- 1Department of Biological and Environmental Sciences, Sefako Makgatho Health Sciences University, Pretoria, South Africa
- 2Faculty of Applied Science, Eduvos, Midrand, South Africa
Cannabinoids, such as Δ9tetrahydrocannabinol (THC) and cannabidiol (CBD), are bioactive compounds with well-documented therapeutic potential, including applications in pain relief, neuroprotection, anti-inflammatory treatments, and seizure control. Traditionally sourced from Cannabis plants, their production remains limited by agricultural constraints, regulatory hurdles, and environmental concerns. In response, recent advances in biotechnology have enabled the microbial biosynthesis of cannabinoids, offering a scalable and sustainable alternative. Engineered fungi, in particular, have gained attention as promising production platforms due to their metabolic flexibility, ease of genetic manipulation, and capacity for synthesizing complex secondary metabolites. This mini-review explores key innovations in synthetic biology and metabolic engineering that have enabled fungal cannabinoid biosynthesis. It highlights strategies such as pathway reconstruction, enzyme optimization, host strain engineering, and the application of CRISPR-Cas9 genome editing. In addition, it examines ongoing challenges, including product toxicity, metabolic burden, and regulatory considerations. Finally, the review outlines future directions in systems biology, the production of rare cannabinoids, and bioprocess optimization. Overall, the development of engineered fungi for cannabinoid biosynthesis represents a major conceptual advance in microbial biotechnology, with far-reaching implications for the pharmaceutical, nutraceutical, and industrial sectors.
1 Introduction
In recent years, there has been a remarkable surge in global interest and research on Cannabis sativa, driven largely by the therapeutic promise and economic value of its active compounds, cannabinoids. Among these, Δ9tetrahydrocannabinol (THC) and cannabidiol (CBD) are the most extensively studied, known for their diverse pharmacological effects, including analgesic, anti-inflammatory, antiseizure, anxiolytic, and neuroprotective properties (Pertwee, 2006; Morales et al., 2017; Pisanti et al., 2017). This surge in popularity has paralleled the rapid growth of the global legal Cannabis market, which is projected to exceed USD 60 billion by 2027 (Grand View Research, 2023). Legalization trends across North America, Europe, Africa, and parts of Asia have contributed to increased consumption and normalization of cannabinoid containing products (Abuhasira et al., 2018). Cannabinoids are currently utilized across multiple industries, including pharmaceuticals, cosmetics, nutraceuticals, and functional foods (Andre et al., 2016; Hanuš et al., 2016). Medical Cannabis is now legal in over 50 countries, while recreational use is permitted in several jurisdictions, leading to increased research, product innovation, and commercialization (European Monitoring Centre for Drugs and Drug Addiction, 2018). Despite this progress, cannabinoid production through Cannabis cultivation presents several challenges—slow growth cycles, environmental variability, land and water use, and strict regulatory controls that limit scalability and standardization (Jin et al., 2017; Chakraborty et al., 2021).
To overcome the challenges of plant-based production, microbial biosynthesis of cannabinoids has emerged as a promising alternative. Fungi are gaining attention due to their fast growth, metabolic versatility, and industrial utility. Synthetic biology tools now enable the expression of cannabinoid pathways in fungi, including enzymes that produce key precursors such as for producing key precursors like olivetolic acid and geranyl pyrophosphate (Keller, 2019; Luo et al., 2019; Gülck and Møller, 2020; Vogt et al., 2021).
Moreover, breakthroughs in genome editing technologies, particularly CRISPR-Cas9, have significantly accelerated strain development and pathway optimization (Nødvig et al., 2015). Notably, recent studies have demonstrated the successful production of cannabinoids in engineered fungal strains, such as Aspergillus niger, laying the foundation for a cost-effective, environmentally friendly, and regulation-compliant production platform (Drysdale et al., 2022). This review provides a comprehensive overview of current advancements in fungal-based cannabinoid biosynthesis, with emphasis on engineering strategies, current challenges, and potential applications.
