- Working Group Sustainable Nutrient and Resource Management in Horticulture, Institute of Horticulture, Weihenstephan-Triesdorf University of Applied Sciences, Freising, Germany
Introduction: Reducing peat in the growing media is one of the challenges currently faced in horticulture. Therefore, the availability of suitable alternative raw materials is a main topic. Separated solid digestate from agricultural biogas plants might be an option. In Germany, separated solid digestates are available in large quantities without a value-adding utilization. Whereas the separated liquid phase is used as an effective organic fertilizer, the nutrient availability of the solid residues is limited. Indeed, some research has been done on the use of separated solid digestate as a growing media constituent. The results revealed that the material has potential. However, there are still many unanswered questions, in particular about the effect of various biogas feedstocks on the quality of the digestate and the development of quality guidelines on how they have been established for aerobically produced compost.
Methods: In the current study, five separated solid digestates from agricultural biogas plants were used. Two of these used only plant-based feedstocks, while two others used 10% and another 30% cattle manure as feedstock. The separated solid digestates were composted aerobically for approximately 6 weeks to break down the phytotoxic compounds and to initiate nitrification. Subsequently, the digestates were mixed with bog peat at ratios of 30%, 50%, and 70% (v/v) respectively, and a plant trial was conducted with French marigold (Tagetes erecta).
Results and discussion: The chemical characterization of the digestates revealed that they comply with the established thresholds for green waste compost suitable as a growing media constituent up to 40% (v/v). However, in the plant trial, some separated solid digestates impaired plant growth already at 30% (v/v). This indicates that the guidelines for green waste compost cannot be transferred one-to-one. Moreover, the growth reduction could not be attributed to a single factor, but was suspected to be the result of the interplay between pH, soluble salts, and the concentration of individual elements. Considering the great heterogeneity of separated solid digestate, it is a potentially suitable but challenging growing media constituent. However, there are various approaches to improve quality, e.g., the use of additives, washing, and the strict selection of feedstocks, which are worth further investigation.
1 Introduction
Due to its favorable chemical and physical properties, peat is currently the most important growing media constituent (Gruda, 2019). However, due to its contribution to climate change and the loss of biodiversity and hydrological functions of drained bogs (Stichnothe, 2022), there is increasing political and social pressure to reduce the use of peat in horticulture (Gruda et al., 2024). Currently, wood fiber and composted green waste, coir, and (composted) bark are the most important peat substitutes in growing media (Blok et al., 2024; IVG/GGS, 2025). In Germany, these four materials have accounted for approximately 90% of all peat substitutes in 2024 (IVG/GGS, 2025). However, in the coming decade, a tightening competition for these materials is to be expected as not only the share of peat in growing media will further decrease and the total demand for growing media is expected to quadruple (Blok et al., 2021) but also the de-carbonization of the economy will increase the overall demand for renewable raw materials (Piotrowski et al., 2015). This is further worsened, e.g., by forest conversion with a growing proportion of hardwoods in the wake of climate change (Fuchs et al., 2024). This further decreases the raw material reserves as hardwood species are less suitable for the production of wood fibers due to their several times higher N immobilization and, thus, reduced crop safety (Beuth et al., 2023).
Thus, new raw materials—preferably those without strong competition for use—are needed. A potential candidate might be separated solid residues from the anaerobic digestion of energy crops and manure. While the liquid phase is used as a fertilizer with similar efficacy to mineral fertilizers, the nutrient availability from separated solid digestates (SSDs) is rather low (Guilayn et al., 2020). A value-added use of SSDs could be as a growing media constituent. However, the chemical properties of SSDs vary considerably depending on the feedstock used in the biogas plant (Jankauskienė et al., 2024). Especially livestock excrements increase the nutrient and ballast salt contents and thus limit the maximum share (Schmitz and Meinken, 2009). In addition to the total content of water-soluble salts, the high concentration of plant available phosphorus (P) is of special concern, particularly in the cultivation of P-sensitive plants. Furthermore, Schmitz and Meinken (2009) stated that SSDs could not be used fresh, but need to be pretreated by aerobic composting in order to stabilize organic matter, thus reducing the N dynamics and breaking down phytotoxic compounds as volatile fatty acids (Brinton, 2006). This is confirmed by others (Torres-Climent et al., 2015; Dubský et al., 2019). Furthermore, composting leads to sanitation, which is not guaranteed for fresh SSD. Therefore, a sufficient thermophilic phase is necessary, which requires proper management of the composting process (Kovačić et al., 2022).
If feedstock is purely plant-based, shares up to 50% (v/v) are possible without significant yield loss (Asp et al., 2022), whereas the maximum share of SSD containing livestock excrements as biogas feedstock is lower. Schmitz and Meinken (2009) outlined the effect of different types of livestock excrements on the possible share of SSD in growing media. Cattle slurry already caused significant yield loss and severe damage at 20% (v/v). Dubský et al. (2019) also described a significant decline in the growth of pelargoniums and petunias when shares exceeded 20% (v/v), which was attributed to the high pH levels and potassium concentrations. However, the authors did not provide details regarding the feedstock used in the biogas plant. Moreover, they assumed that physical properties—in particular a lower content of easily available water—could contribute to more compact growth, which is in line with the results of Crippa et al. (2013). A lower water holding capacity was also reported by Asp et al. (2022), whereby the authors stated that this should not pose any problems for plants, but may require different watering strategies.
The aim of the current study was to evaluate the potential of SSDs with increasing proportions of livestock excrements in the biogas feedstock as a growing media constituent. As the limitations of SSDs have not yet been clearly identified, special focus was on the chemical factors affecting plant growth and resulting in fertilization adjustments to overcome the negative effects.
2 Materials and methods
2.1 Separated solid digestate
The SSDs were procured from five agricultural biogas plants in southern Germany. Two of these only used plant-derived feedstock, while two added approximately 10% and one approximately 30% cattle manure. With the exception of one biogas plant, which used a high percentage of chopped hob bines—composed of steam, leaf, and cone residue—as feedstock, the plant-based feedstocks were typical energy crops (maize, green rye, clover/grass mixture, and cup plant). The SSDs were separated by the respective biogas plants and sampled from heaps. Approximately 0.75 m3 was taken per plant and aerobically composted for 6 weeks at Weihenstephan-Triesdorf University of Applied Sciences according to the procedure described by Schmitz and Meinken (2009). After composting, the residues were dried at ambient temperature in a shaded greenhouse (18 ± 4°C) and stored for approximately 2 weeks. Details of the feedstocks and the chemical characterization of the SSDs right before the start of the experiment are given in Table 1. With the exception of the phosphorus content of SSD E, all values complied at least with the thresholds of the German Federal Quality Association for Compost (BGK, 2025) for green waste compost used as growing media constituent up to a volume of 20%. Furthermore, there is no clear effect of the percentage of cattle manure on the concentrations of soluble salts and plant-available nutrients. Moreover, it is noticeable that only in SSD A were significant amounts of nitrate-N found, indicating that nitrification had not been started during composting in SSD B to E.
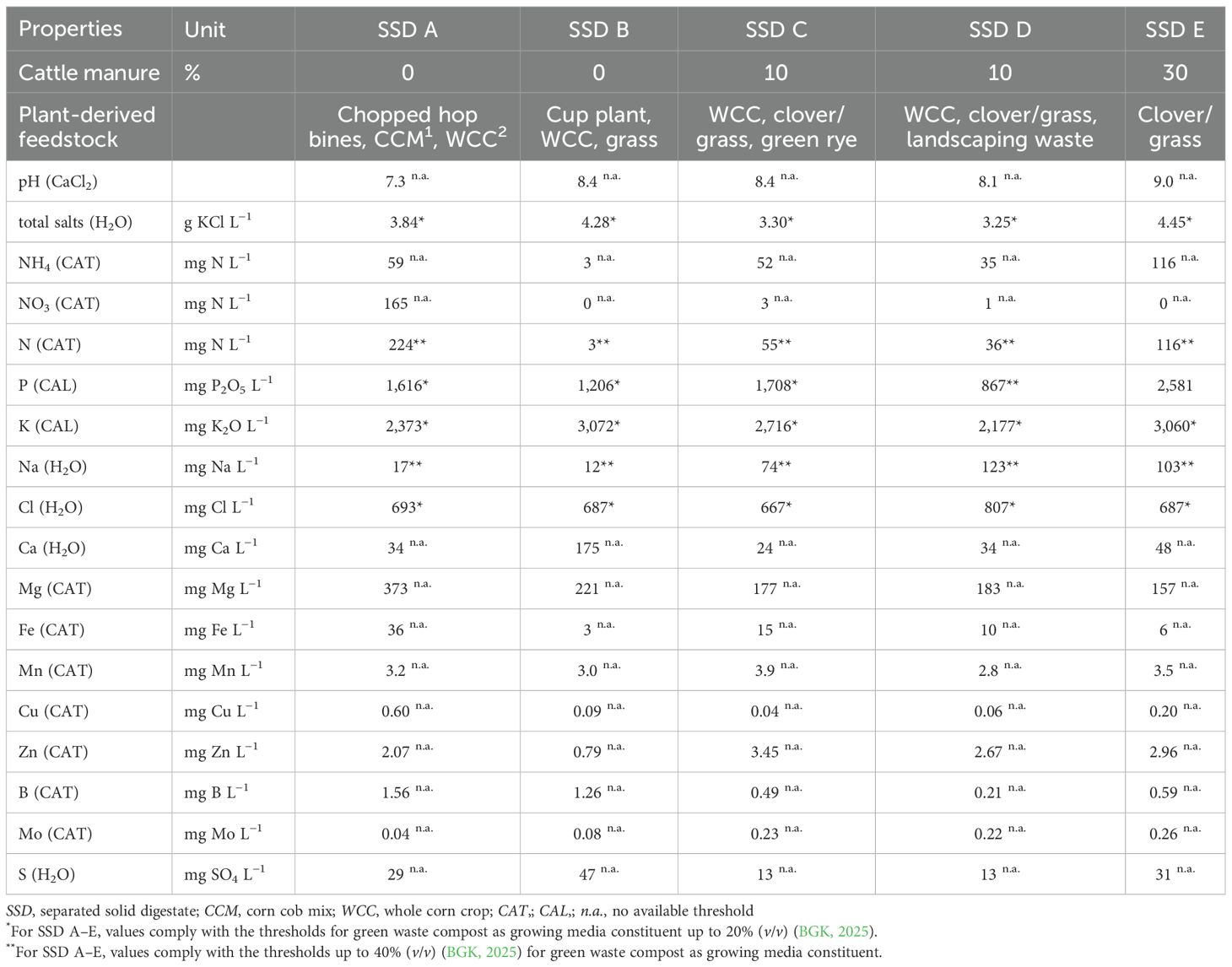
Table 1. Feedstock and chemical characterization (all analysis according to VDLUFA, 2026) of the five separated solid digestates after composting.
