- 1School of Biological Sciences, Victoria University of Wellington | Te Herenga Waka, Wellington, New Zealand
- 2Population Modelling Group, National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand
- 3Department of Marine Science, University of Otago, Dunedin, New Zealand
- 4School of Mathematics and Statistics, Victoria University of Wellington | Te Herenga Waka, Wellington, New Zealand
- 5Department of Conservation, Wellington, New Zealand
The oceans are warming, and marine heatwaves are increasing in frequency, extremity and duration. As ectotherms, fish that experience temperatures above their optimum suffer a host of physiological and demographic impacts, which result in a net negative effect on population biomass and productivity. However, temperatures generally decline with depth, which means that mesophotic ecosystems, found in the ‘twilight zone’ between approximately 30 and 150 m depth, have the potential to act as thermal refuges. While pelagic fishes have flexibility to deepen their distributions in the open ocean, reef fishes are dependent on benthic habitats for structural complexity and food. Mesophotic reefs may therefore be of particular importance as thermal refuges in coastal ecosystems. By analysing 27 years of model-derived temperature-depth data, we found that the intensity, duration and frequency of marine heatwaves were buffered at mesophotic versus euphotic depths at Tawhiti Rahi (the Poor Knights Islands) in Aotearoa, New Zealand. To explore and quantify the importance of this mesophotic thermal refuge we parameterised a temperature-dependent multispecies size-spectrum model for the Poor Knights Islands reef community and ran marine heatwave simulations in the presence and absence of mesophotic reef habitat. Almost all heatwave strengths resulted in biomass and productivity reductions for almost all modelled fish species, but the presence of a mesophotic thermal refuge often reduced or reversed these losses. For the biomass of fish species targeted by fisheries, negative impacts were reversed during a moderate heatwave (28% average difference compared to scenarios lacking a refuge), negated during a strong heatwave (24% difference), and mitigated during severe and extreme heatwaves (21% and 20% respectively). The productivity of fisheries targets was similar with or without a thermal refuge under moderate heatwave conditions, but under strong, severe and extreme heatwaves, refuges became valuable. Average productivity losses were almost negated during strong heatwaves (5% difference), negated during severe heatwaves (17% difference), and mitigated during extreme heatwaves (19% difference). By providing this first estimate of the value of mesophotic reefs as thermal refuges during marine heatwaves we hope to inform conservation and management decisions about the targeted protection of mesophotic reefs.
1 Introduction
The ocean is experiencing long-term warming due to anthropogenic climate change, having absorbed over 90% of the excess heat energy produced by increased greenhouse gases (IPCC, 2021). This long-term increase in ocean temperature is the main factor driving an increase in the frequency, extremity and duration of marine heatwaves (MHWs; Fragkopoulou et al., 2023; Schlegel et al., 2019; Smith et al., 2021), defined as more than five days of ocean temperatures above the 90th percentile of historical observations (Hobday et al., 2016). MHWs have immediate and significant impacts on marine ecosystems (Frölicher et al., 2018; Smith et al., 2021). Consequences for fisheries include biomass reductions (Cavole et al., 2016; Cheung and Frölicher, 2020; Gammelsrød et al., 1998; Mills et al., 2013; Ñiquen and Bouchon, 2004), which can be much greater and more rapid than those caused by decadal-scale warming (Baudron et al., 2014; Cheung and Frölicher, 2020; Givan et al., 2018).
In Aotearoa New Zealand, wild-capture fisheries exports were worth over NZ$1.5 billion in 2023 (MPI, 2023), with inshore finfish contributing approximately $75 million (MPI, 2023). In addition, many coastal species also hold important recreational and cultural value (Bess, 2016; McCarthy et al., 2013, 2014; Parsons et al., 2014). Future increases in ocean temperature are predicted to reduce growth rates of key New Zealand species, such as snapper, Chrysophrys auratus, and tarakihi, Nemadactylus macropterus (Cummings et al., 2021; Morrongiello et al., 2021), and the recent 2017–2018 summer MHW caused mass mortalities of caged coastal aquaculture salmon (Salinger et al., 2019). However, wild fishes can track their preferred thermal niche, unlike sessile species that can only do so on a population-turnover time scale, or aquaculture fish whose range is limited to the cage frame. Many fish species have undergone poleward range shifts in response to warming (García Molinos et al., 2016; Last et al., 2011; Parmesan and Yohe, 2003; Pinsky et al., 2013; Poloczanska et al., 2013), including in New Zealand (Lavin et al., 2023; Middleton, 2022).
Range shifts are not limited to latitudinal movement, however, as the ocean is a three-dimensional space and temperatures decrease with depth (Bongaerts et al., 2010; Frade et al., 2018; Leichter et al., 2006; Riegl and Piller, 2003). Several fish species have demonstrated long-term deepening of their distributions in response to warming (Chaikin et al., 2021; Dulvy et al., 2008; Givan et al., 2018; Nye et al., 2009; Perry et al., 2005; Rutterford et al., 2015), and deeper waters may also offer short-term refuge from thermal stress. Mesophotic ecosystems extend from where light levels are approximately 1% of surface irradiance, up to a maximum depth beyond which primary production cannot occur (Cerrano et al., 2019). These mesophotic conditions can occur across a broad range of depths depending on local conditions but are often found between ~30 and 150 m (Cerrano et al., 2019). The deep reef refuge hypothesis (DRRH), proposed originally for mesophotic coral ecosystems, suggests that mesophotic reefs may be buffered against disturbances that impact the shallows, and may be able to re-seed shallow communities following disturbance (Bongaerts et al., 2010). It is now understood that the DRRH holds only for some coral species under certain disturbances (Bongaerts et al., 2017), but its role in supporting mobile organisms such as fish is less well-studied. Mesophotic reefs could act as ecologically viable thermal refuges from MHWs, particularly for coastal reef fishes that rely on benthic habitat for structural complexity and food. One of the few studies exploring this showed that the subtropical reef fish Lethrinus miniatus on Australia’s Great Barrier Reef demonstrated short-term movement from euphotic to mesophotic reefs when mean daily water temperatures increased (Currey et al., 2015). The potential for a deep reef refuge has not yet been explored for temperate coastal fishes.
While the majority of recent physical oceanography studies found the intensity and duration of MHWs increase with depth (Dayan et al., 2023; Fragkopoulou et al., 2023; Hu et al., 2021; Schaeffer et al., 2023; Schaeffer and Roughan, 2017; Sun et al., 2023; Zhang et al., 2023), several studies investigating the ecological impacts of MHWs across depth have indicated a mesophotic thermal refuge effect, including for canopy-forming macroalgae (Giraldo-Ospina et al., 2020), corals (Frade et al., 2018; Muir et al., 2017), sponges (Perkins et al., 2022) and anemones (Haguenauer et al., 2021). Physical oceanography and ecological impact research into mesophotic MHWs are still somewhat divorced; physical oceanography studies have not yet analysed actual or modelled MHW impacts across depths, and though some ecological studies include temperature-depth data (Frade et al., 2018; Haguenauer et al., 2021), none have yet conducted MHW analyses on those data. Furthermore, to our knowledge, all subsurface MHW studies to date focus on heatwaves according to depth-specific temperature regimes. This is valuable when considering impacts on species which reside within specific depth strata and are acclimatised to their usual temperature regimes, particularly sessile species. However, for mobile organisms with preferred depth ranges, the buffering potential of deeper depths against MHWs (relative to their preferred depth range’s temperature regime) is highly relevant. In addition, depth-driven differences in the maximum absolute temperatures that organisms are exposed to during MHWs might be a more important predictor of stress and physiological response (Cavanaugh et al., 2019).
Exploring the ecosystem-level implications of mesophotic reefs as thermal refuges from MHWs requires consideration of ecosystem dynamics and species interactions. Multispecies size-spectrum models that incorporate temperature effects on physiological rates are well suited for this task. Multispecies size-spectrum models (MSSMs) incorporate species-specific life history traits and well-evidenced size-based feeding theory (Blanchard et al., 2009). They allow for ontogenetic diet shifts, opportunistic predation and cannibalism (Jennings et al., 2001), whilst requiring relatively few parameters compared to data-hungry end-to-end models such as Atlantis (Fulton et al., 2004, 2005, 2011) and Ecopath with Ecosim (Christensen and Walters, 2004). In recent years, especially following the development of the R package mizer (Delius et al., 2023; Scott et al., 2014), MSSMs have been used to investigate fisheries management strategies (Benoit et al., 2022; Blanchard et al., 2014; Robinson et al., 2022; Spence et al., 2021; Szuwalski et al., 2017), blue carbon stocks (Falciani et al., 2022), stock-recruitment relationships (Canales et al., 2020), ecosystem recovery signals and ecological indicators (Clements et al., 2019; Zhang et al., 2018), fisheries impacts (Forestier et al., 2020), and the effects of seasonal processes on dynamics and yields (Datta and Blanchard, 2016). Woodworth-Jefcoats et al. (2019) simulated the impacts of ocean warming and fishing on the bigeye tuna longline fishery in Hawai’i and found that detrimental warming effects could be mediated but not completely mitigated by fisheries management changes. These findings were supported by an Ecopath with Ecosim model of the same system (Howell et al., 2013). Audzijonyte et al. (2022) included size-structured benthic resources as well as temperature effects for a Tasmanian coastal rocky reef ecosystem and found that temperature-driven changes in planktonic and benthic resources affected fish biomasses and yields more strongly than temperature-driven physiological changes did. Lindmark et al. (2022) modelled temperature effects on planktonic resource spectrum dynamics as well as on fish physiology and found that warming resulted in increases in size-at-age and growth rates but decreases in large fish abundance and fisheries yields. In investigating similar warming impacts, Kuo et al. (2022) also incorporated species distribution models, allowing species to respond spatially with horizontal range shifts, habitat shrinkage and changes in species overlap and interactions.
Despite this growing body of research on temperature effects in ecosystem models, the potential of deeper waters to act as a thermal refuge has not yet been explored, although it has been highlighted as an important research avenue (Audzijonyte et al., 2022; Woodworth-Jefcoats et al., 2019). In this study, we use modelled temperature-depth data and develop a temperature-dependent MSSM to test the hypotheses that in temperate ecosystems, (1) MHWs are buffered at mesophotic depths compared to euphotic depths, and (2) that mesophotic thermal refuge use mitigates reductions in fish biomass and productivity during MHWs in temperate coastal ecosystems.
2 Methods
2.1 Study sites
This study focused on euphotic and mesophotic rocky reefs at Tawhiti Rahi (the Poor Knights Islands) located 22 km off the east coast of Northland in Aotearoa, New Zealand (Figure 1). The Poor Knights Islands are surrounded by a Type I (no-take) marine reserve which extends 800 m offshore and covers a 15 km2 area. This area has had restricted commercial fishing since 1981 and has been a no-take marine reserve since 1998. Due to the influence of the warm East Auckland Current (Sim-Smith and Kelly, 2009; Zeldis et al., 2004), the highly diverse fish community comprises typical temperate New Zealand coastal fishes as well as subtropical residents and visitors (Allard, 2020; Brook, 2002; Sim-Smith and Kelly, 2009). As there is no fishing within the marine reserve, fishing effects are not a confounding influence for the MSSM, though many important commercial fisheries species inhabit the site (Fisheries New Zealand, 2023; Sim-Smith and Kelly, 2009).
Figure 1. Map of study sites at the Poor Knights Islands, which are 22 km offshore from the northeast of the North Island of New Zealand.
We chose two sites, Northern Arch and Imagination Point, for temperature analysis and to parameterise the MSSM. These site choices account for possible variation in temperature regimes in this area, as Northern Arch is at the northwestern corner of the northern island, whilst Imagination Point is at the southwestern corner of the southern island. The vertical cliffs at both sites continue underwater to depths of around 85 m (Harris, 2022), considered to be the mid-deep mesophotic zone (Rocha et al., 2018). These steep reef walls result in euphotic and mesophotic rocky reefs that are adjacent to one another, which provides an opportunity for mesophotic reefs to be accessed as thermal refuges for euphotic reef-associated fishes.
2.2 Temperature regimes and marine heatwaves across depth
2.2.1 Model-derived temperature-depth data
We used daily-average temperature data from the Moana Hindcast (Souza et al., 2023), a 27-year (1994 – 2020) three-dimensional, 5 km spatial resolution hydrodynamic model for New Zealand’s ocean region developed using the Regional Ocean Modelling System (ROMS) version 3.9. The model has 50 vertical sigma layers distributed between the surface and seafloor, providing high vertical resolution in coastal and shelf waters. Evaluation of the Moana Hindcast presented in Souza et al. (2023) and Kerry et al. (2023) shows it provides a realistic and skilful representation of upper-ocean (0–1000 m) temperature variability, both locally and regionally, when compared to satellite-derived and in situ temperature observations. This makes the Moana Hindcast, like other well-validated ocean models, a useful tool for assessing the depth-structure of MHWs in locations lacking in situ temperature observations (Kerry et al., 2022; Amaya et al., 2023; Fragkopoulou et al., 2023; Großelindemann et al., 2022; Perelman et al., 2024). The Moana Hindcast has also been used in recent assessments of the characteristics of upper-ocean heat content and sub-surface MHWs around New Zealand (Kerry et al., 2022, Kerry et al., 2023). De Souza et al. (2022) and Kerry et al. (2023) present a comprehensive description of the model configuration and its evaluation.
We extracted daily average temperature data from 10, 30, 50, 70 and 100 m depth from the Moana Hindcast for the Poor Knights from 5 km x 5 km grid cells surrounding the sites Imagination Point (-35.4916647, 174.7390431) and Northern Arch (-35.448466, 174.731481) over the period 1994 – 2020.
