- 1Department of Earth and Environmental Geosciences, Colgate University, Hamilton, NY, United States
- 2Institute for Chemistry and Biology of the Marine Environment, Carl-von-Ossietzky University Oldenburg, Wilhelmshaven, Germany
- 3Department of Physics and Astronomy, Colgate University, Hamilton, NY, United States
Changing climatic conditions can have complex effects on the biomineralized segments of marine organisms, which in turn may influence individual fitness and survival. In nacreous shells, tablet thickness is one microstructural component that has been observed to positively correlate with ocean temperature, though the strength of this relationship is unclear, leaving unresolved whether temperature is a consistent predictor of tablet thickness and, if so, over what scales. Here we investigate the relationship between tablet thickness and temperature in two nacre-producing marine mollusks in the modern ocean. We explore the relationship between nacre tablet thickness and ocean temperature through a global analysis of present-day abalone (Haliotis) nacre, and a temporal analysis of nut clam (Nucula proxima) nacre using shells from individuals that lived before and after 1950 in two regions of the Gulf of Mexico. We document a positive relationship between tablet thickness and ocean temperature within and among closely-related species. For a given temperature, considerable variation was observed in nacre tablet thickness, indicating that other factors also contribute. While increased temperature is likely to cause larger biomineralized units within the calcified segments of marine organisms, other environmental factors might counter those changes, highlighting the need for work exploring the multimodal impact of anthropogenic climate change on biominerals.
1 Introduction
Ocean temperatures are rising globally as a result of increasing greenhouse gas concentrations in the atmosphere (Bindoff et al., 2019; Cheng et al., 2022). Average sea surface temperatures have risen by approximately 0.10°C per decade since the 1950s (Johnson and Lumpkin, 2021), and this rate has accelerated in recent decades (Cheng et al., 2022). Rates of change vary geographically, with certain ocean basins experiencing more rapid warming than others (Johnson and Lumpkin, 2021; Cheng et al., 2022). The oceans are also a major carbon dioxide sink, and CO2 uptake since the late 1980s has reduced the average pH of surface seawater by 0.017 - 0.027 pH units per decade (Bindoff et al., 2019). Declines in pH in many coastal regions are exacerbated by anthropogenic eutrophication, with decomposition of algal blooms reducing the pH (Rabalais et al., 2015; Gobler and Baumann, 2016) and dissolved oxygen concentration (Rabalais et al., 2015; Breitburg et al., 2018; Deutsch et al., 2024) of bottom waters. These changes are especially evident in the Gulf of Mexico, a region that includes one of the world’s largest dead zones (Levin et al., 2009; Rabalais et al., 2015) and where ocean temperatures are rising at twice the rate of the global average (Wang et al., 2023). Anthropogenic changes in the physical and chemical properties of seawater have important implications for marine life across a range of biological scales, from individual organisms to communities. Over the past several decades, considerable work has shown that changes in ocean temperature and pH have complex effects on the biominerals of marine organisms (Ries, 2011; Gilbert et al., 2017; Nardone et al., 2018; McCoy et al., 2018; Latchere et al., 2018; Knights et al., 2020; Muhammad et al., 2020).
While marine organisms produce biominerals for a variety of reasons, many utilize calcium carbonate (CaCO3), most commonly in the form of calcite and aragonite polymorphs (Lowenstam, 1981; Suzuki and Nagasawa, 2013). Mollusks are one of the groups most strongly associated with calcium carbonate biomineralization. Nacre, an ultrastructure found in a number of mollusk species, is the focus of this study. Two nacre-producing mollusks are abalone (Haliotis) and nut clams (Nucula); Haliotis produces columnar nacre (Gilbert et al., 2008) while Nucula produces sheet nacre (Taylor et al., 1969). The aragonitic nacreous region of abalone shells is at the interior of the shell while the exterior generally consists of calcitic prisms. In Nucula, both the inner nacreous layer and the outer prismatic layer are aragonitic. In general, nacre is composed of ~95-99% calcium carbonate and 0.1-5% organic material (polysaccharides, proteins, and lipids) (Jackson et al., 1988; Marin et al., 2012). The cross section of a nacreous shell has layers of largely inorganic calcium carbonate tablets, separated by organic matrices. While each nacre tablet is also a composite of aragonite nanoparticles and an organic matrix, they behave as a single crystal (Rousseau et al., 2005) and thus will be described as a crystal herein, in keeping with other studies (e.g., Olson et al., 2012; Gilbert et al., 2017).
The thickness of nacre layers, or tablet thickness (TT), varies across an individual shell, with TT depending on proximity to the prismatic layer (Gilbert et al., 2008), ontogeny (Salman et al., 2021), environmental conditions (Olson et al., 2012; Linard et al., 2011; Joubert et al., 2014; Muhammad et al., 2017; Latchere et al., 2018; Muhammad et al., 2020), and other yet to be determined factors. Within an individual shell, TT has been found to be thinner closer to the prismatic layer before leveling out to an average thickness ~50 µm from the prismatic layer (Gilbert et al., 2008), with remaining variability scattered around the mean. In addition, recent work has found TT to vary over an organism’s life, with tablets laid down in earlier life stages being thicker than those laid down in later life stages (Salman et al., 2021). TT has also been shown to covary with environmental factors, decreasing with increasing water depth (hydrostatic pressure) (Olson et al., 2012), and varying, albeit inconsistently across studies, with food availability (Linard et al., 2011; Joubert et al., 2014; Muhammad et al., 2017; Latchere et al., 2018; Muhammad et al., 2020).
Water temperature, in particular, has been found in a number of studies of marine environments to be a potentially strong environmental determinant of TT (Gilbert et al., 2017; Latchere et al., 2018; Muhammad et al., 2020, though Olson et al., 2012 documented a relatively weak relationship). Gilbert et al. (2017) observed a positive relationship between TT and ocean temperature in a study of extant and extinct pen shells (different species in the bivalve family Pinnidae) that ranged in geologic age from the Late Cretaceous (66 million years ago) to the present day (Gilbert et al., 2017), and suggested that nacre TT could serve as a paleotemperature proxy. In aquaculture, Muhammad et al. (2020) similarly found that TT depended on temperature in the pearl oyster Pinctada fucata, with thickness decreasing in winter months. In lab-based experiments, Latchere et al. (2018) explored the relationship between temperature, food, and TT in Pinctada margaritifera pearls, and found TT increased with temperature and was independent of food levels. In contrast, two studies on freshwater nacre (shell (Gey et al., 2023) and pearl (Farfan et al., 2021)) found TT did not correspond to temperature changes but rather was associated with other environmental changes (e.g. pH (Gey et al., 2023), lake conductivity (Farfan et al., 2021) and OH/O ratios (Farfan et al., 2021)). In principle, a nacre TT paleotemperature proxy would be valuable, allowing one to detect modest temperature changes in coastal environments using small samples of biogenic carbonate. dO18 values of biogenic carbonate reflect the isotopic composition of ambient seawater and temperature (Johnson et al., 2025), both of which vary tremendously in the coastal zone. Clumped isotope paleothermometry (D47) can disentangle changes in temperature from changes in the dO18 of ambient seawater, but great uncertainty exists when analyzing small samples (Müller et al., 2017; Johnson et al., 2025), thereby limiting applications to the shells of larger-bodied, longer-lived, calcifying organisms. To evaluate the potential for nacre TT to record temperature variation, additional work is needed to determine the existence and strength of the TT-temperature relationship under a range of environmental conditions, and over finer time scales (e.g. decades) than are spanned by the fossil record (e.g., Gilbert et al., 2017), but greater time scales than considered in controlled experiments (e.g., Latchere et al., 2018).
For this study, we had two primary objectives. First, to determine whether present-day TT varies with global sea surface temperature (SST) in the modern ocean by comparing TT from different geographic regions. To investigate this relationship, we generated a global dataset of abalone (genus: Haliotis) TT. Haliotis live in coastal regions today that span a wide range of ocean temperatures, allowing us to analyze the present-day relationship between TT and SST within a single genus of marine mollusk. Second, to determine whether anthropogenic climate change has resulted in recent changes in TT over past decades and centuries. To investigate such recent changes, we analyzed the nacre in nut clam (Nucula proxima) shells from two regions of the northern Gulf of Mexico. Benthic samples of surficial seafloor sediment from these environments include live individuals of N. proxima as well as empty shells derived from historical populations of N. proxima (Calderaro et al., 2024; Harnik et al., 2024). Radiocarbon dates generated for other bivalve species in these settings indicate that empty shells vary in age from the present-day to several millennia ago (Harnik et al., 2017), enabling comparison of TT in contemporary and historical populations. Observing a marked difference in TT between present-day and historical N. proxima shells would indicate that nacre microstructures can respond rapidly to relatively fine-scale temperature shifts.
