- 1Institute of Blue Biotechnology and Development (IBYDA), Experimental Centre Grice Hutchinson, University of Malaga, Malaga, Spain
- 2Doctorate in Sciences Engineering With Specialization in Bioprocesses, Faculty of Engineering and Sciences, University of La Frontera, Temuco, Chile
- 3Laboratory of Coastal Environmental Research, Center of Advanced Studies/HUB Ambiental, University of Playa Ancha, Viña del Mar, Chile
- 4Department of Chemical Engineering, Faculty of Engineering and Sciences, University of La Frontera, Temuco, Chile
Sarcopeltis skottsbergii is an endemic species of the southern region of South America, with R-phycoerythrin (R-PE) as an accessory photosynthetic pigment. The production of S. skottsbergii is around twenty thousand tons of dry alga per year. The evaluation of (R-PE) in a biorefinery model is still incipient in the algal biotechnology area and will be used in the food, pharmaceutical, cosmeceutical, and nutraceutical industries. This work evaluated the cell disruption and separation processes by using two green technologies, ultrasound-assisted extraction (UAE) and high-pressure homogenization (HPH), to obtain an R-phycoerythrin enriched extract from S. skottsbergii. Two-levels three-factor central composite design (CCD) and response surface methodology (RSM) were carried out to optimize the extraction conditions, including the factors for UAE (time, amplitude, and solvent) and HPH (Pressure, number of passes, and solvent). Additionally, a second-order polynomial fit was performed to fit the experimental data by the green method. HPH method was the most efficient extraction method under the conditions obtained of 100-400 MPa pressure power, 2-3 number of passes, and distilled water as solvent. Furthermore, the experimental extraction yields ranged from 4.4-5.7 mg of PE g-1 of dry biomass under the optimal extraction conditions (400 MPa; 2 passes), which agreed with the predictive yield of 4.6-5.5 mg g-1 DW. The ultrafiltration membrane used for the separation process for both methods exhibited a rejection of R-phycoerythrin concentrated at 30 KDa. Furthermore, R-phycoerythrin showed a positive correlation between the antioxidant capacity (ORAC) in the best-selected extractions. After the extraction, the same pattern was observed in Chlorophyll a and total carotenoids with DPPH. Thus, it was an attractive non-aggressive extraction alternative with biological activity of interest for formulating biotechnological products for the food industry is suggested.
Introduction
The phycobiliproteins are water-soluble proteins and pigments found in the cytoplasm or the stroma of the chloroplast (Glazer et al., 1976; Pagels et al., 2019). Red micro and macroalgae, Cyanobacteria, and Cryptophytes are the only sources of reddish-red pigment, R-phycoerythrin (R-PE), and their primary function is to trap light energy between 495 and 650 nm wavelengths and transfer it to chlorophyll a of the photosynthetic reaction center of Photosystem II through other biliproteins as phycocyanin and allophycocyanin (Sekar and Chandramohan, 2008; Castro-Varela et al., 2021; Roy and Pabbi, 2022). R-PE is an oligomeric protein of 240 kDa with three subunits α (about 16 kDa), β (about 21 kDa), and γ (about 39 kDa), and they are bound to specific cysteines by thioether bonds (Li et al., 2019). The phycobiliproteins study mainly focuses on therapeutic applications (as bioactive), i.e., anti-inflammatory, antiviral, hepatoprotective, and anticarcinogenic capacities of R-PE have been reported (Thangam et al., 2015; Senthilkumar et al., 2018; Ulagesan et al., 2021).
The red algae often contain high levels of proteins (Hasan R. and Fao, 2009; Manivannan et al., 2009; Gómez-Ordóñez et al., 2010), in contrast to the brown algae with lower content (Angell et al., 2016). In this sense, the nutritional and the biological properties based on the protein coming from the R-PE from the red algae could be used as functional ingredients in several phases of food fortification, such as Kappaphycus alverezii (fish cutlet), Gelidium amansii (hamburger patties), Porphyra umbilicalis (restructured meats) (Mamatha et al., 2007; Moreira et al., 2010; Jeon and Choi, 2012; Le Guillard et al., 2015; Angell et al., 2016; Ngamnikom et al., 2017). The red macroalga Sarcopeltis skottsbergii (formerly Gigartina skottsbergii), endemic to the southern region of South America (Buschmann et al., 2008), presents a high content of the R-phycoerythrin, sulfated polysaccharide and mycosporine-like amino acids (MAAs) (Roleda et al., 2008).
R-PE is located within the phycobilisomes connected to Photosystem II in the chloroplasts; thus, cell disruption is required for the efficient release during the extraction process. There are several available methods for extraction of phycobiliproteins such as osmotic shock (Kawsar et al., 2011), maceration in the presence of liquid nitrogen in phosphate buffer (Munier et al., 2014), freeze grinding (Galland-Irmouli et al., 2000), freezing and thawing (Senthilkumar et al., 2013), ultrasonication (US) (Le Guillard et al., 2015) and homogenization (Pereira et al., 2020). Due to the complexity of structures and properties of bioactive compounds and the structure of the extracted materials, there is no available universal extraction protocol (Ciko et al., 2018). For this reason, it is essential to carry out extraction studies that allow us to obtain from the biological material high yield and bioactivity (Michalak and Chojnacka, 2014) by applying advanced extraction techniques (Ciko et al., 2018).
Therefore, exploring cost-effective extraction and separation methods for R-PE is necessary. Disruption of the rigid cell wall is a critical step required to increase the availability of algal proteins for extraction (Barba et al., 2015). Recently, extraction technologies, especially those considered green processes such as methods involving extraction with water, have gained attention to exploring marine resources for obtaining functional ingredients.
Ultrasound-Assisted Extraction (UAE) has attracted the attention of researchers for its application in food and allied industries (Michalak and Chojnacka, 2014; Chemat et al., 2017) and acts by creating compression and decompression through sound waves at the frequency of 20 kHz. Thus, several mechanisms for UAE action have been identified, including fragmentation, erosion, sonocapillary effect, sonoporation, local shear stress, and destruction-detexturation of plant cell wall matrix (Le Guillard et al., 2015; Chemat et al., 2017). An overall effect of these mechanisms results in disruption of cell wall. However, a given mechanism’s relative extent of contribution varies with the type of vegetal biomass and process parameters such as amplitude wave or time (Alexandre et al., 2017; Chemat et al., 2017). Another modern non-conventional alternative method to recover intracellular components is high-pressure homogenization (HPH), considered one of the greenest technologies, which showed a considerably shorter extraction time and higher yield than other conventional techniques (Ciko et al., 2018). This method is typically performed by forcing a liquid through a narrow nozzle at high pressure and establishing high shear stress. The use of mild temperatures makes this process especially attractive in extracting thermosensitive bioactive compounds (Alexandre et al., 2017). The membrane technologies are well suited to use with seaweed as part of a biorefinery process to maximize the valorization of all components within algae and avoiding the presence of heavy metals in the final product (Yaich et al., 2011). Some researchers have suggested that the combination of extraction technologies and the incorporation of membrane technologies could be used to isolate algal proteins using the same principles of molecular weight cut-offs used in the dairy industry. Ultrafiltration (UF) membranes constitute a physical barrier that retains all compounds bigger than the membrane molecular weight cut-off. UF could then be used to isolate proteins and other macromolecules between 1 and 200 kDa, as it has been validated on an industrial scale (food and feed industry) to generate enriched fractions less than 10, 5, 3 and 1 kDa. UF was used to isolate R-PE protein from macroalgae Grateloupia turuturu following cell homogenization, retaining about 100% of the protein without denaturation (Denis et al., 2009).
In this regard, determining a behavior pattern between R-phycoerythrin and the extraction method, we could consider a new attribute that reinforces the use of green methods for sustainability and development of a bioprocess. In fact, considering that there is no universal extraction protocol that ensures the quality of the structures and properties of the pigments (Liu et al., 2019). Therefore, it is believed that a specific extraction process must be established for each biological material extracted to obtain extracts with desirable bioactivity and high yield.
Response Surface Methodology (RSM) is a statistical tool used to determine and optimize the optimal experimental conditions to achieve maximum yields with minimum time and resource consumption (Wani et al., 2017). In this way, this study aimed to optimize the extraction process to obtain crude extracts with a higher yield of phycoerythrin from S. skottsbergii using RSM. Two green methods, UAE, and HPH, including the variables for UAE (time, amplitude, and solvent) and HPH (Pressure, number of passes, and solvent), were investigated for their influence on the yield of the extracts. UF technology, antioxidant activity, soluble protein content, and photosynthetic pigments were tested to evaluate the concentration and purity of R-PE in the aqueous extracts.
Material and Methods
Algal Biomass
Biomass of marine macroalgae, S. skottsbergii (Rhodophyta) is distributed in the coast of Chile, from Corral (39° 88’ S) (Westermeyer and Ramírez, 1978) to the Antarctic Peninsula (63° 23’S) (Bischoff-Bäsmann and Wiencke, 1996). In this study, the macroalgae were provided from Magallanes Region by the company Gelymar S.A., Puerto Montt, Chile. The biomass was washed with filtered and distilled water for the removal of sand particles, epiphytes, and other undesirable materials before transporting under cold conditions to the University of La Frontera, Temuco, Chile. Biomass was lyophilized (Biobase BK-FD18PT) for 48h, and substrate samples were carefully milled with a grinder machine (Sindelen Mol165IN, China) until particle size was less than sieve screen number 18 (1 mm openings). The samples were sieved using a Ro-Tap testing sieve shaker (model RX-29-10, W.S. Tyler, Mentor, OH) through a set of sieves (ASTM E11:95). The average particle diameter (dp = 0.70 mm) was determined using Equation 1 of the standard method S319.3 (ASAE S319.3 Method of Determining and Expressing Fineness of Feed Materials by Sieving).
( mm) is the geometric mean diameter of particles on ith sieve, or (di × di+1)1/2 where di is the nominal sieve aperture size of the ith sieve and di+1 is nominal sieve aperture size in next larger than ith sieve wi is the mass of particles with an average diameter of . The biomass was stored at -18 ± 2°C, and desired quantity of biomass was taken out as and when required for the experimentation.
