Abstract
Salt marshes are one of the three blue carbon ecosystems recognized by the Intergovernmental Panel on Climate Change (IPCC). However, coastal salt marshes in China are facing the risk of degradation. To reveal the status of the salt marsh wetland ecosystem in Liaohe Estuary, an Ecopath model composed of 14 functional groups was constructed based on the 2019 ecological survey data. A comprehensive analysis of the system’s food web structure, energy flow processes, and overall ecosystem characteristics was conducted. The results show that the energy flow in the Liaohe Estuary salt marsh wetland ecosystem is mainly distributed in three integrated trophic levels. The utilization rates of trophic levels II and III are low, easily causing blockages in the lower trophic levels of the ecosystem’s energy flow. The total system throughput of the Liaohe Estuary salt marsh wetland ecosystem is 49,099.039 t·km²·a−1;. The system connectivity index and the system omnivory index are 0.207 and 0.109, respectively. Compared with other wetland systems, the ecosystem has a larger scale, but the overall ecosystem characteristic index reveals lower stability and complexity of the Liaohe Estuary salt marsh wetland system.
1 Introduction
The Liaohe Estuary wetland is located at the top of Liaodong Bay, with a total area of more than 80,000 hectares, located at longitude 121°28′24.58″ to 121°58′27.49″E and latitude 40°45′00″ to 41°05′54.13″N. With its unique geographical position and distinctive vegetation types, it serves as a habitat for various rare animal species. In addition to its capabilities in flood prevention, disaster mitigation, and water conservation, the Liaohe Estuary Wetland also plays a crucial role in environmental protection. In recent years, due to human activities such as oil extraction, reed field development, and aquaculture in the Liaohe Estuary area, the wetland area has shrunk, vegetation coverage has decreased, and the wetland has become increasingly arid, leading to a decline in the overall quality of the ecosystem and further affecting the habitat environment of endangered species. The issues of wetland protection and development have become increasingly prominent. Researchers have conducted extensive studies in this area, focusing mainly on environmental organic pollutants (Liu et al., 2022), wetland carbon sequestration (Wan et al., 2018), changes in wetland landscape patterns (Wang et al., 2023),and analysis of wetland degradation driving forces (Li et al., 2022). However, there is still a lack of ecosystem-level understanding of the structure and function of the Liaohe Estuary salt marsh wetland ecosystem and its development degree.
The protection and rational utilization of wetlands need to be conducted at the ecosystem level. Ecological models can study the structure and function of ecosystems, the spatial and temporal evolution of ecosystems, the impact of biological processes on ecosystems, and their feedback mechanisms. Ecological models are a simplification of natural ecosystems, emphasizing system characteristics and concerns based on a thorough understanding of the ecosystem. The Ecopath with Ecosim (EwE) ecological model consists of three modules: Ecopath, Ecosim, and Ecospace. In the early 1980s, Polovina (1984) first proposed the Ecopath model to estimate the biomass and food consumption of aquatic ecosystems. Currently, researchers integrate the latest monitoring data with the EwE model to support ecosystem-based fisheries management (Craig and Link, 2023; Karp et al., 2023), explore the impacts of multiple stressors on the structure and function of marine ecosystems (Papantoniou et al., 2023; Stock et al., 2023), quantify fisheries management strategies under climate change (Yin et al., 2023), assess the impact of environmental pollution on marine food webs (Gao et al., 2024), and simulate changes in current and future scenarios of wetland ecosystems to develop wetland conservation and restoration strategies (Smith et al., 2023).
To understand and grasp the structure and energy flow of the Liaohe Estuary salt marsh wetland ecosystem, this study investigates the food web structure of the Liaohe Estuary salt marsh wetland, constructs an Ecopath model based on the analysis of the food web structure, and quantitatively clarifies the structure and energy flow process of the Liaohe Estuary salt marsh wetland ecosystem. The aim is to provide a scientific basis for the utilization of biological resources in the estuary area and further research on the ecosystem, as well as for the restoration, protection, and rational development of the Liaohe Estuary salt marsh wetland based on the ecosystem.
2 Materials and methods
2.1 Overview of the study area
The study area is located in the undisturbed tidal flat wetlands of Yuanyang Island and its surrounding waters in the Liaohekou National Nature Reserve (Figure 1). Yuanyang Island is a newly formed alluvial island, which was officially included in the national island registry in July 2013 (Hou et al., 2019). The study area is minimally impacted by human activities, maintaining a state relatively close to its natural condition.