2 Materials and methods
2.1 Haliotis specimens from around the world
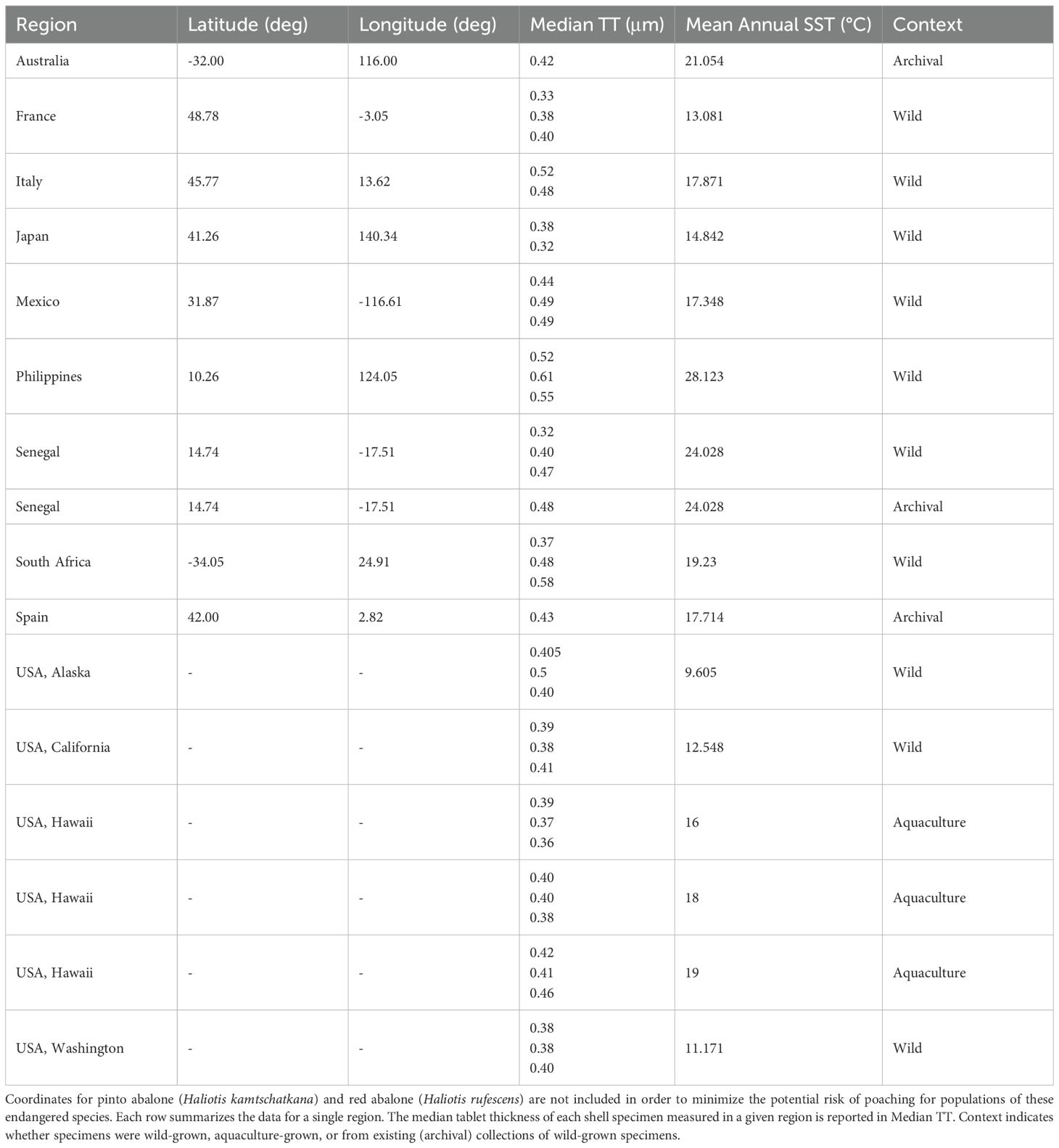
To investigate the relationship between TT and SST in the modern ocean, we generated a global dataset of abalone (genus: Haliotis) nacre. Haliotis shells from wild populations came from several sources (Table 1; Supplementary Table 1), including colleagues at different institutions (see parentheses below) and Conchology, Inc., an online shell supplier (https://www.conchology.be). Specimens sampled offshore of Italy (Stazione Zoologica Anton Dohrn), Alaska (University of California, Davis), California (University of California, Davis), and Washington (Washington Department of Fish and Wildlife) were provided with geographic coordinates for the specific localities where empty shells had been sampled; note, coordinates for pinto abalone (Haliotis kamtschatkana) and red abalone (Haliotis rufescens) are not included in Table 1 and Supplementary Table 1 to minimize poaching of populations of these endangered species at the request of the institutions that provided these shells. Shells obtained from Conchology, Inc. came from the Philippines, Senegal, South Africa, Mexico, Japan, and France; we determined the approximate latitude and longitude for these specimens using the locality descriptions provided by Conchology, Inc. Additional Haliotis shells from Spain, Australia, and Senegal were obtained from the collections of the Department of Earth and Environmental Geosciences at Colgate University. Metadata for these archival specimens did not include the date when they were sampled or the geographic coordinates for the sampling locality; latitude and longitude were approximated, as above, using the available locality information. No information was available about whether specimens obtained from Conchology, Inc. or the Colgate University collections were living at the time of collection. All shells provided by colleagues were empty upon collection. Previous studies have not found a clear correspondence between taphonomic condition and post-mortem shell age (Flessa et al., 1993; Carroll et al., 2003). Consequently, although many of the specimens appeared pristine (e.g., original luster, minimal abrasion) we did not use taphonomic condition to estimate post-mortem shell age. Empty shells in shallow marine environments tend to be dominated numerically by recently-deceased individuals (Kidwell, 2013); Haliotis shells in our study were assumed, on average, to be from recent decades. Empty shells in shallow marine environments also tend to be from species that are sampled live within those same habitats, suggesting minimal out-of-habitat transport after death (Kidwell and Tomašových, 2013). Out-of-habitat transport, when it occurs, tends to affect empty shells < 1 mm, which were not the focus of this study (Kidwell, 2001). Empty Haliotis shells in our study were assumed to have been sampled relatively close to where they lived.
Haliotis discus hannai shells from individuals grown in aquaculture were acquired from Big Island Abalone, located in Kailua-Kona, Hawaii (https://www.bigislandabalone.com). All specimens were raised in tanks set to 19°C for the first six months, then in tanks reduced to 18.5°C for the next six months, and then in tanks further reduced to 18°C for the next six months. After 18 months, the specimens were divided into three temperature regimes: 16°C, 18°C, and 19°C. The specimens grew in these different temperature treatments for approximately one year before they were harvested. All other rearing conditions (e.g. food) were kept constant across temperatures.
Nacre tablet thickness (TT) data were gathered from a total of 31 Haliotis shells sourced from 12 distinct geographic regions with a latitudinal spread from roughly 10° N to 57° N (Figure 1). To account for individual-level variation, we attempted to collect data for three specimens per region, and aquaculture temperature treatment. Only two specimens were analyzed for Japan and Italy, respectively, and only one specimen per region was analyzed for shells sourced from the Colgate University collections (Spain, Australia, and Haliotis marmorata from Senegal), due to the limited availability of Haliotis shells from these various sources.
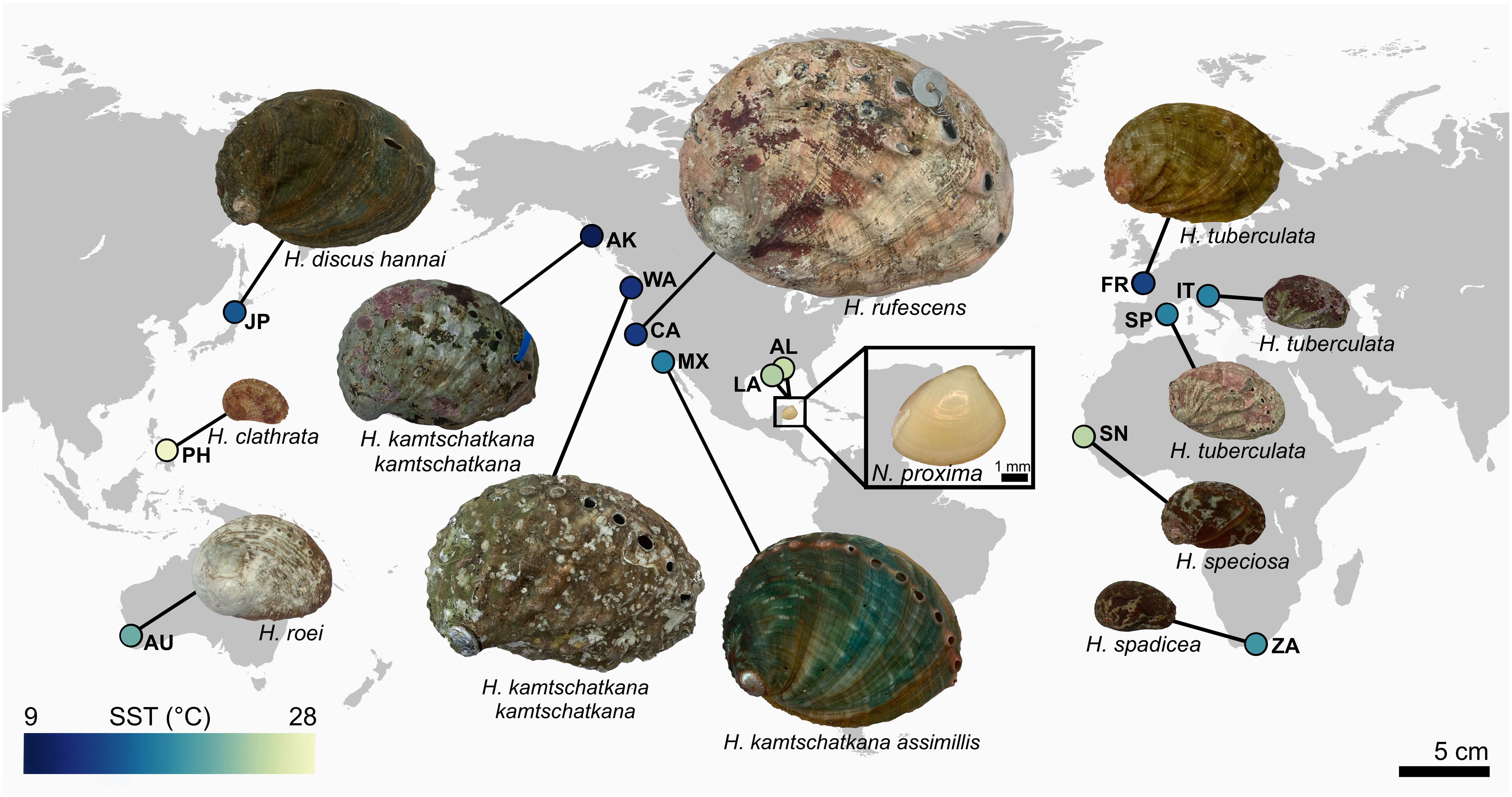
Figure 1. Geographic locations of Haliotis and Nucula proxima specimens analyzed for this study. SST refers to mean annual sea surface temperature for 1991-2020, calculated using data from the World Ocean Atlas. Colors correspond to the mean annual SST at the location where the specimen was collected.
