- 1Department of Sustainable Crop Production (DIPROVES), Faculty of Agricultural, Food and Environmental Science, Università Cattolica del Sacro Cuore, Piacenza, Italy
- 2Department of Animal Science, Food and Nutrition (DIANA), Faculty of Agricultural, Food and Environmental Sciences, Università Cattolica del Sacro Cuore, Piacenza, Italy
Introduction and methods: The distribution of tenuazonic acid (TeA), alternariol (AOH), alternariol monomethyl ether (AME), and tentoxin (TEN) between the pulp and peel was determined in different tomato varieties after artificial inoculation with three Alternaria species (Alternaria alternata, Alternaria tenuissima, and Alternaria solani) and incubation for 3 weeks. The role of heat treatments, similar to pasteurization, in their stability was also investigated.
Results and discussion: Unlike AME that was never detected, TeA, AOH, and TEN were determined at different levels in the pulp and peel. Specifically, AOH remained mainly in the peel, where the inoculation was carried out, while TeA and TEN migrated into the pulp and were also found in the discarded liquid accumulated during the incubation period. Heat treatments reduced TeA, AOH, and TEN to varying degrees. In particular, the TeA level was slightly reduced after treatment both at 100°C (approximately 10%) and 121°C (approximately 20%), while a reduction of approximately 30% was achieved after the double heat treatment (treatment at 100°C followed by treatment at 121°C). AOH was found to be less stable to heat treatments, showing a reduction of around 50% after treatment at 100°C and up to 80% after double heating treatments. TEN was reduced by approximately 50% only after the combined treatment of 100°C + 121°C.
1 Introduction
Alternaria toxins are secondary metabolites produced by fungi that can contaminate cereals, oilseeds, fruits, and vegetables. Their occurrence in tomatoes and derived products has been reported by several authors (da Motta and Soares, 2001; Noser et al., 2011; Bertuzzi et al., 2021; Sanzani et al., 2019; Qin et al., 2022; Ji et al., 2023; Maldonado Haro et al., 2023). In 2016, the European Food Safety Authority (EFSA) published a scientific report evaluating dietary exposure to the main Alternaria toxins—alternariol (AOH), alternariol monomethyl ether (AME), tenuazonic acid (TeA), and tentoxin (TEN)—in the European population (EFSA et al., 2016). In this report, “Tomatoes and tomato-based products” resulted as the main contributors to the dietary exposure to TeA for all age classes, with the only exception of the “Infants” and “Toddlers” classes. Successively, the European Commission published the Recommendation 2022/553 reporting on the indicative limits for AOH, AME, and TeA in certain foods including tomato-derived products. AOH and AME have the ability to induce cell cytotoxicity, interfere with the cell cycle, and cause cell apoptosis. Moreover, they can trigger mutagenicity in either bacterial or mammalian cells and are known to induce primary DNA damage in vitro and in vivo. TeA has been shown to inhibit newly formed proteins from ribosomes, resulting in reduced cell viability across mammalian cell lines. The cytotoxicity effects of TeA involve liver damage, gastrointestinal hemorrhage, cardiovascular collapse, and mortality in various animal species. The toxicological effects of TEN include induction of necrosis, reduction of cell vitality, and interference with plant seedlings (Zhang et al., 2025).
Tomatoes are extremely susceptible to fungal decay due to their thin peel. Alternaria spp. are considered among the main causes of black mold disease in raw tomatoes. During industrial tomato processing for the production of several derived products (i.e., ketchups, purées, juices, and sauces), tomatoes are subjected to several immersions in water and to optical and manual selections in order to discard defective tomatoes. Water immersions occur before entering the production plant as a washing step and after the optical and manual selection for easy transport of the tomatoes to the following processing steps. It is unknown whether, during these immersions, Alternaria toxins could migrate from contaminated fruits to the water and whether the water, if not frequently replaced, could become contaminated. Successively, different heat treatments are carried out, such as for separation of the skin peel from the pulp, in order to obtain cold or hot break products (for the inhibition or inactivation of pectolytic enzymes, respectively) and to produce concentrated tomato paste. To our knowledge, there are no reports on the distribution of Alternaria toxins between the peel and the pulp, while a few studies have indicated their thermal stability during food processing. Estiarte et al. (2018) indicated that AOH and AME are stable during the heat processing of tomatoes, while Siegel et al. (2010) reported stability during bread baking. No results have been reported on the fate of TeA, the main Alternaria toxin present in tomatoes, and TEN during tomato processing.
Considering the limited information available on the distribution of Alternaria toxins during tomato processing and in the waste and finished products, this study aimed to evaluate the distribution of TeA, AOH, AME, and TEN between the pulp and the peel in artificially contaminated tomatoes and their stability to heat treatments at conditions similar to those used during industrial tomato processing.
2 Methods
2.1 Alternaria spp. inoculum preparation
All of the Alternaria strains used for the experiment were purchased from the Westerdijk Fungal Biodiversity Institute (Utrecht, the Netherlands). In particular, one strain of A. alternata (CBS 118814), one strain of A. solani (CBS 109157), and one strain of A. tenuissima (CBS 117.44) were singularly inoculated on Petri dishes (diameter, 9 cm) with potato dextrose agar (PDA; Biolife, Milan, Italy) and incubated at 25°C for 7 days (12-h light/12-h dark photoperiod). After incubation, the developed fungal colonies were washed with sterile water and the spores were collected and mixed together to prepare the inoculum. The concentration of the inoculum was adjusted to –104 CFU/ml.
All of the fungal strains used were tested in previous trials and were shown to be capable of producing the different Alternaria toxins: TeA, AOH, AME, and TEN (Bellotti et al., 2023). Different strains of Alternaria were considered for the inoculum to better represent the field situation, where more strains are present and able to infect plants. A. alternata, A. tenuissima, and A. solani were chosen as they are more frequently isolated in Italy (Sanzani et al., 2021). Only one strain for each Alternaria species was used for the preparation of the artificial inoculum, although the use of more strains would be more representative of species variability. However, this was not crucial to the aim of this study, where only the strains definitely able to produce Alternaria toxins to obtain high contamination were used.
2.2 Artificial fungal inoculation of tomatoes
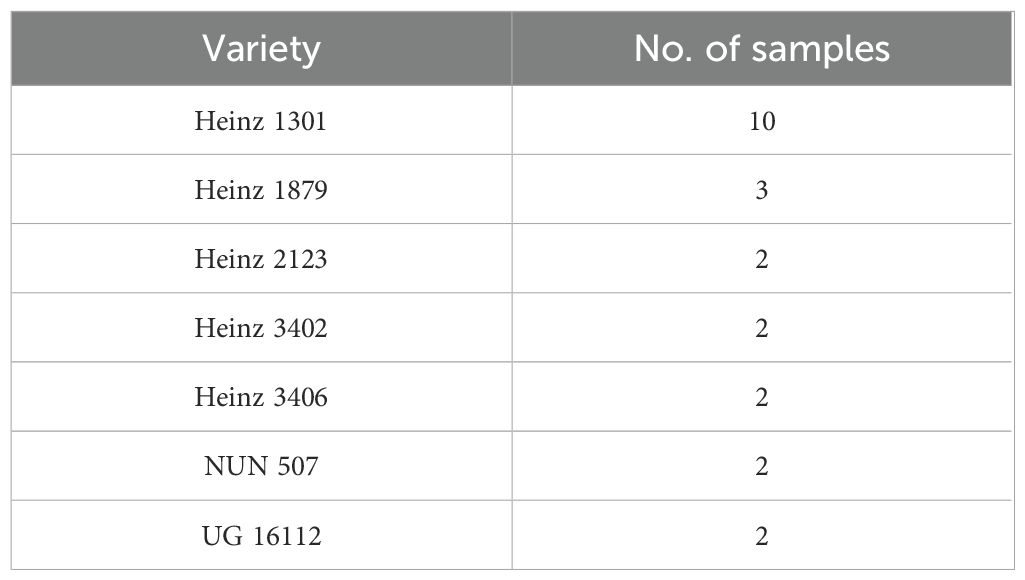
A total of 23 samples belonging to seven different tomato varieties were used for the experiment (Table 1). These samples are from some of the most cultivated varieties in Northern Italy, i.e., the most significant area in Italy for the production of tomato-based products. The number of samples for each variety was based on the incidence of the presence of each variety in the area.
