- 1Département Océanographie et Dynamique des Ecosystèmes, Ifremer, La Seyne sur Mer, France
- 2CoNISMa Local Research Unit, Department of Earth and Environmental Sciences, University of Milano-Bicocca, Milan, Italy
- 3Istituto di Scienze Marine (ISMAR), CNR, Bologna, Italy
- 4Biology Department, Woods Hole Oceanographic Institution, Woods Hole, MA, United States
- 5Stazione Zoologica Anton Dohrn, Naples, Italy
The Santa Maria di Leuca (SML) cold-water coral province (northern Ionian Sea) has the largest occurrence of a living white coral community currently known in the Mediterranean Sea. Madrepora oculata and Lophelia pertusa, identified as marking sensitive habitats of relevance by the General Fisheries Commission for the Mediterranean, have been observed heterogeneously distributed on the summits of several mounds. This particularly patchy and uneven distribution in addition to their importance for regional biodiversity highlights the need to better understand their environmental preferences and predict their distribution. Bathymetric data (40 m resolution) was used to derive seafloor characteristics. A fine scale index quantifying the landscape elevation (Bathymetric Position Index at 120 m resolution) was used to select all the elevated features considered as candidate morphologies for potential coral mounds. Statistics on 22 known coral topped mounds were computed. Two statistical methods were then used to identify other potential coral mounds based on predictive variables. The first method, the Geomorphometric proxies method, consists in computing basic statistics of terrain variables, using them for a step-by-step classification in a quantitative approach to select a subset of candidate morphologies. The second method consists in using a predictive Habitat Suitability Model (Maxent model). The Geomorphometric proxies method identified 736 potential coral mounds while the Maxent method predicted 1,252 potential coral mounds. A subset of 517 potential coral mounds was common to both methods. The analysis of the contribution of each variable with the Maxent method showed that the variable “Vector Ruggedness Measure” at a resolution of 5 pixels (200 m) contributed to 53% of the final Maxent model, followed by the “Terrain Texture” index (31%) at a resolution of 11 pixels (440 m). The common potential coral mounds are mainly located in an area characterized by a mass transport deposit, also called the mounds area because of the roughness of the seafloor, in accordance with the high proportional contribution of the noticeable first roughness index to the Maxent model. The results highlight the importance of the global conservation of the entire Province, with white coral probably widespread over the entire 600 km2 SML area.
Introduction
Cold-Water Corals (CWCs) are considered important marine benthic habitats, providing 3-Dimensional structures as those engineered by the two colonial scleractinians Lophelia pertusa and Madrepora oculata (Tursi et al., 2004). They provide structural niches and nurseries for many organisms, thereby hosting rich ecosystems and biodiversity in all the seas of the globe (Freiwald et al., 2004; Roberts et al., 2006; Roberts and Cairns, 2014), including in the Mediterranean Sea (D'Onghia et al., 2010b; Linley et al., in press). These Vulnerable Marine Ecosystems (VME) are especially fragile and vulnerable as they are characterized by low productivity, low fecundity, older age at first maturity, and high longevity. In addition, they are subject to numerous threats, largely reported in the literature, such as bottom trawling, seafloor exploitation, pollution, and ocean acidification (Freiwald et al., 2004; Guinotte et al., 2006; McCulloch et al., 2012; Fabri et al., 2014; Roberts and Cairns, 2014). The Food and Agricultural Organization (FAO) has formulated management guidelines and criteria for defining VME among which uniqueness and rarity of species or habitat, functional significance, fragility and structural complexity, and life history limit the probability of recovery (FAO, 2009). CWCs have also been identified as sensitive habitats by the General Fisheries Commission for the Mediterranean Sea (GFCM, 2009). International awareness and the management of CWCs have generally increased over the last 20 years and considerable efforts have been made to manage and share information on marine biodiversity and distribution (Costello and Vanden Berghe, 2006; Costello, 2009). The Marine Strategy Framework Directive (MSFD, 2008/56/CE), adopted in 2008, is a European commitment aimed at achieving “Good Environmental Status” (GES) for all marine waters by 2020 and requires each Member State to develop a strategy of knowledge-based sustainable management for its marine waters to preserve biodiversity, including in the deep sea. To ensure efficient management and protection, the first need is good knowledge of the distribution of the species studied, the main factors controlling their dynamics, and the possible impacts of all the different types of anthropogenic pressure. Continuous maps of species distribution are, thus, of great value in the framework of marine ecosystem protection and conservation. However, the exploration of the deep sea to assess species distribution depends on in situ observations, which are still costly and difficult to obtain. The influence of environmental factors on species distribution and growth is, therefore, still only partially understood.
To overcome these difficulties, statistical methods have been applied to build synoptic habitat maps for CWC, using statistical interpolation of observations of species in situ. These methods have the advantage of extrapolating observations in space and time to areas where no information on species occurrence is available, provided that the same predictive variable dataset is used. Habitat Suitability Models (HSMs) have been used frequently over the last few decades. This increasingly popular technique, which is still recent for deep-sea species and ecosystems, is an efficient and cost-effective means of extending the coverage of existing information to the global scale (Vierod et al., 2014). HSMs compare the particular conditions of sites where the species has been observed to the conditions of the entire area studied, in order to identify where suitable environments are distributed in space (Pearson, 2008).
At the global scale, these models have already identified environmental parameters like temperature, depth, slope, aragonite saturation, and oxygen as the main factors influencing CWC distributions (Tittensor et al., 2009; Davies and Guinotte, 2011; Yesson et al., 2012, in press). At the more local scale, however, some terrain attributes, such as morphological features (topographic highs) and roughness, appear to be key contributors to CWC distribution (Dolan et al., 2008; Giusti et al., 2014; Rengstorf et al., 2014; Tong et al., 2016), where suitable environmental parameters for coral growth are present (in terms of temperature, salinity, dissolved oxygen, pH-values, and food availability). The interplay between local hydrodynamics and elevated features indeed promotes moderate to strong hydrodynamics and turbulent mixing, coupled with reduced terrigenous input and constant delivery of organic material, which in turn maintain CWC growth (White et al., 2005; Mienis et al., 2007). At some locations, CWCs are able to form prominent morphologies defined as CWC mounds (Kenyon et al., 2003; Masson et al., 2003; Van Rooij et al., 2003; van Weering et al., 2003; Olu-Le Roy, 2004; Wheeler et al., 2006). Next to these giant mounds, smaller mounds <100 m in diameter in places, and which should not be classified as coral carbonate mounds, strictly speaking, can also be typified by significant coral growth at their tops and/or flanks. Various areas constituted by these numerous small elevations topped by living CWC have already been described in the Mediterranean Sea such as the Chella bank on the Almeria Margin in the eastern Alboran Sea (Lo Iacono et al., 2008) and Santa Maria di Leuca (SML) on the Apulian plateau in the Ionian Sea (Taviani, 2002; Tursi et al., 2004; Taviani et al., 2005a; Vertino et al., 2010; Savini et al., 2016).
The SML, in the northern Ionian Sea, is one of the most famous CWC provinces in the Mediterranean Sea (Tursi et al., 2004; Savini and Corselli, 2010; Taviani et al., 2011). It is indeed the location of a remarkable hotspot of biodiversity where the largest occurrence of a living white coral community is currently known in Mediterranean settings (Freiwald et al., 2009). Early CWC growth in this area is documented as occurring since the late Pleistocene (Malinverno et al., 2010; McCulloch et al., 2010), but they have grown continuously only since the Early Holocene (Fink et al., 2012). In the study area, they thrive between 500 and 1,000 m depth (Budillon et al., 2010) and heterogeneously cover the summits and the north-eastern flanks of sediment blocks (mound-shaped) generated by multiple Pleistocene mass transport events and that are exposed over more than 600 km2 on the northern Ionian margin (Savini et al., 2016). At certain locations, these failure-related blocks lack coral coverage (Rosso et al., 2010; Vertino et al., 2010). This particular patchy and uneven disposition highlights the need to understand the factors driving CWC distribution in this area at the meso-scale (10–1,000 m) in order to precisely predict their distribution and understand their environmental preferences at the mound scale. To date, only one study has attempted to draw a continuous map of the distribution of the mounds suitable for CWC settlement at SML, isolating 5,820 mounds (i.e., sediment blocks) by geomorphometric analysis, among which a total of 1,902 were assumed to be populated by CWC macro-habitats according to their terrain attributes, seismic pattern, and depth-range occurrence (Savini et al., 2014).
Our study intends to go further than the previous study of Savini et al. (2014), using more terrain attributes and two statistical methods to refine the potential topographic features topped by coral: the Geomorphometric proxies method and the Maximum Entropy habitat suitability model (Maxent). The purpose of our study is to generate detailed maps of potential coral mounds in the SML Province and identify the suitable topographic conditions for CWC settlement, thereby classifying the coral preferences of environmental parameters.
Materials and Methods
Study Area
The SML CWC Province is located offshore at the southern limit of the Apulian Peninsula, in the north-eastern Ionian margin (Figures 1A,B). Scarps and ridges are well represented on the western sector of the regional Digital Terrain Model (DTM) (Etiope et al., 2010), with the presence of a major canyon (Freiwald et al., 2009), whereas huge and complex mass-wasting deposits characterize the overall sedimentary setting to the east, where they mask buried faults (Figure 1C; Savini et al., 2016). The reef-forming CWC species of the SML province are L. pertusa and M. oculata, which have been found to live at depths of 500–900 m (Vertino et al., 2010). They predominantly grow on sediment blocks (up to 300 m wide and 25 m high) originating from mass-wasting events and forming coral-topped mounds (Savini et al., 2016). A total of 22 mounds were visually explored (Tursi et al., 2004; Carlier et al., 2009; Freiwald et al., 2009; D'Onghia et al., 2010a, 2011; Vertino et al., 2010; Capezzuto et al., 2012) within the framework of Italian (FIRB2004-2006 APLABES) and European (EUFP6 HERMES) projects, documenting a dominant distribution of CWC habitats on their tops and north-eastern flanks. Previous studies evidenced that the mounds were generated by mass-wasting phenomena that have likely exposed older and harder strata, providing necessary substrates for coral colonization (Savini and Corselli, 2010; Vertino et al., 2010).
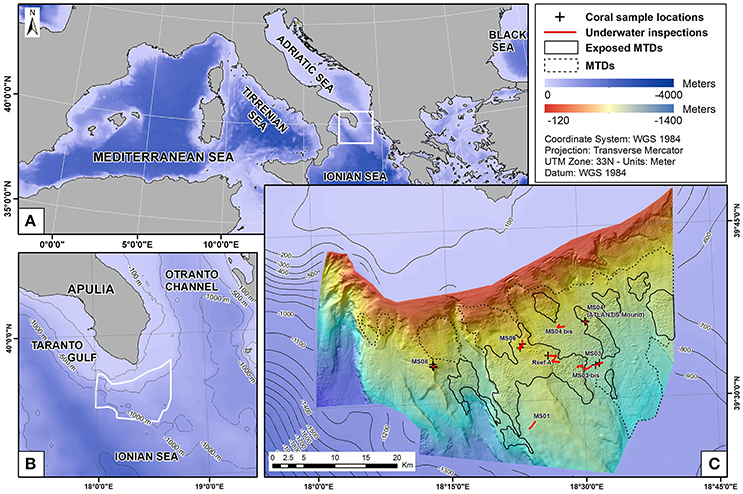
Figure 1. (A,B) Location of the SML Province. (C) Bathymetric map of SML province, with the eight sites with cold-water corals. Underwater inspections (red lines) and coral sample locations (black crosses) are shown as well as the Mass Transport Deposit areas identified by Savini et al. (2016).
