Abstract
The costs and benefits of customary top-down Marine Protected Areas (MPAs) have been studied at length. But the costs and benefits of community-based MPAs –an increasingly common tool in conservation and fisheries management– remain understudied. Here, we quantify the operational costs of maintaining community-based MPA monitoring programs in nine small-scale fishing communities in Mexico. We then compare these costs to the potential extractive use value of invertebrate and fish biomass contained in the reserves. We find that the annual monitoring costs (median: 1,130 MXN/ha; range: 23-3,561 MXN/ha) represent between 0.3% and 55% of the extractive use value of the biomass contained in the reserves (median: 21.31 thousand MXN/ha; 5.22 - 49/12 thousand MXN/ha). These results suggest that the direct monetary benefits of community-based marine conservation can outweigh the costs of monitoring programs, providing further support for these types of management schemes. While further research should explore other mechanisms that would allow fishers to leverage the non-extractive use value of reserves (e.g., tourism) or the non-use value (i.e. existence value of biodiversity) to sustainably finance their conservation efforts, a stop-gap measure to ensuring long-term monitoring costs are covered might include limited extractive use of resources contained in the reserves.
1 Introduction
Marine Protected Areas (MPAs) have become a common tool in the marine conservation and fisheries toolkit, particularly in tropical small-scale fisheries. A rich body of literature has studied the benefits that MPAs can have on fisheries through empirical evaluations (Moland et al., 2013; Lenihan et al., 2021) or numerical simulations (Ovando et al., 2016; Millage et al., 2021), and can be largely summed up by increases in species richness, biomass, and catch-per-unit effort around MPA boundaries (Micheli et al., 2004; Lester et al., 2009; Lenihan et al., 2021; Medoff et al., 2022). Others have empirically evaluated how the costs of establishing MPAs scale with the duration of the planning phase and size of the MPA to be implemented (McCrea-Strub et al., 2011), or combined surveys and national statistics to estimate the recurrent annual expenditure of MPAs and calculate the budgetary requirements for a global network of MPAs (Balmford et al., 2004). A growing body of literature has focused on estimating the socioeconomic costs that MPAs place on resource users (Smith et al., 2010; Rees et al., 2013; Rees et al., 2021). Yet, few (if any) have jointly quantified the relationship between operational costs and socioeconomic benefits of the same MPA, and even fewer have performed such an analysis focusing on community-based MPAs.
Community-based MPAs are areas where fishers voluntarily eliminate fishing effort, or where fisher’s input and knowledge is the main driver of the design, implementation, and management of the areas (White, 1989). An important distinction between these and customary top-down MPAs lies in the distribution of costs and benefits of conservation. Benefits of customary MPAs will mainly accrue to society in general, for example through leisure opportunities, food provisioning, and other ecosystem services (Potts et al., 2014; Leenhardt et al., 2015; Cabral et al., 2019; Johnson et al., 2019), while a smaller portion of the benefits may accrue to a subset of users [e.g. biomass spillover to fishers; Lenihan et al. (2021); Medoff et al. (2022)]. Their operational costs are also generally covered by society thorough national taxation, but we note that some private agents may disproportionately bear some of the opportunity costs [e.g. fishers that are displaced from their fishing grounds; Smith et al. (2010)]. In contrast, community-based MPAs are often implemented within traditional fishing grounds (some of which may be formal territorial user rights for fisheries - TURFs [Afflerbach et al. (2014); Gelcich et al. (2017); Villaseñor-Derbez et al. (2019)], which confer spatial property rights and often result in exclusive access regimes. Therefore, it follows that any benefits that arise from conservation interventions in these private areas will mainly accrue to those who hold the property rights (i.e. the fishers), rather than “society in general”. Communities often resort to philanthropic sources in order to cover the operational costs of their MPAs, which has raised concerns about the long-term feasibility of such endeavors (Johannes, 2002; Ramutsindela et al., 2013; Mallin et al., 2019).
Marine reserves, also known as fully-protected MPAs, are a special type of MPA that do not allow extractive activities (Sala and Giakoumi, 2017). Over the past two decades, some Mexican fishing communities have implemented community-based marine reserves within their fishing grounds (Quintana and Basurto, 2021; Villaseñor-Derbez et al., 2022). Their documented success in maintaining biomass of fishery-relevant species (Smith et al., 2022) and biodiversity more broadly (Micheli et al., 2014; Munguía-Vega et al., 2015) has prompted ambitious commitments to expand coverage of community-based MPAs in Mexico. Yet, little is known about how the costs of implementing and maintaining them will stack up against the benefits that they may provide. Importantly, the existing reserves have received most of their financial support from philanthropic sources (although we recognize that government programs and the communities themselves have also provided some funding). The current funding model is therefore heavily dependent on philanthropic contributions, which cannot guarantee their long-term persistence. This highlights the need to study and develop alternative financing strategies that will be needed to maintain an expanded network of community-based marine reserves, and to better understand the benefits that they may provide.
One proposal discussed in the literature is to allow some amount of commercial fishing within the reserves, and use the proceeds to pay for their monitoring and enforcement. Such a set-up has been explored in the way of “Conservation Finance Areas” [sensuMillage et al. (2021)], where the authors show that in the absence of an exogenous budget it is always optimal to allow for some amount of fishing, and use the proceeds to pay for monitoring and enforcement. Here, we quantify the costs of implementing, monitoring, and maintaining community-based marine reserves in nine small-scale fishing communities in Mexico. We also leverage long-term fisheries and ecological monitoring data to estimate the monetary value of biomass contained in the reserves. We then compare these costs and benefits, and quantify the degree to which limited extraction of biomass contained in the reserves could help cover the costs of conservation. The main contributions of our paper are: We 1) provide the first cost estimates for maintaining community-based marine reserves, 2) quantify the economic benefits that they may provide, and 3) empirically confirm predictions made by previous theoretical work, and show that the value of the biomass within the reserves can help cover the costs of conservation.
2 Data and methods
2.1 Study area
We focus on nine systems of community-based marine reserves implemented in three distinct social-ecological systems (Figure 1). The first three reserve systems (El Rosario, Isla Natividad, and La Bocana) are located along the Pacific coast of the Baja California Peninsula, a temperate upwelling system dominated by kelp forests [mostly Giant Kelp (Macrocystis pyrifera) and Palm Kelp (Ecklonia arborea)]. This area is also known for their successful co-management strategies enabled by systems of TURFs and well-organized fishing cooperatives and federations of cooperatives who mainly target benthic invertebrate species (Lobster, Abalone, Sea Cucumber, Urchins) with a combination of set traps and hookah diving (McCay et al., 2014; McCay, 2017). The next three systems (Puerto Libertad, Isla San Pedro Mártir1, and Isla San Pedro Nolasco) are located along the eastern coast of the Gulf of California, where the predominant habitats are rocky reefs and sandy bottoms. The system is subject to a variety of users with fewer exclusive access rights and more interactions with industrial fisheries (Amador-Castro et al., 2021). Here, fishers target finfish with a variety of fishing gear, while bivalves are collected via hookah diving. Finally, the last three reserve systems (Maria Elena, Punta Herrero, and Banco Chinchorro) are on the Caribbean coastline of the Yucatan Peninsula, where coral reefs and seagrass beds are the predominant habitat types. Fishers also operate under a system of cooperatives and TURFs rooted in historical land-based management practices (Méndez-Medina et al., 2015). They mainly target lobster, which they collect via free diving and hand-held nets and lassos in the open reef and artificial structures (Miller, 1982; Briones-Fourzán and Lozano-Álvarez, 2000). Our set of focal reserve systems are representative of the main marine habitat types, target species, fishing methods, and management regimes typically faced by small-scale fishers in Mexico.
Figure 1
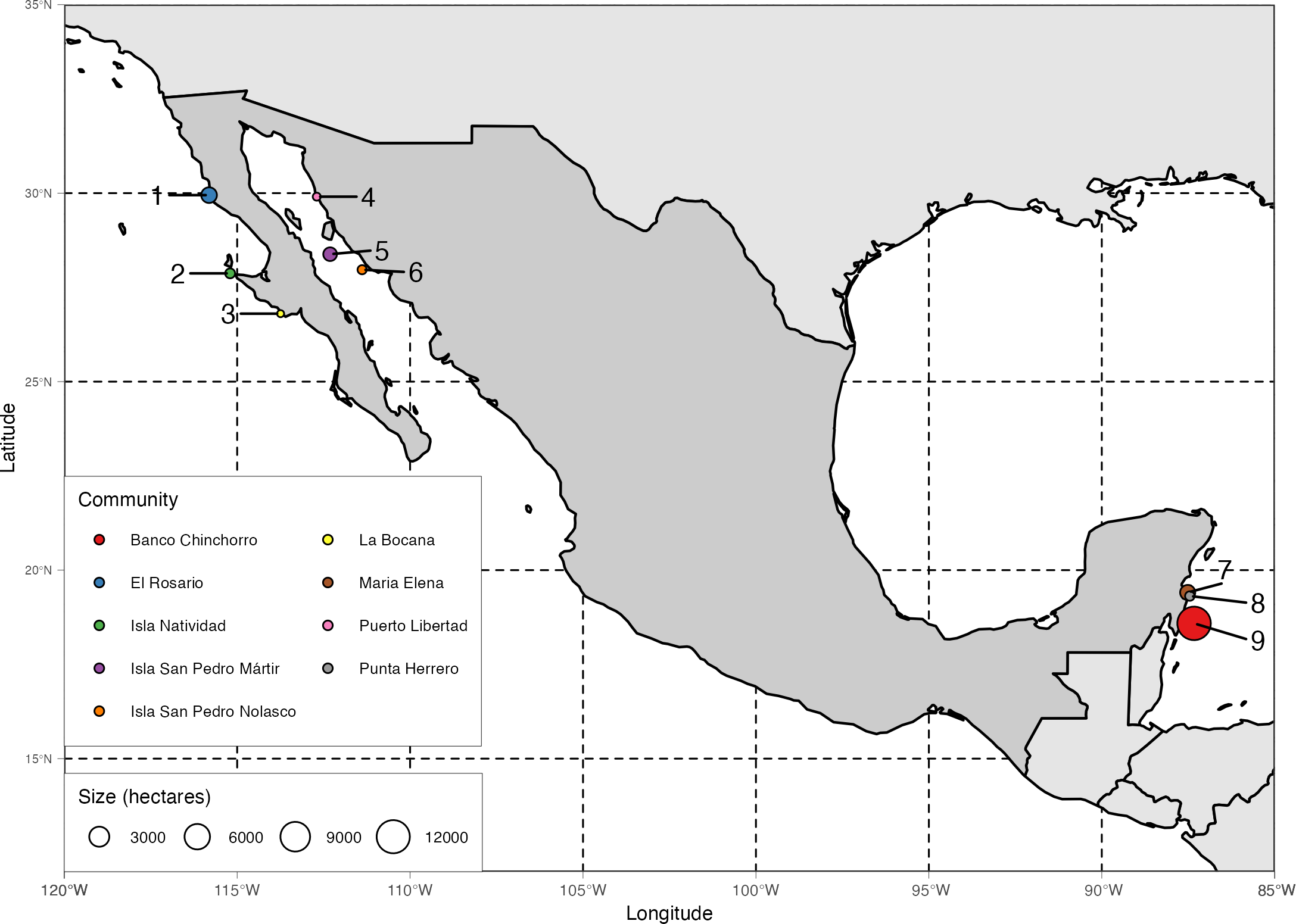
Map of general location of community-based marine reserves included in this analysis. Note that our sample includes three distinct regions: the Pacific coast of the Baja California peninsula, the Gulf of California, and the Mexican Caribbean. Color and numbers indicate the locations of the communities (1 = El Rosario, 2 = Isla Natividad, 3 = La Bocana, 4 = Puerto Libertad, 5 = Isla San Pedro Martir, 6 = Isla San Pedro Nolasco, 7 = Maria Elena, 8 = Punta Herrero, 9 = Banco Chinchorro).
