Abstract
Marine forests are declining worldwide and understanding the ecology of extant forests is crucial for developing practices that best conserve and restore them for the future. In the Mediterranean region, there has been an increasing effort to restore forest forming fucalean seaweeds and to understand the ecological context that supports their persistence. Here, we describe population metrics for a significant extant fucalean forest located in a coastal lagoon on the southern Istrian peninsula (Croatia). In Šćuza Lagoon, Gongolaria barbata settles within two main substrate types, on small stones and pebbles amongst seagrass Cymodocea nodosa and on rocky substrate provided by larger, more exposed boulders within the meadow but where seagrass does not grow. Amongst seagrass, G. barbata grew to a greater maximum height, observed during both its growth and dormant phases. On boulders, any disadvantage in height appeared to be offset by higher recruitment where the overall density was similar between the two areas. Opportunistic recruitment of G. barbata during the senescent period for C. nodosa appeared to contribute to their coexistence in this unique location and seagrasses appeared to reduce the prevalence of cauloid damage for G. barbata. These findings highlight the importance of understanding fine-scale ecological interactions that support the persistence of isolated patches of vulnerable marine forests.
Introduction
The structure and composition of benthic marine habitats are being altered all around the world as groups of foundation species are lost and replaced by groups of lesser ecological complexity. Foundation species create complex physical habitat structure and positively interact with and enhance the resilience of local biological communities (Dayton, 1972; Stachowicz, 2001; Angelini et al., 2011). Marine macrophytes are historically the foundation species found in shallow subtidal temperate environments, large brown seaweeds (i.e. kelps of the orders Laminariales and Fucales) forming forest-like structures on rocky substrates (Teagle et al., 2017) and seagrasses forming vast meadows where sandy substrates dominate. Seaweed and seagrass communities can coexist where rocky and sandy substrates meet and create a unique set of interactions, however, there are relatively few studies that formally test these interactions in temperate regions. Of those that do, the majority present evidence for negative outcomes for either group citing some level of resource competition, with little evidence for facilitation usually (but not exclusively) in favor of the seagrass (reviewed in Richard and Quijón, 2023).
In the Mediterranean Sea, macroalgal species of genera Cystoseira, Ericaria and Gongolaria (order Fucales; hereafter collectively referred to as Cystoseira sensu lato), are considered the dominant canopy forming species for temperate rocky reefs, but in recent decades have dramatically declined (Airoldi and Beck, 2007; Iveša et al., 2016; Mangialajo et al., 2008; Orlando-Bonaca et al., 2021a; Thibaut et al., 2005). The presence of any extant marine forest habitat is considered a positive indicator for ecosystem health (Ballesteros et al., 2007). The importance of conserving these areas is highlighted by research describing the ecological value (Cheminée et al., 2013; Piazzi et al., 2018; Pinna et al., 2020) and by habitat level protection under the Barcelona Convention (United Nations Environment Programme/Mediterranean Action Plan (UNEP/MAP), 2013). Similarly, ecologically valuable seagrass habitats are declining globally (Duarte et al., 2008; Orth et al., 2006; Waycott et al., 2009), including in the Mediterranean Sea (Dunic et al., 2021; Chefaoui et al., 2018). Cymodocea nodosa (Ucria) Ascherson is one of few seagrass species native to the Mediterranean, and forms highly valuable meadows across its distribution along the Mediterranean and Atlantic coasts (Chefaoui et al., 2016) and in the Mediterranean, meadows are considered a protected habitat under the Bern Convention (Council of Europe, 2000). It is projected that a dramatic loss of C. nodosa cover will occur over the coming decades (Chefaoui et al., 2018), despite some potential refuge areas in cooler regions, including the Adriatic Sea, where an increase in cover could occur (Chefaoui et al., 2018), particularly as the species may replace more vulnerable Posidonia oceanica (Linnaeus) Delile meadows under threat (Burgos et al., 2017).
In the northern Adriatic Sea, a unique cold-temperate bioregion of the Mediterranean (Bianchi and Morri, 2000), fucalean forests have declined over the past decade, where only a scattering of remnant populations is documented along the Italian (e.g. Mancuso et al., 2018), Slovenian (e.g. Orlando-Bonaca et al., 2021a) and Croatian (e.g. Iveša et al., 2016) coastlines that make up this region. Among these isolated forests, the species Gongolaria barbata (Stackhouse) Kuntze in particular is commonly observed as a surviving species, an anomaly given a strong regional history of mixed species forests (Iveša and Devescovi, 2014; Iveša et al., 2016). Along the west Istrian Croatian coast, Iveša et al. (2022) have identified a significant remanent population of G. barbata in a costal lagoon where it coexists with the seagrass C. nodosa. Interestingly, the functionally similar fucoid, Cystoseira foeniculacea (Linnaeus) Greville, is also found to persist in a nearby coastal lagoon (Stjuža) in Strunjan Bay, Slovenia where it also coexists amongst a high biomass of seagrasses (C. nodosa and Zostera noltei Hornemann, Battelli and Catra, 2021, 2023). It is therefore perhaps the case that coastal lagoons can represent local refuges for supporting a high biomass of marine macrophytes and/or interactions between seaweed and seagrasses contribute to their simultaneous persistence in these environments.
Šćuza Lagoon is 68.6 ha in area, has a maximum depth of approximately 1.5m and C. nodosa is the dominant habitat forming macrophyte across most of the lagoon as it is dominated by sandy substrate, save for approximately 2 ha along the western edge where a shift to rocky/rubble substrate supports a significant settlement of G. barbata (Iveša et al., 2022). Historically, Šcuza Lagoon was used as an aquaculture basin and thus was heavily fished but the activity was abandoned, and the lagoon is now protected from such activities under the European Union Natura 2000 guidelines. The lagoon is isolated from the sea due to the construction of a pedestrian footbridge and thus there are a range of biotic and abiotic factors that contribute to its unique environment. Importantly, the thermal range of the lagoon is extreme, where the temperature can dip below zero and reach up to 34°C (Iveša et al., 2022). It is therefore quite remarkable that G. barbata, with a previously recorded thermal maxima of 23°C (throughout the Adriatic Sea, Ercegović, 1952), thrives here when it is currently rare elsewhere along the coastline (Iveša et al., 2016). In contrast, C. nodosa is less sensitive to higher temperatures, which was shown experimentally (Olsen et al., 2012) and by its widespread distribution (Chefaoui et al., 2016). The response of both species to extreme cold temperatures is not yet well understood.
It is possible that temporal alternation in the growth cycle of the dominant macrophytes in the lagoon contributes to the coexistence of the two species. G. barbata has a diplontic monophasic lifecycle that is typical of fucalean species (Ercegović, 1952; Rindi et al., 2023 and references therein). Locally, the growth of vegetative branches occurs in the autumn months (Iveša et al., 2022), hypothesized to be triggered by a temperature threshold of ~18°C (Bilajac et al., 2024). During this time, receptacles develop, and fertility is induced in the late winter/early spring when biomass peaks (Iveša et al., 2022; author observations). Annual (branch) material is then lost during the summer months, and the alga enters a “dormant phase” where perennial material remains and prominent, smooth cauloid apexes are evident. During this time, cauloids can become covered in epiphytes (Iveša et al., 2022) and growth is significantly reduced, particularly at high temperatures (Ercegović, 1952; Iveša et al., 2022; Bilajac et al., 2024). C. nodosa however, is a flowering marine angiosperm, capable of both sexual and clonal reproduction (Hemminga and Duarte, 2000), but the strength of either strategy is highly variable and regionally specific (Máñez-Crespo et al., 2020). C. nodosa meadows show a high turnover of biomass (Cunha and Duarte, 2007), can show variations in growth pattern based on depth (Olesen et al., 2002) but generally peak leaf growth is observed in the summer months (Cunha and Duarte, 2007; Olesen et al., 2002; Pérez and Romero, 1994), patterns which have been confirmed for meadows in the northern Adriatic (Bračun et al., 2020; Orlando-Bonaca et al., 2016). Epiphytes recruit heavily on older C. nodosa leaves throughout the year, which are then shed in the months prior to summer (Bračun et al., 2021).
Understanding mechanisms that facilitate the survival of any extant Cystoseira s.l. forest patches across the Mediterranean provides valuable information for formulating effective conservation practices. The identification of this population is important given its documented decline and current rarity along west Istrian coastline (Iveša et al., 2016) and similarly is under threat in wider Mediterranean (e.g. Thibaut et al., 2015; Orlando-Bonaca et al., 2021a; Tamburello et al., 2022). We hypothesize that the population’s unique morphology could provide insight into its persistence here. Falace and Bressan (2006) demonstrated that the morphology of G. barbata can be plastic based on environmental cues. Observationally, Šćuza’s G. barbata population shows obvious morphological distinction from other isolated patches found at other sites outside of the lagoon along the west Istrian coastline, possibly suggesting an evolutionary adaptation to the lagoonal environment (Iveša et al., 2022). This could be partially in response to competitive interactions and/or coexistence with C. nodosa, as we have not observed this interaction amongst remnant patches elsewhere. Within the lagoon itself there are two growth forms, one where a holdfast attaches to the rocky substrate, as is typical for this species and another “detached” form that is free floating where the holdfast is absent or non-functional (Iveša et al., 2022). Despite this free-living lifestyle however, the detached form is spatially restricted to the south-western part of the lagoon where it is primarily found trapped amongst seagrass fronds (Iveša et al., 2022). This observation suggests that there may be an interaction between the two dominant macrophytes, however here we focus on the attached form that is present both amongst seagrass and on exposed boulders for comparison.