Experimental Design
The RSM considering a central composite design (CCD), was employed for the evaluation of the variable’s effect on the phycoerythrin yield extracted with ultrasound (UAE) and high-pressure homogenization (HPH). For the case of UAE method, amplitude percentage (30 to 90%) and time (10 to 30 min) by the solvent (water or phosphate buffer) were tested. For the HPH methods, factors include the pressure (100 to 500 MPa) and the passes (1 to 3) by the solvent (water or phosphate buffer). The experiments were designed according to a 22 factorial and design with three central points for both methods. The range of independent variables, the responding levels, and the results of the complete design composed of 22 experimental runs performed in random order is listed in Tables 1, 2. All the trials were performed in triplicate. Three-dimensional response surface plots were generated by varying the variables within the experimental region. The goodness of fit of the model was evaluated by analysis of variance. The results were analyzed by using Design-Expert version 12.0 (Stat-Ease Inc., Minneapolis, USA). The design was fitted to a reduced quadratic model expressed by a polynomial regression equation
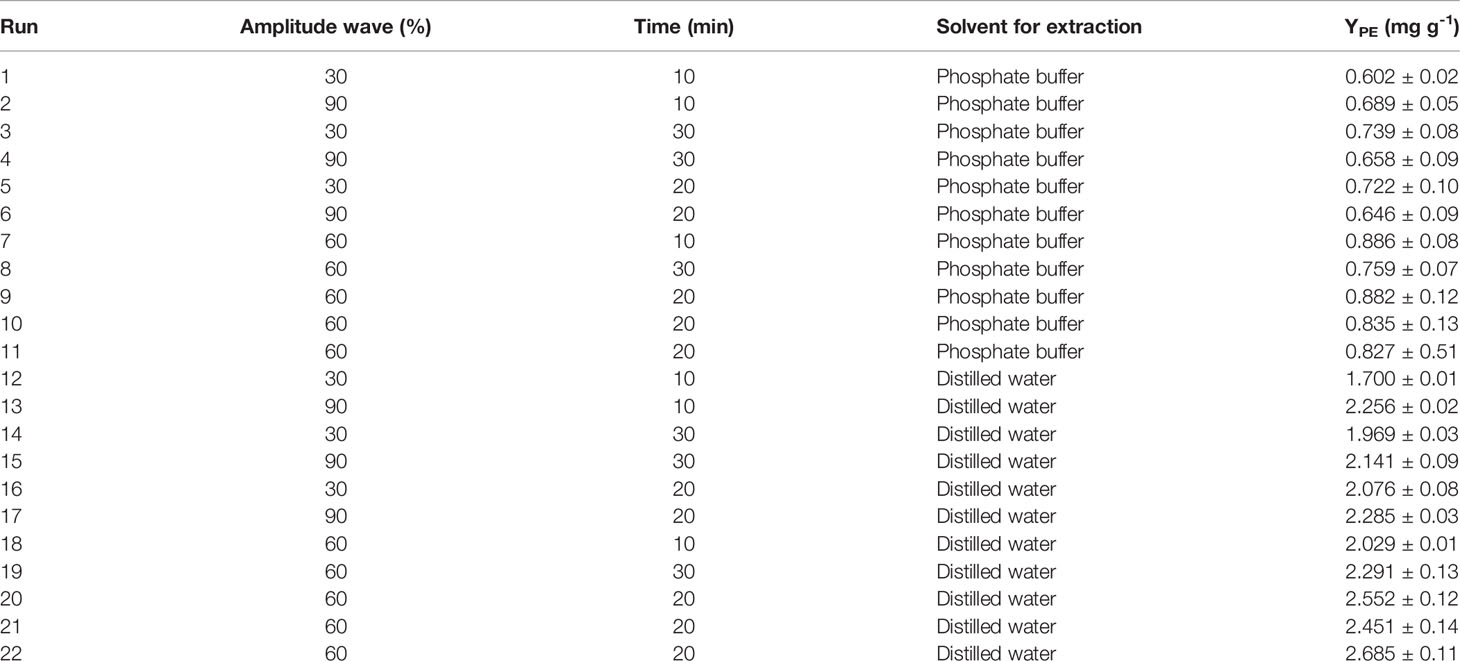
Table 1 Central composite design for ultrasound-assisted method and yield (YPE) results from response surface method analysis.
Different statistical criteria to evaluate the model were considered. Values close to 1 for R2, lower than 10 for Covariance, and higher than 4 for Adequate Precision were considered desirable for model acceptance. Adequate precision measures signal to noise ratio related to the contrast in predicted response concerning its associated error. The statistical significance was based on the total error criteria with a confidence level of 95%.
Extraction Procedure
The procedure followed for R-PE extraction from S. skottsbergii is schematized in Figure 1. For all the runs or experiments, freeze-dried biomass of S. skottsbergii (5 g) was suspended in 500 ml of solvent (buffer phosphate at pH=6.5 or distilled water) and homogenized help of a magnetic stirrer at room temperature for 5 min. Then, the extraction was carried out by UAE or HPP, according to the experimental design. After extraction, the samples were centrifuged (Eppendorf Centrifuge 5810R, Billerica, EUA) at 4.000 rpm for 10 min at 4°C and the pellet was discarded. The supernatant was further filtered through a PTFE 0.45 μm membrane (VWR, North America) and analyzed to determine its R-PE purity, protein content, antioxidant activity, chlorophyll, and carotenoid pigments.
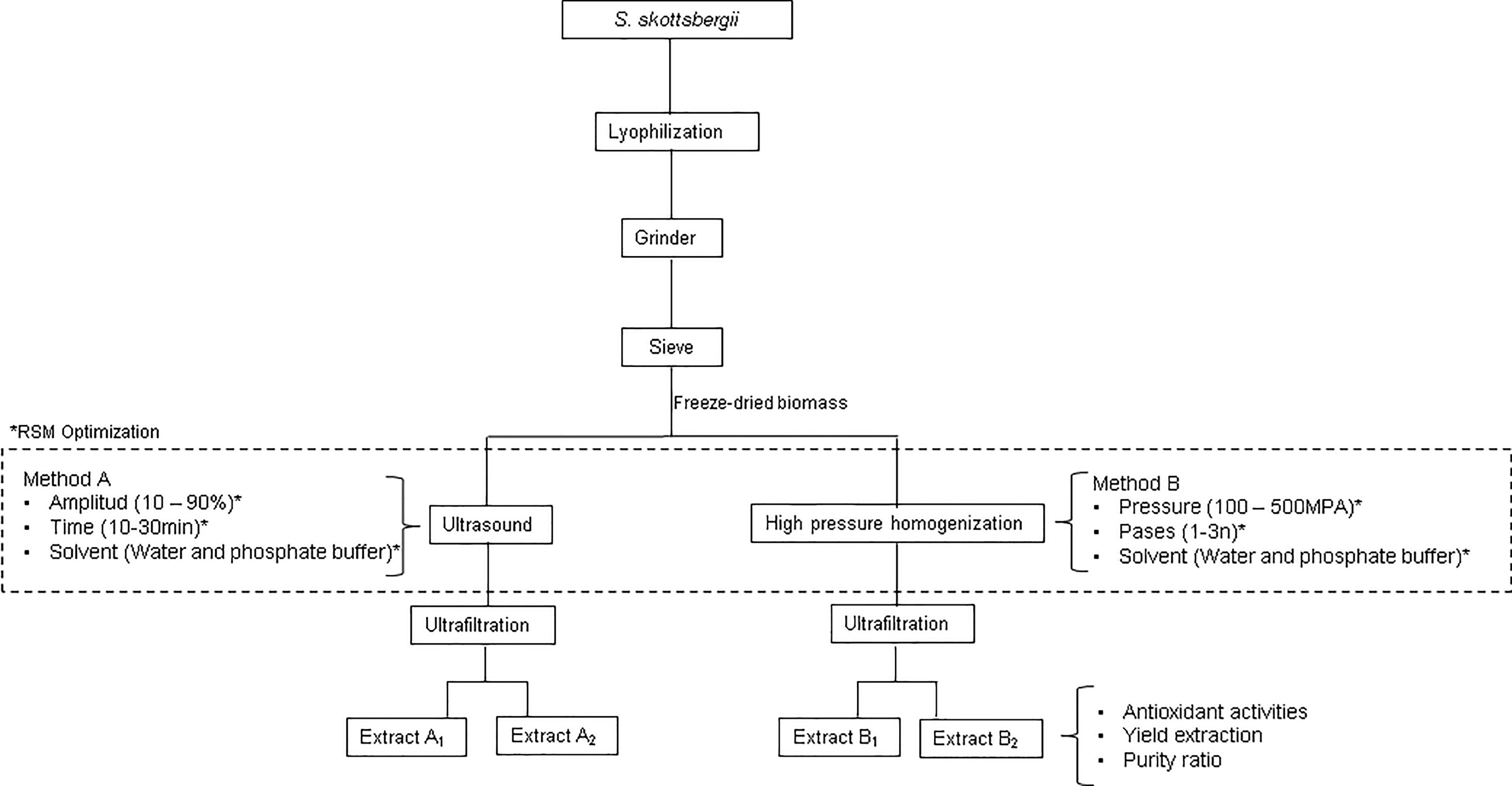
Figure 1 Summarizing scheme of the procedure for the extraction of R-Phycoerythrin from S. skotts. *RSM Optimization.
Ultrasound-Assisted Extraction (UAE)
The biomass/solvent suspension was placed either in an ultrasonic probe (Sonics VC-505 Vibra Cell Digital Ultrasonic with 3/4” (19 mm) probe, 500 W, 20 KHz, Newtown, CT, USA) for different periods (10-30 min) and amplitude (10-90%). The probe was inserted into the sample container at about 0.5 cm from the bottom. After UAE, the sample was immediately cooled in an ice bath to avoid overheating. To ensure a good homogeneity of the sample. After disruption, samples were centrifuged, collected, and analyzed by spectrophotometry at 565 nm (Section 3.3).
High-Pressure Homogenization Assisted (HPH) Extraction
The biomass/solvent suspension was carried out on high-pressure equipment (Gea Niro Soavi, Homogenizer Panda Plus 2000, Germany) for a different number of passes (1-3) at different pressure (100-500 MPa). The cell suspension was well mixed in the supply tank before disruption to ensure a good homogeneity. After disruption, samples were centrifuged, collected, and analyzed by spectrophotometry at 565 nm (Section 2.3).
Ultrafiltration Procedure
The extracts with the best phycoerythrin yields from methods A and B were selected to concentrate phycoerythrin using the ultrafiltration stage. Concentrated preparation was carried out following the method proposed by Denis et al. (2009) with modifications. 500 µL of algal extract solution were taken and transferred into Amicon tubes of 30 kDa porosity. They were centrifuged for 20 min at 4°C. The volumes of the samples (precipitate and permeate) and the phycoerythrin content in both fractions were determined by spectrophotometry at 565 nm (Section 2.3).
Biochemical Analyses Total Carbon, Hydrogen, Nitrogen, and Sulphur in Biomass
Total carbon (C), hydrogen (H), nitrogen (N), and Sulphur (S) were determined from dry biomass (20 mg) using the total combustion technique used in the LECO TruSppec Micro CHNSO-Elemental Analyzer according to a manual. This technique is based on the complete and instantaneous oxidation of the sample by pure combustion with controlled oxygen at a temperature of up to 1050°C (C, H, N, S) and pyrolysis at 1300°C (O) for decomposition of O as CO and oxidation to CO2. The resulting combustion products, CO2, H2O, SO2, and N2, are quantified by a selective IR absorption detector (C, H, S) and TCD (N) differential thermo-conductivity sensor. The result of each element (C, H, N, S) were expressed in % to the weight of the sample.
Total and Soluble Protein Content
Total protein content was calculated by multiplying the total internal nitrogen content by a factor of 4.59 reported by (Lourenço et al., 2002) for the red algae S. skottsbergii. After extraction treatments, the aqueous extract’s soluble protein content (PC) is spectroscopically determined (Bradford, 1976). Briefly, 200 µL of Bradford reagent was diluted with 790 µL distilled water and mixed with 10 µL soluble extracts or bovine serum albumin (BSA, Sigma, MO, USA). The absorbance was read at 730 nm (Biotek Synergy HT) after 5min of incubation at room temperature. Measurements were performed at least in triplicate.
Photosynthetic Pigments
Chla and total carotenoid concentrations were determined through 15 ml aliquots per extraction treatment, using Millipore filters of 0.45 μm. In darkness, the pigment concentration was extracted in 2 mL 100% methanol for 24 h at 4°C. After centrifugation at 5,000 g for 10 min, the absorption was measured by the UV-Vis spectrophotometer Thermo Fisher (Waltham, MA, USA), using the mathematical equation according to (Ritchie, 2008) for Chla (see Equation 1) and for the total carotenoids, the equations reported Parsons and Strickland, 1963 (see Equation 2). The results were expressed by µg mL-1 of volume of extract.
The content of the phycobiliproteins pigments in the phosphate buffer and water extract processed was spectrophotometrically determined at 565 nm, 620 nm, and 650 nm using the dichromatic equations (Eq. 4, 5, and 6) reported by (Bennett and Bogobad, 1973). All the data was normalized against 750 nm. The results were expressed by dry weight of biomass. Triplicate samples were taken from each treatment.
