- 1Graduate School of Agricultural and Life Sciences, The University of Tokyo, Nishitokyo, Japan
- 2Department of Clinical Plant Science, Faculty of Biosciences, Hosei University, Tokyo, Japan
Plant factories using artificial lighting are a promising solution to food security and urban agricultural challenges. However, cultivation of fruit-bearing crops such as tomatoes remains limited due to their high light demands, long growth periods, and tall plant structure. In this study, we aimed to develop an efficient cultivation system for tomatoes in a multilayer plant factory. Mini tomatoes were hydroponically cultivated using white LEDs in a five-tier shelf system under two different cultivation methods. The conventional I-shaped method involved vertical growth on the top tier with downward lighting, while the novel S-shaped method trained each plant horizontally across the second to fourth tiers with lateral lighting on each level. The S-shaped method enabled even light distribution, resulting in consistent photosynthetic rates throughout the canopy. In contrast, the I-shaped method suffered from strong light attenuation in the lower tiers, leading to reduced photosynthetic efficiency in shaded parts. Although total yield did not differ significantly between the two methods, the S-shaped method promoted earlier fruit maturation and improved fruit quality, including higher sugar content. Compared with greenhouse cultivation, plant factory conditions ensured stable temperature and lighting, leading to compact plant morphology, shorter internodes, and higher SPAD values. Moreover, fruit quality was more consistent year-round, with higher lycopene and sugar contents. This study demonstrates that the S-shaped cultivation system offers significant advantages in light use efficiency, plant management, and fruit quality. It represents a scalable approach for enhancing tomato production in plant factories and may facilitate the introduction of other high-light-demanding fruit crops into vertical farming systems.
Introduction
According to a prediction by Benke and Tomkins (2017), the global population is expected to reach 9.7 billion. At the same time, increasing shortages of food and water, along with the loss of arable land due to climate change, continue to pose serious threats to human society, underscoring the urgent need to enhance agricultural productivity (Benke and Tomkins, 2017; Al-Kodmany, 2018; Kwon et al., 2020). Simultaneously, consumer demand for safer and healthier agricultural products is steadily growing (Ali et al., 2020). In response, recent advancements in plant factory technologies have gained significant attention and are emerging as a key component of sustainable agriculture (Levine et al., 2023; Hayashi et al., 2024; Saengtharatip et al., 2021).
Plant factories can be broadly classified into two types (Levine et al., 2024). Sunlight-type plant factories primarily utilize natural sunlight, with supplemental lighting provided under low-light conditions. In contrast, artificial light plant factories (ALPFs) rely entirely on artificial lighting sources. Both types use climate control systems (heating and cooling) and hydroponic techniques to ensure stable, year-round production and to minimize the risk of pests and contamination. Their enclosed environments allow for precise control of growth conditions, including light intensity, humidity, CO2 concentration, and nutrient delivery. As a result, plant factories are not affected by external weather or seasonal fluctuations and can achieve consistent yields (Takatsuji, 2010). Furthermore, their vertical space efficiency enables deployment in urban settings such as rooftops and indoor spaces where conventional farmland is unavailable (Al-Kodmany, 2018; Benke and Tomkins, 2017; SharathKumar et al., 2020). Controlled environments also allow for the optimized cultivation of high-value crops to meet consumer needs (Lee et al., 2014; Kang et al., 2016; Olvera-Gonzalez et al., 2021).
However, plant factories face two major challenges. First, they require high and continuous operational costs for lighting, temperature control, and water circulation (Levine et al., 2024). In Japan, although measures such as replacing fluorescent lights with LEDs and shifting lighting periods to nighttime (to take advantage of lower electricity rates) have been implemented, only 50%–60% of facilities reportedly generate a positive net profit (Ministry of Agriculture, Forestry and Fisheries, 2022). Second, plant factories are primarily designed for leafy vegetables, which have relatively low light requirements. The cultivation of fruit-bearing crops remains rare in ALPFs due to their longer growth periods, greater energy demands, and larger spatial requirements (Yoshida et al., 2013). Nevertheless, recent efforts have explored the feasibility of producing strawberries, blueberries, and figs in plant factories (Yoshida et al., 2013; Aung, 2014; Kawamata et al., 2002). This emerging trend toward fruit crop production in plant factories holds great promise for improving food security and achieving sustainable production.
Tomatoes are among the most widely cultivated and consumed vegetables globally, with annual production exceeding 180 million tons according to the FAO (FAOSTAT, 2020). In addition to fresh consumption, tomatoes are processed into sauces, ketchup, soups, and juices. They are also rich in bioactive compounds such as lycopene and gamma-aminobutyric acid (GABA), which have been linked to health benefits (Palozza et al., 2011; Yamakoshi et al., 2007; Ali et al., 2020). However, tomato production is highly sensitive to environmental fluctuations. Temperatures below 14°C or above 26°C can inhibit stem, leaf, and flower development, reducing both yield and fruit quality (Adams, 2001; Shamshiri et al., 2018). Inconsistent or insufficient lighting can further degrade fruit quality, promote excessive vegetative growth, and reduce overall yield (Gómez and Mitchell, 2016; Paucek et al., 2020; López-Díaz et al., 2020; Hwang et al., 2020; Yoshiyama et al., 2024). These issues can be addressed by growing tomatoes in plant factories, where environmental parameters can be tightly controlled.
Currently, tomato cultivation accounts for approximately 65% of production in sunlight-type plant factories. These facilities achieve stable yields even in regions with limited sunlight, such as the UK and Canada, by supplementing with artificial light (Liu et al., 2019). Numerous studies have reported that the use of LEDs as supplemental lighting enhances tomato growth, yield, and fruit quality (Adams, 2001; Gómez and Mitchell, 2016; Tewolde et al., 2016, 2018; Paucek et al., 2020; López-Díaz et al., 2020; Paponov et al., 2020). Nonetheless, tomato cultivation remains uncommon in ALPFs due to the crop’s high light demand and large spatial footprint. While dwarf tomato varieties have been proposed as a solution for vertical space efficiency and improved yield per unit area (Kwon et al., 2020; Kobayashi and Tabuchi, 2022; Ke et al., 2021), studies have shown that such varieties often produce fruit of lower quality compared to standard cultivars (Tran et al., 2021; Anu et al., 2021). Therefore, exploring viable cultivation methods for conventional tomato cultivars in ALPFs remains an important research priority.
In this study, we developed a new multitier cultivation system for tomato production in an ALPF. Unlike conventional vertical string trellising (I-shaped cultivation), our method employs an S-shaped growth strategy, in which tomato plants are guided through a curved, back-and-forth pattern across multiple layers of LED panels. This structured growth architecture enhances light interception, improves space efficiency, and contributes to improved yield and fruit quality in a closed, artificially lit environment.
Results
Environmental parameters of different cultivation methods
Temperature and light intensity were continuously monitored in the greenhouse. Both parameters exhibited rapid fluctuations across different months and throughout the day (Figure 1A). In contrast, within the plant factory, temperature, light intensity, and air humidity were recorded continuously during the cultivation period at various plant levels. The average temperature in the plant factory showed minimal variation both seasonally and diurnally (Figure 1B), and no significant differences in temperature or humidity were detected among the different plant levels in either the I-shaped or S-shaped cultivation systems (Figure 1B). However, significant differences in light intensity were observed among the upper, middle, and lower plant levels under I-shaped cultivation, while such differences were not evident in the S-shaped system (Figure 1B).
