Abstract
Background: Epidemiologic studies have demonstrated that X-ray repair cross-complementary group 1 (XRCC1) is one of the susceptibility factors in head and neck squamous cell carcinoma (HNSCC) patients. However, its clinical prognostic impact remains controversial. Thus, a meta-analysis was performed to clarify the association between XRCC1 and the survival outcomes in HNSCC patients.
Methods: Following the Preferred Reporting Items or Systematic Reviews Meta Analyses (PRISMA) 2020 guidelines, literature searches were systematically performed in PubMed, EMBASE, Web of Science, Wanfang, and Chinese National Knowledge Infrastructure (CNKI) databases with manual retrieval. Hazard ratios (HRs) and 95% confidence intervals (CIs) were collected to estimate the correlation between XRCC1 and the survival outcomes of HNSCC patients.
Results: Ten studies including 1995 HNSCC patients who satisfied the inclusion and exclusion criteria were included in this meta-analysis. Pooled analysis indicated that XRCC1 Arg399Gln and XRCC1 high protein expression were significantly correlated with poor overall survival with HR of 1.31 (95% CIs: 1.03-1.66, p = 0.027) and 2.32 (95% CIs: 1.55-3.48 p = 0.000) in HNSCC patients. In addition, our results demonstrated that XRCC1 was significantly associated with poor progression-free survival (HR = 1.42, 95% CIs: 1.15-1.75, p = 0.001) in HNSCC patients.
ConclusionThis meta-analysis demonstrated that XRCC1 Arg399Gln and XRCC1 high protein expression increase the risk of poor survival for HNSCC patients. XRCC1 is a potential therapeutic target for HNSCC.
Introduction
Head and neck squamous cell carcinomas (HNSCC), heterogeneous collection of malignancies of the upper aerodigestive tract, salivary glands and thyroid, constitute 90% of cancers that arise in the head and neck (Leemans et al., 2018; Johnson et al., 2020). HNSCC ranks sixth in the most common cancer worldwide, with 890,000 new cases and 450,000 deaths per year (Sung et al., 2021). Although multidisciplinary treatments for HNSCC have great improvement in the past few years. The incidence of HNSCC continues to rise and the overall disease recurrence remains 40%–50%. Epidemiologic studies indicated that tobacco abuse and alcohol consumption are classical etiologic factors for HNSCC (McDermott and Bowles, 2019). Benzo(a)pyrene from tobacco can induce an increase in reactive oxygen species levels that in turn leads to oxidative DNA damage in the epithelial cells of the head and neck region. The DNA repair pathway in human body can generally correct DNA damage caused by endogenous and environmental agents. Defects in the DNA repair system can also lead to genomic instability and cell death and in turn induce tumorigenesis (Jackson and Bartek, 2009).
Two major classes of DNA repair pathways are base excision repair (BER) and single-strand break repair (SSBR). The primary defense mechanism against oxidative DNA damage is BER (Christmann et al., 2003). X-ray repair cross-complementary group 1 (XRCC1) is a DNA repair scaffold that plays a principle role in BER (Beard et al., 2019). XRCC1 gene is approximately 33 kb length, located on the long arm of the 19th chromosome. XRCC1 protein is 69.5 kDa and consists of 17 exons and 633 amino acids (Vasil’eva et al., 2020). The capacity to repair damaged cells with XRCC1 is encoded by polymorphic genes that may modify the expression of encoded proteins. Single-nucleotide polymorphisms (SNPs) are genetic variants that are related to cancer susceptibility (Nissar et al., 2014). The main variant alleles of XRCC1 gene are Arg399Gln (rs25487) and Arg194Trp (rs1799782). Recently, the survival association of XRCC1 SNPs and XRCC1 protein expression have been reported in various human malignant tumors such as thyroid cancer (Liu and Xue, 2020), lung cancer (Schneider et al., 2008), breast cancer (Sanjari Moghaddam et al., 2016), gallbladder cancer (Wu et al., 2020), and hepatocellular carcinoma (Naguib et al., 2020).
Several studies have investigated that XRCC1 gene polymorphisms (Arg399Gln and Arg194Trp) increase the risk of HNSCC (Choudhury et al., 2014; Dutta et al., 2020; Kabzinski et al., 2021) and suggested that high XRCC1 protein expression is associated with poorer survival in patients with HNSCC(Ang et al., 2011). However, some studies indicated that the XRCC1 Arg399Gln polymorphism do not confer a significant risk for HNSCC (Gal et al., 2005; Wu et al., 2014) and revealed that low expression of XRCC1 statistically significant increase the risk of HNSCC (Kumar et al., 2012). Data from current literature are discordance for the association between XRCC1 and HNSCC survival outcomes. Thus, we aim to conduct a meta-analysis to determine the association between XRCC1 gene polymorphisms, protein expression and survival outcomes in HNSCC patients. The present study followed the Preferred Reporting Items or Systematic Reviews Meta Analyses (PRISMA) 2020 guidelines (Page et al., 2021).
Methods
Search strategy
We conducted a systematic computerized search in PubMed, Web of Science, EMBASE, Wanfang, and Chinese National Knowledge Infrastructure (CNKI) databases using the following search terms (XRCC1 or X-ray repair cross-complementing 1) and (prognosis OR outcome OR mortality OR survival OR progression OR recurrence) and (head and neck or laryngeal or tonsil or oropharyngeal or oral or oropharynx or nasopharyngeal) and (squamous cell cancer or carcinoma). Articles published between 1992 and 2022 were considered for study inclusion. The latest search was conducted on 1 June 2022.
