- National Cancer Registration and Analysis Service, National Disease Registration Service, NHS England, London, United Kingdom
Introduction: The development of effective treatment for many childhood cancers has led to dramatic increases in survival rates at the population level, at least in affluent industrialized countries. Studies of survival in numerous populations have been published, but population-based survival estimates that are essential for monitoring and planning are still lacking in many countries. There is no comprehensive account of the type and extent of available information on this topic. A scoping review of population-based studies of childhood cancer in the 21st century was carried out with the aim of repairing this omission.
Methods: The electronic databases PubMed and Web of Science were searched, supplemented by the author's bibliographic files.
Results: The searches produced 5,490 references, of which 303 reported population-based studies containing at least one estimate of 5-year survival for children with cancer diagnosed during a period whose central year was 2001 or later. Overall, 75% of high-income countries with a child population ≥50,000 were represented in these studies, compared with 47% of upper middle income countries, 16% of lower middle income countries and 8% of low income countries. Among countries that were represented in population-based studies, 29% of high income countries were only represented in studies involving multiple countries compared with 75% of those in lower income categories. Similar contrasts were found between countries with very high Human Development Index and those in lower categories of Human Development Index.
Discussion: Wider availability of robust information on survival at population level will be essential for monitoring progress toward the goal set by the World Health Organization's Global Initiative for Childhood Cancer of 60% survival globally for children and adolescents with cancer by the year 2030. Increasing the coverage and quality of cancer registration and death notification in as many lower-resource countries as possible would in turn increase the volume and geographic spread of the data from which survival rates can be estimated for those countries. International collaborations whose results are underpinned by uniform procedures for data validation and analysis will continue to play a vital part in enabling comparison of childhood cancer survival between populations.
1 Introduction
The development of effective treatments for many childhood cancers over the past 60 years is one of the great success stories of oncology (1, 2). In consequence, there have been dramatic increases in survival rates for childhood cancer at the population level, at least in affluent industrialized countries (3, 4). Some of these increases have been shown to occur concurrently with the adoption of successive trial protocols for particular types of childhood cancer (5, 6).
Impressive results have also been reported from specialist centers in many less well-resourced settings. However, population-based estimates of survival of all patients, including those not treated at specialist centers, are essential for monitoring and planning but are still lacking in many countries. This absence prompted Ward et al. (7) to carry out a simulation study to estimate survival for virtually every country in the world including those for which hard data were missing. In the same year, Girardi et al. (8) published a systematic review of worldwide trends in survival from two principal types of childhood brain tumor. Since then, studies of survival from childhood cancer in numerous populations have continued to be published, but there has apparently been no comprehensive delineation of the type and extent of the available information on this topic. The present scoping review was carried out in order to repair this omission.
2 Materials and methods
Eligible publications were defined as those that provided at least one population-based estimate of survival for children diagnosed with cancer according to the following criteria.
Data had to refer to cases arising in a defined population, usually compiled by a population-based cancer registry (PBCR) and were not restricted to patients receiving any particular treatment modality or modalities.
Following Girardi et al. (8), survival estimates had to be at 5 years after diagnosis. Eligibilty was broadened, however, to include endpoints other than death from any cause, thus papers reporting relative survival, net survival, event-free survival, cancer-specific survival or disease-specific survival could also be included.
Age range at diagnosis: lower bound ≤ 14 years and upper bound <20years.
Calendar period of diagnosis: wholly contained within a period whose central year was 2001 or later (including periods of an even number of years in length whose central years were 2000 and 2001).
Two online databases, PubMed and Web of Science, were searched for publications satisfying the above criteria. The search strategies are presented in the Appendix. The results of these searches were supplemented by review of additional publications from 2001 onwards in the author's bibliography files on descriptive epidemiology of childhood cancer. Eligible papers were restricted to those published in peer-reviewed journals. Conference abstracts were excluded. There were no language restrictions.
Throughout this review, the French West Indies have been treated as a single territory and Hong Kong and Macau have been included with China. For analyses by world region, Mexico, which is geographically in North America but more closely aligned socioeconomically and culturally with Latin America, was included in the category of America (Central and Caribbean); for similar reasons, Cyprus, which is geographically in western Asia, was included in the category of Europe.
3 Results
3.1 Literature searches
The searches of PubMed and Web of Science together yielded 5,490 references, with a further 383 from the author's bibliography files giving a grand total of 5,873 (Figure 1). After elimination of 1,010 duplicates and 574 articles published before 2001, 4,289 records remained for further assessment. On the basis of title and abstract, 3,190 references were excluded because they did not satisfy the eligibility criteria listed above. Full text was unavailable for 32 records. The remaining 1,067 were subjected to full-text review, leading to the exclusion of 764 because of failure to satisfy one or more of the eligibility criteria. There were thus a final total of 303 eligible publications, 29 of which were editions of two series of American annual reports.
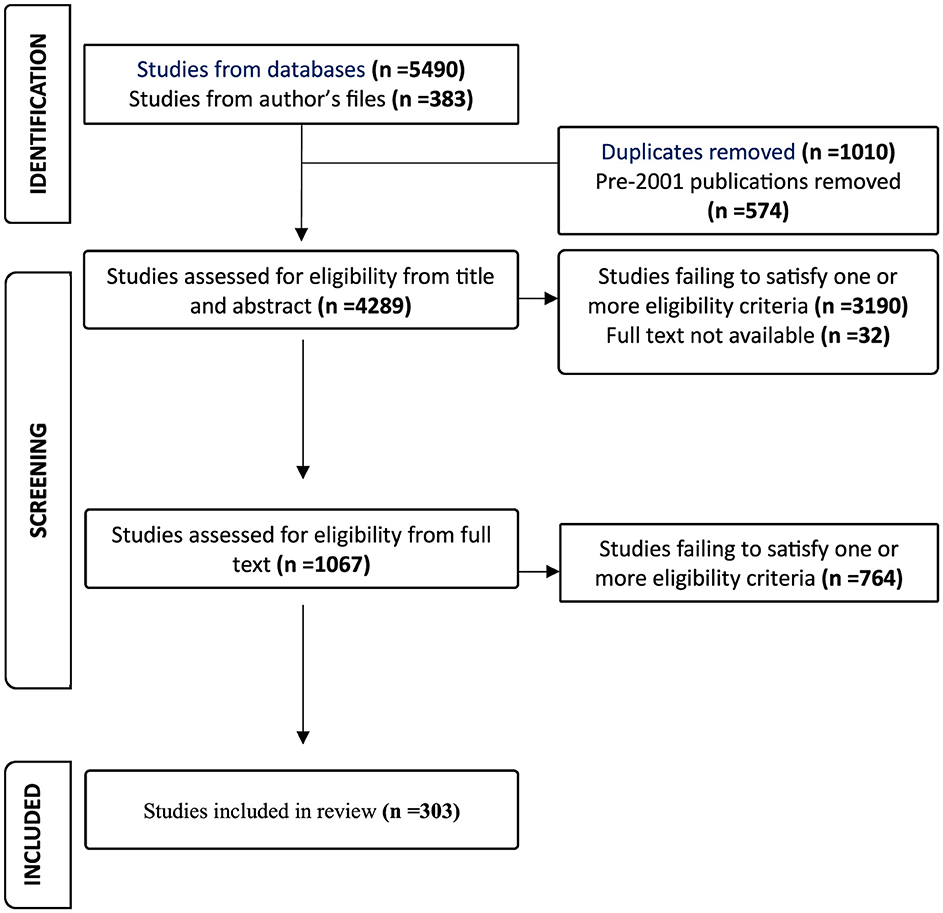
Figure 1. PRISMA flow diagram for scoping review of global population-based childhood cancer survival.
3.2 United States annual series
The American Cancer Society “Cancer Statistics” reports for 2009 onwards have reported 5-year relative survival for childhood cancer diagnosed mainly or entirely in the 21st century, based on data from the Surveillance, Epidemiology and End Results (SEER) Program, thus the 16 reports issued in 2009–2024 were eligible for this review (9–24). The 2009–2016 editions (9–16) documented survival trends from 1975 onwards, with the most recent year of diagnosis being 5 years before the year of publication. Survival data in the editions for 2017 onwards (17–24) have covered a single 7-year period of diagnosis, the most recent year of diagnosis again being 5 years before the year of publication. Diagnoses have been classified according to the International Classification of Childhood Cancer, Third Edition (ICCC-3), in which the categories are defined by ICD-O-3 codes (25). Non-malignant central nervous system (CNS) tumors are excluded with the sole exception of pilocytic astrocytoma, which continued to be defined as malignant for the purposes of SEER when it was downgraded to uncertain behavior in ICD-O-3.
The annual statistical reports of the Central Brain Tumor Registry of the United States (CBTRUS) were initially self-published, but from 2012 they have been issued as supplements to Neuro Oncology. Each of these reports has presented 5-year relative survival for children under 15 years of age who were diagnosed during the 5-year period beginning 7 years before the year of publication, thus all 13 of the reports for 2012–2024 were eligible (26–38). Survival data were derived from the SEER-18 registries in the reports for 2012–2018 (26–32), and from NPCR registries in those for 2019 onwards (33–38). Diagnoses have been classified according to a scheme developed by CBTRUS and based on ICD-O-3 codes. Malignant and non-malignant tumors have been included throughout. Additional CBTRUS reports on childhood and adolescent patients that were published in 2022 and 2023 and are considered under “Other publications” below.
3.3 Other publications
Details of the remaining 274 eligible publications are shown in Table 1. Of these, 245 reported survival within a single country or territory, with 44 different countries represented. Data from the United States were analyzed in 93 (38%) of the 245 single-country studies. The great majority of these United States studies (75/93) were based exclusively on data from the SEER Program, though a substantial number of the analyses were conducted in other countries. Other countries represented by at least five single-country studies were the Netherlands (n = 17), Australia (n = 15), the United Kingdom (n = 11), France (Metropolitan) and Spain (n=8 each), Canada, South Korea and Germany (n = 7 each), Thailand (n = 6), Brazil and Sweden (n = 5 each).

Table 1. Studies included in the review. American Cancer Society “Cancer Statistics” annual series and CBTRUS annual statistical reports are excluded.
The other 29 publications were based on data from more than one country and were mostly products of established international collaborations. Eight papers from successive iterations of EUROCARE and its associated studies RARECARE and HAEMACARE have between them covered all types of childhood cancer in a large number of European countries (39–46). Five papers have presented international results from the CONCORD collaboration on population-based cancer survival worldwide (47–51) and a further paper reported detailed analyses of CONCORD data from 37 states in the United States (52). Leukemia was the only childhood cancer included in CONCORD-2 (50–52). CONCORD-3 expanded its coverage of childhood cancers to leukemia, lymphomas and CNS tumors (47–49). A collaboration between varying numbers of population-based cancer registries in Southern and Eastern Europe reported on survival from various childhood cancers in their respective countries in a series of seven papers, four of which also included United States data from the SEER Program (53–59). Finally, nine papers were from one-off studies that compared results between two, three, or four countries (60–68).
The 29 multinational papers included data not only from many countries that were represented by single-country studies, but also from a further 37 countries that did not have any eligible single-country publications in this review. Thus, a total of 80 countries and territories worldwide had their survival data included in at least one eligible paper, but for 37/80 (46%) this only occurred in the context of multinational studies. The proportion of countries that were only represented in multinational papers varied by continent, from zero in North America and Oceania to 12/35 (34%) in Europe, 5/11 (45%) in the Caribbean, Central and South America, 10/18 (56%) in Asia and 10/12 (83%) in Africa. Transferring Mexico and Cyprus to the groups of North American and Asian countries respectively would make little difference to this pattern.
3.4 Disease classifications and outcome measures
Definition and classification of diagnostic categories included in studies was nearly always by ICCC-3, by the CBTRUS classification of CNS tumors, or by ICD-O-3. Studies covering CNS tumors usually stated whether non-malignant CNS tumors were included in the analyses. Inclusion or exclusion of skin carcinomas or non-melanoma skin cancer was less frequently specified. A considerable variety of outcome measures were used. The most frequent were observed survival and relative or net survival, but some studies reported event-free, cancer-specific or disease-specific survival.
3.5 Characteristics of countries and territories represented in included publications
The only territory with an estimated child population below 50,000 to be represented among the eligible studies was the Faroe Islands (69). Table 2 shows, for all countries and territories with a child population of at least 50,000, their representation in studies covered by this review and in major international cancer survival studies, together with information on World Bank Income Group, Human Development Index, the presence of population-based cancer registration, and completeness of death registration.
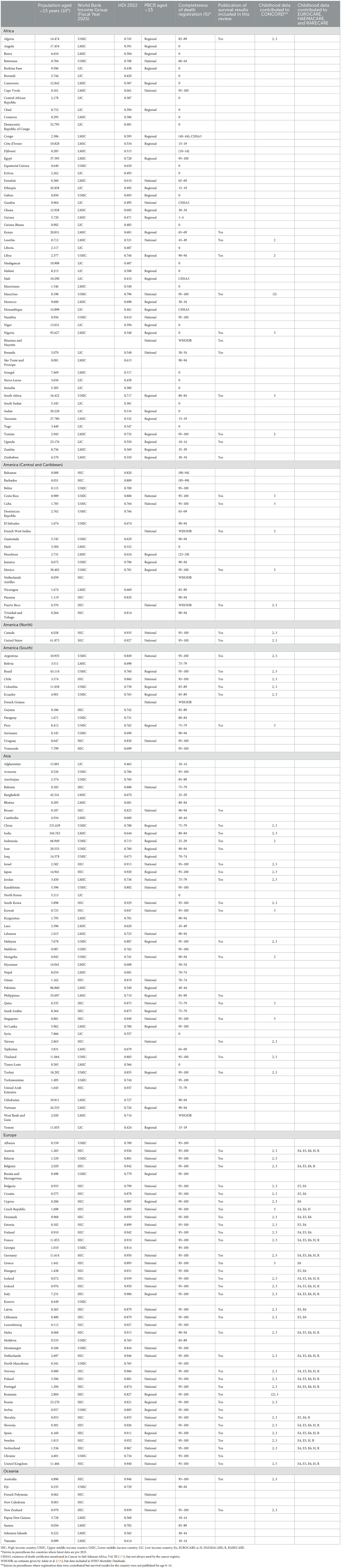
Table 2. Countries and territories with child population of at least 50,000. Size of child population (315), World Bank per capita income category (316), Human Development Index (HDI) (317), presence of population-based cancer registration for age 0–14 during the study period (PBCR) (72, 318, 319), completeness of death registration (320), inclusion of published results in this review, and participation in CONCORD, EUROCARE, HAEMACARE, and RARECARE.
