- Euroapi, Haverhill, United Kingdom
This Perspective reflects on practices that lead to the development of high turnover cross-coupling catalytic cycles involving palladium. These processes are attractive for the manufacture of agrochemical, human or veterinary active ingredients, and in some respects are more sustainable than alternatives that use base metals at much higher loadings. The practices are structured in terms of important elements of the workflow for developing a cross-coupling for commercialization, and are discussed using the key metrics that drive catalytic efficiency and sustainability. Realistic opportunities and challenges for metal recovery and reuse are also among the messages discussed.
1 Introduction
A high level of understanding around the chemistry that characterizes its catalytic cycles has allowed the development of palladium catalysts that turnover at lower temperatures and more efficiently than base metal systems. Since the 1990s, when the switch from the use of nickel catalysis to the more tractable palladium systems gathered pace amongst industrial scientists (Negishi and de Meijere, 2002), the latter have come to dominate the cross-couplings used in active ingredient manufacture (Farina, 2023). Popular such cross-coupling reaction types are sp2−sp2 Suzuki−Miyaura, Sonogashira, Negishi or Kumada couplings, and sp2 Miyaura borylations. Whilst it is not unusual for base metal catalyzed conditions to be used in early development, low levels of side reactions can lead to its later usurping by a palladium-catalyzed variant, with superior functional group tolerance (Andersen et al., 2014; Aydin et al., 2024), or which offers higher turnover.
2 Discussion
2.1 Metrics supporting sustainable palladium-catalyzed cross-couplings
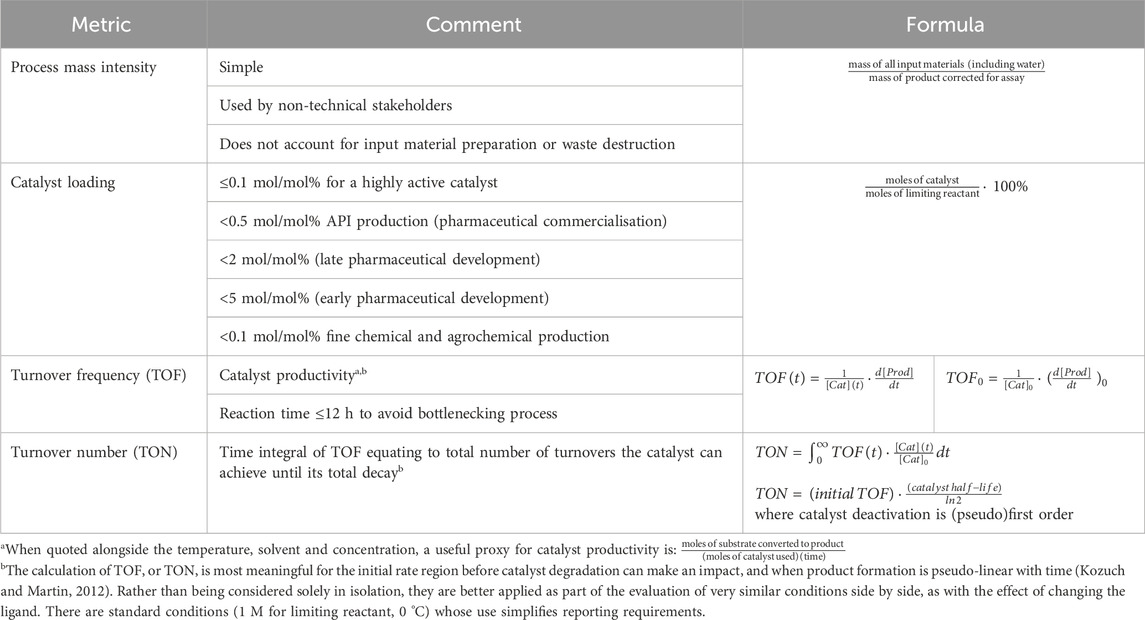
The development of high turnover palladium-catalyzed reactions very much falls under the rubric of green and sustainable chemistry. A high turnover system has been defined as one that does not use more than 0.1 mol/mol% of catalyst, when making human pharmaceuticals (Farina, 2004). Even lower loadings are routinely required for other active ingredients and fine chemicals (Table 1). As well as reducing the footprints associated with metal and ligand use, the lower the catalyst loading, the less resource is required to remove the metal from the product. Unlike the palladium itself, the ligand is an irrecoverable single use item. At a loading of 0.1 mol/mol%, the cost of the ligand makes a small but noticeable contribution to the cost target, albeit just for simple cross-coupling products. If the catalyst loading can be dropped by a further order or magnitude, the ligand makes an insignificant contribution (as does the palladium, even if not recovered), irrespective of its per kilogram cost and the wider cost of making the coupled product. Key metrics, including catalyst loading, used in the development of high turnover systems can be found in Table 1, along with comments on how they are used.
