- 1School of Mathematical and Physical Sciences, The University of Sheffield, Sheffield, United Kingdom
- 2Biology Department, Centre for Novel Agricultural Products (CNAP), University of York, York, United Kingdom
- 3Plants, Photosynthesis and Soil, School of Biosciences, The University of Sheffield, Sheffield, United Kingdom
- 4Department of Earth and Environmental Sciences and Manchester Institute of Biotechnology, The University of Manchester, John Garside Building, Manchester, United Kingdom
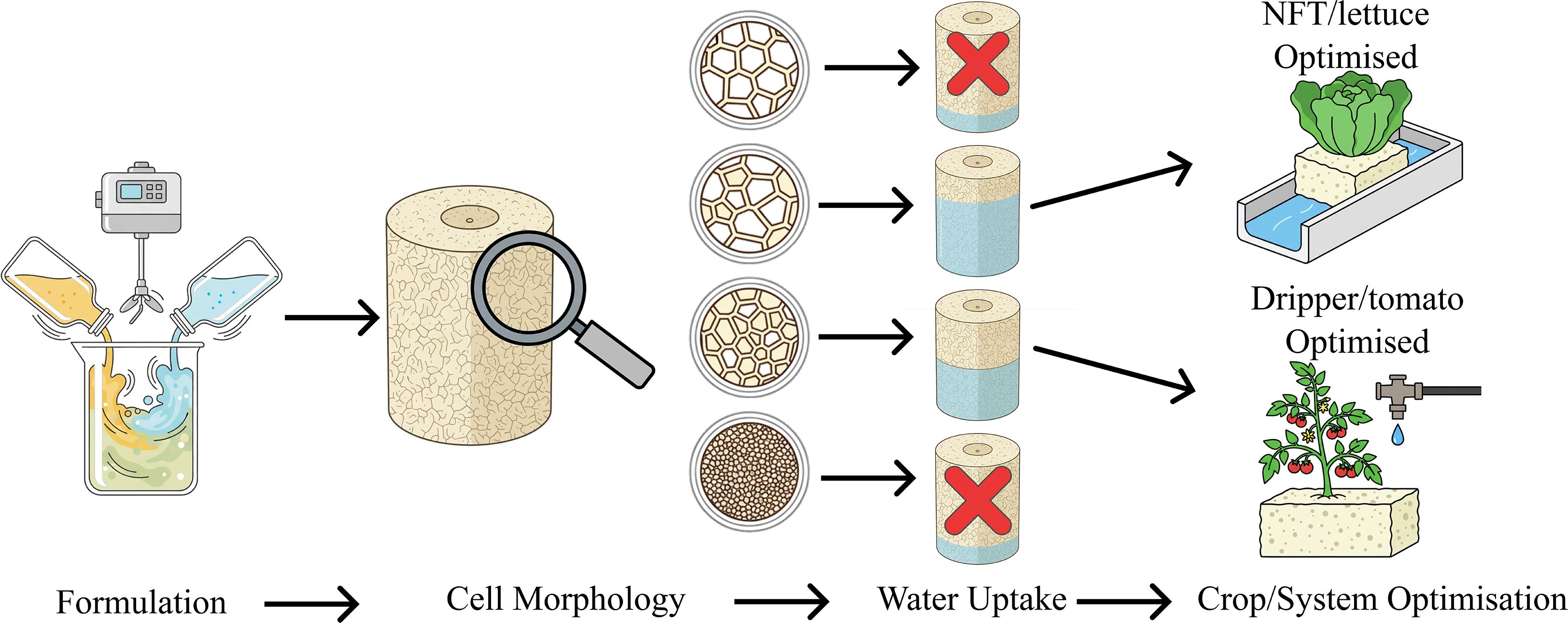
Introduction: Increasing adoption of hydroponics in food production has increased the demand for soilless growing media. Given the variety of crops and cultivation techniques used in soilless systems, optimising the physical properties of novel media for specific crops and systems presents an opportunity, as some growing media, such as mineral wool, provide only a limited set of physical properties. Polyurethane foams (PUFs), a promising soilless growing media, offer flexibility, as foams with a diverse range of physical properties can be produced.
Methods: We examined 10 distinct PUF formulations with a range of physical properties (cell size [d], open cell fraction [peff], maximum water uptake height [Hmax], water uptake rate [Wur]) through germination and growth trials. Additionally, investigations into whether these media influence disease susceptibility were conducted by inoculating tomato plants with Pythium spp.
Results and discussion: Germination trials using lettuce and tomato identified four PUF formulations as unsuitable. A small-scale growth trial demonstrated that the remaining formulations performed comparably to mineral wool (MW). Three of these formulations were tested in trials for lettuce and pak choi in a NFT system and for tomato using a dripper-fed system. Results indicated that two PUF formulations surpassed MW in vegetative growth in tomato trials (F04: d = 669 µm, peff = 0.694, Hmax = 2.94 cm, Wur = 0.023 cm s-1; F08: d = 624 µm, peff = 0.38, Hmax = 2.35 cm, Wur = 0.012 cm s-1) and two PUF formulations matched MW in lettuce yield in NFT trials (F04: properties detailed above; F07: d = 683 µm, peff = 0.897, Hmax = 3.07 cm, Wur = 0.055 cm s-1). Pak choi plants in foam displayed slightly lower yields than those in MW, although differences were not significant. All foam samples suppressed Pythium inoculation, as evidenced by no reduction in germination rates or seedling mass when compared to the uninoculated samples, warranting further investigation into disease suppression. These growth results suggest that growing media physical properties should be optimised according to both hydroponic technique and crop to maximise yields and that PUF media can aid in developing tailored growing media for specific crop and systems.
1 Introduction
The escalating challenges posed by global soil degradation and the increasing unpredictability of weather patterns due to climate change are placing significant pressure on traditional agricultural practices (FAO, 2015). Alternative production systems, such as hydroponics or soilless cultivation, a controlled environment agriculture (CEA) technology, has emerged as a promising method for cultivating crops without soil whilst controlling the crop environment and circumventing many of the limitations associated with traditional farming, especially soil degradation and unpredictable changing weather patterns (Cowan et al., 2022). In addition to challenges like soil degradation and climate change, a significant driver for the adoption of soilless cultivation has been the need to manage the build-up of soil-borne pathogens in intensive monoculture systems. The global phase-out of effective soil fumigants, most notably methyl bromide, accelerated this transition, compelling growers to seek alternatives like hydroponics to ensure crop health and consistent yields (Backstrom, 2002; Shwin et al., 2003).
In hydroponic and soilless cultivation, the soilless growing media serves as the foundational matrix that directly influences plant health and productivity by providing essential support, facilitating aeration, retaining crucial moisture, and enabling the delivery of nutrients to the plant roots (Gruda, 2019). Key physical attributes such as water holding capacity, aeration, porosity, bulk density, and drainage collectively determine the suitability of a media for optimal plant yield (Fields and Gruda, 2021). Water holding is crucial for maintaining adequate hydration of the root system without leading to waterlogged conditions. A media with good water retention ensures that plants can endure periods of reduced irrigation or fluctuating environmental conditions. Aeration, the provision of sufficient airflow around the roots, is equally vital for preventing root rot and promoting healthy plant growth. Inadequate aeration can lead to the buildup of carbon dioxide, which can be toxic to roots, and create an environment conducive to anaerobic pathogens that cause detrimental root diseases (Gliński and Stępniewski, 2018). Porosity, referring to the empty spaces within the growing media, is fundamental for both air circulation and water movement. Total porosity comprises both air porosity and water holding porosity, and an ideal medium typically exhibits a total porosity exceeding 60-70%, with an aeration porosity of at least 20-30% on a volume basis (Caron and Michel, 2021). The distribution of pore sizes is also critical, with macro pores (> 75 µm) facilitating aeration and drainage, while micro pores (<30 µm) are responsible for water retention with available water held in pores between 0.2 µm and 30 µm (Cameron and Buchan, 2017). A balanced distribution ensures both adequate oxygen supply and moisture availability. In loose and packed media, particle size, particle size distribution and particle shape also have an impact on hydrodynamics, water holding capacity, available water and air filled pore space (Durand et al., 2024). Bulk density of a porous material, the measure of a growing medium's mass relative to its volume, influences the media's ability to stabilise container plants and can impact aeration. Lower bulk density is associated with higher total porosity, which is beneficial for root health (Bartley et al., 2022). However, sufficient bulk density is necessary to provide adequate physical support for larger plants, preventing them from toppling over. In addition to physical properties, chemical and biological properties are also important when designing a growing media. However, for this work, we examine chemically and biologically inert media and therefore focus on physical properties.
It is well established that various plant species exhibit distinct preferences for the physical properties of their growing media, particularly concerning water retention and aeration (Gruda, 2019). Furthermore, the requirements for the growing medium can also vary depending on the specific type of hydroponic system employed (Fussy and Papenbrock, 2022). The ability to precisely adjust the physical characteristics of a growing media for specific plant species and hydroponic systems holds significant potential for optimising root zone conditions, enhancing nutrient uptake efficiency, improving water utilisation, and ultimately achieving higher crop yields and superior quality (Fields and Gruda, 2021). For instance, leafy greens might thrive in lightweight, well aerated media, while fruiting plants may require media with higher water retention. Similarly, an ebb and flow system might benefit from a media with good water retention, whereas a deep-water culture system might perform better with a lightweight media that promotes oxygenation. A model growing media platform, based on a single type of material, that allows for the manipulation of physical properties would offer an invaluable tool for researchers and growers to systematically investigate and optimise growing conditions for a diverse range of plants and systems, potentially streamlining both experimentation and commercial production.
Polyurethane (PU) foams, a versatile solid polymeric foam have long found application in horticulture, with the first patent for its use as a synthetic soil issued in 1976 (Bardsley, 1976). Early investigations often utilised PU foam sourced from other industries, which were not specifically designed or optimised for horticultural purposes. However, more recent research endeavours, often in collaboration with polyurethane manufacturers, have focused on developing tailored foam formulations that can match or even surpass the performance of established synthetic media like mineral wool. Notably, PU foam media have demonstrated the potential for reuse over extended periods, lasting up to 10 years (Benoit and Ceustermans, 1995) and can even exhibit improved water holding capacity over successive cropping cycles due to the retention of organic matter from roots (Hardgrave, 1995). Even PUF that have not been optimised for plant growth have been shown to be useable for soilless horticulture, with recycled mattresses being used in NFT systems (Al Meselmani et al., 2020). Polyurethane foam plugs are now commonly employed as collars or supports in solution culture systems, indicating a specific and established role for this material in hydroponics. Polyurethane foams (PUF) possess many of the inherent characteristics required for successful soilless cultivation, including the mechanical strength necessary to anchor plants and a highly porous structure that facilitates the essential transfer of liquids and gases. The promising results observed with early, non-optimised foams spurred further scientific inquiry and development, leading to the creation of specialised formulations designed to enhance plant growth. These specialised formulations now underpin a successful commercial market for PUF plugs, developed through close collaboration between foam manufacturers and growing media experts.