For each sample, approximately 500 g of tomatoes (corresponding to six to eight tomatoes) was placed on a grid in a sterile glass box and artificially inoculated by spraying them with 5 ml of the fungal inoculum, obtained as previously described. To facilitate the fungal inoculation, the peels were wounded at different points with a sterile needle, and 100 ml of sterile water was placed on the bottom of the boxes to maintain high humidity in the chamber. The water on the bottom of the box was kept separate from the tomatoes through a sterile grid and was maintained for the whole incubation period. The boxes were incubated under controlled conditions at 25°C for 21 days (12-h light/12-h dark photoperiod). This incubation time was decided on the basis of a previous in vitro experiment conducted with Alternaria strains and aimed to define the production of Alternaria toxins (Meng et al., 2021; Bagi et al., 2022).
At the same time, 500 g of the same samples was placed in similar boxes, incubated in the same conditions but without artificial fungal inoculation and used as untreated samples for comparison of the results. As all of the tomato samples used were collected from the field at harvest time, possible wounds could have been present on the skin of the untreated samples, as well as possible natural contamination. This could be useful to verify possible differences between natural and artificial contamination in the distribution of Alternaria toxins.
2.3 Sample preparation and heat treatments
After 3 weeks of incubation, in both inoculated and control samples, the peel was manually separated from the pulp. The peel was approximately 2% of the entire tomato samples. For each sample, the peels obtained from all tomatoes were mixed together to improve homogeneity and used for the analysis. The same procedure was also carried out for the pulp. During incubation, infected tomatoes released liquid due to the decay of the fruits (approximately 10 ml), which mixed with the water present on the bottom of box, which was also collected at the end of incubation.
Subsequently, the pulps and peels were homogenized with UltraTurrax (T25, Janke & Kunkel, Labortechnik, Staufen, Germany) before analysis. The volume of water inside the boxes was measured and then analyzed for the presence of Alternaria toxins.
For the control samples, the two parts (peel and pulp) were analyzed for Alternaria toxins (TeA, AOH, AME, and TEN). For the artificially inoculated tomatoes, samples of the peel and pulp were divided into four subsamples and were treated following four different treatments: 1) no heating; 2) heating at 100°C for 10 min; 3) heating at 121°C for 20 min; and 4) heating at 100°C for 10 min, followed by 121°C for 20 min. Although this last double treatment is not normally used in the tomato transformation industry, it was included to determine whether longer and stronger heating treatments could have more effect on Alternaria toxins.
After cooling, both peel and pulp samples were analyzed for Alternaria toxins.
2.4 Analysis of Alternaria toxin determination
After homogenization using UltraTurrax (16,000 × g for 2 min), a volume of 100 ml acetonitrile/water (80 + 20, v/v) was added to 20 g of the sample to extract mycotoxins using a rotary shaker for 60 min (120 rpm), as reported in our previous work (Bertuzzi et al., 2021). The extract was centrifuged at 4,000 rpm, and then 0.2 ml was diluted with 0.8 ml water/acetonitrile (90:10, v/v) in a vial for LC-MS/MS (Vanquish pump and autosampler coupled with a Fortis mass spectrometer; Thermo Fisher Scientific, San Jose, CA, USA) analysis in selected reaction monitoring (SRM) mode. Alternaria toxins were chromatographed on an HSS-T3 RP-18 column (5-µm particle size, 150 × 2.1 mm; Waters, Milford, MA, USA) using a mobile-phase gradient acetonitrile–water (both acidified with 0.2% formic acid) from 30:70 to 65:35 in 6 min, then isocratic for 3 min; gradient to 30:70 in 1 min and isocratic for 6 min (equilibration step). Ionization was carried out with an electrospray ionization (ESI) interface (Thermo-Fisher, San Jose, CA, USA) in positive mode as follows: spray capillary voltage, 4.5 kV; sheath and auxiliary gas, 35 and 14 psi; and temperature of the heated capillary, 270°C. For fragmentation of the [M]+ ions (198 m/z for TeA, 259 m/z for AOH, 273 m/z for AME, and 415 m/z for TEN), the fragment ions were: 125, 139, and 153 m/z (16 V) for TeA; 128, 185, and 213 m/z (35 V) for AOH; 128 and 184 m/z (38 V) and 258 m/z (30 V) for AME; and 132 m/z (37 V) and 135 and 312 m/z (25 V) for TEN. Quantitative determination was performed using the LC_Quan 2.0 software.
To investigate the matrix effect (ME), the slopes of the matrix-matched calibration curves obtained from five spiked blank sample extracts (four replicates for each sample) were compared with those of the solvent-based calibration curves, calculating ME using the following formula: ME (%) = (slope matrix-matched standard curves/slope solvent standard curves) × 100%. The results showed ME values consistently inferior to 11%. Results inferior to ±15% were generally considered as unaffected by the matrix. The limit of detection (LOD) and the limit of quantification (LOQ) were determined using the signal-to-noise approach, defined as those levels resulting in signal-to-noise ratios of 3 and 10, respectively. The analytic response and the chromatographic noise were both measured from the chromatogram of a blank sample extract to which an appropriate volume of the AOH standard solution had been added. The LOD and the LOQ were 5 and 15 µg/kg for TeA, 0.5 and 1.5 µg/kg for AOH and TEN, and 1 and 2 µg/kg for AME, respectively. The linearity of the calibration curves was established through five calibration standards in solvent, showing r values superior to 0.998 (Supplementary Table S1). The concentration levels were 25, 50, 100, 250, and 500 µg/L for TeA and 1, 2.5, 5, 10, and 25 µg/L for AOH, AME, and TEN. Recovery values were determined by spiking the aliquot of an uncontaminated (blank) tomato sample (20 g) with an appropriate volume of the AOH standard solution at three different levels before the extraction. Three replicates were analyzed for each level. The recovery values were between 87.3% and 103.1%. The results were corrected for the mean recovery value.
2.5 Calculation of TeA and TEN migration in water
The percentage of migration of TeA and TEN in the water added in the inoculation box was calculated as: mycotoxin amount in the measured volume of water solution/(mycotoxin amount in 490 g pulp tomato + amount in 10 g peel (2% of entire tomato)) × 100).
2.6 Data analysis
Analysis of variance (ANOVA) on the main factors considered (heat treatments and the distribution of mycotoxins in the fruit) was performed using data from all the samples, despite being from different tomato varieties, and considering them as biological replicates for the statistical analysis. The generalized linear model (GLM) procedure of the IBM SPSS Statistics 27 package (IBM Corp., Armonk, NY, USA) was used. Significant differences were highlighted using Tukey’s test (p ≤ 0.05) for mean separation.
Data on Alternaria toxin production were logarithmically transformed prior to statistical analysis (values +1). Log transformation is generally required for data that cover a wide range from single-digit numbers to numbers in hundreds or thousands (Clewer and Scarisbrick, 2001), such as those obtained for mycotoxin production.
Moreover, data of the presence of Alternaria toxins were analyzed using box plots in order to compare the distribution of data among the different groups (tomato varieties) and to verify consistency of the results obtained.
3 Results
In this section, data are presented as value ± standard deviation (SD).
3.1 Distribution of Alternaria toxins between the pulp and peel
TeA, AOH, and TEN were detected in almost all of the artificially inoculated samples, while AME was never detected in any of them. All of the control samples resulted free from any of the considered Alternaria toxins. For this reason, we did not perform thermal treatments on these samples.
Differences in the contamination of tomatoes were determined between the pulp and peel, independently of the variety. The ANOVA results among all the tomato samples used in the experiment showed that the distribution of mycotoxins between the pulp and peel was statistically different for both AOH (p ≤ 0.01) and TEN (p ≤ 0.05) (Table 2).
