- 1Clinical Pharmacy Center, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Department of Clinical Pharmacy, The Second Clinical Medical College of North Sichuan Medical College, Nanchong, China
Fungal peritonitis represents a significant complication of peritoneal dialysis (PD) and can result in severe consequences. However, fungal peritonitis caused by Fusarium is relatively rare, and there is no standard treatment plan for reference. Consequently, clinical pharmacists participated in a drug therapy for a rare case of fungal peritonitis in PD caused by Fusarium through literature review and therapeutic drug monitoring. Finally, this case received oral voriconazole, and the plasma concentration was maintained above 2 μg/ml. Moreover, the patient achieved favorable outcomes.
Introduction
Peritonitis represents a significant complication of peritoneal dialysis (PD) and can result in severe consequences, including hospitalization, PD catheter extraction, and the necessity for permanent hemodialysis (Htay et al., 2018). The common pathogens of PD-associated peritonitis are predominantly Gram-positive and Gram-negative bacteria (Kim et al., 2004; Whitty et al., 2017). Conversely, fungal infections, particularly those caused by Fusarium spp., are infrequent (Hu et al., 2019; Kanjanabuch et al., 2022). In this study, we report on a case of the use of oral voriconazole for the treatment of PD-associated Fusarium peritonitis.
Case report
A 61-year-old woman weighing 38.1 kg, who works as a farmer and had been undergoing continuous ambulatory peritoneal dialysis (CAPD) for over 8 years, presented with a 5-day history of abdominal pain, diarrhea, and vomiting, and then gradually developed cloudy PD effluent. Laboratory findings revealed a white blood cell (WBC) count of 12.6 × 109/L, a neutrophil (NEU) count of 11.07 × 109/L, and NEU% of 87.5%. Her C-reactive protein (CRP) level was 102.5 mg/L. Analysis of the PD effluent demonstrated turbidity with a WBC count of 4,044/μl (90% NEU). The admission diagnosis was PD-related peritonitis and chronic kidney disease (CKD 5).
Upon admission, empirical treatment with intraperitoneal (IP) cefazolin 0.5 g and amikacin 0.025 g four times daily was initiated. After 3 days, her laboratory tests showed a WBC count of 16.5 × 109/L, NEU% of 94.5%, CRP level of 75.6 mg/L, and procalcitonin (PCT) level of 1.4 ng/ml. Analysis of the PD effluent showed turbidity with a WBC count of 4,210/μl (92% NEU). Due to inadequate response, the anti-infection regimen was adjusted to teicoplanin 0.02 g and meropenem 0.25 g (IP).
On day 7, the patient reported no improvement. Laboratory tests indicated a WBC count of 28.3 × 109/L and NEU% of 93.8%. Her CRP was 131 mg/L and PCT was 2.1 ng/ml. Examination of the PD effluent revealed turbidity with a WBC count of 3,260/μl (91% NEU). Both the blood and PD effluent cultures yielded negative results. In light of the unresponsive nature of the treatment, the abdominal dialysis catheter was removed and changed to hemodialysis. Intravenous administration of meropenem and teicoplanin was initiated.
