- 1Department of Chemistry, Colorado State University, Fort Collins, CO, United States
- 2Department of Biomedical Sciences, Colorado State University, Fort Collins, CO, United States
- 3Unidad de Química Medicinal, Escuela “Dr. Jesús María Bianco” Facultad de Farmacia, Universidad Central de Venezuela, Caracas, Venezuela
- 4Cellular and Molecular Biology Program, Colorado State University, Fort Collins, CO, United States
Introduction: Vanadium and manganese are two biologically relevant redox-active first row transition metals. Both metals have been associated with protective and deleterious effects in the cardiovascular system depending on the biological context, chemical species and metal oxidation state investigated. Many studies have indicated that these metals elicit their effects in part by influencing mitochondrial function, with potential variations due to their redox properties and complexation.
Methods: To better understand these relationships, we investigated the effects of vanadium and manganese salts (VIVOSO4, NaVVO3, MnIICl2) and acetoacetate (Hacac) complexes (VIVO(acac)2 and MnII(acac)2) on murine cardiac mitochondrial function. Metal speciation calculations were performed to predict the chemical species present under biological assay conditions.
Results and Discussion: Both vanadium and manganese salts decreased rates of mitochondrial respiration in a concentration dependent manner, which was attenuated when the metals were complexed to an organic ligand. In contrast, only VIVOSO4 and VIVO(acac)2 induced significant mitochondrial swelling, with greater sensitivity over NaVVO3, MnIICl2, MnII(acac)2 and free Hacac ligand. Swelling induced by both vanadium(IV) species was fully abolished by inhibition of the mitochondrial calcium uniporter and was partially dependent upon the voltage-dependent anion channel. In addition to the simple monomeric form (VIVO(H2O)52+), a second active vanadium species is the dimer (VIVO)2(OH)5−, while for manganese the main active species is Mn2+. In summary, these studies demonstrate distinct effects of vanadium and manganese on cardiac mitochondrial function that vary in part with the chemical speciation and metal oxidation state.
1 Introduction
Mitochondrial function is imperative for cell survival, particularly in organs with high metabolic demands such as the heart. Consequently, mitochondrial dysfunction has been implicated in the pathogenesis of cardiac injury and disease (Zhou and Tian, 2018; Kuznetsov et al., 2019; Bauer and Murphy, 2020; Ramachandra et al., 2020), as well as cancer, diabetes and neurodegenerative diseases (Diaz-Vegas et al., 2020; Wang et al., 2019). Transition metals have the potential to accumulate within cells and impact mitochondrial function to exert protective or pathologic effects by mechanisms that are not fully understood (Gunter et al., 2006; Gavin et al., 1999; Liu et al., 2013; Crans and Kostenkova, 2020). Manganese is an essential element and a co-factor for superoxide dismutase (Hamanaka and Chandel, 2010) and vanadium may have multiple effects on signal transduction as a phosphatase inhibitor (Treviño et al., 2019; Imhoff et al., 2024), but how these effects may impact mitochondrial function is unclear. Vanadium has been shown to have deleterious effects on liver mitochondria (Zhao et al., 2010; Lei et al., 2007), and manganese on cardiac mitochondria (Jiang and Zheng, 2005), but both have also been associated with protective effects against cancer and diabetes (Sun et al., 2025; Huang et al., 2025; Paolillo et al., 2025; Kozieł et al., 2024), as well as cardiac injury and disease (Bhuiyan and Fukunga, 2009; Xiao et al., 2021). Since most studies reported have been on liver mitochondria, we investigated the cardiac mitochondrial function to obtain a better understanding of how the chemical state and speciation of transition metals impacts their effects on mitochondrial function in tissues of various sources.
Metal ions, particularly first-row transition metals, can serve as cofactors in several enzymes necessary for proper biological functioning (Kostenkova et al., 2022). Transition metals are also used in diagnostics and therapeutics, such as for cancer treatment (Wang et al., 2023; Phillips and Pombiero, 2020; Ma et al., 2020; Karges et al., 2021; Kostova, 2009). The example of a particularly successful transition metal therapeutic is cis-platin and other platinum-containing agents to treat several cancers. A significant problem with transition metals is that they can show toxic effects linked to disease pathogenesis (Crans and Kostenkova, 2020; Wang et al., 2023; Phillips and Pombiero, 2020; Ma et al., 2020; Karges et al., 2021; Kostova, 2009; Jaishankar, et al., 2014). Understanding the underlying disease mechanisms induced by transition metals is important due to their widespread use and exploration for potential therapeutic use against cancer, Parkinson’s, Alzheimer’s, and diabetes (Lei et al., 2024; Wang et al., 2024; Souza-Coelho et al., 2024; Kostova, 2009; Prihantono and Raya, 2020; Kowalski et al., 2020; Murakami et al., 2022; Kumar et al., 2024).
Transition metals show different activity based on their specific complexes and speciation which can potentially allow for fine-tuning beneficial or toxic effects. It is well known that different forms (Dinda et al., 2025), oxidation (Buglyó et al., 2005), and protonation states (Buglyó et al., 2005), nuclearity (Samart et al., 2018), coordination to potential metabolites and ligands can dramatically affect their activity (Crans et al., 2013; Kostenkova et al., 2022). For example, organic ligands coordinating to the transition metals forming coordination complexes can alter activity compared against their biological activity of their salts (Willsky et al., 2011). Recent vanadium and manganese complexes have shown activity against cancer cell lines (Prihantono and Raya, 2020; Kowalski et al., 2020; Murakami et al., 2022; Lima et al., 2021); however, little is known about the mechanism of action, but prevailing data indicates trends (Kowalski et al., 2020). Significant amounts of literature indicate mitochondria as potential targets for transition metals and given the redox properties of these materials reported reactive oxygen species (ROS) formation or consumption depending on the specific metal species (Korotkov, 2023; Aureliano et al., 2023; Xiong et al., 2021). Mitochondrial oxidative stress apoptosis induced by a number of metal ions including Hg2+, Cd2+, Pb2+, Al3+, Cr6+, U6+ and other toxic metals may display opening of the mitochondrial permeability transition pore (MPTP), mitochondrial swelling, an increase in the production of ROS and H2O2, lipid peroxidation, or reduced glutathione and oxygen consumption (Korotkov, 2023). Several studies have shown that vanadium complexes have differential impact on mitochondria (Zhao and Zhao, 2013) and some literature shows that oxidation state is critical because Mn2+ is more likely to have a biological effect than Mn3+ (Gunter, 2017). However, little has been done to characterize the effects of Mn salts and complexes that undergo speciation under physiological conditions to understand the role that the manganese ion may play on biological activity (Gunter et al., 2006; Gavin et al., 1999; Liu et al., 2013). Reports show that stable hydrophobic MnII coordination complexes can behave as efficient O2− scavengers and reduce oxidative cell injury (Nistri et at., 2015) and has a direct protective action on H9c2 rat cardiac muscle cells subjected to hypoxia and reoxygenation (Becatti et al., 2019). However, these studies preclude observation of the effects of the free Mn-ions since the hydrophobic complexes were designed for high stability under physiological conditions. Vanadium and manganese have been reported to have similar biological outcomes including the loss of mitochondrial membrane potential, effects on mitochondrial protein, promotion of chronic diseases and cell death (Schell et al., 2025; Avila et al., 2013; Aureliano and Crans, 2009; Bhat et al., 2015; Pajarillo et al., 2021; Ohiomokhare et al., 2020; Ścibior et al., 2023; Kwakye et al., 2015; Guilarte, 2010; He et al., 2020). However, vanadium has also been reported to be potentially protective in some disease contexts (He et al., 2020; Aureliano et al., 2023). In these studies we seek to identify the potential similarities and differences on cardiac mitochondrial function of vanadium(IV) and manganese (II) salts and their respective coordination complexes.
In this study, we observed the effects of different geometries and oxidation states of vanadium and manganese salts and coordination complexes have on cardiac mitochondrial function. We chose to investigate the effects on cardiac mitochondrial respiration and swelling by vanadium species that are structurally analogous to the Mn(II)-salt known to have potent effects on Parkinsons disease and for comparison to the simple salt a known Mn(II)-coordination complex. In Figure 1 we show the structures for the salts vanadyl sulfate (VIVOSO4) and manganese chloride (MnIICl2) as well as the coordination complexes vanadyl acetylacetonate (VIVO(acac)2), and manganese acetylacetonate (MnII(acac)2). The two coordination complexes were chosen because the two metal complexes were structureally similar and VIVO(acac)2 is known to have a greater effect than the simple salt (Reul et al., 1999). Since vanadyl sulfate readily oxidize near neutral pH one would anticipate that some of the vanadium(IV) would oxidize to vanadium(V) under the assay conditions (Crans et al., 1995) so we also investigated the effects of sodium metavanadate (NaVVO3). However, since manganate (K2MnVIO4) and permanganate (KMnVIO4) are strongly oxidative and do not form under the assay conditions the effects of these anions were not included in this study. The studies included investigation of the roles that different mitochondrial pores and channels have in vanadium and manganese uptake. We supported these results with speciation studies to better understand the role that speciation and oxidation state of these metals may have in affecting mitochondrial function.
