- 1Department of Extension, The Ohio State University, Columbus, OH, United States
- 2Department of Horticulture and Crop Science, The Ohio State University, Columbus, OH, United States
- 3US Department of Agriculture, Agricultural Research Service, National Clonal Germplasm Repository, Corvallis, OR, United States
- 4US Department of Agriculture, Agricultural Research Service, Horticultural Crops Production and Genetic Improvement Research Unit, Corvallis, OR, United States
Indoor farming enables consistent production of superior-quality strawberries through optimized conditions. As strawberry growth, production, and quality can be largely affected by both genotype and environment, it is important to identify cultivars with traits desirable for indoor production. Twenty-three publicly available strawberry (Fragaria × ananassa) cultivars were selected from the USDA-ARS National Clonal Germplasm Repository as possible genetic resources for future breeding for indoor production and evaluated in a walk-in growth chamber with sole source electric lighting. Among strawberry cultivars examined, ‘Mara des Bois’ had desirable traits for indoor farming, including long-day photoperiodic response, early production, higher average weekly yield, and low sensitivity to dormancy-inducing photoperiod. Fruit quality traits, including size, calyx area, shape, color, total soluble solid content (Brix), titratable acidity (TA), and firmness were evaluated. ‘Chandler’ produced the largest fruit, ‘Sweet Sunrise’ showed the lowest calyx-to-fruit area ratio, and ‘Benton’, ‘Hood’, ‘Mara des Bois’, ‘NW 90054-37’, and ‘Puget Beauty’ fruit had a relatively high Brix-to-TA ratio. Correlations among productivity, quality, and morphological characteristics revealed the potential to enhance both productivity and quality by optimizing environmental conditions. The information on strawberry plant growth, development, and fruit production provided in this study can assist indoor growers in cultivar selection and potentially contribute to the development of new strawberry cultivars that thrive in indoor production environments.
1 Introduction
Strawberry is one of the most popular fresh fruits consumed around the globe (Simpson, 2018). Global strawberry production, valued at USD 25.6 billion, occurs in 81 countries, with China being the largest producer, followed by the U.S., Turkey, Egypt, and Mexico (FAO, 2022). In the U.S., most strawberries are produced in California and Florida in open-field systems (USDA, 2022). The U.S. is also one of the largest importers of fresh strawberries, and Mexico is its primary supplier, accounting for 99% of the strawberry imports to the U.S (Wu et al., 2018). As the import of strawberries from Mexico continues to grow, the market for U.S. field-grown strawberries will likely be further restricted (Wu et al., 2018).
High-quality strawberries produced in controlled environments can potentially expand the market for fresh strawberries by attracting consumers with specialty berry traits, such as premium flavors and novel fruit colors (Folta, 2019; Kouloumprouka Zacharaki et al., 2024). Additionally, controlled environments provide protection against biotic and abiotic stresses, allowing the production of fresh strawberries in areas where outdoor environments may not be suitable for the production of high-quality strawberries due to extreme climate, disease pressure, and/or limited production resources (Hernández-Martínez et al., 2023; Samtani et al., 2019). Fully controlled indoor environments with sole source electric lighting allow continuous production of strawberry fruit; therefore, off-season and re-planting do not necessarily occur at a certain season or on an annual basis (van Delden et al., 2021). This can be especially beneficial during the off-season of strawberry field production, supplying high-quality strawberries to a less competitive market. From 2009 to 2019, the number of controlled environment operations in the U.S. that grew and sold $10,000 or more of strawberries doubled (USDA, 2009, 2019). The total production under protected environments expanded 3 times, while production in hydroponic systems has grown from 6.7 tons to 93 tons (USDA, 2009, 2019). Among the various controlled environment systems, indoor farming systems allow complete control of the production environment and can therefore provide consistent year-round production of high-quality strawberries (Ketel et al., 2024). However, to compensate high capital and operational expenses, producing high value crops with novel phenotypes, which exclusively expressed in indoor farms, is preferred for the economic sustainability of indoor farms (Banerjee and Adenaeuer, 2014; Hiwasa-Tanase and Ezura, 2016; van Delden et al., 2021).
The desirable characteristics of strawberries in indoor farming can be different compared to field-grown strawberries. The preferred traits for field-grown strawberries typically include high yield, long post-harvest shelf-life, pest and disease resistance, phenotypic stability, and ease of picking (Folta, 2019). Indoor farm production may seek cultivars with compact plant size, rapid growth and production, high resource-use efficiency, and high fruit quality, such as optimal fruit size, novel colors and flavors, and production of compounds with health benefits (Hiwasa-Tanase and Ezura, 2016; Folta, 2019). The U.S. Department of Agriculture Agricultural Research Service National Clonal Germplasm Repository (USDA-ARS-NCGR) maintains a diverse collection of strawberry germplasm that was characterized for desirable traits and parameters for open-field production (Hummer et al., 2022; Zurn et al., 2022). Further evaluation of the strawberry germplasm focusing on the growth, production, and quality parameters under indoor cultivation conditions is needed to identify strawberry cultivars with desirable traits for indoor farming.
Despite the increasing interest in growing strawberries in indoor farms, little information is available on cultivars suitable for fully controlled environment production in indoor farming systems. Most previous trials of cultivar comparisons in controlled environments focused on greenhouse and/or high tunnel production (Chiomento et al., 2021; Paparozzi et al., 2018; Richardson et al., 2022; Wortman et al., 2016). These studies show that strawberry plant growth, production, and fruit quality are largely affected by cultivar and growing conditions (Wortman et al., 2016; Chiomento et al., 2021; Paparozzi et al., 2018; Mathey et al., 2017).
In this study, we analyzed 23 publicly available strawberry (Fragaria × ananassa) cultivars/accessions selected from USDA-ARS-NCGR based on a previously conducted fruit flavor analysis (Mathey et al., 2013). We excluded cultivars protected by patents as the cultivars used in this study are considered as genetic resources for further breeding and genomics studies to generate new cultivars with enhanced quality and productivity. To identify strawberry cultivars with traits desirable for indoor farming, we evaluated plant growth, morphology, productivity, and fruit quality using environmental conditions relevant to indoor farms. We also explored the correlations between fruit quality, fruit production, and plant morphological characteristics.
2 Materials and methods
2.1 Plant materials and transplant growth environment
Twenty-three strawberry (Fragaria × ananassa) cultivars/accessions (Table 1) were selected from the USDA-ARS-NCGR collection in Corvallis, OR, USA. All cultivars except one (‘Mara de Bois’) are short-day (SD) photoperiodic flowering types, due to the limited availability of off-patent long-day cultivars for public breeding. Rooted runner tips were planted in 396-mL black square containers with a coconut coir substrate (Finesse, Jiffy Group, Zwijndrecht, the Netherlands) and maintained under transplant growth conditions for 24 weeks in a walk-in growth chamber (Sankyo Frontier, Kashiwa, Chiba, Japan) at The Ohio State University (Columbus, OH, USA) before transferring to the production environment. Air temperature was maintained at a constant 20°C. An average photosynthetic photon flux density (PPFD) of 347 µmol m−2 s−1 (PAR, 400–700 nm) was provided with white LED light (FAHX30, Panasonic, Kadoma, Osaka, Japan) for a 16-h day/8-h night photoperiod. Locations of the plants in the growth chamber were randomized every other day to ensure even growing conditions. Plants were fertigated by hand every other day using municipal water with a Yamazaki strawberry formula containing (mg L–1) 77 total N (70 NO3-N and 7 NH4-N), 21.4 P, 117.3 K, 40 Ca, 12.2 Mg, 16.1 S, 45.1 Cl, and micronutrients (Yamazaki, 1982). The pH and electrical conductivity (EC) of the nutrient solution were maintained at 6.5 and 1.0 dS m–1.
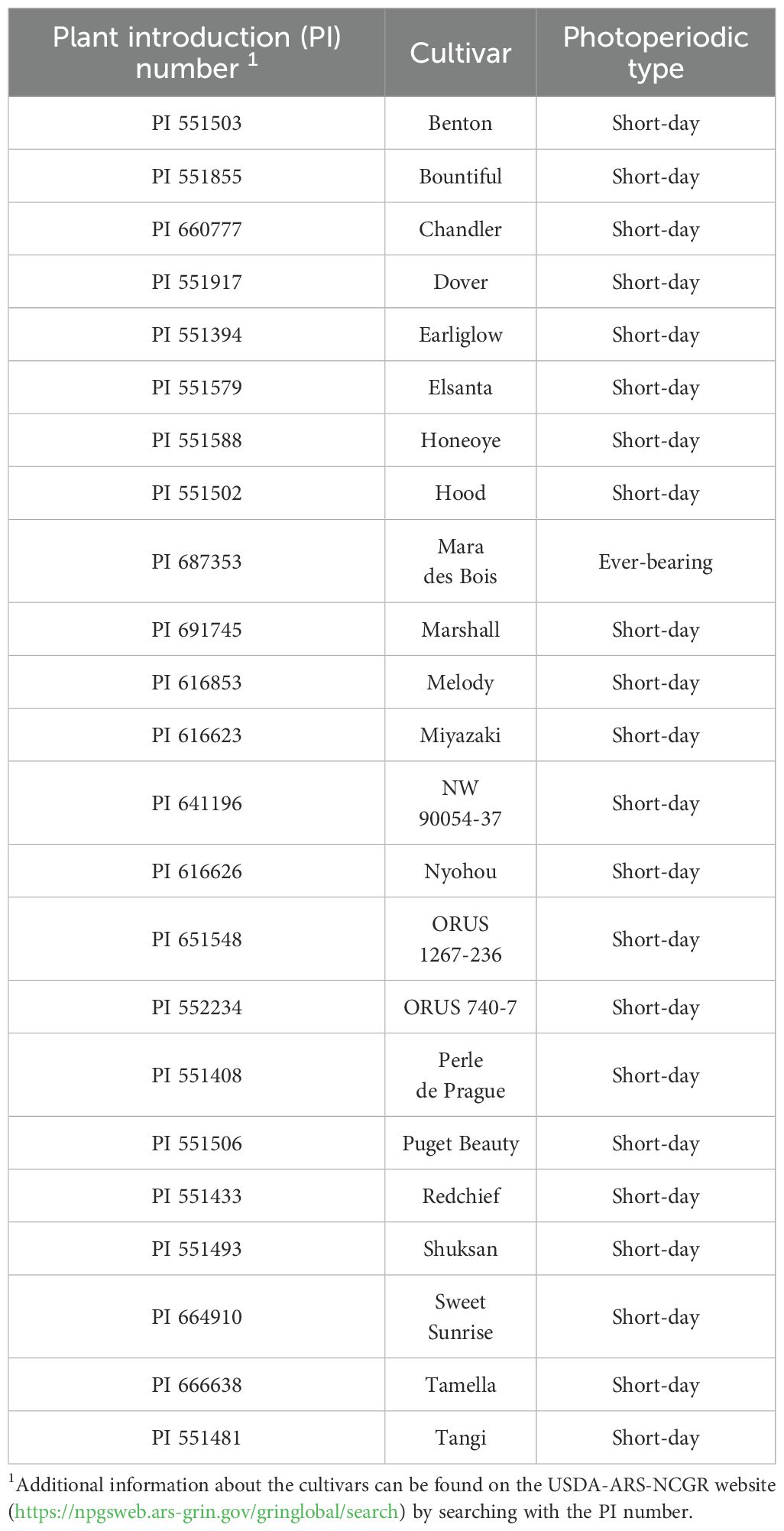
Table 1. List of 23 cultivars selected for this study from the U.S. Department of Agriculture Agricultural Research Service National Clonal Germplasm Repository (USDA-ARS-NCGR).
