Abstract
Hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) are an essential species in marine aquaculture. However, they are susceptible to high temperatures, which can reduce disease resistance, slow growth rates, and decrease production efficiency, resulting in significant economic losses. This study aims to investigate the differences in heat tolerance between hybrid grouper and their parental species, tiger grouper (Epinephelus fuscoguttatus) and giant grouper (E. lanceolatus), and to identify heat stress-related signaling pathways and key genes. Through controlled temperature experiments, we measured the physiological and biochemical parameters of serum (ACP, AKP, TG, COR) and liver (HSP70, HSP90, SOD, CAT) in Hulong hybrid grouper and their parents, followed by liver transcriptome analysis of the three grouper species. The results showed that the lethal temperature of tiger grouper is 41°C, and the lethal temperature of Hulong hybrid grouper and giant grouper is 40°C. Significant changes in antioxidant and heat stress-related indicators were observed in the early stages of stress. Comparative analysis of DEGs related to heat tolerance between Hulong hybrid grouper and their parents revealed common DEGs including the hsp family, danaj family, slc family, pnpla2, magot, actalb, and prodh. Among these, the gene expression trends in hybrids were similar to those of their maternal parent and varied between the same or opposite trends compared to those of their paternal parent. These findings suggest that the hybrids inherit heat regulation genes from both parents, with a higher proportion from the maternal parent, which likely explains their intermediate heat tolerance. This research provides insights into the potential relationship between heat tolerance in Hulong hybrid grouper and their parents and identifies key genetic information affecting heat tolerance.
1 Introduction
Temperature affects many biological processes, with optimal temperatures promoting the growth, development, and reproduction of organisms, and prolonged exposure to extreme temperatures can induce physiological stress and physical damage in humans, livestock, poultry, fish, crops, and other organisms (Asseng et al., 2021). Water temperature is a crucial environmental factor that directly or indirectly threatens the lives of aquatic organisms. Against the backdrop of global climate change, many regions worldwide are experiencing phenomena such as brief extreme heatwaves and more frequent and prolonged periods of high temperatures, and the oceans, acting as the Earth’s heat sink, absorb much of the increased heat, leading to marine heatwaves that jeopardize the stability of marine ecosystems and ultimately endanger the development of aquaculture industries (Frölicher et al., 2018).
Fish are an essential component of aquatic ecosystems. As poikilothermic animals, they lack a mechanism for regulating body temperature; thus, their body temperature fluctuates with changes in water temperature (Clarke and Johnston, 1999). Both increases and decreases in water temperature can have significant impacts on fish, including slow growth, reduced reproductive capacity, and metabolic disruptions (Shahjahan et al., 2017; Howell et al., 2010). Elevated water temperatures can lead to compromised immune function in fish and increased incidence of waterborne diseases. Studies have shown that heat stress in fish can result in severe apoptosis in immune organs such as the head, kidney, and spleen, as well as oxidative stress in the body (Cheng et al., 2015; Sherif et al., 2024). Additionally, levels of lysozyme, immunoglobulins, and other immune-active substances decrease gradually (Mahmoud et al., 2020). High water temperatures may disrupt the microbial community structure in fish organs such as skin and intestines, leading to dysbiosis and increased susceptibility to pathogenic infections (Ghosh et al., 2022; Fang et al., 2023). Furthermore, high water temperatures can affect fish feeding capacity, appetite, metabolic rate, energy balance, and behavior (Volkoff and Rønnestad, 2020). Moreover, high water temperatures can induce stress responses in fish brains. Previous research using transcriptomic analysis of rainbow trout brains exposed to different temperatures has revealed decreased mRNA expression levels of CAT, SOD, GPx, and Bcl2 and increased mRNA expression levels of BDNF, cFOS, apoptosis genes, heat shock genes, and ER-stress genes, indicating physiological changes in fish brains (Topal et al., 2021).
The genus Epinephelus belongs to the family Serranidae within the suborder Percoidei of the order Perciformes. Due to its high nutritional and economic value, Epinephelus species are important marine fish species for aquaculture along the coasts of the Western Pacific Ocean. The optimal overall rearing temperature for different life stages of cultured Epinephelus ranges from 13°C to 35°C, with an average optimal temperature of 26.32°C ± 0.62°C (Das et al., 2021). The tiger grouper requires a temperature of 22 -28°C (Zhang, 2009). The suitable water temperature range of giant grouper is 25-35°C (Chen, 2005). The hatching rate of Plectropomus leopardus is 70-78% at temperatures between 24°C and 30°C, higher than at temperatures below 20°C (Gracia-López et al., 2004). Juvenile Epinephelus coioides H. shows optimal growth at 31.4°C, with feed conversion efficiency initially increasing and then decreasing with rising temperatures (Lin et al., 2008). Hulong hybrid groupers fed with shrimp diets exhibit high red blood cell and total protein counts at 26°C, indicating healthy and less stressed conditions (De et al., 2019). Hulong hybrid groupers, resulting from crosses between Epinephelus fuscoguttatus and E. lanceolatus, thrive within a water temperature range of 26-30°C (De et al., 2016). In recent years, global warming has led to annual increases in seawater temperatures, placing significant pressure on the aquaculture of Hulong hybrid groupers and their parent species, E. lanceolatus and E. fuscoguttatus. This warming trend may affect the production yields of Hulong hybrid groupers, as well as the parental species, which are protogynous hermaphrodites. Temperature plays a crucial role in their growth and metabolism, and exceeding critical temperatures inevitably impacts metabolic processes (Spinks et al., 2021), indirectly affecting the production of Hulong hybrid groupers.
This study employs controlled temperature experiments to investigate the thermal tolerance and physiological responses of Hulong hybrid groupers and their parent species, Epinephelus lanceolatus and E. fuscoguttatus, under heat stress conditions. The research aims to explore the regulatory mechanisms of temperature tolerance in Hulong hybrid groupers and their intrinsic connections with their parents. It seeks to identify relevant signaling pathways and key genes involved in the heat stress response of Hulong hybrid groupers and their parents, providing valuable insights for future breeding applications and genetic analysis of heat tolerance in groupers.
2 Materials and methods
2.1 Temporary rearing management of Hulong hybrid groupers and their parents
The experimental fish used in this study were sourced from enterprises engaged in seedling breeding in Hainan Province, China. In October 2022, giant grouper (E. lanceolatus) specimens approximately 4 cm in size (from Hainan Xinhai Aquatic Products Co., Ltd.) were purchased and temporarily reared until they reached a body length of 11.0 cm ± 2.0 cm. Additionally, Hulong hybrid groupers (crossbred from Epinephelus fuscoguttatus and E. lanceolatus, 12.0 cm ± 1.0 cm in size, from Hengxing Fisheries Technology Co., Ltd.) and tiger grouper (Epinephelus fuscoguttatus, 12.0 cm ± 1.0 cm in size, from Lanliang Technology Co., Ltd.) were purchased. All these groupers were temporarily reared in culture tanks with a cylinder with a radius of 0.4 meters and a height of 1 meter holds 470L of water at the Aquaculture Facility of the College of Marine Biology and Fisheries, Hainan University. The stocking density was maintained at 40-50 individuals per tank. During the temporary rearing period, the water temperature was kept at 28°C, salinity at 33 ppt, with a 12-hour light and 12-hour dark cycle. Tanks were cleaned daily to remove leftover feed and feces, and the water was partially changed every three days. Experimental fish selected for the study had an average weight of 50 g ± 3 g and an average body length of 12 cm ± 1 cm. Experiments were conducted once the groupers had acclimated to the rearing conditions and maintained good health.
2.2 Short-term evaluation of heat tolerance in Hulong hybrid groupers and their parents
A total of 160 individuals each of Hulong hybrid groupers, giant grouper, and tiger grouper, with body lengths of 12.0 ± 1.0 cm and weights of 50 g ± 3 g, were selected for the study. Each species of grouper was divided into four groups: 1 control group maintained at standard temperature and three experimental groups subjected to temperature increase. The stocking density was 40 individuals per tank, and all fish were temporarily reared at 28°C after a 48-hour fasting period. After the temporary rearing period, the water temperature in the culture tanks was adjusted to 28°C as the initial temperature. Heating rods and thermometers were used to control the temperature, increasing it at a rate of 1°C per 4 hours until reaching the temperature at which 50% mortality occurred (LD50). During the temperature increase, judged by the color and spots of the fish, the temperatures at the onset of stress, onset of mortality, and LD50 were recorded, and observe the behavior of the fish at each temperature.
2.3 Long-term heat stress experiment on Hulong hybrid groupers and their parents
A total of 120 individuals, each of Hulong hybrid groupers, giant grouper, and tiger grouper, with body lengths of 12.0 ± 1.0 cm and weights of 50 g ± 3 g, were selected for the study. They were reared at a density of 30 individuals per tank. Among them, the giant grouper had 30 individuals in the control group and 90 individuals in the experimental group. In comparison, the tiger grouper and Hulong hybrid grouper had 60 individuals each in both the control and experimental groups. After a 48-hour fasting period, all fish were temporarily reared at 28°C. Following the temporary rearing period, the water temperature in the culture tanks was gradually increased using heating rods and thermometers at a rate of 1°C per 4 hours until reaching 36°C. The temperature was maintained at 36°C for a period of 60 days to conduct the high-temperature stress-rearing experiment. During the high-temperature stress period, all three species of groupers were fed to satiation daily. On days 1, 2, 3, 7, 11, 15, 30, and 60 of the high-temperature stress experiment, three fish were randomly selected from each group for sampling. Fish were anesthetized using clove oil, and samples of brain, heart, head, kidney, intestines, eyes, gills, muscle, skin, spleen, and liver were collected and immediately frozen in liquid nitrogen and stored at -80°C. Blood samples were collected from the caudal vein into non-anticoagulant tubes and centrifuged at 5000 rpm for 20 minutes, and the serum was collected and aliquoted into sterile 1.5 mL centrifuge tubes.
2.4 Physiological and biochemical analysis of serum and liver in Hulong hybrid groupers and their parents
The levels of triglycerides (TG), cortisol (COR), acid phosphatase (ACP), and alkaline phosphatase (AKP) in the serum were measured according to the instructions provided with the reagent kits (Nanjing Jiancheng Bioengineering Institute). The liver tissue samples were processed and measured using reagent kits provided by Nanjing Jiancheng Bioengineering Institute. The primary indicators tested included catalase (CAT), superoxide dismutase (SOD), heat shock protein 70 (HSP70), and heat shock protein 90 (HSP90). All physiological and biochemical indicators of serum and liver tissues were detected using a microplate reader.
