- Laboratory of Plant Protection, National Institute for Agronomic Research of Tunisia (INRAT), University of Carthage, Ariana, Tunisia
Fruits are susceptible to a diverse range of postharvest rots, which can reduce quality if preventive measures are not taken in time. In this study, samples of orange cv. Maltaise and apple cvs. Golden Delicious and Richared were sorted without infection or injury, treated or not with sodium metabisulfite (SMB), and then placed in cold storage for 20, 42, or 59–61 days, followed by a shelf life of 6 or 15 days. Physicochemical characteristics, degree of fruit infection, and weight loss were analyzed for each storage period. Our results indicated that adequate postharvest storage depends on the type of fruit, duration of cold storage, and shelf life. The heat map grouped ‘Richared’ apples close to its fresh state, without developing rot or perceptible weight loss for 60 days at low temperature (6°C) and 15 days of shelf life. These red apples performed better during storage than ‘Golden’ apples, especially in terms of storability and total flavonoids. Apples of ‘Golden’ showed better storage stability than ‘Maltaise,’ which could be stored properly for up to 20 days at 6°C, followed by a 15-day shelf life, regardless of treatment with sodium metabisulfite. The longer the oranges were stored, the greater the risk of infection and the greater the physicochemical properties; in this case, flavonoids decreased. The chemical criteria (TSS and pH) of apples and oranges were not affected by soaking in SMB, which was similar to that of untreated fruit. However, treating such fruits with SMB is regarded as unlikely because of its low effectiveness in preventing fruit decay during long-term storage. Cluster analysis showed that total polyphenols were linked to poor storability, whereas flavonoids, hardness, and TSS were associated with better storability. This suggests that flavonoids may be more reliable indicators of storage suitability than total polyphenols.
1 Introduction
The annual production of pome fruit and citrus in Tunisia is estimated to be 139,000 tons and 36,500 tons, respectively (GIFruits, 2023). A large quantity of these fruits is usually stored at low temperatures after harvesting, then gradually sold in the local market or exported to various countries. The incidence of postharvest diseases can affect the quality and limit the shelf life of fresh horticultural produce at various stages of the cold chain. Postharvest and pre-retail food losses were estimated at 13.2% of global food production (FAO, 2023), whereas food waste from retail, catering, and households was estimated at 19% of global food production (UNEP, 2024), giving a total food loss and waste index of 32.2%.
Recent attempts have been made to experimentally reduce gray mold that infects table grapes during storage by stimulating the antioxidant system of these fruits using antifungal substances that are considered safe, such as a chitosan/silica nanocomposite formulation (Youssef and Roberto, 2021). Nevertheless, packinghouses generally use synthetic fungicides to treat fruit before storage, in addition to the antifungal treatments carried out in orchards before harvest, to limit waste and losses, first in the field and then during storage. When applied in excessive quantities, these fungicides can impact the environment and pose a risk to human health (Alaoui et al., 2024; Triantafyllidis et al., 2022; Soheilifard et al., 2020; Fantke and Jolliet, 2016). Countries importing fresh fruit apply strict regulations concerning minimum levels of pesticide residues in the edible parts of fresh produce (Sivakumar and Bautista-Baños, 2014). Therefore, a sharp reduction in synthetic pesticide use is essential. For all these reasons, this study is focused on safe and environmentally friendly applications to reduce postharvest deterioration in fruit quality. Some organic and inorganic salts have been considered as promising agents for pre- and post-harvest treatments (Romanazzi et al., 2012). Natural compounds classified as GRAS, such as sodium carbonates/bicarbonates, calcium chloride, and silicate, have in some cases extended the shelf life of fresh fruit after harvesting (Strano et al., 2022; Alaoui et al., 2017; Mehyar et al., 2011). For example, GRAS salts such as calcium chloride, sodium bicarbonate/carbonate and potassium sorbate/bicarbonate/carbonate have been used to test their ability to prevent postharvest gray mold (Karabulut et al., 2005; Nigro et al., 2006). Postharvest diseases in citrus fruits have also been tested using organic salts such as sodium carbonate/bicarbonate/benzoate/parabens and potassium sorbate (Montesinos-Herrero et al., 2016; Moscoso-Ramírez and Palou, 2014; Moscoso-Ramírez et al., 2013; Youssef et al., 2012). Additionally, the efficacy of potassium sorbate against apple blue rot (P. expansum), was evaluated either as a standalone treatment or in combination with thiabendazole. These findings indicated that potassium sorbate exhibited reduced efficacy when used alone (Fadda et al., 2015).
Other groups of salts and sulfites, used as preservatives and/or additives, have been applied to prevent rotting of fruits, such as tomatoes, grapes, raspberries, blueberries, and apricots (de Aguiar et al., 2023; Mühlbeier et al., 2021; Ahmed et al., 2018; Salur-Can et al., 2017; Rodriguez and Zoffoli, 2016). They act as antioxidants by inhibiting non-enzymatic browning and catalyzing various enzymatic reactions (Yan et al., 2022; Treesuwan et al., 2022; Afoakwah, 2020). One sulfite salt is sodium metabisulfite (SMB), which is generally recognized as safe (GRAS).
In this study, semi-commercial-scale experiments were conducted on oranges and apples. The fruits were either treated or untreated with SMB and stored for up to 61 days at 6°C, followed by six or 15 additional days of shelf life at ambient temperature (18°C). Physicochemical analyses, rot incidence, and disease severity were performed initially on fresh fruit and immediately after cold storage or shelf life. In fruit preservation, the first priority should be to maintain the sensory quality of the fruit, as this directly influences consumer purchasing behavior. Sensory qualities are particularly affected by fungal attacks. In Tunisia, the main fungi responsible for postharvest fruit decay on oranges and apples are, in order of importance, Penicillium digitatum, Botrytis cinerea, Penicilllium italicum and Alternaria alternata (Allagui and Ben Amara, 2024; Allagui et al., 2024).
The objectives of this study are as follows:
– Evaluate, under semi-commercial conditions, the effectiveness of SMB in maintaining the quality of apples and oranges that are undamaged and apparently free of infection.
– Comparison of the preservative performance of the three types of fruits during storage.
– Unravel the physicochemical properties and pathological deterioration of the three kinds of fruits as a function of storage period, based on eight attributes, including total phenols and flavonoids, weight loss, and fungal infection.
2 Materials and methods
2.1 Fruit samples
Oranges cv. Maltaise and apples cvs. Golden Delicious and Richared were obtained in 20 January 2023, from the wholesale market of fresh fruit and vegetables of ‘Bir Kasaa’ (Tunis). The fruits were immediately transported to the laboratory and stored at 6°C for 4 days before the experiments began.
In general, the apple harvest begins in September and the ‘Maltaise’ harvest in January. The ‘Maltaise’ fruit was in a fresh state, as some of these fruits still have completely green leaves on the stalk without showing any signs of treatment or wax. The apples were stored cold in refrigerated chambers before being released for sale. On these apples, there was no sign of treatment or wax, although a few fruits showed the beginning of rot (indicating the absence of antifungal treatment) and were immediately removed. Therefore, fruits with no visible defects were sorted and prepared for one of the planned antifungal treatments, and then stored at 6°C for 20, 42, and 59–61 days, plus 6 or 15 days of shelf life after each storage period. It should be noted that the absence of visible rot does not mean that there is no latent infection, which is difficult to detect visually on a fruit-by-fruit basis.
The main reason for using these two apple cultivars was their differential storage behavior, as observed in our preliminary tests, which needs to be confirmed. We used a single citrus cultivar in our experiments, the ‘Maltaise,’ because it is the most popular and exported citrus fruit in the country for which we were looking for answers regarding its storability, in addition to its availability on the market at the time of the experiment.
2.2 Fruit treatments
Oranges and apples were separated into lots, each containing 25 fruits. For each type of fruit, six lots were organized, three of which were untreated (control) apart from the initial sorting, used as a control, and three of which were treated with SMB. For each of the three storage periods, one untreated and one treated lot of each cultivar were used for assessment.
According to an earlier study carried out in the laboratory on fruits that had been inoculated with fungi (Allagui and Ben Amara, 2024), SMB was effective against postharvest rot at 0.5% fruit dip. This concentration was used in the experiments. The treatment consisted of soaking the fruit lot separately in a basin containing 10 L of tap water, in which 50 g of SMB was dissolved (conc. 0.5%). After soaking for 1 min to 2 min, the fruit was left to air dry for 2 h and then stored at 6°C ± 1°C for periods ranging from 20, 42, and 59–61 days, followed by days of shelf life. During storage, the relative humidity inside the cold room was between 90% and 95%.
