- 1Department of Chemical Sciences, University of Lakki Marwat, Lakki Marwat, Pakistan
- 2Department of Chemistry, Women University Swabi, Swabi, Pakistan
- 3Department of Chemistry, Abdul Wali Khan University Mardan, Mardan, Pakistan
- 4Department of Chemical Engineering and Technology, College of Materials Science and Engineering, Beijing University of Technology, Beijing, China
Editorial on the Research Topic
Emerging trends in advanced oxidation processes for water treatment
1 Introduction
Natural water resources are severely exploited by the inevitable addition of emerging contaminants (ECs). ECs are newly identified synthetic or naturally occurring chemicals or biological agents that are released in the environment from various anthropogenic or natural sources. These contaminants, including pharmaceuticals and personal care products (PPCPs), pesticides, dyes, microplastics, per- and polyfluoroalkyl substances (PFAS), industrial chemicals or additives, nanomaterials and microcystin, are ubiquitous, toxic and persistent in the environment, and represent long-term and unpredictable threats to human beings and ecosystem (Shah et al., 2025). Many different techniques, including physical, biological and chemical methods are used for removal of ECs from water. Among them, advanced oxidation processes (AOPs) such as photocatalysis, Fenton, photo-Fenton, ionizing radiation, nonthermal plasmas, sonolysis, photolysis, electrochemical and ozonation have shown promising results in water treatment (Iqbal et al., 2024).
Photocatalysis and UV-based AOPs have gained significant attention in water treatment, owing to their convenient and sustainable nature. Reaction mechanism is a useful tool in understanding the underlying principles of pollutant degradation. Evidence of reactive species generated during AOPs, as well as identification of degradation products provide enormous information on pollutants degradation pathways (Khan et al., 2024). The most common reactive species produced during AOPs include hydroxyl radicals, sulfate radicals, superoxide radical anions, and singlet oxygen. The hydroxyl and sulfate radicals react with organic compounds via addition, hydrogen abstraction and/or electron transfer mechanism. Kinetics studies are useful in evaluating process validity and optimization of reaction conditions in real-world environmental applications. The detection of emerging contaminants in various environmental samples is a growing research area in determining water quality-a desired parameter in water treatment. Biodegradation is an attractive Research Topic in recent years, owing to its cheap and environmentally friendly nature. Tremendous research is going on in developing novel, efficient and sustainable AOPs for water treatment and environmental remediation.
Although AOPs are considered as very effective methods in the field of water purification, these methods face a number of challenges which need to be addressed and overcome to make these processes practically implemented. For example, many AOPs are costly due to the need of expensive reagents and oxidants, high energy consumption and reactor installation. These processes also face limited scalability, formation of toxic byproducts, and hindrance of their efficiency by water constituents especially inorganic ions and organic matter. In photocatalysis, the photocatalyst recovery and leaching is the biggest hurdle in practical implementation of these processes.
Fortunately, researchers are tirelessly trying to develop new strategies to overcome the limitations of AOPs. These strategies include the integration of AOPs with biological treatment methods for cost reduction, integration of different AOPs with each other for synergistic effects and efficiency enhancement, development of visible light active photocatalysts to fully utilize the renewable and freely available solar light, improving catalyst stability, recovery and reusability via film formation and magnetization, optimizing reactor design for better and effective mass transfer, and so on. In conclusion, this field is still open for researchers to utilize their specialized know-how, practical skills and expertise to develop newly and novel integrated AOPs for long-term and large-scale applications at affordable cost.
The Research Topic “Emerging trends in advanced oxidation processes for water treatment” contained four original research articles, expressing the presence of contaminants in environmental compartments and discussing new achievements in the field of AOPs for water treatment. These contributions cover the detection of an emerging pollutant- N-(1,3-dimethylbutyl)-N′-phenyl p-phenylenediamine)-quinone (6PPD-quinone)- in the runoff water samples, the kinetics and mechanism of degradation of emerging contaminants by using more convenient and sustainable photocatalytic and UV-based AOPs, strategy for developing novel immobilized TiO2 photocatalyst using newly designed photoreactor for efficient removal of organic pollutant, and reusability potential of the photocatalysts.