Countries were more likely to be represented in population-based studies of childhood cancer included in this review if they were in higher categories of World Bank income classification and Human Development Index (HDI; Table 3). Overall, 75% of high-income countries (HIC) were represented, compared with 47% of upper-middle-income countries (UMIC), 16% of lower-middle-income countries (LMIC) and 8% of low-income countries (LIC). Similarly, 79% of countries with Very High HDI were represented, compared with 45% of those with High HDI, 11% of those with Medium HDI and 9% of those with Low HDI. This pattern was repeated across all continents except the Caribbean, Central and South America, where there was a marked preponderance of very small countries among those in the highest categories of each of the two indices.
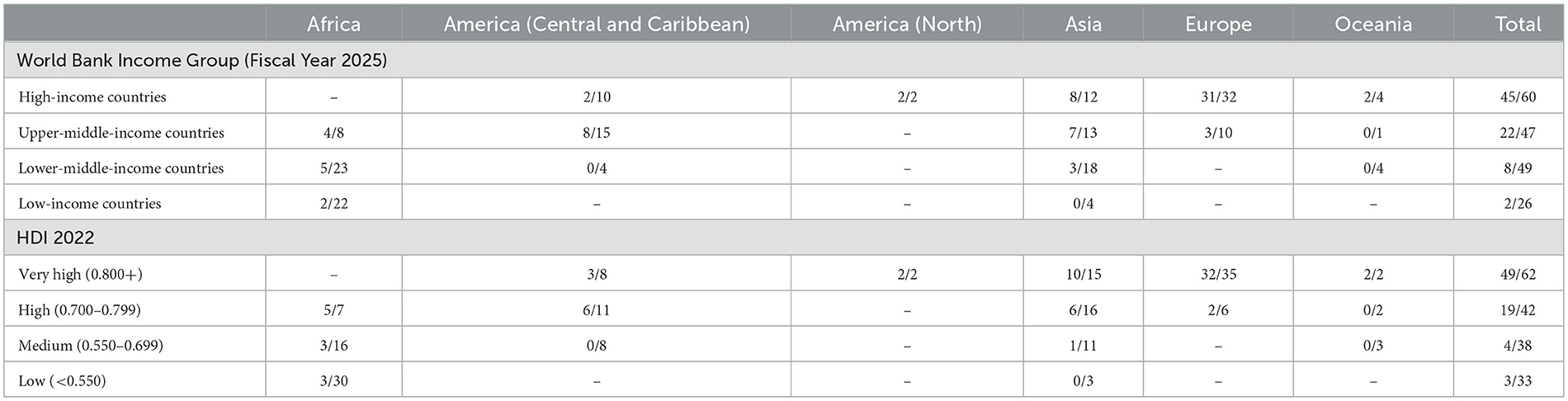
Table 3. Publication of survival results included in this review for countries in each continent with child population of at least 50,000 by World Bank per capita income category and Human Development Index (HDI).
Among countries that were represented in population-based studies, 29% of HICs and 35% of countries with Very High HDI were only represented in multinational papers compared with 75% of those in lower World Bank income categories and 77% of those in the three lower ranges of HDI.
3.6 Coverage of cancer types in included publications
Among all 303 included publications, 84 (28%) covered all cancer types, 60 (20%) were devoted to leukemia and lymphomas, 63 (21%) were devoted to CNS tumors, 74 (24%) were restricted to all or part of a single ICCC-3 main group of non-CNS solid tumors, and 22 (7%) covered a wide range of combinations of cancers, sometimes defined by primary site rather than morphology.
4 Discussion
This review confirms that population-based survival estimates of childhood cancer in the 21st century are available for many affluent countries, although quite often not for all of the principal diagnostic groups. Information is relatively scarce for less well-resourced countries. In many countries it has only been possible to estimate survival for some regions, which may not be representative of the whole country.
The first requisite for calculating cancer survival at the population level is population-based case ascertainment, virtually always by a PBCR. Institutional (including multi-institutional) series often contain richer clinical information than is found in most PBCRs and can yield much valuable information about cancer within a defined population, especially when they contain large numbers of cases (70). However, they necessarily exclude patients from that population who are diagnosed and treated (or remain untreated) outside the participating institutions, and survival estimated from their data will likely be biased upwards compared with the true result in the entire target population.
The second requisite is knowledge of the vital status of individual patients at given time intervals since diagnosis. A large number of countries, predominantly those in the lower categories of per capita income and HDI, are without population-based cancer registration even at sub-national level (Table 2). But even when there is population-based registration of incident cases, follow-up for vital status is not always straightforward. The most efficient method of obtaining this information is passive follow-up by linkage to death registrations, but an acceptable level of accuracy is only achievable if there is a high percentage completeness of death registration in the population. Moreover, for follow-up by a sub-national cancer registry, death registration should ideally be complete not merely within the registry's own territory but nationally, to allow for internal migration. The absence of complete death registration is also more often a feature of less well-resourced countries (Table 2), and follow- up by active tracing of patients becomes necessary. In a recent study of cancer in adults, for example, passive follow-up by linkage with national death registration was possible in only one out of 13 participating PBCRs from Sub-Saharan Africa (71). Active follow-up not only has varying degrees of success, it is also labor intensive, straining already scarce resources. Presumably for this reason, in a study of childhood cancer survival from three PBCRs in Sub-Saharan Africa, active follow-up was carried out by one of the participating registries only for a random sample of patients rather than for the entire patient population (64).
The numerous single-country studies included in this review have made substantial contributions to knowledge concerning population-level survival of children with cancer within their own countries. Multinational studies, however, whether the product of ad hoc collaborations involving a few countries or established consortia of much larger numbers of countries, have several important advantages. They pool data processing and analytical resources which may be scarce in some participating countries. The use of common definitions, data validation standards and procedures, outcome measures, and analytical methods enhances comparability between results for individual countries or for larger groups of countries based on factors including geography, socioeconomic indicators and characteristics of healthcare systems. The contrasts in the proportions of countries represented only in multinational studies between HICs and those in lower income categories and between countries with Very High HDI and those with a lower HDI show that multinational studies present especially valuable opportunities to less well-resourced countries for their results to be made visible and available for comparison.
There should generally be a high degree of comparability of ostensibly similar diagnostic categories between studies because of the widespread use of ICCC-3, other systems defined in terms of ICD-O-3, and ICD-O-3 itself. There are, however, two points on which there may be systematic differences between datasets. The first is uncertainty in most instances as to whether the overall category of all cancers includes skin carcinomas or non-melanoma skin cancer, but this is unlikely to have a substantial influence on survival rates for all cancers combined because these tumors are rare among children (72). More problematic is the continuing exclusion of CNS tumors with non-malignant behavior codes by some PBCRs, although increasing numbers of registries do include non-malignant intracranial and intraspinal tumors. The effect of excluding non-malignant tumors of these sites became more severe when pilocytic astrocytoma, the most frequent of childhood CNS tumors, was downgraded to uncertain behavior in ICD-O-3, having been regarded as malignant in earlier editions of ICD-O. Survival rates could in principle be compared between registries that include non-malignant CNS tumors and those that exclude them by restricting the comparison to malignant tumors, but such comparisons could still be unreliable because astrocytoma, not otherwise specified, is still assigned a malignant behavior code and it is impossible to know how many astrocytomas of unspecified subtype are in fact pilocytic (40, 73). International comparison of survival rates for CNS tumors, whose overall frequency is second only to that of leukemias, would be greatly simplified if non-malignant tumors of intracranial and intraspinal sites were routinely ascertained and followed up by all PBCRs.
The World Health Organization's Global Initiative for Childhood Cancer has the target of achieving 60% survival globally for children and adolescents with cancer by the year 2030, and wider availability of robust information on survival at population level will be essential for monitoring progress toward this goal (74). Reliable population-based information is available and extensively published from a large proportion of the most affluent countries, where 5-year survival is already well above 60%. Increasing the coverage and quality of cancer registration and death notification in as many lower-resource countries as possible would in turn increase the volume and geographic spread of the raw data from which survival rates can be estimated for those countries. International collaborations will continue to play a vital part in enabling comparison of childhood cancer survival between populations with confidence that the results are underpinned by uniform procedures for data validation and analysis.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://pubmed.ncbi.nlm.nih.gov/, https://clarivate.com/academia-government/scientific-and-academic-research/research-discovery-and-referencing/web-of-science/, https://www.cia.gov/the-world-factbook/field/age-structure, https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups, and Human Development Reports. Human Development Index. (2022) https://hdr.undp.org/data-center/human-development-index#/indicies/HDI.
Author contributions
CS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Simone JV. History of the treatment of childhood ALL: a paradigm for cancer cure. Best Pract Res Clin Haematol. (2006) 19:353–9. doi: 10.1016/j.beha.2005.11.003
2. D'Angio GJ. The National Wilms Tumor Study: a 40 year perspective. Lifetime Data Anal. (2007) 13:463–70. doi: 10.1007/s10985-007-9062-0
3. Madanat-Harjuoja LM, Pokhrel A, Kivivuori SM, Saarinen-Pihkala UM. Childhood cancer survival in Finland (1953-2010): a nation-wide population-based study. Int J Cancer. (2014) 135:2129–34. doi: 10.1002/ijc.28844
4. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual report to the Nation on the Status of Cancer, 1975-2014, featuring survival. J Natl Cancer Inst. (2017) 109:djx030. doi: 10.1093/jnci/djx030
5. Pastore G, Viscomi S, Gerov GL, Terracini B, Madon E, Magnani C. Population-based survival after childhood lymphoblastic leukaemia in time periods corresponding to specific clinical trials from 1979 to 1998–a report from the Childhood Cancer Registry of Piedmont (Italy). Eur J Cancer. (2003) 39:952–60. doi: 10.1016/S0959-8049(03)00064-9
6. Stiller CA, Kroll ME, Pritchard-Jones K. Population survival from childhood cancer in Britain during 1978-2005 by eras of entry to clinical trials. Ann Oncol. (2012) 23:2464–9. doi: 10.1093/annonc/mds183
7. Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Girardi F, Atun R. Global childhood cancer survival estimates and priority-setting: a simulation-based analysis. Lancet Oncol. (2019) 20:972–83. doi: 10.1016/S1470-2045(19)30273-6
8. Girardi F, Allemani C, Coleman MP. Worldwide trends in survival from common childhood brain tumors: a systematic review. J Glob Oncol. (2019) 5:1–25. doi: 10.1200/JGO.19.00140
9. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. (2009) 59:225–49. doi: 10.3322/caac.20006
10. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. (2010) 60:277–300. doi: 10.3322/caac.20073
11. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. (2011) 61:212–36. doi: 10.3322/caac.20121
12. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. (2012) 62:10–29. doi: 10.3322/caac.20138
13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. (2013) 63:11–30. doi: 10.3322/caac.21166
14. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. (2014) 64:9–29. doi: 10.3322/caac.21208
15. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. (2015) 65:5–29. doi: 10.3322/caac.21254
16. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. doi: 10.3322/caac.21332
17. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. (2017) 67:7–30. doi: 10.3322/caac.21387
18. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
19. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
20. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
21. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
22. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
23. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
24. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
25. Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. (2005) 103:1457–67. doi: 10.1002/cncr.20910
26. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. (2012) 14:v1–49. doi: 10.1093/neuonc/nos218
27. Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. (2013) 15:ii1–56. doi: 10.1093/neuonc/not151
28. Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. (2014) 16:iv1–63. doi: 10.1093/neuonc/nou223
29. Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. (2015) 17:iv1–62. doi: 10.1093/neuonc/nov189
30. Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. (2016) 18:v1–75. doi: 10.1093/neuonc/now207
31. Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. (2017) 19:v1–88. doi: 10.1093/neuonc/nox158
32. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS, et al. Statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. (2018) 20:iv1–86. doi: 10.1093/neuonc/noy131
33. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. (2019) 21:v1–100. doi: 10.1093/neuonc/noz150
34. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. (2020) 22(12 Suppl 2):iv1–96. doi: 10.1093/neuonc/noaa200
35. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. (2021) 23(Suppl 3):iii1–105. doi: 10.1093/neuonc/noab200
36. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS Statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. (2022) 24:v1–95. doi: 10.1093/neuonc/noac202
37. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2016-2020. Neuro Oncol. (2023) 25:iv1–99. doi: 10.1093/neuonc/noad149
38. Price M, Ballard C, Benedetti J, Neff C, Cioffi G, Waite KA, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2017-2021. Neuro Oncol. (2024) 26:vi1–85. doi: 10.1093/neuonc/noae145
39. Botta L, Gatta G, Capocaccia R, Stiller C, Cañete A, Dal Maso L, et al. Long-term survival and cure fraction estimates for childhood cancer in Europe (EUROCARE-6): results from a population-based study. Lancet Oncol. (2022) 23:1525–36. doi: 10.1016/S1470-2045(22)00637-4
40. Gatta G, Peris-Bonet R, Visser O, Stiller C, Marcos-Gragera R, Sánchez MJ, et al. Geographical variability in survival of European children with central nervous system tumours. Eur J Cancer. (2017) 82:137–48. doi: 10.1016/j.ejca.2017.05.028
41. Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, et al. Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5–a population-based study. Lancet Oncol. (2014) 15:35–47. doi: 10.1016/S1470-2045(13)70548-5
42. Crocetti E, Trama A, Stiller C, Caldarella A, Soffietti R, Jaal J, et al. Epidemiology of glial and non-glial brain tumours in Europe. Eur J Cancer. (2012) 48:1532–42. doi: 10.1016/j.ejca.2011.12.013
43. Gatta G, Ferrari A, Stiller CA, Pastore G, Bisogno G, Trama A, et al. Embryonal cancers in Europe. Eur J Cancer. (2012) 48:1425–33. doi: 10.1016/j.ejca.2011.12.027
44. Visser O, Trama A, Maynadié M, Stiller C, Marcos-Gragera R, De Angelis R, et al. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer. (2012) 48:3257–66. doi: 10.1016/j.ejca.2012.05.024
45. Marcos-Gragera R, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Maynadie M, et al. Survival of European patients diagnosed with lymphoid neoplasms in 2000-2002: results of the HAEMACARE project. Haematologica. (2011) 96:720–8. doi: 10.3324/haematol.2010.034264
46. Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, et al. Survival of European children and young adults with cancer diagnosed 1995-2002. Eur J Cancer. (2009) 45:992–1005. doi: 10.1016/j.ejca.2008.11.042
47. Girardi F, Di Carlo V, Stiller C, Gatta G, Woods RR, Visser O, et al. Global survival trends for brain tumors, by histology: analysis of individual records for 67,776 children diagnosed in 61 countries during 2000-2014 (CONCORD-3). Neuro Oncol. (2023) 25:593–606. doi: 10.1093/neuonc/noac232
48. Ssenyonga N, Stiller C, Nakata K, Shalkow J, Redmond S, Bulliard JL, et al. Worldwide trends in population-based survival for children, adolescents, and young adults diagnosed with leukaemia, by subtype, during 2000-14 (CONCORD-3): analysis of individual data from 258 cancer registries in 61 countries. Lancet Child Adolesc Health. (2022) 6:409–31. doi: 10.1016/S2352-4642(22)00095-5
49. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
50. Bonaventure A, Harewood R, Stiller CA, Gatta G, Clavel J, Stefan DC, et al. Worldwide comparison of survival from childhood leukaemia for 1995-2009, by subtype, age, and sex (CONCORD-2): a population-based study of individual data for 89 828 children from 198 registries in 53 countries. Lancet Haematol. (2017) 4:e202–17. doi: 10.1016/S2352-3026(17)30052-2
51. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. (2015) 385:977–1010. doi: 10.1016/S0140-6736(14)62038-9
52. Tai EW, Ward KC, Bonaventure A, Siegel DA, Coleman MP. Survival among children diagnosed with acute lymphoblastic leukemia in the United States, by race and age, 2001 to 2009: findings from the CONCORD-2 study. Cancer. (2017) 123 Suppl 24:5178–89. doi: 10.1002/cncr.30899
53. Karalexi MA, Servitzoglou M, Papadakis V, Kachanov D, Cesen Mazič M, Baka M, et al. Survival patterns of childhood neuroblastoma: an analysis of clinical data from Southern-Eastern European countries. Eur J Cancer Prev. (2023) 32:254–63. doi: 10.1097/CEJ.0000000000000614
54. Doganis D, Zborovskaya A, Trojanowski M, Zagar T, Bouka P, Baka M, et al. Wilms tumour event-free and overall survival in Southern and Eastern Europe: pooled analyses of clinical data from four childhood cancer registries (1999-2017). Eur J Cancer. (2019) 115:37–46. doi: 10.1016/j.ejca.2019.04.008
55. Doganis D, Panagopoulou P, Tragiannidis A, Vichos T, Moschovi M, Polychronopoulou S, et al. Survival and mortality rates of Wilms tumour in Southern and Eastern European countries: socioeconomic differentials compared with the United States of America. Eur J Cancer. (2018) 101:38–46. doi: 10.1016/j.ejca.2018.06.012
56. Panagopoulou P, Georgakis MK, Baka M, Moschovi M, Papadakis V, Polychronopoulou S, et al. Persisting inequalities in survival patterns of childhood neuroblastoma in Southern and Eastern Europe and the effect of socio-economic development compared with those of the US. Eur J Cancer. (2018) 96:44–53. doi: 10.1016/j.ejca.2018.03.003
57. Georgakis MK, Karalexi MA, Kalogirou EI, Ryzhov A, Zborovskaya A, Dimitrova N, et al. Incidence, time trends and survival patterns of childhood pilocytic astrocytomas in Southern-Eastern Europe and SEER, US. J Neurooncol. (2017) 131:163–75. doi: 10.1007/s11060-016-2284-9
58. Karalexi MA, Georgakis MK, Dessypris N, Ryzhov A, Zborovskaya A, Dimitrova N, et al. Mortality and survival patterns of childhood lymphomas: geographic and age-specific patterns in Southern-Eastern European and SEER/US registration data. Hematol Oncol. (2017) 35:608–18. doi: 10.1002/hon.2347
59. Karalexi MA, Papathoma P, Thomopoulos TP, Ryzhov A, Zborovskaya A, Dimitrova N, et al. Childhood central nervous system tumour mortality and survival in Southern and Eastern Europe (1983-2014): gaps persist across 14 cancer registries. Eur J Cancer. (2015) 51:2665–77. doi: 10.1016/j.ejca.2015.08.018
60. Peirelinck H, Schulpen M, Hoogendijk R, Van Damme A, Pieters R, Henau K, et al. Incidence, survival, and mortality of cancer in children and young adolescents in Belgium and the Netherlands in 2004-2015: a comparative population-based study. Int J Cancer. (2024) 155:226–39. doi: 10.1002/ijc.34918
61. Di Giuseppe G, Youlden DR, Aitken JF, Pole JD. Pediatric hepatic cancer incidence and survival: 30-year trends in Ontario, Canada; the United States; and Australia. Cancer. (2021) 127:769–76. doi: 10.1002/cncr.33319
62. Liu APY, Liu Q, Shing MMK, Ku DTL, Fu E, Luk CW, et al. Incidence and outcomes of CNS tumors in Chinese children: comparative analysis with the surveillance, epidemiology, and end results program. JCO Glob Oncol. (2020) 6:704–21. doi: 10.1200/JGO.19.00378
63. Rostgaard K, Hjalgrim H, Madanat-Harjuoja L, Johannesen TB, Collin S, Hjalgrim LL. Survival after cancer in children, adolescents and young adults in the Nordic countries from 1980 to 2013. Br J Cancer. (2019) 121:1079–84. doi: 10.1038/s41416-019-0632-1
64. Joko-Fru WY, Parkin DM, Borok M, Chokunonga E, Korir A, Nambooze S, et al. Survival from childhood cancers in Eastern Africa: a population-based registry study. Int J Cancer. (2018) 143:2409–15. doi: 10.1002/ijc.31723
65. Nakata K, Ito Y, Magadi W, Bonaventure A, Stiller CA, Katanoda K, et al. Childhood cancer incidence and survival in Japan and England: a population-based study (1993-2010). Cancer Sci. (2018) 109:422–34. doi: 10.1111/cas.13457
66. Demanelis K, Sriplung H, Meza R, Wiangnon S, Rozek LS, Scheurer ME, et al. Differences in childhood leukemia incidence and survival between Southern Thailand and the United States: a population-based analysis. Pediatr Blood Cancer. (2015) 62:1790–8. doi: 10.1002/pbc.25571
67. Pole JD, Darmawikarta D, Alibhai SM, Brandwein JM, Sung L. Differential survival improvement for patients 20-29 years of age with acute lymphoblastic leukemia. Leuk Res. (2013) 37:1258–64. doi: 10.1016/j.leukres.2013.05.001
68. Redaniel MT, Laudico A, Mirasol-Lumague MR, Alcasabas AP, Pulte D, Brenner H. Geographic and ethnic differences in childhood leukaemia and lymphoma survival: comparisons of Philippine residents, Asian Americans and Caucasians in the United States. Br J Cancer. (2010) 103:149–54. doi: 10.1038/sj.bjc.6605703
69. Kristiansen MF, Ósá E, Lyngsie Hjalgrim L, Á Steig B, Andórsdóttir G, Strøm M, et al. Childhood cancer incidence and survival in the Faroe Islands, 1960 to 2019. Acta Oncol. (2024) 63:4–8. doi: 10.2340/1651-226X.2024.27110
70. Ni X, Li Z, Li X, Zhang X, Bai G, Liu Y, et al. Socioeconomic inequalities in cancer incidence and access to health services among children and adolescents in China: a cross-sectional study. Lancet. (2022) 400:1020–32. doi: 10.1016/S0140-6736(22)01541-0
71. Joko-Fru WY, Bardot A, Bukirwa P, Amidou S, N'da G, Woldetsadik E, et al. Cancer survival in sub-Saharan Africa (SURVCAN-3): a population-based study. Lancet Glob Health. (2024) 12:e947–59. doi: 10.1016/S2214-109X(24)00130-X
72. Steliarova-Foucher E, Colombet M, Ries LAG, Hesseling P, Moreno F, Shin HY, et al., editors. International Incidence of Childhood Cancer, Volume III (electronic version). Results. Lyon: International Agency for Research on Cancer. (2017). Available online at: https://iicc.iarc.fr/results/ (Accessed February 3, 2025).
