- 1Leeds Institute of Health Sciences, University of Leeds, Leeds, United Kingdom
- 2Exeter Medical School, University of Exeter, Exeter, United Kingdom
- 3NHS Dorset (Integrated Care Board), Dorset, United Kingdom
- 4Department of Family Medicine, University of Washington, Seattle, WA, United States
Introduction: Point-of-care tests (POCTs) for cancer in primary care have the potential to increase diagnostic certainty, improve triage and enhance patients' experience of diagnosis. However, there is limited evidence to support their adoption, and patient preferences have not previously been investigated. This study aimed to assess the prospective acceptability of POCTs for cancer in primary care based on a hypothetical vignette.
Methods: This was a mixed-methods study based on the Theoretical Framework of Acceptability (TFA) consisting of a quantitative online survey and remote qualitative interviews with the UK public. Quantitative data were reported as frequencies. Qualitative data were analyzed combining inductive and deductive framework analysis.
Results: Two thousand three hundred three adults completed the online survey, and 27 participants were recruited for follow-up interviews. The survey indicated most (92%, 2,116/2,303) participants found the potential use of POCTs for cancer acceptable or very acceptable. There were some small demographic differences in levels of acceptability. Interview findings indicated acceptability was primarily driven by a quick turnaround time for test results, with a preference for testing even when results were indicative and not confirmatory. Participants highlighted the importance of test accuracy, clear communication regarding test limitations, and having a genuine choice in the decision to take the test. Participants also discussed the improved likelihood of adherence to referrals for invasive testing following a positive POCT.
Discussion: The use of POCTs for cancer in primary care is acceptable to the UK public, however important considerations regarding test accuracy and consenting for tests should be considered prior to implementation. Future adoption should evaluate acceptability of specific cancer POCTs, particularly in underserved populations.
1 Introduction
Cancer remains a significant public health burden worldwide (1). Delayed diagnosis has been associated with poorer survival outcomes for many cancers (2–4). In the UK, where patient outcomes fall behind those of other high-income countries (5), efforts have often focused on earlier diagnosis. Most patients with cancer symptoms are initially seen in primary care by general practitioners (GP) (6), with a proportion of cancer diagnoses made following a referral from this setting (7). It is therefore essential to consider how cancer detection can be improved within general practice.
For patients presenting with high-risk cancer symptoms, it usually takes one or two GP consultations before they are referred on urgent suspected cancer pathways (8). This can take three or more consultations for patients with rare cancers or less distinctive characteristics (9), resulting in diagnostic delays. Additionally, about 90% of patients referred via the urgent suspected 2-week wait (2WW) pathway—a service designed to quickly assess patients suspected of having cancer—are not diagnosed with cancer (10). These patients likely have symptoms that the 2WW pathway is not intended to address, impacting both healthcare resource allocation and patient care experience.
The availability of efficient, rapid, diagnostic cancer tests in primary care, such as point-of-care tests (POCTs), can potentially improve triage and reduce diagnostic uncertainty. POCTs are diagnostic tools used during a consultation which can provide results in minutes. They can reduce diagnostic delays by improving certainty and enhance patient experience by minimizing waiting times for tests results, and avoiding unnecessary investigations (11, 12).
POCTs are a dynamic, rapidly evolving industry with ongoing development of new tests (13). Examples of POCTs commonly used in primary care include lateral flow tests and biosensors, such as dipstick urine and glucose meters. They are frequently utilized in general practice to investigate conditions such as diabetes and pregnancy. Commercially available POCTs for cancer include UBC® Rapid Test for bladder cancer, CancerCheck® PSA and PSAwatch for prostate-specific antigen measurement, among others (14). An example of an emerging POCT for lung cancer is the Breath Biopsy®, designed to detect volatile organic compounds (15).
Despite their availability, cancer POCTs are not widely adopted in UK general practice. Several factors may explain this including heterogeneous study designs that hinder comparison with laboratory standards, the accuracy of POCTs often falling short of laboratory tests, and insufficient clinical utility and cost-effectiveness evidence to support adoption. Cancer may not be considered as time-critical enough to warrant immediate testing in general practice, unlike POCTs testing for infections, which can directly influence antibiotic prescribing decisions. Additionally, test developers often prioritize the technical performance of tests, neglecting key issues such as clinical utility (16) and patient acceptability.
Acceptability is a comprehensive concept that reflects how individuals perceive the appropriateness of a healthcare intervention, based on their anticipated or experienced cognitive and emotional responses (17). Assessing patient acceptability is particularly important as interventions considered acceptable, are more likely to result in adherence to treatment and better clinical outcomes (18, 19). The principles of patient-centered care recognize the importance of considering public acceptability (20), especially in the context of testing for cancer.
As the point-of-care testing market expands and new technologies emerge, it is crucial to assess patients' perspectives on point-of-care testing for cancer. Evaluating patient preferences for POCTs will enable test developers, clinicians, and policymakers to address key factors important to patients for their adoption in healthcare. This study aimed to assess the prospective acceptability of the UK public for the use of POCTs for cancer detection in primary care.
2 Methods and materials
2.1 Design
This was a mixed-methods study consisting of a theory-informed quantitative survey and semi-structured qualitative interviews, assessing prospective acceptability for cancer POCTs based on a hypothetical scenario. The widely cited Theoretical Framework of Acceptability (TFA) (17) informed this study, ensuring a rigorous and comprehensive assessment of the concept to capture all aspects of patient acceptability. The study adopted a convergent parallel design where the quantitative and qualitative data were collected and analyzed independently (21). We adhered to the Standard for Reporting Qualitative studies checklist (22).
2.1.1 Survey
2.1.1.1 Participants and recruitment
We hosted an online survey on Qualtrics in phases between October 2023 and August 2024. The study was advertised through a market research panel (Dynata). Adults living in the UK who self-reported being fluent in English and provided informed consent were included. Participants were provided with the study details and consent information online prior to accessing the survey. All participants received Dynata administered incentives following participation.
2.1.1.2 Procedure
All participant-facing materials referred to point-of-care testing as “rapid testing” to simplify the language. However, it was clarified that such testing might also be described as POCTs or near-patient testing elsewhere to avoid confusion. Once participants viewed the study information and consented to the study, they were presented with a 2 minute educational video explaining “rapid testing” with common non-cancer and cancer specific POCT examples (Supplementary material).
