Abstract
Fishing activities alter food web diversity and functioning. Trophic level (TL) has been used as an indicator to assess such impacts on populations and assemblages. We reviewed the scientific literature that examined the relationship between fishing and the trophic aspects of fish species and communities, by focussing on TL. We narrowed the research to the Mediterranean Sea, where fishing is an important economic income for some coastal human populations and might be jeopardised by overfishing and climate change. We collected information on the (i) geographical location; (ii) type of fisheries and (iii) the methodological approach. The 68 collected studies were geographically skewed towards the Western Mediterranean, Adriatic Sea and around Greece. Among the 45 modelling studies, 41 reported TLs for communities or catches. For the field studies, only 6 estimated TLs of species and used stable isotope analysis. Most modelling studies used data from other models, online databases or large-scale monitoring of commercial catches and research surveys, whereas the field studies collected fish locally. Only 6 field and 5 modelling studies used fishing bans or the fully protected zone of marine protected areas as no-fishing control. In these studies, TL values showed different patterns of response to fishing, probably because of differences in environmental factors. Interestingly, recent modelling studies used predictions from the model to explore the impact of different fishing pressure within global change scenarios. The use of trophodynamic modelling is powerful to describe large scale impacts and infer future scenarios, but the in situ approach, the use of stable isotopes and spatial comparisons among areas of different fishing pressure, such as no-take zones in MPA could add insights into local variations of fish TLs in response to perturbations, which might be important to refine the outcomes of the models.
1 Introduction
The impact of fishing activities on marine communities, food webs and seafood supply has been of increasing concern for the last 3 decades (Pauly et al., 1998; Corrales et al., 2015). Fishing may often target large, slow-growing adult fishes that occupy high trophic levels (Farrugio et al., 1993; Watson et al., 2013). When these species become less available and overfished, fishing intensifies the capturing of fishes occupying lower trophic levels; i.e. the so-called ‘fishing down food webs’ (Pauly et al., 1998; Andersen and Pedersen, 2010; Tremblay-Boyer et al., 2011). At an ecosystem level, the removal of high-level predators triggers trophic cascades, affects the biomass, feeding behaviour and diet of intermediate consumers (Guest et al., 2004), thereby altering the structure of the food web (e.g. Libralato et al., 2010; Fry and Davis, 2015; Cardona et al., 2022). This may lead to potential regime shifts (Möllmann et al., 2008; Rocha et al., 2015) and ultimately to the loss of ecosystem functions (Lotze et al., 2006, 2011; Longo et al., 2015). In the Mediterranean Sea, fishing often targets intermediate consumers, which may still impact the low levels of the food web through trophic cascades. For instance, the fishing of the Sparidae Diplodus spp. led to the dramatic increase of sea urchins and the loss of macroalgal cover, causing the shift of subtidal rocky reefs from macroalgal-dominated substrates to coralline barrens (Sala et al., 1998; Guidetti, 2006). These effects may be exacerbated in the proximity of coastal marine areas, where human activities and human-driven climate change strongly impacts biodiversity and impairs important ecosystem services (Giakoumi et al., 2015; Halpern et al., 2019; Zhang et al., 2019; Simeoni et al., 2023).
Elton (1927) provided the fundamental concepts of food chains, trophic pyramids, and trophic levels to define food webs. Since then, estimating the trophic level (TL) occupied by populations and the overall community has become a fundamental component for understanding trophic structure. The TL of populations and of the overall community has been used for indicating the health of food webs affected by human activities (Stergiou and Karpouzi, 2001; Post, 2002; Karachle and Stergiou, 2017; Davis et al., 2019), including the impact of fishing (Shannon et al., 2014; Kytinou et al., 2020) and the status of fisheries (Colloca et al., 2017). At a population level, the TL can allow exploring changes in the diet of the target consumers and thus their role in energy transfer (Cardona et al., 2023), whereas at community level TL may indicate the reduction in biomass of predators and the shift of the food web to a dominance of low trophic level consumers (Pauly et al., 1998; Pauly and Palomares, 2005). The TL definition follows an energy-flow approach and it is based on discrete numbers identifying the place of an organism within a linear food chain (e.g. herbivore, omnivore or predator; Lindeman, 1942; Shannon et al., 2014). This place can be estimated by observing the feeding behaviour and analysing morphological traits related to feeding. However, in nature, what is empirically observed is continuous rather than discrete changes in TL, sometimes called trophic position (TP, Thompson et al., 2012).These changes in TL (or TP) can be estimated by analysing what an organism has ingested (e.g. stomach content analysis, SCA, Hyslop, 1980) or what has been assimilated over time, often using stable isotope analysis (SIA). In this case, SIA is based on the idea that the isotopic ratio of a consumer reflects that of its prey, by taking into account the known isotopic enrichment from the prey to the predator (e.g. fractionation; Post, 2002).
Despite the great progress achieved through the development of new tools and techniques, food web analysis still suffers from important knowledge gaps and limitations of the methodological approaches (Kytinou et al., 2020). Moreover, although fishing exploitation is considered a primary driver to affect food webs, important information such as diet, trophic level estimation, especially for omnivorous species, can be scant (Colloca et al., 2017; Kytinou et al., 2020).
This paper presents a systematic review of studies investigating the relationship between fishing activities and the trophic structure of fish populations and assemblages. It specifically focus on the use of trophic level (TL) as a tool to indicate both the state of fisheries and the healthy status of the fish community. Particular attention is given to the research conducted in the Mediterranean Sea, where fishing activities near the shore account for a large proportion of landings (Lloret et al., 2020, FAO, 2022) and fishing management needs to be improved for a long-term sustainability (Colloca et al., 2017). Understanding the trophic impacts of fishing might provide fundamental information to ecosystem-based management. Our review builds upon previous syntheses of food web dynamics and the impacts of fishing in the Mediterranean (Colloca et al., 2017; Kytinou et al., 2020), by integrating data on Mediterranean fisheries with ecological approaches that assess trophic interactions within coastal fish communities. In details, this systematic review aims to:
(1) Provide a synthesis of the geographical distribution of studies, the types of fishing practices, and the methodological approaches used to estimate TL or trophic position (TP)—which here are considered as an interchangeable terms (Ishikawa et al., 2024).
(2) Identify studies considering different levels of fishing pressure and compare the variations of trophic level or positions in relation to the fishing pressure.
2 Materials and methods
A systematic literature review was conducted following the PRISMA methodology (Moher et al., 2010). The research of relevant articles was performed using the ISI Web of Science core collection database on the 15th of December 2023. Web of Science allows for refined and reproducible searches using detailed filters (e.g., topic, journal, author). This database is also widely used in systematic reviews, making the search strategy reproducible and the methodology consistent with established review standards. We then used Google Scholar as a supplementary quality control to ensure that no relevant studies were missed in the primary search. We first used the string: “(fishing OR fishery OR fisheries) AND (Mediterranean) AND (foodweb OR “food web” OR “food-web” OR “trophic level” OR “trophic interaction” OR “trophic cascade” OR “trophic guild” OR “trophic group” OR “trophic position” OR “trophic length” OR “gut content”) NOT (freshwater OR lake)”, with no restriction on publication year.
Then, by performing the quality control on google scholar we found that the string had left uncovered articles concerning the evaluation of fishing impact on food webs in Marine Protected Areas, considered as no-fishing ground (fully protected zone). We thus added this part to the search, by completing with the following string: “(MPA” OR “marine reserve” OR “marine national park” OR “marine protected area”)”. To ensure scientific rigour and comparability across studies, we restricted our selection to peer-reviewed articles, thereby excluding grey literature. It was indeed difficult not only to access grey literature in an exhaustive manner but also to determine if the found papers could be representative of the study area.
We collected 682 papers, which were first screened through the title and then through the abstract (rounds 1 and 2 in Figure 1). We excluded reviews and research papers that focussed on bioaccumulation or targeted exclusively the pelagic food webs without considering the coastal environment. Specifically, for modelling studies, we selected articles mentioning at least one functional group described as coastal by the authors.