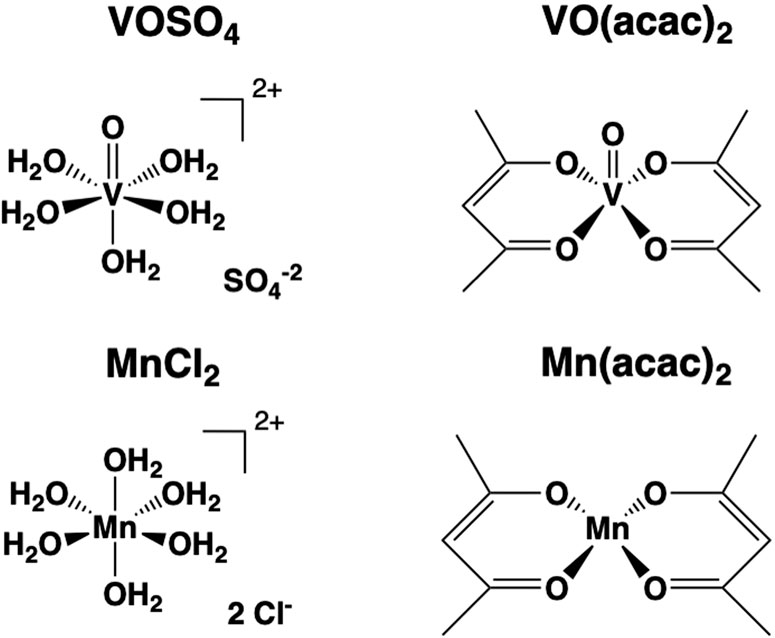
Figure 1. The structures of the compounds formed in solution from the solid compounds used in this study: Two vanadium(IV) compounds (VIVOSO4 and VIVO(acac)2), one vanadium(V) salt NaVVO3) and two manganese(II) compounds (MnIICl2, and MnII(acac)2).
2 Materials and methods
2.1 Materials
Amplex™ UltraRed Reagent, manganese(II) chloride (MnIICl2) (both anhydrous and MnIICl2•4H2O form Mn2+ in aqueous solution), and Vanadyl sulfate (VIVOSO4) was purchased from Thermo Fisher Scientific (Waltham, MA). Manganese(II) acetylacetonate (MnII(acac)2) was purchased from Aldrich Chemical Company (now Sigma Aldrich (Milwaukee, WI)). Acetylacetone was purchased from Oakwood Chemical (Estill, SC). Bis(acetylacetonato) oxovanadium (VIVO(acac)2), sodium metavanadate (NaVVO3), L-(−)-Malic Acid sodium salt, sodium pyruvate, L-Glutamic Acid, sodium succinate dibasic hexahydrate, adenosine 5′-diphosphate monopotassium salt dihydrate (ADP), and horseradish peroxidase (HRP) were purchased from Sigma Aldrich (St. Louis, MO). (3-[3-[[[3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolyl]methyl]amino]benzoyl]-1H-indole-1-butanoic acid (DS16570511) and N-[[1-(1-naphthalenylmethyl)-4-(phenylamino)-4-piperidinyl]carbonyl]-glycine (VBIT-12) were purchased from Cayman Chemicals (Ann Arbor, Michigan) and prepared in DMSO.
2.2 Isolation of cardiac mitochondria
All procedures were approved by Colorado State University Animal Care and Use Committee in accordance with recommendations from the Declaration of Helsinki and the Guiding Principles on the Care and Use of Animals. C57 BL/6 mice (10-28°moths old) were housed in a climate-controlled facility on a traditional 12-h light/dark cycle with ad libitum food and water access. Mice were scarified by lethal carbon dioxide inhalation followed by midline thoracotomy and removal of the heart immediately after confirming the absence of a tail-pinch reflex. Cardiac mitochondria were isolated from heart tissue as previously described (He et al., 2020). All procedures were performed on ice or controlled at 4°C. In brief, hearts were harvested immediately after sacrifice and rinsed with ice-cold Chappell-Perry (CP1) buffer consisting of (in mM) 1 ATP, 100 KCl, 50 MOPS, 1 EDTA, 5 EGTA, and 5 MgSO4·7H2O at pH 7.4 with KOH. Minced tissue was homogenized for 15 s using a polytron, incubated for 7 min in CP1 containing trypsin (∼5 mg/g tissue), quenched with CP2 (CP1+2 mg/mL Bovine Serum Albumin), then subjected to 12 passes with a glass Teflon Potter Elvehjem homogenizer before centrifugation at 600 g. The mitochondria-rich supernatant was collected and centrifuged at 7,000 g. Supernatant was discarded and the pellet resuspended in CP2, before being followed by three 7,000 g clarifying spins in CP2 and then one spin in KME buffer (100 mM KCl, 50 mM MOPS, and 0.5 mM EGTA). Final mitochondrial pellets were suspended in 350°uL of KME. A Bicinchoninic acid protein assay (BCA; Thermo Fisher Scientific, San Jose, CA) was utilized to determined specific protein concentration of mitochondrial isolates before 30°ug protein was added to each respiratory chamber (15°ug/mL).
2.3 Monitoring mitochondrial function by high-resolution respirometry
Mitochondrial respiration was investigated using two Oxygraph O2k high-resolution respirometer (Oroboros Instruments, Innsbruck, AT), which consists of two sealed temperature-controlled chambers that allow for real-time measurement of total O2 flux in the chamber. Samples were run in MiR05 respiration medium containing 0.5 mM EGTA, 3 mM MgCl2 hexahydrate, 60 mM lactobionic acid, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, and 0.1% bovine serum albumin (BSA), pH 7.1 with KOH. The instrument was calibrated using the Datlab software (Oroboros Instruments, Innsbruck, Austria) to accurately measure O2 concentration in the chamber and the rate of oxygen consumption (JO2) which is calibrated prior to sample addition. Each chamber was allowed to equilibrate to room O2 concentration and kept at a temperature of 37°C. Following O2 stabilization, 30 μg of isolated mitochondria were added to the chamber and were treated with substrates (1 mM malate, 5 mM pyruvate, 10 mM glutamate, 10 mM succinate and 10 mM ADP). Each sample was treated separately with titrations of VIVOSO4, MnIICl2, VIVO(acac)2 and MnII(acac)2 from 1 μM to 275 μM.
2.4 Evaluation of mitochondrial swelling induced by VIVOSO4, VIVO(acac)2, NaVVO3, MnIICl2, MnII(acac)2, and Hacac
Changes in the volume of mitochondria induced by shifts in osmotic pressure from metal uptake were measured by ultraviolet absorbance at 540 nm (A540). Mitochondria isolated from the heart of 10–12-month-old mice were diluted in 2RX respiration buffer (500 mM sucrose, 20 mM Tris-MOPS, 0.02 mM EGTA, 10 mM pyruvate, 2 mM malate, adjusted to pH 7.4 with KOH) to a concentration of 60 μg/100 μL RX buffer. In a clear 96 well plate, 100 μL of the mitochondria/RX buffer sample were added VIVOSO4, VIVO(acac)2, NaVVO3, MnIICl2, and MnII(acac)2, at concentrations of 3.2 μM, 20 μM, 200 μM, and 400 μM were prepared fresh in distilled water for each experiment. Acetylacetonate was tested as a control with concentrations of 6.4 μM, 40 μM, 400 μM, and 800 μM. Each well was brought to a final volume of 200 μL with the addition of 100 μL of each metal sample so that the final concentrations of metals were 1.6 μM, 10 μM, 100 μM, 200 μM. Absorbance at 540 nm was measured every 2 min for 60 min with a microplate spectrophotometer (VersaMax, Molecular Devices).
2.5 Effect of inhibiting mitochondrial pores and channels on vanadium and manganese induced swelling
In a 96 well plate 100 μL of master mix of 60 μg/100 μL mitochondria and 2RX buffer (500 mM sucrose, 20 mM Tris-MOPS, 0.02 mM EGTA, 10 mM pyruvate, 2 mM malate, adjusted to pH 7.4 with KOH). Three inhibitors (cyclosporin A, VBIT-12, DS16570511) were tested. Stocks solutions of 4 mM VBIT-12 and DS16570511 as well as 2 mM of CsA were prepared in DMSO. At the bottom of separate wells 1 μL of the 2 mM CsA stock, 5 μL of a 4 mM VBIT-12 stock, and 5 μL of a 4 mM DS16570511 were added followed by the addition of 100 μL of the master mix. The assay was started by the addition of metal compounds (VIVOSO4, NaVVO3, VIVO(acac)2, MnIICl2, MnII(acac)2) to bring the well to a final volume of 200 μL and a concentration of 200 μM metal compound. Each inhibitor was run with an N of 6–9. As a control 400 μM acetylacetonate(acac)-ligand was tested. Absorbance at 450 nm was measured every 2 min for 60 min using a microplate spectrophotometer (VersaMax, Molecular Devices).
2.6 Speciation calculations of VIVOSO4, VIVO(acac)2, NaVVO3, MnIICl2, and MnII(acac)2
Transition metal ions undergo hydrolytic, protonation and redox reactions, and differences have been reported regarding which species interact with proteins, with interphases or with intact cells. In this work the effects of these coordination complexes and their respective salts on the cardiac mitochondria was investigated. However, since most of these studies were carried out at μM concentrations, it is not possible to measure the different species observed because the concentrations of the metal species are present below detection limits by most experimental methods. Thus, we resorted to the estimation of the speciation based on the known reported speciation constants. In such case the speciation will not be exact, but since the comparisons done with speciation constants in different biological systems yielded concentrations within a factor of two (Crans, 1994), the evaluations determined by use of known speciation constants will be representative and provide some information on the systems we have been investigating. In the following how the speciation profiles for each of the two salts and the two coordination complexes were obtained will briefly be described. Some of the considerations, such as the speciation observed in the stock solutions (10 μM) used as well as under the conditions of the study (μM) are provided in the Supplementary Material.