2.2 Fruit production environment
All strawberry transplants were pruned down to three fully expanded leaves and one crown to standardize the transplant size from the long propagation time (24 weeks) before transplanting. Pruned plants were transplanted into 2.37-L black cylindrical plastic containers with the same coconut coir substrate (Finesse, Jiffy Group) covered with a 1-cm layer of rice hulls (PBH Nature’s Media Amendment, Riceland Foods, Stuttgart, AR, USA). The plants were grown in a 24 m2 walk-in growth chamber (GH300, Conviron, Winnipeg, MB, Canada) at the Controlled Environment Food Production Research Complex (CEARC) at The Ohio State University (Supplementary Figure S1A). The plants were arranged in the growth chamber in a randomized complete block design with three blocks. All the cultivars had one plant in each block (n=3). Plants were arranged on table-top gutters with 7.1-7.7 plants m−2 density. Border plants were placed on the gutters against the side walls and are excluded from the data collection.
A spatial average PPFD ± standard deviation of 332 ± 3 µmol m−2 s−1 at canopy level was provided with white LED (Gavita CT1930e, Hawthorne, Port Washington, NY, USA) mounted approximately 2.1 m above the canopy and measured using a quantum sensor (LI-190R sensor with a LI-250A meter, LI-COR Biosciences, Lincoln, NE, USA), and the light distribution is shown in Supplementary Figure S1B. Plants were first grown under a 13-h photoperiod (SD) for initial flower induction. During this period, many plants started showing semi-dormancy symptoms, and we extended the photoperiod to 16-h (LD) using photoperiodic lighting (FOCUS BR30, Focus LED, Deventer, the Netherlands) at 5 µmol m−2 s−1 PPFD with a 0.4 red-to-far-red photon flux ratio, providing a total photon flux density (400–800 nm) of 11 µmol m−2 s−1. The photoperiodic lighting was on for 13 h during SD and 16 h during LD. Daily light integral was 15.5 and 15.6 mol m−2 d−1 during SD and LD, respectively. The spectra with both white LED and photoperiodic lighting during the SD photoperiod and the extended photoperiod were measured using a spectroradiometer (PS-200, Apogee, Logan, UT, USA) and are shown in Supplementary Figure S2. The timeline for photoperiod alternation was 12 weeks of SD, 8 weeks of LD, 5 weeks of SD, and 4 weeks of LD.
The air temperature during the 13-h photoperiod was set at 24°C, while the extended photoperiod and nighttime temperature was set at 12°C. The air temperature was measured at two locations at an approximate height of the plant canopy using Type-T thermocouples (gauge 36) and recorded with a CR23X datalogger (CR23X Micrologger, Campbell Scientific, Logan, UT, USA). The average day and extended-day/night temperatures over the entire production time were 24 ± 0.7°C and 12 ± 0.9°C, respectively. The relative humidity was set at 65%, except for a 6-h period during nighttime before daytime started when it was raised to 93% to prevent tip burn. The actual humidity was 63 ± 2% during the photoperiod and 89 ± 4% during the dark period (HMP60 sensor with a CR23X Micrologger, Campbell Scientific). The average air velocity at the plant canopy level measured perpendicularly to the air circulation direction using an anemometer (A004, Kanomax, Osaka, Japan) was 0.3 ± 0.09 m s–1. The day (13 h) and night (11 h) concentrations of CO2 in the growth chamber were 1000 ± 12 µmol mol−1 and 402 ± 15 µmol mol−1, respectively.
Plants were drip-fertigated daily using Jack’s Strawberry Part A (JR Peters Inc, Allentown, PA, USA) and calcium nitrate (Yara, Oslo, Norway), containing (mg L–1) 80 N (72 NO3-N and 8 NH4-N), 24 P, 121 K, 44 Ca, 13 Mg, 50 S, 10 Cl, and micronutrients. The amount of nutrient solution that plants received was monitored three times a week and adjusted as needed to maintain a 0.1-0.2 drain-to-drip solution volume ratio. The pH and EC of the nutrient solutions were 6.7 ± 0.1 and 1.0 ± 0.1 dS m–1. The pH and EC of the drain solutions were 6.8 ± 0.1 and 1.0 ± 0.2 dS m–1. During production, runners and old leaves were removed weekly. The plants were pollinated by hand, a hand-held leaf blower (CMCBL710, Craftsman, Towson, MD, USA) to create mechanical shaking, and a bumble bee micro-colony of seven worker bees in the growth chamber.
2.3 Plant measurements
To evaluate fruit earliness and productivity, time to first flower (d) and time to first harvest (d) from transplanting were recorded for each plant, and the flower-to-fruit harvest time was calculated. Fruit was deemed harvestable when the redness reached the proximal end row of achenes. Fruit was harvested at least three times a week, and the fresh weight of each harvest and the numbers of fruit were recorded. Average fruit size (g) was calculated as the total fruit fresh weight divided by the total number of fruit. The harvest goal for each plant was 200 g in order to provide enough fruit for fruit sensory analysis in a separate experiment. The number of weeks from the first harvest to when the harvest goal was reached was recorded. Average weekly yield (g) was calculated as the total fruit weight divided by the number of weeks from the week of the first harvest to the week when the harvest goal was reached. Maximum weekly yield (g) was defined as the highest yield produced in one week.
After the strawberry plants were transplanted and moved into the production environment, every month for five months (23 weeks), the plant height (cm) from the top of the substrate to the highest point of the plant shoot was measured using a ruler, and pictures of the plant’s projected canopy were taken. Images of the plant canopies were analyzed in a custom-made image analyzer operated with a Python program (v. 3.8) using the OpenCV library (v. 4.5.4) following an approach described by Kim and van Iersel (2023). Specifically, plant objects and backgrounds in the images were separated using intensity-based thresholding with the saturation channel in the HSV color model. The number of pixels in the plant objects was counted and converted into length (cm) and area (cm2) based on a reference scale. Plant sensitivity to dormancy-inducing SD photoperiod was quantified by the percent change in plant height (%), which was calculated as the percent difference between the average plant height under SD and LD conditions: (heightLD – heightSD)/heightSD, where heightSD was determined using plant height measured after plants were grown five and nine weeks in SD environment, and heightLD was determined using plant height measured after plants were grown seven weeks in LD environment.
To evaluate fruit morphology, three representative fruits, selected for representative color and fruit shape, were collected from each plant. The calyx was separated from the fruit, and the fruit was dissected into two halves lengthwise along the widest side. The calyx and the exterior and the interior of the fruit were scanned using a desktop scanner (CanoScan LiDE 210, Canon, Tokyo, Japan) covered with a black cloth. The scanned RGB images were analyzed using the Python program described above to determine calyx area (cm2), fruit diameter (cm), fruit length (cm), and fruit interior area (cm2). Fruit diameter and length were the maximum width and length of the cross-section, respectively, and the fruit diameter-to-length ratio was calculated. The calyx-to-fruit area ratio was calculated by dividing the calyx area with fruit interior area. The fruit interior redness and fruit exterior redness were quantified using the normalized difference anthocyanin index (NDAI) reported by Kim and van Iersel (2023). Specifically, the pixel intensities of the red and green channels of the scanned images (Ired and Igreen) were used to calculate the NDAI values of the fruit objects using the equation: NDAI = (Ired − Igreen)/(Ired + Igreen). The pixel intensities of the images were adjusted using a standard gray card (50% reflection at all color channels) that has a 50% pixel intensity in each color channel.
To measure the concentration of fruit total soluble solids (TSS, or Brix) and TA, an additional 50 g fruit sample was collected from each plant, frozen at −20°C, and stored at −50°C. The samples were thawed at 4°C, homogenized, and centrifuged at 2490 × g for 10 min. The TSS concentration of the supernatant was analyzed using a digital refractometer (PR-32 alpha, ATAGO, Tokyo, Japan). For TA, 1 mL of the supernatant was diluted to 20 mL with distilled water and gradually titrated with 0.1 N NaOH to pH 8.2, and the pH change was closely monitored using a pH meter (accumet AB150, Thermo Fisher Scientific, Waltham, MA, USA). TA (g L−1) was determined using citric acid as the representative acid, and the Brix-to-TA ratio was calculated.
Fruit firmness (N) was measured with an additional three fruits as sub-samples using a texture analyzer (TA.XTPlus, Stable Micro Systems Texture Technologies, Scarsdale, NY, USA) fitted with a 4 mm probe. The fruits were harvested into a tray with ice and transported immediately to the lab on ice for firmness measurements to eliminate changes of fruit texture. Each fruit was dissected into two halves, and the top 25% of the halved fruit was penetrated at a speed of 1.7 mm s−1. The maximum gram force developed during the test was recorded and converted to Newton (N).
2.4 Statistical analyses
Statistical analyses of plant phenotypic data, hierarchical cluster analysis, and correlation analysis were conducted in R (v. 4.3.2) (R Core Team, 2023). All phenotypic data was analyzed using ANOVA-protected mean separation using Fisher’s least significant difference (LSD) with the R package “agricolae”. For fruit color, shape, firmness analyses, three representative fruits per plant were used as sub-samples. Hierarchical clustering was performed using Ward’s method, and four groups of strawberry cultivars were categorized based on the results of the analysis. The average value in each category was calculated using the mean of each cultivar ± standard errors across the cultivars. Correlation analysis was conducted using Pearson correlation matrix analysis.
3 Results and discussion
3.1 Fruit productivity
Under the indoor growing conditions used in this study, all plants grew well and produced fruit after transplanting. Based on the average weekly yield (200-g yield divided by total number of production weeks) and maximum weekly yield (the maximum yield of a week), we classified the 23 cultivars into four categories of productivity levels using cluster analysis. These categories are 1) high average and maximum yield, 2) medium productivity with higher average yield, 3) medium productivity with higher maximum yield, and 4) low average and maximum yield (Table 2). ‘Benton’, ‘Bountiful’, ‘Earliglow’, ‘Hood’, ‘Shuksan’, and ‘Tamella’ had high average and maximum yield, producing an average weekly yield of 43.0 g and a maximum weekly yield of 92.6 g per plant (Table 2). Among cultivars with medium productivity, ‘Chandler’, ‘Dover’, ‘Mara des Bois’, ‘Marshall’, ‘Miyazaki’, ‘Perle de Prague’, and ‘Sweet Sunrise’ had a relatively higher average weekly yield of 37.4 g and relatively lower maximum weekly yield of 66.7 g (Table 2). ‘Honeoye’, ‘Melody’, ‘Nyohou’, ‘Puget Beauty’, and ‘Tangi’ displayed medium productivity with a relatively lower average weekly yield of 23.9 g and a relatively higher maximum weekly yield of 79.9 g per plant (Table 2). Low average and maximum yields were seen in ‘Elsanta’, ‘NW 90054-37’, ‘ORUS 1267-236’, ‘ORUS 740-7’, and ‘Redchief’, resulting in an average weekly yield of 24.3 g and a maximum weekly yield of 54.5 g per plant (Table 2). When grown under greenhouse conditions, ‘Chandler’, ‘Elsanta’, and ‘Honeoye’ showed similar average weekly yield as in our indoor environment (Fletcher et al., 2002; Paparozzi et al., 2018). Different from our results, when cultivated under open-field conditions in Oregon, USA, ‘Mara des Bois’ and ‘Miyazaki’ had lower yield than ‘ORUS 740-7′ and ‘ORUS 1267-236′, and the yield of ‘NW90054-37′ was higher than ‘Tamella’ (Hummer et al., 2022).
