- Chemistry Department, Delaware State University, Dover, DE, United States
Per- and polyfluoroalkyl substances (PFAS) are highly stable anthropogenic compounds characterized by their persistence in the environment and potential for bioaccumulation. This review investigates the physicochemical properties that underlie their environmental persistence, particularly the robustness of the carbon-fluorine bond. It also examines the synthesis processes of PFAS, their extensive applications across various industries, and the related health risks, including endocrine disruption and carcinogenic effects. The occurrence of PFAS in diverse environmental matrices, such as soil, water, and biota, is analyzed. Additionally, this study assesses advanced oxidation processes (AOPs), with an emphasis on Fenton-based treatments for the oxidative degradation of PFAS. Methods such as Photo-Assisted Anodic Fenton Treatment (P-AAFT) are highlighted for their promise in achieving complete mineralization of PFAS. The findings highlight the critical need for continued research and policy advancement to address the environmental and health challenges posed by PFAS, underscoring the urgent requirement for effective remediation strategies to mitigate PFAS contamination.
1 Introduction
Today, per- and polyfluoroalkyl substances (PFAS) are classified into two primary categories based on their fluorination and functional groups: fully fluorinated perfluoroalkyl substances, which possess entirely fluorinated carbon chains typically terminated with functional groups such as carboxylic acids (perfluoroalkyl carboxylic acids, PFCAs) or sulfonic acids (perfluoroalkyl sulfonic acids, PFSAs), and partially fluorinated polyfluoroalkyl substances, which contain a mix of fluorinated and hydrogenated carbons and include diverse functional groups like ether, phosphate, or sulfonamide moieties. This structural differentiation influences their physicochemical properties, environmental behavior, and persistence. PFAS are anthropogenic compounds referred to as “forever chemicals” due to their persistence in the environment and their ability to bioaccumulate in blood serum (Lau et al., 2007). PFAS are used as a component in various products, including non-stick coatings, fire prevention materials, stain-resistant fabrics, and some cosmetics (Carlson et al., 2022; Carlson and Tupper, 2020).
1.1 PFAS chemical structure and major physicochemical properties
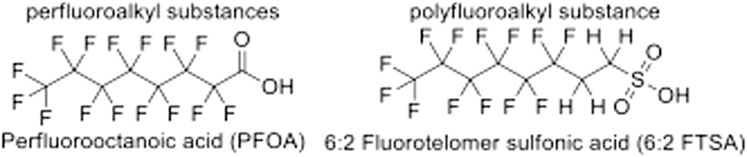
These two series of compounds as shown in Figure 1. Completely saturated perfluoroalkyl substances are represented on the left while partially saturated polyfluoroalkyl substances are represented on the right.
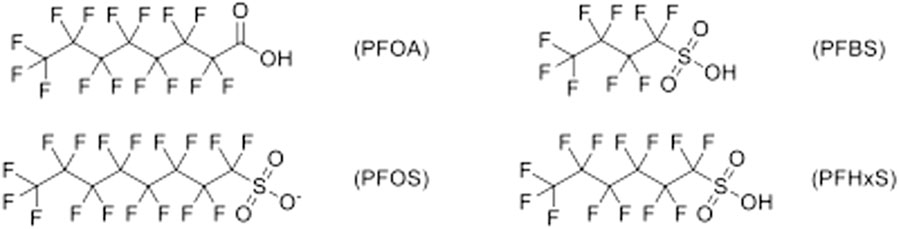
PFAS are further subcategorized into what is referred to as long- and short-chain due to their effective states. Long- and short-chain PFAS are categorized according to the length of the carbon chain and the associated functional group or reactive species. For example, PFAS primarily containing PFCAs with seven or more carbons in the backbone are considered long-chain. In contrast, molecules containing sulfonic acid functional groups or PFSAs with six or more carbons in the backbone are also considered long-chain. PFAS that are classified as PFCA with less than seven carbons and PFSAs with less than six carbons are considered short-chain. Short-chain PFAS are more miscible in water and will have increased mobility in the environment, while long-chain PFAS have been shown to strongly adsorb to soils and have a preference toward organic carbon. That is, long-chain PFAS can be transported by particulate matter and accumulates in soil (Lesmeister et al., 2021). Because their accumulative behavior ultimately will lead these compounds to migrate at a slower rate through shallow surfaces, long-chain compounds tend to be found in soils and groundwater, leaving short-chain compounds to be primarily found in surface waters (Borthakur et al., 2021). More complex PFAS can degrade into long-chain as well as short-chain PFAS and eventually ultra-chain molecules. Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) are considered long-chain while perfluorobutane sulfonic acid (PFBS) and perfluorohexane sulfonic acid (PFHxS) are considered short-chain PFAS, expressed in Figure 2.
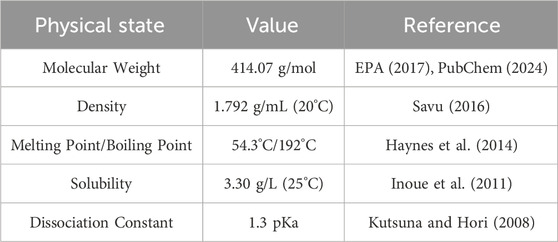
The primary molecular property that gives PFAS detrimental durability is the carbon-fluorine (C-F) bond. The C-F bonding is considered the strongest single type of bond in organic chemistry. These bonds exhibit great strength and unique properties due to the electronegativity of fluorine and its attraction to the partially positive carbon. Discovering ways to break the C-F bond will lead to advancements in managing the decontamination degradation of these compounds in the environment. Common physical properties of PFOA are listed in Table 1.
Fluorine is identified in group 17 of the periodic table, which comprises the halogen elements. The significant influence of fluorine when it replaces hydrogen or other elements in a chemical structure profoundly alters the compound’s properties. This is attributed to fluorine’s reactivity; it is designated as the most reactive of all elements. Additionally, fluorine has the strongest known electronegative value: It has a value of 3.98 according to the Pauling scale, which ranges from 0.7 to 3.98 and measures the tendency of an atom to attract a shared pair of electrons toward itself.
1.2 PFAS synthesis processes and resultant properties
PFAS represent a complex group of synthetic chemicals that have become prevalent in various industries due to their unique physical and chemical properties. Their synthesis involves two main processes: direct fluorination, including methods like electrochemical fluorination, and oligomerization, such as fluorotelomerization. These processes enable the production of chemicals that are extremely stable, resisting degradation and high temperatures and exhibiting distinctive characteristics like water and grease repellency. Initially synthesized in the United States with the creation of polytetrafluoroethylene (PTFE) by Roy J. Plunkett, PFAS have since become known for their persistence in the environment and are nicknamed forever chemicals.
In the context of PFAS, when fluorine replaces other elements, it imparts properties that are quite distinct and valuable in various applications. These properties include stability, repellency, surface tension, persistence, and thermodynamic resistance. PFAS are known for their exceptional chemical stability. The stability of the C-F bond is a direct result of fluorine substitution; it makes PFAS resistant to thermal, chemical, and biological degradation (Olsen, 2015). The fluorine atoms in PFAS provide them with hydrophobic and oleophobic properties. This is due to the low polarity of the carbon-fluorine bond and the size of the fluorine atom, which creates a “shield” around the molecule. These properties explain PFAS’ common use in non-stick coatings for cookware, as well as in stain-resistant fabrics and food packaging (Huang et al., 2022). PFAS can lower the surface tension of water significantly due to their amphiphilic nature—having both hydrophilic and hydrophobic parts. This property is exploited in applications like firefighting foams, where spreading over a surface and penetrating materials is important for extinguishing fires (Wang and Niven, 2021). The substitution of hydrogen by fluorine atoms in PFAS makes them highly persistent in the environment, as they do not readily break down under natural conditions. The C-F bonds confer resistance to environmental degradation processes that would normally break down other chemicals (US Government Accountability Office, 2022). PFAS can withstand extreme temperature ranges without degrading, which is again attributable to the strength of the C-F bond. This property is essential for their use in industrial processes that require performance at high temperatures (Verma et al., 2023). These examples reflect how the incorporation of fluorine dramatically changes the characteristics of PFAS, making them useful in a wide array of products but also raising concerns about their environmental and health impacts due to their long-term persistence.
1.3 Biochemical properties of PFAS
The stability of the C-F bond in PFAS is a key factor in their unique biochemical properties. This stability contributes to their persistence in the environment, as they do not readily break down into simpler compounds. Consequently, PFAS can bioaccumulate in organisms and persist in the environment over extended periods, posing potential risks to ecosystems and human health.
1.3.1 PFAS interaction with human serum albumin
One of the key interactions of PFAS within the human body involves the binding to human serum albumin (hSA), a vital protein in blood plasma. PFAS recognize and bind to specific sites on hSA, influencing its transport and distribution functions. Additionally, PFAS-hSA interactions have been associated with potential adverse health effects, including altered distribution of other small molecules, which may have implications for drug transport and metabolism (Fischer et al., 2024).
1.3.2 PFAS effects on inflammasome activation and neurodegeneration
Studies have indicated that exposure to PFAS can potentially increase inflammasome activation in the brain, leading to synuclein aggregation and dopaminergic degeneration. This interaction highlights the neurotoxic effects of PFAS and their potential role in neurological disorders, creating a significant area of concern for human health (Wang et al., 2021).
1.3.3 PFAS impact on nuclear receptors
PFAS also exert toxic effects through interactions with nuclear receptors, which are proteins responsible for modulating gene expression in response to hormone signals. Peroxisome proliferator-activated receptor alpha (PPARα) is a crucial nuclear receptor in the human body, one that plays a significant role in regulating lipid metabolism and energy homeostasis. This receptor is mainly expressed in metabolically active tissues, such as the liver, heart, kidney, and muscles. That is, because PPARα is fundamentally involved in managing how the body processes fats, it is a vital receptor for energy balance, reducing inflammation, and possibly influencing the risk of metabolic and cardiovascular diseases. Behr et al. (2020) tested several PFAS individually and concluded that all except PFBS can activate human PPARα. Another study investigating Atlantic cod liver slices found that PFAS mixtures led to more differentially expressed genes (DEGs) and impacted pathways related to oxidative stress, cholesterol metabolism, nuclear receptors, and ferroptosis compared to individual compounds, with mainly additive effects and some synergistic responses. PFOS exposure had the most significant transcriptomic effects, indicating potential bioaccumulation and toxicity and highlighting ferroptosis as a potential adverse outcome pathway linked to PFAS exposure (Dale et al., 2022).
1.4 Health hazards of PFAS
Despite an urgent need for additional research into PFAS and their bio-toxicity, clear evidence to date indicates these substances’ detrimental effects. An area of concern is the maternal-fetal gestational and developmental process. PFAS have been linked to several health disruptions including liver disease (Ducatman and Fenton, 2022) as well as fetal and maternal hepatic dysfunctions in CD-1 mice during gestation (Blake et al., 2020; Blake et al., 2022). Fetal thyroid disruptions have also been noted through exposure to PFAS (Yao et al., 2022). Consequently, PFAS’ negative effects on the endocrine system, especially in females, have been detected along with an induced resistance to carboplatin, a type of chemotherapy used to treat ovarian cancer (Rickard et al., 2022). Exposure to PFOA, hexafluoropropylene oxide dimer acid (HFPO-DA), or GenX has also been linked to maternal, placental, and embryonic effects (Blake et al., 2020). Data has revealed concerning levels present in breast milk and infant formula in the United States and Canada (LaKind et al., 2023). Data has also been compiled to conclude that over the past two decades, global low birth weight can be linked to PFAS exposure, especially in parts of Asia (Fan et al., 2022).
