- 1Beijing Research Institute of Chemical Engineering and Metallurgy, China National Nuclear Corporation, Beijing, China
- 2School of Minerals Processing and Bioengineering, Central South University, Changsha, China
Uranium, extensively used in nuclear power generation, military applications, and scientific research, poses significant environmental and human health risks when released into soil due to its radiotoxicity and long half-life. Conventional remediation methods such as chemical leaching, stabilization, and bioremediation often face limitations including high costs, incomplete removal, prolonged treatment durations, and the potential for secondary pollution. In contrast, electrokinetic remediation (EKR) has emerged as a promising in situ technology for addressing uranium-contaminated soils, particularly in low-permeability environments where other methods are less effective. EKR operates by applying an external electric field across the soil matrix, inducing contaminant transport via mechanisms such as electromigration (ionic movement), electroosmosis (bulk fluid flow), and electrophoresis (movement of charged particles). These processes mobilize uranium species toward electrode zones, where they can be collected and removed through various treatment strategies. This review provides a comprehensive overview of recent advances in the application of EKR for uranium remediation, including fundamental transport mechanisms, system design parameters (e.g., electrode materials, electrolyte formulations, voltage gradients), and synergistic approaches such as coupling with phytoremediation and permeable reactive barriers. The role of numerical modeling in predicting system performance and optimizing operational parameters is also highlighted, along with the emerging potential of integrating renewable energy sources to enhance sustainability. Despite encouraging results at laboratory and pilot scales, challenges remain regarding scalability, energy efficiency, electrode longevity, and field deployment under heterogeneous site conditions. Future research should prioritize the development of hybrid systems, site-specific optimization strategies, and robust monitoring frameworks. Overall, EKR represents an environmentally friendly and technically viable solution for the remediation of uranium-contaminated soils, with considerable potential for application in nuclear facility decommissioning and long-term environmental restoration.
1 Introduction
With the rapid development and application of nuclear energy and nuclear technology, uranium-based nuclear industry has been widely used in nuclear power generation, military industry, experimental research, etc. (Usman and Radulescu, 2022). However, the widely use of nuclear technology has produced a certain amount of uranium contaminated soil (Wei et al., 2021). Uranium as radioactive heavy metal have a longer half-life, which will cause serious threats to the surrounding soils, water bodies, and health of humans (Brugge and Buchner, 2011; Shu et al., 2020). After entering the soil, uranium often combines strongly with the soil and has low mobility, making soil remediation difficult, which limits the development and utilization of land after decommissioning of nuclear facilities. Therefore, cost-effective and efficient technologies for remediation of uranium contaminated soil are urgently demanded.
Currently, a variety of methods have been developed to remove uranium from contaminated soils including chemical leaching, stabilization technique, phytoremediation, bioremediation, etc. (Gavrilescu et al., 2009; Sihn et al., 2019; Cheng et al., 2022; Wu et al., 2022; You et al., 2022). However, such conventional methods have drawbacks such as high costs, incomplete removal of contaminants, potential for secondary pollution and long remediation period (Liu et al., 2018). Compared with the above remediation technology, electrokinetic remediation (EKR) technology is widely used in the field of environmental remediation due to the excellent removal ability of heavy metals from the soil, especially soil with poor permeability. Wide application ranges, cost-effectiveness and environmental friendliness are the advantage of EKR for effective removal of pollutants (Vocciante et al., 2021; Sun et al., 2023).
During EKR process, two electrodes are inserted into a medium by applying a voltage and the generated electric field induces direct current or alternating current. Subsequently, the applied potential causes the migration of soluble ions or pore liquid in the soil toward the electrode via electromigration, electroosmosis or electrophoresis (Akansha et al., 2024). The pollutants in the soil migrate to specific locations under the action of electric field and are removed through electroplating, co-precipitation, extraction and ion exchange resin to achieve the purpose of remediating contaminated soil (Gomes et al., 2012). Nowadays, EKR technologies are widely used in the removal of inorganic pollutants (Buchireddy et al., 2009; Wen et al., 2021), organic pollutants (Huang et al., 2012; Chen et al., 2021; Saini et al., 2021; Ganbat et al., 2022; Li et al., 2023) and radioactive substances (Agnew et al., 2011; Purkis et al., 2021). In soil and favorable removal performance is achieved. In this review, we focus only on the application of EKR of uranium contaminated soil. Design strategies, electrolyte composition, electric field strength and treatment effect are fully summarized and compared. EKR of uranium contaminated soil is expected to show great potential in environmental remediation and decommissioning of nuclear facilities.
2 Principles of EKR for contaminated soil
EKR is a promising in situ technique for the removal of contaminants from soil, especially in low-permeability environments. It operates by applying a low-voltage direct current between electrodes inserted into the soil, creating an electric field and current that mobilize contaminants (Wang et al., 2012). Groundwater or an externally added electrolyte solution acts as the conductive medium, allowing the electric current to pass predominantly through the liquid phase of the soil. The effectiveness of EKR stems from the interplay of three fundamental transport mechanisms: electromigration, electrophoresis, and electroosmosis (Wang et al., 2021; Wen et al., 2021).
2.1 Primary electrokinetic transport phenomena
Electromigration refers to the movement of charged ions and ionic complexes under the influence of an electric field. Positively charged species migrate toward the cathode, while negatively charged ones move toward the anode. For uranium, which commonly exists in the hexavalent U(VI) form as uranyl ion (UO22+), electromigration causes migration toward the cathode. Uranium may also form negatively charged carbonate complexes, such as UO2(CO3)34-, that migrate toward the anode. Electromigration is the dominant mechanism for transporting dissolved inorganic uranium species, significantly enhancing their recovery (Maes et al., 2002).
Electrophoresis describes the migration of charged colloidal particles and fine particulates under an electric field. In soils with high organic matter content or where uranium is bound to colloidal particles or nanoparticles, electrophoresis is critical. This mechanism facilitates the movement of uranium in particulate forms, supplementing electromigration to ensure that both ionic and particulate species are mobilized.
Electroosmosis is the movement of the pore fluid, such as groundwater or leaching solutions, through the soil matrix, driven by the interaction between the electric field and the diffuse double layer on soil particles. Electroosmotic flow is particularly significant in fine-grained or low-permeability soils, where traditional hydraulic methods are ineffective. It carries dissolved uranium ions along with the fluid toward the cathode, supporting both contaminant transport and removal.
Under the combined influence of these three processes, contaminants are effectively transported toward the electrodes, where they can be captured by precipitation, extraction, electroplating, or ion exchange. The synergy among these mechanisms is central to the efficacy of EKR in uranium-contaminated soils.
In the EKR process, water near the anode is primarily oxidized and H+ ions are produced, showing low pH. On the other hand, water reduction occurs at the cathode, OH− ions are generated, indicating high pH. The elevated pH in the vicinity of the cathode may impede the migration and dissolution of heavy metals. Metal ions dissolved in the soil may adsorb or precipitate near the cathode, thereby reducing the efficacy of EKR process (Gidudu and Chirwa, 2022).
2.2 Theoretical basis of transport
The transport of ionic species in an electrokinetic system is quantitatively described by the Nernst-Planck equation (Paz-Garcia et al., 2011):
Where
The electric potential φ across the soil is governed by the Poisson equation:
where ρe is the local charge density, and ε is the dielectric permittivity of the soil matrix.
2.3 Electroosmotic flow and velocity
The velocity of electroosmotic flow veo is given by (Kim et al., 2005):
ζ is the zeta potential, μ is dynamic viscosity, and E is electric field strength.
2.4 Adsorption modeling with Langmuir isotherm
The adsorption of uranium onto soil particles can be modeled using the Langmuir isotherm (Vereda-Alonso et al., 2004):
where q is the adsorbed concentration, qmax is the maximum capacity, K is the binding constant, and C is the solution concentration. This model is useful for describing uranium absorption kinetics in numerical simulations.
2.5 Coupled reaction–transport modeling
A comprehensive transport model integrating adsorption and electrokinetic effects is given by (López-Vizcaíno et al., 2017):
where
2.6 Summary and implications
EKR is a versatile and efficient remediation technology that leverages multiple physicochemical processes to remove uranium from contaminated soils. The combination of electromigration, electrophoresis, and electroosmosis enables the movement of both dissolved and particulate uranium species. Accurate modeling using the Nernst–Planck and Langmuir equations allows for optimization and scaling of the technology to various field conditions. Understanding the complex interplay of transport mechanisms, speciation, and soil interactions is essential for advancing EKR applications in uranium remediation.
3 Uranium speciation and mobility in contaminated soils
Understanding uranium speciation and mobility in soil environments is crucial for designing effective remediation strategies. Uranium primarily exists in two oxidation states in the environment: tetravalent U(IV) and hexavalent U(VI). Among these, U(VI), typically present as the uranyl ion (UO22+), is the more mobile and bioavailable species, especially under oxidizing and acidic to neutral pH conditions. In contrast, U(IV), commonly found as insoluble UO2(s), is more stable under reducing conditions and tends to be immobile due to low solubility.
