- 1Division of Hematology and Oncology, Brody School of Medicine at East Carolina University, Greenville, NC, United States
- 2Department of Internal Medicine, University of Toledo, Toledo, OH, United States
Background: Trastuzumab deruxtecan (T-DXd) is an antibody-drug conjugate (ADC) that is effective in treating gastrointestinal (GI) cancers. However, the significant variability in its reported efficacy and safety profiles is likely due to differences in trial designs, patient populations, and clinical settings. This systematic review and meta-analysis aimed to consolidate current evidence on the efficacy and safety of T-DXd in human epidermal growth factor receptor 2 (HER2)-positive GI malignancies.
Methods: We conducted a systematic review and meta-analysis following the Preferred Reporting Items for Systemic Reviews and Meta-Analysis guidelines (PRISMA), utilizing the Medline, Embase, Cochrane Central, and ClinicalTrials.gov databases. Out of 5,594 articles reviewed, 10 studies were ultimately included after both primary and secondary screenings, providing data on the outcomes and safety of T-DXd in HER2-positive GI malignancies. The National Institute of Health quality assessment tool was employed to evaluate the quality of the studies. Pooled analyses were performed using the ‘meta’ package (Schwarzer et al., R programming language), and proportions with 95% confidence intervals (CIs) were calculated.
Results: We identified 653 patients treated with T-DXd for HER2-positive GI malignancies in 10 studies. The median age of the patients was 64.5 years (27–85) and 53% were male. The median follow-up duration was 5.9 months (0.5–30.5). The median overall survival and progression-free survival were 11.15 (1.4–20.8) and 5.6 months (2.6–8.7), respectively. The pooled objective response rate (ORR) was 36.9% (95% CI:31.5%–42.5%, I² = 41%, n = 589), with partial response and complete response rates of 35.2% (95% CI:31.1%–39.5%, I² = 0%, n = 516) and 1.3% (95% CI: 0.0%–4.7%, I² = 73%, n = 516), respectively. The median duration of response (DoR) was 7 months (0.7–22.3). Reported adverse events included anemia, febrile neutropenia, thrombocytopenia, diarrhea, nausea, interstitial lung disease/pneumonitis, heart failure, and hepatitis. For the 5.4 mg/kg dose, grade 3/4 adverse events were reported in 67 patients. For the 6.4 mg/kg dose, 146 grade 3/4 adverse events were reported.
Conclusions: This meta-analysis supports the efficacy of T-DXd in patients with HER2-positive GI malignancies with a moderate ORR, even in patients who have experienced disease progression after multiple lines of therapy. Overall, T-DXd is well-tolerated, with limited severe adverse events. These findings validate existing research and underscore the need for further clinical trials, particularly in earlier lines of treatment.
Highlights
● Antibody-drug conjugates are highly targeted drug delivery systems and T-DXd is one such anti-HER2-directed ADC with a high drug antibody ratio.
● T-DXd has demonstrated effectiveness across various HER2-positive GI cancers.
● T-DXd has a favorable safety profile in various clinical trials.
● ILD is a rare but serious side effect associated with T-DXd.
● This meta-analysis includes data from available clinical trials in HER2-positive GI cancers.
Introduction
Human epidermal growth factor receptor 2 (HER2) is a pivotal protein implicated in the development and progression of various cancers, including gastrointestinal (GI) malignancies. Overexpression of HER2 in GI malignancies is variable with 10%–30% overexpression reported in gastric and gastroesophageal cancers and 3%–5% in colon cancer. The enriched HER2 group varies based on site and subtype with gastro-esophageal junction (GE) junction and intestinal subtypes having a higher percentage of overexpression. HER2 overexpression is more common in left-side and RAS wild-type colon cancer (1). HER2 overexpression has prognostic and predictive significance (2, 3). HER2-targeted therapies such as trastuzumab have revolutionized treatment for HER2-positive cancers, especially breast cancer. The ToGA trial was the first randomized phase III clinical trial that demonstrated the efficacy of trastuzumab with cisplatin and 5FU in advanced/metastatic gastroesophageal cancers. The other HER2-targeted monoclonal antibodies and tyrosine kinase inhibitors have been less effective in HER2-positive GI malignancies compared to breast cancer.
Acquired resistance to trastuzumab often occurs due to activating mutations in the PI3K pathway and reduced HER2 expression, further complicating treatment (3–5). HER2 expression is heterogeneous with variable expression patterns in GI cancers compared to breast cancer, with incomplete membranous staining more common in gastric cancer. The scoring system for HER2 is not standardized for GI malignancies and differs from breast cancer. Antibody-drug conjugates (ADCs) targeting HER2 offer an effective treatment option for this heterogeneous subtype of GI malignancies.
Trastuzumab deruxtecan (T-DXd) is a novel ADC that combines a HER2-targeting monoclonal antibody with a potent topoisomerase I inhibitor linked by a stable connector. This design allows T-DXd to deliver its cytotoxic payload directly to HER2-expressing cancer cells, reducing systemic toxicity (6). T-DXd’s high drug-to-antibody ratio and bystander-killing effect enable it to target tumors with varying HER2 expression levels, making it less dependent on high HER2 expression (7). T-DXd’s topoisomerase I inhibitor payload is effective against resistance mechanisms by inducing DNA damage and cell cycle arrest independent of HER2 signaling. Unlike other ADCs, such as trastuzumab emtansine (T-DM1), T-DXd’s payload is less susceptible to efflux by ATP-binding cassette (ABC) transporters, maintaining its efficacy in resistant cells (8–10) (Figure 1).
