- Department of Chemistry, Colorado State University, Fort Collins, CO, United States
The development of novel diagnostic, theranostic, and therapeutic agents drastically improved human health, human lifespan, and quality of life. In 2024, 15 of the 50 (30%) new drugs approved by the Food and Drug Administration (FDA) were developed for the treatment of cancer. Despite encouraging examples of platinum-based anticancer drugs and many metal-based diagnostic agents for cancer, only a few metal-based drugs have translated to clinical success. Therapeutic drugs share many properties with diagnostic and theranostic agents, such as distribution and uptake, but differ in one key aspect: stability. Stability is key to the action of the potential drug and impact excretion and metabolism, and these properties illustrate the differences between diagnostic and therapeutic agents. That is, diagnostics are inherently stable and not metabolized whereas therapeutics are commonly administered as pro-drugs where metabolism is a common and often important aspect of their mode of action. In this perspective, we point to a novel administration strategy, such as intra-tumoral injections, for which highly reactive compounds, such as metal-based compounds would be desirable as long as the decomposition products are non-toxic. Investigations into a class of vanadium compounds for administration in difficult-to-treat cancers, such as glioblastomas, are briefly described here.
Introduction
Modern medicine has been increasingly successful in treating diseases and genetic disorders, producing a range of pharmaceuticals for various conditions. As a result, pre-clinical studies demonstrating efficacy is no longer sufficient to reflect the clinical success of a drug (Seyhan, 2019). Modern drug development must consider toxicity and side effects, formulation, accessibility and increasingly demanding regulations for a drug to translate to widespread clinical adoption. In this perspective, we aim to highlight the current landscape and recent advances in state-of-the-art cancer drug development (Ronconi and Sadler, 2007; Jomova et al., 2024; Sen et al., 2022; Li et al., 2025; Nidhi et al., 2025; Zahirović et al., 2024; Abdullah et al., 2024; De Sousa-et al., 2023; Wang et al., 2023; Skos et al., 2024; Scattolin et al., 2025; Crans and Kostenkova, 2020; Crans, 2015; Zhang and Sadler, 2017; Gunaydin et al., 2021; Anthony et al., 2020) and diagnostic agents (Wu et al., 2025; Kim and Nimse, 2025; Rex et al., 2025; Stasiuk and Long, 2013; Boswell et al., 2004; Janib et al., 2010; Terreno et al., 2010; Kostelnik and Orvig, 2019; Wahsner et al., 2019; Caravan et al., 1999; Hancu et al., 2010) with a focus on metal complexes used as MRI contrast agents (Janib et al., 2010; Terreno et al., 2010; Wahsner et al., 2019; Caravan et al., 1999; Hancu et al., 2010; Na et al., 2009; Jeon et al., 2021; Runge, 2017; Fraum et al., 2017; Ramalho et al., 2016; Chen et al., 2022; Müssig et al., 2021) and radiopharmaceuticals (Stasiuk and Long, 2013; Boswell et al., 2004; Kostelnik and Orvig, 2019; Rathmann et al., 2019; Yang et al., 2024; Zhang et al., 2025; Holland et al., 2009; Duatti, 2021; Crişan et al., 2022; Kelkar and Reineke, 2011; Gutfilen et al., 2018; Hennrich and Benešová, 2020; Zboralski et al., 2022; Kraus et al., 2022; Krasnovskaya et al., 2023; Kelly et al., 2020). Additionally, we will describe the properties of a few metal-based therapeutics and compare them to a new class of vanadium-based Schiff base catecholate complexes that we have been investigating for potential use for intratumoral administration (Levina et al., 2020; Levina et al., 2022; Bates et al., 2025). Finally, we will compare therapeutic and diagnostic drugs with the aim of gaining a deeper understanding of the desirable properties of successful and potential drugs.
In 2024, 50 new small molecule, biologic, and oligonucleotide therapeutics were approved by the Center for Drug Evaluation and Research in the United States (FDA) (Mullard, 2025). Figure 1 shows the distribution of novel drug approvals in the United States in 2024, indicating cancer therapeutics comprise 30% of newly introduced drugs. Many of the therapeutic areas show a higher number of new drugs in 2024 than the 5-year average (Mullard, 2025). Beyond small molecules, the FDA’s Center for Biologics Evaluation and Research (CBER) added an additional set of cell and gene therapies, vaccines, and blood products which received approvals. These substances provide an alternative approach to cancer treatment which are well tolerated by the immune system and this class of drugs are called T-cell receptor therapy and Afamitresgene autoleucel is an example of a cancer related drug approved in spring of 2025 (Mullard, 2025).
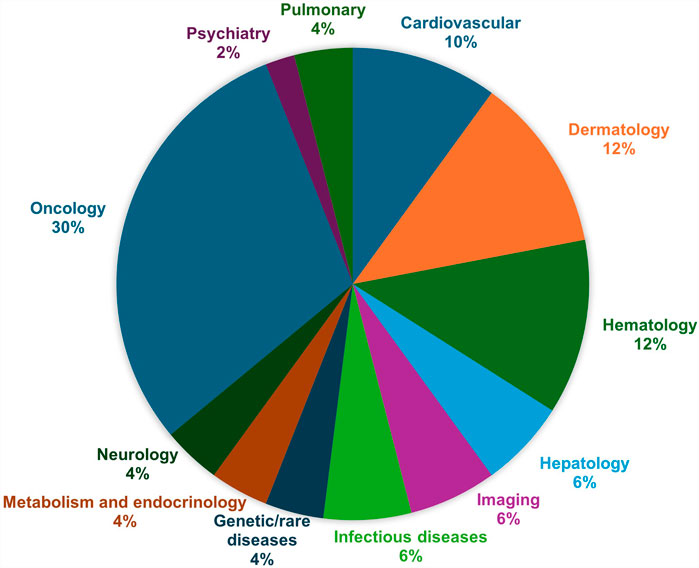
Figure 1. The United States of America FDA approvals by therapeutic areas in 2024 (Mullard, 2025).
Traditionally, small molecule drugs are subject to Lipinski’s rule of five which were developed using computational analysis of successful small molecule drugs and drug candidates (Lipinski, 2016). These guidelines include the following criteria: a molecular weight that is less than 500 Da, a maximum level of hydrophobicity determined by the octanol-water partition coefficient (logP) less than 5; and the molecule must contain no more than 5 hydrogen bond donors, and no more than 10 hydrogen bond acceptors. Other approaches to characterizing drug properties have been reported focusing on structural features and frequency of false positive and negative hits in drug screens (Nelson et al., 2017; Sun et al., 2021). Metal-based therapeutics rarely adhere to Lipinski’s rules because the metal-based drugs are generally less stable in vivo than organic drugs, releasing metal ions, which can potentially create reactive oxygen species when participating in redox chemistry (Dinda et al., 2025; Aurelia et al., 2023).
Many diagnostic agents have a metal ion as part of their chromophore enabling their detection via UV-visible, fluorescence, phosphorescence, or other types of spectroscopic methods. In vivo metal-based diagnostic agents fall into three categories: X-ray contrast agents (Yu and Watson, 1999; Lusic and Grinstaff, 2013), MRI contrast agents (Terreno et al., 2010; Wahsner et al., 2019; Caravan et al., 1999; Hancu et al., 2010; Na et al., 2009; Jeon et al., 2021), and radiotracers (Kostelnik and Orvig, 2019; Rathmann et al., 2019; Yang et al., 2024; Zhang et al., 2025; Holland et al., 2009; Duatti, 2021; Crişan et al., 2022). In 1988, the first metal-based magnetic resonance imaging (MRI) contrast agent was approved by the FDA for clinical use, gadolinium-based contrast agent (GBCA) gadopentetate dimeglumine (Gd-DTPA, Magnevist, Figure 2) (Caravan et al., 1999). Metal-based diagnostic agents must be very stable under physiological conditions to prevent metal leaching and potential cytotoxicity. Furthermore, a new field has emerged combining a diagnostic (“nostic”) and therapeutic (“thera”) called theranostics where the agents both diagnose and treat at the same time (Janib et al., 2010; Kostelnik and Orvig, 2019; Zhang et al., 2025; Crişan et al., 2022; Kelkar and Reineke, 2011; Gutfilen et al., 2018).
