- 1CNISM Unit, Department of Physics and Astronomy, Università degli Studi, Padua, Italy
- 2Istituto Nazionale di Fisica Nucleare, Sezione di Padova, Padua, Italy
- 3Department of Physics and Astronomy, Università degli Studi, Padua, Italy
We report new spectroscopic measurements of the near-infrared fluorescence band of electron-beam excited
1 Introduction
Xenon is chosen in several high-energy physics applications owing to its excellent fluorescence properties. Excitation of the Xe atoms is accomplished by several different techniques, including either electron- (Arai and Firestone, 1969; Lorents, 1976; Koehler et al., 1974)-, proton- (Hurst et al., 1969), ion- (Ulrich, 2012; Khasenov, 2016), or
In the degradation pathway of the energy released in the detector medium, a significant amount of the energy is released in the VUV range by the de-excitation of
The processes leading to excimer fluorescence in the low-density limit have been extensively studied and are now very well-known (Millet et al., 1978; Mulliken, 1970; Salamero et al., 1984; Nowak et al., 1985; Keto et al., 1997). The excimer levels responsible for the emission of the first two VUV continua are the
Eventually, the excimers may radiatively decay toward the repulsive ground state
The first continuum, observed at low pressure
The potential energy curves of these lowest-lying excimer levels in pure Xe have been determined by both the analysis of spectroscopic experiments (Keto et al., 1997; Gornik et al., 1981; Raymond et al., 1984; Castex, 1981; Koeckhoven et al., 1995) and by theoretical calculations (Mulliken, 1970; Ermler et al., 1978; Jonin and Spiegelmann, 2002).
Although less energetic transitions may be involved in the Xe excimer de-excitation processes along the energy degradation pathway, leading to possible infrared (IR) and/or near-infrared (NIR) fluorescence, the investigation of such a possibility has been given less attention than the VUV fluorescence. NIR cathodoluminescence, produced by electron impacts, was first observed in the gaseous phase of pure Xe at a wavelength of approximately
Further investigation of this NIR band produced the de-excitation of
One striking feature of the discovered NIR excimer band is that its central wavelength, i.e., the wavelength of the band maximum,
We have been able to rationalize the experimental findings, i.e., the density-dependent redshift, by setting up a simple theoretical model that takes into account multiple scattering effects (Borghesani et al., 2001). It is only based on the knowledge of the dielectric constant of the gaseous environment and the quantum density-dependent shift
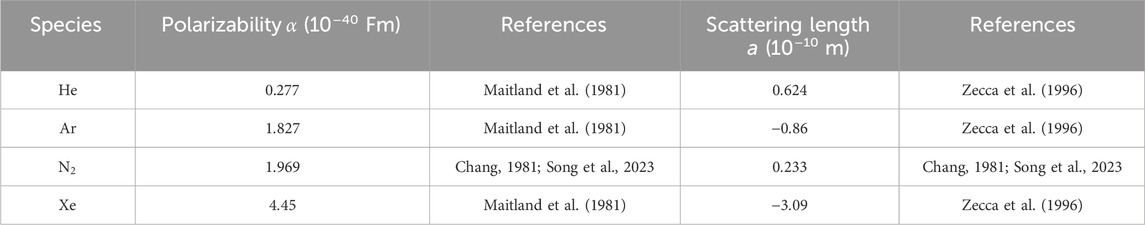
This model does not contain any adjustable parameters and accurately describes the data up to higher densities and will be described in Section 3. As it depends on the properties of the gaseous environment in which the Xe atoms are immersed, we have decided to carry out measurements in gaseous mixtures in which the emitting species Xe is typically the minority component, whose dielectric constants and scattering lengths (i.e.,
This article is organized as follows. The experimental technique is briefly described in Section 2. The phenomenology of the experimental results is presented in Section 3, and their discussion is reported in Section 4. Finally, the conclusions are drawn in Section 5.
2 Experimental details
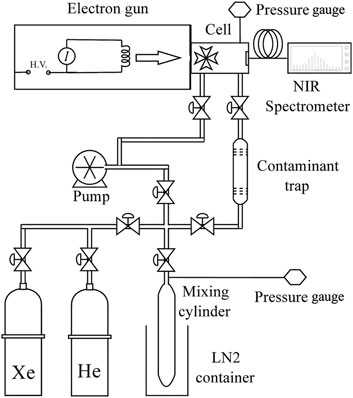
The technique and apparatus have been described accurately in Borghesani et al. (2001). We briefly summarize them here. A simplified schematic of the experimental apparatus is shown in Figure 1. The technique for exciting the gas sample has already been described in the literature (Borghesani et al., 2001). A homemade electron gun (Barcellan et al., 2011) injects
Electrons lose
The emitted light exited the cell through a sapphire window and was analyzed by an NIR spectrometer (Ocean Optics, mod. NIRQuest512).