2.2 Estimating costs of monitoring
Most of these communities have monitored their reserves annually for at least a decade, which allows us to quantify the annual costs of monitoring reserves in each community. We extract information on the costs of the monitoring programs from past budgetary line items of each reserve system. Specifically, we consider payroll (community members participating in the monitoring campaign are compensated at a rate equivalent to an average day of fishing), boat rental, fuel costs, training in SCUBA diving and scientific monitoring, servicing of SCUBA gear, dive insurance, and other costs associated with common field work activities. For each reserve system, we first calculate the total costs of the annual monitoring program and then normalize it by total reserve area (hectares) to generate a cost-per-hectare metric commonly used in the literature [e.g. see Balmford et al. (2004)]. Additionally, it is necessary to standardize costs in this way so that we can compare them to our value-per-hectare metrics developed in the following section. However, whenever relevant, we will also report the absolute costs to reflect the true cost to each community.
2.3 Estimating benefits of conservation
We are concerned with estimating the extractive use value of biomass [i.e. the value derived from depleting the resource (Ninan, 2012)] contained in the reserves, and how this compares to the operational costs. To do this, we will combine a long-term data set of taxa-specific fisheries landings with in situ observations of biomass in the reserves and control sites (See Figure 2).
Figure 2
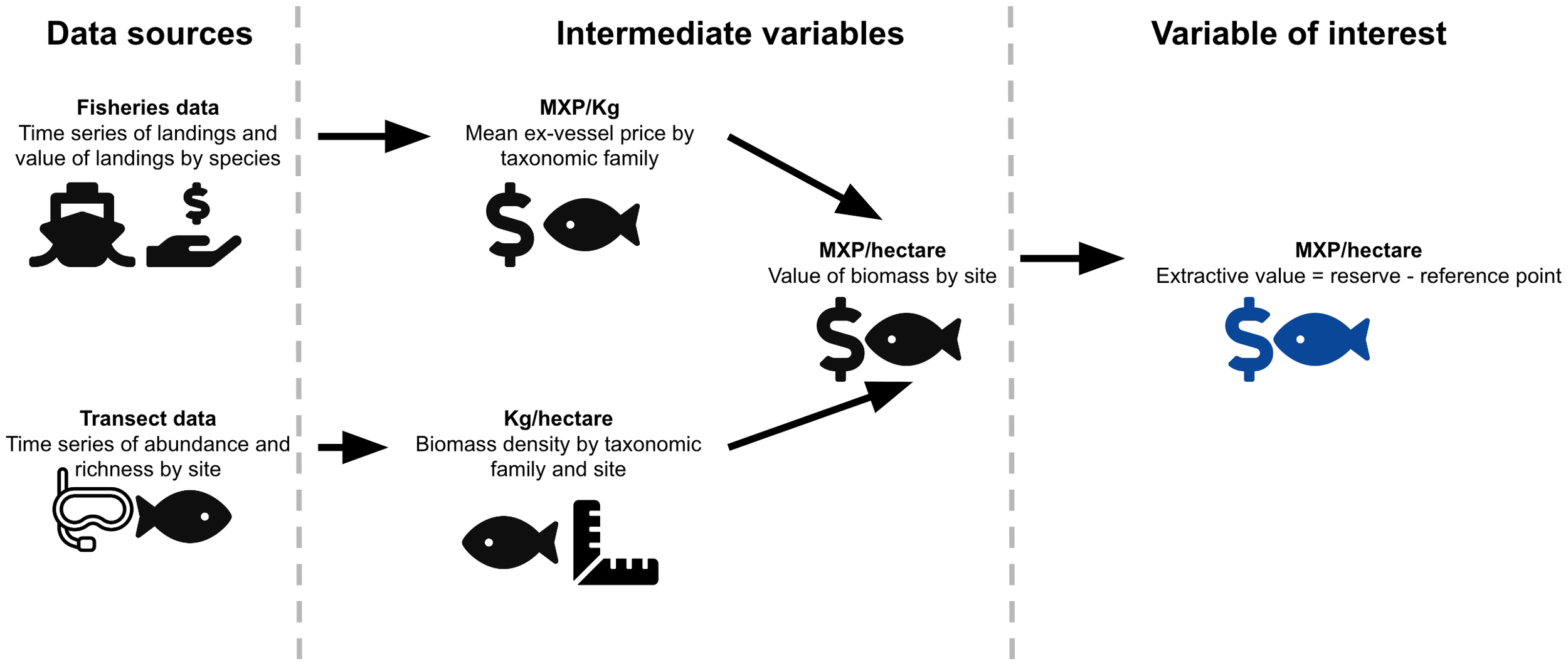
Pictorial representation of our methodological approach. The figure shows the data sources (left column), computed variables (middle column) and final variable of interest (right column).
2.3.1 Ex-vessel prices
The ex-vessel price is the per-kilogram value of catch paid to fishers upon the first transaction (Melnychuk et al., 2017). We use monthly data from landing tickets reported to CONAPESCA (Mexico’s fisheries management agency) between 2001 and 2019, which explicitly report the species or broad taxonomic group (e.g. sometimes the record might indicate “yellowtail jack” and sometimes simply “jack”), type of landed catch [e.g. total weight (recorded “peso vivo”) or gutted weight], weight (in Kg) and value of the total catch (in Mexican Pesos; MXN).
We filter the data to keep only records for which type of catch is recorded as “peso vivo” (total weight), allowing us to exclude records of products with any value-added processes (e.g. filleting, freezing, vacuum sealing, or gutting). Then, we match the reported species or group of species with their respective taxonomic families. For example, both “jack” and “yellowtail jack” would be matched with family Carangidae. We use the same price for families Serranidae and Polyprionidae that contain commercially similar species marketed as “groupers” and “sea bass” (translating from terms like Mero, Pescada, Garropa, and Cabrilla; See Table S1 for a list of main species groups and their respective taxonomic families). For each of these taxonomic families, we calculate the annual mean ex-vessel price (MXN/Kg) by dividing the total value of landed catch by total landed catch. We group our estimates at the family-year level to reduce errors due to species identification or variation in month-to-month price. We then use the Consumer Price Index from the OECD (OECD, 2023) to normalize all values to 2019 MXN, as:
Where is the adjusted ex-vessel price at time t, is the unadjusted ex-vessel price, CPIt is the Consumer Price index for year t, and CPI2019 is the reference consumer price index, in this case for year 2019. Figure 3A shows a time-series of CPI-adjusted mean ex-vessel prices for 12 families of commercial interest in Mexican small-scale fisheries. Since we are concerned with evaluating the current value of the reserves, we must define what the ex-vessel price would be today. We calculate and use the mean ex-vessel price for each family across all years. This mean value better represents the expected ex-vessel price for a given product, compared to using the ex-vessel price from the latest year in record (i.e. 2019, which could introduce bias because the value of a particular group of species might have been abnormally high or abnormally low in 2019). The resulting estimates of ex-vessel price for each of the 12 families are shown in Figure 3B.