In this study, the density and maximum thallus height of G. barbata was measured in-situ using quadrates placed within two distinct settlement habitats (raised submerged rocks outside C. nodosa and pebbles embedded within the C. nodosa seagrass meadow). Based on observation, we expect that G. barbata will grow to a larger maximum height when amongst seagrass compared to when exposed on larger rocky substrates. We aim (1) to test whether the presence of seagrass influences localized settlement and morphology of G. barbata and, (2) to document the presence of a unique marking (characterized by a damaged cauloid apex) that is observed among the G. barbata population largely within Šćuza but occasionally outside of the lagoon as well (author observations). Specifically, this study aims to understand the strength of a seaweed-seagrass interaction in influencing population dynamics of a threatened canopy-forming macroalga.
Materials and methods
Benthic cover
Šćuza Lagoon (44.820377°E, 13.889650°N), is a sandy coastal lagoon located in the south of the Istrian peninsula (Croatia; Figure 1). To characterize the benthic cover representative of the two settlement substrates, three 20 x 20cm quadrats were placed within areas consisting of either boulder substrate or pebble/unconsolidated substrate within C. nodosa meadows (hereafter these groups are referred to as ‘boulder’ and ‘seagrass’ respectively). At seven time points throughout the autumn 2022 – autumn 2023 (02/11/2022, 15/11/2022, 23/05/2023, 11/08/2023, 15/09/2023, 02/10/2023, 17/10/2023) quadrats were photographed. Benthic cover was quantified using a random point count method (n = 20 points) via coral net (coralnet.ucsd.edu), where organisms that fell under each point were assigned one of the following labels: Algae (including Turf, G. barbata, Valonia spp., calcified benthic- and macro-algae, Dictyota spp., Filamentous green algae and Rytiphlaea tinctoria (Clemente) C. Agardh, Ascidian, Seagrass (C. nodosa), Hard substrate (bare rock), Loose substrate (pebble and shell grit), Sand, Silt and Other (including tube worms, hydroids/bryozoans, benthic invertebrates and bivalves, anemones and instances where the point fell on the boarder of the quadrat). Benthic composition within boulder compared to seagrasses was tested via permutational multivariate analysis of variance using the adonis function in vegan R package (Oksanen et al., 2022). The analysis was run specifying 9999 permutations and substrate type (boulder or unconsolidated amongst seagrass) and date (n = 7) were fixed factors. The similarity matrix was generated using the Bray-Curtis method and homogeneity of dispersion was checked via the betadisper function in vegan. Data were visualized as a biplot generated using the princomp function in the R stats package (R Core Team, 2021).
Figure 1
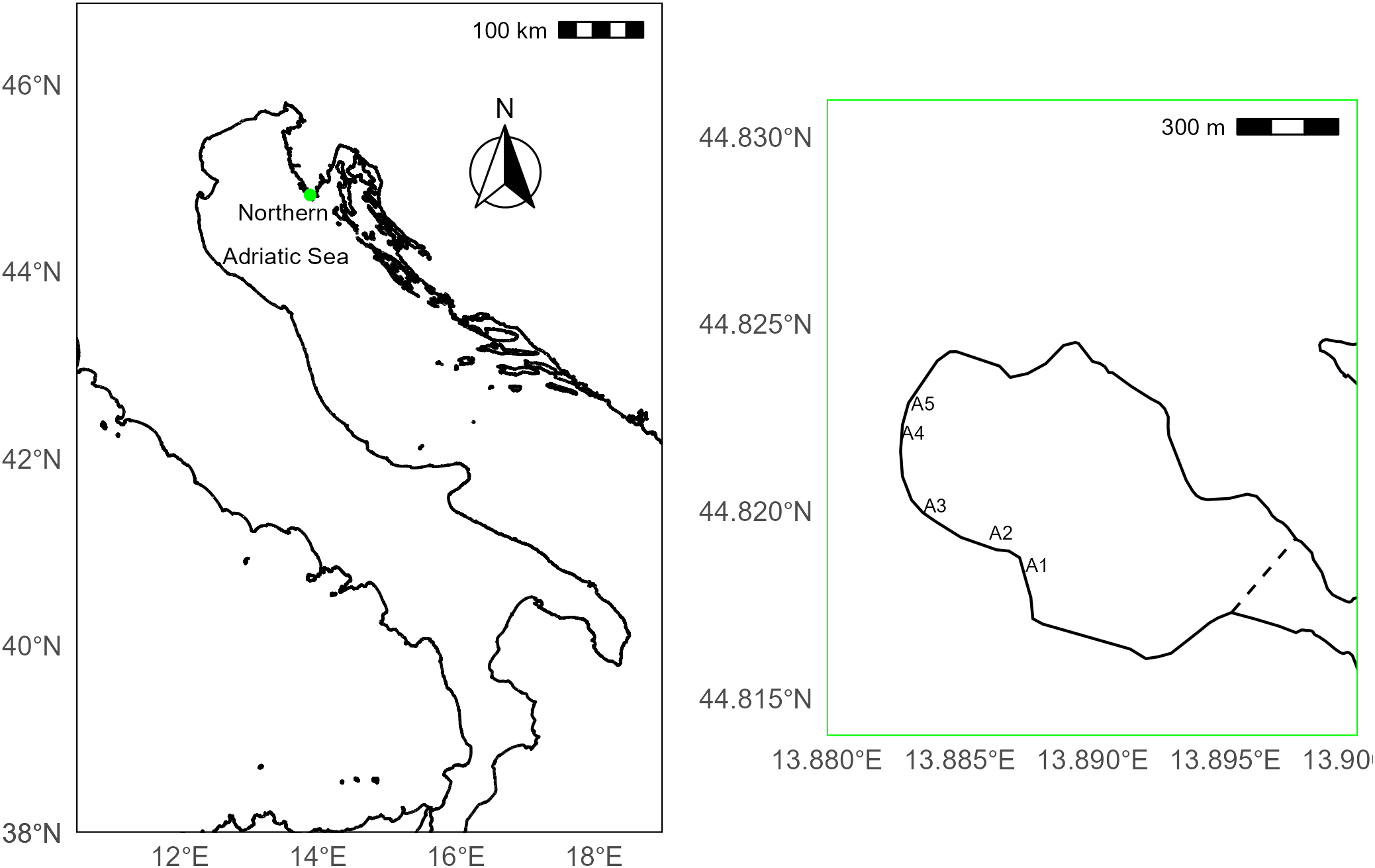
Map showing the northern Adriatic Sea (right plot), highlighting the location of the study site, Šćuza Lagoon (green dot) on the southern Istrian peninsula, Croatia. Within the Lagoon (left plot), the five sub-areas used for sampling are labelled (A1-5) and the location of the pedestrian footbridge that separates the lagoon from the sea is shown as a dashed line.
G. barbata population dynamics
The density and growth of G. barbata was monitored over the period encompassing autumn 2022 – autumn 2023. During the maximum growth period, the population was sampled four times on 04/10/2022, 18/10/2022, 02/11/2022 and 15/11/2022, before anomalously low autumn temperatures and consistently low winter temperatures prevented sampling. We sampled again in the spring of 2023 on 23/05/2023 before unsafe swimming conditions (poor water quality) in the lagoon prevented sampling in early summer. During the later summer during G. barbata’s minimum growth (dormancy) phase, we sampled the population twice on 11/08/2023 and 15/09/2023, with a final early autumn 2023 sampling period on 02/10/2023. During these sampling efforts, 50 x 50cm quadrats were randomly placed either amongst seagrass or on a boulder (n = 3 per group) and the number of G. barbata individuals was counted and the maximum height (base of the holdfast to the tallest point on the algae which could be a vegetative branch or cauloid dependent on season) was recorded following Iveša et al. (2022). To ensure good spatial representation of the G. barbata population and to avoid recounting individuals at each sampling point the lagoon was divided up into five areas that were > 10m apart (Figure 1). At each sampling effort, at least one area was selected, and a full set of quadrats (n = 6) was completed per area.
Analysis of cohorts over the sampling period was conducted as above with height as the response variable. Similarly, when investigating evidence of recruitment, data was filtered to include only individuals that were <3cm and the abundance of individuals that fit this criterion was the model response variable. We consider 3cm as an appropriate cut off to estimate recruitment based on in situ observation and published growth trajectories (Savonitto et al., 2021; Bianchelli et al., 2023; Lokovšek et al., 2023).