The purity of phycoerythrin (extract purity, EP) in the aqueous extract was defined as the ratio between the absorbance measurements at 565 and 280 nm, following the following equation (Sudhakar et al., 2015):
The extraction yield of phycoerythrin (mg g-1 DW) was calculated using the concentration of phycoerythrin (PE, mg ml-1), the volume of the extraction solvent (V, ml), and the mass of the dry biomass defined in the following equation (Sudhakar et al., 2015):
Antioxidant Capacity (DPPH and ORAC Analyses)
The antioxidant activity DPPH (2,2-diphenyl-1-picrylhydrazyil) assay (i.e., EC50) according to (Blois, 1958) was estimated by reducing the stable free radical DPPH. The aqueous supernatant measurements were used for DPPH analysis; 150 mL of DPPH were added to each extract. This solution of DPPH was prepared in 90% methanol (90MeOH: 10H2O) in 20 mL to a concentration of 1.27 mM. The reaction was complete after 30 min in a dark room at ~20°C and the absorbance was read at 517 nm in a spectrophotometer Thermo Fisher (Waltham, MA, USA). A calibration curve made with DPPH was used to calculate the remaining concentration of DPPH in the reaction mixture after incubation. Values of DPPH were expressed as mg DW mL -1. Ascorbic acid was used as a positive control ( Celis-Plá et al., 2014).
The antioxidant capacity was analyzed in aqueous macroalgae extracts determined by the ORAC method described by (Fukumoto and Mazza, 2000). The reaction was carried out in 75 mM phosphate buffer (pH 7.4). Sample (100 µL) and fluorescein (100 µL; 0.082 mM final concentration) solution were placed in the well of the microplate (black 96-well plates; Biotek, Synergy HT, USA). 2,2’-Azobis (-amidino propane) dihydrochloride (AAPH) solution (100 µL; 0.15 M final concentration) was rapidly added using a multichannel pipette. The plate was immediately placed in the plate reader (Biotek, Synergy HT), and the fluorescence was recorded every minute for 150 min at 37°C. Excitation and emission filters were 485-P and 520-P, respectively. The plate was automatically agitated prior to each reading. All reaction mixtures were prepared in triplicate and at least three independent runs were performed for each sample. Blank using phosphate buffer instead of the antioxidant solution and calibration solutions using Trolox (0–8 mM final concentration) as the antioxidant was also performed in the same run. The area under the fluorescence decay curve (AUC) was calculated using the KC4 v.3.4 software, and finally, the ORAC value was expressed as mg Trolox equivalent mL-1 substrate (mg TE mL-1).
Statistical Analysis
The interactive effect on the aqueous extracts selected, purity index, protein content, antioxidant activities, and pigments composition among extraction methods were evaluated through ANOVA (Underwood, 1996). For the ANOVA analysis, two fixed factors were measurements for the solvent extraction: 1) ultrasound-assisted extraction and 2) high pressure-assisted extraction. After significate effects, the interaction was determined with a posteriori test by Student Newman Keuls (SNK) (Underwood, 1996). Homogeneity of variance was evaluated using the Cochran test and visual inspection of the residuals (Underwood, 1996). In addition, the Pearson coefficient was calculated to determine the correlation pattern between antioxidant activity and photosynthetic pigments extracted by using the different mentioned methods. All analyses were performed using SPSS v.21 (IBM, USA).
The general variation patterns between biochemical variables, antioxidant activity and pigments composition measured in S. skottsbergii were explored using a multivariate approach. A Principal Coordinates Analysis (PCA) was performed for this purpose based on Euclidean distance using PERMANOVA+ for the PRIMER 6 package (Anderson et al., 2008). Such multivariate ordination was used to investigate the variation of the content of biochemical responses concurrently from observing the ordination plot.
Results
Chemical Assessment
Total carbon (28.38 ± 0.5) and sulphur contents (5.63 ± 0.05) were higher than the nitrogen content (5.46 ± 0.18) (Table S1). The C: N index in S. skottsbergii. was 23.85 ± 0.49.
Ultrasound-Assisted Extraction Method
The extraction yields of R-PE as a function of the amplitude wave, time, and solvent for extraction (buffer phosphate and distilled water) are shown in Table 1. The YPE ranged from 0.6-0.8 mg PE g-1 DW for buffer phosphate. In comparison, YPE ranged from 1.7-2.6 mg PE g-1 DW in distilled water (Table S2). The mathematical model represents the extraction yield as a function of the independent variables (amplitude wave and time) in each solvent at the chosen ranges; these models are expressed according to the following equations:
where X1 and X2 are the UAE amplitude wave (%) and time (min), respectively. The statistical significance of the regression model was demonstrated through the F-test and p-value (p<0.05).
For buffer phosphate, the ANOVA showed that there are statistical significance differences (p<0.05) in amplitude wave, with R2 0.84 and lack of fit of 4.91 (p-value =0,170) (p>0.05) (Table S3). The most significant effect on R-PE extraction is the quadratic effect of amplitude wave (+0.15). For distilled water, the amplitude wave and time have significant differences in the yield extraction of R-PE, with R2 0.89 and a lack of fit of 1.34 (p-value =0,170) (p>0.05) (Table S2). The most significant effect on R-PE extraction is the linear and quadratic effect of amplitude wave (+0.15 and -0.25, respectively) and the quadratic effect of time (-0.27).
The linear positive and negative interaction effects suggest that the extraction yield increase using distilled water as a solvent with an increase in the amplitude and, in addition, the extraction time is reduced. This effect can be observed in Figure 2B, where the surface response plot illustrates the combined effect of the variables in the extraction yield tendency.
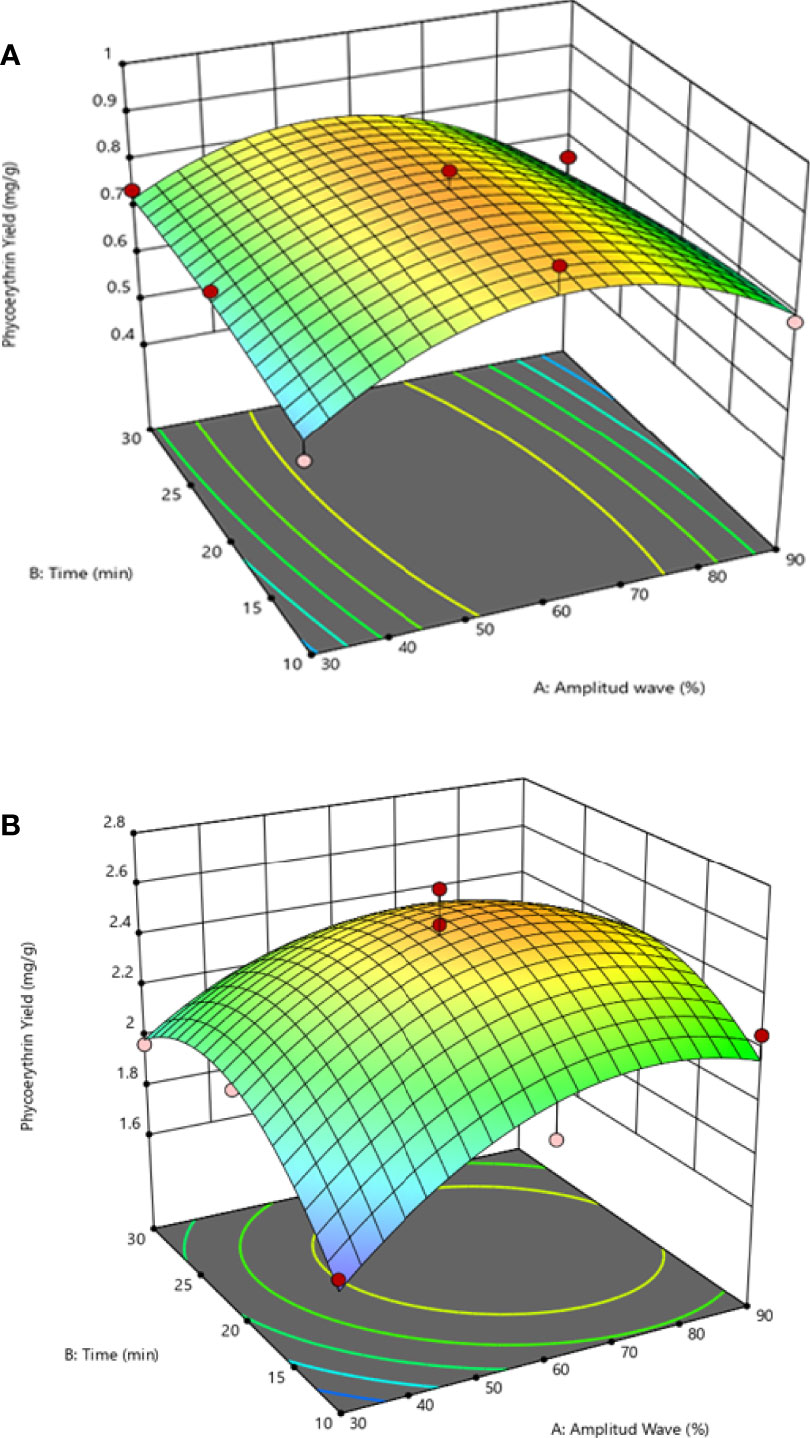
Figure 2 Response surface (3D) plots to display the effect between the amplitude wave (X1) and ultrasonic time (X2) by solvent extraction on the extraction yield: (A) effect between X1 and X2 under buffer phosphate, (B) effect between X1 and X2 under distilled water.
High-Pressure Homogenization Method
The experimental result of the extraction yields of R-PE as a function of the pressure, number of passes and solvent for extraction (buffer phosphate and distilled water) are shown in Table 2. In buffer phosphate, the YPE ranged from 3.1-5.1 mg g-1 of R-PE by dry biomass. The YPE ranged from 4.6-5.7 mg g-1 of R-PE for distilled water by dry biomass. The ANOVA applied to the data and the second-order model selected are summarized in Table 4 (Eq. 12 and 13). The mathematical model represents the extraction yield as a function of the independent variables (pressure and number of passes) for each solvent of extraction in the chosen ranges; the models are written according to the following equations:
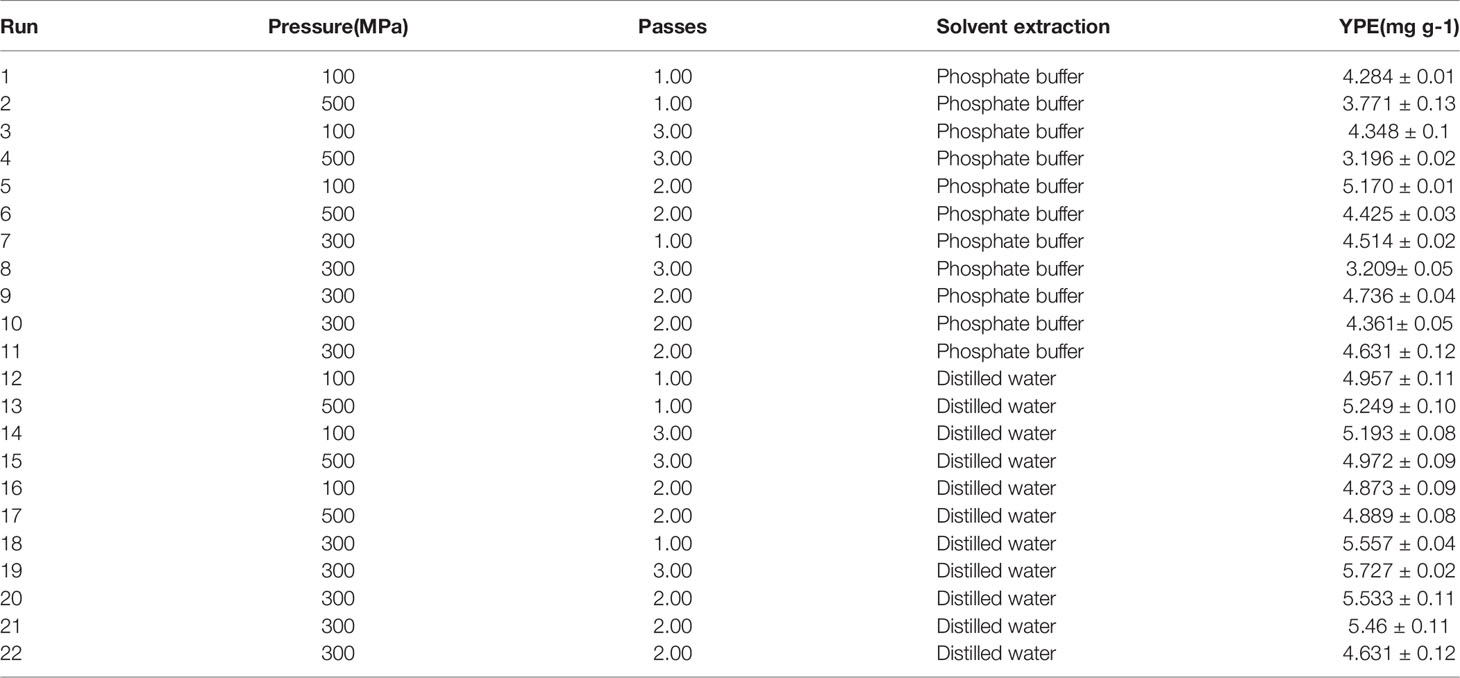
Table 2 Central composite design for high pressure homogenization-assisted method and yield (YPE) from response surface method analysis.
