- 1ICER India, National Institute of Allergy and Infectious Diseases (NIAID) - International Center for Excellence in Research, Chennai, India
- 2Department of Immunology, ICMR - National Institute for Research in Tuberculosis, Chennai, India
- 3Diabetology, Prof. M. Viswanathan Diabetes Research Center, Chennai, India
- 4Medicine, Infectious Diseases, Dignity Health, Chandler, AZ, United States
- 5Medicine, University of Massachusetts Medical School, Worcester, MA, United States
- 6Laboratory of Parasitic Diseases (LPD), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, MD, United States
Introduction: The assessment of tuberculosis (TB) treatment outcomes predominantly relies on sputum culture conversion status. To enhance treatment management, it is crucial to identify non-sputum-based biomarkers that can predict unfavorable outcomes. Cytokines are widely studied as diagnostic biomarkers for active TB. However, their potential as indicators for unfavorable treatment outcomes remains uncertain.
Methodology: This study was conducted within a well-characterized cohort comprising newly diagnosed patients with drug-sensitive pulmonary TB, confirmed through sputum smear and culture positivity. Our objective was to elucidate the TB antigen-stimulated cytokine profile at pre-treatment and at 2 months into anti-TB treatment (ATT) in patients with unfavorable treatment outcomes (cases, n = 27) in comparison to recurrence-free, microbiologically cured controls (n = 31). Whole blood was stimulated with TB antigens using the QuantiFERON In-tube gold method, and plasma supernatants were subjected to a panel of 14 cytokine measurements.
Results: In our study, pre-treatment analysis revealed that eight cytokines (IL-2, IFN-γ, TNF-α, IL-6, IL-10, IL-17A, IL-18, and GM-CSF) were significantly elevated at baseline in cases compared to cured controls, both in unstimulated conditions and following TB antigen (CFP10, ESAT6, and TB7.7) stimulation. A similar pattern was observed at the 2-month mark of ATT, with eight cytokines (IL-2, IL-10, IL-13, IFN-γ, IL-6, IL-12p70, IL-17A, and TNF-α) showing significant differences between the groups. Importantly, no variations were detected following mitogen stimulation, underscoring that these distinctive immune responses are primarily driven by TB-specific antigens.
Conclusion: Our findings indicate that individuals with unfavorable TB treatment outcomes display a characteristic cytokine profile distinct from TB-cured patients, even before commencing ATT. Therefore, the levels of specific cytokine pre-treatment and at the 2-month point in the course of treatment may serve as predictive immune markers for identifying individuals at risk of unfavorable TB treatment outcomes, with these responses being predominantly influenced by TB-specific antigens.
Introduction
Tuberculosis (TB) continues to pose a formidable global health challenge, with India contributing to a staggering 26% of the world’s new TB cases (1). The persistence of latent Mycobacterium tuberculosis (M.tb) infections acts as a reservoir to develop into a disease (2), and the heightened susceptibility conferred by comorbid conditions such as diabetes and HIV contributes to this burden. Furthermore, TB recurrence and treatment failure, occurring at rates of 6.8% and 4.3%, respectively, add significantly to the public health crisis (3).
Given the time-consuming and expensive nature of culture-based TB diagnosis, it is imperative to explore non-sputum-based (4, 5) biomarkers. Several studies have addressed this issue, seeking blood-based biomarkers for monitoring TB treatment response (6–8). While the World Health Organization (WHO) has revised guidelines to shorten drug-susceptible TB regimens from 6 to 4 months, this change raises concerns about increased recurrence risks (9, 10). Nonetheless, shorter treatment regimens promise better disease control and streamlined management, ultimately alleviating the functional burden on national and international TB programs (11, 12). To mitigate the risks associated with shorter treatment durations, predictive biomarkers are urgently needed to identify and stratify patients at risk of unfavorable treatment outcomes, including recurrence, treatment failure, and death. Such markers could be applied either before initiating TB treatment or, minimally, by the 2-month mark.
Cytokines, molecules involved in the immune response (13, 14), have shown promise in differentiating TB infection from active disease (15). Furthermore, specific plasma cytokines exhibit remarkable specificity in distinguishing various stages of TB, from latency to drug sensitivity and drug resistance (16, 17). However, the precise role of these cytokines in treatment outcomes remains incompletely understood. The primary objective of this study was to elucidate TB antigen-specific predictive biomarkers for unfavorable or adverse pulmonary TB (PTB) treatment outcomes. By examining these biomarkers before and during anti-TB treatment (ATT), we aim to provide valuable insights that can inform early interventions and personalized TB management strategies, ultimately enhancing the diagnosis and treatment of this pervasive infectious disease.
Materials and methods
Ethics statement
This study received approval from the Ethics Committee of Prof. M. Viswanathan Diabetes Research Center (ECR/51/INST/TN/2013/MVDRC/01) and the National Institute for Research in Tuberculosis (NIRT IEC No. 2014004). Informed, written consent was obtained from all participants.
Study population
The study subjects were drawn from the EDOTS (Effect of Diabetes on Tuberculosis Severity) cohort, conducted between 2014 and 2019. Patients for this study were enrolled from a TB clinic and screened for diabetes mellitus. Inclusion criteria encompassed newly diagnosed patients with PTB with positive sputum smear and culture results. Exclusion criteria comprised individuals with prior TB episodes, those with drug-resistant TB, those undergoing TB treatment for more than a week, those taking immunosuppressive medication, those with HIV-positive status, and pregnant and lactating individuals. The diagnosis of PTB relied on positive sputum cultures grown on solid media (Lowenstein–Jensen media), with supporting chest x-ray findings. TB treatment adhered to the standards set by the Revised National Tuberculosis Control Program and was managed through government clinics in Chennai. Monthly follow-ups were conducted throughout the 6-month treatment course, with subsequent evaluations at 3-month intervals until 1 year post-treatment completion. This nested case–control study defined cases as individuals with unfavorable treatment outcomes, matched with controls who achieved recurrence-free cures by the study’s end. Cure was defined as having negative sputum cultures at both months 5 and 6 of treatment, without recurrent disease during follow-up. Treatment failure referred to positive sputum cultures at either month 5 or 6 and all were culture positive at month 2. Death included all-cause mortality during TB treatment, which includes cardiorespiratory arrest, sudden cardiac pulmonary arrest, and severe illness, and recurrence indicated an initial cure at month 6 but culture-confirmed disease recurrence before the study’s conclusion. The study included 27 cases (comprising 16 recurrences, 6 treatment failures, and 5 deaths) and 31 cured individuals, matched for age, gender, body mass index (BMI), and diabetic status. Throughout our follow-up of the cohort, we did not record any bacterial infection.
QuantiFERON-TB gold in-tube supernatant ELISA
For TB antigen-specific immune response analysis, the third-generation QuantiFERON (QFT) plasma supernatants were employed. The QFT tubes contained three components: NIL (for immune status without stimulation), TB-Ag (with TB antigens TB 7.7, CFP10, and ESAT6), and mitogen (a positive control for the test). To collect the supernatant, 1 mL of patient whole blood was incubated in the corresponding tubes following the manufacturer’s instructions (QuantiFERON In Tube Gold Kit; Qiagen, Valencia, CA).
Enzyme-linked immunosorbent assay
A multiplex enzyme-linked immunosorbent assay (ELISA) for cytokines was conducted to examine antigen-specific cytokines in unfavorable treatment outcomes (cases, n = 27) and favorable treatment outcomes (controls, n = 31). The assay followed the manufacturer’s instructions (R&D Systems Elisa Kit). Stimulated plasma cytokine levels were measured using Luminex Magpix with xMAP technology (Bio-Rad, Hercules, CA). The lowest detection limits for cytokines were as follows: IL-2, 9.54 pg/mL; IFN-γ, 16.6 pg/mL; TNF-α, 2.56 pg/mL; GM-CSF, 4.21 pg/mL; IL-1α, 1.88 pg/mL; IL-17A, 4.18 pg/mL; IL-5, 2.44 pg/mL; IL-10, 1.41 pg/mL; IL-13, 157.49 pg/mL; IL-18, 5.51 pg/mL; IL-1β, 4.92 pg/mL; IL-4, 5.44 pg/mL; IL-6, 1.4 pg/mL; and IL-12, 48.37 pg/mL, which were analyzed between cases and cured controls.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism Version 9. Significance between cases and controls was assessed using the Mann–Whitney test, while pre- and post-treatment comparisons within related groups were determined using the Wilcoxon test. Median and geometric mean (GM) were used to represent central tendencies, and the range (min and max) was employed to depict data distribution. The chi-square test was used to check the significance for the non-numerical data. Receiver operating characteristic (ROC) analysis was performed using R software, and Combi ROC was conducted through an online tool (http://combiroc.eu/) with the most significant variables.