The overall density of G. barbata within the two substrate types (boulder; examples Figures 2B, D or seagrass; examples Figures 2A, C) was statistically compared using an ANOVA via the aov function in R stats package (R Core Team, 2021) where the number of individuals was the response variable and substrate type (boulder or unconsolidated amongst seagrass) was a fixed factor. Population density throughout the study period was analyzed using a linear mixed effects model via the lmer function in the R package lme4 (Bates et al., 2020). Number of individuals was the response variable, substrate type (exposed boulder or unconsolidated amongst seagrass) and time (n = 8) were fixed factors and area (1-5) was a random factor. For these analyses and those following, a p value of <0.05 was used to infer statistical significance which was calculated using the Anova function in the R package car (Fox et al., 2020). Where applicable and significant, interactions were observed between model factors, pairwise comparisons were calculated via the emmeans function in the emmeans package for R (Lenth et al., 2019). Model assumptions were visually checked via residuals scatter plots.
Figure 2
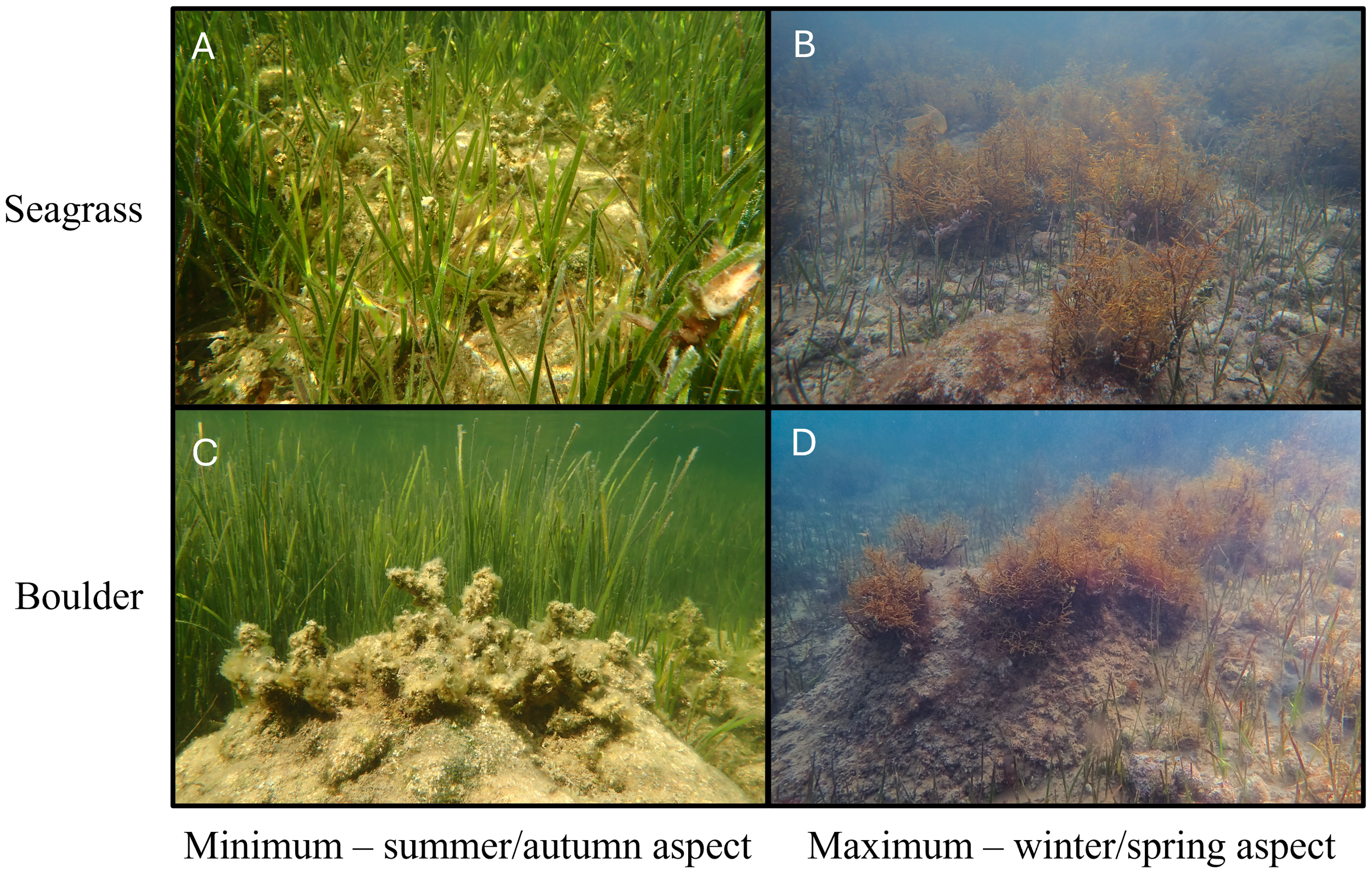
G. barbata growing within the two substrate types: amongst C. nodosa (seagrass) and on boulders within Šćuza Lagoon (per horizontal orientation) and during its minimum vs maximum growth periods in summer/autumn and winter/spring respectively (per vertical orientation). (A)G. barbata amongst seagrass during the minimum growth phase, (B)G. barbata growing amongst seagrass during the maximum growth period, (C)G. barbata on a boulder during the minimum growth phase and, (D)G. barbata on a boulder during the maximum growth phase.
During quadrat surveys it was noted where cauloid apices were absent/damaged for individual alga (Supplementary Figure S1). The total number of alga where apices were damaged on boulders or within seagrass at each sampling time was statistically tested via a linear mixed effects model with the frequency of observation (i.e. proportion of individuals where at least one apex was absent) being the response value, substrate type (exposed boulder or unconsolidated amongst seagrass) and time (n = 8) were fixed factors and area (1-5) was a random factor. All other parameters and procedures were run as described above.
Results
Benthic cover
Boulder substrates were consistently characterized by algae (usually low-lying turfs) and areas of unconsolidated substrate by a high cover of C. nodosa (close to 100% at times) with bare loose substrate and sand being more represented during lower growth periods as expected (Supplementary Figure S2). There was a clear distinction in the benthic cover between the substrate types (exposed boulder and unconsolidated substrate amongst seagrass; Pseudo-F1,75 = 5.005, p = 0.009). Benthic cover also varied based on sampling time (Pseudo-F1, 75 = 9.739, p < 0.001) as expected given the seasonality in growth of C. nodosa, but there was no interaction between substrate type and sampling time (Pseudo-F1, 75 = -0.512, p = 0.971).
G. barbata population dynamics
Overall, the density of G. barbata individuals per 50 x 50cm quadrat was similar for both boulder and unconsolidated substrates, showing a mean density per 0.25m2 of 7(±1) and 6(±1) individuals respectively (Figure 3; F1,118 = 1.55, p = 0.216). When testing for the effect of sampling time however, density was influenced by the interaction between recruitment substrate and sampling time (Figure 4; Substrate type by time interaction: χ2=32.93, p<0.001). During September (summer) sampling there were more G. barbata individuals counted on boulders compared to among seagrass (post hoc pairwise test: p=0.005). The density of G. barbata on boulders during the September sampling period was also higher than the density on boulders in early October 2022 (p=0.031) and higher than that amongst seagrass in May (p<0.001) and August (p=0.035).