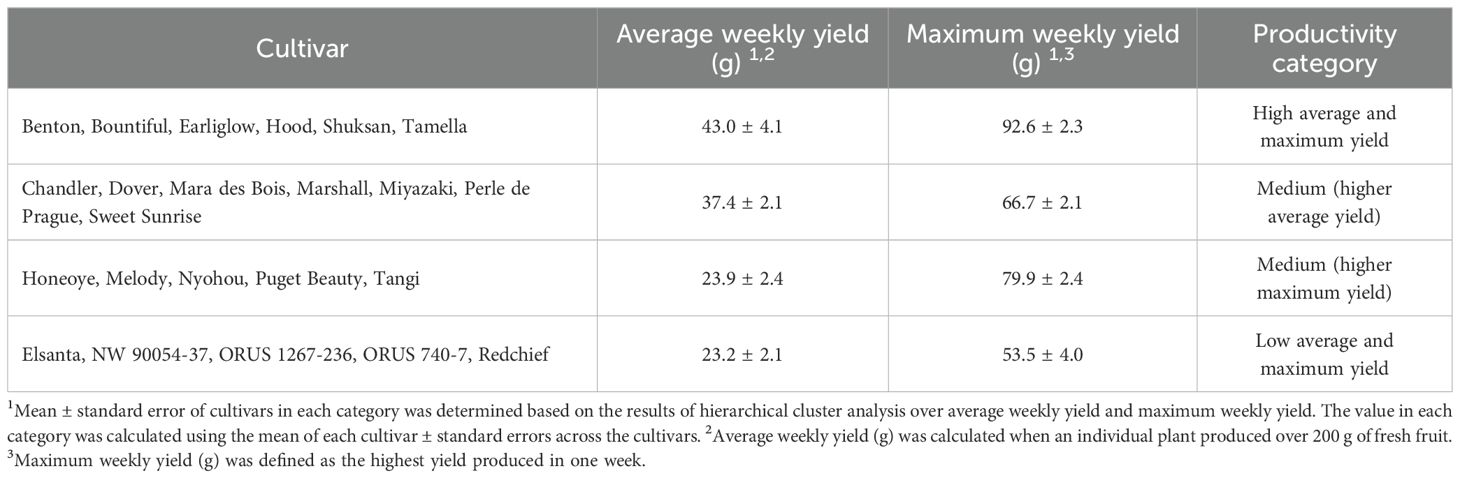
Table 2. Classification of 23 strawberry cultivars in an indoor environment based on fruit productivity quantified by average weekly yield and maximum weekly yield per plant.
3.2 Fruit earliness and ripening time
Early flowering, early harvest, and short ripening time can help accelerate the production process of strawberries. These are valuable traits for indoor farm production as they can reduce operational costs during the non-production time (Hiwasa-Tanase and Ezura, 2016). Our results showed that without receiving chilling treatment, ‘Dover’, ‘Honeoye’, ‘Mara des Bois’, ‘Melody’, ‘Puget Beauty’, and ‘Redchief’ flowered in less than 45 days after transplanting with SD photoperiod, while ‘Hood’, ‘Miyazaki’, and ‘Shuksan’ took more than 90 days to flower (Figure 1A). The earliest fruit harvest was seen in ‘Mara des Bois’ in less than 70 days after transplanting (Figure 1B), likely because it is a long-day strawberry. As long-day strawberries do not require SD conditions for flowering, flower induction and initiation likely occurred during the vegetative growing stage with LD conditions. SD strawberries ‘Dover’, ‘Honeoye’, ‘Melody’, ‘Puget Beauty’, and ‘Redchief’ produced first harvest in less than 80 days after transplanting, while ‘Miyazaki’ and ‘Shuksan’ took more than 130 days to produce the first harvestable fruit (Figure 1B). In addition to cultivar and production environment, the development of flowers and fruit in strawberry can also be affected by pre-transplant chilling (Ariza et al., 2015). Typically, strawberry transplants are treated with chilling to promote early flowering and enhance yield, and chilling requirements are variable among different cultivars (Moreira et al., 2022). Even though none of the strawberry plants in this study received chilling, all of them flowered and produced fruit with SD treatment. ‘Hood’ and ‘Shuksan’ produced fruit relatively late in this study, likely because they are typically cultivated in the Pacific Northwest, including Oregon, Washington, and British Columbia (Hokansan and Finn, 2000). Therefore, chilling may promote earlier flowering and fruit production in these cultivars. Further studies may focus on evaluating the interaction between photoperiod and chilling on the earliness of strawberry production.
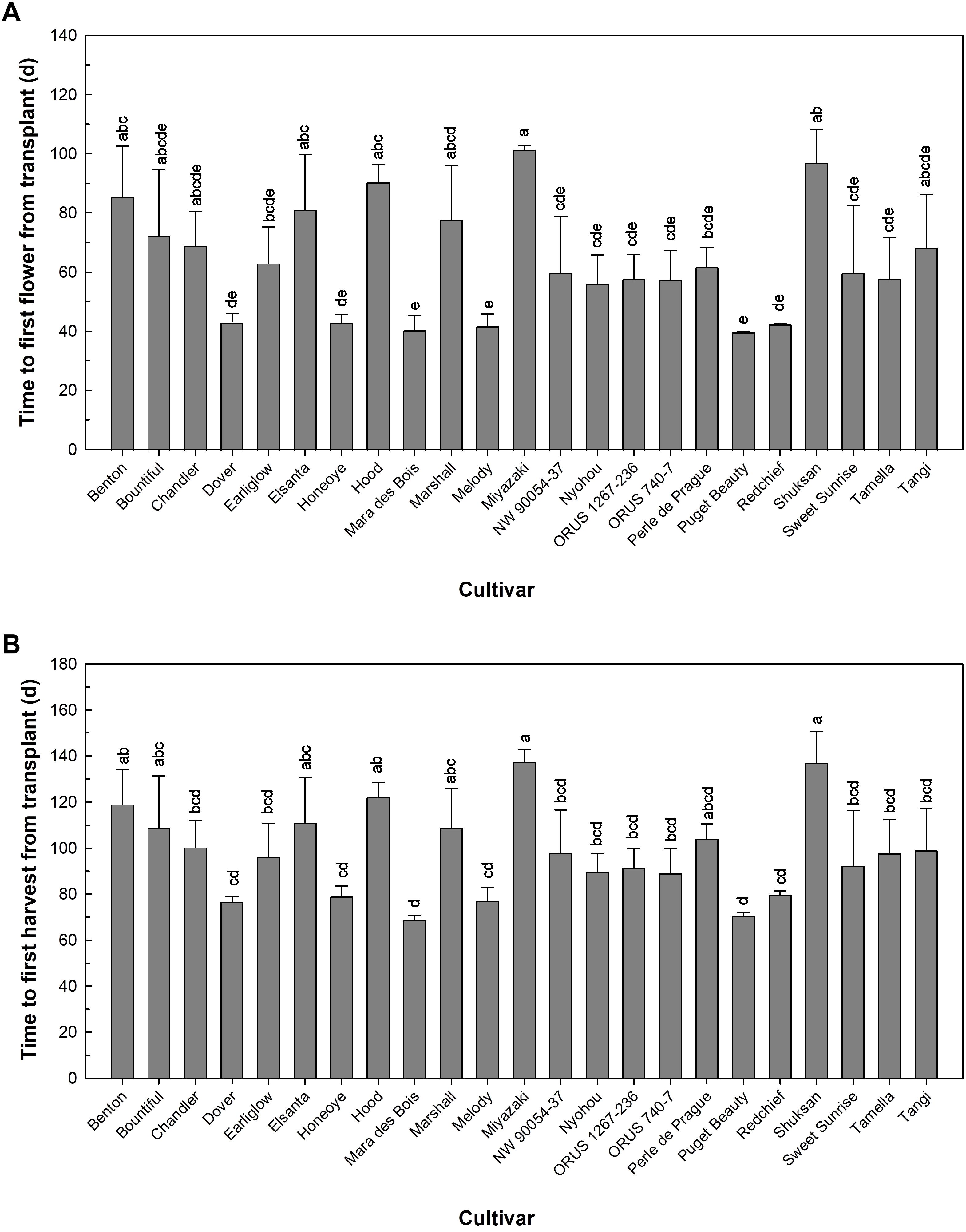
Figure 1. Fruit earliness of 23 strawberry cultivars grown in an indoor environment characterized by (A) Time to first flower and (B) Time to first harvest from transplanting. Bars represent the means of the measurements ± standard error (n = 3), and bars sharing the same letters are not significantly different at p ≤ 0.05.
Flower-to-fruit harvest time of the 23 cultivars ranged from 28 to 42 days (Figure 2). This flower-to-fruit harvest time for ‘Elsanta’ grown under open-field conditions at five different sites in Europe for three production cycles was 29–34 days (Krüger et al., 2012), similar to our observations in the indoor environment. However, Hummer et al. (2022) evaluated ‘Mara des Bois’, ‘Marshall’, ‘Melody’, ‘Miyazaki’, ‘NW 90054-37′, ‘Nyohou’, ‘ORUS 1267-236’, ‘ORUS 740-7’, ‘Puget Beauty’, and ‘Tamella’ under open-field conditions in Oregon, USA, and these cultivars had longer flower-to-fruit harvest time (35–62 days) than what we observed in this study. Based on these comparisons, time required for fruit development and ripening in strawberry varies among genotypes, while environmental factors, such as temperature, have been shown to affect strawberry fruit development and ripening (Krüger et al., 2012; Rosati et al., 1988).
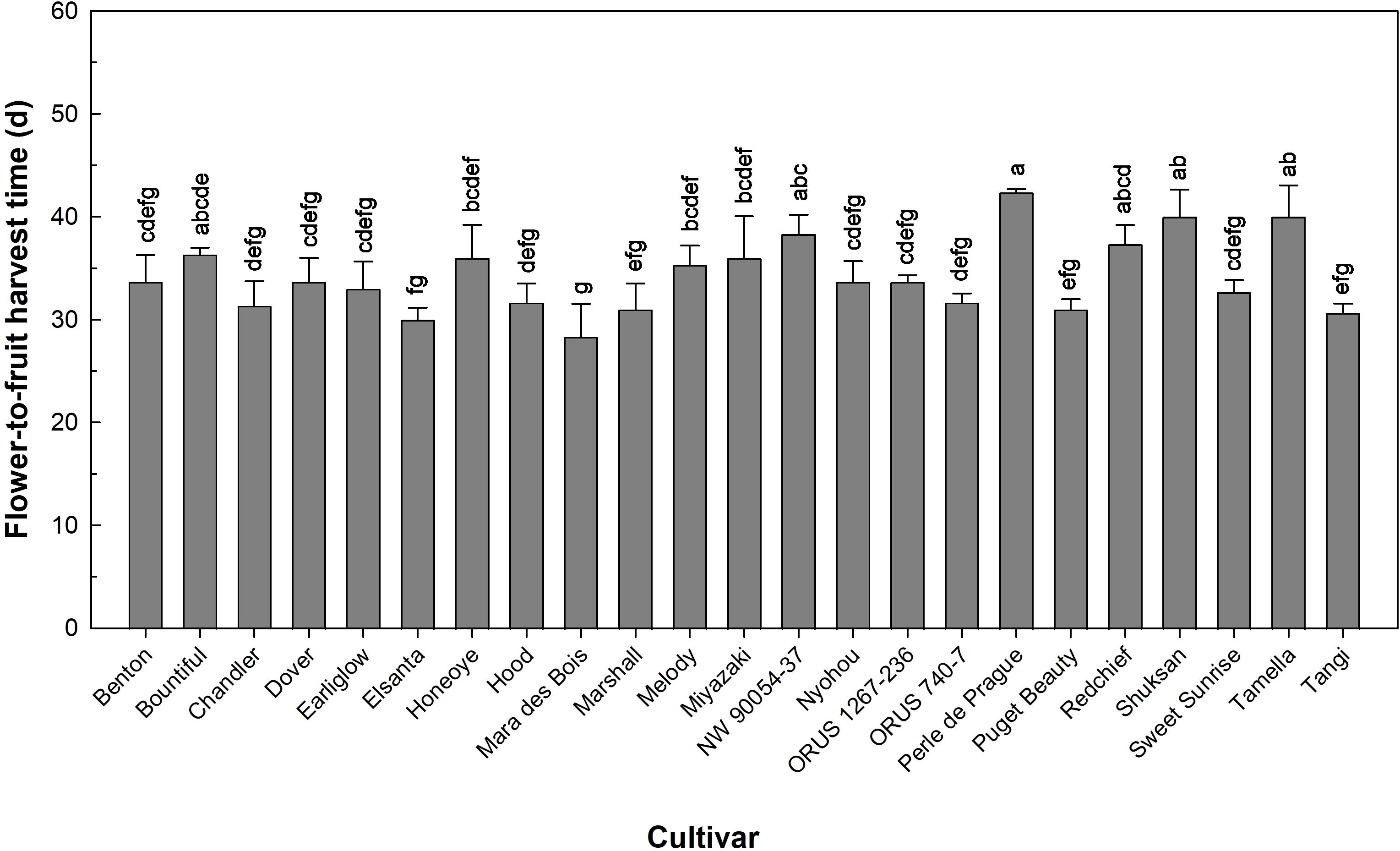
Figure 2. Flower-to-fruit harvest time of 23 strawberry cultivars grown in an indoor environment. Bars represent the means of the measurements ± standard error (n = 3), and bars sharing the same letters are not significantly different at p ≤ 0.05.