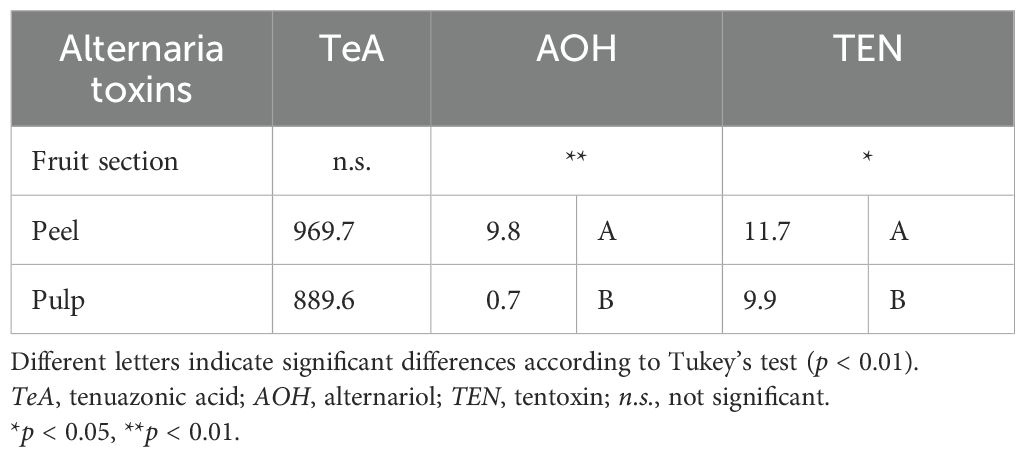
Table 2. Analysis of variance (ANOVA) of the content of Alternaria toxins in the peel and pulp of the 23 tomato samples artificially inoculated with Alternaria species.
The concentrations of TeA ranged from 30 µg/kg (pulp sample) to 4,438 µg/kg (peel sample) (Supplementary Table S2). The distributions between the peel and pulp were similar, with an average concentration ratio of peel/pulp of 1.09 ± 0.36 (Figure 1).
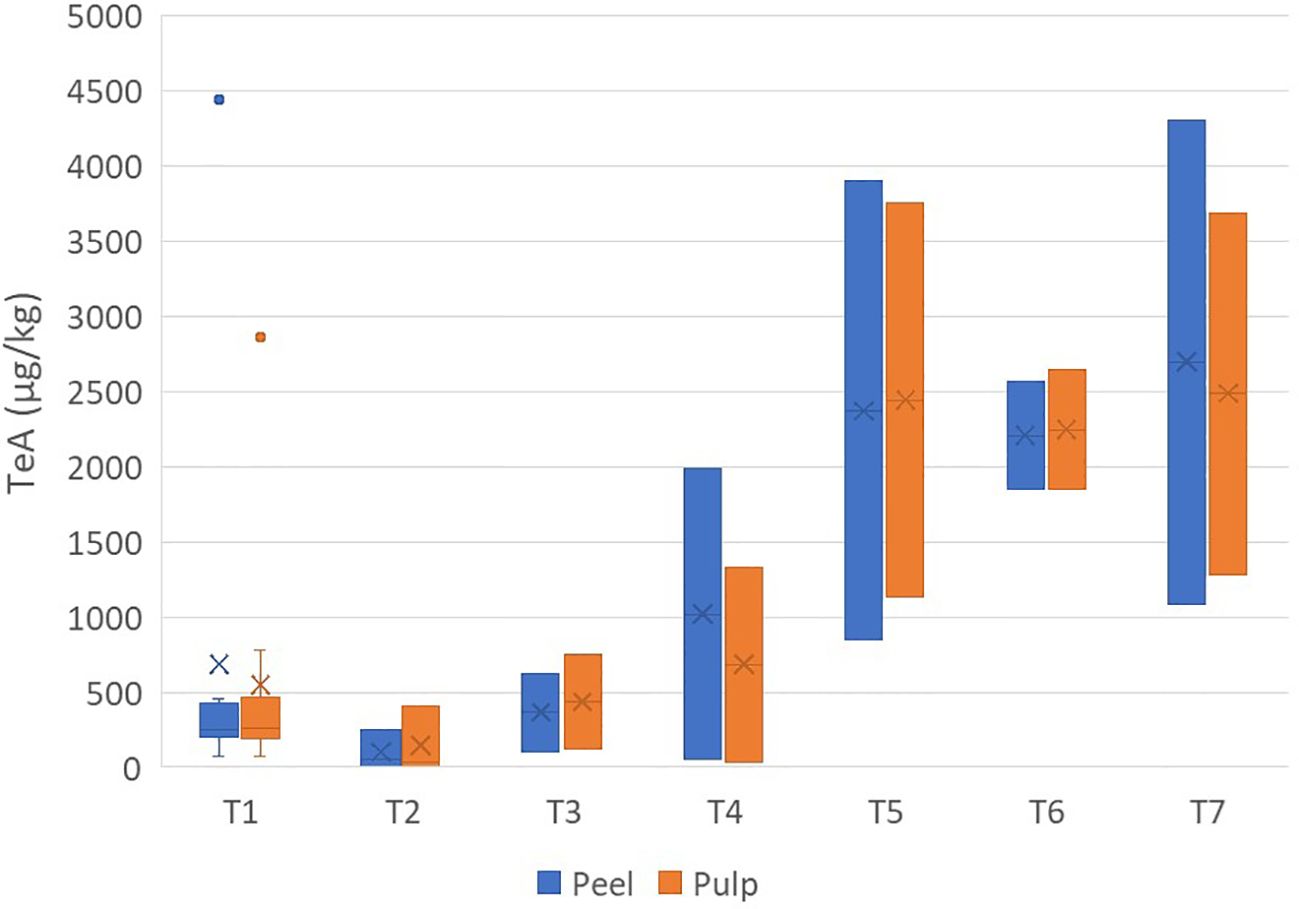
Figure 1. Box plot analysis of the quantity of tenuazonic acid (in micrograms per kilogram) in the peel and pulp of seven different varieties of tomatoes artificially inoculated with Alternaria species. Within each box, horizontal black lines indicate median values, crosses indicate mean values, boxes extend from the 25th to the 75th percentile of each group’s distribution of values, the lower and upper lines denote error lines, and dots denote observations outside the 10th and 90th percentiles. The number of samples considered for each tomato variety is reported in Table 1.
Contamination by AOH was much lower than that by TeA. AOH showed a maximum value of 58.8 µg/kg in a peel sample (in two samples, it was not detected), while only 10 samples of pulp showed contamination (maximum value, 8.0 µg/kg) (Supplementary Table S3). Interestingly, AOH occurred mainly in the peel than in the pulp, with the average concentration ratio of peel/pulp being 12.50 ± 13.41 (Figure 2).