Figure 1
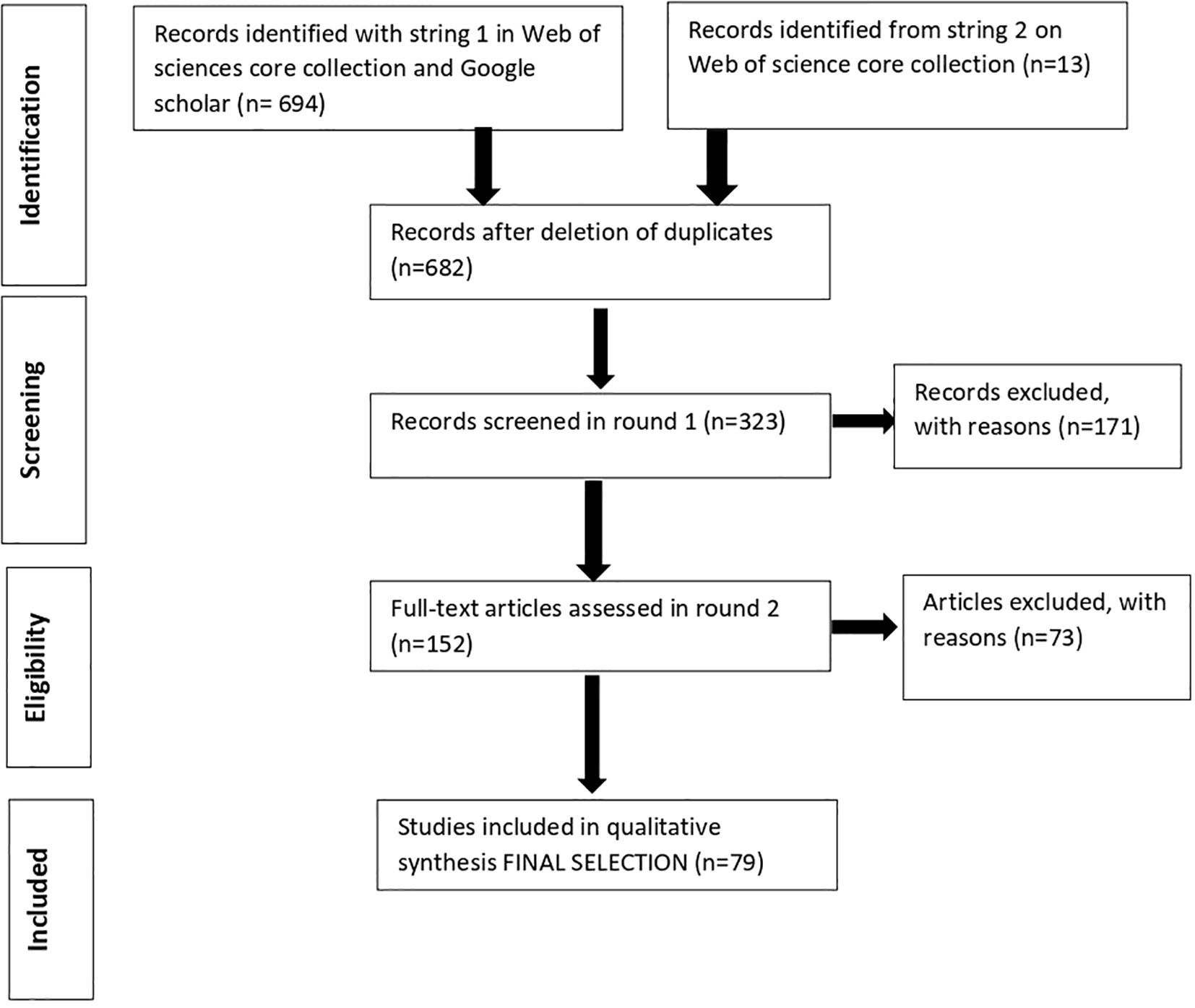
Flow diagram of the selection process used in this review (PRISMA methodology, modified from Moher et al., 2010).
The 152 remaining papers were searched through Introduction, Materials and Methods and Results and retained if they explicitly considered the impacts of fishing activities on trophic aspects of coastal fish populations and assemblages, including trophic interactions, feeding and, of course, trophic level (Figure 1). The final dataset included 68 papers. We recorded: (i) the publication year, (ii) the geographical locations where the study was done (countries, geographical sub-area (GSA; FAO, 2022), (iii) the type of fisheries (trawl and purse seine fisheries, grouped under the label “industrial”, small-scale fisheries (SSFs), and recreational fisheries), (iv) the methodological approach. We focussed on the type of study (Modelling, Field study), how trophic aspects were taken into account and how TL was estimated, including the biological scale of interest (population, community). We also reported the studies directly comparing areas that differed in fishing pressures; e.g. studies that included both areas under the impact of fishing and control areas such as Marine protected areas (MPA) or Fisheries restricted areas (FRA), where fishing activities were excluded or reduced. Of these, 9 studies that specifically evaluated trophic level (TL) were retained to assess how TL values vary between fished and non-fished areas. From those studies comparing fishing and no fishing areas, we extracted the TL values reported in the papers; e.g. population-level TL, mean TL for catches and communities (mTLc and mTLco, respectively).
3 Results and discussion
The 68 collected publications spanned between 2000 and 2023. There was an increasing number of papers through the years and a remarkable inter-annual variability, with a maximum of 8 studies in 2009 and 2021 (Supplementary Figure S1).
3.1 Geographical areas
The Mediterranean Sea, excluding the Black Sea, is divided into 27 fishing areas referred to as Geographical sub-areas (GSAs) and sometimes studies referred to the GSA as the study location. The collected publications referred to 16 GSAs, mostly those situated in the northern Catalan (Spain), the Adriatic Sea (Italy and Croatia), the Ionian and Aegean Sea (Italy, Greece) or the Sicilian Channel (Figure 2; Supplementary Table S1 and Supplementary Reference list). Most studies conducted research in Italy, Spain, and around Greece (Figure 3A). Except for Tunisia, where 2 studies were found, our research did not identify any published paper considering the southern Mediterranean countries outside Europe (e.g. Algeria, Egypt, Libya and Morocco), despite their high biomass of landings (Figure 3B). The lack of information observed in the southern Mediterranean could be explained by the weak institutional capacity to collect and publish fisheries data. However, there could be data available in the grey literature, which we did not consider as explained in the previous section (FAO 2020). Nonetheless, it is necessary to fill in this gap because these regions possess important artisanal fleets and trawlers, comparable to the most studied GSAs. The largest artisanal fleets occur in Greece (Aegean Sea) and Tunisia, while trawlers are mainly concentrated in Egypt, Spain together with the Adriatic Sea (Croatia, Italy) and Algeria (Figure 3B; Colloca et al., 2017; FAO, 2022). They are also countries where artisanal fishing is not only a source of food, but also an important cultural heritage, where landings represent more than a quarter of the total landings of the Mediterranean (Black Sea excluded, FAO, 2022).
Figure 2
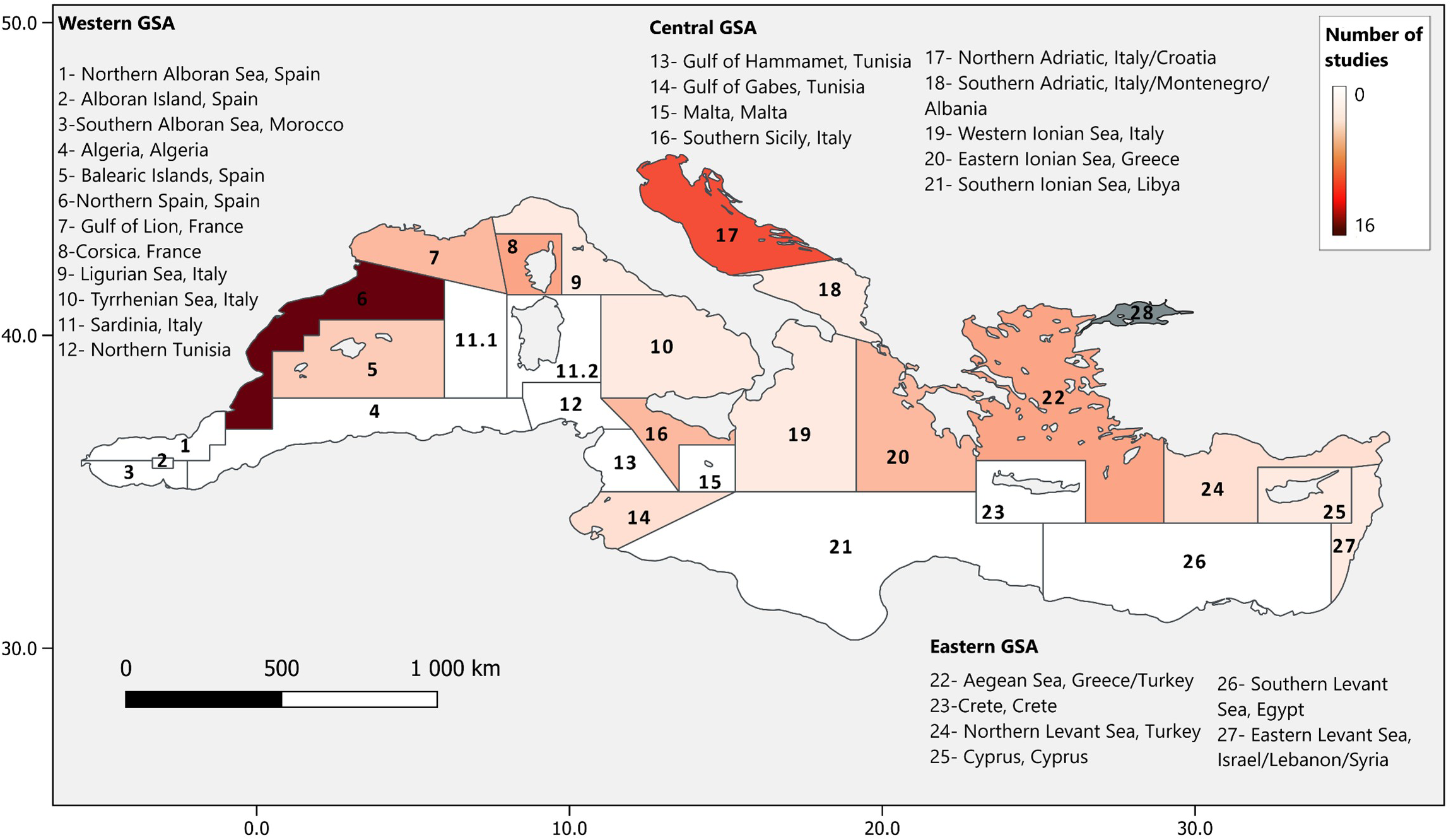
Distribution of the studies across the Mediterranean per GSA.