Therapeutic drug processing and drug formulation
The pharmacological properties of a drug include its pharmacodynamic and pharmacokinetic properties. The pharmacodynamic properties of a drug involve “what a drug does” to a biological system. The potency of a drug is the amount (dose) of drug required to produce the intended effect (intensity/maximum). The efficacy of a drug is its capacity (intensity/maximum) to produce the effect. It is important to recognize that the success of a drug requires much more than high potency (low dose) and favorable efficacy. The pharmacokinetic properties of a drug are the processes which take place upon drug administration. Pharmacokinetics is defined by four critical processes: administration, distribution, metabolism, and excretion, which is abbreviated ADME. Each of these processes is important to the success of a drug and can be impacted by the method of administration and its formulation (delivery vehicle).
There are many different administration methods, and the specific properties of a particular drug must be considered when choosing the administration method and delivery vehicle (Wen et al., 2015). For example, if the drug is administered orally, it must be able to survive the acidic environment in the stomach. Many anticancer drugs are administered intravenously so they must be able to survive circulation and metabolism that can take place in blood. Drug delivery approaches do not change the fundamental pharmacodynamic properties of a drug, but they can modify its pharmacokinetic properties, which can impact its pharmacodynamic performance (Wen et al., 2015; Duan et al., 2016). For example, the initial formulation of chemotherapy drug Vincristine resulted in rapid clearance and was ultimately not approved by the FDA. However, when encapsulated in sphingomyelin/cholesterol liposomes, Vincristine’s clearance rate decreased and resulted in increased efficacy against tumor cells. The modified formulation was approved by the FDA in 2012 (Silverman and Deitcher, 2013), and Vincristine is still administered with high survival rates particularly in protocols with other drugs when treating various leukemias. At this point, few metal-based pharmaceuticals make it to the clinic. However, as novel treatment strategies become more common, the potential benefits of metal-based pharmaceuticals can outweigh the risks, as exemplified by the dramatic increase in number of clinical trials involving intratumoral administration and metal-based drugs (Levina et al., 2022; Bates et al., 2025).
Metal-based therapeutic drugs
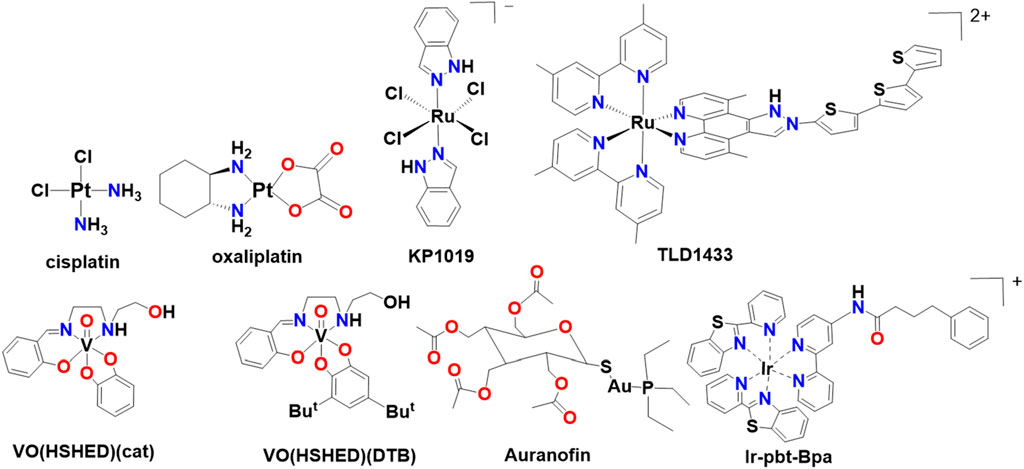
Cis-diamminedichlorido-platinum (II) (cisplatin, Figure 2) has been used in the clinic for >40 years, far beyond the 20-year original patent lifetime. Platinum (Pt)-based drugs are among the most frequently used cancer therapeutic agents (Rottenberg et al., 2020). Pt-based drugs form adducts with DNA, preventing DNA repair enzymes from removing Pt and preventing mitosis (Kartalou and Essigmann, 2001). Cisplatin can form monoadducts, intrastrand, or interstrand cross-links with both single and double-stranded DNA, although research has demonstrated that Pt-compounds engage in multiple other mechanisms of action which result in their antiproliferative properties (Liu et al., 2024; Manioudakis et al., 2019). The 1,2-intrastrand crosslinks with DNA double helices are particularly devastating for cells (Kartalou and Essigmann, 2001), but other mechanisms also cause multiple side effects such as vomiting, nephrotoxicity, and neurotoxicity in patients (Liu et al., 2024; Manioudakis et al., 2019). However, newer Pt-drugs, such as cis-[(1R,2R)-1,2-cyclohexanediamine-N,N'][oxalato (2-)-O,O'] platinum (oxaliplatin, Figure 2), have been developed with increased solubility, improved efficacy, and decreased toxicity compared to cisplatin and both drugs are still used in the clinic. Co-administering cisplatin with lipids reduces its toxicity, and many novel formulations of Pt-drugs are currently being investigated in clinical trials (Duan et al., 2016; Doucette et al., 2016).
Many metal compounds are effective antiproliferative agents and excellent reviews have been written on this topic including coordination complexes with ruthenium, gold, copper, iridium and osmium (Wang et al., 2023; Skos et al., 2024; Scattolin et al., 2025; Anthony et al., 2020; Kozieł et al., 2024), a few representative compounds are shown in Figure 2. Metal ions such as rhodium, rhenium, cobalt, manganese, and vanadium have also been explored and selected vanadium complexes are also shown in Figure 2. We refer the readers to reviews for additional details on these classes of drugs (Ronconi and Sadler, 2007; Jomova et al., 2024; Sen et al., 2022; Li et al., 2025; Nidhi et al., 2025; Zahirović et al., 2024; Abdullah et al., 2024; De Sousa-et al., 2023; Crans and Kostenkova, 2020; Crans, 2015; Zhang and Sadler, 2017; Anthony et al., 2020; Rottenberg et al., 2020; Thota et al., 2018). Suffice to say that two Ru-compounds (KP1019 and TLD1433), shown in Figure 2, are currently in clinical trials (Thota et al., 2018). Both these drugs have activity against Pt-resistant cells and act through a different mechanism than Pt-drugs. In addition, Au-complexes such as Auranofin (Celegato et al., 2015), and other metal-based complexes including Ir-ptb-Bpa (Wang et al., 2023) are being investigated in pre-clinical studies (Figure 2). The success of these compounds supports the current and continued future interest in metal-based anticancer drugs. In the following sections we will describe diagnostic and theranostic agents and conclude this perspective with potential therapeutic vanadium anticancer agents designed for intratumoral injections.
Metal-based diagnostics
Metal complexes have been important tools in the diagnosis of disease for over a century. Metal-based diagnostics share many similarities with therapeutics in that they are designed for imaging a particular target tissue, have sufficient biological half-life, be minimally disruptive to biological processes, show low toxicity, and be excreted or metabolized after imaging is complete. However, there is one major difference from the therapeutics, particularly with metal-based MRI contrast agents and radiopharmaceuticals, they are typically designed to NOT be metabolized. The structures for selected MRI contrast agents and radiopharmaceuticals are shown in Figure 3.
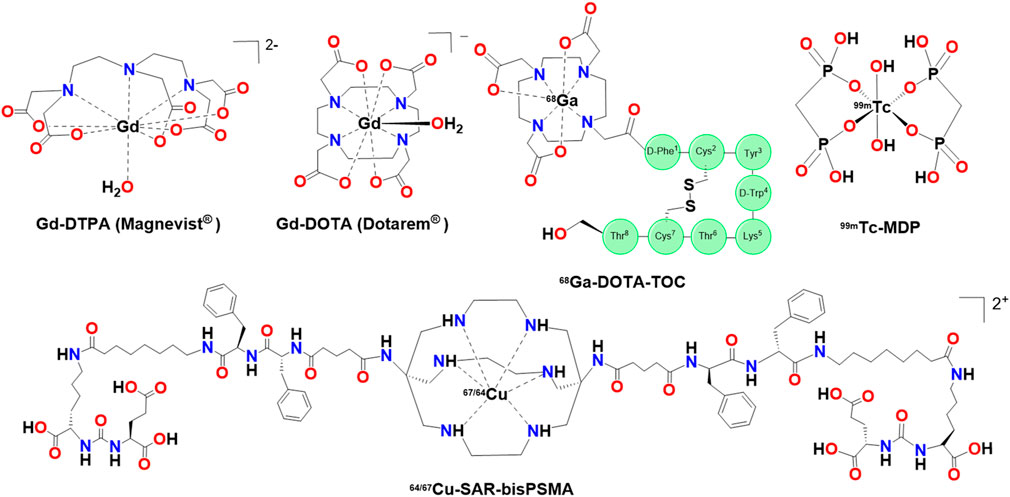
Figure 3. Selected structures of Gadolinium-based MRI contrast agents, a technetium (Tc) radiopharmaceutical and a Cu-radiotheranostic agent.