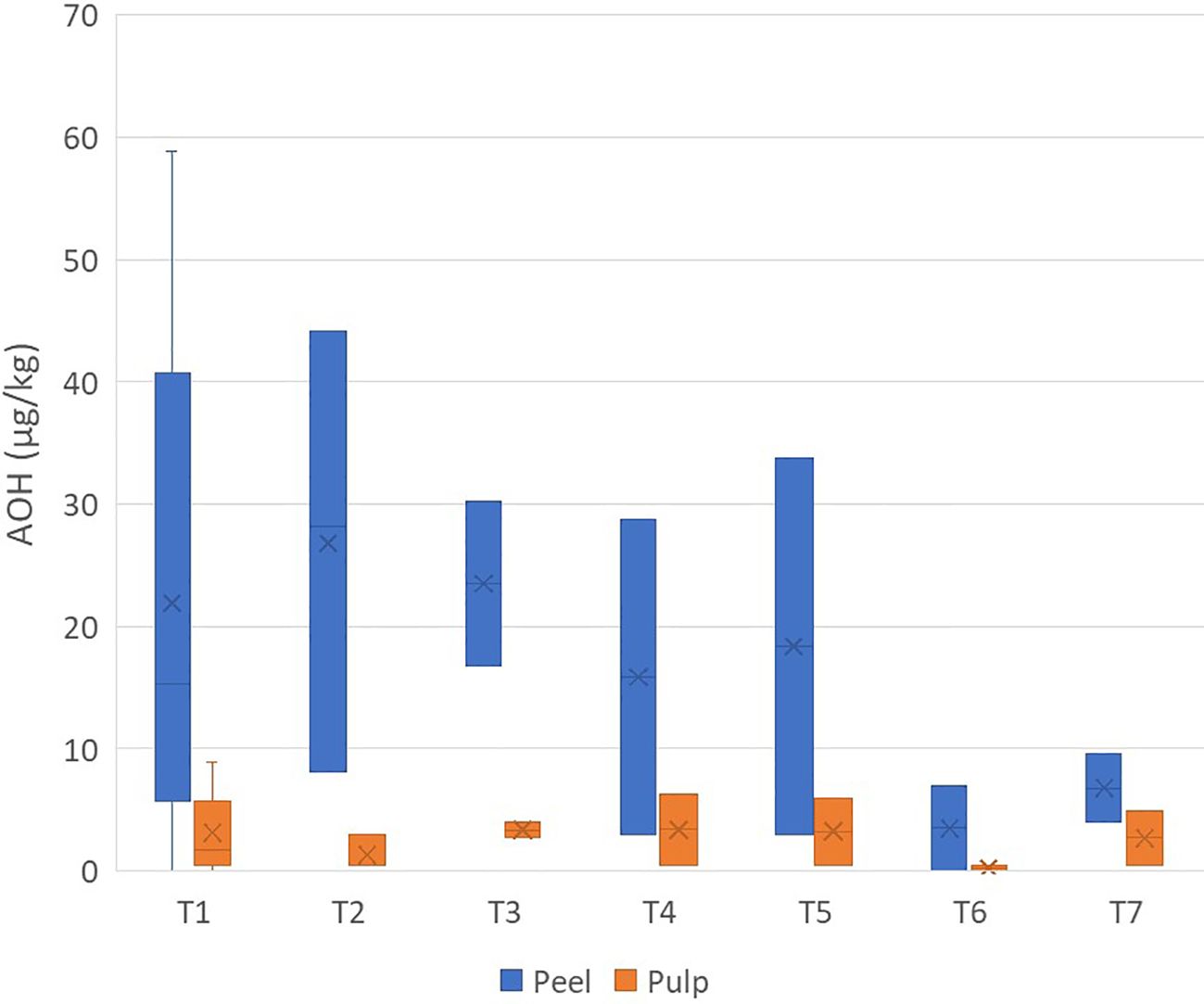
Figure 2. Box plot analysis of the quantity of Alternariol (in micrograms per kilogram) in the peel and pulp of seven different varieties of tomatoes artificially inoculated with Alternaria species. Within each box, horizontal black lines indicate median values, crosses indicate mean values, boxes extend from the 25th to the 75th percentile of each group’s distribution of values, the lower and upper lines denote error lines, and dots denote observations outside the 10th and 90th percentiles. The number of samples considered for each tomato variety is reported in Table 1.
The concentrations of TEN were found in the range <0.5–71.1 µg/kg. It was not detected in one sample of peel and in five samples of pulp (Supplementary Table S4). The average concentration ratio of peel/pulp was 3.83 ± 5.17 and showed a non-constant migration in the pulp (Figure 3).
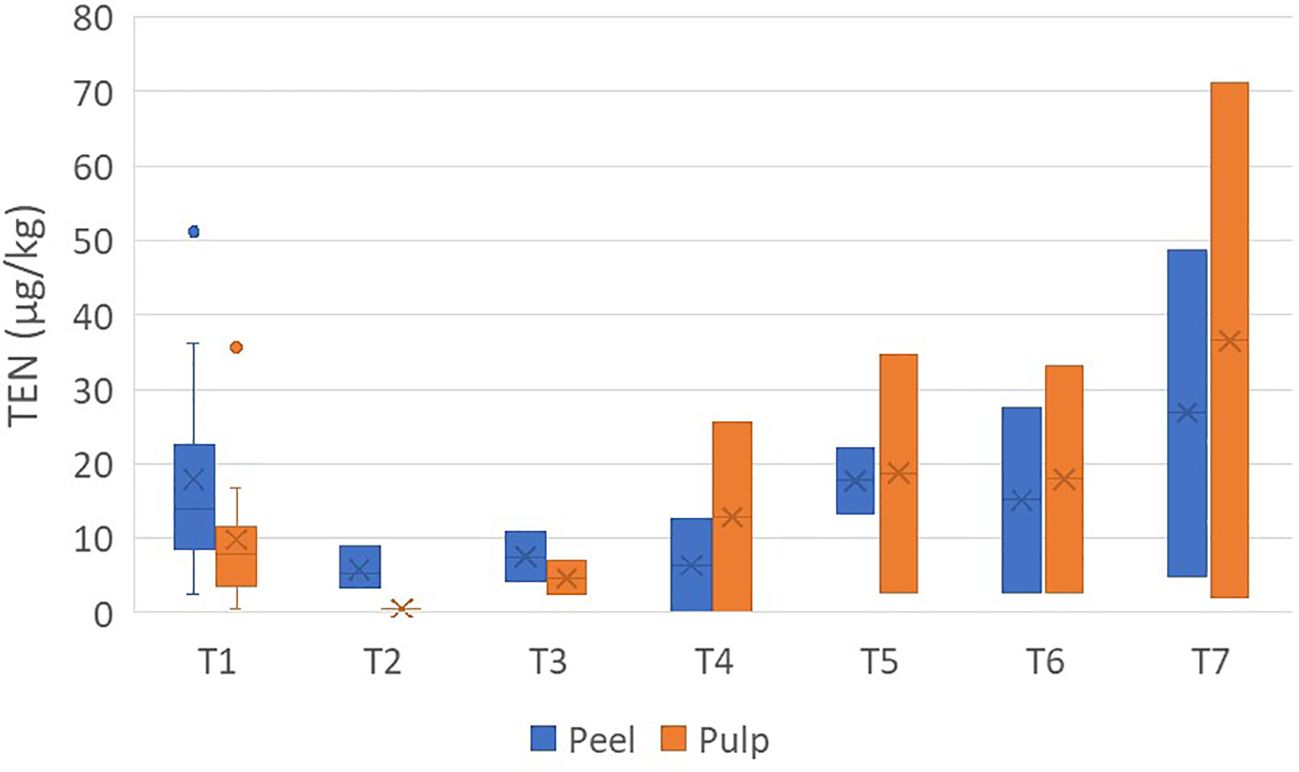
Figure 3. Box plot analysis of the quantity of tentoxin (in micrograms per kilogram) in the peel and pulp of seven different varieties of tomatoes artificially inoculated with Alternaria species. Within each box, horizontal black lines indicate median values, crosses indicate mean values, boxes extend from the 25th to the 75th percentile of each group’s distribution of values, the lower and upper lines denote error lines, and dots denote observations outside the 10th and 90th percentiles. The number of samples considered for each tomato variety is reported in Table 1.
As reported in several studies, TeA is the main toxin detected in tomato products. Our results confirmed this trend. To evaluate whether the level of artificial contamination carried out in the laboratory could be similar to those occurring in the field, a small survey on Alternaria toxins was carried out, which sampled naturally contaminated tomatoes from different truck trailers during the unloading stage at an industrial tomato plant. Moldy tomatoes selected by the operators of the plant were collected from nine different lots on different days. Contamination of Alternaria toxins was similar to those obtained from the artificial infection carried out in this study. TeA showed a contamination level from <5 to 16,414 µg/kg, AOH from <0.5 to 12 µg/kg, AME from 1 to 4 µg/kg, and TEN from <0.5 to 5.5 µg/kg. In all finished tomato products produced by the industrial plant on the same day of the sampling, TeA was consistently below 160 µg/kg, while AOH, AME, and TEN were never detected.
