Abstract
Alzheimer’s disease (AD) is a worldwide neurodegenerative disorder and the leading cause of dementia. Despite decades of research which has improved the understanding of AD, an effective disease-modifying therapy has yet to be developed that can prevent, stop, or reverse neuropathological changes and cognitive deficits of AD. There has been keen interest in targeting the epigenetic protein histone deacetylase 6 (HDAC6) for various human conditions leveraging its pathophysiological functions. Particularly, the pathological hallmarks of AD are aging-related accumulation of β-amyloid (Aβ) plaques and tau protein-related neurofibrillary tangles. In preclinical studies, HDAC6 inhibitors may significantly improve Aβ clearance and decrease tau aggregation and genetic deficiency of HDAC6 ameliorates cognitive deficits in mouse models of AD. While some pan-HDAC inhibitors have been FDA-approved for certain clinical indications, many HDAC6 inhibitors exhibit therapeutic potentials in preclinical studies of AD, for which we prepare this article to review and discuss recent studies and offer prospectives. We envision that the field of drug discovery of HDAC6 in AD may benefit by leveraging multimodal approaches, including structural and computational biology, medicinal chemistry, neuropathology and biomarker discovery. Using these approaches, future research will be better poised to efficiently discover new and potent HDAC6-selective inhibitors with enhanced blood-brain-barrier penetration, desirable safety and anti-AD efficacy. Considering the accumulated findings of HDAC6 and the urgent need in the field of AD, we speculate that many new small molecule inhibitors of HDAC6 will move forward enabling translational and clinical evaluations as potential therapeutics of AD.
Introduction
Alzheimer’s disease (AD) is an aging-associated, progressive neurodegenerative disease and the leading cause of dementia in the elderly. As the global population ages, Alzheimer’s prevalence significantly increases, and it is becoming a major health threat that has not been resolved to date. In 2025, it is estimated that 7.2 million Americans, aged 65 and older, are living with AD, accounting for 11% of that age group. Despite decades of research, currently there are no small molecule therapeutics available that may halt or modify the disease progression. Characterized by progressive memory decline and behavioral disturbance, Alzheimer’s hallmark neuropathology is primarily characterized by extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein (Scheltens et al., 2021) (Figure 1). Considerable evidence supports the amyloid cascade hypothesis, stating that Aβ deposition is primarily the early pathological trigger which leads to the pathological cascade downstream of AD, including NFT formation, neuroinflammation associated with glial cell activation and ultimately neuronal death. Among a variety of mechanisms contributing to AD pathogenesis and progression, histone deacetylase 6 (HDAC6) has emerged as a potentially efficacious and, yet so far, understudied drug target. It is well studied for a range of biological functions of HDAC6, consisting of regulation of microtubule stability, protein aggregation and quality control, as well as innate immunity and neuroinflammation (Figure 1). The functions of HDAC6 have resulted in the discovery of many small molecule inhibitors of HDAC6 for various clinical disorders, primarily cancer. It is only recently that increasing evidence has implicated HDAC6 involved in AD-related pathogenic processes, including tau aggregation, microtubule destabilization, and autophagy dysfunction. While the contribution of HDAC6 to AD pathogenesis is not yet clear and some studies have implicated dual context-dependent roles, there is a clear obligation to further investigate the potential for HDAC6-targeted therapies.
FIGURE 1
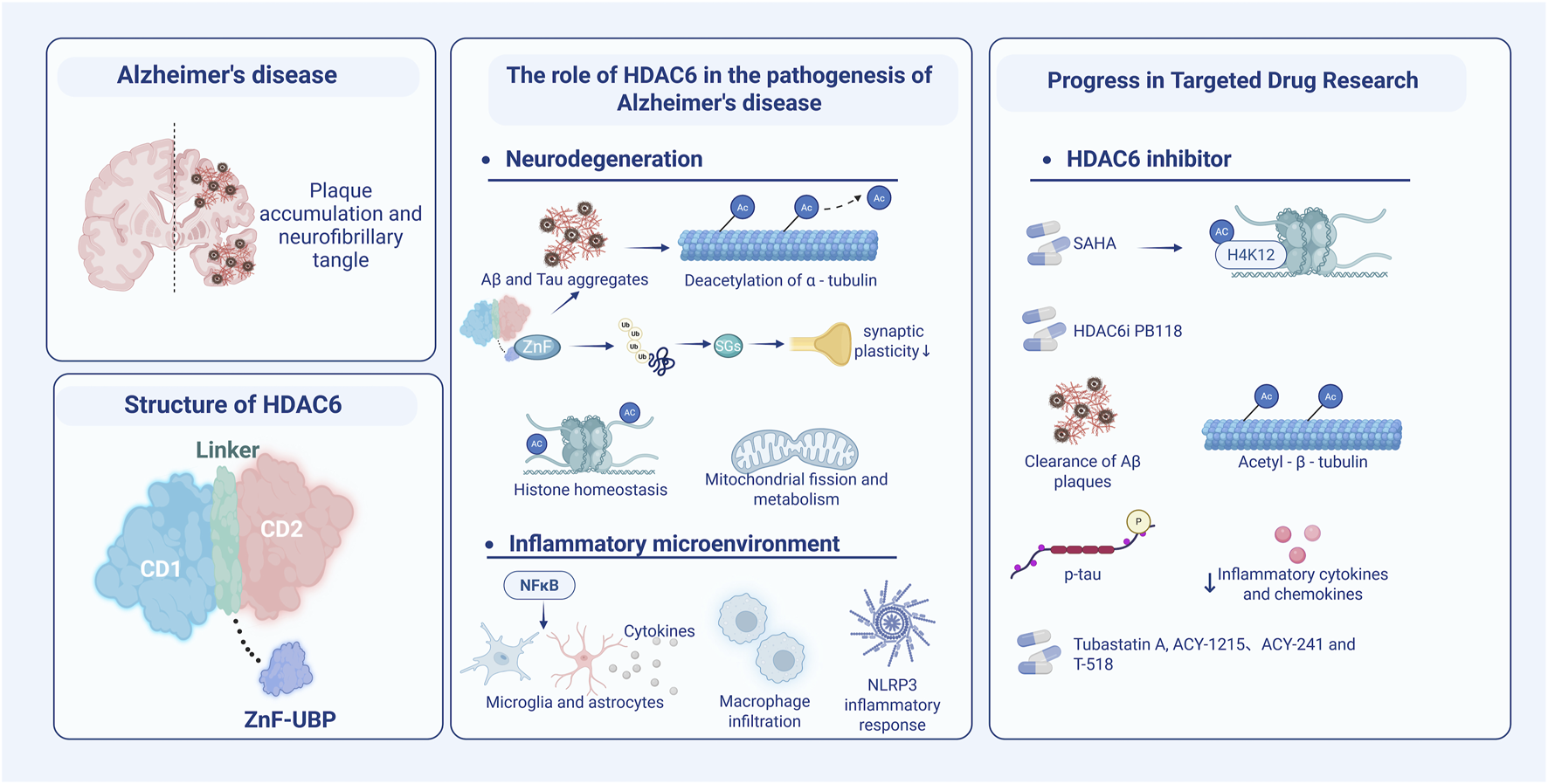
The association of HDAC6 with the pathophysiology of AD and its potential as a therapeutic target for AD are schematically shown. (Left) Accumulated amyloid plaques and neurofibrillary tangles with hyperphosphorylated tau protein are the pathological hallmarks of AD. Structurally, HDAC6 mainly comprises three domains: CD1, CD2, and ZnF UBP. (Center) HDAC6 is involved in amyloid plaque aggregation and neurofibrillary tangles formation, through its multiple functions, e.g., α-tubulin deacetylation, histone homeostasis, and synaptic plasticity disruption, as well as mitochondrial fission and metabolic disorders. Furthermore, HDAC6 regulates the innate immune microenvironment through the NF-κB signaling pathway, cytokine release, macrophage infiltration, activation of microglia and astrocytes, and NLRP3 inflammasome. (Right) The development of selective inhibitors for HDAC6 has received increasing attention, signified by molecules that offer protective effects on AD in multiple dimensions. Abbreviations: ZnF-UBP (a zinc finger ubiquitin-binding domain). Figure created with BioRender.