Magnetic Resonance Imaging (MRI) Contrast Agents
Today, about 40% of all MRI scans utilize Gadolinium-based Contrast Agents (GBCA) and they remain one of the most successful examples of inorganic drugs, particularly for detecting cancer (Wahsner et al., 2019). While other lanthanides have larger magnetic moments, Gd3+ has the maximum number of unpaired electrons (f7) of any stable ion, making it ideal for use as a paramagnetic MRI contrast agent. Coordinated water ligands must be able to rapidly exchange with the cellular environment to shorten T1 relaxation time, providing contrast. As such, there has been some effort made to increase the number of water ligands coordinated to Gd (increasing contrast), but there is significant cost associated with the stability of the complex (Wahsner et al., 2019), another important factor for the success of a GBCA MRI contrast agent.
GBCAs typically belong to two general classes depending on whether they contain linear or macrocyclic ligands. Macrocyclic ligands form significantly more stable metal complexes than the linear complexes as evidenced from the leakage that the latter complexes exhibited in retrospective studies (Grobner, 2006). Additional studies have reported that linear and neutral complexes are less stable under physiological conditions compared to macrocyclic and ionic complexes (Runge, 2017; Fraum et al., 2017). These studies showed that repeated administration of linear GBCAs such as Gd-DTPA led to Gd(III) deposition in the central nervous system (CNS). Gd(III) deposition is associated with nephrogenic systemic fibrosis (NSF), particularly in patients with renal impairment (Ramalho et al., 2016). This discovery has led to macrocyclic GBCAs largely supplanting their linear counterparts in the clinic.
Superparamagnetic Iron Oxide Nanoparticles (SPIONs) and their manganese-based counterparts are composed of a metal oxide core surrounded by a biocompatible surface polymer. While superparamagnetism can lead to a decrease in both T1 and T2 relaxation times, SPIONs have primarily been used as T2 contrast agents (Chen et al., 2022). In SPIONs, smaller core size increases specific surface area, allowing for increased interactions with water protons, increasing longitudinal relaxivity (r1) while decreasing magnetization and transverse relaxivity (r2). Larger r1 values and a smaller r2/r1 ratio results in a better T1 contrast agent (Chen et al., 2022). Careful balancing of core size, polymer coating, hydrodynamic diameter, and several other factors (Jeon et al., 2021) could produce an ultrasmall SPION (<5 nm) that could compete commercially with GBCAs used for T1 contrast or even produce a switchable T1/T2 contrast agent through reversible agglomeration (Chen et al., 2022; Müssig et al., 2021). While SPIONs have primarily been investigated as diagnostics and iron replacement therapeutics, there is significant interest in their development as theranostic agents for cancer therapy, exploiting a variety of cell death mechanisms (Vangijzegem et al., 2023).
Positron Emission Tomography (PET) and Single-Photon Emission Computed Tomography (SPECT) Diagnostics
The general radiotracer 18FDG (fluorodeoxyglucose) is the most prevalent agent used in PET due to increased glucose metabolism in the tumor microenvironment. More specialized, targeted 68Ga tracers are the leading application of radiometals in PET (Kostelnik and Orvig, 2019). SPECT requires a γ-emitting radionuclide while PET requires a β+-emitting (positron) radionuclide. It is generally accepted PET provides better spatial resolution and sensitivity than SPECT, but utilization of 99mTc in SPECT still surpasses all PET radiotracers for the detection of cancer (Duatti, 2021). This can primarily be attributed to comparatively low costs associated with SPECT, an abundance of FDA-approved radiopharmaceuticals targeting a large variety of biological systems, and the accessibility of 99mTc radionuclide production due to convenient 99Mo/99mTc generators (Rathmann et al., 2019). Choice of radionuclide is extremely important for the success of a radiopharmaceutical. Without accessible and affordable cyclotron targets or generators for a radionuclide, a radiopharmaceutical does not have a chance at widespread clinical adoption.
99mTc is a nearly perfect radionuclide for nuclear medicine. It is a γ-emitter with a moderate half-life (t1/2 = 6 h) with the potential to be produced off-site. Many 99mTc radiopharmaceuticals are available in convenient freeze-dried formulation kits which reduce the production burden for administration of Technetium-based radiopharmaceuticals. Success of 68Ga-based radiopharmaceuticals in the diagnosis of neuroendocrine tumors (NETs) and prostate tumors has renewed interest in the use of 99mTc for similar applications (Duatti, 2021; Crişan et al., 2022). When factoring in recent advancements in SPECT technology (Duatti, 2021), 99mTc still has a key role to play in nuclear medicine.
68Ga is a β+-emitter with a relatively short half-life (t1/2 = 68 min). The widespread use of 68Ga is largely attributed to accessibility of 68Ge/68Ga generators and the success of the first 68Ga radiotracers 68Ga-DOTA-TATE and 68Ga-DOTA-TOC (Figure 3) (Hennrich and Benešová, 2020). Like GBCAs, most radiometals necessitate the use of chelators, particularly macrocyclic chelators like DOTA, to maintain required biological stability. 68Ga-DOTA-TATE and 68Ga-DOTA-TOC are peptide-based somatostatin analogs and bind to somatostatin receptors which are highly expressed in NETs. Prostate-specific membrane antigen (PSMA) is expressed on the surface of prostate tumors and 68Ga-PSMA-11 is one of the most successful PSMA imaging agents (Kostelnik and Orvig, 2019). The small molecule drug 68Ga-FAPI (and its derivatives) and the macrocyclic analogue, 68Ga-FAP-2286 are peptide-based radiotracers targeting fibroblast activation protein-α (FAP), which is upregulated in the tumor microenvironment. FAPI and FAP-2286 are promising radioligands due to selective expression of FAP in healthy cells. Additionally, FAPI and FAP-2286 radiotracers outperformed 18FDG in some pre-clinical studies (Zboralski et al., 2022). 68Ga-DOTA-CPCR4-2 (Pentixafor) is a peptide-based radiotracer targeting C-X-C chemokine receptor 4 (CXCR4) which is overexpressed in more than 30 different types of cancer and can be particularly effective for diagnosing various types of blood cancer (Kraus et al., 2022). Though these (FAPI, FAP-2286, and Pentixafor) radiopharmaceuticals are not approved by the FDA, they remain promising and active research areas (Zhang et al., 2025).
64Cu-DOTA-TATE is one of only two copper radiotracers currently approved by the FDA. Studies have shown that 64Cu-DOTA-TATE enables detection of more cancerous lesions (Krasnovskaya et al., 2023) than 68Ga-DOTA-TOC and 64Cu has a significantly longer half-life (t1/2 = 12.7 h) compared to 68Ga. 64Cu radiotracers can be better options for hospitals that do not have cyclotron production facilities and/or transport of radionuclides is necessary (Holland et al., 2009), particularly if a hospital is not located in a major city with third party radionuclide production facilities. Additionally, 64Cu as a radionuclide is appealing for radioimmunodiagnostic applications because the pharmacokinetics tend to be slower (Yang et al., 2024).
89Zr is a kinetically inert isotope with a long half-life (t1/2 = 78.4 h) and has primarily been used as a radiotracer upon conjugation with monoclonal antibodies (mAbs). Trastuzumab was the first FDA-approved companion drug mAb to utilize 89Zr. 89Zr-trastuzumab targets human epidermal growth factor receptor 2 (HER2) which is upregulated in some tumors, particularly breast cancer (Zhang et al., 2025). 89Zr-based mAb radiotracers have also been used to target cluster of differentiation proteins 3, 4, 8, 20, and 30 to diagnose a wide variety of cancers (Zhang et al., 2025). Differentiation proteins are specific for different types of cells, allowing for the design of versatile mAb radiopharmaceuticals. Application of mAb PET radiopharmaceuticals is one of the fastest growing areas in both radiodiagnostics and radiotherapeutics.