The survey utilized branch logic, directing participants to follow-up questions based on their response to a question. Participants were required to answer each question to progress, and incomplete survey responses were not recorded. All survey materials were developed by one author (AAS) and reviewed by co-authors (SS, RN, and MT). A public representative (PW) reviewed and provided feedback on earlier versions of all participant materials. The survey was piloted with a small sample of Dynata participants to ensure its functionality.
2.1.1.3 Demographic and clinical data
Demographic questions included participant age, gender, ethnicity, highest educational or professional qualification obtained, and UK country of residence (Supplementary material). We included additional questions about prior cancer investigations, cancer history and 2WW referrals to identify participants with previous cancer investigation experience.
2.1.1.4 Acceptability of point-of-care tests
The survey was adopted from the previously published TFA questionnaire, with the wording tailored to the context of POCTs as recommended (23). The survey tool included seven items measuring each of the seven constructs of the TFA (Box 1) and one global acceptability item, all rated on a 5-point Likert scale.
2.1.1.5 Data analysis
All survey data were managed and analyzed in IBM SPSS version 29. As incomplete survey responses were not recorded, there were no missing data. Frequencies were extracted for all TFA constructs for total sample, by demographic groups and 2WW status.
2.1.2 Qualitative interview
2.1.2.1 Participants and recruitment
After completing the survey, participants could express interest in follow-up interviews. We sent invitations via email to six participants at a time. For the interviews, we adopted a purposive sampling approach to ensure the inclusion of individuals with diverse ethnicities, educational backgrounds, and varying levels of prior experience with 2WW cancer investigation where possible. We included participants who had previously been referred through the 2WW pathway from primary care to explore potential differences in acceptability reports between those with prior experience and those without. We excluded participants that reported active cancer investigations from the interviews as they may have found the interview distressing or particularly sensitive. We continued recruiting for the interviews until the research team agreed data saturation was reached. Data saturation was determined as the lack of emergence of new codes from the data (24).
2.1.2.2 Interview schedule
The interview schedule was based on the TFA (17) with one question for each construct and an additional question for overall acceptability (Supplementary material). We also included an additional question regarding previous experience of 2WW referral for participants that met this criterion. The interview schedule was approached flexibly, allowing adjustments to the order of questions and inclusion of additional prompts during the interviews to enable participants to highlight aspects of POCT acceptability they deemed important.
Draft interview questions were evaluated by two researchers (SG and NN) independent of the research team through a “back coding” exercise described by Francis and colleagues (25). This process assessed whether each interview question aligned with the relevant TFA construct. We provided the researchers with the constructs of the TFA and the interview topic guide, with questions organized randomly (including opening questions and 2WW question). They were instructed to identify which construct each question addressed and indicate their confidence on a 5-point scale ranging from “absolutely sure” to “not sure at all.” All interview questions were matched correctly with one item related to the “intervention coherence” construct rated as ambivalent. This question was rephrased for clarity.
2.1.2.3 Procedure
Interview participants were requested to re-watch the educational clip on POCTs prior to their interview and were given the opportunity to ask questions at the beginning of the interview. To assess prospective acceptability, participants were presented with a hypothetical scenario in which they visited a GP due to symptoms and were offered a rapid test. Following this description, TFA-related questions were explored within the context of the scenario (Supplementary material).
2.1.2.4 Data analysis
Author (AAS) has previous experience in collecting and analyzing qualitative data. She conducted all interviews either online (via Microsoft Teams) or by telephone. Interviews were audio recorded and professionally transcribed verbatim with identifiable information removed and replaced with non-identifiable codes. Interview data were analyzed combining inductive and deductive approaches using the framework analysis (26). Transcripts were initially inductively coded with similar codes combined and deductively mapped to the constructs of the TFA. This approach allowed for themes outside of the theoretical framework to be identified (26, 27). We found most of the codes aligned with constructs of the TFA. Two authors (AAS and KEL) independently reviewed and coded the same three transcripts. The researchers discussed each coded selection and their interpretations of the data. Based on these discussions, AAS developed the analytical framework, grouping codes into categories and mapping them to the constructs of the TFA. A further two transcripts were independently indexed using the analytical framework by AAS and KEL, which was checked for the emergence of new codes. This version was reviewed and discussed within the wider research team. AAS used this framework to index all remaining transcripts in NVivo software (version 14).
