- 1Greenpeace Research Laboratories, University of Exeter, Exeter, United Kingdom
- 2Hatherly Laboratories, Biosciences, University of Exeter, Exeter, United Kingdom
- 3Greenpeace Greece, Athens, Greece
- 4Greenpeace Bulgaria, Sofia, Bulgaria
The Clarion Clipperton Zone (CCZ) of the Eastern Pacific is an ~ 6 million km2 abyssal area punctuated by seamounts. The CCZ is a focus for potential mining, although this is not, as yet, a commercial reality. Records from online repositories and field guides suggest that up to 30 cetacean species are present in the CCZ, though dedicated surveys have yet to be published. We report the results of a passive acoustic survey for cetaceans conducted over 13 days during summer 2023 in two blocks of the CCZ earmarked for deep seabed mining – NORI-d and TOML-e. The areas surveyed had a mean depth of 4259 m, with no charted seamounts, with 4,328 km of survey effort (273 hours of continuous recordings). In total, there were 74 acoustic detections, with six visual encounters. We report the presence of a sperm whale (Physeter macrocephalus) (one individual), Risso’s dolphins (Grampus griseus) (two groups) and common dolphins (Delphinus delphis) (one group, confirmed by visual sighting). We also acoustically encountered 70 dolphin groups that could not be identified to species level. No baleen whales, kogiids or beaked whales were detected during this short survey. Beaked whales are challenging to detect, such that a lack of detections cannot be taken to confirm the absence of such species. We confirm one threatened species present in these blocks of the CCZ – sperm whales – and suggest that more extensive data are urgently needed to understand the risk of harm to cetaceans that may arise from human activities, including deep-sea mining.
1 Introduction
There is increasing commercial interest in exploiting mineral resources from the deep seafloor, driven, in part, by a perceived need for raw materials for the ‘green transition’ (Miller et al., 2021). The mineral resources of greatest interest are (i) polymetallic nodules on the abyssal plains; (ii) seafloor massive (polymetallic) sulfides at hydrothermal vents; and (iii) ferromanganese crusts on the flanks of seamounts. The Clarion Clipperton Zone (CCZ) is an abyssal region punctuated by seamounts, with an overall average depth of 5500 m spanning approximately 6 million km2 of the Eastern Pacific. Within this area, 17 contracts for exploration of seabed mineral resources have so far been granted by the International Seabed Authority, the United Nations body with responsibility for deep sea mining activities in areas beyond national jurisdiction. Thus far, mining is still not a commercial reality, but its proponents are continuing efforts to develop the industry as quickly as possible, even in the face of mounting concern as to the ecological and other risks it may pose (Miller et al., 2018; Levin et al., 2020; Niner et al., 2018; Crane et al., 2024). One argument put forward by some authors is that deep seabed mining is preferable to terrestrial mining in that it could have lower ecological and ethical impacts (Katona et al., 2022). However, it is unlikely that deep-sea mining would substantially displace terrestrial mining operations, such that, given current demand projections, these two sectors would more likely emerge as competitors rather than substitutes (Miller et al., 2021; Crane et al., 2024). The deposits targeted within the CCZ region are primarily manganese nodules, which would be harvested by seafloor collection vehicles in combination with a vertical transport system to a surface vessel where dewatering of products would facilitate the separation of ore-bearing materials (Miller et al., 2018; Levin et al., 2020). Following initial onboard processing, discharge of sediments and water would likely occur either at the seabed and/or within the water column.
Environmental concerns are diverse, including the direct removal of sessile organisms, alteration of seabed integrity, light pollution, sediment plumes and noise (for examples, see Miller et al., 2018; Christiansen et al., 2020; Drazen et al., 2020; Williams et al., 2022). The ecological impacts of nodule mining on sessile and other deep sea megafauna (typically >1 cm long) have begun to be investigated (for example, Ardron et al., 2019; Simon-Lledó et al., 2019). However, there are still major knowledge gaps relating to ecosystem functioning in the Clarion Clipperton Zone, including how the nodules themselves may be involved in dark oxygen production (Sweetman et al., 2024).
Some of the impacts from future deep seabed mining could potentially result in far-field effects in addition to direct impacts in the mining zones themselves. The potential impacts from deep seabed mining on cetaceans are likely to be varied and could relate to noise emissions, sediment plumes and their effect on food webs and to alteration of seamount ecosystems (Williams et al., 2022; Thompson et al., 2023). Williams et al. (2022) constructed predictive models of mining noise which would likely propagate through the water column and potentially across large distances from the source. The authors suggest that sounds from mid-water pumps, for example, could reach the Sound Fixing and Ranging (SOFAR) channel at ~1000 km deep, and propagate across ocean basins. Gillard et al. (2022) investigated the potential impact of sediment plumes as a result of mining in the German sector of the CCZ. The authors stated that most suspended organic particles in this region are small and settle slowly and an increase in fine lithogenic sediment particles from mining could disturb the benthic-pelagic coupling. Numerous seamounts are present in the CCZ and, given that the region overall is a large area, more information is needed on the distribution of deep water and migratory species, in addition to details of migratory pathways, diet and habitat use by cetaceans.
Few dedicated surveys for cetaceans in this area have been published, but sparse records from online repositories, such as OBIS-Seamaps (https://seamap.env.duke.edu/), show at least 16 cetacean species are present in the CCZ at some time during the year and field guides suggest that there could be up to 30 species found in this region (Carwardine, 2020; Thompson et al., 2023). Niu et al. (2021) investigated the CCZ soundscape using a static hydrophone at 300 m deep (during 2017-2018) and recorded fin whales (Balaenoptera physalus) throughout the year and blue whales (Balaenoptera musculus) during September–December and April–May. These data suggest that there may be several threatened species using this area, for at least at some time during the year.
In this study, we used passive acoustic monitoring from the vessel MY Arctic Sunrise to survey a ~41,000 km2 region of the CCZ for cetaceans in August 2023. A non-systematic visual survey was also conducted to complement the acoustic survey. The survey area comprised two deep-sea mining exploration blocks, NORI-d (Nauru Ocean Resources Inc. – The Metals Company) and TOML-e (Tonga Offshore Mining Ltd. – The Metals Company), both located within the broader CCZ area. The primary aim of the survey was to provide a snapshot of cetacean presence in a region of the CCZ earmarked for future deep-sea mining.
