Abstract
The scalloped hammerhead shark (Sphyrna lewini), a critically endangered species with a decreasing global population, is characterised by its occurrence in large schools. Such schools are still observed today in the Pacific Ocean, but this is generally not the case in the Atlantic Ocean, and in the Cayman Islands not since the 1970s. Here we report a recent record of a school of S. lewini in deep water off Grand Cayman, and describe a recent, concomitant increase in numbers of the species, and its critically endangered congener, the great hammerhead (Sphyrna mokarran), around the Cayman Islands. Relative population trends and seasonal patterns were assessed using data from shallow and deep-water BRUVS, scientific longlining, citizen science projects including the Sharklogger Network and REEF, and social media reports. It appears that S. lewini may be slowly re-occupying the area, selecting and using deeper waters to school, while S. mokarran has also become less scarce than hitherto.
1 Introduction
Large hammerhead sharks are among the most critically endangered shark species globally (Rigby et al., 2019a, b). The scalloped hammerhead shark, Sphyrna lewini, has a maximum size of 370–420 cm total length (TL) (Ebert et al., 2013); females mature at 200–250 cm TL and males at 180–200 cm TL (Branstetter, 1987; Hazin et al., 2001). It is considered a coastal and semi-oceanic pelagic species (Moore and Gates, 2015). The species is currently classed in the IUCN Red List as critically endangered (CR A2bd), since the global population has undergone a steep decline, likely by >80%, and is severely fragmented (Rigby et al., 2019a). The decline is principally the consequence of being caught globally as both target and bycatch in pelagic commercial and small-scale longline, purse seine, and gillnet fisheries, in which it may be retained for both meat and fins (Rigby et al., 2019a). In the Northwest Atlantic and Gulf of Mexico S. lewini appears to have been overfished between 1983 and 2005, and in particular between 1983 and 1995 (Jiao et al., 2011). Since then, the population is showing signs of an increase in this area (Rigby et al., 2019a).
No stock assessment has been undertaken specifically for the Caribbean. However, in the western Atlantic Ocean, Chapman et al. (2009) found that breeding females remain close to or return to their natal area for parturition. Also from genetic work, Pinhal et al. (2020) suggested that population subdivision of S. lewini within the western Atlantic was a product of reproductive philopatry, rather than related to oceanographic or geophysical barriers. Alarmingly, they also estimated an effective population size of only 299 (215-412 CI) for this region and suggested the population’s low genetic diversity may be partly related to the sharks’ philopatric behaviour, in addition to overfishing (Pinhal et al., 2020).
The great hammerhead shark, Sphyrna mokarran, is larger than S. lewini, reaching 610 cm TL, and is also considered a coastal and semi-oceanic pelagic species (Rigby et al., 2019b). It is thought to mature at 224 cm for females and 187 cm for males (Piercy et al., 2010). It occurs globally in tropical and warm temperate seas to depths of 300 m and is currently classed as critically endangered (CR A2bd), having experienced steep population decline, again most likely by >80% through most of its range (Rigby et al., 2019b). Like S. lewini it has been caught both as target and bycatch in coastal and pelagic fisheries, but is more often retained for its fins, which are larger than those of S. lewini (Rigby et al., 2019b). This species appears more sensitive to the stress of capture with a high post-release mortality rate (Gallagher et al., 2014a).
While S. mokarran is almost always encountered as solitary individuals (Miller et al., 2014), S. lewini can be encountered as solitary individuals, in pairs or in schools (Miller et al., 2013). S. lewini is also characterised by occurring in large aggregations (Harned et al., 2022), particularly at oceanic seamounts (Klimley, 1993). Large aggregations continue to be documented in the Pacific Ocean (Ketchum et al., 2014; Aldana-Moreno et al., 2019; Bravo-Ormaza et al., 2023), including in shallow waters (Lòpez et al., 2022) and near known nursery areas (Brown et al., 2016). In the Atlantic, however, there have been no recent records of aggregations except for an oblique reference to an aggregation in the Gulf of Mexico by Hoffmayer et al. (2013). Notably, there have been no recent sightings of schools of S. lewini in the Cayman Islands (western Caribbean) where until the 1970s large schools were regularly encountered by scuba divers at particular sites (MG, pers. comm.).
Given the recent paucity of records of schools of S. lewini or of S. mokarran in the Atlantic Ocean, we took advantage of a recent empirical video survey recording of a school of S. lewini off Grand Cayman made during our ongoing monitoring of sharks in the Cayman Islands. We combined this with other sources of data, including ongoing monitoring of sharks using BRUVS and citizen science reporting (Gore et al., 2020; Kohler, 2022), to examine whether in recent years there has been any evidence of a recovery in numbers of either species. This question was pertinent because, following detailed studies of the more common shark species in the Cayman Islands since 2009 (Ormond et al., 2017; Kohler, 2022), in 2015 all elasmobranchs were given full protection throughout Cayman waters [National Conservation Act, 2013], effectively establishing Cayman Islands territorial waters as a shark sanctuary. In this context marine environment managers were concerned to establish whether species were benefitting from this measure.
2 Materials and methods
2.1 Study area
The study area covered the three Cayman Islands - Grand Cayman (19.344°N, 81.252°W), Little Cayman (19.688°N, 80.044°W) and Cayman Brac (19.721°N, 79.796°W) - which are located on the Cayman Ridge in the centre of the western Caribbean Sea, only 37km to the north of the 7km deep Cayman Trough (Figure 1). For further description of the reefs and coastal zone see Ormond et al. (2017). The isolated location of the islands within the Caribbean and the close proximity and thus connectivity of shallow and very deep coastal waters, provides an unusual habitat for large marine vertebrates (see Gore et al., 2020).
