- 1Institute of Agroecology and Land Management, National University of Water and Environmental Engineering, Rivne, Ukraine
- 2Institute of Civil Engineering, Warsaw University of Life Sciences–SGGW, Warsaw, Poland
This study investigated the feasibility of passively treating acidic Cr6+ wastewater and reducing the migration of toxic Cr3+ using locally sourced, low-cost minerals from the mining industry, which are either waste products or secondary raw materials. Iron-rich aluminosilicates, quartz-glauconite sand (18% glauconite), volcanic tuff (30% chlorite and 10% pyroxene), and glauconite concentrate (70%) were evaluated in combination with limestone. Cr6+ reduction experiments were conducted at varying dosages (10–50 g/L) of the test materials in batch experiments, with changes in pH (initial 3.2), Eh, and total dissolved solids measured. Natural limestone (0–20 mm), sourced from an active limestone quarry in the Ternopil region, Ukraine, was used to assess its feasibility in reducing the migration of Cr3+ formed during Cr6+ reduction. The results demonstrated that the greatest Cr6+ reduction was achieved using a combination of volcanic tuff and quartz-glauconite sand. Both batch and column studies indicated that limestone effectively reduced Cr3+ concentrations by raising the water’s pH to the range of 7.8–8.2, aiding in its immobilization. Overall, the study confirmed the feasibility of utilizing local mining waste as valuable and cost-effective reagents or adsorbents for the highly toxic Cr6+. These findings enabled the development of practical recommendations for employing iron-rich aluminosilicates in combination with limestone.
Introduction
In nature, chromium is most often found in the trivalent form as the mineral chromite (FeCr2O4) and in the hexavalent form as crocoite PbCrO4 (Das et al., 2021). The presence of Cr6+ and Cr3+ in groundwater may be a consequence of natural and anthropogenic processes occurring in different areas. The natural cause of the occurrence of chromium, biological leaching, and others in the environment can be weathering. Such natural processes do not lead to global pollution of the environment, but they should not be omitted during monitoring and assessing the state of water bodies located near deposits of this metal (Ukhurebor et al., 2021).
The leather industry is one of the most polluting industrial sectors (Gautam et al., 2017; Monira and Mostafa, 2023). Almost every leather industrial plant uses a large amount of chemicals in the processing of raw materials into leather. At each technological stage of a tannery, wastewater with different physical and chemical characteristics is generated (Laxmi and Kaushik, 2020; Xu et al., 2024). Thus, pickling and chrome tanning effluents contain sulphuric acid, chromium, chlorides, sodium bicarbonate and sulphates. Such waters often have a temperature of 25°C. Chrome tanning effluents usually have the lowest pH value, i.e. 3.0 ± 0.5 (Chowdhury et al., 2015). The low pH of such effluents may be due to the addition of sulphuric acid during the pickling step to produce a pickled hide (Gibert et al., 2002).
Chromium is a common pollutant introduced into the soil and natural waters due to the discharge of a variety of industrial wastewaters (Shi et al., 2020). Chromium most often exists in the environment as Cr3+ and Cr6+, and the latter is harmful for people and animals (Han et al., 2025). In living organisms, the reduction of Cr6+ takes place at the presence of Fe2+, which is part of haemoglobin. In this case, adverse biochemical reactions occur inside living cells (James and Bartlett, 1983; Naghipour et al., 2020).
The danger of such pollutant lies in the fact that its removal from groundwater and soil is a very difficult process. The traditional technology of Cr6+ removal from water is related to its reduction using a FeSO4 solution and precipitation (Equations 1, 2), resulting in the creation of an insoluble form of Cr3+. Such Cr6+ reduction should be carried out at a minimum concentration of dissolved oxygen, which can oxidize Fe2+ and thereby reduce the efficiency of the process.
The technology of Cr6+ removal is based on the following chemical reactions (Simon et al., 2002; Zheng et al., 2020).
1.Acidic conditions (pH = 3.0 ± 0.5)
2. Hydroxides of trivalent iron and trivalent chromium are produced at pH = 4.0–5.0
Contaminated groundwater can be treated using various methods such as drilling water wells, pumping the polluted water to ground facilities to perform different chemical reagents of treatment (Gheju, 2018; 2011; Noubactep, 2013; Simon et al., 2002). The system of pumping groundwater to the surface, treating it, and reinjecting it into the ground poses several environmental risks. Firstly, it can disrupt the balance of aquifers, causing a decline in groundwater levels and negatively affecting local ecosystems (Seo et al., 2018). Secondly, there is a risk of re-contaminating the aquifer if the treatment process is not sufficiently effective (Meuser and Meuser, 2013; Simon et al., 2002). Additionally, changes in the physicochemical properties of water during treatment may lead to sedimentation, reducing soil permeability (Reddi et al., 2000; Simon et al., 2002). Finally, high energy consumption and potential disruption of natural processes make this system not always a sustainable solution for the environment (Keely, 2019).
In this context, several studies have been conducted to find effective removal chromate by their immobilization (Eary and Rai, 1991; Liu et al., 2025; Naghipour et al., 2020) presented information about using pure minerals, e. g., natural iron and aluminum (oxy)hydroxides (Ajouyed et al., 2010; Madhusudan et al., 2023) and activated carbons (Mohan and Pittman Jr, 2006) were applied. In addition, natural or secondary polymineral mixtures such as soil, claystone, coal, and peat were also used. Also, suitable adsorbents of chromates were organo-silicates. Clay minerals and zeolites modified with quaternary alkylammonium cations were frequently studied because of their potential application as environmental remediation materials (Brigatti et al., 2000b; Jiang et al., 2018; Naghipour et al., 2020; Yin and Ellis, 2009).
Taking into account the disadvantage’s technology of pumping water, scientific research is focused on the possibility of treatment groundwater directly in the ground using the same treatment principles in situ (Hori et al., 2015; Langlois and James, 2015; Li et al., 2005; Simon et al., 2002). The most promising, affordable and environmentally-economically profitable is the application of iron-based technologies (Cundy et al., 2008; Gheju, 2018; 2011; Konadu-Amoah et al., 2022). Different materials of synthetic and natural origin can be used as Cr6+ reducing agents and such synthetic materials include metallic iron Fe0 (Morrison et al., 2002; Zheng et al., 2020; Zuo et al., 2025). A disadvantage of using Fe0 is that very often, groundwater is weakly acidic and contains carbonates in the form of hydrocarbonates (HCO3−) (Eriksson and Khunakasem, 1966; Lai and Lo, 2008). Soil contaminated with chromium-containing wastewater also has a low pH value. In such conditions, which are additionally anaerobic, Fe0 is quantitatively oxidized to Fe2+ and the environment becomes weakly alkaline (Barker et al., 2018; Constantinou et al., 2023). As presented in paper (Eba et al., 2020; Michiels et al., 2015) FeCO3 was formed on the surface of the Fe0 grain, thereby blocking its ability to further participate in the reduction of Сr6+.
Results of using Fe-rich aluminosilicates for the reduction of Cr6+ by goethite are presented in (Luo et al., 2020; Tomaszewski et al., 2017), by chlorite in (Brigatti et al., 2000a; Brookshaw et al., 2014; Carnicelli et al., 1997; Kohut and Warren, 2018) and by glauconite (Bajda and Kłapyta, 2013; Naghipour et al., 2020). A number of authors also observed the reduction of Cr6+ by Fe(II)-rich minerals (Eary and Rai, 1988; Gould, 1982; Ilton and Veblen, 1994; White and Peterson, 1996). In particular, in paper (White and Peterson, 1996) proposed that structural Fe2+ acts as a stronger reducing agent than aqueous Fe2+ under low pH conditions.
Glauconite concentrate is also an aluminosilicate that contains Fe2+ in its crystal structure (Baldermann et al., 2017; McRae, 1972). This mineral is able to adsorb heavy metals and participate in redox reactions (Chayka et al., 2021; Franus et al., 2019). The peculiarity of the presence of glauconite in natural conditions is that it often occurs in sands (Naghipour et al., 2020). Such sands are very common in Ukraine. They can lie at depths from 0 to 50 m, and the content of glauconite can range from 8% to 50% (Natkaniec-Nowak et al., 2017; Trach et al., 2021a). Glauconite can be extracted from sands using wet or dry magnetic separation (Franzosi et al., 2014; Skiba et al., 2014; Tripathy et al., 2017). The process of enrichment of natural minerals is always an economically expensive process. Its feasibility is always determined by the special values of the natural mineral.
