- 1Faculty of Applied Sciences, Uva Wellassa University of Sri Lanka, Badulla, Sri Lanka
- 2Faculty of Engineering, Toyama Prefectural University, Toyama, Japan
- 3Sweco Norge AS, Seljord, Norway
- 4Faculty of Technology, Natural Sciences and Maritime Sciences, University of South-Eastern Norway, Porsgrun, Norway
Introduction: A type of batch electrolysis system comprising a platinum anode and stainless-steel cathode was investigated for the removal of hexavalent chromium (Cr6+) from synthetic wastewater.
Methods: Electrochemical treatment was conducted at a constant current of 0.25 A with NaCl of 1 g/L as the supporting electrolyte.
Results: The highest Cr6+ removal efficiencies achieved were at 100 mg/L metal ion dosage and an initial Cr6+ concentration of 5 mg/L, yielding removal rates of 56.80% for Fe3+, 49.62% for Al3+, and 30.05% for Mg2+.
Discussion: Removal was attributed to the in-situ formation of metal hydroxides (Al(OH)3, Fe(OH)3, Mg(OH)2), which subsequently enhanced the reduction and immobilization of Cr6+ through co-precipitation, Coulomb forces, and electrostatic adsorption. Further increase in Cr6+ removal efficiency was inhibited at higher initial Cr6+ concentrations due to the saturation of hydroxides, which also exhibited competitive behaviour toward ion adsorption. These results confirm the significant role of multivalent cation additives in increasing the remediation of Cr6+ in the electrochemical system, thus lending support to the theory behind the development of scalable additive-assisted electrochemical water treatment technique.
1 Introduction
The term ‘wastewater’ refers to the liquid waste products generated by a community, encompassing typical human waste from residences and workplaces, as well as various waste products and compounds from commercial and industrial activities. Among these contaminants, heavy metals such as lead, nickel, copper, and chromium possess numerous toxic properties that harm humans and the environment.
When considering chromium, it is a metal that can be hazardous and cancer-causing, resulting from both anthropogenic and natural processes. The two primary forms of chromium released into aquatic systems are Trivalent Chromium (Cr3+) and Hexavalent Chromium (Cr6+). Chromium compounds, made using sodium/potassium dichromate as a base material, are released in enormous quantities into the environment (Carneiro et al., 2019). Chromium compounds have gained extensive applications in wood preservation, printing inks, textile dyeing, leather tanning, paints, pigments, and metal plating (Krishnani et al., 2013).
Chromium can be ingested through the skin, consumed, and inhaled by humans. Animal and human tissues are known to collect Cr3+ and Cr6+. Developmental problems, including growth retardation and neurodevelopmental impairments, as well as harm to the skin, respiratory, reproductive, and digestive systems, and an increased risk of cancer, are some of the hazardous effects of chromium compounds on humans and animals (Aitio et al., 1984). Cr6+ has been shown in studies to be hazardous even at low concentrations (Chen et al., 2024; Kerur et al., 2020; Park et al., 2022). These include deadly toxicity, behavioural modifications, and decreased growth, reproduction, and survival in invertebrates (Zhou et al., 2021; Hamadeen et al., 2022). They also include reduced growth and photosynthesis in algae and aquatic plants. Hexavalent chromium exposure in fish has resulted in altered physical and metabolic conditions, longer hatching times, DNA damage, and lower survival rates (Kumar Saha and Ghosh, 2022; Kokkinos et al., 2019; Merical, 2007). Consequently, chromium must be removed from wastewater for environmental conservation and reuse in the material recycling in the existing circular economy systems. Almost all countries, including the World Health Organization (WHO), have set recommended safe discharge limits for chromium (Yan et al., 2023).
Usually, Cr6+ is extremely mobile and soluble throughout a wide pH range, increasing its potential for migration and harmfulness (Chen et al., 2024). Compared to Cr6+, Cr3+ has roughly 500–1,000 times less toxicity and mobility, and is very simple to remove by precipitation and adsorption (Park et al., 2022; Golder et al., 2007; Costa, 2003). Therefore, one essential technique for treating wastewater containing chrome is reducing Cr6+ to Cr3+ (Yogeshwaran and Priya, 2018). Although the traditional adsorption method is easy to use, it can be hard to use on a wide scale because adsorbents' pores (such as activated carbon, zeolite, resin, etc.) are prone to clogging and have low regeneration effectiveness (Mohan and Pittman, 2006). Other methods include solvent extraction, membrane separators, reverse osmosis, ion exchange, biodegradation, adsorption by natural biomaterials like fungi and algae, biosorption, coagulation, and electrochemical treatments such as electrocoagulation, electroreduction, and electrodeionization (Peng et al., 2019; Malinovic et al., 2022; Zongo et al., 2009; Garg et al., 2023).
Electrocoagulation (EC) has been recognized for its efficiency, cost-effectiveness, and environmental compatibility in treating wastewater. It involves the electrolytic dissolution of electrodes (usually aluminum or iron) to generate coagulants that aid in flocculation and contaminant removal. The process utilizes electrons as the primary reagents, making it productive and economically viable (Malinovic et al., 2022; Characterization et al., 2020).
On the other hand, Electrolysis (ELC) has been noted for its ability to generate chlorine gas (Cl2) and hydrogen gas (H2) through an electrolytic process that maintains high pH and low pH solutions in separate compartments via a permeable diaphragm (Amarasooriya and Kawakami, 2020). This technique offers advantages, including reduced electrode dissociation and enhanced process efficiency, through the application of Coulombic forces.
While EC commonly employs sacrificial metal electrodes (e.g., aluminum or iron), releasing coagulants (Malinovic et al., 2022), ELC often utilizes inert electrodes to facilitate redox reactions and coagulant generation, either indirectly or through external addition. Both have proven effective for Cr6+ removal; however, most studies focus on sacrificial electrodes where electrodes serve as reagents.
The use of non-sacrificial electrodes such as platinum anodes and stainless-steel cathodes in combination with externally added coagulants (e.g., Al3+, Fe3+, Mg2+) remains largely unexplored. Although these inert materials do not contribute coagulants directly, they may offer significant advantages in terms of electrode durability, process control, and sustainability. Despite their potential, the impact of such configurations on the performance of EC and ELC systems for Cr6+ removal has not been systematically studied.
This study addresses this critical gap by investigating the removal efficiency of Cr6+ from wastewater using an electrolysis system configured with platinum and stainless-steel electrodes, supplemented with different coagulant agents. The system was assessed under various operational conditions to evaluate the influence of coagulant type and electrochemical configuration.
The novelty of this work lies in employing non-sacrificial electrode materials with externally optimized coagulant dosing, providing new insights into electrochemical process performance, cost-effectiveness, and long-term operational sustainability. These findings contribute to expanding the toolkit for advanced wastewater treatment technologies aligned with circular economy and resource recovery strategies.
2 Materials and methods
2.1 Chemicals and instruments
In this work, different concentrations of synthetic aqueous solutions of Cr6+ and, as a coagulant agent, Fe3+, Al3+, and Mg2+ were used in the ELC reactor. A 1000 mg/L synthetic stock solution of Cr6+ was prepared by dissolving 2.829 g of K2Cr2O4 (HCN Code 28415000- India) in 1 L of distilled water. 5 mg/L, 10 mg/L and 20 mg/L of Cr6+ solutions were prepared by proper dilutions. 1,000 mg/L stock solution of Al3+ was prepared by dissolving 8.970 g of AlCl3.6H2O (HCN Code 2827320000- India) in 1 L of distilled water. 1000 mg/L stock solution of Fe3+ was prepared by dissolving 2.904 g of FeCl3 (HCN Code 282739- India) in 1L of distilled water. 1,000 mg/L stock solution of Mg2+ was prepared by dissolving 8.470 g of MgCl2.6H2O (HSN Code 28273100- India) in 1 L of distilled water. Working concentrations of Fe3+, Al3+, and Mg2+ (5 mg/L to 100 mg/L) were prepared by Cr6+ synthetic stock solution. NaCl 1 g/L was added as an electrolyte to enhance the solution’s conductivity. The pH of the samples was measured by using a laboratory pH meter (HANNA, USA, HI 2020-02). The analytical measurements of chromium, aluminum, iron, and magnesium was performed by the Atomic Absorption Spectrophotometer (Varian AA 240 FS atomic absorption spectrometer- USA). The concentration of chromium in aqueous samples was determined using AAS at a wavelength of 357.9 nm, which corresponds to the most sensitive line for chromium detection. A series of calibration standards was prepared from a 1,000 mg/L certified Chromium stock solution by serial dilution to obtain concentrations of 0.5, 1, 2, 5, 10, and 20 mg/L. The calibration curve exhibited excellent linearity with a correlation coefficient (R2) greater than 0.999, confirming the accuracy of quantification across the working range. To ensure the precision and reliability of measurements, calibration was repeated after every 10 samples to monitor for potential instrumental drift.
2.2 Experimental Setup
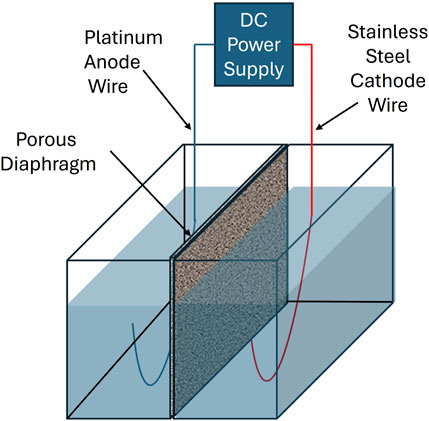
All electrolysis experiments were performed in a square-shaped cell having two equal compartments of a plastic cell (H:7.5 × W:10.5 × L:15.0 cm3). The compartments were named as anode and cathode. The anode and cathode volumes were equally separated by using a permeable clay diaphragm and this diaphragm allows both cations and anions transferred through the anode and cathode without any special ion selectivity (Figure 1). The diaphragm prevents the mixing of sludge produced in the cathode and helps in the separation of anode and cathode solutions. A platinum wire electrode was used as an anode, and a single stainless-steel electrode was used as a cathode at an inter-electrode distance of 5 cm for all experiments with a constant current power source. The Pt electrode was purchased from the Nilaco Corporation, Japan, and the stainless-steel electrode was obtained from the local market in Sri Lanka. Platinum (anode) and stainless steel (cathode) were selected as inert, non-sacrificial electrodes to prevent contamination from electrode dissolution, enhance process stability, and allow precise control over coagulant dosing. This configuration supports long-term sustainability and minimizes maintenance compared to traditional sacrificial electrodes. A constant current of 0.25 A was applied for 30 min during each electrolysis run with 600 mL of synthetic solution. These conditions were selected based on preliminary experimental trials that demonstrated effective Cr6+ removal and stable performance across all test scenarios. Although full optimization was not performed, these parameters provided a consistent and practical baseline for evaluating the effects of different coagulants. After each run, 50 mL of water was collected from each anode and cathode cell to measure the ion concentrations. All experiments were independently performed in triplicate (n = 3) for each condition to ensure reproducibility, and the results are presented as the average of these replicates.
