Abstract
Predation is a major source of mortality in bottom - cultured scallops. To investigate the behavioral, physiological, and molecular mechanisms underlying predator - induced stress responses, an integrative approach was employed on Yesso scallops (Mizuhopecten yessoensis) exposed to the northern Pacific sea star (Asterias amurensis) by combining shell clap behavior quantification, enzyme activity assays, and transcriptomic analysis. Shell clap behavior, recorded using a force gauge system, revealed significantly increased contraction force and frequency following predator exposure, particularly in small and medium-sized scallops. Enzyme assays indicated significant changes in superoxide dismutase (SOD), catalase (CAT), arginine kinase (AK), and octopine dehydrogenase (ODH) across different tissues and size groups, with the most pronounced responses observed in medium - sized individuals. Transcriptomic profiling of adductor muscle in this group identified 514 differentially expressed genes (DEGs), of which 20 remained significant after P - value correction. These included genes such as paramyosin, myosin heavy chain, and Krüppel - like factors, involved in muscle structure, oxidative stress response, and amino acid metabolism. GO and KEGG enrichment analyses revealed significant metabolic shifts associated with oxidative stress and energy regulation. These findings demonstrate coordinated behavioral and molecular adaptations to predator stress in M. yessoensis and provide a foundation for improving predator management and selective breeding strategies in scallop aquaculture.
1 Introduction
The Yesso scallop (M. yessoensis) is a commercially important cold - water bivalve native to Hokkaido and northern Honshu in Japan, as well as the southern waters of the Russian Kuril Islands (Wang et al., 2012). In the 1980s, it was introduced to China for aquaculture, and it has since become a significant industry in northern China (Liu, 1983; Guo and Luo, 2016; Meng et al., 2012), with an annual production of 1, 854, 344 tons (2023 China Fisheries Yearbook). Among various scallop farming methods, bottom culture, in which scallop seed are planted directly onto the seabed, is the dominant practice. However, this approach exposes scallops to high predation pressure, often leading to a high mortality rate.
Among predators, sea stars pose the greatest threat to farmed scallops. These echinoderms use their tube feet and eversible stomachs to pry open scallop shells and digest them externally. Research on the Atlantic sea scallop (Placopecten magellanicus) has shown that predation by sea stars (Astropecten americanus) significantly reduces scallop survival, with vulnerability varying across different life stages (Shank et al., 2012). Similarly, the common sea star Asterias vulgaris has exhibited high capture efficiency for juvenile scallops, particularly at higher prey densities (Barbeau and Scheibling, 1994). In Yesso scallop farming, the northern Pacific sea star (A. amurensis) is one of the most frequently observed predator, particularly the predation by small individual scallops often leads to a rapid decline in survival during the first 1–8 weeks after seed planting (Miyoshi et al., 2019).
Beyond direct mortality, predation pressure influences scallop behavior and physiology, affecting their ability to adapt to environmental stressors while maintaining physiological stability. Scallops have evolved a unique escape mechanism, relying on rapid shell clap and jet-propelled swimming to evade predators (Xia, 2010). The effectiveness of these defense strategies may vary across different life stages and environmental conditions. Understanding the behavioral and physiological responses of M. yessoensis under predation stress is essential for improving survival rates and farming practices. However, such studies remain limited so far. To address this gap, a comprehensive investigation integrating behavioral, physiological, and transcriptomic analyses were conducted. Specifically, the shell clapping behavior under predation stress was quantified, changes in enzymatic activity across different tissues between control and predator-exposed groups were measured, and transcriptome sequencing to identify genes and pathways associated with predator - induced behavioral adaptations was performed. Scallops rely on rapid shell clapping to initiate jet - propelled swimming as an active escape mechanism. Quantitative parameters such as closure force, clap frequency, and duration can serve as indicators of their behavioral response to predator threats, which may vary across individuals or developmental stages. Enzyme activity reflects underlying metabolic and stress - related processes. Measuring key enzymes provides insight into how scallops mobilize energy, manage oxidative stress, and regulate physiological functions during predation stress. These biochemical responses are ultimately driven by changes in gene expression. Transcriptomic analysis provides a global view of molecular responses, enabling the identification of differentially expressed genes and the functional pathways they regulate. Additionally, consistently responsive genes may serve as valuable biomarkers for monitoring scallop health or informing selective breeding programs in aquaculture. Overall, this study provides novel insights into the predator - prey dynamics of bottom - cultured scallops and supports the development of optimized seeding strategies to enhance survival and sustainability in Yesso scallop farming.
2 Materials and methods
2.1 Sample collection and measurement
A total of 200 Yesso scallops were collected from Longwangtang Bay (Lvshunkou District, Dalian, China) in November 2022 and transported to the Key Laboratory of Aquaculture, Ministry of Agriculture and Rural Affairs (MARA), in insulated tanks. Scallops were removed from the substrate and rinsed. They were temporarily raised in a 1,000 L seawater tank at 15°C, with 50% of the water volume replaced daily. Scallops were fed once daily with powdered microalgae feed (Spirulina: Chlorella at 3:1) at a concentration of 10 mg/L. In addition, 30 northern Pacific sea stars were collected from the same location and transported to the laboratory under cool, moist conditions. After cleaning, 15 sea stars with arm lengths of 10–13 cm were selected as predators for the experimental group and temporarily cutured under the same conditions as the scallops, without feeding.
Only scallops that exhibited normal shell-closing behavior were selected for the experiment. Their shell length, height, and width were measured to 0.01 mm using vernier calipers (16EWR[M13] 0–150 mm, Marr Precision Measuring Instruments, Suzhou, China), and wet weight was recorded using an electronic balance (JJ500, Changshu Shuangjie Testing Instrument Factory, China) accurate to 0.01 g. Then they were grouped into three size groups based on shell length (Table 1).
Table 1
| Measurement parameter | Large | Medium | Small |
|---|---|---|---|
| Shell length/mm | 119.85 ± 3.23a | 89.24 ± 3.77b | 60.10 ± 3.23c |
| Shell height/mm | 116.92 ± 6.02 | 87.46 ± 3.55 | 61.39 ± 6.02 |
| Shell width/mm | 26.98 ± 2.98 | 26.98 ± 2.98 | 16.21 ± 2.63 |
| Wet weight/g | 176.50 ± 28.57 | 83.70 ± 13.97 | 30.16 ± 5.29 |
Morphological measurement of M. yessoensis across three size classes.
n = 15 for each group. Values are presented as mean ± standard deviation (x¯ ± SD). Superscript letters (a, b, c) indicate statistically significant differences in shell length between groups (P < 0.05); values sharing the same letter are not significantly different.
2.2 Measurement of shell clap behavior
This behavior involves two phases of adductor muscle contraction (Tremblay et al., 2012). The first is a phasic contraction, involving rapid shell closures driven by striated muscle, resulting in a force peak referred to as the clapping force. A series of rapid claps over a short period constitute the phasic contraction force, which is the cumulative force of multiple claps (n). The second phase is a tonic contraction, involving slower, sustained shell closing (lasting >0.5 s), generated by smooth muscle. The corresponding force is termed the tonic contraction force. In this study, the following parameters were measured using a modified force gauge method (Zhang et al., 2021): the maximum shell closing force (Fmax), the number of phasic contractions (Tphasic), the phasic contraction force (Fphasic), the times of tonic contractions (Ttonic), the tonic contraction force (Ftonic), the duration of tonic contractions (ttonic), and the frequency of shell claps.
The scallop was placed in a glass tank (50 × 30 × 35 cm) with the lower valve fixed to a platform, while the upper valve was connected to a digital force gauge (Nscing SH - III - 100N, Suce, Nanjing, China). The gauge recorded the force, number and time data, which were transmitted to a computer (Figure 1). The experiment was conducted in two groups: a control group (sea star absent) and an experimental group (sea star present). To minimize variability, sea stars of similar size were selected, and each experimental tank contained one sea star and one scallop, with the sea star’s arm in contact with the scallop shell. Each stimulation lasted for 3 minutes. Fifteen scallops from each size class were tested per group as biological replicates.