Selection criteria
According to the PICOS (patients, intervention, comparison, outcomes, and study design) principles, the inclusion criteria of the meta-analysis were as follows: 1) Population: patients of any age diagnosed with HNSCC; 2) Intervention: expression of XRCC1 in HNSCC was assessed by immunohistochemical (IHC) analysis. The target gene polymorphisms for XRCC1 were Arg399Gln (rs25487) and/or Arg194Trp (rs1799782) and assessed by polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP); 3) Comparison: HNSCC patients without concentration on high XRCC1 expression or without concentration on XRCC1 Arg399Gln (rs25487) and/or Arg194Trp (rs1799782); 4) Outcomes: overall survival (OS) between XRCC1 and HNSCC as a primary outcome. Progression-free survival (PFS) between XRCC1 and HNSCC as a secondary outcome; 5) Study design: Any human-based studies; 6) including hazard ratios (HR) and the 95% confidence interval (CI) directly, or p values with Kaplan- Meier survival curves that can be estimated for OS and/or PFS.
The criteria of exclusion included: 1) abstract-only publications, letters, case reports, meta-analyses, comments, conference articles, on-going or unavailable literature; 2) studies with insufficient original data or focused only on odds ratio without HR values. In case of the same population reported by several publications, the latest literature gained the priority.
Data extraction
Two researchers (Jing-cai Chen and Yao Luo) independently conducted the electronic search. Data were carefully extracted and cross-checked by two independent researchers (Liu-qing Zhou and Fan Yang) to minimize variation. If there were divergences, the senior researcher (Yan-Jun Wang) participated in the progress of data extraction for achieving a consensus. To begin with, we removed duplicate literature. After manual screening titles and abstracts, publications were eligible for full-text perusal. Finally, studies that meet the selection criteria were included in this meta-analysis. The following information was extracted from each included study: the name of the first author, year of publication, country, cancer type, sample size, age, gender, stage, follow-up time, survival outcomes, method, HR. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the included publications. A star system (maximum is nine stars) of NOS concentrated in three domains: comparability of study groups, selection of participants and ascertainment of outcomes of interest. Studies with NOS ≥6 were high-quality (Stang, 2010). Reporting recommendations for tumor marker prognostic studies (REMARK) were also applied to evaluate study quality in cancer-related meta-analyses (Sauerbrei et al., 2018).
Statistical analysis
HRs and 95% CIs are effect measures which were obtained directly from the original data in the selected publications or estimated by p values from Kaplan- Meier survival curves following Parmer’s methods (Parmar et al., 1998). OS/PFS were evaluated by pooled HRs and 95% CIs (Tierney et al., 2007). HR = 1 indicates a lack of risk association between XRCC1 and HNSCC. HR > 1 indicates a greater risk of death between XRCC1 and HNSCC. HR < 1 indicates a lower risk of death between XRCC1 and HNSCC. The higher the HR value is, the greater the XRCC1 is related to an increased risk of HNSCC.
Heterogeneity was assessed by the Cochran-based Q test and the I2 test (Higgins et al., 2003). p < 0.1for Q-test was considered statistically significant heterogeneity between-studies. The fixed-effects model was employed for analysis without obvious statistical heterogeneity between studies (p > 0.1, I2 ≤ 50%). Otherwise, the random-effects model was applied (Bagos, 2013). Moreover, we performed subgroup analysis to explore the potential source of heterogeneity. To evaluate the strength of the association results, sensitivity analysis was carried out by removing one article at a time and re-measuring the pooled HR. If the pooled HRs did not change, it suggested that our results were not originated from any certain study (Cumpston et al., 2019). Publication bias was assessed by Begg’s funnel plots. And the asymmetry of funnel plot was assessed by the method of Egger’s linear regression test (Begg and Berlin, 1989). The asymmetric plot of Begg’s test and the p-value of Egger’s test less than 0.05 were considered a significant publication bias. If publication bias exists, “trim and fill” analysis will be used to adjust the effect of publication bias by removing the small studies with the most extreme results (trim) and recalculating the summary effect size at each iteration until the funnel plot becomes symmetric (Duval and Tweedie, 2000). All the statistical tests used in this meta-analysis were performed with Stata version 12.0 (Stata Corporation, College Station, TX, United States).
Results
Study selection and characteristics
As shown in Figure 1, a total of 246 published articles were selected for initial identification. Of these, 131 duplicate articles were excluded. After screening the full texts of the left 48 publications, we discarded 38 studies due to the lack of insufficient original data or unrelated to the high expression of XRCC1 and XRCC1 gene polymorphisms (Arg399Gln and Arg194Trp). Finally, ten studies with 1995 HNSCC patients were selected in this meta-analysis.
FIGURE 1

Flow diagram of the selection of relevant studies included in the meta-analysis. CNKI, Chinese National Knowledge Infrastructure; XRCC1, X-ray repair cross-complementary group 1; HNSCC, head and neck squamous cell carcinoma; HR, hazard ratios.