2.5 Transcriptome analysis of liver in Hulong hybrid groupers and their parents under high-temperature stress
2.5.1 Sample collection and grouping of grouper samples
Based on the physiological and biochemical measurements of liver tissue samples on days 1, 2, 3, 7, 11, 15, 30, and 60, it was observed that the activities of HSP70, HSP90, and CAT in the high-temperature groups of all three species were significantly higher than those in the normal temperature groups during the first three days, with the most pronounced differences occurring on the second day. Therefore, liver tissue samples from the second day were selected for transcriptome sequencing. The samples were labeled as EL-T (high-temperature group of the parental Epinephelus lanceolatus), EF-T (high-temperature group of the parental Epinephelus fuscoguttatus), EE-T (high-temperature group of the hybrid grouper), EL-Z (normal temperature group of the parental Epinephelus lanceolatus), EF-Z (normal temperature group of the parental Epinephelus fuscoguttatus), and EE-Z (normal temperature group of the hybrid grouper), with three replicates per sample (T-1, T-2, T-3; Z-1, Z-2, Z-3), totaling 18 samples. The transcriptome sequencing was conducted by Guangzhou Gene Denovo Biotechnology Co., Ltd.
2.5.2 Library construction and sequence analysis
Construct six cDNA libraries for RNA sample preparation. Process raw reads by removing adapter sequences, ambiguous nucleotides (those with an “N” ratio > 10%), and low-quality sequences (those with more than 50% of bases having a quality score of Q ≤ 20). The expression level of each transcript is measured by the number of clean reads mapped to its sequence. Using the HISAT2 tool, sequences generated from paired-end sequencing are mapped to the reference genome, categorizing the alignment regions into three significant categories: exonic regions, intronic regions, and intergenic regions. By applying necessary normalization to sequencing depth and correcting for differences in gene or transcript lengths, calibrated gene FPKM values are obtained. Principal Component Analysis (PCA) is conducted using R language. Pathways with a Q value ≤ 0.05 are identified as significantly enriched among the differentially expressed genes.
2.6 Statistical analysis
During the physiological and biochemical tests conducted on the serum and liver of the hybrid grouper (Epinephelus fuscoguttatus♀×E. lanceolatus♂) and its parents, all results are presented as the mean ± standard error based on three replicate experiments. To ensure variance homogeneity, the means and standard deviations of these experiments were calculated, followed by comparisons between means using one-way ANOVA. Data were analyzed with GraphPad Prism 5.0 software (GraphPad Software Inc., CA, USA). Multiple group comparisons were conducted using Tukey B and Duncan’s tests, with a p-value of < 0.05 considered statistically significant. All statistical analyses were performed using SPSS 17 (Chicago, IL, USA).
3 Results
3.1 Evaluation of high-temperature tolerance in Hulong hybrid groupers and their parents
3.1.1 Evaluation of high-temperature tolerance
The high-temperature tolerance of the Hulong hybrid grouper and its parent species, the giant grouper and the tiger grouper were evaluated by increasing the water temperature from 28°C to the lethal temperature at a rate of 1°C per 4 hours. The evaluation was based on the stress responses of the three types of groupers under different high-temperature conditions (Figure 1).
Figure 1
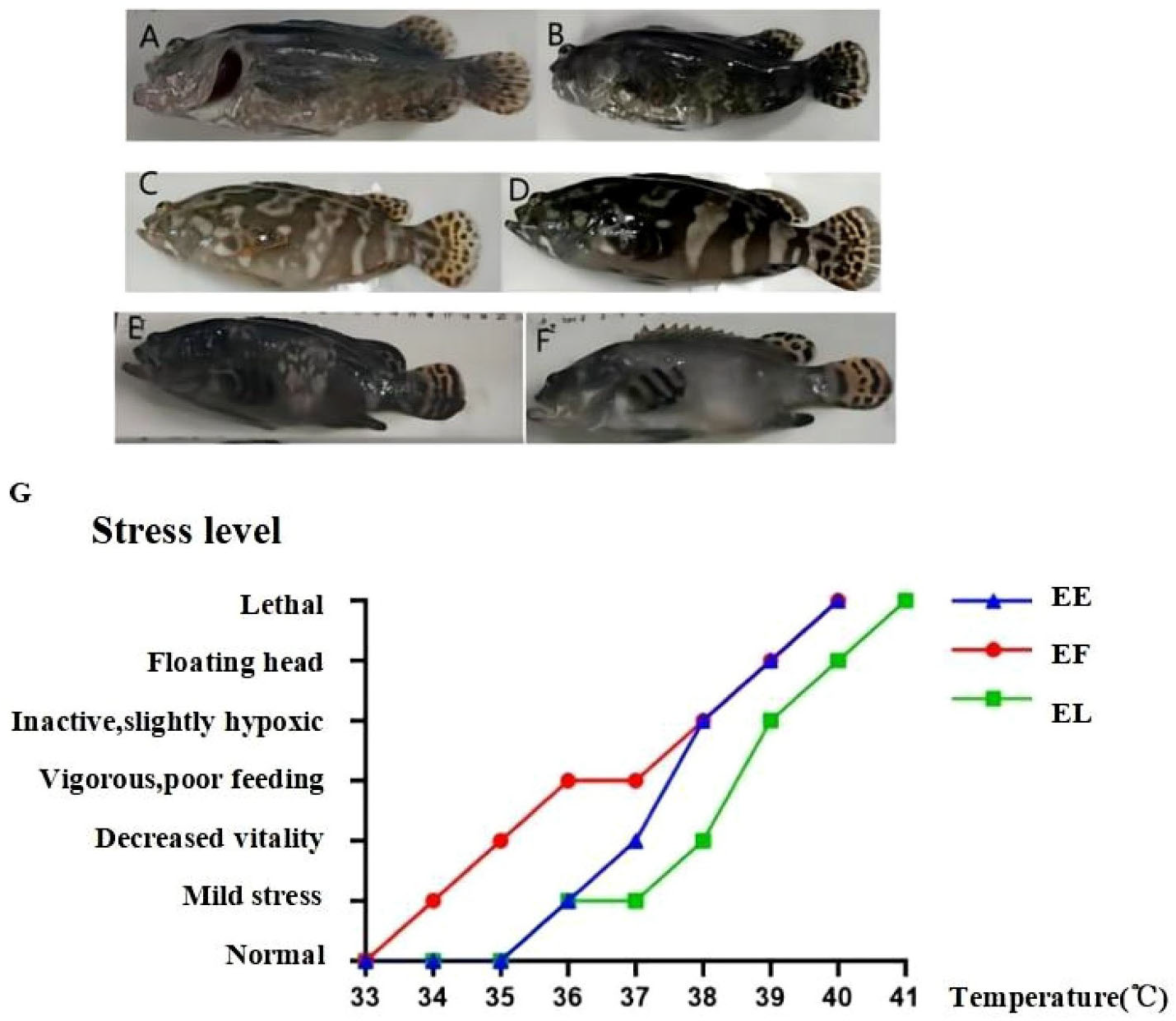
Color Differences in Three Types of Groupers under High-Temperature Stress. Tiger Grouper (A, B), Hybrid Grouper (C, D), Giant Grouper (E, F); (A, C, E) represent the high-temperature group, (B, D, F) represent the normal-temperature group; (G) shows the stress responses of the Hybrid Grouper and its parents under different high-temperature stresses. EE represents the Hybrid Grouper, EL represents the Giant Grouper, and EF represents the Tiger Grouper.
The Hulong hybrid groupers reached 50% lethality after 1.5 hour at 40°C (Table 1; Figure 1G). The giant grouper showed 50% mortality at 40°C after 1 hour (Table 1; Figure 1G). The tiger grouper presented 50% mortality at 41°C after 1.5 hour (Table 1; Figure 1G). The stress tolerance of the Hulong hybrid grouper overlapped with that of the giant grouper at 38-40°C, indicating that the giant grouper is more sensitive to high temperatures than the Hulong hybrid grouper and the tiger grouper.
Table 1
| Temperature (°C) | Hulong hybrid Grouper | Epinephelus septemfasciatus | Epinephelus septemfasciatus |
|---|---|---|---|
| 28 | normalcy | normalcy | normalcy |
| 39 | normalcy | normalcy | normalcy |
| 30 | normalcy | normalcy | normalcy |
| 31 | normalcy | normalcy | normalcy |
| 32 | normalcy | normalcy | normalcy |
| 33 | normalcy | normalcy | normalcy |
| 34 | normalcy | Fish with obvious patches and some white spots faded | normalcy |
| 35 | normalcy | Fish with visible plaques, white spots that fade and turn golden in color | normalcy |
| 36 | Decreased vigor of some fish | Fish with visible plaques, white spots that fade and turn golden in color | Fish are unresponsive, individual fish are stressed |
| 37 | Fish become less vigorous and gradually lighten in color. | Fish with visible and increased golden coloration patches | Fish are unresponsive, individual fish are stressed |
| 38 | Reduced fish vitality, lighter body color and upward swimming, reduced feeding | Fish with visible, gold-colored patches increase and begin to show ambush and reduced feeding | Reduced feeding and vigor of fish |
| 39 | Fish are floating head, bottoming out, sticking to the wall and stopping swimming | Fish are bottomed out, attached to the wall, poor vigor and stop feeding, floating head after 2h |
Fish feeding ability is poor, swimming ability decreases, body color begins to lighten, and mild hypoxia occurs after 2h |
| 40 | Stopped swimming, appeared to roll over and die, half lethal after 1.5h |
Fish stop swimming, half of the wall floating head, half of the bottom, 0.5h later flipped sideways, 1h later half of the lethal | Head floating and body turning sideways after 1h fish body |
| 41 | Fishappeared to float their heads against the wall and ambush the bottom after 1h, and began to show death, and half of them were lethal after 1.5h |
Performance of the hybrid grouper and their parents under different high temperature stress.
During high-temperature stress, both the Hulong hybrid grouper and its parent species showed significant changes in body coloration compared to the normal temperature group. Under high temperatures, the tiger grouper’s body color changed from yellow-black (Figures 1A, B) to yellow-white; the Hulong hybrid grouper’s body color changed from deep black to yellow-black (Figures 1C, D); and the giant grouper’s skin color changed from light black to golden black (Figures 1E, F). Although these color changes can also occur during normal breeding, high-temperature stress clearly accelerated and guided these changes.
3.1.2 Growth detection
During the 60-day rearing period, both the high-temperature group (36°C) and the control group (28°C) of groupers survived normally, with a survival rate of 100%. Significant differences were observed between the experimental and control groups of giant groupers in the liver-body ratio, viscera-body ratio, feed conversion ratio, weight gain rate, specific growth rate, and condition factor.