After each storage period, the respective lots of ‘Maltaise’ fruit were exposed to a shelf life at room temperature (18 ± 1°C) of 15 days (after 20 days of cold storage) and of 6 days (after 42 or 59 days of cold storage). The shelf life of apples was 15 days, regardless of the storage period. All analyses were performed after each cold storage period and shelf life.
2.3 Assessment of fruit quality
Fruit hardness was determined per storage period on three randomized fruits from both equatorial sides using a 3 mm diameter sensor of a fruit hardness tester (LT Lutron FR-5105) and the force was expressed in Newton (N).
Three randomly selected oranges were juiced (without peel) using a domestic blender, and pure juice was used for analysis. For apples, three randomly selected fruits were cut into 1 cm cubic pieces (without stalk or calyx), and these pieces, including the peel, were weighed and juiced by adding the same weight of distilled water (one-time dilution).
One droplet of sample juice was used to determine the TSS, expressed as a percentage, using a digital hand refractometer (Hanna Instruments HI96801 HANNA Woonsocket RI USA). The pH of each juice sample was determined using a pH meter (AZ8651 pH/ORP METER). Each measurement was replicated three times.
The total phenol content (TPC) was determined using the Folin–Ciocalteu (Folin-C) method as described by Singleton et al. (1999) with some modifications. In a cuvette, 100 µL of one-time diluted juice was mixed with 100 µL Folin-C, followed by 2 min of 300 µL of sodium carbonate solution (20%, NaCO3). After 2 h of incubation, the absorbance was read at 750 nm using a UV–visible spectrophotometer (UV-1700 SHIMADZU, PharmaSpec, Jiangsu, China). The absorbance determined was compared with the standard curve for gallic acid (0.02 g L−1–0.10 g L−1) to determine the TPC, which is expressed as the mass of gallic acid equivalent (mg GAE/100 g of apple juice). Each measurement was performed in triplicate.
To determine the total flavonoid content, 250 µL of orange/apple juice was mixed with 1.25 ml of distilled water to which 75 µL of sodium nitrate was added. This mixture was left to react for 5 min, 150 µL of aluminum chloride (2%) was added, and the mixture was left to react for 5 min. This mixture was completed with 500 µL of sodium hydroxide NaOH (1M) and 3 ml of distilled water. The absorbance of this solution was determined at 510 nm using a UV–visible spectrophotometer (UV-1700 SHIMADZU, PharmaSpec, Jiangsu, China) to determine the total flavonoid content expressed in mg catechin/100 mg using a catechin calibration curve.
For each lot, fruit weight was determined, and the weight loss (WL) was calculated as a percentage using the following formula: WL (%) = [(Pi − Pcs)/Pi] × 100, for cold storage. For shelf life after cold storage, WL (%) = [(Pcs − Psl)/Pcs] × 100, where Pi is the initial weight before storage, Pcs is the weight determined immediately after a particular cold storage (20, 42, or 59 days), and Psl is the weight determined after a particular shelf life.
Disease incidence was calculated as follows: DI (%) = [Ncs/N] × 100 for each cold storage period (Singh et al., 2012). For shelf life, DI (%) = [Nsl/(N − Ncs)] × 100, where Ncs is the number of infected fruits detected after cold storage, Nsl is the number of infected fruits newly detected after the respective shelf life, and N is the initial number of fruits. Infected fruits show fungal infection even if the infection is minimal, i.e., less than 1 mm in diameter.
Disease severity (DS) was also estimated for the individual infected fruit following an empirical 0–5 rating scale according to the fruit surface infected (Romanazzi et al., 2013): 0, healthy fruit; 1, 1%–20% infected; 2, 21%–40% infected; 3, 41%–60% infected; 4, 61%–80% infected; and 5, ≥81% infected. This DS allowed the use of McKinney’s disease index (MI) calculated according to McKinney (1923): MI (%) = [(sum of all numerical ratings)/(total number of tested fruits × 5)] × 100.
2.4 Statistical analysis
Data were subjected to analysis of variance (ANOVA) using Statgraphics Centurion 16 software. Mean differences were separated by the honestly significant difference (HSD) procedure using Tukey’s test at p ≤0.05. Hierarchical cluster analysis (Ben Amara et al., 2021) by ‘ClustVis’ (https://biit.cs.ut.ee/clustvis/), a web tool for visualizing clustering of multivariate data (BETA)-custom edition, by the PREDECT project from BoxPlotR, using a defined mean Euclidean distance (Barth et al., 2020).
3 Results
3.1 Quality of oranges after cold storage and shelf life
3.1.1 Physicochemical properties
The initial hardness of the fruit was 15.3 N, varying over the storage periods between 10.4 and 16.2 N, but these averages were not significantly different between treated and untreated fruit for the respective storage periods.
The initial total soluble solids (TSS) content of fresh oranges was 11.5%, showing a slight increase over the different storage periods, varying between 12% and 12.7% in untreated fruit and between 11.6% and 12.6% in treated fruit (Table 1).
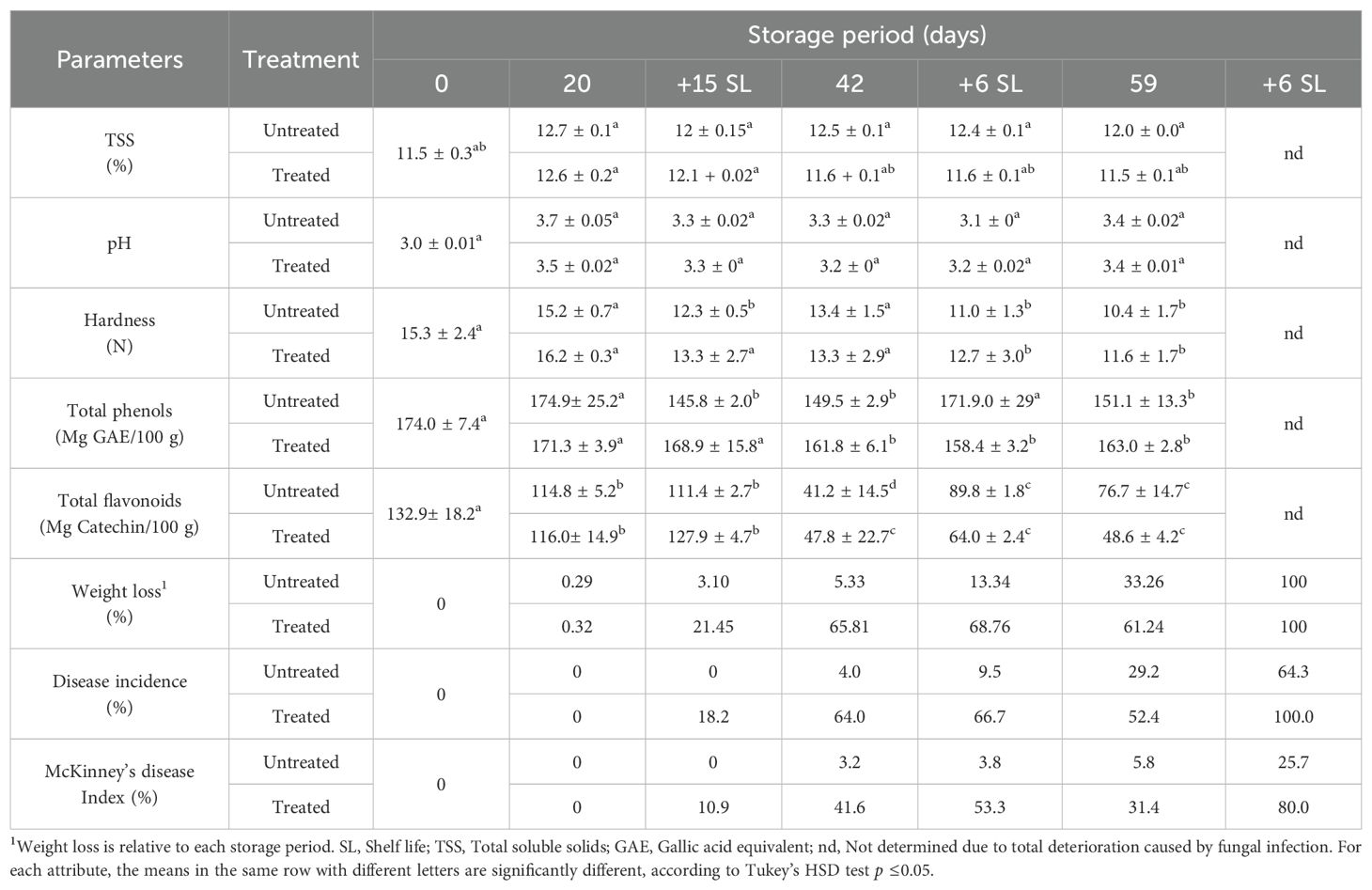
Table 1. Weight loss, decay incidence, disease severity and physicochemical properties of oranges of cv. Maltaise stored at 6°C during 20, 42 or 59 days followed by 15 days shelf life for the first storage period of 20 days and by 6 days shelf life for 42 and 59 cold storage; the fruit were untreated or treated with 0.5% SMB.