Sustainability metrics should not always be taken at face value, without further context, reflecting weaknesses in community-wide interpretations around their use. For example, there is no clear guidance around if and how the calculation of process mass intensity should account for any recycling or downcycling of solvent. Likewise, for a commercial process, an operation will often be in place to recover the palladium that makes up the catalyst loading. This can be credited back to the manufacturer’s account with the catalyst supplier, such that the net use of palladium is small compared to the original loading. Finally, an important source of palladium is as a byproduct of nickel and copper mining in Siberia and Canada (Auronum, 2023). A cradle-to-gate assessment of a cross-coupling process is dependent on the methodology used to assign impact between these coproduced metals, or indeed coproduced platinum group elements for palladium arising from South Africa (Nuss and Eckelman, 2014).
2.2 A high-level overview of catalyst selection
Negotiating the vast cross-coupling literature around palladium catalyst selection should bear some simple principles in mind. With some exceptions (Albino et al., 2023), heterogeneous catalysts rely on the leaching and recapture of palladium (Davies et al., 2001; Vásquez-Céspedes et al., 2021). The leaching rate will be difficult to reproduce with different batches of the catalyst, leading to variable reaction kinetics. These materials are generally unsuitable and should be avoided, as should immobilized homogeneous catalysts (Hübner et al., 2016). The latter react more slowly than their parent versions, wasting the effort used to immobilize the metal.
Where there is more than one catalytic cycle, any chemo- or regioselectivity will change as the reaction progresses, and deconvoluting their kinetics is challenging. Poorly characterized systems should also be approached with care. Palladium nanoparticles fall into this category, as do the cocktail of species arising from the use of palladium N-heterocyclic carbene complexes (Khazipov et al., 2018), and weakly associated dimeric complexes of Pd(0) and Pd(II). Unless stabilizing additives are used, nanoparticles can agglomerate into larger, less active particles that may even precipitate (De Vries, 2006), leading to challenges in reproducing their outstanding turnover numbers over the lifecycle of a manufacture. Where nanoparticles are detected by transmission electron microscopy, the basis on which palladium contributes to turnover numbers or frequencies should be transparently reported, given subsurface palladium might be expected to offer a bystander role.
We instead advocate for the use of a single and clearly defined species that operates via a single significant catalytic cycle. Figure 1 shows this with most possible interconversions, not all of which may be significant in any one system. Twelve-electron-based monocoordinated palladium(0) species are better able to participate in the catalytic cycle than more highly ligated versions (Firsan et al., 2022). If a palladium(0) species is the resting state, it is likely to be both oxygen-sensitive and sensitive to comproportionation with the precursor. In this case, pursuing the adoption of a bulky and sigma-donor rich phosphine offers equity in favoring the monoligated state and speeding up the initial oxidative addition.
