- 1Department of Agriculture, Food and Environment, University of Catania, Catania, Italy
- 2Department of Veterinary Sciences, University of Messina, Messina, Italy
- 3Scuola Superiore Sant’Anna Pisa, Crop Science Research Center, Ghezzano, Pisa, Italy
The Mediterranean Basin is home to one of the highest levels of biodiversity on Earth, with approximately 25,000 plant species. The typical vegetation in this region is predominantly consists of shrubs. Due to their anatomical, morphological, physiological, and biochemical characteristics, these plants can tolerate abiotic stress, particularly drought. Consequently, incorporating them could enhance the sustainability of urban green spaces. However, species native to the Mediterranean Basin are not yet widely represented in green spaces, despite their attractive ornamental traits. To quantify the contribution of Mediterranean native shrubs, a survey was conducted on Italian flora. Using a selection grid, species of potential interest were identified; these species belong to the Mediterranean chorotype and exhibit appealing ornamental characteristics. The overall attributes of these plant species support their ornamental use, owing to their form and the features of their leaves, flowers, or fruits. The investigation identified 369 species that are not currently available in commercial nurseries. The most represented families included Rosaceae, Asteraceae, Fabaceae, Lamiaceae, and Cistaceae. Although the flowering period of each species is relatively short, the blooms of different species are distributed throughout the year. Therefore, by simultaneously utilizing various species, it is possible to ensure a continuous ornamental display.
1 Introduction
The Mediterranean Basin is one of the richest regions in the world in terms of plant diversity, boasting approximately 25,000 plant species (Myers et al., 2000). Due to their high biological diversity, Mediterranean ecosystems have been recognized as biodiversity hotspots and prime targets for conservation efforts (Myers et al., 2000; Tavşanoğlu and Pausas, 2018). However, the climate makes the Mediterranean Basin one of the most vulnerable areas to both abiotic and biotic stress. Drought, combined with extreme temperatures, constitutes abiotic stress that negatively affects the survival, growth, development, and productivity of Mediterranean woody species (Ogaya and Peñuelas, 2003). These environmental constraints are expected to intensify in the near future due to global climate change (Pinheiro et al., 2014).
Mediterranean plants can tolerate stressful abiotic conditions and, therefore, are somewhat resilient to the warming and increased drought associated with climate change (Toscano et al., 2019). However, only species with significant phenotypic plasticity—particularly those capable of rapid evolutionary changes in both their functional traits and plasticity—will persist under rapidly changing environmental conditions induced by global change (Matesanz and Valladares, 2014). These characteristics are especially common in shrubs, which are characterized by having “more active meristems, and more potential points for stem regeneration” (Givnish, 1984). These traits enable shrubs to tolerate abiotic stress better than trees. Shrubs are often associated with disturbed and stressful environments; for these reasons, they are more suitable for green infrastructures in Mediterranean environments, especially where maintenance is limited. The high biodiversity and aesthetic value of these plants suggest that their use is the key component of sustainable landscape (Romano and Scariot, 2021).
Among Mediterranean ecosystems, shrublands represent a characteristic vegetation type that is widespread across various habitats. Due to several factors—including physiological, morphological, reproductive, phenological, and regenerative traits, as well as inter- and intraspecific interactions—each shrub species constitutes a significant component of the plant community. Shrubs play a vital ecological role (Pasalodos-Tato et al., 2015) in multiple ways, such as soil protection (Bochet et al., 2006; Francini et al., 2021), enhancing biodiversity (Mangas et al., 2008), nutrient production, carbon storage (Chapin, 1983), and ecosystem restoration (Rey et al., 2009).
The spread of shrubs in the Mediterranean region is attributed to their adaptability to harsh climatic conditions, characterized by hot summers and low rainfall, resulting in a long, dry season (Paz et al., 2016). The use of native Mediterranean shrub species offers a potential solution to drought (Munné-Bosch and Peñuelas, 2004), salt stress (Cassaniti et al., 2013), and heat stress. These native shrubs can withstand severe drought, a critical factor influencing plant survival and species distribution (Filella et al., 1998). Due to their unique morphophysiological traits, woody plants are well-suited for ornamental purposes. It is no coincidence that shrubs are commonly found in degraded environments where abiotic stresses are frequent. From an ornamental perspective, shrubs exhibit various features—such as a high number of twigs influencing their growth patterns; pulvinate (cushion-like) shapes that reduce transpiration and enhance the aesthetic appeal of green infrastructures; distinctive leaf characteristics; varied flowering periods; diverse flower colors—that contribute interest and variety to the landscape (Leotta et al., 2023a).
Despite widespread appreciation, the vast plant diversity of the Mediterranean region largely consists of neglected and underutilized species (Padulosi et al., 2013). Only a small fraction of these resources is currently employed in the agricultural and ornamental horticulture sectors due to poor documentation of their potential and the lack of coordinated efforts (Rivera et al., 2006; Scariot et al., 2012). Globally, the ornamental horticulture industry seeks new crops—often of exotic origin—that exhibit appealing ornamental traits, adaptability to diverse conditions, and low maintenance costs (Fascetti et al., 2014; Maloupa et al., 2008; Junqueira and Peetz, 2017). Unfortunately, native plants receive insufficient attention, partly because of limited information regarding their performance and propagation.
Studies on the ornamental value of new crops generally focus on the plants’ habit and characteristics, such as morphological and phenological traits, development and growth, ecological characteristics including tolerance to various factors, aesthetic features like flower and leaf attributes, and cultivation methods encompassing agricultural practices such as ease of propagation and adaptability to different cultivation conditions (Krigas et al., 2021). For plants intended for landscaping and gardening, resistance to biotic and abiotic stresses is of particular importance (Krigas et al., 2021).
Another factor supporting the use of native plants in green spaces is the potential to aid populations of declining native species, thereby helping to prevent their extinction. Gardens, which are common in both urban and rural environments, are increasingly recognized for their role in conserving biodiversity (Gratzfeld, 2017). Recently, the concept of conservation gardening has emerged as a novel approach to biodiversity restoration. This approach focuses on cultivating declining native species in private gardens, integrating commercial horticulture with conservation efforts, and promoting public engagement (Segar et al., 2022; Staude, 2024; Munschek et al., 2023). Awareness of the importance of biodiversity and the benefits of incorporating local flora into urban landscape design is growing (Lovell and Johnston, 2009; Ahern, 2013). By doing so, we can safeguard pollinators that have co-evolved with many genera or species of native plants (Hall et al., 2017; Senapathi et al., 2017).
An increasingly informed public is demanding the inclusion of native plants in green spaces, driven by the awareness that this can reduce dependence on resources—primarily water—in urban and suburban environments. Although native flora encompasses a variety of species with diverse water requirements, some species, particularly shrubs, demonstrate greater water-use efficiency (Chaves et al., 2003; Evans et al., 2013; Martinson, 2020).
Recently, the checklist of vascular flora native to Italy has been updated (Bartolucci et al., 2018). The checklist now includes 8,241 species and subspecies, belonging to 1,111 genera and 153 families (Bartolucci et al., 2024). According to Bartolucci et al. (2024), the taxa currently confirmed to occur in Italy number 7,591, as the remaining taxa have not been recently verified, are doubtful, or are affected by errors or incomplete information. In any case, the Italian flora is particularly rich in plant species, which can serve as a valuable resource for revitalizing the biological diversity of ornamental nurseries and for individuating new species better suited for urban greenery.
In this context, the objective of this study was to review the shrub species present in the Italian flora and examine some of their morphological and functional characteristics to conduct a preliminary assessment of their adaptability to various landscape use modalities.
2 Materials and methods
2.1 Literature sources
The survey began with a review of relevant literature sources (Pignatti, 1982; Raimondo et al., 2010; Conti et al., 2005; Bartolucci et al., 2018, 2024), which facilitated the identification of the initial group of species. All data collection and literature searches were performed within the period November 2024 until February 2025. All identified plants belonged to the category commonly referred to as bushes. These plants correspond to chamaephytes, caespitose phanerophytes, and nanophanerophytes, according to Raunkiaer (1934). Although some individual accessions could be classified under other biological forms (e.g., rhizomatous geophytes, caespitose hemicryptophytes, hemicryptophytes with rosettes, scapose hemicryptophytes), all listed taxa fall within these biological categories. The taxa considered were limited to species spread in Italy and classified as indigenous, naturalized archaeophytes, or cryptogenic. From the preliminary list, certain accessions were selected for further study based on specific morpho-biological characteristics, flowering season, and distribution within the national territory. For each accession, synonym names were verified to eliminate duplicates using Plants of the World Online (2025) (https://powo.science.kew.org/). However, a double taxonomic check (accepted name versus heterotypic synonym) has not been performed. Families were listed according to Plants of the World Online (2025). Information on origin and flowering period was referenced from Pignatti (1982) and Acta Plantarum (https://www.actaplantarum.org/). Based on literature data (Pignatti, 1982; Pignatti et al., 2017–2019; Acta Plantarum, 2025), plant height was categorized into classes (< 20 cm; 20–60 cm; 60–100 cm; 100–140 cm; > 140 cm), along with flower color and the organ of greatest showiness (flowers, fruits, or leaves). For the latter information, we relied on iconography available online from reliable websites (Acta Plantarum, 2025; iNaturalist, 2025).