The pressure cell, which was tested with pressure up to
To produce the Xe–
After waiting for enough time for the two gases to inter-diffuse, the mixture is introduced to the cell by flowing it through a trap to further remove residual oxygen and other impurities. At the temperature and pressures of the experiment (
3 Experimental results: phenomenology
In this section, we present the experimental results obtained in the Xe–He, Xe–
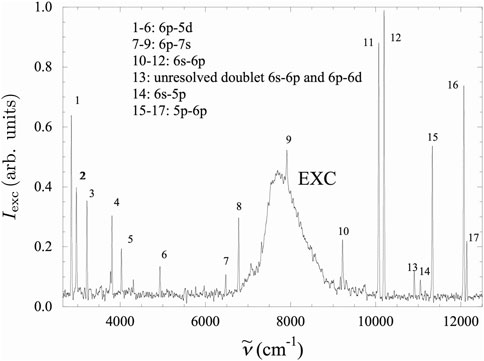
Figure 2. Typical high-resolution spectrum in Xe at
The intensity of the NIR radiation emitted by the
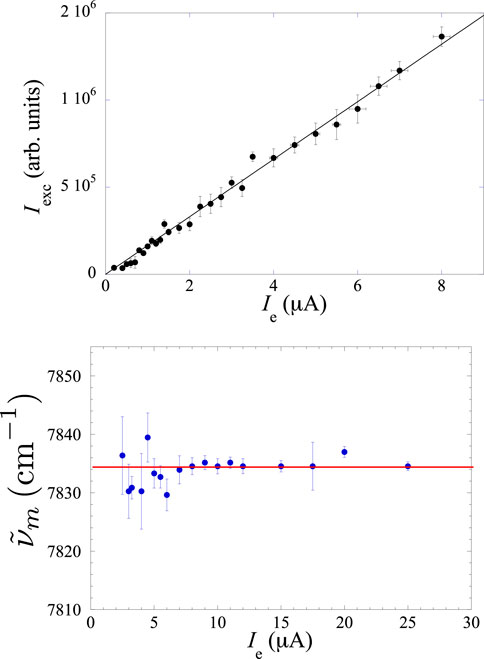
Figure 3. Top panel: linear relationship between the integrated intensity of the excimer spectrum
Another indication that the intensity of the electron current does not influence the microscopic processes leading to excimer formation and decay can be deduced by inspecting the bottom panel of Figure 3, in which the central wavenumber
Finally, we noted that the excimer spectra recorded at different electron current intensities for the same gas pressure do differ only in the intensities and not in their general features. After proper normalization at the unit area, they exactly overlap, provided that the spectra recorded at lower excitation current are less intense and, therefore, much noisier than those recorded at higher excitation current, as shown in Figure 4.
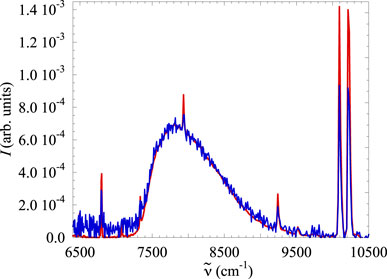
Figure 4. Normalized spectra recorded in pure Xe gas at room temperature for the same pressure
3.1 Pure Xenon gas and the Ar–Xe mixture
In pure Xe gas, the modification of the spectrum as a consequence of the variation of the gas density is the largest among all mixtures. In Figure 5 we show two spectra recorded at the lowest and highest pressures reached in the present experiment, corresponding to densities
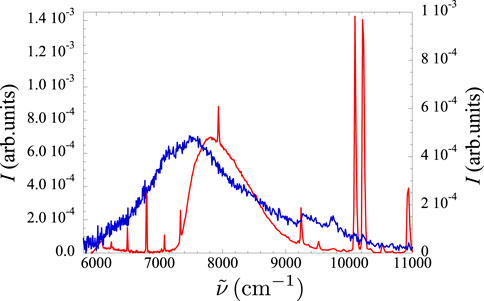
Figure 5. Spectra recorded in pure Xe gas at room temperature at two different pressure values. Red line:
In the Xe–Ar mixture of nominal
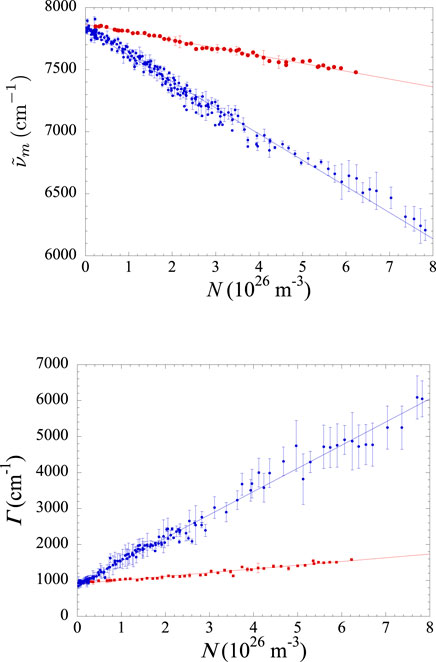
Figure 6. Top panel: density-dependent redshift of the wavenumber
The increase in density is (Equations 2, 4, 5, 6, and 7) expected to result in a higher collision frequency that reduces the lifetime of the excimer species and increases the width of the continuum. Although the excimer spectrum is a band rather than a narrow line, its width is a measure of how much the density affects the excimer lifetime. The density dependence of the full width at half height
in which
The second, and perhaps more interesting, observation is that the spectrum broadening is much larger in the pure gas than in the mixture. Apparently, the
3.2 Xe–He mixtures
We have investigated three Xe–He mixtures of nominal Xe concentrations that were roughly
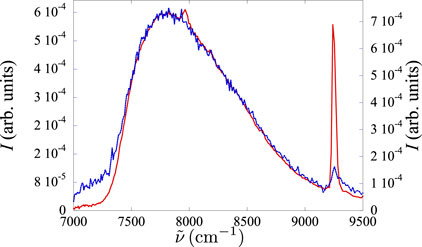
Figure 7. Spectra of a
We immediately note that the redshift in the Xe–He mixture is very weak, as both spectra are almost coincident. The Xe–He mixture requires a much higher density than the pure gas to reach a similar redshift.