where X1 and X2 pressure (MPa) and the number of passes, respectively. The statistical significance of the regression model was demonstrated through the F-test and p-value (p<0.05). The response surfaces of Eq. 12 and 13 are displayed in Table S4 and S5, respectively.
In the analysis of HPH by using buffer phosphate, the ANOVA showed that there are statistically significant differences in pressure and number of passes without interactions, with R2 0.86 and lack of fit 3.65 (p>0.05) (Table S4).
From the HPH model using buffer phosphate, the most significant effects on R-PE extraction are the linear effect of pressure (-0.40) and time (-0.78). While for the water solvent, the model shows similar effects of the pressure (+0.25) and time (-0.42) on R-PE. The variables tested to increase the extraction yield, indicating that the extraction is efficient using a range of pressure between 100-500 MPa and between 1-3 of a number of passes. Figures 3A, B represent the surface response plots illustrating the effects that the combinations of variables have on the extraction yield of phycoerythrin. The range of interest that optimizes the response at a pressure between 100-300 MPa, 2-3 passes with distilled water as solvent is shown in Figure 3B. The maximum predicted optimization point is 390.2 MPa, with two passes with distilled water. This condition was experimentally validated through performance responses of the purity index and had concordance with the predictive yield.
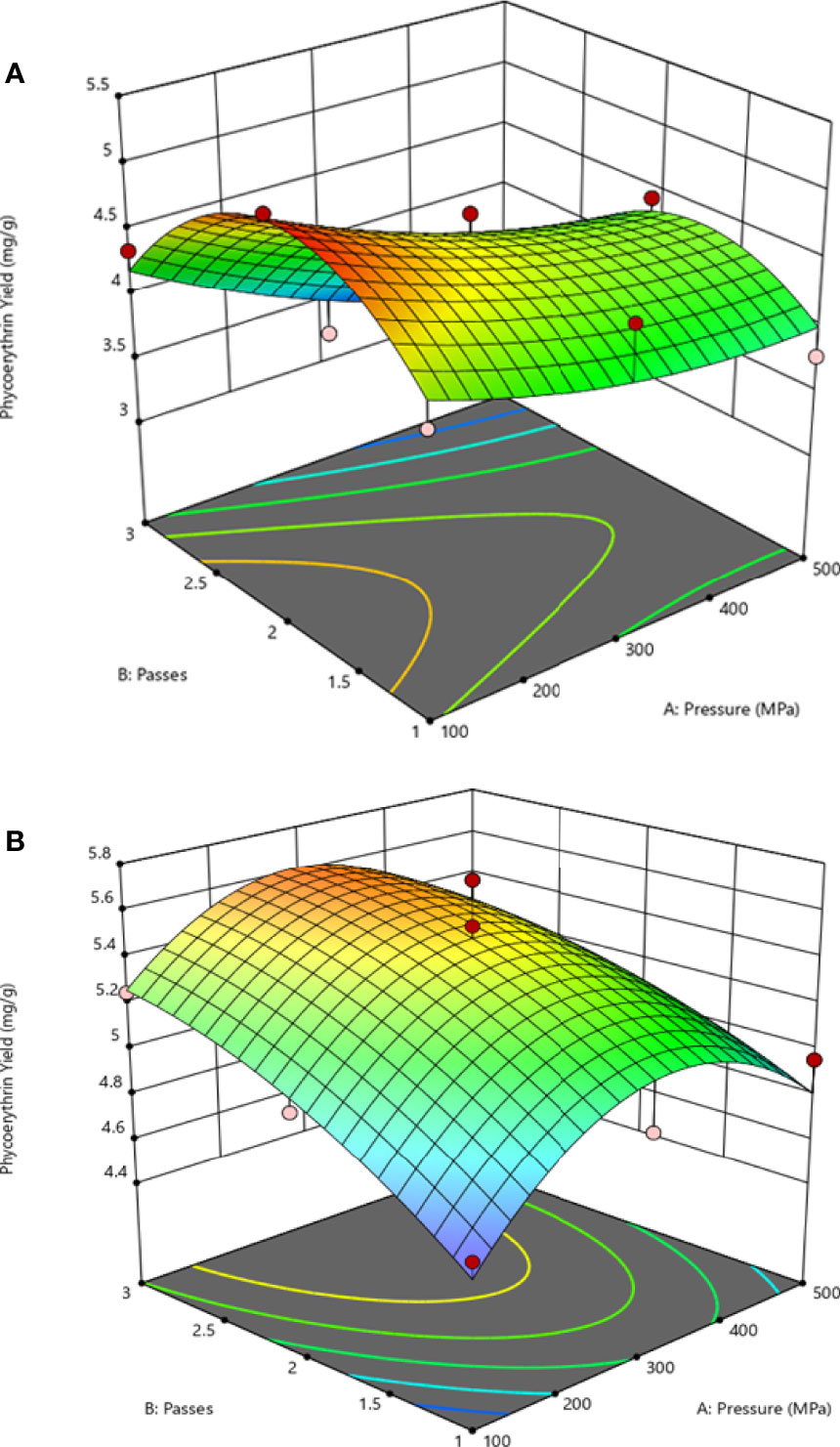
Figure 3 Response surface (3D) plots to display the effect between the pressure (X1) and passes (X2) by solvent extraction on the extraction yield: (A) effect between X1 and X2 under buffer phosphate, (B) effect between X1 and X2 under distilled water.
Characterization of Selected Extracts (Yield of R-Pe, Purity Index, Antioxidant Activities and Soluble Protein)
Yield and Purity Index of R-PE
According to our results, distilled water was the best extraction agent for UAE and HPH (p<0.05; Tables 3, 4). The operational conditions selected for UAE were 60% of amplitude wave and 20 min. For HPH, the conditions selected were 300 MPa and two number of passes. The extraction yields of R-PE for UAE were 2.3 mg g-1 DW and for HPH was 5.7 mg g-1 DW, which were close to the values predicted by the second-order model 2.3 mg R-PE g-1 DW and 5.6 mg R-PE g-1 DW, respectively. Thus, the second-order model was validated by these results. The quality of the phycoerythrin enriched extract of these selected conditions is gathered in Table 3.
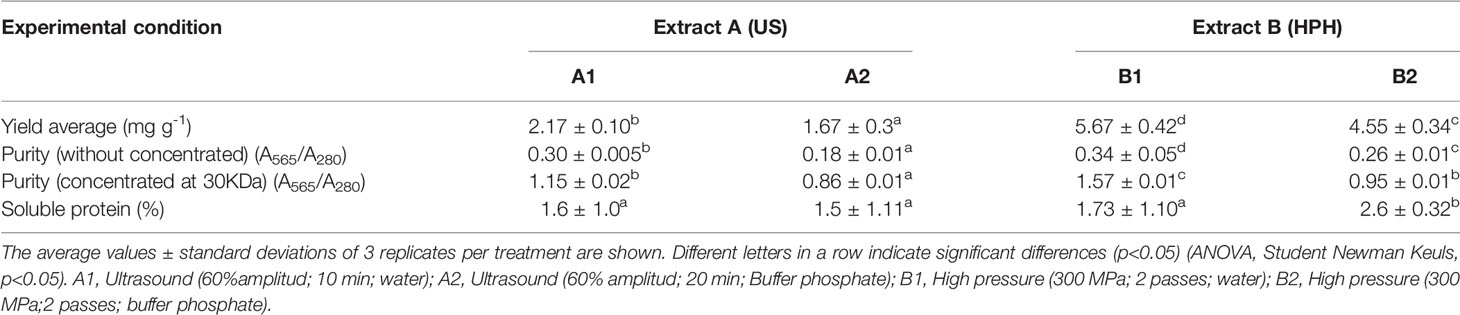
Table 3 Characteristics of extracts obtained at selected conditions for ultrasonication (US) and high-pressure homogenization (HPH) methods.
From the criterium (Yield of R-PE) of selected extracts conditions by methods A (UAE) and B (HPH), the Ultrafiltration process was applicated for both methods. For the UAE, extracts A1 extracts are in distilled water and A2 in buffer phosphate. B1 extracts are in distilled water for the B extraction, and B2 in buffer phosphate. The results showed that the membrane 30 KDa polyethersulfone (PES) allowed 100% of PE recovery in all extracts obtained by UAE (Extracts A1 and A2) and HPH (extracts B1 and B2). R-PE’s purity index (A565/A280) was significantly higher in A2 and B1. Between the treatments, B1 extracts of HPH had the highest extraction yield, which reached 1.57; 0.42 times greater than A1 extract A1 obtained by UAE (Table 3).
Antioxidant Activities
The antioxidant activity by DPPH method ranged from 3.3 to 11.0 (% w/w dry biomass), showing significant differences among the extractions (Figure 4; Table S6). HPH found the highest level of antioxidant activity with distilled water (11.0 ± 2.5%). On the other hand, the ORAC activity differed significantly among the different extraction treatments. The activity increased under both extraction treatments (UAE and HPH) in distilled water concerning the maceration method. However, the distilled water shows the highest activity detected between UAE and HPH. The antioxidant activity ranged between 3.70 ± 0.62 and 10.40 ± 0.77% for UAE conditions evaluated, while under HPH the ORAC was between 3.30 ± 0.32 and 11.0 ± 2.5% (Table 4; S6). The positive correlations were obtained between the antioxidant activity and the analyzed molecules. Chlorophyll a showed a positive correlation (r=0.80 and carotenoids (r=0.73) with antioxidant activity by using DPPH method (Table S7). At the same time, phycoerythrin shows a positive correlation (r=0.75) with ORAC method (Tables S6 and S7).