3 Results and discussion
3.1 Baseline Cr6+ removal by electrolysis without coagulant additives
To establish a performance baseline, control experiments were conducted without the addition of Al3+, Fe3+, or Mg2+ ions. The Cr6+ removal efficiency of the electrolysis system alone was evaluated at initial Cr6+ concentrations of 5, 10, and 20 mg/L under constant charge loading (25 mA for 30 min = 1500 C/L). Removal was primarily driven by cathodic reduction, with cathodic efficiencies of 17.77%, 9.88%, and 14.65%, respectively. Anodic removal was minimal or negative, and appreciable precipitative removal (14.24%) occurred only at the highest Cr6+ concentration (20 mg/L), likely due to localized hydroxide formation enabling Cr(OH)3 precipitation. These results confirm that while electrolysis alone can reduce Cr6+ levels to some extent, the presence of coagulant ions is essential to enhance removal efficiency and support stable precipitation mechanisms.
3.2 Effect of the initial Al3+ concentration on the removal of Cr6+
The removal efficiency of Cr6+ in the synthesized wastewater was investigated by keeping the charge loading (C/L) constant (25 mA for 30 min = 1500 C/L) for initial chromium concentrations of 5 mg/L, 10 mg/L, and 20 mg/L. Further, for different Cr6+ levels, the initial coagulant Al3+ concentration was varied from 0 mg/L to 100 mg/L. The influence of the initial Al3+ concentration on the removal efficiency of Cr6+ at initial Cr6+ concentrations of 5 mg/L, 10 mg/L, and 20 mg/L is depicted in Figures 2a–c, respectively. The respective pH variations in the anode and cathode baths are shown in Figures 3a–c. Equation 1 was employed for calculating Cr6+ removed by precipitation from the total system (Anode + Cathode).
Where,
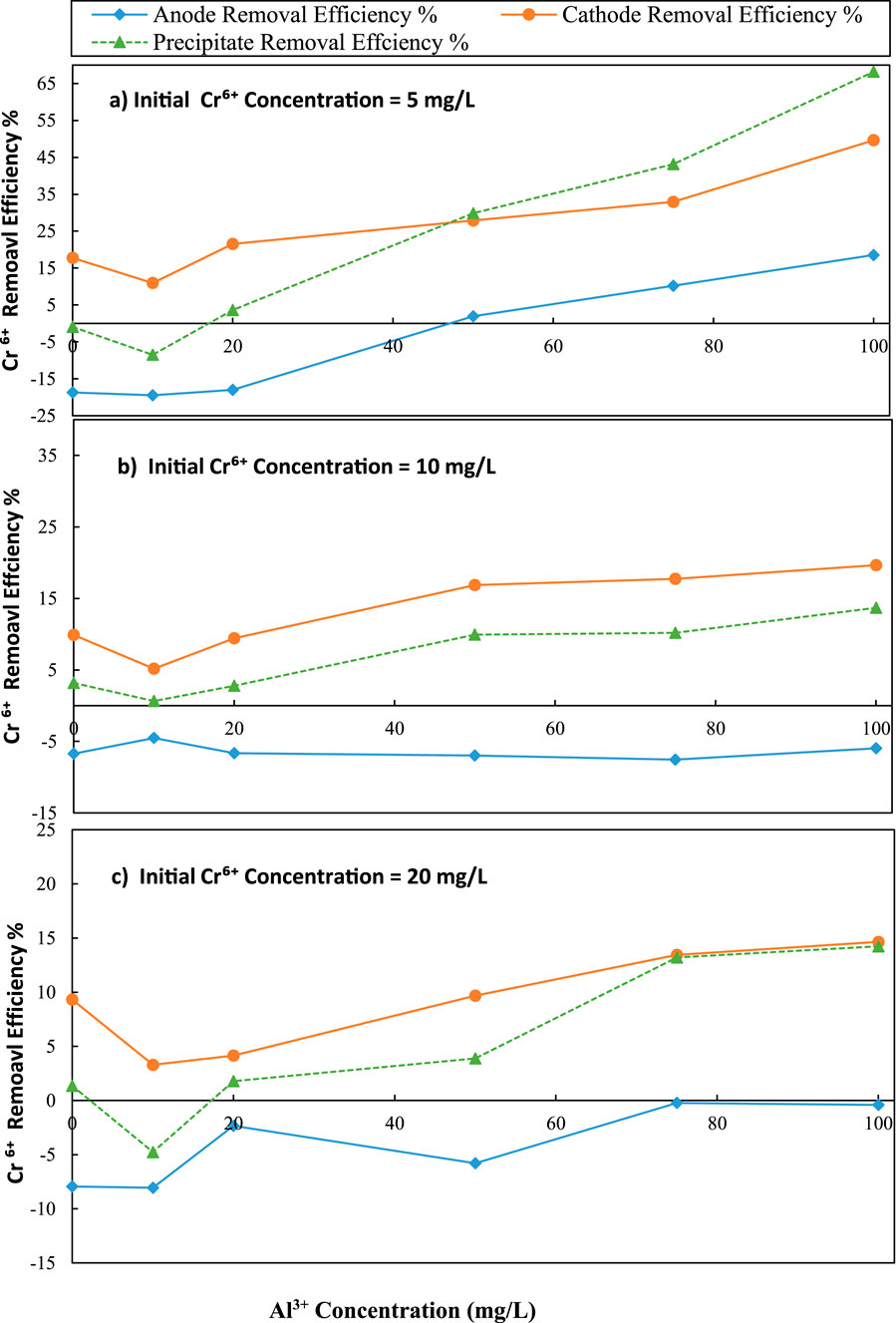
Figure 2. Variation of Cr6+ removal with initial Al3+ concentration over different initial Cr6+ concentration (a) 5 mg/L, (b) 10 mg/L and (c) 20 mg/L; C/L applied at = 1500 C/L (0.25 A)
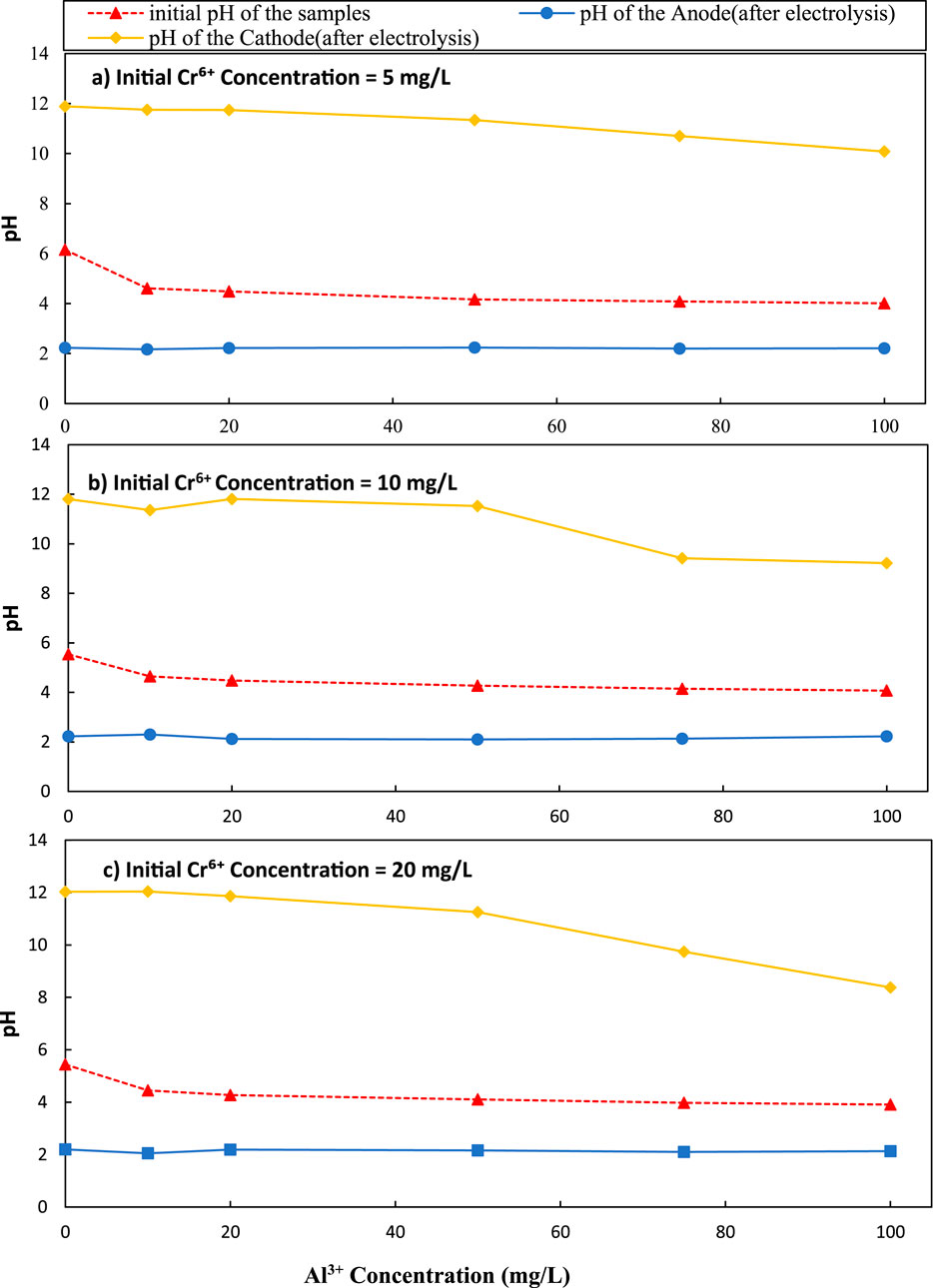
Figure 3. Variation of pH with initial Al3+ concentration over different initial Cr6+ concentration (a) 5 mg/L, (b) 10 mg/L and (c) 20 mg/L C/L applied at = 1500 C/L (0.25A).
Ci: Initial Cr6+ Concentration in the cathode and anode (mg/L).
Ca: Final Cr6+ Concentration in the anode (mg/L).
Cc: Final Cr6+ Concentration in the cathode (mg/L).