Figure 3
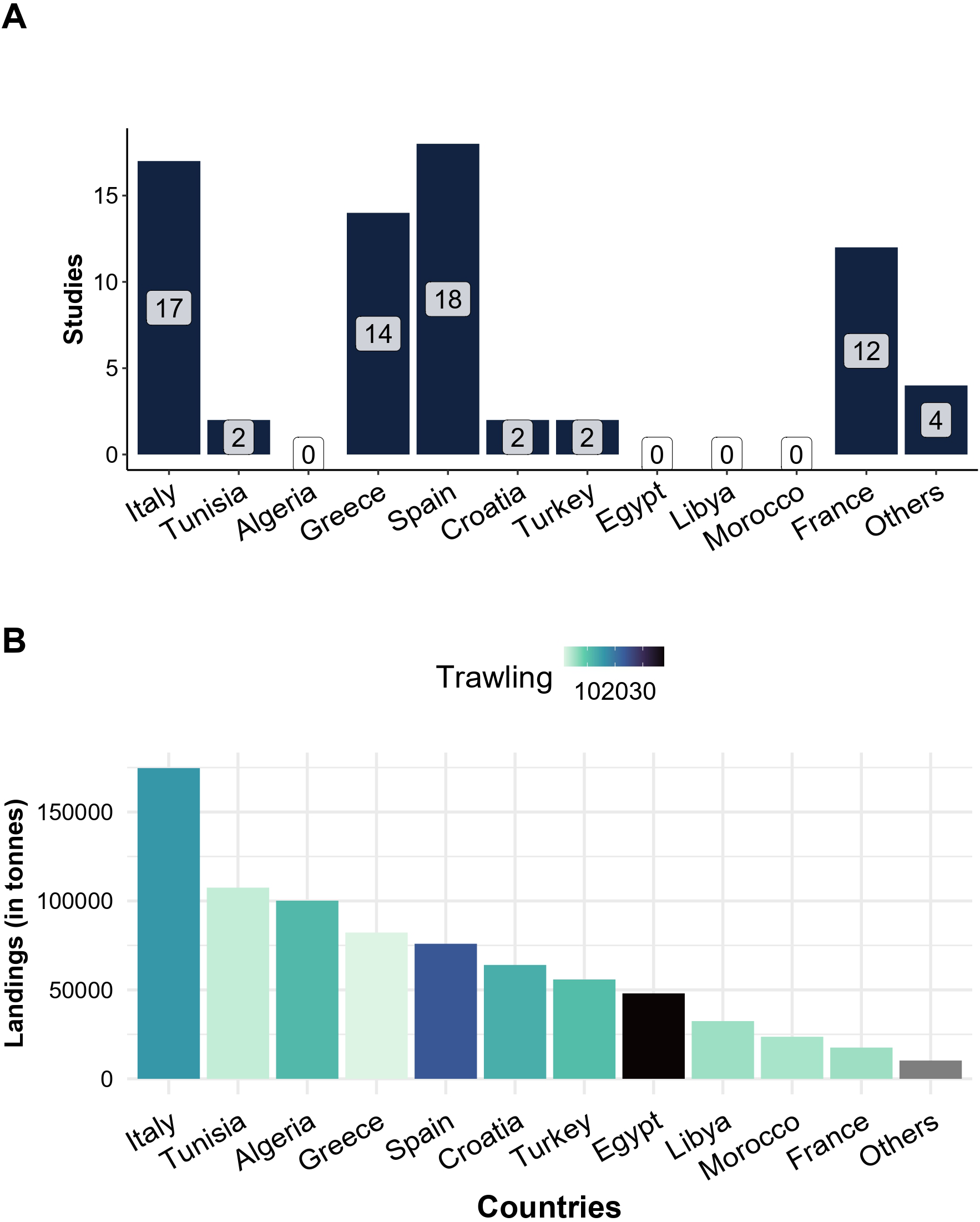
(A) Number of studies per country and (B) Biomass of landings per country and trawlers percentage among the fishing fleet based on Colloca et al. (2017), grey= non-available values.
There is growing concern about the sustainability of the current level of fishing exploitation in relation to the unbalanced and unregulated fishing in several areas of the Mediterranean, and a call for ecosystem-based management of fisheries within the context of future scenarios of global change (Colloca et al., 2017). These scenarios include the increasing presence of alien species, warming, and changes in fishing pressure (Michailidis et al., 2019).
Southern Mediterranean countries are considered hot spots for climate change or invasions and their impacts on fisheries and coastal ecosystems (Farahmand et al., 2023). The understanding of fishing impact in southern countries is thus necessary and it could be improved by collecting well-sounded scientific data and refining existing models of fishing impacts at the scale of the Mediterranean or specifically built for the social and ecological conditions of these countries. This is also important to assist the management of fisheries in rural areas and their evolution in terms of fishing capacity (Maynou, 2020).
3.2 Types of fisheries
Among the reviewed studies, 36 considered a combination of different types of fisheries, 14 did not specify the type of fisheries and only 17 reported to focus exclusively on one type of fisheries; e.g. 8 on industrial, 8 on small-scale fisheries (SSF) and only 2 on recreational fisheries (Figure 4, Supplementary Table S1). SSF in the Mediterranean is multispecies, multigear, and very complex to evaluate in a simple manner. Yet, information on the distinct impacts of SSF, industrial and recreational fishing is needed to implement more effective management in the Mediterranean and worldwide (Goñi et al., 2008; Calò et al., 2022). The different gear types used in SSF are generally considered less destructive to coastal ecosystems than industrial fishing gears (Crowder et al., 2008), but they can have potential ecosystem-wide effects (Stergiou et al., 2007; Crowder et al., 2008). As expected, the papers we collected that reported on the impact of industrial fisheries, particularly trawling, showed a strong decrease in the health of the whole food web (e.g. Coll et al., 2008a; Libralato et al., 2010). They also showed how increasing the selectivity of trawls could mitigate effects on TLs and other trophic indicators (Coll et al., 2008a; Saygu et al., 2020b). These authors showed that using a large mesh size could have a positive effect on the biomass of both commercial and non-commercial species, thereby improving the TL of the community (TLco; Coll et al., 2008a) or the TL of catches (TLc, Saygu et al., 2020b).
Figure 4
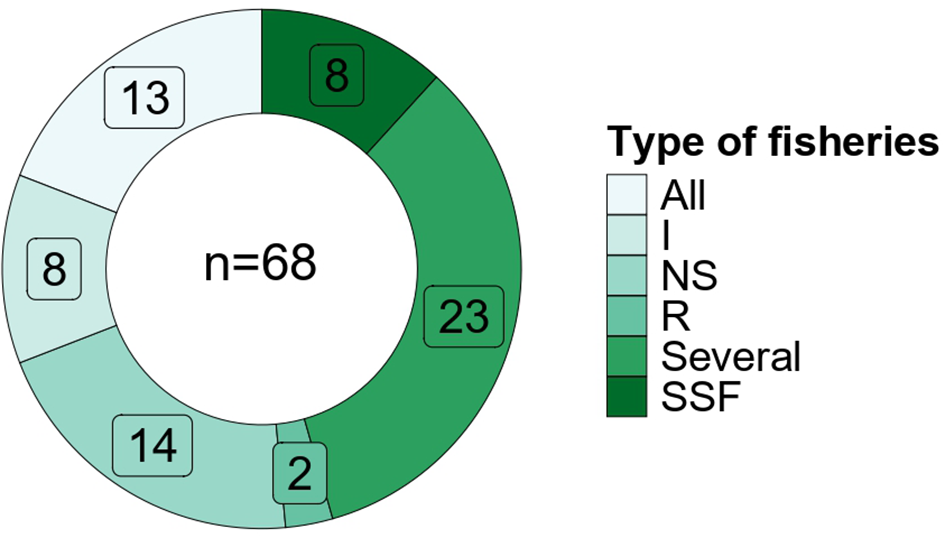
Repartition of the fisheries type among the collected studies. All= Industrial, Small scale and recreational; I= Industrial; NS= Not specified; R= Recreational; Several=Combination of 2 fisheries (e.g. SSF+R or SSF+I or R+I); SSF= Small Scale fishery; More details are in the text and in Supplementary Table S1.
Nevertheless, the authors also emphasise the need of reducing fishing effort in heavily exploited or overexploited fisheries.
The two papers on the impact of SSF in the Corsica island considered fish net, spiny lobster and small trawling. They found SSF to have a very low impact, probably because of the very low level of exploitation of the system. Indeed, evaluation of TL indicators showed small changes in time or considering different fishing pressure scenarios. Nonetheless, they highlighted the negative impact of SSF on functional groups with the highest TLs; e.g. rays and sharks and also on non-targeted groups (Vanalderweireldt et al., 2022; Marengo et al., 2023). The effects of SSF were also variable among geographical areas and strongest in the Ionian and Eastern Mediterranean Sea, probably because of the environmental characteristics of the areas, such as low productivity but also because of the highest number of SSF vessels (Corrales et al., 2018). Similar impacts were reported for recreational fisheries (Lloret et al., 2008). However, recreational fishery was found severely under-represented in the published literature, even if this type of fisheries has been described as important in the Mediterranean Sea (Figure 4; Lloret et al., 2020). This may pose a serious bias in estimating fishing impact on food webs.
