- Caspian Sea Ecology Research Center (CSERC), Iranian Fisheries Science Research Institute (IFSRI), Agricultural Research, Education and Extension Organization (AREEO), Sari, Iran
Introduction: To effectively manage fish stocks, evaluating various aspects of population dynamics is considered crucial. Understanding invasive species is essential for conservation efforts. This study represents the first documentation of population parameters.
Methods: For this purpose, the population dynamics parameters, including growth, mortality, and recruitment of A. boyeri, found in the Coastal Waters of the southeast Caspian Sea of Northern Iran, were analyzed using FiSAT II software.
Result: The analyzed specimens were sampled with a total weight range of 1.2 to 12.51 g and a total length range of 60 to 125 mm. The population has a 5-year life cycle. The length-weight relationship was estimated as W=0.0055 L3.0774 for males and W= 0.0083 L2.9185 for females. The slope value (b) of the length-weight relationships obtained for A. boyeri were between 2.69-3.13 with an average value of 2.91. While populations of A. boyeri showed negative allometric growth patterns, the males of exhibited positive allometric growth patterns, whereas the females had negative allometric growth patterns. The von Bertalanffy growth function fitted to back-calculated size at age data was: Lt = 126.5 [1- exp - 0.34(t + 0.234)] and Lt =131.3[1-exp- 0.35(t + 0.374)] for males and females respectively. The average relative condition factor was reported to be 0.62 and 0.79 for males and females respectively. The growth performance index (∅’) was reported to be 1.74 and 2.10 for males and females respectively. The total mortality of 1.73 year–1, consisting of natural mortality of 1.38 year–1 and fishing mortality of 0.35 year–1 and 1.21 year–1, consisting of natural mortality of 0.90 year–1 and fishing mortality of 0.31 year–1 was reported for males and females respectively. An exploitation rate of 0.20 and 0.26 for males and females respectively, is suggestive of a less exploited state of the fish. For both sexes, the length at first maturity was found to be higher than the length at first capture, a condition that can disturb the stock, as such the utility of a net with a relatively larger mesh size is advisable. The sex ratio was 1:1.34 in favor of females. For both sexes, the reproductive season, evaluated from GSI, extended from March to June, with a peak in April. The average absolute and relative fecundities were 2865 eggs and 684 eggs g-1 of body weight respectively. The diameter of oocytes ranged from 0.028 to 0.25 mm with a mean value of 0.54.
Discussion: The life-history patterns of A. boyeri in the population under study imply that the population of this species in the southeast Caspian Sea differs markedly from those of other localities of its range distribution. The differences were thought to be due to differences in geographical locations. The current research on the population dynamics of A. boyeri can be used as the baseline data for its management stocks.
1 Introduction
The Caspian Sea, as the largest inland body of water in the world, holds special significance in the fields of fisheries and marine resources (Aladin and Plotnikov, 2004; Convention on the Legal Status of the Caspian Sea, 2018). Due to its high biodiversity and rich reserves of fish and other marine resources, this sea has become one of the key areas for the fishing industry. This biodiversity contributes to a variety of fishery products. Despite the economic value of this sea, there are environmental challenges such as pollution, overfishing, and climate change. Therefore, the protection and sustainable management of the Caspian Sea’s marine resources are essential to preserving these values. Scientific research on fish and the ecosystems of the Caspian Sea can aid in improving resource management practices and promoting sustainable fisheries. In general, the Caspian Sea plays a vital role in the fishing industry of the countries bordering it due to its rich economic resources (Convention on the Legal Status of the Caspian Sea. 2018). Increasing evidence indicates that marine species may be placed under threat of local, and ultimately global, extinction due to the direct or indirect effects of fishing (Pitcher, 1998; Roberts and Hawkins, 1999; Wolff, 2000; Reynolds et al., 2001; Dulvy et al., 2003).
The big-scale sand smelt, A. boyeri Risso, 1810, is a small inshore species common in the Mediterranean (along the Mediterranean coasts, and Black, Azov, and Caspian Sea basins) and in the north-east Atlantic (from the Azores to the north-west coast of Scotland) (Bianco et al., 2013: Lorenzoni et al., 2015). This species mainly inhabits coastal and estuarine waters and lagoons, over a wide range of salinities (from freshwater to hypersaline) and, more rarely, freshwaters (Freyhof and Kottelat, 2008). A few permanent freshwater resident populations have also been reported from Santo André lagoon (Iberian Peninsula) and Trichonis Lake (Greece) (Freyhof and Kottelat, 2008). Moreover, this euryhaline species was successfully introduced into many lakes for stock enhancement purposes or due to accidental transfer (Economidis et al., 2000; Leonardos, 2001; Bianco et al., 2013). The Atherina boyeri (Big-scale sand smelt) is classified as “Least Concern” (LC) on the IUCN Red List. This assessment was conducted in 2008 due to its wide distribution and the absence of significant threats to its population (Benzer, 2024; Froese and Pauly, 2024). Atherina boyeri is a highly euryhaline species, tolerating a wide range of salinity. It is commonly found in brackish waters and occasionally in freshwater. Habitats include estuaries, coastal lakes, river mouths, and the sea. In freshwater, it prefers still or slow-flowing waters. The species can grow up to 200 mm in total length and lives up to 4 years. It exhibits negative allometric growth, meaning its weight increases less proportionally compared to its length. This growth pattern may indicate environmental limitations in certain habitats (Benzer, 2024). Atherina boyeri lays demersal eggs that attach to filamentous algae substrates at depths of 2 to 6 meters. The larvae are pelagic and tend to form schools near shorelines (Benzer, 2024; Froese and Pauly, 2024).
Knowledge of invasive species is critical for conservation (Vejan et al., 2023). This study is the first documentation on population parameters of big-scale sand smelt, A. boyeri Risso, 1810, in the southeast Caspian Sea. Growth parameters and Mortality rates studies facilitate the assessment of a fish stock by analyzing three main parameters of a population (Kilduf, 2009; Vivekanandan, 2005). The utility of hard parts for growth assessment faces multiple constraints (especially the sophistication of equipment and time consumption) making length frequency analysis methods a widely used approach to assess age and growth in fishes (Pauly, 1984; Kindong et al., 2018). This has eventually led to the development of various computer programs like the one developed by Fournier et al. (1990) i.e., the MUL-TIFAN, and the other developed by Gayanilo and Pauly (1997) i.e., the FiSAT, both utilizing the length frequency data to assess various population parameters of a fish stock.
Several studies have been conducted on the age, and growth parameters of A. boyeri in marine and brackish environments in different parts of the world (Danilova, 1991). Bartulović, 2003; Gisbert et al., 1996; Pallaoro et al., 2002; Bartulović et al., 2004; Lorenzoni et al., 2015; Benzer and Benzer, 2019; Benzer, 2024; Fahmy Mehana, 2024). However, there is very little information about this species’ population dynamics parameters and reproductive biology in the Southeast Caspian Sea (Patimar et al., 2008).
Differences in environmental conditions undoubtedly affect the life history strategies of the species, causing marked changes in the population parameters (Vejan et al., 2023), which in turn are pivotal to informing conservation decisions. Generally, the life history information is quantified with measurements of growth, mortality, and reproduction. All of these, however, have been regarded as reliable indications of the state of life history strategy of an established population of species. Additionally, detailed data on the population structure of the shrimp are crucial to understanding its place in the functional structure of ecosystems (Vejan et al., 2023). By studying these measurements, the appropriate conservation measures could be determined for species A. boyeri. In some way, this data may aid in inferring the invasion success, which is an important criterion for the species.
This study is the first attempt to assess the stock of A. boyeri in Iran Coastal waters (Southeast Caspian Sea) to manage and conserve the population. Due to the limited research conducted on this species, especially regarding studies of population dynamics, the current study was launched to evaluate the growth, recruitment, exploitation, and mortality rates of A. boyeri from the Southeast Caspian Sea.
2 Methodology
2.1 Study area and location sites
The study was undertaken in the Northern Iran marine waters within the Southeast Caspian Sea, and the Southeast Caspian Sea is one of the most important areas in the sea- Northern Iran (Figure 1). The Caspian Sea area is divided into three, approximately equal, parts: Northern, Middle, and Southern (Aladin and Plotnikov, 2004). The volumes are extremely different. The Southern Caspian has the largest volume - some 64% of the total volume, and its area is 35% of the total sea area. It is the deepest part of the sea with the maximum depth reaching 1025 m. According to I.S. Zonn (2000), the area is from 144690 up to 151018 km2, and the average volume - 48300 km3. The average depth is 300 m.
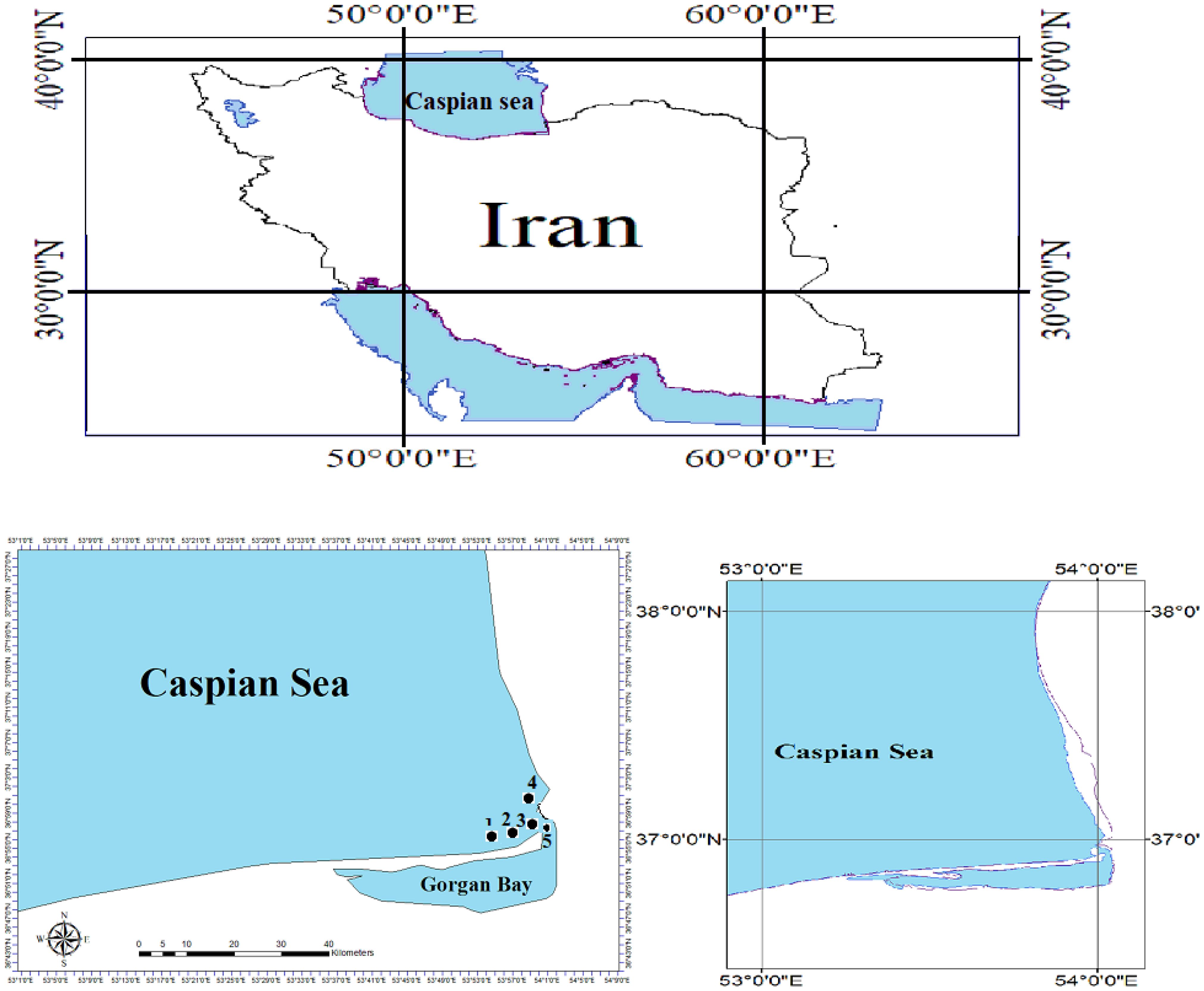
Figure 1. Sampling localities of the big scale sand smelt, A. boyeri in the southeast Caspian Sen-Iran (2022-2023).