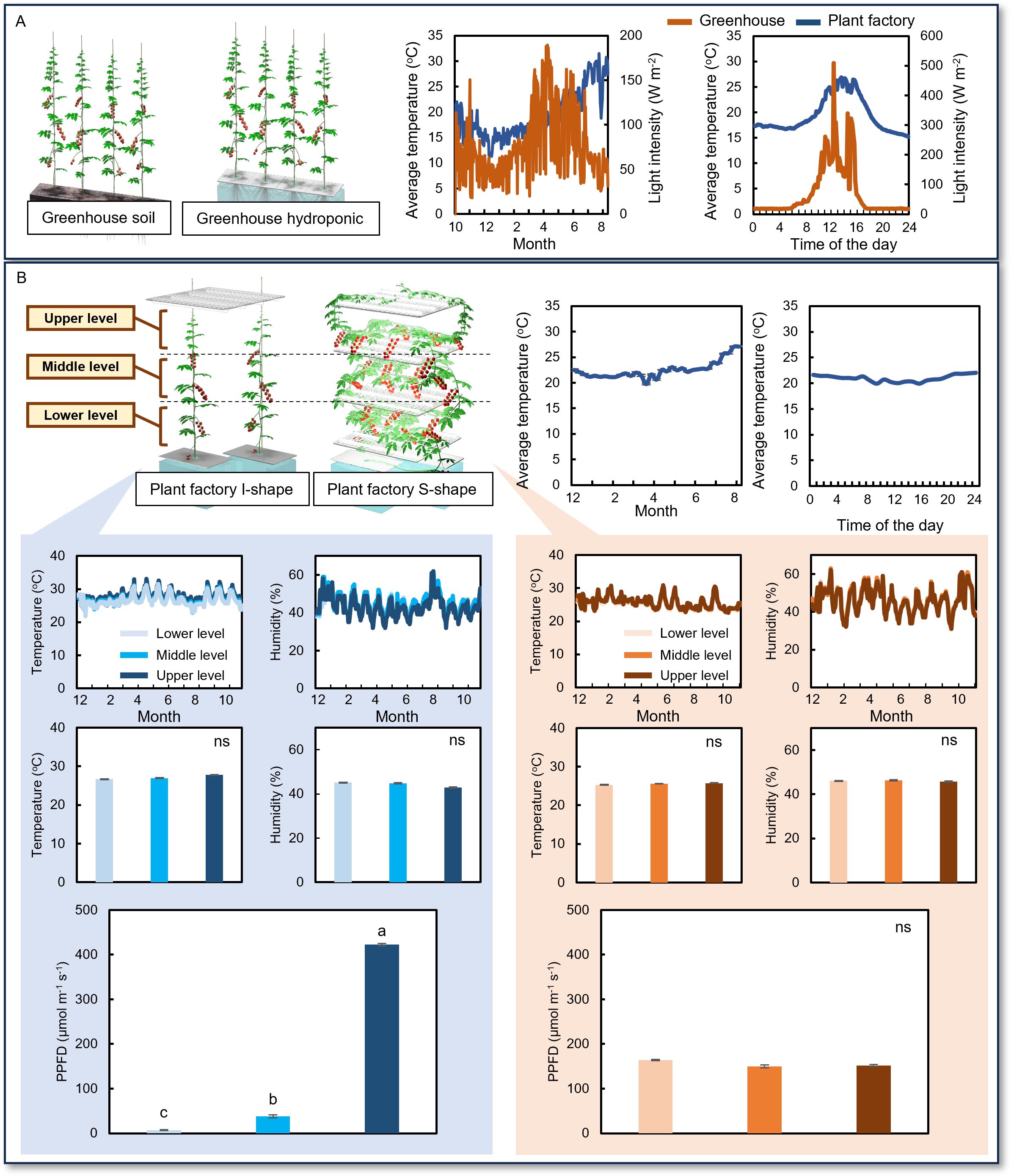
Figure 1. Environmental conditions in greenhouse and plant factory cultivation systems. (A) Average temperature and light intensity in the greenhouse across the entire cultivation period and on a daily basis. (B) Average temperature in the plant factory across the entire cultivation period and on a daily basis. In the plant factory, environmental parameters—temperature, humidity, and light intensity—were recorded separately at the upper, middle, and lower levels of the cultivation racks. Measurements were conducted for both the I-shaped cultivation method, in which plants were trained vertically, and the S-shaped cultivation method, in which plants were trained horizontally along multiple shelf levels to maximize spatial and light use efficiency. Data are mean ± SE. Bars with the same letter are not significantly different; n = 8.
Photosynthetic and growth parameters of plants in different cultivation conditions
Photosynthetic parameters, including electron transport rate (ETR), photochemical quenching (1–qP), and non-photochemical quenching (NPQ), were determined at mature leaves located at the upper, middle, and lower levels across the various cultivation methods.
For greenhouse soil cultivation, clear differences in ETR were observed among the leaf levels: upper leaves exhibited the highest ETR, while lower leaves had the lowest (Figure 2A). In contrast, the 1–qP parameter showed a reverse trend, with lower leaves displaying the highest values and upper leaves the lowest (Figure 2A). However, NPQ did not differ significantly between the various leaf levels (Figure 2A). In greenhouse hydroponic cultivation, the ETR values were similar between the upper and middle leaves, while the lower leaves showed a notably lower ETR (Figure 2A). Similarly, the 1–qP values were higher in the lower leaves, whereas the middle and upper leaves exhibited comparable values (Figure 2A). NPQ remained statistically consistent across all leaf levels in this cultivation method (Figure 2A).
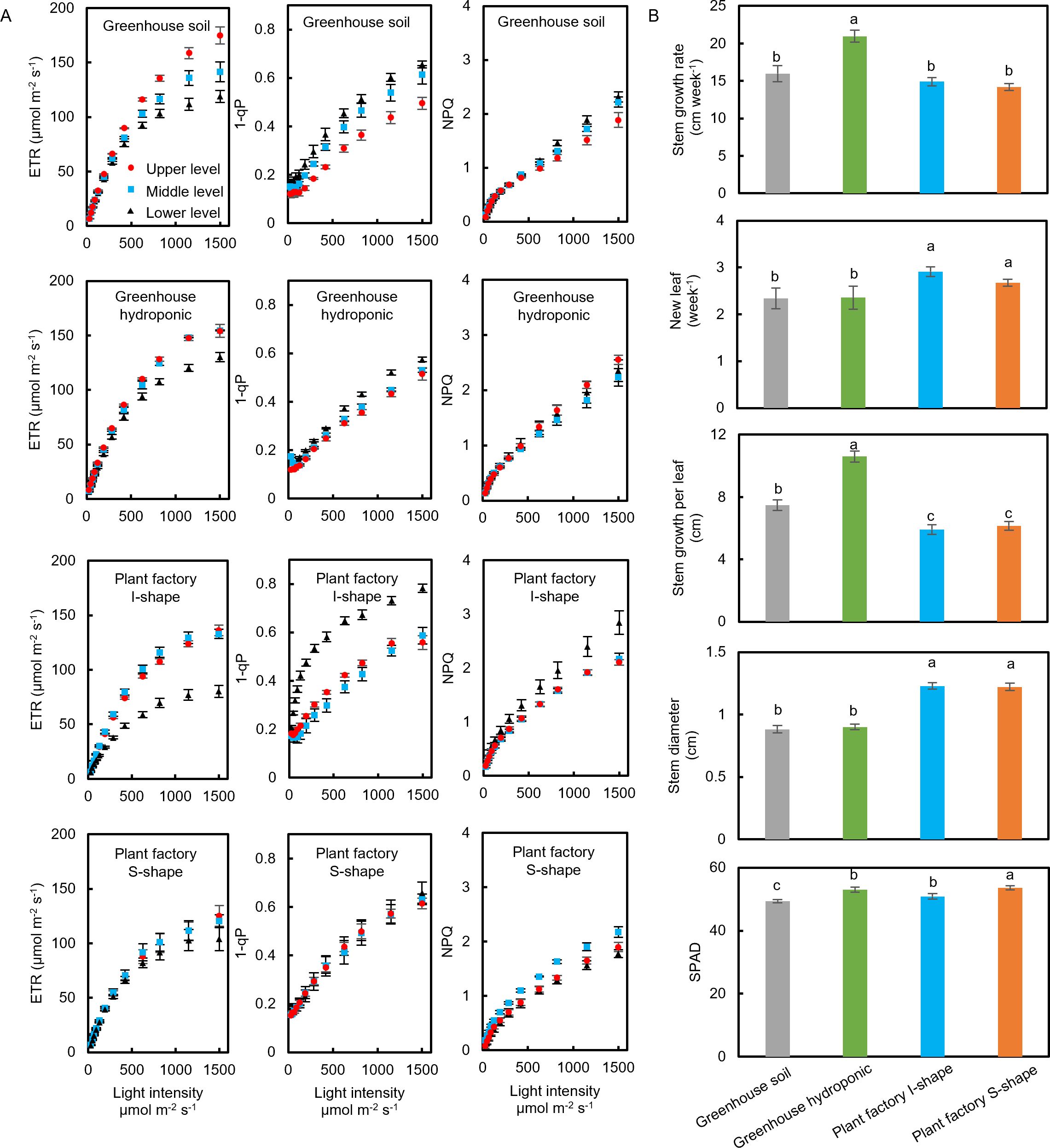
Figure 2. Photosynthetic and growth parameters of tomato plants under different cultivation conditions. (A) Electron transport rate (ETR), photochemical quenching (1–qP), and non-photochemical quenching (NPQ) of tomato plants grown under four cultivation systems: greenhouse soil, greenhouse hydroponics, plant factory with I-shaped cultivation, and plant factory with S-shaped cultivation. (B) Stem elongation rate, number of newly emerged leaves, soil plant analysis development (SPAD) value, and stem diameter of tomato plants cultivated under the same four conditions. Data are mean ± SE. Bars with the same letter are not significantly different; n = 8.