For the tiger grouper, there were no significant differences in specific growth rate and feed conversion ratio over the 60-day period, although the weight gain rate showed significant differences at 30 days. The specific growth rate of the experimental group of tiger grouper was slightly higher than that of the control group, but it gradually approached that of the control group over time, with no significant differences during the stress period.
In the Hulong hybrid grouper, there were no significant differences between the high-temperature group and the control group in terms of weight gain rate, specific growth rate, and feed conversion ratio over the 60-day rearing period. However, numerical differences were observed at different stages. The weight gain rate for both groups was fastest between 30-45 days and slowest between 45-60 days. The specific growth rate consistently declined, with the fastest decline occurring between 15-30 days and the slowest between 30-45 days. The feed conversion ratio steadily increased between 15-45 days, peaking at 45 days, and then decreased at a rate nearly twice as fast as the increase.
3.2 Physiological and biochemical levels of the Hulong hybrid groupers and its parents under high-temperature stress
3.2.1 Serum analysis
Serum samples from the three types of groupers were collected on days 1, 2, 3, 7, 11, 15, 30, and 60 for physiological and biochemical analysis. The results showed the following:
The Giant Grouper: Cortisol (COR) levels in the high-temperature group (36°C) were significantly higher (P<0.005) than those in the control group (28°C) on days 2, 3, 7, 11, 15, 30, and 60, with extremely significant differences on days 15 and 60 (P<0.001) (Figure 2C). No significant difference was observed on day 1. Triglyceride (TG) levels were significantly higher (P<0.001) in the high-temperature group on day one but began to decrease from day two onwards, becoming significantly lower than the control group (P<0.001) (Figure 2D). Acid phosphatase (ACP) activity was significantly lower (P<0.005) in the high-temperature group on day 2, significantly higher on day 15 (P<0.005), and lower again on days 30 and 60 (P<0.001) (Figure 2A). Alkaline phosphatase (AKP) showed no significant difference between the groups on days 1, 3, 7, and 15. However, on day 2, AKP levels in the high-temperature group were significantly higher (P<0.005), and on days 11, 30, and 60, AKP levels were significantly higher in the control group (P<0.005) (Figure 2B).
Figure 2
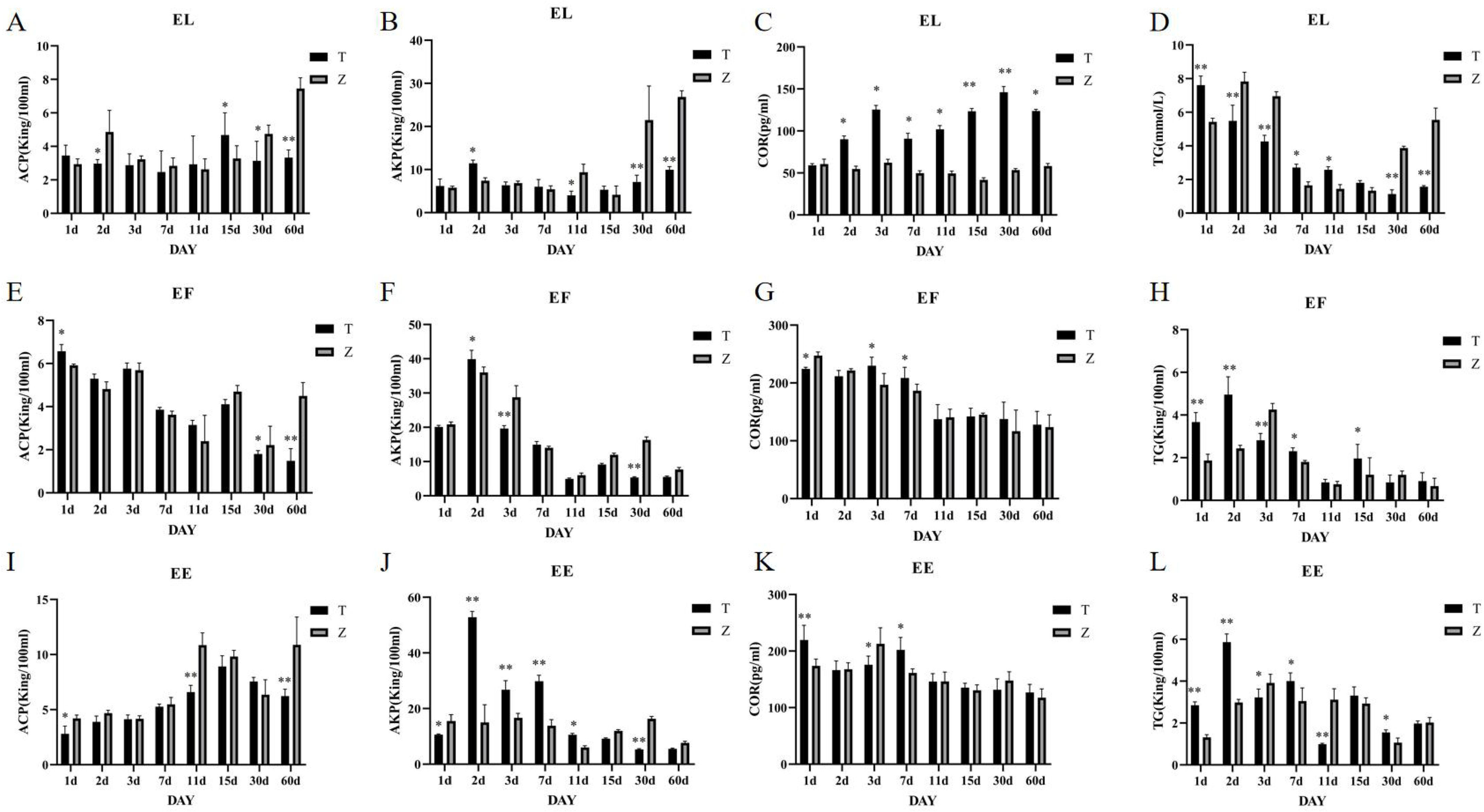
(A–L) Changes in serum ACP, AKP, COR, and TG activities in the three grouper species under different stress days. T represents the high temperature group (36°C), Z represents the normal-temperature group (28-29°C), and samples were taken on days 1, 2, 3, 7, 11, 15, 30, and 60 of cultivation. EE represents the Hybrid Grouper, EL represents the Giant Grouper, and EF represents the Tiger Grouper.
Tiger grouper: Cortisol (COR) levels in the high-temperature group (36°C) showed significant differences (P<0.005) on days 1, 3, and 7, with higher levels in the high-temperature group on days 3 and 7 (Figure 2G). Triglyceride (TG) levels were significantly higher in the high-temperature group on days 1, 2, 7, and 15 but lower on days 3 (P<0.001) (Figure 2H). Acid phosphatase (ACP) levels showed significant differences (P<0.005) on days 1, 30, and 60, with no significant differences on days 2, 3, 7, and 15 (Figure 2E). Alkaline phosphatase (AKP) levels showed extremely significant differences (P<0.001) on days 2 and 3, with higher levels in the high-temperature group on day two and lower on day 3 (Figure 2F).
Hulong hybrid Grouper: Cortisol (COR) levels were significantly higher (P<0.001) in the high-temperature group on days 1 and 7 but were lower on day 3 (P<0.005) (Figure 2K). Triglyceride (TG) levels showed significant differences at most time points, with higher levels in the high-temperature group on days 1, 2, and 7 (P<0.001) but lower levels at other time points (Figure 2L). Acid phosphatase (ACP) levels showed significant differences (P<0.005) on days 1, 11, and 30, with lower levels in the high-temperature group at all these time points (Figure 2I). Alkaline phosphatase (AKP) levels showed significant differences on days 1, 2, 3, 7, 11, and 30, with higher levels in the high-temperature group on days 2, 3, 7, and 11 (P<0.001) (Figure 2J). This study examined the enzymatic activities of acid phosphatase (ACP), alkaline phosphatase (AKP), triglycerides (TG), and cortisol (COR) in serum, as well as the activities of heat shock protein 70 (HSP70), heat shock protein 90 (HSP90), superoxide dismutase (SOD), and catalase (CAT) in liver tissue. The results indicated that these enzymes showed significant differences in serum among the three types of groupers during the first three days, with the temperature-stressed groups generally exhibiting higher activity levels.
In liver tissue, catalase (CAT) showed significant differences at almost all stress time points, while superoxide dismutase (SOD) differences were less pronounced. HSP70 and HSP90 levels were significantly elevated in the high-temperature groups during the first three days. Notably, HSP90 activity remained significantly higher in the high-temperature groups compared to the control groups throughout the stress period. The activity of HSP70 did not show significant differences after the first week of the stress.
Overall, the antioxidant and heat stress-related indicators in the three types of groupers showed significant changes during the first three days of stress.
3.2.2 Liver analysis
The giant grouper: On day 1, catalase (CAT) levels in the liver were significantly lower in the high-temperature group compared to the control group. On day 2, CAT levels significantly increased and surpassed those of the control group (P<0.005) (Figure 3B). There were no significant differences from days 11 to 60. Superoxide dismutase (SOD) levels were significantly higher in the high-temperature group on days 7 and 15 (P<0.001) but significantly lower on days 30 and 60 (P<0.001) (Figure 3A), with no significant differences at other time points. The levels of heat shock proteins HSP70 and HSP90 were significantly higher in the high-temperature group compared to the control group (P<0.001) (Figures 3C, D).