2.6.1 VOSO4 speciation
The speciation diagrams were calculated using the HYSS program and the IUPAC Stability Constants Database Software (Version 5.81) [Data version 4.62] (2000). Speciation profiles were constructed using the following formation constants (Crans et al., 2004): [VIVO(OH)]+ (logβ-1,1 = −5.94), [(VIVO)2(OH)2]2+ (logβ-2,2 = −5.94), [VIVO(OH)3]- (logβ-3,1 = −18.0), [(VIVO)2(OH)5]- (logβ-5,2 = −22.5), {VIVO(OH)2}n (s) solubility product value of Ksp = 6.6 × 10-23 M3, [VIVO(HSO4)]+ (logβ1,1,1 = 1.74), VIVOSO4 (logβ0,1,1 = −2.51).
2.6.2 VO(acac)2 speciation
The speciation diagrams were calculated using the HYSS program and the IUPAC Stability Constants Database Software (Version 5.81) [Data version 4.62] (2000). The speciation profiles illustrates the concentration of VO(acac)2 and hydrolytic species using the following formation constants (Crans et al., 2004; Martell and Smith, 1977): [VIVO(OH)]+ (logβ−1,1 = −5.94), [(VIVO)2(OH)2]2+ (logβ-2,2 = −5.94), [VIVO(OH)3]− (logβ-3,1 = −18.0), [(VIVO)2(OH)5]− (logβ-5,2 = −22.5)[35], {VIVO(OH)2}n (s) solubility product value of Ksp = 6.6 × 10−23 M3, [VIVO(acac)]+ (logβ0,1,1 = 17.67), VIVO(acac)2 (logβ0,1,2 = 33.62).
2.6.3 MnCl2 speciation
The speciation diagrams were calculated using the HYSS program. The diagrams illustrate the concentration of MnCl2 and hydrolytic species. Diagrams have been constructed based on the most abundant species at physiological pH (pH = 7.4) using the following formation constants (Crans et al., 2004; Martell and Smith, 1977): [MnII(OH)]+ (logβ−1,1 = −10.58), MnII(OH)2 (logβ-2,1 = −22.18), [MnII(OH)3]− (logβ-1,1 = −34.34), [MnII(OH)4]2− (logβ-4,1 = −48.28)[37], MnII(OH)2(s) and MnIIO(s) solubility product value of logβ = 15.19 and logβ = 17.94 respectively, [MnIICl]+ (logβ0,1,1 = 3.69), MnIICl2 (logβ0,1,2 = 6.09), [MnIICl3]− (logβ0,1,3 = 10.02), [MnIICl4]2− (logβ0,1,4 = 12.63).
2.6.4 MnII(acac)2 speciation
The speciation diagrams were calculated using the HYSS program. The diagrams illustrate the concentration of MnII(acac)2 and hydrolytic species using the following formation constants (Crans et al., 2004; Martell and Smith, 1977): [MnII(OH)]+ (logβ−1,1 = −10.58), MnII(OH)2 (logβ−2,1 = −22.18), [MnII(OH)3]− (logβ−1,1 = −34.34), [MnII(OH)4]2− (logβ−4,1 = −48.28), MnII(OH)2(s) and MnIIO(s) solubility product value of logβ = 15.19 and logβ = 17.94 respectively, [MnII(acac)]+ (logβ0,1,1 = 13.20), MnII(acac)2 (logβ0,1,2 = 25.28).
3 Results
3.1 MnIICl2 and VIVOSO4 inhibit mitochondrial respiration while Hacac-complexes show mitigated inhibition of respiration
The effects of manganese and vanadium on cardiac mitochondrial respiration varied depending on metal, ligand and oxidation state (Figure 2). To account for individual variations and limitations caused by depleting O2 concentrations in the chamber, the data shown is expressed as a % respiration from the max respiration followed by the addition of ADP. Titrations were stopped after 275 μM addition due to volume displacement effecting mitochondrial concentration in the chamber. VIVOSO4 and MnIICl2 inhibited mitochondrial respiration by nearly 28% at 15 μM concentration but had no significant differences between vanadium or manganese (Figure 2C). Both VIVO(acac)2 and MnII(acac)2 inhibit respiration only 15–20% and begin to differ significantly above 175 μM (p < 0.05) with MnII(acac)2 showing significantly less inhibition. There was a metal dependent effect on Hacac-complex respiration inhibition (p < 0.0001) (Figure 2D). Both manganese compounds inhibited respiration, however, there was a species dependent affect (p < 0.0001) with MnII(acac)2 having greater inhibition of respiration than MnIICl2 above 1 μM concentration (p < 0.05) (Figure 2B). Vanadium compounds showed no significant difference in inhibition of respiration at any concentration but there was a speciation dependent difference on respiration inhibition between VIVOSO4 and VIVO(acac)2 (p < 0.05) (Figure 2A). The metal and type of compound play a significant role in controlling the inhibition of mitochondrial respiration.
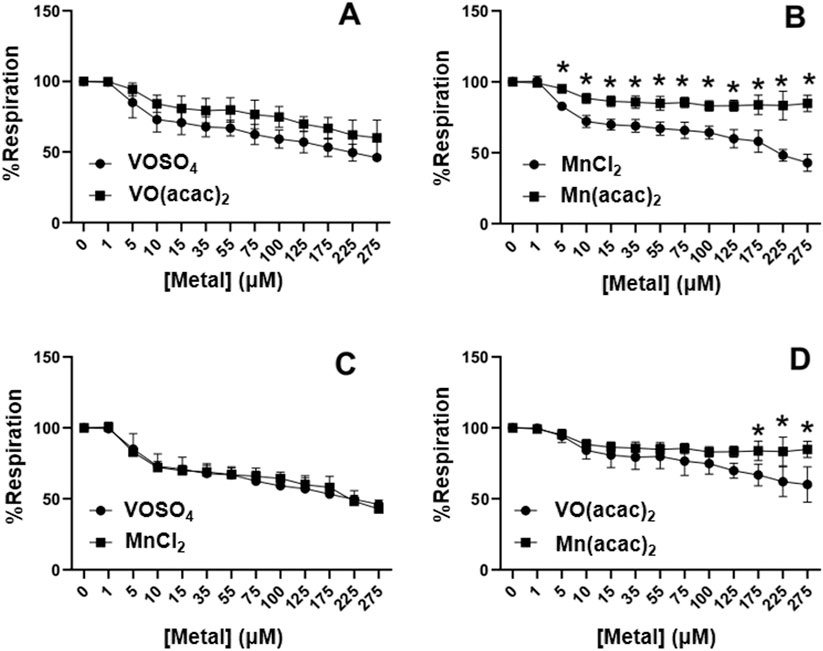
Figure 2. Concentration dependent effects of VIVOSO4, MnIICl2, VIVO(acac)2, and MnII(acac)2 on cardiac mitochondrial respiration. (A) VIVOSO4 and VIVO(acac)2. (B) MnIICl2 and MnII(acac)2. (C) MnIICl2 and VIVOSO4, (D) VIVO(acac)2 and MnII(acac)2. Respiration was measured using two Oxygraphs using a Clark type electrode. Statistical analysis was performed using a 2-Way ANOVA using GraphPad Prism. Each experiment was completed with an N = 3. (*p < 0.05)
3.2 VIVOSO4 and VIVO(acac)2 induce mitochondrial swelling, while NaVVO3, MnIICl2,MnII(acac)2 and free Hacac ligand do not
Mitochondrial swelling refers to the process by which water enters the mitochondria matrix from the surrounding media or cytosol of intact cells due to an increase in osmotic pressure, which is typically observed following excessive uptake of cations capable of crossing the inner mitochondrial membrane. The canonical cation capable of causing mitochondrial swelling is Ca2+ (Matuz-Mares et al., 2022) which accumulates within the mitochondrial matrix during cellular Ca2+ overload associated with pathologic states such as cardiac ischemia (Bertero et al., 2024). Excessive swelling can ultimately cause mitochondria to rupture and lose functionality, and therefore might explain the reduction in mitochondrial respiration observed in response to vanadium and manganese compound titrations. The ability of vanadium salts and selected complexes to induce mitochondrial swelling has been previously reported in liver (Zhao et al., 2010); however, the tissue-specificity impacts of and chemical speciation on these effects are less clear. Though manganese(III) was shown to induce cellular swelling (Ramao Rao et al., 2007) and manganese(II) was shown to induce mitochondrial swelling in animal cells (Mousa and Shehab, 2015), the effect of speciation of manganese compounds was not investigated. Furthermore, the current literature lacks consideration of the different action of manganese salts and complexes on mitochondrial or cellular swelling (Ramao Rao et al., 2007; Mousa and Shehab, 2015).
To address this gap in knowledge, we tested the effect of three vanadium compounds (VIVOSO4, NaVVO3, VIVO(acac)2 and two manganese compounds (MnIICl2, MnII (acac)2 on cardiac mitochondrial swelling (Figure 3). The effects were compared to a sample where an aqueous solution was administered in place of compounds and called “mito control. VIVOSO4 and VIVO(acac)2 allow for direct comparison of V4+ in a salt and a complex while NaVVO3 offers a comparison with a V4+ in case some of the V4+ oxidized. Similarly, MnIICl2 and MnII(acac)2 represent a salt and complex and allows for a comparison of Mn2+ that is comparable to V4+ as well as determine if specific ligands offer similar effects. To study speciation differences, we tested both V4+ and V5+ salts to look at differences in swelling. The V4+ compounds showed rapid and severe mitochondrial swelling starting at 100 μM when compared to the control mitochondrial sample (Figures 3A,B), whereas sodium metavanadate (Figure 3C) and manganese(II) salt and coordination complex (Figures 3D,E) had no significant effects on mitochondrial swelling at 200 μM concentration; the results of all five compounds is summarized in Figure 3F at the 60 min timepoint for convenient comparison. The effect of vanadate and Hacac ligand on the mitochondrial swelling is minimal as is shown in (Supplementary Figures S1, S2) although the Hacac ligand will not prevent the cardiac mitochondrial swelling inhibition by Ca2+ as shown in Supplementary Figure S3.
