Abstract
Introduction:
Disorders of gut-brain interaction (DGBI), including irritable bowel syndrome and functional dyspepsia, are chronic gastrointestinal syndromes characterized by visceral hypersensitivity and altered brain-gut signaling in the absence of known structural pathology. A significant proportion of individuals with DGBI have comorbid psychiatric conditions, especially anxiety and depression, highlighting the biopsychosocial underpinnings of these disorders.
Methods:
This narrative review synthesizes the neurophysiological, psychological, pharmacological, and psychotherapeutic literature related to DGBI. We examined the role of gut-brain axis dysregulation, the prevalence and impact of psychiatric comorbidity, and evaluated current treatment modalities, including neuromodulators, brain-gut behavior therapies (BGBTs), and dietary interventions.
Results:
Neuroimaging and genetic studies support the role of emotional and cognitive circuits in modulating gut sensitivity and symptom perception. Psychiatric comorbidity, particularly anxiety, is bidirectionally linked to DGBI and influences treatment response. Neuromodulators such as tricyclic antidepressants demonstrate modest efficacy. BGBTs—including cognitive behavioral therapy and gut-directed hypnotherapy—exhibit comparable efficacy to pharmacologic treatments, with sustained symptom relief and additional benefit on mood and illness-related beliefs.
Discussion:
DGBI represent complex, stress-sensitive conditions best managed through multidisciplinary care. Integration of pharmacologic neuromodulation, psychotherapeutic interventions, and dietary strategies targeting the brain-gut axis offers the most comprehensive approach. Future research should refine treatment matching based on symptom phenotype, psychological profile, and gut-brain biomarkers to improve long-term outcomes.
Introduction
Dysregulation of the brain-gut axis, or the bidirectional communication system between the central and enteric nervous systems, is the core feature of the disorders of gut-brain interaction (DGBI), a subset of gastrointestinal conditions. The DGBI are syndromes defined by a set of clinical features and symptoms, without demonstrable pathology on testing or biomarkers that enable diagnosis. Conceptualized as stress-sensitive, biopsychosocial disorders, it is estimated that approximately half of all patients with DGBI have a comorbid psychiatric condition, with anxiety disorders being the most common. In this review, we describe the pathophysiology of these conditions, as well as the psychopathological profile often associated with their presentations, and discuss the evidence supporting the benefit of pharmacologic, psychotherapeutic, and dietary interventions for these patients.
Methods
To inform our narrative review, we developed five structured PubMed search strategies targeting key thematic areas related to disorders of gut-brain interaction (DGBIs): neurophysiological mechanisms, psychological comorbidity, pharmacologic treatment, psychotherapy, and dietary interventions (see Appendix 1). Each search string included both umbrella terms (e.g., “functional gastrointestinal disorders,” “IBS,” “functional dyspepsia”) and topic-specific keywords (e.g., “brain-gut axis,” “anxiety,” “SSRIs,” “CBT,” “FODMAP”) as well as filters for publication date (1995–present), English language, human adults, and title/abstract field limits to improve specificity. These searches were designed to identify the most representative, conceptually illustrative, clinically meaningful, and high-yield literature relevant to each domain. Final article selection from each search was guided by the authors’ clinical experience and research expertise.
Neurophysiological basis of gastrointestinal disorders (brain-gut interaction)
The “brain-gut axis” is a bidirectional communication system between the central nervous system (CNS) and the enteric nervous system (ENS). This system signals homeostatic information to the brain through neural (spinal and vagal) and humoral pathways, influenced by the gut microbiome and relying on immune, endocrine, neural, and metabolic pathways (1, 2). Dysregulation of this axis is the core feature of disorders of the DGBI (3).
Visceral pain arises from the conscious perception of gut-brain signals induced by noxious stimuli. These signals are processed in a homeostatic-afferent network (brainstem sensory nuclei, thalamus, posterior insula) and modulated by emotional (amygdala, anterior cingulate cortex) and cognitive circuits (prefrontal cortex, anterior insula). Descending projections modulate pain at the spinal level. Dysfunction in these systems is believed to contribute to visceral hypersensitivity, a hallmark of the DGBI (4). Attempting to elucidate specific areas in the brain associated with this visceral hypersensitivity, structural MRI studies in individuals with IBS have identified some distinctions. These include increased gray matter volume in the primary and secondary somatosensory cortices and subcortical areas, as well as reductions in the posterior insula and superior frontal gyrus (5). Greater cortical thickness in the primary somatosensory cortex has been associated with higher pain intensity ratings during rectal distension, while larger nucleus accumbens volume correlates with lower rectal pain thresholds, suggesting that repeated visceral pain may drive neuroplastic changes in somatosensory and reward-related regions (5). Additional structural abnormalities have been reported in the insular cortex, where reduced cortical thickness and gray matter volume are linked to longer symptom duration and heightened visceral sensitivity (6). Functional neuroimaging further supports the insula’s role as an integrative hub, showing increased activation during rest and in response to visceral stimuli, such as colorectal distension, in both irritable bowel syndrome (IBS) and animal models (7).
Compared to healthy populations, patients with IBS have increased brain activity in homeostatic-afferent regions (8), and individuals with functional dyspepsia (FD) fail to deactivate the amygdala during anticipated gastric distension, which is correlated with higher levels of anxiety (9). Anxiety-related impairment of the descending modulatory system may contribute to why physiological gastric distension is perceived as painful (4).
Animal studies have further clarified the insula’s role in modulating pain: reducing the excitability of pyramidal neurons within this region attenuated both visceral hypersensitivity and anxiety-like behavior associated with pain, highlighting the insular cortex as a potential therapeutic target for DGBIs (10). The neural pathway connecting the insular cortex and the nucleus tractus solitarius (NTS) represents a core component of brain-gut communication. Afferent signals from the gastrointestinal tract travel via the NTS to the insula, influencing central sensitization by encoding visceral discomfort. Descending projections from pyramidal neurons in the insula can influence the medullary vagal complex, thereby modulating gastrointestinal motility and contributing to peripheral pain sensitization (11). This bidirectional circuit may underlie the interplay between emotional regulation and somatic symptom perception in functional gastrointestinal disorders.
There is evidence that diet also plays a key role in DGBI symptomatology; food form and nutrient composition may trigger GI symptoms through various mechanisms, including bacterial fermentation altering gut microbiota, osmotic effects in the intestines, gas production, and immune responses (12). Several microbial- neural mechanisms have been implicated in visceral pain modulation. For instance, both Lactobacillus reuteri supplementation and pharmacologic blockade of IK(Ca) channels have produced similar changes in colonic motility and excitability of myenteric neurons in rodent models, suggesting a shared pathway influencing pain perception (13). In addition, TRPA1 receptors, abundantly expressed in the nodose ganglia (the inferior sensory gangial of the two vagus nerves), act as detectors of chemical irritants and contribute to the neurobiology of inflammatory pain. There is emerging speculation that damage to the intestinal epithelium may allow bacteria such as Edwardsiella tarda to directly activate TRPA1 in the nodose ganglia, potentially initiating neuroplastic changes in the central nervous system and contributing to disease pathogenesis (13–15).
Finally, a recent review of the literature identified three principal routes through which gut microbiota imbalances may influence brain function. First, the vagus nerve serves as a key conduit for signaling between the gut and central nervous system. Second, increased intestinal permeability may allow microbial products to cross into systemic circulation. Finally, certain bacterial metabolites, such as lipopolysaccharides, can trigger neuroinflammatory responses and alter neurotransmitter systems, potentially contributing to cognitive and affective symptoms seen in DGBIs (16).
Psychological comorbidity in gastrointestinal diseases
Psychological comorbidity in gastrointestinal disorders is often misconstrued as being confined to DGBI or as merely a consequence of physical illness (17). Psychological symptoms, however, are not only prevalent in DGBI but are often integral to their development and maintenance (4). A bidirectional two-sample Mendelian randomization study demonstrated that both genetically predicted depression and genetically predicted anxiety were significantly associated with an increased risk of several DGBI. In this context, these psychiatric traits were estimated based on genetic variants identified through large-scale genome-wide association studies (GWAS), which served as instrumental variables to infer potential causal effects while minimizing confounding factors. Specifically, genetically predicted depression was linked to a higher risk of functional dyspepsia (FD), IBS, and functional constipation (FC), while genetically predicted anxiety was associated with an increased risk of IBS (18).