In the plant factory under I-shaped cultivation, the results mirrored those of the greenhouse hydroponic method: the lower-level leaves had a reduced ETR and elevated 1–qP in comparison to the upper and middle leaves, which maintained similar values (Figure 2A). Again, NPQ did not show any significant differences among leaf levels (Figure 2A). Conversely, under plant factory S-shaped cultivation, no substantial differences in ETR or 1–qP were observed among the upper, middle, and lower leaves, although the middle leaves exhibited a slightly higher NPQ compared with the other levels (Figure 2A).
When comparing overall plant growth characteristics among the cultivation methods (Figure 2B), greenhouse hydroponic systems showed the highest stem growth rate. In contrast, the plant factory cultivations (both I-shaped and S-shaped) presented with a higher rate of new leaf emergence and a greater stem diameter than their greenhouse soil and hydroponic counterparts. Consequently, the average distance between the leaves was greatest in the greenhouse hydroponic setup, whereas both plant factory systems had significantly lower interleaf distances. Additionally, the plant factory S-shaped cultivation yielded the highest soil plant analysis development (SPAD) values, while the greenhouse soil cultivation displayed the lowest (Figure 2B).
Productivity and fruit quality parameters of plants in different cultivation conditions
Fruit yield data were collected to evaluate productivity and quality throughout the cultivation period to assess both productivity and fruit quality across all methods. Although no significant differences were found in yield per unit cultivation area, per plant, or harvested cluster per month among the different methods (Figure 3A), both plant factory S-shape and I-shape systems demonstrated a significantly shorter harvest cycle, from the first to the 23rd cluster, compared with the greenhouse cultivations. Among these, the plant factory S-shape system exhibited the shortest harvest cycle (Figure 3A).
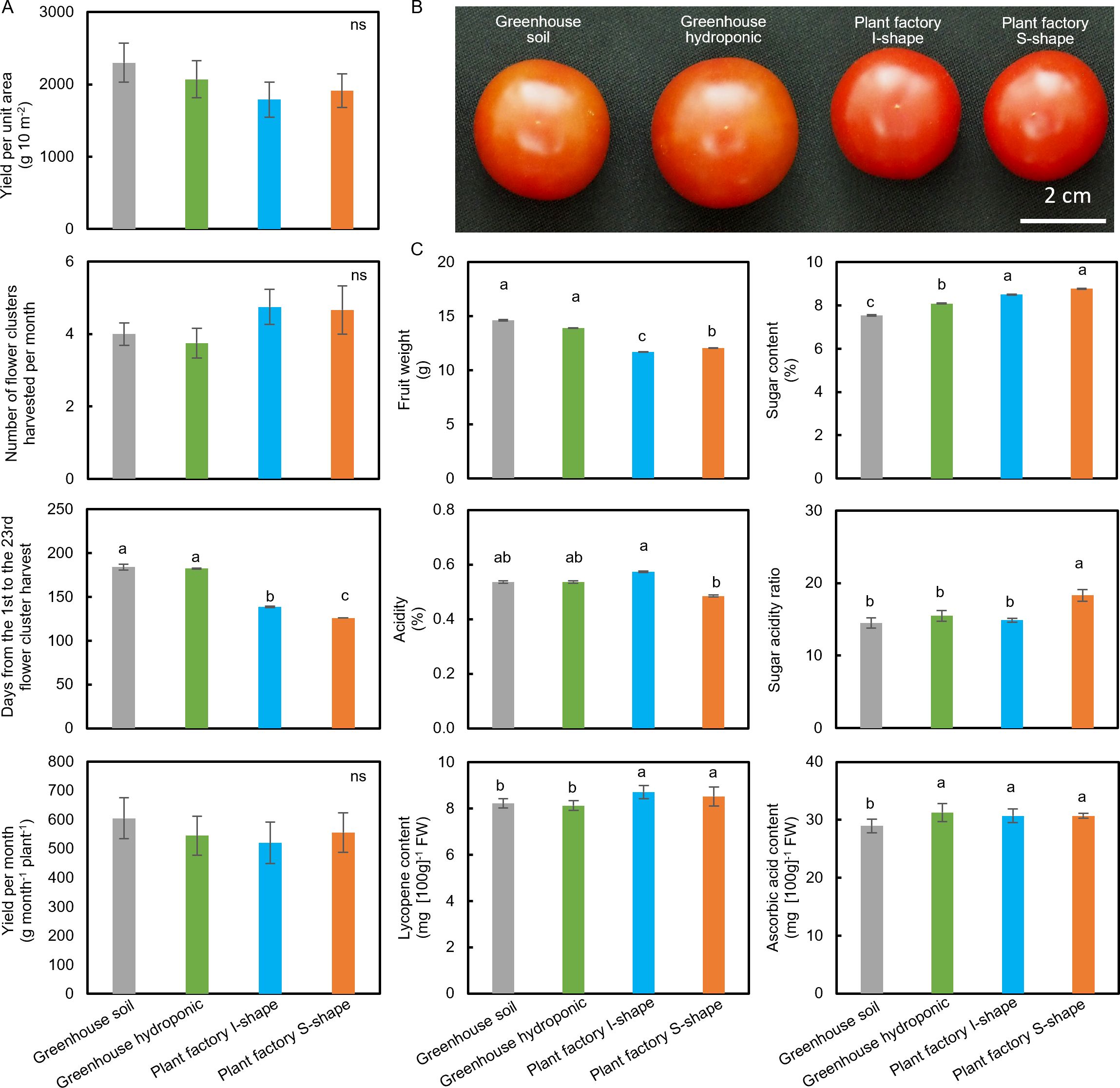
Figure 3. Productivity and fruit quality of tomato plants cultivated under different conditions. (A) Productivity parameters including yield per unit area, number of flower clusters harvested per month, days from the 1st to the 23rd flower cluster harvest, and yield per month per plant across different cultivation systems. (B) Representative images of tomato fruits harvested from each cultivation method. (C) Fruit quality parameters including average fruit weight, acidity, soluble sugar content, lycopene concentration, and ascorbic acid content under each cultivation condition. Data is mean ± SE. Bars with the same letter are not significantly different n = 50 - 90.
In terms of fruit appearance and weight, fruits from plant factory I-shaped and S-shaped systems were smaller and had a deeper red color compared with those from the greenhouse (Figure 3B). Further analysis revealed that fruits harvested from the plant factory systems had significantly lower fruit weights than those from the greenhouse, with the I-shaped system showing the lowest fruit weight overall (Figure 3B).
Regarding fruit quality, plant factory tomatoes exhibited significantly higher sugar content compared with greenhouse tomatoes, with the greenhouse soil method yielding the lowest sugar levels (Figure 3C). Additionally, the plant factory S-shaped system produced tomatoes with the lowest fruit acidity, resulting in the highest sugar–acidity ratio, which is often associated with better flavor, while the other methods showed higher acidity and lower sugar–acidity ratios (Figure 3C). Finally, plant factory tomatoes had significantly higher lycopene content than those from the greenhouse, and ascorbic acid levels were similar across all treatments except for the greenhouse soil cultivation, which had a significantly lower value (Figure 3C).
Key-bioactive compounds within metabolic map
Eight amino acids in the fruit were quantified to assess the effects of different cultivation systems on nutrient composition. Overall, tomatoes grown in the greenhouse exhibited higher amino acid levels, particularly those directly linked to or derived from glycolysis. Within the greenhouse systems, soil cultivation resulted in higher concentrations of phenylalanine and serine, while the hydroponic system produced higher levels of aspartic acid, GABA, glutamic acid, and proline (Figure 4). A notable exception was asparagine: both greenhouse soil and plant factory S-shaped cultivations resulted in elevated asparagine content, whereas greenhouse hydroponic cultivation showed the lowest level among all treatments (Figure 4).
