- 1School of Pharmaceutical Sciences and Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming, China
- 2Yunnan College of Modern Biomedical Industry, Kunming Medical University, Kunming, Kunming, China
- 3Key Laboratory of Tropical Translational Medicine of Ministry of Education, School of Pharmacy, Hainan Medical University, Haikou, China
- 4School of Pharmacy, Lishui University, Lishui, China
- 5Wenshan Center for Disease Control and Prevention, Wenshan, China
- 6Jingdong Center for Disease Control and Prevention, Jingdong, China
Thelephora palmata is a well-known species morphologically characterized by coralloid and leathery basidiomata with numerous fuscous purple to blackish-brown branches. It was once considered to exhibit a wide ecological range and distribution area. However, comprehensive phylogenetic analysis based on four loci (ITS, nrLSU, rpb2, and nrSSU) revealed that T. palmata sensu lato represents a species complex consisting of at least 12 cryptic taxa, with a biogeographic distribution pattern bounded by geographic regions: Asia, Eurasia, Europe, and North America. In this study, we proposed eight new taxa based on available specimens. Of these, seven new species and one forma from China were described here based on phylogenetic analyses, morphological examinations, and environmental niche comparisons, viz., T. apiculata, T. cornu-damae, T. densa, T. esculenta, T. fuscidula, T. sinopalmata, T. truncicola, and T. truncicola f. pallescens.
1 Introduction
The genus Thelephora Ehrh. ex Willd. was initially proposed by Ehrhart in 1785 (Ehrhart, 1789) and later validly published by Willdenow in 1787 and typified by T. terrestris Ehrh. ex Fr (Willdenow, 1787). This genus, along with Amaurodon J. Schröt., Odontia Pers., Polyozellus Murrill., Tomentella Pers. ex Pat., and Tomentellopsis Hjortstam, belongs to Thelephoraceae, Thelephorales (Larsen, 1968; Reid and Larsen, 1976; Stalpers, 1993; Larsen, 1998; Voitk et al., 2017; He et al., 2024).
Recent molecular phylogenetic analyses, nevertheless, cast doubt on the monophyly of the genus Thelephora as previously defined. According to molecular phylogenetic analyses, Thelephora has an exceptionally close phylogenetic affiliation with Tomentella, and both genera have been generally intermingled on the same evolutionary branches and did not form separate monophyletic groups, suggesting that neither Thelephora nor Tomentella is monophyletic (Yorou and Agerer, 2008; Lee et al., 2010; Larsson et al., 2019; Svantesson et al., 2021; Villarruel-Ordaz et al., 2024). Therefore, Kõljalg et al. (2024) re-evaluated the limits of Thelephora and Tomentella, and Tomentella was combined into a more comprehensive Thelephora.
The reconstituted genus Thelephora is characterized macroscopically by annual basidiomata, of two types—resupinate and thin or clavarioid, coralloid to spathulate–rosulate, with various colors and a smooth to granulose surface—and microscopically by subhyaline to brownish, tuberculate, or echinulate basidiospores and monomitic hyphal systems (Fries, 1821; Cunningham, 1963; Corner, 1968, Corner, 1976; Stalpers, 1993; Kõljalg, 1996; Ramírez-López et al., 2015; Tian et al., 2024; Zhang et al., 2024).
This genus is regarded as ectomycorrhizal fungi and is associated with a wide range of angiosperms and gymnosperms, predominantly within Betulaceae, Casuarinaceae, Ericaceae, Fagaceae, and Pinaceae, in a wide range of forest ecosystems, from tropical to temperate, but predominantly temperate (Lentz, 1942; Kõljalg et al., 2000; Roberts and Spooner, 2000; Hilszczanska and Sierota, 2006; Ramírez-López et al., 2013, 2015; Vizzini et al., 2016; Chacón et al., 2018; Das et al., 2018; Liu X. F., et al., 2021; Borovička et al., 2022). Furthermore, some of them hold edible and medicinal values in China. For instance, Thelephora ganbajun M. Zang, a traditional edible mushroom with anticancer and anti-allergic effects distributed in Yunnan Province, is favored by local mushroom enthusiasts as one of the most expensive wild mushrooms (Zang, 1987; Hu and Liu, 2001; Sha et al., 2008; Li et al., 2020; Xu et al., 2016; Yang et al., 2023).
Thelephora palmata (Scop.) Fr. was originally described based on materials from Europe (Carniola) and morphologically characterized by coralloid and leathery basidiomata with numerous fuscous purple to blackish-brown branches (Fries, 1821; Coker, 1921). This name was also broadly applied to the collections from Asia (Dai, 2011) and North America (Coker, 1921). The wide geographical distribution and broad ecological niches of T. palmata, along with the available molecular data, indicate that it could be a species complex consisting of a couple of morphologically similar and phylogenetically related species. In previous studies, these sequences, labeled as “T. palmata” from Asia, Europe, and North America, generally clustered within different evolutionary branches and did not form separate monophyletic groups, indicating that this name harbored at least a couple of different taxa in Eurasia and North America (Ramírez-López et al., 2015; Liu X. F., et al., 2021; Yang et al., 2023; Tian et al., 2024).
This study addresses the species diversity of the T. palmata complex using an integrative taxonomic approach and describes eight new taxa. Our aim was to gain a comprehensive understanding of the taxonomy of this species complex by examining multiple sources of evidence, including phylogenetic analysis, morphological characteristics, geographical distribution, and host preferences.
2 Materials and methods
2.1 Collection sites and sampling
A total of 25 specimens of Thelephora, including 19 samples so-called T. palmata, were collected in the provinces of Anhui, Jilin, and Yunnan, China, from 2019 to 2024 during rainy seasons. These specimens were photographed in situ and dried with an electric dryer at 45–50°C after recording their macroscopic features (Hu et al., 2022). The voucher specimens were deposited in the Mycological Herbarium of Kunming Medical University (MHKMU).
2.2 Morphological studies
Macroscopic morphology was described according to field notes and photographs of basidiomata, and the color codes referred to those of Kornerup and Wanscher (1978). The micromorphological structures were observed under a light microscope (DM2500, Leica Microsystems, Wetzlar, Germany), which were obtained from dried materials, mounted in 5% KOH and stained with 1% (w/v) Congo Red solution, when necessary. Melzer’s reagent was used to examine the amyloidity of basidiospores. Basidiospore ornamentations were examined with a ZEISS Sigma 300 scanning electron microscope (Carl Zeiss AG, Oberkochen, Germany). In this paper, [n/m/p] denotes “n” basidiospores measured from “m” basidiomata of “p” collections; (a)b–c(d) refers to the length and width of basidiospores, with “b–c” indicating the distribution interval of 90% of the measured values and “a” and “d” denoting the minimum and maximum measured values, respectively. “Q” is the length/width ratio of a basidiospore in side view, and “Qm” indicates the mean ± standard deviations of the Q values of all basidiospores.
2.3 DNA extraction, PCR amplification, and sequencing
Extraction of the total genomic DNA from the dried tissue of basidiomata (20 mg) was conducted using the modified CTAB method. Four DNA gene fragments—the nuclear ribosomal DNA internal transcribed spacer (ITS) regions, the large subunit nuclear ribosomal RNA (nrLSU), the RNA polymerase II second largest subunit gene (rpb2), and the small subunit of nuclear ribosomal RNA gene (nrSSU)—were amplified by polymerase chain reaction (PCR) using the primer pairs ITS4/ITS5, LR0R/LR5, rpb2-6F/rpb2-7R, and NS1/NS4, respectively (Vilgalys and Hester, 1990; White et al., 1990; Liu S., et al., 2021).
The PCR amplification reaction system included 2.5 μl of 10× PCR buffer (containing MgCl2), 0.2 μl Taq DNA polymerase (2.5 U/μl), 0.5 μl of deoxyribonucleotide triphosphate (dNTP, 200 M), 1 μl of each primer (5 μM), and 1 μl template DNA, which was then fixed to 25 μl in sterilized double-steamed water. The reaction system was as follows: pre-denaturation at 94°C for 5 min; 35 cycles of denaturation at 94°C for 40 s, annealing at 52/54/58°C for 40 s, and extension at 72°C for 1 min; and extension at 72°C for 10 min. The PCR conditions followed the description of Tang et al. (2014), Tang et al, 2017). The PCR products were examined by electrophoresis on 1% agarose gels. The amplified PCR products were sequenced using an ABI 3730 DNA Analyzer (Sangon, Shanghai, China) with the same primers. The sequences newly generated in the present study were deposited in GenBank (Table 1).
2.4 Phylogenetic analyses
Besides the newly generated sequences for this study, additional related sequences downloaded from the GenBank (two-letter combination) and the European Nucleotide Archive (ENA; four-letter combination) were also incorporated into a combined dataset for phylogenetic analyses. Following previous studies, Odontia ferruginea Pers. and O. fibrosis (Berk. & M.A. Curtis) Kõljalg were chosen as the outgroup taxa (Vizzini et al., 2016; Mu et al., 2021; Tian et al., 2024). The matrices of ITS, nrLSU, rpb2, and nrSSU were separately aligned using MUSCLE v3.6 and manually optimized on BioEdit v7.0.9, when necessary (Hall, 1999; Edler et al., 2021). The concatenated datasets were manually constructed.
The combined dataset (ITS + nrLSU + rpb2 + nrSSU) was analyzed using the maximum likelihood (ML) and Bayesian inference (BI) methods. ML analyses were implemented with raxmlGUI 2.0, and 1,000 rapid bootstrap replicates were performed. GTRGAMMA was set by default as the selected model (Sugawara et al., 2022). For BI analyses, partitioned Bayesian analyses of four Markov chain Monte Carlo (MCMC) chains were run for 5,000,000 generations, with trees sampled every 100 generations until the average standard deviation of split frequencies was <0.01 (Ronquist et al., 2012). The most appropriate substitution models were selected with MrModeltest v2.3 under the Akaike information criterion (AIC) (Nylander, 2004). For the combined dataset, the best-fit likelihood models of ITS, nrLSU, rpb2, and nrSSU were HKY+I+G, GTR+I+G, GTR+G, and GTR+I, respectively. The burn-in was set to discard trees sampled from the first 25% of the generations, and then the Bayesian posterior probabilities (PPs) were calculated for a majority consensus tree of the retained Bayesian trees.
3 Results
3.1 Molecular phylogeny
The combined dataset (ITS + nrLSU + rpb2 + nrSSU) included 156 sequences (68 from GenBank and 88 newly generated: 25 ITS, 25 nrLSU, 19 rpb2, and 19 nrSSU), which were edited and aligned in this study, with 3,374 characters of the alignment length. The alignment was submitted to TreeBASE (accession S31805).
In the phylogeny, the ML and BI approaches showed minimal differences in the evaluation results; thus, only the ML tree is shown here (Figure 1). In a pre-analysis, four DNA gene markers were evaluated: ITS, nrLSU, rpb2, and nrSSU. Of these, ITS, nrLSU, and rpb2 were able to accurately identify different taxa within the T. palmata complex. In contrast, nrSSU proved inadequate for distinguishing some close or sibling taxa.
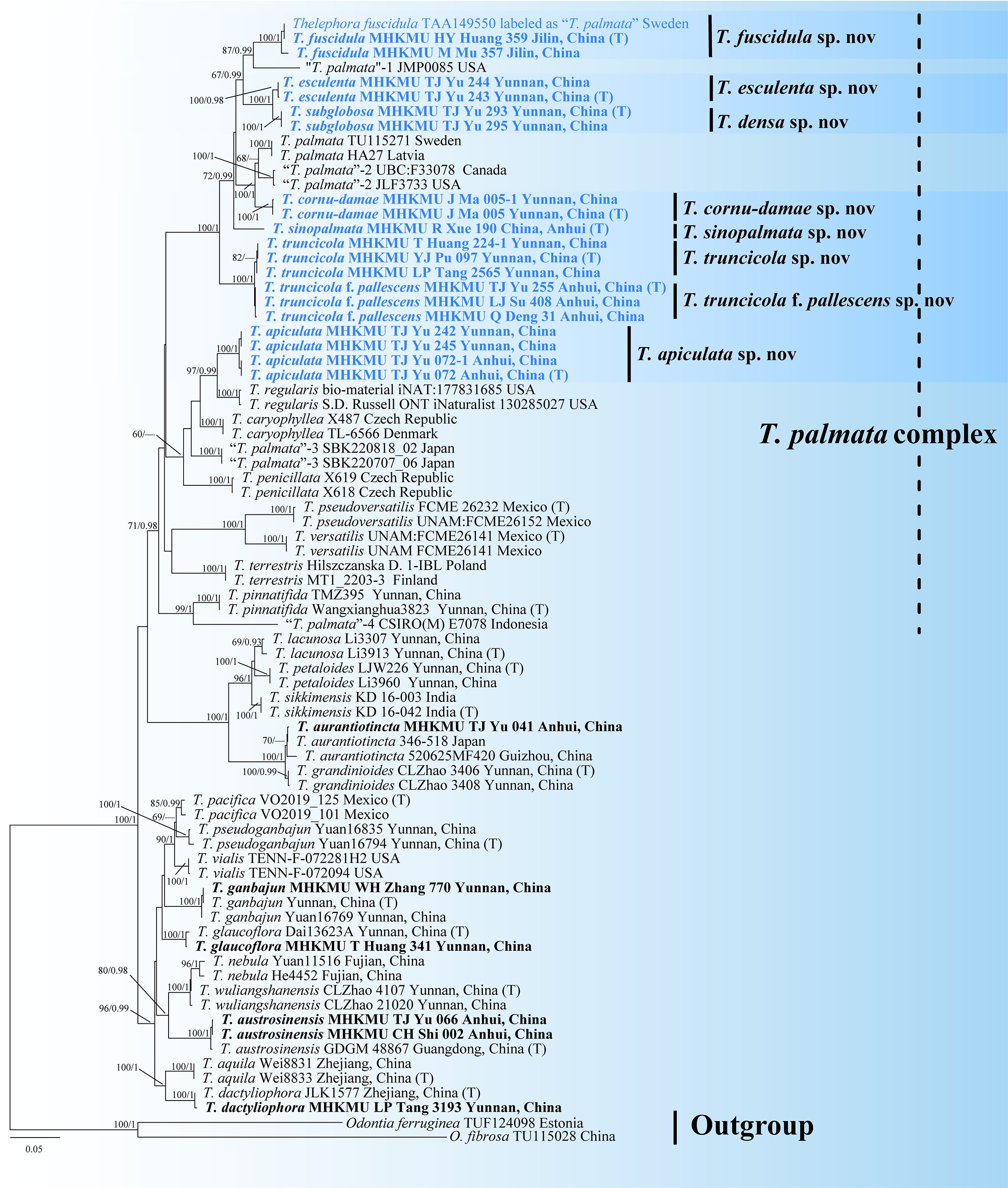
Figure 1. Phylogenetic tree of the genus Thelephora based on the ITS + nrLSU + rpb2 + nrSSU dataset using the maximum likelihood (ML) and Bayesian inference (BI) approaches. ML topology is shown with bootstrap values BS ≥ 50% and Bayesian posterior probabilities PP ≥ 0.9% on the branches. Newly generated sequences are in bold and new species are in blue. T represents the holotype.
