- 1Department of Crop Science, University of Ghana, Accra, Ghana
- 2School of Biological Science, Universiti Sains Malaysia, George, Pulau Pinang, Malaysia
- 3Centre for Biological Control (CBC), Department of Zoology and Entomology, Rhodes University, Makhanda, South Africa
- 4Inbioter – Institute of Biotechnology Rangel, Itatiba, Brazil
- 5Alder’s English Services, São José dos Campos, Brazil
- 6Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Negeri Jakarta, Jakarta, Indonesia
- 7Department of Entomology, Washington State University, Pullman, WA, United States
Chili pepper exports from Ghana are subject to stringent chemical residue regulations in key export destinations. Consequently, microbial biopesticides are urgently needed to complement current nonchemical control options for key pests of chili pepper, particularly the phytosanitary insect, False Codling Moth (FCM). Thus, the search for native entomopathogenic fungi in Ghanaian farms was initiated in 2023. Seven Metarhizium isolates (UGSUHCI, UGJKCS9, UGJKCS10, UGAFMF8, UGAFM F12, UGNAKC1 and UGKAP1), obtained from agricultural soils in Ghana, showed high virulence against the soil-dwelling stages of FCM under laboratory conditions. To facilitate the selection of these virulent isolates for development into a mycoinsecticide for FCM, the UV sensitivity and virulence following UV exposure were investigated for all seven isolates in this study. All isolates exhibited extreme susceptibility to UV radiation in comparison to similar research. Exposure to simulated full-spectrum solar radiation at 0.6 W/m2 for 30 min reduced relative conidial germination by 28–40% 48 h following exposure, while 60 min exposure killed all isolates. High insect mortalities were recorded for four isolates, regardless of UV radiation. The findings suggest that an effective UV-protectant formulation could be required for success in the field against fruit and foliar pests of chili pepper, including those of FCM.
1 Introduction
Chili pepper (Capsicum annuum L.) is a key ingredient in daily diets of Ghanaians, making it the fourth most planted crop in the country, with a current annual average of 140,000 MT (GSS, 2014; MoFa-IFPRI, 2020). Besides the high local demand, this crop is one of Ghana’s top export vegetables to the lucrative European Union (EU) market, which increases annually, especially for the Legon 18 variety, known for its exceptional taste and long shelf life (GEPA, 2021). Chili pepper is therefore cultivated year-round in all 16 regions in Ghana, with the Volta, Eastern and Northern regions of Ghana being the highest producers (GEPA, 2018).
False Codling Moth (FCM) (Thaumatotibia leucotreta Meyrick, Lepidoptera: Tortricidae) is the major impediment to export, as this pest is strictly regulated as a phytosanitary organism in the EU (EPPO, 2013), Ghana’s main chili pepper export market. Local production is significantly constrained by this pest, whose larvae develop within fruits, resulting in immature fruit ripening, dropping of fruits and fruit decay, which lead to yield losses (Adom et al., 2023; Adom et al., 2024). The frequent interceptions of this pest resulted in the EU banning the import of chili peppers from Ghana between 2015 and 2017. This, together with the prohibition of two other vegetables (gourd and eggplant), cost the nation an estimated export revenue loss of USD 30 million (EUROPHYT, 2014; Fening et al., 2017). This has resulted in a drastic reduction in chili pepper exports in Ghana, as demonstrated by decreasing export volumes and values between 2010 to 2014 (984,374–1,079,882 kg with corresponding earnings of USD 350,442–1,184,964 (GEPA, 2021) compared to 2018 to 2021 (USD 351,000–87,000) (GEPA, 2018; GIRSAL, 2025).
The use of synthetic pesticides, which remains the main control method for FCM in Ghana, has not been sufficient, partly due to the narrow window for controlling the inconspicuous eggs and neonates of FCM on fruits. Apart from unsatisfactory control with conventional pesticides, they are also stringently regulated by the key export markets and have adverse effects on the environment, non-target organisms, and human health (Cech et al., 2023; Wan et al., 2025). Therefore, effective and sustainable nonchemical control options are needed to control this pest in Ghana. Although commercially available Bacillus thuringiensis (Bt)-based products in Ghana have been proven useful in the control of the above-ground life stages of FCM (Adom et al., 2023), additional control agents for the non-feeding soil-dwelling life stages of the pest are needed, leading to the search for native entomopathogenic fungi (EPF).
Seven native EPF obtained from agricultural soils in Ghana (Table 1) have shown promise as control agents for the soil-dwelling stages of FCM under laboratory conditions, inducing over 80% pupal mortality of FCM (Acheampong M.A. Unpublished data). However, abiotic environmental constraints, particularly ultraviolet (UV) radiation, are well documented to be among the key efficacy impeding factors of EPF in the field and must be factored into the isolate selection process (Braga et al., 2001a; Posadas et al., 2012; Kaiser et al., 2019; Acheampong et al., 2020a).
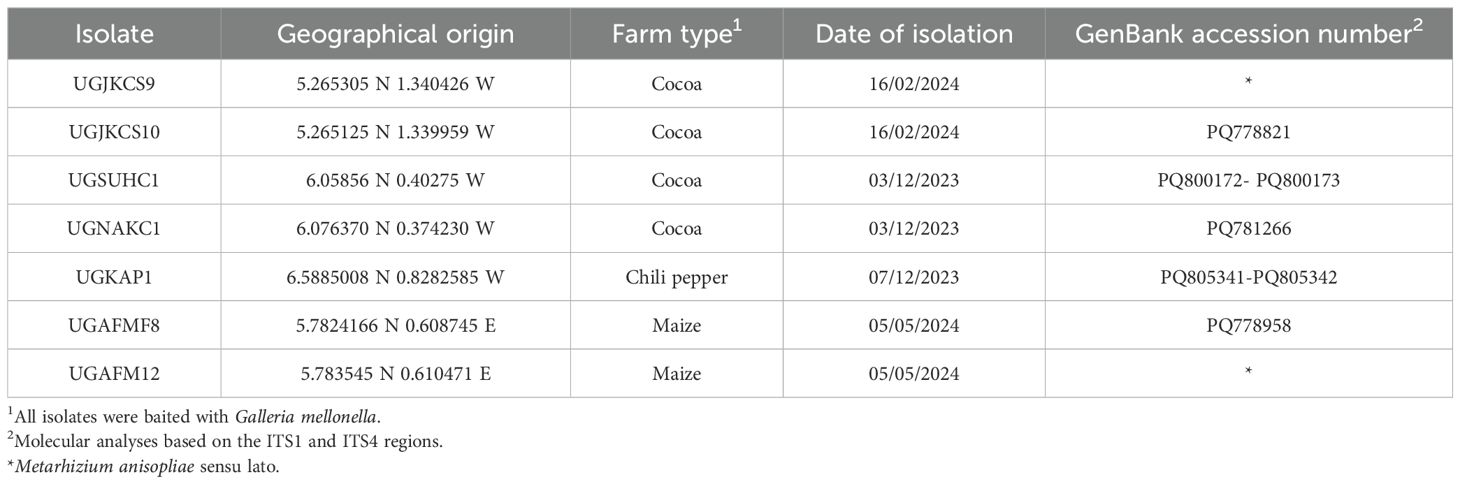
Table 1. Origin and molecular analyses of seven soil-derived Ghanaian Metarhizium spp. used in the study.