3 Results
3.1 Survey findings
3.1.1 Demographic data
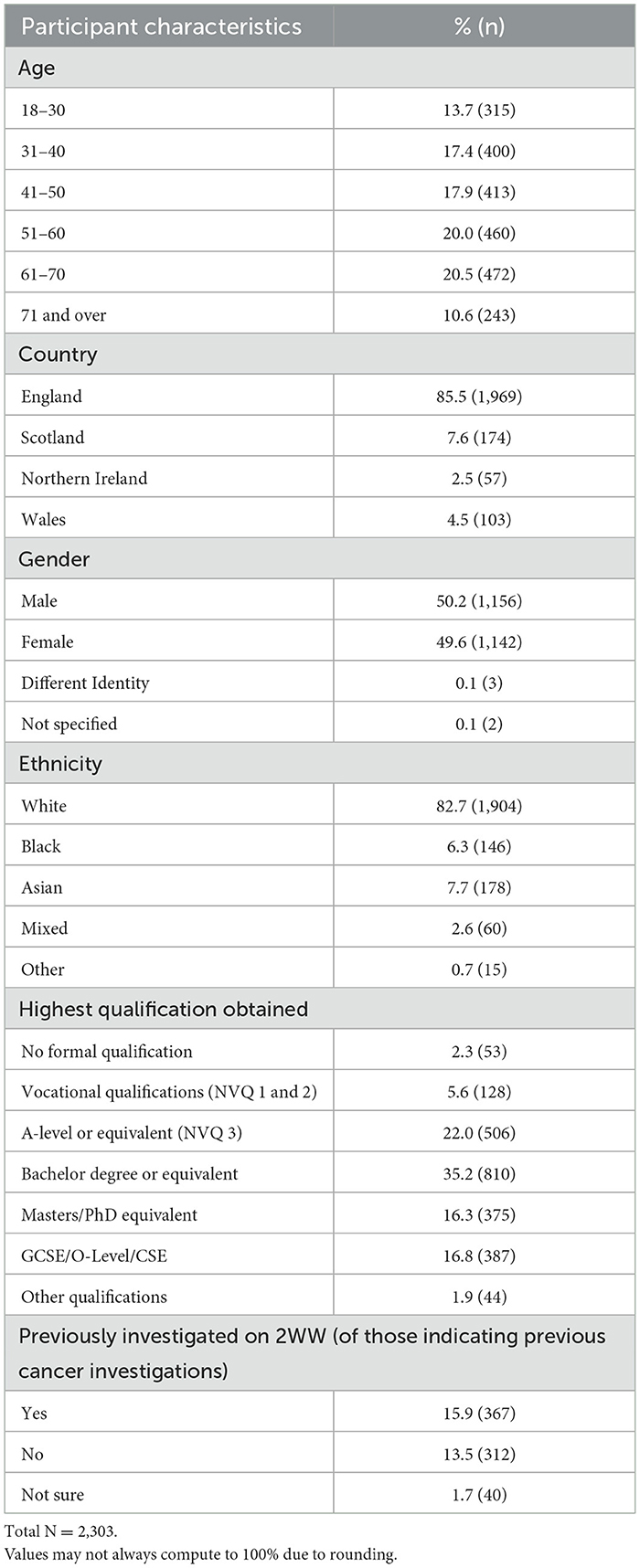
Two thousand three hundred three participants from the UK public completed the survey. Our sample demonstrated a well-distributed age range (Table 1). Most participants were from England (85.5%, n = 1,969). The sample included slightly more men (50.2%, n = 1,156) than women (49.6%, n = 1,142). Participants were predominantly White (82.7%, n = 1,904) and about half (51.5%; n = 1,185) were educated to degree level or above. Approximately one sixth (15.9%; n = 367) of the total sample reported previously receiving an urgent suspected cancer 2WW referral.
3.1.2 Acceptability for point-of care testing by TFA constructs
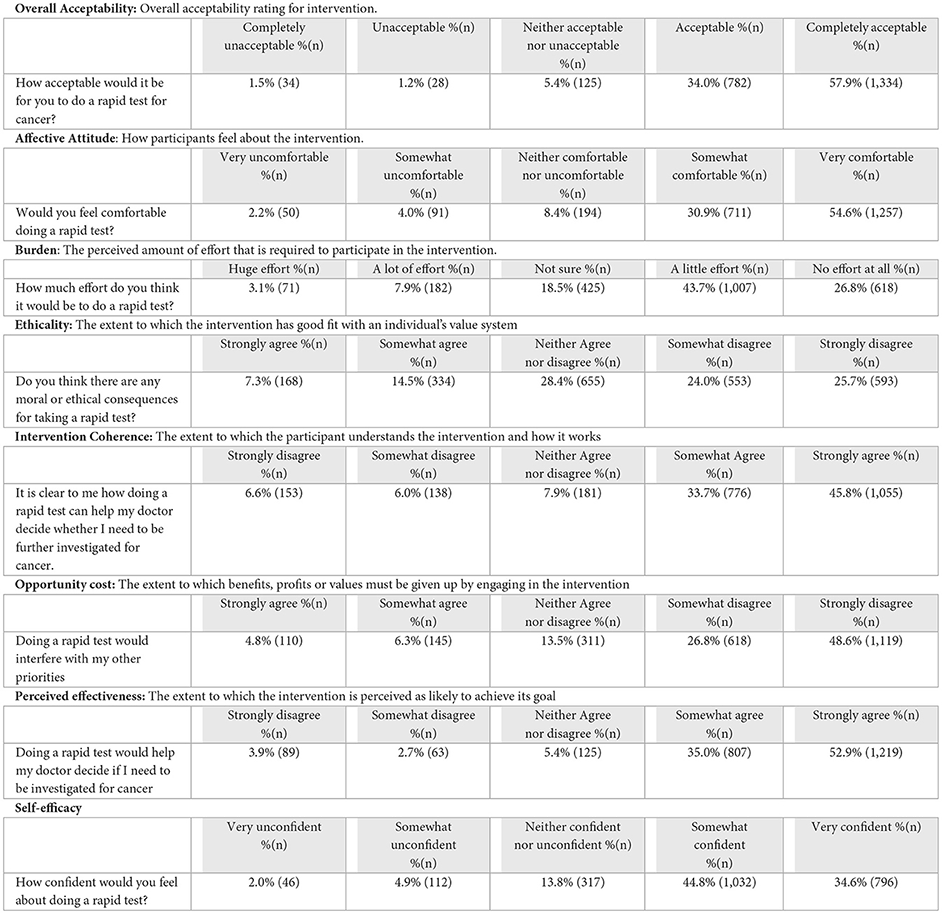
The majority (91.9%, n = 2,116) of participants found the overall use of POCTs for cancer acceptable. Most (85.5%, n = 1,968) believed they would be comfortable doing the test and that it would require a little to no effort from them (70.5%, n = 1,625). Participant views on the ethical implications of using POCTs for cancer varied, with approximately half of the participants either disagreeing that ethical concerns existed (49.7%, n = 1,146), or expressing ambivalence (28.4%, n = 655). Most participants (79.5%, n = 1,831) felt they understood how POCTs can be used by their GP to aid cancer investigations and believed it would help their doctor decide if further investigations were necessary (87.9%, n = 2,026). The majority of people (75.4%, n = 1,737) indicated taking POCTs would not interfere with their other priorities, and they felt confident about taking the test (79.4%, n = 1,828; Box 1).
Box 1 Acceptability scores by Theoretical Framework of Acceptability scale items. Scale adapted from Sekhon et al. (23).