2 Methods
2.1 Survey design and data collection
An equal-spaced complementary zigzag design was created using the ‘dssd’ package (version 1.0.0, Marshall and Rexstad, 2022) in R (version 4.2.2, R Core Team, 2022) following recommendations in Buckland et al. (2004); Strindberg and Buckland (2004) and Buckland et al. (2015). The survey was designed to provide a near-even coverage probability of the survey area whilst also creating an efficient track line for the survey vessel (total design length 4,221 km) (Table 1). Previous acoustic studies using the Arctic Sunrise have estimated the combined hazard rate detection functions for sperm whales and non-narrow band high frequency (non-NBHF) delphinids (Webber et al., 2022). Effective strip half-width (ESHW) estimates for the vessel are 3,277 m for sperm whales and 699 m for non-NBHF delphinids. Coverage probability was simulated using 1,000 sets of transects through the survey area, resulting in a mean coverage score of 0.65 (Sd ± 0.06). Given the open ocean nature of the survey area (mean depth = 4259 m) with no clear stratification, survey lines were oriented for an efficient start and end point for the vessel’s transit from and to ports.
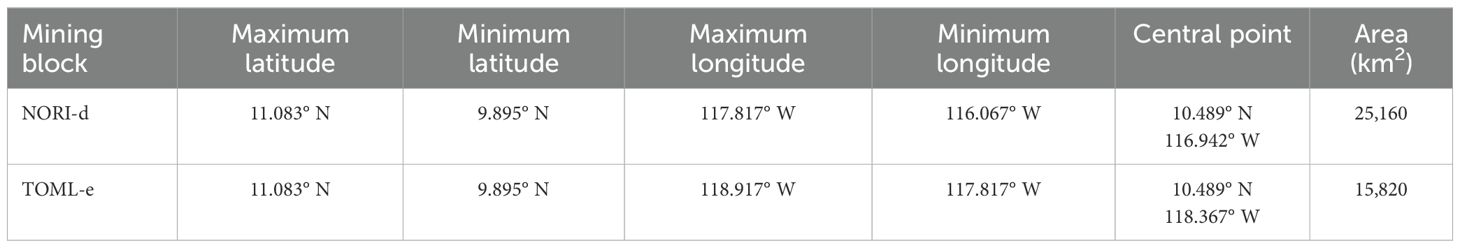
Table 1. Extent and summary of the two exploration blocks within the Clarion-Clipperton Fracture Zone surveyed. Latitudes and longitudes are given in decimal degrees.
Acoustic data were collected from the Arctic Sunrise using a hydrophone array comprising four hydrophone elements (Vanishing Point, United Kingdom) and towed using a 350 m Kevlar-strengthened cable. Two elements formed a medium frequency pair spaced 3 m apart (Benthos AQ4 elements and Magrec HP02 preamplifiers, nominal frequency range 50 Hz to 40 kHz), and two formed the high frequency pair spaced 50 cm apart (Magrec HP03 hydrophone and preamplifier units, nominal frequency range 1 kHz to 200 kHz). Each hydrophone element was connected to a four-channel data acquisition card (St Andrews Instrumentation, UK) where analogue gain and filtering were applied (Medium frequency pair: 10 Hz high pass filter and 6 dB of gain. High frequency pair: 2000 Hz high pass filter and 12 dB of gain). All four channels were digitally sampled at 500 kHz and written to 16-bit lossless ‘.wav’ files using PAMGuard (Gillespie et al., 2009, www.pamguard.org).
A non-systematic visual survey was also conducted during daylight hours (07:00−18:00 hr local time), collecting, where possible, data on species identity, numbers of animals, location and behaviour. Observers performed one-hour watches throughout the survey period, located on the vessel bridge wings, scanning using both binoculars and the naked eye throughout the watch. At the beginning and end of every watch, or if any change was noted, the following environmental and effort variables were recorded: effort status (on or off-effort, depending on whether there was an observer on station), observer identity, vessel position, vessel speed over ground, Beaufort sea state, depth (from chart), water temperature (taken by the bridge staff every four hours using a liquid thermometer), swell height and direction, visibility, glare and rain. Few observers had previous cetacean visual survey experience; therefore, the survey was considered as opportunistic. When cetaceans were observed, the following data were recorded: date, time (local), initial observer identity, effort status, ship’s heading, position, depth, sighting method (naked eye or binoculars), initial sighting cue (blow, surface activity, body), bearing to the animal, closest distance (estimated), group size (minimum/maximum/best guess), presence of calves, species (lowest taxonomic group possible) and confidence of species identity (definite/probable/possible). Species identity was determined using Carwardine (2020), and photographs were taken where possible to aid in species identification.
2.2 Acoustic processing
Acoustic ‘.wav’ files were processed on-shore using PAMGuard based on the methods outlined in Webber et al. (2022). Firstly, a click detector was implemented on data from the high frequency hydrophone pair with a trigger filter of 16 dB and an angle veto of 0° to 20° to remove the majority of click detections originating from the towing vessel. Two narrowband click classifiers with frequency sweeps were used to aid in the identification of beaked whales (test bands of 24–48 kHz and 48–80 kHz), and a third narrowband click classifier was used to aid in the identification of NBHF species such as Kogia spp. (test band of 100 to 150 kHz). Secondly, a whistle and moan detector was run to detect tonal vocalisations of odontocetes and baleen whales up to 24 kHz on the medium frequency hydrophone pair using settings provided in Gillespie et al. (2013). Finally, given its successful performance in the Pacific, a deep learning classifier developed by Allen et al. (2021) was used in an attempt to detect humpback whale vocalisations up to 2 kHz. The deep learning model was implemented within PAMGuard using spectrogram segments with a window length of 3.84 seconds and overlap of 50% as outlined in Allen et al. (2021).
2.3 Manual audit
All acoustic detections were manually verified in PAMGuard Viewer after detectors had run. Clicks were grouped into distinct click trains and assigned to species group, where possible, using click characteristics, for example the upsweep of beaked whale clicks (Griffiths et al., 2020; Yack et al., 2010). Click trains were localised using the target motion analysis module within PAMGuard using the 2D simplex method, where possible. Whistle contours were manually verified using the spectrogram annotation module and assigned to either delphinid or noise. Delphinid whistle contours were then fed into the ROCCA Temperate Pacific whistle classifier to assign whistle detections (those within the time and frequency limits of spectrogram annotations) to a single species (Oswald et al., 2007). Any whistle with a likelihood score below the threshold of 0.8 (out of 1) was determined to be an unidentified delphinid. Species included in this whistle classifier were: common dolphin, Risso’s dolphin (Grampus griseus), short-finned pilot whale (Globicephala macrorhynchus), Pacific white-sided dolphin (Lagenorhynchus obliquidens), killer whale (Orcinus orca), striped dolphin (Stenella coeruleoalba), and bottlenose dolphin (Tursiops truncatus). All delphinid click events were examined for the presence of spectral banding which has been shown to be present in two species present in the survey region (Soldevilla et al., 2008; Soldevilla et al., 2017) – Risso’s dolphin and Pacific white-sided dolphin. In events where spectral banding was present, consistent peaks and notches within the click spectrum of all clicks within the event were examined using a univariate Gaussian Mixed Model (uGMM), along with the inter-click interval (ICI). Using the consistent spectrum values presented by Soldevilla et al (2008; 2017), events with spectral banding characteristics were assigned to the species level. Humpback whale classifications made by the deep learning module which were above the 0.8 threshold were manually verified as being from a humpback whale or not.