Radiotheranostics
Radiotheranostics is a field in which a radiodiagnostic is combined with a radiotherapeutic. While 90Y has been used extensively in radioconjugates with mAbs, 177Lu to-date is the overwhelmingly preferred radiometal for targeted radiotherapy, particularly in terms of pre-clinical studies. 177Lu is a β—-emitter with a long half-life (t1/2 = 6.7 days). 177Lu-DOTA-TATE has been approved by the FDA for use in conjunction with radiotracers such as 68Ga-DOTA-TOC, DOTA-TATE, and 64Cu-DOTA-TATE for the treatment of neuroendocrine tumors. 177Lu-PSMA-617 was approved by the FDA for use in conjunction with radiotracers such as 68Ga-PSMA for the treatment of prostate cancer. Since these approvals, 177Lu-based therapeutics have exploded in popularity and therapeutic complements can be found for many of the 68Ga-based (and 18F) radiotracers currently in development.
Two isotopes of copper, 64Cu and 67Cu, have generated some interest as radiotheranostic agents. While 64Cu has primarily been used in PET as a radiotracer, it also emits β- radiation, enabling theranostic applications (Gutfilen et al., 2018). On 19 February 2025 the FDA approved with a fast-track designation a new radiotheranostic combo 64/67Cu-SAR-bisPSMA for the diagnosis and treatment of prostate cancer. The diagnostic and antineoplastic activity of 64/67Cu-SAR-bisPSMA is achieved through a sarcophagine (SAR)-based chelator bound to either 64Cu for the diagnostic or the β--emitting radioisotope 67Cu which is linked to two PSMA-binding motifs. Cu(II) complexes can be unstable in vivo with some chelators, such as DOTA, but forms more stable complexes with SAR (Kelly et al., 2020). Additionally, 64/67Cu-SAR-bisPSMA contains two PSMA-motifs which increases affinity for the receptor. Even though the combination of 64/67Cu provides many opportunities for the development of versatile radiotheranostic agents due to a single ligand framework for both radionuclides, this is only the third copper radiopharmaceutical approved by the FDA.
Vanadium compounds for use in intratumoral administration
Intratumoral administration is an example of a currently less commonly used method for therapeutic administration. This method avoids circulation of the drug in the blood and any metabolism or degradation that could take place before the drug reaches its target. We have recently been investigating vanadium(V) Schiff base catecholate complexes and their antiproliferative properties to be used for intratumoral administration in difficult-to-treat cancers such as brain cancers (specifically glioblastoma (Levina et al., 2020)) and several other cancers (Levina et al., 2022; Crans et al., 2019). There are multiple classes of vanadium compounds that have been reported to have antiproliferative effects in various types of cancerous cells. Specifically, we are referring to reports describing a range of different classes of vanadium compounds with anticancer activities including dipicolinate oxovanadium(IV) (Choroba et al., 2023), 3-hydroxy-4(1H)-pyridinonate oxovanadium(IV) complexes (Kostenkova et al., 2023), vanadium(III)-L-cysteine (Basu et al., 2017), vanadocene dichloride (Rozzo et al., 2017), acetylacetonateoxydiacetato-oxidovanadium(IV) Complexes coordinated to N-containing aromatic compounds (Chmur et al., 2025), non-oxido vanadium(IV) complexes featuring tridentate ONS chelating S-alkyl/aryl-substituted dithiocarbazate ligands (Banerjee et al., 2023), dioxidovanadium(V) Schiff base complexes ([VVO2(HL)] where HL2 is an ONO Schiff base ligand) (Rana et al., 2025); oxidovanadium(V) complexes, (HNEt3)[VVO2L] and [(VVOL)2-O], with tridentate Schiff base ligand H2L [H2L = 4-((E)-(2-hydroxy-5-nitrophenylimino)-methyl)benzene-1,3-diol] (Sahu et al., 2021); and a review of describing V-complexes with ligands existing in the biosphere (Bera et al., 2025). Unlike most of these vanadium compounds with reported anticancer activity, the vanadium in our complexes is in oxidation V. However, the two vanadium(V) Schiff base complexes listed above show effects on the human cervical cancer cell line HeLa and are significantly more stable than the very reactive non-innocent vanadium Schiff base catecholate complexes that we have been designing for intratumoral administration (Levina et al., 2020; Levina et al., 2022).
Our main design criterion for a desirable antiproliferative complex to be administered intratumorally is that they must quickly decompose (Crans et al., 2004) and the decomposition products are less toxic once they have reacted and killed the tumor tissue in the cancer cells (Figure 4A) (Bates et al., 2025). We have found that the intact vanadium(V) Schiff base catecholate complex [VO(HSHED)(DTB)] is 12 times more potent than cisplatin in the T98G cell lines (glioblastoma, Figure 4B) (Levina et al., 2020). In addition, the data showed that the complex was also more toxic than in non-cancer and normal human cell lines such as HFF-1 (Figure 4B) (Levina et al., 2020). However, as shown in Figure 4B these complexes show antiproliferative effects against several other cancer cell lines including breast (MDA-MB-231), pancreatic (PANC-1) and lung (A549) cancers. Previously, the proliferative effects of this complex were reported in bone cancer cells (human chrondronsarcoma, SW11353 cells) (Levina et al., 2020). The aged ligands and vanadate had a weaker effect than the intact complex. Comparing fresh solutions with aged solutions was used to compare the intact complex and free ligands/vanadate and potential reaction products after the hydrolysis and redox chemistry that take place under the assay conditions. In Figure 4C, the V-uptake in cells was more after treatment by fresh [VO(HSHED)(DTB)] compared to treatment with decomposed and aged [VO(HSHED)(DTB)], which documented that the intact complex is much more effective in entering the cell. Measuring aged solutions in addition to measure the components of the complex it also measure products that forms in the assay during the hydrolysis.
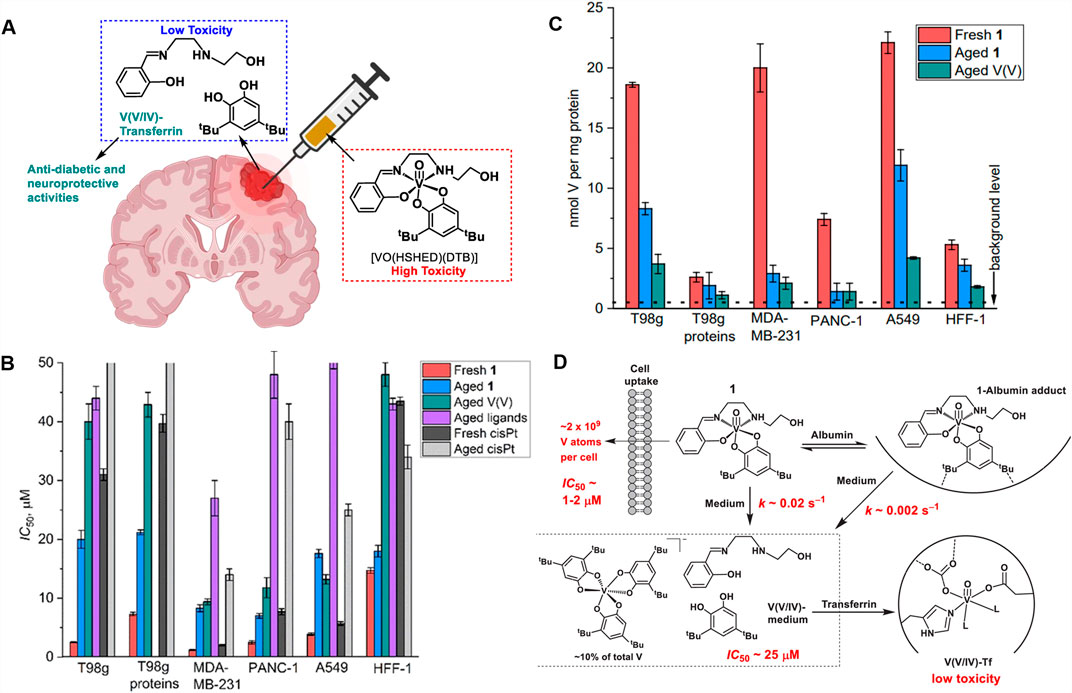
Figure 4. (A) Illustration of the intratumoral drug administration and decomposition into components; (B) the effects of fresh intact complex [VO(HSHED)(DTB)] (red; referred to a 1 in 4B and 4C), the effects of aged [VO(HSHED)(DTB)] hydrolyzed into vanadate, and ligands (coral blue); the effect of aged vanadate (turquis); aged Schiff base and catechol (purple); fresh cisPt abbreviation for cisplatin (black) and aged cisPt (grey); (C) mmol of V per mg of protein and (D) proposed action of the [VO(HSHED)(DTB)]; the complex binds to serum albumin extending its life-time before decomposition; the complex hydrolyzes to form vanadate, ligands, and the proposed [V(DTB)3]-; finally transferrin binds the vanadate that is formed upon hydrolysis.