2 Overview of contributions
This Research Topic features works that strengthen the sample preparation and analytical methods for emerging pollutant detection in water, the kinetics and mechanism of degradation of emerging contaminants by promising AOPs, i.e., UV/TiO2 photocatalysis, UV/H2O2, and UV/Fe2+/H2O2 processes, as well as biodegradation. The concentrations of a low quantity of a widely used and a model organic pollutant, 6-PPD-quinone, in the runoff water from different sources of Norway were detected. Meanwhile, the degradation of a mixture of four quinolone antibiotics of emerging concern, i.e., ofloxacin, norfloxacin, ciprofloxacin and enrofloxacin by biodegradation was investigated using mixed strain bacteria.
Cob-Cantú et al. reported that immobilized TiO2 nanoparticles on mortar spheres is a promising photocatalyst for removal of aniline blue from water. The as-synthesized photocatalyst led to 95%–97% aniline blue removal from water after 150 min UV irradiation. The degradation of dye was attributed to the generation of hydroxyl radicals (•OH), superoxide radical anions Change (O2•−) and holes (h+). The photocatalyst exhibited excellent durability and recyclability as indicated by more than 83% dye removal after 20 cycles, suggesting the prepared photocatalytic reactor could be scaled up for treatment of effluents from dyes or textile industries.
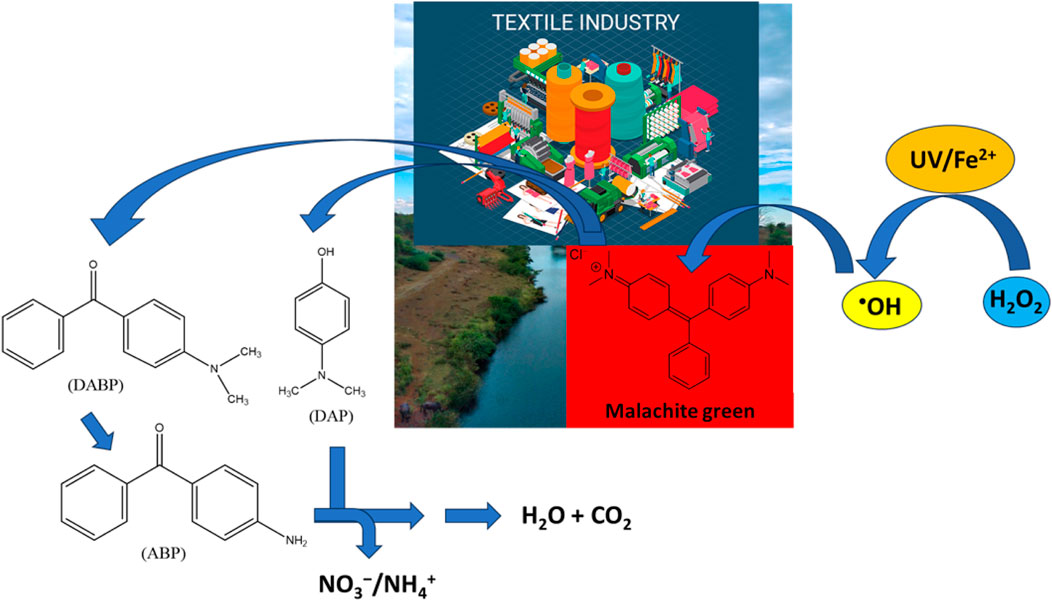
Wilayat et al. achieved 100% degradation of a widely used fungicide and a common coloring agent, malachite green (MG) by UV/H2O2 in 60 min, while, UV/H2O2/Fe2+ achieved 100% dye removal in 30 min only. Furthermore, GC-MS analysis revealed the formation of three degradation products (DPs), i.e., 4-dimethylamino-benzophenone (DABP), 4-amino-benzophenone (ABP), and 4-dimethylamino-phenol (DAP) (Figure 1). It was emphasized that proper focus should be given to the removal of DPs, besides the removal of the target pollutants. Thus, UV/H2O2 and UV/H2O2/Fe2+ were proved to be highly efficient methods for relieving the harmful environmental impacts of the dyes, such as malachite green.