According to the combined molecular tree, the sequences of “T. palmata” formed 12 highly supported clades, and these unique lineages had different distributions in Asia, Europe, and North America. Among them, Chinese collections formed eight monophyletic lineages with strong support, corresponding to seven new species and one forma, herein named Thelephora apiculata, Thelephora cornu-damae, Thelephora densa, Thelephora esculenta, Thelephora sinopalmata, Thelephora truncicola, and Thelephora truncicola f. pallescens, respectively. The “Thelephora palmata” from Jinlin, China, and Sweden formed a single branch representing a new species, herein named Thelephora fuscidula, which is the only species with a Eurasian distribution in this complex. The remaining sequences of “T. palmata” also represented five different taxa: two from North America, one from Europe, one from Indonesia, and one from Japan.
In this study, the sister relationships of some taxa were resolved. The temperate species, T. fuscidula from Eurasia, is a sister to “T. palmata”-1 from North America (GenBank accession: JMP0085), with moderate support values (BS = 87, PP = 0.99). The subtropical species, T. apiculata from Anhui and Yunnan Provinces, China, is a sister to Thelephora regularis Schwein. from North America, with strong support values (BS = 97, PP = 0.99). Another subtropical species, T. cornu-damae from China, grouped with “T. palmata”-2 from Europe and “T. palmata”-3 from North America (GenBank accession: JLF3733 and UBC: F33078), with strong support values (BS = 100, PP = 1). Two tropical species from Yunnan Province, China, T. esculenta and T. densa, formed sister relationship with strong support values (BS = 100, PP = 1). “T. palmata”-5 from Indonesia [GenBank accession: CSIRO(M) E7078], representing a tropical species, is a sister to Thelephora pinnatifida Yan C. Li & Zhu L. Yang from subtropical regions of China, with strong support values (BS = 99, PP = 1).
3.2 Taxonomy
Thelephora apiculata L.P. Tang & T.J. Yu sp. nov.
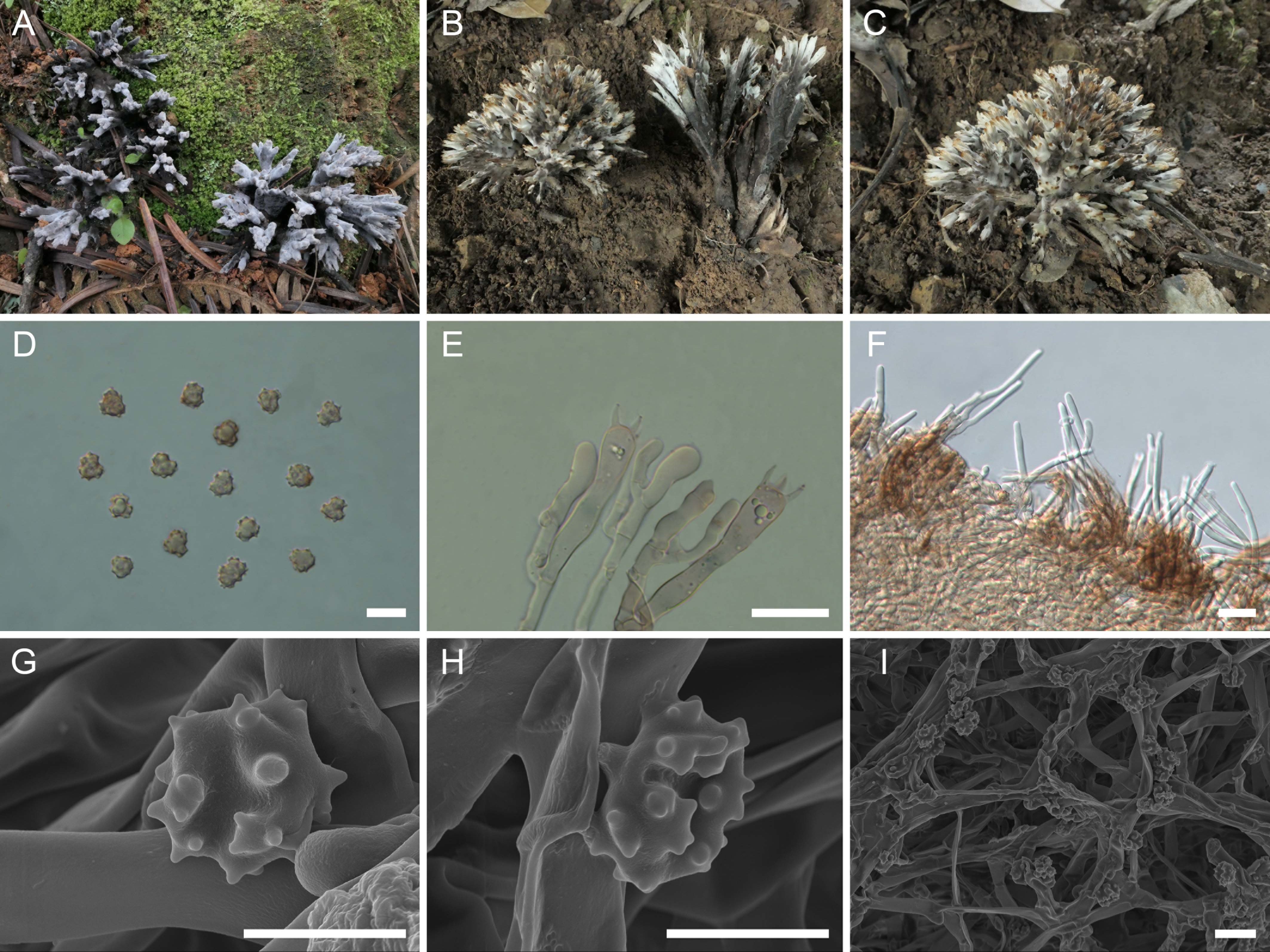
Figure 2. Macroscopic and microscopic features of Thelephora apiculata. (A–C) Basidiomata: from MHKMU TJ Yu 242 (A), and from MHKMU TJ Yu 072, holotype (B, C). (D) Basidiospores. (E) Basidia and basidioles. (F) Hair-like appendages of pointed tips. (G–I) Basidiospores under SEM. Bars, 10 µm (D), 20 µm (E), 25 µm (F), 5 µm (G, H), and 10 µm (I).
MycoBank: 856466
Etymology: apiculata (Latin), refers to the conical and acute terminals of branches.
Diagnosis: Distinct from other species within the T. palmata complex in having grayish to grayish-black tomentose to velvety branches, with grayish-white, conical, and acute terminals, short to insignificant stipe, relatively small basidiospores (6.2–7.1 µm × 5.9–6.7 µm) with short echinulis measuring 0.3–0.5 µm, narrower basidia (46–62.8 µm × 7–10.5 µm), and occurrence under trees of Castanea mollissima and Quercus spp. in subtropical forests.
Type: China—Anhui Province, Shitai County, Dayan Town, Guniujiang National Nature Reserve, 30°04'51" N, 117°28'25" E, elevation 520 m, July 12, 2023, on the ground of broad-leaved forests dominated by Castanea spp. and Quercus spp., Taijie Yu 072 (MHKMU TJ Yu 072, holotype).
Description: Basidiomata solitary, scattered to gregarious, upright, coralloid, and merismatoid, in tufts 5–7 cm high and 3–5 cm broad, coriaceous and moist when fresh, while corky and light in weight when dry; branches arising from a shared center or stipe, branched in three to four ranks, subcylindrical, clavarioid to conical, tomentose to velvety, 0.5–1 cm wide; terminal and margin thin, 0.3–0.5 mm thick, acute and pointed, usually moderately lacerate, forming a small and short sawtooth. Hymenial surface tomentose to velvety, rugulose, azonate, whitish (1A2) to pale grayish white (2B2) initially, dark gray (1E1) to nearly black (2F2) when mature, but pale grayish-white (2B2) at margin, whitish (1A2) after drying. Context 2–5 mm thick, relatively thin at the margin and thick toward the base, gray-brown (4D2). Stipe concolourous with hymenial surface, (0–) 0.2–1.5 cm long, (0–) 0.2–0.6 cm in diameter, irregularly cylindrical to flattened or broadened at the base, surface tomentose, slightly rugose. Odor mild when fresh, slightly sweet and fragrant upon drying. Taste not recorded.
Hymenium predominantly amphigenous. Basidiospores [60/4/2] (6–) 6.2–7.1 (–7.8) × (5.7–) 5.9–6.7 (–7) µm (ornamentations excluded), Q = 1.01–1.15 (–1.37), Qm = 1.08 ± 0.07, globose to subglobose in frontal and lateral views, irregular in outline, slightly thick-walled, inamyloid, pale brownish in 5% KOH, and yellow-brown to brownish in Melzer’s reagent; surface nodulose to verrucose, echinulis numerous and prominent, up to 0.3–0.5 µm high, usually isolated, sometimes in groups of two or three, then bifurcate in shape, subconical, but apex somewhat obtuse; hilar appendage oblique. Basidia 46–62.8 (–74) × 7–10.5 µm, narrow clavate, four-spored, clamped at base, occasionally with finely granulose contents; sterigmata 5–8 µm long. Basidioles numerous, similar to basidia in shape, but slightly smaller. Cystidia absent. Hyphal structure: Hyphal system monomitic, generative hyphae with clamp connections. Hymenophoral trama composed of hyphae in a subparallel to an interwoven pattern; hyphae 2–3.5 µm wide, clamped, moderately branched, generally branched near clamp connections, occasionally flexuous and collapsed. Hair-like appendages of pointed margin composed of subparallel and loosely interwoven hyphae; hyphae cylindrical, thin-walled, clamped, occasionally branched, generally branched near clamp connections, 3–4 µm wide, terminal cell rounded at margin. Clamp connections abundant on hyphae of all tissues.
Habitat: Solitary, scattered to gregarious on the ground of subtropical mixed coniferous–broad forests dominated by broad-leaved trees, C. mollissima, Quercus spp., and a few conifers, Keteleeria evelyniana.
Additional specimens examined: China—Anhui Province, Shitai County, Dayan Town, Guniujiang National Nature Reserve, 30°04'51" N, 117°28'25" E, elevation 520 m, July 12, 2023, on the ground of broad-leaved forests dominated by Castanea spp. and Quercus spp., Taijie Yu 072-1 (MHKMU TJ Yu 072-1). Yunnan Province, Guangnan County, Dongbao Town, Nongdang, 25°12'15" N, 105°05'31" E, elevation 1,400 m, July 7, 2024, on the ground of mixed coniferous–broad forests dominated by C. mollissima and a few K. evelyniana, Taijie Yu 242 (MHKMU TJ Yu 242); same location and date, Taijie Yu 245 (MHKMU TJ Yu 245).
Known distribution: Currently known from Anhui and Yunnan Provinces, China.
Notes: Morphologically, the T. apiculata from the subtropical regions of China is recognized by its tomentose to velvety branches with conical and acute terminals, short to insignificant stipes, and small basidiospores (6.2–7.1 μm × 5.9–6.7 µm) with short echinulis, up to 0.3–0.5 µm. In terms of basidiomatal color, T. apiculata closely resembles T. sinopalmata. Both of them are from the subtropics of China, but the latter is distinguished by its shorter and slender branches, with blunt and round terminals, and larger basidiospores measuring 8.2–10.4 μm × 6.8–8.7 μm. Furthermore, T. apiculata resembles T. cornu-damae, Thelephora dactyliophora Yan C. Li & Zhu L. Yang, Thelephora pseudoversatilis Ramírez-López & M. Villegas, and Thelephora versatilis Ramírez-López & M. Villegas. However, the T. cornu-damae from China is distinguished by its longer basidiomata, with flattened and wide terminals, usually deeply lacerate and serrulate, longer and significant stipe, and relatively larger basidiospores measuring 8.6–10 µm × 7–8.4 µm, with longer echinulis (1.2–1.5 µm). T. dactyliophora, distributed in Yunnan and Zhejiang Provinces, is distinguished by its flattened and thinner branches with lacerate margin, brownish-gray to gray hymenial surface, and orange-white to gray-white context (Tian et al., 2024). T. pseudoversatilis, originally described from Mexico, is distinguished by its brownish-gray to violet-brown branches, longer basidiospores measuring 7−8 μm × 5.5−6 μm, with longer echinulis, up to 1–2 µm, and tropical distribution. Another Mexican species, T. versatilis, is distinguished by its longer basidiomata, up to 9.5 cm, with brownish, flattened, and wider branches, longer echinulis, up to 1–1.5 µm, and tropical distribution (Ramírez-López et al., 2015).
Phylogenetically, T. apiculata formed a sister relationship with T. regularis. However, T. regularis, originally described from North Carolina, USA, is distinguished by its reddish or rusty-buff, infundibuliform or rarely subdimidiate, and smaller basidiomata, buff-colored hymenial surface with a pinkish tinge, longer basidiospores measuring 7.5−8.75 µm × 4.5−6 µm, and temperate distribution in North America (Reid, 1962; Corner, 1968).
Thelephora cornu-damae L.P. Tang & T.J. Yu sp. nov.

Figure 3. Macroscopic and microscopic features of Thelephora cornu-damae (MHKMU J Ma 005, holotype). (A–C) Basidiomata. (D) Basidiospores. (E) Basidia and basidioles. (F) Hair-like appendages of pointed tips. (G–I) Basidiospores under SEM. Bars, 10 µm (D), 20 µm (E), 25 µm (F), 5 µm (G, H), and 10 µm (I).
MycoBank: 856467
Etymology: cornu-damae (Latin), refers to the elkhorn-like branches of this species.