A growing body of evidence supports a high psychological burden among the DGBI. Much of the evidence comes from studies examining the DGBI that are relatively more prevalent (e.g., IBS, FD), as there has been greater research on these. In IBS, for example, a multivariate analysis of 769 patients demonstrated that 44.9% reported significant anxiety, while 25.7% met criteria for depression (19). Furthermore, a large-scale study involving 158,565 cases and 300,995 controls in the United Kingdom identified that stress, anxiety, and depression are potential underlying etiological factors for IBS; individuals with these factors have higher odds of having IBS (OR: 1.06 (95% [C.I]: 1.03-1.08) (20).
Panic disorder is also prevalent in IBS, with studies indicating that close to 55% of IBS patients meet diagnostic criteria for this condition (21).Beyond mood and anxiety disorders, psychological concerns in IBS often include maladaptive cognitive patterns, such as catastrophizing, illness anxiety, and hypervigilance (22). Gastrointestinal-specific anxiety has been identified as a significant mediator between general anxiety symptoms and IBS symptom severity (22–24). Additionally, somatization has been associated with IBS, particularly the mixed subtype (IBS-M), with one study reporting that 32% of these patients exhibited this form of psychological distress (25).
FD is believed to share similar mechanisms with IBS, particularly visceral hypersensitivity, manifested as discomfort with distention of the gastric fundus during meals and even in resting states (26). Numerous studies have shown higher rates of anxiety and depression in individuals with FD, however, comorbidity estimates vary significantly (27). Of the psychiatric disorder, anxiety may have a unique mechanistic role in the development of FD. For instance, a 10-year follow-up study of 887 participants in Sweden found that anxiety at baseline was associated with new-onset FD, but no such association was observed for depression (28). Additionally, a phenotypic and genetic cross-disease analysis involving 10,078 cases identified anxiety disorders as one of the diagnoses most strongly associated with FD (29).
Functional constipation (FC), another DGBI, differs from IBS in that abdominal pain is not a requirement for diagnosis. Similar to IBS, anxiety and depression are more prevalent in patients with FC compared to the general population (30). A recent study involving elderly men and women found that 30% of patients with FC also had depression, while 21% also had anxiety (31). Preclinical research has also implicated serotonin production in the pathogenesis of both constipation and depression, highlighting potential shared pathophysiology and targets for treatment (32) (23, 33–40). In sum, systematic assessment of psychological comorbidities among individuals with DGBI is an important step in the formulation of an effective and comprehensive treatment plan.
Pharmacology
Pharmacologic treatment approaches, including the use of “neuromodulators” or medications targeting the gut-brain axis, to treat GI symptoms and gut motility are increasingly being incorporated into clinical practice. The term neuromodulator refers to medications including antidepressants, antipsychotics, and antiepileptics that exert their effects on the gut-brain axis through serotoninergic, noradrenergic, and dopaminergic pathways (41, 42). Neuromodulators may be particularly useful or relevant for patients with DGBI with comorbid anxiety or depression, though not exclusively. It has been noted that patients with comorbid anxiety are more likely to be treated with a neuromodulator by their gastroenterologist, but paradoxically, may also be less likely to demonstrate the desired treatment response to either neuromodulators or any medication prescribed for their gastrointestinal symptoms. This finding highlights the complex relationship between anxiety and gastrointestinal function, which currently available medications may not adequately or sufficiently target (42). This finding may also reflect what is often observed in clinical practice, that patients with anxiety tend to demonstrate greater sensitivity to their physical sensations, and thus may have more difficulty identifying medications that they can reliably tolerate.
Neuromodulators are increasingly being prescribed for patients with DGBI. A U.S study found that over half (55%) of all gastroenterologists agreed that neuromodulators were key to the management of IBS, for example, with the majority favoring tricyclic antidepressants, though the side effect burden was noted as a common concern (43). Evidence suggests, however, that neuromodulators are largely well tolerated, as, in at least one study, only ~10% of those receiving neuromodulators developed side effects that led to discontinuation (44). Interestingly, there is evidence suggesting that a strong nocebo effect in this patient population could predispose them to early discontinuation (44). Additionally, other studies have demonstrated that certain symptoms attributed by patients to neuromodulators existed prior to treatment and do not correspond to actual drug-related effects (45).
Further research is needed to evaluate the efficacy of neuromodulators to treat GI symptoms specifically (46, 47), though a growing body of research has examined TCAs and SSRIs. A recently published meta analysis evaluated the efficacy of antidepressants in the treatment of IBS demonstrated that those individuals taking antidepressants had three times the odds of experiencing global symptom improvement. Subgroup analyses indicated that both SSRIs and TCAs were associated with roughly two and three times the odds of global symptom improvement, respectively. Notably, this therapeutic effect was also evident in individuals who had not responded to standard initial treatments (48). These findings suggest a robust effect size, and build on earlier estimates from Ford et al. (47), in which the pooled NNT for antidepressants (including both SSRIs and TCAs) was 4.5, highlighting possible shifts in effect size estimates with the inclusion of more recent trials (46, 47). When comparing TCAs to placebo, a recent meta-analysis demonstrated that TCAs carry a relative risk of 0.66 (0.53-0.83) in failing improvement in global IBS symptoms at 4–12 weeks of treatment (49). However, these were also more likely than placebo to lead to side effects with a relative risk of 1.59 (1.26-2.06). In another study, TCAs were associated with a relative risk of 0.70 (0.62-0.80) of failing to improve global IBS symptoms and 0.69 (0.54-0.87) of failing to improve abdominal pain. SSRIs carried a relative risk of 0.74 (0.56-0.99) (50).
Relatively fewer clinical studies have examined serotonin-norepinephrine reuptake inhibitors (SNRIs) such as venlafaxine and duloxetine, in DGBI. The SNRIs, however, have empirical value based on their mechanisms of action (41). Evidence shows that venlafaxine increases colonic compliance, decreases tone, reduces postprandial colonic contractions, and reduces pain intensity ratings during graded distensions (51). SNRIs also carry a relative risk of 0.22 (0.08-0.59) of failing to improve abdominal pain (50). A recent small, retrospective study also demonstrated a likely benefit of SNRIs in targeting symptoms of bloating, though further research is needed (52).
Early research also indicates a likely benefit to other neuromodulators, such as buspirone and gabapentin, in the management of DGBI. In a recent meta-analysis, for example, buspirone improved bloating severity more than placebo but did not improve postprandial fullness or nausea severity (53). Low doses of gabapentin, on the other hand, can improve symptoms of functional dyspepsia, particularly postprandial fullness, upper abdominal pain, and nausea and vomiting (54). Selected studies outlining the evidence for neuromodulators in the management of DGBIs are outlined in Table 1.
Table 1
| Study | Study design | Sample size | Active arm | Comparator | Primary endpoint | Main findings |
|---|---|---|---|---|---|---|
| Ford, 2023 (96) | Placebo controlled RCT | 463 adults with IBS | Amitriptyline, 10 to 30mg daily | Placebo | IBS-SSS score at 6 months | Amitriptyline group with significantly decreased IBS-SSS score as compared to placebo (-27.0 [-46.9 to -7.1]; p=0.0079) |
| Agger, 2017 (97) | Placebo controlled, double blind RCT | 120 adults with multiorgan bodily distress syndrome | Imipramine, 25 to 75mg daily | Placebo | Self-reported overall health improvement on 5 point CGI scale | OR 3.3 (1.6-6.8) favoring treatment with imipramine |
| Ladabaum, 2010 (98) | Placebo controlled, double blind RCT | 54 adults with IBS | Citalopram 20mg daily | Placebo | Overall IBS symptom score | No difference between citalopram and placebo (d=0.398) |
| Houghton, 2025 (99) | Placebo controlled, double blind RCT | 308 adults with IBS | Novel alpha-2-delta ligand | Placebo | adequate relief of abdominal pain/discomfort for ≥ 50% of the active treatment period | Novel medication no more effective than placebo (OR 0.96 [0.65, 1.41]) |
| Cheong, 2018 (100) | Placebo controlled, double blind RCT | 107 adults with functional dyspepsia | Imipramine 50mg daily | Placebo | Self-reported satisfactory relief of global dyspepsia symptoms at 12 weeks | Imipramine more effective than placebo at providing relief of global dyspepsia symptoms, with NNT = 4 |
| Talley, 2015 (101) | Placebo controlled, double blind RCT | 292 adults with functional dyspepsia | Amitriptyline 50mg daily and escitalopram 10mg daily | Placebo | Self-reported adequate relief of symptoms at least 50% of the 10 treatment weeks | Amitriptyline had greater rate of relief of symptoms as compared to placebo (OR 2.1[1.04, 4.36]). No difference for those taking escitalopram as compared to placebo |
| Tack, 2012 (102) | Placebo controlled, double blind RCT crossover | 17 adults with functional dyspepsia | Buspirone 10mg TID before meals | Placebo TID before meals | Self-reported dyspepsia symptom severity (DSS) score | Significantly lower DSS following buspirone, but not placebo, treatment |
| Sharbafchi, 2023 (103) | Placebo controlled, double blind RCT | 37 adults with IBS | Duloxetine 60mg daily | Placebo | Mean IBS-SSS score at 10 weeks of treatment | Duloxetine group with lower IBS-SSS scores at week 10 as compared to placebo |
| Staller, 2019 (54) | Retrospective, open-label, observational study | 62 adults with functional dyspepsia | Gabapentin, dose range not specified however median ending daily dose 954mg ± 1184 mg | N/A | Change in PAGI-SYM score (with -0.3-point decrease being significant) | Mean decrease in PAGI-SYM score -0.44 points |
Select studies of psychopharmacological interventions in DGBIs.
