- 1Laboratory of Ichthyology and Coastal Fisheries, Institute of Oceanography and Fisheries, Split, Croatia
- 2Laboratory of Fisheries Science and Management of Pelagic and Demersal Resources, Institute of Oceanography and Fisheries, Split, Croatia
- 3Laboratory of Chemical Oceanography and Sedimentology of the Sea, Institute of Oceanography and Fisheries, Split, Croatia
Over the past two decades, the field of sclerochronology has been rapidly developing, with scientists devoting significant efforts to studying the physical and chemical variations in hard tissues of aquatic organisms. Most of this research has been limited to certain taxa and geographic areas. Although growth increments in fish otoliths are used for sclerochronology purposes, relatively little has been done in the Mediterranean Sea. According to the literature, the chemical composition of otoliths from Mediterranean fish species has primarily been used for analyzing migration patterns, habitat use, and population structure of commercially important fish species. To the best of our knowledge, there are no studies on fish growth chronology construction conducted in the Mediterranean Sea. In order to identify the opportunities for sclerochronology research on fish from the Mediterranean, we used FishBase to identify potential candidate species with a sufficiently long lifespan and clearly defined growth increments for growth chronology construction and otolith chemistry research. We also present the challenges and limitations for sclerochronology research, including: (i) very few fish species in the Mediterranean Sea have a longevity of several decades; (ii) issues associated with reliable age determination for certain long-lived fish species; (iii) a general lack of understanding and effort to constructed and manage otolith collections; and (iv) limitations imposed by the availability of funding, expertise, and instrumentation. Despite these challenges, fish sclerochronology research has strong potential in the Mediterranean and adjacent seas. Recent studies in the Adriatic Sea have resulted in the construction of bivalve chronologies and the geochemical analysis of shells, providing important time-series data for comparative analysis and a multispecies approach. Furthermore, studies conducted in other parts of the world have demonstrated great potential for the use of fish otoliths in monitoring environmental variability and the effects of pollutants and disturbance.
Introduction
Hard structures of aquatic organisms, including mollusk shells, fish otoliths, corals, and coralline algae, are deposited continuously during the life of the organism, and thereby contain environmental information collected over the organism’s life cycle (e.g., Hudson et al., 1976; Jones, 1983; Black et al., 2008). The field of sclerochronology utilizes these data archives by investigating their morphological (i.e., increment width) and geochemical composition to deduce organismal life history traits as well as to reconstruct records of environmental and climatic change through space and time (Oschmann, 2009). Although many sclerochronology studies have been conducted on sedentary organisms, primarily the bivalve Arctica islandica (e.g., Schöne, 2013; Marali et al., 2017; Reynolds et al., 2018), fish also present a very interesting target taxon for sclerochronology research (e.g., Panfili et al., 2002; Black et al., 2005; Grønkjaer et al., 2013). The objective of this review was to focus primarily on papers that analyzed otoliths in relation to environmental and climatic changes. There are numerous studies in the review that analyzed fish growth and age from growth increment structures in the otoliths (e.g., Gutiérrez and Morales-Nin, 1986; Morales-Nin and Moranta, 1997; Reñones et al., 2007), but they did not directly relate them to the environmental conditions and a detailed review of such studies is beyond the scope of a present paper.
One of the challenges in evaluating the status of sclerochronology research lies within the fact that this term is not always used in publications addressing the morphological and/or geochemical properties of hard structures in aquatic organisms (Gillikin et al., 2019). This is especially the case for research conducted on fish, despite the publication of the very comprehensive Manual of Fish Sclerochronology (Panfili et al., 2002).
Fish possess several hard structures interesting for sclerochronology analysis, including scales, the skeleton, and otoliths (e.g., Chilton and Beamish, 1982; Panfili et al., 2002). Of these, otoliths—calcium carbonate structures located in the inner ear of the fish—are considered the most reliable, as they are metabolically inert, hindering re-absorption (Campana and Neilson, 1985), unlike other structures, such as scales (Simkiss, 1974). Otoliths contain periodically deposited growth increments, from daily to annual, and can thereby provide high temporal resolution data (e.g., Campana, 1999; Morales-Nin, 2000; Black et al., 2008; Elsdon et al., 2008). As fish can attain a maximal life span of several decades, otolith analysis can provide an important window into the past (e.g., Campana, 1999; Black et al., 2008).
Chemical research on otoliths includes analysis of elemental and/or isotopic composition. In 1999, Campana published a review paper on the chemistry and composition of otoliths, presenting in detail the state of the art on this subject at that time and the applications and assumptions of this type of research. The applications of otolith chemistry for describing movements and life-history parameters of fish were comprehensively presented by Elsdon et al. (2008). Numerous publications followed, clearly demonstrating the potential for otolith chemistry as a natural tag of fish stocks (e.g., Trueman et al., 2012; Darnaude and Hunter, 2018; Izzo et al., 2018; Wright et al., 2018). Although most studies focus on stock identification and migration history, the elemental composition of otoliths can also be applied for identifying bioavailable contaminants and establishing long-term trends (e.g., Søndergaard et al., 2015; Andronis et al., 2017; Mounicou et al., 2019). Furthermore, as oxygen isotopes (δ18O) are considered a proxy of water temperatures, analysis of otolith isotopic composition can enable reconstruction of environmental conditions (e.g., West et al., 2012; Willmes et al., 2019).
Use of otolith growth increments to construct fish growth chronology and establishing the relationship with environmental conditions have received increasing attention over the past decade. The methodology for this research has been derived from dendrochronology—the study of growth rings in trees (Black et al., 2005). Primary target organisms are long-living fish species, such as yelloweye rockfish (Sebastes ruberrimus, >70 years; Black et al., 2008), and northern rockfish (Sebastes polyspinis, ∼40 years; Matta et al., 2018). However, development of statistical methods and sample archives have also enabled growth chronology construction for shorter living species. For example, Tanner et al. (2019) constructed half a century chronology for a small, relatively short-lived (<16 years) pelagic fish (Atlantic horse mackerel, Trachurus trachurus).
The main objective of this paper is to present an overview of the sclerochronology related research in the Mediterranean Sea and to present its opportunities and challenges.
Overview of Previous Sclerochronology Related Research in the Mediterranean
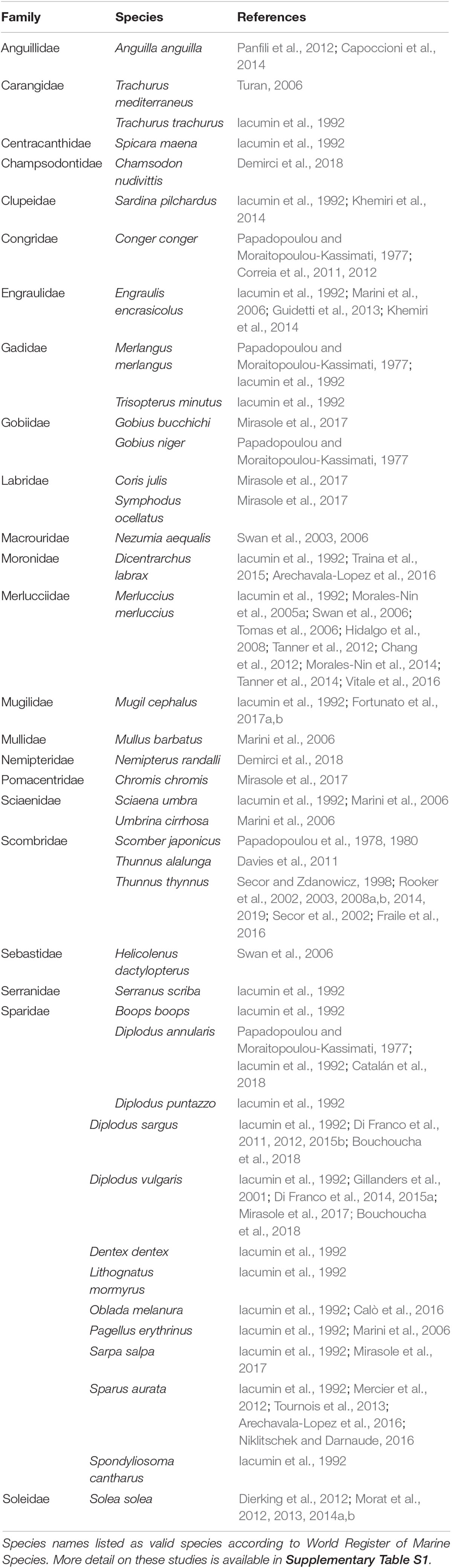
In order to identify relevant publications on fish sclerochronology research in the Mediterranean Sea, we conducted a literature search through the Web of Science database. The keywords “Mediterranean” and “otolith” were used in combination with words “isotope,” “element,” “microchemistry,” “chemistry,” and “chronology.” All publications obtained through this search were read in detail, and only those relating to otolith analysis were included (Table 1 and Supplementary Table S1). Other structures, such as scales and vertebrae, were not considered for the purposes of this review.
Chemical analysis of otoliths has been conducted on over 41 fish species from the Mediterranean, and the most studied species are from the family Sparidae (Table 1). Published studies include data for the entire otolith (e.g., Iacumin et al., 1992; Gillanders et al., 2001; Marini et al., 2006; Arechavala-Lopez et al., 2016), data for a specific area of the otolith (e.g., Tanner et al., 2012; Mirasole et al., 2017; Rooker et al., 2019), and time series data (e.g., Correia et al., 2012; Mercier et al., 2012; Bouchoucha et al., 2018). In most reports, only a single species was analyzed, while Papadopoulou and Moraitopoulou-Kassimati (1977), Iacumin et al. (1992), Marini et al. (2006), Swan et al. (2006), Khemiri et al. (2014), Arechavala-Lopez et al. (2016), Mirasole et al. (2017), Bouchoucha et al. (2018), and Demirci et al. (2018) presented data for 2 to 24 different species. Recently, Chang and Geffen (2013) summarized taxonomic and geographic influences on fish otolith microchemistry based on a number-published paper worldwide and all those related to the Mediterranean are also included in this study.
In total, 12 species from the family Sparidae are listed in Table 1, and most were addressed only by Iacumin et al. (1992). This study analyzed the oxygen and carbon isotope composition of aragonite in fish otoliths with regard to their possible suitability in paleoenvironmental and paleobiological work. This was the first attempt to apply stable isotope analysis to fish otoliths from the Mediterranean Sea. The mostly analyzed species are those from Diplodus genus, particularly D. sargus, and D. puntazzo, regarding possible environmental interpretation of otolith fingerprints related to spatial patterns of population connectivity and dispersal of marine fishes (Di Franco et al., 2011, 2015b), dispersal scales of fish at various life history stages, which is critical for successful design of networks of marine protected areas (Di Franco et al., 2012, 2015a), within-otolith variability enabling its usage as a marker for fish exposure to stressful conditions (Di Franco et al., 2014).