Data of the Eh-pH diagram for chromium-water systems under standard state conditions show that at pH exceeding 5, Cr6+can be transformed into the insoluble form Cr2О3 (Barnhart, 1997). Thus, after the reduction of Cr6+, it is necessary to increase the water pH to reduce the concentration of the formed Cr3+. The use of alkaline reagents, such as NaOH and Ca(OH)2, which are used for this purpose in natural (groundwater, surface water) treatment technologies, is not suitable for technologies applied directly in the environment. This is because when they come into contact with water, the pH of the water rises very quickly. For the environment, a change in pH from 3.5 to nine can lead to various negative phenomena (Antonelli et al., 2017).
Taking into account numerous studies on the properties of natural limestone and its occurrence, the complex solution of the problem of Cr6+ recovery and reduction of the migration of the Cr3+ formed in the environment is of great interest. During the normal operation of the water treatment station, additional use of natural materials is not required. The need to use natural materials may arise in the event of a possible emergency at the treatment water station of an industrial plant. As a result, very large areas of contaminated ground may appear.
The aim of this work was to study the possibility of using inexpensive natural materials of local origin to mitigate the toxic effects of acidic chromium-containing wastewater, which may enter the environment as a result of emergency situations. The relevance of this issue is especially high in the context of Ukraine, where the environmental situation, due to the ongoing war, remains strained in almost all regions, and financial resources for the implementation of complex water treatment technologies are extremely limited.
Materials and methods
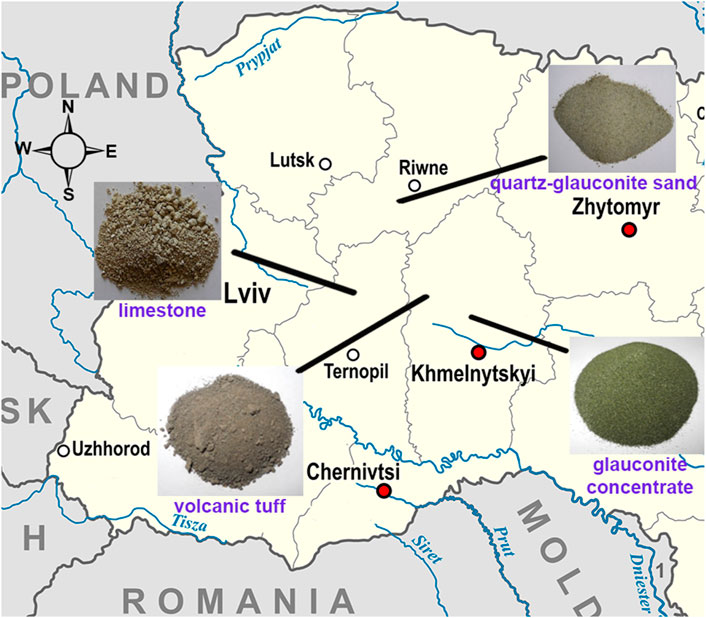
Quartz-glauconite sand, volcanic tuff, and glauconite concentrates were used in experimental studies on the possibility of Cr6+ recovery in acidic waters. Natural limestone was used to increase water pH and decrease the concentration of the formed Cr3+ (as oxide or hydroxide) in the environment. These natural materials have been earlier partially studied and the results have been published.
The studied quartz-glauconite sand contains 15% of glauconite, has grain sizes of 0.1–0.6 mm, and a filtration coefficient of 0.011–0.2 cm/s (9.84–17.28 m/d) (Trach et al., 2021a). Deposits of such sands are not thick (up to 3.5–4.0 m). They very often comprise the overburden, are not used in any way, and stored in special dumps. Their properties were partly studied by (Natkaniec-Nowak et al., 2017; Trach et al., 2021a). As shown in experimental studies performed in static conditions, when such sand is in contact with water (at different initial water pH and mineralization), an increase in the water pH is observed.
Volcanic tuff from the Khmelnitsky region was collected for the experimental study. The volcanic tuff lies at the depth of 0–27 m and has no regional industrial value. Its mineralogical composition is as follows: chlorite 30%, pyroxene 10%, kaolinite 18%, hematite 12%, and quartz 20% (Trach et al., 2021b). It is important to emphasize that among these minerals occurs chlorite and pyroxene, which contain Fe2+, a ion capable of reducing Cr6+.
The glauconite concentrate taken for comparative studies is a commercial product. The product comprises 70% glauconite, 25% sand and 5% limestone. It was purchased at a working quarry in the Khmelnitsky region, Ukraine [https://ua.all.biz/uk/glaukonit-g1619865].
The studied limestone (Ternopil region, Ukraine) is a secondary product of an active limestone quarry and is accumulated every year. According to its petrographic composition, the limestone is very homogeneous. Its properties were partially investigated and the results are presented in the article (Trach et al., 2021c).
In particular sections, the limestone consists of 50%–80% fine crystalline calcite (grain size less than 0.001 mm) and up to 30% of calcite fossils. In some cases, the calcite is more recrystallized and has dimensions between 0.01 and 0.05 mm. The texture is massive, porous and spotted. Recrystallized (dense) limestones are characterized by a CaСО3+MgCO3 content from 85.4% to 99.81% and insoluble residue from 0.89% to 9.5%. The content of magnesium carbonate varies from 0.79% to 3.43%, with an average of 1.91%.
The general appearance of the investigated natural materials and their geographic location are presented in Figure 1.
Study of the recovery Cr6+ by natural materials in static conditions
For conducting experimental studies, the VT volcanic tuff was crushed to the fraction size of less than 0.1 mm. After that, VT, QGS and GC were dried at a temperature of 55°С for 24 h. Knowing in advance that the studied natural materials contain Fe2+, this temperature was selected so that during drying, the oxidation of Fe2+ by air oxygen would not occur.
For the preparation of the solution with a Cr6+ concentration of 0.1 mg/dm3, 99.7% K2Cr2O7 (Kazakhstan) was used. The pH value of the solution was 3.2. Since the low pH value of the tannery wastewater is associated with the presence of H2SO4 in it, the pH value was adjusted with a 0.1 M H2SO4 solution.
The algorithm to carry out the experiment was as follows: masses of 0.25, 0.5, 1.0, 1.5, 2.5 g of VT, QGS and GC were successively added to 50 mL of Cr6+ solutions. Then, the aqueous suspensions were shaken in PVC vials in a shaker at 150 rpm.
The contact of the studied materials with the Cr6+ solution was for 24 h at 20°С. After that, the samples were centrifuged at 5,000 rpm for 10 min and analysed for the content of total chromium and Cr6+.
Determination of total chromium and Cr6+ was carried out using a spectrophotometer V1200 (China) in three repetitions. Determination and control of the significance of pH and TDS of the model solutions were performed using a multimeter (Milwaukee MW802, Rocky Mount, NC, United StatesUnited States). Eh was measured using an ORP meter (Milwaukee MW500 PRO, Rocky Mount, NC, United States).
Determination of Cr6+ was carried out by sequential addition of 1 cm3 of H2SO4 and 2 cm3 of spirit solution of 1,5-diphenylcarbazide.
Determination of the total Cr was performed using successively added chemical reagents, i.e.,: 1M H2SO4 solution, 0.2% (NH4)2S2O8 solution, and 1% AgNO3 solution. After that, the studied water was boiled for 20–25 min (until the excess persulphate was completely decomposed). The solution was evaporated to a volume of approximately 50 cm3, and then, after cooling, transferred to another volumetric flask. 1 cm3 of H2SO4 and 2 cm3 of an ethyl alcohol solution of 1,5-diphenylcarbazide with a mass concentration of 5 g/dm3 were added to the solution (American Public Health Association, American Water Works Association, & Water Environment Federation, 1995).
Determination of Cr3+ was carried out by mathematical calculation of the concentration difference between total chromium and Cr6+.
The method of sampling natural limestones from sites of their occurrence
It is important that the sampling procedure generates a laboratory sample from the parent material that is representative of what was delivered. Such natural materials as limestone and dolomite are difficult to sample due to particle segregation caused by differences in grain size. This is related with the moisture content and variations in the specific gravity of the trace elements of the mineral components. Incorrect sampling may result in the low accuracy of the test results (West, 2012).
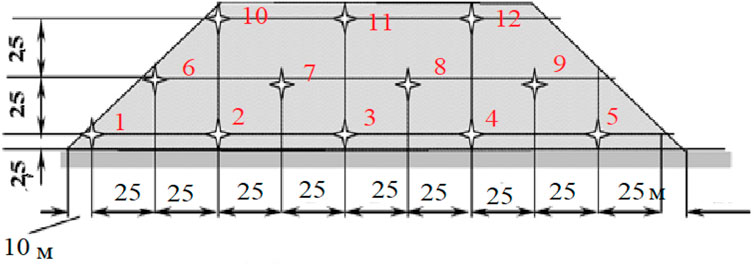
A simple and cheap method of sampling from dumps (without drilling) is the method of collecting samples by the surface scooping method. It provides sufficient accuracy for the experiments. This method is used for testing such minerals as coal, various ores, limestone, etc. (Mladetsky et al., 2019).
