- 1Department of Chemistry, Chemical Technology and Ecology, K. Kulazhanov Kazakh University of Technology and Business, Astana, Kazakhstan
- 2Major Projects Support Department, KMG Engineering LLP, Astana, Kazakhstan
- 3The Laboratory of Engineering Profile of NMR Spectroscopy, Sh. Ualikhanov Kokshetau University, Kokshetau, Kazakhstan
- 4Department of Biology, School of Sciences and Humanities, Nazarbayev University, Astana, Kazakhstan
- 5Institute of Applied Chemistry LLP, Astana, Kazakhstan
Squalene (C30H50), a triterpene hydrocarbon, a critical precursor in the biosynthesis of cell membrane steroids, is enzymatically derived via squalene synthase in both prokaryotic and eukaryotic organisms. Its remarkable physicochemical properties and potent antioxidant characteristics underlie its extensive application across various sectors including nutraceuticals, pharmaceuticals, cosmetics, and fragrance industries. Historically, squalene has been predominantly sourced from shark liver oil and select plant oils. However, contemporary sustainable considerations have spurred pioneering investigations into unconventional reservoirs. This study reports, for the first time, the extraction of squalene from wastewater of Kazakhstan’s Uzen oil field, identifying an abiotic reservoir with significant scientific and industrial potential. In this study, wastewater samples from the Uzen oil field were collected, extracted, and the composition of dissolved volatile compounds in the extract was investigated using gas chromatography-mass spectrometry (GC-MS). Notably, the predominant constituents were hydrocarbons, which was expected. Intriguingly, the analysis also revealed substantial quantities of squalene–a natural biomarker of oil. This unexpected discovery underscores the significant promise of this unconventional source.
1 Introduction
Squalene, a naturally occurring triterpene, holds remarkable health benefits owing to its diverse metabolic properties and potent oxygen scavenging capabilities (Lou-Bonafonte et al., 2018). Squalene was first discovered in shark liver oil in 1906 (Tsujimoto, 1906) and its chemical structure was determined as an unsaturated hydrocarbon in 1916 (Tsujimoto, 1916). It has since been found in olive oil, amaranth, palm, rice bran, wheat germ oil, and other plants (Nergiz and Celikkale, 2011; Shimizu et al., 2019; Permadi and Wilson, 2024). Shark liver oil contains by far the highest content of natural squalene (Popa et al., 2015), and, therefore, it remains the predominant source of squalene extraction. Nonetheless, the adverse impact on shark populations has steered the exploration of alternative avenues and methodologies. This quest encompasses diverse alternatives, including but not limited to plant and microbial sources, alongside innovative strategies like amplifying microbial squalene yield through genetic engineering approaches (Patel et al., 2022; Shalu et al., 2024). Samman et al. (1981) first reported squalene in petroleum asphaltenes; however, its presence in oilfield wastewater has never been expected or investigated.
The Uzen oil field, located in the arid Southern Mangyshlak region of Kazakhstan, employs water injection techniques to displace oil and maintain reservoir pressure. This process has resulted in an increasing volume of co-produced formation water along with oil, leading to challenges including reservoir contamination and suboptimal oil recovery. While industry standards primarily focus on physical and chemical characteristics of injected water, the role of organic substances in the formation water’s composition and its potential impact on oil and gas generation have often been overlooked (Feyzullayev and Lerche, 2019). It is notable that the monitoring of Water Soluble Organics (WSO), a category of dissolved organic compounds, is frequently underscored in literature under the Clean Water Act (Hart, 2003).
In this context, our study presents a quantitative analysis of dissolved volatile compounds present in the wastewater of the Uzen oil field. By shedding light on the composition of these compounds, we aim to unveil unexpected sources of the valuable substance squalene, thus contributing to a deeper understanding of the complex interactions within oil reservoirs.
2 Materials and methods
2.1 Sample collection and preparation
Wastewater samples from the Uzen oil field were collected at the outlet of Preliminary water discharge unit (PWDU-1) prior to Pump-Filter Station. A total volume of 1 L of wastewater was subjected to sequential extraction using three solvents: hexane, followed by ethyl acetate, and finally, dichloromethane (DCM). The sequential use of hexane, ethyl acetate, and dichloromethane established a polarity gradient, maximizing recovery of both non-polar and semi-polar compounds. Samples were collected over three consecutive days in June 2025 at the PWDU-1 outlet, total volume >3 L.