Figure 1
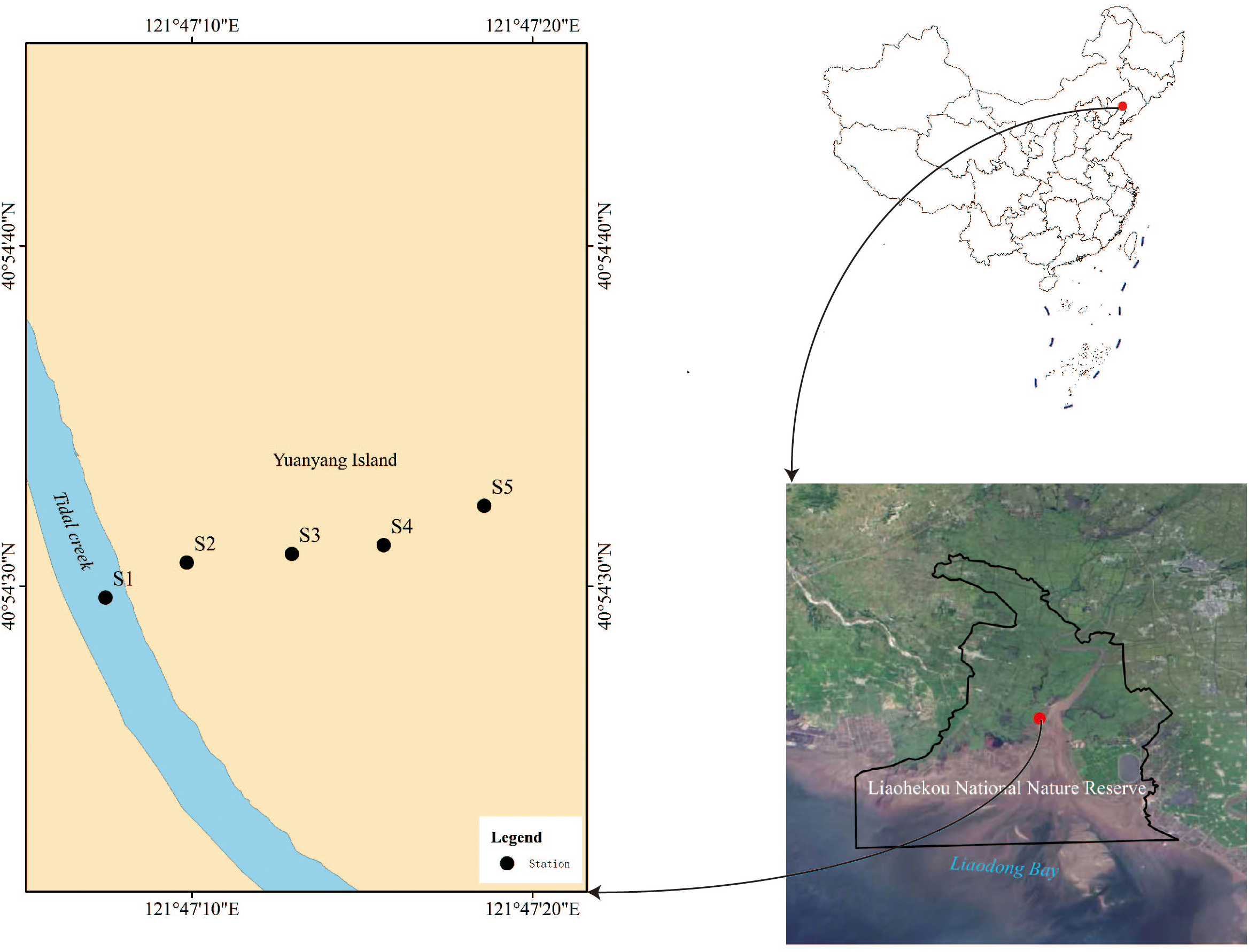
The geographic location and station distribution of the study area.
Based on the current environmental conditions of the tidal flat wetlands in the Liaohe Estuary area and preliminary field surveys, five transect sites were selected perpendicular to the tidal creek. These sites include tidal creek, bare flat zone, Suaeda salsa zone, Suaeda salsa–Phragmites communis transition zone, and Phragmites communis zone. A total of five survey stations were established, with the details of each station’s survey items listed in Table 1. Sampling was conducted during the spring tide periods on April 24, 2019 (spring), August 28, 2019 (summer), and October 31, 2019 (autumn). The collected samples included plankton, benthic microalgae, benthic animals, tidal flat vegetation, and particulate organic matter (POM), all from the 2019 ecological survey of the Liaohekou tidal flat wetlands. Fish samples were primarily acquired from local markets and docks, while seabird samples were collected from the feathers of Chroicocephalus saundersi found along the tidal creek edges in the Liaohe Estuary tidal flat wetlands.
Table 1
| Station | Land Cover | Survey Items |
|---|---|---|
| S1 | Tidal Creek | Zooplankton, Phytoplankton |
| S2 | Bare Flat Zone | Benthic Microalgae, Benthic organisms |
| S3 | Suaeda salsa Zone | Suaeda salsa, Benthic organisms |
| S4 | Suaeda salsa–Phragmites communis Transition Zone | Suaeda salsa, Phragmites communis, Benthic Fauna |
| S5 | Phragmites communis Zone | Phragmites communis |
Land cover and survey items at each station.
2.2 Principle of the Ecopath model
Where Bi is the biomass of functional group i, is the ratio of production to biomass for the functional group i, denotes the ratio of consumption to biomass for the functional group j; EEi is the ecotrophic efficiency of functional group i, DCij is the proportion of prey functional group i in the total food composition of predator functional group j; EXi is the export of functional group i. The parameter is derived from the empirical formula of Palomares and Pauly (1998).
In the Ecopath model, at least three of the four parameters Bi, , , and EEi need to be known, and the unknown parameter can be calculated by the model (Christensen and Pauly, 1992). It is important to input food matrix (DC) data for each functional group because the food matrix is the most influential information for predator–prey interactions and the sole determinant of predator–prey interactions. Traditional methods of measuring diet, such as stomach content analysis, measure the food consumed by the consumer before capture, which cannot exclude the possibility of accidental ingestion and cannot distinguish the digestibility of the food. Therefore, it often reflects an unrealistic snapshot of the consumer’s diet and requires a solid taxonomic foundation. Stable isotopes, as natural markers, can quantify the contribution of various sources to the mixture, reflecting the long-term life activities of organisms and are applied in various biological and ecological studies (Glibert et al., 2019).
2.3 Functional group classification
The functional groups in the Ecopath ecological model should be divided based on the biological species, feeding habits, and biological characteristics of the Liaohe Estuary salt marsh wetland, combined with field survey data, dividing the organisms in the Liaohe Estuary salt marsh wetland into 14 functional groups: seabirds, shrimps, mollusks, Helice tridens tientsinensis, Charybdis japonica, sciaenid fish, benthic organisms, other wetland fish, phytoplankton, benthic microalgae, zooplankton, Suaeda salsa, Phragmites communis, and organic detritus. See Table 2 for details.
