- 1Department of Agriculture Crop Production and Rural Environment, University of Thessaly, Volos, Greece
- 2Agronomy Department of Superior School Engineering, University of Almería, Almería, Spain
- 3Laboratory of Agronomy, Department of Agriculture, University of the Peloponnese, Antikalamos, Greece
The ongoing environmental crisis that takes place during the last years necessitates the adjustment of cultivations practices and their transition to sustainable and eco-friendly cropping systems system. In this context, the adoption of innovative techniques, as well as the integration of wild edible plants (WEPs) in modern farming systems is a promising strategy to cope with modern challenges that the agricultural sector has to face due to climate change. The Mediterranean basin is a valuable hotspot of WEPS and presents an abundant biodiversity of such species with several authors highlighting the potential prospects of valorizing WEPs as complementary/alternative crops due to their wide range of adaptability and the minimal requirements for agriculture inputs. Apart from the agronomic aspects, WEPs are highly appreciated for their numerous health benefits and they could be an interesting addition in the market niche for super and healthy foods that modern consumers are increasingly seeking. Therefore, their exploitation through commercial cropping systems could be a viable solution towards overcoming the ongoing climate crisis while safeguarding food security, especially in the arid and semi-arid regions of the Mediterranean basin where the cultivation of conventional crops is severely compromised. Considering the increasing scientific interest on WEPS during the last years, this review aims to highlight the recent scientific trends regarding the implementation of in vitro techniques for the propagation of these species. Moreover, the optimum cultivation practices and agronomic aspects of selected WEPs and sum up the most up-to date information regarding their integration in modern cropping systems as part of the climate mitigation strategies. The response of WEPS to abiotic stressors (e.g. salinity, heat, drought) is also discussed, considering the capability of these species to adapt under unfavorable conditions, as well as the potential use of WEPS for phytoremediation purposes. Finally, the future challenges and the next steps for further valorization of WEPs will be also discussed.
Introduction
Wild edible plants (WEPs) are plants that are usually found in harsh, coastal, semi-arid or uncultivated areas, while they are collected in the wild for their edible parts such as fruits, leaves, stems, shoots, flowers, roots and seeds that could be a significant alternative source of healthy and nutritious food (Duguma, 2020). These species were valuable in the rural areas during the harsh periods of history where adverse economic conditions and wars made the access to food difficult and for that reason WEPs are characterized as the food of the poor and are entitled as ‘‘famine food’’ or ‘‘poverty food’’ (Corrêa et al., 2020). Although it is estimated that 30,000 species can be used for food purposes only 7,000 of them have been valorized throughout the human history, while nowadays only 20 species provide 90% of the required food on a global scale (Bacchetta et al., 2016). Apart from culinary uses there are several wild edible plants that have played a crucial role to the cultural heritage of different regions of the world, especially in the Mediterranean basin where WEPs are abundant and they constitute an integral part of the so-called Mediterranean diet (Romojaro et al., 2013) (Figure 1). However, nowadays most of these species are treated as noxious weeds in conventional crops and farmers tend to control them with mechanical or chemical means thus resulting in agrobiodiversity loss (Kubiak et al., 2022).
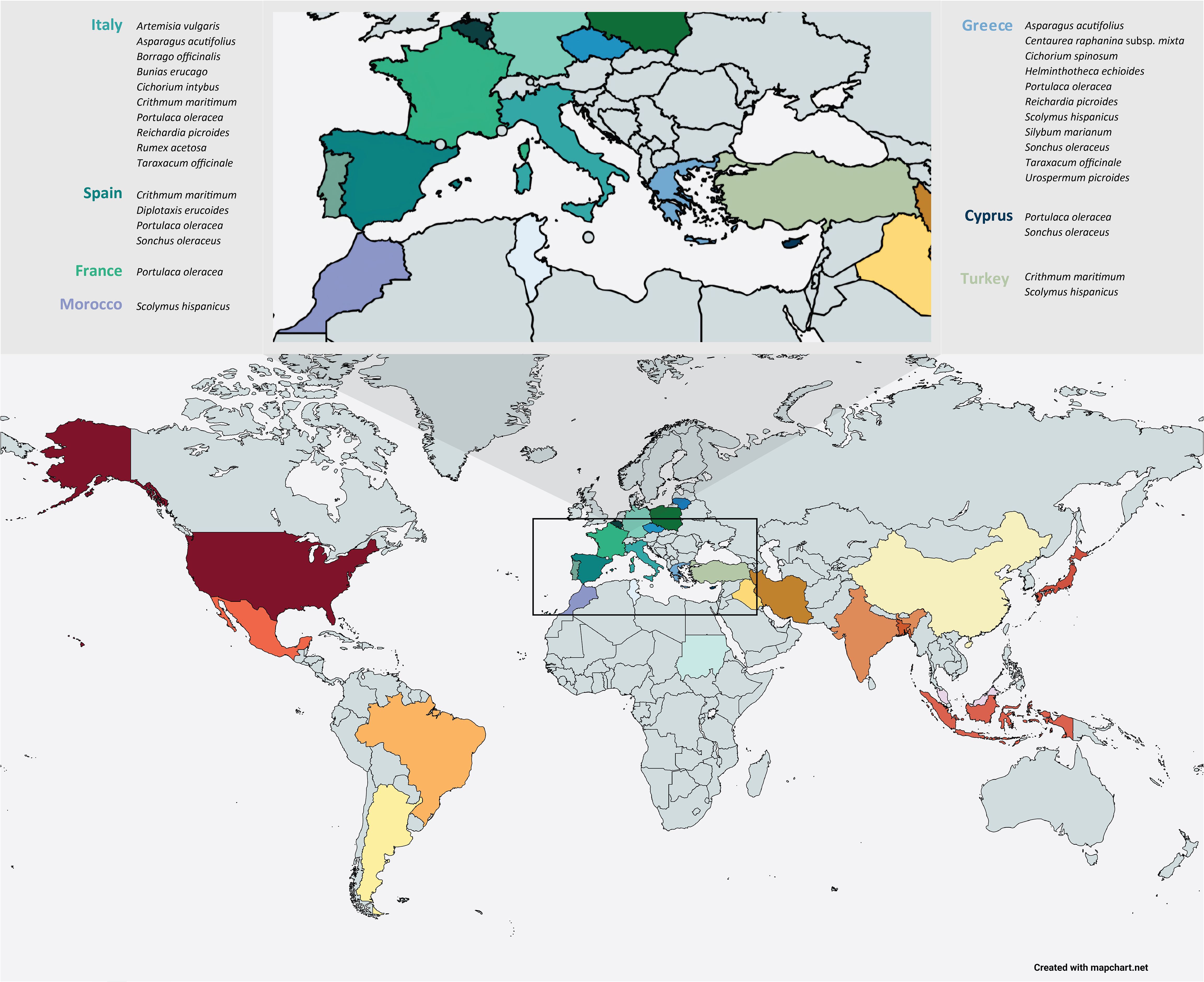
Figure 1. Distribution of wild edible species in the Mediterranean basin. (Photo credits: Dr. Vasiliki Liava).
According to several ethnobotanical surveys, WEPs include more than 7000 species that have been utilized throughout the human history for their medicinal properties (Narzary et al., 2016), while lately the interest of consumers in such species has been rekindled due to their health benefits and their high content in valuable phytochemicals (Carvalho et al., 2016). Several recent reports have highlighted the nutritional profile of WEPs which are a rich source of vitamins, fibers, fatty acids and other health beneficial compounds such as polyphenols, alkaloids, terpenoids, organic acids and other bioactive compounds (Åhlberg, 2021; Kumar et al., 2022; Martins-Noguerol et al., 2023).
Considering that so far the access to such species is mainly through collection from the wild the increasing demands of consumers could be covered with the commercial cultivation of WEPs and their promotion in modern cropping systems, especially in small-scale farms that are the backbone of the cropping sector of the Mediterranean basin (Ceccanti et al., 2020b; Chrysargyris et al., 2023a). Moreover, the current changes in climate conditions combined with land degradation due to anthropogenic activities and the irrational use of fertilizers and natural resources have severe effects on the production of conventional crops thus putting at risk food security and making a necessity the valorization of novel/alternative crops (Molina et al., 2014; Clemente-Villalba et al., 2023; Catarino et al., 2024). Species such as spiny chicory (Cichorium spinosum L.), purslane (Portulaca oleracea L.), sea-fennel or rock-samphire (Crithmum maritimum L.), golden thistle (Scolymus hispanicus L.), common thistle (Sonchus oleraceus L.) are highly appreciated in rural areas of the Mediterranean for their valuable nutritional properties (Ceccanti et al., 2018, 2020a; Petropoulos et al., 2018b; Renna, 2018; Paschoalinotto et al., 2023). Recent research has also highlighted the great potential of utilizing wild species in modern cropping systems within the concept of climate mitigation strategy, since many of them can be adapted to harsh environmental conditions, including high salinity and drought stress, heavy metal toxicity and other abiotic stressors (Ozturk et al., 2021; Lombardi et al., 2022; Vidalis et al., 2023). Moreover, the wild species usually have minimal requirements in agronomic inputs (e.g. irrigation water and fertilizers) which makes them excellent candidates for cropping in sustainable and low input cropping systems and for introducing them as minor crops (Seifzadeh et al., 2020; Amoruso et al., 2022).
However, despite the high potential of utilizing wild species as novel crops further steps are needed to establish efficient cultivation protocols and standardize yield components and the quality of the obtained products (Bacchetta et al., 2016; Pinela et al., 2017; Píoleón et al., 2017). So far, the cultivation of WEPS has taken place in both soil and soilless systems (e.g. floating and hydroponic systems, pot cultivation) aiming to evaluate the response of plants under different conditions and identify the optimum inputs that facilitate high yield and quality of the edible product (Sarrou et al., 2019; Maggini et al., 2021; Chrysargyris and Tzortzakis, 2023; Puccinelli et al., 2024).
Considering the increasing interest in wild edible species during the last few years, the present review aims to gather the latest information regarding the agronomic practices that can be applied under commercial cultivation conditions, the response of those species to different cropping systems and growing conditions and further explore the future requirements for the efficient valorization of this valuable and so far underutilized plant material. For this purpose, databases such as Scopus and Google Scholar were meticulously searched using the combinations of several relevant keywords, including “wild edible plants”, wild edible species”, “farming systems”, “small-scale farming”, “heavy metal toxicity”, “phytoremediation”, “salinity stress”, drought stress”, as well as the scientific and common names of specific wild edible plants, focusing on wild edible greens and on C. spinosum, C. maritimum, S. hispanicus, Sonchus sp. and P. oleracea. The selection of these species was based on the previous experience on our research team through the VALUEFARM project which evaluated the integration of wild edible species in the farming systems of the Mediterranean basin. After the search, the relevant documents were selected including those that referred to seed germination and propagation of WEPS; various cultivation practices such as irrigation, fertilization, etc.; different cropping systems such as field and greenhouse cropping, soil and soilless cultivation, hydroponics; the response to heat, drought and salinity stressors; and the phytoremediation of heavy metals. From this shortlist, the reports of the last decade were mostly included. However, considering the novelty of the subject and the lack of literature reviews we also included some older key reports which provide valuable information for the readers.
Plant propagation
A critical step before introducing the ‘‘domestication’’ of wild species in commercial farms is the proper crop establishment which enables the commercial cultivation and makes economically viable the valorization of such species. Plant propagation in the wild can be achieved through sexual or asexual propagation, depending on the species, while several species are perennial and there is no need for their propagation on an annual base (e.g., C. spinosum L., C. maritimum L., S. hispanicus L., Centaurea raphanina subsp. mixta (DC.) Runemark, Silybum marianum (L.) Gaertn., Urtica dioica L., Asparagus acutifolius L. etc.) (Sánchez-Mata and Tardío, 2016). However, even in the case of perennial species the commercial cultivation could be performed by treating them as annual species with plants being propagated annually, depending on the species, using either seeds and vegetative parts or implementing novel techniques such as in vitro propagation (Figure 2). Therefore, knowing the requirements and the proper conditions for seed germination and/or asexual propagation is essential for the commercial valorization of wild edible greens. To overcome the limitations of the relatively low germination rate and seed dormancy that may occur, pre-germination treatments or vegetative propagation have been suggested as effective techniques for the production of seedlings (Conversa and Elia, 2009).

Figure 2. Seedlings of Cichorium spinosum (left) and Crithmum maritimum (right) (Credits: Prof. Spyridon A. Petropoulos).
Seed germination
Wild plants are usually characterized by limited germination rates compared to conventional and domesticated crops, mostly due to seed dormancy that helps the perpetuation of plants under the harsh and unfavorable conditions where they usually grow (Ensslin et al., 2023). However, although seed dormancy is useful in wild ecosystems since it prevents germination at unfavorable conditions, this trait is undesirable when ex situ cultivation in commercial farms is needed (Benincasa et al., 2007; Ceccanti et al., 2018; Ensslin and Godefroid, 2020).
Environmental conditions such as air and soil temperature, photoperiod and soil moisture content (imposed dormancy) and/or internal factors that are associated with inherent dormancy (e.g., chemical inhibitors present in seed coats and physical barriers associated with seed coats) determine the ability of seeds to germinate under specific conditions (Sari and Tutar, 2009; Lamont and Pausas, 2023). For example, Kashmir et al. (2016) suggested that high and low temperatures (40°C and 15°C, respectively) and salinity levels higher than 100 mM NaCl significantly decreased germination of S. marianum. Moreover, the environmental conditions during seed development and the nutritional status of mother plant may also affect the germination rates of the obtained seeds, depending on the species (Finch-Savage and Leubner-Metzger, 2006; Sergio et al., 2012; Hassan et al., 2014) In order to overcome these limitations, it is required either to subject seeds to scarification that facilitate the breakage of dormancy and/or the prevalence of standard conditions that are necessary to allow seed germination (Lamont and Pausas, 2023). Seed priming is also a useful technique that has been used to increase the seed germination rate in various species where inert or imposed dormancy is present (Sadeghi and Robati, 2015). Lo Porto et al. (2019) reported that the pre-treatment of wild asparagus seeds (A. acutifolius) with cold plasma, stratification at different temperatures and treatments with sterilized water (soaking), polyethylene glycol (PEG) and GA3 had a variable effect on germination rates, mean germination time and 50% of germination time, with plasma treatment for 1 and 15 min showing the best results. Similar results were reported by Katsenios et al. (2019) and Conversa and Elia (2009; 2010) who also suggested that proper pre-treatment of seeds is pivotal in species where dormancy is detected. Torabi et al. (2012) assessed the effects of increasing concentration of exogenous silicon (Na2SiO3) application on germination rate of Borago officinalis L. and they reported that the most effective concentration was 1.5 mM resulting in reduced germination time.