2.2 Data collection
Sampling was carried out using a small beach seine (30 m long, 2 m depth, and mesh size of 3 mm knot to knot), a total of 956 specimens were collected in the southeast Caspian Sea from March 2022 until February 2023. It was set out in such a way that the dragline of one wing remained fastened to the beach, while one wing, the net bag, and then the other wing with its dragline were taken out in a wide arc and then brought back to the beach. In the laboratory, total length (TL) was measured to the nearest 1 mm for all fish sampled. Total weight, the weight of gonads, and their subsamples were recorded with an electronic analytical balance to the nearest 0.01 g. For the age determination scales and opercula were used.
2.2.1 Length-weight relationship
The equation provided by Le Cren (1951) was used to assess the LWRs of the fish.
Where W denotes the total weight of the fish, L denotes the total length of the fish, b represents the slope and a represents the intercept of the length vs weight graph.
2.2.2 Relative condition factor (Kn)
To avoid some drawbacks of the Fulton condition factor, the relative condition factor was introduced by Le Cren (1951). It is the ratio of observed weight to that of calculated weight and is provided below:
2.2.3 Growth parameters and mortality
The two major growth parameters i.e., asymptotic length (L∞) and growth constant (K) were computed using the FiSAT II software (ELEFAN-I module) (Gayanilo et al., 1995), utilizing the equation given below:
The value of the age at zero length (t0) was determined utilizing the equation provided by Pauly (1979), i.e.,
The growth performance index (∅’) was determined using the equation given by Pauly and Munro (1984), i.e.,
The natural mortality (M) was calculated using FiSAT II, utilizing the equation given by Pauly (1980), i.e.,
Where K represents the growth constant, L∞ is the asymptotic length and T is the mean temperature of the water body. The length-converted catch curve was used to determine the fishing mortality (F) and total mortality (Z) as per Pauly (1983). The exploitation ratio (E) was computed using the equation provided by Beverton and Holt (1957) and Ricker (1975) i.e.,
2.2.4 Recruitment pattern, virtual population analysis, Beverton, and Holt analysis
The monthly recruitment pattern of the fish was computed using the FiSAT II software as per Dadzie et al. (2007). Using the growth coefficients of the LWR equation, growth, and mortality parameters, the virtual population analysis (VPA) was carried out as per Gayanilo et al. (2005). The knife edge module of FiSAT II was used for the Beverton and Holt analysis to plot the yield and bio-mass/recruit against the rate of exploitation (Beverton and Holt, 1966; Pauly and Soriano, 1986).
2.2.5 Length at first maturity
The length at first maturity (Lm) describes the length at which 50% population attains reproductive maturity. To determine Lm, the frequencies of occurrence of adult mature specimens were plotted against length class and fitted to a logistic function.
2.3 Reproduction data
Sex was determined by examination of the gonads. Maturity stages were estimated according to the Nikolsky (1963) scale. Gonadosomatic index (GSI %) = (gonad weight/total body weight) - 100 was calculated for each fish and all values were averaged for each sampling date. A. boyeri is a batch spawner, its spawning takes place over a long period of 2-3 months (Creech, 1992). Therefore, to decrease the error due to multiple spawning, we used ovaries of 126 ripe specimens with IV maturity stage, from females caught in March to estimate absolute and relative fecundities. The gonads were removed, weighed, and then placed in Gilson’s fluid for 3-4 weeks to harden eggs and dissolve ovarian membranes. The peritoneum was removed and individual eggs were released from the egg mass. The number of eggs was estimated by gravimetric method, using three pieces removed from the ovary. The relative fecundity index was calculated as RF = AF/TW, where AF is absolute fecundity and TW is total weight (Bagenal and Tesch, 1978). Egg diameter was examined by measuring 20-30 eggs taken randomly from pieces of the ovary of 254 ripe females caught in March 2022-February 2023. Measurements were made to the nearest 0.05 mm with an ocular micrometer microscope. Comparison of GSI between months was carried out by analysis of variance (ANOVA). An analysis of covariance (ANCOVA) was performed to test significant differences in weight-length relationships and GSI values between the sexes. The overall sex ratio was assessed using the Chi-square test (Zar, 1984). Statistical analyses were performed with SPSS-26 software package and a significant level of 0.05 was accepted.
3 Results
3.1 Sex ratio
The overall sex ratio was 1:1.34 (M: F; 569 females, 387 males). The sex ratio of the population was significantly different from the ideal Mendel ratio [X2 (1, N= 956) = 34.65, p < 0.001], yet sex ratio changes were found to be significant within age classes. Males were significantly dominant in the 1 and 2 age groups, whereas females were significantly dominant in 2 and older age groups [X2 age1 (1, N = 141) = 3.32, p > 0.05; X2 age2 (1, N = 112) = 6.00, p> 0.05; X2 age2 (1, N = 176) = 4.64, p > 0.05; X2 age3 (1, N = 147) = 12.62, p > 0.05; X2 age4 (1, N = 144) = 11.26, p > 0.05); X2 age5 (1, N = 6) = 13.01, p > 0.05]. All the specimens were found to be sexually mature in the first spring after hatching.
3.2 Length-weight relationship
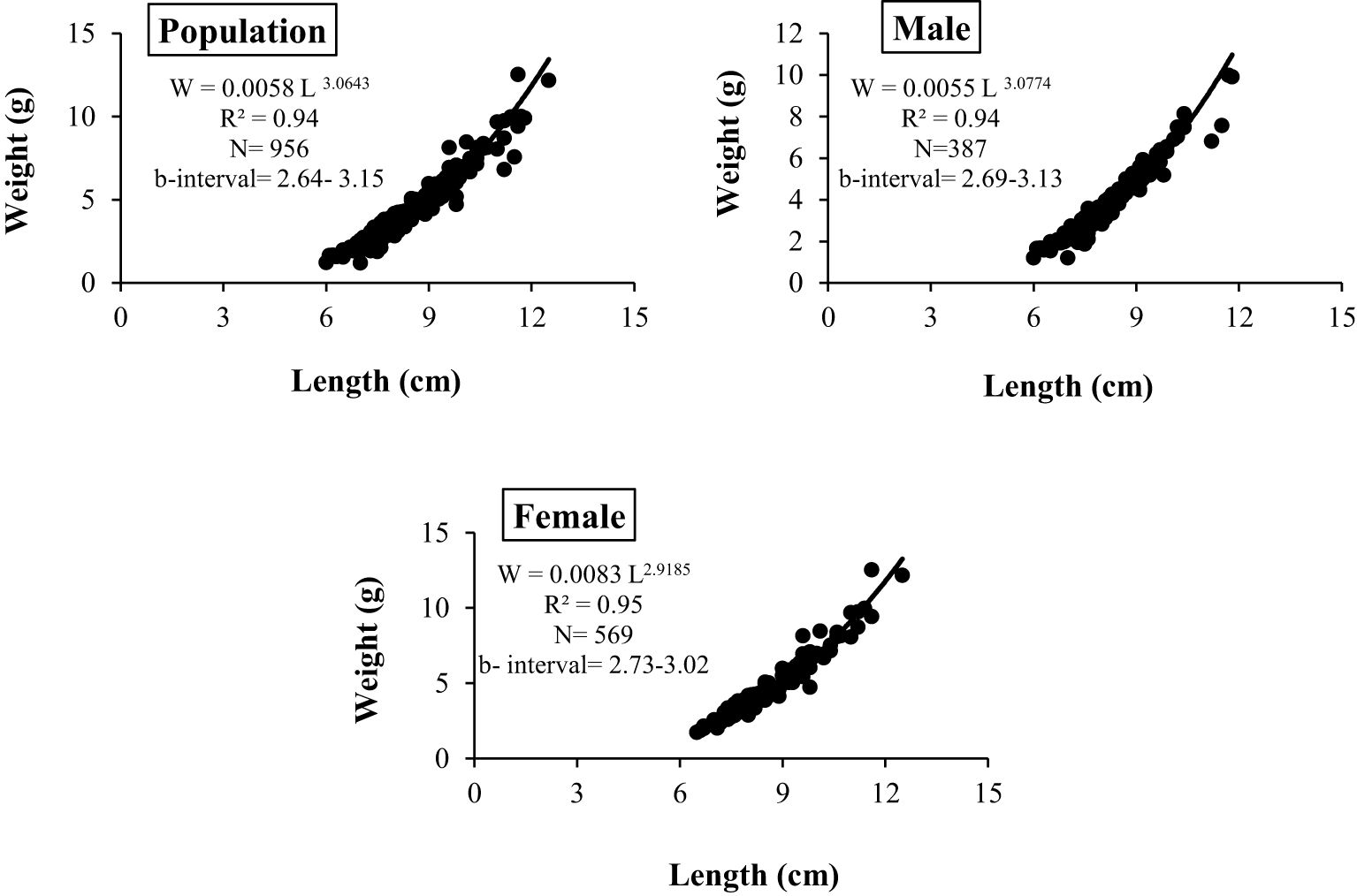
During the study period, A. boyeri showed a minimum length of 60 mm and a maximum length of 125 mm. The total weight fluctuated between a minimum of 1.2 g and a maximum of 12.51 g. The fishes within the length group of 70 to 90 mm were found to be most abundant in the catch. The values of an a (intercept) and b (slope) of the length-weight relationship equation were reported to be 0.0058 and 3.0643 for the population, 0.0055 and 3.0774 for males, and 0.0083 and 2.9185 for females respectively (Figure 2). The highest relative condition factor (Kn) in June was reported to be 0.92 and 0.75 for the males and females respectively (Figure 3).
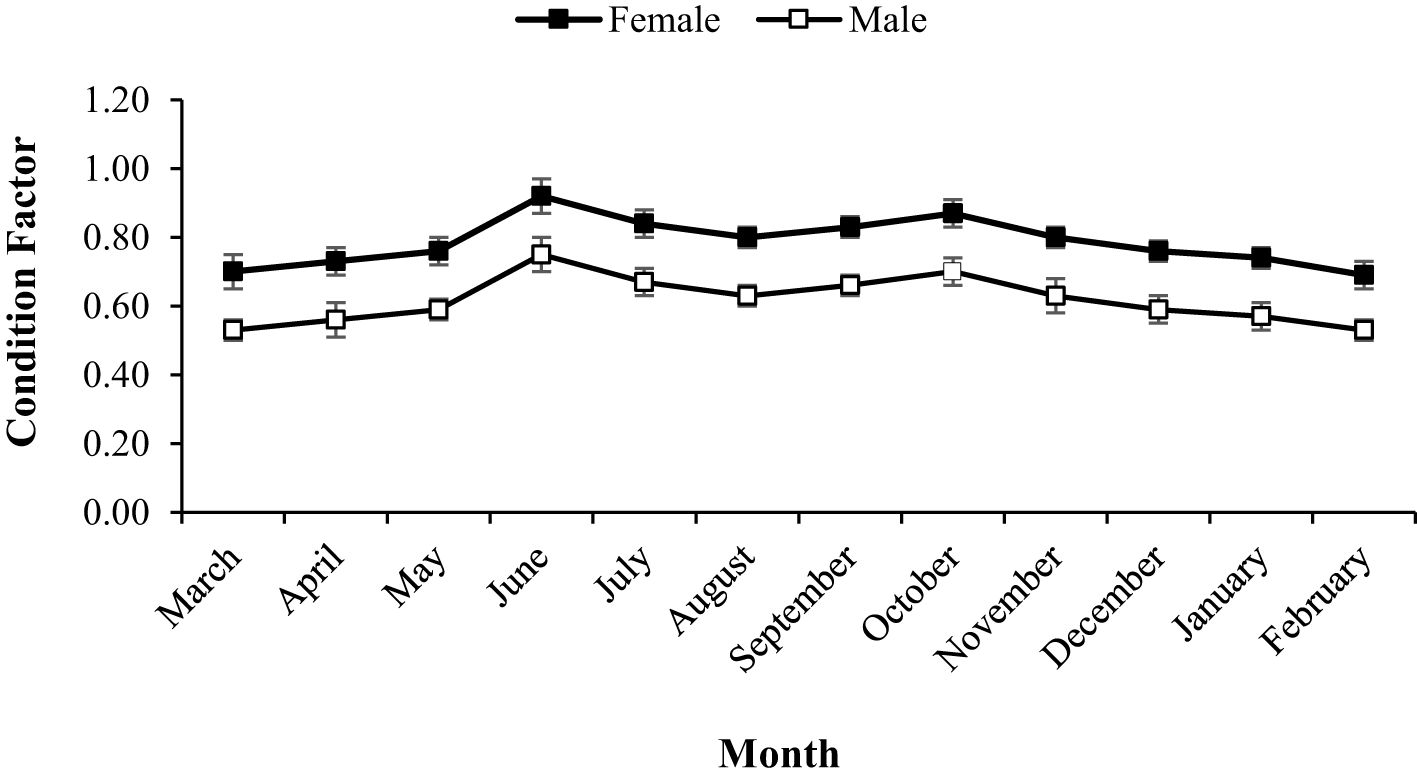
Figure 3. Relative condition factor of males and females of big-scale sand smelt, A. boyeri in the Southeast Caspian Sea-Northen Iran.
