- 1Department of Integrative Marine Ecology, Genoa Marine Centre, Stazione Zoologica Anton Dohrn - Italian National Institute of Marine Biology, Ecology and Biotechnology, Genoa, Italy
- 2Department of Integrative Marine Ecology, Sicily Marine Centre, Stazione Zoologica Anton Dohrn - Italian National Institute for Marine Biology, Ecology and Biotechnology, Palermo, Italy
- 3Department of Biology, University of Pisa, CoNISMa, Pisa, Italy
- 4Department of Integrative Marine Ecology, Stazione Zoologica Anton Dohrn - Italian National Institute for Marine Biology, Ecology and Biotechnology, Naples, Italy
- 5Department of Life and Environmental Sciences, University of Cagliari, Cagliari, Italy
- 6Area for the Protection of Biodiversity, Habitats and Protected Marine Species, Italian National Institute for Environmental Protection and Research, Rome, Italy
- 7Department of Integrative Marine Ecology, Calabria Research Centre for Advanced Marine Infrastructures, Stazione Zoologica Anton Dohrn, Italian National Institute of Marine Biology, Ecology and Biotechnology, Amendolara, Italy
- 8Department of Biology, Ecology and Earth Sciences, University of Calabria, Arcavacata di Rende, Italy
- 9Institute for the Study of Anthropic Impact and Sustainability in the Marine Environment, National Research Council, Genoa, Italy
- 10Department of Physical Sciences, Earth and Environment, University of Siena, Siena, Italy
- 11Department of Life and Environmental Sciences, Polytechnic University of Marche, Ancona, Italy
- 12National Biodiversity Future Center (NBFC), Palermo, Italy
Due to their late maturation, extreme longevity, low fecundity and slow growth rates, deep-sea Chondrichthyes are extremely vulnerable to human impacts. Moreover, assessing the impact of deep-sea fisheries is difficult, as many species (including sharks) are part of the bycatch and are often discarded at sea, and/or landed under generic commercial-species codes. The lack of this information on fishery data sets and the limited availability of species-specific life history data make challenging the management of deep-sea Chondrichthyes. The kitefin shark Dalatias licha is a cosmopolitan elasmobranch, mainly found on continental and insular shelf-breaks and slopes in warm-temperate and tropical waters. This species is a common by-catch of the deep-sea trawling, considered as “Endangered” by the IUCN Red List for all European waters, Mediterranean Sea included. Here we present the results of a study based on a total of 78 specimens of kitefin shark collected over 3 years in the Ligurian Sea (NW Mediterranean) as by-catch from deep-water fisheries. Total length ranged from 380 to 1164 mm, and individual weight ranged from 198 to 8000 g. Immature and mature individuals showed a sex ratio dominated by males. Adult males were observed throughout the year, while mature females were observed only in spring-summer. These data lead to hypothesise a spatial segregation between genders. The kitefin shark diet was dominated by bony fish (mainly Macrouridae) and other small sharks (e.g., Galeus melastomus and Etmopterus spinax), but their gut included plastic items and parasites. Data reported here underline the rarity, complex ecology and the threat for this shark species and support the urgency of promoting initiatives for their monitoring and conservation.
1 Introduction
In the last four decades, human impacts (particularly bottom-contact fisheries) and other multiple stressors have expanded also to the deep-sea (i.e., >200 m depth; Danovaro et al., 2017), and if not adequately managed, can threat several deep-sea species, with cascade effects on the functioning of deep-sea ecosystems (Pusceddu et al., 2014; Clark et al., 2016; Caddell, 2020).
Marine fisheries worldwide have operated at increasing depths since the 1970s, coinciding with declines in shallow-water stocks (Roberts, 2002; Norse et al., 2012; Mejjad and Rovere, 2021). Deep-sea fisheries, in fact, catch species generally characterized by long lifespans, slow growth rates and late maturity (Watson and Morato, 2013). In addition, for most mesopelagic and deep-sea species is very difficult to gather quantitative data to assess their stocks and conservation status (sensu International Union for Conservation of Nature) (Devine et al., 2006). In 2009, the Food and Agriculture Organization (FAO) launched the “International Guidelines for the Management of Deep-sea Fisheries in the High Seas” (FAO, 2009), to recommend the adoption of an ecosystem-based approach for the exploitation of the deep-sea species to States and to Regional Fishery Management Organisations (RFMOs), able to prevent significant adverse impacts on vulnerable marine ecosystems (VMEs).
Chondrichthyes are among the most vulnerable deep-sea taxa due to their extremely long-life histories and limited reproductive potential (Simpfendorfer and Kyne, 2009); in recent years, major declines in shark populations have been observed for several species and maritime sectors (Anderson and Ahmed, 1993; White and Kyne, 2010; Graham et al., 2011; Norse et al., 2012; Barbier et al., 2014; Pacoureau et al., 2021).
Although approximately half of Chondrichthyes species live in the deep-sea, information on the biology and ecology of deep species is extremely limited (Cotton and Grubbs, 2015). This is due to the inherent difficulty associated with investigating deep-sea habitats, and the limited commercial interest for these species (Brooks et al., 2015). Given the apparent rarity of deep-sea species, the lack of sufficient data raises concerns for the sustainability of deep-sea fisheries for the chondrichthyans populations (Shipley et al., 2017).