73. Stiller CA, Bayne AM, Chakrabarty A, Kenny T, Chumas P. Incidence of childhood CNS tumours in Britain and variation in rates by definition of malignant behaviour: population-based study. BMC Cancer. (2019) 19:139. doi: 10.1186/s12885-019-5344-7
74. Piñeros M, Mery L, Soerjomataram I, Bray F, Steliarova-Foucher E. Scaling up the surveillance of childhood cancer: a global roadmap. J Natl Cancer Inst. (2021) 113:9–15. doi: 10.1093/jnci/djaa069
75. Arnett A, Siegel DA Dai S, Thompson TD, Foster J, di Pierro EJ, et al. Incidence and survival of pediatric and adult hepatocellular carcinoma, United States, 2001-2020. Cancer Epidemiol. (2024) 92:102610. doi: 10.1016/j.canep.2024.102610
76. Businge L, Hagenimana M, Motlhale M, Bardot A, Liu B, Anastos K, et al. Stage at diagnosis and survival by stage for the leading childhood cancers in Rwanda. Pediatr Blood Cancer. (2024) 71:e31020. doi: 10.1002/pbc.31020
77. Campbell K, Siegel DA, Umaretiya PJ Dai S, Heczey A, Lupo PJ, et al. A comprehensive analysis of neuroblastoma incidence, survival, and racial and ethnic disparities from 2001 to 2019. Pediatr Blood Cancer. (2024) 71:e30732. doi: 10.1002/pbc.30732
78. de Souza PCF, Espinosa MM, Teixeira MTB, de Lima FCDS, Galvão ND. Survival analysis of haematologic neoplasms in children and adolescents: a population-based study in a state of the Brazilian Legal Amazon. Ecancermedicalscience. (2024) 18:1764. doi: 10.3332/ecancer.2024.1764
79. Elgenidy A, Afifi AM, Gad EF, Atef Abdelsattar Ibrahim H, Khan U, Alomari O, et al. Survival characteristics of Wilms Tumor, a reference developed from a longitudinal cohort study. Ital J Pediatr. (2024) 50:141. doi: 10.1186/s13052-024-01698-7
80. Felix A, Dichamp C, Michaux K, Minard-Colin V, Sarnacki S, Lacour B, et al. Afro-descendant ethnicity does not negatively influence neuroblastoma survival in the French West Indies. Pediatr Blood Cancer. (2024) 71:e31037. doi: 10.1002/pbc.31037
81. Godoy-Casasbuenas N, Gil F, Arias N, Pérez CU, Cruz HMC, Goyes LB, et al. Population-based overall and net survival of childhood leukemia at 1-, 5-, and 10-years of follow-up in three regions of Colombia. Ecancermedicalscience. (2024) 18:1759. doi: 10.3332/ecancer.2024.1759
82. Hoang TT, Rathod RA, Rosales O, Castellanos MI, Schraw JM, Burgess E, et al. Residential proximity to oil and gas developments and childhood cancer survival. Cancer. (2024) 130:3724–33. doi: 10.1002/cncr.35449
83. Hoffman BA, Sanford C Jr, Didier AJ, Lassiter E, Lozano-Calderon SA. Pediatric axial Ewing sarcoma: a retrospective population-based survival analysis. J Am Acad Orthop Surg Glob Res Rev. (2024) 8:e24.00130. doi: 10.5435/JAAOSGlobal-D-24-00130
84. Hou X, Liang F, Lou Y. Clinical features and prognostic factors for malignant parotid tumors in children and adolescents: a population-based study. J Stomatol Oral Maxillofac Surg. (2024) 125:101741. doi: 10.1016/j.jormas.2023.101741
85. Liao ET, Lin HY, Tsai CY. Updated retinoblastoma incidence and outcome in children in Taiwan from 1980 to 2019: a 40-year nationwide study. Eye. (2024) 38:1535–41. doi: 10.1038/s41433-024-02946-0
86. Lin W, Zhu Z, Shang Y. Gastroenteropancreatic neuroendocrine carcinoma in children and adolescents: a population-based study. J Cancer Res Clin Oncol. (2024) 150:4. doi: 10.1007/s00432-023-05568-3
87. Mashtoub S, Ullah S, Collinson A, Singh GR, Clark Adnyamathanha J, Leemaqz S, et al. Childhood cancer incidence and survival in South Australia and the Northern Territory, 1990-2017, with emphasis on Indigenous peoples. Cancers. (2024) 16:2057. doi: 10.3390/cancers16112057
88. Mishra A, Mishra A, Taylor MA, Sharma D, Kubesh M. Poor disease-specific survival in Black pediatric patients diagnosed with Burkitt lymphoma: an analysis of the surveillance, epidemiology, and end results database. Pediatr Blood Cancer. (2024) 71:e31204. doi: 10.1002/pbc.31204
89. Papakonstantinou E, Athanasiadou KI, Markozannes G, Tzotzola V, Bouka E, Baka M, et al. Prognostic factors in high-grade pediatric osteosarcoma among children and young adults: Greek Nationwide Registry for Childhood Hematological Malignancies and Solid Tumors (NARECHEM-ST) data along with a systematic review and meta-analysis. Cancer Epidemiol. (2024) 90:102551. doi: 10.1016/j.canep.2024.102551
90. Pinsuwan C, Santong C, Chainansamit SO, Komvilaisak P, Sirikarn P, Phimha S, et al. Trends in incidence and survival of childhood cancers in Khon Kaen, Thailand (2000-2019): a population-based Khon Kaen Cancer Registry study. BMC Public Health. (2024) 24:1255. doi: 10.1186/s12889-024-18742-0
91. Qi X, Zhu D, Xiong Q, Yan X, Li X. Prognostic factors of primary intrascrotal rhabdomyosarcoma in children: a population-based study. Int Urol Nephrol. (2024) 56:2117–23. doi: 10.1007/s11255-024-03954-5
92. Schulpen M, Haveman LM, van der Heijden L, Kaal SEJ, Bramer JAM, Fajardo RD, et al. The survival disparity between children and adolescents and young adults (AYAs) with Ewing sarcoma in the Netherlands did not change since the 1990s despite improved survival: a population-based study. Eur J Cancer. (2024) 208:114209. doi: 10.1016/j.ejca.2024.114209
93. Schulpen M, Beishuizen A, Chamuleau MED, Dinmohamed AG, Meyer-Wentrup FAG, Vormoor HJ, et al. Survival disparities between children and adolescents and young adults for the major subtypes of non-Hodgkin lymphoma in the Netherlands: a large population-based study. Haematologica. (2024) 109:936–41. doi: 10.3324/haematol.2023.283379
94. Turanzas NJ, Mathiasen R, Heegaard S, Schmiegelow K, Sehested A, Holtz JK, et al. Ophthalmic symptoms, clinical signs and diagnostic delay in infants diagnosed with brain tumours in Denmark between 2007 and 2017. Acta Ophthalmol. (2024) 102:334–41. doi: 10.1111/aos.15745
95. Wang C, Shen CF, Xun LN, Zhang S, Zhang H, Zheng WL, et al. Cancer survival analysis in Tianjin, 2010 to 2016. Zhonghua Zhong Liu Za Zhi. (2024) 46:319–25. doi: 10.3760/cma.j.cn112152-20231024-00236
96. Wang H, Yao B, Tang T, Gong M, Ma Y, Wu X, et al. Racial/ethnic disparities in all-cause and cause-specific death among children with malignant central nervous system tumours: a registry-based cohort retrospective analysis. EClinicalMedicine. (2024) 76:102816. doi: 10.1016/j.eclinm.2024.102816
97. Wellbrock M, Voigt M, Ronckers C, Grabow D, Spix C, Erdmann F. Registration, incidence patterns, and survival trends of central nervous system tumors among children in Germany 1980-2019: an analysis of 40 years based on data from the German Childhood Cancer Registry. Pediatr Blood Cancer. (2024) 71:e30954. doi: 10.1002/pbc.30954
98. Youlden DR, Baade PD, Gottardo NG, Moore AS, Valery PC, Pole JD. Population-level 5-year event-free survival for children with cancer in Australia. Pediatr Blood Cancer. (2024) 71:e31195. doi: 10.1002/pbc.31195
99. Youlden DR, Gupta S, Frazier AL, Moore AS, Gottardo NG, Aitken JF. Incidence and survival for childhood cancer by endorsed non-stage prognostic indicators in Australia. Pediatr Blood Cancer. (2024) 71:e30889. doi: 10.1002/pbc.30889
100. Abdelazeem B, Abbas KS, Shehata J, El-Shahat NA, Eltaras MM, Qaddoumi I, et al. Survival trends for patients with retinoblastoma between 2000 and 2018: what has changed? Cancer Med. (2023) 12:6318–24. doi: 10.1002/cam4.5406
101. Bednarek OL, Seemann N, Brzezinski J, Lorenzo A, Fernandez CV, Romao RLP. Outcomes according to treatment using an established protocol in patients with bilateral Wilms' tumor: a national Canadian population-based study. J Pediatr Surg. (2023) 58:1014–7. doi: 10.1016/j.jpedsurg.2023.01.016
102. Castellanos MI, Oluyomi AO, Chambers TM, Gramatges MM, Winestone LE, Lupo PJ, et al. Ethnic disparities in childhood leukemia survival by border residence: a Texas population-based analysis. Cancer. (2023) 129:1276–86. doi: 10.1002/cncr.34636
103. Chirlaque MD, Peris-Bonet R, Sánchez A, Cruz O, Marcos-Gragera R, Gutiérrez-Ávila G, et al. Childhood and adolescent central nervous system tumours in Spain: incidence and survival over 20 years: a historical baseline for current assessment. Cancers. (2023) 15:5889. doi: 10.3390/cancers15245889
104. Cromie KJ, Hughes NF, Milner S, Crump P, Grinfeld J, Jenkins A, et al. Socio-economic and ethnic disparities in childhood cancer survival, Yorkshire, UK. Br J Cancer. (2023) 128:1710–22. doi: 10.1038/s41416-023-02209-x
105. Gera K, Martir D, Xue W, Wingard JR. Survival after pure (acute) erythroid leukemia in the United States: a SEER-Based study. Cancers. (2023) 15:3941. doi: 10.3390/cancers15153941
106. Goulding D, Arguinchona L, Anderson-Mellies A, Mikkelsen M, Eguchi M, Marinoff H, et al. Sociodemographic disparities in presentation and survival of pediatric bone cancers. J Pediatr Hematol Oncol. (2023) 45:e31–43. doi: 10.1097/MPH.0000000000002531
107. Henau K, Tambuyzer T, Van Gool B, Van Eycken L, Poirel HA. Epidemiological trends of haematological malignancies in Belgium 2004-2018: older patients show the greatest improvement in survival. Cancers. (2023) 15:4388. doi: 10.3390/cancers15174388
108. Hoogendijk R, van der Lugt J, Baugh J, Kline C, Kranendonk M, Hoving E, et al. Sex-related incidence and survival differences in pediatric high-grade glioma subtypes: a population-based cohort study. iScience. (2023) 26:107957. doi: 10.1016/j.isci.2023.107957
109. Kameda-Smith MM, Pond GR, Seow H. Rurality index score and pediatric neuro-oncological outcome in Ontario. J Neurosurg Pediatr. (2023) 31:275–81. doi: 10.3171/2022.12.PEDS22446
110. Karimi A, Ebrahimpour A, Sadighi M, Chehrassan M, Biglari F, Jafari Kafiabadi M, et al. Descriptive epidemiology and survival rate of osteosarcoma: the first national population-based study in the Middle East (2008-2014). Arch Bone Jt Surg. (2023) 11:649–57. doi: 10.22038/ABJS.2023.59676.2945
111. Li PY, Kong YJ, Wan L, Guo J, Li WM, Zhang, et al. Overall survival, late mortality, and cancer-directed surgery among children and adolescents with ultra-rare pediatric pancreatoblastoma in the United States, 1975-2018. J Pancreatol. (2023) 6:61–6. doi: 10.1097/JP9.0000000000000121
112. Liu S, Yin W, Lin Y, Huang S, Xue S, Sun G, et al. Metastasis pattern and prognosis in children with neuroblastoma. World J Surg Oncol. (2023) 21:130. doi: 10.1186/s12957-023-03011-y
113. Liu X, Feng S, Zhao L, Luo L. Clinical characteristics and prognostic models of gonadal and extra-gonadal yolk sac tumors: a population-based analysis in children and adolescents. World J Urol. (2023) 41:3009–17. doi: 10.1007/s00345-023-04616-4
114. McEvoy MT, Siegel DA Dai S, Okcu MF, Zobeck M, Venkatramani R, et al. Pediatric rhabdomyosarcoma incidence and survival in the United States: an assessment of 5656 cases, 2001-2017. Cancer Med. (2023) 12:3644–56. doi: 10.1002/cam4.5211
115. Montes-Rodríguez IM, Soto-Salgado M, Torres-Cintrón CR, Tomassini-Fernandini JC, Suárez E, Clavell LA, et al. Incidence and mortality rates for childhood acute lymphoblastic leukemia in Puerto Rican Hispanics, 2012-2016. Cancer Epidemiol Biomarkers Prev. (2023) 32:1030–7. doi: 10.1158/1055-9965.EPI-22-1227
116. Nissen TN, Rechnitzer C, Albertsen BK, Borgwardt L, Christensen VB, Fallentin E, et al. Epidemiological study of malignant paediatric liver tumours in Denmark 1985-2020. Cancers. (2023) 15:3355. doi: 10.3390/cancers15133355
117. Ohlsen TJD, Doody DR, Mueller BA, Desai AD, Chow EJ. Population-based impact of rurality and neighborhood-level socioeconomic disadvantage on pediatric cancer mortality in Washington State. Cancer Epidemiol Biomarkers Prev. (2023) 32:141–8. doi: 10.1158/1055-9965.EPI-22-0897
118. Price M, Neff C, Kruchko C, Barnholtz-Sloan JS, Cordeiro BB, Penas-Prado M, et al. Capturing evolving definitions of 12 select rare CNS tumors: a timely report from CBTRUS and NCI-CONNECT. J Neurooncol. (2023) 165:279–90. doi: 10.1007/s11060-023-04480-7
119. Pugh G, Bradbeer P, Wood A, Hunter S, Cross S, Denton K, et al. Childhood cancer incidence & survival in Aotearoa, New Zealand 2010-2019. Cancer Epidemiol. (2023) 86:102433. doi: 10.1016/j.canep.2023.102433
120. Wang X, Brown DS, Cao Y, Ekenga CC, Guo S, Johnson KJ. Disparities in survival improvement for U.S. childhood and adolescent cancer between 1995 and 2019: an analysis of population-based data. Cancer Epidemiol. (2023) 85:102380. doi: 10.1016/j.canep.2023.102380
121. Wellbrock M, Spix C, Ronckers CM, Grabow D, Filbert AL, Borkhardt A, et al. Temporal patterns of childhood cancer survival 1991 to 2016: a nationwide register-study based on data from the German Childhood Cancer Registry. Int J Cancer. (2023) 153:742–55. doi: 10.1002/ijc.34556
122. Wu S, Liu Y, Williams M, Aguilar C, Ramirez AG, Mesa R, et al. Childhood cancer survival in the highly vulnerable population of South Texas: a cohort study. PLoS ONE. (2023) 18:e0278354. doi: 10.1371/journal.pone.0278354
123. Wu P, Yang Y, Yu Z, Zhao L, Feng S. Clinical features and survival outcomes in children and adolescents with malignant mediastinal germ cell tumors based on Surveillance, epidemiology, and end results database analysis. J Surg Res. (2023) 288:362–71. doi: 10.1016/j.jss.2023.03.018