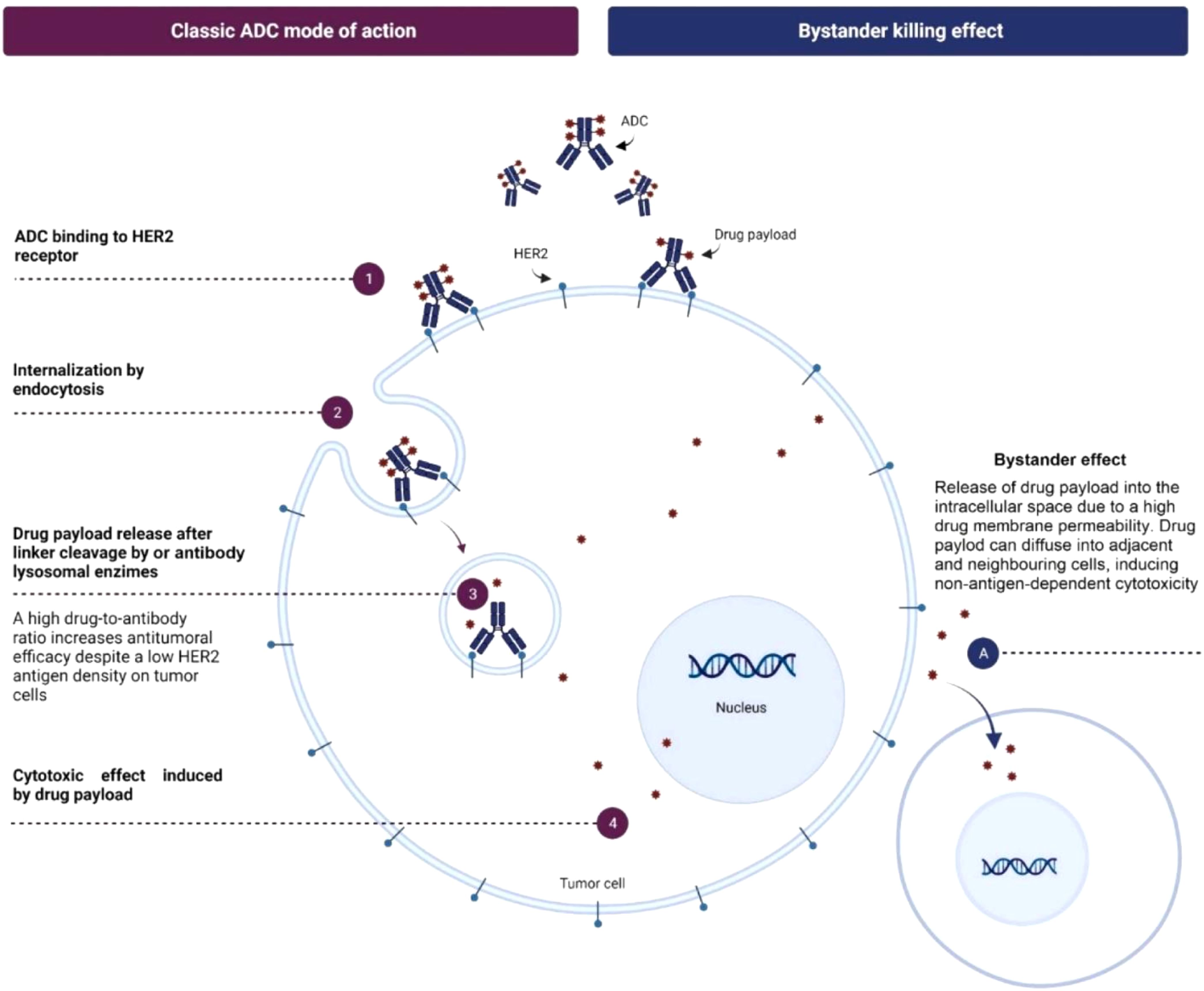
Figure 1. Mechanism of action of T-DXd. Adapted from "Trastuzumab deruxtecan in breast cancer" by Author M Mart�n, 2024, Elsevier. © 2024 The Authors. Published by Elsevier B.V. (Reprinted with permission).
Recent clinical trials have highlighted the impressive efficacy of T-DXd in treating HER2-positive GI malignancies. The DESTINY-Gastric 01 trial established T-DXd’s superiority over standard chemotherapy in patients with metastatic gastric cancer, demonstrating significant improvements in both progression-free survival (PFS) and overall survival (OS) (4). Additionally, the DESTINY-Pan Tumor 02 study supports the applicability of T-DXd across a broader range of HER2-expressing solid tumors, underscoring its potential as a transformative treatment option (11).
Despite its promising efficacy, the safety profile of T-DXd necessitates careful evaluation. Although generally well-tolerated, some patients experience severe adverse events (SAEs), particularly interstitial lung disease (ILD), and myelosuppression with grade 3 or greater adverse events (AEs) occurred in 48.3% of patients (12). Early recognition and timely management of these side effects is essential to optimize patient outcomes.
This systematic review aims to assess the safety and efficacy data of T-DXd in treating GI malignancies from currently available studies.
Methods
Search criteria
This systematic review and meta-analysis were conducted following the methodology outlined in the Cochrane Handbook of Systematic Reviews of Interventions (13) and was conducted according to the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) 2020 guidelines (14). A population, intervention, comparison, and outcome (PICO) table was developed, and Librarian H.R. developed a comprehensive literature search in consultation with the research team. Another librarian peer-reviewed the search using a modified PRESS (15). The search was run from 1/1/2015 to 2/22/2024 in Medline via PubMed, Embase via Embase.com, Cochrane Central via OVID, and ClinicalTrials.gov using keywords and controlled vocabulary terms. The time filter beginning in 2015 was applied after a discussion with the research team given the first T-DXd FDA approval was in December 2019. The entire search strategy can be found at http://hdl.handle.net/10342/13380. The search results were uploaded to the screening software Covidence (https://www.covidence.org/). Duplicates were removed using the automated duplicate checker within Covidence. The reference list of included studies was also checked for additional relevant studies.