Table 2
| Serial Number | Functional Group | Main Species |
|---|---|---|
| 1 | Seabirds | Larus saundersi |
| 2 | Shrimps | Mantis shrimp, Crangon affinis |
| 3 | Mollusks | Busycon canaliculatu, Bullacta exarata |
| 4 | Helice tridens tientsinensis | Helice tridens tientsinensis |
| 5 | Charybdis japonica | Charybdis japonica |
| 6 | Sciaenid fish | Collichthys lucidus |
| 7 | Benthic organisms | Nereis succinea, Periophthalmus cantonensis, Arenicola cristata |
| 8 | Other wetland fish | Sea catfish, Cynoglossus semilaevis, Sphyraenus, Perca fluviatilis, Hypophthalmichthys nobilis |
| 9 | Phytoplankton | Nostoc spongiaeforme, Navicula, Coscinodiscus centralis, etc. |
| 10 | Benthic microalgae | Gyrosigma scalproides, Gyrosigma kuetzingii, Pleurosigma spp, etc. |
| 11 | Zooplankton | Copepods, Nauplius, Paracalanus parvus |
| 12 | Suaeda salsa | Suaeda salsa |
| 13 | Phragmites communis | Phragmites communis |
| 14 | Organic detritus | Particulate organic matter |
Functional groups and dominant species of tidal flat wetland in Liaohe Estuary.
2.4 Sources of biological parameters for functional groups
In the Ecopath model, each functional group’s trophic level is expressed as a fractional value, which simplifies the complex food web relationships through trophic aggregation methods. This approach aids in representing the overall energy distribution across the system and analyzing trophic level flows (Courchamp et al., 2015). Energy flow within the ecosystem is quantified as t·km−2·a−1. The biomass data for each functional group were obtained from the 2019 ecological survey of the Liaohe Estuary salt marsh wetland, fishery resource surveys in the Liaodong Bay area, and relevant literature on the Bohai Sea (Gao et al., 2013; Yan et al., 2017; Lin et al., 2018). The biomass of organic detritus was calculated by combining the mass of particulate organic matter collected per unit volume during field surveys with the average water depth of the Liaohe Estuary area.
In the Ecopath model, P/B, which represents the biomass turnover rate, is the ratio of production to biomass for each functional group. While P/B is typically not directly measurable, it can be estimated using the total mortality rate Z of fish. Q/B, the ratio of consumption to biomass for a given species, is measured in units of 1/year. This parameter for fish functional groups is primarily derived from growth equations and related data, calculated using empirical formulas proposed by Pauly et al (Pauly et al., 1998). The model calculates ecological efficiency. Due to the diversity of fish species within each functional group, determining precise Q/B and P/B values can be challenging. Consequently, this study referenced similar functional group parameters from the Bohai Sea region (Wang et al., 2017; Lin et al., 2018).
2.5 Stomach content analysis
Stable isotopes of each functional group were measured using the elemental analyzer-stable isotope mass spectrometer (vario PYRO cube-isoprime-100, Elementar, Germany). The dietary analysis of consumers in each functional group (Supplementary Table S1) was carried out using the stable isotope mixing model (SIAR) based on R language. Statistical analyses were conducted using Microsoft Excel, SPSS 22.0, and R 2.6.3 software.
2.6 Model balancing and validation
After entering all the initial values, the model is balanced to satisfy the mass-balance assumption. The most common error in the model balancing process is that the predation mortality exceeds the P/B estimate. Therefore, during the model balancing process, adjustments are made to reduce predation events by carnivores (Sagarese et al., 2017). It is essential to ensure that all EE values are less than or equal to 1.0. If one or more EE values exceed 1.0, it indicates that the resource demand in the ecosystem is greater than the available resources. Generally, the P/Q ratio ranges from 0.05 to 0.30, meaning that consumption is 3 to 10 times the production. However, for small, fast-growing fish or bacteria, the P/Q ratio is lower. Parameters must be adjusted to balance the energy in the ecosystem (Christensen and Walters, 2004).
2.7 Model quality evaluation
The Ecopath model requires analysis of the uncertainty in input parameters using the Pedigree Index in the “Pedigree” module (Christensen and Walters, 2004) to evaluate the overall quality of the data and the model. The index for each input value ranges from 0 to 1, with 0 indicating that the input data is not from local data and 1 indicating that the input data is entirely from local data. The overall Pedigree Index is the sum of the Pedigree indices for all input parameters. The overall Pedigree Index can be derived from the following formula:
where the Pedigree Index represents the index value of functional groups and parameters, and the denominator represents the total number of functional groups.
3 Results and analysis
3.1 Food web structure
The schematic diagram of the Liaohe Estuary salt marsh wetland ecosystem structure is shown in Figure 2. The size of the circles represents the biomass of different functional groups, while the connections between them indicate energy transfer processes. According to the food web structure, phytoplankton, benthic microalgae, salt marsh plants, and particulate suspended matter are primary producers at the first trophic level, serving as the energy source for the entire ecosystem. Benthic organisms, and zooplankton are at the second trophic level. Mollusks, Helice tridens tientsinensis, shrimps, sciaenid fish, mollusks, Charybdis japonica, and other wetland fish occupy the second to third trophic levels., with seabirds being the ultimate predators at the top of the food web.