The characteristics of the enrolled studies were summarized in Table 1. Six studies with 1563 patients for HNSCC (Quintela-Fandino et al., 2006; Csejtei et al., 2009; Ang et al., 2011; Azad et al., 2012; Hirakawa et al., 2020; Bold et al., 2021), one study with 134 patients for laryngeal squamous cell carcinoma (LSCC) (Raturi et al., 2020), one study with 98 patients for oral squamous cell carcinomas (OSCC) (Wang et al., 2021), one study with 75 patients for nasopharyngeal carcinoma (NPC) (Jin et al., 2014), and one study with 125 patients for oropharyngeal squamous cell carcinoma (OPSCC) (Costa et al., 2016) were included. Of these, nine studies including 1920 patients reported OS and four studies including 411 patients reported PFS. Sample size of the publications ranged from 75 to 531. Two publications enrolled more than 500 patients. Most of the patients included in this meta-analysis were male and most HNSCC patients were over 45 years old. XRCC1 gene polymorphisms were explored by PCR-RFLP method and IHC method was applied for the expression of XRCC1. Over half of the studies reported the HRs and 95% CIs directly. All the publications’ NOS scores were above 6 and the REMARK scores were between 13 and 16.
TABLE 1
| Author | Year | Country | Cancer type | Sample size | Age | Gender: Male/female | Stage | Follow-up time | Survival | Method | HR | NOS/REMARK score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fandino | 2006 | Canada | HNSCC | 103 | 60.09 (39.09–94.00) | 97/6 | NR | 22.5 (9–51) | OS | PCR-RFLP | Estimated | 8/16 |
| Csejtei | 2009 | Hungary | HNSCC | 108 | 56.7 | 97/11 | I-IV | 60 | OS | PCR-RFLP | Estimated | 6/13 |
| Ang | 2011 | America | HNSCC | 77 | 56 (39–61) | 51/26 | I-IV | 66 (39–87) | OS, PFS | IHC | Reported | 7/14 |
| Azad | 2012 | Canada | HNSCC | 531 | 63 (33–86) | 420/111 | I-II | 100.52 | OS | PCR-RFLP | Reported | 7/14 |
| Jin | 2014 | China | NPC | 75 | 45 (22–72) | 57/18 | II-IV | 25 (5–46) | PFS | PCR-RFLP IHC | Reported | 7/14 |
| Costa1 | 2016 | Brazil | OPSCC | 125 | 57 (33–85) | 113/12 | IV | 24.5 (1.5–116.7) | OS, PFS | PCR-RFLP | Reported | 7/16 |
| Costa2 | 2016 | Brazil | OPSCC | 125 | 57 (33–85) | 113/12 | IV | 24.5 (1.5–116.7) | OS, PFS | PCR-RFLP | Reported | 7/16 |
| Hirakawa | 2020 | Japan | HNSCC | 225 | 67 (41–91) | 200/25 | I-IV | 48 (3–146) | OS | PCR-RFLP | Estimated | 6/14 |
| Raturi | 2020 | Japan | LSCC | 134 | 56 (32–64) | 108/26 | III-IV | 33 | OS, PFS | PCR-RFLP | Reported | 6/14 |
| Bold | 2021 | Germany | HNSCC | 519 | NR | NR | NR | 60 | OS | IHC | Estimated | 6/13 |
| Wang | 2021 | China | OSCC | 98 | 51 (31–76) | 92/6 | I-IV | 40 (2.4–137.4) | OS | IHC | Reported | 7/15 |
Characteristics of the studies included in the meta-analysis. NR, not reported; IHC, Immunohistochemistry; PCR-RFLP, polymerase chain reaction restriction fragment length polymorphism; HNSCC, head and neck squamous cell carcinoma; LSCC, laryngeal squamous cell carcinoma; OSCC, oral squamous cell carcinomas; NPC, nasopharyngeal carcinoma; OPSCC, oropharyngeal squamous cell carcinoma.
Survival association between XRCC1 and HNSCC patients
Ten articles with 1995 patients included in this meta-analysis evaluated the survival association between XRCC1 and HNSCC. Nine studies including 1920 patients evaluated OS between XRCC1 and HNSCC and four studies including 411 patients evaluated PFS between XRCC1 and HNSCC. The results showed that XRCC1 Arg399Gln (HR = 1.31, 95% CIs: 1.03-1.66, p = 0.027) and XRCC1 high protein expression (HR = 2.32, 95% CIs: 1.55-3.48 p = 0.000) were significantly correlated with poor OS in HNSCC patients by the random-effects model. However, XRCC1 Arg194Trp was not a risk for HNSCC patients (HR = 1.56, 95% CIs: 0.86-2.86, p = 0.146). The degrees of heterogeneity are as follows: I2 = 0–25%, no heterogeneity; I2 = 25–50%, moderate heterogeneity; I2 = 50–75%, large heterogeneity; I2 = 75–100%, extreme heterogeneity (Higgins et al., 2003). Moderate heterogeneity was noted between XRCC1 Arg399Gln and OS (I2 = 48.3%, Pheterogeneity = 0.122). Large heterogeneity was noted between XRCC1Arg194Trp and OS (I2 = 70.2%, Pheterogeneity = 0.035). No heterogeneity was noted between high XRCC1 expression and OS (I2 = 0.0%, Pheterogeneity = 0.708). Overall, the pooled heterogeneity of XRCC1 in OS was large (I2 = 56.7%, Pheterogeneity = 0.014) (Figure 2). Significant correlation between XRCC1 and poor PFS (HR = 1.42, 95% CIs: 1.15–1.75, p = 0.001) was observed with no heterogeneity (I2 = 20.1%, Pheterogeneity = 0.286) (Figure 3).
FIGURE 2

Forest plot indicating the overall survival association between XRCC1 gene polymorphisms (Arg194Trp and Arg399Gln)/high XRCC1 protein expression and HNSCC.