In the deep Mediterranean Sea, one the most threatened maritime regions for overfishing (Walls and Dulvy, 2021), 21 species (out of total 73) of cartilaginous fishes are present, (Serena, 2005). Among these only the blackmouth catshark is considered a non vulnerable species (according to IUCN), while the rest are defined “strongly threatened” or “lack enough data for classification” (Dulvy et al., 2016). The kitefin shark Dalatias licha is listed on IUCN Red List (IUCN, 2020) and classified as “Near Threatened” (NT) at global level and “Endangered” (EN) in European and Mediterranean waters (Nieto et al., 2015; Dulvy et al., 2016). It is widely distributed over continental shelves and slopes in either warm temperate and tropical areas, down to 1800 m (Compagno, 1984). In the Mediterranean Sea, the kitefin shark is the second most important top predator inhabiting the deep-sea after the six-gill shark Hexanchus griseus (Serena, 2005). This species is mostly reported from the Western Basin, but it is also present in the Eastern basin (Bradai et al., 2012; Guallart and Walls, 2016; Martin and Mallefet, 2022). It is frequently included in the by-catch of deep-sea fisheries, mainly bottom trawling targeting the shrimps Aristeus antennatus and Aristaeomorpha foliacea (Serena, 2005).
Recent studies have described some life traits of this deep-sea shark, yet the biology and ecology remain unknown, making the development of effective management and conservation actions difficult (Capapé et al., 2008; Navarro et al., 2014; Barría et al., 2015; de Loyola Fernández et al., 2017; Barría et al., 2018; Booth et al., 2020; Mulas et al., 2021).
Here we explored several life traits of the kitefin shark D. licha from the Ligurian Sea through the morphometric analyses, sexual features, and stomach contents to contribute to fill existing gaps on the biology and ecology of this deep-sea species, and to provide insights enabling the development of future conservation initiatives.
2 Materials and methods
2.1 Sampling
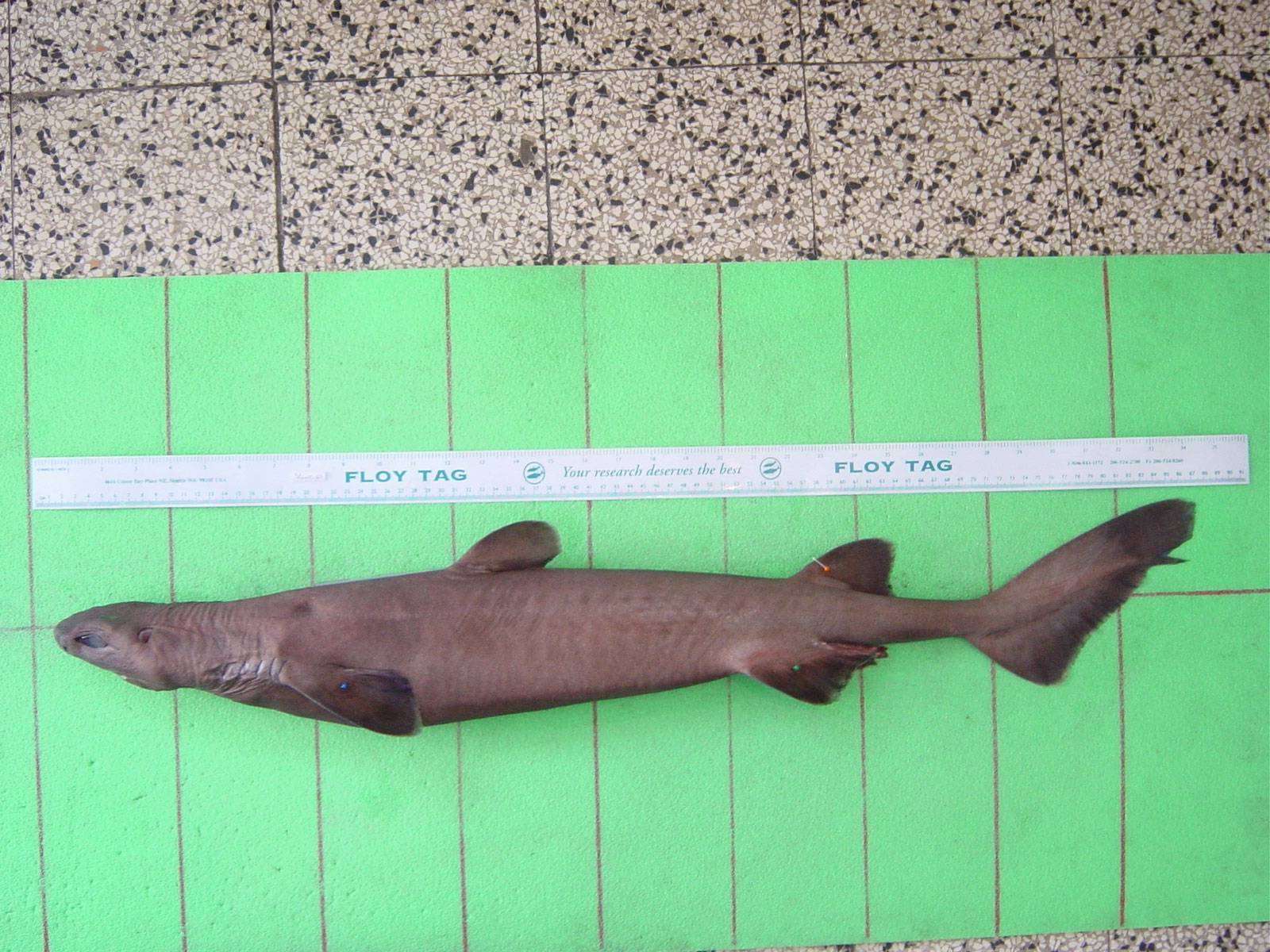
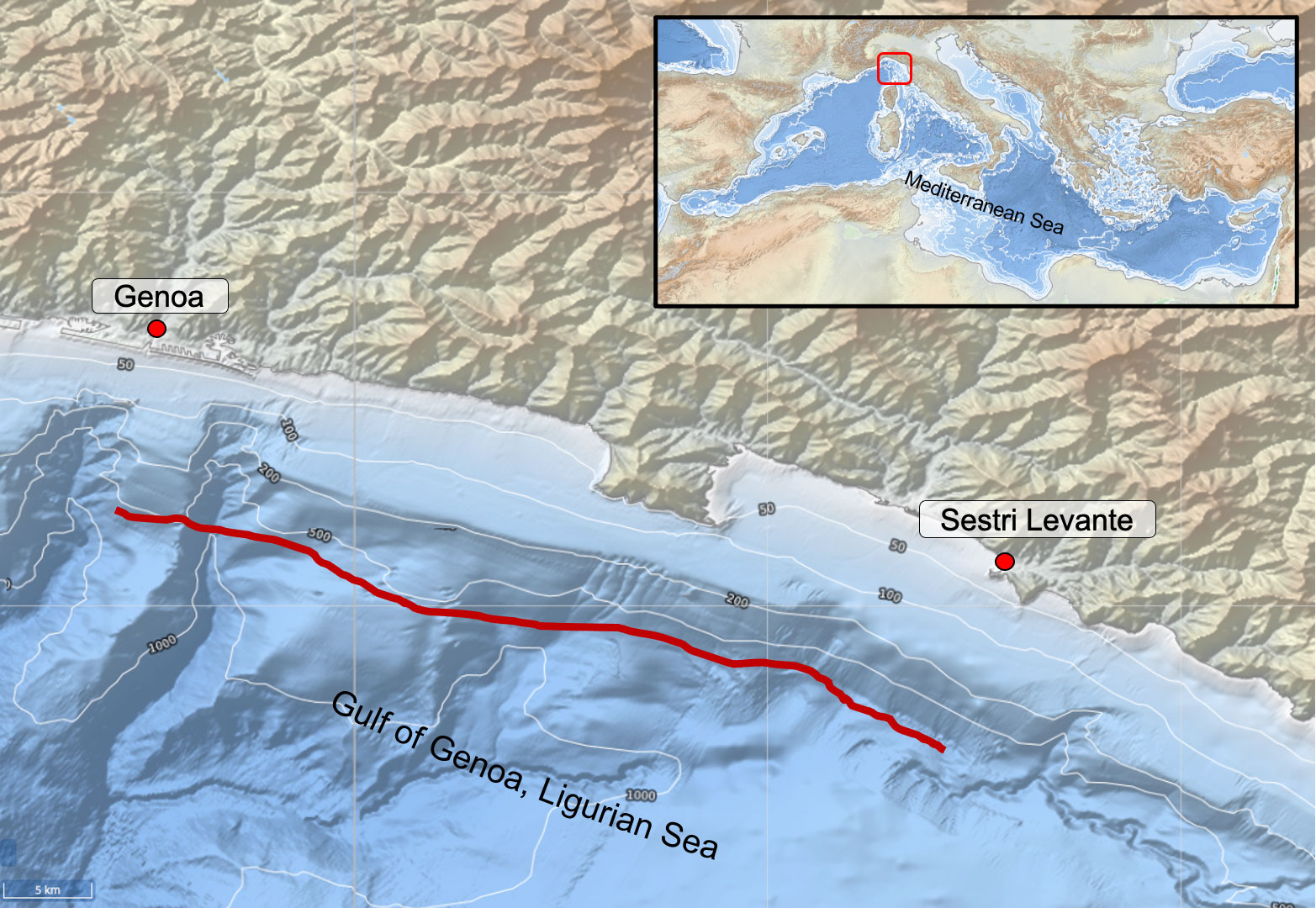
Overall, 78 specimens of Dalatias licha (Figure 1) were collected from June 2001 to September 2003 during activity of monitoring of bottom trawl fishery in the Ligurian sea (NW Mediterranean Sea). The specimens were caught as by-catch by a single professional deep bottom trawler targeting red shrimps and operating on a single fishing ground, between Genoa and Sestri Levante at 600 - 800 m depth (Figure 2).
2.2 Biometric and gravimetric investigations
After landing, all specimens were immediately analysed in the laboratory, or frozen at -28°C until subsequent analysis. In the laboratory, the specimens were identified to species level according to Serena (2005), photographed and examined for the estimation of the following morphometric and gravimetric variables: total length (TL); standard length (SL); clasper length (ClL); total weight (TW); somatic weight (SW); liver weight (LW); gonadal weight (GW) and sex. Meristic characteristics were also collected following the keys reported by Serena (2005).
2.3 Sexual and reproductive variables
Specimens were sexed and the maturity stages were determined following the scales for viviparous Elasmobranchs used in MEDITS project protocol (MEDITS, 2017). The two-sample Kolmogorov-Smirnov (KS) test was used to assess for significant differences in the length frequencies by sex. Sex-Ratio (SR, female to male abundance ratio) was estimated for the whole population. The significance of deviation from the 1:1 null hypothesis was tested by the χ2 test.
2.4 Stomach content analysis
Stomach contents (both individuals, moults and fragments) were identified to the lowest classification level (to the species level whenever possible) to gather information on the shark preys. The eyes’ number, mouth parts, telsons or other anatomical portions were traced and referred to single specimens (Hyslop, 1980). The diet and relative importance of each food item was assessed by the:
● percentage frequency of occurrence (F% = number of stomachs containing prey i item/total number of non-empty stomachs x 100);
● percentage abundance (N% = number of individuals of prey i item/total number of all prey items x 100).
Based on the null hypothesis (i.e., there are no differences in the diet of D. licha among seasons nor among size classes, a PERMANOVA (Permutational Multivariate Analysis of Variance; Anderson, 2001) was performed. Abundance data of stomach content composition (N) was used to obtain a similarity matrix based on a square root transformation using modified Bray-Curtis similarity. The model of analysis included two factors:
1. season, fixed with 3 levels (Spring, Summer and Autumn (no guts useful were found in winter);
2. size classes, fixed and orthogonal with 2 levels (<500 mm; > 500 mm).
3 Results
3.1 Biometric and gravimetric results
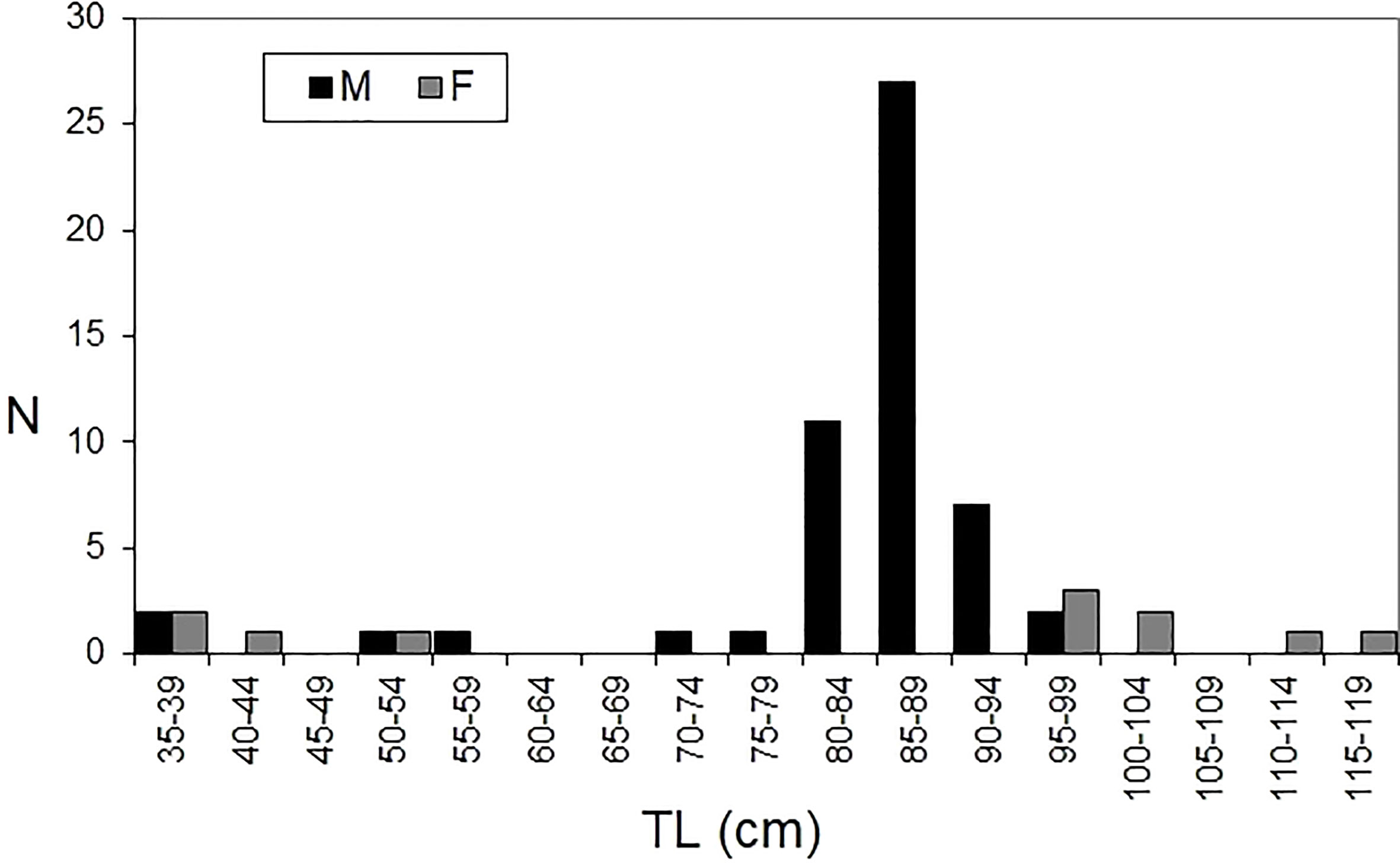
In the present study, 62 males and 16 females were collected. They included both juveniles and adult individuals. The Length frequency distribution (Figure 3) shows a large number of males in the classes from 80 to 94 cm, with a mode in size class 85-89 cm. The length frequency analysis of females showed two main groups: I) from 35 to 54 cm (immature/juveniles) and II) from 95 to 119 cm (i.e., the largest specimens with pregnant sharks; Figure 3). The total length (TL) of Dalatias licha ranged from 34.5 to 116.4 cm (which is also the female range of distribution), while male length ranged from 36.9 to 95.5 cm. Body weight of females and males ranged from 150-187 g to 3591 g, respectively). As a result, significant differences were reported in size distribution between genders (Kolmogorov Smirnov, D=0.4839; P<0.05).