According to the results presented in Figures 2a–c, increasing the initial Al3+ concentrations increased the precipitate and individual removal efficiencies of Cr6+ in both the anode and cathode. In contrast, increased initial Cr6+ reduced the removal percentages. Further increasing the initial Al3+ concentration also increased removal efficiencies in both the anode and the cathode by precipitation. The maximum Cr6+ removal at the cathode (49.62%), was achieved with an initial Al3+ concentration of 100 mg/L at an initial Cr6+ concentration of 5 mg/L (Figure 2a) and the respective pH variation can be seen in Figure 3a. This Cr6+ removal of 49.62% was a 31.85% increase over the initial level of zero Al3+ in the cathode solution ([49.62–31.85] % = coulomb transfer to cathode). However, precipitative removal decreased to 14.24% in the cathode with an initial Al3+ concentration of 100 mg/L and an initial Cr6+ concentration of 20 mg/L (Figure 2c).
According to Figure 2b, when the Cr6+ concentration was 10 mg/L, the maximum Cr6+ removal efficiency in the cathode was 19.64%. This level was a 9.76% increase over the initial level of zero Al3+ in the solution. When the initial Cr6+ concentration was 10 mg/L, the pH was varied from 11.89 to 9.22 for Al3+ concentrations of 0–100 mg/L, at the cathode (Figure 3b). According to Figure 2c, when chromium concentration was 20 mg/L, the maximum removal efficiency of Cr6+ cathode was 14.64%. This level was a 5.31% increase over the initial zero Al3+ level in the solution. When the initial chromium concentration was 20 mg/L, the pH was varied from 11.93 to 8.38 for initial aluminum concentrations of 0–100 mg/L, at the cathode (Figure 3c).
According to Figure 2a, the maximum Cr6+ removal by precipitation (68.17%) was achieved for an initial Al3+ concentration of 100 mg/L for a 5 mg/L Cr6+ concentration. This level was a 69.07% increase over the initial level of Al3+ in the solution. When chromium concentration was increased to 10 mg/L, the maximum Cr6+ removal by precipitation decreased to 13.68%. Though it was a decreased compared to lower initial Cr6+, 5 mg/L, this level was a 10.50% increase over the initial level of zero Al3+ in the solution (Figure 2b). According to Figure 2c, when chromium concentration was 20 mg/L, the maximum Cr6+ removal by precipitation was 14.23% which was achieved in the initial Al3+ concentration as 100 mg/L. This level was a 12.87% increase over the initial level of zero Al3+ in the solution.
The findings indicate that the removal efficiency of Cr6+ is strongly dependent on the initial Al3+ and Cr6+ concentration. At lower Cr6+ concentrations, the addition of Al3+ significantly improved removal, likely owing to massive production of Al(OH)3 flocs that promote adsorption and sweep coagulation. Yet when the initial concentration of Cr6+ is high, the effect becomes less prominent, as a result of the saturation of available binding sites and competitive inhibition by Cr6+. Overall, the results highlight a concentration-dependent synergy between Al3+ dosing and electrochemical treatment, with optimal performance observed at lower Cr6+ loads and elevated Al3+ availability.
Since the overall surface area of Al(OH)3 grows as the initial Al3+ concentration rises, the removal of Cr6+ should eventually lead to an increase in line with the findings of Figures 2a–c. Here, the concentration of Al3+ is increased, and the precipitation of Al(OH)3 occurs in the form of small particles, each of which has a larger surface area to volume ratio. Particulates with a smaller size have greater overall surface area than if the same mass of material is in the form of fewer larger particles. This increase in surface area is important because it determines the number of sites available for binding of Cr6+ ions. The adsorption process entails the trapping of Cr6+ ions by a cationic surface Al(OH)3, and thus removing them from the solution. Due to the electrolytic effect on the water molecule, the anode effluent’s pH reduced as the cathode effluent’s pH significantly increased (Figures 3a–c). An oxygen and hydrogen ion are released into the aqueous system as a result of water oxidation caused by the anode, which lowers pH (Equation 2; Equation 3). The interaction of water molecules and electrons in the cathode causes water molecules to dissociate into hydrogen ions and OH− for high pH (Amarasooriya and Kawakami, 2020; Amarasooriya and Kawakami, 2019).
At the anode
At the cathode
Here, Al3+ ions combined with OH− and generated metallic hydroxides of Al(OH)3; thus hydroxides could remove Cr6+ in water. The predominant species of Al3+ in the pH range of 5.7–6.7 is Al(OH)3 (Equation 4), and Al3+ is predominant at pH less than 5.7.
When the initial chromium concentration was 5 mg/L, the final pH was observed higher than 10, and the value was decreased from 11.80 to 10.08 respectively for initial aluminum concentrations 0–100 mg/L, at the cathode (Figure 3a). At that pH level of, the increment of the pH in cathode lead to generate both Al(OH)4− through Al(OH)3 (Equation 4; Equation 5; Equation 6). Accordingly, Cr6+ removed by precipitation with both Al(OH)3 and Al(OH)4− can be either adsorption or co-precipitation. Electrostatic interactions and surface complexation cause Cr6+ ions to be attracted to and retained on the surface of aluminum or its compounds during adsorption. The adsorbed species are further stabilized by the surface reduction of Cr6+ to Cr3+. In co-precipitation, it is the simultaneous precipitation of aluminum hydroxide produced during electrolysis and Cr3+, which is reduced from Cr6+. This process is especially important in alkaline environments because aluminum hydroxide is soluble and may co-precipitate to absorb Cr3+.
In bases, it acts as a Lewis acid by binding hydroxide ions,
One of the most crucial steps in the treatment process is the reduction of hexavalent chromium (Cr6+) into trivalent chromium (Cr3+). In this stage, dichromate ions (Cr2O72-) are reduced to Cr3+ ions in an acidic reaction medium (Equation 7). This conversion is important because soluble Cr6+ is reduced to insoluble Cr3+, which is less soluble and toxic. After forming, Cr3+ ions combine with hydroxide ions to generate chromium (III)hydroxide (Equation 8). The reduction of Cr6+ to Cr3+ was evaluated using a colorimetric method involving ascorbic acid and EDTA, where a significant decrease in the purple-colored complex at 544 nm indicated effective Cr6+ reduction. Cr ion is also eliminated from the aqueous phase in the following precipitation of Cr(OH)3, which also yields a solid that can be separated from the solution through filtration. Furthermore, adsorption of dichromate ions on aluminum hydroxide plays an important role in the removal process, as described in Equation 9.
Co-precipitation of Cr3+ with Aluminum Hydroxide:
Adsorption of dichromate Ions on Aluminum hydroxide:
Throughout the experiments involving aluminum ions, the initial pH was not externally adjusted or buffered but allowed to vary naturally, reflecting realistic electrochemical treatment conditions. The cathodic pH increased markedly during electrolysis, ranging from 11.89 at 0 mg/L Al3+ to 10.08 at 100 mg/L Al3+ (Figure 3a). This pH elevation critically influences the speciation and precipitation behavior of aluminum hydroxides. At near-neutral pH (5.7–6.7), aluminum hydrolyzes to form amorphous Al(OH)3, which possesses a high specific surface area and a positive surface charge, enhancing its ability to adsorb anionic species like chromate (CrO42-) and dichromate (Cr2O72-). As the pH rises above 9, Al(OH)3 partially dissolves, forming soluble aluminate ions (Al(OH)4-), which decreases solid-phase adsorption capacity but may facilitate secondary complexation or precipitation mechanisms.
Besides, under acidic to neutral conditions, aluminum released from the anode leads to the formation of amorphous Al(OH)3 flocs, which are positively charged and have a greater surface area. Because of their positive charge, hydroxide ions help capture negatively charged Cr6+ oxyanions (such as CrO42- and HCrO4−) by outer-sphere electrostatic attraction. At the same time, when Al(OH)3 and chromate come into contact, ligand exchange between the hydroxyl groups of Al(OH)3 and chromate leads to more specific and stable bonds.
Also, when conditions near the cathode become reducing or intermediates like H2 gas or electrons appear at the surface, Cr6+ adsorbed species usually get reduced to Cr3+, joining the aluminum hydroxide coat or becoming Cr(OH)3. Performing adsorption, reduction, and co-precipitation makes chromium more securely adhered and greatly reduces the release of Cr6+ back into the solution.
Higher pH value means that aluminum becomes more likely to be found in the Al(OH)4- form. Such complexes may interact with Cr3+ ions through ion-pairing or may go back into solid form if close to the cathode, as pH in that area might change. So, keeping the pH in a specific range during electrochemical treatment leads to both a changed appearance and functioning of aluminum hydroxide for taking out chromium.
Because of adsorption and ligand exchange, the reduced Cr6+, with the help of fresh hydroxides, and co-precipitation, the enhanced Cr removal in the electrolysis is easy to explain.
Thermodynamic speciation diagram of aluminum (Al3+), chromium (Cr6+), and their related hydroxide and oxyanion species as a function of pH. Calculations were performed under experimental conditions of 20 mg/L Cr and 100 mg/L Al (Figure 4). The diagram illustrates the dominance of Al(OH)3 and Al(OH)4- species in the pH range six to nine, which coincides with the cathodic pH observed in the Al3+ system, supporting co-precipitation and adsorption mechanisms for Cr removal.
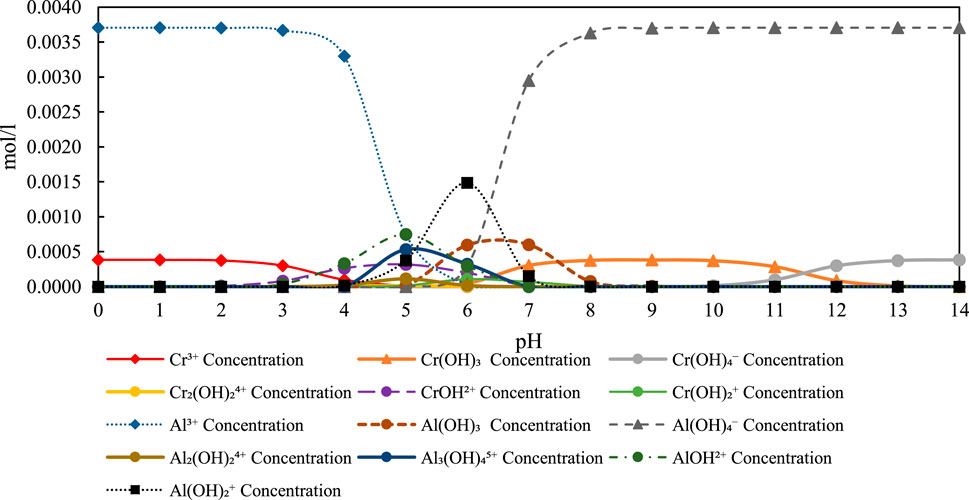
Figure 4. Speciation diagram of Al3+ Concentration (100 mg/L), Cr6+ Concentration (20 mg/L), and related species over pH 0–14 under experimental conditions.