In the top panel of Figure 8 we report the density dependence of the central wavenumber of the spectrum for the three investigated Xe–He mixtures, along with the results obtained in pure Xe gas during the present experimental run.
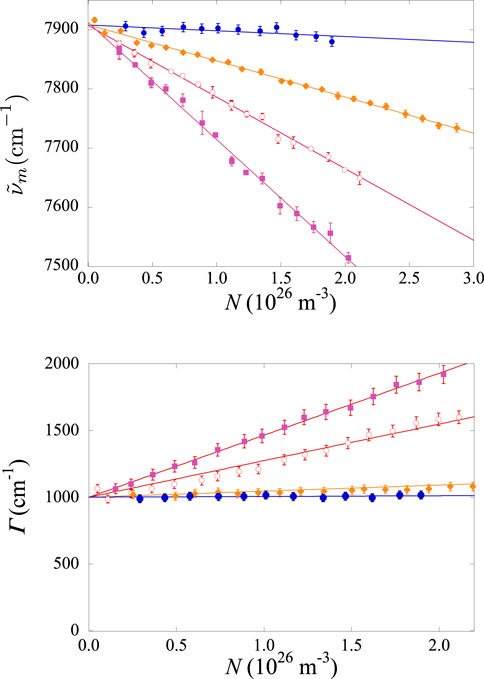
Figure 8. Top panel: density dependence of
We observed that in all mixtures,
The spectra also show collisional broadening. Their full width at half maximum
3.3 Xe–
We investigated several Xe–
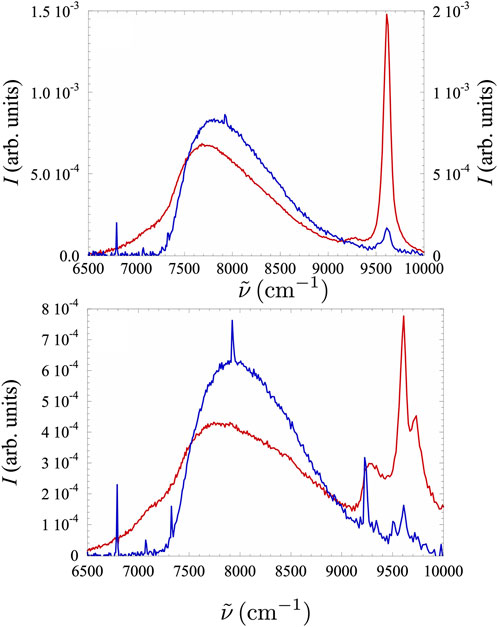
Figure 9. Typical spectra in Xe–
For all Xe concentrations,
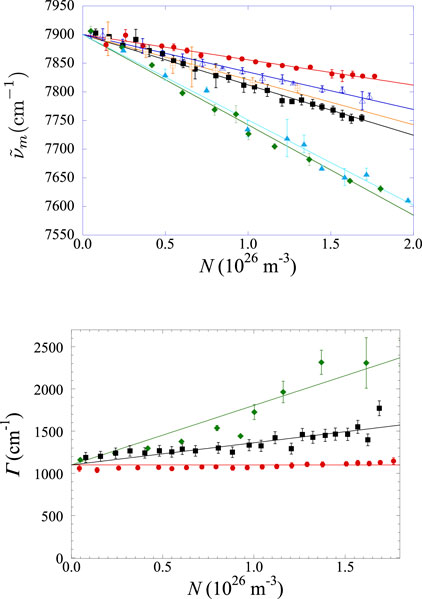
Figure 10. Top panel: density-dependence of
The quantification of the collisional broadening of the spectra is shown in the bottom panel of Figure 10, in which the full width at half height
Finally, we want to provide evidence regarding the large uncertainty on the width at the highest Xe concentration. As previously noted, the spectra in the Xe–
4 Discussion
The most interesting feature of our experimental observation is that the NIR excimer spectrum is always shifted toward lower wavenumbers, i.e., it is redshifted, if the density of the gas is increased. This phenomenon may affect the way in which Xe is used in the field of high-energy particle detection. However, most significantly in our opinion, it may shed light on the (unexpected) correlation between two different realms involving very low-energy electrons in a dense environment: loosely bound electron states in a molecule and quasi-free electron transport in dense gases.
We noted that, in the limit of the negligibly small density,
We were able to set up a theoretical model that explains the experimentally observed redshift in pure Xe gas without any adjustable parameters (Borghesani et al., 2001). We are now in the position to extend this successful model to the case of the mixtures. We will see that all the features of the model maintain their validity and that the extension of the pure gas model to the mixtures is straightforward, provided that the mixtures are dealt with by exploiting the law of the ideal mixtures. Thus, in this section, we will recapitulate the model we have devised and show how it is successfully extended to mixtures.
4.1 Derivation of the model for pure Xe gas
The electronic structure of an isolated homonuclear excimer is accurately described in terms of an ionic core plus a (largely) delocalized electron in a diffuse Rydberg orbital whose radius is much larger than the internuclear separation (Mulliken, 1970). Such a description also retains its validity in a dense gaseous environment, provided that the density is low enough to lead to a negligibly low scattering rate of the electron off the surrounding atoms. Stated differently, the mean free path of a quasi-free electron in the gas must be larger than the size of the Rydberg orbital. This condition is fulfilled in pure Xe gas as the mean free path at the density of our experiment is several nanometers long (Huang and Freeman, 1978). It is also fulfilled in
High-extravalence excitations of atomic impurities in highly polarizable media are known to be redshifted and are assumed to have a probable parentage with Wannier impurity states in liquids (Messing et al., 1977a; Messing et al., 1977b). Thus, the excimer energy spectrum can be computed in analogy with that of these Wannier states.