Figure 1
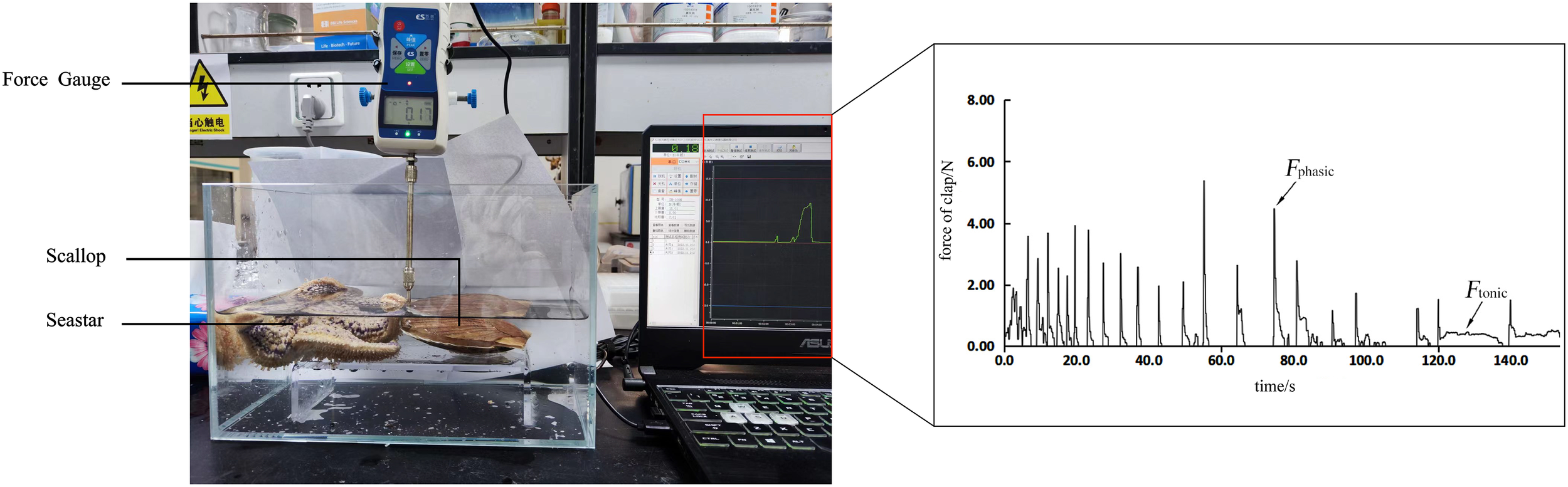
Experimental setup and force response of scallops under predator stress. (Left) A force gauge was used to measure the adductor muscle contraction force of a scallop in response to the presence of a sea star. (Right) Representative force trace over time, showing two distinct phases of muscle contraction: phasic force (Fphasic), characterized by rapid, high-amplitude contractions, and tonic force (Ftonic), defined by sustained low-level force generation.
2.3 Enzyme activity assay
To assess physiological responses to predation stress, the activities of four enzymes, superoxide dismutase (SOD), catalase (CAT), arginine kinase (AK), and octopine dehydrogenase (ODH) were measured. These enzymes are involved in oxidative stress defense, energy metabolism, and muscle function. Three tissues (adductor muscle, gill, and mantle) were sampled from scallops in each size class following the 3 - minute predator exposure. Samples were immediately flash - frozen in liquid nitrogen and stored for analysis. Each group included three biological replicates. Enzyme activities were measured using commercial ELISA kits (Fancovi, Bio - Techne China Co. Ltd., Shanghai), following the manufacturer’s instructions.
2.4 Transcriptome analysis and validation
2.4.1 RNA preparation
The most pronounced and consistent changes in enzyme activity may indicate a strong physiological response to predator stress. This likely reflects metabolic and stress-regulatory pathways are highly active, increasing the likelihood of capturing meaningful gene expression changes and identifying differentially expressed genes associated with predation response. Therefore, in transcriptome study, the scallops were sampled from the size group with most significant difference of enzyme activity.
Adductor muscle tissue was sampled from both control (labeled as mBJ) and predator-exposed groups (labeled as H - mBJ) after 3 minutes of stimulation. Three biological replicates per group were used. Total RNA was extracted using the TRIzol method (Ambion/Invitrogen, USA). RNA concentration and integrity were assessed using the Agilent 2100 Bioanalyzer to ensure quality met sequencing requirements.
2.4.2 Library construction and sequencing
RNA libraries were prepared using the NEBNext® Ultra™ RNA Library Prep Kit (New England Biolabs, USA). mRNA was isolated using poly - T oligo - attached magnetic beads, then fragmented and reverse transcribed into cDNA. Second - strand synthesis was carried out, followed by end repair, 3’ adenylation, adapter ligation, and PCR amplification. The final libraries (250–300 bp) were purified with AMPure XP beads and assessed using the Agilent Bioanalyzer. Sequencing was performed on the Illumina HiSeq platform using 150 bp paired-end reads.
2.4.3 Bioinformatic analysis
Raw sequencing data were filtered to remove adaptors, poly - N sequences, and low-quality reads. Clean reads were mapped to the M. yessoensis reference genome using HISAT2 (v2.0.5; https://www.ncbi.nlm.nih.gov/genome/12193). Gene expression levels were calculated as FPKM (Fragments Per Kilobase of transcript per Million mapped reads). Differential gene expression analysis was conducted using DESeq2. Genes were considered differentially expressed if | log2 (Fold Change) | ≥ 1 and P ≤ 0.05. Multiple testing correction was performed using the False Discovery Rate (FDR). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed for functional annotation.
2.4.4 Quantitative real - time PCR validation
To validate the reliability of RNA - seq data, x differentially expressed genes (DEGs) were randomly selected for qRT - PCR. Primers were designed using Primer Premier 6.0 (Supplementary Table S1) and synthesized by Sangon Biotech (Shanghai, China). RNA was reverse transcribed to cDNA using the TIANGEN® FastKing gDNA Dispelling RT SuperMix. qRT - PCR was performed with TIANGEN® Talent qPCR PreMix (SYBR Green) on a Roche LightCycler® 96 (Switzerland). Each reaction (20 μL) contained 2 μL cDNA, 10 μL 2 × TB Green Premix, 0.4 μL ROX Dye II, 6 μL nuclease-free water, and 0.8 μL of each primer (10 mM). Cycling conditions: 95°C for 600 s, followed by 45 cycles of 95°C for 10 s and 60°C for 60 s. Melt curve analysis confirmed amplification specificity. Relative expression levels were calculated using the 2–ΔΔCt method (Livak and Schmittgen, 2001), with Cytb as the reference gene. Three biological and three technical replicates were performed.
2.5 Statistical analysis
Data were analyzed using SPSS 25.0 (IBM, New York, USA). Mean and standard deviation were calculated for behavioral parameters and enzyme activity. Differences between groups were assessed using independent-sample t-tests. Differences were considered statistically significant at P < 0.05 and highly significant at P < 0.01.
3 Results
3.1 Shell clapping behavior in response to predation
Force gauge recordings of M. yessoensis across different size classes revealed distinct phasic and tonic contraction patterns (Figure 2). In control conditions, shell closure force increased with scallop size. Upon exposure to A. amurensis, scallops of all sizes consistently exhibited higher closure forces compared to their respective controls. Notably, small and medium scallops showed significantly higher phasic contraction counts following predator stimulation (P < 0.05), while large scallops demonstrated significantly longer tonic contraction durations (Figure 2). Over time, all groups exhibited a decline in closure force and frequency, indicating fatigue or habituation.