Modification of the endocrine system leading to a host of biological syndromes and diseases has also been linked to PFAS exposure. Implications have been made that exposure to PFAS has negative effects on cholesterol through data obtained from cross-sectional data sets (Dong et al., 2019). Hyperthyroidism has also been linked to PFAS exposure after animal studies were conducted using temporal comparison in cats (Blake et al., 2018; Wang et al., 2018). Improper thyroid function has relations to cardiovascular disease as well as fertility and fetal neurodevelopment. PFAS have been observed and quantified in several studies involving aquatic invertebrates (Ma et al., 2022; Koch et al., 2019), carp fish (Inoue et al., 2011), fish from China’s Jiulong River (Liu et al., 2022), and undisclosed fish (Langberg et al., 2022). Daphnia carinata, commonly known as water fleas, are used in scientific research for various biological disciplines and have exhibited PFAS accumulation (Logeshwaran et al., 2021). Ulcerative colitis has been linked to high serum levels of PFOA in a study conducted in Ohio, where residents were exposed to the compound issuing from a nearby chemical plant (Steenland et al., 2013). PFAS increase the risk of adverse health effects, including immunotoxicity, dyslipidemia, kidney and testicular cancer, liver damage, decreased fertility, thyroid disruption, and developmental effects (Knutsen et al., 2018). The reported average half-lives of PFOA, PFOS, and PFHxS are 2.7, 3.4, and 5.3 years, respectively (Li et al., 2018).
1.5 PFAS manufacturing phaseout
Materials containing PFAS, produced since the mid-20th century, and because they are toxic to living species and can persist for many years in the environment and internally, they have been the object of increasing attention. Specific PFAS such as PFOA and PFOS have been said to be phased out of manufacturing in the United States. However, other countries around the world are still producing these long-chain PFAS, and their products can still be imported into the United States, meaning they still pose a threat to human health in the United States. As of 2023, the US Environmental Protection Agency (EPA) has proposed drinking water advisory limits for the monitoring and regulation of six PFAS; the agency is also continuing to monitor for any additional PFAS that may need to be regulated (US Environmental Protection Agency, 2023).
2 Contamination, detection, and remediation of PFAS
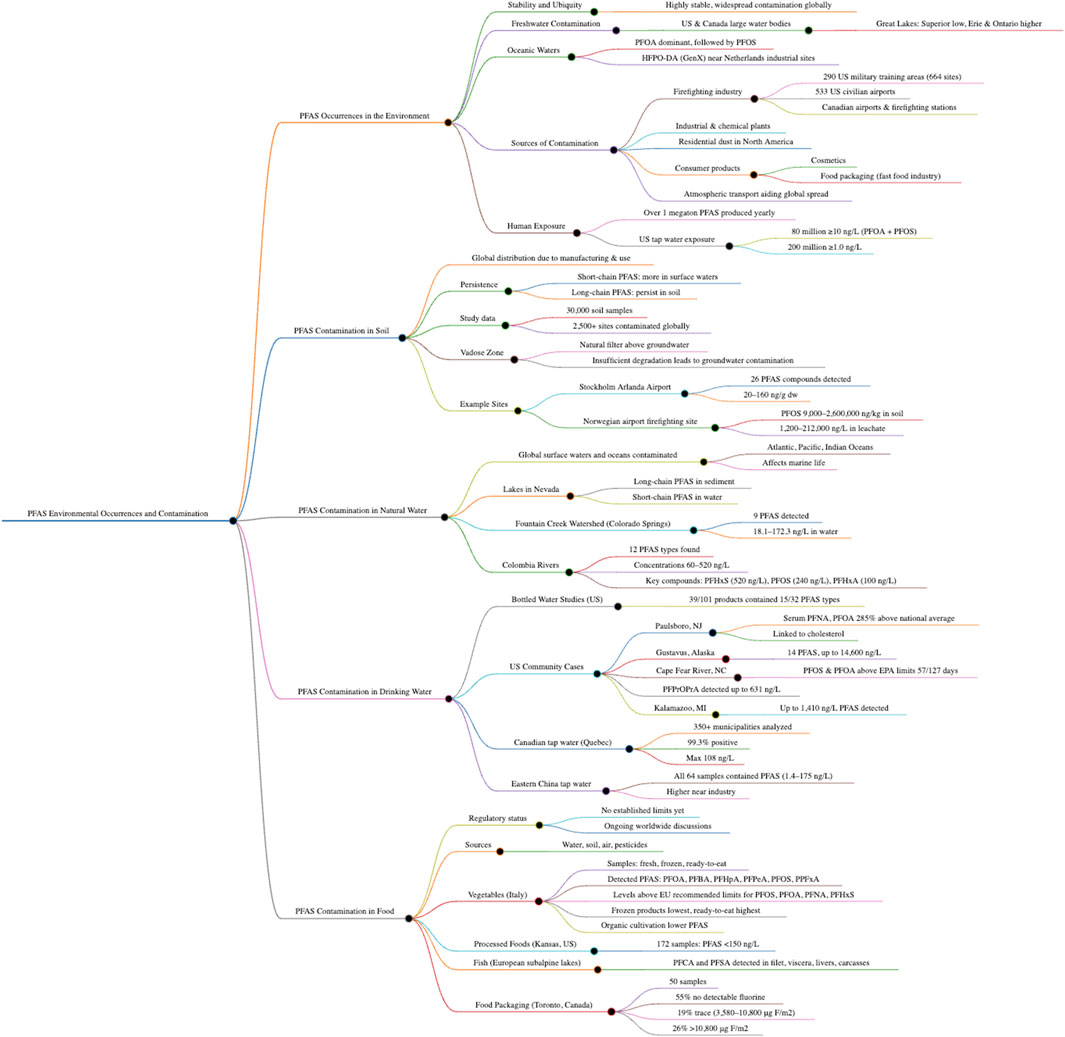
2.1 PFAS occurrences in the environment
Due to the stability of many PFAS, which fosters their ubiquity in the environment, a large cross section of the environment has been contaminated with these substances. PFAS have been found in large freshwater bodies across the United States and Canada (Remucal, 2019). Even in the most pristine region of the Great Lakes Basin, PFAS were detected at low concentrations in all matrixes of Lake Superior; higher concentrations were observed in the more industry-impacted Lakes Erie and Ontario (Remucal, 2019).
PFOA is the major perfluorinated compound detected in oceanic waters; it is followed by PFOS (Yamashita et al., 2004). Even as an alternative to PFOA, HFPO-DA which also goes by the trade name GenX, has been found in soil samples near industrial sites in the Netherlands (Brandsma et al., 2019).
PFAS are commonly used in the firefighting industry due to their excellent fire-retardant properties, but this has led to widespread contamination in many regions of the world. In the United States, 290 military fire training areas (containing 664 training sites), along with 533 civilian airports where firefighting foams have been employed, are contaminated by PFAS degradants (Hu et al., 2016). Airports commonly use PFAS-containing firefighting foams which have also been left behind by Fire Training Academies within four airports located in central and eastern Canada (Liu et al., 2021). Firefighting stations have also been found to have PFAS residues (Blake et al., 2020). Unfortunately, sites near industrial plants and chemical plants are not the only locations where PFAS have been detected; nor are sites where firefighting foams are utilized.
PFAS have been detected in dust samples collected from North American residential homes (Blake et al., 2020). While the concern has arisen from the environment being contaminated with PFAS, researchers have not only found them in the environment but even in popular cosmetics (Whitehead et al., 2021). Food packaging paper, popular in the fast food industry, has also been found to be coated with PFAS (Glenn et al., 2021; Schaider et al., 2017). In terms of global PFAS distribution, atmospheric transport has been shown to play an important role in the spread of contamination (Evich et al., 2022).
Yearly, more than a megaton of PFAS are produced, and thousands of pounds of PFAS eventually wind up in many end-user products (Evich et al., 2022). In the United States, an estimated 80 million people are exposed to tap water containing combined PFOA and PFOS concentrations around 10 ng/L or greater, of and at least 200 million people are estimated to be exposed to concentrations at or above 1.0 ng/L (Andrews and Naidenko, 2020).
2.2 PFAS contamination in soil
Due to widespread manufacturing and distribution, PFAS have unfortunately infiltrated soils around the globe, potentially disrupting ecosystems. As previously mentioned, while short-chain PFAS are more commonly found in surface water samples, long-chain PFAS tend to persist in soil. Based on data compiled from several studies, Brusseau et al. (2020) revealed that 30,000 soil samples collected from more than 2,500 sites over all continents contained PFAS contamination.
The vadose zone, also known as the unsaturated zone, is the part of Earth’s subsurface that lies above the groundwater table. This zone, characterized by the presence of both air and water in the pores of soil, sediment, or rock, acts as a natural filter. Contaminants from the surface can be degraded, transformed, adsorbed, or immobilized in the vadose zone, and this filtration reduces the pollution load reaching the aquifer. However, if contaminants move quickly through this zone without sufficient degradation, they can contaminate the groundwater. PFAS are known to leach into groundwater over decadal time frames (Brusseau et al., 2020; Rovero et al., 2021; Wallis et al., 2022). For instance, contaminated soil samples at Stockholm Arlanda Airport in Sweden were analyzed for PFAS pollutants, and 26 compounds were identified in samples ranging from 20 to 160 ng/g dry weight (dw; Drenning et al., 2023). Hale et al. (2017) analyzed PFAS concentrations from soil samples collected at a Norwegian airport serving as a firefighting training facility. They found total and leachable concentrations of PFOS, which was most prominent, ranging from 9,000 to 2,600,000 ng/kg while those in leachate water ranged from 1,200 to 212,000 ng/L (Hale et al., 2017).
2.3 PFAS contamination in natural water
PFAS have not only reached the soil and groundwater; they have also traveled to large bodies of natural or surface water worldwide. Oceanic waters worldwide have PFAS, from the Atlantic (in conjunction with the Denmark Strait and the Labrador Sea) to the Pacific and Indian oceans, ultimately affecting marine life (Yamashita et al., 2008).
Across four lakes in Nevada, water and sediment were analyzed for 17 long- and short-chain PFAS; the findings showed that long-chain PFAS were likely to be present in sediment samples while short-chain PFAS were found in the water samples (Bai and Son, 2021). In Colorado Springs, the Fountain Creek Watershed is a critical source of natural water for an estimated 100 farms in the vicinity, and for residents’ drinking water. For the duration of 1 year, this watershed was sampled monthly at eight locations; the results revealed that nine PFAS in the water samples ranged from 18.1 to 172.3 ng/L (Davalos et al., 2022).
In Colombia, the Bogotá River flows through the department of Cundinamarca, which is home to more than eleven million residents, eventually reaching the basin of the Magdalena River, a tributary of the Bogotá. The Magdalena River basin is home to 80% of the nation’s 48 million inhabitants; it produces 86% of the nation’s GDP and 75% of the country’s agricultural production. The Bogotá River was analyzed for 38 PFAS at six sampling locations. The analysis detected twelve types of PFAS, and it quantified concentrations ranging from 60 to 520 ng/L. The highest concentration was attributed to PFHxS (520 ng/L), followed by PFOS (240 ng/L) and Perfluorohexanoic Acid (PFHxA; 100 ng/L; Boca Raton et al., 2022).