Patient Assessment of Gastrointestinal Disorders-Symptom Severity Index (PAGI-SYM).
Irritable Bowel Syndrome Symptom Severity Scoring System (IBS-SSS).
Dyspepsia symptom severity (DSS).
Clinical Global Improvement (CGI).
Side effects of pharmacotherapy
Each neuromodulator group is associated with common side effects that clinicians should monitor regularly. TCAs frequently cause sedation, dry mouth, constipation, weight gain, and orthostatic hypotension, particularly at higher doses (55). SSRIs and SNRIs often lead to nausea, insomnia or somnolence, sexual dysfunction, and gastrointestinal disturbances (56). Gabapentinoids, are commonly associated with drowsiness, dizziness, ataxia and rare cases self-harm behaviors (57, 58).
While these side effects are generally manageable, clinicians should also be aware of serious adverse events, especially in vulnerable populations. TCAs have been associated with cardiotoxicity, including QRS/QT prolongation and ventricular arrhythmias due to sodium-channel blockade, even at therapeutic doses (59). A systematic review found that amitriptyline, nortriptyline, and clomipramine carry measurable cardiac risks even with routine use (60).
SSRIs and SNRIs increase the odds of hyponatremia (OR = 3.16; 95% CI 1.91–5.23), with event rates of 7.4% for SNRIs, 5.6% for SSRIs, and 2.7% for TCAs. Evidence suggests this risk is more pronounced during the first two weeks of treatment, particularly among elderly patients or those on diuretics (61).
Gabapentin and pregabalin carry a recognized risk of misuse. They are frequently used to enhance opioid effects or self-manage anxiety, insomnia, or withdrawal, often leading to dose escalation, loss of control, and psychiatric withdrawal symptoms (62). Misuse rates range from 1.6% in the general population to over 60% among individuals with opioid use, with motivations including euphoria and deliberate intoxication (63, 64).
Psychotherapy
Many psychotherapeutic approaches, or brain-gut behavior therapies (BGBTs), have also demonstrated efficacy in reducing the symptom burden of patients with DGBIs. BGBTs are short-term, non-pharmacologic interventions that have been adapted from traditional psychotherapies to specifically target gastrointestinal symptoms, though they may also benefit psychological comorbidity (65). Of the BGBTs currently available, cognitive-behavioral therapy (CBT) and gut-directed hypnotherapy have been the most studied and thus have the most evidence (47).
Most studies examining the efficacy of BGBTs have been completed in individuals with either IBS or FD, given their higher prevalence, though expert consensus is that future research will likely demonstrate efficacy of the BGBTs for other DGBI as well (65). A recent meta-analysis demonstrated that the number needed to treat (NNT) for CBT for IBS is approximately 4, and the NNT for gut-directed hypnotherapy for IBS is approximately 5 (47). Notably, this is comparable to the NNT of 4.5 for antidepressants in patients with IBS.
Compared to CBT and gut-directed hypnotherapy, relatively fewer studies have examined other BGBTs such as exposure-based therapy, mindfulness, disease self-management programs, or psychodynamic psychotherapy, among others. Studies evaluating both CBT and hypnotherapy have yielded promising results, and these will be summarized in subsequent sections. Though fewer studies have examined these other types of BGBTs, a growing body of evidence indicates a likely benefit to their use. For instance, a systematic review and meta-analysis of randomized controlled trials (RCTs) of individuals with IBS suggested that mindfulness, compared to control, was associated with improvement in symptom severity, pain, and quality of life (66). An RCT comparing acceptance and commitment therapy (ACT) to dialectical behavioral therapy (DBT) and mindfulness based stress reduction (MBSR) in individuals diagnosed with IBS demonstrated that the ACT intervention was associated with lower levels of IBS symptoms, anxiety, and depression, as well as higher quality of life when compared to the other groups (67). Finally, based on two RCTs, psychodynamic psychotherapy appears to lead to improvement in IBS symptoms with a NNT of 4 (47).
Further research is needed to elucidate the mechanisms of BGBTs. Early mechanistic work has explored the role of “psychological” factors, such as self-efficacy, a positive treatment expectancy for symptom improvement, or patient-therapist bonding, as potential mediators of treatment (68), as well as a variety of “biological” factors such as gut microbiome composition (69). In the following sections, we summarize the current evidence for gut-directed hypnotherapy and CBT for DGBIs, and selected papers are outlined in Table 2.
Table 2
| Study | Study design | Sample size | Active arm | Comparator | Primary endpoint | Main findings |
|---|---|---|---|---|---|---|
| Lindfors, 2012a (71) | RCT | 90 adults with IBS | Gut directed hypnotherapy | Supportive therapy | GI symptom questionnaire at 3 months | Greater improvement in hypnotherapy group as compared to supportive therapy 3.7 (0.3–7.2); p = 0.03 |
| Lindfors, 2012b (71) | RCT | 48 adults with IBS | Gut directed hypnotherapy | Waitlist | GSRS-IBS at 3 months | Significant reduction in symptoms in active treatment, but no significant difference between treatment and control groups (0.33 [−0.22, –0.91], p=0.22) |
| Lackner, 2018 (76) | RCT | 436 adults with IBS | Standard CBT and minimal contact CBT (MC-CBT) | IBS education | % participants “substantially improved” or “moderately improved” on CGI immediately after treatment | Significantly larger percentage of participants substantially or moderately improved in MC-CBT as compared to IBS education |
| Thakur, 2017 (104) | RCT | 106 adults with IBS | Emotional awareness and expression training (EAET); relaxation training | Waitlist | Change in IBS-SSS at 10 weeks post-treatment | EAET but not relaxation training reduced IBS symptoms severity as compared to waitlist (F (4, 206)=2.43, p = .026) |
| Everitt, 2019 (105) | RCT | 558 adults with IBS | Telephone delivered CBT and Web (TCBT) delivered CBT (WCBT) | Treatment as usual (TAU; no additional psychological therapy) | IBS-SSS at 12 months after initiation | Significantly lower IBS-SSS at 12 months in TCBT (p<0.001) and WCBT (p=0.002) as compared to TAU |
| Hoekman, 2021 (70) | Open-label RCT | 63 adults with IBD in remission and IBS | Gut directed hypnotherapy | Standard medical treatment of various modalities | % participants with reduction of ≥50% on visual analog scale for pain at week 40 | No difference between groups in proportion of participants meeting primary outcome [difference=3% [−19 to 24%], p = 0.81 |
Select studies of psychological interventions in DGBIs.
Gastrointestinal Symptom Rating Scale IBS version (GSRS-IBS).
Irritable Bowel Syndrome Symptom Severity Scoring System (IBS-SSS).
Hypnotherapy
We estimate that there are at least 15 RCTs comparing the effectiveness of hypnotherapy to several interventions including standard care, other forms of therapy, diet, exercise, or education. Collectively, these RCTs demonstrate favorable results for hypnotherapy for several different DGBIs and DGBI-related symptoms.
Hoekman et al., randomized 80 adult participants in an open-label study evaluating the effect of hypnosis versus standard medical treatment (following the Dutch multidisciplinary guideline for diagnosis and management of IBS) on clinical remission and biochemical remission (using fecal calprotectin) in patients with quiescent IBD plus IBS-type symptoms. The primary outcome (≥50% reduction in symptom severity measured with the IBS symptom severity scale (IBS-SSS) at week 40) was met in 30% of the patients randomized to hypnotherapy versus 27% of the ones in standard treatment, without statistical significance. Notably, adequate relief was reported in 60% vs 40% in hypnotherapy and standard treatment, respectively (70).
Lindfors et al., conducted a series of 2 RCTs evaluating the effectiveness of hypnotherapy on IBS. In the first study, participants were randomized to receive the hypnotherapy intervention or supportive therapy in a psychology private practice. In the second study, participants were randomized to receive the intervention or be placed on a waitlist. In both studies, IBS symptoms improved at 3 months in the hypnotherapy group but not in the control group, with effects sustained at 1-year follow-up (71).
