- 11United States Department of Agriculture, Agricultural Research Service, Southern Plains Agricultural Research Center, Pecan Breeding and Genetics, College Stations, TX, United States
- 2Boone Pickens School of Geology, Oklahoma State University, Stillwater, OK, United States
- 3Department of Horticulture and Landscape Architecture, Oklahoma State University, Stillwater, OK, United States
Introduction: Pecan (Carya illinoinensis), native to North America, is the most commercially significant species within the Carya genus, playing a vital role in nut production across the southern United States. Cultivated for its high-quality nuts, pecans are widely utilized in culinary applications, and their increasing global demand underscores the necessity for enhanced cultivation practices that ensure both economic sustainability and long-term viability. One critical factor influencing pecan production is rootstock selection, which affects growth, physiology, and overall orchard performance. This study investigates the impact of 12 different rootstocks on the growth and physiological characteristics of the ‘USDA-ARS-Pawnee’ scion to provide insights into optimal rootstock choices for pecan orchards.
Methods: The study was conducted over multiple years, assessing key traits including budbreak timing, tree morphology, leaf size, leaf retention, photosynthesis, leaf nutrient composition, and soil microbial community structure. Twelve distinct rootstocks were evaluated to determine their influence on scion development. Measurements of photosynthesis rate (PSR) and water use efficiency (WUE) were collected to establish correlations with leaf size. Leaf nutrient content and soil microbial diversity were analyzed to assess rootstock effects on tree health and orchard sustainability.
Results: Significant differences in budbreak timing were observed among the rootstocks, with northern rootstocks, particularly ‘Peruque,’ exhibiting the latest budbreak, while eastern rootstocks demonstrated the earliest budbreak. Growth performance varied across rootstock origins; southern (Mexican) rootstocks produced the tallest trees with the largest trunk diameters and canopy widths, highlighting their potential for enhancing orchard productivity. Leaf size differed among rootstocks, with northern and eastern rootstocks generally producing larger leaves, although statistical significance was not established. Larger leaves correlated positively with increased PSR and WUE, with ‘Giles’ (northern) and ‘VC1-68’ (western) exhibiting the highest values, while ‘Elliott’ (eastern) recorded the lowest. Leaf retention showed no significant differences, but northern and eastern rootstocks retained more foliage into early November than southern and western rootstocks. Nutrient analysis revealed rootstock-dependent variations, with ‘Riverside’ containing the highest Zn levels and ‘Peruque’ the lowest, while ‘Major’ exhibited the highest B content, and ‘Frutoso’ the lowest. Soil microbial analysis identified distinct microbial compositions influenced by rootstock selection, with ‘Peruque’ fostering ectomycorrhizal fungi and ‘87MX5-1.7’ supporting nitrogen-fixing bacteria, suggesting rootstock effects on soil microbial diversity and nutrient cycling.
Discussion: These findings highlight the importance of rootstock selection in optimizing pecan tree growth, physiological performance, and soil health. The superior growth of southern rootstocks suggests their potential for improving orchard productivity, while variations in PSR and WUE underscore the complex interactions between rootstocks and photosynthetic efficiency. Additionally, the microbial differences observed indicate that rootstock selection may influence soil nutrient dynamics, further emphasizing the role of rootstocks in sustainable pecan cultivation. Overall, this study provides valuable insights into rootstock-specific advantages, aiding growers in selecting the most suitable rootstocks to enhance pecan orchard management.
Introduction
Pecan (Carya illinoinensis) is an economically and ecologically valuable nut tree in North America (Thompson and Grauke, 1991). The industry is a major contributor to the agricultural economy, with the United States being one of the largest global producers (USDA-NASS, 2024; INC, 2023). The growing demand for pecans, driven by their nutritional value which is rich in healthy fats, proteins, vitamins, and antioxidants, has increased the need for research to enhance production practices to ensure both economic viability and sustainability (Du et al., 2022; Yusufali et al., 2023).
Pecans thrive across diverse climates and soils in the United States, ranging from the warm, humid climates of the southeast to the arid areas of the southwest (Grauke et al., 2016; Thompson and Grauke, 1991). While their adaptability makes them a valuable crop, differences in cultivation environments poses challenges such as variable rainfall, low water quality, poorly drained soils, and early freeze. Regardless of growing region, one of the primary obstacles to successful pecan production is the long juvenile stage before fruit-bearing, combined with the high costs of establishing and maintaining pecan orchards, making efficient cultivation practices crucial to maximize yield and minimize costs (Fabrizio et al., 2018).
One of the most widely used techniques for improving pecan cultivation is grafting, a method that involves attaching a desired scion cultivar to a rootstock (Melnyk, 2017). By bypassing the lengthy juvenile phase, grafting facilitates faster and more predictable fruit production (Wells, 2024). However, its success relies heavily on selecting appropriate rootstocks (Grauke and O’Barr, 1996; Forner-Giner et al., 2020). Rootstocks influence more than just structural support; they play a central role in water and nutrient uptake, hormonal signaling, and root-to-shoot interactions (Rasool et al., 2020). Their effects on scion performance depend on genetic factors, as well as environmental conditions like soil composition, water availability, and temperature (Mir et al., 2023; Nawaz et al., 2016; Tworkoski and Miller, 2007; Wang et al., 2025b). These influences extend to critical growth aspects, including scion vigor, root development, nutrient uptake, and environmental stress tolerances such as drought, soil salinity, and diseases (Smith et al., 2014; Grauke and Thompson, 1995; Liu et al., 2019; Jamshidi Goharrizi et al., 2020; Rumbaugh et al., 2021; Barone et al., 1998).
Numerous studies have investigated the effects of different rootstocks on pecan tree performance. For instance, rootstock selection has been shown to influence a variety of traits such as scion tree size and growth rate (Grauke et al., 2003; Hasey et al., 2004; Nikpeyma, 2020; Tworkoski and Fazio, 2016). While some rootstocks promote more vigorous growth, others result in a more compact growth habit, which is advantageous for certain orchard management systems (Hayat et al., 2022; Mir et al., 2023; Morales Alfaro et al., 2023). Rootstocks also influence photosynthetic efficiency, leaf size, and overall canopy architecture, which directly affect a tree’s capacity to capture sunlight and produce energy (Fallahi et al., 2001; Mickelbart and Arpaia, 2002). Additionally, they regulate nutrient uptake, which is critical for maintaining healthy tree growth and high-quality nut production (Reig et al., 2018; Ibacache et al., 2020; Brown et al., 1994). Studies have indicated that rootstocks enhanced the ability of pecan trees to acquire essential nutrients, such as nitrogen, phosphorus, and potassium, from the soil, thereby improving overall tree health and increasing nut yields (Walworth, 2020; Miyamoto et al., 1985).
Another emerging focus of rootstock research is their impact on soil microbial communities (Rasool et al., 2020; Ren et al., 2023; Palma et al., 2018). The rhizosphere, or the soil surrounding roots, hosts a diverse microbiome, including bacteria, fungi, and other microbes, which influence nutrient cycling, plant growth, and disease suppression. Studies suggest that rootstocks affect the composition and diversity of these microbial communities, which in turn impact overall tree health and growth (Ren et al., 2024, 2023; Palma et al., 2018). A healthy and balanced soil microbiome enhances nutrient availability, disease resistance, and improves soil structure, contributing to better tree performance (Bokszczanin et al., 2021). Therefore, understanding how different rootstocks shape soil microbial dynamics is crucial for developing sustainable and productive pecan orchard systems.
Pecan trees are highly heterozygous genetically, the open-pollinated seeds are not completely true-to-type of their mother trees. However, growers and nurserymen primarily rely on seedling rootstocks due to the challenges in cloning and micropropagation. The open-pollinated seeds, resulting in genetic variability among rootstocks, which can lead to variability in tree vigor (Fabrizio et al., 2018; Sitton et al., 1939; Wang et al., 2025b), disease resistance (Sanderlin and Sanderlin, 2015), and adaptability to environmental conditions (Mir et al., 2023; Ren et al., 2024).
This study builds on existing knowledge of rootstock-scion interactions by evaluating the effects of 12 different rootstocks on the performance of the ‘USDA-ARS-Pawnee’ scion, a widely planted pecan cultivar in commercial orchards due to its high-quality nuts and early nut maturity. Conducted by the USDA ARS Pecan Breeding Program, the research began with seed germination in 2008 (Wang et al., 2025a), field trials in 2014, and evaluation of grafted scion trees from 2018 to 2023. The trial assessed a range of key parameters, including tree morphology, leaf size, photosynthesis and nutrition, and soil microbial community composition. By examining these factors, this study provides insights into how rootstocks influence pecan tree growth and health, with implications for improved orchard management practices.
Materials and methods
Three-year-old seedlings of 12 open-pollinated rootstocks (Wang et al., 2025a, b) were planted in an orchard in Somerville, TX (30°31’21”N, 96°25’24”W) in 2012. The orchard consisted of four blocks, separated by a border row designed to provide pollination for the scion grafted on these rootstocks. Rows and trees were spaced 9.14 m by 9.14 m. Each block consisted of four rows and a total of 60 trees (Figure 1), arranged in a completely randomized design with five replications. In 2013 and 2014, a scion cultivar ‘USDA-ARS-Pawnee’ was grafted (if rootstock stem diameter was 35 mm or more) or budded (if rootstock diameter was blow 35 mm) onto the rootstocks (Wang et al., 2025b). The irrigation sprinklers were installed near each tree and used as needed. Fertilizers were applied based on the annual soil report. Zinc sprays were applied three times annually in the growing season. Insect control (casebearer) was performed once a year in the spring. The first block was monitored for the selected traits analyzed in this study.
