- 1Department of Biological Sciences, University of South Carolina, Columbia, SC, United States
- 2Department of Oceanography, University of Hawaii, Honolulu, HI, United States
- 3Apex Predators Program, National Marine Fisheries Service, Narragansett, RI, United States
Oceanic whitetip sharks Carcharhinus longimanus are a cosmopolitan epipelagic species that was once prolific throughout the tropics and subtropics but was recently listed as Critically Endangered by the International Union for the Conservation of Nature and as Threatened under the United States Endangered Species Act. Although historically conspicuous in oceanic fisheries catches, relatively little is known about their habitat use, movement, and life history during migration. Given the paucity of data on migratory patterns and lack of age estimate validation available for this species, we evaluated vertebral growth bands for bomb radiocarbon (14C) patterns to derive additional information on these metrics. Individual growth bands (n = 62) were milled from vertebrae of eight individuals caught in the northwestern Atlantic Ocean. Age estimates based on vertebral growth bands ranged 1–13 years, with capture dates spanning 1978–2004. Plots of vertebral Δ14C relative to regional coral, shark, and fish otolith reference curves suggest age estimates based on presumed annual growth bands were accurate, although specimens were not old enough to capture the most informative portion of the bomb radiocarbon reference period. The magnitude of Δ14C varied among individuals, and individual chronologies demonstrated semi-cyclic patterns of Δ14C depletion and subsequent enrichment, which may be indicative of changes to diet as a function of annual migratory patterns and is supported by recently published telemetry, diet, and stable isotope studies. Although these data are preliminary in nature, they provide some evidence that Δ14C patterns in vertebrae can serve as a multi-purpose tool for life history studies of oceanic sharks.
Introduction
Oceanic whitetip sharks, Carcharhinus longimanus, were once among the most prevalent sharks in tropical and temperate surface waters of the world’s equatorial oceans (Compagno, 1984), but are now among the most threatened. Distinctive in appearance with characteristic large, white-tipped dorsal and pectoral fins, this epipelagic, cosmopolitan species has comprised a disproportionate share of fisheries catches over the past 50 years which has resulted in severe depletion of the global population (see Young et al., 2017; Rigby et al., 2019; Young and Carlson, 2020 for reviews). Recently enacted conservation measures include the listing of C. longimanus by the International Union for the Conservation of Nature (IUCN) as Critically Endangered worldwide (Rigby et al., 2019), by the US Endangered Species Act as Threatened in US waters (83 FR 4153; January 30, 2018), and by the Convention on International Trade in Endangered Species (CITES) as prohibited from international trade in accordance with Appendix II classification (CITES, 2013), as well as designation of catch as prohibited across many regional fishery management organizations (Young and Carlson, 2020).
Life history information on C. longimanus is lacking (Rigby et al., 2019, but see Young and Carlson, 2020 for a thorough review) and data are difficult to obtain given the oceanic nature of the species and recent declines in population numbers. Studies published to date suggest C. longimanus exhibit slow to moderate, regionally variable growth rates, and intermediate longevity with maximum ages from direct growth band counts of up to 19 years (Seki et al., 1998; Lessa et al., 1999; Joung et al., 2016; D’Alberto et al., 2017). Annual growth band deposition has been verified with marginal increment analysis (Seki et al., 1998; Lessa et al., 1999; Joung et al., 2016), but aging methods and longevity have not been fully validated. Individuals in the northern Atlantic Ocean are known to undertake philopatric migrations between shallow reef habitats and oceanic waters (Howey-Jordan et al., 2013) as well as to exhibit vertical migrations to depths up to ∼1000 m potentially associated with foraging behavior (Howey-Jordan et al., 2013; Howey et al., 2016). Similar movements and site fidelity have been documented off northeast Brazil (Tolotti et al., 2015). Overall movement patterns elsewhere are less known. As a whole, published information on C. longimanus provides an incomplete picture of longevity, age validation, and migration patterns, all of which affect management ability and conservation potential on both regional and global scales.
Owing to the need for comprehensive life history data on C. longimanus, we set out to evaluate radiocarbon signatures from vertebral growth bands in hopes of validating annual growth band deposition and associated aging methods, as well as to evaluate ontogenetic habitat use patterns via examination of dietary carbon signatures over individual lifespans. Bomb radiocarbon dating has proven to be one of the only true methods of age validation suitable for marine fishes (Campana, 2001; Cailliet et al., 2006) owing to the permanent record of 14C from the environment recorded in calcified tissues. In elasmobranch species, 14C is dietary in origin (Fry, 1988) and can also enable identification of broad habitat shifts via related dietary changes and their effect on 14C (e.g., Kerr et al., 2006; Kneebone et al., 2008; Passerotti et al., 2014). Herein, we present preliminary results of bomb radiocarbon analyses of vertebral growth bands for archival specimens of C. longimanus from the western North Atlantic Ocean (WNA).
Materials and Methods
Vertebral specimens were sourced from the archival collection of the NMFS Apex Predators Program (Narragansett, RI, United States), and were collected from latitudes 38°N to 18°N in the WNA between 1978 and 2004 (Figure 1). At the time of collection, vertebrae were dissected from under the branchial chamber and stored frozen until analysis. To prepare for sectioning, frozen vertebrae were thawed and excess tissue removed. A total of three centra from each shark were sectioned: one for thin sectioning (age reading) and two additional for thick sectioning (radiocarbon analyses). For age reading, one centrum from each specimen was sectioned laterally through the focus to a thickness of 0.5mm using gross sectioning (Natanson et al., 2006), and sections were subsequently stored in capsules in 70% ethanol. Thin sections were imaged while wet using a Nikon DSR121 digital camera (Nikon Corp., Tokyo, Japan) attached to a Nikon SMZ1500 stereo microscope (Nikon Instruments, Inc., Melville, NY, United States). Magnification varied with the size of the section, and a scale was included in each photo. Band pairs (consisting of one opaque and one translucent band; Casey et al., 1985) were counted and marked independently by each author in individual image layers using image editing software (Adobe Photoshop Elements 6, Adobe, Inc., San Jose, CA, United States) following Natanson et al. (2018b). Counts were compared for each sample, and those not in agreement had band assignments compared via image layers to determine consensus on band placement. Additional independent counts were then carried out, and consensus age reached when two of three age reader counts were in agreement.