The initial pH of the orange juice was 3, increasing between 3.1 and 3.7 during storage, with no difference between the pH of the treated and untreated oranges.
The initial total polyphenol content (TPC) of fresh oranges was 174 mg GAE/100 g of orange juice. This content remained unchanged in oranges stored for 20 days at 6°C, with values ranging from 171.2 mg GAE/100 g to 174 mg GAE/100 g, without significant difference (p ≤0.05) between treated and untreated oranges.
Over a longer storage period, including shelf life, TPC showed significantly different levels of decrease and increase, ranging from 148 mg/100 g to 171.8 mg/100 g, with a slightly higher level for the fruit treated with SMB.
The initial total flavonoid content (TFC) determined in fresh fruit was 132.9 mg catechin/100g, which was slightly reduced to 114.8 mg catechin/100 g–116 mg catechin/100 g after 20 days storage at 6°C. These TFC were reduced, after 42 and 59 days of cold storage, to 47.8 mg catechin/100 g–48.6 mg catechin/100 g for treated fruit and 41.2 mg catechin/100 g–76.6 mg catechin/100 g for untreated fruit. During shelf life, the TFC remained low, with a higher content in the untreated fruit, except for (20 + 15SL) (Table 1).
Overall, the physicochemical properties of ‘Maltaise’ remained unaltered by SMB treatment, cold storage period or shelf life. However, there was a notable decline in the TFC and hardness with prolonged storage.
3.1.2 Weight loss, disease incidence and disease severity
After 20 days of storage at 6°C, weight loss was limited to a very low rate of 0.3% for both untreated and treated fruit, but this loss increased with longer cold storage, mainly for SMB-treated fruit (Table 1). Thus, after 42 days, the treated fruit showed a weight loss of 65.8% compared to 5.3% for the untreated oranges, i.e., an excess loss of 60.5% due to the SMB treatment alone. At the end of the 59 day-cold storage period, the highest weight loss was 61.2% for treated oranges compared to 33.3% for untreated oranges. This represents an additional loss of 27.9%, which is linked to the SMB treatment in cold storage. With regard to weight loss during shelf life after discarding the infected fruit from cold storage, it appears that the weight loss varied from 21.4% (20 + 15SL) to 100% (59 + 6SL) for treated fruit and from 3.1% (20 + 15SL) to 100% (59 + 6SL) for untreated oranges.
The incidence of the disease on treated and untreated ‘Maltaise’ was zero during cold storage for 20 days. The absence of fruit infection was also observed in untreated fruits during 15 days of storage at room temperature (shelf life). Fungal infection began on treated fruit after 15 days of storage following 20 days of cold storage, as well as on fruits stored for 42 days or more. The results showed that the incidence of infection during the latter storage periods varied between 4% and 64% in untreated ‘Maltaise’ fruit, compared with 18.2% and 100% in fruit treated with 0.5% SMB. On the other hand, the disease severity determined using the Mckinney’s disease index varied between 3.2% and 25.7% in untreated ‘Maltaise’ and between 10.9% and 80% in those treated with SMB. The results demonstrate that the incidence and severity of infection on ‘Maltaise’ oranges, in addition to weight loss, were minimal and comparable between treated and untreated fruit during the 20-day cold storage period. However, as the storage duration increased, the untreated fruit exhibited diminished susceptibility to these parameters. For the last shelf life (59 + 6SL), the weight loss of the untreated ‘Maltaise’ was 100%, while the disease incidence was 64.3% and not 100%. In fact, at this stage of fruit monitoring, the rest of the uninfected fruit was defective owing to the change in color and texture of the rind, which became pale and dry, and therefore unfit for consumption (lost).
3.2 Quality of red apples cv. Richared after cold storage and shelf life
3.2.1 Physicochemical properties
The initial hardness of the fruit was 16.3 N (Table 2). This attribute decreased to 14.3 N after 20–42 days of cold storage with no significant difference between treated and untreated apples. After 61 days, the hardness reached 13 N for both treated and untreated apples. After 15 days in shelf life following 20, 42 and 61 days of storage at 6°C, the fruit hardness varied between 14.4 and 9.3 N for untreated fruit and between 13.1 and 12.1 N for treated fruit. Overall, the hardness of treated and untreated fruit decreased slowly and similarly during cold storage but was relatively higher during shelf life, particularly for untreated fruit.
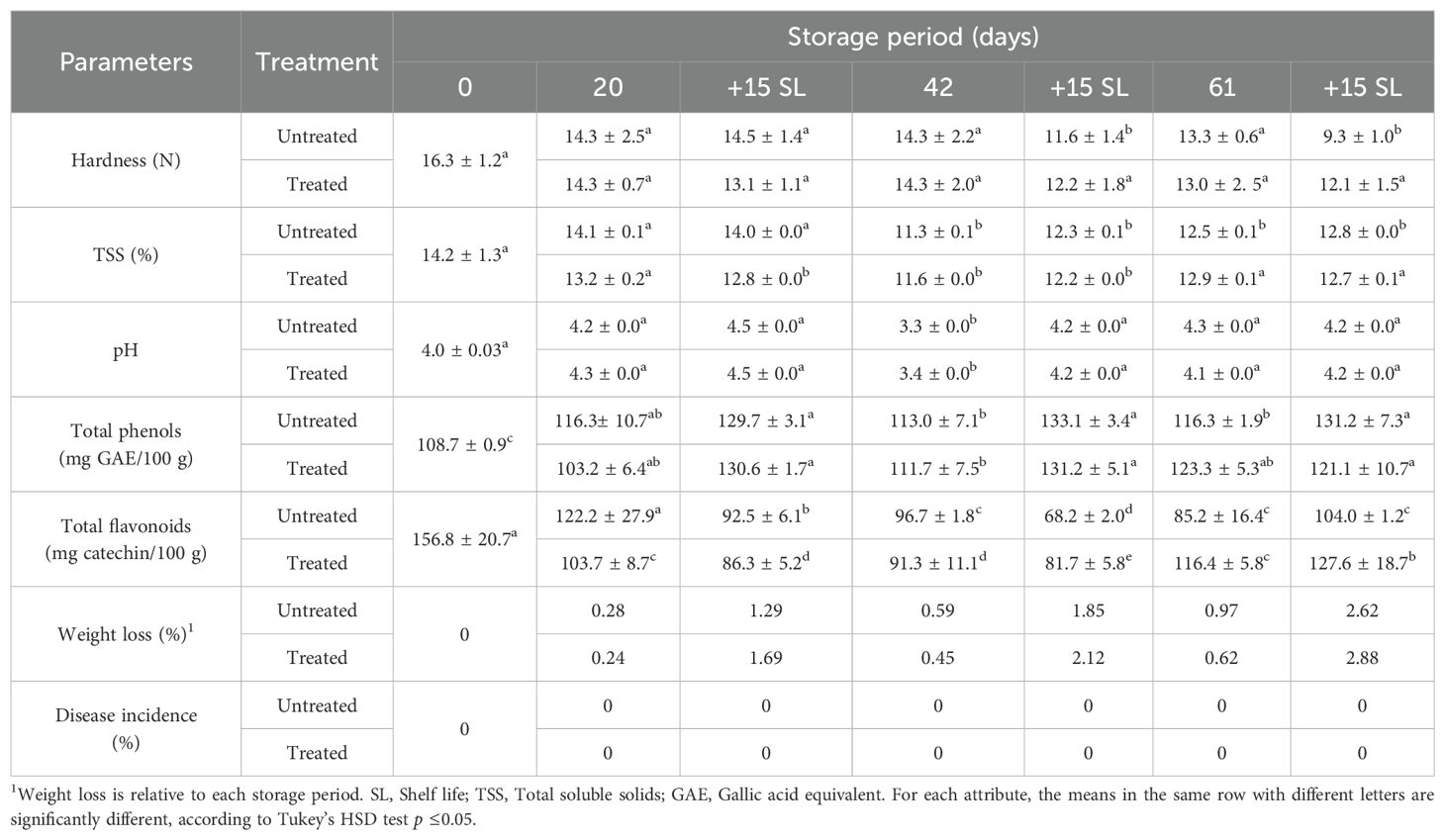
Table 2. Weight loss, disease incidence and physicochemical properties of apple cv. Richared stored at 6°C during 20, 42 or 61 days followed by 15 days shelf life for each cold storage; the fruit were untreated/treated with 0.5% SMB.