Two main effects influence the excimer energy spectrum. The first one is a classical dielectric screening effect. It has been shown that the electron energy eigenvalues in a point-like Coulomb potential immersed in a medium of a relative dielectric constant
In the present case, the excimer band originates from the transition from the high-lying, vibrationally relaxed, bound
As a consequence of dielectric screening, the average energy difference between the initial and final states is reduced by the factor
in which
Owing to the smallness of
In Equation 3
The second, equally relevant contribution to the density dependence of
In Equation 4
By adding this contribution to the first one, we finally get the density-dependent shift of
which turns out to be linear in
The Xe scattering length is
In the case of the Xe
While the dielectric screening effect, although of different strengths in different gases, always acts in the same direction by reducing the transition energy, thereby shifting the excimer spectrum toward longer wavelengths, the contribution of the multiple scattering effect (Equation 4) may be positive or negative depending on the sign of the scattering length
4.2 Extension of the model to Xe-based mixtures
The aim of Section 4.2 is to extend the interpretative model to gaseous mixtures for the redshift. The energy transfer from the lighter to the heavier noble gases is a well-known process (Efthimiopoulos et al., 1997). Therefore, Xe is always excited either by direct or indirect processes, and it is safe to assume that
In a mixture, the
We have to remember that at the temperature and pressures of the present experiment, all gases follow the law of the ideal gas quite accurately1. Therefore, the average density
Let
with the slope
The main result is that
The values of polarizability
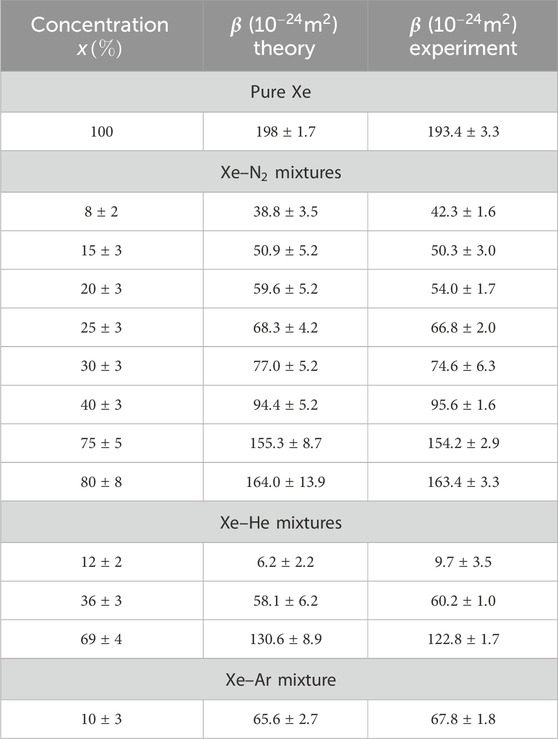
Table 2. Comparison of the experimentally measured values of the linear coefficient
We noted that the proposed extension of the original model for the pure gas accounts for the experimental results for the mixtures in an excellent way. The experimental data, within the uncertainty, are very close to the theoretical values for all mixtures. A visual way to ascertain how the experimental data of the slope are close to the predicted values is shown in Figure 11. Here, the experimental
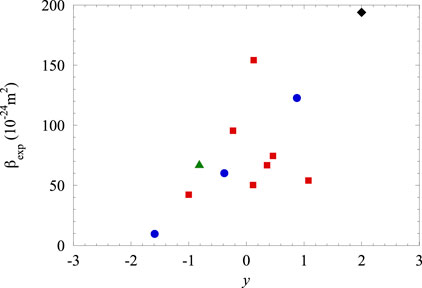
Figure 11. Experimental values of the shift slope
Some additional general remarks can be made by examining the table. The Xe–He mixtures show the lowest
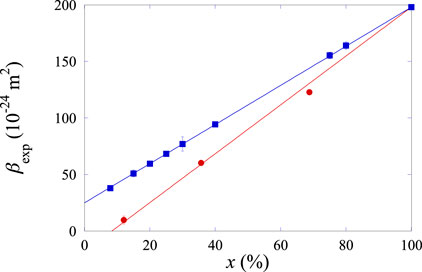
Figure 12. Dependence of the experimental slope
On the contrary, for the Xe–
5 Conclusion
The investigation of the NIR fluorescence of
We have unexpectedly found that in addition to the expected collisional broadening, the excimer band is redshifted, i.e., its centroid linearly shifts to smaller wavenumbers, as the gas density increases. We have been able to rationalize the experimental observations by devising a model in which the optically active electron in the molecule in its high-lying energy state is largely delocalized. In this way, it can be treated almost as if it were a quasi-free electron.
According to our model, two effects act to modify the transition energy. The first one is based on the effect of classical dielectric screening: many surrounding atoms are contained in the extremely extended electron orbit and screen the Coulomb electron–nucleus interaction, thereby reducing the difference between the energy levels.