Figure 2
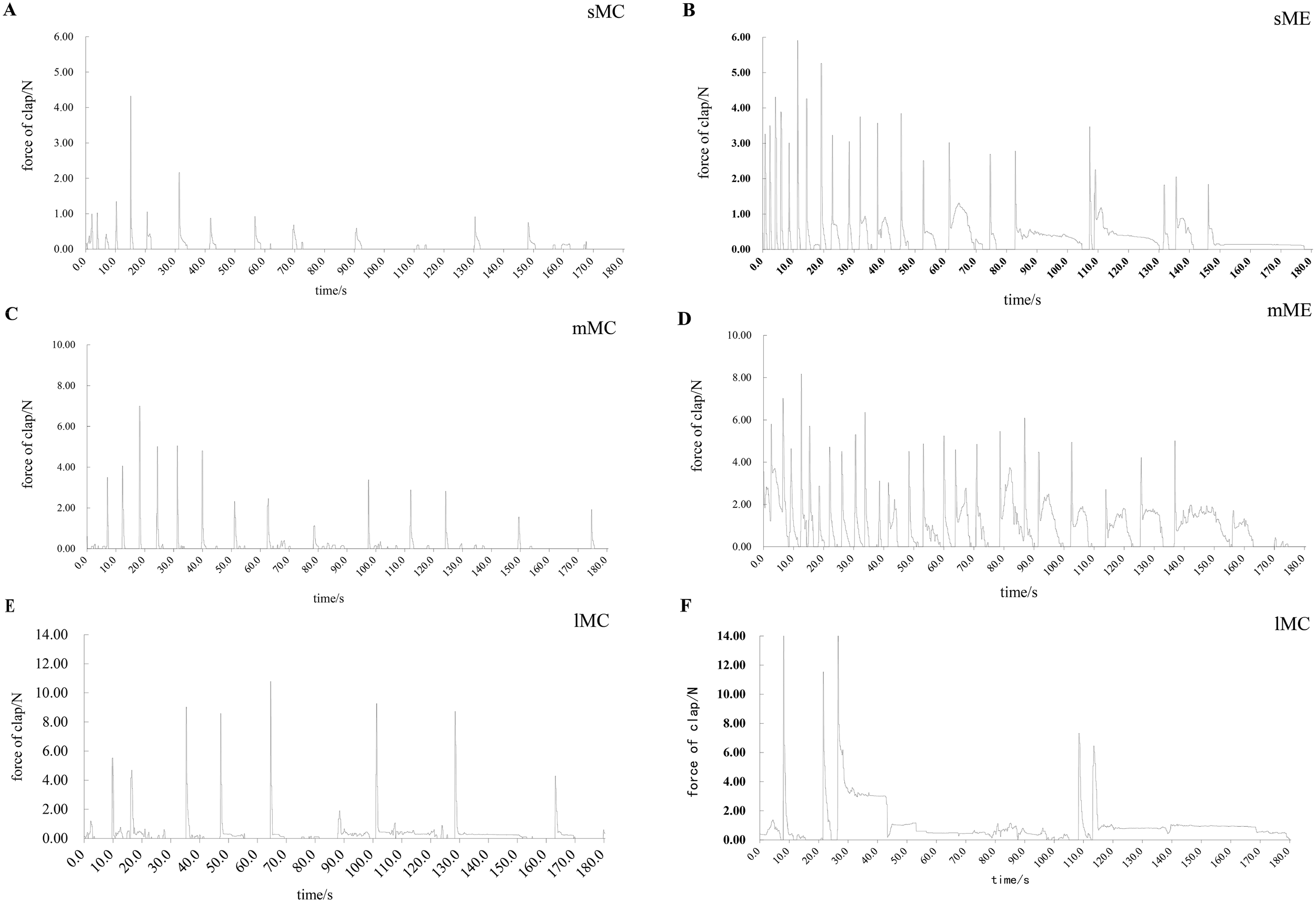
Typical force recordings illustrate differences in both the magnitude and temporal patterns of force production among size classes and between control and experimental conditions. (A) Control group of small size class M. yessoensis (sMC), (B) Experimental group of small size class M. yessoensis (sME), (C) Control group of medium size class M. yessoensis (mMC), (D) Experimental group of medium size class M. yessoensis (mME), (E) Control group of large size class M. yessoensis (lMC), (F) Experimental group of large size class M. yessoensis (lME).
Maximum closure force increased significantly with size across all groups (P < 0.05) and remained consistently higher in predator - exposed individuals (Figure 3A). Phasic contraction force and frequency were significantly elevated in small and medium scallops post - exposure, while large scallops did not show significant differences in these parameters. Tonic contraction force and duration increased significantly in all size groups following stimulation (P < 0.05), with large scallops exhibiting more than a threefold increase in tonic duration. Shell closure frequency was unaffected in small and medium scallops but significantly decreased in large scallops under predator stimulation. During the first 30 seconds of exposure, small scallops displayed the highest closure frequency, significantly greater than medium scallops (P < 0.05), with no significant difference observed between medium and large individuals (Figure 3E).
Figure 3
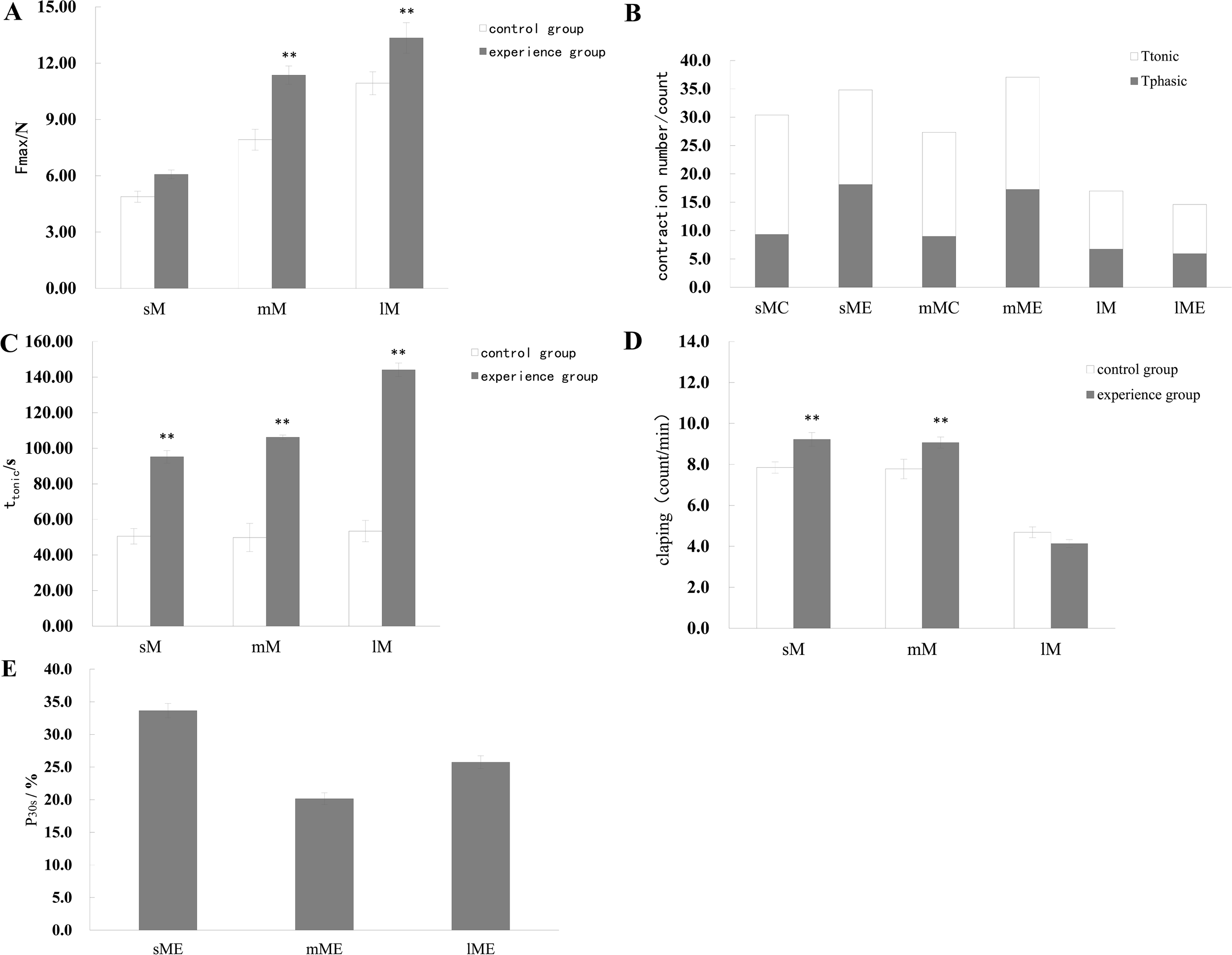
Shell clapping response parameters related to phasic contractions in M. yessoensis in response to predation. (A) Maximum contraction force (Fmax), (B) Total number of contractions, separated into phasic and tonic types, (C) Duration of tonic contractions (ttonic), (D) Frequency of shell clapping (in counts per minute), and (E) Percentage of time within the first 30 seconds that the scallop shell remained closed. Asterisks indicate statistically significant differences between groups: P < 0.05 (*), P < 0.01 (**). Sample size: n = 15, data are presented as mean ± SD.
3.2 Enzyme activity in response to predator stress
Enzymatic activity assays revealed significant alterations in SOD, CAT, AK, and ODH levels across tissues and size classes following predator exposure (Figure 4). In gill tissue, SOD and AK activities were highly significantly altered (P < 0.01), while AK activity in the mantle also showed significant changes (P < 0.05). In adductor muscle tissue, SOD and ODH were significantly elevated (P < 0.05), while CAT and AK exhibited highly significant increases (P < 0.01) following stimulation.