2.2 Preparing the growing media and conduction the plant trial
Each of the five SSDs was mixed with Baltic sod peat (H3–H5; 0–8 mm) (Patzer Erden, Sinntal-Altengronau, Germany) at ratios of 70:30, 50:50, and 30:70 (v/v), respectively. The volume was taken by weight calculated from the bulk density according to VDLUFA (2016). In total, 15 mixtures (five SSDs × three mixing ratios) were first analyzed for pH, water-soluble salts, and CAT-soluble nitrogen (N), phosphorus (P), and potassium (K) and then limed and fertilized on demand. The lower pH limit for liming was set at 5.8. When the pH was higher, no further actions were done. The lower limits for N, P (expressed as P2O5), and K (expressed as K2O) were 250, 250, and 350 mg L−1, respectively. Due to the high P and K concentrations in SSDs (Table 1), all treatments far exceeded the lower limits, and only the treatment with 30% (v/v) SSD A needed to be limed, whereas N fertilization was necessary for all SSD treatments. This was done by BAVARIA CaN (15.4% N; Planta, Regenstauf, Germany). As a control treatment, the Baltic sod peat was limed to pH 5.8 and fertilized up to the listed lower limits of N, P, and K, and additionally with trace elements [BAVARIA CaN (15.4% N), Ferty Basis 1 (0-14-35), and Ferty 10 Spezial] (Planta, Regenstauf, Germany).
The trial was conducted with Marigold (Tagetes erecta ‘Antigua Gold F1’; Beringmeier Samen und Saaten, Volkmarsen, Germany) from the end of October to mid-December 2024. Seedlings (10 days old) were individually pricked into 13-cm plastic pots (approximate volume of 1 L) and arranged randomly (four replications with 15 plants each, giving 60 plants per treatment and 960 plants in total) in a greenhouse. The pots were placed in dishes, and drain water was regularly flushed back into the pots to prevent nutrient loss. The heating temperature was set to 20°C (day/night) during the first 2 weeks, and then lowered to 18°C. Ventilation was 2 K above heating temperature. When the radiation outside the greenhouse was below 10 klx between 6:00–9:00 a.m. and 3:30–10:00 p.m., supplement light was provided (one lamp per 3 m2) (LED-KE 400 VSP; DH Licht, Wülfrath, Germany). Irrigation was done with deionized water on demand per pot. To avoid sulfur deficiency, 50 mg S L−1 as MgSO4·7H2O (analytical grade; AppliChem GmbH, Darmstadt, Germany) was added. Fertilization was continuously adapted based on biweekly analysis of the growing media, whereby the lower limit of N was set to 100 mg N L−1. Nitrogen was applied as ammonium nitrate (technical grade; AppliChem GmbH, Darmstadt, Germany) when the pH was ≥6.5 or as calcium nitrate (BAVARIA CaN; Planta, Regenstauf, Germany) when the pH was <6.5, with both N fertilizers at a dose of 50 mg N per pot. Furthermore, treatments with a growing media pH ≥6.5 were fertilized with Fe-EDDHA (Ferty 72; Planta, Regenstauf, Germany) twice during the experiment. Fertilization of the control was done accordingly, whereby phosphorus, potassium, and trace elements were additionally supplied using water-soluble fertilizers [Ferty Basis 1 (0-14-35) and Ferty 10 Spezial] (Planta, Regenstauf, Germany).
2.3 Data collection and analytical methods
The plant trial ran for 45 days. During the experiment, plant growth was visually rated (e.g., for the occurrence of chlorosis and necrosis). At the end of the experiment, the remaining 12 plants per plot (three of the initial 15 plants per plot were taken out for growing media analysis during the experiment) were cut at the growing media surface, and the fresh and dry mass (drying in a forced-air oven at 60°C until weight was constant) were recorded per replicate. Furthermore, root growth (intensity and health) was visually rated (from 1 = worst to 9 = best) individually per pot, and the indices were calculated as the sum of scores per replicate. Dried plant material was ground (ZM 200 with 0.5-mm mesh; Retsch, Haan, Germany) and analyzed for total N using the dry combustion method (Horneck and Miller, 1998) with a LECO CN 828 (LECO Corporation, St. Joseph, MI, USA) and for total P, K, magnesium (Mg), calcium (Ca), sulfur (S), iron (Fe), zinc (Zn), manganese (Mn), copper (Cu), boron (B), and molybdenum (Mo) using inductively coupled plasma optical emission spectroscopy (ICP-OES) (iCAP PRO X; Thermo Fisher Scientific Inc., Waltham, MA, USA) after microwave-assisted digestion (ecowave; Anton Paar GmbH, Graz, Austria) in a mixture of nitric acid and hydrogen peroxide (Miller, 1998). The SSDs before mixing of growing media and the growing media at the end of the plant trial were analyzed for pH in CaCl2 suspension (method A 5.1.1; VDLUFA, 2016); water-soluble salts (method A 13.4.1; VDLUFA, 2016) and water-soluble sodium (Na), chloride (Cl), Ca, and S (method A 13.4.3; VDLUFA, 2016); CAT-soluble N, P, K, Mg, Fe, Zn, Mn, Cu, B, and Mo (method A 13.1.1; VDLUFA, 2016); and CAL-soluble P and K (method A 6.2.1.1; VDLUFA, 2016). In addition, the growing media were analyzed for pH, water-soluble salts, and CAT-soluble N, P, and K biweekly during the trial for adjustment of fertilization. Analysis of the pH and soluble salts was done using electrodes, and an AA500 continuous flow analyzer coupled with two LED spectrometers (SEAL Analytic, Norderstedt, Germany) was used for NH4– and NO3–N, with Cl measured by potentiometric titration with silver nitrate (TitroLine 5000; SI Analytics, Weilheim, Germany) and the remaining elements by ICP-OES. For analysis during the plant trial, one pot per plot was taken out and the four pots of each treatment were used as a pooled sample. At the final evaluation, each of the remaining 12 pots per plot was sampled and the pooled material analyzed.
2.4 Statistical analysis
For the fresh and dry mass, the concentration of nutrients in plant tissue, and the pH, water-soluble salts, and CAT-soluble nutrients in the growing media, data were tested for normality and homogeneity of variance using the Anderson–Darling test and Levene’s test, respectively. Furthermore, visual inspection was performed according to Kozak and Piepho (2018). Although normality and homoscedasticity were not confirmed in all cases, visual inspection only showed minor deviations. Thus, a one-way ANOVA was first calculated for all parameters, followed by Dunnett’s test against the peat control in case of significant differences. Subsequently, the control was removed from the dataset, and a two-way ANOVA with type and share of SSD as factors was performed. Due to significant interactions between factors in the majority of cases, the datasets were split along type and share of SSD. For each subset, one-way ANOVA was computed, followed by a post-hoc Tukey’s test in case of significant differences.
For the visual rating of root intensity and root health, the sum of scores per replicate was calculated for each treatment and a non-parametric ANOVA (Kruskal–Wallis) calculated. In case of significant differences, the non-parametric ANOVA was followed by a Nemenyi test (n = 4, f = 16).
For all statistical evaluations, the level of significance was set to 5%. Data pretreatment and visualization were conducted in MS Excel, version 2016 (Microsoft Corporation, Redmond, WA, USA), whereas for all statistical calculations, the software package Minitab V22 (Minitab, LLC, State College, PA, USA) was used.