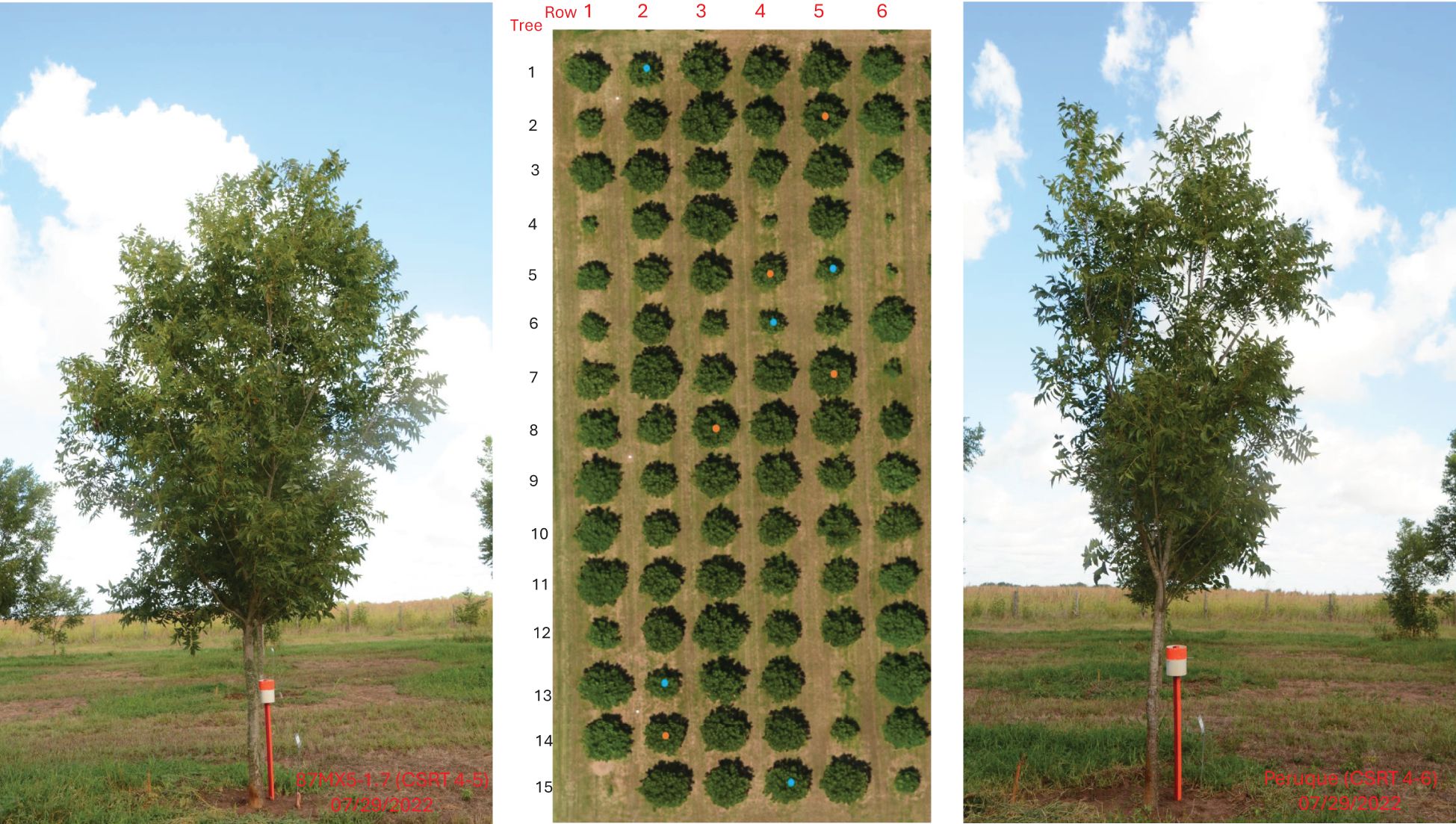
Figure 1. College Station Rootstock Test (CSRT) block one field drone review map (center). Row 1 and 6 are border trees, and rows 2–5 are ‘USDA-ARS-Pawnee’ scion grafted onto 12 different rootstocks. Orange dots indicate the southern rootstock 87MX5-1.7, and blue dots represent the northern rootstock ‘Peruque’. Root and rhizosphere soil samples were collected from these two rootstocks for microbial diversity analysis. One tree of each rootstock is shown on the right and left, respectively.
Budbreak
Budbreak was rated in the spring from 2020 to 2023 using an ordinal scale of 1 to 5, where 1 = dormancy, 2 = swelling, 3 = inner scale splitting, 4 = leaf burst, and 5 = leaflet expansion (Wang et al., 2025b). The date of budbreak scale 3 was transferred to Julian days to compare the length of budbreak time for statistical analysis.
Plant height, canopy width, and trunk diameter
The scion height and canopy width were measured in meters using a laser rangefinder (Vertex Laser Geo, Haglof, Sweden). The trunk diameter was measured 60 cm above ground using a digital caliper. All these three traits were collected in the dormant season in 2020-2023 (between December and January).
Leaf size
The end of July marks the peak of the growing season for pecans each year. On July 27, 2018, and July 27, 2022, ten pairs of the fourth leaflets from the middle compound leaves on a shoot, located in the outer middle section of the canopy, were collected. The leaflets were measured for length and width in millimeters using a digital caliper, and leaflet size (mm2) was calculated by multiplying length by width. Subsequently, the leaflets were dried in an oven at 70°C and ground for nutrient analysis.
Leaf nutrient
Total nitrogen (%) was determined by high-temperature combustion (McGeehan and Naylor, 1988). Plant minerals (B, Ca, Cu, Fe, K, Mg, Mn, Na, P, S, and Zn) (ppm) were analyzed by inductively coupled plasma (ICP) analysis after a nitric acid digest (Havlin and Soltanpour, 1980). Data in 2018 and 2022 were used for analysis.
Photosynthesis
Photosynthesis measurements were taken using LI-COR 6400XT portable photosynthesis system (LI-COR Biosciences, Inc.) from the fourth leaflet pair of a compound leaf located in the middle of a shoot at the outer edge of the middle canopy. Data was collected every 2.5 hours from 7:00 am to 7:30 pm on July 28, 2018.
Root and rhizosphere soil microbial community
The root and rhizosphere soil sampling of two rootstocks, 87MX5-1.7 and ‘Peruque’ (Figure 1) and analysis of microbial components followed the published methods (Ren et al., 2024). Briefly, roots with surrounding soil were collected from a depth of 5–20 cm, one meter away from the tree’s main trunk base in May 2022. Total microbial DNA was extracted separately from roots and soil and then sequenced. The V4 region of bacterial 16S rDNA was amplified to explore the bacterial community, while the ITS2 region of fungi was amplified using universal primer pairs. The bioinformatics analysis was previously published in Ren et al. (2024). The test orchard is part of the USDA ARS Pecan Breeding Program in Somerville, TX (30°31’21”N, 96°25’24”W). Based on U.S. climate data for Somerville, TX in 2022 (accessed on May 1, 2025, via US Climate Data), this region’s annual climate features an average high temperature of 26.3°C (ranging from 16.1–35.6°C), an average low temperature of 12.9°C (ranging from 2.8–22.2°C), and an average annual precipitation of 8.18 cm (ranging from 4.80–11.35 cm). The soil is clay with a pH value of 6.5 (ranging from 5.9–6.9).
Data analysis
Statistical analysis was performed using JMP® Pro 17.0.0 (SAS Institute Inc.). Rootstock effects were tested using a Generalized Linear Model and the overdispersion parameter was estimated by Maximum Likelihood. A one-way analysis of variance (ANOVA) was used to compare the mean values for each trait, with significance determined via the Tukey-Kramer HSD test. The effect of leaf size on photosynthesis rate and water use efficiency was assessed using a nominal logistic model, assuming rootstock independence.
Results
Budbreak
The 12 rootstocks significantly influenced the timing of budbreak in the ‘USDA-ARS-Pawnee’ scion (Table 1), with a 1–4-day difference observed over the four years from 2020 to 2023. The ‘Peruque’ rootstock exhibited a significantly (p = 0.05) later budbreak compared to the other 11 rootstocks, which did not differ significantly from one another (Table 2). Overall, northern rootstocks exhibited the latest budbreak, while eastern rootstocks showed the earliest. Southern and western rootstocks did not show significant differences in budbreak timing (Table 3).
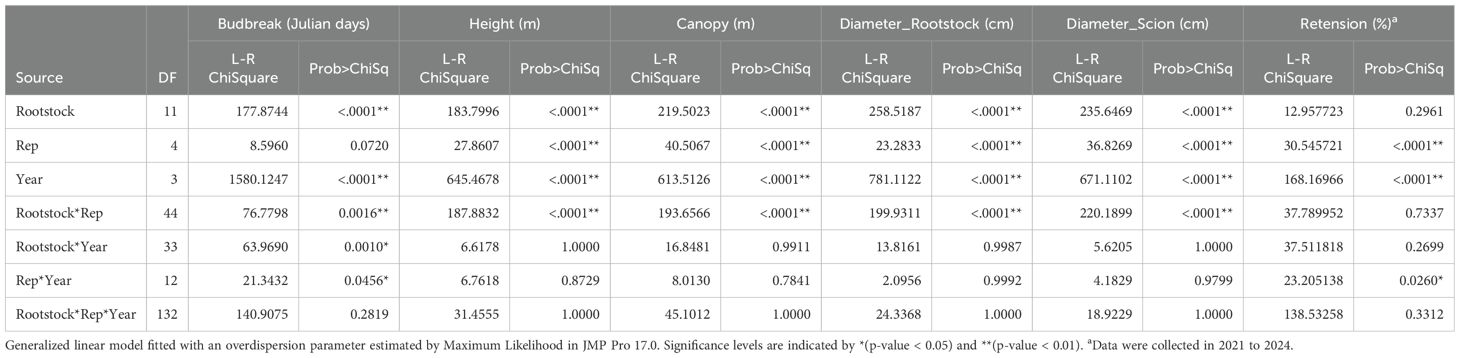
Table 1. Multivariant analysis of the major traits tested in a rootstock test orchard from 2020 to 2023.