Figure 2
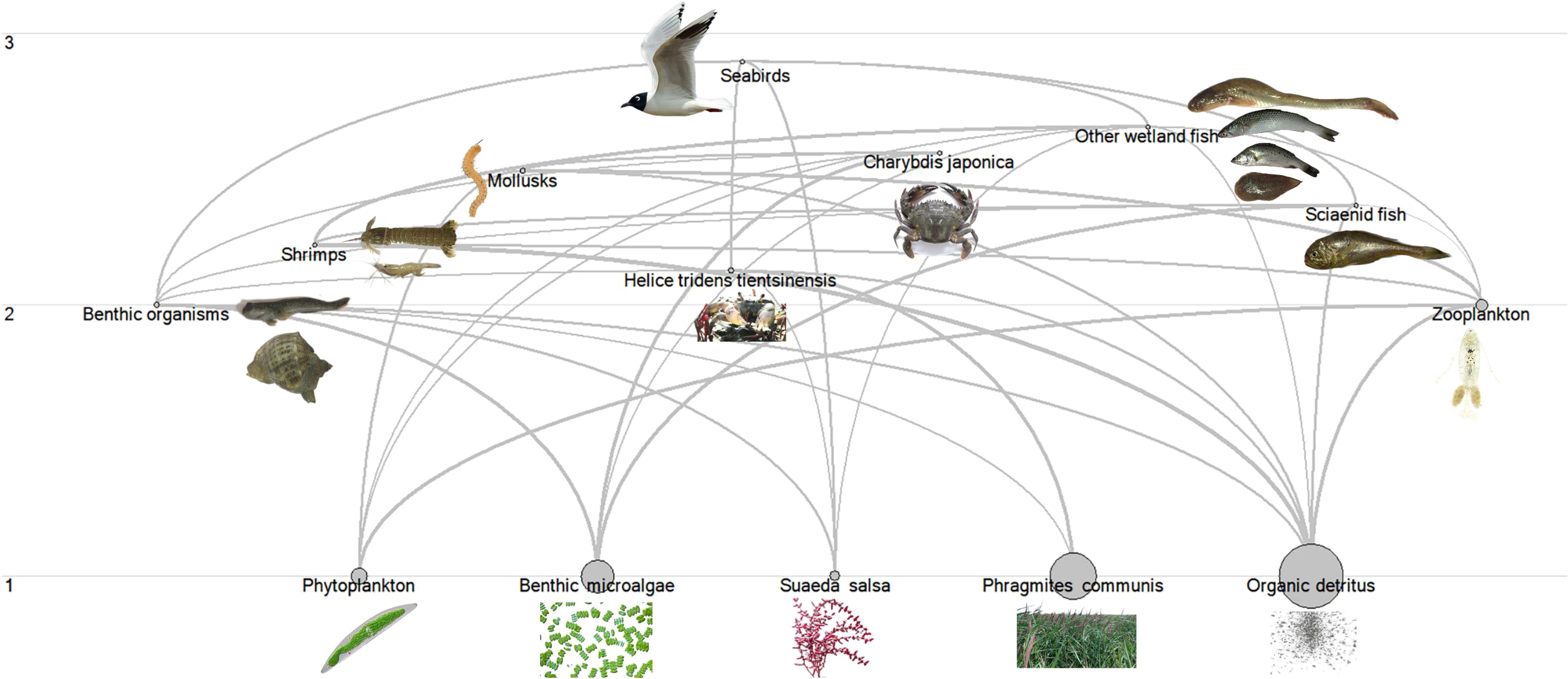
Food web structure of tidal flat wetland in Liaohe Estuary.
In the Liaohe Estuary salt marsh wetland, the primary producers are mainly benthic microalgae and Phragmites communis, with biomasses of 250.6 t·km−2 and 501.0 t·km−2, respectively, far exceeding the biomasses of Suaeda salsa and phytoplankton, which are 25.42 t·km−2 and 68.17 t·km−2, respectively. Among the primary consumers, zooplankton has the highest biomass at 41.63 t·km−2, followed by benthic organisms at 1.797 t·km−2, and mollusks with the lowest biomass at 1.420 t·km−2. Among the secondary consumers, Helice tridens tientsinensis and shrimps have the highest biomasses at 2.772 t·km−2 and 0.0427 t·km−2, respectively, serving as crucial pathways for energy flow to top consumers. The biomass of seabirds, the top consumers, is 0.00120 t·km−2.
3.2 Energy flow between trophic levels
The Ecopath model simplifies the complex food web relationships by analyzing energy flow between trophic levels. In this study, the aggregation method was used to merge the nutrient flows of 14 different functional groups in the Liaohe Estuary salt marsh wetland ecosystem into five integrated trophic levels (Table 3). The biomasses, production, and flows of trophic levels IV and V are relatively low and can be ignored. Thus, the energy flow in the Liaohe Estuary salt marsh wetland ecosystem primarily occurs within three trophic levels, displaying a typical pyramidal distribution where lower trophic levels have higher values and higher trophic levels have lower values, in accordance with the energy pyramid principle.
Table 3
| Trophic Level | Consumption | Flow into Detritus | Respiration | Total Flow | Unit |
|---|---|---|---|---|---|
| V | 0 | 0.000001 | 0.000826 | 0.000827 | t·km−2·a−1 |
| IV | 0.000827 | 0.00931 | 0.0284 | 0.0385 | t·km−2·a−1 |
| III | 0.0385 | 2.869 | 8.891 | 11.8 | t·km−2·a−1 |
| II | 11.8 | 484.6 | 1957 | 2454 | t·km−2·a−1 |
| I | 2454 | 20129 | 0 | 22583 | t·km−2·a−1 |
| Total | 2466 | 20617 | 1966 | 25049 | t·km−2·a−1 |
Total energy flow of Liaohe Estuary ecosystem.
3.3 Energy conversion efficiency between trophic levels
Except for the first trophic level, the total flows of trophic levels II, III, IV, and V decrease with increasing trophic levels, being 2454, 11.8, 0.0385, and 0.000827, respectively (Table 3). The conversion efficiencies of trophic levels II, III, and IV are 0.443%, 0.378%, and 2.034%, respectively (Table 4). The total flow of the Liaohe Estuary salt marsh wetland ecosystem is 25049 t·km−2·a−1, with a total feeding consumption of 2466 t·km−2·a−1 and a total detrital inflow of 20617 t·km−2·a−1.
Table 4
| Energy Source | II | III | IV | Unit |
|---|---|---|---|---|
| Producers | 0.481 | 0.326 | 2.148 | % |
| Detritus | 0.410 | 0.430 | 1.948 | % |
| Total Energy Flow | 0.443 | 0.378 | 2.034 | % |
Transfer efficiencies between trophic levels of the ecosystem.
3.4 Interrelationships between functional groups
The Mixed Trophic Impact (MIT) module is used to study the nutritional relationships between functional groups. The trophic relationships between the functional groups in the Liaohe Estuary ecosystem are shown in Figure 3. In the figure, white represents a positive impact, black represents a negative impact, and the size of the ovals indicates the strength of the impact between different functional groups.