3.3 Length at first sexual maturity (Lm)
The frequency of occurrence of adult female specimens was plotted against length class and fitted to a logistic function to determine the mean fish length at first sexual maturity (Lm). In the case of A. boyeri the length at first sexual maturity was reported to be 70 and 75 mm for both males and females. The various biometric and population parameters of A. boyeri are depicted in Figure 4.
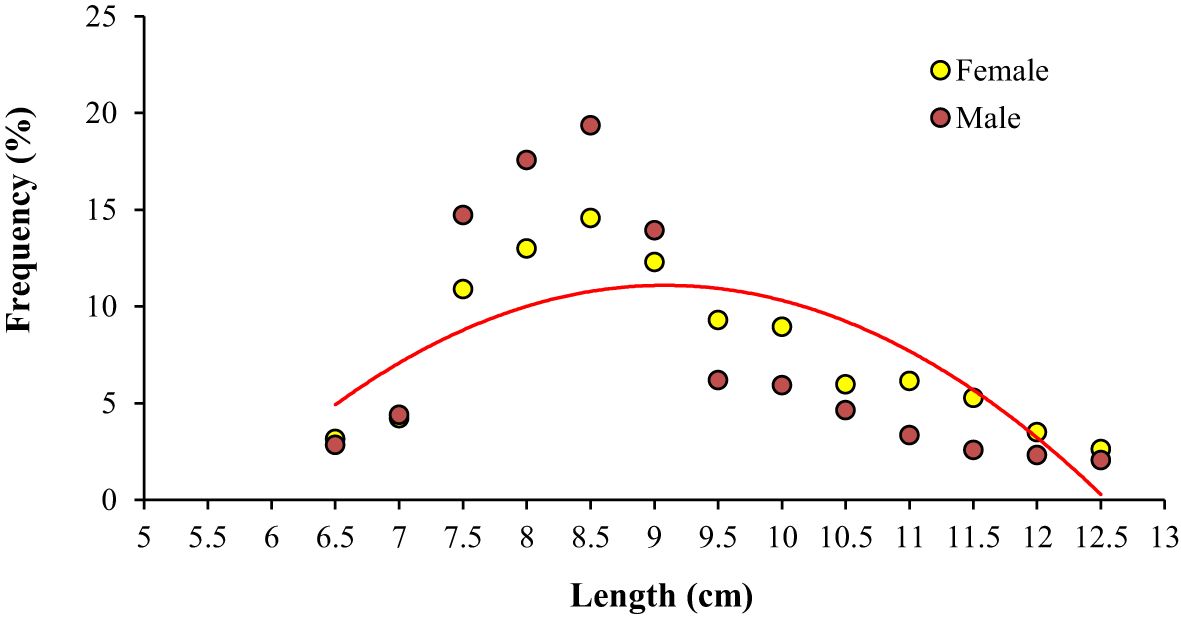
Figure 4. Graph showing length at first matunty in A. boyeri from Southeast Caspian Sea in Northern Iran.
3.4 Growth equations
The growth constant (K) in the population, males and females were reported to be 0.28, 0.34, and 0.35 year-1, and the asymptotic length (L∞) was reported to be 131.0, 126.5, and 131.3 mm respectively. The length frequency of males and females in Figure 5 is presented, indicating the highest abundance in the length classes of 7.5-8 and 8-8.5. The von Bertalanffy growth curve and the length-frequency histograms are presented in Figure 6. The growth performance index of the A. boyeri was reported to be 1.68, 1.74, and 1.78 and the age at zero length (t0) was reported to be -0.28, -0.31, and -0.31 years for Population, males, and females respectively.
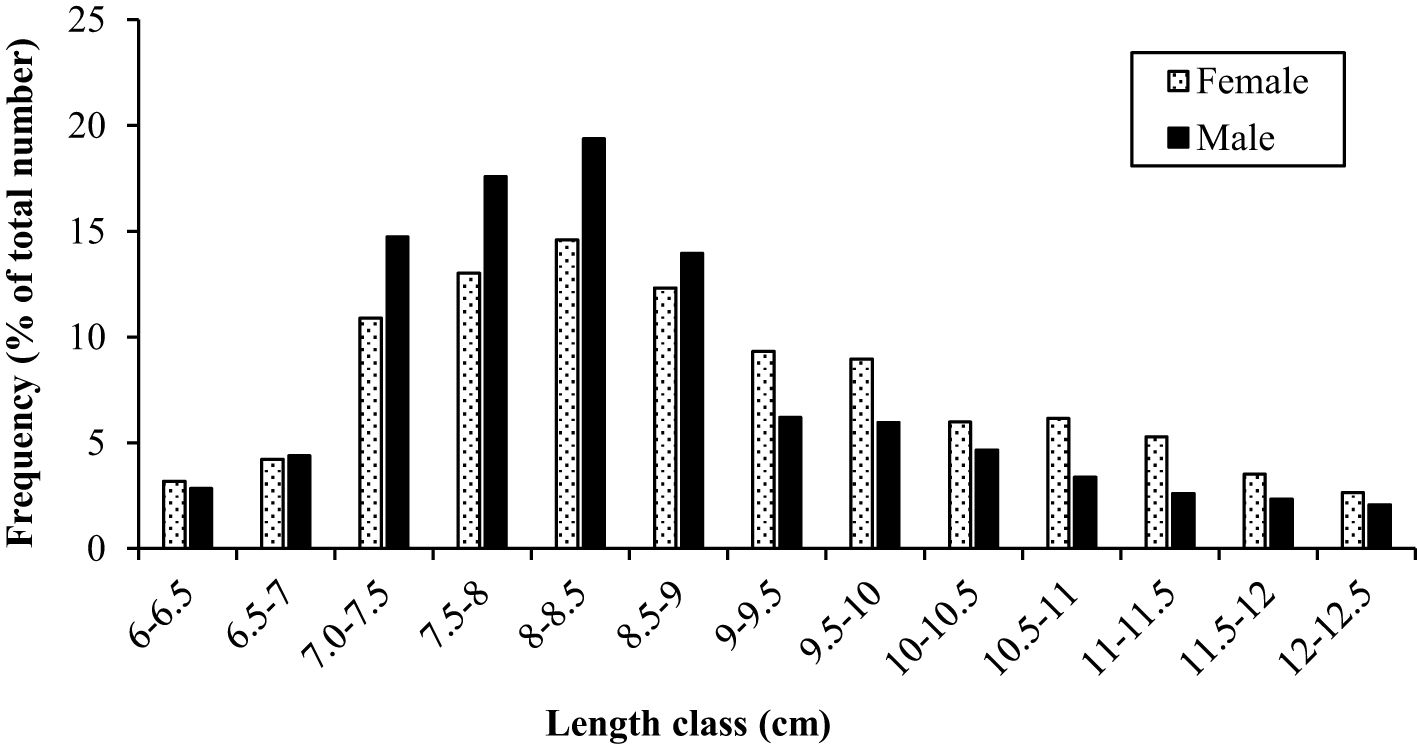
Figure 5. Total length (mm) frequency of males and females of big-scale sand smelt, A. boyeri in the Southeast Caspian Sea-Northen Iran (number of specimens = 956).
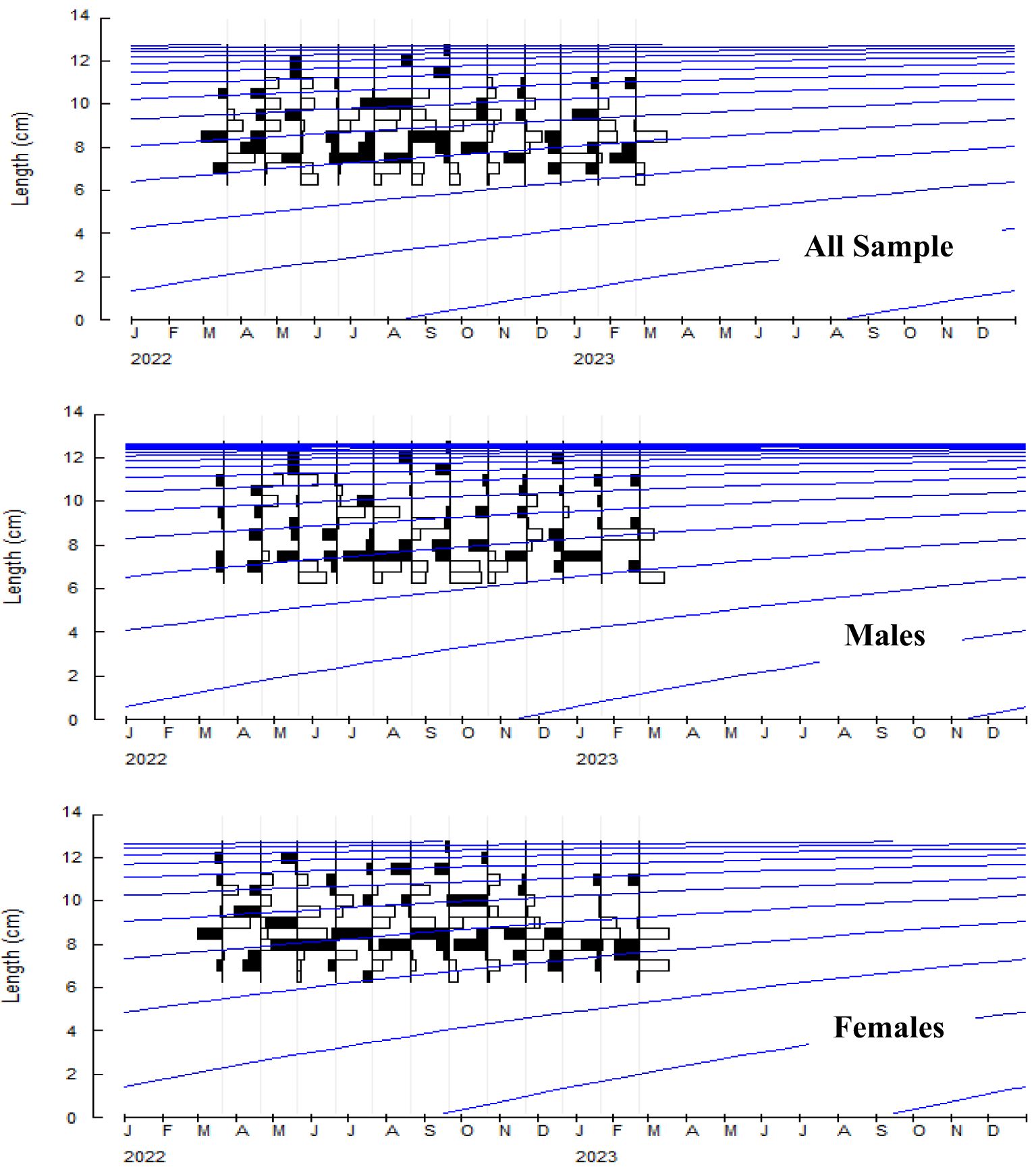
Figure 6. Growth curve of A. boyeri from Southeast Caspian Sea of Northern Iran (Blue line: Cohort).