Selection criteria
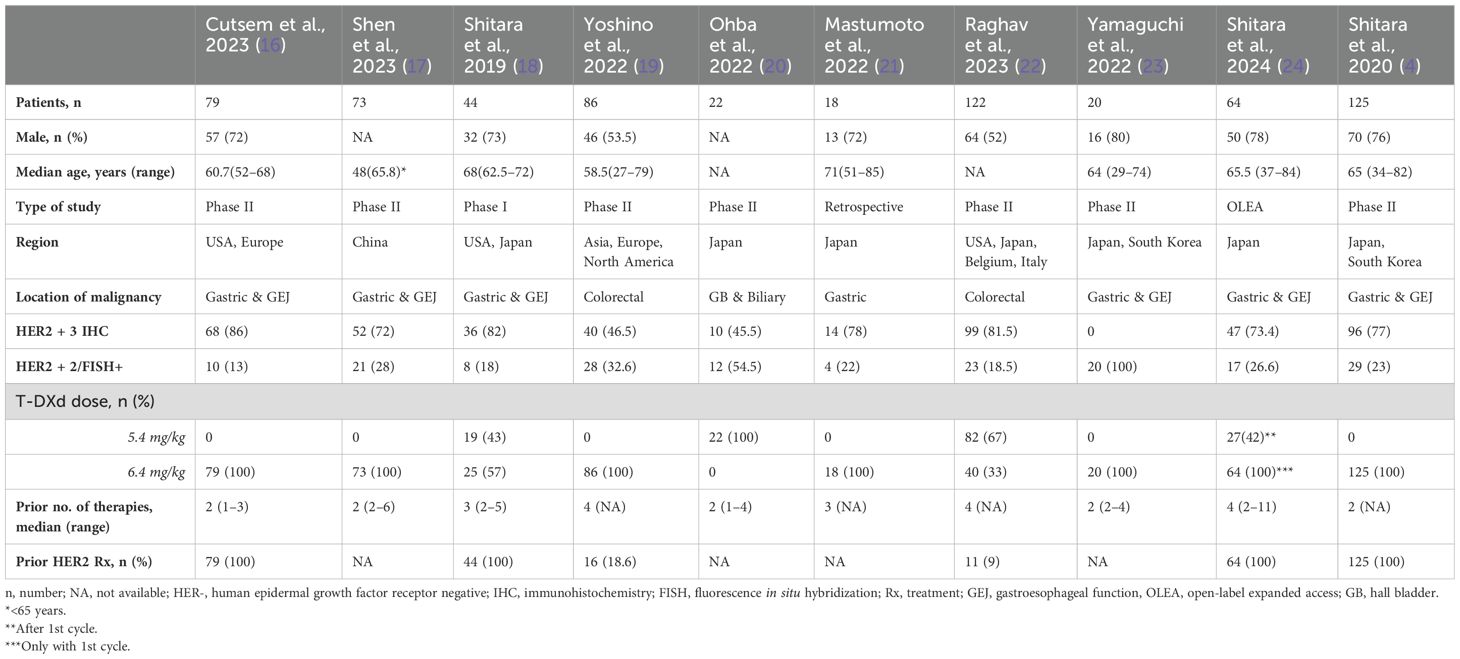
After removing duplicates, 5,594 articles were independently screened in two stages by two reviewers (A.H. and N.M.), with a third acting as a tiebreaker within the screening software Covidence. First, titles and abstracts were screened against preset inclusion criteria after discussion and consensus among all authors and approved by the principal investigator (M.M). The full text of 18 eligible or potentially eligible studies was screened against the inclusion criteria, and 10 articles were included that reported data on the safety and efficacy of T-DXd in GI malignancies (Figure 2), excluding hepatocellular carcinoma (HCC). The inclusion criteria were:
1. Original studies (clinical trials and case-control, retrospective, and prospective cohort studies);
2. Articles reporting data for adult patients aged 18 years and above;
3. Studies reporting the safety and efficacy of T-DXd only in HER2-positive [HER2 + 3 by immunohistochemistry (IHC) or HER2 + 2 by IHC/ISH+] GI malignancies.
Studies with HCC as a diagnosis were excluded, given there was no expression of HER2. Studies with HER2–0 or +1 by IHC were also excluded. Eight studies were excluded in the secondary review, all of which were found to be duplicates or irrelevant and did not report data on T-DXd AEs or outcomes (Figure 1). Supplementary Table S1 lists excluded studies along with the reasons for exclusion.
Data extraction
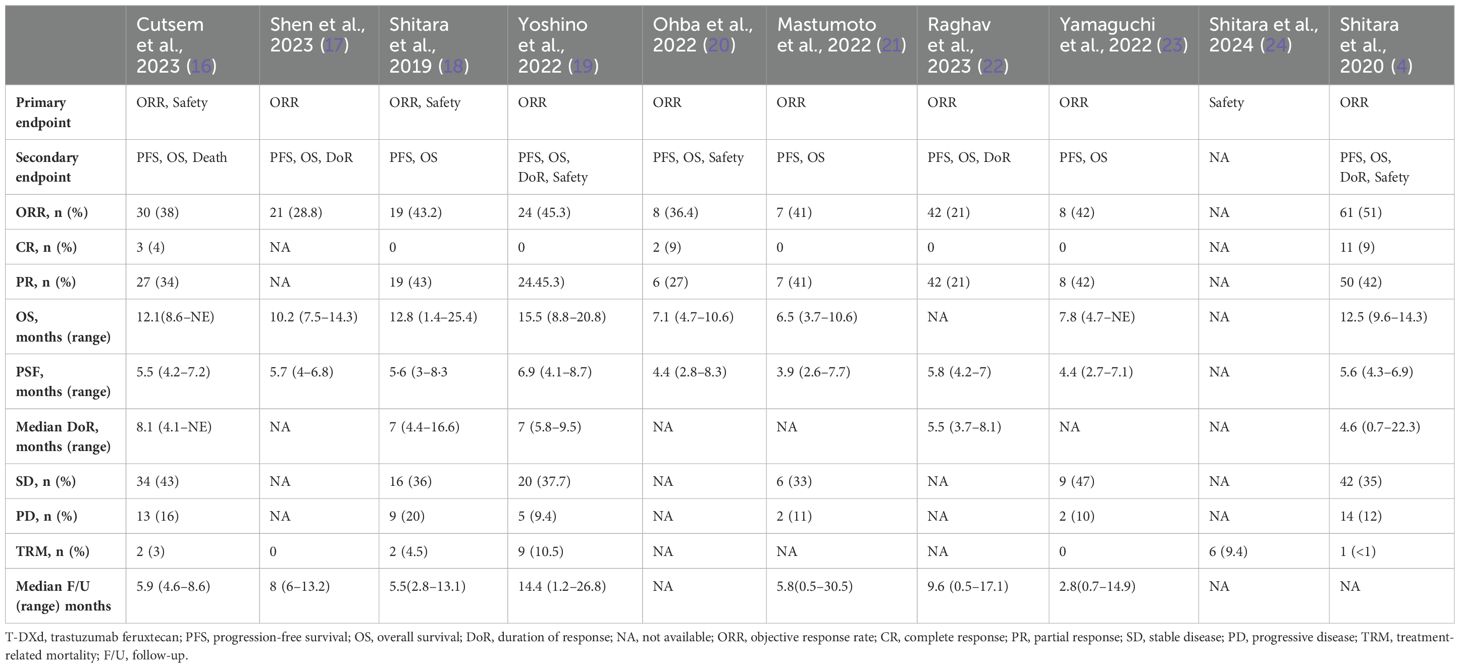
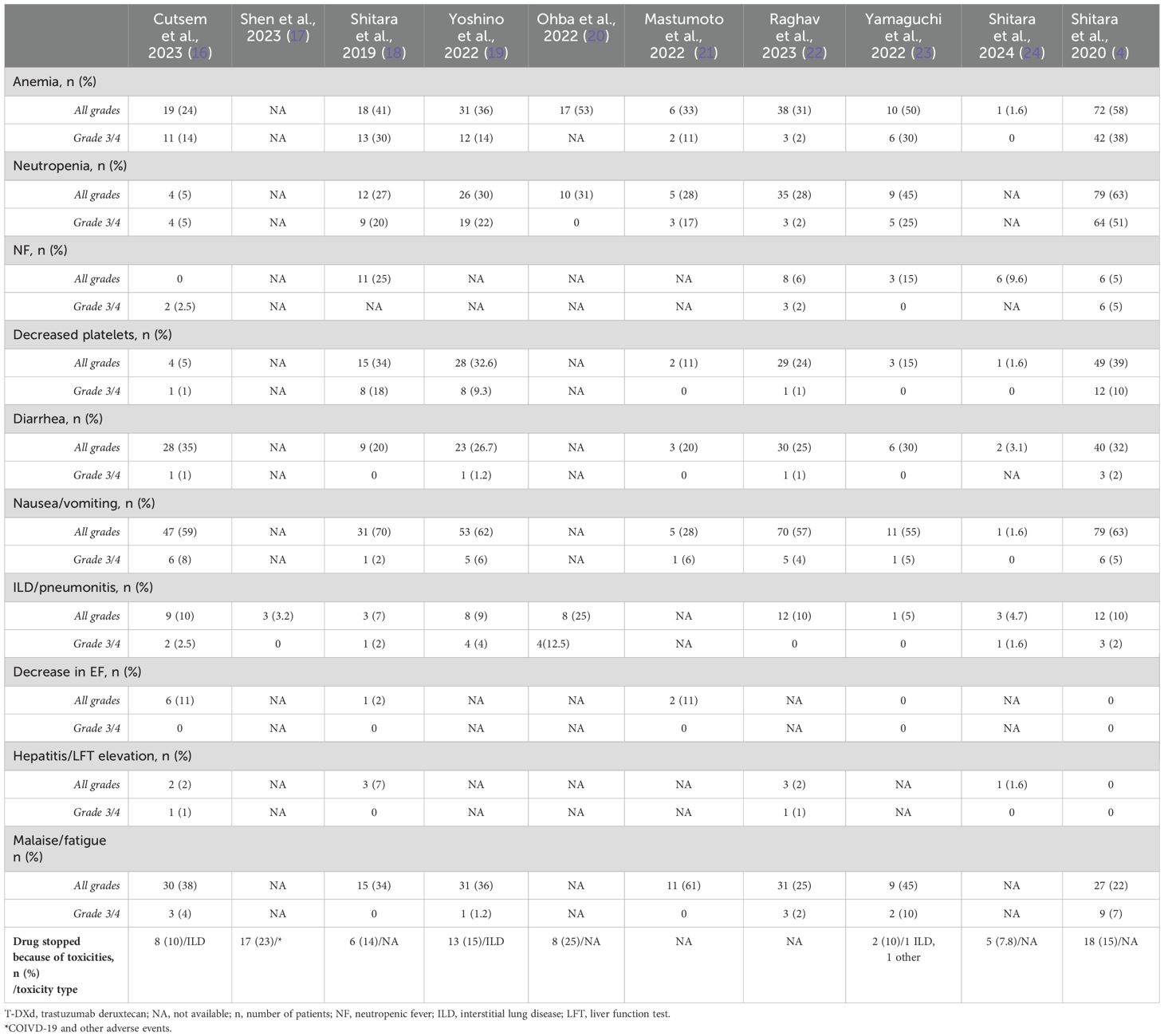
Five authors (M.P., A.T., S.V., A.A., and A.D.) independently extracted data from the 10 selected studies. A.H. and S.I. verified the data for any discrepancies. Data were collected on baseline characteristics (i.e., number of patients, sex, age, diagnosis, HER2-expression status, T-DXd dosing, and prior lines of therapies); efficacy, i.e., ORR, complete response (CR), partial response (PR), median follow-up, OS, PFS, and treatment-related mortality (TRM); and AEs, i.e., anemia, neutropenia, febrile neutropenia, thrombocytopenia, nausea/vomiting, diarrhea, elevation in liver function test, pneumonitis/ILD, heart failure, and malaise/fatigue. The baseline characteristics of each study are listed and tabulated in Table 1.