2.2 Instrumentation and analytical conditions
Two independent GC-MS systems were employed: (i) Clarus-SQ 8 and (ii) Agilent 7890A.
The chemical composition of the wastewater extract was analyzed using a Clarus-SQ 8 gas chromatograph equipped with a mass spectrometric detector (PerkinElmer, Waltham, MA, USA). The chromatographic conditions included a capillary column RestekRxi®-1 ms (0.25 mm × 30 m x 0.25 μm) (Restek Corporation, Bellefonte, PA, USA). A sample volume of 1.0 μL was injected with a helium (He) carrier gas flow rate of 1 mL/min and a split ratio of 1:25. The column temperature was set at 45 °C (held for 2 min), followed by a gradient increase of 1.5 °C/min to 200 °C, then a further gradient increase of 15 °C/min to 280 °C with an isothermal hold at 280 °C for 10 min. The injector temperature was 280 °C, and the mass spectrometric detector source temperature was set at 240 °C with an electron ionization mode (EI+) at 70 eV. The scanning time ranged from 4 to 120 min, and ion scanning was performed within the range of 39–500 m/z.
The chemical composition of the wastewater extract was analyzed using a Agilent 7890A gas chromatograph equipped with a mass spectrometric detector 5975C (Agilent, 5,301 Stevens Creek Blvd. Santa Clara, CA 95051, USA). The chromatographic conditions included a capillary column Rtx®-100-DHA (30 m × 250 μm x 0.5 µm). A sample volume of 2.0 μL was injected with a helium (He) carrier gas flow rate of 0.97 mL/min and a split ratio of 1:20. The column temperature was set at 80 °C (held for 0 min), followed by a gradient increase of 6 °C/min to 300 °C. Run Time was 38.667 min The injector temperature was 280 °C, and the mass spectrometric detector source temperature was set at 230 °C–250 °C with an electron ionization mode (EI+) at 70 eV. The scanning time ranged from 4 to 120 min, and ion scanning was performed within the range of 5–1,000 m/z.
2.3 Quantitative analysis and component identification
The percentage content of components was automatically calculated based on the peak areas of the total ion chromatogram. Component identification was achieved through mass spectra and retention times, utilizing the NIST library. Retention times of components were recalculated relative to the retention times of n-alkanes.
3 Results
The yields of the extracts from 1 L of wastewater by using three solvents sequentially are as follows: hexane 1.48 g, ethyl acetate 0.29 g, and dichloromethane (DCM) 0.21 g.
Analysis of the composition of the wastewater extract by GC-MS (Supplementary Figures S1–S3) resulted in the identification of the following key organic compounds (Supplementary Table S1): (in descending order of major components).
1. The major compounds in the hexane extract were: butyl acetate – 2.7%, N,N-dimethyl-1-dodecanamine – 2.3%, pentacosane – 1.8%, heptadecane – 1.7%, hexadecane – 1.7%, pentadecane – 1.7%, and squalene – 1.4%.
NIST coincidence coefficient of squalene = 950; retention time coincidence with the authentic standard (112.99 min); characteristic ions m/z 69, 81, 410 confirm the identification.
For quantitative analysis, calibration was used according to the squalene standard (Sigma-Aldrich, 99%).
2. The major compounds in ethyl acetate extract were: butyl acetate – 13.2%, pentadecane – 2.9%, hexadecane – 2.7%, tetradecane – 2.6%, heptadecane – 2.5%, tridecane – 2.4%, pentacosane – 2.3%, heptacosane – 2.2%, hexacosane – 2.2%, octadecane – 2.1%, octacosane – 2.0%, tetracosane – 2.0%, genicosane – 2.0%, nonadecane – 2.0%, and dodecane – 2.0%.
3. The major compounds in dichloromethane extract were: xylene – 7.8%, dodecane – 1.7%, and pentadecane – 1.6% (Figure 1).