Among the UV radiation emitted from sunlight, UV-B is the most damaging to entomopathogens (Rangel and Roberts, 2018), inhibiting replication and inducing mutations and cellular mortality (Rangel et al., 2006; Nascimento et al., 2010; Wang et al., 2019), whereas UV-A exposure stimulates the generation of detrimental radicals, which deactivate propagules (Rangel et al., 2006). Nevertheless, the susceptibility of EPF to UV radiation is isolate and species dependent (Fernandes et al., 2007; Posadas et al., 2012; Fernandes et al., 2015; Acheampong et al., 2020a; Licona-Juárez et al., 2023; Rangel et al., 2023). Consequently, identifying UV-resilient EPF strains and formulating them with appropriate UV protectants can enhance their persistence in UV-exposed environments, resulting in greater efficacy against pests (Posadas et al., 2012; Fernandes et al., 2015; Kaiser et al., 2019). While UV-radiation may not be the most inimical abiotic factor for the EPF when applied to control the soil-dwelling stages, the biopesticide product developed will ultimately be used to also target above-ground stages of FCM and other foliar pests of pepper. Thus, this research investigated the UV tolerance of all seven promising native EPF isolates to select the most suitable for the chili pepper environment.
2 Materials and methods
2.1 Source of insects, fungal isolates and culture conditions
All seven native Metarhizium isolates were obtained from the Entomopathology Laboratory of the African Regional Postgraduate Programme in Insect Science (ARPPIS), University of Ghana, where conidia had been stored on Sabouraud dextrose agar (SDA) slants at 4°C. These EPF were isolated from soils from chili pepper, maize and cocoa farms in the Central, Eastern and Greater Accra regions of Ghana using Galleria mellonella (Lepidoptera: Pyralidae) (Goble et al., 2010) (Table 1). All isolates were passed through FCM fifth instar larvae once before use, following the protocol of Acheampong et al. (2020a). Cadaver cultures were maintained on SDA medium supplemented with 50 mg/L chloramphenicol (SDAC) and kept at 4°C, serving as stock cultures for the UV assays. The FCM final (fifth) instar larvae used in this study were obtained from the Centre for Biological Control, Rhodes University, Makhanda, South Africa, where a continuous rearing culture of this insect is held using an artificial larval diet (Moore et al., 2014).
2.2 Simulated solar radiation device
Irradiation tests were carried out in a Q-SUN® Xe-3-HC (Q-Lab Corporation, Westlake, OH, USA) solar radiation simulator. The Q-SUN® reproduces full-spectrum solar radiation from 295 to 780 nm, using three 1800 W Xenon arc lamps and a Daylight-Q filter, which excludes radiation below 295 nm (Dias et al., 2018). The uniform spectral distribution on irradiation surfaces produced by Q-SUN® lamps, enhanced by mirrored walls, facilitates reproducible results. The strong correlation of these lamps to sunlight is well established (https://www.q-lab.com) and recently validated in assessing UV tolerances of EPF (Luo et al., 2017; Dias et al., 2018; Acheampong et al., 2020a).
The Q-SUN® was calibrated to 0.6 W/m2 irradiance at a temperature of 23.2 ± 0.66°C and relative humidity (RH) of 63 ± 10.4% RH. This irradiance set point is less than the average annual daily solar radiation in Ghana of 4–6 kWh/m2 (Edjekumhene and Brew-Hammond, 2001; Aboagye et al., 2021). The Quaite-weighted (biologically effective UV dosage capable of DNA damage in some fungi including EPF) (Quaite et al., 1992; Braga et al., 2001b) irradiance in the Q-SUN® at 0.6 W/m2 is 1335 mW/m2, which also approximates noon irradiance during summer in Sao Jose dos Campos (Luo et al., 2017; Dias et al., 2018), South-Eastern Brazil (Dias et al., 2018), with a similar climate to Southern Ghana. Thus, the irradiance used could be lower than the Quaite-weighted noon irradiance in Ghana’s chili pepper producing regions. In a previous study, 3 h of exposure to simulated full-spectrum solar radiation in the Q-SUN® at 0.6 W/m2 killed over 90% of conidia of 11 tested EPF isolates (ARSEF collections of Aschersonia aleyrodis (Brazil); Beauveria bassiana, Isaria fumosorosea, Metarhizium robertsii and Tolypocladium inflatum from USA; Lecanicillium aphanocladii from Brazil; Marianneae pruinose from China, M. anisopliae s.l. from Mexico, M. brunneum from New Zealand, Simplicillium lanosoniveum from French Guiana, and T. cylindrosporum from Nepal) after 48 h of exposure, whilst germination in their non-irradiated controls exceeded 95% after 24 h (Dias et al., 2018). This result demonstrated that the selected irradiance set point and a maximum exposure duration of 2 h were appropriate for determining the tolerance of these EPF.
2.3 Effect of simulated solar radiation on conidial germination of the isolates
Conidial viability of suspensions to be applied was determined by plating aliquots of conidial suspension [50 mL of 105 conidia/mL from 14-d-old cultures suspended in Tween 20 (0.01% v/v)] onto SDA medium in three replicate Petri plates (Polystyrene, 60 × 15 mm). Plates were incubated at 26 ± 1°C for 12 h, after which the germinated and non-germinated conidia per plate, out of 300 conidia, were evaluated. Germination was assessed at 400× magnification; conidia were considered germinated when the germ tube was longer than the diameter of the conidium (Rangel et al., 2005).
Only three fungal isolates and their control were irradiated on each occasion due to the limited space in the Q-SUN®. Stock cultures of each isolate were sub-cultured on SDAC and incubated for 12–15 days at 27°C, 60% RH, on a 12 h photoperiod. Conidia produced were then harvested from colonies, suspended in sterile distilled water supplemented with 0.01% Tween 20, and adjusted to 1 × 105 conidia/mL. For each isolate, a 50 µL suspension was spread across a 60 mm SDA Petri plate in four replicates for four exposure periods, including controls. Aluminum foil was used to wrap control plates to block UV radiation (Braga et al., 2001a; Acheampong et al., 2020a). The Petri plates were exposed to simulated full-spectrum solar radiation in the Q-SUN® within 30 min after inoculation at 0.6 W/m2 for 15, 30, 60 and 120 min, corresponding to total doses of 1.20, 2.40, 4.81 and 9.61 kJ/m2, respectively. Following irradiation, plates were incubated in the dark at 20 ± 1°C. The number of germinated (conidia with germ tubes) and non-germinated conidia per plate, out of 300 conidia, was assessed 24 h and 48 h following irradiation, using an optical microscope. The entire experiment was repeated three times for each isolate, using fresh conidial suspension.
2.4 Effect of simulated solar radiation on the virulence of fungal isolates
For each isolate, 1.5 mL conidial suspension (106 conidia/mL suspended in 0.01% Tween 20) in a 10 mL sterile glass vial was exposed to the same dose and irradiance period as the UV tolerance assay. After exposure, the vial was vortexed for 1 min, and 0.1 mL of suspension was then pipetted and spread in a 60 mm sterile plastic Petri plates lined with sterile filter paper at four replicates. Ten FCM fifth instar larvae were immediately added to each Petri plate and incubated for 14 days at 25°C. Non-irradiated inoculated vials of each isolate (wrapped with aluminum foil to block UV radiation) and non-inoculated control insects (in sterile Petri plates lined with filter paper and treated with 0.1 mL of 0.01% Tween 20) served as controls. The number of dead and live insects was assessed daily for 14 days after treatment. Death due to mycosis was verified by surface sterilizing cadavers in 0.5% sodium hypochlorite (3.5% active ingredient), followed by 70% ethanol for 2 min each and kept in Petri plates lined with filter papers, and moistened with sterile water for seven days at 25°C. The entire experiment was repeated twice.