FIGURE 3

Forest plot indicating the association between XRCC1 and progression-free survival in HNSCC.
Sensitivity analysis
The sensitivity analysis was conducted to evaluate the effects of each single study on the overall effect. We conducted a leave-one-out sensitivity analysis to evaluate the effect of each single data point against the aggregate. The recalculated outcomes (Figure 4) were not substantially influenced, suggesting that the combined effect size of the meta-analysis results was stable and reliable.
FIGURE 4

The sensitivity analysis was conducted to evaluate the effects of each single study on the overall effect.
Publication bias
Begg’s funnel plot and Egger’s test were applied to validate potential bias from searched publications. We observed that two sides of the Begg’s funnel plot were asymmetric. The Egger’s test indicated publication bias existed (p = 0.03). Therefore, “trim and fill” analysis was further utilized and the pooled HR was 1.246 (95% CIs: 0.967–1.607) (Figure 5).
FIGURE 5
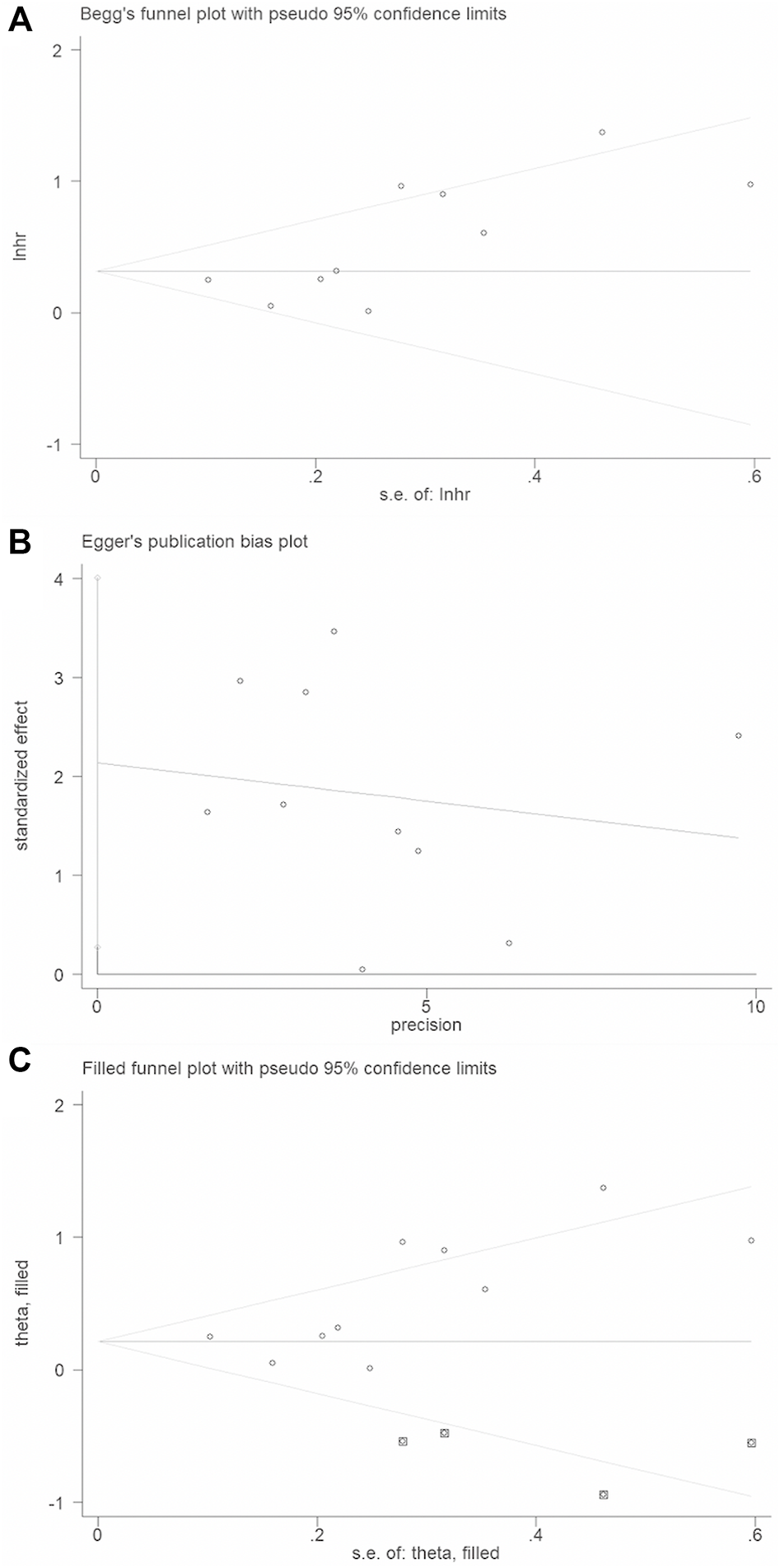
Publication bias and trim and fill analysis of the enrolled analysis: (A). The Begg’s funnel plot; (B). The Egger’s test. (C). Trim and fill analysis.