Figure 3
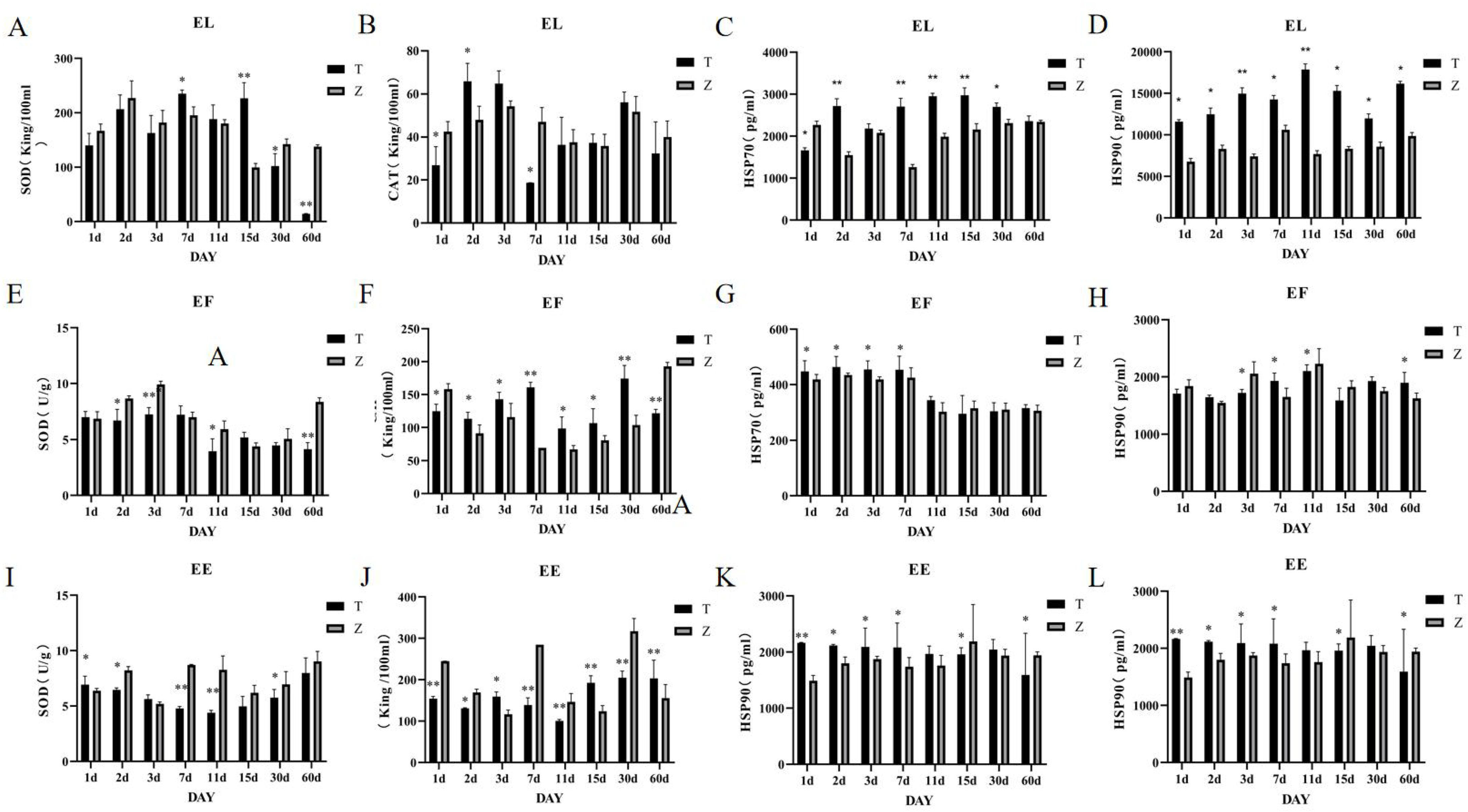
(A–L) Changes in liver SOD, CAT, HSP70, and HSP90 activities in the three grouper species under different stress days. T represents the high temperature group (36°C), Z represents the normal-temperature group (28-29°C), and samples were taken on days 1, 2, 3, 7, 11, 15, 30, and 60 of cultivation.EE represents the Hybrid Grouper, EL represents the Giant Grouper, and EF represents the Tiger Grouper.
Tiger grouper: Significant differences in liver catalase (CAT) levels were observed at all time points. On days 1 and 60, CAT levels were significantly lower in the high-temperature group, whereas at other time points, CAT levels were significantly higher compared to the control group (P<0.005) (Figure 3F). SOD levels in the experimental group were significantly lower on days 2, 3, 11, and 60 (P<0.005), with no significant differences at other time points (Figure 3E). HSP70 levels were significantly higher in the high-temperature group during the first seven days (Figure 3G). HSP90 levels were significantly lower on days 3, 7, and 11 but significantly higher on day 60 (P<0.005) (Figure 3H).
Hulong hybrid Grouper: Significant differences in liver CAT levels were observed at all time points. On days 3 and 15, CAT levels were significantly higher in the high-temperature group (P<0.005), whereas at other time points, CAT levels were significantly lower compared to the control group (Figure 3I).
Except for days 3, 15, and 60, SOD levels were significantly higher in the high-temperature group at all other time points (P<0.005) (Figure 3J). HSP70 levels were significantly higher in the high-temperature group on days 2, 3, and 60, with no significant differences at other time points (Figure 3K). HSP90 levels were significantly higher in the high-temperature group during the first seven days (P<0.005), with a high-to-low shift occurring on day 15 (Figure 3L).
This study measured the enzymatic activities of CAT, SOD, HSP70, and HSP90 in the livers of the three types of groupers under 60 days of high-temperature stress. The results showed a trend of initially increasing and then decreasing enzyme activity, with significant differences compared to the control group. Generally, fish have a system to eliminate oxidative free radicals to maintain a relatively normal level of free radicals. When exposed to stressors such as temperature changes, starvation, or salinity fluctuations, fish produce excess free radicals.
The changes in HSP70 and HSP90 persisted throughout the experiment, while SOD and CAT showed a low-high-low trend. The findings of this study and other experiments suggest that fish exhibit significant adaptive responses in heat shock proteins and antioxidant enzymes under high-temperature stress, with these responses showing correlations with changes in temperature and duration of stress.
3.3 Transcriptome analysis of the Hulong hybrid groupers and its parents under heat stress
3.3.1 Quality control and sequence analysis of transcriptome sequencing data
Transcriptome sequencing was performed on the Hulong hybrid grouper and its parent species. The total clean reads for EE-T, EE-Z, EF-T, EF-Z, EL-T, and EL-Z were 117,226,170; 120,749,748; 127,664,068; 123,773,784; 127,401,606; and 122,893,512, respectively. The average base lengths for the six samples were 5.8 Gb, 6.1 Gb, 6.4 Gb, 6.2 Gb, 6.3 Gb, and 6.2 Gb. The Q30 clean reads exceeded 99% for all samples. With the exception of EF-Z-1, which had a higher proportion of mapped reads, the proportion of unmapped reads exceeded 99.7% in other samples.
High-quality sequencing reads from each sample were aligned to the reference sequence. Using RSEM software to analyze the alignment results, the alignment rates of high-quality sequencing reads for each sample ranged from 84.06% to 95.67%, with the proportion of reads mapping to exonic regions exceeding 75%. These results indicate that the data meet the conditions for subsequent analysis (see Supplementary Material S1).
3.3.2 Differentially expressed genes
3.3.2.1 GO and KEGG enrichment analysis
The transcriptome alignment revealed a total of 262 differentially expressed genes (DEGs) between the high-temperature and normal-temperature groups in the Hulong hybrid grouper. Among these, 168 genes were downregulated, and 98 genes were upregulated. In Giant Grouper, 947 DEGs were identified, with 646 genes downregulated and 301 genes upregulated. Tiger grouper exhibited 245 DEGs, with 173 genes downregulated and 72 genes upregulated (Table 2).
Table 2
| Species | Up-regulated genes |
Down-regulated genes |
|---|---|---|
| Hybrid Grouper | 168 | 98 |
| Giant Grouper | 646 | 301 |
| Tiger Grouper | 173 | 72 |
Differential gene statistics.
GO (Gene Ontology) enrichment analysis was conducted for the DEGs identified in the transcriptomes of the Hulong hybrid grouper, tiger grouper, and giant grouper. The Hulong hybrid grouper and giant grouper used the same reference genome for the analysis. GO annotations were categorized into three main categories: biological process (BP), cellular component (CC), and molecular function (MF) (Figure 4).
Figure 4
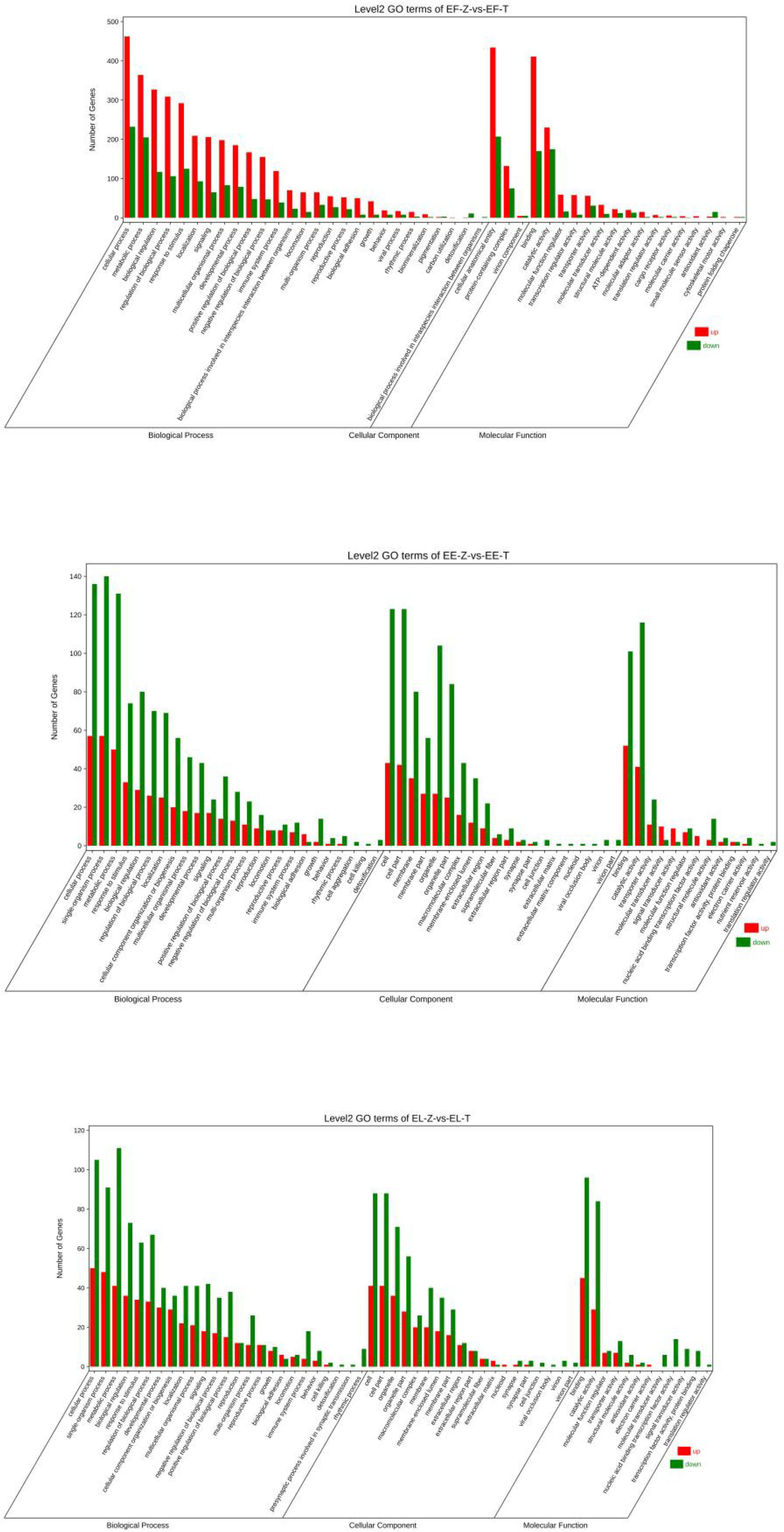
GO classification of the Hulong hybrid Grouper and its parents.