Diagnosis: Distinct from other species within the T. palmata complex in having pale grayish-white branches, with wide spathulate or flabelliform, deeply lacerate and serrulate terminals, relatively long and significant stipe, covered with granular papillate, relatively large and ellipsoid basidiospores (8.6–10 µm × 7–8.4 µm) with long echinulis, up to 1.2–1.5 µm, and occurrence under Pinus yunnanensis in subtropical forests.
Type: China—Yunnan Province, Qujing City, Haizhai Forestry Area, 25°15'01" N, 103°59'19" E, elevation 2,100 m, October 1, 2021, on the ground of mixed coniferous–broad forests dominated by P. yunnanensis and a few Rhododendron spp. and Quercus spp., Jing Ma 005 (MHKMU J Ma 005, holotype).
Description: Basidiomata solitary, scattered to gregarious, upright, coralloid, and merismatoid, in tufts 6–8 cm high and 4–6.5 cm broad, coriaceous and moist when fresh, while firm, brittle, and light in weight when dry; branches arising from a shared stipe, branched in four to five ranks, flattened, finger-like to narrow flabelliform initially, wide spathulate to flabelliform or lobate when mature, subglabrous, 0.4–0.6 cm wide; terminal thin, 0.4–0.6 mm thick, deeply lacerate and serrulate, forming an elkhorn shape, whitish (1A1). Hymenial surface subglabrous, sometimes papillate, pale grayish-white (2B1) to gray (1C2) initially, darkening to brownish-gray (3D2) to grayish-brown (3A2) with age, nearly smooth to somewhat radially rugulose or wrinkled, azonate. Context 2–3 mm thick, relatively thin at the margin and thick toward the base, brown-gray (2D2). Stipe 3–4 cm in length and 0.5–0.7 cm in diameter, irregularly flattened or slightly broadened at base, surface rugose, with granular papillate, dark gray (1E2) to grayish-brown (3D1). Odor mild when fresh, but strong and agreeable upon drying. Taste not recorded.
Hymenium predominantly amphigenous. Basidiospores [80/4/2] (8.3–) 8.6–10 (–10.4) × (6.7–) 7–8.4 (–9.2) µm (ornamentations excluded), Q = (1.01–) 1.07–1.43 (–1.44), Qm = 1.24 ± 0.10, subglobose to ellipsoid in frontal and lateral views, slightly thick-walled, inamyloid, brown in 5% KOH, and yellow-brown to brown in Melzer’s reagent; surface echinulate, echinulis numerous and prominent, up to 1.2–1.5 µm high, usually in groups of two, then bifurcate in shape, subconical, but apex somewhat obtuse; hilar appendage oblique. Basidia 62–74.5 µm × 9.5–14.5 µm, clavate, four-spored, clamped at base, usually with finely granulose contents; sterigmata 6.5–9.7 µm long. Basidioles numerous, similar to basidia in shape, but slightly smaller. Cystidia absent. Hyphal structure: Hyphal system monomitic, generative hyphae with clamp connections. Hymenophoral trama composed of hyphae in a subparallel to an interwoven pattern; hyphae 3.5–5 µm wide, clamped, moderately branched, generally branched near clamp connections, occasionally flexuous and collapsed. Hair-like appendages of pointed margin composed of subparallel and loosely interwoven hyphae; hyphae cylindrical, thin-walled, clamped, occasionally branched, generally branched near clamp connections, 3.5–4.5 µm wide, terminal cell rounded at margin. Clamp connections abundant on hyphae of all tissues.
Habitat: Solitary, scattered to gregarious on the ground of subtropical mixed coniferous–broad forests dominated by P. yunnanensis and a few Quercus spp. and Rhododendron spp.
Additional specimens examined: China—Yunnan Province, Qujing City, Haizhai Forestry Area, 25°15'01" N, 103°59'19" E, elevation 2,100 m, October 1, 2021, on the ground of mixed coniferous–broad forests dominated by P. yunnanensis and a few Quercus spp. and Rhododendron spp., Jing Ma 005-1 (MHKMU J Ma 005-1).
Known distribution: Currently known from Yunnan Province, China.
Notes: Morphologically, the T. cornu-damae from the subtropical regions of China is recognized by its grayish-white and wide spathulate or flabelliform terminals, relatively long and significant stipe, and ellipsoid basidiospores with long echinulis, up to 1.2–1.5 µm. In terms of basidiomatal color, T. cornu-damae is similar to several species from China, such as T. apiculata, T. dactyliophora, and T. pinnatifida. Of these, T. apiculata is distinguished by its velvety to tomentose basidiomata, round branches with acute whitish terminals, grayish-black short stipe, smaller basidiospores measuring 6.2–7.1 μm × 5.9–6.7 μm, with shorter echinulis approximately 0.3–0.5 μm, and a strong odor upon drying. T. dactyliophora is distinguished by its chalky-white to orange-white and thinner branches, relatively shorter and indistinctive stipe, smaller basidiospores measuring 5–7.5 μm × 4–6.5 μm, with shorter echinulis approximately 0.3–0.5 μm, and a sweet odor upon drying (Tian et al., 2024). T. pinnatifida has a geographically overlapping distribution with T. cornu-damae in Yunnan Province. However, T. pinnatifida is distinguished by its brownish-orange to brown branches with orange-white and needle-like margin, smaller basidiospores measuring 6–9.5 μm × 5–8.5 μm, with shorter echinulis approximately 0.3–0.6 μm, and a yeast powder flavor on drying (Tian et al., 2024). Furthermore, T. cornu-damae is reminiscent of Thelephora penicillata (Pers.) Fr. However, T. penicillata is fairly common in wet acidic conifer plantations throughout Europe and North America and is distinguished by its purplish-brown stipe base and white or cream branches, turning brown from the center with age (Corner, 1968).
Phylogenetically, T. cornu-damae has an exceptionally close phylogenetic affiliation with “T. palmata”-2 from Europe (Latvia and Sweden) and “Thelephora palmata”-3 from North America (Canada and USA). However, T. palmata is distinguished by its fuscous purple, chocolate-brown to blackish-brown basidiomata, with flattened, palmatifid, multifid, or dichotomous branches and longer basidiospores measuring 8–12 µm × 6–8 µm (Fries, 1821; Coker, 1921).
Thelephora densa L.P. Tang & T.J. Yu sp. nov.
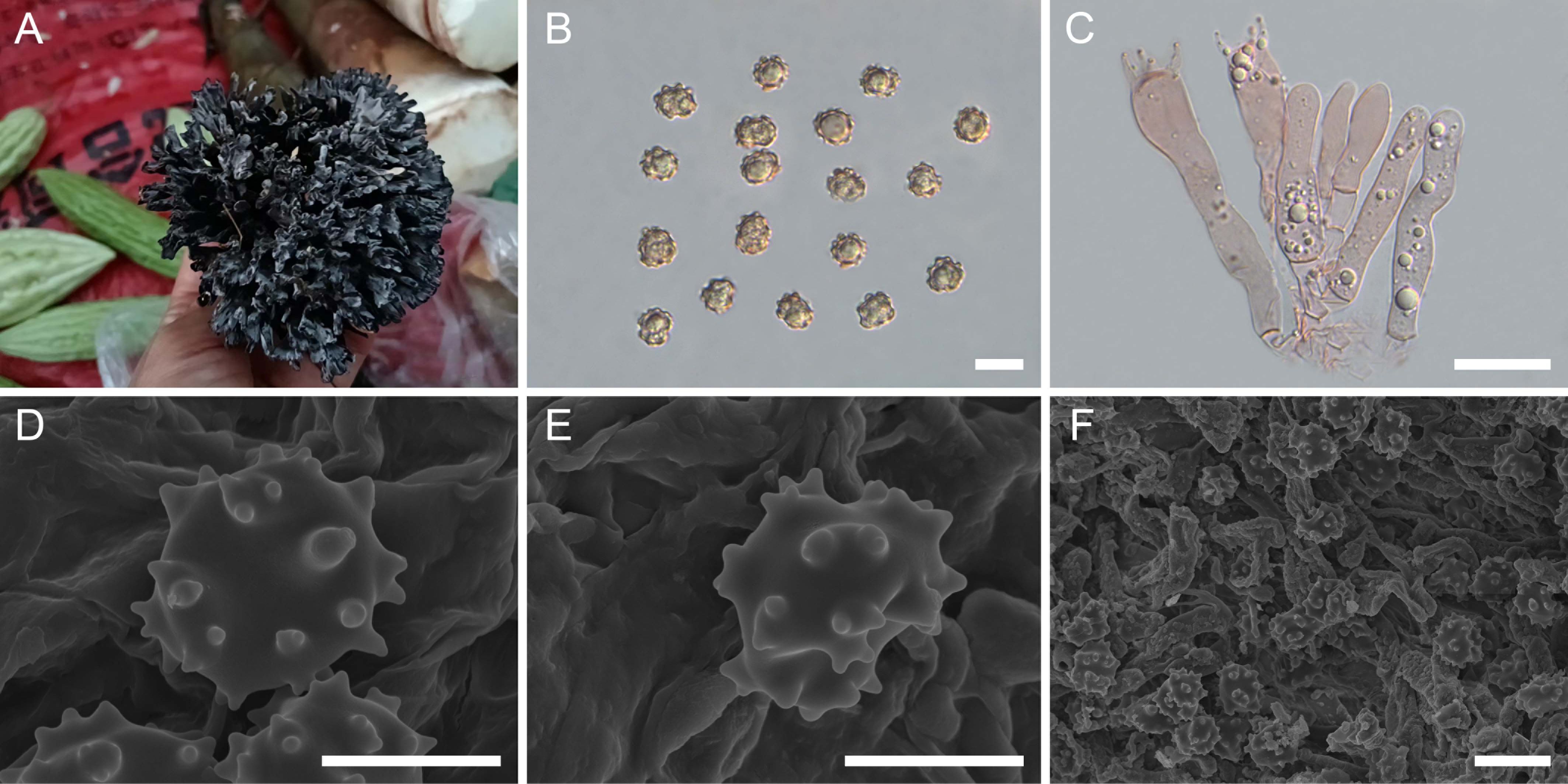
Figure 4. Macroscopic and microscopic features of Thelephora densa (MHKMU TJ Yu 293, holotype). (A) Basidiomata. (B) Basidiospores. (C) Basidia and basidioles. (D–F) Basidiospores under SEM. Bars, 10 µm (B), 20 µm (C), 5 µm (D, E), 10 µm (F).
MycoBank: 856474
Etymology: densa (Latin), refers to the dense branches of this species.
Diagnosis: Distinct from other species within the T. palmata complex in having relatively larger but thin basidiomata, with crowded and irregularly pleated branches, usually several adjacent branches forming a relatively wide complex at terminal, relatively wider basidiospores (8.5–10.1 µm × 8.1–9.5 µm) with short echinulis measuring 0.4–0.6 µm, and relatively longer basidia measuring 63.5–100 µm × 6–14.5 µm.
Type: China—Yunnan Province, Jingdong County, purchased from the local mushroom market, September 15, 2024, Taijie Yu 293 (MHKMU TJ Yu 293, holotype).
Description: Basidiomata solitary, scattered to gregarious, upright, dendritic, and merismatoid, in tufts 4–6 cm high and 4–7 cm broad, coriaceous and moist when fresh, while firm, brittle, and light in weight when dry; branches arising from a shared center or stipe, branched in three to four ranks, arranged in a spherical shape in top view, clavate to narrow spathulate or flabelliform initially, with age becoming more or less flattened, usually several adjacent branches forming a relatively wide complex at terminal, irregularly pleated, subglabrous, crowded, grayish-white (1C1), becoming grayish-black (2F1) when touched, 0.2–0.6 cm wide; terminal thin, 0.5–1 mm thick, rounded, entire or slightly lacerate forming short bifurcations or trifurcations. Hymenial surface grayish-white (1B1), becoming grayish-black (2F1) when touched, subglabrous, azonate. Context 0.1–0.4 cm thick, relatively thin at the margin and thick toward the base, concolourous with hymenial surface. Stipe 0.5–1 cm in length and 0.3–0.6 cm in diameter, irregularly conical to point-like at base, surface subglabrous, nearly black (1F3). Odor strong and agreeable upon drying. Taste not recorded.
Hymenium predominantly amphigenous. Basidiospores [60/2/2] 8.5–10.1 (–10.4) × (7.9–) 8.1–9.5 µm (ornamentations excluded), Q = 1.02–1.21 (–1.24), Qm = 1.10 ± 0.05, subglobose to broadly ellipsoid in frontal and lateral views, slightly thick-walled, inamyloid, brown in 5% KOH, and yellow-brown to brown in Melzer’s reagent; surface nodulose to verrucose, echinulis numerous and prominent, up to 0.4–0.6 µm high, usually in groups of two, occasionally isolated, then bifurcate in shape, subconical, but apex somewhat obtuse; hilar appendage oblique. Basidia 63.5–100 µm × 6–14.5 µm, clavate, four-spored, clamped at base, generally with many granulose contents; sterigmata 3–7.5 µm long. Basidioles numerous, similar to basidia in shape, but slightly smaller. Cystidia absent. Hyphal structure: Hyphal system monomitic, generative hyphae with clamp connections, and tissues turned to olive green in KOH. Hymenophoral trama composed of hyphae in a subparallel to an interwoven pattern; hyphae 3.5–7.5 µm wide, clamped, yellowish-brown, moderately branched, generally branched near clamp connections, occasionally flexuous and collapsed. Hair-like appendages of pointed margin composed of subparallel and loosely interwoven hyphae; hyphae cylindrical, thin-walled, clamped, occasionally branched, generally branched near clamp connections, 4–6 µm wide, terminal cell rounded at margin. Clamp connections abundant on hyphae of all tissues.
Habitat: Solitary, scattered to gregarious on the ground of tropical mixed broad-leaved forests dominated by Castanea spp. and Quercus spp.
Additional specimens examined: China—Yunnan Province, Jingdong County, purchased from the local mushroom market, September 15, 2024, Taijie Yu 295 (MHKMU TJ Yu 295).
Known distribution: Currently only known from Yunnan Province, China.