Discussion
In the present meta-analysis, we included ten studies with 1995 HNSCC patients. Results indicated that the XRCC1 gene polymorphism Arg399Gln and XRCC1 high protein expression were significantly associated with poor OS for HNSCC patients and XRCC1 was significantly associated with poor PFS. There was large heterogeneity in our study (I2 = 56.7%, Pheterogeneity = 0.014). p < 0.10 suggested the heterogeneity is significant and I2>50% indicated the heterogeneity is large across studies. The random-effects mode was employed for analysis with large significant heterogeneity across studies (p < 0.1, I2>50%). The fixed-effects model would be performed for analysis with no obvious heterogeneity between studies (p > 0.1, I2≤50%). Considering the existence of large-significant heterogeneity in our meta-analysis, a random-effects model was chosen for the generation of pooled indexes. The sensitivity analysis confirmed this meta-analysis is stable and reliable. The asymmetric funnel plot and the Egger’s test (p = 0.03) conferred that a significant publication bias existed in our study. Thus, we implemented “Trim and fill” analysis to judge the impact of publication bias. The method of “Trim and fill” analysis was conducted by removing the small studies with the most extreme results (trim) and recalculating the summary effect size at each iteration until the funnel plot becomes symmetric. Results showed that the point estimate of the overall effect size is approximately correct in our study (HR = 1.282, 95% CI: 0.98–1.677).
Anti-cancer drug therapy for HNSCC has been widely used in the clinic and has recently been shown to be effective. In a previous study, oncogene genes in HNSCC were identified through extensive DNA sequencing and genetic analysis (2015). Therefore, it is reasonable to quest potential biomarkers of treatment by analyzing genomic features.
DNA repair genes have been considered driver genes of HNSCC due to their frequent mutation. Defects in DNA repair promote genomic instability and carcinogenesis (Tubbs and Nussenzweig, 2017). DNA repair systems play an indispensable role in protecting cells against carcinogenic agents from internal and external stimuli (Leemans et al., 2011). Statistical analyses indicated that the DNA repair status is associated with poor prognosis in cancers (Birkbak et al., 2011; Azad et al., 2012). XRCC1 is a major DNA repair gene involved in BER for small base lesions resulting from oxidation and alkylation damage (Almeida and Sobol, 2007; Lou et al., 2013). More than 300 validated SNPs in the XRCC1 gene were reported in the dbSNP database (http://www.ncbi.nlm.nih.gov/SNP). Among them, the XRCC1 gene polymorphisms (Arg194Trp and Arg399Gln), which occur within conserved sequences, are the most frequently mutated and the most extensively studied.
Studies have investigated the association between XRCC1 Arg399Gln/XRCC1 Arg194Trp polymorphisms and HNSCC risk (Li et al., 2007; Majumder et al., 2007; Applebaum et al., 2009; Gugatschka et al., 2011; Kostrzewska-Poczekaj et al., 2013; Jin et al., 2014; Raturi et al., 2020). However, these results were contradictory. Wang et al. (2013) conducted a meta-analysis on the association of XRCC1 Arg399Gln polymorphisms with HNSCC risk. Nevertheless, they did not observe any precise estimation of this relationship based on 18 published studies. Sturgis (Sturgis et al., 1999) reported a reduced risk between XRCC1 Arg194Trp and HNSCC while Andrew (Olshan et al., 2002) found a weak elevation between HNSCC risk and the XRCC1 Arg194Trp polymorphism. XRCC1 protein is involved in BER (Thompson and West, 2000; Weaver et al., 2005) and its protein expression alters the sensitivity of cells to radiation and chemotherapeutic agents (Park et al., 2002). It was reported that high protein expression levels of XRCC1 may be a risk factor for HNSCC (Ang et al., 2011; Wang et al., 2021). In addition, XRCC1 protein expression is common in HNSCC and high XRCC1 protein expression may confer poorer survival, regardless of the primary tumor site or stage (Ang et al., 2011; Bold et al., 2021). In summary, these results either contrast with each other or are not accurate conclusions.
This meta-analysis prospectively evaluated XRCC1 as a bio-predictor of survival outcomes in patients with HNSCC. There is no doubt that our study is the first meta-analysis including ten published studies with 1995 patients to comprehensively evaluate the survival value of XRCC1 (SNPs and high protein expression) in HNSCC. It might offer useful information for clinical decision-making in HNSCC.
After analyzing and summarizing all selected data, the results indicated that high protein expression and Arg399Gln SNPs of XRCC1 significantly predicted poor OS in HNSCC patients with HRs of 2.32 and 1.31. These findings confirmed that XRCC1 could be widely applied as a diagnostic marker and therapeutic target in HNSCC patients. As a genetic-associated study, the Hardy-Weinberg (HWE) principle was used to avoid methodological weaknesses, such as biased selection of subjects or genotyping errors. The enrolled studies in our meta-analysis were all in agreement with HWE principle.
This meta-analysis should be interpreted within the context of its limitations. First, all included studies are published with English languages only. Therefore, publication bias is very likely to occur. Second, the number of articles was limited and the sample size was relatively small in the present meta-analysis. False-positive or false-negative findings may have occurred in small sample sizes. Therefore, larger scale and comprehensive studies are needed to achieve a more persuasive conclusion. Third, large heterogeneity was observed in this meta-analysis. The heterogeneity of the included studies was likely due to differences of the baseline characteristics in patients or different sites of HNSCC or tumor treatments or HRs calculated by Parmer’s methods or other parameters. A random-effects model was conducted to minimize the effects of these differences. Forth, studies with positive results are more likely to be published and thus more likely to be enrolled. Hence, the survival association of XRCC1 in HNSCC may to some extent has been overestimated in this meta-analysis.