While some studies focussed specifically on a single fishery type, others took a gear-based approach to infer fishery impacts.—information that can help infer the type of fishery involved (Smith and Basurto, 2019). These studies consistently found that bottom trawling—commonly used in industrial and semi-industrial fisheries—was the main driver of negative impacts on most exploited functional groups. For example, several regional studies assessed the effects of trawling in the southern Catalan Sea (Coll et al., 2008a, 2008b), the Gulf of Gabès (Hattab et al., 2013), and the Eastern Mediterranean Sea (Corrales et al., 2018), all highlighting its impact on demersal community species. A broader study across the entire Mediterranean basin reported similar findings (Piroddi et al., 2015). Additionally, Corrales et al. (2018) also documented a clear increase in community-level trophic indicators (TLc and TLco) after a cessation of trawling.
In contrast, small-scale fisheries (SSF) generally exert a lower ecological impact than trawling (e.g., Bănaru et al., 2013) or recreational fishing (Prato et al., 2016). However, some research highlights that SSF can negatively affect species at the highest trophic levels, particularly non-targeted species such as dolphins, seabirds, and sea turtles (e.g., Michailidis et al., 2019; Keramidas et al., 2022; Sánchez-Zulueta et al., 2023). Similarly, recreational fishing has been shown to impact top predators, including dolphinfish (Sánchez-Zulueta et al., 2023) and sharks (Michailidis et al., 2019). These impacts on upper trophic levels may trigger top-down effects. For instance, Prato et al. (2016) suggest that recreational fishing, which often targets species with a trophic level above 3, could initiate trophic cascades. Additionally, Albouy et al. (2010) emphasise the combined effects of artisanal and recreational fishing fleets, reporting an increase in benthic mollusc feeders biomass, likely linked to intensified fishing pressure on higher trophic levels.
3.3 Methodological approach
In agreement with other reviews on fisheries (Coll and Libralato, 2012; Colloca et al., 2017) and food webs (Kytinou et al., 2020), we found more studies using a modelling than a field approach (45 and 23, respectively). Among these, 16 field and 4 modelling studies did not report any estimates of TLs, while evaluating other aspects of the food web (Supplementary Table S1, Figure 5).
Figure 5
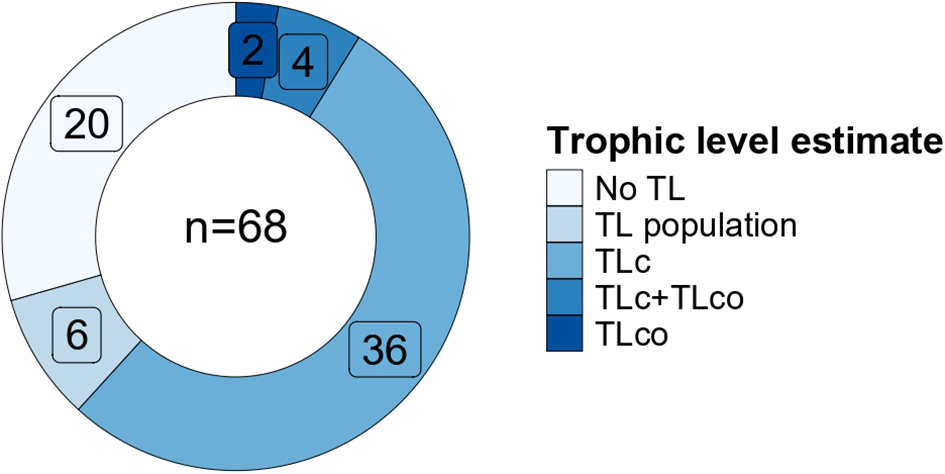
Repartition of the estimate of trophic level (TL) among the different types of TL for the collected studies. TL population= estimate for each fish species calculated using δ15N, see Table 1); TLc = estimates for catches done in modelling studies and in 1 field study (Marengo et al., 2023); TLco= estimates for the whole assemblage of consumers. More details are in the text and in Tables 1 and S1.
The 23 field studies reported results on trophic aspects of target fishes using various methods for in-situ data collection. The most widely used method was underwater visual census (UVC; 9 out of 23 studies) to quantify fish abundance and biomass per trophic group (fishes occupying similar TL, Supplementary Table S1). Other studies (3) used bioassays to estimate the strength of trophic interactions, e.g. predation on intermediate consumers (e.g. Guidetti, 2006; Vergés et al., 2012; Seytre et al., 2013). Ten studies targeted wild fish populations, not necessarily of commercial value, captured with experimental fishing and used either stomach content analysis (2 studies, Bautista-Vega et al., 2008; Zorica et al., 2021), morphological traits (1 study, Alós et al., 2014) or stable isotope analysis (SIA; 6 studies; Supplementary Table S1). Remaining 2 studies took advantage of landings, by interviewing what fishers had captured (Lloret et al., 2008) or using research survey that were independent from fishery data or sampled catches on fish vessels (Marengo et al., 2023). The use of SIA in only 6 out of 23 studies was surprising since SIA is currently the most extensively used method for the identification of trophic levels within foodwebs (Kytinou et al., 2020). These 6 studies using SIA (Supplementary Table S1; Figure 5) estimated TL values for fish population using the δ15N of fish muscles against a trophic baseline (Table 1). The δ15N baseline for estimating TL was usually measured from secondary consumers (Badalamenti et al., 2002, 2008; Deudero et al., 2004; Moranta et al., 2020; Zorica et al., 2021) or derived from the literature (Vizzini and Mazzola, 2009). Instead, Marengo et al. (2023) used the data collected on fish species and the available online database (FishBase) for attributing TL values to each captured species and estimating mean catch TL.
Table 1
| Indicators | Full name | Definition | References |
|---|---|---|---|
| TL population | Trophic level of a population |
TL measured using stable isotope values of δ15N of a species i, based on Post et al., 2002. TLi and TLref are the trophic level of group i and a reference baseline material (usually phytoplankton or bivalves) |
Post, 2002 |
| TLc | Trophic level of landed catches |
where YL is total landings, Yi is the landing of species i, and TLi is the trophic level of species i |
Pauly et al., 1998; Shannon et al., 2014 |
| TLco | TL of the modelled community (from a specific ecosystem excluding primary producers) |
where BMT is total biomass of the modelled ecosystem, BMi is the biomass of each species i in the model, and TLi is the trophic level of species i as an output of the model |
Shannon et al., 2014 |
TL estimates for population using stable isotopes as defined by Post et al. (2002); communities of catches (TLc), as suggested by Pauly et al. (1998) and communities of consumers (TLco; Shannon et al., 2014).
All modelling studies were based on trophodynamic models. Input data of biomass of fishes were derived from other published studies or online databases (Supplementary Table S1). Twenty-one studies used data from research survey which were fishery-independent, such as the MEDITS program (Mediterranean International bottom Trawl Surveys) (Bertrand et al., 2002) and a few studies used fishery-dependent data collected aboard vessels. Other fishery-dependent data were collected using fishers’ logbooks or (inter)national monitoring. Data on TLs of functional groups were in general derived from existing databases or calculated based on literature information on main prey, as described in Stergiou and Karpouzi (2001). The model then combined biomass with TL and produced values relative to a part of the community or to catches. Most modelling studies evaluated the average TL value of catches (TLc or mTLc), while others evaluated community TLs of whole consumers including non-targeted species (TLco in Table 1 or mTLco or TLco>1). Very few model studies evaluated both catch and community TLs (4 out of 45; Tsagarakis et al., 2010; Corrales et al., 2018; García-Rodríguez et al., 2020; Papantoniou et al., 2021, Figure 5).
The population and community TLs as derived from field and modelling studies provides complementary information (McCormack et al., 2019). Field studies are often snapshots of a short-term situation, but those evaluating TL with stable isotopes can determine the trophic position of local fish populations, which can change, in response to perturbations and fishing pressure (Olson et al., 2020). This can become particularly important when evaluating ecosystem-based fisheries impacts, because the response of fish species to environmental changes may reflect a change in their trophic position, particularly important when generalist and omnivore fishes, prone to diet changes, are the most abundant taxa within the food webs and within catches.
In the modelling studies, the use of data considering large temporal and spatial scales, although necessary for covering large-scale food webs over time, does not take into account local variations of trophic position within the same species. In addition, when data comes from landings, there can be a non-negligible source of uncertainty and imprecision (Shannon et al., 2014). Specifically, landings data are not necessarily representative of actual catches, which also include illegal, unregulated and unreported fishing (IUUF) activities as well as discards (Garibaldi, 2012). The IUFF is a serious issue in the Mediterranean Sea (Piroddi et al., 2015) and little progress has been made in the Mediterranean to combat the phenomenon (Srour et al., 2020).