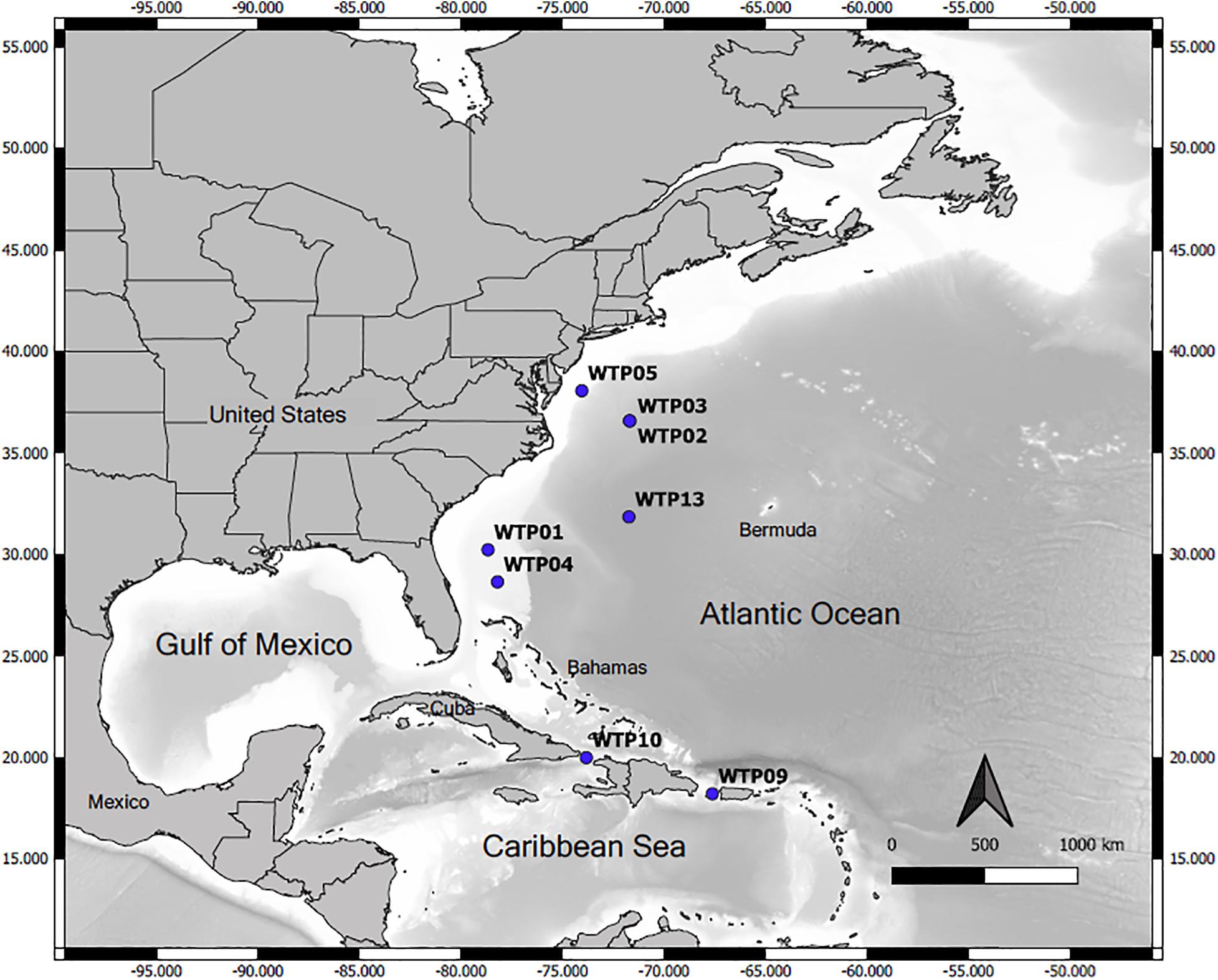
Figure 1. Map of catch locations for sampled Carcharhinus longimanus. Shading of ocean waters denotes rough bathymetry for depth comparison, with light areas indicative of shallow continental shelf waters and darker shading indicative of deeper oceanic waters. WTP 02 and WTP 03 were caught at the same time and location.
For radiocarbon analysis, two additional centra from each shark were sectioned through the core to a thickness of ∼ 1.5 mm each using twin diamond-tipped blades separated by spacers. Sections were pressed between glass slides and air dried overnight to prevent warping and facilitate proper extraction with a micromill. Dried sections were mounted onto double-wide glass slides using two layers of warmed Parafilm, into which the sections were firmly pressed until cool. Prior to micromilling the growth bands visualized from thin sections were used to guide the marking of corresponding bands on all growth axes of both thick sections. Milling was done using a New Wave Research (Elemental Scientific Lasers, LLC, Bozeman, MT, United States) micromilling machine with a 0.5 mm diameter burr (Brasseler, Savannah, GA, United States). For each sample, serial drill holes were made targeting identical years of growth along each of the four growth axes of the corpus calcareum (Figure 2). Depth of milling was just short of passing completely through the section to avoid Parafilm. Resulting powdered material was collected and pooled from other growth axes to comprise a single sample sufficient for 14C analyses. In an effort to evaluate precision of radiocarbon measurements across vertebral centra within the same shark, replicate material was taken from the second thick-section mirroring the growth sampled from the first. Because C. longimanus vertebrae are small and width of growth band pairs decreases with age (and thus available sample material), in many cases it was necessary to pool multiple years of growth per sample. Year(s) of formation (YOF) for each sample were assigned on the basis of estimated shark age and collection year according to the thin section used for age estimation. For samples comprising multiple years of growth, year of formation was plotted as the midpoint of the growth records sampled, including a fractional year adjustment based on month of capture when available. In addition to material beyond the birth band, we also sampled pre-birth material from the core of the vertebrae, near the apex of the section, for four sharks and these samples were not replicated. Growth-band pairs were milled from a total of 14 vertebral sections sampled from 8 sharks. Samples were taken along the section beginning with the band pair immediately following the birth band and ranging from one to nine additional samples for each individual (Figure 2).
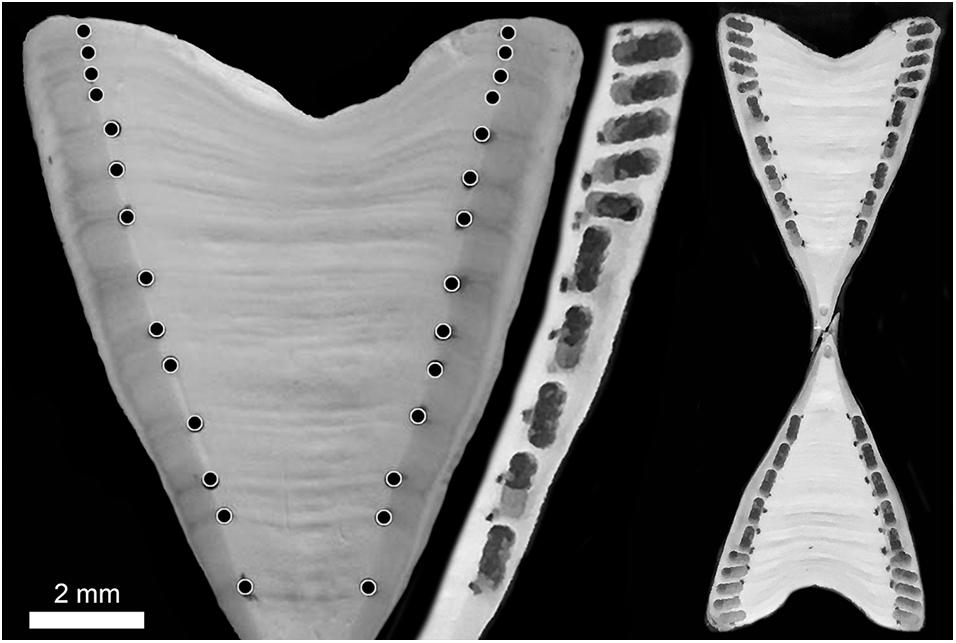
Figure 2. Composite image of a Carcharhinus longimanus vertebra (WTP-05, estimated as 13 years old) showing the marked growth bands (left) on a thick section with milled bands (offset edge of the full section showing age alignments) and the full vertebra after all extractions (right). Milled extractions were pooled across all growth axes for the same age classes or groups.