3.1.3 Acceptability by patient characteristics
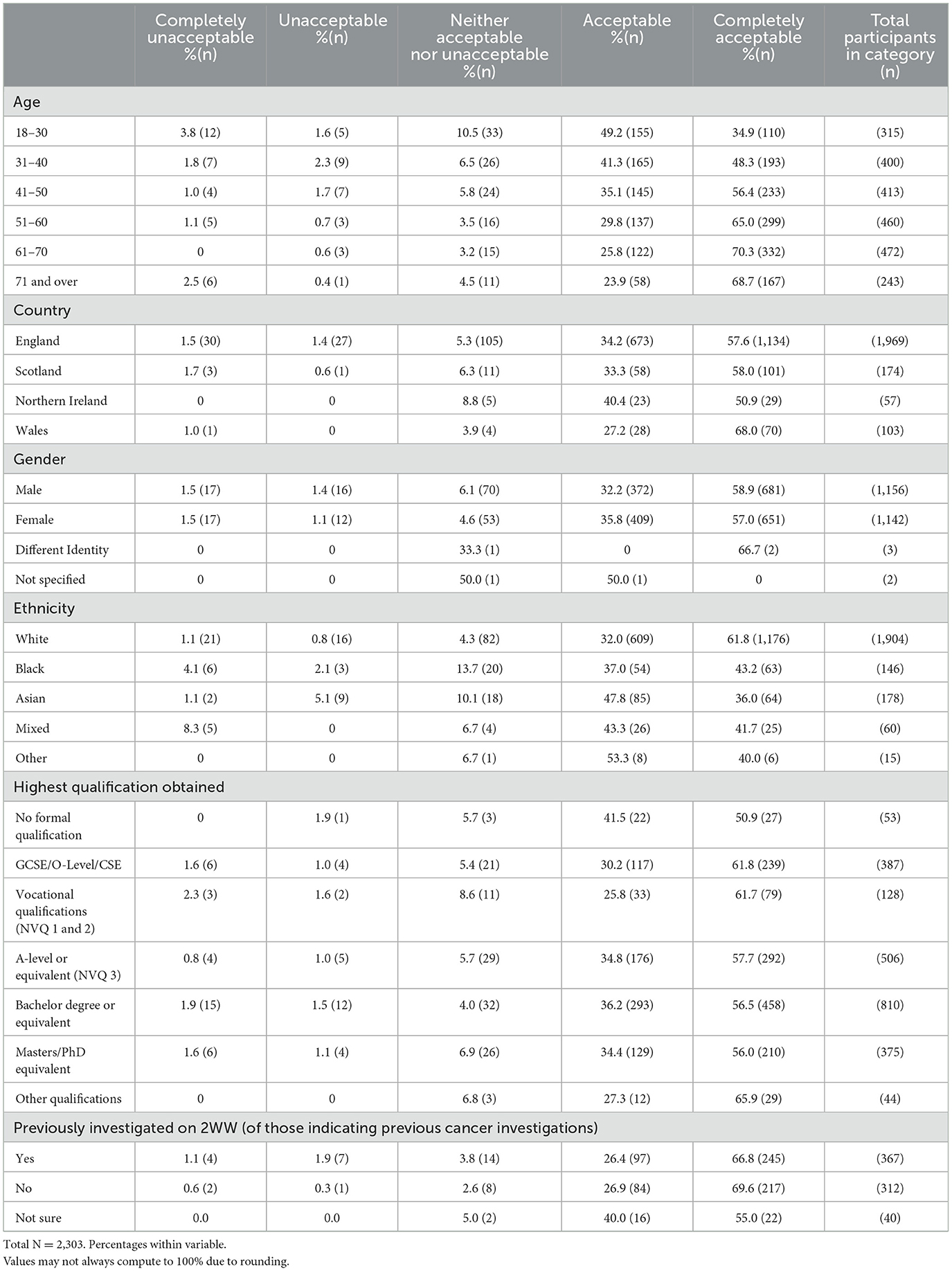
The proportion of participants viewing POCTs as acceptable slightly increased with age between 18 and 70 years, with most people finding POCTs for cancer either acceptable or very acceptable (Table 2). Black participants were the slightly less accepting of POCTs among all reported ethnicities (80.1%, n = 117/146) compared with White (93.8%, n = 1,785/1,904) and Asian (83.7%, n = 149/178) people. Acceptability was also slightly lower in those whose highest qualifications were vocational (NVQ 1 and 2; 87.5%, n = 112/128), compared with participants that were qualified to GCSE (92.0%, 356/387), A- level (92.5% 468/506), Bachelors (92.7%, 751/810) or Masters/PhD level (90.4%, 339/375). For people that indicated their cancer investigation had been following a 2WW referral from their GP, acceptability was lower (93.2%, n = 342/367) compared to those that had no prior experience with 2WW investigations (96.5%, n = 301/312).
3.2 Interview findings
We conducted follow-up interviews with 27 participants. For participants with prior 2WW pathway experience and people with no experience of this pathway, the interviews revealed similar themes across all TFA constructs. However, those with 2WW experience demonstrated a better understanding of investigation pathways, diagnostic processes and were more aware of the limitations of diagnostic tests.
3.2.1 Overall acceptability
Most participants found POCTs for cancer in primary care very acceptable. Participants believed POCTs would be less invasive, and would lead to earlier diagnosis and treatment if necessary:
“Very acceptable … it could save a lot of lives, it'd be a lot less invasive and less waiting around and if you knew you had [cancer] or potentially had it, you could start treatment a lot quicker.” (016AR).
“if the opportunity arose for me to take a rapid test because I had symptoms then I would be very… happy to take one.” (017SR, previous 2WW)
3.2.2 Affective attitude
Participants felt cancer POCTs could provide reassurance if the results were negative. They also felt for patients requiring further investigations, having a positive POCT may motivate them to adhere to further follow-up investigations:
“I'd say having a clearer idea of what was going on, I may be able to get some sort of reassurance that it's unlikely at this stage that it would be cancer.” (034YN, previous 2WW)
“[people] see their GP, express concerns about something and then don't always follow through with the follow up. If they've seen a rapid test that's positive that then may encourage them to actually go through with further investigations …” (025HL)
One participant indicated they would be more willing to make the effort required for attending an invasive diagnostic test if they had a positive POCT, compared to just being informed of an onward referral by their GP during an appointment:
“… if I had someone at the GP's surgery do a test and say … you probably should get this checked, then 100% I would go for the invasive. But if it's just like a meeting and they're like okay, let's go do this invasive … make me … take time off work … I'd probably never do the invasive testing.” (033AN)
Some participants suggested being offered a rapid cancer test during a GP appointment might be unexpected and induce anxiety. This anxiety was attributed to the immediacy of results or, for some, the fear of confronting a cancer diagnosis. However, most participants felt they would still opt to take the test, preferring to know rather than remain unaware:
“Initially quite anxious but … with the word rapid you get your result quite quickly so there wouldn't be any waiting around … I would be anxious but I would do it, I mean your health comes first and the quicker you detect it the better” (016AR)
Other participants felt anxiety would be more manageable with quicker results, as opposed to enduring the prolonged worry associated with waiting for 2WW investigations. They also felt having an initial indication about possible cancer would help them feel more prepared for follow-up appointments.