Following this method, a series of parallel lines is drawn at a distance of 25 m from each other (the lowest one at a distance of 2.5 m from the base of the dump) over the entire surface of the dump, if it is large enough, for example, 200 × 50 m. In the marked points, located perpendicular to the surface of the dump, pits were dug with a shovel to the depth of about 0.5 m and limestone samples were taken from the bottom of the pits for experimental studies. Such scheme presents at Figure 2.
Grain size and fineness modulus of the studied limestone
Determination of the grain-size distribution of the studied natural limestone was carried out in accordance with the DSTU B B.2.1-19: 2009 method. Its essence was to determine the quantitative (mass) distribution of grains of limestone by their size during sieving.
Fineness modulus (FM) is a numerical index of fineness, giving some idea of the mean size of the grains in the entire natural material. To a certain extent, this is a method of standardizing the aggregate grading. Determining the modulus of fineness allows quantifying the average size of natural materials. It is determined by adding the percentage weight of the material retained on each of the standard sieves and dividing it by 100.
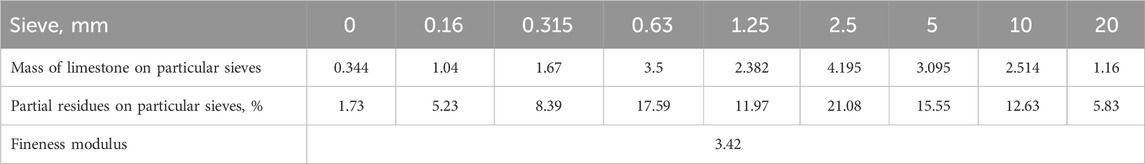
The FM of the studied limestone was determined according to the GOST 8735-88 method. A limestone sample with a mass of 1 kg was sieved at mesh sizes of 20 mm, 10 mm, 5 mm, 2.5 mm, 1.25 mm, 0.63 mm, 0.315 mm, and 0.16 mm. The obtained masses were fixed in percent from 1 kg remaining on the corresponding sieves and the calculation of the modulus of size was carried out according to the following formula:
where Q is the obtained mass of the samples on 7 sieves as a percentage of the total mass of the original sample.
Bulk density
Bulk density is the mass per unit volume of a loose powder. The unit volume includes the voids between the particles, and the inter-particle void volumes. Bulk density can be calculated using formula (“Ukrainian Standard DSTU B V. 2.1–3–96. In Soils. Laboratory Tests. General Provisions,” 1997):
where M - mass in grams; V - apparent volume in millilitres.
Determination of the filtration coefficient
The parameters of water filtration through the studied limestone at different fractions were set to determine their ability to be used as the reaction material for reaction-filtration barriers.
The device for determining the filtration coefficient is KF-OOM. It is intended for the determination of the coefficient of filtration for the sandy soil (State standard specification 25584-90) at a constant gradient from 0 to 1. Determination of the filtration coefficient of limestones was performed in accordance with the requirements of DSTU B B.2.1-23: 2009“Methods of laboratory determination of the filtration coefficient.”
The filtration coefficient (Kf, m/day) was calculated according to the formula:
where Q is the constant water consumption, m3/day; F–cross-sectional area of the cutting cylinder, m2; I–hydraulic gradient; t is water temperature, °C.
Kinetics of Cr3+ removal by the studied natural limestone
The studied limestone with a particle size of 0–20 mm was sieved on a 0.315 mm sieve. The obtained sample, in which the fraction size was less than 0.315 mm, was taken for experimental research. It was dried at 105°C to a constant weight.
The Cr3+ concentration was 1.5 mg/dm3 in the model solution. CrCl3·6H2O and distilled water were taken for preparing the solution. For the experimental studies, solutions of pH 3.2 and pH 5 were used. 0.1M HCl and 0.1M NaOH were taken for the pH correction of the model solution.
The algorithm for conducting the experimental study was as follows. Limestone at a dose of 0.06 g/dm3 was added to 50 mL of the Cr3+ solution. Then the aqueous suspensions were shaken in PVC vials in a shaker at 150 rpm and 20°С. After the contact time of the limestone with the Cr3+ solution, the solution sample was centrifuged at 5,000 rpm for 10 min and the Cr3+ concentration was analysed.
Removal of Cr3+ by limestone filtration in dynamic conditions
The limestone with particle size 0–20 mm was scattered on sieves and two particle size sets at 0.16–20 mm and 1.25–20 mm were obtained. The column experiment was performed at the following conditions: particle size (0–20.0 mm, 0.16–20 mm, 1.25–20 mm) and column dimensions (height-150 mm; diameter-80 mm). Through such filtration columns, water with Cr3+ was filtered at the speed of 0.5 and 1.5 m/h.
The concentration of Cr3+ was 1.5 mg/dm3 in the model solution. CrCl3·6H2O and distilled water were taken for preparing the solution.
Results
The results of the experimental studies showed that at the same initial conditions (С = 0.1 mg/dm3, pH 3.2, TDS = 550 mg/dm3 and the same doses of the applied materials), the effectiveness of Cr6+reduction to Cr3+ by the studied natural materials was different. The lowest efficiency reduction of Cr6+, i.e. 10%, was at the contact of QGS at 0.5 g/50 mL. GC showed the highest efficiency of 86%. The results of the experimental studies are presented in Figure 3.
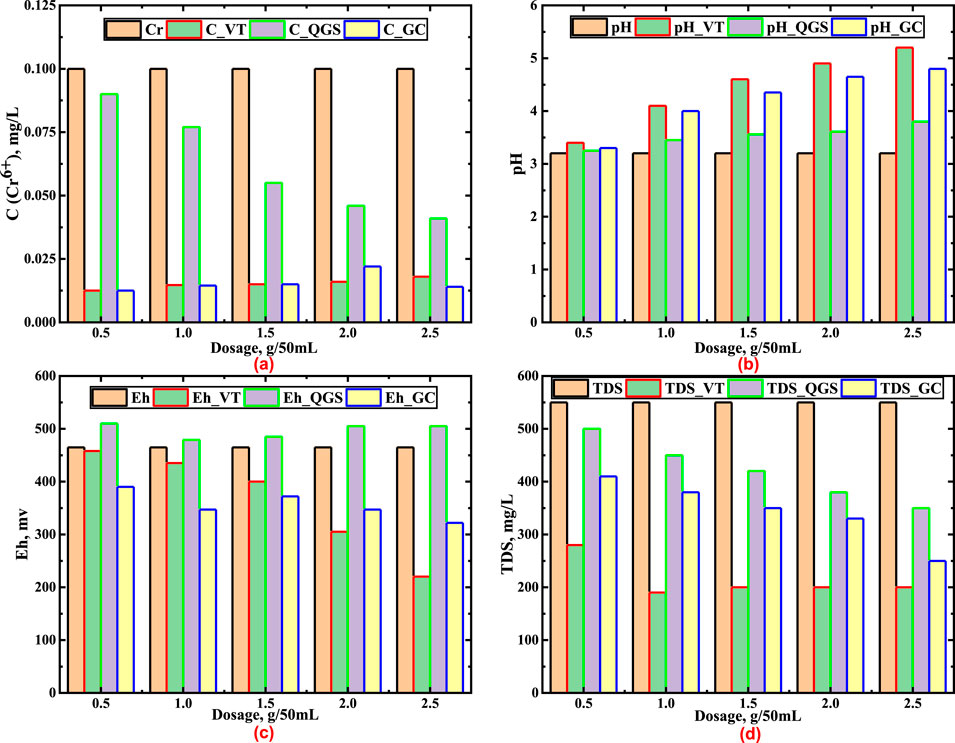
Figure 3. (a) Comparison of changes in Cr6+ concentration (Cr6+), (b) Comparison of changes in pH, (c) Comparison of changes in Eh (mV), (d) Comparison of changes in TDS (mg/L).
Moreover, after 24 h of contact of the studied solution with natural materials, the pH, Eh and TDS were determined. This was done in order to evaluate the change of water parameters after Cr6+ reduction. Figure 3 shows that the pH increase of the solution depended on the doses and type of the applied natural materials. Regarding the increased pH, this process is very important due to the need for the formation of inactive chromium (Cr3+). With increased doses of VT, QGS, GK and at a constant volume of the solution, the pH had always increased. The highest ΔpH = 2 was after the contact with VT, the lowest ΔpH = 0.6 after the contact with QGS.