2.2.2 Marine heatwave analyses
We used R (R Core Team, 2024) in RStudio (Posit Team, 2024) to conduct all analyses in this study. To carry out MHW analyses, we used heatwaveR, an R package based on Hobday et al. (2016)’s methodology. heatwaveR defines a MHW as more than five days of ocean temperatures above the seasonally varying 90th percentile of historical observations. A single MHW event is confirmed if temperatures exceed the threshold for more than five days, and drop below the threshold for less than three days. This method allows for the detection of MHW events outside summer months. It can be as locally specific as the data permit, and has been used in the vast majority of MHW research since it was developed (Bell et al., 2023; Fragkopoulou et al., 2023; Marin, 2021; Oliver et al., 2018, 2019, 2021; Schaeffer and Roughan, 2017; Schlegel et al., 2019; Thoral et al., 2022; Zhang et al., 2023).
For both study sites, we calculated site-specific, seasonally varying climatological means and 90th percentile MHW thresholds using heatwaveR. While a 30-year time series is recommended (Hobday et al., 2016), time series as short as 10 years still result in comparably acceptable MHW metrics (Schlegel et al., 2019) and so our 27 years of data were sufficient. Given that our study focuses on thermal refuges at mesophotic depths, we calculated MHW thresholds for temperatures at 10 m depth (in the euphotic zone) and then applied these thresholds to all other depths. This allowed us to assess when and by how much different depths avoided thermal stress compared to the euphotic zone. From the various MHW metrics calculated by heatwaveR, we analysed the annual number of MHW days, the annual number of MHW events, the annual average MHW duration, the annual average cumulative intensity relative to the MHW threshold, the annual average maximum intensity relative to the MHW threshold, and the annual average mean intensity relative to the MHW threshold. We assigned a zero to years with no MHWs. Cumulative intensity (measured in °C-days) here represents the sum of temperature anomalies relative to the 90th percentile MHW threshold over the duration of a MHW. Maximum intensity (measured in °C) here is the highest temperature anomaly relative to the MHW threshold during a MHW, and mean intensity (measured in °C) is the average temperature anomaly relative to the MHW threshold during a MHW. We used intensities relative to the threshold rather than relative to the climatological mean due to the importance of including years with no MHWs as zeroes.
We chose the best of various regression models for count data using AIC (Supplementary Tables S1, S2) for the number of MHW days and the number of MHW events: a zero-inflated negative binomial regression model and a negative binomial regression model, respectively. These included the effects of site, depth and site-depth interaction and were made using the ‘glmmTMB’ function from the glmmTMB package in R. Diagnostic plots for models were checked using the DHARMa package, and indicated that the residuals followed the expectations of the respective distributions. We then conducted backwards stepwise model selection to select the final model for pairwise Tukey-adjusted comparisons.
For annual average cumulative intensity, maximum intensity, and mean intensity relative to the seasonally-varying MHW threshold as well as for annual average MHW duration, residuals and QQ plots showed strong departures from homogeneity of variances and normality. Non-parametric Kruskal-Wallis tests were therefore used and conducted separately for site and depth, with post-hoc Dunn tests carried out when a variable was significant.
2.3 Size-spectrum modelling with mizer and therMizer
Mizer is a free package available in R (Delius et al., 2023; Scott et al., 2014), and mizer models are dynamic multispecies size-spectrum models which incorporate multispecies predator-prey interactions through size-based feeding, whereby predators eat prey smaller than themselves (Andersen et al., 2016; Sommer et al., 2018). Mizer’s core equation is the McKendrick-von Foerster equation,
which tracks the change through time t in the abundance N of species i at size w (weight in grams), in response to prey-dependent somatic growth and mortality . Individuals enter the model at a constant recruitment rate at egg size . They exit this and every following size class either due to ‘growing out’ of it into the next size class or due to mortality, which is the sum of background, starvation, fishing, and predation mortality (Blanchard et al., 2014; Lindmark et al., 2022; Scott et al., 2014; Zhang et al., 2016).
Additional equations that make up the mizer modelling framework, such as the prey size selection function and those involved in planktonic resource spectrum dynamics and reproduction, are detailed in several other mizer papers (e.g., Blanchard et al., 2014; Datta and Blanchard, 2016; Lindmark et al., 2022; Scott et al., 2014; Zhang et al., 2016).
therMizer is a mizer extension package (https://github.com/sizespectrum/therMizer) which enables temperature-based effects on species’ metabolic rates and aerobic scope. It was developed and first used by Woodworth-Jefcoats et al. (2019). The effect of temperature on metabolic rate, TEM, is represented by the equation
where T is temperature (Kelvin), k is Boltzmann’s constant, and E is activation energy. A scaled form of this equation, (range: 0 – 1), is applied as a multiplier to mizer’s standard metabolic rate, which results in each species’ metabolic rate being lowest at its lower thermal tolerance limit and highest at its upper thermal tolerance limit. The way in which temperature affects predator-prey encounter rates, TER, is modelled by
where T is temperature and and are a species’ lower and upper thermal tolerance limits. A scaled form of this equation, (range: 0 – 1), is applied as a multiplier to mizer’s standard predator-prey encounter rate. This results in species being at peak aerobic performance and encountering the highest amount of prey when at their thermal optimum. Outside the limits of a species’ thermal tolerance, and are set to 0, causing local extinction.
2.3.1 A multispecies size-spectrum model for the Poor Knights Islands
2.3.1.1 Species inclusion and model parameters
Supplementary Table S3 provides key model parameters and their sources for ten specific species and eight functional guilds in the Poor Knights Islands mizer model. The full species list is in Supplementary Table S4. Within a functional guild, the most abundant species was the representative (as in McGregor et al., 2021), and determined life history parameters for that group. We calculated observed guild abundance as the combined average of all species in the group based on observational data.
Observational data were from underwater visual census (UVC) surveys conducted by the Department of Conservation (DOC). Available data were the from the first Poor Knights Islands survey in the austral spring of 1998, the 1992–2002 biannual surveys conducted in spring-summer and autumn, the 2011 summer survey, the 2015 and 2019 autumn surveys, and the 2021 autumn survey. All surveys were conducted at depths between 2.5 and 31 m and incorporated approximately 21 sites throughout the Poor Knights Islands. Nine transects of 25 m x 5 m were assessed at each site, with the aim of three replicates for three depth strata (<10 m, 10–18 m and 18–25+ m). Sampling and replication were similar across survey years (Allard, 2020). We only considered surveys which included rocky reef habitats (rather than soft sediments), in order to best characterise communities on shallow reefs.
We assigned fisheries target species to the model as distinct species, and grouped non-fisheries species into functional guilds with species of similar diet preferences and sizes (McGregor et al., 2021). Note that while mackerels are commercially fished, they are managed as a group (Fisheries New Zealand, 2023), and so we included them as a functional guild. We included the fisheries species butterfish in the functional guild of herbivores rather than as a separate species, as no thermal tolerance data were available for them. Similarly, we included the fisheries species blue cod in the functional guild of small piscivores rather than as a separate species, as they had only two 1999 sightings, with their key fisheries and ranges being in the South Island (Fisheries New Zealand, 2023).
Carpet sharks (Cephaloscyllium isabellum) and rays were present in UVC data, but we did not include them in the model. The life history traits and feeding habits of sharks and rays (i.e., producing very small numbers of young comparative to teleost fishes, hunting over very large areas for sharks, and the lack of existing aging parameters for stingrays) are relatively ill-suited to mizer model mechanics. We also excluded hāpuku (Polyprion oxygeneios) from the model, due to their similar transient predatory behaviour (Beentjes and Francis, 1999) and very low abundance in UVC data.
We calculated a single observed abundance for each species – or the combined average of all guild species for each functional guild – from combined surveys (Supplementary Table S3). We scaled abundances to 1 m2 for standardisation, with the exception of cryptobenthic fishes. Due to the cryptic habits of almost all other triplefins, blennies and gobies, only the representative triplefin species Forsterygion maryannae was recorded during UVC surveys. We therefore set the abundance of this guild as a free parameter that the model could ‘decide’, in order to enable the fish community to persist at equilibrium.
Model parameters not specified in Supplementary Table S3 were assigned mizer’s default values, and those related to reproduction and recruitment were determined during model calibration (Delius, 2022). Given that all modelled species were from the euphotic zone, we left the interaction matrix as its default 1:1 interaction between all species. As the Poor Knights Islands Marine Reserve is an unfished ecosystem, we set fishing effort to zero. The carrying capacity was left as the default 2.05, and the allometric growth and metabolic exponents were both set to the mizer default of 0.75.
2.3.1.2 Model calibration
We iteratively projected the model to steady state, calibrated and matched model abundances to observed abundances, and matched growth rates using the ‘steady’, ‘calibrateNumber’, ‘matchNumbers’ and ‘matchGrowth’ functions in the mizer package (version 2.5.0). Model growth curves for each species were tuned to approximate von Bertalanffy growth curves, and the model was again run to steady state, abundances calibrated and matched, growth rates matched, and steady state converged.
Reproductive parameters were tuned to ensure that species responded appropriately to fishing, using methods described by the mizer course (Delius, 2022). FishBase assigns fishing resilience categories of very low, low, medium and high to species based on life history information. These resilience categories are associated with a maximum intrinsic population growth rate r. The fishing effort that achieves maximum sustainable yield (MSY; which is when the replenishment rate equals the extraction rate) is
and each resilience category has a range within which yield should peak. This relationship was used to adjust reproduction levels so that model resiliencies matched FishBase resiliencies, through testing the impact of various fishing efforts with knife-edge selectivity for different reproduction levels. Chosen reproduction levels were incorporated back into the model (which is an unfished system), and observed and modelled abundances and growth rates were matched again and run to steady state.
We checked full community size spectra (Supplementary Figure S1) and feeding levels (Supplementary Figure S2) to ensure reasonable planktonic resource abundance, approximately flat total community size spectra (according to Sheldon spectrum theory; Sheldon et al., 1972), and feeding levels close to the default 0.6 and above critical thresholds. We also evaluated emergent diets (Supplementary Figure S3) and sources of mortality (Supplementary Figure S4) for all species to ensure ontogenetic shifts from feeding on plankton to feeding on other fishes for all species and guilds for which that should occur.
2.3.2 Euphotic and mesophotic realms in therMizer
We created two therMizer realms: euphotic at 10 m, and mesophotic at 50 m. The vertical migration array (realm × species × size) represents the proportion of time spent in each realm by a given species when it is at a given size. The exposure array (realm × species) has values of 1 for realms a species is exposed to and 0 for realms it is not exposed to. It links the vertical migration array to the ocean temperature array, which contains the realm temperatures through time.
We set up two vertical migration arrays and corresponding exposure arrays: one where the entire modelled fish community inhabited the euphotic realm, and one where they all inhabited the mesophotic realm.
2.3.3 Simulating marine heatwave scenarios
We created the therMizer model using the existing mizer model as a base, with all species set to inhabit the euphotic realm by using the euphotic vertical migration and exposure arrays. The ocean temperature array was set up to contain a year of daily climatological means from the 1994–2020 Moana Hindcast (Souza et al., 2023) at 10 m depth for the euphotic realm and 50 m depth for the mesophotic realm. These temperatures were calculated by averaging across Northern Arch (-35.448466, 174.731481) and Imagination Point (-35.4916647, 174.7390431) grid cells (Figure 1) and then using heatwaveR to calculate daily climatological means. The new therMizer model was re-calibrated and re-matched to observed abundances and iteratively run to steady state. Growth rates, resiliencies, diets, and sources of mortality were checked and did not require re-adjustment.
Depth-specific MHW category thresholds for 10 m depth and 50 m depth were also calculated using heatwaveR. From these, four pairs of new ocean temperature arrays were created for the MHW scenarios: moderate, strong, severe and extreme daily MHW temperatures for the euphotic and mesophotic realms (Figure 2). We then ran eight one-year heatwave simulation scenarios using the therMizer model calibrated to normal euphotic temperatures. For the first pair of simulations, the fish community experienced moderate depth-specific heatwave temperatures for one year on a euphotic versus a mesophotic reef. The next three pairs of simulations were on a euphotic versus a mesophotic reef for each succeeding depth-specific heatwave category level: strong, severe, and extreme.
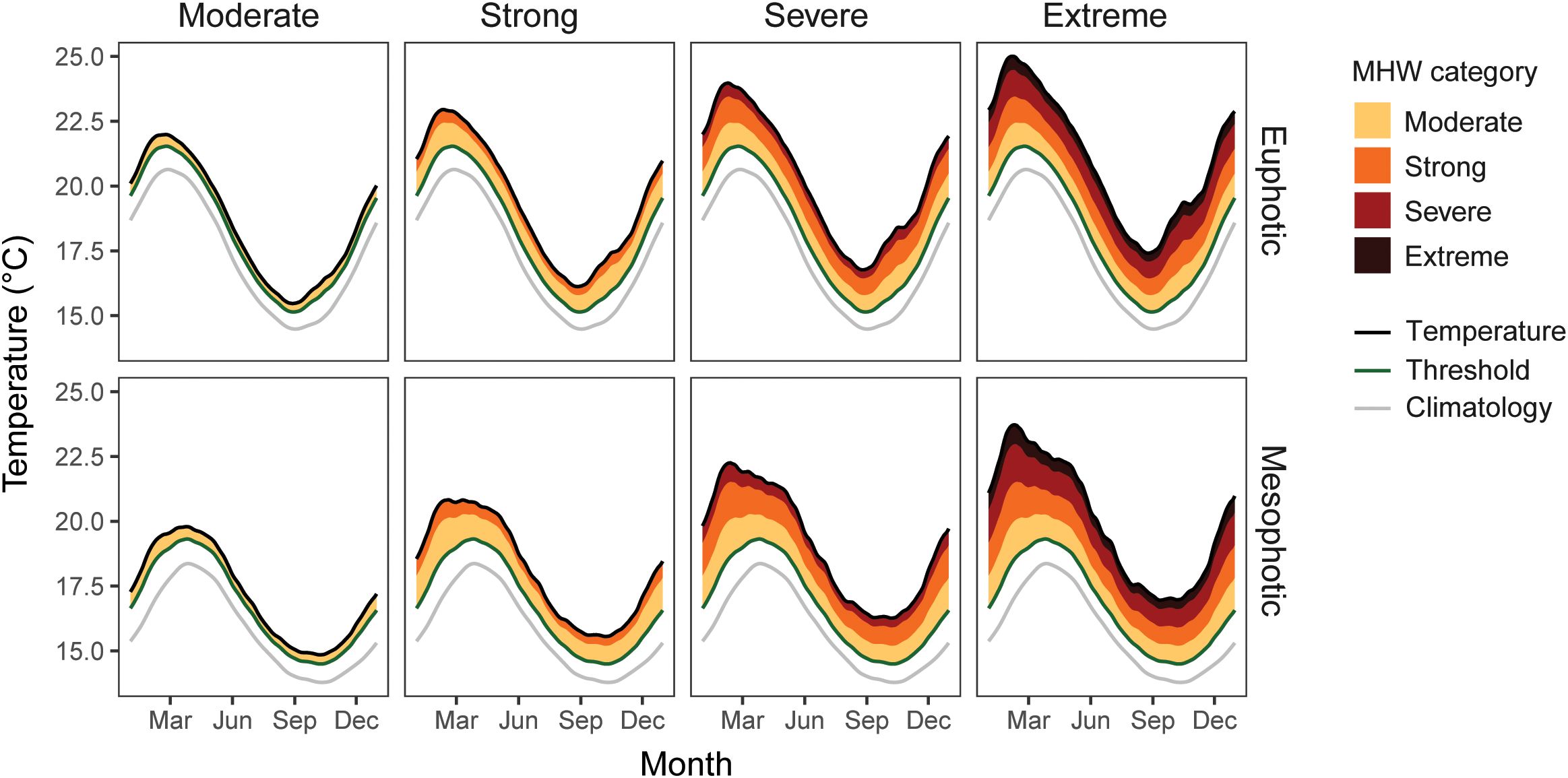
Figure 2. Temperature regimes experienced by the modelled fish community in the one-year heatwave simulations, where the community is either on the euphotic reef or the mesophotic reef. Daily climatological mean temperatures (climatology) and heatwave category thresholds for each depth were calculated using heatwaveR (Hobday et al., 2018).