![A diagram and graph illustrate a catalytic reaction mechanism. The diagram shows a cycle involving a catalyst (LM), catalyst activation and deactivation steps, and intermediate complexes like (LM)AB and (LM)A. Rate constants k1, k2, k3, activation and deactivation constants are marked. There's also an equation for the rate of change of AB concentration over time. Below, a graph plots [A] or [AB] in millimolar (black line) and catalyst concentration in millimolar (red line) over time in minutes. The graph shows catalyst activation and decomposition trends.](https://www.frontiersin.org/files/Articles/1635370/fctls-05-1635370-HTML-r1/image_m/fctls-05-1635370-g001.jpg)
Figure 1. A typical catalytic cycle (top) and reaction profile for a palladium catalyzed cross-coupling that involves the conversion of A + B → AB (bottom).
What follows are selective comments on the different parts of a high-level workflow that support the development of a high turnover and sustainable palladium cross-coupling process. The associated activities make use of the metrics detailed in Table 1.
2.3 Workflow
2.3.1 Familiarization
Performing tables’ worth of screening with a sole focus on yield consumes material whilst providing little understanding around what drives the formation of the product and impurities. Whilst screening supported by medium- and high-throughput automation has an important place to play (Mennen et al., 2019), an extensive period of familiarization with the inherited (baseline) conditions ensures subsequent studies are mechanism-driven. These activities, all performed using scrupulously clean starting materials, can provide quick hits as to how to lower the catalyst loading, modify the reactor design and improve manufacturing reliability, when the resourcing of a full optimization exercise has not been sanctioned.
Prework starts with constructing or reviewing an aspirational profile of the developed reaction. This will likely consist of the catalyst loading, process mass intensity, the reaction time (or otherwise a measure of productivity like turnover frequency) and a cost target for the stage.
Solvents shortlisted for reaction screening are based on solubility data for the reaction components, and allow the product to be crystallized in high purity after cooling, the addition of an antisolvent or a solvent swap. Based on a small number of measurements made in a variety of solvents, solubilities in dozens of solvents and their binary mixtures can be modelled (Dynochem resources, 2024). After winnowing out solvent options whose use presents environmental, toxicological, regulatory or handling issues, actual solubilities for solvents on the refined shortlist of candidates are measured. For products with high manufacturing volumes, the solvent should be chosen so it can be easily recovered in high purity by distillation or organic solvent nanofiltration. Since it is post-reaction solvent use that contributes most to the process mass intensity of most processes (Constable et al., 2007), starting solvent selection with the end in mind leads to modest process mass intensities. This approach extends to the exploration of solubilities in micellar media adulterated with an organic cosolvent, with a view to adopting such a medium for the reaction (Gallou et al., 2016; Lippincott et al., 2019). There are not strong drivers to invest in neoteric solvents or solventless reactions, and biorenewable solvents that are not being used in bulk by other industries are not cost-effective.
Replicates of the inherited (baseline) reaction conditions probe the reproducibility of the reaction and the analytical system. Further repeats, performed without each component in turn, confirm their necessity, and probe impurities and degradants that form in their absence. The reaction is profiled under baseline conditions, and at a lower concentration but with the same molar excess of one of the contributory materials, with and without doped in reaction product. Variable time normalization analysis of these profiles informs whether the catalyst is stable, works better or worse in the presence of the reaction product, or is slow to activate (Nielsen and Burés, 2019). Further “different excess” experimentation to establish working knowledge of the orders of the reaction components, including catalyst, ligand and pseudo(halide) byproduct, allows inferences to be made around the turnover-limiting step and the chemistry leading up to it (Blackmond, 2015). Assessing the impact of doping in 1,000 ppm water can be highly insightful for reactions that do not use bulk water. Where a bisphosphine is used in the baseline conditions, the impact on the rate of using an oxidative addition complex bearing this ligand and its corresponding monoxide should be assessed side-by-side (Yang et al., 2025). This informs whether the reaction is impartial to, activated or hindered by ligand oxidation.