Figure 1
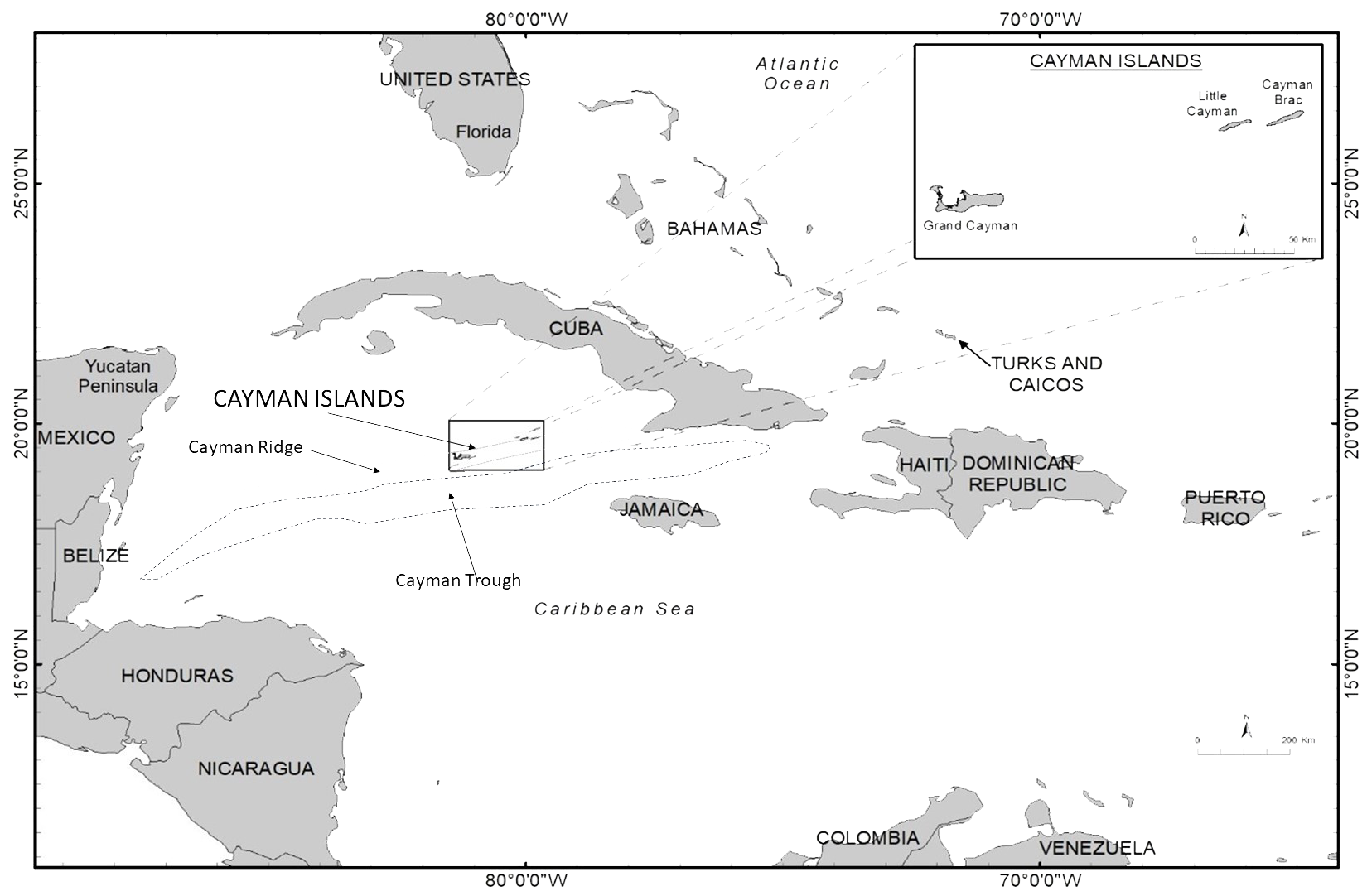
Map showing the location of the Cayman Islands, Cayman Trough and Cayman Ridge within the wider Caribbean region. The white rectangle shows the position of the Cayman Islands, with the inset showing the relative positions of Grand Cayman and the Sister Islands (Little Cayman and Cayman Brac). Main map: reproduced with permission from Esri, USGS | Esri, TomTom, FAO, NOAA, USGS | Sources: Esri; Garmin International, Inc.; U.S. Central Intelligence Agency (The World Factbook); National Geographic Society. Map of the Cayman Islands: reproduced with permission from Department of Environment, Cayman Islands Government.
2.2 Baited remote underwater video system
Between 05 Nov 2009 and 23 Nov 2018, 1473 shallow-water BRUVS were deployed at a series of standard stations approx. 0.5 - 2km apart and up to 20m deep on reefs around large parts of all three islands, though most regularly around Grand Cayman and Little Cayman (see Kohler, 2022). The earliest surveys employed Sony Handycam video cameras enclosed in waterproof housings attached to a heavy metal frame that also carried a bait arm to the end of which was attached a mesh bag containing bait. The bait bag was also used to estimate total length of individuals, which was recorded along with the species and sex. Ebert et al. (2013) was used to determine maturity for all measurements used in this study. When GoPro cameras (gopro.com; Hero3 and Hero4) became available these replaced the Handycams and were set to run for 2 hours, while a lighter weight frame replaced the previous heavy one (see Gore et al., 2020).
In addition, between 08 Mar 2022 and 17 May 2023, as part of a deep-water survey of shark and fish biodiversity, 154 deep-water BRUVS were deployed to record species occurrence and abundance at depths of 50, 100 and 200m around the north-west, west and south-west of both Grand Cayman and Little Cayman. These BRUVS used GoPro Hero4 cameras placed inside GroupB housings (www.GroupBinc.com) attached to a lightweight frame to which a bait arm with bait-bag was also fixed. As with the shallow deployments, cameras for the deep-water surveys were set to run for 2 hours. Following retrieval for both sets of BRUVS, the video recordings were annotated by a first reviewer, with at least 25% of recordings being re-examined by a second reviewer. Identification of hammerhead species was independently checked by at least three authors.
2.3 Scientific longlining
Scientific longlines, consisting of 30 baited circle hooks hung 2m below the surface from a 500m long buoyed line were deployed on 275 occasions at suitable sites around the whole of both Grand and Little Cayman, to catch sharks for scientific tagging. The line was monitored every 30min and any fish caught removed promptly. Sharks were quickly identified and measured and any Sphyrna sp. quickly released without tagging (see Ormond et al., 2017).