2.4 PFAS contamination in drinking water
Today, drinking water is a critical and often overlooked resource. Bottled water is thought of as the most reliable and recommended drinking water source, especially in poverty-stricken areas or during disasters. Chow et al. (2021) screened bottled water products sold in the United States for 32 PFAS. Their results indicated that 39 out of the 101 products had detected 15 out of the 32 PFAS (Chow et al., 2021).
The drinking water in Paulsboro, New Jersey, was found to be severely contaminated with PFAS, prompting a community-wide study that was conducted testing the serum levels of PFNA and PFOA (Graber et al., 2019). The study revealed that residents exposed to the contaminated drinking water had serum levels 285% greater than the rest of the United States population, levels which were additionally linked to increased cholesterol levels (Graber et al., 2019). The residential drinking water in Gustavus, Alaska was discovered to have approximately fourteen PFAS at levels as high as 14,600 ng/L (Babayev et al., 2022).
The Cape Fear River watershed, located in North Carolina, was sampled for 127 days; out of those days, PFOS and PFOA were present and surpassed the EPA’s lifetime health advisory limit on 57 days (Sun et al., 2016). A PFOA replacement, perfluoro-2-propoxypropanoic acid (PFPrOPrA), was found to be present downstream of a North Carolina PFAS manufacturer at levels as high as 631 ng/L (Sun et al., 2016). The Michigan Department of Environmental Quality performed a PFAS analysis for the Kalamazoo community’s water reserve; it revealed that significantly elevated levels, as high as 1,410 ng/L (Matheny, 2018).
In the Canadian province of Munoz et al. (2023) analyzed over 350 municipalities’ tap water for PFAS. More than 99.3% of the samples, drawn from surface water and groundwater, tested positive for PFAS, ranging from below the limit of quantification to 108 ng/L. Tan et al. (2017) evaluated tap water samples from eastern China for PFAS and found that all 64 samples included PFAS, their concentrations ranging from 1.4 to 175 ng/L; the higher concentrations were primarily sampled near industrial sites.
2.5 PFAS contamination in food
Although regulatory limits for PFAS in food do not exist, recent discussions have occurred in the United States, Europe, and China (Brennan et al., 2021). PFAS contamination in food can occur due to contamination in water, soil, the atmosphere, and pesticides.
Researchers in Italy have conducted a study examining 41 samples of fresh, frozen, and readily consumable vegetables; they revealed that PFOA, PFBA, PFHpA, PFPeA, PFOS, and PPFxA were frequently detected in all the vegetables they analyzed (Piva et al., 2023). PFOS, PFOA, and PFNA concentrations were found above the attention limits recommended by the European Commission (2022) for vegetables. According to the recommended limits, PFOS and PFOA concentration in vegetables should not exceed 0.010 ng/g; PFNA should be below 0.005 ng/g, and PFHxS should be below 0.015 ng/g. Piva et al. (2023) found that frozen products contained the lowest amounts of PFAS; readily consumable vegetables showed the highest values for PFAS, although samples derived from organic cultivation showed lower amounts.
Processed foods gathered from local grocery stores in Lenexa, Kansas were evaluated via liquid chromatography with mass spectrometry (LC/MS) and high resolution mass spectrometry. In all 172 samples, PFAS concentrations were below 150 ng/L (Genualdi et al., 2021).
Eight species of fish from lakes in the European subalpine area were analyzed for PFCA and PFSA. Detectable concentrations were present in filets, viscera, livers, and residual carcasses (Valsecchi et al., 2020).
Fifty food packaging samples, sampled repeatedly from the same food retailers in Toronto, Canada; while 55% of the samples contained no detectable fluorine (i.e., <3,580 μg F/m2), 19% contained trace levels ranging from 3,580 to 10,800 μg F/m2, and 26% had >10,800 μg F/m2 (Schwartz-Narbonne et al., 2023).
2.6 Analytical methods for detection of PFAS
The US EPA has issued validated methods that are readily available for analyzing specific PFAS compounds in water via liquid chromatography with tandem mass spectrometry (LC-MS/MS) (Shoemaker and Tettenhorst, 2018). The process of searching for known chemicals refers to targeted analysis, in which researchers have information on chemical characteristics for ease of identification. Targeted analysis does not assess only the chemicals or compounds that researchers have identified, and this targeting leads to an increase in sensitivity and selectivity. Non-target analysis is essentially the opposite of target analysis, as the goal is to identify all chemicals or compounds present. Target analysis should be utilized when analyzing PFAS where there is already a known or specified molecule as the target. Due to the thousands of PFAS already established, most databases should be able to identify them. However, if intermediates of PFAS need to be analyzed, non-target analysis may be the better solution.
Researchers who work on PFAS analysis in water, soil, and food samples have numerous options when it comes to instrumental techniques. Total fluorine determination in samples can be quantified through spectroscopic instruments such as sliding spark spectroscopy, NMR (nuclear magnetic resonance), Raman spectroscopy, PIGE (particle-induced gamma-ray spectroscopy), or chromatographic method CIC (combustion ion chromatography with a conductivity detector; Carnero et al., 2021). An important suggestion when using any type of laboratory equipment for the accurate quantitative analysis of PFAS is that the equipment should contain no fluorinated properties. A common replacement in LC-MS is the use of PTFE filters with Teflon-free filter paper (Choi et al., 2018). The determination of PFAS content is usually performed by LC, ultra performance liquid chromatography, and liquid chromatography high resolution coupled with MS (LC-HRMS), as well as MS/MS; Nason et al., 2021; Yuan et al., 2016; Granby and Tesdal Håland, 2018; Barreca et al., 2018; Davalos et al., 2022). Solid phase extraction-LC-MS/MS has also been employed in the detection of PFAS in bottled water (Chow et al., 2021).
In general, gas chromatography (GC) analysis primarily focuses on volatile, semi-volatile, and neutral PFAS, including compounds such as fluorotelomer alcohols (FTOH) and perfluorinated sulfonamido ethanols (FASE). This selectivity imposes limitations on the method’s applicability. Consequently, the popularity of GC analysis for PFAS is surpassed by that of LC, which offers broader capabilities and is more widely favored for PFAS analysis (Trojanowicz and Koc, 2013).
The analysis of PFAS using high-performance liquid chromatography (HPLC) without MS has demonstrated success in various scientific applications. HPLC alone is capable of separating and quantifying PFAS compounds without the need for MS detection. This method has been particularly effective in environmental monitoring studies where the focus is on identifying and quantifying PFAS in water, soil, and biota. The flexibility of HPLC, catering to the diverse range of PFAS compounds, allows for various types of columns and mobile phases to be employed. While coupling HPLC with MS offers additional specificity and sensitivity, HPLC without MS remains a viable and reliable option for the analysis of PFAS (Amin et al., 2020).
2.7 PFAS remediation methods
2.7.1 PFAS-contaminated soil remediation
Several methods, including phytomanagement, sorbent amendment with activated charcoal, microbial degradation and enzymatic treatment, smoldering combustion, lead peroxide, and nanoscale zero-valent iron, have the ability to result in PFAS degradation and thereby remediate PFAS contamination in soil.
Industrial hemp (Cannabis sativa) has recently gained attention as a promising plant for phytomanagement due to its low cost, energy efficiency, low harmfulness, flexibility, and effectiveness in removing PFAS through controlled contaminated soils. In a recent phytomanagement study using hemp, Pietrini et al. (2019) found arsenic, vanadium, and lead only in the roots; zinc was found in all organs, thus confirming hemp’s phytomanagement capabilities and suggesting applicability for PFAS. In an experimental phytomanagement comparison study, hemp, sunflower, and mustard plants were used to compare their capacities for remediation of PFAS-contaminated soil. Hemp displayed the highest levels of PFAS (14.3 μg/plant, in comparison to 12.9 μg/plant for sunflower and 8.3 μg/plant for mustard; Wu, 2021). Although the idea of phytomanagement to remediate PFAS-contaminated soil locations may be economically ideal, it could take decades to reach optimal contamination levels and, to prevent PFAS from continuously entering the food chain, the crop selected for use needs to be a nonfood crop (Evangelou and Robinson, 2022).
Firefighting foam containing PFAS, also known as aqueous film-forming foams (AFFF) is a prime culprit in soil contamination.
Researchers have immobilized PFAS in soil using an activated carbon (AC) amended sorbent (Hale et al., 2017).
The enzymatic and microbial degradation of PFAS was explored as a green approach due to its economically and environmentally friendly characteristics (Berhanu et al., 2023).
Another experimentally successful remediation technique is the treatment of PFAS-contaminated soil with granular AC smoldering combustion. Through smoldering combustion, the soil is oxidized at temperatures excess of 900°C to degrade PFAS, resulting in PFAS concentrations below detection limits (0.0004 mg/kg; Duchesne et al., 2020).
A unique approach utilizing lead acid batteries removed more than 99% of PFAS by degrading PFAS in lead peroxide (PbO2; Fang et al., 2019).
Nanotechnology has also been employed to degrade PFAS. In a recent study, reduced graphene oxide (rGO) and nanoscale zero-valent iron (nZVI) as a nanohybrid (NH) was used in conjunction with hydrogen peroxide to remove PFAS (H2O2; Masud et al., 2021).
2.7.2 Remediation of PFAS-Contaminated water
The absolute destruction of PFAS is the ultimate goal in the removal of PFAS contamination. Water filtration processes have been employed for the removal of specific PFAS compounds. Nanofiltration (NF) and reverse osmosis (RO) have been proven to successfully remove PFAS from water (Liu et al., 2022; Mastropietro et al., 2021). One promising line of research features ionic fluorogels (IFs) that, given specialized variations, form regenerable building blocks; IFs have demonstrated high efficiency in removing 21 PFAS in water (Manning et al., 2022). On another line of research, an adsorption method was developed utilizing albumin from the Moringa oleifera plant, which was encapsulated in alginate beads and combined with biochar produced from rice straw. This innovative combination was applied to treat water contaminated with PFOS. The hybrid material demonstrated a high removal efficiency, successfully eliminating approximately 87% of PFOS from the water, with a reported margin of error at plus or minus 4.2% (Militao et al., 2023). A hybrid process for the entrapment of PFOA features Fenton reagents (ferric sulfate and H2O2) with humic acid. During the process, humic acid is oxidized by the Fenton reagents, creating a liquid and solid phase that completely separates the PFOA. PFOA was found to remain irreversibly present in the solid phase (Santos et al., 2016).
3 Advanced oxidation processes
3.1 Fenton chemistry background
Henry John Horstman Fenton is responsible for the novel work that has yielded advanced oxidation processes (AOPs). AOPs have made possible the current treatments of water purification systems (Glaze et al., 1987). Techniques that are non-destructive toward PFAS, such as adsorption or removal by RO, are effective in separation or transfer. However, those measures do not solve the contamination issues wholly (Wang et al., 2022). Broadly, AOPs comprise chemical treatments that are designed for the removal or degradation of organic (and, in certain cases, inorganic) chemicals in water and wastewater by oxidizing the inorganic and organic materials through reactions with oxidizing agents such as the hydroxyl radical (•OH). The •OH involved in AOPs traditionally reacts heavily with most organic substances through electrophilic addition to the double bonds or hydrogen abstraction, commonly expected to transform many organic compounds into water, carbon dioxide, and inorganic ions. AOPs include many different technologies, such as photolysis and photocatalysis, sonolysis, electrochemical oxidation, Fenton-based reactions, and ozone-based processes.