2.3.2 Ligand screening
Any working knowledge of the mechanism and the rate expression of the baseline reaction, gained using the familiarization outlined above, allows the ligand to be engineered to accelerate the turnover-limiting step engineering. The mechanistic hypothesis also informs around the selection of the pKa of any base or the pH of an immiscible aqueous phase. Where there is computational modelling capability available, virtual screening of the use of actual or virtual (“dummy”) ligands, to minimize ΔG‡ for the turnover-limiting step, can be used to shortlist ligands for wet lab screening (Lu et al., 2022). A second approach performs wet lab screening on candidate monodentate phosphine, bisphosphine or bisphosphine monoxide ligands selected on the basis of steric and electronic descriptors of representative conformers. These descriptors should be mechanistically relevant, and the use of large off the shelf descriptor sets avoided. Univariate trend analysis, based on the singular importance of a single ligand property, is rarely possible. Instead, multivariate linear regression is used to build a quantitative relationship between the ligand properties and the reaction rate (Niemeyer et al., 2016; Crawford et al., 2022).
The wet lab screening referred to above should take place with at least four datapoints per run, to provide some measure of the reaction rate. To differentiate between moderate and high activity systems, half the amount of palladium used in process familiarization can be used. If the impact of ligand stoichiometry, solvent and base selection are being probed at the same time as the choice of ligand, the use of palladium precursors that rely on different mechanisms to access the on-cycle catalyst complicates control over the concentration and rate of formation of the on-cycle catalysts under study. An emerging solution that is available for some ligands is to charge the palladium and ligand as its oxidative addition complex with another aryl halide (King et al., 2021; Looby et al., 2025). This allows the desired ligand-palladium complex to be released in a controlled and close to quantitative fashion, characteristics that are particularly useful for turnover frequency measurements, given their dependence on the amount of oncycle catalyst. These complexes display good solubility, can be stable to storage, isolation by crystallization or chromatography and a number are available to buy for screening purposes or are increasingly being made inhouse (Sigma-Aldrich, 2025).
2.3.3 Optimization
With a decision made over which ligand, solvent and any base to use, attention shifts to how to access the on-cycle catalyst in the manufacturing environment. The advantages and disadvantages of different palladium precursors have been shared by BMS scientists (Ganley et al., 2025). Where a catalyst manufacturer has a license to supply a proprietary catalyst only required in ppm quantities, its use rapidly pays for itself over the use of a cheaper but less active commodity material. However, the former approach leads to a supply chain dependency on the license holder. Allowing the ligand and metal precursor, charged separately, to react in situ is cheaper on a per mole basis but has disadvantages over the use of a precatalyst in which the ligand is already bound. The latter offers better controlled activation to the on-cycle species. A precatalyst also eradicates the impact of variation in the relative amount of metal and ligand that makes it into the reaction solution, either due to weighing issues, holdup when they are charged to the reactor or differences in dissolution kinetics.
Precatalyst selection is informed by knowledge of the rate of precatalyst activation relative to the rate of the catalytic cycle (Figure 1, lower). The need for control over the rate of precatalyst activation is indicated where turnover increases when the precatalyst is dose-controlled. Alternatively, if we imagine a variant of Figure 1 where the catalyst activates more slowly than shown, by the end of the cross-coupling reaction any unactivated precatalyst is wasted. It is also important to build working knowledge of how, and at what rate, the on-cycle catalyst decomposes (Figure 1, lower). This decomposition process is typically affected by the choice of precatalyst, its activation and loading. For example, if the resting state reacts with itself or is inactivated by reaction with the catalyst precursor, the rate of catalyst activation will need to be controlled. Where catalyst decomposition has not taken place to a significant extent, the catalyst loading can be lowered, if this does not create a fresh bottleneck in the productivity of the overall process, and the reaction still reproducibly reaches completion.
