- 1Department of Research and Conservation, California White Shark Project, Inverness, CA, United States
- 2Coastal Oregon Marine Experiment Station, Oregon State University, Newport, OR, United States
- 3Hopkins Marine Station, Stanford University, Pacific Grove, CA, United States
- 4Department of Marine Science, California State University Monterey Bay, Seaside, CA, United States
1 Introduction
The study of large marine organisms is limited due to the difficulty of making field observations throughout the expansive, deep and relatively opaque ocean environment. While significant advances in electronic tagging and molecular techniques have broadened the scope of questions that can be effectively addressed, major aspects of life history remain unknown. White sharks (Carcharodon carcharias) in central California, for instance, are one of the most studied elasmobranch species with over 21,000 instrumented tracking days (Andrzejaczek et al., 2022), documented regional population trends (Kanive et al., 2021), and a sequenced genome (Marra et al., 2019). Despite decades of dedicated research, targeted tourism, and natural history observation (Jorgensen et al., 2022), significant gaps remain in our basic knowledge of their natural history – for example, no one knows the gestation period of a white shark, where they give birth, when and where they mate, and how they interact with prey, predators and each other (Jorgensen et al., 2022). Over the course of these efforts, a proliferation of photographic devices with increasing miniaturization and ever-improving picture resolution has resulted in vast image libraries documenting individual white sharks repeatedly photographed over decades (Anderson et al., 2011). Our observations of white sharks off Central California, documented in an image library, reveal a large number of injuries, wounds, and scars that occur and heal at regular intervals. The unique patterns and the timing of these marks may provide novel insights into the life history of white sharks that have remained undescribed. Additionally, similar libraries from other regional populations (Towner et al., 2012) can provide insights into region specific as well as broad comparisons of life history patterns. However, before we can use these scars to better understand white shark life history, the source of the wounds must first be determined and systematically classified.
To date, a modest number of studies focused on wounds or scars on sharks and marine mammals have made progress in identifying their causes. Injuries resulting from boat strikes and subsequent healing rates have been described on white sharks (Towner et al., 2012) as well as whale sharks (Speed et al., 2008) and manta rays (McGregor et al., 2019). Diagnostic wounds made by propeller blades are reflected by sets of evenly spaced parallel cuts approximately perpendicular to the direction of vessel travel (Rommel et al., 2007; Towner et al., 2012). Scarring on white sharks consistent with interaction with large squid have also been reported (Becerril-García et al., 2020). Squid marks bear a distinctive pattern with rows of circular scars tapering in diameter consistent with suckers ringed with sharp teeth, that flank the arms and tentacles of larger squid species. Small bites extracted by cookiecutter sharks, common on open ocean cetaceans, have also been identified on white sharks (Hoyos-Padilla et al., 2013). Killer whales are a known white shark predator (Jorgensen et al., 2019), and a failed predatory attempt can leave the signature impression of their tightly spaced pointed teeth (De Maddalena, 2023).
In this paper we propose a systematic classification system to describe the different types of wounds and scars commonly observed on white sharks in the northeastern Pacific associated with prey handling, parasites, conspecific aggression and anthropogenic impacts. The systematic approach described in this study includes a dichotomous key for more efficient and consistent scar classification which may be applicable to numerous other marine organisms where long-term image documentation is obtainable.
2 Methods
From 1987 to 2024, while conducting seasonal research on white sharks (Carcharodon carcharias) around seal rookeries in central California (Año Nuevo Island, Southeast Farallon Island, Point Reyes, Tomales Point, and Monterey Bay), we archived hundreds of hours of underwater video recordings of individual sharks (Anderson et al., 2011; Chapple et al., 2011; Kanive et al., 2015, 2019). White sharks were attracted toward our research boat using a seal decoy made from outdoor carpet and deployed with a standard medium tackle rod and reel. In most cases a small (~2kg) piece of marine mammal blubber (use permitted by National Marine Fisheries Service) was tethered to the boat to provide a localized olfactory attractant to increase the shark’s proximity to the boat once they approached. In Monterey Bay, no decoy or attractant was used and sharks were visually spotted at the surface and approached. With the shark near the boat, we recorded video images using a ‘dip camera’ attached to a 2–4 m pole (camera specifications varied over this period but should not impact the results of this study). Whenever possible, both sides of the shark were imaged, with special attention given to the dorsal fin profile, for individual identification, and the rear ventral area, to determine sex (Anderson et al., 2011).
While individual ID and sex were initially the primary reason for video documentation, many different types of wounds and scars on the white sharks were recorded. To systematically classify the various scars and wounds, we reviewed a selection of clear scar images and matched the wound impressions with candidate causes based on known interactions classes that could result in injury, these included, 1) Conspecific - e.g. these were aggressive bites or mating hold bites, 2) Prey handling - e.g. scratches or bites from their preferred pinniped prey, 3) Environmental – e.g. scrapes from contact with rocky reef, 4) Parasitic - e.g. cookiecutter shark bites or copepod attachment, and 5) Anthropogenic - e.g. gear entanglement or boat strikes. To facilitate standardizing scar classification, we developed a dichotomous key based on a series of yes/no questions about the size, depth and pattern of an observed wound or scar.