Figure 3
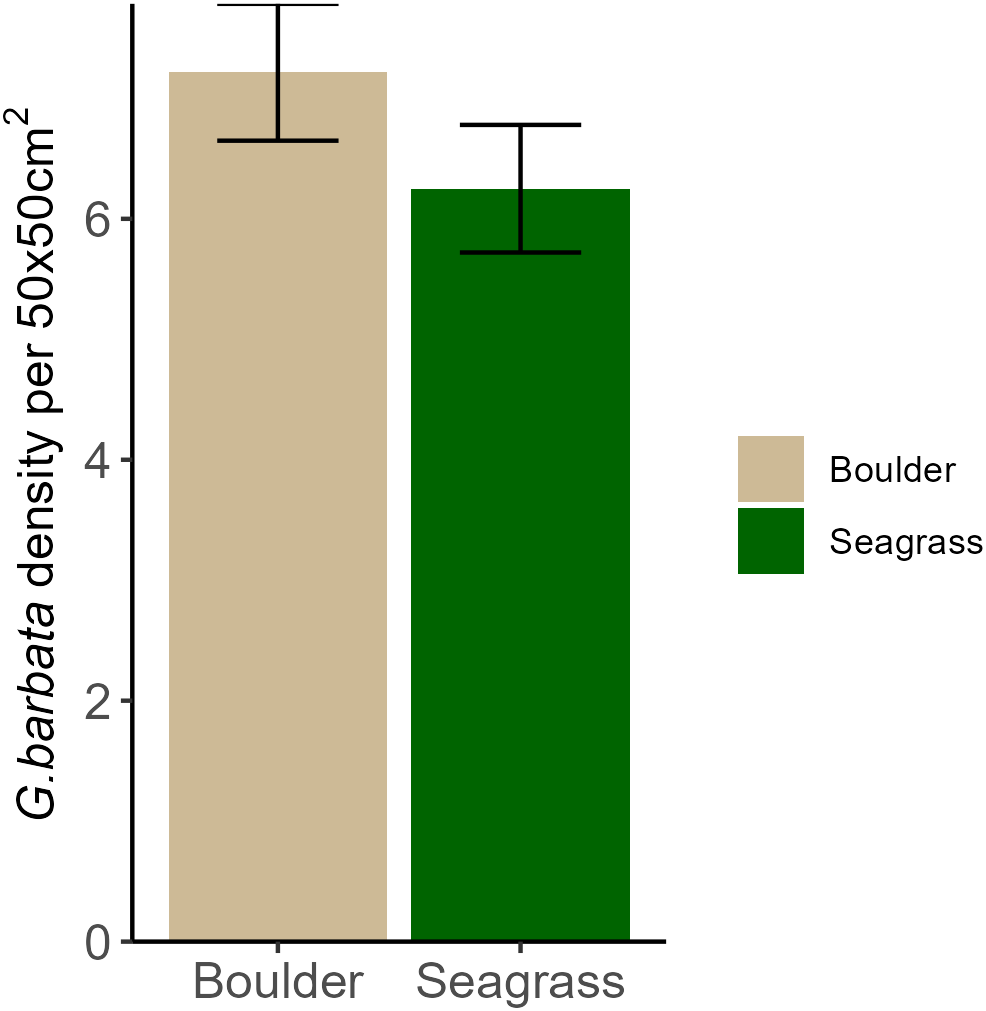
Mean (± se) number of G. barbata individuals per 50 x 50cm quadrat counted across all lagoon areas (n = 5) and across sampling times (n = 8) from autumn 2022 – autumn 2023. Total number of observations = 60 per substrate type; boulder (brown) and unconsolidated amongst seagrass (green).
Figure 4
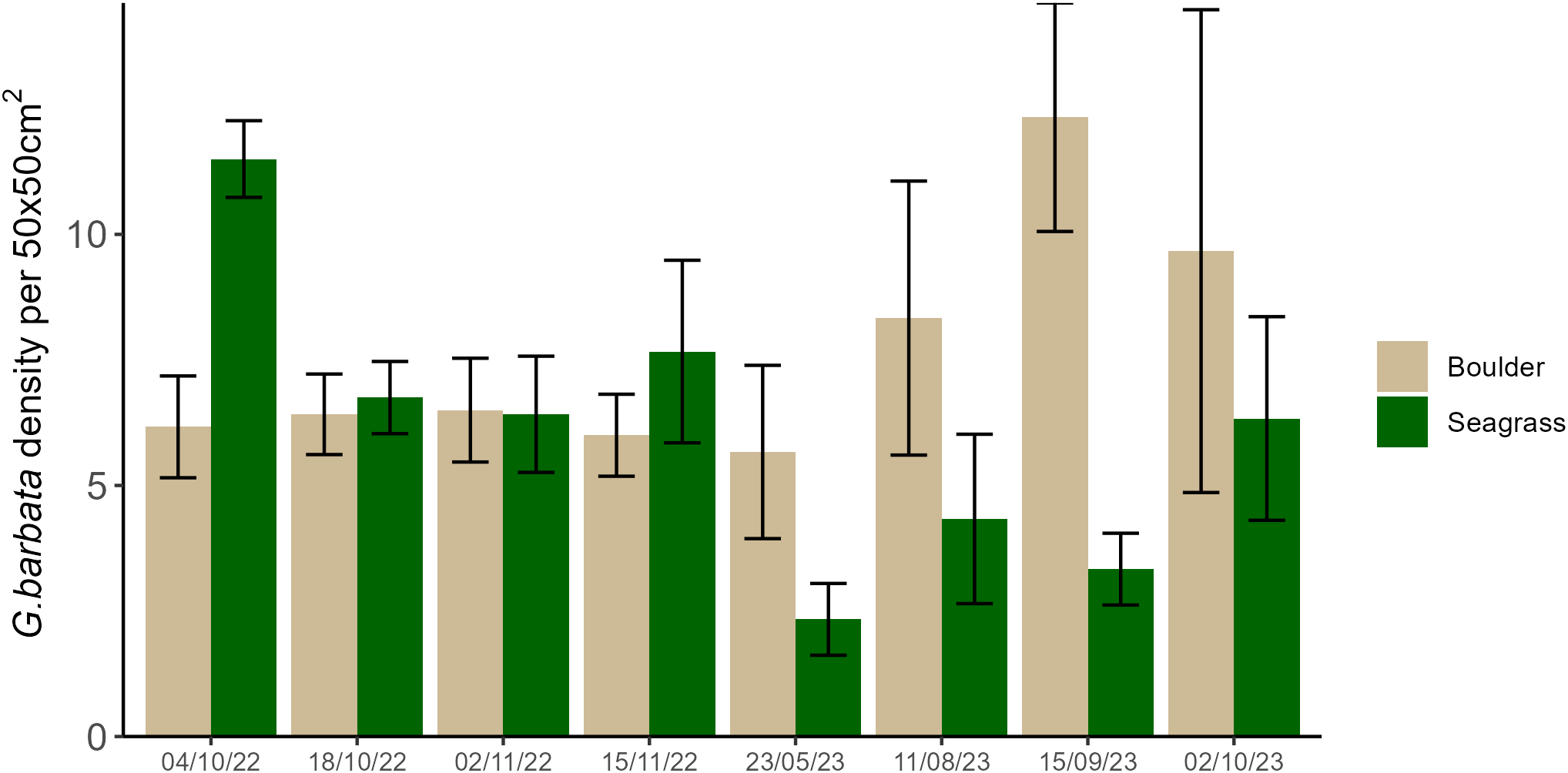
The number of G. barbata individuals per 50 x 50cm quadrat counted for each sampling time (n = 8) on boulders (brown) and amongst seagrass (green). At each sampling time there was a minimum of one area sampled and a maximum of four. Within each area there were three boulder, and three seagrass quadrats sampled. The number of areas sampled for each time point was as follows: 4/10/22 = 2, 18/10/22 = 4, 2/11/22 = 4, 15/11/22 = 3, 23/05/23 = 2, 11/08/23 = 2, 15/09/23 = 2, 2/10/23 = 1. A total of 120 quadrats was sampled over the whole period, 60 on boulders and 60 amongst seagrass.
G. barbata measured in situ ranged in height from a minimum of 0.8cm measured on boulders to 51.5cm measured among seagrass (Figure 5) with a median height of 14.0cm measured across both substrate types. On average, G. barbata measured amongst seagrass grew to almost double the maximum height of those measured on boulders (χ2 = 203.94, p < 0.001), with an observed mean of 21.8 (±0.6) cm and 12.0 (±0.4) cm respectively. As expected by the known lifecycle of G. barbata, height was influenced by sampling time (χ2 = 178.60, p < 0.001) where maximum growth was observed during the autumn/winter months and declined towards spring and summer (Figure 5), independent of substrate type (Substrate type x Sampling date interaction: χ2=13.53, p=0.061).
