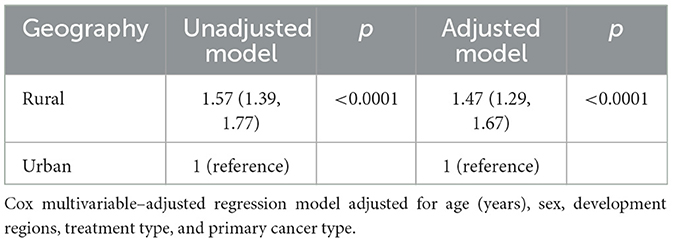
Table 5. Unadjusted/adjusted hazard ratios (95% CIs) by rural/urban designation, number of patients = 4,144.
Contrasted to the patients residing in the North-East, patients residing in Bucharest-Ilfov, Center, and West had 25%, 21%, and 31% reduced risk for dying from cancers (HR for Bucharest-Ilfov =0.75, 95% CI: 0.59, 0.97, HR for center: 0.79, 95% CI: 0.64, 0.98; HR for West: 0.69, 95% CI: 0.53, 0.91 respectively), as presented in Table 6 (p = 0.0359).
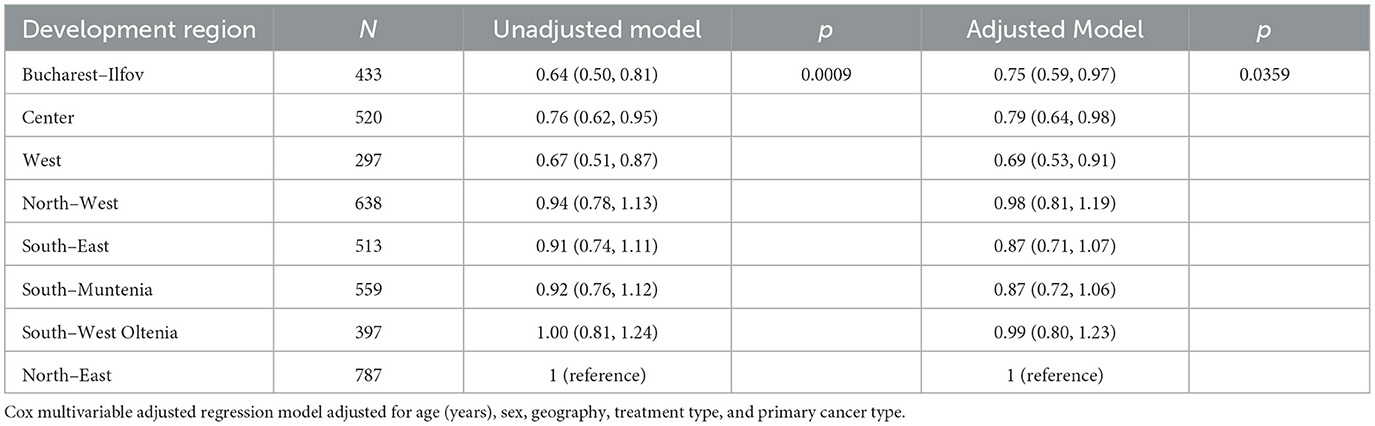
Table 6. Unadjusted/Adjusted hazard ratios (95% CIs) by development region, number of patients = 4,144.
4 Discussion
The present study is the first to report on pediatric cancer survival in Romania based on national cancer registry data, thus allowing for a premiere, comparability to international benchmarks in child cancer outcomes. It examined the overall survival outcomes in different age groups and by selected cancer subtypes, and also explored potential differences in pediatric cancer mortality risk by rural/urban designation, and economic development region, as a prelude to a more in-depth probe into the role of socio-economic determinants of cancer survival chances of Romanian children and adolescents.