Uranium speciation is strongly influenced by several environmental factors including pH, redox potential (Eh), carbonate content, organic matter, and the presence of competing ions such as phosphate, sulfate, or calcium. For instance, in alkaline and carbonate-rich environments, uranyl forms stable aqueous complexes like UO2(CO3)34- and UO2(CO3)22-, which significantly enhance uranium mobility. In contrast, under low pH conditions, uranium forms positively charged species (e.g., UO22+, UO2OH+), which are prone to adsorption on negatively charged mineral surfaces such as clays and oxides, thereby reducing mobility.
Soil texture and mineral composition also affect uranium transport. Fine-textured soils with high clay or organic content exhibit strong adsorption and complexation capacities, reducing uranium migration. In contrast, sandy soils with low organic matter and high permeability facilitate uranium leaching and transport to deeper layers.
These speciation and mobility characteristics have direct implications for EKR. Since EKR relies on electromigration and electroosmosis to mobilize ionic species, uranium must exist in a soluble or weakly adsorbed form to ensure effective removal. Therefore, chemical preconditioning—such as acidification or the addition of chelating agents (e.g., citric acid, EDTA)—is often necessary to increase uranium solubility and promote its migration toward the electrodes. Moreover, redox manipulation through electrochemical methods can convert immobile U(IV) into more extractable U(VI) species.
A comprehensive understanding of uranium’s environmental chemistry thus enables the tailored design of EKR systems, ensuring higher removal efficiency by aligning electric field application with dominant uranium speciation and transport behavior under site-specific conditions.
4 Application of EKR for uranium contaminated soil
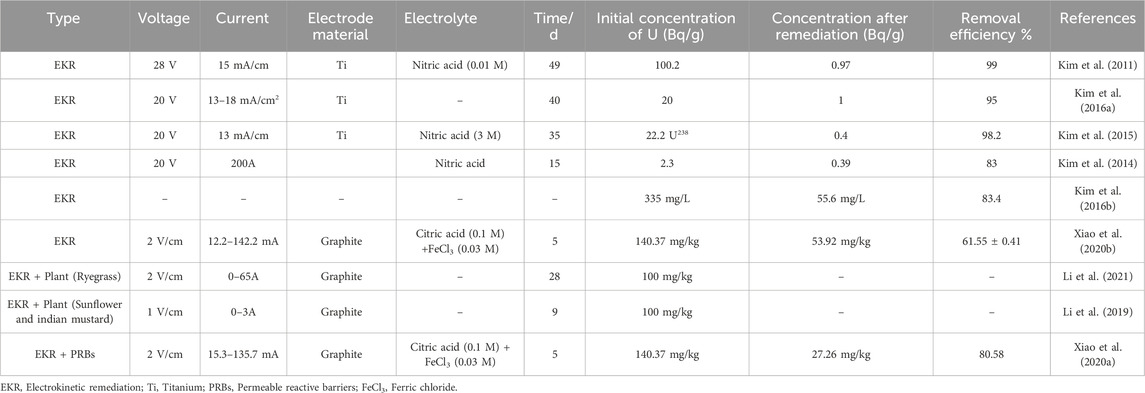
EKR harnesses the application of a low-intensity direct current electric field across contaminated soil to mobilize and transport uranium ions toward collection electrodes. Under the influence of the electric field, uranium, primarily in its mobile hexavalent form (UO22+), migrates through the soil matrix via electromigration, while electroosmosis and electrophoresis further assist in transporting water and charged colloidal particles. This in situ remediation technique is particularly effective in low-permeability soils. To enhance uranium removal efficiency and overcome limitations such as pH gradients and limited extraction depth, EKR systems are increasingly integrated with complementary technologies. Notably, permeable reactive barriers (PRBs) are often installed adjacent to the electrodes to intercept and immobilize mobilized uranium, while phytoremediation leverages plant uptake mechanisms to extract uranium from soil over longer timescales. Table 1 exemplifies a range of EKR design strategies applied to uranium-contaminated soils, highlighting key operational parameters such as electric field strength, electrolyte composition, and treatment duration. Comparative data on removal efficiencies illustrate how combined or enhanced systems outperform standalone EKR in many scenarios, offering valuable insights into practical system optimization.
According to Table 1, EKR for decontamination of uranium contiaminated soil exhibits relatively high removal efficiencies (approximately 61%–99%), whereas longer remediation time is needed. EKR coupling with PRBs (or phytoremediation) achieves shorter remediation durations. Commonly employed electrode materials include graphite and titanium.
4.1 EKR for uranium contaminated soil
EKR were used for soil decontamination around nuclear facilities and radionuclides including 60Co and 137Cs were effectively removed througt EKR (Kim et al., 2008; Kim et al., 2009; Kim et al., 2010). As showed in Figure 1A, Kim et al. developed a pilot-scale EKR equipment for uranium removal (Kim et al., 2011). This EKR equipment included the following parts: anode room, cathode room, electrokinetic soil cell, reagent reservoir, power supply, pH controller. Reasonable design of immersion-washing devices, metal oxide separators and pH control circulation system were conducive to solve the problem of cathode adhering metal oxides. Uranium concentration in the soil dropped from 100 Bq/g to 1.0 Bq/g after 49 days of EKR. Additionally, 1.2 tons of uranium contaminated soil was treated by an indoor electrokinetic decontamination (Kim et al., 2016a). Waste electrolyte was reused for the reduction of waste electrolyte and metal oxide and the optimum pH in the cathode chamber was below 2.35.
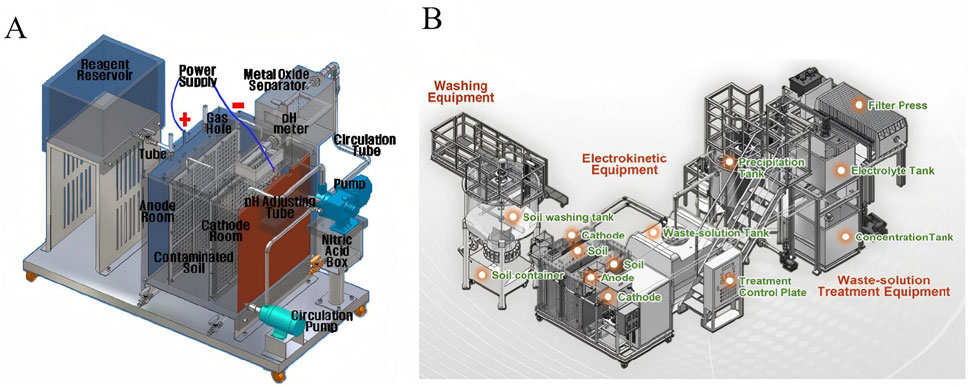
Figure 1. (A) Schematic diagram of EKR equipment for uranium-contaminated soil. Reprinted with permission (Kim et al., 2011), Copyright 2021 Elsevier B.V. (B) EKR system coupled with a washing-based enhancement approach. Reprinted with permission (Kim et al., 2015), Copyright 2015 Elsevier B.V.
Full-sized washing based EKR equipment was manufactured to improve the removal efficiency of uranium as illustrated in Figure 1B (Kim et al., 2015). Soil washing method was used for the pretreatment of uranium contaminated soil before EKR. In order to reduce the generation of metal oxides in the cathode, the pH controller was used to control the pH of the electrolyte solution. Gravels contaminated with uranium were decontaminated by EKR (Kim et al., 2014). Gravel (<20 cm) was treated by gravel washing and EKR. Gravel (>20 cm) was treated by gravel washing method and high concentration of uranium contaminated gravel should be treated by crushing and ball mill washing process. In order to reduce the decontamination time and electric power, advanced remediation of uranium contaminated soil was used (Kim et al., 2016b). After soil washing, muddy solution was removed by using a 100 mesh sieve. Only a small amount of soil collected from the muddy solution required EKR.
Citric acid, a green chelating agent, is suitable for remediation of heavy metal contaminated soils due to the cost-effectiveness and exceptional desorption capacity (Cameselle et al., 2021). Xiao et al. reported a composite electrolyte of citric acid and ferric chloride based EKR approach for uranium contaminated soil (Xiao et al., 2020b). Uranium in soil could be oxidized and leached by FeCl3 under acidic conditions. The removal efficiency was about 61.55% when 0.1 M citric acid and 0.03 M FeCl3 were used and the cumulative energy consumption was 0.2559 kWh.
4.2 EKR coupled phytoremediation
Phytoremediation is an environmentally friendly technology with advantage of low maintenance cost and no secondary pollution. A lot of researches have been reported about the application of phytoremediation for heavy metals contaminated soil (Wu et al., 2015; Huang et al., 2022). A conceptual illustration of the phytoremediation process is shown in Figure 2A (Zhu et al., 2024). The effectiveness of phytoremediation depends on various factors including the type of contaminants, soil conditions, climate, and plant species used (Limmer et al., 2018; Simmer and Schnoor, 2022). Sunflower (Alsabbagh and Abuqudaira, 2017), Tobacco plant (Stojanovic et al., 2012) and Rumex nepalensis (Li et al., 2020) are suitable for uranium remediation. However, phytoremediation is often a slow process and may require several years to achieve significant results.