“… you'd want to know straight away, some kind of indication … otherwise you're full of anxiety waiting for the tests and for the referral to go through, so doing a rapid point-of-care test would really help speed up the process and the diagnosis.” (032BD)
“… at least then you would have a better indication of what the situation was when you went for the further investigation, and you might be able to form certain questions in your mind, that you wouldn't ordinarily be thinking to ask.” (020NX, previous 2WW)
A few participants highlighted the importance of the test being accurate and reliable, with clear information about its limitations. When asked about the possibility of the test merely indicating the need for further investigations rather than confirmation of cancer, they expressed a willingness to take it, valuing any additional information as beneficial.
“… I would want to be clear about… what the results would or wouldn't tell me, … it would just be a case of how useful the information would be, and how accurate it would be in terms of the diagnosis. But any test that provides further information and does it quickly, in my mind would have to be a good thing in the vast majority of cases.” (020NX, previous 2WW)
3.2.3 Burden
Most participants did not view taking a POCT for cancer as burdensome and felt the advantages of taking the test would outweigh any burden:
“I wouldn't think so, I would think the advantages would far outweigh any sort of burden, or any problems that I would have doing the test, so I would say no for that really.” (034YN, previous 2WW)
However, others felt getting positive test results would be a significant mental and emotional burden that would require further support from the GP or signposting to wellbeing services:
“… for it to come back as positive is going to be a huge personal burden … and I would want emotional support, I would want to know who to contact, I would want to have time with my GP to discuss it if that was the case in the appointment...” (032BD)
Despite the emotional burden of a positive result, one participant felt conflicted whether their anticipated affecting response would be unique to POCTs or would apply to any cancer test. Overall, they expressed gratitude for the opportunity to be tested.
“… but I don't know whether it'll be any greater than any other test and I think I would still feel thankful, despite the emotional shock, … that we could get first foot on the ladder, a) of finding out and b) of treating anything.” (053EY)
3.2.4 Ethicality
Overall, participants found it difficult to consider the ethical implications of offering POCTs for cancer in primary care. Some participants suggested there may be ethical issues with offering POCTs to certain religions or cultures, although specific examples were not mentioned:
“I can't see any ethical issues. Unless you're a certain religious group I suppose that don't take tests, I don't know if there are any…” (017SR, previous 2WW).
The need for patient preference and informed consent when offering POCTs was highlighted, with particular consideration for patient's mental and emotional wellbeing. Participants emphasized it was important patients felt they had a genuine choice and were not subjected to indirect pressure to undergo the test:
“…ethically they need to think about the mental wellbeing of the patient in terms of the rapid test, and that may be why it'd be useful to give the patient the option … they could simply wait and have further investigations which would reach the same or a more definitive diagnosis conclusion…”(020NX, previous 2WW).
Participants expressed ethical concerns regarding the storage and handling of their data following a POCT. They sought reassurance their data would be secure, used exclusively within the NHS or for research purposes:
“… when you think about the ethical side of it, people might be worrying about how that data's used and how that data's stored…” (055SM).
“… is it going to be something that the GP submits the data online or where does it go, is it within the NHS or does it go somewhere else … if that information is reported to the NHS if [it] can contribute to research that helps people's health and the system in the long-term then I'm prepared…” (037NS, previous 2WW).
Potential ethical concerns regarding test accuracy, particularly the risk of false negatives, which could provide patients with a false sense of reassurance were raised by some participants. Transparency with patients about the limitations of the test's accuracy was suggested:
“the sensitivity of the test, for example … if it comes back as negative then the patient will be under a false sense of security that okay, they don't have cancer, so it's giving full disclosure to the patient that these are not 100% accurate…” (032BD).
3.2.5 Intervention coherence
Most participants felt they understood the rationale behind a GP offering a rapid test during an appointment, either due to the symptoms they presented or the need for further investigation. Participants believed this would be clear to them, especially if the doctor provided a brief explanation for the test's purpose:
“Yeah, obviously my symptoms … would match with a certain cancer so yeah, I understand.” (016AR, previous 2WW).
“I think it would [make sense], as long as they obviously explained what they were doing the rapid test for. I think they'd need to say “okay, so these symptoms are all something that might need examination, so therefore I'm [going to] do this test just to tell me if I need … to do any other tests”.” (025HL).
3.2.6 Opportunity costs
Most participants did not perceive any opportunity costs associated with taking a POCT during their GP appointment. However, they felt that a positive test result could lead to follow–up hospital appointments and the need to give up immediate plans, such as travel:
“… if you've got the results and they said that you did have cancer, then if you had plans in the future then you think, oh, I can't do that because … you'd have to go to the hospital to have further [tests]…” (023TA).
One participant highlighted that a slightly longer doctor's appointment due to taking a rapid test might cause some inconvenience if they had a busy schedule. However, they stated it was an adjustment they would be willing to make:
“… if I'd gone in and the appointment was going to be longer and I was in a rush to get somewhere else it might be a bit inconvenient but I think I'd just sort it out and … if I had to get to a meeting … I'd just contact someone and say I'm going to be late for a medical reason, … I would give it precedence…” (03NL, previous 2WW).
3.2.7 Perceived effectiveness
Although the majority of participants believed that cancer POCTs would support their GP in determining the next steps with regard to their symptoms, some indicated this would depend on the accuracy of the test:
“It could help toward a diagnosis, it depends on how accurate the POCT is…” (032BD).
Some participants suggested the test would primarily assist the GP in deciding the next steps or serve as a tool to rule out potential diagnoses:
“I think it would be very helpful in maybe ruling out what it is not rather than this is what it is, but it might be “it's definitely not that but there's a possibility it might be this, we need more tests”. (037NS, previous 2WW).