Systematic literature studies on the role of HDAC6 inhibitors in AD and other neurodegenerative disorders have attracted increasing attention (Zhang et al., 2013). Li et al. (2021) published a detailed review article summarizing the development of HDAC6 inhibitors from 2010 to 2020, with an emphasis on chemical structures, selectivity, and effects on AD-related neuropathology. Kumar et al. (2022) expanded on this work by providing a broader overview of the HDAC family, offering a more in-depth discussion of each HDAC member and isoform in the context of neurodegenerative diseases. More recently, Choudhary and colleagues identified panobinostat as a promising HDAC6 inhibitor capable of reducing amyloid pathology by upregulating neprilysin expression, supported by computational modeling and in vitro experiments (Choudhary et al., 2024). Given the rapid progress in HDAC6 inhibitor research, timely and comprehensive literature studies are essential-particularly those incorporating preclinical and clinical trial investigations. These studies may offer valuable insights into molecular mechanisms of HDAC6 inhibitors, such as their new functions associated with neuroinflammation, which may inform strategies for clinical translation. With the aim to highlight recent findings as well as to stimulate future studies, we anticipate that this article may be instrumental in guiding the rational design of next-generation HDAC6 inhibitors with improved pharmacokinetic properties, enhanced target specificity and safety profiles with greater neuroprotective efficacy that may move forward and provide clinical benefits in AD and potentially other neurodegenerative disorders.
HDAC6 and AD: from biological to pathogenic mechanisms
HDAC6 is a cytoplasmic enzyme, which can deacetylate non-histone substrates such as α-tubulin and heat shock protein 90. It is involved in a variety of physiological and pathological processes, including microtubule stability, axonal transport, protein homeostasis, stress response, and autophagic degradation. Furthermore, HDAC6 is a major regulator of aggresome formation and cell survival under stress associated with misfolded protein (Kawaguchi et al., 2003). HDAC6 is a significant component of stress granules (SGs) generated in oxidative or toxic protein stress responses, and it helps remove misfolded proteins through the aggregate-autophagy pathway. On the molecular level, the ZnF-UBP domain of HDAC6 drives the recruitment of ubiquitin and facilitates the formation of aggresomes and stress granules (SGs); this mechanism exacerbates the pathology of neurodegenerative diseases (Wang et al., 2022). SGs are ribonucleoprotein complexes residing in the cytosol and its formation protects mRNA transiently after stress occurs. Nevertheless, prolonged stress-induced persistence of SGs could segregate important RNA-binding proteins and make synaptic plasticity less flexible. In neurons, a malfunction of HDAC6 may exacerbate protein homeostasis, leading to a stressed state. Therapeutics, HDAC-targeting inhibitors (HDACis) can prevent the stressor H2O2 from inducing SGs formation, indicating the promise using these molecules as a therapeutic strategy for treating diseases characterized by oxidative stress and abnormal dysregulated SGs dynamics (Feng et al., 2020).
HDAC6 is intimately connected to the aberrant aggregation of the tau protein (Trzeciakiewicz et al., 2020) and offers the potential to reduce tau pathology in mice (Selenica et al., 2014). HDAC6 inhibition can increase autophagy, help clear tau aggregates and Aβ, and display neuroprotective effects (Shukla and Tekwani, 2020). Genetically, cognitive decline was found to be restored in the HDAC6 knockout mouse model, suggesting its function in physiological conditions (Govindarajan et al., 2013). In the context of tumors and oncology, HDAC6 regulates signaling pathways involving cell growth, metastasis, and invasion. Within the immune system, HDAC6 modulates macrophage infiltration and activates the NLRP3 inflammasome pathway, therefore controlling cytokine release.