The initial TSS content of fresh apple juice was 14.2% (Table 2). This TSS concentration decreased slightly, particularly after 42 days of cold storage (11.3%–11.6%) and after 61 days of cold storage (12.5%–12.9%). Shelving of the fruit did not affect this trait; its content fluctuated between 14% and 12.2%. Fruit treatment was not a distinguishing criterion for TSS content compared to untreated fruit. No significant change was recorded in this trait during shelf life compared with cold storage.
The pH initially recorded in fresh apple juice was 4 and remained at an average of 4.1 for up to 20 days of cold storage. A slight decrease in pH was recorded at 3.4 and 3.3 after 42 days of cold storage for treated and untreated apples respectively, which increased to 4.1–4.3 during 61 days of cold storage. No significant change was detected in this characteristic over the shelf life compared with cold storage.
The initial TPC of fresh apples was 108.8 mg GAE/100 g juice. This trait was maintained without significant change during the different cold storage periods ranging between 103.2 mg GAE/100 g to 113.2 mg GAE/100 g, with no significant differences between untreated and treated fruit, except for treated apples after 61 cold storage days, which was 123.2 mg GAE/100 g. For the shelf life, an increase in this parameter was observed, varying between 121 mg GAE/100 g and 133 mg GAE/100 g, which represents an increase of approximately 20 mg GAE/100 g compared with the values for this parameter in apples (treated or untreated) stored at low temperatures.
The TFC of the fresh apple juice was 156.8 mg catechin/100 g. This initial content decreased with cold storage, reaching 85.2 mg catechin/100 g, the lowest value measured after 61 days of cold storage for untreated apples compared with 116.4 mg catechin/100 g for treated apples. The TFC values ranged from 91.4 mg catechin/100 g, for treated fruit after 42 days of cold storage, to 109.2 mg catechin/100 g, after 20 days of cold storage for untreated fruit. With regard to shelf life, the TFC showed irregular variations ranging from 68.2 mg catechin/100 g (42 d + 15SL) to 127.6 mg catechin/100g (61 d + 15SL). Nevertheless, for the short storage period between 20, 20 + 15SL, and 42, the TFC of the untreated apples was higher than that of the treated apples; however, this situation was reversed for the longer period between 42 + 15SL, 61, and 61 + 15SL showing higher TFC values for the treated apples.
3.2.2 Weight loss and disease incidence
The weight loss of ‘Richared’ apples ranged from 0.24% (after 20 days cold storage) to 2.88% after 75 days (60 days cold storage and 15 days shelf life). This low weight loss after prolonged storage could be attributed to a physiological process and not to fungal infection, as the incidence of disease was zero regardless of the storage period (Table 2).
3.3 Quality of yellow apples cv. Golden after cold storage and shelf life
3.3.1 Physicochemical properties
The hardness values of the cv. ‘Golden’ are reported in Table 3. The initial hardness of the fruit was 12.5 N. This value decreased slightly to approximately 11.1 N (11.0 to 11.2) throughout the cold storage periods for untreated fruit. For treated fruit, values for this characteristic decreased from 13.2 N (20 days of cold storage) to 10.0 N (61 days of cold storage). During shelf life, fruit hardness showed a downward trend, mainly after 61 + 15SL, reaching 7.6 N, but with no difference between treated and untreated fruit.
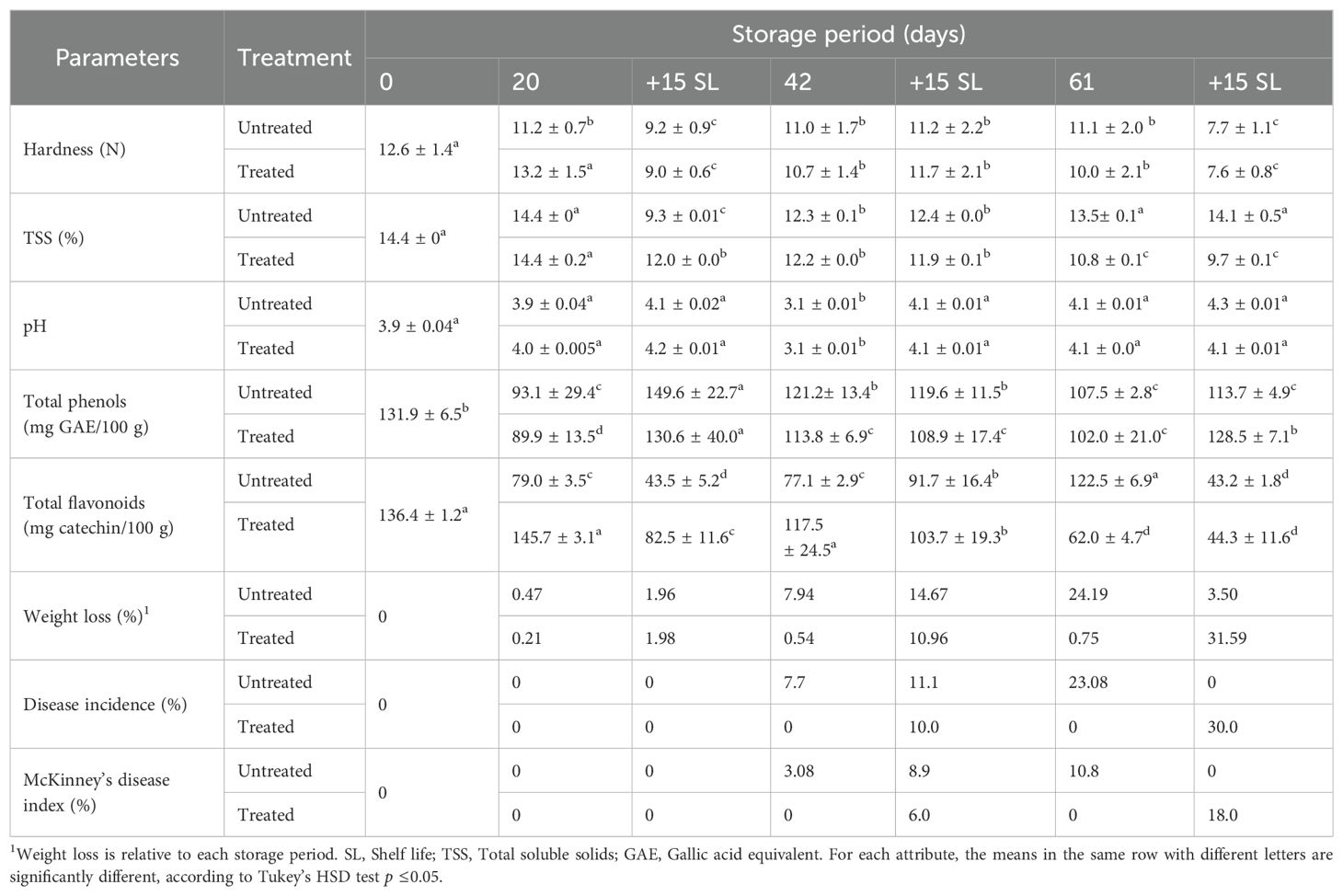
Table 3. Weight loss, disease incidence, disease severity and physicochemical properties of apple cv. Golden stored at 6°C during 20, 42 or 61 days followed by 15 days shelf life for each cold storage; the fruit were untreated/treated with 0.5% SMB.
The initial TSS content of the fresh apple juice was 14.4% (Table 3). This content remained unchanged after 20 days of cold storage in both the untreated and treated fruits. For longer periods of cold storage, untreated fruit showed little change in content, which fluctuated between 12.3% and 13.5%. However, the content of the treated fruit gradually decreased to 12.2% and 10.8%, respectively, after 42 and 61 days of cold storage. For shelf life, the TSS was relatively high in untreated fruit, unlike in treated fruit, which showed decreases in relation to the length of the storage period, decreasing from 12% to 9.7% (Table 3).