Notes: Macroscopically, T. densa from the tropics of China is recognized by its larger basidiomata, with more crowded branches, and wider basidiospores (8.5–10.1 µm × 8.1–9.5 µm) with short echinulis (measuring 0.4–0.6 µm). Morphologically, T. densa is reminiscent of three Chinese species (T. apiculata, T. sinopalmata, and T. truncicola) due to their similar basidiomata color. However, T. apiculata is distinguished by its sparser branches with conical and acute terminals and smaller basidiospores measuring 6.2–7.1 µm × 5.9–6.7 µm. T. sinopalmata is distinguished by its light-colored basidiomata, with whitish, sparser, subcylindrical to clavate papillate terminals and narrower basidiospores measuring 8.2–10.4 µm × 6.8–8.7 µm, with longer echinulis, up to 0.6–0.8 µm. T. truncicola is distinguished by its radially rugose or wrinkled basidiomata, sparser and shorter branches with whitish terminals, and narrower basidiospores measuring 8.3–10.2 µm × 6.7–8.5 µm, with longer echinulis, up to 0.9–1.3 µm.
Phylogenetically, T. densa formed a sister relationship with T. esculenta. Phylogenetically, T. esculenta formed a sister relationship with T. densa. Both of them are from the tropical forests of China and are commonly sold as edible mushrooms in the local mushroom market in Yunnan Province. They share very similar basidiospores and geographical distribution. However, T. esculenta differs in having larger basidiomata, with rounder, sparser, and thicker branches and shorter basidia measuring 50.5–64 µm × 8.5–11 µm.
Thelephora esculenta L.P. Tang & T.J. Yu sp. nov.
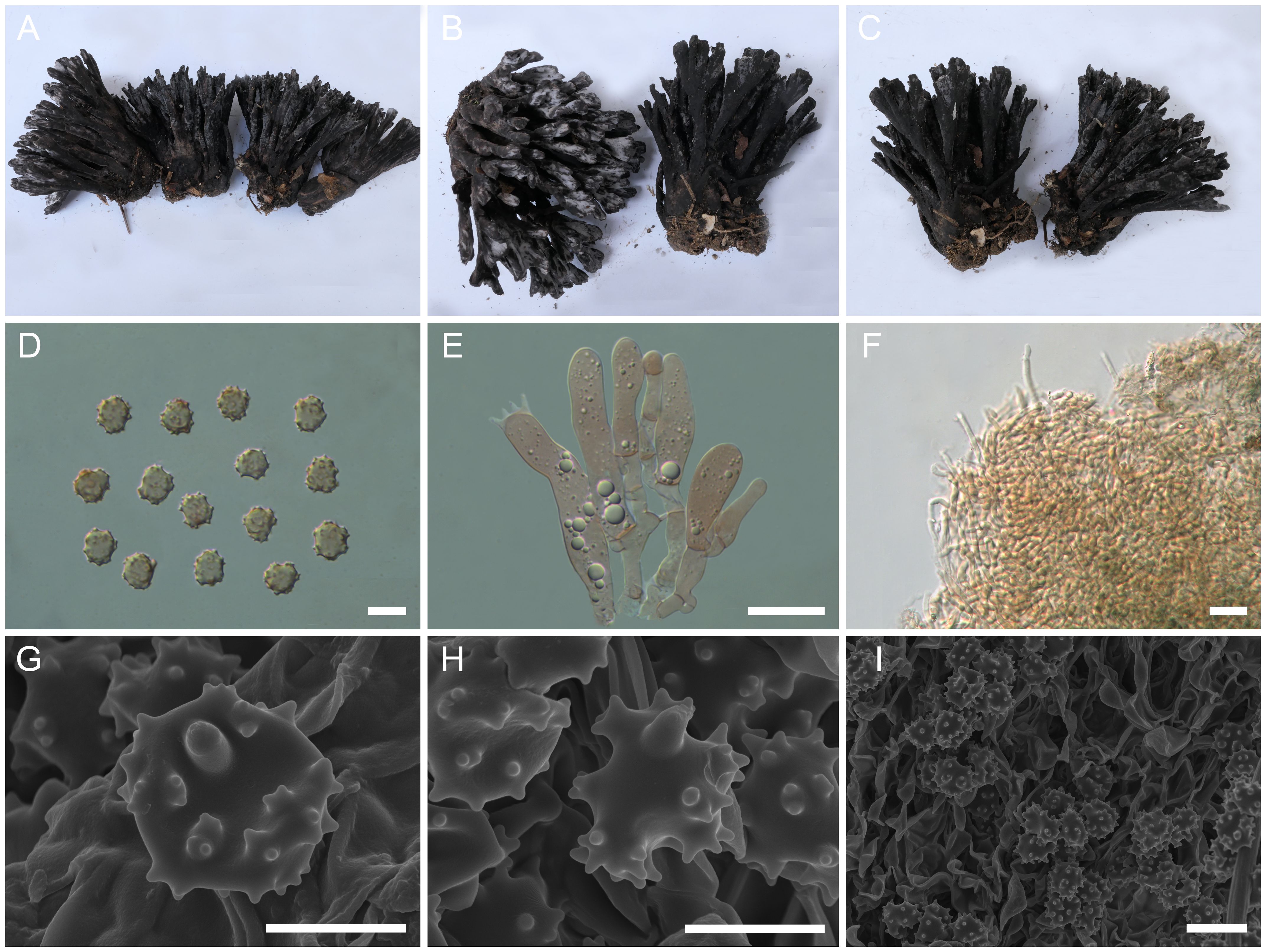
Figure 5. Macroscopic and microscopic features of Thelephora esculenta (MHKMU TJ Yu 243, holotype). (A–C) Basidiomata. (D) Basidiospores. (E) Basidia and basidioles. (F) Hair-like appendages of pointed tips. (G–I) Basidiospores under SEM. Bars, 10 µm (D), 20 µm (E), 25 µm (F), 5 µm (G, H), and 10 µm (I).
MycoBank: 856468
Etymology: esculenta (Latin), refers to the new species being edible.
Diagnosis: Distinct from other species within the T. palmata complex in having relatively large and concrescent basidiomata, with dark grayish, subglabrous, thicker branches, becoming charcoal black when touched, relatively large basidiospores (8.6–10.2 µm × 7.6–9.5 µm) with short echinulis, up to 0.5–0.7 µm, and occurrence under trees of Castanea spp., Quercus spp., and K. evelyniana in tropical forests.
Type: China—Yunnan Province, Guangnan County, purchased from a local mushroom market, July 5, 2024, Taijie Yu 243 (MHKMU TJ Yu 243, holotype).
Description: Basidiomata gregarious, caespitose to concrescent, upright, dendritic, and merismatoid, in tufts 4–6 cm high and 5–8 cm broad, coriaceous and moist when fresh, while firm and light in weight when dry; branches arising from a shared center or stipe, branched in two to three ranks, subcylindrical and narrower spathulate initially, clavate to spathulate when mature, subglabrous, initially grayish-white (1C1), later dark gray (1D1), becoming charcoal black (1F3) when touched, 0.2–0.5 cm wide; terminal thin, 0.5–1 mm thick, rounded, entire or slightly lacerate forming bifurcations or trifurcations. Hymenial surface dark gray (1D1), becoming charcoal black (1F3) when touched, subglabrous, azonate. Context 0.3–0.6 cm thick, relatively thin at the margin and thick toward the base, concolourous with hymenial surface. Stipe 1–1.5 cm in length and 1.5–2 cm in diameter, irregularly conical to point-like at base, surface smooth to subglabrous, charcoal black (1F3). Basal hyphae grayish (1C1). Odor strong but agreeable, especially upon drying. Taste not recorded.
Hymenium predominantly amphigenous. Basidiospores [60/4/2] (8.3–) 8.6–10.2 (–10.7) × (7.2–) 7.6–9.5 µm (ornamentations excluded), Q = (1.01–) 1.02–1.22 (–1.25), Qm = 1.11 ± 0.06, subglobose to broadly ellipsoid in frontal and lateral views, slightly thick-walled, inamyloid, yellowish-brown to pale brown in 5% KOH, and yellow-brown to brown in Melzer’s reagent; surface nodulose to verrucose, echinulis numerous and prominent, up to 0.5–0.7 µm high, usually in groups of two, sometimes isolated, then bifurcate in shape, conical but apex, somewhat obtuse; hilar appendage oblique. Basidia (43.5–) 50.5–64 (–72) × (5.8–) 8.5–11 µm, clavate, four-spored, clamped at the base, generally with many granulose contents; sterigmata 3.3–7.5 µm long. Basidioles numerous, similar to basidia in shape, but slightly smaller. Cystidia absent. Hyphal structure: Hyphal system monomitic, generative hyphae with clamp connections, and tissues turned olive green in KOH. Hymenophoral trama composed of hyphae in a subparallel to an interwoven pattern; hyphae 3–5 µm wide, clamped, brownish, moderately branched, generally branched near clamp connections, occasionally flexuous, and collapsed. Hair-like appendages of pointed margin composed of subparallel and loosely interwoven hyphae; hyphae cylindrical, thin-walled, clamped, occasionally branched, and generally branched near clamp connections, 3–4 µm wide, terminal cell rounded at margin. Clamp connections abundant on hyphae of all tissues.
Habitat: Gregarious, caespitose to concrescent on the ground of tropical mixed coniferous–broad forests dominated by Castanea spp., Quercus spp., and K. evelyniana.
Additional specimens examined: China—Yunnan Province, Guangnan County, purchased from the local mushroom market, July 5, 2024, Taijie Yu 244 (MHKMU TJ Yu 244).
Known distribution: Currently known from Yunnan Province, China.
Notes: Morphologically, the T. esculenta from China is recognized by its relatively large and concrescent basidiomata, with dark grayish-black and thick branches and relatively large basidiospores (8.6–10.2 µm × 7.6–9.5 µm) with short echinulis, up to 0.5–0.7 µm. In terms of basidiomatal color, T. esculenta is reminiscent of two Chinese taxa, T. apiculata and T. truncicola. However, T. apiculata has a relatively smaller basidiomata, usually solitary, rarely concrescent, tomentose to velvety branches with conical and acute margin, and smaller basidiospores measuring 6.2–7.1 µm × 5.9–6.7 µm. T. truncicola is distinguished by its smaller and shorter basidiomata, gray-black to nearly black and thinner branches with radially rugose or wrinkled surface, whitish and flat terminals, and narrower basidiospores (8.3–10.2 µm× 6.7–8.5 µm), with longer echinulis measuring 0.9–1.3 μm.
Phylogenetically, T. esculenta formed a sister relationship with T. densa. For differences in these two taxa, see notes on T. densa.
Thelephora fuscidula L.P. Tang & T.J. Yu sp. nov.
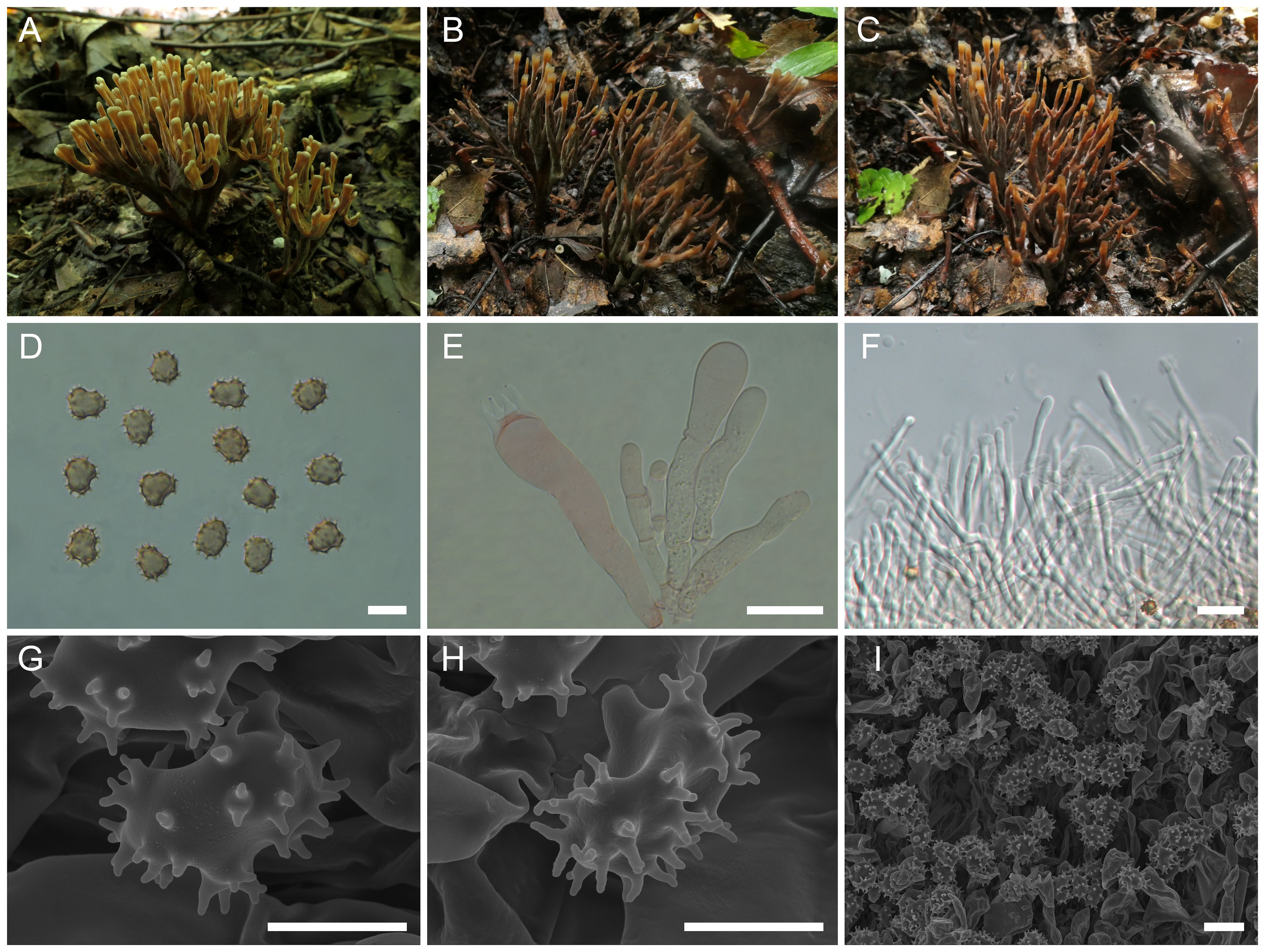
Figure 6. Macroscopic and microscopic features of Thelephora fuscidula. (A–C) Basidiomata: from MHKMU HY Huang 359, holotype (A), and from MHKMU M Mu 357 (B, C). (D) Basidiospores. (E) Basidia and basidioles. (F) Hair-like appendages of pointed tips. (G–I) Basidiospores under SEM. Bars, 10 µm (D), 20 µm (E), 25 µm (F), 5 µm (G, H), and 10 µm (I).