3.2 Alternaria toxins in water
A non-negligible migration of TeA and TEN was found in the water added in the incubation boxes. After 3 weeks of incubation, the water also contained liquid released by the moldy tomatoes due to the natural decay of fruits. Table 3 shows the percentage migration for each variety. The values varied between 9.3% and 26.8% for TeA and between 2.7% and 10.0% for TEN, showing global average percentage migration of 17.7% ± 10.9% and 7.62% ± 6.64% for TeA and TEN, respectively.
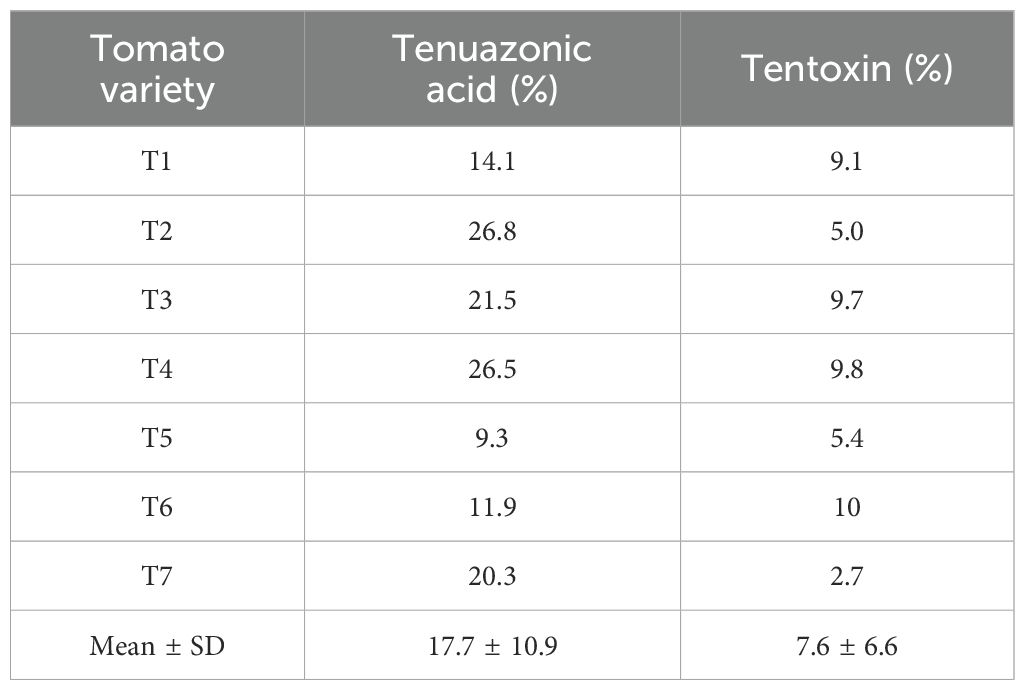
Table 3. Percentage migration of tenuazonic acid (TeA) and tentoxin (TEN) in water added in the incubation boxes to maintain high levels of humidity.
On the other hand, AOH was never detected in these solutions. Nevertheless, a direct contact does not occur between the water and the tomatoes during the incubation period. These data showed that, differently from AOH, TeA and TEN could easily migrate from the contaminated tomatoes during the washing and movement steps in industrial tomato processing.
3.3 Heat stability of TeA, AOH, and TEN
The effects of heat treatments on TeA are reported in Figure 4. TeA showed high stability to heat treatment at 100°C, with average reduction values of 11.23% ± 9.66% and 13.16% ± 10.92% for the peel and pulp, respectively. After treatment at 121°C, the reduction was also not relevant, reaching values of 17.39% ± 10.46% and 20.75% ± 12.83%, while the combined heat treatments (not carried out during tomato processing) decreased the concentration by approximately 30%.
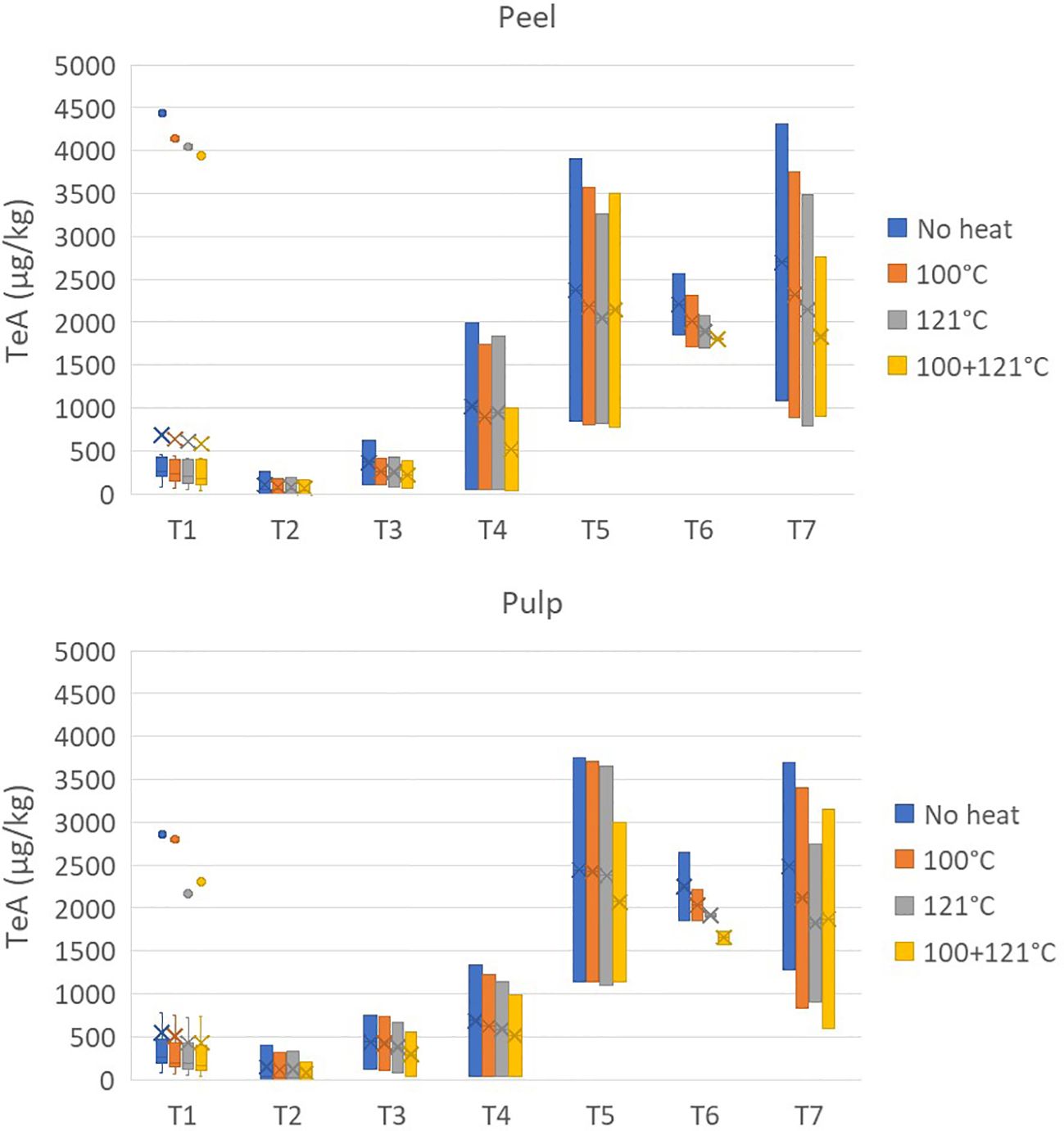
Figure 4. Effect of three different heat treatments on the presence of tenuazonic acid (in micrograms per kilogram) in the peel and pulp of seven different tomato varieties artificially inoculated with Alternaria species. Within each box, horizontal black lines indicate median values, crosses indicate mean values, boxes extend from the 25th to the 75th percentile of each group’s distribution of values, the lower and upper lines denote error lines, and dots denote observations outside the 10th and 90th percentiles. The number of samples considered for each tomato variety is reported in Table 1.