Figure 3
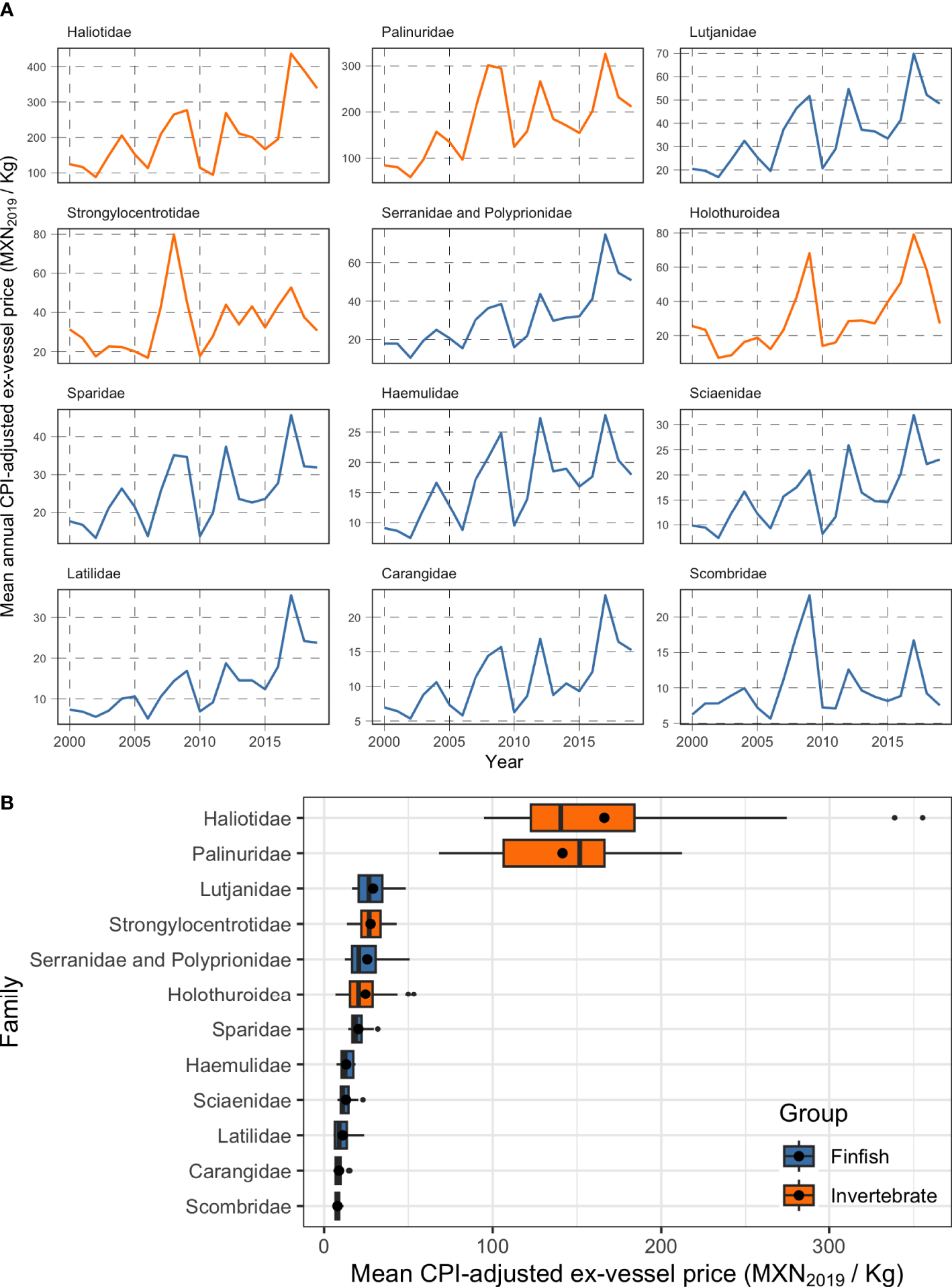
Ex-vessel prices for 12 taxonomic families of commercial importance to Mexican small-scale fisheries (2000 – 2019). Panel (A) shows mean annual ex-vessel prices, and panel (B) shows a boxplot of the ex-vessel price for each taxonomic family across all years. The vertical black line inside each box shows the median value, the black point within the bars shows the mean value (the one used in our analysis), the lower and upper edges of the bars correspond to the first and third quartiles. The upper error bar extends from the quartile to the largest value within 1.5 times the inter-quartile range, and the lower error bar extends from the edge of the first quartile to the smallest value at within 1.5 times the inter-quartile range. Data beyond the end of the whiskers are called “outlying” points and are plotted individually. Colors indicate whether the family contains finfish (blue) or invertebrates (orange).
2.3.2 Biomass density
Each community has implemented annual ecological monitoring programs to track the performance of their reserves. Scientific divers (fishers, community members, and researchers) follow standardized methodologies to record the richness and abundance of fish and invertebrate species along standardized 30-by-2 m belt transects [between 5 and 20 m depth] in each reserve and pre-determined control sites (See Suman et al. (2010); Fulton et al. (2018); Fulton et al., (2019a) for additional details). For fish species, total length (in cm) is also recorded. Sampling effort varies across communities and time (e.g. due to weather events), but on average a total of 70 (± 38) invertebrate and 90 (± 43) fish transects were performed per community each year (See Figure S4 for more details on sampling effort).
We filter monitoring data to keep only species belonging to the 12 families of commercial interest (See Figure 3 and Table S1). For fish survey data, we exclude records from organisms with Total Length ≤ 20 cm to remove juveniles that could sum to a large biomass that is not of commercial size (See Reddy et al. (2013) for a discussion on market-driven size-selective harvesting and the 20 cm cut-off). We then use the standard length-weight relationship () to calculate individual weight using species-specific a and b parameters obtained from FishBase (Froese and Pauly 2010), accessed using the “rfishbase” package in R [Fishbase version 23.01; Boettiger et al. (2012)]. When species-specific data were not available we used the genus-level median. Knowing the mass and number of individuals of each species recorded in each transect, we calculate the total biomass density for each family in each transect and then convert them to Kg/hectare.
The standardized invertebrate surveys do not record body length measurements for invertebrate species [abalone (Haliotidae), lobster (Panulidae), urchins (Strongylocentrotidae) and sea cucumber (Holoturoidea)]. Therefore, we use the species-specific minimum catch size or size at first capture and growth parameters retrieved from scientific literature to calculate individual weight (See Table S2). Other surveys have recorded carapace length of lobster (from a mark-and-recapture experiment in the Yucatan Peninsula) and diameter of abalone shells (during roving diver surveys in El Rosario, Isla Natividad, and La Bocana). These data show that 85.5% of lobster (total N = 173) and 80.24% of abalone (total N = 14,445) are larger than minimum catch sizes, and that the minimum catch sizes are consistently smaller than the mean sizes (Figure S1; See our discussion section for more information on the implications of this choice). As in the case of fish, we calculate the total biomass density for each family in each transect and convert them to Kg/hectare. Fish and invertebrate will continue to be handled separately to avoid confounding our precise estimates of fish biomass with our lower-bound estimates of invertebrate biomass.
2.3.3 Establishing the economic value of the reserves
We now proceed to match ex-vessel prices (MXN/Kg) and biomass density (Kg/ha) for each corresponding family, multiply them to obtain the economic value of the biomass of each family, and then sum across all families to obtain the total per-hectare value of invertebrate or fish biomass (MXN/ha) in the transect. Finally, we calculate the expected per-hectare value of each reserve and control site by taking the average across all transects.
We are interested in determining the immediate2 extractive value of the biomass in the reserves that fishers would perceive by extracting some of the biomass within their reserves. For each reserve, we identify the historical minimum observed in control sites (within the TURFs and fishing grounds, where fishing is allowed) or the reserve sites before they were implemented (when fishing was still allowed) and use them as a reference points (Table S3). We define the extractive value of a reserve as the difference between the value of biomass in the reserve today and value of biomass from the reference point (See Figure 2). This definition of reference points assumes that historical values are both economically and ecologically valuable. We ground this assumption on previous findings from fisheries economics (Gordon, 1954; Costello et al., 2012) and community-based management literature (Gelcich et al., 2008; Gelcich et al., 2015). Greater detail is provided in the discussion section, but it broadly implies two things: 1) That even if a reserve contains high amounts of valuable biomass, only some of it can be extracted (extraction can only be up to the reference point). And 2) that if the value of biomass within the reserve today is lower than the reference point, then no extraction can take place and the economic value of the reserve is zero (even if there is biomass within the reserve).
We can estimate the extractive value of biomass within the reserves via a simple difference-in-means estimation using a linear regression of the form:
Where represents the economic value of biomass in transect at time , is dummy variable that indicates whether an observation comes from the historical reference point (i.e.) or current value (i.e. ), and is an idiosyncratic error term. The interpretation of the coefficients is also convenient: is the mean value of biomass across all transects in the reference point, and the coefficient of interest, , captures the difference in mean value of biomass between the current value () and the reference point (). The null hypothesis is that there is no difference between the value of biomass in the reserves today, and the mean value of biomass in the reserves at the reference point (i.e.). We estimate and for each system of reserves using ordinary least squares with heteroskedastic-robust standard errors (White, 1980). All data were analyzed in R [R version 4.2.3; R Core Team (2023)] using RStudio (RStudio 2023.03.0; Build 386).
3 Results
We divide our results into three brief subsections and leave further interpretation of the results and extended lines of inference for the discussion section. We first report normalized and total costs of monitoring the reserves in each community. We then focus on the temporal patterns observed in fish and invertebrate value of biomass within reserve and control sites, followed by a description of current (2019) value and extractive value of biomass in the reserves. Finally, we turn to our main goal of comparing the costs of monitoring with the potential extractive value of biomass.
3.1 Costs of monitoring
The annual costs of monitoring the reserves studied here range from 23 MXN/ha to 3,561 MXN/ha, with a median value of 1,130 MXN/ha (Figure 4A). La Bocana had the highest per-unit-area costs because they have the smallest reserves (at just 59.76 ha), while Banco Chinchorro has the lowest per-unit-area costs because they have the largest reserve area (12,257 ha). In absolute terms, however, the annual median is of 212,854 MXN/ha (ranging from 95,500 to 458,474 MXN), with the highest value observed for El Rosario and the lowest value observed for Isla San Pedro Martir (Figure 4B).
Figure 4
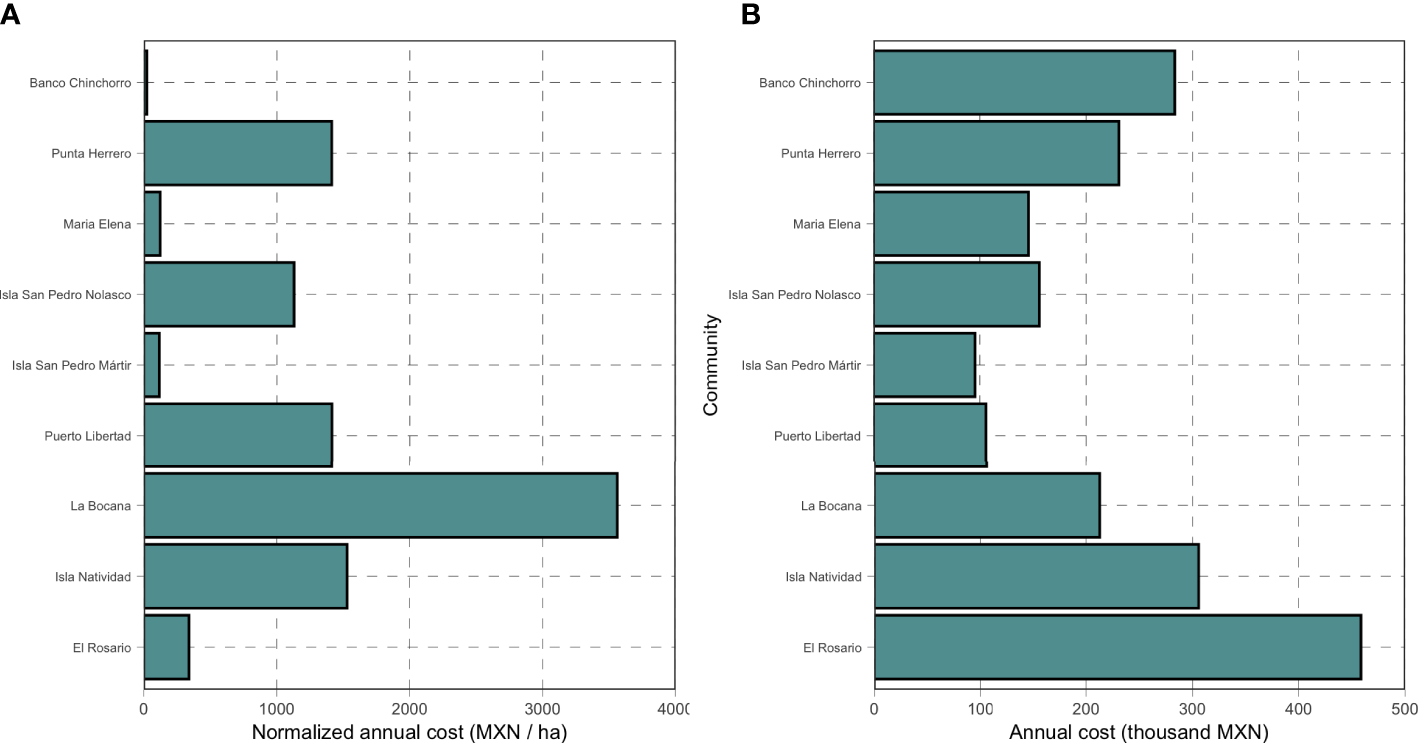
Monitoring costs for nine systems of community-based marine reserves in Mexico. Panel (A) shows the costs normalized by the total reserve area, while panel (B) shows the total annual costs.