3 Results
3.1 Plant growth
Differences between treatments already became noticeable within the first 2 weeks after potting. Plants grown in the mixture with the highest share of SSD E showed reduced growth and severe necrosis (Supplementary Figure S1). Subsequently, nearly all plants in this treatment died. Furthermore, the plants in all mixtures with 50% or 70% (v/v) SSD developed typical symptoms of Fe deficiency. These were most pronounced when SSD B was used. However, the symptoms disappeared completely after fertilization with Fe-EDDHA. During the following weeks, differences between the control and the SSD treatments became more and more clear, in particular with the perceptibly reduced growth in the treatments with SSD B even at a share of 30% (v/v), but also in the treatments with SSDs C and D at the highest share (Supplementary Figure S1). This was confirmed by the fresh and dry mass of the plants at the end of the experiment (Figure 1). Although the fresh and dry mass were highly correlated (r = 0.96***), the results showed a different systematic pattern in relation to the control: While the plants in all SSD treatments, with the exception of SSD B, reached the same fresh mass as the control at a share of 30% or 50% (v/v), none of the SSDs did so for the plant dry mass. Due to the significant interactions between type and share of SSD, the datasets were split along these factors and evaluated separately. For both plant fresh and dry mass, a reduction was found irrespective of whether SSD 70% (v/v) was used, whereas the differences between 30% and 50% (v/v) were only significant for SSD B. However, the decrease in plant fresh and dry mass was lower for SSD A than for the remaining four SSDs. The poorer growth of the plants in the mixtures with SSD B also became obvious from the comparison of SSDs at the same level of share: At 30% and 50% (v/v), there were no significant differences between SSDs A, C, D, and E, whereas the fresh and dry mass of the plants cultivated in the mixtures with SSD B were significantly reduced in almost all cases. However, at the 70% (v/v) level, the differences between SSDs were more pronounced, with SSD A showing the best growth, SSD E the worst, and with SSDs B, C, and D in between.
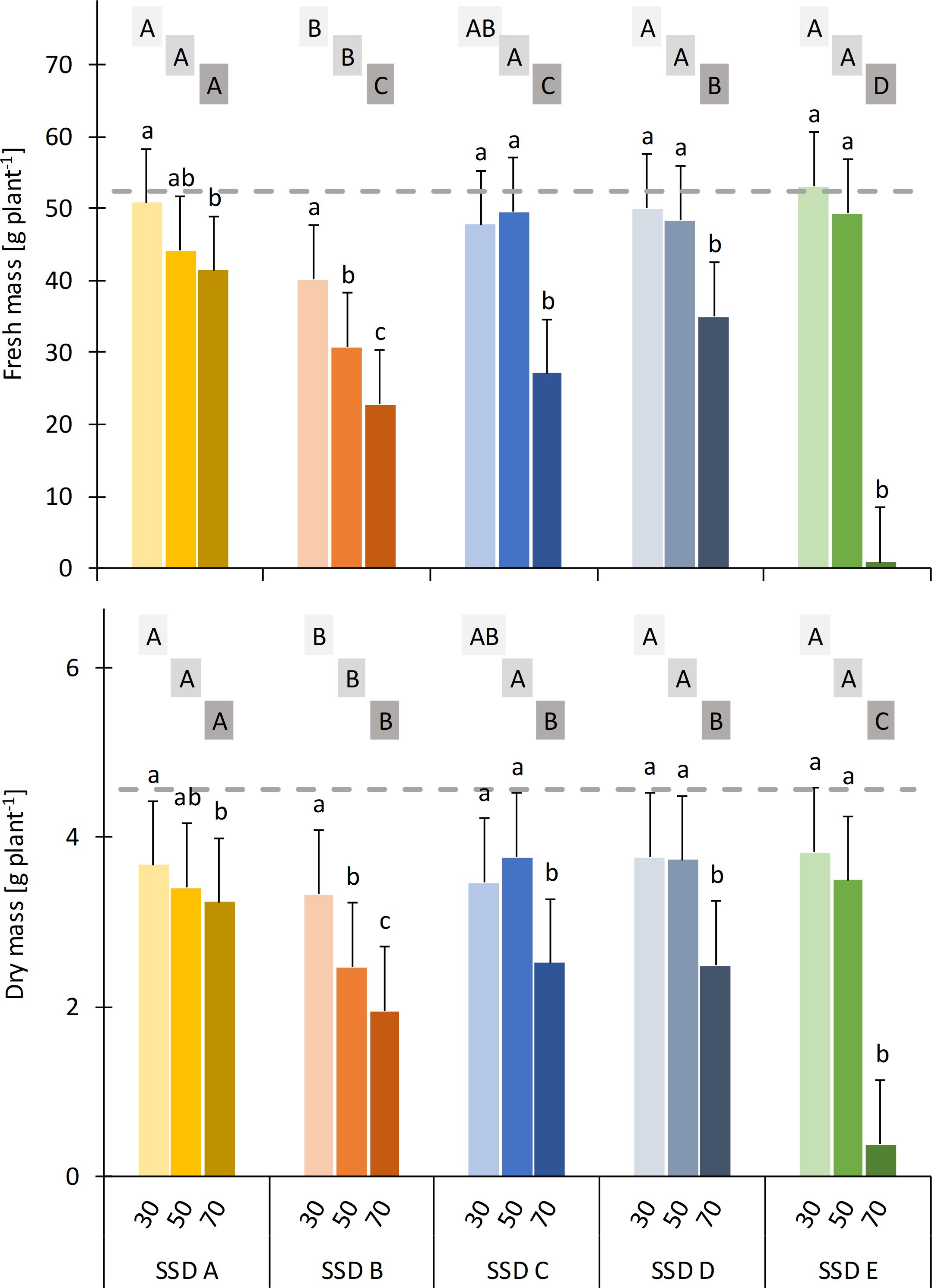
Figure 1. Fresh and dry mass of marigold plants in relation to the type of separated solid digestate (SSD) (for details on SDD A to E, see Table 1) and the share of SSD (30%, 50%, and 70%, v/v) in growing media. Dashed line indicates the fresh and dry mass of the peat control. Error bars indicate the 95% confidence intervals of Dunnett’s test (i.e., treatments do not differ significantly from the control if the error bar intersects with the dashed line, n = 4). Treatments with the same lowercase letters do not differ significantly within the type of SSD, while treatments with the same uppercase letters (the same grayscale field) do not differ significantly within the share of SSD (Tukey’s test, p < 0.05; n = 4).
A similar pattern for aboveground growth was found for root growth (Figure 2), whereas root health was not affected by any SSDs (data not shown). With the exception of SSDs B and C with 30% (v/v) and SSD A with 50% (v/v), the root intensity was significantly reduced by SSD compared with the peat control. Within each SSD, no significant effect of its share on root growth was found for SSD A, whereas for all other SSDs, there was a negative correlation between root growth and the share of SSD. However, the differences were only statistically significant for the highest share. The differences between SSDs become more pronounced with increasing shares, whereby root growth was best in the treatments with SSD A and worst in those with SSD E.
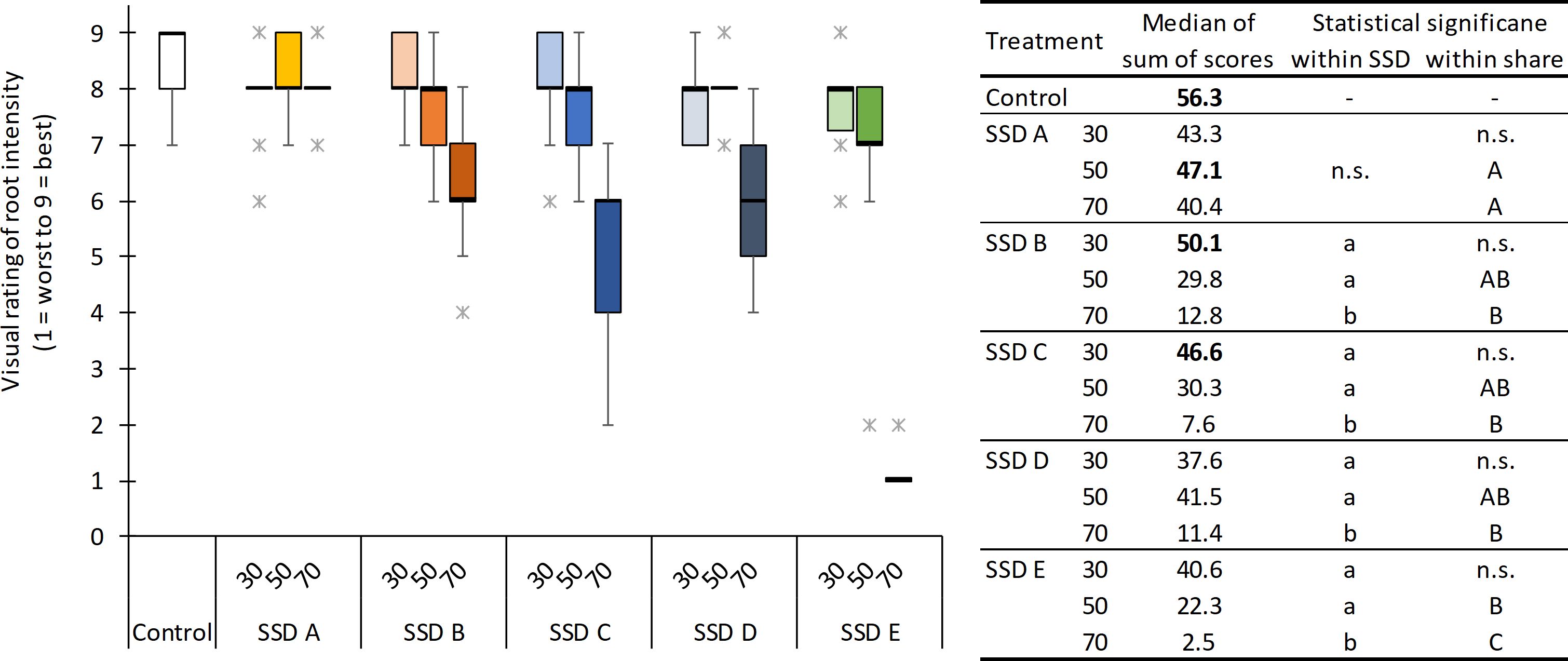
Figure 2. Visual rating of the root intensity (from 1 = worst to 9 = best) of marigold plants in relation to the type of separated solid digestate (SSD) (for details on SSD A to E, see Table 1) and the share of SSD (30%, 50%, and 70%, v/v) in growing media. Horizontal black lines indicate the median, and asterisks indicate outliers. Table on the left: median of the sum of scores marked in bold do not differ significantly from the control. Treatments with the same lowercase letters do not differ significantly within the type of SSD, while treatments with the same uppercase letters do not differ significantly within the share of SSD (Nemenyi test for the sum of scores, p < 0.05, n = 4). n.s., not significant in the Kruskal–Wallis test (p < 0.05).