3.3 Effect of the initial Fe3+ concentration on the removal of Cr6+
This section presents the impact of initial Fe3+ concentration on the removal efficiency of Cr6+ at various Cr6+ concentration levels (5 mg/L, 10 mg/L, and 20 mg/L) with a constant charge loading of 1500 C/L (25 mA for 30 min). Results are shown in Figures 5a–c the initial coagulant (Fe3+) concentrations were varied from 0 mg/L to 100 mg/L for different Cr6+ levels. The corresponding pH variations in the anode and cathode baths are shown in Figures 6a–c, respectively.
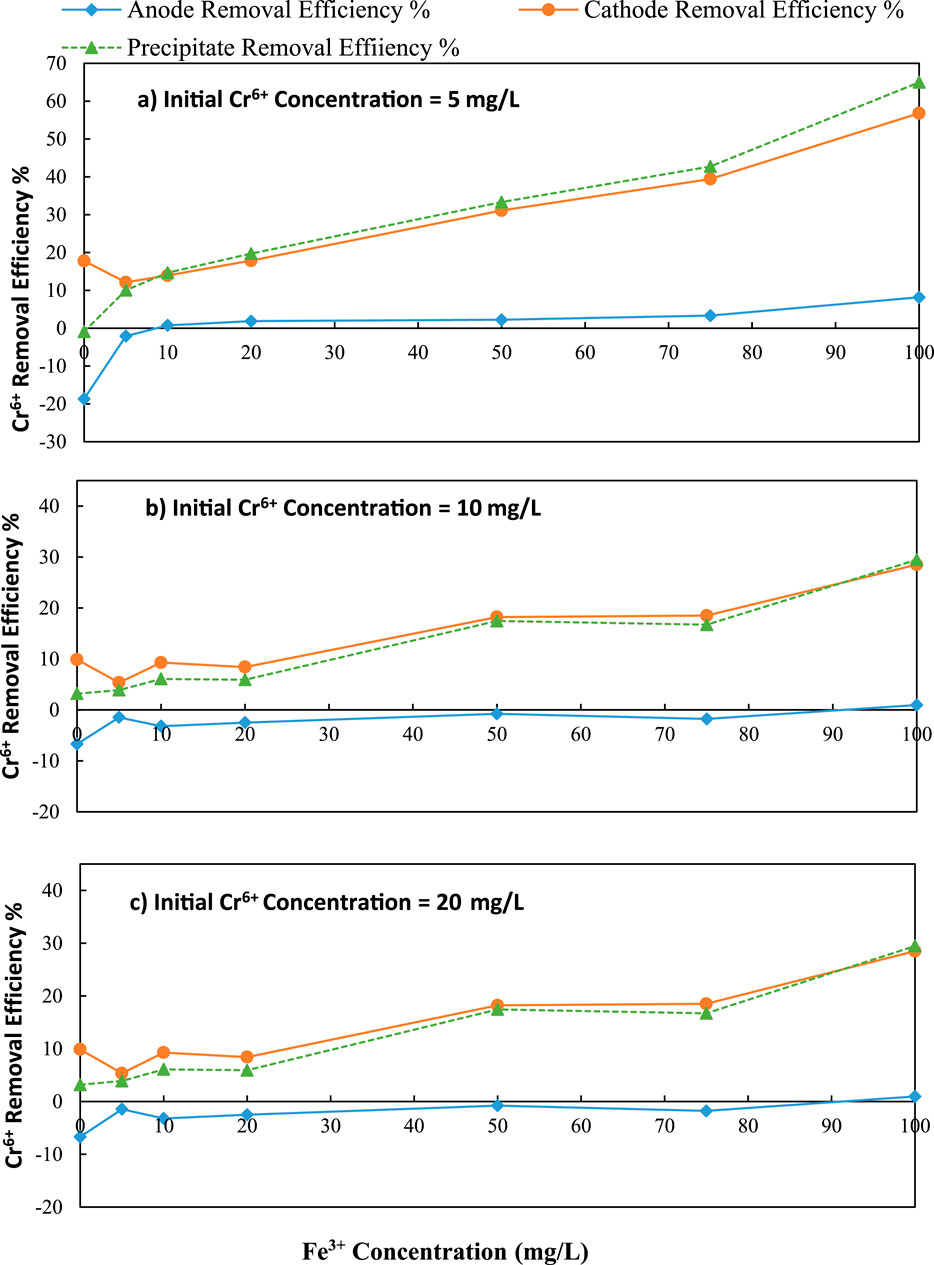
Figure 5. Variation of Cr6+ removal with initial Fe3+ concentration over different initial Cr6+ concentration (a) 5 mg/L, (b) 10 mg/L and (c) 20 mg/L; C/L applied at = 1500 C/L (0.25A).
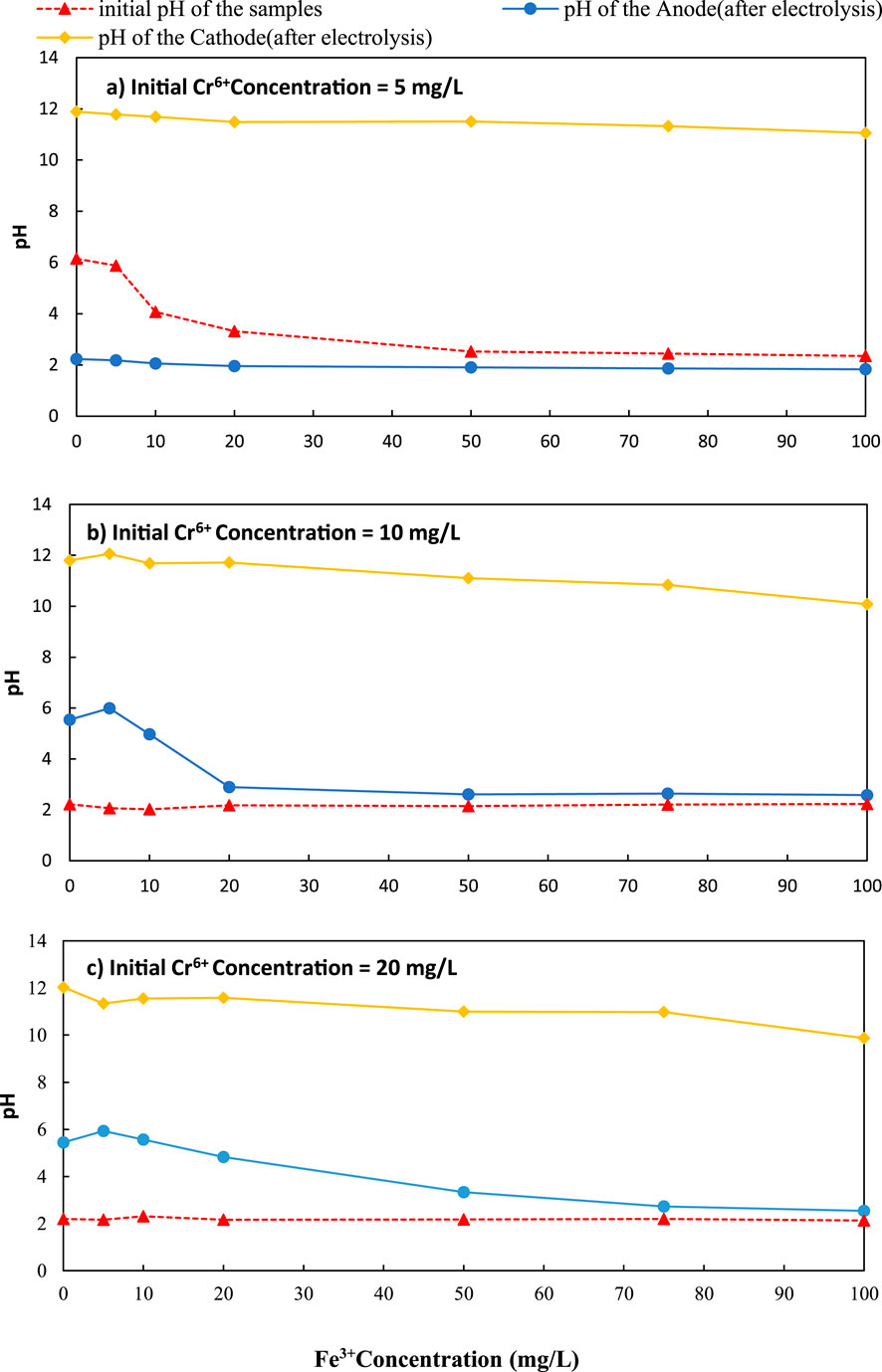
Figure 6. Variation of pH with initial Fe3+ concentration over different initial Cr6+ concentration (a) 5 mg/L, (b) 10 mg/L and (c) 20 mg/L C/L applied at = 1500C/L (0.25A).
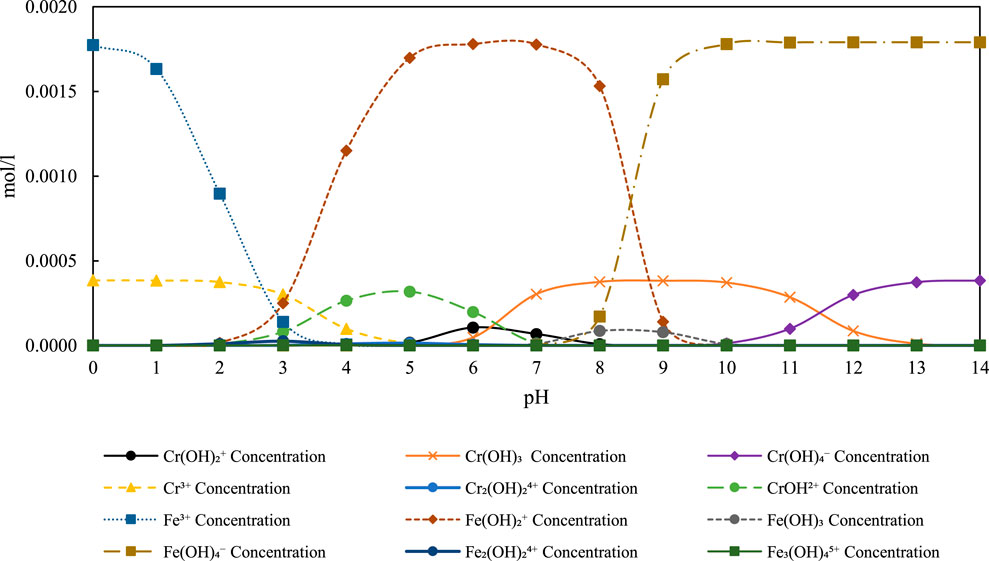
Figure 7. Speciation diagram of Fe3+ Concentration (100 mg/L), Cr6+ Concentration (20 mg/L), and related species over pH 0–14 under experimental conditions.