The structural versatility of PUF offers a platform for modelling plant media interactions due to the nature of their formulation chemistry which offer the ability to synthesise foams with a wide array of physical properties based on the same materials chemistry. By carefully adjusting the quantities of polyols, isocyanates, catalysts, surfactants, and blowing agents during the manufacturing process, the cell size and overall pore architecture of flexible polyurethane foam can be controlled. Polyols and isocyanates make up the backbone of foams, and their chemical backbones can affect hydrophobicity of the polymer. Catalysts and surfactants affect the rates of reactions, varying the rate of bubble formation and surfactants stabilise these bubbles and with the catalysts impact the resulting cell size and open cell fraction. Blowing agents influence the amount of gas formation and also impact resulting cell morphology (Szycher, 2013). Design of Experiment (DoE) techniques have been successfully employed to generate polyurethane foams with a broad spectrum of physical properties by systematically varying the ratios of catalysts, surfactants and additives (Wright et al., 2021a, Wright et al., 2022b). These experimental approaches have also enabled researchers to model the complex relationships between the physical properties of the foam and plant growth (Wright et al., 2021a, Wright et al., 2021b). Of these physical properties the open-cell content was an important parameter for controlling hydrodynamic properties. Commercially, an open-cell structure is often achieved via post-synthesis mechanical crushing, a process that ruptures the cell windows. However, this method is largely binary in nature, resulting in a foam that is either closed-cell or almost fully open-cell, offering little control over intermediate states. In contrast, the chemical synthesis approach utilised in these studies provided precise control over open cell fraction allowing the open-cell fraction to be tuned across a continuous range from nearly closed to fully open. This chemical tunability of polyurethane foam makes it an ideal candidate for a model growing media, allowing researchers to systematically vary specific physical properties, such as water holding capacity and aeration, while maintaining other factors relatively constant. This controlled manipulation enables the isolation and study of the specific impact of these individual properties on plant growth and physiology, providing valuable insights into plant media interactions.
Beyond providing insights into plant media interactions this study provides an opportunity to investigate another critical factor in hydroponic success: disease management. To mitigate the disease risk in soilless systems, preventing spread should always be the primary defence, however, ensuring a disease suppressive root environment will also contribute to a healthy crop (Alsanius and Wohanka, 2019). Damping off, caused by oomycete pathogens of the genus Pythium, is a problematic disease in hydroponic horticulture, particularly for young plants (Amrhein et al., 2025), and is responsible for significant losses in a wide range of crops. In tomato, for example, Pythium spp. can cause seed decay along with pre- and post-emergence damping off (Gravel et al., 2005). Physical properties have been shown to influence Pythium disease severity with media that have high water holding capacity favouring the survival and spread of motile Pythium zoospores (Van Der Gaag and Wever, 2005). Conversely, a well-aerated matrix may physically impede pathogen spread and promote a healthier root system more capable of resisting Pythium infection. The PUF system described previously is therefore the ideal platform to test this hypothesis by isolating the effects of properties like water content and porosity on the progression of Pythium disease.
The aim of this study was to determine whether, by manipulating PUF select physical properties (density, cell size, open cell fraction, water uptake), PUF growing media can be used to demonstrate the importance of optimising the physical properties of soilless growing media, depending on the crop that is being grown and the hydroponic system used.
2 Materials and methods
2.1 Materials
To produce PUF materials, raw ingredients were as follows. DOW Chemical Company (Michigan, United States) kindly supplied Polyols, Voranol™ 3322 (a high propylene oxide content polyether triol), Voranol™ 1447 (a high ethylene oxide content polyether), isocyanate, SpecFlex™ NE 112, a low functionality methylene diphenyl diisocyanate (MDI) and surfactants Vorasurf™ 5906, a medium to high efficiency silicone siloxane and Vorasurf™ 5959, a silicone surfactant to be used as a cosurfactant to introduce finer cells/pneumaticity in production of flexible slabstock polyurethane foams. The catalyst Dabco® T (N-Methyl-N-(N,N-dimethylaminoethyl)-aminoethanol), a non-emissive amine catalyst that promotes the urea reaction, was provided by Evonik Industries (Essen, Germany). The additive, Cloisite® NE 116, a sodium bentonite clay, was provided by BYK-Chemie GmbH (Wesel, Germany). Deionised water was used as the blowing agent and all reagents were used as received.
2.2 Methods
2.2.1 Foam formulation and synthesis
The ten foam formulations are given in Table 1. The amounts of the two surfactants: S1 (Vorasurf™ 5906) and S2 (Vorasurf™ 5959) were varied systematically. This was based on previous work (Wright et al., 2022b) that showed varying these two surfactants produced a set of foams with a large range of physical properties, particularly foams with varying cell size and open to closed cell ratios, the target of this study. For all formulations the isocyanate index (R) was kept constant at 1.2. All masses are reported in parts per hundred polyol (PPHP).
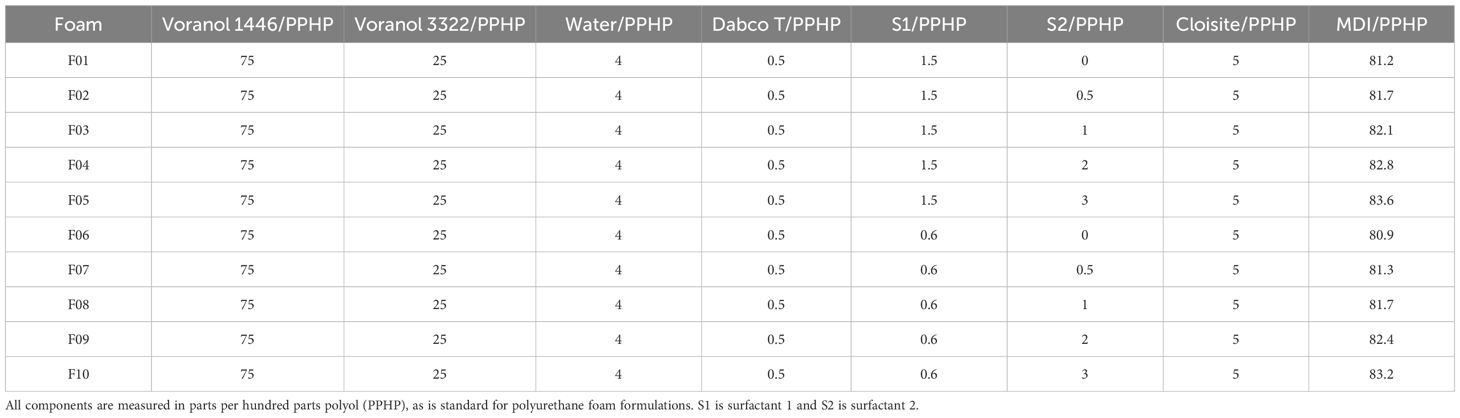
Table 1. Polyurethane formulation for experiments, only the two surfactant concentrations are varied (slight changes in methylene diphenyl diisocyanate, MDI, were made to ensure the isocyanate index, R, remained at 1.2).
Small scale isocyanate conversion foam trials were completed as follows. The polyisocyanate was weighed into a 30 ml syringe. The remaining reaction components were weighed into a 568 ml polypropylene cup and mixed at 3000 RPM for 45 seconds with an overhead mixer with a straight blade disk agitator. This mixture was allowed to debubble in a fume hood for 5 minutes before reacting. The polyisocyanate was added to the polypropylene cup and mixed at 1500 RPM for 6 seconds using the same overhead mixer/stirrer combination. The reacting mixture was transferred immediately from the reaction vessel into a FoamPi (Wright et al., 2022a) an apparatus developed specifically for measuring foam reaction kinetics, for 10 minutes before being transferred to a curing oven at 120 °C for 20 minutes. The FoamPi was employed to ensure full isocyanate conversion, with the calculation detailed in (Wright et al., 2022a.).
For determining all other properties, and for growth trials, PUF synthesis was scaled up by using a 250 mm (L) × 250 mm (W) × 250 mm (H) box as the reaction vessel. This was lined with PTFE film to allow easy removal of the PUF. Mixing and reaction conditions were the same as the small-scale foam experiments.
2.2.2 Foam characterisation methods
PUF density was determined according to ASTM D3574–11 Test A (ASTM Standard D3574-11, 2012). Foam pieces of size 50 mm (L) × 50 mm (W) × 25 mm (H) were cut, from the box block, with the L × W plane perpendicular to the foam rise direction, their dimensions determined using a digital Vernier calliper and their mass recorded. Mass and volume were used to determine PUF density. Analysis was done in triplicate and the mean and standard error are reported.
PUF media cell size was determined according to ASTM D3576-15 (ASTM Standard D3576-15, 2014). A thin piece of foam was cut perpendicular to the rise direction. The surface of the foam was stained using a marker pen and imaged using optical microscopy. ImageJ (Rasband, 1997) software was used to determine the cell size. A minimum of 200 cells was counted and the mean diameter and standard error reported.
Water uptake of media was measured using an adaptation of the apparatus described by (Schulker et al., 2021). Briefly, foam samples were cut from the full box block into 20 mm (L) × 20 mm (W) × 50 mm (H) pieces and placed vertically in a sub irrigation system which irrigated the media for known time intervals (for a final irrigated time of 570 s) to a height of 25 mm (subirrigation height). This irrigation is repeated over several cycles to generate a water uptake curve. Sample mass was determined between each irrigation cycle and used to calculate the capillary height of water rise over time. Triplicate samples were measured, and a water uptake curve is generated from this data with a fitted exponential decay curve. The fitting parameters of this curve give important insight into the water uptake of the media. Equation (1) shows the equation used to fit the capillary rise data.
Where HCR is the capillary height of water rise over time, Hmax is the maximum capillary rise height in cm, WUR is the rate of water uptake in cm s−1 and t is the total sub irrigation time in seconds. The mean and standard error of these two fitting parameters is reported.
Airflow through the foam was calculated according to ASTM D3574–11 test G (ASTM Standard D3574-11, 2012), whereby airflow (in L min-1) was measured through the sample at a standard pressure difference (125 Pa). A calibration curve was generated for determining the linear relationship between airflow and open cell content (Yasunaga et al., 1996) and the effective open cell fraction (peff) is reported. Analysis was done in triplicate and the mean and standard error are reported.
2.2.3 Germination/growth trials
The two germination trials and two vegetative growth trials were performed in a temperature-controlled greenhouse at the Arthur Willis Environmental Centre (AWEC) at the University of Sheffield with a day/night regime of 12 h at 20 °C/12 h at 15 °C. Germination Trial 1 (GT1) was conducted between 2023/02/15 and 2023/02/22. Vegetative growth trial 1 (VGT1) was conducted between 2023/02/22 and 2023/03/29, using seedlings from GT1. Germination trial (GT2) was conducted between 2023/05/17 and 2023/05/25, Vegetative growth Trial 2 (VGT2) was conducted between 2023/05/25 and 2023/06/19 using seedlings from GT2. Tomato Moneymaker (Solanum lycopersicum), lettuce little gem (Lactuca sativa) and pak choi (Brassica rapa var chinensis) seeds were purchased from Suttons seeds (Devon, UK).
Vitalink Hydromax SW two-part hydroponic nutrient was used for all germination and vegetative growth trials. For all germination trials, PUF/MW media plugs were soaked in 4 mL L-1 nutrient solution (2 mL L-1 part A and 2 mL L-1 part B) for 5 minutes before sowing of tomato/lettuce/pak choi seeds. For all vegetative growth trials, the nutrient concentration was 8 mL L-1 (4 mL L-1 part A and 4 mL L-1 part B). All experiments were maintained at pH 6 using dilute phosphoric acid. Concentration of undiluted nutrient solution is given in Supplementary information Supplementary Table S1.