Results
Characteristics of the study population
Table 1 presents the demographic characteristics of the study population. There were no significant differences between the groups concerning age, gender, BMI, diabetes status, cough, smoking, alcohol consumption, or smear and culture grade.
Elevated cytokines in unstimulated plasma of cases before and at month 2 of anti-TB treatment
To estimate the unstimulated cytokine levels in cases and controls, we measured the levels of GM-CSF, IL-2, IL-10, IL-13, IL-18, IFN-γ, IL-1α, IL-4, IL-6, IL-12p70, IL-17A, and TNF-α at pre-treatment (Figure 1A); IL-1α, IL-5, and IL-1β (Supplementary Figure 2A) at month 2 of ATT (Figure 1B); and IL-1α, IL-5, and IL-1β (Supplementary Figure 2B). Unstimulated plasma levels of IL-2 (p = 0.0094, GM of 18.72 pg/mL in cases vs. 14.89 pg/mL in controls), IL-10 (p = 0.0466, GM of 3.74 pg/mL in cases vs. 2.086 pg/mL in controls), IL-18 (p = 0.0310, GM of 463.8 pg/mL in cases vs. 354.2 pg/mL in controls), IFN-γ (p = 0.0014, GM of 40.42 pg/mL in cases vs. 29.32 pg/mL in controls), IL-6 (p = 0.0310, GM of 545.1 pg/mL in cases vs. 314.8 pg/mL in controls), and TNF-α (p = 0.0112, GM of 15.57 pg/mL in cases vs. 9.875 pg/mL in controls) were significantly higher in cases than in controls before treatment. At month 2 of treatment, unstimulated plasma levels of IL-2 (p < 0.0001, GM of 1.055 pg/mL in cases vs. 0.7781 pg/mL in controls), IL-10 (p < 0.0001, GM of 34.47 pg/mL in cases vs. 13.06 pg/mL in controls), IL-13 (p < 0.0001, GM of 3.696 pg/mL in cases vs. 0.060 pg/mL in controls), IL-18 (p = 0.0343, GM of 153.7 pg/mL in cases vs. 65.87 pg/mL in controls), IFN-γ (p = 0.0030, GM of 15.09 pg/mL in cases vs. 10.23 pg/mL in controls), IL-4 (p < 0.0001, GM of 1.076 pg/mL in cases vs. 0.463 pg/mL in controls), IL-12p70 (p = 0.0072, GM of 19.05 pg/mL in cases vs. 16.22 pg/mL in controls), IL-17A (p < 0.0001, GM of 27.95 pg/mL in cases vs. 10.62 pg/mL in controls), and TNF-α (p = 0.0002, GM of 7.387 pg/mL in cases vs. 3.015 pg/mL in controls) were significantly higher in cases than in controls. There were no significant differences in GM-CSF, IL-1α, IL-5, IL-13, IL1-β, IL-4, and IL-12p70 at baseline and GM-CSF, IL1-α, IL-5, IL1-β and IL-6 at month 2 between cases and controls.
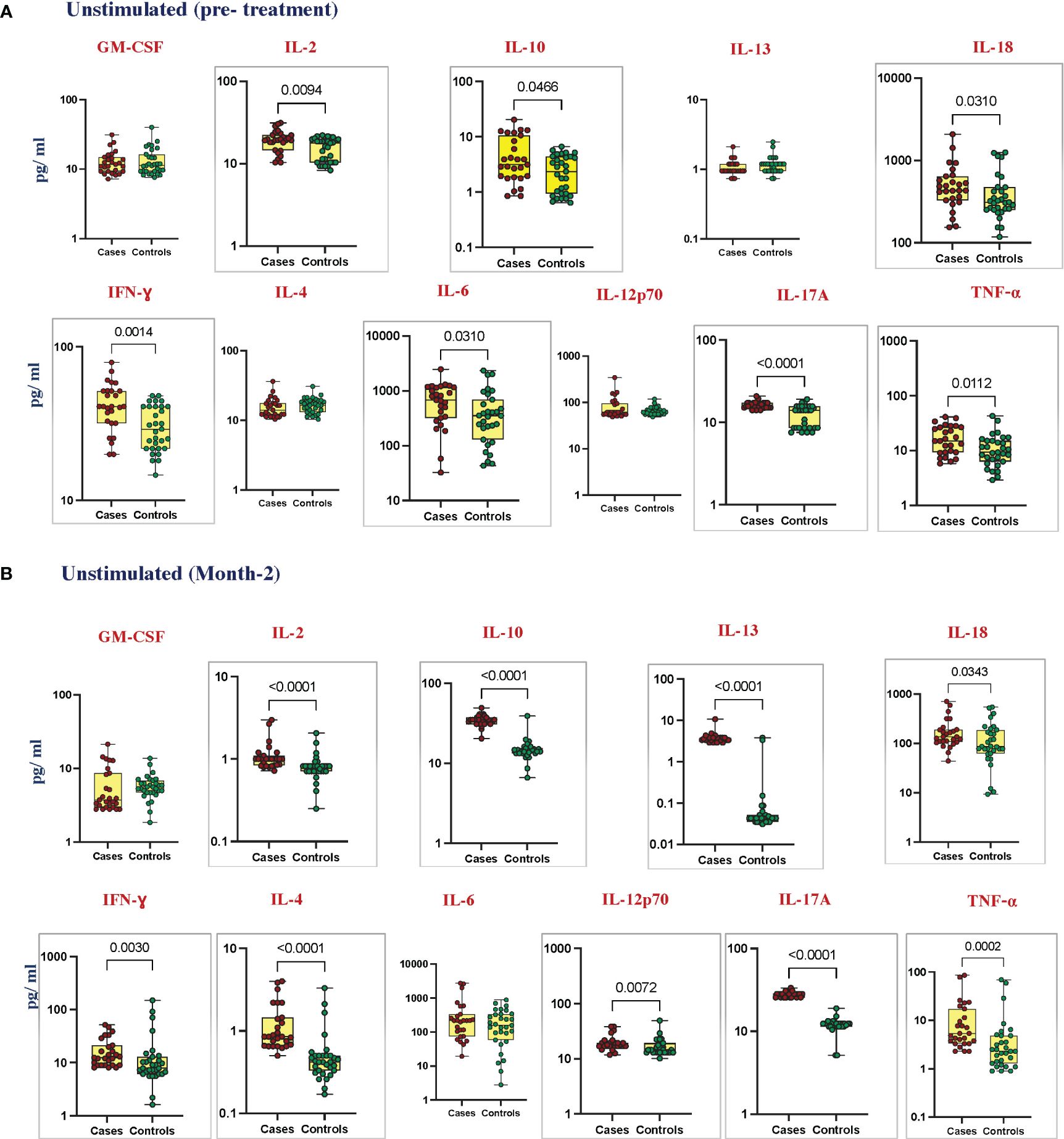
Figure 1 Elevated cytokines in unstimulated plasma can predict unfavorable treatment outcomes before treatment (baseline). Unstimulated plasma levels of cytokines were measured in cases (n = 27) and controls (n = 31) (A) pre-treatment and (B) after 2 months of TB treatment. These data were represented in a box-and-whiskers plot where each dot represents every single participant in its group. The median line was presented at the middle of the box with its error bar at both ends. Mann–Whitney test was used to calculate the p-values of the unrelated groups. Grey boxes refer to the cytokines that were significantly different.