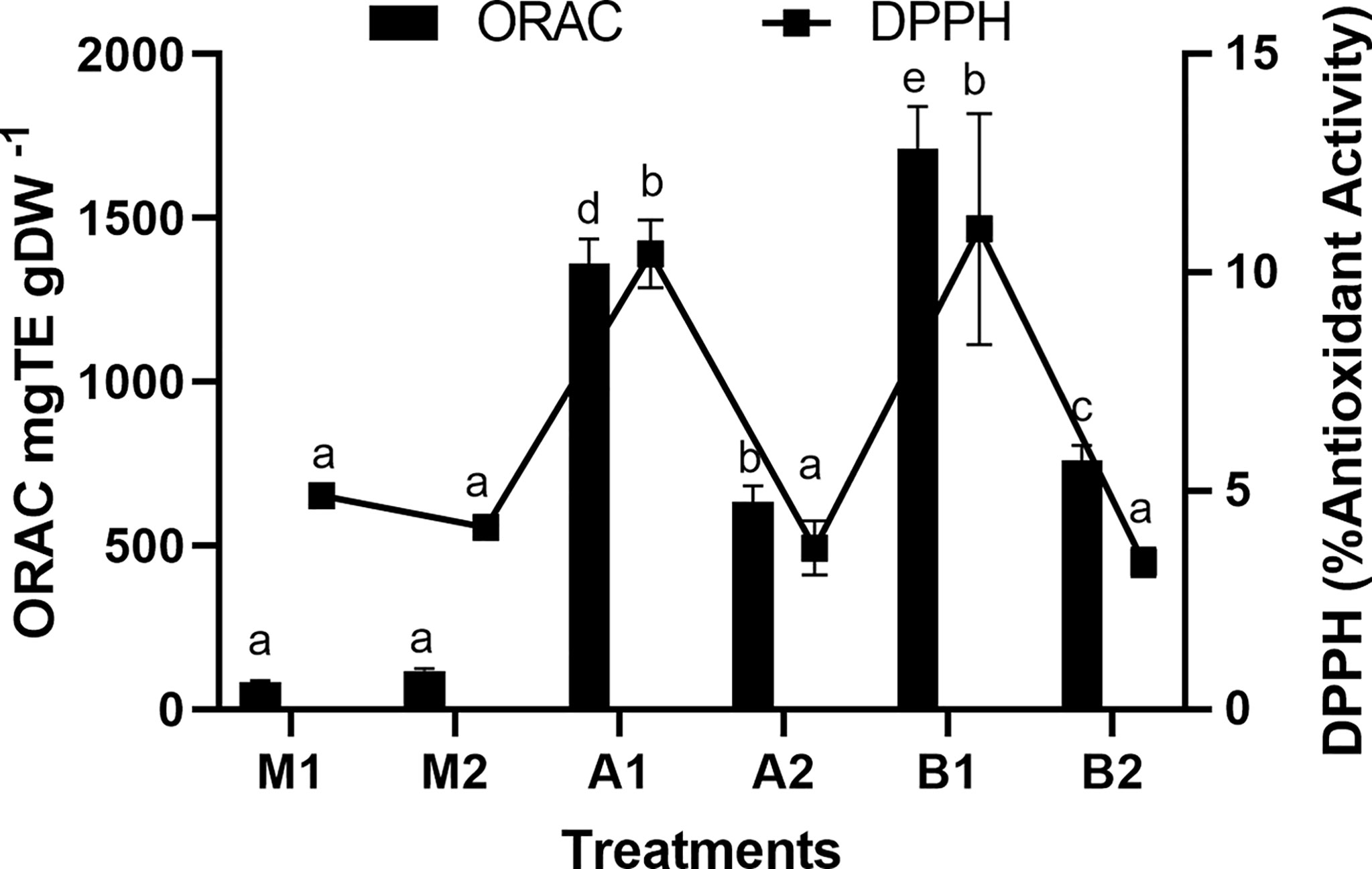
Figure 4 Total antioxidant activities (ORAC and DPPH) of the aqueous extract of S. skottsbergii at selected conditions for ultrasound-assisted extraction (UAE) and high-pressure homogenization (HPH). The average values ± standard deviations of 3 replicates per treatment are shown. Different letters indicate significant differences (p<0.05) (ANOVA, Student Newman Keuls, p<0.05). M1: Maceration by water; M2: Maceration by Buffer phosphate; A1: Ultrasound by water; A2: Ultrasound by Buffer phosphate; B1: High pressure by water; B2: High pressure by Buffer phosphate.
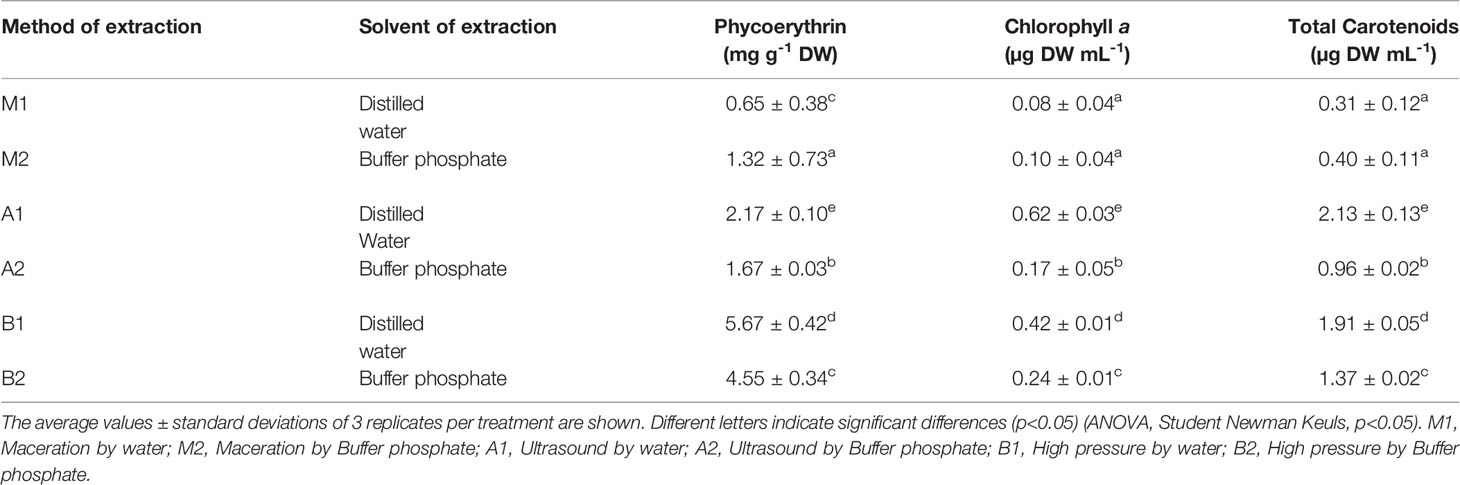
Table 4 Pigmentary composition (Phycoerythrin content, Chlorophyll a and Total of carotenoids of the aqueous extract of S. skottsbergii at selected conditions for ultrasound (US) and high-pressure homogenization (HPH).
Multivariable Analyses
According to the extraction’s treatments, the principal coordinates analyses (PC diagram, according to Figure 5) in the relationship S. skottsbergii extract showed a positive correlation of the first axis (81% of total variation) with the samples for M1, M2, A2 and B2. Conversely, the variables: Phycoerythrin, Carotenoids, ORAC, Chla and DPPH, were highest in the samples of B1 and A1 extraction methods extract and were positively correlated with the second axis (13.3% of total variation), with intermedial values for S. skottsbergii (Figure 5).
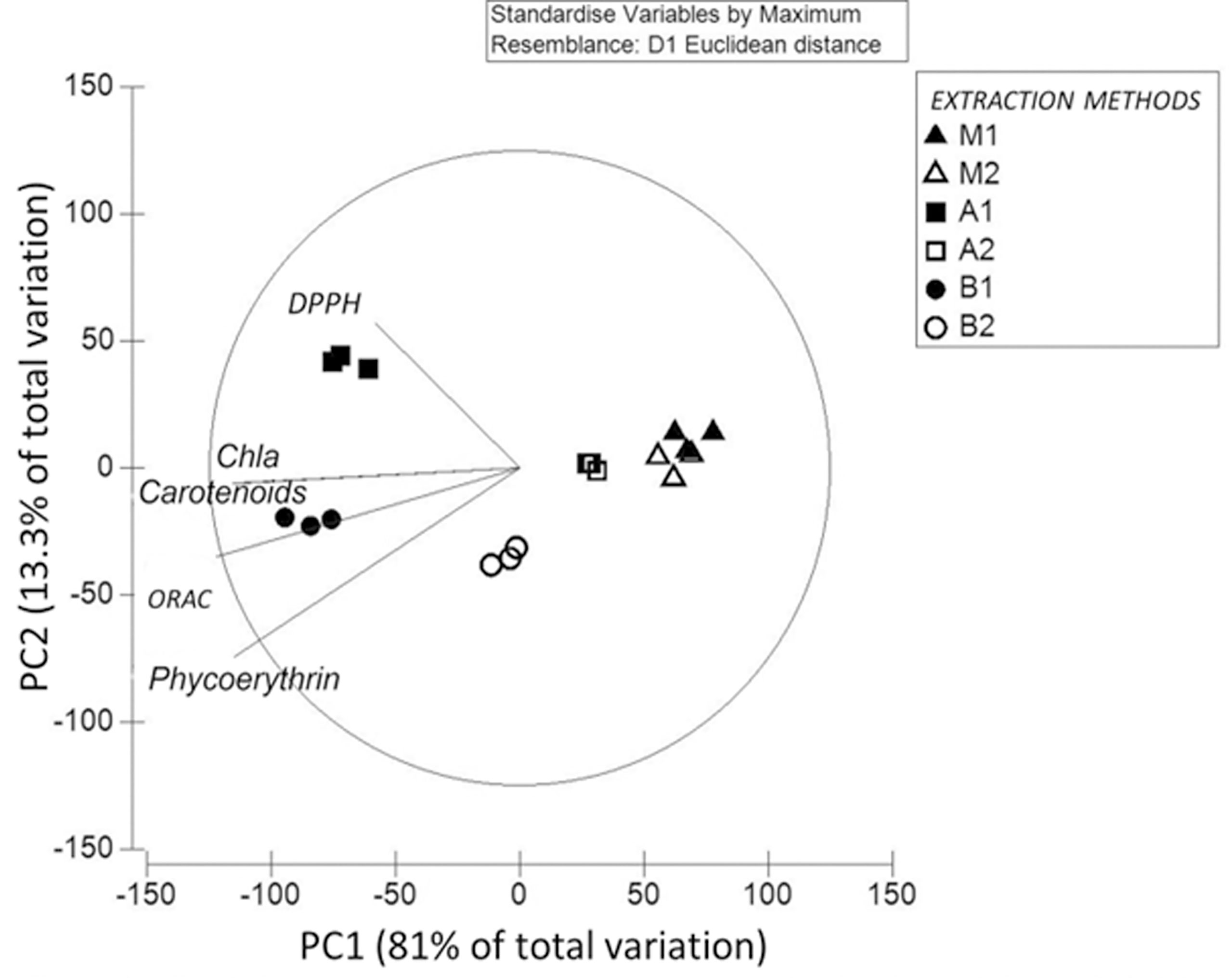
Figure 5 Principal component analysis to pigments quantification and antioxidant activity in S. skottsbergii with respect to the extraction methods. M1: Maceration by water; M2: Maceration by Buffer phosphate; A1: Ultrasound by water; A2: Ultrasound by buffer phosphate; B1: High-pressure by water and B2: High-pressure by Buffer phosphate.
