- 1Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- 2Department of Medical Parasitology and Mycology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
- 3Department of Pharmacology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
Background: The rising prevalence of nosocomial infections, particularly those caused by airborne fungal spores in operating rooms and intensive care units (ICUs), has become a significant public health concern. Fusarium species in water systems pose a severe threat to immunocompromised patients and can disseminate as aerosols through devices such as faucets and showers. This study aimed to isolate and identify Fusarium species from the water systems of the ICUs and transplant units at Imam Khomeini Hospital Complex and Shariati Hospital in Tehran, Iran, as potential sources of future outbreaks. Additionally, the study sought to determine the in vitro susceptibilities of the isolates to conventional antifungal agents.
Methods: Sterile swabs and open plates containing Sabouraud dextrose agar (SDA) with chloramphenicol were used to collect water samples from sink surfaces, shower trays, faucets, and around the drains of sinks, as well as from bathroom areas. Swab samples were cultured, and the open-plate samples were evaluated for the growth of Fusarium species. The validation of all Fusarium sp. isolates was performed using DNA sequencing of the translation elongation factor 1-alpha (TEF-1α) gene. The antifungal susceptibility patterns of each isolate were tested against voriconazole, itraconazole, posaconazole, caspofungin, and amphotericin B using the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method for filamentous fungi.
Results: Fusarium species were recovered from six out of 362 water system samples, representing 1.65% of the total. Five isolates were identified as Fusarium oxysporum from the F. oxysporum complex, while one isolate was identified as Fusarium proliferatum from the Fusarium fujikuroi complex. All isolates were obtained from sinks (three isolates) and faucets (three isolates) at Imam Khomeini Hospital. Antifungal susceptibility testing revealed that posaconazole, voriconazole, and amphotericin B were the most effective drugs against all Fusarium isolates, with no instances of resistance to these antifungal agents observed. However, non-wild-type isolates were noted for the other drugs tested.
Discussion: The isolation of pathogenic Fusarium species from water samples collected in the ICU wards of the Imam Khomeini Hospital Complex underscores the urgent need to implement effective control and prevention measures in hospital water systems.
Introduction
The genus Fusarium consists of hyaline, septate mycelium typically found in plants, soil, and various organic substrates (Ekwomadu and Mwanza, 2023). In recent years, these fungi have emerged as the second most common cause of invasive fungal infections among immunocompromised patients (Nucci and Anaissie, 2023). Fusarium species have the potential to persist in water systems, including those in hospitals, for extended periods (Hino et al., 2020). Some studies have indicated that nosocomial infections can be attributed to Fusarium species (Balmas et al., 2021; Douglas et al., 2023). The prevailing hypothesis suggests that Fusarium species present in water become aerosolized when the water flows through fixtures, such as taps and showers, and are subsequently inhaled by immunocompromised patients who are at an elevated risk for fusariosis (Balmas et al., 2021).
Over 300 species of Fusarium have been identified, with approximately 70 of these species implicated in human infections (Cighir et al., 2023). The species most frequently associated with fusariosis are Fusarium solani (60%) and Fusarium oxysporum (20%) (Kausar et al., 2021). These two species have been classified as species complexes, and several new species have been identified and described recently. The species complexes frequently associated with human infections include the F. solani species complex (FSSC), F. oxysporum species complex (FOSC), Fusarium tricinctum species complex (FTSC), Fusarium incarnatum-equiseti species complex (FIESC), Fusarium fujikuroi species complex (FFSC), Fusarium dimerum species complex (FDSC), and Fusarium chlamydosporum species complex (FCSC) (Abdurakhmonov, 2024).