2.2 Nucula proxima specimens from the Gulf of Mexico
To investigate recent changes in TT, live Nucula proxima, and empty shells of dead N. proxima, were collected from roughly 20 meters water depth at sampling stations located on the continental shelf of the northern Gulf of Mexico (Harnik et al., 2024), offshore of Louisiana and Alabama (Figure 1; Table 2). All specimens were collected in U.S. Federal Waters in accord with federal policies for collecting marine invertebrates that are not listed as at risk. Benthic samples were collected using a Grey O’Hara-type box corer and processed offshore using a 2 mm sieve. Two live-collected, and four dead-collected, N. proxima shells were randomly selected from the collections from each of the two regions (i.e., Louisiana and Alabama). Empty shells were evaluated using a light microscope prior to microstructural analysis; if the shell was very degraded, that specimen was replaced with another dead-collected shell.
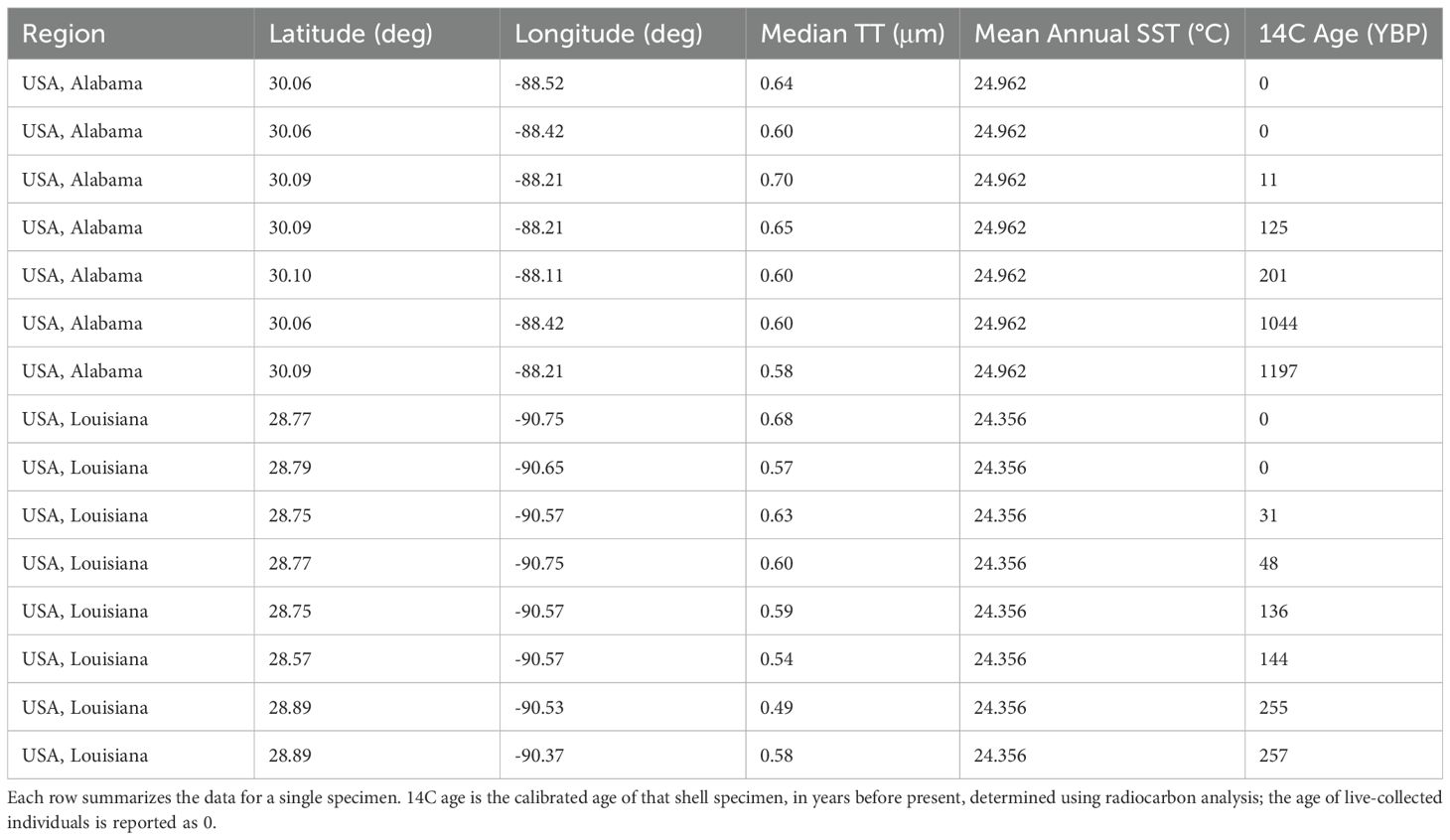
Table 2. Information about nut clam (Nucula proxima) shell specimens that were analyzed for this study.
2.3 Data collection
Cross-sections of Haliotis and Nucula shells were prepared to expose the nacre tablets (Figure 2). Haliotis shells were split with a hammer and chisel while delicate Nucula were split with sharp tweezers. Shell fragments were obtained from the middle of each shell, avoiding the apex (Haliotis)/umbo (Nucula) and aperture (Haliotis)/commissure (Nucula), and were embedded in epoxy (Ultrathin; Pace Technologies) such that the cross-section was perpendicular to the surface to be polished. During the embedding process, fragments were typically held in place with plastic clips to keep them upright; shells embedded at an angle would appear to have thicker tablets than their actual dimensions. Each epoxy puck was examined using an optical microscope to confirm that the embedded shell fragment was not oriented at an angle. Pucks were polished on a Nano-1000T Grinder Polisher (Pace Technologies) with successively finer silicon carbide grits down to a grit size of 2.5 µm, followed by a final polish with 50-nm alumina slurry to remove epoxy covering the cross-section and any surficial scratches.