Currently, several small molecules targeting HDAC6 are being evaluated in clinical trials for treating various cancers. However, their safety and efficacy in neurodegenerative diseases, especially AD, are less well studied and haven’t driven researcher’s attention until recently. Studies have sought to investigate HDAC6 changes in AD brains and showed that HDAC6 expression is significantly increased in AD brains (Bai et al., 2022; Odagiri et al., 2013). Consequently, deacetylation of α-tubulin is associated with increased HDAC6, which may influence the activity of dynamin-related protein Drp1, thereby modulating mitochondrial dynamics and function, including fission and fusion, and indirectly affecting neuronal survival (Chen et al., 2010; Waddell et al., 2021). Oxidative stress and axonal transport dysfunction caused by mitochondrial dysfunction are closely related to the pathogenesis and progression of AD. The antagonistic effect of histone acetyltransferases (HATs) and HDAC maintains histone acetylation homeostasis in the brain, which further position HDAC6 as an essential regulator of AD pathology. Particularly, HAT is beneficial for AD by increasing the level of acetylation: when HAT activity is reduced, it leads to disturbances in neuronal chromatin structure and gene expression. Furthermore, HDAC6 regulates the nuclear factor kB (NF-kB) pathway, controls cytokine release from microglia and astrocytes, and influences the inflammatory microenvironment in AD. Interestingly, HDAC6 inhibition can reduce glial cell activation and subsequent generation and secretion of pro-inflammatory cytokines.
Drug discovery progress that targets HDAC6 for AD
Despite past many years’ of research which has provided new insights of AD pathogenesis, effective therapies require to be developed that can prevent, stop, or reverse neuropathological changes and cognitive deficits of AD. FDA-approved small molecules medications for AD only offer palliative and modest symptomatic relief and do not modify disease neuropathology or progression, which include the acetylcholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor partial antagonists (Andrieu et al., 2015; Livingston et al., 2017; Alzheimer’s Association, 2015). The recently FDA-approved antibodies, e.g., aducanumab (Sevigny et al., 2016) and lecanemab (van Dyck et al., 2023), have brought new medications for AD which however display limited efficacy and adverse effects (Hardy and Mummery, 2023; Tanzi, 2021; Karran and De Strooper, 2022). The field of AD drug discovery requires better solutions of potentially different mechanisms that reduce neuropathology and progression of AD.
Considerable evidence supports HDAC6 as a candidate target for drug development of different human conditions and there has been new evidence in targeting HDAC6 for AD. Particularly, it has become a highly sought-after target for drug development owing to its functions and structural properties enabling strong inhibition. Structurally, HADC6 contains two catalytic deacetylase domains (DD1 and DD2) and a zinc finger ubiquitin-binding domain containing protein (ZnF-UBP). The ZnF-UBP domain is catalytically inactive, CD1 exhibits minimal catalytic activity, and CD2 is the primary catalytic region. HDAC6 expression is elevated in solid tumors and various blood diseases, and is associated with cancer cell proliferation, migration, and invasion. The inhibition of HDAC6 can block α-tubulin deacetylation, disrupt microtubule stability, and reduce cell migration and invasion ability. Moreover, HDAC6 inhibition can inhibit the aggresome formation, inducing endoplasmic reticulum (ER) stress and promoting cell apoptosis (Yang et al., 2020). In terms of tumor immunoregulation, HDAC6 inhibition can improve anti-PD-1 immune checkpoint blockade therapy, regulate Treg cell differentiation by promoting Foxp3 expression (Lee et al., 2022), and increase pro-inflammatory tumor-infiltrating macrophages and CD8 effector T cells. Several HDACis have been authorized by the FDA to treat hematologic cancers, and some have entered clinical trials (Table 1). For example, citarinostat (CC-96241; previously ACY-241), an oral HDAC inhibitor, is being evaluated in a clinical trial (NCT02886065) for smoldering multiple myeloma in combination with vaccine (PVX-410) and lenalidomide, to assess the efficacy and define the appropriate dose. The clinical trial of JBI-802 (LSD1/HDAC6 inhibitor) for the treatment of advanced solid tumors (NCT05268666) has started recruiting. Previous studies (Gajendran et al., 2023) have shown the efficacy of the drug as a monotherapy, as well as synergistic effects when used in combination with standard treatment or immune checkpoint inhibitors. A selective Class III HDACi, nicotinamide regulates mitochondrial energy metabolism. As a non-competitive Sirtuin inhibitor, it also affects multiple regulatory processes in both vivo and in vitro studies. Clinical trials have been initiated to look at its efficacy in AD (NCT 04430517, 05040321) (Cummings et al., 2024). The results of these trials will start to reveal in the coming years which will offer insights for designing trials of AD.