“I appreciate that all tests, nothing is completely 100% foolproof, but I think it's a very good basis to start from, and an indication for further tests…” (055SM).
However, some participants expressed concerns that the use of POCTs in primary care might further strain general practice and GPs:
“If it saves some time, then yes, but if it takes a long time for [GP] to do, then obviously he's going to have a queue building up outside as well for the next, because what are we at now, is it seven or eight minutes with a GP?” (036LE).
3.2.8 Self-Efficacy
Participants felt confident in their ability to undergo cancer POCTs if they were offered by their GP. This confidence was linked to their familiarity with other tests, such as having blood drawn, which provided a sense of understanding about the testing process:
“I took like Covid tests before, I've took vitamin D tests, took smear tests so yeah, I you know, I am familiar with these tests so I would be comfortable doing a cancer one if I had to.” (016AR, previous 2WW)
Participants noted that the type of test may influence their ability to take it, but they expressed particular confidence in completing finger-prick blood, saliva, or urine tests:
“Because it's just either a pinprick for one drop of blood or a urine dip test … Saliva, yes. So stool sample I'd have to come back home to do…” (01HY).
Participants felt anticipating a potential cancer diagnosis may help prepare them for taking a POCT, whereas participants felt being offered a cancer test unexpectedly may require additional time to consider their decision:
“… if you've got an idea in your mind that it could be cancer, you're sort of on the road to being prepared for that surprise rapid test being done. If it's symptoms that you wouldn't know are linked with cancer, then it would be a bit more of a shock … I'd need to go away and digest, … but, no, I would go back [to the doctor]” (045AT).
Receiving clear information from the clinician and having the opportunity to ask questions were considered important factors influencing their ability to take the test:
“I would expect that the GP to explain [in] plain terms to whoever it was, and make sure they understood what the test was, what it would involve, what the results would or wouldn't tell you, and when they would get the results. And also provide an opportunity for them to ask any questions beforehand…” (020NX, previous 2WW).
4 Discussion
In this large mixed methods study of the UK public, the majority of participants considered POCTs for cancer in primary care acceptable. Interview data indicated that participants favored POCTs for the prompt reassurance of a negative result and the quick indication of cancer likelihood. All TFA constructs in the survey, except ethicality, reflected clear acceptability for POCTs in cancer diagnostics. For ethicality, most participants disagreed or expressed ambivalence that offering POCTs raised ethical concerns. Interviews provided further insight, with some participants highlighting potential religious or cultural barriers for testing, while others raised concerns about patient mental health and the implications of using less accurate tests. Most participants preferred being offered a POCT over waiting on a 2WW pathway, even if the test was indicative rather than confirmatory.
Test accuracy was important to participants with an expectation that clinicians would clearly communicate the test's limitations. Participants also valued the opportunity to ask questions. The survey indicated most participants believed POCTs would be minimally burdensome. Interviews revealed that any perceived burden was primarily related to the mental and emotional impact of receiving a positive test result rather than POC testing itself. Through interviews, participants expressed confidence in their ability to undertake a POCT, particularly for sample types such as fingerprick blood or urine. Previous testing experiences appeared to reinforce confidence in taking POCTs for cancer. Overall, participants perceived POCTs as straightforward and less burdensome than invasive testing. We found some TFA constructs elicited more detailed responses from interview participants, such as affective attitude, ethicality, and self-efficacy, while others, like intervention coherence and opportunity costs, generated less discussion. This was likely due to participants finding certain concepts challenging to understand and difficult to address in the context of a hypothetical scenario.
A prominent theme emerging from our interviews was the advantage of receiving the test and results immediately with POCTs. These findings align with previous studies that have shown waiting for cancer tests and results are a primary source of concern for patients (28, 29). Given the sensitivity and profound implications of a cancer diagnosis, patients prefer prompt investigations, as evidenced by studies showing a high willingness to undergo testing, even at a low risk of cancer (30).
Participants in our study emphasized the importance of accurate tests, which is consistent with previous research investigating patient preferences around cancer diagnosis and screening (31, 32). For example, a previous study assessed the acceptability of a non-invasive breast cancer diagnostic test found that women found testing acceptable, or preferable to biopsy if they were equally accurate or nearly equally accurate as a biopsy (31). These findings align with our interview results where participants preferred POCTs over waiting for 2WW investigations but maintained expectations for high accuracy for POCTs. Another study quantifying patient preferences for the use of Multi Early Cancer Detection tests (MCED) reported similar prioritization of accurate test results (32). Given that most POCTs are typically less accurate than laboratory tests (13), policymakers will need to carefully consider the balance of providing the public with rapid testing while maintaining acceptable accuracy standards.
Participants in our study expressed a desire for clear communication from GPs and genuine choice in deciding whether they wanted to take POCTs. This aligns with findings from a study on colorectal cancer screening, which emphasized the importance of shared medical decision-making (33). This is particularly important given that patient and clinician preferences may not always align in cancer care decisions (34).
Several studies have shown that cancer stigma can impact screening and testing uptake in ethnic minority groups, particularly within Black communities (35–37). While our survey indicated high acceptability of POCTs among ethnic minority groups, acceptability was slightly lower in the Black community. Previous research suggests there is a greater stigma surrounding cancer diagnoses in African cultures (38–40). This highlights the importance of cultural differences and barriers in influencing the acceptability and uptake of new tests. Prior to implementation, it is crucial to carefully consider cultural barriers to testing, as failing to do so could exacerbate inequalities in cancer care.
4.1 Strengths and limitations
A key strength of this study is its mixed-methods design. The survey allowed us to assess public acceptability of POCTs for cancer, while the interviews provided deeper insights into the nuances of acceptability. Notably, the interviews identified barriers and facilitators to POCT uptake that the survey could not capture. The use of the TFA (17) to inform both the survey instrument and the interview topic guide facilitated a comprehensive assessment of acceptability by considering its multiple constructs, thereby yielding robust and detailed data. Additionally, considering POCTs broadly as opposed to focussing on a specific exemplar test allowed us to capture wider perspectives applicable to various test (e.g., a preference for fingerprick blood and urine tests). This provides additional insights for test developers to consider when addressing patient acceptability in the design of future tests.