MycoBank: 856469
Etymology: fuscidula (Latin), refers to the yellowish-brown terminals of the branches.
Diagnosis: Distinct from other species within the T. palmata complex in having yellowish-brown, glabrous, spathulate to narrow petaloid branches, sometimes radially rugulose or wrinkled, entire and oblate terminals, relatively long and significant stipe, short basidiospores (7.9–9.6 µm × 6.7–8 µm) with long echinulis, up to 0.9–1.5 µm, and occurrence under trees of Betula platyphylla, Corylus mandshurica, and Q. mongolica in temperate forests.
Type: China—Jilin Province, Antu County, Changbai Mountain, 42°24'03" N, 128°06'01" E, elevation 750 m, August 18, 2019, on the ground of mixed broad-leaved forests dominated by B. platyphylla, C. mandshurica, and Q. mongolica, Hongyan Huang 359 (MHKMU HY Huang 359, holotype).
Description: Basidiomata solitary to gregarious, upright, coralloid, and merismatoid, in tufts 4–5 cm high and 2–3 cm broad, coriaceous and moist when fresh, while firm, brittle, and light in weight when dry; branches arising from a shared stipe, branched in three to four ranks, subcylindrical initially, later flattened, narrow spathulate to narrow clavate when mature, 3–5 mm wide, sulcate; terminal thin, up to 1–2 mm thick, entire, round and flat. Hymenial surface glabrous, pale yellowish brown (2A3), yellowish-brown (2D5) to brown (3D4), radially rugulose or wrinkled, azonate. Context 3–4 mm thick, relatively thin at the margin and thick toward the base, brownish (2B1). Stipe grayish-brown (3D2) to grayish-black (2F1), 1.5–2 cm in length and 0.3–0.5 cm in diameter, irregularly flattened or slightly round at the base, surface smooth to slightly rugose. Odor mild when fresh, strong but agreeable upon drying. Taste not recorded.
Hymenium predominantly amphigenous. Basidiospores [60/3/2] (7.2–) 7.9–9.6 (–9.7) × (6.5–) 6.7–8 (–8.3) µm (ornamentations excluded), Q = (1.07–) 1.09–1.32 (–1.35), Qm = 1.20 ± 0.07, subglobose to broadly ellipsoid in frontal and lateral views, slightly thick-walled, inamyloid, yellowish-brown to brown in 5% KOH, and yellow-brown to brown in Melzer’s reagent; surface echinulate, echinulis numerous and prominent, up to 0.9–1.5 µm high, usually isolated or in groups of two, then bifurcate in shape, conical, but apex somewhat obtuse; hilar appendage oblique. Basidia (51–) 56–77 µm × 9.7–14.8 µm, clavate, four-spored, clamped at base, sometimes with finely granulose contents; sterigmata 5.3–9.7 µm long. Basidioles numerous, similar to basidia in shape, but slightly smaller. Cystidia absent. Hyphal structure: Hyphal system monomitic, generative hyphae with clamp connections. Hymenophoral trama composed of hyphae in a subparallel to an interwoven pattern; hyphae 3–7 µm wide, clamped, moderately branched, generally branched near clamp connections, occasionally flexuous and collapsed. Hair-like appendages of pointed margin composed of subparallel and loosely interwoven hyphae; hyphae cylindrical, thin-walled, clamped, occasionally branched, generally branched near clamp connections, 3.5–4.5 µm wide, terminal cell rounded at margin. Clamp connections abundant on hyphae of all tissues.
Habitat: Solitary to gregarious on the ground of temperate mixed coniferous–broad forests dominated by B. platyphylla, C. mandshurica, and Q. mongolica.
Additional specimens examined: China—Jilin Province, Antu County, Changbai Mountain, 42°21'11" N, 127°58'16" E, elevation 750 m, August 21, 2019, on the ground of mixed broad-leaved forests dominated by B. platyphylla, C. mandshurica, and Q. mongolica, Man Mu 357 (MHKMU M Mu 357).
Known distribution: Currently known from China (Jilin Province) and Sweden.
Notes: Morphologically, the T. fuscidula from the temperate regions of Eurasia is recognized by its yellowish-brown and almost smooth branches with entire terminals, relatively long and significant stipe, and short basidiospores (7.9–9.6 µm × 6.7–8 µm) with long echinulis measuring 0.9–1.5 µm. In terms of basidiomatal colors, T. fuscidula resembles T. pinnatifida. However, T. pinnatifida, so far restricted to southwestern China, has velvety, clavate to pinnatifid or ramiform branches, with chalky white to orange white, deeply lacerate terminals, small basidiospores with shorter echinulis measuring 0.3–0.6 µm, and association with Fagaceae and Pinaceae (Tian et al., 2024). In addition, both species differ in the shape of their basidiomata, with the basidiomata of T. fuscidula being upright, whereas those of T. pinnatifida are rosette-like fans lying low on the forest floor looking as though they have been trodden on (Tian et al., 2024).
Phylogenetically, T. fuscidula formed a sister relationship with “Thelephora palmata”-1 from North America (USA), and both of them have a temperate distribution. Interestingly, a sequence (AF272919), labeled as “T. palmata” from Sweden, was clustered with this new species, indicating T. fuscidula having a wide distribution in temperate areas of Asia and Europe.
Thelephora sinopalmata L.P. Tang & T.J. Yu sp. nov.
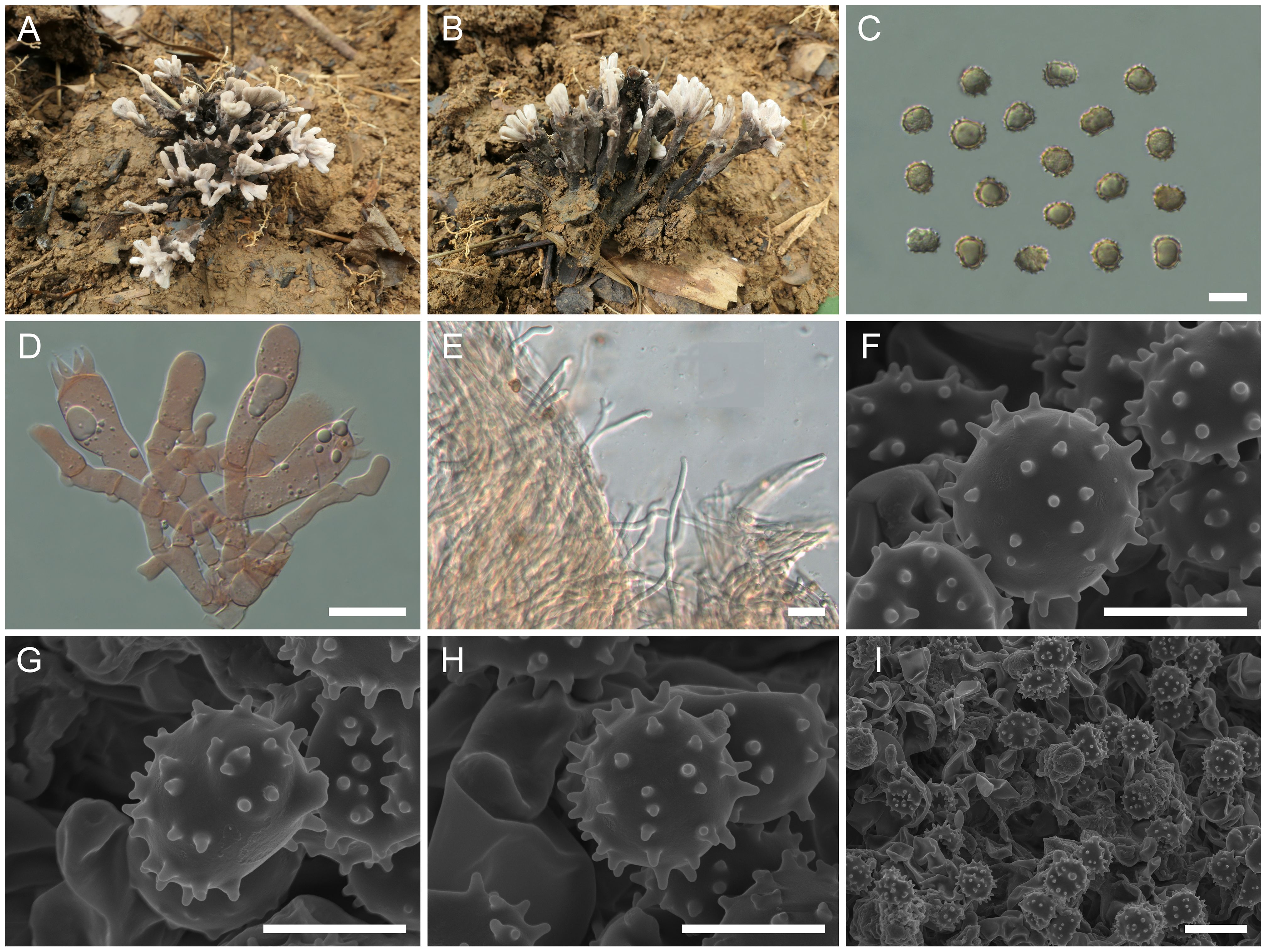
Figure 7. Macroscopic and microscopic features of Thelephora sinopalmata (MHKMU R Xue 190, holotype). (A, B) Basidiomata. (C) Basidiospores. (D) Basidia and basidioles. (E) Hair-like appendages of pointed tips. (F–I) Basidiospores under SEM. Bars, 10 µm (C), 20 µm (D), 25 µm (E), 5 µm (F–H), and 10 µm (I).
MycoBank: 856472
Etymology: sinopalmata (Latin), refers to the similarity of the new species to T. palmata and is found in China.
Diagnosis: Distinct from other species within the T. palmata complex in having dark gray, smooth to papillate, subcylindrical to clavate branches, forming a petal shape, with whitish, entire, blunt, and round terminals, short to insignificant stipe, relatively large basidiospores (8.2–10.4 µm × 6.8–8.7 µm) with medium-length echinulis measuring 0.6–0.8 µm, and occurrence under Fagaceae trees in subtropical forests.
Type: China—Anhui Province, Shitai County, Qidu Town, Jinzhu Mountain, 30°16'01" N, 117°47'08" E, elevation 500 m, July 16, 2024, on the ground of broad-leaved forests dominated by C. seguinii, Phyllostachys heterocycla, and Quercus spp., Rou Xue 190 (MHKMU R Xue 190, holotype).
Description: Basidiomata solitary, gregarious, upright, dendritic, and merismatoid, in tufts 5 cm high and 5 cm broad, coriaceous and moist when fresh, while corky and light in weight when dry; branches arising from a shared center or stipe, branched in two to three ranks, subcylindrical to clavate, smooth to papillate, 0.4–0.8 cm wide; terminal thin, 0.5–0.8 mm thick, subglabrous to subglabrous, flat, whitish (1A2) to pale grayish-white (4D3), forming a petal shape. Hymenial surface subglabrous, velvety, whitish (1A2) initially, darkening to gray (1E1), dark gray (1E1) when fully mature, azonate. Context 1–3 mm thick, relatively thin at the margin and thick toward the base, gray brown (4D2). Stipe concolourous with hymenial surface or slightly darker, (0–) 0.2–0.8 cm in length and (0–) 0.2–0.4 cm in diameter, irregularly subcylindrical to flattened or broadened at the base, surface subglabrous, slightly rugose. Odor mild and slightly fragrant upon drying. Taste not recorded.
Hymenium predominantly amphigenous. Basidiospores [80/1/1] (7.5–) 8.2–10.4 (–10.8) × (6.5–) 6.8–8.7 (–8.9) µm (ornamentations excluded), Q = (1.01–) 1.04–1.31 (–1.37), Qm = 1.16 ± 0.08, subglobose to broadly ellipsoid in frontal and lateral views, echinulate, irregular in outline, slightly thick-walled, inamyloid, yellow-brown to brownish in 5% KOH, and brown in Melzer’s reagent; surface echinulate, echinulis numerous and prominent, up to 0.6–0.8 µm high, usually isolated, occasionally in groups of two, then bifurcate in shape, subconical, but apex somewhat obtuse; hilar appendage oblique. Basidia 51.5–77.5 (–83) µm × 11.5–16.5 µm, clavate, four-spored, clamped at base, occasionally with finely granulose contents; sterigmata 6–7.8 µm long. Basidioles numerous, similar to basidia in shape, but slightly smaller. Cystidia absent. Hyphal structure: Hyphal system monomitic, generative hyphae with clamp connections, and tissues turned olive green in KOH. Hymenophoral trama composed of hyphae in a subparallel to an interwoven pattern; hyphae 4–6.5 µm wide, clamped, moderately branched, generally branched near clamp connections, occasionally flexuous and collapsed. Hair-like appendages of pointed tips composed of subparallel and loosely interwoven hyphae; hyphae cylindrical, thin-walled, clamped, occasionally branched, generally branched near clamp connections, 3.5–5 µm wide, terminal cell rounded at tips, rarely inflating, up to 12 µm wide. Clamp connections abundant on hyphae of all tissues.
Habitat: Solitary to gregarious on the ground of subtropical mixed broad-leaved forests dominated by C. seguinii and Quercus spp.
Known distribution: Currently known from Anhui Province, China.
Notes: Macroscopically, the T. sinopalmata from the subtropical regions of China is recognized by its dark gray and smooth to papillate branches, with whitish entire and subglabrous terminals, short to insignificant stipe, and relatively large basidiospores (8.2–10.4 µm × 6.8–8.7 µm) with medium-length echinulis measuring 0.6–0.8 µm. Morphologically, T. sinopalmata closely resembles T. apiculata and T. palmata. Of them, T. apiculata has a geographically overlapping distribution with T. sinopalmata in Anhui Province. However, T. apiculata is distinguished in having larger branches, with darker conical and acute terminals and small basidiospores measuring 6.2–7.1 µm × 5.9–6.7 µm. The T. palmata from the temperate regions of Europe is distinguished by its fuscous purple, chocolate brown to blackish-brown basidiomata, often with a violet tone at maturity, relatively larger basidiospores measuring 8–12 µm × 6–8 µm, rather fetid flavor, particularly upon drying, and its association with conifers (Fries, 1821; Coker, 1921).