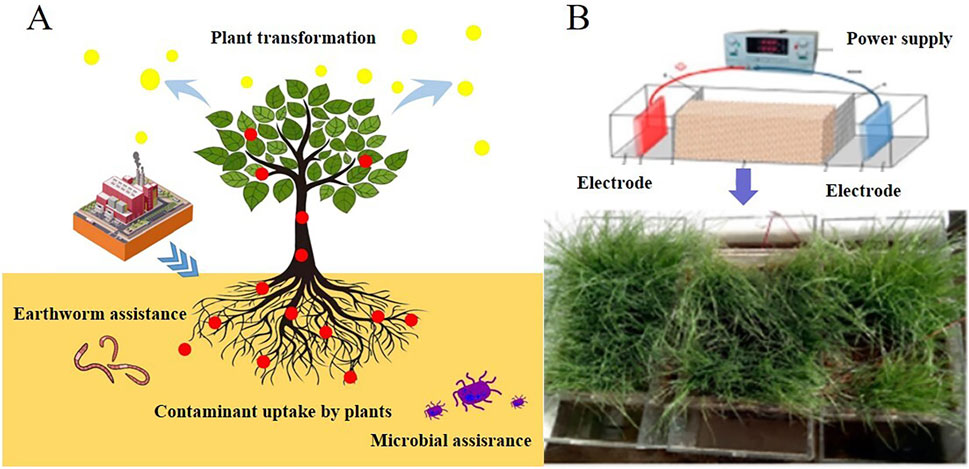
Figure 2. (A) Schematic illustration of phytoremediation for the removal of pollutants from soil. The red elements denote pollutants, while the yellow dots represent contaminants that have been transformed into non-toxic forms Reprinted with permission (Zhu et al., 2024), Copyright 2023, Springer Nature Switzerland AG. (B) EKR coupled phytoremediation system for enhanced uranium extraction from contaminated soil. Reprinted with permission (Li et al., 2021). Copyright 2021, American Chemical Society.
Coupling EKR with phytoremediation offers a synergistic approach that combines the efficiency of EKR with the natural and sustainable aspects of phytoremediation. This integration leads to more effective and environmentally friendly remediation solutions for contaminated sites. EKR coupled phytoremediation was applied by Li et al. as showed in Figure 2B, ryegrass was grown for 2 weeks in the uranium contaminated soil (Li et al., 2021). Subsequently, EKR was applied for 7 days (8 h per day) and polarity reversal was employed for 7 days (8 h per day). EKR significantly increased the uranium uptake and bioaccumulation and more uranium was accumulated in ryegrass plant roots and shoots. Li et al. reported a EKR coupled phytoremediation for decontaminate uranium contaminated soil. Sunflower and indian mustard were used for phytoremediation (Li et al., 2019). After plants grew for 60 days, EKR was applied in soils for 9 days. Uranium was more accumulated in roots than in shoots and EKR treatment was effective near the anode region. EKR increased uranium removal efficiency by 35%–47%, 35%–50%, and 26%–62% from soils with uranyl, UO3, and UO2, respectively. EKR coupled phytoremediation have potential for application in remediating uranium contaminated sites.
4.3 EKR coupling with PRBs
In order to improve the effectiveness of soil decontamination, EKR processes are synergistically coupled with PRBs (Andrade and dos Santos, 2020; Chen et al., 2022; Yang et al., 2024). PRBs is a passive remediation technology used to treat contaminated soil or groundwater. PRBs involves engineered reactive materials placed across the paths of effluents as a treatment zone to retain or adsorb the contaminants (Yu et al., 2019; Li and Liu, 2022; Budania and Dangayach, 2023). Coupling PRBs with EKR fully utilizes the advantages of both methods. An illustration of EKR coupling with PRBs configuration for heavy metals is shown in Figure 3A (Nasiri et al., 2020). The electroosmotic flow of soil pore fluid and electromigration of charged ions driven by the electrical field during EKR process are used to migrate metals out of the contaminated soil. Meanwhile, metal adsorption or in-situ reduction of metals occurred during PRBs process (Yao et al., 2020; Liu et al., 2024).
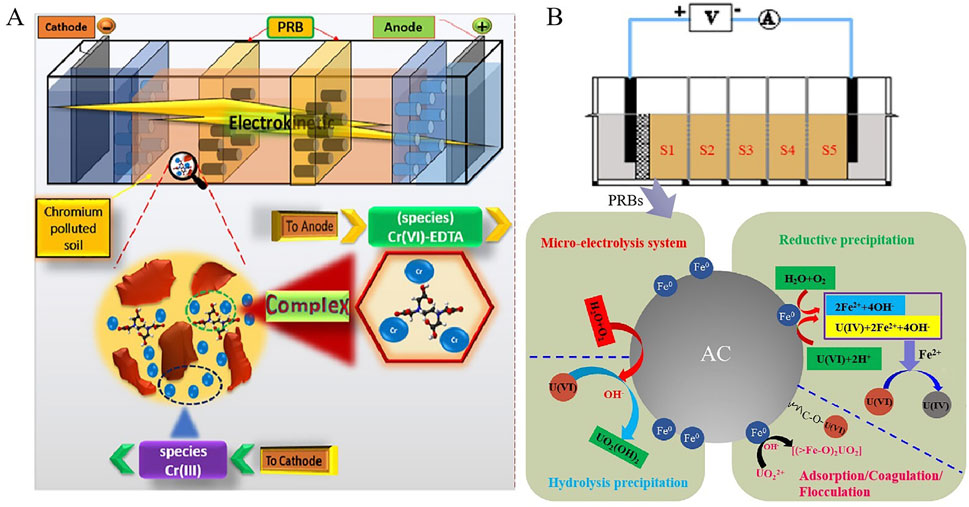
Figure 3. (A) PRBs enhanced EKR system for chromium remediation. Reprinted with permission (Nasiri et al., 2020), Copyright 2020 Elsevier B.V. (B) Mechanistic illustration of uranium removal via EKR coupled with acid-washed ZVI/activated carbon PRBs. Reprinted with permission (Xiao et al., 2020a), Copyright 2020 Elsevier B.V.
Xiao et al. reported a PRBs enhanced EKR for uranium contaminated soil. Zero-valent iron and activated carbon were used as filler of PRBs and citric acid mixed with FeCl3 was selected as composite electrolyte. The optimal removal rate was about 80% (Figure 3B) (Xiao et al., 2020a). Coupling EKR and PRBs technology was also applied to remediate uranium contaminated groundwater (Zheng et al., 2023). Coupling model contained flow and electric field was used for evaluation of the effectiveness of remediation effects. The EKR coupling with PRBs had a better efficiency than the single PRBs system under appropriate electric field settings.
4.4 Numerical simulation for EKR of uranium contaminated soil
Numerical simulation is a computational technique used to model, analyze, and predict the behavior of complex systems or processes by solving mathematical equations. It is a powerful tool employed across various fields including engineering, physics, chemistry, and environmental science. The primary objective of numerical simulation is to gain insights into the underlying mechanisms of a system or process, make predictions, and optimize performance under different conditions (Alobaid et al., 2022; Wu et al., 2023). Section 2 presents the primary electrokinetic transport phenomena and their corresponding mathematical equations during the electrokinetic remediation of U-contaminated soil, facilitating a better interpretation of experimental phenomena by scholars. By simulating the transport of heavy metal ions under various conditions, numerical models predicted the removal efficiency of EKR. This contributed to setting realistic expectations and goals for EKR process (Paz-Garcia et al., 2011; Asadollahfardi et al., 2016; Rezaee et al., 2019; Lu et al., 2022). Numerical simulations enabled sensitivity analyses to understand how changes in various parameters (such as pH, soil conductivity, voltage applied, etc.) to affect the overall efficiency of the remediation process, which was valuable for fine-tuning operational parameters in EKR applications (Kim et al., 2004; Vereda-Alonso et al., 2004; Masi et al., 2017; Rezaee and Asadollahfardi, 2019; Yan et al., 2024).
Numerical simulation was used to evaluate the efficiency of EKR of uranium (Miao and Pan, 2015). Multiple species-driven mechanisms were integrated into one governing partial differential equation characterized by high nonlinearity. The interaction between transporting species and the soil matrix was incorporated into the finite element method model using the Langmuir adsorption isotherm. UO22+ contaminated soil sample (length width and height: 10 cm, 10 cm, 10 cm) could be cleaned in 12 h by applying a level of 8 V direct electric potential between two panel electrodes according to the predicted results.
Numerical simulation can be a powerful tool for understanding and optimizing EKR processes, but it is significant to use appropriate models, validate against experimental data, and interpret results critically to ensure reliable and meaningful outcomes (Sprocati and Rolle, 2020).