TABLE 1
| Agent | Mechanism of action | HDAC6 IC50 | Disease | Therapeutic purpose | Clinical trial | Phase | Study status | Lead sponsor | Start date | Estimated primary completion date | Chemical structure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vorinostat* | pan-HDAC inhibitor(6) | 4.87 nM | Neuroblastoma | Evaluate the feasibility and acute toxicity of using molecularly guided therapy in combination with standard therapy followed by a randomized controlled trial of standard immunotherapy with or without DFMO followed by DFMO maintenance for subjects with newly diagnosed high-risk neuroblastoma | NCT 02559778 |
II | Recruiting | Giselle Sholler | Sep 2015 | Sep 2030 |
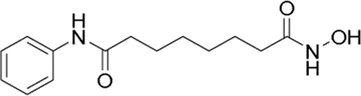
|
| Abexinostat* | pan-HDAC inhibitor | 19.1 nM | Recurrent high grade glioma, anaplastic, astrocytoma, anaplastic, oligodendroglioma, glioblastoma, gliosarcoma | Determine the recommended dose and the side effects of Abexinostat (PCI-24781) with metronomic temozolomide in participants with recurrent high grade glioma | NCT 05698524 |
I | Recruiting | University of Nebraska | Jun 2023 | Mar 2026 |
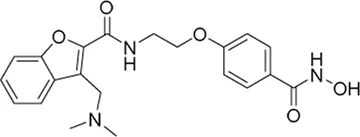
|
| Givinostat# | pan-HDAC inhibitor | 35.02 nM | Duchenne muscular dystrophy | Assess the Pharmacokinetics and Safety of Givinostat in younger DMD Patients | NCT 06769633 |
II | Recruiting | Italfarmaco | Jan 2025 | Dec 2029 |
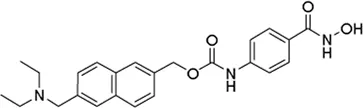
|
| Panobinostat* | pan-HDAC inhibitor(6) | 53.6 nM | Diffuse midline gliomas (DMGs) | Evaluate the feasibility of safely opening the BBB in children with progressive diffuse midline gliomas (DMG) treated with oral panobinostat using focused ultrasound with microbubbles and neuro-navigator-controlled sonication | NCT 04804709 |
I | Active, not recruiting | Cheng-Chia (Fred) Wu | Jul 2021 | Dec 2023 |
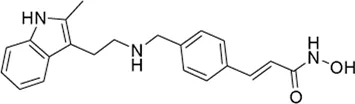
|
| Vorinostat* | Pan-HDAC inhibitor(6) | 4.87 nM | Prostate carcinoma, metastatic prostate adenocarcinoma | Test how well vorinostat works in treating patients with PSMA-low castration-resistant prostate cancer that has spread from where it first started (primary site) to other places in the body (metastatic) | NCT 06145633 |
II | Recruiting | Fred hutchinson cancer center | Sep 2024 | Dec 2025 |
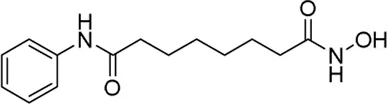
|
| JBI-802 | LSD1/HDAC6/MAO-A inhibitor | 11 nM | Locally advanced solid tumor, metastatic solid tumor | Determine the maximum-tolerated dose (MTD) and recommended Phase 2 dose (RP2D) | NCT 05268666 |
II | Recruiting | Jubilant Therapeutics Inc. | Apr 2022 | Dec 2024 |
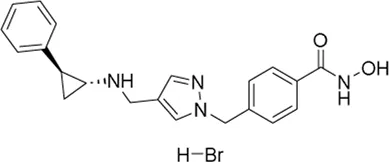
|
| Belinostat* | pan-HDAC inhibitor | 82 nM | Carcinoma, neuroendocrine tumor | Test higher or lower doses of belinostat based on gene variants in people with HGNEC | NCT 06406465 |
II | Recruiting | National Cancer Institute (NCI) | Jun 2025 | Jul 2027 |
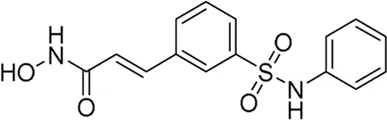
|
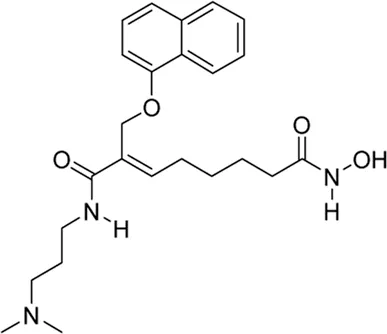
| Ivaltinostat* | pan-HDAC inhibitor | NA | Metastatic pancreatic adenocarcinoma | Assess the efficacy, safety, tolerability, and PK of ivaltinostat in combination with capecitabine and capecitabine monotherapy in patients with metastatic pancreatic adenocarcinoma whose disease has not progressed on a first line fluoropyrimidine-based chemotherapy | NCT 05249101 |
II | Recruiting | CG Pharmaceuticals, Inc. | Aug 2022 | Feb 2026 |
|
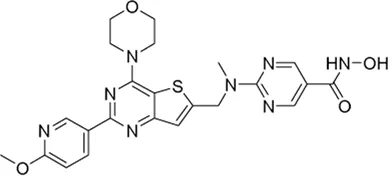
| Fimepinostat# | PI3K/HDAC inhibitor | 27 nM | Cushing disease (CD) | Determine the efficacy and safety of different doses in the treatment of CD and guide dose selection for subsequent, larger studies | NCT 05971758 |
II | Recruiting | University of California, Los Angeles | Jan 2025 | Jan 2029 |
|
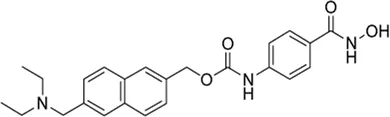
| Givinostat# | Pan-HDAC inhibitor | 35.02 nM | Duchenne muscular dystrophy (DMD) | Investigate the long-term safety, tolerability, and efficacy study of givinostat in DMD patients | NCT 03373968 |
III | Active, not recruiting | Italfarmaco | Oct 2017 | Dec 2029 |
|
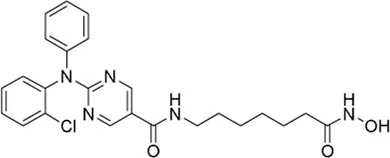
| Citarinostat* | high-selective HDAC6 inhibitor | 2.6 nM | Smoldering multiple myeloma | Test the safety of an investigational intervention and also tries to define the appropriate dose of the investigational intervention to use for further studies | NCT 02886065 |
I | Active, not recruiting | Massachusetts General Hospital | Mar 2017 | Sep 2024 |
|