3.3 Fruit quality
3.3.1 Fruit size
Fruit size is an important quality trait for strawberry production. The 23 cultivars in this study produced strawberries with an average fruit size (total fruit weight divided by total fruit number) ranged from 3.2 to 11.8 g (Figure 3). On the higher end, ‘Chandler’ produced an average fruit size of 11.8 g, and on the lower end, ‘Miyazaki’ produced an average size of 3.2 g (Figure 3). ‘Benton’, ‘Honeoye’, ‘Marshall’, ‘Melody’, Miyazaki’, ‘NW 90054-37′, ‘Nyohou’, ‘ORUS 1267-236’, ‘ORUS 740-7’, ‘Puget Beauty’, ‘Redchief’, and ‘Tamella’ reportedly produced larger fruits in greenhouse (Paparozzi et al., 2018); and open-field (Archbold and Strang, 1986; Hummer et al., 2022; Zhang et al., 1992) studies compared to those produced in the indoor environment in this study. However, the average fruits produced by ‘Chandler’ and ‘Mara des Bois’ in our indoor environment were 64% and 1.5 times larger respectively than those reportedly produced under field (Hummer et al., 2022) and greenhouse (Paparozzi et al., 2018) conditions by the same cultivars, suggesting that strawberry size can be greatly enhanced by modifying growing environment.
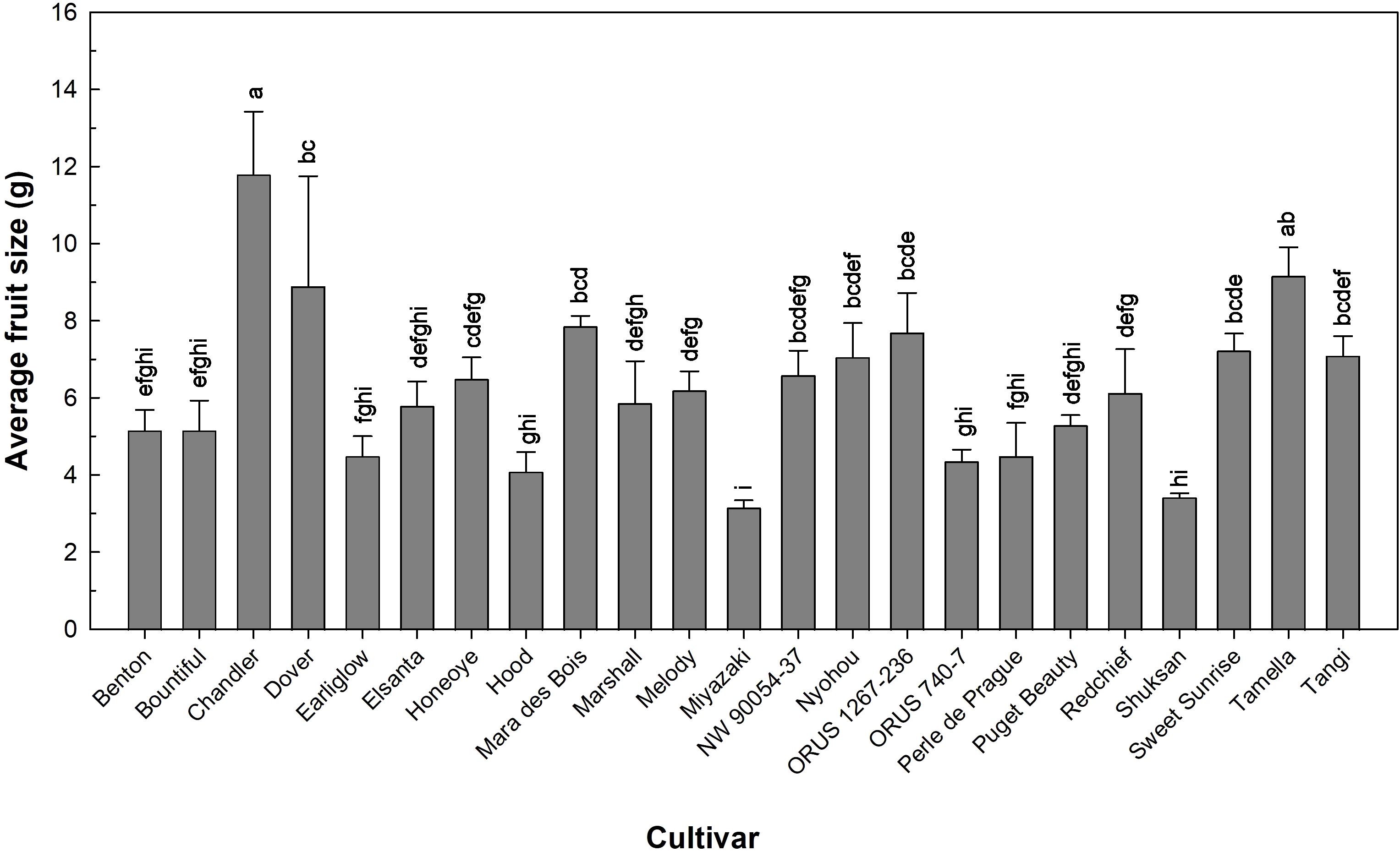
Figure 3. The fruit size of 23 strawberry cultivars grown in an indoor environment. Bars represent the means of the measurements ± standard error (n = 2 or 3), and bars sharing the same letters are not significantly different at p ≤ 0.05.
3.3.2 Calyx area and fruit shape
The relative size of calyx to fruit and fruit shape can affect the appearance of strawberry fruit, contributing to consumers’ perception and liking in strawberries (Bhat et al., 2015; Colquhoun et al., 2012). The fruit of ‘Honeoye’, ‘Marshall’, ‘Sweet Sunrise’, ‘Tamella’, and ‘Tangi’ had calyx-to-fruit area ratio under 0.4, while fruit of ‘Bountiful’, ‘Melody’, ‘Miyazaki’, ‘NW 90054-37’, ‘Nyohou’, ‘Perle de Prague’, and ‘Shuksan’ had a relatively large calyx-to-fruit area ratio over 0.6 (Figure 4A). The fruit shape assessed using fruit diameter-to-length ratio was different across the 23 strawberry cultivars (Figure 4B). ‘Hood’ and ‘Melody’ produced fruit with similar diameter and length (ratio = 1.0) (Figure 4B). Wider fruit was seen in ‘Elsanta’, ‘NW 90054-37′, and ‘Shuksan’, with a diameter-to-length ratio greater than 1.1 (Figure 4B). A longer type of fruit was produced by ‘Tamella’ that had a diameter-to-length ratio of 0.6 (Figure 4B). The diversity in fruit shape and relative size of calyx and fruit area among the 23 cultivars were shown in the pictures of representative strawberry fruit (Figure 5).
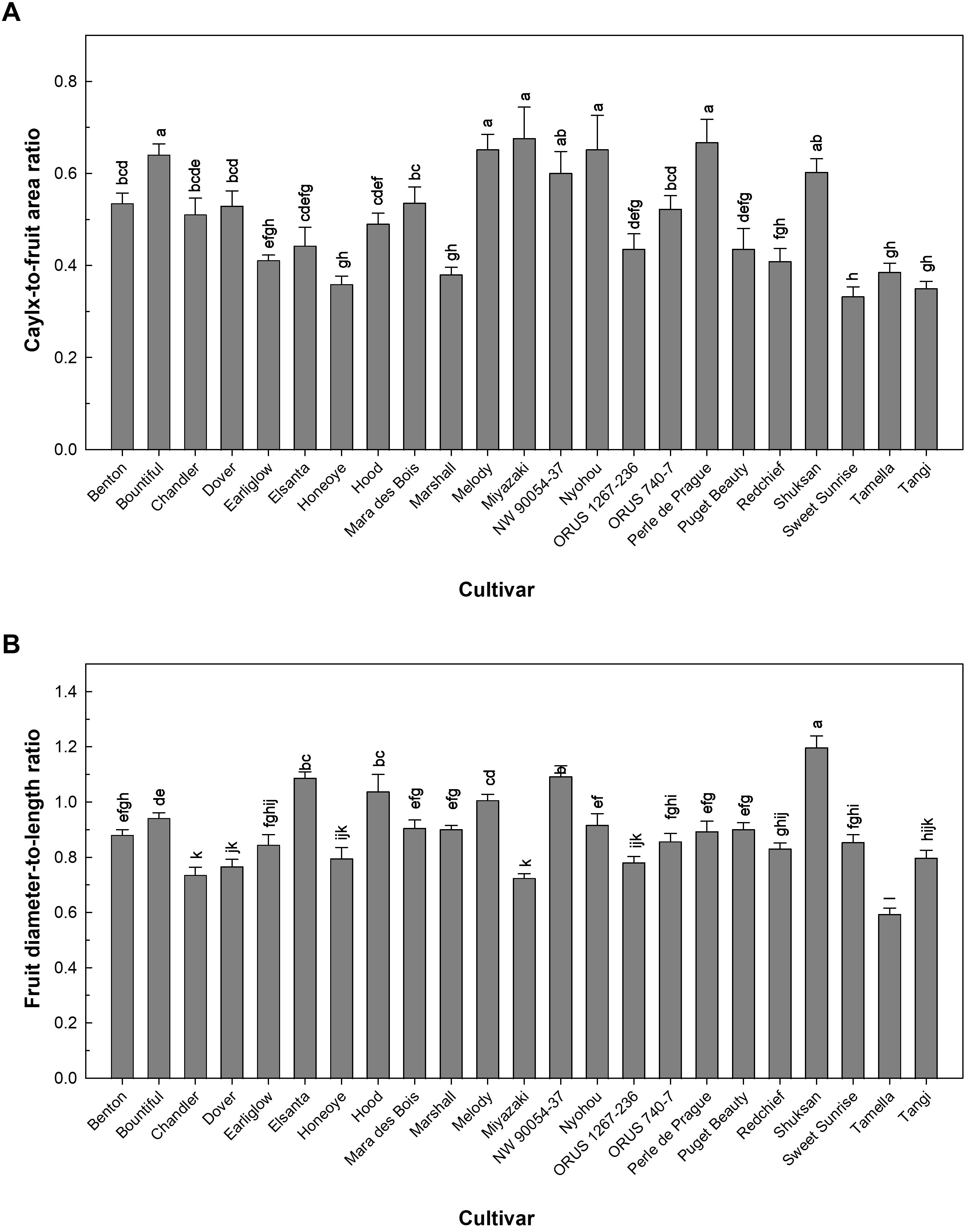
Figure 4. Calyx-to-fruit area ratio (A) and fruit shape characterized by diameter-to-length ratio (B) of 23 strawberry cultivars grown in an indoor environment. Bars represent the means of the measurements ± standard error (n = 3), and bars sharing the same letters are not significantly different at p ≤ 0.05.