Discussion
Red seaweeds, such as Porphyra sp. (Nori), have relatively high protein content (Mouritsen et al., 2018; Abdala-Díaz et al., 2019). However, in our study, the measured content of protein for S. skottsbergii (5.46%DW) was lowest than that of Porphyra sp. (Rhodophyta) (15.6%DW), but high respect to the brown algae Laminaria ochroleuca (Phaeophyceae) (Alginate source) (4.8% DW) reported by Abdala-Díaz et al. (2019). Protein levels may vary in different species, geographical areas, seasons, and extractions methodologies (Pereira et al., 2020). Seaweeds, especially red seaweeds, appear to be an essential source of proteins and biliproteins (Mouritsen et al., 2018; Pereira et al., 2020). On the other hand, it has been seen that the degree of sulphation is related to bioactivity in concordance with protein or polysaccharides. It is unknown if more significant bioactivity is present in this species related to sulfating of organic compounds. Carrageenan-based delivery systems present excellent performance in the delivery of bioactive ingredients, which can improve the stability and bioavailability of bioactive ingredients (Huang et al., 2021).
Generally, the R-PE yield depends on the solvent of extraction. However, the yield decreased above 20 min of exposition amplitude wave. This situation can be attributed to the pigment denaturation due to the amplitude wave, which increases the extracted temperature (48°C; non shown data). In this sense, more than 20 min is not recommended for S. skottsbergii, assuming that chloroplast is slightly damaged, explaining R-PE’s low release (Pereira et al., 2020). Similar conclusions were reported by Simovic et al. (2022), who found a reduction of R-PE over 45°C from macroalgae Porphyra purpurea (Rhodophyta) when using the high-pressure method. Knowing each variable’s individual and combined effects on R-PE extraction, a model was constructed to predict the optimum conditions at which higher R-PE yields can be extracted. The range optimizes the response is an amplitude between 60-90%, 10-20 min of extraction time, and distilled water as solvent. At the same time, the optimal predicted point is 68% of amplitude ultrasound, 18 min with distilled water. These observations agree with Pereira et al. (2020), who found that between 15-20 min under an ultrasound probe would result in a high yield of phycoerythrin from the red algae Gracilaria gracilis. Although they did not include the evaluation of amplitude wave, the extraction was done using buffer phosphate. This suggests the effects of extraction yield depend on the extraction solvent and the species of the macroalgae used. Mittal et al. (2017) analyzed different green technologies and observed that sustainable yield of the phycoerythrin extraction was obtained by a combination of the ultrasound probe with homogenization under buffer phosphate solvent.
However, the extractions yields are reduced (0.16 mg of R-PE g DW-1) compared to this study. As a solvent of extraction, the distilled water is the primary factor that enhances the extraction of phycoerythrin and is less influenced by the combined effects of ultrasound wave (%) and time in S. skottsbergii (Figures 2A, B; Table 2). These observations are in agreement to Jubeau et al. (2013), Sudalkar et al. (2015), and Tan et al. (2020), who there reported higher levels when using distilled water for phycoerythrin extraction. The literature suggests that the UAE has the advantage of direct scalability due to its ability to generate progressively high-intensity cavitation zones and, therefore, suitable for scale-up of the industrial process (Le Guillard et al., 2015; Rodrigues et al., 2018). Previous studies reported the successful extraction of phycobiliproteins using ultrasonic waves from other types of algae, such as the microalgae Porphyridium purpureum (formerly Porphyridium cruentum) (Rhodophyta) and the macroalga Dasysiphonia japonica (formerly Heterosiphonia japonica) (Rhodophyta) (Román et al., 2002; Benavides and Rito-Palomares, 2006; Sun et al., 2009). In this sense, Sharma et al. (2020) found that the duty cycle variables and electrical acoustic intensity have significant exposure for enhancing the C-phycoerythrin from Oscillatoria sp. (Cyanobacteria) whereas Ardiles et al. (2020) found the maximum R-PE yield was obtained under optimal US conditions (Amplitude wave 100% and 15 min for Porphyridium cruentum (Rhodophyta). However, when larger periods of treatment were used (>20 min), a degradation of phycobiliproteins was observed (Figures 2A, B) (decrease in maximal absorbance), in agreement with other studies (Rodrigues et al., 2018; Pereira et al., 2020).
Using high-pressure homogenization (HPH), we observed that the distilled water as a solvent of extraction increases the R-PE yields with pressure and number of passes as a combined effect. Similar results were found by Sudalkar et al. (2015) and Jubeau et al. (2012), showing that water as solvent extraction can increase the yield of phycoerythrin extracts from red algae, as was observed in this study with S. skottsbergii. Our results suggest that the release of phycoerythrin can be promoted with distilled water due to accelerated molecule diffusion and solubility of other proteins. However, heat can also induce the degradation of the extracted bioactive compounds. The high amplitude of UAE (between 75-90% wave amplitude) and HPH (>450MPa) produces a higher temperature (average of 40°C; non shown data).
The macroalgae studied usually grow in the cold water (<10°C) from the Magallanes coast of Chile; thus, the bioactive components could be susceptible to increased heat. These might explain why high pressure and high ultrasound caused a decrease in the phycoerythrin content of the extract. Similar results were observed by Pereira et al. (2020), where the pressure at 600 MPa induced an increase in the extracted temperature, promoting pigment denaturation. Simovic et al. (2022) reported that contrary to elevated temperature, high-pressure (HP) treatment showed significant concentration at 450 MPa had a less destructive effect on R-PE color intensity from macroalgae Porphyra purpurea (Rhodophyta). However, the yields are less even if worked in a range similar to our range optimization of 100-300 MPa. This difference can be attributed to the different species of red algae and geographical latitude because the morphology of the cell wall and accumulation of metabolites can vary despite R-phycoerythrin being the pigment with the highest proportion in red algae.
On the other hand, the lyophilization ice crystals are formed during the freezing step (Soni et al., 2006) that, upon thawing, break down the cellular walls and release the intracellular content directly (Hardouin et al., 2014). Therefore, the treatment with freeze biomass can be used to achieve higher yields. Similar results were observed by Pereira et al. (2020), where the samples that were frozen at -80°C obtained higher R-PE yields than that at room temperature. Ghosh and Mishra (2020) found that the interaction between buffer molarity (0.1 to 1 M) and a number of freeze-thaw cycles (-70 to 27°C) was most significant for modeling the responses optimization of the (C-PE) from Lyngbya sp. (Cyanobacteria). Tan et al. (2020) showed similar results with the combination of freezing at −80°C (2 h) and thawing at 25°C (24 h) as optimal temperature and extraction time to obtain the highest amount of phycobiliproteins for Arthrospira sp. (Cyanobacteria). However, several studies have shown that this increase was not significant due to the high content of cellulose deposited in the cell wall (Kannaujiya et al., 2017; Thoisen et al., 2017).
Regarding other species of red macroalgae, previous studies showed that the extractions by using mortar maceration of Gracilaria corticata and Gracilaria canaliculata (formerly Gracilaria crassa) yielded 0.78 mg R-PE g-1 DW and 0.5 mg R-PE g-1 DW, respectively. These values are still lower than that by using the HPH method but similar to our maceration extraction (0.65 mg R-PE g-1 DW). Previous results by Astorga-España et al. (2017), in the same species of S. skottsbergii by using buffer phosphate under the maceration method showed yields ranging from 0.057-0.078 mg g-1 DW. These differences can be attributed to the extraction method, solvent (phosphate or water), seasonal harvest, and red macroalgae species. However, our work demonstrates high yield levels found in distilled water either by HPH or UAE. In this sense, we believe that the sulfated polysaccharides are released into the matrix because of the increase in temperature, which increases the viscosity of polysaccharides.
Consequently, the cell shear stress is high in this water, allowing the release of a greater quantity to the phosphate buffer. The next step of this work is to study the degree of shear stress at the level of the extraction matrix and comparison between extraction solvents. Therefore, this is a new report of a commercial species where different green extraction methods and solvents for extraction are compared. Considering that S. skottsbergii is a commercial species, the cost of extraction using distilled water is lower than that of buffer phosphate, previous to carrageenan extraction processes, visualizing a possible cost-effective biorefinery of this macroalgae in local industry from Chile.
From the observations in Figures 2, 3, our results could be helping to improve the pigment, which remains at a desirable level, considering its commercial importance and potential applications in the functional food sector or supplement market.
Most of the S. skottsbergii proteins are more soluble in the extracts A1 and B1 than A2 and B2, but not phycoerythrin, which is found in more water solutions (Jubeau et al., 2013; Sudhakar et al., 2015). This explains why the purity ratios are higher in distilled water than in phosphate buffer. The soluble proteins detected reached 2.6 ± 0.32% and 1.73 ± 1.1% using buffer phosphate and distilled water, respectively (p<0.05; Table 5). This result is in agreement with Jubeau et al. (2013) report, where a higher purity ratio (0.79) in distilled water than in a culture medium applying a two-step high-pressure process in microalgae Porphyridium purpureum was detected. Purification yields obtained here and for Denis et al. (2009) were different because of the biomass treatment, but the concentration of the R-PE was the same effect under 30KDa PES membrane. The permeate flux obtained during the filtration on 30 kDa PES membrane was similar in all treatments, allowing for retaining all R-PE without significant accumulation of unwanted molecules. Moreover, from an industrial point of view, polyethersulfone (PES) membranes are more widely used than cellulose regenerated ones due to their lower fragility and higher resistance to chemicals, pH, and temperature variations (Denis et al., 2009; Mittal et al., 2017).
When using HPH in distilled water (pH 7.0), the levels are higher than in phosphate buffer (pH 6.5). It is precisely in distilled water where the high levels of phycoerythrin are found (treatment B1) and chlorophyll and total carotenoids (treatment A1). This could be related to an increase in temperature and the release of polysaccharides that tend to increase the viscosity of the matrix, and so could change the pattern of the cells towards the pressure and the amplitude of the wave, as well as the solubility of PE, being higher in water as solvent by booth methods (UAE and HPH) compared to other solvents.
The R-PE in S. skottsbergii is influenced by HPH with water solvent reached 5.67 mg g−1 by dry biomass. The content pigment in red algae is highly dependent on the perceived light intensity and quality of light (Castro-Varela et al., 2021). However, we suggest the application of HPH to release more content of the R-PE without decreasing the stability, as shown in the purity index (Table 3; Figure 3). Thus, these results reinforce using phycobiliproteins (aqueous extracts) from a new source of S. skottsbergii for nutraceutical or pharmacology applications (Jung et al., 2016; Mittal et al., 2019).
There are still few studies that evaluate the impact of the extractive technique on the bioactive, which makes it difficult to compare our results with other works on pigments process optimization. In this aspect, we consider that our work is the first approach to the state of the pigmentary material of this red macroalgae after method extractions, of high interest as several authors have valued for food applications (Le Guillard et al., 2015; Khanra et al., 2018; Liu et al., 2019; Mittal et al., 2019).
Biliproteins showed a positive correlation with the antioxidant activity in different red algae and cyanobacteria (Sekar and Chandramohan, 2008; Pagels et al., 2019). The pigments and antioxidant capacity of the water extract from the S. skottsbergii show a good relation. Biliproteins concentrations can vary depending on environmental factors like irradiance, light quality, pH or nutrients (Korbee et al., 2005; Celis-Plá et al., 2014; Astorga-España et al., 2017; Pagels et al., 2019). The extraction can also influence the content; e.g., a great number of authors used the freezing and thawing method (Niu et al., 2006). However, our observations showed significant antioxidant properties, which were increased with the enhancement of R-PE concentration in water solvent by UAE and HPH method. Although our study does not use a chromatographic technique for purification and determinate the polyphenols and polysaccharides, the concentred extract of R-PE could be part of a synergy of these compounds in the redox activity can be attributed to this macroalgae.