2 Synthetic biology and metabolic engineering
A major breakthrough in modern biotechnology has been the ability to reconstruct cannabinoid biosynthetic pathways in microbial systems using synthetic biology tools. Central to this achievement is the engineering of fungal hosts to express key enzymes from Cannabis sativa that are responsible for producing the primary cannabinoid precursors—olivetolic acid (OA) and geranyl pyrophosphate (GPP). These precursors combine to form cannabigerolic acid (CBGA), the parent molecule for major cannabinoids including THC, CBD, and CBC (Figure 1). Genes encoding enzymes such as olivetolic acid cyclase (OAC) and geranyl pyrophosphate: olivetolate geranyl transferase (GOT) have been successfully cloned and introduced into fungal platforms, including Aspergillus niger and Penicillium chrysogenum, enabling them to produce cannabinoids under controlled fermentation conditions (Russo, 2011; Qiu et al., 2022). Fungi are naturally suited for this purpose due to their established role in producting of complex secondary metabolites. Their genetic malleability, robust growth profiles, and compatibility with large-scale fermentation processes make them ideal candidates for heterologous production of cannabinoids (Pamplona et al., 2018; Santiago et al., 2019). Recent optimization strategies have included CRISPR-Cas9 genome editing, promoter refinement, and pathway balancing to improve flux toward target compounds. In some systems, these interventions have led to a 40-fold increase in CBGA yield, demonstrating the feasibility of fungal cannabinoid production at a commercially relevant scale (Ceroni et al., 2018).
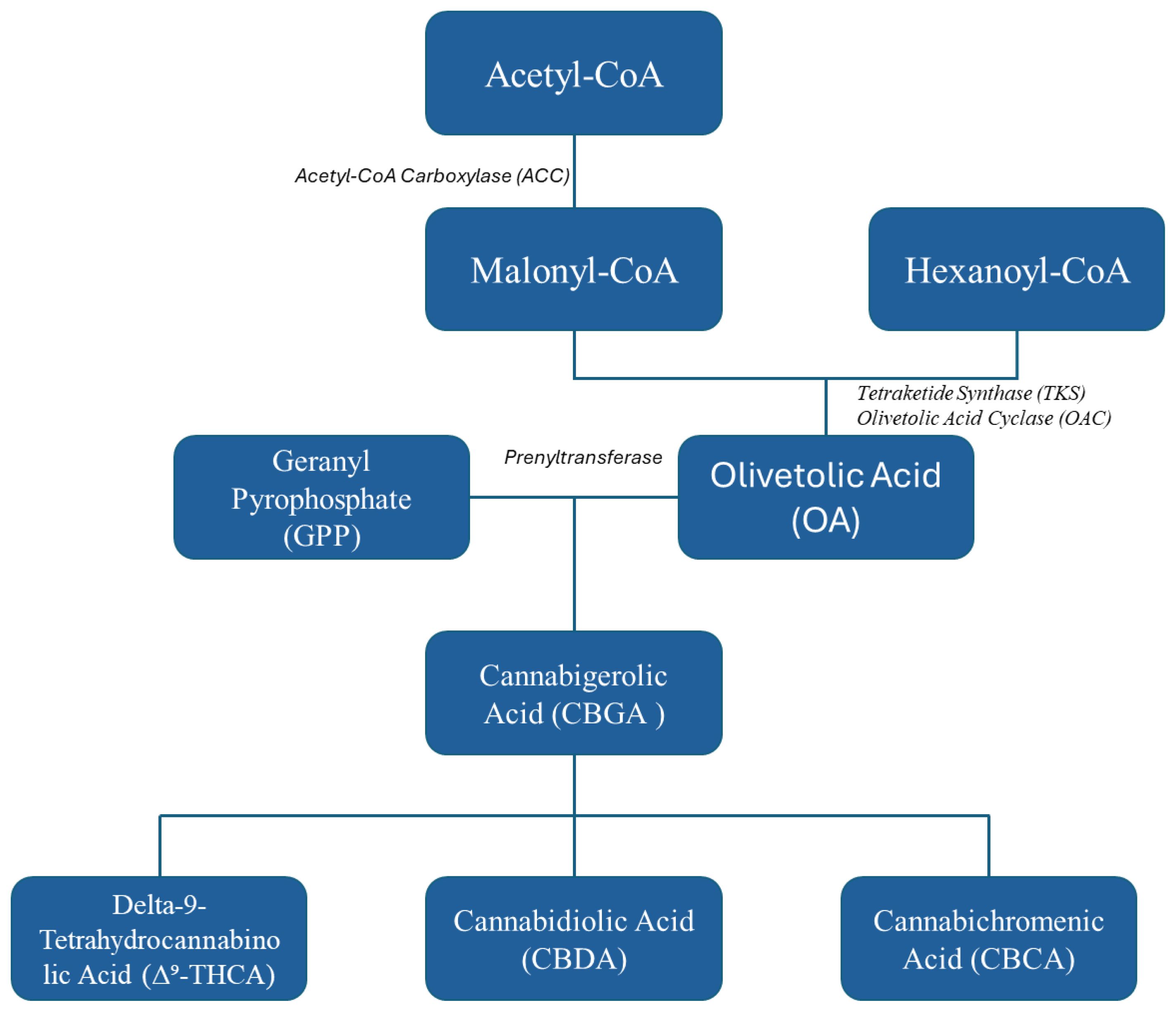
Figure 1. Schematic overview of the engineered biosynthetic pathway for cannabinoid production, illustrating the enzymatic conversion.