Figure 5. Pictures of representative strawberry fruit. Shown in the pictures, from left to right, are the calyx, fruit interior, and fruit exterior of the 23 strawberry cultivars grown in an indoor environment. Scale bar = 2.0 cm.
3.3.3 Fruit color
The color of strawberry fruit can greatly impact consumer preference (Aoki and Akai, 2023; Oliver et al., 2018; Predieri et al., 2021). The redness of strawberry fruit was quantified using NDAI, an index that correlates with the surface concentration of anthocyanins, which are antioxidants that provide health benefits (Kim and van Iersel, 2023; Yoshioka et al., 2013). By analyzing the redness of the interior and the exterior of strawberry fruit, we classified the 23 strawberry cultivars into four redness categories: light, moderate, medium, and dark (Tables 3, 4). ‘Benton’, ‘Hood’, ‘NW 90054-37’, and ‘Tamella’ produced fruit with the highest NDAI on the fruit interior (Table 3). Cultivars with the highest NDAI on the fruit exterior were ‘Earliglow’, ‘Mara des Bois’, and ‘Marshall’, producing dark red fruit exterior surface (Table 4). The fruit of ‘Miyazaki’ and ‘Perle de Prague’ had the lowest NDAI on both the interior and exterior surfaces of the fruit (Tables 3, 4). Pictures of representative strawberry fruit demonstrated visual variations in fruit color among the 23 cultivars (Figure 5). The interior and exterior NDAI of store-bought strawberries from open-field production had a range of 0.19 – 0.50 and 0.37 – 0.62 (Lin et al., unpublished), similar to the fruit NDAI in this study (Tables 3, 4), suggesting that the redness of strawberries produced in the indoor environment was comparable to the marketable fruit from open-field production.
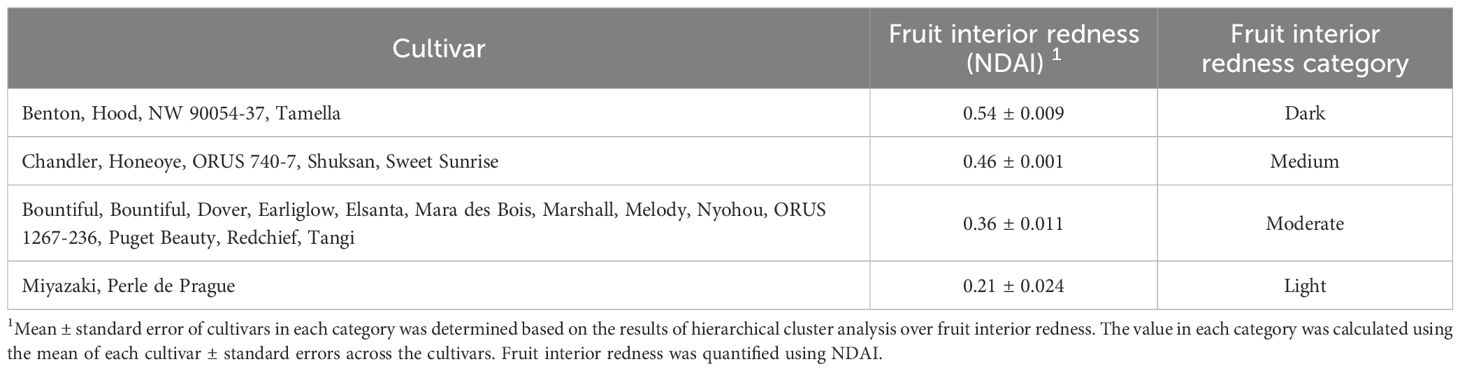
Table 3. Classification of 23 strawberry cultivars in an indoor environment based on fruit interior redness, quantified by fruit interior redness (Normalized Difference Anthocyanin Index, NDAI).
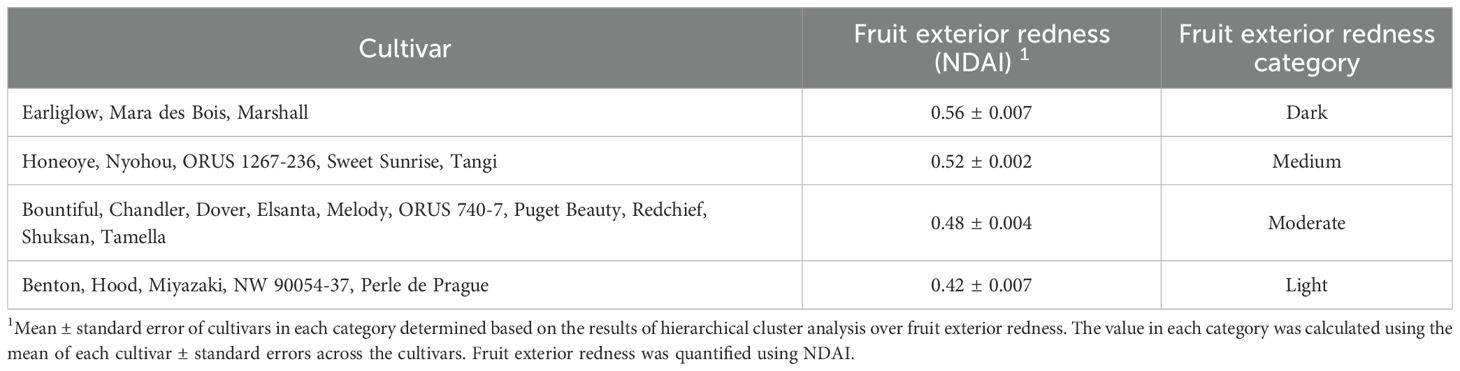
Table 4. Classification of 23 strawberry cultivars in an indoor environment based on fruit exterior redness determined using the Normalized Difference Anthocyanin Index (NDAI, Kim and van Iersel, 2023).
The redness of strawberry fruit can be affected by harvest maturity (Shin et al., 2008). Therefore, an advantage of producing strawberries using indoor farms is that fruit can be harvested when they are fully ripe, as indoor farm systems can be established close to consumers, reducing the time and distance for shipping. The redness of strawberry fruit is also influenced by light quality. Ordidge et al. (2012) showed that strawberry fruit surface color was lighter when the plants were covered by UV-block film compared to non-UV-block plastic in the field. Blue and red LED light treatments can impact the color of strawberries in indoor production (Nadalini et al., 2017). Further studies can focus on optimizing the light spectrum and timing of light treatments during production for the improvement of strawberry fruit color.
3.3.4 Brix, TA, and firmness
Brix and TA are two widely used parameters for evaluating the sweetness and sourness of strawberries (Magwaza and Opara, 2015). For the U.S. market, strawberries with a Brix of 7.0% or higher and a TA of 8 g L−1 or lower are recommended (Cantwell and Suslow, 2002). In our provided environment, the majority of the 23 strawberry cultivars produced fruit with a Brix higher than 7.0%, except for ‘Dover’, ‘Melody’, and ‘Tamella’ (Table 5). ‘NW 90054-37′ produced the highest Brix of 11.9%, followed by ‘Hood’ (11.0%) and ‘Nyohou’ (10.2%) (Table 5). ‘Mara des Bois’, ‘Melody’, and ‘Tamella’ produced fruit with a TA lower than 8 g L−1 (Table 5). Therefore, ‘Mara des Bois’ was the only cultivar whose fruit met the recommended U.S. market standard (Table 5).
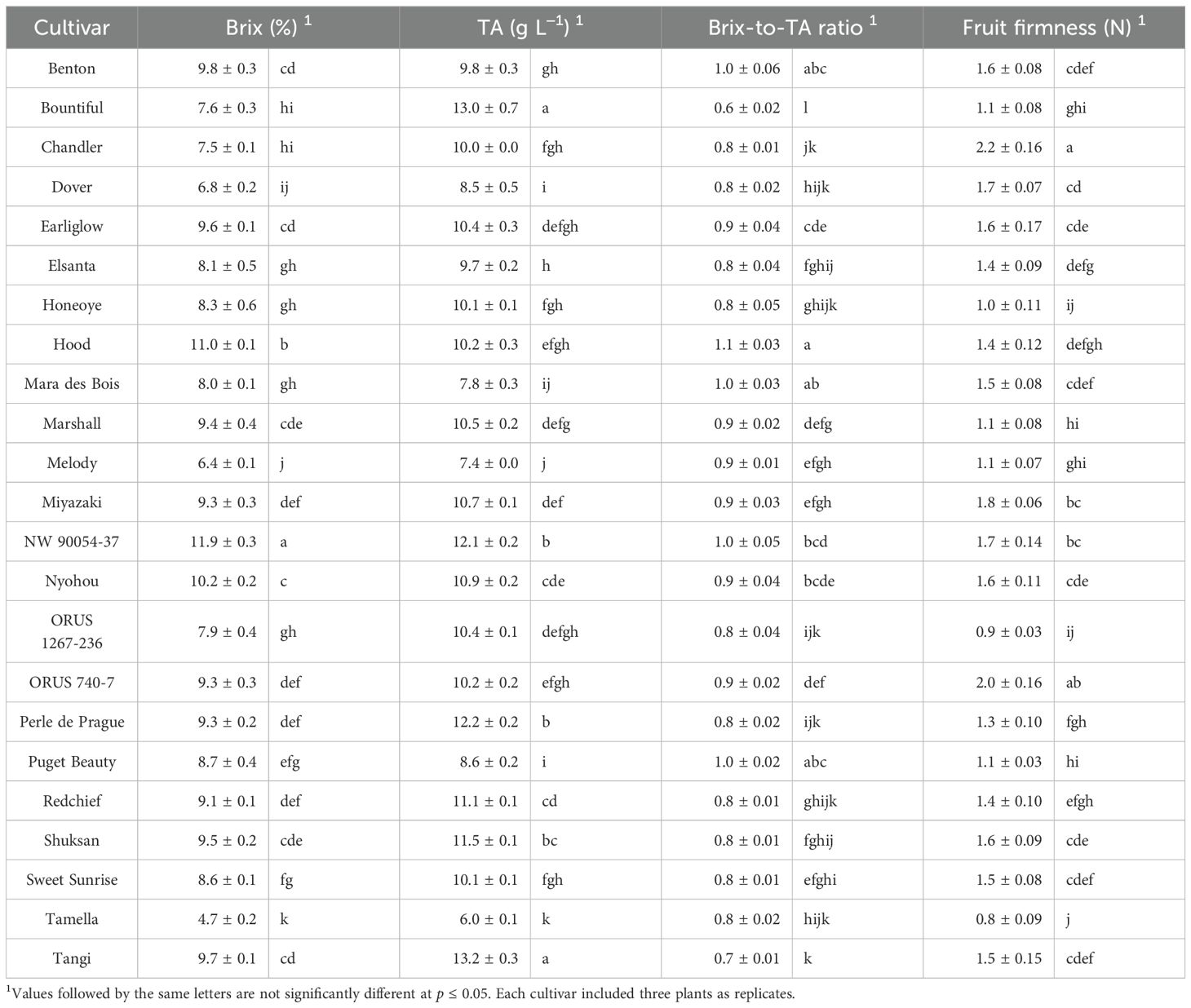
Table 5. Fruit total soluble solid content (Brix), titratable acidity (TA), Brix-to-TA ratio, and fruit firmness of 23 strawberry cultivars grown in an indoor environment.