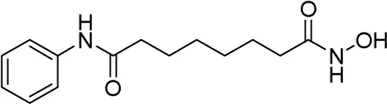
| Vorinostat* | pan-HDAC inhibitor(6) | 4.87 nM | Lymphoblastic leukemia | Improve upon the TINI study treatment test the ability of a type of immunotherapy called blinatumomab to clear persistent leukemia | NCT 05848687 |
II | Recruiting | Tanja Andrea Gruber | Nov 2023 | Dec 2028 |
|
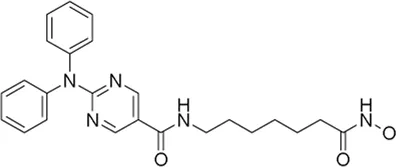
| Ricolinostat* | HDAC6 inhibitor | 6.8 nM | Recurrent chronic lymphoid leukemia (CLL) | Study ricolinostat (ACY-1215) in combination with ibrutinib or idelalisib as a possible treatment for relapsed or refractory CLL | NCT 02787369 |
I | Active, not recruiting | Dana-Farber Cancer Institute | May 2016 | Apr 2026 |
|
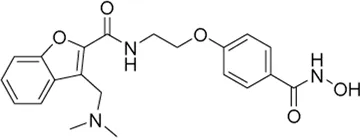
| Abexinostat# | pan-HDAC inhibitor | 19.1 nM | Diffuse large B-cell lymphoma (DLBCL) | Evaluate the efficacy and safety of abexinostat, as monotherapy in patients with relapsed or refractory DLBCL | NCT 03936153 |
II | Recruiting | Xynomic Pharmaceuticals, Inc. | Jan 2020 | Jul 2026 |
|
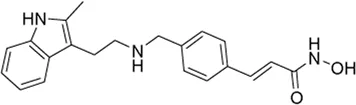
| Panobinostat* | pan-HDAC inhibitor (6) | 53.6 nM | Primary myelofibrosis, polycythemia vera, graft versus host disease, acute myeloid leukemia, thalassemia | It is a long-term safety study for patients that have been treated with either ruxolitinib or a combination of ruxolitinib with panobinostat | NCT 02386800 |
IV | Active, not recruiting | Novartis Pharmaceuticals | Mar 2015 | Sep 2027 |
|
| Belinostat* | Pan-HDAC inhibitor | 82 nM | T-Cell lymphoma | Investigate the efficacy and safety of ITK inhibitor soquelitinib versus physician’s choice standard of care treatment (selected single agent) | NCT 06561048 |
III | Recruiting | Corvus pharmaceuticals, Inc. | Oct 2024 | Nov 2026 |
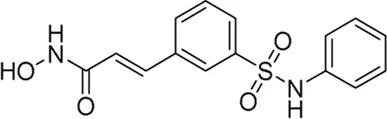
|
| Romidepsin* | pan-HDAC inhibitor | 281 nM | T-Cell lymphoma, relapsed/ refractory T-cell malignancies (TCM) | Test if the combination of romidepsin, CC-486 (5-azacitidine), dexamethasone, and lenalidomide (RAdR) can be given safely to participants with relapsed or treatment refractory TCM | NCT 04447027 |
I | Active, not recruiting | National Cancer Institute (NCI) | Dec 2020 | Jul 2024 |
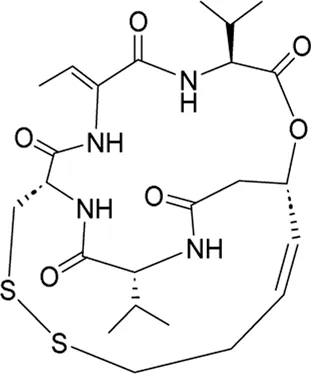
|
| Entinostat* | pan-HDAC inhibitor | 7.35 μM | Lymphoma, malignant solid neoplasm, pancreatic carcinoma/Cancer, non-Hodgkin lymphoma | Tests the safety, side effects, and best dose of entinostat and ZEN003694 in treating patients with solid tumors or lymphoma that has spread to other places in the body (advanced) or does not respond to treatment (refractory) | NCT 05053971 |
II | Recruiting | National Cancer Institute (NCI) | Nov 2022 | Jul 2025 |
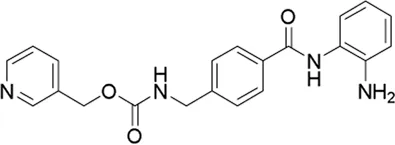
|
| Chidamide* | pan-HDAC inhibitor | >1 μM | Newly diagnosed Philadelphia chromosome-positive acute lymphoblastic Leukemia | Explore the efficacy and safety of ABC regimen(ABC study is a phase 2, single-arm, open-label study of Olverembatinib, CD3/CD19 Bispecific T-cell Engager, and Chidamide in patients with newly diagnosed Philadelphia Chromosome-positive acute lymphoblastic leukemia (Ph + ALL) | NCT 06220487 |
II | Recruiting | Nanfang Hospital, Southern Medical University | Feb 2024 | Dec 2025 |
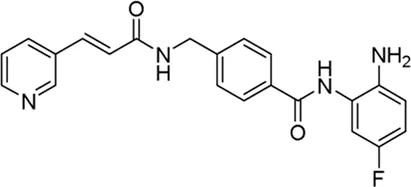
|
Agents of HDAC6 inhibitors in clinical trials.
Note: This Table was generated by searching the keyword “HDAC6 inhibitor” on the website https://clinicaltrials.gov, highlighting representative drugs and disease applications in active or recruiting status, particularly trials initiated since 2020. A small number of pre-2020 trials involving classic inhibitors are included for reference purposes. IC50 values were obtained from the ChEMBL database accessed in 2025. For each compound, data associated with HDAC6 as the target were retrieved. Entinostat is included with a cell-based IC50 value of 7.35 µM due to the absence of single protein data and should not be directly compared. Chidamide (IC50 > 1 µM) indicates low potency at the highest tested concentration. Ivaltinostat lacks published IC50 data for HDAC6 and is marked as unknown or NA. Source: ChEMBL database accessed 2025, and MedChemExpress for JBI-802. Abbreviations DFMO, difluoromethylornithine; DMGs, diffuse midline gliomas; PSMA, prostate-specific membrane antigen; HGNEC, high-grade neuroendocrine carcinomas; DMD, duchenne muscular dystrophy; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; NA, not available; TCM, T-cell malignancies.