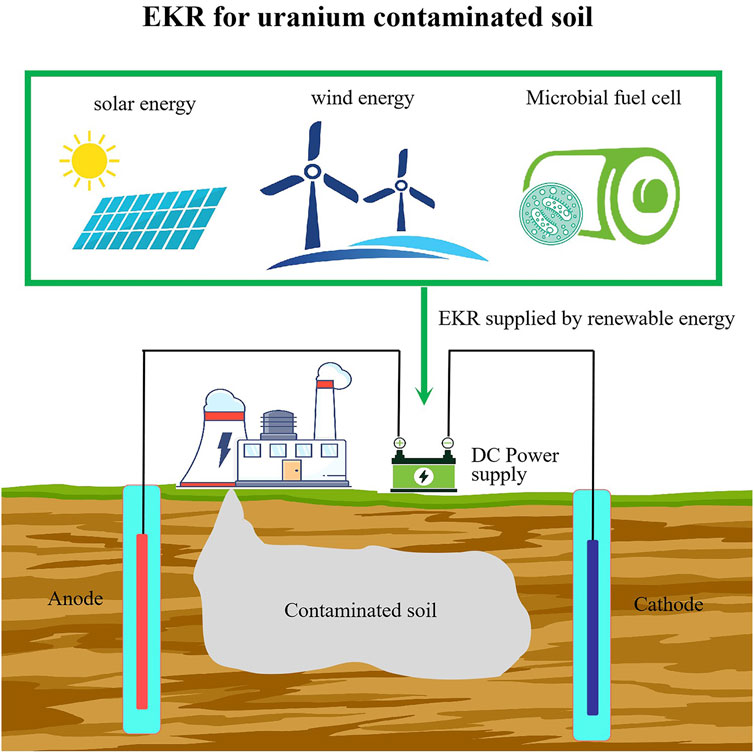
4.5 EKR supplied by renewable energy
The power supply for EKR systems used in soil remediation typically involves the use of direct electricity, which is usually sourced from the power grid. However, renewable energy sources including solar (Jeon et al., 2015; Zhou et al., 2018; Liu et al., 2020) (Figure 4A), wind (Souza et al., 2016), or microbial fuel cell (Chen et al., 2015; Habibul et al., 2016) (Figure 4B) are also applied in EKR. By utilizing renewable energy to supply the electricity required for EKR, the environmental footprint of the remediation process can be minimized. Renewable energy sources offer sustainable alternatives to fossil fuels, reducing greenhouse gas emissions and promoting environmentally friendly remediation practices. Additionally, integrating renewable energy into EKR systems will contribute to long-term cost savings and enhance the overall sustainability of remediation efforts. EKR supplied by renewable energy for uranium contaminated sites has not been reported.
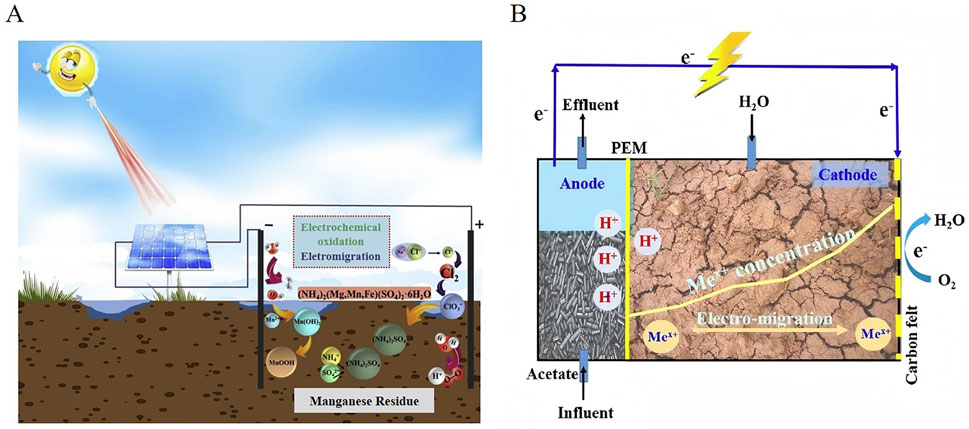
Figure 4. (A) Solar-powered EKR system for remediation of manganese and ammonia nitrogen Reprinted with permission (Liu et al., 2020), Copyright 2020 Elsevier B.V. (B) Microbial fuel cell driving EKR for Cd and Pb contaminated soils. Reprinted with permission (Habibul et al., 2016), Copyright 2016 Elsevier B.V.
4.6 On-site application of EKR for uranium contaminated sites
EKR stands out as a potent solution for sites contaminated with heavy metals. Its key advantage lies in its capacity to address soil contamination without extensive excavation, rendering it a feasible choice for locations where conventional remediation approaches might not be feasible.
There are no reports on the study of on-site application of EKR for heavy metal contaminated sites. Purkis et al. introduced the feasibility of applying EKR to radioactive nuclide contaminated sites including Fukushima-Daiichi Nuclear Power Plant and exclusion zone (Japan), Hanford Site (Washington, United States) and Sellafield, (Cumbria, United Kingdom), showing great potential for decommissioning nuclear facilities (Purkis et al., 2021). The efficacy of EKR is based on the actual condition of the sites. It is crucial to carefully evaluate site attributes and weigh the cost-effectiveness and long-term sustainability of EKR.
Simulations can help in scaling up lab-scale results to field-scale applications, providing theoretical support for the large-scale application of EKR. Which will provide insights into how factors such as soil heterogeneity and electrode spacing affect the overall performance of the remediation system.
5 Comparative analysis of EKR for uranium and other heavy metals
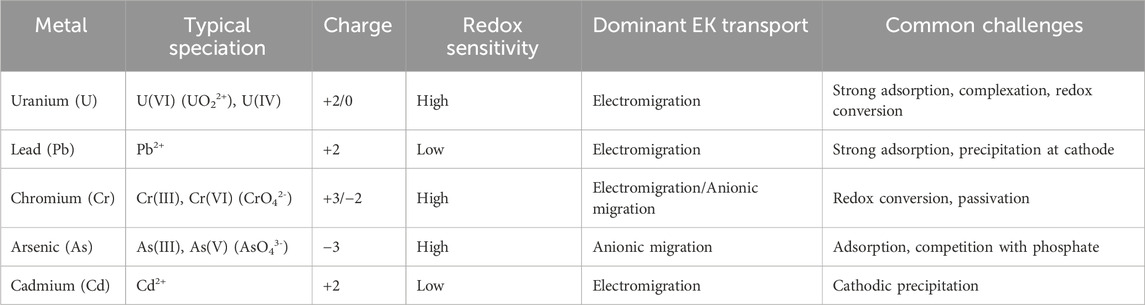
Uranium in soils exhibits complex speciation and migration behavior that is highly dependent on geochemical conditions. In its mobile hexavalent form (UO22+), uranium readily migrates toward the cathode via electromigration under acidic to neutral pH. However, its mobility can be significantly reduced by adsorption onto clay minerals and iron oxides or by forming stable complexes with phosphate. Under neutral to alkaline pH with sufficient carbonate, uranyl predominantly exists as carbonate complexes such as UO2(CO3)34- and UO2(CO3)22-, which are highly mobile and tend to migrate toward the anode. In sulfate-rich environments, uranyl may also form anionic species such as UO2(SO4)34- and UO2(SO4)22-, further enhancing anionic migration. These anionic species coexist with cationic forms such as UO22+, leading to the simultaneous migration of uranium toward both the cathode and anode under an applied electric field. By contrast, tetravalent uranium (U(IV)) is sparingly soluble and largely immobile, often requiring oxidation to U(VI) for effective mobilization. This dual migration behavior, governed by uranium’s redox chemistry and complexation equilibria, is unique compared with most other heavy metals and often necessitates redox conditioning or chemical additives to achieve efficient electrokinetic transport and removal.
Lead is a divalent cation with strong affinity to organic matter and clay minerals. In most soils, Pb demonstrates relatively low mobility due to adsorption and precipitation, particularly at higher pH. EKR of lead often requires acid enhancement (e.g., acetic or citric acid) to mobilize the metal. Compared with uranium, Pb is less sensitive to redox conditions but still requires careful pH management.
Chromium behaves differently depending on its oxidation state. Cr(III) is a cationic species with low mobility, while Cr(VI), in the form of chromate (CrO42-), is highly mobile and toxic. EKR of Cr(VI) is more effective due to its anionic form which migrates toward the anode. However, reduction of Cr(VI) to Cr(III) during remediation often complicates removal. This redox-switching behavior is analogous to uranium’s, highlighting the importance of controlling soil Eh conditions (Buchireddy et al., 2009).
Arsenic also exhibits dual valence behavior, existing as arsenate (AsO43-) or arsenite (AsO33-) under aerobic and anaerobic conditions, respectively. Both forms are anionic and migrate toward the anode during EKR. However, arsenic tends to adsorb onto Fe/Al oxides, requiring phosphate competition or pH adjustment to enhance mobility. Like uranium, As remediation is more efficient when combined with permeable reactive barriers or iron-based additives.
Cadmium is a divalent metal with moderate mobility under acidic conditions. It does not form strong soil complexes, making it easier to mobilize and remove using EKR than uranium. However, Cd is more susceptible to precipitation near the cathode due to local pH increases, a challenge also encountered with uranium (Wang et al., 2021).
Table 2 summarizes the key differences in electrokinetic behavior among uranium and selected heavy metals.