Wang et al. investigated the microbial degradation and co-metabolism of a mixture of four common quinolone antibiotics, i.e., ofloxacin (OFLX), norfloxacin (NOR), ciprofloxacin (CIP) and enrofloxacin (ENR) by mixed strain bacteria. The strains were divided into two groups, i.e., in group mix and ENR, the resistant bacteria played the primary role of microorganisms and degrading bacteria had a secondary role, while in group mix and NOR, the degrading bacteria strains had the major role and resistant bacteria strains had a minor role. The differences in the degradation results were attributed to the type of the antibiotics. The mechanism of NOR degradation suggested the cleavage of carbon-nitrogen bonds on the piperazine ring, followed by oxygenation and deethylation. The environmental factors had no significant effect on the degradation efficiency of the mix and NOR degrading strains, indicating that the mixed bacteria can successfully degrade NOR in diverse environmental samples, such as river, sea and lake water. The article has high research impact on the co-metabolism and biodegradation of mixtures of quinolone antibiotics in real-world environment.
A novel method comprising of liquid-liquid extraction followed by SiO2-based solid phase extraction and subsequently LC-MS/MS analysis was developed by Kryuchkov et al. for the detection of highly toxic compound, 6PPD-quinone, in runoff water of Norway. The samples were collected from different environment, including tunnel-wash water, culvert, water treatment plant, artificial turf pitch and country road puddle. The 6PPD-quinone was likely widespread in Norwegian environment at above LC50 value for some fish species. This warrants the need for an extensive analysis on presence of 6PPD-quinone in more environmental samples, with special focus on toxicity assessments for the key species.
3 Concluding remarks
Overall, the published research articles capture significance and potential of AOPs as promising technologies for water treatment and environmental remediation, in real-world applications. These works, born from high originality, will continue to inspire innovation among the next-generation of researchers in enhancing the scope of AOPs for water treatment.
The published articles were accepted after a rigorous peer review process, and it is believed that the Research Topic will be highly beneficial to the readership in the field of AOPs for environmental sustainability. We are highly thankful to all the authors and reviewers for their valuable contributions in making this Research Topic successful. We also would like to specially thank the editorial board of the Frontiers in Environmental Chemistry for allowing us to publish this Research Topic. In short, the publication of this Research Topic would not have been possible without the collective supports from the authors, reviewers, editorial board, and the publisher.
Author contributions
SK: Writing – review and editing, Writing – original draft. JK: Writing – review and editing, Writing – original draft. ZW: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Iqbal, J., Shah, N. S., Ali Khan, J., Naushad, M., Boczkaj, G., Jamil, F., et al. (2024). Pharmaceuticals wastewater treatment via different advanced oxidation processes: reaction mechanism, operational factors, toxicities, and cost evaluation – a review. Sep. Purif. Technol. 347, 127458. doi:10.1016/j.seppur.2024.127458
Khan, I., Liu, W., Zada, A., Raziq, F., Ali, S., Shah, M. I. A., et al. (2024). Recent progress in emerging materials and hybrid nanocomposites for peroxymonosulfate and peroxydisulfate activation towards solar light-driven photocatalytic degradation of emerging pollutants. Coord. Chem. Rev. 499, 215466. doi:10.1016/j.ccr.2023.215466
Keywords: emerging organic pollutants, occurrence, degradation, advanced oxidation processes, emerging trends, water treatment
Citation: Khan S, Khan JA and Wei Z (2025) Editorial: Emerging trends in advanced oxidation processes for water treatment. Front. Environ. Chem. 6:1693281. doi: 10.3389/fenvc.2025.1693281
Received: 26 August 2025; Accepted: 02 October 2025;
Published: 13 October 2025.
Edited by:
Jia-Qian Jiang, Glasgow Caledonian University, United KingdomCopyright © 2025 Khan, Khan and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanaullah Khan, c3VraGFuM0B3dXMuZWR1LnBr; Javed Ali Khan, amF2ZWRraGFuQGF3a3VtLmVkdS5waw==, a2hhbmphdmVkMjM4MUBnbWFpbC5jb20=
 Sanaullah Khan
Sanaullah Khan Javed Ali Khan
Javed Ali Khan Zhen Wei
Zhen Wei