3.5 Mortality
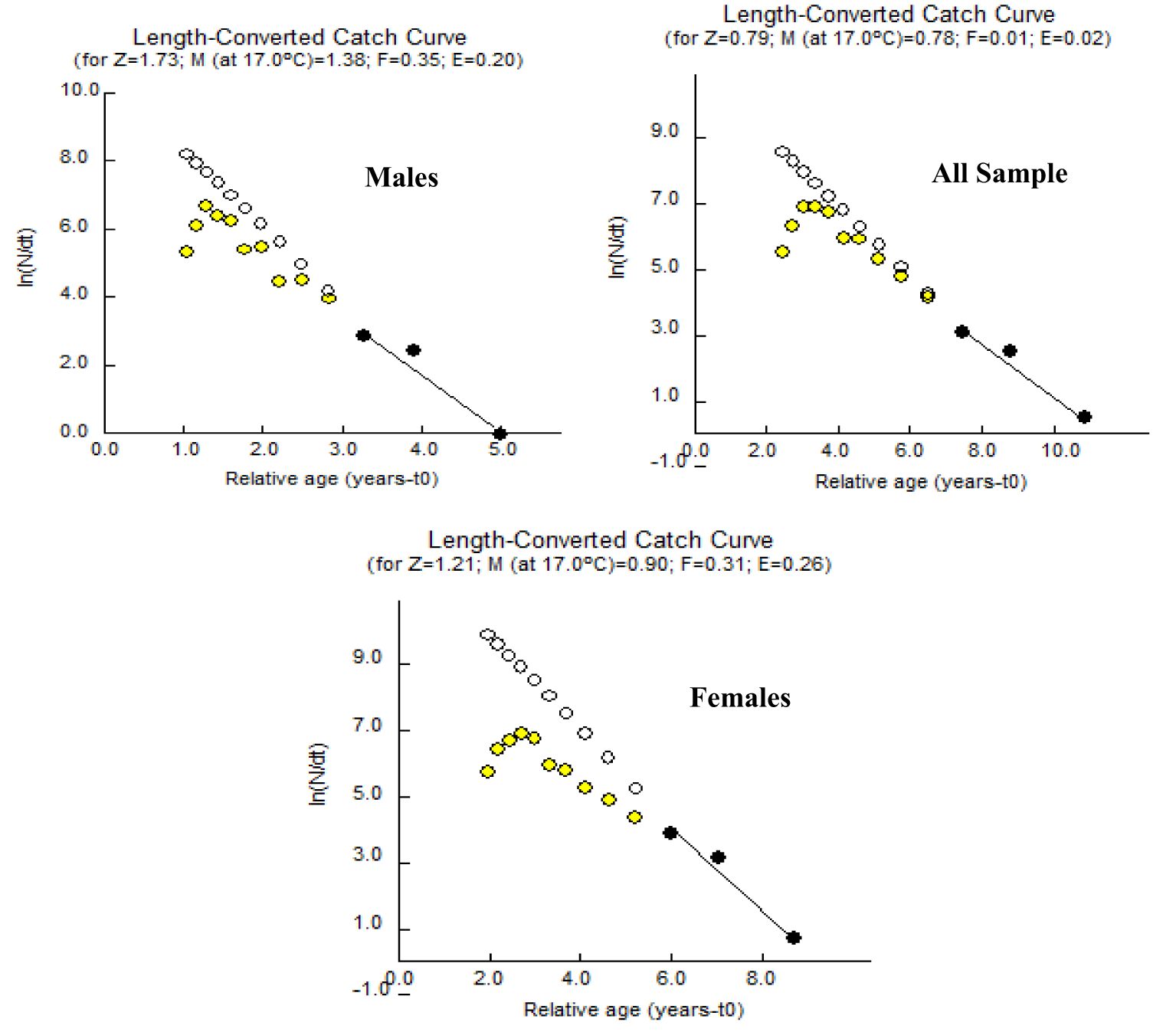
The mortality parameters i.e., the total mortality, the fishing mortality, the natural mortality, and the exploitation ratio for A. boyeri were reported to be 0.79 year-1, 0.78 year-1, 0.01 year-1, and 0.02 for the population, 1.73 year-1, 0.35 year-1, 1.38 year-1 and 0.21 for males and 0.20 year-1, 0.31 year-1, 0.90 year-1 and 0.26 for females respectively (Figure 7).
3.6 Recruitment groups
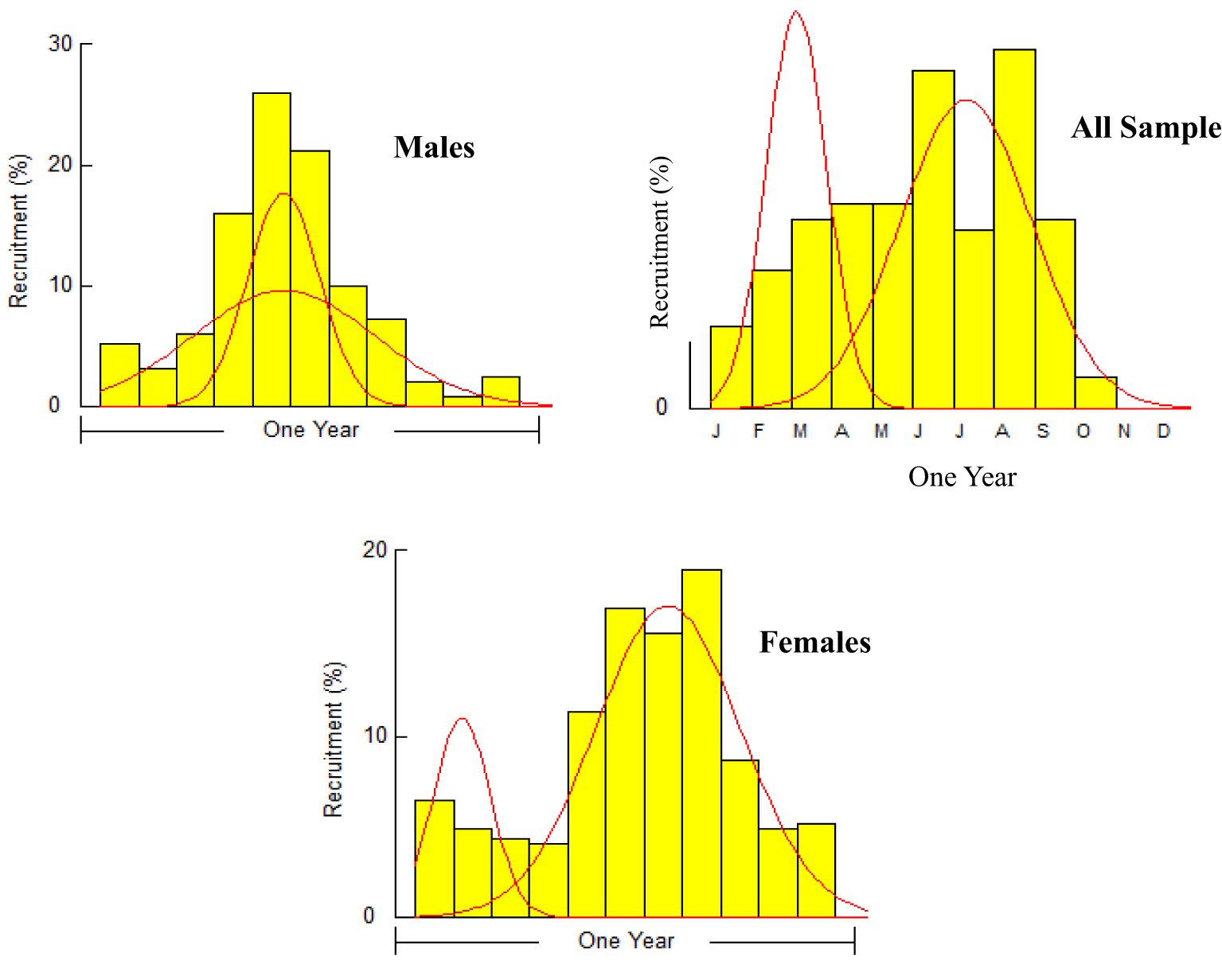
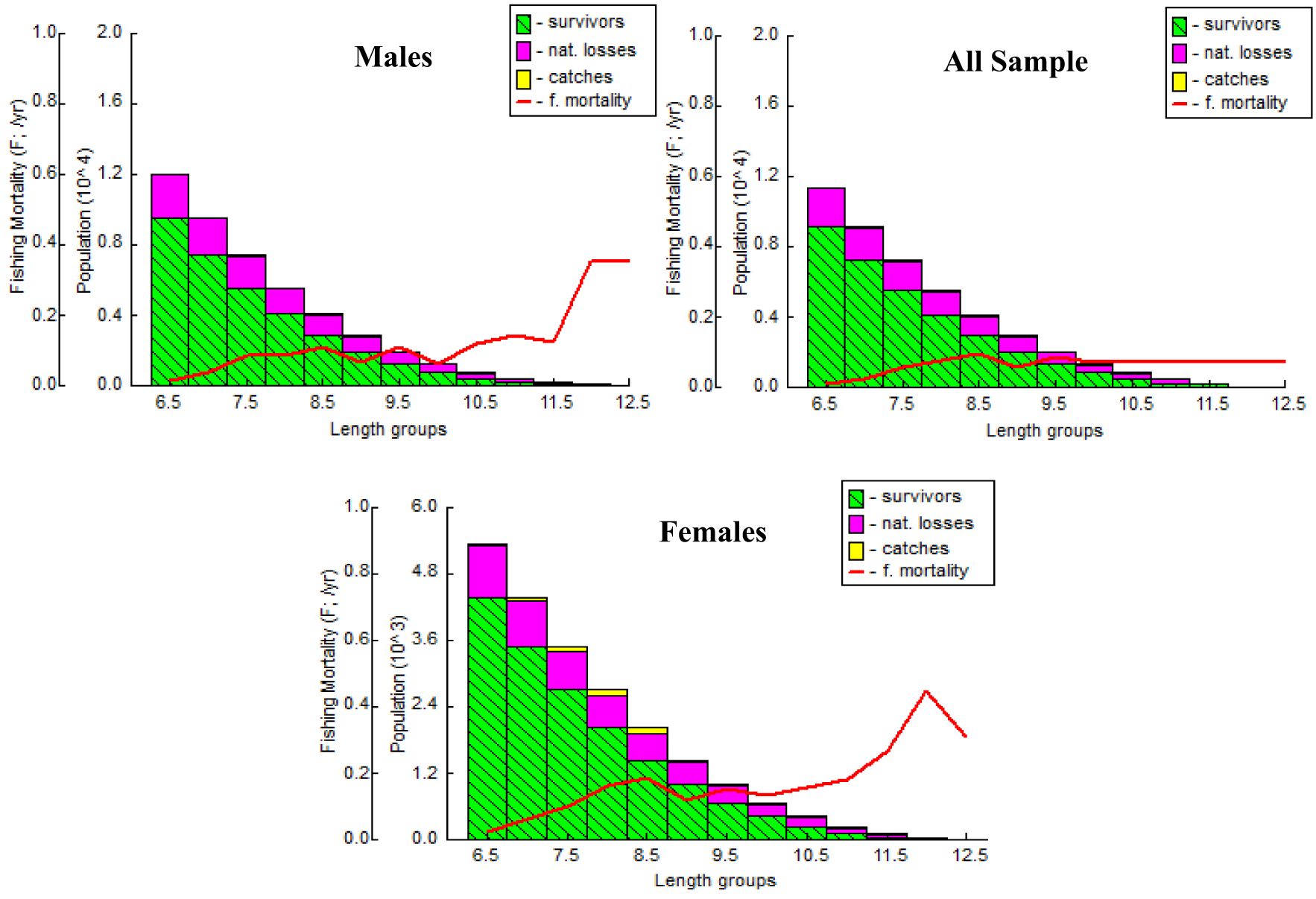
The A. boyeri population from the southeast Caspian Sea exhibits recruitment peaks during late winter, spring, and summer months (March to September) for the entire population; in males, this occurs in spring and early summer (April to July), and in females, it is seen in spring and summer (June to September), collectively contributing approximately 70-85% to total recruitment (Figure 8). The fishing mortality rate (F) for the overall population remains steady beyond a length of 95 mm. However, in males, this rate initially rises after 95 mm until reaching 120 mm, where it then stabilizes. Conversely, in females, this rate also shows a marked increase up to 120 mm in length, as revealed by VPA (Figure 9). The yield per recruit model by Beverton and Holt estimates an exploitation rate at 50% of the unexploited relative biomass per recruit (E0.5) as 0.38, a maximum yield exploitation rate (Emax) of 0.94, and an exploitation rate for which the marginal increase in relative yield per recruit is 10% (E0.1) as 0.80 for the population. The values for males are 0.37, 0.90, and 0.76, and for females, 0.38, 0.80, and 0.67, respectively (Figure 10). The length at first capture (Lc) for both males and females of A. boyeri was found to be 60 mm. The summary of the growth parameters of the Atherina boyeri is presented in Figure 11.