The majority of research on otolith chemical composition has been conducted on commercially important species. Atlantic Bluefin tuna (Thunnus thynnus) was the target of several studies on the element (Secor and Zdanowicz, 1998; Rooker et al., 2003) and isotope composition (Secor et al., 2002; Rooker et al., 2008a, b, 2014, 2019), aiming to reconstruct movement and population exchange. Fraile et al. (2016) observed the depletion in δ13C in T. thynnus otoliths over time, associating this with the oceanic uptake of anthropogenically derived CO2 from the Mediterranean Sea over the past two decades. These studies primarily focused on material deposited in the otolith core or during the first year of life.
The European hake (Mercuccius merluccius) is also an important target species for the analysis of otolith element and isotope composition, and different methodologies have been applied. Morales-Nin et al. (2005a) studied elements in different parts of the otoliths using laser ablation–spot analysis, while in a study from 2014, the same authors used the line scan approach. Laser ablation, as opposed to otolith dissolution that is applied in the analysis of the whole otolith, enables the collection of more data points that can be placed in time (e.g., Elsdon et al., 2008). Tomas et al. (2006) studied composition of the opaque and translucent bands with wavelength dispersive spectrometry (WDS) revealing that annual marks (translucent) were significantly richer in Sr and Ca and significantly poorer in Na than opaque bands. Swan et al. (2006) applied two methods—solution-based inductively coupled plasma mass spectrometry of the whole otolith and laser ablation analysis of the otolith nucleus on hake and bluemonth (Helicolenus dactylopterus). Chang et al. (2012) used hake otoliths to test different widths of ablation lines and evaluate the temporal resolution of data. Hidalgo et al. (2008) and Tanner et al. (2012) applied analysis of δ18O and δ13C to certain sections of the otolith, specifically the core and the edge zone, in determining hake movement and ecology. It is interesting that Tanner et al. (2012) combined stable isotope analysis with analysis of otolith element composition to obtain more comprehensive data. These studies on hake analyzed the migration, population structure, and ecology of this commercially important species. In recent papers, Tanner et al. (2014) accompanied genotype with otolith data to increase the classification accuracy of individuals to their potential natal origins, while Vitale et al. (2016) estimated longevity of 25 years of female hake by applying bomb radiocarbon dating.
Element and isotope composition of otoliths of the common sole (Solea solea) have been analyzed in a series of studies conducted in the Gulf of Lions, in the northwest Mediterranean (Dierking et al., 2012; Morat et al., 2012, 2013, 2014a,b). These studies analyzed dispersion between populations and the use of different habitats.
The chemical composition of otoliths as a proxy of environmental conditions has been analyzed in only a few studies in the Mediterranean Sea, including Traina et al. (2015). They investigated the metal content of European sea bass (Dicentrarchus labrax) otoliths from two fish aquaculture sites. Their results indicated variations in the concentrations of certain metals between locations that were likely due to industrial effluents.
To the best of our knowledge, there is no research in the Mediterranean Sea related to fish growth chronologies constructed from growth increment analysis. The literature search conducted through the Web of Science returned just one publication for the keyword combination “Mediterranean” and “fish” and “sclerochronology”: a report by Prendergast and Schöne (2017) as the preface to the Special Issue on Sclerochronology containing research from different parts of the world including the Mediterranean, but not specifically on fish in the Mediterranean.
Opportunities for Fish Growth Chronology Construction
Over the past two decades, techniques developed in dendrochronology research have been applied for the construction of fish growth chronologies (Black et al., 2005, 2008). They clearly demonstrated the potential for obtaining long term data from growth patterns in otoliths and for identifying environmental drivers (Morrongiello et al., 2012; Rountrey et al., 2014). In a recent review, Black et al. (2019) presented a global list of fish species that have been the subject of sclerochronology studies that included growth chronology construction and applied cross-dating techniques. Their list includes 21 species, none of which were from the Mediterranean Sea. The most studied on the list are cold-water species, such as kelp greenling (Hexagrammos decagrammus) and black rockfish (Sebastes melanops) and other species from the genus Sebastes (S. alutus, S. aurora, S. diploproa, and S. ruberrimus). Other species were from the families: Girellidae, Labridae, Lethrinidae, Lutjanidae, Platycephalidae Pleuronectidae, Polyprionidae, Sciaenidae, and Scombridae. All of these are long-lived, non-migratory, nearshore residents with generalist diets that can be caught easily throughout a wide geographic range (Whitfield and Elliott, 2002).
Identifying target fish species with a sufficiently long life span and clearly defined growth increments is a prerequisite for statistically robust chronology construction. Unlike bivalves and trees that can live for several centuries (e.g., A. islandica, 507 years; Butler et al., 2013) or even millennia (e.g., Pinus longaeva, 4,900 years; Currey, 1965), fish have a shorter lifespan and present a greater challenge for constructing statistically robust chronologies. Another prerequisite for chronology construction is the availability of samples, which needs to take the conservation status of species into account. Although it is scientifically interesting to obtain data from endangered species, sampling such species should be clearly justified and ethically sound. Replication is essential for proper cross-dating that can yield annually resolved chronologies sensitive to environmental stressors (Hudson et al., 1976).
In order to identify possible candidates for fish growth chronology research in the Mediterranean Sea, we conducted a search of the FishBase database. This is a global, scientifically guided, biodiversity information system on fishes that provides a wide range of information (taxonomy, biology, trophic ecology, and life history) on all species currently known in the world, as well as historical data reaching back 250 years1. According to this database, a total of 755 fish species from 174 families inhabit the Mediterranean Sea. We made several reductions to obtain a reasonable pool of potentially interesting target species. Since the database provides data for maximal recorded total length (TL), maximal reported age, trophic level, and habitat p‘nces (demersal, pelagic-neritic, benthopelagic, bathypelagic, bathydemersal, pelagic-oceanic, and reef-associated) and status (endemic, introduced, and native or questionable), we first removed all short-lived (<2 years) and small fishes (TL < 30 cm). This resulted in the removal of species belonging to the families Apogonidae, Atherinidae, Blenniidae, Bregmacerotidae, Callionymidae, and Carapidae. Given their conservation status, Chondrichthyes and the primitive fishes (Myxinidae, Petromyzontidae, Chimaeridae, and Halosauridae) were also excluded. Furthermore, rare or poorly investigated species without any commercial interest or benthopelagic, bathypelagic, and bathydemersal fishes for which no age- related data were provided in FishBase were also excluded.
Finally, a pool of 263 fish species inhabiting Mediterranean Sea was obtained and used in the analysis. The estimated or determined maximum age of 31 fish species from 20 families was over 30 years. However, it is important to note that age was determined by age reading methods on specimens from the Mediterranean Sea for only 12 species (Table 2), while the age of other species was estimated based on growth equation parameters available for that specific species or closely related species from the same family, or age was reported for an area other than the Mediterranean Sea.
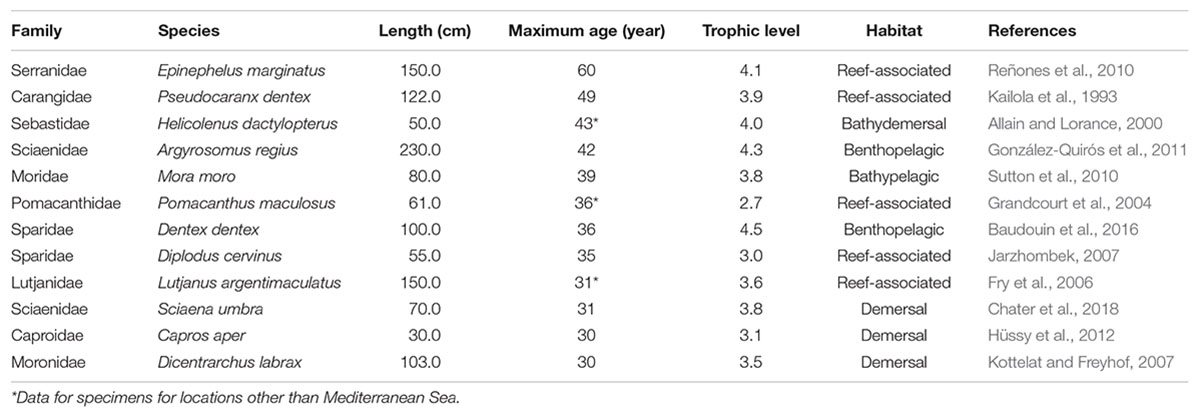
Table 2. The list of long-lived fish species in the Mediterranean Sea according to FishBase. Data sorted by descending maximum reported age.
From these reports, the longest-living species in the Mediterranean Sea is the wreckfish, Polyprion americanus (see Peres and Haimovici, 2004). However, its maximum age of 76 years was reported for specimens from the continental shelf and slope off southern Brazil. Thus, its availability for sclerochronology studies in the Mediterranean is questionable, particularly since this species has been listed as Critically Endangered (CR) on the IUCN Red List (IUCN, 2017). The same is true for the red bream Beryx decadactylus, since the reported maximum age of 61 years was for specimens collected off the southeastern coast of the United States. To the best of our knowledge, there are no relevant data for the maximum age of either species in the Mediterranean.
The dusky grouper, Epinephelus marginatus, lives throughout the Mediterranean Sea and its maximum age of 60 years was reported for specimens from the Balearic Islands (Reñones et al., 2010). Most groupers are solitary, resident fishes. The Mediterranean is the upper limit of their northward distribution, and their growth in the Mediterranean is significantly slower than for groupers in tropical waters (Gracia_López and Castelló-Orway, 2003). Site specificity, a relatively slow growth rate (some species may not be mature until the age of 8 to 10 years) and spawning strategy (synchronic or protogynous hermaphrodites; Sadovy and Shapiro, 1987; Heemstra and Randall, 1993) make them particularly vulnerable (CITES/UNEP-WCMC, 2017). Although the long-life span and resident behavior makes E. marginatus an interesting candidate for construction of growth chronologies, its low abundance and protected status throughout the Mediterranean requires a strategic approach to sample collection extending over time, rather than single on-site sampling action.