We have used the structure-activity relationship to develop more stable and potent antiproliferative complexes, all of which contained sterically hindered catecholates (Kostenkova et al., 2023; Murakami et al., 2022; Haase et al., 2024). Similarly, with structural modification of the Schiff base framework, we have been able to develop complexes with increased stability, resulting in additional potent antiproliferative agents (Bates et al., 2025). When compounds in this class of complexes hydrolyze rapidly, they show similar activity to vanadate (Kostenkova et al., 2023; Murakami et al., 2022; Haase et al., 2024). Importantly, other vanadium compounds have antiproliferative activity against glioblastoma T98G cells, as demonstrated by the hydroxyquinoline vanadium complexes, which are also active against Trypanosoma cruzi (Levina et al., 2024). These compounds do not react quickly with the tumors and form more toxic side products, so although they are antiproliferative agents, they are not well suited for intratumoral administration.
Serum albumin has been reported to enhance other drugs’ efficacies and has even been included in some formulations of drugs in the clinic (Kratz and Elsadek, 2012; Stukan et al., 2025; Gao et al., 2024). The presence of serum albumin in the media when treating triple-negative human breast cancer (MDAMB-231) cells with [VO(HSHED)(cat)] did not change the complex’s antiproliferative effects (Levina et al., 2023). These observations are consistent with the expectation that when the vanadium(V) Schiff base catecholate complex hydrolyzes to form vanadate, such as reported for [VO(HSHED)(cat)], the vanadium complex does not interact with serum albumin (Levina et al., 2023). In contrast, more stable and effective vanadium(V) Schiff base catecholate complexes, which contained sterically hindered substituents on the catechol such as two t-butyl groups or three i-propyl groups, were found to form an adduct with serum albumin (Levina et al., 2023). These observations were supported by UV-vis spectroscopy, demonstrating that a new species was formed in the presence of [VO(HSHED)(DTB)] but not in the presence of [VO(HSHED)(cat)].
Finally, Figure 4D shows proposed pathways which the [VO(HSHED)(DTB)] complex engage in when treating cancer cells under cell culture conditions based on the experiments reported. It shows the superior cellular uptake of the intact complex, and its interaction with serum alhumin that result in stabilization of the complex. The figure also illustrates that upon decomposition the components formed are less toxic, and that the V-atom is bound to transferrin as well as forming a new complex [V(DTB)3]-.
Although intratumoral injections (ITI) are mainly used for palliative care at this time, clinical trials involving intratumoral injections with cisplatin, oxaliplatin, and carboplatin have (Levina et al., 2022) increased by a factor of 5 since 2022 (Bates et al., 2025). In addition, the FDA has approved the use of an oncolytic virus (T-VEC), administered by intratumoral injection to treat unresectable metastatic melanoma (Hamid et al., 2020). Furthermore, similar techniques have been successfully utilized such as convection enhanced delivery (CED), which are intracranial injections of drugs designed to bypass the blood brain barrier, and has been used for the treatment of malignant gliomas (D’Amico et al., 2021; Nwagwu et al., 2021; Kang and Desjardins, 2022). Pressurized intraperitoneal aerosolized chemotherapy (PIPAC) is another administration method under development for treatment of metastatic cancers of the digestive system (Alyami et al., 2019; De Jong et al., 2021). These reports underline the fact that while currently intratumoral procedures are not routine, this method is likely to be much more accessible for cancer treatment in the future. Therefore, it is important to recognize that while novel administration strategies may differ from those more commonly used at the present time they should not be disregarded but further developed for future use. In summary, these results suggest that drugs delivered by intratumoral injections could be effective if they were reactive and immediately destroy tumor cells forming non-toxic compounds in the process and leaving healthy cells unharmed.
Summary
Currently there are only a few metal-based therapeutics approved for and used in the clinic, whereas there are many metal-based diagnostics. Therapeutic and diagnostic drugs share many similarities regarding their pharmacokinetic properties, as upon administration they both must be distributed, taken up into the cells, and excreted properly. However, diagnostic agents and therapeutics differ drastically in their metabolism and their excretion. In vivo diagnostic drugs have been developed to be stable and undergo minimal metabolism, whereas therapeutic agents are designed with a particular target and often are administered as prodrugs, where some metabolism is required for action. In the case of intratumoral agents this is particularly important because they must react immediately with the tumor and upon killing the cancer cells, form non-toxic products. Metal-based radiotheranostic agents are particularly interesting because they, similarly to diagnostic agents, must be exceedingly stable and resist metabolism. This is contrary to typical therapeutics, which are administered as prodrugs and are metabolized in the cell, whereupon they interact with the target potential proteins and other targets. Indeed, as an example, 64/67Cu-SAR-bisPSMA contains both the stable metal radiotracer complex as well as the peptide ligand associated with the target receptor. However, successful development of such agents involves more elaborate ligand design as evidenced by the structure of 64/67Cu-SAR-bisPSMA shown in Figure 3. Importantly, these agents do not comply with the guidelines for traditional drugs as defined by Lipinski, re-emphasizing that such compliance is not a requirement for successful future drugs such as in theranostic agents and drugs designed for unconventional administration strategies such as intratumoral injections.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AM: Conceptualization, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review and editing. DC: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. DCC thank Colorado State and an unnamed donor for funding.
Acknowledgments
We also thank Urszula K. Komarnicka for helpful comments before submitting this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullah, K. M., Kaushal, J. B., Takkar, S., Sharma, G., Alsafwani, Z. W., Pothuraju, R., et al. (2024). Copper metabolism and cuproptosis in human malignancies: unraveling the complex interplay for therapeutic insights. Heliyon 10, e27496. doi:10.1016/j.heliyon.2024.e27496
Alyami, M., Hübner, M., Grass, F., Bakrin, N., Villeneuve, L., Laplace, N., et al. (2019). Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 20, e368–e377. doi:10.1016/S1470-2045(19)30318-3
Anthony, E. J., Bolitho, E. M., Bridgewater, H. E., Carter, O. W. L., Donnelly, J. M., Imberti, C., et al. (2020). Metallodrugs are unique: opportunities and challenges of discovery and development. Chem. Sci. 11, 12888–12917. doi:10.1039/D0SC04082G
Aureliano, M., De Sousa-Coelho, A. L., Dolan, C. C., Roess, D. A., and Crans, D. C. (2023). Biological consequences of vanadium effects on formation of reactive oxygen Species and lipid peroxidation. Int. J. Mol. Sci. 24, 5382. Page 5382 2023;24. doi:10.3390/IJMS24065382