3 Scar categories
Between 1987 and 2022 we recorded over 2500 video clips documenting more than 500 unique individual white sharks in central California. Reviewing every scar was beyond the scope of this study, however from a representative collection of scar observations across a range of age, sex, and locations our sorting by similar scar and wound shapes resulted in 12 observed injury sources, each falling under one of the following five broad interaction classes (Table 1).
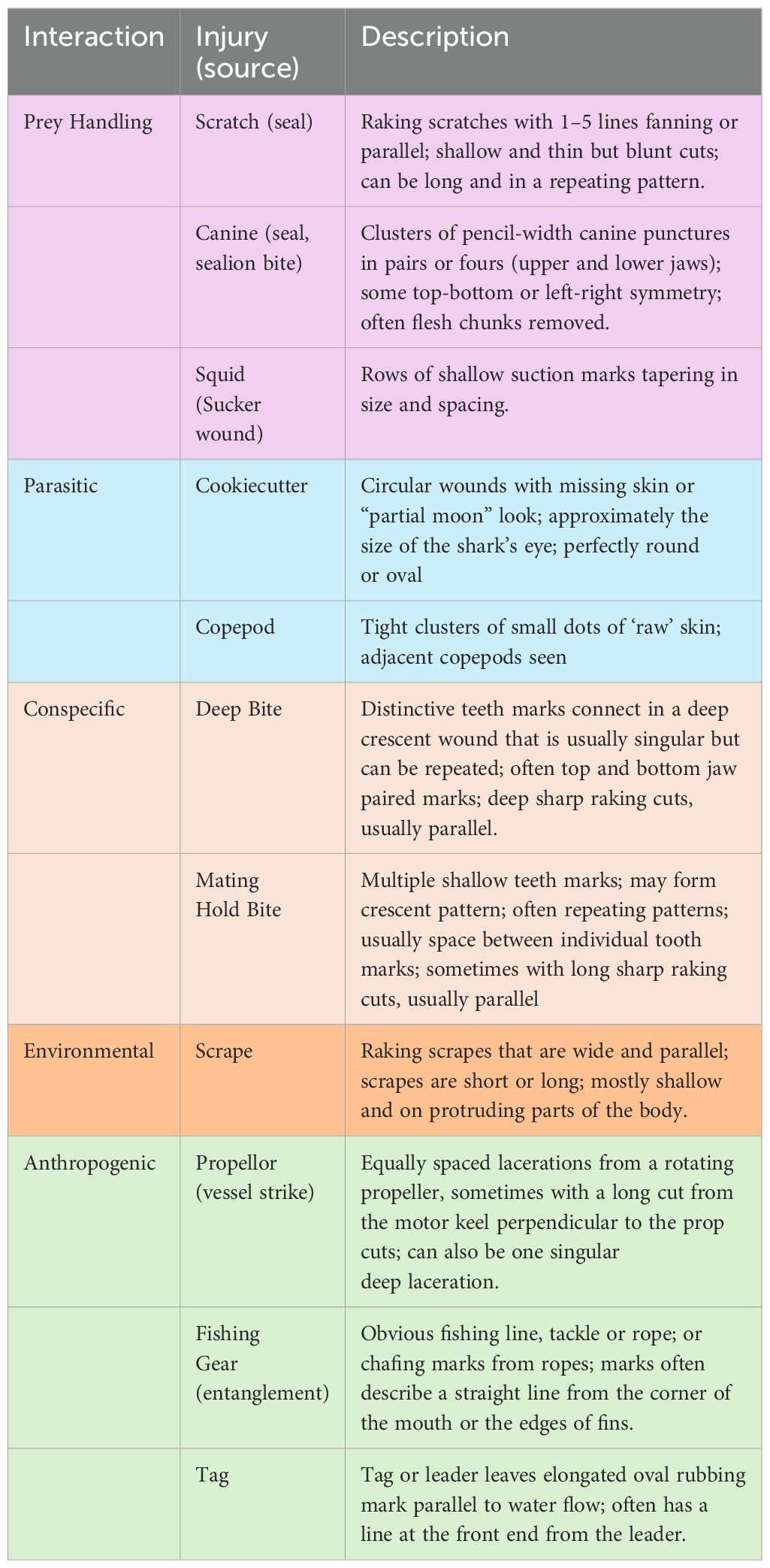
Table 1. A list of the 5 categories of scar types (colored by category) and a general visual description of each to identify the likely source.