Three families listed in Table 2—Sebastidae, Lutjanidae, and Sciaenidae—were identified earlier within the list of globally important fish taxa for sclerochronology research (Black et al., 2019). However, just two species from the Sebastidae family are listed in the Mediterranean Sea, and only H. dactylopterus can attain an age of over 40 years. Certain caution is needed, as this data was reported for individuals caught in the Northeast Atlantic and not in the Mediterranean Sea. According to the available data, H. dactylopterus grows faster and lives longer in the Northeast Atlantic than in the Mediterranean (Ragonese, 1989; Allain and Lorance, 2000; D’Onghia et al., 2004; Consoli et al., 2010). The maximum age reported for H. dactylopterus from the Mediterranean is 21 years (Consoli et al., 2010), questioning the availability of Mediterranean samples for growth analysis for this species.
Two species of Sciaenidae family are listed in Table 2. The maximum reported age for the meagre, Argyrosomus regius, in the Gulf of Cádiz (SW Iberian Peninsula) is 42 years (González-Quirós et al., 2011), while the brown meagre, Sciaena umbra, reached 31 years in the Gulf of Tunis (Chater et al., 2018). The dense calcium carbonate deposition of the large and very thick otoliths in Sciaenids reduces light transmission, making it almost impossible to distinguish hyaline and opaque zones (Arneri et al., 1998; Chater et al., 2018). According to Arneri et al. (1998), growth increments in otoliths of these taxa are more readable in cross-sections. Both species are commercially important and there is the potential for collection of representative otolith samples. However, further development of otolith reading techniques is needed to facilitate identification of growth increment boundaries and enable statistically robust chronology construction.
Two non-native species, yellowbar angelfish (Pomacanthus maculosus) and mangrove red snapper (Lutjanus argentimaculatus), have a lifespan of over 30 years (Grandcourt et al., 2004; Fry et al., 2006) and are interesting candidates for growth chronology construction. Both species entered the Mediterranean via the Suez channel and in recent years have established their populations in the eastern Mediterranean, along the coasts of Israel and Lebanon (Bariche, 2010; Sonin et al., 2019). The maximum reported age for P. maculosus is for specimens from the southern Arabian Gulf, while for L. argentimaculatus the maximum age data is reported for its native range—Papua New Guinea. These two species belong to long-living families (Grandcourt et al., 2004; Piddocke et al., 2015), and although determination of otolith growth patterns present certain challenges for the oldest specimens (Rezende and Ferreira, 2004; Steward et al., 2009), in the context of climate change they are interesting taxa for growth chronology research.
The remaining species listed in Table 2 belong to the families Sparidae and Moronidae. There are total of 31 sparid species in the Mediterranean Sea, which are known to be slow-growing and long-lived (Hanel and Tsigenopoulos, 2011) and susceptible to over-exploitation due to their commercial importance (Comeros-Raynal et al., 2016). Sparid fishes generally have relatively large and easily readable sagittal otoliths, and despite the wealth of literature denouncing the use of whole, unsectioned otoliths in growth studies on sparid fishes (see Winkler et al., 2019), age determination using whole otoliths is still common. According to the information available in Fish Base, the maximum age reported for sparids in the Mediterranean ranges from 5 to 36 years (Table 2). Species with the greatest potential are common dentex Dentex dentex and zebra seabream Diplodus cervinus. Due to its commercial importance, wide distribution, clear growth patterns in otoliths, and lifespan of over 20 years (Kraljević et al., 1998), the gilt head seabream Sparus aurata is also an interesting candidate for sclerochronology research. From the Moronidae family, sea bass, Dicentrarchus labrax can attain age of 30 years (Kottelat and Freyhof, 2007). The species mentioned in this paragraph are economically interesting, and EU Mediterranean countries collect relevant landing and biological data for them [data collection framework (DCF); Regulation (EU), 2017]. It is highly likely, either within monitoring programs or scientific research projects, that otoliths of these species are archived during several years or even decades by different institutions and could be used to extend time series data beyond the maximal reported age.
According to the data presented above, the availability of otoliths for long living species from the Mediterranean is quite limited, as there are only several species reaching a maximum reported age of over 30 years. However, development of statistical techniques enables construction of growth chronologies for shorter living fish species (<15 years) when samples are collected over several years or decades (Coulson et al., 2014). It is possible that, for certain fish species, adequate replicates for chronology construction can be obtained through archive collections.
Opportunities for Otolith Chemistry Research
Clarity of growth rings in otoliths is one of the main factors contributing to sclerochronology research, both for growth increment measurements and for otolith chemical analysis (Campana, 1999). Problems related to interpretation of increments in otolith, including age estimation and validation of periodicity, has been pointed out in number of studies in different parts of the world (e.g., Morales-Nin et al., 2005b; Stransky et al., 2005; Hüssy et al., 2016). This problem should not be underestimated, and interpretation of otolith increments needs to be carefully checked and validated. One of the most appreciated characteristics of otoliths is their lack of resorption. This means that once the material has been deposited, the organism will not use these minerals again, even in periods of starvation. Lack of resorption is not shared with other calcified structure (like scales and bones) in fishes or other vertebrates (Bilton, 1974; cited by Campana and Thorrold, 2001). Another special characteristic of otoliths is that they grow continuously throughout the lifetime of the fish (Campana, 1999).
In order to assign relevant chemistry data to a specific calendar year, it is crucial to distinguish growth increment boundaries (Black et al., 2005; Martino et al., 2019). However, many the most commercially important fish species living in the Mediterranean Sea do not have clearly distinguished growth patterns in their otoliths, which presents a challenge for this type of research. For example, it is still difficult to determine the growth boundaries for the first growth increments in otoliths of Mullus barbatus due slow growth and number of false-growth increments laid down before the annulus (Carbonara et al., 2018) and of Merluccius merluccius due to the fast growth (de Pontual et al., 2003; Piñeiro et al., 2007; Mellon-Duval et al., 2010) and long spawning period of the species (Morales-Nin and Aldebert, 1997) although a number of direct methods to validate age assessment were used, like mark-recapture (de Pontual et al., 2003; Mellon-Duval et al., 2010), first ring appearance (Belcari et al., 2006), or bomb radiocarbon dating (Vitale et al., 2016).
Species from the families Sparidae and Lutjanidae have annual growth rings, that although thin, are clearly visible (Piddocke et al., 2015; Winkler et al., 2019), and represent the most promising target taxa for sclerochronology studies. Interesting target species of Sparidae include Dentex dentex, Diplodus cervinus, and Sparus aurata. The latter species, together with Dicentrarchus labrax (Moronidae), are particularly interesting, as these are the most important fish aquaculture species throughout the Mediterranean region (Lacoue-Labarthe et al., 2016). In addition to these species and those listed in Table 1, another interesting taxon for chemical research of otoliths is Seriola dumerili (Carangidae), a species with a circumglobal distribution (Smith, 1997).
Instrumental restrictions, related to the quantity of material required for the analysis of stable isotopes in otoliths, has been the main limitation for the development of isotope related research in otoliths (Sreemany et al., 2017). Due to the small size of the otolith, this resulted in time averaging of data, and analyses were limited to whole otoliths (e.g., Rooker et al., 2008a), or certain parts of otoliths, e.g., the core (Siskey et al., 2016; Rooker et al., 2019) or edge (Hidalgo et al., 2008; Tanner et al., 2012), without the possibility of obtaining time series data. Development of instruments and methods, including high-resolution laser ablation systems (e.g., Sreemany et al., 2017) and continuous flow isotope ratio mass spectrometry system for ultra-microvolume carbonate samples (Kitagawa et al., 2013; Sakamoto et al., 2017), have opened new opportunities for obtaining time series data from otoliths. Although significant progress was made in instrument development, many are still available only to a limited number of scientists. Therefore, new opportunities for collaborations and research directions related to the Mediterranean are required.
Challenges and Limitations for Fish Sclerochronology Research in the Mediterranean Sea
Alongside the constraints imposed by the biological characteristics of species, there are other challenges to conducting sclerochronology research in the Mediterranean region. Although otoliths are small structures that are easily archived, there still appears to be a general lack of understanding and effort to construct and manage otolith collections. Panfili et al. (2002) clearly indicated the importance of archiving otoliths, highlighting the need to evaluate, catalog, and conserve otolith collections in a way that will make both the otolith and corresponding fish life history information more accessible to all researchers. The Instituto de Ciencias del Mar-CSIC (Spain) maintains an otolith reference collection that includes samples from different parts of the world, including the Western Mediterranean Sea2. One example of an online searchable database of otolith collections from other parts of the world is the otolith collection database housed at the Burke Museum3. Although English is generally accepted as a global scientific language, the Mediterranean is highly politically, economically, cultural, and linguistically diverse region, which impacts sample, data, and knowledge storage and sharing. While online searchable otolith collections are not currently possible for a number of reasons, efforts should be made by different institutions or even scientists themselves to archive otoliths together with relevant collection and biology data. It is highly recommended that collections contain samples for different species, not only commercially most important ones, and that special efforts are made to archive otoliths of rare species. Furthermore, Disspain et al. (2016) pointed out the potential to use otoliths from archeological sites to analyze changes in the environment occurring through human history. Linking archeologists with fish biologist and environmental scientists can provide great potential for sclerochronology research.
In addition to the issues related to the availability of otolith collections, continuous sampling over several decades is also a challenge given the limited availability of funding for long-term studies and the logistics associated with field sampling. Long-term time series data are needed to estimate the real status of exploited resources and their evolution over time (Battaglia et al., 2010) and to analyze climate change effects on marine species and communities (Azzurro et al., 2019). Today, most scientific research projects are short in duration, resulting in difficulties related to securing funds needed for maintaining a longer data time series (Lleonart and Maynou, 2003; Rochet and Trenkel, 2003). Even when data collection takes place over longer periods, it often suffers from inconsistencies in sampling design or sampling methods (Rochet and Trenkel, 2003; Rochet et al., 2005). Sampling designs often tend to be incomplete, lacking either randomization or replication, and as such can never conclusively demonstrate the causes of the observed changes. Sampling and storage protocols are often specific to the institution or project. It is, however, encouraging that all the EU Member States bordering the Mediterranean Sea, eight in total, are required to collect fisheries data using unified methodology [Regulation (EU), 2017].
Human resources present another important segment in the development of all marine-related research in the Mediterranean, including sclerochronology. Although otoliths have been analyzed in relation to age and growth, very few attempts have been made to link the data derived from otoliths with environmental data. Education in methods associated with growth increments analysis, chemical composition of otoliths, and statistical methods related to sclerochronology research is strongly needed, either through personal or workshop-based interactions, to stimulate the involvement of fisheries scientists in sclerochronology studies. Furthermore, sclerochronology includes biological, chemical, and physical aspects, requiring an interdisciplinary research team.