Besides the well-documented distribution of coral-topped mounds associated with widespread mass transport deposits (Vertino et al., 2010; Savini et al., 2014; Figure 1C), CWCs were also sampled or visually investigated within other geomorphic settings (Freiwald et al., 2009; Etiope et al., 2010; Malinverno et al., 2010; Rosso et al., 2010; Savini and Corselli, 2010; D'Onghia et al., 2011, 2012), although their differentiations and relationships with present-day sedimentary processes in those sites have not been subject to thorough investigation. In particular, some small CWC colonies have been observed in the western part of the regional DTM on hard substrata at the top of a fault-related ridge (MS08 site, Figure 1C; Malinverno et al., 2010) and along the erosive and gently-sloping surface of the western sector of a prominent up-thrown regional block (MS01 site, located to the south and west of the main mass transport deposit—Figure 1C), where coral colonies and hard-ground substrates are patchily distributed (Savini and Corselli, 2010). CWC do not form coral-topped mounds at MS08 and MS01 sites in the same way as they have been described in Savini et al. (2016), although elevated features remain associated with their location, forming small mound-like features not originated by mass transport events. Consequently, we will use the general term “coral-mound” to indicate all elevated features hosting CWC in the SML region, whereas the term “candidate morphologies” is used to indicate all those elevated features having a “mound-shape” that may or may not be topped by CWC.
Species Data
The two scleractinian species L. pertusa and M. oculata were considered in this study, with no distinction between the two species as they were observed frequently interlaced one within the other (Mastrototaro et al., 2010; Arnaud-Haond et al., in press). They were considered globally as CWC to study their regional distribution in the SML province.
Presence data points were collected during different oceanographic cruises. They were obtained from video films recorded during underwater inspections with Remotely Operated Vehicles (ROVs) and specimens sampled using box-cores or grabs in the framework of a number of national and European research projects (Table 1).
All the coordinates of the physical samples were directly implemented in a GIS. They were collected in the eight main areas such as MS01, MS03, MS03 bis, MS04 (or Atlantis mound), MS04 bis, MS06, MS08, and Reef A (Figure 1C), which have already been described in the literature (Carlier et al., 2009; Malinverno et al., 2010; Mastrototaro et al., 2010; Rosso et al., 2010; Savini and Corselli, 2010; Savini et al., 2014).
All the videos showing living corals were recorded during three different cruises (Table 1). The videos from the MEDECO cruise were collected at two main stations: Reef A and MS04 (Atlantis mound; Carlier et al., 2009). The videos from the M70-1 cruise were collected at Reef A and around (Freiwald et al., 2009), whereas the videos from Aplabes 2005 cruise were collected at seven different sites including MS01, MS03, MS03bis, MS04, MS04bis, MS06, MS08, and other smaller mounds of the SML province. The georeferenced occurrence points extracted from the videos were plotted in a GIS using Adelie-GIS 2.3 and Adelie-Video 2.18 (©IFREMER) and the ArcGIS 10.2 software suite (©ESRI) depending on the format of data source.
A total of 22 mounds located in eight main areas are known coral mounds. Most of them were located in the center or in the eastern part of the SML province, within the Mass Transport Deposit (MTD) areas described by Savini et al. (2016). Some colonies were also observed in the western part of the central area next to a fault (MS08) and in the southern part (MS01; Figure 1C).
The position of the presence points was finally altered to meet the DTM resolution: CWC presence points were reduced to only one presence point per raster cell (40 × 40 m) or pixel to fit the resolution of the bathymetric raster in order to limit repetition and spatial autocorrelation in further analyses. The working dataset was composed of 228 pixels with CWC presence.
Seafloor Characteristics Derived from Bathymetry
Acoustic data were collected using a RESON SEABAT 8160 Multi Beam Echo Sounder (MBES) during the two main oceanographic cruises (the 2004 APLABES cruise and the 2010 CoralFISH cruise; Table 1). Post-processing of MBES data was carried out using dedicated software (Caris Hips and Sips 6.1) to produce a DTM. A total survey area of 2,000 km2 was covered by multibeam data with a cell size of 40 m resolution (Savini et al., 2014). The DTM was then considered only from −400 to −1,000 m depth (except inside the canyon where the maximum depth is 1,398 m) for further analysis. These values correspond to the shallower limit of the DTM that corresponds to the upper limit of the Adriatic Deep Water (Budillon et al., 2010) and to the maximum depth of CWC distribution in the Mediterranean Sea (Freiwald et al., 2009). This bathymetric water depth range corresponds to the CWC depth range previously observed in the SML Province (Budillon et al., 2010; Savini and Corselli, 2010; Savini et al., 2014). Moreover, the resolution of the bathymetric data decreases with depth and could not be considered below 1,000 m depth in our map.
A set of 13 variables was computed from the DTM, using the Benthic Terrain Modeler (BTM) tool (V.3.0) implemented with ArcGIS 10.2 software (Wright et al., 2005). These variables express different terrain attributes. The slope and the orientation, converted to Northness and Eastness (the cosine and sine of the angle of the slope direction, respectively), were selected for the study. These parameters are often used to approximate the physical processes that can interact between current and topography, sediment stability, seabed structural features, and seabed structural complexity, depending on the scale of analysis (Frederiksen et al., 1992; McArthur et al., 2010; Lecours et al., 2015). The topography of the seafloor was estimated with six Bathymetric position indices (BPI), with an inner radius window size of 1 and outer radius window sizes of 3, 9, 17, 25, 33, and 65 pixels, following a Fibonacci sequence as suggested by Wilson et al. (2007), corresponding to resolutions of 120, 360, 680, 1,000, 1,320, and 2,600 m, respectively. The BPI highlights whether any particular pixel forms topographic features of crests (positive values) or hollows (negative values) within a neighborhood to capture smaller or larger terrain features as a function of window size (Vierod et al., 2014), whereas broad scale BPIs were chosen for analysis, as they are good parameters for capturing terrain variations, and the other indices were computed when different window sizes were required at 3, 5, and 11 surrounding pixels (120, 200, and 440 m resolution, respectively). Window sizes larger than those values smoothed topographic features too much (null variance). Terrain roughness was considered through two indices computed on the BTM. Terrain ruggedness, also called Vector Ruggedness Measure (VRM), which quantifies terrain ruggedness by measuring the dispersion of vectors orthogonal to the terrain surface, was computed with pixel window sizes of 3, 5, and 11 (120, 200, and 440 m resolution, respectively). VRM combines the variability of slope and aspect into a single measure, without being over-correlated to the slope values. The VRM-values are low in both flat and steep areas, but the values are high in areas that are both steep and rugged. The VRM-values range from 0, low values coding for flat terrain or a steep area, to 1 (terrain that is both steep and rugged; Hobson and Chorley, 1972; Sappington et al., 2007). The Surface Area to Planar Area index, which is the ratio of the surface area to the flat area across the neighborhood of a central pixel, was added to the previous indices. The terrain curvature was assessed with the general curvature index (Curv.), defining whether a surface is concave (negative values) or convex (positive values), the profile curvature index (Prof. Curv., parallel to the direction of the maximum slope), and the longitudinal curvature index (Plan Curv., perpendicular to the direction of the maximum slope). These indices may also provide an indication of water interactions with the seafloor, illustrating how water converges or diverges as it flows over the relief (Vierod et al., 2014).
A set of nine additional predictors were computed using the System for Automated Geoscientific Analysis (SAGA, V. 2.1.4), a free open source Geographic Information System (GIS) software application (Conrad et al., 2015). The Local Convexity Index (CX) and the Convergence Index (CI) (both calculated at 3, 5, and 11 pixel window size as mentioned previously) were also computed and used to express the terrain curvature. The CX compares a central cell to the surrounding cells to identify the positive surface curvature (convex surfaces), in percentage of convex-upward cells within a constant radius (Iwahashi and Pike, 2007). The CI, similar to a plane or horizontal curvature with smoother results, highlights topographic features like ridges and channels. This index is based on the terrain aspect to analyze how divergent or convergent the surrounding cells are from this central cell (Conrad et al., 2015). Finally, the terrain roughness was also considered with the Terrain Surface Texture (TEX) (at a window of 3, 5 and, 11 pixels). TEX measures the frequency of peaks and pits, representing terrain “texture” or “grain” (Iwahashi and Pike, 2007). The number of pits and peaks in the specified window size is counted.
Statistical Analyses
The first selection of variables was carried out to prevent the use of correlated variables in the study, as this can lead to problems of parameter identifiability. Correlations between all possible pairs of variables were tested using Spearman's rho coefficient. All pairs with a rho coefficient exceeding 0.6 were considered too correlated to be kept together in the predictive methods. Spearman rank correlations and dendrograms were obtained using the Ade4 package, V.1.7-6 (Dray and Dufour, 2007) on R freeware (R Core team, 2014) and XLSTAT 2015 for Excel (Copyright© 2015 Addinsoft). In order to select the set of non-correlated variables to be used in further analysis, an initial Maxent model (see section Maxent Model Method) was computed with no a priori choice of variables (all the variables included in the model). Variables with the highest explanatory power were sorted in each group of correlated variables previously identified and kept for further analysis.
Boxplots of the non-correlated predictive variable selected were, thus, drawn using OriginPro 2016 (Copyright© 1991–2015 OriginLab Corporation) to study the environmental conditions of the polygons of known coral mounds, and further define the candidate morphologies and the potential coral mounds obtained with the two following statistical methods.
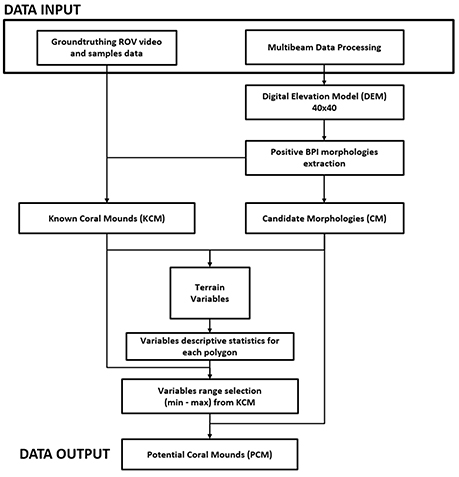
Extraction of Candidate Morphologies
In order to extract elevated features that could be representative of coral mounds, we used a fine scale BPI with a window size of 1 pixel for the inner radius and 3 pixels for the outer radius (BPI 3), corresponding to 120 m. Cells with a BPI3-value > 1 were selected as possible candidate morphologies for further analysis. Cells with a BPI3-value between 1 and 0 were considered as too flat or having over-gentle slope morphologies to be a mound, whereas those with negative BPI3-values were considered as having concave (holes) morphologies (Verfaillie et al., 2007). Each group of a minimum of four adjacent pixels with a BPI3 > 1 was converted into a polygonal feature. The dataset was composed of 7,447 polygonal features called candidate morphologies.