3.1 Conspecific
The impression of white shark teeth is highly distinctive. Their teeth are serrated, extremely sharp, and are capable of inflicting deep cuts that generally form a crescent shape wound where individual tooth cuts are evenly spaced. If the bite is deeper, the individual tooth impressions connect into a single large and slightly jagged crescent cut.
3.1.1 Mating hold bites (shallow bites)
Many scars and wounds clearly formed the crescent shape impression of a second white shark’s jaw. These scars generally fell into two categories; deep injurious bites, or multiple shallow tooth impressions. Lighter tooth impressions were often repeated in proximity resembling multiple light bites, consistent with a ‘hold’ that was repeatedly adjusted. We categorized these as a ‘Hold Bite’ (Figures 1A, 1B). Similar wound patterns have been identified in other species such as nurse sharks (Gangliocytoma cirratum), blacktip reef sharks (Carcharhinus melanopterus) associated with mating activity (Pratt and Carrier, 2001).
3.1.2 Deep bites
These bites are typically large and deep where the individual tooth impressions are connected in a continuous crescent shape (Figures 1C, 1D). These scars can run all the way down the side of the shark, often affecting the gills. What distinguishes these from ‘Hold Bites’ is the depth of the bite and the connected laceration between individual tooth impressions. Unlike Hold Bites, which appear light with multiple repetitions, the Deep Bites appear as one or more single impression deep into the muscle. Whereas Hold Bites appear restrained with a shallow depth of the tooth impressionism, these Deep bites appear more aggressive in nature.
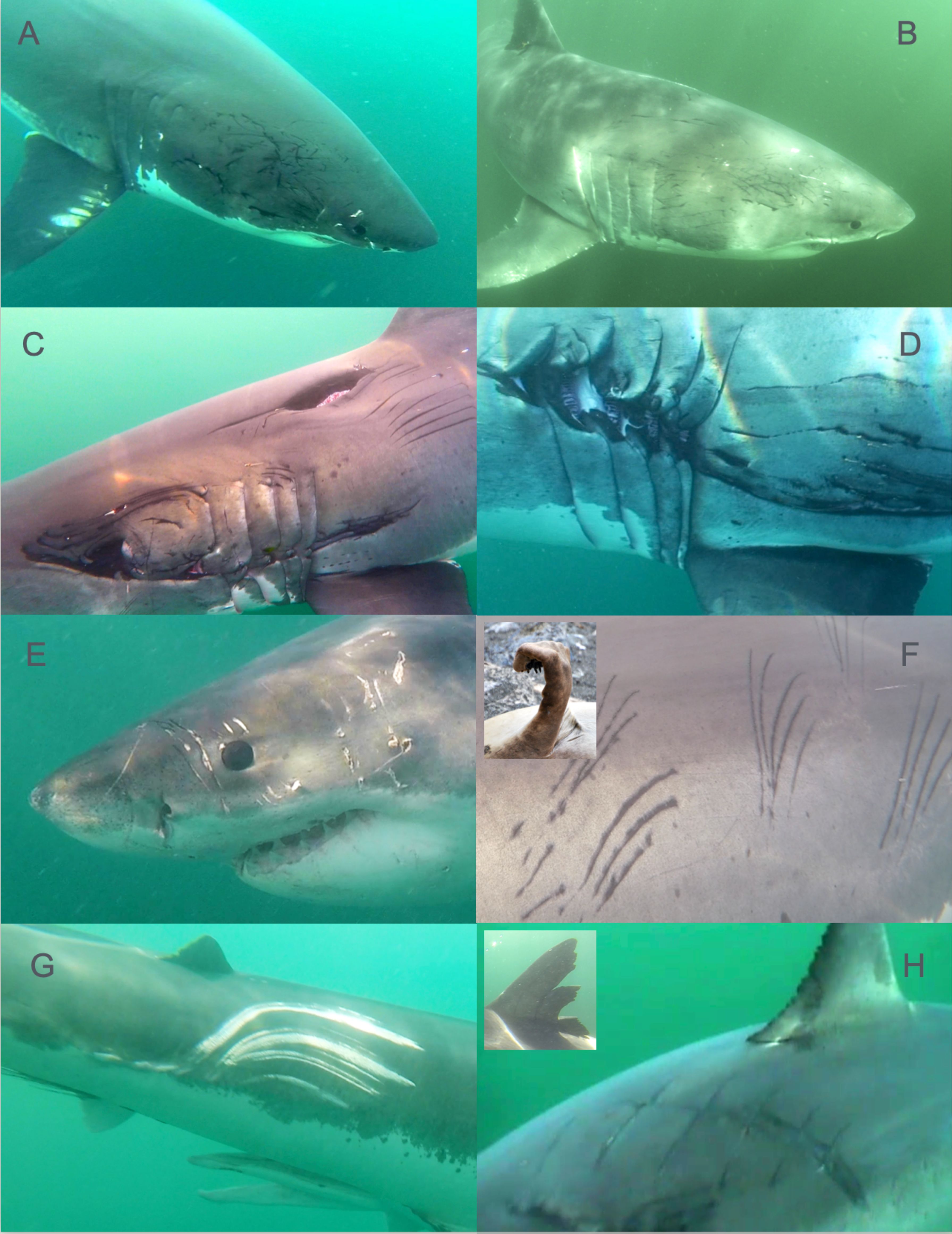
Figure 1. Distinctive wounds and scars on white sharks (C. carcharias) reflect diverse interactions. Conspecific bite marks on white sharks include repeated shallow bites or ‘hold bites’ (A, B) appear restrained and typically occur on large females and presumably result from mating activity. More aggressive ‘deep bites’ (C, D) occur mostly on males and on some females. Paired puncture wounds resulting from seal or sealion canine teeth (E) occur when pinnipeds bite back during prey handling. Another distinctive prey handling injury results from scratches (F) from seal claws (F inset) producing up to 5 parallel or fanning raked cuts. Shallow parallel scrapes (G) running parallel to the shark’s movement result from scraping contact with the reef or other hard substrate. Boat strikes are recognizable from propellor injuries (H) that typically result in a series of deep parallel and perpendicular cuts along the trunk or fins (H inset).