Moreover, Kibar and Kibar (2017) who evaluated the seed properties of some wild edible plants consumed as vegetables in the Middle Black Sea Region of Turkey reported a considerable variation in germination rates between the species Malva neglecta, Polygonum cognatum and Trachystemon orientalis, while significant differences were also recorded between localities of the same species. In the case of S. hispanicus seeds, Sari and Tutar (2009) who evaluated the impact of light-dark cycles (16-hour light and 8-hour dark and continuous dark conditions), cold storage (4°C ± 2°C, and selected temperatures (10 – 25°C) in S. hispanicus seeds collected from three localities suggested the presence of dormancy which varied among the ecotypes and that cold stratification significantly improved germination rates. The same authors also suggested that 20 to 25°C is the optimum range of temperatures where increased germination rates were achieved (Sari and Tutar, 2009). Öğretmen et al. (2014) evaluated the impact of seed treatments (chemical treatments with ethylene, gibberellin (GA3), mannitol, and seaweed extracts and cold pre-treatment) on seed germination and they suggested that the application of 300 ppm of GA3 significantly increased germination rate at 7 days after sowing while cold-pre-treatment at -15°C and GA3 application (at 300 ppm) showed the best germination rates at 27 days after sowing. Similarly, Ceccanti et al. (2018) who performed germination tests in several wild edible species, found a great variation in germination percentage and mean germination time among the studied species. However, they did not record any effect of light conditions (e.g. germination under dark and light conditions) on the studied parameters, except for P. oleracea and Taraxacum officinale where the rate was significantly reduced under dark conditions. Scarification of seed silicles with sand paper was also very effective in the case of Bunias erucago L., while the extraction of seeds and the application of GA3 significantly improved germination rates of raw seeds (Canella et al., 2020).
Sonchus oleraceus L. is a commonly found weed in several crops and its germination requirements have been studied as a means to reduce chemical and mechanical control requirements. Chauhan et al. (2006) suggested that dark conditions, high osmotic potential (> 100 mM NaCl) and high pH (>10) significantly reduced seed germination, while Widderick et al. (2010) recorded reduced germination rates at water potential higher than -0.6 MPa. The latter authors also suggested that Sonchus seeds may germinate well in a broad range of temperatures (5°C to 35°C) which explains the emergence of plants throughout the year (Widderick et al., 2010). According to Lemna and Messersmith (1990), S. arvensis seeds do not exhibit inert dormancy and they are capable for germination directly after maturation. The same authors reported that seeds remain viable for approximately 3 years and they prefer temperatures between 25°C to 30°C for higher germination percentage, especially when imposed in alternating temperatures (Lemna and Messersmith, 1990). Moreover, Lee et al. (2024) who evaluated the germination performance of S. oleraceus and S. asper reported that both species recorded high germination rates at temperatures between 20°C to 35°C during the day and 10°C to 25°C during the night. Finally, increasing osmotic and salt stress reduced germination rates for both species, especially at stress levels higher than – 0.4 MPa and 80 mM of NaCl for osmotic and salt stress, respectively (Chauhan et al., 2006; Lee et al., 2024).
Purslane (P. oleracea) is a cosmopolitan weed which infests several crops throughout the world; therefore, germination requirements of the species have been widely studied aiming to increase the efficiency of control means. Purslane seeds can germinate over a broad range of temperatures (25°C to 35°C during the day and 15°C to 25°C during the night), while they prefer light, pH between 5 and 9 and low osmotic stress (Chauhan and Johnson, 2009). However, germination percentage may differ depending on collection site, the genotype, the time of harvest and the seed age (El-Keblawy and Al-Ansari, 2000), while priming with hot water (50°C), methanol (20%) or GA3 (1000 ppm) may increase germination percentage and decrease mean germination time (Kaur et al., 2021; Akram et al., 2023). Fernández et al. (2008) also suggested that sowing purslane seeds in styrofoam trays at 25°C and 65% relative humidity and then transferring them in floating beds resulted in the highest germination percentage and germination index, although a genotype dependent response was observed. Moreover, Naik and Karadge (2017) suggested that germination was inhibited by high salinity and dark conditions, especially at 100 mM of NaCl or Na2SO4, while at milder stress levels a varied effect of light conditions was recorded depending on the salt source. Similar results were reported by Pham et al. (2023) and Huang et al. (2018) who also observed a negative impact of osmotic stress induced either by PEG or NaCl on germination parameters, although the effect of high levels of NaCl was more prominent than PEG suggesting ion toxicity of Na to purslane seeds. On the other hand, Franco et al. (2011) did not find a significant effect of high salinity on germination percentage, mean germination time and germination index at levels up to 10 dS/m, although the final germination percentage was lower compared to other studies.
Sea fennel (C. maritimum L.) is a halophyte commonly found in coastal areas across the Mediterranean basin. Considering the halophytic nature of the species, several studies have investigated the salinity threshold which may impair seed germination, providing useful information for the valorization of the species through marginal soils reclamation, within the context of saline agriculture. Recently, Martins-Noguerol et al. (2024a). reported that low germination of seeds at high salinity (>150 mM) is due to the presence of salt-induced dormancy, since seeds remain viable and germination may recover at the absence of the stressor. Moreover, Atia et al. (2010) reported that the spongy coat of seeds is responsible for germination inhibition due to high salinity, since Na and Cl are mainly accumulated in this structure, while this protective mechanism allows seeds to survive at saline conditions. According to Atia et al. (2011b), decreasing osmotic potential values (<-0,4 MPa) significantly reduced germination percentage and velocity, although an ion specificity was detected with Na2SO4 and MgCl2 having a stronger negative impact than NaCl, MgSO4 and mannitol. In contrast, seed priming with low concentration of NaCl (50 mM NaCl) may increase germination percentage (Nimac et al., 2018), while supplementation of red light, GA3 and KNO3 (Atia et al., 2009b, a) and L-ascorbic acid and ethanol mitigated the negative effects of salt stress (Meot-Duros and Magné, 2008).
In vitro propagation
Apart from sowing seeds, the production of seedlings through asexual propagation is a valuable technique that may find use in those species where low germination rates and slow emergence of seedlings are observed, as well as in the cases where plant growth is very slow at the initial stages of the vegetative phase (Figure 3). For example, Martins-Noguerol et al. (2021) suggested the use of root cuttings for the propagation of C. maritimum which exhibits very low seed germination rates. Micropropagation is another technique that has been applied in C. maritimum by cutting plant shoot tops and growing them without leaves in Murashige and Skoog (MS) medium with sucrose (20%), agar (0.6%), pH=5.8 at 22 ± 2°C and 16 hours lighting with fluorescent lamps (40 μmol/m2/s) (Grigoriadou and Maloupa, 2008). According to Atia et al. (2011a), MS media seems to be more effective for the in vitro reproduction of C. maritimum, whereas Grigoriadou and Maloupa (2008) reported that B5 media was more effective in rooting. In addition, it has been reported that the production of shoots and rooting are favored by the addition of BA (6-Benzylaminopurine) and NAA (1-Naphthaleneacetic acid) in the growth medium, respectively (Atia et al., 2011a). On the other hand, Mitrofanova et al. (2022) suggested that the use of metatopoline (mT) induced the reaction of explants when added to the growing medium.

Figure 3. In vitro propagation of wild edible species. From upper left to right: Crithmum maritimum seedling obtained from in vitro cultivation of seed; in vitro cultivation of C. maritimum explant. From lower left to right: Cichorium spinosum explant; in vitro cultivation of C. spinosum. (Photo credits: Prof. Alexios A. Alexopoulos).
For S. oleraceus, seeds have been used for the establishment of in vitro cultures after disinfection and immersion in MS nutrient medium with sucrose (87.7 mM) and agar (0.6%) and the use of microplants as explants donors (Mawalagedera and Gould, 2015).
The studies on in vitro propagation of C. spinosum and S. hispanicus are limited. However, reports for other species of the Asteraceae family, and particularly in the Cichorium genus, suggest that such a technique may help to address issues related to seed production, difficulties in seed germination and facilitate the genetic improvement of the plant species (Aldahak et al., 2021). According to Dolinski and Olek (2013) the young leaves of C. intybus can be used for callus production, by implementing combinations of different plant growth regulators (BA, 2,4-Dichlorophenoxyacetic acid (2,4-D), NAA and thiadizuron (TDZ), cytokinin (6-(γ,γ-Dimethylallylamino)Purine-2iP) and auxin (indole-3-acetic acid – IAA)) in the growing medium. In another study, Petrova et al. (2023) used C. intybus seeds for the establishment of in vitro cultures on semi-solid MS nutrient medium and then took stem segments for the production of microplants.
Shekhawat et al. (2015) performed in vitro cultures of P. oleracea using fresh shoots with 1–2 nodes as explants and growing them under aseptic conditions on a semi-solid MS media with cytokinins and auxins. Finally, Sedaghati et al. (2019) used purslane seeds for the establishment of in vitro cultures on semi-solid MS medium with 0.7% agar and 3% sucrose and pH=5.8 and suggested efficient plant regeneration.
Effect of cultivation practices on wild edible plants
Modern agriculture has to face several challenges, including climate change and the rapid increase of world’s population which necessitate the increase in food production despite the yield losses due to unexpected weather events and the pressure from biotic and abiotic stressors (Campbell et al., 2016). Therefore, new strategies have to be adopted, including the introduction of wild edible species in farming systems. In this context, farmers have to reinvent the current agronomic practices and adapt them to the needs of these new crops aiming to establish cultivation protocols that are suitable for their commercial exploitation (Borelli et al., 2020; Singh et al., 2020). Recently, the increasing trend for consumption of foods with high nutritional value and rich content in phytochemicals concerted scientific effort to study the effect of cultivation practices on the yield and quality of various wild edible species (Rakbar et al., 2024; Litskas et al., 2025; Neji et al., 2025).
Therefore, common practices such as nutritional and water inputs, the application of organic amendments, cultivation in growth substrates etc. have been evaluated so far. For example, Petropoulos et al. (2017b, 2018a) reported that nutrient solution composition and the growing season may affect the yield parameters and the quality of C. spinosum, while Karik (2019) found significant differences in the root length and root yield of golden thistle between different harvest dates. Similar results were recorded by Petropoulos et al. (2017a) for C. spinosum aerial biomass when different sowing dates and growing periods were tested and multiple harvests were implemented. Moreover, Benincasa et al. (2007) did not record significant effects of either plant density or genotype on yield and quality parameters of wild asparagus (Asparagus acutifolius L.), while they pointed out that harvest efficiency was significantly improved when weeds were removed prior to harvest. On the other hand, Jankauskienė and Gruzdevienė (2015) suggested that planting density and crop age may affect green biomass production in Urtica dioica L. with lower densities resulting in higher biomass compared to denser planting. Similarly, Ebrahimi et al. (2010) reported that early planting (March 1) combined with low nutrients input through compost and chemical fertilizers significantly increased flower and grain yield and mucilage percentage in Borago officinalis L. plants. According to Andrzejewska et al. (2011) sowing date had no effect on fruit yield in S. marianum but it increased silymarin content, whereas increased sowing rates increased fruit yield without affecting silymarin content. Contradicting results were reported by Bielski (2021) and Rahimi and Kamali (2012) who observed a significant impact of sowing date on fruit yield for the same species.
Although wild species are naturally grown depending on the water which is available through precipitation, knowing the impact of irrigation on such species is crucial when aiming to introduce them in commercial farming systems. Therefore, the establishment of irrigation protocols can facilitate not only higher yield compared to plants grown in the wild but they can also improve the quality of the final edible product, especially through the increase of bioactive phytochemicals content. Recently, Seifzadeh et al. (2020) who tested different irrigation protocols in B. officinalis cultivation reported that drip irrigation is an economically viable practice that may improve not only yield parameters but also the net profit of the crop compared to rain-fed conditions and surface irrigation. Moreover, according to Polyzos et al. (2024) deficit irrigation (at 50% of field capacity) conditions did not impair the aerial biomass yield of S. hispanicus, whereas a negative impact on root growth was recorded. On the other hand, the same authors pointed out that deficit irrigation improved phytochemicals and bioactive compounds content without compromising the yield of fresh leaves (Polyzos et al., 2024).