Geomorphometric Proxies Method
The Geomorphometric proxies method was specifically chosen to identify the seafloor morphologies associated with the presence of coral mounds (i.e., CWC habitats) as defined by Marchese (2016). Terrain variables, previously selected with Spearman's rank correlation, were considered for each candidate morphology (see section Extraction of Candidate Morphologies). The descriptive statistics of each variable [mean, standard deviation (s.d.), maximum (max), and minimum (min)] of each of the 7,447 candidate morphologies (composed of several pixels) were calculated as were those of the 22 known coral mounds. The minimum and the maximum values for each variable characterizing the 22 known coral mounds were chosen as references (Table 2). The statistics of each variable from each of the 7,447 candidate morphologies were compared to these references. Each candidate morphology having all its variable values within these ranges was tagged as a potential coral mound.
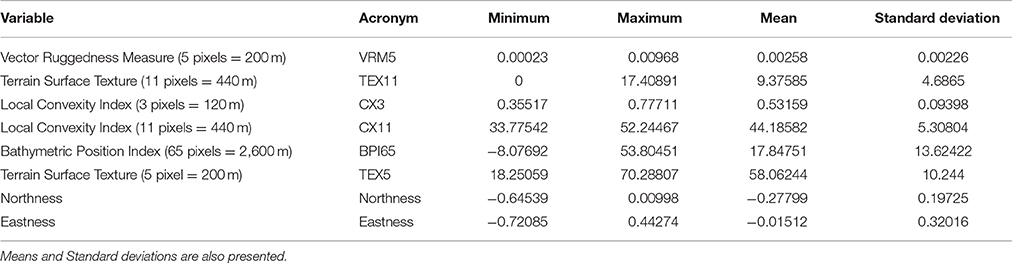
Table 2. Range of values (Minimum and Maximum) used for the selection of the potential coral mounds in the Geomorphometric proxies method deriving from the 22 known coral mound variables.
In brief, the potential coral mound polygons were identified by selecting elevated features with the same range of values, for each variable of the known coral mound polygons with the use of attribute extraction tool in ArcGIS™ (Figure 2).
Maxent Model Method
A HSM was built with the free Maxent software package, version 3.33 (https://www.cs.princeton.edu/~schapire/maxent/, Elith et al., 2006). The Maxent model, a general-purpose machine-learning method aimed at estimating a target probability distribution, by finding the probability distribution of maximum entropy (i.e., that which is the most spread out, or closest to uniform), has been found to surpass other statistical models in assessing species/habitat distribution with presence-only data (Elith et al., 2006; Tittensor et al., 2009). The Maxent model gives good results even with small sample sizes (Pearson et al., 2007; Wisz et al., 2008). It has been frequently used for predictive CWC habitat mapping (Tittensor et al., 2009; O'Hara and Tittensor, 2010; Davies and Guinotte, 2011; Howell et al., 2011; Rengstorf et al., 2012; Brooke and Ross, 2014; Vierod et al., 2014). This method uses the CWC observations and the set of environmental conditions to identify the distribution that conforms to everything known about the distribution of the habitat without making any postulations about what has not already been observed. It also predicts the distribution of the habitat in terms of probability of suitability for the species distribution according to a set of constraints that represent the incomplete information on the target distribution. Maxent formalizes the principle that the estimated distribution must correspond with everything that is known but should avoid introducing any unfounded constraints (Phillips et al., 2004; Elith et al., 2006, 2011). The subset of non-correlated variables composed of the preselected bathymetric indices was used to improve the accuracy and predictive power of the model (Guisan and Zimmermann, 2000; Elith and Leathwick, 2009; Brooke and Ross, 2014; Miller et al., 2015). The default settings were applied (a convergence threshold of 10−5, 500 maximum iterations, 10,000 maximum background points, a regularization multiplier of 1 and a default prevalence of 0.5), as they have proved efficient in many studies (Davies and Guinotte, 2011; Brown et al., 2012; Bentlage et al., 2013; Anderson et al., 2016). However, after visually inspecting the response curves and numerous trials, the regularization multiplier was finally set to 3 to reduce over-fitting, and the prevalence was increased to 0.7 (i.e., the probability of presence at ordinary occurrence points), to calibrate the model toward higher habitat suitability in known mound areas (the sampling stations were not randomly chosen but selected according to previous fishery surveys, described by Taviani et al., 2005b). The selected feature class (transformation of the environmental variables expressing the constraints) was “Hinge only,” which allowed a change in the gradient of the response. This setting provides more succinct approximations of the species' true distribution probability with high predictive power and less complexity in the model (Phillips and Dudik, 2008; Elith et al., 2011). Finally, the logistic outputs, which illustrate the suitability of each pixel of the area from 0 (lowest suitability) to 1 (highest suitability), was selected.
The contribution of each variable to the HSM was also determined. Jackknife plots and variable response curves were, thus, processed to assess the importance of each variable. Jackknife tests compare the predictive performance of the model with only one of each variable and then with all the variables except the variable tested first. The permutation importance values were also considered. These measures assess the contribution for each variable by randomly permuting its values among the training points and measuring the resulting decrease of the training Area Under the receive operator Curve (AUC) (normalized to give a percentage). The AUC-value, ranging from 0 to 1, indicates how well the model fits the data: a test AUC below 0.5 means that the model is no better than random, an AUC of 1 means the model is ideal, whereas an AUC higher than 0.7 can be considered as appropriate (Elith et al., 2011).
Cross-validation was also used to assess the model. Presence data were randomly partitioned into 10 replicates to compare the training datasets and testing datasets for model validation. Thus, nine of the subsamples were used to calibrate the model, whereas the last one was used for validation (around 205 points to run and 23 points for testing). The process was replicated 10 times to use all the subsets as test samples. The global mean AUC of the 10 replicates was computed. The gain value, an estimation of how close the model is to the test samples [if the gain is 2, then it means that the average likelihood of the presence samples is exp(2) ≈7.4 times higher than that of a random background pixel (http://www.cs.princeton.edu/~schapire/maxent)], was also noted as a measure of goodness of fit.
The mean model of the 10 replicates was then applied for habitat suitability mapping. The threshold of the maximum sum of sensitivity (true positive rate) plus the specificity (true negative rate) values (Zweig and Campbell, 1993; Fielding and Bell, 1997) was used to obtain binary maps. This is equivalent to finding a point on the ROC curve whose tangent slope is equal to 1 (Zweig and Campbell, 1993; Fielding and Bell, 1997). This type of reasoned threshold usually gives better results than an arbitrary-fixed threshold approach (Liu et al., 2005). Grid cells with a predicted habitat suitability value above the defined threshold were considered suitable for corals, whereas grid cells with a value below this threshold were considered unsuitable. Suitable areas above the selected threshold were converted into polygons for comparison with the Geomorphometric proxies method. Then, the candidate morphologies intersecting Maxent polygons were considered as potential coral mounds.
The correct classification rate is the percentage of pixels classified well by a model. The correct classification rate of the presence points was computed for the Maxent model by comparing all the presence pixels to the map of potential coral mounds produced with the Maxent model.
Results
Cold-Water Coral Occurrences
The 228 pixels with CWC presence were located between 490 and 815 m depth. All of them were used for the Maxent model, whereas only 106 pixels located inside the candidate morphologies were considered for the Geomorphometric proxies method.
Selection of Non-correlated Seafloor Characteristics
Among the 25 seafloor characteristics computed from the DTM, only the eight non-correlated ones were kept for further analysis (Appendix A in Supplementary Materials). The Eastness and Northness were selected. Regarding the topography, BPI 65 was conserved. The terrain curvature was summarized to CX3 and CX11. Finally, VRM5, Tex5, and Tex11 were chosen to render the terrain roughness. The other indices were over-correlated with each other (Appendix A in Supplementary Materials).
Comparison of Seafloor Characteristics for Known Coral Mounds and Candidate Morphologies
The eight non-correlated seafloor characteristics from the 22 known coral mounds were compared to those from the 7,447 candidate morphology polygons using boxplots (Figure 3). Regarding roughness indices, VRM5, BPI65, Tex5, and Tex11 found on the known coral mounds have higher values than those on the candidate morphologies. As for the terrain curvatures, CX3 and CX11 of known coral mounds and candidates morphologies do not show much difference in their first quartiles (Figure 3). With regards to orientation, Northness was negative on known coral mounds as well as on candidate morphologies and Eastness was negative for more than half of the pixels, which indicates that most part of the pixels are oriented toward the south-western slopes, for both kinds of elevated features (Figure 3).
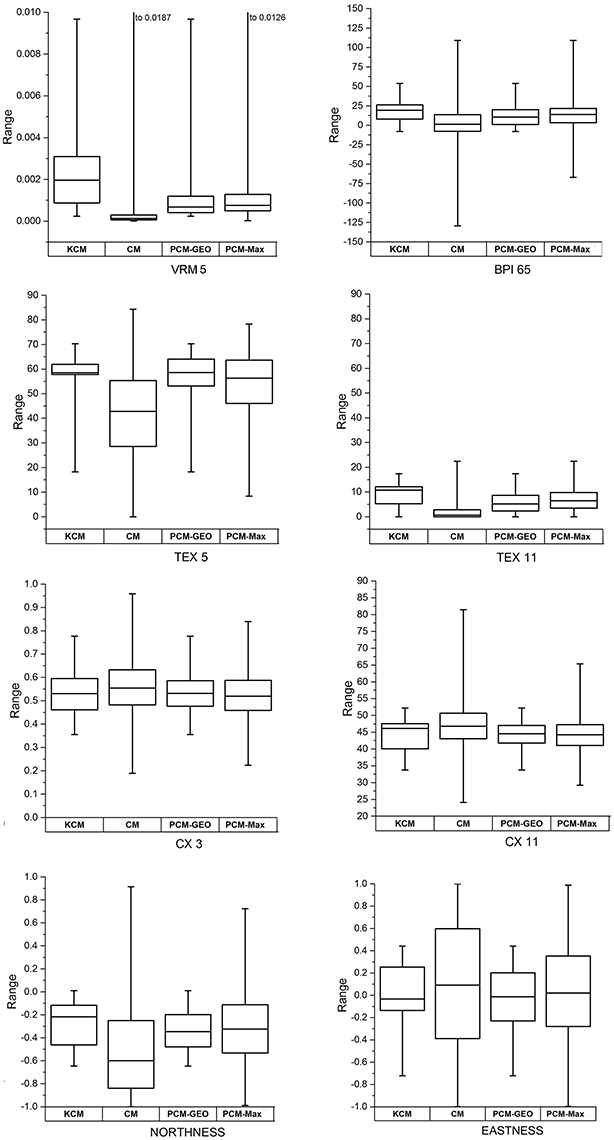
Figure 3. Boxplots of eight non-correlated bathymetric indices used in the study, computed on polygons of known coral mounds (KCM), candidate morphologies (CM), probable coral mounds obtained with the Geomorphometric proxies method (PCM-Geom) and probable coral mounds obtained with the Maxent method (PCM-Maxent).