The initial pH of fresh apple juice was 3.9 (Table 3). This pH was maintained within the range of 4 (3.9 and 4.3), with the exception of the fruit stored for 42 days in cold storage, with a pH of 3.1. There was no difference between the pH values of the treated and untreated fruits.
The total phenolic content was approximately 90 mg GAE/100 g–93 mg GAE/100 g at the end of 20 days of cold storage (Table 3), showing a significant reduction during storage compared with that detected in fresh fruit, which was 132 mg GAE/100 g. Fruits stored for longer periods (42 and 61 days) yielded more phenols, with levels ranging from 102 mg GAE/100 g to 121.2 mg GAE/100 g (Table 3). There was no significant difference between untreated and SMB-treated fruits. During the 15-day shelf life, total phenols accumulated more in untreated fruit than treated fruit after the first 20 days of cold storage, reaching 149.6 mg GAE/100 g (untreated fruit) and 130.6 mg GAE/100 g (treated fruit), as well as after 61 days of cold storage. The observed increase in phenolic compound content over shelf life was relatively low during the cold storage period. On the other hand, the phenolic content of the fruits during shelf life did not show such an increase after 42 days of cold storage, as these levels were relatively high during the 42-day cold storage. In addition, there was an insignificant difference between the phenols of treated and untreated fruits, regardless of storage period.
The initial flavonoid content of ‘Golden’ juice was 136.4 mg catechin/100 g (Table 3). Significant differences were recorded between treated and untreated fruit, with the exception of the last shelf life 61 + 15SL where both contents were low and close (43.2 mg catechin/100 g–44.4 mg catechin/100 g). Thus, the flavonoid content of treated apples was much higher than that of untreated fruit, in some cases twice as high (79 mg catechin/100 g to 145 mg catechin/100 g, 21.8 mg catechin/100 g to 43.6 mg catechin/100 g, and 62 mg catechin/100 g to 122.6 mg catechin/100 g). For each shelf-life periods the flavonoid content of treated and untreated fruits was lower than that of cold-preserved fruit. Thus, TFC was better titrated in fruits that had been previously treated and kept cold.
3.3.2 Weight loss, disease incidence and disease severity
In the absence of disease incidence, the weight loss of the yellow apples ranged from 0.21%–0.47% (20 days after cold storage) to 0.54%–0.75% (42 and 61 days after cold storage for treated fruit) (Table 3). When fungal infections developed on the apples, the weight loss was higher, ranging from 7.94% (42 days after cold storage for untreated fruit) to 31.59% (61 + 15SL for treated fruit) (Table 3).
The extent of weight loss was related to the incidence of disease, which ranged from 7.7% to 30%, and the McKinney disease index, which ranged from 3.08% to 18% (Table 3). Fruit treatment had no positive effect on fruit health at the start of cold storage, as even untreated fruits were unaffected. The effect of treatment only began to appear after 42 days of cold storage, with the exception of the 61 + 15SL period, when treated fruit were much more contaminated (31.59% compared with 3.5% for untreated fruit).
3.4 Heat map inference
Hierarchical cluster analysis was performed in the form of a heat map based on a defined Euclidean metric distance. This exploratory analysis is aimed at determining the possible relationships between the parameters obtained from fruit samples in storage.
The heat map made it possible to group the fruit samples into two main clusters based on fungal infection and fruit quality at the later stages of storage. Group A included fruit from apple cultivars, whereas Group B included orange fruit (Figure 1). Within Group A, there were two distinct subgroups: A1 and A2, with the A1 subgroup being abundant in terms of hardness, TSS, pH, and TFC, while in the A2 subgroup, these quality characteristics were less abundant. The A1 characterizes fresh fruit of ‘Golden’ and ‘Richared,’ the 20 days of cold storage (C1) for ‘Golden’ and ‘Richared,’ the L1 (untreated) shelf life of ‘Richared’ and the C3 and L3 of ‘Richared.’ These results underline that the quality of fruit after short-term storage is similar to that of fresh fruit, and that ‘Richared’ fruit would retain its initial quality as long as cold storage and shelf life are extended. The A2 subgroup includes apple samples subjected to the shelf life L1, L2, and L3 for ‘Golden’ and to L1 (treated) and L2 for ‘Richared’ as well as C3 for ‘Golden.’ Although these parameters in the heat map were less colorful and, therefore, less abundant in A2 than in A1, no fungal disorders were recorded in these samples, mainly in cv. Richared. This sub-group implies that apple fruit quality would be affected particularly during shelf life, especially in the case of ‘Golden.’
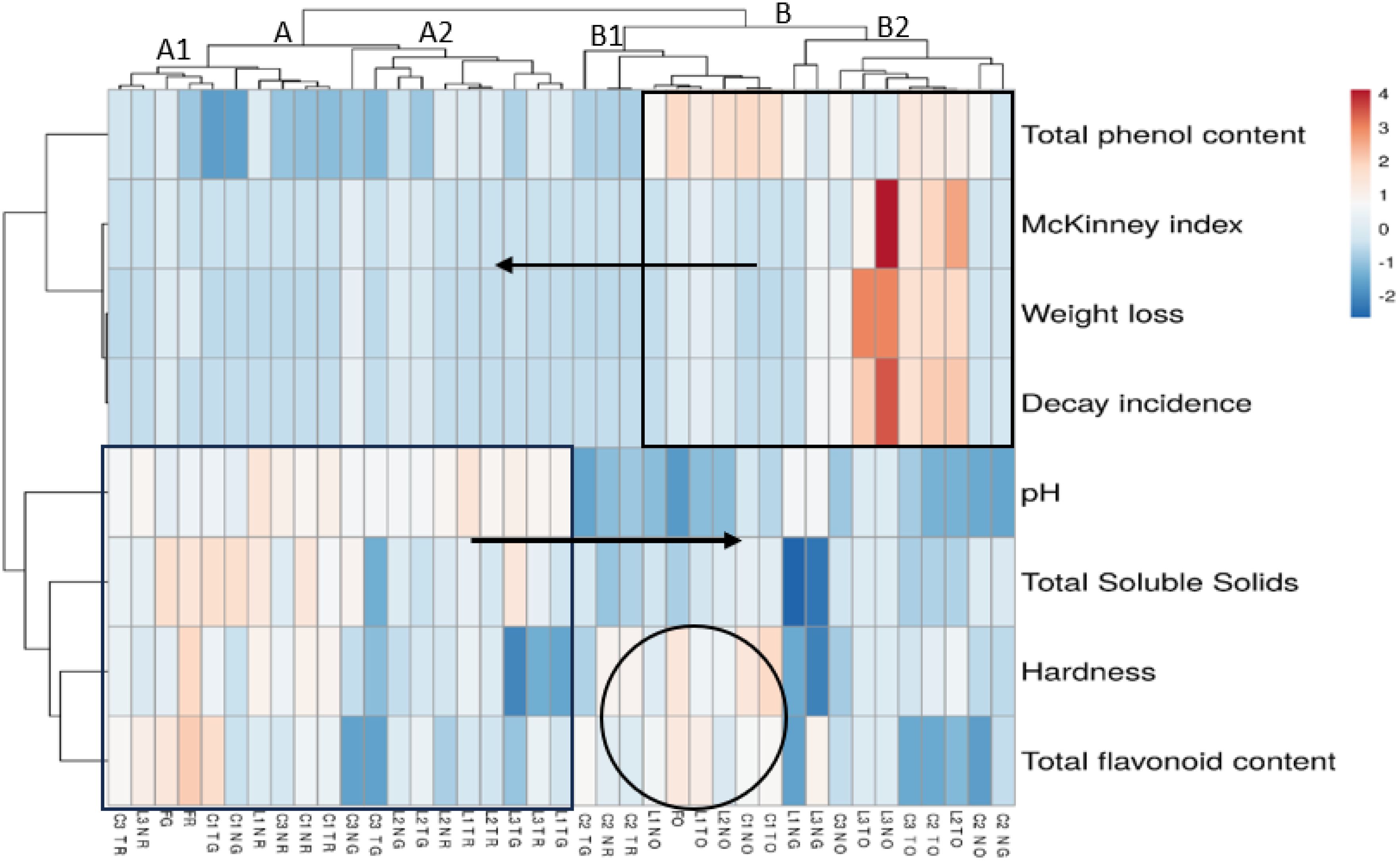
Figure 1. Hierarchical cluster analysis for predicting the link between different physicochemical properties and fungal infection criteria in oranges of cv. Maltaise and apples of both cvs. Richared and Golden during different storage periods. A and B represent cluster code; A1, A2, B1, and B2 represent sub-clusters of A and B, respectively. O, orange; G, apple cv. ‘Golden;’ R, apple cv. ‘Richared;’ FO, fresh orange; FG, fresh ‘Golden;’ FR, fresh ‘Richared;’ C1, C2, and C3 are cold storage periods of 20, 42, and 59–61 days, respectively. L1, L2, and L3 are the storage times in relation to the respective cold storage periods; T, treated; N, untreated. The square of cluster A is in a level of abundance (0–2), the square of cluster B in a level of abundance (0–4) while the circle of sub-cluster B1 is in a level of abundance (0–1).