Figure 5
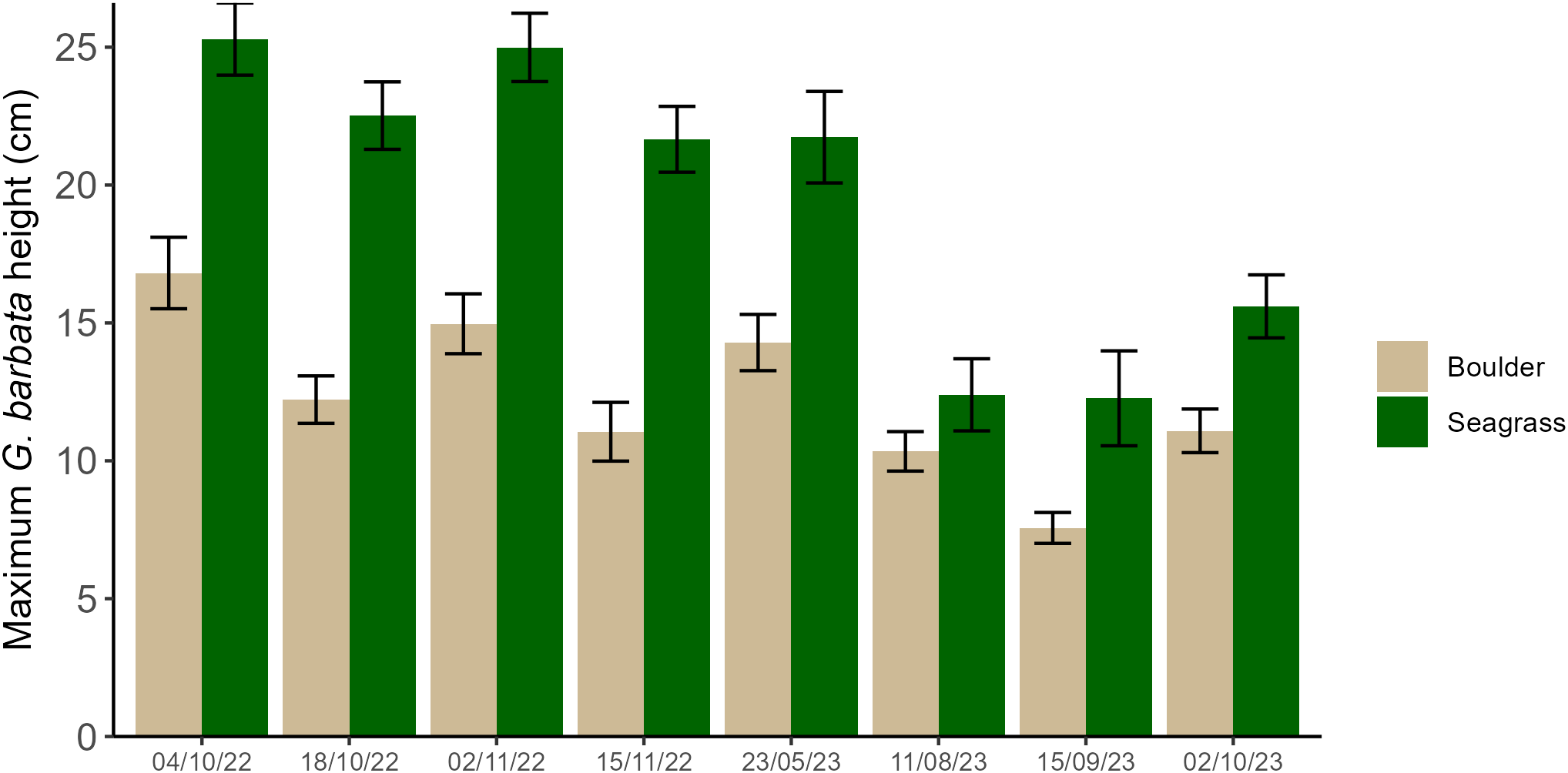
Maximum height measured for G. barbata individuals counted in situ by sampling time (n = 8) on boulders (brown) and amongst seagrass (green). A total of 808 individuals were measured. At each sampling time there was a minimum of one area sampled and a maximum of four. Within each area there were three boulder, and three seagrass quadrats sampled. The number of areas sampled for each time point were as follows: 4/10/22 = 2, 18/10/22 = 4, 2/11/22 = 4, 15/11/22 = 3, 23/05/23 = 2, 11/08/23 = 2, 15/09/23 = 2, 2/10/23 = 1.
During the peak cumulative growth period (early November) there was a clear distinction in the size class distribution of G. barbata measured on boulders versus amongst seagrass (Supplementary Figure S3, p < 0.001). 25% of individuals measured amongst seagrass were > 32cm, compared to 8% for those measured on boulders being within this size class and this coincided with the period of low seagrass cover (Supplementary Figure S2). During the lowest cumulative growth period (September 2023), there was a clear shift in the size class distribution, where 24% of individuals on boulders and 5% of individuals measured amongst seagrass were <4cm, coinciding with high seagrass cover (Supplementary Figure S3).
On boulders there was a higher number of smaller individuals (<3cm) compared to amongst seagrass (χ2 = 51.58, p < 0.001) and the spring/summer time points showed a higher number of smaller individuals compared to the autumn/winter time points (Supplementary Figure S3: χ2 = 10.25, p = 0.001). This difference was particularly evident during September sampling (Supplementary Figure S3; Supplementary Table S6).
Independent of sampling time, a higher percentage of G. barbata observed on boulders (overall mean of 49.9 ±4.4%) consistently showed a damaged cauloid apex compared to those amongst seagrass (13.7 ±2.4%; Figure 6; Substrate type χ2 = 52.87, p < 0.001; substrate type by sampling time interaction χ2 = 2.20, p = 0.139).
Figure 6
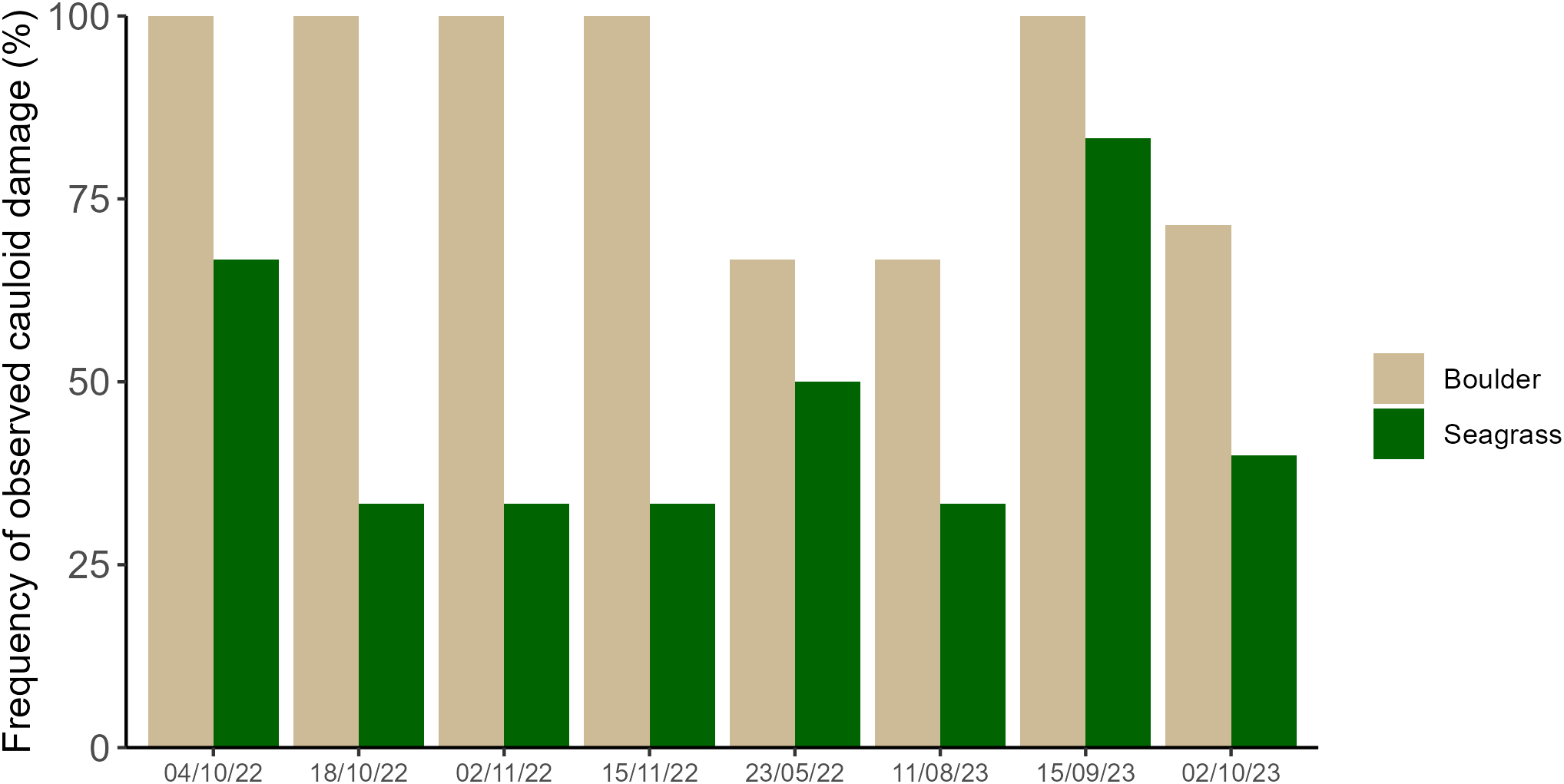
The percentage of G. barbata individuals observed on boulders (brown, n = 433 observations) and amongst seagrass (green, n = 375 observations) showing a damaged cauloid apex. At each sampling time there was a minimum of one area sampled and a maximum of four. Within each area there were three boulder, and three seagrass quadrats sampled. The number of areas sampled for each time point were as follows: 4/10/22 = 2, 18/10/22 = 4, 2/11/22 = 4, 15/11/22 = 3, 23/05/23 = 2, 11/08/23 = 2, 15/09/23 = 2, 2/10/23 = 1.