Nutrients inputs though chemical and organic fertilizers is essential for conventional food crops to achieve high and profitable yields (Di Gioia et al., 2017; Verma and Yadav, 2024). However, this is not always the case for wild plants because they are adapted to unfavorable environmental conditions and minimal nutrient availability that commonly face in their natural habitats. The adoption of fertilization regimes commonly used in conventional leafy vegetables is not appropriate and it could lead in decreased quality of the edible product, as for example increased content of nitrates, as well as in severe implications to agroecosystems due to nutrients leaching, high carbon footprint and degradation of soil quality (Han et al., 2017; Ali and Bijay-Singh, 2025; Wang et al., 2025). Therefore, any fertilization protocols have to be designed having in mind that low amounts of nutrients are usually required to achieve high yields in wild species cultivation without having negative effects on the nutritional and bioactive profile of the edible products (Chatzigianni et al., 2020; Hajisolomou et al., 2024). Parameters such as the total amounts of nutrients applied, the source of nitrogen and the nitrate/ammonium nitrogen ratio should be considered when designing the fertilization protocols for such species. For example, Chatzigianni et al. (2018, 2020) suggested that nitrogen source (low amounts of NH4+ in nutrient solution) and low amounts of applied nitrogen (4 mmol/L of N) may have a significant impact on C. spinosum yield and leaf quality (e.g., low nitrates content), as well as on plant metabolism. Similar results were also reported by Petropoulos et al. (2018c) who tested the impact of nutrient solution with different NH4+ to NO3- ratio on C. spinosum, although a significant combinatorial effect was also detected for growing season and harvesting stage of leaves. Nastou et al. (2021) suggested that increasing fertilization rates in nutrient solution (up to 600 ppm of N) had beneficial effects on growth parameters of pot grown P. oleracea plants in a genotype dependent manner. In contrast, Centaurea raphanina subsp. mixta showed the highest fresh biomass yield when low nitrogen rates were applied (200 ppm of N), whereas increased N rates resulted in high contents of oxalic acid which is considered an antinutritional factor (Petropoulos et al., 2020). Similar results were reported for C. spinosum and S. hispanicus where the highest yield (e.g., biomass of fresh leaves) was achieved when low amounts of N-P-K were applied (200-100–100 ppm and 200-200–200 ppm, for C. spinosum and S. hispanicus, respectively), while quality traits showed a varied response to nutrients amounts (Polyzos et al., 2022). The recent research of Hajisolomou et al. (2024) also suggested that the application of organic fertilization can be equally effective as inorganic fertilizers in terms of plant growth parameters of field grown S. oleraceus and P. oleracea, while having positive effects on soil physicochemical properties. On the other hand, Chrysargyris and Tzortzakis (2025) cultivated purslane in a hydroponic systems and suggested that the application of high P-K rates (105 and 525 mg/L, respectively) significantly increased the above-ground biomass yield compared to the recommended dose of P and K (e.g., 70 and 350 mg/L, respectively), whereas it had a negative impact on antioxidant activity and phenolic compounds content. Moreover, the form of fertilizer may also have an impact on the yield and quality attributes of domesticated wild species, as suggested by Liava et al. (2021) who tested the effect of conventional and controlled-release nitrogen fertilizers and reported that high rates of the latter form significantly increase fruit yield of S. marianum.
Lately, the use of organic waste as growing medium has been suggested as a viable substitute of conventional growing media (e.g., rockwool, peat, perlite etc.). For instance, Ammarellou (2012) tested four media (e.g., field soil, sand, small sand and water) for rooting and growing of Urtica dioica cuttings and found that water was the most efficient medium for root development, while field soil improved the vegetative biomass. In this context, Chrysargyris et al. (2023a) suggested the use of olive and grape-mill waste as a substitute of peat in the growing medium of pot cultivated purslane and common sowthistle plants, while the use of solid waste of essential oil production from aromatic plants was effective as a growing medium for soilless cultivation of purslane when implemented at amounts of 10% (v/v) (Chrysargyris et al., 2023b). Similarly, Montesano et al. (2018) highlighted the use of sea-weed based compost as a growth medium for pot cultivation of C. maritimum plants without negative effects on yield parameters. Moreover, the addition of organic matter through the application of solid municipal waste compost increased the fresh aerial biomass of C. spinosum plants grown in a sandy soil, although a genotype dependent response was recorded.
The application of exogenous chemical compounds is also a cultivation practice of interest between the growers of wild edible plants. In this context, Ebrahimi and Miri (2016) suggested that humic acid application in increased concentrations (up to 30 g/L) significantly improved seed germination and seedling growth of Borago officinalis. Moreover, Si application mitigated the negative effects of salinity on purslane plants by increasing Na accumulation in leaves, while the opposite trend was recorded for K content (Kafi and Rahimi, 2011). In the same line, Mohamed et al. (2024) suggested that putrescine and salicylic acid application on purslane plants (at 200 mg/L) mitigated the negative effects of salinity (up to 171.2 mM of NaCl).
Cropping systems
So far, various cropping systems have been tested for the cultivation of several wild species aiming to domesticate them and valorize them in commercial crop production. Therefore, both soil and soilless systems have been implemented in experimental sites throughout the world in order to establish cultivation protocols and practices that could be extrapolated to commercial conditions and facilitate the promotion and integration of these species in modern agriculture as alternative/complementary minor crops.
Intercropping is the cropping system where the sowing of two or more crops in various layouts (e.g., row intercropping, strip intercropping and mixed intercropping and time periods (e.g., relay intercropping) may take place in the same space at the same growing period (Bavec and Bavec, 2016; Maitra et al., 2021). However, the complexity of these schemes and increasing intensification of cropping systems within the aim of achieving high yield while production is decreased (mostly through the mechanization of cultivation practices) has marginalized them in favor of monocropping (Kokkini et al., 2025). Therefore, the integration of new/alternative intercrops in rotation schemes over long time periods enables the sustainable safeguarding of agrobiodiversity and ecosystem services and secures food production in the ongoing climate change (Aviron et al., 2023).
According to Borelli et al. (2020) the exploitation of “orphan crops” (e.g., domesticated, semi-domesticated and wild species) through their integration in farming systems is of paramount importance towards the diversification of agroecosystems, the sustainable development of rural areas and the reinforcement of crop production against climate change. In this context, Ghamari et al. (2016) tested the intercropping of purslane and dragon’s head (Lallemantia iberica Fisch. and C.A. Mey) at various combinations and they reported that intercropping resulted in higher relative yield total compared to monoculture of purslane, especially when the scheme of 100% purslane and 50% dragon’s head was combined with the application of 50% of inorganic N and nitroxin. Moreover, Carrascosa-Robles et al. (2024) suggested that the inclusion of purslane in crop rotation, intercropping and combinatorial schemes resulted in increased yield for purslane, while crop rotation and the combination of intercropping and crop rotation benefited soil enzymatic activity and modified the soil microbial composition and functionality.
Soilless cultivation is ideal to evaluate nutrient requirements and pH and EC levels that are appropriate for the cultivation of each species or to regulate chemical composition and the content of phytochemicals, either the content of toxic and antinutritional compounds such as nitrates (Santamaria, 2006), or beneficial compounds such polyphenols (Martins-Noguerol et al., 2021). Hydroponic farming is an intensive cropping system that increases water and nutrient use efficiency, while it is ideal for increased yields and better availability of the final product through out of season cultivation (Ceccanti et al., 2018). Moreover, these systems are capable for very high yields due to increased plant density and the shorter cropping period, while less labor is needed for weed management (Zanin et al., 2009). In this context, hydroponic systems have been used for the cultivation of various species, including C. spinosum, S. oleraceus, P. oleracea among others (Alam et al., 2015; Chatzigianni et al., 2018; Chrysargyris and Tzortzakis, 2023; Chrysargyris et al., 2023b). Recently, Vidalis et al. (2023, 2024) tested the cultivation of Urospermum picroides, Reichardia picroides, Plantago coronopus and Hedypnois cretica under three cropping systems, namely pot cultivation indoors and outdoors and in a floating hydroponic system) and they suggested a varied response depending on the species. According to Papadimitriou et al. (2022) S. hispanicus can be cultivated in a hydroponic system and facilitate the out of season production of edible products using minimum amounts of nutrients and irrigation water of marginal quality (e.g., brackish water), while the tailor-made composition of nutrient solution may facilitate the biofortification of the edible parts of plants with health beneficial elements such as Se (Puccinelli et al., 2021).
Recently, various wild edible species have been suggested for microgreens production as novel functional foods due to their nutritional profile, the rich content in bioactive compounds and the attractive sensory attributes (Caracciolo et al., 2020; Rouphael et al., 2021). In this context, purslane was highly appreciated as an ideal species for this cropping system due to its short life cycle, low nutrients requirements and tolerance to saline conditions which facilitates the use of brackish water for microgreens production (Corrado et al., 2021; Plocek et al., 2023; Bonasia et al., 2024). Moreover, Kyriacou et al. (2019) and Giménez et al. (2021) reported that the proper choice of spectral bandwidths in microgreens production under controlled environments may regulate the biosynthetic pathways of bioactive compounds and increase the content of health beneficial compounds (e.g. polyphenols).
Pot cultivation is another option for soilless cultivation when plants are grown in growing media other than soil (Figures 4, 5). For example, Calone et al. (2021) evaluated the cultivation of six underutilized halophytic species (namely, Artemisia absinthium L., A. vulgaris L., Atriplex halimus L., Chenopodium album L., Salsola komarovii Iljin, and Sanguisorba minor Scop.) in pots containing peat moss under saline conditions (up to 600 mM NaCl) and recorded promising results in terms of crop performance for A. halimus and C. album. Moreover, the recent research of Tsoumalakou et al. (2023) and Vlahos et al. (2019) highlighted the potential of cultivating C. spinosum in aquaponics systems within the circular economy approach where aquaculture is combined with hydroponics. Finally, wild edible species have been suggested for green roofs due to their tolerance to drought stress and their limited water requirements (Azeñas et al., 2019; Martini and Papafotiou, 2025).

Figure 4. Field (left) and pot cultivation (right) of Scolymus hispanicus. (Credits: Prof Spyridon A. Petropoulos).
Considering that most of these wild species are usually found as weeds in various agroecosystems, their evaluation under field conditions is of major importance to obtain information about their response to various agronomic practices that could be extrapolated in commercial cropping systems (Figures 4, 5). Recently, Litskas et al. (2025) showcased the cultivation of S. hispanicus under commercial conditions and suggested that the species can contribute to sustainable production of food due to low inputs and environmental impacts. The ongoing climate change necessitates the reduction of inputs in crop production, especially the irrigation water which becomes scarce and water use efficiency of crops has to be improved. For this purpose, Polyzos et al. (2024) suggested that deficit irrigation can be applied in S. hispanicus cultivation without compromising yield parameters while improving phytochemicals and bioactive compounds content. Moreover, Bvenura and Afolayan (2013) suggested that the combined application of goat manure (8.13 t/ha) and inorganic fertilizer (100 kg of N/ha) resulted in the highest yield of Solanum nigrum plants grown under field conditions. In the same line, Zenobi et al. (2021) suggested that drip irrigation alone or combined with fertigation significantly increased fresh biomass yield of field-grown C. maritimum plants compared to the untreated ones. Gómez-Bellot et al. (2021) also suggested the use of recycled wastewater and brine for the irrigation of soil grown C. maritimum plants without affecting plant development and physiological parameters.
The response of wild edible plants to abiotic stressors
Salinity stress
Climate change has led to soil salinization which is associated with devastating effects on crop production around the globe, especially in arid and semi-arid areas in the Mediterranean basin (Raza et al., 2019). Saline water used by farmers for irrigation purposes has led to an increase of Na+ and Cl-, resulting in tremendous morphological, physiological and metabolic changes such as stomatal closure and reduced photosynthetic activity, and consequently in sickly plant growth and development and loss of yield and product quality (Cleydson et al., 2025). Plants present different responses to saline conditions, with most of conventional vegetable crops being considered susceptible to salinity stress (Chele et al., 2021). On the other hand, wild species are adapted to harsh environmental conditions due to protective mechanisms that they have developed over the course of time to safeguard their perpetuation (Qiu et al., 2022), although their response may vary depending on the species or the ecotype within the same species (Bonasia et al., 2017; Alexopoulos et al., 2021). Lately, the tolerance of wild edible plants under biotic and abiotic stressors, including salinity, has been the subject of several studies aiming to reveal the protective mechanisms that lie behind and further introduce them in saline agriculture as cash crops (Alam et al., 2015; Ltaeif et al., 2021; Alexopoulos et al., 2023; Sogoni et al., 2024). However, further research is needed considering the high genetic variability between and within the species, especially when considering the vast number of different ecotypes and the adaptation mechanisms that have been developed at specific growth conditions. According to Gkotzamani et al. (2024), S. oleraceus is a moderately salt-tolerant species since plant growth was impaired only at high salinity levels (e.g., 80 and 100 mM NaCl) where both leaf and root fresh weight was significantly reduced compared to control. Similar results were reported by Sergio et al. (2012) for wild chicory where a reduction in plant growth was recorded at salinity levels higher than 100 mM NaCl, while Poursakhi et al. (2019) reported significant changes in physiological parameters when chicory plants were treated with saline water (130 mM NaCl). Klados and Tzortzakis (2014) also highlighted the tolerance of hydroponically grown spiny chicory (C. spinosum) plants to high salinity (up to 120 mM NaCl), especially when rockwool was the growth medium, while lower salinity levels (40 mM NaCl) did not affect plant growth, regardless of the ecotype (Chatzigianni et al., 2019). Scolymus hispanicus could be also considered as a moderately tolerant wild edible green since according to Papadimitriou et al. (2020) salinity levels up to 3.8 dS/m did not affect plant growth and fresh biomass yield.
Crithmum maritimum is a facultative halophyte which means that saline conditions are not imperative for plant growth and development (Ben Amor et al., 2005), since plants may accumulate high amounts of ions in leaf tissues for osmotic regulation (Ciccarelli et al., 2016). In the study of Ben Hamed et al. (2007) where C. maritimum plants were subjected at salinity levels up to 300 mM NaCl, it was suggested that the biomass of leaves and roots was significantly reduced at 300 mM NaCl and 100 mM NaCl, respectively, while the evaluation of antioxidant enzymes activity indicates that protective mechanisms are more efficient in leaves than in roots. Moreover, Blažević et al. (2025) suggested that plant survival rates significantly decreased when plants were subjected to salinity levels up to 512 mM NaCl.