Quality evaluation
The methodological quality of the included studies was evaluated using the National Institute of Health (NIH) quality assessment tool and all the studies were rated as good. Supplementary Table S2 lists the quality and risk of bias assessment.
Statistical analysis
A pooled analysis was performed using the ‘meta’ package (Schwarzer et al., R programming language), and proportions with 95% confidence intervals (CIs) were computed. The inter-study variance was calculated using the Der Simonian–Laird Estimator.
Results
We identified a total of 10 studies with 653 patients using T-DXd for the treatment of GI malignancies. The median age of patients was 64.5 years (27–85) (4, 16–19, 21, 23, 24), and 53% were male (4, 16, 18, 19, 21–24). Furthermore, 70% of these 653 patients were HER2 + 3 by IHC, and 26% of patients were HER2 + 2 by IHC/FISH. A 5.4 mg/kg T-DXd dose was given to 59.5% (150/252) of the patients (18, 20, 22, 24). Patients in one study received the 5.4mg/kg dose after the first cycle (24). Moreover, a 6.4 mg/kg T-DXd dose was used in 84% (530/631) of the patients (4, 16–19, 21–24). The patients in all studies received at least 2 (1–11) prior therapies and 65% (339/520) of the patients had prior HER2 treatment (4, 16, 18, 19, 22, 24).
Outcomes
The primary endpoints were objective response rate (ORR) and safety in Cutsem et al. (2023) and Shitara et al. (2019) (16, 18). The sole primary endpoint was ORR in seven studies (4, 17, 19–23). Shitara et al. (2024) reported safety as the primary endpoint (24). The median follow-up was 5.9 months (0.5–30.5) (16–19, 21–23). Median OS was 11.15 months (1.4–20.8) (4, 16–21, 23). PFS was 5.6 months (2.6–8.7) (4, 16–23). The median duration of response (DoR) was 7 (0.7–22.3) months (4, 16, 18, 19, 22).