Figure 3
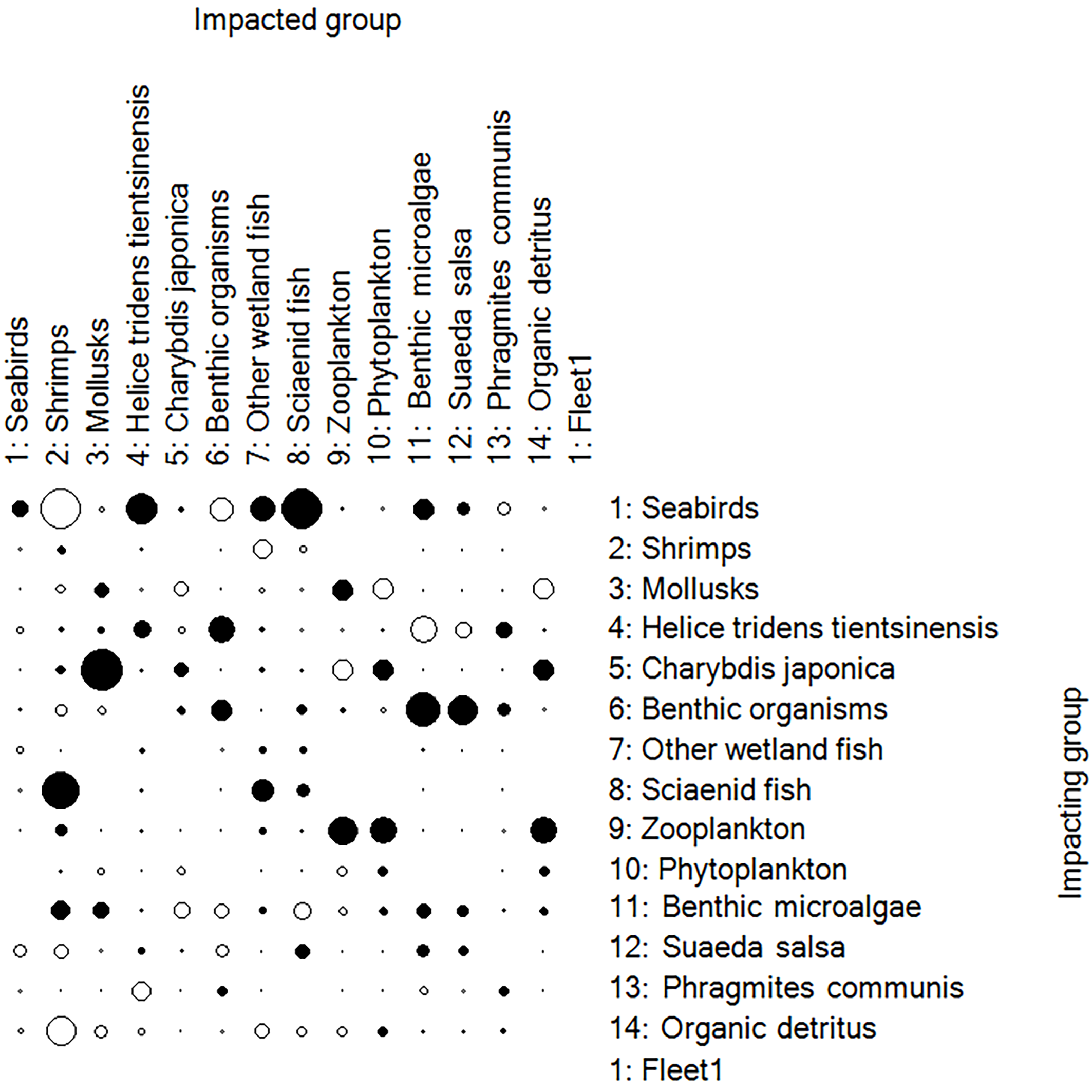
The trophic relationships between the functional groups in the Liaohe Estuary ecosystem.
Shrimp, detritus, Phragmites communis, and Suaeda salsa positively affect most functional groups. Due to the predation relationships, Suaeda salsa, Helice tridens tientsinensis, and other wetland fish positively impact seabirds. Sciaenid fish have a significant negative impact on shrimp. Helice tridens tientsinensis has a noticeable negative impact on benthic organisms, while seabirds negatively impact sciaenid fish and Helice tridens tientsinensis. Most functional groups negatively impact detritus.
3.5 Overall characteristics of the ecosystem
The parameters of the Ecopath model can represent the scale, stability, and maturity of the studied ecosystem. The overall characteristic parameters of the Liaohe Estuary salt marsh wetland ecosystem are shown in Table 5. The total system throughput is a parameter that characterizes the scale of the system, representing the sum of total consumption, total exports, total respiration, and total flow to detritus. The total system throughput of the Liaohe Estuary salt marsh wetland ecosystem is 49099.039 t·km−2·a−1, with 21181.461 t·km−2·a−1 flowing to detritus, indicating that nearly half of the material is not utilized by organisms but is converted to detritus, re-entering the ecosystem cycle. The total exports of the ecosystem are 18324.279 t·km−2·a−1, indicating that a portion of the material leaves the Liaohe Estuary salt marsh wetland ecosystem annually. This high export rate is primarily due to the estuary’s location as a transition zone between land and ocean, where a considerable amount of material is transported into the ocean by tides each year.
Table 5
| Parameter | Value | Unit |
|---|---|---|
| Total Consumption | 5334.605 | t·km−2·a−1 |
| Total Exports | 18324.279 | t·km−2·a−1 |
| Total Respiration | 4258.695 | t·km−2·a−1 |
| Total Flow to Detritus | 21181.461 | t·km−2·a−1 |
| Total System Throughput | 49099.039 | t·km−2·a−1 |
| Total Production | 23658.881 | t·km−2·a−1 |
| Connectance Index | 0.207 | |
| System Omnivory Index | 0.109 | |
| Pedigree Index | 0.750 |
The overall characteristics of tidal flat wetland ecosystem in Liaohe Estuary.