Determination of the Eh of the study solutions has shown the following. After reduction of Cr6+, the value of Eh increased after the contact with QGS, and decreased after the contact with GC and VT (Figure 3). Comparing ΔEh, at increasing dose of VT and GC, the ΔEh was greater for VT. For example, at a dose of 2.5 g/50 mL of VT, the ΔEh was −245, and for GC the ΔEh was −143.
An important water parameter that was monitored after Cr6+ reduction by the tested materials was TDS. The obtained results are graphically presented in Figure 3. The analysis of this chart shows that after 24 h of contact, TDS decreased in all cases, thus showing that the reduction of Cr6+ to Cr3+ was taking place. Hexavalent chromium was in the form of the anion CrО42-, and then turned into the cationic form Cr3+. Therefore, there is a decrease of the mass of the inorganic form of chromium. A higher TDS decrease was observed at the highest dose of VT (2.5 g/50 mL). This was obviously associated with the increase of the solution pH to 5.2, at which the formed Cr3+ partially passed into the inactive form Barnhart (1997).
Simultaneously with the determination of the Cr6+ concentration in each solution, the Cr3+concentration was also determined. Its highest concentration was in the solution after contact with GC, and the lowest after contact with QGS. As shown in Figure 3, the highest efficiency of Cr6+ reduction was for GC at 2.5 g/50 mL, compared to VT in the same conditions. However, the concentration of the formed Cr3+ was significantly lower in the solution that was in contact with 2.5 g/50 mL of VT. This is obviously related to the increase of the solution pH after contact with VT.
After natural limestone sampling, according to the above method, its grain composition and grain size module were determined. Equations 3, 4 was used for the calculation. The results are presented in Table 1.
The next stage in the study of the filtration properties of the investigated limestone was the determination of the filtration coefficient according to the above method. Thus, the filtration coefficient of natural limestone 0–20 mm was Kf = 1.54 m/day; the bulk density was 1,400.5 g/cm3, and the porosity was 48.13%.
The analysis of the grain-size composition of the limestone studied showed that fractions 0–0.16 mm contribute to less than 1.73%. When such limestone comes into contact with acidic water, it will be neutralized and its smallest fractions will dissolve. Thus, over time, the filtration ratio will increase. Therefore, the authors have determined the limestone filtration coefficients for fractions 0.16–20 mm and 1.25–20 mm. For 0.16–20 mm the Kf was 3.9 m/day, the bulk density was 1,394.1 g/cm3, and porosity was 52.31%.
It was experimentally established that in the studied limestone about 70% of the fractions are 1.25–20 mm in size. The filtration coefficient of this fraction was Kf = 12.92 m/day, the bulk density was 1,381.6 g/cm3, and porosity was 58.39%. According to the classification of engineering soils following DSTU B V.2.1-2-96, such limestone belongs to highly permeable media.
During determination of the filtration coefficients for fractions 0–20 mm and 0.16–20 mm by KF-OOM, karst-suffusion process are manifested (Figure 4). Under the pressure of water, particles of calcium carbonate are removed from the filtration column. As a result of the mechanical removal of small particles by the water flow, the silt fraction of limestone I accumulated at the bottom of the outer casing of the device. As a result of suffusion of small limestone particles, a significant shrinkage of 3–5 mm is visible in the inner cup at the height of 100 mm, which corresponds to 3%–5%. Karst holes with a diameter of about 1–3 mm are visible on the surface of the filtered, finely dispersed limestone.

Figure 4. General view of the process of determining the filtration coefficient by KF-OOM and the manifestation of the karst-suffusion process.
Kinetics of Cr3+ removal by limestone in liquid media in static conditions
The further stage of the study was the determination of Cr3+ removal by natural limestone (d ˂ 0.315 mm) in static conditions. It is commonly known than for reduction of the acidity of surface waters, the most frequently used doses of fine-grained limestone are in the range of 750–1,000 kg/ha (for a typical depth of natural lakes 1.0–1.2 m) (Henrikson and Brodin, 1995; Trach et al., 2021c). The dose of limestone depends on the water pH and the type of sediment in the reservoir bottom. In terms of mass concentration, the dose of limestone is approximately 0.025–0.1 g/dm3. For these experimental studies, the average dose of limestone was the mean value, i.e. 0.06 g/dm3. It can be seen in Figure 3 that during the reduction of Cr6+ by different doses of VT, GS, GK, the solution pH either remained unchanged or increased to 5.2. Thus, the next experimental studies of Cr3+ decreased concentration by limestone were carried out at pH levels of 3.2 and 5.2. The results are presented in Figure 5.
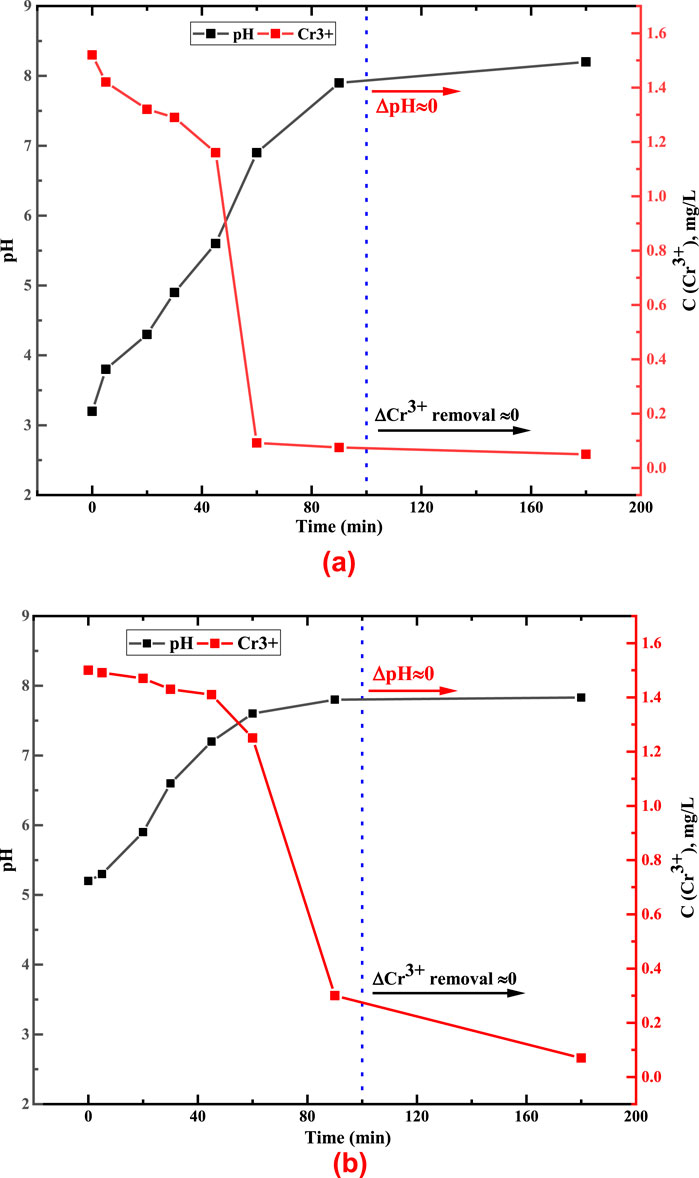
Figure 5. Kinetics of changes in Cr3+ concentration over time depending on the initial pH of the water solution (a) pH 3.2 (b) pH 5.2.
This study shows that the decrease in the Cr3+ concentration over time, when in contact with limestone, depends on the increase of the solution pH. Thus, at initial pH = 3.2, a higher decrease in the concentration of this cation occurred at pH = 8.2 after 90 min of contact. At initial pH = 5.2, the decrease in the Cr3+ concentration was after 90 min at pH = 7.8. This confirms that Cr3+ can remain in water in an ionic form in an acidic or neutral environment. In a weakly alkaline aqueous solution, its concentration reaches insignificant values (< 0.06 mg/dm3).
Kinetics of Cr3+ removal by limestone in dynamic conditions
The process of decrease in the Cr3+ concentration was studied in the filtration column, the parameters of which are presented in the Methods section. For experimental studies, three fractions of limestone were taken, i.e., 0–20 mm, 0.16–20 mm and 1.25–20 mm. The filtering speed was at 0.5 and 1.5 m/h.
During the filtration, changes in the Cr3+ concentration and pH were determined. Thus, when filtering water at pH = 3.2 at the speed of 0.5 m/h through limestone in the fraction of 0–20 mm, the largest increase in pH was up to 7.7 after 4 h. The Cr3+ concentration also stopped changing 4 h after the start of the filtration and reached 0.08 mg/dm3. The efficiency of cation removal was 94.6%. When filtering water at pH = 5.2 at the same rate, the ΔpH was 2.1 and did not increase further. The Cr3+ concentration at chemical equilibrium was 0.15 mg/dm3, i.e., the efficiency of cation removal was 91.6%. Since these two water indicators stopped changing simultaneously after 5.5 h, it can be argued that this is the time when the chemical equilibrium was reached.