Fusarium infections primarily present as keratitis and onychomycosis in immunocompetent individuals, while severe disseminated infections are observed in those with compromised immune systems (Nucci and Anaissie, 2023). Fungemia in patients with significant immunosuppression leads to localized necrosis following secondary dissemination to the skin (Varghese et al., 2023). Multiresistance to antifungal agents is a characteristic feature of all Fusarium species, making these fungi particularly challenging to manage therapeutically (Wiederhold, 2020). Each species exhibits a unique resistance pattern to specific antifungal agents. Furthermore, Fusarium species lack a standardized minimum inhibitory concentration (MIC), complicating the prediction of antifungal susceptibility for individual strains (Wiederhold, 2020). Antifungal susceptibility testing (AFST) should be integrated into standard patient management practices to enhance therapeutic effectiveness, especially in cases of severe infections. To the best of our knowledge, there is limited information regarding the prevalence of Fusarium spp. in hospital water systems, which may serve as potential sources for future outbreaks, as well as the in vitro susceptibilities of isolates to antifungals. This scarcity of data is primarily due to the fact that most laboratories do not routinely perform AFST, combined with the difficulties many laboratories face in accurately identifying Fusarium species. Given the hypothesis that Fusarium species present in water become aerosolized when the water flows through fixtures, subsequently being inhaled by immunocompromised patients at elevated risk for fusariosis, the present study aimed to i) evaluate the prevalence of Fusarium spp. in the water systems of the intensive care units (ICUs) and transplant wards of Imam Khomeini Hospital Complex and Shariati Hospital in Tehran and ii) investigate AFST of the strains to assist clinicians in making optimal antifungal decisions.
Materials and methods
Ethics statement
Ethical approval for this study was obtained from the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.SPH.REC.1402.006).
Sampling, isolation, and growth conditions
This descriptive cross-sectional study was conducted over a period of 19 months (from May 2022 to December 2023) in the transplant wards and ICUs of Imam Khomeini Hospital Complex and Shariati Hospital in Tehran, the capital and largest city of Iran. During the study period, six sterile cotton-tipped applicators were used to collect water samples from the surfaces, shower trays, faucets, and around the sinks with a circular and one-way motion of the swabs (Anaissie et al., 2001). Additionally, to collect water samples from the bathrooms, sampling was performed with the water running for 35 minutes. In this setup, plates containing Sabouraud dextrose agar (SDA) with chloramphenicol (SC, Merck, Germany) culture medium were placed at a height of 1.5 m above the ground, next to the shower, with the plate lid cracked open for 15 minutes. The collected samples were then transferred to the Medical Mycology Laboratory of Tehran University of Medical Sciences (TUMS) for mycological analysis. The SC plates were incubated at 25°C for 72 hours and examined daily for growth of Fusarium spp. The swabs were also cultured on SDA medium and incubated at a temperature of 25°C for 72 hours. The purified colonies were then cultured on subsequently Potato Dextrose Agar medium (PDA; Merck, Germany) for 5–7 days at 25°C. Five to seven Fusarium spp. at 25°C were further identified by colony morphology and lactophenol cotton blue (LPCB) mounts.
Fusarium colonies typically appear fast-growing, with velvety to cottony texture, and can range in color from white to yellow, pink, red, or purple depending on the species; microscopically, Fusarium is characterized by the production of both macroconidia (large, sickle-shaped) and microconidia (smaller, often one-celled spores) (Chidambaram et al., 2017).
Molecular techniques
The validation of all Fusarium spp. isolates was carried out using DNA sequencing of the translation elongation factor 1-alpha (TEF-1α) gene. The genomic DNA was extracted from harvested colonies using the phenol–chloroform method. The amplification of the TEF-1α was performed with a pair of primers, EF1 (5′ ATGGGTAAGGAAGGACAAG 3′) and EF 2 (5′ GGAGAGTACCAGTGCATCAT 3′). Each polymerase chain reaction (PCR) contained 5 μL of 10× reaction buffer, 1.5 μL MgCl, 0.5 μL dNTP mixture, 0.25 μL Taq polymerase (Prime Taq, Genet Bio, South Korea), 0.5 μL of each primer, 1 μL of DNA template solution, and enough distilled water to reach a final volume of 50 μL. PCR was conducted with the following conditions: an initial denaturation step at 95°C for 5 minutes, 35 amplification cycles with denaturation at 94°C for 45 s, primer annealing at 55°C for 1 minute, and elongation at 72°C for 1 minute, followed by a final elongation step at 72°C for 6 minutes. Positive PCR products were examined by staining with DNA safe stain (4 µL) and electrophoresis on 1.5% agarose gel. The PCR products were subjected to sequencing and analyzed using the ChromasPro-Version 2.1.3 software. The EF sequences were compared with those available in the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.gov/) genetic database using the Basic Local Alignment Tool (BLAST) algorithm and using only sequence identity values above 99%.