The connectance index indicates the complexity of interconnections among food chains within the ecosystem. A higher connectance index suggests more complex interconnections among organisms in the food chain, indicating more efficient nutrient utilization and greater ecosystem stability. The system omnivory index represents the complexity of the ecosystem (Christensen and Walters, 2004). In a mature ecosystem, both the connectance index and system omnivory index approach 1. The connectance index of the Liaohe Estuary salt marsh wetland ecosystem is 0.207, and the system omnivory index is 0.109.
4 Discussion
4.1 Model quality evaluation
The shortcomings and limitations of ecosystem models mainly arise from the data sources and quality of the model itself (Christensen et al., 2005). To ensure the reliability and quality of the parameters in the model, the Pedigree index is used to evaluate the overall quality of the input parameters. The Pedigree index for the Liaohe Estuary salt marsh wetland ecological model is 0.75, ranking in the upper-middle level among 150 Ecopath models globally. This indicates that the system has good parameters and high model quality, with high data reliability. Stable isotopes, as natural markers, can quantify the contributions of multiple sources to a mixture, especially in estimating the food sources of consumers. Compared to the method of estimating food matrices based on stomach contents, using stable isotope analysis for verification proves to be more scientific and reasonable (Michener and Kaufman, 2007). In this study, data collected in situ, combined with Bayesian mixing models to calculate the food matrix, helps improve the model’s accuracy.
4.2 Energy flow characteristics of trophic levels
The total energy flow of the system is an indicator of the scale of the ecosystem, and it is proportional to the changes in the system’s productivity. A higher total energy flow indicates higher system productivity. The total system throughput of the Liaohe Estuary salt marsh wetland ecosystem is 49099.039 t·km−2·a−1, higher than that of the Zhushan Bay lake buffer zone wetland (10145.20 t·km−2·a−1) (Li et al., 2019). The reason is that the Liaohe Estuary is a macrotidal estuary influenced by tides, acting as a transitional area between marine and terrestrial ecosystems, regularly or irregularly flooded by tides. The Liao River flows into the Bohai Sea all year round, bringing large amounts of sediment and deposits, and the input of terrestrial detritus increases the total system throughput.
In the trophic flows of trophic level II and trophic level III, the ecological energy conversion efficiencies are 0.443% and 0.378%, respectively, which are much lower than the Lindeman efficiency (10%). The excessively low conversion from primary producers to trophic level II leads to a large amount of primary productivity not being utilized and directly converted into detritus, hindering the flow of energy to higher trophic levels and affecting the healthy development of the Liaohe Estuary salt marsh wetland ecosystem. Previous studies have shown that the low energy conversion efficiency of functional groups is related to low EE values. In this study, the EE value of phytoplankton is 0.140 (Table 6), possibly because the reproduction rate of phytoplankton is greater than the predation rate of secondary consumers. In recent years, the eutrophication of the Liaohe Estuary and its coastal waters has been severe (Wang et al., 2021), providing conditions for the growth of phytoplankton, leading to a decrease in biodiversity and species diversity, and causing inefficient material and energy cycling in the ecosystem.
Table 6
| Functional Group | Biomass(t·km−2) | P/B | Q/B | Trophic Efficiency | Production/Consumption |
|---|---|---|---|---|---|
| Seabirds | 0.0012 | 0.0600 | 61.2400 | 0.0000 | 0.0010 |
| Shrimps | 0.0427 | 8.5000 | 28.0000 | 0.9071 | 0.3036 |
| Mollusks | 1.4200 | 6.0000 | 27.0000 | 0.0014 | 0.2222 |
| Helice tridens tientsinensis | 2.7720 | 3.5000 | 11.0000 | 0.0015 | 0.3182 |
| Charybdis japonica | 0.0037 | 3.5000 | 11.0000 | 0.0000 | 0.3182 |
| Benthic organisms | 1.7970 | 9.0000 | 33.0000 | 0.2447 | 0.2727 |
| Other wetland fish | 0.0121 | 1.7000 | 4.9500 | 0.5640 | 0.3434 |
| Sciaenid fish | 0.0410 | 9.0000 | 33.0000 | 0.0323 | 0.2727 |
| Zooplankton | 41.6300 | 25.0000 | 125.0000 | 0.0185 | 0.2000 |
| Phytoplankton | 68.1700 | 250.0000 | 0.1403 | ||
| Benthic microalgae | 250.6500 | 5.0000 | 0.0185 | ||
| Suaeda salsa | 25.4200 | 1.0000 | 0.0612 | ||
| Phragmites communis | 500.9500 | 1.0000 | 0.0058 | ||
| Organic detritus | 1132.7300 | 1.0000 | 0.1349 |
Input and output of model parameters of tidal flat wetland ecosystem in Liaohe Estuary.
Italicized values represent model-calculated parameters.
4.3 Overall characteristics of the ecosystem
The connectance index of the Liaohe Estuary salt marsh wetland ecosystem is 0.207, and the system omnivory index is 0.109. Both indices are lower than those of most adjacent sea areas and similar types of ecosystems (Lin et al., 2015; Han et al., 2016). Analysis suggests that the complexity of the food web in the study area is relatively low, with loose connections among functional groups, insufficiently complex predation relationships, and low system maturity. Analysis of the system parameter indices indicates that the current Liaohe Estuary salt marsh wetland ecosystem is in an immature stage, indicating that the ecosystem is in an unstable state. The reasons may include environmental pollution in the estuarine waters, overfishing, and reclamation of tidal flats, leading to the destruction of the ecological environment in the Liaohe Estuary area. The area of estuarine wetlands has sharply decreased, the wetland structure is significantly damaged (Yan et al., 2017), biodiversity indices have declined, the number of biological species has decreased annually, benthic organisms have significantly decreased, and aquatic habitats have been destroyed (Chen et al., 2023). Additionally, as the estuary of the Liao River, the area is disturbed by the sediment brought by the Liao River, which is also a reason for the simple and immature ecosystem structure in this region.