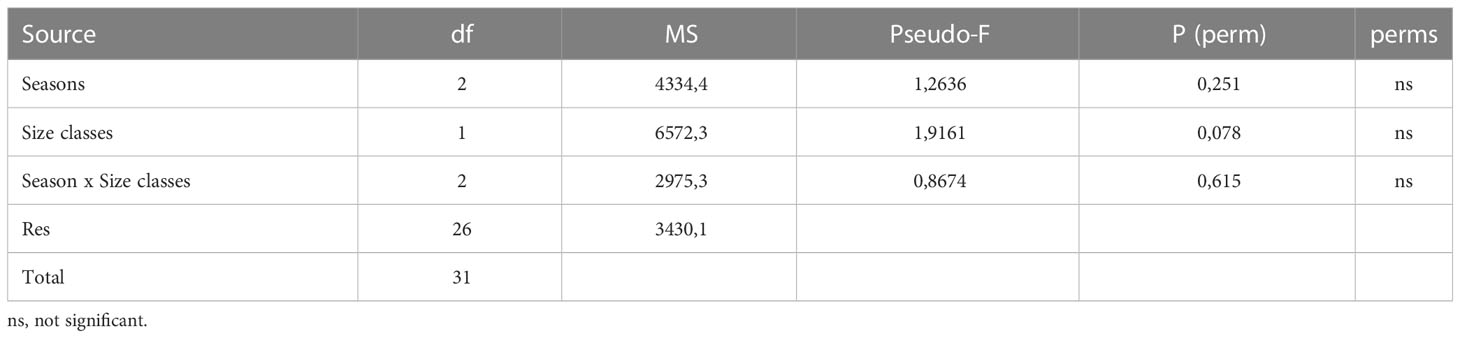
The analysis of the gonadosomatic index (GSI) highlighted a wide variability among adult individuals of both genders (Figures 4A, C).
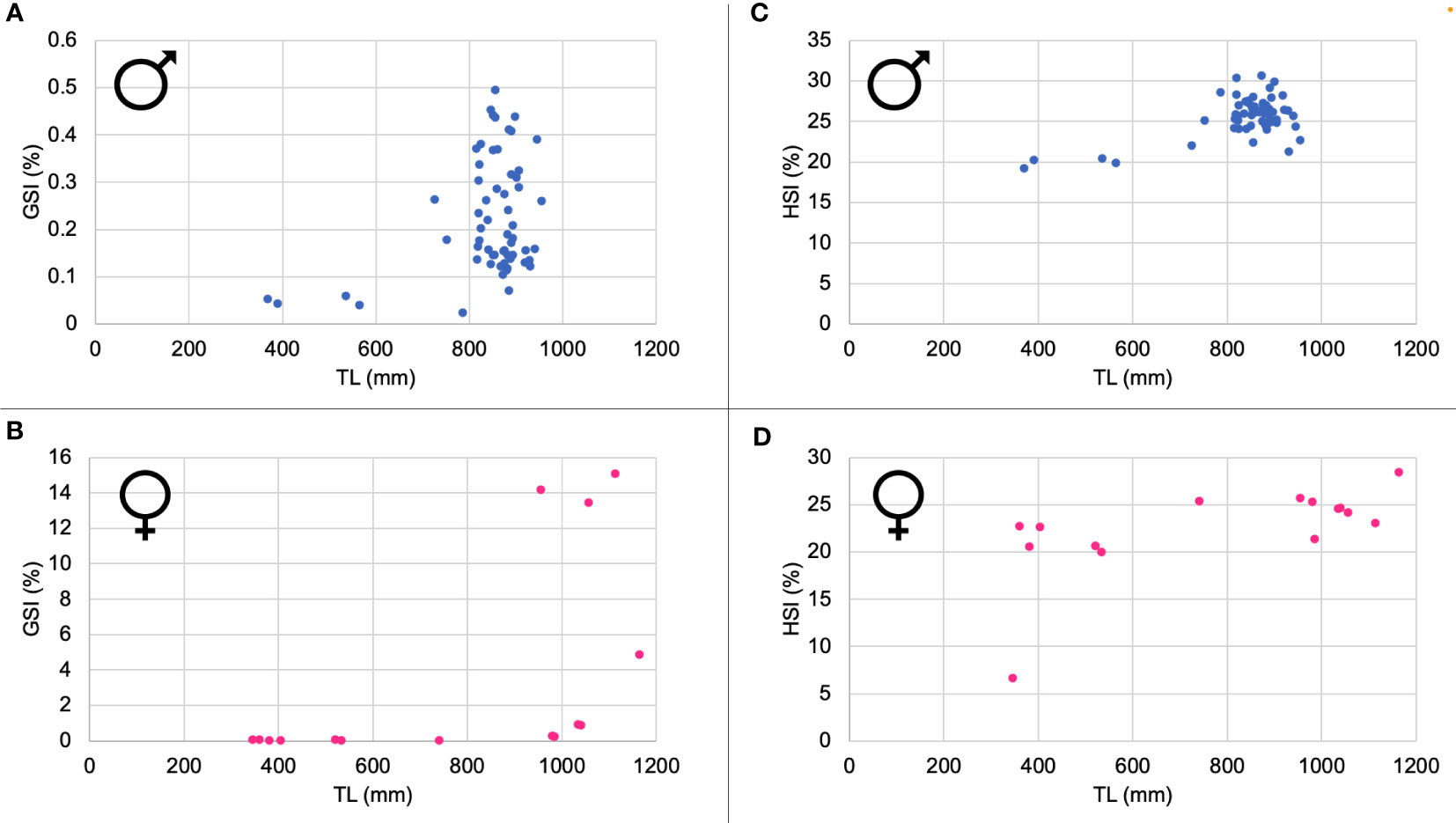
Figure 4 Gonadosomatic Index (GSI) for male (A) and female (B) specimens and Hepatosomatic Index (HSI) for male (C) and female (D).
The hepatosomatic index (HSI) shows an increase directly correlated to size for both the sexes; in particular, it is worth noting higher values for male specimens rather than females among the adult individuals, (Figures 4B, D).
3.2 Sexual and reproductive information
The overall sex ratio (FF/FF*MM) was significantly biased towards males (0.20), as also pointed out by chi-square test (χ2 = 27.128 P<0.05).
The ratio between the length of the claspers and the total length of the male individuals allowed us to highlight the size at first maturity, which is around 70.5 cm (Figure 5).
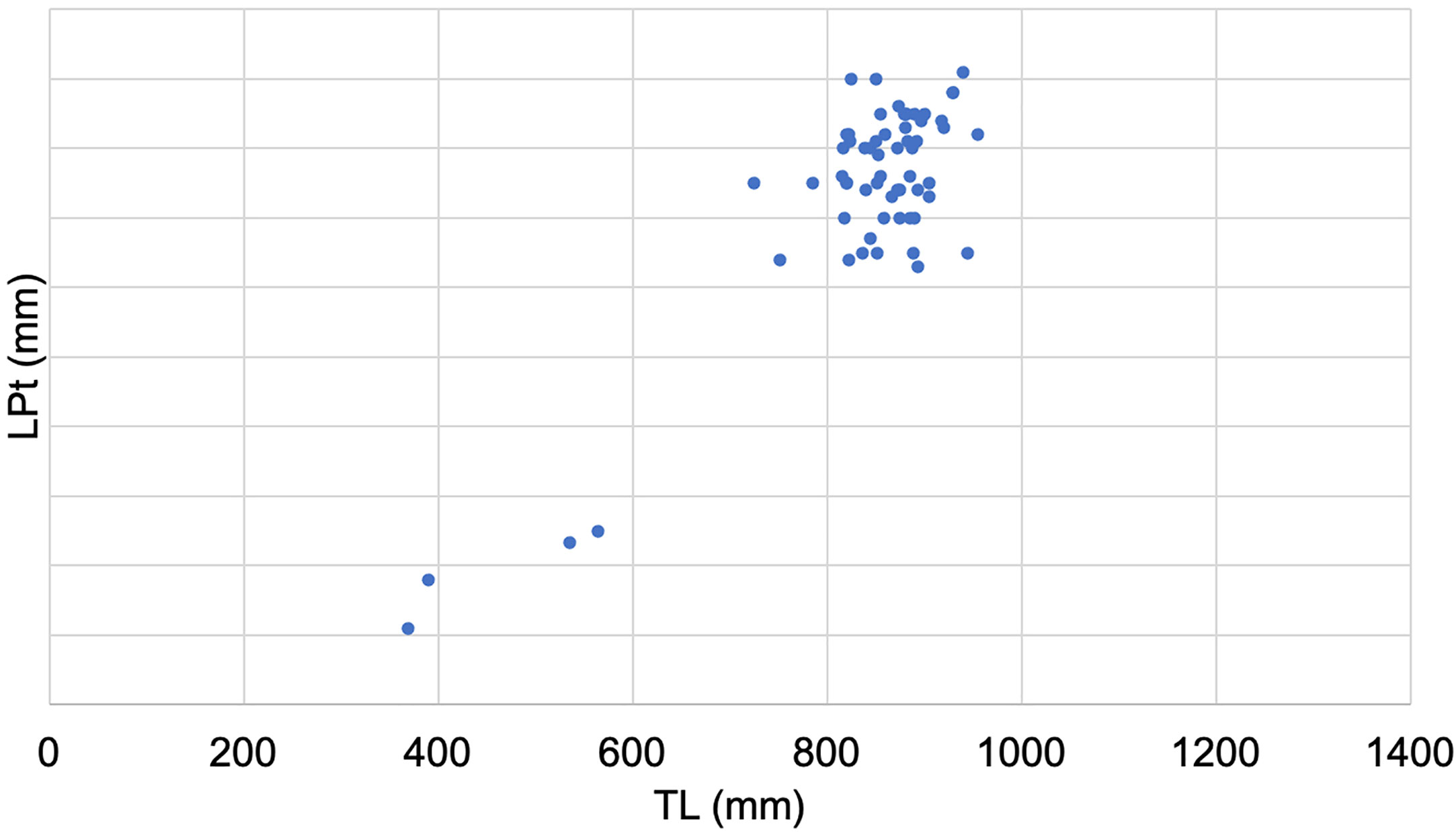
Figure 5 Relationship between the length of the claspers (LPt) and the total length (TL) of the male individuals.