3.2 Nutrient concentration in plant tissue
The concentrations of main and trace elements in plant tissue in relation to the type and share of SSD are displayed in Figure 3. For treatments with 70% (v/v) SSD E, data are presented, but are not discussed as almost all plants died. The N content in plant tissue was nearly the same irrespective of the type and share of SSD and did not differ from the peat control. The same was true for P, with the exception of the SSD B treatment, where the P concentration was approximately half that of the control. The K concentration in the treatments with SSD was two to three times higher than that in the control and reached a maximum of 144 g kg−1 for SSD C with 70% (v/v). Furthermore, a significant increase in the K concentration was found with increasing shares of SSD, and the K content tended to correlate positively with the percentage of cattle manure in the biogas feedstock (SSD A ≈ SSD B ≤ SSD D < SSD C < SSD E). For Ca and Mg, the opposite of that described for K was found, whereby the effect was more pronounced for Ca than for Mg. On the other hand, for Mg at least, the treatments with the lowest SSD share mostly reached the Mg level of the peat control, with the Ca concentration ranging between 2.8 and 18.8 g kg−1 in all SSD treatments compared with 28.9 g kg−1 in the control. For both Ca and Mg, significant negative correlations with the K content were found, with r = −0.94*** and r = −0.80***, respectively. Similarly to Ca, significantly lower S contents were found in all SSD treatments (1.4–2.0 g kg−1) than in the peat control (3.2 g kg−1), but no clear effect was observed for either the type or the share of SSD.
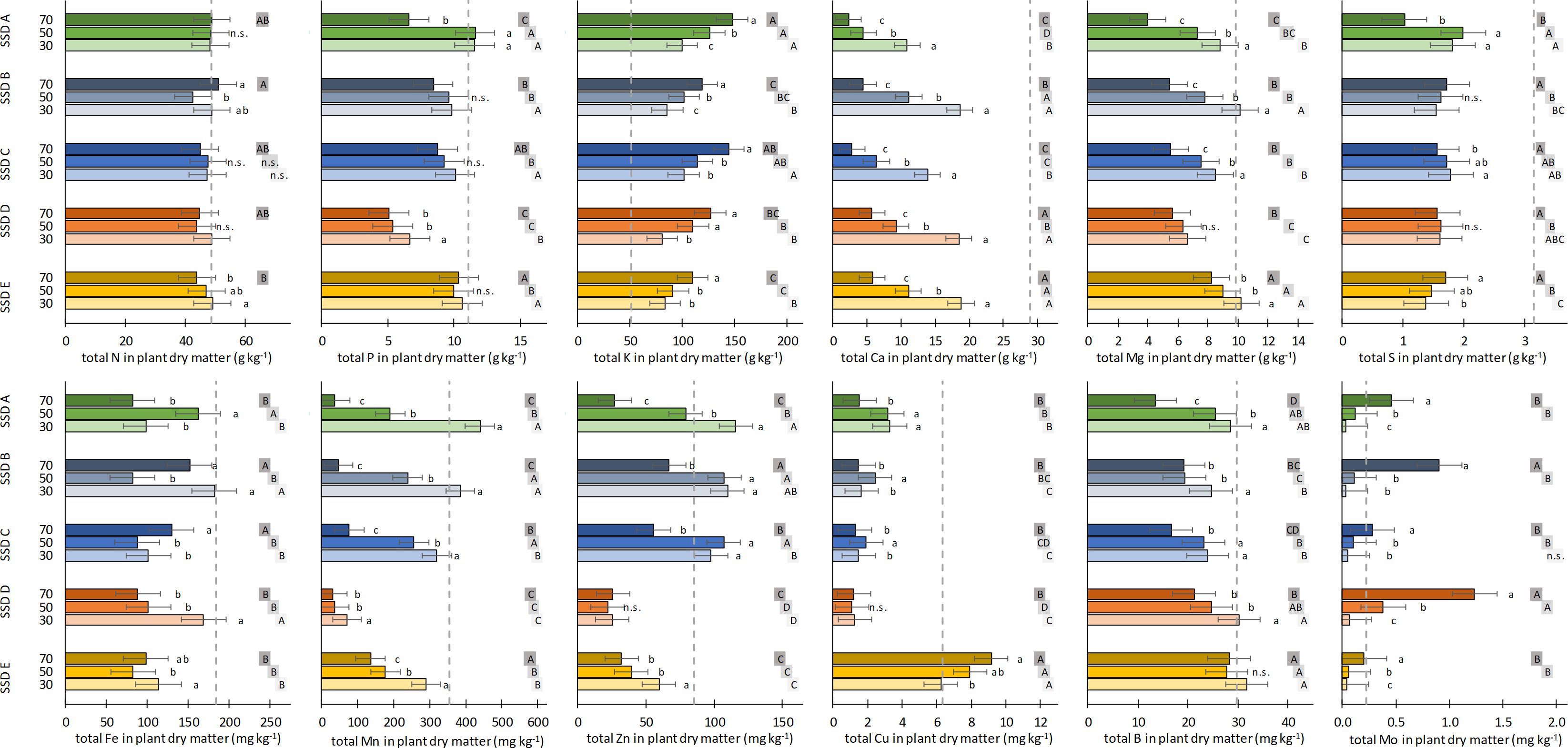
Figure 3. Concentration of the main elements (upper row: N, P, K, Ca, Mg, and S) and the trace elements (lower row: Fe, Mn, Zn, Cu, B, and Mo) in the aboveground biomass of marigold plants in relation to the type of separated solid digestate (SSD) (for details on SSD A to E, see Table 1) and the share of SSD (30%, 50%, and 70%, v/v) in the growing media. Dashed line indicates the respective nutrient concentration in plant tissue of the peat control. Error bars indicate the 95% confidence intervals of Dunnett’s test (i.e., treatments do not differ significantly from the control if the error bar intersects with the dashed line, n = 4). Treatments with the same lowercase letters do not differ significantly within the type of SSD, while treatments with the same uppercase letters (the same grayscale field) do not differ significantly within the share of SSD (Tukey’s test, p < 0.05; n = 4). n.s., not significant in ANOVA (p < 0.05).
The Fe concentration in the SSD treatments was slightly below the control, but no clear effect of the type and share of SSD was recognizable. For Mn and Zn, a similar pattern was found: With the exception of SSD B, a significant decrease of the Mn and Zn concentrations with increasing SSD share was found. In the SSD B treatment, the concentrations of Mn and Zn were the lowest, in particular at shares of 30% and 50% (v/v). For the four remaining SSD treatments, an increase of the Mn and Zn concentrations with increasing percentages of cattle manure in the biogas feedstock was observed at shares of 30% and 50% (v/v) for Mn and irrespective of the share for Zn. For Cu, a distinct difference exists between SSD A and SSDs B to E: Whereas the Cu content in the SSD A treatment was comparable to that of the control and increased with increasing shares of SSD A, the Cu concentrations in the SSDs B to E treatments were less than one-third of the control, and no share effect was found. The B concentrations in the treatments with the lowest SSD share were not significantly different from those in the control for all five SSDs, but tended to decrease when higher shares were used. In contrast to Mn, Zn, and B, a sharp increase of the Mo concentration in plant tissue was found at the highest SSD share, in particular for SSDs B and D. In treatments with 70% (v/v) of these SSDs, the Mo concentration exceeded the control by a factor of 5, but was lower when only 30% (v/v) was used.