2.4 Citizen-science
Data from two citizen-science programmes, that run by the Reef Environmental Education Foundation (REEF, a recreational diver-observation programme, www.reef.org/programs/volunteer-fish-survey-project) and the Cayman-based Sharklogger Network (a local observer programme, see Kohler, 2022) were examined for any records of Sphyrna species between 01 Jan 1993 and 31 Mar 2023 (REEF) and 01 Jan 2017 to 31 Dec 2018 (Sharklogger) for each of the three Cayman Islands. The 365 official recreational dive sites used by the citizen science reporters are distributed around all three islands, their locations being shown in Figure 4.1 in Kohler (2022). The numbers of both Sphyrna species observed were extracted and the number of hours of survey also recorded to provide a measure of the effort involved. Observers in both programmes are trained to identify and distinguish between species and sex and to estimate total length.
2.5 Social media and government database
Data from our project’s Facebook site “Sharks and Cetaceans: the Cayman Islands” and from the Cayman Islands Department of Environment’s (DoE) Sightings Reporting Scheme for large marine vertebrates were reviewed. Data were also gleaned from our “#SpotThatFish” where photos of fish, including sharks, could be uploaded by contributing divers and photographers. Such social media tools not only assist researchers by extending their data gathering, but as we have experienced, also provide an opportunity to raise awareness of related conservation issues with the interested public. Duplicate sightings from the same area or dive and the same time of day were discarded. Since data collected through citizen science does rely on the skill of the observer, a “certainty index” was employed to categorise the reliability of a report, ranging from 1 (very unlikely) to 5 (very likely). Usually the Sphyrna genus is easily recognised as such given their characteristic cephalofoils, however the species can be more difficult to distinguish without relevant experience or observational skills. No effort data were reported by members of the public posting on these social media sites, the observations being largely opportunistic.
3 Results
3.1 Baited remote underwater video systems
Data from the shallow-water BRUVS surveys undertaken between 05 Nov 2009 and 23 Nov 2018 off Grand and Little Cayman, representing 2164.5h of seabed time, included a total of eight S. mokarran but no S. lewini (Table 1). Observations of S. lewini on deep-water BRUVS were made on 6 occasions, with a total of 18 individuals recorded. Of these, one was of a group of at least 11 S. lewini swimming at a depth of >200m adjacent to a vertical wall off North Sound, Grand Cayman on 26 Mar 2022. While individual’s total lengths were difficult to estimate, all were identified as juveniles. These individuals swam close together, moving horizontally across the face of the wall. No other fish were observed while these sharks were visible. There was also one S. mokarran recorded on a deep-water BRUVS on a separate occasion.
Table 1
| Method | Period Start & End | Surveys | Hours | Numbers & Events Recorded | ||
|---|---|---|---|---|---|---|
| S. lewini Number Event |
S. mokarran Number Event |
Sphyrna sp. Number Event |
||||
| /Reports | ||||||
| Shallow-water BRUVS | 05/Nov/2009 | 1,473 | 2,164.5 | 0 | 8 | 0 |
| 23/Nov/2018 | 0 | 8 | 0 | |||
| Deep-water BRUVS | 08/Mar/2022 | 154 | 467 | 27 | 3 | 0 |
| 17/May/2023 | 13 | 13 | 0 | |||
| Scientific longlines | 05/Nov/2009 | 275 | 875 | 0 | 2 | 0 |
| 12/Dec/2016 | 0 | 2 | 0 | |||
| REEF Reports | 01/Jan/1993 | 10,807 | 11,376 | 2 | 4 | 0 |
| 31/Mar/2023 | 2 | 4 | 0 | |||
| Social Media | 29/Jun/1996 | 364 | not available | 9 | 52 | 20 |
| 12/Mar/2023 | 9 | 53 | 20 | |||
| Sharklogger Network | 01/Jan/2017 | 24,442 | 20,536 | 0 | 0 | 103 |
| 31/Dec/2018 | 0 | 0 | 103 | |||
Hammerhead sharks recorded within the various datasets from the Cayman Islands.
The table shows the start (top row) and end (bottom row) dates of each survey period, the number of surveys, the total observation times in hours, and the numbers of individual sharks (top of number pair) and events (bottom of number pair) for each of the datasets: shallow and deep-water BRUVS, scientific longlines, citizen science reports (REEF, 2023), social media (Facebook and DOE sites) and Sharklogger Network reports.
3.2 Scientific longlines
Scientific longline surveys (Table 1) were undertaken between 05 Nov 2009 and 12 Dec 2016 off Grand and Little Cayman, with a total soak time of 875h, resulting in two S. mokarran briefly captured, measured and released (in addition to sharks from other genera).
3.3 Citizen science
Data from REEF (Table 1) indicated that in 10,807 surveys over 11,376 hours were conducted in the three Cayman Islands between 1993 and the end of March 2023 (29 years), resulting in two S. lewini and four S. mokarran sightings (Figure 2). The local Sharklogger Network recorded observation of Sphyrna sp. in all three Cayman Islands in 2017 and 2018 (Kohler, 2022). They were observed largely below 40m off the coastal wall and were relatively abundant. Analyses showed that divers reported seeing 0.004 sharks per dive, with 66% of the individuals observed considered mature (n=24,442 dives) (Table 1).