Identification of Potential Coral Mounds with Geomorphometric Proxies
The Geomorphometric proxies method identified 736 mounds (around 10%) that are defined as potential coral mounds among the 7,447 candidate morphologies (Figure 4). The ranges of values of the eight non-correlated variables computed for the 22 known coral mound polygons and used for the selection of the potential coral mounds in this method are shown in Table 2.
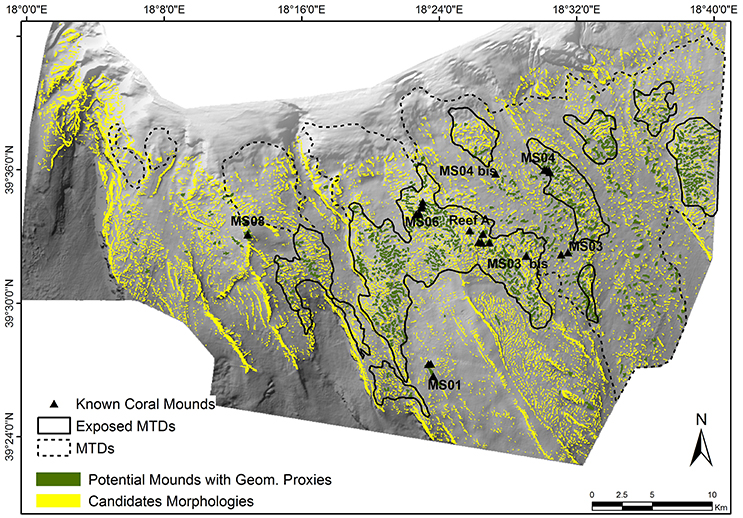
Figure 4. Distribution map of two sets of mound-like features in the SML Province: In yellow the 7,447 candidate morphologies [BPI (3 pixels) > 1] and in green the 736 potential coral mounds identified with the Geomorphometric proxies method.
A considerable proportion of potential coral mounds, about 90%, show a spatial distribution strictly linked to the main MTD areas described by Savini et al. (2016) along the slope in the central and eastern parts of the area investigated (Figure 4).
Maxent Modeling
Model Evaluation
The model performed well using cross-validation and was, thus, significantly better than random, with a mean AUC-value of 0.95 for the 10 replicates (s.d. = 0.01), and a test gain at 2.25, meaning that the average likelihood of the presence samples was 9.49 [exp(2.25)] times higher than that of a random background pixel.
Assessment of Variable Importance within the Model
Terrain roughness generally emerged as the most important factor explaining coral distribution in the SML Province. Firstly, VRM5 appeared to be the most important variable taken into account for Maxent model. Its contribution was 53% in the final Maxent model (Table 3). When looking at the Jackknife results, excluding this variable from the Maxent model significantly decreased the training and test gains, and to a lesser extent the AUC-value (Appendix B in Supplementary Materials). Permutation tests also showed the importance of this variable in the model with a decrease of 35.3%, meaning that the model also depended heavily on this parameter (www.cs.princeton.edu/~schapire/maxent). Tex11 was the second variable explaining coral distribution (31.5% contribution to the final Maxent model, Table 3). The third variable in order of importance for the model was BPI65 (10.1% contribution), whereas CX11, Tex5, CX3, Eastness, and Northness combined explained <6% of the final model (Table 3). The orientation indices (Eastness and Northness) were close to zero. The Jackknife tests provided approximately the same results with a low gain or AUC-values for those variables taken individually. This was also the case for CX3 (Appendix B in Supplementary Materials).
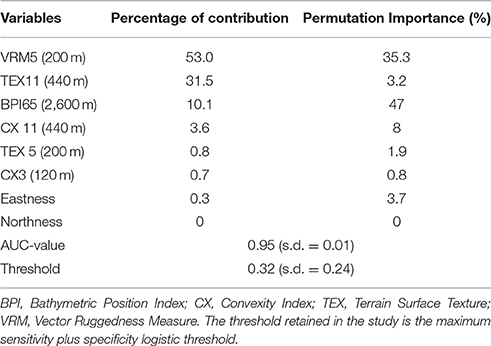
Table 3. Percentage of contribution and permutation importance (in percent) for each variable used in the final Maxent model applied for Cold-Water coral in the SML Province.
Predicted Distribution with the Maxent Model
The Maximum test sensitivity plus specificity logistic threshold obtained was equal to 0.32 (s.d. = 0.17) for Maxent.
When looking at the logistic output map, the highest values obtained were in the central part around the MS-06, Reef-A, and MS-03 sites, as well as around the MS-04 site and along the western canyon (Figure 5A). With a threshold of 0.32, this model allowed identifying 924 suitable polygons for coral settlements. The 924 suitable polygons overlapped several candidate morphologies identified as potential coral mounds for a total of 1,252 (Table 4). These 1,252 mounds were mostly located within the MTD area described by Savini et al. (2016) and along the major crests of the western part of the SML area (Figure 5B).
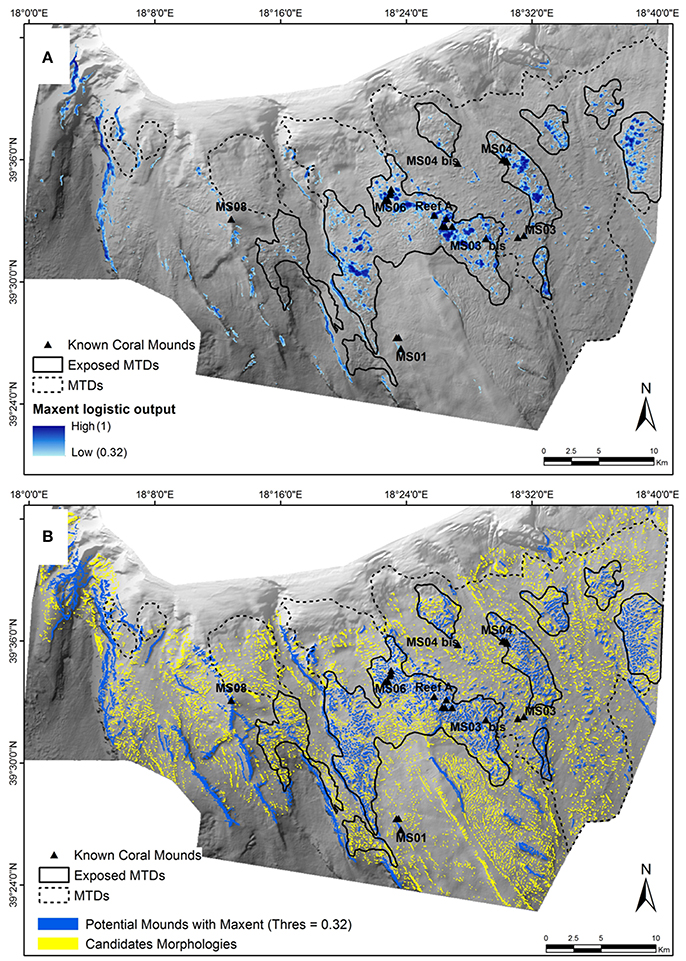
Figure 5. Maps of predicted distributions for Cold-Water Corals in the SML Province using the Maxent method. (A) Distribution of probable coral presence pixels determined by a threshold of logistic outputs of 0.32. (B) Polygons of the 7,447 candidate morphologies [BPI (3 pixels) > 1; in yellow] and the 1,252 potential coral mounds (in blue) intersecting the Maxent polygons obtained with a logistic threshold of 0.32.
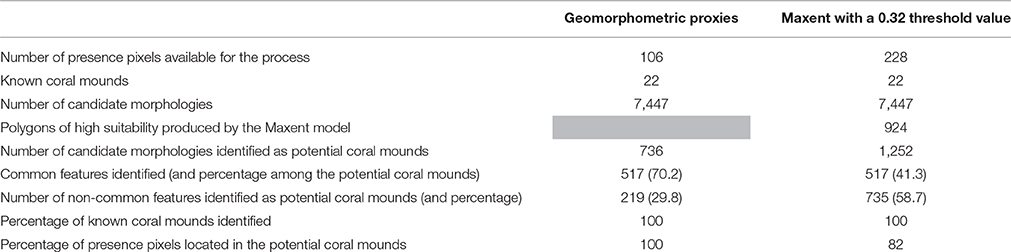
Table 4. Counts of potential coral mounds identified by Geomorphometric proxies and Maxent Method applied on the entire SML area.
The correct classification rate obtained for the known coral mounds was 100% (Table 4). All of them were, therefore, identified as potential coral mounds by the Maxent model. However, we observed that if we had considered a higher logistic threshold, then we would have removed more known coral mounds from the set of suitable predicted ones. Indeed, as can be seen in Figure 5, the known coral mounds at the MS-01 and MS-04 bis sites have logistic threshold values close to 0.32.
Concerning the correct classification rate obtained for the presence points, only 82% of them were well classified (Table 4). These presence points were located between elevated features and not on them. Therefore, they were not located on candidate morphologies and even less on a potential coral mound.
Comparison of Methods
Terrain Attributes Associated with Potential Coral Mounds
The values of the eight non-correlated seafloor characteristics of the 736 potential coral mounds identified by the Geomorphometric proxies method on the one hand and those of the 1,252 mounds identified by the Maxent method on the other hand were compared using boxplots (Figure 3). Globally, the distribution of the values (median and first quartiles) of each variable for the two sets of mounds is similar, except for the roughness variable TEX5 for which the median is similar but the first quartile includes lower values with the Maxent approach (Figure 3). Northness and Eastness are more widely distributed around the median with the Maxent method.
Counts of Potential Coral Mounds
The combination of both methods identified a set of 517 features as potential coral mounds (Table 4). These 517 common features represent 70% of the potential coral mounds identified by the Geomorphometric method but only 41% of those were identified by Maxent.
Distribution of Potential Coral Mounds
Most of the 517 common potential coral mounds are located within the MTD areas described by Savini et al. (2016), within the partially exposed areas and at the MS01 and MS08 sites (Figure 6).