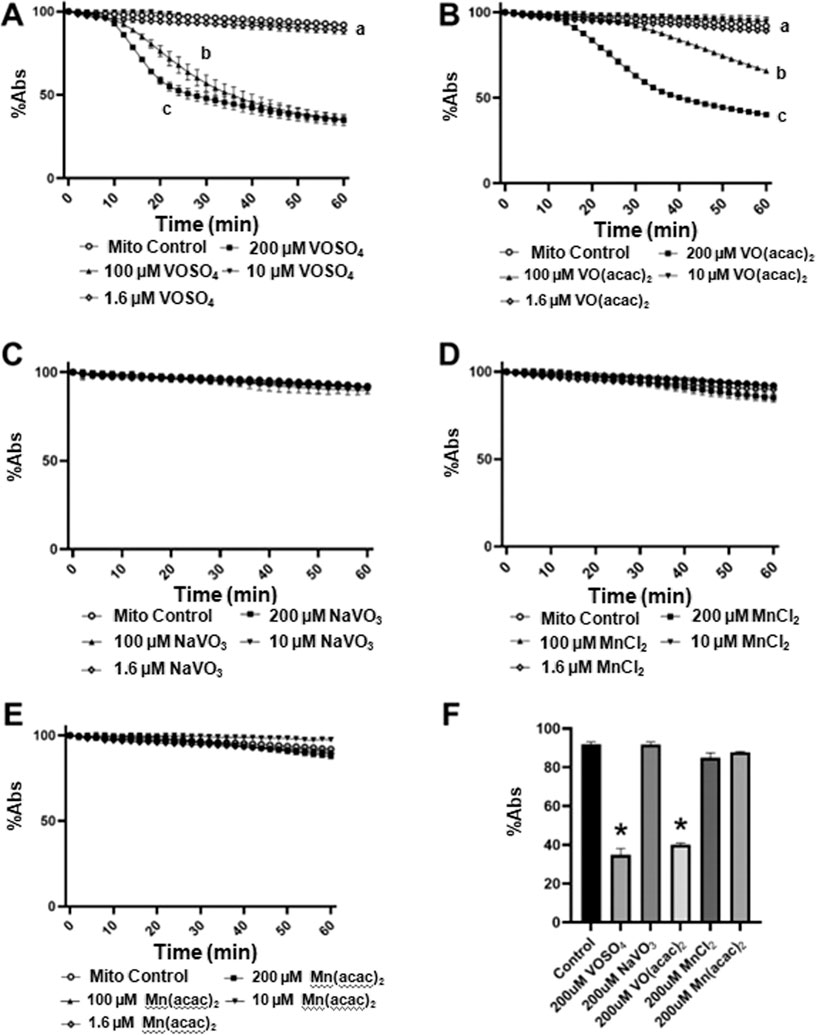
Figure 3. Concentration-dependent effects of vanadium and manganese compounds on cardiac mitochondrial swelling. Swelling was measured as a decrease in absorbance at 540 nm at 25°C in response to increasing concentration of VIVOSO4 (A), VIVO(acac)2 (B), NaVVO2 (C), MnIICl2 (D), or MnII(acac)2 (E). In the control sample (“mito control”) an aqueous blank solution was added in place of compound. The final extents of swelling at 60 min under all conditions are summarized in panel (F). Rates of swelling were compared across concentrations for each compound by one-way ANOVA with repeated measures and Dunnett’s Multiple Comparison Test. Different lowercase letters indicate significant variations in the rate of swelling for each experimental condition. Data represent mean ± SEM of 3 separate experiments per group at each time point. *P < 0.001 vs other groups by post-hoc comparisons at the 60 min time point.
3.3 Mitochondrial calcium uniporter and voltage-gated anion channel impact swelling induced by vanadium compounds in cardiac, but not liver, mitochondria
To investigate the mechanisms by which vanadium compounds mediate mitochondrial swelling, we employed selective inhibitors of two canonical mitochondrial ion transporters: the mitochondrial calcium uniporter (MCU) and the voltage-dependent anion channel (VDAC) (Marchi and Pinton, 2014; Shoshan-Barmatz and De, 2017). Inhibition of the mitochondrial calcium uniporter by 100 μM DS16570511 completely inhibited cardiac mitochondrial swelling induced by 200 μM VIVOSO4 (Figure 4A) and VIVO(acac)2 (Figure 4B), while inhibition of VDAC with 100 μM VBIT-12 partially attenuated swelling induced by both vanadium complexes (Figures 4A,B). Consistent with the findings from the VIVOSO4 and VIVO(acac)2 dose response curves (Figures 3A,B), VIVOSO4 induced an earlier onset of swelling than VIVO(acac)2 (arrows in Figures 4A,B) in cardiac mitochondria. Similar effects are not observed with NaVO3 as shown in Supplementary Figure S4. Given previous evidence for less robust impacts of MCU inhibition on vanadium-induced swelling in liver mitochondria, we repeated experiments to determine any tissue-specific responses and found that MCU inhibition with DS16570511 similarly abolish liver mitochondrial swelling induced by both 200 μM VIVOSO4 (Figure 3C) and VIVO(acac)2 (Figure 3D). However, no effect of VDAC inhibition with 100 μM VBIT-12 was seen, nor any effect of vanadium species on the onset of swelling.
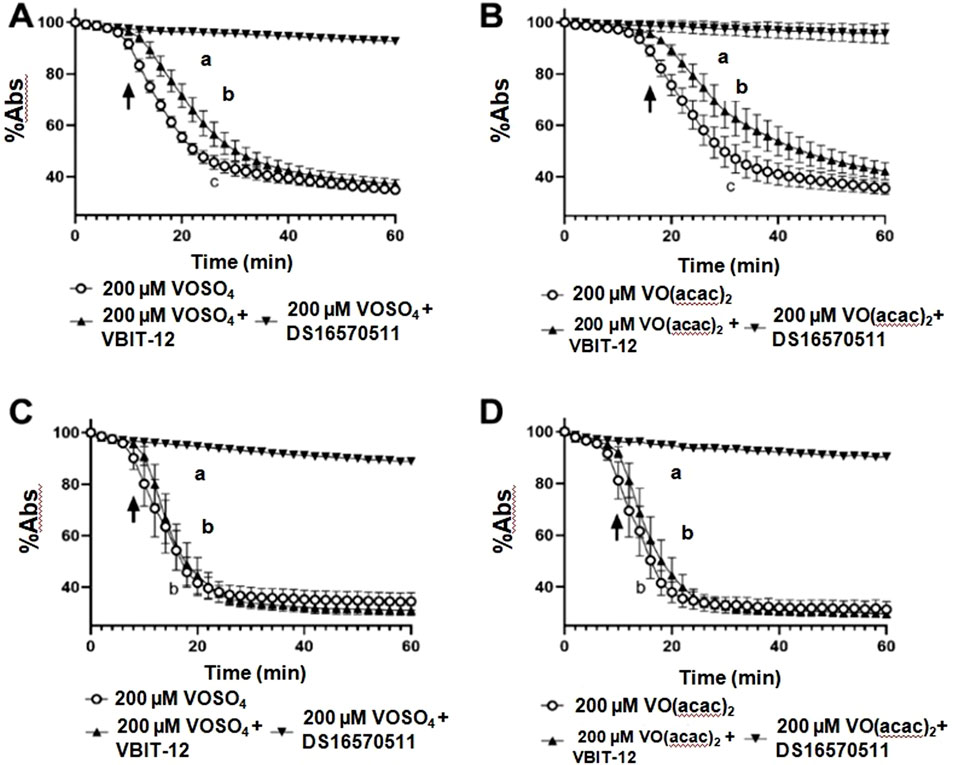
Figure 4. Impacts of MCU and VDAC on vanadium-induced swelling in cardiac and liver mitochondria. Mitochondrial swelling induced by 200 μM VIVOSO4 or VIVO(acac)2 was monitored at 25°C as a decrease in absorbance at 540 nm of intact cardiac (A, B) and liver (C, D) mitochondria, respectively. Impacts of the mitochondrial calcium uniporter (MCU) and voltage-gated anion channel (VDAC) were investigated by co-incubation the MCU-inhibitor DS16570511 or VDAC inhibitor VBIT-12. Extents of mitochondrial swelling were compared by one-way ANOVA with repeated measures and Dunnett’s Multiple Comparison Test. Different lowercase letter indicate significant variations in the rate of swelling for each experimental condition. Data represent mean ± SEM of 6–9 separate experiments per group at each time point.
3.4 Speciation differences in vanadium and manganese salts and complexes
In the Supplementary Material we provide speciation diagrams for the salts and coordination complexes used in this work. These profiles illustrate the diversity of the systems, and the fact that several different species exist varying in protonation states and composition. Both VIVOSO4 and VIVO(acac)2 and the MnIICl2 and MnII(acac)2 contain several species which vary dramatically with pH. Since the studies with the mitochondria are mainly done at pH 7.4, the speciation plots provided here are focusing on the changes as a function of added metal ion concentration in the swelling experiments. The speciation diagrams shown in Figures 5A–D illustrate the distribution of species as a function of concentration used in the swelling assays.