The overall pooled ORR was 36.9% (95% CI 0.315–0.425, I2 = 41%, n = 589) (4, 16–23). The pooled ORR for a 5.4 mg/kg dose of T-DXd was 23.4% (95% CI 0.135–0.350, I2 = 57%, n = 188). The pooled ORR for a 6.4 mg/kg dose of T-DXd was 31.3% (95% CI 0.202–0.435, I2 = 88%, n = 567). The CR and PR rates were 1.3% (95% CI 0.000–0.047, I2 73%, n = 516) and 35.2% (95% CI 0.311–0.395, I2 = 0%, n = 516), respectively (4, 16, 18–23). The pooled rates of stable disease and progressive disease were 34.4% (95% CI 0.276–0.415, I2 = 42%, n = 372) and 11.6% (95% CI 0.075–0.165, I2 = 35%, n = 372), respectively (4, 16, 18, 19, 21, 23). Treatment-related mortality was 2.9% (95% CI 0.004–0.069, I2 = 72%, n = 491) (4, 16–19, 23, 24). The outcomes of each study are listed in Table 2.
Safety analysis
Dose-based adverse events
AEs, including anemia, neutropenia, febrile neutropenia, thrombocytopenia, diarrhea, nausea, ILD/pneumonitis, heart failure, hepatitis, and malaise/fatigue, were reported in the studies. The treatment was discontinued because of toxicities in 77 (15%) patients (4, 16–20, 23, 24). AEs of any grade for a 5.4 mg/kg dose were not specified by any study. A total of 272 AEs of any grade were reported for a 6.4 mg/kg dose. The number of grade 3/4 SAEs for a 5.4 mg/kg dose was 67. There were 146 total grade 3/4 SAEs for a 6.4 mg/kg dose.
Pooled rate of adverse events
The pooled rate for any grade of anemia was 36.6% (95% CI 0.220–0.525, I2 = 93%, n = 580) (4, 16, 18–24), while for grade 3/4 anemia it was 17.4% (95% CI 0.075–0.300, I2 = 90%, n = 494) (4, 16, 18, 19, 21–24). The pooled rates of any grade and grade 3/4 neutropenia were 32.5% (95% CI 0.177–0.493, I2 = 93%, n = 516) and 18.1% (95% CI 0.051–0.363, I2 = 95%, n = 494) (4, 16, 18–23), respectively. The pooled rates of any grade and grade 3/4 thrombocytopenia were 18.5% (95% CI 0.085–0.312, I2 = 91%, n = 558) and 3.3% (95% CI 0.004–0.081, I2 = 80%, n = 558) (4, 16, 18, 19, 21–24), respectively. The pooled rate of any grade diarrhea was 22.7% (95% CI 0.147–0.318, I2 = 81%, n = 558) (4, 16, 18, 19, 21–24), and for grade 3/4 diarrhea it was 0.6% (95% CI 0.000–0.016, I2 = 0%, n = 558) (4, 16, 18, 19, 21–23). The pooled rates of any grade and grade 3/4 nausea were 47.9% (95% CI 0.294–0.667, I2 = 95%, n = 558) and 3.7% (95% CI 0.018–0.060, I2 = 23%, n = 558) (4, 16, 18, 19, 21–24), respectively. The pooled incidences of any grade and grade 3/4 pneumonitis/ILD were 8.8% (95% CI 0.057–0.124, I2 = 49%, n = 635) and 1.6% (95% CI 0.002–0.040, I2 = 58%, n = 635) (4, 16–20, 22–24), respectively. The pooled rate of any grade of heart failure was 2.3% (95% CI 0.000–0.084, I2 = 74%, n = 286) (4, 16, 18, 21, 23). There were no grade 3/4 heart failure events (4, 16, 18, 21, 23). The pooled incidences of any grade and grade 3/4 hepatitis were 1.8% (95% CI 0.002–0.045, I2 = 56%, n = 434) and 0.2% (95% CI 0.000–0.011, I2 = 0%, n = 434) (4, 16, 18, 22, 24), respectively. The total number of events for each study is mentioned in Table 3.
Discussion
HER2, a proto-oncogene expressed on many solid tumors, including those in the breast, kidney, and GI tract, is a therapeutic target (25–27). Apart from breast cancer, there is significant variability in HER2 expression and response to anti-HER2 therapy. Trastuzumab and chemotherapy have demonstrated some effectiveness in GI malignancies, especially esophagogastric cancers. ADCs enable more precise delivery of a high cytotoxic payload combined via a stable linker with specific monoclonal antibodies. T-DXd has shown effectiveness across many different cancer types including HER2-low and -ultra-low breast cancers (28). In our single-arm meta-analysis, T-DXd demonstrated a pooled ORR of 36.9% (31–42) as a second or higher line of treatment for HER2-positive GI malignancies. This evidence underscores the potential of T-DXd to enhance clinical outcomes for this challenging patient population. The highest ORR for gastric and gastroesophageal junction tumors was 51%, according to Shitara et al. (2020) (4). In colon cancer, the best ORR was 45%, as reported by Yoshino et al. (19). In contrast, the ORR for T-DXd in breast and lung cancer has previously been reported at 66.9% and 55.6%, respectively (29). This indicates a lower ORR of T-DXd in GI malignancies when compared to HER2+ breast and lung cancer. The disease control rate (DCR) for gastric tumors ranged from 76% to 89.5%, as noted by Matsumoto and Yamaguchi et al. (21, 23). Conversely, the DCR for breast cancer has been reported at 96.5% (29), suggesting that HER2 GI malignancies have a slightly lower DCR in comparison to breast cancer.
The average age of patients with gastric cancer is 68 years, while colon cancer is typically diagnosed at 70 years (30, 31). Matsumoto et al. reported outcomes in the oldest population with a median age of 71 (51–85) in our included studies (21). Their results showed that age alone did not significantly affect cancer outcomes. Similarly, Yoshino et al. found no significant difference in ORR between patients older than 65 (42.9%) and those younger than 65 (50.0%) (19). This data confirms that age does not appear to affect treatment response adversely. Gastric cancers are more common in men, with a male-to-female ratio ranging from 2:1 to 3:1 (32). In our analysis, the participants diagnosed with gastric cancer were predominantly male, accounting for 72% to 80% of the cases.
Poor functional status, as indicated by an Eastern Cooperative Oncology Group performance status (ECOG PS) greater than 2, is a well-known adverse prognostic factor for GI malignancies (33, 34). In our analysis, Yoshino et al. found that the ORR was significantly better for patients with an ECOG score of 0 at 54% (range 37%–70%) compared to those with an ECOG score of 1, which had an ORR of only 25% (range 7.3%–52.4%) (19). Additionally, Yamaguchi et al. reported that patients with HER2 IHC 1+ experienced more deterioration in ECOG scores over a 12-month period than those with HER2 IHC 2+/ISH- (23). Only Matsumoto et al. focused on patients with ECOG scores of 2 or 3; however, their study had a very small sample size (n=3), leaving recommendations for the use of T-DXd in patients with poorer health and lower performance status unclear (21).