3.2 Overview of fenton treatment
It has been more than 120 years since Fenton first described specific metals’ special oxygen transfer properties, which improve the use of H2O2. From this discovery, the ability to oxidize organic substances using iron (II) or Fe2+ and H2O2 have come to be known as Fenton chemistry or the Fenton process. The Fenton process involves Fenton’s reagent (Fe2+) in the form of iron salt and H2O2 (Equation 1). The reaction generates •OH, mostly occurring under acidic conditions (Fenton, 1894). Unfortunately, Fenton did not get to figure out the mechanism behind the reaction before his death in 1929.
In 1932, Fritz Haber and Joseph Weiss proposed that the responsible intermediate to Fenton’s findings is •OH, leading to what is known as the Haber-Weiss Reaction Equation (Equation 2).
Many of Haber and Weiss’s contemporaries challenged their theory, but the debate was umpired by Donald T. Sawyer, who further proved Fenton’s proposals as well as Haber and Weiss’s. The Haber-Weiss reaction includes supplementary work known as the Haber-Weiss chain reaction, which describes the reaction mechanism in greater detail (Equations 3–5; Koppenol, 2001).
The Fenton reaction initiates the chain:
(Step 1: Initiation)
Then the reaction chain propagates employing two successive steps:
(Step 2: Propagation)
(Step 3: Propagation)
The chain is terminated when a ferrous ion scavenges the hydroxyl radical:
(Step 4: Termination)
3.3 PFAS and hydroxyl radicals
Given the success of PFAS degradation with the use of •OH, Tang et al. (2012) degraded PFOA utilizing a UV-Fenton process, concluding that hydroxyl radicals are the main oxidizing species. An additional article supporting this result confirmed that, in combination with a solar source, •OH degrades a fluorotelomer now used as a replacement for legacy PFAS (Barışçı and Suri, 2021). However, in contrast to the research that has successfully affirmed hydroxyl radicals’ ability to degrade PFAS, other research has claimed otherwise. Olatunde et al. (2020) proposed that •OH radicals alone do not effectively break down PFAS because the perfluorinated compounds lack the desired reactive sites for •OH. Based on experimentation comparing catalyzed H2O2 propagation (CHP) reactions that can generate hydroperoxide anion (HOO•), superoxide anion (
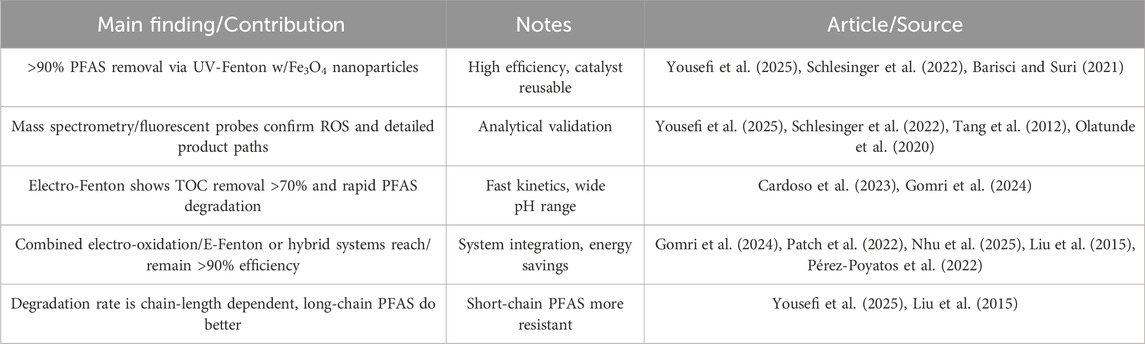
Table 2. Summary of the main findings and contributions related to PFAS degradation with Fenton chemistry.
3.4 Classic fenton treatment
Original work directly using the Fenton reaction is referred to as classic Fenton treatment (CFT). Although CFT has revolutionized many areas in the scientific world, there are disadvantages for CFT when it comes to wastewater treatment. Due to the use of iron, a large volume of ferric sludge is produced, and this may cause environmental pollution (Masood et al., 2023). Also, CFT does not sustain hydroxyl radical production throughout the experiments, therefore the degradation of contaminants is limited. The superiority of Fenton oxidation consists in the benign reagents’ (Fe2+ and H2O2) ease of availability as well as the straightforward process and efficiency of degrading organic contaminants (Bello et al., 2019).
3.5 Anodic Fenton treatment
Anodic Fenton treatment (AFT) is an enhanced electrochemical oxidative process that can also be described as an optimized fusion of CFT and electrochemical Fenton treatment (EFT; Saltmiras and Lemley, 2000). Because it incorporates electrochemical methods to rectify some of CFT drawbacks, AFT represents a significant improvement over CFT and EFT. Primarily, this innovative approach grants improved control over the oxidation of iron. With CFT, the presence of ferrous salts catalyzes the breakdown of H2O2 into radicals that attack organic pollutants, but the ferrous ions quickly turn into less reactive ferric ions and thereby diminish the system’s efficiency. AFT counters this issue through electrochemistry, where the application of an electric current at the cathode enables the conversion of ferric back to ferrous ions and the maintenance of a consistent and effective catalytic cycle.
Moreover, whereas CFT necessitates an external supply of H2O2, thus posing logistic and safety challenges, AFT conveniently generates H2O2 in situ via electrochemical processes. This is achieved through the reduction of oxygen at the cathode, which not only negates the need for H2O2 transport and handling; it also elevates process safety and efficiency. Additionally, AFT tackles the problem of iron sludge formation. Iron sludge, as a prevalent byproduct of CFT due to excessive iron, takes the form of solidified ferric ions after treatment. Through continuous regeneration of ferrous ions in the AFT system, iron sludge production is substantially minimized, and this leads to simplified sludge management and reduced environmental and financial burdens.
In essence, AFT refines the CFT methodology by ensuring a steady reclamation of the ferrous ion catalyst, on-site generation of the necessary H2O2, and a significant reduction in iron sludge production. This culminates in a method that is a more efficient and eco-friendly alternative for the degradation of pollutants (Panizza and Cerisola, 2009). AFT, although a novelty treatment, has only been used to degrade and treat wastewater mainly contaminated with pesticides (Wang and Lemley, 2001; Wang and Lemley, 2002). To date, AFT has not been studied with respect to the degradation of PFAS. The pathways for degrading PFAS include direct electron transfer occurring at the anode, followed by interactions with reactive species that culminate in their complete mineralization (Puttamreddy and Nippatlapalli, 2024).
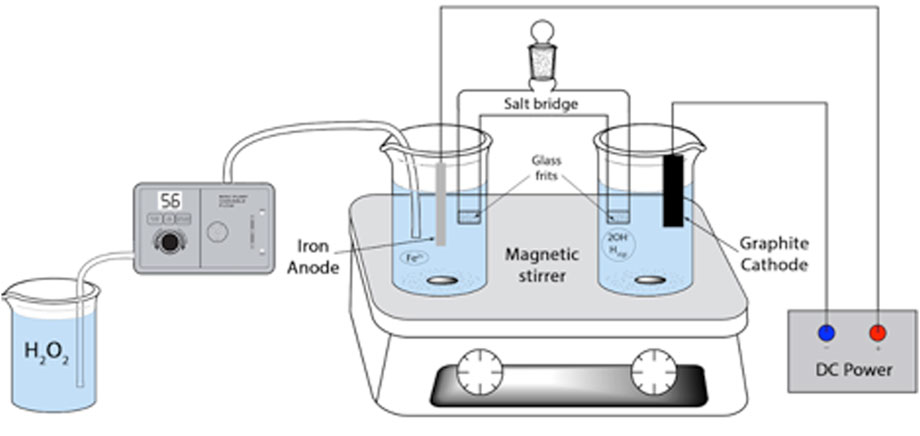
3.6 AFT apparatus
As shown in Figure 3, the AFT apparatus consists of two electrochemical half-cells connected via a salt bridge. It thus differs from EFT, which mainly utilizes one cell for the complete process. The anodic half-cell is where the waste treatment takes place. The ferrous ion is delivered from the sacrificial iron anode (Equation 6).
The other half-cell is considered the cathodic half-cell, which contains the graphite electrode, deionized water (H2O), and generates the •OH. The cathodic half-cell is shown below (Equation 7).
One of the main attributes that distinguishes AFT is the ability to combine the half-cells after treatment to create a partially neutralized treatment effluent; this is possible because the anode half-cell solution is acidic while the cathode solution is alkaline (Saltmiras and Lemley, 2000). It diminishes the step of neutralizing pH upon discharge, as mentioned with CFT and EFT. The salt bridge needs a selected electrolyte added to each half-cell to provide sufficient initial conductivity for constant iron delivery throughout the treatment. Using sodium chloride (NaCl), potassium chloride (KCl), or sodium sulfate (Na2SO4) as the salt bridge electrolyte has been successful during oxidation. However, the use of sodium nitrate (NaNO3) as the electrolyte will result in low efficiency of ferrous ion production and a low oxidation rate of diazinon (Wang and Lemley, 2002). Porous glass frits are often used at the end of the salt bridge to enable electrical or ionic conductivity between the solution and the internal filling solution while preventing the mixing of the solutions. For the degradation of ethylene thiourea (ETU) by AFT, the optimal Fe2+:H2O2 parameters have been determined at 1:10 (Saltmiras and Lemley, 2000).
3.7 Photo-Assisted Anodic Fenton Treatment
Photo-Assisted Anodic Fenton Treatment (P-AAFT) introduces the use of solar light irradiation, which acts as an eco-friendly and low-cost additive to AFT. The inclusion of solar irradiation (Equation 8) improves efficiency through the generation of additional hydroxyl radicals via photolysis of H2O and H2O2 (Equation 9; Patch et al., 2024). Furthermore, it also leads to the enhancement of ferrous ion regeneration (Equation 10; Equation 11; Ganiyu et al., 2022).
P-AAFT will require enhancements and adjustments in comparison to the AFT due to the nature of the molecular properties of most PFAS, as well as the UV light integration may alter the functionality of the Fenton Reagent ratio (FRR) which is comprised of Fe2+ to H2O2 of 1:10 M.
It has been suggested that CFT is inconsistent when it comes to the complete degradation of PFAS; when paired with solar radiation, however, efficiency is enhanced (Hajalifard et al., 2023). Photo radiation includes ultraviolet (UV), visible light, and infrared radiation. UV light can be divided into four bands: UVA (315–400 nm), UVB (280–315 nm), UVC (200–280 nm), and vacuum UV (VUV, 100–200 nm).
Literature states photo-enhanced degradation of PFOA has two steps, the first of which generates a radical species (predominantly •OH) formed by H2O2; the second is directed by ferric iron under UV radiation (Olatunde et al., 2020). PFAS degradation has been explored with the use of UV light in the UVC band at 254 nm using low-pressure mercury lamps (Umar, 2021). Comparing PFOA photolytic degradation under irradiation at 254 nm UVC light and at 185 nm VUV light, Chen et al. (2007) concluded that UVC led to negligible degradation under comparable conditions. Meanwhile, 61.7% PFOA was degraded at 185 nm, and the defluorination ratio reached 17.1% within 2 h (Chen et al., 2007). Schlesinger et al. (2022) investigated the use of nanosized magnetite (Fe3O4) as an additional catalyst in UV-Fenton treatment; they found 90% PFAS destruction, adding that a 30-min UVC exposure time at 254 nm was sufficient to catalyze the Fenton reaction. Another experiment that supports the use of 254 nm UVC for the degradation of PFOA was carried out with the use of humic acid and iodide. After 1.5 h at 254 nm, PFOA decomposition and defluorination ratios were increased to 67.5% and 23.5%, respectively, compared to 8.7% decomposition and 3.3% defluorination without humic acid (Guo et al., 2019).