3.2 Value of biomass
3.2.1 General temporal trends in valuable biomass
The first pattern worth noting is the temporal prevalence of the source of value for each reserve’s community (Figure 5). Fish biomass is consistently more valuable than invertebrate biomass in Banco Chinchorro, Isla San Pedro Martir, Isla San Pedro Nolasco, and Puerto Libertad. Conversely, invertebrate biomass contributes consistently more than fish biomass to the value of reserves in El Rosario and Isla Natividad. In Punta Herrero and María Elena, the values of fish and invertebrate biomass contribute similarly to reserve value. The second temporal pattern of interest is that the value of biomass does not strictly increase in time.
Figure 5
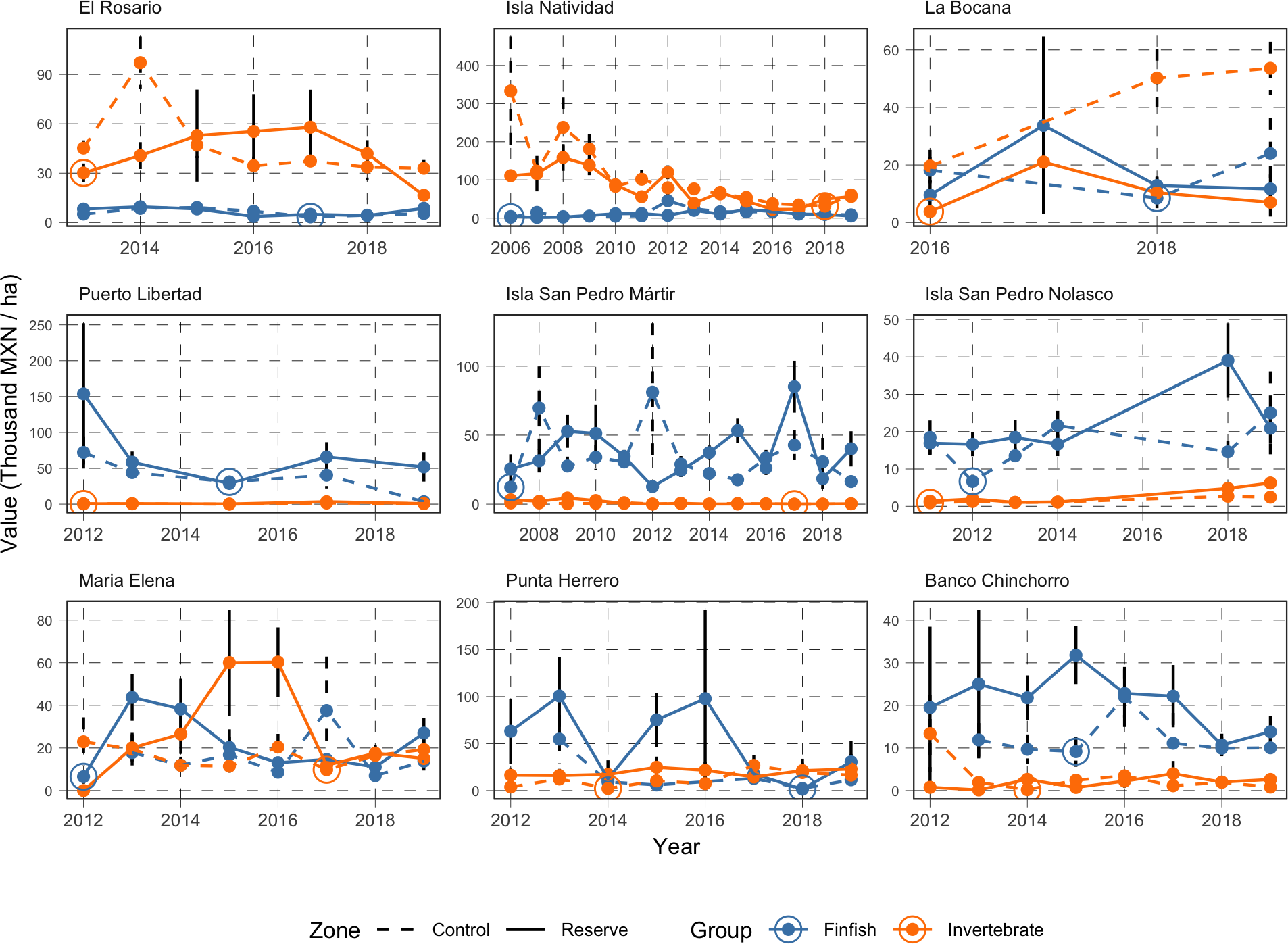
Time series of value of biomass contained in the reserve (solid line) and control sites (dashed line) for finfish (blue) and invertebrate (orange) species. The large circle markers indicate the reference value (historical low) used as benchmark when determining the potential extractive value of each reserve (See Table S3 for details).
3.2.2 Present-day (2019) value of biomass in reserves
All numeric results in this section are reported in thousands of Mexican pesos per hectare (thousand MXN/ha) and accompanied by the standard error around the point estimate. Monitoring data show that the reserves in Isla Natividad (56.3 ± 14.45; mean ± standard error), Punta Herrero (22.9 ± 15.4), and El Rosario (16.54 ± 2.8) have the most valuable mean invertebrate biomass (Figure 6A). On the other hand, the least valuable invertebrate biomass was observed for Isla San Pedro Martir (0.27 ± 0.09), Puerto Libertad (0.75 ± 0.3), and Banco Chinchorro (2.60 ± 1.3). However, when compared against the baseline values identified in the time series (Figure 5, See also Table S3), we find that the extractive value of invertebrate biomass is highest in Isla Natividad (24.9 ± 16.3), Punta Herrero (20.7 ± 15.9), and Isla San Pedro Nolasco (5.3 ± 1.6; Figure 6B). The extractive valuable of invertebrate biomass is lowest in El Rosario where the current (2019) value of biomass was at an all-time low (Figure 5 and Table 1). These patterns are largely driven by spiny lobster (Panulirus argus in the Caribbean and P. interruptus in the Pacific, Figure S2), which are the most abundant and second most valuable species (after abalone; Figure 3).
Figure 6
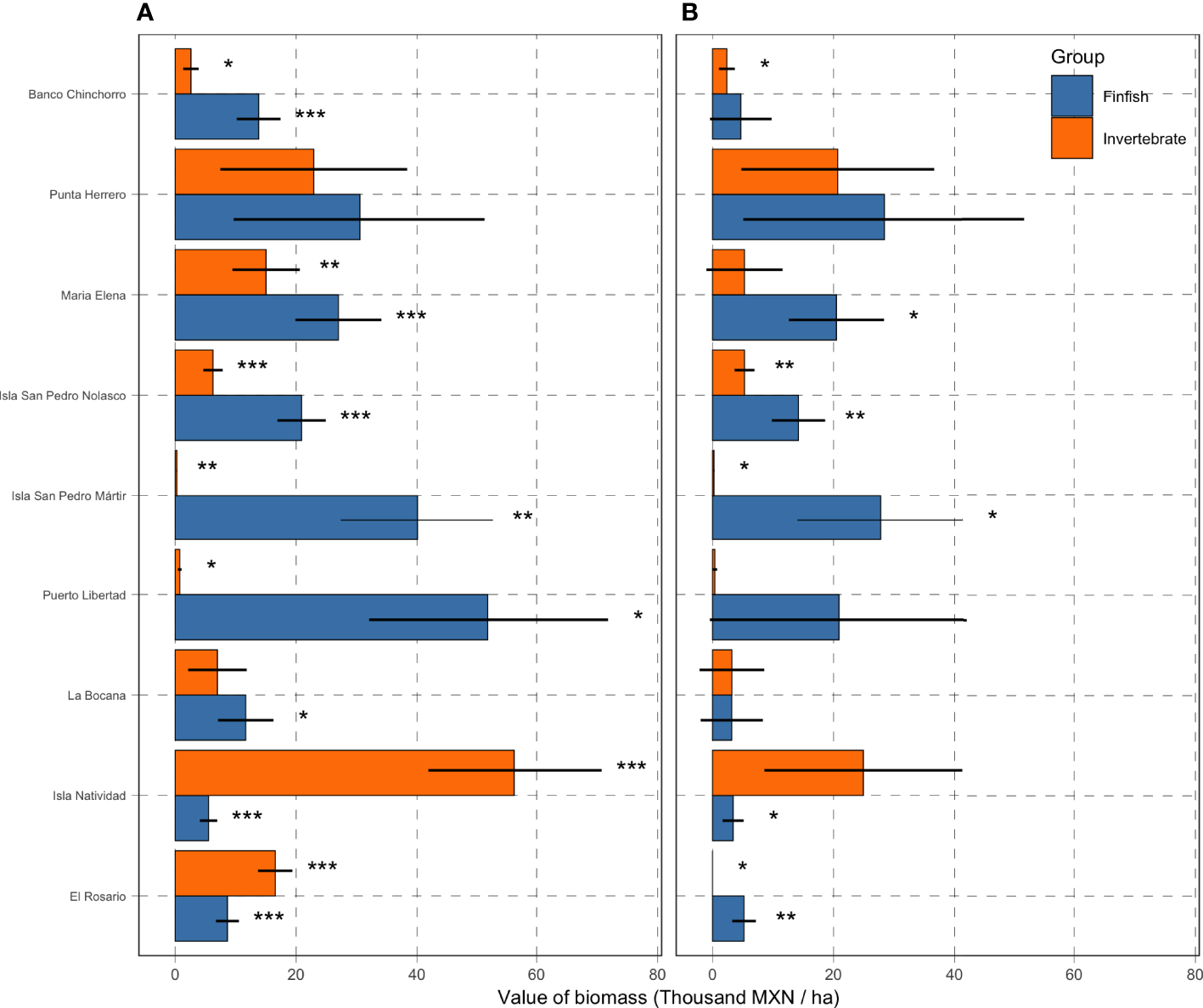
Value of biomass in thousands of MXN per hectare. Panel (A) shows the total value per hectare of reserve, and error bars show Standard Errors. Panel (B) shows the extractive value (i.e. the coefficient in Equation 2), calculated as the difference between values on the left and the reference point (Table S3). Asterisks indicate statistical significance (***: ; **: ; and *: ) on whether the coefficient is different from zero.