4.1 International comparisons
Our results show a significant difference in survival for Romanian children with cancer compared to the European average. It confirms the existence and persistence of childhood cancer survival inequalities among European countries, as illustrated in the Cancer Inequalities Factsheets, published online by the recently launched European Cancer Inequalities Registry (ECIR), a flagship initiative of Europe's Beating Cancer Plan providing data to identify trends, disparities and inequalities on cancer prevention and care across EU member states (8). The December 2023-published ECIR fact sheet on childhood cancers shows relatively large variations in the 5-year survival in the 0–14 population among European countries, based on the EUROCARE−6 study (1, 8).
According to EUROCARE-6, the 5-year survival for all childhood cancers (0–14 years population) combined in Europe in 2010–14 was 81% (95% CI: 81, 82), showing an increase of 3% points compared with 2004–06. The study reported significant progress over time for almost all pediatric cancers, in particular for countries in the Eastern European region, where a trend of an accelerated narrowing of the pediatric cancer survival gap is evident (1). Still, for all cancers combined, inequalities persisted among European countries [with age-adjusted 5-year survival ranging from 71% (95% CI: 60, 79) to 87% (95% CI: 77, 93)]. EUROCARE-6 data were based on 80 population cancer registry reports from 31 countries. Romania was not among the countries that contributed data to EUROCARE (1).
However, our present study, although spanning a slightly longer period (2010–2017) than that covered by the most recent EUROCARE analysis (2010–2014) allows for a relevant comparison of child cancer survival in Romania to the EU average, to the Eastern European profile as well as to other individual member states.
Specifically, 5-year survival in the 0–14 group of our study population is 73% (CI 95% 71, 75), significantly lower than the EU average (81%) and with a major gap to countries such as Denmark (86%), Austria (85%), Slovenia and Belgium (84%), but close to Bulgaria (73%) Lithuania (74%), and other Eastern European countries.
Data published by national registries, covering a longer period, such as the German Childhood Cancer Registry report a 5-year survival of 86% (2009–2018) in the 0–17 years population; while the French National Registry of Childhood Cancers publishes on its website a survival of 83.1% in the 0–14 population, spanning from 2000 to 2016 (22, 23).
Although the EUROCARE study did not note differences in survival among smaller age groups in Europe, around 80% (except for the 0-4 year group: 83%), in our study the 5-year overall survival rates showed a tendency to decrease with age: 75% (0–4 years), 73% (5–9 years) and 69% (10–14 years).
In the 15–19 years group of our study, survival was 69%. No data are available in EUROCARE−6 published analysis on this age group, since many childhood population-based cancer registries do not collect data on patients diagnosed at the ages of 15–19 years (1).
Survival varies considerably by tumor type, with some cancers showing less progress in survival over time than others, a fact that can be observed in all countries, according to the EUROCARE study. For instance, survival rates showed insignificant gains in survival for bone tumors, non-Hodgkin lymphoma and rhabdomyosarcomas (1).
Comparing our crude 5-year observed survival results in the 0–14 age group for cases diagnosed between 2010 and 2017 (Table 3) to EURCARE-6 results (2010–2014) for selected ICCC-3 categories we could note that the gap in survival rates between Romania and the EU average as well as among countries in Eastern Europe vs. other European regions significantly differs by tumor type. In this instance crude rates were used in order to match EUROCARE - 6 individual countries survival data as published in the paper supplement (1).
Hodgkin Lymphomas (92%) and Nephroblastoma (89%) are the tumors with the smallest difference in 5-year survival rates between Romania and the European average as resulted from the EUROCARE-6 study, (97% vs. 92%).Higher differences in survival, up to a 10% gap between Romania and the European average were found in Lymphoid leukemia (80% vs. 90%), Neuroblastoma and ganglioneuroblastoma (67% vs. 75%) and Rhabdomyosarcomas (62% vs. 71%). For these tumors, significant differences are also noted within the Eastern European countries group, with similar survival rates in the lowest end of the range in Romania, Bulgaria, Lithuania and Slovakia.