The transcriptome data included 18,898 BP genes, 17,203 CC genes, and 19,824 MF genes. The DEGs consisted of 213 BP genes, 179 CC genes, and 212 MF genes.
Most DEGs were classified such as cellular process, single-organism process, metabolic process, response to stress, biological regulation, cellular component, single-organism cellular process, binding, and catalytic activity.
The transcriptome data included 19,523 BP genes, 17,445 CC genes, and 20,833 MF genes. The DEGs consisted of 739 BP genes, 647 CC genes, and 764 MF genes.
Most DEGs were classified such as cell process, single-organism process, localization, response to stimulus, biological regulation, development process, membrane part, cell part, organelle part, transporter activity, binding, catalytic activity, and molecular function regulator (see Supplementary Material S3).
The differentially expressed genes are significantly enriched in KEGG pathways, categorized into five main classes and 42 subcategories. The most abundant category is metabolic pathways, followed by disease pathways, organismal systems, cellular processes, genetic information processing, and environmental information processing. In the high-temperature group compared to the control group, the giant grouper and hybrid grouper mainly exhibit differential gene expression focused on lipid metabolism processes, contrasting with the tiger grouper, where differentially expressed genes are predominantly involved in antigen processing and presentation, and endoplasmic reticulum processing pathways.
3.3.3.2 Analysis of differences between hybrid grouper and its parents
In the liver transcriptome sequencing results of Epinephelus fuscoguttatus, E. lanceolatus, and the Hulong hybrid grouper under high-temperature (36°C) and control (28°C) conditions for 48 hours, differentially expressed genes (DEGs) were identified that respond to thermal stress. Comparative analysis of these DEGs revealed genes commonly upregulated in response to high-temperature stress across the three species. These genes are primarily involved in molecular functions (transmembrane transporter activity) and biological processes (ion transport) (see Appendix S4).
Based on Figure 5, the differential analysis among the Hulong hybrid grouper and its parental species revealsseveral categories of common differentially expressed genes (DEGs) and specific differences between the hybrid and each parent.
Genes such as slc, hsp, pnpla2, and magot are commonly differentially expressed. These genes are enriched in pathways such as endoplasmic reticulum protein processing, mineral absorption, antigen processing, adipocytokine signaling, insulin secretion, insulin signaling pathway, and the HIF-1 signaling pathway. Their functions include lipid metabolism for energy production and the clearance of thermal and oxidative stress damage caused by heat stress.
Genes like upp2, ddx5, cdh, and sota1 are shared specifically with the maternal parent. These DEGs are enriched in pathways related to steroid biosynthesis, cholesterol metabolism, vascular smooth muscle contraction, and metabolic pathways. Their functions involve lipid metabolism for energy supply and protection against organismal damage.
Genes enriched in pathways such as fatty acid metabolism, proteasome metabolism, PPAR signaling pathway, unsaturated fatty acid biosynthesis, steroid biosynthesis, peroxisome, fatty acid elongation, and lipid synthesis are specifically shared with the paternal parent. Their roles encompass carbohydrate and lipid metabolism for energy supply, peroxisomal hydrolysis, antioxidant stress response, heat stress resistance, and anti-cell death functions.
This analysis highlights the specific genetic responses and enriched pathways in the Hulong hybrid grouper compared to its parental species under thermal stress conditions.
4 Discussion
4.1 Evaluation of high-temperature tolerance in hybrid grouper and its parental species
High water temperatures significantly affect the life activities of fish, sometimes posing a threat to their survival (Dong, 2022). In this study, we assessed the high-temperature tolerance of the hybrid grouper and its parental species, Epinephelus fuscoguttatus and Epinephelus lanceolatus.
The results indicate that the high-temperature tolerance among the three grouper species ranks from highest to lowest as follows: Epinephelus fuscoguttatus > the hybrid grouper (Epinephelus lanceolatus × Epinephelus fuscoguttatus) > Epinephelus lanceolatus. As the temperature stress increases, the fish exhibit varying degrees of stress responses, including reduced swimming ability, decreased feeding, surface floating, and changes in body coloration. Beyond the maximum tolerance limits, fish mortality occurs.
Similar behavioral changes have been observed in other fish species under temperature gradients. For instance, Monodactylus argenteus eggs showed adverse effects at spawning temperatures (29 ± 1°C), with reduced hatching rates and decreased yolk sac volumes (Thomas et al., 2022). In the case of hybrid groupers (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) at high water temperatures (32°C) compared to normal temperatures (28°C), faster growth rates were observed (Thalib et al., 2021). Temperature acclimatization in different species can lead to significant cardiac remodeling and changes at the molecular level in terms of electrical activity, energy utilization, and structural characteristics (Keen et al., 2017). Fundulus heteroclitus has shown temperature-specific adaptations in hypoxia tolerance under different temperature acclimatization regimes (Ridgway and Scott, 2023). Nile tilapia (Oreochromis niloticus) exhibited significantly increased microplastic ingestion at high temperatures of 36°C (Hasan et al., 2023). Hulong hybrid grouper exposed to different temperature fluctuations (15 ± 1°C, 15 ± 2°C, and 15 ± 3°C) during water deprivation showed decreased physiological function and immune defense capabilities (Mi et al., 2024). These studies collectively demonstrate that fish species undergo various impacts on hatching, growth and development, feeding, and survival capabilities under different temperature regimes. They exhibit long-term temperature adaptability during stress processes, characterized by delayed actions, changes in body coloration, reduced feeding, and ultimately, immobilization, leading to mortality.
In this study, the tiger grouper exhibited the highest heat tolerance, followed by the hybrid grouper (Epinephelus lanceolatus × Epinephelus fuscoguttatus), with the giant grouper displaying the lowest heat tolerance. This pattern is consistent across other hybrid grouper species. For instance, the Hulong hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) has an optimal temperature range of 27-30°C, with mortality observed after 12 hours at 33°C (Liu et al., 2014). As the hybrid offspring of Epinephelus lanceolatus and Epinephelus moara, the hybrid grouper (Epinephelus moara♀ × Epinephelus lanceolatus♂) exhibits high genetic similarity and close genetic distance to the Epinephelus moara it has a significant hybrid vigor (Tang et al., 2018). The high-temperature semi-lethal temperatures for the hybrid grouper (Epinephelus moara♀ × Epinephelus lanceolatus♂) and the hybrid grouper (Epinephelus coioides ♀ × Epinephelus lanceolatus♂) are 34.9°C and 37.9°C, respectively, higher than their maternal parent but lower than their paternal giant grouper (Shao et al., 2017). The heat tolerance of hybrid grouper (Epinephelus moara♀ × Epinephelus lanceolatus♂)reflects hybrid vigor, with heat tolerance closer to the heat-tolerant parent, similar to the findings of this study.
In contrast, hybrids of Clarias gariepinus × Clarias macrocephalus exhibit higher heat tolerance than their parents, with an optimal temperature of 32°C and higher growth rates and feed utilization efficiency (Khieokhajonkhet et al., 2022). Clarias gariepinus has an optimal temperature range of 25°C-30°C (Anderson and Fast, 1991), while Clarias macrocephalus prefers temperatures of 26°C-27°C (Zhao et al., 2009). Unlike our study, hybrids of Clarias gariepinus × Clarias macrocephalus show stronger heat tolerance than their parents, demonstrating hybrid vigor.
Microsatellite analysis indicates a genetic distance of 0.9006 and 1.8195 between hybrids and the maternal yellow catfish and the paternal Pelteobagrus vachelli, respectively, suggesting asymmetrical genetic differences favoring the maternal parent, similar to our study (Zhang et al., 2018). These studies collectively illustrate that hybrid offspring inherit genes for heat tolerance from the parent with higher heat tolerance. In our study, the heat tolerance of the hybrid grouper (Epinephelus lanceolatus × Epinephelus fuscoguttatus) predominantly derives from the maternal parent.
In the high-temperature and normal-temperature conditions observed in the images of the three grouper species, the hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) and the tiger grouper (Epinephelus fuscoguttatus) exhibit noticeable differences in body color intensity, while the giant grouper (Epinephelus lanceolatus) shows variations in its spot pattern. This indicates that all three grouper species experienced stress under 36°C water temperature conditions. Whether this stress level affects long-term survival and reproduction needs further investigation.
Wild tiger groupers generally exhibit lighter colors, leaning towards yellow compared to their counterparts in aquaculture, which tend towards a darker brown color. Under certain temperatures, especially high temperatures, aquaculture tiger groupers may resemble their wild counterparts more closely. The hybrid grouper, being an artificially hybridized species without a natural counterpart (Liao, 2019), shows slightly lighter body coloration in the high-temperature group compared to the normal group, with a contrast similar to its maternal tiger grouper.
Gaint groupers start off yellow with irregular black markings in their youth, which develop white or yellow spots within the black markings as they mature, and adults have black spots on their fins (Zhou et al., 2022). In this study, giant groupers exhibited overall black-grey bodies with white or golden patches and golden fins with brown stripes. Both high-temperature and normal-temperature groups showed changes in body coloration, with the high-temperature group experiencing slightly faster and more frequent color changes. However, the trend of color change in the fish did not show significant differences, contrary to reports by Fernando et al (Fernando et al., 2022), who noted a fading to grey coloration in aquaculture giant groupers, possibly due to differences in fish source and feed. The developmental stages and body shapes of artificially reared giant groupers differ from those of wild juveniles, gradually resembling the growth conditions and body forms of wild fish during aquaculture, with appropriate high temperatures potentially accelerating this transformation to some extent.
4.2 Growth characteristics of Hulong hybrid and its parents under different temperature stress
For most fish species, body temperature closely mirrors the temperature of their habitat (Guderley, 2004). Chronic temperature increases can alter a fish’s ability to cope with additional stressors, while rapid temperature increases can trigger acute stress responses in fish (Alfonso et al., 2021). Studies have shown that fish and other poikilotherms living in warm waters often grow faster during their juvenile stages and mature earlier but tend to be smaller as adults, a pattern known as the Temperature-Size Rule (TSR) (Wootton et al., 2022). Studies by Fan et al. found enhanced metabolic enzyme activity in tiger grouper (Epinephelus fuscoguttatus) at 25°C while the fish maintained lower metabolic levels at 15°C, leading to improved survival capability (Fan et al., 2021). These findings differ from our study, possibly due to differences in temperature ranges adapted by different fish species.