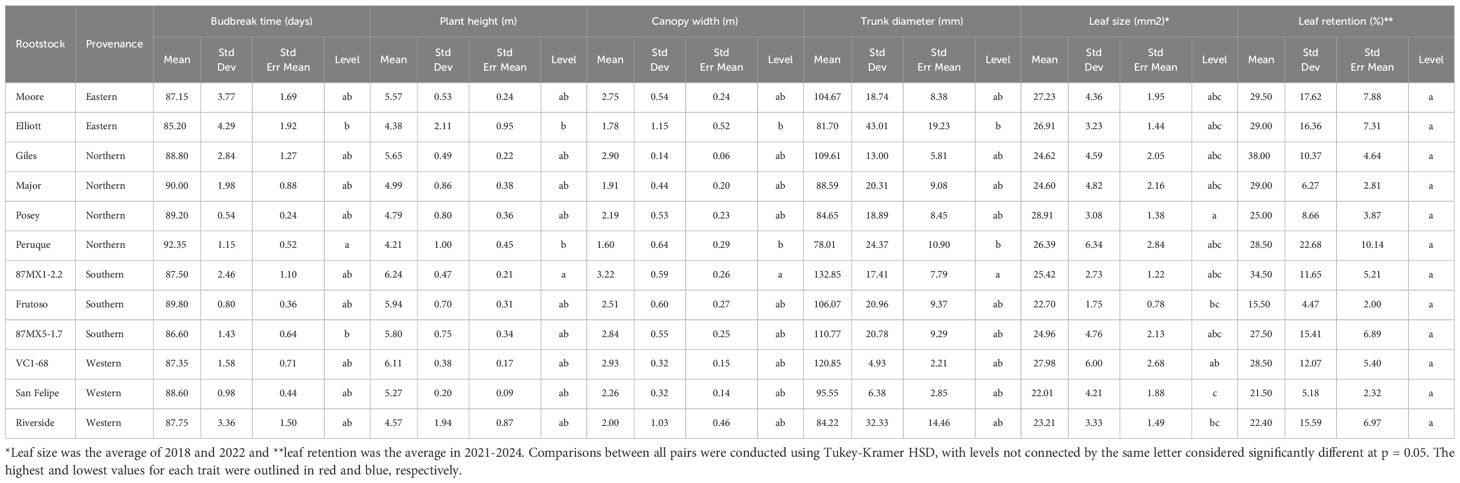
Table 2. Scion growth grafted on 12 different rootstocks from 2020 to 2023 in a replicated testing orchard.
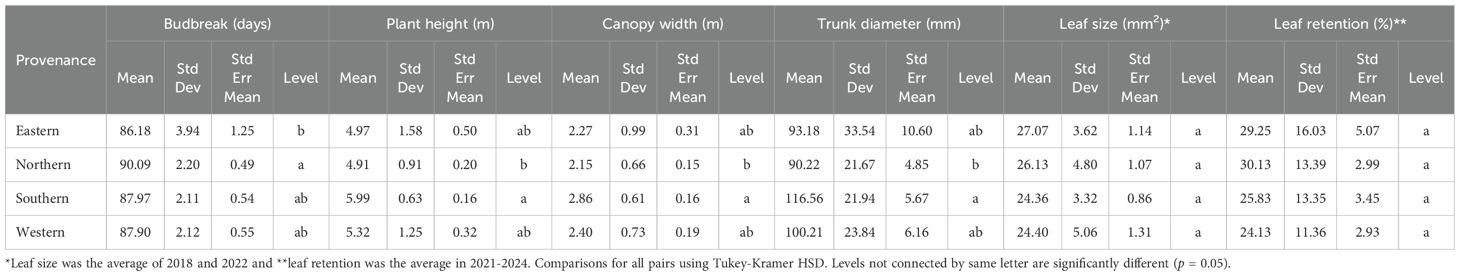
Table 3. Scion growth on different rootstock provenances from 2020 to 2023 in a replicated testing orchard.
Tree size
The scion growth performance varied significantly across the 12 rootstocks in terms of tree size metrics. Southern rootstocks generally outperformed northern ones, with southern (Mexican) rootstocks particularly effective in promoting significant increases in scion plant height, trunk diameter, and canopy width (Table 3). Specifically, the rootstock 87MX1-2.2 demonstrated the greatest vigor when compared to the other rootstocks (Table 2) and was significant (p = 0.05) in comparison to ‘Elliott’ (Eastern) and ‘Peruque’ (Northern). Rootstocks from eastern and western regions showed intermediate performance, with distinct differences when compared to both southern and northern seedstocks (Table 3).
To evaluate the contributions of rootstocks and their origins to scion growth performance, we conducted a principal component analysis (PCA) based on four years of data (2020–2023). The analysis included budbreak, tree height, canopy width, and trunk diameter, accounting for rootstocks and replications. The PCA clarified the above results: northern rootstocks delayed budbreak (resulting in longer Julian days) and slowed tree growth, whereas southern rootstocks (except for Frutoso) advanced budbreak (resulting in shorter Julian days) and enhanced tree growth (Figure 2). The effects of western and eastern rootstocks showed variability (Table 1).
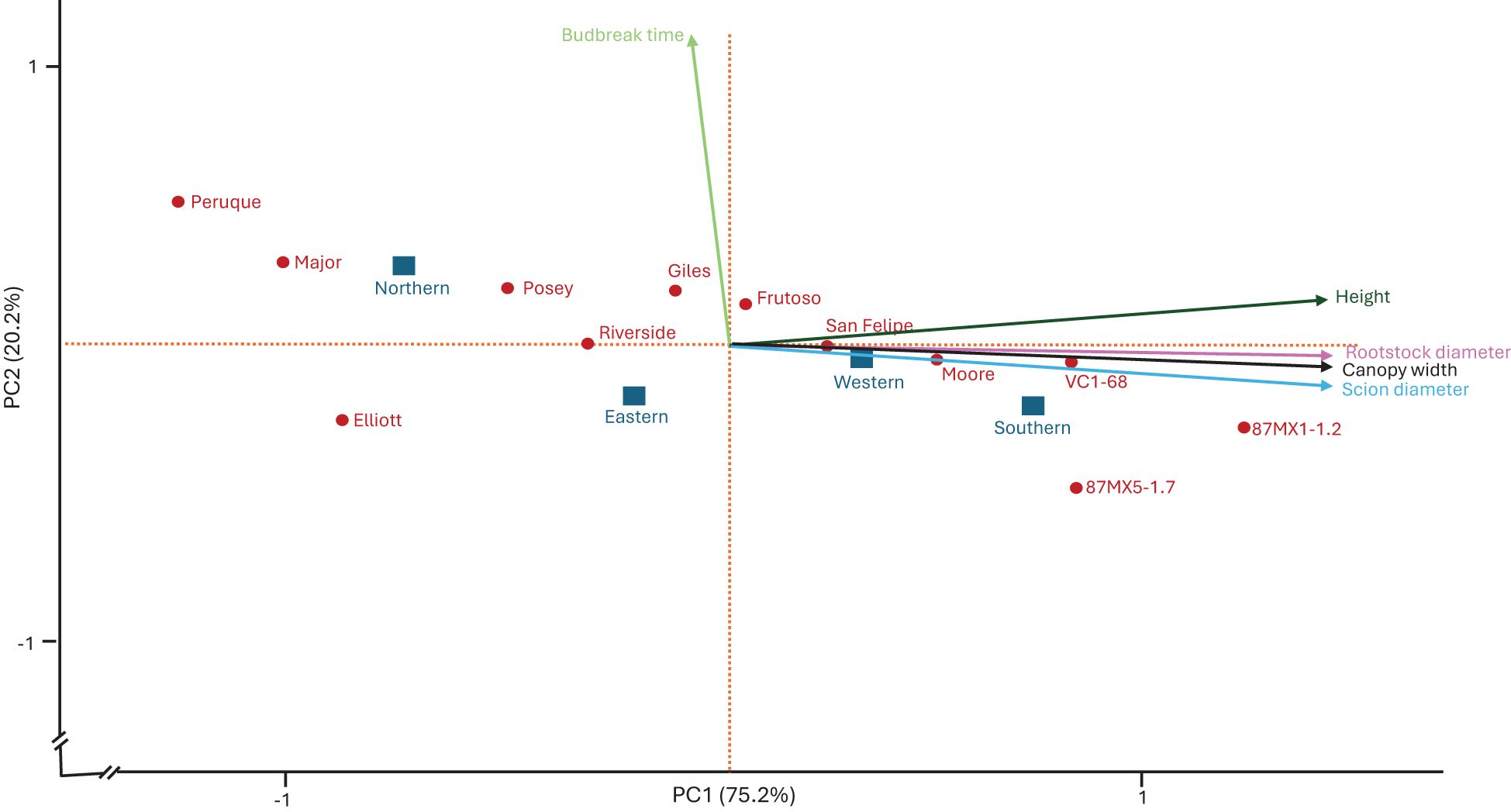
Figure 2. Principal Component Analysis (PCA) illustrating the contributions of 12 rootstocks from four provenances to ‘USDA-ARS-Pawnee’ scion growth performance, including budbreak time, height, canopy width, and trunk diameter.
Leaf size
The average size of the leaflets was variable, with ‘Posey’ exhibiting the largest leaflets and ‘San Felipe’ the smallest (Table 2). On average, rootstocks from eastern and northern provenances produced larger leaflets compared to those from southern and western regions (Table 3). However, these differences were not statistically significant.
Leaf retention
Leaf retention, referring to the duration leaves remaining on the canopy before natural defoliation, was estimated as the percentage of leaves remaining on the canopy during the late growth season (with a maximum of >50% leaf defoliation). The scion leaf retention showed no significant difference across the 12 rootstocks but presented variation in years (Tables 1, 2) (p > 0.05) (. Although rootstock origin did not significantly affect leaf retention, northern and eastern provenances tended to retain more leaves than southern and western rootstocks in early November (Table 3). For instance, the northern rootstock ‘Giles’ had the highest leaf retention (38%), while the southern rootstock ‘Frutoso’ had the lowest leaf retention (15.5%). However, these differences were not statistically significant.