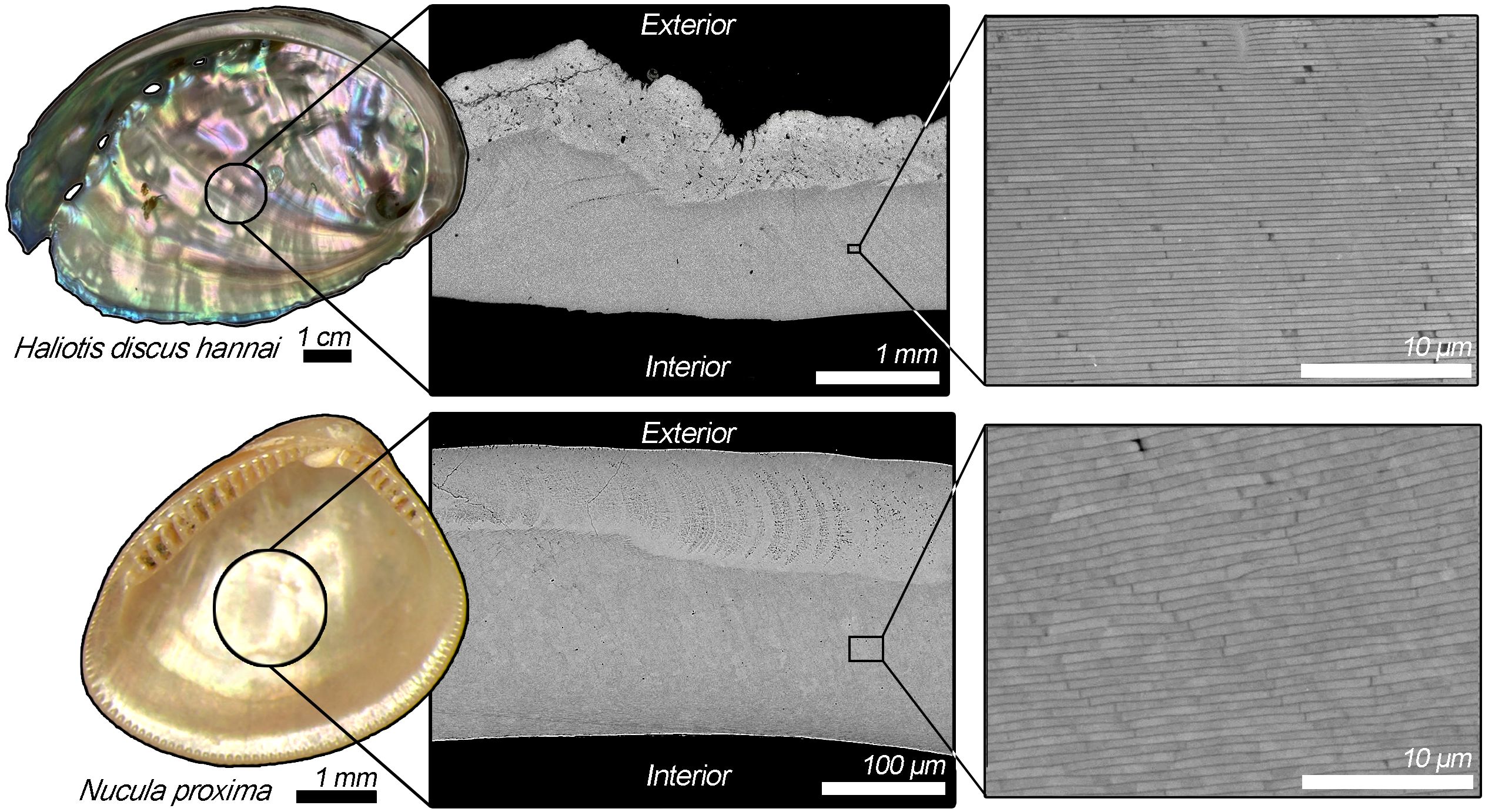
Figure 2. Haliotis (top) and Nucula proxima (bottom) shells and scanning electron micrographs of shell cross-sections highlighting their microstructure at varying magnification. In cross-section (middle), both Haliotis and Nucula shells consist of an exterior prismatic layer immediately adjacent to the interior nacreous layer. While the interior nacreous layer appears iridescent (left), in cross-section (middle) it appears relatively darker in intensity than the exterior prismatic layer located at the top of each image. The individual layers of aragonite tablets that comprise the nacre microstructure can be seen at higher magnification (right). Note the different scales (middle) for Haliotis and Nucula proxima.
Shell cross-sections were imaged in backscatter mode with a Hitachi TM4000 Plus scanning electron microscope (SEM), at a magnification of roughly 3000x to 4000x where the organic layers between tablet layers were clearly visible. High resolution images resolved individual tablet layers (Figure 2); the tablets appeared bright and the organic-layers dark in scanning electron micrographs, allowing the thickness of individual tablets to be systematically measured. We imaged the entire cross-section of each Nucula shell as the nacreous layer of these shells is typically ~150 µm wide (i.e., ~250 tablet layers thick) due to their relatively small size and shell thickness (Supplementary Figure 1). In contrast, the nacreous layer in Haliotis shells could be several mm thick, and consists of thousands of layers of tablets. For Haliotis shells, we measured 100 stacked tablets starting at a random point located towards the middle of each shell’s nacreous cross-section, as previous work has shown TT to decrease within ~50 µm of the nacre-prismatic boundary (Gilbert et al., 2008); for aquaculture shells, tablets were measured further from the nacre-prismatic boundary. For one Haliotis shell (from the Philippines), stacked tablets that spanned the entire nacreous layer were measured in addition to an independent set of 100 stacked tablet measurements from the same shell (Supplementary Figure 2); these data were used to compare finer-scale variation in TT through the nacreous cross-section with mean TT generated from a 100-tablet subsample. For two aquaculture shells in each final temperature regime (16°C, 18°C, and 19°C), stacked tablets that spanned the entire nacreous layer were measured along with an independent set of 100 stacked tablet measurements for one of the shells; for the other shell, the 100-tablet subsample used to calculate the mean was taken from roughly the midpoint of the measurements made through the entire cross-section of the nacreous layer (Supplementary Figure 2). To assess the precision of our TT measurements, we used the Haliotis specimen from Australia to measure 20 consecutive tablets five times, and calculated the mean standard deviation (mean SD = 0.01 µm).
For all samples, scanning electron micrographs of tablets were stitched together in Adobe Photoshop, and tablet thickness was measured by hand with the ImageJ line tool (Schneider et al., 2012). Measurements were made such that the line was drawn perpendicular to either side of the tablet, going from the top of one dark organic-layer to the bottom of the next.
2.4 Radiocarbon dating
Dead-collected Nucula proxima shell fragments were analyzed for radiocarbon at Northern Arizona University’s Arizona Climate and Ecosystems Isotope Laboratory using a MICADAS plus gas interface system (GIS) (Bright et al., 2024). Radiocarbon ages in years-before-present were determined using the Marine20 calibration curve (Heaton et al., 2020), extended to 2022 using a region bomb period radiocarbon curve and regional marine reservoir correction of ΔR=-134 ± 26 yr (Durham et al., 2023). Age calibrations were conducted using OxCal v. 4.4 (Bronk Ramsey, 2009). Nucula proxima are mixed feeders that are capable of deposit feeding and suspension feeding (Mikkelsen and Bieler, 2007). Some previous work has suggested that deposit feeding can bias radiocarbon estimates; specifically, consumption of older sedimentary carbon may result in lower percent Modern Carbon (pMC) values in the shells of deposit feeders (England et al., 2013). Evaluating this potential bias would require comparison of pMC data for co-occurring, live-collected deposit, suspension, and mixed feeders from the northern gulf, which was beyond the scope of the current study. We note this potential bias here to highlight that N. proxima post-mortem shell age estimates in our study may appear older than they actually were because of this species’ feeding mode, however these dates still record relative post-mortem ages (i.e., older versus younger) for these shells.
2.5 Environmental data
Sea surface temperature (SST) data from the World Ocean Atlas were used to calculate mean annual SST at a 1° spatial resolution from 1991-2020 (Locarnini et al., 2024; Reagan et al., 2024). The coordinates for each specimen were matched to the nearest grid point with SST data, based on the shortest geographic distance using the World Geodetic System of 1984 and the R package geosphere (Hijmans et al., 2005, 2021). Closely-spaced localities may have identical SST values because of the 1° spatial resolution. The precision of our locality coordinates varied depending on the source of the specimens, and sampled water depths were not consistently reported. Consequently, we calculated the mean annual ocean temperature at a 1° spatial resolution using surface waters (0 m depth) and assumed that this was a reasonable estimate for the environmental temperatures in which the organism grew. While mean annual ocean temperature varies locally and decreases with depth, we assumed that these offsets in well-mixed, shallow marine environments (e.g., < 50 m) are small relative to the temperature differences among localities in our global Haliotis dataset (Prandle and Lane, 1995). The mean annual SST for localities sampled in the northern Gulf of Mexico offshore of Louisiana and Alabama differ by only ~0.5°C (Table 2). However other environmental conditions (e.g., primary productivity, dissolved oxygen concentration) differ markedly between these two regions due to proximity to the Mississippi River delta (Harnik et al., 2024). Consequently, in our temporal analysis we compared post-1950 versus pre-1950 Nucula proxima nacre tablet thickness separately for each region.
2.6 Statistical analysis
Linear regression was used to investigate the relationship between TT and environmental temperature in our global Haliotis dataset for the modern ocean. We used Analysis of Covariance (ANCOVA) to test for homogeneity of slopes (TT versus SST) among our different populations of samples (i.e., wild, archival, aquaculture). Mean annual SST was used as the predictor for wild-collected and archival shells, whereas temperature of the final growth tank was used for aquaculture shells.
To investigate recent changes in TT in the northern Gulf of Mexico, we grouped Nucula proxima shells in each region (Louisiana versus Alabama) according to their post-mortem age. We used calibrated radiocarbon ages to assign shells to either pre-1950 or post-1950 groups because the rate of anthropogenic environmental change accelerated at that time (Steffen et al., 2015; Kuwae et al., 2024), and to roughly balance the sample size of specimens in each of these temporal groups; the post-1950 group included both live- and dead-collected shells. We used Mann-Whitney U-tests to determine whether median TT differed before and after 1950 in each region, and whether median TT differed between regions. Nucula TT was not plotted as a function of mean annual SST; mean annual SSTs offshore of Louisiana and Alabama, applicable to live-collected Nucula, differ today by ~0.5°C (24.536°C and 24.962°C, respectively), whereas the SST experienced by radiocarbon-dated shells is undetermined.