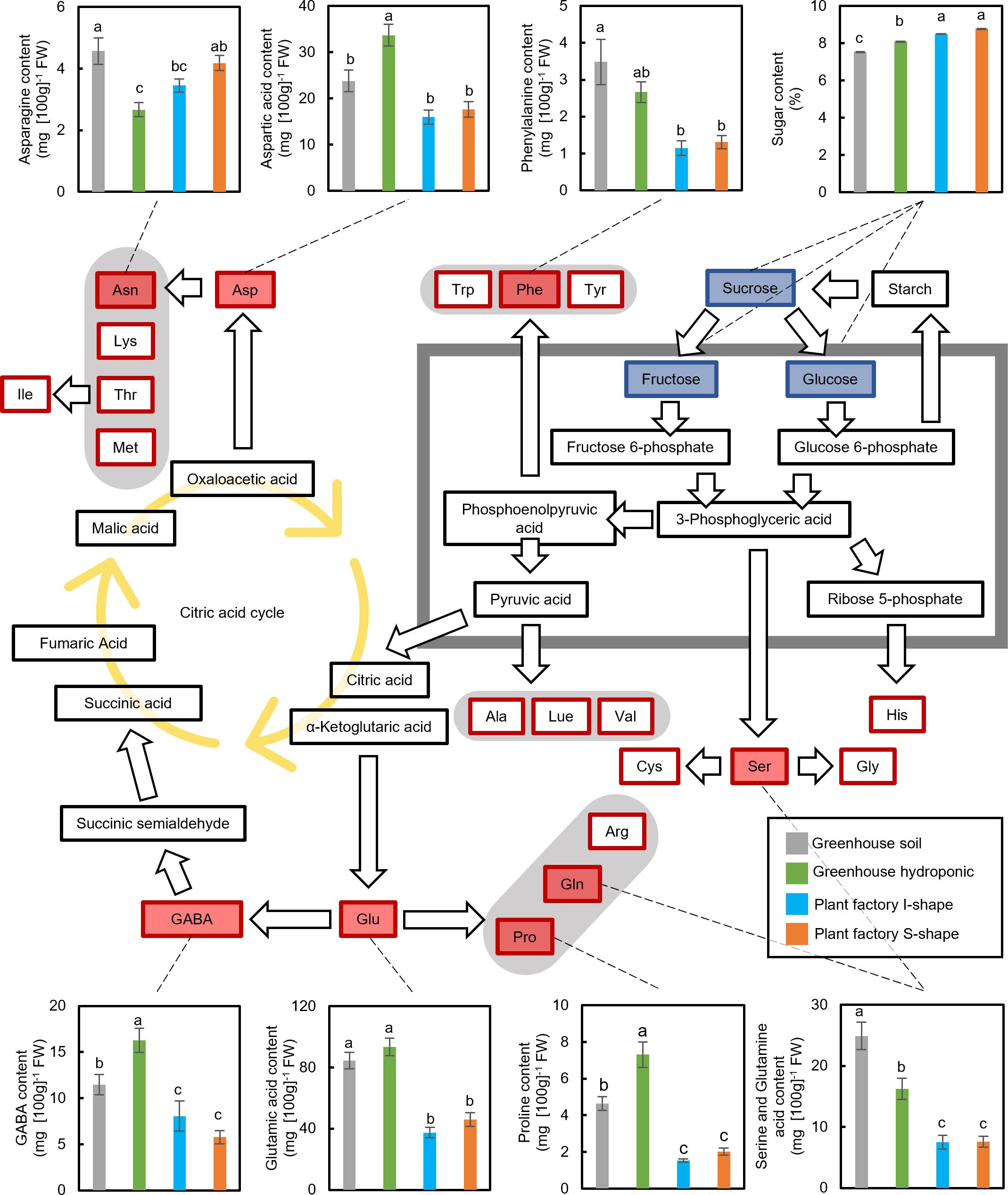
Figure 4. Metabolic map of amino acids and sugars in tomato plants. The contents of amino acids (indicated by red squares) and sugars (indicated by blue squares) were quantified under different cultivation conditions and are shown in the corresponding bar graphs. Data are mean ± SE. Bars with the same letter are not significantly different; n = 50–90.
Discussion
This study aimed to establish a novel tomato cultivation method optimized for closed-type plant factories with artificial lighting. Unlike previous approaches that rely on dwarf cultivars with limited quality (Trien et al., 2021; Anu et al., 2021), we developed a system utilizing high-quality commercial varieties commonly used in greenhouse or open-field cultivation. Two cultivation systems were tested: the conventional vertical “I-shaped” method and a novel space-efficient “S-shaped” system leveraging multilayered cultivation shelves. Compared to greenhouse cultivation, both plant factory methods led to higher inflorescence numbers and increased sugar and lycopene contents, with the S-shaped system further enhancing sugar–acid balance. Generally speaking, plant factory cultivations showed higher growth, ripening speed, and sugar contents, while greenhouse cultivations showed higher amino acid content (Figure 5), which represents interesting trade-off options for people to choose from based on their needs. Our results demonstrate the potential of this cultivation method to improve both yield and fruit quality in plant factories, paving the way for broader application to other fruiting vegetables and even space agriculture.
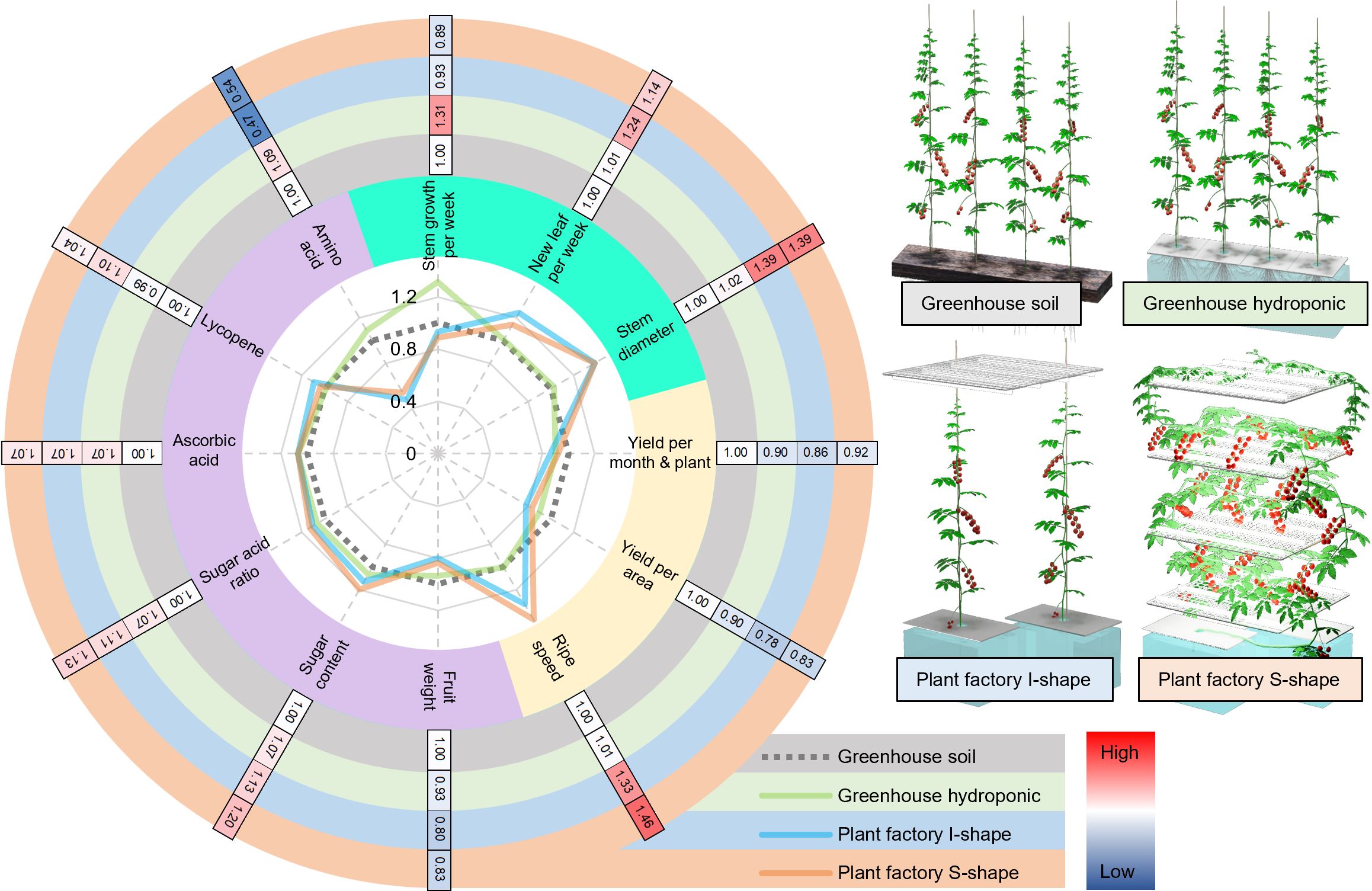
Figure 5. Comparison of key growth, productivity, and fruit quality traits of tomato plants under different cultivation conditions. The radar chart on the left displays relative values of growth parameters (mint green zone), productivity parameters (light yellow zone), and fruit quality parameters (plum zone), normalized against those under greenhouse soil cultivation. The outer heat map shows the actual values for each trait, with colored rings indicating the corresponding cultivation conditions.