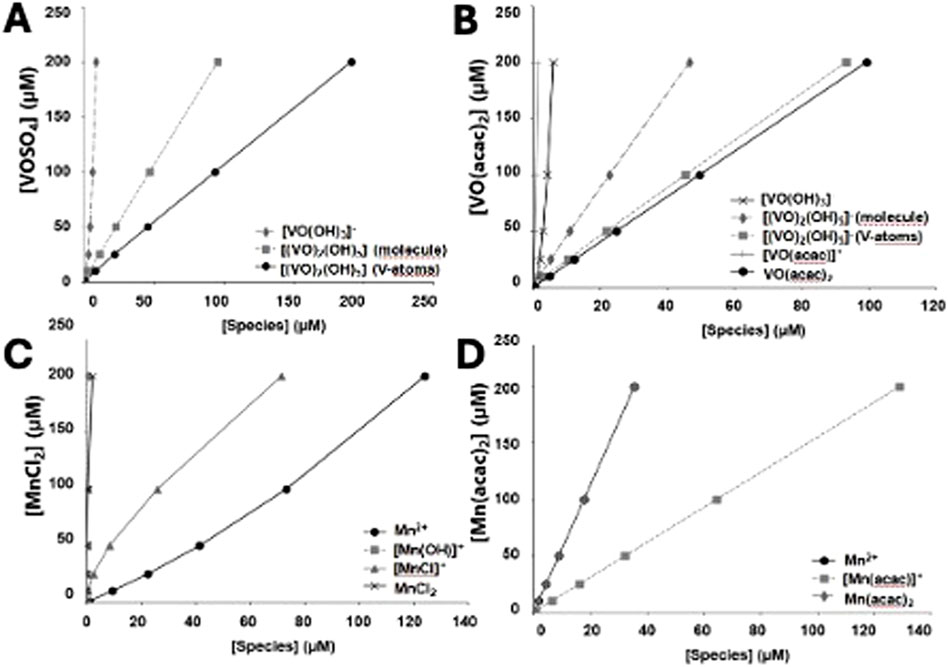
Figure 5. Speciation profiles illustrate the concentration of metal ion species as a function of the overall metal ion concentration. Diagrams have been constructed at physiological pH (pH = 7.4) in order to identy the species present under those conditions (A) VIVOSO4; (B) VIVO(acac)2; (C) MnIICl2; (D) MnII(acac)2.
For VIVOSO4 it is shown that two major species are present, namely, the monomer VIVO(OH)3- and a dimeric species (VIVO)2(OH)5−. The species in solution is mainly a dimeric form of VIV (Figure 5A). Since the dimer contains two vanadium atoms–we show both the concentration of molecule as well as the concentration of V-atoms in the dimeric species (VIVO)2(OH)5−. Calculations from VIVO(acac)2 show that three major species are present, namely, the VIVO(acac)2, the hydrolyzed monomer VIVO(OH)3-, and a dimeric species (VIVO)2(OH)5- shown in Figure 5B. Since the dimer contains two vanadium atoms–we show both the concentration of molecule as well as the concentration of V-atoms in the dimeric species (VIVO)2(OH)5−. Based on these speciation estimations it would be clear that most of the observed effects could be attributed to the hydrolyzed VIVO(acac)2, and thus the monomeric and dimeric forms of VIV.
For MnIICl2 it is shown that two major species and two minor species are present at pH 7.4 in the concentration range up to 200 μM. The two major species are the intact MnIICl2 molecule and the partially hydrolyzed species MnIICl+. The two minor species present are MnII(OH)+ and intact MnIICl2 (Figure 5C). For MnII(acac)2 three major species are present, namely, the MnII(acac)2, the hydrolyzed MnII(acac)+, and the plain Mn2+ cation (Figure 5D). Based on these speciation estimations it would be clear that most of the species present is the hydrolyzed MnII(acac)+ cation, however the two other ions present are in sufficient amounts that any observed effects should be attributed to these forms of Mn2+.
4 Discussion
Transition metals and transition metal complexes play an important role in biological processes and medical applications. Redox-active metals are often used to achieve redox balance in cellular environments and play a key role in enzymatic processes. Previous studies observed that different vanadium compounds exerted varying effects on rat liver mitochondria; this study done in cardiac mitochondria tissue expanded the scope by estimating the speciation of both salts and coordination complexes and by comparing the effects of two redox-active first row transition metal ions. As a result, we assign speciation and suggest active forms (species) which causes mitochondrial swelling.
Speciation differences may account for mechanistic variation between vanadium and manganese and facilitating mitochondrial dysfunction. Therefore, the results from Figure 5 will be discussed first. Comparison of speciation calculation between metals indicates significant differences of species in solution. The primary species in solution for the vanadium compounds at 200 μM is the dimer [(VIVO)2(OH)5]− as well as VIVO(acac)2 for the VIVO(acac)2 (Figures 5A,B). The similarities seen in mitochondrial swelling (Figure 4) may be attributable to the dimeric form of the vanadium in solution, but the existence of VIVO(acac)2 may limit the slightly lowered inhibition of respiration observed (Figure 2A). Below 50 μM, where the most immediate inhibition of respiration took place, the same primary species exists in solution, lending credence to the hypothesis that the inhibition of respiration can be attributed to a dinuclear species (Figures 5A,B). Similarly, the primary species in solution for the manganese compounds show some similarities while also having an outlier. MnIICl2’s primary species below 50 μM and at 200 μM is Mn2+ and MnIICl2 (Figure 5C) while MnII(acac)2’s primary species are Mn2+ and MnII(acac)2 (Figure 5D). The presence of MnII(acac)2 may be the cause for the significant reduction in inhibition of respiration observed (Figure 2B). Therefore, the speciation of the metal compounds may play a role in what biological effects of these compounds are observed on cardiac mitochondria.
Concentration, speciation and oxidation state of metal ion can affect the role the metal ion play in biological systems, and even change the observed effects from therapeutic to toxic responses (Rojas-Lemus et al., 2021). To investigate these potential differences, we monitored mitochondrial function with increasing concentrations of VIVOSO4, VIVO(acac)2, MnIICl2, and MnII(acac)2. Both salts showed similar effects on inhibiting mitochondrial respiration while VIVO(acac)2 and MnII(acac)2 inhibited respiration significantly less when compared to the respective metal salts but not when compared to each other. Earlier reports have shown that cell culture with 100 μM MnIICl2 (Sarkar et al., 2018) or 10–100 μM Mn(II) acetate (Rao et al., 2004) will inhibit mitochondrial function over time. However acute additions of metals with levels under 15 μM MnIICl2 have not previously been reported to inhibit mitochondrial function. In this work we observed a significant amount of the inhibition at concentrations near physiologically relevant concentrations under 15 μM metal ion. Previous reports have shown that to inhibit mitochondrial respiration excessive amounts of Mn2+ ion are needed (Warren et al., 2020), which is contrary to our findings and may be subject to the specific conditions and alteration in speciation; that is specifically complexation of the Mn2+ ion in the medium used in the study where the Mn2+ ion was found to be less active.
Mitochondrial swelling allows for further investigation into the mechanism of metal induced mitochondrial dysfunction in part because the mitochondrial swelling can be caused by rapid influx of calcium ions into the matrix. Calcium ions can enter mitochondria and cause shifts in osmotic pressure. These shifts can occur to such an extreme that mitochondria can burst, leading to a loss of function. This can also lead to the loss of the membrane potential limiting respiratory capacity of the ETS. Inhibition of vanadium and manganese induced swelling by the blocking of ion channels and pores help elucidate potential mechanisms of mitochondrial swelling by vanadium and manganese complexes. DS16570511 is a novel inhibitor of MCU which can block calcium transport into the mitochondria (Belosludtsev et al., 2021) and VBIT-12 acts as an inhibitor for VDAC (Wan et al., 2023), neither of which have been tested with vanadium or manganese. Previous literature has used a range of concentrations for these molecules and to ensure max inhibition we used 100 μM of both agents. DS16570511 completely inhibited vanadium induced swelling while VBIT-12 delayed swelling. There was no significant effect of these inhibitors on swelling by manganese. These findings show that while these ions have many similarities, the mechanism of vanadium and manganese induced cardiac mitochondrial dysfunction are radically different, and we suggest that this can partially be attributed to speciation and the fundamentally different effects of the simple ions.
Heavy metals, as well as transition metals including vanadium and manganese, are identified by the Environmental Protection Agency as toxic byproducts of many industrial processes such as fossil fuel extraction and refinement and thus pose a risk to human health (Queiroz et al., 2021; Hanus-Fajerska et al., 2021; Imtiaz et al., 2015; Markiv et al., 2023). However, some vanadium and manganese species have been shown to have profound effects and potential for medical application when incorporated in therapeutic complexes. The present study emphasizes the importance of metal speciation when considering their biological mechanism and outcome. An important aspect for the therapeutic potential of these complexes is stability of the complex and the organic ligand which can affect uptake and biological activity. If manipulation of the ligand can alter biological effects this could lead toward controllable toxicity and targeted treatments. Further investigation of different vanadium and manganese complexes into their biological effects on cells and mitochondria will be necessary to test the differences in mitochondrial swelling, ROS release, membrane potential and lipid peroxidation damage. Manganese’s inhibition of mitochondrial respiration without impacts on swelling may make it a promising alternative for transition metal-based therapeutics.