3.3 pH, soluble salts, and plant-available nutrients in growing media
Figure 4 shows the time course of pH, water-soluble salts, and plant-available N, P, and K (extractable by CAT). As previously mentioned for nutrient contents in plant tissue, the results of the analyses of the growing media for 70% (v/v) SSD E are presented, but are not discussed as the plants already showed severe damage on the second sampling date (day 12). With the exception of SSD B, which resulted in pH values of 7.0 or higher, the pH values were in an acceptable range between 5.0 and 6.5 up to an SSD share of 50% (v/v). At the highest share of 70% (v/v), the pH values in the treatments with SSDs A, C, and D were between 6.5 and 7.0. During the 45-day cultivation period, the pH values remained stable or tended to increase. The water-soluble salts increased with increasing shares of SSD. At shares of 30% and 50% (v/v), the average values were 2.2(±0.3) and 3.0(±0.4) g KCl L−1, respectively. At a share of 70% (v/v), the differences between SSDs became more noticeable, with the SSD D treatment showing an average water-soluble salt content of 3.0(±0.3) g KCl L−1 compared with 4.2(±0.3) and 4.1(±0.3) g KCl L−1 for the treatments with SSDs B and C, respectively. The time course of the plant-available mineral N (as the sum of NH4– and NO3–N) has to be interpreted with caution as fertilization already started a few days after the second sampling date. Nevertheless, it can be seen that the N concentration dropped during the second half of the experiment, in particular in the control. However, the N supply should be adequate even at such low levels, as plants were fertilized three times a week. The most distinct differences between SSDs were found for CAT-soluble P. However, the share of SSD was of minor importance than the SSD type, e.g., for treatments with SSD B, the CAT-soluble P was, on average, 135(±24), 189(±22), and 178(±25) mg P2O5 L−1 at 30%, 50%, and 70% (v/v), respectively. Moreover, the CAT-soluble P was not significantly different for 50% and 70% (v/v) SSD B at the end of the experiment (Supplementary Table S1). Similar results were found for the four other SSDs. Moreover, it can be noted that the P concentration in the treatments with SSD B, C, and E with shares of 50% and 70% (v/v) remained stable or even increased throughout the experiment, although no P fertilization was performed. In accordance with the CAL-soluble K analyzed in the pure SSDs, the highest values for CAT-soluble K were found in the treatments with SSDs B and E and the lowest in the treatments with SSD D. However, for the lowest share of 30% (v/v) SSD D, the K levels already exceeded 1,000 mg K2O L−1, which was much higher than the target value of the control (250 mg K2O L−1).
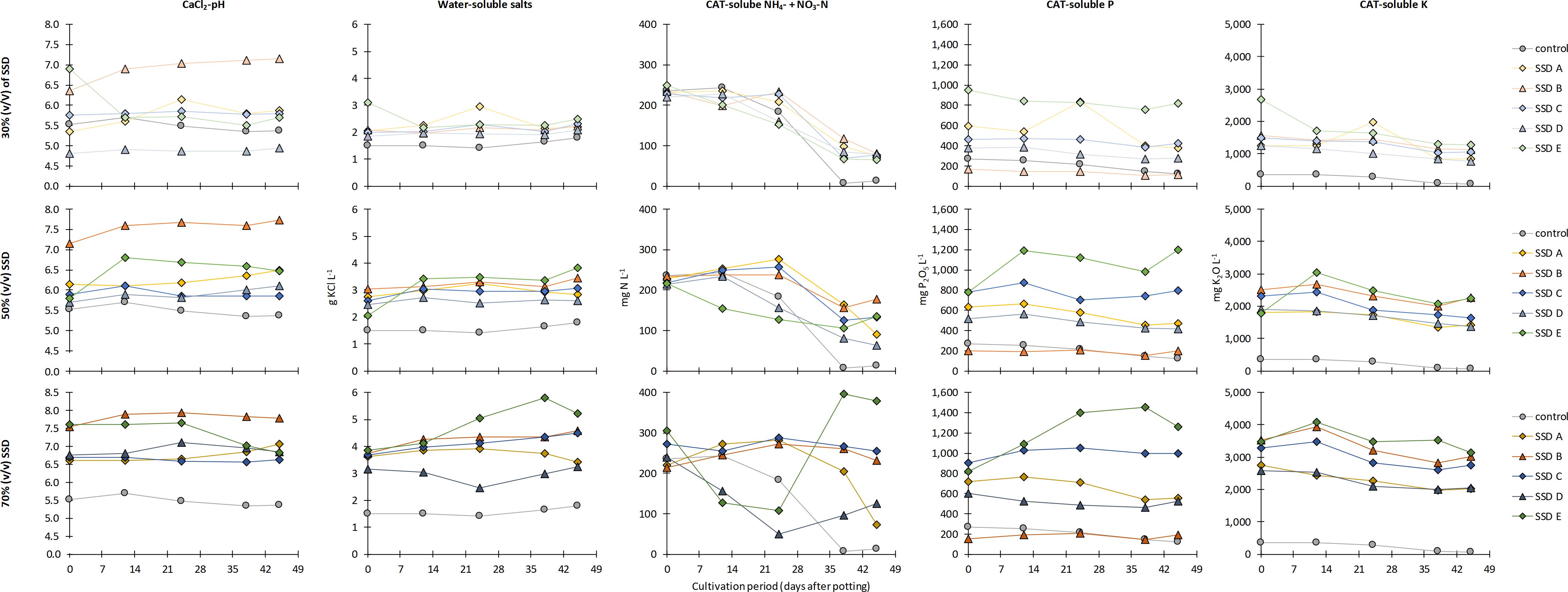
Figure 4. Time course of the pH, the water-soluble salts, and the CAT-soluble N, P, and K in the growing media during a 45-day cultivation period of marigold plants in relation to the type of separated solid digestate (SSD) (for details on SSD A to E, see Table 1) and the share of SSD (30%, 50%, and 70%, v/v) in the growing media compared with the peat control.
Due to the continuous supply of Mg and S with the irrigation water, the values in the growing media were quite high at the end of the experiment (Supplementary Table S1), and the effect of the type and share of SSD was masked. With the exception of SSD B, a systematic increase in water-soluble Ca was found with decreasing shares of SSD. However, this is mainly due to the N fertilization using calcium nitrate in treatments with lower SSD shares because of the lower pH. All treatments with SSD B and treatments with higher shares of the remaining SSDs received N fertilization as ammonium nitrate. For water-soluble Na, a systematic increase with increasing shares of SSD was found, and at the same share, the SSDs without cattle manure showed lower values than those with cattle manure. The highest value of 115 mg Na L−1 at the end of the trial was found for 70% (v/v) SSD D and the lowest for 30% (v/v) SSD A. This corresponds to the Na concentration of the pure SSDs (Table 1). For the CAT-soluble Fe, the effect of SSD share was low and the differences at the end of the experiment not significant in the majority of cases. However, significant differences were found between the SSDs, with the SSD A treatment having the highest (53–58 mg Fe L−1) and SSD B the lowest values (9–17 mg Fe L−1). The values of the other three SSD treatments ranged in between (14–37 mg Fe L−1). An effect of the treatment-specific application of Fe-EDDHA on the CAT-soluble Fe in the growing media was not found. The values for the CAT-soluble Mn were lowest for 30% (v/v) and highest for 70% (v/v) SSD, with the exception of SSD A, where the effect of share was not significant. In relation to the type of SSD, the lowest values were found for SSD treatments B and D (2.7–3.5 and 2.6–4.1 mg Mn L−1, respectively), medium levels for SSD treatments A and C (4.9–5.7 and 4.5–7.8 mg Mn L−1, respectively), and highest for the SSD E treatment (6.4–10.5 mg Mn L−1). Similarly to Mn, an increase of the CAT-soluble Zn and B concentrations in the growing media was found with increasing shares of SSD. As the Zn values tended to increase with increasing proportions of cattle manure in the biogas feedstock, the opposite was true for B. The highest values were found in the treatments with SSD A (up to 1.11 mg B L−1) and SSD B (up to 0.77 mg B L−1), while the maximum values for SSD treatments C, D, and E were 0.39, 0.23, and 0.38 mg B L−1, respectively. For Cu, a tendency toward increasing values in relation to the SSD share was also found. However, the differences between the SSD treatment A with values ranging from 0.33 to 0.50 mg Cu L−1 and the SSD treatments B to E with values between 0.09 and 0.14 mg Cu L−1 were much more pronounced. As previously mentioned for Na, the analysis data of the growing media at the end of the experiment corresponded to the values analyzed in pure SSDs (Table 1) for Zn, B, and Cu, but not for Mn. In all treatments, the levels of CAT-soluble Mo were below the limit of detection (<0.01 mg L−1). The data and the statistical analysis for pH, water-soluble salts, and water-soluble and CAT-soluble nutrients are listed in Supplementary Table S1, whereby statistical analysis was performed only between the SSD treatments, but not in comparison to the control.