3 Results
Between 1 August and 13 August 2023, 273 hours of acoustic recordings were collected. An average speed of 9.5 kts was achieved during acoustic data collection. A total of 74 acoustic detections were made of cetacean groups during the 13 days of the survey (Figure 1). A single sperm whale was detected. Two of the delphinid click events within the CCZ survey region showed the presence of spectral banding. Both events were classified as Risso’s dolphin based on their consistent spectral peaks at 22, 26, 31 and 38 kHz (Soldevilla et al., 2008; Soldevilla et al., 2017) (Figure 2). A single group of common dolphins were acoustically detected, and species identity obtained from a concurrent visual sighting. The ROCCA whistle classifier was unable to provide a robust species classification (likelihood score ≥0.8) for 70 of the acoustic encounters of dolphins; therefore these have been recorded as unidentified delphinids. The deep learning humpback whale classifier did not detect any humpback whale calls with a confidence greater than 0.8, and upon manual checking, all detections were determined to have been triggered by the vessels own propulsion system.
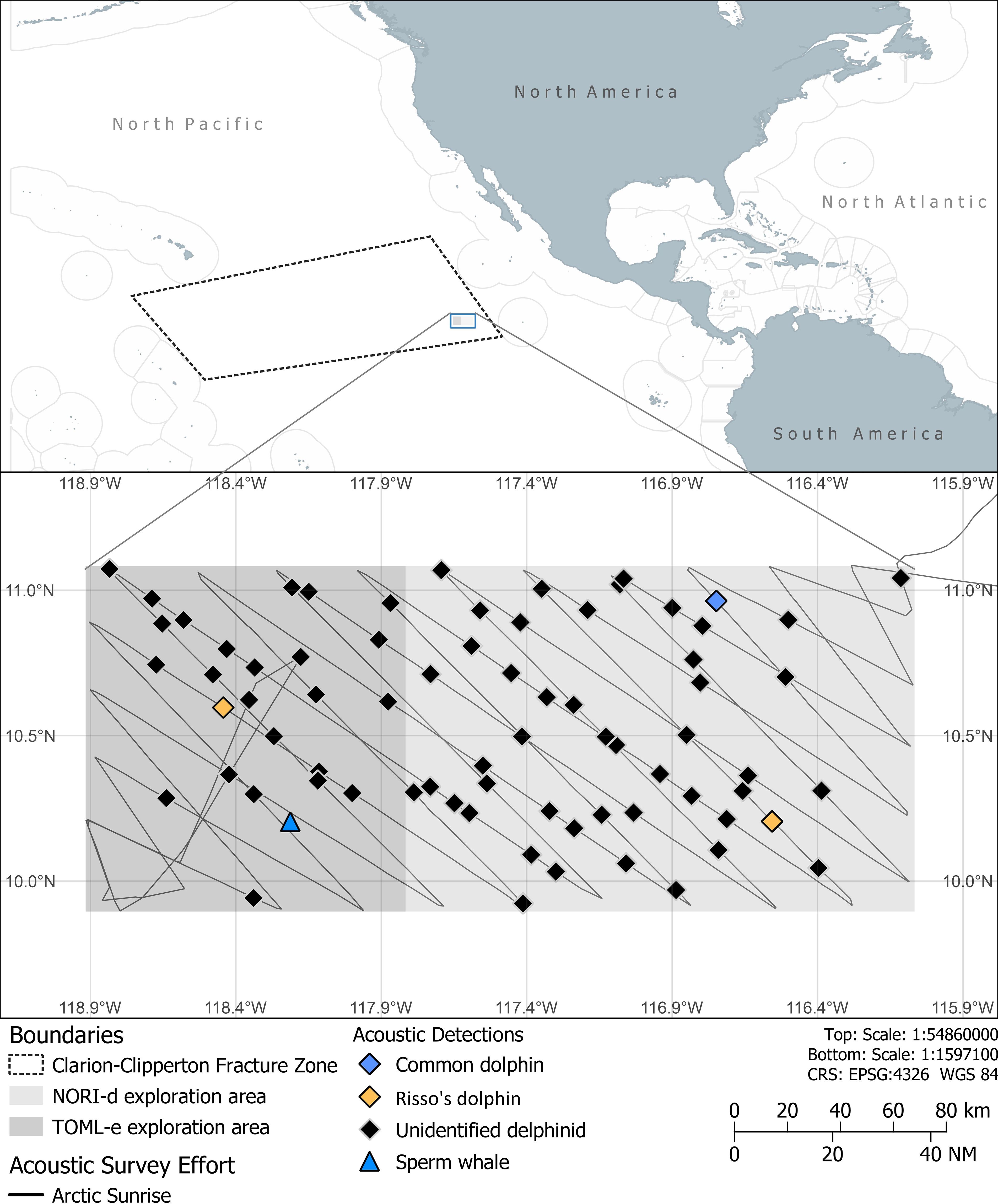
Figure 1. Acoustic detections and visual sightings of cetaceans within the NORI-d and TOML-e explorations zones collected by the MY Arctic Sunrise between the 1 August and 13 August 2023.