Conclusion
In conclusion, our meta-analysis demonstrated that XRCC1 gene polymorphism Arg399Gln and high protein expression of XRCC1 were associated with poor OS in HNSCC patients. And XRCC1 was significantly associated with poor PFS in HNSCC patients. This current meta-analysis might provide favorable data for future application of XRCC1 as bio-predictor for HNSCC treatment. Larger prospective studies should be conducted in the future to further verify the results in the present study.
Statements
Author contributions
JC and YL collected the data. FY and LZ analyzed the data and wrote the paper. LZ and YW conceived and designed this study. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National 12th-Five Year Research Program of China (no.2012BAI12B02) and Health Commission of Hubei Province scientific research project (no.wj 2021M250).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AlmeidaK. H.SobolR. W. (2007). A unified view of base excision repair: Lesion-dependent protein complexes regulated by post-translational modification. DNA Repair (Amst)6, 695–711. 10.1016/j.dnarep.2007.01.009
2
AngM. K.PatelM. R.YinX. Y.SundaramS.FritchieK.ZhaoN.et al (2011). High XRCC1 protein expression is associated with poorer survival in patients with head and neck squamous cell carcinoma. Clin. Cancer Res.17, 6542–6552. 10.1158/1078-0432.CCR-10-1604
3
ApplebaumK. M.MccleanM. D.NelsonH. H.MarsitC. J.ChristensenB. C.KelseyK. T. (2009). Smoking modifies the relationship between XRCC1 haplotypes and HPV16-negative head and neck squamous cell carcinoma. Int. J. Cancer124, 2690–2696. 10.1002/ijc.24256
4
AzadA. K.BairatiI.SamsonE.ChengD.MirshamsM.QiuX.et al (2012). Validation of genetic sequence variants as prognostic factors in early-stage head and neck squamous cell cancer survival. Clin. Cancer Res.18, 196–206. 10.1158/1078-0432.CCR-11-1759
5
BagosP. G. (2013). Genetic model selection in genome-wide association studies: Robust methods and the use of meta-analysis. Stat. Appl. Genet. Mol. Biol.12, 285–308. 10.1515/sagmb-2012-0016
6
BeardW. A.HortonJ. K.PrasadR.WilsonS. H. (2019). Eukaryotic base excision repair: New approaches shine light on mechanism. Annu. Rev. Biochem.88, 137–162. 10.1146/annurev-biochem-013118-111315
7
BeggC. B.BerlinJ. A. (1989). Publication bias and dissemination of clinical research. J. Natl. Cancer Inst.81, 107–115. 10.1093/jnci/81.2.107
8
BirkbakN. J.EklundA. C.LiQ.McclellandS. E.EndesfelderD.TanP.et al (2011). Paradoxical relationship between chromosomal instability and survival outcome in cancer. Cancer Res.71, 3447–3452. 10.1158/0008-5472.CAN-10-3667
9
BoldI. T.SpechtA. K.DrosteC. F.ZielinskiA.MeyerF.ClauditzT. S.et al (2021). DNA damage response during replication correlates with CIN70 score and determines survival in HNSCC patients. Cancers (Basel)13, 1194. 10.3390/cancers13061194
10
ChoudhuryJ. H.ChoudhuryB.KunduS.GhoshS. K. (2014). Combined effect of tobacco and DNA repair genes polymorphisms of XRCC1 and XRCC2 influence high risk of head and neck squamous cell carcinoma in northeast Indian population. Med. Oncol.31, 67. 10.1007/s12032-014-0067-8
11
ChristmannM.TomicicM. T.RoosW. P.KainaB. (2003). Mechanisms of human DNA repair: An update. Toxicology193, 3–34. 10.1016/s0300-483x(03)00287-7
12
CostaE. F.SantosE. S.LiuttiV. T.LealF.SantosV. C.Rinck-JuniorJ. A.et al (2016). Association between polymorphisms in genes related to DNA base-excision repair with risk and prognosis of oropharyngeal squamous cell carcinoma. J. Cancer Res. Clin. Oncol.142, 1917–1926. 10.1007/s00432-016-2202-8
13
CsejteiA.TiboldA.KoltaiK.VargaZ.SzanyiI.GobelG.et al (2009). Association between XRCC1 polymorphisms and head and neck cancer in a Hungarian population. Anticancer Res.29, 4169–4173.