According to the results presented in Figures 5a–c, an increase in initial Fe3+ concentrations increased the precipitation and individual removal efficiencies of Cr6+ in both the anode and cathode. However, as the initial Cr6+ concentration increased, the total removal efficiency of Cr6+ decreased. Furthermore, increasing the initial Fe3+ concentration also increased the removal efficiency in both the anode and cathode. Equation 1 was employed for calculating the Cr6+ removed by precipitation.
The maximum Cr6+ removal (56.80%) in the cathode was achieved with an initial Fe3+ concentration of 100 mg/L when the initial Cr6+ concentration was 5 mg/L. This removal was a 39.03% increase over the initial level of zero Fe3+ in the solution (the coulomb transfer caused Cr6+ ions to transfer from anode to cathode). When the initial chromium concentration was 5 mg/L, the pH was varied from 11.80 to 11.06 for initial Fe concentrations of 0–100 mg/L, at the cathode (Figure 6a).
According to Figure 5b, when chromium concentration was 10 mg/L, the maximum removal efficiency in the cathode was 28.50% with 100 mg/L of initial Fe3+ concentration. This level was an 18.62% increase over the initial level of zero Fe3+ in the solution. When the initial chromium concentration was 10 mg/L, the pH was varied from 11.8 to 10.08 for initial Fe concentrations of 0–100 mg/L, at the cathode (Figure 6b).
According to Figure 5c, when chromium concentration was 20 mg/L, the maximum removal efficiency in the cathode was 16.18% with 100 mg/L of initial Fe3+ concentration. This level was a 6.87% increase over the initial level of zero Fe3+ in the solution. The pH at the cathode was changed from 12.03 to 9.87 for initial Fe concentrations of 0–100 mg/L when the initial concentration of chromium was 20 mg/L (Figure 6c).
The data in Figures 5a,c reveal that the introduction of Fe3+ significantly enhanced the removal of Cr6+ by precipitation and cathodic reduction. At 5 mg/L Cr6+, the highest overall removal percentage (56.80%) was achieved at 100 mg/L Fe3+, demonstrating efficient sweep flocculation mediated by Fe(OH)3 formation. However, when the initial Cr6+ concentration reached 10 mg/L and 20 mg/L, the increase in the removal rate was no longer evident, indicating that the adsorption sites were becoming saturated, and flocs captured decreased. Anodic removal remained consistently low across all conditions, highlighting the dominant role of cathodic and precipitative pathways. These trends reflect a concentration-dependent synergy between Fe3+ addition and electrochemical conditions, where optimal Cr6+ removal occurs at lower Cr6+ loads with sufficient Fe3+ to drive co-precipitation and charge neutralization mechanisms.
Fe(OH)3 is the most common form of Fe in the pH range of 8–12 (Stefánsson, 2007).
In this instance, Fe3+ ions joined with OH− to form metallic hydroxides of Fe(OH)3, which allowed hydroxides to remove Cr6+ from water (Equation 3; Equation 10). Fe(OH)3 is an observed high surface area positive charged material at some pH environments that works well as an adsorbent for negatively charged species such as Cr6+ (typically as CrO42- or HCrO4−). The adsorption of Cr6+ onto its surface can be enhanced by the presence of Fe(OH)3. Cr6+ is frequently converted to Cr3+ during electrolysis, particularly when Fe2+ or Fe3+ ions are present (Equation 7). After that, Cr3+ and Fe(OH)3 might co-precipitate to create a mixed hydroxide precipitate. Finally, the adsorption process can help in the additional improvement of hexavalent chromium removal. In the presence of ferric hydroxide, Cr2O72- ions can be adsorbed onto the ferric hydroxide precipitate (Equation 11).
Experiments with ferric ions similarly employed unbuffered solutions, permitting cathodic pH to increase naturally from 11.06 to 11.80 across Fe3+ concentrations of 0–100 mg/L (Figure 5a). In this alkaline environment, Fe3+ rapidly forms Fe(OH)3, a highly porous solid with abundant positively charged surface sites conducive to strong adsorption of CrO42- and Cr2O72- through Coulombic forces and ligand exchange.
As Fe(OH)3 is more stable and easily formed at pH values of neutral to slightly alkaline, this procedure is especially successful there. According to the results of Figures 5a–c, the removal of Cr6+ should eventually cause an increase as the total surface area of Fe(OH)3 increases as the initial Fe3+ concentration rises.
With an initial Fe3+ concentration of 100 mg/L, the highest removal of Cr6+ by precipitation (64.98%) was achieved in a 5 mg/L Cr6+ solution. Over the initial Fe3+ concentration in the solution, this amount represented an increase of 65.88% (Figure 6a). When the chromium concentration was 10 mg/L, Figure 6b shows that the maximum removal effectiveness of Cr6+ by precipitation was 29.45%. This amount represented a 26.27% increase over the solution’s initial Fe3+ concentration of zero. According to Figure 6c, when chromium concentration was 20 mg/L, the maximum Cr6+ removal by precipitation was 12.24%, achieved in the initial Fe3+ concentration of 100 mg/L. This level was a 10.88% increase over the initial level of zero Fe3+ in the solution.
While the experimental data demonstrate a clear enhancement in Cr6+ removal with increasing Fe3+ concentrations, understanding the underlying chemical pathways is essential to explain this behaviour and optimize treatment performance. Cr6+ exists in aqueous solutions primarily as the oxyanions chromate (CrO42-) and dichromate (Cr2O72-), which are highly mobile and toxic. When Fe3+ ions are introduced into the solution, they rapidly hydrolyse to form Fe(OH)3 precipitates, especially under neutral to slightly alkaline conditions. Because of their large surface area and positive charge in they are suitable for electrostatic reactions.
In addition to adsorption, Fe(OH)3 supports an inner-sphere type of surface complexation between the Cr6+ ions and surface OH groups. Where Fe2+ forms, either from cathodic reactions or the reduction of Fe3+, a redox reaction occurs that reduces Cr6+ to the Cr3+ form. Because Cr6+ is highly toxic, the transformation to Cr3+ and the creation of Cr(OH)3 is necessary, since the new Cr3+ is less dangerous and the Cr(OH)3 easily settles out under the conditions. The freshly created Cr(OH)3 may join Fe(OH)3 particles or adsorb onto its surface, which helps take the CrOH4 out of the solution.
Besides, the exchange of Cr6+ ions with surface hydroxyls on Fe(OH)3 increases the protection of the sorbed complexes. As a result of this process and mixed-metal hydroxide flocs, the structure of the precipitates becomes better, and the possibility of Cr species moving again is reduced. These mixed precipitates may be made up of Fe–Cr hydroxide networks that give more ways for chromium to be captured.
Because of electrostatic adsorption, redox reduction, and co-precipitation, a higher Fe3+ concentration leads to higher removal of Cr6+, which is seen in Figures 6a,c. The process functions best when the solution is somewhat acidic or neutral, since at these levels Fe(OH)3 is both stable and effective. The results validate the experimental findings and show that using active groups of Fe3+ plays a key role in optimizing Cr6+ removal in electrochemical devices.
Thermodynamic speciation diagram of iron (Fe3+), chromium (Cr6), and associated hydroxide and oxyanion species versus pH, at 20 mg/L Cr and 100 mg/L Fe3+. The diagram highlights the formation of Fe(OH)3 predominantly between pH six and 9, consistent with measured cathodic pH values, enabling effective Cr removal through precipitation and redox reactions.
3.4 Effect of the initial Mg2+ concentration on the removal of Cr6+
Additionally, as shown in Figures 8a–c, this study investigates the effect of initial Mg2+ concentration on the removal efficiency of Cr6+ at different Cr6+ concentrations as 5 mg/L, 10 mg/L, and 20 mg/L with a constant charge loading of 1500 C/L (25 mA for 30 min). The initial Mg2+ concentration was varied from 0 mg/L to 100 mg/L. Variation of Cr6+ removal in the cathode is shown in Figures 8a–c as a function of initial Mg2+ concentration. According to the results, when the initial Mg2+ concentrations increased, the removal efficiency of the Cr6+ concentration increased.
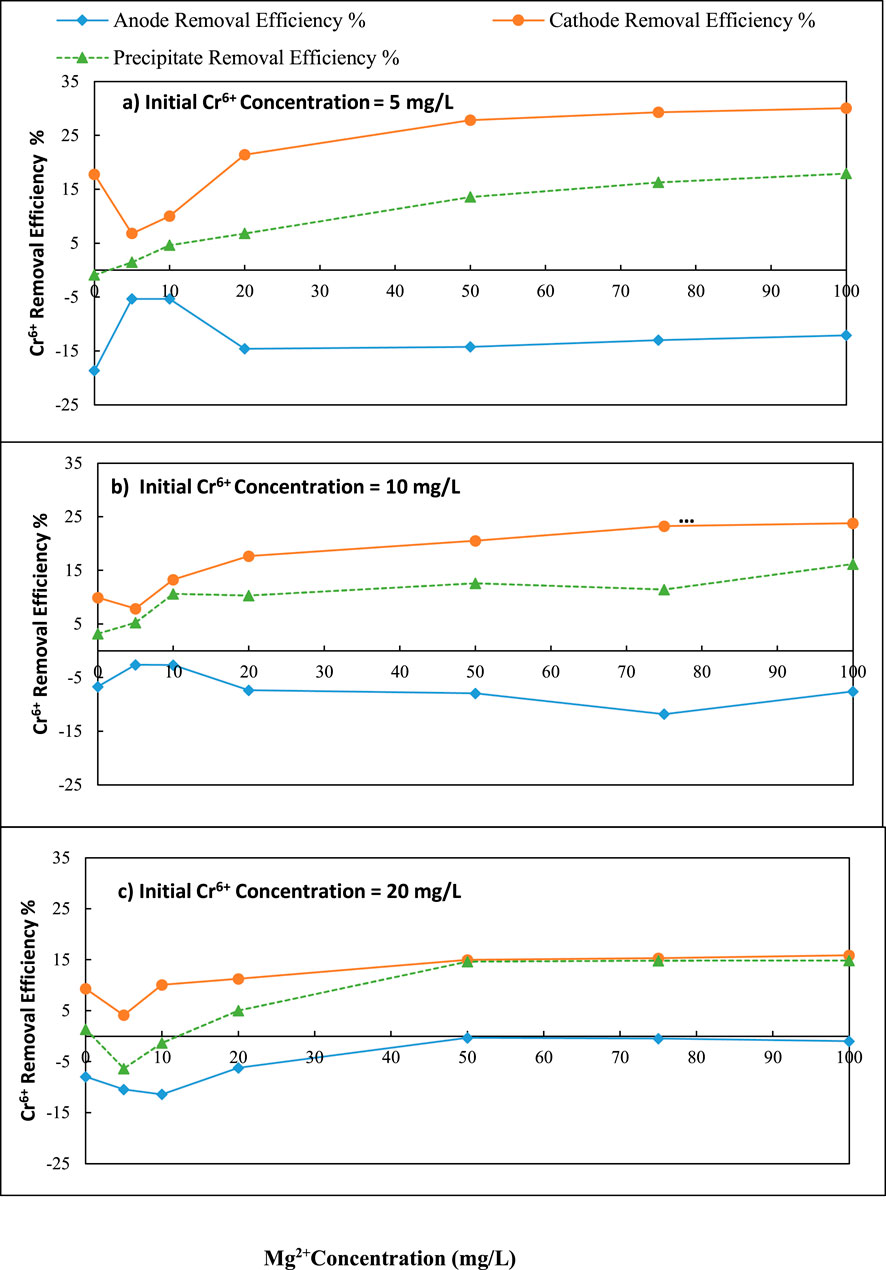
Figure 8. Variation of Cr6+ removal with initial Mg2+ concentration over different initial Cr6+ concentration (a) 5 mg/L, (b) 10 mg/L and (c) 20 mg/L; C/L applied at = 1500 C/L (0.25A).