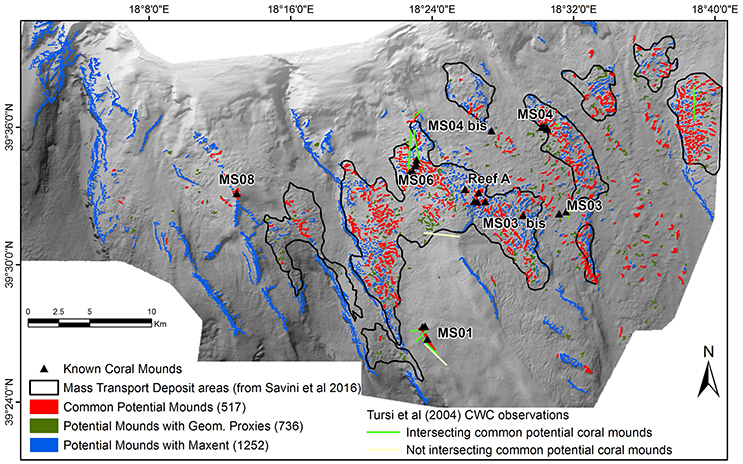
Figure 6. Location of potential coral mounds estimated statistically using the Geomorphometric proxies methods (in dark green) and the Maxent method (in blue). The 517 common potential coral mounds (in red) represent 70.2% of the Geomorphometric proxies and 41.3% of the Maxent features. Mass Transport Deposit areas described by Savini et al. (2016) are delineated by black lines and hauls from Tursi et al. (2004) holding CWC are shown by yellow and green lines.
The potential coral mounds identified only by the Geomorphometric proxies method are mainly distributed inside the global MTD areas, whereas those selected only by the Maxent method are widely distributed both inside and outside the MTD areas especially along the faults and ridges that are not mound-like features but large elevated areas (Figure 6).
Discussion
Identification of Candidate Morphologies
The first step of the approach, represented by the selection of the BPI-value that allowed extracting all the elevated features, was fundamental for representing the distinct mound-like features of the whole DTM and guided the subsequent statistical analysis. These candidate morphologies were highlighted by the BPI at small scale (3 pixels = 120 m resolution). Different scales were, however, also processed but showed that the algorithm's efficiency decreased in detecting typical mound-like features of the surveyed area as the neighboring window size increased.
This decrease in efficiency was due to the size of the mound-like features themselves, as they rarely exceeded 300 m in width and 25 m in height (Savini and Corselli, 2010). These structures were evidenced with a BPI on 3 pixels (120 × 120 m). A higher window size BPI smoothed too many features.
Validations of Geomorphometric Proxies and the Maxent Method
In this study, the AUC-values used for the Maxent method were very high (0.95 ± s.d. 0.01), which indicates that the model predicts suitability areas in the SML Province with good reliability. However, the AUC-value has already been criticized (Austin, 2007; Lobo et al., 2008). As the calibration and the tested data were chosen randomly by the software, they may have been too close to each other and not truly independent, possibly over-estimating the AUC-value (Radosavljevic and Anderson, 2014). However, to avoid possible autocorrelation, the models were run using only one randomly chosen occurrence point for each candidate morphology (22 occurrence points). The results decreased the AUC-value to 0.92, which was still a high value. It also showed the same variable order in the model contribution, with approximately the same order of magnitude. This model presented a small increase in the number of potential coral mounds, which were, however, mostly located at the same place. Nevertheless, the AUC-value is not a sufficient test for model assessment, as proved by Elith et al. (2006), who found different model results with the same AUC-values. The gain obtained in our study was also used for evaluating Maxent. The high gain values obtained suggested that the model performed much better than random. Moreover, the 100% correct classification rates and the selection of all the known coral mounds as potential coral mounds by the Maxent model suggested that this method was consistent.
A more rigorous evaluation will be to test the model as well as the Geomorphometric proxy results with future observations ideally collected at sites other than those used for this study, and if available, absence data in order to compute omission and commission errors as defined by Pearson (2008). In our case, absence data from already existing dives were geographically too close to our presence data to be used in this study.
A study by Tursi et al. (2004) reported 14 occurrences of L. pertusa and/or M. oculata at SML collected from hauls (either bottom trawls or from a home-made iron bar with pieces of old fishing net attached). We compared suitable areas predicted in our study to their observations (Figure 6). Only one of their hauls (n°7) was not located on one of our potential coral mounds (at MS01), and a second haul (n°23) was located on a potential coral mound defined by Maxent but not by the Geomorphemetric proxies method (in the middle SML area, outside a MTD area). This comparison emphasizes the good results obtained by both methods.
Identification of Potential Coral Mounds with the Geomorphometric Proxies Method
The seafloor characteristics used to identify potential coral mounds are directly linked to the DTM resolution (40 m) and to the pixel window analysis size of the geomorphometric variables. The final result allowed us to identify 736 polygons as potential coral mounds, each one selected as an individual feature with its own morphometric properties. Their location (as observed in Figure 4) is definitely more concentrated in some distinct areas of the eastern and central sector of the whole DTM, between 500 and 900 m depth, where extensive mass transport deposits shape the seafloor downslope of a series of arcuate-shaped scars that mark the upper slope at the transition zone of the continental shelf. A description has already been provided of how the complex irregular topography formed by failure events prior to coral settlement created suitable substrates for coral colonization (Taviani et al., 2005b; Savini et al., 2016), especially due to the generation of blocks of partially or completely lithified sediments and/or extensional ridges, forming small-scale elevated locations swept by local bottom currents. From the initial settlement and up to the present time, coral growth also sustained by sediment trapping during accretion (Vertino et al., 2010) has enhanced the small-scale morphology of these sediment blocks which are preferred sites for coral colonization, with the creation of coral-mounds (Savini et al., 2016). Their morphometric parameters present high values for BPI, VRM, and Texture measures in comparison to those characterizing the candidate morphologies (Figure 3). In addition, when observing the results at a broader scale, coral-mounds were not predicted as homogeneously shaped by mass transport deposits over the whole area. Their distribution is instead represented by clusters of mounds (in which known coral-mounds are included—Figure 4) identified in those areas located at the top of regional high points, which likely create regional obstacles to local currents, thereby steering their intensity and direction, and creating suitable environmental conditions for coral growth.
On the contrary, mounds (i.e., sediment blocks) located in more depressed areas were not selected as potential coral mounds. This result is consistent with the higher sediment input to which they are subjected due to sediments bypassing from the continental shelf and the basin-like environment in which they are located, where pelagic/hemipelagic sedimentation and gravity-driven sedimentary fluxes from elevated neighboring areas likely prevent coral-growth, producing mounds with lower reliefs (i.e., low BPI-values), a smoothed morphology (i.e., low terrain and VRMs), and much further part from each other.
Identification of Potential Coral Mounds with Maxent
In habitat suitability mapping studies, choosing a threshold for the probability of suitability is always a key issue. In this study, we used the objective maximum sensitivity plus the specificity threshold value (threshold of 0.32). However, if we consider an arbitrary higher threshold value instead, we observe a decrease in the number of potential coral mounds and thus a more restrictive distribution map. The choice of the threshold is inherently linked to the aims of the analysis (Pearson, 2008). The objective threshold chosen in this study is adequate for comparing both the Geomorphometric method and the Maxent method, whereas the identification of potential coral mounds intended for future dives or for protection measures could use a higher threshold in order to target the more probable suitable mounds. The threshold would have been more accurate if we had used true absence points instead of background points; however, the true absence points available in this study were not used because they were too close to the presence points.
Potential coral mounds identified by the selection of candidate morphologies that intersected Maxent polygons are widely distributed across the area (Figure 5B). Nevertheless, when looking at the distribution map of suitable pixels only (Figure 5A), it is obvious that highly suitable pixels are located inside the MTD areas. The selection of candidate morphologies intersecting Maxent polygons introduced a bias in the results by selecting entire candidate morphologies, although only few pixels had a logistic output higher than 0.32. This can be seen in the western part of the map, where very few pixels have a logistic output threshold of 0.32 (or higher) but the potential coral mounds were defined as large polygons (the entire candidate morphology). If we had used a higher threshold value, then we would have reduced this bias, although not totally. Furthermore, selecting the potential coral mounds instead of selecting the suitable polygons identified by the model considerably increased the total number of probable suitable pixels (from 54,005 to 58,655). This procedure was, however, necessary to compare the potential coral mounds identified by the Geomorphometric method to that selected by the Maxent model.
Using the Maxent method on its own without the first selection of elevated features (BPI3 > 1) would give different results. We tested this and it appeared that if BPI3 had been introduced as a variable in a Maxent model, then its contribution would have been almost null anyway. This means that using BPI3 to pre-select candidate mounds as we did in this study, instead of using it as a variable to build a model, did not penalize our final model.
Some sporadic occurrence points were located outside the suitable areas identified by the Maxent model. They were not located on the candidate morphologies, but between mounds. Thus, they might not have been selected on the final potential coral mounds, resulting in a reduction of the correct classification rate. However, some sparse CWC colonies have already been described living between mounds and not on elevated features, despite the fact these are not their most favorable environments (climax, i.e., at the top and north-eastern flank of single mounds; see Rosso et al., 2010; Vertino et al., 2010; Savini et al., 2014 for a more detailed description of coral distribution over single mounds and inter-mound areas).
Seafloor Characteristics for CWC Settlement
Seafloor characteristics were derived from the DTM. The resolution used was 40 m, which is considered relatively high compared to the resolution used in other studies on coral mounds, i.e., from 50 m in the Galicia Bank (Somoza et al., 2014) to more than 1,000 m at the global or regional scale (Clark et al., 2006; Davies et al., 2008). A HSM of at least 250 m resolution was determined as crucial for detecting topographical features associated with CWC (Rengstorf et al., 2012). The resolution of our DTM provided us with a suitable scale of analysis to focus on coral mounds in SML, with a wide range of terrain attributes (or seafloor characteristics) describing at a scale ranging from 120 (3 pixels) to 2,600 (65 pixels) m.
The potential coral mounds were predicted to be located between 408 and 998 m depth, but our study was limited to a depth of 1,000 m. On the basis of hydroacoustic data and gravity core investigations, Fusi et al. (2006) hypothesized that the lower limit of probable coral mound occurrences could be extended downwards to about 1,600-m water depth (in Freiwald et al., 2009). An extended analysis deeper in the area may lead to the identification of new suitable areas for CWC, but to do this, the dataset must be completed by a deep exploration.
Our analysis provides valuable information on CWC preferences. The results show that VRM5 (200 m resolution) was the most important explanatory variable (53%) for mapping probable suitable areas for CWC in the SML Province. Roughness indices, frequently used as a proxy for hard bottom substrates (Dunn and Halpin, 2009), emphasize the fact that the CWC studied needed hard bottoms or firm grounds to settle, as mentioned by Taviani et al. (2011), Rosso et al. (2010), and Savini et al. (2014). Furthermore, in the SML province, CWC have been observed to enhance seafloor roughness due to their specific growth leading to 3-D structures (Savini et al., 2016). This parameter could, therefore, be both a cause and a consequence of coral presence, and it is a good proxy for assessing coral settlement. The high contribution (30%) of the TEX11 variable (440 m resolution) to the Maxent model confirmed these previous results. It probably contributed to the selection of large suitable areas in the SML province, either on the canyon crests or in the MTD areas. Finally, the low participation of the other variables in the final model means that they were not essential indices for explaining coral distribution in the SML province at this scale of analysis.