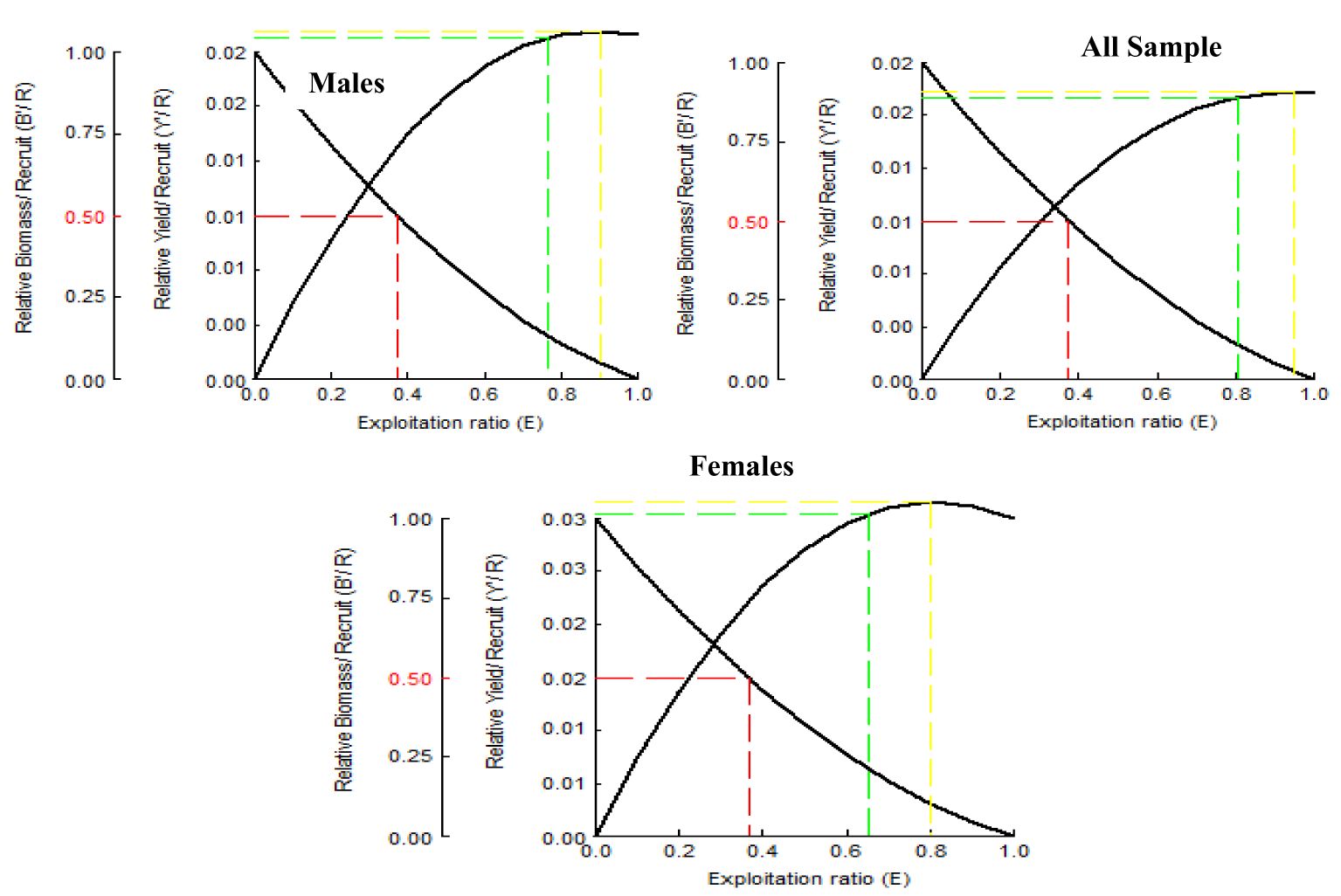
Figure 10. Beverton and Holt analysis curve for A. boyeri from Southeast Caspian Sea in Northern Iran.
3.7 Biology
3.7.1 Gonadosomatic index
The mean GSI ranged from 2.59 to 12.49 in females and 1.03 to 9.47 in males (Figure 12). According to these values, March and April were the ripening phases of gonads, and spawning occurred between the beginning of March and the end of June when the mean GSI of females was 7.41, 12.49, 10.75, and 8.15 respectively. Between July and September was accepted as the quiescence period, rose again in October, and stabilized until January. During spawning, immature, maturing, and mature eggs were in the ovaries. However, mature eggs could only be observed in May, June, and July; other than in those months, mature eggs were not found in the ovaries.
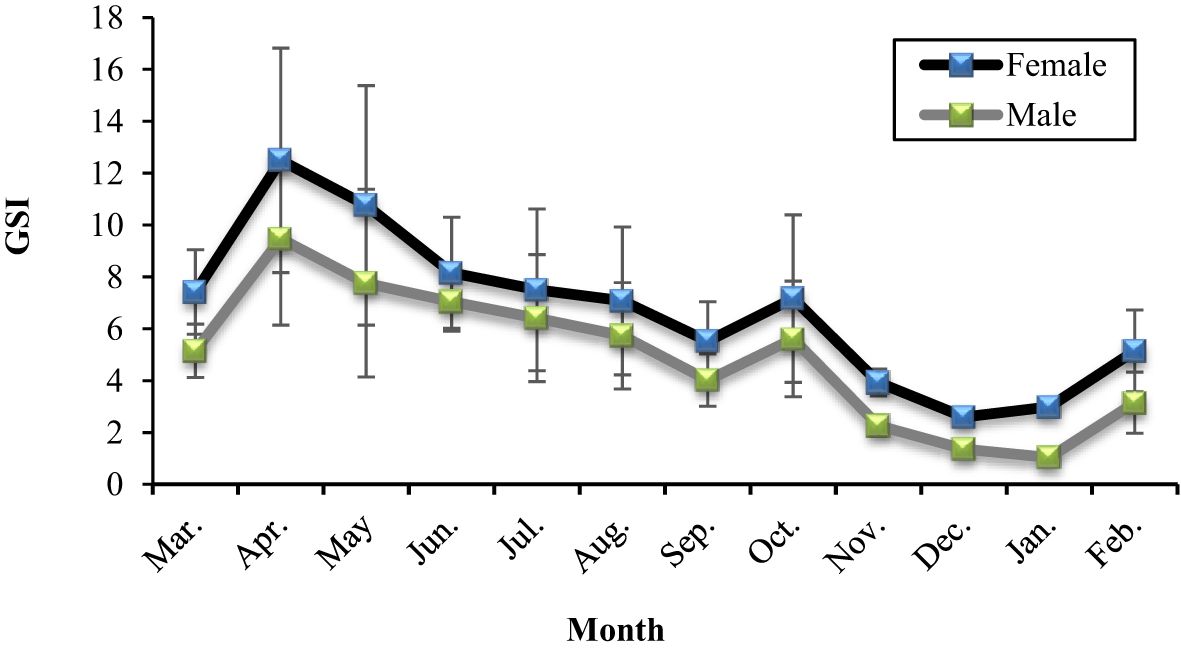
Figure 12. Variation of GSI for females and males of A. boyeri in the Southeast Caspian Sea in Northern Iran.
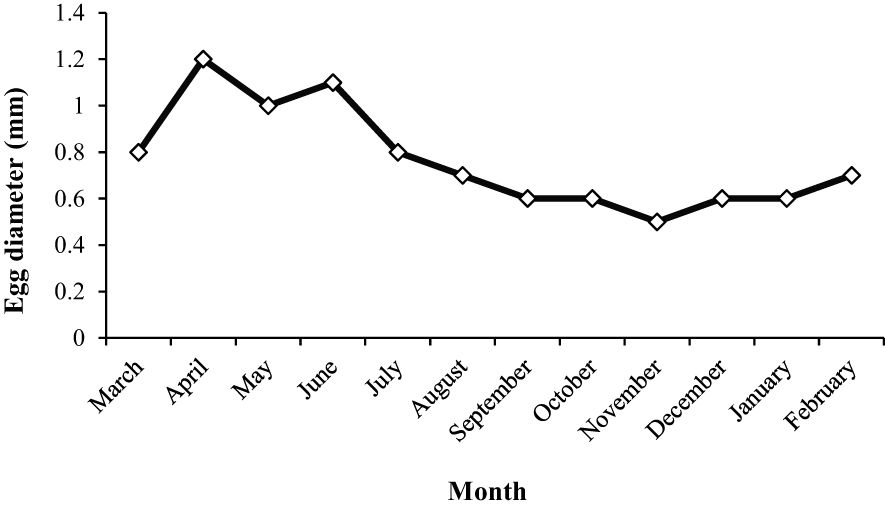
3.7.2 Egg diameter and fecundity
The highest diameter of the egg occurred in April and June, and then it remained constant from August to February (Figure 13). The diameter of the egg, which is correlated with GSI ranged from 6.5-11.2 mm TL and 1.98-11.86 g. The mean mature egg diameter increased during the spawning period, although it did not differ significantly among months (ANOVA, F = 59.84, df= 2, p < 0.001). The mean total fecundity of mature females during the spawning season increased with age; however, the mean relative fecundity decreased. While the mean total fecundity was 498.12 ± 352.94 at age 1, it reached 965.26 ± 517.70 at age 4. The mean relative fecundity was 343.85 ± 212.37 at age 1 and decreased to 143.36 ± 49.21 at age 4. The fecundity relationship with TL and W of the A. boyeri population in the Southeast Caspian Sea was evaluated and no significant relationship was found.
4 Discussions
4.1 Sex ratio
According to the overall sex ratio of the population, females were dominant. This indicates that the sex ratio of the Atherina boyeri population in the Southeast Caspian Sea is not close to the ideal Mendelian ratio. The sex ratio significantly differed within age classes, with females being dominant in age classes 2 and 3, suggesting that male A. boyeri have a shorter lifespan compared to females. In many fish species, females live longer than males, a trend observed in A. boyeri both in sea, brackish waters, and inland waters (Creech, 1992; Sezen, 2005; Gaygusuz, 2006; Tarkan et al., 2007; Ozeren, 2009; Patimar et al., 2008). Our study aligns with several recent studies examining the sex ratio of A. boyeri, which consistently show a female-biased sex ratio across different populations and timeframes. For instance, Bartulović et al. (2004) reported a male-to-female ratio of 1:1.30, with females outnumbering males throughout the year. Similarly, Manzo (2020) found a ratio of 1:1.38, with females predominating, and noted that the sex ratio fluctuated by age group, with females being more dominant in younger populations. Our study also showed female dominance across the year, without significant variation by age. In a study by Yılmaz et al. (2021), the male-to-female ratio was even more skewed at 1:1.75, particularly in June, further confirming the trend of female-biased sex ratios in A. boyeri populations. Likewise, Kovačić et al. (2022) reported a ratio of 1:1.46, reinforcing the general trend of female dominance observed in other studies. These findings suggest that A. boyeri populations tend to exhibit a female-dominant sex ratio across various environments and seasons. The consistent dominance of females in multiple studies indicates that ecological, biological, and environmental factors likely play a significant role in shaping the sex ratio, warranting further research to better understand the underlying causes. In conclusion, our study confirms the female-biased sex ratio in A. boyeri and emphasizes the need for more in-depth investigations into the factors contributing to these patterns.