3.4 Trophic level comparison between fishing and non-fishing areas
Evaluating variation of TLs in relation to fishing activities might be difficult because it requires somehow similar ecological conditions but a different fishing pressure. Among the 21 studies —that considered no-take zones of Marine Protected Areas (MPAs) or Fisheries Restricted Areas (FRAs) as the no-fishing conditions—we found only 5 field studies and 4 modelling studies that compared trophic level (TL) values between fishing and no-fishing areas (Supplementary Table S1). The average (± SD or SE, when available) TL values of those studies are reported in Figure 6). The field studies focussing on population-level TLs showed different patterns of variability in response to protection (Figure 6A). Moranta et al. (2020) showed a positive effect of fishing ban on the rainbow wrasse Coris julis related to its ontogenetic development, when changes in competition and predation occur and might be affected by the level of protection. Vizzini and Mazzola (2009) found a decrease in TL values under full protection for 2 out of the nine species. Since these fishes were invertivores (C. julis and Diplodus annularis), they suggested a possible increase in their predators causing a shift in diet of these species. Badalamenti et al. (2002; 2008), instead, claimed that changes in biomass due to fishing ban were not followed by substantial size-related trophodynamic shifts, which might depend on the availability of food in the area or on the fact that the target species do not shift diet. All these studies were focussed on single species and did not investigate changes at the community level. In the modelling studies focussing on community level, 2 studies found higher TL of catches (TLc) in no-fishing than fishing area (Figure 6B; Valls et al., 2012; Vilas et al., 2020), but the remaining one showed values slightly higher before than after the establishment of a fishery restricted area (2.59 vs 2.50, respectively; Vilas et al., 2021). TLco was similar or slightly higher in no-fishing than fishing areas for the 4 studies reporting it (Libralato et al., 2010; Valls et al., 2012; Vilas et al., 2020, 2021). These variations in the TL response to a fishing ban at both species and community level require further studies in order to gain a better understanding of the pattern of response of TL values under different fishing pressure. While, there is solid evidence of how the removal of human activities, including fishing, may increase diversity and biomass of fishes within food webs (e.g. Stobart et al., 2009; Seytre and Francour, 2014), it is possible that changes in fish trophic ecology and the resulting community shifts could be more related to environmental differences in the study area or to the species composition of the community and those more affected by protection. In particular, fishes occupying high trophic levels are more abundant and show larger sizes in no-take than other zones within MPAs (Micheli et al., 2005; Consoli et al., 2013; Guidetti et al., 2014; Viladrich et al., 2016; Rojo et al., 2021), thereby increasing predation pressure on low-level consumers and variably affecting other species, depending on the trophic level they occupy (Guidetti, 2006; Vergés et al., 2012; Seytre et al., 2013). These effects can also vary with local physical conditions and diversity (Micheli et al., 2005) or geographical scales (Villamor and Becerro, 2012). The papers we found were done in areas characterised by differences in seabed complexity and a different regime of oligotrophy (Supplementary Table S1), which might have affected the results. Studying changes in population and community levels across MPAs with different species composition and environmental attributes could thus represent an important step for testing how changes in fishing activities may modify population and community TLs, within different environmental contexts.
Figure 6
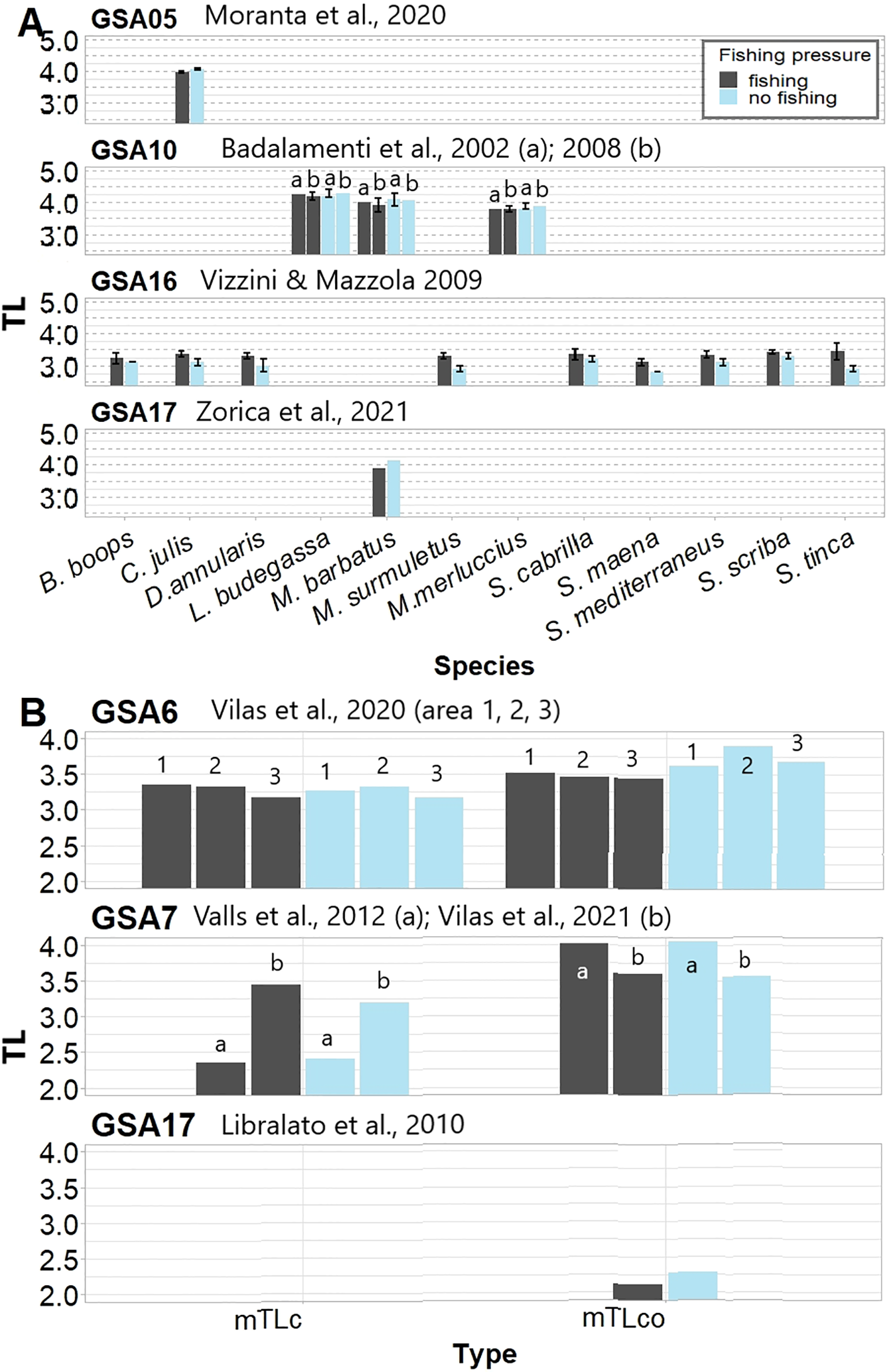
Values of trophic levels as reported in the revised papers (cited within the figure) that compared fishing vs. no-fishing areas. Plots are organised by GSA (as in Figure 2b). (A) Trophic levels (TL) of fish population (mean ± SD or SE, as reported in field studies) based on stable isotopes analysis (SIA) and, (B) TL of catches and community (mTLc and mTLco, respectively) with TL consumers >2 extracted from trophodynamic model studies.
In addition, it should be noted that 20 modelling studies showed how TLs values of community or catches decreased in time by analysing time series (Coll et al., 2008a; Coll et al., 2009a; Fortibuoni et al., 2017; Marengo et al., 2023; Piroddi et al., 2016) or by testing for scenarios of different fishing pressures (e.g. Vanalderweireldt et al., 2022; Papantoniou et al., 2023; Moutopoulos et al., 2018). Some papers also included changes in fishing pressure within global change scenarios by evaluating future catches of alien species (Saygu et al., 2020a, Michailidis et al., 2019) and/or the impact of warming (Corrales et al., 2018). These latter papers showed how changes in environmental conditions could drive impacts of fisheries.
In summary, our review on the literature explicitly dealing with the fishing impact on the trophic level (TL) of Mediterranean fish populations and assemblages highlights not only the geographical skewness of published studies, but also that more studies should focus on comparisons of population and community TL values between different levels of fishing pressure and under different regimes of environmental conditions, including geographical differences. This information would allow us to better understand how TL could be used as an indicator of fishing impact on food webs and to refine predictions on how changes in environmental conditions as those related to warming or invasions could modify fisheries. This is particularly important for food webs occupying the coastal shelf that are supported by a variety of autochtonous sources and under the influence of several human-induced drivers. No-take zones of marine protected areas or temporal fishing bans may offer good opportunities to quantify the impacts of fishing and the changes in TLs, using both field and modelling studies across the Mediterranean (Pinnegar et al., 2000; Grorud-Colvert et al., 2021). A better prediction of fisheries impacts on fish resources is of fundamental value in order to develop more effective conservation and ecosystem-based management measures in light of ongoing climate change and the importance of fishing activities in the Mediterranean Sea.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AM: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Methodology. SB: Writing – review & editing. PG: Writing – review & editing. FR: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreement no. 101002721, acronym: MERMAID). AM was supported by a PhD grant under the MERMAID project.