The Geomorphometric Proxies Method vs. the Maxent Method
Both these methods built predictions based on the seafloor characteristics of the area studied. The Geormophometric proxies method requires the preliminary delimitation of mounds in order to better select individual mounds, whereas the Maxent method allows obtaining a continuous map of suitable habitats in the entire SML Province, and not only of mound morphologies. Nevertheless, we chose to first select candidate morphologies and use them in both methods to focus on individual mounds and identify a common set of 517 potential coral mounds. Both methods used in our study allowed refining the potential coral mound identification performed previously by Savini et al. (2014), who identified 1,902 mounds as potential coral mounds. The two methods used in our study highlighted a distribution of potential coral mounds characterized by clusters, inside the MTD areas themselves, where the VRM 5 and TXT11 present the highest values (at the top of regional highs), and where the topography probably modifies the bottom currents and creates suitable areas for CWC.
In Savini et al. (2014), although the majority of potential coral mounds were located in the exposed MTD areas, the others were spread over the entire Province, including the western area. The Geomorphometric proxies method that was used in the current study was that closest to the method used by Savini et al. (2014) and gave the most comparable results, whereas the Maxent model did not identify the smallest patchy mounds in the western area but selected large areas of topographic highs along ridges. However, these results were influenced by the pre-selection of candidate morphologies. On examining the habitat suitability map produced by the Maxent model alone (Figure 5A), the Maxent method was more selective and the majority of pixels with probable high suitability were located in the exposed MTD areas, around the known coral locations MS06, reef A, MS03bis, and MS04 areas. The Maxent method classified the explanatory variables and gave a weight to all of them to build a model, whereas the Geomorphometric proxies method considered all the seafloor characteristics equally to select the potential coral mounds.
Furthermore, both the Geomorphometric method and the method used in Savini et al. (2014) were specifically designed for the study area, whereas the Maxent model built in this study could potentially be used and transposed in another similar area of the Mediterranean Sea where no coral sampling has been performed, provided that the same type of data (DTM and resolution of seafloor characteristics) are available, which is one of the interesting features of a HSM like Maxent.
Conclusion
Potential coral mounds favorable to CWC settlement were identified in the SML Province using two methods. Before applying these two methods, it was first necessary to select all the elevated features in the study area. Then, the geomorphometric proxies method was used to identify the most suitable single geomorphometric elements associated with CWC settlement (i.e., mound-shaped seafloor morphologies), whereas the statistical model built with the Maxent method predicted the most suitable areas according to the terrain attributes used in the model. In our study, the first method (Geomorphometric proxies) was useful for selecting beforehand candidate morphologies on which the Maxent method was used, and it proved to be an efficient method for identifying important drivers for CWC settlement. Contrary to the Geomorphometric proxies method, which is strictly based on dedicated terrain attributes and dedicated to the area, the Maxent model could be further improved for instance through the use of hydrodynamic factors, when available.
Both these methods highlighted two main areas in the SML Province: (1) the Eastern part of the Province, where the more extensive MTD areas described by Savini et al. (2016) are located, and where the majority of potential coral mounds have been predicted; (2) the Western area shaped by a canyon and several scarps and ridges and where only few potential coral mounds were predicted by both methods.
Nevertheless, the high probability of the presence of more than 500 potential coral mounds in the SML area testifies to the importance of this province for the Mediterranean scleractinian CWC, particularly in the MTD areas already identified. Multiple spatial conservation plans have, thus, been developed in the Mediterranean Sea in recent years and the SML province is among those on which strong consensus has been reached regarding conservation priorities, with more than six conservation initiatives (e.g., Specially Protected Areas of Mediterranean Importance, Marine Protected Area or Natura 2000 site) (Micheli et al., 2013). The probable large distribution of coral mounds predicted in this study should encourage the European Commission and the Italian government to continue these protection measures on this site of rich biodiversity.
Author Contributions
AB and FM performed and designed the methods. AB wrote and coordinated the manuscript. FM wrote the geomorphological proxies section. MF wrote, coordinated and supervised the manuscript and act as corresponding author. All authors contributed substantially to the discussion of the results and revision of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This paper was written in the framework of the Marine Strategy Directive (MSFD). The Habitat Suitability model study was part of a post-doctoral grant funded by the “Agence de l'Eau Rhône Méditerranée & Corse” under Convention Number 2015 0348, Ifremer and ISMAR Bologna. We are grateful to all the participants and the P.I. of the Aplabes 2004; Aplabes 2005, HERMES M70-1, MEDECO 2007 (http://dx.doi.org/10.17600/7030090) and magic CoralFISH 2010 cruises. The authors also benefited from EU FP7 project CoralFish (Grant agreement number: 213144—http://www.eu-fp7-coralfish.net), the Flag Project Ritmare (Ricerca Italiana per il Mare), funded by the Italian Ministry of Universities and Research (MIUR) and the ESF COCARDE Network. This paper is Ismar-Bologna scientific contribution no. 1908 and contributes to EU F.P. VII Projects COCONET, (contract no. 287844), and EVER-EST (contract no. 674907). FM was funded through a Ph. D. fellowship in Earth Sciences at the University of Milano-Bicocca. Furthermore we are indebted to Keith Hodson (http://www.accent-europe.fr) for correcting the English. The authors also thank the reviewers for their helpful comments to improve the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2017.00338/full#supplementary-material
References
Anderson, O. F., Guinotte, J. M., Rowden, A. A., Clark, M. R., Mormede, S., Davies, A. J., et al. (2016). Field validation of habitat suitability models for vulnerable marine ecosystems in the South Pacific Ocean: implications for the use of broad-scale models in fisheries management. Ocean Coast. Manage. 120, 110–126. doi: 10.1016/j.ocecoaman.2015.11.025
Arnaud-Haond, S., Van den Beld, I. M. J., Becheler, R., Orejas, C., Menot, L., Frank, N., et al. (in press). Two “pillars” of cold-water coral reefs along Atlantic European margins: prevalent association of Madrepora oculata with Lophelia pertusa, from reef to colony scale. Deep Sea Res. II Top. Stud. Oceanogr. doi: 10.1016/j.dsr2.2015.07.013
Austin, M. (2007). Species distribution models and ecological theory: a critical assessment and some possible new approaches. Ecol. Model. 200, 1–19. doi: 10.1016/j.ecolmodel.2006.07.005
Bentlage, B., Peterson, A. T., Barve, N., and Cartwright, P. (2013). Plumbing the depths: extending ecological niche modelling and species distribution modelling in three dimensions. Glob. Ecol. Biogeogr. 22, 952–961. doi: 10.1111/geb.12049
Brooke, S., and Ross, S. W. (2014). First observations of the cold-water coral Lophelia pertusa in mid-Atlantic canyons of the USA. Deep Sea Res II Top. Stud. Oceanogr. 104, 245–251. doi: 10.1016/j.dsr2.2013.06.011
Brown, C. J., Sameoto, J. A., and Smith, S. J. (2012). Multiple methods, maps, and management applications: purpose made seafloor maps in support of ocean management. J. Sea Res. 72, 1–13. doi: 10.1016/j.seares.2012.04.009
Budillon, G., Bue, N. L., Siena, G., and Spezie, G. (2010). Hydrographic characteristics of water masses and circulation in the Northern Ionian Sea. Deep Sea Res. II Top. Stud. Oceanogr. 57, 441–457. doi: 10.1016/j.dsr2.2009.08.017
Capezzuto, F., Maiorano, P., Panza, M., Indennidate, A., Sion, L., and D'Onghia, G. (2012). Occurrence and behaviour of Paromola cuvieri (Crustacea, Decapoda) in the Santa Maria di Leuca cold-water coral community (Mediterranean Sea). Deep Sea Res. I Oceanog. Res. Pap. 59, 1–7. doi: 10.1016/j.dsr.2011.10.006
Carlier, A., Le Guilloux, E., Olu, K., Sarrazin, J., Mastrototaro, F., Taviani, M., et al. (2009). Trophic relationships in a deep Mediterranean cold-water coral bank (Santa Maria di Leuca, Ionian Sea). Mar. Ecol. Prog. Ser. 397, 125–137. doi: 10.3354/meps08361
Clark, M., Tittensor, D., Rogers, A., Brewin, P., Schlacher, T., Rowden, A., et al. (2006). Seamounts, Deep-Sea Corals and Fisheries: Vulnerability of Deep-Sea Corals to Fishing on Seamounts Beyond Areas of National Jurisdiction. Cambridge, UK: UNEP-WCMC.
Conrad, O., Bechtel, B., Bock, M., Dietrich, H., Fischer, E., Gerlitz, L., et al. (2015). System for Automated Geoscientific Analyses (SAGA) v. 2.1.4. Geosci. Model Dev. 8, 1991–2007. doi: 10.5194/gmd-8-1991-2015
Costello, M. J. (2009). Distinguishing marine habitat classification concepts for ecological data management. Mar. Ecol. Prog. Ser. 397, 253–268. doi: 10.3354/meps08317
Costello, M. J., and Vanden Berghe, E. (2006). ‘Ocean biodiversity informatics’: a new era in marine biology research and management. Mar. Ecol. Prog. Ser. 316, 203–214. doi: 10.3354/meps316203
Davies, A. J., and Guinotte, J. M. (2011). Global habitat suitability for framework-forming cold-water corals. PLoS ONE 6:e18483. doi: 10.1371/journal.pone.0018483
Davies, A. J., Wisshak, M., Orr, J. C., and Roberts, J. M. (2008). Predicting suitable habitat for the cold-water coral Lophelia pertusa (Scleractinia). Deep Sea Res. I Oceanogr. Res. Pap. 55, 1048–1062. doi: 10.1016/j.dsr.2008.04.010
Dolan, M. F. J., Grehan, A. J., Guinan, J. C., and Brown, C. (2008). Modelling the local distribution of cold-water corals in relation to bathymetric variables: adding spatial context to deep-sea video data. Deep Sea Res. I Oceanogr. Res. Pap. 55, 1564–1579. doi: 10.1016/j.dsr.2008.06.010
D'Onghia, G., Indennidate, A., Giove, A., Savini, A., Capezzuto, F., Sion, L., et al. (2011). Distribution and behaviour of deep-sea benthopelagic fauna observed using towed cameras in the Santa Maria di Leuca cold-water coral province. Mar. Ecol. Progr. Ser. 443, 95–110. doi: 10.3354/meps09432
D'Onghia, G., Maiorano, P., Carlucci, R., Capezzuto, F., Carluccio, A., Tursi, A., et al. (2012). Comparing deep-sea fish fauna between coral and non-coral “Megahabitats” in the Santa Maria di Leuca cold-water coral Province (Mediterranean Sea). PLoS ONE 7:e44509. doi: 10.1371/journal.pone.0044509
D'Onghia, G., Maiorano, P., Sion, L., Giove, A., Capezzuto, F., Carlucci, R., et al. (2010b). Effects of deep-water coral banks on the abundance and size structure of the megafauna in the Mediterranean Sea. Deep Sea Res. II Top. Stud. Oceanogr. 57, 397–411. doi: 10.1016/j.dsr2.2009.08.022
Dray, S., and Dufour, A. B. (2007). The ade4 Package: implementing the duality diagram for ecologists. J. Stat. Softw. 22, 1–20. doi: 10.18637/jss.v022.i04
Dunn, D. C., and Halpin, P. N. (2009). Rugosity-based regional modeling of hard-bottom habitat. Mar. Ecol. Progr. Ser. 377, 1–11. doi: 10.3354/meps07839
Elith, J., and Leathwick, J. R. (2009). Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 40, 677–697. doi: 10.1146/annurev.ecolsys.110308.120159
Elith, J., Graham, C. H., Anderson, R. P., Dudik, M., Ferrier, S., Guisan, A., et al. (2006). Novel methods improve prediction of species' distributions from occurrence data. Ecography 29, 129–151. doi: 10.1111/j.2006.0906-7590.04596.x
Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E., and Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 17, 43–57. doi: 10.1111/j.1472-4642.2010.00725.x
Etiope, G., Savini, A., Lo Bue, N., Favali, P., and Corselli, C. (2010). Deep-sea survey for the detection of methane at the “Santa Maria di Leuca” cold-water coral mounds (Ionian Sea, South Italy). Deep Sea Res. II Top. Stud. Oceanogr. 57, 431–440. doi: 10.1016/j.dsr2.2009.08.020
Fabri, M. C., Pedel, L., Beuck, L., Galgani, F., Hebbeln, D., and Freiwald, A. (2014). Megafauna of the vulnerable marine ecosystems in French Mediterranean Submarine canyons: spatial distribution and anthropogenic impacts. Deep Sea Res. II Top. Stud. Oceanogr. 104, 184–207. doi: 10.1016/j.dsr2.2013.06.016
FAO (2009). Report of the Technical Consultation on International Guidelines for the Management of Dee-Sea Fisheries in the High Seas. Rome: FAO.