In this experiment, juvenile fish of three grouper species were reared for 60 days at 36°C and 28°C. Contrary to expectations, there was no significant deterioration in growth performance observed in the juvenile fish of the 36°C high-temperature treatment group. Similar results have been found in other studies related to temperature and fish growth. For instance, studies by Mai et al. on the effects of water temperature on the growth of sturgeons (Acipenser schrenckii, A. sinensis, and A. baerii) found that growth rates were highest at 24°C, with growth rates increasing between 18°C and 24°C and decreasing between 24°C and 27°C (Mai et al., 2014). Shi et al. demonstrated that juvenile crown dace (Tribolodon brandtii) exhibited the highest growth coefficients in length and weight at 28°C, with the lowest at 24°C, and moderate growth coefficients at 30°C and 26°C (Shi et al., 2017). Deng’s study on grass carp (Ctenopharyngodon idella) found that compared to 18°C and 32°C, juvenile grass carp had higher weight gain rates, specific growth rates, lower feed conversion ratios, and better condition factors and survival rates at 25°C (Deng, 2022) Li’s study on black sea bass (Centropristis striata) showed that under temperature gradients of 18°C, 22°C, 26°C, and 30°C, juvenile black sea bass exhibited trends of increasing and then decreasing final weight, specific growth rate, daily weight gain, gutted body weight, and gutted body length, with peaks at 22°C and 26°C, although the differences were not significant (Li et al., 2021).
Our study aligns with these findings in terms of growth performance, showing advantages in high-temperature growth within a certain temperature range. However, significant differences were not observed between the tiger grouper and the Hulong hybrid grouper in terms of stress, and indicators such as the condition factor did not show significant differences in this experiment.
4.3 Physiological and biochemical analysis of Hulong hybrid grouper and its parents under different temperature stress
Cortisol, produced by the adrenal cortex of vertebrates, is a reliable stress indicator and is considered the primary stress-related hormone in teleost fish (Sadoul and Geffroy, 2019). In this study, cortisol levels in the serum of giant grouper, tiger grouper, and Hulong hybrid grouper (Figure 5) were significantly influenced by high temperatures. Cortisol levels in the high-temperature group consistently exceeded those in the normal-temperature group, whereas alkaline phosphatase levels showed an initial increase followed by a decrease, although the regularity of this pattern was less pronounced compared to the temperature groups.
Figure 5
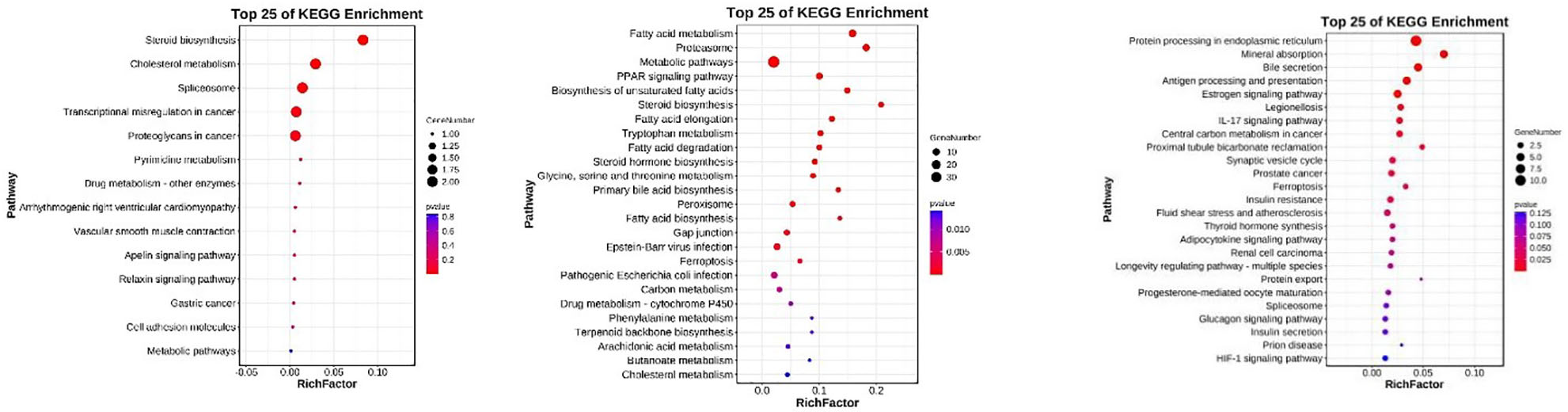
KEGG enrichment of common differential genes between progeny and parents. EE represents the Hulong hybrid Grouper, EL represents the Giant Grouper, and EF represents the Tiger Grouper.
During the masculinization process of Atherinella hubbsi, high temperatures lead to elevated cortisol levels, which is similar to the results of this study (Hattori et al., 2009). Research has shown that four major fish families exhibit significantly increased cortisol levels in response to predation stress (Hughes et al., 2017). In European sea bass (Dicentrarchus labrax), there is a positive correlation between cortisol levels and temperature (Alfonso et al., 2023).
Under 60 days of high-temperature stress, enzyme activity assays for CAT, SOD, HSP70, and HSP90 in the liver of three grouper species exhibited a trend of initial increase followed by a decrease, showing significant differences compared to the control group maintained at a normal temperature. Fish possess a system to scavenge oxidative free radicals generated during physiological metabolism to maintain them at relatively normal levels. However, fluctuations in external factors such as temperature, starvation, and salinity induce stress in fish, resulting in the excessive production of free radicals. Excess reactive oxygen species can damage normal cells and tissues, triggering lipid peroxidation and consequent injury to the fish (Mourente et al., 2002).
In this experiment, the variations in the hsp family were consistently observed throughout the high-temperature stress period. Specifically, levels of HSP90 and HSP70 remained consistently higher in the high-temperature group compared to the control, although the difference in HSP70 was less significant. Additionally, levels of SOD and CAT showed significant differences between the high-temperature and control groups. Specifically, CAT levels in the high-temperature groups of giant grouper and tiger grouper were consistently higher than those in the control group, whereas the opposite trend was observed in the hybrid grouper. As for SOD levels, they were consistently lower in the high-temperature groups of tiger grouper and hybrid grouper compared to the control, whereas giant grouper showed the opposite trend.
Previous studies have indicated that the induction temperature for Hsp70 in the tropical apple snail Pomacea canaliculata is 36°C, with maximum expression at 42°C, and expression slightly decreases at temperatures below 16°C (Song et al., 2014). Similarly, in sublethal heat exposure of the temperate sea cucumber Apostichopus japonicus, green individuals exhibited higher thermal tolerance and higher Hsp70 mRNA levels compared to red individuals (Dong et al., 2010). Research on how water temperature affects the brain of rainbow trout found that high-temperature stress led to decreased mRNA expression levels of CAT, SOD, GPx, and Bcl2, increased mRNA expression of BDNF, cFOS, apoptosis genes (caspase 3, Bax), and heat shock genes (Hsp70 and Hsp90), resulting in physiological changes in the fish brain (Topal et al., 2021). Studies on American shad (Alosa sapidissima) revealed that elevated temperatures increased the activity of SOD, CAT, and cortisol, while MDA, ALP, and LDH initially increased and then decreased, with irregular changes observed in T-AOC and Na-K-ATPase (Luo et al., 2024). In a thermal stimulation study on the rare native fish Gymnocypris przewalskii from Qinghai Lake on the Tibetan Plateau, superoxide dismutase (SOD) and total antioxidant capacity (T-AOC) significantly increased at 20°C (P < 0.05), while Hsp70 and Hsp90 were significantly higher than the control group (16°C) at 18°C and 26°C respectively, reaching a peak at 26°C (Liu et al., 2023). In this experiment, changes in HSP70 and HSP90 were observed throughout the entire process, while SOD and CAT showed a pattern of low-high-low changes, similar to Gymnocypris przewalskii but different from rainbow trout, although similar results were reported by Wang in studies on crucian carp (Wang and Yang, 2021). These cases illustrate that fish exhibit significant changes in the activity of certain enzymes in their serum and liver when exposed to high-temperature stress, primarily involving stress enzymes, antioxidant enzymes, and immune enzymes, showing correlations with temperature and time.
4.4 Genetic analysis of heat tolerance traits in hybrid groupers
In this study, the results indicate that HSPs, SLCs, and DNAJs are the most significant shared differential genes between offspring and parents, primarily inherited from the maternal tiger grouper. Specifically, genes such as HSP90b1, HSPd1, SLC35d2, SLC21a1, and SLC35b1 are classified under the PPAR signaling pathway according to GO annotations. Similarly, Duan et al. conducted a transcriptome analysis on Epinephelus moara under temperature stress, identifying pathways related to binding, catalytic activity, metabolic processes, carbon metabolism, complement and coagulation cascades, AMPK signaling pathway, FoxO signaling pathway, ECM receptor interaction, PPAR signaling pathway, and the differential expression of HSP-related genes such as acsl1, SLC27a2, HSP30, HSP70, HSP90aa1, and HSP90b1; this underscores the significant association of the PPAR signaling pathway with temperature tolerance in groupers, regulated by SLCs and HSPs genes, highlighting their critical roles in temperature regulation in fish (Duan et al., 2023).
Swirplies et al. used fluorescence identification experiments on prickly sculpin to demonstrate that indicators of high-temperature stress are concentrated in the liver and gills, involving heat shock and hypoxia responses, immune activation, energy supply, and developmental pathways (Swirplies et al., 2019), indicating related responses in oxygen and energy supply under high-temperature stress. Similar results are evident in freshwater fish studies, where significant changes in gene expression of hsp families, DNAJ families, and HSD families were observed under high-temperature stress in species like Hucho hucho and Nile tilapia, classified into pathways such as energy metabolism, catalysis, and binding, protein processing, immune stress, enriched in the PPAR signaling pathway, the FoxO signaling pathway, the ECM receptor interaction signaling pathway, the MAPK signaling pathway, and the GnRh signaling pathway. In this study, MAPK, FOX, and PPAR signaling pathways were detected, with HSPs and DNAJB playing crucial roles in the heat stress response, emphasizing the strong influence and potentially critical roles of hsp families, DNAJ families, and SLC families in the strategies employed by fish to cope with high-temperature stress (Feng et al., 2023).
Similarly, Ding et al. conducted a genome-wide association study (GWAS) on acute heat tolerance (AHT) in yellow croaker, identifying 30 candidate genes such as HSP1, DNAJB4, hikeshi protein, and protein disulfide isomerase A3 protein based on their findings (Ding et al., 2022).