Banerjee, A., Patra, S. A., Sahu, G., Sciortino, G., Pisanu, F., Garribba, E., et al. (2023). A series of non-oxido VIVComplexes of dibasic ONS donor ligands: solution stability, chemical transformations, protein interactions, and antiproliferative activity. Inorg. Chem. 62, 7932–7953. doi:10.1021/ACS.INORGCHEM.3C00753
Basu, A., Bhattacharjee, A., Baral, R., Biswas, J., Samanta, A., and Bhattacharya, S. (2017). Vanadium(III)-L-Cysteine enhances the sensitivity of murine breast adenocarcinoma cells to cyclophosphamide by promoting apoptosis and blocking angiogenesis. Tumor Biol. 39, 101042831770575. doi:10.1177/1010428317705759
Bates, A. C., Klugh, K. L., Galaeva, A. O., Patch, R. A., Manganaro, J. F., Markham, S. A., et al. (2025). Optimizing therapeutics for intratumoral cancer treatments: antiproliferative vanadium complexes in glioblastoma. Int. J. Mol. Sci. 26, 994. doi:10.3390/ijms26030994
Bera, A., Sarkar, T., Upadhyay, A., and Hussain, A. (2025). First-row transition metal complexes of naturally occurring anticancer chelators for cancer treatment. Coord. Chem. Rev. 541, 216847. doi:10.1016/J.CCR.2025.216847
Boswell, C. A., Sun, X., Niu, W., Weisman, G. R., Wong, E. H., Rheingold, A. L., et al. (2004). Comparative in vivo stability of Copper-64-Labeled cross-bridged and conventional tetraazamacrocyclic complexes. J. Med. Chem. 47, 1465–1474. doi:10.1021/jm030383m
Caravan, P., Ellison, J. J., McMurry, T. J., and Lauffer, R. B. (1999). Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem. Rev. 99, 2293–2352. doi:10.1021/cr980440x
Celegato, M., Borghese, C., Casagrande, N., Mongiat, M., Kahle, X. U., Paulitti, A., et al. (2015). Preclinical activity of the repurposed drug auranofin in classical hodgkin lymphoma. Blood 126, 1394–1397. doi:10.1182/BLOOD-2015-07-660365
Chen, C., Ge, J., Gao, Y., Chen, L., Cui, J., Zeng, J., et al. (2022). Ultrasmall superparamagnetic iron oxide nanoparticles: a next generation contrast agent for magnetic resonance imaging. WIREs Nanomedicine Nanobiotechnology 14, e1740. doi:10.1002/WNAN.1740
Chmur, K., Brzeski, J., Reghukmar, S., Tesmar, A., Sikorski, A., Inkielewicz-Stępniak, I., et al. (2025). Structural, physicochemical, and biological insights into novel (Acetylacetonate)(Oxydiacetato)Oxidovanadium(IV) complexes with N-Containing aromatic compounds. Chem. – A Eur. J. 31, e202404496. doi:10.1002/chem.202404496
Choroba, K., Filipe, B., Świtlicka, A., Penkala, M., Machura, B., Bieńko, A., et al. (2023). In vitro and in vivo biological activities of dipicolinate Oxovanadium(IV) complexes. J. Med. Chem. 66, 8580–8599. doi:10.1021/acs.jmedchem.3c00255
Crans, D. C. (2015). Antidiabetic, chemical, and physical properties of organic vanadates as presumed transition-state inhibitors for phosphatases. J. Org. Chem. 80, 11899–11915. doi:10.1021/acs.joc.5b02229
Crans, D. C., and Kostenkova, K. (2020). Open questions on the biological roles of first-row transition metals. Commun. Chem. 3 (3), 104–4. doi:10.1038/s42004-020-00341-w
Crans, D. C., Smee, J. J., Gaidamauskas, E., and Yang, L. (2004). The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 104, 849–902. doi:10.1021/cr020607t
Crans, D. C., Koehn, J. T., Petry, S. M., Glover, C. M., Wijetunga, A., Kaur, R., et al. (2019). Hydrophobicity may enhance membrane affinity and anti-cancer effects of schiff base vanadium(V) catecholate complexes. Dalton Trans. 48, 6383–6395. doi:10.1039/C9DT00601J
Crişan, G., Moldovean-cioroianu, N. S., Timaru, D. G., Andrieş, G., Căinap, C., and Chiş, V. (2022). Radiopharmaceuticals for PET and SPECT imaging: a literature review over the last decade. Int. J. Mol. Sci. 23, 5023. doi:10.3390/IJMS23095023/S1
De Jong, L. A. W., Van Erp, N. P., and Bijelic, L. (2021). Pressurized intraperitoneal aerosol chemotherapy: the road from promise to proof. Clin. Cancer Res. 27, 1830–1832. doi:10.1158/1078-0432.CCR-20-4342
De Sousa-Coelho, A. L., Fraqueza, G., and Aureliano, M. (2023). Repurposing therapeutic drugs complexed to vanadium in cancer. Pharmaceuticals 17, 12. doi:10.3390/PH17010012
Dinda, R., Garribba, E., Sanna, D., Crans, D. C., and Costa Pessoa, J. (2025). Hydrolysis, ligand exchange, and redox properties of vanadium compounds: implications of solution transformation on biological, therapeutic, and environmental applications. Chem. Rev. 125, 1468–1603. doi:10.1021/acs.chemrev.4c00475
Doucette, K. A., Hassell, K. N., and Crans, D. C. (2016). Selective speciation improves efficacy and lowers toxicity of platinum anticancer and vanadium antidiabetic drugs. J. Inorg. Biochem. 165, 56–70. doi:10.1016/J.JINORGBIO.2016.09.013
Duan, X., He, C., Kron, S. J., and Lin, W. (2016). Nanoparticle formulations of cisplatin for cancer therapy. WIREs Nanomedicine Nanobiotechnology 8, 776–791. doi:10.1002/WNAN.1390
Duatti, A. (2021). Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 92, 202–216. doi:10.1016/J.NUCMEDBIO.2020.05.005
D’Amico, R. S., Aghi, M. K., Vogelbaum, M. A., and Bruce, J. N. (2021). Convection-enhanced drug delivery for glioblastoma: a review. J. Neurooncol 151, 415–427. doi:10.1007/s11060-020-03408-9
Fraum, T. J., Ludwig, D. R., Bashir, M. R., and Fowler, K. J. (2017). Gadolinium-based contrast agents: a comprehensive risk assessment. J. Magnetic Reson. Imaging 46, 338–353. doi:10.1002/JMRI.25625
Gao, G., Zhou, W., Jiang, X., and Ma, J. (2024). Bovine serum albumin and folic acid-modified aurum nanoparticles loaded with paclitaxel and curcumin enhance radiotherapy sensitization for esophageal cancer. Int. J. Radiat. Biol. 100, 411–419. doi:10.1080/09553002.2023.2281524
Grobner, T. (2006). Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 21, 1104–1108. doi:10.1093/NDT/GFK062
Gunaydin, G., Gedik, M. E., and Ayan, S. (2021). Photodynamic therapy for the treatment and diagnosis of Cancer–A review of the current clinical status. Front. Chem. 9, 686303. doi:10.3389/fchem.2021.686303
Gutfilen, B., Souza, S. A. L., and Valentini, G. (2018). Copper-64: a real theranostic agent. Drug Des. Dev. Ther. 12, 3235–3245. doi:10.2147/DDDT.S170879
Haase, A. A., Markham, S. A., Murakami, H. A., Hagan, J., Kostenkova, K., Koehn, J. T., et al. (2024). Halogenated non-innocent vanadium(V) schiff base complexes: chemical and anti-proliferative properties. New J. Chem. 48, 12893–12911. doi:10.1039/D4NJ01223B
Hamid, O., Ismail, R., and Puzanov, I. (2020). Intratumoral immunotherapy—update 2019. Oncol. 25, e423–e438. doi:10.1634/THEONCOLOGIST.2019-0438
Hancu, I., Dixon, W. T., Woods, M., Vinogradov, E., Sherry, A. D., and Lenkinski, R. E. (2010). CEST and PARACEST MR contrast agents. Acta Radiol. 51, 910–923. doi:10.3109/02841851.2010.502126
Hennrich, U., and Benešová, M. (2020). [68Ga]Ga-DOTA-TOC: the first FDA-approved 68Ga-Radiopharmaceutical for PET imaging. Pharmaceuticals 13 (38 2020), 38–13:38. doi:10.3390/PH13030038
Holland, J. P., Ferdani, R., Anderson, C. J., and Lewis, J. S. (2009). Copper-64 radiopharmaceuticals for oncologic imaging. Pet. Clin. 4, 49–67. doi:10.1016/J.CPET.2009.04.013
Janib, S. M., Moses, A. S., and MacKay, J. A. (2010). Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 62, 1052–1063. doi:10.1016/J.ADDR.2010.08.004
Jeon, M., Halbert, M. V., Stephen, Z. R., and Zhang, M. (2021). Iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging: fundamentals, challenges, applications, and prospectives. Adv. Mater. 33, 1906539. doi:10.1002/ADMA.201906539