Figure 2
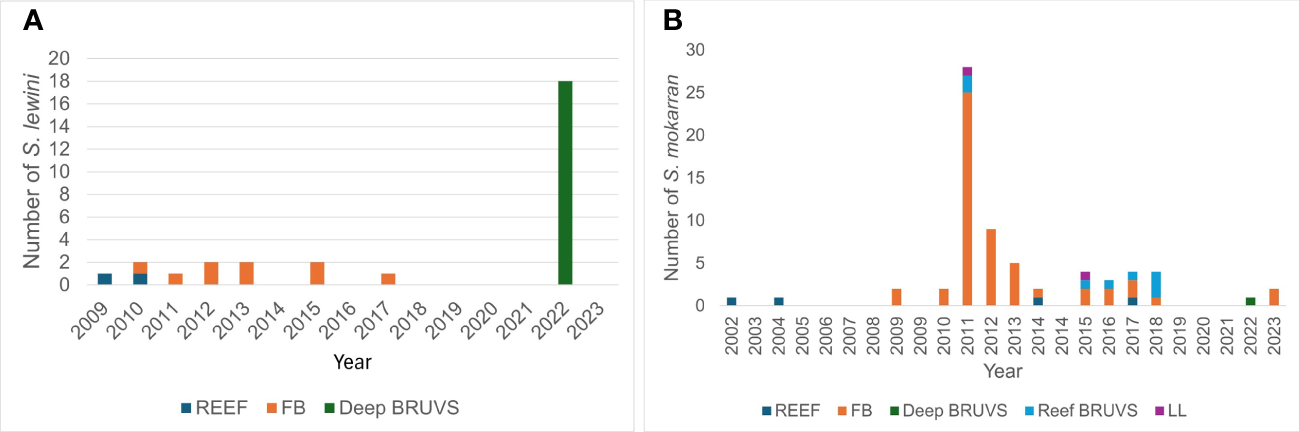
The total numbers of S. lewini(A) and S. mokarran(B) recorded by all data sources for each year from 1993 – 2023. The colour coding represents the survey methods.
3.4 Social media data
From the social media platforms (see Section 2.5) analysed (Table 1), nine S. lewini, 53 S. mokarran and 20 undetermined Sphyrna sp. were reported between the end of 2009 and mid 2023 (Figure 2). All reports were of single sharks, except for one sighting of two S. mokarran swimming together.
3.5 Temporal trends
The numbers S. lewini and S. mokarran recorded annually from all sources between 1993 and 2023 (except Sharklogger) are plotted in Figures 2A, B respectively. There were no records of S. lewini between 1993 and 2008, after which a few were sighted in most years with slowly increasing frequency (Figure 2A) and significant variation across years from 2001 (χ2 = 253.7 <0.001; df = 22)). The large school reported here was detected in 2022. For S. mokarran, there were single sightings in both 2002 and 2004, and occasional sightings each year from 2009 to 2018 (n=62 with a peak in 2011) and in 2022 (n=3). There was no upward trend in sightings of S. mokarran from all sources (Figure 2B), but there was a marked peak in 2011. and significant variation in numbers of sightings across years (χ2 = 270.1; p<0.001; df = 22). The increases in number were not related to any increase in the number of BRUVS deployments or dives reported by citizen science participants.
Variation in the numbers of Sphyrna species recorded with month of the year is shown in Figure 3 (no data for Sharklogger). Number of S. lewini recorded varied from zero to 13 per month, the numbers varying significantly across the year (χ2 = 58.3; p <0.001); there were possible peaks in spring (March/April) and autumn (September/October), but none recorded in August, December or January (Figure 3A). For S. mokarran, numbers were higher in late winter (January/February) and lowest in the period August to November, with none recorded in August (Figure 3B), but any variation between months was not statistically significant (χ2 = 18.8; p >0.05).
Figure 3
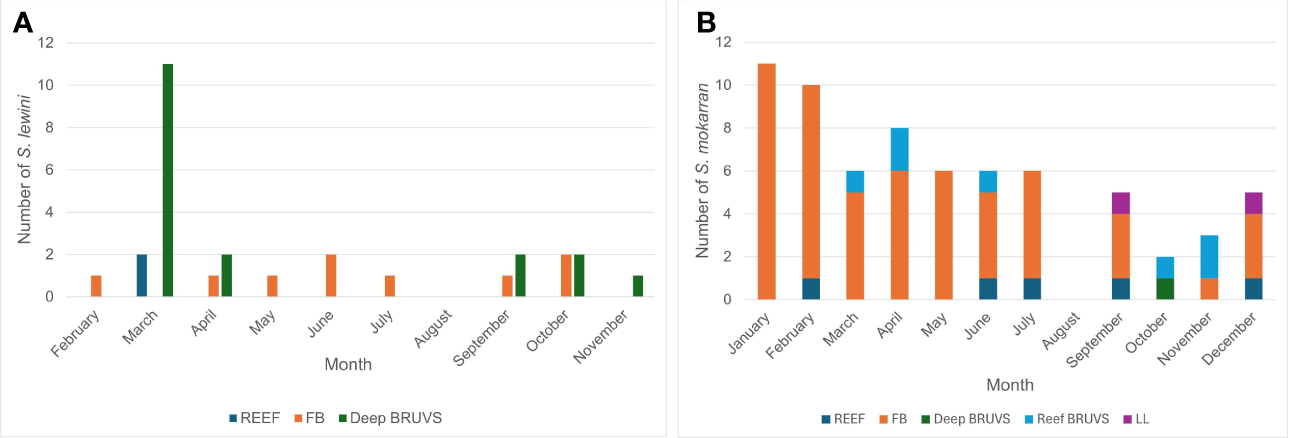
The number of S. lewini(A) and of S. mokarran(B) recorded in each month of the year across all years and from all data sources. The colour coding represents the survey methods.
3.6 Spatial trends
Reports included the island on which Sphyrna sp. were recorded, as well as occasionally information on maturity, size and sex, noting that these variables were used with parsimony. These data are shown in Table 2. The majority of S. lewini, S. mokarran and unidentified Sphyrna sp. were reported from Grand Cayman, where the human population and hence the numbers of participating observers was much higher. 78% (n=9) of S. lewini sexed and 33% of S. mokarran sexed (n=9) were identified as female. One 1.2m juvenile was reported for S. mokarran and also notably a neonate as Sphyrna sp. (likely S. mokarran), both in June.