In addition, this study identified through enrichment analysis that the elov gene series inherited from the paternal parent, along with MOGAT and DGAT genes, are significantly enriched and play roles in fatty acid metabolism. Similarly, Jin’s study on salmon fry transitioning from endogenous to exogenous lipid metabolism found that triacylglycerols can inhibit MOGAT and DGAT genes, leading to the accumulation of monoacylglycerols, which impacts energy supply (Jin et al., 2019). The inhibition of the MOGAT gene results in the accumulation of monoacylglycerols, thereby affecting energy supply, highlighting the regulatory and feedback roles of MOGAT and DGAT in lipid metabolism. For instance, Wang studied the impact of elovl8 deficiency on survival and lipid metabolism under cold stress in zebrafish, and the results indicated that elovl8 deficiency significantly reduced survival rates of zebrafish in cold environments, accompanied by notable lipid deposition and steatosis in the liver. Subsequent fatty acid analysis of elovl8-deficient zebrafish under cold stress revealed a significant accumulation of C20:0 fatty acids in the liver (Wang et al., 2022). Elovl8 likely plays a crucial role in lipid metabolism and provides energy for fish during stress activities. In another study, Lu found high expression of Elovl6 in the northern cold waters of Ruditapes Philippines arum, further demonstrating the important role of elovl genes in biological temperature regulation and energy supply processes. The fatty acid metabolism-related genes discovered in this research provide valuable insights into responses to cold stress (Nie et al., 2023).
Antioxidant genes, heat shock genes, and energy supply in lipid metabolism play crucial roles in responding to high-temperature stress. Antioxidant oxidative stress is primarily regulated by gene families such as SLC, HSP, and DNAJ, while energy supply relies mainly on the ELOVL gene family.
5 Conclusion
The sequencing analysis of three grouper species under high-temperature stress in this experiment, alongside findings from other high-temperature studies, reveals the hybrids inherit heat regulation genes from both parents, with a higher proportion from the maternal parent, which likely explains their intermediate heat tolerance. Specifically, the inheritance of heat tolerance correlates with the parent species exhibiting stronger heat tolerance. Furthermore, genomic studies indicate that these genetic factors are enriched in signaling pathways, including PPAR signaling, FoxO signaling, MAPK signaling, and GnRH signaling. Among these pathways, gene families such as SLC, HSP, and DNAJ exhibit significant regulatory roles.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The animal studies were approved by specific research fund of The Innovation Platform for Academicians of Hainan Province. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
YH: Writing – review & editing, Writing – original draft, Conceptualization. YT: Writing – original draft, Software. JuL: Writing – review & editing, Investigation. HT: Writing – review & editing, Data curation. KW: Writing – review & editing, Data curation. FT: Writing – review & editing, Methodology. XW: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Formal analysis. JiL: Writing – review & editing, Supervision, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the specific research fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202122); Key R&D Project in Hainan (ZDYF2023XDNY046); The Innovation Center of Hainan University(XTCX2022NYC16).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1466656/full#supplementary-material
References
1
Alfonso S. Gesto M. Sadoul B. (2021). Temperature increase and its effects on fish stress physiology in the context of global warming. J. fish Biol.98, 1496–1508. doi: 10.1111/jfb.14599
2
Alfonso S. Houdelet C. Bessa E. Geffroy B. Sadoul B. (2023). Water temperature explains part of the variation in basal plasma cortisol level within and between fish species. J. fish Biol.103, 828–838. doi: 10.1111/jfb.15342
3
Anderson M. J. Fast A. W. (1991). Temperature and feed rate effects on Chinese catfish, Clarias fuscus (Lacepede), growth. Aquac. Res.22, 435–442. doi: 10.1111/j.1365-2109.1991.tb00756.x
4
Asseng S. Spänkuch D. Hernandez-Ochoa I. M. Laporta J. (2021). The upper temperature thresholds of life. Lancet Planetary Health5, e378–e385. doi: 10.1016/S2542-5196(21)00079-6
5
Chen C. (2005). Technical Manual of Main Aquatic Economic Biological Development Vol. 08 (China Agriculture Press), 260–261.
6
Cheng C. H. Yang F. F. Liao S. A. Miao Y. T. Ye C. X. Wang A. L. et al . (2015). High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J. Thermal Biol.53, 172–179. doi: 10.1016/j.jtherbio.2015.08.002
7
Clarke A. Johnston N. M. (1999). Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol.68, 893–905. doi: 10.1046/j.1365-2656.1999.00337.x
8
Das S. K. Xiang T. W. Noor N. M. De M. Mazumder S. K. Goutham-Bharathi M. P. (2021). Temperature physiology in grouper (Epinephelinae: Serranidae) aquaculture: A brief review. Aquac. Rep.20, 100682. doi: 10.1016/j.aqrep.2021.100682
9
De M. Ghaffar M. A. Bakar Y. Das S. K. (2016). Effect of temperature and diet on growth and gastric emptying time of the hybrid, Epinephelus fuscoguttatus♀× E. lanceolatus♂. Aquac. Rep.4, 118–124. doi: 10.1016/j.aqrep.2016.08.002
10
De M. Ghaffar M. A. Noor N. M. Cob Z. C. Bakar Y. Das S. K. (2019). Effects of water temperature and diet on blood parameters and stress levels in hybrid grouper (Epinephelus fuscoguttatus♀× E. lanceolatus♂) juveniles. Aquac. Rep.15, 100219. doi: 10.1016/j.aqrep.2019.100219
11
Deng Z. (2022). Effects of temperature change on growth performance of juvenile Ctenopharyngodon idella. Northern Chin. Fisheries41, 26–28. doi: 10.3969/j.issn.1674-2419.2022.04.008
12
Ding J. Zhang Y. Wang J. Liu C. Gao X. Wu Y. et al . (2022). Genome-wide association study identified candidate SNPs and genes associated with hypoxia tolerance in large yellow croaker (Larimichthys crocea). Aquaculture560, 738472. doi: 10.1016/j.aquaculture.2022.738472
13
Dong F. L. (2022). Physiological and biochemical response and transcriptome analysis of rainbow trout under high temperature stress. Shanghai Ocean Univ.
14
Dong Y. W. Ji T. T. Meng X. L. Dong S. L. Sun W. M. (2010). Difference in thermotolerance between green and red color variants of the Japanese sea cucumber, Apostichopus japonicus Selenka: Hsp70 and heat-hardening effect. Biol. Bull.218, 87–94. doi: 10.1086/BBLv218n1p87
15
Duan P. Tian Y. Qiu Y. Wang X. Ding X. Li Z. et al . (2023). Gastric evacuation and digestive enzyme activity in juveniles of hybrid Epinephelus fuscoguttatus (♀)×Epinephelus tukula (♂) and Hybrid E. fuscoguttatus (♀)×Epinephelus lanceolatus (♂). J. Guangdong Ocean Univ.43, 119–126. doi: 10.3969/j.issn.1673-9159.2023.01.015
16
Fan X. Zhang J. Guo Q. Tu H. Qin X. Liu S. (2021). Effect of CO2 anesthesia on water-free live-transport of the grouper (Epinephelus fuscoguttatus♀ ×Epinephelus laceolatus♂). J. Guangdong Ocean Univ.41, 73–81. doi: 10.3969/j.issn.1673-9159.2021.06.009
17
Fang M. Lei Z. Ruilin M. Jing W. Leqiang D. (2023). High temperature stress induced oxidative stress, gut inflammation and disordered metabolome and microbiome in tsinling lenok trout. Ecotoxicol. Environ. Saf.266, 115607. doi: 10.1016/j.ecoenv.2023.115607
18
Feng C. Liu X. Zhang Y. Lü W. Han S. Zhang Y. et al . (2023). Histological structure and transcriptome characteristics in liver of juvenile Amur grayling (Thymallus arcticus grubei) under high temperature stress. J. Dalian Ocean Univ.38, 603–614. doi: 10.16535/j.cnki.dlhyxb.2022-237
19
Fernando F. Candebat C. L. Strugnell J. M. Andreakis N. Nankervis L. (2022). Dietary supplementation of astaxanthin modulates skin color and liver antioxidant status of giant grouper (Epinephelus lanceolatus). Aquac. Rep.26, 101266. doi: 10.1016/j.aqrep.2022.101266
20
Frölicher T. L. Fischer E. M. Gruber N. (2018). Marine heatwaves under global warming. Nature560, 360–364. doi: 10.1038/s41586-018-0383-9
21
Ghosh S. K. Wong M. K. S. Hyodo S. Goto S. Hamasaki K. (2022). Temperature modulation alters the gut and skin microbial profiles of chum salmon (Oncorhynchus keta). Front. Mar. Sci.9. doi: 10.3389/fmars.2022.1027621
22
Gracia-López V. Kiewek-Martínez M. Maldonado-García M. (2004). Effects of temperature and salinity on artificially reproduced eggs and larvae of the leopard grouper Mycteroperca rosacea. Aquaculture237, 485–498. doi: 10.1016/j.aquaculture.2004.04.018
23
Guderley H. (2004). Metabolic responses to low temperature in fish muscle. Biol. Rev. Cambridge Philos. Soc.79, 409–427. doi: 10.1017/s1464793103006328
24
Hasan J. Siddik M. A. Ghosh A. K. Mesbah S. B. Sadat M. A. Shahjahan M. (2023). Increase in temperature increases ingestion and toxicity of polyamide microplastics in Nile tilapia. Chemosphere327, 138502. doi: 10.1016/j.chemosphere.2023.138502
25
Hattori R. S. Fernandino J. I. Kishii A. Kimura H. Kinno T. Oura M. et al . (2009). Cortisol-induced masculinization: does thermal stress affect gonadal fate in pejerrey, a teleost fish with temperature-dependent sex determination? PloS One4, e6548. doi: 10.1371/journal.pone.0006548
26
Howell P. J. Dunham J. B. Sankovich P. M. (2010). Relationships between water temperatures and upstream migration, cold water refuge use, and spawning of adult bull trout from the Lostine River, Oregon, USA. Ecol. Freshw. Fish19, 96–106. doi: 10.1111/j.1600-0633.2009.00393.x doi: 10.14012/j.cnki.fjsc.2021.04.008 doi: 10.3969/j.issn.1005-3832.2014.04.004
27
Hughes T. P. Kerry J. T. Álvarez-Noriega M. Álvarez-Romero J. G. Anderson K. D. Baird A. H. et al . (2017). Global warming and recurrent mass bleaching of corals. Nature543, 373–377. doi: 10.1038/nature21707
28
Jin Y. Olsen R. E. Østensen M. A. Gillard G. B. Li K. Harvey T. N. et al . (2019). Transcriptional regulation of lipid metabolism when salmon fry switches from endogenous to exogenous feeding. Aquaculture503, 422–429. doi: 10.1016/j.aquaculture.2018.12.089
29
Keen A. N. Klaiman J. M. Shiels H. A. Gillis T. E. (2017). Temperature-induced cardiac remodelling in fish. J. Exp. Biol.220, 147–160. doi: 10.1242/jeb.128496
30
Khieokhajonkhet A. Sangphrom S. Aeksiri N. Tatsapong P. Wuthijaree K. Kaneko G. (2022). Effects of long-term exposure to high temperature on growth performance, chemical composition, hematological and histological changes, and physiological responses in hybrid catfish [♂Clarias gariepinus (Burchell 1822) ×♀C. macrocephalus (Günther 1864). J. Thermal Biol.105, 103226. doi: 10.1016/j.jtherbio.2022.103226
31
Li W. Y. Xu Z. J. Yi X. J. Chen S. Ma X. B. Zhang X. et al . (2021). Effects of temperature on growth, immune factor activity and related gene expression of juvenile Centropristis striata. Oceanol. Et Limnol. Sin.52, 708–717. doi: 10.11693/hyhz20200900257
32
Liao J. (2019). Twenty years of grouper seed industry: from a single seedling to the emergence of improved varieties. Ocean Fishery2019, 78–79. doi: 10.3969/j.issn.1672-4046(s).2019.12.034
33
Lin X. Xie S. Su Y. Cui Y. (2008). Optimum temperature for the growth performance of juvenile orange-spotted grouper (Epinephelus coioides H.). Chin. J. Oceanol. Limnol.26, 69–75. doi: 10.1007/s00343-008-0069-5
34
Liu S. Chen S. Lu C. Qi D. Qi H. Wang Y. et al . (2023). Fatty acid metabolism and antioxidant capacity in Gymnocypris przewalskii (Kessler 1876) response to thermal stress. J. Thermal Biol.116, 103650. doi: 10.1016/j.jtherbio.2023.103650
35
Liu Y. Zhong Y. Zeng Q. Li J. Xie Y. Hu J. et al . (2014). Effects of temperature and salinity on the growth and survivability of young Epinephelus moara. J. Jimei Univ. (Natural Science), 241–246. doi: 10.3969/j.issn.1007-7405.2014.04.001
36
Luo M. Zhu W. Liang Z. Feng B. Xie X. Li Y. et al . (2024). High-temperature stress response: Insights into the molecular regulation of American shad (Alosa sapidissima) using a multi-omics approach. Sci. Total Environ.916, 170329. doi: 10.1016/j.scitotenv.2024.170329
37
Mahmoud S. Sabry A. Abdelaziz A. Shukry M. (2020). Deleterious impacts of heat stress on steroidogenesis markers, immunity status and ovarian tissue of Nile tilapia (Oreochromis niloticus). J. Thermal Biol.91, 102578. doi: 10.1016/j.jtherbio.2020.102578
38
Mai L. Liu X. Pan P. Sun D. (2014). Effects of water temperature on growth performance in juvenile Amur Sturgeon, Sterlet and Siberian Sturgeon. Chin. J. Fisheries, 15–22.