4.2 Length-weight relationship
The growth pattern of a fish population, exploitation intensity, recruitment potential, and mortality are the most vital factors in fish population dynamics. Utilizing the length-frequency data, the current study provides inferences on the population of A. boyeri in the Southeast Caspian Sea. The species, albeit not having the status of a food fish shows strong potential as a native fish as such the study of the various aspects of its population tends to be imperative, which forms the backdrop for the current research problem (Nissar et al., 2024). Length-weight relationship (LWR) is a fundamental concept in fish population studies that facilitates the estimation of the weight of a species using its length data and vice versa. This vital bioecological tool yields data on the general condition of a fish population and has practical applications in fisheries management and assessment of stocks (Ricker, 1975; Arafat and Bakhtiyar, 2022). Analyzing the length and weight data of A. boyeri from the Southeast Caspian Sea revealed that the fish exhibited a length range of 60 to 125 mm and 60 to 113.3 mm and a weight range of 6.0 to 12.50 g and 6.5 to 12.50 g in males and females respectively. The value of LWR growth coefficient b in males and females was found to be 3.0774 and 2.9185 indicating positive and negative allometric growth patterns respectively.
4.2.1 Condition factor
The mean monthly condition factor values for both sexes, calculated from the eviscerated weight, tended to decrease towards the end of the reproduction period (August). Thereafter, A. boyeri gradually recovered during fall and winter, reaching its highest values during spring, when food was abundant. Generally, in freshwater bodies, the diet composition of A. boyeri is dominated by zooplankton (Chrisafi et al., 2007; Doulka et al., 2013), and in the Southeast Caspian Sea, zooplankton density increases in spring (Baykal et al., 2006). Nikolsky (1980) stated that rapid feeding enables many fish to grow rapidly, which is also the case for A. boyeri in the Southeast Caspian Sea. In the Southeast Caspian Sea, the mean monthly condition factor values, calculated using eviscerated weight, varied within two narrow ranges, but the peak was observed in spring. In support of this study, some authors reported more than one peak (Fernandez-Delgado et al., 1988; Andreu-Soler et al., 2006; Koutrakis et al., 2004), which could be attributed to different feeding and environmental conditions.
4.3 Length at first sexual maturity (Lm)
The length at first sexual maturity (Lm) for Atherina boyeri has been reported to range between 70 and 75 mm for both males and females. In marine and lagoon habitats, where this species tends to reach sexual maturity at smaller sizes, environmental factors such as salinity and temperature are believed to play a significant role in determining Lm. However, studies focusing on the length at first maturity of A. boyeri are limited. Reported Lm values vary widely depending on habitat and study: 27 mm in the Suez Canal (Fouda, 1994), 34 mm in the Mesolongi-Etolikon Lagoon (Leonardos and Sinis, 2000), 38 mm in the Southern France Lagoon (Tomasini and Laugier, 2002), 52 mm in the Mala Neretva River (Bartulović et al., 2004), and 45.93 mm in Lake Eğirdir (Küçük et al., 2012). Additionally, Bartulović et al. (2006) reported an Lm of 77.52 mm for females in the Mala Neretva River, while Gaygusuz (2006) found values of 40-44 mm for males and 35-39 mm for females in İznik Lake. Comparing these findings with our results, we observed similarities with Bartulović et al. (2004, 2006), where A. boyeri in Mala Neretva River exhibited Lm values close to our findings. However, significant differences are noted with other studies, particularly those by Küçük et al., 2012 and Gaygusuz (2006). The lower Lm values reported by others may reflect differences in habitat conditions, such as temperature and food availability, in the Mala Neretva River. Conversely, the lower Lm values observed by Gaygusuz (2006) in İznik Lake could be attributed to methodological variations, including sampling size or age structure of the studied populations. These discrepancies highlight three key factors influencing Lm: (i) habitat variability, (ii) differences in methodologies used to assess data, and (iii) variations in sample size and the length ranges examined. Saborido-Rey (2016) proposed that in short-lived species with high mortality rates, maturity curves (ogives) are often best described by a logistic function. These species typically mature over a narrow size range, after which all individuals become sexually mature. Our results align with this framework, showing that A. boyeri completes maturation within a 20 mm length interval (35-55 mm fork length, Figure 4) for both males and females. Accurate estimation of Lm-the length at which 50% of individuals in a population are sexually mature—is vital for sustainable fishery management. As Fontoura et al. (2009) highlighted, Lm forms the scientific foundation for implementing Minimum Landing Sizes (MLS), ensuring that each fish has the opportunity to reproduce at least once before capture. This approach is essential for maintaining the sustainability of fish stocks and supporting ecological balance.
4.4 Growth equations
The asymptotic length was reported to be 129.6 and 131.3 mm in both males and females and happens to be a vital factor for demarcating the mesh size limits of fishing gears (Gebremedhin et al., 2021). In males and females, the von Bertalanffy curvature parameter (growth constant) for A. boyeri of the Southeast Caspian Sea was reported to be 0.34 year–1 and 0.35 year–1 respectively, indicative of a moderate growth rate as per Sparre and Venema (1998). The size distribution of a fish stock can be altered by various external factors, especially the fishing technique and the ambient temperature (Tu et al., 2018). These factors alongside the data models utilized can eventually alter the growth rates and asymptotic lengths of a stock (Etim et al., 1998). Gençoğlu and Ekmekçi (2016) analyzed the population status of A. boyeri from the Hirfanlı Reservoir in Turkey and reported a lower rate of growth (Kmale = 0.15 year–1, Kfemale = 0.20 year–1) than the current study, which was attributed to the Mediterranean conditions of the reservoir. Fish growth in temperate waters is often constrained by lower temperatures. Therefore, it is not surprising that species capable of growing in temperate waters exhibit higher growth rates compared to warmer and Mediterranean regions (Lowe-McConnell, 1987; Henderson, 2005).
4.5 Mortality
The natural mortality of neither sex was found to be higher than the fishing mortality for A. boyeri, indicative of less exploitation of the fish from the Southeast Caspian Sea, since it is not a food fish and the exploitation ratio of 0.20 and 0.26 in males and females respectively, is indicative of the same. The value of K is associated with the longevity of a fish species which in turn is related to mortality (Beverton and Holt, 1959; Saville, 1977). If the natural mortality of a slow-growing fish species is high, it could go extinct (Sparre and Venema, 1998). Mortality and growth rates are connected, with natural mortality showing direct dependence on K and an inverse dependence on asymptotic length (Sparre and Venema, 1998), besides the growth rates also influence the susceptibility of a fish to fishing or predation (Pauly, 1984; Allen and Hightower, 2010). Up to now, there are no published information on the Overall condition and population dynamics of this native species in the southeast Caspian Sea. However, based on the results of this study, natural mortality and fishing mortality significantly differ between the neither sex.
4.6 Recruitment groups
The addition of new individuals to a harvestable stock is denoted by recruitment and happens to be a strong driver of the fish population (Camp et al., 2020). A. boyeri showed recruitment peaks during spring and summer months (April to July), contributing approximately 80% to the total recruitment and coinciding with an increase in the temperature whereas winter months showed the lowest recruitment given a decrease in the temperature (Kindong et al., 2018). The availability of food and favourable weather conditions are the primary factors that could influence the recruitment of fish (Shoji et al., 2011; Okamoto et al., 2012; Tableau et al., 2015; Gebrekiros, 2016; Kripa, 2017). Virtual population analysis provides insights into the proportion of survivors and the losses caused by natural mortality and fishing, which are assessed by plotting various length groups against fishing mortality. In this study, for both males and females, the number of survivors and natural losses decreased with increasing size. Fishing mortality also increased for both sexes after a length of 75 mm. The highest catch rates were observed in the length groups of 65 to 85 mm for males and 70 to 95 mm for females. No previous studies or assessments were available on this parameter in fish stock evaluation, making these findings the first data on the overall status of this species in the study area. The yield per recruit (Y/R) analysis, calculated using the Beverton and Holt model for both sexes, estimated Emax and E0.1 values of 0.90 and 0.80 for males, and 0.76 and 0.67 for females, respectively. These values are higher than the exploitation ratios of 0.20 for males and 0.26 for females, indicating that A. boyeri remains underexploited in the southeast Caspian Sea.
4.7 Biology
The GSI values indicated that the reproductive period lasted around 4 months. In the Southeast Caspian Sea, this period was shorter compared to what other studies have reported in different regions (Table 1). Water temperature plays a crucial role in determining the length of the spawning season for fish (Nikolsky, 1978). In the Southeast Caspian Sea, A. boyeri could reproduce within a year, spawning in the spring after hatching, and by the end of the first summer, the sex of the juveniles could be identified through a simple macroscopic examination of their gonads. A longer reproductive season is a key strategy in the life cycle of A. boyeri, contributing to its ability to invade inland waters (Bogutskaya and Naseka, 2002).
Batch spawning has been observed in A. boyeri populations (Rosecchi and Crivelli, 1992). During the spawning period, eggs at various stages of development-immature, maturing, and mature-were present together within the same ovaries of females in the Southeast Caspian Sea, suggesting the occurrence of asynchronous or batch spawning. As depicted in Figure 12, there is significant variation in the GSI among females throughout the spawning season, indicating that some individuals have already completed spawning, while others are still undergoing maturation. Outside of the spawning period, GSI variation was minimal, with only immature oocytes observed in the ovaries. This further supports the occurrence of batch spawning in A. boyeri in the Southeast Caspian Sea. The diameters of mature eggs in this region were in line with those reported by various studies (Creech, 1992; Rosecchi and Crivelli, 1992; Tomasini et al., 1996).
Fecundity in A. boyeri increased progressively with age. No clear relationship with latitude or environmental conditions emerged from comparing these findings with published data, likely due to differences in the criteria used for assessing fecundity.
A. boyeri, a native species for the inland waters in the Southeast Caspian Sea (Iran), has established abundant populations.
Several features of A. boyeri in the Southeast Caspian Sea, such as their short life-span, rapid growth, extended reproductive period, early sexual maturation, and batch spawning, are typical invasive characteristics ensuring establishment success. However, Bamber and Henderson (1985) stated that many atherinids show a high degree of intraspecific morphological variability, which can be linked to estuarine habitats. Such environments are physically highly variable and this has acted to select for generalist genotypes able to adjust their morphology, physiology, and behavior to a wide range of conditions. Additionally, such plasticity preadapts atherinids to invade and rapidly populate fresh waters containing vacant niches. Our results, obtained from the sand smelt population of the Southeast Caspian Sea, support the hypothesis of Bamber and Henderson (1985).
The limited exploitation of the fishery is attributed to the lack of nutritional value of A. boyeri. Comparable results were observed by Amri Sahebi et al. (2015) regarding A. boyeri. These values are largely determined by fishing intensity, fluctuations in feeding patterns, as well as ecological and physiological factors (Biswas, 1993; Zan-Bi et al., 2022).
Along with refraining from fishing during the spawning season, targeting only mature individuals is essential for sustainable fisheries management. The length at first maturity (Lm) represents a crucial phase in a fish’s life cycle. At this stage, resources previously allocated for growth and survival are redirected toward reproduction (Wootton, 1998). Although Lm is a species-specific characteristic, it can be influenced by factors such as resource availability, fishing pressure, environmental conditions, and biological interactions (Lappalainen et al., 2016; Souza et al., 2019). For A. boyeri, the length at first sexual maturity has been reported as 60 and 65 mm for males and females, respectively. Despite a thorough literature review, no studies have been found providing data on the length at first maturity for A. boyeri. FishBase, a reliable database for fish species, also highlights the absence of data on this parameter for A. boyeri (Froese and Pauly, 2024). Almeida et al. (2018) emphasized that the first sexual maturation is a key milestone in an animal’s life history, which should be considered for effective fisheries management. In this study, the length at first maturity was determined to be 60 and 65 mm for males and females, whereas the length at first capture was found to be 60 mm, indicating that Lm > Lc. This suggests that the stock stability could be disrupted, making the population vulnerable to capture by current fishing gear before reaching maturity. Similar findings indicating Lm > Lc were observed by Wehye et al. (2017) and Rudi et al. (2018). When Lm < Lc, the individuals will at least breed during their lifespan, ensuring stock replenishment and supporting long-term sustainability (Udoh and Ukpatu, 2017; Panda et al., 2018; Mohamed, 2022).