6 Advances in electrode materials for EKR systems
Electrodes are critical components in EKR systems, directly determining electric field distribution, electrochemical reactions, and long-term system stability. An ideal electrode material should exhibit high conductivity, chemical stability under acidic or alkaline conditions, low corrosion rate, and compatibility with the soil-electrolyte interface. In recent years, advances in electrode materials have significantly improved EKR efficiency and broadened its applicability, particularly for challenging contaminants such as uranium (Gidudu and Chirwa, 2022).
6.1 Traditional electrode materials
Conventional electrodes commonly used in EKR include graphite, titanium (Ti), and stainless steel (Raj et al., 2024). Graphite electrodes are inexpensive, widely available, and chemically stable over a broad pH range. However, they are mechanically brittle and may degrade under prolonged use, especially in highly acidic conditions, which can reduce their lifespan and increase maintenance costs (Telepanich et al., 2021). Titanium electrodes, particularly when coated with noble metal oxides such as IrO2 or RuO2, are widely employed as dimensionally stable anodes (DSAs). These electrodes exhibit excellent electrochemical durability, high corrosion resistance, and stable performance under continuous operation, making them suitable for long-term field applications. Nevertheless, the relatively high cost of titanium and noble metal coatings remains a significant limitation, particularly for large-scale remediation projects (Méndez et al., 2012). Stainless steel is more affordable and provides good electrical conductivity, which makes it attractive for cost-sensitive applications. However, under high current densities or extreme pH gradients, stainless steel is prone to corrosion. This can lead to secondary pollution and undermine the overall environmental benefit of the remediation process.
Overall, while traditional electrode materials are widely used due to their availability and proven performance, their long-term stability, cost-effectiveness, and potential environmental impacts must be carefully considered when designing EKR systems for uranium-contaminated soils (Taneja et al., 2023).
6.2 Emerging electrode technologies
To address the limitations of traditional electrode materials, several novel electrode technologies have been explored to enhance the performance and durability of EKR systems. Mixed metal oxide (MMO)-coated electrodes, typically consisting of titanium substrates coated with IrO2, RuO2, or Ta2O5, offer excellent electrochemical stability and catalytic activity for redox reactions, facilitating improved control over pH gradients and redox-sensitive contaminants such as uranium. Carbon-based composites, including graphene, carbon nanotubes (CNTs), and activated carbon, have been integrated into electrode structures to increase surface area and electrical conductivity while also providing additional sorption sites for in situ contaminant capture. In addition, conductive polymers such as polyaniline (PANI) and polypyrrole (PPy) have attracted attention for their environmental compatibility, structural flexibility, and redox reactivity; however, concerns remain regarding their long-term stability and performance under field conditions. These emerging materials present promising opportunities for improving EKR efficiency, particularly in complex and variable soil environments (Qu et al., 2023).
6.3 Electrode design innovations
In addition to advances in electrode materials, structural innovations in electrode design have also played a crucial role in enhancing the performance and adaptability of EKR systems. Reactive electrodes, such as zero-valent iron (ZVI) cathodes, can directly participate in contaminant removal by reducing U(VI) to insoluble U(IV), thus promoting in situ immobilization (Zhang et al., 2021). Meanwhile, modular and replaceable electrode assemblies have been developed to simplify system maintenance and enable performance optimization during long-term remediation projects, particularly in large or heterogeneous sites. Furthermore, the adoption of three-dimensional (3D) electrode configurations including porous or mesh-type structures significantly improves the contact area between electrodes and the soil matrix, enhances electric field uniformity, and reduces ohmic losses, thereby increasing overall treatment efficiency and reducing energy consumption (Xue et al., 2017).
6.4 Challenges and future directions
Despite significant advancements in electrode materials and configurations, several key challenges remain in the development and application of electrodes for EKR. One major issue is the cost-performance trade-off: while high-performance materials such as mixed metal oxides (MMOs) and graphene-based composites offer excellent electrochemical stability and conductivity, their high cost can hinder large-scale or long-term deployment. Another challenge is electrode fouling and passivation, which often results from the precipitation of metal oxides (such as UO2 or Fe(OH)3) near the cathode. These deposits can obstruct current flow, increase system resistance, and ultimately reduce remediation efficiency. Furthermore, compatibility with coupled remediation technologies presents additional complexity; electrodes must maintain performance and structural integrity when integrated with systems like PRBs, phytoremediation modules, or microbial fuel cells. Addressing these challenges is essential for translating laboratory-scale electrode innovations into robust, field-ready EKR systems (Taneja et al., 2024).
Future research should focus on multifunctional electrode systems that combine electrical conductivity, catalytic activity, and contaminant adsorption in a single platform. Moreover, life cycle assessments (LCA) and techno-economic evaluations are needed to guide the selection of materials for site-specific remediation scenarios (Vocciante et al., 2016).
7 Energy consumption and economic evaluation of EKR
Energy consumption is a critical parameter in evaluating the feasibility and sustainability of EKR, particularly for large-scale applications involving uranium-contaminated soils. Since EKR relies on an externally applied electric field to mobilize ionic species, energy use directly influences both the operational cost and the environmental footprint of the process (Akansha et al., 2024).
Energy consumption metrics can reflect the energy efficiency, operational cost, and practical feasibility of EKR for uranium-contaminated soil. The energy required for EKR depends on various factors including applied voltage gradient, treatment duration, soil resistivity, moisture content, and contaminant concentration. A commonly used parameter is specific energy consumption (SEC), defined as:
Where U is voltage (V), I is current (A), t is time (h), and m is the mass of soil treated (kg).
7.1 Cost considerations and economic optimization strategies
EKR operational costs mainly arise from electricity usage, electrode replacement, chemical additives, and labor. Compared to traditional methods like chemical leaching or excavation, EKR avoids large-scale disturbance and can offer lower long-term remediation costs, especially for low-permeability soils (Sun et al., 2023).
To optimize cost and sustainability, several strategies have been proposed: ① Voltage gradient optimization: Intermittent or pulsed application can reduce unnecessary energy input while maintaining contaminant mobilization efficiency. ② Use of renewable energy sources: Solar panels, wind turbines, and microbial fuel cells can provide sustainable power and reduce the carbon footprint of EKR systems. ③ Electrode recycling and regeneration: Extending electrode lifespan lowers material costs and minimizes secondary waste. ④ Coupling with low-cost passive technologies: Integration with PRBs or phytoremediation enhances remediation while reducing overall operational costs.
7.2 Sustainability challenges and outlook
Despite its advantages, EKR is inherently energy-intensive, which remains a barrier for field-scale deployment. Advances in system automation, smart power supply control, and hybrid remediation designs are expected to reduce energy demands in the near future. Recent studies highlight that predictive modeling and numerical simulation can optimize treatment duration and applied voltage, improving cost-effectiveness. Furthermore, integration with renewable energy sources has shown promise in experimental trials, suggesting a viable pathway toward carbon-neutral remediation systems (described in Section 4.5).
Economic feasibility also depends on site-specific conditions such as contaminant concentration, soil resistivity, and regulatory requirements. Comprehensive LCA studies are increasingly recommended to evaluate the environmental trade-offs of EKR relative to conventional technologies. Future research should focus on large-scale pilot demonstrations, combined techno-economic and environmental assessments, and policy incentives to support sustainable implementation of EKR for uranium-contaminated soils.
8 Conclusions and future perspectives
In conclusion, the recent progresses of EKR for uranium-contaminated soil are reviewed, including design methods, removal efficiency, electrode materials, power sources for electric fields, and combined technologies. EKR has demonstrated clear advantages in mobilizing uranium from low-permeability soils under in situ conditions, offering a relatively low-cost and environmentally friendly alternative to conventional remediation methods. Even though good soil remediation effects have been achieved through EKR, further research is still needed to optimize operational parameters such as voltage gradients, electrode configurations, electrolyte formulations, and treatment durations. These optimizations are essential to improve remediation efficiency, reduce energy consumption, and ensure long-term sustainability in field-scale applications.
EKR integrating with complementary remediation methods, such as phytoremediation and PRBs (described in Section 4.2 and 4.3), may enhance overall effectiveness by combining the rapid transport capabilities of EKR with the selective uptake and stabilization capacity of plants or reactive media. Such hybrid approaches not only improve removal efficiency but also help reduce remediation time and minimize secondary pollution. Additionally, numerical simulation can be a powerful tool for understanding and optimizing EKR processes. Models incorporating multi-species transport, electrochemical reactions, and adsorption/desorption dynamics (described in Section 4.4) provide valuable insights into the mechanisms and interactions at play during remediation. The appropriate use of models, validation against experimental data, and critical interpretation of results are crucial to ensure reliable and meaningful conclusions, especially when scaling up to complex and heterogeneous field environments.
Moreover, recent advancements in renewable energy-powered EKR systems and multifunctional electrode materials suggest promising routes toward greener and more efficient remediation technologies (described in Section 4.5). For example, the integration of microbial fuel cells, solar panels, wind, and 3D porous electrodes can not only enhance performance but also reduce dependence on conventional power sources. Despite encouraging results from laboratory and pilot-scale studies, there remains a significant gap in large-scale, long-term field implementation of EKR for uranium-contaminated sites. Future work should prioritize site-specific feasibility assessments, robust monitoring frameworks, and techno-economic evaluations. Regulatory acceptance, stakeholder engagement, and public communication will also be key to accelerating the adoption of EKR in real-world projects, particularly in sensitive or high-risk areas such as former nuclear facilities.