In addition to this study, the fruit Brix of ‘Mara des Bois’, ‘Marshall’, ‘Melody’, ‘Miyazaki’, ‘NW 90054-37′, ‘Nyohou’, ‘ORUS 1267-236’, ‘ORUS 740-7’, ‘Puget Beauty’, and ‘Tamella’ were also evaluated in different open-field environments (Hummer et al., 2022; Mathey et al., 2013). ‘Tamella’ showed consistently low Brix compared to other cultivars, while other cultivars exhibited variations in Brix in different environments (Table 5) (Hummer et al., 2022; Mathey et al., 2013), indicating the critical impact of the growing environment on fruit quality. Strawberry sugar and acid content can be improved by optimizing the growing temperature to enhance the sensory quality of strawberries (Wang and Camp, 2000). Difference between day and night temperatures could also affect fruit sugar accumulation (Wu et al., 2021).
An important parameter used to predict consumer preference in strawberries is the Brix-to-TA ratio (Jayasena and Cameron, 2008). In this study, ‘Benton’, ‘Hood’, ‘Mara des Bois’, ‘NW 90054-37′, and ‘Puget Beauty’ produced fruit with the highest Brix-to-TA ratio equal to or higher than 1.0 (Table 5). Although the Brix-to-TA ratio is commonly used to evaluate strawberry sensation and predict consumer preference, variations in sugar and acid compositions also affect strawberry sensation (Magwaza and Opara, 2015). Additionally, the diverse aroma compounds in strawberries can influence human perception of the sweetness and flavor intensity of the fruit (Schwieterman et al., 2014). Further sensory evaluation will be needed to determine the actual consumer perception of strawberry flavors from strawberry cultivars grown under indoor conditions.
Fruit firmness can also affect strawberry sensory characteristics and consumer liking (Schwieterman et al., 2014). The range of fruit firmness in the cultivars characterized in this study was 0.8–2.2 N (Table 5). Fruit of ‘Chandler’ displayed fruit firmness of over 2 N, while the fruit of ‘Tamella’ and ‘ORUS 1267-236′ had a fruit firmness of less than 1 N (Table 5). The fruit firmness of ‘Bountiful’, ‘Hood’ and ‘ORUS 1267-236’ in open-field environments were 1.1 – 2.2 times as high as the fruits produced in the indoor environment in this study (Finn et al., 2014; Moore, 2001; Stahler et al., 1994).
3.4 Plant morphology
Compared to open fields, indoor farms generally have less available cultivation space. To maximize the use of space without affecting plant growth, it is important to consider plant size when designing an indoor farm with vertical growing structure (Hiwasa-Tanase and Ezura, 2016). Maximum plant height must be considered when deciding the height of growing shelves and the level of lights. Maximum projected canopy area can be used to optimize cultivar-specific planting density, improving space use efficiency. Based on these morphological parameters, we divided the 23 strawberry cultivars in this study into four plant size categories: large, medium, small, and extra small (Table 6). The large plants were ‘Miyazaki’, ‘Nyohou’, and ‘Shuksan’ with an average shoot height of 31.7 cm and an average maximum projected canopy area of 1609.3 cm2 (Table 6). Medium size plants included ‘Benton’, ‘Chandler’, ‘Dover’, ‘Hood’, ‘Marshall’, ‘Sweet Sunrise’, and ‘Tangi’, producing an average shoot height of 26.1 cm and an average maximum projected canopy area of 1333.5 cm2 (Table 6). Small plants, including ‘Bountiful’, ‘Earliglow’, ‘Elsanta’, ‘Honeoye’, ‘Melody’, ‘NW 90054-37’, ‘ORUS 1267-236’, and ‘Perle de Prague’ showed an average shoot height of 23.0 cm and an average maximum projected canopy area of 910.3 cm2 (Table 6). ‘Mara des Bois’, ‘ORUS 740-7’, ‘Puget Beauty’, ‘Redchief’, and ‘Tamella’ were classified as extra small plants with an average shoot height of 19.0 cm and an average maximum projected canopy area of 623.7 cm2 (Table 6). Pictures of representative plant canopies showed variation in plant spread among the 23 cultivars (Figure 6). Among the 23 cultivars, ‘Mara des Bois’ and ‘Tamella’ had high average weekly yields relative to their extra small projected canopy areas (Table 2 and 6).
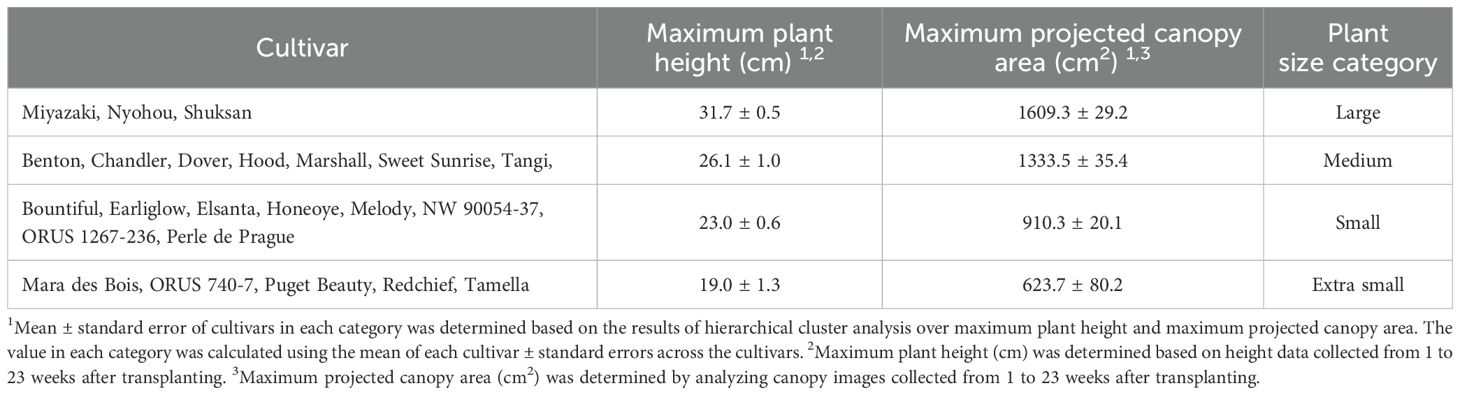
Table 6. Classification of 23 strawberry cultivars in an indoor environment based on plant size, quantified by maximum plant height and maximum projected canopy area.

Figure 6. Pictures of representative plants of 23 strawberry cultivars grown in an indoor environment for 23 weeks. Scale bar = 10.0 cm.
Plant heights of ‘Mara des Bois’, ‘Marshall’, ‘Melody’, ‘Miyazaki’, ‘NW 90054-37′, ‘Nyohou’, ‘ORUS 1267-236’, ‘ORUS 740-7’, ‘Puget Beauty’, and ‘Tamella’ were also measured under field conditions (Hummer et al., 2022). ‘Marshall’, ‘NW 90054-37’, ‘Nyohou’, ‘ORUS 1267-236’, ‘ORUS 740-7’, ‘Puget Beauty’, and ‘Tamella’ displayed similar plant heights in an open-field environment as they were in the provided indoor environment. However, the plants of ‘Mara des Bois’, ‘Melody’, and ‘Miyazaki’ in this study were 2–5 times as tall as those grown in an open-field environment (Hummer et al., 2022). It is thus very important to consider variations of strawberry morphology when changing growing environments/systems.
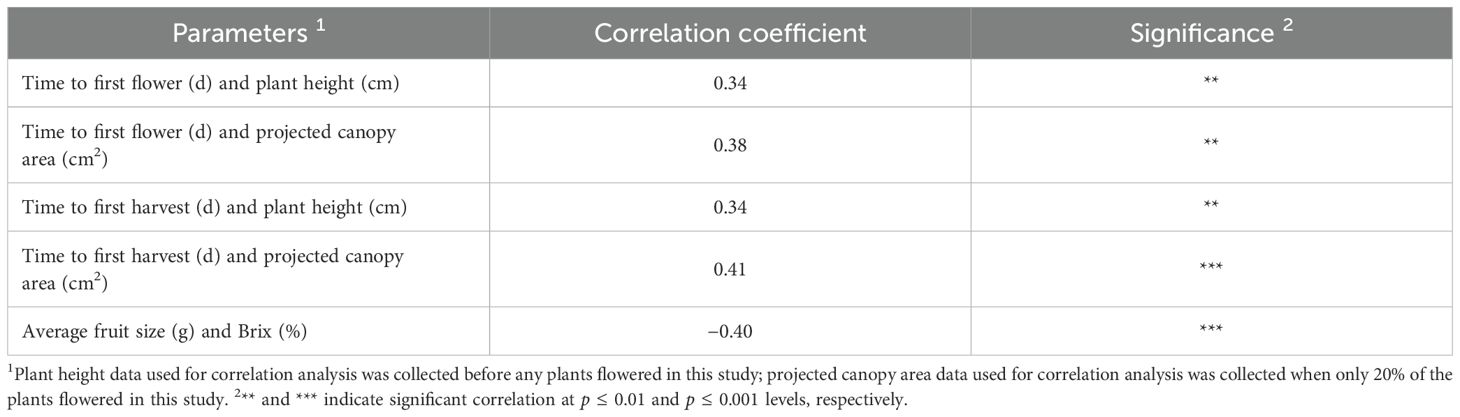
3.5 Correlations among productivity, fruit quality, and plant size characteristics
Understanding the relationship among productivity, fruit quality and plant morphological characteristics of 23 strawberry cultivars can provide information to assist in the improvement of strawberry production. Our results showed that the amount of time to produce the first flower and first harvest from transplanting correlated positively with plant height and projected canopy area (Table 7), indicating that larger plants took a longer time to produce flowers and fruit. This was likely because the plants allocated more photoassimilates to support vegetative growth than promoting the development of reproductive organs. Even though we did not directly measure plant biomass, plant size-related parameters (e.g., canopy area and plant height) have been shown to be highly correlated with strawberry plant dry biomass (Abd-Elrahman et al., 2020; Guan et al., 2020). A similar correlation between vegetative growth and earliness of production was observed in an open-field study with nine strawberry genotypes (Shaw, 1993). A phylogenetic comparative study analyzing 93 perennial herbs from the temperate regions of the Northern Hemisphere also suggests that plant height correlates positively with flower onset time (Bolmgren and Cowan, 2008). These results demonstrated a competitive relationship between plant vegetative growth and reproductive development, although no correlation was identified between plant size and productivity in this study (data not shown).
A negative correlation was identified between average fruit size and Brix (Table 7), similar to what was seen in apple (Malus × domestica) and guava (Psidium guajava), indicating that larger fruit has lower soluble sugar content (De Salvador et al., 2006; Thaipong and Boonprakob, 2006). Reducing irrigation in tomato cultivation reduces fruit size but increases the content of total soluble solids (Chen et al., 2014; Wang and Xing, 2017). However, due to the different compositions of sugars in strawberry cultivars, further studies focusing on the analysis of specific soluble sugars and fruit sensory evaluation can help us better understand the relationships between fruit size, fruit sweetness, and consumer perception. Interestingly, there was no correlation between strawberry productivity parameters (average weekly yield and maximum weekly yield) and any of the fruit quality traits characterized in this study (data not shown), challenging the commonly held belief in the negative relationship between crop yield and quality (Cockerton et al., 2021). Future research in improving controlled environment strawberry production can focus on the enhancement of fruit quality without compromising crop productivity.