Different types of instrumentation are required for sclerochronology analysis. Some instruments need to be readily and continuously available, such as saws and grinding and polishing machines, while those for chemical analysis of otoliths can be offsite. For example, the Laser Ablation System coupled with a High-Resolution Inductively Coupled Plasma Mass Spectrometer (HR-ICPMS) is a sophisticated tool for analysis of elemental composition that is both very expensive and requires specially trained personnel. Careful sample preparation and establishing collaboration with institutions possessing such an instrument can enable the processing of otolith samples at a reasonable cost. Efforts should be made to develop human and research capacities within the framework of different international projects, thereby promoting scientific collaboration and the education of young researchers.
Conclusion
The Mediterranean Sea is a hotspot of marine biodiversity and is also one of the most impacted ecoregions globally (Halpern et al., 2008; Costello et al., 2010), due to increasing levels of human threats that affect all levels of biodiversity (Mouillot et al., 2011; Coll et al., 2012; Micheli et al., 2013) and due to severe impacts from climate change (Lejeusne et al., 2010) and biological invasions (Katsanevakis et al., 2012).
Determining historical changes in marine communities and consequently fisheries (Pauly and Zeller, 2016) allows us to better understand the present, in order to anticipate the future. This is particularly important in relation to the decline of marine resources (Bell et al., 2017). Thus, it is necessary to develop methods to document long-term trends and detect potential stressors. However, establishing causal relationships between a wide range of stressors and effects at the individual, species, or community level in marine ecosystems is a difficult task that requires the use of multiple lines of evidence (Adams, 2005). Fish are excellent candidates for the study of the effects of climate variability (Pörtner and Peck, 2010). In the Mediterranean Sea, besides the phenomenon of a northward shift in population distribution by native Mediterranean species, the arrival of alien species is also playing an important role in carving the faunal assemblages of the Mediterranean Sea. It is presumed that the coldest parts of the Mediterranean Sea (Gulf of Lyon and North Adriatic) could initially serve as a sanctuary for cold-temperate species, though continued warming of these areas could turn them into a cul-de-sac for such species. This is especially important for endemic species that could become extinct due to the trapping effect (Ben Rais Lasram et al., 2010).
Sclerochronology research has the potential to provide insight into environmental changes in the Mediterranean, both at the local and regional scales (Peharda et al., 2019a). Recent research conducted on bivalves in the Adriatic Sea resulted in a construction of bivalve chronologies (Peharda et al., 2018, 2019a) and geochemical analysis of shells (Markulin et al., 2019; Peharda et al., 2019b), providing important time-series data for comparative analysis and a multispecies approach. Such a multispecies approach has been very promising in other parts of the world, including the work by Black (2009), who analyzed growth increments in trees, bivalves and fish to identify climate variability signals. Further development of fish sclerochronology research in the Mediterranean could facilitate a multi-taxa approach, enabling us to gain a better understanding of environmental drivers in marine habitats.
Author Contributions
SM-S and MP analyzed the data and existing literature in collaboration with DV, HU, and KM. SM-S and MP wrote the draft of the manuscript. All authors conceived the research, and participated in the improvement and revision of the document.
Funding
This study was fully supported by the Croatian Science Foundation (HRZZ) under project IP-2016-06-9884 (NurseFish).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00195/full#supplementary-material
TABLE S1 | Published sclerochronology research relating to otolith analysis in the Mediterranean Sea.
Footnotes
References
Adams, S. M. (2005). Assessing cause and effect of multiple stressors on marine systems. Mar. Poll. Bull. 51, 649–657. doi: 10.1016/j.marpolbul.2004.11.040
Allain, V., and Lorance, P. (2000). Age estimation and growth of some deep-sea fish from the Northeast Atlantic Ocean. Cybium 24, 7–16.
Andronis, C., Evans, N. J., McDonald, B. J., Nice, H. E., and Gagnon, M. M. (2017). Otolith microchemistry: insights into bioavailable pollutants in a man-made, urban inlet. Mar. Pollut. Bull. 118, 382–387. doi: 10.1016/j.marpolbul.2017.02.037
Arechavala-Lopez, P., Milošević-González, M., and Sanchez-Jerez, P. (2016). Using trace elements in otoliths to discriminate between wild and farmed European sea bass (Dicentrarchus labrax L.) and Gilthead sea bream (Sparus aurata L.). Int. Aquat. Res. 8, 263–273. doi: 10.1007/s40071-016-0142-1
Arneri, E., Colella, S., and Giannetti, G. (1998). A method for the age determination of two Mediterranean sciaenids, Sciaena umbra (Linnaeus, 1758) and Umbrina cirrhosa (Linnaeus, 1758). Rapp. Comm. Int. Mer Médit. 35, 366–367.
Azzurro, E., Sbragaglia, V., Cerri, J., Bariche, M., Bolognini, L., Souissi, J. B., et al. (2019). Climate change, biological invasions, and the shifting distribution of Mediterranean fishes: a large-scale survey based on local ecological knowledge. Glob. Change Biol. 2019, 2779–2792. doi: 10.1111/gcb.14670
Bariche, M. (2010). First record of the angelfish Pomacanthus maculosus (Teleostei: Pomacanthidae) in the Mediterranean. Aqua 16, 31–33.
Battaglia, P., Romeo, T., Consoli, P., Scotti, G., and Andaloro, F. (2010). Characterization of the artisanal fishery and its socio-economic aspects in the central Mediterranean Sea (Aeolian Islands. Italy. Fish. Res. 102, 87–97. doi: 10.1016/j.fishres.2009.10.013
Baudouin, M., Marengo, M., Pere, A., Culioli, J. M., Santoni, M. C., Marchand, B., et al. (2016). Comparison of otolith and scale readings for age and growth estimation of common dentex Dentex dentex. J. Fish Biol. 88, 760–766. doi: 10.1111/jfb.12816
Belcari, P., Ligas, A., and Viva, C. (2006). Age determination and growth of juveniles of the European hake, Merluccius merluccius (L., 1758), in the northern Tyrrhenian Sea (NW Mediterranean). Fish. Res. 78, 211–217. doi: 10.1016/j.fishres.2006.01.006
Bell, J. D., Watson, R. A., and Ye, Y. (2017). Global fishing capacity and fishing effort from 1950 to 2012. Fish Fish. 18, 489–505. doi: 10.1111/faf.12187
Ben Rais Lasram, F., Guilhaumon, F., Albouy, C., Somot, S., Thuiller, W., and Mouillot, D. (2010). The Mediterranean Sea as a ‘cul-de-sac’ for endemic fishes facing climate change. Glob. Change Biol. 16, 3233–3245. doi: 10.1111/j.1365-2486.2010.02224.x
Bilton, H. (1974). “Effects of starvation and feeding on circulus formation on scales of young sockeye salmon of four racial origins, and of one race of young kokanee, coho and chinook salmon,” in The Ageing of Fish, ed. T. B. Bagenal (Surrey: Unwin Brothers Ltd), 40–70.
Black, B. A. (2009). Climate-driven synchrony across tree, bivalve, and rockfish growth-increment chronologies of the northeast Pacific. Mar. Ecol. Prog. Ser. 378, 37–46. doi: 10.3354/meps07854
Black, B. A., Andersson, C., Butler, P. G., Carroll, M. L., DeLong, K. L., Reynolds, D. J., et al. (2019). The revolution of crossdating in marine palaeoecology and palaeoclimatology. Biol. Lett. 15:20180665. doi: 10.1098/rsbl.2018.0665
Black, B. A., Boehlert, G. W., and Yoklavich, M. M. (2005). Using tree-ring crossdating techniques to validate annual growth increments in long-lived fishes. Can. J. Fish. Aquat. Sci. 62, 2277–2284. doi: 10.1139/f05-142
Black, B. A., Boehlert, G. W., and Yoklavich, M. M. (2008). Establishing climate-growth relationships for yelloweye rockfish (Sebastes ruberrimus) in the northeast Pacific using a dendrochronological approach. Fish. Oceanogr. 17, 368–379. doi: 10.1111/j.1365-2419.2008.00484.x
Bouchoucha, M., Pécheyran, C., Gonzalez, J. L., Lenfant, P., and Darnaude, A. M. (2018). Otolith fingerprints as natural tags to identify juvenile fish life in ports. Estuar. Coast. Shelf Sci. 212, 210–218. doi: 10.1016/j.ecss.2018.07.008
Butler, P. G., Wanamaker, A. D., Scourse, J. D., Richardson, C. A., and Reynolds, D. J. (2013). Variability of marine climate on the North Icelandic Shelf in a 1357-year proxy archive based on growth increments in the bivalve Arctica islandica. Palaeogeogr. Palaeoclimatol. Palaeoecol. 373, 141–151. doi: 10.1016/j.palaeo.2012.01.016
Calò, A., Di Franco, A., De Benedetto, G. E., Pennetta, A., Pérez-Ruzafa, Á, and García-Charton, J. A. (2016). Propagule dispersal and larval patch cohesiveness in a Mediterranean coastal fish. Mar. Ecol. Prog. Ser. 544, 213–224. doi: 10.3354/meps11609
Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 188, 263–297. doi: 10.3354/meps188263
Campana, S. E., and Neilson, J. D. (1985). Microstructure of fish otoliths. Can. J. Fish. Aquat. Sci. 42, 1014–1032. doi: 10.1139/f85-127
Campana, S. E., and Thorrold, S. R. (2001). Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 58, 30–38. doi: 10.1139/f00-177
Capoccioni, F., Lin, D.-Y., Iizuka, Y., Tzeng, W. N., and Ciccotti, E. (2014). Phenotypic plasticity in habitat use and growth of the European eel (Anguilla anguilla) in transitional waters in the Mediterranean area. Ecol. Freshw. Fish 23, 65–76. doi: 10.1111/eff.12049
Carbonara, P., Intini, S., Kolitari, J., Joksimović, A., Milone, N., Lembo, G., et al. (2018). A holistic approach to the age validation of Mullus barbatus L., 1758 in the Southern Adriatic Sea (Central Mediterranean). Sci. Rep. 8:13219. doi: 10.1038/s41598-018-30872-1
Catalán, I. A., Alós, J., Díaz-Gil, C., Pérez-Mayol, S., Basterretxea, G., Morales-Nin, B., et al. (2018). Potential fishing-related effects on fish life history revealed by otolith microchemistry. Fish. Res. 199, 186–195. doi: 10.1016/j.fishres.2017.11.008
Chang, M. Y., and Geffen, A. J. (2013). Taxonomic and geographic influences on fish otolith microchemistry. Fish Fish. 14, 458–492. doi: 10.1111/j.1467-2979.2012.00482.x
Chang, M. Y., Geffen, A. J., Kosler, J., Dundas, S. H., and Maes, G. E. (2012). The effect of ablation pattern on LA-ICPMS analysis of otolith element composition in hake Merluccius merluccius. Environ. Biol. Fish. 95, 509–520. doi: 10.1007/s10641-012-0065-7
Chater, I., Romdhani-Dhahri, A., Dufour, J. L., Mahé, K., and Chakroun-Marzouk, N. (2018). Age, growth and mortality of Sciaena umbra (Sciaenidae) in the Gulf of Tunis. Sci. Mar. 82, 17–25. doi: 10.3989/scimar.04679.21a
Chilton, D. E., and Beamish, R. J. (1982). Age determination methods for fishes studied by the groundfish program at the Pacific Biological Station. Can. Spec. Publ. Fish. Aquat. Sci. 60:102.