A 2007 systematic review including 4 studies comparing hypnotherapy to psychotherapy, placebo, waitlist, or standard medical management, argued that hypnotherapy was superior to waitlist control or standard medical treatment in reducing the severity of IBS symptoms. However, they were unable to pool the data in a meta-analysis given differences in outcome measures and study design, which speaks to the methodological heterogeneity of the included studies (72).
Cognitive behavioral therapy
At least 30 RCTs have examined CBT (most often in patients with IBS) and demonstrated its efficacy in reducing symptoms in a variety of different formats and forms of delivery. It has been also demonstrated that the benefit provided by CBT is often maintained long-term (73). For example, one study randomized patients with IBS to either weekly hour CBT sessions over 10 weeks, 4-hour sessions over 10 weeks, or wait list, and demonstrated that 30% of people receiving CBT were rapid responders and the majority of these maintained the benefit at a 3-month follow-up examination (74). Interestingly, rapid responders were more likely to have higher symptom severity (74). In another study, it was demonstrated that the benefits of using a self-administered and a therapist-administered CBT intervention were comparable (75). A large, 3-arm randomized trial allocating over 400 participants with IBS to either weekly hour-long CBT sessions, 4 home-based CBT sessions with minimal therapist contact, or 4 sessions of IBS education, demonstrated that home CBT is as effective as standard CBT and both of these interventions were slightly superior to education alone at 6 months follow up (76, 77). Furthermore, in a 12-month follow-up analysis from this same study, they concluded that over 30% of the participants receiving either version of CBT sustained the same benefits, compared to only 20% of those receiving IBS education (73). Notably, a multicenter randomized control trial found that the efficacy of CBT is similar when delivered in person or via app, lowering the barrier of delivery of these interventions (78). And a meta-analysis of app-delivered CBTs in IBS found medium to large effect sizes on IBS (79).Another study suggested that the benefits of CBT on these symptoms might be mediated by a positive treatment expectancy for symptom improvement and a good patient-therapist relationship, both early and in the long-term (68).
While BGBTs such as cognitive behavioral therapy and gut-directed hypnotherapy demonstrate efficacy comparable to pharmacologic approaches, their real-world implementation can be limited by accessibility, acceptability, cost, and availability of trained providers. In many settings, neuromodulators may be more readily accessible and affordable, which can influence both clinician recommendations and patient preferences. Acknowledging these practical barriers is essential when translating evidence into individualized treatment planning.
Dietary interventions
Dietary interventions may also play an important role in treatment for some patients with DGBIs, particularly when implemented under the supervision of a dietician. The low fermentable oligo-, di-, monosaccharides, and polyols (FODMAP) diet is perhaps the most well-known and established dietary therapy that has been shown to reduce symptoms of IBS and other DGBIs (80). A recent meta-analysis, for example, found a moderate reduction in symptom severity (standardized mean difference -0.53; 95% CI, -0.68 to -0.38) from the FODMAP diet compared to control diets among patients with IBS (81). Interestingly, a randomized clinical trial compared hypnotherapy, the FODMAP diet, or a combination of both and assessed IBS symptom severity (82). At week 6, all three groups demonstrated similar effectiveness in reduction of symptoms with no notable differences in the effect between groups; ≥20% improvement was achieved in 72%, 71%, and 72% across groups at 6 weeks and 74%, 82%, and 54% at 6 months, respectively. Compared to the two other groups, however, hypnotherapy was superior when assessing anxiety and depression at 6 months (82).
Other dietary interventions have also demonstrated benefit for patients with DGBIs. Certain fibers, for example, can help regulate bowel movements, reduce colonic fermentation to minimize bloating, and benefit gut microbiota. When combined with the low FODMAP diet, psyllium and inulin reduce colonic gas production, and sugarcane bagasse with resistant starch shifts fermentation to the distal colon – both potentially easing GI symptoms (83). Peppermint oil capsules have also been studied and a meta-analysis demonstrated their association with a relative risk of 0.63 (0.48-0.83) for failing to achieve improvement in global IBS symptoms at 4–12 weeks of follow up. And interestingly, there were no significant differences when comparing the effectiveness of peppermint oil capsules to that of TCAs in this meta-analysis (49).
Emerging research highlights how strengthening the gut microbiota and enteric nervous system with prebiotics, probiotics and their metabolites (like tryptophan and short-chain fatty acids [SCFAs]) may improve gut-brain function. Synbiotics –combinations of probiotics (e.g., Lactobacillus, Enterococcus, and Bifidobacterium) and prebiotics (like inulin and resistant starch)– have been shown to boost neurotransmitters and neuropeptides (e.g., gamma-aminobutyric and brain-derived neurotrophic factor), improving CNS activity and psychiatric disease-related functions, such as anxiety, depression, stress, and memory (84). Furthermore, tryptophan, an essential amino acid, play a role in gut-brain signaling by influencing metabolic pathways linked to CNS inflammation. Emerging evidence suggests that microbial metabolites of tryptophan may help reduce neuroinflammation. Disruptions in tryptophan metabolism have been observed in individuals with DGBI (e.g., IBS) and neuropsychiatric conditions (e.g., depression, autism). While human studies are limited, some studies suggest that higher dietary tryptophan intake may help reduce symptoms like anxiety, irritability, and low mood – likely through enhanced levels of serotonin in the brain (85, 86). In mice, tryptophan-rich diets have been shown to improve depression and anxiety behaviors (86, 87).
Digestive enzymes can also serve as a targeted approach for some food sensitivities. Oral lactase, for example, decreases hydrogen levels and symptoms after lactose intake. Similarly, α-galactosidase, which breaks down galactooligosaccharides in legumes, nuts, and soy, has been shown to reduce symptoms in patients sensitive to plant-based protein sources (83).
Though food avoidance and use of exclusion diets can reduce symptoms for some patients, there is growing concern and some preliminary evidence that the use of exclusion diets can increase the risk for development of avoidant/restrictive food intake disorder (ARFID) among patients who follow these diets without sufficient provider guidance (88). Therefore, to reduce the risk of ARFID when using elimination diets, it is recommended that providers (e.g., gastroenterologists, dietitians) routinely monitor the extent of a patient’s food restriction, especially in those with anxiety or rigid eating behaviors. Screening for disordered eating patterns is essential, and involving a behavioral health specialist can provide the added support some patients need. Treatments like exposure-based CBT can gradually help patients feel more comfortable with eating, support better nutrition, and improve their overall quality of life (89). For screening, the Nine-Item ARFID Screen (NIAS) is a brief, validated tool that assesses three core domains of ARFID: sensory sensitivities, fear of aversive consequences, and low interest in eating (90). Using tools like the NIAS in conjunction with clinical judgment can help identify at-risk patients early and guide timely referral to specialized care.
In sum, dietary interventions are often best implemented under the supervision of a dietician, when possible, to mitigate against inadvertent impairment of nutritional status and/or eating-related quality of life.
Emerging treatments
Outside of existing medications and therapies, there are emerging pharmacological and procedural treatments that have demonstrated promising results for the management of DGBIs, such as low dose naltrexone, transcutaneous vagal nerve stimulation (tVNS), and fecal microbiota transplant (FMT). Naltrexone is a peripherally restricted κ-opioid antagonist that has been used for chronic pain syndromes, and one open label study concluded that low dose naltrexone increased number of pain free days in IBS (91). tVNS via the auricular concha in animal models of IBS reversed both gastrointestinal permeability and depression-like symptoms (92), and human studies of tVNS have shown decreased intestinal permeability (93)and trends towards clinical remission in Crohn’s disease (94). FMT is another avenue of active investigation and promise given findings of intestinal dysbiosis in DGBI (95). A placebo controlled RCT of FMT in IBS showed improved IBS symptoms at 12 weeks, though the results diminished over a year (95). Available data in animal and human studies suggest these treatments have therapeutic potential for DGBI, but further robust studies are required before their widespread use.