Purslane is also registered as halophyte with high capacity of ion accumulation (especially Na) (Kafi and Rahimi, 2011) due to adaptation mechanisms related to enhanced antioxidant enzymes activity and physiological changes (e.g., shift from C4 to CAM photosynthesis) and changes in metabolic profile (Camalle et al., 2020; Zaman et al., 2020). On the other hand, Guevara-Olivar et al. (2024) suggested that high salinity (up to 1 M of NaCl) resulted in a significant decrease of fresh biomass, mainly due to a reduction in stems weight, whereas the weight of leaves and roots remained unaffected. Sdouga et al. (2019) reported a variable response to salinity (up to 150 mM NaCl) for four purslane genotypes with the negative effects of stress being evident at the highest salinity level via reduced root length and diameter and smaller and fewer leaves. The same authors suggested that salinity up to 50 mM NaCl has a stimulatory effect on plant growth and could be considered as an eustress for increased yield parameters (Sdouga et al., 2019). Similar results were recorded by Tang et al. (2020) who also observed negative effects on purslane growth at salinity levels higher than 100 mmol/L NaCl, due to reduced length of both shoots and roots. However, according to Alam et al. (2015) there is a genotypic variability of the species to salinity stress which was evidenced in dry matter content and bioactive compounds accumulation in different accessions. Moreover, Mulry et al. (2015) suggested that the increase of proline or betalain content also depends on the genotype.
Heat stress
Climate change implications on plants and crop production are related to extreme temperatures which become more and more frequent throughout the world (Jagadish et al., 2021). Heat stress affects seed germination, photosynthesis rate, water use efficiency and grain yield and quality of cultivated and wild plants and eventually has an impact on agroecosystems and biodiversity (Haider et al., 2021; Semeraro et al., 2023). For example, Mathieu et al. (2014) studied the effects of high temperature (35°C day/28°C night) on the development of C. intybus and recorded reduced growth and early flowering induction, as well as changes in chemical composition of roots (Mathieu et al., 2018). Moreover, high temperatures may reduce or hamper seed germination and seedling growth of several species, including wild ones such as S. marianum (Kashmir et al., 2016), P. oleracea, B. officinalis and Hypericum perforatum (Descamps et al., 2018).
Tolerance to heat stress is associated with the expression of specific genes, as CiXTH29, CiLEA4 and CiFL1 and phytohormones in C. intybus (Mathieu et al., 2020; De Caroli et al., 2023), which protect physiological processes and increase antioxidant compounds content (Delfine et al., 2022). According to Yang et al. (2012), purslane is a thermotolerant species which retains an operation photosynthetic apparatus under high temperature and high humidity due to the overexpression of heat protective proteins.
Drought stress
Water stress in crop production is associated with limited availability of irrigation water during specific time periods or at specific growth stages that are critical for achieving high yields and high quality end-products (Hussain et al., 2018). Water shortage may affect plants at different levels, namely morphology (growth and leaf area reduction), physiology (closure stomata and oxidative stress increase) and biochemistry (proline accumulation and ROS generation) (Seleiman et al., 2021). Wild plants are usually well-adapted to stressed conditions like water scarcity. Nevertheless, there are several reports stating the damages caused by drought in wild plants. For instance, reduced water supply to Cichorium intybus resulted in a drastic decrease in biomass, total leaf area and stomatal conductance, whereas water use efficiency and leaf soluble sugar concentration showed a significant increase (Vandoorne et al., 2012). According to Martins-Noguerol et al. (2024b), drought stress combined with increased temperatures decreased plant growth of different populations of C. maritimum with significant effects on both aerial and root biomass depending on the genotype. Moreover, Azeñas et al. (2019) suggested that C. maritimum plants can be exploited in green roofs when subjected to mild water stress (75% of field capacity), while more severe stress (50% of field capacity) reduced root growth and increased the aerial senescent biomass. Purslane is another hardy succulent species which can tolerate prolonged drought periods and easily recover when rehydrated due to multiple defensive strategies, including physiological and molecular ones (Jin et al., 2015). Similarly, Jia et al. (2018) carried out an experiment with three different wild Sonchus species, e.g. S. oleraceous, S. wightianus, and S. uliginosus subjected to drought conditions and they suggested a varied response among the species due to differences in the activity of antioxidant enzymes such as superoxide dismutase (SOD) and peroxidase (POD).
Phytoremediation
Phytoremediation (PR) is the process according to which marginal problematic soil are improved with the use of plants. This term is mostly used for the improvement of contaminated soils, where the effort is either to (a) introduce hyperaccumulator plants with the aim to absorb high concentrations of toxic compounds from soils (phytoextraction) or (b) introduce tolerant excluder plants with the aim to stabilize toxic compounds in soil while plants are not affected (phytostabilization) (Antoniadis et al., 2017a). However, very often, phytoremediation is linked to the former process rather than the latter, and efforts are concentrated into identifying hyperaccumulators. Over the years PR has gained a lot of credit since it is a non-destructive process, as opposed to other means of soil remediation involving topsoil excavation, ex situ transfer in industrial units for washing and disinfection and then put back to its ecosystem. Such processes are very expensive and can only be applied to limited acreage, while their associated with dramatic aesthetic degradation of the problematic area (Antoniadis et al., 2017a). Moreover, the remediated soil returning back to its former position is the same only in terms of chemical properties and mineral composition, but otherwise it is destroyed concerning its physical properties, like structure and aggregate architecture and stability. This makes it particularly prone to degradation processes, such as erosion and desertification. On the other hand, PR is non-destructing, aesthetically pleasing, requires no energy input (the only energy needed is supplied by sun for plant photosynthesis), and can be applied to vast areas. However, there is an important disadvantage that often seems to slip from the attention of the scientific community: the time needed to be completed is enormous (Muthusaravanan et al., 2018). The reason is that hyperaccumulators are often weeds of low productivity; hence, even if high concentrations of toxic substances (e.g., heavy metals) are absorbed by plants, the net uptake (i.e., mass of absorbed substance, as a product of concentration × yield) is very low. Another issue that proves to be an obstacle towards selecting a plant species as a suitable phytoremediation species is the requirement for a rather unreasonably high root-to-shoot translocation (measured with translocation factor, TF = concentration in shoot over concentration in root) and soil-to-plant transfer (transfer coefficient, TC = concentration in plant over concentration in soil). TF is required to be higher than 1.0 and TC as high and as close to 1.0 as possible. Such requirements are not difficult to be achieved in hydroponics experiments, but they are seldom reported in experiments where plants are grown directly in soil, either in pot or open field tests.
Various WEPs have been used over the years as potential species for phytoremediation of soils contaminate with potentially toxic elements (PTEs, also known referred to as “heavy metals”) (Bacchetta et al., 2016). Antoniadis et al. (2017b) suggested the use of spiny chicory (C. spinosum) for the remediation of a soil spiked with 100 mg/kg of the highly toxic Cr(VI). These authors found that plants absorbed up to 250 mg/kg Cr(VI), and although various toxicity symptoms were evident to physiological functions and plant growth, it was calculated that the time needed to halve the initial added Cr(VI) concentration would be 481 cycles of harvests. Pietrelli et al. (2022) also tested another Cichorium species (C. intybus), among many others, and found that Cr exhibited the higher TC value among all other studied metals, i.e., 0.6, while TF was lower than 1.0. Moreover, Bursztyn Fuentes et al. (2018) evaluated the potential of using C. intybus to remediate a contaminated dredged sediment enriched with Cr (1010), Zn (1023), and Pb (488 mg/kg) and amended with mobilizing agents (like EDTA, citric acids) and they suggested that the Cr TC was 1.2 and TF 3.1. The time needed to reduce Cr levels down to the agricultural limits was 17 years, while the time needed for Zn and Pb was 103 and 81 years, respectively.
Another wild species tested for soil phytoremediation was purslane (P. oleracea). Thalassinos et al. (2021) spiked a pristine soil with 150 mg/kg Cr(VI) and reported a concentration of 4 mg/kg in the aerial parts of purslane plants, while roots had a content of up to 600 mg/kg. Reporting on the same experiment, Thalassinos et al. (2022) calculated a TC of 0.025 and TF of 0.01, while the time needed to absorb all added Cr(VI) was equal to 405 harvest cycles. Hammami et al. (2016) cultivated purslane in a soil spiked with up to 80 mg/kg Cd and they recorded 16.3 mg/kg in shoot and 44.1 mg/kg in root, with TC=0.76 and TF=0.37. Moreover, Thalassinos et al. (2023) tested purslane in Pb-contaminated soil (spiked with up to 900 mg/kg Pb) and they suggested a concentration of up to 1300 mg/kg in roots, while in the aerial biomass the concentration of Pb was ca. 130 mg/kg, with TC and TF values in the range of 0.1 to 0.2. In the same study, the time needed to halve the initial added Pb was 131 harvests. Moreover, Antoniadis et al. (2021) tested three WEPs, e.g. Silene vulgaris (bladder campion), Artemisia vulgaris (mugwort), and Urtica dioica (common nettle) in a soil already contaminated (i.e., not spiked) with a variety of PTEs, among which Cd at 9 mg/kg and Zn at 880 mg/kg. These authors found that Cd TF for A. vulgaris was 1.5, while that of S. vulgaris was 2.3; TC for Cd was 7 for A. vulgaris, 2.5 for S. vulgaris, and only 0.5 for U. dioica; the time needed to halve the current PTE concentrations was only 8 harvests of A. vulgaris for Cd and 104 for Zn, while that of S. vulgaris was 71 for Cd and 347 for Zn; and for U. dioica was 125 for Cd and 644 for Zn. On the other hand, in many reports WEPs have been tested to phytostabilize soil PTEs. For example, Han et al. (2022) added wheat straw biochar in a soil spiked with up to 10 mg/kg Cd, and found a concentration of 30 mg/kg in the aerial parts of purslane, which was reduced to half in the amended soil at 5% biochar. Also, Zanganeh et al. (2022) cultivated purslane in a soil with Cr(VI)=13 and Zn=104 mg/kg and added with wood biochar at concentrations up to 5% in and they found that the concentrations of both metals decreased significantly, and so did TC and TF, which were found well below 1.0.
Cultivation of wild edible plants is also associated with soil improving properties that could be valorized for arable land reclamation, as well as for the increase of yield and quality parameters of conventional crops. In this context, Hassan et al. (2018) incorporated dried aerial parts of S. oleraceus in the soil at two rates (150 and 300 g/m2) and recorded a significant increase in the quality and yield parameters of Phaseolus vulgaris L. plants when supplemented at the highest rate due to better availability of N and P and the increase in total phenolic compounds and the organic carbon and organic matter content.
Future remarks and conclusions
Nowadays, there is a tug of war between food security and increasing world population on the one hand and climate change and agricultural land loss on the other hand. Extreme changes in climate conditions are more and more evident by the farmers as they present devastating effects on crop production in terms of yield loss, land degradation, increased agricultural inputs and limited availability of irrigation water. It is of paramount importance to turn our interest at new complementary/alternative solutions for a modern sustainable agriculture. These species display a remarkable potential to be exploited as novel crops since they present a wide range of adaptability to different soil types, low demands for agrochemical inputs and tolerance to biotic and abiotic factors. Another perspective arises from the global market trends for novel healthy and functional foods, while the high nutritional value, rich content in phytochemicals and bioactive compounds have attracted the intention of pharmaceutical and nutraceutical industries for the design/production of new products.
In this context, the valorization of wild edible species through their integration in farming systems is a sustainable solution to overcome the challenges that modern agriculture has to face. Considering the vast number of species that could be valorized for food purposes and the existing information from ethnobotanical surveys which highlight the recorded uses for such species over the centuries, further research is needed to support the safe use of traditional and new species, as well to establish protocols regarding the growing conditions required for commercial cultivation. Moreover, the great variability among the ecotypes within the same species in terms of growth parameters and chemical profile point out the high potential for selection of elite genotypes that could be used in breeding programs aiming to improve yield parameters, the nutritional profile of the edible parts and the bioactive compounds content of health beneficial phytochemicals.
However, the valorization of wild edible species requires multiple steps to be followed in the near future. Firstly, there is scarce literature regarding the seed germination of wild edible plants and the wide inter- and intra-species variation in germination rates and environmental requirements, which necessitates further research to be conducted to establish germination protocols that will make feasible the successful establishment of commercial cropping of such species. Moreover, future research should focus on the evaluation of agronomic practices such as intercropping and crop rotation schemes, irrigation regimes, biostimulant application and fertilization regimes, as well as the application of various cropping systems aiming to standardize the cultivation protocols required for the cropping of these species under commercial conditions. Another aspect to be considered is the integration of such species in breeding programs to make easily available and affordable to farmers the propagational material needed for crop establishment, while techniques such as in vitro propagation and micropropagation could be useful tools, especially for those species that exhibit low germination rates.
In conclusion, wild edible species are a valuable material that has to be exploited for the alleviation of climate change effects on crop production and the environment through their integration in small-scale farming systems, especially in arid and semi-arid region of the world where the impact on food security and the environment is imminent.