Antifungal susceptibility testing
The Clinical and Laboratory Standards Institute document M38-A2 (CLSI M38-A2) broth microdilution protocol was used as a guideline for AFST of filamentous fungi (Espinel-Ingroff et al., 2009). The susceptibility of the Fusarium spp. isolates to voriconazole (VOR), posaconazole (PSZ), caspofungin (CAS), itraconazole (ITR), and amphotericin B (AMB) (all from Pfizer, Groton, CT, USA) was tested. The following dilutions were developed: 0.06–64 µg/mL for VOR, 0.06–64 µg/mL for ITR, 0.06–64 µg/mL for PSZ, 0.008–8 µg/mL for CAS, and 0.06–64 µg/mL for AMB. A suspension equivalent to 0.5 McFarland was prepared from the Fusarium culture on PDA by suspending the fungal cells in 6 mL of sterile distilled water, and the concentration was spectrophotometrically adjusted according to CLSI M38-A2. Subsequently, 100 µL of fungal inocula was dispensed into wells of microplates, and the results were read after 48 hours of incubation at 35°C. The quality reference strains of Candida parapsilosis (ATCC 22019), Candida krusei (ATCC 6258), and Aspergillus flavus (ATCC 2004304) were used as CLSI control strains.
Statistical analysis
Data were analyzed using the Excel software with descriptive and analytical statistics. Student’s t-test was used to compare the means between the two groups with a 95% confidence interval and p < 0.05 significance level.
Results
During a period of 19 months, a total of 362 water samples were collected, including 144 samples from Imam Khomeini Hospital Complex and 218 samples from Shariati Hospital. Of these, 32 samples were collected using open plates and 330 samples using sterile swabs. After culture, the colony appearance of six samples (1.65%) showed white, cottony colonies with abundant aerial mycelia of Fusarium spp. Five isolates were identified as F. oxysporum from the F. oxysporum complex, and one isolate was identified as Fusarium proliferatum from the F. fujikuroi complex.
All isolates were obtained from sinks (three isolates) and faucets (three isolates) at the Imam Khomeini Hospital Complex. Three isolates were obtained from the general ICU: two from the cardiac ICU (CICU) and one from the thorax and lung transplant ICU (TLTICU) (Table 1).
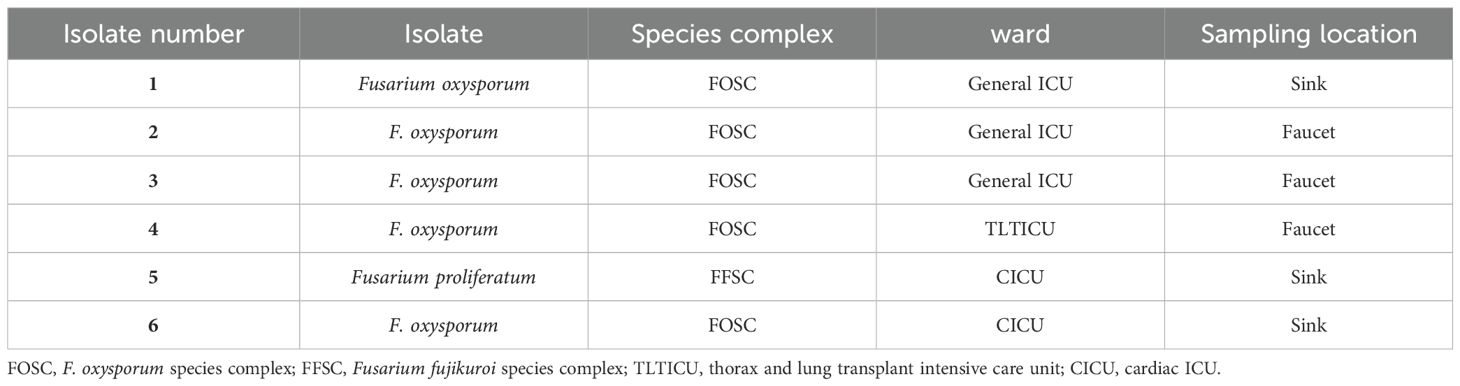
Table 1. Detailed information related to six Fusarium species isolated from the water systems in Imam Khomeini Hospital Complex.
The results of AFST are presented in Table 2. We accessed the susceptibility of six Fusarium species isolates to five antifungal drugs (voriconazole, itraconazole, posaconazole, caspofungin, and amphotericin B) by the epidemiological cutoff values (ECVs) of antifungal agents determined by Espinel-Ingroff et al (WHO (world health organization), 2022).