For GT1 and GT2–20 seeds of each crop were germinated in individual growing media starter cubes of size 25 mm (L) × 25 mm (W) × 40 mm (H). Two separate hydroponic systems were used in VGT1 and VGT2, a recirculating nutrient film technique (NFT) system, for lettuce and pak choi and a dripper fed open system for tomato. Drippers were pressure compensated 2 L h-1 drippers used to provide irrigation for 5 equally spaced one-minute intervals in the daytime. For the dripper trial, tomato seedlings were transferred into larger media blocks of size 100 mm (L) × 100 mm (W) × 65 mm (H). For NFT crops, seedlings were transferred into the channels in their starter media blocks. For VGT1 four replicates were used for the tomato vegetative growth trial and seven replicates for the lettuce trial, the number of replicates was dictated by the maximum number of samples that could be run in the system. The reduced number of media in VGT2 meant more replicates could be completed. For this trial eight replicates were used in the tomato trial and ten replicates were used in the lettuce and pak choi trial. For germination and vegetative growth trials, days after planting is reported (DAP) as the number of days since seeds were sown in the media.
2.2.4 Pythium infection trial
Pythium spp. isolates NIBIO_231095 and NIBIO_231243 were sourced from the Norwegian Institute of Bioeconomy Research (NIBIO) fungal culture collection. Isolates were confirmed as Pythium sp. by sequencing internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2 using the ITS1oo (Riit et al., 2016) and ITS4 (White et al., 1990) primers, producing consensus sequences (Supplementary Table S2) and searching for alignments in GenBank using BLASTN (Benson et al., 2012). Herrero and colleagues (Herrero et al., 2020) conducted ITS (accession No. MK644147) and cytochrome c oxidase subunit I (COI; accession No. MK644144) partial gene sequencing for isolate NIBIO_231095 and concluded that it belongs to Pythium clade B2 (André LéVesque and De Cock, 2004). ITS sequence homology suggests that NIBIO_231243 is Pythium attrantheridium.
Pythium sp. isolates were cultured for 7 days at 25 °C in the dark and without shaking (Postma et al., 2005). Cultures consisted of a glycerol stock (1 mL 15% glycerol and plug of V8 juice agar covered in mycelium), revived from storage at -70 °C, in V8 juice media which contained 4 mL V8 Juice (Campbell Soup Co., Camden, NJ, USA), 16 mL deionised H2O (dH2O) and 60 mg CaCO3. Inoculums were prepared by washing mycelial mats three or four times in dH2O, weighing mats to determine inoculum dose, blitzing for 30 s at low speed with a D-8 homogeniser (MICCRA, Buggingen, Germany) in 50–100 mL dH20 and topping up to a final volume of 200–330 mL with dH2O. The final Pythium inoculum contained 5.5-9.5 g of mycelial mat per L per isolate. Seeds of tomato, lettuce, and pak choi were sown in individual growing media starter cubes (25 mm × 25 mm × 40 mm) pre-soaked in 4 ml per L of PlantStart propagation feed (Vitalink, Coventry, UK). For each crop and medium combination, 24 seeds were sown. Following sowing, starter plugs were inoculated with 1 mL of Pythium inoculum or 1 mL of dH2O as a control. This experiment was repeated three times. To increase pathogen load in repeat three, 1 mL of Pythium inoculum or 1 mL of dH2O were added again at 2 days post sowing. Plugs were maintained for 14 days post sowing at the Arthur Willis Environmental Centre (AWEC), University of Sheffield, under a 12 h day/12 h night regime (20 °C/15 °C) within propagators at 100% relative humidity (RH). Germination was recorded every day and at 14 days post sowing (DAP) fresh weight of above ground tissue was measured.
2.2.5 Statistics
For the analysis of the physical properties of the foams, the mean and standard error were reported. Germination trials, GT1 and GT2, were evaluated using Kaplan-Meier survival analyses for germination and cotyledon expansion, generated with the Lifelines package in Python (Davidson-Pilon, 2019). These survival curves were compared using pairwise log-rank tests, and media that exhibited significant differences from the mineral wool control were reported. For vegetative growth trials, VGT1 and VGT2, shoot fresh mass (SFM) and shoot dry mass (SDM) were analysed using a one-way ANOVA with growing media as the factor. These analyses were performed with the Statsmodels package in Python (Seabold and Perktold, 2010). When the ANOVA indicated significant differences between means, post hoc Tukey HSD tests were conducted using the SciPy stats package (Virtanen et al., 2020). Python 3.12.4 was used for all analyses and figure generation.
3 Results
3.1 PUF physical properties
The density of the foams showed minimal variation, ranging from 24 to 29 kg m-3, relatively low-density, which for flexible PUF can vary depending on formulation and application between 8–100 kg m-3 (Polyurethane Foam Association, 2016). This limited density variation was expected, as only surfactant quantities were altered, which have a minimal impact on foam density (Figure 1A). Foam density decreased with increasing S2 concentration, reaching a minimum when S2 was 1 PPHP. This low density indicates high porosity, which can be estimated using the material density of polyurethane. For all samples total porosity of 0.97-0.98. Cell diameters ranged from 600 µm to 800 µm, typical for polyether flexible foams. Cell diameter decreased with increasing concentrations of either surfactant, reaching a minimum of approximately 600 µm (Figure 1B) Full cell size distribution in supplementary information as Supplementary Figure S1. The ten foams exhibited a uniform distribution of cell diameters, providing a spectrum suitable for testing. Effective open cell content ranged from 0.13 to 1 (zero indicates every cell is closed, and one that every cell is fully open), with several foams exhibiting fully open cells and others nearly completely closed cells (Figure 1C). We have previously demonstrated (Wright et al., 2022b) that these properties are predictive of polyurethane foam hydrodynamic behaviour, a critical factor influencing plant growth (Wright et al., 2021a). Consequently, the primary objective of the formulation was to generate foams with a wide range of cell structures. This variation in cell properties resulted in foams with diverse water uptake capacities, with fully closed cell foams exhibiting minimal water uptake (< 0.5 cm) and partially or fully open cell foams, particularly those with smaller cells, absorbing up to 3 cm of water (Figure 1 D). Similarly, a broad range of water uptake rates (WUR) was achieved. WUR varied from near zero (~ 0 cm s-1), particularly in samples with low effective open cell content due to restricted water ingress, with the majority of samples having rates around 0.01 – 0.1 cm s-1 and a few with the lowest surfactant loadings having higher rates nearing 0.15 cm s-1 (Figure 1E). Full water uptake data as well as fitted curves is given in Supplementary Figure S2. Hmax and WUR for all foams was significantly lower than that of MW, which had a Hmax 3.53 ± 0.237 cm and a WUR of 0.687 ± 0.287 cm s-1. Physical properties for each formulation are summarised in Table 2.
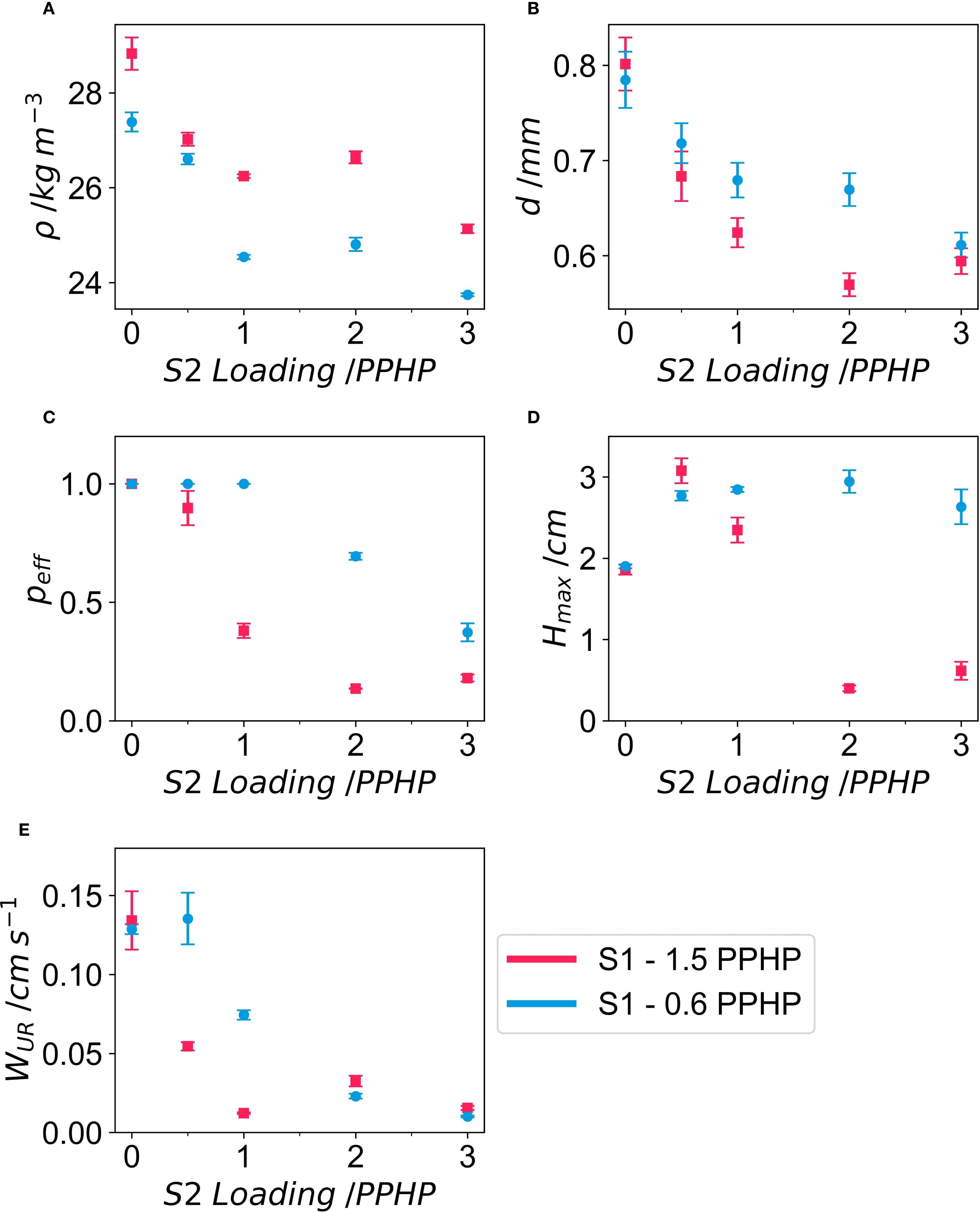
Figure 1. Physical and hydrodynamic properties of PUF growing media at different surfactant-1 (S1) and surfactant-2 loadings (S2). Properties are shown for: density, ρ, (A), cell diameter, d, (B), effective open cell content, Peff, (C), maximum water uptake height, Hmax, (D) and water uptake rate, WUR (E). Results are means +/- SE. Surfactant loadings are given in parts per hundred polyol (PPHP) as is standard in polyurethane formulation chemistry.