3.2 Prey handling
In central California white sharks aggregated around pinniped rookeries where they were repeatedly observed capturing and consuming northern elephant seals (Mirounga angustirostris), harbor seals (Phoca vitulina), and California sea lions (Zalophus californianus) as the pinnipeds transit back and forth between haulouts and adjacent open water. We observed typical and distinctive prey handling behavior consisted of an ambush bite followed by the release of the pinniped prey and eventual return to the prey after it has been mortally wounded, and has been previously described as ‘bite and spit’ or ‘exsanguination’ (McCosker, 1985; Klimley, 1994). As a result, many white sharks appeared to have injuries inflicted by their pinniped prey, typically around the rostrum, gills and pectoral fins in the form of bites or scratches.
3.2.1 Canine punctures
Pinnipeds (seals and sea lions) have retained canine teeth that are similar in shape and size to those of a dog. Wounds that resembled puncture holes often appeared in pairs, consistent with one pair from a pinniped top jaw and another from the lower jaw that are symmetrically opposed from one another (Figure 1E). Sometimes we observed additional scratches potentially caused by the smaller front teeth next to the canine punctures. If a tooth slides across the skin it may leave a line with some skips beginning or ending at a puncture mark. Canine puncture wounds were common and found on the sharks’ head, body and fins, both fresh and healed.
3.2.2 Seal scratches
True seals, both northern elephant seals and harbor seals in the North Pacific, have five sharp claws on the ends of their fore flippers (Figure 1F). Whereas sea lions and fur seals have nails far back from the edge. For defense, sea lions therefore can bite but they can not scratch like a true seal. A common scar pattern encountered resembled a small rake with up to five tines pulled against the shark’s skin, leaving parallel or converging scratches. The spacing between individual scratch marks in these wounds closely resembled a seal claw in scale and occured around the forward portions of white shark’s bodies.
3.2.3 Squid marks
Larger squid species have teeth embedded in their suckers which leave distinctive marks on toothed whales such as sperm whales and have been reported on some white sharks. They appear as a series of circular impressions that taper in size and spacing and can occur in parallel rows. Squid are a potential source of foraging for white sharks while offshore, and scars fitting their description have been reported off Guadalupe Island (Becerril-García et al., 2020), however, the occurrence of scars resembling squid marks on white sharks in central California is very rare.
3.3 Environmental
3.3.1 Scrapes
We recorded evidence of sharks interacting with their physical environment in the form of scrapes that appear to be from abrasive contact with hard substrate (Figure 1G). They appear as large scrapes usually from the first dorsal fin on the flank back to and on the caudal peduncle and fin. These impressions were slightly elongated, with each abrasion much wider than a single pinniped scratch, with frayed wound edges that appear far less clean than raking teeth cuts. They also tended to extend parallel to the body axis, indicating scraping against a hard, relatively dull and stationary object. These were typically not very deep and were mostly observed as white in color unless completely healed and black.
3.4 Parasitic
Although large sharks have formidable dermal denticles that protect their skin, they remain vulnerable to some observable external parasites that produce wounds, scars and other markings.
3.4.1 Cookiecutter sharks
The cookiecutter shark (Isistius brasiliensis) bites have been well described and were occasionally observed in this study. The small (<50cm) squaliform shark lives in the open ocean and is known as an ectoparasite, with prey ranging from the largest apex predators to small, low trophic level species (Carlisle et al., 2021). With large prey, the cookiecutter shark latches onto their body and spins, removing large plugs of tissue. This ice cream scoop-like action removes a very distinctive circular chunk of flesh from the larger ‘host’. The wounds appear as a golf ball to tennis ball size circular bite when successful or like a half circle or a “C” if the parasitic shark fails to remove the bite (Hoyos-Padilla et al., 2013).