The development of HDAC6is for the treatment of AD, on the other hand, has started to show momentum recently with the understanding of the association of HDAC6 in AD and the discovery of new clinically promising HDAC6 inhibitors for AD. Originally authorized for treating cutaneous T-cell lymphoma, pan-HDACi Vorinostat (SAHA) blocks both CD1 and CD2. It has been shown in mouse models to enhance the expression of genes related to memory consolidation by increasing acetylation of histone H4 lysine 12 (H4K12). Then, it improves cognitive function (Peleg et al., 2010). Even though epigenetic drugs have therapeutic potential for AD, the side effects of Vorinostat may limit the application in associated subjects. In vitro studies, where compound 4 exhibited cellular effects in line with targeted inhibition, the designed compound showed high potency (Santini et al., 2024). Pharmacophore study indicated that three primary domains for particular HDAC6is are zinc binding group, surface recognition capping group, and a linker connecting them. Wagner et al. reported a linker-based strategy, BRD9757 as a capless selective HDAC6i, which despite representing high ligand-efficiency (ligE = 0.83), was unable to protect cortical neurons from the deleterious effects of H2O2 (Wagner et al., 2013). Recognizing the surface for binding and brain permeability is mostly achieved by the capping group. We recently created a strong small molecule HDAC6i PB118 based on the structure of a lipophilic capping group, which greatly reduces AD neuropathological changes in mouse microglia model BV2 cells, and our 3D-AD human neural culture models (Mondal et al., 2024). We showed anti-AD multimodal mechanisms of PB118, which may enhance Aβ plaque clearance by upregulating phagocytosis and improve the tubulin/microtubule network by increasing acetylated α-tubulin levels, associated with AD. Furthermore, PB118 significantly lowers pro-inflammatory cytokines and chemokines as well as phosphorated tau levels in cell models linked to AD. These studies warrant future studies focusing on further characterization as well as chemical evolution and validation of the efficacy and safety of PB118 and its analogs for AD. collectively, these findings support that HDAC6 may not only play critical functions in the pathophysiology of AD, but also can offer as a suitable pharmacological target with clinical promise through chemical biology-based drug discovery in AD.
In recent years, FRM-0334 (formerly EVP-0334) emerged as one of the most clinically advanced HDACis investigated for dementia treatment. This brain-penetrant compound, which is Class I and II-selective HDACi, can boost GRN mRNA and PGRN in human GRN−/+ lymphoblasts and in mouse brains. However, the results of the Phase II clinical trial for GRN-related frontotemporal lobar degeneration demonstrated that the compound failed to significantly change pharmacodynamic indicators such as cerebrospinal fluid biomarkers, dementia scores, and FDG-PET (Ljubenkov et al., 2021). The compound was well tolerated, which however displayed an insufficient potency on histone acetylation. The study was discontinued due to the closure of the sponsoring company. A potent Class I and II HDACi, givinostat received an FDA approval for the use of Duchenne muscular dystrophy in 2024, and has been FDA-granted with fast track designation for treating high-risk polycythemia vera earlier this year. Results of the Phase III clinical trial (Mercuri et al., 2024) showed that givinostat was mostly connected to gastrointestinal side effects, including diarrhea (36%) and vomiting (29%), without recorded treatment-related deaths. Reducing the dose revealed no new safety signals following an interim safety analysis. In terms of effectiveness, the drop of the four-stair climb test in the givinostat group was less than in the placebo group. There is another long-term safety and efficacy study presently running (NCT03373968).
Phase I clinical trial of TN-301 (a selective HDAC6i) showed overall well-tolerability and adverse event frequency comparable to placebo in patients with heart failure with preserved ejection fraction (Bexon et al., 2024). Given the neuroprotective potential of HDAC6 inhibition in neurodegenerative diseases, Oryzon Genomics plans to accelerate the creation of its inhibitor ORY-4001 into amyotrophic lateral sclerosis. Besides clinical efforts, several other HDAC6i, such as tubastatin A, ACY-241 (citarinostat), ACY-1215 (ricolinostat), ACY-783, and T-518, have also been tried in animal models for treating neurodegenerative diseases. Efforts have been made by using different mechanisms to increase reduced histone acetylation in the brains of AD animal models. For example, a recent study reported the drug discovery of Tip60 HAT activators as a promising therapeutic neuroepigenetic modulator strategy for AD using the Drosophila AD model. Considering AD-associated reduction of histone acetylation in the brain, the investigators showed the efficacy of Tip60 HAT activators as a therapeutic strategy for AD which restored histone acetylation, improved neurological function and prolonged their lifespan of the Drosophila (Bhatnagar et al., 2025). Further studies will be required to move forward these molecules in clinical translation through additional studies, e.g., the route of administration, blood-brain barrier (BBB) permeability, and off-target toxicity.
Future paths and opportunity
Although HDAC6 has been a well-known regulator of a wide range of biological functions, including clearance and degradation of aggregated proteins, innate immunity and regulation of immunological responses, and neurological pathogenesis, the interest of drug discovery of HDAC6 has been recently switched from the focus on cancer toward AD and other neurological conditions. We speculate that a further understanding of pathological changes of HDAC6 in association with disease progression and clinical evaluation of HDAC6is will be two major areas in the field. These two areas of studies will be critical to not only advancing HDAC6is into clinical trials, but also better predicting the outcome of clinical trials and understanding AD. Below are several specific avenues that we speculate requiring attention to advance HDAC6is toward clinical studies.
1. Studying neuro-immune interactions in combination with HDAC6 pathophysiology to develop highly selective HDACis with therapeutic potential in animal and cellular models. These preclinical studies on the efficacy and safety of HDAC6is may accelerate translational studies toward clinical trials.
2. Understanding HDAC6 dynamic changes throughout different stages in the AD brain. Although positron emission tomography (PET) imaging has been well-utilized to characterize for amyloid and tau pathology changes in AD brains, it remains elusive for HDAC6 changes. It offers an urgent need and valuable opportunity to address HDAC6 changes in the brain with AD progression.
3. Reducing ATN pathology in the AD continuum using clinically promising HDAC6is in combination with potential therapeutics of different mechanisms.