The second one, on the contrary, is a quantum multiple scattering effect. The wavefunction of the delocalized electron has such a wide spatial extension that it spans over several surrounding atoms at a time. As a result, the electron energy is altered by a density-dependent quantum shift
It is extremely interesting to note that
Thus, the spectroscopic measurements may give useful pieces of information on how a delocalized electron interacts with its high-density surroundings. The leading proposal of the present measurements is that the environment could be modified by embedding the probe, i.e., the
The experimental outcome in the mixture turns out to be qualitatively similar to what is observed for pure Xe gas. The excimer band is always redshifted, i.e., its central wavenumber linearly decreases with increasing gas density, but the shift strength depends on the concentration and type of gases in the mixture.
We have extended the equation of the model to the mixtures by simply applying the law of ideal gas mixtures and assuming that the mixture can be treated as a simple gas whose effective scattering length and effective polarizability are given by the contributions of the individual properties of the two species in the mixture, weighted by their relative abundance.
This extended model is able to describe with great accuracy the experimental results without any adjustable parameters and, thus, has great predictive power. For instance, this spectroscopic technique could be used as an alternative way to measure the scattering length of some gases such as, for instance, some highly polarizable gases such as
The outcome of the present experiment could have some implications on liquefied noble gas detectors operated in the two-phase mode, such as for liquid Ar mixed with a small concentration of Xe (Kim et al., 2004; Neumeier et al., 2015a), because redshifted luminescence in the gas phase could help correlate the scintillation signal from the liquid. Additionally, the understanding of the processes leading to the NIR shift of the luminescence in the dense gas could also help shed light on the microscopic processes leading to scintillation in liquefied noble gases and/or their mixtures (Neumeier et al., 2015b; Szydagis et al., 2025).
Some puzzling questions remain unsolved. The most critical one is how the collisional broadening of the excimer band correlates with the properties of the individual gases. Although the full width at half length of the excimer band linearly increases with an increase in the gas density, thereby strongly indicating a collision-induced reduction of the excimer lifetime, nonetheless, it is not clear why the coefficient
Another puzzling question is the effect of nitrogen on some of the Xe I atomic lines, especially at low nitrogen concentrations, as shown in Figure 9. Is their broadening due to the formation of red and blue satellites that are not resolved in our setup? Or is the apparent broadening due to an underlying broad continuum caused by the emission of a possible
Possible future developments of the experiment will be to pursue the possibility of increasing the gas densities to possibly bridge the gap with the liquid, in which similar NIR luminescence has been detected (Neumeier et al., 2014), and to investigate mixtures with different noble gases in order to test the validity of our heuristic model.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Author contributions
AFB: conceptualization, data curation, formal analysis, investigation, methodology, supervision, validation, visualization, writing – original draft, writing – review and editing, resources, and software. GC: conceptualization, investigation, methodology, resources, supervision, writing – review and editing, and project administration. FC: conceptualization, investigation, writing – review and editing, formal analysis, data curation, methodology, and validation, and visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors acknowledge useful discussion with Prof. D. Iannuzzi of Vrije Universiteit Amsterdam (The Netherlands). They also gratefully acknowledge technical assistance by G. Galeazzi, E. Berto, and G. Galet.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1https://webbook.nist.gov/chemistry/fluid/
References
Aalbers, J., AbdusSalam, S. S., Abe, K., Aerne, V., Agostini, F., Ahmed Maouloud, S., et al. (2022). A next-generation liquid xenon observatory for dark matter and neutrino physics. J. Phys. G Nucl. Part. Phys. 50, 013001. doi:10.1088/1361-6471/ac841a
Álvarez, V., Borges, F. I. G., Cárcel, S., Cebrián, S., Cervera, A., Conde, C. A. N., et al. (2013). Ionization and scintillation response of high-pressure xenon gas to alpha particles. JINST 8, P05025. doi:10.1088/1748-0221/8/05/p05025
Anton, G., Badhrees, I., Barbeau, P. S., Beck, D., Belov, V., Bhatta, T., et al. (2020). Measurement of the scintillation and ionization response of liquid xenon at mev energies in the exo-200 experiment. Phys. Rev. C 101, 065501. doi:10.1103/PhysRevC.101.065501
Arai, S., and Firestone, R. F. (1969). Evidence for the formation of neutral rare-gas molecules by electron-beam Pulses. J. Chem. Phys. 50, 4575–4589. doi:10.1063/1.1670934
Barcellan, L., Berto, E., Carugno, G., Galet, G., Galeazzi, G., and Borghesani, A. F. (2011). A battery-operated, stabilized, high-energy pulsed electron gun for the production of rare gas excimers. Rev. Sci. Instrum. 82, 095103–095107. doi:10.1063/1.3636078
Bartels, A. (1975). Density dependence of electron drift velocities in helium and hydrogen at 77.6 K. Appl. Phys. 8, 59–64. doi:10.1007/BF00883671
Belogurov, S., Bressi, G., Carugno, G., Conti, E., Iannuzzi, D., and Meneguzzo, A. (2000). Measurement of the light yield of infrared scintillation in xenon gas. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 452, 167–169. doi:10.1016/S0168-9002(00)00358-2
Borghesani, A., Carugno, G., and Santini, M. (1991). Experimental determination of the conduction band of excess electrons in liquid Ar. IEEE Trans. Elect. Insul. 26, 615–622. doi:10.1109/14.83680
Borghesani, A. F. (2007). Electrons and ions in liquid Helium, International Series of Monographs on physics, 137. Oxford: Oxford University Press.