Leaf photosynthesis
Leaf photosynthesis rate (PSR) and water use efficiency (WUE) were assessed across the 12 rootstocks, revealing significant differences during most of the daylights with exception of no significance at 14:30 pm (Table 4). Two rootstocks, ‘Giles’ (northern region) and VC1-68 (western region)-showed the highest PSR and WUE (Figure 3). In contrast, ‘Elliott’, a rootstock commonly used in southeastern and southern pecan-growing regions, exhibited the lowest PSR and WUE at the time of the highest daily photosynthesis rate (5 p.m.), despite showing higher or at least moderate PSR during other periods of the day (Figure 3). The PSR and WUE had a higher positive correlation (r = 0.752). This study also indicated that scion leaf size has significant positive correlation with PSR (r = 0.270, p <0.05) and WUE (r = 0.300, p < 0.05) during most time of a day (Table 4).
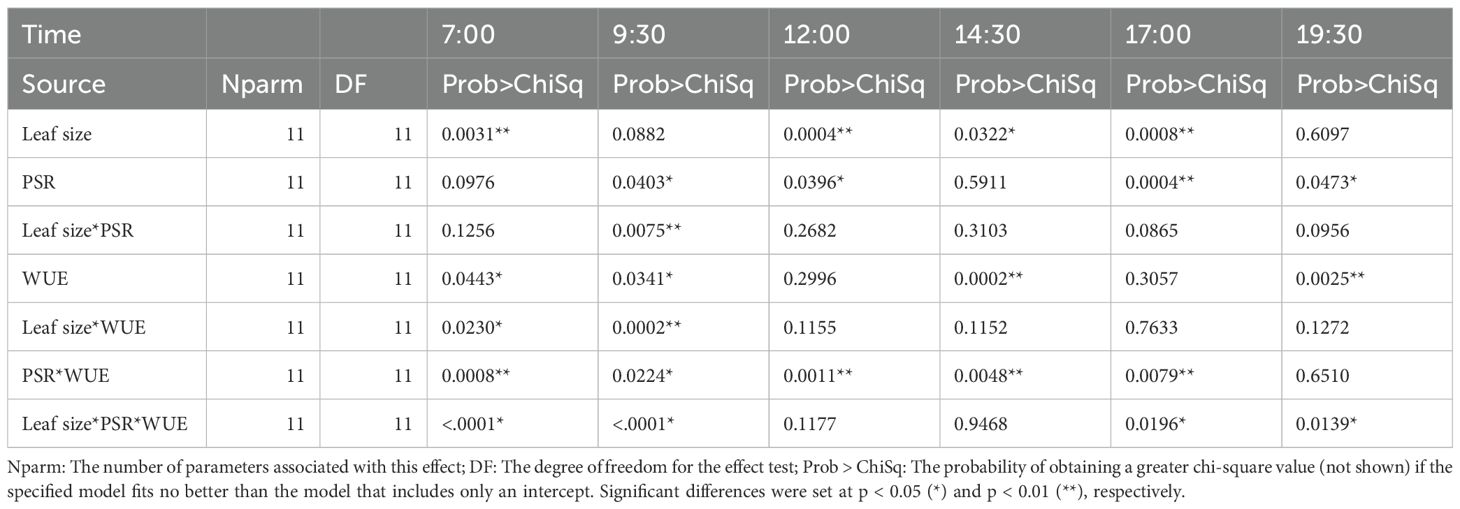
Table 4. The effect tests of leaf size, photosynthesis rate (PSR) and water use efficiency (WUE) of the 12 rootstocks on scion ‘USDA-ARS-Pawnee’ in July 2018.
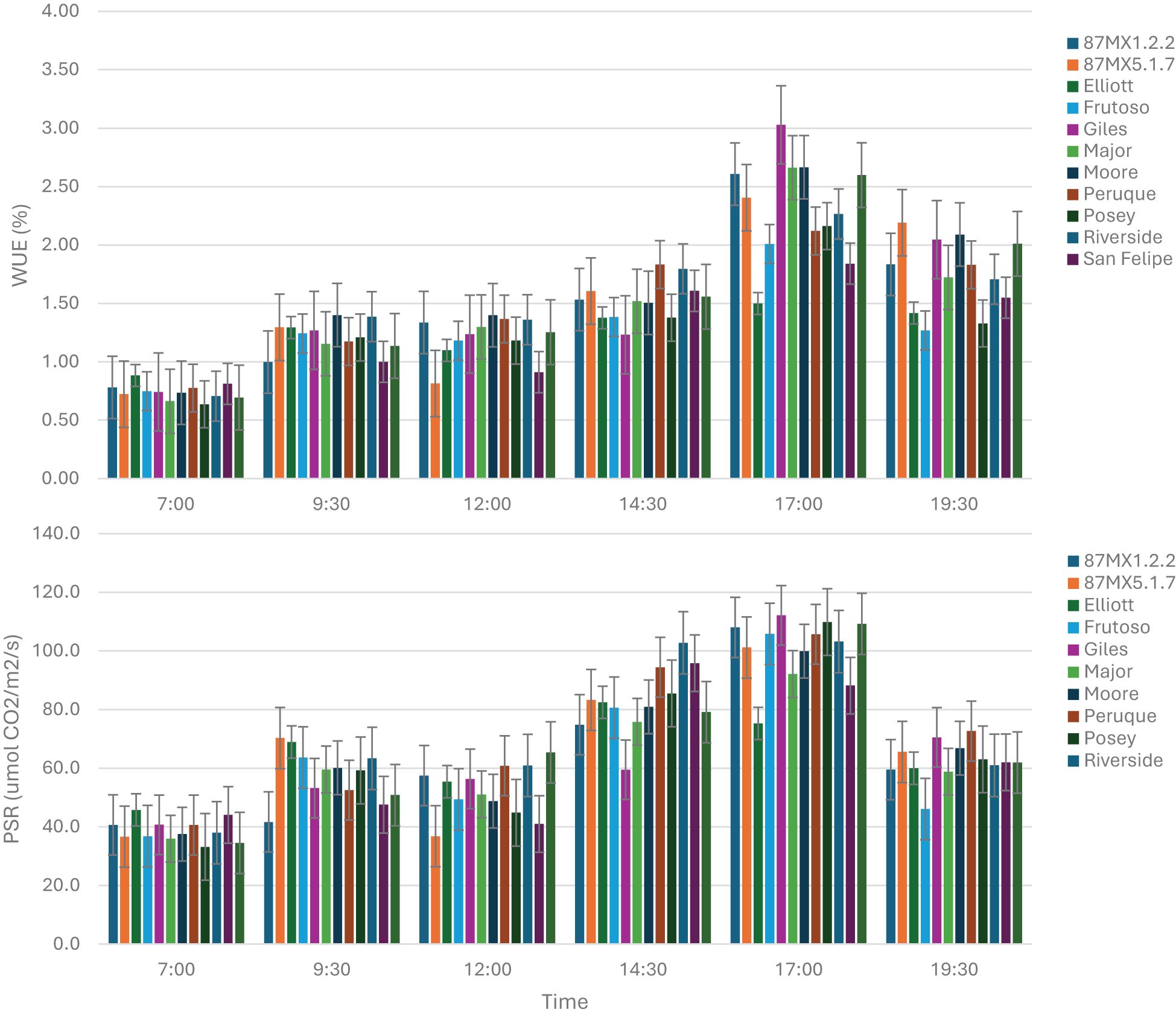
Figure 3. The daily variations in photosynthesis rate (PSR, lower) and water use efficiency (WUE, upper) of the matured leaves of ‘USDA-ARS-Pawnee’ scion grafted onto 12 rootstocks, measured on July 28, 2018.
Leaf nutrition
Nutrient analysis of scion leaves showed variability in most leaf nutrients but no significant differences across the 12 rootstocks, except for zinc (Zn) and boron (B) (Table 5). The rootstock ‘Riverside’ exhibited the highest Zn content (45.95 ppm), while ‘Peruque’ had the lowest Zn content (27.59 ppm). Similarly, ‘Major’ contained the highest B content (180.63 ppm), and ‘Frutoso’ had the lowest (108.40 ppm). Rootstock origin had minimal impact on nutrient content, except for potassium (K), magnesium (Mg), and boron (B). Northern rootstocks had the highest K and B levels but the lowest Mg, while western rootstocks showed the opposite trend (Table 6). These findings demonstrate the role of rootstocks in nutrient uptake and distribution within the scion.