Table 2
| Adult | Adult-Sub-adult | Juvenile | Female | Male | Size range m | GC | LC | CB | |
|---|---|---|---|---|---|---|---|---|---|
| S. lewini | 2 | 5 | 11 | 7 | 2 | 2.4-2.7 | 19 | 2 | 2 |
| S. mokarran | 17 | 8 | 1 | 3 | 6 | 1.2-4.3 | 38 | 3 | 1 |
| Sphyrna sp. | 105 | 67 | 18 | 5 | 0 | 0.2-4.6 | 82 | 23 | 0 |
Location, maturity, size (TL m) and sex (where determined) for S. lewini, S. mokarran and Sphyrna sp. recorded in the Cayman Islands from all data sources.
(GC, Grand Cayman; LC, Little Cayman and CB, Cayman Brac). Additionally, there was a neonate 0.2m Sphyrna caught and released on Grand Cayman.
4 Discussion
The data from a number of sources presented here reveal broadly consistent patterns during this study period for the occurrence of both scalloped (S. lewini) and great hammerhead (S. mokarran) sharks throughout the territorial waters of the Cayman Islands. There was one reported sighting of S. zygaena (Facebook site, see Section 2.5), but we consider this identification uncertain. Through the study period there were less than half as many records of S. lewini as of S. mokarran. This difference is greater if our recent records on the deep-water BRUVS are excluded, since otherwise only two certain sightings of S. lewini were recorded by REEF and six on social media. S. mokarran were recorded comparatively frequently by divers, recorded on BRUVS or caught on longlines in shallower water. In contrast, our deep-water BRUVS recorded a number of S. lewini but only three S. mokarran.
4.1 Depth range
The literature indicates that S. lewini exploit a wide range of depths, mostly between 0 and 275 m (Moore and Gates, 2015), with the maximum recorded depth being 1240 m (Anderson et al., 2022). For example Hoffmayer et al. (2013) satellite tagged a 240 cm (TL) female S. lewini off the Mississippi River and monitored her for 27 d, during which time she was consistently between 0 and 228 m during the day, but from 0 to 964 m at night, when most dives were to >700 m. In the western Gulf of Mexico, Wells et al. (2018) found that 33 satellite tagged S. lewini preferred mid to outer continental shelf within a 200 m isobath. The school of S. lewini off Grand Cayman were observed during the day at >200 m and occasional individuals were also recorded in shallower water by day. The sharks were swimming in a direct manner suggesting potential foraging behaviour, but these individuals may also forage at greater depths by night.
4.2 Spatial range
In general, female S. lewini appear to move regionally, but not between discontinuous continental coastlines (Gallagher and Klimley, 2018), whereas males will cross deep ocean (Duncan, 2006; Daly-Engel et al., 2012). Wells et al. (2018) found that S. lewini in the western Gulf of Mexico did not disperse over long distances, females tending to associate with shelf edge and males with mid-shelf areas. Nalesso et al. (2019) tagged 84 S. lewini at Cocos Island, on the Pacific coast of Costa Rica, and established that these sharks generally showed strong residency. However, Estupiñán-Montaño et al. (2017) reported that S. lewini used the area of Malpelo Island, Columbia, for resting and cleaning, but fed far away from that site. This raises the question of whether the Cayman Islands’ S. lewini are from a distinct stock, or whether they move more widely around the western Caribbean.
The Cayman Islands are placed in a relatively isolated location in the centre of the western Caribbean and largely surrounded by very deep water, with the Yucatan Basin to the north and the Cayman Trough to the south. If the conclusion that females do not normally move between discontinuous continental coastlines (Duncan, 2006; Daly-Engel et al., 2012) is accurate, then this location makes it plausible that the 1970s schools were likely reproducing in the Cayman Islands, as well as feeding there. However, a shallower ocean ridge (~500-1500m deep) runs east-northeast from the Cayman Islands towards south-west Cuba and its adjacent small islands and this might provide a route via which sharks breeding in the Greater Antilles could reach Cayman without swimming over very deep water.
4.3 Cayman Islands S. lewini population
The pattern of sightings of S. lewini since the 1990s (Figure 3A) suggests that the species went unrecorded in the Cayman Islands during the first half of that period. Our data apart, no schools of S. lewini were reported by technical divers (pers. comm. Jo Mikutowicz, DiveTech), or from dives in submersibles run by Atlantic Adventures to 30m depth from 1985, or in dives in submarines run by Atlantis Deep Explorers to 100m between ca. 1983 to 2003. However, a small number of S. lewini appear to have been visiting the area (or possibly to have become semi-resident) since about 2009. It should however be noted that the platforms for public reporting only became available in 2009 and the public response may have been slow to respond until the platform became better known. Equally, a lull in public reports from about 2018 may reflect the fact that the platforms, though still functional, were not being so actively promoted after they became established. Nevertheless, the absence of S. lewini before about 2009, and the small numbers observed since, contrast with historical accounts of diving in the Cayman Islands in the 1970s, when divers described being surrounded by large schools of over 100 S. lewini (MG, pers. comm.).
The loss of the schools of S. lewini observed by divers in Cayman through the 1980s is consistent with the overexploitation of this species elsewhere, since Sphyrna species seem highly vulnerable to anthropogenic exploitation, partly due to their highly specialised ecology and behaviour (Gallagher et al., 2014b). As noted by Lòpez et al. (2022), large aggregations of S. lewini may be targeted by fishers, resulting in severe overfishing. They are also sensitive to other fisheries impacts, Zhang et al. (2022). for example reporting the significant numbers of S. lewini discarded as bycatch by a bottom-longline fishery in the southern US Atlantic and Gulf of Mexico between 2005 and 2019. Further, Morgan and Burgess (2007) found that the annual survival rate for tagged S. lewini, caught on bottom longlines set for sharks between New Jersey and Louisiana, USA, was only 8.6% for all stages of maturity. There was no known targeted exploitation of hammerhead sharks in the Cayman Islands, even though the Cayman Islands supported a traditional shark fishery that is understood to have mainly fished for sharks along the coasts of central America from the 1930s (Zeller and Harper, 2009). However, S. lewini could easily have been subject to capture in targeted fisheries or as bycatch in other fisheries elsewhere in the Caribbean, assuming individuals were crossing into neighbouring regions.