2.5 Statistical analyses
The percentage of germination of all isolates relative to control plates was calculated according to Acheampong et al. (2020a). The 60- and 120-min fungal exposure data for both UV and pathogenicity assays were excluded from the analysis as conidia of all isolates were killed at both periods. The relative germination data were analyzed using a generalized linear model (GLM) with gamma error distribution (link= ‘inverse’), which produced the best goodness of fit (lowest Akaike Information Criteria value) based on Likelihood Ratio Test (LRT) (Acheampong et al., 2020a). A three-way analysis of deviance (ANODEV) was applied to the model and contrasted using the ‘emmeans’ R package (Lenth, 2024), adjusted with Tukey’s HSD test (P ≤ 0.05). The cumulative FCM larval, pupal and adult mortality data at each exposure over the 14-day period were pooled and firstly fitted to a logistic regression in a GLM with binomial error distributions to determine an interaction effect. The mortality data for each exposure period were then subjected to logistic regression followed by pairwise comparison of treatment means using ‘emmeans’, adjusted with Tukey’s HSD test (P ≤ 0.05), where statistical differences were noted. All analyses were done in R version 4.4.2 (R Core Team, 2024).
3 Results
3.1 Effect of simulated solar radiation on conidial germination of the isolates
The three-way ANODEV showed that only fungal isolate (LRT, χ2 = 0.38, df = 6, P < 0.001) and the exposure period (LRT, χ2 = 1.67, df = 1, P < 0.001) significantly influenced conidial germination. The incubation period (LRT, χ2 = 0.01, df = 1, P = 0.182) and associated interactions [fungal isolate × incubation (LRT, χ2 = 0.01, df = 6, P = 0.967), incubation period × exposure period (LRT, χ2 = 0.00, df = 1, P = 0.586), fungal isolate × exposure period × incubation period (LRT, χ2 = 0.00, df = 6, P = 0.999)] were not significant. However, fungal isolate × exposure period (LRT, χ2 = 0.09, df = 6, P = 0.011) was significant.
Exposure to simulated solar radiation for 15 min (1.2 kJ/m2) had little impact on four fungal isolates, with their relative germination exceeding 82% after 24 h of incubation (Figure 1A). UGAFMF8 was the most susceptible isolate at this exposure time, with 72% relative germination 24 h following incubation.
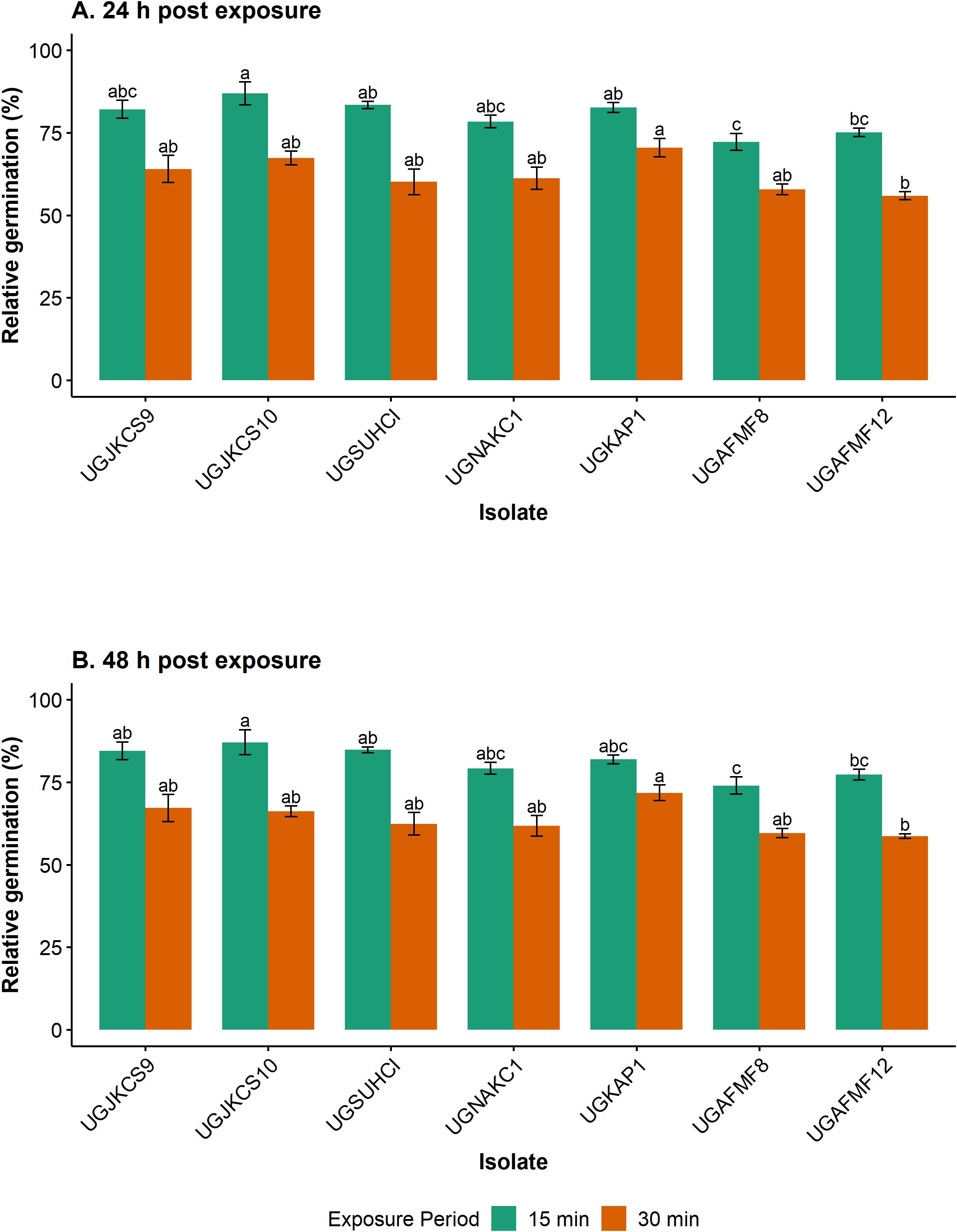
Figure 1. Relative percentage germination of seven Metarhizium isolates after exposure to simulated solar radiation (Xenon arc lamps from 295 to 780 nm at 0.6 W/m2, 28 ± 1°C and 46 ± 3.19% RH) for 0 (control), 15 (1.2 kJ/m2) and 30 (2.4 kJ/m2) min, and incubated for 24 (A) and 48 (B) h at 20 ± 1°C. Error bars are the standard errors of three independent experiments with a fresh batch of conidia. All statistical comparisons were done for each exposure period (but not between each isolate at the two exposure periods). Means within each exposure period with the same lowercase letters are not significantly different (‘emmeans’ adjusted with Tukey’s HSD test, P > 0.05).