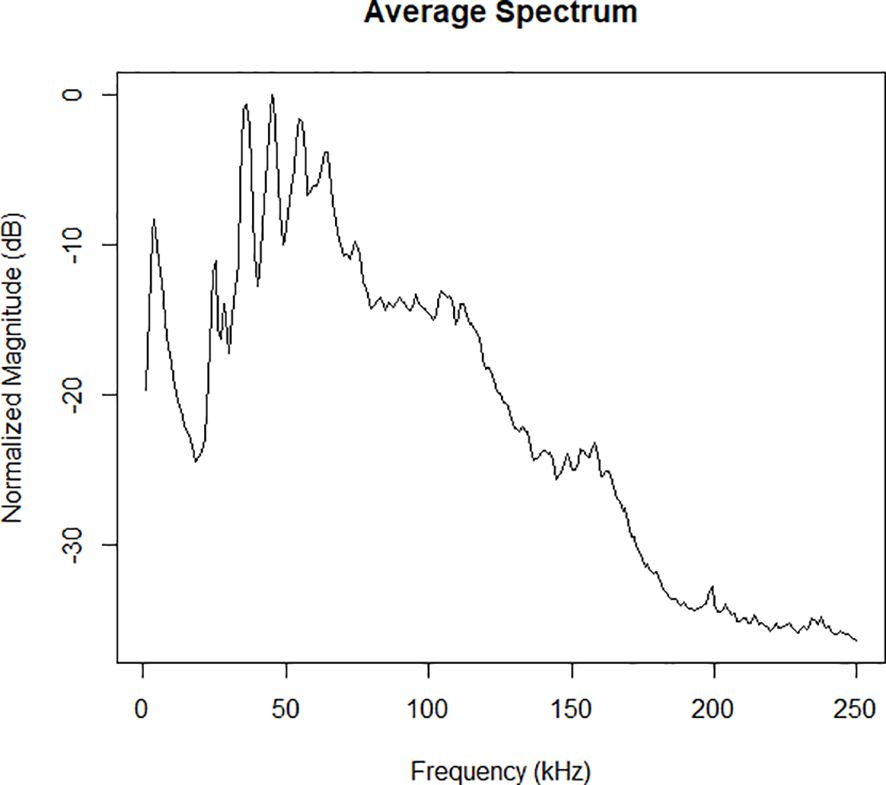
Figure 2. An example of the spectral banding characteristic in an average click spectrum used to classify a delphinid encounter as Risso’s dolphin (Grampus griseus).
There were six sightings of cetaceans during the survey, of which one was classified to species level (Table 2). One of the other five sightings overlapped with acoustic recordings, a group of common dolphins. Throughout the 13 days of survey, weather conditions were typified by sea state of Beaufort 3, low swell height (<2 m), and good visibility (>5 km).
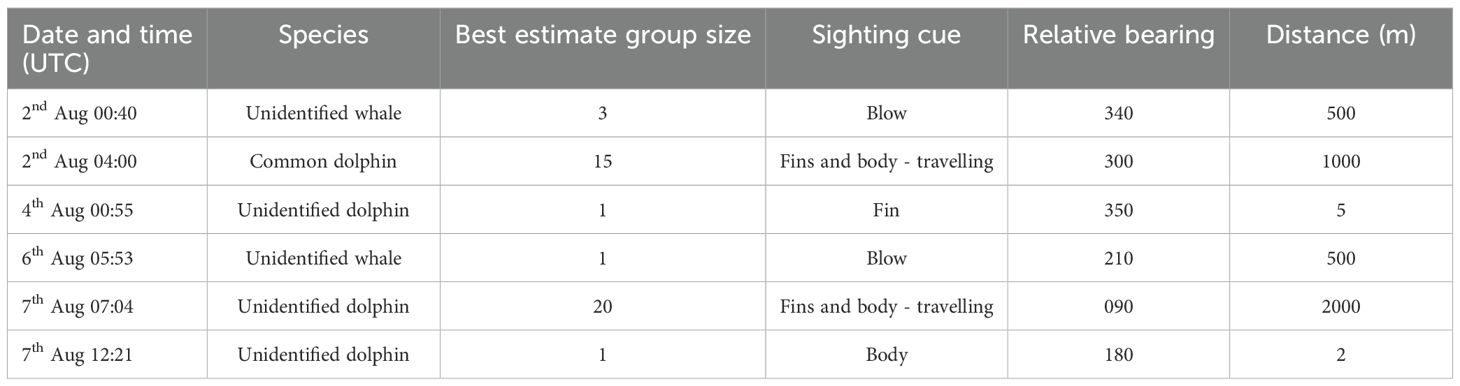
Table 2. Summary of cetacean sightings during the survey of the NORI-d and TOML-e exploration blocks between 1 and 13 August 2023 on board the MY Arctic Sunrise.
4 Discussion
We provide a snapshot of cetacean presence within an ~41,000 km2 of the CCZ earmarked for future commercial scale deep-sea mining, based on acoustic effort over 13 days and 4,328 km of track line during August 2023. A total of 74 acoustic detections included: a single sperm whale, two groups of Risso’s dolphins (based on spectral banding characteristics) and common dolphins. There were no visual sightings to confirm the classification of the two Risso’s dolphin encounters. However, the matching spectral characteristics of these events compared to those described by Soldevilla et al (2008; 2017), along with the typically coastal distribution of Pacific white-sided dolphins, provides a high degree of confidence to these classifications. The remaining 70 acoustic detections were classed as unidentified dolphins, as none achieved a conservative likelihood threshold of ≥0.8 (Webber et al., 2022). No large baleen whale vocalisations were detected, though two sightings of blows were attributed to unidentified whales. No beaked whales or Kogia spp. were detected either visually or acoustically during the survey.
Given previous data on the presence of beaked whales and Kogia spp. in the CCZ, it is surprising that none were detected during the survey in August 2023 (Thompson et al., 2023). The study location has a mean depth of 4259 m with few bathymetric features. Many odontocetes forage in the bentho-pelagic habitats surrounding steep slopes where waters are typically nutrient-rich. These regions may serve as ecotones where pelagic and benthic domains intersect. Beaked whales are thought to occupy such shelf break habitats, with slope being an important habitat feature, including those around oceanic islands and seamounts (Waring et al., 2001; Henderson et al., 2016; MacLeod and Zuur, 2005; Virgili et al., 2022). However, modelling work by Ferguson et al. (2005) indicated that, at least in the Eastern Tropical Pacific, beaked whale distribution may be less confined to these productive regions, and they are predicted to inhabit areas ranging from the continental slope to the deeper waters of the abyssal plains. Therefore, it is not entirely clear whether deep diving species, such as beaked whales, were absent from the area or whether the method used was not effective in detecting them – i.e., whether our result is false-negative or true-negative. The distribution of these species, and any potential seasonal changes in this deep-sea region, are unknown and clearly more survey effort is needed.