3.4.2 Copepods
Copepods are small crustaceans, with parasitic varieties commonly found on many fish and shark species. We observed them on most individuals and on different areas on the shark including the fins, mouth, cloaca and trunk of the body, often appearing clustered in patches. In these patches, they are so close together that they seem to fill the space completely, leaving a pattern of spaced dots where they were attached.
3.5 Anthropogenic
3.5.1 Propeller wounds
Boat propellers cause large traumatic wounds on white sharks which in severe cases are likely to be fatal (Rommel et al., 2007; Towner et al., 2012). While the propeller is rotating, it leaves a series of parallel cuts with even spacing. Propeller cuts are generally clean and straight and evenly spaced (Figure 1H). Most propeller wounds were seen on the middle of the back and dorsal fin, and sometimes the caudal fin as well.
3.5.2 Rope and fishing gear
We have observed a few white sharks with fishing gear with some evidence of entanglement or rolling on a line. A line, if taught, may rub the dermal denticles off the skin leaving a white impression (‘rope burn’) that sometimes reveals the spiral twist of the line, which may resemble squid marks. The line can hang up on the fins and the shark may roll causing the line to chafe the skin. In many cases the gear can be readily identified, for examples sport fishing gear hooked on their fins or mouths.
3.5.3 Tag scars
We have been tagging white sharks in California waters since 1993 with a variety of tag types attached using a subdermal dart (Boustany et al., 2002). Many of these individuals have been reidentified over the years, providing the opportunity to observe and tag individuals repeatedly over long periods. All tags leave some type of scar that remains visible for some time. Marks are typically from the rubbing of the tag or tag assembly (i.e. leaders). In most cases the darted tags shed over time after one to five years (Chapple et al., 2016) including the tag assembly from pop-off satellite tags. Where the tag rubs on the skin, the dermal denticles are worn off, leaving the white skin exposed. Once healed, the area turns black and finally disappears. The most characteristic tag scars are black, featuring two oval shapes in succession with a line in front where the leader pulled out. Since we aim to put the tag in a small area just below (<.25 m) the first dorsal fin the tag scars are typically easy to identify. We have observed reduced rubbing in tags placed closer to the dorsal fin, presumably where flow is more laminar, resulting in a smaller scar.
4 Dichotomous key
We identified five interaction classes of scars/wounds on white sharks in Central California from underwater video (Conspecific, Prey Handling, Environmental, Parasitic, and Anthropogenic), which each described the interaction attributed to one or more of the distinctive scar types. To more efficiently and consistently classify subsequent wounds and scars, our dichotomous key uses a series of yes/no questions about the size, depth and pattern of an observed wound or scar (Figure 2). Some scars will inevitably be less clear because of poor imaging or due to an unusual shape and cannot be classified. In addition, our scheme does not identify all injury types. Still, this work provides a simple and standardized classification system, identifying the most common and recognizable scars/wounds found in these sharks. We further identify their likely causes, either through direct observation or induction, or building on previous work to create a foundation for future studies utilizing scars/wounds to inform our understanding of white shark life history.
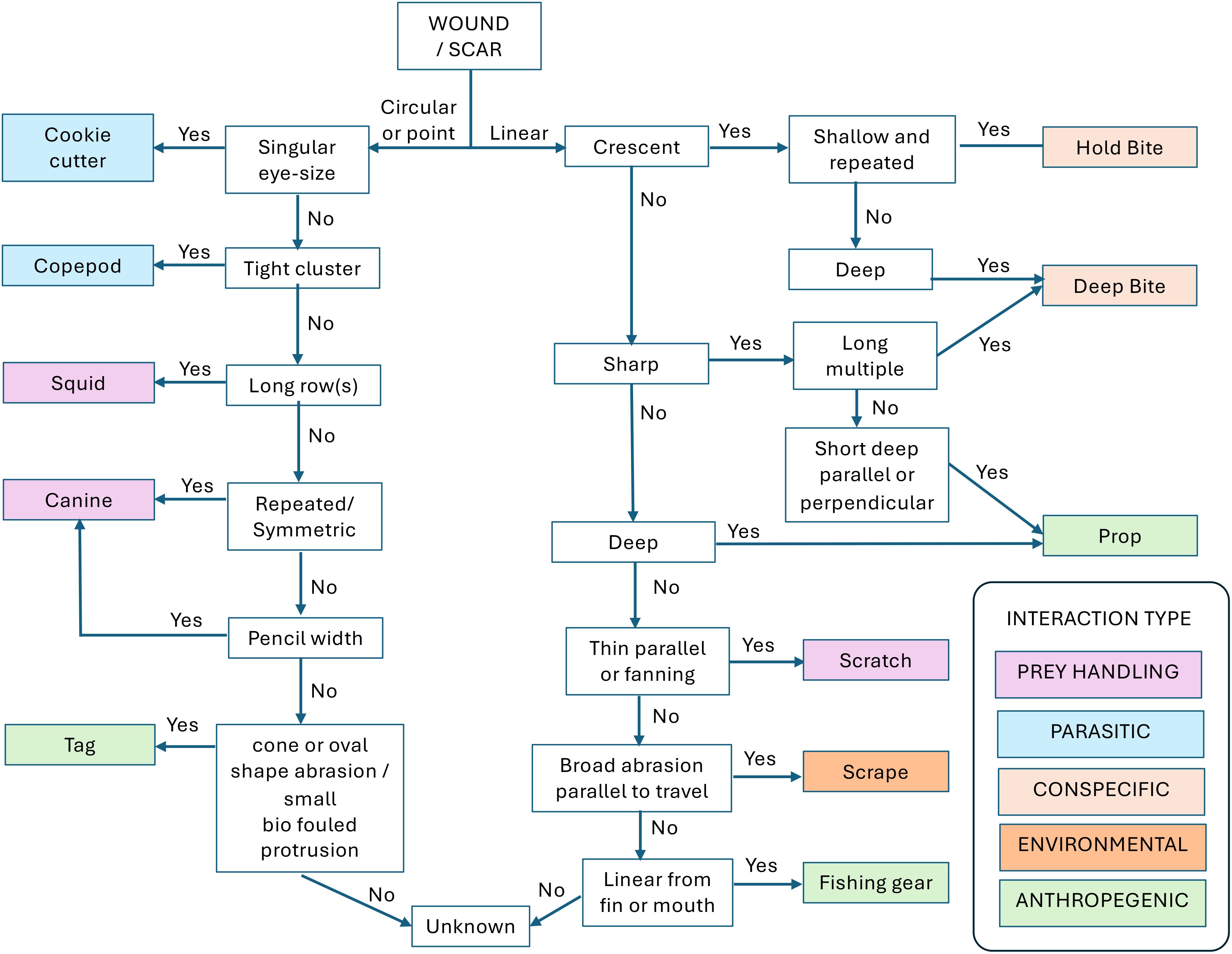