5 Conclusions
This research on the population dynamics of A. boyeri from the Southeast Caspian Sea was conducted to establish foundational data on the growth, mortality, exploitation, and recruitment patterns of the species. Throughout the study, the length-weight relationship (LWR) revealed both positive and negative allometric growth patterns for both sexes. The condition factor of the fish indicated healthy growth within the aquatic environment, largely due to the resilience of the Big-scale sand smelt. The species exhibits moderate growth, high natural mortality, and low exploitation rates. The condition Lm > Lc suggests a potential disruption in the stock, therefore, employing nets with relatively larger mesh sizes is recommended for fishing A. boyeri in this lake. A. boyeri is an essential forage fish in the Southeast Caspian Sea, serving as a crucial food source for aquatic birds, and it also holds significant potential in the ornamental industry, making it a valuable species in the region’s fauna. This study will support the development of more effective management strategies for A. boyeri. Finally, it’s important to closely monitor the population of big-scale sand smelt in the future to ensure a sustainable economic yield. This can be achieved using alternative mathematical approaches, such as Artificial Neural Networks and fuzzy logic in the study area and other inland waters.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Iranian Fisheries Institute in Tehran. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
EH: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. BR: Methodology, Supervision, Writing – original draft. HF: Data curation, Software, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aladin N., Plotnikov I. (2004). “The caspian sea,” in Lake basin management initiative (Thematic Paper, Russia), 29.
Allen M. S., Hightower J. E. (2010). “Fish population dynamics: mortality, growth, and recruitment,” in Inland fisheries management in North America, 3rd edition. Eds. Hubert W. A., Quist M. C. (American Fisheries Society, Bethesda, Maryland), 43–79.
Almeida Z. S., Carvalhob I. F. S., Dinizb A. L. C., Netaa R. N. F. C., Torresc C. L., Serra I. M. R. S. (2018). Models of sexual maturation as a tool for the conservation of commercial fish in a Ramsar site of Brazil. AIP Conf. Proc. 2040, 100004. doi: 10.1063/1.5082301
Amri Sahebi A., Taghavi H., Fazli H. (2015). The diet of big-scale sand smelt Atherina boyeri caspia (Risso 1810) on the southeastern coast of the Caspian Sea. Iranian Sci. Fisheries J. 24, 121–133.
Andreu-Soler A., Oliva-Paterna F. J., Torralva M. (2006). Seasonal variations in somatic condition and gonad activity of sand smelt Atherina boyeri (Teleostei, Atherinidae) in the Mar Menor coastal lagoon (SE Iberian Peninsula). Folia Zool 55, 151–161.
Arafat M. Y., Bakhtiyar Y. (2022). Length-weight relationship, growth pattern and condition factor of four indigenous cypriniform Schizothorax species from Vishav Stream of Kashmir Himalaya. J. Fisheries 10, 101202. doi: 10.17017/j.fish.10.1
Bagenal T. B., Tesch F. W. (1978). “Age and growth,” in Methods for assessment of fish production in fresh waters IBP handbook no. 3, 3rd. Ed. Bagenal T. (Blackwell Science Publications, Oxford).
Bamber R. N., Henderson P. A. (1985). Morphological variation in British atherinids, and the status of Atherina presbyter Cuvier (Pisces: Atherinidae). Biol. J. Linn. SOC. 25, 61–76.
Bartulović V. (2003). Morphometry and population dynamics of sand smelt, Atherina boyeri Risso, ISlO (Pisces) in the estuary of Mala Neretva River. Master thesis Vol. 125 (Croatia: University of Zagreb).
Bartulović B., Glamuzina A., Conides J., Dulcie D., Lucie, Njire J., et al. (2004). Age, growth, mortality and sex ratio of sand smelt, Atherina boyeri Risso (1810) (Pisces: atherinidae) in the estuary of the mala neretva river (middle-eastern adriatic, Croatia). J. Appl. Ichthyol. 20, 427–430.
Bartulović V., Glamuzina B., Conides A., Gavrilović A., Dulčić J. (2006). Maturation, reproduction and recruitment of the sand smelt, Atherina boyeri Risso 1810 (Pisces: Atherinidae) in the estuary of Mala Neretva River (southeastern Adriatic, Croatia). Acta Adriat 47, 5–11.
Baykal T., Salman S., Acıkgoz İ. (2006). The relationship between seasonal variation in phytoplankton and zooplankton densities in Hirfanlı Dam Lake (Kırşehir, Turkey). Turk J. Biol. 30, 217–226.
Benzer S. (2024). First data on big-scale sand smelt Atherina boyeri Risso 1810 (Pisces: Atherinidae) from Kılıçkaya Dam Lake, Turkey. J. Fisheries 12, 122202. doi: 10.17017/j.fish.620
Benzer S., Benzer R. (2019). Artificial neural networks model biometric features of Marine fish Sand smelt. Pakistan J. Mar. Sci. 28, 115–126.
Beverton R. J. H., Holt S. J. (1957). On the dynamics of exploited fish populations. Fisheries Invest. 19, 1–533.
Beverton R. J. H., Holt S. J. (1959). “A review of the lifespans and mortality rates of fishes in nature, and their relation to growth and other physiological characteristics,” in Ciba foundation colloquia on ageing. Eds. Wolstenholme C. E. W., O’Connor M. (J and A Churchill Ltd., London), 142–179.
Beverton R. J. H., Holt S. J. (1966). Manual of methods for fish stock assessment part II tables of yield function. FAO Fish Biol. Tech. Paper 38, 10.
Bianco P. G., Caputo V., Ferrito V., Lorenzoni M., Nonnis Marzano F., Stefani F., et al. (2013).Atherina boyeri Risso. In: Liste Rosse italiane. Available online at: http://www.iucn.it (Accessed 10 September 2014).
Bogutskaya N. G., Naseka A. M. (2002). An overview of nonindigenous fishes in inland waters of Russia. Proc. Zool Inst Russ Acad. Sci. 296, 21–30.
Camp E., Collins A. B., Ahrens R. N., Lorenzen K. (2020). Fish population recruitment: what recruitment means and why it matters. Electronic Data Inf. Source (EDIS) 2020, 1–6. doi: 10.32473/edis-fa222-2020
Chrisafi E., Kaspiris P., Katselis G. (2007). Feeding habits of sand smelt (Atherina boyeri, Risso 1810) in Trichonis Lake (Western Greece). J. Appl. Ichthyol 23, 209–214. doi: 10.1111/j.1439-0426.2006.00824.x
Convention on the Legal Status of the Caspian Sea (2018). "Length-weight relationships and seasonal cycles in the growth of fishes in the tropics," in Proceedings of the International Workshop on Fish Stock Assessment in the Tropics*. p. 1–12.
Creech J. (1992). A multivariate morphometric investigation of Atherina boyeri Risso 1810 and A. presbyter Cuvier, (1829) (Teleostei: Atherinidae): morphometric evidence in support of the two species. J. Fish Biol. 41, 341–353. doi: 10.1111/j.1095-8649.1992.tb02663.x
Dadzie S., Abou-Seedo F., Moreau J. (2007). Population dynamics of Parastromateus Niger in Kuwaiti waters as assessed using length–frequency analysis. J. Appl. Ichthyology 23, 592–597. doi: 10.1111/j.1439-0426.2007.00903.x
Danilova M. M. (1991). Diet of juvenile silversides, Atherina boyeri, from the Black Sea. J. Ichthyology. 31, 137–145.
Doulka E., Kehayias G., Chalkia E., Leonardos I. D. (2013). Feeding strategies of Atherina boyeri (Risso 1810) in a freshwater ecosystem. J. Appl. Ichthyol 29, 200–207. doi: 10.1111/jai.12012
Dulvy N. K., Sadovy Y., Reynolds J. D. (2003). Extinction vulnerability in marine populations. Fish Fisheries 4, 25–64. doi: 10.1046/j.1467-2979.2003.00105.x
Economidis P. S., Dimitriou E., Pagoni R., Michaloudi E., Natsis L. (2000). Introduced and translocated fish species in the inland waters of Greece. Fisheries Manage. Ecol. 7, 239–250. doi: 10.1046/j.1365-2400.2000.00197.x
Etim L., Sankare Y., Brey T., Arntz W. (1998). The dynamics of unexploited population of Corbula trigona (Bivalvia: Corbulidae) in a brackish-water lagoon, Cote d'Ivoire. Arch. Fishery Mar. Res. 46, 253–262.
Fahmy Mehana S. (2024). Fishery characteristics, population dynamics and the impacts of the invasive red swamp crayfish (Procambarus clarkii) in the Nile River, Egypt. J. Fisheries 12, 123204. doi: 10.17017/j.fish.713
Fernandez-Delgado C., Hernando J. A., Herrera M., Bellido M. (1988). Life-history patterns of the sandsmelt Atherina boyeri Risso (1980) in the estuary of the Guadalquivir River, Spain. Estuar. Coast. Shelf Sci. 27, 697–706.
Fontoura N. F., Braun A. S., Milani P. C. C. (2009). Estimating size at first maturity (L50) from Gonadossomatic Index (GSI) data. Neotrop Ichthyology 7, 217–222. doi: 10.1590/S1679-62252009000200013
Fouda M. M. (1994). Life history strategies of four small-size fishes in the Suez Canal, Egypt. J. Fish Biol. 46, 687–702.
Fournier D. A., Sibert J. R., Majkowski J., Hampton J. (1990). MULTIFAN a likelihood-based method for estimating growth parameters and age composition from multiple length frequency data sets illustrated using data for southern bluefin tuna (Thunnus maccoyii). Can. J. Fisheries Aquat. Sci. 47, 301–317. doi: 10.1139/f90-032
Freyhof J., Kottelat M. (2008).Atherina boyeri. In: IUCN red list of threatened species (IUCN). Available online at: www.iucnredlist.org (Accessed 20 August 2014).
Froese R., Pauly D. (2024). FishBase (World Wide Web electronic publication) (Accessed 10 August 2024).
Gayanilo F. C., Pauly D. (1997). FAO-ICLARM fish stock assessment (FiSAT) reference manual Vol. 8 (Rome: FAO Computerized Information Series (Fisheries), 126.
Gayanilo F. C., Sparre P., Pauly D. (1995). FAO-ICLARM stock assessment tools (FiSAT) user’s guide Vol. 8 (Rome: FAO Computerised Information Series (Fisheries), 126.
Gayanilo F. C., Sparre P., Pauly D. (2005). FAO-ICLARM Stock Assessment Tools II (FiSAT II): user’s guide (Rome: FAO Computerized Information Series (Fisheries), 168.
Gaygusuz O. (2006). İznik Golu’nde yaşayan gumuş balığı (Atherina boyeri Riss )’nın ureme ve buyume biyolojisi. MSc (İstanbul, Turkey (in Turkish: İstanbul University).
Gebrekiros S. T. (2016). Factors affecting stream fish community composition and habitat suitability. J. Aquaculture Mar. Biol. 4, 00076. doi: 10.15406/jamb.2016.04.00076
Gebremedhin S., Bruneel S., Getahun A., Anteneh W., Goethals P. (2021). Scientific methods to understand fish population dynamics and support sustainable fisheries management. Water 13, 574. doi: 10.3390/w13040574
Gençoğlu L., Ekmekçi F. G. (2016). Growth and reproduction of a marine fish, Atherina boyeri Risso 1810, in a freshwater ecosystem. Turkish J. Zoology 40, 534–542.
Gisbert E., Cardona L., Castello F. (1996). Resource partitioning among planktivorous fish larvae and fry in a Mediterranean coastal lagoon, Estuary. Coast. Shelf Sci. 43, 723–735. doi: 10.1006/ecss.1996.0099
Henderson P. A. (2005). “The growth of tropical fishes,” in Fish physiology: the physiology of tropical fishes. Eds. Val A. L., de-Almeida-Val F. V. M., Randal D. J. (Elsevier Academics, London), 85–100.
Kilduf P. (2009). Guide to fisheries science and stock assessments (Arlington, USA: The Atlantic States Marine Fisheries Commission).
Kindong R., Gao C., Dai X., Tian S., Wu F. (2018). Population dynamic parameters for Cyprinus carpio in Dianshan Lake. Thalassas 34, 279–288. doi: 10.1007/s41208-017-0062-x
Koutrakis E. T., Kamidis N. I., Leonardos I. D. (2004). Age, growth and mortality of a semi-isolated lagoon population of sand smelt, Atherina boyeri (Risso 1810) (Pisces: Atherinidae) in an estuarine system of northern Greece. J. Appl. Ichthyol 20, 382–388. doi: 10.1111/j.1439-0426.2004.00583.x
Kovačić M., Baršić D., Živanović A. (2022). Temporal and spatial variation of sex ratio of Atherina boyeri in coastal lagoons. Environ. Biol. Fishes 105, 245–259. doi: 10.1007/s10641-021-01196-1
Kripa V. (2017). “Role of environmental variables on spawning and recruitment of small pelagics in an upwelling system,” in course manual summer school on advanced methods for fish stock assessment and fisheries management (CMFRI, Kochi), 289–295.