Discussion
The population dynamics of the ecologically important macroalgal species G. barbata are influenced by the presence or absence of the seagrass C. nodosa in a coastal lagoon of the northern Adriatic Sea. Šćuza Lagoon hosts what is potentially one of the last significant populations of G. barbata along the western Istrian coastline (Croatia). Understanding the ecological context in which it persists here is valuable for informing targeted conservation and restoration efforts in other locations where it is declining or has been lost. G. barbata reached a greater maximum height during its peak growth phase in the autumn/winter months of 2022/23 when living amongst C. nodosa compared to when it had settled on exposed boulders. This height advantage was maintained over the spring/summer months in 2023 when only perennial parts of the algae were present, suggesting an adaptation of the entire growth form that is not limited to the seasonal generation of adventitious branch material. We found evidence that recruitment of G. barbata was influenced by settlement amongst seagrass compared to on boulders, observing more recruitment to the latter. However, further experimental work would be required to understand the overall similarity in density observed between the two settlement strategies beyond the recruitment stage. Our results support previous work describing the high plasticity in growth forms of G. barbata in reponse to localized environmental conditions. In Šćuza Lagoon, an alternation of peak growth periods likely contributes to the persistence and coexistence of significant populations of ecologically valuable marine macrophytes G. barbata and C. nodosa.
Recruitment of G. barbata is highly influenced by benthic topography (Devescovi, 2015), and within Šćuza Lagoon, it is also possible that boulder substrate provides greater recruitment opportunity comparted to areas dominated by unconsolidated substrate interspersed beneath the seagrass canopy. Amongst seagrass, G. barbata settles on small stones and pebbles and other unconsolidated hard substrate that form a secondary benthic substrate layer. A reduced surface area of appropriate recruitment substrate may explain a reduction in the number of small individuals or recruits observed here for the 2022/23 season. Alternatively, low recruitment amongst seagrass could indicate competitive exclusion during the period of peak cover for C. nodosa where boulder substrates may provide refuge from such competition. However, the difference in recruitment was not maintained when comparing the overall density, implying that opportunistic recruitment amongst seagrass, while less common, is not a barrier to survival. Amongst G. barbata that develop amongst seagrass, it is possible that taller cauloids and heightened branch material observed here is driven by light limitation at the recruit stage enforced by the C. nodosa canopy, but this would require further investigation. Nevertheless, such a fine-scale difference in form is consistent with research conducted elsewhere that shows a high degree of morphological variation for G. barbata across relatively small geographic ranges (Falace and Bressan, 2006; Orlando-Bonaca et al., 2022). Additionally, in Šćuza Lagoon, amongst the seagrass there are “detached” individuals that are not fixed to any substrate (author observations). This ecotype was described by Iveša et al. (2022) and is spatially restricted to the south-west of the lagoon but is often found trapped amongst the seagrass meadow or amongst R. tinctoria complexes that are also commonly observed amongst seagrasses. This high adaptability in form, coupled with the demonstrated physiological adaptation to extreme thermal conditions for this population (Iveša et al., 2022; Bilajac et al., 2024), suggests a resilience worth investigating in the context of its long-term survival and its potential as a donor population for fucalean restoration along the broader coastline.
Damage to perennial cauloid apices is an observed feature among Cystoseira s.l. species along the western Istrian coast which was also observed for G. barbata within Šćuza Lagoon (author observations). This feature was consistently recorded more frequently amongst individuals that had settled on boulders compared to those settled amongst seagrass, even in the summer months when cauloids host a high epiphyte biomass that conceal some such markings. It is possible that this feature is a sign of herbivory, however outside of the lagoon, where urchins are present, cauloid damage of this nature is also frequently observed. The herbivorous fish Sarpa salpa is present within and outside of the lagoon and is a voracious grazer of Cystoseira s.l (Vergés et al., 2009; Gianni et al., 2017; Orlando-Bonaca et al., 2022, 2021). However, S. salpa is not strictly herbivorous throughout its lifecycle (Dobroslavić et al., 2012; Havelange et al., 2008) and can also target C. nodosa and its epiphyte load for feeding (Marco-Méndez et al., 2017) and further investigation would be needed to better understand this interaction. It is also possible that herbivorous invertebrates could also graze on G. barbata in Šćuza Lagoon, and this is an important consideration given that early life stage Cystoseira s.l. species are susceptible to invertebrate grazing (Monserrat et al., 2023). However, this would not necessarily explain damaged cauloid apices observed for adult individuals. The ecological significance of damaged cauloid apices, the ecological relevance of seagrasses providing protection from it here and its prevalence amongst Cystoseira s.l populations elsewhere warrants further investigation.
Understanding the mechanisms that allow the survival of remnant marine forests is of increasing conservation importance because harnessing any observable positive interactions within extant forests may benefit conservation efforts. In Šćuza Lagoon it is likely crucial that there are very few sea urchins observed (Iveša et al., 2022), contributing to the observed abundance of both G. barbata and C. nodosa as both species are susceptible to urchin overgrazing (Agnetta et al., 2015; Cvitković et al., 2024; Fernandez et al., 2012; Giakoumi et al., 2012; Iveša et al., 2016; Nikolaou et al., 2023; Thibaut et al., 2005). In the context of at risk marine algal forests, there is increasing evidence for the role of habitat refugia (such as coastal lagoons, rockpools and microstructures) in providing refuge from herbivory and thus supporting the survival of isolated patches of canopy forming seaweeds (Battelli and Catra, 2021; Zarco-Perello et al., 2021; Monserrat et al., 2023) and long-term exclusion of over-abundant grazers is currently a major roadblock to the success of marine forest restoration in the Mediterranean (Verdura et al., 2023) and worldwide (Eger et al., 2022). Furthermore, water quality and excessive nutrient input has been shown to cause large-scale negative impacts on Cystoseira s.l. populations in the past (Iveša et al., 2016), and for Šćuza Lagoon the impact of nutrient input and fluctuations in water chemistry caused by nearby terrestrial activity is unknown yet makes the survival of the forest here more notable. It is important to study the ecology of persistent populations of at-risk species to understand any adaptive mechanisms that may be important considerations for conservation efforts. In the case of Šćuza Lagoon, here we show that the adaptability of G. barbata, even at the micro-environment level may contribute to its survival in a unique lagoonal environment.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SS: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Methodology, Writing – review & editing. EG: Conceptualization, Data curation, Methodology, Writing – review & editing. MN: Methodology, Supervision, Writing – review & editing. LI: Conceptualization, Data curation, Funding acquisition, Methodology, Supervision, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was funded by Croatian Science Foundation project: The response of habitat forming brown macroalgae of the genus Cystoseira on local and global stressors (grant no. IP-2019-04-6984) and by the European Union through the project BRIGANTINE: Chemico-physical and multispectral data fusion for Adriatic Sea monitoring by autonomous vessel (INTERREG Italy-Croatia Program 2021-2027, grant no. ITHR0200237).