Enhanced spatial efficiency and quality stability of tomato via the “S-shaped” system in artificial light-type plant factories
In this study, we demonstrated that a novel S-shaped cultivation system for tomato cultivation in closed-type plant factories under artificial lighting improves plant growth and fruit quality compared to conventional I-shaped systems and greenhouse cultivation. Consistent with previous studies (Adams et al., 2001; Tewolde et al., 2018; Paucek et al., 2020; López-Díaz et al., 2020; Paponov et al., 2020), supplemental lighting in the middle and lower canopy enhanced light use efficiency, resulting in thicker stems, shorter internodes, higher chlorophyll content (SPAD values), and increased inflorescence number (Figure 2B; Supplementary Figure S1). These morphological improvements led to stable fruit development and yield comparable to greenhouse systems, independent of season or weather conditions (Figure 3A; Supplementary Figure S2).
Although our current results showed no significant difference in total yield between the I-shaped and S-shaped cultivation systems, previous studies have reported that increased cumulative light exposure can enhance tomato yield (Palmitessa et al., 2020). Furthermore, since plant productivity is greatly influenced by how light is distributed across individual plants (Zhang et al., 2020; Joshi et al., 2017; Saengtharatip et al., 2021), optimizing both light intensity and its spatial distribution within the S-shaped system may further improve yield in practical applications. In contrast, increasing light intensity at the top of plants in the I-shaped system poses challenges, as it may induce light stress at the shoot apex without effectively enhancing light penetration to the lower canopy. This not only limits the potential for increased productivity but also results in inefficient energy utilization.
The beneficial effect of uniform light intensity on fruit quality has also been reported (Palmitessa et al., 2020). In our current study, high soluble solids content (°Brix) was observed particularly under the S-shaped system (Figure 3C; Supplementary Figures S3, S4), likely due to enhanced photosynthetic activity across the whole plant (Figure 2A; Supplementary Figure S1). This aligns with reports showing that increased light exposure improves sugar accumulation while reducing organic acid levels (Gómez and Mitchell, 2016; Gautier et al., 2009). Although no significant differences in ascorbic acid content were detected among treatments (Figure 3C; Supplementary Figure S5), light exposure to the fruit surface—known to affect ascorbic acid biosynthesis (Gautier et al., 2009; Ntagkas et al., 2019)—could be better optimized in future trials by adjusting leaf arrangement or light positioning.
Lycopene content was higher in plant factory-grown tomatoes than in greenhouse-grown ones (Figure 3C; Supplementary Figures S5A, B), possibly due to the stable light and temperature conditions mitigating the suppressive effects of high fruit surface temperature on lycopene biosynthesis (Helyes et al., 2007; Bianchetti et al., 2020) (Figure 1B; Supplementary Figure S1). However, no significant difference was observed between the I- and S-shaped systems (Figures 3C; Supplementary Figures S5A, B). Future studies using controlled spectral light (e.g., red/blue LEDs) and fresh fruit measurements could further clarify these effects.
Interestingly, the concentrations of key amino acids such as glutamate and GABA were lower in plant factory-grown fruit (Figure 4; Supplementary Figure S5C). One plausible explanation is reduced carbon flux into amino acid biosynthesis due to enhanced sugar retention under controlled lighting. Previous studies have shown that fruit amino acid profiles are sensitive to light intensity, spectral quality, and CO2 levels (Dhakal and Baek, 2014; Ntagkas et al., 2020; Yan et al., 2021), underscoring the need for detailed environmental monitoring around the fruit. It is also noteworthy that the S-shaped cultivation system exhibited faster flower initiation and fruit ripening compared to the I-shaped system (Figure 3A). This acceleration may be attributed to more uniform light distribution or physiological responses triggered by mechanical stress during plant cultivation (Takeno et al., 2016; Cho et al., 2017).
Overall, our findings suggest that the S-shaped cultivation method, by maximizing spatial and light use efficiency, not only improves yield and sugar content but also offers a promising strategy for stable, high-quality tomato production in vertical farming systems and potential applications in resource-limited environments such as space agriculture. Our future studies will focus on optimizing light and nutrient conditions to enhance amino acid accumulation in tomatoes cultivated in artificial light plant factory systems. Such investigations will be important for improving the overall nutritional profile of tomatoes grown in controlled environments.
Environmental and physiological factors affecting fruit quality under artificial lighting conditions
Machine learning models using environmental variables—such as radiation, temperature, humidity, CO2 concentration, and leaf area—have demonstrated the ability to predict fruit quality attributes like soluble solids and glutamate content regardless of tomato cultivar or origin (Xiang et al., 2022; Yoshida et al., 2020). In this study, decision tree regression analysis incorporating plant growth parameters and environmental data revealed that fruit quality traits were primarily influenced by stem diameter and SPAD values (Supplementary Figure S6). Specifically, soluble solids were correlated with stem diameter and SPAD (R² = 0.34), ascorbic acid with SPAD and internode length (R² = 0.31), and glutamate and aspartate with stem diameter (R² = 0.64 and 0.51, respectively) (Supplementary Figure S6).
These findings suggest that improved photosynthetic activity in leaves, facilitated by stable light conditions in plant factories, enhances sugar accumulation, as previously reported (Gómez and Mitchell, 2016; Paucek et al., 2020). Furthermore, fruit-localized light sensing through phytochrome has been shown to suppress starch synthesis and promote sugar accumulation in tomato fruits (Ernesto Bianchetti et al., 2018), emphasizing the potential importance of direct fruit irradiation.
Our analysis also indicated that environmental variables such as cumulative temperature and radiation were strong predictors of plant growth (Supplementary Figure S6), particularly SPAD values and stem thickness (R² = 0.86). However, several fruit metabolites, such as organic acids and lycopene, were not well explained by plant growth traits alone. Previous studies have demonstrated that lycopene accumulation is enhanced by red and blue light (Xie et al., 2019; Zhang et al., 2020) and reduced by elevated fruit surface temperatures (Helyes et al., 2007), highlighting the dual influence of light quality and heat. To directly assess the effect of fruit irradiation, we conducted a light-shielding experiment using neutral density filters (Supplementary Figure S7). While fruit surface temperature was only marginally affected (Supplementary Figures S7A, B), both ascorbic acid concentration and lycopene content significantly increased in fruits exposed to higher light levels (Supplementary Figure S7C). These results provide experimental evidence that light exposure to fruit tissue itself, not just to leaves, is a critical factor for promoting the biosynthesis of health-promoting compounds such as antioxidants and carotenoids.
In conclusion, integrating light distribution models that account for both leaf and fruit exposure may enhance the optimization of controlled environment tomato production systems. Our findings underscore the potential of artificial light-based cultivation not only to stabilize yield but also to improve functional quality, which is particularly relevant for plant factories aiming for high-value crop production.
Yield maximization through sequential planting in vertical S-shaped cultivation
In this study, we demonstrated that the S-shaped cultivation system under artificial lighting conditions achieved tomato yields comparable to those obtained through conventional greenhouse cultivation (Figure 3A). To further enhance productivity per unit area, we conducted a yield simulation incorporating staggered, sequential planting within the same vertical cultivation space. During cultivation, we observed that the lower stem regions beneath the uppermost harvested truss were mostly defoliated and underutilized. We hypothesized that this unoccupied space could be repurposed for additional growth by either cultivating axillary shoots or transplanting a second seedling, both following the same S-shaped pattern (Supplementary Figure S8). This approach aims to maximize spatial and energy efficiency while increasing total yield within a fixed cultivation period.