Both manganese and vanadium are first row transition metals and some similarities would be anticipated. Manganese is an essential element and functions as a cofactor in MnSOD in the conversion of superoxide into H2O2 in mammals. Vanadium is a known cofactor in vanadium peroxidases and vanadium nitrogenase in plants and prokaryotes, but in contrast to manganese is not essential in human beings. However, vanadium has been reported to have favorable biological activity throughout the biosphere and has shown strong biological activity that might be used therapeutically. Each of these metals has two physiologically common oxidation states, and in the case of vanadium can undergo redox cycling between oxidation state IV and V through Fenton chemistry (Avila et al., 2013; Rehder, 2013; Treviño et al., 2019). Manganese also changes redox state under physiological conditions, however, this occurs when it is bound to SOD (as MnSOD) and therefore likely causing a different phenotype change than vanadium compounds induce. A primary cellular location for such redox cycling is the mitochondria and would be consistent with the earlier reports showing a multifaceted effect on mitochondrial function. One of the primary mechanisms of toxicity by both manganese and vanadate are changes in mitochondrial membrane potential and inner membrane protein function (Zhao et al., 2010; Aureliano et al., 2023; Li and Yang, 2018; Hosseini et al., 2013; Galvani iet al., 1995). Because of reports of similar biological effects (Sun et al., 2025; Huang et al., 2025; Paolillo et al., 2025; Kozieł et al., 2024; Korotkov, 2023; Bhuiyan and Fukunga, 2009; Xiao et al., 2021; Becatti et al., 2019; Nistri et at., 2015), we were interested in comparing the speciation of manganese and vanadium while these materials were exerting effects on cardiac mitochondria. The comparison of both salts and complexes allowed us to investigate if a ligand could modify the biological effects through governing speciation under physiological conditions. However, since we found that similar responses were observed on respiration but not on mitochondrial swelling we wondered if the speciation of both Hacac complexes were similar. Indeed, both complexes were found to hydrolyze to form salts, although the species that form are different. Hence, we suggest that the observed effects on the cardiac mitochondria for the metal-acac complexes could be attributed to the formation of salt because limited effect was observed by the ligand Hacac. The major effect of the ligand may thus indicate that the Hacac ligand simply alters the delivery and uptake process of the metal ion. This conclusion is supported by the previous reports of MnII-complexes that were found to scavenge ROS and H2O2 but that this complex was significantly more stable than the MnII(acac)2 complex under physiological conditions ((Nistri et at., 2015; Becatti et al., 2019). Indeed, the report for a number of other metal complexes showing apoptosis which in part was attributed to ROS as well as other effects somewhat specific for each metal ion (Korotkov, 2023).
Further investigation into the differences between effects exerted by these metals should be carried out using multiple speciation states and complexes, lower concentrations and buffers that do not associate with the metal ion (Levina., et al., 2017). The mitochondrial swelling observed occurred above physiologically relevant concentrations and does not explain the inhibition of respiration observed in this work below 15 μM. Cell culture studies tested with these metals could allow for more insight into the mechanistic differences between vanadium and manganese in biological systems, specifically considering oxidation states and speciation. Measuring mitochondrial ROS production and associated damage, intact cellular respiration, and specific enzyme activities and protein expression would provide more in-depth information of the effects that these metals may have on function. This information could prove useful for future therapeutic design by offering a mode of action and a target for future research.
5 Conclusion
Vanadium and manganese both are redox active transition metals, but their redox properties and effects in biological systems can be very different, leading to potentially different capabilities and outcomes in vivo. Despite these differences, similar biological effects have been reported with these elements and in this work both salts and complexes were investigated for their effects on cardiac respiration and mitochondrial swelling. In the present study, both vanadium and manganese inhibited cardiac mitochondrial respiration at low concentrations, however the effect decreased when the metal ions were complexed with a ligand. In contrast, only high concentrations of vanadium induced mitochondrial swelling, which was attenuated by ligand Hacac complexing at 100 μM, but not 200 μM. We attribute this to the speciation chemistry and the fact hydrolysis of the complex occurs much faster at high micromolar concentrations. Further investigation at concentrations under 20 μM using metal complexes that do not undergo rapid hydrolysis and in buffers/media that do not associate with the metal ion (Levina et al., 2017) are suggested to verify this interpretation. Finally, we have shown that vanadium-induced mitochondrial swelling is dependent on the mitochondrial calcium uniporter in both liver and cardiac mitochondria, suggesting possible mechanism of entry into the mitochondrial matrix and/or impacts on mitochondrial calcium handling, corroborating an earlier report of oxidative stress in the liver mitochondria and opening of the mitochondrial permeability transition pore (Zhao et al., 2010). In summary, these studies show that both vanadium and manganese can impair mitochondrial function, but by different mechanisms that depend on the concentration and speciation of metal under the conditions of the system.
Data availability statement
Additional original data contributions obtained are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
All procedures were approved by Colorado State University Animal Care and Use Committee in accordance with recommendations from the Declaration of Helsinki and the Guiding Principles on the Care and Use of Animals.
Author contributions
CD: Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. LW: Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. ED: Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. LR: Investigation, Writing – original draft, Writing – review and editing. AC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Project administration, Supervision, Funding acquisition, Writing – original draft, Writing – review and editing. DC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Project administration, Supervision, Funding acquisition, Writing - original draft, Writing - review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. DC and AC thank Colorado State University for funding (Arthur C. Cope award, University Distinguished Professor grant and private donations).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that DC was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchbi.2025.1602602/full#supplementary-material
Abbreviations
acac, Acetylacetonate (Hacac – protonated acetylacetonate); CsA, Cyclosporin A; DS16570511, (3-[3-[[[3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolyl]methyl]amino]-benzoyl]-1H-indole-1-butanoic acid; ETS, Electron Transfer System; MCU, Mitochondrial calcium uniporter; Mn, Manganese; MnCl2, Manganese(II) Chloride; Mn(acac)2, Manganese(II) acetylacetonate; MnSOD, Manganese superoxide dismutase; MPTP, Mitochondrial permeability transition pore; NaVO3, Sodium metavanadate; ROS, Reactive oxygen species; SOD, Superoxide dismutase; V, Vanadium; V4+, Vanadyl; V5+, Vanadate; VBIT-12, N-[[1-(1-naphthalenylmethyl)-4-(phenylamino)-4-piperidinyl]carbonyl]-glycine; VDAC, Voltage dependent anionic channel; VOSO4, Vanadyl sulfate; VO(acac)2, Bis(acetylacetonato) oxovanadium(IV).
References
Aureliano, M., and Crans, D. C. (2009). Decavanadate (V10O286−) and oxovanadates: oxometalates with many biological activities. J. Inorg. Biochem. 103 (4), 536–546. doi:10.1016/j.jinorgbio.2008.11.010
Aureliano, M., De Sousa-Coelho, A. L., Dolan, C. C., Roess, D. A., and Crans, D. C. (2023). Biological consequences of vanadium effects on formation of reactive oxygen species and lipid peroxidation. Int. J. Molec. Sci. 24 (6), 5382. doi:10.3390/ijms24065382
Avila, D. S., Puntel, R. L., and Aschner, M. (2013). Manganese in health and disease. Metal Ions Life Sci. 13, 199–227. doi:10.1007/978-94-007-7500-8_7
Bauer, T. M., and Murphy, E. (2020). Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circulation Res. 126 (2), 280–293. doi:10.1161/CIRCRESAHA.119.316306
Becatti, M., Bencini, A., Nistri, S., Conti, L., Fabbrini, M. G., Lucarini, L., et al. (2019). Different antioxidant efficacy of two MnII-containing superoxide anion scavengers on hypoxia/reoxygenation-exposed cardiac muscle cells. Sci. Rep. 9, 10320. doi:10.1038/s41598-019-46476-2
Bertero, E., Popoiu, T. A., and Maack, C. (2024). Mitochonrial calcium in cardiac ischemia/reperfusion injury and cardioprotection. Basic Res. Cardiol. 119, 569–585. doi:10.1007/s00395-024-01060-2
Belosludtsev, K. N., Sharipov, R. R., Boyarkin, D. P., Belosludtseva, N. V., Dubinin, M. V., Krasilnikova, I. A., et al. (2021). The effect of DS16570511, a new inhibitor of mitochondrial calcium uniporter, on calcium homeostasis, metabolism, and functional state of cultured cortical neurons and isolated brain mitochondria. Biochimica Biophysica Acta (BBA) - General Subj. 1865 (5), 129847. doi:10.1016/j.bbagen.2021.129847
Bhat, A. H., Dar, K. B., Anees, S., Zargar, M. A., Masood, A., Sofi, M. A., et al. (2015). Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 74, 101–110. doi:10.1016/j.biopha.2015.07.025
Bhuiyan, M. S., and Fukunga, K. (2009). Cardioprotection by vanadium compounds targeting akt-mediated signaling. J. Pharmacol. Sci. 110, 1–13. doi:10.1254/jphs.09R01CR
Buglyó, P., Crans, D. C., Nagy, E. M., Lindo, R. L., Yang, L., Smee, J. J., et al. (2005). Aqueous chemistry of the vanadium III (V III) and the V III −Dipicolinate systems and a comparison of the effect of three oxidation states of vanadium compounds on diabetic hyperglycemia in rats. Inorg. Chem. 44 (15), 5416–5427. doi:10.1021/ic048331q
Crans, D. C. (1994). Aqueous chemistry of labile oxovanadates: relevance to biological studies. Comments Inorg. Chem. 16, 1–33. doi:10.1080/02603599408035850
Crans, D. C., and Kostenkova, K. (2020). Open questions on the biological roles of first-row transition metals. Commun. Chem. 3 (1), 104. doi:10.1038/s42004-020-00341-w
Crans, D. C., Mahroof-Tahir, M., and Keramidas, A. D. (1995). Vanadium chemistry and biochemistry of relevance for use of vanadium compounds as antidiabetic agents. Mol. Cell. Biochem. 153, 17–24. doi:10.1007/BF01075914
Crans, D. C., Smee, J. J., Gaidamauskas, E., and Yang, L. (2004). The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 104 (2), 849–902. doi:10.1021/cr020607t
Crans, D. C., Woll, K. A., Prusinskas, K., Johnson, M. D., and Norkus, E. (2013). Metal speciation in health and medicine represented by iron and vanadium. Inorg. Chem. 52 (21), 12262–12275. doi:10.1021/ic4007873
Diaz-Vegas, A., Sanchez-Aguilera, P., Krycer, J. R., Morales, P. E., Monsalves-Alvarez, M., Cifuentes, M., et al. (2020). Is mitochondrial dysfunction a common root of noncommunicable chronic diseases? Endocr. Rev. 41, bnaa005. doi:10.1210/endrev/bnaa005
Dinda, R., Garribba, E., Sanna, D., Crans, D. C., and Costa Pessoa, J. (2025). Hydrolysis, ligand exchange, and redox properties of vanadium compounds: implications of solution transformation on biological, therapeutic, and environmental applications. Chem. Rev. 125, 1468–1603. doi:10.1021/acs.chemrev.4c00475
Galvani, P., Fumagalli, P., and Santagostino, A. (1995). Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 293 (4), 377–383. doi:10.1016/0926-6917(95)90058-6
Gavin, C. E., Gunter, K. K., and Gunter, T. E. (1999). Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology 20 (2–3), 445–453.