The probability of detecting an acoustically active, deep-diving whale using passive acoustic monitoring is dependent on a combination of factors, including distance of the whale from the receiver, ambient and self-noise, source level of the whale’s clicks (phase of their dive, whether in ascent or descent, group size) and the characteristics of the hydrophone array and receiver (Zimmer et al., 2008). For sperm whales, where detection probabilities have been estimated for the Arctic Sunrise, the ESHW was 3,277 m, indicating that a vocally active sperm whale within 1,500 m of the track line would be detected. Other studies with similar methods have reported ESHW’s of 4.2–10 km, much farther than those of the Arctic Sunrise (Fais et al., 2015; Gordon et al., 2020; Lewis et al., 2018). For beaked whales (and Kogia spp.), the efficiency of detection by the Arctic Sunrise acoustic monitoring system is likely to be much less than that for sperm whales. Zimmer et al. (2008) used data derived from acoustic recording tags on Cuvier’s (Ziphius cavirostris) and Blainville’s beaked whales (Mesoplodon densirostris) to determine variability in detection distances from hydrophones. The study found that whales diving within 0.7 km from the receiver were likely to be detected, but those >4 km were not. Tyack et al. (2006) suggest that foraging Cuvier’s beaked whales echolocate in regular 30-minute bouts during foraging, but can remain silent for up to 110 minutes, meaning that a listening time of 140 minutes is required to improve detection probabilities. In this survey, the Arctic Sunrise averaged 9.5 kts and, therefore, travelled ~22 nm (or 41 km) during a 140-minute period, far beyond the acoustic detection range of a foraging beaked whale. Foraging whales may have been easily missed along the track line if the ship sailed over them during the silent phase of their dive. Also key is that these whales emit regular clicks on the descent phase of a deep dive, circa 400–500 m deep above the foraging layer, with clicks orientated downwards. Therefore, clicks are likely only to be detected by hydrophones below the foraging layer at depth, and/or very close to hydrophones due to their high rate of attenuation.
There are few data on beaked whale foraging behaviour in open ocean abyssal habitats such as those surveyed here. Barlow et al. (2021) analysed the depth profiles of 19 satellite-tagged Cuvier’s beaked whales in habitats of different mean depths. The authors found that the whales generally foraged within 200 m of the bottom in depths <2000 m, but for deeper habitats of 2000–4000 m, foraging depths were ~1200 m deep, similar to those recorded in shallower waters. Several authors, however, have speculated that gouges in the seafloor in deep ocean habitats could be attributed to foraging beaked whales (Woodside et al., 2006; Auster and Watling, 2009), including those found within the CCZ (Marsh et al., 2018). Whether individual beaked whales are foraging at such depths in the CCZ is unknown, but presumably the likelihood of acoustic detection will be highly variable and dependent on the phase of their dive, the depth of the whale in relation to the vessel, ambient noise in terms of other species with overlapping frequencies (e.g. dolphins) and the speed and noise of the survey vessel.
Zimmer et al. (2008) advise that hydrophones mounted on a quiet, slow-moving platform, such as drifters or gliders, rather than a fast-moving vessel, will significantly increase the effectiveness of acoustic detection of beaked whales. The Arctic Sunrise is a relatively noisy ship in comparison to other survey vessels, with a variable pitch propeller that has high levels of cavitation noise at <8 kts (KY, pers. obs.). Survey speeds for the vessel are generally between 8–10 kts to reduce self-noise. Presumably the effect of vessel noise on detection probabilities–similar as for sperm whales—will hold for other species and our ability to detect beaked whales will depend on them being relatively close to the vessel, much less than 0.7 km as determined by Zimmer et al. (2008). They must also be vocalising toward the receiver on the towed array, when other ambient sources of sound, such as dolphins, are not masking their high frequency vocalisations. We suggest that further CCZ surveys specifically tailored to beaked whales are required, potentially using multiple drifting devices or gliders. This would help to elucidate their distributions, densities and habitat use, particularly as this group is known to be highly sensitive to noise (Klinck et al., 2012; Hooker et al., 2019; Barlow et al., 2021; McCullough et al., 2021). Such devices would also be hugely valuable for monitoring Kogia spp.
Regarding delphinid detections, only three were identified to species level with two species – common dolphins and Risso’s dolphins – though there were unidentified delphinids detected across the study area. No baleen whales were acoustically detected during the survey, though there were two sightings of whale blows that could not be identified. Noting the continuous (thoughout the year) acoustic detections of fin whales reported by Niu et al. (2021) for the CCZ, it is plausible that the blows witnessed during our survey could also have been from fin whales, but more data would have been needed to confirm this. No verified detections of humpback whales were found using the PAMGuard detector, although we would not expect significant numbers of these whales, given they would most likely be on their high-latitude summer feeding grounds at the time of the survey (Calambokidis et al., 2001). Niu et al. (2021) also reported a lack of humpback whale acoustic detections in their study of the CCZ. The number of false positive detections of humpbacks using the deep learning humpback whale classifier (Allen et al., 2021) is likely due to the detector being trained on static recorders and, as such, training recordings do not contain the same level of noise in the lower frequency range as the towed survey here.
4.1 Concerns on the impact on cetaceans from deep-sea mining in the Clarion Clipperton Zone
Our survey documented an acoustic encounter with one threatened species, according to the IUCN Red List of Threatened Species – the sperm whale which is listed as Vulnerable (Taylor et al., 2019). Many more species, some with elevated threat status, are likely to be present in the region at least at some time during the year (Niu et al., 2021; Thompson et al., 2023). Our survey did not detect any beaked whales or Kogia spp., and we cannot be sure whether these species are rare or absent from this region at this time of year, or whether the towed array was not effective in detecting them. There are at least eight beaked whale species (Carwardine, 2020) that are thought to be present within the CCZ and it is important that further survey effort is carried out to estimate their distributions and densities.
Cetaceans, including sperm whales and beaked whales, are known to be impacted by anthropogenic noise and may be at risk from sounds emitted by future deep-sea mining operations if they are allowed to become a commercial reality (Williams et al., 2022). Models used by Williams et al. (2022) predict that the acoustic environment surrounding commercial scale deep-sea mining will be significantly altered throughout the water column, from the surface to the seabed, even using the incomplete list of activities modelled. Broadly, the conservative estimates of Williams et al. (2022) suggest that mining noise from a single operation could significantly increase ambient sound levels to a range of ~500 km, with certain sounds potentially interacting with the SOFAR channel to reach across ocean basins. The model further suggests that, within the CCZ alone, there are at least 14 locations where the Level B harassment threshold for continuous sound (NOAA, 2005) for cetaceans (120 dB re 1 μPa) will be exceeded. This increase in sound could impact odontocete navigation or foraging success in these locations, with potential for knock-on effects on individual fitness or vital rates. How these effects on individuals would translate to broader population impacts remains uncertain. A study by Carlucci et al. (2024) indicates that Risso’s dolphins may be sensitive to certain anthropogenic sounds, with ambient noise below 1 kHz and between 20 kHz and 63 kHz altering dolphin click trains, changing inter click intervals and the amplitude of clicks. The authors point out that changes in these vocalisations are concerning as dolphins rely on them for both navigation and searching for prey.