Powdered samples were analyzed at the National Ocean Sciences Accelerator Mass Spectrometry Facility (NOSAMS) at Woods Hole Oceanographic Institution (WHOI), Woods Hole, Massachusetts, for organic combustion, accelerator mass spectrometry (AMS) 14C assay. Radiocarbon measurements are reported as the Fraction modern (Fm, Reimer et al., 2004), which was used to calculate Δ14C with a correction for natural isotopic fractionation (Stuiver and Polach, 1977). Fm is the measured deviation of the 14C:12C ratio from a ‘modern’ sample. This reference is defined as 95% of the radiocarbon concentration of the NBS Oxalic Acid I Standard (SRM 4990B) normalized to δ13C Vienna Pee Dee Belemnite geological standard (VPDB; -19%) in 1950AD (Olsson, 1970). Coral references herein are relevant to the present study because they document mixed layer ocean chemistry of the tropics, similar to the known geographical range of C. longimanus in the WNA (Howey-Jordan et al., 2013; Kohler and Turner, 2019), and because they can be considered the timeliest bomb-produced 14C response for the marine environment—the hermatypic coral 14C records chosen were from southern Florida, Puerto Rico, and Bermuda (Druffel, 1989; Moyer and Grottoli, 2011), as well as validated shark vertebrae 14C data from porbeagle (Lamna nasus, Campana et al., 2002) and a fish otolith reference (Campana et al., 2008), both from the WNA. Measured Δ14C values for the assigned dates were compared with regional Δ14C reference chronologies to assess the accuracy of age estimates from growth band counting. Generally, correctly estimated ages will yield sample formation years that align with the regional reference chronologies when plotted relative to sample Δ14C values. A shift in alignment to the right, relative to reference chronologies, indicates age underestimation, and a shift to the left indicates age overestimation.
Results
A total of 62 samples were available for analysis (Table 1) after the loss of seven measurements during AMS processing and three additional measurements were discarded due to presumed contamination during milling (depleted values likely due to inclusion of paraffin). Consensus age estimates ranged 1–13 years for fish with lengths of 94–247 cm total length (TL), which were consistent with estimated age-at-length from other published growth models for the region (Figure 3). Based on our age estimates, the earliest sampled YOF assigned to post-birth material was 1966.5 for WTP 04-2, meaning the majority of samples analyzed herein did not form during the informative initial rise period of Δ14C (∼1958–1965), and therefore were largely uninformative for the purpose of age validation. Chronologies from sharks aged ≥4 years fell near the peak of the rise period and hence did not exhibit the strong pre- and post-peak environmental signatures evident in reference chronologies. However, because none of the sample chronologies exhibited the sharp 14C rise exhibited by the reference chronologies, it is certain that the largest and oldest specimens were not considerably older. Likewise, the earliest samples from WTP 04-2 show several years of relatively static or slightly increasing values, which may represent the beginning of the plateau after the initial rise period and suggests that over-aging beyond 1–2 years did not occur. Coupled with the favorable fit with published growth curves of the age-length data for estimated ages, we believe annual growth band deposition is occurring over the ages sampled in this study. In total, our results suggest age estimates based on single, annual band pair deposition are accurate, although additional samples to definitively capture the initial rise period are needed to refine age estimates beyond a few years’ accuracy.
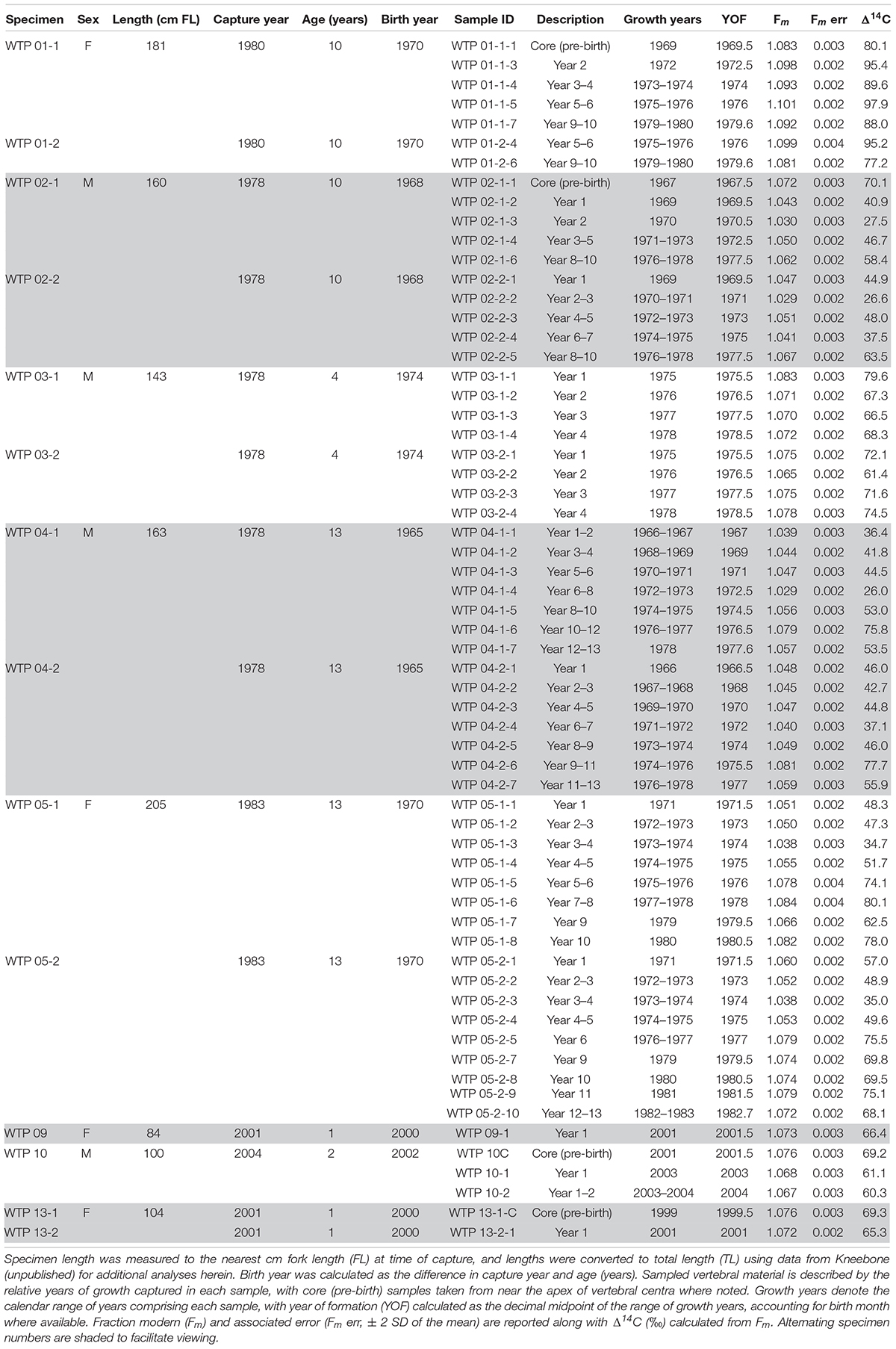
Table 1. Specimen information, sample description, and results of radiocarbon analysis for all Carcharhinus longimanus vertebral samples analyzed in this study.