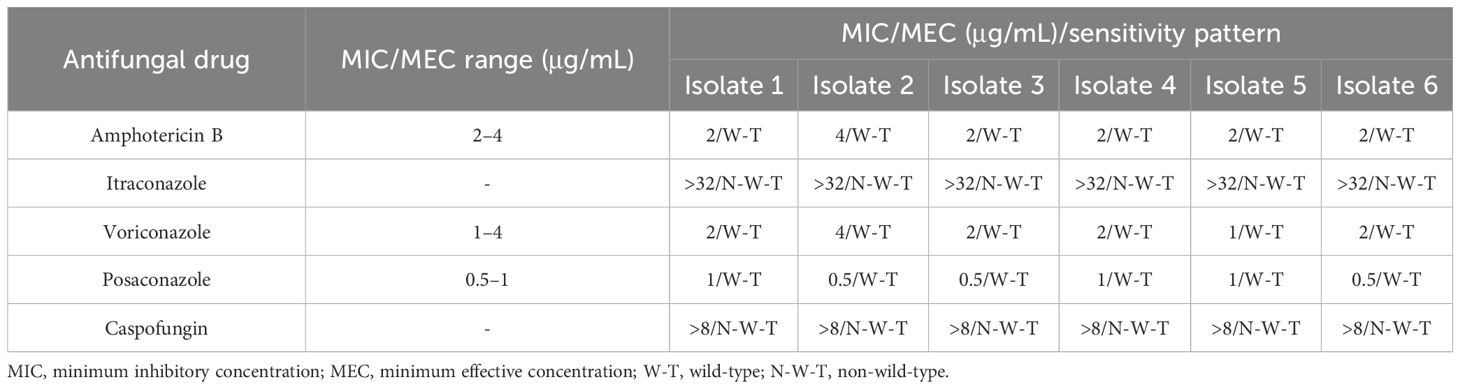
Table 2. In vitro antifungal susceptibility pattern of Fusarium species isolated from the water systems in Imam Khomeini Hospital Complex.
All isolates were wild-type for amphotericin B, posaconazole, and voriconazole, while non-wild-type isolates were observed for the other drugs. AFST showed that posaconazole (MIC range, 0.5–1 μg/mL), voriconazole (MIC range, 1–4 μg/mL), and amphotericin B (MIC range, 2–4 μg/mL) were the most active drugs against all Fusarium isolates, and no case of resistance to these antifungal agents was observed, while non-wild-type isolates were observed for the other drugs.
Discussion
Recent advancements in the treatment of patients admitted to ICUs or those who have undergone organ transplantation have not diminished the severity of fusariosis, and this infection remains a notable contributor to mortality in these patients (Ekwomadu and Mwanza, 2023). The World Health Organization categorized the genus Fusarium as part of the “high priority group” of pathogens that pose significant public health risks and outlined it in the fungal priority pathogens list (FPPL) (WHO (world health organization), 2022). Fusarium spp. may inhabit water distribution systems, including those of hospitals, and can persist in the water system for years (Sautour et al., 2012). The water system has the potential to spread these organisms through aerosols generated by showers and sinks, and the isolates obtained from the water system may also lead to nosocomial infections (Anaissie et al., 2001).
In the present study, five F. oxysporum isolates and one F. proliferatum isolate were recovered from six (1.65%) water system samples. Anaissie et al., for the first time, demonstrated that hospital water systems can be reservoirs for opportunistic fungi, particularly F. oxysporum and F. solani (Anaissie et al., 2001). Numerous reports published in the literature have confirmed the diversity of situations regarding the recovery rates of Fusarium spp. from hospital water distribution systems. No strains of Fusarium were identified in the hospital water systems located in Nijmegen, Netherlands (Warris et al., 2003), nor Athens and Thessaloniki, Greece (Panagopoulou et al., 2002). Furthermore, it was observed that less than 1% of samples tested positive at a hospital located in Thessaloniki, Greece (Arvanitidou et al., 1999). Conversely, molds belonging to the genus Fusarium were detected in 57% of water samples collected from a hospital situated in Houston, USA (Anaissie et al., 2001).