Based on plant growth patterns, we estimated that initiating the growth of an axillary shoot or introducing a secondary seedling approximately 10 weeks after the initial planting would align the shoot apex of the second plant with the height of the uppermost harvested truss of the first plant. At that stage, aged leaves of the primary plant could be pruned following fruit harvest to free up space and enhance light penetration to the developing apex of the second plant (Supplementary Figure S8). This strategy would enable both plants to be trained within the same frame without spatial interference. Simulations based on empirical cultivation data predicted that the introduction of a second plant could increase the total yield per cultivation unit from 2.76 to 4.05 kg, representing a 1.47-fold increase in yield per square meter, from 9.50 to 13.94 kg m-2.
These findings highlight the potential of time-staggered interplanting as a strategy to maximize spatial efficiency in vertical plant factory systems. While this approach shows promise in the S-shaped system by utilizing otherwise underused vertical space, it is difficult to implement in the I-shaped system, where the vertical space is linearly occupied by a single main stem, leaving little room for sequential planting. Further experimental validation is needed to assess the physiological impact of overlapping root and shoot systems, including possible competition for light and nutrients. Moreover, scalability using larger rack systems and optimization of planting intervals could further enhance the productivity of this cultivation method.
Potential application of the S-shaped cultivation method in space agriculture
Controlled environment agriculture using artificial light has been increasingly recognized as a promising solution for future space missions. In long-duration spaceflights, pre-packaged meals often degrade in quality and lack sufficient essential nutrients, which may compromise astronaut health and appetite due to the so-called “menu fatigue” (Khodadad et al., 2020; Sirmons et al., 2020). To address this, NASA’s “VEGGIE” project has conducted several successful experiments growing leafy greens and dwarf tomato cultivars on the International Space Station (Garcia, 2022).
Our study demonstrates a novel S-shaped tomato cultivation method using standard high-quality varieties rather than dwarf types. This system efficiently utilizes vertical space and artificial light (Figure 1B), enabling stable fruit quality and yield under completely controlled conditions. Importantly, our method allows for precise regulation of environmental factors such as light spectrum and intensity, which can be leveraged to optimize growth rates and enhance the accumulation of beneficial compounds such as lycopene and ascorbic acid—key nutrients often limited in space diets.
Given these advantages, the S-shaped cultivation method developed here could serve as a model for high-efficiency crop production systems in extraterrestrial habitats. Furthermore, its applicability to other fruiting crops offers a foundation for developing integrated plant-based life support systems capable of providing a balanced diet for astronauts during extended missions.
Material and methods
Plant material and cultivation
In this study, the mini tomato variety CF Chika (Solanum lycopersicum), developed by Takii Seed Company, was used. For soil-based greenhouse cultivation, a base fertilizer application was conducted using 1 kg of nitrogen–phosphorus–potassium (NPK) mixed fertilizer (N = 12, P = 8, K = 10) and 1 kg of carbonate magnesium lime per 10 m2 (1 m × 10 m) of cultivation area. For hydroponic cultivation in a greenhouse, plants were grown in a substrate of crushed coconut shell, which serves as a sustainable and well-aerated growing medium. The coconut shell is enclosed within hydrophilic textiles that partially contact a nutrient solution reservoir below. This setup allows water and nutrients to be drawn up through capillary action, ensuring a consistent supply to the plant roots. Additionally, two drip irrigation tubes are embedded within the coconut shell medium to provide supplementary nutrient delivery, optimizing moisture distribution. Any excess nutrient solution drains back into the reservoir, where it is collected and recirculated. For hydroponic cultivation in a plant factory, plants are cultivated with their roots fully submerged in a continuously circulated nutrient solution tank. All nutrient solutions mentioned above were made with commercial liquid fertilizer with balanced NPK and maintained at an electrical conductivity (EC) of 1.5 ± 0.05 dS m−1. Nutrient solutions were regularly replaced to remove metabolic waste produced by plants.
For both greenhouse and plant factory, environmental temperature and illumination were recorded every 1 h with TR-76Ui - USB Connectible CO2 Logger (T&D Corporation, Tokyo, Japan) and e-kakashi (PS Solutions Corporation - Agricultural IoT Solutions, Tokyo, Japan). In the greenhouse, temperature was regulated through a combination of passive and active methods. During summer, ventilation fans were activated, roof windows were opened, and shading covers were deployed when temperatures exceeded 30°C. In winter, a kerosene heater was used to maintain temperatures above 10°C. In contrast, the plant factory maintained a stable temperature of approximately 22°C throughout the year using an air conditioning system. In order to study the relationship between illumination and fruit surface temperature, some fruit clusters were covered with shading filters with transparent rates of 69.3% and 6.6% (LEE FILTER). Plant surface temperature was determined with a thermography camera (FLIR C3).
Setup of the I-shaped and S-shaped cultivation systems
Tomato plants in the greenhouse were string-trellised to grow vertically (I-shaped). Tomato plants in the plant factory were cultivated within a 135-cm × 67-cm × 200-cm frame, which was used to install illumination devices and string trellising. For I-shaped cultivation, tomato plants were guided to grow vertically within the frame, and an illumination device was installed at the very top of the frame (Figure 1A). For S-shaped cultivation, tomato plants were guided to grow back and forth through multiple layers within the frame, and illumination devices were installed at each layer (Figure 1B).
Both I-shaped and S-shaped cultivations in the plant factory were illuminated with white light LED at a 16-h/8-h light–dark cycle. For I-shaped cultivation, light intensities 35 cm, 70 cm, and 105 cm below illumination devices were determined to be 422 μmol m−2 s−1, 38 μmol m−2 s−1, and 7 μmol m−2 s−1 during cultivation with the presence of tomato plants. For S-shaped cultivation, illumination devices were installed at every layer between the stems of tomato plants, resulting in a light–plant–light–plant multilayer sandwich structure (Figure 1B). Light intensities in the middle area between each two layers of illumination devices were determined to be 150 ± 3 μmol m−2 s−1 uniformly, with the presence of tomato plants.
Determination of photosynthetic parameters
Real-time photosynthetic fluorenes in PSII were determined with the MICRO-PAM device (Walz, Effeltrich, Germany) in cultivation conditions. Measurements were taken at the upper section (30 cm below the shoot apex), middle section (60 cm below the shoot apex), and lower section (90 cm below the shoot apex). The effective quantum yield of photochemical energy conversion during PSII (Y[II]), NPQ, and photochemical quenching of PSII (qP) were calculated as follows according to previous studies (Ruban, 2017; Tanigawa et al., 2024; Katsuhama et al., 2025). Note that in this study, 1−qP, rather than qP itself, was primarily used to evaluate PSII stress and inefficiency. The ETRs of PSII and PSI were calculated as ETR I (or ETR II) = 0.5 × abs I × Y(I) (or Y[II]), where 0.5 is the fraction of absorbed light between PSII and PSI (assuming they are equal), and abs I is the absorbed irradiance taken as 0.84 of incident irradiance. ETRs were also determined throughout the light period on certain days during cultivation, and data were recorded every minute. The content of chlorophyll was also determined with a hand-held SPAD-502 meter (Spectrum Technologies, Inc., Aurora, United States) at the upper, middle, and lower sections.
Determination of plant growth and fruit quality
Stem length, stem diameter, number of fully expanded leaves (main axis longer than 10 cm), leaf length, and the SPAD value of the leaf immediately above each flower cluster were recorded weekly to assess plant growth.
Ripened fruits were harvested for the determination of fruit quality. Mini tomatoes typically yield 10 fruits per cluster, but the very fruit adjacent to the stem frequently suffers from malformation. Therefore, only nine fruits per cluster were used for fresh weight determination. Three fruits from each cluster were chosen and cut in half. Half of the fruits were used for the determination of sweetness (Brix) and acidity using the ATAGO PAL-BX|ACID3 Pocket Brix-Acidity Meter (ATAGO Co., Ltd., Tokyo, Japan) and for the determination of L-ascorbic acid using a test paper (Reflectoquant, Merck KGaA, Darmstadt, Germany). Lycopene was determined as described by Ito (2014). The remaining half of the fruits were used for the quantification of amino acid including aspartate, glutamate, asparagine, serine, glutamine, proline, GABA, and phenylalanine through pre-column derivatization using phenyl isothiocyanate (PITC), followed by separation and detection via high-performance liquid chromatography (HPLC), as described by Heinrikson and Meredith (1984).