Group B, separating ‘Maltaise’ fruit samples and, to a lesser extent, some apple samples, is divided into two subgroups B1 and B2. Subgroup B1 is abundant with other quality criteria including TPC, TFC and fruit hardness, clustering together fresh oranges with the C1, L1, and L2 untreated oranges, and the C2 treated ‘Golden’ and ‘Richared’ fruit. This indicates that although these apple samples were less well preserved than their counterparts during other storage periods, they remained much longer and in the same category as fresh oranges.
The subgroup B2 associates orange samples conserved during longer time as C2, L2, and C3, L3 except for the sample of untreated ‘Golden’ under C2 storage (Figure 1). In this subgroup, decay incidence and severity, as well as weight loss and TPC, were highly abundant in the heat map, indicating that these fruit samples were the most affected by longer storage. Clearly, the heat map did not show any particular grouping, highlighting the treatment/non-treatment aspect. This indicated that the treatment did not play a distinctive role in differentiating it from the untreated fruit.
4 Discussion
The fruit of cv. Richared was harder than cv. Golden before storage. However, the hardness of fruits from both cultivars, treated or untreated, decreased significantly during prolonged storage. The hardness of oranges was not significantly different between treated and untreated fruits for the respective storage periods, although this characteristic decreased with increasing storage time. This indicates that the interaction between the SMB and the fruit pericarp during storage is unlikely. As the fruit shelf life increases, apples soften, which could be due to reduced water content, increased respiration, changes in pectin content (Billy et al., 2008), and more active starch hydrolysis (Jan and Rab, 2012). Cell wall polysaccharides are among the compounds that are responsible for fruit firmness. Degradation of these compounds by hydrolytic enzymes during ripening leads to softening of the fruit pericarp (Martínez-Blay et al., 2020) and could explain the reduction in fruit hardness as storage time increases, considering the differences in firmness noted between apple cultivars.
TSS and pH are important organoleptic properties of fruits (Lu et al., 2021) that are correlated with the fruit’s texture and composition (Jan and Rab, 2012). In our study, the TSS content of orange juice increased slightly during the first storage period. During subsequent storage periods, the TSS content remained unchanged for treated and untreated oranges, implying an early conversion of available organic acids into sugar, given that the total soluble constituents of citrus juice are 10% organic acids and 80% sugars (Lado et al., 2014). The pH of the fruit juice rose rapidly during the first 20 days of cold storage and then remained stable throughout the rest of the storage period. This increase in pH could be linked to the rapid transformation of organic acids into sugar components (Habibi et al., 2021).
In contrast to oranges, the TSS content in both apple cultivars decreased during storage. Butkeviciute et al. (2021) described a decreasing trend of the soluble solid content of some fruit samples during storage. As the fruit’s shelf life increases, apples soften due to changes in the pectin content of the cells, imparting progressive bitterness to the fruit (Billy et al., 2008) in relation to the balance of sugar and organic acid contents. As a result, pH decreased for ‘Golden’ and ‘Richared’ apples at the end of 40 days of cold storage.
Citrus and apple fruits are rich in bioactive compounds such as polyphenols (Sánchez-Moreno et al., 2003), which are sources of antioxidants that inhibit free radicals (Iturralde-García et al., 2022). Our analyses showed that the TPC of oranges stored at 6°C was lower than that of freshly harvested ‘Maltaise’ fruit. Rapisarda et al. (2008) reported a decrease in TPC in ‘Valencia’ oranges after 40 days storage at 6°C, which was attributed to fruit senescence, loss of astringent flavor and changes in enzyme activity, with phenolic degradation being responsible for the decrease in TPC (Yang et al., 2024). In apples, our findings indicated that the Golden cultivar and, to a lesser extent, the Richared cultivar exhibited a reduction in TPC compared with fresh fruit.
This accumulation of TPC is greater in ‘Golden’ than in ‘Richared,’ although ‘Golden’ seems to be less suitable for long storage than ‘Richared.’ This reverse situation was more pronounced in ‘Maltaise,’ which had more total phenolic content but less storage capacity. This result is corroborated by the heatmap, which groups TPC with traits (disease incidence and severity) that indicate less suitability for storage. This suggests that total polyphenols are indicative of a lower tolerance to prolonged cold storage. Nevertheless, Adyanthaya et al. (2009), working on four apple varieties stored in a cold room (6°C) for 3 months, pointed out that a possible combination of phenolic metabolites and antioxidant enzymes is essential for better postharvest preservation. Sun et al. (2017) hypothesized a possible relationship between the phenolic content of wild apples and their postharvest resistance to blue mold (P. expansum) decay. For example, cold storage has been reported to reduce the phenolic content in different apple cultivars (Napolitano et al., 2004), and the TPC of the four apple cultivars was reduced after 4 months of storage at 2°C. However, other studies have shown that phenolic compounds in apple cultivars remain relatively constant during storage (Carbone et al., 2011).
Flavonoids are plant secondary metabolites with a polyphenolic structure that are widely present in fruits and vegetables (Panche et al., 2016). Flavonoids, generally yellow in color, are responsible for the flavor and color of fruits (Patil and Murumkar, 2024). Our results showed that longer cold storage and shelf life of ‘Maltaise’ significantly reduced the total flavonoid content. With regard to the total flavonoid content of apples, a rapid decrease in this nutritional compound was recorded. The rate of this decrease depended on the apple cultivar, as a sharp reduction in this compound was observed for the cultivar ‘Golden’ after long storage and shelf life, compared with the cultivar Richared, which maintained a relatively higher TFC as the storage time increased. The heat map revealed a strong link between the TFC, hardness, and TSS, which could be indicators of quality preservation during long-term storage. This suggests that flavonoids are putative biomarkers of storability, unlike total phenolics. In a survey conducted by Konstantinou et al. (2011) on apples stored in packaging houses in Greece, four varieties, including ‘Golden Delicious,’ were examined. The findings revealed a negative correlation between susceptibility to B. cinerea and flavonoids, whereas susceptibility to Penicillium expansum was negatively correlated with fruit firmness. These results are consistent with those obtained in the present study.
In terms of fruit health and weight loss, it should be emphasized that for the stored fruit still free of fungal infection, weight loss did not exceed 3% for oranges cv. Maltaise, 2.8% for yellow ‘Golden’ and 0.8% for red apple. For example, the weight loss of cold-preserved apple cultivars was 2.22% for ‘Red Delicious’ and 2.91% for ‘Golden Delicious’ (Jan and Rab, 2012), which is in the same range as our results. This loss of weight, which is difficult to escape or remedy, may be linked to physiological mechanisms, such as the exchange of gas and water between the environment and the fruit’s pericarp during respiration. This limited weight loss is not always the case for different lots and storage periods, as once the fruit is affected by rot, it is considered unfit for consumption and therefore, all of its weight is considered lost. With increasing storage time, no better post-treatment protection was observed for oranges or apples, especially after prolonged storage.
The results obtained under semi-commercial conditions suggest that ‘Maltaise’ oranges can be stored for up to 35 days (20 days at 6°C + 15 d SL at ambient temperature) without showing any signs of deterioration. Then, yellow apples could be stored for up to 61 days in cold storage, and finally red apples for over 75 days (61 days at 6°C +15 d SL). Red apples appear to be good candidates for long-term cold storage. The greater resistance of certain apple cultivars to decay during storage may be explained by the epidermis, which is more resistant to rupture, as reported here for the hardness and flavonoids of cv. Richared. It was highlighted that ‘Golden Delicious’ has a tender epidermal layer and a cortical tissue moderately susceptible to P. expansum (Spotts et al., 1999).