Phylogenetically, T. sinopalmata had no sister to be confirmed in the present study, but it formed a moderately supported (BS = 72, PP = 0.99) clade comprising three Chinese species (T. cornu-damae, T. esculenta, and T. densa), three North American phylogenetic species (“T. palmata”-1, “T. palmata”-2, and “T. palmata”-3), and one Eurasian species (T. fuscidula) and is located at the base of the clade. Among these, T. cornu-damae is distinguished by its pale grayish basidiomata, with spathulate to narrow flabelliform branches, deeply lacerate and serrulate terminals, longer and significant stipe, and basidiospores with longer echinulis, up to 1.2–1.5 µm. T. esculenta is distinguished by its larger and thicker basidiomata, with darker branches becoming charcoal black when touched. T. fuscidula, distributed in temperate regions of Eurasia, has yellowish-brown and flatted terminals, relatively long and significant stipe, and smaller basidiospores (7.9–9.6 µm × 6.7–8 µm) with longer echinulis measuring 0.9–1.5 µm. T. densa, restricted to tropical regions in southwestern China, has a spherical-shaped basidiomata, with more crowded branches, and longer basidiospores (8.5–10.1 µm × 8.1–9.5 µm) with shorter echinulis measuring 0.4–0.6 µm.
Thelephora truncicola L.P. Tang & T.J. Yu sp. nov.
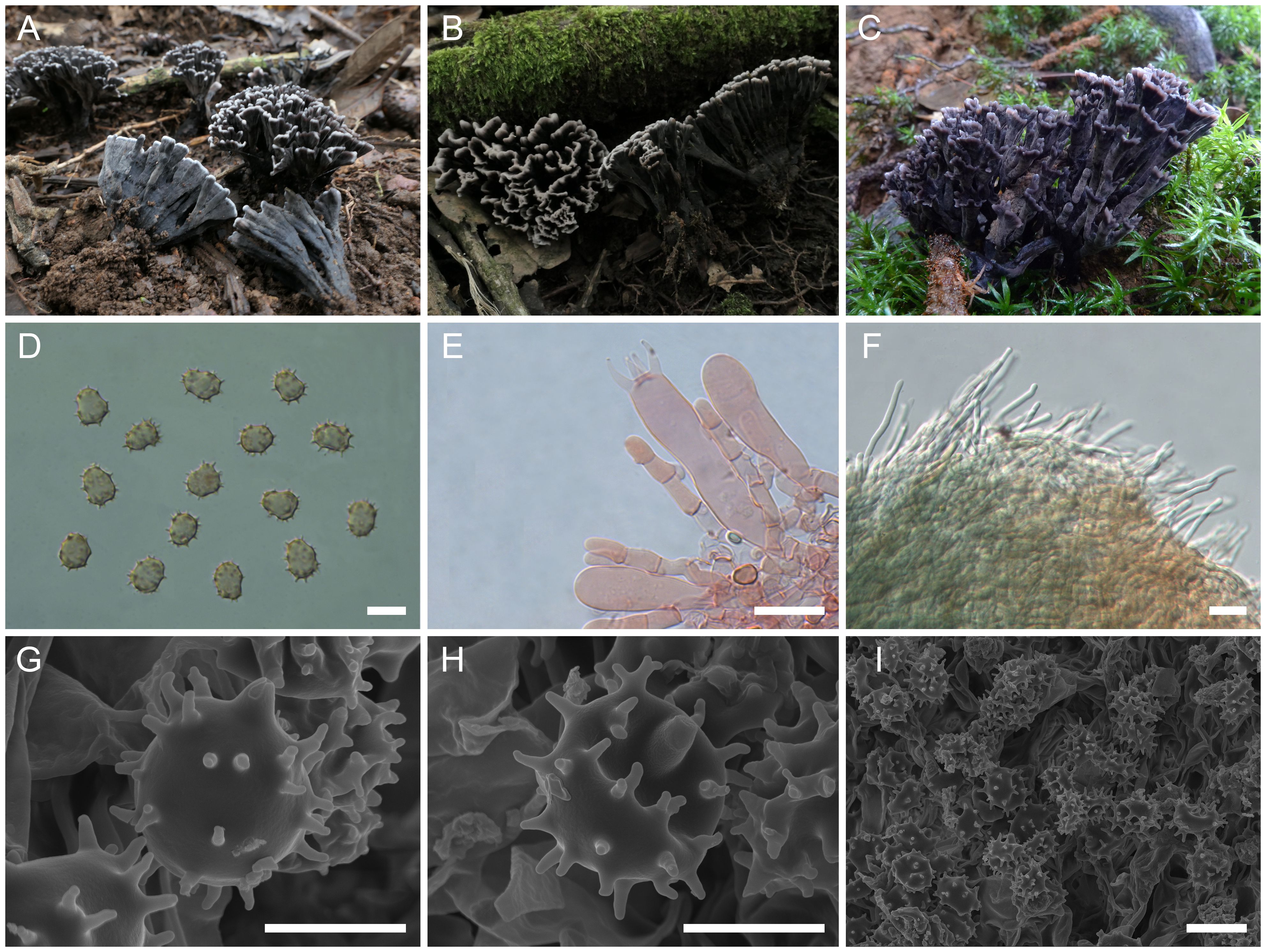
Figure 8. Macroscopic and microscopic features of Thelephora truncicola. (A–C) Basidiomata: from MHKMU YJ Pu 097, holotype (A); from MHKMU T Huang 224-1 (B); and from MHKMU LP Tang 2565 (C). (D) Basidiospores. (E) Basidia and basidioles. (F) Hair-like appendages of pointed tips. (G–I) Basidiospores under SEM. Bars, 10 µm (D), 20 µm (E), 25 µm (F), 5 µm (G, H), and 10 µm (I).
MycoBank: 856471
Etymology: truncicola (Latin), refers to the truncated and flat terminals of the branches.
Diagnosis: Distinct from other species within the T. palmata complex in having small and short basidiomata, with gray-black to nearly black wide spathulate to flabelliform, radially rugulose or wrinkled branches, pale whitish and short terminals, short and inconspicuous stipe, relatively large basidiospores (8.3–10.2 µm × 6.7–8.5 µm) with long echinulis measuring 0.9–1.3 µm, and occurrence under trees of Quercus spp. in subtropical forests.
Type: China—Yunnan Province, Shizong County, Junzi Mountain, 24°38'01" N, 104°09'36" E, elevation 2,300 m, August 10, 2019, on the ground of mixed broad-leaved forests dominated by Quercus spp. and a few Rhododendron spp., Yunju Pu 097 (MHKMU YJ Pu 097, holotype).
Description: Basidiomata caespitose to gregarious, upright, dendritic, and merismatoid, in tufts 2–4 cm high and 3–5 cm broad, coriaceous and more or less moist when fresh, while firm, brittle, and light in weight when dry; branches arising from a shared center or stipe, branched in two to three ranks, clavate to narrow spathulate when young, while wide spathulate to flabelliform when mature, visibly ribbed; terminal thin, 1–2 mm thick, entire, short, flat, and pale grayish-white (2A1). Hymenial surface subglabrous, grayish-black (1F1) to nearly black (4F1), nearly smooth to somewhat radially rugulose or wrinkled, azonate. Context 2–4 mm thick, relatively thin at the margin and thick toward the base, white (1A1), gray-white (3E1). Stipe 0.5–1 cm in length and 0.4–0.6 cm in diameter, irregularly cylindrical to flattened or broadened at the base, surface smooth to slightly rugose, dark gray (3F1) to gray-black (1F1). Odor strong and slightly foul when fresh, especially upon drying. Taste not recorded.
Hymenium predominantly amphigenous. Basidiospores [80/4/3] (8.2–) 8.3–10.2 (–10.7) × (6.5–) 6.7–8.5 (–9) µm (ornamentations excluded), Q = (1.03–) 1.12–1.37 (–1.39), Qm = 1.24 ± 0.07, subglobose to broadly ellipsoid in frontal and lateral views, slightly thick-walled, inamyloid, yellowish-brown to pale brown in 5% KOH, and yellow brown to brown in Melzer’s reagent; surface echinulate, echinulis numerous and prominent, up to 0.9–1.3 µm high, usually isolated or in groups of two, then bifurcate in shape, conical, but apex somewhat obtuse; hilar appendage oblique. Basidia 48.5–68.5 (–74) × 8.5–13 µm, clavate, four-spored, clamped at the base, occasionally with finely granulose contents; sterigmata 6.8–9.5 µm long. Basidioles numerous, similar to basidia in shape, but slightly smaller. Cystidia absent. Hyphal structure: Hyphal system monomitic, generative hyphae with clamp connections, and tissues turned olive green in KOH. Hymenophoral trama composed of hyphae in a subparallel to an interwoven pattern; hyphae 3.5–6 µm wide, clamped, moderately branched, generally branched near clamp connections, occasionally flexuous and collapsed. Hair-like appendages of pointed margin composed of subparallel and loosely interwoven hyphae; hyphae cylindrical, thin-walled, clamped, occasionally branched, generally branched near clamp connections, 3.5–5.5 µm wide, and terminal cell rounded at margin. Clamp connections abundant on hyphae of all tissues.
Habitat: Caespitose to gregarious on the ground of subtropical mixed broad-leaved forests dominated by Quercus spp. and a few Ericaceae.
Additional specimens examined: China—Yunnan Province, Shizong County, Junzi Mountain, 24°38'44" N, 104°09'20" E, elevation 2,300 m, July 23, 2020, on the ground dominated by Quercus spp. in mixed broad-leaved forests, Ting Huang 224-1 (MHKMU T Huang 224-1); same location, 24°31'01" N, 104°09'51" E, elevation 2,250 m, August 23, 2018, on the ground dominated by Quercus spp. and a few Ericaceae in mixed broad-leaved forests, Liping Tang 2565 (MHKMU LP Tang 2565).
Known distribution: Currently known from Yunnan Province, China.
Notes: Morphologically, the T. truncicola from the subtropical regions of China is recognized by its shorter basidiomata, with gray-black to nearly black branches, whitish terminals, short to inconspicuous stipe, and relatively large basidiospores (8.3–10.2 µm × 6.7–8.5 µm) with long echinulis measuring 0.9–1.3 µm. T. truncicola is easy to recognize at maturity and not often confused with other species, except for Thelephora aquila S.R. Yang, Y.L. Wei & H.S. Yuan. However, T. aquila is distinguished by its flabelliform to applanate-lobate basidiomata, with a strongly lobed and wavy margin (Yang et al., 2023). In terms of color, T. truncicola is similar to T. dactyliophora, T. palmata, and T. pinnatifida when not mature. However, T. dactyliophora differs in having spathulate to narrow petaloid basidiomata, with gray to brownish-gray branches, orange-white and deeply lacerate terminals, and smaller basidiospores measuring 5–7.5 μm × 4–6.5 μm, with shorter echinulis (0.3–0.5 μm). T. pinnatifida has a geographically overlapping distribution with T. truncicola in Yunnan Province, but the former differs in having brownish-orange to brown branches, with needle-like and orange-white terminals, and a yeast powder flavor when dried (Tian et al., 2024).
Phylogenetically, T. truncicola had no sister to be confirmed in the present study, but it formed a moderately supported (BS = 100, PP = 1) clade comprising four Chinese species (T. cornu-damae, T. esculenta, T. sinopalmata, and T. densa), three North American phylogenetic species (“T. palmata”-1, “T. palmata”-2, and “T. palmata”-3), and one Eurasian species (T. fuscidula) and is located at the base of the clade.
Thelephora truncicola f. pallescens L.P. Tang & T.J. Yu sp. nov.

Figure 9. Macroscopic and microscopic features of Thelephora truncicola f. pallescens. (A–C) Basidiomata: from MHKMU TJ Yu 255, holotype (A, B), and from MHKMU LJ Su 408 (C). (D) Basidiospores. (E) Basidia and basidioles. (F) Hair-like appendages of pointed tips. (G–I) Basidiospores under SEM. Bars, 10 μm (D), 20 μm (E), 25 μm (F), 5 μm (G, H), and 10 μm (I).
MycoBank: 856470
Etymology: pallescens (Latin), refers to the light-colored branches of this species compared to T. truncicola.
Diagnosis: Distinct from T. truncicola in having pale gray and slightly tomentose basidiomata, with pale gray to pale grayish-brown and longer branches, pale yellowish-gray and narrower terminals, lacerate to entire, forming bifurcate, longer and significant stipe, and occurrence under Lithocarpus spp. trees in subtropical forests.
Type: China—Anhui Province, Shitai County, Renli Town, Shanshan Forest Park, 30°16'58" N, 117°32'44" E, elevation 780 m, July 19, 2024, on the ground dominated by Lithocarpus henryi and a few Camellia spp., C. mollissima, and Quercus spp. in mixed broad-leaved forests, Taijie Yu 255 (MHKMU TJ Yu 255, holotype).
Description: Basidiomata solitary, scattered to gregarious, upright, coralloid, and merismatoid, in tufts 3–5 cm high and 3–4 cm broad, coriaceous and moist when fresh, while firm, brittle, and light in weight when dry; branches arising from a shared stipe, branched in two to four ranks, flattened, clavate to narrow spathulate initially, spathulate to narrow flabelliform or wide petaloid when mature, subglabrous, 0.3–0.5 cm wide; terminal thin, 0.4–0.6 mm thick, moderately lacerate and serrulate, forming a bifurcate to trifurcate shape, pale gray (1C1) to pale grayish-white (1B2). Hymenial surface subglabrous, azonate, surface with powdery adherence, grayish-white (2B1), pale grayish (2C1) to pale grayish-brown (2C2). Context 2–4 mm thick, relatively thin at the margin and thick toward the base, gray (2D1) to grayish-black (2E1). Stipe 1–2 cm in length and 0.2–0.7 cm in diameter, subcylindrical or slightly broadened at the base, surface nearly smooth, with powdery adherence, concolourous with branches or slightly darker. Odor mild when fresh, strawy flavor upon drying. Taste not recorded.