This newly recorded S lewini school in Cayman could either represent a recently protected population that is recovering from a few individuals that survived previous exploitation, or it could represent individuals from a neighbouring stock that have expanded into now vacant Cayman habitat. Re-occupation of a species range following conservation measures has been reported in some other large marine vertebrates, for example with humpback whale (Megaptera novaeangliae) in NE Brazil (Rossi-Santos et al., 2008) and in the Chukotka Peninsula (Melnikov, 2019).
It would be useful to determine whether the S. lewini now observed in Cayman are breeding there, or only feeding. The numbers of observations of S. lewini in the Cayman Islands are distributed through much of the year, but most frequent in spring and autumn, supporting potential seasonal visits by the species. While the timing of parturition has not been recorded in the Caribbean, it has been noted that off Cape Canaveral, Florida, USA, neonates are found in May and June, generally in water <11 m deep (Adams & Paperno, 2007). The higher number of sightings in Cayman from March/April could be related to reproductive behaviour. Alternatively, the greater numbers in spring could be related to the occurrence the spawning aggregations (SPAGs) of grouper and snapper that occur at that time of year at traditional sites off the east or west end of the islands (Whaylen et al., 2004).
4.4 Cayman Islands S. mokarran population
In contrast, S. mokarran appears to be present in Cayman throughout the year, except perhaps in August (Figure 3B). This strongly suggests that the species may be resident on the islands, even though S. mokarran is more usually described as a nomadic, seasonally migratory species (Miller et al., 2014). While a generalist feeder on fishes and crustacea, it is also considered a specialist at feeding on other sharks and rays, especially stingrays (Raoult et al., 2019). It is thus possible that the abundance of stingrays (Hypanus americanus) around the western part of Grand Cayman, particularly around the popular tourist location known as “Stingray City”, (see e.g. https://en.wikipedia.org/wiki/Stingray_City,_Grand_Cayman) may support their year-round presence. Perhaps our most dramatic record of S. mokarran in the Cayman Islands is an image taken from the air by a local helicopter operator (J. Begot) of a S. mokarran chasing down and capturing a large stingray, not far from the Stingray City site. These S. mokarran appear to ingest the stingrays over a period of time, biting one wing on one occasion, another later on and finally the remaining body (pers. comm. J. Begot).
The observation of a neonate Sphyrna sp. (probably S. mokarran) also suggests the species breeds there. S. mokarran females are thought to breed once every two years, giving birth from late spring to summer in the northern hemisphere and from December to January in Australia (Rigby et al., 2019b). If the gestation period is 11 months, as generally assumed (Bester, 2008), the apparent absence of the species in August could perhaps be due to movement of individuals away from the reefs to preferred mating grounds, although this would imply pupping of S. mokarran in Cayman would take place in mid-summer, rather than spring. A report by a local resident (from outside the present dataset) described a hammerhead shark coming into a sound on Little Cayman on a seasonal basis, and apparently pupping; most likely this individual would have been a S. mokarran.
4.5 Protection and management
The return of a school of S. lewini to the Cayman Islands, and the evidence for the persistence locally of S. mokarran, are both significant for the conservation of these two critically endangered species. As noted above, there are indications that, following the introduction of management measures in Cayman and notably the inclusion of hammerhead sharks under the US Endangered Species Act, the population of S. lewini in the Northwest Atlantic and Gulf of Mexico region may be showing signs of stablisation (Rigby et al., 2019a). Likewise, it has been concluded that the population of S. mokarran in the same region may be slowly increasing (Rigby et al., 2019b), even though this trend does not have appear to in continued within the Cayman Islands over the least 5-10 years. In the Cayman Islands, not only do Marine Protected Areas (MPAs) now cover about half of the coastal shelf (to a depth of 46m) of the three islands, but all Cayman waters are in principle protected from fishing for sharks. The coastal MPAs should have afforded protection to any female Sphyrna pupping in the sounds, but it is not known with certainty how effective has been the prohibition on catching sharks elsewhere. Occasionally, a shark is caught as bycatch by inshore fishers; sharks are required to be released unharmed, although not all may survive the stress of capture. In addition, a small number of boats from Honduras were known to fish offshore Cayman waters for sharks prior to the prohibition, although they have not been recorded doing so since sharks were given protection. Nevertheless it seems likely that marine conservation measures in the Cayman Islands have assisted in the tentative recovery of these two hammerhead species.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Heriot-Watt University Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JK: Data curation, Investigation, Methodology, Writing – review & editing. RO: Conceptualization, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. AG: Visualization, Writing – review & editing. TF: Funding acquisition, Writing – review & editing. TA: Funding acquisition, Writing – review & editing. CP-S: Data curation, Formal analysis, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by grants from the UK Department of Environment Food and Rural Affairs (DEFRA) under their Darwin OTEP Programme (CAY601, CAY701) and Darwin Initiative Programme (DPLUS036, DPLUS140), SaveOurSeas Foundation (P145), by funding from CayBrew Ltd. from our “conservation beer” project (the funder was not involved in the study design, collection, analysis, interpretation of data, writing this manuscript or decision to submit for publication), and support from the Cayman Islands’ Department of Environment.
Acknowledgments
The authors thank colleagues in the Cayman Islands Department of Environment for their assistance and support during this study. We also thank all of the volunteers who gave their time and effort to the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MS declared a past co-authorship with the author AG to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adams D. Paperno R. (2007). Preliminary assessment of a nearshore nursery ground for the scalloped hammerhead off the atlantic coast of florida. Am. Fisheries Soc. Symposium50, 165–174.