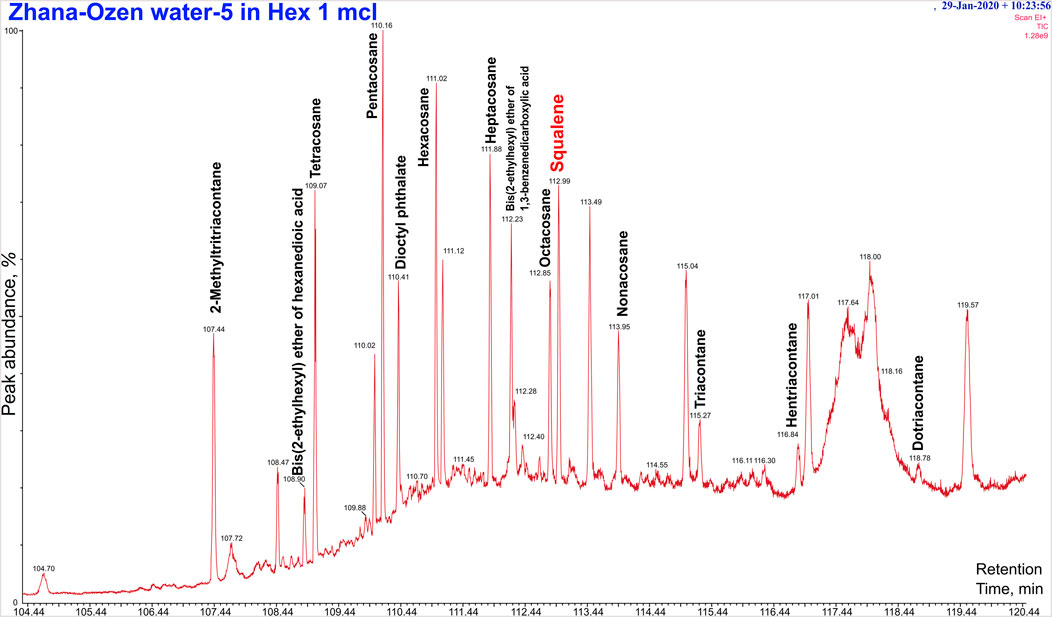
Figure 1. Chromatogram of the chemical composition of wastewater extract during dichloromethane (DCM) extraction, retention time 104.44–120.44 min. Squalene peak is observed at Retention time 112.99 min.
Most of the compounds identified in Uzen Field wastewater extracts belong to hydrocarbons and their derivatives. Some of these compounds have been previously reported in different medicinal plants and have antioxidant, antibacterial, and anti-inflammatory properties (Okechukwu, 2020; Subramanian et al., 2020; Ferdosi et al., 2023). Therefore, the compounds identified from different extracts of Uzen Field wastewater could be used as a valuable source of antioxidants for the food and pharmaceutical industries. More interestingly, the dichloromethane extract of wastewater showed the presence of squalene (Supplementary Figure S3; Supplementary Table S1)—a valuable compound with many health benefits.
From 150 million liters of wastewater processed daily, the estimated hexane-extractable yield is ∼31.5 tons (calculated as 150,000,000 L × 0.21 g/L = 31,500 kg). Consequently, the annual yield reaches approximately 11,500 tons, allowing for the extraction of roughly 160 tons of squalene each year.
The analysis was performed in three independent biological samples (1 L each), each measured in technical duplicates. The concentration of squalene varied from 1.2% to 1.6% of the total extract mass (RSD = 6.4%).
4 Discussion
4.1 Resource identification initiative
The discovery of a significant amount of squalene in the reservoir water of the Uzen oil field holds immense significance, not only as a natural biomarker for petroleum but also as a valuable product in the cosmetics and perfumery industries, and a source of medicinal compounds (Samman et al., 1981; Huang et al., 2009; Lozano-Grande et al., 2018; Albini et al., 2025). The insight shared by renowned chemist Dmitri I. Mendeleev, ‘Burning oil is the same as burning assignments,’ strongly resonates in the context of this study, emphasizing the importance of the potential of oil-associated compounds, such as squalene.
The prospective therapeutic application of squalene could be founded upon its robust antioxidant properties. Administration of a squalene-enriched diet for the therapeutic intervention of prevalent cardiovascular diseases, including hypercholesterolemia, hypertension, and hyperglycemia, demonstrated pronounced quenching activity and potentiation of antioxidant systems by squalene in response to the burst of reactive oxygen and nitrogen species (Ibrahim et al., 2020; Micera et al., 2020).