In summary, EKR holds immense potential as a sustainable and effective solution for the remediation of uranium-contaminated soil. While technical and practical challenges persist, continuous innovations in system design, modeling, and cross-disciplinary integration will help unlock its full potential. With appropriate support from policy, funding, and research communities, EKR may become a mainstream approach in the future landscape of environmental restoration and radiological risk mitigation.
Author contributions
YB: Formal Analysis, Conceptualization, Funding acquisition, Writing – original draft, Writing – review and editing. ZZ: Writing – original draft, Investigation, Formal Analysis. GJ: Validation, Writing – original draft, Data curation, Formal Analysis. SN: Visualization, Resources, Writing – original draft. ZP: Writing – original draft, Data curation, Formal Analysis. LL: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Conflict of interest
Authors YB, ZZ, GJ, SN, ZP, and LL were employed by China National Nuclear Corporation.
The authors declare that this study received funding from China National Nuclear Corporation Basic Research Project (No. 202333, 202429). The funder had the following involvement in the study: study design, collection, analysis, interpretation of data, the writing of this article, the decision to submit it for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agnew, K., Cundy, A. B., Hopkinson, L., Croudace, I. W., Warwick, P. E., and Purdie, P. (2011). Electrokinetic remediation of plutonium-contaminated nuclear site wastes: results from a pilot-scale on-site trial. J. Hazard. Mater. 186, 1405–1414. doi:10.1016/j.jhazmat.2010.12.016
Akansha, J., Thakur, S., Chaithanya, M. S., Gupta, B. S., Das, S., Das, B., et al. (2024). Technological and economic analysis of electrokinetic remediation of contaminated soil: a global perspective and its application in Indian scenario. Heliyon 10, e24293. doi:10.1016/j.heliyon.2024.e24293
Alobaid, F., Almohammed, N., Farid, M. M., May, J., Rossger, P., Richter, A., et al. (2022). Progress in CFD simulations of fluidized beds for chemical and energy process engineering. Prog. Energy Combust. Sci. 91, 100930. doi:10.1016/j.pecs.2021.100930
Alsabbagh, A. H., and Abuqudaira, T. M. (2017). Phytoremediation of Jordanian uranium-rich soil using sunflower. Water Air Soil Pollut. 228, 219. doi:10.1007/s11270-017-3396-3
Andrade, D. C., and Dos Santos, E. V. (2020). Combination of electrokinetic remediation with permeable reactive barriers to remove organic compounds from soils. Curr. Opin. Electrochem. 22, 136–144. doi:10.1016/j.coelec.2020.06.002
Asadollahfardi, G., Rezaee, M., and Mehrjardi, G. T. (2016). Simulation of unenhanced electrokinetic process for lead removal from kaolinite clay. Int. J. Civ. Eng. 14, 263–270. doi:10.1007/s40999-016-0049-7
Brugge, D., and Buchner, V. (2011). Health effects of uranium: new research findings. Rev. Environ. Health 26, 231–249. doi:10.1515/REVEH.2011.032
Buchireddy, P. R., Bricka, R. M., and Gent, D. B. (2009). Electrokinetic remediation of wood preservative contaminated soil containing copper, chromium, and arsenic. J. Hazard. Mater. 162, 490–497. doi:10.1016/j.jhazmat.2008.05.092
Budania, R., and Dangayach, S. (2023). A comprehensive review on permeable reactive barrier for the remediation of groundwater contamination. J. Environ. Manag. 332, 117343. doi:10.1016/j.jenvman.2023.117343
Cameselle, C., Gouveia, S., and Cabo, A. (2021). Enhanced electrokinetic remediation for the removal of heavy metals from contaminated soils. Appl. Sciences-Basel 11, 1799. doi:10.3390/app11041799
Chen, Z., Zhu, B. K., Jia, W. F., Liang, J. H., and Sun, G. X. (2015). Can electrokinetic removal of metals from contaminated paddy soils be powered by microbial fuel cells? Environ. Technol. and Innovation 3, 63–67. doi:10.1016/j.eti.2015.02.003
Chen, Y. X., Zhi, D., Zhou, Y. Y., Huang, A. Q., Wu, S. K., Yao, B., et al. (2021). Electrokinetic techniques, their enhancement techniques and composite techniques with other processes for persistent organic pollutants remediation in soil: a review. J. Industrial Eng. Chem. 97, 163–172. doi:10.1016/j.jiec.2021.03.009
Chen, R., Zhou, L., Wang, W., Cui, D., Hao, D., and Guo, J. (2022). Enhanced electrokinetic remediation of copper-contaminated soil by combining steel slag and a permeable reactive barrier. Appl. Sciences-Basel 12, 7981. doi:10.3390/app12167981
Cheng, C., Chen, L., Guo, K., Xie, J., Shu, Y., He, S., et al. (2022). Progress of uranium-contaminated soil bioremediation technology. J. Environ. Radioact. 241, 106773. doi:10.1016/j.jenvrad.2021.106773
Ganbat, N., Altaee, A., Zhou, J. L., Lockwood, T., Al-Juboori, R. A., Hamdi, F. M., et al. (2022). Investigation of the effect of surfactant on the electrokinetic treatment of PFOA contaminated soil. Environ. Technol. and Innovation 28, 102938. doi:10.1016/j.eti.2022.102938
Gavrilescu, M., Pavel, L. V., and Cretescu, I. (2009). Characterization and remediation of soils contaminated with uranium. J. Hazard. Mater. 163, 475–510. doi:10.1016/j.jhazmat.2008.07.103
Gidudu, B., and Chirwa, E. M. N. (2022). The role of pH, electrodes, surfactants, and electrolytes in electrokinetic remediation of contaminated soil. Molecules 27, 7381. doi:10.3390/molecules27217381
Gomes, H. I., Dias-Ferreira, C., and Ribeiro, A. B. (2012). Electrokinetic remediation of organochlorines in soil: enhancement techniques and integration with other remediation technologies. Chemosphere 87, 1077–1090. doi:10.1016/j.chemosphere.2012.02.037
Habibul, N., Hu, Y., and Sheng, G. P. (2016). Microbial fuel cell driving electrokinetic remediation of toxic metal contaminated soils. J. Hazard. Mater. 318, 9–14. doi:10.1016/j.jhazmat.2016.06.041
Huang, D. Q., Xu, Q., Cheng, J. J., Lu, X. C., and Zhang, H. (2012). Electrokinetic remediation and its combined technologies for removal of organic pollutants from contaminated soils. Int. J. Electrochem. Sci. 7, 4528–4544. doi:10.1016/s1452-3981(23)19558-7
Huang, M., Liu, Z. R., and Li, X. (2022). Phytoremediation of rare tailings-contaminated soil. J. Renew. Mater. 10, 3351–3372. doi:10.32604/jrm.2022.022393
Jeon, E. K., Ryu, S. R., and Baek, K. (2015). Application of solar-cells in the electrokinetic remediation of As-contaminated soil. Electrochimica Acta 181, 160–166. doi:10.1016/j.electacta.2015.03.065
Kim, S. O., Kim, J. J., Kim, K. W., and Yun, S. T. (2004). Models and experiments on electrokinetic removal of Pb(II) from kaolinite clay. Sep. Sci. Technol. 39, 1927–1951. doi:10.1081/ss-120030775
Kim, S. O., Jae-Jin, K., Kyoung-Woong, K., and And Yun, S. T. (2005). Models and experiments on electrokinetic removal of Pb(II) from kaolinite clay. Sep. Sci. Technol. 39, 1927–1951. doi:10.1081/SS-120030775
Kim, G. N., Jung, Y. H., Lee, J. J., Moon, J. K., and Jung, C. H. (2008). Development of electrokinetic-flushing technology for the remediation of contaminated soil around nuclear facilities. J. Industrial Eng. Chem. 14, 732–738. doi:10.1016/j.jiec.2008.05.001
Kim, G. N., Yang, B. I., Choi, W. K., and Lee, K. W. (2009). Development of vertical electrokinetic-flushing decontamination technology to remove 60Co and 137Cs from a Korean nuclear facility site. Sep. Purif. Technol. 68, 222–226. doi:10.1016/j.seppur.2009.05.015
Kim, G. N., Lee, S. S., Shon, D. B., Lee, K. W., and Chung, U. S. (2010). Development of pilot-scale electrokinetic remediation technology to remove 60Co and 137Cs from soil. J. Industrial Eng. Chem. 16, 986–991. doi:10.1016/j.jiec.2010.05.014