124. Wu P, Zhu D, Lou Y, Wang X. Clinical characteristics and prognostic factors for primary pediatric and adolescent Non-Hodgkin Lymphomas of the gastrointestinal tract: a population-based study. World J Surg Oncol. (2023) 21:353. doi: 10.1186/s12957-023-03238-9
125. Youlden DR, Baade PD, Moore AS, Pole JD, Valery PC, Aitken JF. Childhood cancer survival and avoided deaths in Australia, 1983-2016. Paediatr Perinat Epidemiol. (2023) 37:81–91. doi: 10.1111/ppe.12895
126. Youlden DR, Baade PD, Frazier AL, Gupta S, Gottardo NG, Moore AS, et al. Temporal changes in childhood cancer incidence and survival by stage at diagnosis in Australia, 2000-2017. Acta Oncol. (2023) 62:1256–64. doi: 10.1080/0284186X.2023.2251668
127. Zhao L, Liu W, Chu L, Luo L. Factors associated with survival in paediatric and adolescent renal cell carcinoma: a population-based study. ANZ J Surg. (2023) 93:2710–5. doi: 10.1111/ans.18614
128. Caetano dos Santos FL, Michalek IM, Wojciechowska U, Didkowska J, Walewski J. Improved survival of Burkitt lymphoma/leukemia patients: observations from Poland, 1999-2020. Ann Hematol. (2022) 101:1059–65. doi: 10.1007/s00277-022-04758-2
129. Cañete A, Peris-Bonet R, Capocaccia R, Pardo-Romaguera E, Segura V, Muñoz-López A, et al. Neuroblastoma in Spain: linking the national clinical database and epidemiological registries - a study by the Joint Action on Rare Cancers. Cancer Epidemiol. (2022) 78:102145. doi: 10.1016/j.canep.2022.102145
130. Castro-Ríos A, Martínez-Valverde S. Childhood cancer survival, 2006-2012 cohorts of Mexican Institute of Social Security beneficiaries at the Central-South Region of Mexico. Front Oncol. (2022) 12:882501. doi: 10.3389/fonc.2022.882501
131. Cioffi G, Waite KA, Edelson JL, Kruchko C, Ostrom QT, Barnholtz-Sloan JS. Changes in survival over time for primary brain and other CNS tumors in the United States, 2004-2017. J Neurooncol. (2022) 160:209–19. doi: 10.1007/s11060-022-04138-w
132. Cole S, Gianferante DM, Zhu B, Mirabello L. Osteosarcoma: a surveillance, epidemiology, and end results program-based analysis from 1975 to 2017. Cancer. (2022) 128:2107–18. doi: 10.1002/cncr.34163
133. Hart M, Anderson-Mellies A, Beltrami A, Gilani A, Green AL. Population-based analysis of CNS tumor diagnoses, treatment, and survival in congenital and infant age groups. J Neurooncol. (2022) 157:333–44. doi: 10.1007/s11060-022-03967-z
134. Helligsoe ASL, Kenborg L, Henriksen LT, Udupi A, Hasle H, Winther JF. Incidence and survival of childhood central nervous system tumors in Denmark, 1997-2019. Cancer Med. (2022) 11:245–56. doi: 10.1002/cam4.4429
135. Kahla JA, Siegel DA Dai S, Lupo PJ, Foster JH, Scheurer ME, et al. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr Blood Cancer. (2022) 69:e29763. doi: 10.1002/pbc.29763
136. Klangjorhor J, Pongnikorn D, Phanphaisarn A, Chaiyawat P, Teeyakasem P, Suksakit P, et al. An analysis of the incidence and survival rates of bone sarcoma patients in Thailand: reports from population-based cancer registries 2001-2015. Cancer Epidemiol. (2022) 76:102056. doi: 10.1016/j.canep.2021.102056
137. Lee JA, Lim J, Park D, Jin HY, Park M, Park HJ, et al. Incidence patterns and outcomes of Ewing Sarcoma in South Korea (1999-2017): a retrospective analysis using Korea Central Cancer Registry data. Cancer Res Treat. (2022) 54:590–6. doi: 10.4143/crt.2021.311
138. Liu H, Stiller CA, Crooks CJ, Rous B, Bythell M, Broggio J, et al. Incidence, prevalence and survival in patients with Langerhans cell histiocytosis: a national registry study from England, 2013-2019. Br J Haematol. (2022) 199:728–38. doi: 10.1111/bjh.18459
139. Liu YL, Tsai ML, Chen CI, Yar N, Tsai CW, Lee HL, et al. Atypical teratoid/rhabdoid tumor in Taiwan: a nationwide, population-based study. Cancers (Basel). (2022) 14:668. doi: 10.3390/cancers14030668
140. Loap P, Vignon M, Bouscary D, Kirova Y. Pediatric plasma cell neoplasms: a population-based study. Clin Lymphoma Myeloma Leuk. (2022) 22:841–6. doi: 10.1016/j.clml.2022.07.003
141. Lv Z, Yu Y, Luo Y, Lin S, Xiang X, Mao X, et al. Long-term survival outcomes of pediatric adrenal malignancies: an analysis with the upstaged SEER registry during 2000-2019. Front Endocrinol. (2022) 13:977105. doi: 10.3389/fendo.2022.977105
142. Nakata K, Hiyama E, Katanoda K, Matsuda T, Tada Y, Inoue M, et al. Cancer in adolescents and young adults in Japan: epidemiology and cancer strategy. Int J Clin Oncol. (2022) 27:7–15. doi: 10.1007/s10147-021-02064-x
143. Ravaioli A, Crocetti E, Bucchi L, Guzzinati S, Casella C, Falcini F, et al. Update of survival of cancer patients in Italy: geographical comparisons and focus on patients with cancers targeted by screening programmes, childhood cancers, and smoking-associated cancers. Epidemiol Prev. (2022) 46:356–66. doi: 10.19191/EP22.5-6.A489.095
144. Reedijk AMJ, Beishuizen A, Coebergh JWW, Hoeben BAW, Kremer LCM, Hebeda KM, et al. Progress against non-Hodgkin's lymphoma in children and young adolescents in the Netherlands since 1990: stable incidence, improved survival and lower mortality. Eur J Cancer. (2022) 163:140–51. doi: 10.1016/j.ejca.2021.12.010
145. Schulpen M, Roy P, Wijnen MHWA, Tytgat GAM, van den Heuvel-Eibrink MM, van Tinteren H, et al. Incidence and survival of paediatric renal tumours in the Netherlands between 1990 and 2014. Eur J Cancer. (2022) 175:282–90. doi: 10.1016/j.ejca.2022.08.021
146. Schulpen M, Goemans BF, Kaspers GJL, Raaijmakers MHGP, Zwaan CM, Karim-Kos HE. Increased survival disparities among children and adolescents & young adults with acute myeloid leukemia: a Dutch population-based study. Int J Cancer. (2022) 150:1101–12. doi: 10.1002/ijc.33878
147. van de Berg DJ, Kuijpers AMJ, Engelsman AF, Drukker CA, van Santen HM, Terwisscha van Scheltinga SCEJ, et al. Long-term oncological outcomes of papillary thyroid cancer and follicular thyroid cancer in children: a nationwide population-based study. Front Endocrinol. (2022) 13:899506. doi: 10.3389/fendo.2022.899506
148. van der Linde M, van Leeuwen N, Eijkenaar F, Rijneveld AW, Pieters R, Karim-Kos HE. Effect of treatment in a specialized pediatric hemato-oncology setting on 5-year survival in acute lymphoblastic leukemia: a quasi-experimental study. Cancers. (2022) 14:2451. doi: 10.3390/cancers14102451
149. Wellbrock M, Zeeb H, Spix C, Grabow D, Borkhardt A, Erdmann F. Survival in children below the age of 15 years with leukemia: temporal patterns in Eastern and Western Germany since German reunification. Hemasphere. (2022) 6:e755. doi: 10.1097/HS9.0000000000000755
150. Xing H, Yang C, Tan B, Zhang M. Incidence trends and predictive model of hepatic malignant tumors in children: a population-based study. Am J Transl Res. (2022) 14:7268–89.
151. Youlden DR, Baade PD, McBride CA, Pole JD, Moore AS, Valery PC, et al. Changes in cancer incidence and survival among Aboriginal and Torres Strait Islander children in Australia, 1997-2016. Pediatr Blood Cancer. (2022) 69:e29492. doi: 10.1002/pbc.29492
152. Zbitou A, Desandes E, Guissou S, Mallebranche C, Lacour B. Thyroid cancers in children and adolescents in France: incidence, survival and clinical management over the 2000-2018 period. Int J Pediatr Otorhinolaryngol. (2022) 162:111325. doi: 10.1016/j.ijporl.2022.111325
153. Curry SD, Jiang ZY, Jain KS. Population-based survival of pediatric rhabdomyosarcoma of the head and neck over four decades. Int J Pediatr Otorhinolaryngol. (2021) 142:110599. doi: 10.1016/j.ijporl.2020.110599
154. Ellison LF, Xie L, Sung L. Trends in paediatric cancer survival in Canada, 1992 to 2017. Health Rep. (2021) 32:3–15. doi: 10.25318/82-003-x202100200001-eng
155. Hoogendijk R, van der Lugt J, van Vuurden D, Kremer L, Wesseling P, Hoving E, et al. Survival rates of children and young adolescents with CNS tumors improved in the Netherlands since 1990: a population-based study. Neurooncol Adv. (2021) 4:vdab183. doi: 10.1093/noajnl/vdab183
156. Karalexi MA, Servitzoglou M, Moschovi M, Moiseenko R, Bouka P, Ntzani E, et al. Survival and prognostic factors for childhood malignant liver tumors: analysis of harmonized clinical data. Cancer Epidemiol. (2021) 70:101850. doi: 10.1016/j.canep.2020.101850
157. Karalexi MA, Pourtsidis A, Panagopoulou P, Moschovi M, Polychronopoulou S, Kourti M, et al. Overall and event-free survival of childhood lymphoma in Greece: analysis of harmonized clinical data over a 24-year active registration period. Leuk Lymphoma. (2021) 62:2107–19. doi: 10.1080/10428194.2021.1907376
158. Kelm RC, Ali Y, Orrell K, Rangel SM, Kruse L, Wagner AM, et al. Age and sex differences for malignant melanoma in the pediatric population-childhood versus adolescence: analysis of current nationwide data from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program. J Am Acad Dermatol. (2021) 84:862–4. doi: 10.1016/j.jaad.2020.10.050
159. Loizou L, Demetriou A, Erdmann F, Borkhardt A, Brozou T, Sharp L, et al. Increasing incidence and survival of paediatric and adolescent thyroid cancer in Cyprus 1998-2017: a population-based study from the Cyprus Pediatric Oncology Registry. Cancer Epidemiol. (2021) 74:101979. doi: 10.1016/j.canep.2021.101979
160. Miller KD, Ostrom QT, Kruchko C, Patil N, Tihan T, Cioffi G, et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. (2021) 71:381–406. doi: 10.3322/caac.21693
161. Nakata K, Okawa S, Fuji S, Sato A, Morishima T, Tada Y, et al. Trends in survival of leukemia among children, adolescents, and young adults: a population-based study in Osaka, Japan. Cancer Sci. (2021) 112:1150–60. doi: 10.1111/cas.14808
162. Park M, Lim J, Lee JA, Park HJ, Park BK, Lim MC, et al. Incidence and outcomes of malignant ovarian germ cell tumors in Korea, 1999-2017. Gynecol Oncol. (2021) 163:79–84. doi: 10.1016/j.ygyno.2021.07.037
163. Person L, Lacour B, Faure L, Guissou S, Poulalhon C, Orbach D, et al. Childhood head and neck cancer in France: incidence, survival and trends from 2000 to 2015. Int J Pediatr Otorhinolaryngol. (2021) 150:110858. doi: 10.1016/j.ijporl.2021.110858
164. Poulalhon C, Goujon S, Marquant F, Faure L, Guissou S, Bonaventure A, et al. Factors associated with 5- and 10-year survival among a recent cohort of childhood cancer survivors (France, 2000-2015). Cancer Epidemiol. (2021) 73:101950. doi: 10.1016/j.canep.2021.101950
165. Reedijk AMJ, Coebergh JWW, de Groot-Kruseman HA, van der Sluis IM, Kremer LC, Karim-Kos HE, et al. Progress against childhood and adolescent acute lymphoblastic leukaemia in the Netherlands, 1990-2015. Leukemia. (2021) 35:1001–11. doi: 10.1038/s41375-020-01024-0
166. Sasaki K, Jabbour E, Short NJ, Jain N, Ravandi F, Pui CH, et al. Acute lymphoblastic leukemia: a population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980-2017. Am J Hematol. (2021) 96:650–8. doi: 10.1002/ajh.26156
167. Schraw JM, Peckham-Gregory EC, Hughes AE, Scheurer ME, Pruitt SL, Lupo PJ. Residence in a Hispanic enclave is associated with inferior overall survival among children with acute lymphoblastic leukemia. Int J Environ Res Public Health. (2021) 18:9273. doi: 10.3390/ijerph18179273
168. Schulpen M, Visser O, Reedijk AMJ, Kremer LCM, Zwaan CM, Eggermont AMM, et al. Significant improvement in survival of advanced stage childhood and young adolescent cancer in the Netherlands since the 1990s. Eur J Cancer. (2021) 157:81–93. doi: 10.1016/j.ejca.2021.08.001
169. Soon WC, Goacher E, Solanki S, Hayes J, Kapetanstrataki M, Picton S, et al. The role of sex genotype in paediatric CNS tumour incidence and survival. Childs Nerv Syst. (2021) 37:2177–86. doi: 10.1007/s00381-021-05165-0
170. Wang H, Mejia MC, Gonzalez SJ, Zoorob RJ, Chai W, Du XL. Cancer incidence and survival trends among infants in the United States from 1975 to 2014. Pediatr Blood Cancer. (2021) 68:e28917. doi: 10.1002/pbc.28917
171. Youlden DR, Henshaw C, Gottardo NG, Hassall T, Aitken JF. Incidence and survival for childhood central nervous system tumours in Australia, 1983-2016. J Neurooncol. (2021) 155:203–13. doi: 10.1007/s11060-021-03869-6
172. Chen S, Tang W, Yang R, Hu X, Li Z. Pediatric patients with adrenal neuroblastoma: a SEER analysis, 2004-2013. Am Surg. (2020) 86:127–33. doi: 10.1177/000313482008600232
173. de Oliveira MM, Mendonça e Silva DR, Ramos FR, Curado MP. Children and adolescents cancer incidence, mortality and survival a population-based study in Midwest of Brazil. Cancer Epidemiol. (2020) 68:101795. doi: 10.1016/j.canep.2020.101795
174. Desandes E, Faure L, Guissou S, Goujon S, Berger C, Minard-Colin V, et al. Infant cancers in France: incidence and survival (2000-2014). Cancer Epidemiol. (2020) 65:101697. doi: 10.1016/j.canep.2020.101697
175. Fineberg R, Zahedi S, Eguchi M, Hart M, Cockburn M, Green AL. Population-based analysis of demographic and socioeconomic disparities in pediatric CNS cancer survival in the United States. Sci Rep. (2020) 10:4588. doi: 10.1038/s41598-020-61237-2