The reduction of AOH after heat treatments was considered only for the peel as the presence of this Alternaria toxin in the pulp already resulted low prior to the heat treatment (Figure 5). Differently from that on TeA, the heat treatment was more effective on AOH, producing a more relevant result: approximately 50% of the toxin was destroyed after treatment at 100°C (average values of 55.34% ± 30.64% for the peel and 42.00% ± 28.01% for the pulp). The reductions increased up to 70% and 80% with treatment at 121°C and with the combined heat treatment, respectively.

Figure 5. Effect of three different heat treatments on the presence of alternariol (in micrograms per kilogram) in the peel of seven different tomato varieties artificially inoculated with Alternaria species. Within each box, horizontal black lines indicate median values, crosses indicate mean values, boxes extend from the 25th to the 75th percentile of each group’s distribution of values, the lower and upper lines denote error lines, and dots denote observations outside the 10th and 90th percentiles. The number of samples considered for each tomato variety is reported in Table 1.
Finally, the average reductions of TEN after treatment at 100°C were 31.24% ± 21.80% and 22.55% ± 24.75% for the peel and pulp, respectively, and reached values close to 40% and 50% for the 121°C and combined treatments, respectively (Figure 6).
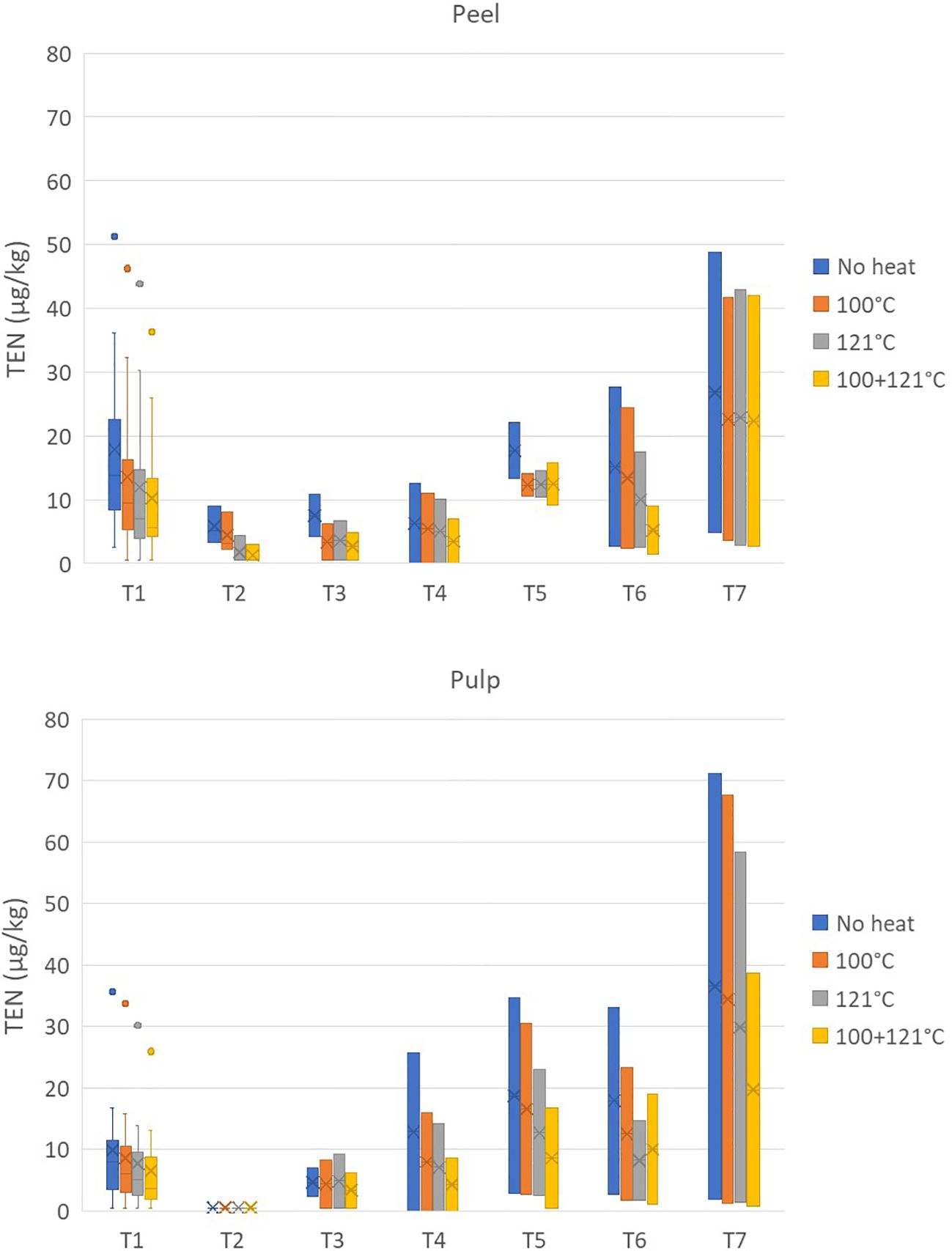
Figure 6. Effect of three different heat treatments on the presence of tentoxin (in micrograms per kilogram) in the peel and pulp of seven different tomato varieties artificially inoculated with Alternaria species. Within each box, horizontal black lines indicate median values, crosses indicate mean values, boxes extend from the 25th to the 75th percentile of each group’s distribution of values, the lower and upper lines denote error lines, and dots denote observations outside the 10th and 90th percentiles. The number of samples considered for each tomato variety is reported in Table 1.
The ANOVA results for all of the tomato samples used for the experiment underlined statistically significant differences (p ≤ 0.01) due to heat treatments only for AOH (Table 4).
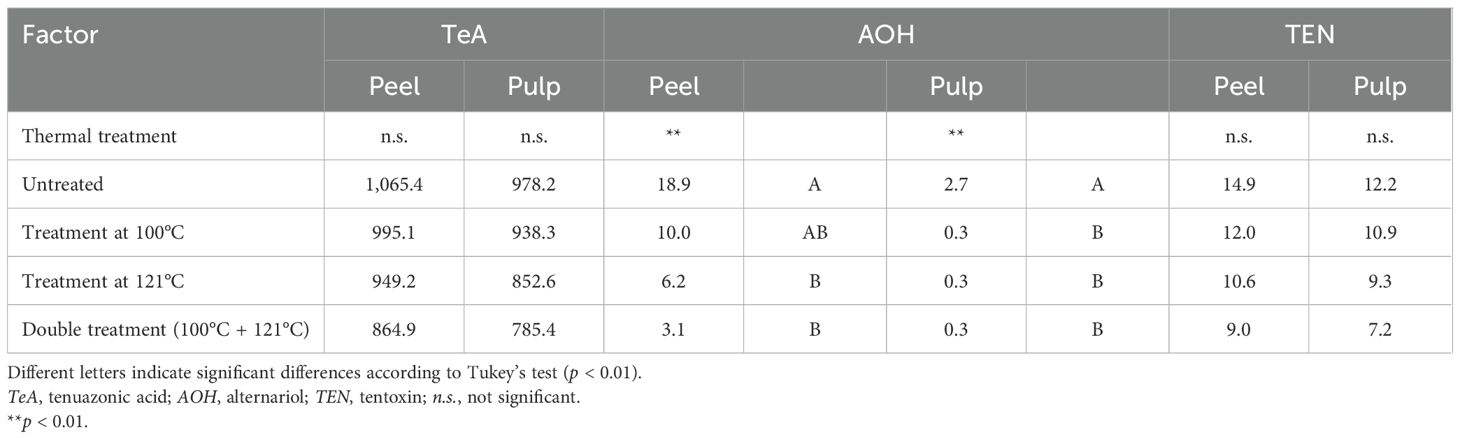
Table 4. Analysis of variance (ANOVA) of the content of Alternaria toxins in the peel and pulp of the 23 tomato samples artificially inoculated with Alternaria species after different thermal treatments.