Recent studies report olivetolic acid (OA) titers of up to 15.79 mg/L in engineered Yarrowia lipolytica (Hong et al., 2025). Engineered Penicillium chrysogenum strains produced CBGA at 0.67 mg/L (supernatant) and 1.51 mg/L (lysate), with olivetolic acid reaching up to 12.23 mg/L (Kosalková et al., 2023). In contrast, Saccharomyces cerevisiae has achieved titers of>100 mg/L 88 CBGA through pathway optimization and precursor feeding strategies. However, filamentous fungi like P. chrysogenum offer distinct advantages, including native polyketide synthase machinery, robust secondary metabolite secretion, and scalable filamentous growth, which make them promising long-term hosts for complex cannabinoid biosynthesis once further optimized. Despite advancements, the therapeutic equivalence of biosynthesized cannabinoids and plant-derived extracts remains a topic of debate. While microbial systems offer precision and scalability, whole-plant Cannabis advocates highlight the “entourage effect” a synergy among cannabinoids, terpenes, and flavonoids that is difficult to replicate with isolated compounds (Nakagawa et al., 2011; Wang et al., 2023). As such, the debate continues over whether purified cannabinoids synthesized in fungi can truly substitute the full-spectrum effects offered by plant-derived products a question that holds significant implications for drug development, regulatory approval, and clinical practice (Karasek and Stasiak, 2021).
3 Optimization of fungal hosts and divergent perspectives
Fungi have emerged as promising platforms for cannabinoid biosynthesis due to their metabolic versatility, industrial compatibility, and ability to produce complex secondary metabolites. Filamentous species such as Aspergillus niger, Penicillium chrysogenum, and Trichoderma reesei are of particular interest, as they are well characterized, genetically tractable, and already utilized in large-scale bioproduction (Geiselmann et al., 2022; Liu et al., 2023). These hosts possess endogenous pathways such as those for terpenoid and polyketide synthesis that align with the biochemical demands of cannabinoid production. To optimize fungal cannabinoid biosynthesis, researchers have targeted both primary and secondary metabolism. Enhancements in precursor availability, including the upregulation of the mevalonate 114 pathway (for GPP) and polyketide synthase pathways (for OA), have resulted in significant yield improvements (Keasling et al., 2022). Metabolic modeling and flux analysis are now standard tools in identifying bottlenecks and optimizing expression levels (Hudalla et al., 2024). Furthermore, synthetic promoter libraries, codon optimization, and dynamic pathway regulation systems are being deployed to fine-tune expression of cannabinoid biosynthetic genes (Stone et al., 2020). However, there is an ongoing scientific debate over the ideal production platform. While synthetic biology advocates view fungi as sustainable, consistent, and scalable, others argue that microbial systems lack the biochemical complexity of the upregulation of the mevalonate 114 pathway (for GPP) and polyketide synthase pathways (for OA), have resulted in the synergistic therapeutic interplay among cannabinoids, terpenes, and flavonoids (Almeida et al., 2023). These differing schools of thought have implications not only for technical development but also for downstream clinical acceptance and regulatory approval.