Elevated cytokines in TB antigen-stimulated plasma of cases before and at month 2 of anti-TB treatment
To elucidate the TB antigen-stimulated cytokine levels in cases and controls, we estimated the production of GM-CSF, IL-2, IL-10, IL-13, IL-18, IFN-γ, IL-4, IL-6, IL-12p70, IL-17A, and TNF-α at pre-treatment (Figure 2A); IL-1α, IL-5, and IL-1β (Supplementary Figure 2C) at month 2 of ATT (Figure 2B); and IL-1α, IL-5, and IL-1β (Supplementary Figure 2D). Upon TB antigen stimulation, the plasma levels of GM-CSF (p = 0.0040, GM of 29.75 pg/mL in cases vs. 16.92 pg/mL in controls), IL-2 (p = 0.0067, GM of 112.2 pg/mL in cases vs. 48.15 pg/mL in controls), IL-10 (p < 0.0001, GM of 8.209 pg/mL in cases vs. 2.202 pg/mL in controls), IL-18 (p = 0.0274, GM of 565.3 pg/mL in cases vs. 486.8 pg/mL in controls), IFN-γ (p = 0.0098, GM of 266.0 pg/mL in cases vs. 135.2 pg/mL in controls), IL-6 (p = 0.0405, GM of 1322 pg/mL in cases vs. 694.7 pg/mL in controls), IL-17A (p < 0.0001, GM of 23.48 pg/mL in cases vs. 14.06 pg/mL in controls), and TNF-α (p = 0.0005, GM of 62.94 in cases vs. 27.06 pg/mL in controls) were significantly higher in cases than in cured controls at pre-treatment. At month 2, TB antigen-stimulated plasma levels of IL-2 (p = 0.0001, GM of 44.67 pg/mL in cases vs. 11.65 pg/mL in controls), IL-10 (p, 0.0001, GM of 36.32 pg/mL in cases vs. 14.27 pg/mL in controls), IL-13 (p < 0.0001, GM of 4.243 pg/mL in cases vs. 0.047 pg/mL in controls), IFN-γ (p = 0.0386, GM of 195.9 pg/mL in cases vs. 106.9 pg/mL in controls), IL-6 (p = 0.283, GM of 613.7 pg/mL in cases vs. 291.9 pg/mL in controls), IL-12p70 (p < 0.0001, GM of 25.84 pg/mL in cases vs. 14.85 pg/mL in controls), IL-17A (p < 0.0001, GM of 30.72 pg/mL in cases vs. 12.58 pg/mL in controls), and TNF-α (p = 0.0047, GM of 60.73 pg/mL in cases vs. 34.38 pg/mL in controls) were elevated in cases compared to controls. There was no significant difference in IL-1α, IL-5, IL-13, IL-1β, IL-4, and IL-12p70 at baseline and GM-CSF, IL-1α, IL-5, IL-18, IL-1β, and IL-4 at month 2 between cases and controls.
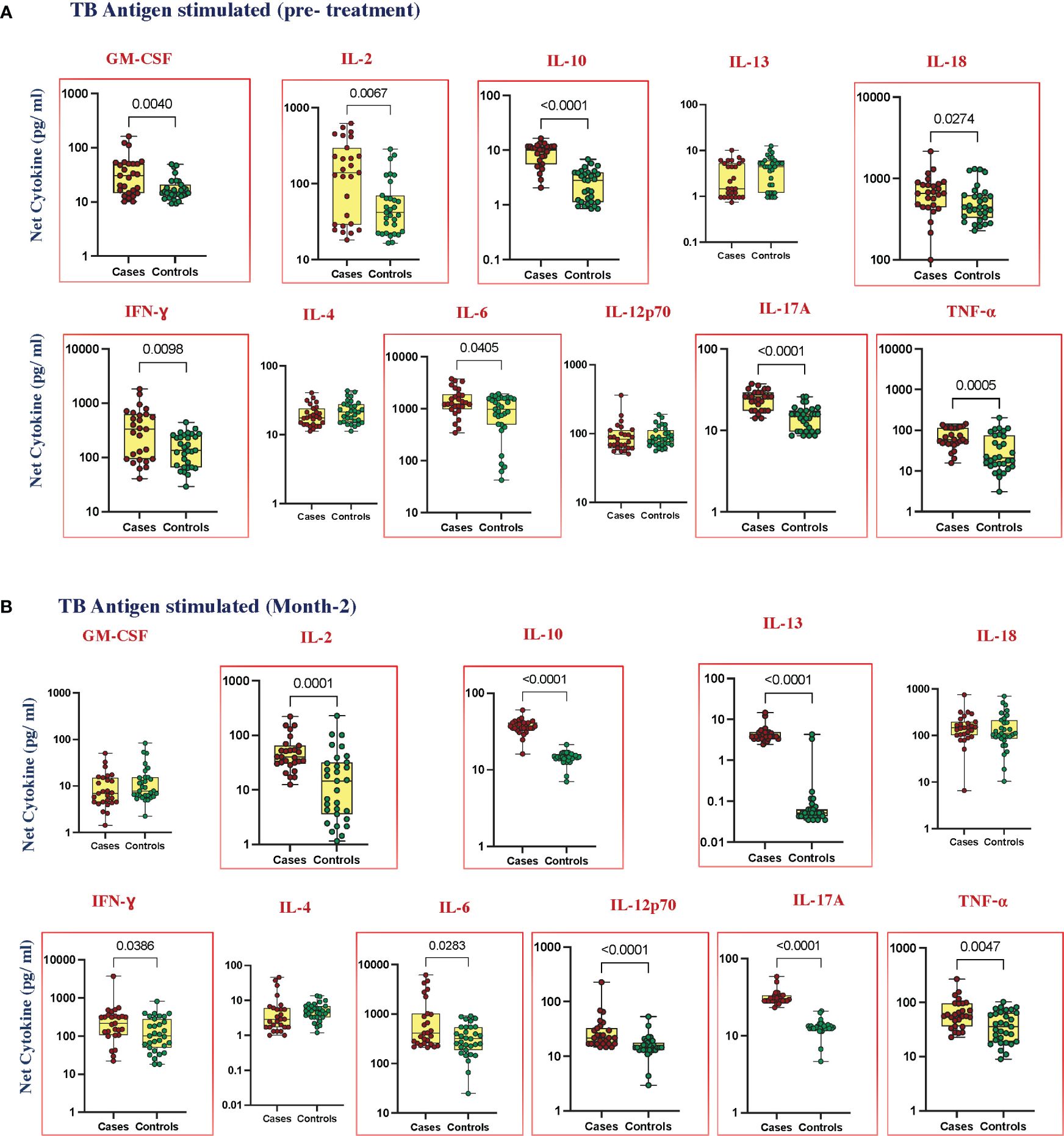
Figure 2 Elevated cytokines in TB antigen-stimulated plasma can predict unfavorable treatment outcomes (A) before treatment (baseline) (B) after treatment at month 2. TB antigen-stimulated plasma levels of cytokines were measured in cases (n = 27) and controls (n = 31) before treatment. This data was represented in a box-and-whiskers plot where each dot represents every single participant in its groups. The median line was presented at the middle of the box with its error bar at both ends. The Mann–Whitney test was used to calculate the p-values of the unrelated groups. Red boxes refer to the cytokines that were significantly different.
No change in cytokine level in mitogen-stimulated plasma
To elucidate the mitogen-stimulated cytokine levels in cases and controls, we estimated the production of GM-CSF, IL-1α, IL-2, IL-5, IL-10, IL-13, IL-18, IFN-γ, IL-1β, IL-4, IL-6, IL-12p70, IL-17A, and TNF-α at pre-treatment (Supplementary Figure 1A) and at month 2 of ATT (Supplementary Figure 1B). There were no significant differences in the cytokine levels between cases and cured controls in pre-treatment as well as at month 2 after treatment.