Guilarte, T. R. (2010). Manganese and Parkinson’s disease: a critical review and new findings. Environ. Health Perspect. 118 (8), 1071–1080. doi:10.1289/ehp.0901748
Gunter, T. E. (2017). “Manganese and mitochondrial function,” in Molecular, genetic, and nutritional aspects of major and trace minerals (Elsevier), 389–396. doi:10.1016/B978-0-12-802168-2.00032-4
Gunter, T. E., Gavin, C. E., Aschner, M., and Gunter, K. K. (2006). Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology 27 (5), 765–776. doi:10.1016/j.neuro.2006.05.002
Hamanaka, R. B., and Chandel, N. S. (2010). Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 35 (9), 505–513. doi:10.1016/j.tibs.2010.04.002
Hanus-Fajerska, E., Wiszniewska, A., and Kamińska, I. (2021). A dual role of vanadium in environmental systems—beneficial and detrimental effects on terrestrial plants and humans. Plants 10 (6), 1110. doi:10.3390/plants10061110
He, Z., Han, S., Zhu, H., Hu, X., Li, X., Hou, C., et al. (2020). The protective effect of vanadium on cognitive impairment and the neuropathology of Alzheimer’s disease in APPSwe/PS1dE9 mice. Front. Mol. Neurosci. 13, 21. doi:10.3389/fnmol.2020.00021
Hosseini, M.-J., Shaki, F., Ghazi-Khansari, M., and Pourahmad, J. (2013). Toxicity of vanadium on isolated rat liver mitochondria: a new mechanistic approach. Metallomics 5 (2), 152. doi:10.1039/c2mt20198d
Huang, R., Wu, Y., Shen, F., Chen, S., Yang, X., Lin, Y., et al. (2025). Manganese-coordinated nanoparticles loaded with CHK1 inhibitor dually activate cGAS-STING pathway and enhance efficacy of immune checkpoint therapy. Biomaterials 319, 123199. doi:10.1016/j.biomaterials.2025.123199
Imhoff, A., Sweeney, N. L., Bongard, R. D., Syrlybaeva, R., Gupta, A., Del Carpio, E., et al. (2024). Structural and kinetic characterization of DUSP5 with a di-phosphorylated tripeptide substrate from the ERK activation loop. Front. Chem. Biol. 3, 1385560. doi:10.3389/fchbi.2024.1385560
Imtiaz, M., Rizwan, M. S., Xiong, S., Li, H., Ashraf, M., Shahzad, S. M., et al. (2015). Vanadium, recent advancements and research prospects: a review. Environ. Int. 80, 79–88. doi:10.1016/j.envint.2015.03.018
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., and Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 7 (2), 60–72. doi:10.2478/intox-2014-0009
Jiang, Y., and Zheng, W. (2005). Cardiovascular toxicities upon manganese exposure. Cardiovasc. Toxicol. 5, 345–354. doi:10.1385/ct:5:4:345
Karges, J., Stokes, R. W., and Cohen, S. M. (2021). Metal complexes for therapeutic applications. Trends Chem. 3 (7), 523–534. doi:10.1016/j.trechm.2021.03.006
Korotkov, S. M. (2023). Mitochondrial oxidative stress is the general reason for apoptosis induced by different-valence heavy metals in cells and mitochondria. Int. J. Mol. Sci. 24, 14459. doi:10.3390/ijms241914459
Kostenkova, K., Scalese, G., Gambino, D., and Crans, D. C. (2022). Highlighting the roles of transition metals and speciation in chemical biology. Curr. Opin. Chem. Biol. 69, 102155. doi:10.1016/j.cbpa.2022.102155
Kostova, I. (2009). Titanium and vanadium complexes as anticancer agents. Anti-Cancer Agents Med. Chem. 9 (8), 827–842. doi:10.2174/187152009789124646
Kowalski, S., Wyrzykowski, D., and Inkielewicz-Stępniak, I. (2020). Molecular and cellular mechanisms of cytotoxic activity of vanadium compounds against cancer cells. Molecules 25 (7), 1757. doi:10.3390/molecules25071757
Kozieł, S., Wojtala, D., Szmitka, M., Sawka, J., and Komarnicka, U. K. (2024). Can Mn coordination compounds be good candidates for medical applications? Front. Chem. Biol. 3, 1337372. doi:10.3389/fchbi.2024.1337372
Kumar, S., Kumari, S., Karan, R., Kumar, A., Rawal, R. K., and Kumar Gupta, P. (2024). Anticancer perspectives of vanadium complexes. Inorg. Chem. Commun. 161, 112014. doi:10.1016/j.inoche.2023.112014
Kuznetsov, A. V., Javadov, S., Margreiter, R., Grimm, M., Hagenbuchner, J., and Ausserlechner, M. J. (2019). The role of mitochondria in the mechanisms of cardiac ischemia-reperfusion injury. Antioxidants 8 (10), 454. doi:10.3390/antiox8100454
Kwakye, G., Paoliello, M., Mukhopadhyay, S., Bowman, A., and Aschner, M. (2015). Manganese-induced parkinsonism and Parkinson’s disease: shared and distinguishable features. Int. J. Environ. Res. Public Health 12 (7), 7519–7540. doi:10.3390/ijerph120707519
Lei, H., Li, Q., Li, G., Wang, T., Lv, X., Pei, Z., et al. (2024). Manganese molybdate nanodots with dual amplification of STING activation for “cycle” treatment of metalloimmunotherapy. Bioact. Mater. 31, 53–62. doi:10.1016/j.bioactmat.2023.07.026
Lei, W., Liu, H., Zhong, L., Yang, X., and Wang, K. (2007). Vanadyl ions binding to GroEL (HSP60) and inducing its depolymerization. Chin. Sci. Bull. 52 (20), 2775–2781. doi:10.1007/s11434-007-0380-0
Levina, A., Crans, D. C., and Lay, P. A. (2017). Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities Coor. Coord. Chem. Rev. 352, 473–498. doi:10.1016/j.ccr.2017.01.002
Li, L., and Yang, X. (2018). The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxidative Med. Cell. Longev. 2018, 7580707. doi:10.1155/2018/7580707
Lima, L. M. A., Murakami, H., Gaebler, D. J., Silva, W. E., Belian, M. F., Lira, E. C., et al. (2021). Acute toxicity evaluation of non-innocent oxidovanadium(V) schiff base complex. Inorganics 9 (6), 42. doi:10.3390/inorganics9060042
Liu, Y., Barber, D. S., Zhang, P., and Liu, B. (2013). Complex II of the mitochondrial respiratory chain is the key mediator of divalent manganese-induced hydrogen peroxide production in microglia. Toxicol. Sci. 132 (2), 298–306. doi:10.1093/toxsci/kfs344
Ma, D.-L., Wu, C., Li, G., Yung, T.-L., and Leung, C.-H. (2020). Transition metal complexes as imaging or therapeutic agents for neurodegenerative diseases. J. Mater. Chem. B 8 (22), 4715–4725. doi:10.1039/C9TB02669J
Marchi, S., and Pinton, P. (2014). The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J. Physiology 592 (5), 829–839. doi:10.1113/jphysiol.2013.268235
Markiv, B., Expósito, A., Ruiz-Azcona, L., Santibáñez, M., and Fernández-Olmo, I. (2023). Environmental exposure to manganese and health risk assessment from personal sampling near an industrial source of airborne manganese. Environ. Res. 224, 115478. doi:10.1016/j.envres.2023.115478
Martell, A. E., and Smith, R. M. (1977). Other organic ligands. Boston, MA: Springer US. doi:10.1007/978-1-4757-1568-2
Matuz-Mares, D., González-Andrade, M., Araiza-Villanueva, M. G., Vilchis-Landero, M. M., and Vázquez-Meza, H. (2022). Mitochondrial calcium: effects of its imbalance in disease. Antioxidants (Basel). 11 (5), 801. doi:10.3390/antiox11050801
Mousa, A., and Shehab, A. (2015). The effect of manganese on the olfactory bulb of adult male albino rat and the role of meloxicam: a histological and immunohistochemical study. J. Microsc. Ultrastruct. 3 (1), 8. doi:10.1016/j.jmau.2014.11.002
Murakami, H. A., Uslan, C., Haase, A. A., Koehn, J. T., Vieira, A. P., Gaebler, D. J., et al. (2022). Vanadium chloro-substituted schiff base catecholate complexes are reducible, lipophilic, water stable, and have anticancer activities. Inorg. Chem. 61 (51), 20757–20773. doi:10.1021/acs.inorgchem.2c02557
Nistri, S., Boccalini, G., Bencini, A., Becatti, M., Valtancoli, B., Conti, L., et al. (2015). A new low molecular weight, MnII-containing scavenger of superoxide anion protects cardiac muscle cells from hypoxia/reoxygenation injury. Free Radic. Res. 49 (1), 67–77. doi:10.3109/10715762.2014.979168
Ohiomokhare, S., Olaolorun, F., Ladagu, A., Olopade, F., Howes, M.-J. R., Okello, E., et al. (2020). The pathopharmacological interplay between vanadium and iron in Parkinson’s disease models. Int. J. Mol. Sci. 21 (18), 6719. doi:10.3390/ijms21186719