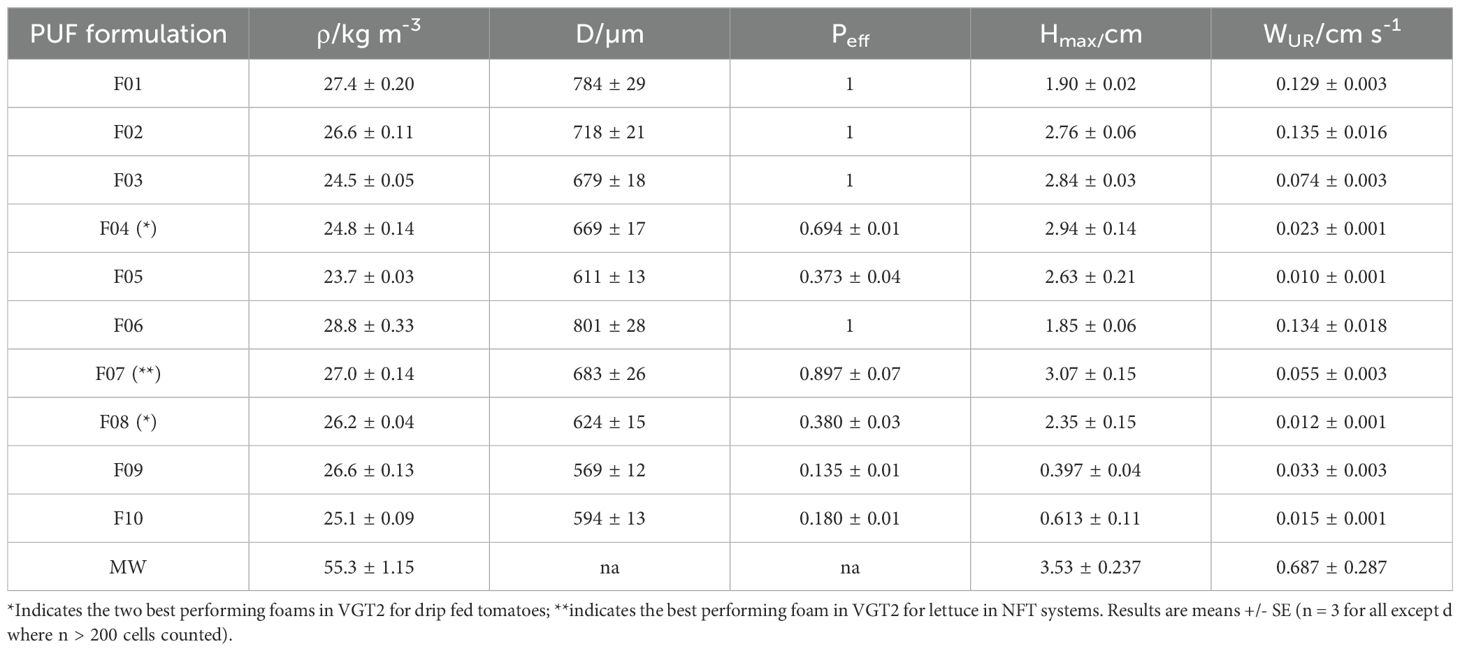
Table 2. Physical properties of PUF formulations as well as select properties of MW; density, ρ, cell diameter, d, effective open cell content, Peff, maximum water uptake height, Hmax, and water uptake rate, WUR.
3.2 Germination trial (GT1)
Germination fraction (Figure 2) for the mineral wool control (MW) were 75% for tomato, and 70% for lettuce seeds germinating within 8 days. Germination fraction and Kaplan-Meier survivability curves for tomato and lettuce in foams F02 - F05, F07 and F08 were not significantly different from MW controls. Germination was notably reduced in four polyurethane foam (PUF) formulations: F01, F06, F09, and F10. This reduction was observed for both tomatoes (Figures 2A, B) and lettuce (Figures 2C, D) with all four Kaplan-Meier curves exhibiting significant deviations from the MW curve (p <.05, pairwise log-rank test). Consequently, vegetative growth trial 1 (VGT1) proceeded with seedlings from the MW control and the six foam formulations (F02-F05, F07, and F08) where the Kaplan-Meier curves did not differ significantly from the MW curve.
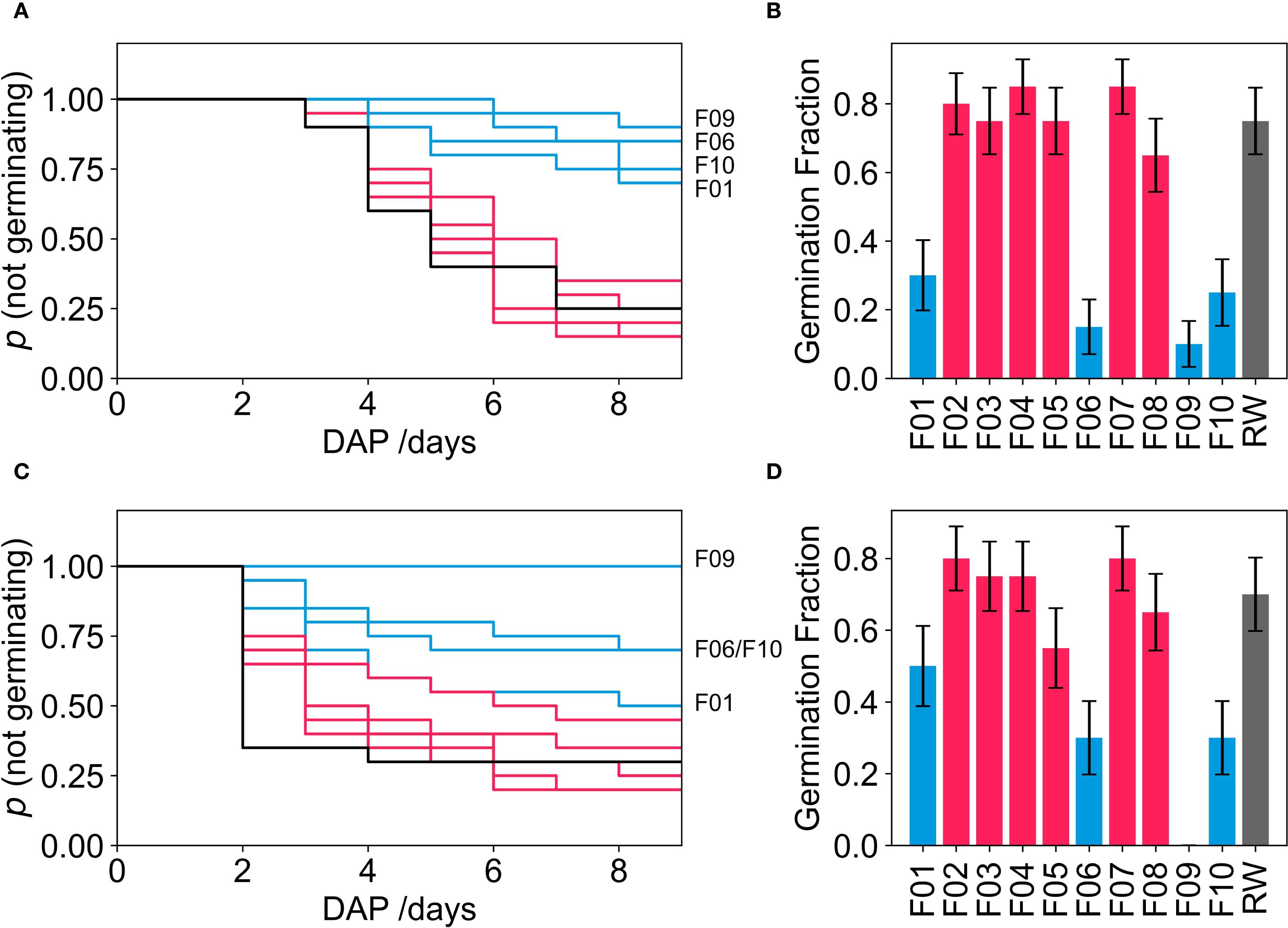
Figure 2. Kaplan-Meier curves for the probability of a seed not germinating over time as well as the germination fraction for tomato seeds (A, B) respectively and lettuce seeds (C, D) respectively. Black lines indicate mineral wool samples, pink indicates PUF samples where p >.05 (pairwise Log-rank test) when compared to mineral wool curves and blue lines indicate where p<.05 (pairwise Log-rank test) when compared to mineral wool curves.
3.3 Vegetative growth trial (VGT1)
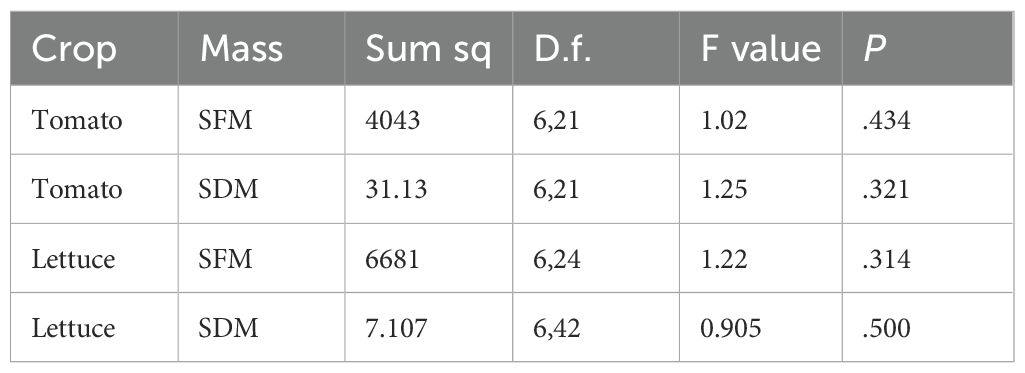
Seedlings from GT1 were transferred into an NFT hydroponic system (lettuce) and a dripper fed hydroponic system (tomato) for vegetative trial (VGT1). Lettuce was grown to yield, and tomatoes were grown until flowering began. The shoot fresh mass (SFM) and shoot dry mass (SDM) was measured for both lettuce and tomato. One way ANOVA indicated that there was no significant effect of media on the SFM or SDM for either of the crops (Figure 3; Table 3).
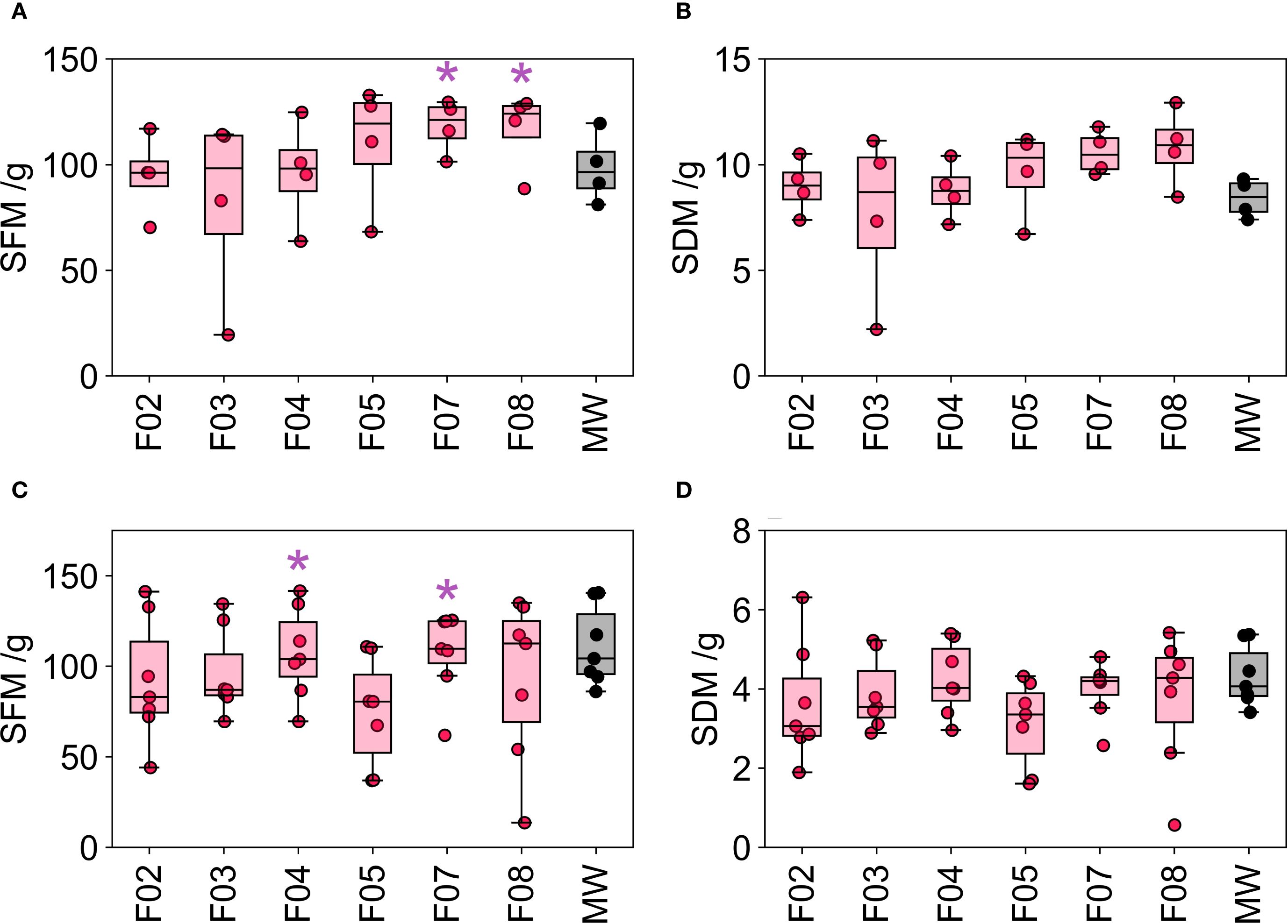
Figure 3. Shoot fresh mass (SFM) and shoot dry mass (SDM) for tomato (A, B) respectively) and lettuce (C, D) respectively) grown in different growing media. Mineral wool is shown in black; pink indicates PUF formulations where p >.05 between the formulation and mineral wool samples and blue indicates PUF formulations where p <.05 between the formulation and mineral wool samples (Tukey's HSD test). The thick black line in the box plots indicate the median, the box indicates the IQR, and the whiskers show 1.5 × IQR. The purple asterisks indicate the two foam media for each crop that had the largest mean SFM value.