Hymenium predominantly amphigenous. Basidiospores [60/3/3] (7.8–) 8.2–10.2 (–10.5) × 6.5–8.8 (–9.5) µm (ornamentations excluded), Q = (1.01–) 1.03–1.33 (–1.42), Qm = 1.19 ± 0.09, subglobose to broadly ellipsoid in frontal and lateral views, slightly thick-walled, inamyloid, brown in 5% KOH, and yellow-brown to brown in Melzer’s reagent; surface echinulate, echinulis numerous and prominent, up to 1–1.5 µm high, usually isolated or in groups of two, then bifurcate in shape, conical, but apex somewhat obtuse; hilar appendage oblique. Basidia 42.5–59 µm× 11.5–14.7 µm, clavate, four-spored, clamped at the base, usually with numerous granulose contents; sterigmata 5–8.2 µm long. Basidioles numerous, similar to basidia in shape, but slightly smaller. Cystidia absent. Hyphal structure: Hyphal system monomitic, generative hyphae with clamp connections. Hymenophoral trama composed of hyphae in a subparallel to an interwoven pattern; hyphae 3–5 µm wide, clamped, moderately branched, generally branched near clamp connections, occasionally flexuous and collapsed. Hair-like appendages of pointed margin composed of subparallel and loosely interwoven hyphae; hyphae cylindrical, thin-walled, clamped, occasionally branched, generally branched near clamp connections, 3–4.5 µm wide, terminal cell rounded at margin. Clamp connections abundant on hyphae of all tissues.
Habitat: Solitary, scattered to gregarious on the ground of subtropical mixed broad-leaved forests dominated by L. henryi and a few Castanea spp. and Quercus spp.
Additional specimens examined: China—Anhui Province, Shitai County, Xianyu Town, Qifeng Village, Xianyu Scenic Area, 30°03'42" N, 117°17'23" E, elevation 200 m, July 27, 2022, on the ground dominated by L. henryi and a few Quercus spp. in mixed broad-leaved forests, Qing Deng 31 (MHKMU Q Deng 31). Shitai County, Jitan Town, near Hongdun Village, 30°15'34" N, 117°24'43" E, elevation 70 m, July 19, 2024, on the ground dominated by L. henryi and a few C. mollissima and Quercus spp. in mixed broad-leaved forests, Linjie Su 408 (MHKMU LJ Su 408).
Known distribution: Currently known from Anhui Province, China.
Notes: Macroscopically, the T. truncicola f. pallescens from the subtropical regions of China is recognized by its pale gray and slightly tomentose basidiomata, with grayish to pale grayish-brown branches and terminals, longer and significant stipe, and large basidiospores (8.2–10.2 µm × 6.5–8.8 µm) with long echinulis measuring 1–1.5 µm. T. truncicola f. pallescens is reminiscent of T. cornu-damae and T. sinopalmata, having light-colored basidiomata. However, T. cornu-damae, associated with P. yunnanensis, is distributed in high elevations of Yunnan Province and has wider and flat branches, and deeply lacerate and serrulate terminals. T. sinopalmata has a geographic overlapping distribution with T. truncicola f. pallescens in Anhui Province, but T. sinopalmata is distinguished by its whitish and thinner terminals, short to insignificant stipe, and shorter echinulis measuring 0.6–0.8 µm. Furthermore, the two species differ in host preferences. T. sinopalmata appears to prefer associating with C. seguinii and Quercus spp., while T. truncicola f. pallescens prefers associating with Lithocarpus spp.
4 Discussion
In the present research, we constructed a four-locus phylogenetic tree (ITS + nrLSU + rpb2 + nrSSU) and demonstrated that what once was considered a single species, T. palmata, actually represents a species complex consisting of at least 12 unknown species. Before new names could be assigned to these undescribed species, the identification of the old name proved to be a challenge, particularly as it has been used for more than 200 years and its original description was not sufficiently detailed (Fries, 1821).
According to our phylogenetic tree, seven new taxa (i.e., T. apiculata, T. cornu-damae, T. densa, T. esculenta, T. sinopalmata, T. truncicola, and T. truncicola f. pallescens) from China and one new taxon (T. fuscidula) from Eurasia are proposed, supported by diagnosable criteria including morphological characteristics, host preferences, and geographical distribution. With the exception of the mentioned new species, there is unrecognized cryptic diversity within the T. palmata complex from Asia, Europe, and North America. The remaining sequences, labeled as “T. palmata”, form five independent lineages, and the difference of the base pairs in the ITS regions is more than 1.5% nucleotides among these taxa; hence, these taxa should represent five different species and are provisionally treated as “T. palmata”-1 and “T. palmata”-3 from North America, “T. palmata”-2 from Europe, “T. palmata”-4 from Japan, and “T. palmata”-5 from Indonesia. Unfortunately, the precise clade representing the true T. palmata lineage needs to be elucidated until the acquisition of collections that correspond to the geographical and ecological origins of the species described by Fries (1821).
The traditional morphological concept of T. palmata agrees with our observations of the species complex. For the identification of species within the T. palmata complex, the following characteristics are quite helpful: microscopic characteristics, host preferences, and geographical distribution (see Table 2 for details). Firstly, SEM characteristics are helpful to distinguish the complex species of T. palmata. For instance, the echinulis of basidiospores are significantly different within the T. palmata complex. Three species with similar macro-morphology, i.e., T. apiculata, T. cornu-damae, and T. sinopalmata, differ significantly in the shape and size of the basidiospores’ echinulis. Among them, T. apiculata has short echinulis measuring 0.3–0.5 µm, usually isolated or in groups of two; T. cornu-damae has long echinulis measuring 1.2–1.5 µm, usually in groups of two; and T. sinopalmata has medium-length and usually isolated echinulis measuring 0.6–0.8 µm.
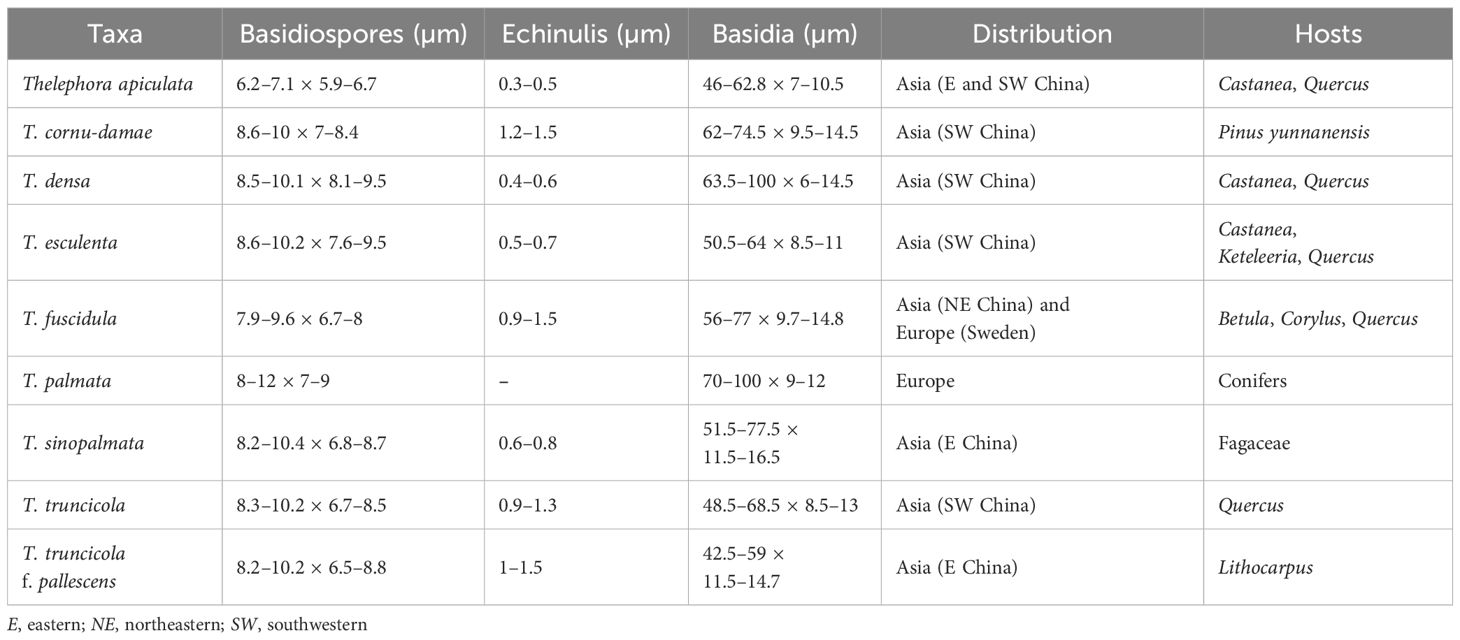
Table 2. Diagnostic morphological characteristics of the species within the Thelephora palmata complex.
Secondly, the host preference and geographical distribution are quite helpful within the T. palmata complex. Indeed, the vast majority of species from China prefer broad-leaved trees (except for T. cornu-damae), predominantly Fagaceae, rather than the conifers described in the literature. Furthermore, species within this species complex have strict distribution patterns. Some phenetically and phylogenetically related species can be well distinguished by their host preferences and geographical distribution. For instance, three Chinese species—T. cornu-damae, T. truncicola, and T. truncicola f. pallescens—have similar morphological features and close phylogenetic affiliation. However, T. cornu-damae is restricted to southwestern China at high elevations (>2,000 m) and prefers P. yunnanensis, T. truncicola prefers Quercus spp. and is restricted to southwestern China at high elevations (>2,000 m), and T. truncicola f. pallescens prefers Lithocarpus spp. and is restricted to eastern China at low elevations (<800 m).
In addition, odor is often overlooked but is quite a useful characteristic within this species complex. For instance, T. palmata is known for its foul odor, particularly on drying (Fries, 1821; Coker, 1921), while the odor of T. apiculata is mild when fresh and is slightly sweet and fragrant after drying. The odor of T. truncicola f. pallescens is strawy after drying. It should be noted, however, that the recording of odor is not only dependent on the subjective feeling of the collector but is also difficult to describe accurately.
Our study confirms that the morphological species concepts of “T. palmata” in Asia, Europe, and North America are, in many cases, problematic, and the host preference and geographical distribution have not been given enough attention. The real host and distribution range of T. palmata is, in fact, more limited than the descriptions in the literature. Similar cases have also been widely reported in other fungal groups, for instance, the Amanita sculpta complex (Huang et al., 2023), Clavariadelphus amplus complex (Huang et al., 2020), Hygrophorus esculentus complex (Huang et al., 2022), Hygrophorus russula complex (Huang et al., 2021), and Rhodotus palmatus complex (Tang et al., 2014). Therefore, careful observation of habitat as well as recording odor and potential partners is essential in the taxonomy of ectomycorrhizal fungi. Hopefully, the recognition that there is more than one species in the T. palmata complex will provide an incentive for future collectors to record detailed macromorphological and habitat information.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, PQ504921 https://www.ncbi.nlm.nih.gov/genbank/, PQ538458 https://www.ncbi.nlm.nih.gov/genbank/, PQ560959 https://www.ncbi.nlm.nih.gov/genbank/, PQ538484 https://www.ncbi.nlm.nih.gov/genbank/, PQ560960 https://www.ncbi.nlm.nih.gov/genbank/, PQ538459 https://www.ncbi.nlm.nih.gov/genbank/, PQ504922 https://www.ncbi.nlm.nih.gov/genbank/, PQ504919 https://www.ncbi.nlm.nih.gov/genbank/, PQ538456 https://www.ncbi.nlm.nih.gov/genbank/, PQ560957 https://www.ncbi.nlm.nih.gov/genbank/, PQ538482 https://www.ncbi.nlm.nih.gov/genbank/, PQ538483 https://www.ncbi.nlm.nih.gov/genbank/, PQ560958 https://www.ncbi.nlm.nih.gov/genbank/, PQ538457 https://www.ncbi.nlm.nih.gov/genbank/, PQ504920 https://www.ncbi.nlm.nih.gov/genbank/, PQ504940 https://www.ncbi.nlm.nih.gov/genbank/, PQ538477 https://www.ncbi.nlm.nih.gov/genbank/, PQ504935 https://www.ncbi.nlm.nih.gov/genbank/, PQ538472 https://www.ncbi.nlm.nih.gov/genbank/, PQ560963 https://www.ncbi.nlm.nih.gov/genbank/, PQ538497 https://www.ncbi.nlm.nih.gov/genbank/, PQ504936 https://www.ncbi.nlm.nih.gov/genbank/, PQ538473 https://www.ncbi.nlm.nih.gov/genbank/, PQ560974 https://www.ncbi.nlm.nih.gov/genbank/, PQ504931 https://www.ncbi.nlm.nih.gov/genbank/, PQ538468 https://www.ncbi.nlm.nih.gov/genbank/, PQ560969 https://www.ncbi.nlm.nih.gov/genbank/, PQ538493 https://www.ncbi.nlm.nih.gov/genbank/, PQ504932 https://www.ncbi.nlm.nih.gov/genbank/, PQ538469 https://www.ncbi.nlm.nih.gov/genbank/, PQ560970 https://www.ncbi.nlm.nih.gov/genbank/, PQ538494 https://www.ncbi.nlm.nih.gov/genbank/, PQ504938 https://www.ncbi.nlm.nih.gov/genbank/, PQ538475.
Author contributions
T-JY: Supervision, Writing – review & editing, Methodology, Data curation, Investigation, Writing – original draft, Software, Formal analysis. RX: Investigation, Writing – review & editing, Data curation, Methodology. L-JS: Methodology, Investigation, Writing – review & editing. XX: Methodology, Writing – review & editing, Investigation. JL: Writing – review & editing, Investigation, Data curation. CX: Methodology, Writing – review & editing, Investigation. H-CL: Writing – review & editing, Investigation. W-HZ: Methodology, Investigation, Writing – review & editing. JM: Investigation, Writing – review & editing. H-YH: Writing – review & editing, Investigation. X-JL: Writing – review & editing, Investigation. Y-XG: Writing – review & editing, Investigation. Z-XZ: Investigation, Writing – review & editing. LL: Investigation, Writing – review & editing. G-LZ: Data curation, Methodology, Investigation, Writing – review & editing. L-PT: Methodology, Writing – review & editing, Visualization, Project administration, Funding acquisition, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was financed by the National Natural Science Foundation of China (No. 32360001, 31960007); digitalization, development, and application of biotic resources (No. 202002AA100007); the program Innovative Research Team in Science and Technology in Yunnan Province (No. 202005AE160004); the Research Project on Undergraduate Educational and Teaching Reforms in Yunnan Province (No. JG2023001); the Hainan Provincial Natural Science Foundation of China (No. 423QN242); and the Hainan Medical University Research Incubation Fund Program (No. XPY200009).