Acknowledgments
We would like to thank Tatiana Theodoropoulou, Emna Ben Lamine and Francesco Colloca for their support and advice on this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1489965/full#supplementary-material
References
1
Albouy C. Mouillot D. Rocklin D. Culioli J. Le Loc’h F. (2010). Simulation of the combined effects of artisanal and recreational fisheries on a Mediterranean MPA ecosystem using a trophic model. Mar. Ecol. Prog. Ser.412, 207–221. doi: 10.3354/meps08679
2
Alós J. Palmer M. Linde-Medina M. Arlinghaus R. (2014). Consistent size-independent harvest selection on fish body shape in two recreationally exploited marine species. Ecol. Evol.4, 2154–2164. doi: 10.1002/ece3.1075
3
Andersen K. H. Pedersen M. (2010). Damped trophic cascades driven by fishing in model marine ecosystems. Proc. R. Soc B.277, 795–802. doi: 10.1098/rspb.2009.1512
4
Badalamenti F. D’Anna G. Pinnegar J. Polunin N. (2002). Size-related trophodynamic changes in three target fish species recovering from intensive trawling. Mar. Biol.141, 561–570. doi: 10.1007/s00227-002-0844-3
5
Badalamenti F. Sweeting C. J. Polunin N. V. C. Pinnegar J. D’Anna G. Pipitone C. (2008). Limited trophodynamics effects of trawling on three Mediterranean fishes. Mar. Biol.154, 765–773. doi: 10.1007/s00227-008-0969-0
6
Bănaru D. Mellon-Duval C. Roos D. Bigot J.-L. Souplet A. Jadaud A. et al . (2013). Trophic structure in the Gulf of Lions marine ecosystem (north-western Mediterranean Sea) and fishing impacts. J. Mar. Syst.111–112, 45–68. doi: 10.1016/j.jmarsys.2012.09.010
7
Bautista-Vega A. A. Letourneur Y. Harmelin-Vivien M. Salen-Picard C. (2008). Difference in diet and size-related trophic level in two sympatric fish species, the red mullets Mullus barbatus and Mullus surmuletus, in the Gulf of Lions (north-west Mediterranean Sea). J. Fish Biol.73, 2402–2420. doi: 10.1111/j.1095-8649.2008.02093.x
8
Bertrand J. Leonori I. Dremière P. Y. Cosimi G. (2002). Depth trajectory and performance of a trawl used for an international bottom trawl survey in the Mediterranean. Scientia Marina66, 169–182. doi: 10.3989/scimar.2002.66s2169
9
Calò A. Di Franco A. Quattrocchi F. Dimitriadis C. Ventura P. Milazzo M. et al . (2022). Multi-specific small-scale fisheries rely on few, locally essential, species: Evidence from a multi-area study in the Mediterranean. Fish Fisheries23, 1299–1312. doi: 10.1111/faf.12689
10
Cardona L. Reñones O. Gouraguine A. Saporiti F. Borrell A. Aguilar A. et al . (2022). Effects of fishing on the trophic structure of carnivorous fish assemblages from shallow rocky bottoms of the Mediterranean Sea and the temperate Atlantic Ocean. ICES J. Mar. Sci.80, fsac229. doi: 10.1093/icesjms/fsac229
11
Cardona L. Reñones O. Gouraguine A. Saporiti F. Borrell A. Aguilar A. et al . (2023). Effects of fishing on the trophic structure of carnivorous fish assemblages from shallow rocky bottoms of the Mediterranean Sea and the temperate Atlantic Ocean. ICES J. Mar. Sci.80, 751–765. doi: 10.1093/icesjms/fsac229
12
Coll M. Libralato S. (2012). Contributions of food web modelling to the ecosystem approach to marine resource management in the Mediterranean Sea. Fish Fisheries13, 60–88. doi: 10.1111/j.1467-2979.2011.00420.x
13
Coll M. Lotze H. K. Romanuk T. N. (2008a). Structural degradation in mediterranean sea food webs: testing ecological hypotheses using stochastic and mass-balance modelling. Ecosystems11, 939–960. doi: 10.1007/s10021-008-9171-y
14
Coll M. Palomera I. Tudela S. Dowd M. (2008b). Food-web dynamics in the South Catalan Sea ecosystem (NW Mediterranean) for 1978–2003. Ecol. Model.217, 95–116. doi: 10.1016/j.ecolmodel.2008.06.013
15
Coll M. Palomera I. Tudela S. (2009). Decadal changes in a NW Mediterranean Sea food web in relation to fishing exploitation. Ecological Modelling220, 2088–2102. doi: 10.1016/j.ecolmodel.2009.04.049
16
Colloca F. Scarcella G. Libralato S. (2017). Recent trends and impacts of fisheries exploitation on mediterranean stocks and ecosystems. Front. Mar. Sci.4. doi: 10.3389/fmars.2017.00244
17
Consoli C. Gianluca S. Gianfranco M. Pietro B. Teresa R. Vincenzo I. et al . (2013). The effects of protection measures on fish assemblage in the Plemmirio marine reserve (Central Mediterranean Sea, Italy): A first assessment 5years after its establishment. J. Sea Res.79, 20–26. doi: 10.1016/j.seares.2013.01.004
18
Corrales X. Coll M. Ofir E. Heymans J. J. Steenbeek J. Goren M. et al . (2018). Future scenarios of marine resources and ecosystem conditions in the Eastern Mediterranean under the impacts of fishing, alien species and sea warming. Sci. Rep.8, 14284. doi: 10.1038/s41598-018-32666-x
19
Corrales X. Coll M. Tecchio S. Bellido J. M. Fernández Á.M. Palomera I. (2015). Ecosystem structure and fishing impacts in the northwestern Mediterranean Sea using a food web model within a comparative approach. J. Mar. Syst.148, 183–199. doi: 10.1016/j.jmarsys.2015.03.006
20
Crowder L. B. Hazen E. L. Avissar N. Bjorkland R. Latanich C. Ogburn M. B. (2008). The impacts of fisheries on marine ecosystems and the transition to ecosystem-based management. Annu. Rev. Ecology Evolution Systematics39, 259–278. doi: 10.1146/annurev.ecolsys.39.110707.173406
21
Davis K. J. Vianna G. M. S. Meeuwig J. J. Meekan M. G. Pannell D. J. (2019). Estimating the economic benefits and costs of highly-protected marine protected areas. Ecosphere10, e02879. doi: 10.1002/ecs2.2879
22
Deudero S. Pinnegar J. K. Polunin N. V. C. Morey G. Morales-Nin B. (2004). Spatial variation and ontogenic shifts in the isotopic composition of Mediterranean littoral fishes. Mar. Biol.145, 971–981. doi: 10.1007/s00227-004-1374-y
23
Elton C. S. (1927). Animal ecology (Sidgewick and Jackson).
24
FAO (2022). The State of Mediterranean and Black Sea Fisheries 2022 (FAO). doi: 10.4060/cc3370en
25
Farahmand S. Hilmi N. Cinar M. Safa A. Lam V. W. Y. Djoundourian S. et al . (2023). Climate change impacts on Mediterranean fisheries: A sensitivity and vulnerability analysis for main commercial species. Ecol. Economics211, 107889. doi: 10.1016/j.ecolecon.2023.107889
26
Farrugio H. Oliver P. Biagi F. (1993). An overview of the history, knowledge, recent and future research trends in Mediterranean fisheries15.