Borghesani, A. F. (2020). Resonant low-energy electron attachment to O2 impurities in dense neon gas. Plasma Sources Sci. Technol. 29, 035024. doi:10.1088/1361-6595/ab708a
Borghesani, A. F. (2021). Accurate electron drift mobility measurements in moderately dense helium gas at several temperatures. Atoms 9, 52–19. doi:10.3390/atoms9030052
Borghesani, A. F., Bressi, G., Carugno, G., Conti, E., and Iannuzzi, D. (2001). Infrared fluorescence of Xe2 molecules in electron/proton beam excited pure Xe gas and in an Ar/Xe gas mixture. J. Chem. Phys. 115, 6042–6050. doi:10.1063/1.1398307
Borghesani, A. F., Bruschi, L., Santini, M., and Torzo, G. (1988). Electron mobility in neon at high densities. Phys. Rev. A 37, 4828–4835. doi:10.1103/PhysRevA.37.4828
Borghesani, A. F., and Carugno, G. (2008). Dielectric screening–, multiple scattering–, and quantum indistinguishability effects in the IR emission spectrum of Xe2 excimers in dense Xe gas. doi:10.48550/arXiv.0802.3602
Borghesani, A. F., Carugno, G., Iannuzzi, D., and Mogentale, I. (2005). Environmental influence on the IR fluorescence of Xe2* molecules in electron beam excited Ar–Xe mixture at high density. Eur. Phys. J. D. 35, 299–306. doi:10.1140/epjd/e2005-00077-9
Borghesani, A. F., Carugno, G., and Mogentale, I. (2007a). Infrared emission spectrum and potentials of 0+u and 0+g states of Xe2 excimers produced by electron impact. J. Phys. B Atomic, Mol. Opt. Phys. 40, 4551–4560. doi:10.1088/0953-4075/40/24/001
Borghesani, A. F., Carugno, G., and Mogentale, I. (2007b). Low- and high-density features of IR luminescence of Xe2 excimers produced by electron impact. Phys. Scr. 76, C84–C89. doi:10.1088/0031-8949/76/3/N13
Borghesani, A. F., Santini, M., and Lamp, P. (1992). Excess electron mobility in high-density argon gas. Phys. Rev. A 46, 7902–7909. doi:10.1103/PhysRevA.46.7902
Carugno, G. (1998). Infrared emission in gaseous media induced by ionizing particles and by drifting electrons. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 419, 617–620. doi:10.1016/S0168-9002(98)00840-7
Castex, M. C. (1981). Experimental determination of the lowest excited Xe2 molecular states from VUV absorption measurements. J. Chem. Phys. 74, 759–771. doi:10.1063/1.441177
Chang, E. S. (1981). Modified effective-range theory for electron scattering from molecules. J. Phys. B Atomic Mol. Phys. 14, 893–901. doi:10.1088/0022-3700/14/5/023
Dutuit, O., Gutcheck, R. A., and Calvé, J. L. (1978). Spectral and kinetic studies of the second continuum fluorescence of xenon excited by synchrotron radiation. Chem. Phys. Lett. 58, 66–72. doi:10.1016/0009-2614(78)80318-2
Efthimiopoulos, T., Zouridis, D., and Ulrich, A. (1997). Excimer emission spectra of rare gas mixtures using either a supersonic expansion or a heavy-ion-beam excitation. J. Phys. D. Appl. Phys. 30, 1746–1754. doi:10.1088/0022-3727/30/12/010
Ermler, W. C., Lee, Y. S., Pitzer, K. S., and Winter, N. W. (1978). Ab initio effective core potentials including relativistic effects. II. Potential energy curves for Xe2. J. Chem. Phys. 69, 976–983. doi:10.1063/1.436650
Fermi, E. (1934). Sopra lo spostamento per pressione delle righe elevate delle serie spettrali. Il Nuovo Cimento 11, 157–166. doi:10.1007/bf02959829
Gómez Cadenas, J. J., Álvarez, V., Borges, F. I. G., Cárcel, S., Castel, J., Cebrián, S., et al. (2014). Present Status and future Perspectives of the NEXT experiment. Adv. High. Energy Phys. 2014, 907067/1–22. doi:10.1155/2014/907067
Gornik, W., Kindt, S., Matthias, E., and Schmidt, D. (1981). Two-photon excitation of xenon atoms and dimers in the energy region of the 5p5-6p configuration. J. Chem. Phys. 75, 68–74. doi:10.1063/1.441856
Hammann, R., Böse, K., Hötzsch, L., Jörg, F., and Marrodán Undagoitia, T. (2024). Investigating the slow component of the infrared scintillation time response in gaseous xenon. JINST 19, C02080/1–7. doi:10.1088/1748-0221/19/02/C02080
Henck, R., and Coche, A. (1967). Studies on noble gas Scintillators. IEEE Trans. Nucl. Sci. 14, 478–486. doi:10.1109/TNS.1967.4324458
Herman, R., Wallis, M. C., and Wallis, R. F. (1956). Intensities of the R1 and R2 bands in KCl crystals. Phys. Rev. 103, 87–93. doi:10.1103/PhysRev.103.87