Approach to integrating pharmacotherapy, psychotherapy, and dietary interventions into treatment
It can be helpful to have a systematic approach for when to integrate these treatment modalities (e.g., pharmacotherapy, psychotherapy, and dietary interventions) into the treatment pathway for each patient. While many systems and individual patient factors will likely impact treatment implementation in practice, we have found the approach suggested by Keefer et al. (65) to be quite helpful (65). This approach suggests using the patient’s most prominent presenting symptom (e.g., gastrointestinal symptom, psychiatric symptom, or behavior/anxiety specific to the gastrointestinal symptom) as a guide. Patients presenting primarily with a gastrointestinal symptom (e.g., nausea, pain) may benefit from earlier treatment with a neuromodulator, to at least quell some symptoms, prior to being referred for a BGBT, and to facilitate more successful BGBT engagement. Patients presenting primarily with a psychiatric symptom (e.g., depression, anxiety) often benefit from earlier referral to psychiatry, ideally for collaboration around selection of a neuromodulating agent. And finally, patients presenting primarily with anxiety or behaviors specific to their gastrointestinal illness, such as gastrointestinal symptom-specific anxiety or illness-specific coping behaviors, would likely benefit from earlier referral for a BGBT. The foundation of treatment for all patients, regardless of their presenting symptom, should likely include a combination of symptom-specific medical treatment, lifestyle modification, stress management, and dietary intervention (65) (Figure 1).
Figure 1
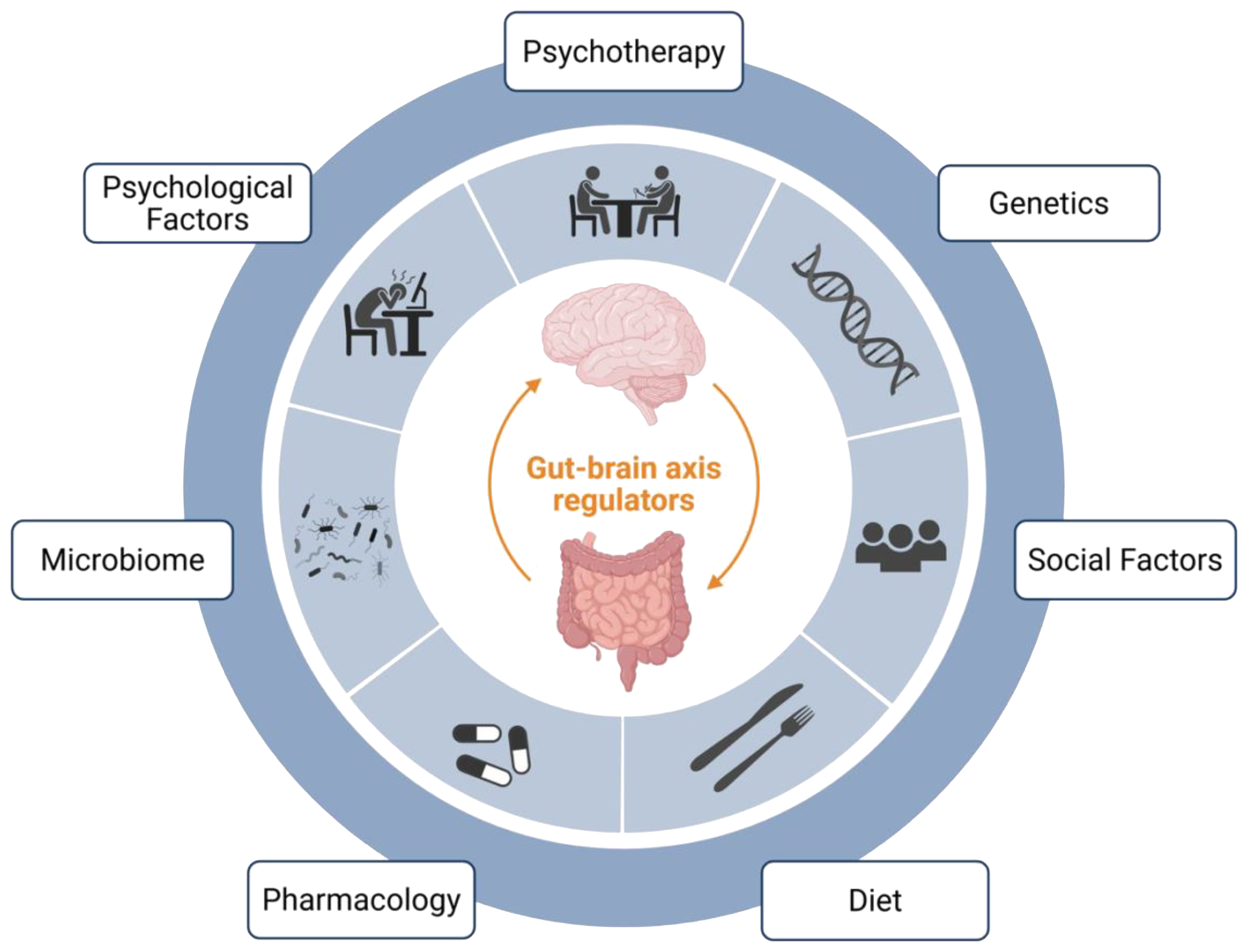
Select gut-brain axis regulators. This figure depicts some of the key regulators of DGBI symptomatology, each of which affects the gut-brain axis. Some of these factors are more easily modifiable (e.g., social factors, diet, microbiome, and psychological factors), and represent potential treatment targets. Finally, direct treatment strategies – which also modulate the gut-brain axis - include pharmacology and psychotherapy. Created in BioRender. Madva, EN. (52) https://BioRender.com/rl3j79f.
Limitations of this study
This narrative review has several limitations. First, even though we developed several search strategies, the final selection of studies was conducted using an approach informed by the authors’ expertise, rather than a systematic methodology, which may have introduced selection bias. Second, publication bias may have influenced the body of literature considered, as studies with negative or null findings are less likely to be published. Third, the heterogeneity of outcome measures across studies—ranging from symptom severity to quality of life—limits the ability to directly compare findings or draw unified conclusions. Fourth, this review mostly focused primarily on IBS and FD due to data availability. As a result, these summarized findings may not be generalizable to other, less-studied DGBIs, such as functional biliary pain or centrally mediated abdominal pain syndrome. Finally, most of the literature reviewed is based on populations in Western, high-income countries, which may reduce the generalizability of conclusions to non-Western or resource-limited settings.
Conclusion
In conclusion, there is a well described and robust connection between the gut and the brain. Dysfunction of the gut-brain connection can manifest as an array of psychiatric and gastrointestinal symptoms. As the DGBI are conceptualized as stress-sensitive biopsychosocial disorders, the most effective treatment approaches are comprehensive, aiming to address the biological, psychological, and social factors contributing to both the development and maintenance of these often-debilitating symptoms. There is a growing body of evidence supporting the use of gut-brain axis medications, BGBTs, and dietary therapies to target DGBI-related symptoms. In sum, a multidisciplinary treatment approach, that benefits from the expertise of gastrointestinal, mental health, and dietary clinicians, has the potential to significantly improve clinical outcomes.