Exposure for 30 min (2.4 kJ/m2) reduced conidial germination of isolates by 29–44% after 24 h of incubation. There were, however, indiscernible differences in susceptibility among six of these isolates at this exposure period. UGAFMF12 was the most susceptible isolate at this exposure period, only differing significantly from UGKAP1.
For both exposure periods, the incubation of isolates for a further 24 h increased germination only marginally. The relative germination of isolates ranged from 74–87% and 60–72% for the 15- and 30-min exposure periods, respectively, after 48 h of incubation (Figure 1B). Exposure to simulated solar radiation for 60 min (4.81 kJ/m2) and 120 min (9.61 kJ/m2) killed conidia of all tested isolates.
3.2 Effect of simulated solar radiation on the virulence of the isolates
Fungal isolate (LRT, χ2 = 675.88, df = 14, P < 0.001) and exposure period (LRT, χ2 = 9.80, df = 1, P = 0.002) significantly influenced pupal mortality. However, their interaction was not significant (LRT, χ2 = 17.82, df = 14, P = 0.215).
For each isolate, insect mortality induced by irradiated conidia for 15 (1.2 kJ/m2) and 30 (2.4 kJ/m2) min was not significantly different from the non-irradiated control, with the exception of UGJKCS10, whose mortality in both treatments differed at the shortest exposure period (Figure 2). High pupal mortalities were recorded for four isolates (UGJKCS9, UGJKCS10, UGAFMF8 and UGAFMF12) regardless of UV radiation. Mortality induced by these isolates ranged from 73–82% and 67–75% at 15- and 30-min exposures, respectively. The mortality of the insects in the control, which was neither irradiated nor inoculated, remained at 5% and was significantly lower than all treatments in all bioassays (Figure 2).
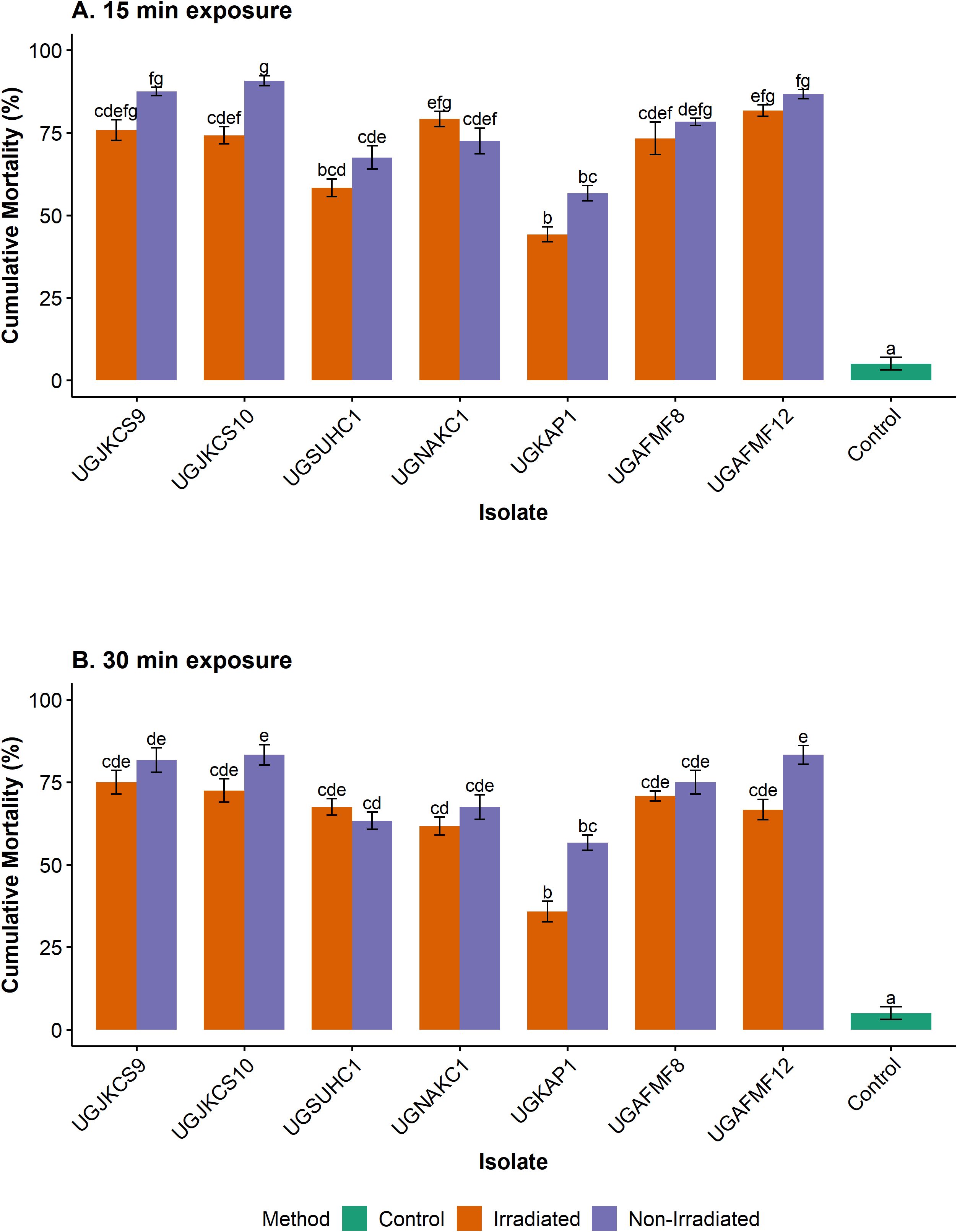
Figure 2. Cumulative percentage mortality of larvae, pupae and adults of T. leucotreta treated with Metarhizium isolates exposed to simulated solar radiation (Xenon arc lamps from 295 to 780 nm at 0.6 W/m2, 28 ± 1°C and 46 ± 3.19% RH) for 0 (control), 15 (A) and 30 (B) min and incubated for 14 days at 25°C. Error bars are the standard errors of three independent experiments with a fresh batch of conidia. Means within each exposure period with the same lowercase letters are not significantly different (‘emmeans’ adjusted with Tukey’s HSD test, P > 0.05).
4 Discussion
UV radiation is well established to be the most important abiotic environmental constraint to the efficacy of biopesticides in the field. However, the location of the targeted insect pest could help prioritize superior UV protectants in the formulation stage of biopesticide development. While UV radiation may not be the most important abiotic efficacy impeding factor for EPF applied to control the soil-dwelling stages (pre-pupating final larvae, pupae) of FCM in this research, other fruit and foliar pests of pepper, particularly thrips, aphids, whiteflies and fruit flies, are equally important and would be targeted. EPF have been used to successfully suppress thrips (Arthurs et al., 2013; Panyasiri et al., 2022), aphids (Mantzoukas et al., 2022) and whiteflies (Avery et al., 2020; Zulfitri et al., 2020) on chili pepper plants in other countries. Furthermore, the above-ground stages of the targeted pest of this research (adults, eggs and neonates) ought to be controlled, hence the need to factor UV resilience in the strain selection.