4 Discussion
The UV-Fenton process represents a significant advancement in PFAS degradation technology, combining hydroxyl radical and ferrous generation with photolytic enhancement. This process operates through a two-step mechanism: first generating radical species (predominantly hydroxyl radicals) from hydrogen peroxide, followed by iron activation under UV radiation. Research by Tang et al. (2012) demonstrated using this method, hydroxyl radicals serve as the primary oxidizing species in PFOA degradation.
The wavelength of UV light significantly impacts treatment efficiency. While 254 nm UVC alone showed limited effectiveness, Schlesinger et al. reported that incorporating nanosized magnetite (Fe3O4) as a catalyst with UV-Fenton treatment achieved impressive 90% PFAS destruction rates with just 30 min of UVC exposure. Additionally, studies comparing 254 nm UVC with 185 nm VUV light found that the latter degraded 61.7% of PFOA with 17.1% defluorination within 2 hours, demonstrating the importance of selecting appropriate UV parameters.
Solar-enhanced Fenton treatment offers a more sustainable alternative to artificial UV sources. Hajalifard et al. observed significantly improved PFAS degradation efficiency when conventional Fenton treatment was paired with solar radiation, suggesting potential for more energy-efficient treatment systems that leverage natural light sources.
UV-Fenton systems offer moderate scalability with balanced cost profiles compared to alternatives. While thermal methods provide the most complete destruction, they carry prohibitive energy costs at scale. Filtration technologies represent the most immediately scalable approach but address contaminant transfer rather than destruction. The UV-Fenton process strikes a balance, offering destruction rather than mere separation while requiring less energy than thermal approaches.
Cost considerations vary significantly across technologies. Nanomaterial-based approaches entail higher material costs but potentially lower operational expenses. UV-Fenton systems require moderate capital investment for UV infrastructure but benefit from widely available and relatively inexpensive iron and hydrogen peroxide reagents. Solar-enhanced versions could substantially reduce operating costs in suitable geographic locations.
Reliability factors strongly favor more established technologies like filtration, which consistently removes PFAS regardless of specific compound properties. Advanced oxidation processes including UV-Fenton show some variability in effectiveness depending on specific PFAS structures, with perfluorinated compounds showing greater resistance to hydroxyl radical attack due to limited reactive sites. However, modified approaches incorporating additional catalysts demonstrate promising improvements in reliability across a broader spectrum of PFAS compounds.
With the increasing urgency to address PFAS contamination in water, Photo-Assisted Anodic Fenton treatment has a particularly promising solution, especially when key operational parameters are optimized. The process relies on the synergistic action of Fenton chemistry, using iron as a catalyst and hydrogen peroxide as the oxidant, enhanced by UV irradiation and supported by a carefully chosen electrolyte. By optimizing the ratio of iron to hydrogen peroxide, the system ensures efficient generation of hydroxyl radicals without excess reagent wastage or undesirable side reactions, as too little iron can limit catalytic cycles, while excessive iron may lead to unwanted radical scavenging. The UV wavelength plays a crucial role as well, which accelerates both the breakdown of hydrogen peroxide into reactive radicals and the regeneration of Fe2+ from Fe3+, thereby maintaining a steady state of catalysis and maximizing oxidative capacity. Additionally, leveraging the right electrolyte improves solution conductivity, stability, and often helps regulate the pH, which is vital for sustaining the optimal performance of the Fenton cycle and minimizing competing reactions that could otherwise hamper PFAS degradation.
When these parameters are carefully balanced, P-AAFT is expected to exhibits remarkable effectiveness, regularly achieving high removal rates of challenging PFAS compounds such as PFCAs and PFSAs within practical reaction times and across a range of real water matrices. This integrated approach overcomes the limitations of classical Fenton and photolysis alone by harnessing the benefits of enhanced radical production, catalyst recycling, and electrochemical control. Ultimately, the robust operational flexibility and proven scalability of this system, along with its adaptability to varying contamination loads and environmental conditions, position P-AAFT as a leading-edge technology for PFAS remediation in water treatment applications.
5 Future work and potential research gaps
Future research into PFAS degradation should address both improvements in Fenton Chemistry-based approaches and the challenges associated with non-Fenton alternatives. It should emphasize overcoming technical barriers and optimizing processes for efficient, safe, and scalable remediation. Research should focus on optimizing key operational parameters, such as reaction time, pH, oxidant dosages, and temperature, since inappropriate settings may result in incomplete degradation and formation of potentially even more toxic or recalcitrant by-products The analytical detection of all possible PFAS and their transformation or degradation byproducts remains unproven; current methods are unable to comprehensively identify and quantify the complexity of PFAS mixtures and their intermediate products.
Research into nanostructured Fenton catalysts should also be evaluated as they can function effectively at neutral pH levels often found in drinking water, in contrast to traditional Fenton processes that perform optimally at acidic pH. By selecting appropriate concentrations of nanomaterials and oxidants (e.g., H2O2), high PFAS degradation can be achieved, broadening real-world applicability and making the process adaptable for varied water matrices. Also, nanomaterials can be engineered with tunable surface charge and structure, enabling enhanced adsorption of negatively charged PFAS, pre-concentrating pollutants near catalytic sites to boost Fenton oxidation effectiveness. This tunability allows selectivity towards specific PFAS molecules and facilitates targeted degradation.
There is significant potential in developing improved electrode materials and alternative reactor architectures (such as tubular or multi-anode systems) to boost degradation kinetics, minimize fouling, and reduce operational costs, particularly for electro-Fenton processes. Strategies to boost the generation and availability of reactive oxygen species (ROS) and hydroxyl radicals, especially in the bulk solution and electrode surface, could address current mass-transfer limitations and improve removal rates. The use of UV-Fenton reactions and nanoparticle-mediated Fenton-like processes has shown high PFAS degradation efficiencies, suggesting additional research is warranted into novel catalyst supports and activation methods. Research should also prioritize the development of sustainable, energy-efficient Fenton-based methods that can be upscaled for real-world application, including strategies to minimize energy consumption and overall cost.
Despite numerous studies, critical gaps remain in the understanding and management of PFAS. Further research is needed to better comprehend the long-term environmental and health impacts of PFAS exposure. While current studies have highlighted associations between PFAS exposure and various health outcomes, such as cancer, liver damage, and thyroid disease, the mechanisms of toxicity remain underexplored. Investigating the molecular pathways through which PFAS exert their effects is crucial for developing targeted mitigation strategies.
Additionally, there is a pressing need for research into effective remediation techniques. Conventional water and soil treatment methods often fall short in removing PFAS due to their strong carbon-fluorine bonds. Innovative approaches, such as advanced oxidation processes (AOPs), electrochemical treatment, and bioremediation, warrant further exploration. Understanding the kinetics and mechanisms of these treatment methods can lead to more efficient and scalable solutions. Technologies that perform well for long-chain PFAS often fail for short-chain varieties or other PFAS classes. These classes tend to be more mobile in the environment and harder to capture or destroy, leading to incomplete remediation in mixed-contaminant settings. Technologies such as adsorption and ion exchange typically generate secondary PFAS-laden wastes (spent sorbents, regeneration brines, membrane concentrates). These residuals require further handling and, ideally, ultimate destruction, compounding the remediation burden. Current detection methods are limited to a narrow range of PFAS out of the thousands identified in commerce, and many precursors or transformation products elude analytics, creating uncertainty about treatment completeness and environmental risk. The impact of co-contaminants or mixed pollution scenarios on PFAS degradation rates, and the possible interactions that inhibit or accelerate PFAS breakdown, are not fully characterized.
Another gap lies in the regulation and monitoring of PFAS. Current regulatory frameworks vary widely across regions, leading to inconsistent standards and enforcement. Comparative studies on the effectiveness of different regulatory approaches and the development of unified guidelines could help harmonize efforts globally.
Moreover, research should focus on the development of alternative, less harmful chemicals to replace PFAS in industrial and consumer products. Identifying and evaluating the safety profiles of these substitutes is essential to prevent future environmental and health crises.
By addressing future research work and gaps, researchers can contribute to a more comprehensive understanding of PFAS and develop strategies to mitigate their impact. Based on the reviewed literature, P-AAFT could play a pivotal role in these efforts.
6 Conclusion
In conclusion, the exploration of PFAS research has underscored the critical need for understanding their long-term environmental and health impacts. The potential associations with severe health outcomes and the persistent nature of PFAS in the environment emphasize the urgency for continued research.
Advanced treatment methods such as AOPs, electrochemical treatment, and bioremediation offer promising avenues for effective degradation The ongoing efforts to unravel the kinetics and mechanisms of these methods are integral to developing scalable solutions that can be widely implemented. PFAS, colloquially referred to as “forever chemicals,” are found globally in air, water, soil, and living organisms, from polar regions to urban centers, largely due to their chemical stability and widespread industrial and commercial applications. Their environmental persistence and tendency to bioaccumulate through ecosystems raise significant concerns about long-term ecological and human health risks. Moreover, their resistance to conventional remediation approaches complicates efforts to mitigate exposure and reduce their environmental footprint.
Efforts to degrade PFAS compounds have increasingly focused on advanced oxidation processes, notably those employing modified Fenton chemistry. The P-AAFT process leverages the generation of powerful reactive oxygen species, often hydroxyl radicals, capable of attacking and breaking the stable carbon-fluorine bonds that make PFAS so recalcitrant.
The research field remains dynamic, urgently needing to bridge the gap between laboratory efficacy and real-world effectiveness. Key directions include improving our understanding of transformation pathways, optimizing treatment conditions for complex and dilute environmental matrices, and marrying oxidative technologies with preventive policies to stem PFAS release at the source. Furthermore, holistic management must account for the safe handling and final destruction of treatment residuals to prevent secondary pollution cycles. While significant hurdles remain, the combined advances in environmental monitoring, regulatory policy, and chemical remediation, epitomized by innovations in modified Fenton treatment, offer hope for gradually mitigating the legacy of PFAS contamination and safeguarding both environmental and public health for future generations.