2.3.4 Thermal tolerance ranges in therMizer
therMizer requires the lower and upper bounds of a species’ thermal tolerance, which are detailed in Supplementary Table S3. Species’ thermal maximums are key in how they are affected by MHWs; the lowest thermal maximum among the modelled species and builds is that of blue moki at 21.4°C, while the highest thermal maximum is that of john dory at 28.5°C. The average thermal maximum across all species and guilds is 24.43°C (23.63°C, 25.24°C 95% CI). Figure 2 shows the temperatures the modelled species will experience in the MHW simulation scenarios, where, for example, the maximum euphotic temperature of the extreme heatwave scenario (25°C) is higher than the thermal maximums of twelve out of eighteen modelled species.
2.3.5 Validating the therMizer model against historical abundance data
To test the predictive skill of our therMizer model, we used the historical UVC survey abundance data. We set up a euphotic ocean temperature array that contained Moana Hindcast yearly temperature averages from 1998 – 2019, as those were the years that overlapped with historical UVC surveys. Species and guilds were set to inhabit the euphotic realm using the euphotic vertical migration and exposure arrays. The twenty-year simulation we ran with these parameters showed that the yearly abundances of modelled species and guilds tended to lie close to the mean UVC survey abundances, as expected (Supplementary Figure S5). There was some divergence between model and historical data abundances (e.g., invertivores), potentially due to the grouping of species into guilds; the individual surveyed species dynamics could diverge while the modelled guilds could not, and hence model fits were poorer for guilds.
2.3.6 Biomass and productivity calculations
For the MHW simulations, we calculated total community biomass and individual species biomasses at the end of each simulation with the ‘getBiomass’ function of the mizer package. We calculated the percentage change in biomass relative to the biomass starting values from the ‘steady state’ therMizer model, which was calibrated to average daily euphotic temperatures.
We also calculated total community productivity and the population productivity of individual species for the final time steps of simulations, using the ‘getProductivity’ function from the mizerReef package which is currently under development (Beese et al., 2024). The function is based on the equation:
where is the productivity P (in grams per m2 per year) of species group i at weight w, is the number of individuals of species group i at weight w, and is the energy rate available for growth for species group i at weight w after accounting for metabolism and movement (Beese et al., 2024). The function requires a mizer object and a size range in length. The size range used here was 1 cm to 173.7 cm, with the latter length as the maximum lmax value of all modelled species (which was the lmax of kingfish).
To calculate the percentage change in productivity between heatwave simulations and normal euphotic temperatures, we ran the ‘steady state’ therMizer model for one year. We then obtained and compared the productivity values for its final time steps to the productivity values of the heatwave simulations’ final time steps.
3 Results
3.1 Temperature regimes and marine heatwaves across depth
3.1.1 Temperature differences across depth
Temperatures at the Poor Knights were distinctly stratified and decreased with depth during the austral summer months (Supplementary Table S5; Figure 3), with the mean summer difference in temperature between adjacent depths as 1.58°C (1.28°C, 1.87°C 95% CI). The mean summer MHW temperature threshold, averaged over the daily varying thresholds, was 20.18°C (20.15°C, 20.21°C 95% CI). The mean summer difference between 10 m and 50 m, a difference which is particularly relevant for the MHW therMizer simulations, was 3.22°C.
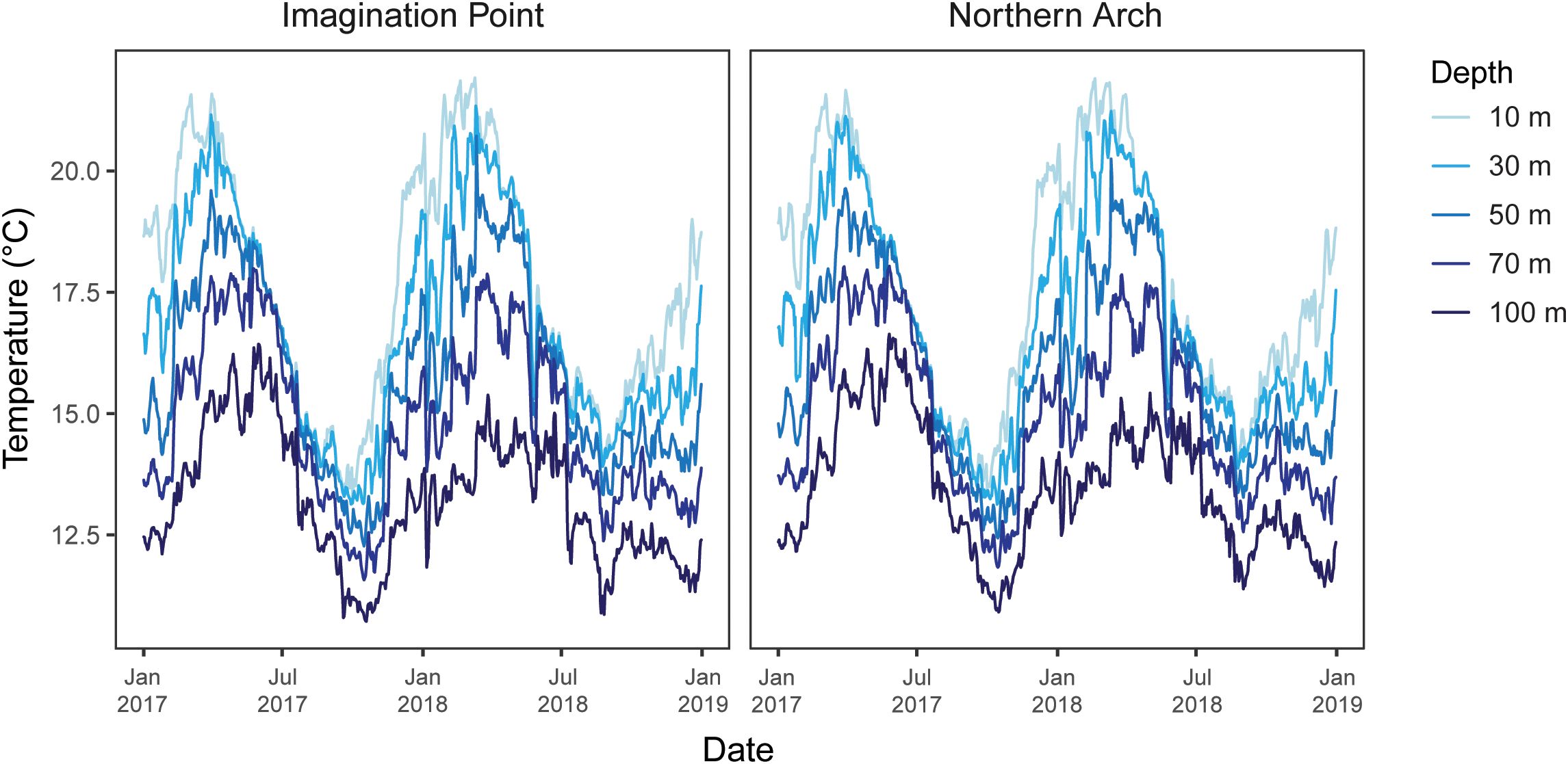
Figure 3. Daily ocean temperatures (°C) at 12 noon by site, depth and time at the Poor Knights Islands, extracted from the Moana Hindcast Model displaying data from January 2017 to January 2019. These dates include the 2017–2018 austral summer heatwave (Salinger et al., 2019).
During the austral winter at the Poor Knights, vertical mixing of the water column resulted in smaller temperature differences between shallower depths. However, differences widened as adjacent depths deepened (Supplementary Table S5), from the mean difference between 10 m and 30 m being only 0.04°C (0.04°C, 0.05°C 95% CI) to the mean difference between 70 m and 100 m being 1.15°C (1.14°C, 1.16°C 95% CI). The mean winter MHW temperature threshold was 16.23°C (16.20°C, 16.25°C 95% CI).
3.1.2 Marine heatwaves are buffered at mesophotic depths relative to euphotic depths
The number of MHW days and MHW counts decreased with depth at both sites, and did not differ between sites (Supplementary Tables S1, S2; Figure 4). The intensities (cumulative, maximum and mean) and durations of MHWs also did not differ between sites (p > 0.05 for all Kruskal-Wallis tests), but decreased with depth. All MHW metrics at 10 m depth were greater than at deeper depths. All MHW metrics at 30 m depth, except for the number of MHW days (which did not differ between 30 and 50 m; p = 0.34 for post-hoc Tukey test), were always less than at 10 m and greater than at deeper depths (p < 0.05 for all post-hoc Tukey and Dunn tests). None of the MHW metrics differed between 50, 70 and 100 m depth (p > 0.05 for all post-hoc tests).
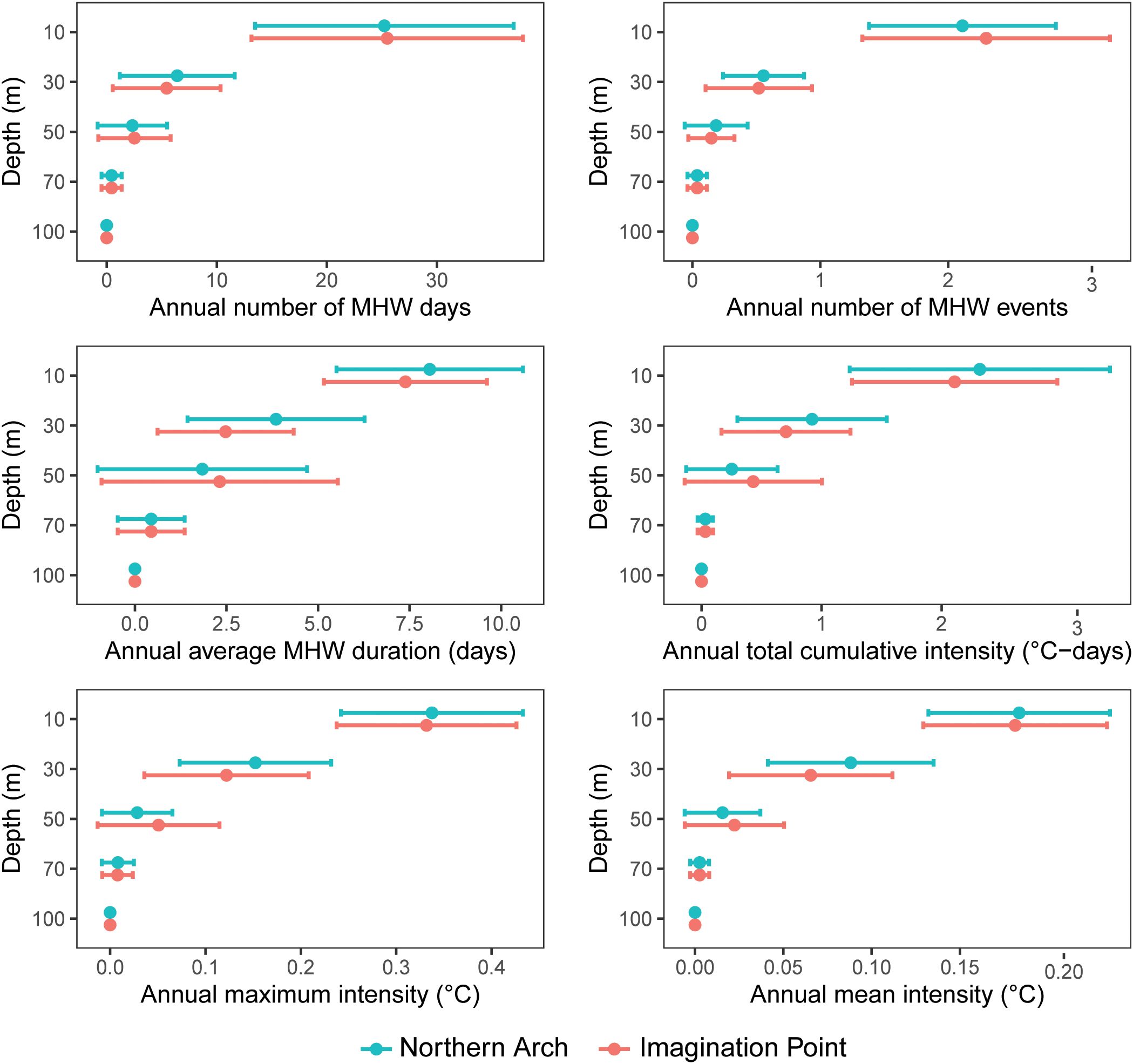
Figure 4. Means and 95% confidence intervals for MHW metrics across depths and sites at the Poor Knights Islands. Daily ocean temperatures (1994 – 2020) were extracted from the Moana Hindcast Model and metrics were calculated using heatwaveR..