Table 1
| Community | Group | Total value | Historical min | Extractive value | Proportion |
|---|---|---|---|---|---|
| El Rosario | Finfish | 8.64 (+ 1.91)*** | 3.42 (+ 0.34)*** | 5.22 (+1.95)** | 60.44% |
| Invertebrate | 16.55 (+2.85)*** | 30.16 (+5.73)*** | 0.00% | ||
| Isla Natividad | Finfish | 5.53 (+ 1.44)*** | 2.12 (+ 0.94)* | 3.41 (+1.74)* | 61.71% |
| Invertebrate | 56.3 (+14.45)*** | 31.36 (+7.49)*** | 24.94 (+ 16.35) | 44.30% | |
| La Bocana | Finfish | 11.67 (+ 4.59)* | 8.47 (+2.06)*** | 3.2 (+5.14) | 27.38% |
| Invertebrate | 6.99 (+4.85) | 3.78 (+2.11)* | 3.21 (+5.36) | 45.98% | |
| Puerto Libertad | Finfish | 51.94 (+19.87)* | 31.03 (+7.25)*** | 20.91 (+21.36) | 40.27% |
| Invertebrate | 0.76 (+ 0.3)* | 0.36 (+0.23) | 0.4 (+0.38) | 52.50% | |
| Isla San Pedro Mártir | Finfish | 40.07 7 (+12.7)** | 12.28 (+4.97)* | 27.8 ( 13.77)* | 69.37% |
| Invertebrate | 0.28 (+0.1)** | 0.04 (+0.04) | 0.24 ( + 0.1)* | 85.71% | |
| Isla San Pedro Nolasco | Finfish | 20.9 (+ 4)*** | 6.68 (+ 1.75)*** | 14.22 (+4.4)** | 68.03% |
| Invertebrate | 6.26 (+ 1.61)*** | 0.96 (+0.38)* | 5.3 (+ 1.66)** | 84.71% | |
| Maria Elena | Finfish | 26.99 (+7.11)*** | 6.51 (+3.06)* | 20.49 (7.86)* | 75.89% |
| Invertebrate | 15.04 (+ 5.58)** | 9.75 (+ 2.81)** | 5.29 (+6.29) | 35.16% | |
| Punta Herrero | Finfish | 30.54 (+20.86) | 2.12 2 (+0.21)*** | 28.42 (+ 23.33) | 93.05% |
| Invertebrate | 22.92 (+15.46) | 2.21 (1.39) | 20.71 (+15.94) | 90.34% | |
| Banco Chinchorro | Finfish | 13.81 (+3.62)*** | 9.14 (+3.53)* | 4.67 (+5.09) | 33.83% |
| Invertebrate | 2.6 (+1.28)* | 0.22 (+0.22) | 2.38 (+1.32)* | 91.53% |
Value of biomass (thousand MXN/ha) for marine reserves in each community.
The columns with numeric values show the total value of biomass contained within the reserve, the historical minimum observed, and the extractive value (difference between total and historical). The last column shows the proportion of the total. Numbers in parentheses are robust standard errors, and asterisks indicate statistical significance (***: p < 0.001; **: p < 0.01; and *: p < 0.1).
The most valuable finfish biomass was observed in Puerto Libertad (51.9 ± 19.9), Isla San Pedro Mártir (40.1 ± 12.7), and Punta Herrero (30.5 ± 20.9). Conversely, Isla Natividad (5.53 ± 1.44), El Rosario (8.64 ± 1.91), and La Bocana (11.7 ± 4.59) had the least valuable finfish biomass. The three communities with the highest valued reserves also exhibit the highest extractive values: Punta Herrero (28.4 ± 23.3) and Isla San Pedro Mártir (27.8 ± 13.8), and Puerto Libertad (20.9 ± 21.4). The lowest value that would allow for some extraction of finfish was observed for La Bocana (3.2 ± 5.14), Isla Natividad (3.41 ± 1.74), and Banco Chinchorro (4.67 ± 5.09). A summary of current total value of biomass, reference point value of biomass, and extractive value of biomass are found in Table 1.
3.3 Costs and benefits of conservation
We find that the costs of monitoring the reserves represent 0.3-55.5% of the extractive value of the reserves, with a median value of 5.5% (Table 2). While Punta Herrero is the community with the most valuable reserves (at 49.12 thousand MXN/ha for finfish and invertebrates combined), it is only the fourth most expensive with costs 1,412 MXN/ha (Figure 4). In this case, the cost of monitoring the reserve is 2.88% of the extractive value of biomass contained in the reserves. El Rosario has the least valuable reserves (5.22 thousand MXN/ha), but the costs of monitoring are only 6.51% of this value (339.89 MXN/ha). The most expensive reserves to monitor are in La Bocana (3,561 MXN/ha), where total extractive value of biomass is one of the lowest (6.410 thousand MXN/ha), and thus costs are 55.57% of extractive value of the reserve. The lowest monitoring costs correspond to Banco Chinchorro (23 MXN/ha), and while this community also has the third lowest valuable reserves (7.06 thousand MXN/ha), costs represent 0.33% of the value of biomass.
Table 2
| Community | Extractive value (Thousand MXN / ha) | Monitoring costs (MXN / ha) | Costs as % of Value |
|---|---|---|---|
| El Rosario | 5.22 | 339.86 | 6.51% |
| Isla Natividad | 28.35 | 1529.47 | 5.39% |
| La Bocana | 6.41 | 3561.81 | 55.57% |
| Puerto Libertad | 21.31 | 1414.15 | 6.64% |
| Isla San Pedro Mártir | 28.03 | 116.43 | 0.42% |
| Isla San Pedro Nolasco | 19.52 | 1130.09 | 5.79% |
| Maria Elena | 25.78 | 122.54 | 0.48% |
| Punta Herrero | 49.12 | 1412.80 | 2.88% |
| Banco Chinchorro | 7.06 | 23.12 | 0.33% |
Extractive value (summing value of invertebrate and fish biomass) and monitoring costs for reserves in each community.
Note that value of biomass is presented in thousands of pesos per hectare, while monitoring costs is in pesos per hectare.
4 Discussion
We begin by providing further interpretation to our results and discussing them in the context of fisheries management and marine conservation in Mexico. We then discuss potential shortcomings in our analysis as it relates to our approach to estimating invertebrate biomass, our measure of extractive value of biomass, and the omission of ancillary economic benefits of marine reserves. We then end with suggestions for further directions in research.
4.1 Interpretation and contextualization of results
Our compilation of cost data shows that the median normalized monitoring cost for community-based MPAs is 1,130 MXN/ha (min: 23 MXN/ha; max: 3,561 MXN/ha), which is higher than what has been reported for customary top-down MPAs around the world, and much larger than the budget typically available for top-down MPAs in Mexico (Figure 7). For example, Balmford et al. (2004) reported a median value of annual recurrent expenses of 155 MXN/ha (they report 775 USD/km2) for a survey on 85 MPAs worldwide, while Hayashida et al. (2021) find that Mexican MPAs receive just 0.17 MXN/ha. If one considers community-based MPAs to receive an optimal amount of funding, one would conclude that many top-down MPAs worldwide –and particularly those in Mexico– remain underfunded (Gill et al., 2017). A counter argument may be that community-based MPAs are simply too costly. Regardless of how one perceives these costs, our analysis shows that the extractive value of biomass often makes up for the large costs. And, importantly, previous work has shown that the financial investment in these long-term monitoring programs has resulted in a series of co-benefits, from allowing fishers to record and understand environmental shocks and resource recovery (Micheli et al., 2012; Smith et al., 2022), to empowering community leaders and promoting social cohesion (Fulton et al., 2019a; Quintana et al., 2020; Quintana et al., 2021).
Figure 7
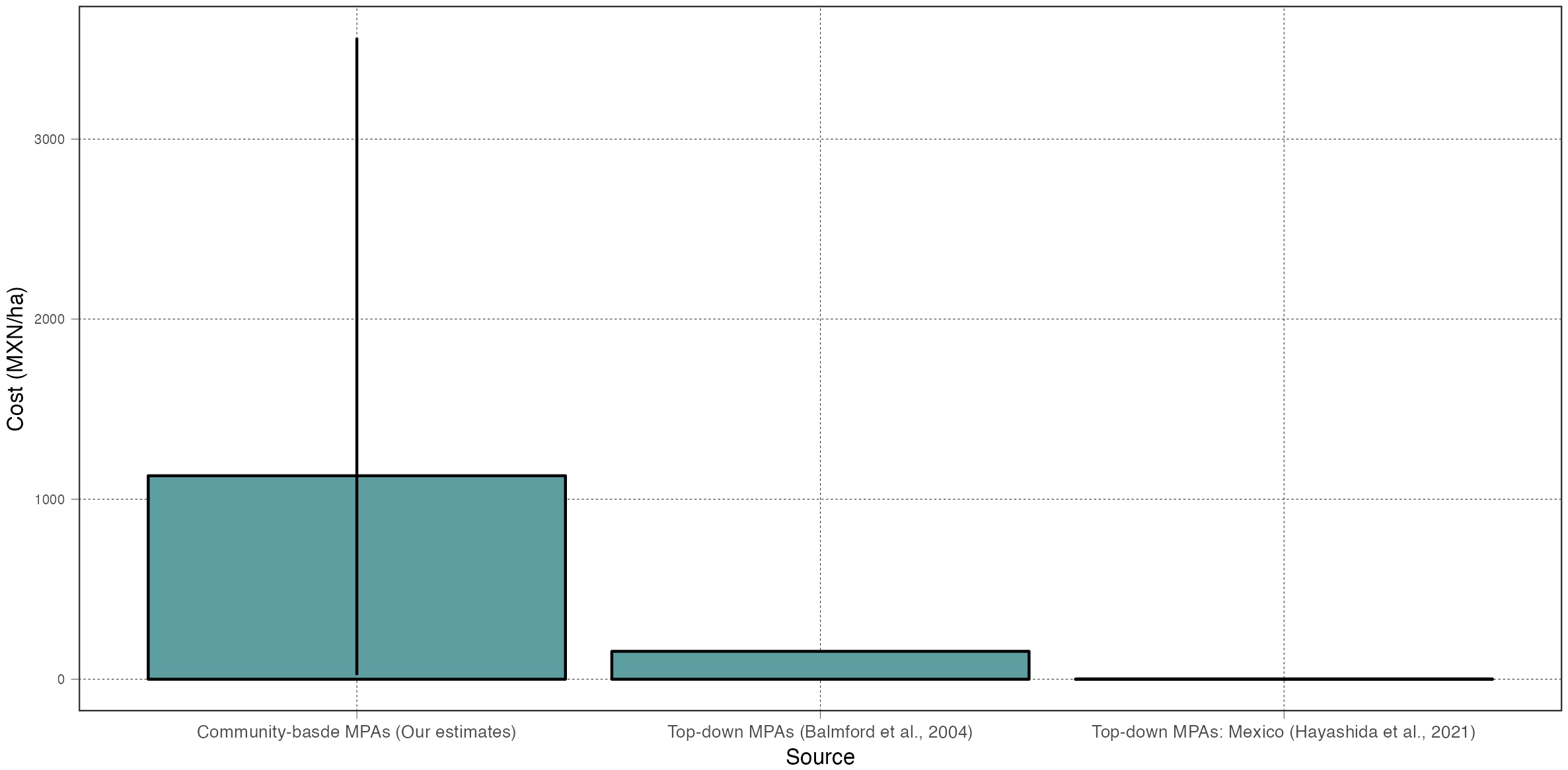
Comparison of per-hectare monitoring costs (MXN/ha). Each column shows the median estimate from our analysis (first column) or from two other relevant sources in the literature. The bars show the range (min, max).