Jomova, K., Alomar, S. Y., Alwasel, S. H., Nepovimova, E., Kuca, K., and Valko, M. (2024). Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Archives Toxicol. 98, 1323–1367. doi:10.1007/S00204-024-03696-4
Kang, J. H., and Desjardins, A. (2022). Convection-enhanced delivery for high-grade glioma. Neuro-Oncology Pract. 9, 24–34. doi:10.1093/NOP/NPAB065
Kartalou, M., and Essigmann, J. M. (2001). Recognition of cisplatin adducts by cellular proteins. Mutat. Research/Fundamental Mol. Mech. Mutagen. 478, 1–21. doi:10.1016/S0027-5107(01)00142-7
Kelkar, S. S., and Reineke, T. M. (2011). Theranostics: combining imaging and therapy. Bioconjugate Chem. 22, 1879–1903. doi:10.1021/bc200151q
Kelly, J. M., Ponnala, S., Amor-Coarasa, A., Zia, N. A., Nikolopoulou, A., Williams, C., et al. (2020). Preclinical evaluation of a high-affinity Sarcophagine-Containing PSMA ligand for 64Cu/67Cu-Based theranostics in prostate cancer. Mol. Pharm. 17, 1954–1962. doi:10.1021/acs.molpharmaceut.0c00060
Kim, J., and Nimse, S. B. (2025). Benzimidazole-scaffold based fluorescent probes for sensing and bioimaging applications. Coord. Chem. Rev. 537, 216690. doi:10.1016/J.CCR.2025.216690
Kostelnik, T. I., and Orvig, C. (2019). Radioactive main group and rare Earth metals for imaging and therapy. Chem. Rev. 119, 902–956. doi:10.1021/acs.chemrev.8b00294
Kostenkova, K., Levina, A., Walters, D. A., Murakami, H. A., Lay, P. A., and Crans, D. C. (2023). Vanadium(V) pyridine-containing schiff base catecholate complexes are lipophilic, redox-active and selectively cytotoxic in glioblastoma (T98G) cells. Chem. – A Eur. J. 29, e202302271. doi:10.1002/CHEM.202302271
Kozieł, S., Wojtala, D., Szmitka, M., Sawka, J., and Komarnicka, U. K. (2024). Can Mn coordination compounds be good candidates for medical applications? Front. Chem. Biol. 3, 1337372. doi:10.3389/FCHBI.2024.1337372
Krasnovskaya, O. O., Abramchuck, D., Erofeev, A., Gorelkin, P., Kuznetsov, A., Shemukhin, A., et al. (2023). Recent advances in 64Cu/67Cu-Based radiopharmaceuticals. Int. J. Mol. Sci. 24, 9154. doi:10.3390/IJMS24119154
Kratz, F., and Elsadek, B. (2012). Clinical impact of serum proteins on drug delivery. J. Control. Release 161, 429–445. doi:10.1016/J.JCONREL.2011.11.028
Kraus, S., Dierks, A., Rasche, L., Kertels, O., Kircher, M., Schirbel, A., et al. (2022). 68Ga-Pentixafor PET/CT for detection of chemokine receptor CXCR4 expression in myeloproliferative neoplasms. J. Nucl. Med. 63, 96–99. doi:10.2967/JNUMED.121.262206
Levina, A., Pires Vieira, A., Wijetunga, A., Kaur, R., Koehn, J. T., Crans, D. C., et al. (2020). A short-lived but highly cytotoxic Vanadium(V) complex as a potential drug lead for brain cancer treatment by intratumoral injections. Angew. Chem. Int. Ed. 59, 15834–15838. doi:10.1002/ANIE.202005458
Levina, A., Crans, D. C., and Lay, P. A. (2022). Advantageous reactivity of unstable metal complexes: potential applications of metal-based anticancer drugs for intratumoral injections. Pharmaceutics 14, 790. doi:10.3390/PHARMACEUTICS14040790
Levina, A., Uslan, C., Murakami, H., Crans, D. C., and Lay, P. A. (2023). Substitution kinetics, albumin and transferrin affinities, and hypoxia all affect the biological activities of anticancer Vanadium(V) complexes. Inorg. Chem. 62, 17804–17817. doi:10.1021/acs.inorgchem.3c02561
Levina, A., Scalese, G., Gambino, D., Crans, D. C., and Lay, P. A. (2024). Solution chemistry and anti-proliferative activity against glioblastoma cells of a vanadium(V) complex with two bioactive ligands. Front. Chem. Biol. 3, 1394645. doi:10.3389/FCHBI.2024.1394645
Li, Y., Duan, Y., Li, Y., Gu, Y., Zhou, L., Xiao, Z., et al. (2025). Cascade loop of ferroptosis induction and immunotherapy based on metal-phenolic networks for combined therapy of colorectal cancer. Exploration 5, 20230117. doi:10.1002/EXP.20230117
Lipinski, C. A. (2016). Rule of five in 2015 and beyond: target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug Deliv. Rev. 101, 34–41. doi:10.1016/J.ADDR.2016.04.029
Liu, Z., Zhang, H., Hong, G., Bi, X., Hu, J., Zhang, T., et al. (2024). Inhibition of Gpx4-mediated ferroptosis alleviates cisplatin-induced hearing loss in C57BL/6 mice. Mol. Ther. 32, 1387–1406. doi:10.1016/j.ymthe.2024.02.029
Lusic, H., and Grinstaff, M. W. (2013). X-ray-computed tomography contrast agents. Chem. Rev. 113, 1641–1666. doi:10.1021/cr200358s
Manioudakis, J., Victoria, F., Thompson, C. A., Brown, L., Movsum, M., Lucifero, R., et al. (2019). Effects of nitrogen-doping on the photophysical properties of carbon dots. J. Mater. Chem. C 7, 853–862. doi:10.1039/c8tc04821e
Mullard, A. (2025). 2024 FDA approvals. Nat. Rev. Drug Discov. 24, 75–82. doi:10.1038/D41573-025-00001-5
Murakami, H. A., Uslan, C., Haase, A. A., Koehn, J. T., Vieira, A. P., Gaebler, D. J., et al. (2022). Vanadium chloro-substituted schiff base catecholate complexes are reducible, lipophilic, water stable, and have anticancer activities. Inorg. Chem. 61, 20757–20773. doi:10.1021/acs.inorgchem.2c02557
Müssig, S., Kuttich, B., Fidler, F., Haddad, D., Wintzheimer, S., Kraus, T., et al. (2021). Reversible magnetism switching of iron oxide nanoparticle dispersions by controlled agglomeration. Nanoscale Adv. 3, 2822–2829. doi:10.1039/D1NA00159K
Na, H. B., Song, I. C., and Hyeon, T. (2009). Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 21, 2133–2148. doi:10.1002/ADMA.200802366
Nelson, K. M., Dahlin, J. L., Bisson, J., Graham, J., Pauli, G. F., and Walters, M. A. (2017). The essential medicinal chemistry of curcumin. J. Med. Chem. 60, 1620–1637. doi:10.1021/acs.jmedchem.6b00975
Nidhi, S., Rao, D. P., Gautam, A. K., Verma, A., and Gautam, Y. (2025). Schiff bases and their possible therapeutic applications: a review. Results Chem. 13, 101941. doi:10.1016/J.RECHEM.2024.101941
Nwagwu, C. D., Immidisetti, A. V., Jiang, M. Y., Adeagbo, O., Adamson, D. C., and Carbonell, A. M. (2021). Convection enhanced delivery in the setting of high-grade gliomas. Pharmaceutics 13, 561. doi:10.3390/PHARMACEUTICS13040561
Ramalho, J., Semelka, R. C., Ramalho, M., Nunes, R. H., AlObaidy, M., and Castillo, M. (2016). Gadolinium-based contrast agent accumulation and toxicity: an update. Am. J. Neuroradiol. 37, 1192–1198. doi:10.3174/AJNR.A4615
Rana, L., Nafisa, M. K., and Mahiya, K. (2025). Synthesis, characterization, catechol oxidase mimics, and anticancer activity of dioxidovanadium(V) and dioxidomolybdenum(VI) complexes. Inorg. Chem. Commun. 172, 113752. doi:10.1016/J.INOCHE.2024.113752
Rathmann, S. M., Ahmad, Z., Slikboer, S., Bilton, H. A., Snider, D. P., and Valliant, J. F. (2019). The radiopharmaceutical chemistry of Technetium-99m. Radiopharm. Chem., 311–333. doi:10.1007/978-3-319-98947-1_18
Rex, T., Mößer, T., Vilela, R. R. C., Hepp, A., Grashoff, C., and Strassert, C. A. (2025). Supramolecular assembly of water-soluble Platinum(II) complexes: from emission modulation to cell imaging in specific organelles. Chem. Weinheim der Bergstrasse, Ger. 31, e202404432. doi:10.1002/CHEM.202404432
Ronconi, L., and Sadler, P. J. (2007). Using coordination chemistry to design new medicines. Coord. Chem. Rev. 251, 1633–1648. doi:10.1016/J.CCR.2006.11.017
Rottenberg, S., Disler, C., and Perego, P. (2020). The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 21 (21), 37–50. doi:10.1038/s41568-020-00308-y
Rozzo, C., Sanna, D., Garribba, E., Serra, M., Cantara, A., Palmieri, G., et al. (2017). Antitumoral effect of vanadium compounds in malignant melanoma cell lines. J. Inorg. Biochem. 174, 14–24. doi:10.1016/J.JINORGBIO.2017.05.010