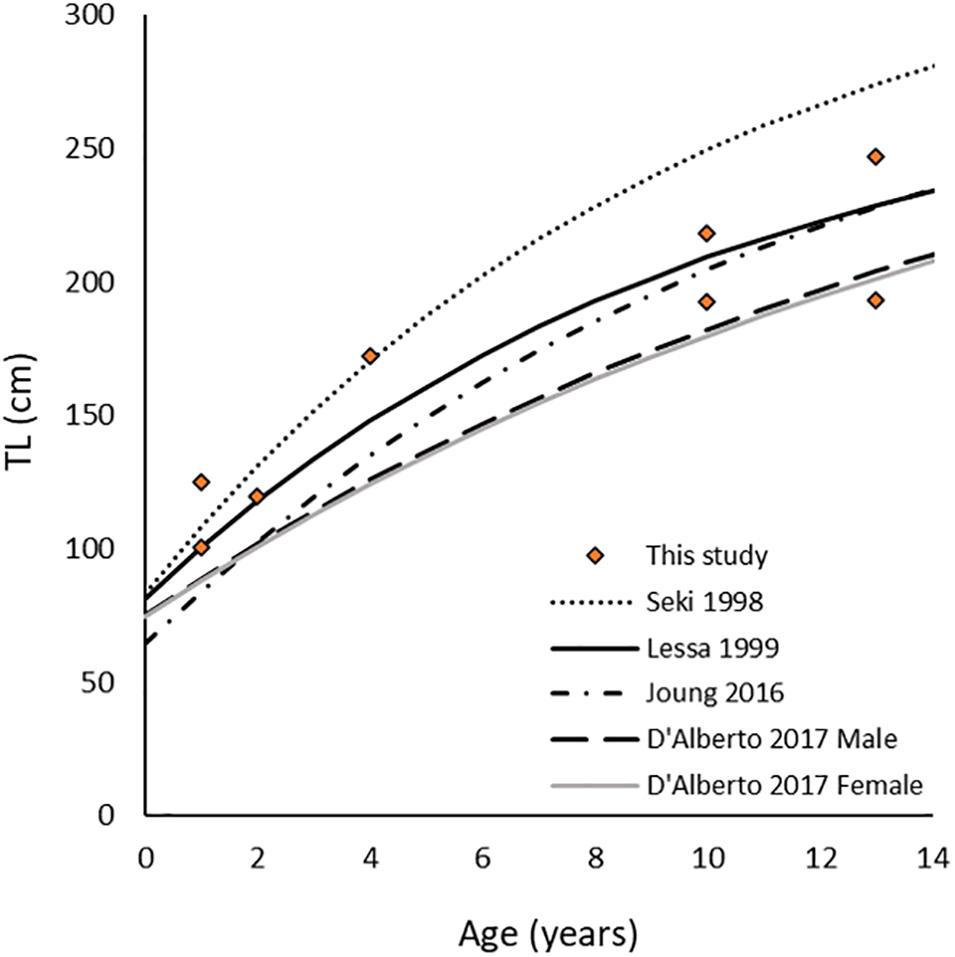
Figure 3. Total length (TL) at age for Carcharhinus longimanus specimens aged in this study plotted against published VB growth curves from Seki et al. (1998); Lessa et al. (1999), Joung et al. (2016), and D’Alberto et al. (2017). Fork length (FL) to TL conversions for samples analyzed in this study were calculated using unpublished length data from the western North Atlantic (Kneebone, unpublished data).
The novel replicate sampling design of our study provides a first estimation of variation in 14C across vertebrae within the same shark, with remarkable precision in 14C estimates, even when multiple years of growth were pooled. Hence, it seems the resulting patterns in Δ14C over time were not spurious variation due to sampling error but instead represent actual fine-scale patterns in 14C uptake that likely reflect migration history via shifts in available prey. Young sharks (aged 4 years or younger) mostly exhibited Δ14C levels near those of coral references, with the exception of WTP 03 which fell more in line with the upper range of porbeagle and NWA otolith references (Figure 4A). Adult sharks also exhibited variation in baseline Δ14C levels: WTP 01 remained intermediate between coral and otolith/porbeagle reference curves for all years sampled (Figure 4B), WTP 02 followed the porbeagle reference (Figure 4B), and both WTP 04 (Figure 4C), and WTP 05 (Figure 4D) exhibited marked increases in Δ14C in later years relative to values near the porbeagle/otolith references in early life. For WTP 04, this increase was followed by a return to more depleted Δ14C, whereas WTP 05 remained less depleted for the remaining years. Additionally, all sharks except WTP 04 exhibited a drop in Δ14C around years 2–3, which could signify ontogenetic dietary shifts and/or initiation of offshore migrations as part of philopatric movements. Continued oscillation in Δ14C patterns of all older sharks seems to support the idea that these shifts are associated with migratory behavior between disparate feeding environments, as reflected in the regional 14C reference records.