ROC analysis reveals that the pre-treatment and month 2 levels of cytokines can serve as predictive biomarkers for unfavorable treatment outcomes
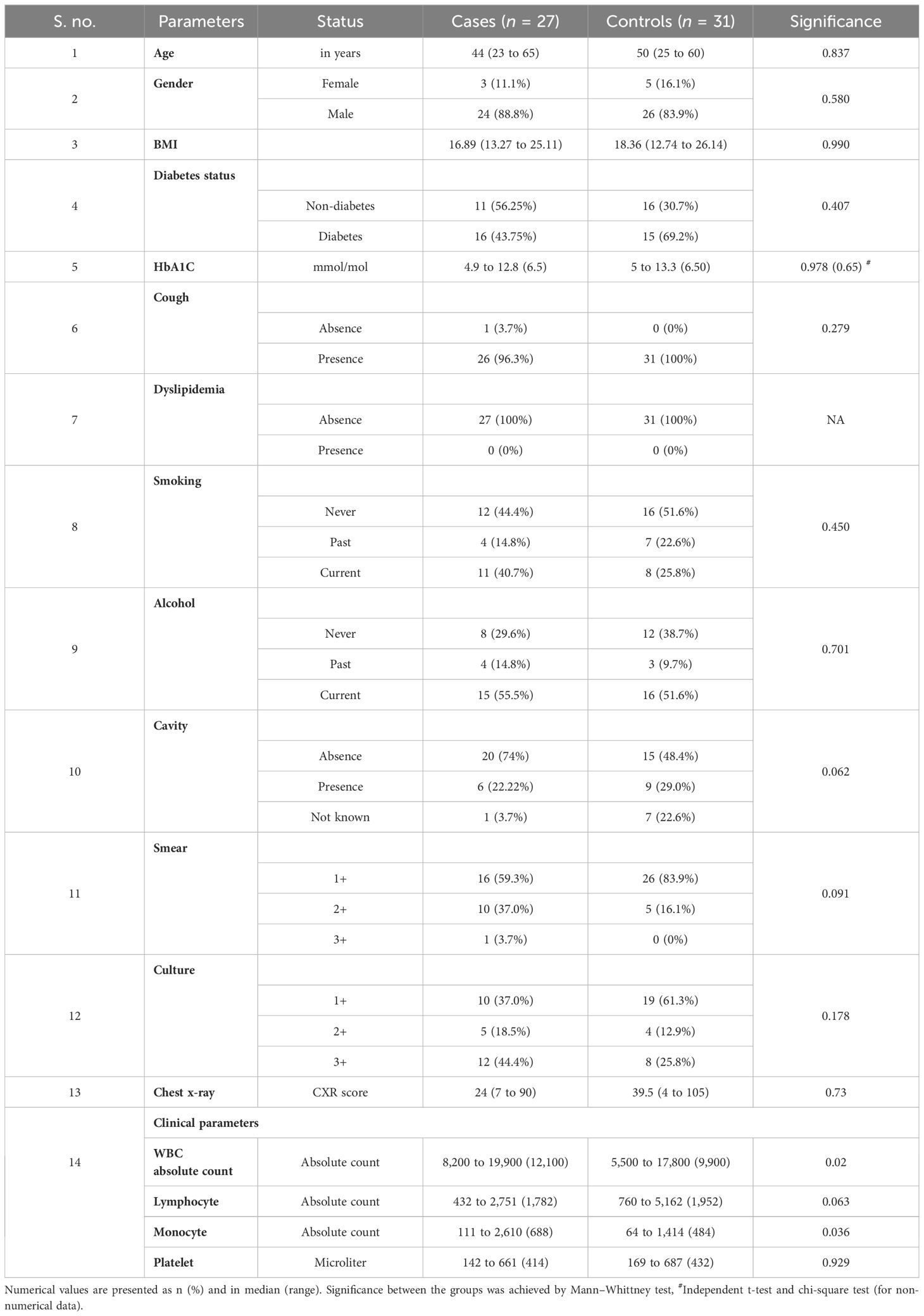
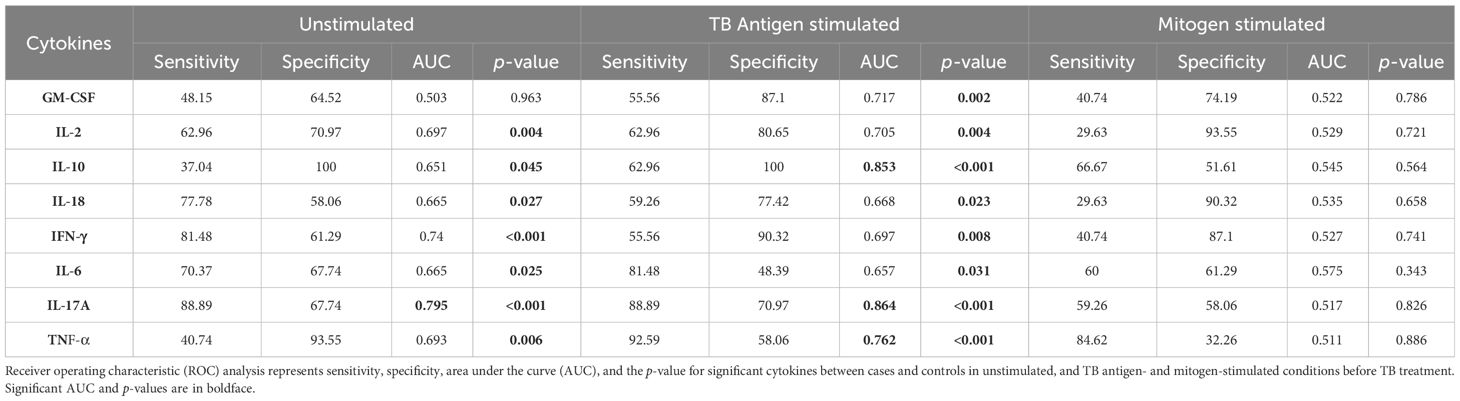
To assess the potential of unstimulated and TB antigen-stimulated cytokine levels as biomarkers for unfavorable treatment outcomes, we conducted ROC analysis to evaluate sensitivity and specificity. Table 2 demonstrates the ROC analysis results for IL-10, IL-17A, and TNF-α upon TB antigen stimulation before treatment, showing good AUC values of 0.85, 0.86, and 0.76, respectively. Sensitivity ranged from 63% to 93%, and specificity varied from 58% to 100%. After treatment at month 2, cytokines such as IL-2, IL-10, and IL-17A exhibited high AUC values (0.78, 0.99, and 1) with corresponding sensitivity (81%, 96%, and 100%) and specificity (71%, 100%, and 100%) in distinguishing cases and controls (as shown in Table 3). Similarly, in the unstimulated condition, IL-17A alone demonstrated a good AUC of 1.0 with 100% sensitivity and specificity before treatment. At month 2, IL-10, IL-17A, and TNF-α presented high AUC values (0.98, 1, and 0.77) with sensitivity (100%, 100%, and 88.9%) and specificity (96.7%, 100%, and 61%) in differentiating cases and controls.
Altered cytokine levels at pre- and post-treatment between cases and controls under unstimulated and TB antigen-stimulated conditions
To examine whether there were any alterations in cytokine levels before and after ATT at month 2 between cases and controls, we found that IL-13 (cases, p < 0.001, GM of 1.05 pg/mL in BL vs. 3.541 pg/mL in M2) (controls, p < 0.001, GM of 1.151 pg/mL in BL vs. 0.045 pg/mL in M2) and IL-17A (cases, p < 0.001, GM of 16.25 pg/mL in BL vs. 27.94 pg/mL in M2) (controls, p = 0.430, GM of 12.01 pg/mL in BL vs. 11.64 pg/mL in M2) were significantly increased in cases at month 2 in the unstimulated condition (Figure 3A). The levels of IL-13 (cases, p = 0.337, GM of 2.25 pg/mL in BL vs. 4.04 pg/mL in M2) (controls, p < 0.001, GM of 3.36 pg/mL in BL vs. 0.07 pg/mL in M2) and IL-17A (cases, p < 0.001, GM of 23.47 pg/mL in BL vs. 30.72 pg/mL of M2) (controls, p = 0.129, GM of 14.05 pg/mL in BL vs. 12.75 pg/mL in M2) continue to be different under TB antigen-stimulated conditions (Figure 3B). Levels of cytokines upon mitogen stimulation did not show significant differences between cases and controls, although they underwent changes pre- and post-treatment (Supplementary Figure 1C).