All indigenous isolates tested in this study exhibited extreme sensitivity to UV radiation, which generally aligns with other EPF-UV sensitivity studies (Braga et al., 2001a; Posadas et al., 2012; Kaiser et al., 2019; Acheampong et al., 2020a). Nonetheless, the total inactivation after only 1 h of exposure contradicts previous findings using the same simulated sunlight device and irradiance (Dias et al., 2018) and others where propagules were completely killed or had a > 50% reduction in viability only after 2–8 h of exposure using monochromatic (Braga et al., 2001a, Braga et al., 2002; Fernandes et al., 2007; Santos et al., 2011; Kaiser et al., 2019) and polychromatic (Alves et al., 1998; Leland and Behle, 2005; Luo et al., 2017) light sources, even at higher irradiances. It is also acknowledged that isolates inherent genetic variability, and geographical origin, in addition to methodological differences (formulation status, culture age, conidial densities and condition in storage and prior to irradiation, amongst others) in some of the aforementioned studies could account for the differences in UV sensitivities compared to the present study.
Exposure to simulated solar radiation for both 15 and 30 min had no impact on the pathogenicity of all seven EPF isolates investigated in this study. These findings corroborate those of Fernández-Bravo et al (2017); Fernández-Bravo et al, 2024) who reported a negative correlation between loss of viability and infection potential against Mediterranean fruit fly (Ceratitis capitata) of three Beauveria bassiana (EABb 10/225-Fi, EABb 09/20-Fi and EABb 09/28-Fil) and a Metarhizium brunneum (EAMa 01/58-Su) isolates, following exposure to UV-B radiation (1200 mW/m2) for 6 h. A similar insignificant effect of UV radiation (UV-A and UV-B) on mortality of FCM was reported with a B. bassiana and three Metarhizium spp. under laboratory conditions by Rossouw et al. (2023). Likewise, 8 h of UV-A exposure (31.514 mW/m2) of Leptolegnia chapmanii zoospores did not affect its in vitro virulence against yellow fever mosquito (Aedes aegypti) larvae (Páramo et al., 2015). However, Rossouw et al. (2023) recorded low persistence and mortalities in field trials with unformulated (aqueous conidial suspension) isolates and highlighted the need for an appropriate UV-protectant formulation to enhance field persistence and efficacy.
Although the most UV-tolerant isolate was elucidated at 30 min, isolates may need to persist for longer than this period to achieve success against insect pests in the phyllosphere environment of chili pepper in Ghana. However, the microclimate within the hypogeal environment of chili pepper could be conducive for infection of the soil-dwelling stages of FCM due to possible shade protection by the canopy of this plant, as reported in other crop systems. For instance, Betabaculovirus cryleucotreta, which is a virus of FCM, exhibited better persistence on the southern side of the trees in Hermitage Farm (33°32’02” S and 25°40’13” E) in the Sunday’s River Valley, Eastern Cape, South Africa, as opposed to the northern sides (which receives higher UV exposure) because of some protection afforded by the trees themselves (shade) (Mwanza, 2015). In recent years, EPF isolates occurring in the phyllosphere environment have been sought after due to their perceived greater tolerance to UV radiation and heat than isolates obtained from soils, with some evidence (Vidal et al., 1997; Bidochka et al., 2001; Braga et al., 2001b; Bidochka et al., 2002; Jaronski, 2010). However, contrary reports exist (Fargues et al., 1996, Fargues et al., 1997; Leland and Behle, 2005; Fernandes et al., 2007; Fernández-Bravo et al., 2016; Acheampong et al., 2020a; Acheampong et al, 2020b). This indicates that UV tolerance could depend more on the isolate than the geoclimatic origins or isolation habitats.
Given the high UV susceptibility of these isolates, a suitable formulation will need to be identified if they are to be used in a commercial setting. Greenyield Ltd (Pato Branco, Paraná, Brazil) has developed a novel adjuvant product, Green Turbo®, for the biopesticide industry. This product, which contains extracts of algae, plants and essential oils, provided excellent in vitro photoprotection of conidia of some entomopathogenic and mycoparasitic fungi (Acheampong, M. A. Unpublished data). The protection of propagules against UV radiation of this formulation, although yet to be established, has been attributed to the potential mycosporines and mycosporine-like amino acids (MAA) produced as secondary metabolites by the algae, coupled with the oils. The exact oils used in Green Turbo® formulation are not known; however, several mineral and vegetable oils are well documented to be capable of protecting propagules of EPF against UV radiation (Leland and Behle, 2005; Posadas et al., 2012; Kaiser et al., 2019; Acheampong et al., 2020a) and are widely utilized in the biopesticide industry. Similarly, mycosporines and MAA are well-known organic sunscreens produced by algae and other organisms (Katoch et al., 2016; Chrapusta et al., 2017; Geraldes and Pinto, 2021; Punchakara et al., 2023) and are widely used in the pharmaceutical and cosmetic industries (Chrapusta et al., 2017; Rosic, 2019; Thiyagarasaiyar et al., 2020). Thus, the new UV-protectant adjuvant formulation, Green Turbo®, is currently being investigated for potential photoprotection of the EPF isolates studied. Nonetheless, the UV sensitivity findings in this study indicate that application of isolates at sunset could ensure persistence on foliage and fruits for infection of nocturnal stages of the main targeted insect pest of this research (FCM), provided attachment of conidia to penetration of the insect host occurs within 24 h as proposed (Jaronski, 2010) and observed in Anastrepha fraterculus (Wiedemann; Diptera: Tephritidae) (Bechara et al., 2011).
5 Conclusions
The manuscript highlights the potential of seven native Metarhizium spp. isolates from Ghana as biological control agents against FCM affecting chili pepper. The main perspective of the study is that, despite their promising virulence against the soil-dwelling stages of FCM, all isolates exhibited extreme sensitivity to UV radiation, with complete conidial inactivation occurring after just one hour of simulated solar exposure. However, the pathogenicity of the isolates remained unaffected at lower exposure durations (15–30 min), suggesting their short-term viability in field conditions. The study emphasizes the importance of developing UV-protectant formulations or applying the isolates during low UV periods (e.g., at sunset) to potentially enhance persistence and infection success against above-ground pests. The key limitation is the poor UV tolerance of the isolates, which significantly hinders their potential as stand-alone biopesticides under natural sunlight, thereby requiring further research on formulation and application strategies to ensure practical field application.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Ethics Committee for Basic and Applied Sciences of the University of Ghana (protocol code ECBAS 075/22-23; approved on 26/09/2023). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PN: Methodology, Investigation, Writing – review & editing, Writing – original draft. VE: Methodology, Supervision, Writing – review & editing, Validation, Writing – original draft. LA-A: Resources, Validation, Writing – review & editing, Writing – original draft, Methodology. MA: Investigation, Writing – original draft, Formal analysis, Writing – review & editing. CC: Writing – original draft, Writing – review & editing, Formal analysis, Resources, Validation, Methodology. DR: Visualization, Writing – original draft, Methodology, Writing – review & editing, Validation. AA-R: Resources, Writing – original draft, Visualization, Writing – review & editing. DS: Writing – original draft, Resources, Methodology, Validation, Writing – review & editing. OA: Validation, Writing – review & editing, Methodology, Formal analysis, Resources, Visualization, Writing – original draft. MA: Writing – original draft, Conceptualization, Supervision, Investigation, Visualization, Project administration, Funding acquisition, Writing – review & editing, Validation, Formal analysis, Resources, Methodology.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was carried out with the aid of a grant from UNESCO and the International Development Research Centre (IDRC), Canada (Grant Number 4500476458). The views expressed herein do not necessarily represent those of UNESCO-TWAS, OWSD (Organization for Women in Science for the Developing World), IDRC or its Board of Governors. The article processing fee was funded by OWSD. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”. We sincerely thank the National Council for Scientific and Technological Development (CNPq) of Brazil for the productivity fellowships PQ1D 302100/2018-0 and PQ1D 302282/2022-0 awarded to DR.