Where the preferred ligand has been changed over studies performed to date, optimization also involves repeating much of the kinetics experimentation detailed above for familiarization. Different excess experimentation is used to establish revised understanding of the turnover-limiting step and the resting state. This understanding is enriched by saturation studies using each reaction component in large excess and spectroscopic characterization of the resting state. For Negishi or Kumada couplings, a full understanding of the kinetics (Figure 1) allows a non-standard reactor design to be proposed that controls impurities and hence the material and plant time that are otherwise spent downstream in removing the impurities from the product. All of the kinetic studies are most informative about relevant interconversions of palladium species, both productive and unproductive, if they can be performed at a process-relevant concentration. This concentration can be as high as 20 wt/wt% with respect to the substrate, where the target profile calls for a high space-time-yield, as with some agrochemicals (Ford and Fairlamb, 2025). When rationalizing any differences in behavior seen at different concentrations, it can be useful to capture the ratio between the number of moles of catalyst and the sum of moles of all species inputted into the reaction (Horbaczewskyj and Fairlamb, 2022).
The impact on catalyst turnover of using plant-grade reagents including nitrogen doped with a controlled level of oxygen should be assessed, before development concludes. This allows meaningful specifications for potential poisons to be set ahead of their use in the manufacturing environment. Where the reaction does not proceed at all below a certain catalyst loading, or if the turnover number drops with dilution, a trace catalyst poison is likely to be present.
2.3.4 Palladium removal and recycling
Contrary to published case studies related to partly optimized processes (Economidou et al., 2023), removing a high turnover palladium catalyst can be straightforward, though the development studies should be performed with levels of oxygen that mimic the plant manufacture. The use of a functionalized scavenger is not necessary, for such a catalyst. It is likely to be unacceptable from a cost of goods perspective and could even contaminate the product with leachables. During the crystallization of the product, the metal mostly remains in the crystallization mother liquors. Small amounts of soluble chelant additives like potassium isopropyl xanthate can be used to compete with the cross-coupling product for coordination to the palladium, where this is a problem (Ren et al., 2017). Where this is not possible, the palladium-bearing solution of product will be scavenged with carbon (Welch et al., 2005). The catalyst manufacturer incinerates the carbon to leave a residue enriched in palladium, which can be reused. It should be able to recover 80%–90% of the palladium charged in this way. Subjecting palladium-bearing waste solvent fractions, to organic solvent nanofiltration through a membrane, as is done at Evonik’s Lafayette facility, also provides a retentate fraction enriched in the metal. Whilst the above processing options limit the impact of palladium remediation on process mass intensity, using a water-soluble chelant to wash out the palladium from the product fraction in any aqueous workup (Economidou et al., 2023), makes a noticeable contribution to this metric. Unlike analogous remediations of a base metal species, the concentration of palladium is likely to be so low, the aqueous waste does not require metal removal prior to release to a watercourse, for the manufacturing site to comply with its environmental permit. A notable alternative to a chelant is to recover the palladium as an insoluble complex following treatment with bisulfite (Tu et al., 2019).
There are significant challenges that apply to the batch-to-batch recycling of a palladium catalyst (Fassbach et al., 2024). For pharmaceuticals, the associated quality system means it is not possible to reuse a catalyst which reacts at slower rate, gives lower yields or produces a slightly different impurity profile versus virgin material (ICH International Council for Harmonisation, 2000). Intricate testing for catalyst activity and impurities between runs could in theory be used to address this, and to address the financial risk of a batch failure using a recycled catalyst, but this testing again builds in costs. The above points become moot anyway in the case of a highly active catalyst. This is reactive and will not meaningfully survive a recovery process, such that it is not suitable for direct recycling. Material retrieved from many literature recycling studies is often catalyst precursor (Farina, 2004), which has failed to activate over the course of the reaction, or another species formed from the on-cycle catalyst.