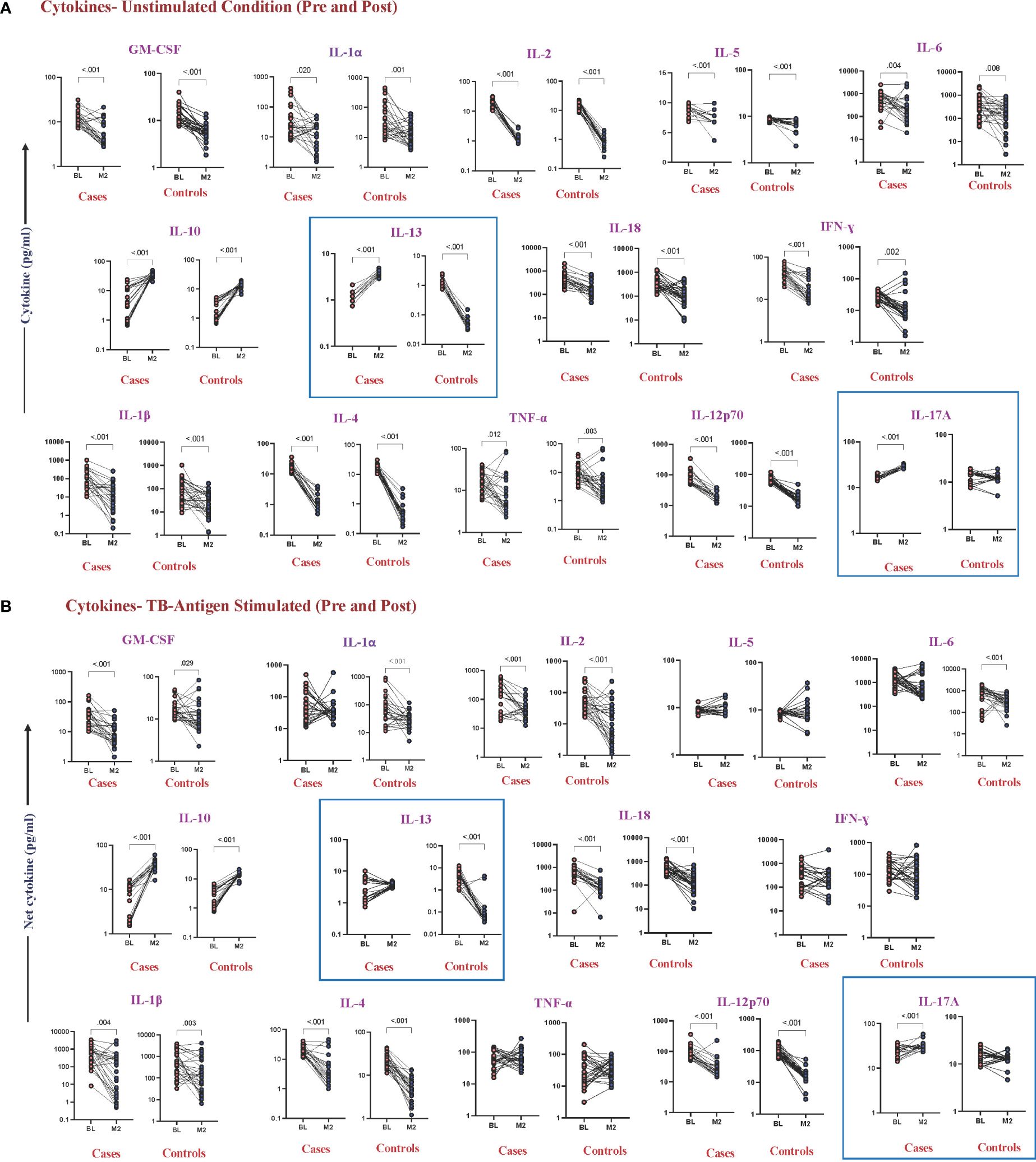
Figure 3 Altered cytokine levels in unstimulated and TB antigen-stimulated plasma were observed in cases and controls before and after 2 months of TB treatment. Wilcoxon rank analysis was performed to determine the p-value in these related groups. (A) Unstimulated. The blue box represents elevated levels of cytokines at month 2 in cases. (B) TB antigens stimulated. The blue box represents altered levels of cytokines between cases and controls in both pre- and post-TB treatment.
Combinatorial ROC analysis revealed the best cytokine combinations as predictive biomarkers for unfavorable treatment outcomes at pre-treatment and at month 2
Combined ROC analysis was conducted to evaluate the efficiency of cytokine combinations before treatment (shown in Figure 4A—NIL and Figure 4B—TB antigen) and after treatment at month 2 (shown in Figure 4C—NIL and Figure 4D—TB antigen). Cytokine signatures for combi-ROC included IL-17A and TNF-α as combo I and IL-10, IL-17A, and TNF-α as combo II. Combo I and II exhibited AUC values of 0.8 and 0.9 with a sensitivity of 96% and 78% and a specificity of 61% and 87%, respectively, in the unstimulated condition (NIL). Under TB antigen-stimulated conditions, AUC values of combo I and II showed 0.8 and 0.9 with a sensitivity of 96% and 78% and a specificity of 68% and 94% before treatment. At month 2 after treatment, AUC values of combo I and II were 0.8 and 0.9 with sensitivity (96% and 78%) and specificity (61% and 87%) in the unstimulated condition, whereas under TB antigen-stimulated conditions, AUC values of combo I and II showed 1 with 100% sensitivity and specificity. Thus, certain cytokine levels in unstimulated and TB antigen stimulation are highly useful in the early prediction of unfavorable treatment outcomes in patients with PTB.
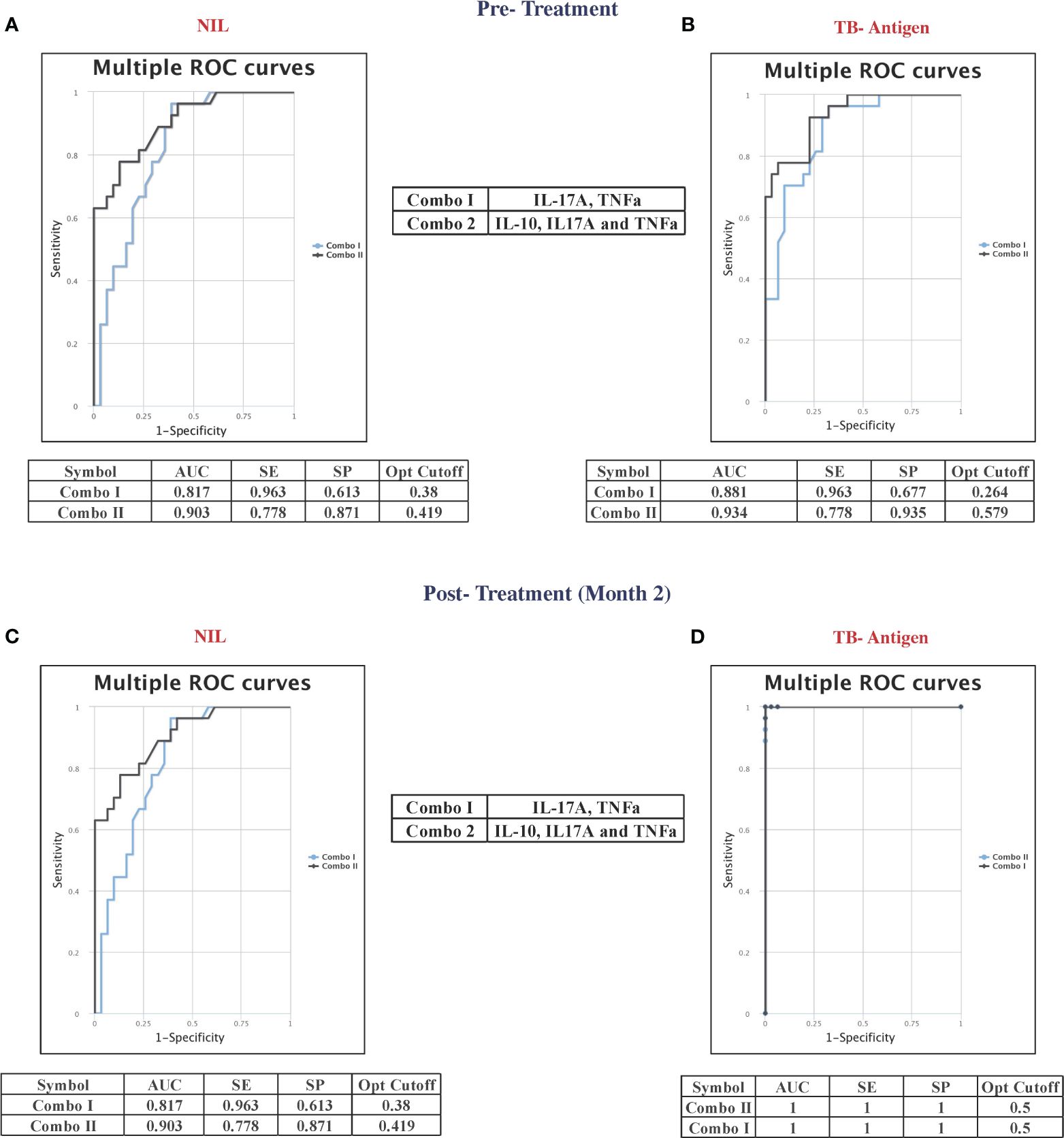
Figure 4 Multiple combinations of cytokines as a predictive biomarker discriminate unfavorable treatment outcomes from cured controls before treatment (baseline) [(A) NIL and (B) TB antigens] and after 2 months of treatment [(C) NIL and (D) TB antigens]. The curve was represented in a combination of receiver’s operator characteristic (ROC). AUC, area under the curve; SE, sensitivity; SP, specificity.