2.2 Morphological traits
Considering morphological traits (height, prominence of flowers, fruits, and leaves) and functional characteristics (origin environments, flowering season), the potential use of these species for ornamental purposes in green areas—such as green roofs, naturalistic gardens, pot cultivation, slopes, xeriscaping, wildflowers, —was proposed. The list of selected plants was then compared with a previous list of species found in twenty-seven nurseries nationwide that marketed ornamental plants used for the arrangement of green spaces (Romano, 2021), in order to identify which of these accessions were available on the market.
3 Results
The review identified 1,137 taxa belonging to 271 genera and 74 different botanical families (Table 1). Based on specific morpho-biological characteristics, flowering season, and distribution within the national territory, a selection process identified 544 taxa belonging to 199 genera and 68 botanical families (Table 2). Among these, 159 taxa were subspecies, accounting for 29.2% of the total (Table 1S). The most represented botanical families are Rosaceae (44 genera and 56 species), Asteraceae (16 genera and 55 species), Fabaceae (17 genera and 50 species), Lamiaceae (16 genera and 45 species), Cistaceae (3 genera and 38 species), and Caryophyllaceae (12 genera and 30 species).
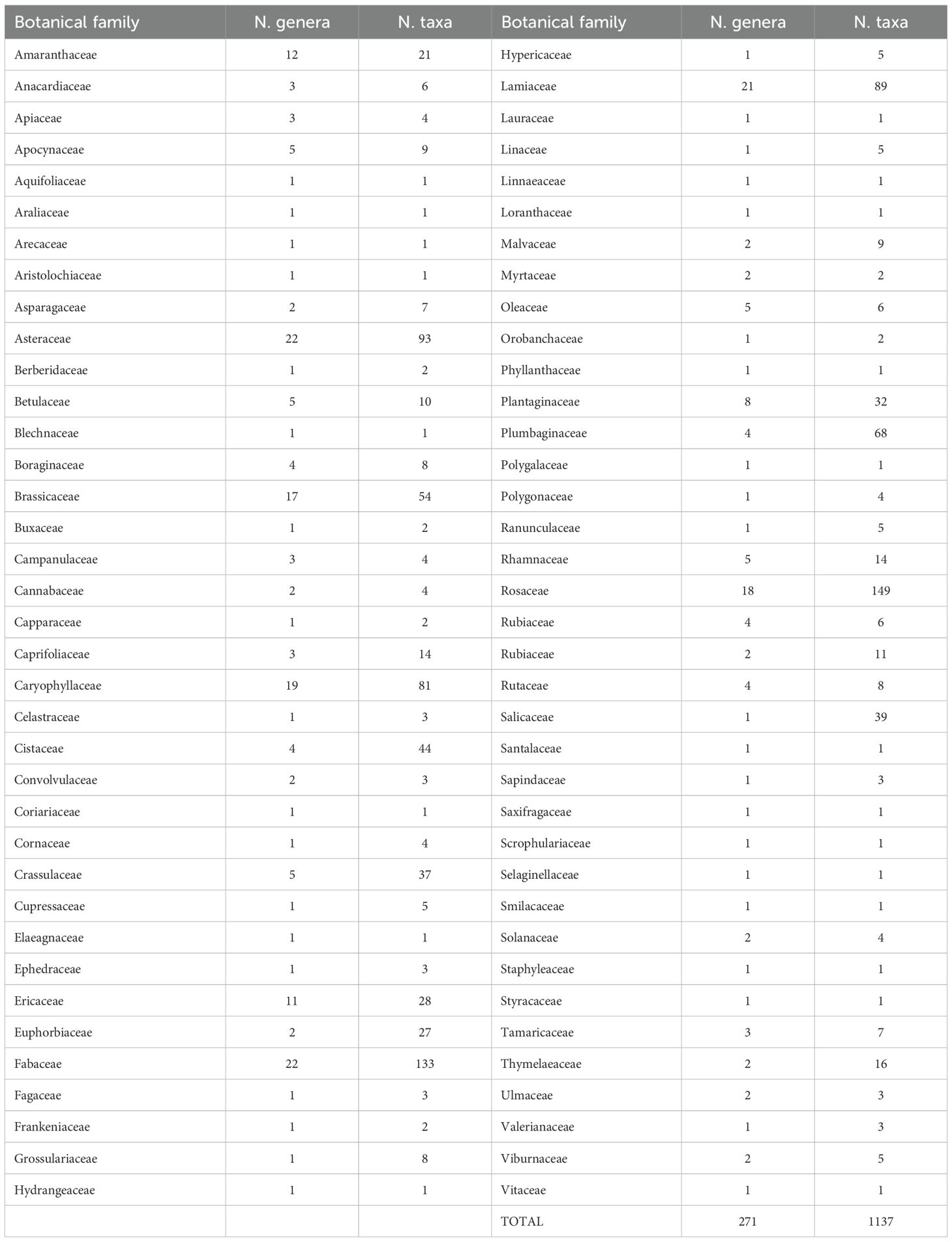
Table 1. Number of taxa and genera for botanical families of Italian flora of potential ornamental value.
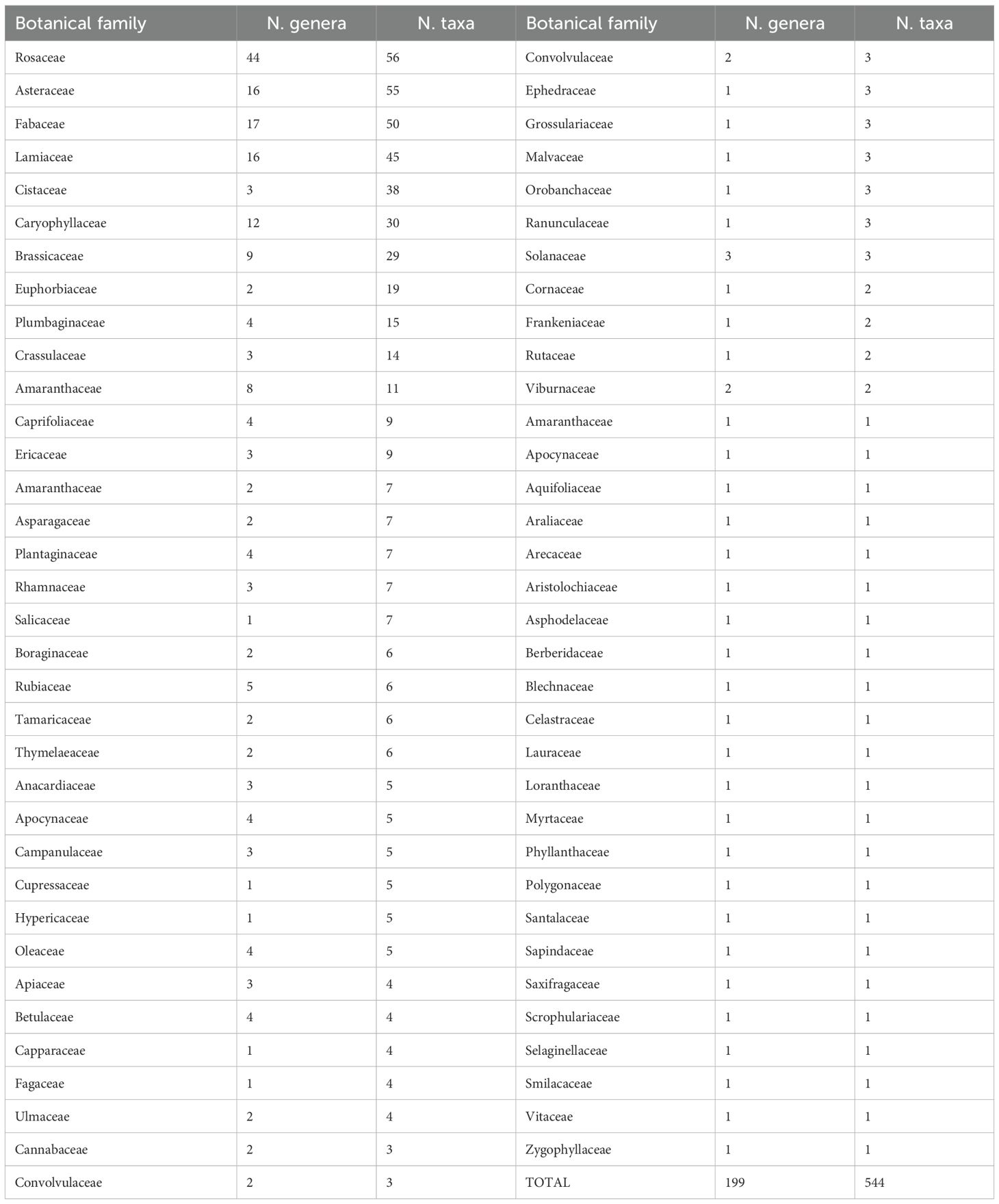
Table 2. Number of taxa and genera for botanical families of selected plants, according the number of taxa.