Following the first vegetative trial (VGT1) the number of growing media formulations was reduced. This step was required to increase the number of replicates for the remaining candidates, thereby enhancing the statistical power of the experiment. However, due to limitations in the number of plants that could be accommodated in each system, increasing the number of replicates necessitated a reduction in the number of growing media tested. The two best performing foam media (based on mean shoot fresh mass) for each crop were carried forward to GT2 and VGT2. The best performing foam media were F07 and F08 for tomato, and F04 and F07 for lettuce with MW as a control as shown by the purple asterisk in Figure 3. Therefore, three foams were taken forward into GT2 and VGT2: F04, F07 and F08.
3.4 Dual-system trial
3.4.1 Germination trial (GT2)
Germination rates in mineral wool (MW) control were >0.85 for all three crops. All three foams matched the performance of MW for each crop. Kaplan-Meier survival analysis, followed by pairwise Log-rank tests, showed no significant differences in germination timing or rates between the foams the MW control in any of the crops (Figure 4). This result was consistent with findings from GT1 and confirms that the foam media also supported pak choi germination comparably to the MW control.
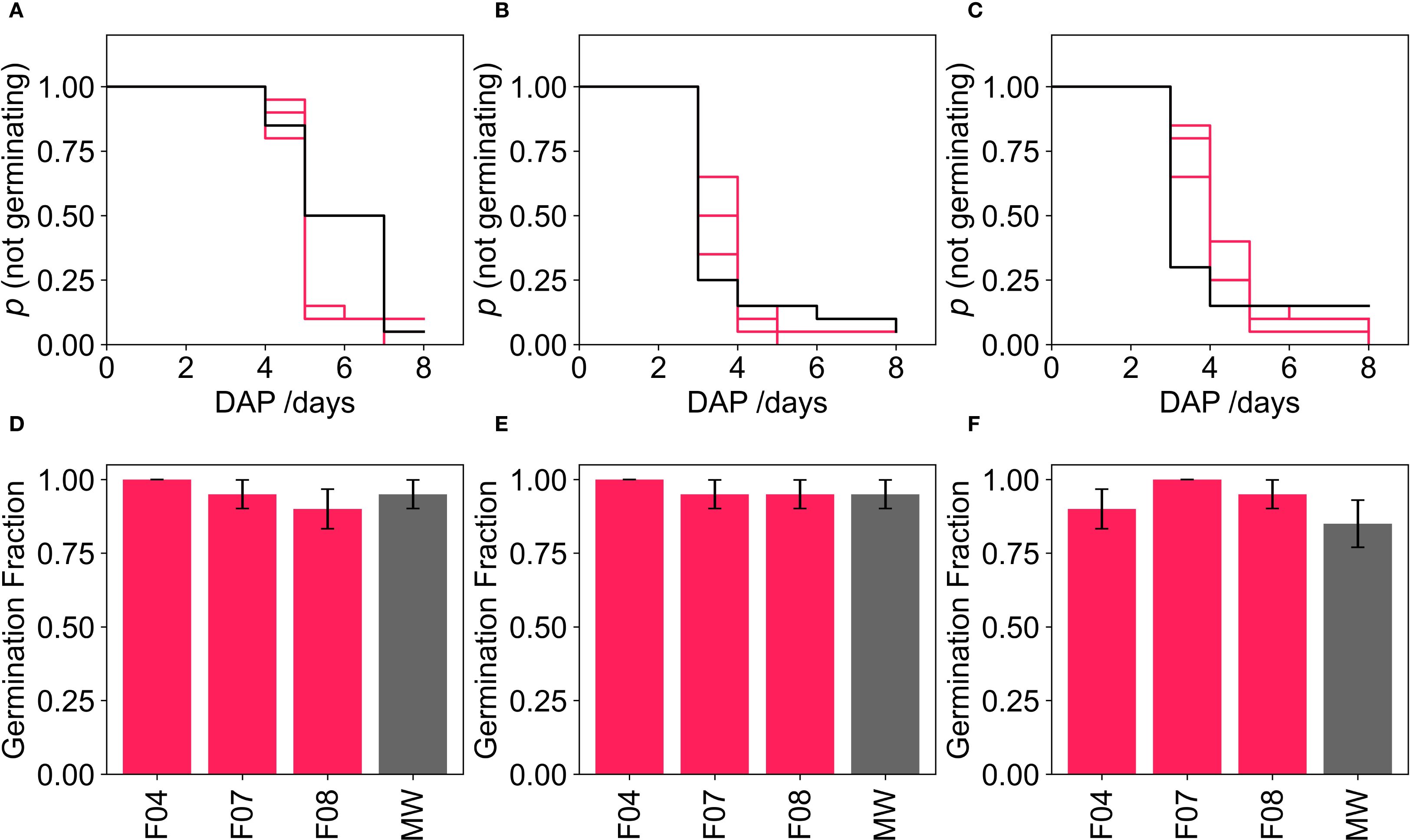
Figure 4. Kaplan-Meier curves for the probability of a seed not germinating over time and the germination fraction for tomato seeds (A, D) respectively). Kaplan-Meier curves for probability of not germinating and germination fraction for lettuce seeds (B, E) respectively) and Kaplan-Meier curves for probability of not germinating and fraction for pak choi seeds (C, F) respectively). Black lines indicate mineral wool samples, pink lines and bars indicate PUF samples where p >.05 (pairwise Log-rank test) when compared to mineral wool curves.
However, pak choi cotyledon emergence differed in the foam media compared to MW. Pak choi seedlings in foam opened their cotyledons under the media surface, likely due to light penetration through the foam structure (Figure 5). This caused a slight initial lag in growth of pak choi grown in foams compared to MW.

Figure 5. Pak Choi seedling grown in mineral wool (A) and foam (B) growing media, showing cotyledons opening under the surface of the foam media.
3.5 Vegetative growth trial (VGT2)
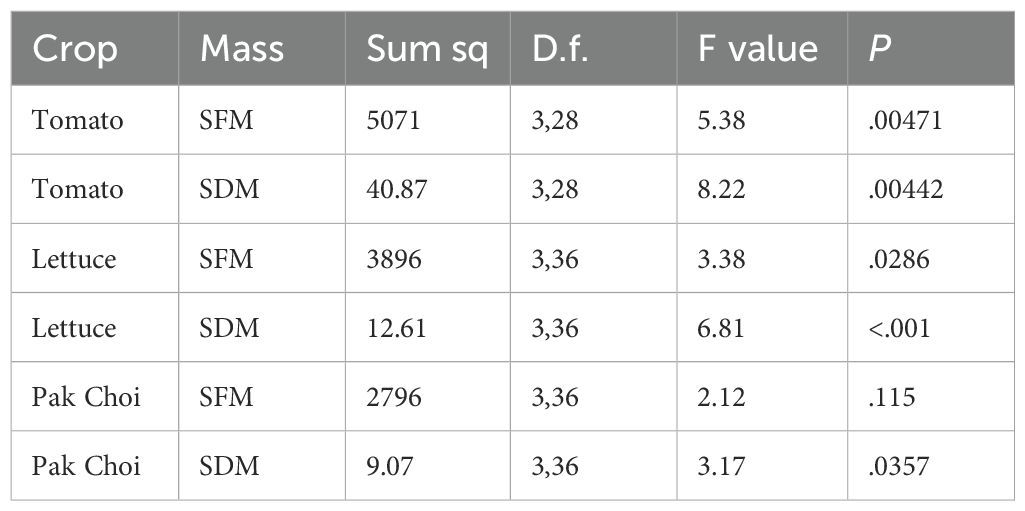
Seedlings from GT2 were transferred into an NFT hydroponic system (lettuce and pak choi) and a dripper fed hydroponic system (tomato) for the vegetative trial (VGT2). Lettuce and pak choi were grown to a harvestable stage, while tomatoes were grown until the onset of flowering. Shoot fresh mass (SFM) and shoot dry mass (SDM) were measured for both lettuce and tomato (Figure 6, Table 4).