Discussion
TB remains a major global health concern, particularly in countries like India, which bears a substantial burden of the disease. The high rates of TB recurrence and treatment failure, coupled with the lack of reliable biomarkers to predict treatment outcomes (17), emphasize the critical need for better tools to guide TB management. This study aimed to investigate cytokines as potential predictive biomarkers for unfavorable TB treatment outcomes, specifically focusing on their response to TB antigens before and during the early phase of ATT.
Our findings revealed that individuals with unfavorable treatment outcomes exhibited distinct cytokine profiles, characterized by heightened levels of several cytokines, both before the initiation of treatment and at the 2-month mark during anti-TB therapy (18, 19). Notably, these differences were particularly pronounced when analyzing the immune responses to TB antigens, such as CFP10, ESAT6, and TB7.7, and were not observed with mitogen stimulation. This suggests that the immune responses driving these observed differences are primarily triggered by the TB infection itself.
Comparing our results to previous studies (20–22), it is evident that altered cytokine levels have been consistently associated with adverse TB treatment outcomes and also with disease severity (19, 23–26). While some studies have focused on baseline cytokine profiles, our investigation extends to the early phase of treatment, providing valuable insights into dynamic changes during therapy. For example, IFN-γ and TNF-α levels have previously been linked to relapse cases (27), but our study demonstrates their elevation both before and during early treatment in individuals with unfavorable outcomes. Importantly, our research highlights that the observed cytokine patterns were not significantly affected by comorbid conditions like diabetes, age, and BMI, underlining their potential as robust predictors of TB treatment response. This aspect is particularly promising for clinical application, as these biomarkers appear to be reliable indicators even in the presence of confounding factors. TB risk factors like low BMI have substantially influenced the cytokine and chemokine levels in both active and latent TB (28, 29).
Our ROC data clearly indicate that IL-10, IL-17A, and TNF-α could serve as possible predictive biomarkers for adverse treatment outcomes even before the start of TB treatment. The combination of IL-17A and TNF-α, with or without the inclusion of IL-10, had an AUC of 1 and 100% sensitivity and specificity in differentiating adverse treatment outcomes from cured individuals. Certain plasma cytokines, such as IL-4, IL-5, IL-10, IL-13, and IL-37, were altered following ATT when compared with latent and healthy individuals (30). Moreover, several other studies showed altered cytokine levels in whole blood plasma from QuantiFERON-TB Gold plus before and after 2 months of TB treatment, which was described to be helpful in assessing the efficiency of ATT (31, 32), as well as the TB treatment response. Although interferon-gamma release withdraws its ability to diagnose disease progression from latency (33, 34), it is essential to analyze its potential in differentiating two extreme treatment outcomes like recurrence and cure. Multiple studies have shown the importance of cytokines in TB disease and treatment outcomes, which are influenced by comorbid conditions like diabetes and HIV (35–37) to a great extent. Additionally, studies have also shown that IL-13 and IL-5 declined in patients who experienced treatment failure (38). In contrast to these results, our results show no significant difference in IL-13 and IL-5 before treatment in adverse TB treatment outcomes. Apart from this, the late reduction of IL-6 plasma level was found to be a promising marker for slow TB treatment responders when compared with fast responders after 6 weeks of ATT (39). These cytokines not only govern the overall efficiency of TB treatment but also help in treatment monitoring. In this study, IL-13 was found to be unaltered between cases and controls before TB treatment but elevated drastically in cases and declined in controls after 2 months of TB treatment when compared with before treatment in the unstimulated condition. Upon TB antigen stimulation, though no significance was observed between pre- and post-levels of IL-13 in cases, controls were marked by their reduced level after 2 months of TB treatment.
While our previous studies have explored various biomarkers for TB treatment outcomes, including chemokines, matrix metalloproteinases (MMPs), tissue inhibitors of matrix metalloproteinases (TIMPs), acute phase proteins (APPs), microbial translocation markers, chitinase, and indoleamine 2,3-dioxygenase (IDO) (40–42), our current work specifically focuses on TB antigen-stimulated cytokines. This emphasis is essential because cytokines play a crucial role in immune responses during TB infection and treatment. Our findings suggest that cytokines hold a great promise as predictive markers, alongside other markers like CXCR3, CXCL9, CXCL10, CXCL11, and TNF-α, which have been implicated in TB diagnosis, treatment monitoring, and outcomes (43–45).
In summary, our study results suggest that measuring IL-17A and TNF-α in QFT supernatants at month 2 of TB treatment accurately discriminates between individuals destined for cure vs. adverse treatment outcomes. Our results further show that only an M.tb antigen-specific response offers predictive efficacy, and that this can conveniently be tested using QFT supernatants. Limitations of our study include the relatively small sample size, the preliminary nature of the study design, and a case–control ratio of only approximately 1. Nonetheless, our findings merit confirmation in larger cohorts at multiple sites, potentially using this test result to stratify patients into those who are likely to achieve cure with 4 months of TB treatment vs. those who will require a longer duration of treatment. These findings offer a potential breakthrough in the field of TB management, enabling early identification of individuals at risk for poor treatment responses. Further research and validation studies are warranted to confirm the utility of these cytokine biomarkers in clinical practice and to explore their role in guiding personalized TB treatment strategies. Ultimately, improving our ability to predict treatment outcomes will help mitigate the burden of TB and enhance the effectiveness of TB control programs worldwide.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Prof. M. Viswanathan Diabetes Research Center and the National Institute for Research in Tuberculosis. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AP: Validation, Writing – review & editing, Data curation, Formal analysis, Methodology, Software. NK: Conceptualization, Data curation, Formal analysis, Supervision, Validation, Writing – original draft, Writing – review & editing. NS: Methodology, Writing – review & editing. SA: Formal analysis, Software, Writing – review & editing. VV: Funding acquisition, Project administration, Resources, Writing – review & editing. KT: Data curation, Formal analysis, Software, Writing – review & editing. SH: Project administration, Resources, Writing – review & editing. SS: Data curation, Methodology, Supervision, Writing – review & editing. RB: Methodology, Resources, Supervision, Writing – review & editing. SN: Data curation, Validation, Writing – review & editing. HK: Writing – review & editing, Conceptualization, Data curation, Funding acquisition, Resources. SB: Writing – review & editing, Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project has been funded in whole or in part with Federal funds from the Government of India’s (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the United States National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), and Office of AIDS Research (OAR), and distributed in part by CRDF Global (grant USB1–31149-XX-13). This work is also funded by CRDF Global RePORT India Consortium Supplemental Funding (grant OISE-17–62911-1). This work was also funded in part by the Division of Intramural Research, NIAID, NIH.