27
Fortibuoni T. Giovanardi O. Pranovi F. Raicevich S. Solidoro C. Libralato S. (2017). Analysis of long-term changes in a Mediterranean marine ecosystem based on fishery landings. Front. Mar. Sci.4, 33. doi: 10.3389/fmars.2017.00033
28
Fry B. Davis J. (2015). Rescaling stable isotope data for standardized evaluations of food webs and species niches. Mar. Ecol. Prog. Ser.528, 7–17. doi: 10.3354/meps11293
29
García-Rodríguez E. Vivas M. Torres M.Á. Esteban A. Bellido J. M. (2020). Revealing environmental forcing in the different trophic guilds of fish communities off the Western Mediterranean Sea. J. Sea Res.166, 101958. doi: 10.1016/j.seares.2020.101958
30
Garibaldi L. (2012). The FAO global capture production database: A six-decade effort to catch the trend. Mar. Policy36, 760–768. doi: 10.1016/j.marpol.2011.10.024
31
Giakoumi S. Halpern B. S. Michel L. N. Gobert S. Sini M. Boudouresque C.-F. et al . (2015). Towards a framework for assessment and management of cumulative human impacts on marine food webs: Vulnerability of Food Webs to Human Threats. Conserv. Biol.29, 1228–1234. doi: 10.1111/cobi.12468
32
Goñi R. Adlerstein S. Alvarez-Berastegui D. Forcada A. Reñones O. Criquet G. et al . (2008). Spillover from six western Mediterranean marine protected areas: evidence from artisanal fisheries. Mar. Ecol. Prog. Ser.366, 159–174. doi: 10.3354/meps07532
33
Grorud-Colvert K. Sullivan-Stack J. Roberts C. Constant V. Horta E Costa B. Pike E. P. et al . (2021). The MPA Guide: A framework to achieve global goals for the ocean. Science373. doi: 10.1126/science.abf0861
34
Guest M. Connolly R. Loneragan N. (2004). Carbon movement and assimilation by invertebrates in estuarine habitats at a scale of metres. Mar. Ecol. Prog. Ser.278, 27–34. doi: 10.3354/meps278027
35
Guidetti P. (2006). Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs. Ecol. Appl.16, 963–976. doi: 10.1890/1051-0761(2006)016[0963:MRRLPI]2.0.CO;2
36
Guidetti P. Baiata P. Ballesteros E. Franco A. D. Hereu B. Macpherson E. et al . (2014). Large-scale assessment of mediterranean marine protected areas effects on fish assemblages. PloS One9, e91841. doi: 10.1371/journal.pone.0091841
37
Halpern B. S. Frazier M. Afflerbach J. Lowndes J. S. Micheli F. O’Hara C. et al . (2019). Recent pace of change in human impact on the world’s ocean. Sci. Rep.9, 11609. doi: 10.1038/s41598-019-47201-9
38
Hattab T. Ben Rais Lasram F. Albouy C. Romdhane M. S. Jarboui O. Halouani G. et al . (2013). An ecosystem model of an exploited southern Mediterranean shelf region (Gulf of Gabes, Tunisia) and a comparison with other Mediterranean ecosystem model properties. J. Mar. Syst.128, 159–174. doi: 10.1016/j.jmarsys.2013.04.017
39
Hyslop E. J. (1980). Stomach contents analysis—a review of methods and their application. J. Fish Biol.17, 411–429. doi: 10.1111/j.1095-8649.1980.tb02775.x
40
Ishikawa N. F. Takashima A. Maruoka H. Kondoh M. (2024). Integrated trophic position as a proxy for food-web complexity. Methods Ecol. Evol.15, 164–177. doi: 10.1111/2041-210X.14256
41
Karachle P. K. Stergiou K. I. (2017). An update on the feeding habits of fish in the Mediterranean Sea, (2002-2015). Mediterr. Mar. Sci.18, 43–52. doi: 10.12681/mms.1968
42
Keramidas I. Dimarchopoulou D. Tsikliras A. C. (2022). Modelling and assessing the ecosystem of the Aegean Sea, a major hub of the eastern Mediterranean at the intersection of Europe and Asia. Regional Stud. Mar. Sci.56, 102704. doi: 10.1016/j.rsma.2022.102704
43
Kytinou E. Sini M. Issaris Y. Katsanevakis S. (2020). Global systematic review of methodological approaches to analyze coastal shelf food webs. Front. Mar. Sci.7. doi: 10.3389/fmars.2020.00636
44
Libralato S. Coll M. Tempesta M. Santojanni A. Spoto M. Palomera I. et al . (2010). Food-web traits of protected and exploited areas of the Adriatic Sea. Biol. Conserv.143, 2182–2194. doi: 10.1016/j.biocon.2010.06.002
45
Lindeman R. L. (1942). The trophic-dynamic aspect of ecology. Ecology23, 399–417. doi: 10.2307/1930126
46
Lloret J. Zaragoza N. Caballero D. Font T. Casadevall M. Riera V. (2008). Spearfishing pressure on fish communities in rocky coastal habitats in a Mediterranean marine protected area. Fisheries Res.94, 84–91. doi: 10.1016/j.fishres.2008.07.002
47
Lloret J. Biton-Porsmoguer S. Carreño A. Di Franco A. Sahyoun R. Melià P. Claudet J. et al . (2020). Recreational and small-scale fisheries may pose a threat to vulnerable species in coastal and offshore waters of the western Mediterranean. ICES J. Mar. Sci.77, 2255–2264. https://academic.oup.com/icesjms/article/77/6/2255/5486184.
48
Longo C. Hornborg S. Bartolino V. Tomczak M. T. Ciannelli L. Libralato S. et al . (2015). Role of trophic models and indicators in current marine fisheries management. Mar. Ecol.-Prog. Ser.538, 257–272. doi: 10.3354/meps11502
49
Lotze H. K. Coll M. Dunne J. A. (2011). Historical changes in marine resources, food-web structure and ecosystem functioning in the adriatic sea, mediterranean. Ecosystems14, 198–222. doi: 10.1007/s10021-010-9404-8
50
Lotze H. K. Lenihan H. S. Bourque B. J. Bradbury R. H. Cooke R. G. Kay M. C. et al . (2006). Depletion, degradation, and recovery potential of estuaries and coastal seas. Science312, 1806–1809. doi: 10.1126/science.1128035
51
Marengo M. Vanalderweireldt L. Horri K. Patrissi M. Santoni M.-C. Lejeune P. et al . (2023). Combining indicator trends to evaluate a typical Mediterranean small-scale fishery: The case study of Corsica. Regional Stud. Mar. Sci.65, 103087. doi: 10.1016/j.rsma.2023.103087
52
Maynou F. (2020). Evolution of fishing capacity in a Mediterranean fishery in the first two decades of the 21st c. Ocean Coast. Manage.192, 105190. doi: 10.1016/j.ocecoaman.2020.105190
53
McCormack S. A. Trebilco R. Melbourne-Thomas J. Blanchard J. L. Fulton E. A. Constable A. (2019). Using stable isotope data to advance marine food web modelling. Rev. Fish Biol. Fisheries29, 277–296. doi: 10.1007/s11160-019-09552-4
54
Michailidis N. Corrales X. Karachle P. K. Chartosia N. Katsanevakis S. Sfenthourakis S. (2019). Modelling the role of alien species and fisheries in an Eastern Mediterranean insular shelf ecosystem. Ocean Coast. Manage.175, 152–171. doi: 10.1016/j.ocecoaman.2019.04.006
55
Micheli F. Benedetti-Cecchi L. Gambaccini S. Bertocci I. Borsini C. Osio G. C. et al . (2005). Cascading human impacts, marine protected areas, and the structure of mediterranean reef assemblages. Ecol. Monogr.75, 81–102. doi: 10.1890/03-4058
56
Moher D. Liberati A. Tetzlaff J. Altman D. G. (2010). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg.8, 336–341. doi: 10.1016/j.ijsu.2010.02.007
57
Möllmann C. Müller-Karulis B. Kornilovs G. St John M. A. (2008). Effects of climate and overfishing on zooplankton dynamics and ecosystem structure: regime shifts, trophic cascade, and feedback loops in a simple ecosystem. ICES J. Mar. Sci.65, 302–310. doi: 10.1093/icesjms/fsm197
58
Moranta J. Reñones O. Gouraguine A. Saporiti F. Cardona L. (2020). The effects of fishing on the ontogeny of trophic position and body condition of a small-sized temperate marine fish. Mar. Environ. Res.161, 105055. doi: 10.1016/j.marenvres.2020.105055
59
Moutopoulos D. K. Tsagarakis K. Machias A. (2018). Assessing ecological and fisheries implications of the EU landing obligation in Eastern Mediterranean. J. Sea Res.141, 99–111. doi: 10.1016/j.seares.2018.08.006
60
Olson A. Frid A. Dos Santos J. Juanes F. (2020). Trophic position scales positively with body size within but not among four species of rocky reef predators. Mar. Ecol. Prog. Ser.640, 189–200. doi: 10.3354/meps13275
61
Papantoniou G. Giannoulaki M. Stoumboudi M. Lefkaditou E. Tsagarakis K. (2021). Food web interactions in a human dominated Mediterranean coastal ecosystem. Mar. Environ. Res.172, 105507. doi: 10.1016/j.marenvres.2021.105507
62
Papantoniou G. Zervoudaki S. Assimakopoulou G. Stoumboudi M. Th. Tsagarakis K. (2023). Ecosystem-level responses to multiple stressors using a time-dynamic food-web model: The case of a re-oligotrophicated coastal embayment (Saronikos Gulf, E Mediterranean). Sci. Total Environ.903, 165882. doi: 10.1016/j.scitotenv.2023.165882
63
Pauly D. Christensen V. Dalsgaard J. Froese R. Torres F. (1998). Fishing down marine food webs. Science279, 860–863. doi: 10.1126/science.279.5352.860
64
Pauly D. Palomares M.-L. (2005). Fishing down marine food web: it is far more pervasive than we thought. Bull. OF Mar. Sci.76.