Sound is far from the only concern in terms of impacts on cetaceans. Deep seabed mining will generate sediment plumes at the seabed and in the water column. Such plumes could have ecological effects on deep midwater (Drazen et al., 2020) and pelagic ecosystems (Gillard et al., 2022; Stenvers et al., 2023). Mesopelagic food webs provision cetaceans and their prey, as well as providing significant ecosystem services for humans. Odontocete prey (for example, tuna, squid and myctophid fish species) make deep dives from the epipelagic to the mesopelagic, where they feed on plankton and micronekton, connecting pelagic food webs (Choy et al., 2013, 2015; Olson et al., 2014; Watanabe et al., 1999, 2006). More focus on understanding how mining plumes could disrupt deep ocean food webs is urgently needed.
5 Conclusions
Fragmented governance of the deep oceans in relation to deep sea mining currently limits coordinated effort to protect cetaceans and other species that inhabit these vast offshore regions (Thompson et al., 2018). Here we provide some of the first survey data on cetacean presence in the CCZ. Considering the triggering of the ‘two-year rule’ within the ISA, and the subsequent push from contractors and sponsoring governments to make commercial scale mining in the CCZ a reality in the near future, we argue that the data available on cetacean distributions, habitat use and densities in this area are currently insufficient to determine the nature and scale of potential impacts (Thompson et al., 2023). Our study is a survey of a data-poor region and should be considered when planning and regulating deep seabed mining. In addition, we highlight opportunities for collaboration across intergovernmental organisations (for example, the International Whaling Commission, Convention on the Conservation of Migratory Species of Wild Animals), scientists, non-governmental organisations and regional seas agreements to provide a coordinated response to prevent future threats of mining on cetaceans. Data on cetacean ecology and conservation are heavily biased to near-shore areas, due to the logistical challenges of studying these species in their offshore habitats. In addition, the designation of Important Marine Mammal Areas is hugely important for cetacean conservation but to fulfil selection criteria regions must be relatively data rich. Importantly, a lack of data on oceanic cetaceans should not result in destructive human activities with chronic effects being permitted to become a commercial reality in distant marine realms.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the study uses passive acoustic monitoring only and therefore is entirely non-invasive. No animals were approached during the survey.
Author contributions
KY: Conceptualization, Data curation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. TW: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. LK: Data curation, Project administration, Writing – review & editing. SM: Data curation, Writing – review & editing. GO: Conceptualization, Writing – review & editing. DS: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing. PJ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The preparation of this manuscript was funded by Greenpeace to provide independent scientific advice and analytic services to that non-governmental organisation.
Acknowledgments
We thank the crew of the MY Arctic Sunrise and Jonathan Gordon, as well as members of the Scientific Committee of the International Whaling Commission, particularly the Subcommittee for Environmental Concerns. We also thank Naomi Rose for her helpful comments on the paper submitted to the committee meeting and the correspondence group on deep sea mining and cetaceans. Many thanks also to Michaela Alksne and Simone Bauman-Pickering at University of California San Diego for their help with distinguishing between Risso’s dolphin and Pacific white-sided dolphin vocalisations. In addition, we thank two reviewers for their helpful comments that improved the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allen A. N., Harvey M., Lauren Harrell L., Jansen A., Karlina P. Merkens K. P., Wall C. C., et al. (2021). A convolutional neural network for automated detection of humpback whale song in a diverse, long-term passive acoustic dataset. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.607321
Ardron J. A., Simon-Lledó E., Jones D. O. B., and Ruhl H. A. (2019). Detecting the effects of deep-seabed nodule mining: Simulations using megafaunal data from the Clarion-Clipperton Zone. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00604
Auster P. J. and Watling L. (2009). Beaked whale foraging areas inferred by gouges in the seafloor. Mar. Mamm. Sci. 26, 226–233. doi: 10.1111/j.1748-7692.2009.00325.x
Barlow J., Fregosi S., Thomas L., Harris D., and Griffiths E. T. (2021). Acoustic detection range and population density of Cuvier’s beaked whales estimated from near-surface hydrophones. J. Acoust. Soc Am. 149, 111–125. doi: 10.1121/10.0002881
Buckland S. T., Anderson D. R., Burnham K. P., Laake J. L., Borchers D. L., and Thomas L. (2004). Advanced Distance Sampling: Estimating abundance of biological populations (Oxford University Press: Oxford, UK).
Buckland S. T., Rexstad E. A., Marques T. A., and Oedekoven C. S. (2015). Distance Sampling: Methods and Applications [Online] (Cham: Springer International Publishing). Available at: http://link.springer.com/10.1007/978-3-319-19219-2.