Kim, G. N., Shon, D. B., Park, H. M., Lee, K. W., and Chung, U. S. (2011). Development of pilot-scale electrokinetic remediation technology for uranium removal. Sep. Purif. Technol. 80, 67–72. doi:10.1016/j.seppur.2011.04.009
Kim, G. N., Park, U. R., Kim, S. S., Kim, W. S., Moon, J. K., and Hyun, J. H. (2014). Decontamination of gravels contaminated with uranium. Ann. Nucl. Energy 72, 367–372. doi:10.1016/j.anucene.2014.05.031
Kim, G. N., Kim, S. S., Moon, J. K., and Hyun, J. H. (2015). Removal of uranium from soil using full-sized washing electrokinetic separation equipment. Ann. Nucl. Energy 81, 188–195. doi:10.1016/j.anucene.2015.01.046
Kim, I., Choi, J.-W., NamKim, G., and Kim, S.-S. (2016a). Removal of uranium from contaminated soil using indoor electrokinetic decontamination. J. Radioanalytical Nucl. Chem. 309, 1175–1181. doi:10.1007/s10967-016-4707-7
Kim, S. S., Han, G. S., Kim, G. N., Koo, D. S., Kim, I. G., and Choi, J. W. (2016b). Advanced remediation of uranium-contaminated soil. J. Environ. Radioact. 164, 239–244. doi:10.1016/j.jenvrad.2016.08.005
Li, H., and Liu, Q. (2022). Reaction medium for permeable reactive barrier remediation of groundwater polluted by heavy metals. Front. Environ. Sci. 10, 968546. doi:10.3389/fenvs.2022.968546
Li, J. X., Zhang, J., Larson, S. L., Ballard, J. H., Guo, K., Arslan, Z., et al. (2019). Electrokinetic-enhanced phytoremediation of uranium-contaminated soil using sunflower and Indian mustard. Int. J. Phytoremediation 21, 1197–1204. doi:10.1080/15226514.2019.1612847
Li, R. F., Dong, F. Q., Yang, G., Zhang, W., Zong, M. R., Nie, X. Q., et al. (2020). Characterization of arsenic and uranium pollution surrounding a uranium mine in Southwestern China and phytoremediation potential. Pol. J. Environ. Stud. 29, 173–185. doi:10.15244/pjoes/103446
Li, J. X., Chen, L. M., Zhang, Q. K., Wu, L. C., Zhang, J., Larson, S. L., et al. (2021). Coupling electrokinetics and phytoremediation to remove uranium from contaminated soil: a laboratory pilot-scale study. Acs Earth Space Chem. 5, 3448–3457. doi:10.1021/acsearthspacechem.1c00286
Li, G., Xu, S. J., Wu, B., Li, F. M., and Guo, S. H. (2023). Removal of organic pollutants via electrochemical oxidation near anodes during electrokinetic remediation: proof of concept. Environ. Eng. Sci. 40, 41–49. doi:10.1089/ees.2022.0106
Limmer, M. A., Wilson, J., Westenberg, D., Lee, A., Siegman, M., and Burken, J. G. (2018). Phytoremediation removal rates of benzene, toluene, and chlorobenzene. Int. J. Phytoremediation 20, 666–674. doi:10.1080/15226514.2017.1413330
Liu, L. W., Li, W., Song, W. P., and Guo, M. X. (2018). Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci. Total Environ. 633, 206–219. doi:10.1016/j.scitotenv.2018.03.161
Liu, R., Wang, H., Liu, Z., and Tao, C. (2020). Electrokinetic remediation with solar powered for electrolytic manganese residue and researching on migration of ammonia nitrogen and manganese. J. Water Process Eng. 38, 101655. doi:10.1016/j.jwpe.2020.101655
Liu, J., Zhao, L., Li, Y., Huang, M., Tong, X., Chen, L., et al. (2024). Important role of cosolvent addition and soil permeability on electrokinetic-persulfate remediation of benzo(a)pyrene. Environ. Technol. and Innovation 33, 103480. doi:10.1016/j.eti.2023.103480
López-Vizcaíno, R., Yustres, A., León, M. J., Saez, C., Cañizares, P., Rodrigo, M. A., et al. (2017). Multiphysics implementation of electrokinetic remediation models for natural soils and porewaters. Electrochimica Acta 225, 93–104. doi:10.1016/j.electacta.2016.12.102
Lu, X. H., Wei, Y. T., Ren, J. L., Zhang, H. T., and Yang, Y. (2022). Study on water-heat-solution transport law in Cr(VI)-Contaminated soil during electric remediation. Sustainability 14, 8136. doi:10.3390/su14138136
Maes, N., Moors, H., Wang, L., Delècaut, G., Cannière, P. D., and Put, M. (2002). The use of electromigration as a qualitative technique to study the migration behaviour and speciation of uranium in the boom clay. Radiochim. Acta 90, 741–746. doi:10.1524/ract.2002.90.9-11_2002.741
Masi, M., Ceccarini, A., and Iannelli, R. (2017). Multispecies reactive transport modelling of electrokinetic remediation of harbour sediments. J. Hazard. Mater. 326, 187–196. doi:10.1016/j.jhazmat.2016.12.032
Méndez, E., Pérez, M., Romero, O., Beltrán, E. D., Castro, S., Corona, J. L., et al. (2012). Effects of electrode material on the efficiency of hydrocarbon removal by an electrokinetic remediation process. Electrochimica Acta 86, 148–156. doi:10.1016/j.electacta.2012.04.042
Miao, T., and Pan, T. Y. (2015). A multiphysics model for evaluating electrokinetic remediation of nuclear waste-contaminated soils. Water Air Soil Pollut. 226, 77. doi:10.1007/s11270-014-2292-3
Nasiri, A., Jamshidi-Zanjani, A., and Khodadadi Darban, A. (2020). Application of enhanced electrokinetic approach to remediate Cr-contaminated soil: effect of chelating agents and permeable reactive barrier. Environ. Pollut. 266, 115197. doi:10.1016/j.envpol.2020.115197
Paz-Garcia, J. M., Johannesson, B., Ottosen, L. M., Ribeiro, A. B., and Miguel Rodriguez-Maroto, J. (2011). Modeling of electrokinetic processes by finite element integration of the Nernst-Planck-Poisson system of equations. Sep. Purif. Technol. 79, 183–192. doi:10.1016/j.seppur.2011.02.023
Purkis, J. M., Warwick, P. E., Graham, J., Hemming, S. D., and Cundy, A. B. (2021). Towards the application of electrokinetic remediation for nuclear site decommissioning. J. Hazard. Mater. 413, 125274. doi:10.1016/j.jhazmat.2021.125274
Qu, Z., Huang, L., Guo, M., Sun, T., Xu, X., and Gao, Z. (2023). Application of novel polypyrrole/melamine foam auxiliary electrode in promoting electrokinetic remediation of Cr(VI)-contaminated soil. Sci. Total Environ. 876, 162840. doi:10.1016/j.scitotenv.2023.162840
Raj, S. K., Carrier, A. J., Youden, B. C., Servos, M. R., Oakes, K. D., and Zhang, X. (2024). Electrochemical techniques for uranium extraction from water. Chem. Eng. J. 492, 152341. doi:10.1016/j.cej.2024.152341
Rezaee, M., and Asadollahfardi, G. (2019). An implicit finite difference model for electrokinetic remediation of Cd-Spiked kaolinite under acid-enhanced and unenhanced conditions. Environ. Model. Assess. 24, 235–248. doi:10.1007/s10666-018-9610-x
Rezaee, M., Asadollahfardi, G., Gomez-Lahoz, C., Villen-Guzman, M., and Paz-Garcia, J. M. (2019). Modeling of electrokinetic remediation of Cd- and Pb-contaminated kaolinite. J. Hazard. Mater. 366, 630–635. doi:10.1016/j.jhazmat.2018.12.034
Saini, A., Bekele, D. N., Chadalavada, S., Fang, C., and Naidu, R. (2021). Electrokinetic remediation of petroleum hydrocarbon contaminated soil (I). Environ. Technol. and Innovation 23, 101585. doi:10.1016/j.eti.2021.101585
Shu, X. Y., Li, Y. P., Huang, W. X., Chen, S. Z., Xu, C., Zhang, S., et al. (2020). Rapid vitrification of uranium-contaminated soil: effect and mechanism. Environ. Pollut. 263, 114539. doi:10.1016/j.envpol.2020.114539
Sihn, Y., Bae, S., and Lee, W. (2019). Immobilization of uranium(VI) in a cementitious matrix with nanoscale zerovalent iron (NZVI). Chemosphere 215, 626–633. doi:10.1016/j.chemosphere.2018.10.073
Simmer, R. A., and Schnoor, J. L. (2022). Phytoremediation, bioaugmentation, and the plant microbiome. Environ. Sci. Technol. 56, 16602–16610. doi:10.1021/acs.est.2c05970
Souza, F. L., Llanos, J., Sáez, C., Lanza, M. R. V., Rodrigo, M. A., and Cañizares, P. (2016). Performance of wind-powered soil electroremediation process for the removal of 2,4-D from soil. J. Environ. Manag. 171, 128–132. doi:10.1016/j.jenvman.2016.01.032