Huang, S. S. S., and Freeman, G. R. (1978). Electron mobilities in gaseous, critical, and liquid xenon: density, electric field, and temperature effects: Quasilocalization. J. Chem. Phys. 68, 1355–1362. doi:10.1063/1.435954
Huang, S. S. S., and Freeman, G. R. (1981). Electron transport in gaseous and liquid argon: effects of density and temperature. Phys. Rev. A 24, 714–724. doi:10.1103/PhysRevA.24.714
Hurst, G. S., Bortner, T. E., and Strickler, T. D. (1969). Proton excitation of the argon atom. Phys. Rev. 178, 4–10. doi:10.1103/PhysRev.178.4
Jonin, C., and Spiegelmann, F. (2002). Pseudopotential hole–particle formalism for excitations in xenon molecules and clusters. II. The electronic structure of Xe2*. J. Chem. Phys. 117, 3059–3073. doi:10.1063/1.1491400
Keto, J. W., Cai, H., Kykta, M., Lei, C., Möller, T., and Zimmerer, G. (1997). Two-photon spectroscopy of xenon dimers in supersonic jets. J. Chem. Phys. 107, 6080–6093. doi:10.1063/1.474276
Khasenov, M. (2016). Emission spectra of noble gases and their mixtures under ion beam excitation. Laser Part. Beams 34, 655–662. doi:10.1017/s0263034616000616
Kim, J., Dardin, S., Kadel, R., Kadyk, J., Peskov, V., and Wenzel, W. (2004). Electron avalanches in liquid argon mixtures. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 534, 376–396. doi:10.1016/j.nima.2004.06.136
Koeckhoven, S. M., Burma, W. J., and de Lange, C. A. (1995). A resonance enhanced multiphoton ionization study of the gerade excited states of Xe2 with a Xe + Xe⋆ 6s[3/2]1 dissociation limit. J. Chem. Phys. 102, 4020–4026. doi:10.1063/1.4366510
Koehler, H. A., Ferderber, L. J., Redhead, D. L., and Ebert, P. J. (1974). Vacuum-ultraviolet emission from high-pressure xenon and argon excited by high-current relativistic electron beams. Phys. Rev. A 9, 768–781. doi:10.1103/PhysRevA.9.768
Krebs, P., and Lang, U. (1996). Electron mobility and multiple scattering effects in dense methanol gas. J. Phys. Chem. 100, 10482–10489. doi:10.1021/jp952470z
Krebs, P., and Wantschik, M. (1980). Mobility of electrons in ammonia vapor at various densities. J. Phys. Chem. 84, 1155–1160. doi:10.1021/j100447a016
Krstic, P. S., and Schultz, D. R. (1999). Elastic scattering and charge transfer in slow collisions: isotopes of H and H+ colliding with isotopes of H and with He. J. Phys. B Atomic, Mol. Opt. Phys. 32, 3485–3509. doi:10.1088/0953-4075/32/14/317
Leardini, S., García, E. S., Amedo, P., Saa-Hernández, A., González-Díaz, D., Santorelli, R., et al. (2022). Time and band-resolved scintillation in time projection chambers based on gaseous xenon. Eur. Phys. J. C 82, 425/1–13. doi:10.1140/epjc/s10052-022-10385-y
Lorents, D. C. (1976). The physics of electron beam excited rare gases at high densities. Phys. B+C 82, 19–26. doi:10.1016/0378-4363(76)90265-5
Maitland, G. C., Rigby, M., Smith, E. B., and Wakeham, W. A. (1981). “Intermolecular Forces. Their Origin and determination,” in International Series of Monographs on chemistry. Oxford: Clarendon Press.
McEachran, R. P., and Stauffer, A. D. (2014). Momentum transfer cross sections for the heavy noble gases. Eur. Phys. J. D. 68, 153–158. doi:10.1140/epjd/e2014-50166-7
Messing, I., Raz, B., and Jortner, J. (1977a). Experimental evidence for Wannier impurity states in doped rare-gas fluids. Chem. Phys. 23, 23–37. doi:10.1016/0301-0104(77)89040-X
Messing, I., Raz, B., and Jortner, J. (1977b). Solvent perturbations of extravalence excitations of atomic xe by rare gases at high pressures. J. Chem. Phys. 66, 4577–4586. doi:10.1063/1.433714
Mikulski, P., Klahn, T., and Krebs, P. (1997). Excess electron mobility in low density CH3CN gas: mShort-lived dipole-bound electron ground states as precursors of localized electron states. Phys. Rev. A 55, 369–377. doi:10.1103/PhysRevA.55.369
Millet, P., Birot, A., Brunet, H., Galy, J., Pons-Germain, B., and Teyssier, J. L. (1978). Time resolved study of the UV and near UV continuums of xenon. J. Chem. Phys. 69, 92–97. doi:10.1063/1.436349
Mimura, M., Kobayashi, S., Masuyama, N., Miyajima, M., and Hasebe, N. (2009). Average numbers of scintillation photons and electrons produced by an alpha particle in high-density xenon gas. Jpn. J. Appl. Phys. 48, 076501. doi:10.1143/JJAP.48.076501
Mogentale, I. (2006). Luminescence of Xe2 molecules in dense Xe gas. Padua, Italy: Università degli Studi di Padova.