To highlight the extensive biodiversity observed, it should be noted that 36 botanical families (52.9% of the total) were represented by a single genus, and 23 botanical families (33.8%) were represented by a single species (Table 1). The highest number of genera was found in the Rosaceae family, followed by Asteraceae, Fabaceae, and Lamiaceae.
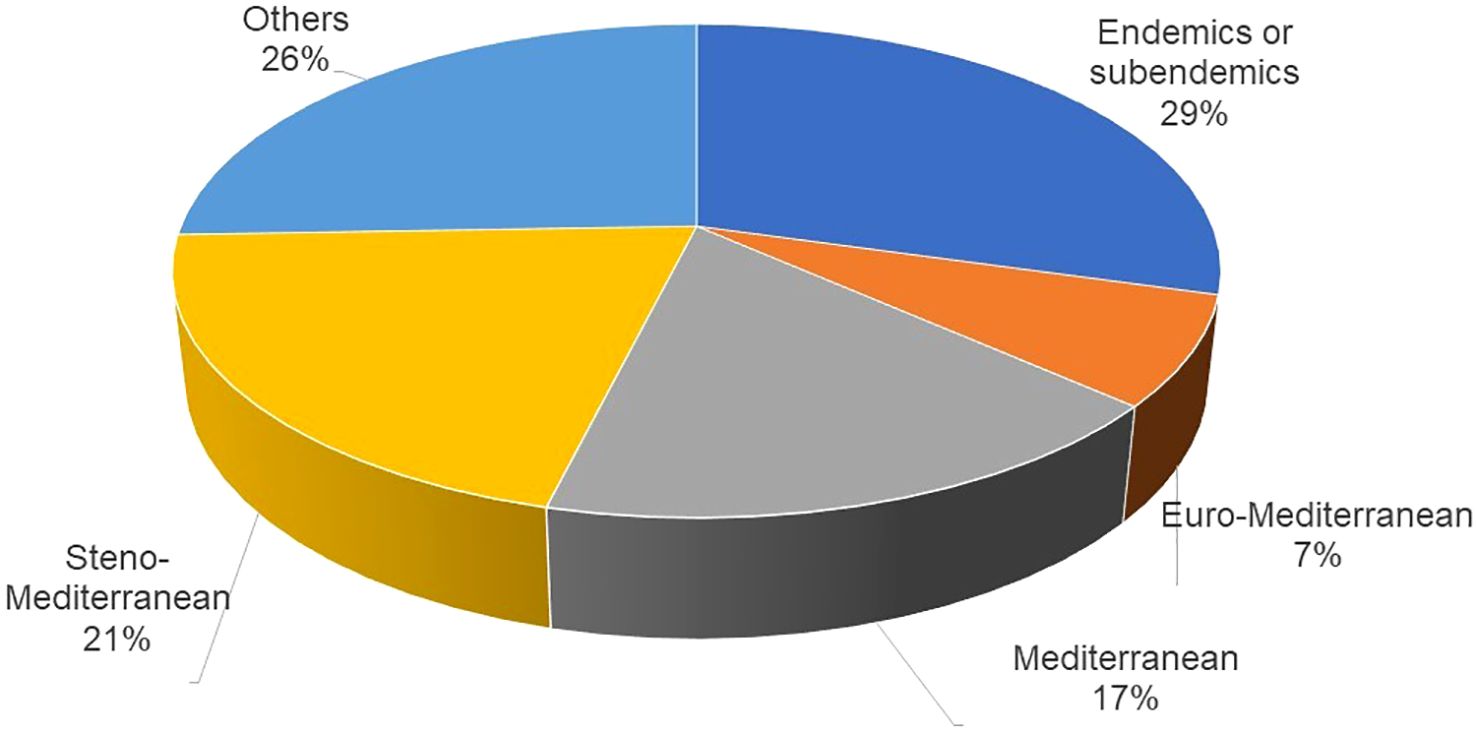
Most of the species were from the Mediterranean area, based on the criteria adopted for inclusion in the list (Figure 1). Endemic or subendemic species account for 29% of the total. Steno-Mediterranean species are also well represented, comprising 21% of the total.
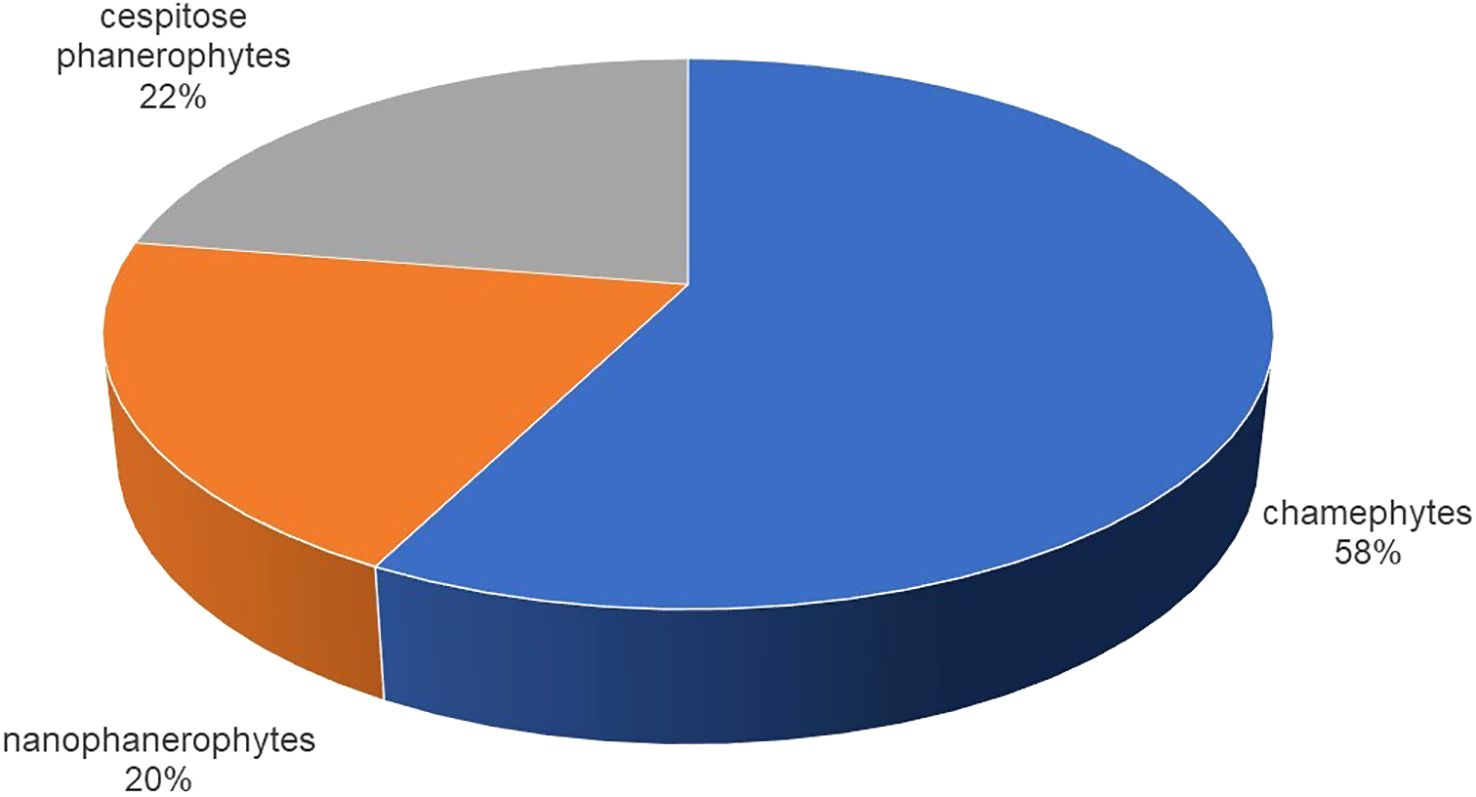
The most common biological form was chamaephytes, which accounted for 58% of the total, followed by cespitose phanerophytes (22%), and nanophanerophytes (20%) (Figure 2).
Over 50% of the plants were under 60 cm in height, while 30% exceeded 100 cm (Figure 3).