Although SMB had no noticeable effect on the physicochemical properties of the fruit, it appears that over time, this treatment may make the fruit more susceptible to fungal infection, in contrast to the untreated fruit, which was better preserved, probably due to an antagonistic microbiome unaffected by the treatment. Our previous studies showed that SMB was highly effective in reducing fungal mycelial growth in vitro and was effective in controlling fruit rot as a curative treatment for wounded and inoculated fruits (Allagui and Ben Amara, 2024). In the present study, the efficacy of SMB was not demonstrated in unwounded or uninoculated fruits. Therefore, this study provides an answer to the question of its efficacy under semi-commercial conditions when the fruit is neither wounded nor inoculated, a result that deserves to be known because, to our knowledge, it does not seem to have been previously reported on fruit under semi-commercial conditions. Future research could focus on the mode of action of SMB on the fruit epidermis, for example by investigating which specific microorganisms are affected and whether these effects are concentration-dependent.
5 Conclusions
Our results indicated that successful postharvest fruit preservation depends on the type of fruit, duration of cold storage, and shelf life. The Richared apple cultivar retained its nutritional and sanitary properties without developing rot or noticeable weight loss for 60 days at a low temperature (6°C) and shelf life of 15 days. This red apple performed better during storage than the yellow Golden cultivar, especially in terms of storability and total flavonoid content. Apples of cv. Golden showed better stability during storage than the oranges of cv. Maltaise. The latter was adequately stored for up to 20 days at 6°C, followed by a 15-day shelf life, irrespective of the sodium metabisulfite treatment. As storage time progressed, the risk of infection of the oranges increased, which was associated with the degradation of certain physicochemical properties, including flavonoids. The nutritional value (TSS and pH) of apples and oranges was not affected by soaking in SMB, which was similar to that of the untreated fruit. However, treatment of fruits with SMB is unlikely because of its minimal effectiveness in preventing fruit rot during long-term storage. It has been proposed that characteristics such as flavonoid content, hardness and total soluble solids (TSS) may serve as markers of the fruit’s storage suitability. Conversely, the polyphenol content has been identified as an indicator of susceptibility to long-term storage.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
MA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MB: Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Ministry of Higher Education and Scientific Research of Tunisia funded this research in the framework of PRIMA, project acronym StopMedWaste ‘Innovative Sustainable technologies TO extend the shelf life of Perishable MEDiterranean fresh fruit, vegetables and aromatic plants and to reduce WASTE,’ Project Number 1556.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adyanthaya I., Kwon Y. I., Apostolidis E., and Shetty K. (2009). Apple postharvest preservation is linked to phenolic content and superoxide dismutase activity. J. Food Biochem. 33, 535–556. doi: 10.1111/j.1745-4514.2009.00236.x
Afoakwah A. N. (2020). Anti-browning methods on fresh-cut fruits and fruit juice. Afr. J. Biol. Sci. 2, 27–32. doi: 10.33472/AFJBS.2.4.2020.27-32
Ahmed S., Roberto S. R., Domingues A. R., Shahab M., Junior O. J. C., Sumida C. H., et al. (2018). Effects of different sulfur dioxide pads on Botrytis mold in ‘Italia’ table grapes under cold storage. Horticulturae 4, 29. doi: 10.3390/horticulturae4040029
Alaoui F. T., Askarne L., Boubaker H., Boudyach E. H., and Aoumar A. A. B. (2017). Control of gray mold disease of tomato by postharvest application of organic acids and salts. Plant Pathol. J. 16, 62–72. doi: 10.3923/ppj.2017.62.72
Alaoui A., Christ F., Silva V., Vested A., Schlünssen V., González N., et al. (2024). Identifying pesticides of high concern for ecosystem, plant, animal, and human health: A comprehensive field study across Europe and Argentina. Sci. Total. Environ. 948, 174671. doi: 10.1016/j.scitotenv.2024.174671
Allagui M. B. and Ben Amara M. (2024). Effectiveness of Several GRAS Salts against Fungal Rot of Fruit after Harvest and Assessment of the Phytotoxicity of Sodium Metabisufite in Treated Fruit. J. Fungi. 10, 359. doi: 10.3390/jof10050359
Allagui M. B., Moumni M., and Romanazzi G. (2024). Antifungal activity of thirty essential oils to control pathogenic fungi of postharvest decay. Antibiotics 13, 28. doi: 10.3390/antibiotics13010028
Barth E., Resende J. T. V. D., Moreira A. F. P., Mariguele K. H., Zeist A. R., Silva M. B., et al. (2020). Selection of experimental hybrids of strawberry using multivariate analysis. Agronomy 10, 598. doi: 10.3390/agronomy10040598
Ben Amara M., Abdelli S., De Chiara M. L. V., Pati S., Amodio M. L., Colelli G., et al. (2021). Changes in quality attributes and volatile profile of ready-to-eat “Gabsi” pomegranate arils as affected by storage duration and temperatures. J. Food Process Preserv. 45, e14415. doi: 10.1111/jfpp.14415
Billy L., Mehinagic E., Royer G., Renard C. M., Arvisenet G., Prost C., et al. (2008). Relationship between texture and pectin composition of two apple cultivars during storage. Postharvest Biol. Technol. 47, 315–324. doi: 10.1016/j.postharvbio.2007.07.011
Butkeviciute A., Viskelis J., Viskelis P., Liaudanskas M., and Janulis V. (2021). Changes in the biochemical composition and physicochemical properties of apples stored in controlled atmosphere conditions. Appl. Sci. 11, 6215. doi: 10.3390/app11136215
Carbone K., Giannin B., Picchi V., Lo Scalzo R., and Cecchini F. (2011). Phenolic composition and free radical scavenging activity of dif ferent apple varieties in relation to the cultivar, tissue type and storage. Food Chem. 127, 493–500. doi: 10.1016/j.foodchem.2011.01.030
de Aguiar A. C., Higuchi M. T., Ribeiro L. T. M., Leles N. R., Bosso B. E. C., Shimizu G. D., et al. (2023). Bio-based and SO2-generating plastic liners to extend the shelf life of ‘Benitaka’table grapes. Postharvest Biol. Technol. 197, 112217. doi: 10.1016/j.postharvbio.2022.112217
Fadda A., Barberis A., D’Aquino S., Palma A., Angioni A., Lai F., et al. (2015). Residue levels and performance of potassium sorbate and thiabendazole and their co-application against blue mold of apples when applied as water dip treatments at 20 or 53°C. Postharvest Biol. Technol. 106, 33–43. doi: 10.1016/j.postharvbio.2015.04.003
Fantke P. and Jolliet O. (2016). Life cycle human health impacts of 875 pesticides. Int. J. Life Cycle Assess. 21, 722–733. doi: 10.1007/s11367-015-0910-y
FAO, Food and Agriculture Organization of the United Nations (2023). SDG Global Food Loss and Waste. Food Loss and Waste Database | Technical Platform on the Measurement and Reduction of Food Loss and Waste (Rome, Italy: Food and Agriculture Organization of the United Nations).
Groupement Interprofessionnel des Fruits (2023). Report of 2023. Available online at: https://gifruits.com/statistiques-production-agrumes/ (Accessed November 10, 2024).
Habibi F., Guillén F., Serrano M., and Valero D. (2021). Physicochemical changes, peel colour, and juice attributes of blood orange cultivars stored at different temperatures. Horticulturae 7, 320. doi: 10.3390/horticulturae7090320
Iturralde-García R. D., Cinco-Moroyoqui F. J., Martínez-Cruz O., Ruiz-Cruz S., Wong-Corral F. J., Borboa-Flores J., et al. (2022). Emerging technologies for prolonging fresh-cut fruits’ quality and safety during storage. Horticulturae 8, 731. doi: 10.3390/horticulturae8080731
Jan I. and Rab A. (2012). Influence of storage duration on physico-chemical changes in fruit of apple cultivars. J. Anim. Plant Sci. 22, 708–714.
Karabulut O. A., Romanazzi G., and Smilanick J. L. (2005). Postharvest ethanol and potassium sorbate treatments of table grapes to control gray mold. Postharvest Biol. Technol. 37, 129–134. doi: 10.1016/j.postharvbio.2005.04.001
Konstantinou S., Karaoglanidis G. S., Bardas G. A., Minas I. S., Doukas E., and Markoglou A. N. (2011). Postharvest fruit rots of apple in Greece: Pathogen incidence and relationships between fruit quality parameters, cultivar susceptibility, and patulin production. Plant Dis. 95, 666–672. doi: 10.1094/PDIS-11-10-0856
Lado J., Rodrigo M. J., and Zacarías L. (2014). Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 10, 1–6.
Lu L., Zuo W., Wang C., Li C., Feng T., Li X., et al. (2021). Analysis of the postharvest storage characteristics of the new red-fleshed apple cultivar ‘meihong’. Food Chem. 354, 129470. doi: 10.1016/j.foodchem.2021.129470
Martínez-Blay V., Taberner V., Pérez-Gago M. B., and Palou L. (2020). Control of major citrus postharvest diseases by sulfur containing food additives. Int. J. Food Microbiol. 330, 108713. doi: 10.1016/j.ijfoodmicro.2020.108713
McKinney H. H. (1923). Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J. Agric. Res. 26, 195–218.