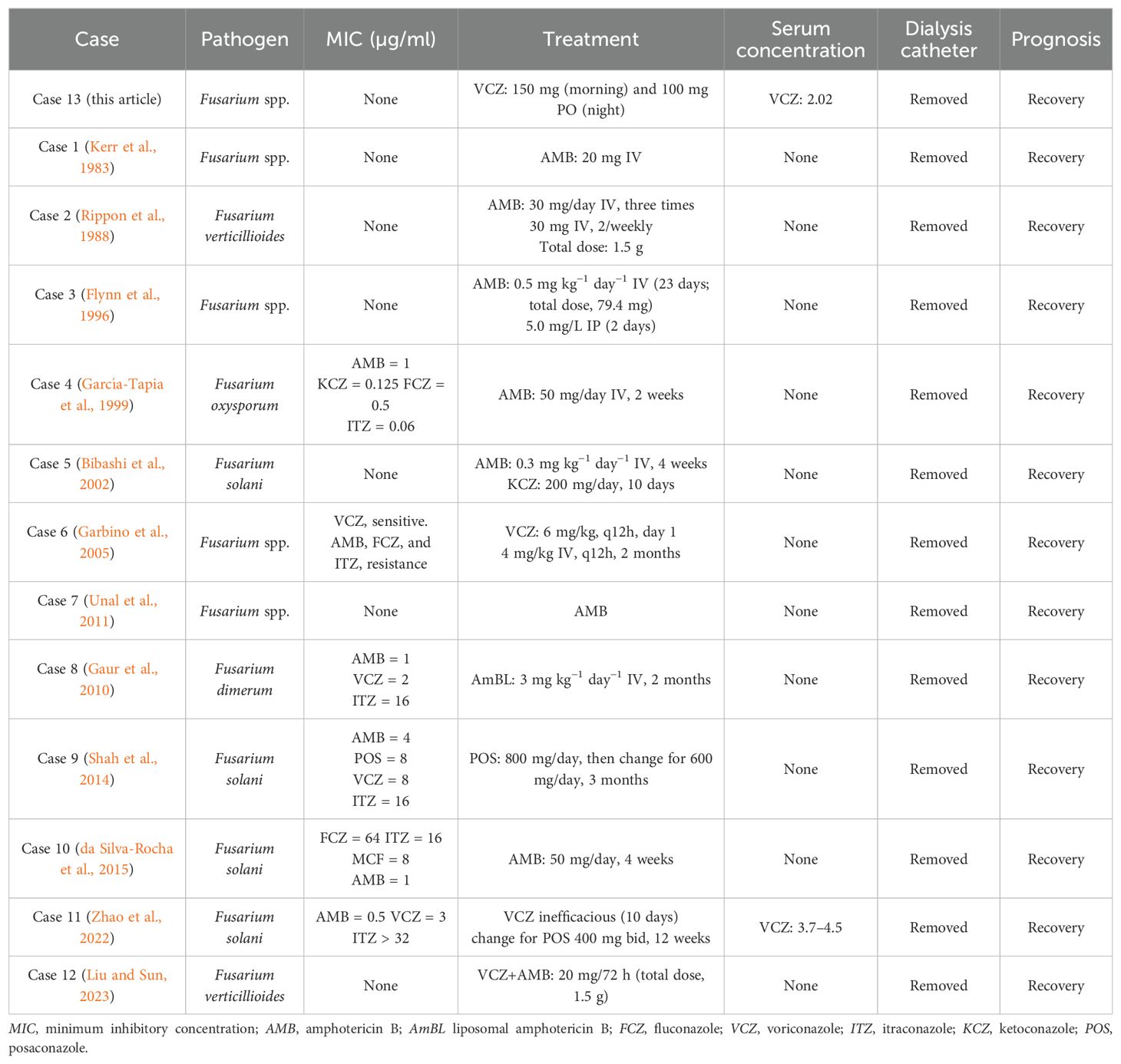
On day 13, with persistent elevation of the infection markers (i.e., CRP and PCT) and suspicion of fungal peritonitis, oral fluconazole 200 mg once daily was initiated and meropenem was discontinued. On day 23, the patient’s WBC count was 13.1 × 109/L, NEU% was 84.1%, CRP was 103 mg/L, and PCT was 1.5 ng/ml. Culture of the PD effluent confirmed the presence of Fusarium spp. by microscopic examination. As a control, the culture of the PD fluid was negative. Teicoplanin and fluconazole were discontinued, and oral voriconazole was commenced with a loading dose of 200 mg on the first day, followed by a maintenance dose of 100 mg every 12 h. After 3 days, given a plasma concentration of voriconazole at 1.38 μg/ml by high-performance liquid chromatography, the maintenance dose was increased to 150 mg in the morning.
On day 37, the patient experienced resolution of abdominal pain. Laboratory analysis revealed a WBC count of 7.79 × 109/L, NEU% of 80.7%, CRP of 64 mg/L, and PCT of 1.7 ng/ml. The plasma concentration of voriconazole measured 2.02 μg/ml. Upon discharge, the patient was prescribed voriconazole at doses of 150 mg in the morning and 100 mg in the evening. At the 2-month post-discharge evaluation, the patient remained afebrile and asymptomatic, with complete normalization of the inflammatory indices, indicating full clinical remission. The alterations in the medication and the key indicators throughout the hospital stay are depicted in Figure 1.