4 Engineering challenges and scientific disagreement
Although significant progress has been made in constructing functional cannabinoid pathways in fungi, several biological and technical challenges persist. One of the primary issues is the cytotoxicity of cannabinoids to fungal cells. These lipid-soluble compounds can integrate into membranes or disrupt cellular signaling, leading to reduced growth and metabolite accumulation (Singh and Bhatia, 2022). To mitigate this, strategies such as efflux pump expression, product sequestration in organelles (e.g., peroxisomes), and the use of tolerance-enhancing mutations are being explored (Almeida et al., 2023). Additionally, the multistep nature of cannabinoid biosynthesis requires tight coordination of gene expression. Even slight imbalances in enzyme activity can lead to precursor buildup or the formation of shunt metabolites, thereby, reducing overall efficiency. Advanced engineering techniques, such as CRISPR137 Cas9-mediated transcriptional tuning and genome-scale pathway balancing, are now being used to fine-tune these systems (Nødvig et al., 2015; Stone et al., 2020). This complexity has sparked another layer of controversy: some researchers believe that microbial systems are ill-suited for full cannabinoid biosynthesis and better suited for producing isolated or rare cannabinoids. They argue that microbial chassis may struggle to match the pharmacodynamic complexity and consumer appeal of full-spectrum Cannabis extracts (Russo, 2011). On the other hand, industrial stakeholders and biopharmaceutical companies see microbial systems as a clean, reproducible, and patentable solutions particularly valuable for producing minor cannabinoids like THCV and CBDV, which occur in low abundance in plants (Karasek and Stasiak, 2021; Hudalla et al., 2024).
5 Success stories, technological advances, and the road ahead
Despite challenges, notable successes have been achieved in engineering fungi for cannabinoid production. In 2022, a landmark study demonstrated the production of cannabigerolic acid (CBGA) in Aspergillus niger, achieved by expressing a full suite of biosynthetic genes and optimizing host metabolism for precursor availability (Drysdale et al., 2022). This work confirmed the feasibility of producing key cannabinoid intermediates at commercially relevant scales through the use of fungal fermentation. Cutting-edge tools, such as CRISPR-Cas9, dynamic biosensors, and synthetic gene circuits, have accelerated 155 the field, enabling researchers to modulate pathways in real-time based on metabolite levels (Ceroni et al., 2018; Geiselmann et al., 2022). These systems also enable rapid testing of cannabinoid analogues and unnatural derivatives, offering opportunities to explore next-generation therapeutic compounds that may surpass the efficacy of natural cannabinoids. Yet, success in the lab does not guarantee acceptance in the clinic or market. A growing divide exists between proponents of “natural” plant-derived cannabinoids and those favoring precision-engineered biosynthetic products. Critics caution that synthetic production might overlook the holistic pharmacology of Cannabis, especially in contexts where whole extract formulations are favored for their perceived broader efficacy (Russo, 2011; Stone et al., 2020). In contrast, supporters of microbial production emphasize purity, traceability, and standardization features that are particularly critical in pharmaceutical development and international regulatory environments (Hudalla et al., 2024).
Synthetic fungi offer a promising platform for producing high-yield, pharmaceutical-grade cannabinoids due to their rapid growth and genetic tractability (Figure 2). Future strategies may combine microbial cannabinoids with plant-derived terpenes to mimic the entourage effect, thereby bridging the gap between scientific and therapeutic perspectives.
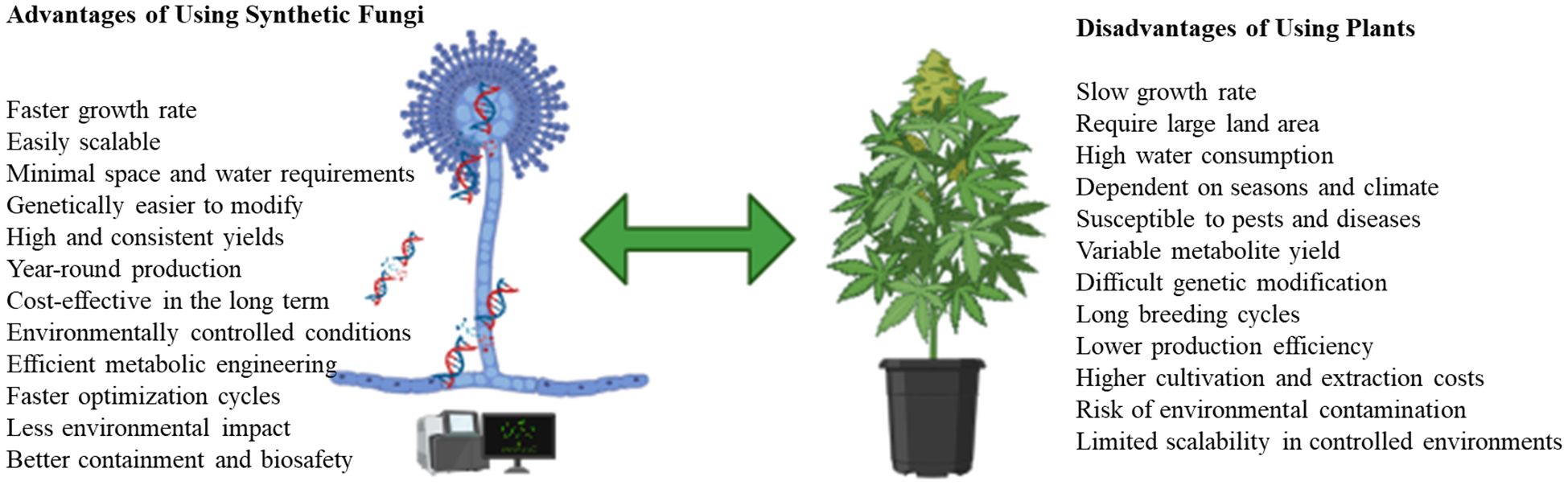
Figure 2. Advantages of synthetic fungi over plants in biotechnology and industrial applications (created by MC Manganyi using BioRender.com).