Author contributions
NP: Writing – original draft, Writing – review & editing. VL: Writing – original draft, Writing – review & editing. VA: Writing – original draft, Writing – review & editing. PG: Writing – original draft, Writing – review & editing. AA: Visualization, Writing – original draft, Writing – review & editing. SP: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Åhlberg M. K. (2021). A profound explanation of why eating green (wild) edible plants promote health and longevity. Food Front. 2, 240–267. doi: 10.1002/fft2.106
Akram W., Hussain A., Roomi I., and Muhammad M. (2023). Effect of different priming agents on germination of purslane (Portulaca oleracea L.) seeds under laboratory conditions. Pakistan J. Bot. 55, 1615–1622. doi: 10.30848/PJB2023-5(14
Alam M. A., Juraimi A. S., Rafii M. Y., Hamid A. A., Aslani F., and Alam M. Z. (2015). Effects of salinity and salinity-induced augmented bioactive compounds in purslane (Portulaca oleracea L.) for possible economical use. Food Chem. 169, 439–447. doi: 10.1016/j.foodchem.2014.08.019
Aldahak L., Salem K. F. M., Al-Salim S. H. F., and Al-Khayri J. M. (2021). Advances in chicory (CIchorium intybusL.) breeding strategies in Advances in Plant Breeding Strategies: Vegetable Crops: Volume 10: Leaves, Flowerheads, Green Pods, Mushrooms and Truffles. Eds. Al-Khayri J. M., Jain S. M., and Johnson D. V. (Springer, Cham, Switzerland), 3–57. doi: 10.1007/978-3-030-66969-0_1
Alexopoulos A. A., Assimakopoulou A., Panagopoulos P., Bakea M., Vidalis N., Karapanos I. C., et al. (2021). Impact of salinity on the growth and chemical composition of two underutilized wild edible greens: Taraxacum officinale and reichardia picroides. Horticulturae 7, 160. doi: 10.3390/horticulturae7070160
Alexopoulos A. A., Assimakopoulou A., Panagopoulos P., Bakea M., Vidalis N., Karapanos I. C., et al. (2023). Hedypnois cretica L. and Urospermum picroides L. Plant Growth, Nutrient Status and Quality Characteristics under Salinity Stress. Horticulturae 9, 65. doi: 10.3390/horticulturae9010065
Ali A. M. and Bijay-Singh (2025). “Environmental pollution and climate change implications of agricultural fertilizer use,” in Agricultural Nutrient Pollution and Climate Change: Challenges and Opportunities. Eds. Hussain N., Hung C.-Y., and Wang L. (Springer Nature Switzerland, Cham), 1–28. doi: 10.1007/978-3-031-80912-5_1
Ammarellou A. (2012). Effects of different culture media on rooting of Urtica dioica L. stem cuttings. J. Soil Sci. Environ. Manage. 3, 172–175. doi: 10.5897/jssem11.029
Amoruso F., Signore A., Gómez P. A., Martínez-Ballesta M. D. C., Giménez A., Franco J. A., et al. (2022). Effect of saline-nutrient solution on yield, quality, and shelf-life of sea fennel (Crithmum maritimum L.) plants. Horticulturae 8, 127. doi: 10.3390/horticulturae8020127
Andrzejewska J., Sadowska K., and Mielcarek S. (2011). Effect of sowing date and rate on the yield and flavonolignan content of the fruits of milk thistle (Silybum marianum L. Gaertn.) grown on light soil in a moderate climate. Ind. Crops Prod. 33, 462–468. doi: 10.1016/j.indcrop.2010.10.027
Antoniadis V., Levizou E., Shaheen S. M., Sik Y., Sebastian A., Baum C., et al. (2017a). Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth-Science Rev. 171, 621–645. doi: 10.1016/j.earscirev.2017.06.005
Antoniadis V., Polyzois T., Golia E. E., and Petropoulos S. A. (2017b). Hexavalent chromium availability and phytoremediation potential of Cichorium spinosum as affect by manure, zeolite and soil ageing. Chemosphere 171, 729–734. doi: 10.1016/j.chemosphere.2016.11.146
Antoniadis V., Shaheen S. M., Stärk H. J., Wennrich R., Levizou E., Merbach I., et al. (2021). Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ. Int. 146, 106233. doi: 10.1016/j.envint.2020.106233
Atia A., Barhoumi Z., Mokded R., Abdelly C., and Smaoui A. (2011a). Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J. Med. Plants Res. 5, 3564–3571.
Atia A., Debez A., Barhoumi Z., Pacini E., Abdelly C., and Smaoui A. (2010). The mericarp of the halophyte Crithmum maritimum (Apiaceae): Structural features, germination, and salt distribution. Biol. (Bratisl). 65, 489–495. doi: 10.2478/s11756-010-0036-4
Atia A., Debez A., Barhoumi Z., Smaoui A., and Abdelly C. (2009a). ABA, GA3, and nitrate may control seed germination of Crithmum maritimum (Apiaceae) under saline conditions. Comptes Rendus - Biol. 332, 704–710. doi: 10.1016/j.crvi.2009.03.009
Atia A., Debez A., Barhoumi Z., Smaoui A., and Abdelly C. (2011b). Effects of different salts and mannitol on seed imbibition, germination and ion content of Crithmum maritimum L. (Apiaceae). J. Biol. Res. 15, 37–45.
Atia A., Debez A., Rabhi M., Smaoui A., and Abdelly C. (2009b). Interactive effects of salinity, nitrate, light, and seed weight on the germination of the halophyte Crithmum maritimum. Acta Biol. Hung. 60, 433–439. doi: 10.1556/ABiol.60.2009.4.9
Aviron S., Berry T., Leroy D., Savary G., and Alignier A. (2023). Wild plants in hedgerows and weeds in crop fields are important floral resources for wild flower-visiting insects, independently of the presence of intercrops. Agric. Ecosyst. Environ. 348, 108410. doi: 10.1016/j.agee.2023.108410
Azeñas V., Janner I., Medrano H., and Gulías J. (2019). Evaluating the establishment performance of six native perennial Mediterranean species for use in extensive green roofs under water-limiting conditions. Urban For. Urban Green. 41, 158–169. doi: 10.1016/j.ufug.2019.04.002
Bacchetta L., Visioli F., Cappelli G., Caruso E., Martin G., Nemeth E., et al. (2016). A manifesto for the valorization of wild edible plants. J. Ethnopharmacol. 191, 180–187. doi: 10.1016/j.jep.2016.05.061
Bavec F. and Bavec M. (2016). “Underutilized crops and intercrops in crop rotation as factors for increasing biodiversity on fields,” in Biodiversity in Ecosystems - Linking Structure and Function. Eds. Lo Y.-H., Blanco J. A., and Shovonlal R. (London UK: IntechOpen), 583–595. Available online at: https://www.intechopen.com/books/advanced-biometric-technologies/liveness-detection-in-biometrics (Accessed May 21, 2025).
Ben Amor N., Ben Hamed K., Debez A., Grignon C., and Abdelly C. (2005). Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci. 168, 889–899. doi: 10.1016/j.plantsci.2004.11.002
Ben Hamed K., Castagna A., Salem E., Ranieri A., and Abdelly C. (2007). Sea fennel (Crithmum maritimum L.) under salinity conditions: A comparison of leaf and root antioxidant responses. Plant Growth Regul. 53, 185–194. doi: 10.1007/s10725-007-9217-8
Benincasa P., Tei F., and Rosati A. (2007). Plant density and genotype effects on wild asparagus (Asparagus acutifolius L.) spear yield and quality. HortScience 42, 1163–1166. doi: 10.21273/HORTSCI.42.5.1163
Bielski S. (2021). Milk thistle (Silybum marianum L. Gaertn.) achene yield had a positive response to nitrogen fertilization, row spacing, sowing date, and weed control methods. Ind. Crops Prod. 160, 113104. doi: 10.1016/j.indcrop.2020.113104
Blažević I., Đulović A., Burčul F., Tomaš J., Brzović P., Radman S., et al. (2025). “Adaptation of the chasmophyte crithmum maritimum to high-salinity conditions,” in Growth and Development in Plants and Their Medicinal and Environmental Impact, vol. 22. (London UK: IntechOpen). doi: 10.1039/C7RA00172J%0Ahttps://www.intechopen.com/books/advanced-biometric-technologies/liveness-detection-in-biometrics%0Ahttp://dx.doi.org/10.1016/j.colsurfa.2011.12.014 (Accessed May 21, 2025).
Bonasia A., Lazzizera C., Elia A., and Conversa G. (2017). Nutritional, biophysical and physiological characteristics of wild rocket genotypes as affected by soilless cultivation system, salinity level of nutrient solution and growing period. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00300
Bonasia A., Lazzizera C., La Rotonda P., Santoro A. M., Botticella L., Elia A., et al. (2024). Productive and qualitative profile of unexploited microgreen genotypes from Brassicaceae, Chenopodiaceae, Asteraceae and Portulacaceae families. Italus Hortus 31, 110–128. doi: 10.26353/j.itahort/2024.1.110128
Borelli T., Hunter D., Padulosi S., Amaya N., Meldrum G., de Oliveira Beltrame D. M., et al. (2020). Local solutions for sustainable food systems: The contribution of orphan crops and wild edible species. Agronomy 10, 231. doi: 10.3390/agronomy10020231
Bursztyn Fuentes A. L., José C., de los Ríos A., do Carmo L. I., de Iorio A. F., and Rendina A. E. (2018). Phytoextraction of heavy metals from a multiply contaminated dredged sediment by chicory (Cichorium intybus L.) and castor bean (Ricinus communis L.) enhanced with EDTA, NTA, and citric acid application. Int. J. Phytoremediation 20, 1354–1361. doi: 10.1080/15226514.2018.1524826
Bvenura C. and Afolayan A. J. (2013). Growth and physiological response to organic and/or inorganic fertilisers of wild Solanum nigrum L. cultivated under field conditions in Eastern Cape Province, South Africa. Acta Agric. Scand. Sect. B Soil Plant Sci. 63, 683–693. doi: 10.1080/09064710.2013.852616
Calone R., Bregaglio S., Sanoubar R., Noli E., Lambertini C., and Barbanti L. (2021). Physiological adaptation to water salinity in six wild halophytes suitable for Mediterranean agriculture. Plants 10, 309. doi: 10.3390/plants10020309
Camalle M., Standing D., Jitan M., Muhaisen R., Bader N., Bsoul M., et al. (2020). Effect of salinity and nitrogen sources on the leaf quality, biomass, and metabolic responses of two ecotypes of Portulaca oleracea. Agronomy 10, 656. doi: 10.3390/agronomy10050656
Campbell B. M., Vermeulen S. J., Aggarwal P. K., Corner-Dolloff C., Girvetz E., Loboguerrero A. M., et al. (2016). Reducing risks to food security from climate change. Glob. Food Sec. 11, 34–43. doi: 10.1016/J.GFS.2016.06.002
Canella M., Rossi G., Mondoni A., and Guzzon F. (2020). Promoting seed germination of Bunias erucago, a Mediterranean leafy vegetable. Seed Sci. Technol. 48, 189–199. doi: 10.15258/sst.2020.48.2.06
Caracciolo F., El-Nakhel C., Raimondo M., Kyriacou M. C., Cembalo L., de Pascale S., et al. (2020). Sensory attributes and consumer acceptability of 12 microgreens species. Agronomy 10, 1043. doi: 10.3390/agronomy10071043
Carrascosa-Robles Á., Pascual J. A., Cuartero J., de Santiago A., Petropoulos S. A., and del Mar Alguacil M. (2024). Optimizing purslane cultivation through legume intercropping and crop rotation: a study on yield and rhizosphere bacterial communities. Plant Soil. doi: 10.1007/s11104-024-07061-3
Carvalho A. M., Barata A. M., Pinela J., Carocho M., Dias M. I., Caleja C., et al. (2016). The consumption of wild edible plants. in Wild Plants Mushrooms Nuts. Eds. Ferreira I. C. F. R., Morales P., and Barros L. Wiley and Sons Ltd, Oxford, England, 159–198. doi: 10.1002/9781118944653.ch6
Catarino L., Romeiras M. M., and Fernandes Â. (2024). Food from the wild—Roles and values of wild edible plants and fungi. Foods 13, 818. doi: 10.3390/foods13060818
Ceccanti C., Landi M., Benvenuti S., Pardossi A., and Guidi L. (2018). Mediterranean wild edible plants: Weeds or “new functional crops”? Molecules 23, 1–15. doi: 10.3390/molecules23092299
Ceccanti C., Landi M., Incrocci L., Pardossi A., and Guidi L. (2020a). Suitability of hydroponically-grown Rumex acetosa L. As fresh-cut produce. Horticulturae 6, 4. doi: 10.3390/horticulturae6010004
Ceccanti C., Landi M., Incrocci L., Pardossi A., Venturi F., Taglieri I., et al. (2020b). Comparison of three domestications and wild-harvested plants for nutraceutical properties and sensory profiles in five wild edible herbs: Is domestication possible? Foods 9, 1065. doi: 10.3390/foods9081065
Chatzigianni M., Aliferis K. A., Ntatsi G., and Savvas D. (2020). Effect of N supply level and N source ratio on Cichorium spinosum L. metabolism. Agronomy 10, 952. doi: 10.3390/agronomy10070952
Chatzigianni M., Alkhaled B., Livieratos I., Stamatakis A., Ntatsi G., and Savvas D. (2018). Impact of nitrogen source and supply level on growth, yield and nutritional value of two contrasting ecotypes of Cichorium spinosum L. grown hydroponically. J. Sci. Food Agric. 98, 1615–1624. doi: 10.1002/jsfa.8636
Chatzigianni M., Ntatsi G., Theodorou M., Stamatakis A., Livieratos I., Rouphael Y., et al. (2019). Functional quality, mineral composition and biomass production in hydroponic spiny chicory (Cichorium spinosum L.) are modulated interactively by ecotype, salinity and nitrogen supply. Front. Plant Sci. 10, 1040. doi: 10.3389/fpls.2019.01040
Chauhan B. S., Gill G., and Preston C. (2009). Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Sci. 54, 854–860. doi: 10.1614/WS-06-047R.1
Chauhan B. S. and Johnson D. E. (2009). Seed germination ecology of Portulaca oleracea L.: an important weed of rice and upland crops. Ann Appl Biol. 155, 61–69. doi: https://doi.org/10.1111/j.1744-7348.2009.00320.x
Chele K. H., Tinte M. M., Piater L. A., Dubery I. A., and Tugizimana F. (2021). Soil salinity, a serious environmental issue and plant responses: A metabolomics perspective. Metabolites 11, 724. doi: 10.3390/metabo11110724
Chrysargyris A., Hajisolomou E., Xylia P., and Tzortzakis N. (2023a). Olive-mill and grape-mill waste as a substitute growing media component for unexploded vegetables production. Sustain. Chem. Pharm. 31, 100940. doi: 10.1016/j.scp.2022.100940
Chrysargyris A., Louka S., Petropoulos S. A., and Tzortzakis N. (2023b). Soilless cultivation of Portulaca oleracea using medicinal and aromatic plant residues for partial peat replacement. Horticulturae 9, 1–15. doi: 10.3390/horticulturae9040474
Chrysargyris A. and Tzortzakis N. (2023). Optimising fertigation of hydroponically grown sowthistle (Sonchus oleraceus L.): The impact of the nitrogen source and supply concentration. Agric. Water Manage. 289, 108528. doi: 10.1016/j.agwat.2023.108528
Chrysargyris A. and Tzortzakis N. (2025). Nitrogen, phosphorus, and potassium requirements to improve Sideritis cypria growth, nutrient and water use efficiency in hydroponic cultivation. Heliyon 11, 1–17. doi: 10.1016/j.heliyon.2024.e40755
Ciccarelli D., Picciarelli P., Bedini G., and Sorce C. (2016). Mediterranean sea cliff plants: Morphological and physiological responses to environmental conditions. J. Plant Ecol. 9, 153–164. doi: 10.1093/jpe/rtv042
Clemente-Villalba J., Burló F., Hernández F., and Carbonell-BarraChina Á.A. (2023). Valorization of wild edible plants as food ingredients and their economic value. Foods 12, 1012. doi: 10.3390/foods12051012
Cleydson J., Silva F., Lima K., MaChado D. G., Flavia A., Silva D. S., et al. (2025). Challenges and opportunities for new frontiers and technologies to guarantee food production. Sustainability 17, 3792. doi: 10.3390/su17093792
Conversa G. and Elia A. (2009). Effect of seed age, stratification, and soaking on germination of wild asparagus (Asparagus acutifolius L.). Sci. Hortic. (Amsterdam). 119, 241–245. doi: 10.1016/j.scienta.2008.08.013
Conversa G., Lazzizera C., and Elia A. (2010). Effects of after-ripening, stratification and GA3 on dormancy release and on germination of wild asparagus (Asparagus acutifolius L.) seeds. Sci. Hortic. (Amsterdam). 125, 196–202. doi: 10.1016/j.scienta.2010.04.025
Corrado G., El-Nakhel C., Graziani G., Pannico A., Zarrelli A., Giannini P., et al. (2021). Productive and morphometric traits, mineral composition and secondary metabolome components of borage and purslane as underutilized species for microgreens production. Horticulturae 7, 211. doi: 10.3390/horticulturae7080211
Corrêa R. C. G., Di Gioia F., Ferreira I. C. F. R., and Petropoulos S. A. (2020). “Wild greens used in the Mediterranean diet,” in The Mediterranean Diet: An Evidence-based Approach. Eds. Preedy V. and Watson R. (Academic Press, London, UK), 209–228.