2
Aldana-Moreno A. Hoyos-Padilla E. González-Armas R. Galván-Magaña F. Hearn A. Klimley A. et al . (2019). Residency and diel movement patterns of the endangered scalloped hammerhead Sphyrna lewini in the Revillagigedo National Park. J. Fish Biol., 1–6. doi: 10.1111/jfb.14239
3
Anderson J. Rex P. Maloney K. Johnston M. Verbeck D. Allen N. et al . (2022). Observations of a species-record deep dive by a central Pacific female scalloped hammerhead shark (Sphyrna lewini). J. Fish Biol.101, 323–327. doi: 10.1111/jfb.15115
4
Bester C. (2008) Biological Profiles: Great Hammerhead. Florida Museum of Natural History Ichthyology Department. Available online at: www.floridamuseum.ufl.edu/discover-fish/species-profiles/sphyrna-mokarran/.
5
Branstetter S. (1987). Age, growth and reproductive biology of the Silky Shark, Carcharhinus falciformis, and the Scalloped Hammerhead, Sphyrna lewini, from the northwestern Gulf of Mexico. Env. Biol. Fishes19, 161–173. doi: 10.1007/BF00005346
6
Bravo-Ormaza E. Arauz R. Bessudo S. Hearn A. Klimley A. Ladino-Archila F. et al . (2023). Scalloped hammerhead shark Sphyrna lewini relative abundance comparison in three offshore marine protected areas of the Eastern Tropical Pacific. Env. Biol. Fishes106, 1767–1784. doi: 10.1007/s10641-023-01454-6
7
Brown K. Seeto J. Lal M. Miller C. (2016). Discovery of an important aggregation area for endangered scalloped hammerhead sharks, Sphyrna lewini, in the Rewa River estuary, Fiji Islands. Pacific Conserv. Biol. 7pp. doi: 10.1071/PC14930
8
Chapman D. Pinhal D. Shivji M. (2009). Tracking the fin trade: genetic stock identification in western Atlantic scalloped hammerhead sharks Sphyrna lewini. ESR9, 221–228. doi: 10.3354/esr00241
9
Daly-Engel T. Seraphin K. Holland K. Coffey J. Nance H. Toonen R. et al . (2012). Global phylogeography with mixed-marker analysis reveals male-mediated dispersal in the endangered scalloped hammerhead shark (Sphyrna lewini). PloS One7, e29986. doi: 10.1371/journal.pone.0029986
10
Duncan K. (2006). Estimation of daily energetic requirements in young scalloped hammerhead sharks, Sphyrna lewini. Environ. Biol. Fish. 12pp. doi: 10.1007/s10641-006-9016-5
11
Ebert D. A. Fowler S. Compagno L. (2013). Sharks of the World. A Fully Illustrated Guide (Plymouth, United Kingdom: Wild Nature Press).
12
Estupiñán-Montaño C. Galván-Magaña F. Tamburin E. Sánchez-González A. Villalobos-Ramírez D. J. Murillo-Bohórquez N. et al . (2017). Trophic inference in two sympatric sharks, Sphyrna lewini and Carcharhinus falciformis (Elasmobranchii: Carcharhiniformes), based on stable isotope analysis at Malpelo Island, Colombia. Acta Ichthyol. Piscat.47, 357–364. doi: 10.3750/AIEP/02177
13
Gallagher A. Hammerschlag N. Schiffman D. Giery S. (2014b). Evolved for extinction: the cost and conservation implications of specialization in hammerhead sharks. Biosci64, 619–624. doi: 10.1093/biosci/biu071
14
Gallagher A. Klimley P. (2018). The biology and conservation status of the large hammerhead shark complex: the great, scalloped, and smooth hammerheads. Rev. Fish Biol. Fisheries. 19pp. doi: 10.1007/s11160-018-9530-5
15
Gallagher A. Serafy J. Cooke S. Hammerschlag N. (2014a). Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. MPES496, 207–218. doi: 10.3354/meps10490
16
Gore M. Ormond R. Clarke C. Kohler J. Millar C. Brooks E. (2020). Application of photo-identification and lengthened deployment periods to baited remote underwater video stations (BRUVS) abundance estimates of coral reef sharks. Oceans1, 274–299. doi: 10.3390/oceans1040019
17
Harned S. Bernard A. Salinas-de-León P. Mehlrose M. Suarez J. Robles Y. et al . (2022). Genetic population dynamics of the critically endangered scalloped hammerhead shark (Sphyrna lewini) in the Eastern Tropical Pacific. Ecol. Evol.12, e9642. doi: 10.1002/ece3.9642
18
Hazin F. Fischer A. Broadhurst M. (2001). Aspects of the reproductive biology of the scalloped hammerhead shark, Sphyrna lewini, off northeastern Brazil. Environ. Biol. Fishes61, 151–159. doi: 10.1023/A:1011040716421
19
Hoffmayer E. Franks J. Driggers W. Howey P. (2013). Diel vertical movements of a scalloped hammerhead, Sphyrna lewini, in the northern Gulf of Mexico. Bull. Mar. Sci.89, 551–557. doi: 10.5343/bms.2012.1048
20
Jiao Y. Cortes E. Andrews K. Guo F. (2011). Poor-data and data-poor species stock assessment using a Bayesian hierarchical approach. Ecol. Appl.21, 2691–2708. doi: 10.1890/10-0526.1
21
Ketchum H. J. Klimley A. Peñaherrera C. Espinoza E. Bessudo S. Soler G. et al . (2014). Inter-island movements of scalloped hammerhead sharks (Sphyrna lewini) and seasonal connectivity in a marine protected area of the eastern tropical Pacific. Mar. Biol. 13pp. doi: 10.1007/s00227-014-2393-y
22
Klimley P. (1993). Highly directional swimming by scalloped hammerhead sharks, Sphyrna lewini, and subsurface irradiance, temperature, bathymetry, and geomagnetic field. Mar. Biol.117, 1–22. doi: 10.1007/BF00346421
23
Kohler J. (2022) The Comparative Abundance and Behaviour of Sharks in the Cayman Islands (BWI) (Edinburgh, Scotland; Heriot-Watt University). Available online at: http://hdl.handle.net/10399/4769.