3 Results
3.1 Global variation in Haliotis tablet thickness and sea surface temperature
Haliotis tablet thickness increased with ocean temperature (Figure 3). A test for homogeneity of slopes (ANCOVA) for wild-grown, archival, and aquaculture-grown Haliotis revealed no significant difference among the TT versus SST regression slopes (F = 0.2935, p = 0.7557). Consequently, data for all samples were combined and a single regression model was fit to the TT versus SST data. While tablet thickness varied within and among shells from the same geographic region or aquaculture regime (Figure 3; Supplementary Figure 2), Haliotis tablet thickness increased with mean annual sea surface temperature overall, at a rate of 0.0063 µm/°C (p < 0.0001; R2 = 0.17; 3998 degrees of freedom; TT = 0.0063SST + 0.3190) (Figure 3).
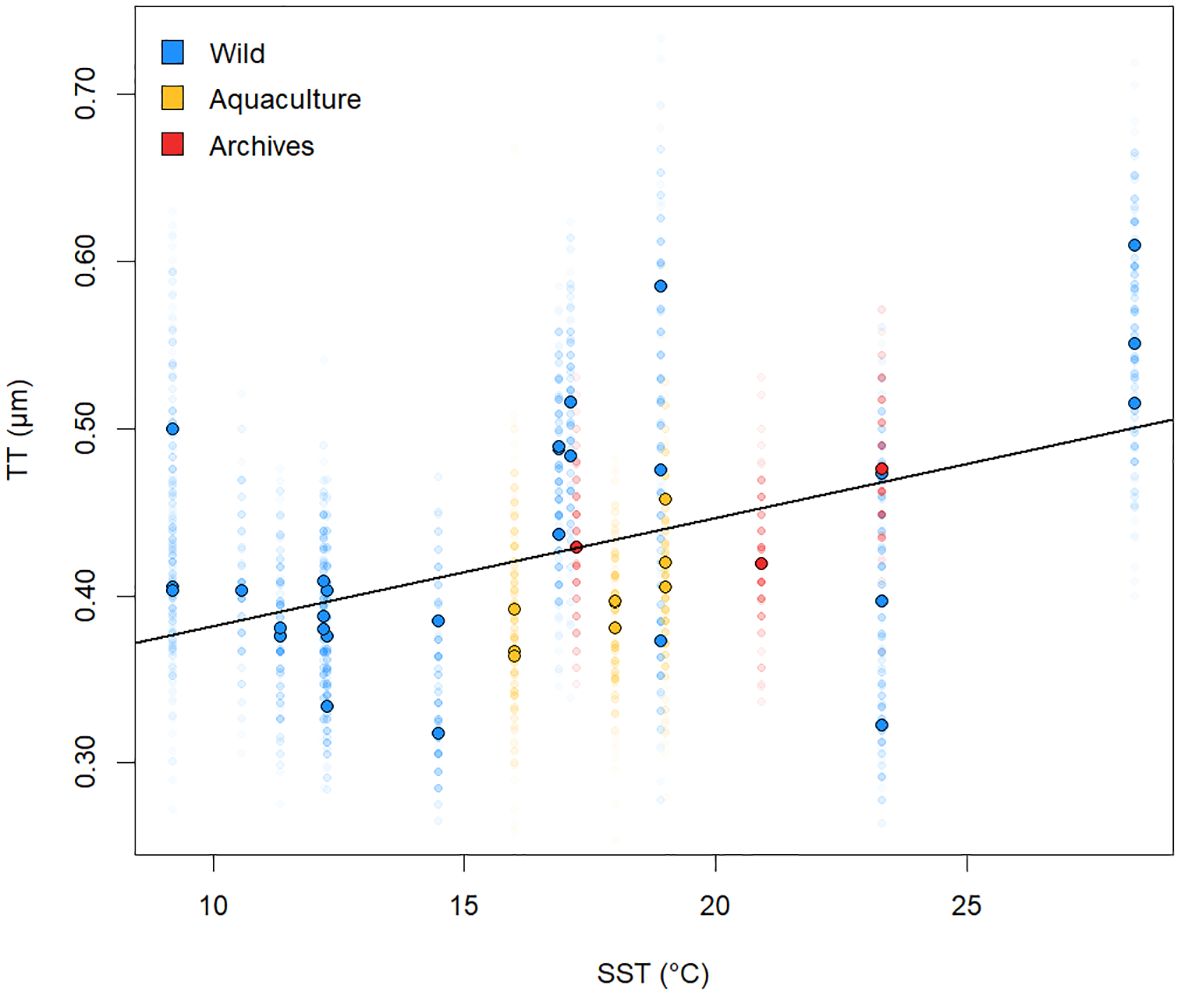
Figure 3. Positive relationship between nacre tablet thickness (TT) and sea surface temperature (SST) globally for all Haliotis shells analyzed in this study; TT measurements for wild-grown specimens are in blue, aquaculture-grown specimens are in yellow, and archival specimens from the Colgate University collections are in red. Darker points with black outlines represent the median tablet thickness of each shell, whereas paler points represent individual tablet measurements. SST for wild-grown and archival shells are mean annual SST measurements calculated using data from the World Ocean Atlas, whereas SST values for shells grown in aquaculture reflect final growth tank temperatures. The solid linear regression line summarizes the relationship between tablet thickness and mean annual SST for all analyzed Haliotis shells (p < 0.0001, R2 = 0.172).
3.2 Temporal variation in Nucula proxima tablet thickness in the Gulf of Mexico
In Nucula proxima, we observed a significant increase in median TT towards the present day, offshore of both Louisiana and Alabama (Figure 4); p < 0.0001 for both Mann-Whitney U-tests. Nacre tablets in post-1950s shells were on average ~ 0.05 µm thicker than pre-1950s shells in each region of the northern gulf. N. proxima TT also varied regionally (Figure 4). Median TT was greater offshore of Alabama than offshore of Louisiana, for both pre-1950s (Mann-Whitney U-test, p < 0.0001) and post-1950s shells (Mann-Whitney U-test, p = 0.002). This difference in Nucula TT between regions of the northern gulf is consistent with the positive TT-temperature relationship we observed in our global analysis of Haliotis TT; mean annual SST in Alabama for 1991–2000 was ~0.5°C greater than offshore of Louisiana (Table 2).
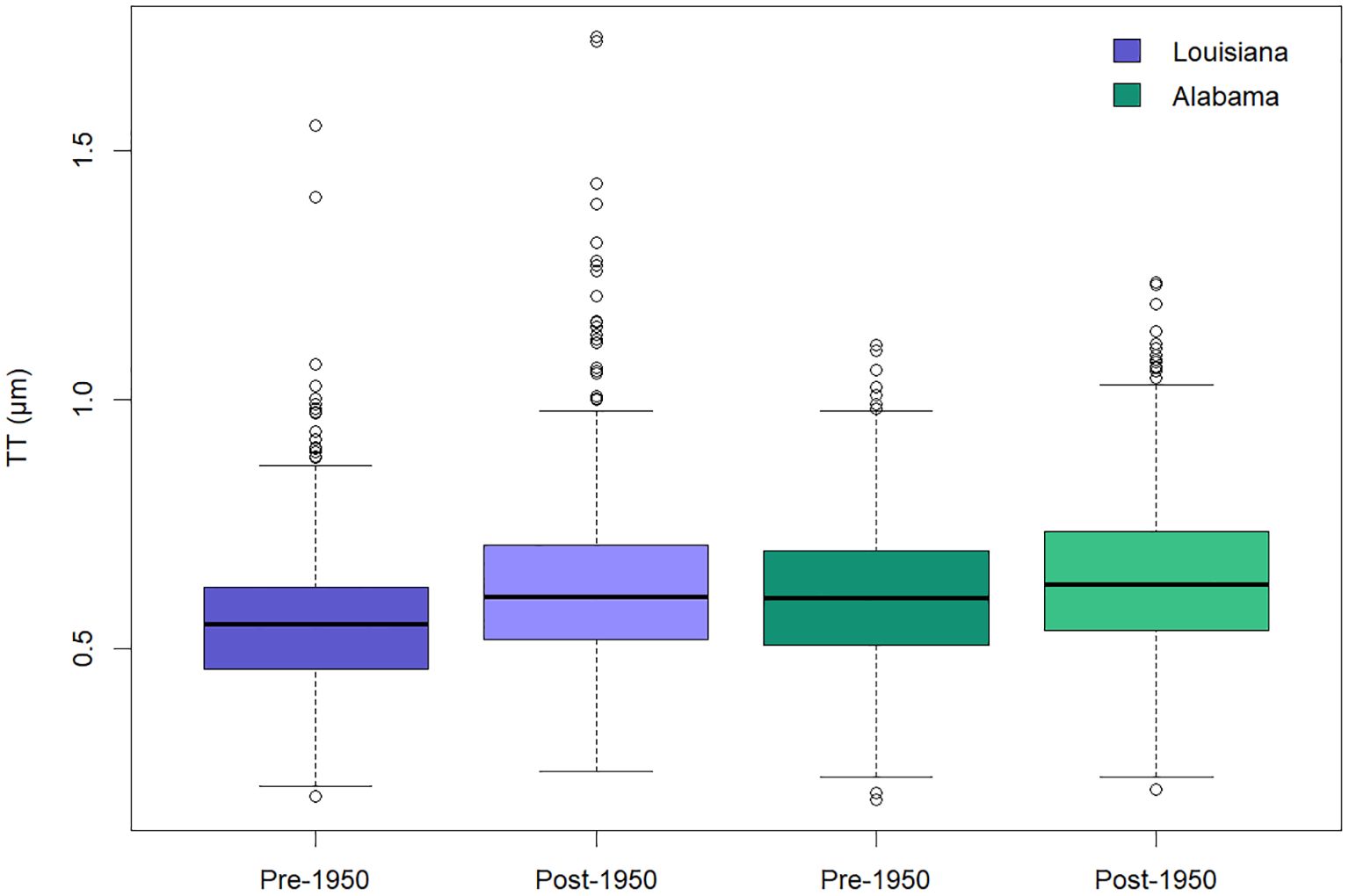
Figure 4. Changes in nacre tablet thickness (TT) over time for Nucula proxima that grew before versus after 1950 in the Gulf of Mexico, offshore of Louisiana (LA, in purple) and Alabama (AL, in green). Median TT is significantly greater for post-1950 shells (lighter color) versus pre-1950 shells (darker color), in both regions (Mann-Whitney U-test, p < 0.0001). Median TT also differs significantly between regions, with greater TT offshore of Alabama where mean annual SST for 1991–2020 is ~ 0.5°C warmer than offshore of Louisiana (Mann-Whitney U-test for pre-1950 LA vs. AL, p < 0.0001; post-1950 LA vs. AL, p = 0.002).