The experimental studies showed the following dependencies. The pH increase of the filtered water depended on the filtration range and the grain size of the filtering material. The lowest efficiency of Cr3+ concentration decrease was observed when filtering water at pH = 5.2 through limestone in the fractions of 1.25–20 mm at a rate of 1.5 m/h.
It should be emphasized that the following phenomena were observed during the experimental studies of water filtration through a laboratory filter column. When filtering water at pH = 3.2, the very fine fraction of the studied limestone was introduced into the suspension and dissolved. The filter column became clear very quickly. When filtering water at pH = 5.2, the fine fraction also entered the suspension, but did not dissolve as quickly. The filter column became clearer much later than when filtering water at pH = 3.2.
Discussion
The rate of Fe2+release is linked with the mineral structure, the strength of the covalent or ionic bond, environmental degradation, particle size, moisture, and the soil pH (Fu et al., 2018). Chlorite, as an iron-rich aluminosilicate occurring in the volcanic tuff studied, is formed mainly as a result of metamorphic changes in biotite, phlogopite, hornblende, and augite. The rate of Fe2+ release is higher in the case of phlogopite and biotite in neutral conditions due to the fusion between soil water and minerals (Feigenbaum et al., 1981). The release of Fe2+ from clay minerals, such as glauconite, takes place at a slower rate under acidic conditions (Baldermann et al., 2017).
Glauconite crystals are characterized by a high content of K+ and Fe2+ (mica layers) and a low content of Fe3+ (smectite layers) (McRae, 1972; Yamashita et al., 2019). The reduction of hexavalent chromium, in contact with this mineral, is due to the presence of Fe2+ in the octahedral layer. Reports suggest that K+ may be bioavailable from glauconite in soil (Rudmin et al., 2020; 2019). This is due to the slightly acidic environment. Since Fe2+ is in the same octahedral leaf, Fe2+ is available at the same time.
GC and VT had a similar effect on Cr6+ reduction. With approximately the same efficiency of hexavalent chromium reduction by glauconite concentrate and volcanic tuff, the increase in the pH value of the solution was different.
This difference in the pH change of the solutions can be explained by the difference in the mineralogical composition of glauconite concentrate and volcanic tuff. As present in (Trach et al., 2021b), volcanic tuff contained up to 10% CaCO3 (calcite). In an acidic solution, this compound is dissolved and thus increases the pH value (Trach et al., 2021c). Regulation (increase) of pH values of the solution, after Cr6+ recovery, is one of the main objective goals of the studied process, since the resulting pH of the solution is one of the most important factors affecting the mobility and migration of Cr3+ in the environment.
The results of experimental studies have shown that the efficiency of Cr6+ reduction was the lowest in contact with quartz-glauconite sand. This is due to the low content (15%) of glauconite. To increase the ability to reduce hexavalent chromium by quartz-glauconite sand, it would be advisable to add volcanic tuff to this medium. VT cannot be used on its own, as it contains a significant amount of clay minerals (Trach et al., 2021b). Their presence hampers water filtration by volcanic tuff.
To increase the efficiency of Cr6+ reduction, the studied glauconite concentrate can also be added to the quartz-glauconite sand. At the same time, the cost of glauconite concentrate should be taken into account. The studied volcanic tuff occurs on the ground surface and has no regional industrial value. Given the proximity of volcanic tuff deposits and quartz-glauconite sands, this can result in a high ecological and economic effect of their application. For Cr6+ reduction, it is advisable to install permeable reactive barriers (PRB) when using Fe-rich aluminosilicates and quartz-glauconite sand.
It is important to note that today there are technologies of Fe3+ reduction to Fe2+ in PRBs. In such technologies, the redox altering reagent is often sodium dithionite (Na2S2O4) (Drits and Manceau, 2000). The dithionite ion is a strong reductant, particularly in strongly alkaline solutions. Reduction reactions with the dithionite anion proceed in two steps: dissociation of the dithionite ion to form a two sulphoxyl radical anion (SO2−); the reaction of these radicals with the oxidized species Fe3+ yields a reduced species Fe2+ and oxidized sulphite (SO32–) or bisulphite (HSO3−). Thus, the use of such materials can be long-term and have an ecological and economic effect.
The peculiarity of the mechanism of Cr6+ reduction, which is in anionic form, is that it passes into Cr3+, which is in cationic form. As commonly known, the extraction of this cation from water is possible by sorption with the help of natural materials, in particular aluminosilicates (Aziz et al., 2008; Laxmi and Kaushik, 2020; Trach et al., 2021b) and by conversion into a stable form of Cr2О3/Cr(OH)3 due to increase in the pH value of the aqueous medium (Barnhart, 1997).
The sorption process can only take place up to a certain point, i.e., until the sorbent’s sorption capacity is exhausted. After that, sorption cannot occur and the desorption process begins. Moreover, the desorption of heavy metals from the sorbent’s surface is possible when the physical and chemical indicators of water quality change (Belousov et al., 2019). Based on this, it can be concluded that the formation of the immobile form Cr3+ is relatively more reliable than its sorption by natural materials. Thus, in this work, experimental studies on the use of natural limestone (CaCO3) were conducted to decrease the migration of Cr3+ in the environment. The use of natural limestone to neutralize or increase the water pH to 7.8–8.2 is a well-known and very common practice in the world (Angeler et al., 2017; Bernes, 1991; Trach et al., 2021c; Yi et al., 2017). This process occurs due to the dissociation of CaCO3 in water and its decomposition into ions (Ca2+ and CO32-). After dissociation, the formed Ca2+ ions are hydrolysed, and CaOH+ and Ca(OH)2 are formed. CO32- anions undergo proton reactions, and form HCO3− and H2CO3.
Considering the above, when natural limestone is added to water containing Cr3+, the following chemical processes occur:
The possibility to decrease Cr3+ concentration by naturally crushed limestone in static conditions, as well as the filtration properties and removal of this cation in dynamic conditions, were studied in this work. The results of such experimental studies have shown its high efficiency. Natural limestone can be used in different ways to achieve the set purpose. The method of its application depends on the topography of the contaminated area. The formed Cr3+ together with the surface runoff can migrate to the lowest point of the terrain and accumulate there. Very often, lakes form in the lowest points of the terrain (Born et al., 1979). The source of their nutrition is surface runoff and atmospheric precipitation. To solve the problem, natural crushed limestone can be used by direct introduction into the polluted lake. The scheme of the possible application of quartz-glauconite sand, volcanic tuff and naturally crushed limestone, taking into account the topographic features of the contaminated area, is presented in Figure 6A.
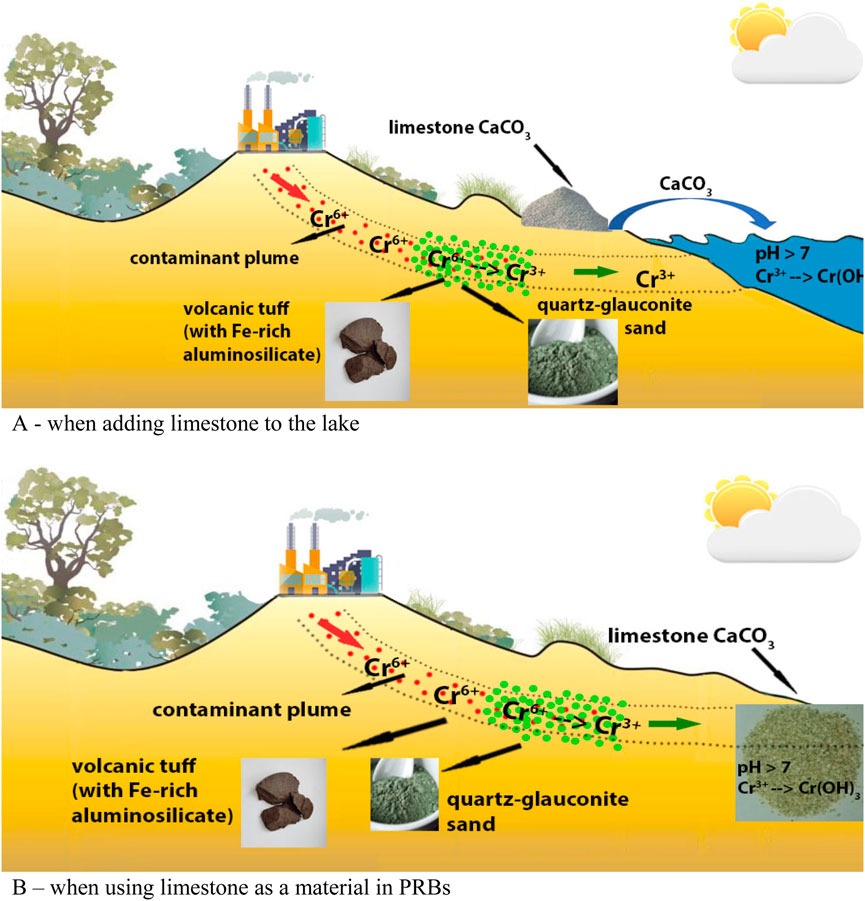
Figure 6. Scheme of the application of the studied Fe-rich natural materials with natural limestone: (A) when adding limestone to the lake; (B) when using limestone as a material in PRBs.