Figure 2. Classification schema and dichotomous key for identifying common wounds and scars observed on white sharks (C. carcharias). A diagnostic description distinguishes each injury type, and the estimated source of each injury type is sorted by general interaction classes associated with broad life history behavioral categories.
5 Conclusion
The categories of this simplified classification system for scars/wounds on white sharks can also be applied to numerous other species where long-term photographic records can be obtained. For example, many large surface-feeding sharks incur boat-strike injuries (Womersley et al., 2022; Chapple et al., 2024) and interact with static fishing gear and most species are subject to parasite and mating scars. Additional categories may also be added in order to further refine species or population specific scar/wound patterns.
This work sets the foundation for continued quantitative analyses of scars/wounds on white sharks with larger datasets and for other populations globally. White sharks heal at generally predictable rates (Jewell et al., 2011; Towner et al., 2012; Chapple et al., 2015), including animals that have experienced significant injury. All wounds that break the skin undergo a visually distinctive healing progression reflected in the coloration ranging from red or pink when fresh, to white and then black as they heal. The black areas fade over many years to the original skin pigmentation. However, if the wound is not obscured by other newer scars, they may remain visible for decades. The white underside of the shark will eventually heal to white accordingly. As such, future studies can capitalize on both the rate of healing and our categorization scheme to identify when, where, and how various scars/wounds occurred thus informing further understanding of their life history. For example, it is uncertain where northeast Pacific white sharks mate (Jorgensen et al., 2010, 2012). Combining known movement data with data on the occurrence and freshness of mating scars and other scar classifications, researchers can infer the timing and location mating is likely to occur (Gallagher et al., 2024). For instance, if mating marks and cookiecutter bites are healed to a similar degree it could indicate those interactions occur at a similar time and place in the migratory cycle - in the open ocean environment. Conversely, if fresh wounds inflicted by coastal pinniped prey coincide with observed fresh mating bites on females then those interactions might be likely to co-occur in space and time. Similarly, identifying prey handling scars/wounds in smaller sharks can indicate the timing of ontogenetic diet shifts. In summary, this work provides a foundational framework for future studies utilizing available data on scars/wounds to inform our understanding of the cryptic life of white sharks and other elasmobranchs more widely.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by California State University Monterey Bay IACUC Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SA: Conceptualization, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. PK: Conceptualization, Investigation, Methodology, Resources, Writing – review & editing. TC: Conceptualization, Investigation, Methodology, Resources, Writing – review & editing. SA: Investigation, Resources, Writing – review & editing. BB: Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing. SJ: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Support of the project provided by the Monterey Bay Aquarium, California White Shark Project, COAST, Sloan, Moore and David and Lucile Packard Foundations and Stanford University. SJJ received support from COAST and the Elakha Alliance, and is thankful for inspiration from the CSUMB Shark Scar project.