4 Discussion
The impact of changing ocean chemistry on the biominerals produced by marine organisms is as complex as the environmental factors contributing to the changes. Understanding the relationship between biomineral structures and the environment can provide insight into both the climatic changes of the past and the impact of future anthropogenic climate change on biomineral structures. Here we present results on biomineral unit (BMU) size that take a step towards untangling these relationships by looking over broad spatio temporal scales at the relationship between sea surface temperature (SST) and nacre tablet thickness (TT). We measured present-day nacre TT for shells of Haliotis (gastropod) collected from globally-distributed locations and aquaculture conditions with known sea surface temperature (SST), and found a positive relationship between TT and SST (Figure 3). A spatial and temporal analysis of nacre TT from Nucula proxima (bivalve) shells from two regions within the Gulf of Mexico found a similar positive relationship between TT and SST (Figure 4). Greater TT was observed in the region characterized today by warmer waters, and TT increased over time in both regions, consistent with documented warming trends (Wang et al., 2023). These results indicate that nacre microstructures, across two classes of marine mollusks (which present columnar vs. sheet nacre, respectively), can respond to rapid and relatively fine-scale changes in environmental conditions, including those caused by ongoing anthropogenic climate change. Both our spatial and temporal analyses indicate the existence of a robust positive relationship between nacre TT and temperature for marine taxa that is taxonomically-independent with respect to the overall positive trend.
The positive relationship observed between TT and SST in both space (Haliotis and Nucula) and time (Nucula) supports previous work on marine nacre response to increased temperature (Olson et al., 2012; Gilbert et al., 2017; Latchere et al., 2018; Muhammad et al., 2020). Our analysis builds on previous work by examining a large set of wild-collected specimens which, due to differences in geographic location (Haliotis globally, Nucula two regions within the Gulf of Mexico) and time of growth (Nucula, pre- versus post-1950s), experienced markedly different mean annual ocean temperatures. Despite differences in geographic scope (broad for Haliotis, narrow for Nucula), a positive relationship between TT and temperature was consistently observed. Similarly, while the temporal scale over which Nucula TT was compared is significantly narrower than that considered in previous analyses of Jurassic through present-day pen shells (Gilbert et al., 2017), the positive relationship between TT and SST remains. Variation in the strength of the TT-temperature relationship across studies (e.g., Gilbert et al., 2017; Latchere et al., 2018; this study) may reflect differences in the scale of environmental change. For example, while ocean temperatures are rising rapidly in the Gulf of Mexico (Wang et al., 2023), the absolute change that has occurred over recent decades to centuries is modest in comparison to the global changes in climate that have occurred over geologic time (Gilbert et al., 2017).
A positive relationship between temperature and the size of individual biomineralized units (BMUs) has also been observed in other marine biomineralizers (Milano et al., 2017; Höche et al., 2020, 2021, 2022). Höche et al. (2021) found an increase in BMU size in the crossed-acicular (needle-like) layer of lab-grown Arctica islandica bivalves with increasing temperature. Milano et al. (2017) found larger prismatic BMUs corresponding to warmer temperatures in wild-grown specimens of the bivalve Cerastoderma edule from different locations. Höche et al. (2020) found the crossed-lamellar BMUs in the bivalve Glycymeris bimaculata increased in length, width and elongation in warmer waters to such an extent that they proposed BMU length could serve as a marine temperature proxy. The combination of our results and those of previous studies indicate that increased temperature plays a role in increasing BMU size for many marine organisms (Gilbert et al., 2017; Muhammad et al., 2017; Höche et al., 2020, 2021; Milano et al., 2017; Höche et al., 2022).
While a positive relationship between individual BMUs and temperature has been found across many marine biomineralizers, such a relationship does not appear in freshwater biominerals (Farfan et al., 2021; Gey et al., 2023). In examining the relationship between temperature, lake conductivity, and OH/O ratios in freshwater pearls produced by the bivalve Megalonaias nervosa, Farfan et al. (2021) found no relationship between temperature and pearl nacre tablet thickness, but rather a dependence of TT on lake conductivity and OH/O ratios. Similarly, Gey et al. (2023) found nacre tablet thickness did not track water temperature in the nacre produced by the freshwater mussel Margaritifera margaritifera in a combination of laboratory and field-based experiments. Our results differ from these studies of freshwater nacre that found no TT-temperature relationship, suggesting, amongst other possibilities, that there may be different homeostasis mechanisms at work for nacre-producing organisms in marine versus freshwater environments. Why relationships between TT and temperature vary between marine and freshwater biomineralizers is currently unknown and more work is needed to untangle the effects of temperature on biomineral formation across a diversity of marine and freshwater organisms.
One aspect of tablet thickness that appears consistent across marine and freshwater nacre is that within a single transect of nacre tablets, tablet thickness varies considerably (Supplementary Figures 1, 2) (Gilbert et al., 2008; Olson et al., 2012; Farfan et al., 2021; Gey et al., 2023). Our comparative study focused on TT variation on a global scale, and its relationship to mean annual SST. We did not assess other potential sources of TT variation, such as ontogeny and seasonality, which would have required higher-resolution sclerochronological and environmental data for individual specimens, at the expense of broader-scale sampling of many specimens that span a range of geographic and environmental contexts. Given the spatial and temporal scale of our analysis, we also did not consider other environmental variables that have been found to influence BMUs [e.g., pH (Nardone et al., 2018; Knights et al., 2020), food availability (Muhammad et al., 2020)]. Despite these limitations, it is unlikely that omitting these variables could have generated the positive TT-temperature relationship that we observed. Similarly, uncertainties regarding the exact locations where specimens were collected and their associated environmental temperatures, may contribute noise to the observed TT-temperature relationship but are unlikely to spuriously generate a positive trend. Overall, these limitations highlight one of the challenges of disentangling the impact of different variables on BMU size; while fine-scale analyses with well-controlled environmental parameters can tease apart the unique contributions of different environmental parameters it is unclear how these observed relationships scale up to natural systems over broader spatial and temporal scales. Thus, while our study does not support the use of nacre TT as a direct temperature proxy, the robust positive relationship between BMU size and temperature observed here and in other studies of marine organisms indicate its usefulness in understanding general temperature trends in the past. The positive TT-SST relationship could allow temperature trends to be detected in coastal settings using the shells of small-bodied marine biomineralizers for which shell mass is insufficient for clumped isotope paleothermometry and where dO18 values conflate changes in the isotopic composition of ambient seawater and temperature.
Biomineral microstructures contain a wealth of information, from the size of the biomineral units making up the material to the orientation of those units relative to one another. Utilizing the information hidden within these structures is complex due to the multiple factors influencing their production. Work looking into the way in which these structural elements change has generally focused on either long time scales, comparing drastically different climates and combining data for multiple species (Olson et al., 2012; Gilbert et al., 2017), or on short time scales, typically for single species (Milano et al., 2017; Muhammad et al., 2017; Latchere et al., 2018; Höche et al., 2020, 2021; Farfan et al., 2021; Höche et al., 2022; Gey et al., 2023). Whether these approaches can provide insight into how the ultrastructures of various taxa change over annual to centennial scales is unclear. Yet, these are the requisite scales that are urgently needed to understand and predict the impact of ongoing anthropogenic climate change on biomineralizing marine organisms. Thus, while our results suggest that warming seawater will influence ultrastructure through the growth of larger biomineral units (nacre tablets), and that these changes may occur within populations over annual to decadal times scales, significantly more work is needed to understand other ultrastructural responses to changing climatic conditions and their combined effects on fitness and survival.