The largest disparities in survival (up to 15% in difference) were recorded for the CNS and miscellaneous intracranial and intraspinal neoplasms (malignant and non-malignant) category: 60% in Romania vs. 73% the European average and in malignant bone tumors: 53% vs. 68% in osteosarcomas and 55% vs. 70% in Ewing tumors. These are also the cancers with the largest variation in survival across countries, irrespective to the European region they belong (1).
The extent to which survival disparities are due to differences in stage distribution (at diagnosis) could not be assessed in our study because of insufficient data completeness on staging and other prognostic factors, a similar barrier being also reported in the EUROCARE studies. However, data suggesting differences in access to treatments have been published in 2021, in a comprehensive review of access to essential anticancer medicines for European children and adolescents by country. The study showed that in some countries less than half of the essential anticancer medicines were always available, with shortages being the main cause of unavailability. The analysis across the different countries showed that the lowest essential medicine availability rates were encountered precisely in those European countries with lower survival rates: Romania, Bulgaria and the Baltic countries (24).
The same pattern of discrepancies is visible in the proportion of oncological clinical trials available to children and adolescents, which varies considerably between countries, ranging from none to over 50%. According to data published on the European Cancer Inequalities Registry portal, the lowest participation in clinical trials is seen in the Eastern European countries with lower survival rates: Romania, Bulgaria, Slovakia, Croatia and the Baltic countries (25).
Still, as noted in the EUROCARE reports, the East-West divide is closing. In Romania's case, the RNPOHR 2024 report includes an analysis of national trends in pediatric cancer survival, comparing 5-year survival outcomes between the 2010–2013 (n = 1,291) diagnosed cohort and the 2014–2017 (n = 1,328) diagnosed cohort. In the 0–14 population group, crude 5-year survival in all cancers has increased from 69% (95% CI: 66, 71) to 74% (95% CI: 72, 76), a 5% increase exceeding that reported by EUROCARE-6 for the European average. Notwithstanding the relatively small number of cases, survival appears to have increased even more markedly for tumors with the lowest survival rates, such as malignant and non-malignant CNS tumors - from 50 % (95% CI: 44, 58) to 65% (95% CI: 59, 72) and malignant bone tumors – from 44 % (95% CI: 34, 58) in the 2010–2013 cohort to 58 % (95% CI: 49, 70) in 2014–2017.
Since the most significant additions to diagnosis and treatment capacity in Romania were made after 2017, the Romanian Society of Pediatric Oncology and Hematology asked the registry to look at the trends in cancer outcomes from 2010 to 2019, by comparing the crude 3-year survival rates between 3 cohorts of cases, diagnosed in 2010–2012 (n = 1,254) vs. 2013–2016 (n = 1638) vs. 2017–2019 (n = 1328). The results appear to support an impact of the improvement in diagnosis (molecular biology tests and precision imagery used on a wider scale) as well as the introduction of new treatments including the availability of targeted therapy on a larger scale. Survival rates shifted from 72% (95% CI: 69, 74) in 2010–12 to 76% (95% CI: 74, 78) for 2013–16 to reach 84% (95% CI: 82, 86) in 2017–2019 (6).
4.2 Geographical variation
Our study also explored potential survival inequalities among population categories in Romania, attributable to socioeconomic and geographical factors such as place or region of residence.
The study reported worse survival evident in the patients residing in rural areas and better survival in Bucharest-Ilfov and West and Center development regions as compared to North-East region.
Previous research revealed that the survival of pediatric cancer was associated with age, late-stage presentation, choice of treatment options, and proximity to pediatric cancer centers (11–13, 26). Rural residents, often characterized as having access to care issues and thus faced with more severe disease presentation for late diagnosis, were largely reported in high-income and middle-income countries to have worse survival than their urban counterparts with some exceptions where sufficient caregiver support is given (27–31). Previous study also reported worse survival of rural pediatric cancer patients in middle-income European countries than in high-income countries (e.g., the U.S.) (32).