Figure 4
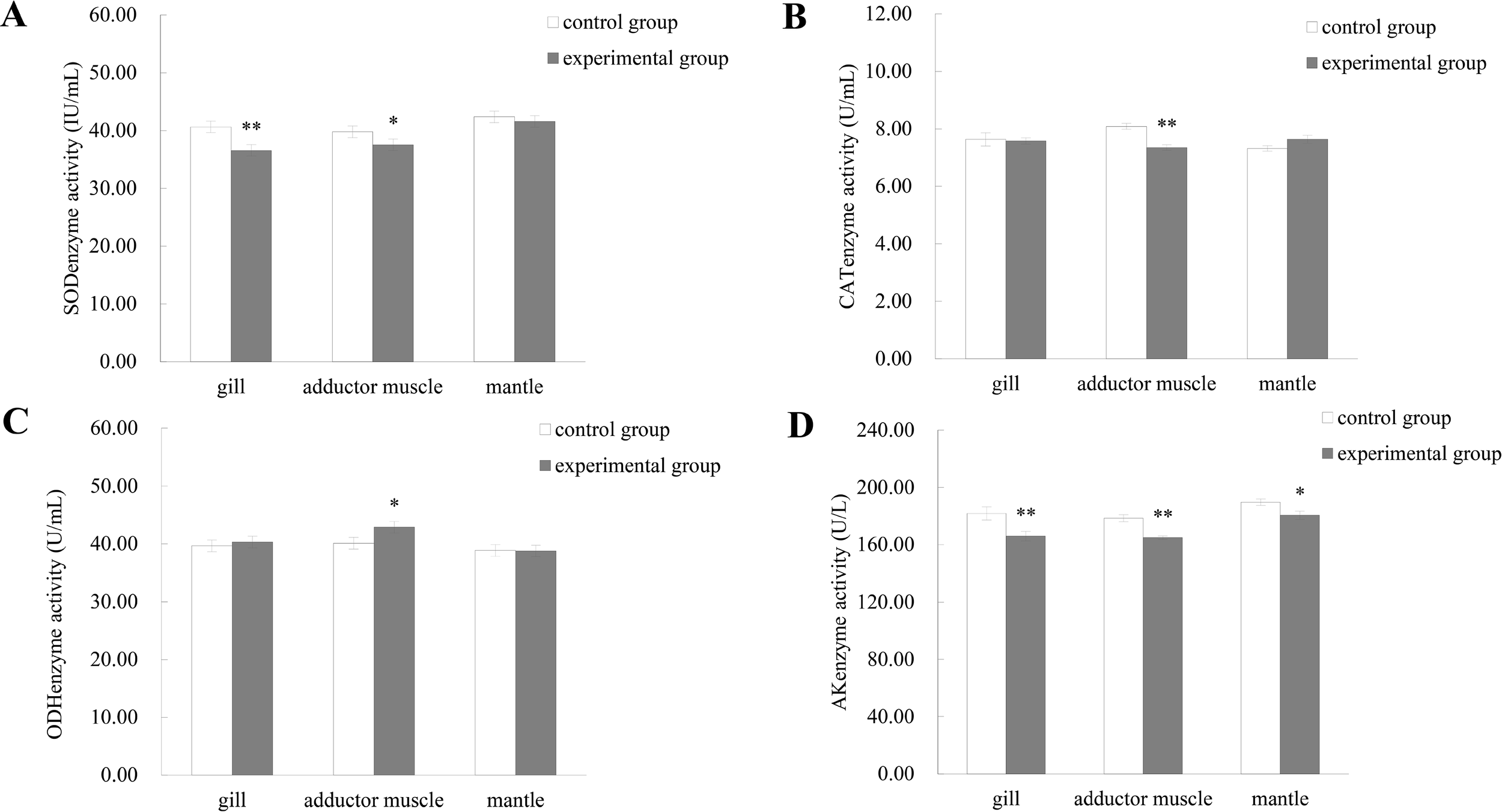
Enzyme activity in the muscle tissue of M. yessoensis in response to predation. (A) Superoxide dismutase (SOD) activity, (B) Catalase (CAT) activity, (C) Octopine dehydrogenase (ODH) activity, and (D) Arginine kinase (AK) activity. Asterisks indicate statistically significant differences between groups: P < 0.05 (*), P < 0.01 (**). Sample size: n = 9, data are presented as mean ± SD.
A more detailed breakdown of enzyme activity in adductor muscles by size class is shown in Figure 5. In large scallops, CAT and ODH levels dropped significantly (P < 0.01), from 7.74 ± 0.21 U/mL and 43.08 ± 1.42 U/mL in controls to 6.98 ± 0.20 U/mL and 42.60 ± 0.62 U/mL, respectively. Medium - sized scallops showed significant changes in all four enzymes: SOD declined from 40.47 ± 1.61 IU/mL to 37.20 ± 1.29 IU/mL (P < 0.05), and CAT, ODH, and AK were all highly significantly reduced (P < 0.01). In small scallops, CAT activity remained unchanged, while SOD, ODH, and AK were significantly altered (P < 0.01).
Figure 5
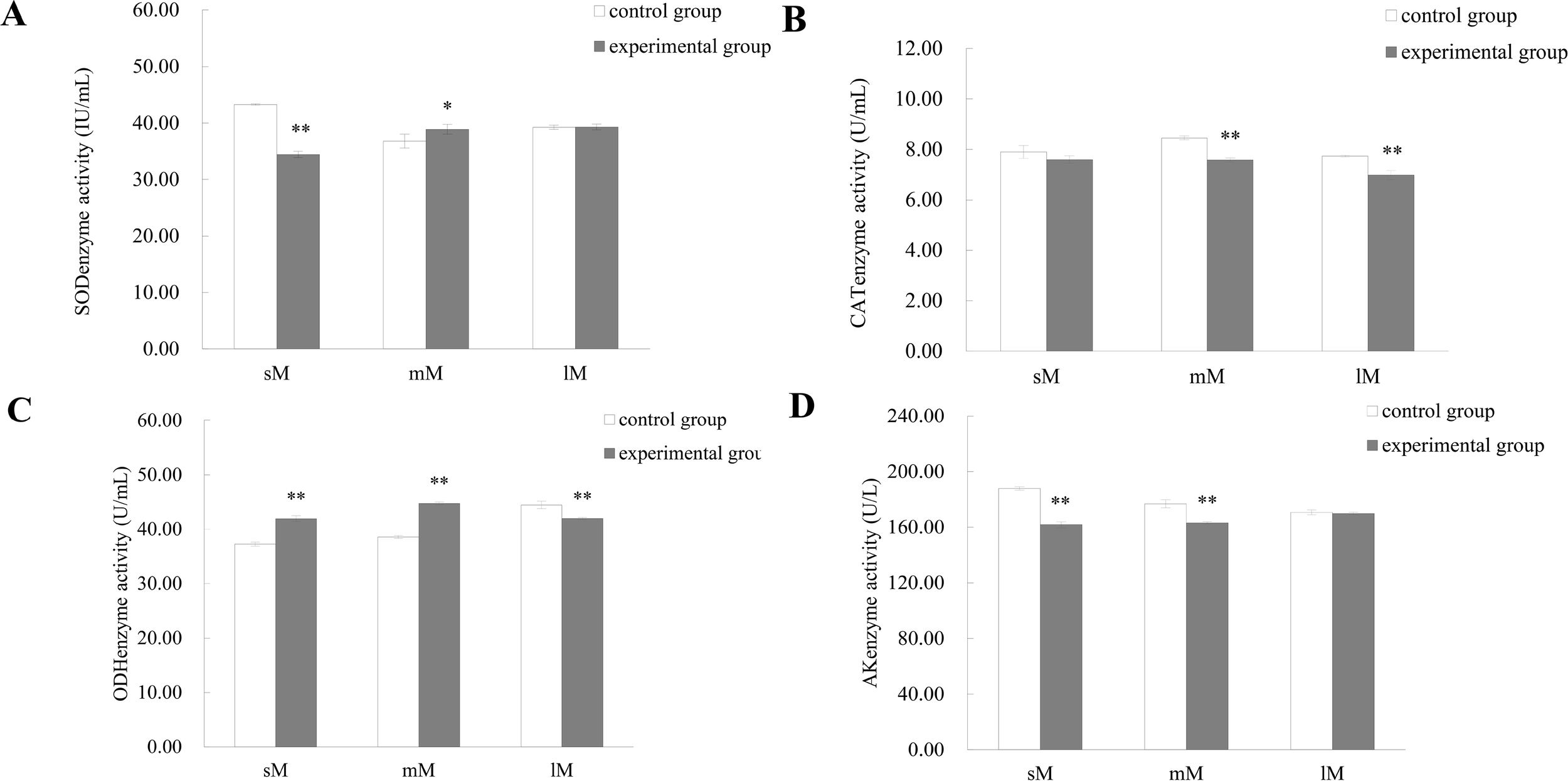
Comparison of enzyme activities in different tissues (gill, adductor muscle, and mantle) of medium - sized M. yessoensis in response to predation. (A) Superoxide dismutase (SOD) activity, (B) Catalase (CAT) activity, (C) Octopine dehydrogenase (ODH) activity, and (D) Arginine kinase (AK) activity. Asterisks indicate statistically significant differences between groups: P < 0.05 (*), P < 0.01 (**). Sample size: n = 3, data are presented as mean ± SD.
Based on these observations, medium - sized scallops exhibited the most pronounced enzymatic responses to predation stress and were therefore selected for transcriptomic analysis.
3.3 Transcriptome analysis of predator - exposed scallops
3.3.1 Sequencing output and quality
Transcriptome sequencing generated between 58.41 and 61.74 million raw reads per sample. After quality filtering, 55.62 - 60.62 million high - quality reads remained, totaling 52.44 Gb of clean data. The GC content ranged from 41.05% to 43.85%, with Q20 and Q30 values exceeding 94.92% and 87.60%, respectively. The maximum single - base error rate was just 0.07%, confirming high sequencing fidelity (Supplementary Table S2). Mapping to the M. yessoensis reference genome yielded alignment rates of 90.9 - 90.97% in control samples and 87.82 - 92.39% in experimental samples (Supplementary Table S2).