4 Discussion
Firstly, it can be stated that the quality guidelines for composted green waste, which have been successfully proven in practice for many years, are not completely valid for SSDs. Although SSD B completely complies with the thresholds for use up to 20% (v/v) (Table 1), an unexpected sharp decline in fresh mass of approximately 20% compared with the peat control was found even at the lowest share of 30% (v/v). One possible reason might be the high pH value of the mixtures with SSD B compared with others (Figure 4), which manifested as symptoms of Fe deficiency within the first 2 weeks of cultivation (Supplementary Figure S1). Although these symptoms disappeared after fertilization with Fe chelate, a latent Fe deficiency might still have been present, leading to a slight growth depression (Venkatraju and Marschner, 1981; Gruber and Kosegarten, 2002). A high pH as a limiting factor for the use of SSDs was also suspected by Dubský et al. (2019). Furthermore, soluble salts and, thus, osmotic stress, which increase with increasing shares of SSD, might have contributed to the growth reduction of both aerial parts (Figure 1) and roots (Figure 2). However, this assumption is not clearly supported by comparisons between SSDs: Although the mixture with SSD D had, by far, the lowest content of soluble salts (approximately 3 g KCl L−1 during the entire experiment) within the treatments with 70% (v/v) SSD, the fresh mass, the dry mass, and the root score index (35.0 g/plant, 2.5 g/plant, and 11.4, respectively) were significantly reduced compared with the mixture with SSD A (41.4 g/plant, 3.3 g/plant, and 40.4, respectively), which had up to 1 g KCl L−1 higher soluble salts, particularly in the first half of the experiment when plants are assumed to be most sensitive to osmotic stress (Läuchli and Grattan, 2007). Moreover, the majority of the plants in the mixture with 70% (v/v) SSD E already died within the first 14 days, but the soluble salts were in the same range (3.5–4.0 g KCl L−1) at the beginning of the experiment as in the other four SSD mixtures. This indicates that soluble salts contribute to the maximum possible share of SSD; however, other factors, in particular the concentration of the individual elements, have to be considered as well.
In contrast to the results of Schmitz and Meinken (2009) and Dubský et al. (2019), no clear negative effect of the percentage of cattle manure, i.e., livestock excrements, was found. This emphasizes the high variability in SSD, which is dependent on the feedstock for biogas production. SSD E, which worked well despite 30% cattle manure up to a share of 50% (v/v), made clear that not the animal-derived feedstock itself but the chemical properties of the SSD must be taken into account. Whereas SSD E contained soluble salts equivalent to 4.45 g KCl L−1, the SSDs with less animal feedstock used by Schmitz and Meinken (2009) had a higher average content of 6.45(±1.52) g KCl L−1. This was probably mainly due to the several times higher amounts of mineral N and CAL-soluble P (Schmitz et al., 2009). The values reported by others (e.g., Asp et al., 2022) cannot be used directly for comparison as different analysis methods were used. Realizing that even SSDs with a higher percentage of livestock excrements in the feedstock can be used is important with regard to the potentially available quantities of SSD, as only a small percentage of biogas plants in Germany (≈7%), the country with by far the highest number of biogas plants in Europe (Pavičić et al., 2022), uses completely plant-based feedstocks (Rensberg et al., 2024).
Matching the fresh and dry mass with the nutrient contents in plant tissue does not indicate a single factor explaining the increasing growth reduction with increasing SSD shares. For nitrogen, no considerable differences were found, neither when compared with the control nor within the type and share of SSD. All values ranged between 43 and 49 g kg−1 (Figure 3). Indeed, a complete N balance could not be calculated as the pots were not filled with a defined amount of growing medium. However, it can be assumed that the N dynamics of SSDs are rather low as the N uptake by plants, the N stock in the growing media, and the N applied by fertilization were similar for all treatments. This is confirmed by the results of incubation experiments (Schmitz et al., 2009) and might be due to the microbial degradation of the easily available carbon sources during biogas production and the subsequent composting (van Midden et al., 2023).
In addition, there are other aspects that might have had an influence on the results and are worth discussing in terms of recommendations for the use of SSD as a growing media constituent: Phosphorus was found to be a limiting factor for the use of SSD in growing media by Schmitz and Meinken (2009). They reported severe damage on Scaevola aemula at CAT-soluble P levels of 300–350 mg L−1, which are lower than the values for the mixtures with 30% (v/v) of SSDs A, C, D, and E (Figure 4 and Supplementary Table S1). In contrast, in the current research, the P content in plant tissue did not exceed the 11 g kg−1 found in the control (Figure 3), even at the highest share of any SSD and even when the CAT-soluble P in the growing media containing SSD exceeded the values in the control by far (Figure 4). The plant damage observed by Meinken and Schmitz (2009) at relatively low P levels in the growing medium might be partly explainable by the high P sensitivity of S. aemula (Schmitz and Meinken, 2009). A second reason might be the mineralization of organic P during plant cultivation, and thus an underestimation of the current P supply by common extraction solutions. This assumption is supported by Grigatti et al. (2015), who reported a higher proportion of organic P in SSDs than in aerobic compost, particularly in those with animal-based feedstock. This is in line with the increase of CAT-soluble P in the growing media with shares of 50% and 70% (v/v) SSD E (Figure 4), which is based on a biogas feedstock containing 30% cattle manure. Due to the higher share (up to 70%) of animal-based feedstock in the SSDs of Schmitz and Meinken (2009), a higher P mineralization and, thus, a stronger underestimation of the P supply can be assumed. Two aspects can be derived from this, which should be addressed in future research: 1) the addition of P-fixing compounds such as Al-associated phosphorus buffers (Tanaka et al., 2006) or clay (Binner, 2014) to reduce plant-available P and thus to avoid plant damage (Caspersen et al., 2023), and 2) the elaboration of reliable methods and limit values for rating P in the quality assurance of SSDs. It is well known that organic P is not extracted by acid-buffered CAL solution (Steffens et al., 2010), which is currently used for the analysis of plant-available P in aerobic compost.
The growth reduction can also be partly attributed to the high potassium content of SSDs (Table 1) as this contributes to an increase of the water-soluble salts in growing media and, thus, to osmotic stress. Moreover, excessive K uptake by plants—the K concentration in plant tissue was two to three times higher than that in the control—probably decreased the Ca and Mg uptake due to ion antagonism (Jakobsen, 1993). In particular, the Ca levels (2.3–18.8 g kg−1) were far below the values of the control (28.9 g kg−1), and that in the 70% (v/v) SSD E treatment also below the damage threshold (3.0 g kg−1) reported by Pitchay (2002). As the symptoms on the plants (Supplementary Figure S1) also fit the author’s description of Ca deficiency (i.e., necrotic spots, wilting of oldest leaves, and dieback of shoot tips), the plants in the 70% (v/v) SSD E treatment most likely died due to Ca deficiency caused by an excessive K supply. As previously mentioned for P, this questions the applicability of the compost guidelines for the assessment of SSDs. In the case of K, this might be due to the lower cation exchange capacity (CEC) of the digestate compared with composts resulting in more dissolved K in the growing medium (Teglia et al., 2011; Binner, 2014). To reduce the risk of K surplus, washing with water or buffering with Ca solution, as is common practice for coir (Carlile et al., 2019), might also be a suitable treatment for SSDs. This can already be done in the biogas plant, followed by a second separation step as the washing solution could be used as a liquid fertilizer or put back into the fermenter, depending on the nutrient concentration (FNR, 2016). Furthermore, maximum usable shares of SSD might be defined in relation to the soluble K levels, as is done for coir and green waste compost by RAL quality assurance in Germany (GGS, 2025; BGK, 2025).
The sulfur concentration in plant tissue in the SSD treatments was only approximately two-thirds that in the peat control, but was in the same range (2–3 mg kg−1) as that reported by Pitchay (2002) for an adequately supplied control and far above the deficiency threshold (<1 mg kg−1). The difference between the SSD treatments and the control was probably due to the additional fertilization of the control with Ferty Basis 1 (0-14-38), which contains potassium sulfate. This highlights the need for a sophisticated adjustment of nutrient management when SSDs are used in growing media. It might be particularly important when SSDs are washed to reduce K, as the already low amounts of sulfate in the SSDs (Table 1) are further lowered. As K might still be sufficient after washing, additional K fertilization, which is typically given as potassium sulfate, is not necessary, and thus no additional S would be supplied either.
As outlined for sulfur, the micronutrient supply must also be adjusted in the growing media with SSD compared to those with green waste compost. At shares of 25%–50% (v/v) green waste compost, an additional base dressing with micronutrients other than Fe is generally not necessary, and higher shares might even result in toxicity (Grigatti et al., 2007). In contrast, the micronutrient supply in the growing media with SSD appears to be insufficient without additional microelement fertilization. This might be due to the high pH values, which is supported by a decrease of the concentrations of Mn, Zn, and B and an increase of the concentration of Mo in plant tissue with increasing shares of SSD (George et al., 2012) and leaching of trace elements to the liquid phase (Romio et al., 2024). However, the comparison of the SSDs at the same level of share indicated that the chemical properties of SSDs other than pH also have to be considered. This becomes most evident for Cu: The Cu concentration of the plants cultivated in the growing media with SSD A was up to five times higher than that of the plants cultivated in the growing media with the remaining four SSDs. This is probably due to the high amounts of Cu in the chopped hop bines used as feedstock in the biogas plant. The accumulation of Cu in hop bines results from the high Cu loads in the topsoils of hop gardens due to excessive spraying of Cu-based fungicides in hop cultivation (Kühne et al., 2017). It has already been indicated by an up to 10 times higher CAT-soluble Cu concentration in this SSD (Table 1). This strengthens the above-mentioned heterogeneity issue of SSD depending on the biogas feedstock, which is a major challenge for growing media manufacturers who rely on the consistent quality of their raw materials (Schmilewski, 2008).