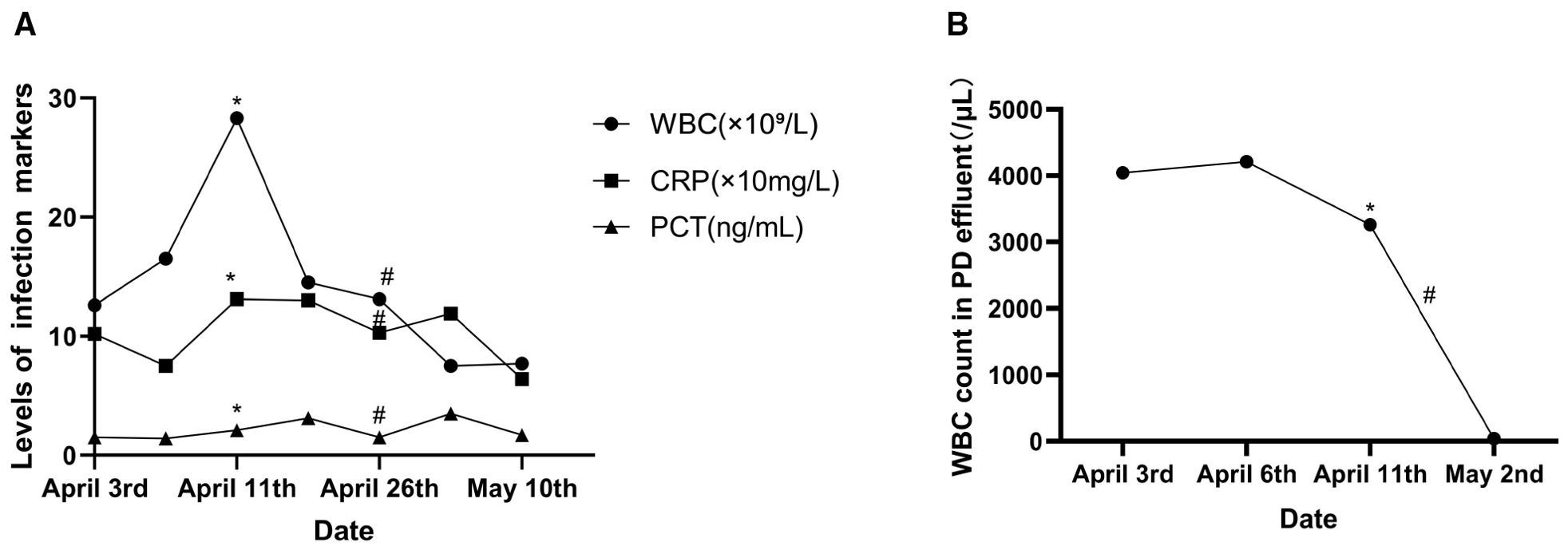
Figure 1. Changes in the infection markers. (A) Serum levels of the infection markers. (B) White blood cell (WBC) count in the peritoneal dialysis (PD) effluent. Asterisk represents removal of the PD catheter and the start of intravenous antimicrobial therapy. Number symbol denotes use of voriconazole. CRP, C-reactive protein; PCT, procalcitonin.
Discussion
Fusarium spp. are filamentous fungi that produce mitospores and are ubiquitously present in the environment, inhabiting the air, water, and soil. They are recognized as significant plant pathogens. In humans, they act as opportunistic pathogens, causing localized or disseminated infection after trauma or weakened immunity. The common clinical pathogenic Fusarium species include Fusarium solani, Fusarium verticillioides, Fusarium oxysporum, and Fusarium proliferatum. Among these, F. solani stands out as the most prevalent and virulent species, accounting for approximately 40%–60% of infections. Management of Fusarium infections poses a challenge due to their multidrug resistance and their ability to cause a range of clinical presentations, such as pneumonia, fungemia, cellulitis, and lymphangitis, following skin trauma (Ledoux et al., 2024; Nucci and Anaissie, 2023). Despite these complexities, PD-related Fusarium peritonitis is a rare occurrence, and the available literature on this topic is limited. The Fusarium spp. infection in this case was likely related to her occupation as a farmer and her living environment. Moreover, as a patient with CKD 5 undergoing PD, she is not only repeatedly exposed to this environment during dialysis but is also immunocompromised, further increasing the risk of infection. Regrettably, neither the infecting subspecies nor the antimicrobial susceptibility profile could be determined.