Statements
Author contributions
FB: Writing – review & editing, Methodology, Validation, Resources, Writing – original draft, Conceptualization. JG: Writing – original draft. TW: Writing – original draft, Writing – review & editing, Validation, Data curation, Formal analysis, Investigation. RB: Writing – original draft, Writing – review & editing. EM: Writing – review & editing, Resources, Validation, Methodology, Writing – original draft, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors have no conflicts of interest to report related to this project. Time for analysis and article preparation was funded by the National Institutes of Health R25MH135837 (Dr. Francisco J. Barrera Flores), and the National Institutes of Health, K23DK138875 (Dr. Elizabeth Madva). The study sponsors had no role in the study design, collection, analysis, or interpretation of the data or writing of the report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgstr.2025.1637172/full#supplementary-material
References
1
Mayer EA Tillisch K . The brain-gut axis in abdominal pain syndromes. Annu Rev Med. (2011) 62:381–96. doi: 10.1146/annurev-med-012309-103958
2
Khlevner J Park Y Margolis KG . Brain-gut axis: clinical implications. Gastroenterol Clin North Am. (2018) 47:727–39. doi: 10.1016/j.gtc.2018.07.002
3
Mertz HR . Overview of functional gastrointestinal disorders: dysfunction of the brain-gut axis. Gastroenterol Clin North Am. (2003) 32:463–76, v. doi: 10.1016/S0889-8553(03)00019-0
4
Van Oudenhove L Crowell MD Drossman DA Halpert AD Keefer L Lackner JM et al . Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology. (2016) 150:1355–67.e2. doi: 10.1053/j.gastro.2016.02.027
5
Grinsvall C Ryu HJ Van Oudenhove L Labus JS Gupta A Ljungberg M et al . Association between pain sensitivity and gray matter properties in the sensorimotor network in women with irritable bowel syndrome. Neurogastroenterol Motil. (2021) 33:e14027. doi: 10.1111/nmo.14027
6
Kano M Dupont P Aziz Q Fukudo S . Understanding neurogastroenterology from neuroimaging perspective: A comprehensive review of functional and structural brain imaging in functional gastrointestinal disorders. J Neurogastroenterol Motil. (2018) 24:512–27. doi: 10.5056/jnm18072
7
Öhlmann H Koenen LR Labrenz F Engler H Theysohn N Langhorst J et al . Altered brain structure in chronic visceral pain: specific differences in gray matter volume and associations with visceral symptoms and chronic stress. Front Neurol. (2021) 12:733035. doi: 10.3389/fneur.2021.733035
8
Tillisch K Mayer EA Labus JS . Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. (2011) 140:91–100. doi: 10.1053/j.gastro.2010.07.053
9
Van Oudenhove L Vandenberghe J Dupont P Geeraerts B Vos R Dirix S et al . Abnormal regional brain activity during rest and (anticipated) gastric distension in functional dyspepsia and the role of anxiety: a H(2)(15)O-PET study. Am J Gastroenterol. (2010) 105:913–24. doi: 10.1038/ajg.2010.39
10
Bai Y Ma LT Chen YB Ren D Chen YB Li YQ et al . Anterior insular cortex mediates hyperalgesia induced by chronic pancreatitis in rats. Mol Brain. (2019) 12:76. doi: 10.1186/s13041-019-0497-5
11
Chang X Zhang H Chen S . Neural circuits regulating visceral pain. Commun Biol. (2024) 7:457. doi: 10.1038/s42003-024-06148-y
12
Moayyedi P Simrén M Bercik P . Evidence-based and mechanistic insights into exclusion diets for IBS. Nat Rev Gastroenterol Hepatol. (2020) 17:406–13. doi: 10.1038/s41575-020-0270-3
13
Han Y Wang B Gao H He C Hua R Liang C et al . Vagus nerve and underlying impact on the gut microbiota-brain axis in behavior and neurodegenerative diseases. J Inflamm Res. (2022) 15:6213–30. doi: 10.2147/JIR.S384949
14
Kunze WA Mao YK Wang B Huizinga JD Ma X Forsythe P et al . Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. (2009) 13:2261–70. doi: 10.1111/j.1582-4934.2009.00686.x
15
Ye L Bae M Cassilly CD Jabba SV Thorpe DW Martin AM et al . Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. (2021) 29:179–96.e9. doi: 10.1016/j.chom.2020.11.011
16
Sabine Kransel MSJZ Juan J Osorio Diago I Becerra Hernandez LV . Depression, anxiety, and intestinal microbiota: neurobiological mechanisms. Acta Neurol Colombiana. (2024) 40. doi: 10.22379/anc.v40i3.1341
17
Wu JC . Psychological co-morbidity in functional gastrointestinal disorders: epidemiology, mechanisms and management. J Neurogastroenterol Motil. (2012) 18:13–8. doi: 10.5056/jnm.2012.18.1.13
18
Liu T Wang Z Kang X Wang X Ren G Lv Y et al . Causal relationships between psychological disorders and functional gastrointestinal disorders: a bidirectional two-sample Mendelian randomization study. Eur J Gastroenterol Hepatol. (2024) 36:1267–74. doi: 10.1097/MEG.0000000000002825
19
Midenfjord I Polster A Sjovall H Tornblom H Simren M . Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol Motil. (2019) 31:e13619. doi: 10.1111/nmo.13619
20
Diao Z Xu W Guo D Zhang J Zhang R Liu F et al . Causal association between psycho-psychological factors, such as stress, anxiety, depression, and irritable bowel syndrome: Mendelian randomization. Medicine (Baltimore). (2023) 102:e34802. doi: 10.1097/MD.0000000000034802
21
Kumano H Kaiya H Yoshiuchi K Yamanaka G Sasaki T Kuboki T . Comorbidity of irritable bowel syndrome, panic disorder, and agoraphobia in a Japanese representative sample. Am J Gastroenterol. (2004) 99:370–6. doi: 10.1111/j.1572-0241.2004.04048.x
22
Person H Keefer L . Psychological comorbidity in gastrointestinal diseases: Update on the brain-gut-microbiome axis. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 107:110209. doi: 10.1016/j.pnpbp.2020.110209
23
Walker JR Ediger JP Graff LA Greenfeld JM Clara I Lix L et al . The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. (2008) 103:1989–97. doi: 10.1111/j.1572-0241.2008.01980.x
24
Labus JS Mayer EA Chang L Bolus R Naliboff BD . The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. (2007) 69:89–98. doi: 10.1097/PSY.0b013e31802e2f24
25
Patel P Bercik P Morgan DG Bolino C Pintos-Sanchez MI Moayyedi P et al . Irritable bowel syndrome is significantly associated with somatisation in 840 patients, which may drive bloating. Aliment Pharmacol Ther. (2015) 41:449–58. doi: 10.1111/apt.13074
26
Mertz H Fullerton S Naliboff B Mayer EA . Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. (1998) 42:814–22. doi: 10.1136/gut.42.6.814
27
Hartono JL Mahadeva S Goh KL . Anxiety and depression in various functional gastrointestinal disorders: do differences exist? J Dig Dis. (2012) 13:252–7. doi: 10.1111/j.1751-2980.2012.00581.x
28
Aro P Talley NJ Johansson SE Agreus L Ronkainen J . Anxiety is linked to new-onset dyspepsia in the swedish population: A 10-year follow-up study. Gastroenterology. (2015) 148:928–37. doi: 10.1053/j.gastro.2015.01.039
29
Garcia-Etxebarria K Carbone F Teder-Laving M Pandit A Holvoet L Thijs V et al . A survey of functional dyspepsia in 361,360 individuals: Phenotypic and genetic cross-disease analyses. Neurogastroenterol Motil. (2022) 34:e14236. doi: 10.1111/nmo.14236
30
Hosseinzadeh ST Poorsaadati S Radkani B Forootan M . Psychological disorders in patients with chronic constipation. Gastroenterol Hepatol Bed Bench. (2011) 4:159–63.
31
Liang J Zhao Y Xi Y Xiang C Yong C Huo J et al . Association between depression, anxiety symptoms and gut microbiota in chinese elderly with functional constipation. Nutrients. (2022) 14:5013. doi: 10.3390/nu14235013
32
Israelyan N Del Colle A Li Z Park Y Xing A Jacobsen JPR et al . Effects of serotonin and slow-release 5-hydroxytryptophan on gastrointestinal motility in a mouse model of depression. Gastroenterology. (2019) 157:507–21 e4. doi: 10.1053/j.gastro.2019.04.022
33
Collaborators GBDIBD . The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2020) 5:17–30. doi: 10.1016/S2468-1253(19)30333-4
34
Neuendorf R Harding A Stello N Hanes D Wahbeh H . Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J Psychosom Res. (2016) 87:70–80. doi: 10.1016/j.jpsychores.2016.06.001
35
Thavamani A Umapathi KK Khatana J Gulati R . Burden of psychiatric disorders among pediatric and young adults with inflammatory bowel disease: A population-based analysis. Pediatr Gastroenterol Hepatol Nutr. (2019) 22:527–35. doi: 10.5223/pghn.2019.22.6.527
36
Valitutti F Cucchiara S Fasano A . Celiac disease and the microbiome. Nutrients. (2019) 11:2403. doi: 10.3390/nu11102403