Fielding, A. H., and Bell, J. F. (1997). A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24, 38–49. doi: 10.1017/S0376892997000088
Fink, H. G., Wienberg, C., Hebbeln, D., McGregor, H. V., Schmiedl, G., Taviani, M., et al. (2012). Oxygen control on Holocene cold-water coral development in the eastern Mediterranean Sea. Deep Sea Res. I Oceanogr. Res. Pap. 62, 89–96. doi: 10.1016/j.dsr.2011.12.013
Frederiksen, R., Jensen, A., and Westerberg, H. (1992). The distribution of the scleractinian coral Lophelia pertusa around the Faroe islands and the relation to internal tidal mixing. Sarsia 77, 157–171. doi: 10.1080/00364827.1992.10413502
Freiwald, A., Beuck, L., Rüggeberg, A., Taviani, M., and Hebbeln, D. (2009). The white coral community in the Central Mediterranean Sea revealed by ROV Surveys. Oceanography 22, 58–74. doi: 10.5670/oceanog.2009.06
Freiwald, A., Fossa, J. H., Grehan, A., Koslow, J. A., and Roberts, J. M. (2004). Cold-water Coral Reefs: Out of Sight - No Longer Out of Mind. Cambridge, UK: UNEP-WCMC.
Fusi, N., Savini, A., and Corselli, C. (2006). Evidence of mud diapirism and coral colonies in the ionian sea (central mediterranean) from high resolution chirp sonar survey. Ann. Geophys. 49, 751–765. doi: 10.4401/ag-3128
GFCM (2009). Criteria for the Identification of Sensitive Habitats of Relevance for the Management of Priority Species. Malaga: General Fisheries Commission for the Mediterranean - Scientific Advisory Committee - Sub-Committee on Marine Environment and Ecosystems (SCMEE) Available online at: www.gfcm.org
Giusti, M., Innocenti, C., and Canese, S. (2014). Predicting suitable habitat for the gold coral Savalia savaglia (Bertoloni, 1819) (Cnidaria, Zoantharia) in the South Tyrrhenian Sea. Cont. Shelf Res. 81, 19–28. doi: 10.1016/j.csr.2014.03.011
Guinotte, J. M., Orr, J., Cairns, S., Freiwald, A., Morgan, L., and George, R. (2006). Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals? Front. Ecol. Environ. 4:141. doi: 10.1890/1540-9295(2006)004[0141:WHCISC]2.0.CO;2
Guisan, A., and Zimmermann, N. E. (2000). Predictive habitat distribution models in ecology. Ecol. Model. 135, 147–186. doi: 10.1016/S0304-3800(00)00354-9
Hobson, R. D., and Chorley, R. J. (1972). “Surface roughness in topography: a quantitative approach,” in Spatial Analysis in Geomorphology, ed R. J. Chorley (New York, NY: Methue & Co), 221–245.
Howell, K. L., Holt, R., Endrino, I. P., and Stewart, H. (2011). When the species is also a habitat: comparing the predictively modelled distributions of Lophelia pertusa and the reef habitat it forms. Biol. Conserv. 144, 2656–2665. doi: 10.1016/j.biocon.2011.07.025
Iwahashi, J., and Pike, R. J. (2007). Automated classifications of topography from DEMs by an unsupervised nested-means algorithm and a three-part geometric signature. Geomorphology 86, 409–440. doi: 10.1016/j.geomorph.2006.09.012
Kenyon, N. H., Akhmetzhanov, A. M., Wheeler, A. J., van Weering, T. C. E., de Haas, H., and Ivanov, M. K. (2003). Giant carbonate mud mounds in the southern Rockall Trough. Mar. Geol. 195, 5–30. doi: 10.1016/S0025-3227(02)00680-1
Lecours, V., Devillers, R., Schneider, D. C., Lucieer, V. L., Brown, C. J., and Edinger, E. N. (2015). Spatial scale and geographic context in benthic habitat mapping: review and future directions. Mar. Ecol. Prog. Ser. 535, 259–284. doi: 10.3354/meps11378
Linley, T. D., Lavaleye, M., Maiorano, P., Bergman, M., Capezzuto, F., Cousins, N. J., et al. (in press). Effects of cold water corals on fish diversity density (European continental margin: Arctic, NE Atlantic Mediterranean Sea): data from three baited lander systems. Deep Sea Res. II Top. Stud. Oceanogr. doi: 10.1016/j.dsr2.2015.12.003
Liu, C., Berry, P. M., Dawson, T. P., and Pearson, R. G. (2005). Selecting thresholds of occurrence in the prediction of species distributions. Ecography 28, 385–393. doi: 10.1111/j.0906-7590.2005.03957.x
Lo Iacono, C., Gràcia, E., Diez, S., Bozzano, G., Moreno, X., Da-obeitia, J., et al. (2008). Seafloor characterization and backscatter variability of the Almería Margin (Alboran Sea, SW Mediterranean) based on high-resolution acoustic data. Mar. Geol. 250, 1–18. doi: 10.1016/j.margeo.2007.11.004
Lobo, J. M., Jiménez-Valverde, A., and Real, R. (2008). AUC: a misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 17, 145–151. doi: 10.1111/j.1466-8238.2007.00358.x
Malinverno, E., Taviani, M., Rosso, A., Violanti, D., Villa, I., Savini, A., et al. (2010). Stratigraphic framework of the Apulian deep-water coral province, Ionian Sea. Deep Sea Res. II Top. Stud. Oceanogr. 57, 345–359. doi: 10.1016/j.dsr2.2009.08.025
Marchese, F. (2016). A Geomorphometric Approach to Assess Multi-Scale Spatial Distribution and Geomorphological Characterization of Benthic Habitats. Università degli Studi di Milano-Bicocca.
Masson, D. G., Bett, B. J., Billett, D. S. M., Jacobs, C. L., Wheeler, A. J., and Wynn, R. B. (2003). The origin of deep-water, coral-topped mounds in the northern Rockall Trough, Northeast Atlantic. Mar. Geol. 194, 159–180. doi: 10.1016/S0025-3227(02)00704-1
Mastrototaro, F., D'Onghia, G., Corriero, G., Matarrese, A., Maiorano, P., Panetta, P., et al. (2010). Biodiversity of the white coral bank off Cape Santa Maria di Leuca (Mediterranean Sea): an update. Deep Sea Res. II Top. Stud. Oceanogr. 57, 412–430. doi: 10.1016/j.dsr2.2009.08.021
McArthur, M. A., Brooke, B. P., Przeslawski, R., Ryan, D. A., Lucieer, V. L., Nichol, S., et al. (2010). On the use of abiotic surrogates to describe marine benthic biodiversity. Estuar. Coast. Shelf Sci. 88, 21–32. doi: 10.1016/j.ecss.2010.03.003
McCulloch, M., Taviani, M., Montagna, P., López Correa, M., Remia, A., and Mortimer, G. (2010). Proliferation and demise of deep-sea corals in the Mediterranean during the Younger Dryas. Earth Planet. Sci. Lett. 298, 143–152. doi: 10.1016/j.epsl.2010.07.036
McCulloch, M., Trotter, J., Montagna, P., Falter, J., Dunbar, R., Freiwald, A., et al. (2012). Resilience of cold-water scleractinian corals to ocean acidification: boron isotopic systematics of pH and saturation state up-regulation. Geochim. Cosmochim. Acta 87, 21–34. doi: 10.1016/j.gca.2012.03.027
Micheli, F., Levin, N., Giakoumi, S., Katsanevakis, S., Abdulla, A., Coll, M., et al. (2013). Setting priorities for regional conservation planning in the Mediterranean Sea. PLoS ONE 8:e59038. doi: 10.1371/journal.pone.0059038
Mienis, F., de Stigter, H. C., White, M., Duineveld, G., de Haas, H., and van Weering, T. C. E. (2007). Hydrodynamic controls on cold-water coral growth and carbonate-mound development at the SW and SE Rockall Trough Margin, NE Atlantic Ocean. Deep Sea Res. I Oceanogr. Res. Pap. 54, 1655–1674. doi: 10.1016/j.dsr.2007.05.013
Miller, R. J., Juska, C., and Hocevar, J. (2015). Submarine canyons as coral and sponge habitat on the eastern Bering Sea slope. Glob. Ecol. Conserv. 4, 85–94. doi: 10.1016/j.gecco.2015.05.009
O'Hara, T. D., and Tittensor, D. P. (2010). Environmental drivers of ophiuroid species richness on seamounts. Mar. Ecol. 31, 26–38. doi: 10.1111/j.1439-0485.2010.00373.x
Olu-Le Roy, K. (2004). Les coraux profonds, une biodiversité à évaluer et à préserver. Vertigo 5, 1–10. Available online at: archimer.ifremer.fr/doc/2004/publication-2364.pdf
Pearson, R. G. (2008). Species Distribution Modeling for Conservation Educators and Practitioners Synthesis. American Museum of Natural History. Available online at: http://ncep.amnh.org
Pearson, R. G., Raxworthy, C. J., Nakamura, M., and Peterson, A. T. (2007). Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr. 34, 102–117. doi: 10.1111/j.1365-2699.2006.01594.x
Phillips, S. J., and Dudik, M. (2008). Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31, 161–175. doi: 10.1111/j.0906-7590.2008.5203.x
Phillips, S. J., Dudik, M., and Schapire, R. E. (2004). “A maximum entropy approach to species distribution modeling,” in Proceedings of the Twenty-First International Conference on Machine Learning (Banff, AB: ACM).