176. Ji J, Luo Z, Chen Y, Xu X, Li X, Liu S, et al. Characteristics and trends of childhood cancer in Pudong, China, 2002-2015. BMC Public Health. (2020) 20:1430. doi: 10.1186/s12889-020-09493-9
177. Jones BC, Youlden DR, Cundy TP, O'Callaghan ME, Karpelowsky J, Aitken JF, et al. Renal tumours in Australian children: 30 years of incidence, outcome and second primary malignancy data from the Australian Childhood Cancer Registry. J Paediatr Child Health. (2020) 56:908–16. doi: 10.1111/jpc.14774
178. Krejci D, Zapletalova M, Svobodova I, Bajciova V, Mudry P, Smelhaus V, et al. Childhood cancer epidemiology in the Czech Republic (1994-2016). Cancer Epidemiol. (2020) 69:101848. doi: 10.1016/j.canep.2020.101848
179. Leong E, Ong SK, Jali F, Ramlee N. Childhood cancer survival in Brunei Darussalam. Asian Pac J Cancer Prev. (2020) 21:3259–66. doi: 10.31557/APJCP.2020.21.11.3259
180. Liu AP, Tung JY, Ku DT, Luk CW, Ling AS, Kwong DL, et al. Outcome of Chinese children with craniopharyngioma: a 20-year population-based study by the Hong Kong Pediatric Hematology/Oncology Study Group. Childs Nerv Syst. (2020) 36:497–505. doi: 10.1007/s00381-019-04480-x
181. Martin E, Coert JH, Flucke UE, Slooff WM, van de Sande MAJ, van Noesel MM, et al. Neurofibromatosis-associated malignant peripheral nerve sheath tumors in children have a worse prognosis: a nationwide cohort study. Pediatr Blood Cancer. (2020) 67:e28138. doi: 10.1002/pbc.28138
182. Mitchell HK, Morris M, Ellis L, Abrahão R, Bonaventure A. Racial/ethnic and socioeconomic survival disparities for children and adolescents with central nervous system tumours in the United States, 2000-2015. Cancer Epidemiol. (2020) 64:101644. doi: 10.1016/j.canep.2019.101644
183. Moreno F, Rose A, Chaplin MA, Cipolla MC, García Lombardi M, Nana M, et al. Childhood liver tumors in Argentina: incidence trend and survival by treatment center. A report from the national pediatric cancer registry, ROHA network 2000-2015. Pediatr Blood Cancer. (2020) 67:e28583. doi: 10.1002/pbc.28583
184. Paapsi K, Baburin A, Mikkel S, Mägi M, Saks K, Innos K. Childhood cancer incidence and survival trends in Estonia (1970-2016): a nationwide population-based study. BMC Cancer. (2020) 20:30. doi: 10.1186/s12885-019-6510-7
185. Siegel DA, Richardson LC, Henley SJ, Wilson RJ, Dowling NF, Weir HK, et al. Pediatric cancer mortality and survival in the United States, 2001-2016. Cancer. (2020) 126:4379–89. doi: 10.1002/cncr.33080
186. Silva FFD, Latorre MDRDO. Survival from acute lymphocytic leukemia in children in the city of São Paulo, Brazil. Cad Saude Publica. (2020) 36:e00008019. doi: 10.1590/0102-311x00008019
187. Tas ML, Reedijk AMJ, Karim-Kos HE, Kremer LCM, van de Ven CP, Dierselhuis MP, et al. Neuroblastoma between 1990 and 2014 in the Netherlands: increased incidence and improved survival of high-risk neuroblastoma. Eur J Cancer. (2020) 124:47–55. doi: 10.1016/j.ejca.2019.09.025
188. Wang X, Feng J, Li Z, Zhang X, Chen J, Feng G. Characteristics and prognosis of embryonal rhabdomyosarcoma in children and adolescents: an analysis of 464 cases from the SEER database. Pediatr Investig. (2020) 4:242–9. doi: 10.1002/ped4.12220
189. Williams LA, Frazier AL, Poynter JN. Survival differences by race/ethnicity among children and adolescents diagnosed with germ cell tumors. Int J Cancer. (2020) 146:2433–41. doi: 10.1002/ijc.32569
190. Youlden DR, Jones BC, Cundy TP, Karpelowsky J, Aitken JF, McBride CA. Incidence and outcomes of neuroblastoma in Australian children: a population-based study (1983-2015). J Paediatr Child Health. (2020) 56:1046–52. doi: 10.1111/jpc.14810
191. Abrahão R, Ribeiro RC, Lichtensztajn DY, Rosenberg AS, Keegan THM. Survival after diffuse large B-cell lymphoma among children, adolescents, and young adults in California, 2001-2014: a population-based study. Pediatr Blood Cancer. (2019) 66:e27559. doi: 10.1002/pbc.27559
192. Bidwell SS, Peterson CC, Demanelis K, Zarins KR, Meza R, Sriplung H, et al. Childhood cancer incidence and survival in Thailand: a comprehensive population-based registry analysis, 1990-2011. Pediatr Blood Cancer. (2019) 66:e27428. doi: 10.1002/pbc.27428
193. Chen X, Pan J, Wang S, Hong S, Hong S, He S. The epidemiological trend of acute myeloid leukemia in childhood: a population-based analysis. J Cancer. (2019) 10:4824–35. doi: 10.7150/jca.32326
194. Drevinskaite M, Patasius A, Kincius M, Jievaltas M, Smailyte G. A population-based analysis of incidence, mortality, and survival in testicular cancer patients in Lithuania. Medicina. (2019) 55:552. doi: 10.3390/medicina55090552
195. Faltermeier C, Chai T, Syed S, Lau N, Elkaim L, Ibrahim G, et al. Survival of infants ≤ 24 months of age with brain tumors: a population-based study using the SEER database. PLoS ONE. (2019) 14:e0223051. doi: 10.1371/journal.pone.0223051
196. Feng J, Polychronidis G, Heger U, Frongia G, Mehrabi A, Hoffmann K. Incidence trends and survival prediction of hepatoblastoma in children: a population-based study. Cancer Commun. (2019) 39:62. doi: 10.1186/s40880-019-0411-7
197. Janz TA, Nagasubramanian R, Wei JL. A survival analysis of pediatric tonsillar malignancies and review of a rare case. Int J Pediatr Otorhinolaryngol. (2019) 118:160–4. doi: 10.1016/j.ijporl.2018.12.033
198. Kang JM, Ha J, Hong EK, Ju HY, Park BK, Shin SH, et al. A nationwide, population-based epidemiologic study of childhood brain tumors in Korea, 2005-2014: a comparison with United States data. Cancer Epidemiol Biomarkers Prev. (2019) 28:409–16. doi: 10.1158/1055-9965.EPI-18-0634
199. Ostrom QT, Gittleman H, Kruchko C, Barnholtz-Sloan JS. Primary brain and other central nervous system tumors in Appalachia: regional differences in incidence, mortality, and survival. J Neurooncol. (2019) 142:27–38. doi: 10.1007/s11060-018-03073-z
200. Quinlan CS, Capra M, Dempsey M. Paediatric malignant melanoma in Ireland: a population study and review of the literature. J Plast Reconstr Aesthet Surg. (2019) 72:1388–95. doi: 10.1016/j.bjps.2019.03.041
201. Reedijk AMJ, Klein K, Coebergh JWW, Kremer LC, Dinmohamed AG, de Haas V, et al. Improved survival for children and young adolescents with acute myeloid leukemia: a Dutch study on incidence, survival and mortality. Leukemia. (2019) 33:1349–59. doi: 10.1038/s41375-018-0314-7
202. Rivas-Vilela S, Rubió-Casadevall J, Fàbrega-Ribas A, Joly-Torta C, Vilardell L, Marcos-Gragera R. Incidence and survival of central nervous system tumors in childhood and adolescence in Girona (Spain) 1990-2013: national and international comparisons. Clin Transl Oncol. (2019) 21:1177–85. doi: 10.1007/s12094-019-02043-9
203. Siegel DA Li J, Ding H, Singh SD, King JB, Pollack LA. Racial and ethnic differences in survival of pediatric patients with brain and central nervous system cancer in the United States. Pediatr Blood Cancer. (2019) 66:e27501. doi: 10.1002/pbc.27501
204. Tabash MA. Characteristics, survival and incidence rates and trends of pilocytic astrocytoma in children in the United States; SEER-based analysis. J Neurol Sci. (2019) 400:148–52. doi: 10.1016/j.jns.2019.03.028
205. Truitt G, Gittleman H, Leece R, Ostrom QT, Kruchko C, Armstrong TS, et al. Partnership for defining the impact of 12 selected rare CNS tumors: a report from the CBTRUS and the NCI-CONNECT. J Neurooncol. (2019) 144:53–63. doi: 10.1007/s11060-019-03215-x
206. Cooney T, Fisher PG, Tao L, Clarke CA, Partap S. Pediatric neuro-oncology survival disparities in California. J Neurooncol. (2018) 138:83–97. doi: 10.1007/s11060-018-2773-0
207. Deng X, Yang Z, Zhang X, Lin D, Xu X, Lu X, et al. Prognosis of pediatric patients with pineoblastoma: a SEER analysis 1990-2013. World Neurosurg. (2018) 118:e871–9. doi: 10.1016/j.wneu.2018.07.079
208. Eggen CAM, Durgaram VVL, van Doorn R, Mooi WJ, Pardo LM, Pasmans SGMA, et al. Incidence and relative survival of melanoma in children and adolescents in the Netherlands, 1989-2013. J Eur Acad Dermatol Venereol. (2018) 32:956–61. doi: 10.1111/jdv.14665
209. González García H, Garrote Molpeceres R, Urbaneja Rodríguez E, Gutiérrez Meléndez P, Herráiz Cristóbal R, Pino Vázquez MA. Differences in incidence and survival to childhood cancer between rural and urban areas in Castilla y León, Spain (2003-2014): a strobe-compliant study. Medicine. (2018) 97:e12797. doi: 10.1097/MD.0000000000012797
210. Khalid SI, Kelly R, Adogwa O, Carlton A, Woodward J, Ahmed S, et al. Pediatric spinal ependymomas: an epidemiologic study. World Neurosurg. (2018) 115:e119–28. doi: 10.1016/j.wneu.2018.03.206
211. Marcos-Gragera R, Solans M, Galceran J, Fernández-Delgado R, Fernández-Teijeiro A, Mateos A, et al. Childhood and adolescent lymphoma in Spain: incidence and survival trends over 20 years. Clin Transl Oncol. (2018) 20:1289–301. doi: 10.1007/s12094-018-1860-1
212. Mazzucco W, Cusimano R, Mazzola S, Rudisi G, Zarcone M, Marotta C, et al. Childhood and adolescence cancers in the Palermo Province (Southern Italy): ten years (2003–2012) of epidemiological surveillance. Int J Environ Res Public Health. (2018) 15:1344. doi: 10.3390/ijerph15071344
213. Parikh PP, Tashiro J, Rubio GA, Sola JE, Neville HL, Hogan AR, et al. Incidence and outcomes of pediatric extremity melanoma: a propensity score matched SEER study. J Pediatr Surg. (2018) 53:1753–60. doi: 10.1016/j.jpedsurg.2018.03.006
214. Ramirez O, Aristizabal P, Zaidi A, Ribeiro RC, Bravo LE; VIGICANCER Working Group. Implementing a childhood cancer outcomes surveillance system within a population-based cancer registry. J Glob Oncol. (2018) 4:1–11. doi: 10.1200/JGO.17.00193
215. Smith L, Glaser AW, Kinsey SE, Greenwood DC, Chilton L, Moorman AV, et al. Long-term survival after childhood acute lymphoblastic leukaemia: population-based trends in cure and relapse by clinical characteristics. Br J Haematol. (2018) 182:851–8. doi: 10.1111/bjh.15424
216. Strahlendorf C, Pole JD, Barber R, Dix D, Kulkarni K, Martineau E, et al. Enrolling children with acute lymphoblastic leukaemia on a clinical trial improves event-free survival: a population-based study. Br J Cancer. (2018) 118:744–9. doi: 10.1038/bjc.2017.462
217. Wu J, Sun H, Li J, Guo Y, Zhang K, Lang C, et al. Increased survival of patients aged 0-29 years with osteosarcoma: a period analysis, 1984-2013. Cancer Med. (2018) 7:3652–61. doi: 10.1002/cam4.1659
218. Zhao Y, Wang Y, Ma S. Racial differences in four leukemia subtypes: comprehensive descriptive epidemiology. Sci Rep. (2018) 8:548. doi: 10.1038/s41598-017-19081-4
219. Bergqvist J, Iderberg H, Mesterton J, Bengtsson N, Wettermark B, Henriksson R. Healthcare resource use, comorbidity, treatment and clinical outcomes for patients with primary intracranial tumors: a Swedish population-based register study. Acta Oncol. (2017) 56:405–14. doi: 10.1080/0284186X.2016.1257864
220. El-Fattah MA. Survival pattern of chronic myeloid leukemia in a pediatric population in the United States. J Pediatr Hematol Oncol. (2017) 39:159–60. doi: 10.1097/MPH.0000000000000693
221. Fuentes-Raspall R, Solans M, Roca-Barceló A, Vilardell L, Puigdemont M, Del Barco S, et al. Descriptive epidemiology of primary malignant and non-malignant central nervous tumors in Spain: results from the Girona Cancer Registry (1994-2013). Cancer Epidemiol. (2017) 50(Pt A):1–8. doi: 10.1016/j.canep.2017.07.005
222. Jakab Z, Juhasz A, Nagy C, Schuler D, Garami M; Hungarian Paediatric Haemato-Oncology Network. Trends and territorial inequalities of incidence and survival of childhood leukaemia and their relations to socioeconomic status in Hungary, 1971-2015. Eur J Cancer Prev. (2017) 26:S183–90. doi: 10.1097/CEJ.0000000000000386
223. Kairiene I, Pasauliene R, Lipunova N, Vaitkeviciene G, Rageliene L, Rascon J. Improved outcome of childhood acute myeloid leukemia in an Eastern European country: Lithuanian experience. Eur J Pediatr. (2017) 176:1329–37. doi: 10.1007/s00431-017-2978-9
224. Khanna V, Achey RL, Ostrom QT, Block-Beach H, Kruchko C, Barnholtz-Sloan JS, et al. Incidence and survival trends for medulloblastomas in the United States from 2001 to 2013. J Neurooncol. (2017) 135:433–41. doi: 10.1007/s11060-017-2594-6
225. Lee SH, Jung KW, Ha J, Oh CM, Kim H, Park HJ, et al. Nationwide population-based incidence and survival rates of malignant central nervous system germ cell tumors in Korea, 2005-2012. Cancer Res Treat. (2017) 49:494–501. doi: 10.4143/crt.2016.129
226. Lins MM, Santos MO, de Albuquerque MFPM, de Castro CCL, Mello MJG, de Camargo B. Incidence and survival of childhood leukemia in Recife, Brazil: a population-based analysis. Pediatr Blood Cancer. (2017) 64:26391. doi: 10.1002/pbc.26391
227. Marcos-Gragera R, Galceran J, Martos C, de Munain AL, Vicente-Raneda M, Navarro C, et al. Incidence and survival time trends for Spanish children and adolescents with leukaemia from 1983 to 2007. Clin Transl Oncol. (2017) 19:301–16. doi: 10.1007/s12094-016-1531-z
228. Moreno F, Cacciavillano W, Cipolla M, Coirini M, Streitenberger P, López Martí J, et al. Childhood osteosarcoma: incidence and survival in Argentina. Report from the National Pediatric Cancer Registry, ROHA Network 2000-2013. Pediatr Blood Cancer. (2017) 64:26533. doi: 10.1002/pbc.26533