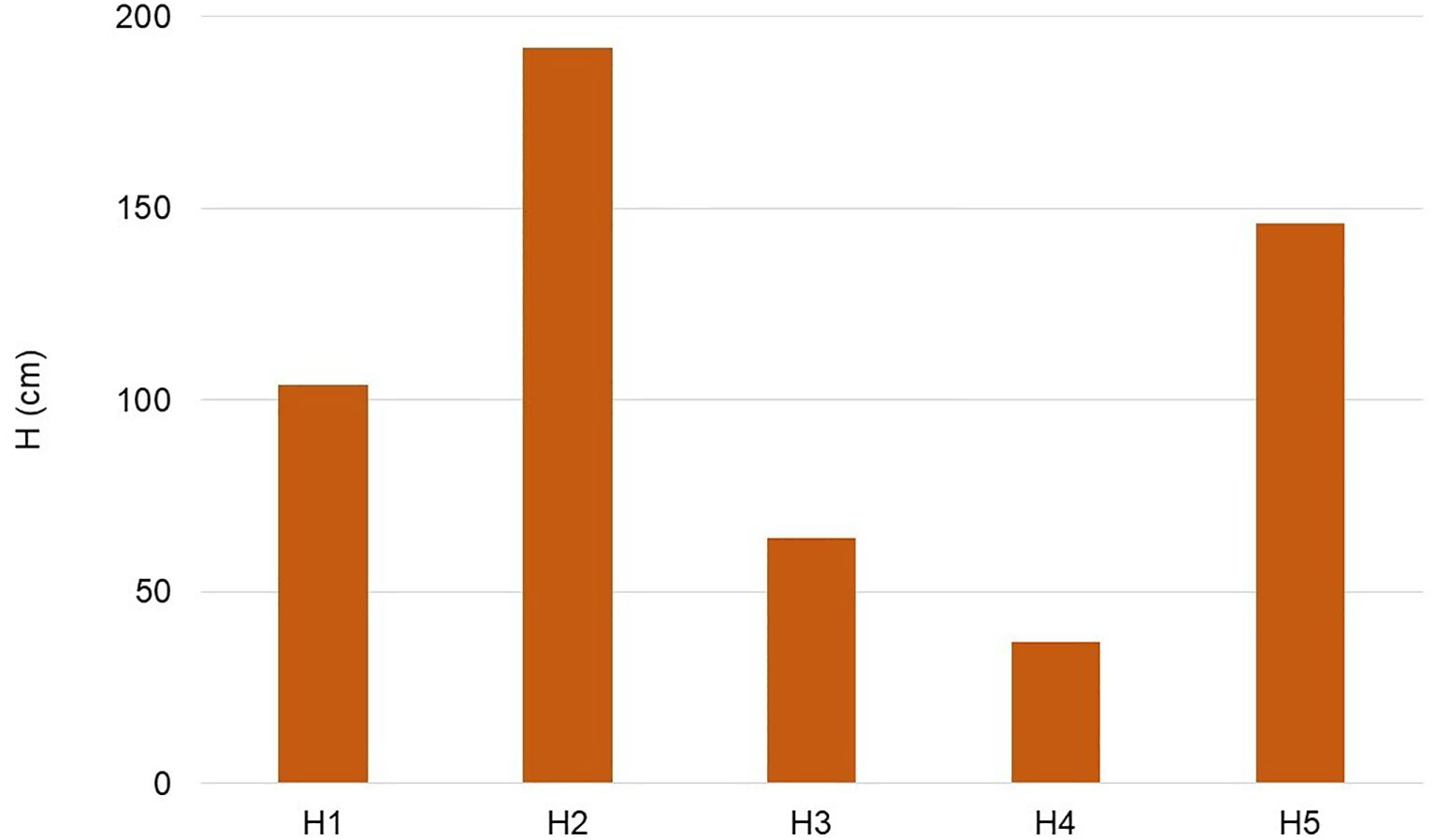
Figure 3. Distribution of shrub for height: H1= < 20 cm; H2 = 20–60 cm; H3 = 60–100 cm; H4 = 100–140 cm; H5= > 140 cm.
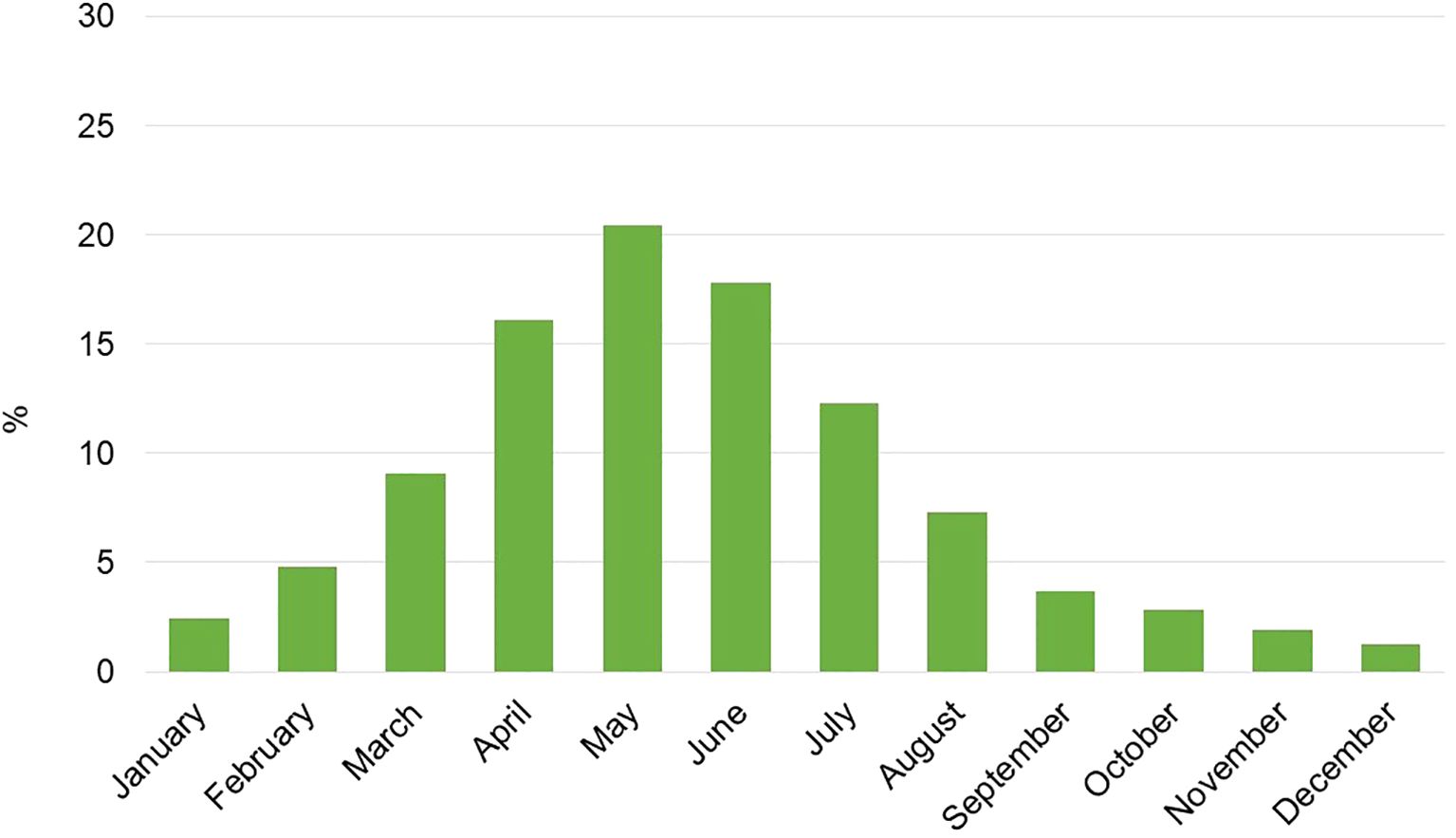
The flowering period of different species lasted an average of 3.7 months. In terms of distribution across the months, the highest concentration of flowering occurred between April and June, accounting for over 50% of the blooms. The lowest concentrations were observed in November and December, at 1.9% and 1.2%, respectively (Figure 4).
The conspicuousness supporting the ornamental characteristics was attributed to the flowers in 86.4% of cases, to the fruit in 20.6%, and to the foliage in 23.7% (Table 1S). In fact, some species were valued for multiple ornamental traits.
The color of the flowers, the most conspicuous organ, was white in 134 species (24.6% of the total), yellow in 196 species (30.0%), pink-red in 103 species (18.9%), and violet-lilac in 46 species (8.5%) (Table 1S).