Mature females occurred mainly in summer, when pregnant specimens with LT > 980 mm presented oviduct glands and uteri fully formed and rounded containing yolk matter (3b- Early pregnancy) or uteri well filled with visible segments and often small embryos (3c- Mid pregnancy) (Figure 6A). Males in the regressing stage were caught in May, June, and September (Figure 6B).
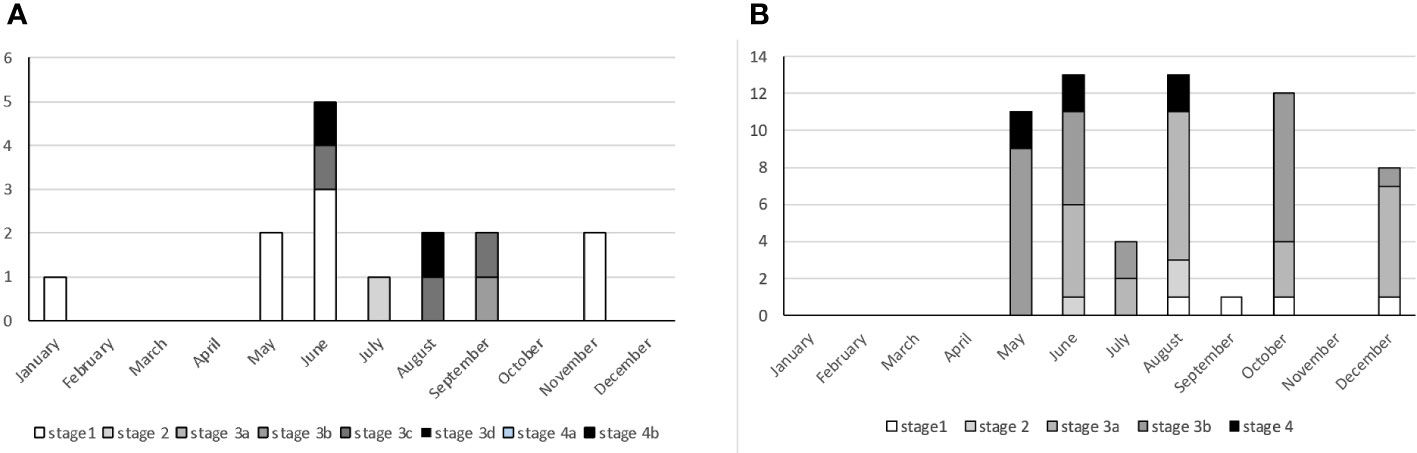
Figure 6 Seasonal distribution of Dalatias licha females (A) and males (B) at each gonadal phase during the sampling period.
In June and August, females with enlarged, flaccid walled uteri and gonads with small follicles of different sizes (stage 4b - Regenerating) were reported (Figure 7).

Figure 7 Female of Dalatias licha in stage 3C – mid pregnancy with small embryo (above) and Stage 4b - resting (below).
Males showed a large reproductive period with respect to the female one (Figure 8). Mature males (stage 3a and 3b) were observed from May to December.
In Table 1, the mean size and weight of female and male D. licha for each maturity stage are represented. A progressive increase of both variables with the development of the maturity process of the gonads can be observed (Table 1).
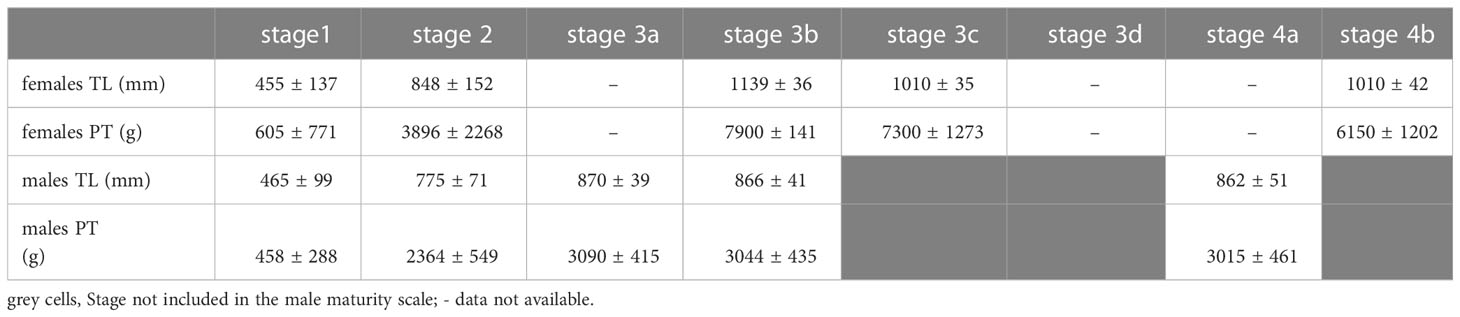
Table 1 Mean total length and weight (± standard deviation) of Dalatias licha females and males in relation to the different gonad phase.
Length and weight were significantly correlated as expected (the Student’s t-test revealed a significant positive allometric growth for the species at P < 0.01) (Figure 9). The equation showed a value of a=0.00045 and 0.0156 respectively for female and male and b= 3.42 and b= 3.14 respectively for female and male.
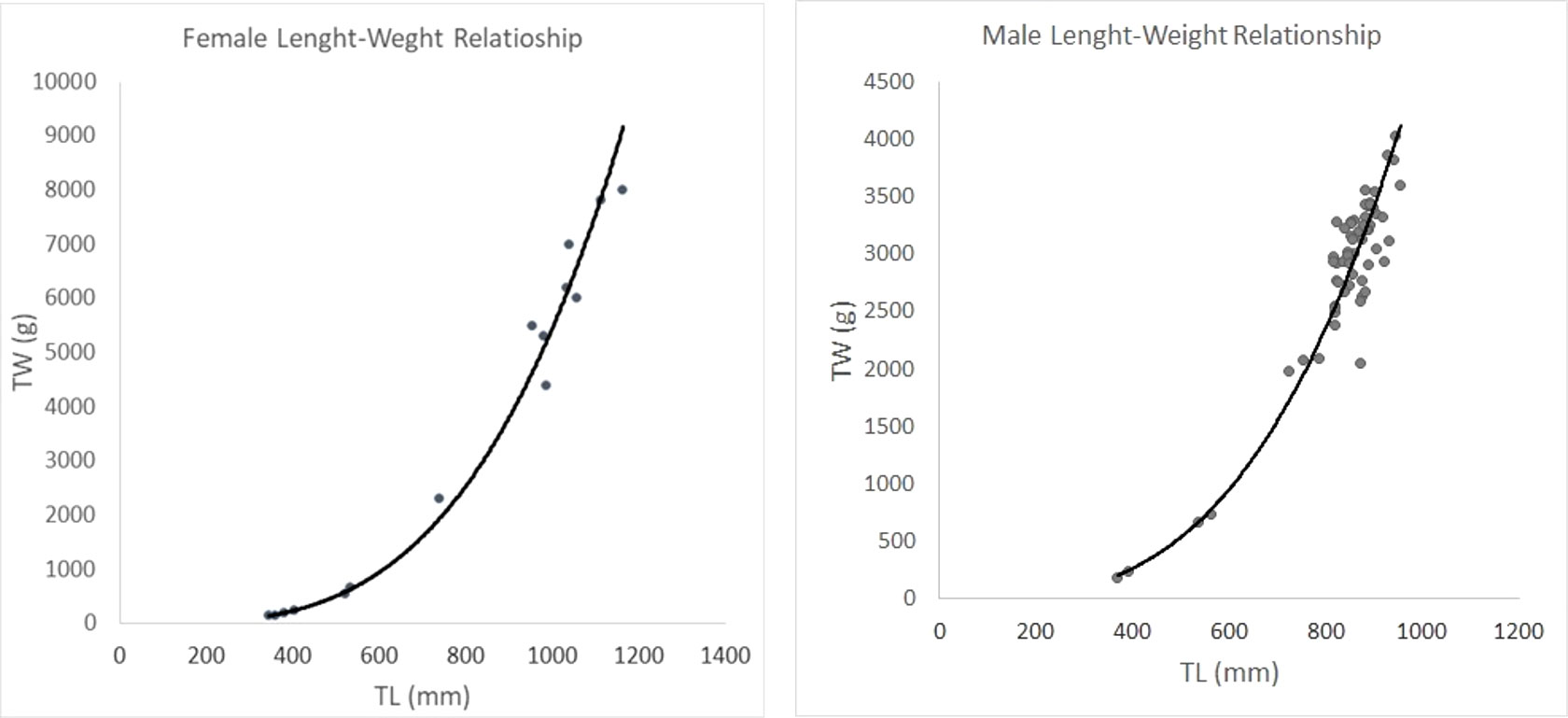
Figure 9 Length-Weight relationship of Dalatias licha caught during the study period. The equation showed a value of a=0.00045 and 0.0156 respectively for Female and Male and b= 3.42 and b= 3.14 respectively for female and male.
3.3 Stomach content data
Gut content analysis (Table 2, Figure 10) reveals a vacuity index of 31.6%. Bony fish were the most abundant preys both in terms of frequency of occurrences (F%) and number (N%) (Table 1). Unidentified bony fish represented a large part (F%=30.2; N%= 16.52) while macrourid fishes were the identified group more represented (F%=7.5; N%= 3.5%). Sharks were the second group found in the stomach of kitefin shark (F%=37.0; N%=18.3): within this group the blackmouth catshark Galeus melastomus was the more present prey in terms of F% (9.4) and N% (4.3). Cephalopods and crustaceans were also present in the gut of D. licha, even if with lower values of F% (9.4; 57 respectively) and N% (6.1; 2.6 respectively). Many parasites (Cestode worms) were found (F%= 60.4; N%=37.4) in the stomachs, along with the presence of plastic items.