Statistical analyses
Data for growth determination including stem length, stem diameter, leaf number, leaf length, and SPAD value were collected throughout 48 weeks of cultivation in the greenhouse and 30 weeks of cultivation in the plant factory. Data for fruit fresh weight and yield determination were collected from 35 clusters under greenhouse soil cultivation, 36 clusters under greenhouse hydroponic cultivation, and 23 clusters under plant factory cultivation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HF: Writing – original draft, Writing – review & editing, Formal analysis, Project administration, Resources. YQ: Writing – original draft, Writing – review & editing, Formal analysis, Resources. DI: Writing – review & editing, Formal analysis, Resources. SK: Writing – review & editing, Formal analysis, Resources. TS: Writing – review & editing, Formal analysis. WY: Writing – review & editing, Funding acquisition, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by KAKENHI (18KK0170, 21H02171, and 24H02277 to WY) from the Japan Society for the Promotion of Science (JSPS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fhort.2025.1633097/full#supplementary-material
References
Adams S. (2001). Effect of temperature on the growth and development of tomato fruits. Ann. Bot. 88, 869–877. doi: 10.1006/anbo.2001.1524
Ali M. Y., Sina A. A. I., Khandker S. S., Neesa L., Tanvir E. M., Kabir A., et al. (2020). Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods 10, 45. doi: 10.3390/foods10010045
Al-Kodmany (2018). He vertical farm: A review of developments and implications for the vertical city. Buildings 8, 24. doi: 10.3390/buildings8020024
Anu B. C., Saha T., Akhtar S., and Kumari K. (2021). Morphological and biochemical constituents influencing aphids and whiteflies tolerance in tomato genotypes. Bangladesh J. Bot. 50, 483–489. doi: 10.3329/bjb.v50i3.55826
Aung T. (2014). Plant growth, fruit quality analysis and consumer evaluation of blueberry grown in an advanced plant factory (Doctoral dissertation, Tokyo University of Agriculture and Technology).
Benke K. and Tomkins B. (2017). Future food-production systems: vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 13, 13–26. doi: 10.1080/15487733.2017.1394054
Bianchetti R., De Luca B., de Haro L. A., Rosado D., Demarco D., Conte M., et al (2020). Phytochrome-dependent temperature perception modulates isoprenoid metabolism. Plant Physiol. 183, 869–882.
Cho L. H., Yoon J., and An G. (2017). The control of flowering time by environmental factors. Plant J. 90, 708–719. doi: 10.1111/tpj.13461
Dhakal R. and Baek K.-H. (2014). Metabolic alternation in the accumulation of free amino acids and γ-aminobutyric acid in postharvest mature green tomatoes following irradiation with blue light. Horticult. Environ. Biotechnol. 55, 36–41. doi: 10.1007/s13580-014-0125-3
Ernesto Bianchetti R., Silvestre Lira B., Santos Monteiro S., Demarco D., Purgatto E., Rothan C., et al. (2018). Fruit-localized phytochromes regulate plastid biogenesis, starch synthesis, and carotenoid metabolism in tomato. J. Exp. Bot. 69, 3573–3586. doi: 10.1093/jxb/ery145
FAOSTAT (2020). Statistical database (Rome: Food and Agriculture Organization of the United Nations).
Garcia M. A. (2022). Astronauts prepare to grow tomatoes, get ready for spacewalk. NASA. Available online at: https://www.nasa.gov/blogs/spacestation/2022/12/01/astronauts-prepare-to-grow-tomatoes-get-ready-for-spacewalk/ (Accessed December 1, 2022).
Gautier H., Massot C., Stevens R., Sérino S., and Génard M. (2009). Regulation of tomato fruit ascorbate content is more highly dependent on fruit irradiance than leaf irradiance. Ann. Bot. 103, 495–504. doi: 10.1093/aob/mcn233
Gómez C. and Mitchell C. A. (2016). Physiological and productivity responses of high-wire tomato as affected by supplemental light source and distribution within the canopy. J. Am. Soc Hortic. Sci. 141, 196–208. doi: 10.21273/JASHS.141.2.196
Hayashi S., Levine C. P., Yu W., Usui M., Yukawa A., Ohmori Y., et al. (2024). Raising root zone temperature improves plant productivity and metabolites in hydroponic lettuce production. Front. Plant Sci. 15, 1352331. doi: 10.3389/fpls.2024.1352331
Heinrikson R. L. and Meredith S. C. (1984). Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal. Biochem. 136, 65–74. doi: 10.1016/0003-2697(84)90307-5
Helyes L., Lugasi A., and Pék Z. (2007). Effect of natural light on surface temperature and lycopene content of vine ripened tomato fruit. Can. J. Plant Sci. 87, 927–929. doi: 10.4141/CJPS07022
Hwang H., An S., Lee B., and Chun C. (2020). Improvement of growth and morphology of vegetable seedlings with supplemental far-red enriched LED lights in a plant factory. Horticulturae 6, 109. doi: 10.3390/horticulturae6040109
Ito H. (2014). New ways to evaluate the quality of vegetables using instruments. Jpn. Agric. Res. Q 48, 111–120. doi: 10.6090/jarq.48.111
Joshi J., Zhang G., Shen S., Supaibulwatana K., Watanabe C. K., and Yamori W.. (2017). A combination of downward lighting and supplemental upward lighting improves plant growth in a closed plant factory with artificial lighting. Horticult Sci. 52, 831–835. doi: 10.21273/HORTSCI11822-17
Kang W. H., Park J. S., Park K. S., and Son J. E. (2016). Leaf photosynthetic rate, growth, and morphology of lettuce under different fractions of red, blue, and green light from light-emitting diodes (LEDs). Hortic. Environ. Biotechnol. 57, 573–579. doi: 10.1007/s13580-016-0093-x
Katsuhama N., Sakoda K., Kimura H., Shimizu Y., Sakai Y., Nagata K., et al. (2025). Proton ATPase Translocation Control1-mediated H+-ATPase translocation boosts plant growth under drought by optimizing root and leaf functions. PNAS Nexus 4, 151. doi: 10.1093/pnasnexus/pgaf151
Kawamata M., et al. (2002). Double Cropping of Fig under Hydroponic culture. Engei Gakkai Zasshi 71, 68–73. doi: 10.2503/jjshs.71.68
Ke X., Yoshida H., Hikosaka S., and Goto E. (2021). Optimization of photosynthetic photon flux density and light quality for increasing radiation-use efficiency in dwarf tomato under LED light at the vegetative growth stage. Plants 11, 121. doi: 10.3390/plants11010121
Khodadad C. L. M., Hummerick M. E., Spencer L. E., Dixit A. R., Richards J. T., Romeyn M. W., et al. (2020). Microbiological and nutritional analysis of lettuce crops grown on the International Space Station. Front. Plant Sci. 11, 199. doi: 10.3389/fpls.2020.00199
Kobayashi T. and Tabuchi T. (2022). Tomato cultivation in a plant factory with artificial light: Effect of UV-A irradiation during the growing period on yield and quality of ripening fruit. Hortic. J. 91, 16–23. doi: 10.2503/hortj.UTD-272
Kwon C.-T., Heo J., Lemmon Z. H., Capua Y., Hutton S. F., Van Eck J., et al. (2020). Rapid customization of Solanaceae fruit crops for urban agriculture. Nat. Biotechnol. 38, 182–188. doi: 10.1038/s41587-019-0361-2
Lee M.-J., Son J. E., and Oh M.-M. (2014). Growth and phenolic compounds of Lactuca sativa L. grown in a closed-type plant production system with UV-A, -B, or -C lamp: Growth and phenolic compounds of Lactuca sativa L. J. Sci. Food Agric. 94, 197–204. doi: 10.1002/jsfa.6227
Levine C. P., Hayashi S., Ohmori Y., Kusano M., Kobayashi M., Nishizawa T., et al. (2023). Controlling root zone temperature improves plant growth and pigments in hydroponic lettuce. Ann. Bot. 132, 455–470. doi: 10.1093/aob/mcad127
Levine C. P., Li L., and Yamori W. (2024). “The Next Evolution of Agriculture: A review of innovations in Plant Factories,” in Handbook of photosynthesis, 4th (Boca Raton, FL: CRC Press), 740–758.