Conclusions
The extraction of R-PE from S. skottsbergii was optimized by RSM, using UAE and HPH. RSM proved to be useful for PE extraction in the tested range of optimization, providing a model with a good agreement between the experimental and predicted results. HPH obtained the most efficient extraction yielding 5.7 mg R-PE g-1 DW biomass at the optimal conditions (300 MPa, 2 passes with distilled water), 50-60% higher than the PE yields obtained with UAE. The most crucial variable in the extraction process was the pressure level, with higher concentrations of R-PE using distilled water. S. skottsbergii revealed an excellent source of R-phycoerythrin and antioxidant activity, visualizing its application as a bioactive ingredient could be suggested for the food industry.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
PC-V, PC-P, FF, and MR conceived and designed the experiments; PC-V performed the experiments; all authors analysed the data and co-wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
The research leading to these results has received funding from National Agency of Development and Research of Chile (PhD Scholarship N°21180257) and Andalusian government, Spain (Project FACCO, UMA18-FEDER JA-162).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We want to express our gratitude to Mr. Jaime Zamorano, Director of Development, from the GELYMAR S.A. for collaborate with this research project. We also want to thank to the Photobiology and Biotechnology of Aquatic Organisms research group (FYBOA, RNM-295) and the Institute of Blue Biotechnology and Developmen (IBYDA), University of Malaga due to the use of laboratory equipments and acknowledgments of the Laboratory of Coastal Environmental Research (LACER, University of Playa Ancha) for the technical support. P.S.M.C-P thanks to CEA 22-20 project of the University of Playa Ancha regular competition research 2019.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.877177/full#supplementary-material
References
Abdala Díaz R. T., Casas Arrojo V., Arrojo Agudo M. A., Cárdenas C., Dobretsov S., Figueroa F. L. (2019). Immunomodulatory and Antioxidant Activities of Sulfated Polysaccharides From Laminaria Ochroleuca, Porphyra Umbilicalis, and Gelidium Corneum. Mar. Biotechnol. 21, 577–587. doi: 10.1007/s10126-019-09905-x
Alexandre E. M. C., Araújo P., Duarte M. F., de Freitas V., Pintado M., Saraiva J. A. (2017). Experimental Design, Modeling, and Optimization of High-Pressure-Assisted Extraction of Bioactive Compounds From Pomegranate Peel. Food Bioproc. Technol. 10, 886–900. doi: 10.1007/s11947-017-1867-6
Anderson M. J., Gorley R. N., Clarke K. R. (2008). PERMANOVA + for PRIMER: Guide to Software and Statistical Methods. PRIMER-E. Plymouth.
Angell A. R., Mata L., de Nys R., Paul N. A. (2016). The Protein Content of Seaweeds: A Universal Nitrogen-to-Protein Conversion Factor of Five. J. Appl. Phycol. 28, 511–524. doi: 10.1007/S10811-015-0650-1/FIGURES/5
Ardiles P., Cerezal-Mezquita P., Salinas-Fuentes F., Órdenes D., Renato G., Ruiz-Domínguez M. C. (2020). Biochemical Composition and Phycoerythrin Extraction From Red Microalgae: A Comparative Study Using Green Extraction Technologies. Processes. 8, 1628. doi: 10.3390/PR8121628
ASAE S319.3. Method of Determining and Expressing Fineness of Feed Materials by Sieving. Available at: https://dokumen.tips/documents/asae-s3193-method-of-determining-and-expressing-fineness-of-feed-materials-by-sieving.html (Accessed January 25, 2022).
Astorga-España M. S., Mansilla A., Ojeda J., Marambio J., Rosenfeld S., Mendez F., et al. (2017). Nutritional Properties of Dishes Prepared With Sub-Antarctic Macroalgae—An Opportunity for Healthy Eating. J. Appl. Phycol. 29, 2399–2406. doi: 10.1007/s10811-017-1131-5
Barba F. J., Grimi N., Vorobiev E. (2015). New Approaches for the Use of Non-Conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals From Microalgae. Food Eng. Rev. 7 (1), 45–62. doi: 10.1007/s12393-014-9095-6
Benavides J., Rito-Palomares M. (2006). Simplified Two-Stage Method to B-Phycoerythrin Recovery from Porphyridium cruentum. J. Chromatogr. B. Anal. Technol. BioMed. Life Sci. 844, 39–44. doi: 10.1016/J.JCHROMB.2006.06.029
Bennett A., Bogobad L. (1973). Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 58, 419–435. doi: 10.1083/jcb.58.2.419
Bischoff-Bäsmann B., Wiencke C. (1996). Temperature Requirements for Growth and Survival of Antarctic Rhodophyta. J. Phycol. 32, 525–535. doi: 10.1111/j.0022-3646.1996.00525.x
Blois M. S. (1958). Antioxidant Determinations by the Use of a Stable Free Radical. Nat. 1958. 181:4617. 181, 1199–1200. doi: 10.1038/1811199a0
Bradford M. M. (1976). A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Buschmann A. H., Hernández-González M. C., Aranda C., Chopin T., Neori A., Halling C., et al. (2008). Mariculture Waste Management. Ency. Ecology. 5, 2211–2217. doi: 10.1016/B978-008045405-4.00045-8
Castro-Varela P. A., Celis-Plá P. S. M., Abdala-Díaz R., Figueroa F. L. (2021). Photobiological Effects on Biochemical Composition in Porphyridium Cruentum (Rhodophyta) With a Biotechnological Application. Photochem and Photobiol. 97, 1032–1042. doi: 10.1111/php.13426
Celis-Plá P. S. M., Korbee N., Gómez-Garreta A., Figueroa F. L. (2014). Seasonal Photoacclimation Patterns in the Intertidal Macroalga Cystoseira Tamariscifolia (Ochrophyta). Scientia. Mar. 78 (3), 377–88. doi: 10.3989/scimar.04053.05a
Chemat F., Rombaut N., Sicaire A. G., Meullemiestre A., Fabiano-Tixier A. S., Abert-Vian M. (2017). Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 34, 540–560. doi: 10.1016/j.ultsonch.2016.06.035
Ciko A. M., Jokić S., Šubarić D., Jerković I. (2018). Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds From Marine Macroalgae. Mar. Drugs 16 (10), 348. doi: 10.3390/md16100348
Denis C., Massé A., Fleurence J., Jaouen P. (2009). Concentration and Pre-Purification With Ultrafiltration of a R-Phycoerythrin Solution Extracted From Macro-Algae Grateloupia Turuturu: Process Definition and Up-Scaling. Separation and Purification Technology 69, 37–42. doi: 10.1016/j.seppur.2009.06.017
Fukumoto L. R., Mazza G. (2000). Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 48 (8), 3597–3604. doi: 10.1021/jf000220w
Galland-Irmouli A., Pons L., Luçon M., Villaume C., Mrabet N. T., Guéant J. L., et al. (2000). One-Step Purification of R-Phycoerythrin From the Red Macroalga Palmaria Palmata Using Preparative Polyacrylamide Gel Electrophoresis. J. Chromatogr. B.: Biomed. Sci. Appl. 739 (1), 117–123. doi: 10.1016/S0378-4347(99)00433-8
Glazer A.N., Apell G.S., Hixson C.S., Bryant D.A., Rimon S., Brown D.M., et al (1976). Biliproteins of Cyanobacteria and Rhodophyta: Homologous Family of Photosynthetic Accessory Pigments. Proc. Natl. Acad. Sci. U.S.A. 73 (2), 428–431. doi: 10.1073/pnas.73.2.428
Ghosh T., Mishra S. (2020). Studies on Extraction and Stability of C-Phycoerythrin From a Marine Cyanobacterium. Front. Sustain. Food Syst. 4, 102. doi: 10.3389/FSUFS.2020.00102/BIBTEX
Gómez-Ordóñez E., Jiménez-Escrig A., Rupérez P. (2010). Dietary Fibre and Physicochemical Properties of Several Edible Seaweeds From the Northwestern Spanish Coast. Food Res. Int. 43 (9), 2289–2294. doi: 10.1016/j.foodres.2010.08.005
Hardouin K., Bedoux G., Burlot A. S., Nyvall-Collén P., Bourgougnon N. (2014). Enzymatic Recovery of Metabolites From Seaweeds: Potential Applications. Adv. Botan. Res. 71, 279–320. doi: 10.1016/B978-0-12-408062-1.00010-X
Hasan M. R., Chakrabarti R. (2009). Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture: A Review. FAO Fisheries and Aquaculture Technical 531, Rome, FAO. 123p.
Huang Y.-Z., Jin Z., Wang Z.-M., Qi L.-B., Song S., Zhu B.-W., et al. (2021). Marine Bioactive Compounds as Nutraceutical and Functional Food Ingredients for Potential Oral Health. Front. Nutr. 8, 1011. doi: 10.3389/FNUT.2021.686663/BIBTEX
Jeon M. R., Choi S. H. (2012). Quality Characteristics of Pork Patties Added With Seaweed Powder. Food Sci. Anim. Resour. 32, 77–83. doi: 10.5851/KOSFA.2012.32.1.71
Jubeau S., Marchal L., Pruvost J., Jaouen P., Legrand J., Fleurence J. (2013). High Pressure Disruption: A Two-Step Treatment for Selective Extraction of Intracellular Components From the Microalga Porphyridium Cruentum. J. Appl. Phycol. 25, 983–989. doi: 10.1007/s10811-012-9910-5
Jung S. M., Park J. S., Shim H. J., Kwon Y. S., Kim H. G., Shin H. S. (2016). Antioxidative Effect of Phycoerythrin Derived From Grateloupia Filicina on Rat Primary Astrocytes. Biotechnol. Bioproc. Eng. 21, 676–682. doi: 10.1007/s12257-016-0369-0
Kannaujiya V. K., Sundaram S., Sinha R. P. (2017). Advances in production technology. In: Kannaujiya VK, Sundaram, Sinha RP (eds) Phycobiliproteins: Recent Developments and Future Applications. Springer, Cham, 83–97. doi: 10.1007/978-981-10-6460-9
Kawsar S., Yuki F., Matsumoto R., Yasumitsu H., Yasuhiro O. (2011). Protein R-Phycoerythrin From Marine Red Alga Amphiroa Anceps: Extraction, Purification and Characterization. Phytologia Balcanica 17 (3), 347–354.
Khanra S., Mondal M., Halder G., Tiwari O. N., Gayen K., Bhowmick T. K. (2018). Downstream Processing of Microalgae for Pigments, Protein and Carbohydrate in Industrial Application: A Review. Food Bioprod. Process. 110, 60–84. doi: 10.1016/j.fbp.2018.02.002
Korbee N., Huovinen P., Figueroa F. L., Aguilera J., Karsten U. (2005). Availability of Ammonium Influences Photosynthesis and the Accumulation of Mycosporine-Like Amino Acids in Two Porphyra Species (Bangiales, Rhodophyta). Mar. Biol. 146, 645–654. doi: 10.1007/s00227-004-1484-6
Le Guillard C., Dumay J., Donnay-Moreno C., Bruzac S., Ragon J. Y., Fleurence J., et al. (2015). Ultrasound-Assisted Extraction of R-Phycoerythrin From Grateloupia Turuturu With and Without Enzyme Addition. Algal. Res. 12, 522–528. doi: 10.1016/j.algal.2015.11.002
Li W., Su H.-N., Pu Y., Chen J., Liu L.-N., Liu Q., et al. (2019). Phycobiliproteins: Molecular Structure, Production, Applications, and Prospects. Biotechnol. Adv. 37, 340–353. doi: 10.1016/j.biotechadv.2019.01.008
Liu X., Luo G., Wang L., Yuan W. (2019). Food and Bioproducts Processing Optimization of Antioxidant Extraction From Edible Brown Algae Ascophyllum Nodosum Using Response Surface Methodology. Food Bioprod. Process. 114, 205–215. doi: 10.1016/j.fbp.2019.01.003
Lourenço S. O., Barbarino E., De-Paula J. C., Pereira L. O. D. S., Lanfer Marquez U. M. (2002). Amino Acid Composition, Protein Content and Calculation of Nitrogen-to-Protein Conversion Factors for 19 Tropical Seaweeds. Phycol. Res. 50, 233–241. doi: 10.1046/j.1440-1835.2002.00278.x
Mamatha B. S., Namitha K. K., Senthil A., Smitha J., Ravishankar G. A. (2007). Studies on Use of Enteromorpha in Snack Food. Food Chem. 101 (4), 1707–1713. doi: 10.1016/j.foodchem.2006.04.032
Manivannan K., Thirumaran G., Devi G. K., Anantharaman P., Balasubramanian T. (2009). Proximate Composition of Different Group of Seaweeds From Vedhai Coastal Waters (Gulf of Mannar): Southeast Coast of India. Middle-East. J. Sci. Res. 4.