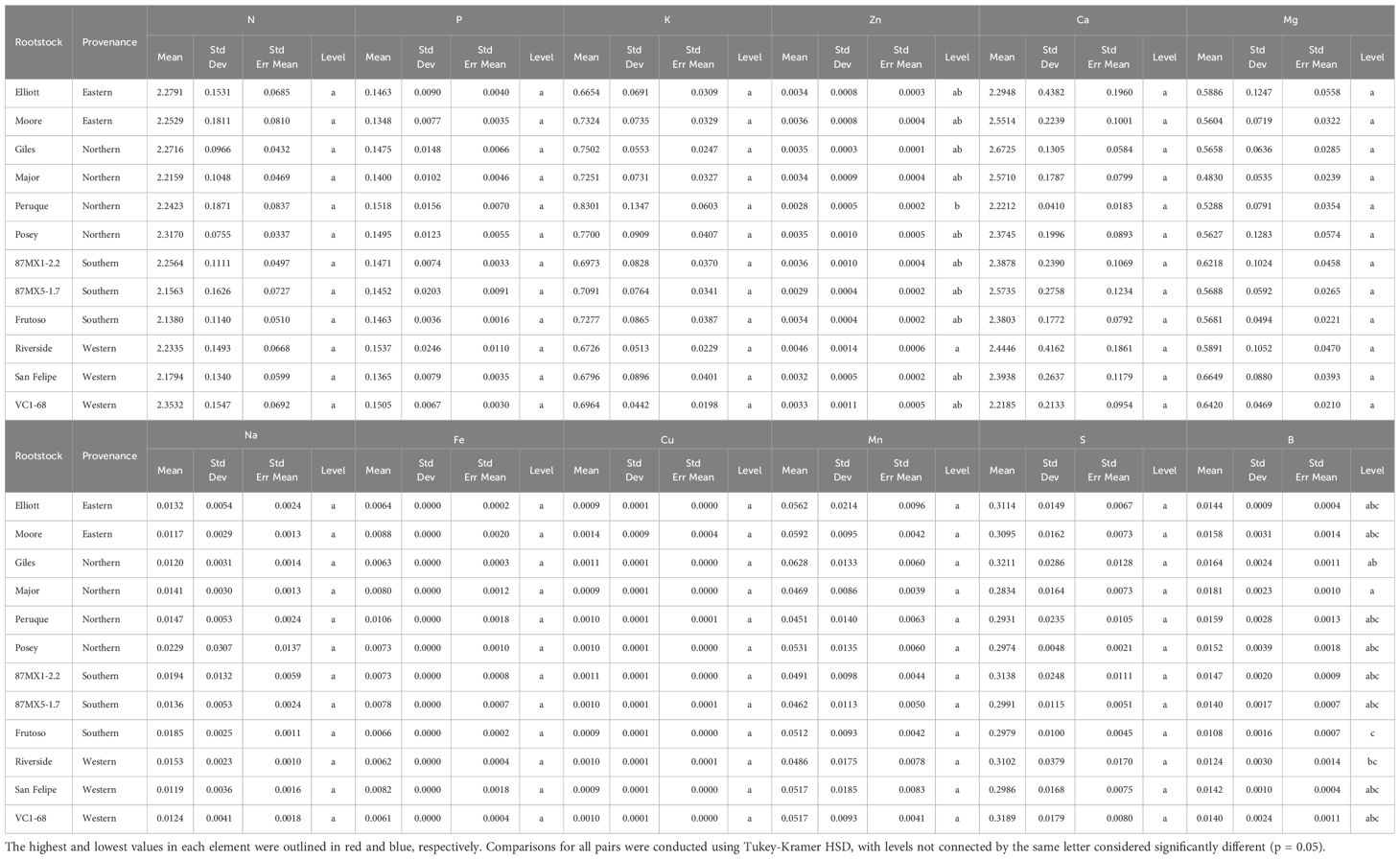
Table 5. Nutrient composition (%) in scion leaves across 12 different rootstocks in a replicated testing orchard (average data in 2018 and 2022).
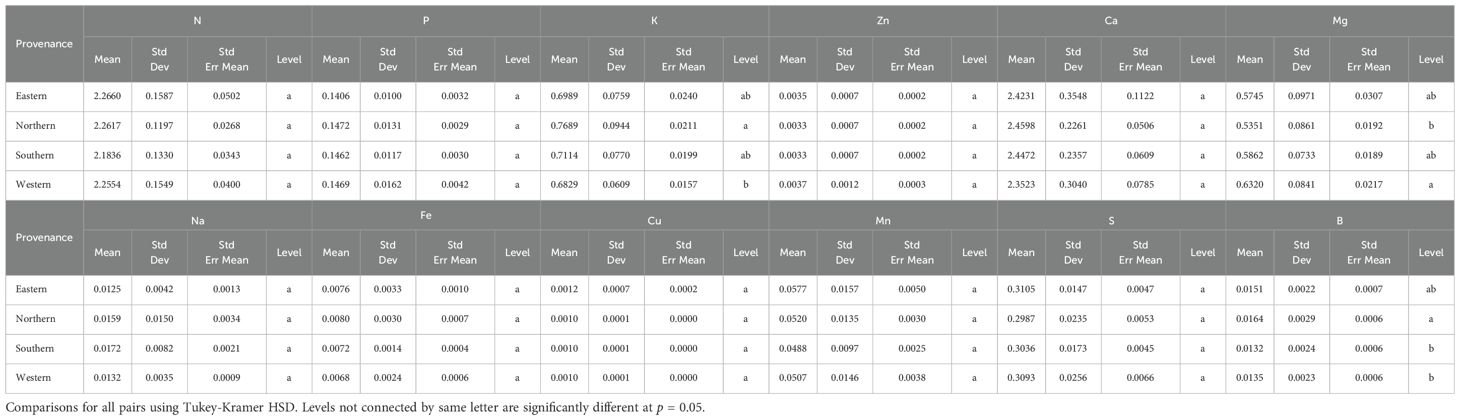
Table 6. Nutrient composition (%) in scion leaves from different rootstock provenances in a replicated testing orchard (average data from 2018 and 2022).
Soil microbial community
The microbial diversity in the roots and rhizosphere soil was examined for two rootstocks: one of the tallest (87MX5-1.7) and one of the shortest (‘Peruque’) (Figure 1). The composition of microbial communities varied significantly between the two rootstocks. ‘Peruque’, a northern rootstock, exhibited a higher abundance of ectomycorrhizal fungi (e.g., Inocybe and Russula), which decompose organic matter and cycling nutrients, particularly phosphorus. In contrast, the southern provenance 87MX5-1.7 showed elevated levels of nitrogen-fixing bacteria (Bradyrhizobium) and phosphorus-absorbing fungi (Tuber) (Table 7).
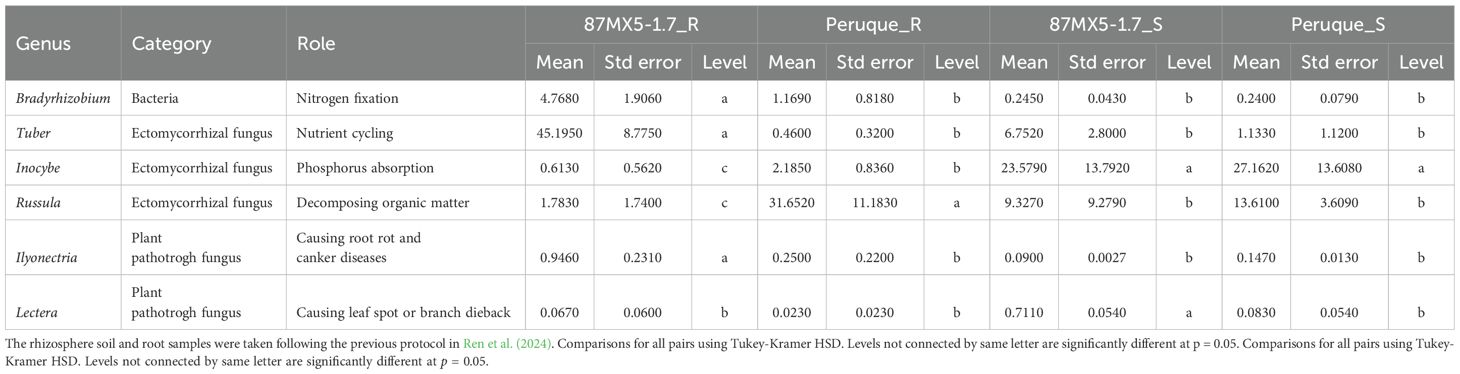
Table 7. Microbial richness in the root and rhizosphere soils of a southern (87MX5-1.7) and a northern ('Peruque') pecan rootstock.
Discussion
The results from this trial evaluating the impact of 12 rootstocks on the ‘USDA-ARS-Pawnee’ scion cultivar provide valuable insights into the role of rootstock provenance in shaping key physiological traits and growth parameters in pecan trees. Several significant trends emerged across different rootstocks, highlighting the importance of selecting the right rootstock to optimize growth, health, and productivity in the pecan orchards. The findings further highlight the pivotal role of rootstock origin in influencing various aspects of tree physiology, including budbreak timing, tree size, leaf development, nutrient uptake, photosynthesis, leaf retention, and soil microbial diversity.
Influence of rootstock on budbreak timing
Budbreak timing is a crucial phenological trait in pecan trees that influences the overall growth cycle and potential for damage from late-season frost (Grauke et al., 1992). In this study, the timing of budbreak in the ‘USDA-ARS-Pawnee’ scion was significantly affected by the rootstock, with a range of 1–4 days difference observed across the four years of the study. Northern rootstocks, such as ‘Peruque’, exhibited later budbreak time compared to southern and eastern rootstocks. This aligns with the natural adaptation of northern provenances to colder climates, where delayed budbreak can serve as a protective mechanism against frost damage (Smith et al., 2001; Wood and Reilly, 2001; Kaur et al., 2024). Conversely, eastern and southern rootstocks showed earlier budbreak, likely due to their adaptation to milder climates with longer growing seasons (Grauke et al., 1992, 2003).
This difference in budbreak timing also suggests that northern rootstocks may have an advantage in regions where early frosts are a concern, while eastern rootstocks could perform better in areas with a longer frost-free period. These findings are consistent with previous studies showing that budbreak is influenced by the rootstock’s climatic adaptation, with early budbreak increasing the risk of frost damage in some regions (Fallah et al., 2022; Grauke et al., 1992; Wood and Reilly, 2001). Moreover, the lack of significant differences in budbreak timing between southern and western rootstocks suggests that these rootstocks may be more similar in their physiological responses to climate variables, such as temperature and day length (Grauke et al., 1992).
Tree size and growth performance
Tree size, including parameters such as plant height, trunk diameter, and canopy width, serves as a critical indicator of tree vigor and productivity. The results from this study indicate that southern rootstocks, particularly those of Mexican (southern) provenance, generally resulted in superior tree growth compared to northern rootstocks. Among the southern rootstocks, 87MX1-2.2 exhibited the most vigorous growth, leading to significant increases in scion plant height, trunk diameter, and canopy width. These findings are consistent with the well-established notion that Mexican pecan rootstocks, which have evolved in regions with hot and dry climates, tend to confer enhanced growth vigor, especially in terms of root development and nutrient uptake (Brown et al., 1994; Mir et al., 2023; Valverdi et al., 2021).