5 Conclusion
Based on the ecological survey data from 2019, an ecological model of the Liaohe Estuary salt marsh wetland, comprising 14 functional groups, was constructed. This model essentially encompasses the overall structure and energy flow processes of the ecosystem in the study area. By integrating stable isotope techniques with the Ecopath model, the food web structure, trophic structure, energy flow, and overall characteristics of the Liaohe Estuary salt marsh wetland were calculated. The following conclusions were drawn from the analysis of the ecosystem characteristics.
The Pedigree index of the Liaohe Estuary salt marsh wetland ecological model is relatively high, indicating good data reliability. The study results on the trophic structure and energy flow of the ecosystem in the study area reveal that the trophic energy in the Liaohe Estuary salt marsh wetland primarily flows through three trophic levels, adhering to the ecological energy pyramid principle. Although the study area, located in an estuarine region, has a high total system throughput, the ecological energy conversion efficiency at each trophic level is relatively low. This low efficiency can cause blockages in the nutrient flow at the lower levels of the ecosystem, leading to a weaker resistance to disturbances. The overall characteristic analysis indicates that the Liaohe Estuary salt marsh wetland ecosystem is in an immature stage, with low system maturity and a simple food web complexity.
However, there are some limitations in this study. First, although the Ecopath model provides an important quantitative tool for analyzing wetland ecosystems, the study relies on ecological survey data from 2019, which has temporal limitations and does not capture the dynamic changes in the wetland system over different years. Secondly, the model primarily focuses on energy flows and the food web structure but does not fully consider external environmental factors, such as climate change, sea-level rise, and pollution, which may significantly affect the wetland ecosystem.
In future research, it is essential to improve the model’s temporal dynamics by incorporating more environmental and anthropogenic factors. This will enable a more comprehensive assessment of the wetland ecosystem’s health and provide more accurate guidance for ecosystem restoration and management.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
DY: Investigation, Writing – original draft. MQ: Software, Writing – original draft. XZ: Conceptualization, Writing – review & editing. YZ: Conceptualization, Writing – review & editing. JZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Liaoning Province Natural Fund(2019-ZD-0729).
Acknowledgments
We extend our sincere gratitude to the foundation for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1487370/full#supplementary-material
Supplementary Table 1Food composition matrix of the Ecopath model in Liaohe estuary tidal flat wetland.
References
1
Chen K. Cong P. Qu L. Liang S. Sun Z. Han J. (2023). Biological connectivity and its driving mechanisms in the Liaohe Delta wetland, China. Ecol. Inform76, 102028. doi: 10.1016/j.ecoinf.2023.102028
2
Christensen V. Pauly D. (1992). ECOPATH II — a software for balancing steady-state ecosystem models and calculating network characteristics. Ecol. Model.61, 169–185. doi: 10.1016/0304-3800(92)90016-8
3
Christensen V. Walters C. J. (2004). Ecopath with Ecosim: methods, capabilities and limitations. Ecol. Model.172, 109–139. doi: 10.1016/j.ecolmodel.2003.09.003
4
Christensen V. Walters C. Pauly D. (2005). Ecopath with Ecosim: a user’s guide (version 5.1) (Vancouver, Canada: Fisheries Centre, University of British Columbia).
5
Courchamp F. Dunne J. A. Le Maho Y. May R. M. Thebaud C. Hochberg M. E. (2015). Fundamental ecology is fundamental. Trends Ecol. Evol.30, 9–16. doi: 10.1016/j.tree.2014.11.005
6
Craig J. K. Link J. S. (2023). It is past time to use ecosystem models tactically to support ecosystem-based fisheries management: Case studies using Ecopath with Ecosim in an operational management context. Fish Fish24, 381–406. doi: 10.1111/faf.12733
7
Gao S. K. Li Z. Zhang S. (2024). Trophic transfer and biomagnification of microplastics through food webs in coastal waters: A new perspective from a mass balance model. Mar. pollut. Bull.200, 116082. doi: 10.1016/j.marpolbul.2024.116082
8
Gao Y. Liu M. Tang Y. Li J. Xing B. (2013). Surveys and analysis of marine fishery resources and ecological environment in Liaodong Bay. J. Dalian Ocean Univ.28, 211–216.
9
Glibert P. M. Middelburg J. J. McClelland J. W. Jake Vander Zanden M. (2019). Stable isotope tracers: Enriching our perspectives and questions on sources, fates, rates, and pathways of major elements in aquatic systems. Limnol. Oceanogr.64, 950–981. doi: 10.1002/lno.11087
10
Han R. Chen Q. Wang L. Tang X. (2016). Preliminary investigation on the changes in trophic structure and energy flow in the Yangtze estuary and adjacent coastal ecosystem due to the Three Gorges Reservoir. Ecol. Inform36, 152–161. doi: 10.1016/j.ecoinf.2016.03.002
11
Hou W. H. Lu W. Z. Zhao K. Y. Zhang J. L. Zhang R. J. Lei W. et al . (2019). Research on the temporal and spatial distribution characteristics of Helice tientsinensis in red beach of the Liaohe estuary. Mar. Environ. Sci.38, 272–277.
12
Karp M. A. Link J. S. Grezlik M. Cadrin S. Fay G. Lynch P. et al . (2023). Increasing the uptake of multispecies models in fisheries management. ICES J. Mar. Sci. 80, 243–257. doi: 10.1093/icesjms/fsad001
13
Li H. F. Su F. L. Guo C. J. Dong L. L. Song F. Wei C. et al . (2022). Landscape ecological risk assessment and driving mechanism of coastal estuarine tidal flats-A case study of the liaohe estuary wetlands. Front. Env. Sci. Switz10, 1070009. doi: 10.3389/fenvs.2022.1070009
14
Li C. Xian Y. Ye C. Wang Y. Wei W. Xi H. et al . (2019). Wetland ecosystem status and restoration using the Ecopath with Ecosim (EWE) model. Sci. Total Environ.658, 305–314. doi: 10.1016/j.scitotenv.2018.12.128
15
Lin Q. Shan X. Wang J. Li Z. (2018). Changes in Chinese shrimp (Fenneropenaeus chinensis) carrying capacity of the Bohai Sea. Prog. Fish. Sci.39, 19–29.