Our analysis of value of biomass in reserves suggests that, generally, total and extractive value are correlated. However, it also highlights the large variability in the value of extractive biomass for finfish and invertebrate species, even within the same community. We can more easily visualize these idiosyncratic responses in Figure 8. While some communities have reserves that have high values of extractive biomass for both groups of species (e.g. Punta Herrero), the extractive value of reserves in most communities is unequally made up from either group. These heterogeneous responses are to be expected since these reserves were designed with the goal of bolstering the biomass of different species targeted by each community. For example, the value of extractive biomass of invertebrate species from reserves in the Gulf of California were relatively low, but these communities largely target finfish and bivalve species. Conversely, in the Pacific, the most important species are abalone (Halliotidae) and spiny lobster (Palinuridae), which make a large portion of the total extractive biomass in the reserves (Figure S2).
Figure 8
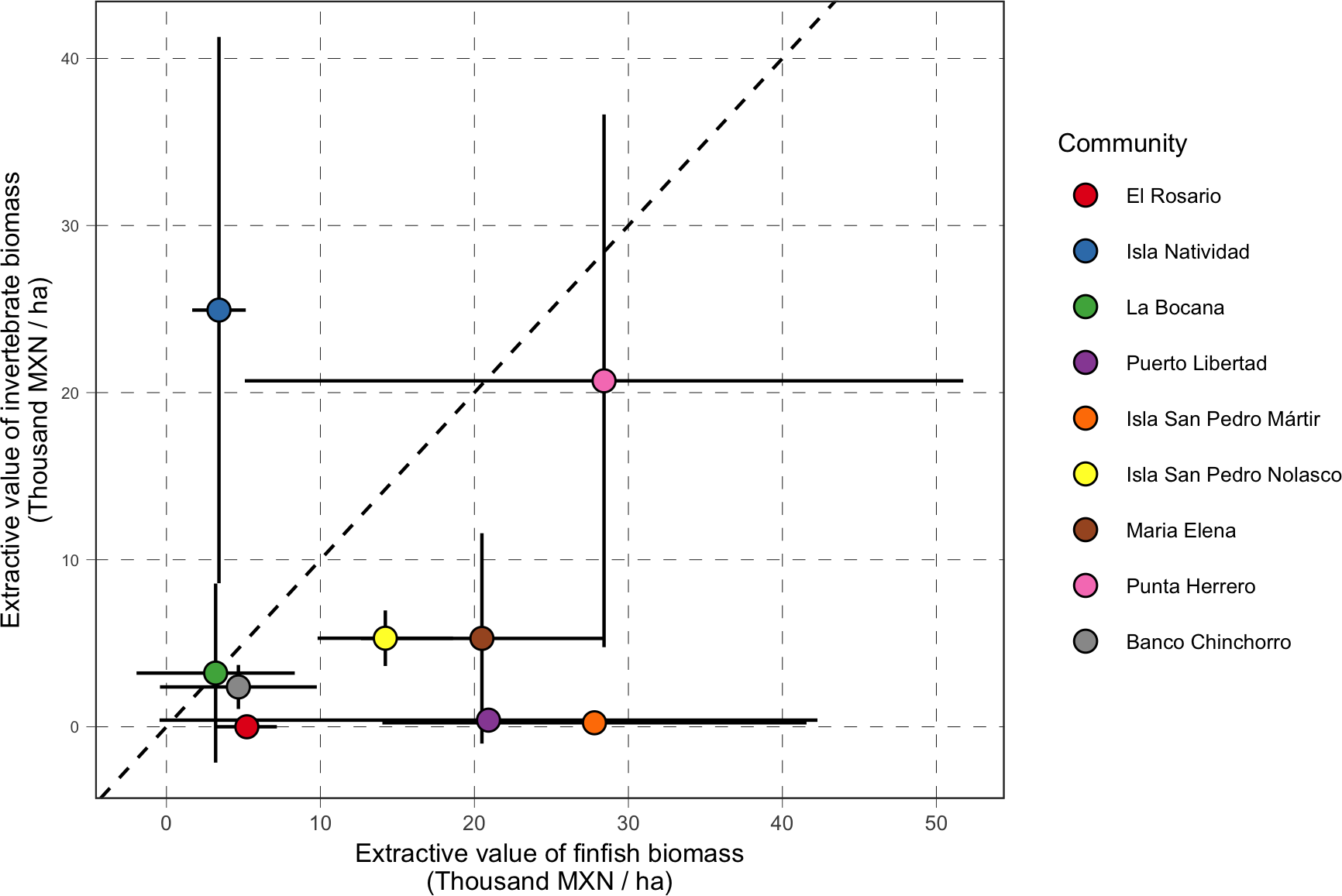
Comparison of extractive value of finfish biomass (x-axis; Thousand MXN/ha) and extractive value of invertebrate biomass (y-axis; Thousand MXN/ha). The dashed red line indicates a 1:1 line. Communities above that line are those with reserves where most of the value comes from invertebrate biomass, while communities below the line are those with reserves where the value comes from finfish biomass.
4.2 Potential shortcomings and recommendations
One of the main limitations of our study is that the standardized invertebrate surveys do not record the size of commercially-relevant organisms, which we attempt to mitigate by taking two steps. First, we assumed that the size of all organisms of each invertebrate species were as big as the minimum catch size. Using the minimum catch sizes assumes most organisms are smaller than they truly are (Figure S1). In our case, this produces a conservative (i.e. lower-bound) estimate of the total biomass, but we note that this may not always be the case elsewhere. And secondly, we kept our estimates of fish biomass separate from our estimates of invertebrate biomass to avoid confounding the total value of the reserves. We chose to still report the invertebrate data due to their importance for some of the communities, but highlight the potential sources of uncertainty to the reader. Going forward, we recommend that the monitoring protocols be modified in order to capture this crucial information.
Our definition of the reference points for the value of biomass assumes they are viable minimums that can be used as benchmarks to determine the value of the reserves. This is a critical assumption that shapes the main results of our study and therefore warrants some attention. We posit that the historical minimums can be used as baselines if they are economically and ecologically viable. We consider them to be economically viable because these are values that we have observed under fishing operations, and that values at or below the observed minima may not be profitable. If it were, standard economic theory predicts that fishers would have fished more and the observed values would have been even lower (Gordon, 1954; Costello et al., 2012). We also consider them to be ecologically viable because these communities operate under well-enforced TURF-managed or limited entry fisheries, which are known to foster higher biomass density of target species than areas operating under open access, and sometimes similar to fully protected no-take zones (Gelcich et al., 2008; Gelcich et al., 2015). In summary, if fishers are willing and able to harvest populations down to historically observed densities, these values are economically viable. And since TURFs are known to have ecologically viable biological densities, even the minimum values we observe are also ecologically viable. In some instances, an alternative may be to use the second lowest value as a conservative reference point (See Figure S5). We note that these assumptions may not hold in places operating under complete open access, severely overfished areas, when destructive or low-selectivity fishing methods are employed, or in particularly vulnerable ecosystems. Regardless of the chosen metric, future research attempting to use a similar approach should carefully scrutinize the data, question the validity of the assumptions, and incorporate best-available knowledge when identifying viable minimums.
Another methodological choice that warrants discussion is that of normalizing the costs of monitoring by reserve area. This is a common approach in the literature [e.g.Balmford et al. (2004); McCrea-Strub et al. (2011)], but fails to account for the fact that the programs should also monitor control sites. In absence of a polygon, a control site does not have an “area” assigned to them and it is difficult to incorporate their cost into our calculations. However, monitoring these control sites allows for robust before-after-control-impact evaluation of the reserves (Ferraro and Pattanayak, 2006; Villaseñor-Derbez et al., 2018; Kerr et al., 2019), and gives fishers the opportunity to monitor their fishing grounds. Thus, allowing some fishing within the reserves and using the proceeds to fund a monitoring program that surveys the reserves and control sites could ensure long-term sustainability of the reserves and the fishery as a whole (Millage et al., 2021; Bergseth et al., 2023).
4.3 Potential future directions
It is important to consider other ways of valuing the biomass contained in the reserves, for example, by valuing the economic benefit of any spillover of commercially important species. Such analyses have been undertaken in similar ecosystems, but for top-down MPAs [e.g.Goñi et al. (2008); Di Lorenzo and Mantua (2016); Lenihan et al. (2021)]. Future research could explore and quantify the spillover benefits (if any) provided by these reserves. Another way to assign a monetary value to the reserves could hinge on the non-extractive use of the biomass (Ninan, 2012). For example, one of the species found in Isla Natividad and El Rosario is the giant sea bass (Stereolepis gigas). The per-kilogram value of the species is 31.41 MXN/Kg (Tab S1), but Guerra et al. (2018) estimate the average value of S. gigas to recreational divers in the order of 46 million MXN per year (they report US$2.3 million). Finally, one might consider the non-use value of the reserves, which would refer to the intrinsic existence value of the biomass and biodiversity contained in them, or society’s willingness to pay to protect the reserve. For traditional top-down MPAs the link may be clear: funding comes from taxpayers’ money. But, under community-based marine reserves, fishers bear all the costs while providing a public benefit. Future research could explore mechanisms that would allow fishers to monetize and capture the public good that arises from their conservation interventions (Gelcich et al., 2019).