3.2 Mesophotic thermal refuge effects on fish biomass and productivity
A one-year simulated heatwave resulted in a decline in total fish community biomass for almost every heatwave category (Figure 5). When fish were restricted to euphotic reef temperatures, biomass reductions increased in severity from –3.62% under the moderate heatwave to –24.66% under the extreme heatwave. However, when exposed only to mesophotic reef temperatures, these biomass reductions were mitigated for every heatwave category level. There were very small increases in biomass of <1% under the moderate and strong heatwaves, and lesser biomass reductions of –2.79% and –7.24% under the severe and extreme heatwaves respectively.
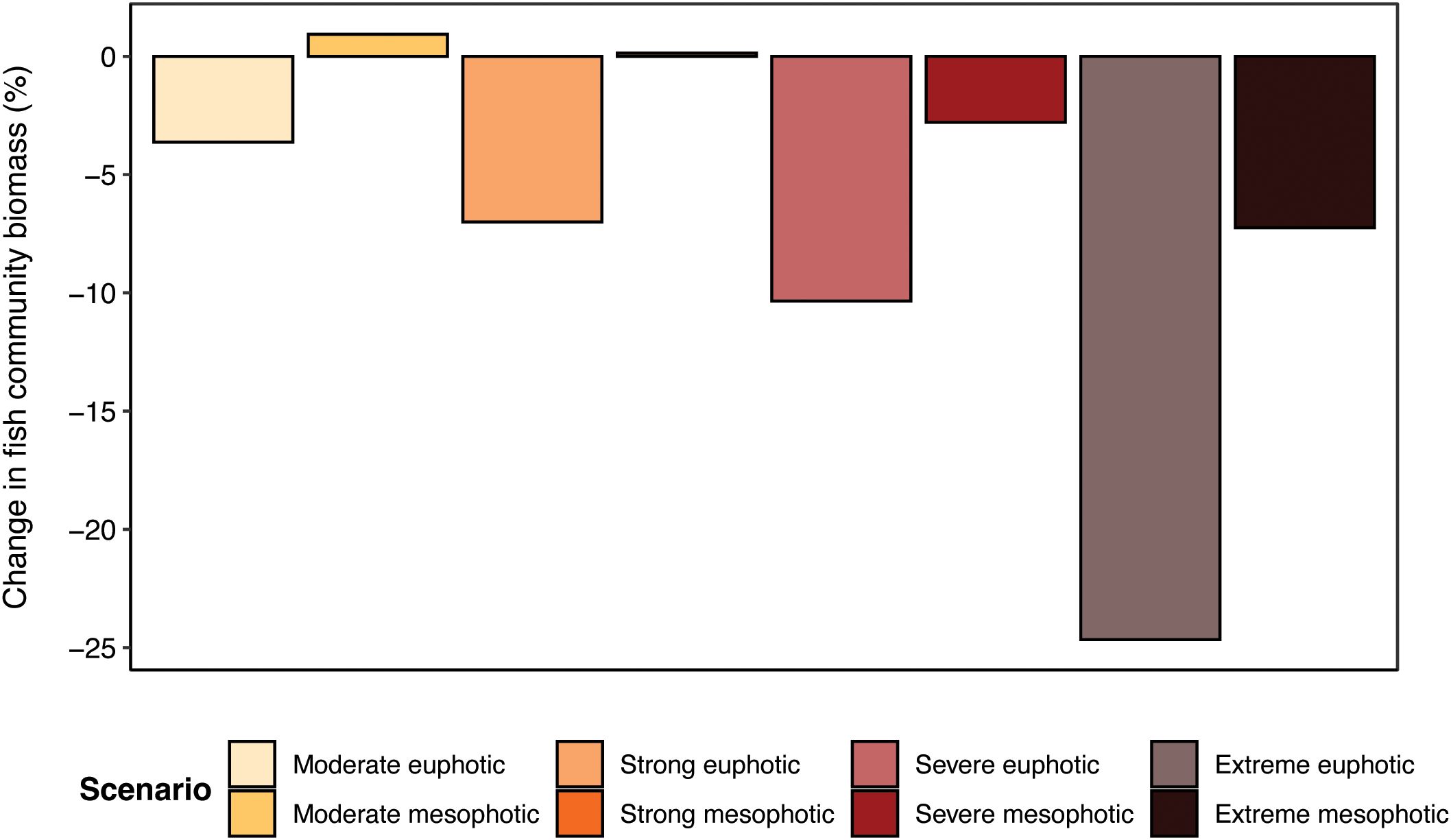
Figure 5. Percentage change in total fish community biomass after year-long simulations of various heatwave scenarios. Paler colour bars represent euphotic reef temperatures, and darker colour bars represent fish utilising mesophotic reefs as thermal refuge. Note that the biomass changes depicted here are from the final time step (i.e., the final day) of each simulation.
Total community productivity increased slightly (<1% increase) during a moderate heatwave at euphotic depths, but reduced by 7.96% under mesophotic temperature conditions (Figure 6). However, all three higher heatwave category levels resulted in progressively larger decreases in fish community productivity at euphotic depths: –1.94%, –8.19% and –19.37% for the strong, severe and extreme heatwaves respectively. Productivity reductions were either greatly mitigated or reversed when fishes were exposed to mesophotic temperature regimes, with productivity changes of only –0.41%, 1.22% and –2.29% for the strong, severe and extreme mesophotic heatwaves.
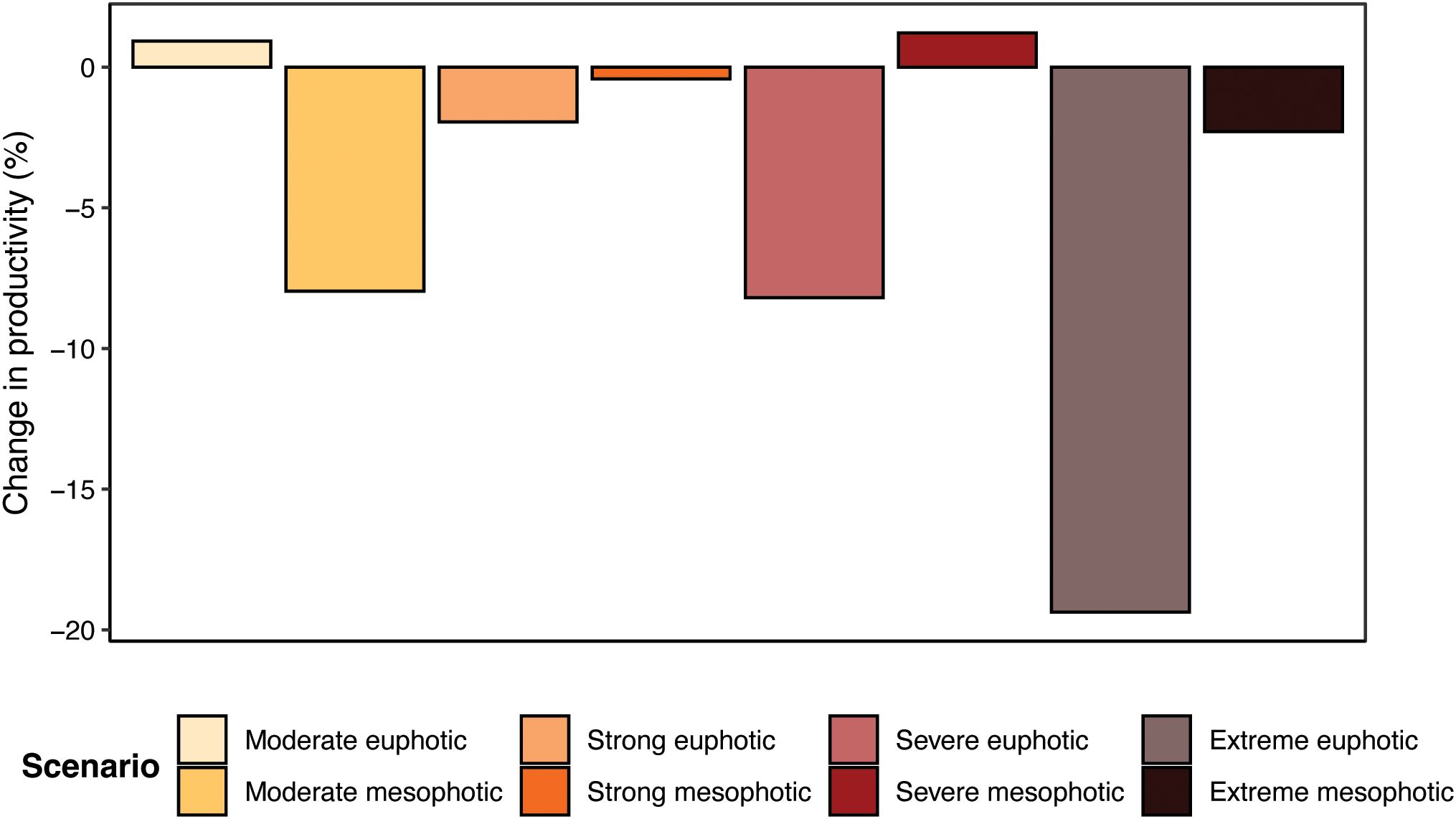
Figure 6. Percentage change in total fish community productivity after year-long simulations of various heatwave scenarios. Paler colour bars are for when the community remained on the euphotic reef, and darker colour bars are for when the community escapes to the mesophotic reef.
All fisheries species, as well as eels, invertivores, small piscivores and planktivores exhibited population biomass responses to heatwaves and thermal refuges similar to those of the total fish community, albeit with variation in absolute impacts (Figure 7). Blue moki, as the species with the lowest thermal maximum (21.4°C, Supplementary Table S3), exhibited the most severe negative response, with an 80.92% biomass reduction after the extreme euphotic heatwave. In contrast, kingfish’s thermal maximum is much higher (26.2°C, Supplementary Table S3), and kingfish biomass reduced by only 4.21%. The greatest biomass decreases for other fisheries species were around 30–50%, and all occurred under extreme heatwave scenarios.
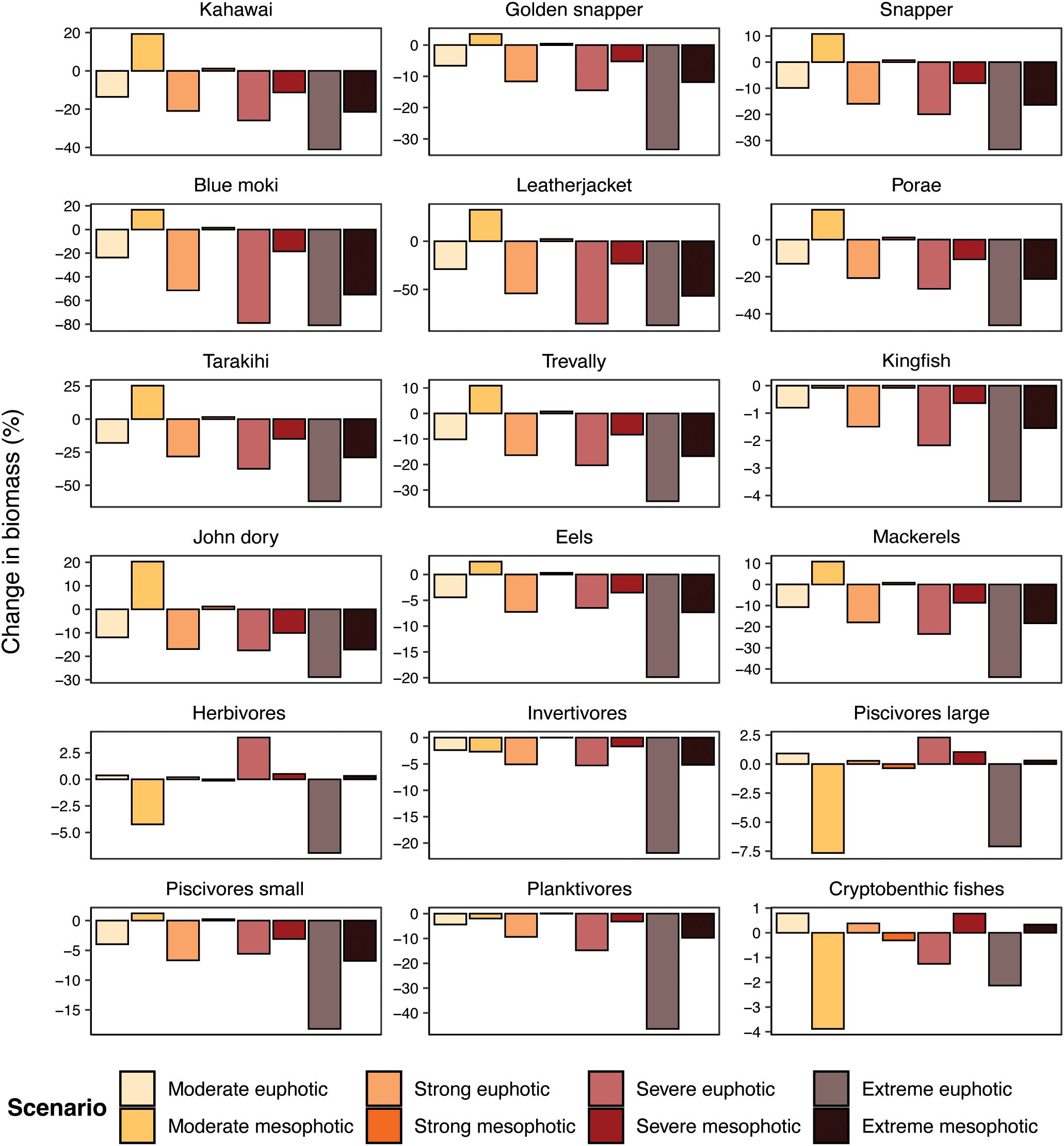
Figure 7. Percentage change in biomass in modelled species and guilds after year-long simulations of various heatwave scenarios. Paler colour bars are for when species remain on the euphotic reef, and darker colour bars are for when species escape to the mesophotic reef.