229. Mukhtar F, Boffetta P, Risch HA, Park JY, Bubu OM, Womack L, et al. Survival predictors of Burkitt's lymphoma in children, adults and elderly in the United States during 2000-2013. Int J Cancer. (2017) 140:1494–502. doi: 10.1002/ijc.30576
230. Nasioudis D, Alevizakos M, Holcomb K, Witkin S. Malignant and borderline epithelial ovarian tumors in the pediatric and adolescent population. Maturitas. (2017) 96:45–50. doi: 10.1016/j.maturitas.2016.11.011
231. Offor UT, Basta NO, James PW, McNally RJQ. Is there a socioeconomic variation in survival from renal tumours in children and young people resident in northern England (1968-2012)? Cancer Epidemiol. (2017) 50(Pt A):92–8. doi: 10.1016/j.canep.2017.08.010
232. Paapsi K, Mägi M, Mikkel S, Saks K, Aareleid T, Innos K. The impact of under-reporting of cases on the estimates of childhood cancer incidence and survival in Estonia. Eur J Cancer Prev. (2017) 26:S147–52. doi: 10.1097/CEJ.0000000000000377
233. Ramiandrisoa J, Jehanne M, Sauvat F, Reguerre Y, Chamouine A, Chirpaz E. Incidence and survival of childhood cancer in the French islands of Reunion and Mayotte (2005-2011). Cancer Epidemiol. (2017) 49:61–5. doi: 10.1016/j.canep.2017.05.009
234. Schindler M, Belle FN, Grotzer MA, von der Weid NX, Kuehni CE, Swiss Paediatric Oncology Group (SPOG). Childhood cancer survival in Switzerland (1976-2013): time-trends and predictors. Int J Cancer. (2017) 140:62–74. doi: 10.1002/ijc.30434
235. Weil AG, Wang AC, Westwick HJ, Ibrahim GM, Ariani RT, Crevier L, et al. Survival in pediatric medulloblastoma: a population-based observational study to improve prognostication. J Neurooncol. (2017) 132:99–107. doi: 10.1007/s11060-016-2341-4
236. Ye X, Torabi M, Lix LM, Mahmud SM. Time and spatial trends in lymphoid leukemia and lymphoma incidence and survival among children and adolescents in Manitoba, Canada: 1984-2013. PLoS ONE. (2017) 12:e0175701. doi: 10.1371/journal.pone.0175701
237. Acharya S, Hsieh S, Shinohara ET, DeWees T, Frangoul H, Perkins SM. Effects of race/ethnicity and socioeconomic status on outcome in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. (2016) 38:350–4. doi: 10.1097/MPH.0000000000000591
238. Batista A, Nogueira-Silva L, Vaz R, Oliveira J. Oncology referral concerning paediatric neurosurgery? Analysis of the situation between 2008-2013 in the North of Portugal. Acta Med Port. (2016) 29:15–23. doi: 10.20344/amp.6432
239. Brennan B, Stiller C, Grimer R, Dennis N, Broggio J, Francis M. Outcome and the effect of age and socioeconomic status in 1318 patients with synovial sarcoma in the English National Cancer Registry: 1985-2009. Clin Sarcoma Res. (2016) 6:18. doi: 10.1186/s13569-016-0058-y
240. Desandes E, Guissou S, Ducassou S, Lacour B. Neonatal solid tumors: incidence and survival in France. Pediatr Blood Cancer. (2016) 63:1375–80. doi: 10.1002/pbc.26006
241. Donnelly DW, Gavin AT. Mortality among children and young people who survive cancer In Northern Ireland. Ulster Med J. (2016) 85:158–63.
242. Fairley L, Picton SV, McNally RJ, Bailey S, McCabe MG, Feltbower RG. Incidence and survival of children and young people with central nervous system embryonal tumours in the North of England, 1990-2013. Eur J Cancer. (2016) 61:36–43. doi: 10.1016/j.ejca.2016.03.083
243. Janitz AE, Campbell JE, Pate A, Anderson A, McNall-Knapp R. Racial, ethnic, and age differences in the incidence and survival of childhood cancer in Oklahoma, 1997-2012. J Okla State Med Assoc. (2016) 109:355–65.
244. Kaatsch P, Strothotte J, Becker C, Bielack S, Dirksen U, Blettner M. Pediatric bone tumors in Germany from 1987 to 2011: incidence rates, time trends and survival. Acta Oncol. (2016) 55:1145–51. doi: 10.1080/0284186X.2016.1195509
245. Kahn JM, Keegan TH, Tao L, Abrahão R, Bleyer A, Viny AD. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. (2016) 122:2723–30. doi: 10.1002/cncr.30089
246. Karim-Kos HE, Hackl M, Mann G, Urban C, Woehrer A, Slavc I, et al. Trends in incidence, survival and mortality of childhood and adolescent cancer in Austria, 1994-2011. Cancer Epidemiol. (2016) 42:72–81. doi: 10.1016/j.canep.2016.03.015
247. Lychou SE, Gustafsson GG, Ljungman GE. Higher rates of metastatic disease may explain the declining trend in Swedish paediatric rhabdomyosarcoma survival rates. Acta Paediatr. (2016) 105:74–81. doi: 10.1111/apa.13172
248. Mogensen H, Modig K, Tettamanti G, Talbäck M, Feychting M. Socioeconomic differences in cancer survival among Swedish children. Br J Cancer. (2016) 114:118–24. doi: 10.1038/bjc.2015.449
249. Moreno F, Lopez Marti J, Palladino M, Lobos P, Gualtieri A, Cacciavillano W. Childhood neuroblastoma: incidence and survival in Argentina. Report from the National Pediatric Cancer Registry, ROHA Network 2000-2012. Pediatr Blood Cancer. (2016) 63:1362–7. doi: 10.1002/pbc.25987
250. Park HJ, Moon EK, Yoon JY, Oh CM, Jung KW, Park BK, et al. Incidence and survival of childhood cancer in Korea. Cancer Res Treat. (2016) 48:869–82. doi: 10.4143/crt.2015.290
251. Rigaud C, Barkaoui MA, Thomas C, Bertrand Y, Lambilliotte A, Miron J, et al. Langerhans cell histiocytosis: therapeutic strategy and outcome in a 30-year nationwide cohort of 1478 patients under 18 years of age. Br J Haematol. (2016) 174:887–98. doi: 10.1111/bjh.14140
252. Schrøder H, Rechnitzer C, Wehner PS, Rosthøj S, Møller JK, Lausen B, et al. Danish childhood cancer registry. Clin Epidemiol. (2016) 8:461–4. doi: 10.2147/CLEP.S99508
253. Wang Y, Huang J, Rong L, Wu P, Kang M, Zhang X, et al. Impact of age on the survival of pediatric leukemia: an analysis of 15083 children in the SEER database. Oncotarget. (2016) 7:83767–74. doi: 10.18632/oncotarget.11765
254. Abrahão R, Lichtensztajn DY, Ribeiro RC, Marina NM, Keogh RH, Marcos-Gragera R, et al. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988-2011: a population-based observational study. Pediatr Blood Cancer. (2015) 62:1819–25. doi: 10.1002/pbc.25544
255. Dolecek TA, Dressler EV, Thakkar JP, Liu M, Al-Qaisi A, Villano JL. Epidemiology of meningiomas post-Public Law 107-206: the Benign Brain Tumor Cancer Registries Amendment Act. Cancer. (2015) 121:2400–10. doi: 10.1002/cncr.29379
256. Dudley RW, Torok MR, Gallegos DR, Mulcahy-Levy JM, Hoffman LM, Liu AK, et al. Pediatric low-grade ganglioglioma: epidemiology, treatments, and outcome analysis on 348 children from the Surveillance, Epidemiology, and End Results database. Neurosurgery. (2015) 76:313–20. doi: 10.1227/NEU.0000000000000619
257. Foresto SA, Youlden DR, Baade PD, Hallahan AR, Aitken JF, Moore AS. The outcomes and treatment burden of childhood acute myeloid leukaemia in Australia, 1997-2008: a report from the Australian Paediatric Cancer Registry. Pediatr Blood Cancer. (2015) 62:1664–166. doi: 10.1002/pbc.25517
258. Kaatsch P, Häfner C, Calaminus G, Blettner M, Tulla M. Pediatric germ cell tumors from 1987 to 2011: incidence rates, time trends, and survival. Pediatrics. (2015) 135:e136–43. doi: 10.1542/peds.2014-1989
259. Katz AJ, Chia VM, Schoonen WM, Kelsh MA. Acute lymphoblastic leukemia: an assessment of international incidence, survival, and disease burden. Cancer Causes Control. (2015) 26:1627–42. doi: 10.1007/s10552-015-0657-6
260. Liu AP, Shing MM, Yuen HL Li CH, Ling SC, Luk CW, et al. Central nervous system tumors in Chinese children under the age of 3: a population study. J Pediatr Hematol Oncol. (2015) 37:94–103. doi: 10.1097/MPH.0000000000000128
261. Olsson DS, Andersson E, Bryngelsson IL, Nilsson AG, Johannsson G. Excess mortality and morbidity in patients with craniopharyngioma, especially in patients with childhood onset: a population-based study in Sweden. J Clin Endocrinol Metab. (2015) 100:467–74. doi: 10.1210/jc.2014-3525
262. Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, et al. Alex's Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. (2015) 16:x1–36. doi: 10.1093/neuonc/nou327
263. Truong B, Green AL, Friedrich P, Ribeiro KB, Rodriguez-Galindo C. Ethnic, racial, and socioeconomic disparities in retinoblastoma. JAMA Pediatr. (2015) 169:1096–104. doi: 10.1001/jamapediatrics.2015.2360
264. Tulla M, Berthold F, Graf N, Rutkowski S, von Schweinitz D, Spix C, et al. Incidence, trends, and survival of children with embryonal tumors. Pediatrics. (2015) 136:e623–32. doi: 10.1542/peds.2015-0224
265. Vlenterie M, Ho VK, Kaal SE, Vlenterie R, Haas R, van der Graaf WT. Age as an independent prognostic factor for survival of localised synovial sarcoma patients. Br J Cancer. (2015) 113:1602–6. doi: 10.1038/bjc.2015.375
266. Wang L, Bhatia S, Gomez SL, Yasui Y. Differential inequality trends over time in survival among US children with acute lymphoblastic leukemia by race/ethnicity, age at diagnosis, and sex. Cancer Epidemiol Biomarkers Prev. (2015) 24:1781–8. doi: 10.1158/1055-9965.EPI-15-0639
267. Waxweiler TV, Rusthoven CG, Proper MS, Cost CR, Cost NG, Donaldson N, et al. Non-rhabdomyosarcoma soft tissue sarcomas in children: a Surveillance, Epidemiology, and End Results analysis validating COG risk stratifications. Int J Radiat Oncol Biol Phys. (2015) 92:339–48. doi: 10.1016/j.ijrobp.2015.02.007
268. Wongmas P, Jetsrisuparb A, Komvilaisak P, Suwanrungruang K, Choeyprasert W, Sriplung H, et al. Incidences, trends and long term outcomes of retinoblastoma in three cancer registries, Thailand. Asian Pac J Cancer Prev. (2015) 16:6899–902. doi: 10.7314/APJCP.2015.16.16.6899
269. Youlden DR, Baade PD, Hallahan AR, Valery PC, Green AC, Aitken JF. Conditional survival estimates for childhood cancer in Australia, 2002-2011: a population-based study. Cancer Epidemiol. (2015) 39:394–400. doi: 10.1016/j.canep.2015.02.008
270. Zheng R, Peng X, Zeng H, Zhang S, Chen T, Wang H, et al. Incidence, mortality and survival of childhood cancer in China during 2000-2010 period: a population-based study. Cancer Lett. (2015) 363:176–80. doi: 10.1016/j.canlet.2015.04.021
271. Desandes E, Guissou S, Chastagner P, Lacour B. Incidence and survival of children with central nervous system primitive tumors in the French National Registry of Childhood Solid Tumors. Neuro Oncol. (2014) 16:975–83. doi: 10.1093/neuonc/not309
272. Gerth DJ, Tashiro J, Thaller SR. Pediatric sinonasal tumors in the United States: incidence and outcomes. J Surg Res. (2014) 190:214–20. doi: 10.1016/j.jss.2014.04.004
273. Golpanian S, Tashiro J, Gerth DJ, Thaller SR. Pediatric histiocytoses in the United States: incidence and outcomes. J Surg Res. (2014) 190:221–9. doi: 10.1016/j.jss.2014.03.063
274. Hassan WM, Alfaar AS, Bakry MS, Ezzat S. Orbital tumors in USA: difference in survival patterns. Cancer Epidemiol. (2014) 38:515–22. doi: 10.1016/j.canep.2014.07.001
275. Ito Y, Miyashiro I, Ito H, Hosono S, Chihara D, Nakata-Yamada K, et al. Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci. (2014) 105:1480–6. doi: 10.1111/cas.12525
276. Kerkhofs TM, Ettaieb MH, Verhoeven RH, Kaspers GJ, Tissing WJ, Loeffen J, et al. Adrenocortical carcinoma in children: first population-based clinicopathological study with long-term follow-up. Oncol Rep. (2014) 32:2836–44. doi: 10.3892/or.2014.3506
277. Lacour B, Goujon S, Guissou S, Guyot-Goubin A, Desmée S, Désandes E, et al. Childhood cancer survival in France, 2000-2008. Eur J Cancer Prev. (2014) 23:449–57. doi: 10.1097/CEJ.0000000000000006
278. Ma H, Sun H, Sun X. Survival improvement by decade of patients aged 0-14 years with acute lymphoblastic leukemia: a SEER analysis. Sci Rep. (2014) 4:4227. doi: 10.1038/srep04227
279. Ostrom QT, Chen Y, M de Blank P, Ondracek A, Farah P, Gittleman H, et al. The descriptive epidemiology of atypical teratoid/rhabdoid tumors in the United States, 2001-2010. Neuro Oncol. (2014) 16:1392–9. doi: 10.1093/neuonc/nou090
280. Park SJ, Woo SJ, Park KH. Incidence of retinoblastoma and survival rate of retinoblastoma patients in Korea using the Korean National Cancer Registry database (1993-2010). Invest Ophthalmol Vis Sci. (2014) 55:2816–21. doi: 10.1167/iovs.14-14078
281. Vaitkevičiene G, Matuzevičiene R, Stoškus M, Žvirblis T, Rageliene L, Schmiegelow K. Cure rates of childhood acute lymphoblastic leukemia in Lithuania and the benefit of joining international treatment protocol. Medicina. (2014) 50:28–36. doi: 10.1016/j.medici.2014.05.005
282. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. (2014) 64:83–103. doi: 10.3322/caac.21219
283. Wiangnon S, Jetsrisuparb A, Komvilaisak P, Suwanrungruang K. Childhood cancer incidence and survival 1985-2009, Khon Kaen, Thailand. Asian Pac J Cancer Prev. (2014) 15:7989–93. doi: 10.7314/APJCP.2014.15.18.7989
284. Yang L, Takimoto T, Fujimoto J. Prognostic model for predicting overall survival in children and adolescents with rhabdomyosarcoma. BMC Cancer. (2014) 14:654. doi: 10.1186/1471-2407-14-654
285. AIRTUM Working Group, CCM, AIEOP Working Group. Italian cancer figures, report 2012: cancer in children and adolescents. Epidemiol Prev. (2013) 37(1 Suppl 1):1–225.