The second option of using limestone is possible by organizing semi-permeable reaction barriers. The scheme of the application of the studied natural materials located close to the contaminated site with a slight difference in relief is presented in Figure 6B.
In practice, it is commonly known that natural limestone can be used as a reaction material of PRB for the extraction of Fe, Mn and neutralization of acidic waters (Wang et al., 2016). The article reported that an increase in water pH and a decrease in the concentration of these pollutants was observed during the three observation years. The use of PRB with limestone is also presented in (Amos and Younger, 2003). It was used to neutralize acidic mine waters and treat coal mine leachates. The filter material was composed of 50% limestone grains at 10 mm and 50% gravel. Such filter material showed that the maximum alkalinity, acidity reduction and heavy metal removal were achieved within 24 h. Due to its filtration properties, natural limestone is used to reduce the concentration of SO42- in acidic mine waters. When such waters come into contact with limestone, partial dissolution of CaСО3 occurs and, as a result, an increase in the water pH and the formation of poorly soluble CaSO4 (O et al., 2002).
Conclusion
In the event of a possible emergency situation in industrial enterprises, it is important to study local deposits of natural materials. In general, it is necessary to have information about possible existing mining waste (overburden, secondary products). Such natural materials can be environmentally friendly and economically beneficial. Thus, to ensure the recovery of Cr6+ and reduce the migration of the formed Cr3+, it is advisable to use iron-containing aluminosilicates mixed with sand and natural limestone.
The results of experimental studies are of great environmental importance in solving the problem of possible emergencies in industrial enterprises that produce wastewater with a high content of hexavalent chromium. This is mainly due to the fact that all the studied natural materials are located close to each other, being waste or by-products of the mining industry.
The studied quartz-glauconite sand, volcanic tuff and glauconite concentrate showed that at pH = 3.2 for 24 h they are able to reduce the Cr6+ concentration. Glauconite concentrate and volcanic tuff showed same efficiency of Cr6+ reduction, while quartz-glauconite sand showed the lowest efficiency. After Cr6+ reduction in all solutions studied, the presence of Cr3+ was established. Its lowest concentration was in a solution, in which Cr6+ was reduced by volcanic tuff. This is due to the fact that the composition of volcanic tuff includes minerals such as kaolinite, calcite, which are able to increase the pH of the solution.
To reduce the migration of the formed Cr3+ in the environment, the properties of natural limestone (0–20 mm), which is a secondary product of limestone quarries, were studied. Experimental studies have shown the ability of limestone to reduce the Cr3+ concentration in static and dynamic conditions. Thus, due to the chemical characteristics and ability to filter water (for 0–20 mm Kf 1.54 m/day, for 0.16–20 mm Kf 3.9 m/day, for 1.25–20 mm Kf 12.92 m/day), the studied accumulated limestone is potentially suitable for the construction of PRBs for the treatment of surface and groundwater or for direct application in surface water bodies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ZVI, zerovalent iron; QGS, quartz-glauconite sand; VT, volcanic tuff; GC, glauconite concentrates; HM, heavy metal; TDS, total dissolved solids, mg/L; FM, fineness modulus; Kf, filtration coefficient, m/d; PRB, permeable reactive barrier; Cr3+, trivalent chromium; Cr6+, hexavalent chromium.
References
Ajouyed, O., Hurel, C., Ammari, M., Allal, L. B., and Marmier, N. (2010). Sorption of Cr (VI) onto natural iron and aluminum (oxy) hydroxides: effects of pH, ionic strength and initial concentration. J. Hazard. Mater. 174, 616–622. doi:10.1016/j.jhazmat.2009.09.096
American Public Health Association, American Water Works Association, & Water Environment Federation. (1995). Standard methods for the examination of water and wastewater (19th edn). Washington, DC: American Public Health Association.
Amos, P. W., and Younger, P. L. (2003). Substrate characterisation for a subsurface reactive barrier to treat colliery spoil leachate. Water Res. 37, 108–120. doi:10.1016/S0043-1354(02)00159-8
Angeler, D. G., Drakare, S., Johnson, R. K., Köhler, S., and Vrede, T. (2017). Managing ecosystems without prior knowledge: pathological outcomes of lake liming. Ecol. Soc. 22, art44. doi:10.5751/ES-09794-220444
Antonelli, M., Wetzel, C. E., Ector, L., Teuling, A. J., and Pfister, L. (2017). On the potential for terrestrial diatom communities and diatom indices to identify anthropic disturbance in soils. Ecol. Indic. 75, 73–81. doi:10.1016/j.ecolind.2016.12.003
Aziz, H. A., Adlan, M. N., and Ariffin, K. S. (2008). Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresour. Technol. 99, 1578–1583. doi:10.1016/j.biortech.2007.04.007
Bajda, T., and Kłapyta, Z. (2013). Adsorption of chromate from aqueous solutions by HDTMA-modified clinoptilolite, glauconite and montmorillonite. Appl. Clay Sci. 86, 169–173. doi:10.1016/j.clay.2013.10.005
Baldermann, A., Dietzel, M., Mavromatis, V., Mittermayr, F., Warr, L. N., and Wemmer, K. (2017). The role of Fe on the formation and diagenesis of interstratified glauconite-smectite and illite-smectite: a case study of Upper Cretaceous shallow-water carbonates. Chem. Geol. 453, 21–34. doi:10.1016/j.chemgeo.2017.02.008
Barker, R., Burkle, D., Charpentier, T., Thompson, H., and Neville, A. (2018). A review of iron carbonate (FeCO3) formation in the oil and gas industry. Corros. Sci. 142, 312–341. doi:10.1016/j.corsci.2018.07.021
Barnhart, J. (1997). Chromium chemistry and implications for environmental fate and toxicity. J. Soil Contam. 6, 561–568. doi:10.1080/15320389709383589
Belousov, P., Semenkova, A., Egorova, T., Romanchuk, A., Zakusin, S., Dorzhieva, O., et al. (2019). Cesium sorption and desorption on glauconite, bentonite, zeolite and diatomite. Minerals 9, 625. doi:10.3390/min9100625
Born, S. M., Smith, S. A., and Stephenson, D. A. (1979). Hydrogeology of glacial-terrain lakes, with management and planning applications. Contemp. Hydrogeol. George Burke Maxey Meml. 43, 7–43. doi:10.1016/0022-1694(79)90163-X
Brigatti, M. F., Franchini, G., Lugli, C., Medici, L., Poppi, L., and Turci, E. (2000a). Interaction between aqueous chromium solutions and layer silicates. Appl. Geochem. 15, 1307–1316. doi:10.1016/S0883-2927(99)00120-1
Brigatti, M. F., Lugli, C., Cibin, G., Marcelli, A., Giuli, G., Paris, E., et al. (2000b). Reduction and sorption of chromium by Fe (II)-bearing phyllosilicates: chemical treatments and X-ray absorption spectroscopy (XAS) studies. Clays Clay Min. 48, 272–281. doi:10.1346/ccmn.2000.0480214
Brookshaw, D. R., Coker, V. S., Lloyd, J. R., Vaughan, D. J., and Pattrick, R. A. D. (2014). Redox interactions between Cr(VI) and Fe(II) in bioreduced biotite and chlorite. Environ. Sci. Technol. 48, 11337–11342. doi:10.1021/es5031849
Carnicelli, S., Mirabella, A., Cecchini, G., and Sanesi, G. (1997). Weathering of chlorite to a low-charge expandable mineral in a spodosol on the apennine mountains, Italy. Clays Clay Min. 45, 28–41. doi:10.1346/CCMN.1997.0450104
Chayka, O., Petrushka, I., Ruda, M., Paranyak, N., and Matskiv, O. (2021). The minimization of impact of oil pollution on soils in the area of railways using glauconite. J. Water Land Dev. 79–84. doi:10.24425/jwld.2021.137099