Runge, V. M. (2017). Critical questions regarding gadolinium deposition in the brain and body after injections of the gadolinium-based contrast agents, safety, and clinical recommendations in consideration of the EMA’s pharmacovigilance and risk assessment committee recommendation for suspension of the marketing authorizations for 4 linear agents. Invest Radiol. 52, 317–323. doi:10.1097/RLI.0000000000000374
Sahu, G., Tiekink, E. R. T., and Dinda, R. (2021). Study of DNA interaction and cytotoxicity activity of oxidovanadium(V) complexes with ONO donor schiff base ligands. Inorganics (Basel) 9, 66. doi:10.3390/inorganics9090066
Scattolin, T., Cavarzerani, E., Alessi, D., Mauceri, M., Botter, E., Tonon, G., et al. (2025). Unlocking the potential of organopalladium complexes for high-grade serous ovarian cancer therapy. Dalton Trans. 54, 4685–4696. doi:10.1039/D5DT00194C
Sen, S., Won, M., Levine, M. S., Noh, Y., Sedgwick, A. C., Kim, J. S., et al. (2022). Metal-based anticancer agents as immunogenic cell death inducers: the past, present, and future. Chem. Soc. Rev. 51, 1212–1233. doi:10.1039/D1CS00417D
Seyhan, A. A. (2019). Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles. Transl. Med. Commun. 4 (4), 18–19. doi:10.1186/S41231-019-0050-7
Silverman, J. A., and Deitcher, S. R. (2013). Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 71, 555–564. doi:10.1007/s00280-012-2042-4
Skos, L., Schmidt, C., Thomas, S. R., Park, M., Geiger, V., Wenisch, D., et al. (2024). Gold-templated covalent targeting of the CysSec-dyad of thioredoxin reductase 1 in cancer cells. Cell Rep. Phys. Sci. 5, 102072. doi:10.1016/J.XCRP.2024.102072
Stasiuk, G. J., and Long, N. J. (2013). The ubiquitous DOTA and its derivatives: the impact of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid on biomedical imaging. Chem. Commun. 49, 2732–2746. doi:10.1039/C3CC38507H
Stukan, I., Żuk, A., Pukacka, K., Mierzejewska, J., Pawłowski, J., Kowalski, B., et al. (2025). Wolf in sheep’s clothing: taming cancer’s resistance with human serum albumin? Int. J. Nanomedicine 20, 3493–3525. doi:10.2147/IJN.S500997
Sun, J., Zhong, H., Wang, K., Li, N., and Chen, L. (2021). Gains from no real PAINS: where ‘Fair Trial Strategy’ stands in the development of multi-target ligands. Acta Pharm. Sin. B 11, 3417–3432. doi:10.1016/J.APSB.2021.02.023
Terreno, E., Castelli, D. D., Viale, A., and Aime, S. (2010). Challenges for molecular magnetic resonance imaging. Chem. Rev. 110, 3019–3042. doi:10.1021/cr100025t
Thota, S., Rodrigues, D. A., Crans, D. C., and Barreiro, E. J. (2018). Ru(II) compounds: next-generation anticancer metallotherapeutics? J. Med. Chem. 61, 5805–5821. doi:10.1021/acs.jmedchem.7b01689
Vangijzegem, T., Lecomte, V., Ternad, I., Van Leuven, L., Muller, R. N., Stanicki, D., et al. (2023). Superparamagnetic iron oxide nanoparticles (SPION): from fundamentals to state-of-the-art innovative applications for cancer therapy. Pharmaceutics 15, 236. doi:10.3390/PHARMACEUTICS15010236
Wahsner, J., Gale, E. M., Rodríguez-Rodríguez, A., and Caravan, P. (2019). Chemistry of MRI contrast agents: current challenges and new frontiers. Chem. Rev. 119, 957–1057. doi:10.1021/acs.chemrev.8b00363
Wang, L., Karges, J., Wei, F., Xie, L., Chen, Z., Gasser, G., et al. (2023). A mitochondria-localized iridium(III) photosensitizer for two-photon photodynamic immunotherapy against melanoma. Chem. Sci. 14, 1461–1471. doi:10.1039/D2SC06675K
Wen, H., Jung, H., and Li, X. (2015). Drug delivery approaches in addressing clinical pharmacology-related issues: opportunities and challenges. AAPS J. 17, 1327–1340. doi:10.1208/s12248-015-9814-9
Wu, S., Liu, Y., Zeng, T., Zhou, T., Sun, Y., Deng, Y., et al. (2025). Enhanced the trans-cleavage activity of CRISPR-Cas12a using metal-organic frameworks as stimulants for efficient electrochemical sensing of circulating tumor DNA. Adv. Sci. 12, 2417206. doi:10.1002/ADVS.202417206
Yang, N., Guo, X. Y., Ding, J., Wang, F., Liu, T. L., Zhu, H., et al. (2024). Copper-64 based PET-Radiopharmaceuticals: ways to clinical translational. Semin. Nucl. Med. 54, 792–800. doi:10.1053/J.SEMNUCLMED.2024.10.002
Yu, S. B., and Watson, A. D. (1999). Metal-based X-ray contrast media. Chem. Rev. 99, 2353–2378. doi:10.1021/cr980441p
Zahirović, A., Fetahović, S., Feizi-Dehnayebi, M., Višnjevac, A., Bešta-Gajević, R., Kozarić, A., et al. (2024). Dual antimicrobial-anticancer potential, hydrolysis, and DNA/BSA binding affinity of a novel water-soluble ruthenium-arene ethylenediamine schiff base (RAES) organometallic. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 318, 124528. doi:10.1016/J.SAA.2024.124528
Zboralski, D., Hoehne, A., Bredenbeck, A., Schumann, A., Nguyen, M., Schneider, E., et al. (2022). Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur. J. Nucl. Med. Mol. Imaging 49, 3651–3667. doi:10.1007/s00259-022-05842-5
Zhang, P., and Sadler, P. J. (2017). Advances in the design of organometallic anticancer complexes. J. Organomet. Chem. 839, 5–14. doi:10.1016/J.JORGANCHEM.2017.03.038
Zhang, S., Wang, X., Gao, X., Chen, X., Li, L., Li, G., et al. (2025). Radiopharmaceuticals and their applications in medicine. Signal Transduct. Target. Ther. 10 (10), 1–51. doi:10.1038/s41392-024-02041-6
Glossary
ADME Administration, Distribution, Metabolism, Excretion
Au Gold
cat Catecholate
CED Convection Enhanced Delivery
CNS Central Nervous System
Cu Copper
CXCR4 C-X-C Chemokine Receptor 4
Da Daltons
DNA Deoxyribonucleic Acid
DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid)
DTB Di-tert-butyl
DTPA Diethylenetriamine Pentaacetate
FAP Fibroblast Activation Protein-α
FAPI Fibroblast Activation Protein-α Inhibitor
FDA Federal Drug Administration
FDG Fluorodeoxyglucose
Ga Gallium
GBCA Gadolinium-based Contrast Agent
Gd Gadolinium
ITI intratumoral injection
Lu Lutetium
mAb Monoclonal Antibody
Mo Molybdenum
MRI Magnetic Resonance Imaging
NET Neuroendocrine Tumor
NSF Nephrogenic Systemic Fibrosis
PET Positron Emission Tomography
PIPAC Pressurized Intraperitoneal Aerosolized Chemotherapy
PSMA Prostate-Specific Membrane Antigen
Pt Platinum
r1 Longitudinal Relaxivity
r2 Transverse Relaxivity
Ru Ruthenium
SAR Sarcophagine
SPECT Single-Photon Emission Computed Tomography
SPION Superparamagnetic Iron Oxide Nanoparticle
T1 Longitudinal Relaxation Time
T2 Transverse Relaxation Time
Tc Technetium
UV-vis Ultraviolet-visible
V Vanadium
Y Yttrium
Zr Zirconium
Keywords: therapeutics, diagnostics, theranostic agents, metal coordination complexes, stability, toxicity, MRI contrast agents, radiopharmaceuticals
Citation: Miller AR and Crans DC (2025) Lessons from metals-containing drugs in diagnostic, and theranostic applications for future development of metal-containing non-conventional therapeutics: vanadium compounds for intratumor administration. Front. Chem. Biol. 4:1639340. doi: 10.3389/fchbi.2025.1639340
Received: 01 June 2025; Accepted: 12 August 2025;
Published: 04 September 2025.
Edited by:
Arthur Tinoco, University of Puerto Rico, Río Piedras Campus, Puerto RicoReviewed by:
Edit Tshuva, Hebrew University of Jerusalem, IsraelLauren Fernández-Vega, Universidad Ana G Mendez, Puerto Rico
Copyright © 2025 Miller and Crans. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Debbie C. Crans, RGViYmllLkNyYW5zQENvbG9zdGF0ZS5lZHU=
 Adam R. Miller
Adam R. Miller Debbie C. Crans
Debbie C. Crans