Three studies [Yoshino et al. (19), Yamaguchi et al. (23), Shitara et al., (4)] evaluated the ORR in relation to HER2 status in tumors. Yoshino et al. found that metastatic colon cancer patients with HER2 IHC 3+ or HER2 2+/ISH positive status exhibited a PR rate of 45%. In contrast, no PR was observed in patients with HER2 2+/ISH negative status. The DoR was significantly longer in the HER2 IHC 3+ or HER2 2+/ISH-positive group, averaging 7 months (ranging from 5.8 to 9.5 months), while the HER2 2+/ISH-negative group showed no response (19). Similarly, Shitara et al. (2020) reported an improved ORR of 58% in patients with HER2 IHC 3+ disease (n=47), compared to a 29% response rate in those with HER2 IHC 2+ and ISH-positive disease (4). Conversely, Yamaguchi et al. noted a PR of 26% in HER2 2+/ISH negative gastric cancer patients, indicating that clinical responses to trastuzumab may vary among different GI malignancies (23). Three studies [Yoshino et al. (19), Yamaguchi et al. (23), Shitara et al., (4)] showed no CR in HER2 + GI malignancies (4, 19, 23). Only Shitara et al. (2020) reported that 9% (n=11) of the participants achieved a CR compared to physician-choice chemotherapy (4). Similarly, Cutsem et al. showed that 5% (n=4) of the patients had a CR (16).
Yoshino et al. reported a better median OS of 15.5 months (8.8 to 20.8 months) for patients in the HER2 3+ IHC or HER2 2+/ISH+ group (n=53). In contrast, patients in the HER2 2+/ISH- group (n=15) had a median OS of only 7.3 months (range: 3.0 to not evaluable). Additionally, Yoshino et al. found that PFS was better for those with HER2 IHC 3+ or HER2 2+/ISH+ at 8.3 months compared to 4.1 months for the HER2 2+/ISH- group (19).
In our analysis, 9 studies (except Ohba et al.) assessed the efficacy and safety of a 6.4 mg/kg dose, while four studies [Shitara et al., (18), Ohba et al., (20), Raghav et al., (22)] assessed a 5.4 mg/kg dose, suggestive greater consensus on utilizing 6.4 mg/kg dose. Raghav et al. reported different ORRs depending on the dose of T-DXd. The author(s) showed a better PR of 38% (27%–49%) among the 5.4 mg/kg arm and 27.5% (14.6%–43.9%) among the 6.4 mg/kg arm. The study also showed worse PFS with a high dose (6.4 mg/kg) at 5.5 months (4.2–7.0) vs. 5.8 months (4.6–7.0) with a lower dose (5.4 mg/kg) (22). Contrary to that, Shitara et al. (2019) showed better ORR with a 6.4 mg/kg dose at 52% (31%–72%) and only 31% (6%–56%) with a 5.4 mg/kg dose (18).
Interestingly, Matsumoto et al. calculated outcomes of HER2+ advanced gastric cancer and prior treatment with immune checkpoint inhibitors (ICIs). The author (s) showed better median PFS/OS among patients with gastric cancer treated with T-DXd and prior ICI treatment. The PFS with ICI exposure (n=11) was 6.5 months and only 2.9 months with no ICI exposure (n=7), p=0.02. OS was 9.2 months with ICI (n=11) and 3.7 months without ICI (n=7), p=0.08. Similarly, ORR was 60% vs. 14%, p=0.059, among patients with prior ICI exposure (21). This finding suggests that a concomitant or earlier treatment with ICIs among HER2+ GI malignancies could potentiate the beneficial effects of T-DXd. Matsumoto et al. showed a DCR of 76%; this DCR was higher in patients with prior ICI treatment at 90% vs. 57% with no prior ICIs, p=0.11 (21).
Left-sided colon cancer has a slightly better 5-year survival at 68.4% compared to 65.6% in right-sided colon cancer (35, 36). In our analysis, PFS and ORR depending on the location of colon cancer were reported by Yoshino et al. The author(s) showed that a left-sided HER2+ colon tumor had better PFS (7.3 months vs. 3.5 months) compared to a right-sided tumor (cecum and ascending and transverse colon) along with better ORR at 46.8% compared to 33% (4.3%–77.7%), respectively (19).
The presence of ascites in GI malignancies is regarded as a poor prognostic factor (37). In our included studies, the role of ascites was not evaluated due to the nature of phase 1 and 2 studies, except in the case of Matsumoto et al. The authors reported that 44% (n=8) of the patients had ascites, with 7 out of 8 experiencing massive ascites related to HER2+ advanced gastric cancer. The PFS was shorter for these patients at 2.75 months compared to 6.5 months for those without ascites (p=0.01). Similarly, the OS was also significantly worse, averaging 3.9 months for patients with ascites vs. 9.4 months for those without (p=0.04) (21).
In our analysis, most participants discontinued T-DXd treatment due to disease progression. According to Cutsem et al., approximately 75% of patients stopped T-DXd because their disease worsened (16). Shitara et al. (2020) found that 15% of patients discontinued therapy due to side effects (4). Additionally, Shitara et al. (2019) and Yoshino et al. reported 75% and 70% treatment discontinuation due to disease progression, respectively (18, 19).
We also analyzed the safety profile of T-DXd among HER2+ GI malignancies. Our analysis showed better tolerability of T-DXd treatment among HER2+ GI malignancy participants. The most reported side effect of T-DXd treatment was nausea, with a pooled incidence of 47.9%. Shitara et al. (2020) reported nausea among 63% (n=79) of the participants. Fortunately, grade 3/4 nausea was only reported by 5% (n=6) (4). Dowling et al. showed that the prevalence of all-grade nausea caused by T-DXd in HER2+ breast cancer patients was 76% (38). ILD/pneumonitis is a less common but serious side effect of monoclonal antibody therapy against the HER2 receptor. Trastuzumab causes ILD in approximately 9.9% of patients. Similarly, T-DM1 causes ILD in only 1.1% of cases. Lapatinib showed an ILD incidence of 0.2% (39). Raghav et al. showed drug-related ILD to be more frequent with a 6.4 mg/kg dose (12.8%) compared to 8.4% with a 5.4 mg/kg dose. The incidence of ILD seems to correlate with higher doses of T-DXd (22). Matsumoto et al. showed drug-induced grade 2 heart failure in 6% of the patients (21). Shitara et al., 2019 reported one case of decreased EF (grade 2) and one with prolonged QT interval (grade 3) (18). Overall, the included patients had no significant cardiac side effects.