6 Applications and implications
The biosynthesis of cannabinoids in engineered fungi represents a transformative shift in the cannabinoid supply chain. Unlike traditional Cannabis cultivation, which requires vast amounts of land, energy, and water, fungal fermentation enables sustainable, high throughput production in controlled bioreactors (Liu et al., 2023). These systems drastically reduce environmental burdens and allow year-round production, independent of agricultural constraints such as climate or soil quality. Furthermore, fungi can be engineered to bypass the time-consuming maturation periods associated with Cannabis sativa, leading to faster turnaround times and lower production costs (Keasling et al., 2022).
Fungal platforms enable the selective expression of biosynthetic pathways for rare cannabinoids, such as cannabigerol (CBG), tetrahydrocannabivarin (THCV), and 192 cannabichromene (CBC), which are known for their diverse therapeutic potential. For instance, THCV shows promise in appetite suppression and glycaemic regulation, making it a potential candidate for obesity and type 2 diabetes treatments (Pertwee, 2006). Meanwhile, CBC exhibits strong anti-inflammatory and neuroprotective properties, which are being explored in models of neurodegenerative diseases and chronic pain (Almeida et al., 2023). The ability to engineer fungi for the precise production of such molecules accelerates drug discovery pipelines and reduces dependency on Cannabis biomass extraction.
Microbial cannabinoid production ensures consistency and safety but introduces new regulatory challenges. Authorities, such as the FDA and EMA, must assess the purity, bioequivalence, and long-term safety of fungal-derived cannabinoids. Ongoing debates surrounding classification and labeling may impact clinical use and global commercialization (Hudalla et al., 2024).
7 Future directions
Fungal engineering for cannabinoid biosynthesis is a relatively new field, but advancements in CRISPR-based genome editing, chassis development, and metabolic flux analysis are rapidly accelerating progress (Stone et al., 2020). Recent breakthroughs in utilizing of Aspergillus oryzae and Trichoderma reesei as high-yielding hosts have demonstrated enhanced cannabinoid titers through pathway optimization and precursor feeding strategies (Singh and Bhatia, 2022). Future research is expected to delve deeper into system-level modeling, enabling dynamic control over metabolic nodes to maximize cannabinoid yield while minimizing toxic byproducts. Efforts are also underway to engineer fungal strains that can utilize low-cost, renewable substrates such as agricultural waste or lignocellulose hydrolysates further enhancing sustainability (Singh and Bhatia, 2022). Integrating AI driven bioinformatics with omics data will play a pivotal role in identifying novel enzymes, regulatory elements, and gene circuits to fine-tune cannabinoid biosynthetic routes.
8 Conclusion
The convergence of synthetic biology, fungal biotechnology, and cannabinoid research is paving the way for a new era in pharmaceutical manufacturing. Engineered fungi not only offer a scalable and ecoconscious alternative to traditional Cannabis cultivation but also enable the tailored production of pharmacologically relevant cannabinoids. While regulatory frameworks and technical challenges remain, the trajectory of this field suggests that fungi may soon become central players in the biomanufacturing of cannabinoid-based therapeutics.