There is a growing, though still limited, body of evidence that squalene can modulate neuroinflammatory and oxidative-stress pathways relevant to neuroprotection. Chronic injections of Aurantio chytrium extract have been demonstrated to reduce neuroinflammation and exhibit antistress and antidepressant effects, associated with the modulation of neurotransmitter brain systems. Squalene has also been identified as one of the active compounds within the A. chytrium extract (Sasaki et al., 2019). Additionally, chronic squalene injections have shown the ability to prevent oxidative damage in the striatum and the toxicity induced by 6-hydroxydopamine (6-OHDA) in a mouse model of Parkinson’s disease (Kabuto et al., 2013). Mechanistic work on squalene emulsions also shows robust innate and adaptive immune modulation–features that may intersect with neuroimmune pathways (Kim et al., 2020; Mendes et al., 2022). It is recognized that biallelic loss-of-function in squalene synthase (FDFT1) can lead to a neurodevelopmental disorder with early-life seizures and intellectual disability (Coman et al., 2018; 1993). At the same time, no definitive direct anticonvulsant activity for squalene has been established in animal studies or human experiments available to date. Nonetheless, since the roles for neuroinflammation and redox imbalance in epileptogenesis are well established and described, this makes a rationale for testing the anti-seizure activity of squalene (Vezzani et al., 2013; Geronzi et al., 2018; Perry et al., 1988). Although several medicinal plants that biosynthesize triterpenes, including squalene, such as Ricinus communis L. and Artemisia vulgaris L., have yielded extracts with reported anticonvulsant activity (Faheem et al., 2022; Ragasa et al., 2008), attribution to squalene has not been established and may reflect rather a result of multi-component phytochemistry (Tripathi et al., 2011). Overall, these data suggest that squalene’s neuroprotective and anti-seizure potential needs to be studied in targeted experiments. It is also important to note that any extrapolation of neuroactive properties of biological source-derived squalene to squalene obtained from petroleum or oil-field wastewater should be considered hypothetical until direct equivalence is demonstrated empirically. Emulsion adjuvant performance is sensitive to oil purity and oxidation products, which can influence ROS signaling, antigen uptake, and immunomodulatory properties in general (Iyer et al., 2015; Ondrejková et al., 2017; Huang et al., 2018). While adjuvant equivalence has been shown for high-purity plant- and yeast-derived squalene relative to shark-derived controls (Brito et al., 2011; Tateno et al., 2020; Mendes et al., 2022), we were unable to find studies comparing the neuroprotective, anti-inflammatory, or adjuvant properties of wastewater-recovered squalene to biological sources under identical conditions. Based on the principle of chemical identity and the source-agnostic framework used for small-molecule pharmaceutical equivalence (Raw et al., 2011; Dunne et al., 2013), we propose future work to test side-by-side whether petroleum/wastewater-derived squalene that meets pharmacopeial identity/purity criteria exhibits the same level of bioactivity as biologically derived squalene.
The significance of squalene has been further heightened by the global COVID-19 pandemic. Notably, this compound has emerged as an important adjuvant, effectively mitigating the negative effects associated with the administration of coronavirus vaccines (Dormont et al., 2020). Remarkably, among the forefront developers of SARS-CoV-2 vaccines, 17 out of 27 prominently incorporate squalene as an adjuvant, thus exemplifying its multifaceted applications. It is of significance to note that squalene is an inherent component of various plant oils, as evidenced, including oils derived from sources such as amaranth and celery (He et al., 2002). However, despite these alternatives, the most economically viable source for squalene extraction remains the liver of sharks, despite the severe ecological risks their populations face (Mendes et al., 2022).
The race to develop effective COVID-19 vaccines has placed enormous pressure on the availability of squalene sourced from shark livers. The estimates provided by shark conservation groups indicate that the pursuit of an effective coronavirus vaccine could result in the death of around 500,000 sharks annually, that could potentially be saved by utilizing the Uzen oil field wastewater, considering that approximately 3,000 of sharks are required to obtain 1 ton of squalene (Yarkent and Oncel, 2022). This dire predicament underscores the urgent need for sustainable alternatives that do not threaten already vulnerable species (He et al., 2002). The environmental trade-offs between oilfield wastewater extraction and shark liver harvesting remain to be critically evaluated; nonetheless, the proposed approach warrants serious consideration and quantitative assessment.