The importance of trophic cascades and species interactions in heatwave responses could be seen in the responses of herbivores, large piscivores and cryptobenthic fishes. While all species and guilds experienced a mitigation (and very occasionally, a reversal) in biomass reductions when exposed to mesophotic temperature regimes, these three groups differed in their responses to other heatwave categories; sometimes their biomasses increased, and sometimes they did not differ to biomasses under normal conditions. However, none of these responses were very large and so they did not have a strong impact on the total community response; the greatest response was –7.66%, for large piscivores under the moderate mesophotic heatwave.
Productivity responses of individual species to heatwaves were almost entirely negative (Figure 8), and the pattern of responses very similar to that of the total community (Figure 6). During the moderate heatwave, productivity of all species was very similar to the daily average euphotic baseline when on the euphotic reef, and productivity decreased when species moved to the mesophotic reef. All higher heatwave category levels had progressively larger negative impacts on population productivity for almost all species when they were forced to remain on the euphotic reef, and escaping to the mesophotic reef was more beneficial for productivity for all species. Productivity reductions were almost entirely negated or reversed for all species during the strong and severe heatwaves when they were allowed to escape to the mesophotic reef, and greatly mitigated even during the extreme heatwave.
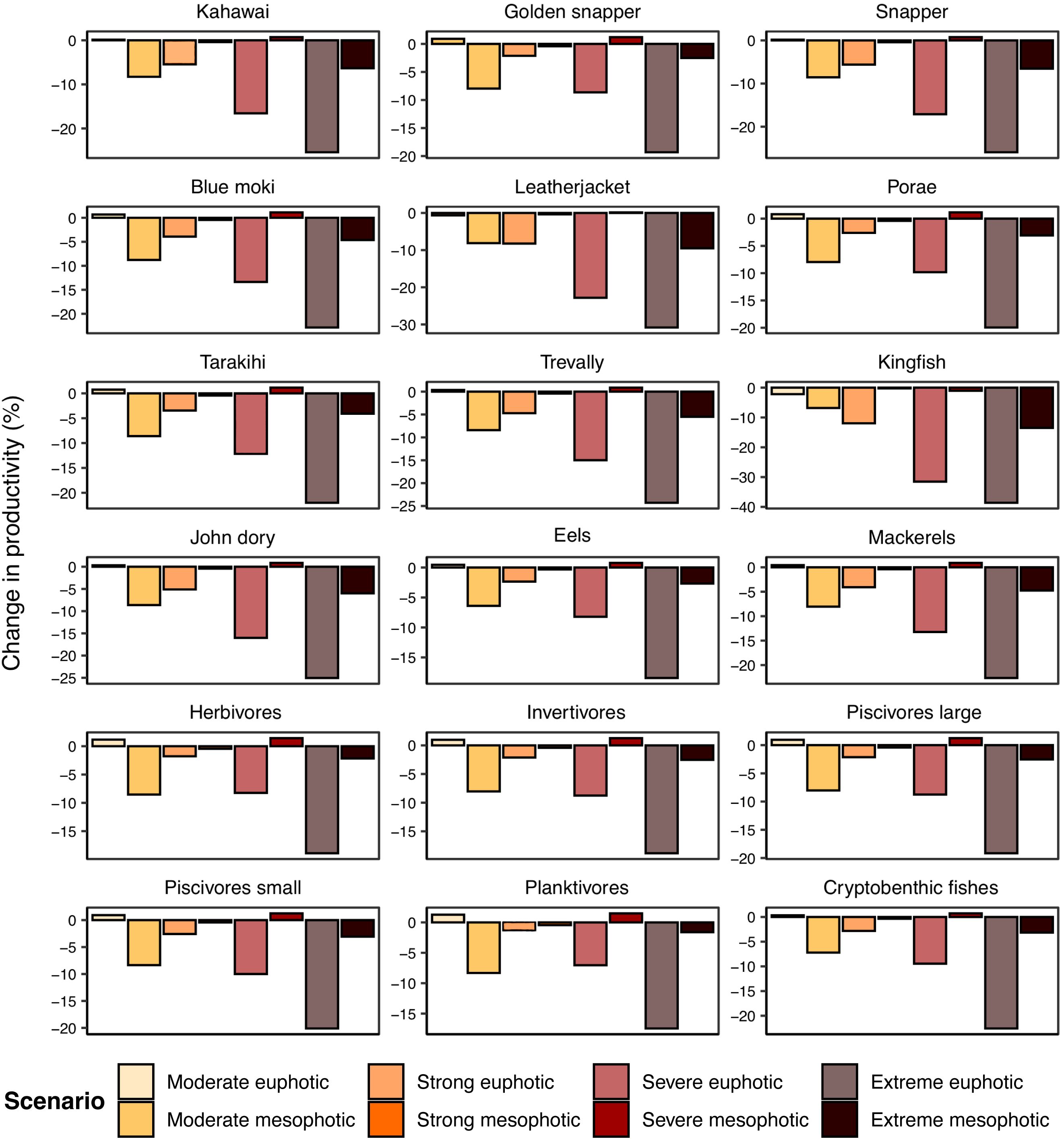
Figure 8. Percentage change in productivity in modelled species and guilds after year-long simulations of various heatwave scenarios. Paler colour bars are for when species remain on the euphotic reef, and darker colour bars are for when species escape to the mesophotic reef. and a Centre for Biodiversity and Restoration Ecology (CBRE) Student Award.
4 Discussion
Mesophotic reefs at the Poor Knights Islands have the potential to act as refuges from MHWs for mobile species. During summer, when temperatures are most likely to approach the upper bounds of coastal fish species’ thermal tolerance ranges (Shultz et al., 2016), mesophotic depths were cooler than euphotic depths; for example, 50 m was 3.22°C cooler than 10 m. Temperatures were more vertically mixed during winter, consistent with the typical seasonal pattern observed in shelf seas (Frade et al., 2018; Rippeth, 2005; Sallée et al., 2021; Simpson and Rippeth, 1993). Importantly, the duration, intensity and frequency of MHWs, which are considered the most dangerous present and future source of heat stress for marine species (Bell et al., 2023; Frölicher et al., 2018), were buffered from 30 m depth and below at the Poor Knights relative to MHWs at euphotic depths (Figure 4). In contrast, recent studies using depth-specific MHW thresholds have found that subsurface MHWs are only occasionally less intense at mesophotic depths (Zhang et al., 2023) and are often equally or more intense and have longer durations than at euphotic depths (Amaya et al., 2023; Elzahaby and Schaeffer, 2019; Fragkopoulou et al., 2023; Großelindemann et al., 2022; Hu et al., 2021; Scannell et al., 2020; Schaeffer et al., 2023; Schaeffer and Roughan, 2017; Zhang et al., 2023). Part of the explanation for these contrasting results is due to our definition of the MHW, which is determined by threshold temperatures at euphotic, shallow depths. However, differences in scale and the oceanographic processes at play compared to other studies may also play a role in this result. Mesophotic reefs at the Poor Knights potentially receive a regular (twice-daily) input of cool water at depth due to storms at sea and shoaling internal waves and/or bores occurring at tidal frequencies, which are a well-known feature of the northeast continental shelf of New Zealand (Sharples et al., 2001; Sharples and Zeldis, 2019) and the Poor Knights Islands specifically (Stevens et al., 2005). The Moana Hindcast, like similar regional ocean configurations using ROMS, is expected to resolve the cooling associated with internal waves (Suanda et al., 2017; Fagundes et al., 2020). This process is unlikely to be relevant to studies such as Elzahaby and Schaeffer (2019); Fragkopoulou et al. (2023); Großelindemann et al. (2022); Hu et al. (2021); Scannell et al. (2020), or Zhang et al. (2023), all of which focus on MHWs in the open, deep (>1000 m) ocean. Shoaling internal waves are, however, well-recognised as a process that buffers heat exposure at depths similar to those considered here on coral reefs (Storlazzi et al., 2020; Wyatt et al., 2020), and may be a key process explaining the buffering effect of MHWs at mesophotic depths at the Poor Knights Islands. Further investigation of this possibility would require dedicated observations and/or numerical modelling that are beyond the scope of the present research.
Some studies of the ecological impacts of MHWs by depth – as opposed to solely oceanographic studies – have found that sessile mesophotic species, having acclimatised to mesophotic temperature regimes, experience similar thermal stress to their euphotic counterparts (Bongaerts and Smith, 2019; Smith et al., 2016: Bell et al., 2024). However, often MHW impacts on sessile species at mesophotic depths are less than those at euphotic depths (Frade et al., 2018; Giraldo-Ospina et al., 2020; Haguenauer et al., 2021; Muir et al., 2017; Perkins et al., 2022; Bell et al., 2024). This, combined with our findings of positive mesophotic thermal refuge effects for mobile fishes, warrants the consideration of incorporating assessments of some measure of euphotic- or surface-relative temperature anomalies or absolute temperatures alongside depth-specific temperature anomalies in subsurface MHW analyses that intend to predict ecological impacts.
Under temperature-dependent multispecies size-spectrum model simulations of various heatwave scenarios, most species and guilds responded with progressively larger biomass reductions with each increase in heatwave category level, but the thermal refuge effect mitigated these reductions. For fisheries species, average biomass losses were reversed (28% difference) during the moderate heatwave, negated (24% difference) during the strong heatwave, and mitigated by 21% and 20% during the severe and extreme heatwaves. The responses of herbivores, large piscivores and cryptobenthic fishes varied, however, with mesophotic refuges having a positive, neutral, or negative impact on biomass depending on heatwave category level; when the effects were positive it was likely because the temperatures of a given heatwave resulted in fewer predators and/or competitors, while not yet exceeding their own thermal tolerance limits. Population productivity, a rate which is more relevant to fisheries than biomass alone, increased slightly or was similar during historic daily average euphotic temperatures for all species during the moderate euphotic heatwave, likely due to faster growth (Carozza et al., 2018; Pepin, 1991). Temperature increases have been shown to result in there being smaller, faster-growing fishes in a population (Baudron et al., 2014). However, productivity decreased during strong, severe, and extreme heatwaves. For fisheries species, moving to a mesophotic thermal refuge almost negated average productivity losses during the strong heatwave (5% difference), negated them during the severe heatwave (17% difference) and mitigated them by 19% during the extreme heatwave.
These findings emphasise the well-evidenced negative impacts of thermal stress on fish population biomass and productivity (Capitani et al., 2022; Carozza et al., 2018; Free et al., 2019; Wilson et al., 2021), as well as the importance of species interactions in determining responses to warming (Baum and Worm, 2009; Kirby and Beaugrand, 2009; Steneck, 2012) and thermal refuge availability. Reductions in biomass and fisheries yields with ocean warming have been observed using other temperature-dependent mizer models (Audzijonyte et al., 2022; Kuo et al., 2022; Lindmark et al., 2022; Woodworth-Jefcoats et al., 2019), and Kuo et al. (2022) highlighted evidence of warming-induced trophic cascades as changes in spatial overlap and decreases in fish biomass resulted in species having to feed more on background resources. However, the community-wide negative response to the extreme euphotic heatwave highlights that the impacts of approaching or exceeding thermal tolerance limits outweigh the benefits of release from competition and predation. Of particular interest is the fact that a mesophotic thermal refuge mitigates even the effects of this year-long extreme heatwave for all species, given that by 2100 under RCP8.5, marine ecosystems will be under constant heatwave conditions relative to current thresholds and the majority of events will be extreme (Oliver et al., 2019).
This study demonstrates the utility and potential of using multispecies size-spectrum models to explore the impacts of thermal refuges on fish community biomass and productivity under climate change scenarios, but there are several features that would increase the accuracy of this size-spectrum model’s representation of a coastal rocky reef ecosystem that could be incorporated in future research. Mizer models in general are currently particularly well-suited to modelling open-ocean pelagic ecosystems, but developments have been occurring for realistically representing coastal ecosystems. Rather than having herbivores feed on the plankton spectrum, algae could be modelled explicitly and adjustments could be made to therMizer’s mechanics to incorporate the feeding on benthic resources allowed for in the mizer extension package mizerMR (Audzijonyte et al., 2022). The response of herbivores in the simulations run using this study’s model should be treated with some caution. Though the effects of temperature and predation were represented in herbivores’ response, so too was the effect of competition for the plankton resource, which is not applicable to them in a real system. Temperature effects on the planktonic (Woodworth-Jefcoats et al., 2019) and benthic resource spectra (Audzijonyte et al., 2022) could also be included, which would allow for a more complete picture of heatwave impacts. How to realistically incorporate large transient predators such as sharks, which do not act within model boundaries and whose life history traits are very different to those of teleost fishes (and hence were excluded from the model presented here), is also a valuable future research avenue.