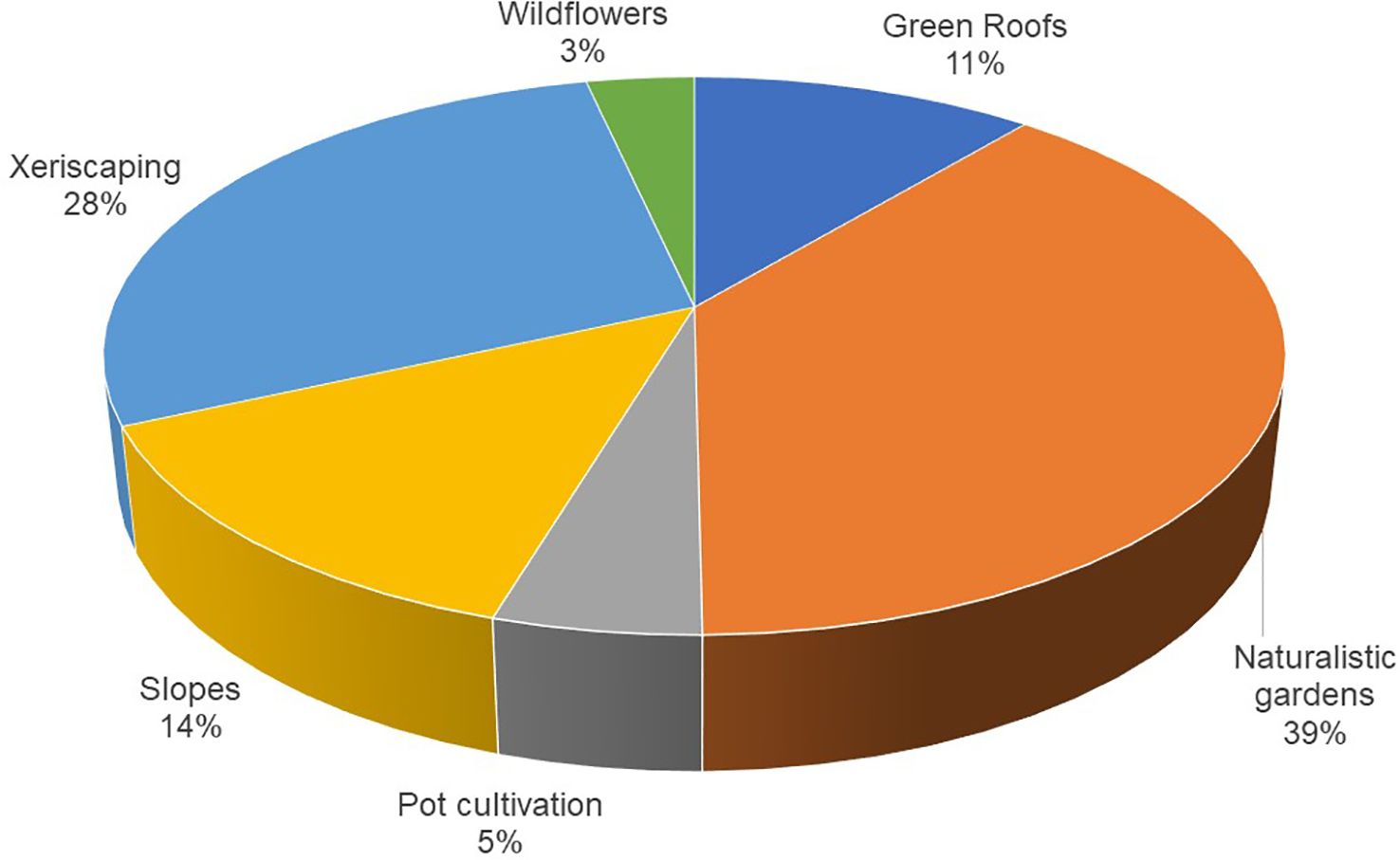
Based on their morphological and phenological traits, the species can be primarily used in naturalistic gardens (39%), xeriscaping (28%), slope stabilization (14%), and green roofs (11%). Their use for pot cultivation and wildflowers is marginal (Figure 5). Each species supports multiple applications, averaging 2.1 uses per species, highlighting their potential value in green spaces.
Despite the small size of the flowers, many species are appreciated for their abundant blooms and, occasionally, for the distinctive characteristics of their fruits and foliage, highlighting the aesthetic appeal of many recorded taxa (Figure 6).
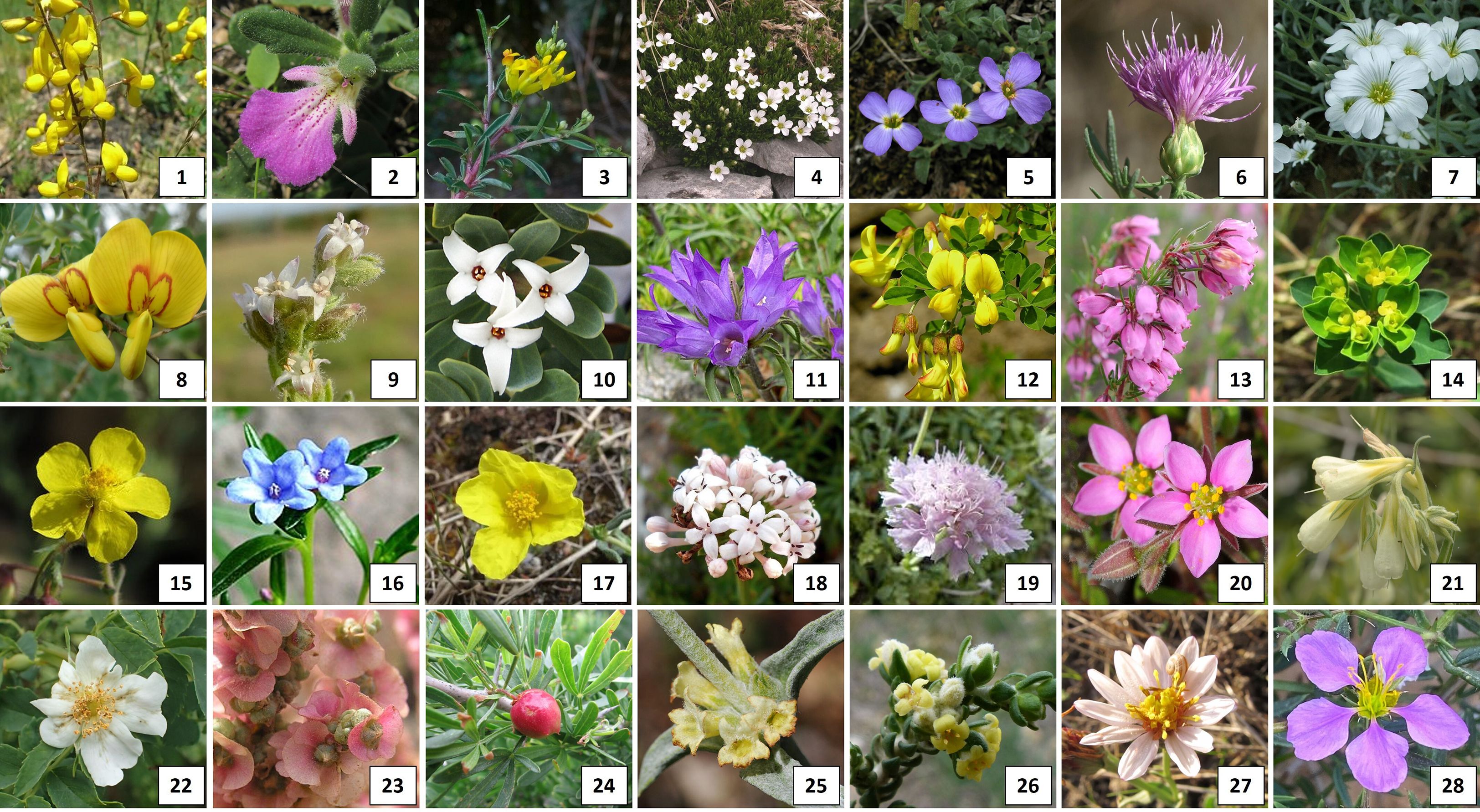
Figure 6. Top left to right: 1) Adenocarpus complicatus subsp. bivonae (C.Presl) Peruzzi; 2) Ajuga iva subsp. iva; 3) Anthyllis hermanniae L.; 4) Arenaria grandiflora subsp. grandiflora; 5) Aubrieta columnae Guss.; 6) Centaurea aeolica Guss. Ex Lojac.; 7) Cerastium tomentosum L.; 8) Colutea arborescens L.; 9) Cressa cretica L.; 10) Daphne oleoides Schreb.; 11) Edraianthus graminifolius A.DC.; 12) Emerus major Mill.; 13) Erica cinerea L.; 14) Euphorbia gasparrinii Boiss.; 15) Fumana laevipes Spach; 16) Glandora rosmarinifolia (Ten.) D.C.Thomas; 17) Helianthemum croceum (Desf.) Pers.; 18) Hexaphylla rupestris (Tineo) P.Caputo & Del Guacchio; 19) Lomelosia crenata (Cirillo) Greuter & Burdet; 20) Minuartia geniculate Thell.; 21) Onosma echioides (L.) L.; 22) Rosa caesia Sm.; 23) Salsola vermiculata L.; 24) Searsia pentaphylla (Jacq.) F.A.Barkley ex Moffett, 25) Sideritis italica (Miller) Greuter & Burdet; 26) Thymelaea hirsuta Endl.; 27) Tripolium sorrentinoi (Tod.) Raimondo & Greuter; 28) Zygophyllum creticum (L.) Christenh. & Byng.
Many of the ornamental species surveyed are not listed in the catalogs of major Italian commercial nurseries. A total of 369 plant species, representing 67.8% of the recorded accessions, were absent from these commercial nursery catalogs. However, it cannot be ruled out that some of these unlisted species are cultivated on a marginal scale.