3 Closing remarks
Key to a palladium-catalyzed cross-coupling used in active ingredient manufacture becoming sustainable and commercially viable is a high turnover catalyst that operates via a single catalytic cycle. This naturally allows access to a low loading of catalyst, and controls the footprint associated with the ligand, which will inevitably be the product of a multistep synthesis and, unlike the palladium, cannot be recovered after use. We also emphasize that it is better to develop outright a catalyst with a high turnover, than to try and recycle one with moderate activity (Gladysz, 2001). There is also merit, in the first instance, in decoupling the identification of the on-cycle catalyst, from the selection of conditions that lead to its practical formation from a shelf-stable precursor in the reaction environment. Achieving a low catalyst loading, without the need for a “kicker charge” of catalyst, requires intimate knowledge of the interconversion of the different palladium species present, including activation of a precursor material and catalyst deactivation. Regarding the last point, access to high turnover palladium catalysts requires more research into what and how they deactivate. Metrics that support the development of a high turnover system, alongside catalyst loading, are process mass intensity, catalyst turnover number and frequency. The most informative means of characterizing a palladium-catalyzed cross-coupling is not tabulated in Table 1. This is of course a form of the rate law, as shown in Figure 1, and knowledge of the observed rate constant and its dependence on temperature. On this note, we reflect that as process chemists, catalysis and automation specialists and data scientists collaborate on the development of the active ingredients of the future, their workflows are best informed by mechanism-driven hypotheses (Farina, 2023).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
AS: Conceptualization, Funding acquisition, Writing – review and editing, Writing – original draft, Visualization, Resources.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The author thanks Vittorio Farina for useful discussions.
Conflict of interest
Author AS was employed by Euroapi.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fctls.2025.1720991.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albino, M., Burden, T. J., Piras, C. C., Whitwood, A. C., Fairlamb, I. J. S., and Smith, D. K. (2023). Mechanically robust hybrid gel beads loaded with “naked” palladium nanoparticles as efficient, reusable, and sustainable catalysts for the Suzuki-Miyaura reaction. ACS Sustain. Chem. Eng. 11, 1678–1689. doi:10.1021/acssuschemeng.2c05484
Andersen, S. M., Bollmark, M., Berg, R., Fredriksson, C., Karlsson, S., Liljeholm, C., et al. (2014). A scalable route to 5-substituted 3-isoxazolol fibrinolysis inhibitor AZD6564. Org. Process Res. Dev. 18, 952–959. doi:10.1021/op500193s
Auronum (2023). Canary in the palladium mine – when the best can’t survive, prices are too low. Available online at: https://auronum.co.uk/canary-in-the-palladium-mine-when-the-best-cant-survive-prices-are-too-low/(Accessed July 26, 2025).
Aydin, G., Ball, M., Bishop, T. G., Burns, M., Campbell, A. D., Cox, R. J., et al. (2024). Metal-catalyzed C–N bond forming reaction selection and process development for the manufacture of AZD7594. Org. Process Res. Dev. 28, 559–576. doi:10.1021/acs.oprd.3c00393
Blackmond, D. G. (2015). Kinetic profiling of catalytic organic reactions as a mechanistic tool. J. Am. Chem. Soc. 137, 10852–10866. doi:10.1021/jacs.5b05841
Constable, D. J. C., Jimenez-Gonzalez, C., and Henderson, R. K. (2007). Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 11, 133–137. doi:10.1021/op060170h
Crawford, J. M., Gensch, T., Sigman, M. S., Elward, J. M., and Steves, J. E. (2022). Impact of phosphine featurization methods in process development. Org. Process Res. Dev. 26, 1115–1123. doi:10.1021/acs.oprd.1c00357
Davies, I. W., Matty, L., Hughes, D. L., and Reider, P. J. (2001). Are heterogeneous catalysts precursors to homogeneous catalysts? J. Am. Chem. Soc. 123, 10139–10140. doi:10.1021/ja016877v
De Vries, J. G. (2006). A unifying mechanism for all high-temperature Heck reactions. The role of palladium colloids and anionic species. Dalton Trans., 421–429. doi:10.1039/b506276b
Dynochem Resources (2024). Predict solubility and select solvents using Dynochem. Available online at: https://dcresources.scale-up.com/?v=5#EVT___6803 (Accessed July 26, 2025).