Author contributions
TW: Conceptualization, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review and editing. KM: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Title III, HBGI, supported this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amin, M. A., Sobhani, Z., Liu, Y., Dharmaraja, R., Chadalavada, S., Naidu, R., et al. (2020). Recent advances in the analysis of per- and polyfluoroalkyl substances (PFAS)—a review. Environ. Technol. and Innovation 19, 100879. doi:10.1016/j.eti.2020.100879
Andrews, D. Q., and Naidenko, O. V. (2020). Population-wide exposure to per- and polyfluoroalkyl substances from drinking water in the United States. Environ. Sci. and Technol. Lett. 7, 931–936. doi:10.1021/acs.estlett.0c00713
Babayev, M., Capozzi, S. L., Miller, P. S., McLaughlin, K. R., Medina, S. S., Byrne, S., et al. (2022). PFAS in drinking water and serum of the people of a southeast Alaska community: a pilot study. Environ. Pollut. 305, 119246. doi:10.1016/j.envpol.2022.119246
Bai, X., and Son, Y. (2021). Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA. Sci. Total Environ. 751, 141622. doi:10.1016/j.scitotenv.2020.141622
Barisci, S., and Suri, R. (2021). Electrooxidation of short- and long-chain perfluoroalkyl substances (PFASs) under different process conditions. J. Environ. Chem. Eng. 9 (4), 105323. doi:10.1016/j.jece.2021.105323
Barışçı, S., and Suri, R. P. (2021). Removal of polyfluorinated telomer alcohol by advanced oxidation processes (AOPs) in different water matrices and evaluation of degradation mechanisms. J. Water Process Eng. 39, 101745. doi:10.1016/j.jwpe.2020.101745
Barreca, S., Busetto, M., Vitelli, M., Colzani, L., Clerici, L., and Dellavedova, P. (2018). Online solid-phase extraction LC-MS/MS: a rapid and valid method for the determination of perfluorinated compounds at sub ng•L−1 level in natural water. J. Chem. 2018 (1), 1–9. doi:10.1155/2018/3780825
Behr, A., Plinsch, C., Braeuning, A., and Buhrke, T. (2020). Activation of human nuclear receptors by perfluoroalkylated substances (PFAS). Toxicol. Vitro 62, 104700. doi:10.1016/j.tiv.2019.104700
Bello, M. M., Raman, A. A., and Asghar, A. (2019). A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Chem. Eng. Res. and Des. 126, 119–140. doi:10.1016/j.psep.2019.03.028
Berhanu, A., Mutanda, I., Taolin, J., Qaria, M. A., Yang, B., and Zhu, D. (2023). A review of microbial degradation of per- and polyfluoroalkyl substances (PFAS): biotransformation routes and enzymes. Sci. Total Environ. 859 (Pt 1), 160010. doi:10.1016/j.scitotenv.2022.160010
Blake, B. E., Cope, H. A., Hall, S. M., Keys, R. D., Mahler, B. W., McCord, J., et al. (2020). Evaluation of maternal, embryo, and placental effects in CD-1 mice following gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide dimer acid (HFPO-DA or GenX). Environ. Health Perspect. 128 (2), 27006. doi:10.1289/ehp6233
Blake, B. E., Miller, C. N., Nguyen, H., Chappell, V. A., Phan, T. P., Phadke, D. P., et al. (2022). Transcriptional pathways linked to fetal and maternal hepatic dysfunction caused by gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) in CD-1 mice. Ecotoxicol. Environ. Saf. 248, 114314. doi:10.1016/j.ecoenv.2022.114314
Blake, B. E., Pinney, S. M., Hines, E. P., Fenton, S. E., and Ferguson, K. K. (2018). Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ. Pollut. 242 (Pt A), 894–904. doi:10.1016/j.envpol.2018.07.042
Boca Raton, F. L., Herrera, F., Ramírez-Canon, A., and Becerra-Quiroz, A. (2022). Perfluoroalkyl and polyfluoroalkyl substances (PFAS): first survey in water samples from the Bogotá River, Colombia. Environ. Adv. 8, 100223. doi:10.1016/j.envadv.2022.100223
Borthakur, A., Wang, M., He, M., Ascencio, K., Blotevogel, J., Adamson, D. T., et al. (2021). Perfluoroalkyl acids on suspended particles: significant transport pathways in surface runoff, surface waters, and subsurface soils. J. Hazard Mater. 417, 126159. doi:10.1016/j.jhazmat.2021.126159
Brandsma, S. H., Koekkoek, J. C., van Velzen, M. J. M., and de Boer, J. (2019). The PFOA substitute GenX detected in the environment near a fluoropolymer manufacturing plant in The Netherlands. Chemosphere 220, 493–500. doi:10.1016/j.chemosphere.2018.12.135
Brennan, N. M., Evans, A. T., Fritz, M. K., Peak, S. A., and Von Holst, H. E. (2021). Trends in the regulation of per- and polyfluoroalkyl substances (PFAS): a scoping review. Int. J. Environ. Res. Public Health 18 (20), 10900. doi:10.3390/ijerph182010900
Brusseau, M. L., Anderson, R. H., and Guo, B. (2020). PFAS concentrations in soils: background levels versus contaminated sites. Sci. Total Environ. 740, 140017. doi:10.1016/j.scitotenv.2020.140017
Cardoso, I. M. F., Da Silva, L. P., and Da Silva, J. C. G. E. (2023). Nanomaterial-based advanced oxidation/reduction processes for the degradation of PFAS. Nanomaterials 13, 1668. doi:10.3390/nano13101668
Carlson, G. L., and Tupper, S. (2020). Ski wax use contributes to environmental contamination by per- and polyfluoroalkyl substances. Chemosphere 261, 128078. doi:10.1016/j.chemosphere.2020.128078
Carlson, L. M., Angrish, M., Shirke, A. V., Radke, E. G., Schulz, B., Kraft, A., et al. (2022). Systematic evidence map for over one hundred and fifty per- and polyfluoroalkyl substances (PFAS). Environ. Health Perspect. 130 (5), 56001. doi:10.1289/ehp10343
Carnero, A. R., Lestido-Cardama, A., Loureiro, P. V., Barbosa-Pereira, L., De Quirós, A. R., and Sendón, R. (2021). Presence of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in food contact materials (FCM) and its migration to food. Foods 10 (7), 1443. doi:10.3390/foods10071443
Chen, J. M., Zhang, P., and Liu, J. (2007). Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light. J. Environ. Sci. 19 (4), 387–390. doi:10.1016/s1001-0742(07)60064-3
Choi, H., Bae, I., Choi, J. C., Park, S., and Kim, M. (2018). Perfluorinated compounds in food simulants after migration from fluorocarbon resin-coated frying pans, baking utensils, and non-stick baking papers on the Korean market. Food Addit. Contam. Part B 11 (4), 264–272. doi:10.1080/19393210.2018.1499677
Chow, S., Ojeda, N., Jacangelo, J. G., and Schwab, K. J. (2021). Detection of ultrashort-chain and other per- and polyfluoroalkyl substances (PFAS) in U.S. bottled water. Water Res. 201, 117292. doi:10.1016/j.watres.2021.117292
Dale, K., Yadetie, F., Horvli, T., Zhang, X., Frøysa, H. G., Karlsen, O. A., et al. (2022). Single PFAS and PFAS mixtures affect nuclear receptor- and oxidative stress-related pathways in precision-cut liver slices of Atlantic cod (Gadus morhua). Sci. Total Environ. 814, 152732. doi:10.1016/j.scitotenv.2021.152732
Davalos, J. C. Q., Michaud, M. A., Lowe, L. E., Hanson, E. N., and Owens, J. E. (2022). Per- and polyfluoroalkyl substances (PFASs) in the Fountain Creek watershed, Colorado Springs, CO, USA: a yearlong investigation of PFAS levels in water, soils, and sediments. ACS ES&T Water 3 (1), 96–105. doi:10.1021/acsestwater.2c00440
Dong, Z., Wang, H., Yu, Y. Y., Li, Y. B., Naidu, R., and Liu, Y. (2019). Using 2003–2014 U.S. NHANES data to determine the associations between per- and polyfluoroalkyl substances and cholesterol: trend and implications. Ecotoxicol. Environ. Saf. 173, 461–468. doi:10.1016/j.ecoenv.2019.02.061
Drenning, P., Volchko, Y., Ahrens, L., Rosen, L., Soderqvist, T., and Norrman, J. (2023). Comparison of PFAS soil remediation alternatives at a civilian airport using cost-benefit analysis. Sci. Total Environ. 882, 163664. doi:10.1016/j.scitotenv.2023.163664
Ducatman, A., and Fenton, S. E. (2022). Erratum: invited perspective: PFAS and liver disease: bringing all the evidence together. Environ. Health Perspect. 130 (6), 69001. doi:10.1289/ehp11560
Duchesne, A. L., Brown, J. K., Patch, D. J., Major, D., Weber, K. P., and Gerhard, J. I. (2020). Remediation of PFAS-contaminated soil and granular activated carbon by smoldering combustion. Environ. Sci. Technol. 54 (19), 12631–12640. doi:10.1021/acs.est.0c03058
European Commission (2022). The monitoring of perfluoroalkyl substances in food. J. Eur. Union 221, 105–109. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022H1431.
Evangelou, M. W. H., and Robinson, B. H. (2022). The phytomanagement of PFAS-contaminated land. Int. J. Environ. Res. Public Health 19, 6817. doi:10.3390/ijerph19116817
Evich, M. G., Davis, M. J. B., McCord, J. P., Acrey, B., Awkerman, J. A., Knappe, D. R. U., et al. (2022). Per- and polyfluoroalkyl substances in the environment. Science 375 (6580), eabg9065. doi:10.1126/science.abg9065
Fan, X., Tang, S., Wang, Y., Fan, W., Ben, Y., Naidu, R., et al. (2022). Global exposure to per- and polyfluoroalkyl substances and associated burden of low birthweight. Environ. Sci. Technol. 56 (7), 4282–4294. doi:10.1021/acs.est.1c08669
Fang, C., Sobhani, Z., Niu, J., and Naidu, R. (2019). Removal of PFAS from aqueous solution using PbO(2) from lead-acid battery. Chemosphere 219, 36–44. doi:10.1016/j.chemosphere.2018.11.206
Fenton, H. J. H. (1894). LXXIII.—oxidation of tartaric acid in presence of iron. J. Chem. Soc. 65, 899–910. doi:10.1039/ct8946500899
Fischer, F. C., Ludtke, S., Thackray, C. P., Pickard, H. M., Haque, F., Dassuncao, C., et al. (2024). Binding of per- and polyfluoroalkyl substances (PFAS) to serum proteins: implications for toxicokinetics in humans. Environ. Sci. and Technol. 58 (2), 1055–1063. doi:10.1021/acs.est.3c07415
Ganiyu, S. O., Nidheesh, P. V., and Oturan, M. A. (2022). “Synthesis and application of nanostructured iron oxides heterogeneous catalysts for environmental applications,” in Advanced materials for sustainable environmental remediation: terrestrial and aquatic environments. Editors D. Giannakoudakis, L. Meili, and I. Anastopoulos (Amsterdam, Netherlands: Elsevier), 583–608.
Genualdi, S., Beekman, J. K., Carlos, K. S., Fisher, C. M., Young, W. B., DeJager, L., et al. (2021). Analysis of per- and poly-fluoroalkyl substances (PFAS) in processed foods from FDA’s Total Diet Study. Anal. and Bioanal. Chem. 414 (3), 1189–1199. doi:10.1007/s00216-021-03610-2
Glaze, W. H., Kang, J. W., and Chapin, D. H. (1987). The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. and Eng. 9 (4), 335–352. doi:10.1080/01919518708552148
Glenn, G., Shogren, R., Jin, X., Orts, W., Hart-Cooper, W., and Olson, L. (2021). Per- and polyfluoroalkyl substances and their alternatives in paper food packaging. Compr. Rev. Food Sci. Food Saf. 20 (3), 2596–2625. doi:10.1111/1541-4337.12726
Gomri, C., Makhoul, E., Koundia, F. N., Petit, E., Raffy, S., Bechelany, M., et al. (2024). Electrochemical advanced oxidation combined to electro-Fenton for effective treatment of perfluoroalkyl substances “PFAS” in water using a Magnéli phase-based anode. Nanoscale Adv. 7, 261–268. doi:10.1039/d4na00626g
Graber, J. M., Alexander, C., Laumbach, R., Black, K., Strickland, P. O., Georgopoulos, P. G., et al. (2019). Per and polyfluoroalkyl substances (PFAS) blood levels after contamination of a community water supply and comparison with 2013–2014 NHANES. J. Expo. Sci. and Environ. Epidemiol. 29 (2), 172–182. doi:10.1038/s41370-018-0096-z
Granby, K., and Tesdal Håland, J. (2018). Per- and polyfluorinated alkyl substances (PFAS) in paper and board food contact materials—selected samples from the Norwegian market 2017. Kongens Lyngby, Denmark: Technical University of Denmark.