Mehyar G. F., Al-Qadiri H. M., Abu-Blan H. A., and Swanson B. G. (2011). Antifungal effectiveness of potassium sorbate incorporated in edible coatings against spoilage molds of apples, cucumbers, and tomatoes during refrigerated storage. J. Food Sci. 76, 210–217. doi: 10.1111/j.1750-3841.2011.02059.x
Montesinos-Herrero C., Moscoso-Ramírez P. A., and Palou L. (2016). Evaluation of sodium benzoate and other food additives for the control of citrus postharvest green and blue molds. Postharvest Biol. Technol. 115, 72–80. doi: 10.1016/j.postharvbio.2015.12.022
Moscoso-Ramírez P. A., Montesinos-Herrero C., and Palou L. (2013). Characterization of postharvest treatments with sodium methylparaben to control citrus green and blue molds. Postharvest Biol. Technol. 77, 128–137. doi: 10.1016/j.postharvbio.2012.10.007
Moscoso-Ramírez P. A. and Palou L. (2014). Preventive and curative activity of postharvest potassium silicate treatments to control green and blue molds on orange fruit. Eur. J. Plant Pathol. 138, 721–732. doi: 10.1007/s10658-013-0345-x
Mühlbeier D. T., Ribeiro L. T., Higuchi M. T., Khamis Y., Junior O. J. C., Koyama R., et al. (2021). SO2-generating pads reduce gray mold in clamshell-packaged ‘Rubi’table grapes grown under a two-cropping per year system. Semina: Ciências Agrárias 42, 1069–1086. doi: 10.5433/1679-0359.2021v42n3p1069
Napolitano A., Cascone A., Graziani G., Ferracane R., Scalfi L., Di Vaio C., et al. (2004). Influence of variety and storage on the polyphenol composition of apple flesh. J. Agric. Food Chem. 52, 6526–6531. doi: 10.1021/jf049822w
Nigro F., Schena L., Ligorio A., Pentimone I., Ippolito A., and Salerno M. G. (2006). Control of table grape storage rots by pre-harvest applications of salts. Postharvest Biol. Technol. 42, 142–149. doi: 10.1016/j.postharvbio.2006.06.005
Panche A. N., Diwan A. D., and Chandra S. R. (2016). Flavonoids: an overview. J. Nutr. Sci. 5, e47. doi: 10.1017/jns.2016.41
Patil M. and Murumkar C. (2024). “The Classes and Biosynthesis of Flavonoids,” in Flavonoids as Nutraceuticals, 1st Edition. Eds. Kesharwani R. K., Saini D., Keservani R. K., and Kumar A. (Apple Academic Press, New York), 2023 233–251. doi: 10.1201/9781003412441
Rapisarda P., Bianco M. L., Pannuzzo P., and Timpanaro N. (2008). Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck. Postharvest Biol. Technol. 49, 348–354. doi: 10.1016/j.postharvbio.2008.02.002
Rodriguez J. and Zoffoli J. P. (2016). Effect of sulfur dioxide and modified atmosphere packaging on blueberry postharvest quality. Postharvest Biol. Technol. 117, 230–238. doi: 10.1016/j.postharvbio.2016.03.008
Romanazzi G., Feliziani E., Santini M., and Landi L. (2013). Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol. Technol. 75, 24–27. doi: 10.1016/j.postharvbio.2012.07.007
Romanazzi G., Lichter A., Gabler F. M., and Smilanick J. L. (2012). Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol.Technol. 63, 141–147. doi: 10.1016/j.postharvbio.2011.06.013
Salur-Can A., Türkyılmaz M., and Özkan M. (2017). Effects of sulfur dioxide concentration on organic acids and β-carotene in dried apricots during storage. Food Chem. 221, 412–421. doi: 10.1016/j.foodchem.2016.10.081
Sánchez-Moreno C., Plaza L., de Ancos B., and Cano M. P. (2003). Quantitative bioactive compounds assessment and their relative contribution to the antioxidant capacity of commercial orange juices. J. Sci. Food Agric. 83, 430–439. doi: 10.1002/jsfa.v83:5
Singh P., Mishra A. K., and Tripathi N. N. (2012). Assessment of mycoflora associated with postharvest losses of papaya fruits. J. Agric. Technol. 8, 961–968.
Singleton V. L., Orthofer R., and Lamuela-Raventos R. M. (1999). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Meth. Enzymol. 299, 152–178. doi: 10.1016/S0076-6879(99)99017-1
Sivakumar D. and Bautista-Baños S. (2014). A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 64, 27–37. doi: 10.1016/j.cropro.2014.05.012
Soheilifard F., Marzban A., Raini M. G., Taki M., and van Zelm R. (2020). Chemical footprint of pesticides used in citrus orchards based on canopy deposition and off-target losses. Sci. Environ. 732, 139118. doi: 10.1016/j.scitotenv.2020.139118
Spotts R. A., Cervantes L. A., and Mielke E. A. (1999). Variability in postharvest decay among apple cultivars. Plant Dis. 83, 1051–1054. doi: 10.1094/PDIS.1999.83.11.1051
Strano M. C., Altieri G., Allegra M., Di Renzo G. C., Paterna G., Matera A., et al. (2022). Postharvest technologies of fresh citrus fruit: Advances and recent developments for the loss reduction during handling and storage. Hortic. 8, 612. doi: 10.3390/horticulturae8070612
Sun J., Janisiewicz W. J., Nichols B., Jurick W. M. II, and Chen P. (2017). Composition of phenolic compounds in wild apple with multiple resistance mechanisms against postharvest blue mold decay. Postharvest Biol. Technol. 127, 68–75. doi: 10.1016/j.postharvbio.2017.01.006
Treesuwan K., Jirapakkul W., Tongchitpakdee S., Chonhenchob V., Mahakarnchanakul W., and Tongkhao K. (2022). Sulfite-free treatment combined with modified atmosphere packaging to extend trimmed young coconut shelf life during cold storage. Food Control 139, 109099. doi: 10.1016/j.foodcont.2022.109099
Triantafyllidis V., Kosma C., Karabagias I. K., Zotos A., Pittaras A., and Kehayias G. (2022). Fungicides in Europe during the twenty-first century: a comparative assessment using agri-environmental indices of EU27. Water Air Soil Pol. 233, 52. doi: 10.1007/s11270-022-05529-5
United Nation Environment Program (UNEP), Food Waste Index Report (2024). Food Waste Index Report 2024 (Nairobi, Kenya: UNEP - UN Environment Programme).
Yan F., Cui J., Wang C., Tian X., Li D., Wang Y., et al. (2022). Real-time quantification for sulfite using a turn-on NIR fluorescent probe equipped with a portable fluorescence detector. Chin. Chem. Lett. 33, 4219–4222. doi: 10.1016/j.cclet.2022.03.006
Yang M., Zheng E., Lin Z., Miao Z., Li Y., Hu S., et al. (2024). Melatonin rinsing treatment associated with storage in a controlled atmosphere improves the antioxidant capacity and overall quality of lemons. Foods 13, 3298. doi: 10.3390/foods13203298
Youssef K., Ligorio A., Nigro F., and Ippolito A. (2012). Activity of salts incorporated in wax in controlling postharvest diseases of citrus fruit. Postharvest Biol. Technol. 65, 39–43. doi: 10.1016/j.postharvbio.2011.10.006
Keywords: postharvest, pathogen, severity, physicochemical, bioactive compounds
Citation: Allagui MB and Ben Amara M (2025) Unravelling during cold storage and shelf life the pathological and physicochemical characteristics of postharvest apples and oranges. Front. Hortic. 4:1567906. doi: 10.3389/fhort.2025.1567906
Received: 28 January 2025; Accepted: 16 April 2025;
Published: 21 May 2025.
Edited by:
Sergio Ruffo Roberto, State University of Londrina, BrazilReviewed by:
Khamis Youssef, Agricultural Research Center, EgyptAntonio Ippolito, University of Bari Aldo Moro, Italy
Copyright © 2025 Allagui and Ben Amara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Bechir Allagui, YWxsYWd1aS5iZWNoaXJAZ21haWwuY29t
 Mohamed Bechir Allagui
Mohamed Bechir Allagui Mouna Ben Amara
Mouna Ben Amara