The current results not only revealed the potential of SSDs as a growing media constituent but also highlighted the challenges regarding the chemical properties and the resulting adjustments of fertilization. Furthermore, quality issues, in particular the inconsistent chemical properties and the lack of suitable thresholds, were stressed. However, there are several other potential risks and limitations that need to be addressed in further research. Some of them are more relevant, i.e., regarding plant growth, while others are more important for user safety, and some affect both. Regarding plant growth, the stability of the organic matter and the physical properties have to be investigated in detail. In the case of organic matter stability, special focus should be placed on the pretreatment of SSDs and the elaboration of suitable thresholds for maturity assessment. Moreover, the microbiome of SSDs and its effect on plant growth need to be considered. As outlined in recent research on green waste compost (Lutz et al., 2020; Pot et al., 2021; Pot et al., 2022), the microbiome is probably one of the most important and at the same time the least understood factor of compost quality. Therefore, not only the positive effects of the microbiome on suppressing plant diseases but also the contamination with both human and plant pathogens must be considered. As shown in a meta-analysis by Álvarez-Fraga et al. (2025), the reduction performance of anaerobic digestion widely differs depending on the microbial species and strongly relies on the process conditions. In addition to pathogens, organic and inorganic pollutions are also a potential safety risk (Nkoa, 2014; Czatzkowska et al., 2025). The two main groups of organic pollutants are antibiotics and other active pharmaceutical ingredients from animal husbandry (Lehmann and Bloem, 2021; Nesse et al., 2022), as well as pesticide residues such as pyridine carboxylic acids (Tremblay et al., 2014). Moreover, contamination with heavy metals, e.g., Cu and Zn, has been reported (Alburquerque et al., 2012). Therefore, not only the total loads but also the bioavailability—which may be increased during digestion—have to be considered (Zheng et al., 2022).
5 Conclusion
Even when a higher percentage of livestock excrement is used as a feedstock in the biogas plant, SSDs can be suitable as a growing media constituent with a substantial share. This share might be increased by pretreating the SSDs similarly to coir and by using additives such as clay to avoid the negative effects of high potassium and phosphorus loads. The most challenging issue for both growing media manufacturers and growers is likely to be the heterogeneity of SSDs, which requires a sophisticated adjustment of the base dressing and the subsequent nutrient management. However, a big advantage of SSDs appears to be the lack of N dynamics. Thus, the targeted digestion of carefully selected renewable raw materials in biogas plants might be a possible approach for the production of high-quality growing media constituents, whereby the costs for raw material and processing might be covered by the gas yield. However, even when the feedstock is strictly controlled and nutrient management is optimized, there are still a lot of challenges and safety risks that need to be addressed before SSD can be used as a growing media constituent without restrictions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MM: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. EM: Writing – review & editing. DL: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The research was done within the Junior Research Group: Regionally accumulating residual materials and renewable raw materials as peat substitutes: preparation – use – assessment; funded by the Federal Ministry of Agriculture, Food and Regional Identity by resolution of the German Bundestag (grant no. 2222MT014A).
Acknowledgments
The authors would like to thank Alexander Maidl, Naomi Malisi and Sonja Wolke for the careful execution of the experimental work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fhort.2025.1665721/full#supplementary-material
References
Alburquerque J. A., de la Fuente C., Ferrer-Costa A., Carrasco L., Cegarra J., Abad M., et al. (2012). Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 40, 181–189. doi: 10.1016/j.biombioe.2012.02.018
Álvarez-Fraga L., Capson-Tojo G., Sanglier M., Hamelin J., Escudié R., Wéry N., et al. (2025). A meta-analysis of pathogen reduction data in anaerobic digestion. Renewable Sustain. Energy Rev. 207, 114982. doi: 10.1016/j.rser.2024.114982
Asp H., Bergstrand K. J., Caspersen S., and Hultberg M. (2022). Anaerobic digestate as peat substitute and fertiliser in pot production of basil. Biolog Agric. Hortic. 38, 247–257. doi: 10.1080/01448765.2022.2064232
Beuth E., Schreiner M., Meinken E., and Lohr D. (2023). Time course of N immobilization of wood fibers made from different raw materials. DGG-Proceedings 11, 1–8. doi: 10.5288/dgg-pr-11-11-eb-2023
BGK (2025). Qualitätsanforderungen Substratkompost (Bundesgütegemeinschaft Kompost). Available online at: https://www.kompost.de/fileadmin/user_upload/Dateien/Guetesicherung/Dokumente_Kompost/Dok._251-006-3_Qualitaetskrit._SK.pdf. Dok. 251-006-3 (Accessed October 14, 2025).
Binner I. (2014). Clay characteristics affecting the P, K and Mn dynamics in peat-clay substrates. Leibniz University Hannover, Germany.
Blok C., Beerling E., Barbagli T., and Eveleens B. (2024). Renewable Raw Materials for Growing Media: Basic data for the environmental impact of potting soil and substrates agreement (Wageningen, Netherlands: Wageningen University and Research). Report WPR-1354. doi: 10.18174/673902
Blok C., Eveleens B., and van Winkel A. (2021). Growing media for food and quality of life in the period 2020-2050. Acta Hortic. 1305, 341–356. doi: 10.17660/ActaHortic.2021.1305.46
Carlile W. R., Raviv M., and Prasad M. (2019). “Chapter 8: organic soilless media components,” in Soilless culture: Theory and practice, 2nd Ed. Eds. Raviv M., Lieth J. H., and Bar-Tal A. (Elsevier, Amsterdam, The Netherlands), 303–378.
Caspersen S., Oskarsson C., and Asp H. (2023). Nutrient challenges with solid-phase anaerobic digestate as a peat substitute-Storage decreased ammonium toxicity but increased phosphorus availability. Waste Manag 165, 128–139. doi: 10.1016/j.wasman.2023.04.032
Crippa L., Zaccheo P., and Orfeo D. (2013). Utilization of the solid fraction of digestate from anaerobic digestion as container media substrate. Acta Hortic. 1013, 367–373. doi: 10.17660/ActaHortic.2013.1013.45
Czatzkowska M., Rolbiecki D., Korzeniewska E., and Harnisz M. (2025). Heavy metal and antimicrobial residue levels in various types of digestate from biogas plants—a review. Sustainability 17, 416. doi: 10.3390/su17020416
Dubský M., Chaloupková Š., Kaplan L., Vondráčková S., and Tlustoš P. (2019). Use of solid phase of digestate for production of growing horticultural substrates. Hortic. Sci. 46, 34–42. doi: 10.17221/221/2016-HORTSCI
FNR (2016). Leitfaden Biogas - Von der Gewinnung zur Nutzung. 7th edi (Gülzow, Germany: Fachagentur Nachwachsende Rohstoffe e.V).
Fuchs Z., Vacek Z., Vacek S., Cukor J., Šimůnek V., Štefančík I., et al. (2024). European beech (Fagus sylvatica L.): A promising candidate for future forest ecosystems in Central Europe amid climate change. Cent. Europ Fores J. 70, 62–76. doi: 10.2478/forj-2023-0020
George E., Horst W. J., and Neumann E. (2012). “Chapter 17: adaptation of plants to adverse chemical soil conditions,” in Marschner’s Mineral Nutrition of Higher Plants, 3td Ed. Ed. Marschner P. (Elsevier, Amsterdam, The Netherlands), 409–472.
GGS (2025). Quality Parameters for Coir Products as Growing Media Constituent (RAL-GZ 250/5-4) (Hannover, Germany: RAL-Gütegemeinschaft Substrate für Pflanzen e.V.).
Grigatti M., Boanini E., Cavani L., Ciavatta C., and Marzadori C. (2015). Phosphorus in digestate-based compost: chemical speciation and plant-availability. Waste Biomass Valor 6, 481–493. doi: 10.1007/s12649-015-9383-2
Grigatti M., Giorgioni M. E., and Ciavatta C. (2007). Compost-based growing media: Influence on growth and nutrient use of bedding plants. Biores Technol. 98, 3526–3534. doi: 10.1016/j.biortech.2006.11.016
Gruber B. and Kosegarten H. (2002). Depressed growth of non-chlorotic vine grown in calcareous soil is an iron deficiency symptom prior to leaf chlorosis. J. Plant Nutr. Soil Sci. 165, 111–117. doi: 10.1002/1522-2624(200202)165:1<111::AID-JPLN111>3.0.CO;2-B
Gruda N. S. (2019). Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 9, 298. doi: 10.3390/agronomy9060298
Gruda N. S., Hirschler O., and Stuart J. (2024). Peat reduction in horticulture – an overview of Europe. Acta Hortic. 1391, 545–560. doi: 10.17660/ActaHortic.2024.1391.75
Guilayn F., Rouez M., Crest M., Patureau D., and Jimenez J. (2020). Valorization of digestates from urban or centralized biogas plants: a critical review. Rev. Environ. Sci. Biotechnol. 19, 419–462. doi: 10.1007/s11157-020-09531-3
Horneck D. A. and Miller R. O. (1998). “Chapter 9: Determination of total nitrogen in plant tissue,” in Handbook of reference methods for plant analysis. Ed. Kalra Y. P. (CRC Press, Boca Raton (FL), USA), 75–83.