In contrast, northern rootstocks, such as ‘Peruque’, resulted in smaller trees, likely due to their adaptation to colder climates where growth is naturally more limited. This may also reflect differences in the rootstock’s ability to access and utilize soil nutrients, particularly in warmer climates, where higher nutrient availability promotes faster growth. We measured the common nutrient contents in soil samples taken annually at depths of 6 inches and 12 inches and found no significant difference (p = 0.05; data not shown), indicating that soil nutrient levels were consistent across all rootstocks. Interestingly, rootstocks from the eastern and western regions showed intermediate performance, suggesting that these rootstocks might be better suited for regions with moderate climatic conditions, offering balanced growth characteristics that could be beneficial in more variable environments.
The growth advantages associated with southern rootstocks have practical implications for orchard management strategies aimed at maximizing tree size and canopy development before tree maturity. Larger trees are often associated with higher nut production, greater canopy coverage for improved pest and disease management, and better overall orchard performance (Westwood et al., 1973; Caruso et al., 2020; Valverdi et al., 2021). These results suggest that pecan growers in warmer climates may benefit from selecting Mexican rootstocks to improve orchard productivity (Wang et al., 2025b).
Leaf size and growth conditions
Leaf size is an important trait that can serve as an indirect indicator of a plant’s overall physiological condition, including its capacity for photosynthesis and environmental adaptation. Leaflet size is influenced by a variety of factors, including genetic traits, environmental conditions, and the efficiency of the photosynthetic apparatus (Salazar et al., 2019; Sun et al., 2020; Ferdous et al., 2023; Kawai et al., 2023). Larger leaves, particularly in the early stages of growth, may indicate better photosynthetic capacity, which could contribute to improved tree growth. The results of this study showed significant variation in leaflet size across the evaluated rootstocks, with ‘Posey’ exhibiting the largest leaflets and ‘San Felipe’ the smallest. While these differences were not statistically significant, the observed trend suggests that rootstock provenance may influence leaf size. Additionally, larger leaves showed the higher photosynthesis rate and water use efficiency (Table 4). The lack of statistical significance, however, indicates that other factors such as soil conditions and environmental stresses may have a greater impact on leaf size than the rootstock origin alone. Generally, rootstocks from eastern and northern regions were associated with larger leaflets compared to those from southern and western regions.
The variation in leaf size observed in this study likely reflects genetic differences among the rootstocks, with northern and eastern provenances potentially having more robust leaf growth characteristics. The differences may ultimately affect the tree’s ability to capture light and produce energy, potentially impacting overall tree health and nut production in the long term. Nevertheless, further research is needed to better understand the direct impact of leaf size on yield and quality.
Leaf retention and environmental adaptation
Leaf retention is an important physiological trait, particularly due to its implications for tree energy conservation during the dormant season (Zhao et al., 2023; Koundinya et al., 2023). The results indicated that rootstock origin did not significantly influence leaf retention during the first two weeks of November. However, northern and eastern provenances tended to retain more leaves compared to southern and western rootstocks. This pattern suggests that northern rootstocks, which are adapted to more temperate climates, may have a greater capacity for leaf retention, potentially conserving energy and prolonging photosynthesis before leaf falls.
The trend observed in this study is consistent with previous research indicating that leaf retention can vary depending on environmental factors, such as temperature, light, and moisture availability (Steinparzer et al., 2023; Lv et al., 2024). For instance, rootstocks from cooler climates may retain leaves longer, which could help the tree absorb more sunlight and store energy for the winter months. Conversely, southern and western rootstocks may shed their leaves earlier as a response to hot and dry conditions.
Despite these trends, the lack of statistically significant variation suggests that leaf retention may not be a primary factor influencing tree performance in this study. However, in regions with less favorable growing conditions, such as late-season droughts or early frosts, retaining more foliage on the canopy before frosts could potentially provide an advantage in optimizing photosynthetic activity during the late-growing season (Mickelbart and Arpaia, 2002; Marquard, 1987).
Leaf photosynthesis and water use efficiency
Photosynthesis is a vital process for tree growth, and its efficiency can be influenced by both genetic and environmental factors. In this study, photosynthetic rates (PSR) and water use efficiency (WUE) were measured for the same scion cultivar grafted onto each of the 12 rootstocks. Two rootstocks - ‘Giles’ (northern provenance) and ‘VC1-68’ (western provenance) - showed the highest PSR and WUE, indicating their superior efficiency in utilizing water and converting it into energy through photosynthesis. This is particularly important as higher WUE is often associated with better drought tolerance and improved growth in arid conditions (Mickelbart and Arpaia, 2002; Marquard, 1987; Fallahi et al., 2001).
In contrast, ‘Elliott’, a rootstock commonly used in southeastern and southern regions, showed high or at least a moderate PSR and WUE compared to other rootstocks during most period of the day. However, at 5 p.m., when PSR and WUE peaked, ‘Elliott’ exhibited the lowest values, suggesting that it may not be optimal for maximizing photosynthetic efficiency in certain environments, especially in south Texas. In addition, these findings carry practical implications, as optimizing photosynthesis and WUE is crucial for increasing overall tree productivity, particularly in areas prone to drought or water stress.
The results highlight the potential for selecting rootstocks based on their photosynthetic efficiency and WUE, particularly in areas where water availability is a limiting factor for tree growth. Growers in areas with unpredictable rainfall or prolonged droughts might benefit from selecting rootstocks like ‘Giles’ or VC1–68 to maximize photosynthetic performance and ensure better water utilization.
Leaf nutrient composition and rootstock influence
Nutrient content in the leaves of pecan trees is critical factor influencing overall tree health and productivity. This study found that most leaf nutrients showed no significant differences across the 12 rootstocks, except for Zinc (Zn) and Boron (B). ‘Riverside’ exhibited the highest Zn content, while ‘Peruque’ had the lowest, ‘Major’ had the highest B content, whereas ‘Frutoso’ showed the lowest levels. Notably, differences in nutrient content were generally not associated with rootstock origin, except for Potassium (K), Magnesium (Mg), and Boron (B).
Rootstocks play a significant role in shaping leaf nutrient composition, as the availability of these nutrients is essential for tree health, growth, and productivity. Zn deficiency is common and frequently limits productivity in commercial pecan orchards, especially those established in soils with low Zn availability (Fenn et al., 1990; Ojeda-Barrios et al., 2012; Sparks and Payne, 1982). Poor zinc uptake requires multiple foliar application per year (Cruz-Alvarez et al., 2024; Liu et al., 2021; Ojeda-Barrios et al., 2012; Smith et al., 2022; Walworth et al., 2006). Rootstocks with higher levels of Zn and B may offer advantages such as enhanced enzyme activity, improved disease resistance, and more efficient nutrient cycling (Smith et al., 2022; Barone et al., 1998; Fallahi et al., 2001; Amiri et al., 2014). Conversely, variations in K and Mg content, which were higher in northern rootstocks and lower in western rootstocks, could have important implications for nutrient management in pecan orchards. For instance, higher K content in northern rootstocks may improve the tree’s ability to tolerate stress, while lower Mg content in southern rootstocks could negatively impact photosynthetic efficiency and plant growth.
Soil microbial community and rootstock effects
This study investigated the influence of rootstocks on soil microbial diversity within the roots and the rhizosphere. The findings revealed that a northern rootstock, ‘Peruque’, exhibited a higher relative abundance of ectomycorrhizal fungi, such as Inocybe and Russula. These fungi are critical for decomposing organic matter and facilitating phosphorus cycling. In contrast, the southern provenance, 87MX5-1.7, demonstrated elevated levels of nitrogen-fixing bacteria, such as Bradyrhizobium, and fungi like Tuber, which are actively involved in nutrient absorption, particularly phosphorus. These distinct microbial communities play a crucial role in improving soil fertility and supporting plant growth by enhancing nutrient uptake and creating a balanced soil ecosystem (Ren et al., 2023, 2024).
The findings emphasize the potential of rootstocks to influence soil health by shaping microbial community. Rootstocks originating from different geographical regions appear to foster unique microbial populations that may be beneficial for nutrient cycling and overall tree performance. Understanding these interactions offers valuable insights for orchard managers, aiding in the selection of rootstocks that not only promote tree growth but also enhance soil fertility and long-term orchard sustainability (Palma et al., 2018).
Conclusions
This study underscores the significant influence of rootstock provenance on the growth and physiological traits of ‘USDA-ARS-Pawnee’ pecan trees. Based on four years of data (2020–2023), results revealed that northern rootstocks delayed budbreak (resulting in longer Julian days) and slowed tree growth, which may be beneficial in regions prone to early-season frost risks. In contrast, southern rootstocks (excluding Frutoso) promoted earlier budbreak (shorter Julian days) and enhanced tree growth, making them ideal for warmer climates where tree vigor is critical for productivity. Western and eastern rootstocks showed varying effects, reflecting region-specific adaptations. Northern rootstocks, such as ‘Peruque’ and ‘Giles,’ were associated with higher concentrations of essential nutrients like zinc and boron, which could enhance tree health and stress resilience. Additionally, they exhibited higher photosynthetic rates, suggesting greater efficiency in energy conversion under specific environmental conditions. Southern rootstock displayed superior growth metrics, potentially due to their capacity to foster robust root development and nutrient uptake.