Coll, M., Piroddi, C., Albouy, C., Ben Rais Lasram, F., Cheung, W. W. L., Christensen, V., et al. (2012). The Mediterranean under siege: spatial overlap between marine biodiversity, cumulative threats and marine reserves. Glob. Ecol. Biogeogr. 21, 465–481.
Comeros-Raynal, M. T., Polidoro, B., Broatch, J., Mann, B. Q., Gorman, C., Buxton, C., et al. (2016). Key predictors of extinction risk in sea breams and porgies (Family: Sparidae). Biol. Conserv. 202, 88–98. doi: 10.1016/j.biocon.2016.08.027
Consoli, P., Battaglia, P., Castriota, L., Esposito, V., Romeo, T., and Andaloro, F. (2010). Age, growth and feeding habits of the bluemouth rockfish, Helicolenus dactylopterus dactylopterus (Delaroche 1809) in the central Mediterranean (southern Tyrrhenian Sea). J. Appl. Ichthyol. 26, 583–591. doi: 10.1111/j.1439-0426.2010.01467.x
Correia, A. T., Barros, F., and Sial, A. N. (2011). Stock discrimination of European conger eel (Conger conger L.) using otolith stable isotope ratios. Fish. Res. 108, 88–94. doi: 10.1016/j.fishres.2010.12.002
Correia, A. T., Ramos, A. A., Barros, F., Silva, G., Hamer, P., Morais, P., et al. (2012). Population structure and connectivity of the European conger eel (Conger conger) across the north-eastern Atlantic and western Mediterranean: integrating molecular and otolith elemental approaches. Mar. Biol. 159, 1509–1525. doi: 10.1007/s00227-012-1936-3
Costello, M. J., Coll, M., Danovaro, R., Halpin, P., Ojaveer, H., and Miloslavich, P. (2010). A census of marine biodiversity knowledge, resources and future challenges. PLoS One 5:e12110. doi: 10.1371/journal.pone.0012110
Coulson, P. G., Black, B. A., Potter, I. C., and Hall, N. G. (2014). Sclerochronological studies reveal that patterns of otolith growth of adults of two co-occurring species of Platycephalidae are synchronised by water temperature variations. Mar. Biol. 161, 383–393. doi: 10.1007/s00227-013-2343-0
Currey, D. R. (1965). An ancient bristlecone pine stand in eastern Nevada. Ecology 46, 564–566. doi: 10.2307/1934900
Darnaude, A. M., and Hunter, E. (2018). Validation of otolith d18O values as effective natural tags for shelf-scale geolocation of migrating fish. Mar. Ecol. Prog. Ser. 598, 167–185. doi: 10.3354/meps12302
Davies, C. A., Brophy, D., Jeffries, T., and Gosling, E. (2011). Trace elements in the otoliths and dorsal spines of albacore tuna (Thunnus alalunga, Bonnaterre, 1788): an assessment of the effectiveness of cleaning procedures at removing post mortem contamination. J. Exp. Mar. Bio. Ecol. 396, 162–170. doi: 10.1016/j.jembe.2010.10.016
de Pontual, H., Bertignac, M., Battaglia, A., Bavouzet, G., Moguedet, P., and Groison, A.-L. (2003). A pilot tagging experiment on European hake (Merluccius merluccius): methodology and preliminary results. ICES J. Mar. Sci. 60, 1318–1327. doi: 10.1016/S1054-3139(03)00149-8
Demirci, S., Özyılmaz, A., Öksüz, A., Nadir, R. S., and Şimşek, E. (2018). Otolith chemistry of Champsodon nudivittis (Ogilby, 1895) and Nemipterus randalli (Russell, 1986) in Iskenderun Bay, Turkey. J. Appl. Ichthyol. 34, 1131–1135. doi: 10.1111/jai.13761
Di Franco, A, Bulleri, F., Pennetta, A., De, Benedetto G, Clarke, K. R., and Guidetti, P. (2014). Within-otolith variability in chemical fingerprints: implications for sampling designs and possible environmental interpretation. PLoS One 9:e101701. doi: 10.1371/journal.pone.0101701
Di Franco, A., de Benedetto, G., de Rinaldis, G., Raventos, N., Sahyoun, R., and Guidetti, P. (2011). Large scale-variability in otolith microstructure and microchemistry: the case study of Diplodus sargus sargus (Pisces: Sparidae) in the Mediterranean Sea. Ital. J. Zool. 78, 182–192. doi: 10.1080/11250003.2011.566227
Di Franco, A., Calò, A., Pennetta, A., De Benedetto, G., Planes, S., and Guidetti, P. (2015a). Dispersal of larval and juvenile seabream: implications for Mediterranean marine protected areas. Biol. Conserv. 192, 361–368. doi: 10.1016/j.biocon.2015.10.015
Di Franco, A., Gianni, F., and Guidetti, P. (2015b). Mismatch in early life traits between settlers and recruits in a Mediterranean fish: clue of the relevance of the settlement tail? Acta Ichthyol. Piscat. 45, 153–159. doi: 10.3750/AIP2015.45.2.05
Di Franco, A., Gillanders, B. M., De Benedetto, G., Pennetta, A., De Leo, G. A., and Guidetti, P. (2012). Dispersal patterns of coastal fish: implications for designing networks of marine protected areas. PLoS One 7:e31681. doi: 10.1371/journal.pone.0031681
Dierking, J., Morat, F., Letourneur, Y., and Harmelin-Vivien, M. (2012). Fingerprints of lagoonal life: migration of the marine flatfish Solea solea assessed by stable isotopes and otolith microchemistry. Estuar. Coast. Shelf Sci. 10, 23–32. doi: 10.1016/j.ecss.2011.03.018
Disspain, M. C. F., Ulm, S., and Gillanders, B. M. (2016). Otoliths in archaeology: methods, applications and future prospects. J. Archaeol. Sci. Rep. 6, 623–632. doi: 10.1016/j.jasrep.2015.05.012
D’Onghia, G., Lloris, D., Politou, C.-Y., Sion, L., and Dokos, J. (2004). New records of deep-water teleost fishes in the Balearic Sea and Ionian Sea (Mediterranean Sea). Sci. Mar. 68, 171–183. doi: 10.3989/scimar.2004.68s3171
Elsdon, T. S., Wells, B., Campana, S., Gillanders, B., Jones, C., Limburg, K., et al. (2008). Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences in Oceanography and marine biology: an annual review, Vol. 46. Boca Raton: CRC Press-Taylor & Francis Group, 297–330.
Fortunato, C. R., Durà, B. V., and Volpedo, A. (2017a). Otolith morphometry and microchemistry as habitat markers for juvenile Mugil cephalus Linnaeus 1758 in nursery grounds in the Valencian community, Spain. J. Appl. Ichthyol. 33, 163–167. doi: 10.1111/jai.13291
Fortunato, C. R., Galán, A. R., Alonso, G. I., Volpedo, A., and Durà, B. V. (2017b). Environmental migratory patterns and stock identification of Mugil cephalus in the Spanish Mediterranean Sea, by means of otolith microchemistry. Estuar. Coast. Shelf Sci. 188, 174–180. doi: 10.1016/j.ecss.2017.02.018
Fraile, I., Arrizabalaga, H., Groeneveld, J., Kölling, M., Santos, M. N., Macías, D., et al. (2016). The imprint of anthropogenic CO2 emissions on Atlantic bluefin tuna otoliths. J. Mar. Syst. 158, 26–33. doi: 10.1016/j.jmarsys.2015.12.012
Fry, G. C., Brewer, D. T., and Venables, W. N. (2006). Vulnerability of deepwater demersal fishes to commercial fishing: evidence from a study around a tropical volcanic seamount in Papua New Guinea. Fish. Res. 81, 126–141. doi: 10.1016/j.fishres.2006.08.002
Gillanders, B. M., Sanchez-Jerez, P., Bayle-Sempere, J., and Ramos-Espla, A. (2001). Trace elements in otoliths of the two-banded bream from a coastal region in the south-west Mediterranean: are there differences among locations? J. Fish Biol. 59, 350–363. doi: 10.1006/jfbi.2001.1643
Gillikin, D. P., Wanamaker, A. D., and Andrus, C. F. T. (2019). Chemical sclerochronology. Chem. Geol. 526, 1–6. doi: 10.1016/j.chemgeo.2019.06.016
González-Quirós, R., Del Árbol, J., García-Pacheco, M. del M, Silva-García, A. J., Naranjo, J. M., Nin, B. M., et al. (2011). Life-history of the meagre Argyrosomus regius in the Gulf of Cádiz (SW Iberian Peninsula). Fish. Res. 109, 140–149. doi: 10.1016/j.fishres.2011.01.031
Gracia_López, V., and Castelló-Orway, F. (2003). Preliminary data on the culture of juveniles of the dusky grouper, Epinephelus marginatus (Lowe, 1834). Hidrobiológica 13, 321–327.