4 Discussion
Tomato plants can be infected by several pathogens during their cultivation in the field. Among fungi, Alternaria spp. have been commonly reported during the tomato growing season and are considered responsible for the visible and severe damages on the plants and fruits, causing important yield losses when environmental conditions are particularly conducive for their development (Nash and Gardner, 1988; Parvin et al., 2021). The infection of tomatoes by Alternaria spp., as well as for other fungal infections, is very climate-dependent. To our knowledge, there are no monitoring studies on the presence of Alternaria toxins throughout the entire growing season in tomato fields; moreover, the level of mycotoxin contamination in a restricted area can be very different from field to field (Locatelli et al., 2022). For this reason, in order to have different samples with similar infections, an artificial inoculation of tomatoes was preferred and was performed.
The most common Alternaria species isolated from Italian tomatoes are A. solani, A. alternata, and A. tenuissima (Garganese et al., 2019; Sanzani et al., 2019). For this reason, it was decided to use isolates belonging to these strains to artificially inoculate samples. Moreover, the strains used have already been tested in previous experiments in order to ensure that they could produce all of the four Alternaria toxins reported as important in terms of human exposure and toxicity: AOH, AME, TeA, and TEN (Aichinger et al., 2021; Lin et al., 2023).
Recently, many studies have been conducted in the field and in vitro to examine possible methods for containment of Alternaria species and Alternaria toxins, also considering innovative biological products such as lipopeptides (Zhang et al., 2024), curcumin (Qi et al., 2024), and ginger (Hyder et al., 2024); the use of microorganisms such as bacteria (Bellotti et al., 2023; Bertuzzi et al., 2022; Hyder et al., 2024) or Trichoderma species (Bassant et al., 2024); and the use of new technologies such as the nanosystem approach (Khatoon et al., 2024; Xu et al., 2024), demonstrating the emerging interest linked to this fungal pathology able to produce mycotoxins in tomato products worldwide (Bertuzzi et al., 2021; Xu et al., 2024) and with possible health risks to consumers. The results obtained from this study underlined a different distribution of Alternaria toxins in tomato peel and pulp.
In particular, the findings of this work can indicate that the widespread occurrence of TeA in tomato-based products is certainly due to the high contamination in raw moldy tomatoes; however, it can also be favored by migration from the peel, where the fungal contamination starts in the field and is normally considered as a by-product, to the pulp. The different distribution of the Alternaria toxins in the peel and pulp of the tomato samples could be explained by their different chemical properties. Unlike AOH and TEN, TeA is a monobasic acid with pKa = 3.5 (EFSA, 2011), which can then easily move in the acidic water-rich pulp fraction of tomatoes. On the other hand, the limited occurrence of AOH in tomato products can also be due to its limited migration in the pulp, remaining in the skin waste.
This study has some limitations due to not being a complete replication of field conditions. In particular, as we wanted to achieve a very high contamination in tomatoes, we used a high concentration of Alternaria spores for the preparation of the artificial inoculum, a concentration normally never present in the field. However, as we wanted to have a high amount of mycotoxins, a high level of inoculum was necessary. Tomatoes were also damaged in order to favor the fungal infection and make it more speedy; this parameter is also not always present in the field, where fruits can also be contaminated without any damage. Moreover, detached tomatoes were used without consideration of the possible role of the plant in disease containment. However, the results obtained in this study can be considered preliminary, but also very important in order to determine the distribution of the different Alternaria toxins in fruits to better define possible measures for their containment during tomato transformation.
Among the Alternaria toxins present in the water after the incubation of tomatoes, only AOH was never detected. TeA and TEN have been reported to be easily removed from the tomato surface through washing (Estiarte et al., 2018). However, the water taken into consideration was not derived from the tomato washing, but is distilled and sterilized water placed inside the glass boxes for maintenance of high humidity. Furthermore, the water was not in contact with the tomatoes. However, during the incubation time (3 weeks), the tomatoes decayed, releasing little quantity of liquid that contaminated the water. It is interesting that the TeA levels in the water were very high, indicating that its hydrophilic properties can favor migration in water solutions. This trend could indicate that the water used for tomato washing and/or for fruit movement during the industrial processing can become contaminated if not frequently replaced or when not using large volumes of water. The high mobility of TeA in the pulp and in aqueous solutions may facilitate cross-contamination from a limited number of highly contaminated tomatoes to whole derived products, obtained from different lots of tomatoes processed simultaneously.
Similarly to other mycotoxins, Alternaria toxins are not completely destroyed during heat processing. Siegel et al. (2010) reported a limited degradation of AOH, AME, and altenuene during wet bread baking, but a significant degradation upon dry baking. Estiarte et al. (2018) reported that heating of tomato samples at 100°C and 110°C significantly affected the stability of AOH. Our results confirmed this last study. Approximately 50% of AOH was destroyed after heat treatments at conditions close to those used in industrial tomato processing. AOH belongs to the family of isocoumarins and derivatives. To our knowledge, there is no information available on the thermal stability of AOH in different matrices. It is probable that both the acidic conditions of the matrix (the pH of tomato is approximately 3.5) and the high temperature, as well as the limited migration into the pulp (in our experiments, the inner temperature of tomatoes was lower than that in the peel; data not shown), contribute to the reduction in AOH. It will be important to recognize its metabolites and their toxicity. The reduction of TeA and TEN contamination was extremely limited, showing high stability to heat treatments. The best results were obtained with the double heat treatment (100°C + 121°C). However, this type of treatment is not used in industrial tomato processing as it greatly worsens the quality of the final products, modifying the color and decreasing the nutrient content.
The findings of this work improve our understanding of the behavior of Alternaria species during the infection phase and in the definition of protocols useful for reducing the presence of Alternaria toxins in tomato-derived products. In particular, the different distribution of toxins in the pulp and the skin during the infection cycle of the fungus needs to be investigated further, while the washing and the sorting steps appear to be two of the most important points during tomato processing.
The use of clean and abundant water can help in reducing the level of certain mycotoxins such as TeA and TEN in the final product, while the removal of visibly defective fruits could be essential in reducing the initial contamination levels of tomatoes as heat treatments are not able to greatly reduce the presence of Alternaria toxins in the final derived products. Indeed, the EU Recommendation 2022/553 reported a single indicative limit for all tomato-derived products, independently of the concentration factor occurring during the processing with respect to raw tomatoes. It will be important to evaluate both the concentration factor of the different final products (i.e., sauce, pulp, and paste) and the different values of migration in the pulp and heat stability for each Alternaria toxin, as reported in this study.
Further studies are needed to understand the critical control points of tomato processing and possible corrective measures to reducing the risk of contamination of the final products and protecting consumers’ health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
PG: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. EB: Investigation, Writing – original draft. TB: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors wish to thank Giovanna Balordi for her valuable collaboration in tomato sampling.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffunb.2025.1516557/full#supplementary-material
References
Aichinger G., Del Favero G., Warth B., and Marko D. (2021). Alternaria toxins - Still emerging? Compr. Rev. Food Sci. Food Saf. 20, 4390–4406. doi: 10.1111/1541-4337.12803
Bagi F. F., Ilicic R. M., Jevtic R. M., Orbovic B. R., Savic Z. N., Suman M., et al. (2022). Toxigenic potential of Alternaria species from cereals. Matica Srpska J. Nat. Sci. 142, 39–45. doi: 10.2298/ZMSPN2242039B
Bassant P., Behiry S. I., Salem M. Z. M., Amer M. A., El-Samra I. A., Abdelkhalek A., et al. (2024). Trichoderma afroharzianum TRI07 metabolites inhibit Alternaria alternata growth and induce tomato defense-related enzymes. Sci. reports. 14, 1874. doi: 10.1038/s41598-024-52301-2
Bellotti G., Guerrieri M. C., Giorni P., Bulla G., Fiorini A., Bertuzzi T., et al. (2023). Enhancing plant defense using rhizobacteria in processing tomatoes: a bioprospecting approach to overcoming Early Blight and Alternaria toxins. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1221633
Bertuzzi T., Leni G., Bulla G., and Giorni P. (2022). Reduction of mycotoxigenic fungi growth and their mycotoxins production by Bacillus subtilis QST 713. Toxins 14, 797. doi: 10.3390/toxins14110797
Bertuzzi T., Rastelli S., Pietri A., and Giorni P. (2021). Alternaria toxins in tomato products in Northern Italy in the period 2017-2019. Food Addit. Contam. B. 14, 170–176. doi: 10.1080/19393210.2021.1895325
Clewer A. G. and Scarisbrick D. H. (2001). Practical statistics and experimental design for plant and crop science (England: John Wiley & Sons, Ltd).