3.6 Challenge of dormancy in strawberry indoor farming
The majority of the cultivars evaluated in this study required SD conditions to induce flowering. However, SD conditions also induce semi-dormancy in strawberry plants. Semi-dormancy is a state of plant when plant growth is strongly inhibited but not completely ceased (Sønsteby and Heide, 2011). The symptoms of semi-dormancy include stunted growth, compacted shoot canopy, and reduced flowering and fruit productivity (Bodson and Verhoeven, 2005; Sønsteby and Heide, 2011, Sønsteby and Heide, 2021). After prolonged exposure to SD in this study, all cultivars of strawberry plants showed typical semi-dormancy symptoms. To recover plant vigor and productivity, LD extension lighting was introduced 12 weeks after transplanting. Plant sensitivity to dormancy-inducing SD photoperiod was quantified based on the percentage difference of plant height under SD and LD conditions (Table 8). Twenty-three strawberry cultivars were classified into four different categories using cluster analysis: extra high, high, medium, and low sensitivity to dormancy-inducing SD (Table 8). The cultivars that were most sensitive to SD photoperiod were ‘Benton’, ‘Earliglow’, ‘Elsanta’, ‘Honeoye’, ‘Hood’, and ‘Miyazaki’ (Table 8). ‘Chandler’, ‘Dover’, ‘Mara des Bois’, and ‘Perle de Prague’ showed the lowest sensitivity to SD (Table 8). Solutions to prevent semi-dormancy symptoms include alternation of SD and LD photoperiods (as used in this study), chilling, and cultivation of cultivars insensitive to a dormancy-inducing SD environment (Hytönen and Kurokura, 2020; Melke, 2015; Vince-Prue et al., 1976). Our classification of the 23 strawberry cultivars based on their sensitivity to dormancy-inducing SD photoperiod can provide information to assist in production management in strawberry indoor farming.
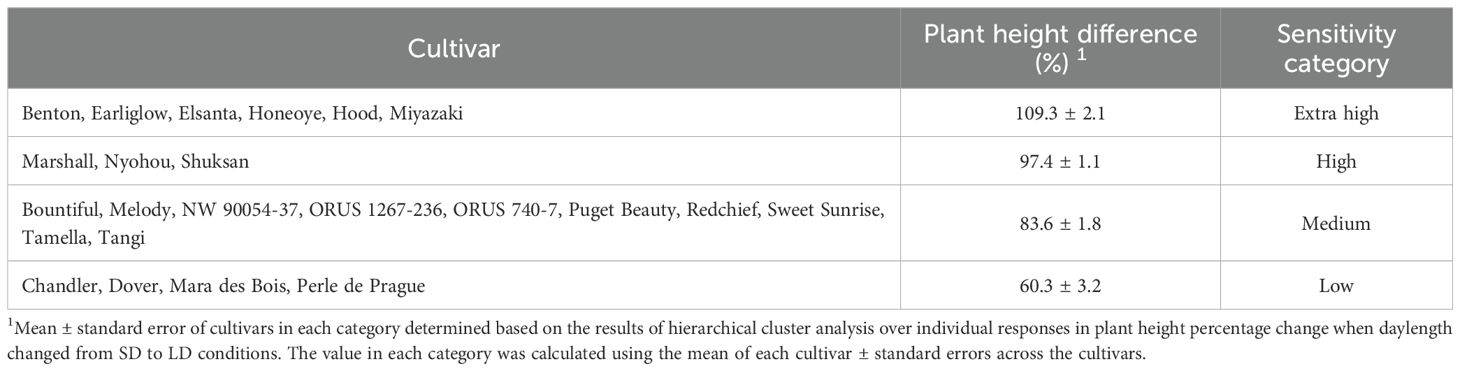
Table 8. Classification of 23 strawberry cultivars in an indoor environment based on sensitivity to dormancy-inducing short-day photoperiod, quantified by the percentage change of plant height from short-day (SD) to long-day (LD) conditions.
4 Conclusion
By examining the growth, morphology, productivity, and fruit quality of 23 strawberry cultivars in a fully controlled indoor environment with sole source electric lighting, significant variations of phenotypes relevant to indoor production were observed among different cultivars, suggesting the importance of cultivar selection for indoor farming. Among the strawberry cultivars evaluated in this study, ‘Mara des Bois’ with characteristics suitable for indoor cultivation with sole source electric lighting was recommended. Analysis of fruit quality, including fruit size, shape, color, firmness, TSS (Brix), and TA can assist indoor strawberry growers in cultivar selection based on market preference. The information provided in this study can support breeders in the development of new cultivars suitable for indoor vertical farm production. Information on plant size can aid in the design of vertical farms. Correlations linked fruit earliness with plant size characteristics, demonstrating crop dynamics in photoassimilate allocation between vegetative growth and fruit production. No correlation was found between strawberry productivity and quality, suggesting that future research can focus on improving both crop productivity and fruit quality by optimizing environmental conditions in indoor strawberry production.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: Controlled Environment Agriculture Open Data, ceaod.github.io.
Author contributions
YL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CKi: Investigation, Methodology, Software, Validation, Writing – review & editing. NB: Conceptualization, Funding acquisition, Resources, Writing – review & editing. JO: Conceptualization, Funding acquisition, Resources, Writing – review & editing. MH: Conceptualization, Funding acquisition, Resources, Writing – review & editing. CKu: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research reported in this publication was supported in part by the Foundation for Food and Agriculture Research (FFAR) grant number 21-000051.
Acknowledgments
We are grateful to Mark Kroggel for his assistance in establishing the irrigation and sensor systems in the growth chamber, his guidance in developing an integrated pest management plan for the production study, and his expertise in managing strawberry crops in controlled environments. We thank Pooja Tripathi for rooting and maintaining the strawberry transplants and Alex Davis for assisting with plant maintenance, fruit harvest, and data collection. We also thank Matt Chrusciel for facilitating the fruit firmness measurement. We appreciate James Strange and Morgan Christman for providing bumble bee micro-colonies to support flower pollination. We are thankful to David Francis and Jonathan Fresnedo Ramirez for reviewing the preliminary drafts of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the Foundation for Food and Agriculture Research.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fhort.2025.1621763/full#supplementary-material
References
Abd-Elrahman A., Guan Z., Dalid C., Whitaker V., Britt K., Wilkinson B., et al. (2020). Automated canopy delineation and size metrics extraction for strawberry dry weight modeling using raster analysis of high-resolution imagery. Remote Sens. 12, 3632. doi: 10.3390/rs12213632
Aoki K. and Akai K. (2023). A comparison between Spain and Japan with respect to the color, expected taste scale, and sustainability of strawberries: A choice experiment. Food Qual. Prefer. 103, 104671. doi: 10.1016/j.foodqual.2022.104671
Archbold D. D. and Strang J. G. (1986). Effect of BA on growth and yield of ‘Redchief’ Strawberry. HortScience. 21, 1377–1379. doi: 10.21273/HORTSCI.21.6.1377
Ariza M. T., Soria C., and Martínez-Ferri E. (2015). Developmental stages of cultivated strawberry flowers in relation to chilling sensitivity. AoB Plants 7, plv012. doi: 10.1093/aobpla/plv012
Banerjee C. and Adenaeuer L. (2014). Up, up and away! The economics of vertical farming. J. Agric. Stud. 2, 40–60. doi: 10.5296/jas.v2i1.4526
Bhat R., Geppert J., Funken E., and Stamminger R. (2015). Consumers perceptions and preference for strawberries—A case study from Germany. Int. J. Fruit Sci. 15, 405–424. doi: 10.1080/15538362.2015.1021408
Bodson M. and Verhoeven B. (2005). Characteristics of dormancy of June-bearing strawberry (Fragaria × ananassa Duch. cv. Elsanta). Int. J. Fruit Sci. 5, 51–58. doi: 10.1300/J492v05n01_05
Bolmgren K. and Cowan P. D. (2008). Time–size tradeoffs: A phylogenetic comparative study of flowering time, plant height and seed mass in a north-temperate flora. Oikos. 117, 424–429. doi: 10.1111/j.2007.0030-1299.16142.x
Cantwell M. and Suslow T. (2002). Lettuce, crisphead: recommendations for maintaining postharvest quality. Available online at: https://postharvest.ucdavis.edu/produce-facts-sheets/strawberry (Accessed January 28, 2025).
Chen J., Kang S., Du T., Guo P., Qiu R., Chen R., et al. (2014). Modeling relations of tomato yield and fruit quality with water deficit at different growth stages under greenhouse condition. Agric. Water Manage. 146, 131–148. doi: 10.1016/j.agwat.2014.07.026
Chiomento J. L. T., Júnior E. P. L., D’Agostini M., De Nardi F. S., dos Santos Trentin T., Dornelles A. G., et al. (2021). Horticultural potential of nine strawberry cultivars by greenhouse production in Brazil: A view through multivariate analysis. Sci. Hortic. 279, 109738. doi: 10.1016/j.scienta.2020.109738
Cockerton H. M., Karlström A., Johnson A. W., Li B., Stavridou E., and Harrison R. J. (2021). Genomic informed breeding strategies for strawberry yield and fruit quality traits. Front. Plant Sci. 12, 724847. doi: 10.3389/fpls.2021.724847
Colquhoun T. A., Levin L. A., Moskowitz H. R., Whitaker V. M., Clark D. G., and Folta K. M. (2012). Framing the perfect strawberry: an exercise in consumer-assisted selection of fruit crops. J. Berry Res. 2, 45–61. doi: 10.3233/JBR-2011-027
De Salvador F. R., Fisichella M., and Fontanari M. (2006). Correlations between fruit size and fruit quality in apple trees with high and standard crop load levels. J. Fruit Ornam. Plant Res. 14, 113.
Finn C. E., Strik B. C., Yorgey B. M., Mackey T. A., Moore P. P., Dossett M., et al. (2014). ‘Sweet sunrise’ Strawberry. HortScience 49, 1088–1092. doi: 10.21273/HORTSCI.49.8.1088
Fletcher J. M., Tatsiopoulou A., Hadley P., Davis F. J., and Henbest R. G. C. (2002). Growth, yield and development of strawberry cv. ‘Elsanta’ under novel photoselective film clad greenhouses. In Proceedings of the XXVI International Horticultural Congress: Protected Cultivation 2002: In Search of Structures, Systems and Plant Materials for Sustainable Greenhouse Production. Toronto, Canada, 11 August 2002, Papadopoulos A. P. Eds., International Society for Horticultural Science: Toronto, Canada 633, pp. 99–106.
Folta K. M. (2019). Breeding new varieties for controlled environments. Plant Biol. 21, 6–12. doi: 10.1111/plb.2019.21.issue-S1
Food and Agriculture Organization of the United Nations (FAO) Data from 2022 strawberry production and value.2022 Available online at: http://www.fao.org/faostat/en/data (Accessed January 28, 2025).