Acknowledgments
The authors thank three reviewers for their valuable feedback on an earlier version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1493581/full#supplementary-material
References
1
Agnetta D. Badalamenti F. Ceccherelli G. Di Trapani F. Bonaviri C. Gianguzza P. (2015). Role of two co-occurring Mediterranean sea urchins in the formation of barren from Cystoseira canopy. Estuarine Coastal Shelf Sci.152, 73–77. doi: 10.1016/j.ecss.2014.11.023
2
Airoldi L. Beck M. W. (2007). Loss, status and trends for coastal marine habitats of Europe. In Oceanography Marine Biology: an Annu. Rev.GibsonR. N.AtkinsonR. J. A.GordonJ. D. M. (Boca Raton, FL: CRC Press), 45, 345–405. doi: 10.1201/9781420050943
3
Angelini C. Altieri A. H. Silliman B. R. Bertness M. D. (2011). Interactions among Foundation species and their consequences for community organization, biodiversity and conservation. BioScience61, 782–789. doi: 10.1525/bio.2011.61.10.8
4
Ballesteros E. Torras X. Pinedo S. García M. Mangialajo L. de Torres M. (2007). A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Marine Pollution Bull.55, 172–180. doi: 10.1016/j.marpolbul.2006.08.038
5
Bates D. M. Maechler M. Bolker B. Walker S. Christensen R. H. B. Signmann H. et al . (2020). Package ‘lme4’: Linear mixed-effects models using ‘Eigen’ and S4. doi: 10.32614/CRAN.package.lme4
6
Battelli C. Catra M. (2021). First report of Cystoseira aurantia (Sargassaceae, Fucophyceae) from the Lagoon of Strunjan (Gulf of Trieste, Northern Adriatic). Annales Ser. Hist. Naturalis31, 139–146. doi: 10.19233/ASHN.2021.16
7
Battelli C. Catra M. (2023). Morphological and reproductive phenology of Cystoseira foeniculacea f. tenuiramosa (Phaeophyceae, Fucales) from the lagoon of Strunjan (Gulf of Trieste, northern Adriatic). Acta Marina64, 33–44. doi: 10.32582/aa.64.1.9
8
Bianchelli S. Martini F. Lo Martire M. Danovaro R. Corinaldesi C. (2023b). Combining passive and active restoration to rehabilitate a historically polluted marine site. Fronteirs Marine Science.10. doi: 10.3389/fmars.2023.1213118
9
Bianchi C. N. Morri C. (2000). Marine biodiversity of the mediterranean sea: situation, problem and prospects for future research. Marine Pollution Bulletin.40, 367–376. doi: 10.1016/S0025-326X(00)00027-8
10
Bilajac A. Gljušćić E. Smith S. M. Najdek M. Iveša L. (2024). Effects of extreme temperatures and recovery potential of Gongolaria barbata from a coastal lagoon in the northern Adriatic Sea: An ex situ approach. Ann. Botany.134, 415–426. doi: 10.1093/aob/mcae038
11
Bračun S. Wagner M. Koblmüller S. (2021). Spatio-temporal occurrence patterns of epibiota along the leaves of the seagrass Cymodocea nodosa in the Northern Adriatic Sea. Marine Biol. Res.17, 592–602. doi: 10.1080/17451000.2021.2015389
12
Bračun S. Wagner M. Sefc K. M. Koblmüller S. (2020). Seasonal growth patterns of Cymodocea nodosa and diversity of its epibiota in the northern Adriatic Sea. Annales: Ser. Hist. Naturalis301, 69–84. doi: 10.19233/ashn.2020.10
13
Burgos E. Montefalcone M. Ferrari M. Paoli C. Vassallo P. Morri C. et al . (2017). Ecosystem functions and economic wealth: Trajectories of change in seagrass meadows. J. Cleaner Production168, 1108–1119. doi: 10.1016/j.jclepro.2017.09.046
14
Chefaoui R. M. Assis J. Duarte C. M. Serrão E. A. (2016). Large-scale prediction of seagrass distribution integrating landscape metrics and environmental factors: The case of Cymodocea nodosa (Mediterranean-Atlantic). Estuaries Coasts39, 123–137. doi: 10.1007/s12237-015-9966-y
15
Chefaoui R. M. Duarte C. M. Serrão E. A. (2018). Dramatic loss of seagrass habitat under projected climate change in the Mediterranean Sea. Global Change Biol.24, 4919–4928. doi: 10.1111/gcb.14401
16
Cheminée A. Sala E. Pastor J. Bodilis P. Thirilet P. Mangialajo L. et al . (2013). Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. J. Exp. Marine Biol. Ecology.442, 70–79. doi: 10.1016/j.jembe.2013.02.003
17
Council of Europe (2000). Convention of the Conservation of European Wildlife and Natural Habitats, Bern 19.IX.1979, Annex I – Strictly protected flora species, European Treaty Series No. 104. CETS 104 - Annex I - Convention on the Conservation of European Wildlife and Natural Habitats. Bern, Switzerland: Council of Europe.
18
Cunha A. H. Duarte C. M. (2007). Biomass and leaf dynamics of Cymodocea nodosa in the Ria Formosa lagoon, South Portugal. Botanica Marina50, 1–7. doi: 10.1515/bot.2007.001
19
Cvitković I. Despalatović M. Žuljević A. Vučić I. Lučić P. Nejašmić J. (2024). Distribution of sea urchin barrens in shallow algal communities along the eastern Adriatic coast. Mediterranean Marine Science.25, 213–219. doi: 10.12681/mms.33553
20
Dayton P. K. (1972). Towards an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In Proc. colloquium conservations problems Antarctica. ed. ParkerB. C. (Blacksburg, Virginia), 81–96.
21
Devescovi M. (2015). Effects of bottom topography and anthropogenic pressure on northern Adriatic Cystoseira spp. (Phaeophyceae, Fucales). Aquat. Bot.121, 26–32. doi: 10.1016/j.aquabot.2014.10.009
22
Dobroslavić T. Zlatović A. Bartulović V. Lučić D. Glamuzina B. (2012). Diet overlap of the juvenile salema (Sarpa salpa), bouge (Boops boops) and common two-banded sea bream (Diplodus vulgaris) in the south-eastern Adriatic. J. Appl. Icthyology29, 181–185. doi: 10.1111/j.1439-0426.2012.02046.x
23
Duarte C. M. Borum J. Short F. T. Walker D. I. (2008). Seagrass ecosystems: their global status and prospects. Aquat. Ecosystems: Trends Global Prospects, ed. PoluninN. V. C. (New York: Cambridge University Press) 281–294. doi: 10.1017/CBO9780511751790.025
24
Dunic J. C. Brown C. J. Connolly R. M. Turschwell M. P. Côte I. M. (2021). Long-term declines and recovery of meadow area across the world’s seagrass bioregions. Global Change Biol.27, 4096–4109. doi: 10.1111/gcb.15684
25
Eger A. M. Marzinelli E. M. Christie H. Fagerli C. W. Fujita D. Gonzalez A. P. et al . (2022). Global kelp forest restoration: past lessons, present status, and future directions. Biol. Rev.97, 1449–1475. doi: 10.1111/brv.12850
26
Ercegović A. (1952). Jadranske cistozire. Njihova morfologija, ekologija i razvitak. Sur les Cystoseira adriatiques. Leur morphologie, ecologie et evolution. Fauna Flora Adriatica IOR Split212, 212.
27
Falace A. Bressan G. (2006). Seasonal variations of Cystoseira barbata (Stackhouse) C. Agardh frond architecture. Hydrobiologia555, 193–206. doi: 10.1007/s10750-005-1116-2
28
Fernandez C. Ferrat L. Pergent G. Pasqualini V. (2012). Sea uchin-seagrass interactions: trophic links in a benthic ecosystem from a coastal lagoon. Hydrobiologia699, 21–33. doi: 10.1007/s10750-012-1151-8
29
Fox J. Weisberg S. Price B. Adler D. Bates D. Baud-Bovy G. et al . (2020). Package “Car”: Companion to applied regression. doi: 10.32614/CRAN.package.car
30
Giakoumi S. Cebrian E. Kokkoris G. D. Ballesteros E. Sala E. (2012). Relationships between fish, sea urchins and macroalgae: The structure of shallow rocky sublittoral communities in the Cyclades, Eastern Mediterranean. Estuarine Coastal Shelf Science.109, 1–10. doi: 10.1016/j.ecss.2011.06.004
31
Gianni F. Bartolini F. Pey A. Laurent M. Martins G. M. Airoldi L. et al . (2017). Threats to large brown algal forests in temperate seas: the overlooked role of native herbivorous fish. Sci. Rep.7, 6012. doi: 10.1038/s41598-017-06394-7
32
Havelange S. Lepont G. Dauby P. Bouquegneau J. M. (2008). Feeding of the sparid fish Sarpa salpa in a seagrass ecosystem: Diet and carbon flux. Marine Ecol.18, 289–297. doi: 10.1111/j.1439-0485.1997.tb00443.x
33
Hemminga M. A. Duarte C. M. (2000). Seagrass Ecology (United Kingdom: Cambridge University Press).
34
Iveša L. Bilajac A. Gljušćić E. Najdek M. (2022). Gongolaria barbata forest in the shallow lagoon on the southern Istrian Coast (norther Adriatic Sea). Botanica Marina65, 255–268. doi: 10.1515/bot-2022-0021
35
Iveša L. Devescovi M. (2014). Distribution and composition of Cystoseira strands along the west Istrian coast (northern Adriatic, Croatia) and comparison with historical data. 5th Mediterranean Symposium Marine Vegetation, ed. LangerH.BouafifC.OuerghiA., Portorož, Slovenia2014, 102–107.