De Caroli M., Rampino P., Curci L. M., Pecatelli G., Carrozzo S., and Piro G. (2023). CiXTH29 and ciLEA4 role in water stress tolerance in Cichorium intybus varieties. Biol. (Basel). 12, 444. doi: 10.3390/biology12030444
Delfine S., Fratianni A., D’Agostino A., and Panfili G. (2022). Influence of drought stress on physiological responses and bioactive compounds in chicory (Cichorium intybus L.): opportunity for a sustainable agriculture. Foods 11, 3725. doi: 10.3390/foods11223725
Descamps C., Quinet M., Baijot A., and Jacquemart A. L. (2018). Temperature and water stress affect plant–pollinator interactions in Borago officinalis (Boraginaceae). Ecol. Evol. 8, 3443–3456. doi: 10.1002/ece3.3914
Di Gioia F., Gonnella M., Buono V., Ayala O., and Santamaria P. (2017). Agronomic, physiological and quality response of romaine and red oak-leaf lettuce to nitrogen input. Ital. J. Agron. 12, 47–58. doi: 10.4081/ija.2017.806
Dolinski R. and Olek A. (2013). Micropropagation of wild chicory (Cichorium intybus L. var. silvestre Bisch.) from leaf explants. Acta Sci. Pol. Hortorum Cultus 12, 33–44.
Duguma H. T. (2020). Wild edible plant nutritional contribution and consumer perception in Ethiopia. Int. J. Food Sci. 2020, 2958623. doi: 10.1155/2020/2958623
Ebrahimi M. and Miri E. (2016). Effect of Humic Acid on Seed Germination and Seedling Growth of Borago officinalis and Cichorium intybus. Ecopersia 4, 1239–1249. doi: 10.18869/modares.ecopersia.4.1.1239
Ebrahimi A., Moaveni P., and Farahani H. A. (2010). Effects of planting dates and compost on mucilage variations in borage (Borago officinalis L.) under different chemical fertilization systems. Int. J. Biotechnol. Mol. Biol. Res. 1, 58–61. Available online at: http://www.academicjournals.org/ijbmbr (Accessed May 21, 2025).
El-Keblawy A. and Al-Ansari F. (2000). Effects of site of origin, time of seed maturation, and seed age on germination behavior of Portulaca oleracea from the Old and New Worlds. Can. J. Bot. 78, 279–287. doi: 10.1139/cjb-78-3-279
Ensslin A. and Godefroid S. (2020). Ex situ cultivation impacts on plant traits and drought stress response in a multi-species experiment. Biol. Conserv. 248, 108630. doi: 10.1016/j.biocon.2020.108630
Ensslin A., Sandner T. M., and Godefroid S. (2023). Does the reduction of seed dormancy during ex situ cultivation affect the germination and establishment of plants reintroduced into the wild? J. Appl. Ecol. 60, 685–695. doi: 10.1111/1365-2664.14354
Fernández J. A., Navarro A., Vicente M. J., Peà ± apareja D., and Plana V. (2008). “Effect of seed germination methods on seedling emergence and earliness of purslane (Portulaca oleracea L.) cultivars in a hydroponic floating system,” in Acta Horticulturae (International Society for Horticultural Science (ISHS, Leuven, Belgium), 207–212. doi: 10.17660/ActaHortic.2008.782.24
Finch-Savage W. E. and Leubner-Metzger G. (2006). Seed dormancy and the control of germination. New Phytol. 171, 501–523. doi: 10.1111/j.1469-8137.2006.01787.x
Franco J. A., Cros V., Vicente M. J., and Martínez-Sánchez J. J. (2011). Effects of salinity on the germination, growth, and nitrate contents of purslane (Portulaca oleracea L.) cultivated under different climatic conditions. J. Hortic. Sci. Biotechnol. 86, 1–6. doi: 10.1080/14620316.2011.11512716
Ghamari H., Shafagh Kollvanagh J., Sabaghpour S. H., and Dabbagh Mohammadi Nassab A. (2016). The Effect of Intercropping and Nitroxin Biofertilizer on Yield Components and Relative Yield Total of Purslane (Portulaca oleracea L.) and Dragon’s Head (Lallemantia iberica Fisch. & C.A. Mey). Not. Sci. Biol. 8, 472–476. doi: 10.15835/nsb849936
Giménez A., Martínez-Ballesta M. D. C., Egea-Gilabert C., Gómez P. A., Artés-Hernández F., Pennisi G., et al. (2021). Combined effect of salinity and led lights on the yield and quality of purslane (Portulaca oleracea L.) microgreens. Horticulturae 7, 180. doi: 10.3390/horticulturae7070180
Gkotzamani A., Ipsilantis I., Menexes G., Katsiotis A., Mattas K., and Koukounaras A. (2024). The impact of salinity in the irrigation of a wild underutilized leafy vegetable, Sonchus oleraceus L. Plants 13, 1–13. doi: 10.3390/plants13111552
Gómez-Bellot M. J., Lorente B., Ortuño M. F., Medina S., Gil-Izquierdo Á., Bañón S., et al. (2021). Recycled wastewater and reverse osmosis brine use for halophytes irrigation: Differences in physiological, nutritional and hormonal responses of Crithmum maritimum and Atriplex halimus plants. Agronomy 11:627. doi: 10.3390/agronomy11040627
Grigoriadou K. and Maloupa E. (2008). Micropropagation and salt tolerance of in vitro grown Crithmum maritimum L. Plant Cell. Tissue Organ Cult. 94, 209–217. doi: 10.1007/s11240-008-9406-9
Guevara-Olivar B. K., Gómez-Merino F. C., Ruíz-Posadas L., del M., Fernández-Pavía Y. L., Escalante-Estrada J. A., et al. (2024). Morphological, biochemical and nutritional variations in a Mexican purslane (Portulaca oleracea L.) variety exposed to salt stress during the vegetative stage. Not. Bot. Horti Agrobot. Cluj-Napoca 52, 13434. doi: 10.15835/nbha52213434
Haider S., Iqbal J., Naseer S., Yaseen T., Shaukat M., Bibi H., et al. (2021). Molecular mechanisms of plant tolerance to heat stress: current landscape and future perspectives. Plant Cell Rep. 40, 2247–2271. doi: 10.1007/s00299-021-02696-3
Hajisolomou E., Neofytou G., Petropoulos S. A., and Tzortzakis N. (2024). The application of conventional and organic fertilizers during wild edible species cultivation: A case study of purslane and common sowthistle. Horticulturae 10, 1222. doi: 10.3390/horticulturae10111222
Hammami H., Parsa M., Mohassel M. H. R., Rahimi S., and Mijani S. (2016). Weeds ability to phytoremediate cadmium-contaminated soil. Int. J. Phytoremediation 18, 48–53. doi: 10.1080/15226514.2015.1058336
Han X., Cui L., and Yan J. (2022). Effects of biochar on purslane-mediated transfer and uptake of soil bioavailable cadmium. Water. Air. Soil pollut. 233, 1–10. doi: 10.1007/s11270-022-05952-8
Han J., Shi J., Zeng L., Xu J., and Wu L. (2017). Impacts of continuous excessive fertilization on soil potential nitrification activity and nitrifying microbial community dynamics in greenhouse system. J. Soils Sediments 17, 471–480. doi: 10.1007/s11368-016-1525-z
Hassan M., Pacurar A., Gaspar A., Vicente O., and Boscaiu M. (2014). Growth and reproductive success under saline conditions of three Plantago species with different levels of stress tolerance. Not. Bot. Horti Agrobot. Cluj-Napoca 42, 180–186. doi: 10.15835/nbha4219349
Hassan M. O., Saleh A. M., and Abdelgawad H. (2018). Sonchus oleraceus Residue Improves Nutritive and Health-Promoting Value of Common Bean (Phaseolus vulgaris L.): A Metabolic Study. J. Agric. Food Chem. 66, 2092–2100. doi: 10.1021/acs.jafc.7b05821
Huang Y., Guo M., Zhang H. R., Zhou Y., Li H. M., Gao Z. M., et al. (2018). Effects of salt stress on seed germination and seedling growth in Portulaca. Acta Prataculturae Sin. 23, 13340–13349. doi: 10.11686/cyxb2020027
Hussain H. A., Hussain S., Khaliq A., Ashraf U., Anjum S. A., Men S., et al. (2018). Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00393
Jagadish S. V. K., Way D. A., and Sharkey T. D. (2021). Plant heat stress: Concepts directing future research. Plant Cell Environ. 44, 1992–2005. doi: 10.1111/pce.14050
Jankauskienė Z. and Gruzdevienė E. (2015). Changes in the productivity of wild and cultivated stinging nettle (Urtica dioica L.) as influenced by the planting density and crop age. Zemdirbyste- 102, 31–40. doi: 10.13080/z-a.2015.102.004
Jia P. Y., Zhang L. X., Huang Z., Tian F. P., Hu Y., and Wu G. L. (2018). Physiological characteristics of three wild Sonchus species to prolonged drought tolerance in arid regions. Pakistan J. Bot. 50, 9–17.
Jin R., Shi H., Han C., Zhong B., Wang Q., and Chan Z. (2015). Physiological changes of purslane (Portulaca oleracea L.) after progressive drought stress and rehydration. Sci. Hortic. (Amsterdam). 194, 215–221. doi: 10.1016/j.scienta.2015.08.023
Kafi M. and Rahimi Z. (2011). Effect of salinity and silicon on root characteristics, growth, water status, proline content and ion accumulation of purslane (Portulaca oleracea L.). Soil Sci. Plant Nutr. 57, 341–347. doi: 10.1080/00380768.2011.567398
Karik U. (2019). The effect of different harvest dates on the yield and quality of the golden thistle (Scolymus hispanicus L.). Turkish J. F. Crop 24, 230–236. doi: 10.17557/tjfc.655129
Kashmir S., Khan M. A., Shad A. A., Marwat K. B., and Khan H. (2016). Temperature and salinity affect the germination and growth of Silybum marianum Gaertn and Avena fatua L. Pakistan J. Bot. 48, 469–476.