39
Mi H. Zhang T. Lu Y. Chen J. Li X. (2024). Effect of temperature fluctuation on the physiological stress response of hybrid pearl gentian grouper during waterless keeping alive. Fish Physiol. Biochem.50, 927–939. doi: 10.1007/s10695-024-01307-8
40
Mourente G. Dıaz-Salvago E. Bell J. G. Tocher D. R. (2002). Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: attenuation by dietary vitamin E. Aquaculture214, 343–361. doi: 10.1016/s0044-8486(02)00064-9
41
Nie H. Lu Z. Li D. Dong S. Li N. Chen W. et al . (2023). The potential roles of fatty acid metabolism-related genes in Manila clam Ruditapes philippinarum under cold stress. Aquaculture562, 738750. doi: 10.1016/j.aquaculture.2022.738750
42
Ridgway M. R. Scott G. R. (2023). Constant temperature and fluctuating temperature have distinct effects on hypoxia tolerance in killifish (Fundulus heteroclitus). J. Exp. Biol.226, jeb245425. doi: 10.1242/jeb.245425
43
Sadoul B. Geffroy B. (2019). Measuring cortisol, the major stress hormone in fishes. J. fish Biol.94, 540–555. doi: 10.1111/jfb.13904
44
Shahjahan M. Kitahashi T. Ando H. (2017). Temperature affects sexual maturation through the control of kisspeptin, kisspeptin receptor, GnRH and GTH subunit gene expression in the grass puffer during the spawning season. Gen. Comp. Endocrinol.243, 138–145. doi: 10.1016/j.ygcen.2016.11.012
45
Shao Y. Chen C. Zhang T. Li Y. Zhang M. Li W. (2017). Influence of high temperature stress on survival rate and serum biochemical indexes of 2 Epinephelus Hybrids. J. Guangdong Ocean Univ.37, 89–95. doi: 10.3969/j.issn.1673-9159.2017.06.014
46
Sherif A. H. Farag E. A. H. Mahmoud A. E. (2024). Temperature fluctuation alters immuno-antioxidant response and enhances the susceptibility of Oreochromis niloticus to Aeromonas hydrophila challenge. Aquacult. Int.32, 2171–2184. doi: 10.1007/s10499-023-01263-9
47
Shi J. Wang Y. Wang F. Xie B. Yang L. Feng C. (2017). Influence of temperature on the growth of Juvenile Chromobotia macracanthus. Freshw. Fisheries, 102–105. doi: 10.13721/j.cnki.dsyy.2017.05.016
48
Song H. M. Mu X. D. Gu D. E. Luo D. Yang Y. X. Xu M. et al . (2014). Molecular characteristics of the HSP70 gene and its differential expression in female and male golden apple snails (Pomacea canaliculata) under temperature stimulation. Cell Stress Chaperones19, 579–589. doi: 10.1007/s12192-013-0485-0
49
Spinks R. K. Bonzi L. C. Ravasi T. Munday P. L. Donelson J. M. (2021). Sex- and time-specific parental effects of warming on reproduction and offspring quality in a coral reef fish. Evolutionary Appl.14, 1145–1158. doi: 10.1111/eva.13187
50
Swirplies F. Wuertz S. Baßmann B. Orban A. Schäfer N. Brunner R. M. et al . (2019). Identification of molecular stress indicators in pikeperch Sander lucioperca correlating with rising water temperatures. Aquaculture501, 260–271. doi: 10.1016/j.aquaculture.2018.11.043
51
Tang J. Tian Y. Li Z. Cheng M. Chen Z. Mao D. et al . (2018). Analysis of genetic characters in Epinephelus moara, E. lanceolaus and their hybrids. J. Agric. Biotechnol., 819–829.
52
Thalib Y. A. Razali R. S. Mohamad S. Zainuddin R. Rahmah S. Ghaffar M. A. et al . (2021). Environmental changes affecting physiological responses and growth of hybrid grouper - The interactive impact of low pH and temperature. Environ. pollut. (Barking Essex: 1987)271, 116375. doi: 10.1016/j.envpol.2020.116375
53
Thomas D. Rekha M. U. Angel J. R. J. Sreekanth G. B. Sukumaran K. Sandeep K. P. et al . (2022). The effect of acclimation temperature and optimal temperature gradient for egg and larvae of silver moony (Monodactylus argenteus) during the early ontogenesis. Environ. Sci. pollut. Res.29, 35422–35433. doi: 10.1007/s11356-021-18329-x
54
Topal A. Özdemir S. Arslan H. Çomaklı S. (2021). How does elevated water temperature affect fish brain? (A neurophysiological and experimental study: Assessment of brain derived neurotrophic factor, cFOS, apoptotic genes, heat shock genes, ER-stress genes and oxidative stress genes). Fish shellfish Immunol.115, 198–204. doi: 10.1016/j.fsi.2021.05.002
55
Volkoff H. Rønnestad I. (2020). Effects of temperature on feeding and digestive processes in fish. Temp. (Austin Tex.)7, 307–320. doi: 10.1080/23328940.2020.1765950
56
Wang Y. Sun S. He J. Yang G. Gao J. (2022). Effects of elovl8 deletion on survival and lipid metabolism under cold stress of zebrafish. Acta Hydrobiologica Sin.46, 303–315. doi: 10.7541/2022.2021.063
57
Wang X. Yang T. (2021). Effects of acute hypoxia stress on blood biochemical indices of Carassius crassius. Fishery Modernization48, 36. doi: 10.3969/j.issn.1007-9580.2021.06.005
58
Wootton H. F. Morrongiello J. R. Schmitt T. Audzijonyte A. (2022). Smaller adult fish size in warmer water is not explained by elevated metabolism. Ecol. Lett.25, 1177–1188. doi: 10.1111/ele.13989
59
Zhang X. (2009). Technology of factory farming of tiger spot in northern China. Sci. Fish Farming02, 25.
60
Zhang J. Li J. Zhang G. Wang T. Yi S. Tang Z. et al . (2018). Microsatellite-based Analysis of Genetic Diversity of Parent and Hybrid of Yellow Catfish Pelteobagrus fulvidraco (♀)×P. vachelli (♂). Fisheries Sci.37, 612–621. doi: 10.16378/j.cnki.1003-1111.2018.05.006
61
Zhao H. Han D. Xie S. Zhu X. & Yang Y. (2009). Effect of water temperature on the growth performance and digestive enzyme activities of Chinese longsnout catfish (Leiocassis longirostris Günther). Aquac. Res.40, 1864–1872. doi: 10.1111/j.1365-2109.2009.02292.x
62
Zhou K. Zhang K. Fan X. Zhang W. Liang Y. Wen X. et al . (2022). The skin-color is associated with its physiological state: A case study on a colorful variety, hybrid grouper (Epinephelus fuscoguttatus× Epinephelus lanceolatus). Aquaculture549, 737719. doi: 10.1016/j.aquaculture.2021.737719
Summary
Keywords
hybrid grouper, high temperature tolerance, genetic analysis, gene expression, physiological analysis
Citation
Hu Y, Tan Y, Liu J, Tang H, Wang K, Tang F, Luo J and Wen X (2024) Analysis of the response to high temperature stress in hybrid grouper (Epinephelus fuscoguttatus♀×E. lanceolatus♂). Front. Mar. Sci. 11:1466656. doi: 10.3389/fmars.2024.1466656
Received
18 July 2024
Accepted
05 September 2024
Published
29 October 2024
Volume
11 - 2024
Edited by
Mohammed Fouad El Basuini, Tanta University, Egypt
Reviewed by
Akram Shehata, Alexandria University, Egypt
Amit Ranjan, Tamil Nadu Fisheries University, India
Updates
Copyright
© 2024 Hu, Tan, Liu, Tang, Wang, Tang, Luo and Wen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Wen, wenxin@hainanu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.