Acknowledgments
We express our gratitude to our colleagues at Kunming Medical University for data collection and analyses; to Mr. Zhi-jia Gu from the Kunming Institute of Botany for conducting scanning electron microscope (SEM) photography; and to Guangnan County Center for Disease Control and Prevention, Shitai County Forestry Bureau, Mr. Cheng-yu Zhang, and the forest rangers for their help in specimen collection. We also show our gratitude to the reviewers for their constructive advice and corrections to improve this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffunb.2025.1599905/full#supplementary-material
References
Borovička J., Braeuer S., Walenta M., Hršelová H., Leonhardt T., Sácký J., et al. (2022). A new mushroom hyperaccumulator: Cadmium and arsenic in the ectomycorrhizal basidiomycete Thelephora penicillata. Sci. Total Environ. 826, 154227. doi: 10.1016/j.scitotenv.2022.154227
Chacón S., Tapia F., and Jarvio D. (2018). Four interesting aphyllophoroid species in the tropical northern region of Veracruz, Mexico. Mycotaxon 133, 153–163. doi: 10.5248/133.153
Coker W. C. (1921). Notes on the thelephoraceae of north carolina. J. Elisha Mitchell Sci. Soc 36, 146–196.
Corner E. J. H. (1976). Futher notes on cantharelloid fungi and Thelephora. Nova Hedwig 27, 325–342.
Cunningham G. H. (1963). The thelephoraceae of Australia and New Zealand. New zealand department of scientific and industrial research. Bulletin 145, 228–239.
Dai Y. C. (2011). A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 52, 69–79. doi: 10.1007/s10267-010-0068-1
Das K., Manoj E., Ghosh A., Parihar A., and Kuhar F. (2018). Thelephora sikkimensis sp. nov. (Thelephoraceae) from the eastern himalayas (India). Nova Hedwig 107, 337–347. doi: 10.1127/nova_hedwigia/2018/0475
Edler D., Klein J., Antonelli A., and Silvestro D. (2021). raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 12, 373–377. doi: 10.1111/2041-210X.13512
Ehrhart J. F. (1789). 1785–1795: Plantae Cryptogamae Linneae, quas in Locis Earum Natalibus Collegit et Exsiccavit Fridericus Ehrhart. Lower Saxony Germany pp, 1–320.
Fries E. M. (1821). Systema Mycologicum: Sistens Fungorum Ordines, Genera et Species, huc Usque Cognitas, quas ad Normam Methodi Naturalis Determinavit. Lundae (Lund, Swedish) 1, 432.
Hall T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98. doi: 10.1021/bk-1999-0734.ch008
He M., Cao B., Liu F., Boekhout T., Denchev T. T., Schoutteten N., et al. (2024). Phylogenomics, divergence times and notes of orders in Basidiomycota. Fungal Divers. 126, 127–406. doi: 10.1007/s13225-024-00535-w
Hilszczanska D. and Sierota Z. (2006). Persistence of ectomycorrhizas by Thelephora terrestris on outplanted Scots pine seedlings. Acta Mycol. 41, 313–318. doi: 10.5586/am.2006.032
Hu Y., Karunarathna S. C., Li H., Galappaththi M. C., Zhao C. L., Kakumyan P., et al. (2022). The impact of drying temperature on basidiospore size. Diversity 14, 239. doi: 10.3390/d14040239
Hu L. and Liu J. K. (2001). Two novel phenylacetoxylated p-terphenyls from Thelephora ganbajun Zang. Z. Naturforsch. C. J. Biosci. 56, 983–987. doi: 10.1515/znc-2001-11-1213
Huang T., Su L. J., Zeng N. K., Lee S. M. L., Lee S. S., Thi B. K., et al. (2023). Notes on amanita section validae in hainan island, China. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1087756
Huang H. Y., Zhang W. H., Huang T., Gabriel M., Liu T. Z., and Tang L. P. (2021). Hygrophorus russula complex (Hygrophoraceae, Agaricales) in China. Mycol. Prog. 20, 1115–1134. doi: 10.1007/s11557-021-01715-7
Huang H. Y., Zhang W. H., Huang T., Moreno G., Pu Y. J., Fan Y. G., et al. (2022). Phylogeny and species diversity in Hygrophorus section Aurei in China. Mycol. Prog. 21, 79. doi: 10.1007/s11557-022-01825-w
Huang H. Y., Zhao J., Zhang P., Ge Z. W., Li X., and Tang L. P. (2020). The genus clavariadelphus (Clavariadelphaceae, gomphales) in China. MycoKeys 70, 89–121. doi: 10.3897/mycokeys.70.54149
Kõljalg U. (1996). Tomentella (Basidiomycota) and related genera in temperate Eurasia. Synopsis Fungorum. 9, 1–213.
Kõljalg U., Dahlberg A., Taylor A. F., Larsson E., Hallenberg N., Stenlid J., et al. (2000). Diversity and abundance of resupinate thelephoroid fungi as ectomycorrhizal symbionts in Swedish boreal forests. Mol. Ecol. 9, 1985–1996. doi: 10.1046/j.1365-294x.2000.01105.x
Kõljalg U., Saar I., and Svantesson S. (2024). Merging the genus tomentella with thelephora (Fungi, thelephorales). Folia Cryptog. Est. 61, 67–86. doi: 10.12697/fce.2024.61.09
Kornerup A. and Wanscher J. H. K. (1978). The methuen handbook of colour. 3rd ed (London, UK: Eyre Methuen Ltd).
Larsen M. J. (1968). Tomentelloid fungi of North America; State University College of Forestry at Syracuse University Technical Publication Vol. 93 (NY, USA: Syracuse).
Larsen M. (1998). Tomentella (Basidiomycota) and related genera in temperate eurasia, synopsis fungorum 9. Mycologia 90, 738. doi: 10.2307/3761235
Larsson K. H., Svantesson S., Miscevic D., Kõljalg U., and Larsson E. (2019). Reassessment of the generic limits for Hydnellum and Sarcodon (Thelephorales, Basidiomycota). MycoKeys 54, 31–47. doi: 10.3897/mycokeys.54.35386
Lee S. S., Thi B. K., and Patahayah M. (2010). An ectomycorrhizal thelephoroid fungus of Malaysian dipterocarp seedlings. J. Trop. For. Sci. 22, 355–363.
Li T., Li T. H., Song B., and Hosen I. (2020). Thelephora austrosinensis (Thelephoraceae), a new species close to T. ganbajun from southern China. Phytotaxa 471, 208–220. doi: 10.11646/phytotaxa.471.3.3
Liu S., Shen L. L., Wang Y., Xu T. M., Gates G., and Cui B. K. (2021). Species diversity and molecular phylogeny of Cyanosporus (Polyporales, Basidiomycota). Front. Microbiol. 12. doi: 10.3389/fmicb.2021.631166
Liu X. F., Tibpromma S., Xu J. C., Kumla J., Karunarathna S. C., and Zhao C. L. (2021). Taxonomy and phylogeny reveal two new potential edible ectomycorrhizal mushrooms of Thelephora from east Asia. Diversity 13, 646. doi: 10.3390/d13120646
Mu Y. H., Yu J. R., Cao T., Wang X. H., and Yuan H. S. (2021). Multi-gene phylogeny and taxonomy of Hydnellum (Bankeraceae, Basidiomycota) from China. J. Fungi 7, 818. doi: 10.3390/jof7100818
Nylander J. A. A. (2004). MrModeltest v2. Program distributed by the author (Uppsala, Sweden: Uppsala University, Evolutionary Biology Centre;), 1–2.
Ramírez-López I., Villegas-Ríos M., and Cano-Santana Z. (2013). Phenotypic plasticity of the basidiomata of Thelephora sp. (Thelephoraceae) in tropical forest habitats. Rev. Biol. Trop. 61, 343–350. doi: 10.15517/rbt.v61i1.11133
Ramírez-López I., Villegas-Ríos M., and Salas-Lizana R. (2015). Thelephora versatilis and Thelephora pseudoversatilis: two new cryptic species with polymorphic basidiomes inhabiting tropical deciduous and sub-perennial forests of the Mexican Pacific coast. Mycologia 107, 346–358. doi: 10.3852/14-151
Reid D. A. (1962). Notes on fungi which have been referred to the Thelephoraceae senso lato. Persoonia 2, 109–170.
Reid D. A. and Larsen M. (1976). Contribution to the taxonomy of the genus tomentella. Kew Bull. 31, 195. doi: 10.2307/4109015
Roberts P. J. and Spooner B. M. (2000). Cantharelloid, clavarioid and thelephoroid fungi from Brunei Darussalam. Kew Bull. 55, 843–851. doi: 10.2307/4113629
Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Sha T., Xu J. P., Palanichamy M. G., Zhang H. B., Li T., Zhao Z. W., et al. (2008). Genetic diversity of the endemic gourmet mushroom Thelephora ganbajun from south-western China. Microbiology 154, 3460–3468. doi: 10.1099/mic.0.2008/020495-0
Stalpers J. A. (1993). The Aphyllophoraceous fungi I. Keys to the species of the Thelephorales. Stud. Mycol. 35, 1–168.
Sugawara R., Aoki W., Yamada A., Nakagiri A., and Endo N. (2022). Ecological speciation of Japanese hedgehog mushroom: Thelephora subalpinum sp. nov. is distinguished from its sister species H. repando-orientale by means of integrative taxonomy. Mycol. Prog. 21, 94. doi: 10.1007/s11557-022-01844-7
Svantesson S., Kõljalg U., Wurzbacher C., Saar I., Larsson K. H., and Larsson E. (2021). Polyozellus vs. Pseudotomentella: generic delimitation with a multi-gene dataset. Fungal Syst. Evol. 8, 143–154. doi: 10.3114/fuse.2021.08.11
Tang L. P., Hao Y. J., Cai Q., Tolgor B., and Yang Z. L. (2014). Morphological and molecular evidence for a new species of Rhodotus from tropical and subtropical Yunnan, China. Mycol. Prog. 13, 45–53. doi: 10.1007/s11557-013-0890-x
Tang L. P., Lee S. S., Zeng N. K., Cai Q., Zhang P., and Yang Z. L. (2017). Notes on amanita section caesareae from Malaysia. Mycologia 109, 557–567. doi: 10.1080/00275514.2017.1394789
Tian M. Z., Xia H. B., Gao Z. L., Zhao C. Y., Ma D., Yang Z. L., et al. (2024). Four new species and one new record of thelephora from China. J. Fungi 10, 300. doi: 10.3390/jof10040300
Vilgalys R. and Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Villarruel-Ordaz J. L., Maldonado-Bonilla L. D., Sánchez-Espinoza A. C., Garibay-Orijel R., and Álvarez-Manjarrez J. (2024). Thelephora pacifica (Basidiomycota: Thelephorales), a new species of the tropical forests in the Mexican Pacific coast. Phytotaxa 634, 204–214. doi: 10.11646/phytotaxa.634.3.2
Vizzini A., Angelini C., Losi C., and Ercole E. (2016). Thelephora Dominicana (Basidiomycota, Thelephorales), a new species from the Dominican Republic, and preliminary notes on thelephoroid genera. Phytotaxa 265, 27–38. doi: 10.11646/phytotaxa.265.1.2
Voitk A., Saar I., Trudell S., Spirin V., Beug M., and Kõljalg U. (2017). Polyozellus multiplex (Thelephorales) is a species complex containing four new species. Mycologia 109, 975–992. doi: 10.1080/00275514.2017.1416246
White T. J., Bruns T., Lee S., and Taylor J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR - protocols and applications - a laboratory manual. Eds. Bruns T., Lee S., and Taylor J. (Academic Press, Cambridge, MA, USA), 315–322.
Willdenow C. L. (1787). “Florae berolinensis prodromus: secundum systema linneanum ab illustr,” in Viro ac eq. C. P. Thunbergio emendatum conscriptus (Classic reprint) (Impensis Wilhelmi Viewegii Berolini;, Berlin, Germany), 1–476.
Xu D. P., Zheng. J., Zhou Y., Li Y., Li S., and Li H. B. (2016). Extraction of natural antioxidants from the Thelephora ganbajun mushroom by an ultrasound-assisted extraction technique and evaluation of antiproliferative activity of the extract against human cancer cells. Int. J. Mol. Sci. 17, 1664. doi: 10.3390/ijms17101664
Yang S. R., Wei Y. L., and Yuan H. S. (2023). Molecular phylogeny and morphology reveal four new species of Thelephora (Thelephorales, Basidiomycota) from subtropical regions of China, closely related to T. ganbajun. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1109924
Yorou N. S. and Agerer R. (2008). Tomentella africana, a new species from Benin (West Africa) identified by morphological and molecular data. Mycologia 100, 68–80. doi: 10.3852/mycologia.100.1.68
Zang M. (1987). Some new and noteworthy higher fungi from eastern himalayas. Acta Botanica Yunnanica. 9, 81–88.
Keywords: ectomycorrhiza, edible mushroom, cryptic species, molecular systematics, taxonomy
Citation: Yu T-J, Xue R, Su L-J, Li J, Xia X, Xu C, Lei H-C, Zhang W-H, Ma J, Huang H-Y, Li X-J, Gao Y-X, Zhang Z-X, Li L, Zhang G-L and Tang L-P (2025) Multi-loci phylogeny reveals unexpected novelty of the Thelephora palmata complex (Thelephoraceae, Thelephorales) from China. Front. Fungal Biol. 6:1599905. doi: 10.3389/ffunb.2025.1599905
Received: 26 March 2025; Accepted: 08 July 2025;
Published: 14 August 2025.
Edited by:
Rajesh Jeewon, University of Mauritius, MauritiusReviewed by:
Samantha Chandranath Karunarathna, Qujing Normal University, ChinaYan-Chun Li, Chinese Academy of Sciences (CAS), China
Copyright © 2025 Yu, Xue, Su, Li, Xia, Xu, Lei, Zhang, Ma, Huang, Li, Gao, Zhang, Li, Zhang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Li Zhang, WmhhbmdndW9saTIwMDFAMTYzLmNvbQ==; Li-Ping Tang, dGFuZ2xpcGluZ0BrbW11LmVkdS5jbg==
 Tai-Jie Yu
Tai-Jie Yu Rou Xue1,2,3
Rou Xue1,2,3