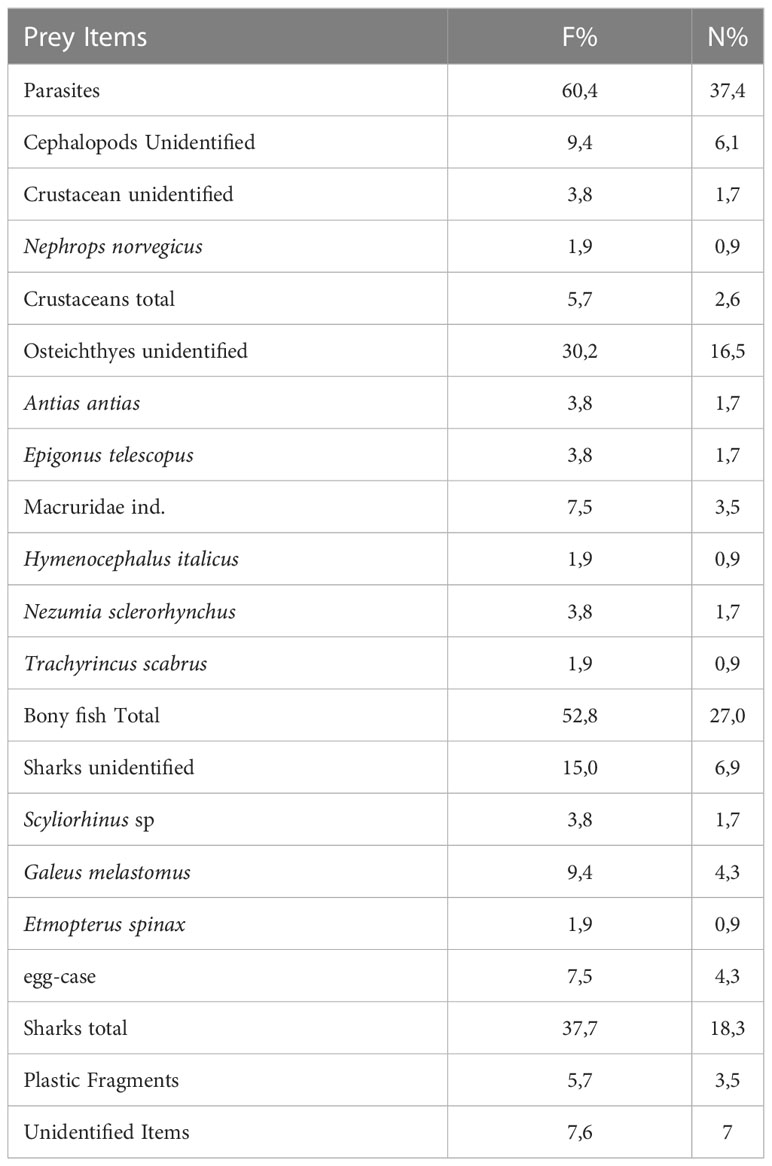
Table 2 Gut content analysis of Dalatias licha. Frequency of occurrence (F%) and percent number (N%) of prey items are reported.

Figure 10 Images of preys found in the stomach contents of some specimens sampled in this study. (A) teleosts and shrimps (B) Galeus melastomus remains and egg capsules, (C) Etmopterus spinax remains (D) cephalopods remains, (E) parasites and (F) plastic fragments.
PERMANOVA showed no significant differences associated with seasons and size classes in the diet of D. licha (Table 3).
4 Discussion
The kitefin shark Dalatias licha is a deep-sea shark incidentally caught by bottom-contact fisheries down to 800 m depth at mid latitudes. According to the International Union for Conservation of Nature (IUCN) D. licha is an endangered species (Nieto et al., 2015; Dulvy et al., 2016). Recently the observations of this shark are becoming so rare that the European Council set a quota zero for certain European maritime sectors (EU, 2021).
Here we provide new insights on the biology and ecology of the shark D. licha from the Ligurian Sea. Specimens were collected at 600-800m depth, which represent the deepest depths recorded so far for this species in the Mediterranean Sea (Macpherson, 1980; Matallanas, 1982; Kabasakal and Kabasakal, 2002; Capapé et al., 2008). Previous investigations reported the presence of this species at high depths only in the southern Sardinian waters and the Gulf of Lion (Follesa et al., 2011; Navarro et al., 2014). This batymetric range corresponds to the deepest depths of trawling in the Mediterranean Sea, but it cannot be excluded the presence of this shark at deeper depths.
Consistently with previous information available for this species, the maximum size observed here was larger for females than for males, yet the longest specimens remain considerably smaller than those reported in South Africa (maximum total length of 1820 mm; Compagno, 1984). However, we have insufficient information on this species to assess whether the smaller size in the Mediterranean Sea is due to the intensive by-catch on this species or the result of a miniaturization of the body size, as previously reported for another deep-sea sharks (Centroscymnus coelolepis) in the Mediterranean Sea (Catarino et al., 2015).
The allometric growth observed in the present study is consistent to values reported in previous studies and confirms that females show a significantly higher body weight compared to males, which is a typical feature of elasmobranchs (Carrier et al., 2004; López Calero, 2013; Barría et al., 2015; de Loyola Fernández et al., 2017).
D. licha is a piscivorous predator species, specialised in hunting fast fish preys, either strictly demersal and bentho-pelagic (Navarro et al., 2014). The hepatosomatic index of this species shows significant higher values in smaller individuals, which could be related to a remarkable swimming capacity associated to the predatory behaviour (Martin and Mallefet, 2022). As observed in other deep-sea shark species, the slight increase in the gonadosomatic index reported in the present study for males could be related to their deeper bathymetric distribution than the females (Moura et al., 2014) and the lower values observed in mature females could be related to the vitellogenesis (Clarke et al., 2001).
The results of this study pointed out a sex ratio significantly shifted towards males. A similar unbalanced ratio was also reported in previous studies, but could be due to a sexually-driven spatial segregation between the two genders, possibly displacing females at depth beyond the trawling depths (Munoz-Chapuli, 1984; Yano and Tanaka, 1988; Wetherbee, 1996; Girard and Buit, 1999; Clarke et al., 2001; Jakobsdóttir, 2001; Mclaughlin and Morrissey, 2005; Moura et al., 2014; Finucci et al., 2018). Such spatial segregation has been proposed as a mechanism to increase the efficiency of the reproductive effort, while minimising the risk of cannibalism and depredation of juveniles and sub-adults by mature individuals (Moura et al., 2014). Such spatial segregation would also explain the dominance of females at deeper depths in Sardinian waters, (Mulas et al., 2021).
The few female specimens investigated in the present study were juveniles or pregnant with embryos in the early stage of development. Previous studies conducted on Centrophorus squamosus and C. coelolepis reported pregnant females in shallower and warmer waters, suggesting that this might be a common feature of several deep-sea shark species (Yano and Tanaka, 1988; Moura et al., 2014) and it could be explained by the fact that these conditions can satisfy specific requirement for embryonic development and/or trophic feedings of pregnant specimens (Robbins, 2007; Moura et al., 2014). The absence of females in later stages of pregnancy could suggest that this species moves to greater depths for the parturition, as observed other elasmobranchs (Bansemer and Bennett, 2009), to reduce the risk of predation on the new-born sharks.
Males investigated in the present study included individuals with different sexual maturity stages during the four seasons suggesting that the investigated area is a mating area. Data reported here also suggest that mature males of D. licha might have an extended reproductive period (i.e., the lack of a seasonality for the reproductive period), similarly to what was reported for most deep-sea shark species (Girard and Buit, 1999) and for other ovoviviparous sharks (Kyne and Simpfendorfer, 2010).
The size of first maturity in Mediterranean males is around 70 cm (see also Capapé et al., 2008), yet this size is much lower than that reported from the Atlantic Ocean, as previously observed for the Mediterranean in the Portuguese dogfish C. coelolepis (Compagno, 2001; Serena, 2005; Catarino et al., 2015), suggesting that the oligotrophic conditions of the Deep Mediterranean Sea promote the dwarfism in deep shark species.
The analysis of the stomach contents of kitefin shark confirms that this species is a carnivorous predator, preferring fish and highly mobile animals (Macpherson, 1980; Matallanas, 1982; Kabasakal and Kabasakal, 2002; Capapé et al., 2008; Dunn et al., 2010; Dunn et al., 2013; Navarro et al., 2014; Mulas et al., 2021). The importance of bony fish (e.g., macrourids) in the diet of this species is confirmed by other studies conducted in other areas of the Mediterranean Sea (Macpherson, 1980; Matallanas, 1982; Capapé et al., 2008; Mulas et al., 2021) and reflects the common availability of these preys within the distribution range of the kitefin shark (Sinopoli et al., 2012). Our results confirm also the importance of small sharks (such as Galeus melastomus) in the diet of D. licha (Macpherson, 1980; Matallanas, 1982; Kabasakal and Kabasakal, 2002; Capapé et al., 2008; Dunn et al., 2010; Dunn et al., 2013; Navarro et al., 2014; Mulas et al., 2021). There are various hypotheses to explain the relevance of these elasmobranchs in the kitefin shark diet: the first is that the high concentration of fatty acids in their liverscan support the energetic requirements and the buoyancy of their predators (Corner et al., 1997; Navarro et al., 2014). The second is that preying upon other sharks reduces the interspecific competition for the common preys (e.g., macrourids; Lourenço et al., 2014). The predation among sharks remains and intriguing enigma in the scientific community (Cortés, 1999), and further studies are needed to elucidate these predation preferences (Lotze et al., 2006; Navarro et al., 2014). Finally, the large abundance of crustaceans and cephalopods in the diet of kitefin shark reflects the relative abundance of these preys in the deep-sea consistently with the optimal foraging theory (Pyke, 1984; Kabasakal and Kabasakal, 2002; Mulas et al., 2021) as reported in other shark species and in general for fish species (Valls et al., 2011; Sinopoli et al., 2012; Navarro et al., 2014; Martin and Mallefet, 2022).