While the observed abundances to which this size-spectrum model was calibrated were from euphotic depths, the depth ranges of a number of species captured in the model do already include upper mesophotic depths (Allen, 1991; Heemstra and Randall, 1993; Fisheries New Zealand, 2023; McMillan et al., 2011; Mundy, 2005; Paulin, 1990; Randall, 1999; Stevenson, 2004). Future models could incorporate existing depth ranges, and then allow individuals in the upper portions of the depth ranges to deepen during MHWs while individuals already in the mesophotic would remain there. However, this would require fish survey data at mesophotic depths, which are beyond recreational diving limits and require either technical divers, remotely operated vehicles or autonomous underwater vehicles to survey (Armstrong et al., 2019; Munoz et al., 2017; Pyle, 2019; Turner et al., 2018). In mizer models of fished systems, biomasses at depth are usually taken from catch data (Blanchard et al., 2014; Jacobsen et al., 2017; Szuwalski et al., 2017; Woodworth-Jefcoats et al., 2019), and it would be interesting to explore the impact of mesophotic thermal refuge availability on fisheries yields specifically. In models encompassing large areas, it would also be pertinent to incorporate latitudinal range shifts as a simulation possibility (Kuo et al., 2022). Furthermore, site-attached species such as triplefins and eels (Abrams et al., 1983; Nagelkerken et al., 2016) would be less likely to move to a mesophotic reef during warming, even if one were available. Herbivores would likely only be able to utilise the upper limit of the mesophotic as a thermal refuge, as their food is light limited; macroalgal cover reduces from approximately 15% at 30 m to 2% at 40 m and below at the Poor Knights (Harris et al., 2021). Other factors that could limit the vertical migration of different fish species include the effects of density and prior residency, and potential local niche saturation (Pinheiro et al., 2023). With some species remaining and some escaping, species interactions would likely play an even larger role in thermal refuge availability responses than they did here. However, as interesting, complex and more accurate mizer models incorporating transient predators, algal and benthic resources, existing depth ranges, latitudinal range shifts, complex species interactions, and temperature impacts on planktonic, algal and benthic resources would be, such models are beyond the scope of this study. There is also the principle of parsimony and the issue of requiring sufficient data to accurately model all these processes and interactions. Simpler models do have the benefit of being able to clearly and succinctly explore one main research question, especially when data are available but not extensive.
This study provides clear evidence supporting the hypothesis that mesophotic reefs can act as an effective thermal refuge from MHWs for commercially and recreationally important coastal New Zealand fish species. Species that responded positively to heatwave events in this study were almost always non-fisheries species, while those that suffered the greatest losses were almost always species targeted by fisheries. If these fisheries species do in fact use mesophotic refuges during marine heatwaves, it would heighten their resilience, and support the sustainability of fisheries in the face of climate change. This has significant conservation and management implications, as mesophotic reefs are often only incidentally protected when they happen to occur in marine protected areas (Bell et al., 2022). Though often buffered from thermal stress, as shown here and by several other studies (Giraldo-Ospina et al., 2020; Haguenauer et al., 2021; Muir et al., 2017; Perkins et al., 2022), mesophotic reefs are not invulnerable to anthropogenic impacts (Bell et al., 2022; Frade et al., 2018; Rocha et al., 2018), and targeting mesophotic reefs specifically for protection could improve the future climate resilience of New Zealand’s coastal fisheries.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Victoria University of Wellington Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft. SD: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – review & editing. RS: Methodology, Resources, Writing – review & editing. LW: Formal Analysis, Methodology, Writing – review & editing. ML: Resources, Writing – review & editing. JB: Conceptualization, Resources, Writing – review & editing. AR: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The ocean model data was sourced from the Moana Project, www.moanaproject.org, funded by the New Zealand Ministry of Business Innovation and Employment, contract number METO1801. The model data is freely available from De Souza et al (2022), https://doi.org/10.5281/zenodo.5895265. Hiromi Beran’s MSc was supported by a Wellington Master’s by Thesis Scholarship, a Fisheries New Zealand/NIWA Masters Scholarship in Quantitative Fisheries Science, the Sarah Anne Rhodes Research Scholarship and a Centre for Biodiversity and Restoration Ecology (CBRE) Student Award. Data collection was supported by a Victoria University of Wellington Returning Carers Research Fund to Alice Rogers.
Acknowledgments
The ocean model data was sourced from the Moana Project, www.moanaproject.org, funded by the New Zealand Ministry of Business Innovation and Employment, contract number METO1801. The model data is freely available from De Souza et al (2022), https://doi.org/10.5281/zenodo.5895265. We acknowledge and thank funding from a Wellington Master’s by Thesis Scholarship, a Fisheries New Zealand/NIWA Masters Scholarship in Quantitative Fisheries Science, the Sarah Anne Rhodes Research Scholarship
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1540055/full#supplementary-material
References
Abrams R. W., Abrams M. D., and Schein M. W. (1983). Diurnal observations on the behavioral ecology of Gymnothorax moringa (Cuvier) and Muraena miliaris (Kaup) on a Caribbean coral reef. Coral Reefs 1, 185–192. doi: 10.1007/BF00571196
Allard H. (2020). The direct and indirect effects of marine reserve protection on reef fish assemblages (Auckland, New Zealand: The University of Auckland).
Allen G. R. (1991). Damselfishes of the world: Contains the descriptions of 16 new species of the family Pomacentridae. Mergus, Melle, Germany.
Amaya D. J., Jacox M. G., Alexander M. A., Scott J. D., Deser C., Capotondi A., et al. (2023). Bottom marine heatwaves along the continental shelves of North America. Nat. Commun. 14, 1038. doi: 10.1038/s41467-023-36567-0
Andersen K. H., Blanchard J. L., Fulton E. A., Gislason H., Jacobsen N. S., and van Kooten T. (2016). Assumptions behind size-based ecosystem models are realistic. ICES J. Marine Sci. 73, 1651–1655. doi: 10.1093/icesjms/fsv211
Armstrong R. A., Pizarro O., and Roman C. (2019). “Underwater robotic technology for imaging mesophotic coral ecosystems,” in Mesophotic Coral Ecosystems. Eds. Loya Y., Puglise K. A., and Bridge T. C. L. (Switzerland: Springer International Publishing), 973–988. doi: 10.1007/978-3-319-92735-0_51
Audzijonyte A., Delius G., Stuart-Smith R. D., Novaglio C., Edgar G. J., Barrett N. S., et al. (2022). Changes in benthic and pelagic production interact with warming to drive responses to climate change in a temperate coastal ecosystem (bioRxiv). p. 2022.06.20.496925. doi: 10.1101/2022.06.20.496925
Baudron A. R., Needle C. L., Rijnsdorp A. D., and Tara Marshall C. (2014). Warming temperatures and smaller body sizes: Synchronous changes in growth of North Sea fishes. Global Change Biol. 20, 1023–1031. doi: 10.1111/gcb.12514
Baum J. K. and Worm B. (2009). Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 78, 699–714. doi: 10.1111/j.1365-2656.2009.01531.x
Beentjes M. P. and Francis M. P. (1999). Movement of hapuku (Polyprion oxygeneios) determined from tagging studies. New Z. J. Marine Freshwater Res. 33, 1–12. doi: 10.1080/00288330.1999.9516852
Beese C., Delius G., Mumby P. J., and Rogers A. (2024). mizerReef: Mizer Models with Benthic Coupling for Coral Reefs. (Version R package version 1.0.1) (Wellington, New Zealand: Computer software). Available at: https://sizespectrum.org/mizerReef/ (Accessed January 2024).
Bell J. J., Micaroni V., Harris B., Strano F., Broadribb M., and Rogers A. (2022). Global status, impacts, and management of rocky temperate mesophotic ecosystems. Conserv. Biol. 38, e13945. doi: 10.1111/cobi.13945
Bell J. J., Micaroni V., Strano F., Ryan K. G., Mitchell K., Mitchell P., et al. (2024). Marine heatwave-driven mass mortality and microbial community reorganisation in an ecologically important temperate sponge. Global Change Biol. 30, e17417. doi: 10.1111/gcb.17417
Bell J. J., Smith R. O., Micaroni V., Strano F., Balemi C. A., Caiger P. E., et al. (2023). Marine heat waves drive bleaching and necrosis of temperate sponges. Curr. Biol. 33, 158–163.e2. doi: 10.1016/j.cub.2022.11.013
Benoit D. M., Chu C., Giacomini H. C., and Jackson D. A. (2022). Size spectrum model reveals importance of considering species interactions in a freshwater fisheries management context. Ecosphere 13, e4163. doi: 10.1002/ecs2.4163
Bess R. (2016). What’s the catch? The state of recreational fisheries management in New Zealand (New Zealand) (The New Zealand Initiative). Available at: https://apo.org.au/node/68174 (Accessed January 2024).
Blanchard J. L., Andersen K. H., Scott F., Hintzen N. T., Piet G., and Jennings S. (2014). Evaluating targets and trade-offs among fisheries and conservation objectives using a multispecies size spectrum model. J. Appl. Ecol. 51, 612–622. doi: 10.1111/1365-2664.12238
Blanchard J. L., Jennings S., Law R., Castle M. D., McCloghrie P., Rochet M.-J., et al. (2009). How does abundance scale with body size in coupled size-structured food webs? J. Anim. Ecol. 78, 270–280. doi: 10.1111/j.1365-2656.2008.01466.x
Bongaerts P., Ridgway T., Sampayo E. M., and Hoegh-Guldberg O. (2010). Assessing the ‘deep reef refugia’ hypothesis: Focus on Caribbean reefs. Coral Reefs 29, 309–327. doi: 10.1007/s00338-009-0581-x
Bongaerts P., Riginos C., Brunner R., Englebert N., Smith S. R., and Hoegh-Guldberg O. (2017). Deep reefs are not universal refuges: Reseeding potential varies among coral species. Sci. Adv. 3, e1602373. doi: 10.1126/sciadv.1602373
Bongaerts P. and Smith T. B. (2019). “Beyond the “Deep reef refuge” Hypothesis: A conceptual framework to characterize persistence at depth,” in Mesophotic Coral Ecosystems. Eds. Loya Y., Puglise K. A., and Bridge T. C. L. (Switzerland: Springer International Publishing), 881–895. doi: 10.1007/978-3-319-92735-0_45
Brook F. J. (2002). Biogeography of near-shore reef fishes in northern New Zealand. J. R. Soc. New Z. 32, 243–274. doi: 10.1080/03014223.2002.9517694
Canales T. M., Delius G. W., and Law R. (2020). Regulation of fish stocks without stock–recruitment relationships: The case of small pelagic fish. Fish Fisheries 21, 857–871. doi: 10.1111/faf.12465
Capitani L., de Araujo J. N., Vieira E. A., Angelini R., and Longo G. O. (2022). Ocean warming will reduce standing biomass in a tropical western atlantic reef ecosystem. Ecosystems 25, 843–857. doi: 10.1007/s10021-021-00691-z
Carozza D. A., Bianchi D., and Galbraith E. D. (2018). Metabolic impacts of climate change on marine ecosystems: Implications for fish communities and fisheries. Global Ecol. Biogeography 28, 158–169. doi: 10.1111/geb.12832
Cavanaugh K. C., Reed D. C., Bell T. W., Castorani M. C. N., and Beas-Luna R. (2019). Spatial variability in the resistance and resilience of giant kelp in southern and Baja California to a multiyear heatwave. Front. Marine Sci. 6. doi: 10.3389/fmars.2019.00413
Cavole L. M., Demko A. M., Diner R. E., Giddings A., Koester I., Pagniello C. M. L. S., et al. (2016). Biological impacts of the 2013–2015 warm-water anomaly in the northeast pacific: winners, losers, and the future. Oceanography 29, 273–285. doi: 10.5670/oceanog.2016.32
Cerrano C., Bastari A., Calcinai B., Di Camillo C., Pica D., Puce S., et al. (2019). Temperate mesophotic ecosystems: Gaps and perspectives of an emerging conservation challenge for the Mediterranean Sea. Eur. Zoological J. 86, 370–388. doi: 10.1080/24750263.2019.1677790
Chaikin S., Dubiner S., and Belmaker J. (2021). Cold-water species deepen to escape warm water temperatures. Global Ecol. Biogeography 31, 75–88. doi: 10.1111/geb.13414
Cheung W. W. L. and Frölicher T. L. (2020). Marine heatwaves exacerbate climate change impacts for fisheries in the northeast Pacific. Sci. Rep. 10, 6678. doi: 10.1038/s41598-020-63650-z
Christensen V. and Walters C. J. (2004). Ecopath with Ecosim: Methods, capabilities and limitations. Ecol. Modelling 172, 109–139. doi: 10.1016/j.ecolmodel.2003.09.003
Clements C. F., McCarthy M. A., and Blanchard J. L. (2019). Early warning signals of recovery in complex systems. Nat. Commun. 10, 1681. doi: 10.1038/s41467-019-09684-y
Cummings V. J., Lundquist C. J., Dunn M. R., Francis M., Horn P. L., Law C., et al. (2021). Assessment of potential effects of climate-related changes in coastal and offshore waters on New Zealand’s seafood sector. (Wellington, New Zealand: Ministry for Primary Industries).
Currey L. M., Heupel M. R., Simpfendorfer C. A., and Williams A. J. (2015). Assessing environmental correlates of fish movement on a coral reef. Coral Reefs 34, 1267–1277. doi: 10.1007/s00338-015-1318-7
Datta S. and Blanchard J. L. (2016). The effects of seasonal processes on size spectrum dynamics. Can. J. Fisheries Aquat. Sci. 73, 598–610. doi: 10.1139/cjfas-2015-0468
Dayan H., McAdam R., Juza M., Masina S., and Speich S. (2023). Marine heat waves in the Mediterranean Sea: An assessment from the surface to the subsurface to meet national needs. Front. Marine Sci. 10. doi: 10.3389/fmars.2023.1045138
Delius G. (2022). mizer course—Online mizer course. Mizer: Multi-Species Size Spectrum Modelling in R. Available online at: https://mizer.course.aug22.sizespectrum.org/.
Delius G., Scott F., Blanchard J., and Andersen K. H. (2023). mizer: Dynamic Multi-Species Size Spectrum Modelling (Version R package version 2.5.0) (Computer software). Available at: https://github.com/sizespectrum/mizerhttps://sizespectrum.org/mizer/ (Accessed June 2022).