Acknowledgments
We are grateful for project assistance including vessel operations by Ron Elliott, Em Homer, Pat Conroy, Tom O’leary, Tom Baty, and Shawn Rhodes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anderson S. D., Chapple T. K., Jorgensen S. J., Klimley A. P., and Block B. A. (2011). Long-term individual identification and site fidelity of white sharks, Carcharodon carcharias, off California using dorsal fins. Mar. Biol. 158, 1233–1237. doi: 10.1007/s00227-011-1643-5
Andrzejaczek S., Lucas T. C. D., Goodman M. C., Hussey N. E., Armstrong A. J., Carlisle A., et al. (2022). Diving into the vertical dimension of elasmobranch movement ecology. Sci. Adv. 8, eabo1754. doi: 10.1126/sciadv.abo1754
Becerril-García E. E., Bernot-Simon D., Arellano-Martínez M., Galván-Magaña F., Santana-Morales O., and Hoyos-Padilla E. M. (2020). Evidence of interactions between white sharks and large squids in Guadalupe Island, Mexico. Sci. Rep. 10, 17158. doi: 10.1038/s41598-020-74294-4
Boustany A. M., Davis S. F., Pyle P., Anderson S. D., Le Boeuf B. J., Block, et al. (2002). Expanded niche for white sharks. Nature 415, 35–36. doi: 10.1038/415035b
Carlisle A. B., Allan E. A., Kim S. L., Meyer L., Port J., Scherrer S., et al. (2021). Integrating multiple chemical tracers to elucidate the diet and habitat of Cookiecutter Sharks. Sci. Rep. 11, 11809. doi: 10.1038/s41598-021-89903-z
Chapple T. K., Cade D. E., Goldbogen J., Massett N., Payne N., and McInturf A. G. (2024). Behavioral response of megafauna to boat collision measured via animal-borne camera and IMU. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1430961
Chapple T. K., Chambert T., Kanive P. E., Jorgensen S. J., Rotella J. J., Anderson S. D., et al. (2016). A novel application of multi-event modeling to estimate class segregation in a highly migratory oceanic vertebrate. Ecology 97, 3494–3502. doi: 10.1002/ecy.1589
Chapple T. K., Gleiss A. C., Jewell O. J. D., Wikelski M., and Block B. A. (2015). Tracking sharks without teeth: a non-invasive rigid tag attachment for large predatory sharks. Anim. Biotelemetry 3, 1–8. doi: 10.1186/s40317-015-0044-9
Chapple T. K., Jorgensen S. J., Anderson S. D., Kanive P. E., Klimley A. P., Botsford L. W., et al. (2011). A first estimate of white shark, Carcharodon carcharias, abundance off Central California. Biol. Lett. 7, 581–583. doi: 10.1098/rsbl.2011.0124
De Maddalena А. (2023). Evidence of a failed predatory attempt by an orca, Orcinus orca (Linnaeus 1758), on a great white shark, Carcharodon carcharias (Linnaeus 1758). Marine Biol. J. 8, 51–55. doi: 10.21072/mbj.2023.08.1.04
Gallagher A. J., de Silva C., Delaney D., Harris S. D., Phillips B. T., Shipley O. N., et al. (2024). Novel behavioral observations and body scarring for the bluntnose sixgill shark (Hexanchus griseus) offer clues to reproductive patterns and potential mating events. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1305487
Hoyos-Padilla M., Papastamatiou Yannis P., O’Sullivan J., and Lowe Christopher G. (2013). Observation of an Attack by a Cookiecutter Shark (Isistius brasiliensis) on a White Shark (Carcharodon carcharias). Pac. Sci. 67, 129–134. doi: 10.2984/67.1.10
Jewell O. J. D., Wcisel M. A., Gennari E., Towner A. V., Bester M. N., Johnson R. L., et al. (2011). Effects of smart position only (SPOT) tag deployment on white sharks carcharodon carcharias in South Africa. PloS One 6, e27242. doi: 10.1371/journal.pone.0027242
Jorgensen S. J., Arnoldi N. S., Estess E. E., Chapple T. K., Rückert M., Anderson S. D., et al. (2012). Eating or meeting? Cluster analysis reveals intricacies of white shark (Carcharodon carcharias) migration and offshore behavior. PloS One 7, e47819. doi: 10.1371/journal.pone.0047819
Jorgensen S. J., Anderson S., Ferretti F., Tietz J. R., Chapple T., Kanive P., et al. (2019). Killer whales redistribute white shark foraging pressure on seals. Scientific Reports 9 (1), 6153. doi: 10.1038/s41598-019-39356-2