Sprocati, R., and Rolle, M. (2020). Charge interactions, reaction kinetics and dimensionality effects on electrokinetic remediation: a model-based analysis. J. Contam. Hydrology 229, 103567. doi:10.1016/j.jconhyd.2019.103567
Stojanovic, M. D., Mihajlovic, M. L., Milojkovic, J. V., Lopicic, Z. R., Adamovic, M., and Stankovic, S. (2012). Efficient phytoremediation of uranium mine tailings by tobacco. Environ. Chem. Lett. 10, 377–381. doi:10.1007/s10311-012-0362-6
Sun, Z., Zhao, M., Chen, L., Gong, Z., Hu, J., and Ma, D. (2023). Electrokinetic remediation for the removal of heavy metals in soil: limitations, solutions and prospection. Sci. Total Environ. 903, 165970. doi:10.1016/j.scitotenv.2023.165970
Taneja, S., Karaca, O., and Haritash, A. K. (2023). Combined effects of high voltage gradient and electrolyte conditioning on electrokinetic remediation for chromium (VI)-Contaminated soils. Rendiconti Lincei. Sci. Fis. Nat. 34, 635–646. doi:10.1007/s12210-023-01159-z
Taneja, S., Karaca, O., and Haritash, A. K. (2024). Electrokinetic remediation: past experiences and future roadmap for sustainable remediation of metal-contaminated soils. J. Geochem. Explor. 259, 107437. doi:10.1016/j.gexplo.2024.107437
Telepanich, A., Marshall, T., Gregori, S., Marangoni, A. G., and Pensini, E. (2021). Graphene-alginate fluids as unconventional electrodes for the electrokinetic remediation of Cr(VI). Water, Air Soil Pollut. 232, 334. doi:10.1007/s11270-021-05278-x
Usman, M., and Radulescu, M. (2022). Examining the role of nuclear and renewable energy in reducing carbon footprint: does the role of technological innovation really create some difference? Sci. Total Environ. 841, 156662. doi:10.1016/j.scitotenv.2022.156662
Vereda-Alonso, C., Miguel Rodrı́Guez-Maroto, J., Garcı́a-Delgado, R. A., Gómez-Lahoz, C., and Garcı́a-Herruzo, F. (2004). Two-dimensional model for soil electrokinetic remediation of heavy metals: application to a copper spiked kaolin. Chemosphere 54, 895–903. doi:10.1016/j.chemosphere.2003.09.002
Vocciante, M., Caretta, A., Bua, L., Bagatin, R., and Ferro, S. (2016). Enhancements in ElectroKinetic remediation technology: environmental assessment in comparison with other configurations and consolidated solutions. Chem. Eng. J. 289, 123–134. doi:10.1016/j.cej.2015.12.065
Vocciante, M., Dovì, V. G., and Ferro, S. (2021). Sustainability in ElectroKinetic remediation processes: a critical analysis. Sustainability 13, 770. doi:10.3390/su13020770
Wang, J. X., Feng, X. B., Anderson, C. W. N., Xing, Y., and Shang, L. H. (2012). Remediation of Mercury contaminated sites - a review. J. Hazard. Mater. 221, 1–18. doi:10.1016/j.jhazmat.2012.04.035
Wang, Y. C., Li, A., and Cui, C. W. (2021). Remediation of heavy metal-contaminated soils by electrokinetic technology: mechanisms and applicability. Chemosphere 265, 129071. doi:10.1016/j.chemosphere.2020.129071
Wei, G. L., Han, W. H., Shu, X. Y., Luo, F., Tang, H. X., Chen, S. Z., et al. (2021). Heavy-ion irradiation effects on uranium-contaminated soil for nuclear waste. J. Hazard. Mater. 405, 124273. doi:10.1016/j.jhazmat.2020.124273
Wen, D., Fu, R., and Li, Q. (2021). Removal of inorganic contaminants in soil by electrokinetic remediation technologies: a review. J. Hazard. Mater. 401, 123345. doi:10.1016/j.jhazmat.2020.123345
Wu, Z. L., Bañuelos, G. S., Lin, Z. Q., Liu, Y., Yuan, L. X., Yin, X. B., et al. (2015). Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 6, 136. doi:10.3389/fpls.2015.00136
Wu, F., Wei, P., Li, X., Huang, M., Zhou, L., and Liu, Z. (2022). Research progress of rhizosphere effect in the phytoremediation of uranium-contaminated soil. J. Radioanalytical Nucl. Chem. 331, 5493–5505. doi:10.1007/s10967-022-08630-5
Wu, L., Hou, Z. M., Luo, Z. F., Xiong, Y., Zhang, N. L., Luo, J. S., et al. (2023). Numerical simulations of supercritical carbon dioxide fracturing: a review. J. Rock Mech. Geotechnical Eng. 15, 1895–1910. doi:10.1016/j.jrmge.2022.08.008
Xiao, J., Pang, Z. H., Zhou, S. K., Chu, L. P., Rong, L. S., Liu, Y. J., et al. (2020a). The mechanism of acid-washed zero-valent iron/activated carbon as permeable reactive barrier enhanced electrokinetic remediation of uranium-contaminated soil. Sep. Purif. Technol. 244, 116667. doi:10.1016/j.seppur.2020.116667
Xiao, J., Zhou, S. K., Chu, L. P., Liu, Y. J., Li, J. L., Zhang, J., et al. (2020b). Electrokinetic remediation of uranium(VI)-contaminated red soil using composite electrolyte of citric acid and ferric chloride. Environ. Sci. Pollut. Res. 27, 4478–4488. doi:10.1007/s11356-019-06990-2
Xue, F., Yan, Y., Xia, M., Muhammad, F., Yu, L., Xu, F., et al. (2017). Electro-kinetic remediation of chromium-contaminated soil by a three-dimensional electrode coupled with a permeable reactive barrier. RSC Adv. 7, 54797–54805. doi:10.1039/C7RA10913J
Yan, K., Chai, Z., Li, T., Duan, B., Xiao, C., Liu, X., et al. (2024). Effect of voltage gradients on EK-PRB remediation: experimental and molecular dynamics simulations. Environ. Res. 252, 119085. doi:10.1016/j.envres.2024.119085
Yang, Z., Tang, J., Feng, H., Liu, X., Zhuang, X., Wang, H., et al. (2024). Research progress on remediation of heavy metal contaminated soil by electrokinetic-permeable reactive barrier. Chem. Eng. J. 490, 151548. doi:10.1016/j.cej.2024.151548
Yao, W. K., Cai, Z. P., Sun, S. Y., Romantschuk, M., Sinkkonen, A., Sun, Y., et al. (2020). Electrokinetic-enhanced remediation of actual arsenic-contaminated soils with approaching cathode and Fe0 permeable reactive barrier. J. Soils Sediments 20, 1526–1533. doi:10.1007/s11368-019-02459-4
You, Y., Dou, J., Xue, Y., Jin, N., and Yang, K. (2022). Chelating agents in assisting phytoremediation of uranium-contaminated soils: a review. Sustainability 14, 6379. doi:10.3390/su14106379
Yu, X., Muhammad, F., Yan, Y. J., Yu, L., Li, H. L., Huang, X., et al. (2019). Effect of chemical additives on electrokinetic remediation of Cr-contaminated soil coupled with a permeable reactive barrier. R. Soc. Open Sci. 6, 182138. doi:10.1098/rsos.182138
Zhang, Q., Wang, Y., Wang, Z., Zhang, Z., Wang, X., and Yang, Z. (2021). Active biochar support nano zero-valent iron for efficient removal of U(VI) from sewage water. J. Alloys Compd. 852, 156993. doi:10.1016/j.jallcom.2020.156993
Zheng, F. X., Zhai, Y. Z., Yue, W. F., and Teng, Y. G. (2023). Coupling flow and electric fields to simulate migration and remediation of uranium in groundwater remediated by electroosmosis and a permeable reactive bio-barrier. J. Environ. Manag. 346, 118947. doi:10.1016/j.jenvman.2023.118947
Zhou, M., Xu, J. M., Zhu, S. F., Wang, Y. J., and Gao, H. (2018). Exchange electrode-electrokinetic remediation of Cr-contaminated soil using solar energy. Sep. Purif. Technol. 190, 297–306. doi:10.1016/j.seppur.2017.09.006
Keywords: environmental protection, uranium, electrokinetic remediation, contaminated soil, radioactive pollution
Citation: Bai Y, Zhang Z, Jiang G, Niu S, Peng Z and Liu L (2025) Electrokinetic remediation technology for uranium contaminated soil: from fundamental principles to application challenges and breakthroughs. Front. Chem. Eng. 7:1687808. doi: 10.3389/fceng.2025.1687808
Received: 18 August 2025; Accepted: 22 September 2025;
Published: 10 October 2025.
Edited by:
Leandro Goulart de Araujo, UMR5256 Institut de Recherches sur la Catalyse et l'Environnement de Lyon (IRCELYON), FranceReviewed by:
Savan Raj, Université du Québec, CanadaYongjin Xu, University of Chinese Academy of Science, China
Copyright © 2025 Bai, Zhang, Jiang, Niu, Peng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlong Bai, YmFpeXVubG9uZ0Bjbm1zdGMuY29t; Longcheng Liu, bGl1bG9uZ2NoZW5nQGNubXN0Yy5jb20=
 Yunlong Bai
Yunlong Bai Zhean Zhang1
Zhean Zhang1