24
Lòpez N. Mcauley R. Meeuwig J. (2022). Identification of the southernmost aggregation of scalloped hammerhead sharks (Sphyrna lewini) in Australia. Austral Ecol.0, 1–6. doi: 10.1111/aec.13149
25
Melnikov V. V. (2019). Observation of humpback whales (Megaptera novaeangliae) in the waters adjacent to the chukotka peninsula with comparisons to historical sighting data. Open Access Library J.6, e5407. doi: 10.4236/oalib.1105407
26
Miller M. H. Carlson J. Cooper P. Kobayashi D. Nammack M. Wilson J. (2013). Status review report: scalloped hammerhead shark (Sphyrna lewini) (USA: Report to National Marine Fisheries Service, Office of Protected Resources), 131.
27
Miller M. H. Carlson J. Hogan L. Kobayashi D. (2014). Status Review Report: Great Hammerhead Shark (Sphyrna mokarran) (USA: Report to National Marine Fisheries Service, Office of Protected Resources), 116.
28
Moore A. B. M. Gates A. R. (2015). Deep-water observation of scalloped hammerhead Sphyrna lewini in the western Indian Ocean off Tanzania. Mar. Biodiv. Rec.8, 15. doi: 10.1017/S1755267215000627
29
Morgan A. Burgess G. (2007). At-vessel fishing mortality for six species of sharks caught in the northwest Atlantic and Gulf of Mexico. Gulf Carib. Res.19, 123–129. doi: 10.18785/gcr.1902.15
30
Nalesso E. Hearn A. Sosa-Nishizaki O. Steiner T. Antoniou A. Reid A. et al . (2019). Movements of scalloped hammerhead sharks (Sphyrna lewini) at Cocos Island, Costa Rica and between oceanic islands in the Eastern Tropical Pacific. PloS One14, e0213741. doi: 10.1371/journal.pone.0213741
31
National Conservation Law (2013). Supplement No. 1 Published with Extraordinary Gazette No. 9. Cayman Islands Government, Grand Cayman.
32
Ormond R. Gore M. Bladon A. Dubock O. Kohler J. Millar C. (2017). “Protecting cayman island sharks: monitoring, movement and motive,” in Proceedings of the 69th Gulf and Caribbean Fisheries Institute, Grand Cayman, Cayman Islands, November 7 - 11, 2016.
33
Piercy A. Carlson J. Passerrotti M. (2010). Age and growth of the great hammerhead shark, Sphyrna mokarran, in the north-western Atlantic Ocean and Gulf of Mexico. Marine and Freshwater Research61, 992–998.
34
Pinhal D. Domingues R. Bruels C. Ferrette B. Gadig O. Shivji M. et al . (2020). Restricted connectivity and population genetic fragility in a globally endangered Hammerhead Shark. Rev. Fish Biol. Fisheries. 17pp. doi: 10.1007/s11160-020-09607-x
35
Raoult V. Broadhurst M. Peddemors V. Williamson J. Gaston T. (2019). Resource use of great hammerhead sharks (Sphyrna mokarran) off eastern Australia. J. Fish Biol.95, 1430–1440. doi: 10.1111/jfb.14160
36
REEF (2023). Reef Environmental Education Foundation Volunteer Fish Survey Project Database (USA: WWW electronic publication). Available at: www.REEF.org.
37
Rigby C. L. Barreto R. Carlson J. Fernando D. Fordham S. Francis M. P. et al . (2019b) Sphyrna Mokarran (The IUCN Red List of Threatened Species 2019) (Accessed 07 October 2023).
38
Rigby C. L. Dulvy N. K. Barreto R. Carlson J. Fernando D. Fordham S. et al . (2019a) Sphyrna Lewini (The IUCN Red List of Threatened Species 2019) (Accessed on 23 June 2023).
39
Rossi-Santos M. Neto E. Baracho C. Cipolotti S. Marcovaldi E. Engel M. (2008). Occurrence and Distribution of Humpback Whales (Megaptera novaeangliae) on the North Coast of the State of Bahia, Brazil 2000–2006 (Brazil: ICES. Published by Oxford Journals), P667–P673.
40
Wells R. TinHan T. Dance M. Drymon M. Falterman B. Ajemina M. et al . (2018). Movement, behavior, and habitat use of a marine apex predator, the scalloped hammerhead. Front. Mar. Sci.5. doi: 10.3389/fmars.2018.00321
41
Whaylen L. Pattengill-Semmens C. V. Semmens B. X. Bush P. G. Boardman M. R. (2004). Observations of a Nassau grouper, Epinephelus striatus, spawning aggregation site in Little Cayman, Cayman Islands, including multi-species spawning information. Env. Biol. Fishes70, 305–313. doi: 10.1023/B:EBFI.0000033341.57920.a8
42
Zeller D. Harper S. (2009). Fisheries Catch Reconstructions: Islands, Part I. Fisheries Centre Research Reports 17 (5) (Fisheries Centre, University of British Columbia, Canada), 3–12.
43
Zhang X. Carlson J. Corteś E. Babcock E. Latour R. (2022). Revised bycatch estimates of scalloped and great hammerhead shark in the shark bottom longline fishery. SEDAR77-DW37.SEDAR, North Charleston, SC. 24 pp.
Summary
Keywords
hammerhead sharks, deep sea, Caribbean, BRUVS, citizen science, schooling
Citation
Gore M, Kohler J, Ormond R, Gallagher A, Fernandes T, Austin T and Pattengill-Semmens C (2024) Renewed occurrence of schooling scalloped hammerhead (Sphyrna lewini) and of great hammerhead (S. mokarran) sharks in the Cayman Islands. Front. Mar. Sci. 11:1347285. doi: 10.3389/fmars.2024.1347285
Received
01 December 2023
Accepted
29 February 2024
Published
18 March 2024
Volume
11 - 2024
Edited by
J. Marcus Drymon, Mississippi State University, United States
Reviewed by
Ornella Céline Weideli, Labormedizinische Zentrum Dr Risch, Liechtenstein
Matthew Smukall, Bimini Biological Field Station Foundation, Bahamas
Updates
Copyright
© 2024 Gore, Kohler, Ormond, Gallagher, Fernandes, Austin and Pattengill-Semmens.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mauvis Gore, mauvis.gore.mci@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.