Liu N., Ji F., Xu L., and He D. (2019). Effects of LED light quality on the growth of pepper seedling in plant factory. Int. J. Agric. Biol. Eng. 12, 44–50. doi: 10.25165/j.ijabe.20191205.4847
López-Díaz G., Carreño-Ortega A., Fatnassi H., Poncet C., and Díaz-Pérez M. (2020). The effect of different levels of shading in a photovoltaic greenhouse with a north–south orientation. Appl. Sci. (Basel) 10, 882. doi: 10.3390/app10030882
Ministry of Agriculture, Forestry and Fisheries For farming photovoltaic power plants. Maff.go.jp. Available online at: https://www.maff.go.jp/j/shokusan/renewable/energy/einou.html (Accessed April 1, 2025).
Ntagkas N., de Vos R. C., Woltering E. J., Nicole C. C., Labrie C., Marcelis L. F., et al. (2020). Modulation of the tomato fruit metabolome by LED light. Metabolites 10, 266. doi: 10.3390/metabo10060266
Ntagkas N., Woltering E., Nicole C., Labrie C., and Marcelis L. F. M. (2019). Light regulation of vitamin C in tomato fruit is mediated through photosynthesis. Environ. Exp. Bot. 158, 180–188. doi: 10.1016/j.envexpbot.2018.12.002
Olvera-Gonzalez E., Escalante-Garcia N., Myers D., Ampim P., Obeng E., Alaniz-Lumbreras D., et al. (2021). Pulsed LED-lighting as an alternative energy savings technique for vertical farms and plant factories. Energies 14, 1603. doi: 10.3390/en14061603
Palmitessa O. D., Paciello P., and Santamaria P. (2020). Supplemental LED increases tomato yield in mediterranean semi-closed greenhouse. Agronomy 10, 1353. doi: 10.3390/agronomy10091353
Palozza P., Simone R. E., Catalano A., and Mele M. C. (2011). Tomato lycopene and lung cancer prevention: from experimental to human studies. Cancers (Basel) 3, 2333–2357. doi: 10.3390/cancers3022333
Paponov M., Kechasov D., Lacek J., Verheul M. J., and Paponov I. A. (2020). Supplemental light-emitting diode inter-lighting increases tomato fruit growth through enhanced photosynthetic light use efficiency and modulated root activity. Front. Plant Sci. 10, 1656.
Paucek I., Pennisi G., Pistillo A., Appolloni E., Crepaldi A., Calegari B., et al. (2020). Supplementary LED interlighting improves yield and precocity of greenhouse tomatoes in the Mediterranean. Agron. (Basel) 10, 1002. doi: 10.3390/agronomy10071002
Ruban A. V. (2017). Quantifying the efficiency of photoprotection. Philos. Trans. R. Soc Lond. B Biol. Sci. 372, 20160393. doi: 10.1098/rstb.2016.0393
Saengtharatip S., Joshi J., Zhang G., Takagaki M., Kozai T., and Yamori W.. (2021). Optimal light wavelength for a novel cultivation system with a supplemental upward lighting in plant factory with artificial lighting. Environ. Control Biol. 59, 21–27. doi: 10.2525/ecb.59.21
Shamshiri R. R., Jones J. W., Thorp K. R., Ahmad D., Man H. C., and Taheri S.. (2018). Review of optimum temperature, humidity, and vapour pressure deficit for microclimate evaluation and control in greenhouse cultivation of tomato: a review. Int. Agrophys 32, 287–302. doi: 10.1515/intag-2017-0005
SharathKumar M., Heuvelink E., and Marcelis L. F. (2020). Vertical farming: moving from genetic to environmental modification. Trends Plant Sci. 25, 724–727.
Sirmons T. A., Roma P. G., Whitmire A. M., Smith S. M., Zwart S. R., Young M., et al. (2020). Meal replacement in isolated and confined mission environments: Consumption, acceptability, and implications for physical and behavioral health. Physiol. Behav. 219, 112829. doi: 10.1016/j.physbeh.2020.112829
Takatsuji M. (2010). Present status of completely-controlled plant factories. Shokubutsu Kankyo Kogaku 22, 2–7. doi: 10.2525/shita.22.2
Takeno K. (2016). Stress-induced flowering: the third category of flowering response. J. Exp. Bot. 67, 4925–4934.
Tanigawa K., Yuchen Q., Katsuhama N., Sakoda K., Wakabayashi Y., Tanaka Y., et al. (2024). C4 monocots and C4 dicots exhibit rapid photosynthetic induction response in contrast to C3 plants. Physiol Plantarum 176, e14431. doi: 10.1111/ppl.14431
Tewolde F. T., Lu N., Shiina K., Maruo T., Takagaki M., Kozai T., et al. (2016). Nighttime supplemental LED inter-lighting improves growth and yield of single-truss tomatoes by enhancing photosynthesis in both winter and summer. Front. Plant Sci. 7, 448. doi: 10.3389/fpls.2016.00448
Tewolde F. T., Shiina K., Maruo T., Takagaki M., Kozai T., and Yamori W.. (2018). Supplemental LED inter-lighting compensates for a shortage of light for plant growth and yield under the lack of sunshine. PLoS One 13, e0206592. doi: 10.1371/journal.pone.0206592
Tran L. T., Nguyen A. T., Nguyen M. H., Nguyen L. T., Nguyen M. T., Trinh L. T., et al. (2021). Developing new parthenocarpic tomato breeding lines carrying iaa9–3 mutation. Euphytica 217, 139. doi: 10.1007/s10681-021-02853-5
Xiang Y., Chen Q., Su Z., Zhang L., Chen Z., Zhou G., et al. (2022). Deep learning and hyperspectral images based tomato soluble solids content and firmness estimation. Front. Plant Sci. 13, 860656. doi: 10.3389/fpls.2022.860656
Xie B.-X., Wei J. J., Zhang Y. T., Song S. W., Wei S. U., Sun G. W., et al. (2019). Supplemental blue and red light promote lycopene synthesis in tomato fruits. J. Integr. Agric. 18, 590–598. doi: 10.1016/S2095-3119(18)62062-3
Yamakoshi J., Fukuda S., Satoh T., Tsuji R., Saito M., Obata A., et al. (2007). Antihypertensive and natriuretic effects of less-sodium soy sauce containing γ-aminobutyric acid in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 71, 165–173. doi: 10.1271/bbb.60424
Yan L., et al. (2021). UV-C treatment enhances organic acids and GABA accumulation in tomato fruits during storage. Food Chem. 338, 128126. doi: 10.1016/j.foodchem.2020.128126
Yoshida H., Hikosaka S., Goto E., Takasuna H., and Kudo T. (2013). Effects of continuous lighting and time of initiation of treatments on the flowering time and growth of everbearing strawberry nursery plants in a closed plant factory. Shokubutsu Kankyo Kogaku 25, 77–82. doi: 10.2525/shita.25.77
Yoshida K., Oba M., and Takamori M. (2020).Development of prediction system for sugar content of cherry tomatoes using machine learning. Jst.go.jp. Available online at: https://jglobal.jst.go.jp/detail?JGLOBAL_ID=202002291636969424 (Accessed February 21, 2020).
Yoshiyama Y., et al. (2024). Natural genetic variation in dynamic photosynthesis is correlated with stomatal anatomical traits in diverse tomato species across geographical habitats. J. Exp. Bot. 75, 6762–6777. doi: 10.1093/jxb/erae082
Keywords: plant factory, tomato, hydroponics, vertical farming, light distribution
Citation: Furuta H, Qu Y, Ishizuka D, Kawabata S, Sano T and Yamori W (2025) A novel multilayer cultivation strategy improves light utilization and fruit quality in plant factories for tomato production. Front. Hortic. 4:1633097. doi: 10.3389/fhort.2025.1633097
Received: 22 May 2025; Accepted: 20 June 2025;
Published: 30 July 2025.
Edited by:
Tiejun Zhao, Niigata Agro-Food University, JapanReviewed by:
Yiting Zhang, South China Agricultural University, ChinaDedong Kong, Zhejiang Academy of Agricultural Sciences, China
Copyright © 2025 Furuta, Qu, Ishizuka, Kawabata, Sano and Yamori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wataru Yamori, eWFtb3JpQGcuZWNjLnUtdG9reW8uYWMuanA=
†These authors have contributed equally to this work
 Hanaka Furuta1†
Hanaka Furuta1† Yuchen Qu
Yuchen Qu Saneyuki Kawabata
Saneyuki Kawabata Wataru Yamori
Wataru Yamori