37
Slim M Rico-Villademoros F Calandre EP . Psychiatric comorbidity in children and adults with gluten-related disorders: A narrative review. Nutrients. (2018) 10:875. doi: 10.3390/nu10070875
38
Javadi S Shafikhani AA . Anxiety and depression in patients with gastroesophageal reflux disorder. Electron Physician. (2017) 9:5107–12. doi: 10.19082/5107
39
Jansson C Nordenstedt H Wallander MA Johansson S Johnsen R Hveem K et al . Severe gastro-oesophageal reflux symptoms in relation to anxiety, depression and coping in a population-based study. Aliment Pharmacol Ther. (2007) 26:683–91. doi: 10.1111/j.1365-2036.2007.03411.x
40
Nakada K Oshio A Matsuhashi N Iwakiri K Kamiya T Manabe N et al . Causal effect of anxiety and depression status on the symptoms of gastroesophageal reflux disease and functional dyspepsia during proton pump inhibitor therapy. Esophagus. (2023) 20:309–16. doi: 10.1007/s10388-022-00960-3
41
Drossman DA Tack J Ford AC Szigethy E Törnblom H Van Oudenhove L . Neuromodulators for functional gastrointestinal disorders (Disorders of gut-brain interaction): A rome foundation working team report. Gastroenterology. (2018) 154:1140–71.e1. doi: 10.1053/j.gastro.2017.11.279
42
Madva EN Staller K Huffman JC Kuo B Garcia-Fischer I Atkins M et al . Psychiatric comorbidities among adult patients with disorders of gut-brain interaction: Prevalence and relationships to treatment outcomes. Neurogastroenterol Motil. (2023) 35:e14493. doi: 10.1111/nmo.14493
43
Nulsen B LeBrett W Drossman DA Chang L . A survey of gastroenterologists in the United States on the use of central neuromodulators for treating irritable bowel syndrome. Aliment Pharmacol Ther. (2021) 54:281–91. doi: 10.1111/apt.16467
44
Glissen Brown JR Sanayei A Proctor S Flanagan R Ballou S Bain PA et al . Examining the nocebo effect in trials of neuromodulators for use in disorders of gut-brain interaction. Am J Gastroenterol. (2023) 118:692–701. doi: 10.14309/ajg.0000000000002108
45
Thiwan S Drossman DA Morris CB Dalton C Toner BB Diamant NE et al . Not all side effects associated with tricyclic antidepressant therapy are true side effects. Clin Gastroenterol Hepatol. (2009) 7:446–51. doi: 10.1016/j.cgh.2008.11.014
46
Ford AC Talley NJ Schoenfeld PS Quigley EM Moayyedi P . Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. (2009) 58:367–78. doi: 10.1136/gut.2008.163162
47
Ford AC Lacy BE Harris LA Quigley EMM Moayyedi P . Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. (2019) 114:21–39. doi: 10.1038/s41395-018-0222-5
48
Temido MJ Cristiano M Gouveia C Mesquita B Figueiredo P Portela F . Antidepressants in irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterol. (2025) 38:284–93. doi: 10.20524/aog.2025.0962
49
Black CJ Yuan Y Selinger CP Camilleri M Quigley EMM Moayyedi P et al . Efficacy of soluble fibre, antispasmodic drugs, and gut-brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. (2020) 5:117–31. doi: 10.1016/S2468-1253(19)30324-3
50
Khasawneh M Mokhtare M Moayyedi P Black CJ Ford AC . Efficacy of gut-brain neuromodulators in irritable bowel syndrome: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2025) 10:537–49. doi: 10.1016/S2468-1253(25)00051-2
51
Chial HJ Camilleri M Ferber I Delgado-Aros S Burton D McKinzie S et al . Effects of venlafaxine, buspirone, and placebo on colonic sensorimotor functions in healthy humans. Clin Gastroenterol Hepatol. (2003) 1:211–8. doi: 10.1016/S1542-3565(03)70038-X
52
Madva EN Varma S Small V Dekel M Salamone S Burton-Murray H et al . Pharmacologic neuromodulation for bloating. Scand J Gastroenterol. (2025) 60:1–5. doi: 10.1080/00365521.2025.2544306
53
Mohamedali Z Amarasinghe G Hopkins CWP Moulton CD . Effect of buspirone on upper gastrointestinal disorders of gut-brain interaction: A systematic review and meta-analysis. J Neurogastroenterol Motil. (2025) 31:18–27. doi: 10.5056/jnm24115
54
Staller K Thurler AH Reynolds JS Dimisko LR McGovern R Skarbinski KF et al . Gabapentin improves symptoms of functional dyspepsia in a retrospective, open-label cohort study. J Clin Gastroenterol. (2019) 53:379–84. doi: 10.1097/MCG.0000000000001034
55
Schneider J Patterson M Jimenez XF . Beyond depression: Other uses for tricyclic antidepressants. Cleve Clin J Med. (2019) 86:807–14. doi: 10.3949/ccjm.86a.19005
56
Kearns B Cooper K Orr M Essat M Hamilton J Cantrell A . The incidence and costs of adverse events associated with antidepressants: results from a systematic review, network meta-analysis and multi-country economic model. Neuropsychiatr Dis Treat. (2022) 18:1133–43. doi: 10.2147/NDT.S356414
57
Athavale A Murnion B . Gabapentinoids: a therapeutic review. Aust Prescr. (2023) 46:80–5. doi: 10.18773/austprescr.2023.025
58
Quintero GC . Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol. (2017) 9:13–21. doi: 10.2147/JEP.S124391
59
Mladěnka P Applová L Patočka J Costa VM Remiao F Pourová J et al . Comprehensive review of cardiovascular toxicity of drugs and related agents. Med Res Rev. (2018) 38:1332–403. doi: 10.1002/med.21476
60
Taylor D Poulou S Clark I . The cardiovascular safety of tricyclic antidepressants in overdose and in clinical use. Ther Adv Psychopharmacol. (2024) 14:20451253241243297. doi: 10.1177/20451253241243297
61
Leth-Møller KB Hansen AH Torstensson M Andersen SE Ødum L Gislasson G et al . Antidepressants and the risk of hyponatremia: a Danish register-based population study. BMJ Open. (2016) 6:e011200. doi: 10.1136/bmjopen-2016-011200
62
McNeilage AG Sim A Nielsen S Murnion B Ashton-James CE . Experiences of misuse and symptoms of dependence among people who use gabapentinoids: A qualitative systematic review. Int J Drug Policy. (2024) 133:104605. doi: 10.1016/j.drugpo.2024.104605
63
Goodman CW Brett AS . A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med. (2019) 179:695–701. doi: 10.1001/jamainternmed.2019.0086
64
Smith RV Havens JR Walsh SL . Gabapentin misuse, abuse and diversion: a systematic review. Addiction. (2016) 111:1160–74. doi: 10.1111/add.13324
65
Keefer L Ballou SK Drossman DA Ringstrom G Elsenbruch S Ljótsson B . A rome working team report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. (2022) 162:300–15. doi: 10.1053/j.gastro.2021.09.015
66
Baboș CI Leucuța DC Dumitrașcu DL . Meditation and irritable bowel syndrome, a systematic review and meta-analysis. J Clin Med. (2022) 11:6516. doi: 10.3390/jcm11216516
67
Taghvaeinia A Karami M Azizi A . Comparison of the effect of dialectical behavior therapy, acceptance and commitment therapy mindfulness-based stress reduction on irritable bowel syndrome symptoms, quality of life, anxiety and depression: A pilot randomized controlled trial. Psychiatr Q. (2024) 95:53–68. doi: 10.1007/s11126-023-10058-3
68
Lackner JM Jaccard J . Specific and common mediators of gastrointestinal symptom improvement in patients undergoing education/support vs. cognitive behavioral therapy for irritable bowel syndrome. J Consult Clin Psychol. (2021) 89:435–53. doi: 10.1037/ccp0000648
69
Jacobs JP Gupta A Bhatt RR Brawer J Gao K Tillisch K et al . Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome. (2021) 9:236. doi: 10.1186/s40168-021-01188-6
70
Hoekman DR Vlieger AM Stokkers PC Mahhmod N Rietdijk S de Boer NK et al . Hypnotherapy for irritable bowel syndrome-type symptoms in patients with quiescent inflammatory bowel disease: A randomized, controlled trial. J Crohns Colitis. (2021) 15:1106–13. doi: 10.1093/ecco-jcc/jjaa241
71
Lindfors P Unge P Arvidsson P Nyhlin H Björnsson E Abrahamsson H et al . Effects of gut-directed hypnotherapy on IBS in different clinical settings-results from two randomized, controlled trials. Am J Gastroenterol. (2012) 107:276–85. doi: 10.1038/ajg.2011.340
72
Webb AN Kukuruzovic RH Catto-Smith AG Sawyer SM . Hypnotherapy for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. (2007) 4:Cd005110. doi: 10.1002/14651858.CD005110.pub2
73
Lackner JM Jaccard J Radziwon CD Firth RS Gudleski GD Hamilton F et al . Durability and decay of treatment benefit of cognitive behavioral therapy for irritable bowel syndrome: 12-month follow-up. Am J Gastroenterol. (2019) 114:330–8. doi: 10.1038/s41395-018-0396-x
74