IVG/GGS (2025). Produktionsstatistik Consumer-Erden und Kultursubstrate - Produktionsjahr 2024 (Industrieverband Garten e. V. (IVG) and Gütegemeinschaft Substrate für Pflanzen e. V. (GGS). Available online at: https://erden-substrate.info/wp-content/uploads/2025/04/IVG-Produktionsstatistiken_2024.pdf (Accessed October 14, 2025).
Jakobsen S. T. (1993). Interaction between plant nutrients: III. Antagonism between potassium, magnesium and calcium. Acta Agri Scandi Sect B - Soil Plant Sci. 43, 1–5. doi: 10.1080/09064719309410223
Jankauskienė J., Laužikė K., and Kaupaitė S. (2024). The use of anaerobic digestate for greenhouse horticulture. Agronomy 14, 2437. doi: 10.3390/agronomy14102437
Kovačić Đ., Lončarić Z., Jović J., Samac D., Popović B., and Tišma M. (2022). Digestate management and processing practices: a review. Appl. Sci. 12, 9216. doi: 10.3390/app12189216
Kozak M. and Piepho H. P. (2018). What’s normal anyway? Residual plots are more telling than significance tests when checking ANOVA assumptions. J. Agron. Crop Sci. 204, 86–98. doi: 10.1111/jac.12220
Kühne S., Roßberg D., Röhrig P., Von Mehring F., Weihrauch F., Kanthak S., et al. (2017). The use of copper pesticides in Germany and the search for minimization and replacement strategies. Org Farm 3, 66–75. doi: 10.12924/of2017.03010066
Läuchli A. and Grattan S. (2007). “Plant growth and development under salinity stress,” in Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops. Eds. Jenks M. A., Hasegawa P. M., and Jain S. M. (Springer, Dordrecht, The Netherlands). doi: 10.1007/978-1-4020-5578-2_1
Lehmann L. and Bloem E. (2021). Antibiotic residues in substrates and output materials from biogas plants–Implications for agriculture. Chemosphere 278, 130425. doi: 10.1016/j.chemosphere.2021.130425
Lutz S., Thuerig B., Oberhaensli T., Mayerhofer J., Fuchs J. G., Widmer F., et al. (2020). Harnessing the microbiomes of suppressive composts for plant protection: from metagenomes to beneficial microorganisms and reliable diagnostics. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.01810
Miller R. O. (1998). “Chapter 8:Microwave digestion of plant tissue in a closed vessel,” in Handbook of reference methods for plant analysis. Ed. Kalra Y. P. (CRC Press, Boca Raton (FL), USA), 69–74.
Nesse A. S., Aanrud S. G., Lyche J. L., Sogn T., and Kallenborn R. (2022). Confirming the presence of selected antibiotics and steroids in Norwegian biogas digestate. Environ. Sci. pollut. Res. 29, 86595–86605. doi: 10.1007/s11356-022-21479-1
Nkoa R. (2014). Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agron. Sustain. Dev. 34, 473–492. doi: 10.1007/s13593013-0196-z
Pavičić J., Novak Mavar K., Brkić V., and Simon K. (2022). Biogas and biomethane production and usage: technology development, advantages and challenges in Europe. Energies 15, 2940. doi: 10.3390/en15082940
Piotrowski S., Essel R., Carus M., Dammer L., and Engel L. (2015). Nachhaltig nutzbare Potenziale für Biokraftstoffe in Nutzungskonkurrenz zur Lebens- und Futtermittelproduktion, Bioenergie sowie zur stofflichen Nutzung in Deutschland, Europa und der Welt. Final report (Grant No 22501112 and 12BMU011).
Pitchay D. S. (2002). Impact of 11 elemental nutrient deficiencies on shoot and root growth, and foliar analysis standards of 13 ornamental taxa with emphasis on Ca and B control of root apical meristem development. North Carolina State University, Raleigh (NC), USA.
Pot S., De Tender C., Ommeslag S., Delcour I., Ceusters J., Gorrens E., et al. (2021). Understanding the shift in the microbiome of composts that are optimized for a better fit-for-purpose in growing media. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.643679
Pot S., Tender C. D., Ommeslag S., Delcour I., Ceusters J., Vandecasteele B., et al. (2022). Elucidating the microbiome of the sustainable peat replacers composts and nature management residues. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.983855
Rensberg N., Denysenko V., and Daniel-Gromke J. (2024). Biogaserzeugung und -nutzung in Deutschland: Report zum Anlagenbestand Biogas und Biomethan. DBFZ-Report 50. doi: 10.48480/zptb-yy32
Romio C., Ward A. J., and Møller H. B. (2024). Characterization and valorization of biogas digestate and derived organic fertilizer products from separation processes. Front. Sustain Food Syst. 8. doi: 10.3389/fsufs.2024.1415508
Schmilewski G. (2008). The role of peat in assuring the quality of growing media. Mires Peat 3, 2–8.
Schmitz H.-J. and Meinken E. (2009). Composts from residues of anaerobically treated renewable resources and their suitability in growing media. Acta Hortic. 819, 361–366. doi: 10.17660/ActaHortic.2009.819.43
Schmitz H.-J., Sprau G., Schumacher H.-J., and Meinken E. (2009). Umweltverträgliche Restnährstoffverwertung aus Biogasanlagen als Torfersatzstoffe im Gartenbau. Final report (DBU Grant No AZ 16002).
Steffens D., Leppin T., Luschin-Ebengreuth N., Min Yang Z., and Schubert S. (2010). Organic soil phosphorus considerably contributes to plant nutrition but is neglected by routine soil-testing methods. J. Plant Nutr. Soil Sci. 173, 765–771. doi: 10.1002/jpln.201000079
Stichnothe H. (2022). Life cycle assessment of peat for growing media and evaluation of the suitability of using the Product Environmental Footprint methodology for peat. Int. J. Life Cycle Asses 27, 1270–1282. doi: 10.1007/s11367-022-02106-0
Tanaka M., Snyder R., Boateng J. K., Lamont W. J., Orzolek M. D., Brown K. M., et al. (2006). Utility of alumina-buffered phosphorus fertilizer for vegetable production. HortScience 41, 775–779. doi: 10.21273/HORTSCI.41.3.775
Teglia C., Tremier A., and Martel J. L. (2011). Characterization of solid digestates: Part 2, assessment of the quality and suitability for composting of six digested products. Waste Biomass Valor 2, 113–126. doi: 10.1007/s12649-010-9059-x
Torres-Climent A., Martin-Mata J., Marhuenda-Egea F., Moral R., Barber X., Perez-Murcia M. D., et al. (2015). Composting of the solid phase of digestate from biogas production: optimization of the moisture, C/N ratio, and pH conditions. Comm Soil Sci. Plant Anal. 46, 197–207. doi: 10.1080/00103624.2014.988591
Tremblay L. A., Gielen G., and Northcott G. L. (2014). Organic Materials Guidelines–Organic Contaminants Review Vol. 23 (Hamilton, New Zealand: Centre for Integrated Biowaste Research).
van Midden C., Harris J., Shaw L., Sizmur T., and Pawlett M. (2023). The impact of anaerobic digestate on soil life: A review. Appl. Soil Ecol. 191, 105066. doi: 10.1016/j.apsoil.2023.105066
VDLUFA (2016). VDLUFA method book Vol. I - Analysis of soils. 4th edi (Darmstadt, Germany: VDLUFA-Verlag). with 1st to 7th supplement.
Venkatraju K. and Marschner H. (1981). Inhibition of iron-stress reactions in sunflower by bicarbonate. J. Plant Nutr. Soil Sci. 144, 339–355. doi: 10.1002/jpln.19811440403
Keywords: peat substitute, soilless cultivation, feedstock, residue, manure
Citation: Muser M, Meinken E and Lohr D (2025) Separated solid digestate from biogas production as a growing media constituent for potted ornamentals. Front. Hortic. 4:1665721. doi: 10.3389/fhort.2025.1665721
Received: 14 July 2025; Accepted: 29 September 2025;
Published: 17 October 2025.
Edited by:
Chris Blok, Wageningen University and Research, NetherlandsReviewed by:
Lin Ouyang, Chinese Academy of Agricultural Sciences, ChinaAdrie Veeken, Wageningen University and Research, Netherlands
Copyright © 2025 Muser, Meinken and Lohr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dieter Lohr, ZGlldGVyLmxvaHJAaHN3dC5kZQ==
 Michael Muser
Michael Muser Elke Meinken
Elke Meinken Dieter Lohr
Dieter Lohr