Data availability statement
All analyses were conducted in R; all data used in this study are available from the Dryad Digital Repository https://doi.org/10.5061/dryad.kd51c5bjb.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
JC: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft. MR: Investigation, Data curation, Formal Analysis, Writing – review & editing. PH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. RM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work described here was supported by Colgate University's Picker Interdisciplinary Science Institute (to P.G.H., R.A.M., and D. McHugh), the National Science Foundation (NSF DMR-1905619 to R.A.M.; NSF EAR-2041667 to P.G.H.) and the German Research Foundation (DFG) Cluster of Excellence ‘The Ocean Floor -Earth’s Uncharted Interface’ (EXC 2077, grant no. 390741603, M.C.R.).
Acknowledgments
We are grateful to the following individuals for providing Haliotis shells: Paolo Albano (Italy), Taylor White and Ashley Bolwerk (Alaska), Kathleen Sowul (Washington), Laura Rogers-Bennett (California), and Cecilia Viljoen (Hawaii). For assistance in collecting the Nucula samples used for this study, we thank the captains, crew, and marine technical support staff at the Louisiana Universities Marine Consortium and the Dauphin Island Sea Lab, and undergraduate research students in the Paleo Lab. Quan Hua generously provided the 14C calibration codes used in the study by Durham et al. (2023). Thank you to the reviewers whose comments helped to clarify and strengthen this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1517327/full#supplementary-material
Supplementary Figure 1 | Variation in nacre tablet thickness (TT) from the nacre-prismatic boundary to the shell interior for two specimens of Nucula proxima from offshore of Alabama (top) and two specimens from offshore of Louisiana (bottom). Each colored point is an individual tablet measurement, the moving average of 50 tablets is denoted by the varying line, whereas the straight horizontal lines are median TT calculated from 100 tablets centered on the mid-point of each shell.
Supplementary Figure 2 | Variation in nacre tablet thickness (TT) from the nacre-prismatic boundary to the shell interior for Haliotis specimens from the Philippines (top; wild) and each of the aquaculture temperature regimes (middle and bottom); specimens are ordered from the warmest (top) to coolest (bottom) temperature Each colored point is an individual tablet measurement, the moving average of 100 tablets is denoted by the varying line, whereas each straight horizontal line is the median TT calculated from a sample of 100 tablet measurements. The median TT for the Philippines specimen and one specimen from each of the aquaculture treatments was calculated using an independent subsample of 100 stacked tablet measurements from the middle of the nacreous layer. The median TT for the other specimens in each of the aquaculture treatments was calculated using a subsample of 100 tablets centered on the mid-point of the shell.
References
Bindoff N. L., Cheung W. W.L., Kairo J. G., Arístegui J., Guinder V. A., Hallberg R., et al. (2019). “Changing ocean, marine ecosystems, dependent communities,” in IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, eds Pörtner H. O., Roberts D. C., Masson-Delmotte V., Zhai P., Tignor M., Poloczanska E., Mintenbeck K., Alegría A., Nicolai M., Okem A., Petzold J., Rama B, Weyer N. M., et al. (Geneva: Intergovernmental Panel on Climate Change (IPCC)), 448–587.
Breitburg D., Levin L. A., Oschlies A., Grégoire M., Chavez F. P., Conley D. J., et al. (2018). Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240. doi: 10.1126/science.aam7240
Bright J., Ebert C., Flores C., Harnik P. G., Huntley J. W., Kowalewski M., et al. (2024). Comparing MICADAS gas source, direct carbonate, and standard graphite 14C determinations of biogenic carbonate. Radiocarbon 66, 295–305. doi: 10.1017/RDC.2024.45
Bronk Ramsey C. (2009). Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360. doi: 10.1017/S0033822200033865
Calderaro L. A., Harnik P. G., and Rillo M. C. (2024). Environmental correlates of molluscan predator–prey body size in the northern Gulf of Mexico. Paleobiology 50, 70–84. doi: 10.1017/pab.2023.22
Carroll M., Kowalewski M., Simões M. G., and Goodfriend G. A. (2003). Quantitative estimates of time-averaging in terebratulid brachiopod shell accumulations from a modern tropical shelf. Paleobiology 29, 381–402. doi: 10.1666/0094-8373(2003)029<0381:QEOTIT>2.0.CO;2
Cheng L., von Schuckmann K., Abraham J. P., Trenberth K. E., Mann M. E., Zanna L., et al. (2022). Past and future ocean warming. Nat. Rev. Earth Environ. 3, 776–794. doi: 10.1038/s43017-022-00345-1
Deutsch C., Penn J. L., and Lucey N. (2024). Climate, oxygen, and the future of marine biodiversity. Annu. Rev. Mar. Sci. 16, 217–245. doi: 10.1146/annurev-marine-040323-095231
Durham S. R., Dietl G. P., Hua Q., Handley J. C., Kaufman D., and Clark C. P. (2023). Age variability and decadal time-averaging in oyster reef death assemblages. Geology 51, 1067–1071. doi: 10.1130/G50778.1
England J., Dyke A. S., Coulthard R. D., Mcneely R., and Aitken A. (2013). The exaggerated radiocarbon age of deposit-feeding molluscs in calcareous environments. Boreas 42, 362–373. doi: 10.1111/j.1502-3885.2012.00256.x
Farfan G. A., Zhou C., Valley J. W., and Orland I. J. (2021). Coupling mineralogy and oxygen isotopes to seasonal environmental shifts recorded in modern freshwater pearl nacre from Kentucky lake. Geochemistry Geophysics Geosystems 22, e2021GC009995. doi: 10.1029/2021GC009995
Flessa K. W., Cutler A. H., and Meldahl K. H. (1993). Time and taphonomy: Quantitative estimates of time-averaging and stratigraphic disorder in a shallow marine habitat. Paleobiology 19, 266–286. doi: 10.1017/S0094837300015918
Gey C. J., Thielen F., Pfister L., Hissler C., Türk G., Baier S., et al. (2023). Disturbed by pH? Nacre tablet thickness of freshwater pearl mussels (Margaritifera margaritifera) is a poor temperature proxy. Mar. Freshw. Res. 74, 1129–1144. doi: 10.1071/MF23058
Gilbert P. U. P. A., Bergmann K. D., Myers C. E., Marcus M. A., DeVol R. T., Sun C.-Y., et al. (2017). Nacre tablet thickness records formation temperature in modern and fossil shells. Earth Planetary Sci. Lett. 460, 281–292. doi: 10.1016/j.epsl.2016.11.012
Gilbert P. U. P. A., Metzler R. A., Zhou D., Scholl A., Doran A., Young A., et al. (2008). Gradual ordering in Red Abalone nacre. J. Am. Chem. Soc. 130, 17519–17527. doi: 10.1021/ja8065495
Gobler C. J. and Baumann H. (2016). Hypoxia and acidification in ocean ecosystems: Coupled dynamics and effects on marine life. Biol. Lett. 12, 20150976. doi: 10.1098/rsbl.2015.0976
Harnik P. G., Chao A., Collins K. S., and Rillo M. C. (2024). Declining bivalve species and functional diversity along a coastal eutrophication-deoxygenation gradient in the northern Gulf of Mexico. Continental Shelf Res. 282, 105339. doi: 10.1016/j.csr.2024.105339
Harnik P. G., Torstenson M. L., and Williams M. A. (2017). Assessing the effects of anthropogenic eutrophication on marine bivalve life history in the northern Gulf of Mexico. Palaios 32, 678–688. doi: 10.2110/palo.2017.033
Heaton T. J., Köhler P., Butzin M., Bard E., Reimer R. W., Austin W. E. N., et al. (2020). Marine20—The marine radiocarbon age calibration curve (0–55,000 cal BP). Radiocarbon 62, 779–820. doi: 10.1017/RDC.2020.68
Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., and Jarvis A. (2005). Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatology 25, 1965–1978. doi: 10.1002/(ISSN)1097-0088
Hijmans R. J., Karney C., Williams E., and Vennes C. (2021). geosphere: Spherical trigonometry, R package version 1. Available online at: http://cran.r-project.org/package=geosphere. (Accessed November 26, 2024).