4 Discussion
The survey highlighted the floristic diversity of the Mediterranean region, which is influenced by various factors such as topographical, biogeographical, ecological, and climatic heterogeneity, as well as human interference that has significantly impacted the vegetation. The current levels of endemism—many of the taxa identified in the review are endemic—and biodiversity are the result of these interactions (Rundel and Cowling, 2013; FAO and Plan Bleu, 2019). Italy’s geographical position as a peninsula with numerous islands is a key factor explaining its biodiversity (Conti et al., 2005; Tavşanoğlu and Pausas, 2018).
Urban populations rely on various ecosystem services provided by natural areas. However, the expansion of urban environments and the consequent destruction of natural habitats have increased the reliance on ornamental green spaces to deliver ecosystem services for the benefit of city residents (Francini et al., 2022). Among these services, biodiversity conservation plays a crucial role. Introducing native plants can contribute significantly to conservation efforts, especially when these plants are rare or threatened. Many species identified in our study possess these characteristics, which enhances the value of their use. Incorporating native plants into urban landscapes not only boosts biodiversity but also creates environments capable of providing essential ecosystem goods and services within cities.
Public awareness of the importance of biodiversity and the benefits derived from incorporating regional flora into urban landscape design is now well established (Lovell and Johnston, 2009; Ahern, 2013; Phondani et al., 2016).
The demand for native plants in landscaping and gardening, driven by ecological concerns, is increasing. Therefore, research is necessary to identify suitable native plants and to evaluate whether their use can enhance biodiversity resilience and improve the capacity of native plant landscapes to provide ecosystem services for a growing urban population (Martinson, 2020). The most common benefits of green spaces include the reduction of pollution (particulate matter, heavy metals, ozone), mitigation of high temperatures, reduction of surface runoff by preventing erosion, slope stabilization, carbon dioxide sequestration, and oxygen emission (Francini et al., 2021, 2022; Leotta et al., 2023a).
Green infrastructure (GI) plays a crucial role in biodiversity conservation. Conservation gardening has recently been promoted as a strategy to mitigate the decline of native plant species (Affolter, 1997; Gratzfeld, 2017; Ismail et al., 2021; Mounce et al., 2017). This concept has emerged as a participatory approach to restoring biodiversity by cultivating declining native species in private gardens, integrating commercial horticulture with conservation efforts, and encouraging public engagement (Munschek et al., 2023; Segar et al., 2022). Investigating the role of gardens as dispersal corridors for native and endangered plant species could provide valuable insights for collectively addressing the biodiversity crisis (Staude, 2024).
The species identified in our review could therefore contribute to enhancing the native biodiversity of cities; however, the feasibility of their actual inclusion still needs to be evaluated.
The long-term sustainability of landscapes utilizing native plant resources requires management to consider not only ornamental purposes but also ecological and environmental values (e.g., soil, water, biodiversity) and socio-economic factors (Muhtaman et al., 2000; Burlando et al., 2025).
The biodiversity of urban green spaces is crucial because it mitigates the risks posed by pests, diseases, and climate change, thereby enhancing the resilience of the ecosystem services provided by green infrastructure (Leotta et al., 2023a).
Depending on their hardiness, shrubs play a fundamental ecological role in green areas (Pasalodos-Tato et al., 2015). In natural environments, the shrub stage represents a secondary succession that can stimulate, facilitate, or positively interact with community dynamics. In fact, shrub species, which act as nurse plants, can promote the germination and growth of woody forest species beneath their canopy by improving the water status of seedlings through reducing radiation, lowering soil temperature, conserving moisture, and protecting them from herbivore damage. These effects are also influenced by the shrubs’ functional traits and morphological characteristics (Bruno et al., 2003; Castro et al., 2004; Manaut et al., 2011). Shrubs are pioneer, branched plant species that are highly adaptable and strongly interactive with other species (Götmark et al., 2016).
In Mediterranean ecosystems, shrubs are a characteristic type of vegetation, widespread across various habitats. Due to multiple factors—including physiological, morphological, reproductive, phenological, and regenerative traits, as well as inter- and intraspecific interactions—each shrub species constitutes an important component of the plant community and fulfills a specific ecological role (Lombardo et al., 2020). The most critical morphological traits for abiotic stress tolerance are leaf morphology and functionality, particularly the ability to reduce gas exchange and enhance metabolite accumulation (Toscano et al., 2020; Leotta et al., 2023a).
The high biodiversity potential offered by the identified plant species—over 540 taxa belonging to 68 botanical families—can ensure the goal of enhancing the sustainability of green spaces. Native shrubs, due to their hardiness, are well-suited for new urban green design systems that reduce maintenance costs, partly through more naturalistic planting (Nam and Dempsey, 2019). This approach enables an increase in biodiversity even within urban environments (Capotorti et al., 2020; Bonthoux et al., 2019; Pomatto et al., 2023).
Biological form helps us understand the characteristics of plants and their ability to adapt to marginal conditions. In our review, the most common type was chamaephytes. The specific morphological traits of chamaephytes can vary greatly depending on the species and environmental factors. Chamaephyte plants are characterized by low growth close to the ground, which allows them to survive in harsh conditions such as cold climates, where the warmth of the soil aids their survival, or drought conditions, due to reduced transpiration resulting from the plant’s cushion-like shape. Many chamaephytes possess adaptations that enable them to endure these challenging environments. Additionally, survival in harsh conditions is facilitated by a reduction in leaf size, which minimizes water loss.
The list is well represented by caespitose phanerophytes, which are plants characterized by the formation of dense clumps or groups of stems arising from a common base. Nanophanerophytes, typically low-growing woody plants that usually reach only a few meters in height, are also included in the list compiled by our study.
The potential for domesticating endemic plants for ornamental purposes is underscored by the inadequate ex situ conservation of local endemic species in botanical gardens and seed banks (Krigas et al., 2021). Another challenge is the lack of studies on assessment systems for the rapid identification of suitable landscapes and garden plants. Key characteristics to consider include plant habit and height, vegetative and flowering periods, flower traits, modes of reproduction, tolerance to biotic and abiotic stresses, and associated survival rates (Liu et al., 2016). Utilizing these plants in landscapes and gardens can also contribute to the conservation of endemic species at risk of extinction.
An interesting aspect that emerged from this study was the variability in the heights attained. In many cases, the plants did not exceed 20 cm in height; therefore, the species identified can be used as ground cover. Ground cover plants serve as components or connecting elements in other green infrastructure interventions (Woods Ballard et al., 2015), such as rain gardens, green roofs, or rainwater harvesting tanks (Nur Hannah Ismail et al., 2023).
Their small size—over 54% of the species surveyed do not exceed 60 cm—makes them particularly suitable for new types of greenery, such as extensive green roofs (Savi et al., 2016; Leotta et al., 2023b). Their limited height is linked to their biological form: in 58% of cases, they are chamaephytes. The height of the plants surveyed, often less than one meter, allows for a wide variety of uses and plays an important ecological role in plant communities. The cushion shape, which minimizes the exposed surface area of the plant relative to its volume, contributes to their tolerance of abiotic stresses, as observed in the Mediterranean region.
Based on the information available for each of the accessions surveyed, we can only hypothesize about their possible uses, which appear to be quite diverse. The different species had an average of 2.1 uses. However, only experimental trials conducted in representative contexts will be able to accurately assess the actual uses of the accessions surveyed.
Aesthetic aspects play a crucial role in urban landscape design. The beauty of plants is primarily conveyed through their visual appearance, which is inherently linked to color. Ornamental plants contribute vibrant color to urban green spaces (Wang, 2021). Additionally, the landscape created by ornamental plants evolves over time, meaning that the color of plants across different seasons has a significant chromatic impact. The color of plants, particularly their flowers, is therefore a key element in crafting an appealing urban landscape (Wang, 2021). The survey revealed that plants typically flower for several months; notably, it is possible to have varying blooms—and thus different colors—throughout the year. Many of the species identified were also valued for their combined aesthetic qualities related to flowers, foliage, and fruit.
Today, ornamental plants are valued not only for their aesthetic appeal but also for their ability to enhance the environment and improve quality of life (Savé, 2009). A large number of Mediterranean-origin species, particularly shrubs, are well-suited for sustainable landscaping due to their capacity to thrive in harsh conditions and tolerate drought stress. Unlike agriculture, the success of an ornamental landscape is not measured by yield but by its ability to satisfy user expectations while requiring minimal maintenance. In degraded environments, plant survival may be the primary objective of cultivation (Toscano et al., 2019). Many of these plants, owing to their origin, resilience in marginal settings, distinctive morpho-biometric traits, and attractive ornamental qualities, appear well-equipped to meet the current demands of urban landscaping.