Calambokidis J., Steiger G. H., Straley J. M., Herman L. M., Cerchio S., Salden D. R., et al. (2001). Movements and population structure of humpback whales in the North Pacific. Mar. Mamm. Sci. 17, 769–794. doi: 10.1111/j.1748-7692.2001.tb01298.x
Carlucci R., Cipriano G., Bonato M., Buscaino G., Crugliano R., Fanizza C., et al. (2024). Anthropogenic noise effects on Risso’s dolphin vocalizations in the Gulf of Taranto (Northern Ionian Sea, central Mediterranean Sea). Ocean Coast. Manage 254, 107177. doi: 10.1016/j.ocecoaman.2024.107177
Choy C. A., Popp B. N., Hannides C. C. S., and Drazen J. C. (2015). Trophic structure and food resources of epipelagic and mesopelagic fishes in the North Pacific subtropical Gyre ecosystem inferred from nitrogen isotopic compositions. Limnol. Oceanogr. 60, 1156–1171. doi: 10.1002/lno.10085
Choy C. A., Portner E., Iwane M., and Drazen J. C. (2013). Diets of five important predatory mesopelagic fishes of the central North Pacific. Mar. Ecol. Prog. Ser. 492, 169–184. doi: 10.3354/meps10518
Christiansen B., Denda A., and Christiansen S. (2020). Potential effects of deep seabed mining on pelagic and benthopelagic biota. Mar. Pol. 114, 103442. doi: 10.1016/j.marpol.2019.02.014
Crane R., Laing C., Littler K., Moore K., Roberts C., Thompson K. F., et al. (2024). Deep sea mining poses and unjustifiable environmental risk. Nat. Sustain. 7, 836–838. doi: 10.1038/s41893-024-01326-6
Drazen J. C., Smith C. R., Gjerde K. M., Haddock S. H., Carter G. S., Choy C. A., et al. (2020). Midwater ecosystems must be considered when evaluating environmental risks of deep-sea mining. PNAS 117, 17455–17460. doi: 10.1073/pnas.2011914117
Fais A., Aguilar Soto N., Johnson M., Pérez-González C., Miller P. J. O., and Madsen P. T. (2015). Sperm whale echolocation behaviour reveals a directed, prior-based search strategy informed by prey distribution. Behav. Ecol. Sociobiol. 69, 663–674. doi: 10.1007/s00265-015-1877-1
Ferguson M. C., Barlow J., Reilly S. B., and Gerrodette T. (2005). Predicting Cuvier’s (Ziphius cavirostris) and Mesoplodon beaked whale population density from habitat characteristics in the eastern tropical Pacific Ocean. J. Cetacean Res. Manage. 7, 287–299. doi: 10.47536/jcrm.v7i3.738
Gillard B., Harbour R. P., Nowald N., Thomsen L., and Iversen M. H. (2022). Vertical distribution of particulate matter in the Clarion Clipperton Zone (German sector)—potential impacts from deep-sea mining discharge in the water column. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.820947
Gillespie D., Caillat M., Gordon J., and White P. (2013). Automatic detection and classification of odontocete whistles. J. Acoust. Soc Am. 134, 2427–2437. doi: 10.1121/1.4816555
Gillespie D., Mellinger D., Gordon J., McLaren D., Redmond P., McHugh R., et al. (2009). PAMGuard: semiautomated, open source software for real-time acoustic detection and localisation of cetaceans. J. Acoust. Soc Am. 125, 2547. doi: 10.1121/1.4808713
Gordon J., Gillespie D., Leaper R., Lee A., Porter L., O’Brien J., et al. (2020). A first acoustic density estimate for sperm whales in Irish offshore waters. J. Cetacean Res. Manage. 21, 123–133. doi: 10.47536/jcrm.v21i1.187
Griffiths E. T., Archer F., Rankin S., Keating J. L., Keen E., Barlow J., et al. (2020). Detection and classification of narrow-band high frequency echolocation clicks from drifting recorders. J. Acoust. Soc Am. 147, 3511–3522. doi: 10.1121/10.0001229
Henderson E. E., Martin S. W., Manzano-Roth R., and Matsuyama B. M. (2016). Occurrence and habitat use of foraging Blainville’s beaked whales (Mesoplodon densirostris) on a US navy range in Hawaii. Aquat. Mamm. 42, 549. doi: 10.1578/AM.42.4.2016.549
Hooker S. K., De Soto N. A., Baird R. W., Carroll E. L., Claridge D., Feyrer L., et al. (2019). Future directions in research on beaked whales. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00514
Katona S., Paulikas D., and Stone G. S. (2022). Ethical opportunities in deep-sea collection of polymetallic nodules from the Clarion-Clipperton Zone. Integr. Environ. Asses. 18, 634–654. doi: 10.1002/ieam.4554
Klinck H., Mellinger D. K., Klinck K., Bogue N. M., Luby J. C., Jump W. A., et al. (2012). Near-real-time acoustic monitoring of beaked whales and other cetaceans using a Seaglider™. PloS One 7, e36128. doi: 10.1371/journal.pone.0036128
Levin L. A., Amon D. J., and Lily H. (2020). Challenges to the sustainability of deep-seabed mining. Nat. Sustain. 3, 784–794. doi: 10.1038/s41893-020-0558-x
Lewis T., Boisseau O., Danbolt M., Gillespie D., Lacey C., Leaper R., et al. (2018). Abundance estimates for sperm whales in the Mediterranean Sea from acoustic line-transect surveys. J. Cetacean Res. Manage. 18, 103–117. doi: 10.47536/jcrm.v18i1.437
MacLeod C. D. and Zuur A. F. (2005). Habitat utilization by Blainville’s beaked whales off Great Abaco, northern Bahamas, in relation to seabed topography. Mar. Biol. 147, 1–11. doi: 10.1007/s00227-004-1546-9
Marsh L., Huvenne V. A., and Jones D. O. (2018). Geomorphological evidence of large vertebrates interacting with the sea - floor at abyssal depths in a region designated for deep sea mining. R. Soc Open Sci. 5, 180286. doi: 10.1098/rsos.180286
Marshall L. and Rexstad E. (2022). ddsd: Distance Sampling Survey Design. Available online at: https://cran.asia/web/packages/dssd/dssd.pdf (Accessed July 01, 2013).
McCullough J. L., Wren J. L., Oleson E. M., Allen A. N., Siders Z. A., and Norris E. S. (2021). An acoustic survey of beaked whales and Kogia spp. in the Mariana Archipelago using drifting recorders. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.664292
Miller K. A., Brigden K., Santillo D., Currie D., Johnston P., and Thompson K. F. (2021). Challenging the need for deep seabed mining from the perspective of metal demand, biodiversity, ecosystem services, and benefit sharing. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.706161
Miller K. A., Thompson K. F., Johnston P., and Santillo D. (2018). An overview of seabed mining including the current state of development, environmental impacts, and knowledge gaps. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00418
Niner H. J., Ardron J. A., Escobar E. G., Gianni M., Jaeckel A., Jones D. O., et al. (2018). Deep-sea mining with no net loss of biodiversity—an impossible aim. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00053
Niu F., Xue R., Yang Y., Chen B., Ruan H., and Luo K. (2021). Baseline assessment of ocean ambient noise in the western Clarion Clipperton Zone, Pacific Ocean. Mar. Poll. Bull. 173, 113057. doi: 10.1016/j.marpolbul.2021.113057
NOAA (2005). Endangered Fish and Wildlife; Notice of Intent to Prepare an Environmental Impact Statement. Available online at: https://www.federalregister.gov/documents/2005/01/11/05-525/endangered-fish-and-wildlife-notice-of-intent-to-prepare-an-environmental-impact-statement (Accessed 2 May 2025).
Olson R. J., Duffy L. M., Kuhnert P. M., Galván-Magaña F., Bocanegra-Castillo N., and Alatorre-Ramírez V. (2014). Decadal diet shift in yellowfin tuna Thunnus albacares suggests broad-scale food web changes in the eastern tropical Pacific Ocean. Mar. Ecol. Prog. Ser. 497, 157–178. doi: 10.3354/meps10609
Oswald J. N., Rankin S., Barlow J., and Lammers M. O. (2007). A tool for real-time acoustic species identification of delphinid whistles. J. Acoust. Soc Am. 122, 587–595. doi: 10.1121/1.2743157
R Core Team. (2022). R: A language and environment for statistical computing (R Foundation for Statistical Computing). Available at: https://www.r-project.org/.