One limitation of this study was assessing prospective acceptability using a hypothetical scenario for POC testing in the interviews. This approach may have been challenging for participants, as a vignette might not elicit the same level of detail as reflecting on the actual experience of undergoing POC testing. Additionally, the underrepresentation of individuals with education below degree level, particularly in the follow-up interviews was also a study weakness. This may have contributed to a greater awareness of diagnostic test limitations and cancer pathways among the sample. Moreover, the exclusion of participants who were not fluent in English may have resulted in the omission of important perspectives on the acceptability of POCTs among non-English speaking communities in the UK. Prior to potential implementation of POCTs, the acceptability of POCTs within these communities should be explored further, particularly given that findings indicated there may be cultural or religious ethical considerations to address.
5 Conclusion
POCTs for cancer were acceptable and desired by the UK public, primarily due to the immediacy of their results. Future test developers should consider patients' preference for tests with accuracy comparable to laboratory tests. When using cancer POCTs in primary care, GPs should prioritize clear communication regarding the test and its limitations, while fostering shared decision-making with patients. We recommend that the uptake of any cancer POCT in the future should involve evaluating the acceptability of the specific test, particularly in underrepresented populations.
Data availability statement
The datasets presented in this article are not readily available because of lack of resources to prepare data for sharing, however may be made available following publication. Requests to access the datasets should be directed to YW5hbS5heWF6LnNoYWhAZ21haWwuY29t.
Ethics statement
The studies involving humans were approved by School of Medicine Research Ethics Committee at the University of Leeds (MREC 22-014). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AA-S: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing. RN: Funding acquisition, Supervision, Writing – review & editing, Conceptualization. ZH: Writing – review & editing, Supervision. KL: Methodology, Writing – review & editing. SG: Methodology, Writing – review & editing. NN: Methodology, Writing – review & editing. MT: Supervision, Writing – review & editing. SS: Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Cancer Research UK as part of the CanTest collaborative (reference: C8640/A23385). This report is independent research supported by the National Institute for Health Research NIHR Advanced Fellowship, Professor Samuel Smith NIHR300588. The funders had no role in the design of the study, data collection, analysis, interpretation of data, and in the writing of this manuscript. Smith also acknowledges funding support from a Yorkshire Cancer Research University Academic Fellowship (L389SS).
Acknowledgments
The authors would like to express their gratitude to all the participants that took part in this study. We would also like to thank Pete Wheatstone for supporting in the design of this study.
Conflict of interest
SS declares consulting fees from Lilly for participation in an advisory board. RN declares consulting fees from GRAIL relating to his role as Co-Chief Investigator of the NHS-Galleri trial.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. For checking spelling and grammatical errors in the final draft.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcacs.2025.1568916/full#supplementary-material
References
1. Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer (2024). Available online at: https://gco.iarc.who.int/today (accessed January 15, 2025).
2. Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. (2015) 112:S92–S107. doi: 10.1038/bjc.2015.48
3. Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. (2012) 23:2731–7. doi: 10.1093/annonc/mds101
4. Tørring ML, Frydenberg M, Hansen RP, Olesen F, Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur J Cancer. (2013) 49:2187–98. doi: 10.1016/j.ejca.2013.01.025
5. Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TML, Myklebust TÅ, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. (2019) 20:1493–505. doi: 10.1016/S1470-2045(19)30456-5
6. Hamilton W. Five misconceptions in cancer diagnosis. Br J Gen Pract. (2009) 59:441–7. doi: 10.3399/bjgp09X420860
7. NHS Digital. Available online at: https://digital.nhs.uk/ndrs/data/data-outputs/cancer-data-hub/cancer-routes-to-diagnosis (accessed January 15, 2025).
8. Rubin G, Berendsen A, Crawford SM, Dommett R, Earle C, Emery J, et al. The expanding role of primary care in cancer control. Lancet Oncol. (2015) 16:1231–72. doi: 10.1016/S1470-2045(15)00205-3
9. Lyratzopoulos G, Abel GA, McPhail S, Neal RD, Rubin GP. Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer. (2013) 108:686–90. doi: 10.1038/bjc.2013.1
10. Round T, Ashworth M, L'Esperance V, Møller H. Cancer detection via primary care urgent referral and association with practice characteristics: a retrospective cross-sectional study in England from 2009/2010 to 2018/2019. Br J Gen Pract. (2021) 71:e826–e35. doi: 10.3399/BJGP.2020.1030
11. Al-Ansary L, Farmer A, Hirst J, Roberts N, Glasziou P, Perera R, et al. Point-of-care testing for Hb A1c in the management of diabetes: a systematic review and metaanalysis. Clin Chem. (2011) 57:568–76. doi: 10.1373/clinchem.2010.157586
12. Elrobaa IH, Khan K, Mohamed E. The role of point-of-care testing to improve acute care and health care services. Cureus. (2024) 16:e55315. doi: 10.7759/cureus.55315
13. Khan AR, Hussain WL, Shum HC, Hassan SU. Point-of-care testing: a critical analysis of the market and future trends. Front Lab Chip Technol. (2024) 3:1394752. doi: 10.3389/frlct.2024.1394752
14. Pezzuto F, Scarano A, Marini C, Rossi G, Stocchi R, Di Cerbo A, et al. Assessing the reliability of commercially available point of care in various clinical fields. Open Public Health J. (2019) 12:342–68. doi: 10.2174/1874944501912010342
15. Owlstone Medical (2024). Available online at: https://www.owlstonemedical.com/science-technology/cancer/
16. Lingervelder D, Koffijberg H, Kusters R, IJzerman MJ. Point-of-care testing in primary care: a systematic review on implementation aspects addressed in test evaluations. Int J Clin Pract. (2019) 73:e13392. doi: 10.1111/ijcp.13392
17. Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. (2017) 17:88. doi: 10.1186/s12913-017-2031-8
18. Fisher P, McCarney R, Hasford C, Vickers A. Evaluation of specific and non-specific effects in homeopathy: feasibility study for a randomised trial. Homeopathy. (2006) 95:215–22. doi: 10.1016/j.homp.2006.07.006
19. Hommel KA, Hente E, Herzer M, Ingerski LM, Denson LA. Telehealth behavioral treatment for medication nonadherence: a pilot and feasibility study. Eur J Gastroenterol Hepatol. (2013) 25:469–73. doi: 10.1097/MEG.0b013e32835c2a1b
20. Forster AS, Rubin G, Emery JD, Thompson M, Sutton S, Wit Nd, et al. Measuring patient experience of diagnostic care and acceptability of testing. Diagnosis. (2021) 8:317–21. doi: 10.1515/dx-2020-0112