Consistently, the current study reported the rural-urban disparities in economic development region distribution. Rural Romanian patients were more likely to be in the North-Eastern region where the net income per capita is lowest across all eight regions. The rural population in the North-Eastern region is located remotely distant from the center of the region, making it difficult for them to timely access pediatric cancer treatment facilities and imaging services that are most available in the center of the region (9). Rural areas in Romania have also endured inadequate infrastructure in basic areas such as public services, medical facilities, roads, railways, water, and sanitary sewerage (33). This inadequacy may aggravate the access to care issue and therefore cause delayed diagnosis (34), contributing to late disease presentation and requiring complicated multimodal treatment in rural patients.
Our findings show a lower survival rate and a higher risk of premature death in rural pediatric cancer patients than their urban counterparts, supporting all previous findings from high- and middle-income countries where rural residents are also more likely to face financial and emotional challenges and to have barriers in access to comprehensive cancer treatment centers and imaging services (35). Due to the poor occupational diversification, insufficient entrepreneurial attraction, and high dependence on agriculture, lower employment rates in rural areas could also exacerbate the access to care issues (33, 36).
The regional differences in survival found in this study could be reflective of the limited financial resources and health services centered in certain regions where rural residents were more clustered. For example, the North-Eastern region where the most rural population reside was found to have the highest premature mortality risk. While national income levels in Romania converge toward neighboring European countries, regional disparities in income levels have widened since the 2000s in Romania (37). This economic inequality further weakens rural residents' financial capabilities within these regions (38), affecting the rural residents' quality of life and their survival probability. Notably, up to 85% Romanian citizen is entitled with cost-free basic package of medical services through public healthcare system and the treatment of pediatric cancer is totally free (39). However, obstacles to receiving adequate medical services and the full knowledge about the health benefits remain to be addressed because of the shortage of medical personnel and well-round medical delivery (34).
We were among the first to characterize the survival outcomes of pediatric cancer population in Romania using specifically designed national pediatric cancer registry data that are well-validated and organized, informative, and well-linked to reliable and accepted coding system for rural-urban designation and development regions (6).
However, the study may be subject to limitations such as potential incompleteness of cancer cases that were underreported in the registry, in particular adolescents receiving treatment in adult care facilities and the pediatric population that seeks cross-border care without reaching the national pediatric oncology network. Still, although not applicable to the totality of cancer cases in the Romanian pediatric population, the results comprehensively reflect the outcomes of the Romanian pediatric oncology care system (6). Also, our study could have benefited from a relatively longer follow-up time and complete stage information which was not made available in the registry. Not least, potential variation in the standards of care among centers in the different regions of Romania may also warrant a more in-depth exploration.
Improvement in access to and quality of care is a central focus of current reforms in Romania, under the 2022 adopted National Cancer Control Plan, which dedicates an entire chapter to pediatric cancers and is currently developing a new framework regulating the national pediatric oncology network with a specific aim of increasing coverage with optimal services of the entire national territory (40).
5 Conclusion
This study for the first time reported the overall, rural-urban, regional, and cancer-subtype-specific disparities in survival and mortality risk of the Romanian pediatric cancer population using a national-level cancer registry. Although narrowing at an accelerated pace, a significant gap remains between Romania and other European countries, in particular in cancers that showed an evident increase in survival over the past 10 years such as CNS tumors, neuroblastoma and rhabdomyosarcomas. Urban-rural and regional differences may also partially contribute to the pediatric cancer survival disparities between Romania and other EU members.
The findings suggest collective efforts at regional and national levels should be made to mitigate these disparities to promote the better survival of patients with pediatric cancers in Romania and any middle-income or high-income countries alike where the disparity exists.