The gut of kitefin sharks investigated in the present study contained a rich endoparasitic helminthofauna. The presence of other parasites, such as nematodes (Anisakis simplex and Raphidascaris sp.), has been previously reported in other Mediterranean kitefin sharks (Henderson et al., 2003) as well as the presence of Hexabothriids (Kheddam et al., 2016), monogeneans (Septitrema lichae in nasal tissue; Kheddam et al., 2020), and digenean trematodes (Otodistomum veliporum; Sperone and Milazzo, 2018). However, this is the first study reporting the presence of flatworms as endoparasites of the kitefin shark.
We report here also that the gut of the D. licha contained plastic items. This result confirms that plastics are impacting also large predatory deep-sea species and point out the potential risk of this form of contamination for the conservation of this vulnerable species (Chiba et al., 2018; Valente et al., 2020).
4.1 Possible management and conservation measures
Our study stresses that, besides the industrial fishery, also local-scale fisheries, as those utilised for the present study, can significantly impact this endangered deep-sea shark species, and underline the urgency of planning adequate and effective fishery management measures for its conservation (King and McFarlane, 2003; Young et al., 2006; McCluskey and Lewison, 2008).
In Mediterranean waters (as in the other European seas), deep-sea cartilaginous fishes are not primary fishing targets, but represent in most cases victims of the by-catch. Most of these species monitored by the International Union for Conservation of Nature (IUCN) are reported as endangered or data deficient (Nieto et al., 2015; Dulvy et al., 2016). The track record of European fisheries is clearly unsustainable also for deep-sea sharks (Villasante et al., 2012), as evident from the remarkable decline of the abundance of deep-sea sharks (Danovaro et al., 2010; Eigaard et al., 2017). At the same time, in the last 30 years, some deep-sea sharks started having a commercial interest especially in the northeast Atlantic (Alves et al., 2020). The global market for elasmobranch products is also increasing and diversifying in different sectors: food, pharmaceutical, and even material (clothing), with industries now able of processing and selling meat, liver oil, cartilage, skin, and other shark related products (Dent and Clarke, 2015).
To mitigate the impact of the fisheries on deep-sea Chondrichthyes and on deep-sea biodiversity, the General Fisheries Commission for the Mediterranean (GFCM) in 2005 banned bottom trawling at depths beyond 1000 m. In addition, the European Union has restricted the total allowable catches (EU, 2021) announced the prohibition of bottom trawling in a large area of the North Atlantic (EU, 2022) and is planning further management efforts with the creation of Fisheries Restricted Areas (FRAs) for the deep-sea fisheries (EU, 2019).
Despite all of these efforts, deep-sea sharks do not show signs of recovery, as their high mobility and migrations at shallower depths exposes these species to a significant risk of by catch (Musick and Cotton, 2015; Treberg and Speers-Roesch, 2016). In this regard, the obligation to release deep-sea Chondrichthyes incidentally caught is not always effective as many of these large animals do not survive once fished (Talwar et al., 2017). Extending no-fishing zones or marine protected areas from the shelf break down to the continental slopes (thus able to cover the whole bathymetric range of their movements across life stages and encompassing the spatial segregations between genders) could represent an important tool for conservation of these threatened species. However, enforcing the protection of marine areas is difficult, due to the conflicting economic interests (Hilborn et al., 2004; Botsford et al., 2009; Pérez-Ruzafa et al., 2017).
A complementary solution could be the active engagement of fishermen to endorse improved fishing practices (Ellis et al., 2017), and the use of devices able to reduce the by-catch (Brčić et al., 2015; Nuez et al., 2023). Currently, experimental trials the use by-catch reduction devices are showing remarkable positive effects, with a significant decrease of the incidental captures of deep-water elasmobranchs without negative effects on commercial yields (see the results of the LIFE ELIFE project).
Another solution is intensifying the controls against illegal fisheries that are often extending trawling beyond the depth limits (1000 m for the Mediterranean Sea) also using new technologies and satellite sensors (Clarke et al., 2015). Finally, a better fishers’ knowledge of this deep-sea Chondrichthyes and the direct involvement of local fisheries for the use of low impact fishing gears may contribute significantly to preserve these vulnerable deep-sea Chondrichthyes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Animal Welfare Body of SZN (OBA).
Author contributions
MB: conceptualization; acquisition, analysis and interpretation of data, drafting the work and revising the final version. MS: analysis and interpretation of data, drafting the work and revising the final version. IB: analysis and interpretation of data, drafting the work. MCF: analysis and interpretation of data, drafting the work. AC: analysis and interpretation of data. IC: acquisition and analysis of data. FS: analysis of data. ES: analysis and interpretation of data. MV: conceptualization, revising the final version. LM: analysis of data. GC: analysis and interpretation of data and drafting the work. RD: conceptualization, interpretation of data, revising the final version. All the authors provide approval for publication of the content. All authors contributed to the article and approved the submitted version.
Funding
This study was partially supported and carried out in the framework of the LIFE ELIFE Project - Elasmobranchs Low-Impact Fishing Experience (LIFE18 NAT/IT/000846; https://webgate.ec.europa.eu/life/publicWebsite/index.cfm?fuseaction=search.dspPage&n_proj_id=7246). GC was supported by a PhD grant co-funded by the Stazione Zoologica Anton Dohrn and the University of Siena.
Acknowledgments
The authors are grateful to the crew of F/V “Maria Rosa” (Genoa) for providing specimens of Dalatias licha from the Ligurian Sea. Special thanks to Edoardo Mostarda and Peter N. Psomadakis (both of the Food and Agriculture Organization of the United Nations) for the relevant suggestions and to Mario Santoro (Stazione Zoologica Anton Dohrn) for taxonomic identification of the parasites. Martina Arpaia, Manfredi Madia, Ilaria Di Lauro, Francesco L. Leonetti and Lorenzo Minoia (Stazione Zoologica Anton Dohrn), as well as Umberto Scacco and Leonardo Tunesi (Italian National Institute for Environmental Protection and Research) are acknowledged for their assistance. Thanks also to Makenna Mahrer (Claremont McKenna College, USA) for her readily collaboration and to the reviewers for the useful comments, which greatly improved this paper. The present study complies with the current EU laws of bioethics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MG declared a shared affiliation with the author MV to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alves L. M. F., Correia J. P. S., Lemos M. F. L., Novais S. C., Cabral H. (2020). Assessment of trends in the Portuguese elasmobranch commercial landings over three decades, (1986–2017). Fish. Res. 230, 105648. doi: 10.1016/j.fishres.2020.105648
Anderson M. J. (2001). Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 58 (3), 626–639. doi: 10.1139/f01-004
Anderson R., Ahmed H. (1993). The shark fisheries of the Maldives (Male, Maldives: FAO, Rome, and Ministry of Fisheries).
Bansemer C. S., Bennett M. B. (2009). Reproductive periodicity, localised movements and behavioural segregation of pregnant carcharias taurus at wolf rock, southeast Queensland, Australia. Mar. Ecol. Prog. Ser. 374, 215–227. doi: 10.3354/meps07741
Barbier E. B., Moreno-Mateos D., Rogers A. D., Aronson J., Pendleton L., Danovaro R., et al. (2014). Ecology: protect the deep sea. Nature 505 (7484). doi: 10.1038/505475a
Barría C., Coll M., Navarro J. (2015). Unravelling the ecological role and trophic relationships of uncommon and threatened elasmobranchs in the western Mediterranean Sea. Mar. Ecol. Prog. Ser. 539, 225–240. doi: 10.3354/meps11494
Barría C., Navarro J., Coll M. (2018). Feeding habits of four sympatric sharks in two deep-water fishery areas of the western Mediterranean Sea. Deep Sea Res. Part I: Oceanogr. Res. Pap. 142, 34–43. doi: 10.1016/j.dsr.2018.09.010
Booth H., Squires D., Milner-Gulland E. J. (2020). The mitigation hierarchy for sharks: a risk-based framework for reconciling trade-offs between shark conservation and fisheries objectives. Fish Fish (Oxf) 21 (2), 269–289. doi: 10.1111/faf.12429
Botsford L. W., Brumbaugh D. R., Grimes C., Kellner J. B., Largier J., O’Farrell M. R., et al. (2009). Connectivity, sustainability, and yield: bridging the gap between conventional fisheries management and marine protected areas. Rev. Fish Biol. Fish. 19 (1), 69–95. doi: 10.1007/s11160-008-9092-z
Bradai M. N., Saidi B., Enajjar S. (2012). “Studies and reviews - general fisheries commission for the Mediterranean,” in Elasmobranchs of the Mediterranean and black Sea: status, ecology and biology bibliographic analysis. Available at: https://www.cabdirect.org/cabdirect/abstract/20133082654.
Brčić J., Herrmann B., De Carlo F., Sala A. (2015). Selective characteristics of a shark-excluding grid device in a Mediterranean trawl. Fish. Res. 172, 352–360. doi: 10.1016/j.fishres.2015.07.035
Brooks E. J., Brooks A. M. L., Williams S., Jordan L. K. B., Abercrombie D., Chapman D. D., et al. (2015). First description of deep-water elasmobranch assemblages in the exuma sound, the Bahamas. Deep Sea Res. Part II: Top. Stud. Oceanogr. 115, 81–91. doi: 10.1016/j.dsr2.2015.01.015
Caddell R. (2020). Deep-Sea bottom fisheries and the protection of seabed ecosystems: problems, progress and prospects. Ed. Nijhoff B., 255–284. doi: 10.1163/9789004391567_014
Capapé C., Hemida F., Quignard J.-P., Amor M. M. B., Reynaud C. (2008). Biological observations on a rare deep-sea shark, dalatias licha (Chondrichthyes: dalatiidae), off the maghreb coast (south-western Mediterranean). Pan-Am. J. Aquat. Sci. 3 (3), 355–360.
Carrier J. C., Musick J. A., Heitaus M. R. (2004). Biology of sharks and their relatives (CRC Press: Boca Raton, USA).