Grandcourt, E. M., Al Abdessalaam, T. Z., Francis, F., and Al Shamsi, A. T. (2004). Biology and stock assessment of the Sparids, Acanthopagrus bifasciatus and Argyrops spinifer (Forsskål, 1775), in the Southern Arabian Gulf. Fish. Res. 69, 7–20. doi: 10.1016/j.fishres.2004.04.006
Grønkjaer, P., Pedersen, J. B., Ankjaerø, T. T., Kjeldsen, H., Heinemeier, J., Steingrund, P., et al. (2013). Stable N and C isotopes in the organic matrix of fish otoliths: validation of a new approach for studying spatial and temporal changes in the trophic structure of aquatic ecosystems. Can. J. Fish. Aquat. Sci. 70, 143–146. doi: 10.1139/cjfas-2012-0386
Guidetti, P., Petrillo, M., De Benedetto, G., and Albertelli, G. (2013). The use of otolith microchemistry to investigate spawning patterns of European anchovy: a case study in the eastern Ligurian Sea (NW Mediterranean). Fish. Res. 139, 1–4. doi: 10.1016/j.fishres.2012.10.015
Gutiérrez, E., and Morales-Nin, B. (1986). Time series analysis of daily growth in Dicentrarchuslabrax L. otoliths. J. Exp. Mar. Biol. Ecol. 103, 163–179. doi: 10.1016/0022-0981(86)90139-5
Halpern, B. S., Walbridge, S., Selkoe, K. A., Kappel, C. V., Micheli, F., D’Agrosa, C., et al. (2008). A global map of human impact on marine ecosystems. Science 319, 948–952. doi: 10.1126/science.1149345
Hanel, R., and Tsigenopoulos, C. S. (2011). “Phylogeny, Evolution and Taxonomy of sparids with some notes on their Ecology and Biology in Sea bream,” in Biology & Aquaculture of Sparidaen, eds M. Pavlidis and C. C. Mylonas (Hoboken, NJ: Wiley-Blackwell), 51–74.
Heemstra, P. C., and Randall, J. E. (1993). FAO Species Catalogue Cephalopods. An Annotated and Illustrated Catalogue of the Grouper, Rockcod, Hind, Coral, Grouper, and Lyretail Species Known to Date. Rome: FAO Fish.
Hidalgo, M., Tomás, J., Høie, H., Morales-Nin, B., and Ninnemann, U. S. (2008). Environmental influences on the recruitment process inferred from otolith stable isotopes in Merluccius merluccius off the Balearic Islands. Aquat. Biol. 3, 195–207. doi: 10.3354/ab00081
Hudson, J. H., Shinn, E. A., Halley, R. B., and Lidz, B. (1976). Sclerochronology: a tool for interpreting past environments. Geology 4, 361–364. doi: 10.1130/0091-761319764<361:SATFIP>2.0.CO;2
Hüssy, K., Coad, J. O., Farrell, E. D., Clausen, L. W., and Clarke, M. W. (2012). Sexual dimorphism in size, age, maturation, and growth characteristics of boarfish (Capros aper) in the Northeast Atlantic. ICES J. Mar. Sci. 69, 1729–1735. doi: 10.1093/icesjms/fss156
Hüssy, K., Radtke, K., Plikshs, M., Oeberst, R., Baranova, T., Krumme, U., et al. (2016). Challenging ICES age estimation protocols: lessons learned from the eastern Baltic cod stock. ICES J. Mar. Sci. 73, 2138–2149. doi: 10.1093/icesjms/fsw107
Iacumin, P., Bianucci, G., and Longinelli, A. (1992). Oxygen and carbon isotopic composition of fish otoliths. Mar. Biol. 113, 537–542. doi: 10.1007/BF00349696
IUCN (2017). IUCN Red List of Threatened Species. Version 2017–2. Available online at: www.iucnredlist.org (accessed December 17, 2019).
Izzo, C., Reis-Santos, P., and Gillanders, B. M. (2018). Otolith chemistry does not just reflect environmental conditions: a meta-analytic evaluation. Fish Fish. 19, 441–454. doi: 10.1111/faf.12264
Jarzhombek, A. A. (2007). Compilation of studies on the growth of Acanthopterygii. Russian Federal Res. Ins. Fish. Oceanogr. (VNIRO). 86.
Jones, D. S. (1983). Sclerochronology: reading the record of the molluscan shell. Am. Sci. 71, 384–391.
Kailola, P. J., Williams, M. J., Stewart, P. C., Reichelt, R. E., McNee, A., and Grieve, C. (1993). Australian Fisheries Resources. Canberra: Bureau of Resource Sciences, 422.
Katsanevakis, S., Bogucarskis, K., Gatto, F., Vandekerkhove, J., Deriu, I., and Cardoso, A. C. (2012). Building the European Alien Species Information Network (EASIN): a novel approach for the exploration of distributed alien species data. Bioinvasions Rec 1, 235–245. doi: 10.3391/bir.2012.1.4.01
Khemiri, S., Labonne, M., Gaamour, A., Munaron, J. M., and Morize, E. (2014). The use of otolith chemistry to determine stock structure of Sardina pilchardus and Engraulis encrasicolus in Tunisian Coasts. Cah. Biol. Mar. 55, 21–29.
Kitagawa, T., Ishimura, T., Uozato, R., Shirai, K., Amano, Y., Shinoda, A., et al. (2013). Otolith δ18O of Pacific bluefin tuna Thunnus orientalis as an indicator of ambient water temperature. Mar. Ecol. Prog. Ser. 481, 199–209. doi: 10.3354/meps10202
Kottelat, M., and Freyhof, J. (2007). Handbook of European Freshwater Fishes. Berlin: Publications Kottelat, Cornol and Freyhof.
Kraljević, M., Dulčić, J., and Tudor, M. (1998). Growth parameters of the gilt-head sea bream Sparus aurata L. in the eastern Adriatic (Croatian waters). Period. Biol. 100, 87–91.
Lacoue-Labarthe, T., Nunes, P. A. L. D., Ziveri, P., Cinar, M., Gazeau, F., Hall-Spencer, J. M., et al. (2016). Impacts of ocean acidification in a warming Mediterranean Sea: an overview. Reg. Stud. Mar. Sci. 5, 1–11. doi: 10.1016/j.rsma.2015.12.005
Lejeusne, C., Chevaldonne, P., Pergent-Martini, C., Boudouresque, C. F., and Perez, T. (2010). Climate change effects on a miniature ocean: the highly diverse, highly impacted Mediterranean Sea. Trends Ecol.Evol. 25, 250–260. doi: 10.1016/j.tree.2009.10.009
Lleonart, J., and Maynou, F. (2003). Fish stock assessment in Mediterranean: state of the art. Sci. Mar. 67, 37–49. doi: 10.3989/scimar.2003.67s137
Marali, S., Schöne, B. R., Mertz-Kraus, R., Griffin, S. M., Wanamaker, A. D., Matras, U., et al. (2017). Ba/Ca ratios in shells of Arctica islandica - Potential environmental proxy and crossdating tool. Palaeogeogr. Palaeoclimatol. Palaeoecol. 465, 347–361. doi: 10.1016/j.palaeo.2015.12.018
Marini, M., Campanelli, A., and Abballe, F. (2006). Measurement of alkaline and earthy ions in fish otolith and sea water using a high performance ion chromatography. Mar. Chem. 99, 24–30. doi: 10.1016/j.marchem.2005.01.009
Markulin, K., Peharda, M., Mertz-Kraus, R., Schöne, B. R., Uvanović, H., and Kovač, Ž, et al. (2019). Trace and minor element records in aragonitic bivalve shells as environmental proxies. Chem. Geol. 507, 120–133. doi: 10.1016/j.chemgeo.2019.01.008
Martino, J. C., Fowler, A. J., Doubleday, Z. A., Grammer, G. L., and Gillanders, B. M. (2019). Using otolith chronologies to understand long-term trends and extrinsic drivers of growth in fisheries. Ecosphere 10:e02553. doi: 10.1002/ecs2.2553
Matta, M. E., Helser, T. E., and Black, B. A. (2018). Intrinsic and environmental drivers of growth in an Alaskan rockfish: an otolith biochronology approach. Environ. Biol. Fish. 101, 1571–1587. doi: 10.1007/s10641-018-0801-8
Mellon-Duval, C., De Pontual, H., Metral, L., and Quemener, L. (2010). Growth of European hake (Merluccius merluccius) in the Gulf of Lions based on conventional tagging. ICES J. Mar. Sci. 67, 62–70. doi: 10.1093/icesjms/fsp215
Mercier, L., Mouillot, D., Bruguier, O., Vigliola, L., and Darnaude, A. M. (2012). Multi-element otolith fingerprints unravel sea-lagoon lifetime migrations of gilthead sea bream Sparus aurata. Mar. Ecol. Prog. Ser. 444, 175–194. doi: 10.3354/meps09444
Micheli, F., Halpern, B. S., Walbridge, S., Ciriaco, S., Ferretti, F., Fraschetti, S., et al. (2013). Cumulative human impacts on Mediterranean and Black Sea marine ecosystems: assessing current pressures and opportunities. PLoS One 8:e79889. doi: 10.1371/journal.pone.0079889
Mirasole, A., Gillanders, B. M., Reis-Santos, P., Grassa, F., Capasso, G., Scopelliti, G., et al. (2017). The influence of high pCO2 on otolith shape, chemical and carbon isotope composition of six coastal fish species in a Mediterranean shallow CO2 vent. Mar. Biol. 164, 1–15. doi: 10.1007/s00227-017-3221-y
Morales-Nin, B. (2000). “Daily increments in otoliths: endogenous versus exogenous growth regulation,” in Proceedings of the Second International Symposium on Fish Otolith Research and Application (Norway: Fisheries Research Special Publication), 53–68.
Morales-Nin, B., and Aldebert, Y. (1997). Growth of juvenile Merluccius merluccius in the Gulf of Lions (NW Medterranean) based on otolith microstructure and length-frequency analysis. Fish. Res. 30, 77–85. doi: 10.1016/s0165-7836(96)00553-x
Morales-Nin, B., and Moranta, J. (1997). Life history and fishery of the common dentex (Dentex dentex) in Mallorca (Balearic Islands, western Mediterranean). Fish. Res. 30, 67–76. doi: 10.1016/s0165-7836(96)00560-7
Morales-Nin, B., Pérez-Mayol, S., Palmer, M., and Geffen, A. J. (2014). Coping with connectivity between populations of Merluccius merluccius: an elusive topic. J. Mar. Syst. 138, 211–219. doi: 10.1016/j.jmarsys.2014.04.009
Morales-Nin, B., Swan, S. C., Gordon, J. D. M., Palmer, M., Geffen, A. J., Shimmield, T., et al. (2005a). Age-related trends in otolith chemistry of Merluccius merluccius from the north-eastern Atlantic Ocean and the western Mediterranean Sea. Mar. Freshw. Res. 56, 599–607. doi: 10.1071/MF04151
Morales-Nin, B., Tores, G. J., Lombarte, A., and Recasens, L. (2005b). Otolith growth and age estimation in the European hake. J. Fish Biol. 53, 1155–1168. doi: 10.1111/j.1095-8649.1998.tb00239.x
Morat, F., Blamart, D., Candaudap, F., and Letourneur, Y. (2013). Differences in elemental chemistry and c-o stable isotope composition between left and right otoliths of a flatfish, the common sole Solea solea. Vie Milieu 63, 169–179.