5.1 Future directions
The study findings have significant public health implications and set the foundation for making necessary policy shifts to prioritize economic and medical resources for special pediatric cancer populations, including rural populations and economically and healthcare-underserved populations in some regions. Collective efforts at regional and national level should be made to effectively increase healthcare expenditure to establish healthcare services and improve healthcare access in rural areas and certain economic development regions. It is also necessary in the future to improve coverage of the Romanian pediatric population by RNPOHR by diversifying and complementing data sources and optimizing the clinical information capture by the Registry in order to cope with the current limitations in potential missing cases faced by the national-level cancer registry.
Further research is necessary for a more in-depth characterization of the survival determinants and their specific interplay in the outcomes of pediatric cancer in Romania.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was obtained from the Romanian Institutional Review Board of the Romanian Society for Pediatric Oncology and Hematology and the CUNY Institutional Review Boards. Written informed consent from the patients or patients' next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
MB: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing, Writing – original draft. LZ: Formal analysis, Methodology, Resources, Validation, Visualization, Writing – original draft. BW: Formal analysis, Investigation, Resources, Writing – original draft. AS: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing. AN: Data curation, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. CEESP is a research training Program funded by the National Cancer Institute of the National Institute of Health of the United States (U.S.) since 2006 through an educational grant (R25 CA112383). CEESP provides funding, research opportunities, and mentorship to public health graduate students from different universities in the U.S. to collaborate and conduct research under the mentorship of leaders from global cancer centers, cancer registries, and non–governmental organizations to analyze existing data, conduct research studies, and disseminate information under the mentorship of global sites' mentors.
Acknowledgments
We acknowledge the kind contribution of the Daruieste Aripi Association (Romania) as a supporter of the Romanian National Pediatric Oncology and Hematology Registry.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcacs.2025.1546879/full#supplementary-material
References
1. Botta L, Gatta G, Capocaccia R, Stiller C, Cañete A, Dal Maso L, et al. Long-term survival and cure fraction estimates for childhood cancer in Europe (EUROCARE-6): results from a population-based study. Lancet Oncol. (2022) 23:1525–36. doi: 10.1016/S1470-2045(22)00637-4
2. EC. Europe's Beating Cancer Plan. Communication from the commission to the European Parliament and the Council (2021).
3. INS. Romanian Statistical Yearbook. (2023). Available online at: https://insse.ro/cms/sites/default/files/field/publicatii/anuarul_statistic_al_romaniei_carte_ed_2023-ro.pdf (accessed December 4, 2024).
4. Bank W. România—Prezentare generala. (2024). Available online at: https://www.worldbank.org/ro/country/romania/overview (accessed April 1, 2024).
5. Ferlay JEM, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today: Lyon, France: International Agency for Research on Cancer. (2024). Available online at: https://gco.iarc.who.int/today (accessed December 6, 2024).
6. RNPOHR. Executive Report. 2010–2021 (2024). Available online at: https://srohp.ro/wp-content/uploads/2024/02/RNOHP-cu-studiu-2024-1.pdf (accessed December 6, 2024).
7. Steliarova-Foucher E, Colombet M, Ries LA, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. (2017) 18:719–31. doi: 10.1016/S1470-2045(17)30186-9
8. ECIR. Childhood Cancers Every Child and Adolescent Deserves an Equal Chance. (2023). Available online at: https://cancer-inequalities.jrc.ec.europa.eu/sites/default/files/ECIR-inequalities-factsheet-childhood-cancer-Dec2023.pdf (accessed December 6, 2024).