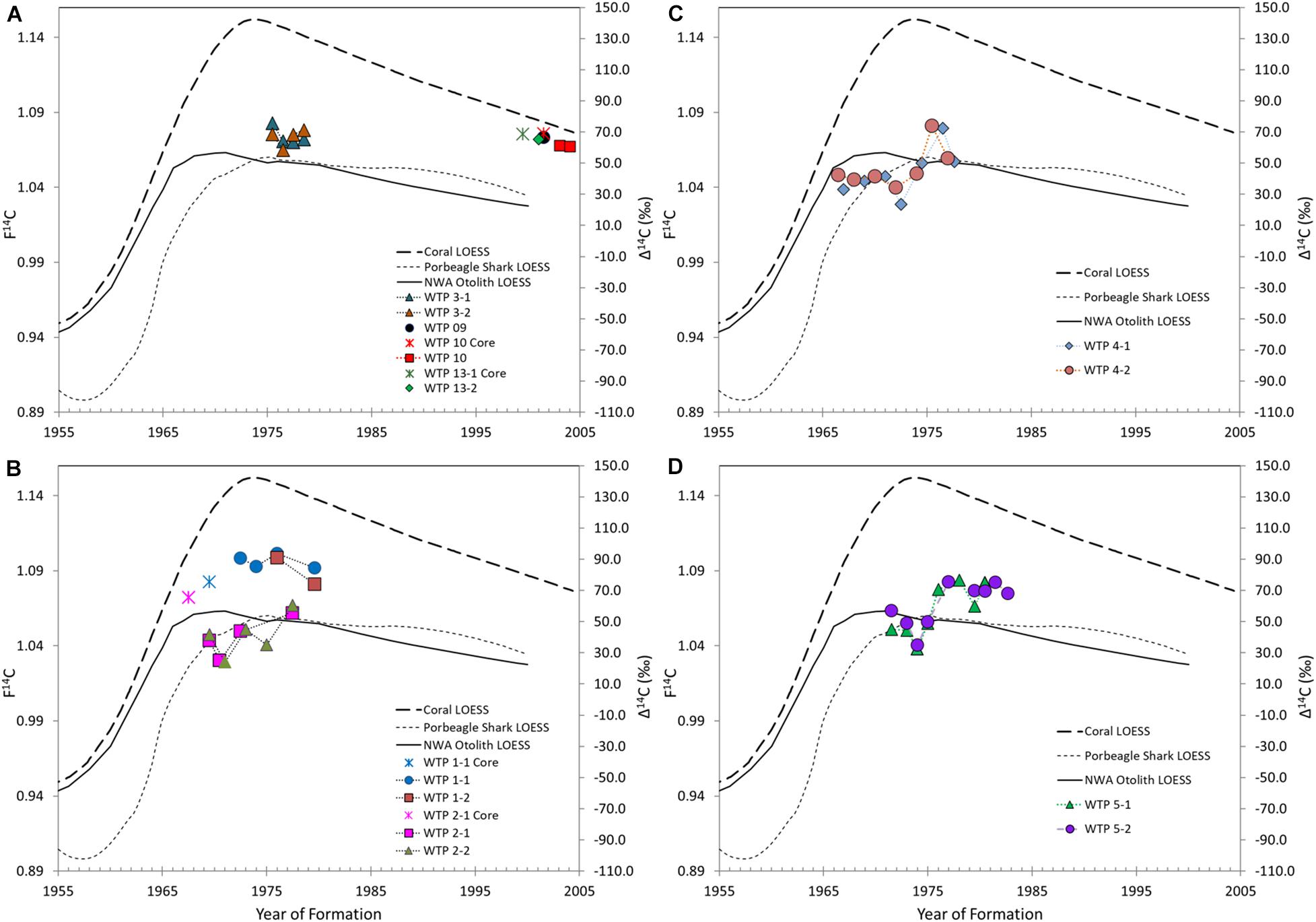
Figure 4. Vertebral radiocarbon results for Carcharhinus longimanus plotted over the estimated life span of each individual and relative to the regional 14C reference records (Loess curves for coral, porbeagle, and otoliths). The year of formation for each sample is plotted based on age estimates from growth band counting in the vertebral thin sections. Plots for C. longimanus aged ≤4 years are included in panel (A), followed by sharks aged 10 years in panel (B), and aged 13 years in (C,D). Samples corresponding to pre-birth material are denoted by star shaped icons in panels (A,B). Complete sample information can be found according to specimen ID in Table 1.
Pre-birth tissue sampled from the apex of centra from WTP 1, 2, 10, and 13 had Δ14C levels between ∼60 and 75‰ for all specimens. Relative to timing, pre-birth values fell near the mean Δ14C of the corresponding individual chronology, meaning they were closest to the coral reference in WTP 10 and 13 and to the otolith reference in WTP 01 and 02, although aging error of 1-2 years in older sharks would alternatively place them closer to the coral reference. Gestation in C. longimanus is characterized as viviparous placental, meaning resources used to form tissues in utero are derived at least in part from the maternal blood supply (Buddle et al., 2019). Hence, all pre-birth material ostensibly reflects maternal dietary carbon sourced during gestation, although the narrow Δ14C range of these samples might suggest more influence from the ambient radiocarbon levels (i.e., closer alignment to coral records that trace DIC in the mixed layer environment) while in utero.
Discussion
Preliminary analysis of vertebral radiocarbon from C. longimanus herein suggests annual band-pair deposition to at least 13 years of age, although further confirmation of this as well as maximum lifespan are needed. By employing a novel, multi-centra approach to measuring replicate growth bands within individual sharks, we have demonstrated that the fine-scale patterns in vertebral Δ14C apparent for this species are genuine records of Δ14C variation, which are conserved across centra within the individual, and may allow for precise reconstruction of dietary shifts corresponding to movement patterns over the lifespan of the shark. Previous documentation of philopatric movement patterns for C. longimanus support these findings.
Vertebral radiocarbon is typically used to determine growth band periodicity and to test the validity of age reading protocol, with the potential to determine maximum lifespan in sharks (Kalish and Johnston, 2001; Campana et al., 2002; Natanson et al., 2018a). Its use has led to discoveries of “missing time” in the vertebrae due to cessation of growth in later years of life (Francis et al., 2007; Andrews et al., 2011; Passerotti et al., 2014; Andrews and Kerr, 2015) and in many cases significant underestimation of age (Harry, 2018). The sharks sampled for this study were the oldest/largest available from archival material, yet YOF for the earliest formed material did not fall early enough to validate annual band-pair deposition with certainty. However, species found to have “missing time” generally have ages validated from early life through maturity, with a loss of years documented later in life (Harry, 2018). Hence, given indirect verification of annual deposition of growth bands in C. longimanus using marginal increment analysis (Seki et al., 1998; Lessa et al., 1999; Joung et al., 2016), and that size-at-age using our age estimates fell as expected along the published growth curve for Atlantic C. longimanus (Lessa et al., 1999) and within range of all published growth curves (Figure 3), the assumption of annual growth band deposition to 13 years of age is supported.