Our results suggest that allowing some level of biomass to be extracted from the reserves could help cover the costs of the monitoring programs in some communities. As an example, one of the communities included in our study (which has asked to remain anonymous) conducted limited extraction (three days of fishing) of one high-value species from one of their reserves to create liquidity during the COVID-19 pandemic. The reported earnings show that this limited extraction could have covered the biological monitoring of all the community’s reserves for nearly a decade (Hernández-Velasco et al., 2020). Extracting accumulated biomass to fund long-term monitoring of reserves is likely a controversial proposal, especially when accounting for the well-documented benefits of full-protection (Lester et al., 2009; Sala et al., 2018). However, one must consider that the true choice is not between a fully-protected area and a partial-take area, but between a self-financed partial-take area, an externally-funded no-take area, or no conservation at all. Evidence from rotational closures suggest that alternating between protection and harvest can have long-term benefits (Plagányi et al., 2015), but further research should focus on evaluating biomass before and after any extraction occurs and determine whether this can be sustained in the long-term.
5 Conclusion
Our analysis determined the costs and potential benefits of the extractive use value of the invertebrate and fish biomass contained in marine reserves from nine small-scale fishing communities in Mexico. We show that community-based marine reserves accumulate enough commercially-important biomass to allow for some limited extraction, and that the proceeds could help cover the costs of monitoring the reserves and control sites. The creation of a marine reserve monitoring fund operated by the fishing organization and periodically funded from proceeds of limited (and monitored) biomass extraction could be a viable option to ensure the long-term financial sustainability of community-based marine reserves.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/jcvdav/reserve_economic_value.
Author contributions
JV-D conceived the project, analyzed data, and wrote the first draft. SF secured funding, conceived the project, and edited the drafts. AH-V and IA-C collected the data, helped secure funding, and edited the drafts. All authors contributed to the article and approved the submitted version.
Funding
Authors of this paper were supported by the Walton Family Foundation (00104754), Summit Foundation (20220233), Packard Foundation (2021-73213), Sandler Family Foundation (20220429), Marisla Foundation (20220131), the National Science Foundation (2108566 and 2206739), and Oceans 5.
Acknowledgments
This study would have not been possible without the efforts of members of the fishing communities mentioned herein who have undertaken underwater monitoring in their marine reserves over multiple years. We are grateful for helpful feedback provided by two reviewers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1180920/full#supplementary-material
Footnotes
1.^Isla San Pedro Mártir was implemented by the government with significant input from fishers. However, the monitoring program is still led by the a group of community members (Fulton et al., 2019b).
2.^ i.e. we do not account for the value of escapement and subsequent somatic growth and reproduction.
References
1
Afflerbach J. C. Lester S. E. Dougherty D. T. Poon S. E. (2014). A global survey of “TURF-reserves”, territorial use rights for fisheries coupled with marine reserves. Global Ecol. Conserv.2, 97–106. doi: 10.1016/j.gecco.2014.08.001
2
Amador-Castro I. G. Melo F. J. F.-R. Torre J. (2021). Marine diversity in the biosphere reserve of the most oceanic island in the gulf of california: San pedro mártir. Zookeys1062, 177–201. doi: 10.3897/zookeys.1062.67964
3
Balmford A. Gravestock P. Hockley N. McClean C. J. Roberts C. M. (2004). The worldwide costs of marine protected areas. Proc. Natl. Acad. Sci. U. S. A.101, 9694–9697. doi: 10.1073/pnas.0403239101
4
Bergseth B. J. Arias A. Barnes M. L. Caldwell I. Datta A. Gelcich S. et al . (2023). Closing the compliance gap in marine protected areas with human behavioural sciences. Fish Fish24(4), 695–704. doi: 10.1111/faf.12749
5
Boettiger C. Temple Lang D. Wainwright P. (2012). rfishbase: exploring, manipulating and visualizing FishBase data from R. J. Fish Biol81(6), 2030–2039. doi: 10.1111/j.1095-8649.2012.03464.x
6
Briones-Fourzán P. Lozano-Álvarez E. (2000). “The spiny lobster fisheries in mexico,” in Spiny lobsters: fisheries and culture (Oxford, United Kingdom: Fishing News Books, Blackwell Science), 169–188.
7
Cabral R. B. Halpern B. S. Lester S. E. White C. Gaines S. D. Costello C. (2019). Designing MPAs for food security in open-access fisheries. Sci. Rep.9, 1–10. doi: 10.1038/s41598-019-44406-w
8
Costello C. Ovando D. Hilborn R. Gaines S. D. Deschenes O. Lester S. E. (2012). Status and solutions for the world’s unassessed fisheries. Science338, 517–520. doi: 10.1126/science.1223389
9
Di Lorenzo E. Mantua N. (2016). Multi-year persistence of the 2014/15 north pacific marine heatwave. Nat. Clim. Change6, 1042–1047. doi: 10.1038/nclimate3082
10
Ferraro P. J. Pattanayak S. K. (2006). Money for nothing? a call for empirical evaluation of biodiversity conservation investments. PloS Biol.4, e105. doi: 10.1371/journal.pbio.0040105
11
Froese R. Pauly D. Editors. (2010). FishBase. World Wide Web electronic publication. www.fishbase.org, ( 02/2023 ). https://www.fishbase.se/summary/citation.php
12
Fulton S. Caamal-Madrigal J. Aguilar-Perera A. Bourillón L. Heyman W. D. (2018). Marine conservation outcomes are more likely when fishers participate as citizen scientists: case studies from the mexican mesoamerican reef. Citizen Science: Theory Pract.3(1), 7, pp. 1–12. doi: 10.5334/cstp.118
13
Fulton S. Hernández-Velasco A. Suarez-Castillo A. Fernández-Rivera Melo F. Rojo M. Sáenz-Arroyo A. et al . (2019a). “From fishing fish to fishing data: The role of artisanal fishers in conservation and resource management in mexico,” in Viability and Sustainability of Small-Scale Fisheries in Latin America and The Caribbean. Eds. SalasS.Barragán-PaladinesM. J.ChuenpagdeeR. (Cham: Springer International Publishing), 151–175.
14
Fulton S. López-Sagástegui C. Weaver A. H. Fitzmaurice-Cahluni F. Galindo C. Fernandez-Rivera Melo F. et al . (2019b). Untapped potential of citizen science in mexican small-scale fisheries. Front. Mar. Sci.517. doi: 10.3389/fmars.2019.00517
15
Gelcich S. Cinner J. Donlan C. J. Tapia-Lewin S. Godoy N. Castilla J. C. (2017). Fishers’ perceptions on the chilean coastal TURF system after two decades: problems, benefits, and emerging needs. Bull. Mar. Sci.93, 53–67. doi: 10.5343/bms.2015.1082
16
Gelcich S. Godoy N. Prado L. Castilla J. C. (2008). Add-on conservation benefits of marine territorial user rights fishery policies in central chile. Ecol. Appl.18, 273–281. doi: 10.1890/06-1896.1
17
Gelcich S. Martínez-Harms M. J. Tapia-Lewin S. Vasquez-Lavin F. Ruano-Chamorro C. (2019). Comanagement of small-scale fisheries and ecosystem services. Conserv. Lett.12, e12637. doi: 10.1111/conl.12637
18
Gelcich S. Peralta L. Donlan C. J. Godoy N. Ortiz V. Tapia-Lewin S. et al . (2015). Alternative strategies for scaling up marine coastal biodiversity conservation in chile. Marit. Stud.14, 5. doi: 10.1186/s40152-015-0022-0
19
Gill D. A. Mascia M. B. Ahmadia G. N. Glew L. Lester S. E. Barnes M. et al . (2017). Capacity shortfalls hinder the performance of marine protected areas globally. Nature543, 665–669. doi: 10.1038/nature21708
20
Goñi R. Adlerstein S. Alvarez-Berastegui D. Forcada A. Reñones O. Criquet G. et al . (2008). Spillover from six western mediterranean marine protected areas: evidence from artisanal fisheries. Mar. Ecol. Prog. Ser.366, 159–174. doi: 10.3354/meps07532
21
Gordon H. S. (1954). The economic theory of a Common-Property resource: The fishery. J. Polit. Econ.62, 124–142. doi: 10.1086/257497
22
Guerra A. S. Madigan D. J. Love M. S. McCauley D. J. (2018). The worth of giants: The consumptive and non-consumptive use value of the giant sea bass (stereolepis gigas). Aquat. Conserv.28, 296–304. doi: 10.1002/aqc.2837
23
Hayashida H. Cisneros A. Palmeros M. (2021) esLas extensas áreas marinas “desprotegidas” de méxico. Available at: https://causanatura.org/wiki-cn/las-extensas-areas-marinas-desprotegidas-de-mexico (Accessed 2023-3-1).
24
Hernández-Velasco A. Flores A. Précoma de la Mora M. Romero A. (2020). Reporte: Pesca en una reserva marina como medida de adaptación ante cambios. (Guaymas, Sonora, Mexico: Comunidad y Biodiversidad).
25
Johannes R. E. (2002). The renaissance of community-based marine resource management in oceania. Annu. Rev. Ecol. Syst.33, 317–340. doi: 10.1146/annurev.ecolsys.33.010802.150524
26
Johnson D. N. van Riper C. J. Chu M. Winkler-Schor S. (2019). Comparing the social values of ecosystem services in US and australian marine protected areas. Ecosystem Serv.37, 100919. doi: 10.1016/j.ecoser.2019.100919
27
Kerr L. A. Kritzer J. P. Cadrin S. X. (2019). Strengths and limitations of before–after–control–impact analysis for testing the effects of marine protected areas on managed populations. ICES Journal of Marine Science76 (4), 1039–1051. doi: 10.1093/icesjms/fsz014
28
Leenhardt P. Low N. Pascal N. Micheli F. Claudet J. (2015). “Chapter 9 - the role of marine protected areas in providing ecosystem services,” in Aquatic Functional Biodiversity. Eds. BelgranoA.WoodwardG.JacobU. (San Diego: Academic Press), 211–239.