4. Developing BBB-penetrant HDAC6is. It has been a challenge developing BBB-penetrant small molecule drugs for AD. PET imaging has provided a useful to precisely evaluate BBB penetrance of molecules of intertest. Several strategies may be helpful to increase BBB penetrance, e.g., carrier-mediated prodrug strategies targeting the BBB, or delivery systems based on brain-targeted nanoparticles.
5. Employing computational and AI-technology to enhance the accuracy of drug design for HDAC6is. These may include the use to high-resolution crystal structure to facilitate the design of high-affinity and selective compounds, to evaluate the absorption, distribution, metabolism, excretion, and toxicity (ADMET) characteristics of potential drugs methods. The machine learning algorithms offer a potential tool to assess the HDAC6i datasets to extract key features enabling drug discovery.
Conclusion
In summary, recent years have witnessed new insights into AD pathogenesis and FDA-approved new medications offered to the healthcare field of AD, as well as the discovery of new HDAC6 inhibitors. These findings highlight the need and opportunity of future multi-modal studies which may integrate medicinal chemistry, structural biology, biomarker-based patient stratification and precise medicine. Considering the accumulated findings of HDAC6 and the urgent need in the field of AD, we envision that many more new small molecule inhibitors of HDAC6 will be synthesized and move forward soon enabling further preclinical and clinical evaluations as potential therapeutics of AD.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WZ: Writing – original draft, Data curation. YZ: Writing – review and editing. YW: Writing – review and editing. CW: Writing – review and editing, Supervision. CZ: Writing – review and editing, Funding acquisition, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Cure Alzheimer’s Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Alzheimer's Association (2015). 2015 Alzheimer's disease facts and figures. Alzheimer's and dementia11, 332–384. 10.1016/j.jalz.2015.02.003
2
Andrieu S. Coley N. Lovestone S. Aisen P. S. Vellas B. (2015). Prevention of sporadic Alzheimer's disease: lessons learned from clinical trials and future directions. Lancet. Neurology14, 926–944. 10.1016/S1474-4422(15)00153-2
3
Bai P. Mondal P. Bagdasarian F. A. Rani N. Liu Y. Gomm A. et al (2022). Development of a potential PET probe for HDAC6 imaging in Alzheimer's disease. Acta Pharm. Sin. B12, 3891–3904. 10.1016/j.apsb.2022.05.017
4
Bexon M. Argast G. Robertson L. Hoey T. J. V. Tingley W. et al (2024). Phase 1 clinical trial of TN-301, A highly selective HDAC6 inhibitor with potential in HFPEF, shows target engagement. J. Cardiac Fail.30, 295. 10.1016/j.cardfail.2023.10.426
5
Bhatnagar A. Thomas C. M. Nge G. G. Zaya A. Dasari R. Chongtham N. et al (2025). Tip60 HAT activators as therapeutic modulators for Alzheimer's disease. Nat. Commun.16, 3347. 10.1038/s41467-025-58496-w
6
Chen S. Owens G. C. Makarenkova H. Edelman D. B. (2010). HDAC6 regulates mitochondrial transport in hippocampal neurons. PLoS One5, e10848. 10.1371/journal.pone.0010848
7
Choudhary G. Prajapat M. Kaur G. Singh H. Mahendiratta S. Prakash A. et al (2024). Integrated in-silico and in-vitro assessments of HDAC6 inhibitor efficacy in mitigating amyloid beta pathology in Alzheimer's disease. J. Biomol. Struct. Dyn.42, 9720–9730. 10.1080/07391102.2023.2274518
8
Cummings J. Zhou Y. Lee G. Zhong K. Fonseca J. Cheng F. (2024). Alzheimer's disease drug development pipeline: 2024. Alzheimers Dement. (N Y)10, e12465. 10.1002/trc2.12465
9
Feng S. Daw J. N. Chen Q. M. (2020). Histone deacetylase inhibitors prevent H(2)O(2) from inducing stress granule formation. Curr. Res. Toxicol.1, 141–148. 10.1016/j.crtox.2020.10.004
10
Gajendran C. Tantry S. J. M N. S. Mohammed Z. Dewang P. Hallur M. et al (2023). Novel dual LSD1/HDAC6 inhibitor for the treatment of cancer. PLoS One18, e0279063. 10.1371/journal.pone.0279063
11
Govindarajan N. Rao P. Burkhardt S. Sananbenesi F. Schluter O. M. Bradke F. et al (2013). Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer's disease. EMBO Mol. Med.5, 52–63. 10.1002/emmm.201201923
12
Hardy J. Mummery C. (2023). An anti-amyloid therapy works for Alzheimer's disease: why has it taken so long and what is next?Brain a J. neurology146, 1240–1242. 10.1093/brain/awad049
13
Karran E. De Strooper B. (2022). The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat. Rev. Drug Discov.21, 306–318. 10.1038/s41573-022-00391-w
14
Kawaguchi Y. Kovacs J. J. McLaurin A. Vance J. M. Ito A. Yao T. P. (2003). The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell115, 727–738. 10.1016/s0092-8674(03)00939-5
15
Kumar V. Kundu S. Singh A. Singh S. (2022). Understanding the role of histone deacetylase and their inhibitors in neurodegenerative disorders: current targets and future perspective. Curr. Neuropharmacol.20, 158–178. 10.2174/1570159X19666210609160017
16
Lee J. H. Kim H. S. Jang S. W. Lee G. R. (2022). Histone deacetylase 6 plays an important role in TGF-β-induced murine treg cell differentiation by regulating cell proliferation. Sci. Rep.12, 22550. 10.1038/s41598-022-27230-7