9. Surd V, Kassai I, Giurgiu L. Romania disparities in regional development. Procedia-Soc Behav Sci. (2011) 19:21–30. doi: 10.1016/j.sbspro.2011.05.102
10. Eurostat. EU Regions by GDP, Eurostat (2024). Available online at: www.ec.europa.eu
11. Pruteanu DP, Olteanu ED, Cosnarovici R, Mihut E, Ecea R, Todor N, et al. Early deaths in childhood cancer in Romania—a single institution study. Children. (2021) 8:814. doi: 10.3390/children8090814
12. Cosnarovici MM, Cosnarovici R, Piciu D. Therapeutic results in children with brain tumors–a single center experience over 18 years. Med Pharm Rep. (2024) 97:56. doi: 10.15386/mpr-2571
13. Radu L-E, Colita A, Pasca S, Tomuleasa C, Popa C, Serban C, et al. Day 15 and day 33 minimal residual disease assessment for acute lymphoblastic leukemia patients treated according to the BFM ALL IC 2009 protocol: single-center experience of 133 cases. Front Oncol. (2020) 10:923. doi: 10.3389/fonc.2020.00923
14. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
15. ECIR. Death Rate of Malignant Neoplasms (2021) By Country And Age Groups: European Comission. (2021). Available online at: https://cancer-inequalities.jrc.ec.europa.eu/ (accessed December 9, 2024).
16. ACTIVITATEA REELEI SANITARE I DE OCROTIRE A SANATAII ÎN ANUL 2021 (2022). Available online at: https://www.insse.ro (accessed July 1, 2022).
17. ACTIVITATEA REELEI SANITARE I DE OCROTIRE A SANATAII ÎN ANUL 2022 (2023). Available online at: https://www.insse.ro (accessed July 3, 2023).
19. WHO. International Classification of Diseases for Oncology, 3rd Edition. (ICD-O-3 Available from: https://www.who.int/standards/classifications/other-classifications/international-classification-of-diseases-for-oncology (accessed December 4, 2024).
20. Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer. Cancer. (2005) 103:1457–67. doi: 10.1002/cncr.20910
21. MO. Hotarari ale Guvernului Romaniei. Hotarare privind aprobarea programului statistic anual pentru anul 2009 (2009). Available online at: https://insse.ro/cms/files/legislatie/programe%20si%20strategii/PSA%202009%20MO.pdf (accessed December 4, 2024).
22. Spix C, Erdmann F, Grabow D, Ronckers C. Childhood and adolescent cancer in Germany–an overview. J Health Monitor. (2023) 8:79. doi: 10.25646/11438
23. RNCE. Registre National des Cancer de l'Enfant-Les chiffres (2021) Available online at: https://rnce.inserm.fr/rnce/les-chiffres/ (accessed December 9, 2024).
24. Vassal G, Kozhaeva O, Griskjane S, Arnold F, Nysom K, Basset L, et al. Access to essential anticancer medicines for children and adolescents in Europe. Ann Oncol. (2021) 32:560–8. doi: 10.1016/j.annonc.2020.12.015
25. ECIR. Proportion of Oncology Clinical Trials Available to Children (2022) By Country. (2022). Available online at: https://cancer-inequalities.jrc.ec.europa.eu/data-tool-by-countryind=ONCTCHLD&ft=TOTAL (accessed December 12, 2024).