Acknowledgments
We thank the staff of the Department of Clinical Research and the Department of Bacteriology, NIRT for valuable assistance in bacterial cultures and radiology and the staff of MVDRC, RNTCP, and Chennai Corporation for valuable assistance in recruiting the patients for this study. Data in this manuscript were collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the DBT, the ICMR, the NIH, or CRDF Global.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1392256/full#supplementary-material
Supplementary Figure 1 | Cytokine levels in mitogen-stimulated plasma between favorable and unfavorable treatment outcomes (A) before treatment and (B) after treatment at month 2. Mitogen-stimulated plasma levels of cytokines were measured in cases (n = 27) and controls (n = 31) before TB treatment. These data were represented in a box-and-whiskers plot where each dot represents every single participant in its group. The median line was presented at the middle of the box with its error bar at both ends. The Mann–Whitney test was used to calculate the p-values of the unrelated groups. No significant difference in cytokine levels upon mitogen stimulation before TB treatment and after ATT at month 2. (C) Cytokine levels in mitogen-stimulated plasma in cases and controls before and after 2 months of TB treatment. Wilcoxon rank analysis was performed to determine the p-value in these related groups. A similar trend of cytokines in both groups in pre- and post-TB treatment represents the non-influential pattern upon mitogen stimulation.
Supplementary Figure 2 | Cytokine levels in unstimulated plasma [(A) before treatment and (B) after treatment at month 2] and TB antigen-stimulated plasma [(C) before treatment and (D) after treatment at month 2] between favorable and unfavorable treatment. These are the common cytokines (IL-1α, IL-5, and IL-1β), which are not significant between the groups in both unstimulated and TB antigen-stimulated conditions.
References
2. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med. (2016) 13:e1002152. doi: 10.1371/journal.pmed.1002152
3. Central Tuberculosis Division GoI. India TB Report 2022. New Delhi: Central TB Division, Ministry of Health and Family Welfare (2022).
4. Heyckendorf J, Georghiou SB, Frahm N, Heinrich N, Kontsevaya I, Reimann M, et al. Tuberculosis treatment monitoring and outcome measures: new interest and new strategies. Clin Microbiol Rev. (2022) 35:e0022721. doi: 10.1128/cmr.00227-21
5. Walzl G, McNerney R, du Plessis N, Bates M, McHugh TD, Chegou NN, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. (2018) 18:e199–210. doi: 10.1016/S1473-3099(18)30111-7
6. Mihret A, Bekele Y, Bobosha K, Kidd M, Aseffa A, Howe R, et al. Plasma cytokines and chemokines differentiate between active disease and non-active tuberculosis infection. J Infect. (2013) 66:357–65. doi: 10.1016/j.jinf.2012.11.005
7. Wallis RS, Kim P, Cole S, Hanna D, Andrade BB, Maeurer M, et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis. (2013) 13:362–72. doi: 10.1016/S1473-3099(13)70034-3
8. Goletti D, Lee MR, Wang JY, Walter N, Ottenhoff THM. Update on tuberculosis biomarkers: From correlates of risk, to correlates of active disease and of cure from disease. Respirology. (2018) 23:455–66. doi: 10.1111/resp.13272
9. WHO. Consolidated guidelines on tuberculosis, Module 4: Treatment - drug-susceptible tuberculosis treatment. Geneva: WHO (2022).
10. Goletti D, Lindestam Arlehamn CS, Scriba TJ, Anthony R, Cirillo DM, Alonzi T, et al. Can we predict tuberculosis cure? What tools are available? Eur Respir J. (2018) 52. doi: 10.1183/13993003.01089-2018
11. Dorman SE, Nahid P, Kurbatova EV, Phillips PPJ, Bryant K, Dooley KE, et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med. (2021) 384:1705–18. doi: 10.1056/NEJMoa2033400
12. Grace AG, Mittal A, Jain S, Tripathy JP, Satyanarayana S, Tharyan P, et al. Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis. Cochrane Database Syst Rev. (2019) 12:CD012918. doi: 10.1002/14651858.CD012918.pub2
13. Dinarello CA. Historical insights into cytokines. Eur J Immunol. (2007) 37 Suppl 1:S34–45. doi: 10.1002/(ISSN)1521-4141
14. Domingo-Gonzalez R, Prince O, Cooper A, Khader SA. Cytokines and chemokines in mycobacterium tuberculosis infection. Microbiol Spectr. (2016) 4(5). doi: 10.1128/microbiolspec.TBTB2-0018-2016
15. Yu Y, Zhang Y, Hu S, Jin D, Chen X, Jin Q, et al. Different patterns of cytokines and chemokines combined with IFN-gamma production reflect Mycobacterium tuberculosis infection and disease. PloS One. (2012) 7:e44944. doi: 10.1371/journal.pone.0044944
16. Yao X, Liu Y, Liu Y, Liu W, Ye Z, Zheng C, et al. Multiplex analysis of plasma cytokines/chemokines showing different immune responses in active TB patients, latent TB infection and healthy participants. Tuberculosis (Edinb). (2017) 107:88–94. doi: 10.1016/j.tube.2017.07.013
17. Mirsaeidi M, Sadikot RT. Patients at high risk of tuberculosis recurrence. Int J Mycobacteriol. (2018) 7:1–6. doi: 10.4103/ijmy.ijmy_164_17
18. Nikolayevskyy V, Balabanova Y, Kontsevaya I, Ignatyeva O, Skenders G, Vasiliauskiene E, et al. Biomarkers of treatment success in fully sensitive pulmonary tuberculosis patients: a multicenter longitudinal study. biomark Med. (2020) 14:1439–52. doi: 10.2217/bmm-2020-0246
19. Gupte AN, Kumar P, Araujo-Pereira M, Kulkarni V, Paradkar M, Pradhan N, et al. Baseline IL-6 is a biomarker for unfavourable tuberculosis treatment outcomes: a multisite discovery and validation study. Eur Respir J. (2022) 59. doi: 10.1183/13993003.00905-2021
20. Kumar NP, Moideen K, Nancy A, Viswanathan V, Thiruvengadam K, Nair D, et al. Plasma chemokines are baseline predictors of unfavorable treatment outcomes in pulmonary tuberculosis. Clin Infect Dis. (2021) 73:e3419–e27. doi: 10.1093/cid/ciaa1104
21. Zimmer AJ, Lainati F, Aguilera Vasquez N, Chedid C, McGrath S, Benedetti A, et al. Biomarkers that correlate with active pulmonary tuberculosis treatment response: a systematic review and meta-analysis. J Clin Microbiol. (2022) 60:e0185921. doi: 10.1128/jcm.01859-21
22. Kumar NP, Moideen K, Nancy A, Viswanathan V, Shruthi BS, Sivakumar S, et al. Plasma chemokines are biomarkers of disease severity, higher bacterial burden and delayed sputum culture conversion in pulmonary tuberculosis. Sci Rep. (2019) 9:18217. doi: 10.1038/s41598-019-54803-w
23. Kumar NP, Moideen K, Banurekha VV, Nair D, Babu S. Plasma proinflammatory cytokines are markers of disease severity and bacterial burden in pulmonary tuberculosis. Open Forum Infect Dis. (2019) 6:ofz257. doi: 10.1093/ofid/ofz257