Morat, F., Lecomte-Finiger, R., Blamart, D., Robert, M., and Letourneur, Y. (2012). Indicios preliminares de variaciones ontogenéticas y espaciales en las señales isotópicas y elementales de otolitos de Solea solea del Golfo de León (Mediterráneo noroccidental). Sci. Mar. 76, 647–657. doi: 10.3648/scimar.03648.09B
Morat, F., Letourneur, Y., Blamart, D., Pécheyran, C., Darnaude, A. M., and Harmelin-Vivien, M. (2014a). Offshore-onshore linkages in the larval life history of sole in the Gulf of Lions (NW-Mediterranean). Estuar. Coast. Shelf Sci. 149, 194–202. doi: 10.1016/j.ecss.2014.08.023
Morat, F., Letourneur, Y., Dierking, J., Pećheyran, C., Bareille, G., Blamart, D., et al. (2014b). The great melting pot. Common sole population connectivity assessed by otolith and water fingerprints. PLoS One 9:e0086585. doi: 10.1371/journal.pone.0086585
Morrongiello, J., Thresher, R., and Smith, D. (2012). Aquatic biochronologies and climate change. Nat. Clim. Change 2, 849–857. doi: 10.1038/nclimate1616
Mouillot, D., Albouy, C., Guilhaumon, F., Ben Rais Lasram, F., Coll, M., Devictor, V., et al. (2011). Protected and threatened components of fish biodiversity in the Mediterranean Sea. Curr. Biol. 21, 1044–1050. doi: 10.1016/j.cub.2011.05.005
Mounicou, S., Frelon, S., Le Guernic, A., Eb-Levadoux, Y., Camilleri, V., Février, L., et al. (2019). Use of fish otoliths as a temporal biomarker of field uranium exposure. Sci. Total Environ. 690, 511–521. doi: 10.1016/j.scitotenv.2019.06.534
Niklitschek, E. J., and Darnaude, A. M. (2016). Performance of maximum likelihood mixture models to estimate nursery habitat contributions to fish stocks: a case study on sea bream Sparus aurata. Peer J. 4:e2415. doi: 10.7717/peerj.2415
Oschmann, W. (2009). Sclerochronology: editorial. Int. J. Earth Sci. 98, 1–2. doi: 10.1007/s00531-008-0403-3
Panfili, J., Darnaude, A. M., Lin, Y. J., Chevalley, M., Iizuka, Y., Tzeng, W. N., et al. (2012). Habitat residence during continental life of the European eel Anguilla anguilla investigated using linear discriminant analysis applied to otolith Sr:Ca ratios. Aquat. Biol. 15, 175–185. doi: 10.3354/ab00414
Panfili, J., Meunier, F. J., Mosegaard, H., Troadec, H., Wright, P. J., and Geffen, A. J. (2002). Manual of Fish Sclerochronology. Brest: Ifremer-Ird coedition.
Papadopoulou, C., Kanias, G. D., and Moraitopoulou Kassimati, E. (1978). Zinc content in otoliths of mackerel from the Aegean. Mar. Pollut. Bull. 9, 106–108. doi: 10.1016/0025-326x(78)90482-4
Papadopoulou, C., Kanias, G. D., and Moraitopoulou-Kassimati, E. (1980). Trace element content in fish otoliths in relation to age and size. Mar. Pollut. Bull. 11, 68–72. doi: 10.1016/0025-326X(80)90546-9
Papadopoulou, C., and Moraitopoulou-Kassimati, E. (1977). Stable elements in skeletal formations of fish species from Greek waters. Thalassia Jugoslavica 13, 187–192.
Pauly, D., and Zeller, D. (2016). Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 7:10244. doi: 10.1038/ncomms10244
Peharda, M., Vilibić, I., Black, B., Uvanović, H., Markulin, K., and Mihanović, H. (2019a). A network of bivalve chronologies from semi-enclosed seas. PLoS One 14:e0220520. doi: 10.1371/journal.pone.0220520
Peharda, M., Walliser, E. O., Markulin, K., Purroy, A., Uvanović, H., Janeković, I., et al. (2019b). Glycymeris pilosa (Bivalvia) – A high-potential geochemical archive of the environmental variability in the Adriatic Sea. Mar. Env. Res. 150:104759. doi: 10.1016/j.marenvres.2019.104759
Peharda, M., Vilibić, I., Black, B. A., Markulin, K., Dunić, N., Džoić, T., et al. (2018). Using bivalve chronologies for quantifying environmental drivers in a semi-enclosed temperate sea. Sci. Rep. 8:5559. doi: 10.1038/s41598-018-23773-w
Peres, M. B., and Haimovici, M. (2004). Age and growth of southwestern Atlantic wreckfish Polyprion americanus. Fish. Res. 66, 157–169. doi: 10.1016/s0165-7836(03)00207-8
Piddocke, T. P., Butler, G. L., Butcher, P. A., Purcell, S. W., Butcher, J. D., and Christidis, L. L. (2015). Age validation in the Lutjanidae: a review. Fish. Res. 167, 48–63. doi: 10.1016/j.fishres.2015.01.016
Piñeiro, C., Rey, J., de Pontual, H., and Goñi, R. (2007). Tag and recapture of European hake (Merluccius merluccius L.) off the Northwest Iberian Peninsula: first results support fast growth hypothesis. Fish. Res. 88, 150–154. doi: 10.1016/j.fishres.2007.08.015
Pörtner, H. O., and Peck, M. A. (2010). Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J. Fish Biol. 77, 1745–1779. doi: 10.1111/j.1095-8649.2010.02783.x
Prendergast, A. L., and Schöne, B. R. (2017). Oxygen isotopes from limpet shells: implications for palaeothermometry and seasonal shellfish foraging studies in the Mediterranean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 484, 33–47. doi: 10.1016/j.palaeo.2017.03.007
Ragonese, S. (1989). L‘Applicazione dell’equazione di von Bertalanffy generale: il caso di Helicolenus dactylopterus (Delar.) (Pisces: Scorpaenidae) del Tirreno Settentrionale. Oebalia 15, 753–762.
Regulation (EU) (2017). 2017/1004 of the European Parliament and of the Council of 17 May 2017 on the establishment of a Union framework for the Collection, Management and Use of Data in the Fisheries Sector and Support for Scientific Advice Regarding the Common Fisheries Policy and Repealing Council Regulation (EC) No 199/2008. Brussels: EU.
Reñones, O., Grau, A., Mas, X., Riera, F., and Saborido-Rey, F. (2010). Reproductive pattern of an exploited dusky grouper Epinephelus marginatus (Lowe 1834) (Pisces: Serranidae) population in the western Mediterranean. Sci. Mar. 74, 523–537. doi: 10.3989/scimar.2010.74n3523
Reñones, O., Pineiro, C., Mas, X., and Goni, R. (2007). Age and growth of the dusky grouper Epinephelus marginatus (Lowe 1834) in an exploited population of the western Mediterranean Sea. J. Fish Biol. 71, 346–362. doi: 10.1111/j.1095-8649.2007.01482.x
Reynolds, D. J., Hall, I. R., Slater, S. M., Mette, M. J., Wanamaker, A. D., Scourse, J. D., et al. (2018). Isolating and reconstructing key components of North Atlantic Ocean variability from a sclerochronological spatial network. Paleoceanogr. Paleoclimatol. 33, 1086–1098. doi: 10.1029/2018PA003366
Rezende, S. M., and Ferreira, B. P. (2004). Age, growth and mortality of dog snapper Lutjanus jocu (Bloch & Schneider, 1801) in the northeast coast of Brazil. Braz. J. Oceanogr. 52, 107–121. doi: 10.1590/S1679-87592004000200003
Rochet, M. J., and Trenkel, V. M. (2003). Which community indicators can measure the impact of fishing? A review and proposals. Can. J. Fish Aquat. Sci. 60, 86–99. doi: 10.1139/f02-164
Rochet, M.-J., Trenkel, V. M., Bellail, R., Coppin, F., Le Pape, O., Mahé, J. C., et al. (2005). Combining indicator trends to assess ongoing changes in exploited fish communities: diagnostic of communities off the coasts of France. ICES J. Mar. Sci. 62:1647e1664. doi: 10.1016/j.icesjms.2005.06.009
Rooker, J. R., Arrizabalaga, H., Fraile, I., Secor, D. H., Dettman, D. L., Abid, N., et al. (2014). Crossing the line: migratory and homing behaviors of Atlantic bluefin tuna. Mar. Ecol. Prog. Ser. 504, 265–276. doi: 10.3354/meps10781
Rooker, J. R., Fraile, I., Liu, H., Abid, N., Dance, M. A., Itoh, T., et al. (2019). Wide-ranging temporal variation in transoceanic movement and population mixing of bluefin tuna in the North Atlantic Ocean. Front. Mar. Sci. 6:398. doi: 10.3389/fmars.2019.00398
Rooker, J. R., Secor, D. H., DeMetrio, G., Kaufman, A. J., Ríos, A. B., and Tičina, V. (2008a). Evidence of trans-Atlantic movement and natal homing of bluefin tuna from stable isotopes in otoliths. Mar. Ecol. Prog. Ser. 368, 231–239. doi: 10.3354/meps07602
Rooker, J. R., Secor, D. H., De Metrio, G., Schloesser, R., Block, B. A., and Neilson, J. D. (2008b). Natal homing and connectivity in Atlantic Bluefin Tuna populations. Science 322, 742–744. doi: 10.1126/science.1161473
Rooker, J. R., Secor, D. H., DeMetrio, G., Kaufman, A. J., Ríos, A. B., and Tičina, V. (2008a). Evidence of trans-Atlantic movement and natal homing of bluefin tuna from stable isotopes in otoliths. Mar. Ecol. Prog. Ser. 368, 231–239. doi: 10.3354/meps07602
Rooker, J. R., Secor, D. H., Zdanowicz, V. S., De Metrio, G., and Relini, L. O. (2003). Identification of Atlantic bluefin tuna (Thunnus thynnus) stocks from putative nurseries using otolith chemistry. Fish. Oceanogr. 12, 75–84. doi: 10.1046/j.1365-2419.2003.00223.x
Rooker, J. R., Secor, D. H., Zdanowicz, V. S., Relini, L. O., Santamaria, N., Deflorio, M., et al. (2002). Otolith elemental fingerprints of Atlantic bluefin tuna from eastern and western nurseries. ICCAT Collect. Vol. Sci. Papers 54, 498–506.