Michalak I., Chojnacka K. (2014). Algal Extracts: Technology and Advances. Eng. Life Sci. 14, 581–591. doi: 10.1002/elsc.201400139
Mittal R., Sharma R., Raghavarao K. S. M. S. (2019). Aqueous Two-Phase Extraction of R-Phycoerythrin From Marine Macro-Algae, Gelidium Pusillum. Biores. Technol. 280, 277–286. doi: 10.1016/j.biortech.2019.02.044
Mittal R., Tavanandi H. A., Mantri V. A., Raghavarao K. S. M. S. (2017). Ultrasound Assisted Methods for Enhanced Extraction of Phycobiliproteins From Marine Macro-Algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 38, 92–103. doi: 10.1016/j.ultsonch.2017.02.030
Moreira A. S., González-Torres L., Olivero-David R., Bastida S., Benedi J., Sánchez-Muniz F. J. (2010). Wakame and Nori in Restructured Meats Included in Cholesterol-Enriched Diets Affect the Antioxidant Enzyme Gene Expressions and Activities in Wistar Rats. Plant Food. Hum. Nutr. 65, 290–298. doi: 10.1007/s11130-010-0179-z
Mouritsen O. G., Rhatigan P., Pérez-Lloréns J. L. (2018). World Cuisine of Seaweeds: Science Meets Gastronomy. Int. J. Gastro. Food Sci. 14, 55–65. doi: 10.1016/J.IJGFS.2018.09.002
Munier M., Jubeau S., Wijaya A., Morançais M., Dumay J., Marchal L., et al. (2014). Physicochemical Factors Affecting the Stability of Two Pigments: R-Phycoerythrin of Grateloupia Turuturu and B-Phycoerythrin of Porphyridium cruentum. Food Chem. 150, 400–407. doi: 10.1016/j.foodchem.2013.10.113
Ngamnikom P., Phawaphuthanon N., Kim M., Boonsupthip W., Shin I. S., Chung D. (2017). Fabrication of Core–Shell Structured Macrocapsules by Electro-Coextrusion With Agar–Hydrocolloid Mixtures for Precooked Food Applications: Textural and Release Characteristics. Int. J. Food Sci. Technol. 52, 2538–2546. doi: 10.1111/ijfs.13539
Niu J. F., Wang G. C., Tseng C. K. (2006). Method for Large-Scale Isolation and Purification of R-Phycoerythrin From Red Alga Polysiphonia Urceolata Grev. Protein Expression Purificat. 49 (1), 23–31. doi: 10.1016/j.pep.2006.02.001
Pagels F., Guedes A. C., Amaro H. M., Kijjoa A., Vasconcelos V. (2019). Phycobiliproteins From Cyanobacteria: Chemistry and Biotechnological Applications. Biotechnol. Adv. 37, 422–443. doi: 10.1016/J.BIOTECHADV.2019.02.010
Parsons T.T., Strickland J. D. H. (1963). Discussion of Spectrophotometric Determination of Marine-Plant Pigments, with Revised Equations for Ascertaining Chlorophylls and Carotenoids. J. Mar. Res. 21, 155–63.
Pereira T., Barroso S., Mendes S., Amaral R. A., Dias J. R., Baptista T., et al. (2020). Optimization of Phycobiliprotein Pigments Extraction From Red Algae Gracilaria Gracilis for Substitution of Synthetic Food Colorants. Food Chem. 321, 126688. doi: 10.1016/j.foodchem.2020.126688
Ritchie R. J. (2008). Universal Chlorophyll Equations for Estimating Chlorophylls a, B, C, and D and Total Chlorophylls in Natural Assemblages of Photosynthetic Organisms Using Acetone, Methanol, or Ethanol Solvents. Photosynthetica 46, 115–126. doi: 10.1007/s11099-008-0019-7
Rodrigues R. D. P., de Castro F. C., Santiago-Aguiar R.S.de, Rocha M. V. P. (2018). Ultrasound-Assisted Extraction of Phycobiliproteins From Spirulina (Arthrospira) Platensis Using Protic Ionic Liquids as Solvent. Algal. Res. 31, 454–462. doi: 10.1016/j.algal.2018.02.021
Roleda M. Y., Dethleff D., Wiencke C. (2008). Transient Sediment Load on Blades of Arctic Saccharina Latissima Can Mitigate UV Radiation Effect on Photosynthesis. Pol. Biol. 31, 765–769. doi: 10.1007/s00300-008-0434-z
Román R. B., Alvárez-Pez J. M., Fernández F. G. A., Grima E. M. (2002). Recovery of Pure B-Phycoerythrin From the Microalga Porphyridium Cruentum. J. Biotechnol. 93 (1), 73–85. doi: 10.1016/S0168-1656(01)00385-6
Roy D., Pabbi S. (2022). A Simple Improved Protocol for Purification of C-Phycocyanin From Overproducing Cyanobacteria and its Characterization. J. Appl. Phycol. 34, 799–810. doi: 10.1007/S10811-021-02649-Z/TABLES/5
Sekar S., Chandramohan M. (2008). Phycobiliproteins as a Commodity: Trends in Applied Research, Patents and Commercialization. J. Appl. Phycol. 20, 113–136. doi: 10.1007/s10811-007-9188-1
Senthilkumar N., Suresh V., Thangam R. (2013). International Journal of Biological Macromolecules Isolation and Characterization of Macromolecular Protein R-Phycoerythrin From Portieria Hornemannii. Int. J. Biol. Macromol. 55, 150–160. doi: 10.1016/j.ijbiomac.2012.12.039
Senthilkumar N., Thangam R., Murugan P., Suresh V., Kurinjimalar C., Kavitha G., et al. (2018). Hepato-Protective Effects of R-Phycoerythrin-Rich Protein Extract of Portieria Hornemannii (Lyngbye) Silva Against DEN-Induced Hepatocellular Carcinoma. J. Food Biochem. 42, 1–11. doi: 10.1111/jfbc.12695
Sharma R., Bhunia B., Mondal A., Kanti Bandyopadhyay T., Devi I., Oinam G., et al. (2020). Statistical Optimization of Process Parameters for Improvement of Phycobiliproteins (PBPs) Yield Using Ultrasound-Assisted Extraction and its Kinetic Study. Ultrason. Sonochem. 60, 1350–4177. doi: 10.1016/J.ULTSONCH.2019.104762
Simovic A., Combet S., Cirkovic Velickovic T., Nikolic M., Minic S. (2022). Probing the Stability of the Food Colourant R-Phycoerythrin From Dried Nori Flakes. Food Chem. 374, 131780. doi: 10.1016/J.FOODCHEM.2021.131780
Soni B., Kalavadia B., Trivedi U., Madamwar D. (2006). Extraction, Purification and Characterization of Phycocyanin From Oscillatoria Quadripunctulata-Isolated From the Rocky Shores of Bet-Dwarka, Gujarat, India. Proc. Biochem. 41, 2017–2023. doi: 10.1016/j.procbio.2006.04.018
Sudhakar M. P., Jagatheesan A., Perumal K., Arunkumar K. (2015). Methods of Phycobiliprotein Extraction From Gracilaria Crassa and Its Applications in Food Colourants. Algal. Res 8, 115–120. doi: 10.1016/j.algal.2015.01.011
Sun L., Wang S., Gong X., Zhao M., Fu X., Wang L. (2009). Isolation, Purification and Characteristics of R-Phycoerythrin From a Marine Macroalga Heterosiphonia Japonica. Protein Expression Purificat. 64, 146–154. doi: 10.1016/j.pep.2008.09.013
Tan H. T., Khong N. M. H., Khaw Y. S., Ahmad S. A., Yusoff F. M. (2020). Optimization of the Freezing-Thawing Method for Extracting Phycobiliproteins from Arthrospira Sp. Molecules 25, 3894. doi: 10.3390/MOLECULES25173894
Thangam R., Sundarraj S., Vivek R., Suresh V., Sivasubramanian S., Paulpandi M., et al. (2015). Theranostic Potentials of Multifunctional Chitosan-Silver-Phycoerythrin Nanocomposites Against Triple Negative Breast Cancer Cells. RSC. Adv. 5, 12209–12223. doi: 10.1039/c4ra14043e
Thoisen C., Hansen B. W., Nielsen S. L. (2017). A Simple and Fast Method for Extraction and Quantification of Cryptophyte Phycoerythrin. MethodsX 4, 209–213. doi: 10.1016/j.mex.2017.06.002
Ulagesan S., Nam T.-J., Choi Y.-H. (2021). Extraction and Purification of R-Phycoerythrin Alpha Subunit From the Marine Red Algae Pyropia yezoensis and Its Biological Activities. Molecules 26, 6479. doi: 10.3390/molecules2621647
Underwood A. J. (1996). Experiments in Ecology (Cambridge, Cambridge University Press). doi: 10.1017/cbo9780511806407
Wani S. M., Jan N., Wani T. A., Ahmad M., Masoodi F. A., Gani A. (2017). Full Length Article. J. Saudi. Soc. Agric. Sci. 2, 119–126. doi: 10.1016/J.JSSAS.2015.03.006
Westermeyer R., Ramirez C (1978). Algas marinas de Niebla y Mehuín (Valdivia-Chile). Medio Ambiente 3, 44–49.
Keywords: R-phycoerythrin, Sarcopeltis skottsbergii, ultrasound, high-pressure homogenization, red algae
Citation: Castro-Varela P, Celis-Pla PSM, Figueroa FL and Rubilar M (2022) Highly Efficient Water-Based Extraction of Biliprotein R-Phycoerythrin From Marine the Red-Macroalga Sarcopeltis skottsbergii by Ultrasound and High-Pressure Homogenization Methods. Front. Mar. Sci. 9:877177. doi: 10.3389/fmars.2022.877177
Received: 16 February 2022; Accepted: 19 May 2022;
Published: 16 June 2022.
Edited by:
Maja Berden Zrimec, AlgEn, algal technology centre, SloveniaReviewed by:
Leonel Pereira, University of Coimbra, PortugalTonmoy Ghosh, Indian Institute of Technology Indore, India
Copyright © 2022 Castro-Varela, Celis-Pla, Figueroa and Rubilar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pablo Castro-Varela, cGFibG8uY2FzdHJvQHVtYS5lcw==
 Pablo Castro-Varela
Pablo Castro-Varela Paula S.M. Celis-Pla
Paula S.M. Celis-Pla Felix L. Figueroa
Felix L. Figueroa Monica Rubilar4
Monica Rubilar4