The maximum Cr6+ (Initial Cr6+ = 5 mg/L) removal (30.05%) was achieved at the cathode when the initial Mg2+ concentration was 100 mg/L. This level was a 12.28% increase over the initial level of zero Mg2+ in the solution ([30.05–12.28] % = coulomb transfer to cathode) (Figure 8a). The pH levels of 11.79–11.04 were observed for initial magnesium concentrations of 0–100 mg/L, in an initial chromium concentration was 10 mg/L (Figure 9a).
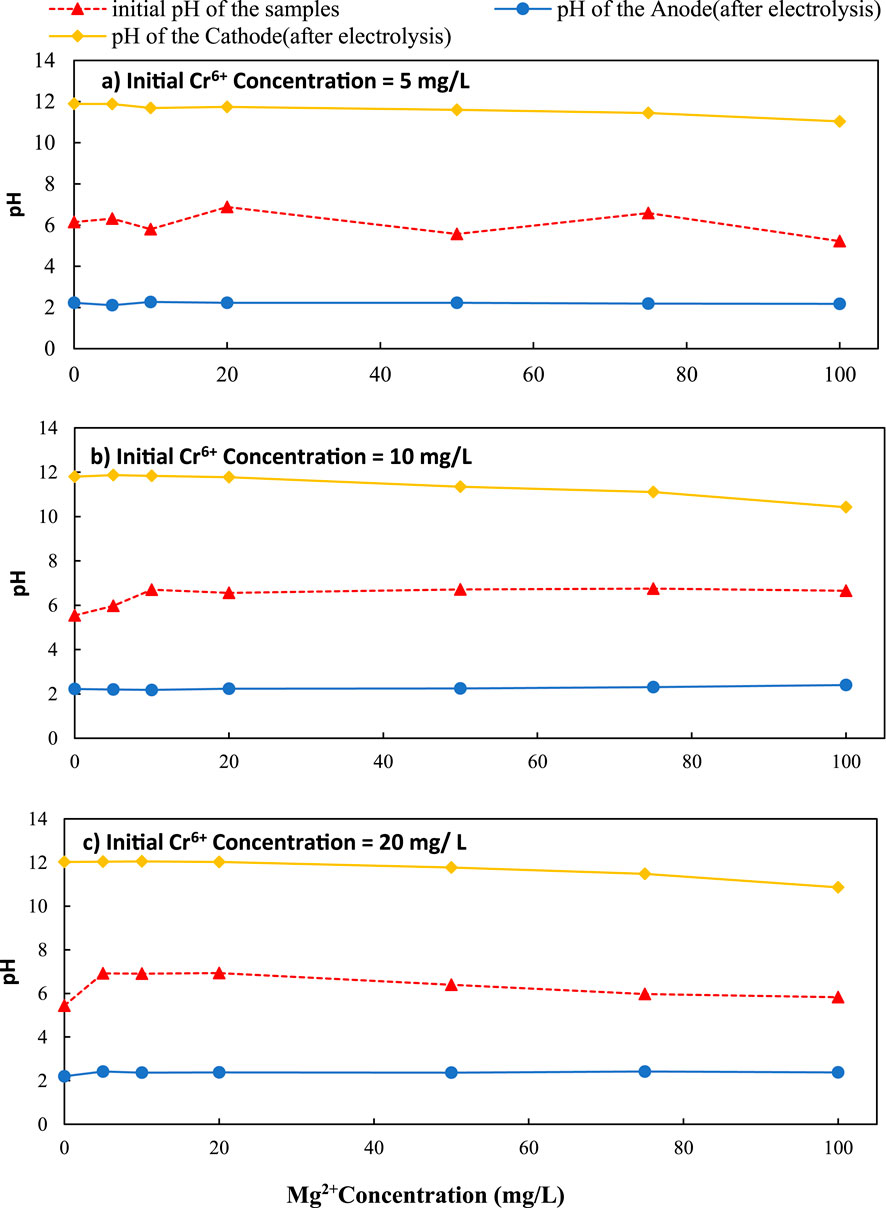
Figure 9. Variation of pH with initial Mg2+ concentration over different initial Cr6+ concentration (a) 5 mg/L, (b) 10 mg/L and (c) 20 mg/L C/L applied at = 1500 C/L (0.25A)
According to Figure 8b, when the solution chromium concentration was 10 mg/L, the maximum removal efficiency at the cathode was found as 23.78% at 100 mg/L initial Mg2+ concentration. This level was a 13.90% increase over the initial level of zero Mg2+ in the solution. When the initial chromium concentration was 10 mg/L, the pH was varied from 11.80 to 10.42 for initial magnesium concentrations of 0–100 mg/L, at the cathode (Figure 9b).
According to Figure 8c, when chromium concentration was 20 mg/L, the maximum removal efficiency was 15.87% at 100 mg/L initial Mg2+ concentration at the cathode. This level was a 6.56% increase over the initial level of zero Mg2+ in the solution. According to the results, the chromium removal efficiency decreased as the initial chromium concentration increased. The pH was varied from 11.93 to 10.86 for initial magnesium concentrations of 0–100 mg/L, and the initial chromium concentration was 20 mg/L at the cathode (Figure 9c).
The Cr6+ removal efficiency increased with rising initial Mg2+ concentrations at all initial Cr6+ levels (5, 10, and 20 mg/L), but the magnitude of improvement diminished as the Cr6+ concentration increased. At lower Cr6+ concentrations (e.g., 5 mg/L), Mg2+ effectively formed Mg(OH)2 flocs that promoted Cr6+ removal via adsorption and sweep coagulation, resulting in a maximum removal of 30.05%. However, as initial Cr6+ increased to 10 and 20 mg/L, the available Mg(OH)2 became insufficient relative to Cr6+ load, leading to floc surface saturation and reduced removal efficiency. Additionally, the gradual decrease in pH observed with increasing Mg2+ may have reduced the formation and stability of hydroxide flocs, further impacting performance. These findings highlight a concentration-dependent trend, where Mg2+ dosing is most effective at lower Cr6+ concentrations and is limited by charge imbalance and floc capacity at higher contaminant loads.
The predominant species of Mg2+ in the pH range of 8.7–12.5 is Mg(OH)2.
Magnesium experiments were conducted under similar unbuffered conditions, with cathodic pH rising from 10.86 to 12.03 as Mg2+ concentrations increased from 0 to 100 mg/L (Figure 9c). Above pH 9, Mg2+ precipitates as Mg(OH)2, a crystalline phase characterized by lower surface charge and adsorption capacity compared to amorphous Al(OH)3 and Fe(OH)3.
Here, Mg2+ ions interacted with OH− to generate Mg(OH)3 metallic hydroxides, which allowed hydroxides to remove Cr6+ from water (Equation 3; Equation 12).
The pH change for different initial Mg2+ concentrations is shown in Figures 9a–c. The pH levels of 11.79–11.04 were observed for initial magnesium concentrations of 0–100 mg/L; hence predominant species is Mg(OH)2. The formation of Mg(OH)2 could lead to Cr co-precipitation or adsorption on the surface (Equation 8; Equation 13).
Adsorption of Dichromate Ions on Magnesium Hydroxide:
The overall surface area of Mg(OH)2 grows as the initial Mg2+ concentration rises. Thus, the removal of Cr6+ should eventually lead to an increase in line with the findings of Figures 8a–c, Mg(OH)2 generation increased with increasing initial Mg2+ concentration, increasing Cr6+ removal as a form of precipitate in the cathode, and increasing the gradient of Cr6+ ion concentration between the cathode and anode. Hence, the Cr6+ removal by co-precipitation was increased. The maximum Cr6+ removal by precipitation (17.91%) was achieved with an initial Mg concentration of 100 mg/L (Figure 8a). This level was an 18.81% increase over the initial level of zero Mg2+ in the solution. According to Figure 8b, when chromium concentration was 10 mg/L, the maximum Cr6+ removal by precipitation removal efficiency was 16.18%. This level was a 13.00% increase over the initial level of zero Mg2+ in the solution. According to Figure 8c, when chromium concentration was 20 mg/L, the maximum Cr6+ removal by precipitation was 14.86%, achieved in an initial Mg2+ concentration of 100 mg/L. This level was a 13.50% increase over the initial level of zero Mg2+ in the solution.