Guo, C., Zhang, C., Sun, Z., Zhao, X. Y., Zhou, Q., and Hoffmann, M. R. (2019). Synergistic impact of humic acid on the photo-reductive decomposition of perfluorooctanoic acid. Chem. Eng. J. 360, 1101–1110. doi:10.1016/j.cej.2018.10.204
Hajalifard, Z., Kazemi, S., Eftekhari, S., Rezaei, S., Mousazadeh, M., and Usman, M. (2023). Per- and polyfluoroalkyl substances degradation using hydroxyl- and sulphate-radical-based advanced oxidation from water matrices: which one is the best approach? Int. J. Environ. Anal. Chem. 104 (20), 9128–9152. doi:10.1080/03067319.2023.2225412
Hale, S. E., Arp, H. P. H., Slinde, G. A., Wade, E. J., Bjorseth, K., Breedveld, G. D., et al. (2017). Sorbent amendment as a remediation strategy to reduce PFAS mobility and leaching in a contaminated sandy soil from a Norwegian firefighting training facility. Chemosphere 171, 9–18. doi:10.1016/j.chemosphere.2016.12.057
Haynes, W. M., Lide, D. R., and Bruno, T. J. (2014). “Physical constants of organic compounds,” in CRC handbook of chemistry and physics. 95th ed., 579.
Hu, X. C., Andrews, D. Q., Lindstrom, A. B., Bruton, T. A., Schaider, L. A., Grandjean, P., et al. (2016). Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. Drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ. Sci. and Technol. Lett. 3 (10), 344–350. doi:10.1021/acs.estlett.6b00260
Huang, S., Xi-Yun, W., Zhang, Y., Meng, Y., Hua, F., and Xia, X. (2022). Cellulose nanofibers/polyvinyl alcohol blends as an efficient coating to improve the hydrophobic and oleophobic properties of paper. Sci. Rep. 12 (1), 16148. doi:10.1038/s41598-022-20499-8
Inoue, Y., Hashizume, N., Yakata, N., Murakami, H., Suzuki, Y., Kikushima, E., et al. (2011). Unique physicochemical properties of perfluorinated compounds and their bioconcentration in common carp Cyprinus carpio L. Archives Environ. Contam. Toxicol. 62 (4), 672–680. doi:10.1007/s00244-011-9730-7
Jones, B., and Malley, J. P. (2023). Focus on water | destruction of PFAS by magnetite nanoparticle-catalyzed UV-Fenton reaction. UV Solutions Innovations for Industry, Public Health Environment.
Knutsen, H. K., Alexander, J., Barregård, L., Bignami, M., Brüschweiler, B., Ceccatelli, S., et al. (2018). Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J. 16 (12), e05194. doi:10.2903/j.efsa.2018.5194
Koch, A., Karrman, A., Yeung, L. W. Y., Jonsson, M., Ahrens, L., and Wang, T. (2019). Point source characterization of per- and polyfluoroalkyl substances (PFASs) and extractable organofluorine (EOF) in freshwater and aquatic invertebrates. Environ. Sci. Process Impacts 21 (11), 1887–1898. doi:10.1039/c9em00281b
Koppenol, W. H. (2001). The Haber-Weiss cycle—70 years later. Redox Rep. 6 (4), 229–234. doi:10.1179/135100001101536373
Kutsuna, S., and Hori, H. (2008). Experimental determination of Henry’s law constant of perfluorooctanoic acid (PFOA) at 298k by means of an inert-gas stripping method with a helical plate. Atmos. Environ. 42 (39), 8883–8892. doi:10.1016/j.atmosenv.2008.09.008
LaKind, J. S., Verner, M. A., Rogers, R. D., Goeden, H., Naiman, D. Q., Marchitti, S. A., et al. (2023). Erratum: current breast milk PFAS levels in the United States and Canada: after all this time, why don’t we know more? Environ. Health Perspect. 131 (3), 39001. doi:10.1289/ehp12915
Langberg, H. A., Hale, S. E., Breedveld, G. D., Jenssen, B. M., and Jartun, M. (2022). A review of PFAS fingerprints in fish from Norwegian freshwater bodies subject to different source inputs. Environ. Sci. Process Impacts 24 (2), 330–342. doi:10.1039/d1em00408e
Lau, C., Anitole, K., Hodes, C. S., Lai, D. Y., Pfahles-Hutchens, A., and Seed, J. (2007). Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol. Sci. 99 (2), 366–394. doi:10.1093/toxsci/kfm128
Lesmeister, L., Lange, F. T., Breuer, J., Biegel-Engler, A., Giese, E., and Scheurer, M. (2021). Extending the knowledge about PFAS bioaccumulation factors for agricultural plants—a review. Sci. Total Environ. 766, 142640. doi:10.1016/j.scitotenv.2020.142640
Li, Y., Fletcher, T., Mucs, D., Scott, K., Lindh, C. H., Tallving, P., et al. (2018). Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 75 (1), 46–51. doi:10.1136/oemed-2017-104651
Liu, C., Zhao, X., De Faria, A. F., Quiñones, K. Y. D., Zhang, C., He, Q., et al. (2022). Evaluating the efficiency of nanofiltration and reverse osmosis membrane processes for the removal of per- and polyfluoroalkyl substances from water: a critical review. Sep. Purif. Technol. 302, 122161. doi:10.1016/j.seppur.2022.122161
Liu, M., Munoz, G., Vo Duy, S., Sauvé, S., and Liu, J. (2021). Per- and polyfluoroalkyl substances in contaminated soil and groundwater at airports: a Canadian case study. Environ. Sci. and Technol. 56 (2), 885–895. doi:10.1021/acs.est.1c04798
Liu, Y., Chen, S., Quan, X., Yu, H., Zhao, H., and Zhang, Y. (2015). Efficient mineralization of perfluorooctanoate by electro-fenton with H2O2 electro-generated on hierarchically porous carbon. Environ. Sci. and Technol. 49 (22), 13528–13533. doi:10.1021/acs.est.5b03147
Logeshwaran, P., Sivaram, A. K., Surapaneni, A., Kannan, K., Naidu, R., and Megharaj, M. (2021). Exposure to perfluorooctanesulfonate (PFOS) but not perflurorooctanoic acid (PFOA) at ppb concentration induces chronic toxicity in Daphnia carinata. Sci. Total Environ. 769, 144577. doi:10.1016/j.scitotenv.2020.144577
Ma, T., Ye, C., Wang, T., Li, X., and Luo, Y. (2022). Toxicity of per- and polyfluoroalkyl substances to aquatic invertebrates, planktons, and microorganisms. Int. J. Environ. Res. Public Health 19 (24), 16729. doi:10.3390/ijerph192416729
Manning, I. N., Guan Pin Chew, H., Macdonald, K., Miller, M., Strynar, O., Coronell, F., et al. (2022). Hydrolytically stable ionic fluorogels for high-performance remediation of per- and polyfluoroalkyl substances (PFAS) from natural water. Angew. Chem. 61 (41), e202208150. doi:10.1002/anie.202208150
Masood, A. S., Ali, M. S., Manzar, M. S., Khan, N. A., and Khan, A. H. (2023). Current situation of pharmaceutical wastewater around the globe. Elsevier eBooks.
Mastropietro, T. F., Bruno, R., Pardo, E., and Armentano, D. (2021). Reverse osmosis and nanofiltration membranes for highly efficient PFASs removal: overview, challenges and future perspectives. Dalton Trans. 50 (16), 5398–5410. doi:10.1039/d1dt00360g
Masud, A., Guardian, M. G. E., Travis, S. C., Chavez Soria, S. G., Jarin, M., Aga, D. S., et al. (2021). Redox-active rGO-nZVI nanohybrid-catalyzed chain shortening of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS). J. Hazard. Mater. Lett. 2, 100007. doi:10.1016/j.hazl.2020.100007
Matheny, K. (2018). DEQ: harmful PFAS might contaminate more than 11,000 sites statewide. Detroit Free Press. Available online at: https://www.freep.com/story/news/local/michigan/2018/07/30/deq-pfas-chemical-contamination-pollution-michigan/851152002/.
Militao, I. M., Roddick, F., Fan, L., Zepeda, L. C., Parthasarathy, R., and Bergamasco, R. (2023). PFAS removal from water by adsorption with alginate-encapsulated plant albumin and rice straw-derived biochar. J. Water Process Eng. 53, 103616. doi:10.1016/j.jwpe.2023.103616
Mitchell, S. G., Ahmad, M., Teel, A. L., and Watts, R. A. (2013). Degradation of perfluorooctanoic acid by reactive species generated through catalyzed H2O2 propagation reactions. Environ. Sci. Technol. Lett. 1 (1), 117–121. doi:10.1021/ez4000862
Munoz, G., Liu, M., Duy, S. V., Liu, J., and Sauvé, S. (2023). Target and nontarget screening of PFAS in drinking water for a large-scale survey of urban and rural communities in Québec, Canada. Water Res. 233, 119750. doi:10.1016/j.watres.2023.119750
Nason, S. L., Koelmel, J. P., Zuverza-Mena, N., Stanley, C. J., Tamez, C., Bowden, J. A., et al. (2021). Software comparison for nontargeted analysis of PFAS in AFFF-contaminated soil. J. Am. Soc. Mass Spectrom. 32 (4), 840–846. doi:10.1021/jasms.0c00261
Nhu, H. D. H., Van Tri, D., Luu, T. L., Trippel, J., and Wagner, M. (2025). Degradation of 29 per- and poly-fluoroalkyl substances (PFAS) in water using fenton-assisted electrochemical oxidation process. Sep. Purif. Technol. 362, 131908. doi:10.1016/j.seppur.2025.131908
Olatunde, O. C., Kuvarega, A. T., and Onwudiwe, D. C. (2020). Photo enhanced degradation of polyfluoroalkyl and perfluoroalkyl substances. Heliyon 6 (12), e05614. doi:10.1016/j.heliyon.2020.e05614
Olsen, G. W. (2015). “PFAS biomonitoring in higher exposed populations,” in Toxicological effects of perfluoroalkyl and polyfluoroalkyl substances. Editor J. C. DeWitt (Cham, Switzerland: Springer International), 77–125.
Panizza, M., and Cerisola, G. (2009). Electro-Fenton degradation of synthetic dyes. Water Res. 43 (2), 339–344. doi:10.1016/j.watres.2008.10.028
Patch, D., O’Connor, N., Ahmed, E., Houtz, E., Bentel, M., Ross, I., et al. (2024). Advancing PFAS characterization: development and optimization of a UV-H2O2-TOP assay for improved PFCA chain length preservation and organic matter tolerance. Sci. Total Environ. 946, 174079. doi:10.1016/j.scitotenv.2024.174079
Patch, D., O’Connor, N., Koch, I., Cresswell, T., Hughes, C., Davies, J. B., et al. (2022). Elucidating degradation mechanisms for a range of per- and polyfluoroalkyl substances (PFAS) via controlled irradiation studies. Sci. Total Environ. 832, 154941. doi:10.1016/j.scitotenv.2022.154941
Pérez-Poyatos, L. T., Morales-Torres, S., Maldonado-Hódar, F. J., and Pastrana-Martínez, L. M. (2022). Magnetite nanoparticles as solar Photo-Fenton catalysts for the degradation of the 5-Fluorouracil cytostatic drug. Nanomaterials 12 (24), 4438. doi:10.3390/nano12244438
Pietrini, F., Passatore, L., Patti, V., Francocci, F., Giovannozzi, A., and Zacchini, M. (2019). Morpho-physiological and metal accumulation responses of hemp plants (Cannabis sativa L.) grown on soil from an agro-industrial contaminated area. Water 11, 808. doi:10.3390/w11040808
Piva, E., Fais, P., Ioime, P., Cecchetto, G., Viel, G., Forcato, M., et al. (2023). Per- and polyfluoroalkyl substances (PFAS) presence in food: comparison among fresh, frozen and ready-to-eat vegetables. Food Chem. 410, 135415. doi:10.1016/j.foodchem.2023.135415
PubChem (2024). PubChem compound summary for CID 9554, perfluorooctanoic acid. Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/Perfluorooctanoic-acid (Accessed September 12, 2024).