Also, in the current investigation, all isolates were obtained from sinks and faucets at Imam Khomeini Hospital Complex, the largest hospital in the Middle East. Considering the fact that there are many old buildings in this hospital, the construction and renovation of different buildings of this hospital can be an important source for Fusarium conidia (Sautour et al., 2012). We hypothesize that variations in hospital infrastructure, maintenance practices, and water quality may have contributed to the significant difference in isolate recovery. For instance, the water management protocols and the sanitation practices in place at each hospital could impact the presence of airborne fungal spores. Therefore, we point out the need for further studies that include a comparison of these factors across institutions to better understand their influence on the prevalence of Fusarium species. Additionally, there is the possibility of sampling bias. The specific locations sampled (sinks, faucets, and showers) may not be uniformly contaminated across both hospitals or may have been affected by variable water flow and usage patterns. Future research could benefit from a randomized sampling strategy and an extended duration to capture temporal variations in fungal presence more comprehensively.
Also, the presence of biofilms in the sinks and faucets could be another source of water contamination in the present study (Sautour et al., 2012). In accordance with the present investigation, in a previous study, the sink was reported to be the most isolated place of Fusarium spp., and from 92 sink drains tested, 72 (88%) yielded contamination with Fusarium spp (Sautour et al., 2012).
Fusarium species are commonly resistant to many antifungal drugs currently available in clinical settings. The intrinsic resistance of Fusarium spp. to azoles, along with high MICs/minimum effective concentrations (MECs) to polyenes and echinocandins, has been reported, although some studies have reported successful treatment with antifungals (Sav et al., 2018).
In the present study, the in vitro activity of five common antifungal drugs including voriconazole, itraconazole, posaconazole, amphotericin B, and caspofungin against Fusarium isolates was investigated. Posaconazole showed greater in vitro activity than other tested antifungal drugs against the Fusarium isolates. Therefore, the results showed its superiority to amphotericin B as the primary therapy of invasive fusariosis.
In a study conducted by Paphitou et al (Paphitou et al., 2002), the sensitivity of various Fusarium species (n = 22) against five antifungal drugs (voriconazole, fluconazole, amphotericin B, posaconazole, and ravuconazole) was investigated. According to their findings, posaconazole and voriconazole exhibited promising antifungal activities, which is completely consistent with the findings of the present study.
Therefore, in severely immunocompromised patients, it is imperative to take all necessary precautions to minimize their exposure to Fusarium spp. This may include putting high-risk patients in rooms equipped with High-Efficiency Particulate Air (HEPA) filters and maintaining positive pressure, avoiding contact with potential sources of Fusarium spp., such as tap water, and ensuring that showers are thoroughly cleaned before use by high-risk patients (Nucci and Anaissie, 2007).
The results of the present study showed that among the Fusarium spp. isolated from water samples, five isolates were identified as F. oxysporum from the F. oxysporum complex, and one isolate was identified as F. proliferatum from the F. fujikuroi complex. Additionally, it will be useful to conduct future studies to show how the picture will change after the necessary protective measures. Also, it is recommended to pursue further studies with an expanded sample size to validate our findings and enhance the applicability of our results.
Furthermore, the present study identified environmental isolates of Fusarium species, but it did not investigate their direct association with patient infections. This is a significant limitation of our research that we must emphasize. Therefore, further research is essential to establish the clinical relevance of these environmental isolates through a comprehensive epidemiological approach, possibly involving patient screening and clinical data correlation.
Conclusion
The isolation of pathogenic Fusarium spp. in water samples gathered from ICU wards of the Imam Khomeini Hospital Complex highlights the necessity of implementing appropriate control and prevention methods in hospital water systems to reduce the infection risks associated with these potentially dangerous microorganisms.
The presence of Fusarium species in ICUs can indeed be complex. While Fusarium infections are often considered less common than those caused by other fungi, such as Aspergillus, this perception may be influenced by several factors:
1. The methodology used in culture and identification can significantly affect the reported prevalence of Fusarium. Traditional culture techniques may not effectively capture all fungal species present in the environment, especially if their growth conditions differ from those preferred by Aspergillus or if they require specific media for optimal growth. Molecular methods, such as PCR, can improve detection rates of Fusarium and other fungi that are underreported with standard culturing techniques.