5 Conclusions
The Mediterranean region is a rich source of biodiversity for the ornamental sector, hosting a wide variety of plant species suitable for urban and peri-urban landscapes. Although morphological and physiological traits vary among botanical families, they remain of significant interest. The ornamental value is derived from leaves, flowers, and fruits, each contributing to aesthetic appeal at different times or seasons. Consequently, combining various ornamental species can extend the visual appeal of an area throughout the year. In this study, the most important genera and species were identified for use in successive experimental tests aimed at selecting native plants capable of adapting to urban environments. This approach aims to enhance the sustainability of urban green spaces while revitalizing the biological diversity of ornamental floriculture.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
LL: Writing – original draft. ST: Writing – review & editing. AF: Writing – review & editing, Conceptualization, Writing – original draft. DR: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fhort.2025.1652517/full#supplementary-material
Supplementary Table 1 | Mediterranean shrub species selected d for the potential landscape use.
References
Acta Plantarum (2025). Available online at: https://www.actaplantarum.org/ (Accessed February 2025).
Affolter J. M. (1997). Essential role of horticulture in rare plant conservation. HortScience 32, 29–34. doi: 10.21273/HORTSCI.32.1.29
Ahern J. (2013). Urban landscape sustainability and resilience: the promise and challenges of integrating ecology with urban planning and design. Landsc. Ecol. 28, 1203–1212. doi: 10.1007/s10980-012-9799-z
Bartolucci F., Peruzzi L., Galasso G., Albano A., Alessandrini A. N. M. G., Ardenghi N. M. G., et al. (2018). An updated checklist of the vascular flora native to Italy. Plant Biosyst 152 (2), 179–303. doi: 10.1080/11263504.2017.1419996
Bartolucci F., Peruzzi L., Galasso G., Alessandrini A., Ardenghi N. M. G., Bacchetta G., et al. (2024). A second update to the checklist of the vascular flora native to Italy. Plant Biosyst 158 (2), 219–296. doi: 10.1080/11263504.2024.2320126
Bochet E., Poesen J., and Rubio J. L. (2006). Runoff and soil loss under individual plants of a semi-arid Mediterranean shrubland: influence of plant morphology and rainfall intensity. Earth Surf. Process. Landf. 31, 536–549. doi: 10.1002/esp.1351
Bonthoux S., Voisin L., Bouché-Pillon S., and Chollet S. (2019). More than weeds: Spontaneous vegetation in streets as a neglected element of urban biodiversity. Landsc. Urban. Plan. 185, 163–172. doi: 10.1016/j.landurbplan.2019.02.009
Bruno J. F., Stachowicz J. J., and Bertness M. D. (2003). Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18, 119–125. doi: 10.1016/S0169-5347(02)00045-9
Burlando P., Ferrante A., and Vagge I. (2025). Multiscale and multidisciplinary method for plant selection to design green urban and peri-urban areas. Front. Hortic. 4. doi: 10.3389/fhort.2025.1354764
Capotorti G., Bonacquisti S., Abis L., Aloisi I., Attorre F., Bacaro G., et al. (2020). More nature in the city. Plant Biosyst. 154, 1003–1006. doi: 10.1080/11263504.2020.1837285
Cassaniti C., Romano D., Hop M. E. C. M., and Flowers T. J. (2013). Growing floricultural crops with brackish water. Environ. Exp. Bot. 92, 165–175. doi: 10.1016/j.envexpbot.2012.08.006
Castro J., Zamora R., Hodar J. A., Gomez J. M., and Gómez-Aparicio L. (2004). Benefits of using shrubs as nurse plants for reforestation in Mediterranean mountains: a 4-year study. Restor. Ecol. 12, 352–358. doi: 10.1111/j.1061-2971.2004.0316.x
Chapin III, F.S. (1983). Nitrogen and phosphorus nutrition and nutrient cycling by evergreen and deciduous understory shrubs in an Alaskan black spruce forest. Can. J. For. Res. 13, 773–781. doi: 10.1139/x83-107
Chaves M. M., Maroco J. P., and Pereira J. S. (2003). Understanding plant responses to drought—from genes to the whole plant. Funct. Plant Biol. 30, 239–264. doi: 10.1071/FP02076
Conti F., Abbate G., Alessandrini A., and Blasi C. (2005). An annotated checklist of the Italian vascular flora (Roma: Palombi Editori).
Evans T. L., Mata-González R., Martin D. W., McLendon T., and Noller J. S. (2013). Growth, water productivity, and biomass allocation of Great Basin plants as affected by summer watering. Ecohydrology 6, 713–721. doi: 10.1002/eco.1292
FAO and Plan Bleu (2019). State of Mediterranean forests 2018 (Rome and Plan Bleu, Marseille: Food and Agriculture Organization of the United Nations).
Fascetti S., Potenza G., Castronuovo D., and Candido V. (2014). Wild geophytes of ornamental interest in the native flora of southern Italy. Ital. J. Agron. 9, 595. doi: 10.4081/ija.2014.595
Filella I., Llusia J., Piñol J., and Peñuelas J. (1998). Leaf gas exchange and fluorescence of Phillyrea latifolia, Pistacia lentiscus and Quercus ilex saplings in severe drought and high temperature conditions. J. Exp. Bot. 39, 213–220. doi: 10.1016/S0098-8472(97)00045-2
Francini A., Romano D., Toscano S., and Ferrante A. (2022). The contribution of ornamental plants to urban ecosystem services. Earth 3, 1258–1274. doi: 10.3390/earth3040071
Francini A., Toscano S., Romano D., Ferrini F., and Ferrante A. (2021). Biological contribution of ornamental plants for improving slope stability along urban and suburban areas. Horticulturae 7, 310. doi: 10.3390/horticulturae7090310
Givnish T. J. (1984). “Leaf and canopy adaptations in tropical forests,” in Physiological Ecology of Plants of the Wet Tropics: Tasks for Vegetation Science, vol. 12 . Eds. Medina E., Mooney H. A., and Vázquez-Yánes C. (Springer, Dordrecht, The Netherlands), 51–84.
Götmark F., Götmark E., and Jensen A. M. (2016). Why be a shrub? A basic model and hypotheses for the adaptive values of a common growth form. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01095
Gratzfeld J. (2017). What is conservation horticulture? BGjournal 14, 14–17. Available online at: https://www.jstor.org/stable/26369188 (Accessed April 22, 2025).
Hall D. M., Camilo G. R., Tonietto R. K., Ollerton J., Ahrné K., Arduser M., et al. (2017). The city as a refuge for insect pollinators. Biol. Conserv. 31, 24–29. doi: 10.1111/cobi.12840
iNaturalist (2025). Available online at: https://share.google/MVBE48HWL3u7zGmtY (Accessed February 2025).
Ismail S. A., Pouteau R., van Kleunen M., Maurel N., and Kueffer C. (2021). Horticultural plant use as a so-far neglected pillar of ex situ conservation. Conserv. Lett. 14, e12825. doi: 10.1111/conl.12825
Junqueira A. H. and Peetz M. S. (2017). Intellectual property rights in Brazilian floriculture: Innovations for the growth and development of the market. Ornam. Hortic. 23, 296–306. doi: 10.14295/oh.v23i3.1071
Krigas N., Tsoktouridis G., Anestis I., Khabbach A., Libiad M., Megdiche-Ksouri W., et al. (2021). Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 3, 2539. doi: 10.3390/su13052539
Leotta L., Toscano S., Ferrante A., Romano D., and Francini A. (2023a). New strategies to increase the abiotic stress tolerance in woody ornamental plants in Mediterranean climate. Plants 12, 2022. doi: 10.3390/plants12102022
Leotta L., Toscano S., and Romano D. (2023b). Which plant species for green roofs in the mediterranean environment? Plants 12, 3985. doi: 10.3390/plants12233985
Liu J., Gao Y., Zhang Q., Bai W., Wang Y., and Yang M. (2016). Plant evaluation and selection for rain gardens in China. Acta Hortic. 1108, 201–212. doi: 10.17660/ActaHortic.2016.1108.26
Lombardo E., Bancheva S., Domina G., and Venturella G. (2020). Distribution, ecological role and symbioses of selected shrubby species in the Mediterranean Basin: a review. Plant Biosyst. 154, 438–454. doi: 10.1080/11263504.2020.1727988
Lovell S. T. and Johnston D. M. (2009). Designing landscapes for performance based on emerging principles in landscape ecology. Ecol. Soc 14, 44. Available online at: https://www.jstor.org/stable/26268059 (Accessed April 23, 2025).
Maloupa E., Krigas N., Grigoriadou K., Lazari D., and Tsoktouridis G. (2008). “Conservation strategies for native plant species and their sustainable exploitation: Case of the Balkan Botanic Garden of Kroussia, N. Greece,” in Floriculture Ornamental Plant Biotechnology: Advances and Topical Issues, vol. 5 . Ed. Teixeira da Silva J. A. (Global Science Books, Isleworth, UK), 37–56.
Manaut N., Chaffii K., Ouhammou A., Ouahmane L., Thioulouse J., Mohamed H., et al. (2011). “Shrub plants and associated mycorrhizal fungi sustainably improve the native tree establishment in Mediterranean ecosystems,” in Mediterranean Ecosystems: Dynamics, Management and Conservation. Ed. Williams G. S. (Nova Science Publishers, New York), 151–166.