De Souza J. M., Suanda S. H., Couto P. P., Smith R. O., Kerry C., and Roughan M. (2022). Moana Ocean Hindcast – A 25+ year simulation for New Zealand waters using the Regional Ocean Modeling System (ROMS) v3.9 model. EGUsphere 16 (1), 1–34. doi: 10.5194/egusphere-2022-41
Dulvy N. K., Rogers S. I., Jennings S., Stelzenmüller V., Dye S. R., and Skjoldal H. R. (2008). Climate change and deepening of the North Sea fish assemblage: A biotic indicator of warming seas. J. Appl. Ecol. 45, 1029–1039. doi: 10.1111/j.1365-2664.2008.01488.x
Elzahaby Y. and Schaeffer A. (2019). Observational insight into the subsurface anomalies of marine heatwaves. Front. Marine Sci. 6. doi: 10.3389/fmars.2019.00745
Fagundes M., Litvin S. Y., Micheli F., De Leo G., Boch C. A., Barry J. P., et al. (2020). Downscaling global ocean climate models improves estimates of exposure regimes in coastal environments. Sci. Rep. 10, 14227. doi: 10.1038/s41598-020-71169-6
Falciani J. E., Grigoratou M., and Pershing A. J. (2022). Optimizing fisheries for blue carbon management: Why size matters. Limnology Oceanography 67, S171–S179. doi: 10.1002/lno.12249
Fisheries New Zealand (2023). Fisheries Assessment Plenary, May 2023: Stock assessments and stock status. (Wellington, New Zealand: Fisheries Science Team, Fisheries New Zealand).
Forestier R., Blanchard J. L., Nash K. L., Fulton E. A., Johnson C., and Audzijonyte A. (2020). Interacting forces of predation and fishing affect species’ maturation size. Ecol. Evol. 10, 14033–14051. doi: 10.1002/ece3.6995
Frade P. R., Bongaerts P., Englebert N., Rogers A., Gonzalez-Rivero M., and Hoegh-Guldberg O. (2018). Deep reefs of the Great Barrier Reef offer limited thermal refuge during mass coral bleaching. Nat. Commun. 9, 3447. doi: 10.1038/s41467-018-05741-0
Fragkopoulou E., Sen Gupta A., Costello M. J., Wernberg T., Araújo M. B., Serrão E. A., et al. (2023). Marine biodiversity exposed to prolonged and intense subsurface heatwaves. Nat. Climate Change 13, 1114–1121. doi: 10.1038/s41558-023-01790-6
Free C. M., Thorson J. T., Pinsky M. L., Oken K. L., Wiedenmann J., and Jensen O. P. (2019). Impacts of historical warming on marine fisheries production. Science 363, 979–983. doi: 10.1126/science.aau1758
Frölicher T. L., Fischer E. M., and Gruber N. (2018). Marine heatwaves under global warming. Nature 560, 360–364. doi: 10.1038/s41586-018-0383-9
Fulton E. A., Link J. S., Kaplan I. C., Savina-Rolland M., Johnson P., Ainsworth C., et al. (2011). Lessons in modelling and management of marine ecosystems: The Atlantis experience. Fish Fisheries 12, 171–188. doi: 10.1111/j.1467-2979.2011.00412.x
Fulton E. A., Parslow J. S., Smith A. D. M., and Johnson C. R. (2004). Biogeochemical marine ecosystem models II: The effect of physiological detail on model performance. Ecol. Modelling 173, 371–406. doi: 10.1016/j.ecolmodel.2003.09.024
Fulton E. A., Smith A. D. M., and Punt A. E. (2005). Which ecological indicators can robustly detect effects of fishing? ICES J. Marine Sci. 62, 540–551. doi: 10.1016/j.icesjms.2004.12.012
Gammelsrød T., Bartholomae C. H., Boyer D. C., Filipe V. L. L., and O’Toole M. J. (1998). Intrusion of warm surface water along the Angolan-Namibian coast in February–March 1995: The 1995 Benguela Nino. South Afr. J. Marine Sci. 19, 41–56. doi: 10.2989/025776198784126719
García Molinos J., Halpern B. S., Schoeman D. S., Brown C. J., Kiessling W., Moore P. J., et al. (2016). Climate velocity and the future global redistribution of marine biodiversity. Nat. Climate Change 6, 83–88. doi: 10.1038/nclimate2769
Giraldo-Ospina A., Kendrick G. A., and Hovey R. K. (2020). Depth moderates loss of marine foundation species after an extreme marine heatwave: Could deep temperate reefs act as a refuge? Proc. R. Soc. B: Biol. Sci. 287, 20200709. doi: 10.1098/rspb.2020.0709
Givan O., Edelist D., Sonin O., and Belmaker J. (2018). Thermal affinity as the dominant factor changing Mediterranean fish abundances. Global Change Biol. 24, e80–e89. doi: 10.1111/gcb.13835
Großelindemann H., Ryan S., Ummenhofer C. C., Martin T., and Biastoch A. (2022). Marine heatwaves and their depth structures on the Northeast U.S. Continental shelf. Front. Climate 4. doi: 10.3389/fclim.2022.857937
Haguenauer A., Zuberer F., Siu G., Cortese D., Beldade R., and Mills S. C. (2021). Deep heat: A comparison of water temperature, anemone bleaching, anemonefish density and reproduction between shallow and mesophotic reefs. Fishes 6 (3), 37. doi: 10.3390/fishes6030037
Harris B. (2022). The distribution and feeding ecology of temperate marine sponges through shallow and mesophotic habitats. Open Access Te Herenga Waka-Victoria University of Wellington, Wellington, New Zealand. doi: 10.26686/wgtn.19669398
Harris B., Davy S. K., and Bell J. J. (2021). Benthic community composition of temperate mesophotic ecosystems (TMEs) in New Zealand: Sponge domination and contribution to habitat complexity. Marine Ecol. Prog. Ser. 671, 21–43. doi: 10.3354/meps13758
Heemstra P. C. and Randall J. E. (1994). FAO Species Catalogue. Vol. 16. Groupers of the world (Family serranidae, subfamily epinephelinae). An annotated and illustrated catalogue of the grouper, rockcod, hind, coral grouper, and lyretail species known to date. FAO Fisheries Synopsis (Rome, FAO). 382 p.
Hobday A. J., Alexander L. V., Perkins S. E., Smale D. A., Straub S. C., Oliver E. C. J., et al. (2016). A hierarchical approach to defining marine heatwaves. Prog. Oceanography 141, 227–238. doi: 10.1016/j.pocean.2015.12.014
Hobday A. J., Oliver E., Sen Gupta A., Benthuysen J., Burrows M., Donat M., et al. (2018). Categorizing and naming marine heatwaves. Oceanography 31 (2), 162–173. doi: 10.5670/oceanog.2018.205
Howell E. A., Wabnitz C. C. C., Dunne J. P., and Polovina J. J. (2013). Climate-induced primary productivity change and fishing impacts on the Central North Pacific ecosystem and Hawaii-based pelagic longline fishery. Climatic Change 119, 79–93. doi: 10.1007/s10584-012-0597-z
Hu S., Li S., Zhang Y., Guan C., Du Y., Feng M., et al. (2021). Observed strong subsurface marine heatwaves in the tropical western Pacific Ocean. Environ. Res. Lett. 16, 104024. doi: 10.1088/1748-9326/ac26f2
IPCC (2021). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Masson-Delmotte V., Zhai P., Pirani A., Connors S.L., Péan C., Berger S., et al. (eds.)] Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press. doi: 10.1017/9781009157896
Jacobsen N. S., Burgess M. G., and Andersen K. H. (2017). Efficiency of fisheries is increasing at the ecosystem level. Fish Fisheries 18, 199–211. doi: 10.1111/faf.12171
Jennings S., Pinnegar J. K., Polunin N. V. C., and Boon T. W. (2001). Weak cross-species relationships between body size and trophic level belie powerful size-based trophic structuring in fish communities. J. Anim. Ecol. 70, 934–944. doi: 10.1046/j.0021-8790.2001.00552.x
Kerry C., Roughan M., and Azevedo Correia De Souza J. (2022). Drivers of upper ocean heat content extremes (marine heatwaves) around New Zealand revealed by Adjoint Sensitivity Analysis. Front. Climate 28. doi: 10.3389/fclim.2022.980990
Kerry C., Roughan M., and De Souza J. (2023). Characterizing the variability of boundary currents and ocean heat content around New Zealand using a multi-decadal high-resolution regional ocean model. J. Geophysical Research: Oceans 128, e2022JC018624. doi: 10.1029/2022JC018624
Kirby R. R. and Beaugrand G. (2009). Trophic amplification of climate warming. Proc. R. Soc. B: Biol. Sci. 276, 4095–4103. doi: 10.1098/rspb.2009.1320
Kuo C.-Y., Ko C.-Y., and Lai Y.-Z. (2022). Assessing warming impacts on marine fishes by integrating physiology-guided distribution projections, life-history changes and food web dynamics. Methods Ecol. Evol. 13, 1343–1357. doi: 10.1111/2041-210X.13846
Last P. R., White W. T., Gledhill D. C., Hobday A. J., Brown R., Edgar G. J., et al. (2011). Long-term shifts in abundance and distribution of a temperate fish fauna: A response to climate change and fishing practices. Global Ecol. Biogeography 20, 58–72. doi: 10.1111/j.1466-8238.2010.00575.x
Lavin C. P., Pauly D., Dimarchopoulou D., Liang C., and Costello M. J. (2023). Fishery catch is affected by geographic expansion, fishing down food webs and climate change in Aotearoa, New Zealand. PeerJ 11, e16070. doi: 10.7717/peerj.16070
Leichter J., Helmuth B., and Fischer A. (2006). Variation beneath the surface: Quantifying complex thermal environments on coral reefs in the Caribbean, Bahamas and Florida. J. Marine Res. 64 (4). doi: 10.1357/002224006778715711
Lindmark M., Audzijonyte A., Blanchard J. L., and Gårdmark A. (2022). Temperature impacts on fish physiology and resource abundance lead to faster growth but smaller fish sizes and yields under warming. Global Change Biol. 28, 6239–6253. doi: 10.1111/gcb.16341
Marin M. (2021). A global, multiproduct analysis of coastal marine heatwaves: distribution, characteristics, and long-term trends. J. Geophysical Research: Oceans 126. doi: 10.1029/2020JC016708
McCarthy A., Garden C., Flack B., Bragg C., Meadows S., Hepburn C., et al. (2013). Who is catching what? A survey of recreational fishing effort and success on the East Otago Taiāpure. Available online at: https://ourarchive.otago.ac.nz/handle/10523/5368 (Accessed January 2024).
McCarthy A., Hepburn C., Scott N., Schweikert K., Turner R., and Moller H. (2014). Local people see and care most? Severe depletion of inshore fisheries and its consequences for Māori communities in New Zealand. Aquat. Conservation: Marine Freshwater Ecosystems 24, 369–390. doi: 10.1002/aqc.2378
McGregor V. L., Horn P., Dutilloy A., Datta S., Rogers A., Porobic J., et al. (2021). From data compilation to model validation: Comparing three ecosystem models of the Tasman and Golden Bays, New Zealand. PeerJ 9, e11712. doi: 10.7717/peerj.11712
McMillan P. J., Francis M., James G. D., Paul L., Marriott P. J., Mackay E., et al. (2011). New Zealand Fishes Volume 1: A field guide to common species caught by bottom and midwater fishing (Wellington, New Zealand: New Zealand Aquatic Environment and Biodiversity Report No. 68), 329.
Middleton I. (2022). Range shifts and the population dynamics of tropical, subtropical, and rare fishes in New Zealand: A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Ecology at Massey University, Auckland, New Zealand. Auckland, New Zealand: Massey University. Available at: http://hdl.handle.net/10179/17491 (Accessed January 2024).
Mills K. E., Pershing A. J., Brown C. J., Chen Y., Chiang F.-S., Holland D. S., et al. (2013). Fisheries management in a changing climate: lessons from the 2012 ocean heat wave in the Northwest Atlantic. Oceanography 26, 191–195. doi: 10.5670/oceanog.2013.27
Morrongiello J. R., Horn P. L., Ó Maolagáin C., and Sutton P. J. H. (2021). Synergistic effects of harvest and climate drive synchronous somatic growth within key New Zealand fisheries. Global Change Biol. 27, 1470–1484. doi: 10.1111/gcb.15490
MPI (2023). Situation and Outlook for Primary Industries. (Wellington, New Zealand: Ministry for Primary Industries). Available at: https://www.mpi.govt.nz/dmsdocument/60526-Situation-and-Outlook-for-Primary-Industries-SOPI-December-2023 (Accessed January 2024).
Muir P. R., Marshall P. A., Abdulla A., and Aguirre J. D. (2017). Species identity and depth predict bleaching severity in reef-building corals: Shall the deep inherit the reef? Proc. R. Soc. B: Biol. Sci. 284, 20171551. doi: 10.1098/rspb.2017.1551
Mundy B. C. (2005). Checklist of the fishes of the Hawaiian Archipelago. Bishop Museum Bulletins Zoology 6, 1–704.