14
CumpstonM.LiT.PageM. J.ChandlerJ.WelchV. A.HigginsJ. P.et al (2019). Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev.10, Ed000142. 10.1002/14651858.ED000142
15
DuttaD.AbarnaR.ShubhamM.SubbiahK.DuraisamyS.ChinnusamyR.et al (2020). Effect of Arg399Gln single-nucleotide polymorphism in XRCC1 gene on survival rate of Indian squamous cell head-and-neck cancer patients. J. Cancer Res. Ther.16, 551–558. 10.4103/jcrt.JCRT_476_18
16
DuvalS.TweedieR. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics56, 455–463. 10.1111/j.0006-341x.2000.00455.x
17
GalT. J.HuangW. Y.ChenC.HayesR. B.SchwartzS. M. (2005). DNA repair gene polymorphisms and risk of second primary neoplasms and mortality in oral cancer patients. Laryngoscope115, 2221–2231. 10.1097/01.mlg.0000183736.96004.f7
18
GugatschkaM.DehchamaniD.WascherT. C.FriedrichG.RennerW. (2011). DNA repair gene ERCC2 polymorphisms and risk of squamous cell carcinoma of the head and neck. Exp. Mol. Pathol.91, 331–334. 10.1016/j.yexmp.2011.03.004
19
HigginsJ. P.ThompsonS. G.DeeksJ. J.AltmanD. G. (2003). Measuring inconsistency in meta-analyses. Bmj327, 557–560. 10.1136/bmj.327.7414.557
20
HirakawaH.IkegamiT.AzechiS.AgenaS.UezatoJ.KinjyoH.et al (2020). ERCC1 C8092A polymorphism predicts fair survival outcome in Japanese patients with pharyngo-laryngeal squamous cell carcinoma. Eur. Arch. Otorhinolaryngol.277, 601–610. 10.1007/s00405-019-05731-y
21
JacksonS. P.BartekJ. (2009). The DNA-damage response in human biology and disease. Nature461, 1071–1078. 10.1038/nature08467
22
JinH.XieX.WangH.HuJ.LiuF.LiuZ.et al (2014). ERCC1 Cys8092Ala and XRCC1 Arg399Gln polymorphisms predict progression-free survival after curative radiotherapy for nasopharyngeal carcinoma. PLoS One9, e101256. 10.1371/journal.pone.0101256
23
JohnsonD. E.BurtnessB.LeemansC. R.LuiV. W. Y.BaumanJ. E.GrandisJ. R. (2020). Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim.6, 92. 10.1038/s41572-020-00224-3
24
KabzinskiJ.MaczynskaM.KaczmarczykD.MajsterekI. (2021). Influence of Arg399Gln, Arg280His and Arg194Trp XRCC1 gene polymorphisms of Base Excision Repair pathway on the level of 8-oxo-guanine and risk of head and neck cancer in the Polish population. Cancer Biomark.32, 317–326. 10.3233/CBM-203163
25
Kostrzewska-PoczekajM.GawęckiW.IllmerJ.RydzaniczM.GajeckaM.SzyfterW.et al (2013). Polymorphisms of DNA repair genes and risk of squamous cell carcinoma of the head and neck in young adults. Eur. Arch. Otorhinolaryngol.270, 271–276. 10.1007/s00405-012-1993-8
26
KumarA.PantM. C.SinghH. S.KhandelwalS. (2012). Reduced expression of DNA repair genes (XRCC1, XPD, and OGG1) in squamous cell carcinoma of head and neck in North India. Tumour Biol.33, 111–119. 10.1007/s13277-011-0253-7
27
LeemansC. R.BraakhuisB. J.BrakenhoffR. H. (2011). The molecular biology of head and neck cancer. Nat. Rev. Cancer11, 9–22. 10.1038/nrc2982
28
LeemansC. R.SnijdersP. J. F.BrakenhoffR. H. (2018). The molecular landscape of head and neck cancer. Nat. Rev. Cancer18, 269–282. 10.1038/nrc.2018.11
29
LiC.HuZ.LuJ.LiuZ.WangL. E.El-NaggarA. K.et al (2007). Genetic polymorphisms in DNA base-excision repair genes ADPRT, XRCC1, and APE1 and the risk of squamous cell carcinoma of the head and neck. Cancer110, 867–875. 10.1002/cncr.22861
30
LiuS. Y.XueW. (2020). XRCC1 Arg194Trp polymorphism and thyroid cancer. J. Endocrinol. Invest.43, 749–753. 10.1007/s40618-019-01155-x
31
LouY.PengW. J.CaoD. S.XieJ.LiH. H.JiangZ. X. (2013). DNA repair gene XRCC1 polymorphisms and head and neck cancer risk: An updated meta-analysis including 16344 subjects. PLoS One8, e74059. 10.1371/journal.pone.0074059
32
MajumderM.SikdarN.GhoshS.RoyB. (2007). Polymorphisms at XPD and XRCC1 DNA repair loci and increased risk of oral leukoplakia and cancer among NAT2 slow acetylators. Int. J. Cancer120, 2148–2156. 10.1002/ijc.22547
33
McdermottJ. D.BowlesD. W. (2019). Epidemiology of head and neck squamous cell carcinomas: Impact on staging and prevention strategies. Curr. Treat. Options Oncol.20, 43. 10.1007/s11864-019-0650-5
34
NaguibM.HelwaM. M.SolimanM. M.Abdel-SamieeM.EljakyA. M.HammamO.et al (2020). XRCC1 gene polymorphism increases the risk of hepatocellular carcinoma in Egyptian population. Asian pac. J. Cancer Prev.21, 1031–1037. 10.31557/APJCP.2020.21.4.1031
35
NissarS.SameerA. S.RasoolR.RashidF. (2014). DNA repair gene--XRCC1 in relation to genome instability and role in colorectal carcinogenesis. Oncol. Res. Treat.37, 418–422. 10.1159/000364898
36
OlshanA. F.WatsonM. A.WeisslerM. C.BellD. A. (2002). XRCC1 polymorphisms and head and neck cancer. Cancer Lett.178, 181–186. 10.1016/s0304-3835(01)00822-9
37
PageM. J.MckenzieJ. E.BossuytP. M.BoutronI.HoffmannT. C.MulrowC. D.et al (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg.88, 105906. 10.1016/j.ijsu.2021.105906
38
ParkS. Y.LamW.ChengY. C. (2002). X-ray repair cross-complementing gene I protein plays an important role in camptothecin resistance. Cancer Res.62, 459–465.