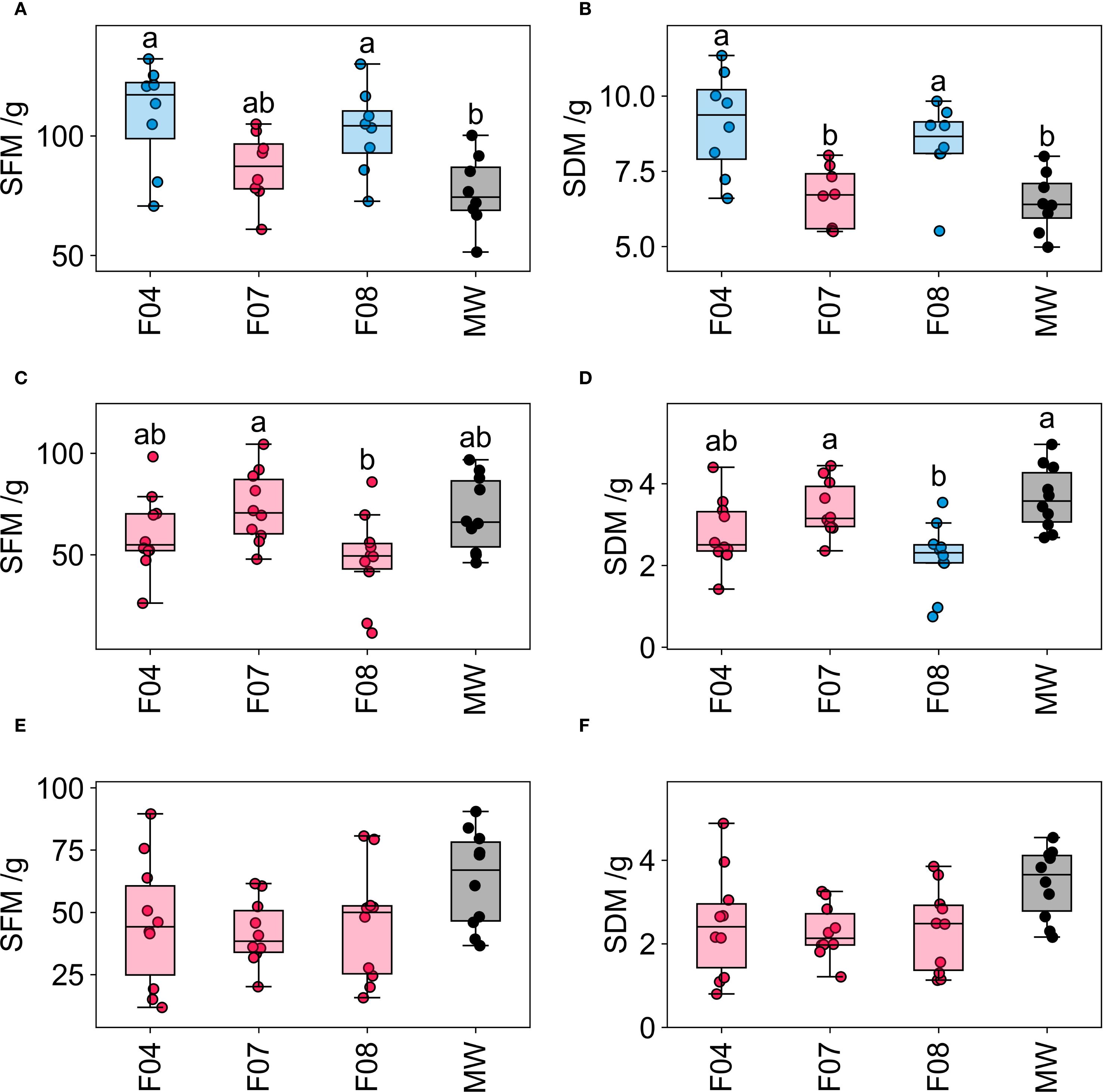
Figure 6. Shoot fresh mass (SFM) and shoot dry mass (SDM) for tomato (A, B) respectively), lettuce (C, D) respectively) and pak choi (E, F) respectively) grown in different growing media. Differing letters indicate p<.05 (Tukey's HSD test), mineral wool is shown in black, pink indicates where PUF formulations where p >.05 between the formulation and mineral wool samples and blue indicates PUF formulations where p <.05 between the formulation and mineral wool samples (Tukey's HSD test). The thick black line in the box plots indicate the median, the box indicates the IQR, and the whiskers show 1.5 × IQR.
Growing media significantly affected SFM (ANOVA; F[3,28] = 5.38, p = .0047) and SDM (ANOVA; F[3,28] = 8.22, p = .0044) in the dripper fed tomato growth trial. Post-hoc analysis revealed that two foam based media (F04 and F08) yielded significantly higher SFM and SDM than the MW control (Tukey HSD test; p <.05). In contrast, F07 did not perform significantly differently from the MW control in VGT2, this is consistent with the results from VGT1. Consequently, two of the three foam media tested (F04 and F08) resulted in tomato growth that met or exceeded that observed in the MW control within this dripper fed system. Furthermore, the variation in both SFM and SDM for the tomato crops grown in the foams was similar to that of the MW crops. Notably, F04 produced the highest mean biomass (mean SFM and mean SDM). F08 yielded the second highest mean SFM and SDM, performing consistently with performance in VGT1.
For the NFT system, growing media significantly affected the SFM (ANOVA; F[3,36] = 3.38, p = .029) and the SDM (ANOVA; F[3,36] = 6.81, p < 0.001) of the lettuce crop in VGT2. Post-hoc analysis showed that all three-foam media yielded similar SFM compared to the MW control; however, F08 showed slightly suppressed SFM, with two replicates having particularly low values. Post-hoc analysis of SDM indicated that F08 produced significantly lower SDM than the other two foam samples (F04, F07) and the MW control. The results of the VGT2 lettuce trial showed good agreement with VGT1 results compared to the tomato trial, particularly as F04 and F07 were the best performing media in terms of mean biomass in both VGT1 and VGT2. Consistent with this, F08, which was the fourth best performing medium in VGT1, was the lowest performing of the foam media in the VGT2 lettuce trial and was only selected for this trial due to performing well in tomato growth trials.
For pak choi NFT trials, growing media did not significantly affect SFM (ANOVA; F[3,36] = 2.12, p = .115). However, growing media significantly affected SDM (ANOVA; F[3,36] = 3.17, p = .0357). Further post-hoc analysis on SDM indicated that there were no statistically significant differences between any two groups. All three-foam media exhibited a marginal decrease in total biomass (mean SFM and mean SDM) when compared to mineral wool. This reduction was likely a combination of suppressed early growth due to cotyledon emergence below the surface of the foam media and the pak choi exhibiting a preference for the root zone conditions provided by mineral wool, potentially due to differences in aeration or moisture retention.
3.6 Pythium infection trial
To evaluate whether the physical properties of soilless growing media influences disease susceptibility in hydroponic systems, we inoculated plants grown in MW, F04, F07 or F08 with an inoculum containing two Pythium isolates. Pythium can cause seed decay along with pre- and post-emergence damping off (Gravel et al., 2005), therefore, we monitored germination and cotyledon emergence along with post emergence growth. No effects of Pythium inoculation were observed with lettuce or Pak Choi, indicating that the Pythium isolates used in this study do not infect these crops (Supplementary Figure S3) For tomato, a marked decrease in cotyledon emergence was observed in mineral wool (MW), dropping from 0.95 (control) to 0.33 when challenged with Pythium. In contrast, the three-foam media (F04, F07, F08) inoculated with Pythium showed little change in the total fraction and timing of cotyledon emergence when compared to their respective controls. Pairwise Log-rank tests comparing the Pythium challenged groups indicated that the Kaplan-Meier survival curve for MW differed significantly from those for F07 and F08. No significant difference was found between the curves for the Pythium challenged F04 and MW media (Figures 7A–D; Supplementary Figure S4, S5).
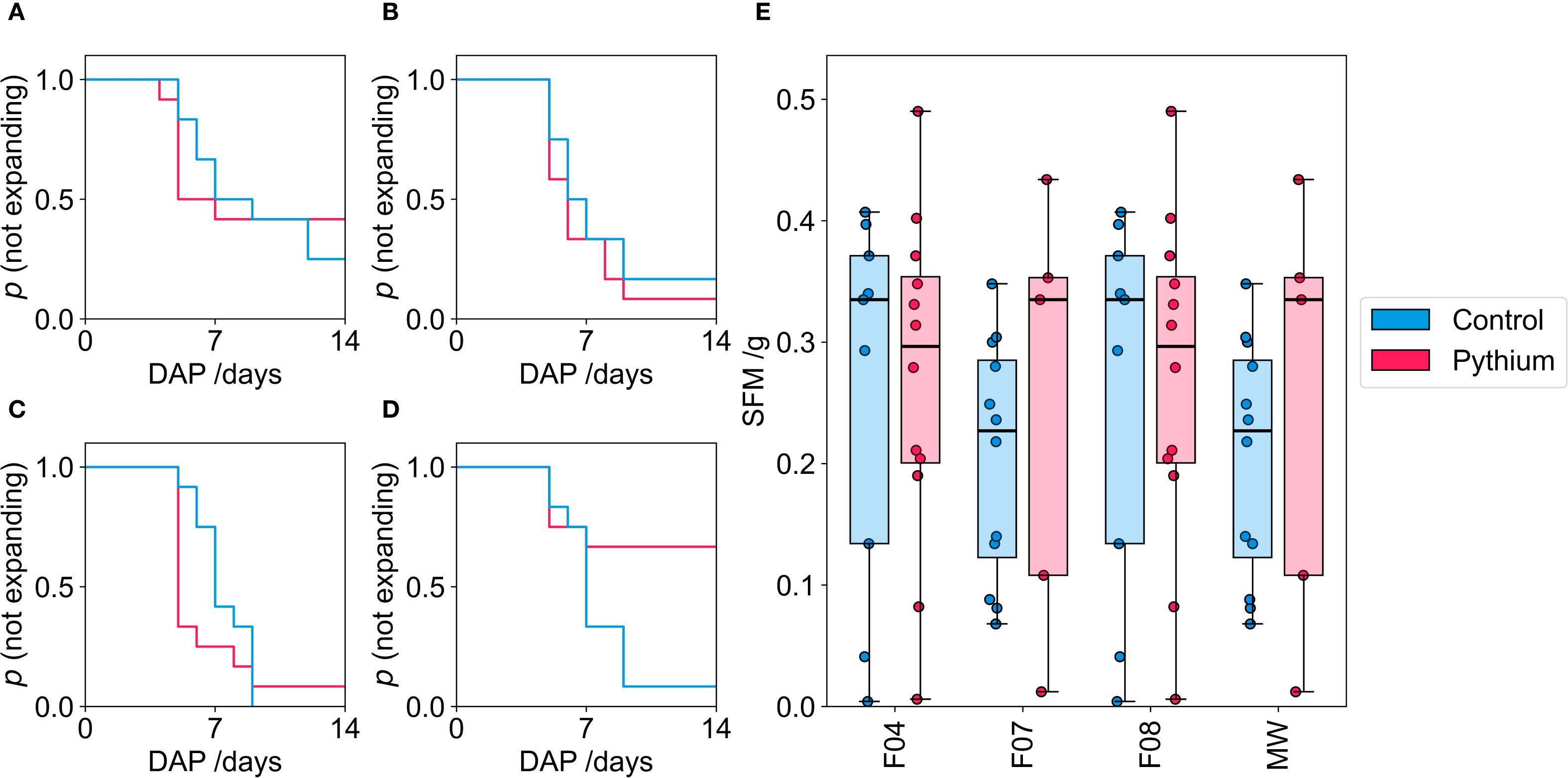
Figure 7. Kaplan-Meier plots showing the probability of cotyledons not emerging, for the three-foam media, F04 (A); F07 (B); F08 (C) and the MW control (D), in the Pythium germination trial. The pink line indicates plants inoculated with Pythium and the blue indicates those dosed with distilled water control. The mass of the SFM harvested at 14 DAP for the four media as well as the MW control (E). The thick black line in the box plots indicate the median, the box indicates the IQR, and the whiskers show 1.5 × IQR. The results shown are representative of three experimental replicates.
After 14 days, tomato plants which had emerged were harvested, and weighed. Shoot fresh mass (SFM) exhibited high variance across all samples, and mean SFM was comparable across all media types and treatments (Pythium vs. control) for the surviving plants (Figure 7E). The reduced germination in MW but comparable SFM values among surviving plants across treatments (Figures 7D, E) suggests that pathogen induced mortality primarily occurred pre-emergence (damping off or seed decay), with minimal effects attributable to post-emergence damping off in this experiment.
4 Discussion
To investigate the potential of polyurethane foams (PUF) as customisable hydroponic growing media, a set of ten foams exhibiting a range of physical properties was synthesised. The objective was to determine if optimising these properties could benefit specific crops or hydroponic systems compared to a 'one-size-fits-all' growing media such as mineral wool. Formulation and synthesis were successful in generating variation among the ten foams regarding key target properties: open cell fraction, cell size, water uptake rate (WUR), and maximum water uptake (Hmax). PUF media all had significantly lower maximum capillary rise (Hmax) and water uptake rates (WUR) than the MW control. This agrees with previous works investigating PUF as alternative growing media where water holding capacity was lower in PUF than MW (Huber, 2006). These foams subsequently underwent two germination and two vegetative growth trials to evaluate their performance against a standard mineral wool (MW) control.