We hypothesize that the considerable presence of squalene in the oil fields of Western Kazakhstan’s Mangyshlak region is attributed to the historical presence of the Tethys Ocean in this area, which hosted numerous aquatic organisms. Around five to ten million years ago, natural upheavals resulted in the emergence of the Ustyurt Plateau, forcing the oceanic domain to recede and seep into the Earth’s depths, ultimately forming the contemporary Mangyshlak Peninsula. Ongoing scientific discoveries, such as ancient shark and mollusk remains, continue to underscore this transition (Bratishko and Udovichenko, 2013).
Microbial biosynthesis of triterpenes including squalene is well-documented in diverse microorganisms such as bacteria, fungi, and algae, indicating that biotic contributions cannot be fully excluded in petroleum reservoir environments (Shalu et al., 2024). Moreover, engineered microbes including yeasts and bacteria are capable of synthesizing squalene, demonstrating the biosynthetic potential under varied conditions (Ghimire et al., 2016; Chai et al., 2024; Huang et al., 2025). Geochemically, hopanoids—structurally related C30 triterpenoids—are known to preserve well in subsurface petroleum matrices and serve as robust biomarkers. This supports the hypothesis that squalene may also be preserved via analogous diagenetic stabilization pathways.
The annual potential to extract up to 160 tons of squalene from the reservoir water of the Uzen oil field highlights the potential economic viability of this discovery. In case of finding an effective method of extracting the valuable substance, the extraction of squalene from the associated water of the oil field will give a new impetus and look at this unconventional source.
These projections highlight both the economic value of squalene recovery and its potential contribution to regional job creation in Kazakhstan.
In light of these considerations, it is paramount to acknowledge the significance of Yerlan Suleimen’s discovery of squalene in the reservoir water of the Uzen oil field. The subsequent development of technology for its extraction, solidified through intellectual property protection in the form of a patent # 35837 issued by the Republic of Kazakhstan, serves as a testament to the potential for responsible utilization of this invaluable resource. This discovery marks a pivotal milestone in sustainable resource management, balancing economic gains with environmental stewardship.
5 Conclusion
In this study, we have conducted, for the first time, an analysis of the compositional profile of volatile dissolved organic compounds in the wastewater of the Uzen field using gas chromatography-mass spectrometry (GC-MS). The investigation revealed that the primary constituents of the samples are hydrocarbons. Our attention was particularly drawn to the identification of a valuable component, squalene, which was found to be significantly present in the samples.
This finding holds crucial practical implications, as squalene not only possesses valuable industrial applications but also demonstrates substantial medical potential. Its utilization as an adjuvant in coronavirus vaccines, enhancing immune responses, underscores the current relevance and promise of this compound. Moreover, considering the ongoing global efforts to combat various health challenges, including pandemics, the significance of squalene as a potential component in future vaccine formulations becomes increasingly evident.
In conclusion, this study expands our understanding of the chemical composition of Uzen oil field wastewater and emphasizes the importance of exploring squalene as a valuable industrial and biomedical resource.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. AT: Data curation, Formal Analysis, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. GM: Writing – original draft. DK: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – review and editing. BA: Visualization, Writing – review and editing. TT: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grants Nos AP19679527 and BR24992761) and by the Collaborative Research Program of the Nazarbayev University, Republic of Kazakhstan, grant no. 20122022CRP1616 (for TT).
Conflict of interest
Author YS was employed by KMG Engineering LLP. Authors YS, GM, and DK were employed by Institute of Applied Chemistry LLP.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fenvc.2025.1717331.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvc.2025.1672872/full#supplementary-material
References
Albini, A., Corradino, P., Morelli, D., Albini, F., and Noonan, D. (2025). Cosmeceutical and dermatological potential of olive mill wastewater: a sustainable and eco-friendly source of natural ingredients. Cosmetics 12, 142. doi:10.3390/cosmetics12040142
Bratishko, A., and Udovichenko, M. (2013). Fish otoliths from the early oligocene of Mangyshlak, Kazakhstan. N. Jb. Geol. Pal. A 270, 195–208. doi:10.1127/0077-7749/2013/0366
Brito, L. A., Chan, M., Baudner, B., Gallorini, S., Santos, G., O’Hagan, D. T., et al. (2011). An alternative renewable source of squalene for use in emulsion adjuvants. Vaccine 29, 6262–6268. doi:10.1016/j.vaccine.2011.06.067
Chai, L., Che, J., Qi, Q., and Hou, J. (2024). Metabolic engineering for squalene production: advances and perspectives. J. Agric. Food Chem. 72 (50), 27715–27725. doi:10.1021/acs.jafc.4c09608
Coman, D., Vissers, L., Waterham, H., Christodoulou, J., Wevers, R. A., and Pitt, J. (1993). “Squalene synthase deficiency,” in GeneReviews®. Editors M. P. Adam, H. H. Ardinger, R. A. Pagon, S. E. Wallace, L. J. Bean, K. Stephenset al. (Seattle (WA): University of Washington, Seattle).