Munoz R. C., Buckel C. A., Whitfield P. E., Viehman S., Clark R., Taylor J. C., et al. (2017). Conventional and technical diving surveys reveal elevated biomass and differing fish community composition from shallow and upper mesophotic zones of a remote United States coral reef. PLoS One 12, e0188598–e0188598. doi: 10.1371/journal.pone.0188598
Nagelkerken I., Russell B. D., Gillanders B. M., and Connell S. D. (2016). Ocean acidification alters fish populations indirectly through habitat modification. Nat. Climate Change 6, 89–93. doi: 10.1038/nclimate2757
Ñiquen M. and Bouchon M. (2004). Impact of El Niño events on pelagic fisheries in Peruvian waters. Deep Sea Res. Part II 51, 563–574. doi: 10.1016/j.dsr2.2004.03.001
Nye J., Link J., Hare J., and Overholtz W. (2009). Changing spatial distribution of fish stocks in relation to climate and population size on the Northeast United Sates continental shelf. Marine Ecol. Prog. Ser. 393, 111–129. doi: 10.3354/meps08220
Oliver E. C. J., Benthuysen J. A., Darmaraki S., Donat M. G., Hobday A. J., Holbrook N. J., et al. (2021). Marine heatwaves. Annu. Rev. Marine Sci. 13, 313–342. doi: 10.1146/annurev-marine-032720-095144
Oliver E. C. J., Burrows M. T., Donat M. G., Sen Gupta A., Alexander L. V., Perkins-Kirkpatrick S. E., et al. (2019). Projected marine heatwaves in the 21st century and the potential for ecological impact. Front. Marine Sci. 6. doi: 10.3389/fmars.2019.00734
Oliver E. C. J., Donat M. G., Burrows M. T., Moore P. J., Smale D. A., Alexander L. V., et al. (2018). Longer and more frequent marine heatwaves over the past century. Nat. Commun. 9. doi: 10.1038/s41467-018-03732-9
Parmesan C. and Yohe G. (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. doi: 10.1038/nature01286
Parsons D., Sim-Smith C., Cryer M., Francis M., Hartill B., Jones E., et al. (2014). Snapper (Chrysophrys auratus): A review of life history and key vulnerabilities in New Zealand. New Z. J. Marine Freshwater Res. 48, 256–283. doi: 10.1080/00288330.2014.892013
Paulin C. D. (1990). Pagrus auratus, a new combination for the species known as “snapper” in Australasian waters (Pisces: Sparidae). New Z. J. Marine Freshwater Res. 24, 259–265. doi: 10.1080/00288330.1990.9516422
Pepin P. (1991). Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fish. Can. J. Fisheries Aquat. Sci. 48, 503–518. doi: 10.1139/f91-065
Perelman J. N., Tanaka K. R., Smith J. N., Barkley H. C., and Powell B. S. (2024). Subsurface temperature estimates from a Regional Ocean Modelling System (ROMS) reanalysis provide accurate coral heat stress indices across the Main Hawaiian Islands. Sci. Rep. 14, 6620. doi: 10.1038/s41598-024-56865-x
Perkins N. R., Monk J., Soler G., Gallagher P., and Barrett N. S. (2022). Bleaching in sponges on temperate mesophotic reefs observed following marine heatwave events. Climate Change Ecol. 3, 100046. doi: 10.1016/j.ecochg.2021.100046
Perry A. L., Low P. J., Ellis J. R., and Reynolds J. D. (2005). Climate change and distribution shifts in marine fishes. Science 308, 1912–1915. doi: 10.1126/science.1111322
Pinheiro H. T., MacDonald C., Quimbayo J. P., Shepherd B., Phelps T. A., Loss A. C., et al. (2023). Assembly rules of coral reef fish communities along the depth gradient. Curr. Biol. 33, 1421–1430.e4. doi: 10.1016/j.cub.2023.02.040
Pinsky M. L., Worm B., Fogarty M. J., Sarmiento J. L., and Levin S. A. (2013). Marine taxa track local climate velocities. Science 341, 1239–1242. doi: 10.1126/science.1239352
Poloczanska E. S., Brown C. J., Sydeman W. J., Kiessling W., Schoeman D. S., Moore P. J., et al. (2013). Global imprint of climate change on marine life. Nat. Climate Change 3, 919–925. doi: 10.1038/nclimate1958
Posit Team (2024). RStudio: Integrated Development Environment for R. (Boston, MA: Posit Software, PBC.). Available at: http://www.posit.co/ (Accessed October 2023).
Pyle R. L. (2019). “Advanced technical diving,” in Mesophotic Coral Ecosystems. Eds. Loya Y., Puglise K. A., and Bridge T. C. L. (Switzerland: Springer International Publishing), 959–972. doi: 10.1007/978-3-319-92735-0_50
Randall J. E. (1999). Revision of the Indo-Pacific labrid fishes of the genus Coris, with descriptions of five new species. Bernice Pauahi Bishop Museum 29.
R Core Team (2024). R: A Language and Environment for Statistical Computing. (Version 4.3.2 (2023-10–31 ucrt)) (R Foundation for Statistical Computing). Available at: https://www.R-project.org/ (Accessed February 2024).
Riegl B. and Piller W. E. (2003). Possible refugia for reefs in times of environmental stress. Int. J. Earth Sci. 92, 520–531. doi: 10.1007/s00531-003-0328-9
Rippeth T. P. (2005). Mixing in seasonally stratified shelf seas: A shifting paradigm. Philos. Trans. R. Soc. A 363, 2837–2854. doi: 10.1098/rsta.2005.1662
Robinson J. P. W., Nash K. L., Blanchard J. L., Jacobsen N. S., Maire E., Graham N. A. J., et al. (2022). Managing fisheries for maximum nutrient yield. Fish Fisheries 23, 800–811. doi: 10.1111/faf.12649
Rocha L. A., Pinheiro H. T., Shepherd B., Papastamatiou Y. P., Luiz O. J., Pyle R. L., et al. (2018). Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361, 281–284. doi: 10.1126/science.aaq1614
Rutterford L. A., Simpson S. D., Jennings S., Johnson M. P., Blanchard J. L., Schön P.-J., et al. (2015). Future fish distributions constrained by depth in warming seas. Nat. Climate Change 5, 569–573. doi: 10.1038/nclimate2607
Salinger M. J., Renwick J., Behrens E., Mullan A. B., Diamond H. J., Sirguey P., et al. (2019). The unprecedented coupled ocean-atmosphere summer heatwave in the New Zealand region 2017/18: Drivers, mechanisms and impacts. Environ. Res. Lett. 14, 044023. doi: 10.1088/1748-9326/ab012a
Sallée J.-B., Pellichero V., Akhoudas C., Pauthenet E., Vignes L., Schmidtko S., et al. (2021). Summertime increases in upper-ocean stratification and mixed-layer depth. Nature 591, 592–598. doi: 10.1038/s41586-021-03303-x
Scannell H. A., Johnson G. C., Thompson L., Lyman J. M., and Riser S. C. (2020). Subsurface evolution and persistence of marine heatwaves in the northeast pacific. Geophysical Res. Lett. 47, e2020GL090548. doi: 10.1029/2020GL090548
Schaeffer A. and Roughan M. (2017). Subsurface intensification of marine heatwaves off southeastern Australia: The role of stratification and local winds. Geophysical Res. Lett. 44, 5025–5033. doi: 10.1002/2017GL073714
Schaeffer A., Sen Gupta A., and Roughan M. (2023). Seasonal stratification and complex local dynamics control the sub-surface structure of marine heatwaves in Eastern Australian coastal waters. Commun. Earth Environ. 4, 304. doi: 10.1038/s43247-023-00966-4
Schlegel R. W., Oliver E. C. J., Hobday A. J., and Smit A. J. (2019). Detecting marine heatwaves with sub-optimal data. Front. Marine Sci. 6. doi: 10.3389/fmars.2019.00737
Scott F., Blanchard J. L., and Andersen K. H. (2014). mizer: An R package for multispecies, trait-based and community size spectrum ecological modelling. Methods Ecol. Evol. 5, 1121–1125. doi: 10.1111/2041-210X.12256
Sharples J., Moore C. M., and Abraham E. R. (2001). Internal tide dissipation, mixing, and vertical nitrate flux at the shelf edge of NE New Zealand. J. Geophysical Res. 106, 14069–14081. doi: 10.1029/2000JC000604
Sharples J. and Zeldis J. R. (2019). Variability of internal tide energy, mixing and nitrate fluxes in response to changes in stratification on the northeast New Zealand continental shelf. New Z. J. Marine Freshwater Res. 55, 94–111. doi: 10.1080/00288330.2019.1705357
Sheldon R. W., Prakash A., and Sutcliffe W. H. Jr (1972). The size distribution of particles in the ocean1. Limnology Oceanography 17, 327–340. doi: 10.4319/lo.1972.17.3.0327
Shultz A. D., Zuckerman Z. C., and Suski C. D. (2016). Thermal tolerance of nearshore fishes across seasons: Implications for coastal fish communities in a changing climate. Marine Biol. 163, 83. doi: 10.1007/s00227-016-2858-2
Simpson J. H. and Rippeth T. P. (1993). The clyde sea: A model of the seasonal cycle of stratification and mixing. Estuarine Coastal Shelf Sci. 37, 129–144. doi: 10.1006/ecss.1993.1047
Sim-Smith C. and Kelly M. (2009). A literature review on the Poor Knights Islands Marine Reserve. Whangarei, New Zealand: Dept. of Conservation, Northland Conservancy.
Smith K. E., Burrows M. T., Hobday A. J., Sen Gupta A., Moore P. J., Thomsen M., et al. (2021). Socioeconomic impacts of marine heatwaves: Global issues and opportunities. Science 374, eabj3593. doi: 10.1126/science.abj3593
Smith T. B., Gyory J., Brandt M. E., Miller W. J., Jossart J., and Nemeth R. S. (2016). Caribbean mesophotic coral ecosystems are unlikely climate change refugia. Global Change Biol. 22, 2756–2765. doi: 10.1111/gcb.13175
Sommer U., Charalampous E., Scotti M., and Moustaka-Gouni M. (2018). Big fish eat small fish: Implications for food chain length? Community Ecol. 19, 107–115. doi: 10.1556/168.2018.19.2.2
Souza J. M. A. C., Felsing M., Jakoboski J., Gardner J. P. A., and Hudson M. (2023). Moana Project: lessons learned from a national scale transdisciplinary research project. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1322194
Spence M. A., Thorpe R. B., Blackwell P. G., Scott F., Southwell R., and Blanchard J. L. (2021). Quantifying uncertainty and dynamical changes in multi-species fishing mortality rates, catches and biomass by combining state-space and size-based multi-species models. Fish Fisheries 22, 667–681. doi: 10.1111/faf.12543
Steneck R. S. (2012). Apex predators and trophic cascades in large marine ecosystems: Learning from serendipity. Proc. Natl. Acad. Sci. 109, 7953–7954. doi: 10.1073/pnas.1205591109
Stevens C. L., Abraham E. R., Moore C. M., Boyd P. W., and Sharples J. (2005). Observations of small-scale processes associated with the internal tide encountering an island. J. Phys. Oceanography 35, 1553–1567. doi: 10.1175/JPO2754.1
Stevenson M. L. (2004). Trawl survey of the west coast of the South Island and Tasman and Golden Bays, March-April 2003 (KAH0304). (Wellington, New Zealand: New Zealand Fisheries Assessment Report).
Storlazzi C. D., Cheriton O. M., van Hooidonk R., Zhongxiang Z., and Brainard R. (2020). Internal tides can provide thermal refugia that will buffer some coral reefs from future global warming. Sci. Rep. 10, 13435. doi: 10.1038/s41598-020-70372-9
Suanda S. H., Feddersen F., and Kumar N. (2017). The effect of barotropic and baroclinic tides on coastal stratification and mixing. J. Geophysical Res. 122, 10,156–10,173. doi: 10.1002/2017JC013379
Sun D., Li F., Jing Z., Hu S., and Zhang B. (2023). Frequent marine heatwaves hidden below the surface of the global ocean. Nat. Geosci. 16. doi: 10.1038/s41561-023-01325-w
Szuwalski C. S., Burgess M. G., Costello C., and Gaines S. D. (2017). High fishery catches through trophic cascades in China. Proc. Natl. Acad. Sci. 114, 717–721. doi: 10.1073/pnas.1612722114
Thoral F., Montie S., Thomsen M. S., Tait L. W., Pinkerton M. H., and Schiel D. R. (2022). Unravelling seasonal trends in coastal marine heatwave metrics across global biogeographical realms. Sci. Rep. 12, 7740. doi: 10.1038/s41598-022-11908-z
Turner J. A., Babcock R. C., Hovey R., and Kendrick G. A. (2018). AUV-based classification of benthic communities of the Ningaloo shelf and mesophotic areas. Coral Reefs 37, 763–778. doi: 10.1007/s00338-018-1700-3
Wilson R. J., Sailley S. F., Jacobs Z. L., Kamau J., Mgeleka S., Okemwa G. M., et al. (2021). Large projected reductions in marine fish biomass for Kenya and Tanzania in the absence of climate mitigation. Ocean Coastal Manage. 215, 105921. doi: 10.1016/j.ocecoaman.2021.105921
Woodworth-Jefcoats P. A., Blanchard J. L., and Drazen J. C. (2019). Relative impacts of simultaneous stressors on a pelagic marine ecosystem. Front. Marine Sci. 6. doi: 10.3389/fmars.2019.00383
Wyatt A. S. J., Leichter J. J., Toth L. T., Miyajima T., Aronson R. B., and Nagata T. (2020). Heat accumulation on coral reefs mitigated by internal waves. Nat. Geoscience. 13, 28–34. doi: 10.1038/s41561-019-0486-4
Zeldis J. R., Walters R. A., Greig M. J. N., and Image K. (2004). Circulation over the northeastern New Zealand continental slope, shelf and adjacent Hauraki Gulf, during spring and summer. Continental Shelf Res. 24, 543–561. doi: 10.1016/j.csr.2003.11.007
Zhang C., Chen Y., Thompson K., and Ren Y. (2016). Implementing a multispecies size-spectrum model in a data-poor ecosystem. Acta Oceanologica Sin. 35, 63–73. doi: 10.1007/s13131-016-0822-0
Zhang C., Chen Y., Xu B., Xue Y., and Ren Y. (2018). Sampling effects on the effectiveness of ecological indicators in detecting fishery-induced community changes. Can. J. Fisheries Aquat. Sci. 75, 1485–1494. doi: 10.1139/cjfas-2017-0199
Keywords: coastal fisheries, marine ecosystem, simulation, mizer, thermal refuge, marine heatwave
Citation: Beran H, Datta S, Smith RO, Woods L, Ladds M, Bell JJ and Rogers A (2025) Mesophotic thermal refuge modelling shows mitigated reductions in fisheries productivity during marine heatwaves. Front. Mar. Sci. 12:1540055. doi: 10.3389/fmars.2025.1540055
Received: 05 December 2024; Accepted: 20 May 2025;
Published: 06 June 2025.
Edited by:
Samuel Starko, University of Victoria, CanadaReviewed by:
Wei Cheng, University of Washington, United StatesChancey Macdonald, Newcastle University, United Kingdom
Copyright © 2025 Beran, Datta, Smith, Woods, Ladds, Bell and Rogers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alice Rogers, YWxpY2Uucm9nZXJzQHZ1dy5hYy5ueg==
 Hiromi Beran
Hiromi Beran Samik Datta2
Samik Datta2 Robert O. Smith
Robert O. Smith Alice Rogers
Alice Rogers