2. Fusarium species are widely distributed in soil and plants, and they can be found in healthcare settings, especially in environments with plant material or organic matter. However, their actual counts in ICU air and surfaces compared to those of other fungi like Aspergillus may vary based on local environmental conditions, cleaning practices, and the specific design of the ICU.
3. Research indicates that Aspergillus is more commonly associated with invasive fungal infections in immunocompromised patients, particularly in hospital settings. In contrast, while Fusarium can cause serious infections, particularly in patients with weakened immune systems, it is considered less prevalent overall in clinical settings, although reports of fusariosis are increasing, and studying its presence can provide insights into potential sources of infection and transmission routes within ICUs and other high-risk areas. By focusing on Fusarium, researchers can contribute valuable knowledge to the field, helping to ensure early detection, appropriate treatment, and effective prevention strategies against this and other fungal pathogens.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Ethical approval for this study was obtained from the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.SPH.REC.1402.006).
Author contributions
FM: Data curation, Investigation, Writing – original draft, Writing – review & editing. RD-G: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. S-JH: Supervision, Validation, Conceptualization, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. SK: Validation, Visualization, Conceptualization, Investigation, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing. PA: Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. ZR: Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. DR: Conceptualization, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. HB: Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. FA-G: Investigation, Validation, Writing – original draft, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was part of the master’s thesis of FM and was supported by the Tehran University of Medical Sciences (TUMS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were editorial board members of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdurakhmonov I. Y. (2024). “Fusarium-recent studies: recent studies,” in BoD–books on demand. London, United Kingdom: IntechOpen
Anaissie E. J., Kuchar R. T., Rex J. H., Francesconi A., Kasai M., Müller F. M., et al. (2001). Fusariosis associated with pathogenic Fusarium species colonization of a hospital water system: a new paradigm for the epidemiology of opportunistic mold infections. Clin. Infect. Diseases. 33, 1871–1878. doi: 10.1086/324501
Arvanitidou M., Kanellou K., Constantinides T. C., Katsouyannopoulos V. (1999). The occurrence of fungi in hospital and community potab le waters. Lett. Appl. Microbiol. 29, 81–84. doi: 10.1046/j.1365-2672.1999.00583.x
Balmas V., Fancellu F., Sanna S., Scherm B., Migheli Q., Malbrán I. (2021). Water distribution systems in Sardinian hospitals host invasive clonal lineages of the Fusarium oxysporum and Fusarium solani species complexes. Mycologia. 113, 725–733. doi: 10.1080/00275514.2021.1905497
Chidambaram J. D., Prajna N. V., Larke N., Macleod D., Srikanthi P., Lanjewar S., et al. (2017). In vivo confocal microscopy appearance of Fusarium and Aspergillus species in fungal keratitis. Br. J. Ophthalmology. 101, 1119–1123. doi: 10.1136/bjophthalmol-2016-309656
Cighir A., Mare A. D., Vultur F., Cighir T., Pop S. D., Horvath K., et al. (2023). Fusarium spp. in human disease: exploring the boundaries between commensalism and pathogenesis. Life. 13, 1440. doi: 10.3390/life13071440
Douglas A. P., Stewart A. G., Halliday C. L., Chen S. C. (2023). Outbreaks of fungal infections in hospitals: epidemiology, detection, and management. J. Fungi. 9, 1059. doi: 10.3390/jof9111059
Ekwomadu T. I., Mwanza M. (2023). Fusarium fungi pathogens, identification, adverse effects, disease management, and global food security: A review of the latest research. Agriculture. 13, 1810. doi: 10.3390/agriculture13091810