Katsenios N., Roussis I. E., Efthimiadou A., Kakabouki I., and Bilalis D. (2019). Seed treatment techniques to improve germination of wild asparagus (Asparagus acutifolius L.), a potential new crop. Not. Bot. Horti Agrobot. Cluj-Napoca 47, 995–1000. doi: 10.15835/nbha47311554
Kaur H., Kaur N., and Bhullar M. S. (2021). Germination ecology and management of Portulaca oleracea L. - A weed of summer vegetable crops in punjab. Agric. Res. J. 58, 51–59. doi: 10.5958/2395-146X.2021.00007.7
Kibar B. and Kibar H. (2017). Determination of the nutritional and seed properties of some wild edible plants consumed as vegetable in the Middle Black Sea Region of Turkey. South Afr. J. Bot. 108, 117–125. doi: 10.1016/j.sajb.2016.10.011
Klados E. and Tzortzakis N. (2014). Effects of substrate and salinity in hydroponically grown Cichorium spinosum. J. Soil Sci. Plant Nutr. 14, 211–222. doi: 10.4067/S0718-95162014005000017
Kokkini M., Gazoulis I., Danaskos M., Kontogeorgou V., Kanatas P., and Travlos I. (2025). Enhancing ecosystem services in agriculture: the special role of legume intercropping. Front. Sustain. Food Syst. 9. doi: 10.3389/fsufs.2025.1547879
Kubiak A., Wolna-Maruwka A., Niewiadomska A., and Pilarska A. A. (2022). The problem of weed infestation of agricultural plantations vs. the assumptions of the European biodiversity strategy. Agronomy 12, 1808. doi: 10.3390/agronomy12081808
Kumar A., Sreedharan S., Kashyap A. K., Singh P., and Ramchiary N. (2022). A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 8, e08669. doi: 10.1016/j.heliyon.2021.e08669
Kyriacou M. C., El-Nakhel C., Pannico A., Graziani G., Soteriou G. A., Giordano M., et al. (2019). Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01501
Lamont B. B. and Pausas J. G. (2023). Seed dormancy revisited: Dormancy-release pathways and environmental interactions. Funct. Ecol. 37, 1106–1125. doi: 10.1111/1365-2435.14269
Lee Y., Mahajan G., Beregszaszi R., and Chauhan B. S. (2024). Seed Germination Ecology of Sonchus asper and Sonchus oleraceus in Queensland Australia. Plants 13, 3451. doi: 10.3390/plants13233451
Lemna W. K. and Messersmith C. G. (1990). The biology of Canadian weeds. 94. Sonchus arvensis L. Can. J. Plant Pathol. 70, 509–532. doi: 10.4141/cjps90-060
Liava V., Karkanis A., and Tsiropoulos N. (2021). Yield and silymarin content in milk thistle (Silybum marianum (L.) Gaertn.) fruits affected by the nitrogen fertilizers. Ind. Crops Prod. 171, 113955. doi: 10.1016/j.indcrop.2021.113955
Litskas V. D., Chrysargyris A., Tzortzakis N., Stavrinides M. C., and Petropoulos S. A. (2025). Can the commercial cultivation of wild edible species contribute to sustainable food production? A case study of golden thistle (Scolymus hispanicus L.). Int. J. Life Cycle Assess., 446–461. doi: 10.1007/s11367-024-02418-3
Lombardi T., Bertacchi A., Pistelli L., Pardossi A., Pecchia S., Toffanin A., et al. (2022). Biological and agronomic traits of the main halophytes widespread in the Mediterranean region as potential new vegetable crops. Horticulturae 8, 195. doi: 10.3390/horticulturae8030195
Lo Porto C., Sergio L., Boari F., Logrieco A. F., and Cantore V. (2019). Cold plasma pretreatment improves the germination of wild asparagus (Asparagus acutifolius L.) seeds. Sci. Hortic. (Amsterdam). 256, 108554. doi: 10.1016/j.scienta.2019.108554
Ltaeif H. B., Sakhraoui A., González-Orenga S., Landa Faz A., Boscaiu M., Vicente O., et al. (2021). Responses to salinity in four plantago species from Tunisia. Plants 10, 1392. doi: 10.3390/plants10071392
Maggini R., Benvenuti S., Leoni F., Incrocci L., and Pardossi A. (2021). Effects of NaCl on hydroponic cultivation of Reichardia picroides (L.) Roth. Agronomy 11, 2352. doi: 10.3390/agronomy11112352
Maitra S., Hossain A., Brestic M., Skalicky M., Ondrisik P., Gitari H., et al. (2021). Intercropping—A low input agricultural strategy for food and environmental security. Agronomy 11, 343. doi: 10.3390/agronomy11020343
Martini A. N. and Papafotiou M. (2025). Comparative Evaluation of Crithmum maritimum and Origanum dictamnus Cultivation on an Extensive Urban Green Roof. Land 14, 195. doi: 10.3390/land14010195
Martins-Noguerol R., Gallego-Tévar B., Pérez-Ramos I. M., Matías L., Davy A. J., and Cambrollé J. (2024a). Evaluation of direct and transgenerational influences of salinity on germination and early seedling growth in an edible halophyte, Crithmum maritimum. Ann. Bot. 134, 1177–1190. doi: 10.1093/aob/mcae168
Martins-Noguerol R., Matías L., Pérez-Ramos I. M., Moreira X., Francisco M., Pedroche J., et al. (2023). Soil physicochemical properties associated with the yield and phytochemical composition of the edible halophyte Crithmum maritimum. Sci. Total Environ. 869, 161806. doi: 10.1016/j.scitotenv.2023.161806
Martins-Noguerol R., Matías L., Pérez-Ramos I. M., Moreira X., Muñoz-Vallés S., Mancilla-Leytón J. M., et al. (2021). Differences in nutrient composition of sea fennel (Crithmum maritimum) grown in different habitats and optimally controlled growing conditions. J. Food Compos. Anal 106, 104266. doi: 10.1016/j.jfca.2021.104266
Martins-Noguerol R., Rico-Jiménez D., Matías L., Pérez-Ramos I. M., Moreira X., Francisco M., et al. (2024b). Effects of drought and increased temperature on phytochemical traits of the edible halophyte Crithmum maritimum: Perspectives for future climatic scenarios. Environ. Exp. Bot. 226, 105924. doi: 10.1016/j.envexpbot.2024.105924
Mathieu A. S., Dobrev P. I., Tarkowská D., Pospíšil J., Motyka V., Jacquemin G., et al. (2020). Phytohormone profile and CiFL1 expression in young seedlings of Cichorium intybus L. var sativum exposed to high temperature in relation to vernalization and de-vernalization processes. Environ. Exp. Bot. 178, 104127. doi: 10.1016/j.envexpbot.2020.104127
Mathieu A. S., Lutts S., Vandoorne B., Descamps C., Périlleux C., Dielen V., et al. (2014). High temperatures limit plant growth but hasten flowering in root chicory (Cichorium intybus) independently of vernalisation. J. Plant Physiol. 171, 109–118. doi: 10.1016/j.jplph.2013.09.011
Mathieu A. S., Tinel C., Dailly H., Quinet M., and Lutts S. (2018). Impact of high temperature on sucrose translocation, sugar content and inulin yield in Cichorium intybus L. var. sativum. Plant Soil 432, 273–288. doi: 10.1007/s11104-018-3802-7
Mawalagedera S. M. M. R. and Gould K. S. (2015). Chilling and salinity increase extractable antioxidants in cell suspension cultures of the sow thistle, Sonchus oleraceus L. Plant Cell. Tissue Organ Cult. 121, 35–44. doi: 10.1007/s11240-014-0676-0
Meot-Duros L. and Magné C. (2008). Effect of salinity and chemical factors on seed germination in the halophyte Crithmum maritimum L. Plant Soil 313, 83–87. doi: 10.1007/s11104-008-9681-6
Mitrofanova I. V., Lesnikova-Sedoshenko N. P., Chelombit S. V., Zhdanova I. V., Ivanova N. N., and Mitrofanova O. V. (2022). The effect of plant growth regulators on the in vitro regeneration capacity in some horticultural crops and rare endangered plant species. Acta Hortic. 1339, 181–189. doi: 10.17660/ActaHortic.2022.1339.24
Mohamed M. H. M., Ali M. M. E., Zewail R. M. Y., Liava V., and Petropoulos S. A. (2024). The mitigating effects of biostimulant amendments on the response of purslane plants grown under drought stress conditions. Horticulturae 10, 1–14. doi: 10.3390/horticulturae10080858
Molina M., Tardío J., Aceituno-Mata L., Morales R., Reyes-García V., and Pardo-de-Santayana M. (2014). Weeds and food diversity: natural yield assessment and future alternatives for traditionally consumed wild vegetables. J. Ethnobiol. 34, 44–67. doi: 10.2993/0278-0771-34.1.44
Montesano F. F., Gattullo C. E., Parente A., Terzano R., and Renna M. (2018). Cultivation of potted sea fennel, an emerging Mediterranean halophyte, using a renewable seaweed-based material as a peat substitute. Agriculture 8, 96. doi: 10.3390/agriculture8070096
Mulry K. R., Hanson B. A., and Dudle D. A. (2015). Alternative strategies in response to saline stress in two varieties of Portulaca oleracea (Purslane). PloS One 10, 1–18. doi: 10.1371/journal.pone.0138723
Muthusaravanan S., Sivarajasekar N., Vivek J. S., Paramasivan T., Naushad M., Prakashmaran J., et al. (2018). Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environ. Chem. Lett. 16, 1339–1359. doi: 10.1007/s10311-018-0762-3
Naik V. V. and Karadge B. A. (2017). Effect of NaCl and Na2SO4 salinities and light conditions on seed germination of purslane (Portulaca oleracea Linn.). J. Plant Stress Physiol. 3, 1. doi: 10.19071/jpsp.2017.v3.3142
Narzary H., Islary A., and Basumatary S. (2016). Phytochemicals and antioxidant properties of eleven wild edible plants from Assam, India. Med. J. Nutr. Metab. 9, 191–201. doi: 10.3233/MNM-16116
Nastou E., Thalassinos G., Polyzos N., Antoniadis V., and Petropoulos S. A. (2021). The effect of nitrogen fertilization rate on growth and physiological parameters of three purslane genotypes grown in a soilless cultivation system. Acta Hortic. 1321, 125–132. doi: 10.17660/ActaHortic.2021.1321.16
Neji M., Bagues M., Hessini K., and Nagaz K. (2025). Clinal adaptation and phenotypic plasticity drive seed germination and morphology in response to salinity in Tunisian populations of the invasive weed Portulaca oleracea. Euro-Mediterranean J. Environ. Integr. doi: 10.1007/s41207-025-00761-x
Nimac A., Lazarević B., Petek M., Vidak M., Šatović Z., and Carović-Stanko K. (2018). Effects of salinity and seed priming on germination of sea fennel (Crithmum maritimum L.). Agric. Conspec. Sci. 83, 181–185.
Öğretmen N. G., Arabaci O., Tan U., and Yaşar F. (2014). Determination of different seed germination applications on Scolymus hispanicus. Turkish J. Agric. Nat. Sci. 1, 1779–1783.
Ozturk M., Altay V., and Güvensen A. (2021). Portulaca oleracea: A vegetable from saline habitats. in Grigore M. -N. (ed). Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture, (Springer Cham: Cham), 2319–32.
Papadimitriou D. M., Daliakopoulos I. N., Kontaxakis E., Sabathianakis M., Manios T., and Savvas D. (2022). Effect of moderate salinity on Golden Thistle (Scolymus hispanicus L.) grown in a soilless cropping system. Sci. Hortic. (Amsterdam). 303, 111182. doi: 10.1016/j.scienta.2022.111182
Papadimitriou D., Kontaxakis E., Daliakopoulos I., Manios T., and Savvas D. (2020). Effect of N:K ratio and electrical conductivity of nutrient solution on growth and yield of hydroponically grown golden thistle (Scolymus hispanicus L.). Proceedings 30, 1. doi: 10.3390/proceedings2019030087
Paschoalinotto B. H., Polyzos N., Compocholi M., Rouphael Y., Alexopoulos A., Dias M. I., et al. (2023). Domestication of wild edible species: the response of Scolymus hispanicus plants to different fertigation regimes. Horticulturae 9, 1–14. doi: 10.3390/horticulturae9010103
Petropoulos S. A., Fernandes Â., Antoniadis V., Ntatsi G., Barros L., and Ferreira I. C. F. R. (2018a). Chemical composition and antioxidant activity of Cichorium spinosum L. leaves in relation to developmental stage. Food Chem. 239, 946–952. doi: 10.1016/j.foodchem.2017.07.043
Petropoulos S. A., Fernandes Â., Dias M. I., Pereira C., Calhelha R. C., Ivanov M., et al. (2020). The effect of nitrogen fertigation and harvesting time on plant growth and chemical composition of Centaurea raphanina subsp. mixta (DC.) Runemark. Molecules 25, 1–21. doi: 10.3390/molecules25143175
Petropoulos S., Fernandes Â., Karkanis A., Antoniadis V., Barros L., and Ferreira I. C. F. R. (2018c). Nutrient solution composition and growing season affect yield and chemical composition of Cichorium spinosum plants. Sci. Hortic. (Amsterdam). 231, 97–107. doi: 10.1016/j.scienta.2017.12.022
Petropoulos S., Fernandes Â., Karkanis A., Ntatsi G., Barros L., and Ferreira I. (2017a). Successive harvesting affects yield, chemical composition and antioxidant activity of Cichorium spinosum L. Food Chem. 237, 83–90. doi: 10.1016/j.foodchem.2017.05.092
Petropoulos S. A., Karkanis A., Martins N., and Ferreira I. C. F. R. (2018b). Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 74, 69–84. doi: 10.1016/j.tifs.2018.02.006
Petropoulos S., Levizou E., Ntatsi G., Fernandes Â., Petrotos K., Akoumianakis K., et al. (2017b). Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 214, 129–136. doi: 10.1016/j.foodchem.2016.07.080
Petrova M., Nikolova M., Dimitrova L., Dimitrova M., and Sergiev I. (2023). In vitro multiplication and GC/MS-based metabolic profiles of Cichorium intybus L. J. Microbiol. Biotechnol. Food Sci. 13, e9688. doi: 10.55251/jmbfs.9688
Pham A. C., Vo T. C., Vu H. D., and Tran D. Q. (2023). Effects of salinity and drought stress on seed germination of common purslane (Portulaca oleracea). Biol. Life Sci. Forum 27, 31. doi: 10.3390/iecag2023-14974
Pietrelli L., Menegoni P., and Papetti P. (2022). Bioaccumulation of heavy metals by herbaceous species grown in urban and rural sites. Water. Air. Soil pollut. 233, 1–19. doi: 10.1007/s11270-022-05577-x
Pinela J., Carvalho A. M., and Ferreira I. C. F. R. (2017). Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today ‘s society. Food Chem. Toxicol. 110, 165–188. doi: 10.1016/j.fct.2017.10.020
Píoleón J. F., Delgadovargas F., La Luz J. L. L., and Ortegarubio A. (2017). Prioritizing wild edible plants for potential new crops based on deciduous forest traditional knowledge by a rancher community. Bot. Sci. 95, 47–59. doi: 10.17129/botsci.772
Plocek G., Kathi S., and Simpson C. (2023). Effects of eustress induced by low concentrations of salinity on broccoli (Brassica oleracea) and purslane (Portulaca oleracea) microgreens. Technol. Hortic. 3, 1–7. doi: 10.48130/tih-2023-0004
Polyzos N., Paschoalinotto B. H., Compocholi M., Pinela J., Heleno S. A., Calhelha R. C., et al. (2022). Fertilization of pot-grown Cichorium spinosum L.: How it can affect plant growth, chemical profile, and bioactivities of edible parts? Horticulturae 8, 1–22. doi: 10.3390/horticulturae8100890
Polyzos N., Paschoalinotto B. H., Pires T. C. S. P., Añibarro-Ortega M., Calhelha R., Ferreira I. C. F. R., et al. (2024). The impact of deficit irrigation on the agronomic performance and chemical composition of Scolymus hispanicus L. Horticulturae 10, 479. doi: 10.3390/horticulturae10050479
Poursakhi N., Razmjoo J., and Karimmojeni H. (2019). Interactive effect of salinity stress and foliar application of salicylic acid on some physiochemical traits of chicory (Cichorium intybus L.) genotypes. Sci. Hortic. (Amsterdam). 258, 108810. doi: 10.1016/j.scienta.2019.108810
Puccinelli M., Marchioni I., Botrini L., Carmassi G., Pardossi A., and Pistelli L. (2024). Growing salicornia europaea L. with saline hydroponic or aquaculture wastewater. Horticulturae 10, 1–13. doi: 10.3390/horticulturae10020196
Puccinelli M., Pezzarossa B., Pintimalli L., and Malorgio F. (2021). Selenium biofortification of three wild species, Rumex acetosa L., Plantago coronopus L., and Portulaca oleracea L., grown as microgreens. Agronomy 11, 1155. doi: 10.3390/agronomy11061155
Qiu Q. S., Melino V. J., Zhao Z., Qi Z., Sweetman C., and Roessner U. (2022). Editorial: Salinity tolerance: From model or wild plants to adapted crops. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.985057
Rahimi A. and Kamali M. (2012). Different planting date and fertilizing system effects on the seed yield, essential oil and nutrition uptake of Milk thistle (Silybum marianum (L.) Gaertn). Adv. Environ. Biol. 6, 1789–1796.