da Motta S. and Soares L. (2001). Survey of Brazilian tomato products for alternariol, alternariol monomethyl ether, tenuazonic acid and cyclopiazonic acid. Food Addit. Contam. 18, 630–634. doi: 10.1080/02652030117707
Estiarte N., Crespo-Sempere A., Marín S., Ramos A. J., and Worobo R. W. (2018). Stability of alternariol and alternariol monomethyl ether during food processing of tomato products. Food Chem. 245, 951–957. doi: 10.1016/j.foodchem.2017.11.078
European Food Safety Authority (EFSA) (2011). Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 9, 2407. doi: 10.2903/j.efsa.2011.2407
European Food Safety Authority (EFSA), Arcella D., Eskola M., and Gómez Ruiz J. A. (2016). Scientific report on the dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 14, 32. doi: 10.2903/j.efsa.2016.4654
Garganese F., Sanzani S. M., Di Rella D., Schena L., and Ippolito A. (2019). Pre- and postharvest application of alternative means to control Alternaria Brown spot of citrus. Crop Prot. 121, 73–79. doi: 10.1016/j.cropro.2019.03.014
Hyder S., Gondal A. S., Sehar A., Khan A. R., Riaz N., Rizvi Z. F., et al. (2024). Use of ginger extract and bacterial inoculants for the suppression of Alternaria solani causing early blight disease in tomato. BMC Plant Biol. 24, 131. doi: 10.1186/s12870-024-04789-z
Ji X., Deng T., Xiao Y., Jin C., Lyu W., Wu Z., et al. (2023). Emerging Alternaria and Fusarium mycotoxins in tomatoes and derived tomato products from the China market: Occurrence, methods of determination, and risk evaluation. Food Control. 145, 109464. doi: 10.1016/j.foodcont.2022.109464
Khatoon J., Mehmood A., ur Rehman Khalid A., Khan M. A. R., Ahmad K. S., Amjad M. S., et al. (2024). Green-fabricated silver nanoparticles from Quercus incana leaf extract to control the early blight of tomatoes caused by Alternaria solani. BMC Plant Biol. 24, 302. doi: 10.1186/s12870-024-05008-5
Lin H., Jia B., and Wu A. (2023). Cytotoxicities of Co-occurring alternariol, alternariol monomethyl ether and tenuazonic acid on human gastric epithelial cells. Food Chem. Toxicol. 171, 113524. doi: 10.1016/j.fct.2022.113524
Locatelli S., Scarpino V., Lanzanova C., Romano E., and Reyneri A. (2022). Multi-mycotoxin long-term monitoring survey on north-italian maize over an 11-year period, (2011-2021): the co-occurrence of regulated, masked and emerging mycotoxins and fungal metabolites. Toxins 14, 520. doi: 10.3390/toxins14080520
Maldonado Haro M. L., Cabrera G., Fernandez Pinto V., and Patriarca A. (2023). Alternaria toxins in tomato products from the Argentinean market. Food Control. 147, 109607. doi: 10.1016/j.foodcont.2023.109607
Meng J., Guo W., Zhao Z., Zhang Z., Nie D., Tangni E. K., et al. (2021). Production of Alternaria toxins in yellow peach (Amygdalus persica) upon artificial inoculation with Alternaria alternate. Toxins 13, 656. doi: 10.3390/toxins13090656
Nash A. F. and Gardner R. G. (1988). Tomato early blight resistance in a breeding line derived from Lycopersicon hirsutum PI 126445. Plant Dis. 72, 206–209. doi: 10.1094/PD-72-0206
Noser J., Schneider P., Rother M., and Schmutz H. (2011). Determination of six Alternaria toxins with UPLC-MS/MS and their occurrence in tomatoes and tomato products from the Swiss market. Mycotoxin Res. 27, 265–271. doi: 10.1007/s12550-011-0103-x
Parvin I., Mondal C., Sultana S., Sultana N., and Aminuzzaman F. M. (2021). Pathological survey on early leaf blight of tomato and in vitro effect of culture media, temperature and pH on growth and sporulation of Alternaria solani. Open Access Library J. 8, 1–17. doi: 10.4236/oalib.1107219
Qi C., Zhang H., Chen W., and Liu W. (2024). Curcumin: an innovative approach for postharvest control of Alternaria alternata induced black rot in cherry tomatoes. Fungal Biol. 128, 1691–1697. doi: 10.1016/j.funbio.2024.02.005
Qin Q., Fan Y., Jia Q., Duan S., Liu F., Jia B., et al. (2022). The potential of alternaria toxins production by A. alternata in processing tomatoes. Toxins 14, 827. doi: 10.3390/toxins14120827
Sanzani S., Djenane F., Incerti O., Admane N., Mincuzzi A., and Ippolito A. (2021). Mycotoxigenic fungi contaminating greenhouse-grown tomato fruit and their alternative control. Eur. J. Plant Pathol. 160, 287–300. doi: 10.1007/s10658-021-02240-9
Sanzani S., Gallone T., Garganese F., Caruso A., Amenduni M., and Ippolito A. (2019). Contamination of fresh and dried tomato by Alternaria toxins in southern Italy. Food Addit. Contam. A. 36, 789–799. doi: 10.1080/19440049.2019.1588998
Siegel D., Feist M., Proske M., Koch M., and Nehls I. (2010). Degradation of the Alternaria mycotoxins alternariol, alternariol monomethyl ether, and altenuene upon bread baking. J. Agric. Food Chem. 58, 9622–9630. doi: 10.1021/jf102156w
Xu S., Ge S., Xie Y., Cheng J., Ding K., Li H., et al. (2024). Stabilizing methyl ferulate with nanosystem enhances its antifungal activity against Alternaria alternata in vitro and in vivo. Postharvest Biol. Technol. 216, 113057. doi: 10.1016/j.postharvbio.2024.113057
Xu W., Han X., Zhang J., Xu J., and Bai L. (2024). Occurrence and co-occurrence of Alternaria toxins in tomato based products collected in China. Food Control. 155, 110030. doi: 10.1016/j.foodcont.2023.110030
Zhang Y., Fan Y., Dai Y., Jia Q., Guo Y., Wang P., et al. (2024). Crude lipopeptides produced by Bacillus amyloliquefaciens could control the growth of Alternaria alternata and production of Alternaria toxins in processing tomato. Toxins 16, 65. doi: 10.3390/toxins16020065
Keywords: tomato, Alternaria spp., Alternaria toxins, tenuazonic acid, alternariol, tentoxin, heat treatments, early blight
Citation: Giorni P, Barato E and Bertuzzi T (2025) Distribution of Alternaria toxins in tomato pulp and peel and their stability to heat treatments. Front. Fungal Biol. 6:1516557. doi: 10.3389/ffunb.2025.1516557
Received: 24 October 2024; Accepted: 15 May 2025;
Published: 06 June 2025.
Edited by:
Alessandra Marcon Gasperini, University of Hertfordshire, United KingdomReviewed by:
Magdalena Cara, Agricultural University of Tirana, AlbaniaMario Masiello, National Research Council (CNR), Italy
Copyright © 2025 Giorni, Barato and Bertuzzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Terenzio Bertuzzi, dGVyZW56aW8uYmVydHV6emlAdW5pY2F0dC5pdA==
 Paola Giorni
Paola Giorni Erica Barato2
Erica Barato2 Terenzio Bertuzzi
Terenzio Bertuzzi