Author contributions
MM: Writing – review & editing, Writing – original draft, Conceptualization. CK: Conceptualization, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank their respective institutions for supporting this work: Sefako Makgatho Health Sciences University (Madira Coutlyne Manganyi) and Eduvos (Christ Donald Kaptchouang Tchatchouang).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The author(s) verify and take full responsibility for the use of generative AI in preparing this manuscript. AI tools were used to assist with language editing, improve clarity, and support structural organization. All outputs were critically reviewed and approved to ensure scientific accuracy and integrity.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abuhasira R., Shbiro L., and Landschaft Y. (2018). Medical use of Cannabis and cannabinoids containing products – regulations in Europe and North America. Eur. J. Intern. Med. 49, 2–6. doi: 10.1016/j.ejim.2018.01.001
Almeida J. R., Kallscheuer N., and Wriessnegger T. (2023). Engineering filamentous fungi for the production of plantderived terpenoids and cannabinoids. Curr. Opin. Biotechnol. 79, 102927. doi: 10.1016/j.copbio.2023.102927
Andre C. M., Hausman J. F., and Guerriero G. (2016). Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00019
Ceroni F., Boo A., Furini S., Gorochowski T. E., Borkowski O., Ladak Y. N., et al. (2018). Burdendriven feedback control of gene expression. Nat. Methods 15, 387–393. doi: 10.1038/nmeth.4635
Chakraborty S., Datta S., and Saha T. (2021). Cannabis biotechnology: Recent advances and future prospects. Plant Cell Rep. 40, 437–462. doi: 10.1007/s00299020026203
Drysdale M., McLean D., and Johnson S. R. (2022). Engineering Aspergillus Niger for the heterologous production of cannabinoids. Metab. Eng. 70, 88–97. doi: 10.1016/j.ymben.2022.05.007
European Monitoring Centre for Drugs and Drug Addiction (2018). Medical use of Cannabis and cannabinoids: Questions and answers for policymaking (Lisbon: EMCDDA).
Geiselmann J., Ribardière L., Ropers D., and Hersen P. (2022). Unraveling the interplay between feedback regulation and noise in synthetic gene networks. ACS Synth Biol. 11, 2487–2495. doi: 10.1021/acssynbio.2c00083
Grand View Research. (2023). Cannabis market size, share & Trends analysis report, 2021–2028. Available online at: https://www.grandviewresearch.com/industryanalysis/legalmarijuanamarket.
Gülck T. and Møller B. L. (2020). Phytocannabinoids: origins and biosynthesis. Trends Plant Sci. 25, 985–1004. doi: 10.1016/j.tplants.2020.05.005
Hanuš L. O., Meyer S. M., Muñoz E., Taglialatela-Scafati O., and Appendino G. (2016). Phytocannabinoids: a unified critical inventory. Nat. Prod Rep. 33, 1357–1392. doi: 10.1039/C6NP00074F
Hong Y., Gu Y., Lin D., Wu Z., Chen W., Lu T., et al. (2025). De novo biosynthesis of cannabinoid and its analogs in Yarrowia lipolytica. BioDesign Res. 7, 100021. doi: 10.1016/j.bidere.2025.100021
Hudalla C. J., Raber J. C., and Sotiropoulos S. (2024). Regulatory perspectives on cannabinoids produced by engineered microorganisms. Trends Biotechnol. 42, 29–35. doi: 10.1016/j.tibtech.2023.08.003
Jin D., Dai K., Xie Z., and Chen J. (2017). Secondary metabolism in filamentous fungi: regulators, genes and enzymes. Appl. Microbiol. Biotechnol. 101, 261–272. doi: 10.1007/s0025301679411
Karasek D. and Stasiak A. (2021). THCV and CBDV – novel minor cannabinoids with potential therapeutic applications. Eur. J. Pharmacol. 911, 174524. doi: 10.1016/j.ejphar.2021.174524
Keasling J. D., Smolke C. D., and Ro D. K. (2022). Engineered biosynthesis of plant secondary metabolites in microorganisms. Nat. Rev. Microbiol. 20, 245–260. doi: 10.1038/s41579022006525
Keller N. P. (2019). Fungal secondary metabolism: regulation, function and drug discovery. Nat. Rev. Microbiol. 17, 167–180. doi: 10.1038/s4157901801211