Rountrey, A. N., Coulson, P. G., Meeuwig, J. J., and Meekan, M. (2014). Water temperature and fish growth: otoliths predict growth patterns of a marine fish in a changing climate. Glob. Chang. Biol. 20, 2450–2458. doi: 10.1111/gcb.12617
Sadovy, Y., and Shapiro, D. Y. (1987). Criteria for the diagnosis of hermaphroditism in fishes. Copeia 1987, 136–156.
Sakamoto, T., Komatsu, K., Yoneda, M., Ishimura, T., Higuchi, T., Shirai, K., et al. (2017). Temperature dependence of δ18O in otolith of juvenile Japanese sardine: laboratory rearing experiment with micro-scale analysis. Fish. Res. 194, 55–59. doi: 10.1016/j.fishres.2017.05.004
Schöne, B. R. (2013). Arctica islandica (Bivalvia): a unique paleoenvironmental archive of the northern North Atlantic Ocean. Glob. Planet. Change 111, 199–225. doi: 10.1016/j.gloplacha.2013.09.013
Secor, D. H., Campana, S. E., Zdanowicz, V. S., Lam, J. W. H., Yang, L., and Rooker, J. R. (2002). Inter-laboratory comparison of Atlantic and Mediterranean bluefin tuna otolith microconstituents. ICES J. Mar. Sci. 59, 1294–1304. doi: 10.1006/jmsc.2002.1311
Secor, D. H., and Zdanowicz, V. S. (1998). Otolith microconstituent analysis of juvenile bluefin tuna (Thunnus thynnus) from the Mediterranean Sea and Pacific Ocean. Fish. Res. 36, 251–256. doi: 10.1016/S0165-7836(98)00103-9
Simkiss, K. (1974). “Calcium metabolism of fish in relation to aging,” in The Ageing of Fish, ed. T. B. Bagenal (London: Unwin Brothers Ltd), 1–12.
Siskey, M. R., Wilberg, M. J., Allman, R. J., Barnett, B. K., and Secor, D. H. (2016). Forty years of fishing: changes in age structure and stock mixing in northwestern Atlantic bluefin tuna (Thunnus thynnus) associated with size-selective and long-term exploitation. ICES J. Mar. Sci. J. du Cons. 73, 2518–2528. doi: 10.1093/icesjms/fsw115
Smith, C. L. (1997). National Audubon Society field guide to tropical marine fishes of the Caribbean, the Gulf of Mexico, Florida, the Bahamas, and Bermuda. New York, NY: Alfred A. Knopf, Inc, 720.
Søndergaard, J., Halden, N., Bach, L., Gustavson, K., Sonne, C., and Mosbech, A. (2015). Otolith chemistry of common sculpins (Myoxocephalus scorpius) in a mining polluted Greenlandic fiord (Black Angel Lead-Zinc Mine. West Greenland). Water. Air Soil Pollut. 226:336. doi: 10.1007/s11270-015-2605-1
Sonin, O., Edelist, D., and Golani, D. (2019). The occurrence of the Lessepsian migrant Lutjanus argentimaculatus in the Mediterranean, (Actinopterygii: Perciformes: Lutjanidae) first record from the coast of Israel. Acta Adriat. 60, 99–102. doi: 10.32582/aa.60.1.11
Sreemany, A., Kumar Bera, M., and Sarkar, A. (2017). Rapid and high-resolution stable isotopic measurement of biogenic accretionary carbonate using an online CO2 laser ablation system: standardization of the analytical protocol. Rapid Commun. Mass Spec. 31, 2109–2117. doi: 10.1002/rcm.7992
Steward, C. A., De Maria, K. D., and Shenker, J. M. (2009). Using otolith morphometrics to quickly and inexpensively predict age in the gray angelfish (Pomacanthus arcuatus). Fish. Res. 99, 123–129. doi: 10.1016/j.fishres.2009.05.011
Stransky, C., Gudmundsdóttir, S., Sigurdsson, T., Lemvig, S., Nedreaas, K., and Saborido-Rey, F. (2005). Age determination and growth of Atlantic redfish (Sebastes marinus and S. mentella): bias and precision of age readers and otolith preparation methods. ICES J. Mar. Sci. 62, 655–670. doi: 10.1016/j.icesjms.2005.01.018
Sutton, C. P., Tracey, D. M., Andrews, A. H., Hart, A. C., and MacGibbon, D. J. (2010). Validated age and growth of ribaldo (Mora moro). New Zealand Fisheries Assessesment Report 2010/24. Available online at: M:\SCIPOL\FARs\Electronic copies of Published FARs\2010 FARs\10_24_FAR.pdf (accessed August 19, 2010).
Swan, S. C., Geffen, A. J., Morales-Nin, B., Gordon, J. D. M., Shimmield, T., Sawyer, T., et al. (2006). Otolith chemistry: an aid to stock separation of Helicolenus dactylopterus (bluemouth) and Merluccius merluccius (European hake) in the Northeast Atlantic and Mediterranean. ICES J. Mar. Sci. 63, 504–513. doi: 10.1016/j.icesjms.2005.08.012
Swan, S. C., Gordon, J. D. M., Morales-Nin, B., Shimmield, T., Sawyer, T., and Geffen, A. J. (2003). Otolith microchemistry of Nezumia aequalis (Pisces: Macrouridae) from widely different habitats in the Atlantic and Mediterranean. J. Mar. Biol. Assoc. U. K. 83, 883–886. doi: 10.1017/S0025315403007987h
Tanner, S. E., Perez, M., Presa, P., Thorrold, S. R., and Cabral, H. N. (2014). Integrating microsatellite DNA markers and otolith geochemistry to assess population structure of European hake (Merluccius merluccius). Estuar. Coast. Shelf Sci. 142, 68–75. doi: 10.1016/j.ecss.2014.03.010
Tanner, S. E., Vasconcelos, R. P., Cabral, H. N., and Thorrold, S. R. (2012). Testing an otolith geochemistry approach to determine population structure and movements of European hake in the northeast Atlantic Ocean and Mediterranean Sea. Fish. Res. 12, 198–205. doi: 10.1016/j.fishres.2012.02.013
Tanner, S. E., Vieira, A. R., Vasconcelos, R. P., Dores, S., Azevedo, M., Cabral, H. N., et al. (2019). Regional climate, primary productivity and fish biomass drive growth variation and population resilience in a small pelagic fish. Ecol. Indic. 103, 530–541. doi: 10.1016/j.ecolind.2019.04.056
Tomas, J., Geffen, A. J., Millner, R. S., Pineiro, C. G., and Tserpes, G. (2006). Elemental composition of otolith growth marks in three geographically separated populations of European hake (Merluccius merluccius). Mar. Biol. 148, 1399–1413. doi: 10.1007/s00227-005-0171-6
Tournois, J., Ferraton, F., Velez, L., McKenzie, D. J., Aliaume, C., Mercier, L., et al. (2013). Temporal stability of otolith elemental fingerprints discriminates among lagoon nursery habitats. Estuar. Coast. Shelf Sci. 131, 182–193. doi: 10.1016/j.ecss.2013.07.006
Traina, A., Oliveri, E., Salvagio Manta, D., Barra, M., Mazzola, S., and Cuttitta, A. (2015). Metals content in otoliths of Dicentrarchus labrax from two fish farms of Sicily. Environ. Monit. Assess. 187:360. doi: 10.1007/s10661-015-4434-5
Trueman, C. N., Mackenzie, K. M., and Palmer, M. R. (2012). Identifying migrations in marine fishes through stable-isotope analysis. J. Fish Biol. 81, 826–847. doi: 10.1111/j.1095-8649.2012.03361.x
Turan, C. (2006). The use of otolith shape and chemistry to determine stock structure of Mediterranean horse mackerel Trachurus mediterraneus (Steindachner). J. Fish Biol. 69, 165–180. doi: 10.1111/j.1095-8649.2006.01266.x
Vitale, S., Andrews, A. H., Rizzo, P., Gancitano, S., and Fiorentino, F. (2016). Twenty-five-year longevity of European hake (Merluccius merluccius) from novel use of bomb radiocarbon dating in the Mediterranean Sea. Mar. Freshw. Res. 67, 1077–1080. doi: 10.1071/MF15376
West, C. F., Wischniowski, S., and Johnston, C. (2012). Pacific cod (Gadus macrocephalus) as a paleothermometer: Otolith oxygen isotope reconstruction. J. Archaeol. Sci. 39, 3277–3283. doi: 10.1016/j.jas.2012.05.009
Whitfield, A. K., and Elliott, M. (2002). Fishes as indicators of environmental and ecological changes within estuaries: a review of progress and some suggestions for the future. J. Fish Biol. 61, 229–250. doi: 10.1111/j.1095-8649.2002.tb01773.x
Willmes, M., Lewis, L. S., Davis, B. E., Loiselle, L., James, H. F., Denny, C., et al. (2019). Calibrating temperature reconstructions from fish otolith oxygen isotope analysis for California’s critically endangered Delta Smelt. Rapid Commun. Mass Spectrom. 33, 1207–1220. doi: 10.1002/rcm.8464
Winkler, A. C., Duncan, M. I., Farthing, M. W., and Potts, W. M. (2019). Sectioned or whole otoliths? A global review of hard structure preparation techniques used in ageing sparid fishes. Rev. Fish Biol. Fisheries 29, 605–661. doi: 10.1007/s11160-019-09571-1
Keywords: otolith, sclerochronology, growth increments, geochemical fingerprints, stable isotopes, longevity, Mediterranean Sea
Citation: Matić-Skoko S, Peharda M, Vrdoljak D, Uvanović H and Markulin K (2020) Fish and Sclerochronology Research in the Mediterranean: Challenges and Opportunities for Reconstructing Environmental Changes. Front. Mar. Sci. 7:195. doi: 10.3389/fmars.2020.00195
Received: 07 January 2020; Accepted: 12 March 2020;
Published: 17 April 2020.
Edited by:
Alejandra Vanina Volpedo, University of Buenos Aires, ArgentinaReviewed by:
Audrey J. Geffen, University of Bergen, NorwayBeatriz Morales-Nin, Instituto Mediterráneo de Estudios Avanzados (IMEDEA), Spain
Copyright © 2020 Matić-Skoko, Peharda, Vrdoljak, Uvanović and Markulin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanja Matić-Skoko, c2FuamFAaXpvci5ocg==
 Sanja Matić-Skoko
Sanja Matić-Skoko Melita Peharda
Melita Peharda Dario Vrdoljak
Dario Vrdoljak Hana Uvanović
Hana Uvanović Krešimir Markulin
Krešimir Markulin