Economidou, M., Mistry, N., Wheelhouse, K. M. P., and Lindsay, D. M. (2023). Palladium extraction following metal-catalyzed reactions: recent advances and applications in the pharmaceutical industry. Org. Process Res. Dev. 27, 1585–1615. doi:10.1021/acs.oprd.3c00210
Farina, V. (2004). High-turnover palladium catalysts in cross-coupling and Heck chemistry: a critical overview. Adv. Synth. Catal. 346, 1553–1582. doi:10.1002/adsc.200404178
Farina, V. (2023). How to develop organometallic catalytic reactions in the pharmaceutical industry. Org. Process Res. Dev. 27, 831–846. doi:10.1021/acs.oprd.3c00086
Fassbach, T. A., Ji, J.-M., Vorholt, A. J., and Leitner, W. (2024). Recycling of homogeneous catalysts─basic principles, industrial practice, and guidelines for experiments and evaluation. ACS Catal. 14, 7289–7298. doi:10.1021/acscatal.4c01006
Firsan, S. J., Sivakumar, V., and Colacot, T. J. (2022). Emerging trends in cross-coupling: twelve-electron-based L1Pd(0) catalysts, their mechanism of action, and selected applications. Chem. Rev. 122, 16983–17027. doi:10.1021/acs.chemrev.2c00204
Ford, M. J., and Fairlamb, I. J. S. (2025). An agrochemical perspective on Pd-catalyzed cross-coupling chemistry. Chem. Catal. 5, 101256. doi:10.1016/j.checat.2024.101256
Gallou, F., Isley, N. A., Ganic, A., Onken, U., and Parmentier, M. (2016). Surfactant technology applied toward an active pharmaceutical ingredient: more than a simple green chemistry advance. Green Chem. 18, 14–19. doi:10.1039/c5gc02371h
Ganley, J. M., Joe, C. L., and Simmons, E. M. (2025). Development of robust, efficient and scalable transition metal catalyzed transformations: translation of reactions from micromole to multi-kilogram scale processes. ACS Catal. 15, 8317–8336. doi:10.1021/acscatal.5c00836
Gladysz, J. A. (2001). Recoverable catalysts. Ultimate goals, criteria of evaluation, and the green chemistry interface. Pure Appl. Chem. 73, 1319–1324. doi:10.1351/pac200173081319
Horbaczewskyj, C. S., and Fairlamb, I. J. S. (2022). Pd-Catalyzed cross-couplings: on the importance of the catalyst quantity descriptors, mol % and ppm. Org. Process Res. Dev. 26, 2240–2269. doi:10.1021/acs.oprd.2c00051
Hübner, S., De Vries, J. G., and Farina, V. (2016). Why does industry not use immobilized transition metal complexes as catalysts? Adv. Synth. Catal. 358, 3–25. doi:10.1002/adsc.201500846
ICH International Council for Harmonisation (2000). ICH harmonised tripartite guideline: good manufacturing practice guide for active pharmaceutical ingredients Q7. U.S. Food and Drug Administration. Available online at: https://www.fda.gov/media/71518/download (Accessed July 26, 2025).
Khazipov, O. V., Shevchenko, M. A., Chernenko, A. Y., Astakhov, A. V., Pasyukov, D. V., Eremin, D. B., et al. (2018). Fast and slow release of catalytically active species in Metal/NHC systems induced by aliphatic amines. Organometallics 37, 1483–1492. doi:10.1021/acs.organomet.8b00124
King, R. P., Krska, S. W., and Buchwald, S. L. (2021). A ligand exchange process for the diversification of palladium oxidative addition complexes. Org. Lett. 23, 6030–6034. doi:10.1021/acs.orglett.1c02101
Kozuch, S., and Martin, J. M. L. (2012). “Turning Over” definitions in catalytic cycles. ACS Catal. 2, 2787–2794. doi:10.1021/cs3005264
Lippincott, D. J., Landstrom, E., Cortes-Clerget, M., Lipshutz, B. H., Buescher, K., Schreiber, R., et al. (2019). Surfactant technology: with new rules, designing new sequences is required. Org. Process Res. Dev. 24, 841–849. doi:10.1021/acs.oprd.9b00454
Looby, A. P., Venigalla, L., Hou, W., Xiao, M., Yang, Y., Chen, H., et al. (2025). On-Demand access to palladium oxidative addition complexes (OACs) from a stable organopalladate salt. Organometallics 44, 704–711. doi:10.1021/acs.organomet.5c00039
Lu, J., Donnecke, S., Paci, I., and Leitch, D. C. (2022). A reactivity model for oxidative addition to palladium enables quantitative predictions for catalytic cross-coupling reactions. Chem. Sci. 13, 3477–3488. doi:10.1039/d2sc00174h
Mennen, S. M., Alhambra, C., Allen, C. L., Barberis, M., Berritt, S., Brandt, T. A., et al. (2019). The evolution of high-throughput experimentation in pharmaceutical development and perspectives on the future. Org. Process Res. Dev. 23, 1213–1242. doi:10.1021/acs.oprd.9b00140
E. Negishi, and A. De Meijere (2002). Handbook of organopalladium chemistry for organic synthesis (New York: Wiley), 1, 2.