Küçük F., Güçlü S. S., Gülle İ., Güçlü Z., Çiçek L., Diken G. (2012). Reproductive features of big scale-sand smelt, atherina boyeri (Risso (1810)), an exotic fish in lake eğirdir (Isparta, Turkey). Turkish J. Fisheries Aquat. Sci. 12, 729–733.
Lappalainen A., Saks L., Šuštar M., Heikinheimo O., Jürgensm K., Vetemaam M. (2016). Length at maturity as a po-tential indicator of fishing pressure effects on coastal pikeperch (Sander lucioperca) stocks in the northern Baltic Sea. Fisheries Res. 174, 47–57. doi: 10.1016/j.fishres.2015.08.013
Le Cren E. D. (1951). The length relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 20, 210–218.
Leonardos I. D. (2001). Ecology and exploitation pattern of a landlocked population of sand smelt, Atherina boyeri (Risso 1810), in Trichonis Lake (western Greece). J. Appl. Ichthyology 17, 1–5. doi: 10.1046/j.1439-0426.2001.00296.x
Leonardos I. D., Sinis A. (2000). Age, growth and mortality atherina boyeri risso (1810)(Pisces: atherinidae) in the mesolongi and etolikon lagoons (W. Greece). J. Appl. Ichthyology 45, 81–91. doi: 10.1016/S0165-7836(99)00097-1
Lorenzoni M., Giannetto D., Carosi A., Dolciami R., Ghetti L., Pompei L. (2015). Age, growth and body condition of big-scale sand smelt Atherina boyeri Risso (1810)inhabiting a freshwater environment: Lake Trasimeno (Italy). Knowl. Manage. Aquat. Ecosyst. 416, 09. doi: 10.1051/kmae/2015005
Lowe-McConnell R. H. (1987). Ecological studies in tropical fish communities (Cambridge: Cambridge University Press), 382.
Manzo C. (2020). Sex ratio and growth of Atherina boyeri in different Mediterranean environments. Mar. Ecol. Prog. Ser. 646, 133–142. doi: 10.3354/meps13342
Mohamed A. R. M. (2022). Stock assessment and virtual pop-ulation analysis of river Shad, Tenualosa ilisha (Bloch & Schneider 1801) in the Shatt Al-Arab River, Iraq. Arch. Agric. Environ. Sci. 7, 199–208. doi: 10.26832/24566632.2022.070208
Nikolsky G. V. (1980). Theory of fish population dynamics as the biological background for rational exploitation and management of fishery resources (Edinburgh, UK: Oliver and Boyd).
Nissar S., Bakhtiyar Y., Yousuf T., Bhat A., Zuber S. M. (2024). Stock assessment of rosy barb, Pethia conchonius (Hamilton 1822) in Dal Lake of Kashmir Himalayas. J. Fisheries 12, 123201. doi: 10.17017/j.fish.572
Okamoto D. K., Schmitt R. J., Holbrook S. J., Reed D. C. (2012). Fluctuations in food supply drive recruitment variation in a marine fish. Proc. R. Soc. B: Biol. Sci. 279, 4542–4550. doi: 10.1098/rspb.2012.1862
Ozeren S. C. (2009). Age, growth and reproductive biology of the sand smelt Atherina boyeri, Risso 1810 (Pisces: Atherinidae) in Lake Iznik, Turkey. J. Fish Int. 4, 34–39.
Pallaoro A., Franicevic M., Matic S. (2002). Age, growth and mortality of big-scale sand smelt, Atherina (Hepsetia) boyeri Risso 1810 in the Pantana Lagoon, Croatia. Period. BioI. 104, 175–183.
Panda D., Mohanty S. K., Pattnaik A. K., Das S., Karna S. K. (2018). Growth, mortality and stock status of mullets (Mugilidae) in Chilika Lake, India. Lakes Reservoirs, 1–13. doi: 10.1111/lre.2018.23.issue-1
Patimar R., Yousefi M., Hoseini S. M. (2008). Age, growth and reproduction of the sand smelt Atherina boyeri Risso 1810 in the Gomishan wetland – southeast Caspian Sea. Estuar. Coast. Shelf Sci. 81, 457–462.
Pauly D. (1979). “Theory and management of tropical multispecies stocks: a review, with emphasis on the Southeast Asian demersal fisheries,” in ICLARM studies and review no. 1 (International Centre for Living Aquatic Resources Management, Manila, Philippines), 35.
Pauly D. (1980). On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 39, 175–192. doi: 10.1093/icesjms/39.2.175
Pauly D. (1983). Some simple methods for the assessment of tropical fish stocks Vol. 52 (FAO Fisheries and Aquaculture Technical).
Pauly D. (1984). “Fish population dynamics in tropical waters: a manual for use with programmable calculators,” in ICLARM studies and review no. 8, 325.
Pauly D., Munro J. L. (1984). Once more on the composition of growth in fish and invertebrates. Fishbyte WorldFish Centre 2, 1–21.
Pauly D., Soriano M. L. (1986). “Some practical extensions to Beverton and Holt’s relative yield-per-recruit model,” in First asian fisheries forum. Eds. Maclean J. L., Lb D., Hosillos L. V. (Manila, Philippines: Asian Fisheries Society), 491–496.
Pitcher T. J. (1998). A cover story: fisheries may drive stocks to extinction. Rev. Fish Biol. Fisheries 8, 367–370. doi: 10.1023/A:1008804029850
Reynolds J. D., Jennings S., Dulvy N. K. (2001). “Life histories of fishes and population responses to exploitation,” in Conservation of exploited species. Eds. Reynolds J. D., Mace G. M., Redford K. H., Robinson J. G. (Cambridge University Press, Cambridge), 147–169.
Ricker W. E. (1975). Computation and interpretation of biological statistics of fish populations. Bull. Fisheries Res. Board Canada 191, 1–382.
Roberts C. M., Hawkins J. P. (1999). Extinction risk in the sea. Trends Ecol. Evol. 14 , 241–246. doi: 10.1016/S0169-5347(98)01584-5
Rosecchi E., Crivelli A. J. (1992). Study of a sand smelt (Atherina boyeri Risso (1810)) population reproducing in fresh water. Ecol. Freshw. Fish 1, 77–85. doi: 10.1111/j.1600-0633.1992.tb00076.x
Rudi S., Zainul A. M., Silvester S. (2018). Some biology aspects of oxeye scad, Selar boops caught from Bitung wa-ters within Molluccas Sea of Indonesia. Russian J. Agric. Socio-Economic Sci. 6, 403–413.
Saborido-Rey F. (2016). “Fish reproduction,” in Encyclopedia of ocean sciences, 3rd. Eds. Cochran J. K., Bokuniewicz H. J., Yager P. L. (Academic Press, Oxford), 232–245.
Saville A. (1977). Survey methods of appraising fisheries resources. FAO Fisheries Tech. Paper 171, 76.
Sezen B. (2005). İzmir Homa Lagunu gumuş balığı (Atherina boyeri Risso 1810) populasyonunun biyolojik ozelliklerinin incelenmesi. MSc (İzmir, Turkey: Ege University).
Shoji J., Toshito S. I., Mizuno K. I., Kamimura Y., Hori M., Hirakawa K. (2011). Possible effects of global warming on fish recruitment: shifts in spawning season and latitudinal distribution can alter growth of fish early life stages through changes in daylength. ICES Jour-nal Mar. Sci. 68, 1165–1169. doi: 10.1093/icesjms/fsr059
Souza A. C. V., Costa R. S., Novaes J. L. C. (2019). Estimation of the length at first maturity of fish species of the Apodi/Mossoró River reservoirs in the Brazilian semi-arid region. Acta Ichthyologica Piscatoria 49, 195–198. doi: 10.3750/AIEP/02547
Sparre P., Venema S. C. (1998). Introduction to tropical fish stock assessment. Part 1. Manual (Rome: FAO Fisheries Technical paper), 306.
Tableau A., Brind'Amour A., Woillez M., Le Bris H. (2015). Influence of food availability on the spatial distribution of juvenile fish within soft sediment nursery habitats. J. Sea Res. 111, 76–87. doi: 10.1016/j.seares.2015.12.004
Tarkan S., Bilge G., Sezen B., Tarkan A., Gaygusuz O., Gursoy C., et al. (2007). Variations in growth and life history traits of sand smelt, Atherina boyeri, populations from different water bodies of Turkey: influence of environmental factors. Rapp Comm Int. Mer Medit 38, 611.
Tomasini J. A., Collart D., Quignard J. P. (1996). Female reproductive biology of the sand smelt in brackish lagoons of southern France. J. Fish Biol. 49, 594–612. doi: 10.1111/j.1095-8649.1996.tb00057.x
Tomasini J. A., Laugier T. (2002). Male reproductive strategy and reserve allocation in sand smelt from brackish lagoons of southern France. J. Fish Biol. 60, 521–531. doi: 10.1111/j.1095-8649.2002.tb01681.x
Tu C. Y., Chen K. T., Hsieh C. H. (2018). Fishing and temperature effects on the size structure of exploited fish stocks. Sci. Rep. 8, 1–10. doi: 10.1038/s41598-018-25403-x
Udoh J. P., Ukpatu J. E. (2017). First estimates of growth, re-cruitment pattern and length-at-first-capture of Nematopalaemon hastatus (Aurivilliuo (1810)) in okoro river estuary, southeast Nigeria. AACL Bioflux 10, 1074–1084.
Vejan A., Patimar R., Jafaryan H., Gholizadeh M., Adineh H., Aghilinezhad S. M. (2023). Population parameters of the non-indigenous invasive shrimp Palaemon macrodactylus Rathbun 1902 (Caridea: Palaemonidae) from the southeastern Caspian Sea, with implications for range expansions, threats and conservation. Mar. Environ. Res. 190, 106078. doi: 10.1016/j.marenvres.2023.106078
Vivekanandan E. (2005). Stock assessment of tropical marine fishes (New Delhi: Indian Council of Agricultural Research).
Wehye A. S., Amponsah S. K. K., Jueseah A. S. (2017). Growth, mortality and exploitation of Sardinella maderensis (Lowe 1838) in the Liberian coastal waters. Fisheries Aquaculture J. 8, 1–5.
Wolff W. J. (2000). The south-eastern North Sea: losses of vertebrate fauna during the past 2000 years. Biol. Conserv. 95, 209–217. doi: 10.1016/S0006-3207(00)00035-5
Wootton R. J. (1998). “Ecology of teleost fishes,” in Fish and fisheries series 24 (Kluwer Academic Publishers, Dordrecht, Boston, London).
Yılmaz M., Aydın M., Altın A., A.k S. (2021). Sex ratio of Atherina boyeri in Hirfanlı Reservoir, Turkey. Limnology Oceanography 66, 497–507. doi: 10.1002/lno.11656
Zan-Bi T. T., Sylla S., Arra S., Amponsah S. K. K. (2022). Population dynamics parameters of Bigeye grunt Brachy-deuterus auratus (Pisces, Haemulidae) from the continental shelf of Côte d’Ivoire (West Africa). J. Wildlife Biodiversity 6, 63–77.
Keywords: A. boyeri, Caspian sea, stock assessment, FiSAT II, growth and mortality
Citation: Hajiradkouchak E, Rahnama B and Fazli H (2025) First documentation of population dynamics and reproductive biology of big-scale sand smelt, A. boyeri Risso, 1810 in the coastal waters of the Southeast Caspian Sea- Northern Iran. Front. Mar. Sci. 12:1530184. doi: 10.3389/fmars.2025.1530184
Received: 18 November 2024; Accepted: 31 January 2025;
Published: 24 February 2025.
Edited by:
Shulian Wang, Hubei University of Technology, ChinaReviewed by:
José Pedro Andrade, University of Algarve, PortugalLu Cai, Ministry of Water Resources and Chinese Academy of Sciences, China
Copyright © 2025 Hajiradkouchak, Rahnama and Fazli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eisa Hajiradkouchak, ZWlzYWhhamlyYWRAZ21haWwuY29t
 Eisa Hajiradkouchak
Eisa Hajiradkouchak Behzad Rahnama
Behzad Rahnama Hasan Fazli
Hasan Fazli