3.3.2 Differential gene expression
Differential gene expression analysis based on FPKM values identified 514 genes with raw P - values < 0.05, of which 243 were upregulated and 271 were downregulated following predator exposure (Figure 6; Supplementary Table S3). After multiple testing correction, 20 genes remained statistically significant. These included genes associated with muscle structure and function such as phenylalanine hydroxylase, ammonium transporter, β - galactosidase, branched - chain amino acid aminotransferase, Krüppel - like factor, paramyosin, transmembrane protein, Fanconi anemia protein, complement C1q, methyltransferase subunit, RING finger protein, and myosin heavy chain. Among them, paramyosin (Li et al., 2021), myosin heavy chain (Funabara et al., 2013), and Krüppel - like factor (Prosdocimo et al., 2015) have been previously reported to play significant roles in bivalve adductor muscle function and locomotor activity.
Figure 6
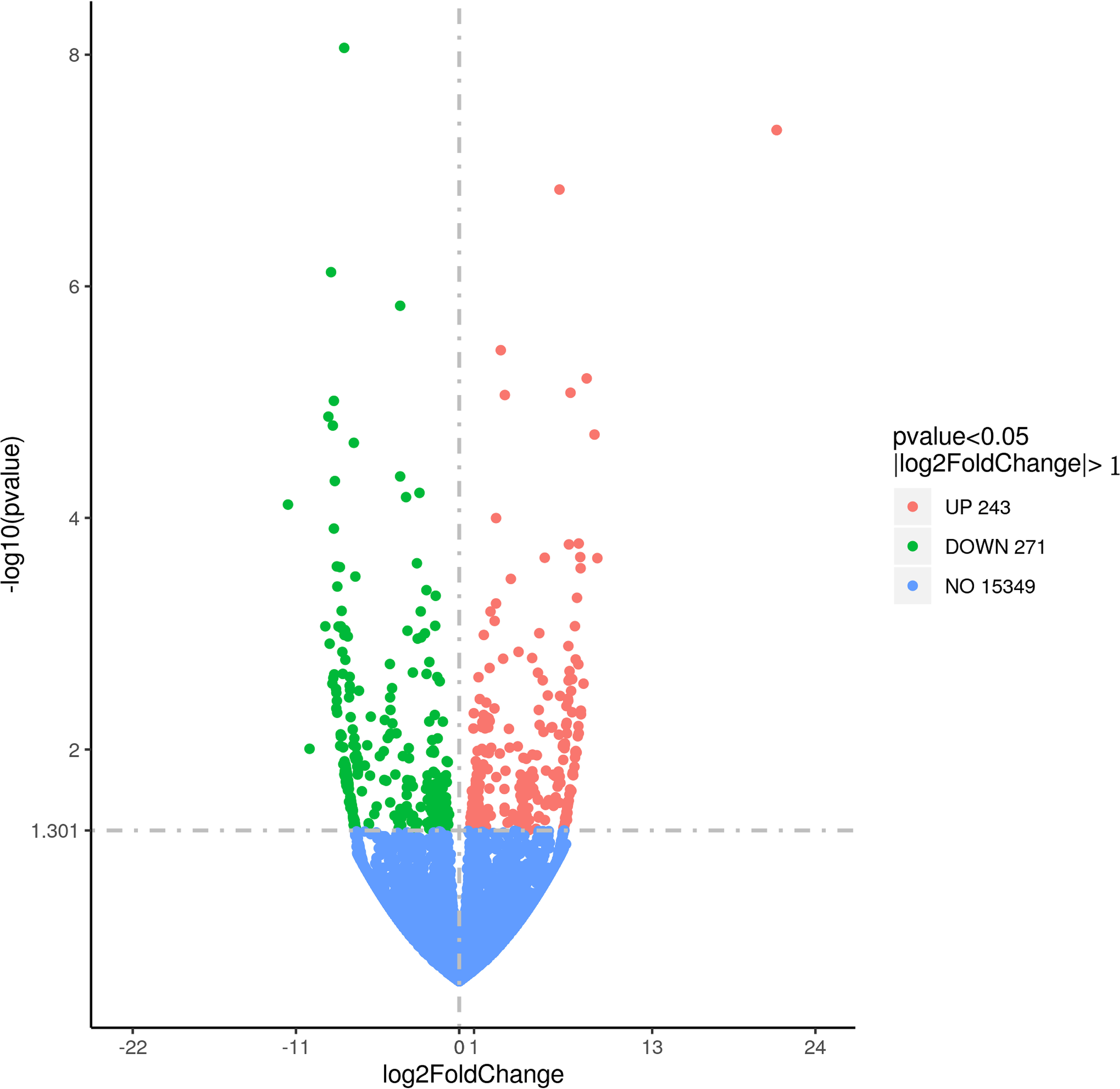
Volcano plot of the differentially expressed genes in M. yessoensis in response to predation by A. amurensis.
3.3.3 Gene ontology and KEGG pathway enrichment
Gene Ontology (GO) terms were classified into biological processes (BP), cellular components (CC), and molecular functions (MF). Figure 7 presents the top ten enriched GO terms in each category. These included oxidoreductase activity (MF), oxidation-reduction process (BP), and intracellular membrane-bounded organelle (CC). Although most GO terms were not statistically significant (P > 0.05), 14 GO terms achieved significance (P < 0.05), notably including carboxylic acid metabolic process, organic acid metabolic process, oxoacid metabolic process (BP), and coenzyme binding (MF).
Figure 7
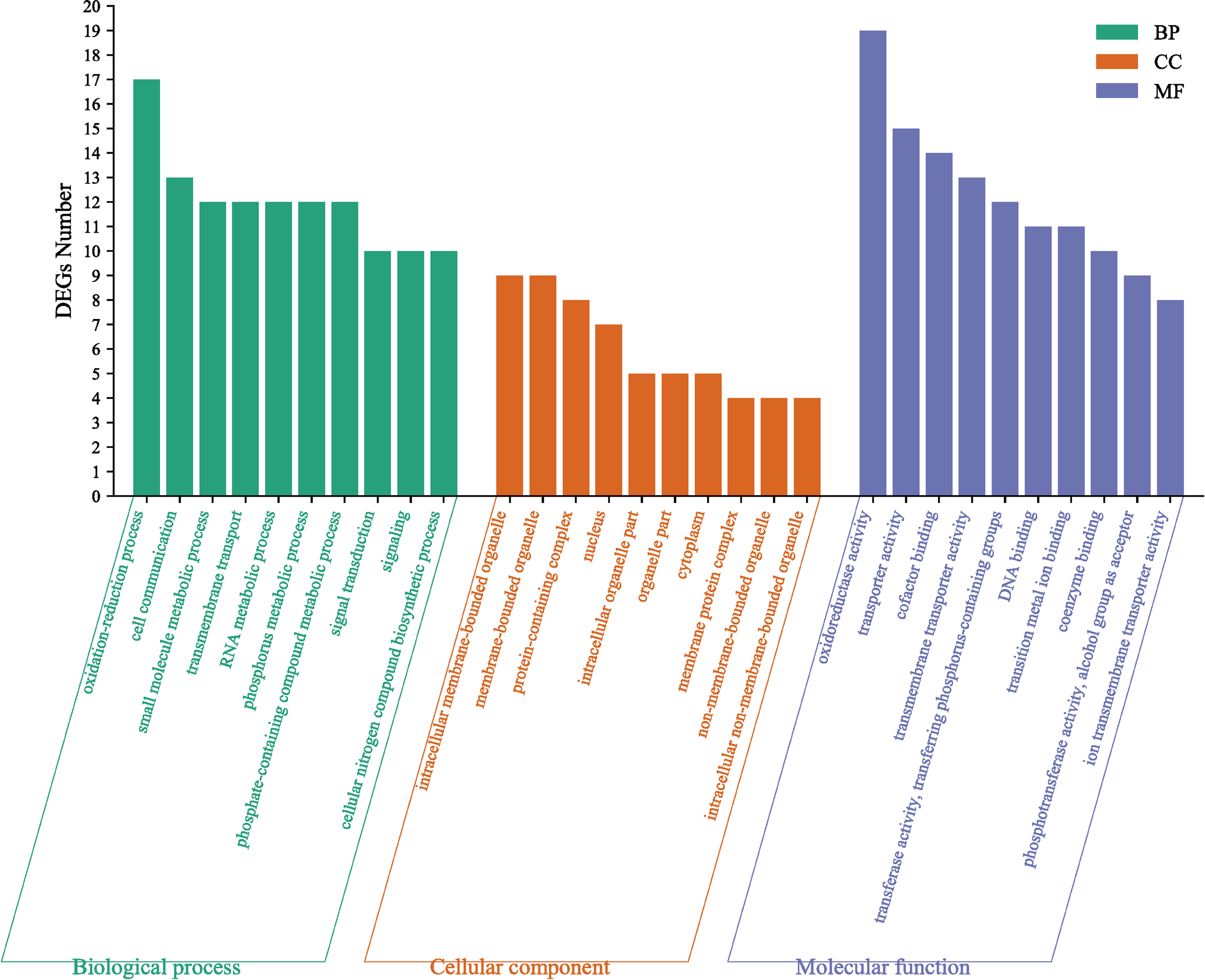
Gene ontology classification of the differentially expressed genes in M. yessoensis in response to predation by A. amurensis.