Chowdhury, M., Mostafa, M. G., Biswas, T. K., Mandal, A., and Saha, A. K. (2015). Characterization of the effluents from leather processing industries. Environ. Process. 2, 173–187. doi:10.1007/s40710-015-0065-7
Constantinou, D., Samanides, C. G., Koutsokeras, L., Constantinides, G., and Vyrides, I. (2023). Hydrogen generation by soluble CO2 reaction with zero-valent iron or scrap iron and the role of weak acids for controlling FeCO3 formation. Sustain. Energy Technol. Assess. 56, 103061. doi:10.1016/j.seta.2023.103061
Cundy, A. B., Hopkinson, L., and Whitby, R. L. D. (2008). Use of iron-based technologies in contaminated land and groundwater remediation: a review. Sci. Total Environ. 400, 42–51. doi:10.1016/j.scitotenv.2008.07.002
Das, P. K., Das, B. P., and Dash, P. (2021). Chromite mining pollution, environmental impact, toxicity and phytoremediation: a review. Environ. Chem. Lett. 19, 1369–1381. doi:10.1007/s10311-020-01102-w
Drits, V. A., and Manceau, A. (2000). A model for the mechanism of Fe3+ to Fe2+ reduction in dioctahedral smectites. Clays Clay Min. 48, 185–195. doi:10.1346/CCMN.2000.0480204
Eary, L., and Rai, D. (1988). Chromate removal from aqueous wastes by reduction with ferrous ion. Environ. Sci. Technol. 22, 972–977. doi:10.1021/es00173a018
Eary, L. E., and Rai, D. (1991). Chromate reduction by subsurface soils under acidic conditions. Soil Sci. Soc. Am. J. 55, 676–683. doi:10.2136/sssaj1991.03615995005500030007x
Eba, H., Takahashi, M., and Sakurai, K. (2020). Progress of hydrogen gas generation by reaction between iron and steel powder and carbonate water in the temperature range near room temperature. Int. J. Hydrog. Energy 45, 13832–13840. doi:10.1016/j.ijhydene.2020.03.087
Feigenbaum, S., Edelstein, R., and Shainberg, I. (1981). Release rate of potassium and structural cations from micas to ion exchangers in dilute solutions. Soil Sci. Soc. Am. J. 45, 501–506. doi:10.2136/sssaj1981.03615995004500030012x
Franus, M., Bandura, L., and Madej, J. (2019). Mono and poly-cationic adsorption of heavy metals using natural glauconite. Minerals 9, 470. doi:10.3390/min9080470
Franzosi, C., Castro, L. N., and Celeda, A. M. (2014). Technical evaluation of glauconies as alternative potassium fertilizer from the salamanca formation, patagonia, southwest Argentina. Nat. Resour. Res. 23, 311–320. doi:10.1007/s11053-014-9232-1
Fu, J., Wang, C., Chen, X., Huang, Z., and Chen, D. (2018). Classification research and types of slow controlled release fertilizers (SRFs) used-a review. Commun. Soil Sci. Plant Anal. 49, 2219–2230. doi:10.1080/00103624.2018.1499757
Gautam, S., Kaithwas, G., Bharagava, R., and Saxena, G. (2017). “Pollutants in tannery wastewater: pharmacological effects, and bioremediation approaches for human Health protection and environmental safety,” in Environmental pollutants and their bioremediation approaches. Editor R. Bharagava (Boca Raton: CRC Press), 369–396. doi:10.1201/9781315173351-14
Gheju, M. (2011). Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water. Air. Soil Pollut. 222, 103–148. doi:10.1007/s11270-011-0812-y
Gheju, M. (2018). Progress in understanding the mechanism of CrVI Removal in Fe0-based filtration systems. Water 10, 651. doi:10.3390/w10050651
Gibert, O., de Pablo, J., Cortina, J. L., and Ayora, C. (2002). Treatment of acid mine drainage by sulphate-reducing bacteria using permeable reactive barriers: a review from laboratory to full-scale experiments. Rev. Env. Sci. Biotechnol. 1, 327–333. doi:10.1023/A:1023227616422
Gould, J. (1982). The kinetics of hexavalent chromium reduction by metallic iron. Water Res. 16, 871–877. doi:10.1016/0043-1354(82)90016-1
Han, G., Shi, Y., Huang, Y., Liu, C., Xia, H., and Sun, S. (2025). Guidelines for inorganic arsenic (Ⅲ, V, and Total) and chromium (Ⅲ, Ⅵ, and Total) in Chinese soils. J. Hazard. Mater. 484, 136753. doi:10.1016/j.jhazmat.2024.136753
Henrikson, L., and Brodin, Y. W. (1995). Liming of acidified surface waters: a Swedish synthesis. Berlin; New York: Springer.
Hori, M., Shozugawa, K., and Matsuo, M. (2015). Reduction process of Cr(VI) by Fe(II) and humic acid analyzed using high time resolution XAFS analysis. J. Hazard. Mater. 285, 140–147. doi:10.1016/j.jhazmat.2014.11.047
Ilton, E. S., and Veblen, D. R. (1994). Chromium sorption by phlogopite and biotite in acidic solutions at 25 C: Insights from X-ray photoelectron spectroscopy and electron microscopy. Geochim. Cosmochim. Acta 58, 2777–2788. doi:10.1016/0016-7037(94)90113-9
James, B. R., and Bartlett, R. J. (1983). Behavior of chromium in soils: VII. Adsorption and reduction of hexavalent forms. J. Environ. Qual. 12, 177–181. doi:10.2134/jeq1983.00472425001200020005x
Jiang, N., Shang, R., Heijman, S. G. J., and Rietveld, L. C. (2018). High-silica zeolites for adsorption of organic micro-pollutants in water treatment: a review. Water Res. 144, 145–161. doi:10.1016/j.watres.2018.07.017
Keely, J. F. (2019). “Performance evaluations of pump-and-treat remediations 1,” in EPA environmental engineering sourcebook (Boca Raton: CRC Press), 400.
Kohut, C. K., and Warren, C. J. (2018). “Chlorites,” in SSSA book series. Editors J. B. Dixon, and D. G. Schulze (Madison, WI: Soil Science Society of America), 531–553. doi:10.2136/sssabookser7.c17
Konadu-Amoah, B., Hu, R., Ndé-Tchoupé, A. I., Gwenzi, W., and Noubactep, C. (2022). Metallic iron (Fe0)-based materials for aqueous phosphate removal: a critical review. J. Environ. Manage. 315, 115157. doi:10.1016/j.jenvman.2022.115157
Lai, K. C. K., and Lo, I. M. C. (2008). Removal of chromium (VI) by acid-washed zero-valent iron under various groundwater geochemistry conditions. Environ. Sci. Technol. 42, 1238–1244. doi:10.1021/es071572n
Langlois, C. L., and James, B. R. (2015). Chromium oxidation-reduction chemistry at soil horizon interfaces defined by iron and manganese oxides. Soil Sci. Soc. Am. J. 79, 1329–1339. doi:10.2136/sssaj2014.12.0476
Laxmi, V., and Kaushik, G. (2020). “Toxicity of hexavalent chromium in environment, Health threats, and its bioremediation and detoxification from tannery wastewater for environmental safety,” in Bioremediation of industrial waste for environmental safety. Editors G. Saxena, and R. N. Bharagava (Singapore, Singapore: Springer), 223–243. doi:10.1007/978-981-13-1891-7_11
Li, L., Benson, C. H., and Lawson, E. M. (2005). Impact of mineral fouling on hydraulic behavior of permeable reactive barriers. Ground Water 43, 582–596. doi:10.1111/j.1745-6584.2005.0042.x
Liu, B., Cai, J., Mao, X., Xie, F., and Zhu, X. (2025). Extreme chromium transformation and immobilization via ferrous coupling surfactant-enhanced nanofiltration process. Desalination 606, 118771. doi:10.1016/j.desal.2025.118771
Luo, Y., Ding, J., Hai, J., Tan, W., Hao, R., and Qiu, G. (2020). Interaction mechanism of dissolved Cr (VI) and manganite in the presence of goethite coating. Environ. Pollut. 260, 114046. doi:10.1016/j.envpol.2020.114046
Madhusudan, P., Lee, C., and Kim, J.-O. (2023). Synthesis of Al2O3@Fe2O3 core–shell nanorods and its potential for fast phosphate recovery and adsorption of chromium (VI) ions from contaminated wastewater. Sep. Purif. Technol. 326, 124691. doi:10.1016/j.seppur.2023.124691
Meuser, H., and Meuser, H. (2013). Groundwater, soil vapour and surface water treatment. Soil remediat. Rehabil. Treat. Contam. Disturb. Land, 279–346. doi:10.1007/978-94-007-5751-6_7
Michiels, K., Spooren, J., and Meynen, V. (2015). Production of hydrogen gas from water by the oxidation of metallic iron under mild hydrothermal conditions, assisted by in situ formed carbonate ions. Fuel 160, 205–216. doi:10.1016/j.fuel.2015.07.061
Mladetsky, I., Pilov, P., Levchenko, K., and Kuvaev, Ya. (2019). Testing and control of mineral enrichment processes. Dnipro: Zhurfond.