16
Lin Q. Wang J. Li Z. Wu Q. (2015). Assessment of ecosystem energy flow and carrying capacity of swimming crab enhancement in the Yellow River estuary and adjacent waters. Ying yong sheng tai xue bao = J. Appl. Ecol.26, 3523–3531.
17
Liu G. Z. Ye J. Q. Chen Y. Yang X. L. Gu Y. B. (2022). Analysis of Water Pollution Causes and Control Countermeasures in Liaohe Estuary via Support Vector Machine Particle Swarm Optimization under Deep Learning. CMES Comp. Model. Eng.130, 315–329. doi: 10.32604/cmes.2022.016224
18
Michener R. H. Kaufman L. (2007). “Stable isotope ratios as tracers in marine food webs: an update,” in Stable isotopes in ecology and environmental science (Oxford, UK: Blackwell Publishing), 238–282.
19
Palomares M. L. D. Pauly B. D. (1998). Predicting food consumption of fish populations as functions of mortality, food type, morphometrics, temperature and salinity. Mar. Freshw. Res. 49 (5), 447–453. doi: 10.1071/MF98015
20
Papantoniou G. Zervoudaki S. Assimakopoulou G. Stoumboudi M. T. Tsagarakis K. (2023). Ecosystem-level responses to multiple stressors using a time-dynamic food-web model: The case of a re-oligotrophicated coastal embayment (Saronikos Gulf, E Mediterranean). Sci. Total Environ.903, 165882.
21
Pauly D. Trites A. W. Capuli E. Christensen V. (1998). Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci.55, 467–481. doi: 10.1006/jmsc.1997.0280
22
Polovina J. J. (1984). Model of a coral reef ecosystem: I. The ECOPATH model and its application to French Frigate Shoals. Coral Reefs3, 1–11. doi: 10.1007/BF00306135
23
Sagarese S. R. Lauretta M. V. Walter J. F. I. (2017). Progress towards a next-generation fisheries ecosystem model for the northern Gulf of Mexico. Ecol. Model.345, 75–98. doi: 10.1016/j.ecolmodel.2016.11.001
24
Smith M. Chagaris D. Paperno R. Markwith S. (2023). Troipcal estuarine ecosystem change under the interacting influences of future climate and ecosystem restoration. Global Change Biol.29, 5850–5865. doi: 10.1111/gcb.v29.20
25
Stock A. Murray C. C. Gregr E. J. Steenbeek J. Woodburn E. Micheli F. et al . (2023). Exploring multiple stressor effects with Ecopath, Ecosim, and Ecospace: Research designs, modeling techniques, and future directions. Sci. Total Environ.869, 161719.
26
Wan S. Mou X. J. Liu X. T. (2018). Effects of reclamation on soil carbon and nitrogen in coastal wetlands of Liaohe River Delta, China. Chin. Geogr. Sci.28, 443–455. doi: 10.1007/s11769-018-0961-7
27
Wang X. Jiang H. Zhang Y. Chen L. Song C. Li Y. (2017). Diet composition of Saunders’s Gull (Larus saundersi) determined using stable isotope analysis at the Shuangtaihekou National Nature Reserve, China. Acta Ecol. Sin.37, 1796–1804.
28
Wang S. Liu Y. Chen L. Yang H. Wang G. Wang C. et al . (2021). Effects of excessive nitrogen on nitrogen uptake and transformation in the wetland soils of Liaohe estuary, northeast China. Sci. Total Environ.791, 148228. doi: 10.1016/j.scitotenv.2021.148228
29
Wang G. X. Pan J. Y. Yu J. Yan W. W. Gu D. Q. Du J. (2023). Soil organic carbon storage in Liaohe River Estuary Wetlands under restoration and multiple management strategies, based on landscape patterns. Front. Mar. Sci.10, 1100208. doi: 10.3389/fmars.2023.1100208
30
Yan X. Hu Y. Chang Y. Zhang D. Liu M. Guo J. et al . (2017). Monitoring wetland changes both outside and inside reclamation areas for coastal management of the Northern Liaodong Bay, China. WETLANDS37, 885–897. doi: 10.1007/s13157-017-0922-4
31
Yin J. Xue Y. Li Y. Z. Zhang C. L. Xu B. D. Liu Y. W. et al . (2023). Evaluating the efficacy of fisheries management strategies in China for achieving multiple objectives under climate change. Ocean Coast. Manage245, 106870. doi: 10.1016/j.ocecoaman.2023.106870
Summary
Keywords
Ecopath, isotope, ecological model, ecosystem, Liaohe Estuary
Citation
Yuan D, Qiu M, Zhou X, Zhang Y and Zhao J (2024) Study on the structure and energy flow of the salt marsh wetland ecosystem in Liaohe Estuary based on the Ecopath model. Front. Mar. Sci. 11:1487370. doi: 10.3389/fmars.2024.1487370
Received
28 August 2024
Accepted
23 September 2024
Published
15 October 2024
Volume
11 - 2024
Edited by
Shuping Wang, Chinese Research Academy of Environmental Sciences, China
Reviewed by
Cui Wang, State Oceanic Administration, China
Anning Suo, Chinese Academy of Sciences (CAS), China
Updates
Copyright
© 2024 Yuan, Qiu, Zhou, Zhang and Zhao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xushen Zhou, jnsszbp@163.com; Yan Zhang, 58726147@qq.com; Jianhua Zhao, jhzhao77@163.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.