Acknowledgments
We are grateful for the technical assistance with the Q-SUN® provided by Mr. Osei Ofosu and Miss Bulelwa Mntanya of the Center for Nano-structured and Advanced Materials (CeNAM) in the Chemicals Cluster Group of CSIR, Pretoria, South Africa during the trials. We thank Dr. Honest Machekano of the Faculty of Natural and Agricultural Sciences, University of Pretoria for his invaluable help with logistics for the trials.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aboagye B., Gyamfi S., Ofosu E. A., and Djordjevic S. (2021). Status of renewable energy resources for electricity supply in Ghana. Sci. Afr. 11, e00660. doi: 10.1016/j.sciaf.2020.e00660
Acheampong M. A., Hill M. P., Moore S. D., and Coombes C. A. (2020a). UV sensitivity of Beauveria bassiana and Metarhizium anisopliae isolates under investigation as potential biological control agents in South African citrus orchards. Fungal Biol. 124, 304–310. doi: 10.1016/j.funbio.2019.08.009, PMID: 32389292
Acheampong M. A., Hill M. P., Moore S. D., and Coombes C. A. (2020b). Temperature tolerance and humidity requirements of select entomopathogenic fungal isolates for future use in citrus IPM programmes. J. Invertebr. Pathol. 174, 10743. doi: 10.1016/j.jip.2020.107436, PMID: 32619548
Adom M., Fening K. O., Billah M. K., Aigbedion-Atalor P. O., Acheampong. M. A., and Wilson D. D. (2024). Susceptibility of Capsicum varieties to Thaumatotibia leucotreta (Lepidoptera: Tortricidae) infestation for production optimization. J. Econ. Entomol. 117, 2567–2576. doi: 10.1093/jee/toae213, PMID: 39350334
Adom M., Fening K. O., Billah M. K., Aigbedion-Atalor P. O., and Wilson D. D. (2023). Efficacy of selected biopesticides on key pests of chilli pepper for increased productivity in Ghana. Crop Prot. 176, 106497. doi: 10.1016/j.cropro.2023.106497
Alves R. T., Bateman R. P., Prior C., and Leather S. R. (1998). Effects of simulated solar radiation on conidial germination of Metarhizium anisopliae in different formulations. Crop Prot. 17, 675–679. doi: 10.1016/S0261-2194(98)00074-X
Arthurs S. P., Fernando A. L., and Bruce A. P. (2013). Evaluation of entomopathogenic fungi against chilli thrips, Scirtothrips dorsalis. J. Insect Sci. 13, 31. doi: 10.1673/031.013.3101, PMID: 23895429
Avery P. B., Kumar V., Antonio F., McKenzie C. L., and Osborne L. S. (2020). Compatibility of the predatory beetle, Delphastus catalinae, with an entomopathogenic fungus, Cordyceps fumosorosea, for biocontrol of invasive pepper whitefly, Aleurothrixus trachoides, in Florida. I. nsects 11, 590. doi: 10.3390/insects11090590, PMID: 32882941
Bechara I. J., Destéfano R. H. R., Bresil C., and Messias C. L. (2011). Histopathological events and detection of Metarhizium anisopliae using specific primers in infected immature stages of the fruit fly Anastrepha fraterculus (Wiedeman) (Diptera: Tephritidae). Braz. J. Biol. 71, 91–98. doi: 10.1590/S1519-69842011000100014, PMID: 21437404
Bidochka M. J., Kamp A. M., Lavender T. M., Dekoning J., and De Croos J. N. (2001). Habitat association in two genetic groups of the insect pathogenic fungus Metarhizium anisopliae: uncovering cryptic species? Appl. Environ. Microbiol. 67, 1335–1342. doi: 10.1128/AEM.67.3.1335-1342.2001, PMID: 11229929
Bidochka M. J., Menzies F. V., and Kamp A. M. (2002). Genetic groups of the insect-pathogenic fungus Beauveria bassiana are associated with habitat and thermal growth preferences. Arch. Microbiol. 178, 531–537. doi: 10.1007/s00203-002-0490-7, PMID: 12420176
Braga G. U., Flint S. D., Messias C. L., Anderson A. J., and Roberts D. W. (2001a). Effect of UV-B on conidia and germlings of the entomopathogenic hyphomycete Metarhizium anisopliae. Mycol. Res. 105, 874–882. doi: 10.1017/S0953756201004270
Braga G. U. L., Flint S. D., Miller C. D., Anderson A. J., and Roberts D. W. (2001b). Variability in response to UV-B among species and strains of Metarhizium isolated from sites at latitudes from 61°n to 54°s. J. Invertebr. Pathol. 78, 98–108. doi: 10.1006/jipa.2001.5048, PMID: 11812112
Braga G. U. L., Rangel D. E. N., Flint S. D., Miller C. D., Anderson A. J., and Roberts D. W. (2002). Damage and recovery from UV-B exposure in conidia of the entomopathogens Verticillium lecanii and Aphanocladium album. Mycologia 94, 912e920. doi: 10.2307/3761859, PMID: 21156565
Cech R., Zaller J. G., Lyssimachou A., Clausing P., Hertoge K., and Linhart C. (2023). Pesticide drift mitigation measures appear to reduce contamination of non-agricultural areas, but hazards to humans and the environment remain. Sci. Total Environ. 854, 158814. doi: 10.1016/j.scitotenv.2022.158814, PMID: 36115411
Chrapusta E., Kaminski A., Duchnik K., Bober B., Adamski M., and Bialczyk J. (2017). Mycosporine-like amino acids: potential health and beauty ingredients. Mar. Drugs 15, 326. doi: 10.3390/md15100326, PMID: 29065484
Dias L. P., Araújo C. A. S., Pupin B., Ferreira P. C., Braga G. U. L., and Rangel D. E. N. (2018). The Xenon test chamber Q-SUN® for testing realistic tolerances of fungi exposed to simulated full spectrum solar radiation. Fungal Biol. 122, 592e601. doi: 10.1016/j.funbio.2018.01.003, PMID: 29801804
Edjekumhene I. and Brew-Hammond A. (2001)Barriers to the use of RET for sustainable development in Ghana in Proceedings of the african high-level regional meeting on energy and sustainable development for the 9th session of the commission on sustainable development, roskilde, vol. 2001)Ed. Wamukonya N.. Roskilde, UNEP Collaborating Centre on Energy and Environment.
EPPO (2013). Pest risk analysis for Thaumatotibia leucotreta (Paris: EPPO). Available online at: http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm.
EUROPHYT (European Union Notification System for Plant Health Interceptions) (2014). Annual report. Available online at: https://foodlawlatest.com/2015/10/07/europhyt-annual-report-2014-plant-health-interceptions-in-eu/ (Accessed June 30, 2025).