21. Creswell JW, Clark VLP. Designing and Conducting Mixed-Methods Research. 2nd ed. Sage publishers Inc. (2011).
22. O'Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. (2014) 89:1245–51. doi: 10.1097/ACM.0000000000000388
23. Sekhon M, Cartwright M, Francis JJ. Development of a theory-informed questionnaire to assess the acceptability of healthcare interventions. BMC Health Serv Res. (2022) 22:279. doi: 10.1186/s12913-022-07577-3
24. Naeem M, Ozuem W, Howell K, Ranfagni S. A step-by-step process of thematic analysis to develop a conceptual model in qualitative research. J Qual Methods. (2023) 22:16094069231205789. doi: 10.1177/16094069231205789
25. Francis JJ, Grimshaw JM, Zwarenstein M, Eccles MP, Shiller S, Godin G, et al. Testing a theory-inspired message ('TRY-ME'): a sub-trial within the Ontario printed educational message (OPEM) trial. Implement Sci. (2007) 2:39. doi: 10.1186/1748-5908-2-39
26. Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. (2013) 13:117. doi: 10.1186/1471-2288-13-117
27. McGowan LJ, Powell R, French DP. How can use of the theoretical domains framework be optimized in qualitative research? A rapid systematic review. Br J Health Psychol. (2020) 25:677–94. doi: 10.1111/bjhp.12437
28. Piano M, Black G, Amelung D, Power E, Whitaker KL. Exploring public attitudes towards the new faster diagnosis standard for cancer: a focus group study with the UK public. Br J Gen Pract. (2019) 69:e413–e21. doi: 10.3399/bjgp19X702677
29. Venning B, Pearce A, De Abreu Lourenco R, Hall R, Bergin RJ, Lee A, et al. Patient preferences for investigating cancer-related symptoms in Australian general practice: a discrete-choice experiment. Br J Gen Pract. (2024) 74:e517–e26. doi: 10.3399/BJGP.2023.0583
30. Banks J, Hollinghurst S, Bigwood L, Peters TJ, Walter FM, Hamilton W. Preferences for cancer investigation: a vignette-based study of primary-care attendees. Lancet Oncol. (2014) 15:232–40. doi: 10.1016/S1470-2045(13)70588-6
31. Liang W, Lawrence WF, Burnett CB, Hwang YT, Freedman M, Trock BJ, et al. Acceptability of diagnostic tests for breast cancer. Breast Cancer Res Treat. (2003) 79:199–206. doi: 10.1023/A:1023914612152
32. Gelhorn H, Ross MM, Kansal AR, Fung ET, Seiden MV, Krucien N, et al. Patient preferences for multi-cancer early detection (MCED) screening tests. Patient. (2023) 16:43–56. doi: 10.1007/s40271-022-00589-5
33. Zhu X, Weiser E, Jacobson DJ, Griffin JM, Limburg PJ, Finney Rutten LJ. Patient preferences on general health and colorectal cancer screening decision-making: results from a national survey. Patient Educ Couns. (2022) 105:1034–40. doi: 10.1016/j.pec.2021.07.033
34. Zhang M, He X, Wu J, Xie F. Differences between physician and patient preferences for cancer treatments: a systematic review. BMC Cancer. (2023) 23:1126. doi: 10.1186/s12885-023-11598-4
35. Marlow L, Vrinten C, Waller J. Cancer stigma among ethnic minority women. Eur J Surg Oncol. (2016) 42:S238. doi: 10.1016/j.ejso.2016.07.086
36. Vrinten C, Gallagher A, Waller J, Marlow LAV. Cancer stigma and cancer screening attendance: a population based survey in England. BMC Cancer. (2019) 19:566. doi: 10.1186/s12885-019-5787-x
37. Vapiwala N, Miller D, Laventure B, Woodhouse K, Kelly S, Avelis J, et al. Stigma, beliefs and perceptions regarding prostate cancer among Black and Latino men and women. BMC Public Health. (2021) 21:758. doi: 10.1186/s12889-021-10793-x
38. White HL, Mulambia C, Sinkala M, Mwanahamuntu MH, Parham GP, Moneyham L, et al. ‘Worse than HIV' or ‘not as serious as other diseases'? Conceptualization of cervical cancer among newly screened women in Zambia. Soc Sci Med. (2012) 74:1486–93. doi: 10.1016/j.socscimed.2012.01.028
39. De Ver Dye T, Bogale S, Hobden C, Tilahun Y, Hechter V, Deressa T, et al. A mixed-method assessment of beliefs and practice around breast cancer in Ethiopia: Implications for public health programming and cancer control. Glob Public Health. (2011) 6:719–31. doi: 10.1080/17441692.2010.510479
Keywords: point-of-care tests, rapid tests, early cancer diagnosis, primary care, patient acceptability, patient experience
Citation: Ayaz-Shah AA, Neal RD, Haider ZF, Lloyd KE, Green SMC, Nasir N, Thompson MJ and Smith SG (2025) Acceptability of using point-of-care tests for cancer in primary care: a UK public mixed-methods study. Front. Cancer Control Soc. 3:1568916. doi: 10.3389/fcacs.2025.1568916
Received: 30 January 2025; Accepted: 17 March 2025;
Published: 15 April 2025.
Edited by:
Lily Taylor, University of Cambridge, United KingdomReviewed by:
Anurima Baidya, Johns Hopkins University, United StatesJerome Gnanaraj, Johns Hopkins University, United States
Copyright © 2025 Ayaz-Shah, Neal, Haider, Lloyd, Green, Nasir, Thompson and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anam A. Ayaz-Shah, YW5hbS5heWF6LnNoYWhAZ21haWwuY29t
 Anam A. Ayaz-Shah
Anam A. Ayaz-Shah Richard D. Neal2
Richard D. Neal2 Kelly E. Lloyd
Kelly E. Lloyd