Catarino D., Knutsen H., Veríssimo A., Olsen E., Jorde P., Menezes G., et al. (2015). “The pillars of Hercules as a bathymetric barrier to gene-flow promoting isolation in a global deep-sea shark” (Centroscymnus coelolepis). Mol. Ecol. doi: 10.1111/mec.13453
Chiba S., Saito H., Fletcher R., Yogi T., Kayo M., Miyagi S., et al. (2018). Human footprint in the abyss: 30 year records of deep-sea plastic debris. Mar. Policy 96, 204–212. doi: 10.1016/j.marpol.2018.03.022
Clark M. R., Althaus F., Schlacher T. A., Williams A., Bowden D. A., Rowden A. A. (2016). The impacts of deep-sea fisheries on benthic communities: a review. ICES J. Mar. Sci. 73 (suppl_1), i51–i69. doi: 10.1093/icesjms/fsv123
Clarke M. W., Connolly P. L., Bracken J. J. (2001). Aspects of reproduction of the deep water sharks Centroscymnus coelolepis and Centrophorus squamosus from west of Ireland and Scotland. J. Mar. Biol. Assoc. U. K. 81 (6), 1019–1029. doi: 10.1017/S0025315401005008
Clarke J., Milligan R. J., Bailey D. M., Neat F. C. (2015). A scientific basis for regulating deep-Sea fishing by depth. Curr. Biol. 25 (18), 2425–2429. doi: 10.1016/j.cub.2015.07.070
Compagno L. J. V. (1984) FAO species catalogue. Vol.4. sharks of the world. an annotated and illustrated catalogue of shark species known to date. part 1. hexanchiformes to lamniformes. Available at: https://policycommons.net/artifacts/1422268/fao-species-catalogue/2036355/.
Compagno L. J. V. (2001). Bullhead, mackerel and carpet sharks: heterodontiformes, lamniformes and orectolobiformes (Rome: Food and Agriculture Organization of the United Nations).
Corner E. D. S., Denton E. J., Forster G. (1997). On the buoyancy of some deep-sea sharks. Proc. R. Soc.B:Biol.Sci. 171 (1025), 415–429. doi: 10.1098/rspb.1969.0003
Cortés E. (1999). Standardized diet compositions and trophic levels of sharks. ICES J. Mar. Sci. 56 (5), 707–717. doi: 10.1006/jmsc.1999.0489
Cotton C. F., Grubbs R. D. (2015). Biology of deep-water chondrichthyans: introduction. Deep Sea Res. Part II Top. Stud. Oceanog. 115, 1–10. doi: 10.1016/j.dsr2.2015.02.030
Danovaro R., Company J. B., Corinaldesi C., D’Onghia G., Galil B., Gambi C., et al. (2010). Deep-Sea biodiversity in the Mediterranean Sea: the known, the unknown, and the unknowable. PloS One 5 (8), e11832. doi: 10.1371/journal.pone.0011832
Danovaro R., Corinaldesi C., Dell’Anno A., Snelgrove P. V. R. (2017). The deep-sea under global change. Curr. Biol. 27 (11), R461–R465. doi: 10.1016/j.cub.2017.02.046
de Loyola Fernández I., Báez J. C., García-Barcelona S., Camiñas J. A., de Urbina M. O., Macías D. (2017). Length–weight relationships of kitefin shark (Dalatias licha), and little sleeper shark (Somniosus rostratus) from the Western Mediterranean Sea, and long snouted lancetfish (Alepisaurus ferox) from the Eastern north Atlantic ocean. Turkish J. Fish. Aquat. Sci. 17 (5), 1073–1076. doi: 10.4194/1303-2712-v17_5_24
Dent F., Clarke S. (2015). State of the global market for shark products (Rome: FAO Fisheries and Aquaculture technical paper). Available at: https://www.proquest.com/openview/3b5c990099f5140bc44e63e7e691e271/1?cbl=237320&pq-origsite=gscholar&parentSessionId=rP%2BcXqbwR2kaNjPqMJraPHMB9SKVNfFmxnb7PvWdemc%3D.
Devine J. A., Baker K. D., Haedrich R. L. (2006). Deep-sea fishes qualify as endangered. Nature 439 (7072). doi: 10.1038/439029a
Dulvy N. K., Allen D. J., Ralph G. M., Walls R. H. L. (2016). The conservation status of sharks, rays and chimaeras in the Mediterranean Sea. doi: 10.13140/RG.2.2.22020.53129
Dunn M. R., Stevens D. W., Forman J. S., Connell A. (2013). Trophic interactions and distribution of some squaliforme sharks, including new diet descriptions for deania calcea and squalus acanthias. PloS One 8 (3), e59938. doi: 10.1371/journal.pone.0059938
Dunn M. R., Szabo A., McVeagh M. S., Smith P. J. (2010). The diet of deepwater sharks and the benefits of using DNA identification of prey. Deep Sea Res. Part I Oceanogr. Res. Pap. 57 (7), 923–930. doi: 10.1016/j.dsr.2010.02.006
Eigaard O. R., Bastardie F., Hintzen N. T., Buhl-Mortensen L., Buhl-Mortensen P., Catarino R., et al. (2017). The footprint of bottom trawling in European waters: distribution, intensity, and seabed integrity. ICES J. Mar. Sci. 74 (3), 847–865. doi: 10.1093/icesjms/fsw194
Ellis J. R., McCully Phillips S. R., Poisson F. (2017). A review of capture and post-release mortality of elasmobranchs. J.Fish Biol. 90 (3), 653–722. doi: 10.1111/jfb.13197
EU (2019) ). fisheries and aquaculture measures in the general fisheries commission for the Mediterranean (GFCM) area. Available at: https://eur-lex.europa.eu/EN/legal-content/summary/fisheries-and-aquaculture-measures-in-the-general-fisheries-commission-for-the-mediterranean-gfcm-area.html.
EU (2021) COUNCIL REGULATION (EU) 2021/91 of 28 January 2021. fixing, for the years 2021 and 2022, the fishing opportunities for union fishing vessels for certain deep-sea fish stocks. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32021R0091&rid=9.
EU (2022) COMMISSION IMPLEMENTING REGULATION (EU) 2022/1614 of 15 September 2022 determining the existing deep-sea fishing areas and establishing a list of areas where vulnerable marine ecosystems are known to occur or are likely to occur. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ%3AL%3A2022%3A242%3ATOC.
FAO (2009). International guidelines for the management of deep-sea fisheries in the high seas (Rome: FAO).
Finucci B., Dunn M. R., Jones E. G., Bartolino V. (2018). Aggregations and associations in deep-sea chondrichthyans. ICES J. Mar. Sci. 75 (5), 1613–1626. doi: 10.1093/icesjms/fsy034
Follesa M. C., Porcu C., Cabiddu S., Mulas A., Deiana A. M., Cau A. (2011). Deep-water fish assemblages in the central-western Mediterranean (south sardinian deep-waters). J. Appl. Ichthyol. 27 (1), 129–135. doi: 10.1111/j.1439-0426.2010.01567.x
Girard M., Buit M.-H. D. (1999). Reproductive biology of two deep-water sharks from the British isles, centroscymnus coelolepis and centrophorus squamosus (Chondrichthyes: squalidae). J. Mar. Biol. Assoc. U. K. 79 (5), 923–931. doi: 10.1017/S002531549800109X
Graham K. J., Daley R. K., Graham K. J., Daley R. K. (2011). Distribution, reproduction and population structure of three gulper sharks (Centrophorus, centrophoridae) in south-east Australian waters. Mar. Freshw. Res. 62 (6), 583–595. doi: 10.1071/MF10158
Guallart J., Walls R. (2016) IUCN red list of threatened species: dalatias licha. IUCN red list of threatened species. Available at: https://www.iucnredlist.org/en.
Henderson A. C., Flannery K., Dunne J. (2003). Biological observations on shark species taken in commercial fisheries to the West of Ireland. Biol. Environ. 103B (1), 1–7. doi: 10.3318/BIOE.2003.103.1.1
Hilborn R., Stokes K., Maguire J.-J., Smith T., Botsford L. W., Mangel M., et al. (2004). When can marine reserves improve fisheries management? Ocean Coast. Manag. 47 (3), 197–205. doi: 10.1016/j.ocecoaman.2004.04.001
Hyslop E. J. (1980). Stomach contents analysis–a review of methods and their application. J. Fish Biol. 17 (4), 411–429. doi: 10.1111/j.1095-8649.1980.tb02775.x
IUCN (2020). The IUCN red list of threatened species (Switzerland: International Union for Conservation of Nature). Available at: https://www.iucnredlist.org/.
Jakobsdóttir K. B. (2001). Biological aspects of two deep-water squalid sharks: centroscyllium fabricii (Reinhardt 1825) and etmopterus princeps (Collett 1904) in icelandic waters. Fish. Res. 51 (2), 247–265. doi: 10.1016/S0165-7836(01)00250-8
Kabasakal H., Kabasakal E. (2002). Morphometrics of young kitefin sharks, dalatias licha (Bonnaterre 1788), from the northeastern Aegean Sea, with notes on its biology. Ann. Ser. Hist. Nat. 12 (2), 161–166.
Kheddam H., Chisholm L. A., Tazerouti F. (2020). Septitrema lichae n. g., n. sp. (Monogenea: monocotylidae) from the nasal tissues of the deep-sea kitefin shark, dalatias licha (Bonnaterre) (Squaliformes: dalatiidae), off Algeria. Syst. Parasitol. 97 (6), 553–559. doi: 10.1007/s11230-020-09942-4
Kheddam H., Justine J. L., Tazerouti F. (2016). Hexabothriid monogeneans from the gills of deep-sea sharks off Algeria, with the description of squalonchocotyle euzeti n. sp (Hexabothriidae) from the kitefin shark dalatias licha (Euselachii, dalatiidae). Helminthologia 53 (4), 354. doi: 10.1515/helmin-2016-0034
King J. R., McFarlane G. A. (2003). Marine fish life history strategies: applications to fishery management. Fish. Manage. Ecol. 10 (4), 249–264. doi: 10.1046/j.1365-2400.2003.00359.x
Kyne P. M., Simpfendorfer C. A. (2010). “Deepwater chondrichthyans,” in Sharks and their relatives II, vol. 1. , 78. Available at: https://www.taylorfrancis.com/chapters/edit/10.1201/9781420080483-7/deepwater-chondrichthyans-peter-kyne-colin-simpfendorfer.
López Calero L. (2013) Trophic strategies and basic morphology of the rare demersal kitefin shark dalatias licha in the northwestern Mediterranean Sea. Available at: https://digital.csic.es/handle/10261/113071.