65
Pinnegar J. K. Polunin N. V. C. Francour P. Badalamenti F. Chemello R. Harmelin-Vivien M.-L. et al . (2000). Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Envir. Conserv.27, 179–200. doi: 10.1017/S0376892900000205
66
Piroddi C. Coll M. Steenbeek J. Macias Moy D. Christensen V. (2015). Modelling the Mediterranean marine ecosystem as a whole: addressing the challenge of complexity. Mar. Ecol. Prog. Ser.533, 47–65. doi: 10.3354/meps11387
67
Piroddi C. Moutopoulos D. K. Gonzalvo J. Libralato S. (2016). Ecosystem health of a Mediterranean semi-enclosed embayment (Amvrakikos Gulf, Greece): Assessing changes using a modeling approach. Cont. Shelf Res.121, 61–73. doi: 10.1016/j.csr.2015.10.007
68
Post D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology83, 703–718. doi: 10.2307/3071875
69
Prato G. Barrier C. Francour P. Cappanera V. Markantonatou V. Guidetti P. et al . (2016). Assessing interacting impacts of artisanal and recreational fisheries in a small Marine Protected Area (Portofino, NW Mediterranean Sea). Ecosphere7, e01601. doi: 10.1002/ecs2.1601
70
Rocha J. Yletyinen J. Biggs R. Blenckner T. Peterson G. (2015). Marine regime shifts: drivers and impacts on ecosystems services. Phil. Trans. R. Soc B370, 20130273. doi: 10.1098/rstb.2013.0273
71
Rojo I. Anadón J. D. García-Charton J. A. (2021). Exceptionally high but still growing predatory reef fish biomass after 23 years of protection in a Marine Protected Area. PloS One16, e0246335. doi: 10.1371/journal.pone.0246335
72
Sala E. Boudouresque C. F. Harmelin-Vivien M. (1998). Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos82, 425–439. doi: 10.2307/3546364
73
Sánchez-Zulueta P. Valls M. Guijarro B. Ángeles Torres M. Ángeles Zapata M. Coll M. et al . (2023). Trophic structure and fishing impacts on an oligotrophic ecosystem in the Western Mediterranean: the Balearic Islands. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1166674
74
Saygu İ. Heymans J. J. Fox C. J. Özbilgin H. Eryaşar A. R. Gökçe G. (2020a). The importance of alien species to the food web and bottom trawl fisheries of the Northeastern Mediterranean, a modelling approach. J. Mar. Syst.202, 103253. doi: 10.1016/j.jmarsys.2019.103253
75
Saygu İ. Heymans J. J. Fox C. Özbilgin H. Bentley J. W. Eryaşar A. R. et al . (2020b). Community-level impacts of trawl selectivity in the Eastern Mediterranean Sea assessed using an ecosystem modelling approach. ICES J. Mar. Sci.77, 2918–2932. doi: 10.1093/icesjms/fsaa167
76
Seytre C. Francour P. (2014). A long-term survey of Posidonia oceanica fish assemblages in a Mediterranean Marine Protected Area: Emphasis on stability and no-take area effectiveness. Mar. Freshw. Res.65, 244–254. doi: 10.1071/MF13080
77
Seytre C. Vanderklift M. A. Bodilis P. Cottalorda J.-M. Gratiot J. Francour P. (2013). Assessment of commercial and recreational fishing effects on trophic interactions in the Cap Roux area (north-western Mediterranean): COMMERCIAL AND RECREATIONAL FISHING EFFECTS ON TROPHIC INTERACTIONS. Aquat. Conserv: Mar. Freshw. Ecosyst.23, 189–201. doi: 10.1002/aqc.2309
78
Shannon L. Coll M. Bundy A. Gascuel D. Heymans J. Kleisner K. et al . (2014). Trophic level-based indicators to track fishing impacts across marine ecosystems. Mar. Ecol. Prog. Ser.512, 115–140. doi: 10.3354/meps10821
79
Simeoni C. Furlan E. Pham H. V. Critto A. de Juan S. Trégarot E. et al . (2023). Evaluating the combined effect of climate and anthropogenic stressors on marine coastal ecosystems: Insights from a systematic review of cumulative impact assessment approaches. Sci. Total Environ.861, 160687. doi: 10.1016/j.scitotenv.2022.160687
80
Smith H. Basurto X. (2019). Defining small-scale fisheries and examining the role of science in shaping perceptions of who and what counts: A systematic review. Front. Mar. Sci.6. doi: 10.3389/fmars.2019.00236
81
Srour A. Ferri N. Carlson A. (2020). The General Fisheries Commission for the Mediterranean and the Fight against Illegal, Unreported, and Unregulated Fishing through Better Compliance. Ocean Yearbook Online34, 412–427. doi: 10.1163/9789004426214_018
82
Stergiou K. I. Karpouzi V. S. (2001). Feeding habits and trophic levels of Mediterranean fish. Rev. Fish Biol. Fisheries11, 217–254. doi: 10.1023/A:1020556722822
83
Stergiou K. Moutopoulos D. Casal H. Erzini K. (2007). Trophic signatures of small-scale fishing gears: implications for conservation and management. Mar. Ecol. Prog. Ser.333, 117–128. doi: 10.3354/meps333117
84
Stobart B. Warwick R. González C. Mallol S. Díaz D. Reñones O. et al . (2009). Long-term and spillover effects of a marine protected area on an exploited fish community. Mar. Ecol. Prog. Ser.384, 47–60. doi: 10.3354/meps08007
85
Thompson R. M. Brose U. Dunne J. A. Hall R. O. Hladyz S. Kitching R. L. et al . (2012). Food webs: reconciling the structure and function of biodiversity. Trends Ecol. Evol.27, 689–697. doi: 10.1016/j.tree.2012.08.005
86
Tremblay-Boyer L. Gascuel D. Watson R. Christensen V. Pauly D. (2011). Modelling the effects of fishing on the biomass of the world’s oceans from 1950 to 2006. Mar. Ecol. Prog. Ser.442, 169–185. doi: 10.3354/meps09375
87
Tsagarakis K. Coll M. Giannoulaki M. Somarakis S. Papaconstantinou C. Machias A. (2010). Food-web traits of the North Aegean Sea ecosystem (Eastern Mediterranean) and comparison with other Mediterranean ecosystems. Estuarine Coast. Shelf Sci.88, 233–248. doi: 10.1016/j.ecss.2010.04.007
88
Valls A. Gascuel D. Guénette S. Francour P. (2012). Modeling trophic interactions to assess the effects of a marine protected area: case study in the NW Mediterranean Sea. Mar. Ecol. Prog. Ser.456, 201–214. doi: 10.3354/meps09701
89
Vanalderweireldt L. Albouy C. Le Loc’h F. Millot R. Blestel C. Patrissi M. et al . (2022). Ecosystem modelling of the Eastern Corsican Coast (ECC): Case study of one of the least trawled shelves of the Mediterranean Sea. J. Mar. Syst.235, 103798. doi: 10.1016/j.jmarsys.2022.103798
90
Vergés A. Tomas F. Ballesteros E. (2012). Interactive effects of depth and marine protection on predation and herbivory patterns. Mar. Ecol. Prog. Ser.450, 55–65. doi: 10.3354/meps09599
91
Viladrich N. Rossi S. López-Sanz A. Orejas C. (2016). Nutritional condition of two coastal rocky fishes and the potential role of a marine protected area. Mar. Ecol.37, 46–63. doi: 10.1111/maec.12247
92
Vilas D. Coll M. Corrales X. Steenbeek J. Piroddi C. Calò A. et al . (2020). The effects of marine protected areas on ecosystem recovery and fisheries using a comparative modelling approach. Aquat. Conservation: Mar. Freshw. Ecosyst.30, 1885–1901. doi: 10.1002/aqc.3368
93
Vilas D. Coll M. Corrales X. Steenbeek J. Piroddi C. Macias D. et al . (2021). Current and potential contributions of the Gulf of Lion Fisheries Restricted Area to fisheries sustainability in the NW Mediterranean Sea. Mar. Policy123, 104296. doi: 10.1016/j.marpol.2020.104296
94
Villamor A. Becerro M. A. (2012). Species, trophic, and functional diversity in marine protected and non-protected areas. J. Sea Res.73, 109–116. doi: 10.1016/j.seares.2012.07.002
95
Vizzini S. Mazzola A. (2009). Stable isotopes and trophic positions of littoral fishes from a Mediterranean marine protected area. Environ. Biol. Fish84, 13–25. doi: 10.1007/s10641-008-9381-3
96
Watson R. A. Cheung W. W. L. Anticamara J. A. Sumaila R. U. Zeller D. Pauly D. (2013). Global marine yield halved as fishing intensity redoubles. Fish Fisheries14, 493–503. doi: 10.1111/j.1467-2979.2012.00483.x
97
Zhang C. Chen Y. Xu B. Xue Y. Ren Y. (2019). How to predict biodiversity in space? An evaluation of modelling approaches in marine ecosystems. Diversity Distributions25, 1697–1708. doi: 10.1111/ddi.12970
98
Zorica B. Ezgeta-Balić D. Vidjak O. Vuletin V. Šestanović M. Isajlović I. et al . (2021). Diet composition and isotopic analysis of nine important fisheries resources in the eastern adriatic sea (Mediterranean). Front. Mar. Sci.8. doi: 10.3389/fmars.2021.609432
Summary
Keywords
trophic position, trophic interactions, trophic cascade, fishery, foodweb, Mediterranean Sea
Citation
Marguin A, Bussotti S, Guidetti P and Rossi F (2025) A systematic review of fishing impacts on the trophic level of fish populations and assemblages in the Mediterranean Sea. Front. Mar. Sci. 12:1489965. doi: 10.3389/fmars.2025.1489965
Received
02 September 2024
Accepted
21 July 2025
Published
13 August 2025
Volume
12 - 2025
Edited by
Elena Gissi, National Research Council (CNR), Italy
Reviewed by
Fabio Fiorentino, National Research Council (CNR), Italy
Britas Klemens Eriksson, University of Groningen, Netherlands
Updates
Copyright
© 2025 Marguin, Bussotti, Guidetti and Rossi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Audrey Marguin, marguinaudrey@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.