Fargues J., Goettel M. S., Smits N., and Ouedraogo A. (1997). Rougier M. Effect of temperature on vegetative growth of Beauveria bassiana isolates from different origins. Mycologia 89, 383–392. doi: 10.1080/00275514.1997.12026797
Fargues J., Goettel M. S., Smits N., Ouedraogo A., Vidal C., Lacey L. A., et al. (1996). Variability in susceptibility to simulated sunlight of conidia among isolates of entomopathogenic hyphomycetes. Mycopathologia 135, 171–181. doi: 10.1007/BF00632339, PMID: 20882453
Fening K. O., Billah M. K., and Kukiriza C. N. (2017). “Roadmap for pest reduction in Ghana’s export vegetab le sector,” in GhanaVeg Sector Reports 2017 (GhanaVeg, Accra, Ghana).
Fernandes É.K., Rangel D. E., Braga G. U., and Roberts D. W. (2015). Tolerance of entomopathogenic fungi to ultraviolet radiation: a review on screening of strains and their formulation. Curr. Genet. 61, 427–440. doi: 10.1007/s00294-015-0492-z, PMID: 25986971
Fernandes E. K., Rangel D. E., Moraes A. M., Bittencourt V. R., and Roberts D. W. (2007). Variability in tolerance to UV-B radiation among Beauveria spp. isolates. J. Invertebr. Pathol. 96, 237–243. doi: 10.1016/j.jip.2007.05.007, PMID: 17610892
Fernández-Bravo M., Bonnet J., Quesada-Moraga E., and Garrido-Jurado I. (2024). Imperfect match between radiation exposure times required for conidial viability loss and infective capacity reduction attenuate UV-B impact on Beauveria bassiana. Pest Manage. Sci. 80, 1557–1565. doi: 10.1002/ps.7889, PMID: 37964642
Fernández-Bravo M., Flores-León A., Calero-López S., Gutiérrez-Sánchez F., Valverde-García P., and Quesada-Moraga E. (2017). UV-B radiation-related effects on conidial inactivation and virulence against Ceratitis capitata (Wiedemann) (Diptera; Teph-ritidae) of phylloplane and soil Metarhizium sp. strains. J. Invertebr. Pathol. 148, 142–151. doi: 10.1016/j.jip.2017.06.012, PMID: 28668256
Fernández-Bravo M., Garrido-Jurado I., Valverde-García P., Enkerli J., and Quesada-Moraga E. (2016). Responses to abiotic environmental stresses among phylloplane and soil isolates of Beauveria bassiana from two holm oak ecosystems. J. Invertebr. Pathol. 141, 6–17. doi: 10.1016/j.jip.2016.09.007, PMID: 27693652
GEPA (Ghana Export Promotion Authority) (2018). Sector capabilities-Chili pepper from Ghana. Available online at: https://www.gepaGhana.org/import/Ghana-product/chili-pepper-Ghana/ (Accessed June 30, 2025).
GEPA (Ghana Export Promotion Authority) (2021). Statistics on performance of exportable e fruits and vegeta bles annual report 2010–2021 (Accra, Ghana: Ghana Export Promotion Authority).
Geraldes V. and Pinto E. (2021). Mycosporine-like amino acids (MAAs): Biology, chemistry and identification features. Pharmaceuticals 14, 63. doi: 10.3390/ph14010063, PMID: 33466685
GIRSAL (Ghana Incentive-Based Risk Sharing System. for Agricultural Lending) (2025). Reviving Ghana’s fresh chili pepper export industry: Commercial chili production trial net houses by GIRSAL and partners. Available online at: https://www.girsal.com/2023/04/12/reviving-Ghanas-fresh-chili-pepper-export-industry-a-commercial-trial-of-production-of-chili-in-net-houses-by-girsal-and-partners/ (Accessed June 30, 2025).
Goble T. A., Dames J. F., Hill M. P., and Moore S. D. (2010). The effects of farming system, habitat type and bait type on the isolation of entomopathogenic fungi from citrus soils in the Eastern Cape Province, South Africa. BioControl 55, 399–412. doi: 10.1007/s10526-009-9259-0
GSS (Ghana Statistical Service) (2014). Ghana living standards survey report of the sixth round (GLSS6). Available online at: https://statsGhana.gov.gh/gssmain/fileUpload/Living%20conditions/GLSS6_Main%20Report.pdf (Accessed June 30, 2025).
Jaronski S. T. (2010). Ecological factors in the inundative use of fungal entomopathogens. BioControl 55, 159–185. doi: 10.1007/s10526-009-9248-3
Kaiser D., Bacher S., Mène-Saffrané L., and Grabenweger G. (2019). Efficiency of natural substances to protect Beauveria bassiana conidia from UV radiation. Pest Manage. Sci. 75, 556–563. doi: 10.1002/ps.5209, PMID: 30221461
Katoch M., Mazmouz R., Chau R., Pearson L. A., Pickford R., and Neilan B. A. (2016). Heterologous production of cyanobacterial mycosporine-like amino acids mycosporine-ornithine and mycosporine-lysine in Escherichia coli. Appl. Environ. Microbiol. 82, 6167–6173. doi: 10.1128/AEM.01632-16, PMID: 27520810
Leland J. E. and Behle R. W. (2005). Coating Beauveria bassiana with lignin for protection from solar radiation and effects on pathogenicity to Lygus lineolaris (Heteroptera: Miridae). Biocontrol Sci. Technol. 15, 309–320. doi: 10.1080/09583150400016936
Lenth R. V. (2024). “emmeans: Estimated marginal means, aka least-squares means,” in R package version 1.10.5-0900003, vol. 2024. . Available online at: https://rvlenth.github.io/emmeans/.
Licona-Juárez K. C., Andrade E. P., Medina H. R., Oliveira J. N., Sosa-Gómez D. R., and Rangel D. E. (2023). Tolerance to UV-B radiation of the entomopathogenic fungus Metarhizium rileyi. Fungal Biol. 127, 250–1258. doi: 10.1016/j.funbio.2023.04.004, PMID: 37495315
Luo Z., Ren H., Mousa J. J., Rangel D. E. N., Zhang Y., Bruner S. D., et al. (2017). The PacC transcription factor regulates secondary metabolite production and stress response, but has only minor effects on virulence in the insect pathogenic fungus Beauveria bassiana. Environ. Microbiol. 19, 788e802. doi: 10.1111/1462-2920.13648, PMID: 28083986
Mantzoukas S., Tamez-Guerra P., Zavala-Garcia F., Lagogiannis I., and Ek-Ramos. M. J. (2022). Entomopathogenic fungi tested in planta on pepper and in field on sorghum, to control commercially important species of aphids. World J. Microb. Biot. 38, 84. doi: 10.1007/s11274-022-03268-7, PMID: 35378608
MoFa-IFPRI (2020). “Ghana’s chilli market,” in MoFA-IFPRI market brief No. 4, vol. 2020. Accra, MoFA-IFPRI.
Moore S. D., Richards G. I., Chambers C., and Hendry D. (2014). An improved larval diet for commercial mass rearing of the False Codling Moth, Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae). Afr. Entomol. 22, 216–219. doi: 10.4001/003.022.0125
Mwanza P. (2015). Determination of the effects of sunlight and UV irradiation on the structure, viability and reapplication frequency of the biopesticide Cryptophlebia leucotreta granulovirus in the protection against False Codling Moth infestation of citrus crops. Master’s Thesis (Gqeberha, South Africa: Nelson Mandela University).