Guan Z., Abd-Elrahman A., Fan Z., Whitaker V. M., and Wilkinson B. (2020). Modeling strawberry biomass and leaf area using object-based analysis of high-resolution images. ISPRS J. Photogramm. Remote Sens. 163, 171–186. doi: 10.1016/j.isprsjprs.2020.02.021
Hernández-Martínez N. R., Blanchard C., Wells D., and Salazar-Gutiérrez M. R. (2023). Current state and future perspectives of commercial strawberry production: A review. Sci. Hortic. 312, 111893. doi: 10.1016/j.scienta.2023.111893
Hiwasa-Tanase K. and Ezura H. (2016). Molecular breeding to create optimized crops: From genetic manipulation to potential applications in plant factories. Front. Plant Sci. 7, 165199. doi: 10.3389/fpls.2016.00539
Hokansan S. C. and Finn C. E. (2000). Strawberry cultivar use in North America. HortTechnology. 10, 94–106. doi: 10.21273/HORTTECH.10.1.94
Hummer K. E., Bassil N. V., Zurn J. D., and Amyotte B. (2022). Phenotypic characterization of a strawberry (Fragaria × ananassa Duchesne ex Rosier) diversity collection. Plants People Planet. 5, 209–224. doi: 10.1002/ppp3.10316
Hytönen T. and Kurokura T. (2020). Control of flowering and runnering in strawberry. Hortic. J. 89, 96–107. doi: 10.2503/hortj.UTD-R011
Jayasena V. and Cameron I. (2008). °Brix/acid ratio as a predictor of consumer acceptability of Crimson Seedless tab le grapes. J. Food Qual. 31, 736–750. doi: 10.1111/j.1745-4557.2008.00231.x
Ketel E., van Hoogdalem M., and Janse J. (2024). The added value of indoor products: the strawberry case. (Report/Stichting Wageningen Research, Wageningen Plant Research, Business Unit Greenhouse Horticulture; No. WPR-1322). Wageningen Plant Res.
Kim C. and van Iersel M. W. (2023). Image-based phenotyping to estimate anthocyanin concentrations in lettuce. Front. Plant Sci. 14,1155722. doi: 10.3389/fpls.2023.1155722
Kouloumprouka Zacharaki A., Monaghan J. M., Bromley J. R., and Vickers L. H. (2024). Opportunities and challenges for strawberry cultivation in urban food production systems. Plants People Planet. 6, 611–621. doi: 10.1002/ppp3.10475
Krüger E., Josuttis M., Nestby R., Toldam-Andersen T. B., Carlen C., and Mezzetti B. (2012). Influence of growing conditions at different latitudes of Europe on strawberry growth performance, yield and quality. J. Berry Res. 2, 143–157. doi: 10.3233/JBR-2012-036
Magwaza L. S. and Opara U. L. (2015). Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. 184, 179–192. doi: 10.1016/j.scienta.2015.01.001
Mathey M. M., Mookerjee S., Gündüz K., Hancock J. F., Iezzoni A. F., Mahoney L. L., et al. (2013). Large-scale standardized phenotyping of strawberry in RosBREED. J. Am. Pomol. Soc 67, 205–216.
Mathey M. M., Mookerjee S., Mahoney L. L., Gündüz K., Rosyara U., Hancock J. F., et al. (2017). Genotype by environment interactions and combining ability for strawberry families grown in diverse environments. Euphytica. 213, 112. doi: 10.1007/s10681-017-1892-6
Melke A. (2015). The physiology of chilling temperature requirements for dormancy release and bud-break in temperate fruit trees grown at mild winter tropical climate. J. Plant Stud. 4, 110–156. doi: 10.5539/jps.v4n2p110
Moore P. P. (2001). Firmness and drained weight of fruit of 19 strawberry clones. HortScience 36, 116–117. doi: 10.21273/HORTSCI.36.1.116
Moreira A. F. P., de Resende J. T. V., Shimizu G. D., Hata F. T., do Nascimento D., Oliveira L. V. B., et al. (2022). Characterization of strawberry genotypes with low chilling requirement for cultivation in tropical regions. Scientia Hortic. 292, 110629. doi: 10.1016/j.scienta.2021.110629
Nadalini S., Zucchi P., and Andreotti C. (2017). Effects of blue and red LED lights on soilless cultivated strawberry growth performances and fruit quality. Eur. J. Hortic. Sci. 82, 12–20. doi: 10.17660/eJHS.2017/82.1.2
Oliver P., Cicerale S., Pang E., and Keast R. (2018). Identifying key flavors in strawberries driving liking via internal and external preference mapping. J. Food Sci. 83, 1073–1083. doi: 10.1111/jfds.2018.83.issue-4
Ordidge M., García-Macías P., Battey N. H., Gordon M. H., John P., Lovegrove J. A., et al. (2012). Development of colour and firmness in strawberry crops is UV light sensitive, but colour is not a good predictor of several quality parameters. J. Sci. Food Agric. 92, 1597–1604. doi: 10.1002/jsfa.v92.8
Paparozzi E. T., Meyer G. E., Schlegel V., Blankenship E. E., Adams S. A., Conley M. E., et al. (2018). Strawberry cultivars vary in productivity, sugars and phytonutrient content when grown in a greenhouse during the winter. Sci. Hortic. 227, 1–9. doi: 10.1016/j.scienta.2017.07.048
Predieri S., Lippi N., and Daniele G. M. (2021). “What can we learn from consumers’ perception of strawberry quality?,” in Proceedings of the international strawberry symposium IX, rimini, Italy, 1–5 may 2021, vol. 1309 . Eds. Mezzetti B., Battino M., and Baruzzi G. (International Society for Horticultural Science, Leuven, Belgium), 987–994.
R Core Team (2023). “R: A language and environment for statistical computing,” in R foundation for statistical computing(Vienna, Austria). Available at: https://www.R-project.org.
Richardson M. L., Arlotta C. G., and Lewers K. S. (2022). Yield and nutrients of six cultivars of strawberries grown in five urban cropping systems. Sci. Hortic. 294, 110775. doi: 10.1016/j.scienta.2021.110775
Rosati P., Dradi G., and Faedi W. (1988). “Genotype-environment relation affecting the ripening time in strawberry,” in Proceedings of the international strawberry symposium, cesena, Italy, 22–27 may 1988, vol. 256 . Eds. Galletta G. J., Maas J. L., and Rosati P. (International Society for Horticultural Science, Leuven, Belgium), 113–122.
Samtani J. B., Rom C. R., Friedrich H., Fennimore S. A., Finn C. E., Petran A., et al. (2019). The status and future of the strawberry industry in the United States. HortTechnology 29, 11–24. doi: 10.21273/HORTTECH04135-18
Schwieterman M. L., Colquhoun T. A., Jaworski E. A., Bartoshuk L. M., Gilbert J. L., Tieman D. M., et al. (2014). Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PloS One 9, e88446. doi: 10.1371/journal.pone.0088446
Shaw D. V. (1993). Genetic correlations between vegetative growth traits and productivity at different within-season intervals for strawberries (Fragaria × ananassa). Theor. Appl. Genet. 85, 1001–1009. doi: 10.1007/BF00215040
Shin Y., Ryu J. A., Liu R. H., Nock J. F., and Watkins C. B. (2008). Harvest maturity, storage temperature and relative humidity affect fruit quality, antioxidant contents and activity, and inhibition of cell proliferation of strawberry fruit. Postharvest Biol. Technol. 49, 201–209. doi: 10.1016/j.postharvbio.2008.02.008
Simpson D. (2018). “The economic importance of strawberry crops,” in The genomes of rosaceous berries and their wild relatives, 1st ed, vol. 2018 . Eds. Hytönen T., Graham J., and Harrison R. (Springer, Cham, Switzerland), 1–7.
Sønsteby A. and Heide O. M. (2011). Environmental regulation of dormancy and frost hardiness in Norwegian populations of wood strawberry (Fragaria vesca L.). Eur. J. Plant Sci. Biotechnol. 5, 42–48.
Sønsteby A. and Heide O. M. (2021). Dynamics of dormancy regulation in ‘Sonata’ strawberry and its relation to flowering and runnering. CABI Agric. Biosci. 2, 4. doi: 10.1186/s43170-021-00026-x
Stahler M. M., Lawrence F. J., Moore P. P., Martin L. W., Varseveld G. W., and Sheets W. A. (1994). ‘Redgem’ and ‘Bountiful’ strawberries. HortScience 29, 1203–1205. doi: 10.21273/HORTSCI.29.10.1203
Thaipong K. and Boonprakob U. (2006). Repeatability, optimal sample size of measurement and phenotypic correlations of quantitative traits in guava. Kasetsart J. Nat. Sci. 40, 11–19.
U.S. Department of Agriculture (USDA) Census of agriculture: 2019 census of horticultural specialties.2019 Available online at: https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/Census_of_Horticulture_Specialties/HORTIC.pdf (Accessed January 22, 2025).
U.S. Department of Agriculture (USDA) Census of agriculture: 2009 census of horticultural specialties.2009 Available online at: https://agcensus.library.cornell.edu/wp-content/uploads/2007-Census-of-Horticultural-Specialties-HORTIC.pdf (Accessed January 22, 2025).
U.S. Department of Agriculture (USDA) (2022). Census of agriculture. Available online at: https://www.nass.usda.gov/Publications/AgCensus/2022/Full_Report/Volume_1,_Chapter_1_US/usv1.pdf (Accessed January 22, 2025).
van Delden S. H., SharathKumar M., Butturini M., Graamans L. J. A., Heuvelink E., Kacira M., et al. (2021). Current status and future challenges in implementing and upscaling vertical farming systems. Nat. Food. 2, 944–956. doi: 10.1038/s43016-021-00402-w
Vince-Prue D., Guttridge C. G., and Buck M. W. (1976). Photocontrol of petiole elongation in light-grown strawberry plants. Planta. 131, 109–114. doi: 10.1007/BF00389978
Wang S. Y. and Camp M. J. (2000). Temperatures after bloom affect plant growth and fruit quality of strawberry. Sci. Hortic. 85, 183–199. doi: 10.1016/S0304-4238(99)00143-0
Wang X. and Xing Y. (2017). Evaluation of the effects of irrigation and fertilization on tomato fruit yield and quality: A principal component analysis. Sci. Rep. 7, 350. doi: 10.1038/s41598-017-00373-8
Wortman S. E., Douglass M. S., and Kindhart J. D. (2016). Cultivar, growing media, and nutrient source influence strawberry yield in a vertical, hydroponic, high tunnel system. HortTechnology 26, 466–473. doi: 10.21273/HORTTECH.26.4.466
Wu F., Guan Z., and Whidden A. (2018). An overview of the US and Mexico strawberry industries. EDIS., FE971. doi: 10.32473/edis-fe1014-2017
Wu X., Han W., Yang Z., Zhang Y., and Zheng Y. (2021). The difference in temperature between day and night affects the strawberry soluble sugar content by influencing the photosynthesis, respiration and sucrose phosphatase synthase. Hortic. Sci. 48, 174–182. doi: 10.17221/169/2020-HORTSCI
Yoshioka Y., Nakayama M., Noguchi Y., and Horie H. (2013). Use of image analysis to estimate anthocyanin and UV-excited fluorescent phenolic compound levels in strawberry fruit. Breed. Sci. 63, 211–217. doi: 10.1270/jsbbs.63.211
Zhang L., Strik B. C., and Martin L. W. (1992). Date of renovation affects fall and summer fruiting of June-bearing strawberry. Adv. Strawberry Res. 11, 47–50.
Keywords: controlled environment agriculture (CEA), Fragaria × ananassa, hydroponics, plant factory, soilless cultivation
Citation: Lin Y, Kim C, Bassil NV, Oliphant JM, Hardigan MA and Kubota C (2025) Characterizing the growth, morphology, productivity, and fruit quality of twenty-three strawberry cultivars in an indoor environment with sole source electric lighting. Front. Hortic. 4:1621763. doi: 10.3389/fhort.2025.1621763
Received: 01 May 2025; Accepted: 04 June 2025;
Published: 26 June 2025.
Edited by:
Li Zhang, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Genhua Niu, Texas A&M University, United StatesWen He, Sichuan Agricultural University, China
Copyright © 2025 Lin, Kim, Bassil, Oliphant, Hardigan and Kubota. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chieri Kubota, a3Vib3RhLjEwQG9zdS5lZHU=
 Yiyun Lin
Yiyun Lin Changhyeon Kim
Changhyeon Kim Nahla V. Bassil
Nahla V. Bassil James M. Oliphant3
James M. Oliphant3 Chieri Kubota
Chieri Kubota