Moutard, P., Laporte, P., Subtil, J. L., Damany, N., and Damany, H. (1988). Pressure effects on kinetics and decay processes in xenon after selective photoexcitation. J. Chem. Phys. 88, 7485–7500. doi:10.1063/1.454313
Mulliken, R. S. (1970). Potential curves of diatomic rare-gas molecules and their ions, with particular reference to Xe2. J. Chem. Phys. 52, 5170–5180. doi:10.1063/1.1672756
Neumeier, A., Dandl, T., Heindl, T., Himpsl, A., Hagn, H., Hofmann, M., et al. (2014). Intense infrared scintillation of liquid Ar-Xe mixtures. Eur. Phys. Lett. 106, 32001/1–5. doi:10.1209/0295-5075/106/32001
Neumeier, A., Dandl, T., Heindl, T., Himpsl, A., Oberauer, L., Potzel, W., et al. (2015a). Intense vacuum ultraviolet and infrared scintillation of liquid Ar-Xe mixtures. EPL Europhys. Lett. 109, 12001–12006. doi:10.1209/0295-5075/109/12001
Neumeier, A., Dandl, T., Himpsl, A., Oberauer, L., Potzel, W., Schönert, S., et al. (2015b). Attenuation of vacuum ultraviolet light in pure and xenon-doped liquid argon —an approach to an assignment of the near-infrared emission from the mixture. EPL Europhys. Lett. 111, 12001–12006. doi:10.1209/0295-5075/111/12001
Nowak, G., Frey, L., and Fricke, J. (1985). Ar2*, Kr2* and Xe2* excimer formation from metastable and alpha -particle impact on solid rare-gas layers. J. Phys. B Atomic Mol. Phys. 18, 2851–2860. doi:10.1088/0022-3700/18/14/010
Piotter, M., Cichon, D., Hammann, R., Jörg, F., Hötzsch, L., and Marrodán Undagoitia, T. (2023). First time-resolved measurement of infrared scintillation light in gaseous xenon. Eur. Phys. J. C 83, 482. doi:10.1140/epjc/s10052-023-11618-4
Raymond, T. D., Böwering, N., Kuo, C. Y., and Keto, J. W. (1984). Two–photon laser spectroscopy of xenon collision pairs. Phys. Rev. A 29, 721–734. doi:10.1103/PhysRevA.29.721
Reininger, R., Asaf, U., Steinberger, I. T., and Basak, S. (1983). Relationship between the energy V0 of the quasi-free-electron and its mobility in fluid argon, krypton, and xenon. Phys. Rev. B 28, 4426–4432. doi:10.1103/PhysRevB.28.4426
Salamero, Y., Birot, A., Brunet, H., Galy, J., and Millet, P. (1984). Kinetic study of the VUV xenon emissions using selective multiphoton excitation. J. Chem. Phys. 80, 4774–4780. doi:10.1063/1.446550
Song, M. Y., Cho, H., Karwasz, G. P., Kokoouline, V., and Tennyson, J. (2023). Electron scattering on molecular nitrogen: common gas, uncommon cross sections. Eur. Phys. J. D 77, 105–108. doi:10.1140/epjd/s10053-023-00687-5
Suzuki, M., and Kubota, S. (1979). Mechanism of proportional scintillation in argon, krypton and xenon. Nucl. Instrum. Methods 164, 197–199. doi:10.1016/0029-554X(79)90453-1
Szydagis, M., Balajthy, J., Block, G. A., Brodsky, J. P., Brown, E., Cutter, J. E., et al. (2025). A review of NEST models for liquid xenon and an exhaustive comparison with other approaches. Front. Detect. Sci. Technol. 2. doi:10.3389/fdest.2024.1480975
Tanaka, Y., and Zelikoff, M. (1954). Continuous emission spectrum of xenon in the vacuum ultraviolet region. J. Opt. Soc. Am. 44, 254_1–255. doi:10.1364/JOSA.44.0254_1
Ulrich, A. (2012). Light emission from particle beam induced plasma: an overview. Laser Part. Beams 30, 199–205. doi:10.1017/S0263034611000838
Wada, T., and Freeman, G. R. (1981). Temperature, density, and electric-field effects on electron mobility in nitrogen vapor. Phys. Rev. A 24, 1066–1076. doi:10.1103/PhysRevA.24.1066
Wenck, H. D., Hasnain, S. S., Nikitin, M. M., Sommer, K., Zimmerer, G. F. K., and Haaks, D. (1979). Time and spectrally resolved fluorescence of Xe2 molecules excited with synchrotron radiation. Chem. Phys. Lett. 66, 138–143. doi:10.1016/0009-2614(79)80384-X
Wojciechowski, K., and Foryś, M. (1999). The mechanism of three-body process of energy transfer from excited xenon atoms to molecules. Radiat. Phys. Chem. 54, 1–10. doi:10.1016/S0969-806X(98)00217-5
Zecca, A., Karwasz, G. P., and Brusa, R. S. (1996). One century of experiments on electron-atom and molecule scattering: a critical review of integral cross-sections. La Riv. del Nuovo Cimento 19, 1–146. doi:10.1007/bf02742990
Keywords: Xe excimers, infrared cathodoluminescence, density-induced redshift, collision broadening, dense gas mixtures
Citation: Borghesani AF, Carugno G and Chiossi F (2025) Near-infrared cathodoluminescence of
Received: 20 February 2025; Accepted: 16 April 2025;
Published: 19 May 2025.
Edited by:
Saša Dujko, Institute of Physics, University of Belgrade, SerbiaReviewed by:
Robert Hammann, Max Planck Institute for Nuclear Physics, GermanyGrzegorz Karwasz, Nicolaus Copernicus University in Toruń, Poland
Copyright © 2025 Borghesani, Carugno and Chiossi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. F. Borghesani, YXJtYW5kb2ZyYW5jZXNjby5ib3JnaGVzYW5pQHVuaXBkLml0
†Present address: F. Chiossi, Chimie ParisTech, PSL University, CNRS, Institut de Recherche de Chimie, Paris, France
 A. F. Borghesani
A. F. Borghesani G. Carugno
G. Carugno F. Chiossi3†
F. Chiossi3†