Aside from age information, vertebral 14C has also been interpreted to reflect shifts in dietary patterns, related to habitat use across the lifespan. Because carbon uptake in elasmobranchs is accomplished via feeding (dietary source; Fry, 1988)—as opposed to direct uptake of dissolved inorganic carbon from seawater, as in teleosts (Kalish, 1993)—changes in prey composition occurring due to habitat shifts can often be detected in vertebral radiocarbon (Natanson et al., 2018a). Depth-related changes and the consequent change in prey items was well-supported as the reason for an attenuated and phase-lagged bomb 14C signal for porbeagle shark (Campana et al., 2002). In contrast, 14C from early growth of tiger shark, Galeocerdo cuvier, (Kneebone et al., 2008) and sand tiger shark, Carcharias taurus, (Passerotti et al., 2014) demonstrated an affinity for nearshore habitat by having well-constrained 14C values from young sharks aligned with coral references, while the vertebrae from older sharks indicated there was a dietary shift to more depleted 14C values that may indicate life in offshore waters and thus consumption of prey from these deeper waters. Additional studies have hypothesized that post-rise differences in 14C magnitude between adults can signal individual differences in diet or location, as in white sharks, Carcharodon carcharias, (Kerr et al., 2006; Hamady et al., 2014). Young C. longimanus caught in shallow nearshore areas (WTP 09 and 10) provided similar time-constrained references for residence in shallow waters possibly near where they were pupped, while WTP 03 caught offshore at higher latitude shows a more depleted Δ14C signature. We could similarly hypothesize that WTP 13, caught offshore between Bermuda and the US mainland but exhibiting enriched Δ14C, may have been pupped in shallow waters before moving offshore. The overall range of vertebral Δ14C among sampled sharks was narrow relative to that measured across habitats, hence complete validation of habitat-specific influence on vertebral Δ14C patterns cannot be made without additional samples. However, the relative correlation of young shark habitat with Δ14C evident in our study samples suggests that the oscillations seen in Δ14C for older C. longimanus reflect annual to biennial migrations.
Published tagging, diet, and stable isotope studies for C. longimanus provide empirical evidence to support the interpretation of radiocarbon signatures to reflect movement patterns. Catch locations of most sampled sharks in this study overlapped C. longimanus movement tracked near Cat Island, Bahamas, by Howey-Jordan et al. (2013) as well as diet studies for individuals tagged in the Bahamas including stable isotope and stomach contents analyses (Madigan et al., 2015). Howey-Jordan et al. (2013) documented philopatric movements for many sharks, but disparate movement patterns among individuals were also evident despite most being the same sex and maturity (mature females). Some exhibited philopatric movements to the original tagging location over a scale of months, relatively fewer initiated longer (to ∼1900 km) offshore migrations (including to near Bermuda), while several others remained near the tagging location for the duration of the tracking period (up to ∼150 days). Repeated annual sightings of individuals are also reported from the Bahamas (Madigan et al., 2015). Vertical migration patterns also varied among individuals across multiple tagging studies, with some sharks initiating deep dives to >1000 m potentially associated with foraging behavior or thermoregulation (Howey-Jordan et al., 2013; Tolotti et al., 2017; Andrzejaczek et al., 2018).
The Bahamas population of C. longimanus is well-established, and this region provides access to abundant epipelagic prey such as billfish and tunas which are an important component of diet for sharks in the area (Madigan et al., 2015). Short term (blood plasma) stable isotope data showed higher proportions of large pelagic teleosts in the diet while sharks were in the Bahamas, while squid and smaller planktivorous teleosts were more prevalent in the diet in the long term (muscle) and appear to be important prey during oceanic portions of the life cycle (Madigan et al., 2015). Other diet studies have reported mammals in the diet of C. longimanus (Bass et al., 1973; Cortés, 1999). Squid are depleted in δ13C (∼−18 per mil) relative to pelagic teleosts (∼−16.5 per mil, Madigan et al., 2015), and mammals tend to be relatively depleted in δ13C and Δ14C (Stewart et al., 2006; Madigan et al., 2015). Post-bomb Δ14C of Atlantic seawater is variable and ranges to −100‰ across depths dependent on residence time and mixing rates (Druffel et al., 1992); hence, any planktivorous prey taken during deep dives would likely reflect the depleted profile of the deeper water column. A recent observation of a C. logimanus in the Pacific with scars from an interaction with a large, deep-dwelling cephalopod (likely either Architeuthis, Thysanoteuthis or Megalocranchia; Papastamatiou et al., 2020) lend further evidence to the theory that foraging occurs in deep waters. While sub-annual patterns of offshore-onshore movements might not cause year-to-year oscillations in Δ14C, the sharks undertaking longer offshore migrations may remain offshore substantially beyond the time recorded by tagging, and thus consume proportionally more depleted prey items for the year as a whole. Alternatively, even a small proportion of highly depleted prey in the diet can likely cause depleted vertebral Δ14C (Kerr et al., 2006). The porbeagle reference chronology (Campana et al., 2002) lends solid evidence of the effect that deep foraging can have on Δ14C signatures in sharks.
The maternally derived pre-birth Δ14C measurements reported herein for C. longimanus are only the second species to be reported in the literature. White shark pre-birth Δ14C was reported from the Pacific (Kerr et al., 2006), and Atlantic (Hamady et al., 2014). Aside from reporting the values as part of the larger studies, the pre-birth data were not used to inform any conclusions, although Natanson and Skomal (2015) used the Atlantic pre-birth value to align the sample chronology for age validation. Our results are likewise presented for informational purposes, with hopes that future work will determine applicability of these measurements to life history research. If pre-birth vertebral material can be used as a proxy for maternal 14C during gestation, then chronologies including both pre- and post-birth samples may be useful for exploring movement and diet related to gestation, parturition, and ontogeny of pups.
An important aspect of this study is the replication of 14C measurements for most vertebral growth bands, which provides insight on the variation of 14C uptake across vertebrae. These findings, coupled with empirical evidence, support life history insights about ontogenetic movements gained from fine-scale patterns in vertebral 14C. These analyses are generally expensive and studies involving large numbers of samples (and thus replicates) are usually cost-prohibitive. Hence, this study provides novel insights into the measurement precision attainable for vertebral 14C and its potential utility for applications outside of age validation, such as deciphering life history ecology in the years following the more informative bomb-produced 14C rise period.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Author Contributions
LN provided archival vertebrae and sectioned and photographed vertebrae for aging. MP sectioned and marked vertebrae, performed all laboratory analyses, data analysis, and prepared the first draft of the manuscript. AA milled vertebrae for radiocarbon analysis, contributed to data analysis, and provided images. All authors designed the study, aged vertebrae, and contributed to the final version of the manuscript.
Funding
This work was made possible by a Graduate Student Fellowship from the National Ocean Sciences Accelerator Mass Spectrometry (NOSAMS) laboratory at Woods Hole Oceanographic Institute, Woods Hole, Massachusetts. Funds for open access publication fees were provided by the University of South Carolina.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Our overwhelming thanks go to Kathryn Elder and Mark Roberts of NOSAMS for their advisory role in this work, as well as to Tess Walther and other NOSAMS staff for assistance in sample processing. We also thank Bryan Frazier and the South Carolina Department of Natural Resources for providing lab space and equipment for sample preparation. Additional thanks go to the NOAA staff and fisheries observers who collected the specimens used in this study. NOAA Pacific Islands Fisheries Science Center provided infrastructural support.
References
Andrews, A. H., and Kerr, L. A. (2015). Estimates of maximum age for white sharks of the northeasternPacific Ocean: altered perceptions of vertebral growth shed light on complicated bomb Δ14Cresults. Environ. Biol. Fish. 98, 971–978. doi: 10.1007/s10641-014-0326-8
Andrews, A. H., Natanson, L. J., Kerr, L. A., Burgess, G. H., and Cailliet, G. M. (2011). Bomb radiocarbon andtag-recapture dating of sandbar shark (Carcharhinus plumbeus). Fish. Bull. 109, 454–465.