This study also highlights the role of rootstocks in influencing soil microbial diversity. Northern rootstocks fostered greater populations of ectomycorrhizal fungi, while southern rootstocks supported higher levels of nitrogen-fixing bacteria. These microbial communities contribute to nutrient cycling and soil health, which are essential for sustainable orchard management. Overall, these findings provide practical guidance for selecting rootstocks tailored to specific environmental conditions, enhancing tree growth, productivity, and soil health in pecan orchards. Key choices include ‘Giles’ and ‘Peruque’ for northern regions, ‘VC1-68’ and ‘Riverside’ for western areas, and ‘Elliott’ for the southeastern United States and beyond. Future research should explore the long-term effects of rootstock-scion interactions on nut yield and quality while investigating the genetic and physiological mechanisms underlying these variations to refine rootstock selection for diverse growing regions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. KK: Conceptualization, Data curation, Writing – review & editing. WC: Writing – review & editing. AH: Writing – review & editing. BT: Data curation, Writing – review & editing. TX: Formal Analysis, Validation, Writing – review & editing. LZ: Formal Analysis, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the U.S. Department of Agriculture – Agriculture Research Service National Programs through CRIS project 3091-21000-046-000-D (Crop Germplasm Research Unit, TX). The agency was not involved in the study design, collection, analysis, interpretation of data and the writing of this article. However, this manuscript was approved by the agency before submission for publication. This article reports on the results of the research only. Mention of a trademark or proprietary product is solely to provide specific information and does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products that may also be suitable.
Acknowledgments
The authors highly appreciate the efforts of Dr. LJ Grauke, a retired Horticulturist in the USDA-ARS Pecan Breeding and Genetics Program, for establishing this project, and retired senior technician Lynn Johnson for his efforts in seed measurement, germination, seedling management, and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amiri M., Fallahi E., and Safi-Songhorabad M. (2014). Influence of rootstock on mineral uptake and scion growth of ‘Golden Delicious’ and ‘Royal Gala’ apples. J. Plant Nutr. 37, 16–29. doi: 10.1080/01904167.2013.792838
Barone E., Sottile F., Palazzolo E., and Caruso T. (1998). Effect of rootstock on trunk growth and foliar mineral content in cv. Bianca pistachio (Pistacia vera L.) trees. Acta Hortic. 470, 394–401. doi: 10.17660/ActaHortic.1998.470.54
Bokszczanin K. L., Wrona D., and Przybylko S. (2021). The effect of microbial inoculation under various nitrogen regimes on the uptake of nutrients by apple trees. Agronomy-Basel 11, 2348. doi: 10.3390/agronomy11112348
Brown P. H., Zhang Q., and Ferguson L. (1994). Influence of rootstock on nutrient acquisition by pistachio. J. Plant Nutr. 17, 1137–1148. doi: 10.1080/01904169409364794
Caruso M., Continella A., Modica G., Pannitteri C., Russo R., Salonia F., et al. (2020). Rootstocks influence yield precocity, productivity, and pre-harvest fruit drop of mandared pigmented mandarin. Agronomy 10, 1305. doi: 10.3390/agronomy10091305
Cruz-Alvarez O., Sanchez-Chavez E., Benavides-Mendoza A., Hernandez-Rodriguez O. A., Parra-Quezada R. A., Ciscomani-Larios J. P., et al. (2024). Foliar applications of zinc oxide nanoparticles and boric acid affect leaf oxidative metabolism and productivity in young pecan trees. Heliyon 10, e34742. doi: 10.1016/j.heliyon.2024.e34742
Du X. F., Wang X. W., Muniz A., and Kubenka K. (2022). Consumer hedonic ratings and associated sensory characteristics and emotional responses to fourteen pecan varieties grown in Texas. Plants-Basel 11, (14). doi: 10.3390/plants11141814
Fabrizio G. C., Elmarie V. D. W., and Gesine M. C. (2018). Propagation of pecan (carya illinoensis): a review. Afr. J. Biotechnol. 17, 586–605. doi: 10.5897/AJB2017.16183
Fallah M., Vahdati K., Hassani D., Rasouli M., and Sarikhani S. (2022). Breeding of Persian walnut: Aiming to introduce late-leafing and early-harvesting varieties by targeted hybridization. Scientia Hortic. 295, 110885. doi: 10.1016/j.scienta.2022.110885
Fallahi E., Chun I.-J., Neilsen G., and Colt W. (2001). Effects of three rootstocks on photosynthesis, leaf mineral nutrition, and vegetative growth of “BC-2 Fuji” apple trees. J. Plant Nutr. 24, 827–834. doi: 10.1081/PLN-100103776
Fenn L. B., Malstrom H. L., Riley T., and Horst G. L. (1990). Acidification of calcareous soils improves zinc-absorption of pecan trees. J. Am. Soc. Hortic. Sci. 115, 741–744. doi: 10.21273/Jashs.115.5.741
Ferdous J., Islam M., and Rahman M. (2023). The role of tree size, wood anatomical and leaf stomatal traits in shaping tree hydraulic efficiency and safety in a South Asian tropical moist forest. Global Ecol. Conserv. 43, e02453. doi: 10.1016/j.gecco.2023.e02453
Forner-Giner M. A., Continella A., and Grosser J. W. (2020). “Citrus rootstock breeding and selection,” in The Citrus Genome. Eds. Gentile A., La Malfa S., and Deng Z. (Springer International Publishing, Springer, Cham), 49–74. doi: 10.1007/978-3-030-15308-3_5
Grauke L. and O’Barr R. (1996). Initial survival of pecan grafts on seedling rootstock of pecan, water hickory, and their interspecific hybrid. HortTechnology 6, 45–48. doi: 10.21273/HORTTECH.6.1.45
Grauke L. J. and Thompson T. E. (1995). Patterns of rootstock usage in the pecan industry. HortScience 30, 431. doi: 10.21273/HORTSCI.30.3.431f
Grauke L. J., Pratt J. W., Grauke L. J., and Pratt J. W. (1992). Pecan bud growth and freeze damage are influenced by rootstock. J. Am. Soc. Hortic. Sci. 117, (3). doi: 10.21273/JASHS.117.3.404
Grauke L., Thompson T., Wood B., and Storey J. (2003). Rootstock influence on tree performance. Proc. Texas Pecan Growers Assoc. 70, 20–24.
Grauke L. J., Wood B. W., and Harris M. K. (2016). Crop vulnerability: carya. HortScience 51, 653–663. doi: 10.21273/HORTSCI.51.6.653
Hasey J., Westerdahl B., Lampinen B., and Conant J. (2004). Long-term performance of own-rooted ‘chandler’ walnut compared to ‘chandler’ walnut on paradox rootstock. Acta Horticulturae. 636, 83–87. doi: 10.17660/ActaHortic.2004.636.9
Havlin J. L. and Soltanpour P. N. (1980). A nitric acid plant tissue digest method for use with inductively coupled plasma spectrometry. Commun. Soil Sci. Plant Anal. 11, (10). doi: 10.1080/00103628009367096
Hayat F., Li J., Iqbal S., Peng Y., Hong L., Balal R. M., et al. (2022). A mini review of citrus rootstocks and their role in high-density orchards. Plants (Basel) 11, 2876. doi: 10.3390/plants11212876
Ibacache A., Verdugo-Vásquez N., and Zurita-Silva A. (2020). Chapter 21 - Rootstock: Scion combinations and nutrient uptake in grapevines, Editor(s): A.K. Srivastava, Chengxiao Hu. Fruit Crops, Elsevier, 2020, 297–316, ISBN 9780128187326. doi: 10.1016/B978-0-12-818732-6.00021-6
INC (2023). “Pecans crop progress report,” in International Nuts & Dried Fruits Statistical Yearbook, International Nut and Dried Fruit. vol. 2023, 34–37. Available online at: https://inc.nutfruit.org/pecans-crop-progress-report/.
Jamshidi Goharrizi K., Amirmahani F., and Salehi F. (2020). Assessment of changes in physiological and biochemical traits in four pistachio rootstocks under drought, salinity and drought + salinity stresses. Physiologia Plantarum 168, 973–989. doi: 10.1111/ppl.13042
Kaur A., Zhang L., Maness N. O., Ferguson L., Graham C. J., Sun Y., et al. (2024). Dormant carbohydrate reserves enhance pecan tree spring freeze tolerance: controlled environment observations. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1393305
Kawai K., Kenzo T., Ito S., and Kanna K. (2023). Size-related changes in leaf, wood, and bark traits in even-aged Falcataria falcata trees. Tropics 32, 15–27. doi: 10.3759/tropics.MS22-06
Koundinya A. V. V., Ajeesh B. R., Lekshmi N. S., Hegde V., and Sheela M. N. (2023). Classification of genotypes, leaf retention, pith density and carbohydrate dynamics in cassava under water deficit stress conditions. Acta Physiologiae Plantarum 45, 83. doi: 10.1007/s11738-023-03557-0
Liu J. P., Deng Q. J., Shang Y. J., Yao X. W., Wang H. K., Tang Y. J., et al. (2021). Effects of zinc application on the growth and photosynthetic characteristics of pecan at the seedling stage. Plant Biol. 23, 1149–1156. doi: 10.1111/plb.13307
Liu B., Liang J., Tang G., Wang X., Liu F., and Zhao D. (2019). Drought stress affects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks. Scientia Hortic. 250, 230–235. doi: 10.1016/j.scienta.2019.02.056
Lv H. L., Dermann A., Dermann F., Petridis Z., Kohler M., and Saha S. (2024). Comparable diameter resulted in larger leaf area and denser foliage in the park trees than in street trees: A study on Norway maples of Karlsruhe city, Germany. Heliyon 10, e23647. doi: 10.1016/j.heliyon.2023.e23647
Marquard R. D. (1987). Influence of leaf to fruit ratio on nut quality, shoot carbohydrates, and photosynthesis of pecan. HortScience 22, 256–257. doi: 10.21273/HORTSCI.22.2.256
McGeehan S. L. and Naylor D. V. (1988). Automated instrumental analysis of carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plant Anal. 19, 493–505. doi: 10.1080/00103628809367953
Melnyk C. W. (2017). Plant grafting: insights into tissue regeneration. Regeneration (Oxf) 4, 3–14. doi: 10.1002/reg2.71
Mickelbart M. V. and Arpaia M. L. (2002). Rootstock influences changes in ion concentrations, growth, and photosynthesis of `Hass’ Avocado trees in response to salinity. J. Am. Soc. Hortic. Sci. 127, 649–655. doi: 10.21273/JASHS.127.4.649
Mir M. M., Parveze M. U., Iqbal U., Munib Ur R., Kumar A., Simnani S. A., et al. (2023). “Development and selection of rootstocks,” in Temperate Nuts. Eds. Mir M. M., Rehman M. U., Iqbal U., and Mir S. A. (Springer Nature, Singapore), 45–78. doi: 10.1007/978-981-19-9497-5_3
Miyamoto S., Gobran G. R., and Piela K. (1985). Salt effects on seedling growth and ion uptake of three pecan rootstock cultivars. Agron. J. 77, 383–388. doi: 10.2134/agronj1985.00021962007700030008x
Morales Alfaro J., Bermejo A., Navarro P., Quiñones A., and Salvador A. (2023). Effect of rootstock on citrus fruit quality: A review. Food Rev. Int. 39, 2835–2853. doi: 10.1080/87559129.2021.1978093
Nawaz M. A., Imtiaz M., Kong Q., Cheng F., Ahmed W., Huang Y., et al. (2016). Grafting: A technique to modify ion accumulation in horticultural crops. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01457
Nikpeyma Y. (2020). The effect of different pistacia rootstocks on yield and tree performances of several pistachio (p. vera) cultivars. Sylwan 2020, 347.