KEGG pathway analysis identified 101 biological pathways, among which cysteine and methionine metabolism, pantothenate and CoA biosynthesis, branched - chain amino acid degradation (valine, leucine, isoleucine), and tyrosine metabolism were significantly enriched (P < 0.01) (Figure 8).
Figure 8
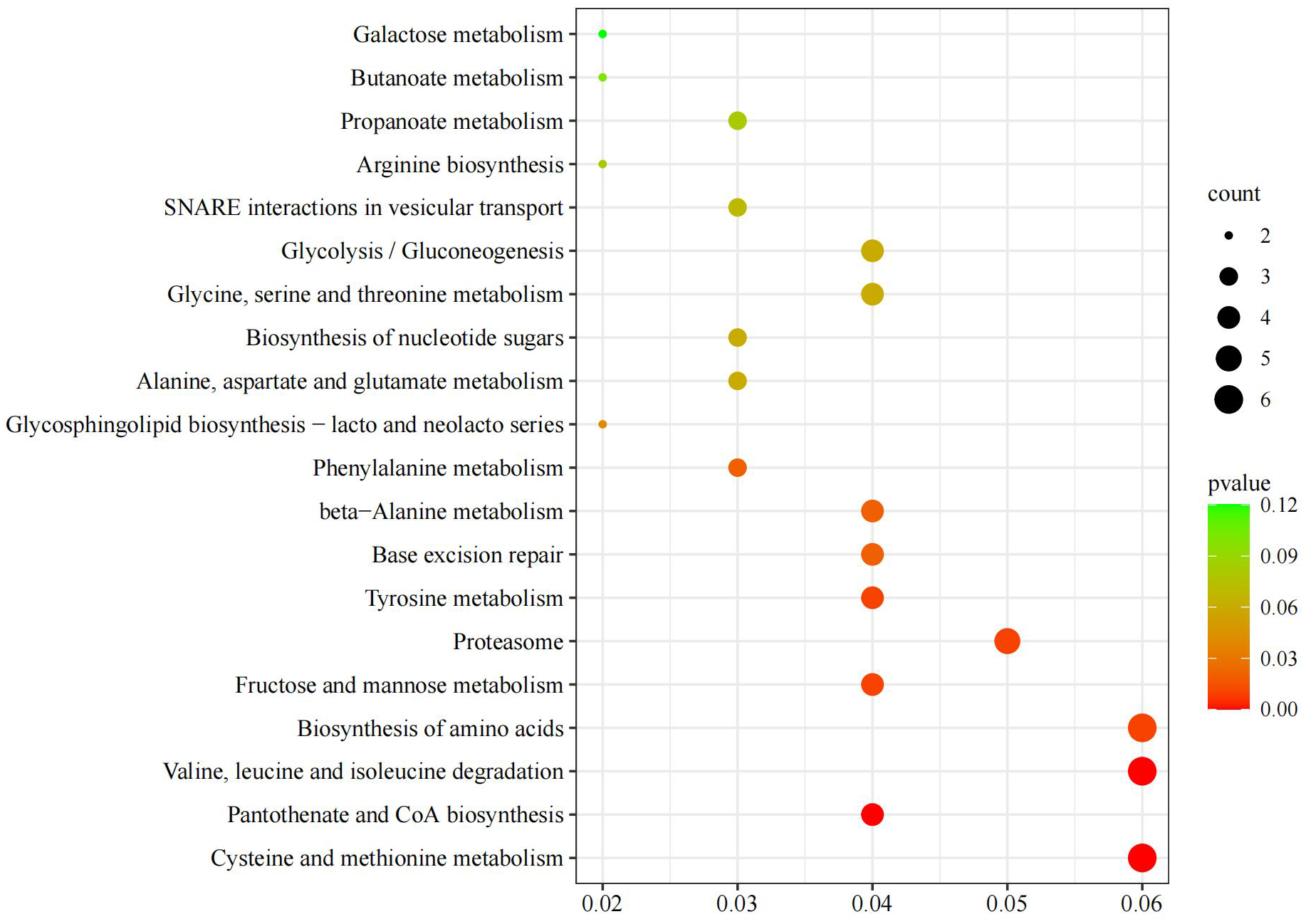
Kyoto encyclopedia of genes and genomes bubble chart of M. yessoensis in response to predation by A. amurensis.
3.3.4 qRT - PCR validation
To validate the RNA - seq results, six DEGs were randomly selected for qRT - PCR analysis: kinesin protein 13B (KIF13B, LOC110458105), cytochrome P450 (P450, LOC110459241), thioredoxin (TRXL, LOC110449874), cell cycle checkpoint protein 17 (RAD17, LOC110466699), otoferlin (OTOF, LOC110467166), NMDA receptor synaptonuclear signaling and neuronal migration factor (NSMF, LOC110459652), and an uncharacterized methyltransferase (C25B8.10, LOC110461631). The qRT - PCR results were consistent with RNA - seq data, showing a strong correlation (R² = 0.81) in expression trends, thereby validating the transcriptome analysis (Figure 9).
Figure 9
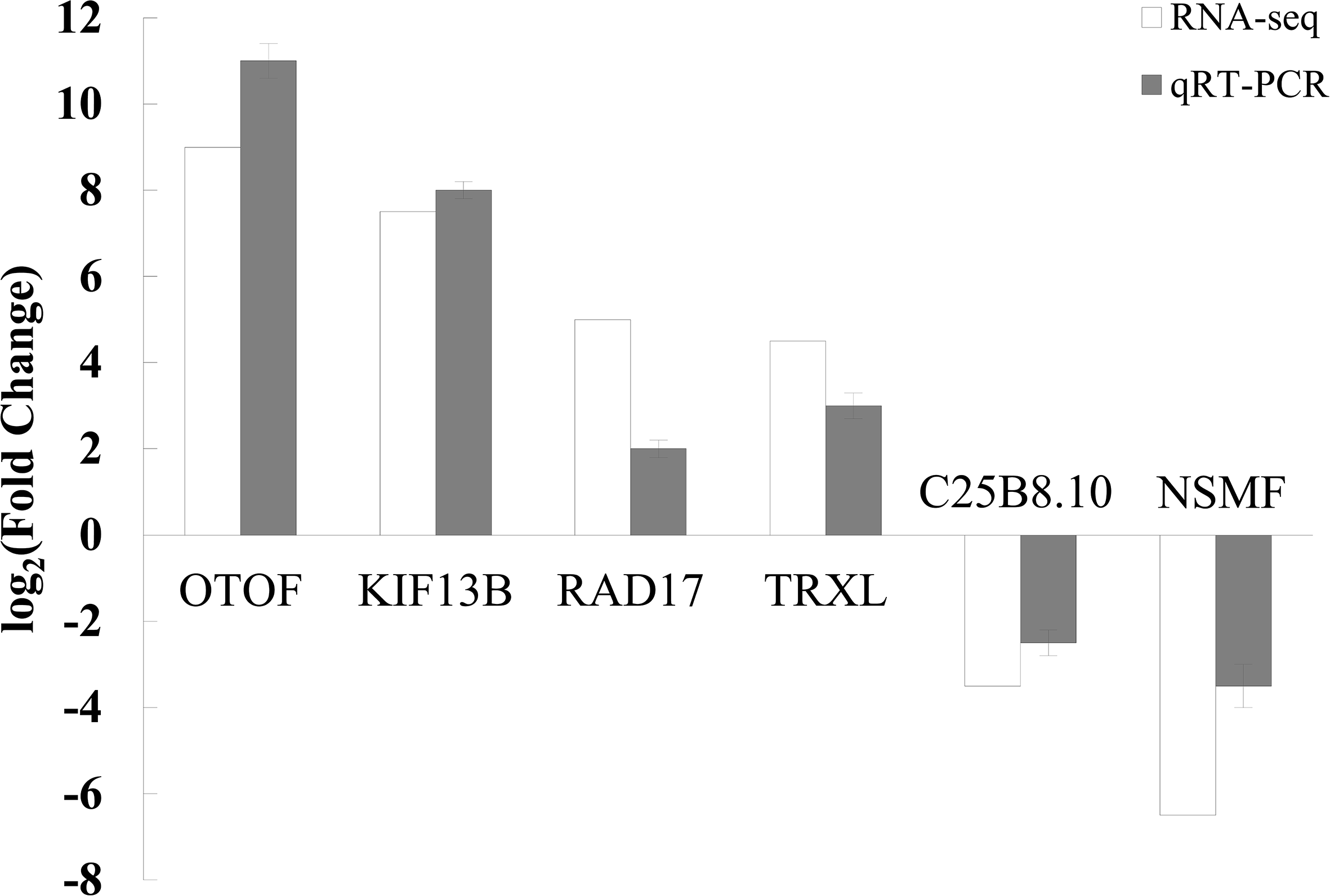
Comparison of gene expression levels in M. yessoensis exposed to A. amurensis predation as measured by RNA - Seq and qRT - PCR.