Following the initial germination trial, four foam formulations were excluded from further testing due to significantly lower germination and/or germination rates compared to the MW control. Examination of the normalised physical properties of these four unsuccessful formulations provides insight into potential reasons for their poor performance. Analysis revealed that these four foams had properties at the extremes of the range. They included the two formulations with the largest and the two with the smallest cell sizes as well as those with the highest and lowest effective open cell fractions (Table 2). As previously demonstrated (Wright et al., 2022b) cell size and open cell fraction strongly influence water uptake characteristics. Correspondingly, these four foams exhibited the lowest Hmax values among all ten formulations. Furthermore, PCA analysis (Figure 8) revealed that this group was made up of two subgroups, each consisting of two foams (Figure 8; pink dots). These two groups differed along the axes that correlated with the vectors Wur and peff. Two had very high-water uptake rates, and two had low water uptake rates. These findings suggest two distinct failure modes related to inadequate water management within the root zone. Firstly, the two foams characterised by small, predominantly closed cells likely impeded capillary action, resulting in both slow water uptake rates and low maximum capillary rise (Hmax). Secondly, the two foams with large, mostly open cells, while potentially allowing rapid initial water uptake, likely also exhibited excessive drainage, ultimately leading to similarly low Hmax values. In both scenarios, the resulting suboptimal water availability (either too little retention or too rapid drainage) likely contributed directly to the observed poor germination rates.
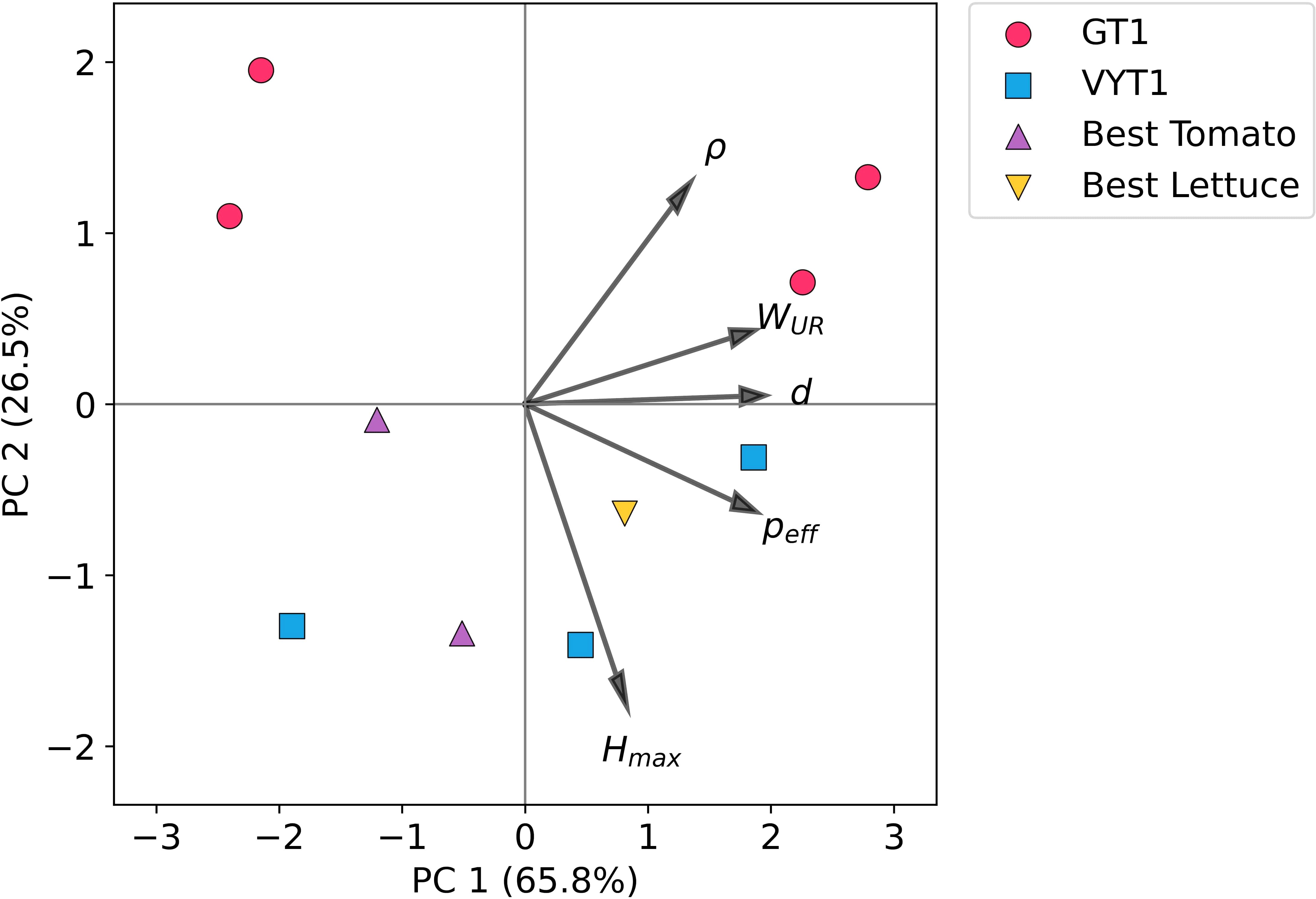
Figure 8. Principal component analysis (PCA) of the physical properties of the PUF growing media. The plot visualises the relationships between media based on their physical properties and shows their progression through the experimental selection process. PC1 (65.8%) and PC2 (26.5%) are displayed. Each point represents a unique PUF foam (means of physical properties used) and they are categorised as follows. Pink dots are formulations that were dropped after GT1, blue squares are formulations dropped after VGT1, purple triangles are the best performing formulations for tomato in VGT2 and yellow upside-down triangles is the best performing formulation for lettuce in VGT2. The vectors show the loadings of the measured physical properties.
The poor performance of these four formulations provides important insights into the boundary conditions for designing effective PUF based growing media. Notably, these results suggest that successful PUF media require a sufficient minimum water retention (Hmax). Achieving this appears dependent on attaining a balanced pore structure, likely characterised by partially interconnected pores (avoiding extremes of fully open or closed structures) and moderate cell sizes.
In the initial vegetative growth trial (VGT1), tomato and lettuce yields were comparable across the tested media. Based on performance in the initial germination (GT1) and vegetative growth (VGT1) trials, the three most promising foam formulations (F04, F07, and F08) were selected for further evaluation. In subsequent germination tests (GT2), these three selected foams demonstrated germination and rates comparable to MW for all three tested crops: tomato, lettuce, and pak choi.
A second vegetative growth trial (VGT2) directly compared these three selected foams against MW using two distinct hydroponic systems tailored to different crops: a dripper fed system for tomatoes and a nutrient film technique (NFT) system for lettuce and pak choi. The experimental design for VGT2 was refined to include fewer media types and a greater number of replicates. This modification enhanced the study's statistical power, allowing for the detection of statistically significant differences in growth performance between media in both the tomato and lettuce trials. F04 and F08 outperformed MW in tomato trials and F08 underperformed MW in lettuce trials. Yield in the pak choi trial was similar between the three-foam media and the MW control, with all three foams performing slightly worse than the MW control, likely due to crops germinating below the surface of the foam, hampering early growth. Further studies into optimal planting depth in foam, as well as the option of germinating seedlings in the dark, should be explored to mitigate this effect.
Analysis of the relationship between hydroponic performance and material properties provides insights into optimisation for specific systems. Examination of the hydrodynamic properties associated with the highest yielding foams in the tomato/dripper system (F04 and F08; Figure 8; purple triangles and asterisks in Table 2) reveals relatively slow water uptake rates coupled with high maximum capillary rise. These characteristics are advantageous for an intermittent dripper system, as slower water uptake and potentially slower release (implied by high capillary retention) help maintain media moisture and nutrient availability between irrigation events. Structurally, these foams (F04, F08) possessed moderate cell sizes and effective open cell contents relative to the full range synthesised.
In contrast, the best performing foam for lettuce in the NFT system (F07) exhibited faster water uptake rates compared to F04/F08, slightly higher maximum capillary rise, a more open cell structure (higher open cell content), but similarly moderate cell sizes (Figure 8; yellow inverted triangle and asterisks in Table 2). This more open structure is likely advantageous in an NFT system, where the media's role in water retention is less critical due to continuous nutrient solution flow. Furthermore, open cell structure of F07 may facilitate easier root anchorage and subsequent penetration into the NFT channel. This high porosity enhances airflow through the substrate, improving gas exchange within the root zone. Considering inadequate aeration is a known cause of yield reduction in NFT systems, with inorganic media with higher aeration have been shown to improve crop performance (Jackson et al., 1984), this may aid in explaining why F07 was best performing in NFT lettuce trials.
These initial trials indicate that a single growing media approach is unlikely to be crop and system optimised and furthermore that PUF media can be used as a platform to determine growing media physical property recipes that are both crop and system optimised.
We acknowledge that testing multiple substrates under a single irrigation strategy can limit the applicability of the results, as optimal irrigation is media-specific (Raviv et al., 2019). However, our methodology intentionally uses an irrigation regime optimised for MW. This approach was chosen because growers are often reluctant to modify established infrastructure for novel media, making it critical to demonstrate that foam can perform effectively as a direct replacement within existing systems.
PUF appeared to reduce the effect of Pythium, a common hydroponic pathogen (Zlnnen, 1988), on germination of tomato plants. Compared to the MW medium, the reduced early mortality observed in two of the foam media (F07 and F08) under Pythium challenge was an unexpected finding. This superior germination may be due to the large differences in capillarity between the MW and PUF samples. High water uptake by MW has been shown to be a major contributor to root rot proliferation in hydroponic systems (Juneau et al., 2006) and increasing the height of MW block in these systems, and therefore the reducing water content in the root zone decreases the chance of yield loss due to the disease (Van Der Gaag and Wever, 2005). In addition to the large differences in hydrodynamics, the lack of disease symptoms in PUF could also be explained by the surface properties of the foam which may be less conducive to Pythium proliferation compared to MW; or a chemical component or property of the foam might possess antimicrobial activity effective against the oomycete pathogen. Trials with phenolic foams, oasis cubes, examining root rot in different soilless growing media, also found that their foams had the lowest disease incidence, hypothesising that this was due to different physicochemical parameters, changes in microbiomes or due to chemical components in the foams (DeGenring and Poieatewich, 2023).
Further investigation of the mechanism of this disease suppression is required to understand how microbes interact with the foam media before utilising these foams as model growing platforms in experiments involving biological factors, such as pathogen screening or the application of beneficial plant health or growth promoting microorganisms.
In conclusion, this study demonstrates that a single growing media formulation is not optimal for different crops and hydroponic systems. Furthermore, it demonstrates the potential of PUFs as a versatile platform for developing tailored growing media recipes, allowing for the identification of physical property attributes optimal for a given crop and system requirements. It is important to acknowledge the broader market context and sustainability challenges around growing media development. The PUFs synthesised here were petroleum-based, which may face headwinds from retailers and consumers who are increasingly favouring bio-based or biodegradable materials. Therefore, a next step for this research is to explore the development of bio-based and biodegradable alternatives; by replacing fossil-based reagents and adding specific sites for degradation This would combine the customisable physical properties demonstrated here with a significantly improved environmental profile, aligning the technology with the future sustainability goals of the horticultural industry.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: 10.15131/shef.data.29314193.