In a multicenter study involving 88 patients with Fusarium infection, an analysis was conducted on the correlation between the minimum inhibitory concentration (MIC) of antifungal agents and the treatment outcomes. The mean MIC50 values of voriconazole against F. solani and F. oxysporum were determined to be 8 and 4 μg/ml, respectively, while those of amphotericin B were 2 and 1 μg/ml, respectively. In a comparative analysis, it was observed that there was no statistically significant variance in the mortality rates between voriconazole and amphotericin B liposomes. Conversely, amphotericin B deoxycholate exhibited a notably elevated mortality rate of 60% (Nucci et al., 2021). In addition, a retrospective multicenter investigation that included 233 instances of invasive Fusarium infection revealed similar 90-day survival rates for voriconazole and amphotericin B liposomes, while amphotericin B deoxycholate demonstrated a distinctly inferior outcome (Nucci et al., 2014). The 2021 Global Guidelines robustly advocate intravenous voriconazole or amphotericin B liposomes, either as monotherapy or in combination, as the primary therapeutic approach. Isavuconazonium or posaconazole is recommended as a second-line treatment. Notably, amphotericin B deoxycholate is not recommended in this context (Hoenigl et al., 2021).
At present, there exists a lack of consensus regarding the optimal management of peritonitis related to Fusarium spp. in patients with PD. Table 1 displays a compilation and summary of the reported cases of Fusarium peritonitis from the references (Bibashi et al., 2002; da Silva-Rocha et al., 2015; Flynn et al., 1996; Garbino et al., 2005; García-Tapia et al., 1999; Gaur et al., 2010; Kerr et al., 1983; Liu and Sun, 2023; Rippon et al., 1988; Shah et al., 2014; Unal et al., 2011; Zhao et al., 2022). For drug selection, liposomal amphotericin B was unavailable, and amphotericin B deoxycholate was excluded due to its pronounced toxicity and poor prognosis. Intravenous voriconazole was withheld to avoid sulfobutyl-ether-β-cyclodextrin accumulation in CKD. Oral voriconazole, with equivalent bioavailability, was used instead and attained effective concentrations. Clinical pharmacists recommended the initial regimen of oral voriconazole for this patient, with a measured plasma concentration of 1.05 μg/ml. However, it is noteworthy that the MIC for Fusarium is relatively elevated. Studies have indicated that the MIC of the commonly obtained voriconazole is at least 2 μg/ml (Espinel-Ingroff et al., 2016), with a maximum of 5 μg/ml (Chen et al., 2018). Therefore, clinical pharmacists recommended an escalation in the voriconazole dosage to 150 mg in the morning. Subsequent monitoring revealed an increase in the plasma concentration to 2.02 μg/ml. This adjusted treatment regimen resulted in a notable alleviation of the patient’s abdominal pain and a substantial decrease in the infection markers.
Conclusion
PD-related peritonitis caused by Fusarium spp. is a rare occurrence. The diagnosis was confirmed for this case due to meeting all three of three diagnostic criteria: abdominal pain and cloudy effluent; effluent WBC count >100 × 106/L (4,460 × 106/L) with >90% NEU; and effluent culture positive for Fusarium spp. However, the pathogen was not cultured until 13 days after admission, which delayed the treatment to some extent. Rapid assays such as the (1→3)-β-d-glucan test, the galactomannan test, and next-generation sequencing of the blood or peritoneal dialysate samples can accelerate the identification of rare fungal peritonitis.