Mohan, D., and Pittman Jr, C. U. (2006). Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J. Hazard. Mater. 137, 762–811. doi:10.1016/j.jhazmat.2006.06.060
Monira, U., and Mostafa, M. (2023). Leather industrial effluent and environmental concerns: a review. Sustain. Water Resour. Manag. 9, 181. doi:10.1007/s40899-023-00969-1
Morrison, S. J., Metzler, D. R., and Dwyer, B. P. (2002). Removal of as, mn, mo, se, u, v and zn from groundwater by zero-valent iron in a passive treatment cell: reaction progress modeling. J. Contam. Hydrol. 56, 99–116. doi:10.1016/s0169-7722(01)00205-4
Naghipour, D., Taghavi, K., Ashournia, M., Jaafari, J., and Arjmand Movarrekh, R. (2020). A study of Cr(VI) and NH4+ adsorption using greensand (glauconite) as a low-cost adsorbent from aqueous solutions. Water Environ. J. 34, 45–56. doi:10.1111/wej.12440
Natkaniec-Nowak, L., Dumańska-Słowik, M., Naglik, B., Melnychuk, V., Krynickaya, М. B., Smoliński, W., et al. (2017). Depositional environment of Paleogen amber-bearing quartz-glauconite sands from Zdolbuniv (Rivne region, NW Ukraine): mineralogical and petrological evidences. Gospod. Surowcami Min. 33, 45–62. doi:10.1515/gospo-2017-0041
Noubactep, C. (2013). Metallic iron for water treatment: a critical review. Clean. – Soil Air Water 41, 702–710. doi:10.1002/clen.201200502
Reddi, L. N., Xiao, M., Hajra, M. G., and Lee In, Mo (2000). Permeability reduction of soil filters due to physical clogging. J. Geotech. Geoenviron. Eng. 126, 236–246. doi:10.1061/(ASCE)1090-0241(2000)126:3(236)
Rudmin, M., Banerjee, S., and Makarov, B. (2020). Evaluation of the effects of the application of glauconitic fertilizer on oat development: a two-year field-based investigation. Agronomy 10, 872. doi:10.3390/agronomy10060872
Rudmin, M., Banerjee, S., Makarov, B., Mazurov, A., Ruban, A., Oskina, Y., et al. (2019). An investigation of plant growth by the addition of glauconitic fertilizer. Appl. Clay Sci. 180, 105178. doi:10.1016/j.clay.2019.105178
Seo, S. B., Mahinthakumar, G., Sankarasubramanian, A., and Kumar, M. (2018). Assessing the restoration time of surface water and groundwater systems under groundwater pumping. Stoch. Environ. Res. Risk Assess. 32, 2741–2759. doi:10.1007/s00477-018-1570-9
Shi, J., McGill, W. B., Chen, N., Rutherford, P. M., Whitcombe, T. W., and Zhang, W. (2020). Formation and immobilization of Cr (VI) species in long-term tannery waste contaminated soils. Environ. Sci. Technol. 54, 7226–7235. doi:10.1021/acs.est.0c00156
Simon, F. G., Meggyes, T., and McDonald, C. (2002). Advanced groundwater remediation: active and passive technologies. London, United Kingdom: Telford, 200.
Skiba, M., Maj-Szeliga, K., Szymański, W., and Błachowski, A. (2014). Weathering of glauconite in soils of temperate climate as exemplified by a Luvisol profile from Góra Puławska, Poland. Geoderma 235–236, 212–226. doi:10.1016/j.geoderma.2014.07.013
Tomaszewski, E. J., Lee, S., Rudolph, J., Xu, H., and Ginder-Vogel, M. (2017). The reactivity of Fe (II) associated with goethite formed during short redox cycles toward Cr (VI) reduction under oxic conditions. Chem. Geol. 464, 101–109. doi:10.1016/j.chemgeo.2017.01.029
Trach, Y., Melnychuk, V., Melnychuk, G., Mazur, Ł., Podlasek, A., Vaverková, M., et al. (2021a). Using local mineral materials for the rehabilitation of the Ustya River – a case study. Desalination Water Treat. 232, 346–356. doi:10.5004/dwt.2021.27559
Trach, Y., Melnychuk, V., Michel, M. M., Reczek, L., Siwiec, T., and Trach, R. (2021b). The characterization of Ukrainian volcanic tuffs from the khmelnytsky region with the theoretical analysis of their application in construction and environmental technologies. Materials 14, 7723. doi:10.3390/ma14247723
Trach, Y., Trach, R., Kalenik, M., Koda, E., and Podlasek, A. (2021c). A study of dispersed, thermally activated limestone from Ukraine for the safe liming of water using ANN models. Energies 14, 8377. doi:10.3390/en14248377
Tripathy, S. K., Banerjee, P. K., Suresh, N., Murthy, Y. R., and Singh, V. (2017). Dry high-intensity magnetic separation in mineral industry—a review of present status and future prospects. Min. Process. Extr. Metall. Rev. 38, 339–365. doi:10.1080/08827508.2017.1323743
Ukhurebor, K. E., Aigbe, U. O., Onyancha, R. B., Nwankwo, W., Osibote, O. A., Paumo, H. K., et al. (2021). Effect of hexavalent chromium on the environment and removal techniques: a review. J. Environ. Manage. 280, 111809. doi:10.1016/j.jenvman.2020.111809
Ukrainian Standard DSTU B V. 2.1–3–96 (1997). In Soils. Laboratory tests (Kyiv: General Provisions).
Wang, Y., Pleasant, S., Jain, P., Powell, J., and Townsend, T. (2016). Calcium carbonate-based permeable reactive barriers for iron and manganese groundwater remediation at landfills. Waste Manag. 53, 128–135. doi:10.1016/j.wasman.2016.02.018
West, M. (2012). Practical guide for the assessment of glass making limestones and dolomites: Part 1-Procedures for sampling and physical testing. Glass technol.-eur. J. Glass Sci. Technol. Part A 53, 198–201.
White, A. F., and Peterson, M. L. (1996). Reduction of aqueous transition metal species on the surfaces of Fe (II)-containing oxides. Geochim. Cosmochim. Acta 60, 3799–3814. doi:10.1016/0016-7037(96)00213-x
Xu, H., Zhang, H., Qin, C., Li, X., Xu, D., and Zhao, Y. (2024). Groundwater Cr (VI) contamination and remediation: a review from 1999 to 2022. Chemosphere 360, 142395. doi:10.1016/j.chemosphere.2024.142395
Yamashita, S., Mukai, H., Tomioka, N., Kagi, H., and Suzuki, Y. (2019). Iron-rich smectite formation in subseafloor basaltic lava in aged oceanic crust. Sci. Rep. 9, 11306–11308. doi:10.1038/s41598-019-47887-x
Yi, Y., Wen, J., Zeng, G., Zhang, T., Huang, F., Qin, H., et al. (2017). A comparative study for the stabilisation of heavy metal contaminated sediment by limestone, MnO2 and natural zeolite. Environ. Sci. Pollut. Res. 24, 795–804. doi:10.1007/s11356-016-7839-y
Yin, S., and Ellis, D. E. (2009). DFT studies of Cr(VI) complex adsorption on hydroxylated hematite surfaces. Surf. Sci. 603, 736–746. doi:10.1016/j.susc.2009.01.015
Zheng, Y., Liu, S., Dai, C., Duan, Y., Makhinov, A. N., Hon, L. K., et al. (2020). Study on the influence mechanism of underground mineral element Fe(II) on Cr(VI) transformation under subsurface and groundwater interaction zones. Environ. Sci. Eur. 32, 62. doi:10.1186/s12302-020-00332-7
Keywords: chlorite, glauconite, hexavalent chromium, reduction, limestone, volcanic tuff
Citation: Trach Y (2025) Assessing the potential of low-cost minerals for the removal of hexavalent chromium from groundwater: a case study from Ukraine. Front. Environ. Chem. 6:1512237. doi: 10.3389/fenvc.2025.1512237
Received: 16 October 2024; Accepted: 12 June 2025;
Published: 06 August 2025.
Edited by:
Nguyen Nhat Huy, Ho Chi Minh City University of Technology, VietnamReviewed by:
Chicgoua Noubactep, University of Göttingen, GermanyRajesh Kumar Meena, University of Delhi, India
Rizwan Arif, Lingayas University, India
Warren Raymond Lee Cairns, National Research Council (CNR), Italy
Copyright © 2025 Trach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuliia Trach, eS5wLnRyYWNoQG51d20uZWR1LnVh
 Yuliia Trach
Yuliia Trach