Ojeda-Barrios D., Abadia J., Lombardini L., Abadia A., and Vazquez S. (2012). Zinc deficiency in field-grown pecan trees: changes in leaf nutrient concentrations and structure. J. Sci. Food Agric. 92, 1672–1678. doi: 10.1002/jsfa.5530
Palma L. E., Piñn H., Tarango S. H., Duran R., Muñoz L., González E., et al. (2018). Variability of microbial communities associated to pecan tree rhizosphere with organic fertilization. New Biotechnol. 44, S81–S81. doi: 10.1016/j.nbt.2018.05.913
Rasool A., Mansoor S., Bhat K. M., Hassan G. I., Baba T. R., AlYemeni M. N., et al. (2020). Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.590847
Reig G., Lordan J., Fazio G., Grusak M. A., Hoying S., Cheng L., et al. (2018). Horticultural performance and elemental nutrient concentrations on ‘Fuji’ grafted on apple rootstocks under New York State climatic conditions. Scientia Hortic. 227, 22–37. doi: 10.1016/j.scienta.2017.07.002
Ren W., Zhang L., Maness N., Wang X. W., Tang M., and Xu T. Y. (2023). Changes in the diversity of pecan rhizosphere microbial community with different nitrogen fertilization, a case study in Oklahoma pecan orchard. Scientia Hortic. 321, 112365. doi: 10.1016/j.scienta.2023.112365
Ren W., Zhang L., Tondre B., Wang X., and Xu T. (2024). The rootstock genotype shapes the diversity of pecan (Carya illinoinensis) rhizosphere microbial community. Front. Microbiologyl 15. doi: 10.3389/fmicb.2024.1461685
Rumbaugh A. C., Girardello R. C., Cooper M. L., Plank C., Kurtural S. K., and Oberholster A. (2021). Impact of rootstock and season on red blotch disease expression in cabernet sauvignon (v. vinifera). Plants (Basel) 10, 1583. doi: 10.3390/plants10081583
Salazar P. C., Navarro-Cerrillo R. M., Grados N., Cruz G., Barrón V., and Villar R. (2019). Tree size and leaf traits determine the fertility island effect in Prosopis pallida dryland forest in Northern Peru. Plant Soil 437, 117–135. doi: 10.1007/s11104-019-03965-7
Sanderlin R. S. and Sanderlin R. S. (2015). Susceptibility of some common pecan rootstocks to infection by Xylella fastidiosa. HortScience 50, 1183–1186. doi: 10.21273/HORTSCI.50.8.1183
Sitton B. G., Dodge F. N., Sitton B. G., and Dodge F. N. (1939). Growth and fruiting of three varieties of pecans on different seedling rootstocks. Proc. Am. Soc. Hortic. Sci. 36, 121–125.
Smith M. W., Cheary B. S., and Carroll B. L. (2001). Rootstock and scion affect cold injury of young pecan trees. J. Am. Pomological Soc. 55, 124–128. Available at: https://www.pubhort.org/aps/55/v55_n2_a20.htm (Accessed June 13, 2025).
Smith M. W., Goff W. D., Smith M. W., and Goff W. D. (2014). Patch budding pecan: girdling, tipping, age, and size of budwood and rootstock for budding; girdling, 2,3,5-Triodobenzoic acid and 6-benzylaminopurine for bud forcing. HortTechnology 24, (5). doi: 10.21273/HORTTECH.24.5.512
Smith C. A., VanLeeuwen D., Heerema R. J., Sherman J. D., Comeau M. J., and Walworth J. L. (2022). Zinc variability in pecan orchards: implications for leaf sampling and nutrient recommendations. HortScience 57, 550–557. doi: 10.21273/HORTSCI16463-21
Sparks D. and Payne J. A. (1982). Zinc concentration in pecan leaflets associated with zinc-deficiency symptoms. HortScience 17, 670–671. doi: 10.21273/HORTSCI.17.4.670
Steinparzer M., Schaubmayr J., Godbold D. L., and Rewald B. (2023). Particulate matter accumulation by tree foliage is driven by leaf habit types, urbanization- and pollution levels. Environ. pollut. 335, 122289. doi: 10.1016/j.envpol.2023.122289
Sun J., Chen X. P., Wang M. T., Li J. L., Zhong Q. L., and Cheng D. L. (2020). Application of leaf size and leafing intensity scaling across subtropical trees. Ecol. Evol. 10, 13395–13402. doi: 10.1002/ece3.6943
Thompson T. E. and Grauke L. J. (1991). Pecans and other hickories (Carya). Acta Hortic. 290), 839–906. doi: 10.17660/ActaHortic.1991.290.19
Tworkoski T. and Fazio G. (2016). Hormone and growth interactions of scions and size-controlling rootstocks of young apple trees. Plant Growth Regul. 78, 105–119. doi: 10.1007/s10725-015-0078-2
Tworkoski T. and Miller S. (2007). Rootstock effect on growth of apple scions with different growth habits. Scientia Hortic. 111, 335–343. doi: 10.1016/j.scienta.2006.10.034
USDA-NASS (2024). Pecan production. Available online at: https://usda.library.cornell.edu/concern/publications/5425kg32f (Accessed January 24, 2024).
Valverdi N. A., Kalcsits L., Valverdi N. A., and Kalcsits L. (2021). Rootstock affects scion nutrition and fruit quality during establishment and early production of ‘Honeycrisp’ apple. HortScience 56, (2). doi: 10.21273/HORTSCI15488-20
Walworth J. L. (2020). Tree-to-tree nutrient variability in pecan orchards. Crops Soils 53, (1). doi: 10.1002/crso.20008
Walworth J. L. P., Sower A. P., Kilby G. J., and M. W. (2006). Fall-applied foliar zinc for pecans. HortScience 41, 275–276. doi: 10.21273/HORTSCI.41.1.275
Wang X., Kubenka K., Hilton A., Chatwin W., Cox T., and Tondre B. (2025a). The effects of freezing and stratification on pecan Carya illinoinensis seed germination and seedling growth. Technol. Horticulture. 5, e002. doi: 10.48130/tihort-0024-0030
Wang X., Kubenka K., Hilton A., Chatwin W., Pisani C., Bock C., et al. (2025b). Effect of twelve rootstocks on the scion ‘Pawnee’ in a 10-year-old orchard. Acta Hortic. 1420, 55–62. doi: 10.17660/ActaHortic.2025.1420.8
Wells L. (2024). Budding and grafting of pecan. Bulletin 1376 (University of Georgia Extension Publication). Available online at: https://extension.uga.edu/publications/detail.html?number=B1376&title=budding-and-grafting-of-pecan (Accessed June 13, 2025).
Westwood M. N., Chaplin M. H., and Roberts A. N. (1973). Effects of rootstock on growth, bloom, yield, maturity, and fruit quality of prune (prunus domestica l.). J. Am. Soc. Hortic. Sci. 98, 352–357. doi: 10.21273/JASHS.98.4.352
Wood B. and Reilly C. (2001). A typical symptoms of cold damage to pecan. HortScience 36, 298–301. doi: 10.21273/HORTSCI.36.2.298
Yusufali Z., Liu X. J., Wang X. W., Kubenka K., and Du X. F. (2023). Texture properties, crude fat, fatty acid profiles, total soluble solids, and total polyphenols for 21 pecan varieties and the effects of the harvest year. ACS Food Sci. &Technology 3, 1663–1679. doi: 10.1021/acsfoodscitech.3c00216
Keywords: Carya illinoinensis, provenance, seedstock, scion, horticultural traits, microbial diversity
Citation: Wang X, Kubenka K, Chatwin W, Hilton A, Tondre B, Xu T and Zhang L (2025) Rootstock impacts on ‘USDA-ARS-Pawnee’ pecan growth, physiological traits, and soil microbial communities. Front. Hortic. 4:1603031. doi: 10.3389/fhort.2025.1603031
Received: 31 March 2025; Accepted: 04 June 2025;
Published: 26 June 2025.
Edited by:
Manuel Jamilena, University of Almeria, SpainReviewed by:
Michail Michailidis, Aristotle University of Thessaloniki, GreeceIoana Grozea, Banat University of Agricultural Sciences and Veterinary Medicine, Romania
Copyright © 2025 Wang, Kubenka, Chatwin, Hilton, Tondre, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinwang Wang, eGlud2FuZy53YW5nQHVzZGEuZ292
‡ORCID: Warren Chatwin, orcid.org/0000-0003-1769-7417
 Xinwang Wang
Xinwang Wang Keith Kubenka1
Keith Kubenka1 Angelyn Hilton
Angelyn Hilton Tingying Xu
Tingying Xu Lu Zhang
Lu Zhang