Coman, D., Vissers, L. E. L. M., Riley, L. G., Kwint, M. P., Hauck, R., Koster, J., et al. (2018). Squalene synthase deficiency: clinical, biochemical, and molecular characterization of a defect in cholesterol biosynthesis. Am. J. Hum. Genet. 103, 125–130. doi:10.1016/j.ajhg.2018.05.004
Dormont, F., Brusini, R., Cailleau, C., Reynaud, F., Peramo, A., Gendron, A., et al. (2020). Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation in rodents. Sci. Adv. 6, eaaz5466. doi:10.1126/sciadv.aaz5466
Dunne, S., Shannon, B., Dunne, C., and Cullen, W. (2013). A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacol. Toxicol. 14, 1. doi:10.1186/2050-6511-14-1
Faheem, M., Ameer, S., Khan, A. W., Haseeb, M., Raza, Q., Ali Shah, F., et al. (2022). A comprehensive review on antiepileptic properties of medicinal plants. Arabian J. Chem. 15, 103478. doi:10.1016/j.arabjc.2021.103478
Ferdosi, M. F. H., Khan, I. H., and Javaid, A. (2023). Bioactive components of ethyl acetate extract of cassia fistula flowers. JAPS 33, 511–517. doi:10.36899/JAPS.2023.3.0643
Feyzullayev, A. A., and Lerche, I. (2019). Organic matter in rock–water systems of petroliferous basins: interrelationships (a case study: south Caspian Basin). Oil Gas. Sci. Technol. – Rev. IFP Energies Nouv. 74, 74. doi:10.2516/ogst/2019046
Geronzi, U., Lotti, F., and Grosso, S. (2018). Oxidative stress in epilepsy. Expert Rev. Neurother. 18, 427–434. doi:10.1080/14737175.2018.1465410
Ghimire, G. P., Thuan, N. H., Koirala, N., and Sohng, J. K. (2016). Advances in biochemistry and microbial production of squalene and its derivatives. J. Microbiol. Biotechnol. 26 (3), 441–451. doi:10.4014/jmb.1510.10039
Hart, P. R. (2003). “Removal of water Soluble organics from produced brine without formation of scale,” in All days (SPE). doi:10.2118/80250-MS
He, H.-P., Cai, Y., Sun, M., and Corke, H. (2002). Extraction and purification of squalene fromAmaranthus grain. J. Agric. Food Chem. 50, 368–372. doi:10.1021/jf010918p
Huang, Z.-R., Lin, Y.-K., and Fang, J.-Y. (2009). Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules 14, 540–554. doi:10.3390/molecules14010540
Huang, C.-H., Huang, C.-Y., and Huang, M.-H. (2018). Unsaturated squalene content in emulsion vaccine adjuvants plays a crucial role in ROS-mediated antigen uptake and cellular immunity. Mol. Pharm. 15, 420–429. doi:10.1021/acs.molpharmaceut.7b00800
Huang, C., Song, X., Li, J., Cui, Q., Gu, P., and Feng, Y. (2025). Optimization of squalene production by pseudozyma sp. P4-22. Molecules 30 (7), 1646. doi:10.3390/molecules30071646
Ibrahim, N. ’I., Fairus, S., Zulfarina, M. S., and Naina Mohamed, I. (2020). The efficacy of squalene in cardiovascular disease risk-A systematic review. Nutrients 12, 414. doi:10.3390/nu12020414
Iyer, V., Cayatte, C., Guzman, B., Schneider-Ohrum, K., Matuszak, R., Snell, A., et al. (2015). Impact of formulation and particle size on stability and immunogenicity of oil-in-water emulsion adjuvants. Hum. Vaccin. Immunother. 11, 1853–1864. doi:10.1080/21645515.2015.1046660
Kabuto, H., Yamanushi, T. T., Janjua, N., Takayama, F., and Mankura, M. (2013). Effects of squalene/squalane on dopamine levels, antioxidant enzyme activity, and fatty acid composition in the striatum of Parkinson^|^rsquo;s disease mouse model. J. Oleo Sci. 62, 21–28. doi:10.5650/jos.62.21