At a mechanistic level, the interaction between Cr6+ species and Mg(OH)2 involves a combination of surface chemistry, solid-phase equilibria, and ionic strength effects under alkaline conditions. Mg(OH)2, crystallizing in a brucite-type structure, has surface hydroxyl moieties (≡Mg–OH) that are subject to protonation–deprotonation under the influence of the pH of the solution, imparting surface charge. At a pH level above 10, the surface exhibits either a neutral or slightly positive charge, which enables a selective adsorption of Cr6+ anions through inner-sphere complexation, wherein the dichromate or chromate species displace surface hydroxyls via ligand exchange. The interaction is thermodynamically favoured in high-pH, low-buffering environments in that the dissolution of Mg(OH)2 is kept very low to the extent of occurrence of a stable solid phase. The interaction can also take place through partial incorporation of chromium into the lattice of Mg(OH)2, and at least in the early stages of precipitation, under very fast nucleation, hydroxide precipitation is capable of entrapping Cr6+ ions. Although Mg(OH)2 has a lower charge density on the surface relative to Fe(OH)3 or Al(OH)3, the structural stability of Mg(OH)2 increases, together with its surface area as the concentrations of Mg2+ increase, thereby providing potential removal power continually and cumulatively. The enhancement of the overall driving force for both adsorption and entrapment could also be attributed to the creation of local microenvironments around the cathode that subsequently give rise to a diffusion gradient favouring Cr6+ migration and immobilization.
Thermodynamic speciation diagram of magnesium (Mg2+), chromium (Cr6+), and their related hydroxide and complex species as a function of pH, modeled for 20 mg/L Cr and 100 mg/L Mg (Figure 10). The figure shows that Mg(OH)2 becomes dominant above pH 10, in agreement with cathodic pH measurements, facilitating Cr removal via surface complexation and entrapment mechanisms.
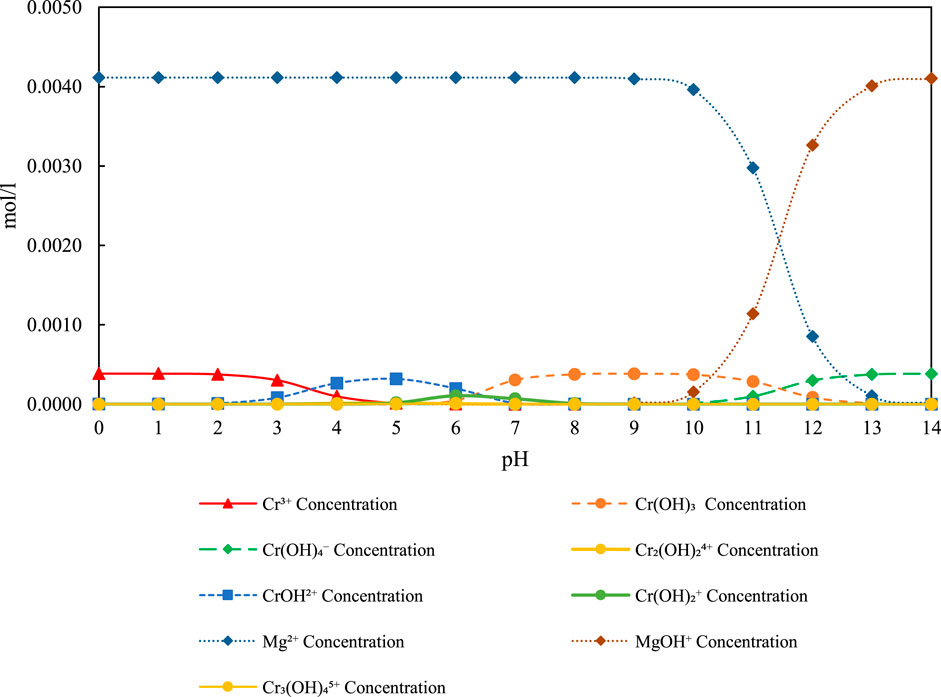
Figure 10. Speciation diagram of Mg2+ Concentration (100 mg/L), Cr6+ Concentration (20 mg/L), and related species over pH 0–14 under experimental conditions.
3.5 Comparison of maximum removal efficiencies
From the study, according to Figure 11, it is demonstrated that at the lowest Cr6+ concentration of 5 mg/L, Al3+ (100 mg/L) is highly preferred in the precipitation of Cr6+ with nearly 70% precipitation efficiency. This high performance indicates that Al3+ ions may also contribute greatly to the enhancement of Cr6+ removal via the formation of soluble complexes or co-precipitate. Similarly, to Fe3+ (100 mg/L), the removal efficiency increases to a maximum of over 65% at this concentration, proving that it could be an effective candidate for chromium precipitation. However, Mg2+ (100 mg/L) has a significantly poor performance as its removal efficiency is nearly 15%, which could mean that its contribution towards the Cr6+ precipitation is not as efficient as other ions at this concentration.
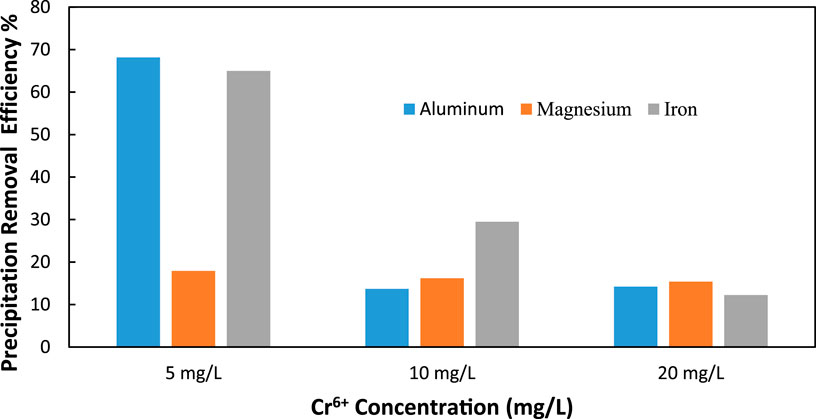
Figure 11. Comparison of Al3+ (100 mg/L), Mg2+ (100 mg/L), and Fe3+ (100 mg/L) Ion Additives on Cr6+ Precipitation Removal Efficiency at Different Cr6+ Levels (5 mg/L, 10 mg/L, and 20 mg/L) and c) 20 mg/L (C/L applied at = 1500 C/L (0.25 A).
When the concentration of Cr6+ reaches 10 mg/L, it can be observed that the removal percentage of each ion additive decreases. Thus, Fe3+ remains the most efficient ion, even though its efficiency is only 35% compared to the maximum values. This indicates that although Fe3+ is still involved in the removal of Cr6+, its effectiveness depends on its concentration. At this concentration, both Al3+ and Mg2+ have nearly the same efficiency, which varies between 10%–15%. This decrease, especially in Al3+, means that, as the concentration increases, the ion’s ability to promote Cr6+ precipitation decreases, possibly because of saturation and other impacts on competitive precipitation mechanisms that may occur after adding more Cr6+ into the solution.
When the Cr6+ ion concentration increases to the highest level of 20 mg/L, the removal efficiencies of all ions are reduced. As for the efficiency of Al3+, Mg2+, and Fe3+, the values defined and calculated reach almost the same numbers, equal to 10% and less than 10%, respectively, for every ion. At these higher concentrations, therefore, the present results indicate that the ability of each ion to precipitate Cr6+ may be constrained. The marked decrease in efficiency, especially in Al3+ and Fe3+, suggests that increased Cr6+ concentrations present certain difficulties to the precipitation process, either due to the lower availability of reactive sites or due to the enhanced complexity of interactions in solution.
Among the cations, Al3+ and Fe3+ are found to be the most effective ions to be used as additives for chromium ions at low initial concentrations of 5 mg/L of Cr6+ and varying pH levels, with Al3+ being the most efficient. Al3+ and Fe3+ removal rate decreases significantly with the increase in the concentration of Cr6+ in the solution, and reveals a strong concentration dependency of Cr6+. At the same time, the data clearly illustrate that Mg2+ has the lowest removal efficiency in all the concentrations and, therefore, appears to be the least efficient in promoting Cr6+ precipitation under the tested conditions.
The relevance of these outcomes is emphasized by the importance of enhancing the electrolysis-based Cr6+ removal processes. With the high performance of Al3+ at lower concentrations, it could be used as an additive where the trace of Cr6+ is present or in areas where Cr6+ concentrations are still relatively low. Currently, for high chromium concentration, it may be useful to develop new techniques or apply more than one additive to facilitate the improvement of Cr6+ removal efficiency.
Compared to existing studies on this study provides a unique assessment of Cr6+ removal by systematically evaluating and comparing the electrocoagulation removal efficiencies of potential coagulant additive ions (Al3+, Fe3+, and Mg2+) under the same conditions, rather than conducting simple operational comparisons and other constraints discussed earlier. Prior studies were able to attain high Cr6+ removal efficiencies through electrocoagulation or chemical precipitation processes individually, but none were easily found as explicit side-by-side comparisons of different coagulant ions in a single electrolysis treatment study. In this study, Al3+-mediated precipitation offers some of the highest Cr6+ removal, which also includes cathodic precipitation at 68.17% and 49.62%, respectively, with comparable treatment times (30 min) and charge loadings (1500 C/L) for other experimental ions. The reported Cr6+ removal efficiencies were not from the samples using either coagulant, nor adjusting sample volumes, and therefore the voltages and overall treatment times were higher, which are presumably inefficient and potentially not commercially scalable. Additionally, the concentration-dependent removal efficiencies and mechanisms described here, along with the influence of pH, coagulant dose, and Cr6+ loading, elaborated more detailed and diverse studies to provide a thorough review of the conditions influencing the conductivity of Cr6+ with various coagulant ions, which other works do not typically consider. It is expected that such coupling of an electrochemical process can be designed in such a way as to produce even better results. Therefore, this study has many distinct advantages and is a notably comprehensive and practically useful reference to optimize Cr6+ removal issues related to electrochemical-coagulant coupling.
3.6 Practical implications and scalability for industrial application
The batch electrolysis system demonstrated effective Cr6+ removal at the laboratory scale, indicating promising potential for industrial wastewater treatment applications. However, applying these techniques in real-world applications requires careful consideration of several technical and economic factors. Energy consumption is a key determinant of feasibility; although the current applied here (0.25 A) is modest, scaling to higher volumes will proportionally increase power requirements. The energy consumption for Cr6+ removal was estimated to be between 1.4 and 3.5 kWh per cubic meter of treated water, corresponding to an operational cost of approximately USD 0.25 to USD 0.63 (LKR 75–189) per cubic meter. These findings demonstrate the process’s potential for energy-efficient electrochemical remediation. Optimizing current density and cell design can improve energy efficiency, but requires pilot-scale studies. The selection of electrode materials and their longevity have a significant impact on operational costs and maintenance frequency. The use of a platinum anode, while effective and inert, is expensive, potentially limiting commercial viability; thus, exploring alternative, cost-effective anode materials with similar stability is recommended. Stainless steel cathodes offer durability but may experience corrosion or fouling issues over extended use, which can affect removal efficiency. The generation and management of metal hydroxide sludge (Al(OH)3, Fe(OH)3, Mg(OH)2) pose environmental and operational challenges. Proper handling, dewatering, and safe disposal or recovery of chromium-laden sludge must be integrated into the treatment workflow to avoid secondary pollution. Wastewater variability, including fluctuating Cr6+ concentrations, pH, and presence of competing ions, can influence system performance; thus, adaptive operational controls will be necessary. Integration with existing treatment processes, such as sedimentation tanks or filtration units, could enhance overall system efficacy and feasibility. To advance from laboratory to industrial scale, future research should focus on continuous-flow reactor designs, long-term electrode stability testing, energy consumption optimization, and comprehensive cost-benefit analyses. Such efforts will clarify the method’s practical potential and guide engineering development for large-scale chromium remediation.