29
Lenihan H. S. Gallagher J. P. Peters J. R. Stier A. C. Hofmeister J. K. K. Reed D. C. (2021). Evidence that spillover from marine protected areas benefits the spiny lobster (panulirus interruptus) fishery in southern california. Sci. Rep.11, 2663. doi: 10.1038/s41598-021-82371-5
30
Lester S. E. Halpern B. S. Grorud-Colvert K. Lubchenco J. Ruttenberg B. I. Gaines S. D. et al . (2009). Biological effects within no-take marine reserves: a global synthesis. Mar. Ecol. Prog. Ser.384, 33–46. doi: 10.3354/meps08029
31
Mallin M.-A. F. Stolz D. C. Thompson B. S. Barbesgaard M. (2019). In oceans we trust: Conservation, philanthropy, and the political economy of the phoenix islands protected area. Mar. Policy107, 103421. doi: 10.1016/j.marpol.2019.01.010
32
McCay B. J. (2017). Territorial use rights in fisheries of the northern pacific coast of mexico. Bull. Mar. Sci.93, 69–81. doi: 10.5343/bms.2015.1091
33
McCay B. J. Micheli F. Ponce-Díaz G. Murray G. Shester G. Ramirez-Sanchez S. et al . (2014). Cooperatives, concessions, and co-management on the pacific coast of mexico. Mar. Policy44, 49–59. doi: 10.1016/j.marpol.2013.08.001
34
McCrea-Strub A. Zeller D. Rashid Sumaila U. Nelson J. Balmford A. Pauly D. (2011). Understanding the cost of establishing marine protected areas. Mar. Policy35, 1–9. doi: 10.1016/j.marpol.2010.07.001
35
Medoff S. Lynham J. Raynor J. (2022). Spillover benefits from the world’s largest fully protected MPA. Science378, 313–316. doi: 10.1126/science.abn0098
36
Melnychuk M. C. Clavelle T. Owashi B. Strauss K. (2017). Reconstruction of global ex-vessel prices of fished species. ICES J. Mar. Sci.74, 121–133. doi: 10.1093/icesjms/fsw169
37
Méndez-Medina C. Schmook B. McCandless S. R. (2015). The punta allen cooperative as an emblematic example of a sustainable small-scale fishery in the mexican caribbean. Marit. Stud.14, 12. doi: 10.1186/s40152-015-0026-9
38
Micheli F. De Leo G. Butner C. Martone R. G. Shester G. (2014). A risk-based framework for assessing the cumulative impact of multiple fisheries. Biol. Conserv.176, 224–235. doi: 10.1016/j.biocon.2014.05.031
39
Micheli F. Halpern B. S. Botsford L. W. Warner R. R. (2004). Trajectories and correlates of community change in no-take marine reserves. Ecol. Appl.14, 1709–1723. doi: 10.1890/03-5260
40
Micheli F. Saenz-Arroyo A. Greenley A. Vázquez L. Espinoza Montes J. A. Rossetto M. et al . (2012). Evidence that marine reserves enhance resilience to climatic impacts. PloS One7, e40832. doi: 10.1371/journal.pone.0040832
41
Millage K. D. Villaseñor-Derbez J. C. Bradley D. Burgess M. G. Lenihan H. S. Costello C. (2021). Self-financed marine protected areas. Environmental Research Letters16(12), 125001. doi: 10.1088/1748-9326/ac3439
42
Miller D. L. (1982). “Construction of shallow water habitat to increase lobster production in mexico,” in Gulf and Caribbean Fisheries Institute, vol. 34, pp. 168–79.
43
Moland E. Olsen E. M. Knutsen H. Garrigou P. Espeland S. H. Kleiven A. R. et al . (2013). Lobster and cod benefit from small-scale northern marine protected areas: inference from an empirical before–after control-impact study. Proc. R. Soc. B: Biol. Sci.280, 20122679. doi: 10.1098/rspb.2012.2679
44
Munguía-Vega A. Sáenz-Arroyo A. Greenley A. P. Espinoza-Montes J. A. Palumbi S. R. Rossetto M. et al . (2015). Marine reserves help preserve genetic diversity after impacts derived from climate variability: Lessons from the pink abalone in baja california. Global Ecol. Conserv.4, 264–276. doi: 10.1016/j.gecco.2015.07.005
45
Ninan K. N. (2012). The economics of biodiversity conservation: valuation in tropical forest ecosystems (New York, USA: Routledge).
46
OECD (2023) enPrices - inflation (CPI) - OECD data. Available at: https://data.oecd.org/price/inflation-cpi.htm (Accessed 2023-2-18).
47
Ovando D. Dougherty D. Wilson J. R. (2016). Market and design solutions to the short-term economic impacts of marine reserves. Fish Fish. 17(4), 939–954. doi: 10.1111/faf.12153
48
Plagányi É.E. Skewes T. Murphy N. Pascual R. Fischer M. (2015). Crop rotations in the sea: Increasing returns and reducing risk of collapse in sea cucumber fisheries. Proc. Natl. Acad. Sci. U. S. A.112, 6760–6765. doi: 10.1073/pnas.1406689112
49
Potts T. Burdon D. Jackson E. Atkins J. Saunders J. Hastings E. et al . (2014). Do marine protected areas deliver flows of ecosystem services to support human welfare? Mar. Policy44, 139–148. doi: 10.1016/j.marpol.2013.08.011
50
Quintana A. C. E. Basurto X. (2021). Community-based conservation strategies to end open access: The case of fish refuges in mexico. Conserv. Sci. Pract.3(1), e283. doi: 10.1111/csp2.283
51
Quintana A. Basurto X. Rodriguez Van Dyck S. Weaver A. H. (2020). Political making of more-than-fishers through their involvement in ecological monitoring of protected areas. Biodivers. Conserv.29, 3899–3923. doi: 10.1007/s10531-020-02055-w
52
Quintana A. C. E. Giron-Nava A. Urmy S. Cramer A. N. Domínguez-Sánchez S. Dyck R.-V. et al . (2021). Positive social-ecological feedbacks in community-based conservation. Front. Mar. Sci.8, 428. doi: 10.3389/fmars.2021.652318
53
Ramutsindela M. Spierenburg M. Wels H. (2013). Sponsoring nature: Environmental philanthropy for conservation (London: Routledge).
54
R Core Team (2023). R: A language and environment for statistical computing. (Vienna, Austria: R Foundation for Statistical Computing)
55
Reddy S. M. W. Wentz A. Aburto-Oropeza O. Maxey M. Nagavarapu S. Leslie H. M. (2013). Evidence of market-driven size-selective fishing and the mediating effects of biological and institutional factors. Ecol. Appl.23, 726–741. doi: 10.1890/12-1196.1
56
Rees S. E. Ashley M. Evans L. Mangi S. Sheehan E. V. Mullier T. et al . (2021). An evaluation of the social and economic impact of a marine protected area on commercial fisheries. Fish. Res.235, 105819. doi: 10.1016/j.fishres.2020.105819
57
Rees S. E. Attrill M. J. Austen M. C. Mangi S. C. Rodwell L. D. (2013). enA thematic cost-benefit analysis of a marine protected area. J. Environ. Manage.114, 476–485. doi: 10.1016/j.jenvman.2012.10.048
58
Sala E. Giakoumi S. (2017). No-take marine reserves are the most effective protected areas in the ocean. ICES J. Mar. Sci.75, 1166–1168. doi: 10.1093/icesjms/fsx059
59
Sala E. Lubchenco J. Grorud-Colvert K. Novelli C. Roberts C. Sumaila U. R. (2018). Assessing real progress towards effective ocean protection. Mar. Policy91, 11–13. doi: 10.1016/j.marpol.2018.02.004
60
Smith A. Aguilar J. D. Boch C. De Leo G. Hernández-Velasco A. Houck S. et al . (2022). Rapid recovery of depleted abalone in Isla Natividad, Baja California, Mexico. Ecosphere13(3), e4002. doi: 10.1002/ecs2.4002
61
Smith M. D. Lynham J. Sanchirico J. N. Wilson J. A. (2010). Political economy of marine reserves: Understanding the role of opportunity costs. Proc. Natl. Acad. Sci.107, 18300–18305. doi: 10.1073/pnas.0907365107
62
Suman C. S. Saenz-Arroyo A. Dawson C. Luna M. C. (2010). Manual de Instrucción de Reef Check California: Guia de instrucción para el monitoreo del bosque de sargazo en la Peninsula de Baja California (Pacific Palisades, CA, USA: Reef Check Foundation).
63
Villaseñor-Derbez J. C. Aceves-Bueno E. Fulton S. Suarez A. Hernández-Velasco A. Torre J. et al . (2019). An interdisciplinary evaluation of community-based TURF-reserves. PloS One14, e0221660. doi: 10.1371/journal.pone.0221660
64
Villaseñor-Derbez J. C. Amador-Castro I. G. Hernández-Velasco A. Torre J. Fulton S. (2022). Two decades of Community-Based marine conservation provide the foundations for future action. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.893104
65
Villaseñor-Derbez J. C. Faro C. Wright M. Martínez J. Fitzgerald S. Fulton S. et al . (2018). A user-friendly tool to evaluate the effectiveness of no-take marine reserves. PloS One13, e0191821. doi: 10.1371/journal.pone.0191821
66
White H. (1980). A Heteroskedasticity-Consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica48, 817–838. doi: 10.2307/1912934
67
White A. T. (1989). “Two community-based marine reserves: lessons for coastal management,” in Coastal area management in Southeast Asia: Policies, management strategies and case studies (Society in New York), vol. 138. Available at: https://www.jstor.org/publisher/econosoc.
Summary
Keywords
bottom-up conservation, small-scale fisheries, conservation financing, marine protected areas, sustainable development goals
Citation
Villaseñor-Derbez JC, Fulton S, Hernández-Velasco A and Amador-Castro IG (2023) Biomass accrual benefits of community-based marine protected areas outweigh their operational costs. Front. Mar. Sci. 10:1180920. doi: 10.3389/fmars.2023.1180920
Received
06 March 2023
Accepted
22 June 2023
Published
18 August 2023
Volume
10 - 2023
Edited by
Pablo Pita, University of Santiago de Compostela, Spain
Reviewed by
Stephen Mangi, MRAG Ltd., United Kingdom; Natali Lazzari, University of Barcelona, Spain
Updates
Copyright
© 2023 Villaseñor-Derbez, Fulton, Hernández-Velasco and Amador-Castro.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Carlos Villaseñor-Derbez, juancvd@stanford.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.