Jorgensen S. J., Micheli F., White T. D., Houtan K. S. V., Alfaro-Shigueto J., Andrzejaczek S., et al. (2022). Emergent research and priorities for shark and ray conservation. Endanger. Species Res. 47, 171–203. doi: 10.3354/esr01169
Jorgensen S. J., Reeb C. A., Chapple T. K., Anderson S., Perle C., Van Sommeran S. R., et al. (2010). Philopatry and migration of Pacific white sharks. Proc. R. Soc B Biol. Sci. 277, 679–688. doi: 10.1098/rspb.2009.1155
Kanive P. E., Rotella J. J., Chapple T. K., Anderson S. D., White T. D., Block B. A., et al. (2021). Estimates of regional annual abundance and population growth rates of white sharks off central California. Biological Conservation 257, 109104. doi: 10.1016/j.biocon.2021.109104
Kanive P. E., Rotella J. J., Jorgensen S. J., Chapple T. K., Anderson S. D., Klimley A. P., et al. (2015). Estimating apparent survival of sub-adult and adult white sharks (Carcharodon carcharias) in central California using mark-recapture methods. Mar. Megafauna 2, 109104. doi: 10.3389/fmars.2015.00019
Kanive P. E., Rotella J. J., Jorgensen S. J., Chapple T. K., Hines J. E., Anderson S. D., et al. (2019). Size-specific apparent survival rate estimates of white sharks using mark–recapture models. Can. J. Fish. Aquat. Sci. 76, 1–8. doi: 10.1139/cjfas-2018-0142
Marra N. J., Stanhope M. J., Jue N. K., Wang M., Sun Q., Bitar P. P., et al. (2019). White shark genome reveals ancient elasmobranch adaptations associated with wound healing and the maintenance of genome stability. Proc. Natl. Acad. Sci. 116, 4446–4455. doi: 10.1073/pnas.1819778116
McCosker J. E. (1985). White shark attack behavior: observations of and speculations about predator and prey strategies. Mem. South. Calif. Acad. Sci. 9, 123–135.
McGregor F., Richardson A. J., Armstrong A. J., Armstrong A. O., and Dudgeon C. L. (2019). Rapid wound healing in a reef manta ray masks the extent of vessel strike. PloS One 14, e0225681. doi: 10.1371/journal.pone.0225681
Pratt H. L. and Carrier J. C. (2001). A review of elasmobranch reproductive behavior with a case study on the nurse shark, Ginglymostoma cirratum. Environ. Biol. Fishes 60, 157–188. doi: 10.1023/A:1007656126281
Rommel S. A., Costidis A. M., Pitchford T. D., Lightsey J. D., Snyder R. H., and Haubold E. M. (2007). Forensic methods for characterizing watercraft from watercraft-induced wounds on the florida manatee (trichechus manatus latirostris). Mar. Mammal Sci. 23, 110–132. doi: 10.1111/j.1748-7692.2006.00095.x
Speed C. W., Meekan M. G., Rowat D., Pierce S. J., Marshall A. D., and Bradshaw C. J. A. (2008). Scarring patterns and relative mortality rates of Indian Ocean whale sharks. J. Fish Biol. 72, 1488–1503. doi: 10.1111/j.1095-8649.2008.01810.x
Towner A., Smale M. J., and Jewell O. (2012). “Boat strike wound healing in Carcharodon carcharias,” in Global perspectives on the biology and life history of the great white shark. Ed. Domeier M. L. (CRC Press, Boca Raton, FL), 77–84.
Keywords: shark, behavior, ecology, observation, interaction
Citation: Anderson SD, Kanive PE, Chapple TK, Andrzejaczek S, Block BA and Jorgensen SJ (2025) A classification system for wounds and scars observed on white sharks (Carcharodon carcharias). Front. Mar. Sci. 12:1520348. doi: 10.3389/fmars.2025.1520348
Received: 31 October 2024; Accepted: 22 April 2025;
Published: 03 June 2025.
Edited by:
Austin Gallagher, Beneath the Waves, Inc., United StatesReviewed by:
Mariana Díaz-Santana-Iturrios, Instituto Mexicano de Investigación en Pesca y Acuacultura Sustentables (IMIPAS), MexicoMassimiliano Bottaro, Anton Dohrn Zoological Station Naples, Italy
Copyright © 2025 Anderson, Kanive, Chapple, Andrzejaczek, Block and Jorgensen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scot D. Anderson, c2hhcmttYW4xMTM3QGVhcnRobGluay5uZXQ=
 Scot D. Anderson
Scot D. Anderson Paul E. Kanive
Paul E. Kanive Taylor K. Chapple
Taylor K. Chapple Samantha Andrzejaczek
Samantha Andrzejaczek Barbara A. Block
Barbara A. Block Salvador J. Jorgensen
Salvador J. Jorgensen