Large-scale investigations focusing on antifungal interventions for Fusarium peritonitis are scarce, with the current therapeutic strategies predominantly documented through isolated case reports. A key limitation is the absence of Fusarium susceptibility data, leaving antifungal selection empirical rather than targeted. Fortunately, in this case, clinical pharmacists conducted a comprehensive review of the pertinent literature, evaluated the epidemiological aspects of Fusarium infections, appraised the pharmacological profiles of therapeutic agents, and collaborated with clinicians to devise personalized antifungal regimens incorporating therapeutic drug monitoring. Finally, despite the eventual recovery, the patient and her family lamented the delayed identification of the pathogen that needlessly prolonged hospitalization. This case underscores the significance of oral voriconazole combined with therapeutic drug monitoring in the management of peritonitis associated with Fusarium in PD and serves as a valuable reference regarding its efficacy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author contributions
QP: Writing – original draft. WW: Writing – original draft, Writing – review & editing. LD: Writing – original draft. HT: Writing – original draft. HW: Conceptualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by Scientific Research Fund of Guangdong Pharmaceutical Association (2024KP04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bibashi E., Kokolina E., Sigler L., Sofianou D., Tsakiris D., Visvardis G., et al. (2002). Three cases of uncommon fungal peritonitis in patients undergoing peritoneal dialysis. Perit. Dial. Int. 22, 523–525. doi: 10.1177/089686080202200415
Chen K., Zhang X., Ke X., Du G., Yang K., Zhai S., et al. (2018). Individualized medication of voriconazole: a practice guideline of the division of therapeutic drug monitoring, Chinese Pharmacological Society. Ther. Drug Monit. 40, 663–674. doi: 10.1097/ftd.0000000000000561
da Silva-Rocha W. P., Zuza-Alves D. L., de Azevedo Melo A. S., and Chaves G. M. (2015). Fungal peritonitis due to Fusarium solani species complex sequential isolates identified with DNA sequencing in a kidney transplant recipient in Brazil. Mycopathologia 180, 397–401. doi: 10.1007/s11046-015-9929-7
Espinel-Ingroff A., Colombo A., Cordoba S., Dufresne P., Fuller J., Ghannoum M., et al. (2016). International evaluation of MIC distributions and epidemiological cutoff value (ECV) definitions for Fusarium species identified by molecular methods for the CLSI broth microdilution method. Antimicrob. Agents Chemother. 60, 1079–1084. doi: 10.1128/AAC.02456-15
Flynn J. T., Meislich D., Kaiser B. A., Polinsky M. S., and Baluarte H. J. (1996). Fusarium peritonitis in a child on peritoneal dialysis: case report and review of the literature. Perit. Dial. Int. 16, 52–57. doi: 10.1177/089686089601600113
Garbino J., Uckay I., Rohner P., Lew D., and Delden C. V. (2005). Fusarium peritonitis concomitant to kidney transplantation successfully managed with voriconazole: case report and review of the literature. Transpl. Int. 18, 613–618. doi: 10.1111/j.1432-2277.2005.00102.x
García-Tapia A., Aznar E., García-Martos P., Marín P., Márquez A., Lozano C., et al. (1999). Fusarium peritonitis in a patient on peritoneal dialysis. Rev. Iberoam. Micol. 16, 166–167.