Rakbar S., Jabbarzadeh Z., and Barin M. (2024). Impact of putrescine and arbuscular mycorrhizal fungi on nutrient uptake, growth, and post-harvest performance of Gerbera (Gerbera jamesonii cv. Dune) cut flowers. Acta Physiol. Plant 46, 1–12. doi: 10.1007/s11738-024-03674-4
Raza A., Razzaq A., Mehmood S. S., Zou X., Zhang X., Lv Y., et al. (2019). Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 8, 34. doi: 10.3390/plants8020034
Renna M. (2018). Reviewing the prospects of sea fennel (Crithmum maritimum L.) as emerging vegetable crop. Plants 7, 92. doi: 10.3390/plants7040092
Romojaro A., Botella M.Á., Obón C., and Pretel M. T. (2013). Nutritional and antioxidant properties of wild edible plants and their use as potential ingredients in the modern diet. Int. J. Food Sci. Nutr. 64, 944–952. doi: 10.3109/09637486.2013.821695
Rouphael Y., Colla G., and De Pascale S. (2021). Sprouts, microgreens and edible flowers as novel functional foods. Agronomy 11, 2568. doi: 10.3390/agronomy11122568
Sadeghi H. and Robati Z. (2015). Response of Cichorium intybus L. @ to eight seed priming methods under osmotic stress conditions. Biocatal. Agric. Biotechnol. 4, 443–448. doi: 10.1016/j.bcab.2015.08.003
Sánchez-Mata M.de C. and Tardío J. (2016). Mediterranean Wild Edible Plants. Eds. Sánchez-Mata M.de C. and Tardío J. (New York: Springer).
Santamaria P. (2006). Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 86, 10–17. doi: 10.1002/jsfa.2351
Sari A. O. and Tutar M. (2009). Effects of light, cold storage, and temperature on seed germination of golden thistle (Scolymus hispanicus L.). J. Herbs Spices Med. Plants 15, 318–325. doi: 10.1080/10496470903507858
Sarrou E., Siomos A. S., Riccadona S., Aktsoglou D. C., Tsouvaltzis P., Angeli A., et al. (2019). Improvement of sea fennel (Crithmum maritimum L.) nutritional value through iodine biofortification in a hydroponic floating system. Food Chem. 296, 150–159. doi: 10.1016/j.foodchem.2019.05.190
Sdouga D., Ben Amor F., Ghribi S., Kabtni S., Tebini M., Branca F., et al. (2019). An insight from tolerance to salinity stress in halophyte Portulaca oleracea L.: Physio-morphological, biochemical and molecular responses. Ecotoxicol. Environ. Saf. 172, 45–52. doi: 10.1016/j.ecoenv.2018.12.082
Sedaghati B., Haddad R., and Bandehpour M. (2019). Efficient plant regeneration and Agrobacterium-mediated transformation via somatic embryogenesis in purslane (Portulaca oleracea L.): an important medicinal plant. Plant Cell. Tissue Organ Cult. 136, 231–245. doi: 10.1007/s11240-018-1509-3
Seifzadeh A. R., Khaledian M. R., Zavareh M., Shahinrokhsar P., and Damalas C. A. (2020). European borage (Borago officinalis L.) yield and profitability under different irrigation systems. Agriculture 10, 1–13. doi: 10.3390/agriculture10040136
Seleiman M. F., Al-Suhaibani N., Ali N., Akmal M., Alotaibi M., Refay Y., et al. (2021). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10, 259. doi: 10.3390/plants10020259
Semeraro T., Scarano A., Leggieri A., Calisi A., and De Caroli M. (2023). Impact of climate change on agroecosystems and potential adaptation strategies. Land 12, 1117. doi: 10.3390/land12061117
Sergio L., de Paola A., Cantore V., Pieralice M., Cascarano N. A., Bianco V. V., et al. (2012). Effect of salt stress on growth parameters, enzymatic antioxidant system, and lipid peroxidation in wild chicory (Cichorium intybus L.). Acta Physiol. Plant 34, 2349–2358. doi: 10.1007/s11738-012-1038-3
Shekhawat S. M., Kannan N., and Manokari M. (2015). Propagation of Portulaca oleracea L. @ in liquid medium: implications of plant growth regulators in culture. J. Microbiol. Biotechnol. Food Sci. 4, 332–335. doi: 10.15414/jmbfs.2015.4.4.332-335
Singh A., Dubey R. K., Bundela A. K., and Abhilash P. C. (2020). The trilogy of wild crops, traditional agronomic practices, and un-sustainable development goals. Agronomy 10, 648. doi: 10.3390/agronomy10050648
Sogoni A., Jimoh M. O., Mngqawa P., Ngxabi S., Le Roes-Hill M., Kambizi L., et al. (2024). Comparing the nutritional, phytochemical, and anti-microbial potential of wild and cultivated Tetragonia decumbens Mill.: A promising leafy vegetable for bio-saline agriculture in South Africa. J. Agric. Food Res. 18, 20220368. doi: 10.1016/j.jafr.2024.101419
Tang N., Zhang B., Chen Q., Yang P., Wang L., and Qian B. (2020). Effect of salt stress on photosynthetic and antioxidant characteristics in purslane (Portulaca oleracea). Int. J. Agric. Biol. 24, 1309–1314. doi: 10.17957/IJAB/15.1564
Thalassinos G., Nastou E., Petropoulos S. A., and Antoniadis V. (2021). Nitrogen effect on growth-related parameters and evaluation of Portulaca oleracea as a phytoremediation species in a Cr(VI)-spiked soil. Horticulturae 7, 192. doi: 10.3390/horticulturae7070192
Thalassinos G., Nastou E., Petropoulos S. A., and Antoniadis V. (2022). Soil dynamics of Cr(VI) and responses of Portulaca oleracea L. grown in a Cr(VI)-spiked soil under different nitrogen fertilization regimes. Environ. Sci. pollut. Res. 29, 14469–14478. doi: 10.1007/s11356-021-16413-w
Thalassinos G., Petropoulos S. A., and Antoniadis V. (2023). The response of purslane (Portulaca oleracea) to soil-added Pb: is it suitable as a potential phytoremediation species? Toxics 11, 153. doi: 10.3390/toxics11020153
Torabi F., Majd A., and Enteshari S. (2012). Effect of exogenous silicon on germination and seedling establishment in Borago officinalis L. J. Med. Plants Res. 6, 1896–1901. doi: 10.5897/JMPR11.1307
Tsoumalakou E., Mente E., Vlahos N., and Levizou E. (2023). Cultivating the mediterranean wild edible species cichorium spinosum L. @ in aquaponics: functional and growth responses to minimal nutrient supplementation. Sustainability 15, 5572. doi: 10.3390/su15065572
Vandoorne B., Mathieu A.-S., Ende W., Vergauwen R., Périlleux C., Javaux M., et al. (2012). Water stress drastically reduces root growth and inulin yield in Cichorium intybus (var. sativum) independently of photosynthesis. J. Exp. Bot. 63, 4359–4373. doi: 10.1093/jxb/err313
Verma A. K. and Yadav M. K. (2024). An overview of the effect of integrated nutrient management on vegetable crops. Agric. Biol. Res. 40, 1222–1225. doi: 10.35248/0970-1907.24.40.1222-1225
Vidalis N., Kourkouvela M., Argyris D. C., Liakopoulos G., Alexopoulos A., Petropoulos S. A., et al. (2023). The Impact of Salinity on Growth, Physio-Biochemical Characteristics, and Quality of Urospermum picroides and Reichardia picroides Plants in Varied Cultivation Regimes. Agriculture 13, 1852. doi: 10.3390/agriculture13091852
Vidalis N., Pentotis E., Thanos N., Alexopoulos A., Tsouvaltzis P., Petropoulos S. A., et al. (2024). Effect of Salinity on the Growth and Biochemical Profile of Hedypnois cretica and Plantago coronopus Plants in Relation to the Cropping System and Growth Environment. Horticulturae 10, 1148. doi: 10.3390/horticulturae10111148
Vlahos N., Levizou E., Stathopoulou P., Berillis P., Antonopoulou E., Bekiari V., et al. (2019). An experimental brackish aquaponic system using juvenile gilthead sea bream (Sparus aurata) and rock samphire (Crithmum maritimum). Sustainability 11, 4820. doi: 10.3390/su11184820
Wang J., Zhang L., Liu K., Zhou B., Gao H., Han X., et al. (2025). Minimizing the potential risk of soil nitrogen loss through optimal fertilization practices in intensive agroecosystems. Agron. Sustain. Dev. 45, 1–14. doi: 10.1007/s13593-025-01006-5
Widderick M. J., Walker S. R., Sindel B. M., and Bell K. L. (2010). Germination, emergence, and persistence of Sonchus oleraceus, a major crop weed in subtropical Australia. Weed Biol. Manage. 10, 102–112. doi: 10.1111/j.1445-6664.2010.00370.x
Yang Y., Chen J., Liu Q., Ben C., Todd C. D., Shi J., et al. (2012). Comparative proteomic analysis of the thermotolerant plant Portulaca oleracea acclimation to combined high temperature and humidity stress. J. Proteome Res. 11, 3605–3623. doi: 10.1021/pr300027a
Zaman S., Bilal M., Du H., and Che S. (2020). Morphophysiological and Comparative Metabolic Profiling of Purslane Genotypes (Portulaca oleracea L.) under Salt Stress. BioMed. Res. Int. 2020, 4827045. doi: 10.1155/2020/4827045
Zanganeh F., Heidari A., Sepehr A., and Rohani A. (2022). Bioaugmentation and bioaugmentation–assisted phytoremediation of heavy metal contaminated soil by a synergistic effect of cyanobacteria inoculation, biochar, and purslane (Portulaca oleracea L.). Environ. Sci. pollut. Res. 29, 6040–6059. doi: 10.1007/s11356-021-16061-0
Zanin G., Ponchia G., and Sambo P. (2009). Yield and quality of vegetables grown in a floating system for readyto-eat produce. Acta Hortic. 807, 433–438. doi: 10.17660/ActaHortic.2009.807.61
Keywords: wild edible species, agrobiodiversity, farming systems, minor crops, climate change, small-scale farming
Citation: Polyzos N, Liava V, Antoniadis V, Garcia P, Alexopoulos AA and Petropoulos SA (2025) The new trends in wild edible plants valorization: commercial cultivation protocols, agronomic practices and future challenges. Front. Hortic. 4:1638703. doi: 10.3389/fhort.2025.1638703
Received: 31 May 2025; Accepted: 30 June 2025;
Published: 23 July 2025.
Edited by:
Antonio Ferrante, Sant’Anna School of Advanced Studies, ItalyReviewed by:
Bozidar Benko, University of Zagreb, CroatiaAna A Feregrino-Perez, Autonomous University of Queretaro, Mexico
Copyright © 2025 Polyzos, Liava, Antoniadis, Garcia, Alexopoulos and Petropoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Spyridon A. Petropoulos, c3BldHJvcG91bG9zQHV0aC5ncg==
 Nikolaos Polyzos
Nikolaos Polyzos Vasiliki Liava1
Vasiliki Liava1 Vasileios Antoniadis
Vasileios Antoniadis Pedro Garcia
Pedro Garcia Alexios A. Alexopoulos
Alexios A. Alexopoulos Spyridon A. Petropoulos
Spyridon A. Petropoulos