Nascimento E., Da Silva S. H., Marques E. R., Roberts D. W., and Braga G. U. L. (2010). Quantification of cyclobutane pyrimidine dimers induced by UVB radiation in conidia of the fungi Aspergillus fumigatus, Aspergillus nidulans, Metarhizium acridum and Metarhizium robertsii. Photochem. Photobiol. 86, 1259e1266. doi: 10.1111/j.1751-1097.2010.00793.x, PMID: 20860693
Panyasiri C., Supothina S., Veeranondha S., Chanthaket R., Boonruangprapa T., and Vichai V. (2022). Control efficacy of entomopathogenic fungus Purpureocillium lilacinum against chili thrips (Scirtothrips dorsalis) on chili plant. Insects 13, 684. doi: 10.3390/insects13080684, PMID: 36005309
Páramo M. E. R., Lastra C. C. L., García J. J., Fernandes É.K., Marreto R. N., and Luz C. (2015). Effect of ultraviolet-A radiation on the production of Leptolegnia chapmanii (Saprolegniales: Saprolegniaceae) zoospores on dead Aedes aEgypti (Diptera: Culicidae) larvae and their larvicidal activity. J. Invertebr. Pathol. 130, 133–135. doi: 10.1016/j.jip.2015.08.002, PMID: 26259676
Posadas J. B., Maricel A. L., Mini Jorge I., and Lecuona Roberto E. (2012). Natural tolerance to UV-B and assessment of photoprotectants in conidia of six native isolates of Beauveria bassiana (Bals-Criv) Vuillemin. World Appl. Sci. J. 20, 1024–1030. doi: 10.5829/idosi.wasj.2012.20.07.2469
Punchakara A., Prajapat G., Bairwa H. K., Jain S., and Agrawal A. (2023). Applications of mycosporine-like amino acids beyond photoprotection. Appl. Environ. Microbiol. 89, e0074023. doi: 10.1128/aem.00740-23, PMID: 37843273
Quaite F. E., Sutherland B. M., and Sutherland J. C. (1992). Action spectrum for DNA damage in alfalfa lowers predicted impact of ozone depletion. Nature 358, 576–578. doi: 10.1038/358576a0
Rangel D. E., Acheampong M. A., Bignayan H. G., Golez H. G., and Roberts D. W. (2023). Conidial mass production of entomopathogenic fungi and tolerance of their mass-produced conidia to UV-B radiation and heat. Fungal Biol. 127, 1524–1533. doi: 10.1016/j.funbio.2023.07.001, PMID: 38097326
Rangel D. E. N., Braga G. U. L., Anderson A. J., and Roberts D. W. (2005). Variability in conidial thermotolerance of Metarhizium anisopliae isolates from different geographic origins. J. Invertebr. Pathol. 88, 116–125. doi: 10.1016/j.jip.2004.11.007, PMID: 15766928
Rangel D. E. N., Butler M. J., Torabinejad J., Anderson A. J., Braga G.Ú.L., Day A. W., et al. (2006). Mutants and isolates of Metarhizium anisopliae are diverse in their relationships between conidial pigmentation and stress tolerance. J. Invertebr. Pathol. 93, 170–182. doi: 10.1016/j.jip.2006.06.008, PMID: 16934287
Rangel D. E. and Roberts D. W. (2018). Possible source of the high UV-B and heat tolerance of Metarhizium acridum (isolate ARSEF 324). J. Invertebr. Pathol. 157, 32–35. doi: 10.1016/j.jip.2018.07.011, PMID: 30017952
R Core Team (2024). R: A language and environment for statistical computing R foundation for statistical computing (Vienna, Austria). Available online at: https://www.R-project.org/.
Rosic N. N. (2019). Mycosporine-like amino acids: making the foundation for organic personalised sunscreens. Mar. Drugs 17, 638. doi: 10.3390/md17110638, PMID: 31726795
Rossouw S., Mathulwe L. L., Stokwe N. F., and Malan A. P. (2023). Effect of visible light and ultraviolet light on the pathogenicity of entomopathogenic fungi to False Codling Moth, Thaumatotibia leucotreta (Lepidoptera: Tortricidae) larvae. Afr. Entomol. 31, 1–8. doi: 10.17159/2254-8854/2023/a13141
Santos P. D. S., Da Silva M. A. Q., Monteiro A. C., and Gava C. A. T. (2011). Improving photoprotection of Beauveria bassiana conidia for biological control of the cactus pest Dactylopius opuntiae in the semiarid region northeast of Brazil. Biocontrol Sci. Technol. 21, 893–902. doi: 10.1080/09583157.2011.586022
Thiyagarasaiyar K., Goh B. H., Jeon Y. J., and Yow Y. Y. (2020). Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 18, 323. doi: 10.3390/md18060323, PMID: 32575468
Vidal C., Fargues J., and Lacey L. A. (1997). Intraspecific variability of Paecilomyces fumosoroseus: effect of temperature on vegetative growth. J. Invertebr. Pathol. 70, 18–26. doi: 10.1006/jipa.1997.4658
Wan N. F., Fu L., Dainese M., Kiær L. P., Hu Y. Q., and Xin F. (2025). Pesticides have negative effects on non-target organisms. Nat. Commun. 16, 1360. doi: 10.1038/s41467-025-56732-x, PMID: 39948065
Wang D. Y., Fu B., Tong S. M., Ying S. H., and Feng M. G. (2019). Two photolyases repair distinct DNA lesions and reactivate UVB-inactivated conidia of an insect mycopathogen under visible light. Appl. Environ. Microbiol. 85, e02459–e02418. doi: 10.1128/AEM.02459-18, PMID: 30552186
Keywords: chili pepper, entomopathogenic fungi, False Codling Moth, Metarhizium spp., simulated solar radiation, UV tolerance, virulence
Citation: Nyahe PAS, Eziah VY, Al-Ani LKT, Akumyoungta M, Coombes CA, Rangel DEN, Alder-Rangel A, Sukmawati D, Aidoo OF and Acheampong MA (2025) Extreme UV sensitivity of native Metarhizium spp. as potential biocontrol agent for False Codling Moth (Thaumatotibia leucotreta Meyrick) on chili pepper in Ghana. Front. Fungal Biol. 6:1660692. doi: 10.3389/ffunb.2025.1660692
Received: 06 July 2025; Accepted: 05 August 2025;
Published: 25 August 2025.
Edited by:
Nicolas Pedrini, National University of La Plata, ArgentinaReviewed by:
Manuel Rueda-Páramo, CEPAVE - Centro de Estudios Parasitológicos y de Vectores, ArgentinaRonaldo Alves Pereira-Junior, Federal University of Uberlandia, Brazil
Copyright © 2025 Nyahe, Eziah, Al-Ani, Akumyoungta, Coombes, Rangel, Alder-Rangel, Sukmawati, Aidoo and Acheampong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mavis Agyeiwaa Acheampong, bWFhY2hlYW1wb25nQHVnLmVkdS5naA==
 Patricia Akua Sitsofe Nyahe
Patricia Akua Sitsofe Nyahe Vincent Yao Eziah1
Vincent Yao Eziah1 Laith Khalil Tawfeeq Al-Ani
Laith Khalil Tawfeeq Al-Ani Monica Akumyoungta
Monica Akumyoungta Candice Anne Coombes
Candice Anne Coombes Drauzio Eduardo Naretto Rangel
Drauzio Eduardo Naretto Rangel Owusu Fordjour Aidoo
Owusu Fordjour Aidoo Mavis Agyeiwaa Acheampong
Mavis Agyeiwaa Acheampong