Author contributions
HW: Funding acquisition, Writing – original draft, Formal analysis, Visualization, Writing – review & editing, Methodology, Conceptualization, Validation, Investigation, Data curation. SW: Formal analysis, Methodology, Writing – review & editing, Conceptualization, Visualization, Validation, Investigation. CM: Data curation, Methodology, Formal analysis, Investigation, Writing – review & editing. SR: Supervision, Conceptualization, Funding acquisition, Writing – review & editing. DC: Funding acquisition, Writing – review & editing, Supervision. AR: Supervision, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work is supported by the 'Transforming UK food systems' research programme via UKRI's Strategic Priorities Fund (BB/V004719/1) as well as BBSRC Protected and Controlled Environment horticulture research programme (BB/Z514433/1).
Acknowledgments
This work has been hosted on a preprint server that is available here https://doi.org/10.21203/rs.3.rs-6890616/v1.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI (Gemini 2.5 Pro, a large language model from Google) was used to produce the icons used in the graphical abstract. The authors provided text prompts to the AI to create the individual visual assets. The final graphical abstract was then assembled by the authors using these icons.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fhort.2025.1646783/full#supplementary-material
References
Al Meselmani M. A., Wright H. C., Cameron D. D., and Ryan A. J. (2020). How scientists and refugees brought green to the Desert Garden. Nat. Rev. Earth Environ. 1, 439. doi: 10.1038/s43017-020-0081-7
Alsanius B. W. and Wohanka W. (2019). “Root zone microbiology of soilless cropping systems,” in Soilless culture (Oxford: Elsevier), 149–194. doi: 10.1016/B978-0-444-63696-6.00005-0
Amrhein J. J., Rotondo F., Kubota C., Miller S. A., and Testen A. L. (2025). Diagnostic guide for pythium root rot in hydroponic leafy green and herb production. Plant Heal. Prog. 26, 87–95. doi: 10.1094/PHP-07-24-0070-DG
André LéVesque C. and De Cock A. W. A. M. (2004). Molecular phylogeny and taxonomy of the genus Pythium. Mycol. Res. 108, 1363–1383. doi: 10.1017/S0953756204001431
ASTM Standard D3574-11 (2012). Standard test methods for flexible cellular materials—Slab, bonded, and molded urethane foams (West Conshohocken: ASTM International). doi: 10.1520/D3574-11.2
ASTM Standard D3576-15 (2014). Standard test method for cell size of rigid cellular plastics (West Conshohocken: ASTM International). doi: 10.1520/D2842-12.2
Backstrom M. J. (2002). Methyl bromide: the problem, the phase out, and the alternatives. Drake J. Agric. Law 7, 213.
Bartley P. C., Amoozegar A., Fonteno W. C., and Jackson B. E. (2022). Particle densities of horticultural substrates. HortScience 57, 379–383. doi: 10.21273/HORTSCI16319-21
Benoit F. and Ceustermans N. (1995). A decade of research on on ecologically sound substrates. Acta Hortic. 408, 17–30. doi: 10.17660/ActaHortic.1995.408.2
Benson D. A., Cavanaugh M., Clark K., Karsch-Mizrachi I., Lipman D. J., Ostell J., et al. (2012). GenBank. Nucleic Acids Res. 41, D36–D42. doi: 10.1093/nar/gks1195
Cameron K. C. and Buchan G. D. (2017). “Porosity: pore size distribution,” in Encyclopedia of soil science (Boca Raton: R. Lal (CRC Press), 1782–1785.
Caron J. and Michel J. (2021). Understanding and optimizing the physical properties of growing media for soilless cultivation, in Advances in horticultural soilless culture (London: Burleigh Dodds Science Publishing), 107–137. doi: 10.1201/9781003048206-6
Cowan N., Ferrier L., Spears B., Drewer J., Reay D., and Skiba U. (2022). CEA systems: the means to achieve future food security and environmental sustainability? Front. Sustain. Food Syst. 6. doi: 10.3389/fsufs.2022.891256
Davidson-Pilon C. (2019). lifelines: survival analysis in Python. J. Open Source Software 4, 1317. doi: 10.21105/joss.01317
DeGenring L. and Poieatewich A. (2023). The effect of propagation substrate on pythium root rot severity and the efficacy of biopesticides. Plant Heal. Prog. 24, 306–311. doi: 10.1094/PHP-01-23-0010-SC
Durand S., Fonteno W. C., and Michel J. C. (2024). A review and analysis of particle size parameters and their relationships to physical properties of growing media. Soil Sci. Soc Am. J. 88, 652–666. doi: 10.1002/saj2.20661
FAO (2015). Status of the world's soil resources: main report (Rome, Italy). Available online at: http://www.fao.org/3/i5199e/I5199E.pdf (Accessed June 28, 2025).
Fields J. S. and Gruda N. S. (2021). “Developments in inorganic materials, synthetic organic materials and peat in soilless culture systems” in Advances in horticultural soilless culture (Burleigh Dodds Science Publishing), 45–72. doi: 10.19103/as.2020.0076.02
Fussy A. and Papenbrock J. (2022). Techniques — Chances, challenges and the neglected question of sustainability. Plants 11, 1–32. doi: 10.3390/plants11091153
Gliński J. and Stępniewski W. (2018). Soil aeration and its role for plants (Boca Raton: CRC Press). doi: 10.1201/9781351076685
Gravel V., Martinez C., Antoun H., and Tweddell R. J. (2005). Antagonist microorganisms with the ability to control Pythium damping-off of tomato seeds in rockwool. BioControl 50, 771–786. doi: 10.1007/s10526-005-1312-z
Gruda N. (2019). Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 9, 298. doi: 10.3390/agronomy9060298
Hardgrave M. (1995). An evaluation of polyurethane foam as a reusable substrate for hydroponic cucumber production. Acta Hortic. 401, 201–208. doi: 10.17660/ActaHortic.1995.401.24
Herrero M. L., Brurberg M. B., Ojeda D. I., and Roleda M. Y. (2020). Occurrence and pathogenicity of Pythium (Oomycota) on Ulva species (Chlorophyta) at different salinities. Algae 35, 79–89. doi: 10.4490/algae.2020.35.2.25
Huber J. J. (2006).Development of a urethane-based plant growth substrate for hydroponic greenhouse vegeta ble production. Available online at: http://atrium.lib.uoguelph.ca/xmlui/handle/10214/22050 (Accessed May 24, 2025).
Jackson M. B., Blackwell P. S., Chrimes J. R., and Sims T. V. (1984). ). Poor aeration in NFT and a means for its improvement. J. Hortic. Sci. 59, 439–448. doi: 10.1080/00221589.1984.11515216
Juneau V., Caron J., Martinez C., Gravel V., and Allaire S. (2006). Growing media, greenhouse tomato yield and Pythium root rot. Can. J. Soil Sci. 86, 501–512. doi: 10.4141/s05-038
Polyurethane Foam Association (2016). The importance of density. Available online at: https://www.pfa.org/wp-content/uploads/2019/02/InTouch_v1.2a.pdf (Accessed October 6, 2025).
Postma J., Geraats B. P. J., Pastoor R., and Van E. J.D. (2005). Characterization of the microbial community involved in the suppression of pythium aphanidermatum in cucumber grown on rockwool. Phytopathology 95, 808–818. doi: 10.1094/PHYTO-95-0808
Rasband W. S. (1997). Available online at: https://imagej.net/ij/ (Accessed October 6, 2025).
Raviv M., Lieth J. H., and Bar-Tal A. (2019). “Growing plants in soilless culture,” in Soilless culture (Oxford: Elsevier), 637–669. doi: 10.1016/B978-0-444-63696-6.00014-1
Riit T., Tedersoo L., Drenkhan R., Runno-Paurson E., Kokko H., and Anslan S. (2016). Oomycete-specific ITS primers for identification and metabarcoding. MycoKeys 14, 17–30. doi: 10.3897/mycokeys.14.9244
Schulker B. A., Jackson B. E., and Fonteno W. C. (2021). A practical method for determining substrate capillary water sorption. Acta Hortic. 1317, 327–334. doi: 10.17660/ActaHortic.2021.1317.38
Seabold S. and Perktold J. (2010). Statsmodels: econometric and statistical modeling with python. Proc. Of The 9th Python in Science Conf. 92–96. doi: 10.25080/Majora-92bf1922-011
Shwin A., Aranjpe V. P., Aniel D., Antliffe J. C., Lizabeth E., Amb M. L., et al. (2003). Winter strawberry production in greenhouses using soilless substrates: an alternative to methyl bromide soil fumigation. Proc. Fla. State Hortic. Soc Proc. Fla. State Hortic. Soc. 116, 98–105.
Szycher M. (2013). Szycher's handbook of polyurethanes. 2nd Edn (Boca Raton, FL: CRC Press). Available online at: http://www.crcnetbase.com/isbn/9781439839584.
Van Der Gaag D. J. and Wever G. (2005). Conduciveness of different soilless growing media to Pythium root and crown rot of cucumber under near-commercial conditions. Eur. J. Plant Pathol. 112, 31–41. doi: 10.1007/s10658-005-1049-7
Virtanen P., Gommers R., Oliphant T. E., Haberland M., Reddy T., Cournapeau D., et al. (2020). SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272. doi: 10.1038/s41592-019-0686-2
White T. J., Bruns T., Lee S., and Taylor J. (1990). “Amplification and direct sequencing of fungal ribosomal rna genes for phylogenetics,” in PCR protocols (San Diego: Elsevier), 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wright H. C., Cameron D. D., and Ryan A. J. (2021a). Experimental design as a framework for optimising polyurethane foam as a soilless growing media. Acta Hortic. 1317, 125–132. doi: 10.17660/ActaHortic.2021.1317.15
Wright H. C., Cameron D. D., and Ryan A. J. (2022a). FoamPi: An open-source raspberry Pi based apparatus for monitoring polyurethane foam reactions. HardwareX 12, e00365. doi: 10.1016/j.ohx.2022.e00365
Wright H. C., Cameron D. D., and Ryan A. J. (2022b). Rational design of a polyurethane foam. Polymers (Basel). 14, 5111. doi: 10.3390/polym14235111
Wright H. C., Zhu J., Cameron D. D., and Ryan A. J. (2021b). Flexible polyurethane foam with sodium bentonite: improving the properties of foams for use as a synthetic growing media. Acta Hortic. 1305, 47–54. doi: 10.17660/ActaHortic.2021.1305.7
Yasunaga K., Neff R. A., Zhang X. D., and Macosko C. W. (1996). Study of cell opening in flexible polyurethane foam. J. Cell. Plast. 32, 427–448. doi: 10.1177/0021955X9603200502
Keywords: growing media, horticulture, hydroponic, mineral wool, polyurethane foam, soilless
Citation: Wright HC, Wilkinson SW, Morgan CW, Rolfe SA, Cameron DD and Ryan AJ (2025) Polyurethane foam as a soilless growing media: demonstrating the importance of physical property optimisation. Front. Hortic. 4:1646783. doi: 10.3389/fhort.2025.1646783
Received: 13 June 2025; Accepted: 29 September 2025;
Published: 16 October 2025.
Edited by:
Chris Blok, Wageningen University and Research, NetherlandsReviewed by:
Jane Debode, Institute for Agricultural, Fisheries and Food Research (ILVO), BelgiumZienab F R Ahmed, United Arab Emirates University, United Arab Emirates
Copyright © 2025 Wright, Wilkinson, Morgan, Rolfe, Cameron and Ryan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harry C. Wright, aGFycnkud3JpZ2h0QHNoZWZmaWVsZC5hYy51aw==
 Harry C. Wright
Harry C. Wright Samuel W. Wilkinson
Samuel W. Wilkinson Caleb W. Morgan
Caleb W. Morgan Stephen A. Rolfe
Stephen A. Rolfe Duncan D. Cameron
Duncan D. Cameron Anthony J. Ryan1
Anthony J. Ryan1