Lackner JM Gudleski GD Keefer L Krasner SS Powell C Katz LA . Rapid response to cognitive behavior therapy predicts treatment outcome in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. (2010) 8:426–32. doi: 10.1016/j.cgh.2010.02.007
75
Lackner JM Jaccard J Krasner SS Katz LA Gudleski GD Holroyd K . Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clin Gastroenterol Hepatol. (2008) 6:899–906. doi: 10.1016/j.cgh.2008.03.004
76
Lackner JM Jaccard J Keefer L Brenner DM Firth RS Gudleski GD et al . Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. (2018) 155:47–57. doi: 10.1053/j.gastro.2018.03.063
77
Lackner JM Keefer L Jaccard J Firth R Brenner D Bratten J et al . The Irritable Bowel Syndrome Outcome Study (IBSOS): rationale and design of a randomized, placebo-controlled trial with 12 month follow up of self- versus clinician-administered CBT for moderate to severe irritable bowel syndrome. Contemp Clin Trials. (2012) 33:1293–310. doi: 10.1016/j.cct.2012.07.013
78
Pathipati MP Scott LL Griser AC Staller K . Real-world outcomes for a digital prescription mobile application for adults with irritable bowel syndrome. Neurogastroenterol Motil. (2024) 36:e14811. doi: 10.1111/nmo.14811
79
Kim H Oh Y Chang SJ . Internet-delivered cognitive behavioral therapy in patients with irritable bowel syndrome: systematic review and meta-analysis. J Med Internet Res. (2022) 24:e35260. doi: 10.2196/35260
80
Popa SL Dumitrascu DI Pop C Surdea-Blaga T Ismaiel A Chiarioni G et al . Exclusion diets in functional dyspepsia. Nutrients. (2022) 14:2057. doi: 10.3390/nu14102057
81
Hahn J Choi J Chang MJ . Effect of low FODMAPs diet on irritable bowel syndromes: A systematic review and meta-analysis of clinical trials. Nutrients. (2021) 13:2460. doi: 10.3390/nu13072460
82
Peters SL Yao CK Philpott H Yelland GW Muir JG Gibson PR . Randomised clinical trial: the efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. (2016) 44:447–59. doi: 10.1111/apt.13706
83
So D Tuck C . Innovative concepts in diet therapies in disorders of gut-brain interaction. JGH Open. (2024) 8:e70001. doi: 10.1002/jgh3.70001
84
Ansari F Neshat M Pourjafar H Jafari SM Samakkhah SA Mirzakhani E . The role of probiotics and prebiotics in modulating of the gut-brain axis. Front Nutr. (2023) 10:1173660. doi: 10.3389/fnut.2023.1173660
85
Badrasawi MM Shahar S Abd Manaf Z Haron H . Effect of Talbinah food consumption on depressive symptoms among elderly individuals in long term care facilities, randomized clinical trial. Clin Interv Aging. (2013) 8:279–85. doi: 10.2147/CIA.S37586
86
Gao K Mu CL Farzi A Zhu WY . Tryptophan metabolism: A link between the gut microbiota and brain. Adv Nutr. (2020) 11:709–23. doi: 10.1093/advances/nmz127
87
Wang D Wu J Zhu P Xie H Lu L Bai W et al . Tryptophan-rich diet ameliorates chronic unpredictable mild stress induced depression- and anxiety-like behavior in mice: The potential involvement of gut-brain axis. Food Res Int. (2022) 157:111289. doi: 10.1016/j.foodres.2022.111289
88
Atkins M Zar-Kessler C Madva EN Staller K Eddy KT Thomas JJ et al . History of trying exclusion diets and association with avoidant/restrictive food intake disorder in neurogastroenterology patients: A retrospective chart review. Neurogastroenterol Motil. (2023) 35:e14513. doi: 10.1111/nmo.14513
89
Burton Murray H Staller K . When food moves from friend to foe: why avoidant/restrictive food intake matters in irritable bowel syndrome. Clin Gastroenterol Hepatol. (2022) 20:1223–5. doi: 10.1016/j.cgh.2021.09.017
90
Zickgraf HF Ellis JM . Initial validation of the Nine Item Avoidant/Restrictive Food Intake disorder screen (NIAS): A measure of three restrictive eating patterns. Appetite. (2018) 123:32–42. doi: 10.1016/j.appet.2017.11.111
91
Kariv R Tiomny E Grenshpon R Dekel R Waisman G Ringel Y et al . Low-dose naltreoxone for the treatment of irritable bowel syndrome: a pilot study. Dig Dis Sci. (2006) 51:2128–33. doi: 10.1007/s10620-006-9289-8
92
Yan Q Chen J Ren X Song Y Xu J Xuan S et al . Vagus nerve stimulation relives irritable bowel syndrome and the associated depression via α7nAChR-mediated anti-inflammatory pathway. Neuroscience. (2023) 530:26–37. doi: 10.1016/j.neuroscience.2023.08.026
93
Mogilevski T Rosella S Aziz Q Gibson PR . Transcutaneous vagal nerve stimulation protects against stress-induced intestinal barrier dysfunction in healthy adults. Neurogastroenterol Motil. (2022) 34:e14382. doi: 10.1111/nmo.14382
94
Bonaz B Sinniger V Hoffmann D Clarençon D Mathieu N Dantzer C et al . Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol Motil. (2016) 28:948–53. doi: 10.1111/nmo.12792
95
Holvoet T Joossens M Vázquez-Castellanos JF Christiaens E Heyerick L Boelens J et al . Fecal microbiota transplantation reduces symptoms in some patients with irritable bowel syndrome with predominant abdominal bloating: short- and long-term results from a placebo-controlled randomized trial. Gastroenterology. (2021) 160:145–57.e8. doi: 10.1053/j.gastro.2020.07.013
96
Ford AC Wright-Hughes A Alderson SL Ow P-L Ridd MJ Foy R et al . Amitriptyline at Low-Dose and Titrated for Irritable Bowel Syndrome as Second-Line Treatment in primary care (ATLANTIS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 402:1773–85. doi: 10.1016/S0140-6736(23)01523-4
97
Agger JL Schröder A Gormsen LK Jensen JS Jensen TS Fink PK . Imipramine versus placebo for multiple functional somatic syndromes (STreSS-3): a double-blind, randomised study. Lancet Psychiatry. (2017) 4:378–88. doi: 10.1016/S2215-0366(17)30126-8
98
Ladabaum U Sharabidze A Levin TR Zhao WK Chung E Bacchetti P et al . Citalopram provides little or no benefit in nondepressed patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. (2010) 8:42–8.e1. doi: 10.1016/j.cgh.2009.09.008
99
Houghton LA Gao S Gilbert SA Coffin B Simren M Gale JD et al . Clinical trial: study to investigate the efficacy and safety of the alpha-2-delta ligand PD-217,014 in patients with irritable bowel syndrome. Alimen Pharmacol Ther. (2025) 61:803–13. doi: 10.1111/apt.18487
100
Cheong PK Ford AC Cheung CKY Ching JYL Chan Y Sung JJY et al . Low-dose imipramine for refractory functional dyspepsia: a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. (2018) 3:837–44. doi: 10.1016/S2468-1253(18)30303-0
101
Talley NJ Locke GR Saito YA Almazar AE Bouras EP Howden CW et al . Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology. (2015) 149:340–9.e2. doi: 10.1053/j.gastro.2015.04.020
102
Tack J Janssen P Masaoka T Farré R Van Oudenhove L . Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. (2012) 10:1239–45. doi: 10.1016/j.cgh.2012.06.036
103
Sharbafchi MR Afshar Zanjani H Saneian Z Feizi A Daghaghzadeh H Adibi P . Effects of duloxetine on gastrointestinal symptoms, depression, anxiety, stress, and quality of life in patients with the moderate-to-severe irritable bowel syndrome. Adv Biomed Res. (2023) 12:249. doi: 10.4103/abr.abr_379_21
104
Thakur ER Holmes HJ Lockhart NA Carty JN Ziadni MS Doherty HK et al . Emotional awareness and expression training improves irritable bowel syndrome: A randomized controlled trial. Neurogastroenterol Motil. (2017) 29:e13143. doi: 10.1111/nmo.13143
105
Everitt HA Landau S O’Reilly G Sibelli A Hughes S Windgassen S et al . Assessing telephone-delivered cognitive–behavioural therapy (CBT) and web-delivered CBT versus treatment as usual in irritable bowel syndrome (ACTIB): a multicentre randomised trial. Gut. (2019) 68:1613. doi: 10.1136/gutjnl-2018-317805
Summary
Keywords
DGBI, psychotherapy, psychopharmacology, IBS, neuromodulators
Citation
Barrera Flores FJ, Guerrero Tamez JA, Winkelman T, Barrera Flores R and Madva EN (2025) A multidisciplinary approach to the management of disorders of gut-brain interaction: psychopharmacology, psychotherapy, and diet. Front. Gastroenterol. 4:1637172. doi: 10.3389/fgstr.2025.1637172
Received
28 May 2025
Accepted
08 September 2025
Published
07 October 2025
Volume
4 - 2025
Edited by
Fabio Grizzi, Humanitas Research Hospital, Italy
Reviewed by
Tai Zhang, Peking University Health Science Center, China
Jonathan Soldera, University of Caxias do Sul, Brazil
Updates
Copyright
© 2025 Barrera Flores, Guerrero Tamez, Winkelman, Barrera Flores and Madva.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisco J. Barrera Flores, fbarreraflores@mgh.harvard.edu; Elizabeth N. Madva, emadva@mgb.org
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.