24. Sampath P, Rajamanickam A, Thiruvengadam K, Natarajan AP, Hissar S, Dhanapal M, et al. Cytokine upsurge among drug-resistant tuberculosis endorse the signatures of hyper inflammation and disease severity. Sci Rep. (2023) 13:785. doi: 10.1038/s41598-023-27895-8
25. Bai XJ, Li HM, Yang YR, Zhang JX, Liang Y, Wu XQ. Cytokine and soluble adhesion molecule profiles and biomarkers for treatment monitoring in Re-treated smear-positive patients with pulmonary tuberculosis. Cytokine. (2018) 108:9–16. doi: 10.1016/j.cyto.2018.03.009
26. Kumar NP, Moideen K, Viswanathan V, Sivakumar S, Ahamed SF, Ponnuraja C, et al. Heightened microbial translocation is a prognostic biomarker of recurrent tuberculosis. Clin Infect Dis. (2022) 75:1820–6. doi: 10.1093/cid/ciac236
27. Waghmare PJ, Lende T, Goswami K, Gupta A, Gupta A, Gangane N, et al. Immunological host responses as surveillance and prognostic markers in tubercular infections. Int J Mycobacteriol. (2019) 8:190–5. doi: 10.4103/ijmy.ijmy_48_19
28. Kumar NP, Nancy AP, Moideen K, Menon PA, Banurekha VV, Nair D, et al. Low body mass index is associated with diminished plasma cytokines and chemokines in both active and latent tuberculosis. Front Nutr. (2023) 10:1194682. doi: 10.3389/fnut.2023.1194682
29. Anuradha R, Munisankar S, Bhootra Y, Kumar NP, Dolla C, Kumaran P, et al. Coexistent malnutrition is associated with perturbations in systemic and antigen-specific cytokine responses in latent tuberculosis infection. Clin Vaccine Immunol. (2016) 23:339–45. doi: 10.1128/CVI.00009-16
30. Moideen K, Kumar NP, Bethunaickan R, Banurekha VV, Nair D, Babu S. Heightened systemic levels of anti-inflammatory cytokines in pulmonary tuberculosis and alterations following anti-tuberculosis treatment. Cytokine. (2020) 127:154929. doi: 10.1016/j.cyto.2019.154929
31. Mvungi HC, Mbelele PM, Buza JJ, Mpagama SG, Sauli E. Blood cytokine responses to early secreted protein antigen-6/culture filtrate protein-10 tuberculosis antigens 2 months after antituberculosis treatment among patients with drug-susceptible pulmonary tuberculosis. Int J Mycobacteriol. (2019) 8:53–9. doi: 10.4103/ijmy.ijmy_30_19
32. Leem AY, Song JH, Lee EH, Lee H, Sim B, Kim SY, et al. Changes in cytokine responses to TB antigens ESAT-6, CFP-10 and TB 7.7 and inflammatory markers in peripheral blood during therapy. Sci Rep. (2018) 8:1159. doi: 10.1038/s41598-018-19523-7
33. Metcalfe JZ, Everett CK, Steingart KR, Cattamanchi A, Huang L, Hopewell PC, et al. Interferon-gamma release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. (2011) 204 Suppl 4:S1120–9. doi: 10.1093/infdis/jir410
34. Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. (2014) 27:3–20. doi: 10.1128/CMR.00034-13
35. Kumar NP, Moideen K, Nancy A, Viswanathan V, Shruthi BS, Sivakumar S, et al. Heterogeneity in the cytokine profile of tuberculosis - diabetes co-morbidity. Cytokine. (2020) 125:154824. doi: 10.1016/j.cyto.2019.154824
36. Sivro A, McKinnon LR, Yende-Zuma N, Gengiah S, Samsunder N, Abdool Karim SS, et al. Plasma cytokine predictors of tuberculosis recurrence in antiretroviral-treated human immunodeficiency virus-infected individuals from Durban, South Africa. Clin Infect Dis. (2017) 65:819–26. doi: 10.1093/cid/cix357
37. Thobakgale C, Naidoo K, McKinnon LR, Werner L, Samsunder N, Karim SA, et al. Interleukin 1-beta (IL-1beta) production by innate cells following TLR stimulation correlates with TB recurrence in ART-treated HIV-infected patients. J Acquir Immune Defic Syndr. (2017) 74:213–20. doi: 10.1097/QAI.0000000000001181
38. Ronacher K, Chegou NN, Kleynhans L, Djoba Siawaya JF, du Plessis N, Loxton AG, et al. Distinct serum biosignatures are associated with different tuberculosis treatment outcomes. Tuberculosis (Edinb). (2019) 118:101859. doi: 10.1016/j.tube.2019.101859
39. Vivekanandan MM, Adankwah E, Aniagyei W, Acheampong I, Yeboah A, Arthur JF, et al. Plasma cytokine levels characterize disease pathogenesis and treatment response in tuberculosis patients. Infection. (2023) 51:169–79. doi: 10.1007/s15010-022-01870-3
40. Kumar NP, Moideen K, Nancy A, Viswanathan V, Thiruvengadam K, Sivakumar S, et al. Association of plasma matrix metalloproteinase and tissue inhibitors of matrix metalloproteinase levels with adverse treatment outcomes among patients with pulmonary tuberculosis. JAMA Netw Open. (2020) 3:e2027754. doi: 10.1001/jamanetworkopen.2020.27754
41. Kumar NP, Moideen K, Nancy A, Viswanathan V, Thiruvengadam K, Sivakumar S, et al. Acute phase proteins are baseline predictors of tuberculosis treatment failure. Front Immunol. (2021) 12:731878. doi: 10.3389/fimmu.2021.731878
42. Kumar NP, Nancy A, Viswanathan V, Sivakumar S, Thiruvengadam K, Ahamed SF, et al. Chitinase and indoleamine 2, 3-dioxygenase are prognostic biomarkers for unfavorable treatment outcomes in pulmonary tuberculosis. Front Immunol. (2023) 14:1093640. doi: 10.3389/fimmu.2023.1093640
43. Chung W, Lee K, Jung Y, Kim Y, Park J, Sheen S, et al. Serum CXCR3 ligands as biomarkers for the diagnosis and treatment monitoring of tuberculosis. Int J Tuberc Lung Dis. (2015) 19:1476–84. doi: 10.5588/ijtld.15.0325
44. Sivakumaran D, Jenum S, Vaz M, Selvam S, Ottenhoff THM, Haks MC, et al. Combining host-derived biomarkers with patient characteristics improves signature performance in predicting tuberculosis treatment outcomes. Commun Biol. (2020) 3:359. doi: 10.1038/s42003-020-1087-x
Keywords: tuberculosis, recurrence, TB treatment failure, cytokines, TB treatment cure
Citation: Pandiarajan AN, Kumar NP, Selvaraj N, Ahamed SF, Viswanathan V, Thiruvengadam K, Hissar S, Shanmugam S, Bethunaickan R, Nott S, Kornfeld H and Babu S (2024) Distinct TB-antigen stimulated cytokine profiles as predictive biomarkers for unfavorable treatment outcomes in pulmonary tuberculosis. Front. Immunol. 15:1392256. doi: 10.3389/fimmu.2024.1392256
Received: 27 February 2024; Accepted: 29 April 2024;
Published: 03 June 2024.
Edited by:
Ramcés Falfán-Valencia, National Institute of Respiratory Diseases-Mexico (INER), MexicoReviewed by:
Harriet Mayanja-Kizza, Makerere University, UgandaNorma A. Tellez-Navarrete, National Institute of Respiratory Diseases-Mexico (INER), Mexico
Leslie Chavez-Galan, National Institute of Respiratory Diseases-Mexico (INER), Mexico
Copyright © 2024 Pandiarajan, Kumar, Selvaraj, Ahamed, Viswanathan, Thiruvengadam, Hissar, Shanmugam, Bethunaickan, Nott, Kornfeld and Babu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Subash Babu, sbabu@icerindia.org
 Arul Nancy Pandiarajan
Arul Nancy Pandiarajan Nathella Pavan Kumar
Nathella Pavan Kumar Nandhini Selvaraj1
Nandhini Selvaraj1 Shaik Fayaz Ahamed
Shaik Fayaz Ahamed Kannan Thiruvengadam
Kannan Thiruvengadam Sivakumar Shanmugam
Sivakumar Shanmugam Ramalingam Bethunaickan
Ramalingam Bethunaickan Hardy Kornfeld
Hardy Kornfeld Subash Babu
Subash Babu