Höche N., Peharda M., Walliser E. O., and Schöne B. R. (2020). Morphological variations of crossed-lamellar ultrastructures of Glycymeris bimaculata (Bivalvia) serve as a marine temperature proxy. Estuarine Coast. Shelf Sci. 237, 106658. doi: 10.1016/j.ecss.2020.106658
Höche N., Walliser E. O., de Winter N. J., Witbaard R., and Schöne B. R. (2021). Temperature-induced microstructural changes in shells of laboratory-grown Arctica islandica (Bivalvia). PloS One 16, e0247968. doi: 10.1371/journal.pone.0247968
Höche N., Walliser E. O., and Schöne B. R. (2022). Microstructural mapping of Arctica islandica shells reveals environmental and physiological controls on biomineral size. Front. Earth Sci. 9, 781305. doi: 10.3389/feart.2021.781305
Jackson A. P., Vincent J. F. V., and Turner R. M. (1988). The mechanical design of nacre. Proc. R. Soc. B: Biol. Sci. 234, 415–440. doi: 10.1098/rspb.1988.0056
Johnson G. C. and Lumpkin R. (2021). State of climate in 2020: Global oceans. Bull. Am. Meteor. Soc. 102, S143–S198. doi: 10.1175/BAMS-D-21-0083.1
Johnson A. L., Schöne B. R., Petersen S. V., de Winter N. J., Dowsett H. J., Cudennec J. F., et al. (2025). Molluscan isotope sclerochronology in marine palaeoclimatology: Taxa, technique and timespan issues. Quaternary Sci. Rev. 350, 109068. doi: 10.1016/j.quascirev.2024.109068
Joubert C., Linard C., Le Moullac G., Soyez C., Saulnier D., Teaniniuraitemoana V., et al. (2014). Temperature and food influence shell growth and mantle gene expression of shell matrix proteins in the Pearl Oyster Pinctada margaritifera. PloS One 9, e103944. doi: 10.1371/journal.pone.0103944
Kidwell S. M. (2001). Preservation of species abundance in marine death assemblages. Science 294, 1091–1094. doi: 10.1126/science.1064539
Kidwell S. M. (2013). Time-averaging and fidelity of modern death assemblages: Building a taphonomic foundation for conservation palaeobiology. Palaeontology 56, 487–522. doi: 10.1111/pala.2013.56.issue-3
Kidwell S. M. and Tomašových A. (2013). Implications of time-averaged death assemblages for ecology and conservation biology. Annu. Rev. Ecology Evolution Systematics 44, 539–563. doi: 10.1146/annurev-ecolsys-110512-135838
Knights A. M., Norton M. J., Lemasson A. J., and Stephen N. (2020). Ocean acidification mitigates the negative effects of increased sea temperatures on the biomineralization and crystalline ultrastructure of Mytilus. Front. Mar. Sci. 7, 567228. doi: 10.3389/fmars.2020.567228
Kuwae M., Yokoyama Y., Tims S., Froehlich M., Fifield L. K., Aze T., et al. (2024). Toward defining the Anthropocene onset using a rapid increase in anthropogenic fingerprints in global geological archives. Proc. Natl. Acad. Sci. U.S.A. 121, e2313098121. doi: 10.1073/pnas.2313098121
Latchere O., Mehn V., Gaertner-Mazouni N., Le Moullac G., Fievet J., Belliard C., et al. (2018). Influence of water temperature and food on the last stages of cultured pearl mineralization from the black-lip pearl oyster Pinctada margaritifera. PloS One 13, e0193863. doi: 10.1371/journal.pone.0193863
Levin L. A., Ekau W., Gooday A. J., Jorissen F., Middelburg J. J., Naqvi S. W. A., et al. (2009). Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 6, 2063–2098. doi: 10.5194/bg-6-2063-2009
Linard C., Gueguen Y., Moriceau J., Soyez C., Hui B., Raoux A., et al. (2011). Calcein staining of calcified structures in pearl oyster Pinctada margaritifera and the effect of food resource level on shell growth. Aquaculture 313, 149–155. doi: 10.1016/j.aquaculture.2011.01.008
Locarnini R. A., Mishonov A. V., Baranova O. K., Reagan J. R., Boyer T. P., Seidov D., et al. (2024). Temperature. World Ocean Atlas 2023, 1. doi: 10.25923/54bh-1613
Lowenstam H. A. (1981). Minerals formed by organisms. Science 211, 1126–1131. doi: 10.1126/science.7008198
Marin F., Le Roy N., and Marie B. (2012). The formation and mineralization of mollusk shell. Front. Bioscience S4, 1099–1125. doi: 10.2741/s321
McCoy S. J., Kamenos N. A., Chung P., Wootton T. J., and Pfister C. A. (2018). A mineralogical record of ocean change: Decadal and centennial patterns in the California mussel. Global Change Biol. 24, 2554–2562. doi: 10.1111/gcb.2018.24.issue-6
Mikkelsen P. M. and Bieler R. (2007). Seashells of southern florida: living marine mollusks of the florida keys and adjacent regions: bivalves Princeton, New Jersey: Princeton University Press. doi: 10.1515/9780691239453
Milano S., Schöne B. R., and Witbaard R. (2017). Changes of shell microstructural characteristics of Cerastoderma edule (Bivalvia) — A novel proxy for water temperature. Palaeogeography Palaeoclimatology Palaeoecol. 465, 395–406. doi: 10.1016/j.palaeo.2015.09.051
Muhammad G., Atsumi T., and Komaru A. (2020). The influence of water temperature, salinity and food availability on nacre deposition rates in shells and pearls of Japanese and hybrid pearl oyster, Pinctada fucata (Gould 1850). Aquaculture 528, 735512. doi: 10.1016/j.aquaculture.2020.735512
Muhammad G., Atsumi T., Sunardi, and Komaru A. (2017). Nacre growth and thickness of Akoya pearls from Japanese and Hybrid Pinctada fucata in response to the aquaculture temperature condition in Ago Bay, Japan. Aquaculture 477, 35–42. doi: 10.1016/j.aquaculture.2017.04.032
Müller I. A., Fernandez A., Radke J., Van Dijk J., Bowen D., Schwieters J., et al. (2017). Carbonate clumped isotope analyses with the long-integration dual-inlet (LIDI) workflow: Scratching at the lower sample weight boundaries. Rapid Commun. Mass Spectrometry 31, 1057–1066. doi: 10.1002/rcm.7878
Nardone J. A., Patel S., Siegel K. R., Tedesco D., McNicholl C. G., O’Malley J., et al. (2018). Assessing the impacts of ocean acidification on adhesion and shell formation in the barnacle Amphibalanus amphitrite. Front. Mar. Sci. 5, 369. doi: 10.3389/fmars.2018.00369
Olson I. C., Kozdon R., Valley J. W., and Gilbert P. U. P. A. (2012). Mollusk shell nacre ultrastructure correlates with environmental temperature and pressure. J. Am. Chem. Soc. 134, 7351–7358. doi: 10.1021/ja210808s
Prandle D. and Lane A. (1995). Stability of the annual temperature cycle in shelf seas. J. Thermal Biol. 20, 111–120. doi: 10.1016/0306-4565(94)00039-L
Rabalais N. N., Cai W.-J., Carstensen J., Conley D. J., Fry B., Hu X., et al. (2015). Eutrophication-driven deoxygenation in the coastal ocean. Oceanography 27, 172–183. doi: 10.5670/oceanog.2014.21
Reagan J. R., Boyer T. P., García H. E., Locarnini R. A., Baranova O. K., Bouchard C., et al. (2024). World Ocean Atlas 2023, NOAA National Centers for Environmental Information. Dataset: NCEI Accession 0270533. Available online at: https://www.ncei.noaa.gov/access/metadata/landing-page/bin/iso?id=gov.noaa.nodc:0270533. (Accessed November 26, 2024).
Ries J. B. (2011). Skeletal mineralogy in a high-CO2 world. J. Exp. Mar. Biol. Ecol. 403, 54–64. doi: 10.1016/j.jembe.2011.04.006
Rousseau M., Lopez E., Stempflé P., Brendlé M., Franke L., Guette A., et al. (2005). Multiscale structure of sheet nacre. Biomaterials 26, 6254–6262. doi: 10.1016/j.biomaterials.2005.03.028
Salman J., Stifler C. A., Shahsafi A., Sun C.-Y., Weibel S. C., Frising M., et al. (2021). Hyperspectral interference tomography of nacre. Proc. Natl. Acad. Sci. 118, e2023623118. doi: 10.1073/pnas.2023623118
Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Steffen W., Broadgate W., Deutsch L., Gaffney O., and Ludwig C. (2015). The trajectory of the anthropocene: the great acceleration. Anthropocene Rev. 2, 81–98. doi: 10.1177/2053019614564785
Suzuki M. and Nagasawa H. (2013). Mollusk shell structures and their formation mechanism. Can. J. Zoology 91, 349–366. doi: 10.1139/cjz-2012-0333
Taylor J. D., Kennedy W. J., and Hall A. (1969). The shell structure and mineralogy of the Bivalvia (Nuculacea-Trigonacea).. Bull. Br. Mus. Nat. Hist. Zool. 3 (Suppl.), 1–125.
Keywords: nacre, biomineral, mollusk, ocean temperature, conservation paleobiology, scanning electron microscopy, climate, global change biology
Citation: Carskaddan JS, Rillo MC, Harnik PG and Metzler RA (2025) Nacre microstructure records spatiotemporal variation in temperature in the modern ocean. Front. Mar. Sci. 12:1517327. doi: 10.3389/fmars.2025.1517327
Received: 29 October 2024; Accepted: 08 July 2025;
Published: 31 July 2025.
Edited by:
Menghong Hu, Shanghai Ocean University, ChinaReviewed by:
Ruth H. Carmichael, Dauphin Island Sea Lab, United StatesAlejandro Rodriguez-Navarro, University of Granada, Spain
Copyright © 2025 Carskaddan, Rillo, Harnik and Metzler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul G. Harnik, cGhhcm5pa0Bjb2xnYXRlLmVkdQ==; Rebecca A. Metzler, cm1ldHpsZXJAY29sZ2F0ZS5lZHU=
 Jane S. Carskaddan
Jane S. Carskaddan Marina C. Rillo
Marina C. Rillo Paul G. Harnik
Paul G. Harnik Rebecca A. Metzler
Rebecca A. Metzler