17
Li Y. Sang S. Ren W. Pei Y. Bian Y. Chen Y. et al (2021). Inhibition of histone deacetylase 6 (HDAC6) as a therapeutic strategy for Alzheimer's disease: a review (2010-2020). Eur. J. Med. Chem.226, 113874. 10.1016/j.ejmech.2021.113874
18
Livingston G. Sommerlad A. Orgeta V. Costafreda S. G. Huntley J. Ames D. et al (2017). Dementia prevention, intervention, and care. Lancet London, Engl.390, 2673–2734. 10.1016/s0140-6736(17)31363-6
19
Ljubenkov P. A. Edwards L. Iaccarino L. La Joie R. Rojas J. C. Koestler M. et al (2021). Effect of the histone deacetylase inhibitor FRM-0334 on progranulin levels in patients with progranulin gene haploinsufficiency: a randomized clinical trial. JAMA Netw. Open4, e2125584. 10.1001/jamanetworkopen.2021.25584
20
Mercuri E. Vilchez J. J. Boespflug-Tanguy O. Zaidman C. M. Mah J. K. Goemans N. et al (2024). Safety and efficacy of givinostat in boys with Duchenne muscular dystrophy (EPIDYS): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol.23, 393–403. 10.1016/s1474-4422(24)00036-x
21
Mondal P. Bai P. Gomm A. Bakiasi G. Lin C. J. Wang Y. et al (2024). Structure-based discovery of A small molecule inhibitor of histone deacetylase 6 (HDAC6) that significantly reduces Alzheimer's Disease neuropathology. Adv. Sci. (Weinh)11, e2304545. 10.1002/advs.202304545
22
Odagiri S. Tanji K. Mori F. Miki Y. Kakita A. Takahashi H. et al (2013). Brain expression level and activity of HDAC6 protein in neurodegenerative dementia. Biochem. Biophys. Res. Commun.430, 394–399. 10.1016/j.bbrc.2012.11.034
23
Peleg S. Sananbenesi F. Zovoilis A. Burkhardt S. Bahari-Javan S. Agis-Balboa R. C. et al (2010). Altered histone acetylation is associated with age-dependent memory impairment in mice. Science328, 753–756. 10.1126/science.1186088
24
Santini A. Tassinari E. Poeta E. Loi M. Ciani E. Trazzi S. et al (2024). First in class dual Non-ATP-Competitive glycogen synthase kinase 3β/Histone deacetylase inhibitors as a potential therapeutic to treat Alzheimer's Disease. ACS Chem. Neurosci.15, 2099–2111. 10.1021/acschemneuro.4c00061
25
Scheltens P. De Strooper B. Kivipelto M. Holstege H. Chételat G. Teunissen C. E. et al (2021). Alzheimer's disease. Lancet397, 1577–1590. 10.1016/S0140-6736(20)32205-4
26
Selenica M. L. Benner L. Housley S. B. Manchec B. Lee D. C. Nash K. R. et al (2014). Histone deacetylase 6 inhibition improves memory and reduces total tau levels in a mouse model of tau deposition. Alzheimers Res. Ther.6, 12. 10.1186/alzrt241
27
Sevigny J. Chiao P. Bussiere T. Weinreb P. H. Williams L. Maier M. et al (2016). The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature537, 50–56. 10.1038/nature19323
28
Shukla S. Tekwani B. L. (2020). Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front. Pharmacol.11, 537. 10.3389/fphar.2020.00537
29
Tanzi R. E. (2021). FDA approval of aduhelm paves a new path for Alzheimer's Disease. ACS Chem. Neurosci.12, 2714–2715. 10.1021/acschemneuro.1c00394
30
Trzeciakiewicz H. Ajit D. Tseng J. H. Chen Y. Ajit A. Tabassum Z. et al (2020). An HDAC6-dependent surveillance mechanism suppresses tau-mediated neurodegeneration and cognitive decline. Nat. Commun.11, 5522. 10.1038/s41467-020-19317-4
31
van Dyck C. H. Swanson C. J. Aisen P. Bateman R. J. Chen C. Gee M. et al (2023). Lecanemab in early Alzheimer's Disease. N. Engl. J. Med.388, 9–21. 10.1056/NEJMoa2212948
32
Waddell J. Banerjee A. Kristian T. (2021). Acetylation in Mitochondria dynamics and neurodegeneration. Cells10, 3031. 10.3390/cells10113031
33
Wagner F. F. Olson D. E. Gale J. P. Kaya T. Weïwer M. Aidoud N. et al (2013). Potent and selective inhibition of histone deacetylase 6 (HDAC6) does not require a surface-binding motif. J. Med. Chem.56, 1772–1776. 10.1021/jm301355j
34
Wang L. Moreira E. A. Kempf G. Miyake Y. Oliveira Esteves B. I. Fahmi A. et al (2022). Disrupting the HDAC6-ubiquitin interaction impairs infection by influenza and Zika virus and cellular stress pathways. Cell Rep.39, 110736. 10.1016/j.celrep.2022.110736
35
Yang J. Li D. Zhou J. (2020). Histone deacetylase 6 as a therapeutic target in B cell-associated hematological malignancies. Front. Pharmacol.11, 971. 10.3389/fphar.2020.00971
36
Zhang L. Sheng S. Qin C. (2013). The role of HDAC6 in Alzheimer's disease. J. Alzheimers Dis.33, 283–295. 10.3233/jad-2012-120727
Summary
Keywords
Alzheimer’s disease, neurodegenaration, HDAC, HDAC6, drug discovery, translational medicine
Citation
Zhang W, Zhang Y, Wang Y, Wang C and Zhang C (2025) Advancing histone deacetylase 6 (HDAC6) as a promising therapeutic target for Alzheimer’s disease: from molecular insights to clinical prospects. Front. Drug Discov. 5:1662691. doi: 10.3389/fddsv.2025.1662691
Received
09 July 2025
Accepted
15 August 2025
Published
03 September 2025
Volume
5 - 2025
Edited by
Swati S. More, University of Minnesota Twin Cities, United States
Reviewed by
Robin Polt, University of Arizona, United States
Riccardo Petrelli, University of Camerino, Italy
Updates
Copyright
© 2025 Zhang, Zhang, Wang, Wang and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Can Zhang, Zhang.Can@mgh.harvard.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.