4 Conclusion
This study investigated the electrolysis system to optimize the removal of Cr6+ from wastewater using a platinum anode and a stainless-steel cathode. The effectiveness of the electrolysis system in removing Cr6+ at various concentrations (5mg/L-100 mg/L) of Al3+, Fe3+, and Mg2+ was examined. Results indicated that even in the absence of Al3+, Fe3+, and Mg2+, the system could remove Cr6+ through Coulomb forces and precipitation. However, the presence of these additives markedly enhanced removal efficiency through co-precipitation with their respective hydroxides (Al(OH)3, Fe(OH)3, and Mg(OH)2). The observed decline in removal efficiency at higher initial Cr6+ concentrations is attributed to the saturation of hydroxide binding sites and limited charge transfer capacity inherent to the system.
With an initial Al3+ concentration of 100 mg/L and an initial Cr6+ concentration of 5 mg/L, the maximum Cr6+ removal achieved was 49.62%, representing a 31.85% increase compared to the solution with zero initial Al3+ concentration. Similarly, with an initial Fe3+ concentration of 100 mg/L and an initial Cr6+ concentration of 5 mg/L, the maximum Cr6+ removal observed was 56.80%, a 39.03% increase over the solution with zero initial Fe3+ concentration. The highest removal of Cr6+ (30.05%) was achieved with an initial Mg2+ concentration of 100 mg/L and an initial Cr6+ concentration of 5 mg/L, showing a 12.28% increase compared to the solution with zero initial Mg2+ concentration.
Building upon these findings, future research should explore a wider spectrum of multivalent additives such as Ca2+, Zn2+, Mn2+, and Cu2+, which may form hydroxides with distinct surface properties conducive to improved adsorption and coagulation. Studies on synergistic effects of the binary or ternary combinations of additives will also highlight removal efficiencies that surpass those obtained using single additives. In another direction, optimization of operational parameters such as current density, pH, and electrolyte composition, through modern statistical tools like response surface methodology, may provide other possible improvements.
Given the similarities in removal mechanisms, most notably involving adsorption, precipitation, and redox transformations, the presented electrochemical platform would also be promising for the removal of the heavy metals lead (Pb2+), cadmium (Cd2+), nickel (Ni2+), and arsenic (As5+). More importantly, validation of this system for the treatment of real industrial wastewater, which has complex matrices and competing ions among others, will be critical in assessing its robustness and hence, applicability in a practical scenario.
For system scalability and field deployment, stepping up from batch experiments to flows or pilots is crucial when it comes to testing energy recovery efficiency, electrode stability, sludge disposal and long-term operational feasibility. Another promising prospect for sustainable decentralized wastewater treatment is supposed to be the integration with renewable power generation, i.e., solar or wind. This study confirmed the removal feasibility of Cr6+ by additive-enhanced electrolysis and laid down an exhaustive basis for future studies towards improving treatment efficiency, broadening the scope to other contaminants, and advancing scalable and environmentally sustainable wastewater remediation technologies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ST: Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review and editing, Formal Analysis. AG: Conceptualization, Formal Analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review and editing. TK: Supervision, Writing – review and editing. VS: Writing – original draft, Writing – review and editing, Formal Analysis. GS: Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Author VS was employed by Sweco Norge AS.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aitio, A., Järvisalo, J., Kiilunen, M., Tossavainen, A., and Vaittinen, P. (1984). Urinary excretion of chromium as an indicator of exposure to trivalent chromium sulphate in leather tanning. Int. Archives Occup. Environ. Health 54 (3), 241–249. doi:10.1007/BF00379053
Amarasooriya, A. A. G. D., and Kawakami, T. (2019). Removal of fluoride, hardness and alkalinity from groundwater by electrolysis. Groundw. Sustain. Dev. 9, 100231. doi:10.1016/j.gsd.2019.100231
Amarasooriya, A. A. G. D., and Kawakami, T. (2020). Removal of co-existing fluoride, calcium, magnesium, and carbonates, by non-chemical induced electrolysis system for drinking and industrial purposes. H2Open J. 3 (1), 10–31. doi:10.2166/h2oj.2020.022
Carneiro, J., Tobaldi, D. M., Capela, M. N., Seabra, M. P., and Labrincha, J. A. (2019). Waste-based pigments for application in ceramic glazes and stoneware bodies. Materials 12 (20), 3396. doi:10.3390/ma12203396
Characterization, S., Genawi, N. M., Ibrahim, M. H., and El-naas, M. H. (2020). Chromium removal from tannery wastewater by electrocoagulation: optimization and.
Chen, Z., Chen, Y., Liang, J., Sun, Z., Zhao, H., and Huang, Y. (2024). The release and migration of Cr in the soil under alternating wet–dry conditions. Toxics 12 (2), 140. doi:10.3390/toxics12020140
Costa, M. (2003). Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 188 (1), 1–5. doi:10.1016/S0041-008X(03)00011-5
Garg, R., Garg, R., Sillanpää, M. A., Khan, M. A., Mubarak, N. M., Tan, Y. H., et al. (2023). Rapid adsorptive removal of chromium from wastewater using walnut-derived biosorbents. Sci. Rep. 13 (1), 6859–12. doi:10.1038/s41598-023-33843-3
Golder, A. K., Samanta, A. N., and Ray, S. (2007). Removal of trivalent chromium by electrocoagulation. Sep. Purif. Technol. 53 (1), 33–41. doi:10.1016/j.seppur.2006.06.010
Hamadeen, H. M., Elkhatib, E. A., and Moharem, M. L. (2022). Optimization and mechanisms of rapid adsorptive removal of chromium (VI) from wastewater using industrial waste derived nanoparticles. Sci. Rep. 12 (1), 14174–12. doi:10.1038/s41598-022-18494-0
Kerur, S. S., Bandekar, S., Hanagadakar, M. S., Nandi, S. S., Ratnamala, G. M., and Hegde, P. G. (2020). Removal of hexavalent Chromium-Industry treated water and Wastewater: a review. Mater. Today Proc. 42, 1112–1121. doi:10.1016/j.matpr.2020.12.492
Kokkinos, E., Proskynitopoulou, V., and Zouboulis, A. (2019). Chromium and energy recovery from tannery wastewater treatment waste: Investigation of major mechanisms in the framework of circular economy. J. Environ. Chem. Eng. 7 (5), 103307. doi:10.1016/j.jece.2019.103307
Krishnani, K. K., Srinives, S., Mohapatra, B. C., Boddu, V. M., Hao, J., Meng, X., et al. (2013). Hexavalent chromium removal mechanism using conducting polymers. J. Hazard. Mater. 252–253, 99–106. doi:10.1016/j.jhazmat.2013.01.079
Kumar Saha, S., and Ghosh, D. (2022). A comprehensive review on toxicity of chromium in freshwater fishes. Appl. Ecol. Environ. Sci. 10 (8), 527–533. doi:10.12691/aees-10-8-5
Malinovic, B., Djuricic, T., and Bjelic, D. (2022). Electrochemical removal of hexavalent chromium by. January. doi:10.5281/zenodo.6918321
Merical, J. (2007). The effects and processes for removal of chromium in activated sludge treatment, 1–11.
Mohan, D., and Pittman, C. U. (2006). Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 137 (2), 762–811. doi:10.1016/j.jhazmat.2006.06.060
Park, J. E., Shin, J. H., Oh, W., Choi, S. J., Kim, J., Kim, C., et al. (2022). Removal of hexavalent chromium(VI) from wastewater using chitosan-coated iron oxide nanocomposite membranes. Toxics 10 (2), 98. doi:10.3390/toxics10020098
Peng, H., Leng, Y., and Guo, J. (2019). Electrochemical removal of chromium (VI) from wastewater. Appl. Sci. Switz. 9 (6), 1156. doi:10.3390/app9061156
Stefánsson, A. (2007). Iron (III) hydrolysis and solubility at 25 degrees C. Environ. Sci. and Technol. 41 (17), 6117–6123. doi:10.1021/es070174h
Yan, G., Gao, Y., Xue, K., Qi, Y., Fan, Y., Tian, X., et al. (2023). Toxicity mechanisms and remediation strategies for chromium exposure in the environment. Front. Environ. Sci. 11 (February), 1–10. doi:10.3389/fenvs.2023.1131204
Yogeshwaran, V., and Priya, A. K. (2018). Removal of hexavalent chromium (Cr6) using different natural adsorbents A review. SSRN Electron. J., 1–20. doi:10.2139/ssrn.3090245
Zhou, J., Du, N., Li, D., Qin, J., Li, H., and Chen, G. (2021). Combined effects of perchlorate and hexavalent chromium on the survival, growth and reproduction of Daphnia carinata. Sci. Total Environ. 769, 144676. doi:10.1016/j.scitotenv.2020.144676
Keywords: electrolysis, Al3+, Fe3+, Mg2+: co-precipitation, coulomb forces
Citation: Jayasinghe T, Amarasooriya G, Kawakami T, Sivalingam V and Samarakoon G (2025) Hexavalent chromium (Cr6+) removal from wastewater through electrolysis: Influence of Al3+, Fe3+, and Mg2+ ion additives on treatment efficiency. Front. Chem. Eng. 7:1580201. doi: 10.3389/fceng.2025.1580201
Received: 20 February 2025; Accepted: 01 July 2025;
Published: 20 August 2025.
Edited by:
Chirangano Mangwandi, Queen’s University Belfast, United KingdomReviewed by:
Jayato Nayak, Mahindra University, IndiaJarosław Serafin, University of Barcelona, Spain
Copyright © 2025 Jayasinghe, Amarasooriya, Kawakami, Sivalingam and Samarakoon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gamunu Samarakoon, Z2FtdW51LmFyYWNoY2hpZ2VAdXNuLm5v
 Thilini Jayasinghe
Thilini Jayasinghe Gayan Amarasooriya
Gayan Amarasooriya Tomonori Kawakami
Tomonori Kawakami Vasan Sivalingam
Vasan Sivalingam Gamunu Samarakoon
Gamunu Samarakoon