4 Discussion
This study presents a comprehensive analysis of behavioral, biochemical, and transcriptomic responses of Yesso scallops to predator stress induced by the northern Pacific sea star. The findings provide new insights into the mechanisms of predator avoidance and physiological stress in this economically significant species, with important implications for scallop aquaculture practices.
4.1 Behavioral adaptation and size - dependent escape strategies
The shell closure behavior of scallops serves as a primary escape response against benthic predators. Our results demonstrated that the maximum shell closing force (Fmax) increased significantly with scallop size, supporting previous findings that larger scallops possess stronger adductor muscles (Tremblay et al., 2012). When exposed to A. amurensis, all size classes showed elevated shell closure force compared to controls, indicating an acute defensive response. Notably, small and medium - sized scallops exhibited significantly higher phasic contraction frequency and force, while large scallops relied on prolonged tonic contractions with reduced closure frequency. This suggests a size - dependent behavioral adaptation: smaller scallops respond with rapid and repeated shell clapping to deter predators, whereas larger individuals depend on sustained force and shell thickness for protection. These findings are consistent with escape strategies observed in other scallop species, such as Aequipecten opercularis, which exhibit more frequent closures in juveniles compared to adults (Schmidt et al., 2008).
4.2 Enzymatic responses reflecting oxidative and metabolic stress
Predator - prey interactions elicit acute stress responses in prey species, often triggering physiological and biochemical changes. In shellfish, antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) are critical for mitigating oxidative stress by regulating reactive oxygen species (ROS) levels (Gao, 2016; Liu et al., 2003). Excessive ROS, generated during stress or high metabolic activity, can damage cellular structures unless effectively neutralized. Our results showed a size - dependent pattern of antioxidant response in Yesso scallops exposed to A. amurensis. Specifically, SOD activity increased significantly in medium and large scallops, while small individuals exhibited a marked decrease. Conversely, CAT activity declined across all size groups, with medium - sized scallops showing the most substantial drop. This pattern may reflect a rapid initial surge followed by depletion of enzymatic activity in small scallops, possibly due to their limited physiological reserves. The increase in SOD activity in larger scallops suggests a more robust antioxidant defense, aligning with prior studies that associate greater body size or age with enhanced stress resistance and immune capacity (Hao et al., 2014; Le et al., 1999).
Energy metabolism also plays a crucial role in stress response. Arginine kinase (AK), a key enzyme for ATP buffering in invertebrate muscle, supports rapid energy turnover during sudden escape movements (Morris et al., 2005; Yao et al., 2008). Our data revealed significant downregulation of AK activity in the adductor muscle of small and medium - sized scallops following predator exposure. This downregulation likely reflects acute energy demand during swimming behavior and subsequent depletion of ATP stores. Previous studies have demonstrated similar decreases in AK activity following intensive muscle activity in bivalves (Gäde et al., 1978; Smits et al., 2010), supporting the idea that predator stress imposes a high metabolic load on scallops. Octopine dehydrogenase (ODH), an enzyme involved in anaerobic glycolysis, was also significantly affected across all size groups. ODH catalyzes the formation of octopine as a terminal product of anaerobic metabolism, analogous to lactic acid formation in vertebrates (Livingstone et al., 1981; Bailey et al., 2003; Cavallini et al., 1966). Increased ODH activity likely supports short-term bursts of swimming and is consistent with earlier studies showing higher ODH levels in scallops following predator exposure (Guerra et al., 2013). The observed changes in AK and ODH activities are likely directly linked to the energetic demands of escape behavior, indicating that these enzymes may serve as reliable physiological markers of predator-induced locomotion in scallops.
4.3 Molecular insights into predator - induced stress responses
Transcriptome analysis revealed that exposure to A. amurensis led to significant changes in gene expression within the adductor muscle of medium - sized Yesso scallops. A total of 514 differentially expressed genes (DEGs) were identified (raw P < 0.05), with 20 remaining significant after multiple testing correction. These DEGs included genes encoding muscle structure and function proteins such as paramyosin and myosin heavy chain, as well as KLF, which is known to regulate muscle development and stress response (Funabara et al., 2013; Prosdocimo et al., 2015). The upregulation of paramyosin and myosin heavy chain genes supports the observed enhancement of muscle contraction during predator exposure. These proteins play essential roles in maintaining the structural integrity and contraction dynamics of the adductor muscle, which are critical for the scallop’s escape response. The increased expression suggests a transcriptional reprogramming aimed at boosting locomotor capacity under acute stress conditions (Li et al., 2021). Krüppel - like factor (KLF), a transcription factor, has been implicated in muscle differentiation and cellular stress responses in various species. Its differential expression in scallops exposed to predators suggests that it may be involved in modulating muscle function or repair under stress. This aligns with physiological findings that indicate altered enzymatic activity supporting increased energy demand and oxidative stress management during predator evasion (Prosdocimo et al., 2015).
Additionally, several metabolic pathway-related genes, including those involved in amino acid metabolism (e.g., valine, leucine, isoleucine degradation), coenzyme biosynthesis, and oxidative metabolism (e.g., cysteine and methionine metabolism), were significantly enriched in KEGG pathway analysis. These findings reflect the underlying metabolic reorganization in response to predation stress, supporting rapid energy mobilization and reactive oxygen species (ROS) detoxification. Although GO enrichment did not identify significant terms related to muscle structure directly, several biological processes associated with organic acid metabolism and oxidoreductase activity were enriched, highlighting shifts in biochemical processes crucial for immediate stress adaptation. Overall, the transcriptomic responses corroborate behavioral and enzymatic data, confirming that predator - induced stress in Yesso scallops triggers a coordinated response involving enhanced muscle performance, metabolic adaptation, and stress signaling. These molecular signatures offer potential biomarkers for assessing scallop fitness and could inform selective breeding strategies aimed at enhancing resilience to environmental stressors, including predation.
4.4 Implications for aquaculture
Understanding predator - induced responses in scallops has direct or indirect applications for improving survival in bottom culture systems. Our findings show that scallops, particularly juveniles, undergo substantial physiological changes when confronted with predators. These responses, while adaptive, incur metabolic costs that may compromise long - term health and growth. Early detection of predation stress and the use of predator deterrents or protective seeding strategies (e.g., larger initial seed size, temporary netting) could help reduce mortality during critical early grow - out phases. The enzymatic assays revealed consistent patterns of stress-induced changes, especially in SOD, CAT, AK, and ODH levels. These enzymes reflect oxidative stress and energy demands in scallops under predation or other stresses. Such biomarkers may be used to monitor stress levels in real time and assess scallop health. Moreover, the identified genes could be used for development of molecular markers associated with stronger locomotor ability, supporting a selected breeding to develop scallop strains with enhanced predator resistance and stress tolerance.
5 Conclusion
This study reveals that M. yessoensis exhibits distinct behavioral, physiological, and molecular responses to predation by A. amurensis, which vary with scallop size. Small and medium scallops employ rapid shell clapping supported by heightened metabolic enzyme activity and stress - responsive gene expression, whereas large scallops rely on prolonged contraction and shell strength. These results enhance our understanding of predator-prey dynamics in bottom culture systems and provide a scientific basis for improving scallop aquaculture practices through size - selective seeding, stress biomarker development, and integrated predator management strategies.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1035919.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because M. yessoensis utilized in this study was cultured marine species available commercially, and was not part of an endangered or protected species.
Author contributions
XTL: Data curation, Formal analysis, Methodology, Validation, Writing – original draft. XAL: Data curation, Methodology, Project administration, Writing – review & editing. DL: Data curation, Software, Validation, Writing – original draft. DZ: Data curation, Investigation, Methodology, Writing – review & editing. XZ: Data curation, Investigation, Methodology, Writing – review & editing. XW: Formal analysis, Validation, Writing – original draft. JM: Conceptualization, Data curation, Investigation, Writing – original draft. YT: Formal analysis, Project administration, Writing – original draft. ML: Formal analysis, Funding acquisition, Writing – review & editing. YC: Funding acquisition, Project administration, Resources, Visualization, Writing – original draft. ZH: Funding acquisition, Project administration, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by funds from Marine Economy Development Special Project of Liaoning Province Department of Natural Resources, the Central Government Subsidy Project for Liaoning Fisheries (2023) and the Science and Technology Foundation of Dalian (2021JB11SN035), Liaoning Provincial Nature Foundation Project (2023 -MS -271), Tarim University President Fund (Master) Hu Yang Talent Project TDZKSS202310, Liaoning Provincial Modern Agricultural Industry Technology System for Shellfish, Liaoning Provincial Major Special Project (2024JH1/11700010), Eamark Fund for Morgan State University’s PEARL Lab Student Research Enhancements.