Simon-Lledó E., Bett B. J., Huvenne V. A., Schoening T., Benoist N. M., and Jones D. O. (2019). Ecology of a polymetallic nodule occurrence gradient: Implications for deep-sea mining. Limnol. Oceanogr. 64, 1883–1894. doi: 10.1002/lno.11157
Soldevilla M. S., Henderson E. E., Campbell G. S., Wiggins S. M., Hildebrand J. A., and Roch M. A. (2008). Classification of Risso’s and Pacific white-sided dolphins using spectral properties of echolocation clicks. J. Acoust. Soc Am. 124, 609–624. doi: 10.1121/1.2932059
Soldevilla M. S., Hildebrand J. A., Frasier K. E., Aichinger Dias L., Martinez A., Mullin K. D., et al. (2017). Spatial distribution and dive behavior of Gulf of Mexico Bryde’s whales: potential risk of vessel strikes and fisheries interactions. Endang. Species Res. 32, 533–550. doi: 10.3354/esr00834
Stenvers V. I., Hauss H., Bayer T., Havermans C., Hentschel U., Schmittmann L., et al. (2023). Experimental mining plumes and ocean warming trigger stress in a deep pelagic jellyfish. Nat. Commun. 14, 7352. doi: 10.1038/s41467-023-43023-6
Strindberg S. and Buckland S. T. (2004). Zigzag survey designs in line transect sampling. JABES 9, 443. doi: 10.1198/108571104X15601
Sweetman A. K., Smith A. J., de Jonge D. S. W., Hahn T., Schroedl P., Silverstein M., et al. (2020). Evidence of dark oxygen production at the abyssal seafloor. Nat. Geosci. 17, 737–739. doi: 10.1038/s41561-024-01480-8
Taylor B. L., Baird R., Barlow J., Dawson S. M., Ford J., Mead J. G., et al. (2019). Physeter macrocephalus (amended version of 2008 assessment). IUCN Red List Threat. Species 2019, e.T41755A160983555. doi: 10.2305/IUCN.UK.2008.RLTS.T41755A160983555.en
Thompson K. F., Miller K. A., Currie D., Johnston P., and Santillo D. (2018). Seabed mining and approaches to governance of the deep seabed. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00480
Thompson K. F., Miller K. A., Wacker J., Derville S., Laing C., Santillo D., et al. (2023). Urgent assessment needed to evaluate potential impacts on cetaceans from deep seabed mining. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1095930
Tyack P. L., Johnson M. P., Zimmer W. M., De Soto N. A., and Madsen P. T. (2006). “Acoustic behavior of beaked whales, with implications for acoustic monitoring,” in OCEANS, Boston, MA, USA, IEEE, pp. 1–6. doi: 10.1109/OCEANS.2006.307120
Virgili A., Teillard V., Dorémus G., Dunn T. E., Laran S., Lewis M., et al. (2022). Deep ocean drivers better explain habitat preferences of sperm whales, Physeter macrocephalus, than beaked whales in the Bay of Biscay. Sci. Rep. 12, 9620. doi: 10.1038/s41598-022-13546-x
Waring G. T., Hamazaki T., Sheehan D., Wood G., and Baker S. (2001). Characterization of beaked whale (Ziphiidae) and sperm whale (Physeter macrocephalus) summer habitat in shelf-edge and deeper waters off the northeast US. Mar. Mamm. Sci. 17, 703–717. doi: 10.1111/j.1748-7692.2001.tb01294.x
Watanabe H., Kubodera T., Moku M., and Kawaguchi K. (2006). Diel vertical migration of squid in the warm core ring and cold water masses in the transition region of the western North Pacific. Mar. Ecol. Progr. Ser. 315, 187–197. doi: 10.3354/meps315187
Watanabe H., Moku M., Kawaguchi K., Ishimaru K., and Ohno A. (1999). Diel vertical migration of myctophid fishes (Family Myctophidae) in the transitional waters of the western North Pacific. Fish. Oceanogr. 8, 115–127. doi: 10.1046/j.1365-2419.1999.00103.x
Webber T., Gillespie D., Lewis T., Gordon J., Ruchirabha T., and Thompson K. F. (2022). Streamlining analysis methods for large acoustic surveys using automatic detectors with operator validation. Methods Ecol. Evol. 13, 1765–1777. doi: 10.1111/2041-210X.13907
Williams R., Erbe C., Duncan A., Nielsen K., Washburn T., and Smith C. (2022). Noise from deep-sea mining may span vast ocean areas. Science 377, 157–158. doi: 10.1126/science.abo2804
Woodside J., David L., Frantzis A., and Hooker S. (2006). Gouge marks on deep-sea mud volcanoes in the eastern Mediterranean: Caused by Cuvier’s beaked whales? Deep Sea Res. I 53, 1762–1771. doi: 10.1016/j.dsr.2006.08.011
Yack T. M., Barlow J., Roch M. A., Klinck H., Martin S., Mellinger D. K., et al. (2010). Comparison of beaked whale detection algorithms. Appl. Acoust. 71, 1043–1049. doi: 10.1016/j.apacoust.2010.04.010
Keywords: deep sea mining, minerals, passive acoustic monitoring, sperm whale, oceanic dolphin, Pacific Ocean, CCZ, impact
Citation: Young KF, Webber T, Karantzas L, Miteva S, Oakes G, Santillo D and Johnston P (2025) Threatened cetaceans in a potential deep seabed mining region, Clarion Clipperton Zone, Eastern Pacific, August 2023. Front. Mar. Sci. 12:1511075. doi: 10.3389/fmars.2025.1511075
Received: 14 October 2024; Accepted: 19 May 2025;
Published: 24 June 2025.
Edited by:
Xuelei Zhang, Ministry of Natural Resources, ChinaReviewed by:
Mark Meekan, University of Western Australia, AustraliaEwan Edwards, Xodus Group, United Kingdom
Copyright © 2025 Young, Webber, Karantzas, Miteva, Oakes, Santillo and Johnston. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsten F. Young, ay5mLnlvdW5nQGV4ZXRlci5hYy51aw==
 Kirsten F. Young
Kirsten F. Young Thomas Webber
Thomas Webber Leonidas Karantzas
Leonidas Karantzas Severina Miteva4
Severina Miteva4 David Santillo
David Santillo