Espinel-Ingroff A., Canton E., Peman J. (2009). Updates in antifungal susceptibility testing of filamentous fungi. Curr. Fungal Infection Reports. 3, 133–141. doi: https://doi.org/10.1007/S12281-009-0017-7
Hino Y., Muraosa Y., Oguchi M., Yahiro M., Yarita K., Watanabe A., et al. (2020). Drain outlets in patient rooms as sources for invasive fusariosis: An analysis of patients with haematological disorders. J. Hosp. Infection. 105, 518–526. doi: 10.1016/j.jhin.2020.04.029
Kausar R., Iram S., Ahmad K. S., Jaffri S. B. (2021). Molecular characterization of Fusarium solani and Fusarium oxysporum phyto-pathogens causing mango maturity malconformation. Arch. Phytopathol. Plant Protection. 54, 1372–1390. doi: 10.1080/03235408.2021.1910417
Nucci M., Anaissie E. (2007). Fusarium infections in immunocompromised patients. Clin. Microbiol Rev. 20, 695–704. doi: 10.1128/CMR.00014-07
Nucci M., Anaissie E. (2023). Invasive fusariosis. Clin. Microbiol. Rev. 36, e00159–e00122. doi: 10.1128/cmr.00159-22
Panagopoulou P., Filioti J., Petrikkos G., et al. (2002). Environmental surveillance of fi lamentous fungi in three tertiary care hospitals in Greece. J. Hosp Infect. 52, 185–191. doi: 10.1053/jhin.2002.1298
Paphitou N. I., Ostrosky-Zeichner L., Paetznick V. L., Rodriguez J. R., Chen E., Rex J. H. (2002). In vitro activities of investigational triazoles against Fusarium species: effects of inoculum size and incubation time on broth microdilution susceptibility test results. Antimicrob Agents Chemother. 46, 3298–3300. doi: 10.1128/AAC.46.10.3298-3300.2002
Sautour M., Edel-Hermann V., Steinberg C., Sixt N., Laurent J., Dalle F., et al. (2012). Fusarium species recovered from the water distribution system of a French university hospital. Int. J. hygiene Environ. Health 215, 286–292. doi: 10.1016/j.ijheh.2011.11.003
Sav H., Rafati H., Öz Y., Dalyan-Cilo B., Ener B., Mohammadi F., et al. (2018). Biofilm formation and resistance to fungicides in clinically relevant members of the fungal genus Fusarium. J. Fungi. 4, 16. doi: 10.3390/jof4010016
Varghese J. A., Guhan S., Zheng L. (2023). Emerging fungal infections and cutaneous manifestations in immunosuppressed patients. Curr. Dermatol. Reports. 12, 69–81. doi: 10.1007/s13671-023-00386-9
Warris A., Klaassen C. H., Meis J. F., De Ruiter M. T., De Valk H. A., Abrahamsen T. G., et al. (2003). Molecular epidemiology of Aspergillus fumigatus isolates recovered from water, air, and patients shows two clusters of genetically distinct strains. J. Clin. Microbiol. 41, 4101–4106. doi: 10.1128/JCM.41.9.4101-4106.2003
WHO (world health organization). WHO fungal priority pathogens list to guide research, in Development and public health action. (Geneva, Switzerland: WHO (world health organization)) (2022). Available at: https://www.who.int/publications/i/item/9789240060241.
Keywords: Fusarium oxysporum complex, Fusarium fujikuroi complex, water system, hospital, antifungal susceptibility testing
Citation: Mirhasani F, Daie-Ghazvini R, Hashemi SJ, Khodavaisy S, Ardi P, Rafat Z, Roostaei D, Bakhshi H and Amirzadeh-Ghasemi F (2025) Isolation and identification of Fusarium species from the water systems of ICUs and transplant wards of hospitals and determination of the in vitro susceptibilities of isolates to conventional antifungals. Front. Fungal Biol. 6:1564237. doi: 10.3389/ffunb.2025.1564237
Received: 07 February 2025; Accepted: 17 April 2025;
Published: 14 May 2025.
Edited by:
Dongmei Li, Georgetown University, United StatesReviewed by:
Maurizio Sanguinetti, Catholic University of the Sacred Heart, ItalyNuri Kiraz, Istanbul University-Cerrahpasa, Türkiye
Liyan Xi, Sun Yat-sen University, China
Copyright © 2025 Mirhasani, Daie-Ghazvini, Hashemi, Khodavaisy, Ardi, Rafat, Roostaei, Bakhshi and Amirzadeh-Ghasemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roshanak Daie-Ghazvini, cmRhaWVAdHVtcy5hYy5pcg==
 Fatemeh Mirhasani1
Fatemeh Mirhasani1 Roshanak Daie-Ghazvini
Roshanak Daie-Ghazvini Seyed Jamal Hashemi
Seyed Jamal Hashemi Sadegh Khodavaisy
Sadegh Khodavaisy Pegah Ardi
Pegah Ardi Zahra Rafat
Zahra Rafat Davoud Roostaei
Davoud Roostaei Heidar Bakhshi
Heidar Bakhshi