Our single-arm meta-analysis has several limitations. The diverse range of GI malignancies among the included studies makes it challenging to generalize our pooled data. Additionally, most of the studies were phase 1 and phase 2 trials with relatively small sample sizes. The absence of significant randomization and potential selection bias may compromise the quality of the data in our analysis.
This systemic review shows that T-DXd is an effective and relatively safe therapeutic option for HER2-positive advanced GI malignancies. T-DXd demonstrates a moderate ORR and a clinically meaningful benefit even in patients who have experienced disease progression after multiple lines of therapies for GI malignancies. The safety data shows no new signals. ILD is a rare but serious side effect that can be managed effectively with early diagnosis and prompt intervention. Further studies are required to leverage the potential of ADCs, which demonstrate effectiveness in HER 2-low and -ultra-low GI cancers, ADC combinations, and optimal sequencing of these agents in advanced GI cancers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AH: Data curation, Methodology, Writing – original draft, Writing – review & editing, Conceptualization, Project administration. QI: Writing – original draft, Writing – review & editing. SI: Data curation, Writing – review & editing. FS: Formal Analysis, Writing – original draft. ET: Formal Analysis, Writing – original draft. HA: Data curation, Writing – review & editing. NM: Data curation, Writing – review & editing. HR: Methodology, Writing – review & editing. AA: Data curation, Writing – review & editing. AD: Data curation, Writing – review & editing. AT: Data curation, Writing – review & editing. SV: Data curation, Writing – review & editing. MP: Data curation, Writing – review & editing. MH: Writing – review & editing. HB: Writing – review & editing. MM: Conceptualization, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgstr.2025.1559934/full#supplementary-material
References
1. Djaballah SA, Daniel F, Milani A, Ricagno G, Lonardi S. HER2 in colorectal cancer: the long and winding road from negative predictive factor to positive actionable target. Am Soc Clin Oncol Educ Book. (2022) 42:219–32. doi: 10.1200/EDBK_351354
2. Ma C, Wang X, Guo J, Yang B, Li Y. Challenges and future of HER2-positive gastric cancer therapy. Front Oncol. (2023) 13. doi: 10.3389/fonc.2023.1080990
3. Varghese R, Soman N, Karsiya J, Bafna N, Nag S. Fam-trastuzumab deruxtecan-nxki (Enhertu®): A narrative drug review. Cancer Research Statistics Treat. (2022) 5:701–9. doi: 10.4103/crst.crst_302_22
4. Shitara K, Bang Y-J, Iwasa S, Sugimoto N, Ryu M-H, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. New Engl J Med. (2020) 382:2419–30. doi: 10.1056/NEJMoa2004413
5. Vivekanandhan S, Knutson KL. Resistance to trastuzumab. Cancers. (2022) 14:5115. doi: 10.3390/cancers14205115
6. Okamoto H, Oitate M, Hagihara K, Shiozawa H, Furuta Y, Ogitani Y, et al. Pharmacokinetics of trastuzumab deruxtecan (T-DXd), a novel anti-HER2 antibody-drug conjugate, in HER2-positive tumour-bearing mice. Xenobiotica. (2020) 50:1242–50. doi: 10.1080/00498254.2020.1755909
7. Takegawa N, Tsurutani J, Kawakami H, Yonesaka K, Kato R, Haratani K, et al. [fam-] trastuzumab deruxtecan, antitumor activity is dependent on HER2 expression level rather than on HER2 amplification. Int J Cancer. (2019) 145:3414–24. doi: 10.1002/ijc.v145.12
8. Park J, Kang SK, Kwon WS, Jeong I, Kim TS, Yu SY, et al. Novel HER2-targeted therapy to overcome trastuzumab resistance in HER2-amplified gastric cancer. Sci Rep. (2023) 13:22648. doi: 10.1038/s41598-023-49646-5
9. Takegawa N, Nonagase Y, Yonesaka K, Sakai K, Maenishi O, Ogitani Y, et al. DS-8201a, a new HER2-targeting antibody-drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int J Cancer. (2017) 141:1682–9. doi: 10.1002/ijc.v141.8
10. Martín M, Pandiella A, Vargas-Castrillón E, Díaz-Rodríguez E, Iglesias-Hernangómez T, Martínez Cano C, et al. Trastuzumab deruxtecan in breast cancer. Crit Rev Oncol Hematol. (2024) 198:104355. doi: 10.1016/j.critrevonc.2024.104355
11. Meric-Bernstam F, Makker V, Oaknin A, Oh D-Y, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-panTumor02 phase II trial. J Clin Oncol. (2024) 42:47–58. doi: 10.1200/JCO.23.02005
12. Hong R, Xia W, Wang L, Lee K, Lu Q, Jiang K, et al. Safety, tolerability, and pharmacokinetics of BAT8001 in patients with HER2-positive breast cancer: An open-label, dose-escalation, phase I study. Cancer Commun (Lond). (2021) 41:171–82. doi: 10.1002/cac2.12135