Kim, E. H., Woodruff, M. C., Grigoryan, L., Maier, B., Lee, S. H., Mandal, P., et al. (2020). Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. eLife 9, e52687. doi:10.7554/eLife.52687
Lou-Bonafonte, J. M., Martínez-Beamonte, R., Sanclemente, T., Surra, J. C., Herrera-Marcos, L. V., Sanchez-Marco, J., et al. (2018). Current insights into the biological action of squalene. Mol. Nutr. Food Res. 62, e1800136. doi:10.1002/mnfr.201800136
Lozano-Grande, M. A., Gorinstein, S., Espitia-Rangel, E., Dávila-Ortiz, G., and Martínez-Ayala, A. L. (2018). Plant sources, extraction methods, and uses of squalene. Int. J. Agron. 2018, 1–13. doi:10.1155/2018/1829160
Mendes, A., Azevedo-Silva, J., and Fernandes, J. C. (2022). From sharks to yeasts: squalene in the development of vaccine adjuvants. Pharm. (Basel) 15, 265. doi:10.3390/ph15030265
Micera, M., Botto, A., Geddo, F., Antoniotti, S., Bertea, C. M., Levi, R., et al. (2020). Squalene: more than a step toward sterols. Antioxidants (Basel) 9, 688. doi:10.3390/antiox9080688
Nergiz, C., and Celikkale, D. (2011). The effect of consecutive steps of refining on squalene content of vegetable oils. J. Food Sci. Technol. 48, 382–385. doi:10.1007/s13197-010-0190-2
Okechukwu, P. N. (2020). Evaluation of anti-inflammatory, analgesic, antipyretic effect of eicosane, pentadecane, octacosane, and heneicosane. Asian J. Pharm. Clin. Res., 29–35. doi:10.22159/ajpcr.2020.v13i4.36196
Ondrejková, A., Süli, J., Harvanová, J., Ondrejka, R., and Prokeš, M. (2017). Antioxidative protection of squalene adjuvant and rabies vaccine with adjuvant. Biol. Pharm. Bull. 40, 1029–1034. doi:10.1248/bpb.b17-00026
Patel, A., Bettiga, M., Rova, U., Christakopoulos, P., and Matsakas, L. (2022). Microbial genetic engineering approach to replace shark livering for squalene. Trends Biotechnol. 40, 1261–1273. doi:10.1016/j.tibtech.2022.03.008
Permadi, A., and Wilson, M. (2024). Review: exploration of squalene from natural materials as its potential in health and food fields. Indonesian J. Chem. Eng. 2, 79–89. doi:10.26555/ijce.v2i2.1423
Perry, S. B., McAuliffe, J., Balschi, J. A., Hickey, P. R., and Ingwall, J. S. (1988). Velocity of the creatine kinase reaction in the neonatal rabbit heart: role of mitochondrial creatine kinase. Biochemistry 27, 2165–2172. doi:10.1021/bi00406a052
Popa, O., Băbeanu, N. E., Popa, I., Niță, S., and Dinu-Pârvu, C. E. (2015). Methods for obtaining and determination of squalene from natural sources. Biomed. Res. Int. 2015, 1–16. doi:10.1155/2015/367202
Ragasa, C. Y., de Jesus, J. P., Apuada, M. J., and Rideout, J. A. (2008). A new sesquiterpene from Artemisia vulgaris. J. Nat. Med. 62, 461–463. doi:10.1007/s11418-008-0253-0
Raw, A. S., Lionberger, R., and Yu, L. X. (2011). Pharmaceutical equivalence by design for generic drugs: modified-release products. Pharm. Res. 28, 1445–1453. doi:10.1007/s11095-011-0397-6
Samman, N., Ignasiak, T., Chen, C. J., Strausz, O. P., and Montgomery, D. S. (1981). Squalene in petroleum asphaltenes. Science 213, 1381–1383. doi:10.1126/science.213.4514.1381