R Core team (2014). R: A Language and Environment for Statistical Computing. (Vienna: R Foundation for Statistical Computing).
Radosavljevic, A., and Anderson, R. P. (2014). Making better Maxent models of species distributions: complexity, overfitting and evaluation. J. Biogeogr. 41, 629–643. doi: 10.1111/jbi.12227
Rengstorf, A. M., Grehan, A., Yesson, C., and Brown, C. (2012). Towards high-resolution habitat suitability modeling of vulnerable marine ecosystems in the deep-sea: resolving terrain attribute dependencies. Mar. Geodesy 35, 343–361. doi: 10.1080/01490419.2012.699020
Rengstorf, A. M., Mohn, C., Brown, C., Wisz, M. S., and Grehan, A. J. (2014). Predicting the distribution of deep-sea vulnerable marine ecosystems using high-resolution data: considerations and novel approaches. Deep Sea Res. I Oceanogr. Res. Pap. 93, 72–82. doi: 10.1016/j.dsr.2014.07.007
Roberts, J. M., and Cairns, S. D. (2014). Cold-water corals in a changing ocean. Curr. Opin. Environ. Sustain. 7, 118–126. doi: 10.1016/j.cosust.2014.01.004
Roberts, J. M., Wheeler, A. J., and Freiwald, A. (2006). Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312, 543–547. doi: 10.1126/science.1119861
Rosso, A., Vertino, A., Di Geronimo, I., Sanfilippo, R., Sciuto, F., Di Geronimo, R., et al. (2010). Hard- and soft-bottom thanatofacies from the Santa Maria di Leuca deep-water coral province, Mediterranean. Deep Sea Res II Top. Stud. Oceanogr. 57, 360–379. doi: 10.1016/j.dsr2.2009.08.024
Sappington, J. M., Longshore, K. M., and Thompson, D. B. (2007). Quantifying landscape ruggedness for animal habitat analysis: a case study using bighorn sheep in the mojave desert. J. Wildl. Manage. 71, 1419–1426. doi: 10.2193/2005-723
Savini, A., and Corselli, C. (2010). High-resolution bathymetry and acoustic geophysical data from Santa Maria di Leuca Cold Water Coral province (Northern Ionian Sea-Apulian continental slope). Deep Sea Res II Top. Stud. Oceanogr. 57, 326–344. doi: 10.1016/j.dsr2.2009.08.014
Savini, A., Marchese, F., Verdicchio, G., and Vertino, A. (2016). “Submarine Slide Topography and the Distribution of Vulnerable Marine Ecosystems: a Case Study in the Ionian Sea (Eastern Mediterranean),” in Submarine Mass Movements and their Consequences: 7th International Symposium, eds G. Lamarche, J. Mountjoy, S. Bull, T. Hubble, S. Krastel, E. Lane, A. Micallef, L. Moscardelli, C. Mueller, I. Pecher, and S. Woelz (Cham: Springer International Publishing), 163–170.
Savini, A., Vertino, A., Marchese, F., Beuck, L., and Freiwald, A. (2014). Mapping Cold-Water Coral Habitats at Different Scales within the Northern Ionian Sea (Central Mediterranean): an assessment of coral coverage and associated vulnerability. PLoS ONE 9:e87108. doi: 10.1371/journal.pone.0087108
Somoza, L., Ercilla, G., Urgorri, V., León, R., Medialdea, T., Paredes, M., et al. (2014). Detection and mapping of cold-water coral mounds and living Lophelia reefs in the Galicia Bank, Atlantic NW Iberia margin. Mar. Geol. 349, 73–90. doi: 10.1016/j.margeo.2013.12.017
Taviani, M. (2002). The Mediterranean benthos from late Miocene up to present: ten million years of dramatic climatic and geologic vicissitudes. Biol. Mar. Mediterr 9, 445–463. Available online at: https://www.academia.edu/1875064
Taviani, M., Angeletti, L., Antolini, B., Ceregato, A., Froglia, C., Lopez Correa, M., et al. (2011). “Geo-biology of Mediterranean deep-water coral ecosystems,” in Marine Research at CNR, ed E. E. A. Brugnoli (Rome: National Research Council of Italy), 705–719.
Taviani, M., Freiwald, A., and Zibrowius, H. (2005a). “Deep coral growth in the Mediterranean Sea: an overview,” in Cold-Water Corals and Ecosystems, ed A. R. Freiwald (Heidelberg: Springer), 137–156.
Taviani, M., Remia, A., Corselli, C., Freiwald, A., Malinverno, E., Mastrototaro, F., et al. (2005b). First geo-marine survey of living cold-water Lophelia reefs in the Ionian Sea (Mediterranean basin). Facies 50, 409–417. doi: 10.1007/s10347-004-0039-0
Tittensor, D. P., Baco, A. R., Brewin, P. E., Clark, M. R., Consalvey, M., Hall-Spencer, J., et al. (2009). Predicting global habitat suitability for stony corals on seamounts. J. Biogeogr. 36, 1111–1128. doi: 10.1111/j.1365-2699.2008.02062.x
Tong, R., Purser, A., Guinan, J., Unnithan, V., Yu, J., and Zhang, C. (2016). Quantifying relationships between abundances of cold-water coral Lophelia pertusa and terrain features: a case study on the Norwegian margin. Cont. Shelf Res. 116, 13–26. doi: 10.1016/j.csr.2016.01.012
Tursi, A., Mastrototaro, F., Matarrese, A., Maiorano, P., and D'Onghia, G. (2004). Biodiversity of the white coral reefs in the Ionian Sea (Central Mediterranean). Chem. Ecol. 20(3 Supp. 1), 107–116. doi: 10.1080/02757540310001629170
Van Rooij, D., De Mol, B., Huvenne, V., Ivanov, M., and Henriet, J. P. (2003). Seismic evidence of current-controlled sedimentation in the Belgica mound province, upper Porcupine slope, southwest of Ireland. Mar. Geol. 195, 31–53. doi: 10.1016/S0025-3227(02)00681-3
van Weering, T. C. E., de Haas, H., Akhmetzanov, A. M., and Kenyon, N. H. (2003). “Giant Carbonate Mounds along the porcupine and SW rockall trough margins,” in European Margin Sediment Dynamics: Side-Scan Sonar and Seismic Images, eds J. Mienert and P. Weaver (Berlin: Springer Berlin Heidelberg), 211–216.
Verfaillie, E., Doornenbal, P., Mitchell, A. J., White, J., and Van Lancker, V. (2007). “The bathymetric position index (BPI) as a support tool for habitat mapping,” in Worked Example for the MESH Final Guidance. 14. Available online at: http://www.emodnet-seabedhabitats.eu/PDF/GMHM4_Bathymetric_position_index_(BPI).pdf
Vertino, A., Savini, A., Rosso, A., Di Geronimo, I., Mastrototaro, F., Sanfilippo, R., et al. (2010). Benthic habitat characterization and distribution from two representative sites of the deep-water SML Coral Province (Mediterranean). Deep Sea Res. II Top. Stud. Oceanogr. 57, 380–396. doi: 10.1016/j.dsr2.2009.08.023
Vierod, A. D. T., Guinotte, J. M., and Davies, A. J. (2014). Predicting the distribution of vulnerable marine ecosystems in the deep sea using presence-background models. Deep Sea Res. II Top. Stud. Oceanogr. 99, 6–18. doi: 10.1016/j.dsr2.2013.06.010
Wheeler, A. J., Beyer, A., Freiwald, A., Haas, H., Huvenne, V. A. I., Kozachenko, M., et al. (2006). Morphology and environment of cold-water coral carbonate mounds on the NW European margin. Int. J. Earth Sci. 96, 37–56. doi: 10.1007/s00531-006-0130-6
White, M., Mohn, C., de Stigter, H., and Mottram, G. (2005). “Deep-water coral development as a function of hydrodynamics and surface productivity around the submarine banks of the Rockall Trough, NE Atlantic,” in Cold-Water Corals and Ecosystems, eds A. Freiwald and J. M. Roberts (Berlin: Springer Berlin Heidelberg), 503–514.
Wilson, M. F. J., O'Connell, B., Brown, C., Guinan, J. C., and Grehan, A. J. (2007). Multiscale terrain analysis of multibeam bathymetry data for habitat mapping on the continental slope. Mar. Geodesy 30, 3–35. doi: 10.1080/01490410701295962
Wisz, M. S., Hijmans, R. J., Li, J., Peterson, A. T., Graham, C. H., Guisan, A., et al. (2008). Effects of sample size on the performance of species distribution models. Divers. Distrib. 14, 763–773. doi: 10.1111/j.1472-4642.2008.00482.x
Wright, D. J., Lundblad, E. R., Larkin, E. M., Rinehart, R. W., Murphy, J., Cary-Kothera, L., et al. (2005). ArcGIS benthic terrain modeler,” in Davey Jones Locker Seafloor Mapping (Corvallis, OR: Oregon State University; Marine GIS Laboratory and NOAA Coastal Services Center).
Yesson, C., Bedford, F., Rogers, A. D., and Taylor, M. L. (in press). The global distribution of deep-water Antipatharia habitat. Deep Sea Res. II Top. Stud. Oceanogr. doi: 10.1016/j.dsr2.2015.12.004
Yesson, C., Taylor, M. L., Tittensor, D. P., Davies, A. J., Guinotte, J., Baco, A., et al. (2012). Global habitat suitability of cold-water octocorals. J. Biogeogr. 39, 1278–1292. doi: 10.1111/j.1365-2699.2011.02681.x
Keywords: predictive habitat mapping, maxent, cold-water coral, ecological proxies, Santa Maria di Leuca, Mediterranean Sea
Citation: Bargain A, Marchese F, Savini A, Taviani M and Fabri M-C (2017) Santa Maria di Leuca Province (Mediterranean Sea): Identification of Suitable Mounds for Cold-Water Coral Settlement Using Geomorphometric Proxies and Maxent Methods. Front. Mar. Sci. 4:338. doi: 10.3389/fmars.2017.00338
Received: 02 May 2017; Accepted: 12 October 2017;
Published: 27 October 2017.
Edited by:
Cinzia Corinaldesi, Università Politecnica delle Marche, ItalyReviewed by:
Alexander David Tinsley Vierod, Bangor University, United KingdomVincent Lecours, University of Florida, United States
Copyright © 2017 Bargain, Marchese, Savini, Taviani and Fabri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie-Claire Fabri, bWFyaWUuY2xhaXJlLmZhYnJpQGlmcmVtZXIuZnI=
 Annaëlle Bargain
Annaëlle Bargain Fabio Marchese
Fabio Marchese Alessandra Savini
Alessandra Savini Marco Taviani
Marco Taviani Marie-Claire Fabri
Marie-Claire Fabri