26. Ohlsen TJ, Doody DR, Mueller BA, Desai AD, Chow EJ. Population-based impact of rurality and neighborhood-level socioeconomic disadvantage on pediatric cancer mortality in Washington state. Cancer Epidemiol Biomarkers Prev. (2023) 32:141–8. doi: 10.1158/1055-9965.EPI-22-0897
27. Puthenpura V, Canavan ME, Poynter JN, Roth M, Pashankar FD, Jones BA, et al. racial/ethnic, socioeconomic, and geographic survival disparities in adolescents and young adults with primary central nervous system tumors. Pediatr Blood Cancer. (2021) 68:e28970. doi: 10.1002/pbc.28970
28. Doganis D, Panagopoulou P, Tragiannidis A, Vichos T, Moschovi M, Polychronopoulou S, et al. Survival and mortality rates of Wilms tumor in Southern and Eastern European countries: socioeconomic differentials compared with the United States of America. Eur J Cancer. (2018) 101:38–46. doi: 10.1016/j.ejca.2018.06.012
29. Tarnasky AM, Olivere LA, Ledbetter L, Tracy ET. Examining the effect of travel distance to pediatric cancer centers and rurality on survival and treatment experiences: a systematic review. J Pediatr Hematol Oncol. (2021) 43:159–71. doi: 10.1097/MPH.0000000000002095
30. Delavar A, Johnson KJ. Place of residence and childhood cancer survival. Oncotarget. (2019) 10:1864–5. doi: 10.18632/oncotarget.26717
31. González García H, Garrote Molpeceres R, Urbaneja Rodríguez E, Gutiérrez Meléndez P, Herráiz Cristóbal R, Pino Vázquez MA. Differences in incidence and survival to childhood cancer between rural and urban areas in Castilla y León, Spain (2003–2014): a strobe-compliant study. Medicine. (2018) 97:e12797. doi: 10.1097/MD.0000000000012797
32. Panagopoulou P, Georgakis MK, Baka M, Moschovi M, Papadakis V, Polychronopoulou S, et al. Persisting inequalities in survival patterns of childhood neuroblastoma in Southern and Eastern Europe and the effect of socio-economic development compared with those of the US. Eur J Cancer. (2018) 96:44–53. doi: 10.1016/j.ejca.2018.03.003
33. Ignat R, Stoian M, Roşca V. Socio-economic aspects of rural Romania. Procedia Econ Financ. (2014) 15:1331–8. doi: 10.1016/S2212-5671(14)00596-6
34. Petre I, Barna F, Gurgus D, Tomescu LC, Apostol A, Furau C, et al. Analysis of the healthcare system in Romania: a brief review. Healthcare. (2023) 11:2069. doi: 10.3390/healthcare11142069
35. Olson AL, Boyle WE, Evans MW, Zug LA. Overall function in rural childhood cancer survivors. The role of social competence and emotional health. Clin Pediatr. (1993) 32:334–42. doi: 10.1177/000992289303200603
36. Georgeta ES. The economic and social situation in Romania. wwweesceuropaeu: European Economic and Social Committee (2015).
37. IMF. Romania: Selected Issues (2023). Available online at: https://www.elibrary.imf.org/view/journals/002/2023/396/002.2023.issue-396-en.xmlrskey=rZaRIJ&result=8 (accessed December 6, 2024).
38. Fina S, Heider B, Rat C. Unequal Romania- Regional Social Economic Disparities in Romania (2021).
39. Vladescu C, Scintee SG, Olsavszky V, Hernandez-Quevedo C, Sagan A. Romania: health system review. Health Syst Transit. (2016) 18:1–170.
40. Sanatatii M. Planul Naional de Combatere i Control al Cancerului (2023). Available online at: https://ms.ro/media/documents/Planul_Naional_de_Combatere_i_Control_al_Cancerului_RIQiTXG.pdf (acesses December 12, 2024).
Keywords: pediatric cancer, disparity, rural, urban, survival, mortality, Eastern-Europe
Citation: Bucurenci M, Zhang L, Wayne B, Soliman A and Neaga A (2025) Childhood cancer survival in Romania—A national pediatric registry perspective. Front. Cancer Control Soc. 3:1546879. doi: 10.3389/fcacs.2025.1546879
Received: 17 December 2024; Accepted: 03 April 2025;
Published: 09 May 2025.
Edited by:
Vesna Zadnik, Institute of Oncology Ljubljana, SloveniaReviewed by:
Vinit Nalawade, Duke University Health System, United StatesMohamed Saad Zaghloul, Cairo University, Egypt
Copyright © 2025 Bucurenci, Zhang, Wayne, Soliman and Neaga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mihaela Bucurenci, bWloYWVsYS5idWN1cmVuY2lAc3JvaHAucm8=
 Mihaela Bucurenci
Mihaela Bucurenci Li Zhang2
Li Zhang2 Breana Wayne
Breana Wayne Alexandra Neaga
Alexandra Neaga