39
ParmarM. K.TorriV.StewartL. (1998). Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med.17, 2815–2834. 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8
40
Quintela-FandinoM.HittR.MedinaP. P.GamarraS.MansoL.Cortes-FunesH.et al (2006). DNA-repair gene polymorphisms predict favorable clinical outcome among patients with advanced squamous cell carcinoma of the head and neck treated with cisplatin-based induction chemotherapy. J. Clin. Oncol.24, 4333–4339. 10.1200/JCO.2006.05.8768
41
RaturiV.HojoH.BhattM. L. B.SuhelM.WuC. T.BeiY.et al (2020). Prospective evaluation of XRCC-1 Arg194Trp polymorphism as bio-predictor for clinical outcome in locally advanced laryngeal cancer undergoing cisplatin-based chemoradiation. Head. Neck42, 1045–1056. 10.1002/hed.26083
42
Sanjari MoghaddamA.NazarzadehM.NorooziR.DarvishH.Mosavi JarrahiA. (2016). XRCC1 and OGG1 gene polymorphisms and breast cancer: A systematic review of literature. Iran. J. Cancer Prev.9, e3467. 10.17795/ijcp-3467
43
SauerbreiW.TaubeS. E.McshaneL. M.CavenaghM. M.AltmanD. G. (2018). Reporting recommendations for tumor marker prognostic studies (REMARK): An abridged explanation and elaboration. J. Natl. Cancer Inst.110, 803–811. 10.1093/jnci/djy088
44
SchneiderJ.ClassenV.HelmigS. (2008). XRCC1 polymorphism and lung cancer risk. Expert Rev. Mol. diagn.8, 761–780. 10.1586/14737159.8.6.761
45
StangA. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol.25, 603–605. 10.1007/s10654-010-9491-z
46
SturgisE. M.CastilloE. J.LiL.ZhengR.EicherS. A.ClaymanG. L.et al (1999). Polymorphisms of DNA repair gene XRCC1 in squamous cell carcinoma of the head and neck. Carcinogenesis20, 2125–2129. 10.1093/carcin/20.11.2125
47
SungH.FerlayJ.SiegelR. L.LaversanneM.SoerjomataramI.JemalA.et al (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin.71, 209–249. 10.3322/caac.21660
48
ThompsonL. H.WestM. G. (2000). XRCC1 keeps DNA from getting stranded. Mutat. Res.459, 1–18. 10.1016/s0921-8777(99)00058-0
49
TierneyJ. F.StewartL. A.GhersiD.BurdettS.SydesM. R. (2007). Practical methods for incorporating summary time-to-event data into meta-analysis. Trials8, 16. 10.1186/1745-6215-8-16
50
TubbsA.NussenzweigA. (2017). Endogenous DNA damage as a source of genomic instability in cancer. Cell168, 644–656. 10.1016/j.cell.2017.01.002
51
Vasil'EvaI. A.MoorN. A.LavrikO. I. (2020). Effect of human XRCC1 protein oxidation on the functional activity of its complexes with the key enzymes of DNA base excision repair. Biochemistry.85, 288–299. 10.1134/S0006297920030049
52
WangY.ChuX.MengX.ZouF. (2013). Association of X-ray repair cross complementing group 1 Arg399Gln polymorphisms with the risk of squamous cell carcinoma of the head and neck: Evidence from an updated meta-analysis. PLoS One8, e77898. 10.1371/journal.pone.0077898
53
WangY. Y.FangP. T.SuC. W.ChenY. K.HuangJ. J.HuangM. Y.et al (2021). Excision repair cross-complementing group 2 upregulation is a potential predictive biomarker for oral squamous cell carcinoma recurrence. Oncol. Lett.21, 450. 10.3892/ol.2021.12711
54
WeaverD. A.CrawfordE. L.WarnerK. A.ElkhairiF.KhuderS. A.WilleyJ. C. (2005). ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol. Cancer4, 18. 10.1186/1476-4598-4-18
55
WuW.LiuL.YinZ.GuanP.LiX.ZhouB. (2014). Association of X-ray repair cross-complementing group 1 Arg194Trp, Arg399Gln and Arg280His polymorphisms with head and neck cancer susceptibility: A meta-analysis. PLoS One9, e86798. 10.1371/journal.pone.0086798
56
WuZ.MiaoX.ZhangY.LiD.ZouQ.YuanY.et al (2020). XRCC1 is a promising predictive biomarker and facilitates chemo-resistance in gallbladder cancer. Front. Mol. Biosci.7, 70. 10.3389/fmolb.2020.00070
Summary
Keywords
X-ray repair cross-complementing 1, polymorphism, head and neck squamous cell carcinomas, survival, meta-analysis
Citation
Yang F, Zhou L, Chen J, Luo Y and Wang Y (2023) Survival association of XRCC1 for patients with head and neck squamous cell carcinoma: A systematic review and meta-analysis. Front. Genet. 13:1035910. doi: 10.3389/fgene.2022.1035910
Received
03 September 2022
Accepted
29 November 2022
Published
05 January 2023
Volume
13 - 2022
Edited by
Alfredo Mauricio Batista De Paula, Unimontes, Brazil
Reviewed by
Gh Rasool Bhat, Virginia Commonwealth University, United States
Giuseppe Spriano, Humanitas University, Italy
Updates
Copyright
© 2023 Yang, Zhou, Chen, Luo and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjun Wang, yjwang@hust.edu.cn
†These authors have contributed equally to this work
This article was submitted to Cancer Genetics and Oncogenomics, a section of the journal Frontiers in Genetics
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.