36
Iveša L. Djakovac T. Devescovi M. (2016). Long-term fluctuations in Cystoseira populations along the west Istrian Coast (Croatia) related to eutrophication patterns in the northern Adriatic Sea. Marine Pollution Bull.106, 162–173. doi: 10.1016/j.marpolbul.2016.03.010
37
Lenth R. V. Buerkner P. Herve M. Love J. Miguez F. Riebl H. et al . (2019). Package “emmeans”: Estimated Marginal Means, aka Least-Squares Means. doi: 10.32614/CRAN.package.emmeans
38
Lokovšek A. Pitacco V. Trkov D. Lojze Zamuda L. Falace A. Orlando-Bonaca M. (2023). Keep it simple: improving the ex situ culture of cystoseira s.l. to restore macroalgal forests. Plants12, 2615. doi: 10.3390/plants12142615
39
Mancuso F. P. Strain E. M. A. Piccioni E. De Clerck O. Sarà G. Airoldi L. (2018). Status of vulnerable Cystoseira populations along the Italian infralittoral fringe, and relationships with environmental and anthropogenic variables. Marine Pollution Bull.129, 762–771. doi: 10.1016/j.marpolbul.2017.10.068
40
Máñez-Crespo J. Tuya F. Fernández-Torquemada Y. Royo L. del Pilar-Ruso Y. Espino F. et al . (2020). Seagrass Cymodocea nodosa across biogeographical regions and times: Differences in abundance, meadow structure and sexual reproduction. Marine Environ. Res.162, 105159. doi: 10.1016/j.marenvres.2020.105159
41
Mangialajo L. Chiantore M. Cattaneo-Vietti R. (2008). Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Marine Ecol. Prog. Ser.358, 63–74. doi: 10.3354/meps07400
42
Marco-Méndez C. Ferrero-Vicente L. M. Prada P. Sánchez-Lizaso J. L. (2017). Epiphytes and nutrient contents influence Sarpa salpa herbivory on Caulerpa spp va. Seagrass species in Mediterranean meadows. Estuarine Coastal Shelf Sci.184, 54–66. doi: 10.1016/j.ecss.2016.11.005
43
Monserrat M. Verdura J. Comeau S. Cottalorda J. Priouzeau F. Romero G. et al . (2023). The role of grazers in early-life stages of Cystoseira sensu lato can be crucial for the restoration of marine forests. Front. Marine Sci.10. doi: 10.3389/fmars.2023.1176780
44
Nikolaou A. Tsirinatanis K. Rilov G. Katsanevakis S. (2023). Invasive fish and sea urchins drive the status of canopy forming macroalgae in the eastern Mediterranean. Biology12, 763. doi: 10.3390/biology12060763
45
Oksanen J. Simpson G. L. Blanchet F. G. Kindt R. Legendre P. Minchin P. R. et al . (2022). Package “Vegan”: Community Ecology Package. doi: 10.32614/CRAN.package.vegan
46
Olesen B. Enríquez S. Duarte C. M. Sand-Jensen K. (2002). Depth-acclimation of photosynthesis, morphology and demography of Posidonia oceanica and Cymodocea nodosa in the Spanish Mediterranean Sea. Marine Ecol. Prog. Ser.236, 89–97. doi: 10.3354/meps236089
47
Olsen Y. S. Sánchez-Camacho M. Marbà N. Duarte C. M. (2012). Mediterranean seagrass growth and demography responses to experimental warming. Estuaries Coasts35, 1205–1213. doi: 10.1007/s12237-012-9521-z
48
Orlando-Bonaca M. Lipej L. Francé J. (2016). The most suitable time and depth to sample Cymodocea nodosa (Ucria) Ascherson meadows in the shallow coastal area. Experiences from the northern Adriatic Sea. Acta Adriatica57, 251–262.
49
Orlando-Bonaca M. Pitacco V. Lipej L. (2021a). Loss of canopy-forming algal richness and coverage in the northern Adriatic Sea. Ecol. Indic.125, 107501. doi: 10.1016/j.ecolind.2021.107501
50
Orlando-Bonaca M. Pitacco V. Slavinec P. Šiško M. Makovec T. Falace A. (2021b). First restoration experiment for Gongolaria barbata in Slovenian coastal waters. What can go wrong? Plants10, 239. doi: 10.3390/plants10020239
51
Orlando-Bonaca M. Savonitto G. Asnaghi V. Trkov D. Pitacco V. Šiško M. et al . (2022). Where and how – new insight for brown algal forest restoration in the Adriatic. Front. Marine Sci. 9, 988584. doi: 10.3389/fmars.2022.988584
52
Orth R. J. Carruthers T. J. B. Dennison W. C. Duarte C. M. Fourqurean J. W. Heck K. L. et al . (2006). A global crisis for seagrass ecosystems. Bioscience56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
53
Pérez M. Romero J. (1994). Growth, dynamics, production, and nutrient status of the seagrass Cymodocea nodosa in a Mediterranean semi-estuarine environment. Marine Ecol.15, 51–64. doi: 10.1111/j.1439-0485.1994.tb00041.x
54
Piazzi L. Bonaviri C. Castelli A. Ceccherelli G. Costa G. Curini-Galetti M. et al . (2018). Biodiveristy in canopy-forming algae: Structure and spatial variability of the Mediterranean Cystoseira assemblages. Estuarine Coastal Shelf Sci.207, 132–141. doi: 10.1016/j.ecss.2018.04.001
55
Pinna S. Piazzi L. Ceccherelli G. Castelli A. Costa G. Curini-Galletti M. et al . (2020). Macroalgal forest vs sea urchin barren: Patterns of macro-zoobenthic diversity in a large-scale Mediterranean study. Marine Environ. Res.159, 104955. doi: 10.1016/j.marenvres.2020.104955
56
R Core Team (2021). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
57
Richard M. Quijón P. A. (2023). Seagrass-macroalgal interactions in a changing ocean. Front. Climate5. doi: 10.3389/fclim.2023.1283305
58
Rindi F. Vergés A. Zuchegna I. Bianchelli S. de Caralt S. Galobart C. et al . (2023). Strandardised protocol for reproductive phenology monitoring of fucalean algae of the genus Cystoseira s.l. with potential for restoration. Fronteirs Marine Science.10. doi: 10.3389/fmars.2023.1250642
59
Savonitto G. de la Fuente G. Tordoni E. Ciriaco S. Srijemsi M. Bacaro G. et al . (2021). Addressing reproductive stochasticity and grazing impacts in the restoration of a canopy-forming brown alga by implementing mitigation solutions. Aquat. Conserv.31, 1611–1623. doi: 10.1002/aqc.3555
60
Stachowicz J. (2001). Mutualism, facilitation, and the structure of ecological communities: positive interactions play a critical, but underappreciated, role in ecological communities by reducing physical or biotic stresses in existing habitats and by creating new habitats on which many species depend. BioScience51, 235–246. doi: 10.1641/0006-3568(2001)051[0235:MFATSO]2.0.CO;2
61
Tamburello L. Chiarore A. Fabbrizzi E. Colletti A. Franzitta G. Grech D. et al . (2022). Can we preserve and restore overlooked macroalgal forests. Sci. Total Environ.806, 150855. doi: 10.1016/j.scitotenv.2021.150855
62
Teagle H. Hawkins S. J. Moore P. J. Smale D. A. (2017). The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Marine Biol. Ecol.491, 81–98. doi: 10.1016/j.jembe.2017.01.017
63
Thibaut T. Blanfune A. Boudouresque C.-F. Verlaque M. (2015). Decline and local extinction of Fucales in the French Riviera: the harbinger of future extinctions? Mediterranean Marine Science.16, 206–224. doi: 10.12681/mms.1032
64
Thibaut T. Pinedo S. Torras X. Ballesteros E. (2005). Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, north-western Mediterranean). Marine Pollution Bull.50, 1472–1489. doi: 10.2016/j.marpolbul.2005.06.014
65
United Nations Environment Programme/Mediterranean Action Plan (UNEP/MAP) (2013). Protocol Concerning Specially Protected Areas and Biological Diversity in the Mediterranean. List of Endangered Species (Athens, Greece: UNEP/MAP. Specially Protected Areas Protocol / SPA and Biodiversity Protocol | UNEPMAP).
66
Verdura J. Rehues L. Mangialajo L. Fraschetti S. Belattmania Z. Bianchelli S. et al . (2023). Distribution, health and threats to Mediterranean macroalgal forests: defining the baselines for their conservation and restoration. Front. Marine Sci.10. doi: 10.3389/fmars.2023.1258842
67
Vergés A. Alcoverro T. Ballesteros E. (2009). Role of fish herbivory in structuring the vertical distribution of canopy algae Cystoseira spp. in the Mediterranean Sea. Marine Ecol. Prog. Ser.375, 1–11. doi: 10.3354/meps07778
68
Waycott M. Duarte C. M. Carruthers T. J. B. Orth R. J. Dennison W. C. Olyarnik S. et al . (2009). Accelerating loss of seagrass across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci.106, 12377–12381. doi: 10.1073/pnas.0905620106
69
Zarco-Perello S. Bosch N. E. Bennett S. Vanderklift M. A. Wernberg T. (2021). Persistence of tropical herbivores in temperate reefs constrains kelp resilience to cryptic habitats. J. Ecol.109, 2081–2094. doi: 10.1111/1365-2745.13621
Summary
Keywords
Adriatic sea, coastal lagoon, coexistence, Cymodocea nodosa , Gongolaria barbata , marine forests
Citation
Smith SM, Bilajac A, Gljušćić E, Najdek M and Iveša L (2025) Development amongst the seagrass Cymodocea nodosa influences the morphology of the brown algae Gongolaria barbata in a coastal lagoon of the northern Adriatic Sea. Front. Mar. Sci. 12:1493581. doi: 10.3389/fmars.2025.1493581
Received
09 September 2024
Accepted
12 May 2025
Published
18 June 2025
Volume
12 - 2025
Edited by
Susana Carvalho, King Abdullah University of Science and Technology, Saudi Arabia
Reviewed by
Edson A. Vieira, Federal University of Rio Grande do Norte, Brazil
Felipe Ribeiro, Universidade Federal Fluminense, Brazil
Updates
Copyright
© 2025 Smith, Bilajac, Gljušćić, Najdek and Iveša.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shannen M. Smith, shannen.m.smith@outlook.com
†Present address: Shannen M. Smith, Aquatic Sciences, South Australian Research and Development Institute, Adelaide, SA, Australia
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.