Gaur S., Rajgopal A., and Ashbee R. (2010). A successfully treated case of peritonitis due to Fusarium dimerum. J. Infect. 61, 86–88. doi: 10.1016/j.jinf.2010.03.020
Hoenigl M., Salmanton-García J., Walsh T. J., Nucci M., Neoh C. F., Jenks J. D., et al. (2021). Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect. Dis. 21, e246–e257. doi: 10.1016/s1473-3099(20)30784-2
Htay H., Cho Y., Pascoe E. M., Darssan D., Nadeau-Fredette A.-C., Hawley C., et al. (2018). Center effects and peritoneal dialysis peritonitis outcomes: analysis of a national registry. Am. J. Kidney Dis. 71, 814–821. doi: 10.1053/j.ajkd.2017.10.017
Hu S., Tong R., Bo Y., Ming P., and Yang H. (2019). Fungal peritonitis in peritoneal dialysis: 5-year review from a North China center. Infection 47, 35–43. doi: 10.1007/s15010-018-1204-7
Kanjanabuch T., Nopsopon T., Chatsuwan T., Purisinsith S., Johnson D. W., Udomsantisuk N., et al. (2022). Predictors and outcomes of peritoneal dialysis-related infections due to filamentous molds (MycoPDICS). PloS One 17, e0268823. doi: 10.1371/journal.pone.0268823
Kerr C. M., Perfect J. R., Craven P. C., Jorgensen J. H., Drutz D. J., Shelburne J. D., et al. (1983). Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Ann. Intern. Med. 99, 334–337. doi: 10.1046/j.0933-7407.2003.00922.x
Kim D. K., Yoo T.-H., Ryu D.-R., Xu Z.-G., Kim H. J., Choi K. H., et al. (2004). Changes in causative organisms and their antimicrobial susceptibilities in CAPD peritonitis: a single center’s experience over one decade. Perit. Dial. Int. 24, 424–432. doi: 10.1177/089686080402400506
Ledoux M.-P., Dicop E., Sabou M., Letscher-Bru V., Castelain V., Danion F., et al. (2024). Fusarium, scedosporium and other rare mold invasive infections: over twenty-five-year experience of a european tertiary-care center. J. Fungi 10, 289. doi: 10.3390/jof10040289
Liu Y. and Sun F. (2023). Peritoneal dialysis-related peritonitis and bacteremia caused by Fusarium verticillioides: a case report and literature review. Chin. J. Nephrol. 39, 935–938. doi: 10.3760/cma.j.cn441217-20230321-00329
Nucci M. and Anaissie E. (2023). Invasive fusariosis. Clin. Microbiol. Rev. 36, e00159–e00122. doi: 10.1128/eissn.1098-6618
Nucci M., Jenks J., Thompson G. R. III, Hoenigl M., Dos Santos M. C., Forghieri F., et al. (2021). Do high MICs predict the outcome in invasive fusariosis? J. Antimicrob. Chemother. 76, 1063–1069. doi: 10.1093/jac/dkaa516
Nucci M., Marr K., Vehreschild M., De Souza C., Velasco E., Cappellano P., et al. (2014). Improvement in the outcome of invasive fusariosis in the last decade. Clin. Microbiol. Infect. 20, 580–585. doi: 10.1111/1469-0691.12409
Rippon J. W., Larson R. A., Rosenthal D. M., and Clayman J. (1988). Disseminated cutaneous and peritoneal hyalohyphomycosis caused by Fusarium species: three cases and review of the literature. Mycopathologia 101, 105–111. doi: 10.1007/bf00452895
Shah P. J., Bergman S., Vegi S., and Sundareshan V. (2014). Fusarium peritonitis successfully managed with posaconazole and catheter removal. Perit. Dial. Int. 34, 566–568. doi: 10.3747/pdi.2013.00142
Unal A., Kocyigit I., Sipahioglu M. H., Tokgoz B., Oymak O., and Utas C. (2011). Fungal peritonitis in peritoneal dialysis: an analysis of 21 cases. Int. Urol. Nephrol. 43, 211–213. doi: 10.1007/s11255-010-9763-2
Whitty R., Bargman J. M., Kiss A., Dresser L., and Lui P. (2017). Residual kidney function and peritoneal dialysis–associated peritonitis treatment outcomes. Clin. J. Am. Soc Nephrol. 12, 2016–2022. doi: 10.2215/cjn.00630117
Keywords: Fusarium, peritonitis, peritoneal dialysis, voriconazole, therapeutic drug monitoring
Citation: Peng Q, Wu W, Deng L, Tong H and Wu H (2025) Peritoneal dialysis-related peritonitis caused by Fusarium: a case report and literature review. Front. Fungal Biol. 6:1637498. doi: 10.3389/ffunb.2025.1637498
Received: 29 May 2025; Accepted: 26 August 2025;
Published: 16 September 2025.
Edited by:
Raquel Sabino, Universidade de Lisboa, PortugalReviewed by:
Nandini Sethuraman, Apollo Hospitals, IndiaNuri Kiraz, Istanbul University-Cerrahpasa, Türkiye
Copyright © 2025 Peng, Wu, Deng, Tong and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiyi Wu, aHVpeWkwMjE0QHllYWgubmV0
†These authors have contributed equally to this work and share first authorship
 Qin Peng
Qin Peng Wenfeng Wu1†
Wenfeng Wu1† Huiyi Wu
Huiyi Wu