Acknowledgments
Chat-GPT 4o (https://chatgpt.com) is used for polishing the language and correcting the English grammar mistakes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1493892/full#supplementary-material
Supplementary Table 1Primers used for the qRT-PCR validation.
Supplementary Table 2Summary of sample sequencing data quality.
Supplementary Table 3The list of differentially expressed genes (P<0.05).
References
1
Bailey D. M. Peck L. S. Bock C. Pörtner H. O. (2003). High - energy phosphate metabolism during exercise and recovery in temperate and Antarctic scallops: an in vivo31P - NMR study. Physiol. Biochem. Zool.76, 622–633. doi: 10.1086/376920
2
Barbeau M. A. Scheibling R. E. (1994). Behavioral mechanisms of prey size selection by sea stars (Asterias vulgaris Verrill) and crabs (cancer irroratus say) preying on juvenile sea scallops (Placopecten magellanicus (Gmelin). J. Exp. Mar. Biol. Ecol.180, 103–136. doi: 10.1016/0022-0981(94)90082-5
3
Cavallini D. de Marco C. Scandurra R. Dupreí S. Graziani M. T. (1966). The enzymatic oxidation of cysteamine to hypotaurine. Purification properties enzyme. J. Biol. Chem.241, 3189–3196. doi: 10.1016/S0021-9258(18)96514-2
4
Funabara D. Watanabe D. Satoh N. Kanoh S. (2013). Genome - wide survey of genes encoding muscle proteins in the pearl oyster, Pinctada fucata. Zool. Sci.30, 817–825. doi: 10.2108/zsj.30.817
5
Gäde G. Weeda E. Gabbott P. A. (1978). Changes in the level of octopine during the escape responses of the scallop, Pecten maximus (L.). J. Comp. Physiol.124, 121–127. doi: 10.1007/BF00689172
6
Gao Z. K. (2016). Effects of environmental stresses on physiological, immunological parameters and behavioral characteristics of Patinopecten yessoensis. Shanghai Ocean University, Shanghai.
7
Guerra C. Zenteno - Savín T. Maeda - Martínez A. N. Abele D. Philipp E. E. R. (2013). The effect of predator exposure and reproduction on oxidative stress parameters in the Catarina scallop Argopecten ventricosus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology165 (1), 89–96. doi: 10.1016/j.cbpa.2013.02.006
8
Guo X. Luo Y. (2016). Scallops and scallop aquaculture in China. Developments Aquaculture Fisheries Sci.40, 937–952. doi: 10.1016/B978-0-444-62710-0.00022-5
9
Hao Z. L. Tang X. J. Ding J. Ben Y. Chang Y. Q. (2014). Survival rate, oxygen consumption rate and immune enzymetic activity of Mizuhopecten yessoensis at high temperature. Chin. J. Ecology.33, 1580.
10
Le Y. X. Zhou J. W. Ai X. H. Ke F. E. (1999). Influence of age, size and nutrition of Trionyx sinensis on the immune response. 375–380.
11
Li H. Li Q. Yu H. Du S. (2021). Characterization of paramyosin protein structure and gene expression during myogenesis in Pacific oyster (Crassostrea gigas). Comp. Biochem. Phys. B.255, 110594. doi: 10.1016/j.cbpb.2021.110594
12
Liu Y. F. (1983). Scallop (Patinopecten yessoensis) farming in Japan. Fish. Sci.1), 14–19.
13
Liu Z. H. Mou H. J. Wang Q. Y . (2003). Research progress of immune related enzymes in mollusca. Mar. Fish. Rev.24 (3), 86–90.
14
Livak K. J. Schmittgen T. D. (2001). Analysis of relative gene expression data using real - time quantitative PCR and the 2–ΔΔCT method. methods.25, 402–408. doi: 10.1006/meth.2001.1262
15
Livingstone D. R. Zwaan A. D. Thompson R. J. (1981). Aerobic metabolism, octopine production and phosphoarginine as sources of energy in the phasic and catch adductor muscles of the giant scallop Placopecten magellanicus during swimming and the subsequent recovery period. CBPB70, 35–44. doi: 10.1016/0305-0491(81)90120-6
16
Meng Q. Bao Z. Wang Z. Wang S. Hu J. Hu X. et al . (2012). Growth and reproductive performance of triploid yesso scallops (Patinopecten yessoensis) induced by hypotonic shock. J. Shellfish Res.31, 1113–1122. doi: 10.2983/035.031.0422
17
Miyoshi K. Kuwahara Y. Chiba S. (2019). Interactions between predatory sea stars (Asterias amurensis and Distolasterias nipon) and Japanese scallops (Mizuhopecten yessoensis) and implications for scallop seeding in mariculture. Aquaculture Res.50, 2419–2428. doi: 10.1111/are.14195
18
Morris S. Van Aardt W. J. Ahern M. D. (2005). The effect of lead on the metabolic and energetic status of the Yabby, Cherax destructor, during environmental hypoxia. Aquat. Toxicol.75, 16–31. doi: 10.1016/j.aquatox.2005.07.001
19
Prosdocimo D. A. Sabeh M. K. Jain M. K. (2015). Kruppel - like factors in muscle health and disease. Trends Cardiovas Med.25, 278–287. doi: 10.1016/j.tcm.2014.11.006
20
Schmidt M. Philipp E. E. R. Abele D. (2008). Size and age - dependent changes of escape response to predator attack in the Queen scallop Aequipecten opercularis. Mar. Biol. Res.4, 442–450. doi: 10.1080/17451000802270346
21
Shank B. V. Hart D. R. Friedland K. D. (2012). Post - settlement predation by sea stars and crabs on the sea scallop in the Mid - Atlantic Bight. Mar. Ecol. Prog. Ser.468, 161–177. doi: 10.3354/meps09974
22
Smits S. H. J. Tatu M. Andre M. Nadine V. O. Matthias S. Dieter W. et al . (2010). Insights into the mechanism of ligand binding to octopine dehydrogenase from pecten maximus by NMR and crystallography. PloS One5, e12312. doi: 10.1371/journal.pone.0012312
23
Tremblay I. Guderley H. E. Himmelman J. H. (2012). Swimming away or clamming up: the use of phasic and tonic adductor muscles during escape responses varies with shell morphology in scallops. J. Exp. Biol.215, 4131–4143. doi: 10.1242/jeb.075986
24
Wang W. M. Ji Y. E. Liu H. L. Sun P. F. (2012). Culture technology of scallop (Patinopecten yessoensis) in Haiyang sea area. J. Aquaculture.33, 37–38.
25
Xia Y. Y. (2010). Effects of Environmental Stress on the Survival, Behavior Metabolism and Immunity of Scallops. Shanghai Ocean University, Shanghai.
26
Yao C. L. Wang Z. Y. Xiang J. H. (2008). Structure and function of arginine kinase in crustacean. Chin. J. Biochem. Mol. Biol.24, 203–208. doi: 10.13865/j.cnki.cjbmb.2008.03.009
27
Zhang J. H. Xia Y. Y. Gao Z. K. Liu Y. Wu W. G. Zhang Z. X. (2021). Force production during shell clap of scallop Pationopecten yessoensis and its response to predator starfish. J. Fishery Sci. China28, 871–877. doi: 10.122674/JFSC2020-0510
Summary
Keywords
Yesso scallop, Mizuhopecten yessoensis , northern Pacific sea star, Asterias amurensis , shell clap behavior, enzyme activity, transcriptome analysis
Citation
Li X, Li X, Li D, Zhang D, Zhai X, Wang X, Mao J, Tian Y, Liu M, Chang Y and Hao Z (2025) Behavioral, physiological and genetic responses of the Yesso scallop Mizuhopecten yessoensis to the predation of sea star. Front. Mar. Sci. 12:1493892. doi: 10.3389/fmars.2025.1493892
Received
09 September 2024
Accepted
29 April 2025
Published
03 June 2025
Volume
12 - 2025
Edited by
Hongbo Jiang, Shenyang Agricultural University, China
Reviewed by
Jinghui Fang, Chinese Academy of Fishery Sciences (CAFS), China
Zhichuang Lu, Liaoning Ocean and Fisheries Research Institute, China
Updates
Copyright
© 2025 Li, Li, Li, Zhang, Zhai, Wang, Mao, Tian, Liu, Chang and Hao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenlin Hao, haozhenlin@dlou.edu.cn; Ming Liu, ming.liu@morgan.edu; Yaqing Chang, yqchang@dlou.edu.cn
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.