Nielsen, C. D., and Burés, J. (2019). Visual kinetic analysis. Chem. Sci. 10, 348–353. doi:10.1039/c8sc04698k
Niemeyer, Z. L., Milo, A., Hickey, D. P., and Sigman, M. S. (2016). Parameterization of phosphine ligands reveals mechanistic pathways and predicts reaction outcomes. Nat. Chem. 8, 610–617. doi:10.1038/nchem.2501
Nuss, P., and Eckelman, M. J. (2014). Life cycle assessment of metals: a scientific synthesis. PLoS One 9, e101298. doi:10.1371/journal.pone.0101298
Ren, H., Strulson, C. A., Humphrey, G., Xiang, R., Li, G., Gauthier, D. R., et al. (2017). Potassium isopropyl xanthate (PIX): an ultra-efficient palladium scavenger. Green Chem. 19, 4002–4006. doi:10.1039/c7gc01765k
Sigma-Aldrich (2025). Buchwald G6 precatalysts and oxidative addition complexes. Available online at: https://www.sigmaaldrich.com/GB/en/technical-documents/technical-article/chemistry-and-synthesis/cross-coupling/buchwald-g6-precatalysts-oxidative-addition-complexes (Accessed July 26, 2025).
Tu, S., Yusuf, S., Muehlfeld, M., Bauman, R., and Vanchura, B. (2019). The destiny of palladium: development of efficient palladium analysis techniques in enhancing palladium recovery. Org. Process Res. Dev. 23, 2175–2180. doi:10.1021/acs.oprd.9b00204
Vásquez-Céspedes, S., Betori, R. C., Cismesia, M. A., Kirsch, J. K., and Yang, Q. (2021). Heterogeneous catalysis for cross-coupling reactions: an underutilized powerful and sustainable tool in the fine chemical industry? Org. Process Res. Dev. 25, 740–753. doi:10.1021/acs.oprd.1c00041
Welch, C. J., Albaneze-Walker, J., Leonard, W. R., Biba, M., Dasilva, J., Henderson, D., et al. (2005). Adsorbent screening for metal impurity removal in pharmaceutical process research. Org. Process Res. Dev. 9, 198–205. doi:10.1021/op049764f
Keywords: palladium, cross-coupling, turnover, catalysis, metrics, workflow
Citation: Steven A (2025) How to develop a sustainable palladium-catalyzed cross-coupling reactions for active ingredient manufacture. Front. Catal. 5:1635370. doi: 10.3389/fctls.2025.1635370
Received: 26 May 2025; Accepted: 19 September 2025;
Published: 01 October 2025; Corrected: 07 November 2025.
Edited by:
Pablo Domínguez de María, Sustainable Momentum, SL, SpainReviewed by:
Mohsen Ahmadi, Leibniz Institute for Plasma Research and Technology e.V. (INP), GermanyCopyright © 2025 Steven. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alan Steven, a2p3eDEwOUBnbWFpbC5jb20=
 Alan Steven
Alan Steven