Sasaki, K., Othman, M. B., Ferdousi, F., Yoshida, M., Watanabe, M., Tominaga, K., et al. (2019). Modulation of the neurotransmitter systems through the anti-inflammatory and antidepressant-like effects of squalene from Aurantiochytrium sp. PLoS ONE 14, e0218923. doi:10.1371/journal.pone.0218923
Shalu, S., Karthikanath, P. K. R., Vaidyanathan, V. K., Blank, L. M., Germer, A., and Balakumaran, P. A. (2024). Microbial squalene: a sustainable alternative for the cosmetics and pharmaceutical industry - a review. Eng. Life Sci. 24, e202400003. doi:10.1002/elsc.202400003
Shimizu, N., Ito, J., Kato, S., Eitsuka, T., Miyazawa, T., and Nakagawa, K. (2019). Significance of squalene in rice bran oil and perspectives on squalene oxidation. J. Nutr. Sci. Vitaminol. 65, S62–S66. doi:10.3177/jnsv.65.S62
Subramanian, S., Dowlath, M. J. H., Karuppannan, S. K., Saravanan M, S., and Arunachalam, K. D. (2020). Effect of solvent on the phytochemical extraction and GC-MS analysis of gymnema sylvestre. PJ 12, 749–761. doi:10.5530/pj.2020.12.108
Tateno, M., Stone, B. J., Srodulski, S. J., Reedy, S., Gawriluk, T. R., Chambers, T. M., et al. (2020). Synthetic Biology-derived triterpenes as efficacious immunomodulating adjuvants. Sci. Rep. 10, 17090. doi:10.1038/s41598-020-73868-6
Tripathi, A. C., Gupta, R., and Saraf, S. K. (2011). Phytochemical investigation characterisation and anticonvulsant activity of Ricinus communis seeds in mice. Nat. Prod. Res. 25, 1881–1884. doi:10.1080/14786419.2010.551753
Tsujimoto, M. (1906). About kuroko-zame shark oil. J. Soc. Chem. Industry 9, 953–958. Available online at: https://scholar.google.com/scholar_lookup?journal=Journal%20of%20the%20Society%20of%20Chemical%20Industry&title=About%20kuroko-zame%20shark%20oil&author=M.%20Tsujimoto&volume=9&issue=104&publication_year=1906&pages=953-958&#d=gs_cit&t=1758747464615&u=%2Fscholar%3Fq%3Dinfo%3AfYmLtjfi6CoJ%3Ascholar.google.com%2F%26output%3Dcite%26scirp%3D0%26hl%3Dru.
Tsujimoto, M. (1916). A highly unsaturated hydrocarbon in shark liver oil. J. Ind. Eng. Chem. 8, 889–896. doi:10.1021/i500010a005
Vezzani, A., Friedman, A., and Dingledine, R. J. (2013). The role of inflammation in epileptogenesis. Neuropharmacology 69, 16–24. doi:10.1016/j.neuropharm.2012.04.004
Keywords: squalene, Uzen oil field, wastewater, gas chromatography-mass spectrometry (GC-MS), compositional analysis, SARS-CoV-2 vaccine adjuvant
Citation: Suleimen YM, Trofimov AN, Mamytbekova GK, Kurbanaliyeva D, Akbay B and Tokay T (2025) Squalene recovery from Uzen oil field wastewater: a novel abiotic resource. Front. Environ. Chem. 6:1672872. doi: 10.3389/fenvc.2025.1672872
Received: 25 July 2025; Accepted: 22 September 2025;
Published: 01 October 2025; Corrected: 17 October 2025.
Edited by:
Naser A. Anjum, Aligarh Muslim University, IndiaReviewed by:
Han Zhang, Shenzhen University, ChinaOluwabamise Lekan Faboya, Federal University Oye-Ekiti, Nigeria
Copyright © 2025 Suleimen, Trofimov, Mamytbekova, Kurbanaliyeva, Akbay and Tokay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tursonjan Tokay, dHVyc29uamFuLnRva2F5QG51LmVkdS5reg==
†These authors have contributed equally to this work
 Yerlan M. Suleimen
Yerlan M. Suleimen Alexander N. Trofimov
Alexander N. Trofimov Gulnur K. Mamytbekova1,5
Gulnur K. Mamytbekova1,5 Burkitkan Akbay
Burkitkan Akbay Tursonjan Tokay
Tursonjan Tokay