13. Higgins JPT, T J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons (2019).
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. (2016) 75:40–6. doi: 10.1016/j.jclinepi.2016.01.021
16. Van Cutsem E, di Bartolomeo M, Smyth E, Chau I, Park H, Siena S, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. (2023) 24:744–56. doi: 10.1016/S1470-2045(23)00215-2
17. Shen L, Chen P, Lu J, Wan Y, Zheng Y, Ye F, et al. 172P Trastuzumab deruxtecan (T-DXd) in Chinese patients (pts) with previously treated HER2-positive locally advanced/metastatic gastric cancer (GC) or gastroesophageal junction adenocarcinoma (GEJA): Primary efficacy and safety from the phase II single-ar. Ann Oncol. (2023) 34:S1542–S3. doi: 10.1016/j.annonc.2023.10.307
18. Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. (2019) 20:827–36. doi: 10.1016/S1470-2045(19)30088-9
19. Yoshino T, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, et al. Final results of DESTINY-CRC01 investigating trastuzumab deruxtecan in patients with HER2-expressing metastatic colorectal cancer. Nat Commun. (2023) 14:3332. doi: 10.1038/s41467-023-38032-4
20. Ohba AM, Kawamoto Y, Komatsu Y, Ueno M, Kobayashi S, Ikeda M, et al. J Clin Oncol. (2022) 40(16). doi: 10.1200/JCO.2022.40.16_suppl.4006
21. Matsumoto T, Yamamura S, Ikoma T, Kurioka Y, Doi K, Boku S, et al. Real-world data of trastuzumab deruxtecan for advanced gastric cancer: A multi-institutional retrospective study. J Clin Med. (2022) 11:2247. doi: 10.3390/jcm11082247
22. Raghav K, Siena S, Takashima A, Kato T, Van den Eynde M, Pietrantonio F, et al. Trastuzumab deruxtecan in patients with HER2-positive advanced colorectal cancer (DESTINY-CRC02): primary results from a multicentre, randomised, phase 2 trial. Lancet Oncol. (2024) 25:1147–62. doi: 10.1016/S1470-2045(24)00380-2
23. Yamaguchi K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in anti-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2-low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II trial. J Clin Oncol. (2023) 41:816–25. doi: 10.1200/JCO.22.00575
24. Shitara K, Yamaguchi K, Muro K, Yasui H, Sakai D, Oshima T, et al. Trastuzumab deruxtecan in patients with locally advanced or metastatic HER2-positive gastric cancer: a multicenter, open-label, expanded-access study. Int J Clin Oncol. (2024) 29:27–35. doi: 10.1007/s10147-023-02422-x
25. Grivas PD, Antonacopoulou A, Tzelepi V, Sotiropoulou-Bonikou G, Kefalopoulou Z, Papavassiliou AG, et al. HER-3 in colorectal tumourigenesis: from mRNA levels through protein status to clinicopathologic relationships. Eur J Cancer. (2007) 43:2602–11. doi: 10.1016/j.ejca.2007.08.019
26. Li Q, Zhang R, Yan H, Zhao P, Wu L, Wang H, et al. Prognostic significance of HER3 in patients with Malignant solid tumors. Oncotarget. (2017) 8:67140–51. doi: 10.18632/oncotarget.18007
27. Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. (1986) 232:1644–6. doi: 10.1126/science.3012781
28. Curigliano G, Hu X, Dent RA, Yonemori K, Barrios CH, O’Shaughnessy J, et al. Trastuzumab deruxtecan (T-DXd) vs physician’s choice of chemotherapy (TPC) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-low or HER2-ultralow metastatic breast cancer (mBC) with prior endocrine therapy (ET): Primary results from DESTINY-Breast06 (DB-06). J Clin Oncol. (2024) 42:LBA1000–LBA. doi: 10.1200/JCO.2024.42.17_suppl.LBA1000
29. Kou L, Chen X, Xie X, Wen Q, Li J, Li Y. The efficacy and safety of trastuzumab deruxtecan (T-DXd) in HER2-expressing solid tumours: a single-arm meta-analysis. Jpn J Clin Oncol. (2023) 53:722–9. doi: 10.1093/jjco/hyad036
30. Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. (2016) 7:105–14.
31. American Cancer Society. Cancer Facts & Figures 2024. Atlanta: American Cancer Society (2024). Available at: https://www.cancer.org/cancer/types/stomach-cancer/about/key-statistics.html (Accessed November 7, 2024).
32. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. (2019) 14:26–38. doi: 10.5114/pg.2018.80001
33. Abdel-Rahman O. ECOG performance score 0 versus 1: impact on efficacy and safety of first-line 5-FU-based chemotherapy among patients with metastatic colorectal cancer included in five randomized trials. Int J Colorectal Dis. (2019) 34:2143–50. doi: 10.1007/s00384-019-03430-y
34. Demirelli B, Babacan NA, Ercelep Ö, Öztürk MA, Kaya S, Tanrıkulu E, et al. Modified glasgow prognostic score, prognostic nutritional index and ECOG performance score predicts survival better than sarcopenia, cachexia and some inflammatory indices in metastatic gastric cancer. Nutr Cancer. (2021) 73:230–8. doi: 10.1080/01635581.2020.1749290
35. Janssens K, Boeckx N, Van Camp G, De Beeck KO, Fransen E, Calay F, et al. Comparing survival in left-sided and right-sided colorectal carcinoma: A Belgian population-based study. Ann Oncol. (2018) 29:v98. doi: 10.1093/annonc/mdy150.017
36. Asghari-Jafarabadi M, Wilkins S, Plazzer JP, Yap R, McMurrick PJ. Prognostic factors and survival disparities in right-sided versus left-sided colon cancer. Sci Rep. (2024) 14:12306. doi: 10.1038/s41598-024-63143-3
37. Sangisetty SL, Miner TJ. Malignant ascites: A review of prognostic factors, pathophysiology and therapeutic measures. World J Gastrointest Surg. (2012) 4:87–95. doi: 10.4240/wjgs.v4.i4.87
38. Dowling GP, Daly GR, Keelan S, Boland F, Toomey S, Hill ADK, et al. Efficacy and safety of trastuzumab deruxtecan in breast cancer: A systematic review and meta-analysis. Clin Breast Cancer. (2023) 23:847–55.e2. doi: 10.1016/j.clbc.2023.09.005
Keywords: trastuzumab deruxtecan (T-DXd), antibody-drug conjugate (ADC), gastrointestinal malignancies, safety, efficacy, HER2-positive
Citation: Hussain A, Iqbal Q, Isaac S, Shariff F, Tariq E, Awais H, Mainkar N, Reis HL, Arora A, Deotare A, Tasleem A, Valasapalli S, Paracha M, Hashmi M, Battah H and Muzaffar M (2025) Efficacy and safety of trastuzumab deruxtecan in gastrointestinal malignancies: a systemic review and meta-analysis. Front. Gastroenterol. 4:1559934. doi: 10.3389/fgstr.2025.1559934
Received: 13 January 2025; Accepted: 02 April 2025;
Published: 29 April 2025.
Edited by:
Ali H Zaidi, Allegheny Health Network, United StatesReviewed by:
Ryan Varghese, Saint Joseph’s University, United StatesCristiane Tefé-Silva, Barão de Mauá University Center, Brazil
Copyright © 2025 Hussain, Iqbal, Isaac, Shariff, Tariq, Awais, Mainkar, Reis, Arora, Deotare, Tasleem, Valasapalli, Paracha, Hashmi, Battah and Muzaffar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahvish Muzaffar, bXV6YWZmYXJtQGVjdS5lZHU=
 Ali Hussain
Ali Hussain Qamar Iqbal
Qamar Iqbal Sangeetha Isaac1
Sangeetha Isaac1 Ezza Tariq
Ezza Tariq Heidi Lynn Reis
Heidi Lynn Reis Maya Hashmi
Maya Hashmi Hamdi Battah
Hamdi Battah Mahvish Muzaffar
Mahvish Muzaffar