286. Bravo LE, García LS, Collazos P, Aristizabal P, Ramirez O. Descriptive epidemiology of childhood cancer in Cali: Colombia 1977-2011. Colomb Med. (2013) 44:155–64. doi: 10.25100/cm.v44i3.1243
287. Njoku K, Basta N, Mann KD, McNally RJ, Pearce MS. Socioeconomic variation in survival from childhood leukaemia in northern England, 1968-2010. Br J Cancer. (2013) 108:2339–45. doi: 10.1038/bjc.2013.222
288. Rabinowicz R, Barchana M, Liphshiz I, Linn S, Futerman B, Ben-Arush MW. Cancer incidence and survival among infants in Israel, 1998-2007. Pediatr Hematol Oncol. (2013) 30:646–54. doi: 10.3109/08880018.2013.813099
289. Valery PC, Youlden DR, Baade PD, Ward LJ, Green AC, Aitken JF. Cancer survival in Indigenous and non-Indigenous Australian children: what is the difference? Cancer Causes Control. (2013) 24:2099–106. doi: 10.1007/s10552-013-0287-9
290. Villano JL, Parker CK, Dolecek TA. Descriptive epidemiology of ependymal tumours in the United States. Br J Cancer. (2013) 108:2367–71. doi: 10.1038/bjc.2013.221
291. Bao PP, Zheng Y, Wu CX, Peng P, Gong YM, Huang ZZ, et al. Population-based survival for childhood cancer patients diagnosed during 2002-2005 in Shanghai, China. Pediatr Blood Cancer. (2012) 59:657–61. doi: 10.1002/pbc.24043
292. Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. (2012) 119:34–43. doi: 10.1182/blood-2011-04-347872
293. Goggins WB, Lo FF. Racial and ethnic disparities in survival of US children with acute lymphoblastic leukemia: evidence from the SEER database 1988-2008. Cancer Causes Control. (2012) 23:737–43. doi: 10.1007/s10552-012-9943-8
294. Jung KW, Yoo H, Kong HJ, Won YJ, Park S, Lee SH. Population-based survival data for brain tumors in Korea. J Neurooncol. (2012) 109:301–7. doi: 10.1007/s11060-012-0893-5
295. Kachanov DY, Dobrenkov KV, Abdullaev RT, Shamanskaya TV, Varfolomeeva SR. Incidence and survival of pediatric soft tissue sarcomas in Moscow Region, Russian Federation, 2000-2009. Sarcoma. (2012) 2012:350806. doi: 10.1155/2012/350806
296. Pui CH, Pei D, Pappo AS, Howard SC, Cheng C, Sandlund JT, et al. Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children's Research Hospital, 1992 through 2007. J Clin Oncol. (2012) 30:2005–12. doi: 10.1200/JCO.2011.40.8617
297. Rabinowicz R, Barchana M, Liphshiz I, Futerman B, Linn S, Weyl-Ben-Arush M. Cancer incidence and survival among children and adolescents in Israel during the years 1998 to 2007. J Pediatr Hematol Oncol. (2012) 34:421–9. doi: 10.1097/MPH.0b013e31826157ce
298. Wiromrat P, Jetsrisuparb A, Komvilaisak P, Sirichativapee W, Kamsa-Ard S, Wiangnon S. Incidence and survival rates among pediatric osteogenic sarcoma cases in Khon Kaen, Thailand, 1985-2010. Asian Pac J Cancer Prev. (2012) 13:4281–4. doi: 10.7314/APJCP.2012.13.9.4281
299. AIRTUM Working Group. Italian cancer figures, report 2011: survival of cancer patients in Italy. Epidemiol Prev. (2011) 35(5–6 Suppl 3):1–200.
300. Feltbower RG, Siller C, Woodward E, McKinney PA, Picton SV, Joffe J, et al. Treatment and survival patterns for germ cell tumors among 13- to 24-year olds in Yorkshire, UK. Pediatr Blood Cancer. (2011) 56:282–8. doi: 10.1002/pbc.22794
301. Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. (2011) 103:714–376. doi: 10.1093/jnci/djr077
302. Ljungman G, Jakobson A, Behrendtz M, Ek T, Friberg LG, Hjalmars U, et al. Incidence and survival analyses in children with solid tumours diagnosed in Sweden between 1983 and 2007. Acta Paediatr. (2011) 100:750–7. doi: 10.1111/j.1651-2227.2010.02122.x
303. Perez EA, Kassira N, Cheung MC, Koniaris LG, Neville HL, Sola JE. Rhabdomyosarcoma in children: a SEER population based study. J Surg Res. (2011) 170:e243–51. doi: 10.1016/j.jss.2011.03.001
304. Walsh PM, Byrne J, Capra M, Comber H. Childhood cancer survival in Ireland: temporal, regional and deprivation-related patterns. Eur J Cancer. (2011) 47:1852–62. doi: 10.1016/j.ejca.2011.03.021
305. Youlden DR, Baade PD, Valery PC, Ward LJ, Green AC, Aitken JF. Differentials in survival for childhood cancer in Australia by remoteness of residence and area disadvantage. Cancer Epidemiol Biomarkers Prev. (2011) 20:1649–56. doi: 10.1158/1055-9965.EPI-11-0432
306. Baade PD, Youlden DR, Valery PC, Hassall T, Ward L, Green AC, et al. Population-based survival estimates for childhood cancer in Australia during the period 1997-2006. Br J Cancer. (2010) 103:1663–70. doi: 10.1038/sj.bjc.6605985
307. Peris-Bonet R, Salmerón D, Martínez-Beneito MA, Galceran J, Marcos-Gragera R, Felipe S, et al. Childhood cancer incidence and survival in Spain. Ann Oncol. (2010) 21(Suppl 3):iii103–10. doi: 10.1093/annonc/mdq092
308. Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. (2010) 28:2625–34. doi: 10.1200/JCO.2009.27.0421
309. Sultan I, Qaddoumi I, Rodríguez-Galindo C, Nassan AA, Ghandour K, Al-Hussaini M. Age, stage, and radiotherapy, but not primary tumor site, affects the outcome of patients with malignant rhabdoid tumors. Pediatr Blood Cancer. (2010) 54:35–40. doi: 10.1002/pbc.22285
310. Ellison LF De P, Mery LS, Grundy PE; Canadian Cancer Society's Steering Committee for Canadian Cancer Statistics. Canadian cancer statistics at a glance: cancer in children. CMAJ. (2009) 180:422–4. doi: 10.1503/cmaj.081155
311. Juárez-Ocaña S, Palma-Padilla V, González-Miranda G, Siordia-Reyes AG, López-Aguilar E, Aguilar-Martínez M, et al. Epidemiological and some clinical characteristics of neuroblastoma in Mexican children (1996-2005). BMC Cancer. (2009) 9:266. doi: 10.1186/1471-2407-9-266
312. Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990-2004. J Natl Cancer Inst. (2008) 100:1301–9. doi: 10.1093/jnci/djn276
313. Spix C, Spallek J, Kaatsch P, Razum O, Zeeb H. Cancer survival among children of Turkish descent in Germany 1980-2005: a registry-based analysis. BMC Cancer. (2008) 8:355. doi: 10.1186/1471-2407-8-355
314. Ellison LF, Pogany L, Mery LS. Childhood and adolescent cancer survival: a period analysis of data from the Canadian Cancer Registry. Eur J Cancer. (2007) 43:1967–75. doi: 10.1016/j.ejca.2007.05.014
315. Central Intelligence Agency. The World Factbook. Field Listing - Age Structure. (2025). Available online at: https://www.cia.gov/the-world-factbook/field/age-structure (Accessed January 22, 2025).
316. World Bank Data helpdesk. World Bank Country and Lending Groups. (2025). Available online at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (Accessed January 24, 2025).
317. Human Development Reports. Human Development Index. (2022). Available online at: https://hdr.undp.org/data-center/human-development-index#/indicies/HDI (Accessed January 22, 2025).
318. International Agency for Research on Cancer. Global Cancer Observatory. Cancer Today. Data & Methods. (2025). Available online at: https://gco.iarc.fr/today/en/data-sources-methods (Accessed January 24, 2025).
319. Parkin DM, Jemal A, Bray F, Korir AR, Kamaté B, Singh E, et al., editors. Cancer in Sub-Saharan Africa, Volume III. Geneva: Union for International Cancer Control (2019).
320. Adair T, Mikkelsen L, Hooper J, Badr A, Lopez AD. Assessing the policy utility of routine mortality statistics: a global classification of countries. Bull World Health Organ. (2023) 101:777–85. doi: 10.2471/BLT.22.289036
Appendix
PubMed search strategy
(infant, newborn[mh] OR newborn Infant*[tw] OR newborn*[tw] OR neonate*[tw] OR infant[mh] OR infan*[tw] OR child[mh] OR child*[tw] OR pediatric[tw] OR paediatric[tw])
AND
(neoplasms[mh] OR neoplas*[tw] OR tumor*[tw] OR tumour*[tw] OR cancer*[tw] OR malignan*[tw] OR benign neoplasm*[tw] OR leukaemia[tw] OR leukemia[tw] OR lymphoma[tw] OR sarcoma[tw] OR carcinoma[tw])
AND
(survival rate[mh] OR survival rate*[tw])
AND
nationwide[tw]
Web of Science search strategy
TS=((infants, newborn OR newborn infant* OR newborn* OR neonate* OR infant* OR child*)
AND
(neoplas* OR tumo$r* OR cancer* OR malignan* OR benign neoplasm*)
AND
(survival* OR survival stud* OR studies, survival OR survival rate*)
AND
(registr* OR population registr* OR population based OR populational based))
Keywords: childhood cancer, leukemia, survival, population-based, cancer registry, international
Citation: Stiller CA (2025) Global population-based childhood cancer survival in the 21st century: a scoping review. Front. Cancer Control Soc. 3:1572317. doi: 10.3389/fcacs.2025.1572317
Received: 06 February 2025; Accepted: 14 July 2025;
Published: 11 August 2025.
Edited by:
Vesna Zadnik, Institute of Oncology Ljubljana, SloveniaReviewed by:
Stefan Heinze, Nova Scotia Health Authority, CanadaJaime Shalkow, Cancer Center, ABC Medical Center, Mexico
Copyright © 2025 Stiller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles A. Stiller, Y2hhcmxlcy5zdGlsbGVyMUBuaHMubmV0
 Charles A. Stiller
Charles A. Stiller