Andrzejaczek, S., Gleiss, A. C., Jordan, L. K., Pattiaratchi, C. B., Howey, L. A., Brooks, E. J., et al. (2018). Temperature and the vertical movements of oceanic whitetip sharks. Carcharhinuslongimanus. Sci. Rep. 8, 1–12.
Bass, A. J., D’ Aubrey, J. D., and Kistnasamy, N. (1973). Sharks of the east coast of southern Africa. I. The genus Carcharhinus (Carcharhinidae). Oceanogr. Res. Inst. 33:168.
Buddle, A. L., Van Dyke, J. U., Thompson, M. B., Simpfendorfer, C. A., and Whittington, C. M. (2019). Evolution of placentotrophy: using viviparous sharks as a model to understand vertebrateplacental evolution. Mar. Freshw. Res. 70, 908–924. doi: 10.1071/mf18076
Cailliet, G. M., Smith, W. D., Mollet, H. F., and Goldman, K. J. (2006). Age and growth studies ofchondrichthyan fishes: the need for consistency in terminology, verification, validation, andgrowth function fitting. Env. Biol. Fish. 77, 211–228. doi: 10.1007/S10641-0069105-5
Campana, S. E. (2001). Accuracy, precision and quality control in age determination, including a reviewof the use and abuse of age validation methods. J. Fish Biol. 59, 197–242. doi: 10.1111/J.1095-8649.2001.TB00127.X
Campana, S. E., Casselman, J. M., and Jones, C. M. (2008). Bomb radiocarbon chronologies in the Arctic, with implications for the age validation of lake trout (Salvelinus namaycush) and other Arcticspecies. Can. J. Fish. Aquat. Sci. 65, 733–743. doi: 10.1139/f08-012
Campana, S. E., Natanson, L. J., and Myklevoll, S. (2002). Bomb dating and age determination of largepelagic sharks. Can. J. Fish. Aquat. Sci. 59, 450–455. doi: 10.1139/f02-027
Casey, J. G., Pratt, H. L.Jr, and Stillwell, C. E. (1985). Age and growth of the sandbar shark (Carcharhinus plumbeus) from the western North Atlantic. Can. J. Fish. Aquat. Sci. 42, 963–975.
CITES (2013). “Convention on International Trade in Endangered Species of Wild Fauna and Floraconsideration of proposals for amendment of Appendices I and II”. in Sixteenth Meeting of the Conference of the Parties Bangkok (Thailand), 3– 14, 2013. (Thailand: CITES).
Compagno, L. J. V. (1984). FAO Species Catalogue Vol 4. Sharks of the world: an annotated and illustratedcatalogue of shark species known to date. Parts 1 and 2. FAO Fisheries Synopsis No. 125. Italy: FAO, 655.
Cortés, E. (1999). Standardized diet compositions and trophic levels of sharks. ICES J. Mar. Sci. 56, 707–717. doi: 10.1006/jmsc.1999.0489
D’Alberto, B. M., Chin, A., Smart, J. J., Baje, L., White, W. T., and Simpfendorfer, C. A. (2017). Age, growthand maturity of oceanic whitetip shark (Carcharhinus longimanus) from Papua New Guinea.Mar. Freshw. Res. 68, 1118–1129. doi: 10.1071/mf16165
Druffel, E. M. (1989). Decadal time scale variability of ventilation in the North Atlantic: high-precisionmeasurements of bomb radiocarbon in banded corals. J. Geoph. Res. 94, 3271–3285. doi: 10.1029/jc094ic03p03271
Druffel, E. M., Williams, P. M., Bauer, J. E., and Ertel, J. R. (1992). Cycling of dissolved and particulateorganic matter in the open ocean. J. Geophys. Res 97, 15639–15659. doi: 10.1029/92jc01511
Francis, M. P., Campana, S. E., and Jones, C. M. (2007). Age under-estimation in New Zealand porbeaglesharks (Lamna nasus): Is there an upper limit to ages that can be determined from sharkvertebrae? Mar. Freshw. Res. 58, 10–23. doi: 10.1071/mf06069
Fry, B. (1988). Food web structure on Georges Bank from stable C. N and S isotopic compositions. Limnol.Oceanogr. 33, 1182–1190. doi: 10.4319/lo.1988.33.5.1182
Hamady, L. L., Natanson, L. J., Skomal, G. B., and Thorrold, S. R. (2014). Vertebral bomb radiocarbonsuggests extreme longevity in white sharks. PloS One 9:e84006. doi: 10.1371/journal.pone.0084006
Harry, A. V. (2018). Evidence for systemic age underestimation in shark and ray ageing studies. Fish Fisheries 19, 185–200. doi: 10.1111/faf.12243
Howey, L. A., Tolentino, E. R., Papastamatiou, Y. P., Brooks, E. J., Abercrombie, D. L., Watanabe, Y. Y., et al. (2016). Into the deep: the functionality of mesopelagic excursions by an oceanicapex predator. Ecol. Evol. 6, 5290–5304. doi: 10.1002/ece3.2260
Howey-Jordan, L. A., Brooks, E. J., Abercrombie, D. L., Jordan, L. K., Brooks, A., Williams, S., et al. (2013). Complex movements, philopatry and expanded depth range of aseverely threatened pelagic shark, the oceanic whitetip (Carcharhinus longimanus) in thewestern North Atlantic. PloS One 8:e56588. doi: 10.1371/journal.pone.0056588
Joung, S. J., Chen, N. F., Hsu, H. H., and Liu, K. M. (2016). Estimates of life history parameters of theoceanicwhitetip shark, Carcharhinus longimanus, in the western North Pacific Ocean. Mar.Biol. Res. 12, 758–768. doi: 10.1080/17451000.2016.1203947
Kalish, J. M. (1993). Pre-and post-bomb radiocarbon in fish otoliths. Earth Plan. Sci. Lett. 114, 549–554. doi: 10.1016/0012-821x(93)90082-k
Kalish, J. M., and Johnston, J. (2001). “Determination of school shark age based on analysis of radiocarbonin vertebral collagen,” in Use of the bomb radiocarbon chronometer to validatefish age. Final Report. FDRC Project 93/109, ed. J. M. Kalish (Canberra: Fisheries Research and Development Corporation), 116–122.