Pajarillo, E., Nyarko-Danquah, I., Adinew, G., Rizor, A., Aschner, M., and Lee, E. (2021). Neurotoxicity mechanisms of manganese in the central nervous system. Adv. Neurotoxicology 5, 215–238. doi:10.1016/bs.ant.2020.11.003
Paolillo, M., Ferraro, G., Sahu, G., Pattanayak, P. D., Garribba, E., Halder, S., et al. (2025). Interaction of VVO2-hydrazonates with lysozyme. J. Inorg. Biochem. 264, 112787. doi:10.1016/j.jinorgbio.2024.112787
Phillips, A. M. F., and Pombeiro, A. J. L. (2020). Transition metal-based prodrugs for anticancer drug delivery. Curr. Med. Chem. 26 (41), 7476–7519. doi:10.2174/0929867326666181203141122
Prihantono, I. R., Irfandi, R., Raya, I., and Warsinggih, W. (2020). Potential anticancer activity of Mn (II) complexes containing arginine dithiocarbamate ligand on MCF-7 breast cancer cell lines. Ann. Med. Surg. (Lond.) 60, 396–402. doi:10.1016/j.amsu.2020.11.018
Queiroz, H. M., Ying, S. C., Abernathy, M., Barcellos, D., Gabriel, F. A., Otero, X. L., et al. (2021). Manganese: the overlooked contaminant in the world largest mine tailings dam collapse. Environ. Int. 146, 106284. doi:10.1016/j.envint.2020.106284
Ramachandra, C. J. A., Hernandez-Resendiz, S., Crespo-Avilan, G. E., Lin, Y.-H., and Hausenloy, D. J. (2020). Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine 57, 102884. doi:10.1016/j.ebiom.2020.102884
Rama Rao, K. V., Reddy, P. V. B., Hazell, A. S., and Norenberg, M. D. (2007). Manganese induces cell swelling in cultured astrocytes. NeuroToxicology 28 (4), 807–812. doi:10.1016/j.neuro.2007.03.001
Rao, K. V. R., and Norenberg, M. D. (2004). Manganese induces the mitochondrial permeability transition in cultured astrocytes. J. Biol. Chem. 279 (31), 32333–32338. doi:10.1074/jbc.m402096200
Rehder, D. (2013). Vanadium its role for humans. Metal Ions Life Sci. 13, 139–169. doi:10.1007/978-94-007-7500-8_5
Reul, B. A., Amin, S. S., Buchet, J. P., Ongemba, L. N., Crans, D. C., and Brichard, S. M. (1999). Effects of vanadium complexes with organic ligands on glucose metabolism: a comparison study in diabetic rats. Br. J. Pharmacol. 126, 467–477. doi:10.1038/sj.bjp.0702311
Rojas-Lemus, M., López-Valdez, N., Bizarro-Nevares, P., González-Villalva, A., Ustarroz-Cano, M., Zepeda-Rodríguez, A., et al. (2021). Toxic effects of inhaled vanadium attached to particulate matter: a literature review. Int. J. Environ. Res. Pub. Health 18 (16), 8457. doi:10.3390/ijerph18168457
Samart, N., Arhouma, Z., Kumar, S., Murakami, H. A., Crick, D. C., and Crans, D. C. (2018). Decavanadate inhibits mycobacterial growth more potently than other oxovanadates. Front. Chem. 6, 519. doi:10.3389/fchem.2018.00519
Sarkar, S., Malovic, E., Harischandra, D. S., Ngwa, H. A., Ghosh, A., Hogan, C., et al. (2018). Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology 64, 204–218. doi:10.1016/j.neuro.2017.05.009
Schell, J. R., Wei, S.-J., Zhang, J., Trevino, R., Chen, W. H., Aguilar, L., et al. (2025). MnSOD non-acetylation mimic knock-in mice exhibit dilated cardiomyopathy. Free Radic. Biol. Med. 229, 58–67. doi:10.1016/j.freeradbiomed.2025.01.028
Ścibior, A., Llopis, J., Dobrakowski, P. P., and Męcik-Kronenberg, T. (2023). CNS-related effects caused by vanadium at realistic exposure levels in humans: a comprehensive overview supplemented with selected animal studies. Int. J. Mol. Sci. 24 (10), 9004. doi:10.3390/ijms24109004
Shoshan-Barmatz, V., and De, S. (2017). Mitochondrial VDAC, the Na+/Ca2+ exchanger, and the Ca2+ uniporter in Ca2+ dynamics and signaling. Adv. Exp. Med. Biol. 981, 323–347. doi:10.1007/978-3-319-55858-5_13
Sousa-Coelho, A. L. D., Fraqueza, G., and Aureliano, M. (2024). Repurposing therapeutic drugs complexed to vanadium in cancer. Pharmaceuticals 17 (1), 12. doi:10.3390/ph17010012
Sun, X., Xu, X., Li, F., Wang, H., Sun, Y., Yang, H., et al. (2025). Immunity-modulating metal-based nanomaterials for cancer immunotherapy. Adv. Funct. Mater. doi:10.1002/adfm.202502646
Treviño, S., Díaz, A., Sánchez-Lara, E., Sanchez-Gaytan, B. L., Perez-Aguilar, J. M., and González-Vergara, E. (2019). Vanadium in biological action: chemical, pharmacological aspects, and metabolic implications in diabetes mellitus. Bio.Trace ele. Res 188 (1), 68–98. doi:10.1007/s12011-018-1540-6
Wan, H., Yan, Y., Hu, X., Shang, L., Chen, Y., Huang, Y., et al. (2023). Inhibition of mitochondrial VDAC1 oligomerization alleviates apoptosis and necroptosis of retinal neurons following OGD/R injury. Ann. Anat. - Anatomischer Anzeiger 247, 152049. doi:10.1016/j.aanat.2023.152049
Wang, W., Mo, W., Hang, Z., Huang, Y., Yi, H., Sun, Z., et al. (2023). Cuproptosis: harnessing transition metal for cancer therapy. ACS Nano 17 (20), 19581–19599. doi:10.1021/acsnano.3c07775
Wang, Y., Xu, E., Musich, P. R., and Lin, F. (2019). Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 25 (7), 816–824. doi:10.1111/cns.13116
Wang, Y., Xu, Y., Song, J., Liu, X., Liu, S., Yang, N., et al. (2024). Tumor cell-targeting and tumor microenvironment-responsive nanoplatforms for the multimodal imaging-guided photodynamic/photothermal/chemodynamic treatment of cervical cancer. Int. J. Nanomedicine. 19, 5837–5858. doi:10.2147/IJN.S466042
Warren, E. B., Bryan, M. R., Morcillo, P., Hardeman, K. N., Aschner, M., and Bowman, A. B. (2020). Manganese-induced mitochondrial dysfunction is not detectable at exposures below the acute cytotoxic threshold in neuronal cell types. Toxicol. Sci. 176 (2), 446–459. doi:10.1093/toxsci/kfaa079
Willsky, G. R., Chi, L.-H., Godzala, M., Kostyniak, P. J., Smee, J. J., Trujillo, A. M., et al. (2011). Anti-Diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes. Coord. Chem. Rev. 255 (19–20), 2258–2269. doi:10.1016/j.ccr.2011.06.015
Xiao, S., Zhou, Y., Liu, T., Hu, Y., Wu, Q., Pan, Q., et al. (2021). The association between manganese exposure with cardiovascular disease in older adults: NHANES 2011-2018. J. Environ. Sci. Health, Part A 56, 1221–1227. doi:10.1080/10934529.2021.1973823
Xiong, Z., Xing, C., Xu, T., Yang, Y., Liu, G., Hu, G., et al. (2021). Vanadium induces oxidative stress and mitochondrial quality control disorder in the heart of ducks. Front. Vet. Sci. 8, 756534. doi:10.3389/fvets.2021.756534
Zhao, Y., Ye, L., Liu, H., Xia, Q., Zhang, Y., Yang, X., et al. (2010). Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J. Inorg. Biochem. 104 (4), 371–378. doi:10.1016/j.jinorgbio.2009.11.007
Zhao, Y., and Zhao, B. (2013). Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2013, 1–10. doi:10.1155/2013/316523
Keywords: cardiac mitochondrial function, vanadium, manganese, salts, coordination complexes, mitochondrial calcium uniporter, mitochondrial swelling, speciation
Citation: Dolan CC, Whitcomb LA, Del Carpio E, Rose L, Chicco AJ and Crans DC (2025) How vanadium and manganese compounds impact cardiac mitochondrial function. Front. Chem. Biol. 4:1602602. doi: 10.3389/fchbi.2025.1602602
Received: 30 March 2025; Accepted: 16 May 2025;
Published: 17 June 2025.
Edited by:
Sotiris K. Hadjikakou, University of Ioannina, GreeceReviewed by:
Francesco Paolo Fanizzi, University of Salento, ItalyAnastasios Keramidas, University of Cyprus, Cyprus
Christina Banti, University of Ioannina, Greece
Copyright © 2025 Dolan, Whitcomb, Del Carpio, Rose, Chicco and Crans. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam J. Chicco, YWRhbS5jaGljY29AY29sb3N0YXRlLmVkdQ==; Debbie C. Crans, ZGViYmllLmNyYW5zQGNvbG9zdGF0ZS5lZHU=
 Connor C. Dolan1
Connor C. Dolan1 Edgar Del Carpio
Edgar Del Carpio Adam J. Chicco
Adam J. Chicco Debbie C. Crans
Debbie C. Crans