Puttamreddy, S., and Nippatlapalli, N. (2024). Advanced insights into sustainable electrooxidation technique and futuristic strategies: multifaceted approach for PFAS degradation. J. Environ. Chem. Eng. 12 (5), 113307. doi:10.1016/j.jece.2024.113307
Remucal, C. K. (2019). Spatial and temporal variability of perfluoroalkyl substances in the Laurentian Great Lakes. Environ. Sci. Process Impacts 21 (11), 1816–1834. doi:10.1039/c9em00265k
Rickard, B. P., Tan, X., Fenton, S. E., and Rizvi, I. (2022). Select per- and polyfluoroalkyl substances (PFAS) induce resistance to carboplatin in ovarian cancer cell lines. Int. J. Mol. Sci. 23 (9), 5176. doi:10.3390/ijms23095176
Rovero, M., Cutt, D., Griffiths, R., Filipowicz, U., Mishkin, K., White, B., et al. (2021). Limitations of current approaches for predicting groundwater vulnerability from PFAS contamination in the vadose zone. Ground Water Monit. remediat. 41 (4), 62–75. doi:10.1111/gwmr.12485
Saltmiras, D. A., and Lemley, A. T. (2000). Degradation of ethylene thiourea (ETU) with three Fenton treatment processes. J. Agric. Food Chem. 48 (12), 6149–6157. doi:10.1021/jf000084v
Santos, A., Rodríguez, S., Pardo, F., and Romero, A. (2016). Use of Fenton reagent combined with humic acids for the removal of PFOA from contaminated water. Sci. Total Environ. 563–564, 657–663. doi:10.1016/j.scitotenv.2015.09.044
Savu, P. M. (2016). “Fluorinated higher carboxylic acids,” in Kirk-othmer encyclopedia of chemical technology (New York: John Wiley and Sons).
Schaider, L. A., Balan, S. A., Blum, A., Andrews, D. Q., Strynar, M. J., Dickinson, M. E., et al. (2017). Fluorinated compounds in U.S. fast food packagin. Environ. Sci. Technol. Lett. 4 (3), 105–111. doi:10.1021/acs.estlett.6b00435
Schlesinger, D. R., McDermott, C., Le, N. Q., Ko, J. S., Johnson, J. K., Demirev, P. A., et al. (2022). Destruction of per/poly-fluorinated alkyl substances by magnetite nanoparticle-catalyzed UV-Fenton reaction. Environ. Sci. Water Res. Technol. 8 (11), 2732–2743. doi:10.1039/d2ew00058j
Schwartz-Narbonne, H., Xia, C., Shalin, A., Whitehead, H. D., Yang, D., Peaslee, G. F., et al. (2023). Per- and polyfluoroalkyl substances in Canadian fast food packaging. Environ. Sci. Technol. Lett. 10 (4), 343–349. doi:10.1021/acs.estlett.2c00926
Shoemaker, J., and Tettenhorst, D. (2018). Method 537.1: determination of selected per- and polyfluorinated alkyl substances in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS). Cincinnati, OH: US EPA.
Steenland, K., Zhao, L., Winquist, A., and Parks, C. (2013). Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio valley. Environ. Health Perspect. 121 (8), 900–905. doi:10.1289/ehp.1206449
Sun, M., Arevalo, E., Strynar, M. J., Lindstrom, A. B., Richardson, M. K., Kearns, B., et al. (2016). Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear River watershed of North Carolina. Environ. Sci. and Technol. Lett. 3 (12), 415–419. doi:10.1021/acs.estlett.6b00398
Tan, K., Lu, G., Piao, H., Chen, S., Jiao, X., Gai, N., et al. (2017). Current contamination status of perfluoroalkyl substances in tapwater from 17 cities in the eastern China and their correlations with surface waters. Bull. Environ. Contam. Toxicol. 99 (2), 224–231. doi:10.1007/s00128-017-2109-3
Tang, H., Xiang, Q., Lei, M., Yan, J., Zhu, L., and Zou, J. (2012). Efficient degradation of perfluorooctanoic acid by UV–Fenton process. Chem. Eng. J. 184, 156–162. doi:10.1016/j.cej.2012.01.020
Trojanowicz, M., and Koc, M. (2013). Recent developments in methods for analysis of perfluorinated persistent pollutants. Mikrochim. Acta 180 (11–12), 957–971. doi:10.1007/s00604-013-1046-z
Umar, M. (2021). Reductive and oxidative UV degradation of PFAS—status, needs and future perspectives. Water 13 (22), 3185. doi:10.3390/w13223185
US Environmental Protection Agency (2023). Per- and polyfluoroalkyl substances (PFAS): final PFAS national primary drinking water regulation. Available online at: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas#:∼:text=The%20proposed%20PFAS%20NPDWR%20does,of%20serious%20PFAS%2Dattributable%20illnesses.
US Environmental Protection Agency (2017). Technical fact sheet - Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). Available online at: https://19january2021snapshot.epa.gov/sites/static/files/2017-12/documents/ffrrofactsheet_contaminants_pfos_pfoa_11-20-17_508_0.pdf.
US Government Accountability Office (2022). Persistent chemicals: technologies for PFAS assessment, detection, and treatment. Available online at: https://www.gao.gov/products/gao-22-105088.
Valsecchi, S., Babut, M., Mazzoni, M., Pascariello, S., Ferrario, C., De Felice, B., et al. (2020). Per- and polyfluoroalkyl substances (PFAS) in fish from European lakes: current contamination status, sources, and perspectives for monitoring. Environ. Toxicol. Chem. 40 (3), 658–676. doi:10.1002/etc.4815
Verma, S., Lee, T., Sahle-Demessie, E., Ateia, M., and Nadagouda, M. N. (2023). Recent advances on PFAS degradation via thermal and nonthermal methods. Chem. Eng. J. Adv. 13, 100421. doi:10.1016/j.ceja.2022.100421
Wallis, I., Hutson, J., Davis, G., Kookana, R., Rayner, J., Prommer, H., et al. (2022). Model-based identification of vadose zone controls on PFAS mobility under semi-arid climate conditions. Water Res. 222, 119096. doi:10.1016/j.watres.2022.119096
Wang, J., and Niven, R. K. (2021). Unification of surface tension isotherms of PFOA or GenX salts in electrolyte solutions by mean ionic activity. Chemosphere 280, 130715. doi:10.1016/j.chemosphere.2021.130715
Wang, L., Liu, T., Yang, S., Sun, L., Zhao, Z., and Li, L. (2021). Perfluoroalkyl substance pollutants activate the innate immune system through the AIM2 inflammasome. Nat. Commun. 12 (1), 2915. doi:10.1038/s41467-021-23201-0
Wang, M., Guo, W., Gardner, S., Petreas, M., and Park, J. S. (2018). Per- and polyfluoroalkyl substances in Northern California cats: temporal comparison and a possible link to cat hyperthyroidism. Environ. Toxicol. Chem. 37 (10), 2523–2529. doi:10.1002/etc.4239
Wang, Q., and Lemley, A. T. (2001). Kinetic model and optimization of 2,4-D degradation by anodic Fenton treatment. Environ. Sci. Technol. 35 (22), 4509–4514. doi:10.1021/es0109693
Wang, Q., and Lemley, A. T. (2002). Oxidation of diazinon by anodic Fenton treatment. Water Res. 36 (13), 3237–3244. doi:10.1016/s0043-1354(02)00041-6
Wang, S., Cai, Y., Ma, L., Lin, X., Li, Q., Li, Y., et al. (2022). Perfluoroalkyl substances in water, sediment, and fish from a subtropical river of China: environmental behaviors and potential risk. Chemosphere 288 (Pt 1), 132513. doi:10.1016/j.chemosphere.2021.132513
Whitehead, H. D., Venier, M., Wu, Y., Eastman, E., Urbanik, S., Diamond, M. L., et al. (2021). Fluorinated compounds in North American cosmetics. Environ. Sci. and Technol. Lett. 8 (7), 538–544. doi:10.1021/acs.estlett.1c00240
Wu, T. C. (2021). Phytoremediation potential for poly-and perfluoroalkyl substances (PFAS) using various plant species. (Master thesis). Environmental science, Swedish University of Agricultural Sciences. SLU. Available online at: http://urn.kb.se/resolve?urn=urn:nbn:se:slu:epsilon-s-17146
Yamashita, N., Kannan, K., Taniyasu, S., Horii, Y., Okazawa, T., Petrick, G., et al. (2004). Analysis of perfluorinated acids at parts-per-quadrillion levels in seawater using liquid chromatography-tandem mass spectrometry. Environ. Sci. Technol. 38 (21), 5522–5528. doi:10.1021/es0492541
Yamashita, N., Taniyasu, S., Petrick, G., Wei, S., Gamo, T., Lam, P. K., et al. (2008). Perfluorinated acids as novel chemical tracers of global circulation of ocean waters. Chemosphere 70 (7), 1247–1255. doi:10.1016/j.chemosphere.2007.07.079
Yao, Q., Vinturache, A., Lei, X., Wang, Z., Pan, C., Shi, R., et al. (2022). Prenatal exposure to per- and polyfluoroalkyl substances, fetal thyroid hormones, and infant neurodevelopment. Environ. Res. 206, 112561. doi:10.1016/j.envres.2021.112561
Yousefi, A., Omi, F. R., Yang, L., Ganiyu, S. O., Ullah, A., El-Din, M. G., et al. (2025). Innovative hybrid approach for enhanced PFAS degradation and removal: integrating membrane distillation, cathodic electro-Fenton, and anodic oxidation. J. Environ. Manag. 379, 124818. doi:10.1016/j.jenvman.2025.124818
Keywords: per-and polyfluoroalkyl substances (PFAS), environmental remediation, oxidative degradation, advanced oxidation processes (AOPs), fenton chemistry, water contamination, endocrine disruption
Citation: Walker T and Milligan KA (2025) From persistence to progress: assessing per- and polyfluoroalkyl substances (PFAS) environmental impact and advances in photo-assisted fenton chemistry for remediation. Front. Environ. Chem. 6:1591290. doi: 10.3389/fenvc.2025.1591290
Received: 10 March 2025; Accepted: 26 May 2025;
Published: 14 July 2025.
Edited by:
Muhammad Ahmad, Peking University, ChinaReviewed by:
Guangming Li, Tongji University, ChinaHector Alfredo Calderon, National Polytechnic Institute (IPN), Mexico
Copyright © 2025 Walker and Milligan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tasia Walker, VHN3YWxrZXJAZGVzdS5lZHU=
 Tasia Walker
Tasia Walker Kimberly A. Milligan
Kimberly A. Milligan