Lotze H. K., Lenihan H. S., Bourque B. J., Bradbury R. H., Cooke R. G., Kay M. C., et al. (2006). Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312 (5781), 1806–1809. doi: 10.1126/science.1128035
Lourenço R., Penteriani V., Rabaça J. E., Korpimäki E. (2014). Lethal interactions among vertebrate top predators: a review of concepts, assumptions and terminology. Biol. Rev. 89 (2), 270–283. doi: 10.1111/brv.12054
Macpherson E. (1980). Morphometrics of young kitefin sharks, dalatias licha (Bonnaterre 1788), from northeastern Aegean Sea, with notes on its biology. Vie Milieu.
Martin U., Mallefet J. (2022). The diet of deep-water sharks. Deep Sea Res. Part I Oceanogr. Res. Pap., 103898. doi: 10.1016/j.dsr.2022.103898
Matallanas J. (1982). Feeding habits of scymnorhinus licha in Catalan waters. J. Fish Biol. 20 (2), 155–163. doi: 10.1111/j.1095-8649.1982.tb03916.x
McCluskey S. M., Lewison R. L. (2008). Quantifying fishing effort: a synthesis of current methods and their applications. Fish Fish (Oxf) 9 (2), 188–200. doi: 10.1111/j.1467-2979.2008.00283.x
Mclaughlin D. M., Morrissey J. F. (2005). Reproductive biology of centrophorus cf. uyato from the cayman trench, jamaica. J. Mar. Biol. Assoc. U. K. 85 (5), 1185–1192. doi: 10.1017/S0025315405012282
Mejjad N., Rovere M. (2021). Understanding the impacts of blue economy growth on deep-Sea ecosystem services. Sustainability 13 (22). doi: 10.3390/su132212478
Moura T., Jones E., Clarke M. W., Cotton C. F., Crozier P., Daley R. K., et al. (2014). Large-Scale distribution of three deep-water squaloid sharks: integrating data on sex, maturity and environment. Fish. Res. 157, 47–61. doi: 10.1016/j.fishres.2014.03.019
Mulas A., Bellodi A., Carbonara P., Cau A., Marongiu M. F., Pesci P., et al. (2021). Bio-ecological features update on eleven rare cartilaginous fish in the central-Western Mediterranean Sea as a contribution for their conservation. Life 11 (9). doi: 10.3390/life11090871
Munoz-Chapuli R. (1984). Ethologie de la reproduction chez quelques requins de l’Atlantique nord-est. Cybium 8, 3, 1–14.
Musick J. A., Cotton C. F. (2015). Bathymetric limits of chondrichthyans in the deep sea: a re-evaluation. Deep Sea Res. Part II Top. Stud. Oceanog. 115, 73–80. doi: 10.1016/j.dsr2.2014.10.010
Navarro J., López L., Coll M., Barría C., Sáez-Liante R. (2014). Short- and long-term importance of small sharks in the diet of the rare deep-sea shark dalatias licha. Mar. Biol. 161 (7), 1697–1707. doi: 10.1007/s00227-014-2454-2
Nieto A., Ralph G. M., Comeros-Raynal M. T., Kemp J., Criado M. G., Allen D. J., et al. (2015). European Red list of marine fishes (Luxembourg: Publications Office of the European Union). Available at: https://data.europa.eu/doi/10.2779/082723.
Norse E. A., Brooke S., Cheung W. W. L., Clark M. R., Ekeland I., Froese R., et al. (2012). Sustainability of deep-sea fisheries. Mar. Policy 36 (2), 307–320. doi: 10.1016/j.marpol.2011.06.008
Nuez I., Giovos I., Tiralongo F., Penadés-Suay J., Cetkovic I., Di Lorenzo M., et al. (2023). Assessing the current status of hexanchus griseus in the Mediterranean Sea using local ecological knowledge. Mar. Policy 147, 105378. doi: 10.1016/j.marpol.2022.105378
Pacoureau N., Rigby C. L., Kyne P. M., Sherley R. B., Winker H., Carlson J. K., et al. (2021). Half a century of global decline in oceanic sharks and rays. Nature 589 (7843). doi: 10.1038/s41586-020-03173-9
Pérez-Ruzafa A., García-Charton J. A., Marcos C. (2017). North East Atlantic vs. Mediterranean marine protected areas as fisheries management tool. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00245
Pusceddu A., Bianchelli S., Martín J., Puig P., Palanques A., Masqué P., et al. (2014). Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. PNAS 111 (24), 8861–8866. doi: 10.1073/pnas.1405454111
Pyke G. H. (1984). Optimal foraging theory: a critical review. Annu. Rev. Ecol. Evol. Syst. 15, 523–575. doi: 10.1146/annurev.es.15.110184.002515
Robbins R. L. (2007). Environmental variables affecting the sexual segregation of great white sharks carcharodon carcharias at the Neptune islands south Australia. J. Fish Biol. 70 (5), 1350–1364. doi: 10.1111/j.1095-8649.2007.01414.x
Roberts C. M. (2002). Deep impact: the rising toll of fishing in the deep sea. Trends Ecol. Evol. 17 (5), 242–245. doi: 10.1016/S0169-5347(02)02492-8
Serena F. (2005). Field identification guide to the sharks and rays of the Mediterranean and black Sea (Rome: Food & Agriculture Org).
Shipley O. N., Brooks E. J., Madigan D. J., Sweeting C. J., Dean Grubbs R. (2017). Stable isotope analysis in deep-sea chondrichthyans: recent challenges, ecological insights, and future directions. Rev. Fish Biol. Fish. 27 (3), 481–497. doi: 10.1007/s11160-017-9466-1
Simpfendorfer C. A., Kyne P. M. (2009). Limited potential to recover from overfishing raises concerns for deep-sea sharks, rays and chimaeras. Environ. Conserv. 36 (2), 97–103. doi: 10.1017/S0376892909990191
Sinopoli M., Fanelli E., D’Anna G., Badalamenti F., Pipitone C. (2012). Assessing the effects of a trawling ban on diet and trophic level of hake, Merluccius merluccius, in the southern tyrrhenian Sea. Sci. Mar. doi: 10.3989/scimar.03564.29A
Sperone E., Milazzo C. (2018). On the occurrence of the digenean otodistomum veliporum in the spiracle of the kitefin shark dalatias licha. Acta Adriat. 59 (1). doi: 10.32582/aa.59.1.11
Talwar B., Brooks E. J., Mandelman J. W., Grubbs R. D. (2017). Stress, post-release mortality, and recovery of commonly discarded deep-sea sharks caught on longlines. Mar. Ecol. Prog. Ser. 582, 147–161. doi: 10.3354/meps12334
Treberg J. R., Speers-Roesch B. (2016). Does the physiology of chondrichthyan fishes constrain their distribution in the deep sea? J. Exp. Biol. 219 (5), 615–625. doi: 10.1242/jeb.128108
Valente T., Scacco U., Matiddi M. (2020). Macro-litter ingestion in deep-water habitats: is an underestimation occurring? Environ. Res. 186, 109556. doi: 10.1016/j.envres.2020.109556
Valls M., Quetglas A., Ordines F., Moranta J. (2011). Feeding ecology of demersal elasmobranchs from the shelf and slope off the Balearic Sea (western Mediterranean). Sci. Mar. 75 (4), 633–639. doi: 10.3989/scimar.2011.75n4633
Villasante S., Morato T., Rodriguez-Gonzalez D., Antelo M., Österblom H., Watling L., et al. (2012). Sustainability of deep-sea fish species under the European union common fisheries policy. Ocean Coast. Manag. 70, 31–37. doi: 10.1016/j.ocecoaman.2012.07.033
Walls R. H. L., Dulvy N. K. (2021). Tracking the rising extinction risk of sharks and rays in the northeast Atlantic ocean and Mediterranean Sea. Sci. Rep. 11 (1). doi: 10.1038/s41598-021-94632-4
Watson R. A., Morato T. (2013). Fishing down the deep: accounting for within-species changes in depth of fishing. Fish. Res. 140, 63–65. doi: 10.1016/j.fishres.2012.12.004
Wetherbee B. M. (1996). Distribution and reproduction of the southern lantern shark from new Zealand. J.Fish Biol. 49 (6), 1186–1196. doi: 10.1111/j.1095-8649.1996.tb01788.x
White W. T., Kyne P. M. (2010). The status of chondrichthyan conservation in the indo-Australasian region. J.Fish Biol. 76 (9), 2090–2117. doi: 10.1111/j.1095-8649.2010.02654.x
Yano K., Tanaka S. (1988). Size at maturity, reproductive cycle, fecundity, and depth segregation of the deep Sea squaloid sharks Centroscymnus owstoni and C. coelolepis in suruga bay, Japan. Nippon Suisan Gakkaishi 54 (2), 167–174. doi: 10.2331/suisan.54.167
Keywords: Chondrichthyes, conservation, deep-sea fisheries, life traits, Mediterranean Sea
Citation: Bottaro M, Sinopoli M, Bertocci I, Follesa MC, Cau A, Consalvo I, Scarcelli F, Sperone E, Vacchi M, Marsili L, Consales G and Danovaro R (2023) Jaws from the deep: biological and ecological insights on the kitefin shark Dalatias licha from the Mediterranean Sea. Front. Mar. Sci. 10:1155731. doi: 10.3389/fmars.2023.1155731
Received: 31 January 2023; Accepted: 27 June 2023;
Published: 21 July 2023.
Edited by:
Daniele Tibullo, University of Catania, ItalyReviewed by:
Michele Gristina, ISA Rome, National Research Council (CNR), ItalySara Ignoto, University of Catania, Italy
Copyright © 2023 Bottaro, Sinopoli, Bertocci, Follesa, Cau, Consalvo, Scarcelli, Sperone, Vacchi, Marsili, Consales and Danovaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimiliano Bottaro, bWFzc2ltaWxpYW5vLmJvdHRhcm9Ac3puLml0; Mauro Sinopoli, bWF1cm8uc2lub3BvbGlAc3puLml0
 Massimiliano Bottaro
Massimiliano Bottaro Mauro Sinopoli
Mauro Sinopoli Iacopo Bertocci
Iacopo Bertocci Maria Cristina Follesa
Maria Cristina Follesa Alessandro Cau
Alessandro Cau Ivan Consalvo1,6
Ivan Consalvo1,6 Faustino Scarcelli
Faustino Scarcelli Emilio Sperone
Emilio Sperone Marino Vacchi
Marino Vacchi Letizia Marsili
Letizia Marsili Guia Consales
Guia Consales Roberto Danovaro
Roberto Danovaro