Kerr, L. A., Andrews, A. H., Cailliet, G. M., Brown, T. A., and Coale, K. H. (2006). “Investigations of Δ14C,δ13C, and δ15N in vertebrae of white shark (Carcharodon carcharias) from the eastern NorthPacific Ocean,” in Special Issue: Age and Growth of Chondrichthyan Fishes: New Methods, Techniques and Analysis, eds John K Carlson and Kenneth J Goldman (Dordrecht: Springer), 337–353. doi: 10.1007/978-1-4020-5570-6_14
Kneebone, J., Natanson, L. J., Andrews, A. H., and Howell, W. H. (2008). Using bomb radiocarbonanalyses to validate age and growth estimates for the tiger shark, Galeocerdo cuvier, in thewestern North Atlantic. Mar. Biol. 154, 423–434. doi: 10.1007/S00227-008-0934-Y
Kohler, N. E., and Turner, P. A. (2019). Distributions and Movements of Atlantic Shark Species: A 52-YearRetrospective Atlas of Mark and Recapture Data. Mar. Fish. Rev. 81, 1–94. doi: 10.7755/mfr.81.2.1
Lessa, R., Santana, F. M., and Paglerani, R. (1999). Age, growth and stock structure of the oceanicwhitetip shark, Carcharhinus longimanus, from the southwestern equatorial Atlantic. Fish. Res. 42, 21–30. doi: 10.1016/s0165-7836(99)00045-4
Madigan, D. J., Brooks, E. J., Bond, M. E., Gelsleichter, J., Howey, L. A., Abercrombie, D. L., et al. (2015). Diet shift and site-fidelity ofoceanicwhitetip sharks Carcharhinus longimanus along the Great Bahama Bank. Mar. Ecol. Prog.Ser. 529, 185–197. doi: 10.3354/meps11302
Moyer, R. P., and Grottoli, A. G. (2011). Coral skeletal carbon isotopes (δ13C and Δ14C) record the deliveryof terrestrial carbon to the coastal waters of Puerto Rico. Coral Reefs 30:791.
Natanson, L. J., and Skomal, G. B. (2015). Age and growth of the white shark, Carcharodon carcharias, inthe western North Atlantic Ocean. Mar. Freshw. Res. 66, 387–398. doi: 10.1071/mf14127
Natanson, L. J., Andrews, A. H., Passerotti, M. S., and Wintner, S. P. (2018a). “History and Mystery of Ageand Growth Studies in Elasmobranchs,” in Shark Research: Emerging Technologies and Applications forthe Field and Laboratory, eds J. C. Carrier, M. R. Heithaus, and C. A. Simpfendorfer (Boca Raton, FL: CRC Press), 177–200.
Natanson, L. J., Skomal, G. B., Hoffmann, S. L., Porter, M. E., Goldman, K. J., and Serra, D. (2018b). Age andgrowth of sharks: do vertebral band pairs record age? Mar. Freshw. Res. 69, 1440–1452. doi: 10.1071/mf17279
Natanson, L., Kohler, N., Ardizzone, D., Cailliet, G., Wintner, S., and Mollet, S. (2006). Validated age andgrowth estimates for the shortfin mako, Isurus oxyrinchus, in the North Atlantic Ocean. Env. Biol.Fish. 77, 367–383. doi: 10.1007/S10641-006-9127-Z
Olsson, I. U. (1970). “The use of oxalic acid as a standard,” in ‘Radiocarbon Variations and AbsoluteChronology. Proceedings of the 12th Nobel Symposium.’, ed. I. U. Olsson (New York, NY: John Wiley &Sons), 17.
Passerotti, M. S., Andrews, A. H., Carlson, J. K., Wintner, S. P., Goldman, K. J., and Natanson, L. J. (2014). Maximum age and missing time in the vertebrae of sand tiger shark (Carcharias taurus):validated lifespan frombomb radiocarbon dating in the western North Atlantic andsouthwestern Indian Oceans. Mar. Freshw. Res. 65, 1131–1140. doi: 10.1071/MF13214
Papastamatiou, Y. P., Verbeck, D., Hutchinson, M., Bracken-Grissom, H. D., and Chapman, D. (2020). An encounter between a pelagic shark and giant cephalopod. J. Fish Biol. 97, 588–589. doi: 10.1111/jfb.14415
Reimer, P. J., Brown, T. A., and Reimer, R. W. (2004). Discussion: reporting and calibration of post-bomb 14C data. Radiocarbon 46, 1299–1304.
Rigby, C. L., Barreto, R., Carlson, J., Fernando, D., Fordham, S., Francis, M. P., et al. (2019). Carcharhinus longimanus. The IUCN Red List of Threatened Species 2019: e.T39374A2911619. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T39374A2911619.en (accessed May 26, 2020).
Seki, T., Taniuchi, T., Nakano, H., and Shimizu, M. (1998). Age, growth and reproduction of the oceanicwhitetip shark from the Pacific Ocean. Fish. Sci. 64, 14–20. doi: 10.2331/fishsci.64.14
Stewart, R. E. A., Campana, S. E., Jones, C. M., and Stewart, B. E. (2006). Bomb radiocarbon datingcalibrates beluga (Delphinapterus leucas) age estimates. Can. J. Zool. 84, 1840–1852. doi: 10.1139/z06-182
Tolotti, M. T., Bach, P., Romanov, E., and Dagorn, L. (2015). Interactions of oceanic whitetip sharks with the tuna purse seine fishery in the Indian Ocean. IOTC-2015-WPEB11-29. Italy, RM: Food and Agriculture Organization of the United Nations.
Tolotti, M., Bauer, R., Forget, F., Bach, P., Dagorn, L., and Travassos, P. (2017). Fine-scale vertical movementsof oceanic whitetip sharks (Carcharhinus longimanus). Fish. Bull. 115, 380–395. doi: 10.7755/FB.115.3.8
Young, C. N., and Carlson, J. K. (2020). The biology and conservation status of the oceanic whitetip shark(Carcharhinus longimanus) and future directions for recovery. Rev. Fish Biol. Fisheries 30, 293–312. doi: 10.1007/s11160-020-09601-3
Young, C. N., Carlson, J. K., Hutchinson, M., Hutt, C., Kobayashi, D., McCandless, C. T., et al. (2017). Status review report: oceanic whitetip shark (Carcharhinius longimanus). Final Report to the National Marine Fisheries Service, Office of Protected Resources. December 2017. Silver Spring: National Marine Fisheries Service.
Keywords: carbon-14, age validation, migration, diet, vertebrae, family Carcharhinidae
Citation: Passerotti MS, Andrews AH and Natanson LJ (2020) Inferring Life History Characteristics of the Oceanic Whitetip Shark Carcharhinus longimanus From Vertebral Bomb Radiocarbon. Front. Mar. Sci. 7:581775. doi: 10.3389/fmars.2020.581775
Received: 09 July 2020; Accepted: 21 October 2020;
Published: 12 November 2020.
Edited by:
David Wells, Texas AM University at Galveston, United StatesReviewed by:
Paul Brickle, South Atlantic Environmental Research Institute, Falkland IslandsPhillip Sanchez, Texas A&M University at Galveston, United States
Copyright © 2020 Passerotti, Andrews and Natanson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle S Passerotti, bXBhc3Nlcm90dGlAZ21haWwuY29t
 Michelle S. Passerotti
Michelle S. Passerotti Allen H. Andrews
Allen H. Andrews Lisa J. Natanson3
Lisa J. Natanson3