Kosalková K., Barreiro C., Sánchez-Orejas I. C., Cueto L., and García-Estrada C. (2023). Biotechnological fungal platforms for the production of biosynthetic cannabinoids. J. Fungi. 9, 234. doi: 10.3390/jof9020234
Liu J., Zhang W., and Chen X. (2023). Engineering microbial cell factories for cannabinoid production: From synthetic biology to fermentation scaleup. Metab. Eng. 75, 101–116. doi: 10.1016/j.ymben.2023.02.006
Luo X., Reiter M. A., d’Espaux L., Wong J., Denby C. M., Lechner A., et al. (2019). Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature. 567, 123–126. doi: 10.1038/s4158601909789
Morales P., Reggio P. H., and Jagerovic N. (2017). An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front. Pharmacol. 8. doi: 10.3389/fphar.2017.00422
Nakagawa A., Minami H., Kim J. S., Koyanagi T., Katayama T., Sato F., et al. (2011). A bacterial platform for fermentative production of plant alkaloids. Nat. Commun. 2, 326. doi: 10.1038/ncomms1327
Nødvig C. S., Nielsen J. B., Kogle M. E., and Mortensen U. H. (2015). A CRISPR-Cas9 system for genetic engineering of filamentous fungi. PloS One 10, e0133085. doi: 10.1371/journal.pone.0133085
Pamplona F. A., da Silva L. R., and Coan A. C. (2018). Potential clinical benefits of CBDrich Cannabis extracts over purified CBD in treatmentresistant epilepsy. Front. Neurol. 9. doi: 10.3389/fneur.2018.00759
Pertwee R. G. (2006). Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol. 147, S163–S171. doi: 10.1038/sj.bjp.0706406
Pisanti S., Malfitano A. M., Ciaglia E., Lamberti A., Ranieri R., Cuomo G., et al. (2017). Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 175, 133–150. doi: 10.1016/j.pharmthera.2017.02.041
Qiu J., Hou K., Li Q., Chen J., Li X., Hou H., et al. (2022). Boosting the cannabidiol production in engineered Saccharomyces cerevisiae by harnessing the vacuolar transporter BPT1. J. Agric. Food Chem. 70, 12055–12064.
Russo E. B. (2011). Taming THC: potential Cannabis synergy and phytocannabinoidterpenoid entourage effects. Br. J. Pharmacol. 163, 1344–1364. doi: 10.1111/j.14765381.2011.01238.x
Santiago M., Sachdev S., Arnold J. C., and McGregor I. S. (2019). Absence of entourage: terpenoids commonly found in Cannabis sativa do not modulate Δ9THC activity at human CB1 and CB2 receptors. Cannabis Cannabinoid Res. 4, 165–176. doi: 10.1089/can.2018.0058
Singh R. and Bhatia S. K. (2022). Valorization of agricultural waste for bioproducts: A strategy toward circular bioeconomy. Bioresour Technol. Rep. 19, 101132. doi: 10.1016/j.biteb.2022.101132
Stone N. L., Murphy A. J., England T. J., and O’Sullivan S. E. (2020). A systematic review of minor phytocannabinoids with promising neuroprotective potential. Bri J. Pharm. 177, 4330–4352. doi: 10.1111/bph.15185
Vogt V., Arentshorst M., and Meyer V. (2021). Genetic engineering of fungal cell factories for natural product biosynthesis. Fungal Biol. Biotechnol. 8, 3. doi: 10.1186/s40694021001170
Keywords: biotechnology, cannabinoid biosynthesis, CRISPR-Cas9, engineered fungi, synthetic biology
Citation: Manganyi MC and Kaptchouang Tchatchouang CD (2025) Biotechnological advancements enabling cannabinoid biosynthesis in engineered fungi: a mini review. Front. Fungal Biol. 6:1660661. doi: 10.3389/ffunb.2025.1660661
Received: 06 July 2025; Accepted: 07 October 2025;
Published: 24 October 2025.
Edited by:
Samantha Chandranath Karunarathna, Qujing Normal University, ChinaReviewed by:
Yuxiang Hong, Technion Israel Institute of Technology, IsraelCopyright © 2025 Manganyi and Kaptchouang Tchatchouang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madira Coutlyne Manganyi, bWFkaXJhLm1hbmdhbnlpQHNtdS5hYy56YQ==
 Madira Coutlyne Manganyi
Madira Coutlyne Manganyi Christ Donald Kaptchouang Tchatchouang2
Christ Donald Kaptchouang Tchatchouang2