Mangas J. G., Lozano J., Cabezas-Díaz S., and Virgós E. (2008). The priority value of scrubland habitats for carnivore conservation in Mediterranean ecosystems. Biodivers. Conserv. 17, 43–51. doi: 10.1007/s10531-007-9229-8
Martinson R. (2020). Native plants in urban landscapes: a biological imperative. Native. Plants J. 21, 275–280. doi: 10.3368/npj.21.3.275
Matesanz S. and Valladares F. (2014). Ecological and evolutionary responses of Mediterranean plants to global change. Environ. Exp. Bot. 103, 53–67. doi: 10.1016/j.envexpbot.2013.09.004
Mounce R., Smith P., and Brockington S. (2017). Ex situ conservation of plant diversity in the world’s botanic gardens. Nat. Plants 3, 795–802. doi: 10.1038/s41477-017-0019-3
Muhtaman D. R., Siregar C. A., and Hopmans P. (2000). Criteria and indicators for sustainable plantation forestry in Indonesia (Bagor, Indonesia: Center for International Forestry Research (Cifor).
Munné-Bosch S. and Peñuelas J. (2004). Drought-induced oxidative stress in strawberry tree (Arbutus unedo L.) growing in Mediterranean field conditions. Plant Sci. 166, 1105–1110. doi: 10.1016/j.plantsci.2003.12.034
Munschek M., Witt R., Kaltofen K., Segar J., Wirth C., Weigelt A., et al. (2023). Putting conservation gardening into practice. Sci. Rep. 13, 12671. doi: 10.1038/s41598-023-39432-8
Myers N., Mittermeier R. A., Mittermeier C. G., Da Fonseca G. A., and Kent J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Nam J. and Dempsey N. (2019). Understanding stakeholder perceptions of acceptability and feasibility of formal and informal planting in Sheffield’s district parks. Sustainability 11, 360. doi: 10.3390/su11020360
Nur Hannah Ismail S., Stovin V., and Cameron R. W. F. (2023). Functional urban ground-cover plants: identifying traits that promote rainwater retention and dissipation. Urban. Ecosyst. 26, 1709–1724. doi: 10.1007/s11252-023-01417-w
Ogaya R. and Peñuelas J. (2003). Comparative field study of Quercus ilex and Phillyrea latifolia: photosynthetic response to experimental drought conditions. Environ. Exp. Bot. 50, 137–148. doi: 10.1016/S0098-8472(03)00019-4
Padulosi S., Thompson J., and Rudebjer P. G. (2013). Fighting poverty, hunger and malnutrition with neglected and underutilized species: needs, challenges and the way forward (Rome, Italy: Biodiversity International).
Pasalodos-Tato M., Ruiz-Peinado R., Del Río M., and Montero G. (2015). Shrub biomass accumulation and growth rate models to quantify carbon stocks and fluxes for the Mediterranean region. Eur. J. For. Res. 134, 537–553. doi: 10.1007/s10342-015-0870-6
Paz S., Negev M., Clermont A., and Green M. S. (2016). Health aspects of climate change in cities with Mediterranean climate, and local adaptation plans. Int. J. Environ. Res. Public Health 13, 438. doi: 10.3390/ijerph13040438
Phondani P. C., Bhatt A., Elsarrag E., Alhorr Y. M., and El-Keblawy A. (2016). Criteria and indicator approach of global sustainability assessment system for sustainable landscaping using native plants in Qatar. Ecol. Indic. 69, 381–389. doi: 10.1016/j.ecolind.2016.05.003
Pignatti S., Guarino R., and La Rosa M. (2017–2019). Flora d’Italia Vol. 1-IV (Bologna, Italy: Edagricole).
Pinheiro C., Guerra-Guimaraes L., David T. S., and Vieira A. (2014). Proteomics: State of the art to study Mediterranean woody species under stress. Environ. Exp. Bot. 103, 117–127. doi: 10.1016/j.envexpbot.2014.01.010
Plants of the World Online (2025). Available online at: https://powo.science.kew.org/ (Accessed February 2025).
Pomatto E., Larcher F., Caser M., Gaino W., and Devecchi M. (2023). Evaluation of different combinations of ornamental perennials for sustainable management in urban greening. Plants 12, 3293. doi: 10.3390/plants12183293
Raimondo F. M., Domina G., and Spadaro V. (2010). Checklist of the vascular flora of Sicily. Quad. Bot. Amb. Appl. 21, 189–252.
Raunkiaer C. (1934). The Life Forms of Plants and Statistical Plant Geography (London, UK: Oxford University Press).
Rey P. J., Siles G., and Alcántara J. M. (2009). Community-level restoration profiles in Mediterranean vegetation: nurse-based vs. traditional reforestation. J. Appl. Ecol. 46, 937–945. doi: 10.1111/j.1365-2664.2009.01680.x
Rivera D., Obón C., Heinrich M., Inocencio C., Verde A., and Fajardo J. (2006). “Gathered Mediterranean food plants—Ethnobotanical investigations and historical development,” in Local Mediterranean Food Plants and Nutraceuticals, vol. 59 . Eds. Heinrich M., Müller W. E., and Galli C. (Karger Publishing, Basel, Switzerland), 18–74.
Romano D. and Scariot V. (2021). Woody ornamentals: a review of genetic resources in the Mediterranean area. Acta Hortic. 1331, 325–334. doi: 10.17660/ActaHortic.2021.1331.43
Romano D. (2021). “Il progetto QUAPROVER. Un’ipotesi di lavoro per migliorare le conoscenze sulla gestione del verde urbano,” In VerdeCittà. Il rinnovo delle alberate nelle città: verde, bellezza e salute. Il made in Italy del florovivaismo italiano, ed. Burchi G. (CREA Consiglio per la ricerca in agricoltura e l’analisi dell’economia agraria, Roma), 263–274.
Rundel P. W. and Cowling R. M. (2013). “Mediterranean-climate ecosystems,” in Encyclopedia of Biodiversity. Ed. Levin (Academic Press, Cambridge), 212–222.
Savé R. (2009). What is stress and how to deal with it in ornamental plants? Acta Hortic. 813, 241–254. doi: 10.17660/ActaHortic.2009.813.31
Savi T., Dal Borgo A., Love V. L., Andri S., Tretiach M., and Nardini A. (2016). Drought versus heat: What’s the major constraint on Mediterranean green roof plants? Sci. Total. Environ. 566, 753–760. doi: 10.1016/j.scitotenv.2016.05.100
Scariot V., Seglie L., Gaino W., and Devecchi M. (2012). Evaluation of European native bluebells for sustainable floriculture. Acta Hortic. 937, 273–279. doi: 10.17660/ActaHortic.2012.937.33
Segar J., Callaghan C. T., Ladouceur E., Meya J. N., Pereira H. M., Perino A., et al. (2022). Urban conservation gardening in the decade of restoration. Nat. Sustain. 5, 649–656. doi: 10.1038/s41893-022-00882-z
Senapathi D., Goddard M. A., Kunin W. E., and Baldock K. C. (2017). Landscape impacts on pollinator communities in temperate systems: evidence and knowledge gaps. Funct. Ecol. 31, 26–37. doi: 10.1111/1365-2435.12809
Staude I. R. (2024). Gardens as drivers of native plant species dispersal and conservation. People Nat. 6, 1220–1228. doi: 10.1002/pan3.10627
Tavşanoğlu Ç. and Pausas J. G. (2018). A functional trait database for Mediterranean Basin plants. Sci. Data 5, 180135. doi: 10.1038/sdata.2018.135
Toscano S., Branca F., Romano D., and Ferrante A. (2020). An evaluation of different parameters to screen ornamental shrubs for salt spray tolerance. Biology 9, 250. doi: 10.3390/biology9090250
Toscano S., Ferrante A., and Romano D. (2019). Response of Mediterranean ornamental plants to drought stress. Horticulturae 5, 6. doi: 10.3390/horticulturae5010006
Wang D. (2021). Seasonal color matching method of ornamental plants in urban landscape construction. Open Geosci. 13, 594–605. doi: 10.1515/geo-2020-0261
Keywords: biodiversity, ornamentals, native plants, sustainable landscape, new crops, conservation gardening
Citation: Leotta L, Toscano S, Ferrante A and Romano D (2025) The use of Mediterranean native shrubs for improving the sustainability of urban environments. Front. Hortic. 4:1652517. doi: 10.3389/fhort.2025.1652517
Received: 23 June 2025; Accepted: 08 October 2025;
Published: 27 October 2025.
Edited by:
Valentina Scariot, University of Turin, ItalyReviewed by:
Sonia Cacini, Council for Agricultural and Economics Research (CREA), ItalyPablo Marinangeli, National University of the South, Argentina
Copyright © 2025 Leotta, Toscano, Ferrante and Romano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Ferrante, YW50b25pby5mZXJyYW50ZUBzYW50YW5uYXBpc2EuaXQ=
 Luca Leotta
Luca Leotta Stefania Toscano
Stefania Toscano Antonio Ferrante
Antonio Ferrante Daniela Romano
Daniela Romano